Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | gnca-20200730.htm |

GEN-009 Ph1/2a Part B Discussion of Initial Clinical Data July 30, 2020

This presentation contains “forward-looking” statements that are within the meaning of federal securities laws and are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, clinical trials and pre-clinical studies, regulatory approval of our product candidates, liquidity position and capital needs, financing plans, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “expects,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or combination of which, could materially affect our results of operations and whether the forward- Disclaimer looking statements ultimately prove to be correct. Factors that may materially affect our results of operations include, among other things, our ability to progress product candidates in preclinical and clinical trials, the ability of ATLAS™ to identify promising oncology vaccine and immunotherapy product candidates, the scope, rate and progress of our preclinical and clinical trials and other research and development activities, anticipated timing of IND applications and new clinical trials, the amount of funds that we may require to conduct our clinical trials for our product candidates, the timing of, and ability to, obtain and maintain necessary regulatory approvals for our product candidates, and those listed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2019 and other filings with the Securities and Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings for free by visiting EDGAR on the SEC website at http://www.sec.gov. 2

Agenda • ATLAS overview – Jessica Flechtner, Ph.D., Chief Scientific Officer, Genocea • GEN-009 introduction – Tom Davis, M.D., Chief Medical Officer, Genocea • GEN-009 initial Part B data review – Maura Gillison, M.D, Ph.D., Professor, Dept. of Thoracic/Head and Neck Medical Oncology, MD Anderson Cancer Center • Summary – Chip Clark, Chief Executive Officer, Genocea • Q&A 3
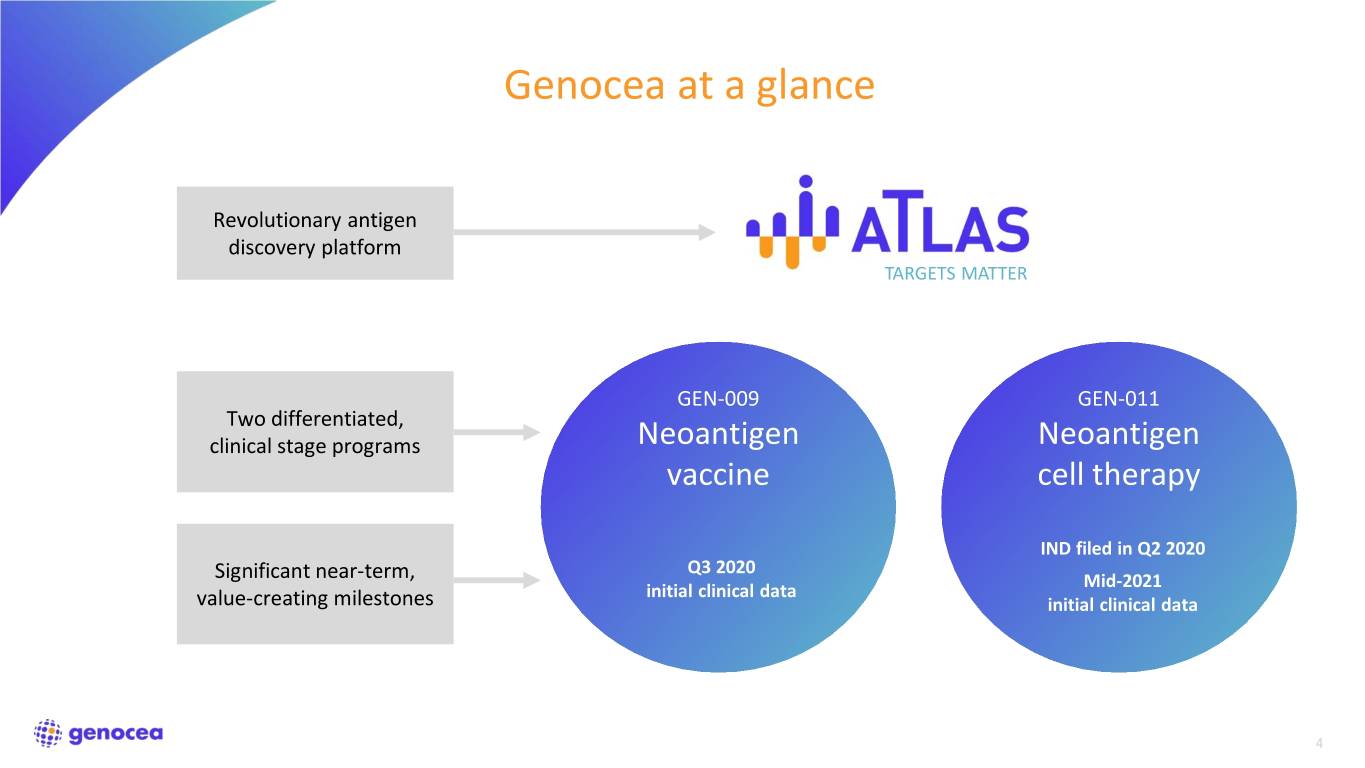
Genocea at a glance Revolutionary antigen discovery platform TARGETS MATTER GEN-009 GEN-011 Two differentiated, clinical stage programs Neoantigen Neoantigen vaccine cell therapy IND filed in Q2 2020 Q3 2020 Significant near-term, Mid-2021 initial clinical data value-creating milestones initial clinical data 4

