Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Kiniksa Pharmaceuticals, Ltd. | tm2023613d3_ex99-1.htm |
| 8-K - FORM 8-K - Kiniksa Pharmaceuticals, Ltd. | tm2023613d3_8k.htm |

Exhibit 99.2Every Second Counts!™RHAPSODY Top-Line Results June 2020

WelcomeMark Ragosa VP of Investor Relations and FinanceEvery Second Counts!TM2

Forward Looking StatementsThis presentation (together with any other statements or information that we may make in connection herewith) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 with respect to Kiniksa Pharmaceuticals, Ltd. (and its consolidated subsidiaries, collectively, unless context otherwise requires, “Kiniksa,” “we,” “us” or “our”). In some cases, you can identify forward looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “goal,” “design,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these identifying words. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation, statements regarding our strategy; potential acquisitions and collaborations; potential value drivers; potential indications; potential market opportunities and competitive position; on going, planned and potential clinical trials and other studies; timing and potential impact of clinical data; regulatory and other submissions, applications and approvals; commercial strategy and pre-commercial activities; expected run rate for our cash, cash equivalents and short-term investments; expected funding of our operating plan; and capital allocation.These statements involve known and unknown risks, uncertainties, and other important factors that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements, including without limitation potential delays or difficulties with our clinical trials; potential inability to demonstrate safety or efficacy or otherwise producing negative, inconclusive or uncompetitive results; potential for changes in final data from preliminary or interim data; potential inability to replicate in later clinical trials positive results from earlier trials and studies; our reliance on third parties for manufacturing and conducting clinical trials, research and other studies; potential changes in our strategy, operating plan and funding requirements; drug substance and/or drug product shortages; substantial new or existing competition; potential impact of the COVID-19 pandemic, and measures taken in response to the pandemic, on our business and operations as well as the business and operations of our manufacturers, CROs upon whom we rely to conduct our clinical trials, and other third parties with whom we conduct business or otherwise engage, including the FDA and other regulatory authorities; and our ability to attract and retain qualified personnel. These and the other important factors are discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (the “SEC”) on May 4, 2020 and other filings subsequently filed with the SEC. These forward-looking statements reflect various assumptions of Kiniksa's management that may or may not prove to be correct. No forward- looking statement is a guarantee of future results, performance, or achievements, and one should avoid placing undue reliance on such statements. Except as otherwise indicated, this presentation speaks as of the date of this presentation. We undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.This presentation also contains estimates, projections, and/or other information regarding our industry, our business and the markets for certain of our product candidates, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, clinical trials, studies and similar data prepared by market research firms and other third parties, from industry, medical and general publications, and from government data and similar sources. Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information.3

IntroductionSanj K. Patel CEO and Chairman of the BoardEvery Second Counts!TM4
Summary of Pivotal Phase 3 Trial of Rilonacept in Recurrent Pericarditis• Prespecified primary and all major secondary efficacy endpoints were highly statistically significant.• The primary efficacy endpoint of median time-to-first adjudicated pericarditis recurrence in the randomized withdrawal period was highly statistically significant.o Median [95% CI] time to pericarditis recurrence for rilonacept recipients in the randomized withdrawal period could not be estimated due to the low number of recurrences in the rilonacept treatment arm. The median time-to-recurrence for placebo recipients was 8.6 [4.0-11.7] weeks (Hazard Ratio = 0.04, p<0.0001).o Rilonacept recipients experienced a 96% reduction in risk of recurrent pericarditis events.