Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - CRISPR Therapeutics AG | crsp-ex991_7.htm |
| 8-K - 8-K - CRISPR Therapeutics AG | crsp-8k_20200612.htm |

Exhibit 99.2
Initial Safety and Efficacy Results with a Single Dose of Autologous CRISPR-Cas9-Modified CD34+ Hematopoietic Stem and Progenitor Cells in Transfusion-Dependent β-Thalassemia and Sickle Cell Disease Selim Corbacioglu1, Maria Domenica Cappellini2, John Chapin3, Nicole Chu-Osier4, Christine Marie Fernandez3, Juergen Foell1, Josu de la Fuente5, Stephan Grupp6, Tony W. Ho3, Antonis Kattamis7, Julie Lekstrom-Himes4, Franco Locatelli8, Yimeng Lu4, Mariane de Montalembert9, Damiano Rondelli10, Ainsley Ross3, Niraj Shanbhag4, Sujit Sheth11, Sandeep Soni12, Martin H. Steinberg13, Donna A. Wall14, Haydar Frangoul15 June 12, 2020 Session topic: 25. Gene therapy, cellular immunotherapy and vaccination - Clinical 1Paediatric Haemotology, Oncology and Stem Cell Transplantation, Regensburg University Hospital, Clinic and Polyclinic for Paediatric and Adolescent Medicine, Regensburg, Germany; 2Department of Clinical Sciences and Community, University of Milan, IRCCS Ca’ Granda Foundation Maggiore Policlinico Hospital, Milan, Italy; 3CRISPR Therapeutics, Cambridge, United States; 4Vertex Pharmaceuticals Incorporated, Boston, United States; 5Imperial College Healthcare NHS Trust, Hammersmith Hospital, London, United Kingdom; 6Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, United States; 7Division of Pediatric Hematology-Oncology, First Dept of Pediatrics, University of Athens, Athens, Greece; 8IRCCS Ospedale Pediatrico Bambino Gesù, Rome, Italy; 9Hôpital Universitaire Necker-Enfants Malades, Paris, France; 10University of Illinois at Chicago, Chicago, United States; 11Division of Pediatric Hematology / Oncology, Weill Cornell Medicine, New York, United States; 12Lucile Packard Children’s Hospital, Palo Alto, United States; 13Boston University, Boston, United States; 14Blood and Marrow Transplant/Cellular Therapy, Division of Haematology / Oncology, The Hospital for Sick Children and the University of Toronto, Toronto, Canada; 15The Children’s Hospital at TriStar Centennial Medical Center / Sarah Cannon Center for Blood Cancers, Nashville, United States

