Attached files
| file | filename |
|---|---|
| 10-Q - 10-Q - Zentalis Pharmaceuticals, Inc. | zntl-20200630.htm |
| EX-32.2 - EX-32.2 - Zentalis Pharmaceuticals, Inc. | zentalis10-qq22020exhi1.htm |
| EX-32.1 - EX-32.1 - Zentalis Pharmaceuticals, Inc. | zentalis10-qq22020exhi.htm |
| EX-31.2 - EX-31.2 - Zentalis Pharmaceuticals, Inc. | zentalis10-qq22020exhi2.htm |
| EX-31.1 - EX-31.1 - Zentalis Pharmaceuticals, Inc. | zentalis10-qq22020exhi3.htm |

Exhibit 10.3 [***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. GREATER CHINA AMENDMENT TO THE SECOND AMENDED AND RESTATED LICENSE AGREEMENT between Recurium IP Holdings, LLC, and Zeno Management, Inc. Dated: May 19, 2020

[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. GREATER CHINA AMENDMENT TO THE SECOND AMENDED AND RESTATED LICENSE AGREEMENT THIS GREATER CHINA AMENDMENT TO THE SECOND AMENDED AND RESTATED LICENSE AGREEMENT (“Amendment”), dated May 19, 2020, by and between Recurium IP Holdings, LLC (f/k/a Zeno Royalties & Milestones, LLC), a Delaware Limited Liability Company (“LICENSOR”) and Zeno Management, Inc., a corporation organized and existing under the laws of Delaware (“LICENSEE”) and shall only become effective if and when Zentera Therapeutics (Cayman) Ltd. (“ZTCL”) Series A financing has its initial closing (the “Effective Date”). LICENSOR and LICENSEE may, from time-to-time, be individually referred to as a “Party” and collectively referred to as the “Parties”. RECITALS WHEREAS, ZIP Pharma, Inc. merged into LICENSEE, effective as of September 3, 2019; WHEREAS, LICENSOR and LICENSEE entered into that certain Second Amended and Restated License Agreement, dated September 6, 2019 (the “Agreement”); WHEREAS, LICENSEE entered into each of those certain Amended and Restated Sublicense Agreements with each of Zeno Alpha, Inc., Zeno Beta, Inc., K-Group Alpha, Inc. and K-Group Beta, Inc., each dated September 6, 2019, each as amended by that certain Greater China Amendment, dated as of the date hereof, (collectively, the “Sublicense Agreements”); WHEREAS, each of Zeno Alpha, Inc., K-Group Alpha, Inc. and K-Group Beta, Inc. entered into each of those certain Collaboration and License Agreements with ZTCL and Zeno Beta, Inc. entered into that certain Option Agreement for a Collaboration and License with ZTCL, each dated as of the date hereof (collectively, the “Greater China Sublicense Agreements”); WHEREAS, LICENSEE and ZTCL entered into that certain Option Agreement for Collaboration and License, dated as of the date hereof and (the “Greater China Option Agreement”); and WHEREAS, LICENSOR and LICENSEE desire to amend certain payment terms in the Agreement with respect to milestone, royalty and sublicensing fee payments to be made with respect to activities in the People’s Republic of China, Macau, Hong Kong, and Taiwan (collectively, “Greater China”). NOW, THEREFORE, in consideration of the mutual agreements and covenants set forth herein and other good and valuable consideration, the receipt and sufficiency of which the Parties hereby acknowledge, the Parties, intending to be legally bound hereby, agree to amend the Agreement as follows: AMENDMENT 1. Capitalized terms used but not defined herein will have the meaning ascribed to them in the Agreement. 2. First Amendment to the Agreement. Section 4.1.3 of the Agreement is hereby replaced in its entirety with the following:

