Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Forte Biosciences, Inc. | d89447d8k.htm |

Forte biosciences TOPICAL live biotherapeutic for the treatment of Inflammatory skin disease Corporate PRESENTATION June 2020 Exhibit 99.1

Certain statements contained in this presentation regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation Act of 1995, known as the PSLRA. These include statements regarding management’s intention, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Forte Biosciences, Inc. (“we”, the “Company” or “Forte”) undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. We use words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, risks relating to the sufficiency of the Company’s cash balance to fund the Company’s activities, and the expectation with respect thereto; the business and prospects of the Company; Forte’s plans to develop and potentially commercialize its product candidates, including FB-401; the timing of initiation of Forte’s planned clinical trials; the timing of the availability of data from Forte’s clinical trials; the timing of any planned investigational new drug application or new drug application; Forte’s plans to research, develop and commercialize its current and future product candidates; Forte’s ability to successfully enter into collaborations, and to fulfill its obligations under any such collaboration agreements; the clinical utility, potential benefits and market acceptance of Forte’s product candidates; Forte’s commercialization, marketing and manufacturing capabilities and strategy; Forte’s ability to identify additional products or product candidates with significant commercial potential; developments and projections relating to Forte’s competitors and its industry; the impact of government laws and regulations; Forte’s ability to protect its intellectual property position; Forte’s estimates regarding future