Attached files
| file | filename |
|---|---|
| EX-23.1 - OPINION OF CENTURION ZD CPA & CO. - 4DMed, Ltd. | ex23-1.htm |
| EX-5.1 - LEGAL OPINION AND CONSENT OF LANE & ASSOCIATES, P.A. - 4DMed, Ltd. | ex5-1.htm |
| EX-3.2 - BYLAWS - 4DMed, Ltd. | ex3-2.htm |
| EX-3.1 - CERTIFICATE OF INCORPORATION - 4DMed, Ltd. | ex3-1.htm |
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
| 4DMED, LTD. |
| (Name of registrant as specified in its charter) |
| Nevada | 8099 | 82-2523472 | ||
| (State
or jurisdiction of incorporation or organization) |
(Primary
Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
| 11/F Capital Centre, |
| 151 Gloucester Road, Wanchai, |
| Hong Kong |
| (852) 93841733 |
| (Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices) |
| Resident Agency National |
| 4650 Wedekind Road, #2 |
| Sparks, Nevada 89431 |
| (775) 882-7549 |
| With a copy to: |
| Lane & Associates, P.A. |
| c/o Paul Camp Lane |
| 5401 S. Kirkman Road |
| Suite 310 |
| Orlando, FL 32819 |
| (407) 316-0343 |
| (Name, address, including zip code, and telephone number, including area code, of agent for service) |
Approximate date of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | o | Accelerated filer | o |
| Non-accelerated filer | o (Do not check if a smaller reporting company) | Smaller reporting company | x |
| Emerging growth company | x | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. x
| Title of Each Class of Securities to be Registered | Amount to be Registered | Proposed Maximum Offering Price per Security(1) ($) | Proposed Maximum Aggregate Offering Price(1) ($) | Amount of Registration Fee ($) | ||||||||||||
| Selling Shareholders | 400,000 | $ | 1.00 | $ | 400,000 | $ | 51.92 | |||||||||
| (1) | Estimated solely for purposes of calculating the registration fee in accordance with Rule 457 of the Securities Act of 1933. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said section 8(a), may determine.
1
The information in this prospectus is not complete and may be amended. The Registrant may not sell these securities until the Registration Statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION DATED ___________, 2019
PRELIMINARY PROSPECTUS
4DMED,
LTD.
400,000 SHARES OF COMMON STOCK
OFFERING PRICE $1.00 PER SHARE
This prospectus relates to the offering (the Offering”) of 400,000 shares of our common stock by our Selling Shareholders at a fixed offering price of $1.00 per share until our shares are quoted on the Over-the-Counter Bulletin Board, and thereafter at prevailing market prices or privately negotiated prices. We will pay all expenses incurred in this Offering (other than transfer taxes), and the Selling Shareholders will receive all of the net proceeds from this Offering.
AN INVESTMENT IN OUR SECURITIES IS SPECULATIVE. INVESTORS SHOULD BE ABLE TO AFFORD THE LOSS OF THEIR ENTIRE INVESTMENT. YOU SHOULD CAREFULLY CONSIDER THE FACTORS DESCRIBED UNDER THE HEADING “RISK FACTORS” BEGINNING ON PAGE 6 BEFORE INVESTING IN OUR COMMON STOCK.
Prior to this Offering, there has been no public market for our common stock and we have not applied for the listing or quotation of our common stock on any public market. We have arbitrarily determined the offering price of $1.00 per share in relation to this Offering. The offering price bears no relationship to our assets, book value, earnings or any other customary investment criteria. After the effective date of the registration statement, we intend to seek a market maker to file an application with the Financial Industry Regulatory Authority (“FINRA”) to have our common stock quoted on the OTC Bulletin Board. We may never be approved for trading on any exchange. We currently have no market maker who is willing to list quotations for our stock. There is no assurance that an active trading market for our shares will develop or will be sustained if developed.
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this Prospectus. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common shares.
The Company is an emerging growth company under the Jumpstart Our Business Startups Act.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is _______, 2020
2
TABLE OF CONTENTS
The following table of contents has been designed to help you find information contained in this prospectus. We encourage you to read the entire prospectus.
3
This Prospectus and any supplement to this Prospectus include “forward-looking statements”. To the extent that the information presented in this Prospectus discusses financial projections, information or expectations about our business plans, results of operations, products or markets, or otherwise makes statements about future events, such statements are forward-looking. Such forward-looking statements can be identified by the use of words such as “intends”, “anticipates”, “believes”, “estimates”, “projects”, “forecasts”, “expects”, “plans” and “proposes”. Although we believe that the expectations reflected in these forward-looking statements are based on reasonable assumptions, there are a number of risks and uncertainties that could cause actual results to differ materially from such forward-looking statements. These include, among others, the cautionary statements in the “Risk Factors” section beginning on page 6 of this Prospectus and the “Management’s Discussion and Analysis of Financial Position and Results of Operations” section beginning on page 28 of this Prospectus.
This summary only highlights selected information contained in greater detail elsewhere in this Prospectus. This summary does not contain all of the information that you should consider before investing in our common stock. You should carefully read the entire Prospectus, including “Risk Factors” beginning on page 6, and the consolidated financial statements, before making an investment decision.
Corporate Background and Business Overview
4DMed, Ltd. (“4DMed”, the “Company” “we” or “us”) is a development stage company. We were incorporated under the laws of the state of Nevada on November, 2016. We are a medical software and medical device development company that intends to design, patent, and market a medical virtual reality operating system with integrated, customary haptic and position sensing interfaces for the health care education research, software and medical device markets.
Our Hong Kong business office is located at 11/F, Capital Centre, 151 Gloucester Road, Wanchai, Hong Kong. This office is provided to us by our Chief Executive Officer, President and Director, Yuen May Cheung, at no cost to our Company. Our telephone number is (852) 93841733.
We have one (1) executive officer, Yuen May Cheung, our Chief Executive Officer and President, Chief Financial Officer and Secretary. Our two (2) Directors are Yuen May Cheung and Hau Tin Cheung.
We are a development stage company that has generated no revenues and has had limited operations to date. At June 30, 2020, the Company had not yet recorded any revenues, has working capital of $132,969 and has an accumulated deficit of $3,676,041. As of June 30, 2020, we had $4,319,153 in current assets and current liabilities of $35,581. Through June 30, 2020, we have issued an aggregate of 25,250,703 shares of our common stock since our inception. We issued 21,000,000 shares of our common stock to one (1) founding shareholder for services. We issued a total of 4,250,703 shares to 188 separate accredited foreign shareholders from July 20, 2017 through August 5, 2020, pursuant to private placements of our common stock exempt from registration under Regulation S of the Securities Act of 1933, for total proceeds of approximately $7,508,324.26. Since our inception we have not made any significant purchase or sale of assets, nor have we been involved in any mergers, acquisitions or consolidations.
Due to the uncertainty of our ability to meet our current operating and capital expenses, our independent auditors have included a going concern opinion in their report on our audited financial statements for the period December 31, 2019. The notes to our financial statements contain additional disclosure describing the circumstances leading to the issuance of a going concern opinion by our auditors.
Implications of Being an Emerging Growth Company
As a company with less than $1 billion in revenue in our last fiscal year, we are defined as an “emerging growth company” under the Jumpstart Our Business Startups (“JOBS”) Act. We will retain “emerging growth company” status until the earliest of:
| ● | The last day of the fiscal year during which our annual revenues are equal to or exceed $1 billion; |
| ● | The last day of the fiscal year following the fifth anniversary of our first sale of common stock pursuant to a registration statement filed under the Securities Act of 1933, as amended, which we refer to in this document as the Securities Act; |
| ● | The date on which we have issued more than $1 billion in nonconvertible debt in a previous three-year period; or |
| ● | The date on which we qualify as a large accelerated filer under Rule 12b-2 adopted under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) (i.e., an issuer with a public float of $700 million that has been filing reports with the U.S. Securities and Exchange Commission (“SEC”) under the Exchange Act for at least 12 months). |
4
As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to SEC reporting companies. For so long as we remain an emerging growth company we will not be required to:
| ● | have an auditor report on our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Wall Street Reform and Consumer Protection Act of 2002; |
| ● | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); |
| ● | submit certain executive compensation matters to stockholder non-binding advisory votes; |
| ● | submit for stockholder approval golden parachute payments not previously approved; |
| ● | disclose certain executive compensation related items, as we will be subject to the scaled disclosure requirements of a smaller reporting company with respect to executive compensation disclosure; and |
| ● | present more than two years of audited financial statements and two years of selected financial data in this registration statement and future filings, instead of the customary three years for audited financial statements and five years for selected financial data. |
We have not elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2) of The JOBS Act.
Because the worldwide market value of our common stock held by non-affiliates, or public float, is below $75 million, we are also a “smaller reporting company” as defined under the Exchange Act. Some of the foregoing reduced disclosure and other requirements are also available to us because we are a smaller reporting company and may continue to be available to us even after we are no longer an emerging growth company under the JOBS Act but remain a smaller reporting company under the Exchange Act. As a smaller reporting company we are not required to:
| ● | have an auditor report on our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; and |
| ● | present more than two years of audited financial statements in our registration statements and annual reports on Form 10-K and present any selected financial data in such registration statements and annual reports. |
Summary of the Offering
| Shares of common stock being offered by the Registrant: | 400,000 shares (the “Maximum Offering”) of the Selling Shareholder’s common stock. | |
| Offering price: | $1.00 per share of common stock. | |
| Number of shares outstanding before the Offering: | As of August 5, 2020, we had 25,250,703 shares of our common stock issued and outstanding, and no issued and outstanding convertible securities. | |
| Number of shares outstanding after the Offering | 25,250,703 if all of the shares being offered are sold | |
| Market for the common stock: | There is no public market for our common stock. After the effective date of the registration statement of which this prospectus is a part, we intend to seek a market maker to file an application on our behalf to have our common stock quoted on the Over-the-Counter Bulletin Board. We may never be approved for trading on any exchange. We currently have no market maker who is willing to list quotations for our stock. There is no assurance that a trading market for our stock will develop be sustained if developed | |
| Use of Proceeds: | No proceeds to the Company. | |
| Risk Factors: | See the “Risk Factors” beginning on page 6 and the other information in this prospectus for a discussion of the factors you should consider before deciding to invest in shares of our common stock. | |
| Dividend Policy: | We have not declared or paid any dividends on our common stock since our inception, and we do not anticipate paying any such dividends for the foreseeable future. |
5
Summary Financial Data
The following summary financial information for the period from January 1, 2019, through June 30, 2020, includes statement of expenses and balance sheet data from our audited financial statements. The information contained in this table should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operation” and the financial statements and accompanying notes included in this prospectus.
Statement of Operations
| Period from January 1, 2019 To December 31, 2019 | Six Months Ended June 30, 2020 (Unaudited) | |||||||
| Revenues | $ | 0 | 0 | |||||
| Total expenses | $ | (2,336,135 | ) | $ | (799,080 | ) | ||
| Net loss | $ | (2,336,135 | ) | $ | (799,080 | ) | ||
Balance Sheet Data
| June 30, 2020 (Unaudited) | ||||
| Total assets | $ | 4,319,153 | ||
| Total liabilities | $ | 35,581 | ||
| Total liabilities and stockholders’ equity (deficit) | $ | 4,319.153 | ||
An investment in our common stock involves a high degree of risk. You should carefully consider the following risk factors and other information in this prospectus before deciding to invest in our Company. If any of the following risks actually occur, our business, financial condition, results of operations and prospects for growth could be seriously harmed. As a result, the trading price of our common stock could decline and you could lose all or part of your investment.
Risks Relating to Our Business
There is uncertainty regarding our ability to continue as a going concern, indicating the possibility that we may be required to curtail or discontinue our operations in the future. If we discontinue our operations, you may lose all of your investment.
We have incurred a net loss of ($799,080) for the six months ended June 30, 2020, and an accumulated deficit of $2,876,961 as of June 30, 2020, and have completed only the preliminary stages of our business plan. We anticipate incurring additional losses before realizing any revenues and will depend on additional financing in order to meet our continuing obligations and ultimately, to attain profitability. The financial statements do not include any adjustments that might result from the uncertainty about our ability to continue our business. If we are unable to obtain additional financing from outside sources and eventually produce enough revenues, we may be forced to sell our assets, or curtail or discontinue our operations. If this happens, you could lose all or part of your investment.
We are in an early stage of development. If we are not able to develop our business as anticipated, we may not be able to generate revenues or achieve profitability and you may lose your investment.
We were incorporated on November 2, 2016. We are in an early stage of development and have not earned any revenues to date. Our business prospects are difficult to predict because of our limited operating history, early stage of development, and unproven business strategy. For the six month period ending June 30, 2020, we have incurred accumulated net losses of ($799,080). We expect to continue to incur operating losses for the foreseeable future, and such losses may be substantial. Given our history of operating losses, we cannot assure you that we will be able to achieve or maintain operating profitability on an annual or quarterly basis or at all. If we are unable to execute our business plan as anticipated, we may not be able to generate revenues or achieve profitability and you may lose your investment.
6
We expect to suffer losses in the immediate future that may cause us to curtail or discontinue our operations.
We expect to incur operating losses in future periods. These losses will occur because we do not yet have any revenues to offset the expenses associated with the development of our business operations, generally. We cannot guarantee that we will ever be successful in generating revenues in the future. We recognize that if we are unable to generate revenues, we will not be able to earn profits or continue operations. There is no history upon which to base any assumption as to the likelihood that we will prove successful, and we can provide investors with no assurance that we will generate any operating revenues or ever achieve profitable operations. If we are unsuccessful in addressing these risks, our business will almost certainly fail.
We have limited sales and marketing experience, which increases the risk that our business will fail.
Our officers, who will be responsible for marketing, have only nominal sales and marketing experience. There can be no assurance that our efforts will be successful. Further, if our initial efforts to create a market are not successful, there can be no assurance that we will be able to attract and retain qualified individuals with marketing and sales expertise to attract customer. Our future success will depend, among other factors, upon whether our products and services can be sold at a profitable price and the extent to which consumers acquire, adopt, and continue to use them.
We may not be able to execute our business plan or stay in business without additional funding.
Our ability to generate future operating revenues depends in part on whether we can obtain the financing necessary to implement our business plan. We will likely require additional financing through the issuance of debt and/or equity in order to establish profitable operations, and such financing may not be forthcoming. As widely reported, the global and domestic financial markets have been extremely volatile in recent months. If such conditions and constraints continue or if there is no investor appetite to finance our specific business, we may not be able to acquire additional financing through credit markets or equity markets. Even if additional financing is available, it may not be available on terms favorable to us. At this time, we have not identified or secured sources of additional financing. Our failure to secure additional financing when it becomes required will have an adverse effect on our ability to remain in business.
If our estimates related to future expenditures are erroneous or inaccurate, our business will fail and you could lose your entire investment.
Our success is dependent in part upon the accuracy of our management’s estimates of our future cost expenditures for legal and accounting services (including those we expect to incur as a publicly reporting company), for research and development, construction, marketing, and for administrative expenses over the next twelve (12) months. If such estimates are erroneous or inaccurate, or if we encounter unforeseen costs, we may not be able to carry out our business plan, which could result in the failure of our business and the loss of your entire investment.
Our auditor has raised substantial doubts about our ability to continue as a going concern and if we are unable to continue our business, our shares may have little or no value.
Our ability to become a profitable operating company is dependent upon our ability to generate revenues and/or obtain financing adequate to fulfill its research and market introduction activities, and achieving a level of revenues adequate to support our cost structure has raised substantial doubts about our ability to continue as a going concern. We plan to attempt to raise additional equity capital by selling shares, if necessary, through one or more private placement or public offerings. However, the doubts raised, relating to our ability to continue as a going concern, may make our shares an unattractive investment for potential investors. These factors, among others, may make it difficult to raise any additional capital.
We are a small company with limited resources relative to our competitors and we may not be able to compete effectively.
Our competitors have longer operating histories, greater resources and name recognition, and a larger base of customers than we have. As a result, these competitors will have greater credibility with our potential customers. They also may be able to adopt more aggressive pricing policies and devote greater resources to the development, promotion, and sale of their services than we may be able to devote to our services. Therefore, we may not be able to compete effectively and our business may fail.
7
The loss of the services of any of our officers or our failure to timely identify and retain competent personnel could negatively impact our ability to develop our website and sell our services.
The development of our business will continue to place a significant strain on our limited personnel, management, and other resources. Our future success depends upon the continued services of our executive officer, Yuen May Cheung, our Chief Executive Officer, President, and Chief Financial Officer, who is developing our business, and on our ability to identify and retain competent consultants and employees with the skills required to execute our business objectives. The loss of the services of any of our officers or our failure to timely identify and retain competent personnel could negatively impact our ability to develop our website and sell our services, which could adversely affect our financial results and impair our growth.
Our Chief Executive Officer and Chief Financial Officer has conflicts of interest in that she has other time commitments that will prevent her from devoting full-time to our operations, which may affect our operations.
Because our Chief Executive Officer and Chief Financial Officer, Yuen May Cheung, only devote approximately fifty percent (50%) of her full working time to operation and management of us, the implementation of our business plans may be impeded. Ms. Cheung has limited time in which to devote to our operations, which may slow our operations and may reduce our financial results and as a result, we may not be able to continue with our operations. Additionally, if Ms. Cheung became unable to handle her daily duties of the Chief Executive Officer and Chief Financial Officer on her own, we may not be able to hire additional qualified personnel to replace her in a timely manner. If this event should occur, we may not be able to reach profitability, which might result in the loss of some or all of your investment in our common stock.
We are subject to financing and interest rate exposure risks.
Our future success depends on our ability to access capital markets and obtain financing on reasonable terms. Our ability to access financial markets and obtain financing on commercially reasonable terms in the future is dependent on a number of factors, many of which we cannot control, including changes in:
| ● | our credit rating; |
| ● | interest rates; |
| ● | the structured and commercial financial markets; |
| ● | market perceptions of us and the oil and natural gas exploration and production industry; and |
| ● | tax burdens due to new tax laws. |
Any amounts due under our credit facility will bear interest at a variable rate. Any increases in our interest rates, or our inability to access the debt or equity markets on reasonable terms, could have an adverse impact on our financial condition, results of operations and growth prospects.
Risks Relating to Our Common Stock
There is no active trading market for our common stock and if a market for our common stock does not develop, our investors will be unable to sell their shares.
There has been no public market for our securities and there can be no assurance that an active trading market for the securities offered herein will develop or be sustained after this Offering. After the effective date of the registration statement of which this prospectus is a part, we intend to identify a market maker to file an application with the Financial Industry Regulatory Authority (“FINRA”) to have our common stock quoted on the Over-the-Counter Bulletin Board. We must satisfy certain criteria in order for our application to be accepted. We may never be approved for trading on any exchange. We do not currently have a market maker willing to participate in this application process, and even if we identify a market maker, there can be no assurance as to whether we will meet the requisite criteria or that our application will be accepted. Our common stock may never be quoted on the Over-the-Counter Bulletin Board or a public market for our common stock may not materialize if it becomes quoted.
If our securities are not eligible for initial or continued quotation on the Over-the-Counter Bulletin Board or if a public trading market does not develop, purchasers of the common stock in this Offering may have difficulty selling or be unable to sell their securities should they desire to do so, rendering their shares effectively worthless and resulting in a complete loss of their investment.
If we do not file a Registration Statement on Form 8-A to become a mandatory reporting company under Section 12(g) of the Securities Exchange Act of 1934, we will continue as a reporting company and will not be subject to the proxy statement requirements, and our officers, directors and 10% stockholders will not be required to submit reports to the SEC on their stock ownership and stock trading activity, all of which could reduce the value of your investment and the amount of publicly available information about us.
As a result of this Offering as required under Section 15(d) of the Securities Exchange Act of 1934, we will file periodic reports with the Securities and Exchange Commission once this registration statement is declared effective. Once this registration statement is declared effective, we intend voluntarily to file a registration statement on Form 8-A which will subject us to all of the reporting requirements of the 1934 Act. This will require us to file quarterly and annual reports with the SEC and will also subject us to the proxy rules of the SEC. In addition, our officers, directors and 10% stockholders will be required to submit reports to the SEC on their stock ownership and stock trading activity. We are not required under Section 12(g) or otherwise to become a mandatory 1934 Act filer unless we have more than 500 shareholders and total assets of more than $10 million on March 31, 2020. If we do not file a registration statement on Form 8-A, we will continue as a reporting company and will not be subject to the proxy statement requirements of the 1934 Act, and our officers, directors and 10% stockholders will not be required to submit reports to the SEC on their stock ownership and stock trading activity.
8
Because we will be subject to “penny stock” rules once our shares are quoted on the Over-the-Counter Bulletin Board, the level of trading activity in our stock may be reduced.