[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. “4.1.3 Sublicense Fees. In consideration of the licenses and rights granted to LICENSEE hereunder, LICENSEE shall pay to LICENSOR the applicable percentage of all Third Party Fees payable from any of LICENSEE’s and/or its Affiliates’ sublicensees, assignees and other transferees (including without limitation the PRC Sublicensee but excluding any sublicensee, assignee or transferee that is an Affiliate of LICENSEE immediately following the applicable sublicense, assignment or transfer) (the “Sublicense Fees”) as set forth below. As used herein, “Third Party Fees” means any and all consideration in any form provided by sublicensees, assignees and other transferees (including without limitation the PRC Sublicensee) hereunder for rights under the Licensed Technology related to the Royalty & Milestone Products, excluding: (a) Royalties (which shall be subject to Section 4.1.2 above); (b) reimbursement of actual research and Development expenses for Royalty & Milestone Product; (c) manufacturing costs for the Royalty & Milestone Product; (d) payments for prosecution, enforcement or maintenance of any Licensed Technology; (e) Milestone Payments which are less than the Milestone Payments due to LICENSOR hereunder, if for achievement of the same Milestone event; and (f) any consideration received in connection with a Change in Control of LICENSEE and/or its Affiliates. LICENSEE shall pay all Sublicense Fees received during each Calendar Quarter within [***] following the expiration of each such Calendar Quarter. All payments shall be accompanied by a report that includes a calculation of all Sublicense Fees payable to LICENSOR for the applicable Calendar Quarter. Sublicense Fees Percentage by LICENSOR’s Product Family Equity Percentage of Third Party Fees By LICENSOR Ownership LICENSOR’s Product [***] [***] [***] Family Equity Percentage of Third [***] [***] [***] Party Fees For clarity, all sublicense fees due under the Agreement resulting from activity concerning each and every sublicensee, assignee and transferee of LICENSEE and/or its Affiliates anywhere in the Territory, including the sublicensees pursuant to the Greater China Sublicense Agreements, shall be determined pursuant to Section 4.1.3 as amended herein. 3. Second Amendment to Agreement. Article 14 of the Agreement is hereby replaced in its entirety with the following: “14. DISPUTE RESOLUTION/DAMAGES 14.1 General. Except for disputes for which injunctive or other equitable relief is sought to prevent the unauthorized use or disclosure of proprietary materials or information or prevent the infringement or misappropriation of a Party’s Intellectual Property Rights, the following procedures shall be used to resolve any dispute arising out of or in connection with this Agreement. 2

[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. 14.2 Meeting. Promptly after the written request of either Party, each of the Parties shall appoint a designated representative to meet in person or by telephone to attempt in good faith to resolve any dispute arising out of or resulting from this Agreement (“Dispute”). If such designated representatives do not resolve such Dispute within [***] of such written request, then the Executive Officer of each Party shall meet in person or by telephone to review and attempt to resolve such Dispute in good faith, and such Executive Officers shall have [***] to attempt to resolve such dispute (such total [***] the “Dispute Resolution Period”). If the Parties are unable to resolve a Dispute within a Dispute Resolution Period then such Dispute shall be resolved in accordance with Sections 14.3 and 14.4 or Section 14.5, as applicable. 