Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - EYEGATE PHARMACEUTICALS INC | tm2021443d1_8k.htm |
Exhibit 99.1

NASDAQ: EYEG Corporate Presentation

2 Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s OBG and EGP - 437 product candidates , for the timing and outcome of the Company’s clinical trials, the potential approval to market OBG and EGP - 437 , and the Company’s capital needs . Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation . Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful . The Company may consume more cash than it currently anticipates and faster than projected . Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates . If the U . S . Food and Drug Administration or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them . Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities . If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations . Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on March 04 , 2020 . The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law . The Company uses its website ( www . EyeGatePharma . com ), Facebook page ( https : //www . facebook . com/ EyeGatePharma / ), corporate Twitter account ( https : //twitter . com/EyeGatePharma ), and LinkedIn page ( https : //www . linkedin . com/company/ 135892 / ) as channels of distribution of information about the Company and its product candidates . Such information may be deemed material information, and the Company may use these channels to comply with its disclosure obligations under Regulation FD . Therefore, investors should monitor the Company’s website and its social media accounts in addition to following its press releases, SEC filings, public conference calls, and webcasts . The social media channels that the Company intends to use as a means of disclosing the information described above may be updated from time to time as listed on the Company’s investor relations website . Forward Looking Statements 2
3 EyeGate Pharma: Company Highlights Nasdaq: EYEG » EyeGate is NASDAQ listed; went public in 2015 » Product under development, OBG, is an eye drop formulation of modified hyaluronic acid (HA) ▪ Currently being developed for two indications: wound healing and dry eye » HA is a natural substance in the body that promotes wound healing and provides hydration and lubrication » OBG has recently completed clinical development for the first indication, wound healing, by demonstrating superiority over standard - of - care in a pivotal study » OBG has recently completed a follow - on phase 2 dry eye study and continues to demonstrate healing of the corneal surface

OBG: A Therapeutic Lubricant » OBG supports corneal health and protects/treats the eye » OBG is a crosslinked or chemically modified version of the natural polymer hyaluronic acid (“HA”) ▪ HA has a high viscosity that promotes wound healing and provides hydration and lubrication ▪ Crosslinking stabilizes the HA molecule and prevents degradation while on the ocular surface » Crosslinking provides a prolonged retention (up to 2 hrs ) on the corneal surface without causing blurriness (supporting clinical data) ▪ The viscosity of OBG decreases with blinking due to its high shear thinning properties » Will be the only HA eye drop in the U.S. where HA is the active ingredient ▪ HA at 0.75% concentration is preservative free; only other ingredients are water and PBS (pH and osmolarity) 4 OBG being developed as a class II device (de novo filing)

OBG: Wound Healing and Dry Eye » Recently, demonstrated statistical significance in a pivotal study against a bandage contact lens for the acceleration of wound healing in patients undergoing PRK surgery (9mm epi defects) ▪ Development for this indication complete; preparing de novo filing » OBG has now demonstrated that it effectively treats patients with dry eye ▪ A uniquely designed study confirmed the ability of OBG eye drops to improve the ocular surface for several important ophthalmic endpoints ▪ Outperforming the positive control, Allergan’s Refresh Preservative - Free lubricant ▪ Focus is on Moderate Dry Eye patient population (greatest opportunity) » Next step: pre - submission meeting with FDA’s CDRH Division to confirm pivotal study design 5 Dry Eye de novo filing only requires: • ONE pivotal study and • ONE endpoint

» OBG Stand - Alone Device ▪ Wound Healing: Development completed for wound healing (post PRK surgery) ▪ Completing manufacturing testing required to file de novo application ▪ Targeting filing de novo application by end of 2020 ▪ Dry Eye: FDA meeting scheduled for July 13 to discuss moving into pivotal study ▪ Targeting initiation of pivotal study by end of 2020 » OBG Plus Active ▪ First combination product is OBG plus an antibiotic - 505(b)(2) filing ▪ Pre - IND Meeting scheduled for Aug 10 ▪ Targeting end of 2020 1 for IND filing ▪ Same concentration, dosing regimen, indication as Vigamox (expect only ONE Ph 3 study 1 ) The OBG Franchise 1. Will confirm at pre - IND meeting 6