Why do targets matter? Lesson from infectious Lesson from cancer Targeting the right disease vaccines immunotherapy neoantigens delivers clinical responses Wrong antigens no protection Responses to neoantigens Challenge: identifying drive checkpoint inhibitor surface-presented and (CPI), TIL therapy outcomes immunogenic neoantigens 5

The ATLAS™ antigen selection difference output All surface-presented True peptides eliciting anti- antigens tumor T cell responses T cell Are peptides surface- ATLAS identifies antigens TCR presented? of pre-existing responses Peptide Limit of Not MHC 1st-gen Are peptides recognized necessarily Our goal: Dramatically prediction by T cells? good strengthen anti-tumor platforms antigens immune activity by amplifying these Do T cells respond responses appropriately to antigen? Tumor cell 6

ATLAS: high throughput platform harnessing the immune system for antigen selection Tumor NGS biopsy analysis Unique Bacterial plasmids for vectors Autologous every expressing each dendritic cell candidate candidate neoantigen Autologous T cell Blood sample 7

Inhibigen identification and exclusion is critical to ensure efficacy 1,600 Adjuvant only 1,000 ) 1,400 Protective vaccine Adjuvant only 3 ) 3 1,200 Protective vaccine + Inhibigen 800 Inhibigens + Adjuvant 1,000 600 800 400 600 400 200 (mm volume Tumor 200 Tumor volume (mm volume Tumor 0 0 0 6 8 10 12 14 16 18 0 3 6 8 10 12 14 16 18 20 22 24 Day Day Therapeutic mouse vaccination with a pool of ATLAS-identified The presence of Inhibigens in an otherwise protective Inhibigens drives tumor hyperprogression mouse vaccine completely abrogates protection Inhibigen-specific T cells may be responsible for hyperprogression after CPI therapy1,2 Experiments run in B16F10 melanoma model 1. Champiat et al., Clin Can Res (2017) 2. Ferrara et al., JAMA (2018) 8
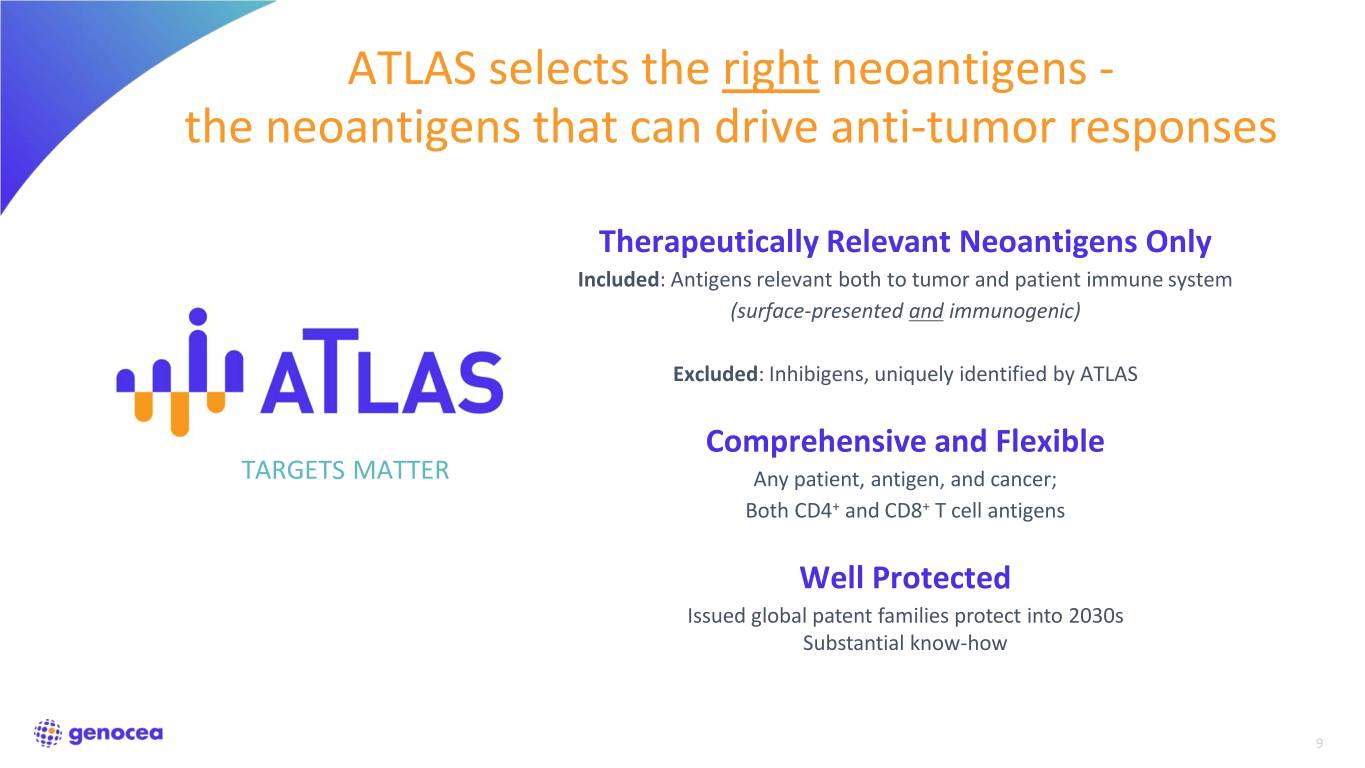
ATLAS selects the right neoantigens - the neoantigens that can drive anti-tumor responses Therapeutically Relevant Neoantigens Only Included: Antigens relevant both to tumor and patient immune system (surface-presented and immunogenic) Excluded: Inhibigens, uniquely identified by ATLAS Comprehensive and Flexible TARGETS MATTER Any patient, antigen, and cancer; Both CD4+ and CD8+ T cell antigens Well Protected Issued global patent families protect into 2030s Substantial know-how 9

