Attached files
| file | filename |
|---|---|
| 8-K - VITRO DIAGNOSTICS, INC. - VITRO DIAGNOSTICS INC | vitro_8k.htm |
Vitro Biopharma 2nd Quarter ended April 30th 2020 Financial Results of Operations
Golden, Colorado-June 25th, 2020-Vitro Diagnostics, Inc. (OTCQB: VODG), dba Vitro Biopharma, announced its 2nd quarter ended April 30th 2020 financial results of operations.
Vitro Diagnostics Inc. (“Vitro Biopharma”) announced a reduction in 2nd quarter revenues across all its revenue categories. Vitro Biopharma recorded 2nd quarter revenues of $128,631 vs $211,900 a decrease of 39% over the same comparative quarter last year. Prior to the lockdowns which began at different times for different jurisdictions Vitro had reported increasing revenues across all its revenue categories for 13 consecutive quarters. We expect to see revenue returning in the 4th quarter of 2020 and into the first half of 2021. Preliminary feedback from our customers indicates that patients awaiting treatments at our partner clinic in the Cayman Islands www.DVCstem.com
are not dropping off but merely postponing their treatments and as such a backlog is building
rather than an outright customer lost. The cosmetic clinics www.Infinivive.com have started to open up but only with reduced occupancy and variations by state and hence reduced revenue into the 3rd quarter with expectations of a revival of revenue in the 4th quarter of 2020 and into the first half of 2021.
Overall operating expenses increased in the quarter by $114,178 to $281,485 from $167,307 in the prior year’s comparative quarter. The increase in expenses reflects the increased costs of FDA regulatory, legal, consulting, business and product development expenses. The company added extra resources to turn its attention to the world wide challenge of finding therapies to fight the Covid-19. Vitro filed an Investigational New Drug (“IND”) application and also received emergency use authorization from the FDA for use of AlloRx Stem Cells ® in the treatment of COVID-19 patients. A single patient was treated subsequent to the end of the second quarter. There were no adverse events demonstrating safety and the patient showed evidence of efficacy including improved lung and kidney function. We also entered into an MOU with GIOSTAR, a leading global stem cell research operating multiple international stem cell clinics. www.giostar.com
During and subsequent to the quarter the company achieved and pursed the following objectives:
Series A Convertible Preferred Stock Offering:
During the quarter and subsequent to the quarter the company continued with its Series A Convertible Preferred Stock offering to accredited investors under the SEC Regulation D exemption. The preferred Stock is priced at $25 per share which is convertible at $0.25 cents per share for a total of 100 shares. The minimum investment is $50,000 per unit. The company sold $550,000 of the Series A Convertible Preferred Stock during and subsequent to the quarter. The offering was sold out at $1,000,000 and the company is considering expanding it to ensure sufficient working capital during the Coronavirus pandemic and to start the regulatory process of current reporting audits and funding for its expanded clinical trial activities with the FDA.
Filed Investigational New Drug (IND) Application with the FDA
As a part of our overall strategy to target both global and US stem cell markets, Vitro submitted a Phase I IND application to the FDA to assess safety of AlloRx Stem Cells® in the treatment of COVID-19 patients in the US. Recent umbilical cord stem cell therapies in China to fight the Coronavirus are producing encouraging safety and efficacy results. We are establishing strong communication channels with FDA officials to facilitate and expedite review of our application as well as subsequent steps to gain full FDA approval of AlloRx Stem Cells®. The application is presently under review and we are working closely with FDA reviewers to gain authorization to enroll patients. Several clinical centers have expressed interest in our stem cell therapy. We are also pursuing other avenues for Emergency Use Authorization (EUA). The FDA has thus far authorized three separate EUA applications for compassionate use of AlloRx Stem Cells® in COVID-19 patients. Unfortunately, two patients died prior to treatment. A single patient has been treated by three separate dosages of AlloRx Stem Cells® through an authorized EUA by Giostar. There were no adverse events and the patient who has various comorbidities stabilized and exhibited enhanced pulmonary and renal functions during the six weeks following AlloRx Stem Cell® Therapy. While presently intubated and hospitalized in the ICU, this patient is exhibiting gradual improvement. We are presently pursuing additional EUA applications through our collaboration with GIOSTAR The data obtained from these studies corroborates our studies of safety and efficacy. MSCs block the cytokine storm that occurs in COVID-19 patients in acute respiratory distress through their powerful anti-inflammatory effects. The cytokine storm leads to the need for assisted breathing by ventilators, transfer to ICU and tremendous burdens on the US health care system. It is important to note that AlloRx Stem Cells® are therapy for other viral attacks including influenza since stem cells block acute respiratory distress and damage to other major organs including cardiovascular, pulmonary and renal systems. AlloRx Stem Cells® are very likely to assist in recovery from failure of various organ systems in COVID-19 survivors, as our case study is demonstrating.
