Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Blueprint Medicines Corp | bpmc-20200529xex99d2.htm |
| 8-K - 8-K - Blueprint Medicines Corp | bpmc-20200529x8k.htm |
Exhibit 99.1
| Pralsetinib data review and registration program update MAY 29, 2020 ASCO 2020 VIRTUAL MEETING Linnea, living with lung cancer |
| Blueprint Medicines call participants Introduction Jeff Albers, Chief Executive Officer Pralsetinib clinical data review Andy Boral, MD, PhD, Chief Medical Officer Pralsetinib commercial strategy Christy Rossi, Chief Commercial Officer Q&A All 2 PREPARED REMARKS |
| Forward-looking statements 3 This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words "aim," "may," "will," "could," "would," "should," "expect," "plan," "anticipate," "intend," "believe," "estimate," "predict," "project," "potential," "continue," "target" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. In this presentation, forward-looking statements include, without limitation, statements regarding plans and timelines for the development of avapritinib and pralsetinib, including the timing, designs, implementation, enrollment, plans and announcement of results regarding the ongoing and planned clinical trials of Blueprint Medicines Corporation (the “Company”); plans and timelines for submitting marketing applications for avapritinib and pralsetinib and, if approved, for commercializing pralsetinib; the potential benefits of the Company’s current and future approved drugs or drug candidates in treating patients; and the Company’s strategy, goals and anticipated milestones, business plans and focus. The Company has based these forward-looking statements on management's current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks, uncertainties and other important factors, many of which are beyond the Company's control and may cause actual results, performance or achievements to differ materially from those expressed or implied by any forward-looking statements. These risks and uncertainties include, without limitation, risks and uncertainties related to the impact of the COVID-19 