Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - MOMENTA PHARMACEUTICALS INC | tm2022594d1_ex99-1.htm |
| 8-K - FORM 8-K - MOMENTA PHARMACEUTICALS INC | tm2022594d1_8k.htm |
Exhibit 99.2

Vivacity - MG Phase 2 Interim Analysis Topline Results Investor and Analyst Conference Call June 15, 2020

Introduction • Craig Wheeler, President and Chief Executive Officer Trial Design and Overview of Results • Santiago Arroyo, SVP and Chief Medical Officer Closing Remarks • Craig Wheeler, President and Chief Executive Officer Question & Answer Session Agenda 2

Statements in this presentation regarding management's future expectations, beliefs, intentions, goals, strategies, plans or p rospects, are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including but not lim ited to statements about our novel drug candidates for immune - mediated disorders, which include M281; the design, timing, enrollment, strategy and goals of clinical trials and the availability, timing and announcement of data and results; the use, efficacy, s afe ty, potency, dosing, tolerability, convenience, differentiation and commercial potential of our products and product candidates, including th eir potential as best - in - class agents; and our development timelines. Forward - looking statements may be identified by words such a s “anticipate” "believe," "continue," expect”, “intend” "plan to,", objectives”, “building”, “developing”, "potential," "will," an d other similar words or expressions, or the negative of these words or similar words or expressions. Such forward - looking statements in volve known and unknown risks, uncertainties and other important factors, including final and quality controlled verification of in ter im data and the related analyses; the impact of the COVID - 19 pandemic on the status, enrollment, timing and results of our clinical tria ls, the supply of our manufactured drug materials and our business; the unpredictable nature of early stage development efforts for o ur product candidates; safety, efficacy or tolerability problems with our product candidates; unexpected adverse clinical trial res ults; and those referred to under the section "Risk Factors" in the Company’s Quarterly Report on Form 10 - Q for the quarter ended March 31 , 2020 filed with the Securities and Exchange Commission, as well as other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. As a result of such risks, uncertainties and factors, the Company's actual res ults may differ materially from any future results, performance or achievements discussed in or implied by the forward - looking statements contained herein. The Company is providing the information in this presentation as of this date and assumes no obligations to up date the information included in this presentation or revise any forward - looking statements, whether as a result of new information, future events or otherwise. Forward Looking Statements 3

Myasthenia Gravis, a Rare Autoimmune Neuromuscular Disease 4 • Caused by circulating autoantibodies, most commonly against acetylcholine receptors ( AChR ) or the muscle - specific receptor tyrosine kinase ( MuSK ) • Autoantibodies disrupt these receptors at post - synaptic neuromuscular junctions, thus functionally blocking the excitatory action of acetylcholine • Bimodal age distribution: younger women and older men prototypical patients 60,000 MG patients in the U.S., 85% with generalized myasthenia gravis ( gMG ) Neuromuscular Junctions Source: MGFA and Momenta research

Nipocalimab (M281): Attributes of a potential best - in - class FcRn Antagonist 5 Dosing Dose - dependent IgG reduction Rapidly infused IV Weekly SC option Highest IgG reduction observed, >80% Ability to maintain 100% receptor occupancy drives IgG lowering and ability to maintain low IgG levels Efficacy Effectorless antibody design minimizes effector function related AEs Strong safety profile Safety 5

Nipocalimab was well tolerated, safe and efficacious in patients with gMG 6 Dosing Efficacy with monthly dosing and seen as early as two weeks Supports continued clinical development in gMG and subcutaneous formulation dose selection Efficacy Nipocalimab demonstrated efficacy at all doses tested Statistically significant relationship between IgG reduction and clinical benefit Safety Study supports best - in - class attributes of efficacy, safety and patient convenience Nipocalimab was well tolerated No infusion related reactions No clinically relevant changes in albumin or creatine phosphokinase No AEs leading to discontinuation, severe AEs, or nipocalimab related SAEs

• Data set includes all patients through Day 57 (week 8) • Analysis is for the primary efficacy outcome (MG - ADL change) and safety and laboratory data available up to day 113 (week 16) • The study was designed to detect a dose responsive efficacy in MG - ADL with at least an 80% power and experiment - wise one - sided type I error of 5% • COVID - 19 did not impact the primary safety and efficacy data • Expect additional study results to be available later in the year Topline Results Represent an Interim Analysis on the Study 7 &UHDWHGE\:LOVRQ-RVHSK IURPWKH1RXQ3URMHFW &UHDWHGE\SUL\DQND IURPWKH 1RXQ3URMHFW