Disclosures This study was sponsored by Vertex Pharmaceuticals Incorporated and CRISPR Therapeutics AG JC is a shareholder of CRISPR Therapeutics and was an employee of CRISPR Therapeutics at the time this research was conducted. NC-O, JL-H, YL, and NS are employees of Vertex Pharmaceuticals Incorporated and hold stock and / or stock options in that company. CMF, TWH, and AR are employees of CRISPR Therapeutics and hold stock and / or stock options in that company. SG receives study support from Novartis, Kite, and Servier, consults for Novartis, Roche, GSK, Cure Genetics, Humanigen, CBMG, and Janssen / J&J, participates in study steering committees or scientific advisory boards for Jazz, Adaptimmune, TCR2, Eureka, Cellectis, Juno, and Vertex, and has a patent (Toxicity management for anti-tumor activity of CARs, WO2014011984A1) that is managed according to the University of Pennsylvania patent policy. AK has participated in advisory boards for Vertex Pharmaceuticals Incorporated / CRISPR Therapeutics, Novartis, Vifor, Ionis, and BMS / Celgene, has participated in a steering committee for Vertex Pharmaceuticals Incorporated / CRISPR Therapeutics, has received research support from Novartis, and has received speaker fees from BMS / Celgene. MM has participated in advisory boards for Addmedica, Bluebird Bio, and Novartis. MHS has participated in advisory boards for Vertex Pharmaceuticals Incorporated / CRISPR Therapeutics, Fulcrum Therapeutics, DSMB, and Imara. S. Sheth has served as a consultant for Acceleron, Agios, Bluebird Bio, Celgene, and Novartis, has received research support from Agios, Celgene, Dispersol, LaJolla, Novartis, and Terumo, and has participated in a steering committee for Vertex Pharmaceuticals Incorporated / CRISPR Therapeutics. S. Soni and HF have participated in a steering committee for Vertex Pharmaceuticals Incorporated / CRISPR Therapeutics. SC, MDC, JF, J de la F, FL, DR, and DAW have no conflicts to disclose Medical writing support was provided by Katie L. Beski, PhD of Complete HealthVizion, Inc., Chicago, IL, USA, funded by Vertex Pharmaceuticals Incorporated. Development and review coordination was provided by Leah Eardley, PhD of Vertex Pharmaceuticals Incorporated, who holds stock and / or stock options in that company