revenue, expenses, capital requirements and need for additional financing following the proposed transaction; and the impact of COVID-19 on the Company, the Company’s industry or the economy generally. The known risks and uncertainties are described in detail under the caption “Risk Factors” and elsewhere in the Company’s Annual Report on Form 10-K for the year ending December 31, 2019, Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, and the Registration Statement/Proxy Statement/Prospectus filed on Form S-4 (Registration No. 333-237371), initially filed on March 25, 2020, as amended, and declared effective by the Securities and Exchange Commission, or SEC, on May 13, 2020. Additional information will also be set forth in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2020 and our subsequent reports filed with the SEC and other documents filed from time to time with the SEC. Forward-looking statements included in this presentation are based on information available to Forte as of the date of this presentation. Accordingly, our actual results may materially differ from our current expectations, estimates and projections. Forte undertakes no obligation to update such forward-looking statements to reflect events or circumstances after the date of this presentation. Cautionary note regarding forward-looking statements
Summary OF Forte Biosciences: FB-401 – POTENTIAL first-in-class topical live biotherapeutic for the treatment of inflammatory Skin disease Late-stage CLINICAL Asset Phase 2a trial in atopic dermatitis completed including pediatrics 3 years and older, FB-401 was well tolerated and demonstrated clinical improvement Randomized Phase 2 initiation in adults and pediatrics 2 years and older expected in mid-2020 in atopic dermatitis (AD) -data readout expected in mid-2021 10-20% of children in industrialized countries develop atopic dermatitis (AD) In the U.S. , AD affects 17 