Broker-dealer practices in connection with transactions in “penny stocks” are regulated by penny stock rules adopted by the Securities and Exchange Commission. Penny stocks generally are equity securities with a price of less than $5.00 (other than securities registered on some national securities exchanges). The penny stock rules require a broker-dealer to deliver to its customers a standardized risk disclosure document that provides information about penny stocks and the nature and level of risks in the penny stock market prior to carrying out a transaction in a penny stock not otherwise exempt from the rules,. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and, if the broker-dealer is the sole market maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market, and monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, broker-dealers who sell these securities to persons other than established customers and “accredited investors” must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. Consequently, these requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security subject to the penny stock rules. If a trading market does develop for our common stock, these regulations will likely be applicable, and investors in our common stock may find it difficult to sell their shares.
Financial Industry Regulatory Authority (FINRA) sales practice requirements may also limit your ability to buy and sell our stock, which could depress our share price.
FINRA rules require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares, depressing our share price.
State securities laws may limit secondary trading, which may restrict the states in which you can sell the shares offered by this prospectus.
If you purchase shares of our common stock sold pursuant to this Offering, you may not be able to resell the shares in a certain state unless and until the shares of our common stock are qualified for secondary trading under the applicable securities laws of such state or there is confirmation that an exemption, such as listing in certain recognized securities manuals, is available for secondary trading in such state. There can be no assurance that we will be successful in registering or qualifying our common stock for secondary trading, or identifying an available exemption for secondary trading in our common stock in every state. If we fail to register or qualify, or to obtain or verify an exemption for the secondary trading of, our common stock in any particular state, the shares of common stock could not be offered or sold to, or purchased by, a resident of that state. In the event that a significant number of states refuse to permit secondary trading in our common stock, the market for the common stock will be limited which could drive down the market price of our common stock and reduce the liquidity of the shares of our common stock and a stockholder’s ability to resell shares of our common stock at all or at current market prices, which could increase a stockholder’s risk of losing some or all of his investment.
Our Company has a concentration of stock ownership and control, which may have the effect of delaying, preventing, or deterring a change of control.
Our common stock ownership is highly concentrated. Through ownership of shares of our common stock, three shareholders, Yuen May Cheung, who is also our Chief Executive Officer, Chief Financial Officer and Director, beneficially owns approximately 83.2% of our total outstanding shares of common stock before this Offering. As a result of the concentrated ownership of the stock, Ms. Cheung, who owns 21,000,000 shares of our common stock, acting alone, will be able to control all matters requiring stockholder approval, including the election of directors and approval of mergers and other significant corporate transactions. This concentration of ownership may have the effect of delaying, preventing or deterring a change in control of our Company. It could also deprive our stockholders of an opportunity to receive a premium for their shares as part of a sale of our company and it may affect the market price of our common stock. Moreover, such concentration of our common stock in Ms. Cheung could create potential or actual conflicts of interests detrimental to our stockholders and have a negative impact on our ability to maintain effective internal controls.
9
If quoted, the price of our common stock may be volatile, which may substantially increase the risk that you may not be able to sell your shares at or above the price that you may pay for the shares.
Even if our shares are quoted for trading on the Over-the-Counter Bulletin Board following this Offering and a public market develops for our common stock, the market price of our common stock may be volatile. It may fluctuate significantly in response to the following factors:
| ● | variations in quarterly operating results; |
| ● | our announcements of significant contracts and achievement of milestones; |
| ● | our relationships with other companies or capital commitments; |
| ● | additions or departures of key personnel; |
| ● | sales of common stock or termination of stock transfer restrictions; |
| ● | changes in financial estimates by securities analysts, if any; and |
| ● | fluctuations in stock market price and volume. |
Your inability to sell your shares during a decline in the price of our stock may increase losses that you may suffer as a result of your investment.
The stock market has experienced extreme price and volume fluctuations and if we face a class action suit due to the volatility of the price of our common stock, regardless of the outcome, such litigation may have an adverse impact on our financial condition and business operations.
The market price of the securities offered herein, if any, may decline below the initial public offering price. The stock market has experienced extreme price and volume fluctuations. In the past, securities class action litigation has often been instituted against various companies following periods of volatility in the market price of their securities. If instituted against us, regardless of the outcome, such litigation would result in substantial costs and a diversion of management’s attention and resources, which would increase our operating expenses and affect our financial condition and business operations.
Because we do not intend to pay any dividends on our common stock; holders of our common stock must rely on stock appreciation for any return on their investment.
We have not declared or paid any dividends on our common stock since our inception, and we do not anticipate paying any such dividends for the foreseeable future. Accordingly, holders of our common stock will have to rely on capital appreciation, if any, to earn a return on their investment in our common stock.
Any future additional issuances of our common stock may result in immediate dilution to existing shareholders.
We are authorized to issue up to 75,000,000 shares of common stock, of which 25,250,703 shares are issued and outstanding as of the date of this prospectus. Our Board of Directors has the authority, without the consent of any of our stockholders, to cause us to issue additional shares of common stock, and to determine the rights, preferences and privileges attached to such shares. The sale of our common stock pursuant to this prospectus, and any future additional issuances of our common stock will result in immediate dilution to our existing shareholders’ interests, which may have a dilutive impact on our existing shareholders, and could negatively affect the value of your shares.
We have not voluntarily implemented various corporate governance measures, in the absence of which, shareholders may have more limited protections against interested director transactions, conflicts of interest and similar matters.
Recent federal legislation, including the Sarbanes-Oxley Act of 2002, has resulted in the adoption of various corporate governance measures designed to promote the integrity of the corporate management and the securities markets. Some of these measures have been adopted in response to legal requirements; others have been adopted by companies in response to the requirements of national securities exchanges, such as the NYSE or the NASDAQ Stock Market, on which their securities are listed. Among the corporate governance measures that are required under the rules of national securities exchanges and NASDAQ, are those that address the board of Directors independence, audit committee oversight, and the adoption of a code of ethics. While our Board of Directors has adopted a Code of Ethics and Business Conduct, we have not yet adopted any of these corporate governance measures, and since our securities are not listed on a national securities exchange or NASDAQ, we are not required to do so. It is possible that if we were to adopt some or all of these corporate governance measures, shareholders would benefit from somewhat greater assurances that internal corporate decisions were being made by disinterested directors and that policies had been implemented to define responsible conduct. For example, in the absence of audit, nominating and compensation committees comprised of at least a majority of independent directors, decisions concerning matters such as compensation packages to our senior officers and recommendations for director nominees, may be made by a majority of directors who have an interest in the outcome of the matters being decided. Prospective investors should bear in mind our current lack of corporate governance measures in formulating their investment decisions.
10
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements and information relating to our business that are based on our beliefs, on assumptions made by us, or upon information currently available to us. These statements reflect our current views and assumptions with respect to future events and are subject to risks and uncertainties. Forward-looking statements are often identified by words like: “believe,” “expect,” “estimate,” “anticipate,” “intend,” “project” and similar expressions or words which, by their nature, refer to future events. In some cases, you can also identify forward-looking statements by terminology such as “may,” “will,” “should,” “plans,” “predicts,” “potential” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks in the section entitled Risk Factors beginning on page 6, that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. In addition, you are directed to factors discussed in the Management’s Discussion and Analysis of Financial Condition and Results of Operation section beginning on page 28 and the section entitled “Description of Our Business” beginning on page 12, and as well as those discussed elsewhere in this prospectus. Other factors include, among others: general economic and business conditions; industry capacity; industry trends; competition; changes in business strategy or development plans; project performance; availability, terms, and deployment of capital; and availability of qualified personnel.
These forward-looking statements speak only as of the date of this prospectus. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, or achievements. Except as required by applicable law, including the securities laws of the United States, we expressly disclaim any obligation or undertaking to disseminate any update or revisions of any of the forward-looking statements to reflect any change in our expectations with regard thereto or to conform these statements to actual results.
The Company will receive no proceeds from this offering by the selling shareholders.
As this is a selling shareholders offering there is no dilution in the price of the stock as a result of this registration.
DETERMINATION OF THE OFFERING PRICE
There is no established public market for our shares of common stock. The offering price of $1.00 per share was determined by us arbitrarily. We believe that this price reflects the appropriate price that a potential investor would be willing to invest in our Company at this initial stage of our development. This price bears no relationship whatsoever to our business plan, the price paid for our shares by our founders, our assets, earnings, book value or any other criteria of value. The offering price should not be regarded as an indicator of the future market price of the securities, which is likely to fluctuate.
See “Plan of Distribution” for additional information.
We have not paid any dividends since our incorporation and do not anticipate the payment of dividends in the foreseeable future. At present, our policy is to retain any earnings to develop and market our services. The payment of dividends in the future will depend upon, among other factors, our earnings, capital requirements, and operating financial conditions.
Market Information
There is no established public market for our common stock.
After the effective date of the registration statement of which this prospectus is a part, we intend to seek a market maker to file an application with the Financial Industry Regulatory Authority, Inc., or FINRA, to have our common stock quoted on the Over-the-Counter Bulletin Board. We will have to satisfy certain criteria in order for our application to be accepted. We do not currently have a market maker who is willing to participate in this application process, and even if we identify a market maker, there can be no assurance as to whether we will meet the requisite criteria or that our application will be accepted. Our common stock may never be quoted on the Over-the-Counter Bulletin Board, or, even if quoted, a public market may not materialize. There can be no assurance that an active trading market for our shares will develop, or, if developed, that it will be sustained.
We have issued 25,250,703 shares of our common stock since our inception on November 2, 2016. There are no outstanding options, warrants, or other securities that are convertible into shares of common stock.
Holders
There were 189 holders of record of our common stock as of August 5, 2020.
Securities Authorized for Issuance under Equity Compensation Plans
We do not have any compensation plan under which equity securities are authorized for issuance.
11
Overview
Company Summary
4DMed, Ltd. (“4DMed”, the “Company” “we” or “us”) is a development stage company. We were incorporated under the laws of the state of Nevada on November, 2016. We are a medical software and medical device development company that intends to design, patent, and market a medical virtual reality operating system with integrated, customary haptic and position sensing interfaces for the health care education research, software and medical device markets.
We are a development stage company that has generated no revenues and has had limited operations to date. At June 30, 2020, the Company had not yet recorded any revenues, has working capital of $132,969 and has an accumulated deficit of $3,676,041. As of June 30, 2020, we had $4,319,153 in current assets and current liabilities of $35,581. Through June 30, 2020, we have issued an aggregate of 25,250,703 shares of our common stock since our inception. We issued 21,000,000 shares of our common stock to one (1) founding shareholder for services. We issued a total of 4,250,703 shares to 188 separate accredited foreign shareholders from July 20, 2017 through August 5, 2020, pursuant to private placements of our common stock exempt from registration under Regulation S of the Securities Act of 1933, for total proceeds of approximately $7,508,324.26. Since our inception we have not made any significant purchase or sale of assets, nor have we been involved in any mergers, acquisitions or consolidations.
Business Overview
Company Mission, Vision and Values
4DMED’s mission is to create a medical Virtual Reality (VR) operating system that will provide top-notch training for medical practitioners and learning institutions, and also make the provision of medical services, including surgeries, more available and affordable.
4DMED’s vision is the placement of at least one of its VR operating systems in every hospital and medical clinic in which surgeries are performed, and every medical school throughout the world.
4DMED’s values are seen by the company to be critical to success. They are the strong foundation of the company, define who the company is, and set it apart from all competitors. They underlie the company’s vision of the future. These values include:
| ● | Product Quality |
| 4DMED acts with utmost responsibility, always seeking to meet or exceed expectations. |
| ● | Teamwork |
| 4DMED acts as a team with all of its partners, committed to each other, and bound by trust and loyalty. |
| ● | Integrity |
| 4DMED treats all of its partners, and all other stakeholders with dignity and respect and honesty. Ethical behavior, and integrity are fundamental characteristics of its business conduct. |
12
Implementaton of Business Plan
We intend to implement our business plan through four (4) divisions with our Company. These divisions are. MedVR, MedXR, MedSLS and MedAI.
MedVR
The MedVR mission is to educate, motivate, and activate people to achieve the best possible health program for their environment and it will be responsible for developing the educational component of 4DMED activities. We are currently developing two training programs:
One is surgeon training to be given by senior, experienced trainers, the other creates medical simulation (“SIM”) environments for training nurses or other junior trainees. In designing the 4DMED Simulator, the Company employs both the “far” and “close” approaches - to make it easier for training in hospitals or universities. MedVR is also building a complete anatomy library to support the VR program, with assistance from educational and clinical organizations around the globe.
MedXR
MedXR will develop the treatment component of 4DMED activities. These will include virtual environments to clinically treat severe mental and behavioral health problems including…drug and alcohol abuse, schizophrenia, post-traumatic stress disorder, mood disorders, mild cognitive impairment, autism spectrum disorder, ADHD and others. MedXR creates experiences that activate cognitive, emotional and physiological responses with the goal of improving health. These VR experiences empower individuals and lower the barriers to good health with personalized content and treatment, by creating emotional regulation skills and stress resilience techniques. MedXR has built a breakthrough, comprehensive platform for activating healthy behaviors and improving health outcomes. The platform enables clinical organizations to extend their brand into new virtual patient care experiences.
MedSLS
MedSLS will create the hardware units for 4DMED. One of the first such products is the 4DMED Simulator which is being developed in concert with a Swiss company. Trainees remain engaged and learn more quickly with the progressively more complex cases offered by the VR program with the double screens that are a feature of the simulator…a first. Double screens can help the trainers to get familiar with the surgery room and to working with the assistants. With simulator, techniques complete procedures can be practiced again and again at increasing levels of difficulty, with thousands of new challenges, scenarios and complications to make each experience unique. Each exercise is digitally recorded with detailed metrics, statistics and video debriefing, providing both immediate and long-term skill development feedback.
MedSLS is also working with a development company in Florida on a VR glove with which the trainer can feel more reality in the VR exercise. The user…hospital or university…will have more choice and the trainers can ‘feel their hands’ during the VR application.
MedAI
MedAI will be responsible for the surgical element of 4DMED activities. 4DMED software development kit (SDK) is the Company’s own proprietary product used to develop all of its surgical operations. Users can also use this proprietary SDK to create their own applications, or other VR teaching applications together with support from the MedVR library program.
Streaming interactive, cloud-based VR experiences over upcoming high-speed 5G mobile networks may be possible with a combination of a low latency encoder and optimized client decoding. This will help to achieve VR experiences that deliver presence without simulation sickness. 5G refers to the fifth generation of mobile networks. Currently, many countries have adopted 4G as the standard for high-speed mobile data, allowing users to browse the web, stream media, and make video calls almost effortlessly. The next generation, 5G, will bring improvements to all of these areas.
Corporate Strategy
Virtual simulation technology has come a long way since the Sensorama Simulator was first introduced in 1962. Over the past few decades, VR and simulation technology have become intertwined and implemented in various industries…in particular healthcare. Surgery simulators have been invaluable for physician training, and hospitals have paid …and will continue to pay…large sums of money for this specialized equipment.
With visual simulation combined with force-feedback technology, the surgeon can experience both visual and physical feedback when practicing a procedure. Companies developing cutting-edge simulators for the system. It is unlikely that current HMD technology will be able to replace these sophisticated simulators, but the cost should come down with new sensor and display technologies now available.
13
Beyond surgery, VR is a cost-effective, safe and engaging method for clinical education and training of healthcare professionals, such as nurses, physicians, surgeons, counsellors, dentists, paramedics and even patients. Practitioners can receive training on procedures, techniques, equipment and patient interactions in a far more immersive and realistic environment than using traditional videos and paperwork.
Training in VR allows physicians a risk-free environment in which to practice life-saving procedures, particularly ones that are not commonly performed.
Based on the need to enhance rapid market penetration along with the distribution and adaptation of new medical technology 4DMED is working to build up strategic associations with medical schools, medical software developers, and medical device manufacturers. In view of its solid comprehension of the present market, 4DMED has already created integral arrangements, some which use patent pending innovation which are set in place to serve as income producing answers to existing market needs.
All of this will help 4DMED achieve its main goal to become the industry leader for developing, marketing, and licensing medical VR technology.
The basic 4DMED strategy is embodied in our 4DMED.net program. 4DMED.net is a joint venture with universities and hospitals around the world with a focus on developing program content. The components of the 4DMED strategy are:
Content
4DMED.net is a joint venture with universities and hospitals to develop content. The joint venturers include hospitals in Asia such as Shanghai Hospital, Shanghai, China; Hungary University, Budapest, Hungary.

Cameras
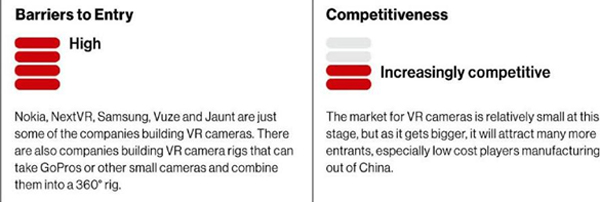
VR cameras are differentiating themselves in a few ways — some are stereoscopic, and some don’t have top and bottom cameras or only cover 1800. Some can live stream, while others just record to disc. One new and interesting technology is volumetric capture, where multiple cameras are arranged facing inward to capture video within an area as opposed to 360-degree cameras that capture video from one point facing outward. The market is currently small but growing. There will be professional, prosumer, and consumer camera rigs, but like the action camera market, there will be a couple of primary players for each target customer, along with a number of cheaper knockoffs or highly specialized cameras. The market will likely only sustain a handful of manufacturers. 4DMED.net is currently evaluating potential partners for developing our cameras.
14
Platforms
Platforms are the “glue” of VR. The companies that will succeed in this space are those that can build innovative and differentiated value added features, along with a delivery mechanism.

VR Headsets
At this point the VR Headset component is in a bit of turmoil with participating manufacturers not fully decided on how they are going to approach the market. New features might give some headset manufacturers an edge (for example FOVE is supposed to include eye tracking), but a good headset manufacturer needs plenty of funding in order to solve problems with the current headset devices. Oculus and HTC are already likely planning version 2 of their headsets and, like the camera market, the dedicated headset market will only sustain a limited number of manufacturers, unless they’re niche products like medical applications. The next round of VR headsets is likely to include higher resolutions, better head tracking, and better interaction between physical and virtual environments (e.g. better controllers) – all of which will have high costs for the first players in this space. In addition, mobile phone manufacturers will build ever more powerful devices, with higher-resolution displays, that will be used to power VR headsets. While dedicated headsets are likely to provide a higher-end experience, every mobile phone user will potentially have a VR-capable device. 4DMED has recognized this potential dilemma and is focused on overcoming it through careful adherence to its values, its strategy and its keys to success.

The Market
The VR/AR healthcare market has been valued at approximately US$748.3 Million in 2017 and is anticipated to enjoy healthy growth over the forecast period 2019-2026. The market is conservatively estimated to reach a value US$6.91 Billion by 2026, according to a March 2019 publication by Reports and Data. This can be mainly associated with the increasing demand for innovative diagnostic techniques, the presence of many more neurological disorders, and increasing disease awareness. The market is driven mainly by applications in surgical simulation, diagnostic imaging, patient care management, rehabilitation and health management. The advancements being made in the field of information technology such as computers of all types, internet connectivity and mobile applications, are also a significant factor stimulating market demand.
15
Because of the constant technological advancement of related products, prevalence of neurological & psychological disorders, and presence of a sophisticated healthcare infrastructure, the North America region accounts for the largest market share of 44.21% in 2018. It is expected to retain the dominant position in 2026 but because of accelerating growth in other regions, its overall market penetration will likely decrease.
| ● | The overall market is projected to grow at a compound annual growth rate (CAGR) of 16.2% in terms of value and reach US$6.91 billion by 2026. |
| ● | The software component is expected to grow at a CAGR of 15.4% during the forecast period. |
| ● | The hardware component is expected to grow at a CAGR of 14.8% during the forecast period. |
| ● | Fully immersive VR is projected to grow at a CAGR of 16.6% during the forecast period. Different medical training institutes have combined fully immersive VR, with medical data and highly advanced medical simulation technologies, to enable modern medical education and training globally. The adaptability offered by the institutions assist as a practical teaching tool today and will continue to do in the future. This will serve as an excellent vehicle for promoting the development to the next level of medical practice. |
| ● | The patient care management component is estimated to grow at a CAGR of 16.6 % during the forecast period and is expected to lead the market for VR in the healthcare segment. The use of VR/AR in therapies and rehabilitation, including brain injury, stroke, and physical therapy among others, will be a significant addition to the healthcare market. |
The main market participants are:
| ● | individuals (retail customers) accounting for more than 90 percent of sales, and |
| ● | local businesses (corporate customers) which, in terms of purchase orders, typically make larger orders for their employees and business needs. |
VR technology is beneficial for not just doctors but also for patients; this technology offers great convenience to patients by enabling them to visit physicians virtually. This has also driven the growth of healthcare VR. According to March 2019 Reports and Data research, VR is ahead of AR because of the advanced offerings of VR technology. VR allows a person to experience their presence inside a different world and interact with it in real time, whereas AR builds a visual world around but does not facilitate any interaction with this world.