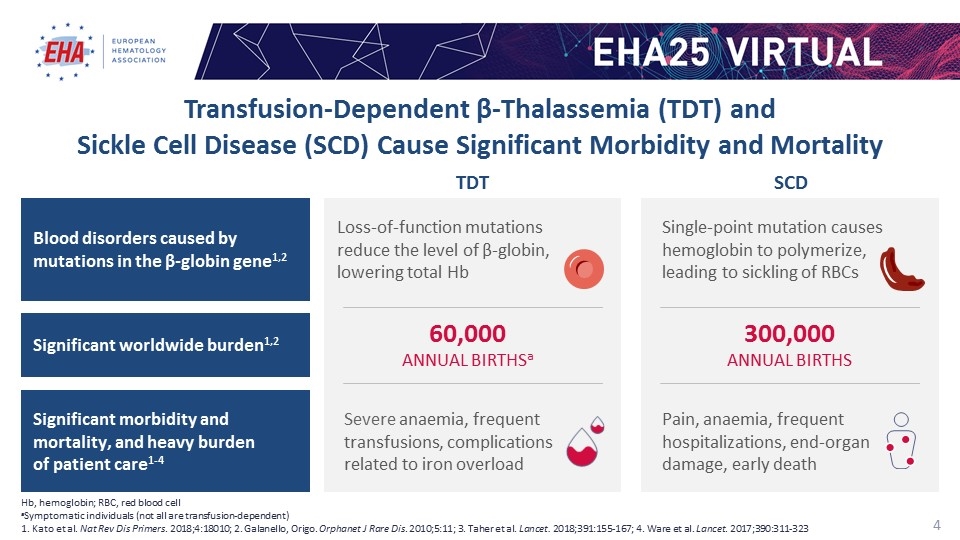
Severe anaemia, frequent transfusions, complications related to iron overload 60,000 ANNUAL BIRTHSa TDT Loss-of-function mutations reduce the level of β-globin, lowering total Hb 300,000 ANNUAL BIRTHS Pain, anaemia, frequent hospitalizations, end-organ damage, early death SCD Single-point mutation causes hemoglobin to polymerize, leading to sickling of RBCs Hb, hemoglobin; RBC, red blood cell aSymptomatic individuals (not all are transfusion-dependent) 1. Kato et al. Nat Rev Dis Primers. 2018;4:18010; 2. Galanello, Origo. Orphanet J Rare Dis. 2010;5:11; 3. Taher et al. Lancet. 2018;391:155-167; 4. Ware et al. Lancet. 2017;390:311-323 Transfusion-Dependent β-Thalassemia (TDT) and Sickle Cell Disease (SCD) Cause Significant Morbidity and Mortality Blood disorders caused by mutations in the β-globin gene1,2 Significant worldwide burden1,2 Significant morbidity and mortality, and heavy burden of patient care1-4
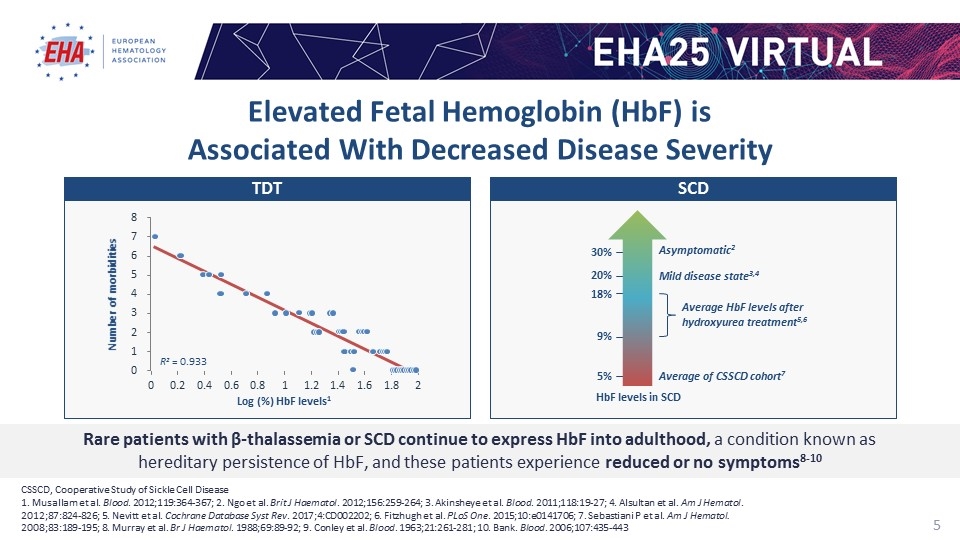
CSSCD, Cooperative Study of Sickle Cell Disease 1. Musallam et al. Blood. 2012;119:364-367; 2. Ngo et al. Brit J Haematol. 2012;156:259-264; 3. Akinsheye et al. Blood. 2011;118:19-27; 4. Alsultan et al. Am J Hematol. 2012;87:824-826; 5. Nevitt et al. Cochrane Database Syst Rev. 2017;4:CD002202; 6. Fitzhugh et al. PLoS One. 2015;10:e0141706; 7. Sebastiani P et al. Am J Hematol. 2008;83:189-195; 8. Murray et al. Br J Haematol. 1988;69:89-92; 9. Conley et al. Blood. 1963;21:261-281; 10. Bank. Blood. 2006;107:435-443 Rare patients with β-thalassemia or SCD continue to express HbF into adulthood, a condition known as hereditary persistence of HbF, and these patients experience reduced or no symptoms8-10 Log (%) HbF levels1 Number of morbidities R2 = 0.933 HbF levels in SCD 5% Average of CSSCD cohort7 Average HbF levels after hydroxyurea treatment5,6 9% 20% Mild disease state3,4 18% 30% Asymptomatic2 TDT SCD Elevated Fetal Hemoglobin (HbF) is Associated With Decreased Disease Severity
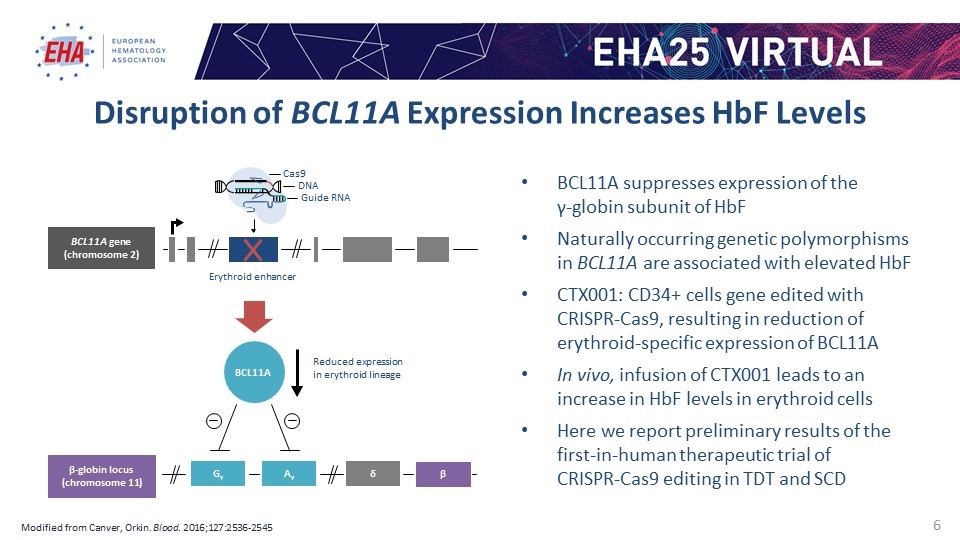
