Attached files
Exhibit 10.11
CERTAIN INFORMATION IDENTIFIED BY BRACKETED ASTERISKS ([* * *]) HAS BEEN OMITTED FROM THIS EXHIBIT BECAUSE IT IS BOTH NOT MATERIAL AND WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
CLINICAL TRIAL COLLABORATION AND SUPPLY AGREEMENT
This CLINICAL TRIAL COLLABORATION AND SUPPLY AGREEMENT (this “Agreement”), made as of August 22, 2018 (the “Effective Date”), is by and between Ares Trading S.A., Z.I de l’Ouriettaz, CH-1170 Aubonne, Switzerland (“Merck”), an affiliate of Merck KGaA, Darmstadt, Germany, and Pfizer Inc., having a place of business at 235 East 42nd Street, New York, NY 10017 USA (“Pfizer”) on the one side, and Checkmate Pharmaceuticals, Inc., having a place of business at One Broadway, 14th floor, Cambridge, MA 02142 (“Checkmate”) on the other side. Merck and Pfizer together are referred to herein as the “Alliance”. The Alliance and Checkmate are each referred to herein individually as “Party” and collectively as “Parties”.
RECITALS
A. Under a separate agreement between Merck and Pfizer the Alliance is developing the Alliance Compound (as defined below) for the treatment of certain tumor types.
B. Checkmate is developing the Checkmate Compound (as defined below) for the treatment of certain tumor types.
C. Pfizer is developing the Pfizer Compounds (as defined below) for the treatment of certain tumor types.
D. Pfizer desires to sponsor a clinical trial in which the Checkmate Compound, a Pfizer Compound and the Alliance Compound would be dosed concurrently or in combination.
E. The Alliance, Pfizer and Checkmate, consistent with the terms of this Agreement, desire to collaborate as more fully described herein, including by providing the Alliance Compound, Pfizer Compounds and the Checkmate Compound for the Study (as defined below).
NOW, THEREFORE, in consideration of the premises and of the following mutual promises, covenants and conditions, the Parties, intending to be legally bound, mutually agree as follows:
| 1. | Definitions. |
For all purposes of this Agreement, the capitalized terms defined in this Article 1 and throughout this Agreement shall have the meanings herein specified.
1.1 “Affiliate” means, with respect to a Party, a firm, corporation or other entity which directly or indirectly owns or controls said Party, or is owned or controlled by said Party, or is
under common ownership or control with said Party. The word “control” means (i) the direct or indirect ownership of fifty percent (50%) or more of the outstanding voting securities of a legal entity, or (ii) possession, directly or indirectly, of the power to direct the management or policies of a legal entity, whether through the ownership of voting securities, contract rights, voting rights, corporate governance or otherwise.
1.2 “Agreement” means this agreement, as amended by the Parties from time to time, and as set forth in the preamble.
1.3 “Alliance” has the meaning set forth in the preamble.
1.4 “Alliance Class Compound” means any small or large molecule that inhibits PD-1 or PD-L1 activity, including the Alliance Compound and any other anti-PD-L1 (programmed death-ligand 1) mono-clonal antibody and any other antibody that blocks binding of PD-L1 to PD-1, and any formulations thereof.
1.5 “Alliance Compound” means the antibody known as MSB0010718C referred to by the Alliance as “avelumab”.
1.6 “Alliance Compound Inventions” has the meaning set forth in Section 10.3.
1.7 “Alliance Liability” has the meaning set forth in Section 14.2.2.
1.8 “Alliance Manager” has the meaning set forth in Section 3.7.
1.9 “Applicable Law” means all international, federal, state, local, national and regional statutes, laws, rules, regulations and directives applicable to a particular activity hereunder, including performance of clinical trials, medical treatment and the processing and protection of personal and medical data, that may be in effect from time to time, including those promulgated by the United States Food and Drug Administration (“FDA”), state and national regulatory authorities, the European Medicines Agency (“EMA”) and any successor agency to the FDA or EMA or any agency or authority performing some or all of the functions of the FDA or EMA in any jurisdiction outside the United States or the European Union (each a “Regulatory Authority” and collectively, “Regulatory Authorities”), and including without limitation cGMP and GCP (each as defined below); all data protection requirements such as those specified in the EU General Data Protection Regulation and the regulations issued under the United States Health Insurance Portability and Accountability Act of 1996, as amended (“HIPAA”); export control and economic sanctions regulations which prohibit the shipment of United States-origin products and technology to certain restricted countries, entities and individuals; anti-bribery and anti-corruption laws pertaining to interactions with government agents, officials and representatives; laws and regulations governing payments to healthcare providers; and any United States or other country’s or jurisdiction’s successor or replacement statutes, laws, rules, regulations and directives relating to the foregoing.
1.10 “Business Day(s)” means any day other than a Saturday, Sunday or any public holiday in the country where the applicable obligations are to be performed.
1.11 “Calendar Quarter” means a three-month period beginning on January, April, July or October 1st.
1.12 “Calendar Year” means a one-year period beginning on January 1st and ending on December 31st.
1.13 “cGMP” means the current Good Manufacturing Practices officially published and interpreted by EMA, FDA and other applicable Regulatory Authorities that may be in effect from time to time and are applicable to the Manufacture of the Compounds.
1.14 “Change of Control” means, with respect to a Party, a transaction with a Third Party(ies) involving (a) the acquisition, merger or consolidation, directly or indirectly, of such Party, and, immediately following the consummation of such transaction, the shareholders of such Party immediately prior thereto hold, directly or indirectly, as applicable, shares of capital stock of the surviving company representing less than fifty percent (50%) of the outstanding shares of such surviving or continuing company, (b) the sale of all or substantially all of the assets or business of such Party, or (c) a person, or group of persons acting in concert, acquire more than fifty percent (50%) of the voting equity securities or management control of such Party.
1.15 “Checkmate” has the meaning set forth in the preamble.
1.16 “Checkmate Class Compound” means a TLR-9-agonist which stimulates anti-tumor CTLs including the Checkmate Compound and any formulations thereof.
1.17 “Checkmate Compound” means CMP-001.
1.18 “Checkmate Compound Inventions” has the meaning set forth in Section 10.1.5.
1.19 “Checkmate Liability” has the meaning set forth in Section 14.2.
1.20 “Claims” has the meaning set forth in Section 14.2.
1.21 “Clinical Data” means all data (including raw data) and results generated under the Study, including any analysis thereof; excluding, however, Sample Testing Results.
1.22 “Clinical Quality Agreement” means that certain clinical quality assurance agreement being entered into by the Parties in conjunction herewith prior to the start of the Study.
1.23 “CMC” means Chemistry Manufacturing and Controls.
1.24 “Class Combination” means the Alliance Class Compound, the Checkmate Class Compound, or the Pfizer Class Compound in pairwise combination or a combination of all three combined, as applicable, whether in a single composition or separate compositions, including the use or method of using the Alliance Class Compound and the Checkmate Class Compound and the Pfizer Class Compound in concomitant or sequential administration.
1.25 “Class Compound” means either the Alliance Class Compound or the Checkmate Class Compound, or the Pfizer Class Compound, as applicable.
1.26 “Compounds” means the Alliance Compound and the Checkmate Compound and the Pfizer Compounds. A “Compound” means either the Alliance Compound or the Checkmate Compound or the Pfizer Compounds, as applicable.
1.27 “Compound Combination” means (a) the Alliance Compound, (b) a Pfizer Compound and (c) the Checkmate Compound in pairwise combination, or a combination of all three combined, as applicable, whether in a single composition or separate compositions, including the use or method of using or method of treatment using (a) the Alliance Compound, (b) the Pfizer Compound, and (c) the Checkmate Compound in concomitant or sequential administration.
1.28 “Confidential Information” means any information, Know-How or other proprietary information or materials furnished to one Party by the other Party pursuant to this Agreement, except to the extent that such information or materials: (a) was already known to the receiving Party, other than under an obligation of confidentiality, at the time of disclosure by the other Party, as demonstrated by competent evidence; (b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party; (c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement; (d) was disclosed to the receiving Party by a Third Party who had no obligation to the disclosing Party not to disclose such information to others; or (e) was subsequently developed by the receiving Party without use of the Confidential Information, as demonstrated by competent evidence.
1.29 “Continuing Party” has the meaning set forth in Section 10.1.1.
1.30 “CTA” means an application to a Regulatory Authority for purposes of requesting the ability to start or continue a clinical trial, which CTA may consist of, or include, an IND or IMPD, as applicable.
1.31 “Data Sharing and Sample Testing Schedule” means the schedule describing each Party’s data sharing and sample testing obligations which shall be finalized in writing by mutual agreement of the Parties and prior to the enrollment of the first patient in the Study.
1.32 “Defending Party” has the meaning set forth in Section 14.2.2.
1.33 “Delivery” has the meaning set forth in Section 8.3 with respect to the Alliance Compound and in Section 8.3.1 with respect to the Checkmate Compound.
1.34 “Direct Manufacturing Cost” shall include the sum of manufacturing fees; raw materials; direct labor; quality, release and in-process control costs; charges for reasonable spoilage, scrap or rework costs; freight and duty, and factory overhead costs that can be directly attributed to such Compound, including but not limited to equipment maintenance and repair, supplies, ongoing stability program costs, other plant services, indirect labor and depreciation on direct capital assets.
1.35 “Disposition Package” has the meaning set forth in Section 8.7.
1.36 “Dispute” has the meaning set forth in Section 21.
1.37 “Effective Date” has the meaning set forth in the preamble.
1.38 “Exclusivity Period” has the meaning set forth in Section 3.8.
1.39 “EMA” has the meaning set forth in the definition of Applicable Law.
1.40 “FDA” has the meaning set forth in the definition of Applicable Law.
1.41 “First Press Release” has the meaning set forth in Section 12.
1.42 “Force Majeure” has the meaning set forth in Section 0.
1.43 “GCP” means the Good Clinical Practices officially published by EMA, FDA and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) that may be in effect from time to time and are applicable to the testing of the Compounds.
1.44 “GDP” means the Good Distribution Practices officially published and interpreted by EMA, FDA and other applicable Regulatory Authorities that may be in effect from time to time and are applicable to the Distribution of the Compounds.
1.45 “Government Official” means: (a) any officer or employee of a government or any department, agency or instrument of a government; (b) any person acting in an official capacity for or on behalf of a government or any department, agency, or instrument of a government; (c) any officer or employee of a company or business owned in whole or part by a government; (d) any officer or employee of a public international organization such as the World Bank or United Nations; (e) any officer or employee of a political party or any person acting in an official capacity on behalf of a political party; and/or (f) any candidate for political office; who, when such Government Official is acting in an official capacity, or in an official decision-making role, has responsibility for performing regulatory inspections, government authorizations or licenses, or otherwise has the capacity to make decisions with the potential to affect the business of either of the Parties.
1.46 “HIPAA” has the meaning set forth in the definition of Applicable Law.
1.47 “IMPD” means an Investigational Medicinal Product Dossier which includes all data required by Regulatory Authorities in the European Union for the performance of clinical trials in one or more European Union member states.
1.48 “IND” means the Investigational New Drug Application filed or to be filed with the FDA as described in Title 21 of the U.S. Code of Federal Regulations, Part 312, and the equivalent application in the jurisdictions outside the United States.
1.49 “Indication” means squamous cell carcinoma of the head and neck.
1.50 “Indirect Manufacturing Costs” shall include allocations of indirect factory overhead and site support costs, including but not limited to utilities, quality, planning, engineering, maintenance, safety, site science and technology, and depreciation on indirect capital
assets, procurement, warehousing, and corporate services; shipping costs; all costs incurred by a Party in connection with audits conducted pursuant to the Clinical Quality Agreements; any non-refundable or non-creditable indirect taxes, customs and excise duties, or similar taxes paid or payable by any Third Party or Affiliate in relation to the Manufacture of any portion of such Compound. Allocations shall be based on such Compound’s utilization relative to a manufacturing site’s total activity.
1.51 “Intellectual Property Rights” or “IP Rights” means any provisional patent application, pending patent application whether or not patentable, granted or allowed patent, divisional patent application, continuation, reissue, utility model, design, trademark and Know-How as defined in Section 1.58, as well as any equivalent of said rights in any jurisdiction.
1.52 “Inventions” means all inventions and discoveries which are made or conceived in the design or performance of the Study and/or which are made or conceived by a Party through use of the Clinical Data and/or Sample Testing Results.
1.53 “Joint Combination Study Committee” or “JCSC” has the meaning set forth in Section 3.7.
1.54 “Joint IP” means Jointly Owned Inventions, Joint Patent Applications and Joint Patents.
1.55 “Jointly Owned Invention” has the meaning set forth in Section 10.1.
1.56 “Joint Patent Application” has the meaning set forth in Section 10.1.1.
1.57 “Joint Patent” means a patent that issues from a Joint Patent Application.
1.58 “Know-How” means any proprietary invention, innovation, improvement, development, discovery, computer program, device, trade secret, method, know-how, process, technique or the like, including manufacturing, use, process, structural, operational and other data and information, whether or not written or otherwise fixed in any form or medium, regardless of the media on which contained and whether or not patentable or copyrightable, that is not generally known or otherwise in the public domain.
1.59 “Liability” (including “Checkmate Liability”, “Alliance Liability” and “Pfizer Liability”) has the meaning set forth in Section 14.2.
1.60 “Lead Prosecuting Party” has the meaning set forth in Section 10.1.2.
1.61 “Manufacture,” “Manufactured,” or “Manufacturing” means all stages of the manufacture of a Compound, including planning, purchasing, manufacture, processing, compounding, storage, filling, packaging, waste disposal, labeling, leafleting, testing, quality assurance, sample retention, stability testing, release, dispatch and supply, as applicable.
1.62 “Manufacturer’s Release” shall mean that a manufacturer, represented by its qualified person, after careful testing, draws up a certificate of analysis (CoA) and certificate of compliance (BC) that provides information on the manufacture, testing, packaging and labeling in
accordance with the requirements of the principles and guidelines of EC Good Manufacturing Practice or the good manufacturing practice of a third country recognized as equivalent under a mutual recognition agreement and any other relevant legal requirement and in the event that a defect needs to be investigated, ensures that the Qualified Person who certified the batch and the relevant records are readily identifiable.
1.63 “Manufacturing Cost” shall mean the Direct Manufacturing Costs and the Indirect Manufacturing Costs on a per vial basis.
1.64 “Manufacturing Quality Agreement(s)” means all quality assurance agreements being entered into by the Parties in conjunction herewith prior to the shipment of the Merck Compound, covering all quality assurance agreements being entered into by the Parties in conjunction with manufacturing needs of the Compounds.
1.65 “Manufacturing Site” means the facilities where a Compound is Manufactured by or on behalf of a Party, as such Manufacturing Site may change from time to time in accordance with Section 8.6 (Changes to Manufacturing).
1.66 “Merck” has the meaning set forth in the preamble.
1.67 “Non-Conformance” means, with respect to a given unit of Compound, an event that deviates from an approved cGMP requirement with respect to the applicable Compound, such as a procedure, Specification, or operating parameter (including shelf life as specified in Section 8.2 and the applicable Specifications), or that requires an investigation to assess impact to the quality of the applicable Compound. Classification of the Non-Conformance is detailed in the Quality Agreements.
1.68 “Non-Conformance Event” has the meaning set forth in Section 8.7.2.2.
1.69 “Other Party” has the meaning set forth in Section 14.2.4.