Vivacity - MG Phase 2 Study Design 8 Placebo Q2W M281 5 mg/kg/Q4W M281 30 mg/kg/Q4W M281 60 mg/kg/Q2W M281 60 mg/kg single dose Open Label Extension Trial R a n d o m i z a t I o n Screening up to 4 weeks Week s Treatment Follow - Up 0 4 2 6 1 8 8 8 Primary Endpoint Assessment Dosing (M281 or placebo) given Q2W Age >18 years of age Documented history of gMG (Class II, III, or IVa ); both AChR and anti - MuSK enrolled QMG score of >=12 & MG - ADL score of >=4 On stable MG therapy with no changes during treatment period No plasmapheresis or IVIG within 6 weeks of randomization 68 Subjects Enrolled Key Eligibility Criteria:

9 Vivacity - MG: Subject Disposition 5 mg/kg Q4W 14 30 mg/kg Q4W 13 60 mg/kg single dose 13 60 mg/kg Q2W 14 Placebo 14* 1 (7.1%) Total completed @ D57 68 Randomized Discontinued treatment due to pandemic Discontinued treatment for other reasons 0 13 (92.9%) 2 (15.4%) 1 (7.7%) 10 (76.9%) 3 (23.1%) 0 10 (76.9%) 1 (7.1%) 0 13 (92.9%) 0 3 (21.4%) 11 (78.6%)

Nipocalimab (n=54) Placebo (n=13) Age mean years (SD) 54.3 (17.0) 58.7 (18.2) Female n (%) 29 (53.7) 7 (53.8) Time since diagnosis mean months (SD) 80.0 (83.3) 139.9 (114.7) MG - ADL score mean (SD) 8.0 (2.8) 7.0 (2.7) QMG score mean (SD) 16.5 (3.5) 17.7 (4.4) MGFA class n (%) II 20 (37.1) 5 (38.5) III 32 (59.3) 7 (53.9) IVa 2 (3.7) 1 (7.7) Anti - AChR positive n (%) 51 (94.4) 12 (92.3) Anti - MuSK positive n (%) 3 (5.6) 1 (7.7) Baseline Characteristics 10

Treatment Emergent Adverse Event Overview 11 There were no clinically relevant CK elevations Nipocalimab (n=54) Placebo (n=14) Patients with Adverse Event (AE) n (%) 44 (81.5) 11 (78.6) Patients with AE grade ≥3 n (%) 0 4 (28.6) Most frequent AEs n (%) Exacerbation of MG 0 2 (14.3) Headache 6 (11.1) 1 (7.1) Nasopharyngitis 6 (11.1) 0 Diarrhea 6 (11.1) 1 (7.1) Patients who discontinued due to AEs n (%) 0 2 (14.3) Patients with Serious Adverse Event (SAE) n (%) 1 (1.9)* 2 (14.3)* Patients with AEs deemed related by investigator n (%) 21 (38.9) 1 (7.1) *SAE deemed unrelated to study drug

12 Average Albumin Concentrations Were Within Normal Limits 1 patient had an asymptomatic Grade 2 Hypoalbuminemia in the 60 mg/kg Q2W 30 35 40 45 50 Serum Albumin (g/L) Baseline (N=67) Day 8 (N=27) Day 15 (N=66) Day 29 (N=61) Day 43 (N=63) Day 57 (N=59) Day 85 (N=54) Day 113 (N=46) Lower Limit of Normal Placebo 5 mg/kg Q4W 30 mg/kg Q4W 60 mg/kg single dose 60 mg/kg Q2W Serum Albumin Concentrations Treatment Period Follow - up Period

• Dose dependent IgG decreases • Rapid onset significant lowering of IgG within 1 st week • Maximal decrease achieved at 60 mg/kg Q2W • Similar reductions were seen in: - IgG subclasses - Anti - AChR antibodies • No changes in IgA or IgM concentrations Rapid and Dose Related Reduction of IgG as Predicted Serum Total IgG Concentrations 13 0 10 20 30 40 50 60 70 80 90 100 110 120 130 Serum Total IgG (% of baseline) Baseline (N=67) Day 8 (N=27) Day 15 (N=66) Day 29 (N=61) Day 43 (N=63) Day 57 (N=59) Day 85 (N=54) Day 113 (N=46) Placebo 5 mg/kg Q4W 30 mg/kg Q4W 60 mg/kg single dose 60 mg/kg Q2W Q4W Q2W Based on patients who completed all dosing treatments

Robust and Statistically Significant Relationship Between IgG Reduction and Clinical Benefit • MG - ADL improvement is highly correlated with serum IgG reduction (p<0.0001) • MG - ADL and Anti - AChR receptor binding antibodies are also highly correlated (p<0.0001) 14 ,J* 5DWLR;IURP %DVHOLQH &KDQJH IURP %DVHOLQH LQ0* $'/ S YDOXH IRU VORSH Comparison of MG - ADL Score and IgG Levels

