Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Tempest Therapeutics, Inc. | tm2023160d1_ex99-1.htm |
| EX-10.2 - EXHIBIT 10.2 - Tempest Therapeutics, Inc. | tm2023160d1_ex10-2.htm |
| EX-10.1 - EXHIBIT 10.1 - Tempest Therapeutics, Inc. | tm2023160d1_ex10-1.htm |
| 8-K - FORM 8-K - Tempest Therapeutics, Inc. | tm2023160-1_8k.htm |
Exhibit 99.2
 |
Corporate Presentation June 2020 |
 |
Cautionary Statement Regarding Forward-Looking Statements Certain statements contained in this presentation regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These include statements with respect to Millendo’s strategic review and results thereof, Millendo’s financial condition, Millendo’s future capital needs, the impact of Millendo’s plan to not further invest in the nevanimibe program, Millendo’s timeline for the continued development of MLE-301 for menopausal vasomotor symptoms, and, therefore, you are cautioned not to place undue reliance on them. Such forward-looking statements are based on Millendo’s expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including that Millendo has incurred significant losses since inception, Millendo has a limited operating history and has never generated any revenue from product sales, Millendo will require additional capital to finance its operations, Millendo's future success is dependent on the successful clinical development, regulatory approval and subsequent commercialization of current and any future product candidates, preclinical studies or earlier clinical trials are not necessarily predictive of future results and the results of Millendo's clinical trials may not support Millendo's product candidate claims, Millendo may encounter substantial delays in its clinical trials or Millendo may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities, enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside Millendo's control, Millendo's product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, or limit their commercial potential, Millendo faces substantial competition and Millendo’s business, preclinical studies and clinical development programs and timelines, its financial condition and results of operations could be materially and adversely affected by the