million people (over 50% are children) Significant unmet need for safe and effective AD therapy for pediatrics Large market with unmet need Clinical data demonstrates FB-401 was well-tolerated and active Phase 2a study, including pediatrics, demonstrates potential for acceptable safety profile and significant reduction in atopic dermatitis disease and pruritus, as well as control of S. aureus while tapering/eliminating steroid use Potential First-in-class topical live biotherapeutic Exclusive license to NIH-owned patent families as well as Forte owned IP. Coverage includes composition and method of use patents Patent coverage through at least 2037 (7 U.S. patents issued) Intellectual Property Experienced life science investor base including Alger, ArrowMark, BVF Partners LP, Franklin Templeton and OrbiMed Cash runway expected to be sufficient through Phase 2 data readout in mid-2021 Management team with significant drug development, innovation and corporate strategy experience Financing / Management
FORTE BIOSCIENCES: Overview of FB-401 FB-401 drug product consists of 3 therapeutic bacterial strains of commensal gram negative R. mucosa specifically selected based on screening for impact on inflammatory skin disease parameters Topical application of the specifically selected therapeutic bacterial strains of R. mucosa drug product: Drives immune pathways that are defective Suppresses Staphylococcus aureus growth Improves skin barrier function Clinical data demonstrates FB-401 live biotherapeutic therapy was well-tolerated and active in both adults and pediatric Phase 2a study, including pediatrics, well tolerated and demonstrated clinical improvement in atopic dermatitis disease and pruritus, as well as control of S. aureus while tapering/eliminating steroid use Randomized Phase 2 trial initiation expected in mid-2020