MOU with Giostar
We entered into an exclusive Memorandum of Understanding (MOU) with Global Institute of Stem Cell Therapy and Research, Inc. (“GIOSTAR”) a leading stem cell research institute based in San Diego, California to jointly partner together for a separate COVID-19 Investigational New Drug (“IND”) application to the FDA using Vitro Biopharma’s umbilical cord mesenchymal stem cell product AlloRx Stem Cells™ in a clinical trial to treat Covid-19 patients. GIOSTAR is a worldwide leader in the in the field of stem cell research and has stem cell research and treatment facilities around the world. GIOSTAR is leading the way for filling the joint IND application for the Covid-19 with the FDA while Vitro will provide its AlloRx Stem Cells® for use in the study and post-approval stages through a supply agreement with GIOSTAR. Giostar has already obtained EUAs from the FDA for using stem cell treatment for severe Covid-19 hospitalized patients using AlloRx Stem Cells®.
Vitro will continue to seek FDA authorization of its pending IND. As the approval process proceeds, Vitro will seek AlloRx Stem Cells® FDA approval through Phase 2/3 IND filings for
indications other than COVID-19 such as osteoarthritis while at the same time continuing to supply GIOSTAR AlloRx Stem Cells® for treatment of COVID-19 patients in global markets.
GIOSTAR in collaboration with government of Gujarat, India is building one of the world’s largest stem cell hospital. This is a dream project of India’s Prime Minister Narendra Modi. The MOU stated the intended discussions regarding use of AlloRx Stem Cells® at GIOSTAR’s various international stem cell facilities that would provide quality and economic advantages.
Continues expansion of its manufacturing capacity
The company is doubling its laboratory and manufacturing facilities and expanding its clean room by 100% in size and capacity. This new facility is expected to be online during the 1st quarter of next year. This represents approximately $6M of AlloRx Stem Cell™ Vitro Biopharma revenue capacity per year. Furthermore, the completion of the 2nd clean room processing facility at the beginning of the 2021 year will expand our capacity to approximately 100 Billion AlloRx Stem Cell™ s a month or approximately $1.7 Million of AlloRx Stem Cell™ Vitro Biopharma revenue capacity per month. This would give Vitro Biopharma a revenue run rate capacity of $20M a year.
Our increased capacity is rigorously controlled by our Quality Management System, now certified to the ISO9001 Quality Standard and the ISO13485 Medical Device Standard as well. This provides GMP-compliant manufacturing of the highest quality stem cells/medical devices for clinical trial testing to provide further evidence of safety and efficacy for treatment of a wide variety of indications. Highly regulated GMP biologics manufacturing within an FDA-compliant facility provides numerous opportunities to the Company to drive strong revenue growth. We are presently focused on our partnerships in the Caribbean with DVC Stem in Grand Cayman Island, Infinivive MD in the US and emerging opportunities in the Commonwealth of the Bahamas. We are actively pursuing other partnership opportunities as well.
STEMulize™ manufacturing update:
We have reformulated with our CMO to produce STEMulize™ in large quantity manufacturing runs. STEMulize™ contains natural substances that activate the body’s own stem cells to enhance recovery from injury such as TBI, stroke, MS, PD and other autoimmune, inflammatory and neurological diseases. The STEMulize™ product will be offered as a private label product to Infinivive MD™ clinics and is being implemented as supplemental support to clinical treatments now ongoing in the Cayman Islands. Patients report positive benefits from STEMulize™ therapy following stem cell transplants including increased overall energy and enhancement of improved motor function in MS patients. We are currently pursing licensing arrangements with nutraceutical companies that can scale our formulation under their own private label.
Reduced Revenue from Infinivive MD™ Serum due to closure of the cosmetic clinics
The Company’s cosmetic stem cell serum private labelled as Infinivive MD Serum is being applied as a topical cosmetic serum in medical spas and plastic surgery offices.. Infinivive MD™ revenue was reduced by the Coronavirus pandemic and as a result, revenues declined by 50% in the quarter to approximately $50,000 vs $100,000 in the prior quarter. This also compares to $50,000 in the current quarter of 2020 vs $130,000 in the prior comparative quarter of 2019.