current COVID-19 pandemic. You should refer to the risk factor disclosure set forth in the periodic reports and other documents Millendo files with the Securities and Exchange Commission available at www.sec.gov, including without limitation Millendo’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2020. New factors emerge from time to time and it is not possible for Millendo to predict all such factors, nor can Millendo assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements included in this presentation are based on information available to Millendo as of the date hereof. Millendo disclaims any obligation to update such forward-looking statements to reflect events or circumstances after the date of this press release, except as required by applicable law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and Millendo's own internal estimates and research. While Millendo believes these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of Millendo's internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. 2 |
 |
Millendo Therapeutics (Nasdaq: MLND) Strategic evaluation underway to reposition company for success Evaluating strategic options from position of financial strength with cash balance of $58.9M as of March 31, 2020* MLE-301, a novel NK3R antagonist for the treatment of menopausal vasomotor symptoms, expected to initiate first-in-human clinical studies in 3Q20 Seeking opportunities for potential pipeline expansion by leveraging our demonstrated experience in acquiring and in-licensing product candidates Experienced leadership team with deep development expertise to evaluate and execute on company strategy * Includes cash, cash equivalents and restricted cash 3 |
 |
MLE-301 for Menopausal Vasomotor Symptoms |
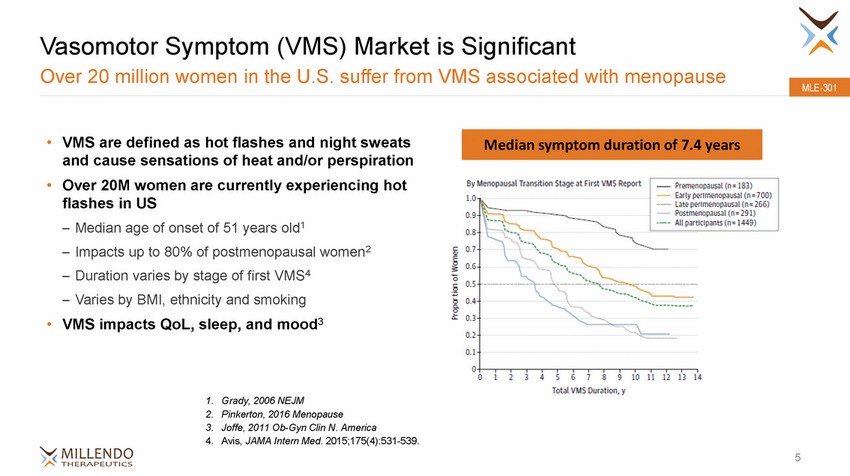 |
Vasomotor Symptom (VMS) Market is Significant Over 20 million women in the U.S. suffer from VMS associated with menopause MLE-301 • VMS are defined as hot flashes and night sweats and cause sensations of heat and/or perspiration Over 20M women are currently experiencing hot flashes in US – Median age of onset of 51 years old1 – Impacts up to 80% of postmenopausal women2 – Duration varies by stage of first VMS4 – Varies by BMI, ethnicity and smoking VMS impacts QoL, sleep, and mood3 • • 1. 2. 3. 4. Grady, 2006 NEJM Pinkerton, 2016 Menopause Joffe, 2011 Ob-Gyn Clin N. America Avis, JAMA Intern Med. 2015;175(4):531-539. 5 Median symptom duration of 7.4 years |