EXPERIENCED management and advisory Teams Paul Wagner, Ph.D. – CEO Forte BioSciences team has extensive experience in microbial manufacturing, quality, regulatory and clinical development in dermatology Dan Burge, MD – Head of Clinical Development Eric Emery – Head of Downstream Manufacturing Hank Talbot, Ph.D. – Head of Process Development and Quality Scientific Advisory Board (SAB) Prof. Amy Paller, MD – Chair, Department of Dermatology Northwestern University Feinberg School of Medicine Prof. Lawrence Eichenfield, MD – Chief of Pediatric and Adolescent Dermatology at Rady Children's Hospital-San Diego, Editor in Chief of Pediatric Dermatology Prof. Eric Simpson, MD –Professor of Dermatology, Oregon Health & Science University, Portland Dr. Patricia Walker, MD, Ph.D. – Former CMO of Allergan Medical and Dermatology TA Head Tony Riley – Chief Financial Officer
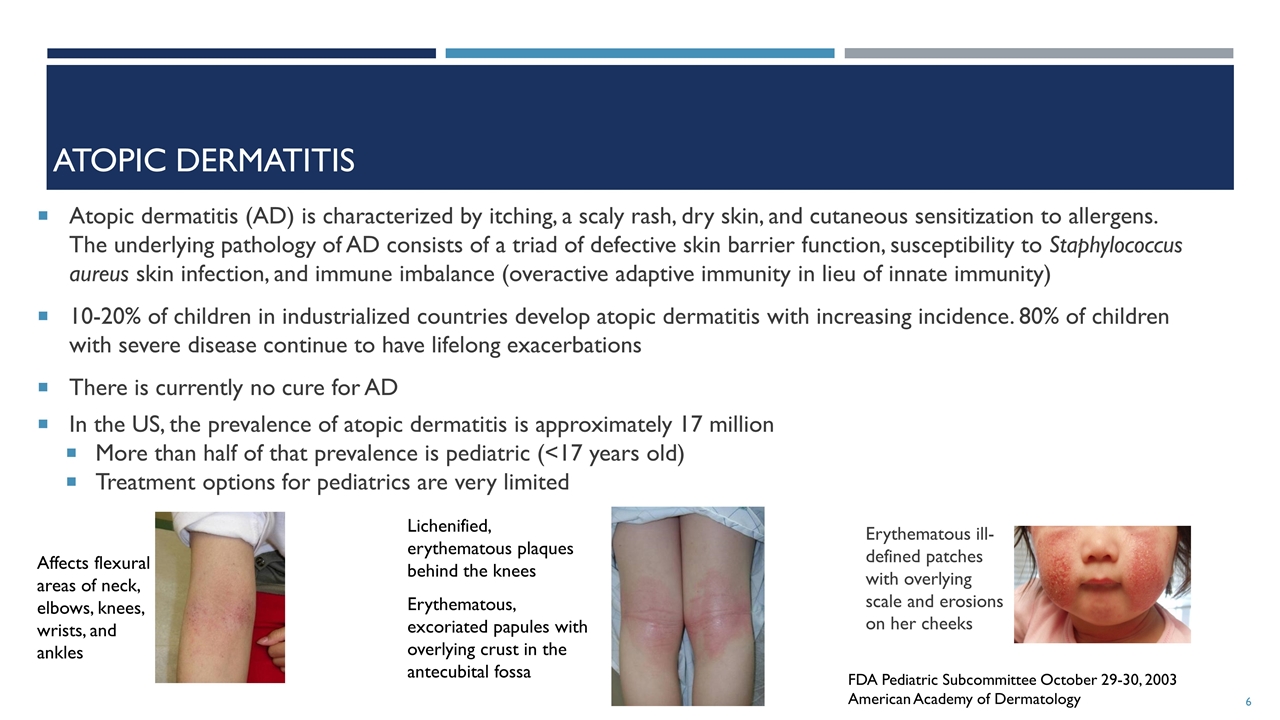
Atopic dermatitis Atopic dermatitis (AD) is characterized by itching, a scaly rash, dry skin, and cutaneous sensitization to allergens. The underlying pathology of AD consists of a triad of defective skin barrier function, susceptibility to Staphylococcus aureus skin infection, and immune imbalance (overactive adaptive immunity in lieu of innate immunity) 10-20% of children in industrialized countries develop atopic dermatitis with increasing incidence. 80% of children with severe disease continue to have lifelong exacerbations There is currently no cure for AD In the US, the prevalence of atopic dermatitis is approximately 17 million More than half of that prevalence is pediatric (<17 years old) Treatment options for pediatrics are very limited FDA Pediatric Subcommittee October 29-30, 2003 American Academy of Dermatology Affects flexural areas of neck, elbows, knees, wrists, and ankles Lichenified, erythematous plaques behind the knees Erythematous, excoriated papules with overlying crust in the antecubital fossa Erythematous ill-defined patches with overlying scale and erosions on her cheeks
