Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aridis Pharmaceuticals, Inc. | a20-23558_18k.htm |
Novel Anti-infectives Focusing on Respiratory Health APEXTM mAb Discovery Platform Cantor Fitzgerald Fireside Chat 25June2020 Aridis Pharmaceuticals 1
Forward-Looking Statements These forward-looking statements relate to future events or future financial performance of the Company. All such forward-looking statements involve risks and uncertainties and are not guaranties of future performance. An investment in the securities of Aridis is speculative in nature, involves a high degree of risk, and should not be made by an investor who cannot bear the economic risk of its investment for an indefinite period of time and who cannot afford the loss of its entire investment. These in clude many important factors that affect our ability to achieve our stated objectives including, but not limited to: • • • • • • • • • • • • • • • • The timing of regulatory submissions; Our ability to obtain and maintain regulatory approval of our existing product candidates and any other product candidates we may develop, and the labeling under any approval we may obtain; Approvals for clinical trials may be delayed or withheld by regulatory agencies; Pre-clinical and clinical studies will not be successful or confirm earlier results or meet expectations or meet regulatory requirements or meet performance thresholds for commercial success; The timing and costs of clinical trials, the timing and costs of other expenses; Our ability to obtain funding from third parties; Management and employee operations and execution risks; Loss of key personnel; Competition; Market acceptance of products; Intellectual property risks; Assumptions regarding the size of the available market, benefits of our products, product pricing, timing of product launches ; The uncertainty of future financial results; Risks associated with this offering; Our ability to attract collaborators and partners; Our reliance on third party organizations. We operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any fac tor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations. Aridis Pharmaceuticals 2

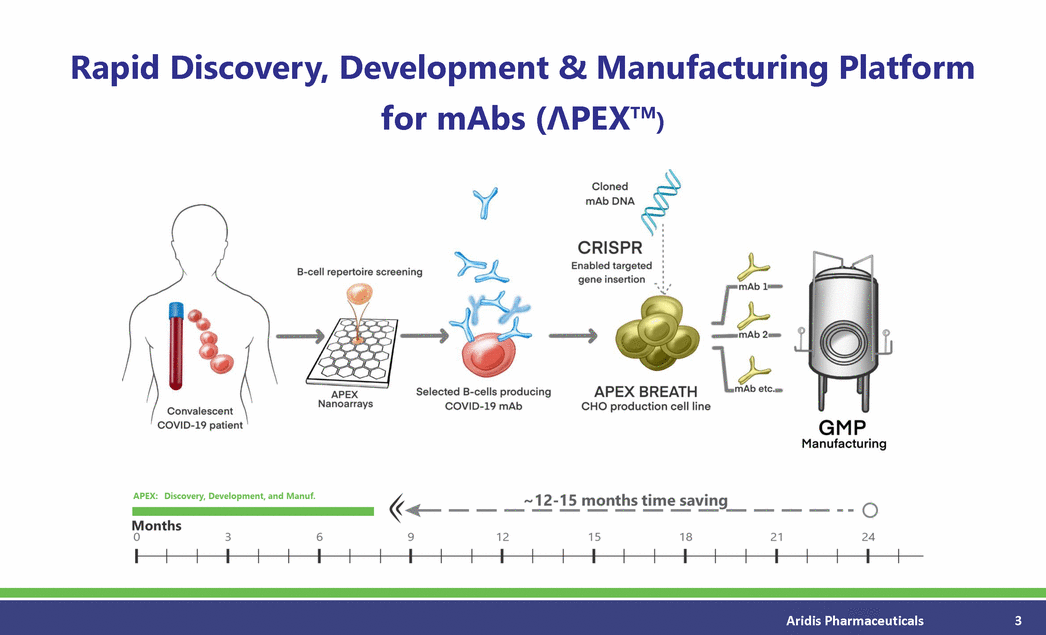
Rapid Discovery, Development & Manufacturing Platform for mAbs (PEXTM) Aridis Pharmaceuticals 3
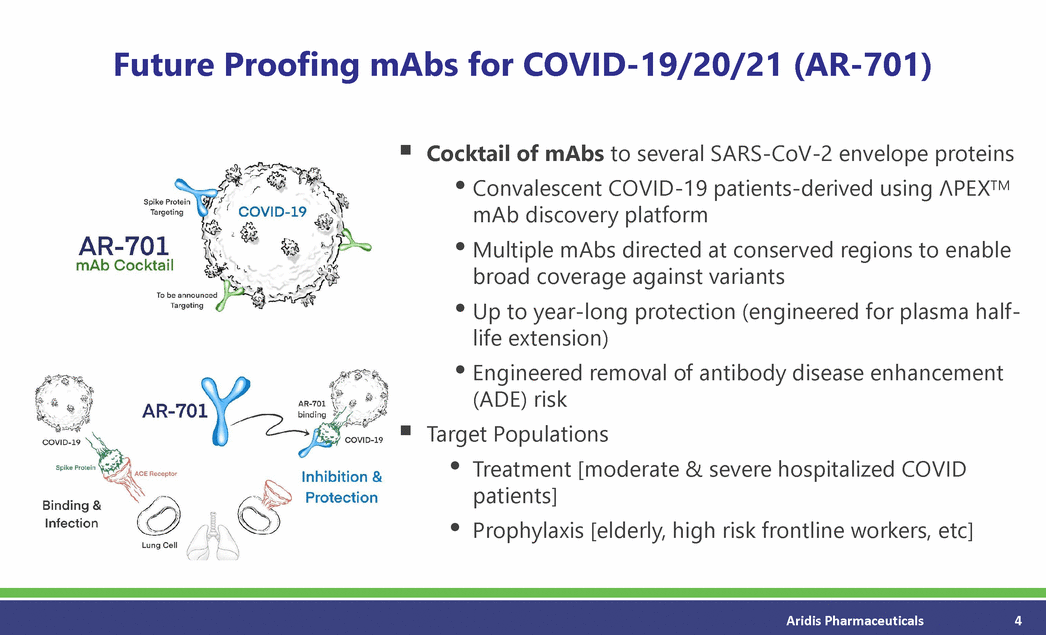