Disruption of BCL11A Expression Increases HbF Levels Modified from Canver, Orkin. Blood. 2016;127:2536-2545 BCL11A suppresses expression of the γ-globin subunit of HbF Naturally occurring genetic polymorphisms in BCL11A are associated with elevated HbF CTX001: CD34+ cells gene edited with CRISPR-Cas9, resulting in reduction of erythroid-specific expression of BCL11A In vivo, infusion of CTX001 leads to an increase in HbF levels in erythroid cells Here we report preliminary results of the first-in-human therapeutic trial of CRISPR-Cas9 editing in TDT and SCD Cas9 DNA BCL11A gene (chromosome 2) Erythroid enhancer BCL11A Reduced expression in erythroid lineage β-globin locus (chromosome 11) Gγ Aγ δ β Guide RNA

Phase 1 / 2 Studies in Patients with TDT and SCD Target enrollment 45 patients aged between 18 and 35 years with TDT, including β0 / β0 genotypes, defined as a history of at least 100 mL/kg/year or 10 units/year of packed RBC transfusions in the previous 2 years 45 patients aged between 18 and 35 years with severe SCD and a history of ≥2 vaso-occlusive crises (VOCs)/year over the previous 2 years Primary endpoint Proportion of patients achieving sustained transfusion reduction of 50% for at least 6 months starting 3 months after CTX001 infusion Proportion of patients with HbF ≥20% sustained for at least 3 months starting 6 months after CTX001 infusion Design Phase 1 / 2, international, multicenter, open-label, single-arm study (NCT03655678) Phase 1 / 2, international, multicenter, open-label, single-arm study (NCT03745287)
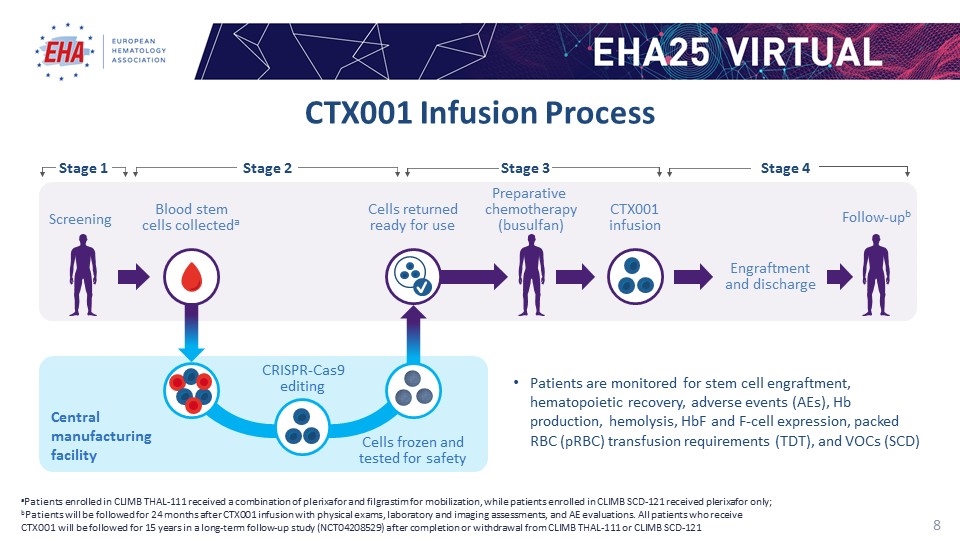
CTX001 Infusion Process aPatients enrolled in CLIMB THAL-111 received a combination of plerixafor and filgrastim for mobilization, while patients enrolled in CLIMB SCD-121 received plerixafor only; bPatients will be followed for 24 months after CTX001 infusion with physical exams, laboratory and imaging assessments, and AE evaluations. All patients who receive CTX001 will be followed for 15 years in a long-term follow-up study (NCT04208529) after completion or withdrawal from CLIMB THAL-111 or CLIMB SCD-121 Patients are monitored for stem cell engraftment, hematopoietic recovery, adverse events (AEs), Hb production, hemolysis, HbF and