GEN-009 Unprecedented immune responses; Neoantigen Vaccine Emerging clinical efficacy 10

Simple, scalable production and delivery 11

GEN-009 phase 1/2a trial design Part A • Vaccine monotherapy in patients with no evidence of disease • Multiple tumor types with CPI approval • Objectives: safety, immunogenicity • Status: all patients in long-term follow-up
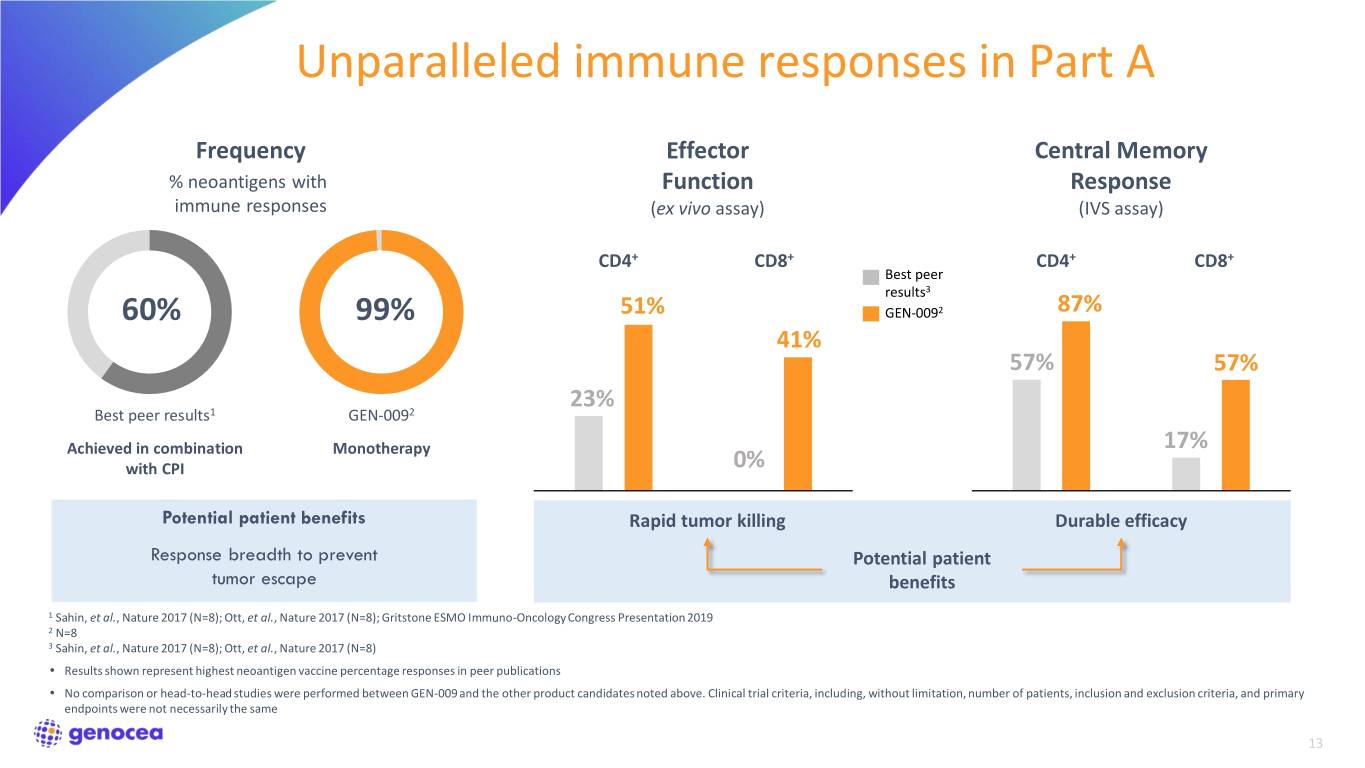
Unparalleled immune responses in Part A Frequency Effector Central Memory % neoantigens with Function Response immune responses (ex vivo assay) (IVS assay) CD4+ CD8+ CD4+ CD8+ Best peer results3 60% 99% 51% GEN-0092 87% 41% 57% 57% 23% Best peer results1 GEN-0092 Achieved in combination Monotherapy 17% with CPI 0% Potential patient benefits Rapid tumor killing Durable efficacy Response breadth to prevent Potential patient tumor escape benefits 1 Sahin, et al., Nature 2017 (N=8); Ott, et al., Nature 2017 (N=8); Gritstone ESMO Immuno-Oncology Congress Presentation 2019 2 N=8 3 Sahin, et al., Nature 2017 (N=8); Ott, et al., Nature 2017 (N=8) • Results shown represent highest neoantigen vaccine percentage responses in peer publications • No comparison or head-to-head studies were performed between GEN-009 and the other product candidates noted above. Clinical trial criteria, including, without limitation, number of patients, inclusion and exclusion criteria, and primary endpoints were not necessarily the same 13