There are a lot of factors contributing to the growth of the healthcare VR/AR market including new start-ups that are inclined towards capitalizing on highly prevalent technology. With a positive growth approach of the global market these start-ups are widening their scope of VR/AR applications by using this technology in new applications, thereby providing new functionalities in their products. As these start-ups continue to increase, new products will keep getting developed, increasing the market numbers significantly. The easy availability of VR/AR products, and the need to gain competitive advantage, mean that a growing number of companies has, or will, lower the prices of products, making them affordable for healthcare providers.
VR/AR services are likely to witness higher demand compared to hardware and software and are expected to account for more than 42% share of the total revenue by the end of 2026. Applications of healthcare VR/AR in the medical training segment will reportedly remain the highest, followed by the surgery planning segment. They are anticipated to present the most lucrative opportunities in coming years. Hospitals and clinics are foreseen to retain the top end-user position with more than 40% value share estimated by the end of 2026, followed by medical research organizations.
Competition
There are many worldwide competitors for 4DMED in the VR arena. The following brief summaries of some of the larger, more well-known companies focuses only on their activities in the healthcare industry. The list identifies a number of potential partners that 4DMED can work with to pursue its goals.
Phillips Healthcare (Netherlands)
Koninklijke Philips N.V. (KPNV) was founded in 1891 and is headquartered in Amsterdam, the Netherlands. KPNV operates as a health technology company worldwide. It operates through Diagnosis & Treatment, Connected Care & Health Informatics, and Personal Health segments. The company offers mother, child-care, and oral healthcare products; male grooming and beauty products; food preparation and home care products; and sleep and respiratory care. It provides diagnostic X-ray, integrated clinical, magnetic resonance imaging, computed tomography, and molecular imaging solutions. The company also offers interventional X-ray systems, and imaging and therapy devices for treatment of coronary artery and peripheral vascular disease; imaging products focus on diagnosis, treatment planning, and guidance for cardiology, general imaging, obstetrics/gynecology, and point-of-care applications; and proprietary software to enable diagnostics and intervention. Further, it provides patient monitoring solutions; patient analytics, precision diagnosis, and clinical decision support systems; therapeutic care products; patient monitoring and therapeutic care consumables; and customer services. Additionally, the company offers healthcare information technology, clinical, and visualization and quantification informatics solutions for radiology, cardiology, and oncology departments; universal data management solutions, picture archiving and communication systems, and integrated electronic medical record systems; clinical and hospital IT platforms; technology-enabled monitoring and intervention, actionable program, cloud-based, and population health management software solutions. It also provides digital frameworks that connect consumers, patients, and healthcare providers in a cloud-based connected health ecosystem of devices, apps, and tools.
16
CAE Healthcare (Canada)
CAE Healthcare is committed to improving clinical education and patient safety through simulation-based training solutions. With a mission to Improve clinical competency and performance, we develop evidence-based curriculum and innovative learning technologies for healthcare education.
Firsthand Technology (USA)
Firsthand has helped pioneer VR as a digital therapeutic for pain and rehab, mental health, and promoting healthy lifestyles. The company believes that VR has an Firsthand enormous potential to improve health and wellness.
EON Reality (USA)
EON Reality is the world leader in Virtual and Augmented Reality based knowledge transfer for industry and education. EON believes that knowledge is a human right, and its goal is to make knowledge available, affordable, and accessible for every human on the planet. To do this, it is creating the next generation of Virtual and Augmented Reality tools to increase the world’s knowledge transfer capabilities.
GE Healthcare (USA)
GE Healthcare expertise in medical imaging and information technologies, medical diagnostics, patient monitoring systems, performance improvement, drug discovery, and biopharmaceutical manufacturing technologies is helping clinicians around the world re-imagine new ways to predict, diagnose, inform and treat disease, so their patients can live their lives to the fullest.
Intuitive Surgical Inc. (USA)
Intuitive was founded in 1995 to create innovative, robotic-assisted systems that help empower doctors and hospitals to make surgery less invasive than an open approach. Since da Vinci® became one of the first robotic-assisted systems cleared by the FDA for general laparoscopic surgery, it’s taken robotic-assisted surgery from “science fiction” to reality. Working with doctors and hospitals, Intuitive is continuing to develop new, minimally invasive surgical platforms and future diagnostic tools, including VR, to help solve complex healthcare challenges around the world.
Medtronic plc (USA)
Medtronic plc is the world’s largest medical device company that generates the majority of its sales and profits from the U.S. healthcare system. The company is a leading innovator of healthcare VR.
Mimic Technologies Inc. (USA)
Mimic has a proven programmatic approach that leverages “best-in-class” simulation, cloud-based data collection, advanced analytics that enable objective decisions on privileging and credentialing and the latest VR techniques. By facilitating training outside of the operating room, Mimic enables a rapid climb up the learning curve without wasting valuable OR time. Surgical quality and efficiency are emphasized, while training costs and risk are minimized.
Siemens Healthineers (Germany)
Siemens is a leading global healthcare company, which is continually refining and expanding its healthcare offerings, from medical imaging and laboratory diagnostics, to consulting, healthcare IT, and VR development – as well as further technologies for therapeutic and molecular diagnostics. It is very successful and has an abundance of capital to undertake its operations and research.
17
Surgical Science AB (Sweden)
TeamSim® is a dynamic platform for inter-professional education development taking surgery simulation into the real-world. Using the validated VR training system LapSim® as base, the TeamSim® package is the element that lifts a team training to new levels and immerse into realistic surgery scenarios While innovations in laparoscopic surgical simulators have made an incredible impact on individualized training, there has been a gap between the experience of a single professional standing at the simulator and stepping into a collaborative OR staffed with multiple team members. TeamSim® erases that gap by giving the whole OR team the opportunity to practice and refine their communication and non-technical skills without any patient risk.
Virtual Realities (USA)
Virtual Realities is a leading world distributor of 3D peripheral products and output devices, software, and systems, primarily for the educational and healthcare industries. Its primary goal is to deliver integrated turnkey solution with professional VR applications.
Virtually Better Inc. (USA)
Virtually Better has over 20 years’ experience developing VR to treat mental health disorders. Its corporate motto is “Virtual Reality for a Healthier You” and it focuses on the VR environment almost exclusively.
Vital Images Inc. (USA)
Vital, a Canon Group company, has a legacy of leadership in healthcare imaging using smart algorithms and VR techniques spanning 30 years. As the premier provider of an enterprise imaging (EI) solution focused on interoperability, Vital transforms and seamlessly connects disparate PACS and other data into an efficient, perceptive and interoperable EI solution. Through modular and scalable enterprise message orchestration, enterprise visualization and enterprise analytics solutions, Vital’s Vitrea® Enterprise Imaging solution makes data accessible across the entire enterprise anytime, anywhere, and in any standardized form.
Vuzix Corporation (USA)
Vuzix is a leading supplier of Smart-Glasses and AR technologies and products for the consumer and healthcare markets. The Company’s products include personal display and wearable computing devices that offer users a portable high-quality viewing experience, provide solutions for mobility, wearable displays and both VR and AR. Vuzix holds 66 patents and 43 additional patents pending and numerous IP licenses in the near-eye display field.
WorldViz LLC (USA)
WorldViz makes virtual reality hardware and software for professionals, ranging from comprehensive development platforms to drag-and-drop VR creation and collaboration tools. It has 17 years of building a VR knowledge base, creation VR projection systems. WorldViz is an industry-leading provider of VR solutions for non-entertainment applications, primarily in the healthcare industry. It has patent pending VR products used widely in that industry.
zSpace Inc. (USA)
zSpace provides an immersive learning platform for medical training, allowing students to examine virtual living body structures with accurate anatomical representation, plan procedures and present findings.
The foregoing is not intended to be an exhaustive list of 4DMED’s competitors in the VR healthcare market. Nor is it intended to focus on their history in the market, their financial strength or their respective position in the market.
What it is intended to do is illustrate that there are many participants in the industry who provide products or services that are complimentary to 4DMED’s activities and may be able to be relied upon as a provider to help 4DMED achieve its goals. This is consistent with the 4DMED strategy of using external partners as much as possible.
18
SWAT Analysis
SWOT is the acronym for “Strengths, Weaknesses, Opportunities, Threats”. The analysis is a framework used to evaluate a company’s competitive position and to develop strategic planning. SWOT analysis assesses internal and external factors, as well as current and future potential.
The SWOT analysis for 4DMED looks like:
| Strengths | Weaknesses | ||
| ● | relationship with medical and educational facilities | ● | small – lack of financial resources |
| ● | working with top end providers of products and services | ● | no profile in VR industry |
| ● | understand VR technology | ● | no well-established base |
| ● | understand VR market | ||
| ● | small and focused | ||
| ● | addressing all healthcare VR components | ||
| Opportunities | Threats | ||
| ● | rapidly changing industry initiatives | ● | large, financially secure competition |
| ● | collaboration with industry participants | ● | many participants in a “hot” industry |
| ● | increasing demand for VR | ● | economic/political change |
| ● | increased pressure for healthcare provider cost reductions | ● | lack of public funding for purchasers |
| ● | pressure to reduce healthcare costs | ||
4DMED is admittedly small and has no strong profile in the VR industry yet compared to companies that are more stablished and better funded. But we are also participating in an industry that appears to have an insatiable demand for VR products and processes where there are opportunities for meaningful collaboration with well-established industry participants.
Healthcare VR is largely paid for from the public purse in most environments and there are always pressures to reduce costs, coupled with ongoing economic and political changes. Public funding is not always available, and expenditures get delayed.
Property and Facilities
We do not own or lease any real property or facilities. Our Hong Kong business office is located at 11/F, Capital Centre, 151 Gloucester Road, Wanchai, Hong Kong. This office is provided to us by our Chief Executive Officer, President and Director, Yuen May Cheung, at no cost to our Company.
Dependence on One or a Few Major Customers
We do not anticipate dependence on one or a few major customers for at least the next twelve (12) months or the foreseeable future.
Environmental Regulations
Environmental regulations have had no materially adverse effect on our operations to date, but no assurance can be given that environmental regulations will not, in the future, result in a curtailment of service or otherwise have a materially adverse effect on our business, financial condition or results of operation. Public interest in the protection of the environment has increased dramatically in recent years. The trend of more expansive and stricter environmental legislation and regulations could continue. To the extent that laws are enacted or other governmental action is taken that imposes environmental protection requirements that result in increased costs, our business and prospects could be adversely affected.
Patents, Trademarks and Licenses
We currently do not have any patents or trademarks; and we are not party to any license, franchise, concession, or royalty agreements or any labor contracts.
Legal Proceedings
As of the date of this prospectus, we know of no material pending legal proceedings to which we are a party or of which any of our property is the subject. There are no proceedings in which any of our directors, executive officers or affiliates, or any registered or beneficial stockholder, is an adverse party or has a material interest adverse to our interest.
Employees
Our only employee is our one (1) executive officer.
19
The name, age and position of each of our directors and executive officers are as follows:
| Name | Age | Position | ||
| Yuen May Cheung | 54 | CEO, President, CFO, Secretary and Director | ||
| Hau Tin Cheung | 52 | Director |
Yuen May Cheung, Age 54, Chief Executive Officer, President, Chief Financial Officer, Secretary and Director
Ms. Cheung, is our founder and our Chief Executive Officer, President, Chief Financial Officer, Secretary and Director and has served in in such capacities since our inception in November of 2016. Ms. Cheung currently devotes 50% of her working time to the management and operations of our Company. Ms. Cheung was the co-founder of GuangNing ChangRong Bamboo & Wood Handicraft Products Co. Ltd, a factory and manufacturer, served as Director and owned by Ms. Cheung, since 2007, Ms. Cheung served as Director of Zhong Cui Investments Ltd., a marketing company, owned by Ms. Cheung since 2006. Ms. Cheung also serves as President and sole Director of AIFam, Ltd. Ms. Cheung provides hands-on leadership, strategic direction and operations management with a focus on business development, exceptional quality management and fiscal accountability. Ms. Cheung attended Centennial College in Toronto, Canada from 1989-1991 where she received a Certificate of Accounting, and she also attended Chui Hai College in Hong Kong from 1984-1988 where she received a degree in Business Management. Ms. Cheung has served as an officer and director of AIFarm. Ltd., a company formerly required to file reports with the Securities and Exchange Commission.
Hau Tin Cheung, Age 52, Director
Prior to 4DMed Ltd, Mr. Cheung was involved for over 20 years as a private investor, business man, and merchant banker, managing and coordinating the financing of tens of millions of dollars of transaction proceeds, in numerous domestic and overseas business opportunities, across diverse sectors of industries including; natural gas, oil, mining, pharmaceuticals and technology. Mr. Cheung attended Centennial College in Toronto, Canada where he received a Certificate of Finance Management in 1990. Mr. Cheung does not, and has not served as an officer or director of any other company required to file reports with the Securities and Exchange Commission.
Board Composition
Our Bylaws provide that the Board of Directors shall consist of no less than 1, but not more than 9 directors. Each director serves until his successor is elected and qualified.
Committees of the Board of Directors
We do not presently have a separately constituted audit committee, compensation committee, nominating committee, executive committee or any other committees of our Board of Directors. Nor do we have an audit committee “financial expert.” As such, our entire Board of Directors acts as our audit committee and handles matters related to compensation and nominations of directors.
Potential Conflicts of Interest
Since we do not have an audit or compensation committee comprised of independent directors, the functions that would have been performed by such committees are performed by our directors. Thus, there is a potential conflict of interest in that our directors and officers have the authority to determine issues concerning management compensation and audit issues that may affect management decisions. We are not aware of any other conflicts of interest with any of our executives or directors.
Director Independence
We are not subject to listing requirements of any national securities exchange or national securities association and, as a result, we are not at this time required to have our board comprised of a majority of “independent directors.” Our determination of independence of directors is made using the definition of “independent director” contained in Rule 4200(a) (15) of the Marketplace Rules of the NASDAQ Stock Market (“NASDAQ”), even though such definitions do not currently apply to us because we are not listed on NASDAQ. We have determined that none of our directors currently meet the definition of “independent” as within the meaning of such rules as a result of their current positions as our executive officers.
20
Significant Employees
We have no significant employees other than the executive officers/directors described above.
Family Relationships
Yuen May Cheung and Hau Tin Cheung are siblings.
Involvement in Certain Legal Proceedings
No director, person nominated to become a director, executive officer, promoter or control person of our company has, during the last ten years: (i) been convicted in or is currently subject to a pending a criminal proceeding (excluding traffic violations and other minor offenses); (ii) been a party to a civil proceeding of a judicial or administrative body of competent jurisdiction and as a result of such proceeding was or is subject to a judgment, decree or final order enjoining future violations of, or prohibiting or mandating activities subject to any federal or state securities or banking or commodities laws including, without limitation, in any way limiting involvement in any business activity, or finding any violation with respect to such law, nor (iii) any bankruptcy petition been filed by or against the business of which such person was an executive officer or a general partner, whether at the time of the bankruptcy or for the two years prior thereto.
Stockholder Communications with the Board
We have not implemented a formal policy or procedure by which our stockholders can communicate directly with our Board of Directors. Nevertheless, every effort has been made to ensure that the views of stockholders are heard by the Board of Directors or individual directors, as applicable, and that appropriate responses are provided to stockholders in a timely manner. We believe that we are responsive to stockholder communications, and therefore have not considered it necessary to adopt a formal process for stockholder communications with our Board. During the upcoming year, our Board will continue to monitor whether it would be appropriate to adopt such a process.
Section 16(a) Beneficial Ownership Reporting Compliance
16(a) of the Securities Exchange Act of 1934 requires the Company directors and executive officers, and persons who own more than ten percent of the Company’s common stock, to file with the Securities and Exchange Commission initial reports of ownership and reports of changes of ownership of our common stock. Officers, directors and greater than ten percent shareholders are required by SEC regulation to furnish the Company with copies of all Section 16(a) forms they file. The Company intends to ensure to the best of our ability that all Section 16(a) filing requirements applicable to its officers, directors and greater than ten percent (10%) beneficial owners are complied with in a timely fashion.
We have not paid since our inception, nor do we owe, any compensation to our executive officers or directors. There are no arrangements or employment agreements with our executive officer or directors pursuant to which they will be compensated now or in the future for any services provided as an executive officer, and we do not anticipate entering into any such arrangements or agreements with them in the foreseeable future.
Outstanding Equity Awards
We do not currently have a stock option plan or any long-term incentive plans that provide compensation intended to serve as incentive for performance. No individual grants of stock options or other equity incentive awards have been made to any executive officer or any director since our inception; accordingly, none were outstanding at August 5, 2020, and through the date of this Report.
Employment Contracts, Termination of Employment, Change-in-Control Arrangements
There are currently no employments or other contracts or arrangements with our executive officers. There are no compensation plans or arrangements, including payments to be made by us, with respect to our officers, directors or consultants that would result from the resignation, retirement or any other termination of such directors, officers or consultants from us. There are no arrangements for directors, officers, employees or consultants that would result from a change-in-control.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
As June 30, 2020, 4DMED Limited, a Hong Kong incorporated company under common control of the president of the Company, owed $4,186,184 to the Company, which is non-interest bearing, unsecured, and due on demand.
21
The following table sets forth information regarding the beneficial ownership of our common stock as of August 5, 2020 for:
| ● | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; |
| ● | each of our executive officers; |
| ● | each of our directors; and |
| ● | all of our executive officers and directors as a group. |
We have determined beneficial ownership in accordance with the rules of the Securities and Exchange Commission. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. The person is also deemed to be a beneficial owner of any security of which that person has a right to acquire beneficial ownership within 60 days. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws, and the address for each person listed in the table is c/o 4DMed, Ltd. _________________________________
The percentage ownership information shown in the table below is calculated based on 25,250,703 shares of our common stock issued and outstanding as of August 5, 2020. We do not have any outstanding options, warrants or other securities exercisable for or convertible into shares of our common stock.
| Title
of Class of Beneficial Ownership |
Name of Beneficial Owner | Amount and Nature | Percentage
of Class | |||
| Common Stock | Yuen May Cheung | 21,000,000 | 83.2% | |||
| Direct | ||||||
| All officers and directors as a group | 21,000,000 | 83.2% |
We are unaware of any contract or other arrangement the operation of which may at a subsequent date result in a change in control of our Company.
We do not have any issued and outstanding securities that are convertible into common stock. None of our stockholders are entitled to registration rights.
There Is No Current Market for Our Shares of Common Stock
There is currently no market for our shares of common stock. We cannot give you any assurance that the shares you purchase will ever have a market or that if a market for our shares ever develops, that you will be able to sell your shares. In addition, even if a public market for our shares develops, there is no assurance that a secondary public market will be sustained.
The shares you purchase are not traded or listed on any exchange or quotation medium. After the effective date of the registration statement, we intend to seek a market maker to file an application with the Financial Industry Regulatory Authority, Inc., or FINRA, to have our common stock quoted on the Over-the-Counter Bulletin Board. We will have to satisfy certain criteria in order for our application to be accepted. We do not currently have a market maker who is willing to participate in this application process, and even if we identify a market maker, there can be no assurance as to whether we will meet the requisite criteria or that our application will be accepted. Our common stock may never be quoted on the Over-the-Counter Bulletin Board, or, even if quoted, a public market may not materialize. There can be no assurance that an active trading market for our shares will develop, or, if developed, that it will be sustained.
The Over-the-Counter Bulletin Board is maintained by the Financial Industry Regulatory Authority, Inc. The securities traded on the Over-the-Counter Bulletin Board are not listed or traded on the floor of an organized national or regional stock exchange. Instead, these securities transactions are conducted through a telephone and computer network connecting dealers in stocks. Over-the-counter stocks are traditionally smaller companies that do not meet the financial and other listing requirements of a regional or national stock exchange.
22
Even if our shares are quoted on the Over-the-Counter Bulletin Board, a purchaser of our shares may not be able to resell the shares. Broker-dealers may be discouraged from effecting transactions in our shares because they will be considered penny stocks and will be subject to the penny stock rules. Rules 15g-1 through 15g-9 promulgated under the Securities Exchange Act of 1934, as amended, impose sales practice and disclosure requirements on brokers-dealers who make a market in a “penny stock.” A penny stock generally includes equity securities (other than securities registered on some national securities exchanges) that have a market price of less than $5.00 per share. Under the penny stock regulations, a broker-dealer selling penny stock to anyone other than an established customer or “accredited investor” (generally, an individual with net worth in excess of $1,000,000 or an annual income exceeding $200,000, or $300,000 together with his or her spouse) must make a special suitability determination for the purchaser and must receive the purchaser’s written consent to the transaction prior to sale, unless the broker-dealer or the transaction is otherwise exempt. In addition, the penny stock regulations require the broker-dealer to deliver, prior to any transaction involving a penny stock, a disclosure schedule prepared by the SEC relating to the penny stock market, unless the broker-dealer or the transaction is otherwise exempt. A broker-dealer is also required to disclose commissions payable to the broker-dealer and the registered representative and current quotations for the securities. Finally, a broker-dealer is required to send monthly statements disclosing recent price information with respect to the penny stock held in a customer’s account and information with respect to the limited market in penny stocks.
The additional sales practice and disclosure requirements imposed upon broker-dealers may discourage broker-dealers from effecting transactions in our shares, which could severely limit the market liquidity of the shares and impede the sale of our shares in the secondary market, assuming one develops.