• All major secondary efficacy endpoints in the randomized withdrawal period were also highly statistically significant.o 81% of rilonacept recipients maintained clinical response at Week 16 of the randomized withdrawal period, compared to 20% of placebo recipients (p=0.0002). Consistent results were observed at Week 8 and Week 24 and were also highly statistically significant (p<0.0001 and p=0.0022, respectively).o The proportion of rilonacept recipients with absent or minimal pericarditis symptoms at Week 16 of the randomized withdrawal period was 81% compared to 25% for placebo recipients (p=0.0006). Consistent results were observed at Week 8 and Week 24 and were also highly statistically significant (p<0.0001 and p=0.0002, respectively).o Rilonacept recipients experienced no or minimal pain for 95% of trial days through Week 16 compared to 47% of trial days for placebo recipients (p<0.0001). Consistent results were observed at Weeks 8 and 24 and were also highly significant (p<0.0001 and p<0.0001, respectively).• Patients are continuing to receive open label rilonacept in the Long-Term Extension.• Rilonacept was well-tolerated, with a safety profile consistent with the existing ARCALYST® label.• Based on the Phase 3 RHAPSODY data, Kiniksa plans to submit an sBLA with the FDA later this year. 5

RHAPSODY Top-Line ResultsJohn F. Paolini Chief Medical OfficerEvery Second Counts!TM6
RilonaceptIndication1 Recurrent Pericarditis: Painful and debilitating autoinflammatory cardiovascular diseaseMechanism of Action2IL-1α and IL-1β cytokine trapScientific Rationale2 IL-1α and IL-1β are cytokines shown to play key role in inflammatory diseasesPrevalence3~40k prevalent in U.S.; addressable opportunity of ~14k in U.S.Competition4StatusNo FDA-approved therapies for recurrent pericarditisBreakthrough Therapy designation granted in December 2019; pivotal Phase 3 data reported in June 2020Economics Regulatory milestones; 50/50 profit split upon commercialization excluding certain expensesRightsBLA transfers to Kiniksa after receipt of positive Phase 3 clinical data and an acceptable safety profile; upon approval Kiniksa has the rights to recurrent pericarditis worldwide (excluding MENA)1) Rilonacept (ARCALYST®) is approved and marketed for cryopyrin-associated periodic syndrome (CAPS), in the United States by Regeneron Pharmaceuticals, Inc.; 2) Brucato et al. JAMA. 2016, 316 (18): 1906-1912; Arcalyst Prescribing Information; 3) IQVIA PharMetrics Plus Claims Data 1/1/2013-3/31/2018; ClearView Analysis, UptoDate, Trinity Partners, Mayo Clin Proc. 2010 ;85 (6): 572-593; New Diagnostic Criteria for Acute Pericarditis: A Cardiac MRI Perspective, 2015 American College of Cardiology; 4) Drugs@FDA: Arcalyst Prescribing Information, Ilaris Prescribing Information, Kineret Prescribing Information; Kaiser et al. Rheumatol Int (2012) 32:295–299; Theodoropoulou et al. Pediatric Rheumatology 2015, 13(Suppl 1):P155 ; Fleischmann et al, 2017 ACR/ARHP Abstract 1196; Kosloski et al, J of Clin Pharm 2016, 56 (12) 1582-1590; Cohen et al. Arthritis Research & Therapy 2011, 13:R125; Cardiel et al. Arthritis Research & Therapy 2010, 12:R192; Hong et al. Lancet Oncol 2014, 15: 656-666;
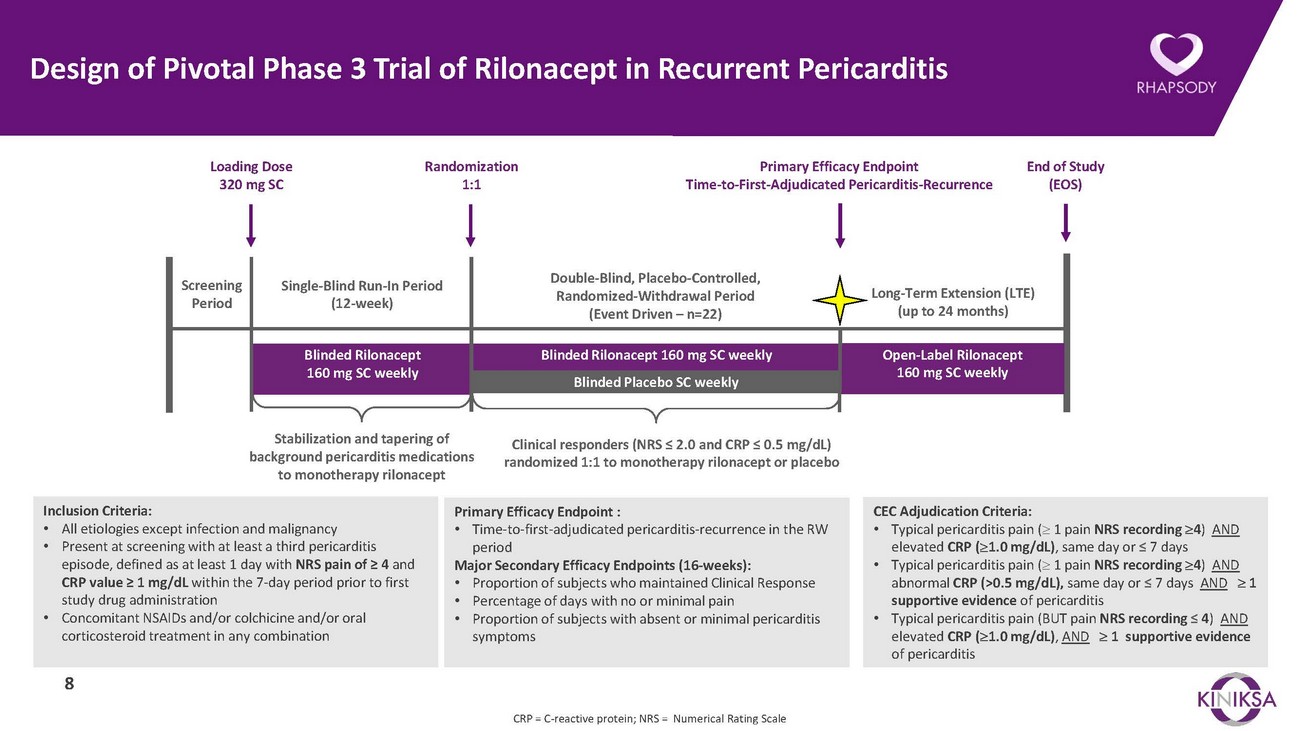
Design of Pivotal Phase 3 Trial of Rilonacept in Recurrent PericarditisLoading Dose 320 mg SCRandomization 1:1Primary Efficacy Endpoint Time-to-First-Adjudicated Pericarditis-RecurrenceEnd of Study (EOS)Screening PeriodSingle-Blind Run-In Period (12-week)Double-Blind, Placebo-Controlled, Randomized-Withdrawal Period (Event Driven – n=22)Long-Term Extension (LTE) (up to 24 months)Blinded Rilonacept 160 mg SC weeklyBlinded Rilonacept 160 mg SC weeklyBlinded Placebo SC weeklyOpen-Label Rilonacept 160 mg SC weeklyStabilization and tapering of background pericarditis medications to monotherapy rilonaceptClinical responders (NRS ≤ 2.0 and CRP ≤ 0.5 mg/dL) randomized 1:1 to monotherapy rilonacept or placeboInclusion Criteria: • All etiologies except infection and malignancy • Present at screening with at least a third pericarditis episode, defined as at least 1 day with NRS pain of ≥ 4 and CRP value ≥ 1 mg/dL within the 7-day period prior to first study drug administration • Concomitant NSAIDs and/or colchicine and/or oral corticosteroid treatment in any combination8Primary Efficacy Endpoint : • Time-to-first-adjudicated pericarditis-recurrence in the RW period Major Secondary Efficacy Endpoints (16-weeks): • Proportion of subjects who maintained Clinical Response • Percentage of days with no or minimal pain • Proportion of subjects with absent or minimal pericarditis symptomsCEC Adjudication Criteria: • Typical pericarditis pain (≥ 1 pain NRS recording ≥4) AND elevated CRP (≥1.0 mg/dL), same day or ≤ 7 days • Typical pericarditis pain (≥ 1 pain NRS recording ≥4) AND abnormal CRP (>0.5 mg/dL), same day or ≤ 7 days AND ≥ 1 supportive evidence of pericarditis • Typical pericarditis pain (BUT pain NRS recording ≤ 4) AND elevated CRP (≥1.0 mg/dL), AND ≥ 1 supportive evidence of pericarditisCRP = C-reactive protein; NRS = Numerical Rating Scale