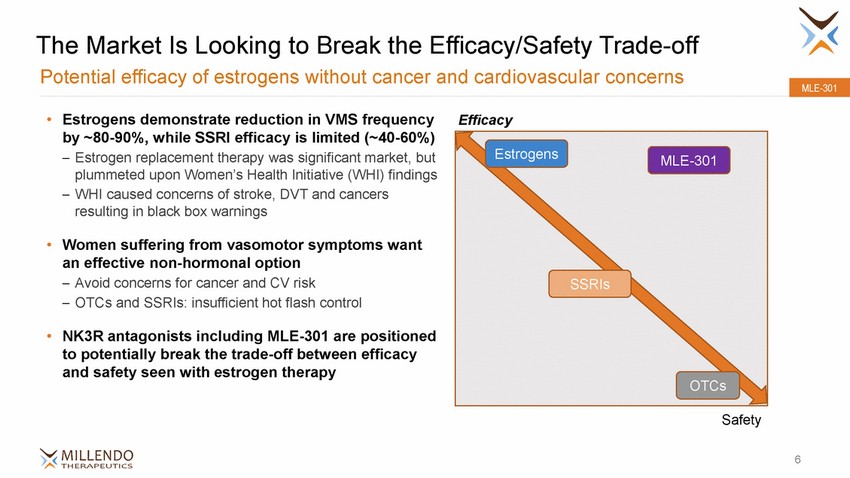 |
The Market Is Looking to Break the Efficacy/Safety Trade-off Potential efficacy of estrogens without cancer and cardiovascular concerns MLE-301 • Estrogens demonstrate reduction in VMS frequency by ~80-90%, while SSRI efficacy is limited (~40-60%) – Estrogen replacement therapy was significant market, but plummeted upon Women’s Health Initiative (WHI) findings – WHI caused concerns of stroke, DVT and cancers resulting in black box warnings Efficacy • Women suffering from vasomotor symptoms want an effective non-hormonal option – Avoid concerns for cancer and CV risk – OTCs and SSRIs: insufficient hot flash control • NK3R antagonists including MLE-301 are positioned to potentially break the trade-off between efficacy and safety seen with estrogen therapy Safety 6 EstrogensMLE-301 SSRIs OTCs |
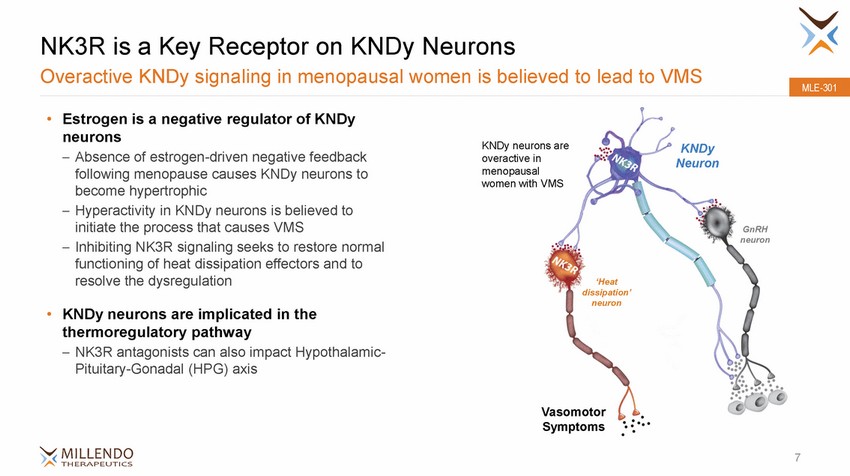 |
NK3R is a Key Receptor on KNDy Neurons Overactive KNDy signaling in menopausal women is believed to lead to VMS MLE-301 • Estrogen is a negative regulator of KNDy neurons – Absence of estrogen-driven negative feedback following menopause causes KNDy neurons to become hypertrophic – Hyperactivity in KNDy neurons is believed to initiate the process that causes VMS – Inhibiting NK3R signaling seeks to restore normal functioning of heat dissipation effectors and to resolve the dysregulation KNDy neurons are overactive in menopausal women with VMS KNDy Neuron GnRH neuron ‘Heat dissipation’ neuron • KNDy neurons are implicated in the thermoregulatory pathway – NK3R antagonists can also impact Hypothalamic-Pituitary-Gonadal (HPG) axis Vasomotor Symptoms 7 |
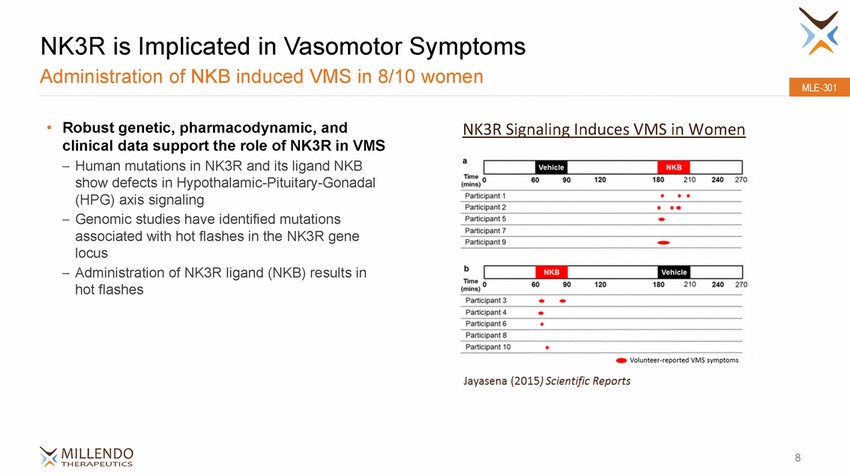 |
NK3R is Implicated in Vasomotor Symptoms Administration of NKB induced VMS in 8/10 women MLE-301 • Robust genetic, pharmacodynamic, and clinical data support the role of NK3R in VMS NK3R Signaling Induces VMS in Women Human mutations in NK3R and its ligand NKB show defects in Hypothalamic-Pituitary-Gonadal (HPG) axis signaling Genomic studies have identified mutations associated with hot flashes in the NK3R gene locus Administration of NK3R ligand (NKB) results in hot flashes – – – 8 |