Future Proofing mAbs for COVID-19/20/21 (AR-701) Cocktail of mAbs to several SARS-CoV-2 envelope proteins • • • • Convalescent COVID-19 patients-derived using PEXTM mAb discovery platform Multiple mAbs directed at conserved regions to enable broad coverage against variants Up to year-long protection (engineered for plasma half-life extension) Engineered removal of antibody disease enhancement (ADE) risk Target Populations • • Treatment [moderate & severe hospitalized COVID patients] Prophylaxis [elderly, high risk frontline workers, etc] Aridis Pharmaceuticals 4
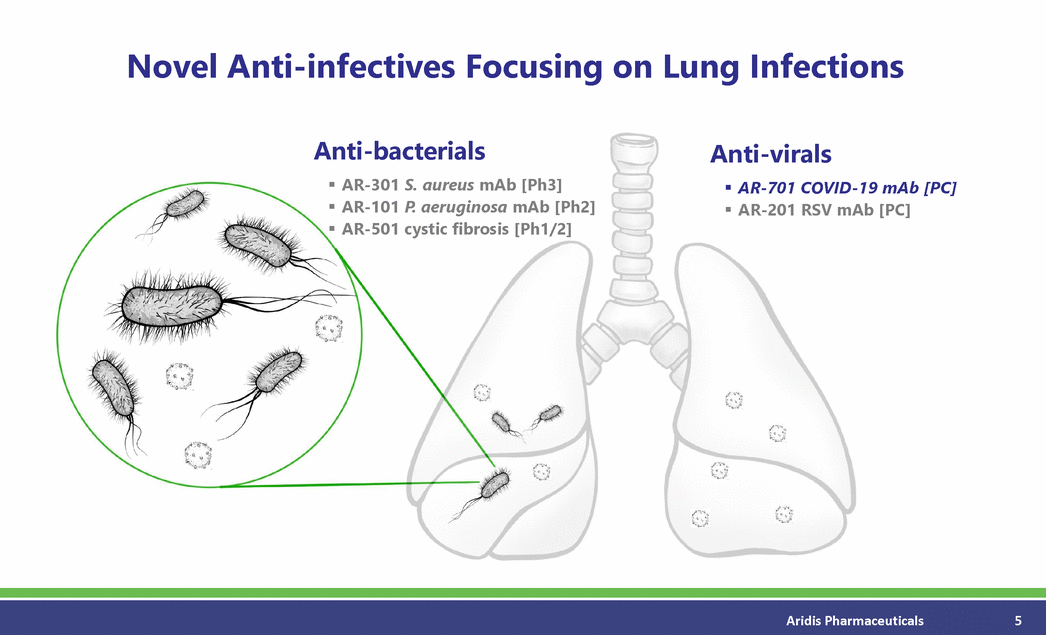
Novel Anti-infectives Focusing on Lung Infections Anti-bacterials Anti-virals AR-301 S. aureus mAb [Ph3] AR-101 P. aeruginosa mAb [Ph2] AR-501 cystic fibrosis [Ph1/2] AR-701 COVID-19 mAb [PC] AR-201 RSV mAb [PC] Aridis Pharmaceuticals 5
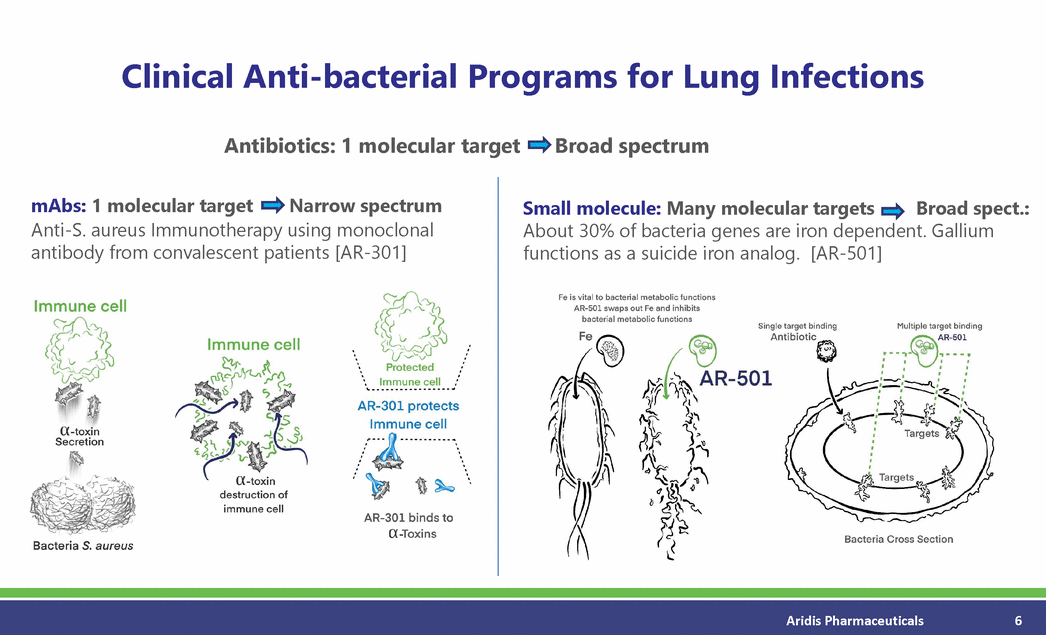
Clinical Anti-bacterial Programs for Lung Infections Antibiotics: 1 molecular target Broad spectrum mAbs: 1 molecular target Narrow spectrum Small molecule: Many molecular targets Broad spect.: Anti-S. aureus Immunotherapy using monoclonal antibody from convalescent patients [AR-301] About 30% of bacteria genes are iron dependent. Gallium functions as a suicide iron analog. [AR-501] Aridis Pharmaceuticals 6
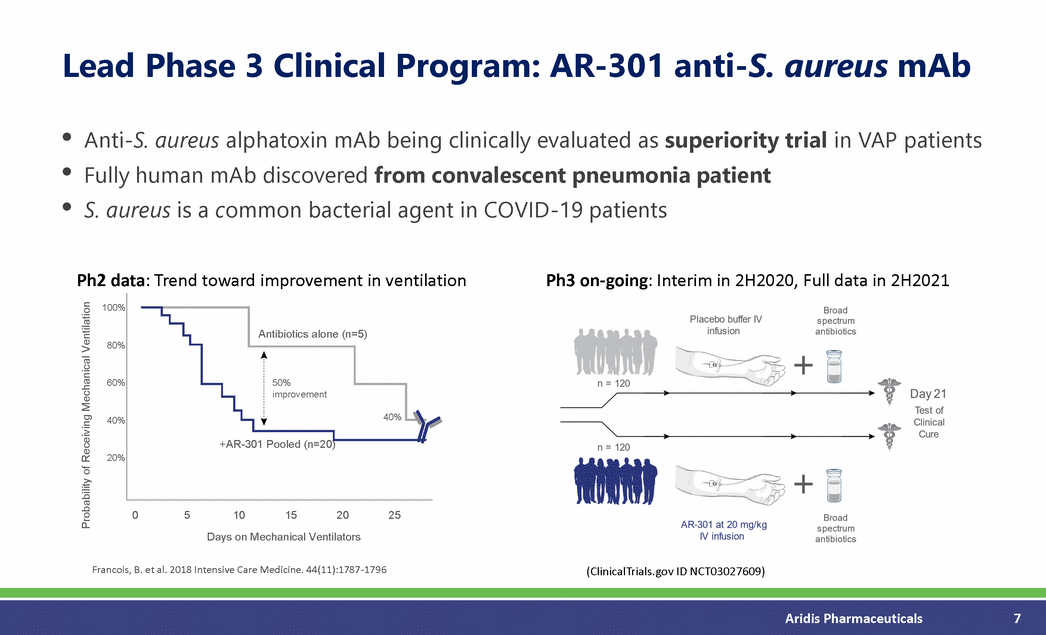