F-cell expression, packed RBC (pRBC) transfusion requirements (TDT), and VOCs (SCD) Engraftment and discharge Screening Blood stem cells collecteda Cells returned ready for use Preparative chemotherapy (busulfan) CTX001 infusion Follow-upb Cells frozen and tested for safety Central manufacturing facility CRISPR-Cas9 editing Stage 2 Stage 3 Stage 4 Stage 1
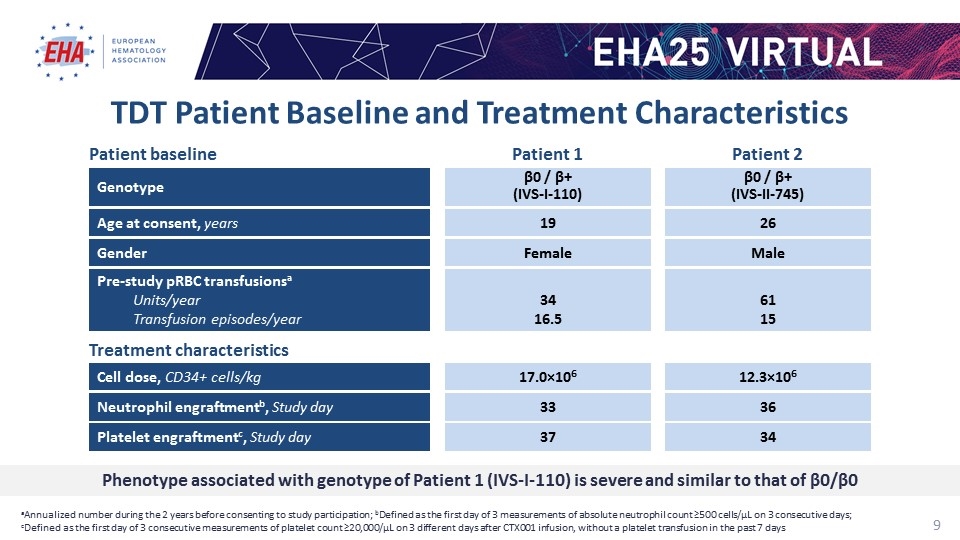
TDT Patient Baseline and Treatment Characteristics aAnnualized number during the 2 years before consenting to study participation; bDefined as the first day of 3 measurements of absolute neutrophil count ≥500 cells/µL on 3 consecutive days; cDefined as the first day of 3 consecutive measurements of platelet count ≥20,000/µL on 3 different days after CTX001 infusion, without a platelet transfusion in the past 7 days Cell dose, CD34+ cells/kg 17.0×106 12.3×106 Patient baseline Treatment characteristics Genotype β0 / β+ (IVS-I-110) β0 / β+ (IVS-II-745) Gender Female Male Age at consent, years 19 26 Pre-study pRBC transfusionsa Units/year Transfusion episodes/year 34 16.5 61 15 Neutrophil engraftmentb, Study day 33 36 Platelet engraftmentc, Study day 37 34 Patient 1 Patient 2 Phenotype associated with genotype of Patient 1 (IVS-I-110) is severe and similar to that of β0/β0
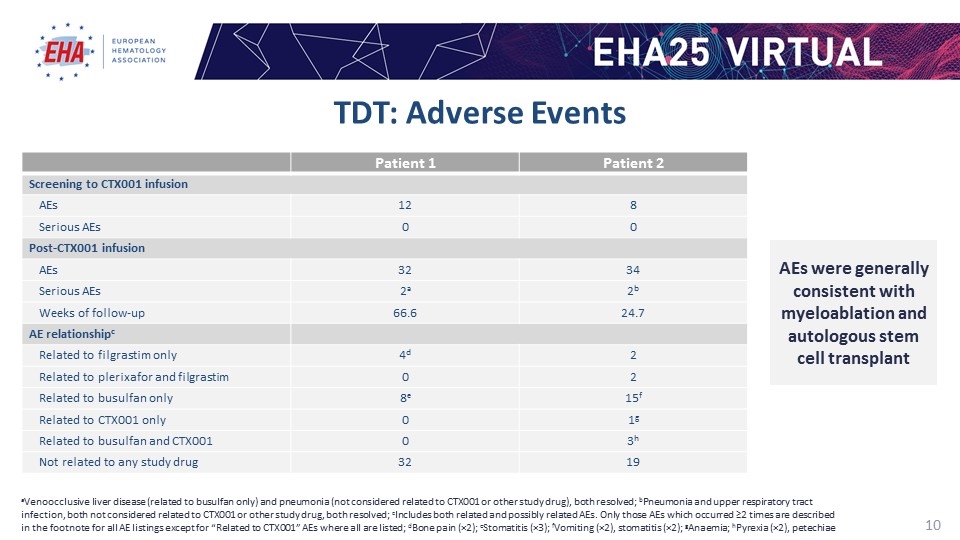
TDT: Adverse Events Patient 1 Patient 2 Screening to CTX001 infusion AEs 12 8 Serious AEs 0 0 Post-CTX001 infusion AEs 32 34 Serious AEs 2a 2b Weeks of follow-up 66.6 24.7 AE relationshipc Related to filgrastim only 4d 2 Related to plerixafor and filgrastim 0 2 Related to busulfan only 8e 15f Related to CTX001 only 0 1g Related to busulfan and CTX001 0 3h Not related to any study drug 32 19 aVenoocclusive liver disease (related to busulfan only) and pneumonia (not considered related to CTX001 or other study drug), both resolved; bPneumonia and upper respiratory tract infection, both not considered related to CTX001 or other study drug, both resolved; cIncludes both related and possibly related AEs. Only those AEs which occurred ≥2 times are described in the footnote for all AE listings except for “Related to CTX001” AEs where all are listed; dBone pain (×2); eStomatitis (×3); fVomiting (×2), stomatitis (×2); gAnaemia; hPyrexia (×2), petechiae AEs were generally consistent with myeloablation and autologous stem cell transplant