SKIN MICROBIOME Dreno et al, European Academy of Dermatology and Venerology 2016, 30, 2038-2047 Kong HH et al. Genome research. 2012;22(5):850-859 The skin is a complex barrier organ characterized by symbiotic relationship between microbial communities and host tissue via complex signals provided by the innate and the adaptive immune systems Exposure to various endogenous and exogenous factors impact the system balance potentially leading to inflammatory skin conditions comprising infections, allergies or autoimmune diseases Researchers in microbiology and dermatology identified and characterized the microorganisms present on the skin, to evaluate the bacterial diversity and their relative abundance and to understand how microbial diversity may contribute to skin health and dermatological conditions Recent work has revealed that the skin microbiome is significantly different between healthy controls and patients with AD and that symptoms are associated with a loss of commensal diversity
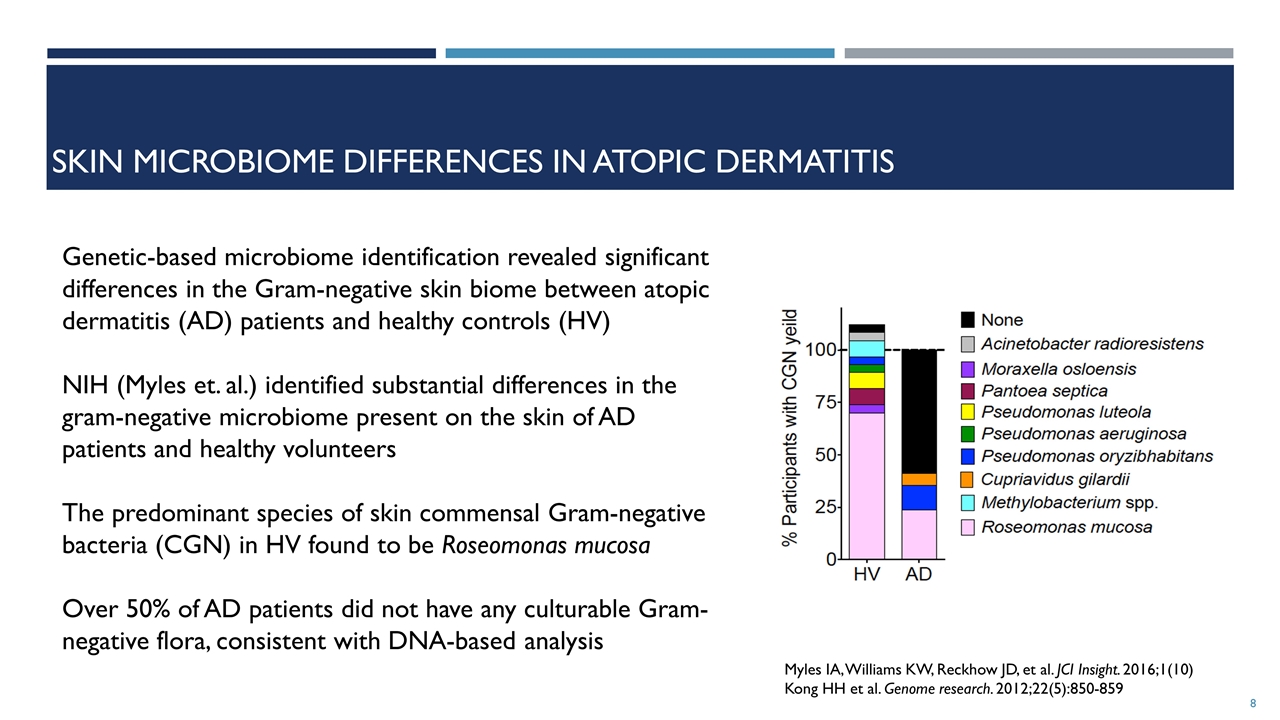
SKIN MICROBIOME differences in atopic dermatitis Myles IA, Williams KW, Reckhow JD, et al. JCI Insight. 2016;1(10) Kong HH et al. Genome research. 2012;22(5):850-859 Genetic-based microbiome identification revealed significant differences in the Gram-negative skin biome between atopic dermatitis (AD) patients and healthy controls (HV) NIH (Myles et. al.) identified substantial differences in the gram-negative microbiome present on the skin of AD patients and healthy volunteers The predominant species of skin commensal Gram‑negative bacteria (CGN) in HV found to be Roseomonas mucosa Over 50% of AD patients did not have any culturable Gram-negative flora, consistent with DNA-based analysis