• Follow - on Pilot Study OBG: Dry Eye
Punctate Epitheliopathy and Dry Eye » Punctates are a sign of epithelial compromise (corneal barrier disruption) and is characterized by a breakdown of the epithelium of the cornea and an increased permeability to fluorescein dye » Corneal barrier disruption is associated with an increased risk of corneal ulceration, corneal haze and decreased vision » Corneal permeability to fluorescein dye is used to clinically evaluate severity of corneal barrier disruption » PE is associated with many pathologic ocular inflammatory conditions, which can include: ▪ dry eye , conjunctivitis, trauma/corneal abrasions, contact lens wear, and chemical irritation and burns » Nothing currently approved for the treatment of PE in the U.S. 8 Planned “indications for use”: improvement of punctate epitheliopathy in patients with dry eye
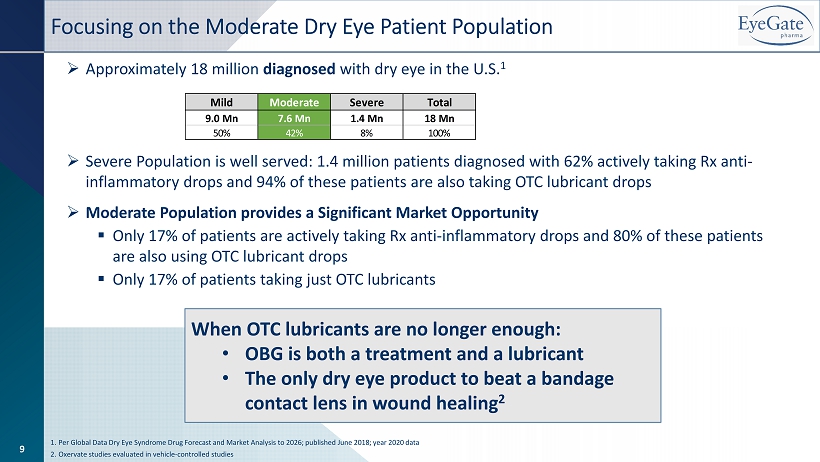
Focusing on the Moderate Dry Eye Patient Population » Approximately 18 million diagnosed with dry eye in the U.S. 1 » Severe Population is well served: 1.4 million patients diagnosed with 62% actively taking Rx anti - inflammatory drops and 94% of these patients are also taking OTC lubricant drops » Moderate Population provides a Significant Market Opportunity ▪ Only 17% of patients are actively taking Rx anti - inflammatory drops and 80% of these patients are also using OTC lubricant drops ▪ Only 17% of patients taking just OTC lubricants 1. Per Global Data Dry Eye Syndrome Drug Forecast and Market Analysis to 2026; published June 2018; year 2020 data 2. Oxervate studies evaluated in vehicle - controlled studies Mild Moderate Severe Total 9.0 Mn 7.6 Mn 1.4 Mn 18 Mn 50% 42% 8% 100% 9 When OTC lubricants are no longer enough: • OBG is both a treatment and a lubricant • The only dry eye product to beat a bandage contact lens in wound healing 2
» 20 patients and 40 eyes as both eyes must qualify for study randomization » Investigator masked with positive - control » Patient acted as own control; one eye randomized to OBG and one eye randomized to Refresh® » 7 day run - in period with all eyes taking Refresh QID, followed by 14 day QID treatment period » Main Inclusion Criteria: ▪ Fluorescein staining of cornea using NEI scale (≥4, max is 15) and ▪ Slit lamp Tear Film Break - Up Time (≤7 seconds) » Effectiveness Outcomes: ▪ Treatment assessments: at time zero on visit days ▪ Post - application assessments: at 30 and 60 minutes after drops applied ▪ Drop applied to respective eye after all treatment assessments completed Dry Eye Study Design (PE - 042) 10