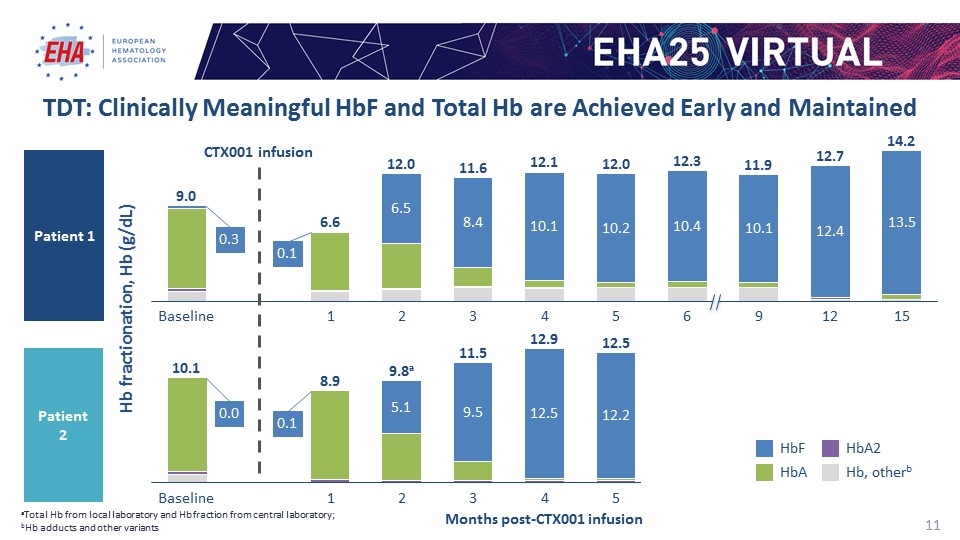
Months post-CTX001 infusion TDT: Clinically Meaningful HbF and Total Hb are Achieved Early and Maintained aTotal Hb from local laboratory and Hb fraction from central laboratory; bHb adducts and other variants Hb fractionation, Hb (g/dL) CTX001 infusion Patient 1 Patient 2 a Hb, otherb 0.3 0.1 0.1 0.0
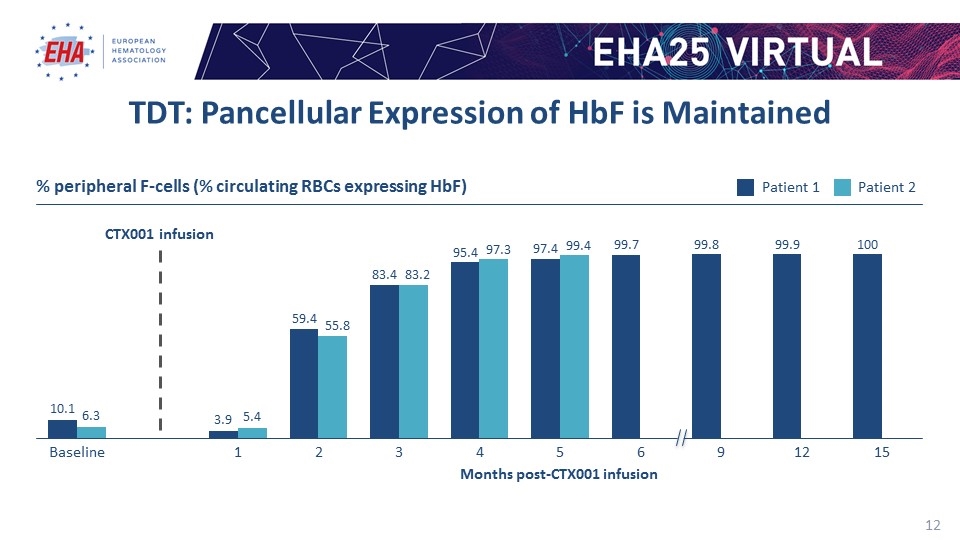
TDT: Pancellular Expression of HbF is Maintained % peripheral F-cells (% circulating RBCs expressing HbF) Months post-CTX001 infusion CTX001 infusion
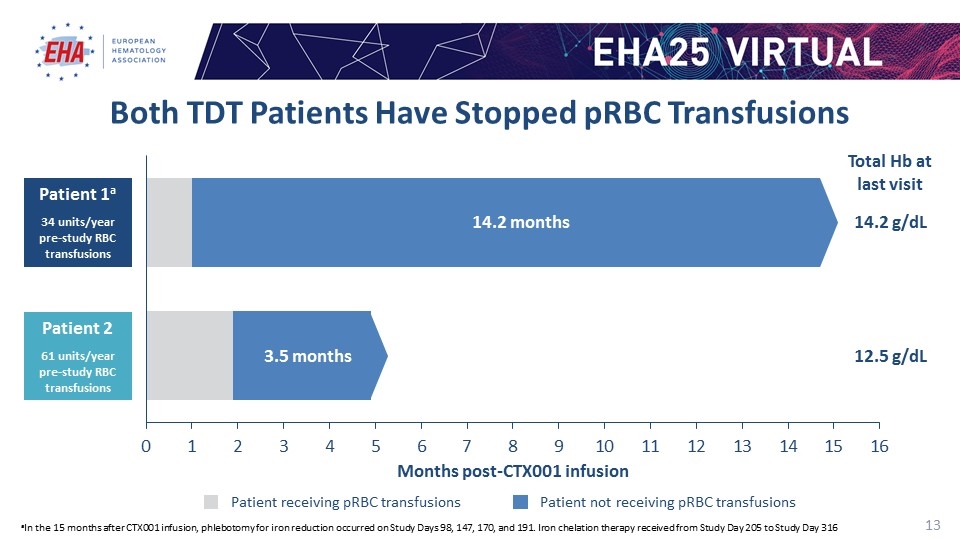
Both TDT Patients Have Stopped pRBC Transfusions aIn the 15 months after CTX001 infusion, phlebotomy for iron reduction occurred on Study Days 98, 147, 170, and 191. Iron chelation therapy received from Study Day 205 to Study Day 316 Patient receiving pRBC transfusions Patient not receiving pRBC transfusions Patient 1a 34 units/year pre-study RBC transfusions 14.2 months months Months post-CTX001 infusion Total Hb at last visit 14.2 g/dL 12.5 g/dL Patient 2 61 units/year pre-study RBC transfusions
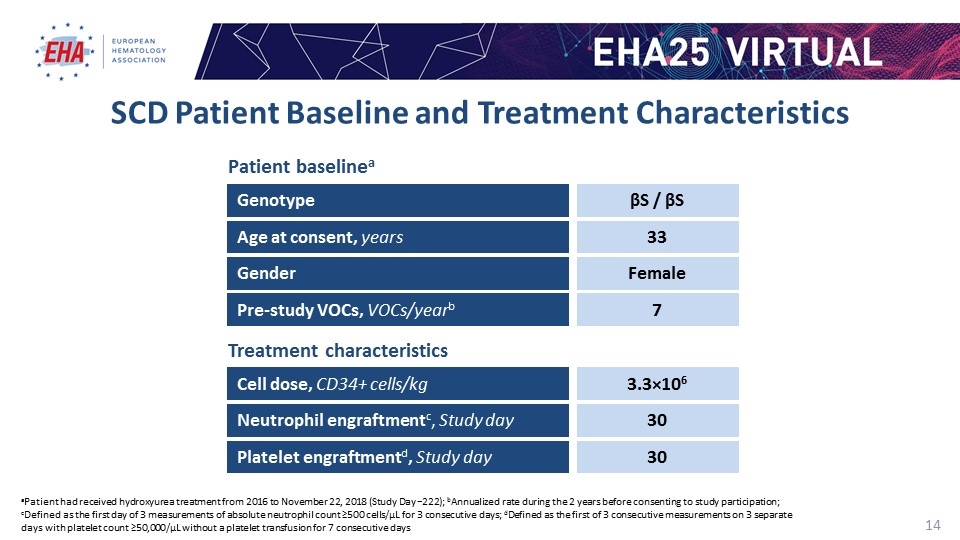
SCD Patient Baseline and Treatment Characteristics aPatient had received hydroxyurea treatment from 2016 to November 22, 2018 (Study Day −222); bAnnualized rate during the 2 years before consenting to study participation; cDefined as the first day of 3 measurements of absolute neutrophil count ≥500 cells/µL for 3 consecutive days; dDefined as the first of 3 consecutive measurements on 3 separate days with platelet count ≥50,000/µL without a platelet transfusion for 7 consecutive days Cell dose, CD34+ cells/kg Neutrophil engraftmentc, Study day Platelet engraftmentd, Study day 3.3×106 Genotype βS / βS Gender Female Age at consent, years 33 Pre-study VOCs, VOCs/yearb 7 30 30 Patient baselinea Treatment characteristics
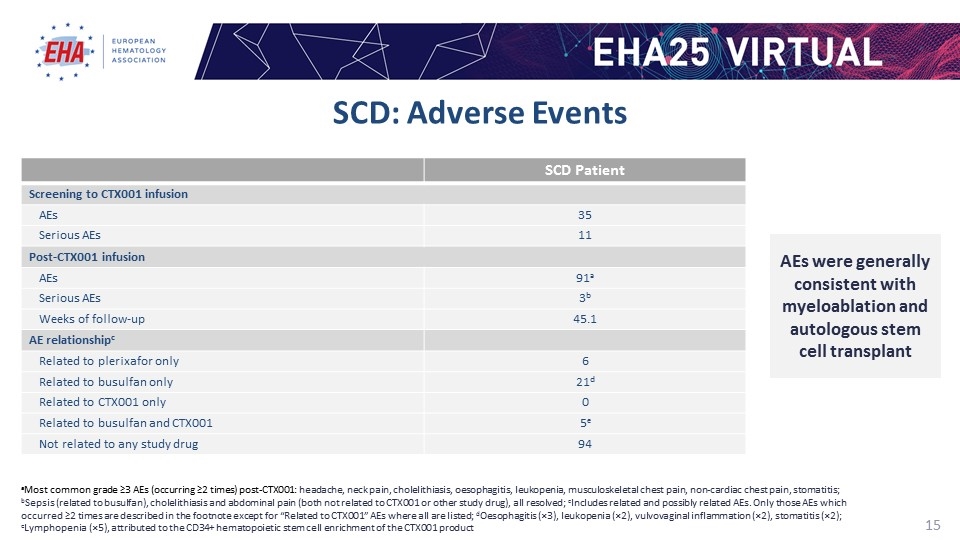