By Selling Stockholders
We are registering shares of our common stock on behalf of the selling shareholders. The selling shareholders will offer and sell the shares of our common stock to which this prospectus relates for their own accounts. We will not receive any proceeds from the sale of those shares. We will pay all fees and expenses in connection with the registration of those shares. Fees and expenses of any attorneys or other advisors retained by the selling shareholders in connection with the registration will be paid by the selling shareholders.
The selling shareholders may sell some or all of their shares of our common stock registered hereby at a fixed price of $1.00 per share. Prior to those prices being quoted on the OTCBB, the selling shareholders may sell their shares of our common stock registered hereby in private transactions to other individuals. Although our common stock is not listed on a public exchange, we intend to apply for participation on the OTCBB concurrently with the filing of this registration statement. In order to be quoted on the OTCBB, a market maker must file an application on our behalf in order to make a market for our common stock. There can be no assurance that a market maker will agree to file the necessary documents with FINRA, which operates the OTCBB, nor can there be any assurance that such an application for quotation will be approved. However, sales by selling shareholders of their shares of our common stock registered hereby must be made at the fixed price of $1.00 until the prices of our common stock are quoted on the OTCBB.
When prices for our common stock are quoted on the OTCBB, the shares of our common stock registered hereby may be sold or distributed from time to time by the selling shareholders directly to one or more purchasers or through brokers or dealers who act solely as agents, at market prices prevailing at the time of sale, at prices related to such prevailing market prices, at negotiated prices or at fixed prices, which may be changed. The distribution of those shares may be effected in one or more of the following methods:
| ● | ordinary brokers transactions, which may include long or short sales; |
| ● | transactions involving cross or block trades on any securities or market where our common stock is trading; |
| ● | through direct sales to purchasers or sales effected through agents; |
| ● | through transactions in options, swaps or other derivatives (whether exchange listed or otherwise); or |
| ● | any combination of the foregoing; |
In addition, the selling shareholders may enter into hedging transactions with broker-dealers who may engage in short sales, if short sales were permitted, of those shares in the course of hedging the positions they assume with the selling stockholders. The selling shareholders may also enter into option or other transactions with broker-dealers that require the delivery by such broker-dealers of those shares, which shares may be resold thereafter pursuant to this prospectus. To our best knowledge, none of the selling shareholders are broker-dealers or affiliates of broker dealers.
We will inform the selling shareholders that the anti-manipulation rules of Regulation M under the Securities Exchange Act of 1934 may apply to sales of those shares in the market and to the activities of the selling shareholders and their affiliates. In addition, we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling shareholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act of 1933. The selling shareholders may indemnify any broker-dealer that participates in transactions involving the sale of those shares against certain liabilities, including liabilities arising under the Securities Act of 1933.
Brokers, dealers, or agents participating in the distribution of those shares may receive compensation in the form of discounts, concessions or commissions from the selling shareholders and/or the purchasers of shares for whom such broker-dealers may act as agent or to whom they may sell as principal, or both (which compensation as to a particular broker-dealer may be in excess of customary commissions). Neither the selling shareholders nor we can presently estimate the amount of such compensation. We know of no existing arrangements among the selling shareholders and any other shareholder, broker, dealer or agent relating to the sale or distribution of those shares.
23
Our affiliates and/or promoters, if any, who are offering their shares of our common stock for sale and any broker-dealers who act in connection with the sale of the shares of our common stock hereunder will be deemed to be “underwriters” of this offering within the meaning of the Securities Act of 1933, and any commissions they receive and proceeds of any sale of the shares may be deemed to be underwriting discounts and commissions under the Securities Act of 1933.
The selling shareholders and any purchasers of our common stock should be aware that any market that develops for our common stock will be subject to “penny stock” rules.
Insofar as indemnification for liabilities occurring pursuant to the Securities Act of 1933 may be permitted to our directors, officers, and controlling persons, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act of 1933 and, therefore, unenforceable.
If any of the selling shareholders enter into an agreement after the effectiveness of the registration statement of which this prospectus is a part to sell all or a portion of his or her shares of our common stock registered hereby to a broker-dealer as principal and that broker-dealer acts as underwriter, we will file a post-effective amendment to this registration statement identifying that broker-dealer, providing the required information regarding the plan of distribution, revising disclosures in that registration statement, as required, and filing a copy of that agreement as an exhibit to that registration statement.
24
All of the shares of common stock issued are being offered by the selling stockholders listed in the table below. None of the selling stockholders are broker-dealers or affiliated with broker-dealers. We issued the shares of common stock pursuant to a private placement exempt from registration under Section 4(2) and Regulation S of the Securities Act of 1933.
The selling stockholders may offer and sell, from time to time, any or all of the common stock issued. Because the selling stockholders may offer all or only some portion of the 400,000 shares of common stock to be registered, no estimate can be given as to the amount or percentage of these shares of common stock that will be held by the selling stockholders upon termination of the offering.
The following table sets forth certain information regarding the beneficial ownership of shares of common stock by the selling stockholders as of August 5, 2020, and the number of shares of common stock covered by this prospectus. The number of shares in the table represents an estimate of the number of shares of common stock to be offered by the selling stockholders.
| Selling Shareholders | Shares of Common Stock Owned Prior to Offering | Shares of Common Stock to be Offered for Sale | Shares of Common Stock Owned After the Offering | Percentage of Common Stock Owned Before the Offering | Percentage of Common Stock Owned After the Offering | |||||
| ULRICH ZWISSLER | 625000 | 10000 | 615000 | 2.48% | 2.44% | |||||
| ALOIS KNIEWALLNER | 374000 | 10000 | 364000 | 1.49% | 1.45% | |||||
| CLAUS NECKEL | 353500 | 10000 | 343500 | 1.40% | 1.36% | |||||
| JOZEF BALOGH | 320000 | 10000 | 310000 | 1.27% | 1.23% | |||||
| REINHOLD JANACH | 198442 | 10000 | 188442 | 0.79% | 0.75% | |||||
| SIEGFRIED HEGER | 150000 | 10000 | 140000 | 0.60% | 0.56% | |||||
| RAINER LACHMANN | 137000 | 10000 | 127000 | 0.54% | 0.50% | |||||
| JUERGEN KNOBLAUCH | 130200 | 10000 | 120200 | 0.52% | 0.48% | |||||
| MICHAEL KRAFT | 120600 | 10000 | 110600 | 0.48% | 0.44% | |||||
| FRIEDHELM FINKE | 118400 | 10000 | 108400 | 0.47% | 0.43% | |||||
| MANFRED BAUMANN | 80000 | 10000 | 70000 | 0.32% | 0.28% | |||||
| BERND WEICHERDING | 71000 | 10000 | 61000 | 0.28% | 0.24% | |||||
| EBERHARD BEHREND | 50000 | 10000 | 40000 | 0.20% | 0.16% | |||||
| EDWIN PIRZER | 50000 | 10000 | 40000 | 0.20% | 0.16% | |||||
| HERBERT ROSENEDER | 50000 | 10000 | 40000 | 0.20% | 0.16% | |||||
| SIEGFRIED MORATH | 46000 | 10000 | 36000 | 0.18% | 0.14% | |||||
| KARL HUTTER | 40000 | 10000 | 30000 | 0.16% | 0.12% | |||||
| EDWIN DORNISCH | 36000 | 10000 | 26000 | 0.14% | 0.10% | |||||
| HELMUT LANTZBERG | 33000 | 10000 | 23000 | 0.13% | 0.09% | |||||
| HOLLY RUDIGER | 30000 | 10000 | 20000 | 0.12% | 0.08% | |||||
| JOSEF REINKOBER | 30000 | 10000 | 20000 | 0.12% | 0.08% | |||||
| REINHOLD EISENBRAUN | 30000 | 10000 | 20000 | 0.12% | 0.08% | |||||
| ROLF SCHRAFT | 30000 | 10000 | 20000 | 0.12% | 0.08% | |||||
| WOLFGANG HOFFMAN | 29000 | 10000 | 19000 | 0.12% | 0.08% | |||||
| CHRISTOPH GASSMAN | 26500 | 10000 | 16500 | 0.11% | 0.07% | |||||
| SIEGFRIED HOELLE | 26200 | 10000 | 16200 | 0.10% | 0.06% | |||||
| HELMUT ROSCHLAK | 25000 | 10000 | 15000 | 0.10% | 0.06% | |||||
| ROBERT BAUER | 22900 | 10000 | 12900 | 0.09% | 0.05% | |||||
| HORST BOLTEN | 20435 | 10000 | 10435 | 0.08% | 0.04% | |||||
| ERICH SPERER | 15000 | 10000 | 5000 | 0.06% | 0.02% | |||||
| BERND DANKELMANN | 14000 | 10000 | 4000 | 0.06% | 0.02% | |||||
| EKKEHARD ADY | 14000 | 10000 | 4000 | 0.06% | 0.02% | |||||
| HELMUT LACHMANN | 13000 | 10000 | 3000 | 0.05% | 0.01% | |||||
| GERHARD WAGNER | 12800 | 10000 | 2800 | 0.05% | 0.01% | |||||
| DIETER HANKIEWICZ | 11000 | 10000 | 1000 | 0.04% | 0.00% | |||||
| ARMIN WAGNER | 10000 | 10000 | 0 | 0.04% | 0.00% | |||||
| HANS ERLACHER | 10000 | 10000 | 0 | 0.04% | 0.00% | |||||
| HAUF ERNST | 10000 | 10000 | 0 | 0.04% | 0.00% | |||||
| HELMUT PASCHER | 10000 | 10000 | 0 | 0.04% | 0.00% | |||||
| MARIA SEMMELROCK-PICEJ | 10000 | 10000 | 0 | 0.04% | 0.00% | |||||
| 3382977 | 400000 | 2982977 |
25
Common Stock
Our authorized capital stock consists of 75,000,000 shares of common stock, par value $0.001 per share.
The holders of our common stock:
| ● | Have equal rateable rights to dividends from funds legally available therefore, when, as and if declared by our Board of Directors; |
| ● | Are entitled to share rateably in all of our assets available for distribution to holders of common stock upon liquidation, dissolution or winding up of our affairs; |
| ● | Do not have pre-emptive, subscription or conversion rights and there are no redemption or sinking fund provisions or rights; and |
| ● | Are entitled to one non-cumulative vote per share on all matters on which stockholders may vote. |
The shares of common stock are not subject to any future call or assessment and all have equal voting rights. There are no special rights or restrictions of any nature attached to any of the common shares and they all rank at equal rate or pari passu , each with the other, as to all benefits, which might accrue to the holders of the common shares. All registered stockholders are entitled to receive a notice of any general annual meeting to be convened by our Board of Directors.
At any general meeting, subject to the restrictions on joint registered owners of common shares, every stockholder who is present in person or by proxy and entitled to vote has one vote, and on a poll every stockholder has one vote for each share of common stock of which he is the registered owner and may exercise such vote either in person or by proxy. To the knowledge of our management, at the date hereof, our officers and directors are the only persons to exercise control, directly or indirectly, over more than 10% of our outstanding common shares. See “Security Ownership of Certain Beneficial Owners and Management.”
We refer you to our Articles of Incorporation and Bylaws, copies of which were filed with the registration statement of which this prospectus is a part, and to the applicable statutes of the State of Nevada for a more complete description of the rights and liabilities of holders of our securities.
As of August 5, 2020, there were 25,250,703 shares of our common stock issued and outstanding.
Preferred Stock
We are not authorized to issue any preferred stock.
Options, Warrants and Rights
There are no outstanding options, warrants, or similar rights to purchase any of our securities.
Non-cumulative Voting
Holders of shares of our common stock do not have cumulative voting rights, which means that the holders of more than 50% of the outstanding shares, voting for the election of directors, can elect all of the directors to be elected, if they so choose, and, in such event, the holders of the remaining shares will not be able to elect any of our directors.
Cash Dividends
As of the date of this prospectus, we have not paid any cash dividends to stockholders. The declaration of any future cash dividend will be at the discretion of our Board of Directors and will depend upon our earnings, if any, our capital requirements and financial position, our general economic and other pertinent conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, into our business.
Transfer Agent
The transfer agent and registrar for our common stock is Sedona Equity Registrar & Transfer, Inc., 12601 N. Cave Creek Road, Suite 118, Phoenix, AZ 85022. The transfer agent is responsible for all record-keeping and administrative functions in connection with our issued and outstanding common stock.
SHARES ELIGIBLE FOR FUTURE SALE
There is no public market for our common stock. We cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock. Sales of substantial amounts of our common stock in the public market could adversely affect the market prices of our common stock and could impair our future ability to raise capital through the sale of our equity securities.
26
Upon completion of this Offering, based on our outstanding shares as of August 5, 2020, we will have outstanding an aggregate of 25,250,703 shares of common stock outstanding. Of these shares, upon effectiveness of the registration statement of which this prospectus forms a part, all shares covered hereby and sold under the Offering will be freely transferable without restriction or further registration under the Securities Act.
Rule 144
In general, under Rule 144 as currently in effect, a person who is not one of our affiliates and who is not deemed to have been one of our affiliates at any time during the three months preceding a sale and who has beneficially owned shares of our common stock that are deemed restricted securities for at least six months would be entitled after such six-month holding period to sell the common stock held by such person, subject to the continued availability of current public information about us (which current public information requirement is eliminated after a one-year holding period).
A person who is one of our affiliates, or has been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned shares of our common stock that are deemed restricted securities for at least six months would be entitled after such six-month holding period to sell his or her securities, provided that he or she sells an amount that does not exceed 1% of the number of shares of our common stock then outstanding (or approximately 251,787 shares) immediately after this Offering (or, if our common stock is listed on a national securities exchange, the average weekly trading volume of the shares during the four calendar weeks preceding the sale), subject to the continued availability of current public information about us and compliance with certain manner of sale provisions.
Rule 144 is not available for resale of restricted securities of shell companies or former shell companies until one year elapses from the time that such company is no longer considered a shell company.
We know of no existing or pending legal proceedings against us, nor are we involved as a plaintiff in any proceeding or pending litigation. There are no proceedings in which any of our directors, officers or any of their respective affiliates, or any beneficial stockholder, is an adverse party or has a material interest adverse to our interest. Our address for service of process in Nevada is 4650 Wedekind Road, #2, Sparks, Nevada 89431.
Lane & Associates, P.A., c/o Paul Camp Lane, Esq. will pass upon the validity of the common stock offered hereby.
The financial statements included in this prospectus, and in the registration statement of which this prospectus is a part, have been audited by Centurion ZD CPA & Co., an independent registered public accounting firm, to the extent and for the period set forth in their report appearing elsewhere herein and in the registration statement, and are included in reliance upon such report given upon the authority of said firm as experts in auditing and accounting. No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis or had, or is to receive, in connection with the offering, a substantial interest, directly or indirectly, in the Company, nor was any such person connected with the Company as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES LIABILITIES
Our Bylaws, subject to the provisions of Nevada law, contain provisions which allow us to indemnify any person against liabilities and other expenses incurred as the result of defending or administering any pending or anticipated legal issue in connection with service to us if it is determined that person acted in good faith and in a manner which he reasonably believed was in the best interest of the corporation. Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
There have not been any changes in or disagreements with accountants on accounting, financial disclosure or any other matter.
27
WHERE YOU CAN GET MORE INFORMATION
In accordance with the Securities Act of 1933, we are filing with the SEC a registration statement on Form S-1, of which this prospectus is a part, covering the securities being offered by the Registrant. As permitted by rules and regulations of the SEC, this prospectus does not contain all of the information set forth in the registration statement. For further information regarding both our Company and our common stock, we refer you to the registration statement, including all exhibits and schedules, which you may inspect without charge at the public reference facilities of the SEC’s Washington, D.C. office, 100 F Street, N.E., Washington, D.C. 20549, on official business days during the hours of 10am and 3pm, and on the SEC Internet site at http:\\www.sec.gov. Information regarding the operation of the public reference rooms may be obtained by calling the SEC at 1-800-SEC-0330.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion of our financial condition and results of operation should be read in conjunction with the financial statements and related notes that appear elsewhere in this prospectus. This discussion contains forward-looking statements and information relating to our business that reflect our current views and assumptions with respect to future events and are subject to risks and uncertainties, including the risks in the section entitled Risk Factors beginning on page 6, that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
These forward-looking statements speak only as of the date of this prospectus. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, or achievements. Except as required by applicable law, including the securities laws of the United States, we expressly disclaim any obligation or undertaking to disseminate any update or revisions of any of the forward-looking statements to reflect any change in our expectations with regard thereto or to conform these statements to actual results.
Plan of Operations
4DMed, Ltd. (“4DMed”, the “Company” “we” or “us”) is a development stage company. We were incorporated under the laws of the state of Nevada on November 2, 2016. 4DMed, Ltd. (“4DMed”, the “Company” “we” or “us”) is a development stage company. We were incorporated under the laws of the state of Nevada on November, 2016. We are a medical software and medical device development company that intends to design, patent, and market a medical virtual reality operating system with integrated, customary haptic and position sensing interfaces for the health care education research, software and medical device markets.
Milestones
Months 1 through 3
During the first three (3) months we plan to:
| o | Complete development on the second generation of 4dmed Scene in hospital |
| o | Complete development on the first scene for coronavirus inspection in hospital. |
| o | Apply for Trademark registration in USA, Singapore, and Canada for the scenes design |
| o | Enter contract with a hospital and the education institutions for the first VR course development in Singapore |
| o | Set up the Show room in Singapore and Toronto |
| o | Design the first new scene and guideline of surgery room for virtual reality course |
| o | Hire 2-3 VR engineering staff in Canada |
4DMed-MedVR
We design MedVR to educate, motivate, and activate people to achieve the best possible health program for their environment and it will be responsible for developing the educational component of 4DMED activities. It is currently developing two different training programs.
One is surgeon training to be given by senior, experienced trainers, the other creates medical simulation (“SIM”) environments for training nurses or other junior trainees. In designing the 4DMED Simulator, the company employs both the “far” and “close” approaches - to make it easier for training in hospitals or universities.
Development the Second Generation of the 4dmed software.
We presently are working with few VR medial companies to develop the new medical scenes for education industries. During this period, we intend on securing all work in process inventory, design documentation, prototypes and all intellectual property.
28
Financial/Sales/Engineering Staff
We plan on hiring three VR engineers. Two engineers for software, one and one for mechanical. If in the event, we are unable to secure engineers with the required skills necessary we will continue to outsource such functions. We believe, however, that having in house engineers will significantly reduce the amount of time we spend going back and forth with outsourced service providers.
In addition, we expect that during months one (1) through three (3) that we will hire a VP of sales to handle product sales to distributors and retailers for the vegetable markets.
Months 4 through 6
During the following three (3) months, we expect to achieve the following:
| o | Hiring the industrial designing company for the new design for VR headset with brain mapping |
| o | Seek more suppliers for the materials for VR groves and sensors |
| o | Hiring the marketing company of expansion in Singapore Office |
| o | Engage the universities to design the surgery education VR program |
| o | Developing second generation of MedXR eco Systems. |
| o | Developing the IT marketing Program for education media and other social media platforms. |
4dMED -MedXR
We will develop the treatment component of 4DMED activities. These will include virtual environments to clinically treat severe mental and behavioral health problems including…drug and alcohol abuse, schizophrenia, post-traumatic stress disorder, mood disorders, mild cognitive impairment, autism spectrum disorder, ADHD and others. MedXR creates experiences that activate cognitive, emotional and physiological responses with the goal of improving health. These VR experiences empower individuals and lower the barriers to good health with personalized content and treatment, by creating emotional regulation skills and stress resilience techniques. MedXR has built a breakthrough, comprehensive platform for activating healthy behaviors and improving health outcomes. The platform enables clinical organizations to extend their brand into new virtual patient care experiences.
Months 7 through 9
During the following three (3) months, we expect to achieve the following:
| o | Set up the New App and start to send sample to end users |
| o | Finish the design in Canada project and start to order the materials for our 4dmed Box |
| o | Finish the second level of MedXR and Virtual Reality Platform for the Senior Living Industry |
| o | Finish the 4dmed SDK box for MedXR and MedVR |
| o | Starting the develop the computer system to brain mapping record of the New Headset system |
MedAI will be responsible for the surgical element of 4DMED activities. 4DMED software development kit (SDK) is the company’s own proprietary product used to develop all of its surgical operations. Users can also use this proprietary SDK to create their own applications, or other VR teaching applications together with support from the MedVR library program.
Streaming interactive, cloud-based VR experiences over upcoming high-speed 5G mobile networks may be possible with a combination of a low latency encoder and optimized client decoding. This will help to achieve VR experiences that deliver presence without simulation sickness.
Distributor Demo
If we are successful in previous months, it is anticipated that in months seven (7) through nine (9) we will have the first three scenes and equipment set( 4dmed Box) and send samples to education institutions and senior home managements companies as well as certain University and Hospital. During our discussions with distributors, such as Big Corporation, our understanding is that new products are evaluated and tested by a committee and then taken to retailers to gauge interest. Retailer interest determines initial order levels.
