Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - F-star Therapeutics, Inc. | sbph-ex991_117.htm |
| EX-10.5 - EX-10.5 - F-star Therapeutics, Inc. | sbph-ex105_8.htm |
| EX-10.4 - EX-10.4 - F-star Therapeutics, Inc. | sbph-ex104_7.htm |
| EX-10.3 - EX-10.3 - F-star Therapeutics, Inc. | sbph-ex103_6.htm |
| EX-10.2 - EX-10.2 - F-star Therapeutics, Inc. | sbph-ex102_319.htm |
| EX-10.1 - EX-10.1 - F-star Therapeutics, Inc. | sbph-ex101_318.htm |
| EX-2.1 - EX-2.1 - F-star Therapeutics, Inc. | sbph-ex21_202.htm |
| 8-K - 8-K - F-star Therapeutics, Inc. | sbph-8k_20200729.htm |

30th July 2020 Spring Bank Pharmaceuticals and F-star Therapeutics Combination Redirecting T Cells . Overcoming Cancer . Exhibit 99.2

Certain statements contained in this communication regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, known as the PSLRA. These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Spring Bank and F-star undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. We use words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, risks relating to the completion of the combination, including the need for stockholder approval and the satisfaction of closing conditions; the anticipated financing to be completed immediately prior to the closing of the combination; the cash balances of the combined company following the closing of the combination and the F-star financing; the ability of Spring Bank to remain listed on the Nasdaq Capital Market; and expected restructuring-related cash outlays, including the timing and amount of those outlays. Risks and uncertainties related to F-star that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to F-star’s status as a clinical stage immuno-oncology company and its need for substantial additional