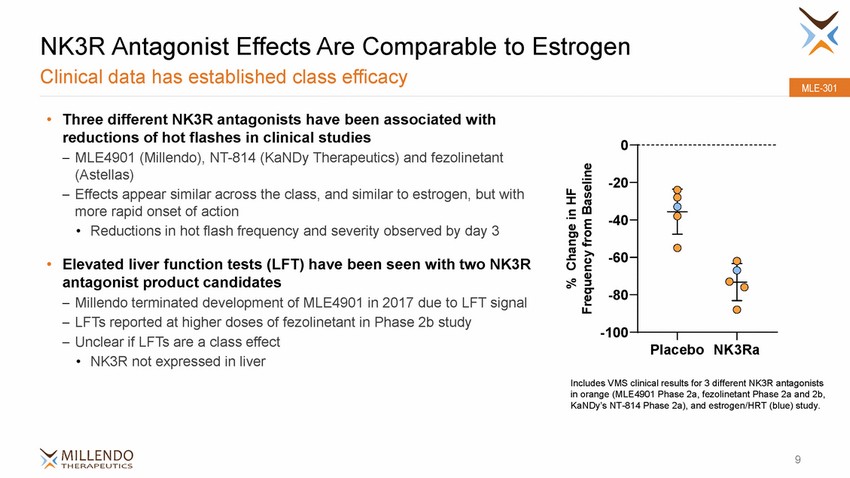 |
NK3R Antagonist Effects Are Comparable Clinical data has established class efficacy to Estrogen MLE-301 • Three different NK3R antagonists have been associated with reductions of hot flashes in clinical studies – MLE4901 (Millendo), NT-814 (KaNDy Therapeutics) and fezolinetant (Astellas) – Effects appear similar across the class, and similar to estrogen, but with more rapid onset of action •Reductions in hot flash frequency and severity observed by day 3 0 -20 -40 -60 • Elevated liver function tests (LFT) have been seen with two NK3R antagonist product candidates -80 Millendo terminated development of MLE4901 in 2017 due to LFT signal LFTs reported at higher doses of fezolinetant in Phase 2b study Unclear if LFTs are a class effect •NK3R not expressed in liver – – – -100 PlaceboNK3Ra Includes VMS clinical results for 3 different NK3R antagonists in orange (MLE4901 Phase 2a, fezolinetant Phase 2a and 2b, KaNDy’s NT-814 Phase 2a), and estrogen/HRT (blue) study. 9 % Change in HF Frequency from Baseline |
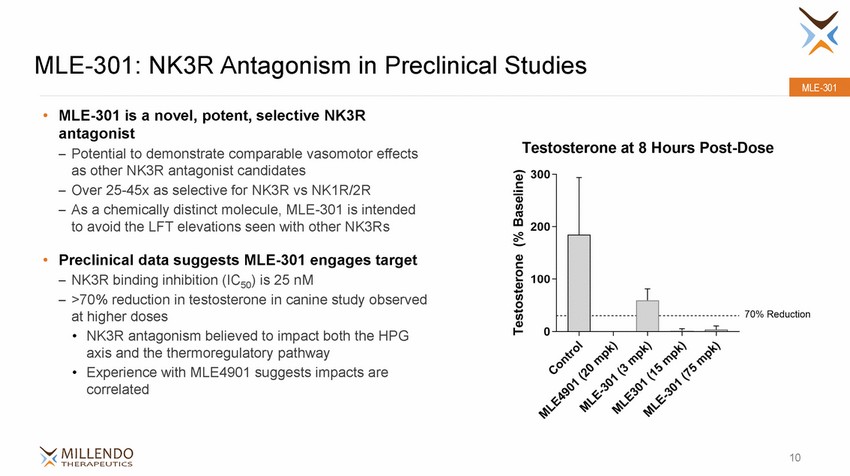 |
MLE-301: NK3R Antagonism in Preclinical Studies MLE-301 • MLE-301 is a novel, potent, selective NK3R antagonist – Potential to demonstrate comparable vasomotor effects as other NK3R antagonist candidates – Over 25-45x as selective for NK3R vs NK1R/2R – As a chemically distinct molecule, MLE-301 is intended to avoid the LFT elevations seen with other NK3Rs • Preclinical data suggests MLE-301 engages target NK3R binding inhibition (IC50) is 25 nM >70% reduction in testosterone in canine study observed at higher doses •NK3R antagonism believed to impact both the HPG axis and the thermoregulatory pathway •Experience with MLE4901 suggests impacts are correlated – – 10 |
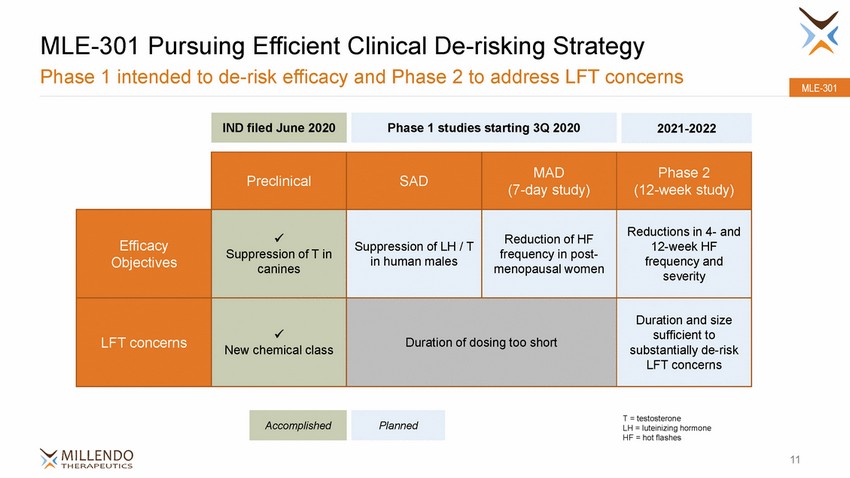 |