14.3 Mediation. If the Parties are unable to resolve a Dispute (other than a Dispute subject to Section 14.5) within a Dispute Resolution Period in accordance with Section 14.2, then either Party may submit such Dispute (other than a Dispute subject to Section 14.5) for resolution by mediation pursuant to the Center for Public Resources Model Procedure for Mediation of Business Disputes as then in effect. The mediation shall be conducted in San Diego County, California. At the request of either Party, the mediator will be asked to provide an evaluation of the Dispute and the Parties’ relative positions. Each Party shall bear its own costs with respect to the mediation effort. The Parties shall have [***] to attempt to resolve the dispute through mediation. 14.4 Arbitration. 14.4.1. Any Disputes (other than a Disputes subject to Section 14.5) that are not resolved by the Parties in accordance with Section 14.2 and 14.3 shall be submitted to binding arbitration with the office of the American Arbitration Association (“AAA”) in San Diego County, California in accordance with the then-prevailing commercial arbitration rules of the American Arbitration Association. Such Dispute shall be heard by a panel of three (3) arbitrators appointed in accordance with such rules. 14.4.2. All such arbitration proceedings shall be held in English and a transcribed record shall be prepared in English. The Party submitting the Dispute to arbitration shall select the first of the three (3) arbitrators and shall provide notice of the same at the time it submits the Dispute to arbitration. The non-initiating Party shall then have [***] to select the second arbitrator. Thereafter, the first and second arbitrators shall have [***] to choose the third arbitrator. If no arbitrator is appointed within the times herein provided or any extension of time which is mutually agreed upon, the AAA shall make such appointment of the first two (2) arbitrators within [***] of such failure who shall thereafter pick the third as set forth herein. Each Party in any arbitration proceeding commenced hereunder shall initially bear such Party’s own costs and expenses (including expert witness and attorneys’ fees) of investigating, preparing and pursuing such arbitration claim. The fees and expenses of the arbitrators, will be shared equally by the Parties. Nothing in this Agreement shall be deemed as preventing either Party from seeking injunctive relief (or any other provisional remedy) from any court having jurisdiction over the Parties and the subject matter of the Dispute as necessary to protect either Party’s name, Confidential Information, Intellectual Property Rights or any other proprietary rights. If the Dispute involves scientific or technical matters, each arbitrator chosen hereunder shall have educational training and 3

[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. experience relevant to the field of pharmaceuticals. The award rendered by the arbitrators shall be written, final and non-appealable, and judgment upon the award rendered by the arbitrator may be entered in any court having jurisdiction thereof. The prevailing Party shall be entitled to recover from the losing Party the prevailing Party’s attorneys’ fees and costs. The arbitrator shall have the right to apportion liability between the Parties, but will not have the authority to award any damages or remedies not available under the express terms of this Agreement. The arbitration award will be presented to the Parties in writing, and upon the request of either Party, will include findings of fact and conclusions of law. The award may be confirmed and enforced in any court of competent jurisdiction. 