The Joint Development and Supply Agreement dated May 15th 2018 between Vitro Biopharma and Jack Zamora is being renegotiated due to the Coronavirus pandemic and as such the minimum exclusivity requirements have been delayed by approximately a year.
Infinivive MD™ Cosmetic Serum is revolutionizing the cosmetic industry. Patients are experiencing unparalleled improvements in the appearance of fine lines and wrinkles. This is one of the fastest growing revenue streams for Vitro Biopharma. . We work with a variety of regulatory experts to assist us in the appropriate regulatory pathway. At this point it is regulated as a cosmetic topical productbut may be reclassified based on regulatory input.
www.jackzamoramd.comwww.infinivivemd.com
Infinivive MD™ also has an exclusive agreement to distribute AlloRx Stem Cells™ into the countries of Saudi Arabia, U.A.E., and Colombia. The first trial run clinic in Dubai is scheduled for the fourth quarter of 2020. The agreement calls for minimum commitments to maintain exclusivity and provides for minimum revenue of $250,000 annually in 2020. However due to the worldwide Corona Virus lockdown of business and customers the agreement for performance requirements have been delayed by approximately a year.
Vitro Biopharma’s OEM cosmetic topical serum is being distributed exclusively by Infinivive MD™ into cosmetic clinics that are providing the topical treatment as a beautification product. To date the company’s product is being offered quartering a number of clinics throughout the United States and soon internationally ; but with the clinics just opening again for business and with limited occupancy rules we do not expect this revenue to recover back to peak levels with growth until the first half of 2021.
Update on the Clinical Trial of Musculoskeletal Conditions in the Bahamas
This initiative broadens Vitro Biopharma's expansion into highly regulated stem cell trials in collaboration with the Nassau-based Medical Pavilion of the Bahamas (TMPB).
http://www.tmp-bahamas.com
We will now be able to extend stem cell therapy based on our novel, patent-pending AlloRx Stem Cell™ product to a variety of musculoskeletal conditions. These include OA of any joint, ACL/MCL tear, Achilles tendon rupture, rotator cuff injury, tennis elbow and herniated disc that are highly prevalent and have few disease-modifying options. It is important to note that many stem cell treatments now performed are problematic due to limited potency and failure to meet basic criteria of stem cells. Also, contamination due to poor production methods that are not in
compliance with FDA regulations can cause serious complications. Vitro Biopharma operates a highly regulated, FDA-compliant commercial biologics manufacturing operation for several years and is cGMP compliant, ISO 9001Certified, ISO 13485 Certified, CLIA Certified and FDA registered. All manufacturing occurs in a certified sterile clean room with extensive and advanced testing to assure the absence of contamination. Furthermore, in numerous patients treated to date by IV infusion of AlloRx Stem Cells™ there have been no significant adverse events.''
The company is partnered with Dr. Conville Brown, MD, MBBS, FACC, FESC, PhD, the founder and CEO of the Medical Pavilion of the Bahamas who is the Principal Investigator of this trial and director of its clinical administration. Dr Brown was instrumental in the establishment of the NSCEC in the Bahamas.
About the Medical Pavilion of the Bahamas: TMPB operates within a 40,000 square foot building as a partnered care specialty medical facility with 10 different centers in various areas including cardiology, cancer, clinical research and kidney disease. One of the centers is the Partners Stem Cell Centre, where the present trial will be conducted. The Partners Stem Cell Centre provides an environment to conduct stem cell research and clinical trials under the model of ''FDA rigor in a Non-FDA Jurisdiction'' TMPB employs 20 medical specialists in various fields. See www.tmp-bahamas.com for additional information.
The company expects to begin patient enrollment for the clinical trial in late QIV but does not expect to realize revenue until QI/QII of 2021.
Temporary hold on revenues from the clinical trials in the Cayman Islands:
Due to the Corona virus pandemic the Cayman Islands closed itself and its businesses down for the majority of the quarter and next quarter, the current status is listed as locked down until Sept. 1st 2020. However, our partner reports that customers are staying on the waiting list and will return for their treatments as soon as the island opens back up. There currently is a backlog of patients of over 40 treatments pending which exceeds all of the treatments performed in 2019. We expect to see a surge in revenues from this backlog to bring back our revenue stream in the fourth quarter of 2020 and into the first half of 2021.
Expanded our Patent & Intellectual Property Portfolio
The Company has several patent applications (11) pending in the US and foreign jurisdictions. These patents cover our AlloRx Stem Cell™ line and various aspects of our STEMulize™ stem cell activation products & processes as well as specific diagnostic tests of stem cell activity and therapeutic effectiveness. During the quarter, the Company has responded to office actions and continues to vigorously prosecute & expand its patent filings.