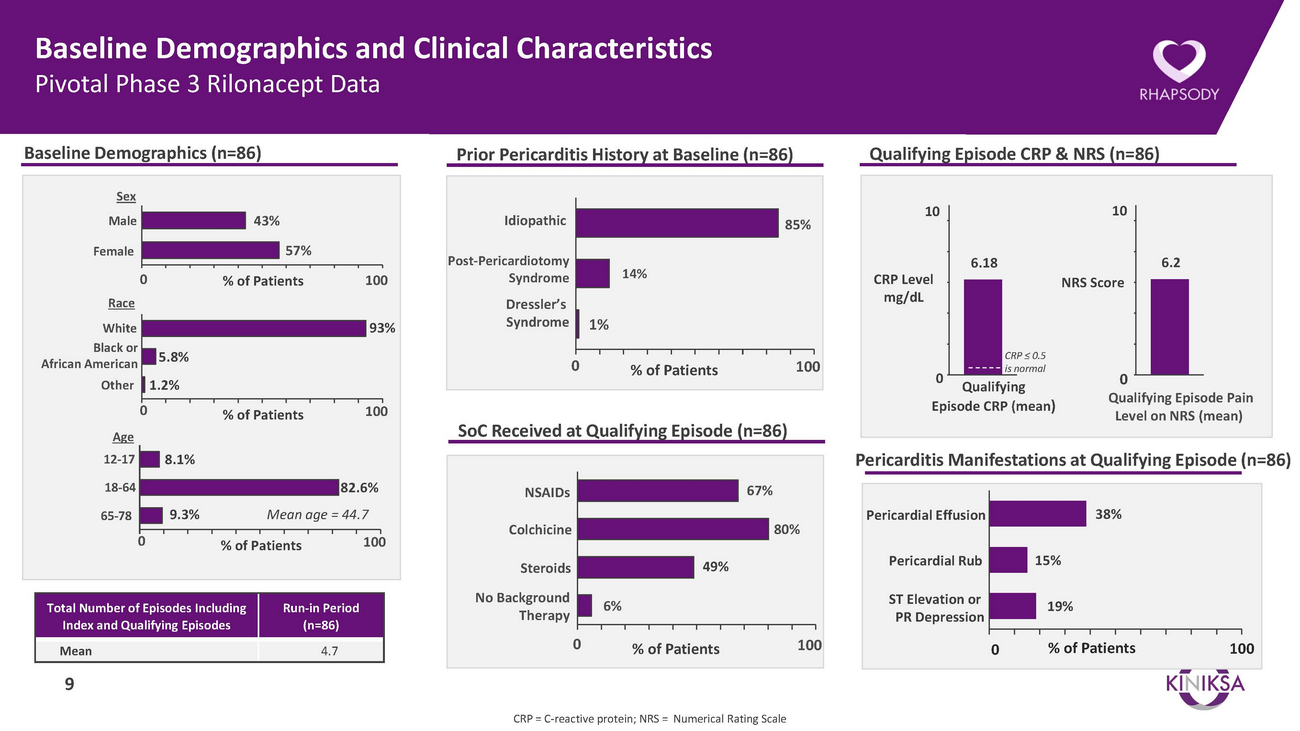
Baseline Demographics and Clinical Characteristics Pivotal Phase 3 Rilonacept DataBaseline Demographics (n=86)Prior Pericarditis History at Baseline (n=86)Qualifying Episode CRP & NRS (n=86)SexMaleFemale43%57%IdiopathicPost-Pericardiotomy85%106.18106.20 RaceWhite Black or African American5.8%% of Patients 10093%SyndromeDressler’s Syndrome 1%014%% of Patients 100CRP Level mg/dL0CRP ≤ 0.5 is normalNRS Score0OtherAge1.2%0% of Patients 100SoC Received at Qualifying Episode (n=86)9 Qualifying Episode CRP (mean)Qualifying Episode Pain Level on NRS (mean)12-1718-648.1%82.6%NSAIDs67%Pericarditis Manifestations at Qualifying Episode (n=86)65-7809.3%Mean age = 44.7% of Patients 100ColchicineSteroids49%80%Pericardial EffusionPericardial Rub15%38%Total Number of Episodes Including Index and Qualifying EpisodesRun-in Period (n=86)No Background 6% TherapyST Elevation or PR Depression19%Mean 4.790 % of Patients 1000 % of Patients100CRP = C-reactive protein; NRS = Numerical Rating Scale
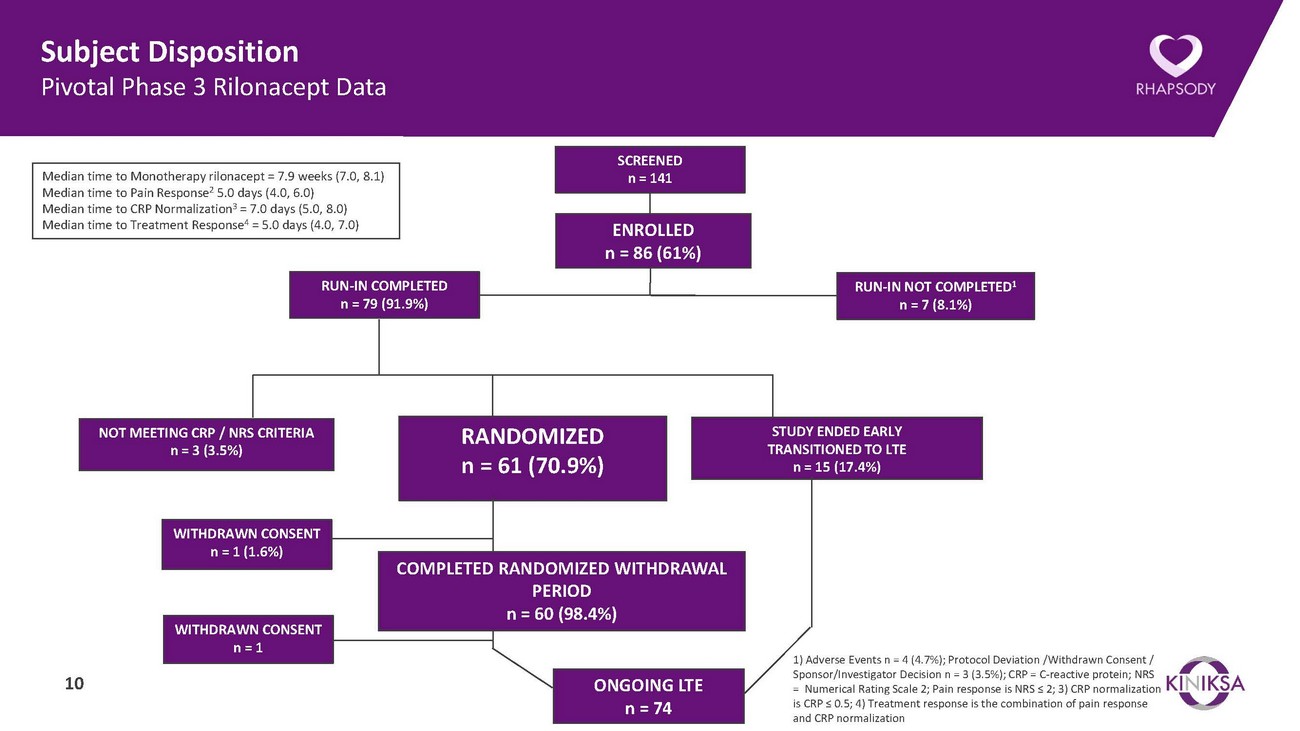
Subject Disposition Pivotal Phase 3 Rilonacept DataMedian time to Monotherapy rilonacept = 7.9 weeks (7.0, 8.1) Median time to Pain Response2 5.0 days (4.0, 6.0) Median time to CRP Normalization3 = 7.0 days (5.0, 8.0) Median time to Treatment Response4 = 5.0 days (4.0, 7.0)RUN-IN COMPLETED n = 79 (91.9%)SCREENED n = 141ENROLLED n = 86 (61%)RUN-IN NOT COMPLETED1 n = 7 (8.1%)NOT MEETING CRP / NRS CRITERIA n = 3 (3.5%)RANDOMIZED n = 61 (70.9%)STUDY ENDED EARLY TRANSITIONED TO LTE n = 15 (17.4%)WITHDRAWN CONSENT n = 1 (1.6%)WITHDRAWN CONSENT n = 110COMPLETED RANDOMIZED WITHDRAWAL PERIOD n = 60 (98.4%)ONGOING LTE n = 741) Adverse Events n = 4 (4.7%); Protocol Deviation /Withdrawn Consent / Sponsor/Investigator Decision n = 3 (3.5%); CRP = C-reactive protein; NRS = Numerical Rating Scale 2; Pain response is NRS ≤ 2; 3) CRP normalization is CRP ≤ 0.5; 4) Treatment response is the combination of pain response and CRP normalization
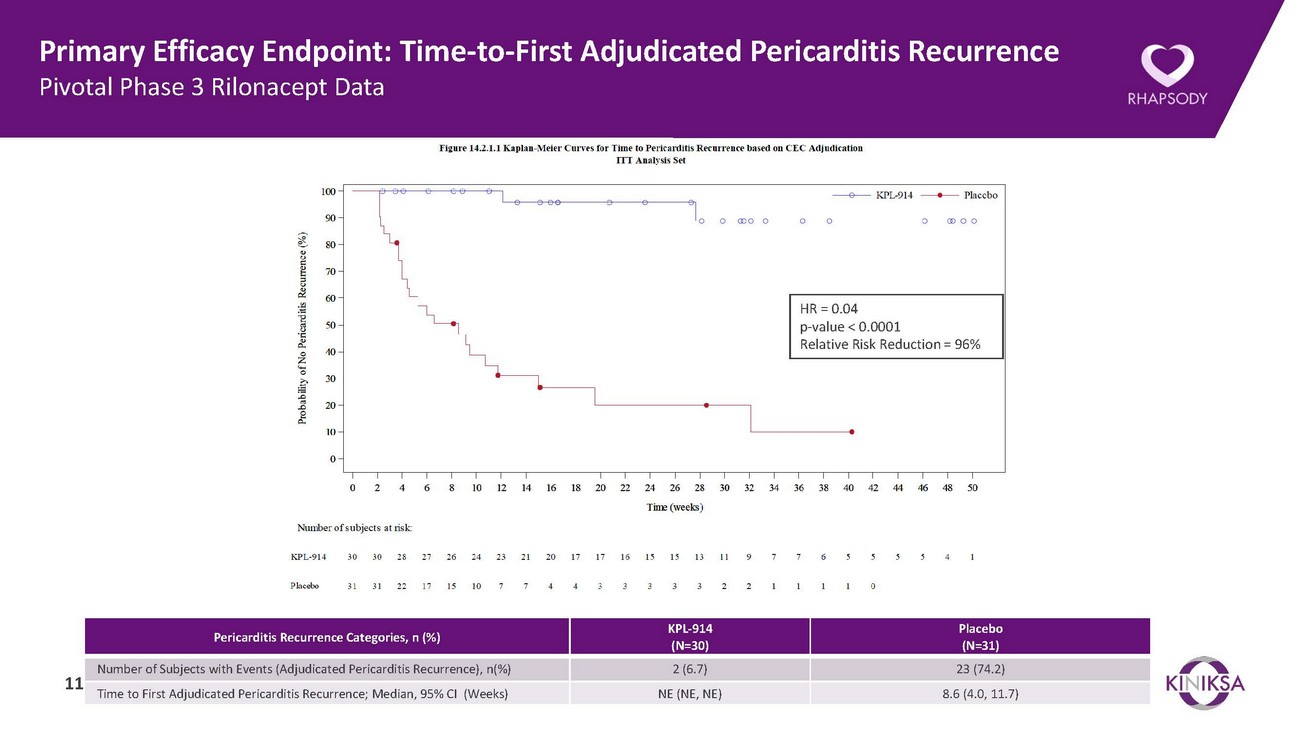
Primary Efficacy Endpoint: Time-to-First Adjudicated Pericarditis Recurrence Pivotal Phase 3 Rilonacept DataHR = 0.04 p-value < 0.0001 Relative Risk Reduction = 96%Pericarditis Recurrence Categories, n (%) KPL-914 (N=30)Placebo (N=31)Number of Subjects with Events (Adjudicated Pericarditis Recurrence), n(%) 2 (6.7) 23 (74.2) 11 Time to First Adjudicated Pericarditis Recurrence; Median, 95% CI (Weeks) NE (NE, NE) 8.6 (4.0, 11.7)
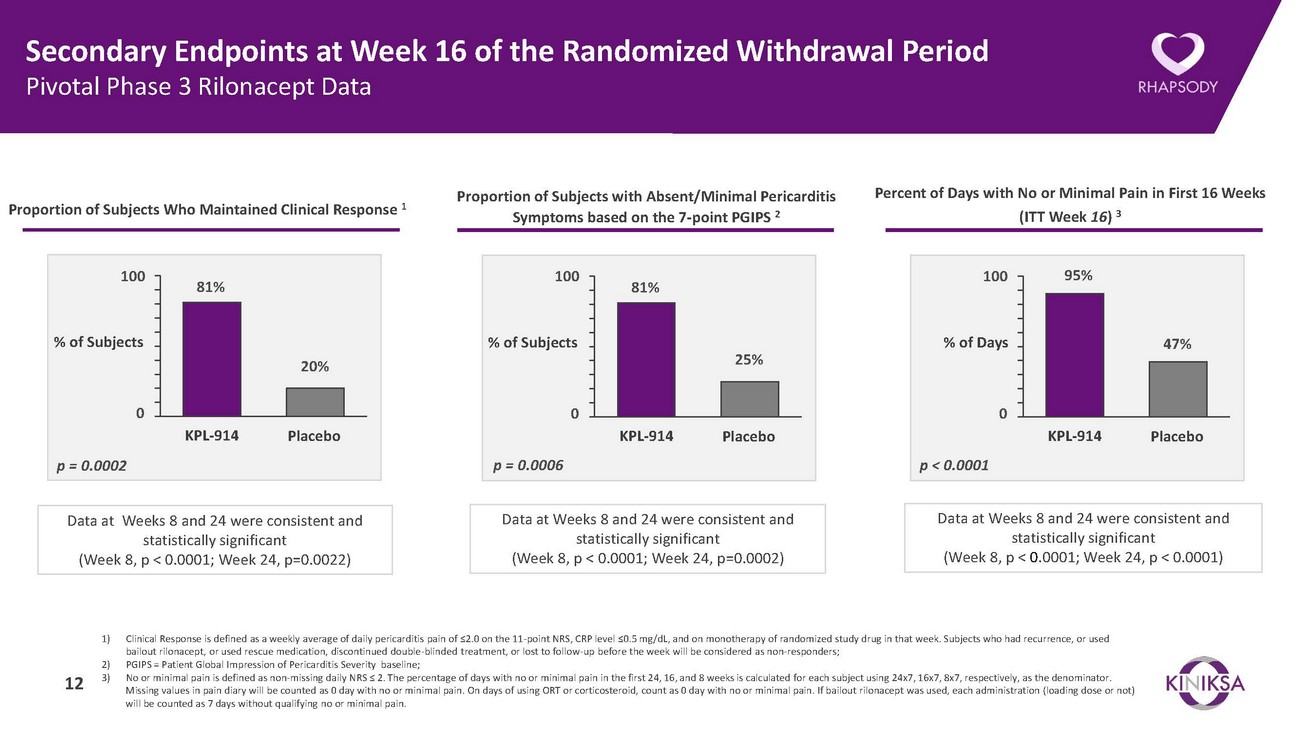
Secondary Endpoints at Week 16 of the Randomized Withdrawal Period Pivotal Phase 3 Rilonacept DataProportion of Subjects Who Maintained Clinical Response 1Proportion of Subjects with Absent/Minimal Pericarditis Symptoms based on the 7-point PGIPS 2Percent of Days with No or Minimal Pain in First 16 Weeks (ITT Week 16) 310081%10081%10095%% of Subjects20%% of Subjects25%% of Days47%0 KPL-914 Placebo0 KPL-914 Placebo0 KPL-914 Placebop = 0.0002 p = 0.0006 p < 0.0001Data at Weeks 8 and 24 were consistent and statistically significant (Week 8, p < 0.0001; Week 24, p=0.0022)Data at Weeks 8 and 24 were consistent and statistically significant (Week 8, p < 0.0001; Week 24, p=0.0002)Data at Weeks 8 and 24 were consistent and statistically significant (Week 8, p < 0.0001; Week 24, p < 0.0001)1) Clinical Response is defined as a weekly average of daily pericarditis pain of ≤2.0 on the 11-point NRS, CRP level ≤0.5 mg/dL, and on monotherapy of randomized study drug in that week. Subjects who had recurrence, or used bailout rilonacept, or used rescue medication, discontinued double-blinded treatment, or lost to follow-up before the week will be considered as non-responders; 2) PGIPS = Patient Global Impression of Pericarditis Severity baseline; 3) No or minimal pain is defined as non-missing daily NRS ≤ 2. The percentage of days with no or minimal pain in the first 24, 16, and 8 weeks is calculated for each subject using 24x7, 16x7, 8x7, respectively, as the denominator. Missing values in pain diary will be counted as 0 day with no or minimal pain. On days of using ORT or corticosteroid, count as 0 day with no or minimal pain. If bailout rilonacept was used, each administration (loading dose or not) will be counted as 7 days without qualifying no or minimal pain.