Phase 2A First HUMAN study of FB-401 - cutaneous live biotherapeutic for the treatment of atopic dermatitis Drug product: FB-401 (3 specifically selected therapeutic R. mucosa strains) lyophilized and reconstituted with sterile water in single-use, self-administered spray Design: Phase 1/2a enrolled 2 cohorts: Initial cohort enrolled 10 adult atopic dermatitis patients 18 years and older Following positive safety assessment from cohort I, the second cohort of 20 pediatric patients was enrolled Primary Objective: To evaluate the safety and activity of R mucosa as a live biotherapeutic for treatment of AD Secondary Objective: To evaluate the effect of R mucosa live biotherapy on quality of life of participants with AD Exploratory Objectives Measure trans epidermal water loss (TEWL) Characterize changes to total and specific IgE Evaluate potential changes to pre-diagnosed asthma and/or food allergies Evaluate incidence of S aureus infections that require treatment Persistence of R mucosa colonization after treatment
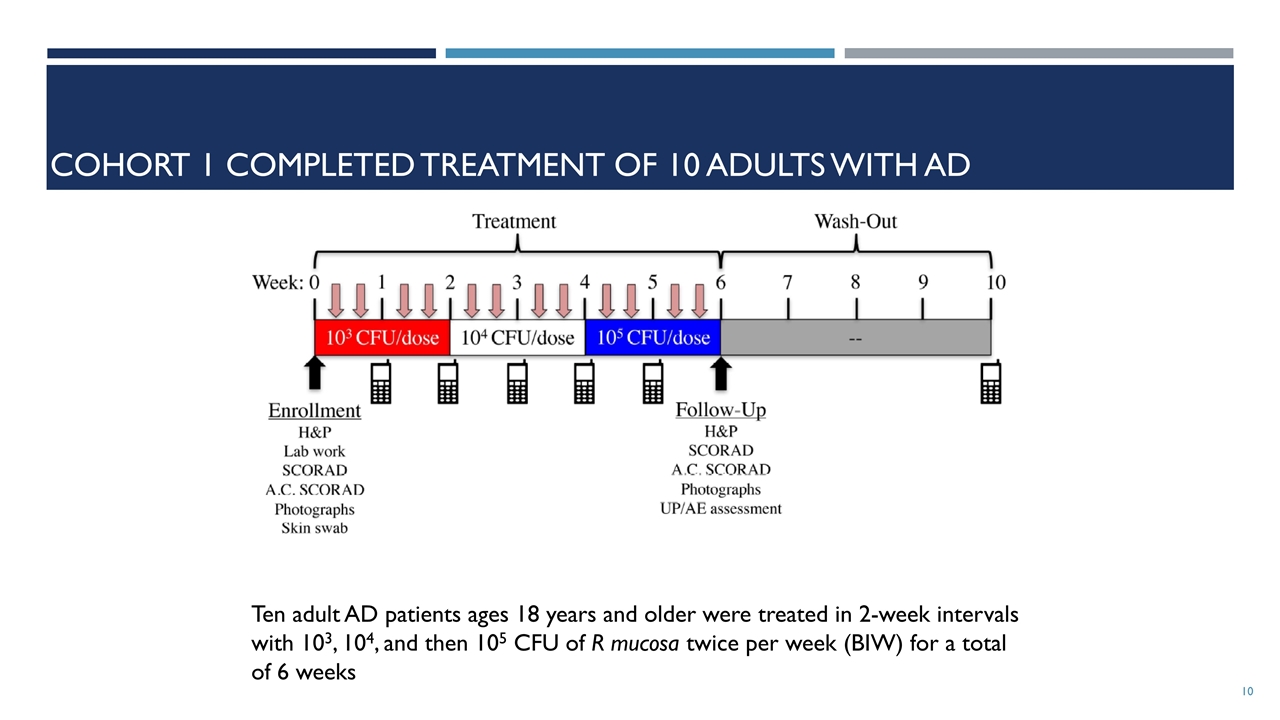
Cohort 1 completed treatment of 10 adults with AD Ten adult AD patients ages 18 years and older were treated in 2-week intervals with 103, 104, and then 105 CFU of R mucosa twice per week (BIW) for a total of 6 weeks
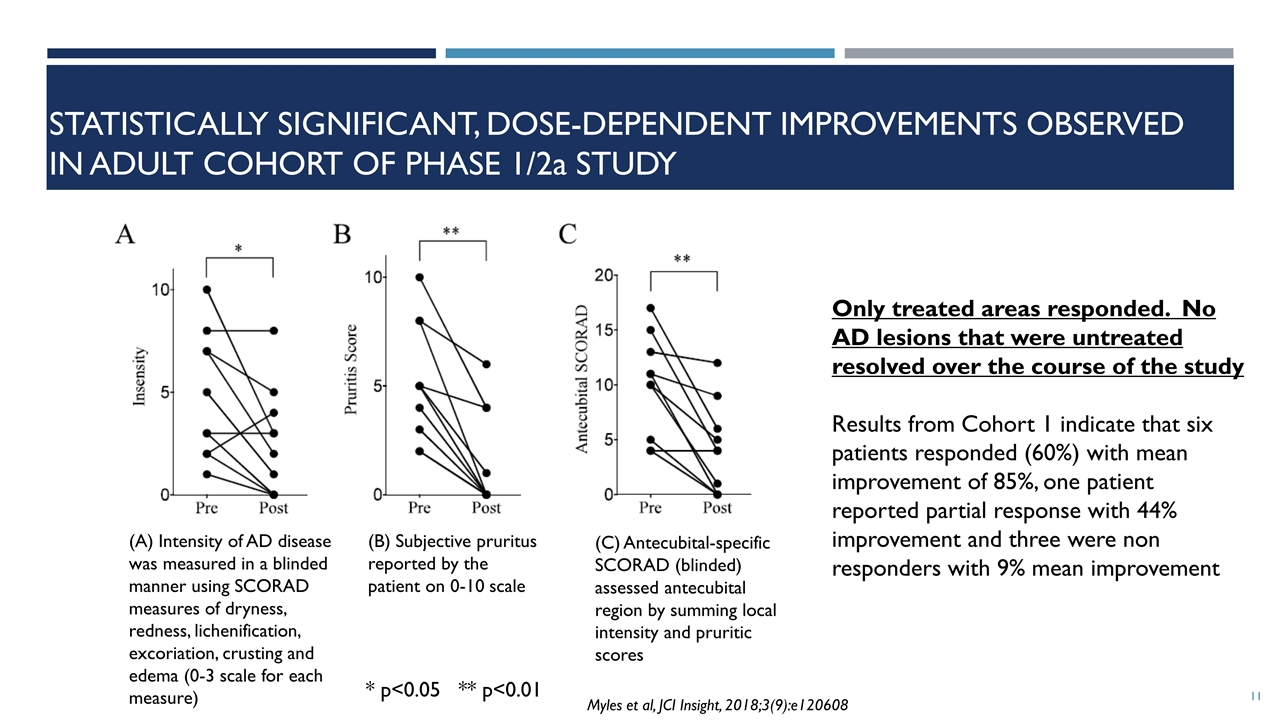
Statistically significant, Dose-dependent improvements observed in adult cohort of PHASE 1/2a Study (A) Intensity of AD disease was measured in a blinded manner using SCORAD measures of dryness, redness, lichenification, excoriation, crusting and edema (0-3 scale for each measure) (B) Subjective pruritus reported by the patient on 0-10 scale (C) Antecubital-specific SCORAD (blinded) assessed antecubital region by summing local intensity and pruritic scores Only treated areas responded. No AD lesions that were untreated resolved over the course of the study Results from Cohort 1 indicate that six patients responded (60%) with mean improvement of 85%, one patient reported partial response with 44% improvement and three were non responders with 9% mean improvement * p<0.05 ** p<0.01 Myles et al, JCI Insight, 2018;3(9):e120608