funding in order to complete the development and commercialization of its product candidates, that F-star may experience delays in completing, or ultimately be unable to complete, the development and commercialization of its product candidates, that F-Star’s clinical trials may fail to adequately demonstrate the safety and efficacy of its product candidates, that preclinical drug development is uncertain, and some of F-star’s product candidates may never advance to clinical trials, that results of preclinical studies and early stage clinical trials may not be predictive of the results of later state clinical trials, that F-star relies on patents and other intellectual property rights to protect its product candidates, and the enforcement, defense and maintenance of such rights may be challenging and costly, and that F-star faces significant competition in its drug discovery and development efforts. New factors emerge from time to time and it is not possible for us to predict all such factors, nor can we assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. These risks, as well as other risks associated with the combination, will be more fully discussed in the proxy statement/prospectus that will be included in the registration statement that will be filed with the SEC in connection with the proposed transaction. Additional risks and uncertainties are identified and discussed in the “Risk Factors” section of Spring Bank’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other documents filed from time to time with the SEC. Forward-looking statements included in this press release are based on information available to Spring Bank and F-star as of the date of this communication. Neither Spring Bank nor F-star undertakes any obligation to update such forward-looking statements to reflect events or circumstances after the date of this communication. Cautionary Note Regarding Forward-Looking Statements