pandemic to the Company's business, operations, strategy, goals and anticipated milestones, including the Company's ongoing and planned research and discovery activities, ability to conduct ongoing and planned clinical trials, clinical supply of current or future drug candidates, commercial supply of current or future approved drugs, and launching, marketing and selling current or future approved drugs; the delay of any current or planned clinical trials or the development of the Company's drug candidates or the licensed drug candidate; the Company's advancement of multiple early-stage efforts; the Company's ability to successfully demonstrate the efficacy and safety of its drug candidates and gain approval of its drug candidates on a timely basis, if at all; the preclinical and clinical results for the Company's drug candidates, which may not support further development of such drug candidates; actions or decisions of regulatory agencies or authorities, which may affect the initiation, timing and progress of clinical trials or marketing applications; the Company's ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing or AYVAKIT; the Company' ability and plans for maintaining a commercial infrastructure, and successfully launching, marketing and selling its current or future approved drugs; the Company's ability to develop and commercialize companion diagnostic tests for any of the Company's current or future approved drugs or drug candidates; and the success of the Company's current and future collaborations, partnerships and licenses. These and other risks and uncertainties are described in greater detail under "Risk Factors" in the Company's filings with the Securities and Exchange Commission ("SEC"), including its most recent Annual Report on Form 10-K, as supplemented by its most recent Quarterly Report on Form 10-Q, and any other filings it has made or may make with the SEC in the future. The Company cannot guarantee future results, outcomes, levels of activity, performance, developments, or achievements, and there can be no assurance that its expectations, intentions, anticipations, beliefs, or projections will result or be achieved or accomplished. The forward-looking statements in this presentation are made only as of the date hereof, and except as required by law, the Company undertakes no obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or otherwise. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company's industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of the Company's future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. |
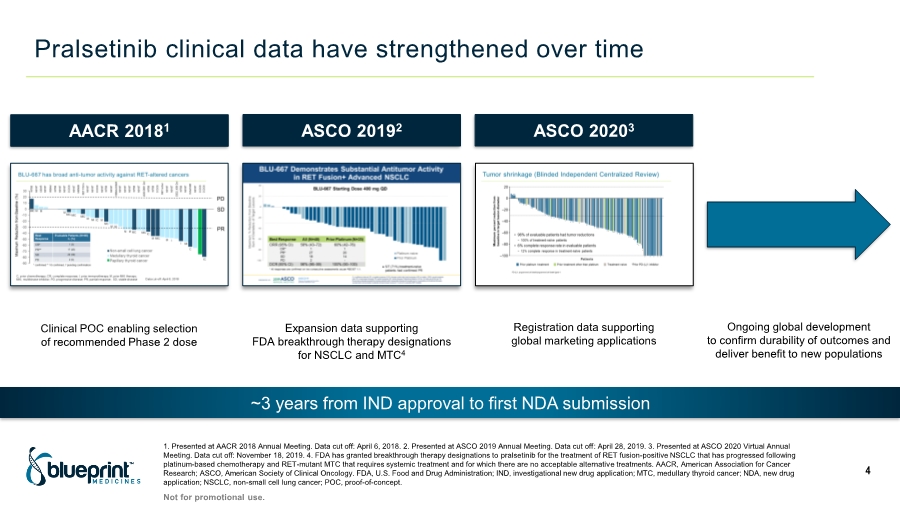
| Pralsetinib clinical data have strengthened over time 1. Presented at AACR 2018 Annual Meeting. Data cut off: April 6, 2018. 2. Presented at ASCO 2019 Annual Meeting. Data cut off: April 28, 2019. 3. Presented at ASCO 2020 Virtual Annual Meeting. Data cut off: November 18, 2019. 4. FDA has granted breakthrough therapy designations to pralsetinib for the treatment of RET fusion-positive NSCLC that has progressed following platinum-based chemotherapy and RET-mutant MTC that requires systemic treatment and for which there are no acceptable alternative treatments. AACR, American Association for Cancer Research; ASCO, American Society of Clinical Oncology. FDA, U.S. Food and Drug Administration; IND, investigational new drug application; MTC, medullary thyroid cancer; NDA, new drug application; NSCLC, non-small cell lung cancer; POC, proof-of-concept. 4 ~3 years from IND approval to first NDA submission AACR 20181 ASCO 20192 ASCO 20203 Clinical POC enabling selection of recommended Phase 2 dose Expansion data supporting FDA breakthrough therapy designations for NSCLC and MTC4 Registration data supporting global marketing applications Not for promotional use. Ongoing global development to confirm durability of outcomes and deliver benefit to new populations |
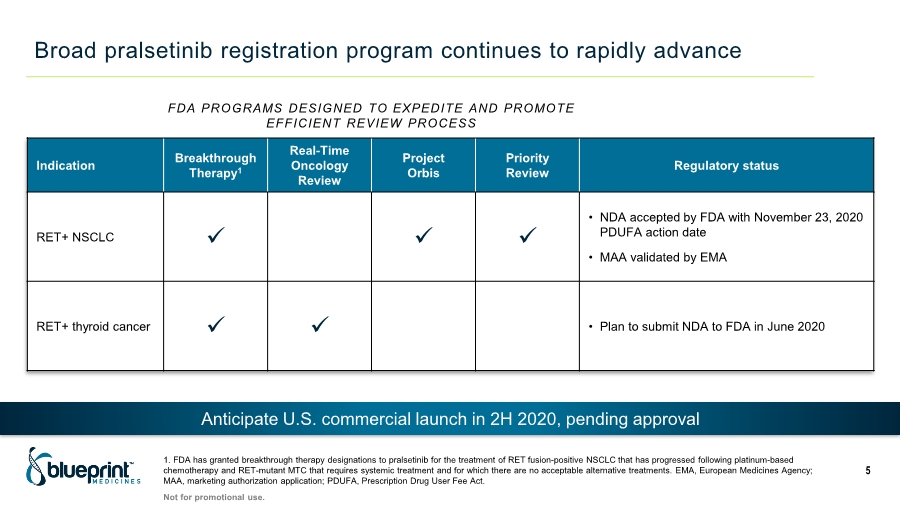