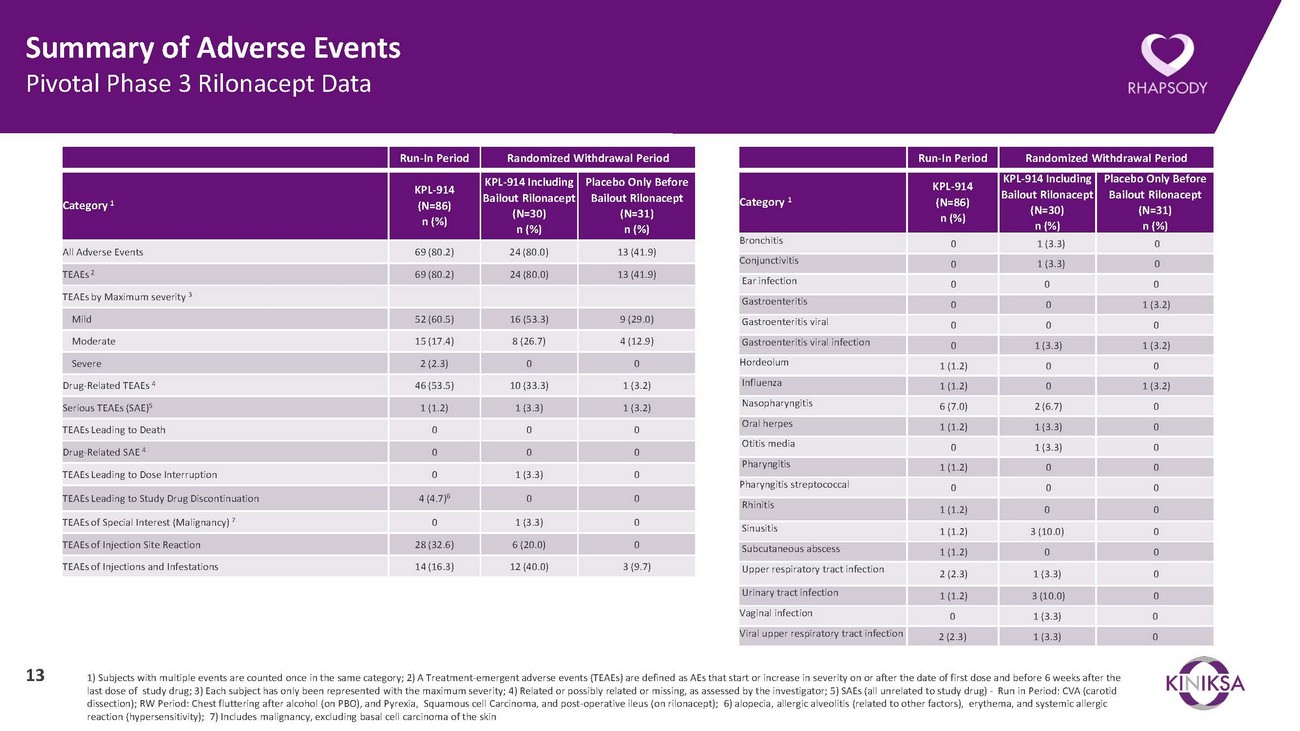
Summary of Adverse Events Pivotal Phase 3 Rilonacept DataRun-In Period Randomized Withdrawal PeriodRun-In Period Randomized Withdrawal PeriodCategory 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Category 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)All Adverse Events 69 (80.2) 24 (80.0) 13 (41.9) TEAEs 269 (80.2) 24 (80.0) 13 (41.9)Bronchitis 0 1 (3.3) 0 Conjunctivitis 0 1 (3.3) 0 Ear infectionTEAEs by Maximum severity 3Gastroenteritis0 0 00 0 1 (3.2)Mild 52 (60.5) 16 (53.3) 9 (29.0) Moderate 15 (17.4) 8 (26.7) 4 (12.9) Severe 2 (2.3) 0 0 Drug-Related TEAEs 4 46 (53.5) 10 (33.3) 1 (3.2) Serious TEAEs (SAE)51 (1.2) 1 (3.3) 1 (3.2) TEAEs Leading to Death 0 0 0 Drug-Related SAE 40 0 0TEAEs Leading to Dose Interruption 0 1 (3.3) 0TEAEs Leading to Study Drug Discontinuation 4 (4.7)6 0 0Gastroenteritis viral 0 0 0 Gastroenteritis viral infection 0 1 (3.3) 1 (3.2) Hordeolum 1 (1.2) 0 0 Influenza 1 (1.2) 0 1 (3.2) Nasopharyngitis 6 (7.0) 2 (6.7) 0 Oral herpes 1 (1.2) 1 (3.3) 0 Otitis media 0 1 (3.3) 0 Pharyngitis 1 (1.2) 0 0 Pharyngitis streptococcal 0 0 0 RhinitisTEAEs of Special Interest (Malignancy) 7 0 1 (3.3) 0Sinusitis1 (1.2) 0 01 (1.2) 3 (10.0) 0TEAEs of Injection Site Reaction 28 (32.6) 6 (20.0) 0TEAEs of Injections and Infestations 14 (16.3) 12 (40.0) 3 (9.7)Subcutaneous abscess 1 (1.2) 0 0 Upper respiratory tract infection 2 (2.3) 1 (3.3) 0 Urinary tract infection 1 (1.2) 3 (10.0) 0 Vaginal infection 0 1 (3.3) 0 Viral upper respiratory tract infection 2 (2.3) 1 (3.3) 013 1) Subjects with multiple events are counted once in the same category; 2) A Treatment-emergent adverse events (TEAEs) are defined as AEs that start or increase in severity on or after the date of first dose and before 6 weeks after the last dose of study drug; 3) Each subject has only been represented with the maximum severity; 4) Related or possibly related or missing, as assessed by the investigator; 5) SAEs (all unrelated to study drug) - Run in Period: CVA (carotid dissection); RW Period: Chest fluttering after alcohol (on PBO), and Pyrexia, Squamous cell Carcinoma, and post-operative ileus (on rilonacept); 6) alopecia, allergic alveolitis (related to other factors), erythema, and systemic allergic reaction (hypersensitivity); 7) Includes malignancy, excluding basal cell carcinoma of the skin
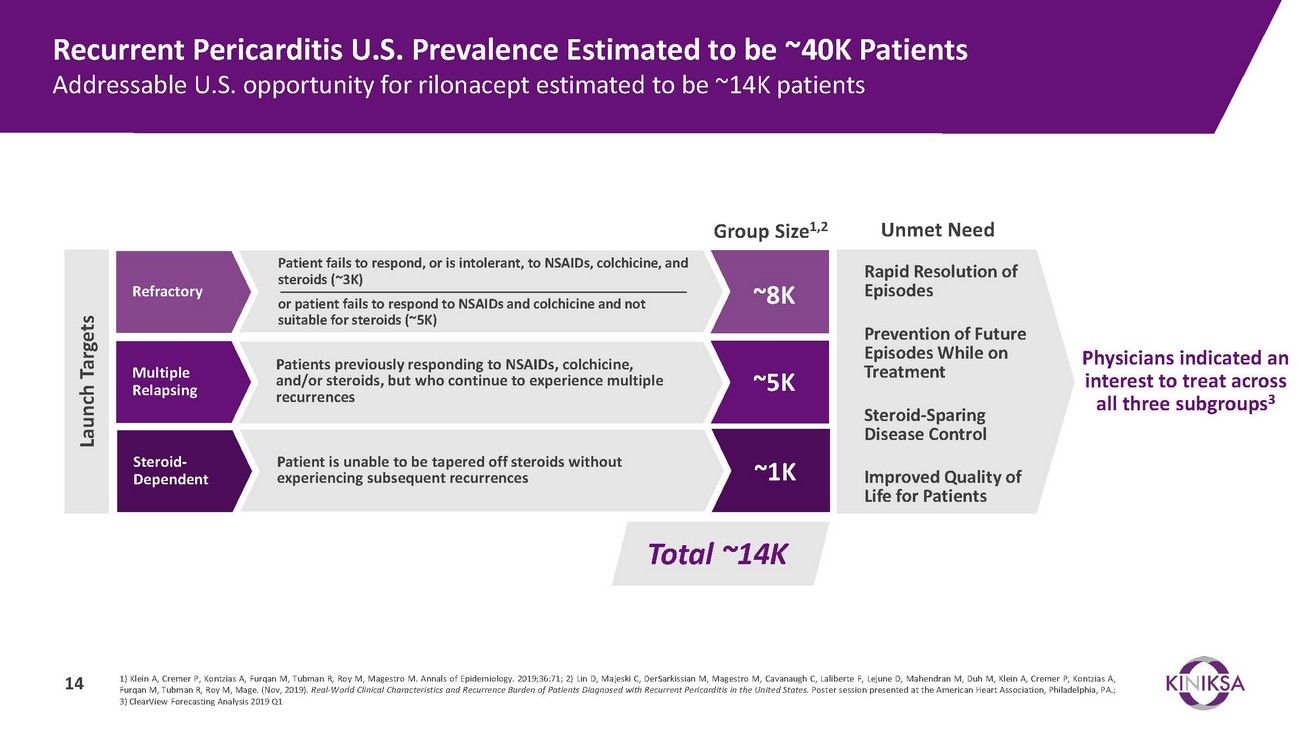
Recurrent Pericarditis U.S. Prevalence Estimated to be ~40K Patients Addressable U.S. opportunity for rilonacept estimated to be ~14K patientsRefractoryMultiple RelapsingSteroid- DependentPatient fails to respond, or is intolerant, to NSAIDs, colchicine, and steroids (~3K)or patient fails to respond to NSAIDs and colchicine and not suitable for steroids (~5K)Patients previously responding to NSAIDs, colchicine, and/or steroids, but who continue to experience multiple recurrencesPatient is unable to be tapered off steroids without experiencing subsequent recurrencesGroup Size1,2~8K~5K~1KUnmet NeedRapid Resolution of EpisodesPrevention of Future Episodes While on TreatmentSteroid-Sparing Disease ControlImproved Quality of Life for PatientsPhysicians indicated an interest to treat across all three subgroups3Total ~14K1) Klein A, Cremer P, Kontzias A, Furqan M, Tubman R, Roy M, Magestro M. Annals of Epidemiology. 