1.70 “Opting-out Party” has the meaning set forth in Section 10.1.2.
1.71 “Party” has the meaning set forth in the preamble.
1.72 “Payer” has the meaning set forth in Section 8.17.
1.73 “Payee” has the meaning set forth in Section 8.17.
1.74 “Payments” has the meaning set forth in Section 8.17.
1.75 “Permitted Use” means (i) seeking Regulatory Approval for the use of its respective Compound in the Compound Combination; (ii) filing and prosecuting patent applications for Jointly Owned Inventions and enforcing any resulting patents in accordance with Article 10 (iii) conducting the Study, (iv) complying with requirements of Applicable Law, including applicable pharmacovigilance and safety reporting obligations or (v) exercising its rights and performing its obligations under this Agreement.
1.76 “Pfizer” has the meaning set forth in the preamble.
1.77 “Pfizer Class Compound” means any small or large molecule that is an OX- 40 agonist or a 4-1BB agonist, including any anti OX-40 agonist or 4-1BB agonist monoclonal antibody and any other anti OX-40 or 4-1BB agonist antibody.
1.78 “Pfizer Compound” means PF-04518600, an anti OX-40 agonist monoclonal antibody or PF-05082566, also known as utomilumab, an anti-4-1BB agonist monoclonal antibody.
1.79 “Pfizer Compound Inventions” has the meaning set forth in Section 10.4.
1.80 “Pfizer Liability” has the meaning set forth in Section 14.2.1.
1.81 “Protocol” means the written documentation that describes the Study as a sub-study within the Javelin Medley Clinical Protocol and sets forth specific activities to be performed as part of the Study conduct, a summary of which is attached hereto as Appendix A.
1.82 “Quality Agreements” means the Manufacturing Quality Agreement(s) and the Clinical Quality Agreement(s).
1.83 “Regulatory Approvals” means any and all permissions (other than the Manufacturing approvals) required to be obtained from Regulatory Authorities and any other competent authority for the development, registration, marketing, importation and distribution of a Compound Combination in the United States, Europe or other applicable jurisdictions for use in humans.
1.84 “Regulatory Authorities” has the meaning set forth in the definition of Applicable Law.
1.85 “Related Agreements” means the Safety Data Exchange Agreement and the Quality Agreements.
1.86 “Replacement Threshold” has the meaning set forth in Section 8.7.2.3.
1.87 “Safety Data Exchange Agreement” means that certain pharmacovigilance agreement regarding the Compounds that shall be entered into by the Parties prior to the enrollment of the first patient in the Study.
1.88 “Samples” means urine, blood and tissue samples from patients participating in the Study.
1.89 “Sample Testing” means the analyses to be performed by each Party using the applicable Samples, as described in the Data Sharing and Sample Testing Schedule.
1.90 “Sample Testing Results” means those data and/or results arising from the Sample Testing which are to be shared between the Alliance and Checkmate, as set forth in the Data Sharing and Sample Testing Schedule.
1.91 “Specifications” means, with respect to a given Compound, the set of requirements for such Compound as set forth in the Quality Agreements.
1.92 “Study” means clinical studies, performed in accordance with the Protocol, as further described in Appendix A.
1.93 “Study Completion” has the meaning set forth in Section 3.5.
1.94 “Study Results” means the results generated under the Study.
1.95 “Subcontractors” has the meaning set forth in Section 2.2.
1.96 “Team Leader” has the meaning set forth in Section 3.7.
1.97 “Term” has the meaning set forth in Section 6.1.
1.98 “Territory” means anywhere in the world.+
1.99 “Third Party” means any person or entity other than Checkmate, Pfizer, Merck, or their respective Affiliates.
| 2. | Scope of the Agreement. |
2.1 Each Party shall contribute to the Study such resources as are necessary to fulfill its obligations set forth in this Agreement.
2.2 Each Party agrees to act in good faith in performing its obligations under this Agreement and each Related Agreement, and shall notify the other Party as promptly as possible in the event of any Manufacturing delay that is likely to adversely affect supply of its Compound as contemplated by this Agreement.
2.3 Each Party shall have the right to subcontract any portion of its obligations hereunder to Third Party subcontractors (“Subcontractors”). Each Party shall remain solely and fully liable for the performance of its Subcontractors. Each Party shall ensure that each of its Subcontractors performs its obligations pursuant to the terms of this Agreement, including the Appendices attached hereto. To the extent that a Party has an obligation under this Agreement to perform an action or to meet a standard, and such Party subcontracts such obligation, such Party shall be responsible for any failure by such Party’s Subcontractor to perform the action or meet the standard. Each Party shall use reasonable efforts to obtain and maintain copies of documents relating to the obligations performed by such Subcontractors that are held by or under the control of such Subcontractors and that are required to be provided to the other Party under this Agreement.
2.4 This Agreement does not create any obligation on the part of the Alliance to provide the Alliance Compound for any activities other than the Study, nor does it create any obligation on the part of Checkmate to provide the Checkmate Compound for any activities other than the Study, nor does it create any obligation on the part of Pfizer to provide the Pfizer Compound for any activities other than the Study.
2.5 Subject to Section 3.8 below, nothing in this Agreement shall (i) prohibit any Party from performing clinical studies other than the Study relating to its own Compound, either individually or in combination with any other compound or product, in any therapeutic area, or (ii) create an exclusive relationship between the Parties with respect to any Compound.
| 3. | Conduct of the Study. |
3.1 Notwithstanding anything to the contrary herein, Pfizer shall act as the sponsor of the Study and shall own and hold the IND and/or CTA, as applicable, for the Study; provided, however, that in no event shall Pfizer file a separate IND or CTA for the Study unless required by Regulatory Authorities to do so. If a Regulatory Authority requests a separate IND or CTA for the Study, the JCSC will promptly meet and mutually agree on an approach to address such requirement. In the event that the JCSC cannot agree on an approach to address such requirement the matter is elevated in accordance with Section 3.7 for resolution.
3.2 Pfizer shall ensure that the Study is performed in accordance with this Agreement, the Protocol, the informed consent and all Applicable Law, including GCP. After the completion of each arm in the Study Protocol, the Parties will jointly determine whether to commence a new arm as outlined in the Protocol. The Parties agree that if they jointly determine to commence the F3 arm in the Protocol that this Agreement will need to be amended to include the additional Pfizer Compound and update the budget.
3.3 Pfizer shall ensure that all directions from any Regulatory Authority and/or ethics committee with jurisdiction over the Study are followed. The Alliance and Checkmate each shall fully cooperate with Pfizer to comply with such directions, including the Alliance with respect to supply of the Alliance Compound. Pfizer shall participate in and lead all discussions with any Regulatory Authority regarding the Study, provided, however, that to the extent practicable (e.g. ad hoc conversations with Regulatory Authorities requiring an immediate response will be excluded) and if not prohibited by such Regulatory Authority, Checkmate shall have the right (but no obligation) to participate in any discussions with a Regulatory Authority regarding matters related to the Checkmate Compound; provided further that the Parties acknowledge and agree that such right does not apply to discussions regarding general Study matters that are not related to the Checkmate Compound. Each Party grants to the other Party a non-exclusive, non-transferable (except in connection with a permitted assignment, sublicense or subcontract) “right of reference” (as defined in US FDA 21 CFR 314.3(b)), or similar “right of reference” as defined in applicable regulations in the relevant part of the Territory (only if possible, i.e., a CTA for the respective Compound was already submitted to the local Health Authorities), with respect to Study Results and results related to Compounds, solely as necessary for the other Party to prepare, submit and maintain regulatory submissions of the Study related to the other Party’s Compound and Regulatory Approvals. In all other cases, where a “right of reference” is not possible, the parties will promptly discuss in good faith on how to provide the required documentation for CTA of the Study. Further, each Party shall provide to the other a cross-reference letter or similar communication to the applicable Regulatory Authority to effectuate such right of reference on a need-to-know basis with respect to the confidential part of the Drug Master Files of both Parties. Notwithstanding anything to the contrary in this Agreement, no Party shall have any right to access the other Party’s CMC data with respect to its Compound.
3.4 Pfizer shall maintain reports and all related documentation with respect to the Study in good scientific manner and in compliance with Applicable Law. Each Party shall provide to the other Parties all Study information and documentation (excluding information and documentation relating to the Sample Testing other than the Sample Testing Results themselves) reasonably requested by any such other Party to enable it to (i) comply with any of its legal and regulatory obligations, or any request by any Regulatory Authority, in each case, to the extent related to the Study or such Party’s Compound, (ii) conduct the Sample Testing, (iii) satisfy any contractual obligation to a Subcontractor engaged pursuant to Section 2.3 hereof, and (iv) in the case of the Alliance or Checkmate, determine whether the Study has been performed by Pfizer in accordance with this Agreement.
3.5 Upon request of the Alliance or Checkmate, Pfizer shall provide to the requesting Party copies of all Clinical Data to the extent generated by such Party, in an agreed electronic form or other mutually agreeable alternate form, and on the timelines specified in the Data Sharing and Sample Testing Schedule (if applicable) or upon mutually agreeable timelines; provided, however, that Clinical Data shall be provided to each of the Alliance or Checkmate in written format no more frequently than once every 3 months or as otherwise mutually agreed by the Parties. Checkmate and Pfizer shall orally exchange on a monthly basis data and information (for example, enrollment numbers, dropouts, safety/tolerability findings, translational data, efficacy, other relevant clinical information) related to the Study and to on-going studies being conducted by Checkmate, as applicable. A complete copy of the Clinical Data shall be provided to the Alliance and Checkmate no later than thirty (30) days following completion of the final Study report. “Study Completion” shall be deemed to occur upon either (a) the lock of the Study database or (b) if the Parties jointly determine, upon the completion of an arm in the Study Protocol, not to commence and enroll patients in a new arm as outlined in the Protocol. Pfizer shall use commercially reasonable efforts to ensure that all patient authorizations and consents required under HIPAA, the EU General Data Protection Regulation or subsequent revised versions thereof or any other similar Applicable Law in connection with the Study permit such sharing of Clinical Data with Checkmate and the Alliance.
3.6 Pfizer shall provide Samples to the Alliance and Checkmate as specified in the Protocol or as agreed to by the JCSC; provided that the patients consented to such provision or provided that the Samples are anonymized according to applicable data privacy laws including the EU General Data Protection Regulation. Each Party shall use the Samples only for the Sample Testing and each Party shall be responsible for conducting the Sample Testing as set forth on the Data Sharing and Sampling Testing Schedule, including all expenses related thereto. The Alliance shall own all Sample Testing Results arising from the Sample Testing conducted by or on behalf of the Alliance except that (i) Sample Testing Results pertaining to the Class Combination shall be jointly owned by the Parties, (ii) Sample Testing Results pertaining solely to Pfizer’s Compound shall be owned solely by Pfizer and (iii) Sample Testing Results pertaining solely to Checkmate’s Compound shall be owned solely by Checkmate. Checkmate shall own all Sample Testing Results arising from the Sample Testing conducted by or on behalf of the Checkmate except that (i) Sample Testing Results pertaining to the Class Combination shall be jointly owned by the Parties, (ii) Sample Testing Results pertaining solely to Pfizer’s Compound shall be owned solely by Pfizer and (iii) Sample Testing Results pertaining solely to the Alliance Compound shall be owned solely by the Alliance. Pfizer shall own all Sample Testing Results arising from the Sample Testing conducted by or on behalf of the Pfizer except that (i) Sample Testing Results pertaining to the
Class Combination shall be jointly owned by the Parties, (ii) Sample Testing Results pertaining solely to Checkmate’s Compound shall be owned solely by Checkmate and (iii) Sample Testing Results pertaining solely to the Alliance Compound shall be owned solely by the Alliance. Each Party shall provide to the other Parties the Sample Testing Results for the Sample Testing conducted by or on behalf of such Party, in electronic form or other mutually agreeable alternate form, and on the timelines specified in the Data Sharing and Sample Testing Schedule or other mutually agreed timelines. Likewise, Checkmate shall own all Sample Testing Results arising from the Sample Testing conducted by or on behalf of Checkmate except that (i) Sample Testing Results pertaining to the Class Combination shall be jointly owned by the Parties and (ii) Sample Testing Results pertaining solely to Alliance’s Compound shall be owned solely by the Alliance. If applicable, Checkmate shall provide to the Alliance the Sample Testing Results for the Sample Testing conducted by or on behalf of Checkmate, in electronic form or other mutually agreeable alternate form, and on the timelines specified in the Data Sharing and Sample Testing Schedule or other mutually agreed timelines. Except to the extent otherwise agreed in writing signed by authorized representatives of each Party, prior to publication or other public disclosure permitted under this Agreement, each Party shall use or disclose the other Party’s Sample Testing Results only for the purposes of the Permitted Use. All Clinical Data and Sample Testing Results pertaining to the Class Combination that are generated under this Agreement shall be jointly owned by Checkmate, Pfizer and the Alliance with each of Checkmate, Pfizer and the Alliance having an undivided one-third ownership interest therein. It is understood and acknowledged by the Parties that the Parties shall have the right to use Clinical Data from the Study report to obtain the original label or label changes for their respective Compounds. In such event, the Parties will enter into good faith negotiations to determine a regulatory submission strategy for the Compounds, and cost sharing of the next part of the Study and/or future study(ies) that may be needed for regulatory submission for the Compounds. Prior to the publication of a particular item of Clinical Data and/or Sample Testing Results pursuant to Section 12 or other public disclosure permitted under this Agreement or as otherwise agreed by the Parties, neither Party shall use or disclose such item of Clinical Data and/or Sample Testing Results other than for the Permitted Use, except to the extent otherwise agreed in writing signed by authorized representatives of each Party. Checkmate may use or disclose such item of Clinical Data and/or Sample Testing Results (a) to a third party engaged in merger and acquisition negotiations with Checkmate that has provided a term sheet to Checkmate in connection with such negotiation, (b) to potential investors of at least 10% of the financing round in a Checkmate financing round or (c) to any third party if the Alliance has declined to enter into a new collaboration, a broader collaboration or a new clinical trial with Checkmate after completion of the Study, provided that the recipients of such data and information are bound to maintain the confidentiality of such data and information by written obligations of confidentiality and non-use at least as restrictive as the obligations contained herein.