Share Exchange Agreement announced on 29th July 2020 Expected to be completed in Fall 2020 New company: F-star Therapeutics, Inc. expected to trade on NASDAQ under FSTX Dr. Eliot Forster to serve as President and CEO Combined company Board of Directors proportional to ownership spilt Pre-closing financing round anticipated to be $25M Combined company cash at closing expected to fund through multiple milestones Proforma ownership: Current Spring Bank stockholders will own approximately 39% of the combined company and F-star shareholders will own 61% Overview of the Merger Transaction
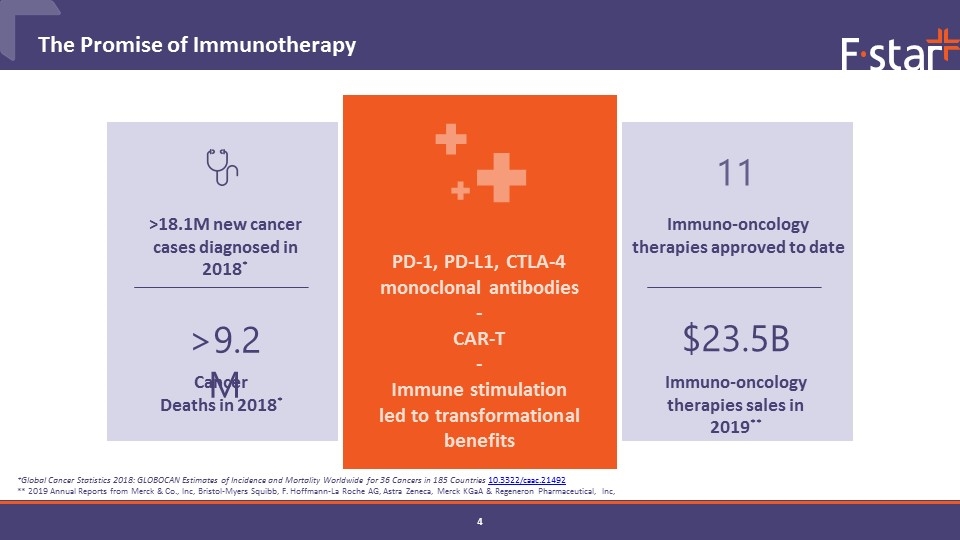
The Promise of Immunotherapy $23.5B >9.2M Immuno-oncology therapies sales in 2019** Immuno-oncology therapies approved to date 11 PD-1, PD-L1, CTLA-4 monoclonal antibodies - CAR-T - Immune stimulation led to transformational benefits >18.1M new cancer cases diagnosed in 2018* Cancer Deaths in 2018* *Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries 10.3322/caac.21492 ** 2019 Annual Reports from Merck & Co., Inc, Bristol-Myers Squibb, F. Hoffmann-La Roche AG, Astra Zeneca, Merck KGaA & Regeneron Pharmaceutical, Inc,
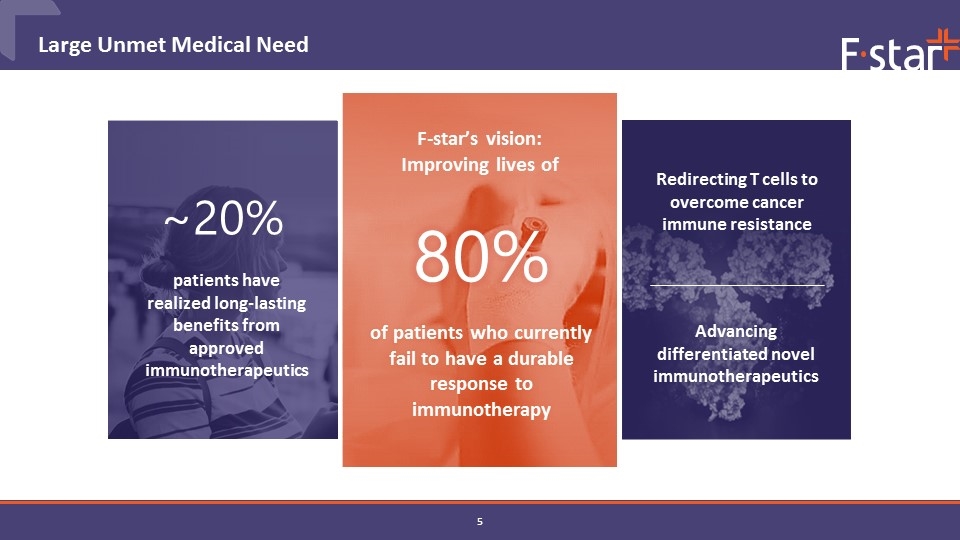
Large Unmet Medical Need F-star’s vision: Improving lives of Advancing differentiated novel immunotherapeutics Redirecting T cells to overcome cancer immune resistance of patients who currently fail to have a durable response to immunotherapy 80% patients have realized long-lasting benefits from approved immunotherapeutics ~20%
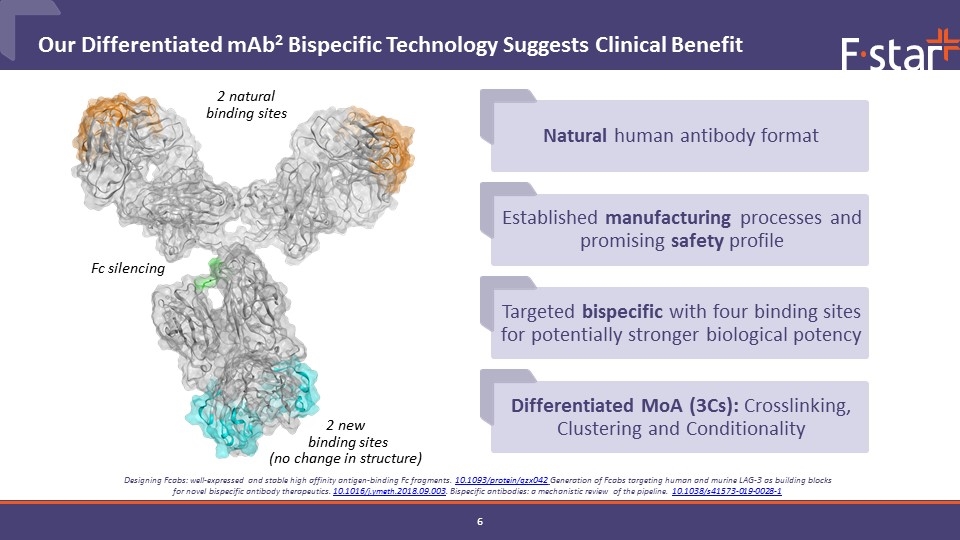