2019;36:71; 2) Lin D, Majeski C, DerSarkissian M, Magestro M, Cavanaugh C, Laliberte F, Lejune D, Mahendran M, Duh M, Klein A, Cremer P, Kontzias A, Furqan M, Tubman R, Roy M, Mage. (Nov, 2019). Real-World Clinical Characteristics and Recurrence Burden of Patients Diagnosed with Recurrent Pericarditis in the United States. Poster session presented at the American Heart Association, Philadelphia, PA.; 3) ClearView Forecasting Analysis 2019 Q1
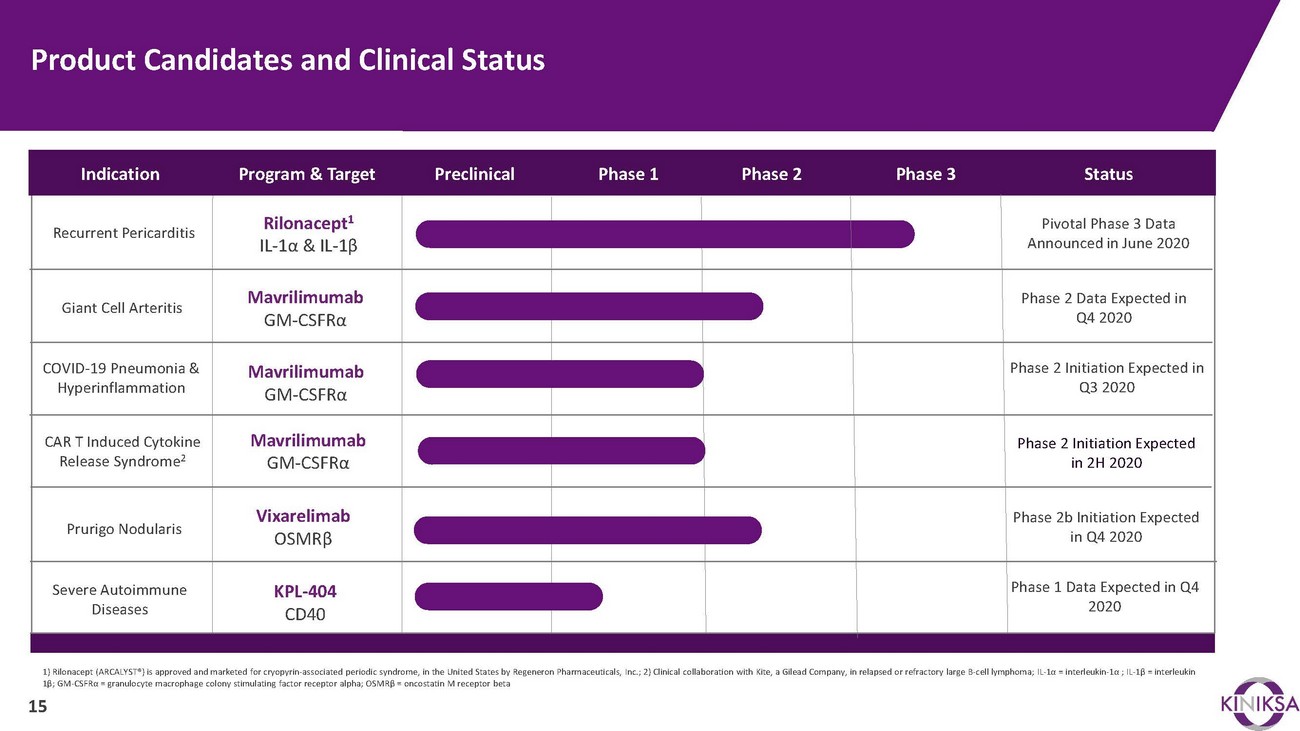
Product Candidates and Clinical StatusIndicationRecurrent PericarditisGiant Cell ArteritisProgram & TargetRilonacept1 IL-1α & IL-1βMavrilimumab GM-CSFRαPreclinicalPhase 1 Phase 2 Phase 3StatusPivotal Phase 3 Data Announced in June 2020Phase 2 Data Expected in Q4 2020COVID-19 Pneumonia & HyperinflammationMavrilimumab GM-CSFRαPhase 2 Initiation Expected in Q3 2020CAR T Induced Cytokine Release Syndrome2Mavrilimumab GM-CSFRαPhase 2 Initiation Expected in 2H 2020Prurigo NodularisSevere Autoimmune DiseasesVixarelimab OSMRβKPL-404 CD40Phase 2b Initiation Expected in Q4 2020Phase 1 Data Expected in Q4 20201) Rilonacept (ARCALYST®) is approved and marketed for cryopyrin-associated periodic syndrome, in the United States by Regeneron Pharmaceuticals, Inc.; 2) Clinical collaboration with Kite, a Gilead Company, in relapsed or refractory large B-cell lymphoma; IL-1α = interleukin-1α ; IL-1β = interleukin 1β; GM-CSFRα = granulocyte macrophage colony stimulating factor receptor alpha; OSMRβ = oncostatin M receptor beta 15

Every Second Counts!™

AppendixEvery Second Counts!TM17
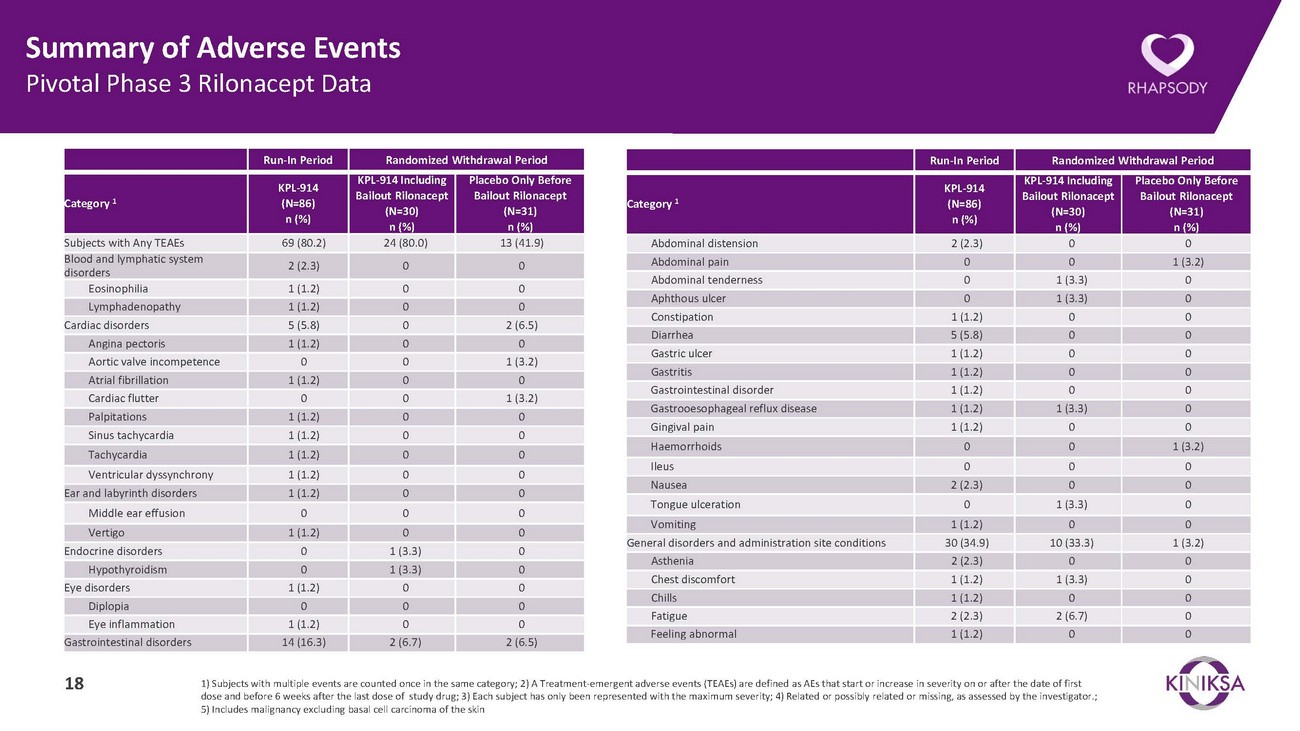
Summary of Adverse Events Pivotal Phase 3 Rilonacept DataRun-In Period Randomized Withdrawal PeriodRun-In Period Randomized Withdrawal PeriodCategory 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Category 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Subjects with Any TEAEs 69 (80.2) 24 (80.0) 13 (41.9) Blood and lymphatic system disorders 2 (2.3) 0 0 Eosinophilia 1 (1.2) 0 0 Lymphadenopathy 1 (1.2) 0 0 Cardiac disorders 5 (5.8) 0 2 (6.5) Angina pectoris 1 (1.2) 0 0 Aortic valve incompetence 0 0 1 (3.2) Atrial fibrillation 1 (1.2) 0 0 Cardiac flutter 0 0 1 (3.2) Palpitations 1 (1.2) 0 0 Sinus tachycardia 1 (1.2) 0 0 Tachycardia 1 (1.2) 0 0 Ventricular dyssynchrony 1 (1.2) 0 0 Ear and labyrinth disorders 1 (1.2) 0 0 Middle ear effusion 0 0 0 Vertigo 1 (1.2) 0 0 Endocrine disorders 0 1 (3.3) 0 Hypothyroidism 0 1 (3.3) 0 Eye disorders 1 (1.2) 0 0 Diplopia 0 0 0 Eye inflammation 1 (1.2) 0 0 Gastrointestinal disorders 14 (16.3) 2 (6.7) 2 (6.5)Abdominal distension 2 (2.3) 0 0 Abdominal pain 0 0 1 (3.2) Abdominal tenderness 0 1 (3.3) 0 Aphthous ulcer 0 1 (3.3) 0 Constipation 1 (1.2) 0 0 Diarrhea 5 (5.8) 0 0 Gastric