» POSITIVE RESULTS ▪ Objectives – corneal health, tear film and vision • All OBG assessments support central cornea healing (Staining, HOA, TFBUT and BCVA) ▪ OBG outperforms in central cornea staining with a 25% improvement from baseline vs only 15% for Refresh® • Supports data seen in first study where OBG improvement over vehicle (saline solution) at Day 14 was 19% • Xiidra demonstrated a 6.9% difference over vehicle at Day 84 in the Opus 3 study (inferior region) • Xiidra (~$400 million 2018 sales) sold for $3.4 billion (upfront) in 2019 to Novartis (additional $1.9 bn milestones) • Also, OBG works quickly with a 10% improvement at Day 7 vs NO improvement for Refresh® Overview » STRONG DESIGN ▪ Small N: Only 20 patients – investigator masked with patient as their own control • Both eyes of patient had to qualify; one eye was randomized to OBG and the other eye to a positive control • Reduces interpatient variability ▪ High Standard: OBG against a positive control; Allergan’s Refresh® Preservative Free lubricant • Drugs developed for dry eye go against placebo (vehicle) 11

OBG: Dry Eye Study Treatment Assessments • Corneal Staining • High Order Aberrations (HOA) • Best Corrected Visual Acuity (BCVA)
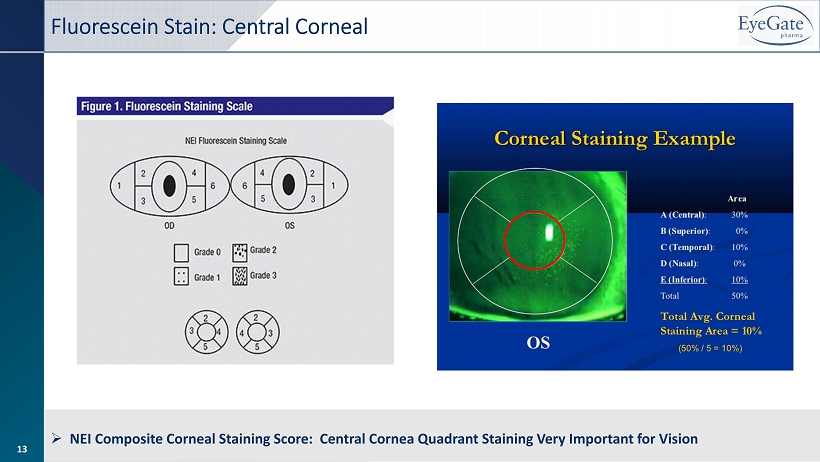
13 Fluorescein Stain: Central Corneal » NEI Composite Corneal Staining Score: Central Cornea Quadrant Staining Very Important for Vision 13
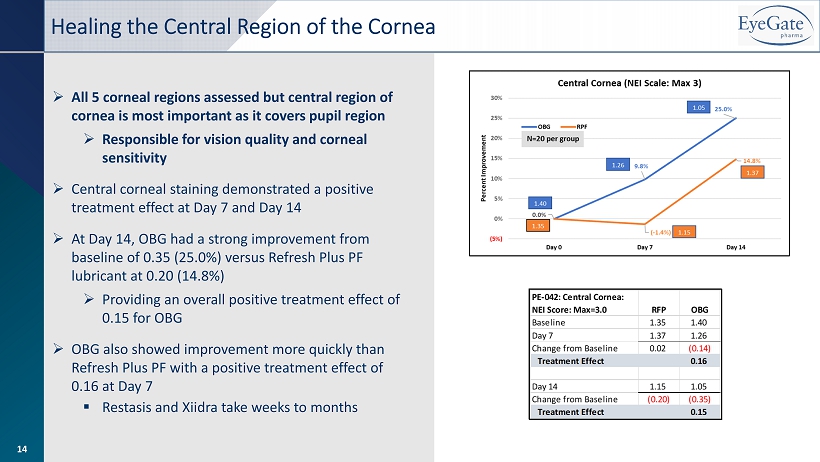
» All 5 corneal regions assessed but central region of cornea is most important as it covers pupil region » Responsible for vision quality and corneal sensitivity » Central corneal staining demonstrated a positive treatment effect at Day 7 and Day 14 » At Day 14, OBG had a strong improvement from baseline of 0.35 (25.0%) versus Refresh