Lead Phase 3 Clinical Program: AR-301 anti-S. aureus mAb • • • Anti-S. aureus alphatoxin mAb being clinically evaluated as superiority trial in VAP patients Fully human mAb discovered from convalescent pneumonia patient S. aureus is a common bacterial agent in COVID-19 patients Ph2 data: Trend toward improvement in ventilation Ph3 on-going: Interim in 2H2020, Full data in 2H2021 100% 80% 60% 40% 20% 0 5 10 15 20 25 Days on Mechanical Ventilators Francois, B. et al. 2018 Intensive Care Medicine. 44(11):1787-1796 (ClinicalTrials.gov ID NCT03027609) Aridis Pharmaceuticals 7 Probability of Receiving Mechanical Ventilation 50% +AR-301 Pooled (n=20) Antibiotics alone (n=5) improvement 40%
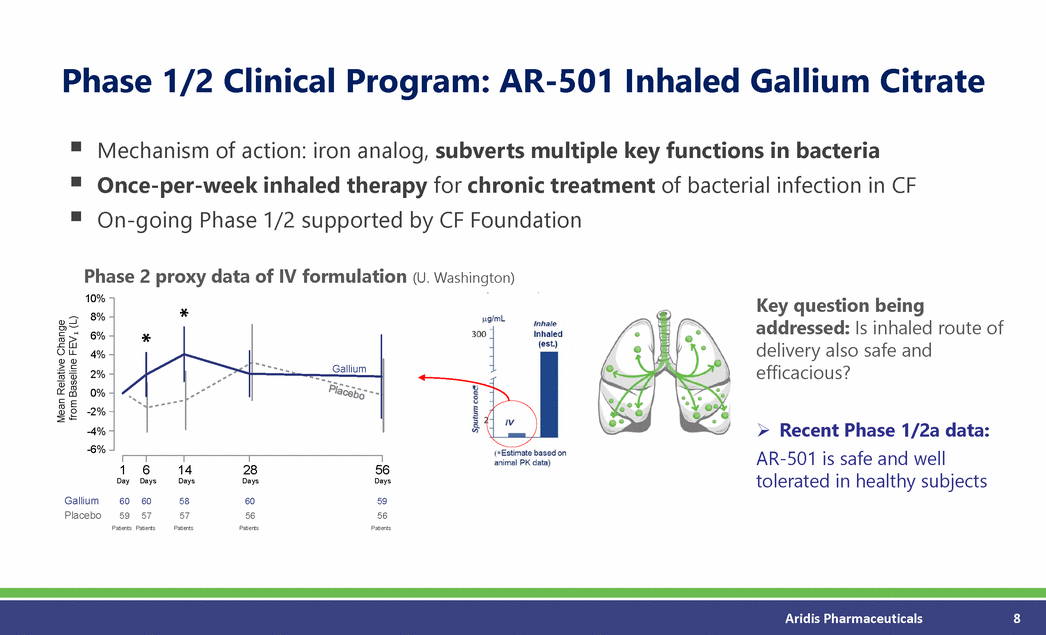
Phase 1/2 Clinical Program: AR-501 Inhaled Gallium Citrate Mechanism of action: iron analog, subverts multiple key functions in bacteria Once-per-week inhaled therapy for chronic treatment On-going Phase 1/2 supported by CF Foundation of bacterial infection in CF Phase 2 proxy data of IV formulation (U. Washington) Key question being addressed: Is inhaled route of delivery also safe and efficacious? Recent Phase 1/2a data: AR-501 is safe and well tolerated in healthy subjects Aridis Pharmaceuticals 8