14.5 Baseball Arbitration for Certain Sublicensing Fee Related Disputes. In the event of any Dispute arising under Section 4.1.3 (including if the Parties fail to agree on apportionment of the amount of proceeds that are and are not Sublicense Fees subject to Section 4.1.3), the Parties shall submit such Dispute to mediation and binding baseball arbitration pursuant to the mediation and baseball arbitration process set forth under this Section 14.5. The purpose of the mediation and baseball arbitration shall be to resolve only those issues that remain in dispute under Section 4.1.3 following good faith negotiations within a Dispute Resolution Period in accordance with Section 14.2. The mediation and baseball arbitration shall be conducted in San Diego County, California under the applicable AAA rules (except as modified by this Section 14.5 below) and the proceedings shall be held in English. Each Party shall bear its own costs with respect to the mediation and baseball arbitration proceedings and share the cost of the Third Party Expert (defined below). 14.5.1. Any Dispute arising under Section 4.1.3 that the Parties are unable to resolve within a Dispute Resolution Period in accordance with Section 14.2 shall, on the written request of either Party, be submitted to a Third Party expert (a “Third Party Expert”) mutually acceptable to the Parties having relevant expertise with respect to the Dispute and who is independent, conflict-of-interest-free, and not affiliated or consulting with either Party or its Affiliates, (or in the event that the Parties fail to agree on the selection of such Third Party Expert within [***] of the submission of such matter to resolution in accordance with this Section 14.5, by an appropriately qualified, independent, conflict-of-interest-free individual not affiliated or consulting with either Party or its Affiliates, and appointed by AAA). The Parties shall use reasonable efforts to mutually agree on the Third Party Expert within [***] after either Party designates the Dispute for resolution under this Section 14.5. The Third Party Expert shall initially attempt to resolve the Dispute through non-binding mediation. At the request of either Party, the mediator will be asked to provide an evaluation of the Dispute and the Parties’ relative positions. If the Third Party Expert is unable to resolve the Dispute through non-binding mediation within [***] of submission of such Dispute to mediation, the Dispute will, upon the written request of either Party, be resolved through Section 14.5.2. 14.5.2. Within [***] days of completion of non-binding mediation, each Party will deliver to both the Third Party Expert and the other Party a detailed written proposal setting forth its proposed terms for the resolution of the Dispute (the “Proposed Terms”) and a memorandum (the “Support Memorandum”) in support thereof, not exceeding [***] in length each. The Parties will also provide the Third Party Expert with a copy of this Agreement, as amended through such date. Within [***] 4

[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. after receipt of the other Party’s Proposed Terms and Support Memorandum, each Party may submit to the Third Party Expert (with a copy to the other Party) a response to the other Party’s Proposed Terms and Support Memorandum, such response not exceeding [***] in length. Neither Party may have any other communications (either written or oral) with the Third Party Expert; provided that the Third Party Expert may, in its discretion, convene a hearing to ask questions of the Parties and hear oral argument and discussion regarding each Party’s Proposed Terms and Support Memorandum and response to the other Party’s Proposed Terms and Support Memorandum, at which time each Party shall have an agreed upon time to argue and, if requested by the Third Party Expert, present witnesses in support of its Proposed Terms. 