3.7 Joint Combination Study Committee. The Parties shall form a joint development team (the “Joint Combination Study Committee” or “JCSC”), made up of an equal number of representatives of the Merck, Pfizer and Checkmate, which shall have the following responsibility for coordinating all activities under, and pursuant to, this Agreement:
3.7.1 Reviewing and approving the Study Protocol and changes thereto for the Compounds in accordance with Section 4 of this Agreement;
3.7.2 Discussing and overseeing regulatory related activities to ensure regulatory compliance and timely management of responses to any regulatory authority queries during regulatory review processes;
3.7.3 Approving publication strategies for Clinical Data and Sample Testing Results arising out of the Study in accordance with Section 12 of this Agreement;
3.7.4 Facilitating the exchange of information in compliance with this Agreement in order to ensure that significant issues concerning adverse event information and safety issues are addressed consistently and in a timely manner;
3.7.5 Reviewing the background of delays in the recruitment of patients and deciding on mitigation measures;
3.7.6 Reviewing and approving all Study reports in accordance with Sections 3.8 and 12 of this Agreement.
3.8 The Alliance, Pfizer and Checkmate shall each designate a Team Leader (the “Team Leader”) who shall be responsible for implementing and coordinating activities, and facilitating the exchange of scientific information between the Parties with respect to the Study. The JCSC is chaired by one of the Team Leaders. The JCSC chair is rotating in the following order: 2018 Checkmate, 2019 the Alliance, 2020 Pfizer. Other JCSC members will be chosen by each Party for itself with an equal number of representatives (such number to be agreed by all of the Parties) of the Alliance, Pfizer and Checkmate. The JCSC shall meet as soon as practicable after the Effective Date and then no less than twice yearly, and more often as reasonably considered necessary at the request of either Party, to provide an update on Study progress. Each Party shall be responsible for its expenses, including travel costs incurred for attending the JCSC. The JCSC may meet in person or by means of teleconference, Internet conference, videoconference or other similar communications equipment. In the event the JCSC agrees to meet in person, the geographical location of such meeting shall be decided by each of the Parties at its own discretion rotating in the following order: 1st Checkmate, 2nd the Alliance, 3rd Pfizer and then back to Checkmate and the rotating order above-described. One week prior to any such meeting, the Pfizer Team Leader shall provide an update in writing to the Team Leader, which update shall contain information about overall Study progress, recruitment status, interim analysis (if results are available), final analysis and other information relevant to the conduct of the Study. The Alliance, Pfizer and Checkmate will each appoint a compliance representative who will be an ad-hoc member of the JCSC and who will sign-off on all JCSC meeting minutes.
3.9 Immediately after the Effective Date, the Alliance, Pfizer and Checkmate shall each appoint a person, who possesses a general understanding of this Agreement and of matters relating to the development of the respective Compounds, to act as alliance manager (each an “Alliance Manager”), who shall oversee interactions between the Parties between meetings of the JCSC. The role of Alliance Manager is to act as a key point of contact between the Parties to facilitate a successful collaboration hereunder and resolution of deadlocks or disputes that may arise hereunder. The Alliance Managers shall attend all JCSC meetings on an agenda driven basis and may bring to the attention to the JCSC any matters or issues either of them reasonably believes should be discussed, and shall have such other responsibilities as the Parties may mutually agree in writing. Each Party may in its sole discretion replace its Alliance Manager at any time by notice in writing to the other Party.
3.10 In the event that an issue arises and the Alliance Managers cannot or do not, after good-faith efforts, reach agreement on such issue, the issue shall be elevated to the Senior Vice President of External Innovation of Merck KGaA, Darmstadt, Germany (or delegate), the President, Oncology of Pfizer (or delegate) and the Chief Executive Officer for Checkmate (or delegate).
3.11 Within one hundred and twenty (120) days of Study Completion, Pfizer shall provide the Alliance and Checkmate with an electronic draft of the clinical Study report for the Alliance and Checkmate to each to provide comments to Pfizer. Pfizer shall consider in good faith any comments provided by the Alliance and Checkmate on the draft of the clinical Study report, provided that such comments are received by Pfizer within thirty (30) days after the Alliance’s and Checkmate’s respective receipt of such draft clinical Study report. Pfizer shall provide the Alliance and Checkmate with the final version of the clinical Study report promptly following such review and comment period of the draft clinical Study report by the Alliance and Checkmate.
3.12 Each Party acknowledges and agrees that the other Parties may have present or future business activities or opportunities, including business activities or opportunities with Third Parties, involving the Alliance Compound, in the case of the Alliance, the Pfizer Compound in the case of Pfizer or the Checkmate Compound, in the case of Checkmate, or other similar products, programs, technologies or processes. Accordingly, but subject to Section 3.8, each Party acknowledges and agrees that nothing in this Agreement shall be construed as a representation or inference that the each other Party will not develop for itself, or enter into business relationships with Third Parties regarding, any products, programs, studies (including combination studies), technologies or processes that are similar to or that may compete with the Class Combination or any other product, program, technology or process, provided that any unpublished Clinical Data, Sample Testing Results, Jointly Owned Inventions, and Confidential Information of the other Parties is not used or disclosed in connection therewith in violation of Sections 3.5, 3.6, 9.1 or 10 (as applicable) of this Agreement.
3.13 Nothing in this Agreement shall prohibit or restrict a Party from licensing, assigning or otherwise transferring to an Affiliate or Third Party its Compound and the related Clinical Data, Confidential Information, Sample Testing Results or Joint IP and the rights associated thereto; provided, however, that the licensee, assignee or transferee may only use such Clinical Data, Confidential Information, Sample Testing Results or Joint IP for the Permitted Use and, with respect to Joint IP, the other uses permitted under Section 10, and such Party shall be responsible for compliance by the licensee, assignee or transferee with the applicable terms and conditions of this Agreement.
| 4. | Protocol and Related Documents. |
4.1 A synopsis of the initial Protocol, entitled a combination of CMP-001 with avelumab and/or Pfizer compounds, has been agreed to by the Parties as of the Effective Date, and is attached as Appendix A (the “Protocol Summary”). Pfizer as sponsor shall finalize the contents of such Protocol consistent with the Synopsis. Any material deviations of such Protocol from the
Synopsis that Pfizer as Study sponsor may determine are advisable shall require prior written approval of all Parties which approval shall not be unreasonably withheld or delayed. Pfizer shall send in writing its proposed final Protocol to the Alliance’s Alliance Manager for the Alliance’s review and comment and to Checkmate’s Alliance Manager for Checkmate’s review and comment. The Parties shall agree to a final Protocol within thirty (30) days from the date such proposed final Protocol is disclosed to the Alliance’s Alliance Manager and to Checkmate’s Alliance Manager, failing which the Protocol approval shall be elevated in accordance with Section 3.8 for final resolution.
4.2 After finalization of the Protocol, any proposed amendments to the Protocol will be sent in writing to the Alliance’s Alliance Manager and to Checkmate’s Alliance Manager and require prior written approval of all Parties which shall not be unreasonably withheld or delayed. In the event that the Parties cannot agree in writing on amendments to the Protocol within fifteen (15) days from the date such proposed the matter is elevated to the Alliance Managers in accordance with Section 3.8 for final resolution.
4.3 In the event that the Alliance Managers cannot reach agreement on changes or amendments to the Protocol solely related to the dosing of the Alliance Compound, the Checkmate Compound or the Pfizer Compound after elevating the matter in accordance with Section 3.8, the Alliance, Checkmate or Pfizer, as the case may be, shall have the final decision on such Protocol amendments.
4.4 Pfizer shall prepare the patient informed consent forms for the Study (which shall include any required consent for the Sample Testing and sharing of patient data with the Alliance and Checkmate) in consultation with the Alliance and Checkmate (it being understood and agreed that the portion of the informed consent form relating to the Alliance Compound will be provided to Pfizer by the Alliance and that the portion of the informed consent form relating to the Checkmate Compound will be provided to Pfizer by Checkmate). Any changes to such form that relate to the Sample Testing, the Checkmate Compound or the Alliance Compound or the sharing of data shall be subject respectively to Checkmate’s and the Alliance’s review and prior written consent. Any such proposed changes will be sent in writing to the Alliance’s Project Manager and Checkmate’s Alliance Manager.
| 5. | Adverse Event Reporting. |
Pfizer will be solely responsible for compliance with all Applicable Law pertaining to safety reporting for the Study and related activities. The Parties shall execute the Safety Data Exchange Agreement prior to initiating any clinical activities implicating pharmacovigilance responsibilities to ensure the exchange of relevant safety data within appropriate timeframes and in an appropriate format to enable the Parties to fulfill local and international regulatory reporting obligations and to facilitate appropriate safety reviews. Serious Adverse Event (SAE) and adverse event reports and other information arising from any aspect of the Study where a patient has been exposed to the Alliance Compound or the Checkmate Compound will be exchanged in accordance with the Safety Data Exchange Agreement.
| 6. | Term and Termination. |
6.1 The term of this Agreement shall commence on the Effective Date and shall continue in full force and effect until completion of all of the obligations of the Parties hereunder or until terminated by a Party pursuant to this Article 6 (the “Term”).
6.2 In the event that the Alliance or Checkmate reasonably and in good faith believes that the Alliance Compound or Checkmate Compound, as applicable, is being used in the Study in an unsafe manner or reasonably believes that there is imminent danger to patients and notifies Pfizer in writing of the grounds for such belief, and Pfizer fails to promptly incorporate (subject to approval by applicable Regulatory Authorities or Institutional Review Boards) changes into the Protocol reasonably and in good faith requested by the Alliance or Checkmate, as applicable, to address such issue or to otherwise reasonably and in good faith address such issue, the Alliance or Checkmate, as applicable, may terminate this Agreement and the supply of the Alliance Compound or Checkmate Compound, as applicable, effective upon written notice to Pfizer.
Subject to Section 6.10, a Party may terminate this Agreement if another Party commits a material breach of this Agreement, and such material breach continues for thirty (30) days after receipt of written notice thereof from the non-breaching Party; provided that if such material breach is capable of cure and cannot reasonably be cured within thirty (30) days, the breaching Party shall be given a reasonable period of time to cure such breach.
6.3 If a Party reasonably determines in good faith, based on a review of the Clinical Data, Sample Testing Results and/or other Study-related Know-How or other information, that the Study may unreasonably affect patient safety, such Party shall promptly notify the other Party of such determination. The Party receiving such notice may propose modifications to the Protocol to address the safety issue identified by the other Party and, if the notifying Party agrees, shall act to immediately implement such modifications; provided, however, that if the notifying Party, in its sole discretion, reasonably believes that there is imminent danger to patients, such Party needs not wait for the other Party to propose modifications and may instead terminate this Agreement immediately upon written notice to such other Party. Furthermore, if the notifying Party, in its sole discretion, reasonably believes that any modifications proposed by the other Party will not resolve the patient safety issue, such Party may terminate this Agreement effective upon written notice to such other Party.
6.4 Subject to Section 6.11, any Party may terminate this Agreement immediately upon written notice to the other Parties in the event that any Regulatory Authority takes any action, or raises any objection, that prevents the terminating Party from supplying its Compound for purposes of the Study. Additionally, any Party shall have the right to terminate this Agreement immediately upon written notice to the other Parties in the event that it determines in its sole discretion to discontinue development of its Compound, for safety, medical, scientific, legal, regulatory or other reasons.
6.5 In the event that this Agreement is terminated,
6.5.1 Pfizer shall, at Checkmate’s sole discretion, and, solely if the Agreement is terminated for a Checkmate breach, at Checkmate’s cost, promptly either return or destroy all
unused Checkmate Compound pursuant to Checkmate’s instructions. If Checkmate requests that Pfizer destroys the unused Checkmate Compound, Pfizer shall provide written certification of such destruction.
6.5.2 Pfizer shall, at the Alliance’s sole discretion, and, solely if the Agreement is terminated for an Alliance breach, at the Alliance’s cost, promptly either return or destroy all unused Alliance Compound pursuant to the Alliance’s instructions. If the Alliance requests that Pfizer destroy the unused Alliance Compound, Pfizer shall provide written certification of such destruction.
6.6 A Party shall be entitled to terminate this Agreement upon thirty (30) days advance written notice to the other Parties, if such other Parties fail to perform any of its obligations under Section 13.3 or breaches any representation or warranty contained in Section 13.3. The non-terminating Party shall have no claim against the terminating Party for compensation for any loss of whatever nature by virtue of the termination of this Agreement in accordance with this Section 6.5.2.
6.7 The provisions of this Section 6.7 and Sections 3.6 (other than the first, fourth and sixth sentences thereof), 3.7, 3.9, 6.7, 6.9, 6.10 13.2, 13.5.5, 13.6, 14.2 (Indemnification), 14.3 (Limitation of Liability), and Articles 1 (Definitions), 7 (Costs of Study), 9 (Confidentiality), 10 (Intellectual Property), 11 (Reprints; Rights of Cross-Reference), 12 (Press Releases and Publications), 15 (Use of Name), 19 (Invalid Provision), 20 (No Additional Obligations), 21 (Dispute Resolution and Jurisdiction), 22 (Notices), 23 (Relationship of the Parties) and 25 (Construction) shall survive the expiration or termination of this Agreement.
6.8 Termination of this Agreement shall be without prejudice to any claim or right of action of a Party against the other Parties for any prior breach of this Agreement.
6.9 Upon termination of this Agreement, each Party and its Affiliates shall promptly return to the other Party or destroy any Confidential Information of the other Party (other than Clinical Data, Sample Testing Results and Inventions) furnished to the receiving Party by the other Party, except that the receiving Party shall have the right to retain one copy solely for record-keeping purposes which shall remain subject to the confidentiality and nonuse provisions set forth herein. Pfizer shall also be responsible for all costs associated with the Termination of this Agreement.
6.10 Upon receipt by a Party of a termination notice of this Agreement, subject to the terms of this Article 6, Pfizer shall submit a wind-down plan to the Alliance and Checkmate, setting forth the tasks reasonably necessary or required in connection with the orderly termination of the Study and the proper plan for managing the patients enrolled in the Study, including any actions reasonably required to safely close out the Study, or required by Applicable Laws. If patient safety considerations require more time to safely close out the Study than the termination periods set forth herein, then the Parties agree that the Agreement shall be extended to the extent necessary to ensure patient safety, after which the Agreement shall terminate immediately in accordance with the terms of the applicable section in this Article 6.
| 7. | Costs of Study; Recruitment. |
7.1 The Parties agree that (i) the Alliance shall provide the Alliance Compound for use in the Study, as described in Article 8 below, [***] to either Pfizer or Checkmate; (i) Checkmate shall provide Checkmate Compound for use in the Study, as described in Article 8 below, at no cost to either Pfizer or the Alliance, (iii) Pfizer shall provide the Pfizer Compound for use in the Study, as described in Article 8 below, [***] to the Alliance or Checkmate. The Study costs will be shared equally by Checkmate and the Alliance, with Checkmate reimbursing [***] of all Study costs incurred on a Calendar Quarter basis, as set forth in this Article 7. An estimate of the total expected Study costs as of the Effective Date is attached hereto as Appendix C. Within thirty (30) days of the end of each Calendar Quarter following the first patient first dose in the Study, the Alliance shall provide Checkmate an invoice, in reasonable detail, setting forth the incurred Study costs, on the basis of the actual Study costs for such Calendar Quarter, including, for the first such invoice any Study costs incurred during, or prior to, such Calendar Quarter. Any changes to the agreed Study costs more than ten percent (10%) in excess of the amounts set forth on Appendix C shall be reviewed and approved in writing by both Parties prior to payment of any amounts outlined in this Section 7.1. Within forty-five (45) days following receipt of each such invoice by Checkmate, Checkmate shall reimburse the Alliance for [***] of the total Study costs incurred by the Alliance during such Calendar Quarter. Concurrently with each such Calendar Quarter invoice, the Alliance shall describe in writing any deviations in the total Study costs from the original estimate.
7.2 For the avoidance of doubt, Pfizer will not be required to reimburse Checkmate for any costs or expenses incurred by Checkmate or its Affiliates in connection with the Study and Checkmate will not be required to reimburse Pfizer for any costs or expenses incurred by Pfizer or its Affiliates in connection with the Study (other than the Study costs).
| 8. | Supply and Use of the Compounds. |
8.1 Supply of the Compounds. Checkmate, the Alliance and Pfizer shall each supply, or cause to be supplied, the quantities of its respective Compound set forth on Appendix B on the timelines set forth in Appendix B, in each case, for use in the Study. In the event that Pfizer determines that the quantities of Compounds set forth on Appendix B are not sufficient to complete the Study (due, for example, to the addition of Study sites or countries), Pfizer shall so notify the Alliance and Checkmate, and the Parties shall discuss in good faith regarding additional quantities of Compounds to be provided and the schedule on which such additional quantities may be provided. Each Party shall also provide to the other Party a contact person for the supply of its Compound under this Agreement. Notwithstanding the foregoing, or anything to the contrary herein, in the event that either Party is not supplying its Compound in accordance with the terms of this Agreement, or is allocating under Section 8.10, then the other Party shall have no obligation to supply its Compound, or may allocate proportionally.