Dr. Jim Musick, CEO of Vitro Biopharma, said, “We are pleased to report our activities in fighting the Covid-19 with filings of our eIND and INDs and partnership with Giostar. While we are disappointed in the extraordinary events of the Corona Virus pandemic and its results on our operations, we have taken the time to advance our clinical applications and partnerships in further preparation for realized growth in 2021 as a result of these activities.
Our stem cell products are distinctly superior to stem cell transplants in the USA. The latter usually involve use of impure products lacking validation as stem cells and containing insufficient numbers of stem cells to achieve therapeutic benefits. These are produced without regulatory oversight and have been known to cause serious adverse effects. Hence the use of highly purified and well characterized stem cells (AlloRx Stem Cells™) is needed to provide safety and efficacy in regenerative medicine therapies.
In summary, Vitro Biopharma is advancing as a key player in regenerative medicine with 10+ years’ experience in the development and commercialization of stem cell products for research, recognized by a Best in Practice Technology Innovation Leadership award for Stem Cell Tools and Technology and a growing track record of successful translation to therapy. We plan to leverage our proprietary technology platform to the establishment of international Stem Cell Centers of Excellence and regulatory approvals in the US and worldwide.
Vitro Biopharma has supplied major biopharmaceutical firms, elite university laboratories and clinical trials worldwide with its Umbilical Cord Mesenchymal Stem Cells (AlloRx Stem Cells™), and it's MSC-Grow™ Brand of cell culture media along with advanced stem cell diagnostic services. www.vitrobiopharma.com
Sincerely yours,
James R. Musick, PhD.
President, CEO & Chairman of the Board
www.vitrobiopharma.com
Forward-Looking Statements