| Broad pralsetinib registration program continues to rapidly advance FDA PROGRAMS DESIGNED TO EXPEDITE AND PROMOTE EFFICIENT REVIEW PROCESS Indication Breakthrough Therapy1 Real-Time Oncology Review Project Orbis Priority Review Regulatory status RET+ NSCLC ✓ ✓ ✓ • NDA accepted by FDA with November 23, 2020 PDUFA action date • MAA validated by EMA RET+ thyroid cancer ✓ ✓ • Plan to submit NDA to FDA in June 2020 Anticipate U.S. commercial launch in 2H 2020, pending approval Not for promotional use. 5 1. FDA has granted breakthrough therapy designations to pralsetinib for the treatment of RET fusion-positive NSCLC that has progressed following platinum-based chemotherapy and RET-mutant MTC that requires systemic treatment and for which there are no acceptable alternative treatments. EMA, European Medicines Agency; MAA, marketing authorization application; PDUFA, Prescription Drug User Fee Act. |
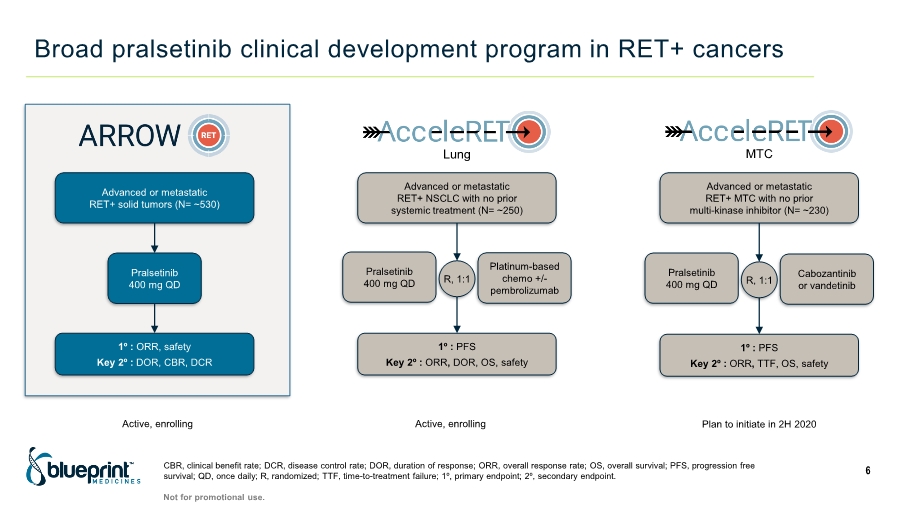
| Broad pralsetinib clinical development program in RET+ cancers CBR, clinical benefit rate; DCR, disease control rate; DOR, duration of response; ORR, overall response rate; OS, overall survival; PFS, progression free survival; QD, once daily; R, randomized; TTF, time-to-treatment failure; 1º, primary endpoint; 2º, secondary endpoint. 6 Advanced or metastatic RET+ solid tumors (N= ~530) Advanced or metastatic RET+ NSCLC with no prior systemic treatment (N= ~250) Advanced or metastatic RET+ MTC with no prior multi-kinase inhibitor (N= ~230) Pralsetinib 400 mg QD 1º : ORR, safety Key 2º : DOR, CBR, DCR Pralsetinib 400 mg QD Platinum-based chemo +/- pembrolizumab 1º : PFS Key 2º : ORR, DOR, OS, safety R, 1:1 Pralsetinib 400 mg QD Cabozantinib or vandetinib 1º : PFS Key 2º : ORR, TTF, OS, safety R, 1:1 Not for promotional use. Lung MTC Plan to initiate in 2H 2020 Active, enrolling Active, enrolling |
| Pralsetinib clinical data review Andy Boral, MD, PhD, Chief Medical Officer |
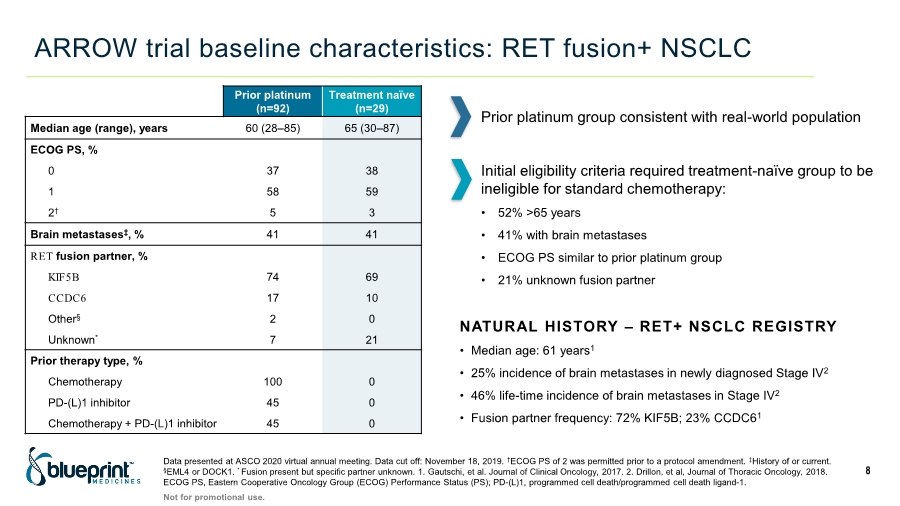
| ARROW trial baseline characteristics: RET fusion+ NSCLC 8 Prior platinum (n=92) Treatment naïve (n=29) Median age (range), years 60 (28–85) 65 (30–87) ECOG PS, % 0 37 38 1 58 59 2† 5 3 Brain metastases‡, % 41 41 RET fusion partner, % KIF5B 74 69 CCDC6 17 10 Other§ 2 0 Unknown* 7 21 Prior therapy type, % Chemotherapy 100 0 PD-(L)1 inhibitor 45 0 Chemotherapy + PD-(L)1 inhibitor 45 0 Data presented at ASCO 2020 virtual annual meeting. Data cut off: November 18, 2019. †ECOG PS of 2 was permitted prior to a protocol amendment. ‡History of or current. §EML4 or DOCK1. * Fusion present but specific partner unknown. 