8.2 Minimum Shelf Life Requirements. Each Party shall supply its Compound hereunder with an adequate remaining shelf life at the time of Delivery to meet the Study’s requirements. The shelf life for each Compound to continue to be conforming and meet Specifications shall at a minimum be three (3) months from the time of Delivery; provided that the Compound is handled and stored according to the specified handling and storage conditions.
8.3 Provision of Compounds.
8.3.1 The Alliance and Checkmate will each deliver the Alliance Compound and Checkmate Compound, as applicable, to Pfizer Ex Works (Alliance Compound Manufacturing Site or Checkmate Compound Manufacturing Site, as applicable) (Incoterms 2010) (“Delivery”) with respect to such Alliance Compound and Checkmate Compound, as applicable. Title and risk of loss for the Alliance Compound and Checkmate Compound, as applicable, shall transfer from the Alliance and Checkmate, as applicable to Pfizer at Delivery. All costs associated with the subsequent transportation, warehousing and distribution of the Alliance Compound and Checkmate Compound, as applicable, shall be borne by Pfizer. Pfizer will, or will cause its designee to: (i) take delivery of the Alliance Compound and Checkmate Compound, as applicable, supplied hereunder; (ii) perform the acceptance procedures allocated to it under the Quality Agreements; (iii) subsequently label and pack (in accordance with Section 8.4) and promptly ship the Alliance Compound and Checkmate Compound, as applicable, to the Study sites, in compliance with cGMP, GDP, GCP and other Applicable Law and the Quality Agreements; and (iv) provide, from time to time at the reasonable request of the Alliance and Checkmate, as applicable, the following information with respect to Alliance Compound and Checkmate Compound, as applicable, shipped by Pfizer: any applicable chain of custody forms; in-transport temperature recorder(s); records and receipt verification documentation; such other transport or storage documentation as may be reasonably requested by the Alliance and Checkmate, as applicable, (to the extent within Pfizer’s possession or control); and usage and inventory reconciliation documentation related to the Alliance Compound and Checkmate Compound, as applicable.
8.3.2 Pfizer is solely responsible, at its own cost, for supplying (including all Manufacturing, acceptance and release testing) the Pfizer Compound for the Study, and the subsequent handling, storage, transportation, warehousing and distribution of the Pfizer Compound supplied hereunder. Pfizer shall ensure that all such activities are conducted in compliance with cGMP, GDP, GCP and other Applicable Law and the Quality Agreements. For purposes of this Agreement, the Delivery of a given quantity of the Pfizer Compound shall be deemed to occur when such quantity is packaged for shipment to the Study site.
8.4 Labeling and Packaging; Use, Handling and Storage.
8.4.1 The Parties’ obligations with respect to the labeling and packaging of the Compounds are as set forth in the Quality Agreements. Notwithstanding the foregoing or anything to the contrary contained herein, the Alliance shall provide the Alliance Compound and Checkmate shall provide the Checkmate Compound to Pfizer in the form of unlabeled vials, and Pfizer shall be responsible for labeling, packaging and leafleting such Alliance Compound and Checkmate Compound and the Pfizer Compound, as applicable, in accordance with the terms and conditions of the Quality Agreements and otherwise in accordance with all Applicable Law, including cGMP, GDP, GCP, and health, safety and environmental protections.
8.4.2 Pfizer shall (i) use the Alliance Compound and Checkmate Compound, as applicable, solely for purposes of performing the Study; (ii) not use the Alliance Compound or Checkmate Compound, as applicable, in any manner inconsistent with this Agreement or for any commercial purpose other than conduct of the Study; and (iii) use, store, transport, handle and
dispose of the Alliance Compound and Checkmate Compound, as applicable, in compliance with Applicable Law and the Quality Agreements. Pfizer shall not reverse engineer, reverse compile, disassemble or otherwise attempt to derive the composition or underlying information, structure or ideas of the Alliance Compound or Checkmate Compound, as applicable, and in particular shall not analyze the Alliance Compound and Checkmate Compound, as applicable, by physical, chemical or biochemical means except as necessary to perform its obligations under the Quality Agreements and/or the Protocol.
8.5 Product Specifications. A certificate of analysis shall accompany each shipment of the Alliance Compound and Checkmate Compound, as applicable, to Pfizer. Upon request, Pfizer shall provide the Alliance or Checkmate, as applicable, with a certificate of analysis covering each shipment of Pfizer Compound used in the Study.
8.6 Changes to Manufacturing. Each Party may make changes from time to time to its Compound or the Manufacturing Site; provided that such changes shall be in accordance with the Quality Agreements.
8.7 Product Testing; Noncompliance.
8.7.1 After Manufacturer’s Release. After Manufacturer’s Release of the Alliance Compound and Checkmate Compound, as applicable, and concurrently with Delivery of the Compound to Pfizer, the Alliance and Checkmate shall provide Pfizer with such certificates and documentation as are described in the Quality Agreements (“Disposition Package”). Pfizer shall, within the time defined in the Quality Agreements, perform (i) with respect to the Alliance Compound and Checkmate Compound, as applicable, the acceptance procedures allocated to it under the Quality Agreements, and (ii) with respect to the Alliance Compound and Checkmate Compound, as applicable, the testing and release procedures allocated to it under the Quality Agreements. Pfizer shall take all steps necessary in its reasonable discretion to determine that the Alliance Compound, Checkmate Compound or Pfizer Compound, as applicable, is suitable for distribution before making such Alliance Compound, Checkmate Compound or Pfizer Compound, as applicable, available for human use, and the Alliance and Checkmate, as applicable, shall provide cooperation or assistance as reasonably requested by Pfizer in connection with such determination with respect to the Alliance Compound and Checkmate Compound, as applicable. Pfizer shall be responsible for (a) storage and maintenance of the Alliance Compound and Checkmate Compound until it is tested and/or released, which storage and maintenance shall be in compliance with the Specifications for the Alliance Compound or Checkmate Compound, as applicable, the Quality Agreements and Applicable Law; and (b) any failure of the Alliance Compound or Checkmate Compound, as applicable, to meet the applicable Specifications to the extent caused by shipping, storage or handling conditions after Delivery to Pfizer hereunder.
8.7.2 Non-Conformance.
8.7.2.1 In the event that a Party becomes aware that any Compound may have a Non-Conformance, despite testing and quality assurance activities (including any activities conducted by the Parties under Sections 8.7.1 (After Manufacturer’s Release)), such Party shall immediately notify the other Parties in accordance with the procedures of the Quality Agreements. The Parties shall investigate any Non-Conformance in accordance with Section 8.9 (Investigations) and any discrepancy between them shall be resolved in accordance with Section 8.8 (Resolution of Discrepancies).
8.7.2.2 In the event that any proposed or actual shipment of the Alliance Compound or Checkmate Compound, as applicable, (or portion thereof) shall be agreed to have a Non-Conformance at the time of Delivery to Pfizer or through no fault of Pfizer during the shelf life set forth in Section 8.2 (in either case, a “Non-Conformance Event”), then unless otherwise agreed to by the Parties, the Alliance or Checkmate, as applicable, shall replace such Alliance Compound or Checkmate Compound, as applicable, as is found to have a Non-Conformance (with respect to Alliance Compound or Checkmate Compound, as applicable, that has not yet been administered in the course of performing the Study). Unless otherwise agreed to by the Parties in writing, the sole and exclusive remedies of Pfizer with respect to any Alliance Compound or Checkmate Compound, as applicable, that is found to have a Non-Conformance at the time of Delivery shall be (i) replacement of such Alliance Compound or Checkmate Compound, as applicable, as set forth in this Section 8.7.2(b), (ii) indemnification under Section 14.2 (to the extent applicable) and (iii) termination of this Agreement pursuant to Section 6.3 (to the extent applicable, but subject to the applicable cure periods set forth therein); provided, for clarity, that no Party shall be deemed to be waiving any rights under Section 8.15. The Alliance or Checkmate, as applicable, shall be responsible for any costs incurred by Pfizer in connection with the return or destruction of any Alliance Compound or Checkmate Compound, as applicable, supplied hereunder that is found to have a Non-Conformance caused by the Alliance or Checkmate, as applicable.
8.7.2.3 In the event that Alliance Compound or Checkmate Compound, as applicable, is lost or damaged after Delivery, the Alliance or Checkmate, as applicable, may provide additional Alliance Compound or Checkmate Compound, as applicable, to Pfizer, if available for the Study. Such replaced Alliance Compound or Checkmate Compound, as applicable, shall be provided to Pfizer, so long as the amount replaced does not in the aggregate exceed five percent (5%) of the total quantity of Alliance Compound or Checkmate Compound, as applicable, to be provided by the Alliance or Checkmate, as applicable, pursuant to Appendix B (the “Replacement Threshold”). For the avoidance of doubt, the Alliance shall have no obligation to provide replacement Alliance Compound for any Alliance Compound supplied hereunder other than such Alliance Compound as has been agreed or determined to have a Non-Conformance at the time of Delivery to Checkmate shall be responsible for any costs incurred by the Alliance in connection with the return or destruction of any Checkmate Compound supplied hereunder that is found to have a Non-Conformance caused by Checkmate.
8.7.2.4 The Alliance shall be responsible for, and Checkmate shall have no obligations or liability with respect to, any Alliance Compound supplied hereunder that is found to have a Non-Conformance. The Alliance shall replace any Alliance Compound as is found to have a Non-Conformance (with respect to Alliance Compound that has not yet been administered in the course of performing the Study). Pfizer shall be responsible for, and Checkmate and the Alliance shall have no obligations or liability with respect to, any Pfizer Compound supplied hereunder that is found to have a Non-Conformance. Pfizer shall replace any Pfizer Compound as is found to have a Non-Conformance (with respect to Pfizer Compound that has not yet been administered in the course of performing the Study). Checkmate shall be responsible for, and Pfizer and the Alliance shall have no obligations or liability with respect to, any Checkmate
Compound supplied hereunder that is found to have a Non-Conformance. Checkmate shall replace any Checkmate Compound as is found to have a Non-Conformance (with respect to Checkmate Compound that has not yet been administered in the course of performing the Study). Unless otherwise agreed to by the Parties in writing, the sole and exclusive remedies of the Alliance and Checkmate with respect to any Pfizer Compound that is found to have a Non-Conformance at the time of Delivery shall be (i) replacement of such Pfizer Compound as set forth in this Section 8.7.2.4, (ii) indemnification under Section 14.2 (to the extent applicable) and (iii) termination of this Agreement pursuant to Article 6 (to the extent applicable, but subject to the applicable cure periods set forth therein); provided, for clarity, that the no Party shall be deemed to be waiving any rights under Section 8.15.
8.8 Resolution of Discrepancies. Disagreements regarding any determination of Non-Conformance by Pfizer shall be resolved in accordance with the provisions of the Quality Agreements.
8.9 Investigations. The process for investigations of any Non-Conformance shall be handled in accordance with the Quality Agreements.
8.10 Shortage; Allocation. In the event that a Party’s Compound is in short supply as a result of a manufacturing disruption, manufacturing difficulties or other similar event such that a Party reasonably believes in good faith that it will not be able to fulfill its supply obligations hereunder with respect to its Compound, such Party will provide prompt written notice to the other Party thereof (including the shipments of Compound hereunder expected to be impacted and the quantity of its Compound that such Party reasonably determines it will be able to supply) and the Parties will promptly discuss such situation (including how the quantity of Compound that such Party is able to supply hereunder will be allocated within the Study). In such event, the Party experiencing such shortage shall (i) use its commercially reasonable efforts both to remedy the situation giving rise to such shortage and to take action to minimize the impact of the shortage on the Study, and (ii) to allocate to the other Party an amount of Compound at least proportionate to the total amount of the Compound shipments hereunder expected to be impacted by the shortage divided by the total demand for the Compound for the impacted time period.
8.11 Records. Each Party shall maintain complete and accurate records in all material respects pertaining to its Manufacture of its Compound supplied hereunder, and, upon the reasonable prior request of the other Party, will make such records available to review by such other Party in accordance with the Quality Agreements solely for the purpose of confirming such Party’s compliance with this Agreement with respect to its Manufacturing obligations hereunder.
8.12 Quality. Quality matters related to the Manufacture of the Compounds shall be governed by the terms of the Quality Agreements in addition to the relevant quality provisions of this Agreement.
8.13 Quality Control. Each Party shall implement and perform operating procedures and controls for sampling, stability and other testing of its Compound, and for validation, documentation and release of its Compound and such other quality assurance and quality control procedures as are required by the Specifications, cGMPs and the Quality Agreements.
8.14 Audits and Inspections. The Parties’ audit and inspection rights under this Agreement shall be governed by the terms of the Quality Agreements.
8.15 Recalls. Recalls of the Compounds shall be governed by the terms of the Quality Agreements.
8.16 VAT and other indirect taxes. All payments under the Agreement are deemed exclusive of VAT or any other indirect taxes; The invoicing Party shall, if required under applicable laws & regulations, add VAT or any other indirect taxes to the price at the prevailing rate under applicable laws & regulations; the invoicing Party shall also fulfill all material and formal conditions required from the invoicing Party under applicable laws & regulations to ensure a refund of the VAT or any other indirect taxes charged to the invoiced Party provided a refund is available to the invoiced Party under applicable laws & regulations.
8.17 Withholding Taxes. The amounts payable by one Party (the “Payer”) to another Party (the “Payee”) pursuant to this Agreement (“Payments”) shall not be reduced on account of any Taxes unless required by Law. The Payee alone shall be responsible for paying any and all Taxes (other than withholding Taxes required to be paid by the Payer) levied on account of, or measured in whole or in part by reference to, any Payments it receives. The Payer shall deduct or withhold from the Payments any Taxes that it is required by Law to deduct or withhold. Notwithstanding the foregoing, if the Payee is entitled under any applicable Tax treaty to a reduction of rate of, or the elimination of, or recovery of, applicable withholding Tax, it shall promptly deliver to the Payer or the appropriate governmental body (with the assistance of the Payer to the extent that this is reasonably required and is expressly requested in writing) the prescribed forms necessary to reduce the applicable rate of withholding or to relieve the Payer of its obligation to withhold Tax, and the Payer shall apply the reduced rate of withholding, or dispense with the withholding, as the case may be. If, in accordance with the foregoing, the Payer withholds any amount, it shall make timely payment to the proper Taxing authority of the withheld amount, and send to the Payee reasonable proof of such payment within 60 days following that payment. If Taxes are paid to a tax authority, each Party will provide the other such assistance as is.
| 9. | Confidentiality. |
9.1 Subject to Section 13.6.7, Checkmate, Pfizer and Merck agree to hold in confidence any Confidential Information provided by the other Party, and no Party shall use Confidential Information of another Party except for the performance of the Study and for the Permitted Use. No Party shall, without the prior written permission of the providing Party, disclose any Confidential Information of the providing Party to any Third Party, except to such Party’s directors, officers, employees, consultants, Affiliates and/or legal and financial advisors who have a need to know such Confidential Information for the purpose of this Agreement and are bound to maintain the confidentiality of the Confidential Information by written obligations of confidentiality and non-use at least as restrictive as the obligations contained herein. Notwithstanding the foregoing, nothing herein shall prohibit any disclosure to the extent such disclosure (i) is required by Applicable Law; (ii) is pursuant to the terms of this Agreement; or (iii) is necessary for the conduct of the Study, and in each case ((i) through (iii)) provided that the disclosing Party shall provide reasonable advance notice to the applicable other Party before
making such disclosure and, at the request of such applicable other Party, cooperate with such applicable other Party in obtaining a protective order or similar relief that prevents or limits the scope of, or delays, such disclosure. For the avoidance of doubt, Pfizer may, without the Alliance’s or Checkmate’s consent, disclose Confidential Information to clinical trial sites, CROs and clinical trial investigators performing the Study, other vendors (including Subcontractors) directly working on the Study, the data safety monitoring and advisory board relating to the Study, and Regulatory Authorities working with Pfizer on the Study, in each case to the extent necessary for the performance of the Study and provided that such persons (other than governmental entities) are bound by an obligation of confidentiality at least as stringent as the obligations contained herein.