ulcer 1 (1.2) 0 0 Gastritis 1 (1.2) 0 0 Gastrointestinal disorder 1 (1.2) 0 0 Gastrooesophageal reflux disease 1 (1.2) 1 (3.3) 0 Gingival pain 1 (1.2) 0 0 Haemorrhoids 0 0 1 (3.2) Ileus 0 0 0 Nausea 2 (2.3) 0 0 Tongue ulceration 0 1 (3.3) 0 Vomiting 1 (1.2) 0 0 General disorders and administration site conditions 30 (34.9) 10 (33.3) 1 (3.2) Asthenia 2 (2.3) 0 0 Chest discomfort 1 (1.2) 1 (3.3) 0 Chills 1 (1.2) 0 0 Fatigue 2 (2.3) 2 (6.7) 0 Feeling abnormal 1 (1.2) 0 018 1) Subjects with multiple events are counted once in the same category; 2) A Treatment-emergent adverse events (TEAEs) are defined as AEs that start or increase in severity on or after the date of first dose and before 6 weeks after the last dose of study drug; 3) Each subject has only been represented with the maximum severity; 4) Related or possibly related or missing, as assessed by the investigator.; 5) Includes malignancy excluding basal cell carcinoma of the skin
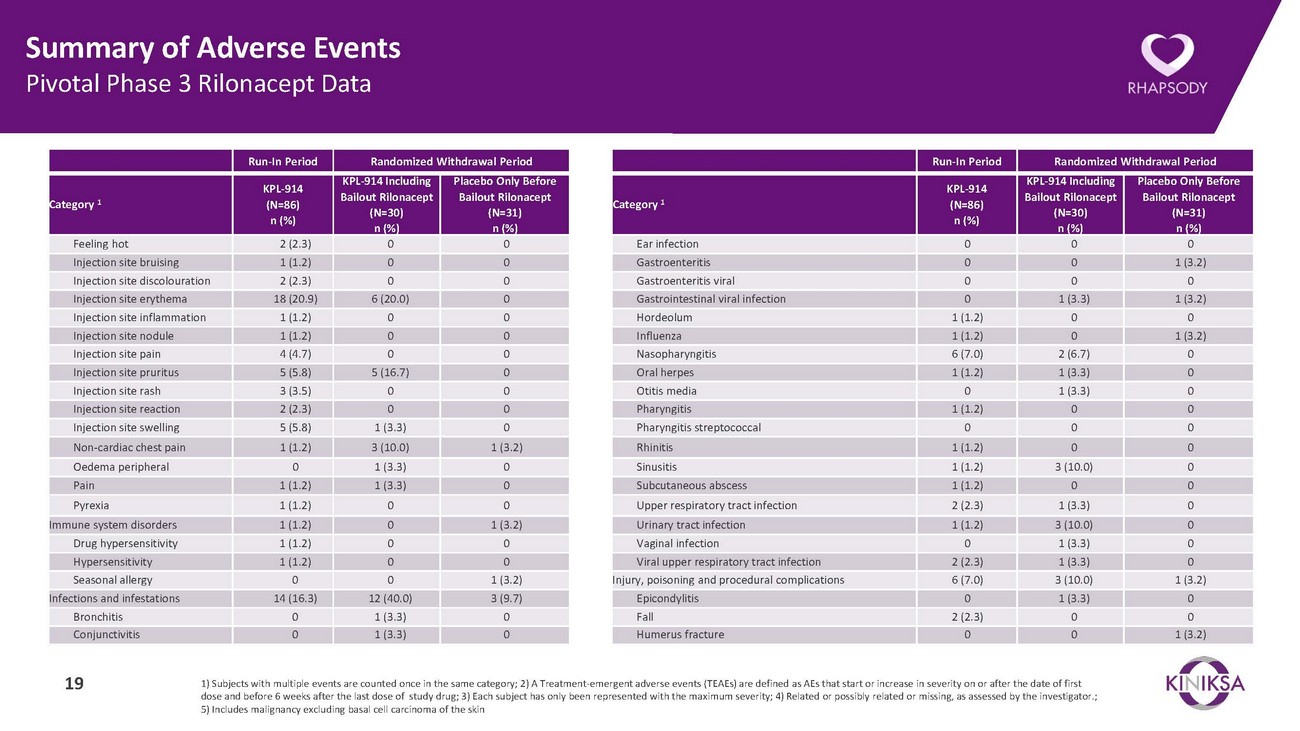
Summary of Adverse Events Pivotal Phase 3 Rilonacept DataRun-In Period Randomized Withdrawal PeriodRun-In Period Randomized Withdrawal PeriodCategory 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Category 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Feeling hot 2 (2.3) 0 0 Injection site bruising 1 (1.2) 0 0 Injection site discolouration 2 (2.3) 0 0 Injection site erythema 18 (20.9) 6 (20.0) 0 Injection site inflammation 1 (1.2) 0 0 Injection site nodule 1 (1.2) 0 0 Injection site pain 4 (4.7) 0 0 Injection site pruritus 5 (5.8) 5 (16.7) 0 Injection site rash 3 (3.5) 0 0 Injection site reaction 2 (2.3) 0 0 Injection site swelling 5 (5.8) 1 (3.3) 0 Non-cardiac chest pain 1 (1.2) 3 (10.0) 1 (3.2) Oedema peripheral 0 1 (3.3) 0 Pain 1 (1.2) 1 (3.3) 0 Pyrexia 1 (1.2) 0 0 Immune system disorders 1 (1.2) 0 1 (3.2) Drug hypersensitivity 1 (1.2) 0 0 Hypersensitivity 1 (1.2) 0 0 Seasonal allergy 0 0 1 (3.2) Infections and infestations 14 (16.3) 12 (40.0) 3 (9.7) Bronchitis 0 1 (3.3) 0 Conjunctivitis 0 1 (3.3) 0Ear infection 0 0 0 Gastroenteritis 0 0 1 (3.2) Gastroenteritis viral 0 0 0 Gastrointestinal viral infection 0 1 (3.3) 1 (3.2) Hordeolum 1 (1.2) 0 0 Influenza 1 (1.2) 0 1 (3.2) Nasopharyngitis 6 (7.0) 2 (6.7) 0 Oral herpes 1 (1.2) 1 (3.3) 0 Otitis media 0 1 (3.3) 0 Pharyngitis 1 (1.2) 0 0 Pharyngitis streptococcal 0 0 0 Rhinitis 1 (1.2) 0 0 Sinusitis 1 (1.2) 3 (10.0) 0 Subcutaneous abscess 1 (1.2) 0 0 Upper respiratory tract infection 2 (2.3) 1 (3.3) 0 Urinary tract infection 1 (1.2) 3 (10.0) 0 Vaginal infection 0 1 (3.3) 0 Viral upper respiratory tract infection 2 (2.3) 1 (3.3) 0 Injury, poisoning and procedural complications 6 (7.0) 3 (10.0) 1 (3.2) Epicondylitis 0 1 (3.3) 0 Fall 2 (2.3) 0 0 Humerus fracture 0 0 1 (3.2)19 1) Subjects with multiple events are counted once in the same category; 2) A Treatment-emergent adverse events (TEAEs) are defined as AEs that start or increase in severity on or after the date of first dose and before 6 weeks after the last dose of study drug; 3) Each subject has only been represented with the maximum severity; 4) Related or possibly related or missing, as assessed by the investigator.; 5) Includes malignancy excluding basal cell carcinoma of the skin