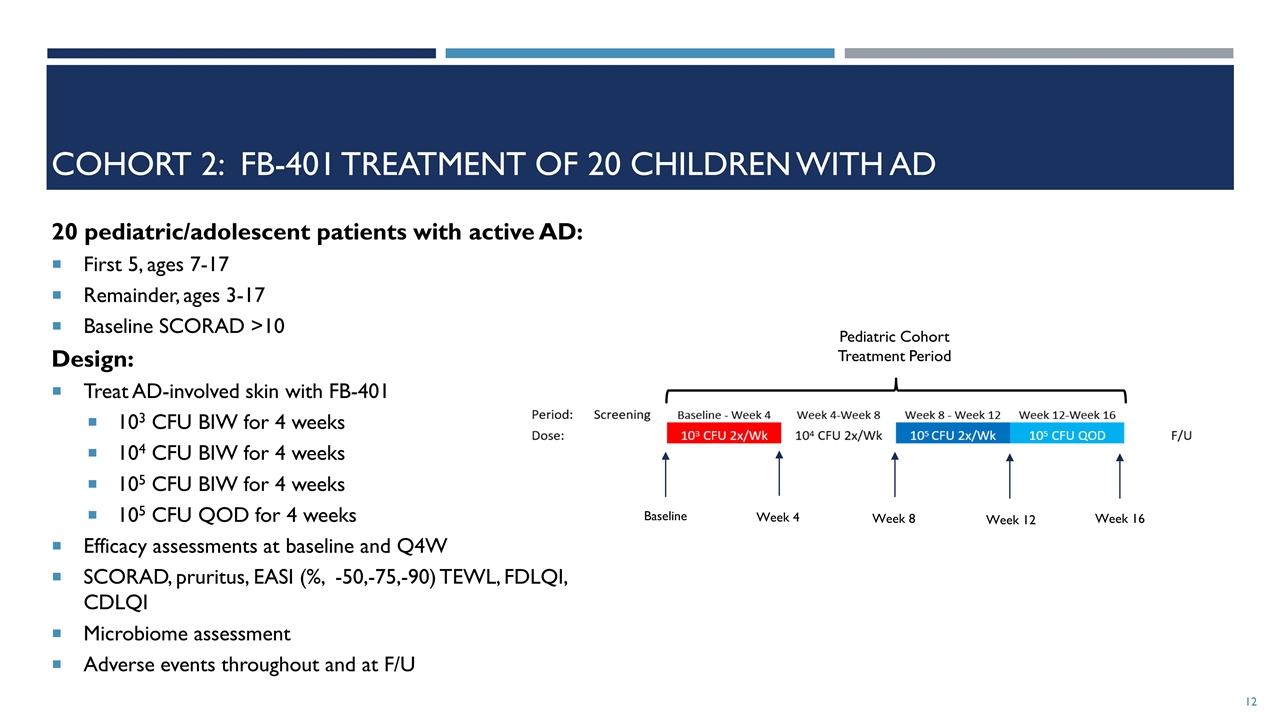
Cohort 2: FB-401 Treatment of 20 Children with AD 20 pediatric/adolescent patients with active AD: First 5, ages 7-17 Remainder, ages 3-17 Baseline SCORAD >10 Design: Treat AD-involved skin with FB-401 103 CFU BIW for 4 weeks 104 CFU BIW for 4 weeks 105 CFU BIW for 4 weeks 105 CFU QOD for 4 weeks Efficacy assessments at baseline and Q4W SCORAD, pruritus, EASI (%, -50,-75,-90) TEWL, FDLQI, CDLQI Microbiome assessment Adverse events throughout and at F/U Baseline Week 4 Week 8 Week 12 Week 16 Pediatric Cohort Treatment Period
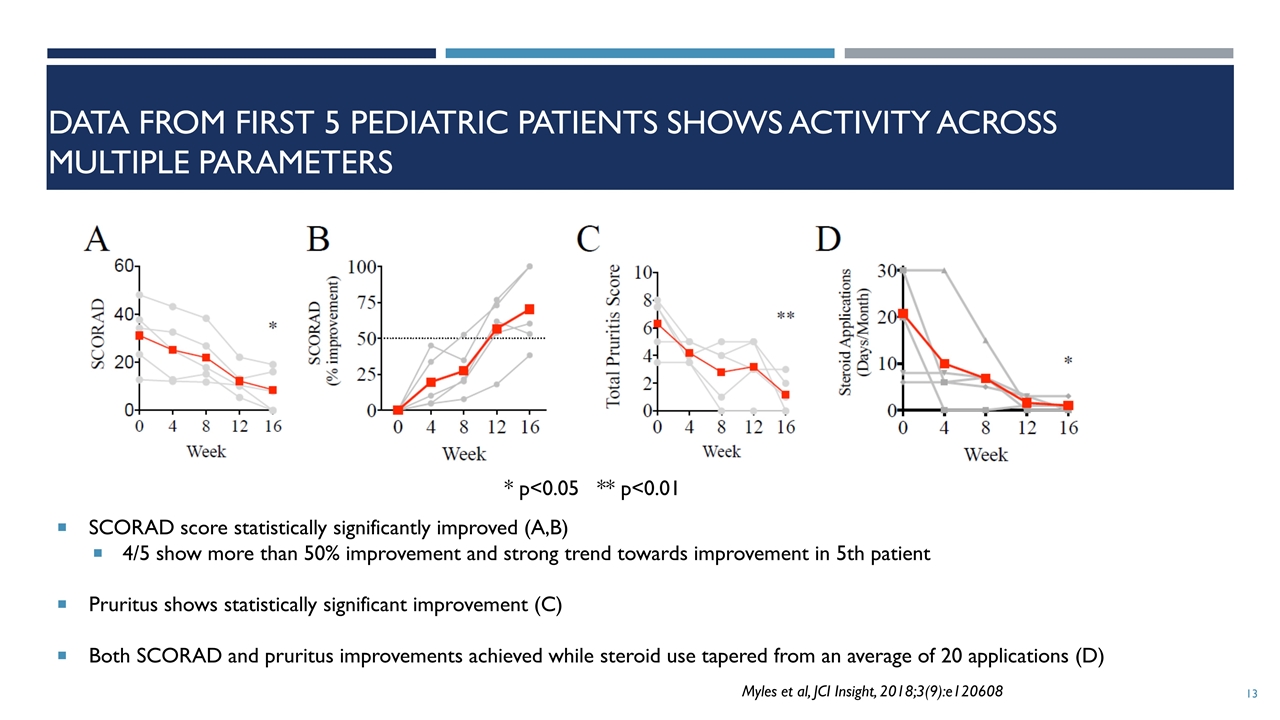
Data from First 5 pediatric patients Shows Activity Across multiple parameters SCORAD score statistically significantly improved (A,B) 4/5 show more than 50% improvement and strong trend towards improvement in 5th patient Pruritus shows statistically significant improvement (C) Both SCORAD and pruritus improvements achieved while steroid use tapered from an average of 20 applications (D) * p<0.05 ** p<0.01 Myles et al, JCI Insight, 2018;3(9):e120608
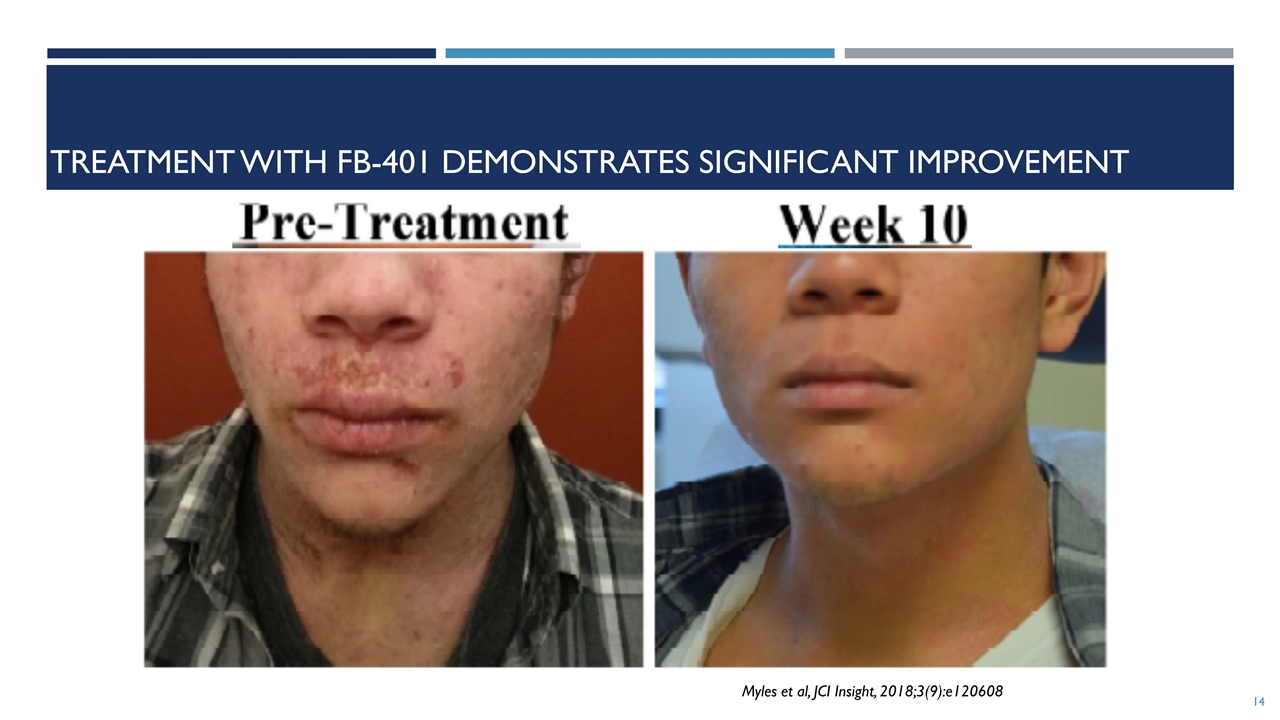
treatment with FB-401 demonstrates significant improvement Myles et al, JCI Insight, 2018;3(9):e120608
Planned FB-401 Randomized Phase 2 Study CONFIDENTIAL Randomized, placebo-controlled, multi-center study Includes children (2 years and older), adolescents and adults with mild to moderate atopic dermatitis Approximately 124 subjects randomized 1:1 to receive FB-401 or placebo for 16 weeks Endpoints include Eczema Area and Severity Index (EASI), Investigator Global Assessment (IGA), pruritus