9.2 Notwithstanding the foregoing, (i) Jointly Owned Inventions shall constitute the Confidential Information of all Parties and each Party shall have the right to use and disclose such Confidential Information only as consistent with Articles 10, 11 and 12; (ii) Inventions and are solely owned by one Party shall constitute the Confidential Information of that Party and each Party shall have the right to use and disclose such Confidential Information only as consistent with Articles 10, 11 and 12; (iii) use and disclosure of Sample Testing Results shall be governed by Section 3.6 and Article 10, and (iv) use and disclosure of Clinical Data shall be governed by Section 3.6 and Article 10.
9.3 All Confidential Information containing personal identifiable data shall be handled in accordance with all data protection and privacy laws, rules and regulations applicable to such Party.
| 10. | Intellectual Property. |
10.1 Joint Ownership and Prosecution.
10.1.1 Subject to Sections 10.2 and 10.3, all rights to all Inventions relating to the Class Combination (each a “Jointly Owned Invention”) shall belong jointly to the Parties whose respective Compounds make up the applicable Class Combination. Each Party shall and hereby does assign to the other Party sufficient rights, title and interest in each Jointly Owned Invention so that: (a) each of Checkmate and Merck and Pfizer owns an undivided one-third interest in Inventions directed to the Class Combination of the Class Compounds of all three Parties; (b) each of Pfizer and Checkmate owns an undivided one-half interest in the Inventions directed to the Class Combination of their respective Class Compounds; (c) each of Pfizer and Merck owns an undivided one-half interest in Inventions directed to the Class Combination of their respective Class Compounds; (d) each of Checkmate, Pfizer and Merck owns an undivided one-third interest in the Inventions directed to the Class Combination of the Checkmate Class Compound and Alliance Class Compound. Unless otherwise mutually agreed, each Party shall have the right to freely exploit the Joint IP to which it has an undivided interest pursuant to the immediately preceding sentence, both within and outside the scope of the Study, without accounting to or any other obligation to the other Party, and each Party may grant licenses (with a right to sublicense) to Third Parties under such Party’s interest in the Joint IP, in each case subject to the restrictions in Sections 3.6, 3.10, Article 9 and this Article 10. For those countries where a specific license is required for a joint owner of a Jointly Owned Invention to practice such Jointly Owned Invention in such countries, (i) Merck hereby grants to Pfizer a perpetual, irrevocable, nonexclusive, worldwide, [***] license, [***], under Merck’s right, title and interest in and to all
Jointly Owned Inventions that are jointly owned by Merck and Pfizer to use such Jointly Owned Inventions for any use, (ii) Merck hereby grants to Checkmate a perpetual, irrevocable, non-exclusive, worldwide, [***] license, [***], under Merck’s right, title and interest in and to all Jointly Owned Inventions that are jointly owned by Merck and Checkmate to use such Jointly Owned Inventions for any use, (iii) Checkmate hereby grants to Merck a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, fully paid-up license, transferable and sublicensable, under Checkmate’s right, title and interest in and to all Jointly Owned Inventions that are jointly owned by Merck and Checkmate to use such Jointly Owned Inventions for any use, (iv) Checkmate hereby grants to the Pfizer a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, fully paid-up license, transferable and sublicensable, under Checkmate’s right, title and interest in and to all Jointly Owned Inventions that are jointly owned by Pfizer and Checkmate to use such Jointly Owned Inventions for any use, (v) Pfizer hereby grants to Checkmate a perpetual, irrevocable, nonexclusive, worldwide, royalty-free, fully paid-up license, transferable and sublicensable, under Pfizer’s right, title and interest in and to all Jointly Owned Inventions that are jointly owned by Pfizer and Checkmate to use such Jointly Owned Inventions for any use,, and (vi) Pfizer hereby grants to Merck a perpetual, irrevocable, non-exclusive, worldwide, royalty- free, fully paid-up license, transferable and sublicensable, under Pfizer’s right, title and interest in and to all Jointly Owned Inventions that are jointly owned by Merck and Pfizer to use such Jointly Owned Inventions for any use, in each case of the foregoing (i) through (vi) subject to the restrictions in Sections 3.6, 3.10, Article 9 and this Article 10. The terms of this Agreement do not provide Checkmate, Pfizer or Merck any rights to use or commercialize the other Party’s Class Compounds, alone or in combination with that Party’s Class Compounds, or with any rights, title or interest or any license to the other Party’s background intellectual property except as necessary to conduct the Study and as expressly set forth in Section 10.5. The Parties shall discuss and mutually agree on disclosures towards a patent authority with respect to the Compound Combination of the Checkmate Compound, a Pfizer Compound and the Alliance Compound, in pairwise combination or a combination of all three, with each Party’s consent not to be unreasonably withheld. Until such consent is reached, (x) Checkmate shall not disclose to a patent authority the Protocol, any Clinical Data relating to the Combination of the Checkmate Compound, the Pfizer Compound and the Alliance Compound, in pairwise combination or a combination of all three, or any Sample Testing Results relating to the Alliance Compound or the Pfizer Compound in or in connection with any patent application (relating to any Invention or otherwise), (y) Pfizer shall not disclose to a patent authority the Protocol, any Clinical Data relating to the Combination of the Checkmate Compound, the Pfizer Compound and the Alliance Compound, in pairwise combination or a combination of all three, or any Sample Testing Results relating to the Alliance Compound or the Checkmate Compound in or in connection with any patent application (relating to any Invention or otherwise), and (z) Merck shall not disclose to a patent authority the Protocol, any Clinical Data relating to the Compound Combination of the Checkmate Compound, the Pfizer Compound and the Alliance Compound, in pairwise combination or a combination of all three, or any Sample Testing Results relating to the Checkmate Compound or the Pfizer Compound in or in connection with any patent application (relating to any Invention or otherwise).
10.1.2 Following the Effective Date, patent representatives of each of the Parties shall meet (in person or by telephone) to discuss the patenting strategy for any Jointly Owned Inventions that may arise (including any Jointly Owned Inventions owned by only two of the Parties), including deciding on (A) the timing for filing of any provisional or regular patent application, if any; and (B) the countries in which any patent applications should be filed, subject
to the opt-out procedure described below, provided that any decision in part (B) shall be determined by only the owning Parties. The Parties hereby agree that Pfizer will take the lead in prosecuting, maintaining and/or defending Joint IP (the “Lead Prosecuting Party”) (it being understood that the Parties shall mutually agree to conduct some or all prosecution, maintenance and/or defense jointly through a patent counsel acceptable to both Parties). The Parties acknowledge and agree that unless otherwise agreed and subject to Section 10.1.1, following agreement and consent of all Parties that have an interest in a Jointly Owned Invention for which an applicable application for a Joint Patent Application is proposed to be filed that such a patent application should be filed and an agreement by all Parties on timing of filing and any disclosure of each Party’s Confidential Information, the Lead Prosecuting Party shall have the first right (but not the obligation) to file itself or through its selected patent counsel, which is reasonably acceptable to the other Parties, a patent application (including any provisional, substitution, divisional, continuation, continuation in part, reissue, renewal, reexamination, extension, supplementary protection certificate and the like) in respect of any Jointly Owned Invention (each, a “Joint Patent Application”). In any event, the Parties shall consult and reasonably cooperate with one another (i) in the preparation, filing, prosecution (including prosecution strategy, which shall also include procedures before the Unified Patent Court) and maintenance of such Joint Patent Application and in the maintenance and defense of any Joint Patent, and (ii) subject to the opt-out procedure described below the Parties that have an interest in the Jointly Owned Invention for which the applicable application for a Joint Patent Application is filed shall equally share the expenses associated therewith. For the avoidance of doubt both the Lead Prosecuting Party and the other Party or Parties (as applicable) shall be fully and equally considered as the beneficial owners of the rights derived from the Joint IP, subject to the optout procedure described below. If an applicable Party (the “Opting-out Party”) has consented to the filing but does not want to share expenses for a patent application for a Jointly Owned Invention (either generally or with respect to a particular country) or at any point after the initial filing wishes to discontinue the prosecution, maintenance or defense of a Joint Patent Application or Joint Patent or sharing expenses therefor, the other Party or Parties. As applicable, at its or each of their sole option (each a “Continuing Party”), may continue such prosecution, maintenance or defense at its or their sole expense. In such event, at the Continuing Party’s request the Opting-out Party shall execute such documents and perform such acts at the Opting-out Party’s expense as may be reasonably necessary in a timely manner to effect an assignment of such Joint IP to the Continuing Party or Parties (in such country or all countries, as applicable) to allow the Continuing Party to continue to prosecute, maintain or defend such Joint IP. Any Joint IP so assigned shall thereafter be owned solely by the Continuing Party or Parties, provided however, that the Opting-out Party (including its successors and assigns) shall retain a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, sublicensable, transferable, and fully paid-up license for all purposes under any patent claims arising from such Jointly Owned Invention in any applicable countries with respect to a product discovered, developed, or commercialized by the Opting- out Party.
10.1.3 Except as expressly provided in Section 10.1.2 and in furtherance and not in limitation of Section 9.1, each Party agrees it will not make or support any patent application that includes the other Party’s Confidential Information, and will not provide assistance to any Third Party for any such application, without the other Party’s prior written authorization.
10.1.4 Subject to this Section 10.1.4:
10.1.4.1 Pfizer shall have the first right (but not the obligation) to initiate legal action to enforce all Joint Patents in which it has an ownership interest against infringement, and to protect all Jointly Owned Inventions in which it has an ownership interest from misappropriation, by any Third Party where such infringement or misappropriation results from the development or sale of a Pfizer Compound or a Pfizer Class Compound, or to defend any declaratory judgment or inter partes review actions (or foreign equivalents thereof) relating thereto, at its sole expense; provided that in the event that Pfizer fails to initiate or defend such action within thirty (30) days after being first notified of such infringement or misappropriation, Checkmate shall have the second right (but not the obligation) to do so at its sole expense with respect to any such Joint Patent in which Checkmate has an ownership interest, and further provided that if Checkmate fails to initiate or defend such action within thirty (30) days following the earlier of notice from Pfizer that it will not initiate or defend such action or expiration of the thirty (30) day period allotted to Pfizer, then Merck shall have the third right (but not the obligation) to do so at its sole expense with respect to any such Joint Patent in which Merck has an ownership interest;
10.1.4.2 Merck shall have the first right (but not the obligation) to initiate legal action to enforce all Joint Patents in which it has an ownership interest against infringement, and to protect all Jointly Owned Inventions from misappropriation, by any Third Party where such infringement or misappropriation results from the development or sale of the Alliance Compound or an Alliance Class Compound, or to defend any declaratory judgment or inter partes review actions (or foreign equivalents thereof) relating thereto, at its sole expense; provided that in the event that Merck fails to initiate or defend such action within thirty (30) days after being first notified of such infringement or misappropriation, Checkmate shall have the second right (but not the obligation) to do so at its sole expense with respect to any such Joint Patent in which Checkmate has an ownership interest, and further provided that if Checkmate fails to initiate or defend such action within thirty (30) days following the earlier of notice from Merck that it will not initiate or defend such action or expiration of the thirty (30) day period allotted to Merck, then Pfizer shall have the third right (but not the obligation) to do so at its sole expense with respect to any such Joint Patent in which Pfizer has an ownership interest; and
10.1.4.3 Checkmate shall have the first right (but not the obligation) to initiate legal action to enforce all Joint Patents in which it has an ownership interest against infringement, and to protect all Jointly Owned Inventions from misappropriation, by any Third Party where such infringement or misappropriation results from the development or sale of the Checkmate Compound or a Checkmate Class Compound, or to defend any declaratory judgment or inter partes review actions (or foreign equivalents thereof) relating thereto, at its sole expense; provided that in the event that Checkmate fails to initiate or defend such action within thirty (30) days after being first notified of such infringement or misappropriation, Pfizer shall have the second right (but not the obligation) to do so at its sole expense with respect to any such Joint Patent in which Pfizer has an ownership interest, and further provided that if Pfizer fails to initiate or defend such action within thirty (30) days following the earlier of notice from Checkmate that it will not initiate or defend such action or expiration of the thirty (30) day period allotted to Checkmate, then Merck shall have the third right (but not the obligation) to do so at its sole expense with respect to any such Joint Patent in which Merck has an ownership interest.
10.1.5 If one Party exercises its right to initiate or defend legal action against a Third Party as set forth in Section 10.1.4 above, such initiating/defending Party shall keep the other Party or Parties who have an ownership interest in the applicable Joint Paten reasonably and regularly informed of the status and progress of the action. Each such interested non- initiating/non-defending Party agrees to be joined as a party plaintiff where necessary for purposes of legal standing and to give the initiating/defending Party reasonable assistance and authority to file and prosecute the suit. In such case, the costs and expenses of the non- initiating/non-defending Party or Parties shall be borne by the initiating/defending Party, and the initiating/defending Party shall indemnify the non-initiating/non-defending Party or Parties against any claims, suits, losses, or liabilities incurred as a result of being joined as plaintiff, except to the extent arising from the negligence or willful misconduct of the applicable non-initiating/non-defending Party. In any event, each non-initiating/non- defending Party shall have the right to be represented in the action by counsel of its choice and at its own expense. Any damages or other monetary awards recovered in the action shall be retained by the initiating/defending Party; provided, however, that in the event that the Parties agree to share the cost of the action as part of a cost-sharing arrangement, such damages or other monetary awards shall be shared by the Parties in proportion to their relative contributions to the total costs and expenses of the action, or as otherwise agreed by the applicable Parties in writing. A settlement or consent judgment or other voluntary final disposition of a suit under this Section 10.1.5 may not be entered into without the consent of each involved Parties, which consent shall not be unreasonably withheld, conditioned or delayed.