SCD: Adverse Events SCD Patient Screening to CTX001 infusion AEs 35 Serious AEs 11 Post-CTX001 infusion AEs 91a Serious AEs 3b Weeks of follow-up 45.1 AE relationshipc Related to plerixafor only 6 Related to busulfan only 21d Related to CTX001 only 0 Related to busulfan and CTX001 5e Not related to any study drug 94 aMost common grade ≥3 AEs (occurring ≥2 times) post-CTX001: headache, neck pain, cholelithiasis, oesophagitis, leukopenia, musculoskeletal chest pain, non-cardiac chest pain, stomatitis; bSepsis (related to busulfan), cholelithiasis and abdominal pain (both not related to CTX001 or other study drug), all resolved; cIncludes related and possibly related AEs. Only those AEs which occurred ≥2 times are described in the footnote except for “Related to CTX001” AEs where all are listed; dOesophagitis (×3), leukopenia (×2), vulvovaginal inflammation (×2), stomatitis (×2); eLymphopenia (×5), attributed to the CD34+ hematopoietic stem cell enrichment of the CTX001 product AEs were generally consistent with myeloablation and autologous stem cell transplant
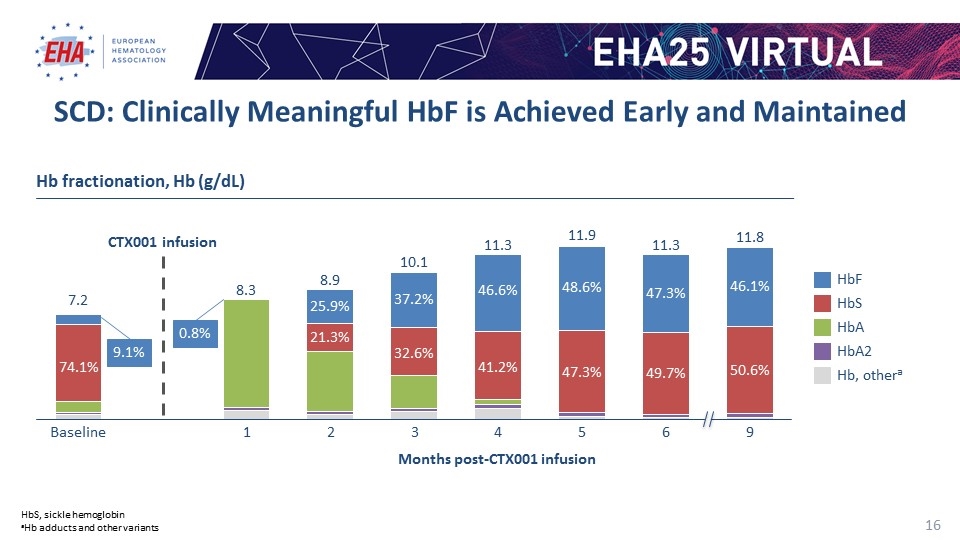
CTX001 infusion Months post-CTX001 infusion Hb fractionation, Hb (g/dL) HbS HbA 2 Hb, othera SCD: Clinically Meaningful HbF is Achieved Early and Maintained HbS, sickle hemoglobin aHb adducts and other variants 9.1% 0.8%
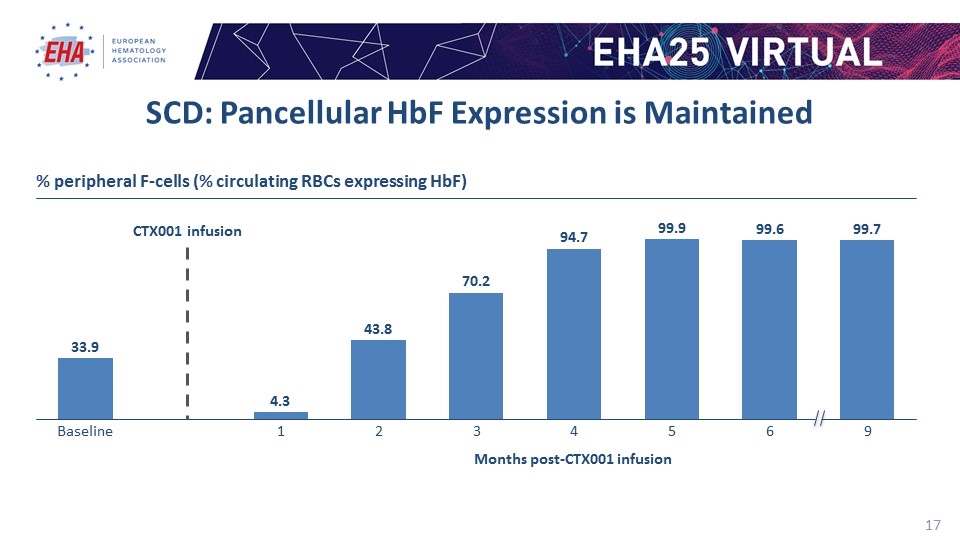
CTX001 infusion SCD: Pancellular HbF Expression is Maintained % peripheral F-cells (% circulating RBCs expressing HbF) Months post-CTX001 infusion 33.9 4.3 43.8 70.2 94.7 99.9 99.6 99.7
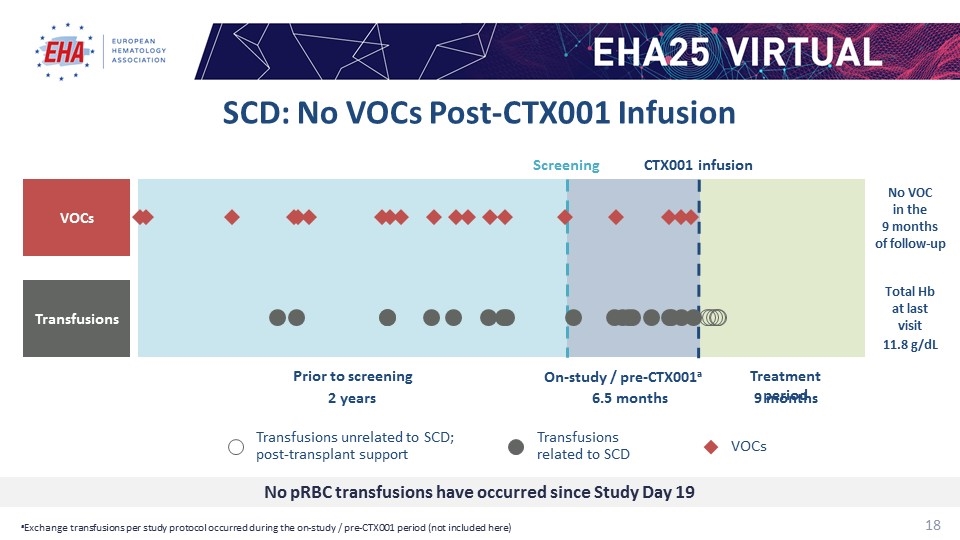
Screening CTX001 infusion 6.5 months 9 months On-study / pre-CTX001a Treatment period 2 years Prior to screening Total Hb at last visit 11.8 g/dL No VOC in the 9 months of follow-up No pRBC transfusions have occurred since Study Day 19 VOCs Transfusions VOCs Transfusions related to SCD Transfusions unrelated to SCD; post-transplant support SCD: No VOCs Post-CTX001 Infusion aExchange transfusions per study protocol occurred during the on-study / pre-CTX001 period (not included here)
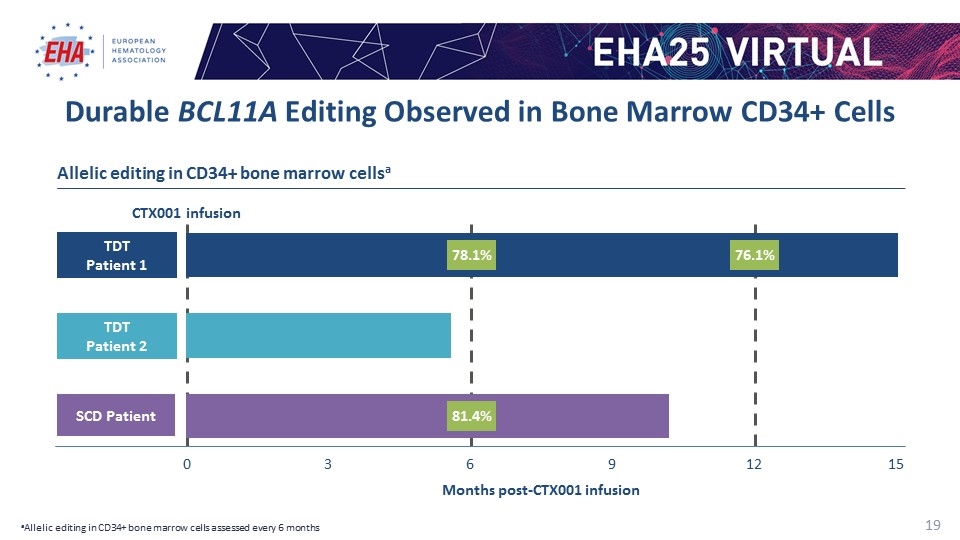
CTX001 infusion Durable BCL11A Editing Observed in Bone Marrow CD34+ Cells Months post-CTX001 infusion TDT Patient 1 SCD Patient 0 3 Allelic editing in CD34+ bone marrow cellsa TDT Patient 2 aAllelic editing in CD34+ bone marrow cells assessed every 6 months 78.1% 81.4% 76.1%