No disease progression in almost all Part A patients Patient Tumor Type Recurrence-free Survival of Part A Patients Median follow up of 14 months A NSCLC Compares favorably with expected relapse rates in these malignancies B Urothelial C Melanoma Patient H had low ex vivo immune responses compared to rest of Part E Urothelial A cohort F NSCLC G Urothelial Legend H Urothelial Last imaging, no measurable disease Recurrence K SCCHN Second primary unrelated to vaccine 0 6 12 18 Months 14

GEN-009 phase 1/2a trial design Part B • Combination of GEN-009 and standard-of-care PD-1-based regimens in patients with advanced disease • Tumor types: Melanoma, NSCLC, SCCHN, Urothelial, RCC • Objectives: safety, immunogenicity, efficacy • Vaccination contribution to be determined after CPI response established • Initial data from full Part B cohort expected in September 2020
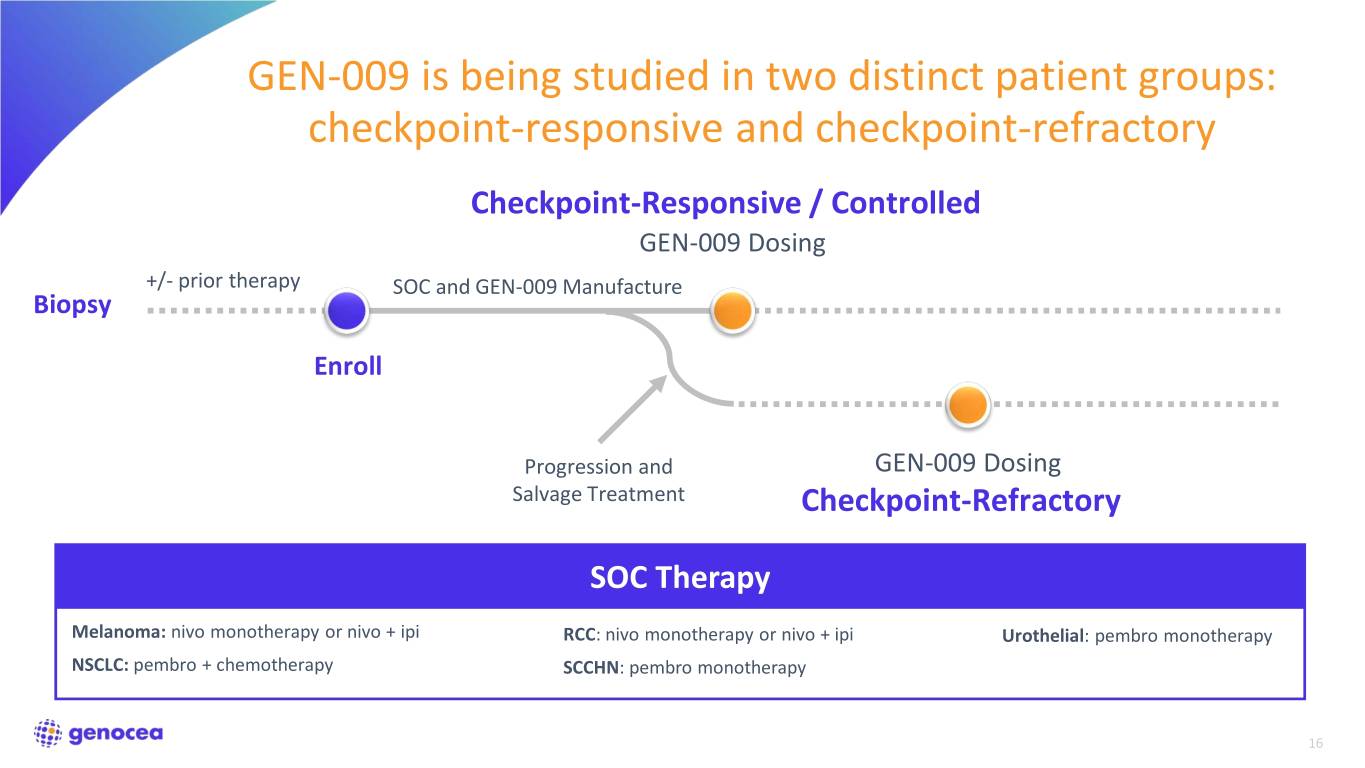
GEN-009 is being studied in two distinct patient groups: checkpoint-responsive and checkpoint-refractory Checkpoint-Responsive / Controlled GEN-009 Dosing +/- prior therapy SOC and GEN-009 Manufacture Biopsy Enroll Progression and GEN-009 Dosing Salvage Treatment Checkpoint-Refractory SOC Therapy Melanoma: nivo monotherapy or nivo + ipi RCC: nivo monotherapy or nivo + ipi Urothelial: pembro monotherapy NSCLC: pembro + chemotherapy SCCHN: pembro monotherapy 16

Part B patients Subject ID Data Available* Histology Ranking by predicted ORR to 1st line CPI SOC Checkpoint-Responsive / Controlled 058 ✓ NSCLC, adeno PD-L1: 60% 057 ✓ RCC Intermediate risk 105 ✓ SCCHN PD-L1: 20% 001 ✓ UC PD-L1: 20% 031 NSCLC, adeno PD-L1: 95% 030 NSCLC, squamous PD-L1 positive 078 RCC Intermediate risk 060 RCC Intermediate risk 204 SCCHN PD-L1: 100% Checkpoint-Refractory 106 ✓ SCCHN Refractory 108 SCCHN Refractory 109 SCCHN Refractory 110 SCCHN Refractory 027 UC Refractory 059 Melanoma Refractory Ranking by predicted ORR to 1st line CPI SOC – red: <30%, yellow: 30-50%, green: >50% *Patients for whom at least one post-vaccination scan was available Data Cutoff Date: June 30, 2020 17

Safety profile for GEN-009 dosing GEN-009 is very well tolerated without additive toxicity Related Serious Events Related to GEN-009 Events 8 / 8 patients Part A Grade 1 and Grade 2 injection site reactions, 0 Grade 2 fever 6 / 11 patients Part B 0 Grade 1 injection site reactions Data Cutoff Date: July 14, 2020 18

Maura L. Gillison, MD, PhD Professor of Medicine, Department of Thoracic/Head and Neck Medical Oncology MD Anderson Cancer Center Member, National Academy of Medicine