10.2 Inventions Owned by Checkmate. Notwithstanding Section 10.1, the Parties agree that all rights to Inventions relating solely to the Checkmate Compound, or an Checkmate Class Compound, but not to a Class Combination (collectively, “Checkmate Compound Inventions”), are the sole and exclusive property of Checkmate. Checkmate shall be entitled to file in its own name relevant patent applications and to own resultant patent rights for any such Checkmate Compound Invention, subject to Checkmate’s obligations under Sections 3.6, 3.10, Article 9 and this Article 10 and Checkmate’s cooperation with Merck and Pfizer regarding timing of, and disclosure in, the filing of any such patent applications vis-a-vis filing of any Joint IP. For the avoidance of doubt, any Checkmate Compound Invention generically encompassing a Checkmate Class Compound (and not an Alliance Class Compound or Pfizer Class Compound) within its scope, even where the Checkmate Compound is not disclosed per se, is a Checkmate Compound Invention and the sole and exclusive property of Checkmate. Merck and Pfizer shall and hereby do assign to Checkmate its respective entire right, title and interest in any such Checkmate Class Compound Inventions the assignment of which Checkmate herewith accepts.
10.3 Inventions Owned by Merck. Notwithstanding Section 10.1, the Parties agree that all rights to Inventions relating solely to the Alliance Compound or an Alliance Class Compound but not to a Class Combination (collectively, “Alliance Compound Inventions”) are the sole and exclusive property of Merck and Pfizer. Merck and Pfizer shall be entitled to file in their own name relevant patent applications and to own resultant patent rights for any such Alliance Compound Invention, subject to Merck’s and Pfizer’s obligations under Sections 3.6, 3.10, Article 9 and this Article 10 and Merck’s and Pfizer’s cooperation with Checkmate regarding timing of, and disclosure in, the filing of any such patent applications vis-a-vis filing of any Joint IP. For the avoidance of doubt, any Alliance Compound Invention generically encompassing an Alliance Class Compound (and not a Checkmate Class Compound or Pfizer Class Compound)
within its scope, even where the Alliance Compound is not disclosed per se, is an Alliance Compound Invention and the sole and exclusive property of Merck and Pfizer. Checkmate shall and hereby do assign to Merck and Pfizer its respective entire right, title and interest in any such Alliance Class Compound Inventions the assignment of which Merck and Pfizer herewith accepts.
10.4 Inventions Owned by the Pfizer. Notwithstanding Section 10.1, the Parties agree that all rights to Inventions relating solely to a Pfizer Compound or a Pfizer Class Compound but not to a Class Combination (collectively, “Pfizer Compound Inventions”) are the sole and exclusive property of Pfizer. Pfizer shall be entitled to file in its own name relevant patent applications and to own resultant patent rights for any such Pfizer Compound Invention, subject to Pfizer’s obligations under Sections 3.6, 3.10, Article 9 and this Article 10 and Pfizer’s cooperation with Checkmate and Merck regarding timing of, and disclosure in, the filing of any such patent applications vis-a-vis filing of any Joint IP. For the avoidance of doubt, any Pfizer Compound Invention generically encompassing a Pfizer Class Compound (and not a Checkmate Class Compound or Alliance Class Compound) within its scope, even where the Pfizer Compound is not disclosed per se, is a Pfizer Compound Invention and the sole and exclusive property of Pfizer. Checkmate and Merck shall and hereby do assign to Pfizer its respective entire right, title and interest in any such Pfizer Class Compound Inventions the assignment of which Pfizer herewith accepts.
10.5 Mutual Freedom to Operate for Combination Inventions.
10.5.1 [***], Checkmate shall grant, and hereby does grant to Pfizer a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, fully paid-up license, transferable and sublicensable, under any particular claims in any patent owned or controlled by Checkmate that was filed or includes a priority claim to an application that was filed prior to the initiation of the Study (i.e., first dosing of the first patient in the Study), or issues from any patent applications filed at any time and relating to an invention conceived of and owned or controlled by Checkmate prior to initiation of the Study, that specifically recite a product combining, or a use or method of use of (i) a Checkmate Class Compound with a respective Pfizer Class Compound or (ii) a Checkmate Class Compound, a respective Pfizer Class Compound and an Alliance Class Compound, in each case to practice the Class Combination for all purposes.
10.5.2 [***], Checkmate shall grant and hereby grants to Merck and Pfizer a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, fully paid-up license, transferable and sublicensable, under any particular claims in any patent owned or controlled by Checkmate that was filed or includes a priority claim to an application that was filed prior to the initiation of the Study (i.e., first dosing of the first patient in the Study), or issues from any patent applications filed at any time and relating to an invention conceived of and owned or controlled by Checkmate prior to initiation of the Study, that specifically recite a product combining, or a use or method of use of, (i) a Checkmate Class Compound with an Alliance Class Compound or (ii) a Checkmate Class Compound, a respective Pfizer Class Compound and an Alliance Class Compound, in each case to practice the Class Combination for all purposes.
10.5.3 [***], Pfizer shall grant and hereby grants to Checkmate a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, fully paid-up license, transferable and sublicensable, under any particular claims in any patent owned or controlled by Pfizer that was
filed or includes a priority claim to an application that was filed prior to the initiation of the Study (i.e., first dosing of the first patient in the Study), or issues from any patent applications filed at any time and relating to an invention conceived of and owned or controlled by Pfizer prior to initiation of the Study, that specifically recite a product combining, or a use or method of use of, (i) a Checkmate Class Compound with a respective Pfizer Class Compound or (ii) a Checkmate Class Compound, a respective Pfizer Class Compound and an Alliance Class Compound, in each case to practice the Class Combination for all purposes.
10.5.4 [***], Pfizer shall grant and hereby grants to Merck a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, fully paid-up license, transferable and sublicensable, under any particular claims in any patent owned or controlled by Pfizer that was filed or includes a priority claim to an application that was filed prior to the initiation of the Study (i.e., first dosing of the first patient in the Study), or issues from any patent applications filed at any time and relating to an invention conceived of and owned or controlled by Pfizer prior to initiation of the Study, that specifically recite a product combining, or a use or method of use of a Checkmate Class Compound, a respective Pfizer Class Compound and an Alliance Class Compound to practice the Class Combination for all purposes.
10.5.5 [***], Merck shall grant and hereby grants to Pfizer a [***] license, [***], under any particular claims in any patent owned or controlled by Merck that was filed or includes a priority claim to an application that was filed prior to the initiation of the Study (i.e., first dosing of the first patient in the Study), or issues from any patent applications filed at any time and relating to an invention conceived of and owned or controlled by Merck prior to initiation of the Study, that specifically recite a product combining, or a use or method of use of a Checkmate Class Compound, a respective Pfizer Class Compound and an Alliance Class Compound to practice the Class Combination for all purposes.
10.5.6 [***], Merck shall grant and hereby grants to Checkmate a [***] license, [***], under any particular claims in any patent owned or controlled by Merck that was filed or includes a priority claim to an application that was filed prior to the initiation of the Study (i.e., first dosing of the first patient in the Study), or issues from any patent applications filed at any time and relating to an invention conceived of and owned or controlled by Merck prior to initiation of the Study, that specifically recite a product combining, or a use or method of use of, (i) a Checkmate Class Compound with an Alliance Class Compound or (ii) a Checkmate Class Compound, a respective Pfizer Class Compound and an Alliance Class Compound, in each case to practice the Class Combination for all purposes.
10.5.7 For clarity, the terms of this Section 10.5 do not, expressly or by necessity or by implication, provide Merck, Pfizer or Checkmate with any rights, title or interest in, or any license to, the other Party’s Intellectual Property Rights which do not claim the Class Combination (except that each Party grants to the other Party a non-exclusive license under its applicable intellectual property as necessary to conduct the Study) and do not grant any rights to Merck, Pfizer or Checkmate to use, manufacture, have manufactured, offer for sale or sell the other Party’s Compound or other compounds or products controlled by the other Party other than an Alliance Class Compound (where Merck or Pfizer is the licensee, a Pfizer Class Compound (where Pfizer is the licensee) or a Checkmate Class Compound (where Checkmate is the licensee).
10.5.8 For purposes of Section 10.5.1 to 10.5.6, use in “combination” means the use or method of using an Alliance Class Compound, a Checkmate Class Compound and a Pfizer Class Compound, pairwise or all three as applicable, in concomitant or sequential administration.
| 11. | Reprints; Rights of Cross-Reference. |
Consistent with applicable copyright and other laws and subject to Article 12 below, each Party may use, refer to, and disseminate reprints of scientific, medical and other published articles and materials from journals, conferences and/or symposia relating to the Study which disclose the name of a Party, provided such use does not constitute an endorsement of any commercial product or service by the other Party.
| 12. | Press Releases and Publications. |
12.1 No Party shall publicly disclose the terms of this Agreement without the prior written consent of each of the other Parties provided that a Party may disclose the terms on a need to know basis in connection with the Study to maintain its compliance to the obligations stated herein, as required, or as needed to comply with applicable laws, including any reporting obligations with the Securities and Exchange Commission. If the Parties agree to issue a press release, following a review by all Parties, at least five (5) days after the Effective Date, it shall only generally describe the clinical collaboration set forth hereunder. After the First Press Release, the Parties agree to seek prior written approval from each other for any press release regarding the collaboration under this Agreement, and a Party will provide the others with the draft press releases at least seven (7) Business Days prior to distribution. Notwithstanding the forgoing, in the event that a planned press release references (1) any Compound Combination, (2) any Class Combination, or (3) any Party’s Compound in connection with any Class Combination, which has not previously been published, any Party whose Compound(s) or Class Compound(s) are implicated shall have no less than fifteen (15) Business Days to review and provide comments and if requested by such Party, the press release should be delayed for sixty (60) days from the intended release date to permit the Party to prepare and file a patent application.
12.2 To the extent required by Applicable Law or Pfizer’s policies, Pfizer will register the Study with the Clinical Trials Registry located at www.clinicaltrials.gov and any other local clinical registry if locally legally required. Pfizer agrees to provide any proposed registration to Alliance and Checkmate for review at least ten (10) days prior to registering the Study. Pfizer is committed to timely publication of the final results of the Study following Study Completion, after taking appropriate action to secure intellectual property rights (if any) arising from the Study, in accordance with Section 3.8 and the review process described in Section 12.3. The publication of the final results of the Study will be in accordance with the Protocol.
12.3 It is the Parties intention that the seminal results of the Study be published jointly; however and in any case, any publication or presentation of one Party relating to Jointly Owned Inventions, Protocol, Sample Testing Results that pertain to a Class Combination, and Clinical Data requires prior written approval of the other Party or Parties whose Class Compound(s) are implicated by such publication or presentation. This includes, but is not limited to, all medical publications in peer-reviewed journals and abstracts and presentations at scientific or medical congresses. Any proposed publication or presentation of either Party shall be consistent with the
other Party’s scientific standards. This will be achieved by (i) applying the highest industry standards, including but not limited to the Good Publication Practice and the Recommendations for Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals of the International Committee of Medical Journal Editors (ICMJE) in their current version and (ii) publishing primary data manuscripts before any non-primary data (e.g. secondary analyses, case studies). Each publishing Party agrees to submit any proposed publication or presentation to the other Party or Parties (as applicable) as follows:
To the Alliance: email address: medical.publication@merckgroup.com
To Checkmate: email address: akrieg@checkmatepharma.com.
To Pfizer: email address: contractnotices@pfizer.com and john.deyoung@pfizer.com
for review at least forty-five (45) days prior to submitting any such proposed publication to a publisher or proceeding with such proposed presentation. Within forty-five (45) days of its receipt, each applicable other Party shall advise the publishing Party, as the case may be, in writing of any information contained therein which is Confidential Information (other than Clinical Data and Sample Testing Results) or which may impair the availability of patent protection for Inventions. Each applicable other Party shall have the right to require the publishing Party, as applicable, to remove specifically identified Confidential Information (other than Clinical Data and Sample Testing Results) and/or to delay the proposed publication or presentation for an additional forty-five (45) days to enable such other Party to seek patent protection for Inventions.
| 13. | Representations and Warranties; Disclaimers. |
13.1 Each of Checkmate, Pfizer and the Alliance represents and warrants to the other that (a) it has the full right and authority to enter into this Agreement and to perform its obligations hereunder (including its Compound supply obligations); (b) it has the full right and authority to grant the licenses hereunder that it purports to grant; and (c) subject to Section 3.9, it has not entered into, and will not enter into, any agreement or arrangement with any Third Party which would (i) prevent the Parties from performing the Study; or (ii) otherwise prevent a Party or all Parties from pursuing any additional studies with respect to the Compound Combination; or (iii) would violate the exclusivity obligation during the Exclusivity Period.
13.2 Pfizer agrees to Manufacture and supply the Pfizer Compound for purposes of the Study as set forth in Article 8, and Pfizer hereby represents and warrants to the Alliance and Checkmate that, at the time of Delivery of the Pfizer Compound, such Pfizer Compound shall have been Manufactured and supplied in compliance with: (i) the Specifications for the Pfizer Compound; (ii) the Clinical Quality Agreements; and (iii) all Applicable Law, including cGMP and health, safety and environmental protections. The Alliance agrees to Manufacture and supply the Alliance Compound for purposes of the Study as set forth in Article 8, and the Alliance hereby represents and warrants to Checkmate and Pfizer that, at the time of Delivery of the Alliance Compound, such Alliance Compound shall have been Manufactured and supplied in compliance with: (a) the Specifications for the Alliance Compound; (b) the Clinical Quality Agreements; and (c) all Applicable Law, including cGMP and health, safety and environmental protections.
13.3 Without limiting the foregoing, each Party is responsible for obtaining all regulatory approvals (including facility licenses) that are required to Manufacture its Compound in accordance with Applicable Law (provided that for clarity, Checkmate shall be responsible for obtaining Regulatory Approvals for the Study as set forth in Section 3.3).
13.4 Pfizer does not undertake that the Study shall lead to any particular result, nor is the success of the Study guaranteed. No Party accepts any responsibility for any use that the other Party may make of the Clinical Data and/or Sample Testing Results nor for advice or information given in connection therewith.
13.5 Anti-Corruption.
13.5.1 In performing their respective obligations hereunder, the Parties acknowledge that the corporate policies of Checkmate, Pfizer and the Alliance and their respective Affiliates require that each Party’s business be conducted within the letter and spirit of the law. By signing this Agreement, each Party agrees to conduct the business contemplated herein in a manner which is consistent with all Applicable Law, including the U.S. Foreign Corrupt Practices Act, good business ethics, and its ethics and other corporate policies, and to abide by the spirit of the other Party’s applicable ethics and compliance guidelines which may be provided by such other Party from time to time. Specifically, each Party agrees that it has not, and covenants that it, its Affiliates and its Affiliates’ directors, employees, officers, and anyone acting on its behalf, will not, in connection with the performance of this Agreement, directly or indirectly, make, promise, authorize, ratify or offer to make, or take any action in furtherance of, any payment or transfer of anything of value for the purpose or intent of influencing, inducing or rewarding any act, omission or decision to secure an improper advantage; or improperly assisting it in obtaining or retaining business for it or the other Party, or in any way with the purpose or effect of public or commercial bribery.
13.5.2 Each Party shall not contact, or otherwise knowingly meet with, any Government Official for the purpose of discussing activities arising out of or in connection with this Agreement, without the prior written approval of the other Party, except where such meeting is consistent with the purpose and terms of this Agreement and in compliance with Applicable Law.
13.5.3 Each Party represents that: (i) it has no impediment to enter into the transaction contemplated in this Agreement; and (ii) it is not excluded, debarred, suspended, proposed for suspension or debarment, or otherwise ineligible for government programs.