14.5.3. Within [***] after the Third Party Expert is appointed, the Third Party Expert shall select one of the two Proposed Terms (without modification) provided by the Parties which most closely reflects a commercially reasonable interpretation of the terms of this Agreement. In making its selection, (i) the Third Party Expert shall only have the authority to accept one or the other Party’s Proposed Terms and shall not modify the terms or conditions of either Party’s Proposed Terms nor shall the Third Party Expert combine provisions from both Proposed Terms and (ii) the Third Party Expert shall consider the terms and conditions of this Agreement, the relative merits of the Proposed Terms, the Support Memorandums and, if applicable, the oral arguments of the Parties. Subject to the foregoing, the Third Party Expert shall make its decision known to both Parties as promptly as possible by delivering written notice to both Parties. The decision of the Third Party Expert shall be final and binding on the Parties, and specific performance may be ordered by any court of competent jurisdiction. 14.6 Confidentiality of Disputes. The existence, content and/or results of any Dispute, as well as any mediation or arbitration proceedings conducted under this Section 14, shall be the Confidential Information of both Parties.” 4. Greater China Milestones and Royalties. For all Product Families sublicensed to ZTCL under the Greater China Sublicense Agreements or the Greater China Option Agreement, all milestone and royalty payments due under the Agreement resulting from activity anywhere in the Territory shall be determined pursuant to this Amendment, notwithstanding anything to the contrary in Sections 4.1.1 or 4.1.2 of the Agreement. 5. Any Milestone First Accrued Outside Greater China. For any Product Family that is sublicensed to ZTCL under the Greater China Sublicense Agreements or the Greater China Option Agreement, if a Milestone under Section 4.1.1 of the Agreement is achieved in the Territory outside of Greater China before it is achieved in Greater China, the corresponding Milestone Payment in Section 4.1.1 of the Agreement shall be due to LICENSOR and, if and when, the corresponding Greater China Milestone (as defined in Paragraph 7 of this Amendment) is achieved no Greater China Milestone Payment (as defined in Paragraph 7 of this Amendment) shall be due to LICENSOR. 6. Any Milestone First Accrued Inside Greater China. For any Product Family that is sublicensed to ZTCL under the Greater China Sublicense Agreements or the Greater China Option Agreement, if a Greater China Milestone under Paragraph 7 of this Amendment is achieved in Greater China before the corresponding Milestone is achieved in the Territory outside of Greater China, the Greater China Milestone Payment in Paragraph 7 of this Amendment shall be due to LICENSOR as set forth in 5
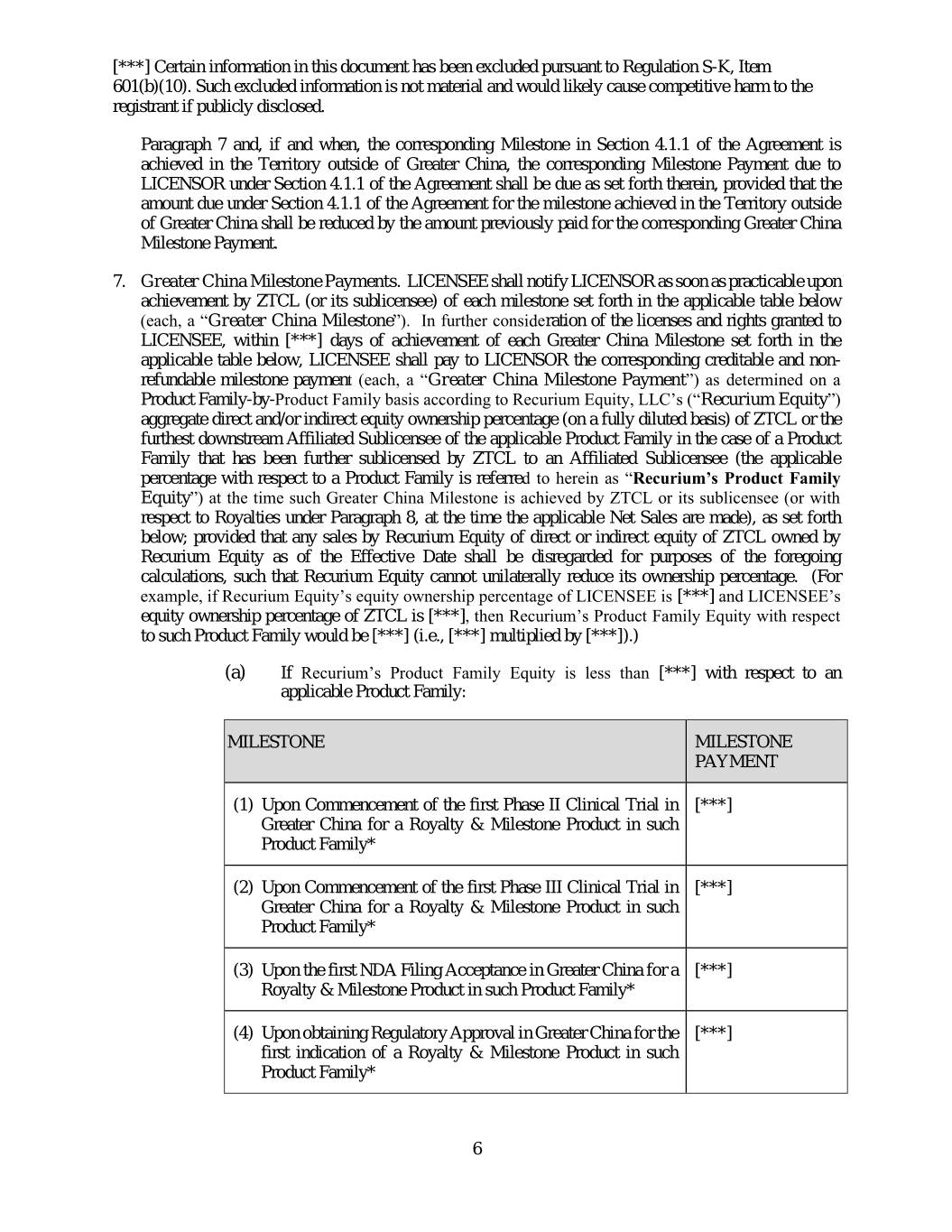