Plus PF lubricant at 0.20 (14.8%) » Providing an overall positive treatment effect of 0.15 for OBG » OBG also showed improvement more quickly than Refresh Plus PF with a positive treatment effect of 0.16 at Day 7 ▪ Restasis and Xiidra take weeks to months Healing the Central Region of the Cornea 14
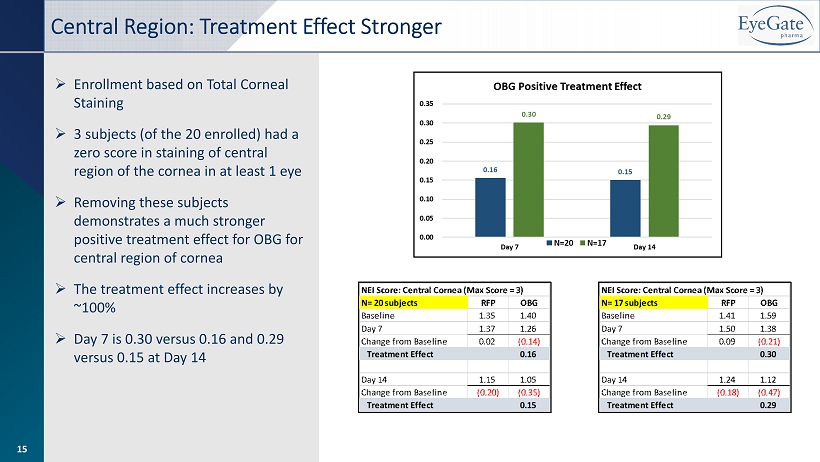
» Enrollment based on Total Corneal Staining » 3 subjects (of the 20 enrolled) had a zero score in staining of central region of the cornea in at least 1 eye » Removing these subjects demonstrates a much stronger positive treatment effect for OBG for central region of cornea » The treatment effect increases by ~100% » Day 7 is 0.30 versus 0.16 and 0.29 versus 0.15 at Day 14 Central Region: Treatment Effect Stronger 15
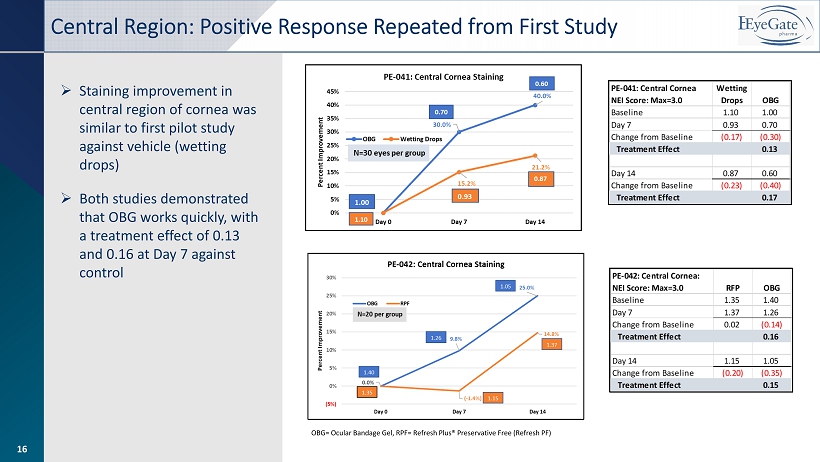
16 OBG= Ocular Bandage Gel, RPF= Refresh Plus® Preservative Free (Refresh PF) » Staining improvement in central region of cornea was similar to first pilot study against vehicle (wetting drops) » Both studies demonstrated that OBG works quickly, with a treatment effect of 0.13 and 0.16 at Day 7 against control Central Region: Positive Response Repeated from First Study 16
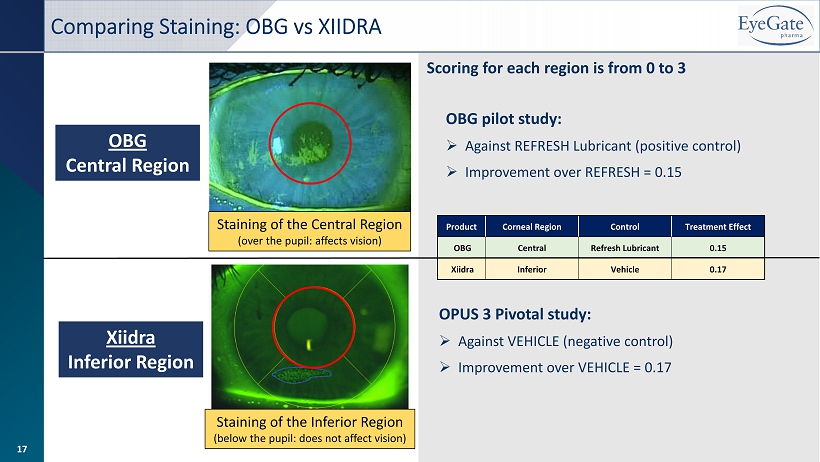
OBG Central Region Xiidra Inferior Region Staining of the Inferior Region (below the pupil: does not affect vision) Staining of the Central Region (over the pupil: affects vision) Product Corneal Region Control Treatment Effect OBG Central Refresh Lubricant 0.15 Xiidra Inferior Vehicle 0.17 OBG pilot study: » Against REFRESH Lubricant (positive control) » Improvement over REFRESH = 0.15 OPUS 3 Pivotal study: » Against VEHICLE (negative control) » Improvement over VEHICLE = 0.17 Scoring for each region is from 0 to 3 Comparing Staining: OBG vs XIIDRA 17