1. Gautschi, et al. Journal of Clinical Oncology, 2017. 2. Drillon, et al, Journal of Thoracic Oncology, 2018. ECOG PS, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS); PD-(L)1, programmed cell death/programmed cell death ligand-1. Not for promotional use. NATURAL HISTORY – RET+ NSCLC REGISTRY • Median age: 61 years1 • 25% incidence of brain metastases in newly diagnosed Stage IV2 • 46% life-time incidence of brain metastases in Stage IV2 • Fusion partner frequency: 72% KIF5B; 23% CCDC61 Initial eligibility criteria required treatment-naïve group to be ineligible for standard chemotherapy: • 52% >65 years • 41% with brain metastases • ECOG PS similar to prior platinum group • 21% unknown fusion partner Prior platinum group consistent with real-world population |
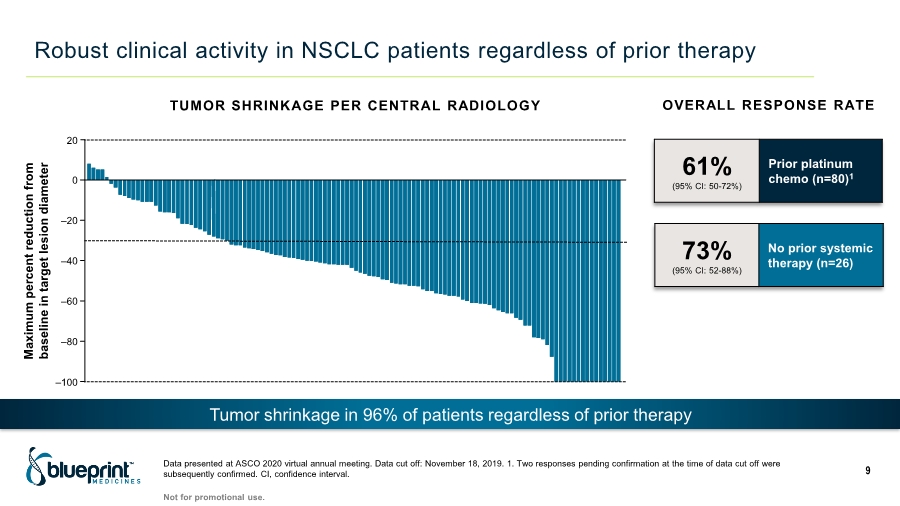
| Robust clinical activity in NSCLC patients regardless of prior therapy Data presented at ASCO 2020 virtual annual meeting. Data cut off: November 18, 2019. 1. Two responses pending confirmation at the time of data cut off were subsequently confirmed. CI, confidence interval. 9 61% (95% CI: 50-72%) Prior platinum chemo (n=80)1 73% (95% CI: 52-88%) No prior systemic therapy (n=26) Maximum percent reduction from baseline in target lesion diameter ‒100 20 ‒20 ‒40 ‒60 ‒80 0 TUMOR SHRINKAGE PER CENTRAL RADIOLOGY OVERALL RESPONSE RATE Not for promotional use. Tumor shrinkage in 96% of patients regardless of prior therapy |
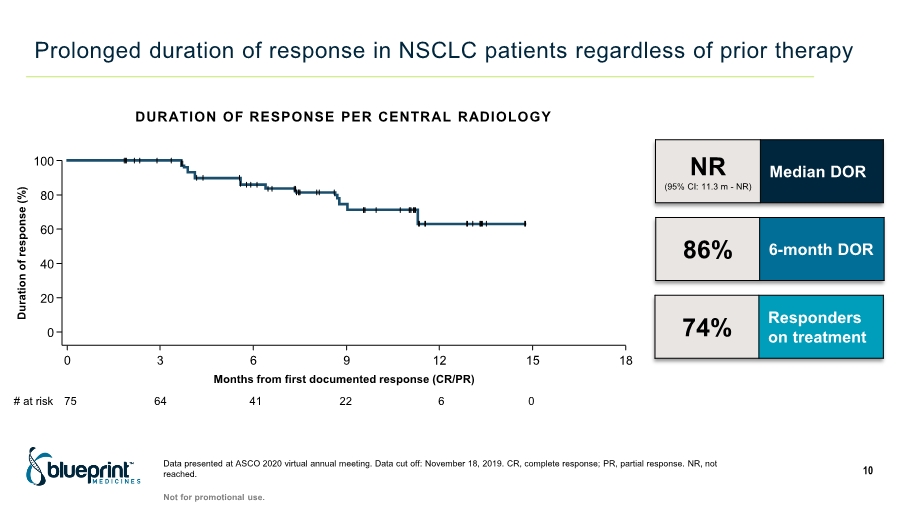
| DURATION OF RESPONSE PER CENTRAL RADIOLOGY Prolonged duration of response in NSCLC patients regardless of prior therapy Data presented at ASCO 2020 virtual annual meeting. Data cut off: November 18, 2019. CR, complete response; PR, partial response. NR, not reached. 10 Not for promotional use. NR (95% CI: 11.3 m - NR) Median DOR 86% 6-month DOR 74% Responders on treatment Months from first documented response (CR/PR) # at risk 75 64 41 22 6 0 100 80 60 40 20 0 0 3 6 9 12 15 Duration of response (%) 18 |