Differentiated MoA (3Cs): Crosslinking, Clustering and Conditionality Established manufacturing processes and promising safety profile 2 natural binding sites 2 new binding sites (no change in structure) Fc silencing Natural human antibody format Targeted bispecific with four binding sites for potentially stronger biological potency Our Differentiated mAb2 Bispecific Technology Suggests Clinical Benefit Designing Fcabs: well-expressed and stable high affinity antigen-binding Fc fragments. 10.1093/protein/gzx042 Generation of Fcabs targeting human and murine LAG-3 as building blocks for novel bispecific antibody therapeutics. 10.1016/j.ymeth.2018.09.003. Bispecific antibodies: a mechanistic review of the pipeline. 10.1038/s41573-019-0028-1
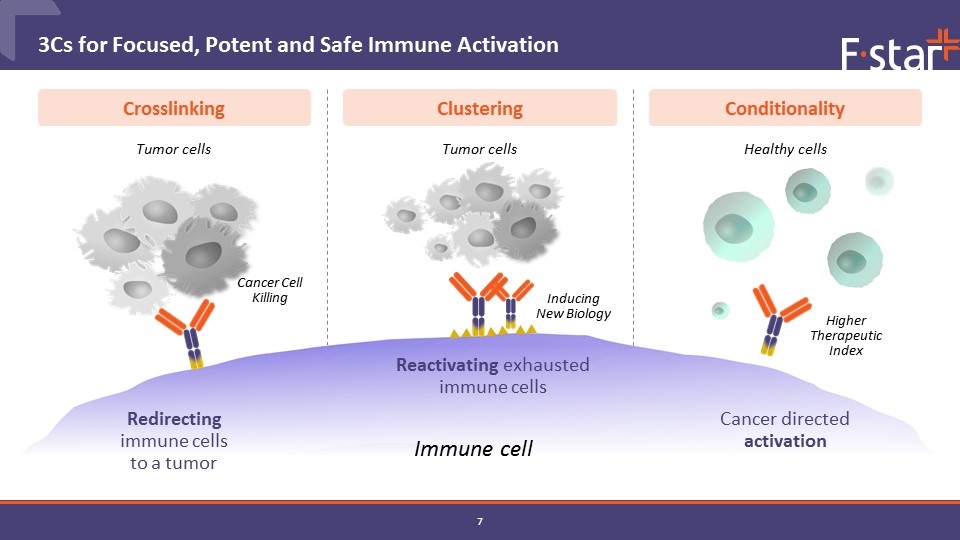
Tumor cells Immune cell Clustering Crosslinking Conditionality Redirecting immune cells to a tumor Tumor cells Healthy cells 3Cs for Focused, Potent and Safe Immune Activation Reactivating exhausted immune cells Cancer directed activation Cancer Cell Killing Inducing New Biology Higher Therapeutic Index
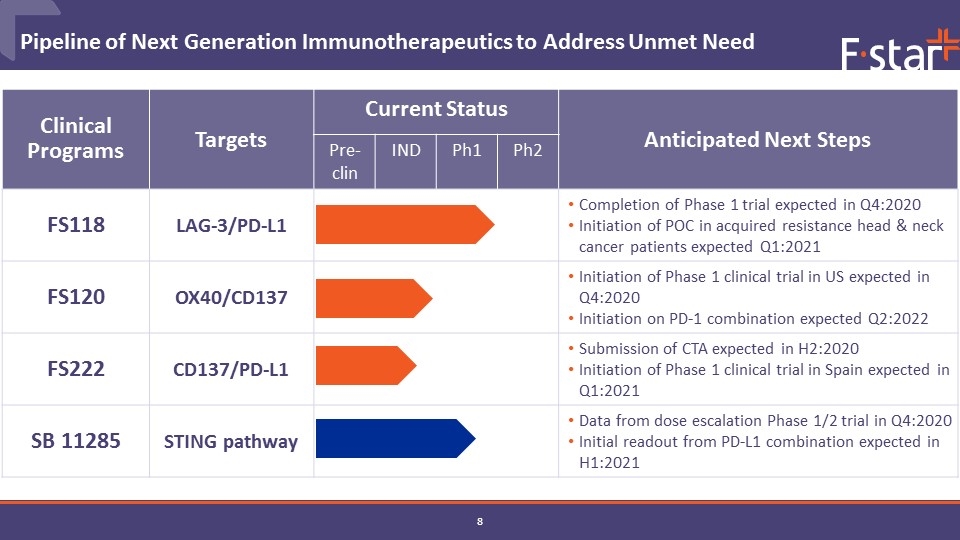
Pipeline of Next Generation Immunotherapeutics to Address Unmet Need 2020 mAb2 candidates 2021 Clinical Programs Targets Current Status Anticipated Next Steps Pre-clin IND Ph1 Ph2 FS118 LAG-3/PD-L1 Completion of Phase 1 trial expected in Q4:2020 Initiation of POC in acquired resistance head & neck cancer patients expected Q1:2021 FS120 OX40/CD137 Initiation of Phase 1 clinical trial in US expected in Q4:2020 Initiation on PD-1 combination expected Q2:2022 FS222 CD137/PD-L1 Submission of CTA expected in H2:2020 Initiation of Phase 1 clinical trial in Spain expected in Q1:2021 SB 11285 STING pathway Data from dose escalation Phase 1/2 trial in Q4:2020 Initial readout from PD-L1 combination expected in H1:2021