13.5.4 Each Party represents and warrants that except as disclosed to the other in writing prior to the commencement of this Agreement: (1) it does not have any interest which directly or indirectly conflicts with its proper and ethical performance of this Agreement; and (2) it shall maintain arm’s length relations with all Third Parties with which it deals for or on behalf of the other in performance of this Agreement. Each Party shall make all further disclosures as necessary to the other Party to ensure the information provided remains complete and accurate throughout the term of this Agreement. Subject to the foregoing, each Party agrees that it shall not hire or retain any Government Official to assist in its performance of this Agreement, with the sole exception of conduct of or participation in clinical trials under this Agreement, provided that such
hiring or retention shall be subject to the completion by the hiring or retaining Party of a satisfactory anti-corruption and bribery (e.g., FCPA) due diligence review of such Government Official. Each Party further covenants that any future information and documentation submitted to the other Party as part of further due diligence or a certification shall be complete and accurate.
13.5.5 Each Party shall have the right during the term of this Agreement, and for a period of two (2) years following termination of this Agreement, to conduct an investigation and audit of the other Party’s activities, books and records, to the extent they relate to that other Party’s performance under this Agreement, to verify compliance with the terms of this Section 13.6. Such other Party shall cooperate fully with such investigation or audit, the scope, method, nature and duration of which shall be at the sole reasonable discretion of the Party requesting such audit.
13.5.6 Each Party shall ensure that all transactions under the Agreement are properly and accurately recorded in all material respects on its books and records and that each document upon which entries in such books and records are based is complete and accurate in all material respects. Each Party further represents, warrants and covenants that all books, records, invoices and other documents relating to payments and expenses under this Agreement are and shall be complete and accurate and reflect in reasonable detail the character and amount of transactions and expenditures. Each Party must maintain a system of internal accounting controls reasonably designed to ensure that no off-the-books or similar funds or accounts will be maintained or used in connection with this Agreement.
13.5.7 Each Party agrees that in the event that the other Party believes in good faith that there has been a possible violation of any provision of this Section 13.6, such other Party may make full disclosure of such belief and related information needed to support such belief at any time and for any reason to any competent government bodies and its agencies, and to whoever such Party determines in good faith has a legitimate need to know.
13.5.8 Each Party shall comply with its own ethical business practices policy and any Corporate Integrity Agreement to which it is subject, and shall conduct its Study-related activities in accordance with Applicable Law. Each Party agrees to ensure that all of its employees involved in performing its obligations under this Agreement are made specifically aware of the compliance requirements under this Section 13.5. In addition, each Party agrees to ensure that all such employees participate in and complete mandatory compliance training to be conducted by each Party, including specific training on anti-bribery and corruption, prior to his/her performance of any obligations or activities under this Agreement. Each Party further agrees to certify its continuing compliance with the requirements under this Section 13.5 on a periodic basis during the term of this Agreement in such form as may be reasonably requested by the other Party.
13.6 EXCEPT AS EXPRESSLY PROVIDED HEREIN, THE ALLIANCE MAKES NO WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH RESPECT TO THE ALLIANCE COMPOUND, AND CHECKMATE MAKES NO WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH RESPECT TO THE CHECKMATE COMPOUND, AND PFIZER MAKES NO WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH RESPECT TO THE PFIZER COMPOUND.
| 14. | Insurance; Indemnification; Limitation of Liability. |
14.1 Insurance. Each Party warrants that it maintains a policy or program of insurance or self-insurance at levels sufficient to support the indemnification obligations assumed herein. Upon request, a Party shall provide evidence of such insurance.
14.2 Indemnification.
14.2.1 Indemnification by Checkmate. Checkmate agrees to defend, indemnify and hold harmless each of the Alliance, Pfizer, and each of its Affiliates, and its and their employees, directors, subcontractors and agents from and against any loss, damage, reasonable costs and expenses (including reasonable attorneys’ fees and expenses) incurred in connection with any claim, proceeding, or investigation by a Third Party (collectively, the “Claims”) to the extent arising out of this Agreement or the Study (an “Checkmate Liability’ ), except to the extent that such Checkmate Liability (A) was directly caused by (i) negligence or willful misconduct on the part of the Alliance or Pfizer, as applicable, (or any of its Affiliates, or its and their employees, directors, subcontractors or agents); (ii) a breach on the part of the Alliance or Pfizer, as applicable, of any of its representations and warranties or any other covenants or obligations of the Alliance or Pfizer, as applicable, under this Agreement; or (iii) a breach of Applicable Law by the Alliance or Pfizer, as applicable; or (B) is determined to be attributable to the Alliance Compound or Pfizer Compound, as applicable.
14.2.2 Indemnification by the Alliance. The Alliance agrees to defend, indemnify and hold harmless each of Checkmate and Pfizer, and each of its Affiliates, and its and their employees, directors, subcontractors and agents from and against any Claims to the extent arising out of this Agreement or the Study (an “Alliance Liability”), except to the extent that such Alliance Liability (A) was directly caused by (i) negligence or willful misconduct on the part of Checkmate or Pfizer, as applicable, (or any of its Affiliates, or its and their employees, directors, subcontractors or agents); (ii) a breach on the part of Checkmate or Pfizer, as applicable, of any of its representations and warranties or any other covenants or obligations of Checkmate or Pfizer, as applicable, under this Agreement; or (iii) a breach of Applicable Law by Checkmate or Pfizer, as applicable,; or (B) is determined to be attributable to the Checkmate Compound or the Pfizer Compound, as applicable.
14.2.3 Indemnification by Pfizer. Pfizer agrees to defend, indemnify and hold harmless each of Checkmate and the Alliance, and each of its Affiliates, and its and their employees, directors, subcontractors and agents from and against any Claims to the extent arising out of this Agreement or the Study (an “Pfizer Liability”), except to the extent that such Pfizer Liability (A) was directly caused by (i) negligence or willful misconduct on the part of Checkmate or the Alliance, as applicable, (or any of its Affiliates, or its and their employees, directors, subcontractors or agents); (ii) a breach on the part of Checkmate or the Alliance, as applicable, of any of its representations and warranties or any other covenants or obligations of Checkmate or the Alliance, as applicable, under this Agreement; or (iii) a breach of Applicable Law by Checkmate or the Alliance, as applicable,; or (B) is determined to be attributable to the Checkmate Compound or the Alliance Compound, as applicable.
14.2.4 Procedure. The obligations of the Alliance, Pfizer and Checkmate under this Section 14.2 are conditioned upon the delivery of written notice to the Alliance, Pfizer or Checkmate, as the case might be, of any potential Liability within a reasonable time after a Party becomes aware of such potential Liability. A Party will have the right to assume the defense of any suit or claim related to the Liability (using counsel reasonably satisfactory to the other Party) if it has assumed responsibility for the suit or claim in writing. The other Party may participate in (but not control) the defense thereof at its sole cost and expense. The Party controlling such defense (the “Defending Party”) shall keep the other Party (the “Other Party”) advised of the status of such action, suit, proceeding or claim and the defense thereof and shall consider recommendations made by the Other Party with respect thereto. The Defending Party shall not agree to any settlement of such action, suit, proceeding or claim without the prior written consent of the Other Party, which shall not be unreasonably withheld. The Defending Party shall not agree to any settlement of such action, suit, proceeding or claim or consent to any judgment in respect thereof that does not include a complete and unconditional release of the Other Party from all liability with respect thereto or that imposes any liability or obligation on the Other Party without the prior written consent of the Other Party.
14.2.5 Study Subjects. Pfizer shall not offer compensation on behalf of the Alliance or Checkmate to any Study subject or bind the Alliance or Checkmate to any indemnification obligations in favor of any Study subject. Checkmate shall not offer compensation on behalf of the Alliance or Pfizer to any Study subject or bind the Alliance or Pfizer to any indemnification obligations in favor of any Study subject. Likewise, the Alliance shall not offer compensation on behalf of Checkmate or Pfizer to any Study subject or bind Checkmate or Pfizer to any indemnification obligations in favor of any Study subject.
14.3 LIMITATION OF LIABILITY. OTHER THAN WITH RESPECT TO DAMAGES ARISING OUT OF OR RELATED TO A PARTY’S BREACH OF ITS OBLIGATIONS UNDER THIS AGREEMENT TO USE, DISCLOSE, LICENSE, ASSIGN OR OTHERWISE TRANSFER SAMPLE TESTING RESULTS, CLINICAL DATA, CONFIDENTIAL INFORMATION AND JOINT IP ONLY FOR THE USE HEREIN, IN NO EVENT SHALL A PARTY (OR ANY OF ITS AFFILIATES OR SUBCONTRACTORS) BE LIABLE TO ANY OTHER PARTY FOR, NOR SHALL ANY INDEMNIFIED PARTY HAVE THE RIGHT TO RECOVER, ANY SPECIAL, INDIRECT, INCIDENTAL, PUNITIVE OR CONSEQUENTIAL DAMAGES (INCLUDING LOST PROFITS OR DAMAGES FOR LOST OPPORTUNITIES), WHETHER IN CONTRACT, WARRANTY, NEGLIGENCE, TORT, STRICT LIABILITY OR OTHERWISE, ARISING OUT OF (x) THE MANUFACTURE OR USE OF ANY COMPOUND SUPPLIED HEREUNDER OR (y) ANY BREACH OF OR FAILURE TO PERFORM ANY OF THE PROVISIONS OF THIS AGREEMENT OR ANY REPRESENTATION, WARRANTY OR COVENANT CONTAINED IN OR MADE PURSUANT TO THIS AGREEMENT, EXCEPT THAT SUCH LIMITATION SHALL NOT APPLY TO DAMAGES PAID OR PAYABLE TO A THIRD PARTY BY AN INDEMNIFIED PARTY FOR WHICH THE INDEMNIFIED PARTY IS ENTITLED TO INDEMNIFICATION HEREUNDER.
| 15. | Use of Name. |
Except as expressly provided herein or with the other Party’s written approval, neither Party shall have any right, express or implied, to use in any manner the name or other designation of the other Party or any other trade name, trademark or logo of the other Party for any purpose in connection with the performance of this Agreement.
| 16. | Force Majeure. |
If in the performance of this Agreement, one of the Parties is prevented, hindered or delayed by reason of any cause beyond such Party’s reasonable control (e.g., war, riots, fire, strike, governmental laws), such Party shall be excused from performance to the extent that it is necessarily prevented, hindered or delayed (“Force Majeure”). The non-performing Party will notify the other Party of such Force Majeure within ten (10) Business Days after such occurrence by giving written notice to the other Party stating the nature of the event, its anticipated duration, and any action being taken to avoid or minimize its effect. The suspension of performance will be of no greater scope and no longer duration than is necessary and the non-performing Party will use commercially reasonable efforts to remedy its inability to perform.
| 17. | Entire Agreement; Modification. |
The Parties agree to the full and complete performance of the mutual covenants contained in this Agreement. This Agreement, together with the Related Agreements, constitutes the sole, full and complete agreement by and between the Parties with respect to the subject matter of this Agreement, and all prior agreements, understandings, promises and representations, whether written or oral, with respect thereto are superseded by this Agreement. No amendments, changes, additions, deletions or modifications to or of this Agreement shall be valid unless reduced to writing and signed by the Parties hereto.
| 18. | Assignment and Sub-Contracting. |
Neither Party shall assign or transfer this Agreement without the prior written consent of the other Party; provided, however, that no such consent shall be required in connection with a Change of Control of a Party. Notwithstanding the foregoing, either Party may assign all or any part of this Agreement to one or more of its Affiliates without the other Party’s consent, and any and all rights and obligations of either Party may be exercised or performed by its Affiliates, provided that such Affiliates agree to be bound by this Agreement. In the event of a Change of Control of a Party, such Party undergoing the Change of Control shall notify the other Party in writing at least thirty (30) days prior to completion of such Change of Control (to the extent such notification is legally permissible prior to completion of such Change of Control, and if such notification is not legally permissible prior to such Change of Control, then such notification shall be provided to the other Party in writing simultaneously with the first public announcement with respect to such Change of Control). Any permitted assignee of a Party (which assignee shall include the Third Party in a Change of Control situation under Section 1.20) shall, in writing to the non-assigning Party, expressly assume the obligation to perform this Agreement. Any attempted assignment not in accordance with this Section 18 shall be null and void and of no legal effect. The terms and conditions of this Agreement shall be binding upon, and shall inure to the benefit of, the Parties and their respected successors and permitted assigns. For the avoidance of doubt, nothing in this Section limits the provisions of Section 3.10.
| 19. | Invalid Provision. |
If any provision of this Agreement is held to be illegal, invalid or unenforceable, the remaining provisions shall remain in full force and effect and will not be affected by the illegal, invalid or unenforceable provision. In lieu of the illegal, invalid or unenforceable provision, the Parties shall negotiate in good faith to agree upon a reasonable provision that is legal, valid and enforceable to carry out as nearly as practicable the original intention of the entire Agreement.
| 20. | No Additional Obligations. |
Checkmate, Pfizer and the Alliance have no obligation to renew this Agreement or apply this Agreement to any clinical trial other than the Study. No Party is under any obligation to enter into another type of agreement at this time or in the future.
| 21. | Dispute Resolution and Jurisdiction. |
21.1 The Parties shall attempt in good faith to settle all disputes arising out of or in connection with this Agreement in an amicable manner. Any claim, dispute or controversy arising out of or relating to this Agreement, including the breach, termination or validity hereof or thereof (each, a “Dispute”), shall be governed by and construed in accordance with the substantive laws of New York, without giving effect to its choice of law principles.
21.2 Nothing contained in this Agreement shall deny either Party the right to seek injunctive or other equitable relief from a court of competent jurisdiction in the context of a bona fide emergency or prospective irreparable harm, and such an action may be filed or maintained notwithstanding any ongoing discussions between the Parties.
| 22. | Notices. |
All notices or other communications that are required or permitted hereunder shall be in writing and delivered personally, sent by facsimile (and promptly confirmed by personal delivery or overnight courier), or sent by internationally-recognized overnight courier addressed as follows:
If to Checkmate, to:
Checkmate Pharmaceuticals, Inc.
One Broadway, 14th Floor
Cambridge, MA 02142
Attention: General Counsel
With a copy to: President and CEO
If to the Alliance, to:
Ares Trading S.A.
Attention: Legal Department
Z.I de l’Ouriettaz,
CH-1170 Aubonne,
Switzerland
With a copy to:
Merck KGaA
Attention: Merck Healthcare Legal
Frankfurter Strasse 250
64293 Darmstadt, Germany
Pfizer Inc.
235 East 42nd Street
New York, NY 10017
Attention: VP, Oncology Alliance Manager
With a copy to:
Pfizer Inc.
235 East 42nd Street
New York, NY 10017
Attention: Jason Smith, Chief Counsel, Oncology Business Unit
and to:
Pfizer Inc.