Broad and potent immunogenicity demonstrated in all five patients Antigen-specific CD4+ and CD8+ T cell responses detected in 100% of patients (N=5) • Every patient is responding to multiple antigens • Generated both ex vivo and after in vitro stimulation • Strong magnitude of response Amplification of T cell responses due to GEN-009 and not induced by CPI treatment is also apparent More detailed analysis of GEN-009 immune responses is underway 20
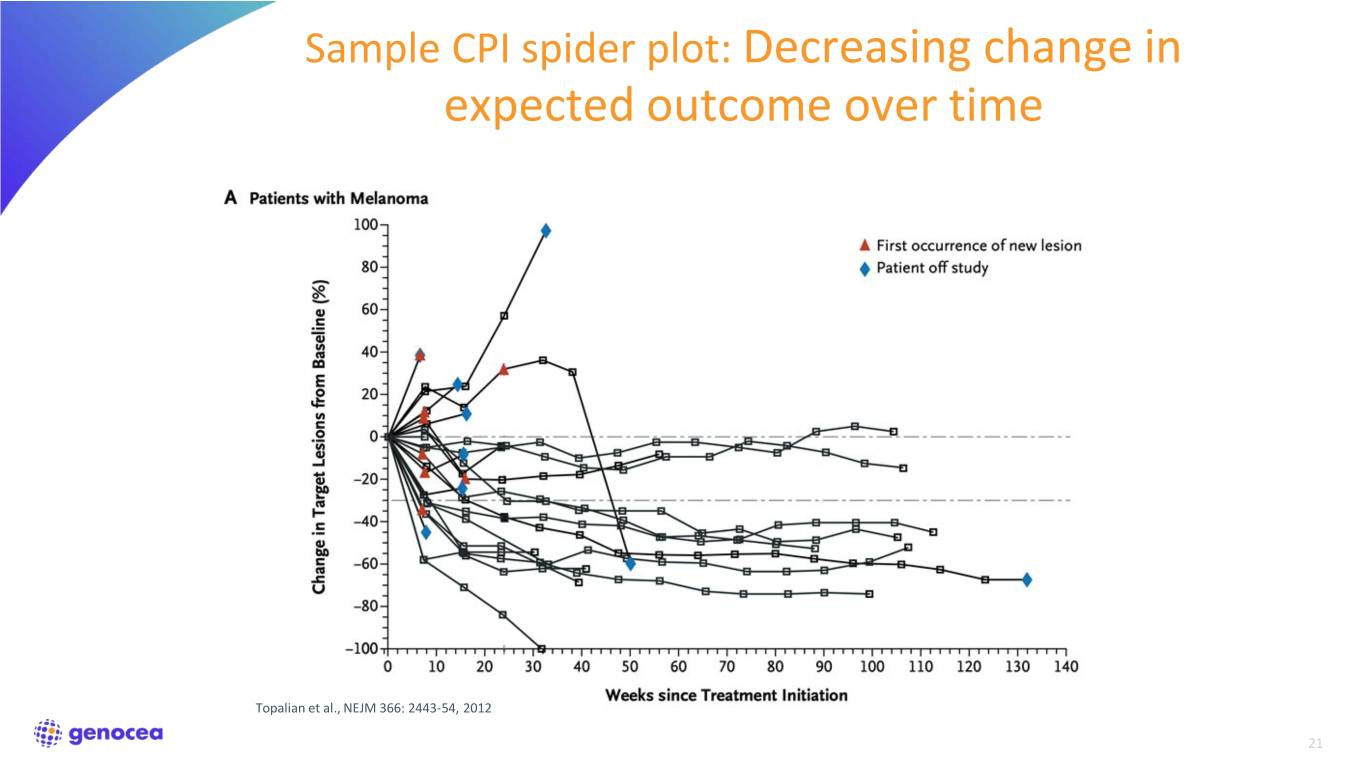
Sample CPI spider plot: Decreasing change in expected outcome over time Topalian et al., NEJM 366: 2443-54, 2012 21

Trial design enables assessment of vaccine specific contribution to clinical responses Each patient can serve as own control Pre-Vaccination Post-Vaccine Dose 1 20 PD (+20%) Scan 0 Baseline -20 PR (-30%) -40 -60 Effect attributable % Change in Target Lesion Sum Target in Change% to vaccine -80 Vaccine Dose 1 2 3 CR (-100%) -100 -100 -50 0 50 100 Days from Vaccine Dose 1 Therapy SOC SOC & Vaccine 22

Patient 057 Ex Vivo Immunogenicity 40 Scan 500 Pool 4 20 PD (+20%) 400 Pool 3 PBMC 6 300 Pool 2 0 Baseline Pool 1 200 -20 100 SFC per 1x10 PR (-30%) 0 -40 Baseline Post-Checkpoint Post-Vaccine -60 • 68 yo female with stage 3 RCC diagnosed at % Change in Target Lesion Sum Target in Change% resection Jun ’19, high risk with progression Sep -80 GEN-009 ‘19 Dose 1 • Received nivo+ipi Oct ‘19 through Dec ’19 then -100 CR (-100%) nivo as single agent -150 -100 -50 0 50 100 • Achieved CR prior to GEN-009 PD-1 SOC Treatment SOC + GEN-009 # GEN-009 antigens: 8 Actual Days from GEN-009 Dose 1 • 23