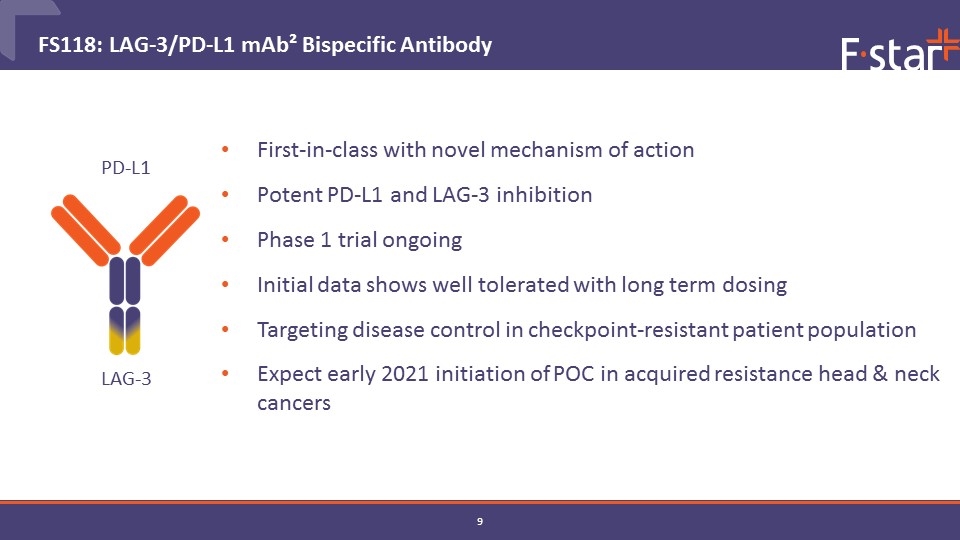
First-in-class with novel mechanism of action Potent PD-L1 and LAG-3 inhibition Phase 1 trial ongoing Initial data shows well tolerated with long term dosing Targeting disease control in checkpoint-resistant patient population Expect early 2021 initiation of POC in acquired resistance head & neck cancers FS118: LAG-3/PD-L1 mAb² Bispecific Antibody PD-L1 LAG-3
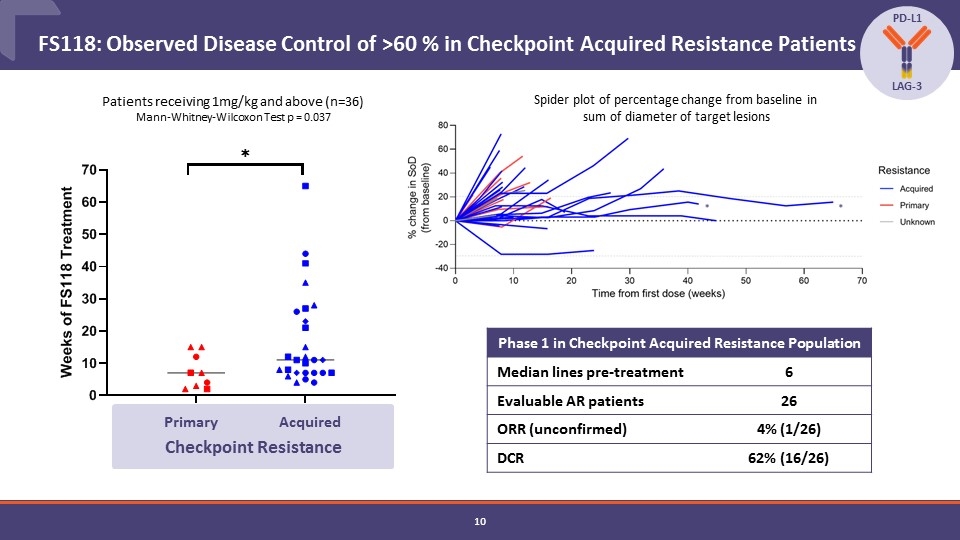
FS118: Observed Disease Control of >60 % in Checkpoint Acquired Resistance Patients Phase 1 in Checkpoint Acquired Resistance Population Median lines pre-treatment 6 Evaluable AR patients 26 ORR (unconfirmed) 4% (1/26) DCR 62% (16/26) PD-L1 LAG-3 Primary Acquired Patients receiving 1mg/kg and above (n=36) Mann-Whitney-Wilcoxon Test p = 0.037 Checkpoint Resistance Spider plot of percentage change from baseline in sum of diameter of target lesions
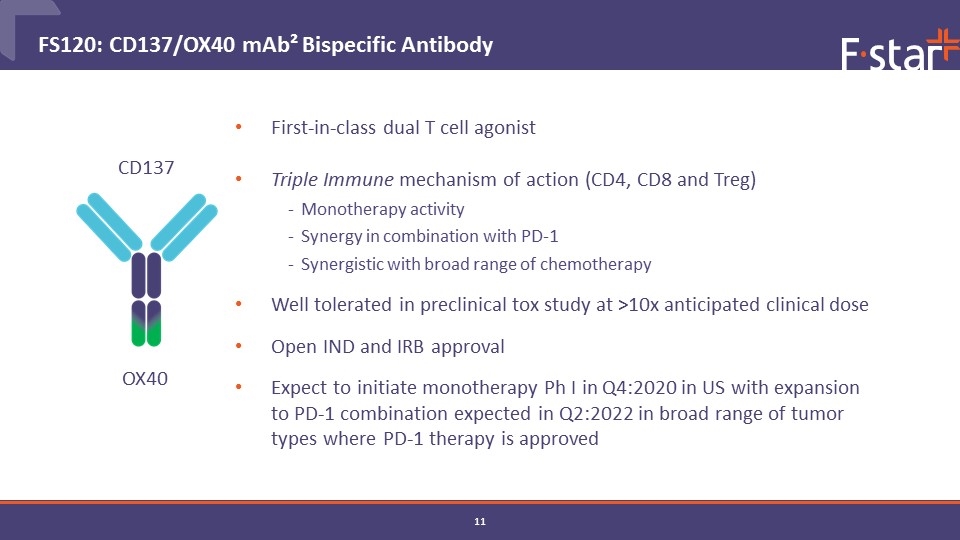
First-in-class dual T cell agonist Triple Immune mechanism of action (CD4, CD8 and Treg) Monotherapy activity Synergy in combination with PD-1 Synergistic with broad range of chemotherapy Well tolerated in preclinical tox study at >10x anticipated clinical dose Open IND and IRB approval Expect to initiate monotherapy Ph I in Q4:2020 in US with expansion to PD-1 combination expected in Q2:2022 in broad range of tumor types where PD-1 therapy is approved FS120: CD137/OX40 mAb² Bispecific Antibody CD137 OX40
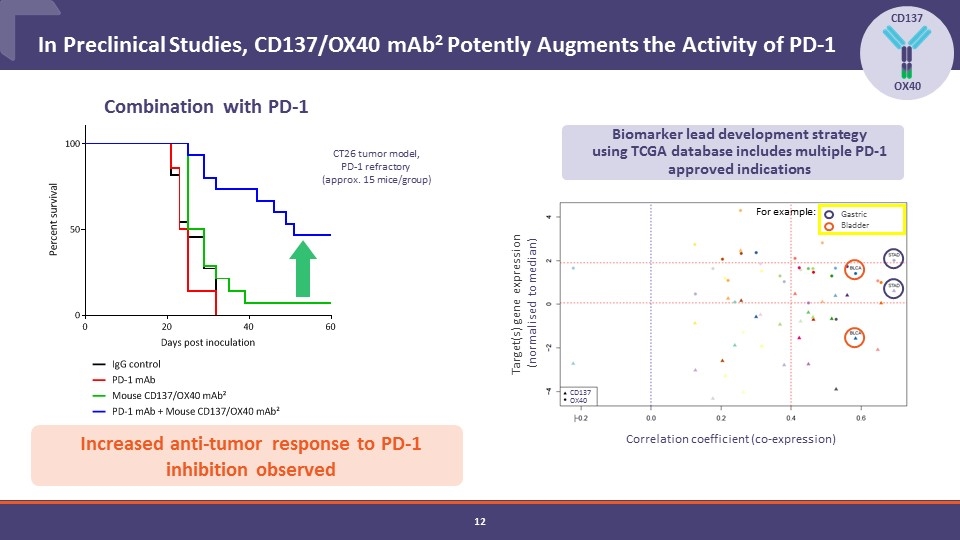
In Preclinical Studies, CD137/OX40 mAb2 Potently Augments the Activity of PD-1 CD137 OX40 CT26 tumor model, PD-1 refractory (approx. 15 mice/group) Combination with PD-1 Increased anti-tumor response to PD-1 inhibition observed Correlation coefficient (co-expression) Biomarker lead development strategy using TCGA database includes multiple PD-1 approved indications Target(s) gene expression (normalised to median) Gastric Bladder CD137 OX40 For example:
Redirecting activated immune cells to the tumor Potential for Best-in-class Potential for wider therapeutic window than mAb combinations or other bispecific approaches Potential for clinical differentiation in PD-L1 low setting Potential to move to earlier lines of treatment replacing current checkpoint inhibitors Well tolerated in preclinical tox study at >10x anticipated clinical dose CTA submission expected in H2:2020 Initiation of Phase I expected in early 2021 in Spain FS222: CD137/PD-L1 mAb² Bispecific Antibody PD-L1 CD137
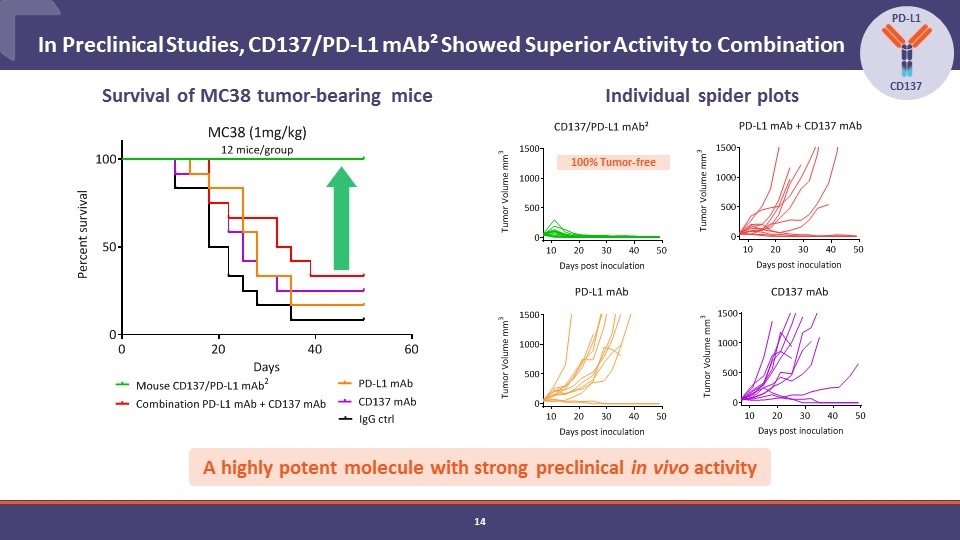
In Preclinical Studies, CD137/PD-L1 mAb² Showed Superior Activity to Combination PD-L1 CD137 Survival of MC38 tumor-bearing mice Individual spider plots A highly potent molecule with strong preclinical in vivo activity 100% Tumor-free
SB 11285: An Intravenously Administered Next Generation STING Agonist Second generation intravenous STING agonist Preclinical studies indicate potential advantages over intratumoral STING agonists Phase 1/2 trial including combination with PD-L1 ongoing in US Currently patients are at the 3rd dose level for monotherapy and the first dose level in combination with atezolizumab (Tecentriq®) No drug related Serious Adverse Events (SAEs) or DLTs have been reported Data readout expected in Q4:2020 STING Agonist