[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. Paragraph 7 and, if and when, the corresponding Milestone in Section 4.1.1 of the Agreement is achieved in the Territory outside of Greater China, the corresponding Milestone Payment due to LICENSOR under Section 4.1.1 of the Agreement shall be due as set forth therein, provided that the amount due under Section 4.1.1 of the Agreement for the milestone achieved in the Territory outside of Greater China shall be reduced by the amount previously paid for the corresponding Greater China Milestone Payment. 7. Greater China Milestone Payments. LICENSEE shall notify LICENSOR as soon as practicable upon achievement by ZTCL (or its sublicensee) of each milestone set forth in the applicable table below (each, a “Greater China Milestone”). In further consideration of the licenses and rights granted to LICENSEE, within [***] days of achievement of each Greater China Milestone set forth in the applicable table below, LICENSEE shall pay to LICENSOR the corresponding creditable and non- refundable milestone payment (each, a “Greater China Milestone Payment”) as determined on a Product Family-by-Product Family basis according to Recurium Equity, LLC’s (“Recurium Equity”) aggregate direct and/or indirect equity ownership percentage (on a fully diluted basis) of ZTCL or the furthest downstream Affiliated Sublicensee of the applicable Product Family in the case of a Product Family that has been further sublicensed by ZTCL to an Affiliated Sublicensee (the applicable percentage with respect to a Product Family is referred to herein as “Recurium’s Product Family Equity”) at the time such Greater China Milestone is achieved by ZTCL or its sublicensee (or with respect to Royalties under Paragraph 8, at the time the applicable Net Sales are made), as set forth below; provided that any sales by Recurium Equity of direct or indirect equity of ZTCL owned by Recurium Equity as of the Effective Date shall be disregarded for purposes of the foregoing calculations, such that Recurium Equity cannot unilaterally reduce its ownership percentage. (For example, if Recurium Equity’s equity ownership percentage of LICENSEE is [***] and LICENSEE’s equity ownership percentage of ZTCL is [***], then Recurium’s Product Family Equity with respect to such Product Family would be [***] (i.e., [***] multiplied by [***]).) (a) If Recurium’s Product Family Equity is less than [***] with respect to an applicable Product Family: MILESTONE MILESTONE PAYMENT (1) Upon Commencement of the first Phase II Clinical Trial in [***] Greater China for a Royalty & Milestone Product in such Product Family* (2) Upon Commencement of the first Phase III Clinical Trial in [***] Greater China for a Royalty & Milestone Product in such Product Family* (3) Upon the first NDA Filing Acceptance in Greater China for a [***] Royalty & Milestone Product in such Product Family* (4) Upon obtaining Regulatory Approval in Greater China for the [***] first indication of a Royalty & Milestone Product in such Product Family* 6
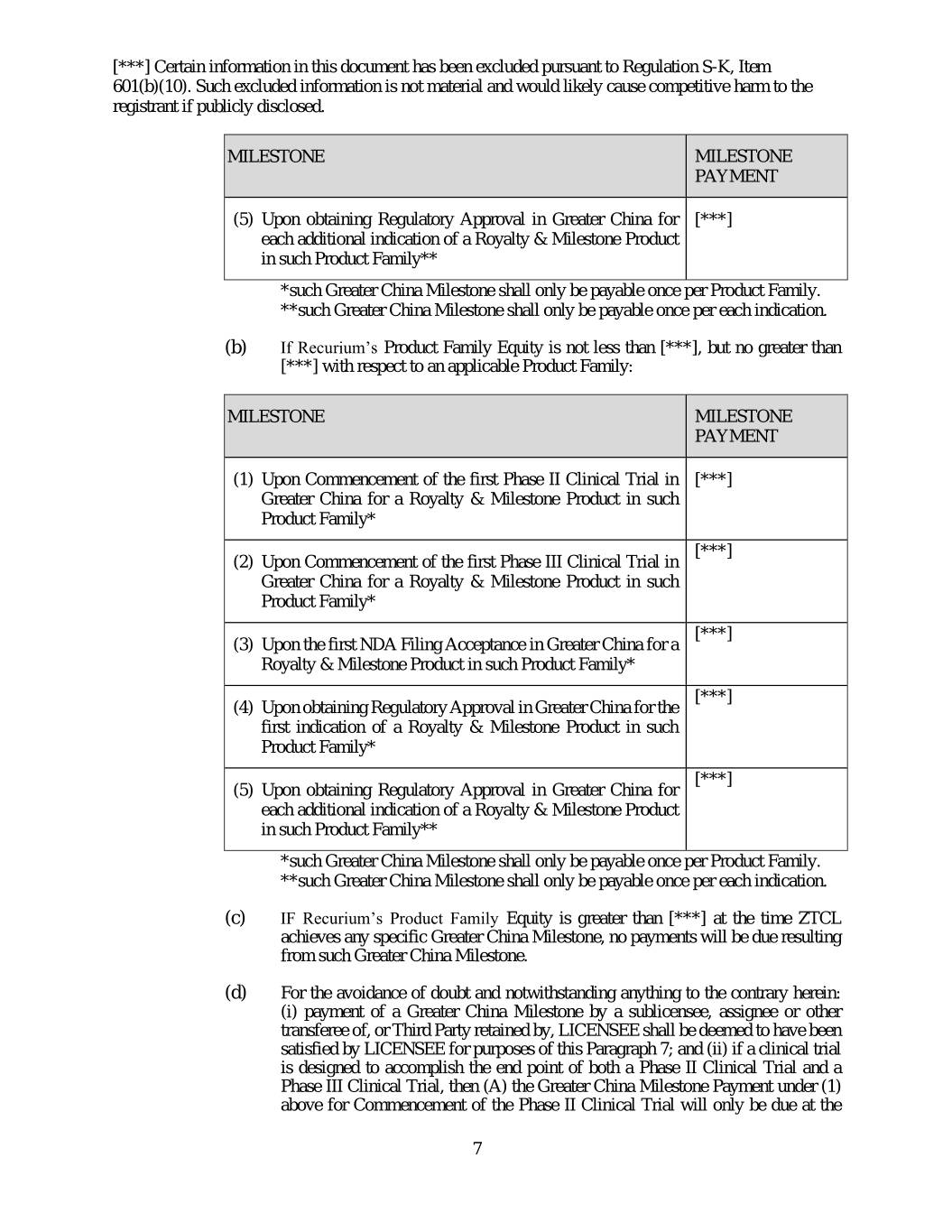
[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. MILESTONE MILESTONE PAYMENT (5) Upon obtaining Regulatory Approval in Greater China for [***] each additional indication of a Royalty & Milestone Product in such Product Family** *such Greater China Milestone shall only be payable once per Product Family. **such Greater China Milestone shall only be payable once per each indication. (b) If Recurium’s Product Family Equity is not less than [***], but no greater than [***] with respect to an applicable Product Family: MILESTONE MILESTONE PAYMENT (1) Upon Commencement of the first Phase II Clinical Trial in [***] Greater China for a Royalty & Milestone Product in such Product Family* [***] (2) Upon Commencement of the first Phase III Clinical Trial in Greater China for a Royalty & Milestone Product in such Product Family* [***] (3) Upon the first NDA Filing Acceptance in Greater China for a Royalty & Milestone Product in such Product Family* [***] (4) Upon obtaining Regulatory Approval in Greater China for the first indication of a Royalty & Milestone Product in such Product Family* [***] (5) Upon obtaining Regulatory Approval in Greater China for each additional indication of a Royalty & Milestone Product in such Product Family** *such Greater China Milestone shall only be payable once per Product Family. **such Greater China Milestone shall only be payable once per each indication. (c) IF Recurium’s Product Family Equity is greater than [***] at the time ZTCL achieves any specific Greater China Milestone, no payments will be due resulting from such Greater China Milestone. (d) For the avoidance of doubt and notwithstanding anything to the contrary herein: (i) payment of a Greater China Milestone by a sublicensee, assignee or other transferee of, or Third Party retained by, LICENSEE shall be deemed to have been satisfied by LICENSEE for purposes of this Paragraph 7; and (ii) if a clinical trial is designed to accomplish the end point of both a Phase II Clinical Trial and a Phase III Clinical Trial, then (A) the Greater China Milestone Payment under (1) above for Commencement of the Phase II Clinical Trial will only be due at the 7
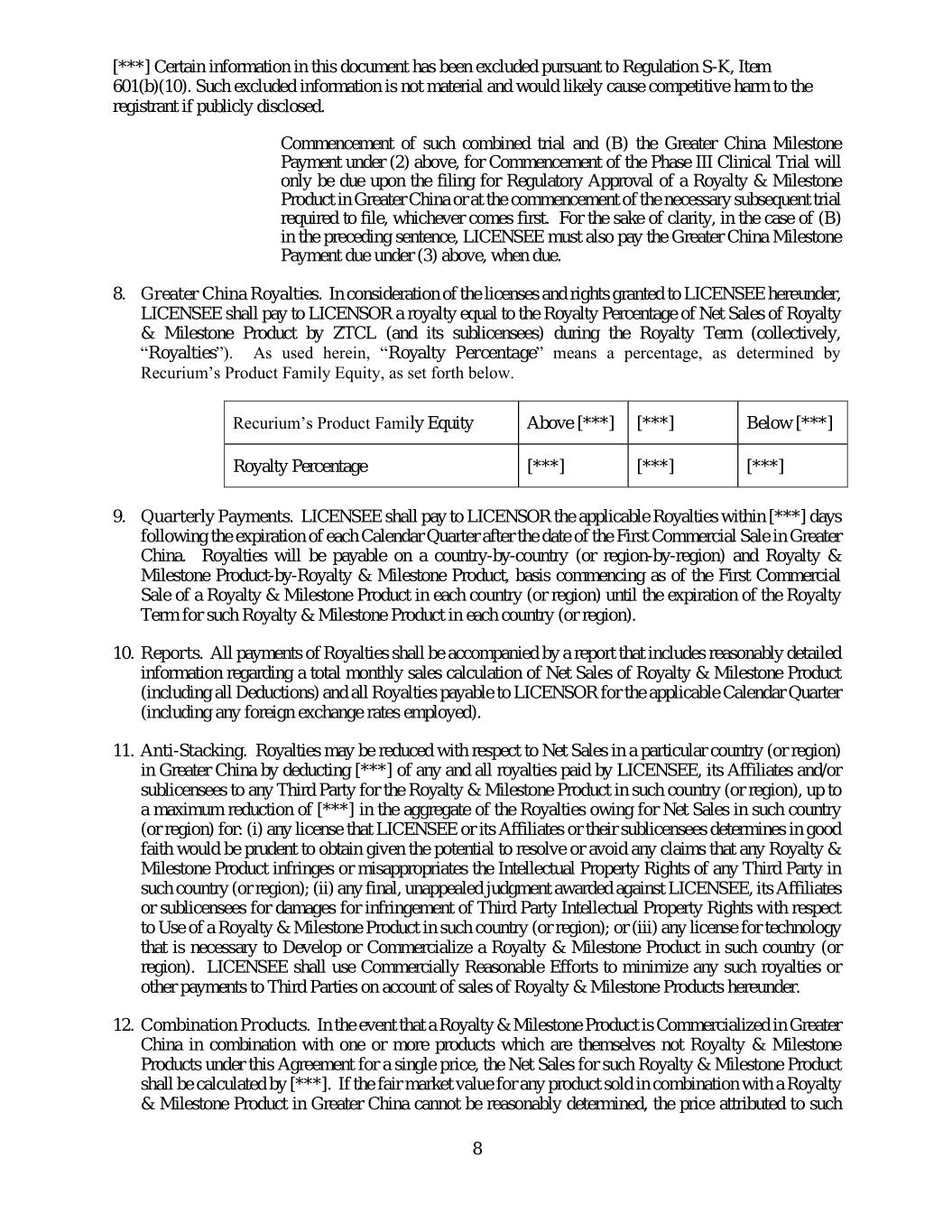
[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. Commencement of such combined trial and (B) the Greater China Milestone Payment under (2) above, for Commencement of the Phase III Clinical Trial will only be due upon the filing for Regulatory Approval of a Royalty & Milestone Product in Greater China or at the commencement of the necessary subsequent trial required to file, whichever comes first. For the sake of clarity, in the case of (B) in the preceding sentence, LICENSEE must also pay the Greater China Milestone Payment due under (3) above, when due. 8. Greater China Royalties. In consideration of the licenses and rights granted to LICENSEE hereunder, LICENSEE shall pay to LICENSOR a royalty equal to the Royalty Percentage of Net Sales of Royalty & Milestone Product by ZTCL (and its sublicensees) during the Royalty Term (collectively, “Royalties”). As used herein, “Royalty Percentage” means a percentage, as determined by Recurium’s Product Family Equity, as set forth below. Recurium’s Product Family Equity Above [***] [***] Below [***] Royalty Percentage [***] [***] [***] 9. Quarterly Payments. LICENSEE shall pay to LICENSOR the applicable Royalties within [***] days following the expiration of each Calendar Quarter after the date of the First Commercial Sale in Greater China. Royalties will be payable on a country-by-country (or region-by-region) and Royalty & Milestone Product-by-Royalty & Milestone Product, basis commencing as of the First Commercial Sale of a Royalty & Milestone Product in each country (or region) until the expiration of the Royalty Term for such Royalty & Milestone Product in each country (or region). 10. Reports. All payments of Royalties shall be accompanied by a report that includes reasonably detailed information regarding a total monthly sales calculation of Net Sales of Royalty & Milestone Product (including all Deductions) and all Royalties payable to LICENSOR for the applicable Calendar Quarter (including any foreign exchange rates employed). 