Finish the design for MedXR project and start to plug in our SDK program
Senior program can Improve quality of life for seniors by deploying cutting edge technology to help decrease pain, anxiety, depression and more. The result is happier clients, increased mental activity and reduced operational cost.
Transform our recreational program by providing a completely new way to interact, explore and provide unforgettable fulfilling experiences. Marketing add-on allows for the virtual tours of seniors will give them the opportunity to discover and experience the world in a whole new way.
29
Months 10 through 12
| o | Start to install the 1st Senior program in the 4dmed set |
| o | Design the package for the products for the North America market |
| o | Seeking the products seeds sources for Asia markets |
| o | Testing the Med SLS and start to design programs for mental health, exposure therapy, mindfulness, pain management, |
| o | Begin advertising / promotion campaign |
During the following three (3) months, we expect to achieve the following:
Start to instore our product – 4DMed Box set in North America and Asia.
Design the package for the products for the Online Customers.
Begin advertising / promotion campaign.
Seek on-going associations and industry group approvals and marketing support. We intend to get needed industry and association and government approvals and seek their help in securing potential industry and government access as well as any needed sponsorships and support.
Work with other firms to assist in optimizing its products for certain virtual reality medial where needed.
Sign additional sub-license agreements or joint venture agreements with strategic partners. Central to the Company’s strategy is to sign large “education of medical” and “elderly care “agreements with commercial partners.
We do not currently have any arrangements for financing, and we can provide no assurance to investors we will be able to find such financing. There can be no assurance that additional financing will be available to us, or on terms that are acceptable. Consequently, we may not be able to proceed with our intended business plans or complete the development and commercialization of our product.
Liquidity and Results of Operations
Six months results – for the six months ended June 30, 2020 and 2019; Liquidity and Capital Resources Comparison of the Years Ended Results – For the Years Ended December 31, 2019 and 2018
Revenues and Gross Profit
Revenues and Gross Profit for the six months ended June 30, 2020 and June 20, 2019; and the years ended December 31, 2019 and December 31, 2018 are zero. The Company is a development stage company and has incurred significant costs in research and development activities. See discussion below for further information. At June 30, 2020, the Company had incurred an accumulated deficit of $3,676,041 since inception.
Costs and Expenses
Costs and Expenses
Total operating cost and expenses decreased to $799,080 for the six months ended June 30, 2020, as compared to $1,415,471 for the six months ended June 30, 2019. These decreases were primarily due to decreasing costs associated with General Administrative Expenses, mainly Marketing expenses
Total operating cost and expenses increased to $2,336,135 for the year ended December 31, 2019, as compared to $514,583 for the year ended December 31, 2018. These increases were primarily due to increasing costs associated with Marketing and Consulting expenses.
Other Income and Expenses
Income Taxes
The Company had no income tax expenses or income tax benefit for the years ended December 31, 2019 and 2018, due to incurrence of net operating loss in each of these periods. There are no income tax refund opportunities currently available.
30
Effect of Inflation
Inflation has not had a significant impact on the Company’s operations or cash flows.
Liquidity and Capital Resources
Cash Flow Information
As of June 30, 2020, the Company had cash and cash equivalents of approximately $132,969 as compared to cash and cash equivalents of $569,285 at June 30, 2019. This represents a slight decrease in cash of $463,316.
As of December 31, 2019, the Company had cash and cash equivalents of approximately $3,074 as compared to cash and cash equivalents of $343,622 at December 31, 2018. This represents a slight decrease in cash of $340,548.
Cash used in Operating Activities
The Company used approximately $806,596 of cash for operating activities for the six months ended June 30, 2020 as compared to use $1,415,471 of cash for operating activities for the six months ended June 30, 2019. This resulted in a decrease in cash used in operating activities of $608,875. The expenses consisted of filing fees, professional fees and other general expenses.
The Company used $2,314,587 of cash for operating activities in the year ended December 31, 2019 as compared to use $493,035 of cash for operating activities in the year ended December 31, 2018. This resulted in an increase in cash used in operating activities of $1,821,552. The expenses consisted of filing fees, professional fees and marketing expenses.
Cash provided by Investing Activities
The Company provided $(602,612) of cash by investing activities for the six months ended June 30, 2020 as compared to use $1,308,407 of cash for investing activities for the six months ended June 30, 2019.
The Company provided $2,946,997 of cash by investing activities in the year ended December 31, 2019 as compared to use $1,309,745 of cash for investing activities in the years ended December 31, 2018.
Cash (Used in) Provided by Financing Activities
The Company provided $333,879 of cash by investing activities in the six months ended June 30, 2020 as compared to provided $2,949,541 of cash for investing activities in the six months ended June 30, 2019.
Financing activities in the years ended December 31, 2019, used approximately $4,921,036 of cash provided as compared to $2,146,402 of cash provided in the year ended December 31, 2018.
The Company’s principal sources and uses of funds are investments from accredited investors. The Company would need to raise additional capital in order to meet its business plan. Management intends to secure additional funds using borrowing or the further sale of securities to accredited investors in the future. There is no assurance that we may secure funding, or whether it can do so on terms acceptable to us, or at all, and its liquidity would be severely compromised.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern which contemplates, amongst other things, the realization of assets and satisfaction of liabilities in the course of business.
We anticipate that our future liquidity requirements will arise from the need to fund our growth, pay our current obligations and future capital expenditures. The primary sources of funding for such requirements are expected to be cash generated from operations and raising additional funds from private sources and/or debt financing.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
31
Going Concern Qualification
Our independent auditors included an explanatory paragraph in their report on the accompanying financial statements expressing concerns about our ability to continue as a going concern. Our financial statements contain additional note disclosures describing the circumstances that lead to this disclosure by our independent auditors.
We anticipate that our future liquidity requirements will arise from the need to fund our growth, pay our current obligations and future capital expenditures. The primary sources of funding for such requirements are expected to be cash generated from operations and raising additional funds from private sources and/or debt financing.
Critical Accounting Policies
The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, and expenses and related disclosures about contingent assets and liabilities. We base these estimates and assumptions on historical experience and on various other information and assumptions that are believed to be reasonable under the circumstance. Estimates and assumptions about future events and their effects cannot be perceived with certainty and, accordingly, these estimates may change as additional information is obtained, as more experience is acquired, as our operating environment changes and as new events occur. Our critical accounting policies are listed in the notes to our audited financial statements included in of this Registration Statement.
32
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of 4DMed, Ltd.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of 4DMed, Ltd.(the “Company”) as of December 31, 2019 and 2018, and the related statements of operations and comprehensive losses, stockholders’ equity and cash flows for the years ended December 31, 2019 and 2018, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for the year ended December 31, 2019 and 2018 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ Centurion ZD CPA & Co. |
| Centurion ZD CPA & Co. |
| Hong Kong |
| June 11, 2020 |
We have served as the Company’s auditor since 2020.
F-2
| 4DMED, LTD. |
| Balance Sheets |
| December 31, | December 31, | |||||||
| 2019 | 2018 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Amount due from a related party | $ | 4,788,796 | $ | 1,841,799 | ||||
| Cash and cash equivalents | 3,074 | 343,622 | ||||||
| Total current assets | 4,791,870 | 2,185,421 | ||||||
| Total assets | $ | 4,791,870 | $ | 2,185,421 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Accrual | $ | 43,097 | $ | 21,549 | ||||
| Total current liabilities | 43,097 | 21,549 | ||||||
| Total liabilities | $ | 43,097 | $ | 21,549 | ||||
| Stockholders’ equity | ||||||||
| Common stock at $0.001 par value; 75,000,000 shares authorized; 24,821,003 and 22,861,434 shares issued and outstanding at December 31, 2019 and 2018, respectively. | 24,821 | 22,861 | ||||||
| Additional paid-in capital | 6,708,268 | 2,656,314 | ||||||
| Common stock subscribed | 892,645 | 25,523 | ||||||
| Accumulated deficit | (2,876,961 | ) | (540,826 | ) | ||||
| Total Stockholders’ equity | 4,748,773 | 2,163,872 | ||||||
| Total liabilities and Stockholders’ equity | $ | 4,791,870 | $ | 2,185,421 | ||||
The accompanying notes are an integral part of these financial statements.
F-3
| 4DMED, LTD. |
| Statements of Operations and Comprehensive Loss |
| Year ended December 31, 2019 | Year ended December 31, 2018 | |||||||
| Revenue | $ | - | $ | - | ||||
| Cost of sales | - | - | ||||||
| Gross profit | - | - | ||||||
| Operating expenses | (2,336,135 | ) | (514,583 | ) | ||||
| Loss from operations | (2,336,135 | ) | (514,583 | ) | ||||
| Income tax expense | - | - | ||||||
| Net loss | $ | (2,336,135 | ) | (514,583 | ) | |||
| Other comprehensive loss | ||||||||
| Foreign currency translation adjustments | - | - | ||||||
| Comprehensive loss | - | - | ||||||
The accompanying notes are an integral part of these financial statements.
F-4
4DMED, LTD.
Statements of Stockholders’ Equity
| Number
of Shares | Amount | Additional
Paid-in Capital | Common
Stock Subscribed | Accumulated Deficit | Stockholders’ Equity | |||||||||||||||||||
| Balance as of December 31, 2017 and January 1, 2018 | 21,397,950 | $ | 21,397 | $ | 528,634 | $ | 8,265 | $ | (26,243 | ) | $ | 532,053 | ||||||||||||
| Issuance of common stock | 1,463,484 | 1,464 | 2,127,680 | - | - | 2,129,144 | ||||||||||||||||||
| Stock subscriptions | - | - | - | 17,258 | - | 17,258 | ||||||||||||||||||
| Net loss for the year | - | - | - | - | (514,583 | ) | (514,583 | ) | ||||||||||||||||
| Balance as of December 31, 2018 and January 1, 2019 | 22,861,434 | $ | 22,861 | $ | 2,656,314 | $ | 25,523 | $ | (540,826 | ) | $ | 2,163,872 | ||||||||||||
| Issuance of common stock | 1,965,569 | 1,966 | 4,069,128 | - | - | 4,071,094 | ||||||||||||||||||
| Cancellation of common stock | (6,000 | ) | (6 | ) | (17,174 | ) | - | - | (17,180 | ) | ||||||||||||||
| Stock subscriptions | - | - | - | 867,122 | - | 867,122 | ||||||||||||||||||
| Net loss for the year | - | - | - | - | (2,336,135 | ) | (2,336,135 | ) | ||||||||||||||||
| Balance as of December 31, 2019 | 24,821,003 | $ | 24,821 | $ | 6,708,268 | $ | 892,645 | $ | (2,876,961 | ) | $ | 4,748,773 | ||||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
| 4DMED, LTD. |
| Statements of Cash Flows |
| Year ended December 31, 2019 | Year ended December 31, 2018 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss from operations | $ | (2,336,135 | ) | $ | (514,583 | ) | ||
| Changes in operating assets and liabilities: | ||||||||
| Accrual | 21,548 | 21,548 | ||||||
| Net cash used in operating activities | (2,314,587 | ) | (493,035 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Advanced to a related party | (2,952,525 | ) | (2,060,565 | ) | ||||
| Repayment from a related party | 5,528 | 750,820 | ||||||
| Net cash used in investing activities | (2,946,997 | ) | (1,309,745 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceed from issuance of common stock | 4,071,094 | 2,129,144 | ||||||
| Redemption of common stock | (17,180 | ) | - | |||||
| Proceed from stock subscription | 867,122 | 17,258 | ||||||
| Net cash provided by financing activities | 4,921,036 | 2,146,402 | ||||||
| Net (decreased) increased in cash and cash equivalents | (340,548 | ) | 343,622 | |||||
| Cash and cash equivalents, beginning of year | 343,622 | - | ||||||
| Cash and cash equivalents, end of year | $ | 3,074 | $ | 343,622 | ||||
| Supplemental cash flow disclosure: | ||||||||
| Cash paid for interest expense | $ | - | $ | - | ||||
| Cash paid for income taxes | $ | - | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
F-6
4DMED, LTD.
FOR THE YEAR ENDED DECEMBER 31, 2019 AND 2018
NOTE 1 - ORGANIZATION AND BUSINESS DESCRIPTION
4DMed, Ltd. (the “Company”) was incorporated on November 2, 2016 in the State of Nevada, USA. The Company is a medical software and medical device development company that intends to design, patent, and market a medical virtual reality operating system with integrated, customary haptic and position sensing interfaces for the health care education research, software and medical device markets. The purpose of the Company is to be a holding company for Hong Kong and Canadian operating companies.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
The financial statements have been prepared on a historical cost basis to reflect the financial position and results of operations of the Company in accordance with the accounting principles generally accepted in the United States of America (“US GAAP”).
USE OF ESTIMATES
The preparation of the Company’s financial statements in conformity with generally accepted accounting principles of the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management makes its best estimate of the ultimate outcome for these items based on historical trends and other information available when the financial statements are prepared. Actual results could differ from those estimates.
FAIR VALUE MEASUREMENTS
ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. Certain current assets and current liabilities are financial instruments. Management believes their carrying amounts are a reasonable estimate of fair value because of the short period of time between the origination of such instruments and their expected realization and, if applicable, their current interest rates are equivalent to interest rates currently available. The three levels of valuation hierarchy are defined as follows:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
F-7
The carrying amounts of financial assets and liabilities, such as balance with related parties approximate their fair values because of the short maturity of these instruments or the rate of interest of these instruments approximate the market rate of interest.
CASH AND CASH EQUIVALENTS
Cash and cash equivalents represent cash on hand, demand deposits, and other short-term highly liquid investments placed with banks, which have original maturities of three months or less and are readily convertible to known amounts of cash.
INCOME TAXES
The Company uses the asset and liability method of accounting for income taxes in accordance with Accounting Standards Codification (“ASC”) 740, “Income Taxes” (“ASC 740”). Under this method, income tax expense is recognized as the amount of: (i) taxes payable or refundable for the current year and (ii) future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of available evidence it is more likely than not that some portion or all of the deferred tax assets will not be realized.
RECENT ACCOUNTING PRONOUNCEMENTS
In March 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) ASU No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting (“ASU 2020-04”). The amendments in this update provide optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. The amendments in this ASU apply only to contracts, hedging relationships, and other transactions that reference LIBOR or another reference rate that is expected to be discontinued because of reference rate reform. The expedients and exceptions provided by the amendments do not apply to contract modifications made and hedging relationships entered into or evaluated after December 31, 2022, except for hedging relationships existing as of December 31, 2022, that an entity has elected certain optional expedients for and that are retained through the end of the hedging relationship. The amendments in this ASU are effective for all entities as of March 12, 2020 through December 31, 2022. The Company did not modify any material contracts due to reference rate reform during fiscal 2020. The Company will continue to evaluate the impact this guidance will have on financial statements for all future transactions affected by reference rate reform during the time permitted.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”). The FASB issued this update as part of its initiative to reduce complexity in accounting standards. The amendments in this ASU simplify the accounting for income taxes by removing certain exceptions to the general principles in Topic 740 and also improve consistent application of other areas by clarifying and amending existing guidance. ASU 2019-12 is effective for the Company in fiscal 2022 and early adoption is permitted. Certain amendments of this ASU may be adopted on a retrospective basis, modified retrospective basis or prospective basis. The Company is currently evaluating the impact this guidance will have on its financial statements.
F-8
In June 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments, which establishes ASC 326, Financial Instruments - Credit Losses. The ASU revises the measurement of credit losses for financial assets measured at amortized cost from an incurred loss methodology to an expected loss methodology. The ASU affects trade receivables, debt securities, net investment in leases, and most other financial assets that represent a right to receive cash. Additional disclosures about significant estimates and credit quality are also required. In November 2018, the FASB issued ASU No. 2018-19, Codification Improvements to Topic 326, Financial Instruments - Credit Losses. This ASU clarifies that receivables from operating leases are accounted for using the lease guidance and not as financial instruments. In May 2019, the FASB issued ASU No. 2019-05, Targeted Transition Relief, which amends ASC 326. This ASU provides an option to irrevocably elect to measure certain individual financial assets at fair value instead of amortized cost. The Company is currently evaluating the impact this guidance will have on its financial statements.
In August 2018, the FASB issued ASU 2018-14, Compensation - Retirement Benefits - Defined Benefit Plans - General (Subtopic 715-20): Disclosure Framework - Changes to the Disclosure Requirements for Defined Benefit Plans (“ASU 2018-14”), which updates the standard to remove disclosures that no longer are considered cost beneficial, clarifies the specific requirements of disclosures, and adds disclosure requirements identified as relevant. ASU 2018-14 is effective for the Company in fiscal 2021 on a retroactive basis. This guidance will have no impact on the Company’s financial statements upon adoption other than with respect to the updated disclosure requirements.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842). In July 2018, the FASB issued ASU No. 2018-10, Codification Improvements to Topic 842, Leases, which provides narrow amendments to clarify how to apply certain aspects of the new lease standard, and ASU 2018-11, Leases (Topic 842): Targeted Improvements, which addressed implementation issues related to the new lease standard. These and certain other lease-related ASUs have generally been codified in ASC 842, Leases (“ASC 842”). ASC 842 supersedes the lease accounting requirements in ASC 840, Leases (“ASC 840”). ASC 842 establishes a right-of-use model that requires a lessee to record a right-of-use (“ROU”) asset and a lease liability on the balance sheet for all leases. Under ASC 842, leases are classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The standard also requires disclosures around the amount, timing and uncertainty of cash flows arising from leases. The Company is currently evaluating the impact of adoption of this new standard and does not believe that the adoption of this ASU will have a significant impact on its financial statements.
Note 3 - GOING CONCERN
The accompanying financial statements have been prepared assuming the Company will continue as a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. The Company had an accumulated deficit of $2,876,961 and $540,826 at December 31, 2019 and 2018, respectively, and had a net loss of $2,336,135 and $514,583 for the years ended December 31, 2019 and 2018, respectively.
The Company’s continuation as a going concern is dependent on its ability to obtain additional financing to fund operations, implement its business model, and ultimately, attain profitable operations. The Company will need to secure additional funds through various means, including equity and debt financing or any similar financing. There can be no assurance that the Company will be able to obtain additional equity or debt financing, if and when needed, on terms acceptable to the Company, or at all. Any additional equity or debt financing may involve substantial dilution to the Company’s stockholders, restrictive covenants or high interest costs. The Company’s long-term liquidity also depends upon its ability to generate revenues and achieve profitability.
F-9
NOTE 4 - ACCRUAL
Accrual consisted of the following:
| December 31, 2019 | December 31, 2018 | |||||||
| Accrued accounting fee | $ | 3,097 | $ | 1,549 | ||||
| Accrued audit fee | 40,000 | 20,000 | ||||||
| $ | 43,097 | $ | 21,549 | |||||
NOTE 5 - COMMON STOCK
The Company entered into certain private placements with different investors. During the years ended December 31, 2019 and 2018, the Company issued 1,965,569 and 1,463,484 shares of common stock, and cancelled 6,000 and nil of shares of common stock, respectively. As of December 31, 2019 and 2018, 24,821,003 and 22,861,434 shares respectively, were issued and outstanding.
In addition, the Company has received $892,645 and $25,523, respectively as of December 31, 2019 and 2018 from the stock subscription and the shares of common stock had not been issued.
NOTE 6 - INCOME TAXES
The Company is incorporated in the State of Nevada in the U.S., and is subject to a gradual U.S. federal corporate income tax of 21%. The Company did not generate taxable income for the years ended December 31, 2019 and 2018.
The Company has not recognized an income tax benefit for its operating losses generated based on uncertainties concerning its ability to generate taxable income in future periods. The tax benefit for the periods presented is offset by a valuation allowance established against deferred tax assets arising from the net operating losses and other temporary differences, the realization of which could not be considered more likely than not. In future periods, tax benefits and related deferred tax assets will be recognized when management considers realization of such amounts to be more likely than not. A valuation allowance will be maintained until sufficient positive evidence exists to support the reversal of any portion or all of the valuation allowance
| December 31, 2019 | December 31, 2018 | |||||||
| Deferred tax asset from operating losses carry-forwards | $ | 490,588 | $ | 108,062 | ||||
| Valuation allowance | (490,588 | ) | (108,062 | ) | ||||
| Deferred tax asset, net | $ | - | $ | - | ||||
F-10
NOTE 7 - RELATED PARTY TRANSACTIONS AND BALANCES
| a. | Related party |
| Name of related party | Relationship with the Company | |
| 4DMED Limited (Hong Kong Incorporated Company) | Common Director, Cheung Yuen May |
| b. | Related party balances |
The Company had the following related party balances at December 31, 2019 and 2018:
| December 31, 2019 | December 31, 2018 | |||||||
| Due from a related party: | ||||||||
| 4DMED Limited | (i) | 4,788,796 | 1,841,799 | |||||
| (i) | As of December 31, 2019 and 2018, the above amount due from related party is without interest and due on demand. |
NOTE 8 - COMMITMENTS AND CONTINGENCIES
Operating lease
The Company did not have any operating lease as of December 31, 2019 and 2018.