Difference vs Placebo: 27.5% 30.8% 38.5% 48.9% p - value: 0.1044 0.1008 0.0484 0.0092 Durable MG - ADL Responses at All Doses 15 0 10 20 30 40 50 60 70 15.4% N=2/13 42.9% N=6/14 46.2% N=6/13 53.9% N=7/13 64.3% N=9/14 % Patients Pooled nipocalimab arms showed a 51.9% durable MG - ADL response vs 15.4% in placebo (p - value: 0.017) Durable response is defined as improvement in MG - ADL >= 2 points for at least 4 consecutive weeks during the 1st 8 weeks; p - valu es are one sided Placebo 5 mg/kg Q4W 30 mg/kg Q4W 60 mg/kg single dose 60 mg/kg Q2W

16 Significant Durable Reductions in MG - ADL Across All Doses 30 mg/kg Q4W 5 mg/kg Q4W Durable Response 6 5 4 3 2 14 7 0 7 14 7% 7% 14% 29% 15% 43% 15% Durable Response 6 5 4 3 2 14 7 0 7 14 8% 8% 15% 8% 15% 8% 23% 15% 46% 15% 8% 8% 8% Durable response is defined as improvement in MG - ADL >=2, 3, 4 ... points for at least 4 consecutive weeks 60 mg/kg Q2W 60 mg/kg single dose Durable Response 6 5 4 3 2 14 7 0 7 14 8% 8% 8% 8% 23% 8% 46% 54% Durable Response 6 5 4 3 2 14 7 0 7 14 14% 21% 29% 43% 64% 15% 15% 15% 15% 8% 8% 8% Placebo M281 5 mg/kg Q4W M281 30 mg/kg Q4W M281 60 mg/kg single dose M281 60 mg/kg Q2W

Early Onset Responders (response within the first 2 weeks) 17 Rapid Onset of Action with Clinical Response within First Two Weeks 15.4 % 42.9 % 38.5 % 46.2 % 42.9 % 0 5 10 15 20 25 30 35 40 45 50 Placebo 5 mg/kg Q4W 30 mg/kg Q4W 60 mg/kg single dose 60 mg/kg Q2W MG - ADL % Response

Robust and Dose Responsive MG - ADL Improvement from Baseline 18 Day 57 Day 29 Placebo 5 mg/kg Q4W 30 mg/kg Q4W - 1.4 - 1.7 - 2.9 - 3.9 - 3.1 -4.5 -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 - 1.8 - 2.5 - 3.9 - 1.5 - 3.9 -4.5 -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 MG - ADL Change from Baseline MG - ADL Change from Baseline 60 mg/kg single dose 60 mg/kg Q2W
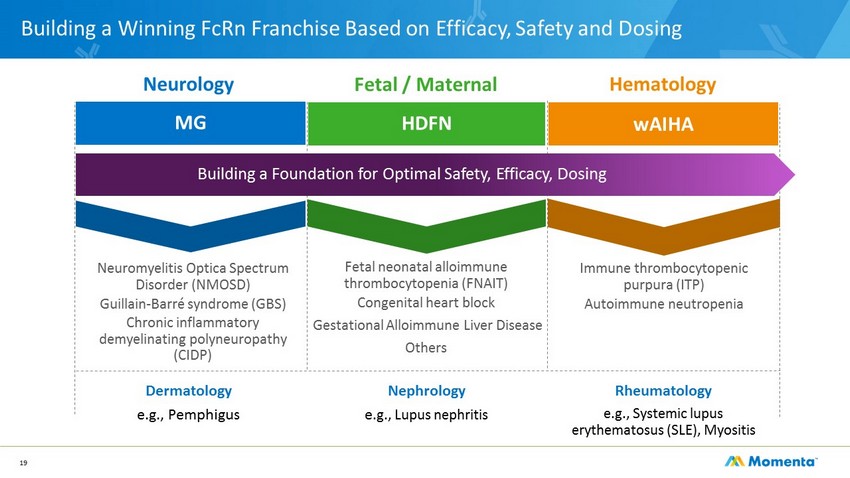
Building a Winning FcRn Franchise Based on Efficacy, Safety and Dosing 19 Dermatology e .g., Pemphigus Rheumatology e .g., Systemic lupus erythematosus (SLE), Myositis Nephrology e .g., Lupus nephritis MG Neurology Fetal / Maternal HDFN Fetal neonatal alloimmune thrombocytopenia (FNAIT) Congenital heart block Gestational Alloimmune Liver Disease Others Neuromyelitis Optica Spectrum Disorder (NMOSD) Guillain - Barré syndrome (GBS) Chronic inflammatory demyelinating polyneuropathy (CIDP) Immune thrombocytopenic purpura (ITP) Autoimmune neutropenia Hematology wAIHA Building a Foundation for Optimal Safety, Efficacy, Dosing

Vivacity - MG Phase 2 Interim Analysis Topline Results Investor and Analyst Conference Call June 15, 2020