11. Anti-Stacking. Royalties may be reduced with respect to Net Sales in a particular country (or region) in Greater China by deducting [***] of any and all royalties paid by LICENSEE, its Affiliates and/or sublicensees to any Third Party for the Royalty & Milestone Product in such country (or region), up to a maximum reduction of [***] in the aggregate of the Royalties owing for Net Sales in such country (or region) for: (i) any license that LICENSEE or its Affiliates or their sublicensees determines in good faith would be prudent to obtain given the potential to resolve or avoid any claims that any Royalty & Milestone Product infringes or misappropriates the Intellectual Property Rights of any Third Party in such country (or region); (ii) any final, unappealed judgment awarded against LICENSEE, its Affiliates or sublicensees for damages for infringement of Third Party Intellectual Property Rights with respect to Use of a Royalty & Milestone Product in such country (or region); or (iii) any license for technology that is necessary to Develop or Commercialize a Royalty & Milestone Product in such country (or region). LICENSEE shall use Commercially Reasonable Efforts to minimize any such royalties or other payments to Third Parties on account of sales of Royalty & Milestone Products hereunder. 12. Combination Products. In the event that a Royalty & Milestone Product is Commercialized in Greater China in combination with one or more products which are themselves not Royalty & Milestone Products under this Agreement for a single price, the Net Sales for such Royalty & Milestone Product shall be calculated by [***]. If the fair market value for any product sold in combination with a Royalty & Milestone Product in Greater China cannot be reasonably determined, the price attributed to such 8

[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed. product will be based on the relative cost of goods for such product, as determined in accordance with GAAP. In addition, in the event that a Royalty & Milestone Product is sold in Greater China with any other product(s) or if any giveaways, discounts, rebates or charge-backs (whether as part of a customer loyalty, bundling or “loss leader” program, or otherwise) are provided for a Royalty & Milestone Product to promote or sell other products or otherwise, the Net Sales for such Royalty & Milestone Product shall be no less than the fair market value of such Royalty & Milestone Product on a stand- alone basis (excluding any such discounts, rebates or charge-backs). 13. Downstream Sublicense Fee Disputes. LICENSOR hereby acknowledges and agrees that, in the event that a dispute arises concerning Sublicense Fees under a. any Sublicense Agreements, it acknowledges and agrees that to the extent it participates in any such dispute brought pursuant to Section 14.5 of the applicable Sublicense Agreements, (i) it will comply with the provisions of Sections 14.5 and 14.6 of the applicable Sublicense Agreement and (ii) it will be bound by any binding baseball arbitration proceeding brought pursuant to Section 14.5 of the applicable Sublicense Agreement; or b. any of the Greater China Sublicense Agreements or the Greater China Option Agreement, it acknowledges and agrees that to the extent it participates in any such dispute brought pursuant to Section 16.5 of the applicable Greater China Sublicense Agreements or the Greater China Option Agreement, (i) it will comply with the provisions of Sections 16.5 and 16.6 of the applicable agreement and (ii) it will be bound by any binding baseball arbitration proceeding brought pursuant to Section 16.5 of the applicable Greater China Sublicense Agreements or the Greater China Option Agreement. 14. General Provisions. Article 15 of the Agreement is incorporated herein by reference in its entirety. [Signatures on next page] 9

IN WITNESS WHEREOF, the parties have duly executed this Amendment as of the Effective Date. LICENSOR: LICENSEE: RECURIUM IP HOLDINGS, LLC ZENO MANAGEMENT, INC. By: /s/ Ned A. Israelsen By: /s/ Anthony Y. Sun, M.D. Ned A. Israelsen Anthony Y. Sun, M.D.