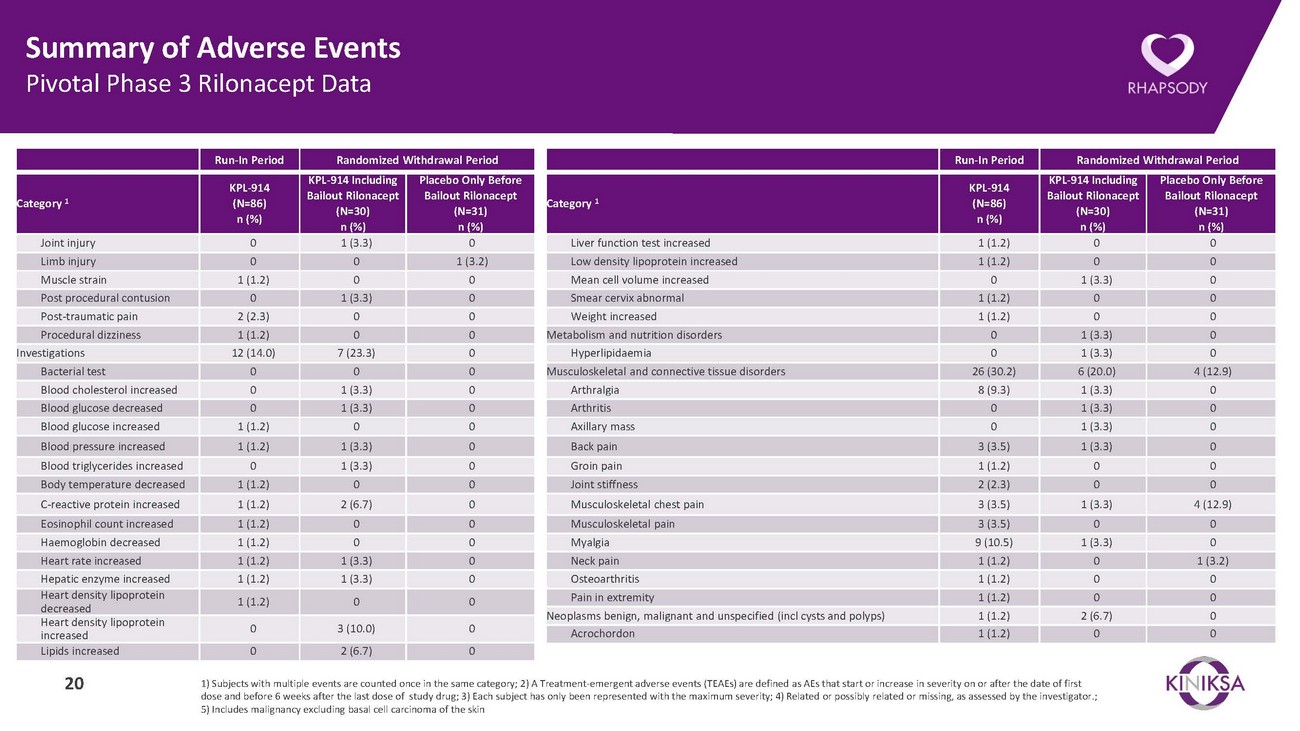
Summary of Adverse Events Pivotal Phase 3 Rilonacept DataRun-In Period Randomized Withdrawal PeriodRun-In Period Randomized Withdrawal PeriodCategory 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Category 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Joint injury 0 1 (3.3) 0 Limb injury 0 0 1 (3.2) Muscle strain 1 (1.2) 0 0 Post procedural contusion 0 1 (3.3) 0 Post-traumatic pain 2 (2.3) 0 0 Procedural dizziness 1 (1.2) 0 0 Investigations 12 (14.0) 7 (23.3) 0 Bacterial test 0 0 0 Blood cholesterol increased 0 1 (3.3) 0 Blood glucose decreased 0 1 (3.3) 0 Blood glucose increased 1 (1.2) 0 0 Blood pressure increased 1 (1.2) 1 (3.3) 0 Blood triglycerides increased 0 1 (3.3) 0 Body temperature decreased 1 (1.2) 0 0 C-reactive protein increased 1 (1.2) 2 (6.7) 0 Eosinophil count increased 1 (1.2) 0 0 Haemoglobin decreased 1 (1.2) 0 0 Heart rate increased 1 (1.2) 1 (3.3) 0 Hepatic enzyme increased 1 (1.2) 1 (3.3) 0 Heart density lipoprotein decreased 1 (1.2) 0 0 Heart density lipoprotein increased 0 3 (10.0) 0 Lipids increased 0 2 (6.7) 0Liver function test increased 1 (1.2) 0 0 Low density lipoprotein increased 1 (1.2) 0 0 Mean cell volume increased 0 1 (3.3) 0 Smear cervix abnormal 1 (1.2) 0 0 Weight increased 1 (1.2) 0 0 Metabolism and nutrition disorders 0 1 (3.3) 0 Hyperlipidaemia 0 1 (3.3) 0 Musculoskeletal and connective tissue disorders 26 (30.2) 6 (20.0) 4 (12.9) Arthralgia 8 (9.3) 1 (3.3) 0 Arthritis 0 1 (3.3) 0 Axillary mass 0 1 (3.3) 0 Back pain 3 (3.5) 1 (3.3) 0 Groin pain 1 (1.2) 0 0 Joint stiffness 2 (2.3) 0 0 Musculoskeletal chest pain 3 (3.5) 1 (3.3) 4 (12.9) Musculoskeletal pain 3 (3.5) 0 0 Myalgia 9 (10.5) 1 (3.3) 0 Neck pain 1 (1.2) 0 1 (3.2) Osteoarthritis 1 (1.2) 0 0 Pain in extremity 1 (1.2) 0 0 Neoplasms benign, malignant and unspecified (incl cysts and polyps) 1 (1.2) 2 (6.7) 0 Acrochordon 1 (1.2) 0 020 1) Subjects with multiple events are counted once in the same category; 2) A Treatment-emergent adverse events (TEAEs) are defined as AEs that start or increase in severity on or after the date of first dose and before 6 weeks after the last dose of study drug; 3) Each subject has only been represented with the maximum severity; 4) Related or possibly related or missing, as assessed by the investigator.; 5) Includes malignancy excluding basal cell carcinoma of the skin
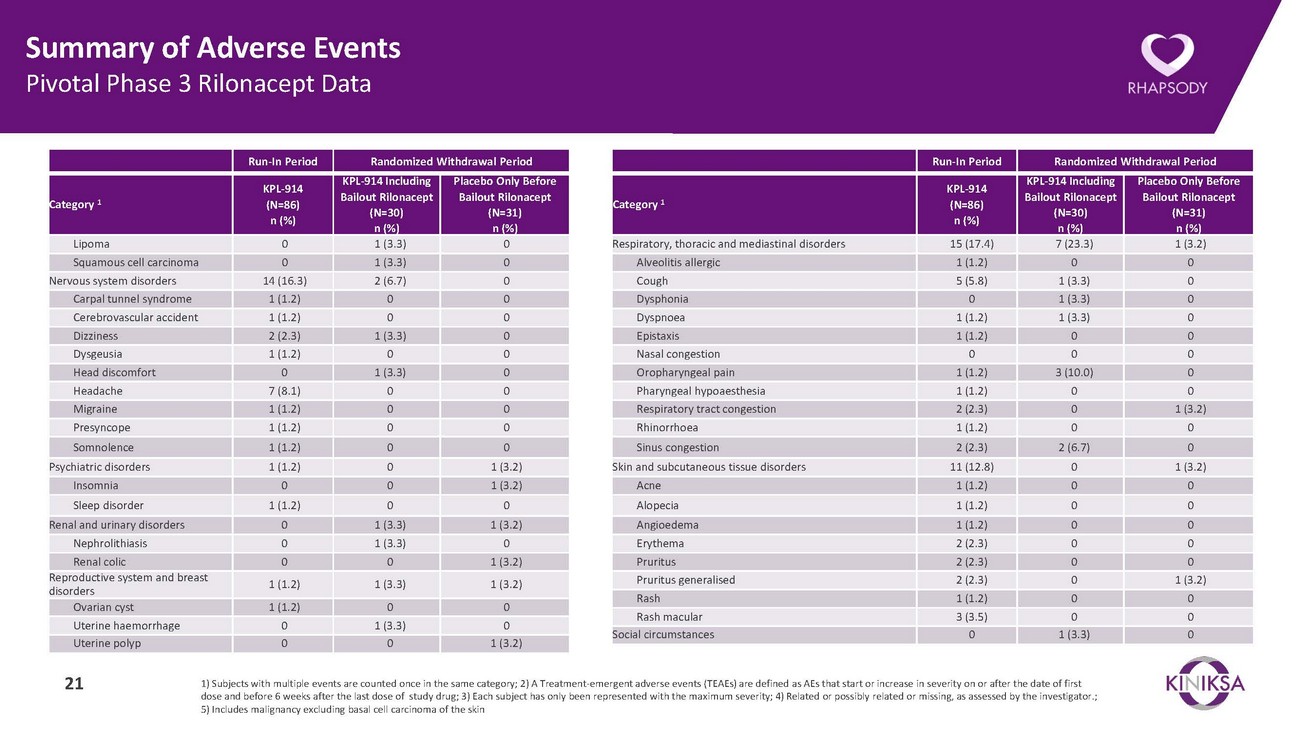