Intellectual property overview Exclusive license to NIH-owned patent family focused on treatment of AD with a consortia of gram negative bacteria from healthy donors Patent coverage through at least 2037 7 US patents issued Entered in 10 Ex-US jurisdictions Europe, China, Canada, Japan, South Korea, Australia, Singapore, Mexico, Israel, and Hong Kong Company-owned IP directed to compositions for treatment of skin conditions associated with dysbiosis Broadly covers use of gram negative bacteria for treatment of atopic disorders
Summary OF Forte Biosciences: FB-401 – POTENTIAL first-in-class topical live biotherapeutic for the treatment of inflammatory Skin disease Late-stage CLINICAL Asset Phase 2a trial in atopic dermatitis completed including pediatrics 3 years and older, FB-401 was well tolerated and demonstrated clinical improvement Randomized Phase 2 initiation in adults and pediatrics 2 years and older expected in mid-2020 in atopic dermatitis (AD) -data readout expected in mid-2021 10-20% of children in industrialized countries develop atopic dermatitis (AD) In the U.S. , AD affects 17 million people (over 50% are children) Significant unmet need for safe and effective AD therapy for pediatrics Large market with unmet need Clinical data demonstrates FB-401 was well-tolerated and active Phase 2a study, including pediatrics, demonstrates potential for acceptable safety profile and significant reduction in atopic dermatitis disease and pruritus, as well as control of S. aureus while tapering/eliminating steroid use Potential First-in-class topical live biotherapeutic Exclusive license to NIH-owned patent families as well as Forte owned IP. Coverage includes composition and method of use patents Patent coverage through at least 2037 (7 U.S. patents issued) Intellectual Property Experienced life science investor base including Alger, ArrowMark, BVF Partners LP, Franklin Templeton and OrbiMed Cash runway expected to be sufficient through Phase 2 data readout in mid-2021 Management team with significant drug development, innovation and corporate strategy experience Financing / Management