OBG: Dry Eye Study Post Application Assessments • Vision Break - Up Time (VBUT) • Best Corrected Visual Acuity (BCVA)
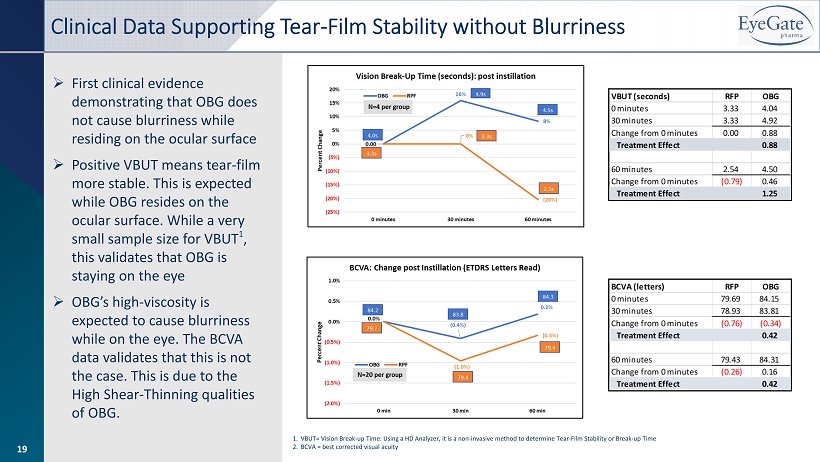
19 » First clinical evidence demonstrating that OBG does not cause blurriness while residing on the ocular surface » Positive VBUT means tear - film more stable. This is expected while OBG resides on the ocular surface. While a very small sample size for VBUT 1 , this validates that OBG is staying on the eye » OBG’s high - viscosity is expected to cause blurriness while on the eye. The BCVA data validates that this is not the case. This is due to the High Shear - Thinning qualities of OBG. Clinical Data Supporting Tear - Film Stability without Blurriness 1. VBUT= Vision Break - up Time: Using a HD Analyzer, it is a non - invasive method to determine Tear - Film Stability or Break - up Time 2. BCVA = best corrected visual acuity
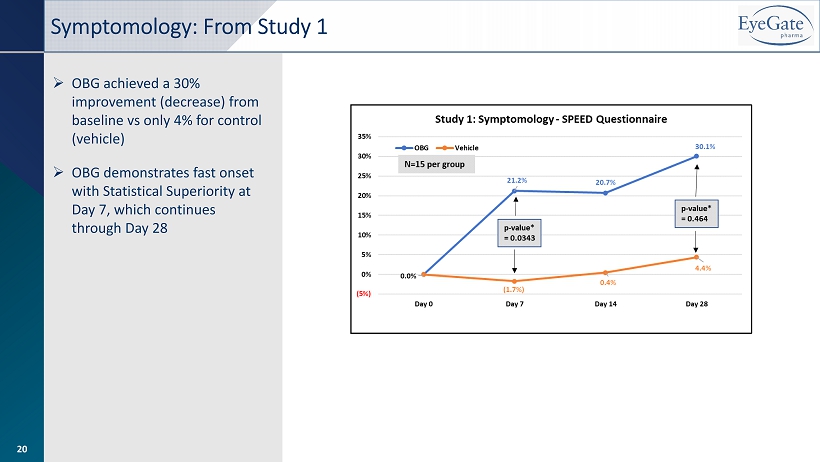
» OBG achieved a 30% improvement (decrease) from baseline vs only 4% for control (vehicle) » OBG demonstrates fast onset with Statistical Superiority at Day 7, which continues through Day 28 Symptomology: From Study 1 20