Patient 105 Ex Vivo 20 PD (+20%) Immunogenicity Scan 1000 Pool 4 0 Baseline 750 Pool 3 PBMC 6 Pool 2 Sum −20 500 Pool 1 Lesion PR (-30%) 250 −40 SFC per 1x10 Target 0 Baseline Post-Checkpoint Post-Vaccine −60 % % Change in • 66 yo male diagnosed with stage 1 oropharyngeal −80 cancer Nov ‘18 • Received neoadjuvant cisplatin + XRT, progressed −100 CR (-100%) May ‘19 −100 −50 0 50 100 GEN-009 Dose 1 • SOC - On continuous single agent pembro every PD-1 SOC Treatment SOC + GEN-009 3 weeks since Sept ‘19 with little CPI toxicity Actual Days from GEN-009 Dose 1 • # GEN-009 antigens: 5 24

Patient 058 20 PD (+20%) IVS Immunogenicity Scan 2000 500 cells cells + 0 Baseline + 400 1500 CD8 CD4 4 4 300 1000 200 Sum −20 500 100 PR (-30%) SFC per 2 x 10 0 SFC per 2 x 10 0 Lesion −40 Baseline Post-Vaccine Baseline Post-Vaccine Target • 68 yo female diagnosed with stage 4 adeno of lung Oct −60 ‘19 GEN-009 Dose 1 • Underwent biopsy and brain XRT to one met % % Change in −80 • SOC - received pembro, pemetrexed and carbo from Dec ‘19 to Feb ‘20, then single agent pembro with little CR (-100%) −100 −100 −50 0 50 CPI toxicity PD-1 SOC Treatment SOC + GEN-009 • # GEN-009 antigens: 15 Actual Days from GEN-009 Dose 1 • Ex vivo fluorospot assay for immune response magnitude assessment not conducted as D50 leukopak not collected due to COVID 25

Patient 001 20 PD (+20%) Ex Vivo Immunogenicity Scan 125 0 Baseline Pool 4 100 Pool 3 PBMC 6 Sum 75 Pool 2 −20 Pool 1 50 Lesion PR (-30%) 25 −40 Target SFC per 1x10 0 Baseline Post-Checkpoint Post-Vaccine −60 % % Change in • 68 yo male diagnosed with bladder carcinoma June ‘19 −80 GEN-009 Dose 1 • Prior to SOC therapy, underwent resection but rapidly progressed CR (-100%) −100 −100 −50 0 50 • SOC - received pembro July ‘19 through Sept ’20 PD-1 SOC Treatment SOC + GEN-009 • # GEN-009 antigens: 8 Actual Days from GEN-009 Dose 1 26