235 East 42nd Street
New York, NY 10017
Attention: Paul Schneider
Assistant General Counsel, Business Transactions
| 23. | Relationship of the Parties. |
The relationship between the Parties is and shall be that of independent contractors, and does not and shall not constitute a partnership, joint venture, agency or fiduciary relationship. Neither Party shall have the authority to make any statements, representations or commitments of any kind, or take any actions, which are binding on the other Party, except with the prior written consent of the other Party to do so. All persons employed by a Party will be the employees of such Party and not of the other Party and all costs and obligations incurred by reason of any such employment shall be for the account and expense of such Party.
| 24. | Counterparts and Due Execution. |
This Agreement and any amendment may be executed in two (2) or more counterparts (including by way of facsimile or electronic transmission), each of which shall be deemed an original, but all of which together shall constitute one and the same instrument, notwithstanding any electronic transmission, storage and printing of copies of this Agreement from computers or printers. When executed by the Parties, this Agreement shall constitute an original instrument, notwithstanding any electronic transmission, storage and printing of copies of this Agreement from computers or printers. For clarity, facsimile signatures and signatures transmitted via PDF shall be treated as original signatures.
| 25. | Construction. |
Except where the context otherwise requires, wherever used, the singular will include the plural, the plural the singular, the use of any gender will be applicable to all genders, and the word “or” is used in the inclusive sense (and/or). Whenever this Agreement refers to a number of days, unless otherwise specified, such number refers to calendar days. The captions of this Agreement are for convenience of reference only and in no way define, describe, extend or limit the scope or intent of this Agreement or the intent of any provision contained in this Agreement. The term “including” as used herein shall be deemed to be followed by the phrase “without limitation” or like expression. The term “will” as used herein means shall. References to “Article,” “Section” or “Appendix” are references to the numbered sections of this Agreement and the appendices attached to this Agreement, unless expressly stated otherwise. Except where the context otherwise requires, references to this “Agreement” shall include the appendices attached to this Agreement. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties and no rule of strict construction will be applied against either Party hereto.
IN WITNESS WHEREOF, the respective representatives of the Parties have executed this Agreement as of the Effective Date.
| Ares Trading S.A. | ||
| By: | /s/ Cedric Hyde | |
| Name: | Cedric Hyde | |
| Title: | Authorized Representative | |
| Ares Trading S.A. | ||
| By: | /s/ Luigia Bocola | |
| Name: | Luigia Bocola | |
| Title: | Authorized Representative | |
| Pfizer Inc. | ||
| By: | /s/ Andy Schmeltz | |
| Name: | Andy Schmeltz | |
| Title: | Global President, Oncology | |
| Checkmate Pharmaceuticals, Inc. | ||
| By: | /s/ Charles E. Yon | |
| Name: | Charles E. Yon | |
| Title: | General Counsel | |
Appendix A
PROTOCOL SUMMARY:

Combination of CMP-001 with avelumab and/or Pfizer compounds
Javelin Medley, Combination F
Appendix A
PROTOCOL SUMMARY:
CMP-001 will be incorporated into the Javelin Medley study (B9991004), with avelumab as a backbone therapy for patients with PDx (anti-PD-1/L1) refractory SCCHN (squamous cell carcinoma of the head and neck). Upon successful achievement of the specific “Go” criterion for this doublet, separate patient cohorts will be evaluated with triplets of utomilumab/CMP-001/avelumab or PF-04518600/CMP-001/avelumab.
Study Type: Phase 1b safety lead-in followed by Phase 2 expansion cohorts
Rationale:
For Combination F1, CMP-001 will be combined with avelumab. Since the combination of CMP-001 with pembrolizumab (anti-PD-1) showed significant anti-tumor activity in the setting of PDx-refractory melanoma, the goal of this study will be to assess the activity of CMP-001 in combination with avelumab as Combination F, Arm F in patients with PDx- refractory SCCHN. This disease has been selected for Combination F firstly because SCCHN is a type of tumor that responds to avelumab as a single agent, in a PDx pretreatment naive setting. However, after treatment with single agent PDx agents many of these patients are either found to have refractory disease or develop resistance. This may be in part due to a lack of an active immune tumor microenvironment. TLR9-responsive plasmacytoid dendritic cells have been observed in SCCHN, and injection of TLR9 agonist may “heat up” otherwise PDx refractory tumors. The anatomic location upon local recurrence as well sites of metastases are frequently feasible for intratumoral injections in this disease as well.
If anti-tumor activity is noted in Combination F1, it is likely that the targets for 4-1BB or OX40 agonist antibodies will be induced on activated anti-tumor T cells in tumor-draining lymph nodes. There are scientific data to support the addition of utomilumab (4-1BB agonist) or an OX-40 agonist in combination with a TLR9 agonist (CMP-001) plus avelumab. TLR9 agonists will stimulate tumor DCs and these cells will traffic to tumor draining lymph nodes, where they
will promote anti-tumor T cell activation. DCs can present tumor peptides to T cells in different ways and this may determine if OX40 or 4-1BB is upregulated. OX40 and 4-1BB agonists have been observed in tumor model systems to significantly boost anti-tumor immunity in combination with a TLR agonist and a PD-1/PD-L1 inhibitor. Therefore, if a tolerable safety profile of the CMP-001 plus avelumab doublet is observed, the JDC may elect to combine Pfizer’s OX40 agonist, PF-404518600, and/or utomilumab with avelumab and CMP-001 as triplets in in separate Combination F study arms in order to understand if the agonist triplets are both safe and potentially more effective in PDx refractory SCCHN.
Arm F1: Avelumab + CMP-001 (SC to IT) (N=20)
Sequenced sub cutaneous (SC) followed intra-tumoral (IT) administration of CMP-001 added to avelumab therapy (6-12 safety lead-in followed by 8-14 expansion patients).
Arms F2 and/or F3 may be initiated upon successfully meeting the criteria outlined later in this summary. Patients will be randomly assigned to either Arm F2 or F3.
Arm F2: Avelumab + CMP-001 + OX-40 (N=20)
The study arm will enroll 20 patients (6 safety lead-in followed by 14 expansion patients). The arm will be initiated after efficacy threshold for Arm F.
Arm F3: Avelumab + CMP-001 + Utomilumab (N=20)
The study arm will enroll 20 patients (6 safety lead-in followed by 14 expansion patients). The arm will be initiated after efficacy threshold for Arm F1 is met.
Tumor type: SCCHN post PDx (other tumor types can be considered based on clinical benefit observed in SCCHN patients and may be added as a part of a an amendment to the agreement)
Total Number of Patients: Up to approximately 60. Each arm F1-3 will enroll 20 patients each. Actual number of enrolled patient will vary based on need of replacements for patients ineligible for safety and efficacy assessment.
Principal Investigator: N/A (The B9991004 basket study has many PIs based on the number of sites participating); Number of sites anticipated to participate: 6 for the lead-in based on 6-12 patients in the lead-in portion of F1, followed by allowing all active Javelin Medley sites enrolling SCCHN patients in the USA to participate in the expansion phase.
Description: Each study arm will begin with a 6-12 patient safety lead-in, followed by an expansion of patients up to 20 at a tolerable dose of CMP-001.
As shown in the schematic below:
Arm F1 will include a safety lead-in cohort of 6 to 12 patients treated with 2 weekly doses of SC CMP-001 on Cycle 1 Day -14 and Day -7, followed by 3rd week intratumoral (IT) injections of CMP-001 on Cycle 1 Day 1, with avelumab administered on Day 2 of Cycle 1. CMP-001 will be switched to every 2 week IT dosing to match avelumab dosing starting with Cycle 2. Upon clearing the safety lead-in, the JDC may elect to initiate Arm F2 and /or F3.
Arm F2 will consist of the regimen used in arm F1 in combination with utomilumab (4-1BB agonist) as a triplet therapy. Utomilumab will be injected intravenously at a recommended dose once, monthly.
Arm F3 will consist of the regimen used in arm F1 in combination with PF-04518600 (OX- 40 agonist) in a triplet therapy.
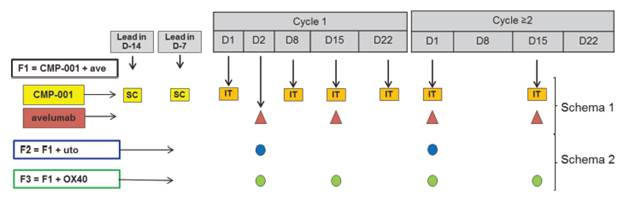
Statistical approach:
If in safety lead-in portion < 2 of 6 patients experience DLT, the cohort will be expanded to enroll up to a total of 20 patients;
The primary endpoint for Phase 1b lead-in and the Phase 2 cohort expansion combined is the confirmed objective response (OR) as assessed by the Investigator using RECIST v1.1.
OR is defined as a CR or PR per RECIST v1.1 from the first dose of study treatment until disease progression or death due to any cause. Both CR and PR must be confirmed by repeat assessments performed no less than 4 weeks after the criteria for response are first met. Objective response rate (ORR) is defined as the proportion of patients with a confirmed CR or PR per Investigator’s assessment according to RECIST v.1.1. Confirmed responses are those that persist on repeat tumor assessments for at least 4 weeks after initial documentation or response. Otherwise, the patient will be counted as a non-responder in the assessment of ORR. Additionally, patients with inadequate data for tumor assessment (e.g., no baseline assessment or no follow up assessments) will be considered as non-responders in the assessment of ORR. The two-sided exact 90% CI for ORR will be calculated. Go criterion from Arm F1 to Arm F2/F3 will be based on the observation of a tolerable safety profile for the CMP-001 + avelumab combination.
Dosing: Avelumab 10 mg/kg q2w + CMP-001 at 5 mg (or another dose as suggested by Checkmate). CMP-001 can be injected into 1 or 2 target tumors. Subcutaneous administration is suggested to be within the lymphatic bed draining into target tumors, when feasible.
Primary endpoints:
Lead-ins: DLTs (DLT period will be proposed to the FDA as 4 weeks to match the period of weekly CMP-001 administration)
Expansion phase: ORR
Secondary endpoints as standard for the Javelin Medley study (e.g. PFS, TTP, OS, biomarkers)
Patients will be required to provide a baseline tumor biopsy for the above biomarker analyses in addition to other biomarker exploratory analyses, as standard for the Javelin Medley study (e.g. tumor mutation status, TCR analyses, IDO1 and PD-L1 expression).
Dosing of study drugs will continue until loss of tolerability or loss of clinical benefit.
Dosing may be stopped upon confirmation of a CR and may be re-initiated if a patient with a CR discontinues treatment and subsequently relapses.
Key Enrollment Criteria:
| • | Histologically confirmed diagnosis of metastatic or recurrent SCCHN who has received up to 3 lines of prior therapies. |
| • | Patient should be refractory to treatment with single agent PDx therapy (Refractory is defined as best response PD or SD and progression on treatment within 4 months of starting the treatment). |
| • | Patient must be candidate for intralesional therapy administration defined as at least 1 injectable tumor lesion >10 mm in longest diameter. |
| • | Patient should have at least 1 target lesion different from lesions selected for IT therapy. |
Key Exclusion Criteria (in addition to standard protocol exclusion criteria):
| • | Patient should not have received previous treatment with talimogene laherparepvec, other oncolytic virus therapies, or other TLR9 agonists. Patient should not have received prior intra-tumoral anticancer treatment. |
| • | Lesions that directly contact or encase a maj or blood vessel. |
Possible additional arm pending JDC approval
Arm F4: Avelumab + CMP-001 (SC) (N=20)
SC CMP-001 added to avelumab therapy, to be initiated after data supporting SC doses are available and concept is endorsed by JDC. Arm F2 will include CMP-001 administered SC only in the same frequency as used for Arm F1 combined with avelumab standard dosing (subject to change in schedule based on emerging Checkmate SC data).
Appendix B
SUPPLY OF COMPOUNDS
Schedule of Deliveries for Alliance Compound
| Delivery Date |
Quantity of [**] |
Quantity of [**] | ||
| Total |
DRUG RESPONSIBILITY MATRIX
| Task |
Responsibility of Pfizer |
Responsibility of the Alliance |
Responsibility of Checkmate | |||
| On a quarterly basis, provide the Alliance with a 24-month rolling forecast of Alliance Compound drug supply needs by country | X | |||||
| Monitor Alliance Compound clinical trial supply inventory and submit orders for Alliance Compound to the Alliance 16 weeks prior to date that drug is needed | X | |||||
| Confirm receipt of order and provide Pfizer with estimated shipment/delivery date | X | |||||
| Conduct monthly (or other frequency as needed) conference call to review any drug supply issues for the Alliance Compound | X | X | ||||
| Provide the Alliance with quarterly reports of the number of vials of Alliance Compound that Checkmate ships to each site | X | |||||
| Produce clinical supply label proof for the Alliance Compound | X | |||||
| Review Alliance Compound clinical supply label to ensure it meets Alliance Compound regulatory and safety requirements | X | |||||
| Provide unlabeled vials of Alliance Compound in quantities determined by Checkmate to be used in the Study and through completion of the Study | X | |||||
| Provide Qualified Person (QP) approval/ release certificate for all batches of commercially packaged Alliance Compound provided for use in the study | X | |||||
| Review the executed clinical packaging/labeling records and issue a GMP release
* NOTE: For EU territories, health authority / ethics committee approval documentation in the local language as well as an English translation must be provided for GMP release |
X | |||||
| Provide the following information to Pfizer with respect to the Alliance Compound: | X | |||||
|
☐ MSDS
☐ Certificate of Compliance/Conformance where the Certificate of Conformance generated should contain the following at a minimum: |
||||||
|
• Product name
• Lot or batch number
• Expiry date
• Date of manufacturing |
||||||
|
☐ Certificate of Analysis
☐ SmPC
☐ BSE/TSE Certificate
☐ Qualified Person GMP declaration for CTA filings of the Alliance Compound |
||||||
| Distribution of clinically packaged and labeled Alliance Compound to Checkmate clinical sites | X | |||||
| Return/destruction of unused Alliance Compound Study drug at depots/clinical sites | X | |||||
| Provide the Alliance with destruction certificate for any returns and/or unused Alliance Compound Study drug | X | |||||
| On a quarterly basis, provide Checkmate with a 24-month rolling forecast of Checkmate Compound drug supply needs by country | X | |||||
| Monitor Checkmate Compound clinical trial supply inventory and submit orders for Checkmate Compound to Checkmate 16 weeks prior to date that drug is needed | X | |||||
| Confirm receipt of order and provide Pfizer with estimated shipment/delivery date | X | |||||
| Conduct monthly (or other frequency as needed) conference call to review any drug supply issues for Checkmate Compound | X | X | ||||
| Provide Checkmate with quarterly reports of the number of vials of Checkmate Compound that Checkmate ships to each site | X | |||||
| Produce clinical supply label proof for Checkmate Compound | X | |||||
| Review Checkmate Compound clinical supply label to ensure it meets Checkmate Compound regulatory and safety requirements | X | |||||
| Provide unlabeled vials of Checkmate Compound in quantities determined by Checkmate to be used in the Study and through completion of the Study | X | |||||
| Provide Qualified Person (QP) approval/ release certificate for all batches of commercially packaged Checkmate Compound provided for use in the study | X | |||||
| Review the executed clinical packaging/labeling records and issue a GMP release
* NOTE: For EU territories, health authority / ethics committee approval documentation in the local language as well as an English translation must be provided for GMP release |
X | |||||
| Provide the following information to Pfizer with respect to the Alliance Compound: | X | |||||
|
☐ MSDS
☐ Certificate of Compliance/Conformance where the Certificate of Conformance generated should contain the following at a minimum: |
||||||
|
• Product name |
||||||
| • Lot or batch number
• Expiry date
• Date of manufacturing |
||||||
|
☐ Certificate of Analysis
☐ SmPC
☐ BSE/TSE Certificate
☐ Qualified Person GMP declaration for CTA filings of the Alliance Compound |
||||||
| Distribution of clinically packaged and labeled Checkmate Compound to Checkmate clinical sites | X | |||||
| Return/destruction of unused Checkmate Compound Study drug at depots/clinical sites | X | |||||
| Provide Checkmate with destruction certificate for any returns and/or unused Checkmate Compound Study drug | X | |||||