OBG: Wound Healing

» PRK is a surgical correction of refractive errors for patients who are not suitable candidates for LASIK due to: ▪ Inadequate corneal thickness, larger pupil size, dry eye, and anterior basement membrane disease » PRK involves controlled mechanical removal of corneal epithelium with subsequent lasering of stroma » Although PRK yields excellent visual results, common complications include: ▪ Post - operative pain, risk of infection, corneal haze, decreased contrast sensitivity, and slower visual recovery » Enabling the epithelium to heal faster may mitigate the immediate peri - operative complications as well as improve the longer visual term outcomes » PRK population ideal for clinical development: ▪ Large population (~700,000 LASIK/PRK surgeries per year in the U.S.) ▪ Large wound (9mm), same size for all patients and know time zero ▪ Healthy eyes required and time to healing well established » Standard - of - care is a Bandage Contact Lens (BCL); can result in subsequent erosion of epithelium Wound Healing: Refractive Market 22
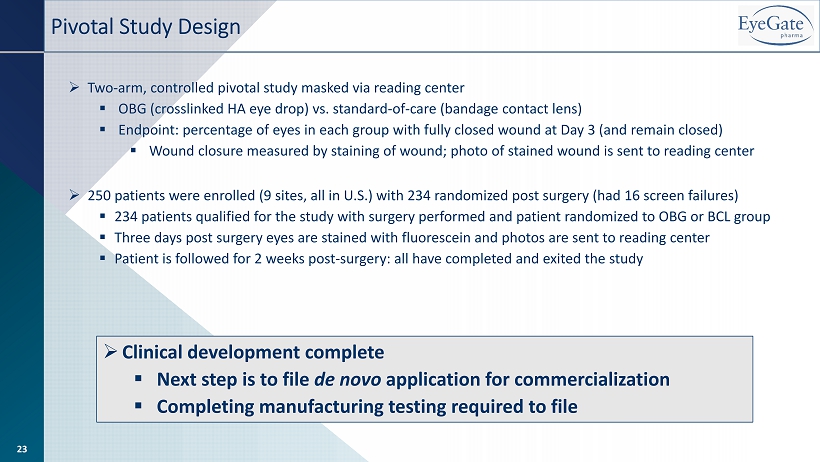
» Clinical development complete ▪ Next step is to file de novo application for commercialization ▪ Completing manufacturing testing required to file Pivotal Study Design » Two - arm, controlled pivotal study masked via reading center ▪ OBG (crosslinked HA eye drop) vs. standard - of - care (bandage contact lens) ▪ Endpoint: percentage of eyes in each group with fully closed wound at Day 3 (and remain closed) ▪ Wound closure measured by staining of wound; photo of stained wound is sent to reading center » 250 patients were enrolled (9 sites, all in U.S.) with 234 randomized post surgery (had 16 screen failures) ▪ 234 patients qualified for the study with surgery performed and patient randomized to OBG or BCL group ▪ Three days post surgery eyes are stained with fluorescein and photos are sent to reading center ▪ Patient is followed for 2 weeks post - surgery: all have completed and exited the study 23
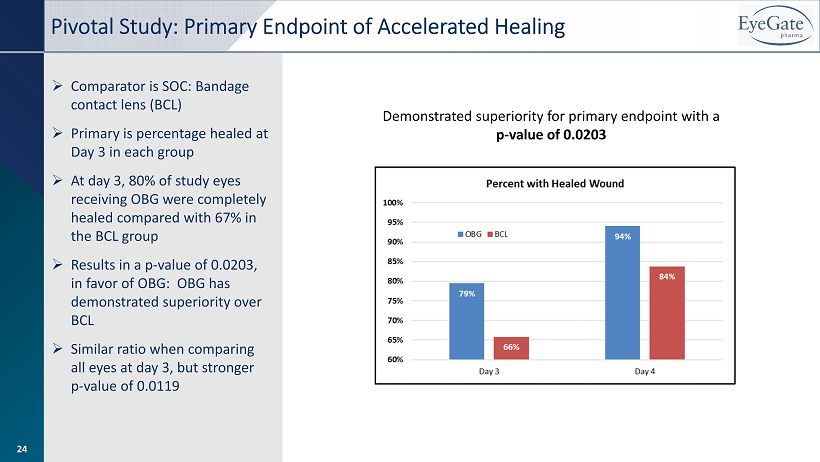
» Comparator is SOC: Bandage contact lens (BCL) » Primary is percentage healed at Day 3 in each group » At day 3, 80% of study eyes receiving OBG were completely healed compared with 67% in the BCL group » Results in a p - value of 0.0203, in favor of OBG: OBG has demonstrated superiority over BCL » Similar ratio when comparing all eyes at day 3, but stronger p - value of 0.0119 Pivotal Study: Primary Endpoint of Accelerated Healing Demonstrated superiority for primary endpoint with a p - value of 0.0203 24
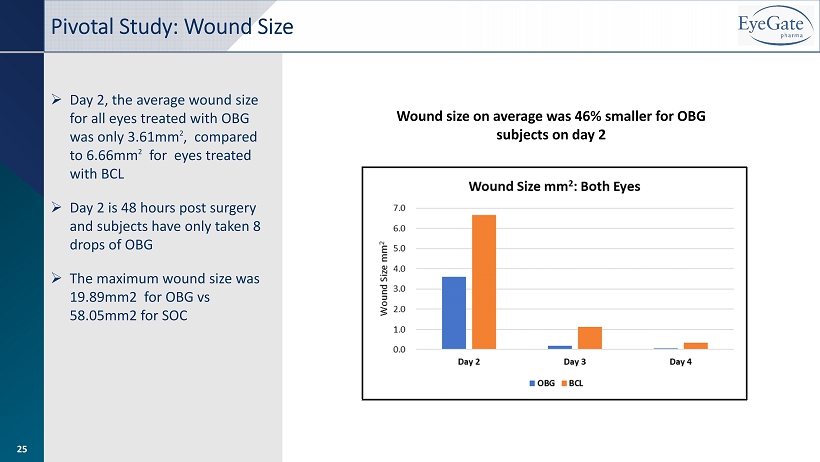
Pivotal Study: Wound Size Wound size on average was 46% smaller for OBG subjects on day 2 » Day 2, the average wound size for all eyes treated with OBG was only 3.61mm 2 , compared to 6.66mm 2 for eyes treated with BCL » Day 2 is 48 hours post surgery and subjects have only taken 8 drops of OBG » The maximum wound size was 19.89mm2 for OBG vs 58.05mm2 for SOC 25