Conclusions These studies are the first demonstration of the clinical impact of CRISPR-Cas9-based gene editing for hemoglobinopathies and establish proof of concept for TDT Overall safety is consistent with myeloablative conditioning and autologous transplant Clinically meaningful HbF and total Hb levels, as well as pancellular expression of HbF in red blood cells, are observed early and maintained in TDT and SCD First 2 TDT patients have been free of pRBC transfusions for >14 and >3 months respectively; first SCD patient has had no VOCs in >9 months Sustained engraftment of edited hematopoietic stem cells is supportive of long-term clinical efficacy Enrollment and manufacturing of CTX001 for TDT and SCD are ongoing with further dosing planned in 2020 CTX001 has been granted Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA, Orphan Drug Designation from both the FDA and the EMA, and Fast Track Designation from the FDA
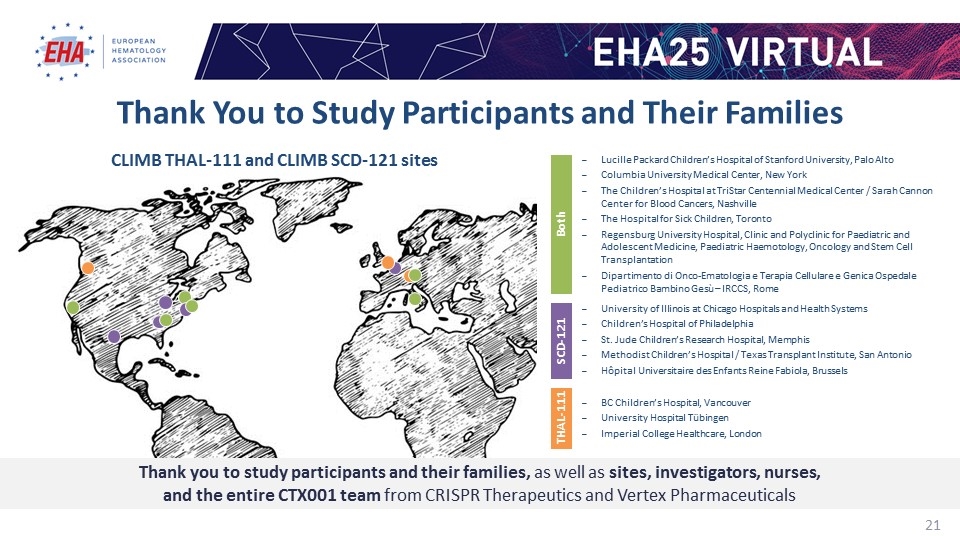
Thank You to Study Participants and Their Families CLIMB THAL-111 and CLIMB SCD-121 sites Lucille Packard Children’s Hospital of Stanford University, Palo Alto Columbia University Medical Center, New York The Children’s Hospital at TriStar Centennial Medical Center / Sarah Cannon Center for Blood Cancers, Nashville The Hospital for Sick Children, Toronto Regensburg University Hospital, Clinic and Polyclinic for Paediatric and Adolescent Medicine, Paediatric Haemotology, Oncology and Stem Cell Transplantation Dipartimento di Onco-Ematologia e Terapia Cellulare e Genica Ospedale Pediatrico Bambino Gesù – IRCCS, Rome Both University of Illinois at Chicago Hospitals and Health Systems Children’s Hospital of Philadelphia St. Jude Children’s Research Hospital, Memphis Methodist Children’s Hospital / Texas Transplant Institute, San Antonio Hôpital Universitaire des Enfants Reine Fabiola, Brussels SCD-121 BC Children’s Hospital, Vancouver University Hospital Tübingen Imperial College Healthcare, London THAL-111 Thank you to study participants and their families, as well as sites, investigators, nurses, and the entire CTX001 team from CRISPR Therapeutics and Vertex Pharmaceuticals