Legal proceedings
There has been no legal proceeding in which the Company is a party as of December 31, 2019 and 2018.
NOTE 9 - SUBSEQUENT EVENT
There were no events or transactions that would require recognition or disclosure in financial statements for the year ended December 31, 2019.
F-11
| 4DMED, LTD. |
| INDEX TO CONDENSED FINANCIAL STATEMENTS |
F-12
| 4DMED, LTD. |
| Condensed Balance Sheets |
| June 30, | December 31, | |||||||
| 2020 | 2019 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Amount due from a related party | $ | 4,186,184 | $ | 4,788,796 | ||||
| Cash and cash equivalents | 132,969 | 3,074 | ||||||
| Total current assets | 4,319,153 | 4,791,870 | ||||||
| Total assets | $ | 4,319,153 | $ | 4,791,870 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Accrual | $ | 5,581 | $ | 43,097 | ||||
| Deposit received | 30,000 | - | ||||||
| Total current liabilities | 35,581 | 43,097 | ||||||
| Total liabilities | $ | 35,581 | $ | 43,097 | ||||
| Stockholders’ equity | ||||||||
| Common stock at $0.001 par value; 75,000,000 shares authorized; 25,250,703 and 24,821,003 shares issued and outstanding at June 30, 2020 and December 31, 2019, respectively. | 25,251 | 24,821 | ||||||
| Additional paid-in capital | 7,504,074 | 6,708,268 | ||||||
| Common stock subscribed | 430,288 | 892,645 | ||||||
| Accumulated deficit | (3,676,041 | ) | (2,876,961 | ) | ||||
| Total Stockholders’ equity | 4,283,572 | 4,748,773 | ||||||
| Total liabilities and Stockholders’ equity | $ | 4,319,153 | $ | 4,791,870 | ||||
The accompanying notes are an integral part of these condensed financial statements.
F-13
| 4DMED, LTD. |
| Condensed Statements of Operations and Comprehensive Loss |
| For the Three Months Ended June 30, | For the Six Months Ended June 30, | |||||||||||||||
| 2020 | 2019 | 2020 | 2019 | |||||||||||||
| (Unaudited) | (Unaudited) | (Unaudited) | (Unaudited) | |||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| Cost of sales | - | - | - | - | ||||||||||||
| Gross profit | - | - | - | - | ||||||||||||
| Operating expenses | (423,801 | ) | (771,669 | ) | (799,080 | ) | (1,415,471 | ) | ||||||||
| Loss from operations | (423,801 | ) | (771,669 | ) | (799,080 | ) | (1,415,471 | ) | ||||||||
| Income tax expense | - | - | - | - | ||||||||||||
| Net loss | $ | (423,801 | ) | $ | (771,669 | ) | $ | (799,080 | ) | $ | (1,415,471 | ) | ||||
| Other comprehensive loss | ||||||||||||||||
| Foreign currency translation adjustments | - | - | - | - | ||||||||||||
| Comprehensive loss | $ | - | $ | - | $ | - | $ | - | ||||||||
The accompanying notes are an integral part of these condensed financial statements.
F-14
4DMED, LTD.
Condensed Statements of Stockholders’ Equity
| Number
of Shares | Amount | Additional
Paid-in Capital | Common
Stock Subscribed | Accumulated Deficit | Stockholders’ Equity | |||||||||||||||||||
| Balance as of December 31, 2019 | 24,821,003 | $ | 24,821 | $ | 6,708,268 | $ | 892,645 | $ | (2,876,961 | ) | $ | 4,748,773 | ||||||||||||
| Issuance of common stock | 289,700 | 290 | 638,445 | (637,35 | ) | - | - | |||||||||||||||||
| Stock subscriptions | - | - | - | 176,378 | - | 176,378 | ||||||||||||||||||
| Net loss for the period | - | - | - | - | (375,279 | ) | (375,279 | ) | ||||||||||||||||
| Balance as of March 31, 2020 | 25,110,703 | $ | 25,111 | $ | 7,346,713 | $ | 430,288 | $ | (3,252,240 | ) | $ | 4,549,872 | ||||||||||||
| Issuance of common stock | 140,000 | 140 | 157,361 | - | - | 157,501 | ||||||||||||||||||
| Net loss for the period | - | - | - | - | (423,801 | ) | (423,801 | ) | ||||||||||||||||
| Balance as of June 30, 2020 | 25,250,703 | 25,251 | 7,504,074 | 430,288 | (3,676,041 | ) | 4,283,572 | |||||||||||||||||
| Number
of Shares | Amount | Additional
Paid-in Capital | Common
Stock Subscribed | Accumulated Deficit | Stockholders’ Equity | |||||||||||||||||||
| Balance as of December 31, 2018 | 22,861,434 | $ | 22,861 | $ | 2,656,314 | $ | 25,523 | $ | (540,826 | ) | $ | 2,163,872 | ||||||||||||
| Issuance of common stock | 267,630 | 268 | 604,602 | (25,523 | ) | - | 579,347 | |||||||||||||||||
| Stock subscriptions | - | - | - | 852,263 | - | 852,263 | ||||||||||||||||||
| Net loss for the period | - | - | - | - | (643,802 | ) | (643,802 | ) | ||||||||||||||||
| Balance as of March 31, 2019 | 23,129,064 | 23,129 | 3,260,916 | 852,263 | (1,184,628 | ) | 2,951,680 | |||||||||||||||||
| Stock subscriptions | - | - | - | 1,517,931 | - | 1,571,931 | ||||||||||||||||||
| Net loss for the period | - | - | - | - | (771,669 | ) | (771,669 | ) | ||||||||||||||||
| Balance as of June 30, 2019 | 23,129,064 | 23,129 | 3,260,916 | 2,370,194 | (1,956,297 | ) | 3,697,942 | |||||||||||||||||
The accompanying notes are an integral part of these condensed financial statements.
F-15
| 4DMED, LTD. |
| Condensed Statements of Cash Flows |
| Six Months Ended June 30, 2020 | Six Months Ended June 30, 2019 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss from operations | $ | (799,080 | ) | $ | (1,415,471 | ) | ||
| Changes in operating assets and liabilities: | ||||||||
| Accrual | (37,516 | ) | - | |||||
| Deposit received | 30,000 | - | ||||||
| Net cash used in operating activities | (806,596 | ) | (1,415,471 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Advanced to a related party | (333,878 | ) | (1,308,407 | ) | ||||
| Repayment from a related party | 936,490 | - | ||||||
| Net cash provided by (used in) investing activities | 602,612 | (1,308,407 | ) | |||||
| Cash flows from financing activities: | ||||||||
| Proceed from issuance of common stock | 796,236 | 604,870 | ||||||
| Proceed from stock subscription | - | 2,344,671 | ||||||
| Redemption of stock subscription | (462,357 | ) | - | |||||
| Net cash provided by financing activities | 333,879 | 2,949,541 | ||||||
| Net increased in cash and cash equivalents | 129,895 | 225,663 | ||||||
| Cash and cash equivalents, beginning of period | 3,074 | 343,622 | ||||||
| Cash and cash equivalents, end of period | $ | 132,969 | $ | 569,285 | ||||
| Supplemental cash flow disclosure: | ||||||||
| Cash paid for interest expense | $ | - | $ | - | ||||
| Cash paid for income taxes | $ | - | $ | - | ||||
The accompanying notes are an integral part of these condensed financial statements.
F-16
4DMED, LTD.
FOR SIX MONTHS ENDED MARCH 31, 2020 AND 2020
NOTES TO CONDENSED FINANCIAL STATEMENTS
NOTE 1 - ORGANIZATION AND BUSINESS DESCRIPTION
4DMed, Ltd. (the “Company”) was incorporated on November 2, 2016 in the State of Nevada, USA. The Company is a medical software and medical device development company that intends to design, patent, and market a medical virtual reality operating system with integrated, customary haptic and position sensing interfaces for the health care education research, software and medical device markets. The purpose of the Company is to be a holding company for Hong Kong and Canadian operating companies.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
The condensed financial statements have been prepared on a historical cost basis to reflect the financial position and results of operations of the Company in accordance with the accounting principles generally accepted in the United States of America (“US GAAP”).
USE OF ESTIMATES
The preparation of the Company’s condensed financial statements in conformity with generally accepted accounting principles of the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management makes its best estimate of the ultimate outcome for these items based on historical trends and other information available when the financial statements are prepared. Actual results could differ from those estimates.
FAIR VALUE MEASUREMENTS
ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. Certain current assets and current liabilities are financial instruments. Management believes their carrying amounts are a reasonable estimate of fair value because of the short period of time between the origination of such instruments and their expected realization and, if applicable, their current interest rates are equivalent to interest rates currently available. The three levels of valuation hierarchy are defined as follows:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
F-17
The carrying amounts of financial assets and liabilities, such as balance with related parties approximate their fair values because of the short maturity of these instruments or the rate of interest of these instruments approximate the market rate of interest.
CASH AND CASH EQUIVALENTS
Cash and cash equivalents represent cash on hand, demand deposits, and other short-term highly liquid investments placed with banks, which have original maturities of three months or less and are readily convertible to known amounts of cash.
INCOME TAXES
The Company uses the asset and liability method of accounting for income taxes in accordance with Accounting Standards Codification (“ASC”) 740, “Income Taxes” (“ASC 740”). Under this method, income tax expense is recognized as the amount of: (i) taxes payable or refundable for the current year and (ii) future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of available evidence it is more likely than not that some portion or all of the deferred tax assets will not be realized.
RECENT ACCOUNTING PRONOUNCEMENTS
In June 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) ASU No. 2020-05, Revenue from Contracts with Customers (Topic 606) and Leases (Topic 842): Effective Dates for Certain Entities (“ASU 2020-05”). The amendments in this update provide optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. The amendments in this ASU apply only to contracts, hedging relationships, and other transactions that reference LIBOR or another reference rate that is expected to be discontinued because of reference rate reform. The expedients and exceptions provided by the amendments do not apply to contract modifications made and hedging relationships entered into or evaluated after December 31, 2022, except for hedging relationships existing as of December 31, 2022, that an entity has elected certain optional expedients for and that are retained through the end of the hedging relationship. The amendments in this ASU are effective for all entities as of March 12, 2020 through December 31, 2022. The Company did not modify any material contracts due to reference rate reform during fiscal 2020. The Company will continue to evaluate the impact this guidance will have on financial statements for all future transactions affected by reference rate reform during the time permitted.
In December 2020, the FASB issued ASU No. 2020-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2020-12”). The FASB issued this update as part of its initiative to reduce complexity in accounting standards. The amendments in this ASU simplify the accounting for income taxes by removing certain exceptions to the general principles in Topic 740 and also improve consistent application of other areas by clarifying and amending existing guidance. ASU 2020-12 is effective for the Company in fiscal 2022 and early adoption is permitted. Certain amendments of this ASU may be adopted on a retrospective basis, modified retrospective basis or prospective basis. The Company is currently evaluating the impact this guidance will have on its financial statements.
F-18
In June 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments, which establishes ASC 326, Financial Instruments - Credit Losses. The ASU revises the measurement of credit losses for financial assets measured at amortized cost from an incurred loss methodology to an expected loss methodology. The ASU affects trade receivables, debt securities, net investment in leases, and most other financial assets that represent a right to receive cash. Additional disclosures about significant estimates and credit quality are also required. In November 2019, the FASB issued ASU No. 2019-19, Codification Improvements to Topic 326, Financial Instruments - Credit Losses. This ASU clarifies that receivables from operating leases are accounted for using the lease guidance and not as financial instruments. In May 2020, the FASB issued ASU No. 2020-05, Targeted Transition Relief, which amends ASC 326. This ASU provides an option to irrevocably elect to measure certain individual financial assets at fair value instead of amortized cost. The Company is currently evaluating the impact this guidance will have on its financial statements.
In August 2019, the FASB issued ASU 2019-14, Compensation - Retirement Benefits - Defined Benefit Plans - General (Subtopic 715-20): Disclosure Framework - Changes to the Disclosure Requirements for Defined Benefit Plans (“ASU 2019-14”), which updates the standard to remove disclosures that no longer are considered cost beneficial, clarifies the specific requirements of disclosures, and adds disclosure requirements identified as relevant. ASU 2019-14 is effective for the Company in fiscal 2021 on a retroactive basis. This guidance will have no impact on the Company’s financial statements upon adoption other than with respect to the updated disclosure requirements.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842). In July 2019, the FASB issued ASU No. 2019-10, Codification Improvements to Topic 842, Leases, which provides narrow amendments to clarify how to apply certain aspects of the new lease standard, and ASU 2019-11, Leases (Topic 842): Targeted Improvements, which addressed implementation issues related to the new lease standard. These and certain other lease-related ASUs have generally been codified in ASC 842, Leases (“ASC 842”). ASC 842 supersedes the lease accounting requirements in ASC 840, Leases (“ASC 840”). ASC 842 establishes a right-of-use model that requires a lessee to record a right-of-use (“ROU”) asset and a lease liability on the balance sheet for all leases. Under ASC 842, leases are classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The standard also requires disclosures around the amount, timing and uncertainty of cash flows arising from leases. The Company is currently evaluating the impact of adoption of this new standard and does not believe that the adoption of this ASU will have a significant impact on its financial statements.
NOTE 3 - GOING CONCERN
The accompanying financial statements have been prepared assuming the Company will continue as a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. The Company had an accumulated deficit of $3,676,041 and $2,876,961 at June 30, 2020 and December 31, 2019, respectively, and had a net loss of $799,080 and $1,415,471 for six months ended June 30, 2020 and 2019, respectively, and had a net loss of $423,801 and $771,669 for three months ended June 30, 2020 and 2019, respectively.
The Company’s continuation as a going concern is dependent on its ability to obtain additional financing to fund operations, implement its business model, and ultimately, attain profitable operations. The Company will need to secure additional funds through various means, including equity and debt financing or any similar financing. There can be no assurance that the Company will be able to obtain additional equity or debt financing, if and when needed, on terms acceptable to the Company, or at all. Any additional equity or debt financing may involve substantial dilution to the Company’s stockholders, restrictive covenants or high interest costs. The Company’s long-term liquidity also depends upon its ability to generate revenues and achieve profitability.
F-19
NOTE 4 - ACCRUAL
Accrual consisted of the following:
| June 30, 2020 | December 31, 2019 | |||||||
| (Unaudited) | ||||||||
| Accrued accounting fee | $ | 2,581 | $ | 3,097 | ||||
| Accrued audit fee | 3,000 | 40,000 | ||||||
| $ | 5,581 | $ | 43,097 | |||||
NOTE 5 - COMMON STOCK
The Company entered into certain private placements with different investors. During six months ended June 30, 2020 and 2019, the Company issued 429,700 and 267,630 shares of common stock, respectively. As of June 30, 2020 and December 31, 2019, 25,250,703 and 24,821,003 shares respectively, were issued and outstanding.
In addition, the Company has received $430,288 and $892,645, respectively as of June 30, 2020 and December 31, 2019 from the stock subscription and the shares of common stock had not been issued.
NOTE 6 - INCOME TAXES
The Company is incorporated in the State of Nevada in the U.S., and is subject to a gradual U.S. federal corporate income tax of 21%. The Company did not generate taxable income for six months ended June 30, 2020 and 2019.
The Company has not recognized an income tax benefit for its operating losses generated based on uncertainties concerning its ability to generate taxable income in future periods. The tax benefit for the periods presented is offset by a valuation allowance established against deferred tax assets arising from the net operating losses and other temporary differences, the realization of which could not be considered more likely than not. In future periods, tax benefits and related deferred tax assets will be recognized when management considers realization of such amounts to be more likely than not. A valuation allowance will be maintained until sufficient positive evidence exists to support the reversal of any portion or all of the valuation allowance.
| June 30, 2020 | December 31, 2019 | |||||||
| (Unaudited) | ||||||||
| Deferred tax asset from operating losses carry-forwards | $ | 167,807 | $ | 490,588 | ||||
| Valuation allowance | (167,807 | ) | (490,588 | ) | ||||
| Deferred tax asset, net | $ | - | $ | - | ||||
F-20
NOTE 7 - RELATED PARTY TRANSACTIONS AND BALANCES
| a. | Related party |
| Name of related party | Relationship with the Company | |
| 4DMED Limited (Hong Kong Incorporated Company) | Common Director, Cheung Yuen May |
| b. | Related party balances |
The Company had the following related party balances at June 30, 2020 and December 31, 2019:
| June 30, 2020 | December 31, 2019 | |||||||
| (Unaudited) | ||||||||
| Due from a related party: | ||||||||
| 4DMED Limited | (i) | 4,186,184 | 4,788,796 | |||||
| (i) | As of June 30, 2020 and December 31, 2019, the above amount due from related party is without interest and due on demand. |
NOTE 8 - COMMITMENTS AND CONTINGENCIES
Operating lease
The Company did not have any operating lease as of June 30, 2020 and December 31, 2019.
Legal proceedings
There has been no legal proceeding in which the Company is a party as of June 30, 2020 and December 31, 2019.
NOTE 9 - SUBSEQUENT EVENT
There were no events or transactions that would require recognition or disclosure in financial statements for the six months ended June 30, 2020.
F-21
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. Other Expenses of Issuance and Distribution
The following table sets forth the expenses in connection with the issuance and distribution of the securities being registered hereby. All such expenses will be borne by the registrant.
| Name of Expense | Amount | |||
| Securities and Exchange Commission registration fee | $ | 52 | ||
| Legal, accounting fees and expenses(1) | $ | 15,000 | ||
| Edgar filing, printing and engraving fees(1) | $ | 1,500 | ||
| Total | $ | 16,552 | ||
| (1) | Estimated. |
ITEM 14. Indemnification of Directors and Officers
Our officers and directors are indemnified as provided by the Nevada Statutes and by our Bylaws.
Under the Nevada Revised Statutes, director immunity from liability to a company or its stockholders for monetary liabilities applies automatically unless it is specifically limited by a company’s Articles of Incorporation. Our Articles of Incorporation do not specifically limit our directors’ immunity. Excepted from that immunity are: (a) a willful failure to deal fairly with the company or its stockholders in connection with a matter in which the director has a material conflict of interest; (b) a violation of criminal law, unless the director had reasonable cause to believe that his or her conduct was lawful or no reasonable cause to believe that his or her conduct was unlawful; (c) a transaction from which the director derived an improper personal profit; and (d) willful misconduct.
Our Bylaws provide that we will indemnify our directors and officers to the fullest extent not prohibited by Nevada law; provided, however, that we may modify the extent of such indemnification by individual contracts with our directors and officers; and, provided, further, that we shall not be required to indemnify any director or officer in connection with any proceeding, or part thereof, initiated by such person unless such indemnification: (a) is expressly required to be made by law, (b) the proceeding was authorized by our Board of Directors, (c) is provided by us, in our sole discretion, pursuant to the powers vested in us under Nevada law or (d) is required to be made pursuant to the Bylaws.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and control persons pursuant to the foregoing provisions or otherwise, we have been advised that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy, and is, therefore, unenforceable.