Summary of Adverse Events Pivotal Phase 3 Rilonacept DataRun-In Period Randomized Withdrawal PeriodRun-In Period Randomized Withdrawal PeriodCategory 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Category 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Lipoma 0 1 (3.3) 0 Squamous cell carcinoma 0 1 (3.3) 0 Nervous system disorders 14 (16.3) 2 (6.7) 0 Carpal tunnel syndrome 1 (1.2) 0 0 Cerebrovascular accident 1 (1.2) 0 0 Dizziness 2 (2.3) 1 (3.3) 0 Dysgeusia 1 (1.2) 0 0 Head discomfort 0 1 (3.3) 0 Headache 7 (8.1) 0 0 Migraine 1 (1.2) 0 0 Presyncope 1 (1.2) 0 0 Somnolence 1 (1.2) 0 0 Psychiatric disorders 1 (1.2) 0 1 (3.2) Insomnia 0 0 1 (3.2) Sleep disorder 1 (1.2) 0 0 Renal and urinary disorders 0 1 (3.3) 1 (3.2) Nephrolithiasis 0 1 (3.3) 0 Renal colic 0 0 1 (3.2) Reproductive system and breast disorders 1 (1.2) 1 (3.3) 1 (3.2) Ovarian cyst 1 (1.2) 0 0 Uterine haemorrhage 0 1 (3.3) 0 Uterine polyp 0 0 1 (3.2)Respiratory, thoracic and mediastinal disorders 15 (17.4) 7 (23.3) 1 (3.2) Alveolitis allergic 1 (1.2) 0 0 Cough 5 (5.8) 1 (3.3) 0 Dysphonia 0 1 (3.3) 0 Dyspnoea 1 (1.2) 1 (3.3) 0 Epistaxis 1 (1.2) 0 0 Nasal congestion 0 0 0 Oropharyngeal pain 1 (1.2) 3 (10.0) 0 Pharyngeal hypoaesthesia 1 (1.2) 0 0 Respiratory tract congestion 2 (2.3) 0 1 (3.2) Rhinorrhoea 1 (1.2) 0 0 Sinus congestion 2 (2.3) 2 (6.7) 0 Skin and subcutaneous tissue disorders 11 (12.8) 0 1 (3.2) Acne 1 (1.2) 0 0 Alopecia 1 (1.2) 0 0 Angioedema 1 (1.2) 0 0 Erythema 2 (2.3) 0 0 Pruritus 2 (2.3) 0 0 Pruritus generalised 2 (2.3) 0 1 (3.2) Rash 1 (1.2) 0 0 Rash macular 3 (3.5) 0 0 Social circumstances 0 1 (3.3) 021 1) Subjects with multiple events are counted once in the same category; 2) A Treatment-emergent adverse events (TEAEs) are defined as AEs that start or increase in severity on or after the date of first dose and before 6 weeks after the last dose of study drug; 3) Each subject has only been represented with the maximum severity; 4) Related or possibly related or missing, as assessed by the investigator.; 5) Includes malignancy excluding basal cell carcinoma of the skin
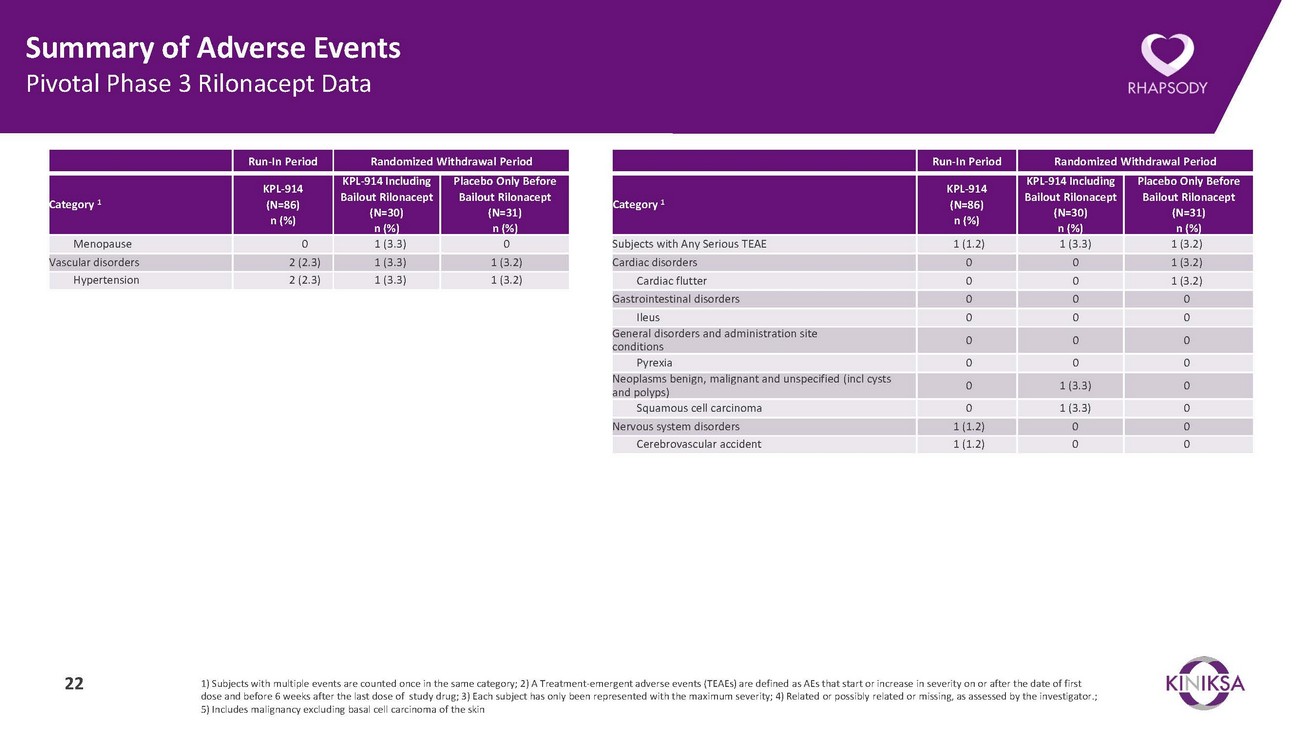
Summary of Adverse Events Pivotal Phase 3 Rilonacept DataRun-In Period Randomized Withdrawal PeriodRun-In Period Randomized Withdrawal PeriodCategory 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Category 1KPL-914 (N=86) n (%)KPL-914 Including Bailout Rilonacept (N=30) n (%)Placebo Only Before Bailout Rilonacept (N=31) n (%)Menopause 0 1 (3.3) 0 Vascular disorders 2 (2.3) 1 (3.3) 1 (3.2) Hypertension 2 (2.3) 1 (3.3) 1 (3.2)Subjects with Any Serious TEAE 1 (1.2) 1 (3.3) 1 (3.2) Cardiac disorders 0 0 1 (3.2) Cardiac flutter 0 0 1 (3.2) Gastrointestinal disorders 0 0 0 Ileus 0 0 0 General disorders and administration site conditions 0 0 0 Pyrexia 0 0 0 Neoplasms benign, malignant and unspecified (incl cysts and polyps) 0 1 (3.3) 0 Squamous cell carcinoma 0 1 (3.3) 0 Nervous system disorders 1 (1.2) 0 0 Cerebrovascular accident 1 (1.2) 0 022 1) Subjects with multiple events are counted once in the same category; 2) A Treatment-emergent adverse events (TEAEs) are defined as AEs that start or increase in severity on or after the date of first dose and before 6 weeks after the last dose of study drug; 3) Each subject has only been represented with the maximum severity; 4) Related or possibly related or missing, as assessed by the investigator.; 5) Includes malignancy excluding basal cell carcinoma of the skin