Statements herein regarding financial performance have not yet been reported to the SEC nor reviewed by the Company’s auditors. Certain statements contained herein and subsequent statements made by and on behalf of the Company, whether oral or written may contain “forward-looking statements”. Such forward looking statements are identified by words such as “intends,” “anticipates,” “believes,” “expects” and “hopes” and include, without limitation, statements regarding the Company’s plan of business operations, product research and development activities, potential contractual arrangements, receipt of working capital, anticipated revenues and related expenditures. Factors that could cause actual results to differ materially include, among others, acceptability of the Company’s products in the market place, general economic conditions, receipt of additional working capital, the overall state of the biotechnology industry and other factors set forth in the Company’s filings with the Securities and Exchange Commission. Most of these factors are outside the control of the Company. Investors are cautioned not to put undue reliance on forward-looking statements. Except as otherwise required by applicable securities statutes or regulations, the Company disclaims any intent or obligation to update publicly these forward-looking statements, whether as a result of new information, future events or otherwise.
CONTACT:
Dr. James Musick
Chief Executive Officer
Vitro BioPharma
(303) 999-2130 Ext. 1
E-mail: jim@vitrobiopharma.com
Source: Vitro Diagnostics, Inc.
www.vitrobiopharma.com
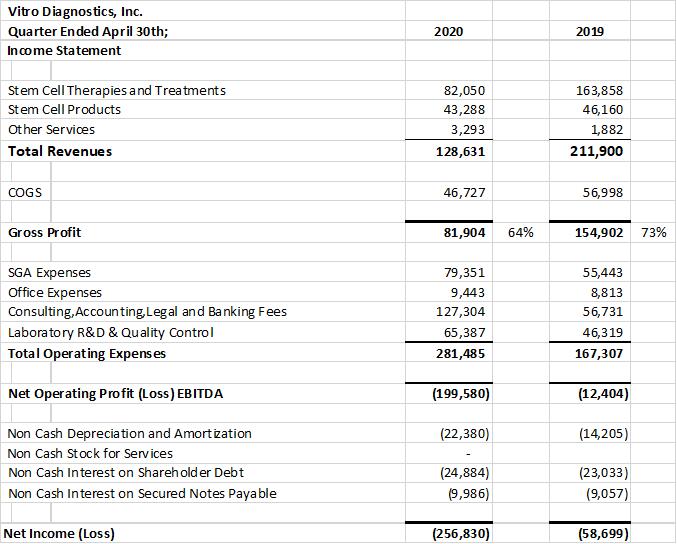
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
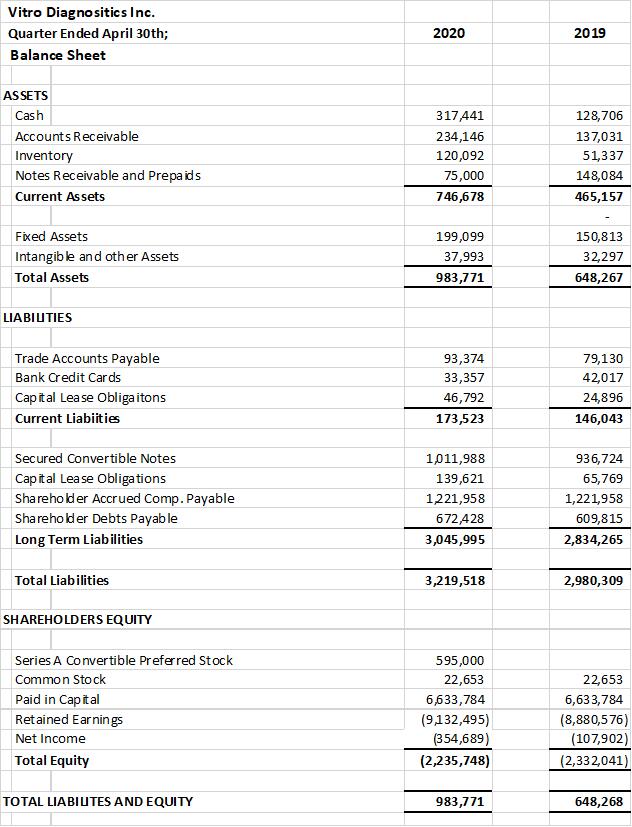
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant
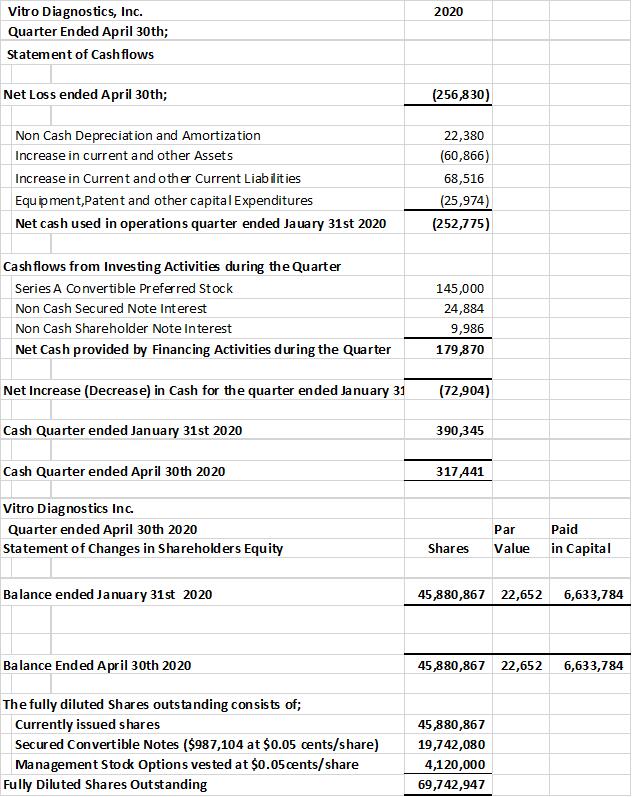
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
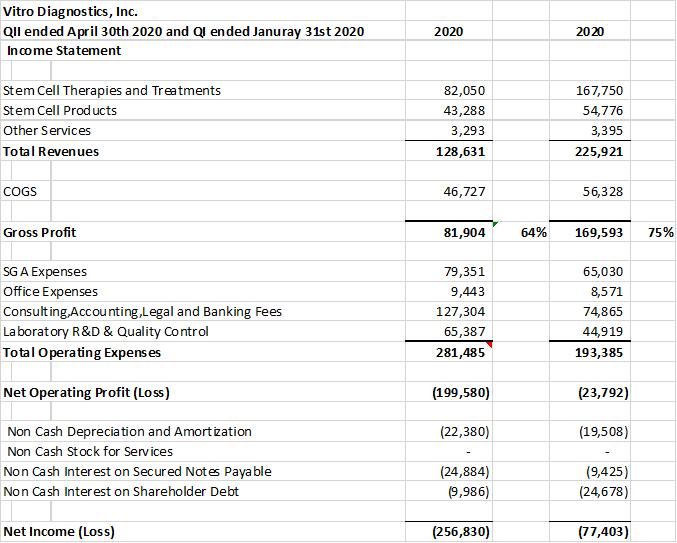
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