Experienced Management Team Medical Oncologist with 25+ years' experience in clinical research in Europe and US Experience in registrational studies, registration strategy and drug approval Louis Kayitalire CMO, MD 25 years leading financial operations in public and private companies Proven track record in finance strategy, capital raising, M&A and strategic partnerships Darlene Deptula-Hicks CFO, MBA 28+ years’ experience in the pharmaceutical and biotechnology industries Senior leadership roles in drug development Eliot Forster CEO, PhD MBA 25+ years' experience in antibody engineering and drug discovery Advanced novel platforms from inception to clinical proof of concept in oncology Neil Brewis CSO, PhD DSc Management team has successfully brought 19 drugs to market

Board of Directors Nessan Bermingham PhD, Chairman Co-Founder and previously CEO of Intellia Therapeutics, leading it through IPO (NASDAQ: NTLA). Founder and CEO of Triplet Therapeutics, Founder and Exec Chair of Korro Bio and venture partner at Atlas Venture. Previously Chair of Ex Nihlio, and independent advisory board member of MerckSerono. Eliot Forster PhD MBA, President and CEO Geoffrey Race FCMA MBA EVP, CFO and CBO, Minerva Neurosciences (NASDAQ: NERV). CEO of PanGenetics during its sale to Abbvie. Edward Benz MD President and CEO Emeritus, Dana-Farber Cancer Institute, Boston, MA. Faculty Dean Emeritus for Oncology at Harvard Medical School. Patrick Krol MBA Managing Partner, Aescap Venture. Former Director of Healthcare at Fleishman-Hillard, London and member of Omnicom group (NYSE: OMC). David Arkowitz MBA CFO of Flexion Therapeutics Inc. (NASDAQ: FLXN). A member of the board at Proteostasis Therapeutics, Inc. (NASDAQ: PTI) Pamela Klein MD Principal and founder of PMK BioResearch, Previously CMO of Intellikine and Research Director at NCI-Navy Breast Care Center. A member of the board at argenx SE. Todd Brady MD PhD President and Chief Executive Officer of Aldeyra Therapeutics, Inc. A member of the board at Aldeyra Therapeutics, Inc. and Evoke Pharma, Inc. Combined Company Board of Directors