22
ITEM 15. Recent Sales of Unregistered Securities
We issued a total of 4,250,703 shares to 189 separate accredited foreign shareholders from July 20, 2017 through August 5, 2020, pursuant to private placements of our common stock exempt from registration under Regulation S of the Securities Act of 1933, for total proceeds of approximately $7,508,324.26. We issued an additional 21,000,000 shares to our founding shareholder for services rendered in connection with the formation and organization of the Company issued pursuant to a private placement of our common stock exempt from registration under Regulation S of the Securities Act of 1933 for a total value of approximately $21,000. The following table sets forth the Shareholders, Shares Purchased, Purchase Price and the Date Purchased.
| Shareholder Name | Shares Purchased | Purchase Price $ Per shares | Amounts received | Date Purchased | ||||||||||||
| 1 | Achim Brossmann | 2,000 | $ | 2.752 | 5,504.00 | 25-Nov-19 | ||||||||||
| 2 | Albert Alwin | 1,000 | $ | 2.781 | 2,781.36 | 15-Aug-18 | ||||||||||
| 2 | Albert Alwin | 1,000 | $ | 2.702 | 2,702.00 | 23-Nov-18 | ||||||||||
| 3 | Alexander Haindorff | 1,000 | $ | 2.730 | 2,730.45 | 22-Jan-20 | ||||||||||
| 4 | Alois Kniewallner | 20,000 | $ | 0.573 | 11,455.22 | 4-Dec-17 | ||||||||||
| 4 | Alois Kniewallner | 236,000 | $ | 1.401 | 330,586.42 | 8-Aug-18 | ||||||||||
| 4 | Alois Kniewallner | 110,000 | $ | 1.879 | 206,731.38 | 12-Feb-19 | ||||||||||
| 4 | Alois Kniewallner | 80,000 | $ | 1.681 | 134,499.94 | 13-Mar-19 | ||||||||||
| 5 | Andreas Loecher | 2,000 | $ | 2.778 | 5,555.73 | 28-Jun-19 | ||||||||||
| 5 | Andreas Loecher | 2,083 | $ | 2.618 | 5,454.11 | 23-Oct-19 | ||||||||||
| 6 | Andreas Oleksow | 2,000 | $ | 2.858 | 5,715.79 | 20-Oct-17 | ||||||||||
| 6 | Andreas Oleksow | 3,000 | $ | 2.731 | 8,193.17 | 7-Feb-18 | ||||||||||
| 7 | Andreas Pippig | 1,000 | $ | 2.914 | 2,913.86 | 26-Jun-18 | ||||||||||
| 7 | Andreas Pippig | 2,000 | $ | 2.625 | 5,250.34 | 15-Feb-19 | ||||||||||
| 8 | Armin Gemmer | 10,000 | $ | 2.734 | 27,340.02 | 15-Nov-19 | ||||||||||
| 8 | Armin Gemmer | 5,000 | $ | 2.219 | 11,092.59 | 22-Jan-20 | ||||||||||
| 9 | Armin Wagner | 10,000 | $ | 2.436 | 24,362.46 | 29-Jun-18 | ||||||||||
| 10 | Bernhard Hoehnke | 2,000 | $ | 1.280 | 2,560.01 | 16-Aug-17 | ||||||||||
| 11 | Bernd Dankelmann | 2,000 | $ | 2.824 | 5,647.66 | 27-Jul-18 | ||||||||||
| 11 | Bernd Dankelmann | 12,000 | $ | 2.411 | 28,930.44 | 4-Apr-19 | ||||||||||
| 12 | Bernd Fricke | 1,000 | $ | 2.675 | 2,674.79 | 29-Aug-19 | ||||||||||
| 12 | Bernd Fricke | 4,000 | $ | 2.215 | 8,861.99 | 22-Jan-20 | ||||||||||
| 13 | Bernd Klack | 2,000 | $ | 2.761 | 5,521.14 | 14-Nov-19 | ||||||||||
| 14 | Bernd Knorr | 2,000 | $ | 1.841 | 3,682.29 | 2-May-18 | ||||||||||
| 14 | Bernd Knorr | 2,000 | $ | 2.733 | 5,465.28 | 19-Feb-19 | ||||||||||
| 15 | Bernd Weicherding | 10,000 | $ | 1.054 | 10,538.80 | 29-Dec-17 | ||||||||||
| 15 | Bernd Weicherding | 31,666 | $ | 1.466 | 46,409.12 | 24-Apr-18 | ||||||||||
| 15 | Bernd Weicherding | 1,834 | $ | 1.623 | 2,977.00 | 21-Dec-18 | ||||||||||
| 15 | Bernd Weicherding | 17,500 | $ | 0.848 | 14,834.73 | 25-Apr-19 | ||||||||||
| 15 | Bernd Weicherding | 10,000 | $ | 1.985 | 19,848.14 | 11-Dec-19 | ||||||||||
| 16 | Carsten Bechtel | 2,000 | $ | 2.715 | 5,430.00 | 6-Dec-18 | ||||||||||
| 17 | Christian Dubi | 7,500 | $ | 0.788 | 5,908.61 | 4-Aug-17 | ||||||||||
| 18 | Christoph Gassman | 20,000 | $ | 1.768 | 24,277.10 | 27-Jul-18 | ||||||||||
| 18 | Christoph Gassman | 6,500 | $ | 1.768 | 22,581.00 | 23-Nov-18 | ||||||||||
| 19 | Christoph Magnusson | 4,000 | $ | 2.915 | 11,661.30 | 20-Jul-18 | ||||||||||
| 20 | Claus Neckel | 22,000 | $ | 2.535 | 55,775.61 | 5-Feb-19 | ||||||||||
| 20 | Claus Neckel | 205,500 | $ | 1.601 | 329,077.88 | 8-Jul-19 | ||||||||||
| 20 | Claus Neckel | 76,000 | $ | 2.308 | 175,434.75 | 18-Sep-19 | ||||||||||
| 20 | Claus Neckel | 50,000 | $ | 1.662 | 83,106.00 | 21-Aug-19 | ||||||||||
| 21 | Colin Weiss | 4,500 | $ | 1.318 | 5,930.06 | 31-Jan-18 | ||||||||||
| 21 | Colin Weiss | 2,500 | $ | 2.392 | 5,980.00 | 13-Dec-18 | ||||||||||
| 22 | Daniel Gisiger | 1,000 | $ | 2.715 | 2,715.01 | 2-Apr-19 | ||||||||||
| 23 | Daniel Runden | 1,000 | $ | 2.717 | 2,717.00 | 21-Dec-18 | ||||||||||
| 24 | Detlef Lorenz | 2,000 | $ | 2.763 | 5,526.66 | 2-Oct-19 | ||||||||||
| 25 | Dieter Borner | 3,000 | $ | 0.885 | 2,655.61 | 20-Jul-17 | ||||||||||
| 25 | Dieter Borner | 3,000 | $ | 2.135 | 6,406.17 | 6-Feb-18 | ||||||||||
| 26 | Dieter Hankiewicz | 7,000 | $ | 0.735 | 5,146.57 | 16-Aug-17 | ||||||||||
| 26 | Dieter Hankiewicz | 2,000 | $ | 2.575 | 5,149.75 | 2-Mar-18 | ||||||||||
| 26 | Dieter Hankiewicz | 2,000 | $ | 2.071 | 4,142.00 | 5-Feb-19 | ||||||||||
| 27 | Dieter Held | 1,000 | $ | 2.733 | 2,733.00 | 5-Feb-19 | ||||||||||
| 28 | Dieter Herkenrath | 8,000 | $ | 2.776 | 22,210.62 | 9-Dec-19 | ||||||||||
| 29 | Dr Kurt Grabner | 1,000 | $ | 2.845 | 2,844.90 | 4-Dec-17 | ||||||||||
| 30 | Dr Walter Sturm | 4,000 | $ | 2.774 | 11,095.14 | 12-Dec-17 | ||||||||||
23
| Shareholder Name | Shares Purchased | Purchase Price $ Per shares | Amounts received | Date Purchased | ||||||||||||
| 31 | Eberhard Behrend | 5,000 | $ | 1.333 | 6,666.00 | 6-Nov-18 | ||||||||||
| 31 | Eberhard Behrend | 9,546 | $ | 2.398 | 22,893.00 | 5-Feb-19 | ||||||||||
| 31 | Eberhard Behrend | 35,454 | $ | 2.308 | 81,837.30 | 18-Apr-19 | ||||||||||
| 32 | Eckbert Kraft | 4,000 | $ | 2.576 | 10,303.76 | 23-Aug-17 | ||||||||||
| 33 | Edwin Dornisch | 4,000 | $ | 2.788 | 11,150.84 | 28-Aug-18 | ||||||||||
| 33 | Edwin Dornisch | 16,000 | $ | 2.084 | 33,347.19 | 7-Feb-19 | ||||||||||
| 33 | Edwin Dornisch | 15,000 | $ | 2.252 | 36,036.47 | 22-Jul-19 | ||||||||||
| 33 | Edwin Dornisch | 1,000 | $ | 2.252 | - | 24-Nov-19 | ||||||||||
| 34 | Edwin Pirzer | 1,000 | $ | 2.715 | 2,714.54 | 14-Jun-19 | ||||||||||
| 34 | Edwin Pirzer | 14,000 | $ | 2.213 | 30,975.74 | 11-Oct-19 | ||||||||||
| 34 | Edwin Pirzer | 35,000 | $ | 2.102 | 73,564.30 | 4-Dec-19 | ||||||||||
| 35 | Edwin Sebenring | 4,000 | $ | 2.145 | 8,581.75 | 27-Mar-18 | ||||||||||
| 36 | Ekkehard Ady | 4,000 | $ | 2.764 | 11,054.93 | 11-Jun-19 | ||||||||||
| 36 | Ekkehard Ady | 10,000 | $ | 2.376 | 23,758.68 | 28-Nov-19 | ||||||||||
| 37 | Ellen Kluthe | 5,000 | $ | 2.750 | 13,750.07 | 5-Nov-19 | ||||||||||
| 37 | Ellen Kluthe | 2,000 | $ | 2.764 | 5,528.29 | 26-Dec-19 | ||||||||||
| 38 | Erich Sperer | 10,000 | $ | 2.290 | 22,896.33 | 14-Feb-19 | ||||||||||
| 38 | Erich Sperer | 5,000 | $ | 2.167 | 10,836.28 | 8-Apr-19 | ||||||||||
| 39 | Erwin Vaih | 2,500 | $ | 2.687 | 6,716.44 | 25-Sep-19 | ||||||||||
| 40 | Eugen Preuss | 2,000 | $ | 2.960 | 5,919.41 | 13-Nov-17 | ||||||||||
| 41 | Falko Hammermuller | 2,000 | $ | 2.566 | 5,131.18 | 1-Aug-17 | ||||||||||
| 41 | Falko Hammermuller | 3,000 | $ | 2.756 | 8,267.92 | 26-Mar-19 | ||||||||||
| 42 | Frank Kramer | 1,000 | $ | 2.740 | 2,739.69 | 21-Nov-19 | ||||||||||
| 43 | Frank Sauer | 2,000 | $ | 2.552 | 5,103.94 | 29-Jun-18 | ||||||||||
| 44 | Frank Schumann | 5,000 | $ | 2.757 | 13,786.80 | 28-Oct-19 | ||||||||||
| 45 | Frank Zimmermann | 1,000 | $ | 2.773 | 2,773.43 | 6-Feb-19 | ||||||||||
| 46 | Franz Burger | 2,000 | $ | 2.715 | 5,430.00 | 2-Jan-19 | ||||||||||
| 47 | Franz Georg Baaden | 2,000 | $ | 2.858 | 5,715.73 | 18-Dec-17 | ||||||||||
| 47 | Franz Georg Baaden | 2,000 | $ | 2.965 | 5,929.64 | 31-Jan-18 | ||||||||||
| 48 | Franz Leitner | 1,500 | $ | 2.757 | 4,134.88 | 24-Oct-19 | ||||||||||
| 49 | Friedhelm Finke | 2,000 | $ | 2.881 | 5,761.78 | 21-Sep-17 | ||||||||||
| 49 | Friedhelm Finke | 5,000 | $ | 2.841 | 14,206.58 | 18-Oct-17 | ||||||||||
| 49 | Friedhelm Finke | 50,000 | $ | 0.553 | 27,633.98 | 16-Mar-18 | ||||||||||
| 49 | Friedhelm Finke | 45,000 | $ | 2.705 | 121,715.11 | 13-Dec-18 | ||||||||||
| 49 | Friedhelm Finke | 11,000 | $ | 1.472 | 16,187.00 | 5-Feb-19 | ||||||||||
| 49 | Friedhelm Finke | 5,400 | $ | 2.130 | 11,500.00 | 18-Apr-19 | ||||||||||
| 50 | Friedhelm Noerr | 4,000 | $ | 1.344 | 5,374.82 | 19-Dec-17 | ||||||||||
| 51 | Friedrich Hochleitner | 6,000 | $ | 2.778 | 16,668.13 | 31-Jan-19 | ||||||||||
| 52 | Georg Sterz | 4,000 | $ | 2.854 | 11,417.91 | 16-Dec-17 | ||||||||||
| 53 | George Hammerschmid | 5,000 | $ | 2.057 | 10,283.02 | 19-Dec-17 | ||||||||||
| 54 | Gerd-Ruediger Schroeder | 1,200 | $ | 2.722 | 3,266.00 | 6-Nov-18 | ||||||||||
| 55 | Gerhard Siekmann | 1,000 | $ | 2.685 | 2,685.14 | 18-Sep-19 | ||||||||||
| 56 | Gerhard Wagner | 4,000 | $ | 1.429 | 5,715.63 | 30-Oct-17 | ||||||||||
| 56 | Gerhard Wagner | 3,800 | $ | 2.729 | 10,369.50 | 2-Feb-18 | ||||||||||
| 56 | Gerhard Wagner | 5,000 | $ | 2.570 | 12,851.81 | 5-Feb-19 | ||||||||||
| 57 | Greim Bernd | 1,000 | $ | 2.750 | 2,750.28 | 17-Apr-19 | ||||||||||
| 58 | Gunter Dally | 1,000 | $ | 2.773 | 2,773.43 | 6-Feb-19 | ||||||||||
| 59 | Hanno Schnabel | 1,000 | $ | 2.845 | 2,844.93 | 21-Dec-17 | ||||||||||
24
| Shareholder Name | Shares Purchased | Purchase Price $ Per shares | Amounts received | Date Purchased | ||||||||||||
| 60 | Hans Erlacher | 2,000 | $ | 2.858 | 5,715.80 | 24-Nov-17 | ||||||||||
| 60 | Hans Erlacher | 8,000 | $ | 1.248 | 9,980.16 | 13-Apr-18 | ||||||||||
| 61 | Hans Floeter | 1,000 | $ | 2.755 | 2,754.79 | 9-Oct-18 | ||||||||||
| 62 | Hans Hugo Schneider | 1,000 | $ | 2.757 | 2,756.70 | 4-Oct-19 | ||||||||||
| 63 | Hans Juergen Heyer | 1,000 | $ | 2.950 | 2,949.72 | 23-Apr-18 | ||||||||||
| 64 | Hans Lutz | 1,500 | $ | 2.726 | 4,089.73 | 8-Aug-19 | ||||||||||
| 65 | Hans Wilhelm Fricke | 2,000 | $ | 2.762 | 5,523.45 | 2-Jul-19 | ||||||||||
| 65 | Hans Wilhelm Fricke | 5,000 | $ | 2.212 | 11,057.75 | 3-Oct-19 | ||||||||||
| 66 | Harald Maier | 8,000 | $ | 2.721 | 21,771.08 | 18-Sep-19 | ||||||||||
| 67 | Hartmut and Roswitha | 10,000 | $ | 2.331 | 23,309.87 | 17-Dec-19 | ||||||||||
| 68 | Hauf Ernst | 4,000 | $ | 2.708 | 10,830.00 | 13-Nov-18 | ||||||||||
| 68 | Hauf Ernst | 6,000 | $ | 2.770 | 16,622.84 | 14-Feb-19 | ||||||||||
| 69 | Heini Herbstler | 2,000 | $ | 2.716 | 5,431.68 | 30-Apr-19 | ||||||||||
| 69 | Heini Herbstler | 2,000 | $ | 2.750 | 5,499.02 | 9-Oct-19 | ||||||||||
| 70 | Heinich Conen | 1,000 | $ | 2.713 | 2,712.53 | 2-May-19 | ||||||||||
| 70 | Heinich Conen | 1,000 | $ | 2.757 | 2,756.70 | 15-Oct-19 | ||||||||||
| 71 | Heinz Berger | 1,000 | $ | 2.951 | 2,951.31 | 17-Mar-18 | ||||||||||
| 72 | Helga Albert | 4,000 | $ | 2.721 | 10,885.01 | 17-Sep-19 | ||||||||||
| 72 | Helga Albert | 3,000 | $ | 2.433 | 7,298.28 | 15-Nov-19 | ||||||||||
| 73 | Helmut Backes | 3,000 | $ | 1.817 | 5,452.00 | 29-Nov-18 | ||||||||||
| 73 | Helmut Backes | 2,000 | $ | 2.624 | 7,871.42 | 5-Feb-19 | ||||||||||
| 74 | Helmut Gebert | 4,125 | $ | 2.058 | 8,489.02 | 15-Aug-18 | ||||||||||
| 74 | Helmut Gebert | 2,084 | $ | 2.623 | 5,466.00 | 5-Feb-19 | ||||||||||
| 75 | Helmut Lachmann | 13,000 | $ | 2.547 | 33,109.00 | 26-Oct-18 | ||||||||||
| 76 | Helmut Lantzberg | 9,200 | $ | 0.621 | 5,715.88 | 29-Dec-17 | ||||||||||
| 76 | Helmut Lantzberg | 20,800 | $ | 1.277 | 26,554.81 | 14-May-18 | ||||||||||
| 76 | Helmut Lantzberg | 3,000 | $ | 3.044 | 9,132.00 | 13-Dec-18 | ||||||||||
| 77 | Helmut Lubinski | 2,000 | $ | 2.734 | 5,468.00 | 21-Dec-18 | ||||||||||
| 78 | Helmut Pascher | 5,000 | $ | 2.736 | 13,679.52 | 24-Apr-19 | ||||||||||
| 78 | Helmut Pascher | 5,000 | $ | 2.278 | 11,388.28 | 23-Aug-19 | ||||||||||
| 79 | Helmut Roschlak | 16,000 | $ | 2.496 | 39,936.00 | 5-Feb-19 | ||||||||||
| 79 | Helmut Roschlak | 9,000 | $ | 1.391 | 12,519.50 | 28-Jun-19 | ||||||||||
| 80 | Herbert Roseneder | 30,000 | $ | 0.778 | 23,330.11 | 15-Sep-17 | ||||||||||
| 80 | Herbert Roseneder | 20,000 | $ | 1.880 | 37,590.77 | 17-Mar-18 | ||||||||||
| 81 | Hermann Geller | 1,000 | $ | 2.702 | 2,702.00 | 23-Nov-18 | ||||||||||
| 82 | Hermann Kreis | 1,000 | $ | 2.705 | 2,704.58 | 3-May-19 | ||||||||||
| 83 | Holly Rudiger | 8,000 | $ | 2.720 | 21,761.00 | 13-Dec-18 | ||||||||||
| 83 | Holly Rudiger | 22,000 | $ | 2.364 | 51,998.35 | 29-May-19 | ||||||||||
| 84 | Horst Bolten | 10,000 | $ | 2.380 | 4,034.71 | 6-Apr-18 | ||||||||||
| 84 | Horst Bolten | 10,000 | $ | 2.380 | 19,426.00 | 6-Nov-18 | ||||||||||
| 84 | Horst Bolten | 435 | $ | 2.380 | 25,169.01 | 25-Feb-19 | ||||||||||
| 85 | Horst Donhauser | 1,000 | $ | 2.730 | 2,729.85 | 5-Dec-19 | ||||||||||
| 86 | Ingo Schimke | 2,000 | $ | 2.737 | 5,474.56 | 3-Jul-19 | ||||||||||
| 87 | Irmer Rudiger | 1,000 | $ | 2.709 | 2,709.00 | 13-Dec-18 | ||||||||||
| 88 | Ismail Karaca | 1,000 | $ | 2.725 | 2,724.55 | 9-Jul-19 | ||||||||||
| 89 | Joachim Frey | 2,000 | $ | 2.735 | 5,469.99 | 4-Jul-19 | ||||||||||
| 90 | Joachim Mueller | 1,000 | $ | 2.723 | 2,722.51 | 28-May-19 | ||||||||||
| 91 | Joachim Putz | 1,000 | $ | 2.726 | 2,726.00 | 19-Nov-19 | ||||||||||
25
| Shareholder Name | Shares Purchased | Purchase Price $ Per shares | Amounts received | Date Purchased | ||||||||||||
| 92 | Johannes Bayer | 2,000 | $ | 2.360 | 4,720.98 | 11-Aug-17 | ||||||||||
| 92 | Johannes Bayer | 3,000 | $ | 2.850 | 8,550.57 | 27-Mar-19 | ||||||||||
| 93 | Johann Obehammer | 2,000 | $ | 2.724 | 5,447.40 | 15-Mar-18 | ||||||||||
| 94 | Josef Reinkober | 1,000 | $ | 2.645 | 2,644.67 | 16-Nov-18 | ||||||||||
| 94 | Josef Reinkober | 4,000 | $ | 2.850 | 11,400.00 | 1-Feb-19 | ||||||||||
| 94 | Josef Reinkober | 25,000 | $ | 2.261 | 56,519.51 | 19-Jun-19 | ||||||||||
| 95 | Jozef Balogh | 30,000 | $ | 0.923 | 27,675.33 | 28-Jul-17 | ||||||||||
| 95 | Jozef Balogh | 50,000 | $ | 1.146 | 57,308.89 | 9-Nov-17 | ||||||||||
| 95 | Jozef Balogh | 240,000 | $ | 1.191 | 285,775.81 | 10-Apr-18 | ||||||||||
| 96 | Juergen Bergheim | 1,000 | $ | 2.769 | 2,769.33 | 29-Jan-20 | ||||||||||
| 97 | Juergen Ellinger | 19,000 | $ | 2.319 | 44,057.40 | 30-Sep-19 | ||||||||||
| 97 | Juergen Ellinger | 35,000 | $ | 2.206 | 77,224.26 | 12-Nov-19 | ||||||||||
| 98 | Juergen Knoblauch | 75,000 | $ | 1.004 | 75,281.28 | 13-Jul-18 | ||||||||||
| 98 | Juergen Knoblauch | 32,500 | $ | 2.667 | 86,688.74 | 13-Dec-18 | ||||||||||