Patient 106 40 Ex Vivo Immunogenicity 400 Pool 4 20 PD (+20%) 300 Pool 3 PBMC 6 Pool 2 0 Baseline Sum 200 Pool 1 100 Lesion −20 PR (-30%) SFC per 1x10 N.T. 0 Target −40 Scan Baseline Post-Checkpoint Post-Vaccine • 62 yo male with stage 4, P16+ oropharyngeal carcinoma −60 Jul ’19 % % Change in GEN-009 • Prior to SOC therapy, underwent resection but rapidly −80 Dose 1 progressed SOC - received pembro Sep ‘19 through Apr ’20 −100 CR (-100%) • −150 −100 −50 0 50 • Received cisplatin, docetaxel, and pembro after PD-1 SOC Salvage SOC + Treatment Treatment GEN-009 progression • # GEN-009 antigens: 10 Actual Days from GEN-009 Dose 1 N.T. = Not yet tested 27
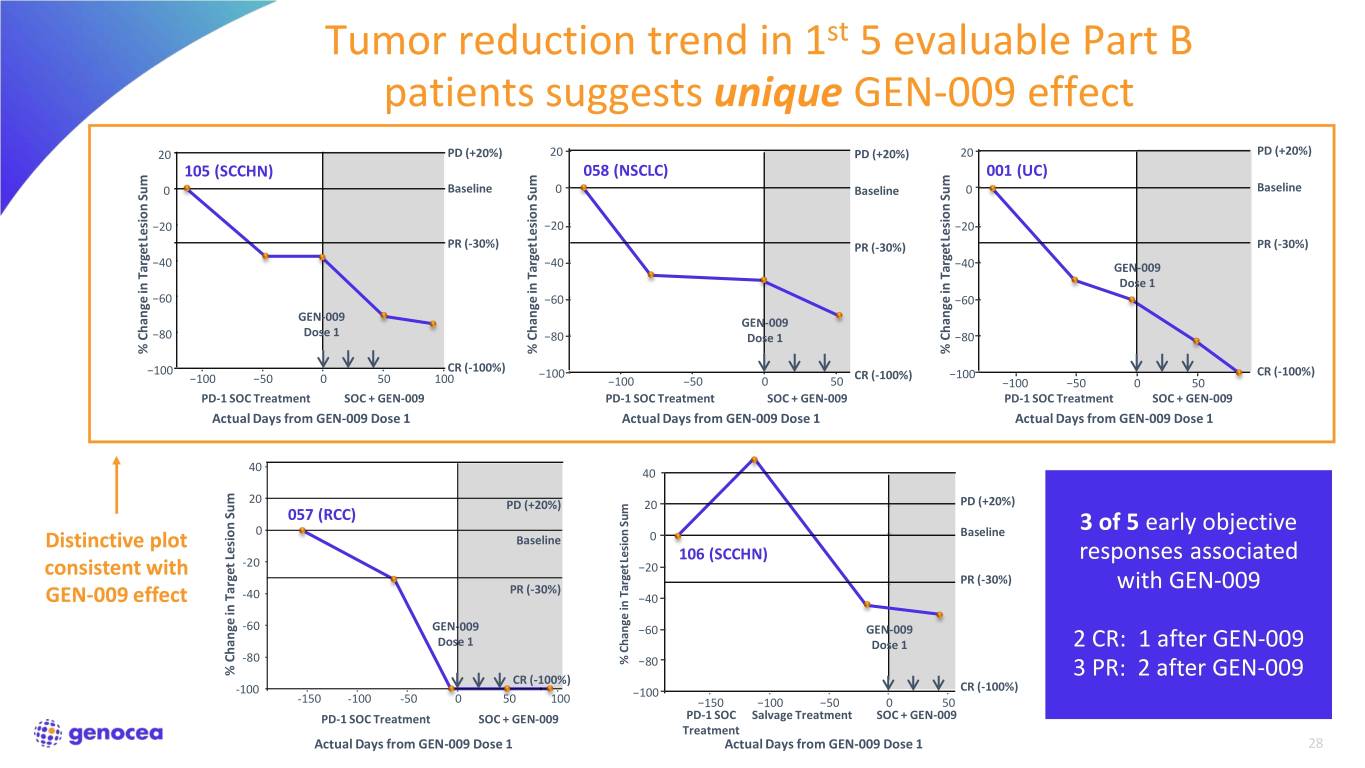
Tumor reduction trend in 1st 5 evaluable Part B patients suggests unique GEN-009 effect 20 PD (+20%) 20 PD (+20%) 20 PD (+20%) 105 (SCCHN) 058 (NSCLC) 001 (UC) 0 Baseline 0 Baseline 0 Baseline Sum Sum Sum −20 −20 −20 Lesion Lesion Lesion PR (-30%) PR (-30%) PR (-30%) −40 −40 −40 GEN-009 Target Target Target Target Dose 1 −60 −60 −60 GEN-009 GEN-009 −80 Dose 1 −80 Dose 1 −80 % Change in % in Change % in Change % in Change CR (-100%) −100 −100 CR (-100%) −100 CR (-100%) −100 −50 0 50 100 −100 −50 0 50 −100 −50 0 50 PD-1 SOC Treatment SOC + GEN-009 PD-1 SOC Treatment SOC + GEN-009 PD-1 SOC Treatment SOC + GEN-009 Actual Days from GEN-009 Dose 1 Actual Days from GEN-009 Dose 1 Actual Days from GEN-009 Dose 1 40 40 20 PD PD (+20%) 20 (+20%) PD (+20%) 057 (RCC) Sum 0 Baseline Baseline 3 of 5 early objective Distinctive plot Baseline 0 Lesion 106 (SCCHN) responses associated -20 −20 consistent with PR (−30%) PR (-30%) with GEN-009 PR (-30%) Target GEN-009 effect -40 −40 -60 GEN-009 −60 GEN-009 Dose 1 Dose 1 2 CR: 1 after GEN-009 -80 % Change in −80 % Change in Target Lesion Sum Lesion % in Target Change CR (-100%) 3 PR: 2 after GEN-009 -100 −100 CR (-100%) -150 -100 -50 0 50 100 −150 −100 −50 0 50 PD-1 SOC Treatment SOC + GEN-009 PD-1 SOC Salvage Treatment SOC + GEN-009 Treatment Actual Days from GEN-009 Dose 1 Actual Days from GEN-009 Dose 1 28

GEN-009: Delivering on the promise of neoantigen therapies Compelling early signal Clear anti-tumor activity and immunogenicity; More data to come “Targets matter” Highly differentiated results Powerful readthrough to GEN-011 ATLAS enables unique breadth and specificity 29

Q&A