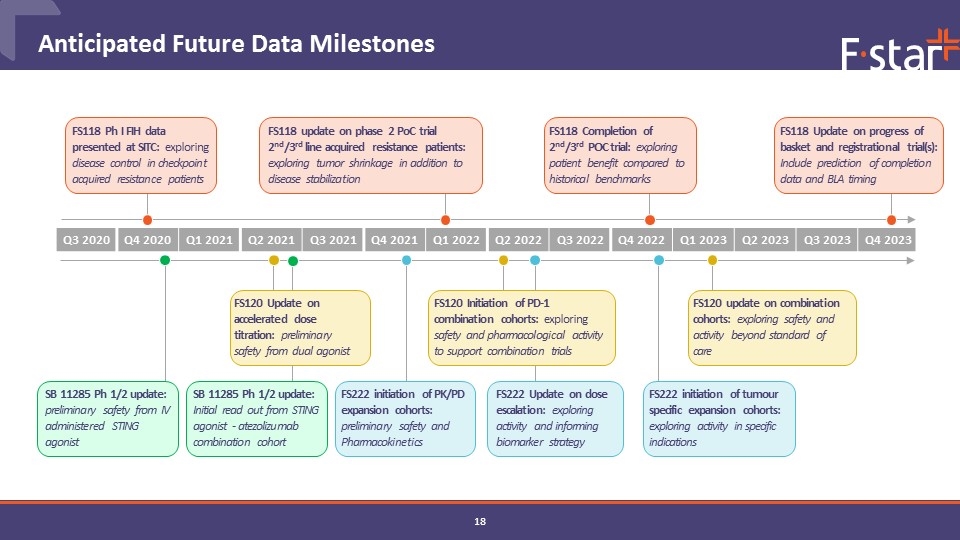
Anticipated Future Data Milestones SB 11285 Ph 1/2 update: preliminary safety from IV administered STING agonist FS222 initiation of PK/PD expansion cohorts: preliminary safety and Pharmacokinetics FS222 Update on dose escalation: exploring activity and informing biomarker strategy Q3 2020 Q4 2020 Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 Q2 2023 Q3 2023 Q4 2023 FS118 Completion of 2nd/3rd POC trial: exploring patient benefit compared to historical benchmarks FS120 Update on accelerated dose titration: preliminary safety from dual agonist FS118 Ph I FIH data presented at SITC: exploring disease control in checkpoint acquired resistance patients FS118 update on phase 2 PoC trial 2nd/3rd line acquired resistance patients: exploring tumor shrinkage in addition to disease stabilization FS222 initiation of tumour specific expansion cohorts: exploring activity in specific indications FS118 Update on progress of basket and registrational trial(s): Include prediction of completion data and BLA timing FS120 update on combination cohorts: exploring safety and activity beyond standard of care FS120 Initiation of PD-1 combination cohorts: exploring safety and pharmacological activity to support combination trials SB 11285 Ph 1/2 update: Initial read out from STING agonist - atezolizumab combination cohort
Highlights of F-star Therapeutics Multiple, anticipated near-term value adding clinical data milestones across four novel programs Proprietary, highly differentiated, bispecific antibody platform delivering multiple clinical programs Addressing fast growing market and burgeoning patients' needs in immuno-oncology

Thank you .