| 98 | Juergen Knoblauch | 22,700 | $ | 1.918 | 43,539.48 | 26-Apr-19 | ||||||||||
| 99 | Juergen Koegel | 4,000 | $ | 2.773 | 11,090.19 | 6-Dec-17 | ||||||||||
| 99 | Juergen Koegel | 4,445 | $ | 2.378 | 10,572.00 | 21-Dec-18 | ||||||||||
| 100 | Juergen Rist | 1,000 | $ | 2.757 | 2,756.86 | 26-Nov-19 | ||||||||||
| 101 | Juergen Schlate-Ditgens | 1,000 | $ | 2.718 | 2,718.00 | 6-Nov-18 | ||||||||||
| 102 | Juergen Volkmar | 2,000 | $ | 2.761 | 5,521.14 | 14-Oct-19 | ||||||||||
| 103 | Jurgen Pawliczek | 1,000 | $ | 2.845 | 2,844.89 | 24-Nov-17 | ||||||||||
| 103 | Jurgen Pawliczek | 3,000 | $ | 2.969 | 8,907.97 | 7-Feb-18 | ||||||||||
| 104 | Karl Bader | 1,000 | $ | 2.878 | 2,877.62 | 18-Jul-18 | ||||||||||
| 105 | Karl Dreschler | 1,000 | $ | 2.742 | 2,742.14 | 16-Apr-19 | ||||||||||
| 106 | Karl Heinz Hesse | 1,000 | $ | 2.764 | 2,764.44 | 8-Oct-19 | ||||||||||
| 107 | Karl Heinz Steeg | 2,000 | $ | 2.543 | 5,086.40 | 6-Jul-18 | ||||||||||
| 107 | Karl Heinz Steeg | 2,200 | $ | 2.777 | 6,109.67 | 28-Feb-19 | ||||||||||
| 108 | Karl Hettwer | 1,000 | $ | 2.734 | 2,734.10 | 26-Jun-19 | ||||||||||
| 109 | Karl Hutter | 5,000 | $ | 2.724 | 13,620.00 | 29-Nov-18 | ||||||||||
| 109 | Karl Hutter | 5,000 | $ | 2.785 | 13,923.08 | 20-Mar-19 | ||||||||||
| 109 | Karl Hutter | 10,000 | $ | 2.301 | 23,013.09 | 28-Aug-19 | ||||||||||
| 109 | Karl Hutter | 20,000 | $ | 1.802 | 36,031.31 | 5-Feb-20 | ||||||||||
| 110 | Klaus Kuehnelt | 4,000 | $ | 2.763 | 11,050.01 | 16-Oct-19 | ||||||||||
| 110 | Klaus Kuehnelt | 4,000 | $ | 2.774 | 11,097.70 | 3-Dec-19 | ||||||||||
| 111 | Klausdieter Schwabe | 2,000 | $ | 2.837 | 5,674.36 | 6-Dec-17 | ||||||||||
| 112 | Knud Jansen | 4,000 | $ | 2.758 | 11,032.32 | 11-Oct-19 | ||||||||||
| 112 | Knud Jansen | 8,000 | $ | 2.761 | 22,090.63 | 15-Nov-19 | ||||||||||
| 113 | Kurt Schaefer | 1,000 | $ | 2.696 | 2,695.88 | 28-Oct-19 | ||||||||||
| 114 | Lidia Schaar | 4,000 | $ | 2.572 | 20,579.45 | 23-Aug-17 | ||||||||||
| 115 | Ludwig Grote | 1,000 | $ | 2.764 | 2,764.44 | 3-Oct-19 | ||||||||||
| 115 | Ludwig Grote | 5,000 | $ | 2.206 | 11,028.06 | 12-Nov-19 | ||||||||||
| 116 | Lushan Venture Consult Gmbh | 4,000 | $ | 2.753 | 11,013.52 | 30-Sep-19 | ||||||||||
| 117 | Maik Lasar | 1,000 | $ | 2.764 | 2,764.44 | 6-Nov-19 | ||||||||||
| 118 | Manfred Ammon | 4,000 | $ | 2.730 | 10,918.58 | 4-Feb-20 | ||||||||||
| 119 | Manfred Baumann | 8,000 | $ | 3.070 | 24,563.54 | 21-Feb-18 | ||||||||||
| 119 | Manfred Baumann | 32,000 | $ | 2.751 | 88,020.01 | 24-May-19 | ||||||||||
| 119 | Manfred Baumann | 40,000 | $ | 1.944 | 77,757.48 | 25-Sep-19 | ||||||||||
| 120 | Manfred Brauning | 1,000 | $ | 2.819 | 2,819.28 | 7-Feb-19 | ||||||||||
26
| Shareholder Name | Shares Purchased | Purchase Price $ Per shares | Amounts received | Date Purchased | ||||||||||||
| 121 | Manfred Brunner | 10,000 | $ | 2.221 | 22,213.76 | 28-Nov-19 | ||||||||||
| 121 | Manfred Brunner | 2,000 | $ | 2.763 | 5,526.94 | 23-Jan-20 | ||||||||||
| 122 | Manfred Engel | 1,000 | $ | 2.940 | 2,939.59 | 6-Dec-17 | ||||||||||
| 123 | Manfred Glaschke | 4,000 | $ | 2.363 | 9,452.10 | 22-May-19 | ||||||||||
| 124 | Manfred Klose | 2,000 | $ | 2.771 | 5,541.49 | 26-Aug-19 | ||||||||||
| 125 | Manfred Meiborn | 1,000 | $ | 2.751 | 2,751.31 | 14-Mar-19 | ||||||||||
| 126 | Manfred Sadeler | 1,000 | $ | 2.675 | 2,675.31 | 12-Sep-19 | ||||||||||
| 127 | Marcel Beerli | 18,000 | $ | 2.718 | 48,931.14 | 4-Jan-19 | ||||||||||
| 128 | Maria Semmelrock-Picej | 10,000 | $ | 2.573 | 25,726.31 | 29-Aug-17 | ||||||||||
| 129 | Mario Kubler | 5,000 | $ | 2.816 | 14,079.00 | 29-Mar-19 | ||||||||||
| 130 | Martin Sonderegger | 2,000 | $ | 2.753 | 5,506.41 | 19-Feb-19 | ||||||||||
| 131 | Matthias Baumgart | 2,000 | $ | 2.793 | 5,586.50 | 9-Aug-19 | ||||||||||
| 132 | Matthias Wehl | 1,000 | $ | 2.646 | 2,645.91 | 1-Oct-19 | ||||||||||
| 133 | Michael Baranowski | 1,000 | $ | 2.820 | 2,820.00 | 22-Jul-19 | ||||||||||
| 134 | Michael Boehm | 2,000 | $ | 2.969 | 5,938.23 | 27-Mar-18 | ||||||||||
| 134 | Michael Boehm | 4,000 | $ | 2.733 | 10,930.00 | 5-Feb-19 | ||||||||||
| 135 | Michael Essenwanger | 1,000 | $ | 2.705 | 2,705.04 | 26-Aug-19 | ||||||||||
| 136 | Michael Kraft | 12,000 | $ | 2.404 | 28,850.48 | 8-Feb-19 | ||||||||||
| 136 | Michael Kraft | 61,600 | $ | 1.129 | 69,577.17 | 18-Jun-19 | ||||||||||
| 136 | Michael Kraft | 47,000 | $ | 2.208 | 103,783.50 | 25-Sep-19 | ||||||||||
| 137 | Mike Brugger | 1,000 | $ | 2.776 | 2,776.33 | 27-Dec-19 | ||||||||||
| 138 | Norbert Hellenkamp | 2,000 | $ | 2.746 | 5,491.81 | 13-May-19 | ||||||||||
| 139 | Norbert Schindler | 1,000 | $ | 2.951 | 2,951.31 | 16-Mar-18 | ||||||||||
| 140 | Oliver Debus | 1,000 | $ | 2.951 | 2,951.44 | 20-Dec-17 | ||||||||||
| 141 | Paulssen Uta | 1,000 | $ | 2.711 | 2,710.50 | 25-Apr-19 | ||||||||||
| 142 | Peter Bodenstein | 1,000 | $ | 3.026 | 3,025.55 | 8-Feb-18 | ||||||||||
| 143 | Peter Jansen | 4,000 | $ | 2.750 | 11,000.42 | 28-Nov-19 | ||||||||||
| 144 | Peter Juergensen | 3,000 | $ | 0.927 | 2,781.36 | 8-Aug-18 | ||||||||||
| 144 | Peter Juergensen | 5,000 | $ | 2.405 | 12,026.00 | 5-Feb-19 | ||||||||||
| 145 | Peter Voecklinghaus | 4,000 | $ | 2.958 | 11,832.00 | 8-Feb-18 | ||||||||||
| 146 | Rainer Gaenssle | 6,000 | $ | 2.745 | 16,468.31 | 22-Oct-19 | ||||||||||
| 147 | Rainer Lachmann | 8,334 | $ | 2.126 | 17,718.63 | 21-Sep-17 | ||||||||||
| 147 | Rainer Lachmann | 95,916 | $ | 0.722 | 69,254.88 | 21-Sep-17 | ||||||||||
| 147 | Rainer Lachmann | 7,900 | $ | 2.107 | 16,641.80 | 15-Mar-19 | ||||||||||
| 147 | Rainer Lachmann | 14,850 | $ | 2.201 | 32,692.13 | 24-Dec-18 | ||||||||||
| 147 | Rainer Lachmann | 10,000 | $ | 2.219 | 22,190.56 | 29-Jan-20 | ||||||||||
| 148 | Reinhold Eisenbraun | 2,000 | $ | 2.727 | 5,454.00 | 6-Nov-18 | ||||||||||
| 148 | Reinhold Eisenbraun | 8,000 | $ | 2.790 | 22,321.42 | 11-Feb-19 | ||||||||||
| 148 | Reinhold Eisenbraun | 13,000 | $ | 2.545 | 33,088.00 | 20-Jun-19 | ||||||||||
| 148 | Reinhold Eisenbraun | 7,000 | $ | 2.284 | 15,988.15 | 30-Jul-19 | ||||||||||
| 149 | Reinhold Janach | 40,442 | $ | 1.621 | 65,538.60 | 13-Apr-18 | ||||||||||
| 149 | Reinhold Janach | 60,000 | $ | 0.704 | 42,228.63 | 6-Dec-18 | ||||||||||
| 149 | Reinhold Janach | 48,000 | $ | 2.403 | 115,329.83 | 21-Feb-19 | ||||||||||
| 149 | Reinhold Janach | 50,000 | $ | 1.955 | 97,747.40 | 2-May-19 | ||||||||||
| 150 | Rene Faak | 1,000 | $ | 2.781 | 2,781.36 | 8-Aug-18 | ||||||||||
| 151 | Richard Selbach | 5,000 | $ | 2.270 | 11,352.33 | 18-Sep-19 | ||||||||||
| 152 | Rita Hussong | 8,000 | $ | 1.535 | 12,281.77 | 15-Jan-18 | ||||||||||
| 153 | Robert Bauer | 4,000 | $ | 2.771 | 11,085.43 | 11-Feb-19 | ||||||||||
| 153 | Robert Bauer | 14,100 | $ | 2.461 | 34,700.86 | 2-Jul-19 | ||||||||||
| 153 | Robert Bauer | 4,800 | $ | 2.282 | 10,955.36 | 21-Jul-19 | ||||||||||
27
| Shareholder Name | Shares Purchased | Purchase Price $ Per shares | Amounts received | Date Purchased | ||||||||||||
| 154 | Rolf Hinderer | 2,000 | $ | 2.750 | 5,499.02 | 6-Nov-19 | ||||||||||
| 155 | Rolf Mauti | 4,000 | $ | 2.827 | 11,307.01 | 7-Feb-18 | ||||||||||
| 155 | Rolf Mauti | 4,000 | $ | 2.656 | 10,624.77 | 21-Oct-19 | ||||||||||
| 156 | Rolf Schraft | 12,000 | $ | 1.259 | 15,106.42 | 29-Nov-17 | ||||||||||
| 156 | Rolf Schraft | 4,000 | $ | 2.673 | 10,693.74 | 7-Feb-18 | ||||||||||
| 156 | Rolf Schraft | 6,000 | $ | 1.890 | 11,340.00 | 13-Dec-18 | ||||||||||
| 156 | Rolf Scharft | 8,000 | $ | 1.887 | 15,095.18 | 3-May-19 | ||||||||||
| 157 | Rudiger Karsten | 2,000 | $ | 2.733 | 5,466.10 | 13-Jun-19 | ||||||||||
| 158 | Ruscio Marcello | 3,000 | $ | 2.764 | 8,293.43 | 26-Jun-19 | ||||||||||
| 158 | Ruscio Marcello | 2,000 | $ | 2.577 | 5,154.82 | 22-Aug-19 | ||||||||||
| 159 | Sandra Flick | 1,000 | $ | 2.702 | 2,701.71 | 26-Jul-19 | ||||||||||
| 160 | Scheel Dirk | 1,000 | $ | 2.872 | 2,871.95 | 21-Sep-17 | ||||||||||
| 161 | Siegfried Heger | 2,000 | $ | 2.510 | 5,020.30 | 8-Aug-18 | ||||||||||
| 161 | Siegfried Heger | 98,000 | $ | 1.336 | 130,909.00 | 21-Dec-18 | ||||||||||
| 161 | Siegfried Heger | 50,000 | $ | 1.700 | 84,982.09 | 2-Jan-19 | ||||||||||
| 162 | Siegfried Hoelle | 4,200 | $ | 2.673 | 11,228.49 | 18-Apr-18 | ||||||||||
| 162 | Siegfried Hoelle | 22,000 | $ | 2.707 | 59,560.55 | 20-Jun-19 | ||||||||||
| 163 | Siegfried Morath | 1,000 | $ | 2.718 | 2,718.00 | 6-Nov-18 | ||||||||||
| 163 | Siegfried Morath | 19,500 | $ | 2.529 | 49,324.31 | 11-Mar-19 | ||||||||||
| 163 | Siegfried Morath | 12,000 | $ | 2.173 | 26,070.12 | 12-Sep-19 | ||||||||||
| 163 | Siegfried Morath | 13,500 | $ | 2.453 | 33,117.81 | 4-Feb-20 | ||||||||||
| 164 | Siegfried Schmiedener | 1,000 | $ | 2.752 | 2,752.06 | 2-Dec-19 | ||||||||||
| 165 | Sieghard Woehr | 1,000 | $ | 2.703 | 2,702.56 | 21-Aug-19 | ||||||||||
| 166 | Steffen Mueller | 4,000 | $ | 2.783 | 11,130.17 | 25-Jun-19 | ||||||||||
| 167 | Teichmann Jens | 1,000 | $ | 2.872 | 2,871.95 | 21-Sep-17 | ||||||||||
| 168 | Thomas Frey | 6,000 | $ | 2.778 | 16,670.11 | 15-Jul-19 | ||||||||||
| 169 | Thomas Pfadenhauer | 8,167 | $ | 2.672 | 21,825.73 | 23-Aug-19 | ||||||||||
| 170 | Toresten Grossmann | 1,000 | $ | 2.755 | 2,755.10 | 9-Oct-18 | ||||||||||
| 171 | Ulrich Mang | 1,200 | $ | 2.744 | 3,292.46 | 15-Nov-19 | ||||||||||
| 172 | Ulrich Zwissler | 8,000 | $ | 2.774 | 22,191.56 | 25-Oct-17 | ||||||||||
| 172 | Ulrich Zwissler | 242,000 | $ | 1.014 | 245,436.83 | 4-Jul-18 | ||||||||||
| 172 | Ulrich Zwissler | 250,000 | $ | 1.753 | 438,275.04 | 30-Apr-19 | ||||||||||
| 172 | Ulrich Zwissler | 125,000 | $ | 1.883 | 235,396.88 | 23-Jul-19 | ||||||||||
| 173 | Walter Hillebrand | 4,000 | $ | 2.692 | 10,768.48 | 24-Sep-19 | ||||||||||
| 173 | Walter Hillebrand | 16,000 | $ | 2.653 | 42,454.19 | 25-Nov-19 | ||||||||||
| 174 | Waltraud Scheld-Fehren | 1,000 | $ | 2.845 | 2,844.74 | 10-Nov-17 | ||||||||||
| 175 | Werner Jantke | 1,000 | $ | 2.845 | 2,844.82 | 25-Oct-17 | ||||||||||
| 176 | Werner Preugschat | 1,000 | $ | 2.555 | 2,555.24 | 14-Aug-17 | ||||||||||
| 177 | Wilfried Buesch | 4,000 | $ | 2.718 | 10,872.35 | 25-Jul-19 | ||||||||||
| 178 | Wilfried Lahmann | 108,000 | $ | 2.225 | 240,342.77 | 31-Oct-19 | ||||||||||
| 178 | Wilfried Lahmann | 140,000 | $ | 1.125 | 157,501.04 | 20-Feb-20 | ||||||||||
| 179 | Willi Seelig | 2,000 | $ | 2.639 | 5,277.36 | 30-Aug-19 | ||||||||||
| 180 | Willy Mattes | 2,000 | $ | 2.360 | 4,720.98 | 11-Aug-17 | ||||||||||
| 181 | Wolf Dieter Windgassen | 1,000 | $ | 2.845 | 2,844.93 | 12-Dec-17 | ||||||||||
| 182 | Wolfgang Freitag | 2,222 | $ | 2.542 | 5,647.66 | 19-Jul-18 | ||||||||||
| 183 | Wolfgang Hoffman | 20,000 | $ | 1.168 | 23,361.88 | 9-Aug-17 | ||||||||||
| 183 | Wolfgang Hoffman | 9,000 | $ | 1.431 | 12,878.66 | 26-Mar-18 | ||||||||||
| 184 | Wolfgang Luebbe | 1,000 | $ | 2.740 | 2,740.11 | 18-Oct-19 | ||||||||||
| 185 | Wolfgang Melber | 1,000 | $ | 2.951 | 2,951.31 | 7-Mar-18 | ||||||||||
| 186 | Wolfgang Unterholler | 4,000 | $ | 2.884 | 11,535.48 | 21-Sep-17 | ||||||||||
| 186 | Wolfgang Unterholler | 2,000 | $ | 2.753 | 5,506.06 | 10-Dec-19 | ||||||||||
| 187 | Wolfgang von Plessen | 1,000 | $ | 2.735 | 2,735.00 | 18-Oct-18 | ||||||||||
| 188 | Wolfgang-Hans Tischler | 2,000 | $ | 2.730 | 9,460.36 | 19-Jul-18 | ||||||||||
| 188 | Wolfgang-Hans Tischler | 2,000 | $ | 2.733 | 5,466.00 | 5-Feb-19 | ||||||||||
| 188 | Wolfgang-Hans Tischler | 6,000 | $ | 2.714 | 16,282.48 | 10-Dec-19 | ||||||||||
| 189 | Yuen May Cheung | 1,000,000 | $ | 0.001 | 1,000.00 | 10-Dec-19 | ||||||||||
| 189 | Yuen May Cheung | 19,000,000 | $ | 0.001 | 19,000.00 | 10-Dec-19 | ||||||||||
| 189 | Yuen May Cheung | 1,000,000 | $ | 0.001 | 1,000.00 | 5-Jan-17 | ||||||||||
| 25,250,703 | 7,529,324.26 | |||||||||||||||
28
ITEM 16. Exhibits and Financial Statement Schedules
| (a) | Exhibits: |
The following exhibits are filed as part of this registration statement:
| Exhibit No. | Description |
| Ex. 3.1 | Certificate of Incorporation |
| Ex. 3.2 | Bylaws |
| Ex. 5.1 | Legal Opinion and Consent of Lane & Associates, P.A. |
| Ex. 23.1 | Opinion of Centurion ZD CPA & Co. |
Undertakings
The undersigned Registrant hereby undertakes:
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (a) | To include any prospectus required by Section 10(a) (3) of the Securities Act of 1933; |
| (b) | To reflect in the prospectus any facts or events arising after the effective date of this registration statement, or most recent post-effective amendment, which, individually or in the aggregate, represent a fundamental change in the information set forth in this registration statement; and |
| (c) | To include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in the registration statement. |
That, for the purpose of determining any liability under the Securities Act, each post-effective amendment shall be deemed to be a new registration statement relating to the securities offered herein, and the Offering of such securities at that time shall be deemed to be the initial bona fide Offering thereof.
To remove from registration by means of a post-effective amendment any of the securities being registered hereby which remain unsold at the termination of the Offering.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions described above, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
29
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Sparks on August 5, 2020.
| 4DMED, LTD. |
| By: /s/ Yuen May Cheung |
| Name: Yuen May Cheung |
| Title: Chief Executive Officer, President and Director |
| (Principal executive officer) |
| By: /s/Yuen May Cheung |
| Name: Yuen May Cheung |
| Title: Chief Financial Officer |
| (Principal financial officer) |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| /s/ Yuen May Cheung | August 5, 2020 |
| Name: Yuen May Cheung | |
| Chief Executive Officer, President and Director | |
| (Principal executive officer) | |
| /s/ Yuen May Cheung | August 5, 2020 |
| Name: Yuen May Cheung | |
| Chief Financial Officer | |
| (Principal accounting officer) | |
| /s/ Hau Tin Cheung | August 5, 2020 |
| Name: Hau Tin Cheung | |
| Director |
30