OBG: Combination Product

Large market opportunity: • 16 million Rx’s written annually in the U.S. 1 • Highly - differentiated product (wound healing device & antibiotic) Combining Wound Healing with an Antibiotic » OBG plus Moxifloxacin Hydrochloride ( Vigamox ) ▪ A 505(b)(2) filing requiring only one pivotal study for registration (superiority against placebo) ▪ Will mirror Vigamox label: same concentration and dosing regimen for the treatment of bacterial conjunctivitis » Expected IND filing by end of year 2020 ▪ Preparing for 28 day GLP tox study ▪ pre - IND meeting to confirm IND and NDA filing requirements scheduled for Aug 10 » Antibiotic eye drops are widely prescribed including conjunctivitis and trauma ▪ ALL ocular wounds and surgical procedures (e.g. cataract surgery, PRK and LASIK) 1. Per IMS Health (Division of IQVIA) 27

Next Steps » Three FDA Meetings Requested • June 9 : Meeting with CDRH to discuss final packaging (manufacturing) • July 13 : Meeting with CDRH to discuss Dry Eye Pivotal Study • Aug 10 : pre - IND meeting requested with CDER to discuss combination product » PRK de novo filing : Completing manufacturing testing required to file • Targeting filing of de novo application by end of 2020 » Dry Eye : Targeting initiation of pivotal by end of 2020 » Combination Product : Targeting filing IND by end of 2020 » Finance : Cash into Q 1 2021 28