MLE-301 Pursuing Efficient Clinical De-risking Strategy Phase 1 intended to de-risk efficacy and Phase 2 to address LFT concerns MLE-301 (12-week study) 12-week HF severity substantially de-risk T = testosterone LH = luteinizing hormone HF = hot flashes 11 Accomplished Planned Preclinical SAD MAD (7-day study) Phase 2 Efficacy Objectives x Suppression of T in canines Suppression of LH / T in human males Reduction of HF frequency in post-menopausal women Reductions in 4-and frequency and LFT concerns x New chemical class Duration of dosing too short Duration and size sufficient to LFT concerns IND filed June 2020 Phase 1 studies starting 3Q 2020 2021-2022 |
 |
Nevanimibe for Congenital Adrenal Hyperplasia (CAH) |
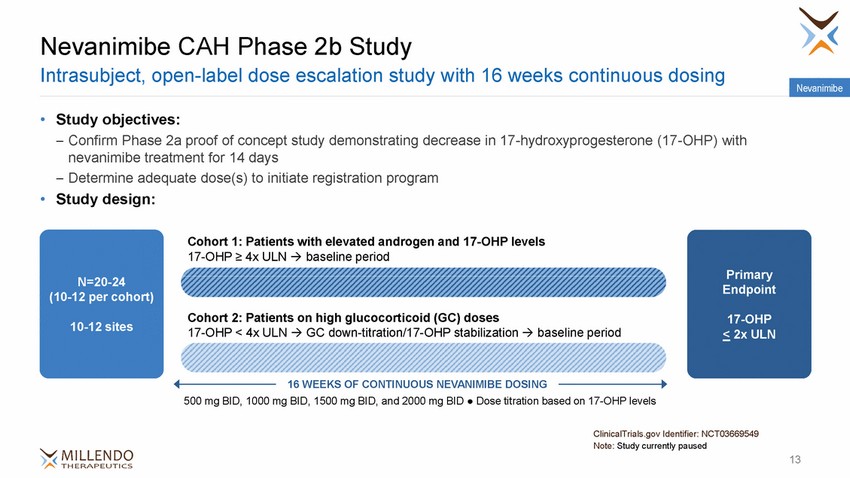 |
Nevanimibe CAH Phase 2b Study Intrasubject, open-label dose escalation study with 16 weeks continuous dosing Nevanimibe • Study objectives: – Confirm Phase 2a proof of concept study demonstrating decrease in 17-hydroxyprogesterone nevanimibe treatment for 14 days – Determine adequate dose(s) to initiate registration program Study design: (17-OHP) with • Cohort 1: Patients with elevated androgen and 17-OHP levels 17-OHP ≥ 4x ULN baseline period Primary Endpoint N=20-24 (10-12 per cohort) Cohort 2: Patients on high glucocorticoid (GC) doses 17-OHP < 4x ULN GC down-titration/17-OHP stabilization baseline period 17-OHP < 2x ULN 10-12 sites 16 WEEKS OF CONTINUOUS NEVANIMIBE DOSING 500 mg BID, 1000 mg BID, 1500 mg BID, and 2000 mg BID ● Dose titration based on 17-OHP levels ClinicalTrials.gov Identifier: NCT03669549 Note: Study currently paused 13 |
 |
Nevanimibe CAH Phase 2b: Interim Data Analysis Analyzed 10 subjects (9 cohort 1, 1 cohort 2) with at least 12 weeks of treatment Nevanimibe • Efficacy: – 50% of patients achieved a ≥ 50% decrease in 17-OHP, confirming nevanimibe activity in decreasing 17-OHP levels in patients with CAH – 1/10 (10%) of patients achieved the primary endpoint of reaching 17-OHP levels ≤ 2x upper limit of normal (ULN) – Decreases in 17-OHP did not consistently translate into decreases in androstenedione • Safety: – Starting dose of 500 mg BID was better tolerated than a 1000 mg BID starting dose – Adverse events reported were mostly gastrointestinal, skin and urinary events – Most adverse events were mild to moderate • Based on the observed level of nevanimibe activity and the changing competitive further investment in the nevanimibe program is currently planned environment, no 14 |
|
[LOGO] |