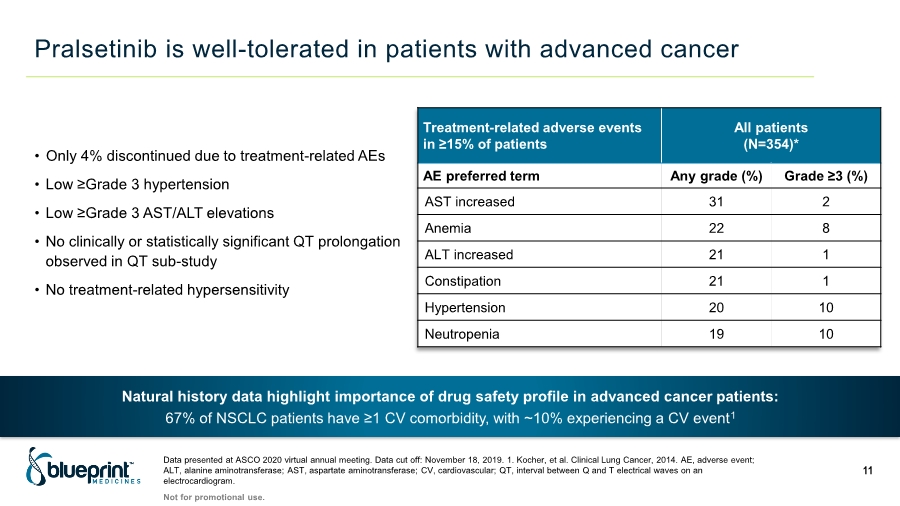
| Pralsetinib is well-tolerated in patients with advanced cancer Data presented at ASCO 2020 virtual annual meeting. Data cut off: November 18, 2019. 1. Kocher, et al. Clinical Lung Cancer, 2014. AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CV, cardiovascular; QT, interval between Q and T electrical waves on an electrocardiogram. 11 Treatment-related adverse events in ≥15% of patients All patients (N=354)* AE preferred term Any grade (%) Grade ≥3 (%) AST increased 31 2 Anemia 22 8 ALT increased 21 1 Constipation 21 1 Hypertension 20 10 Neutropenia 19 10 • Only 4% discontinued due to treatment-related AEs • Low ≥Grade 3 hypertension • Low ≥Grade 3 AST/ALT elevations • No clinically or statistically significant QT prolongation observed in QT sub-study • No treatment-related hypersensitivity Natural history data highlight importance of drug safety profile in advanced cancer patients: 67% of NSCLC patients have ≥1 CV comorbidity, with ~10% experiencing a CV event1 Not for promotional use. |
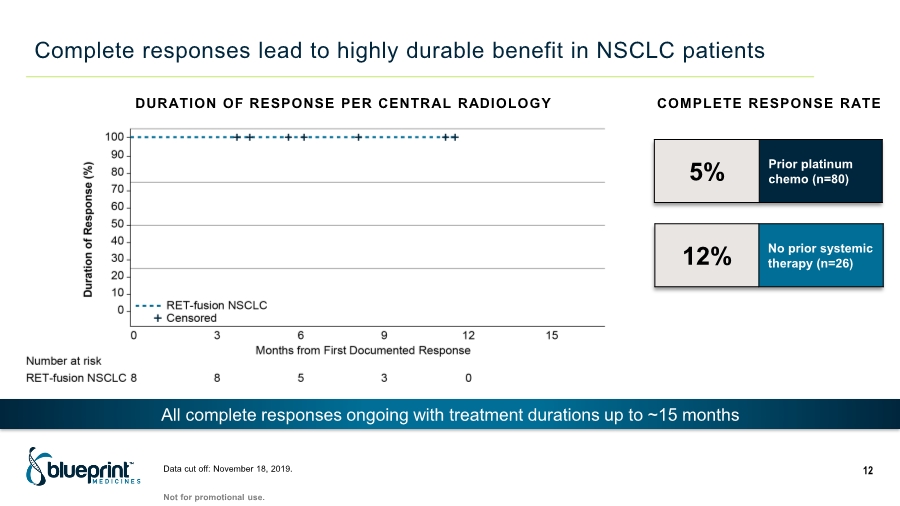
| Complete responses lead to highly durable benefit in NSCLC patients Data cut off: November 18, 2019. 12 COMPLETE RESPONSE RATE 5% Prior platinum chemo (n=80) 12% No prior systemic therapy (n=26) DURATION OF RESPONSE PER CENTRAL RADIOLOGY All complete responses ongoing with treatment durations up to ~15 months Not for promotional use. |
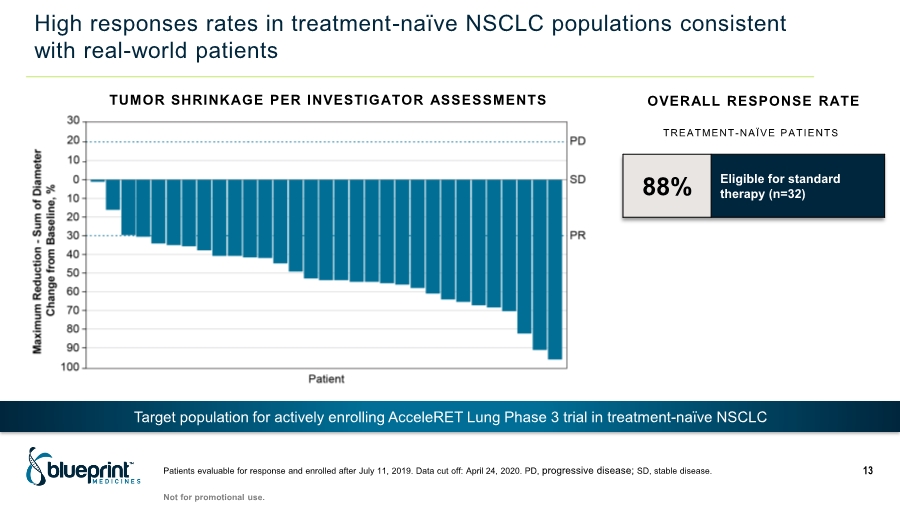
| High responses rates in treatment-naïve NSCLC populations consistent with real-world patients 88% Eligible for standard therapy (n=32) OVERALL RESPONSE RATE TUMOR SHRINKAGE PER INVESTIGATOR ASSESSMENTS Target population for actively enrolling AcceleRET Lung Phase 3 trial in treatment-naïve NSCLC TREATMENT-NAÏVE PATIENTS Patients evaluable for response and enrolled after July 11, 2019. Data cut off: April 24, 2020. PD, progressive disease; SD, stable disease. 13 Not for promotional use. |
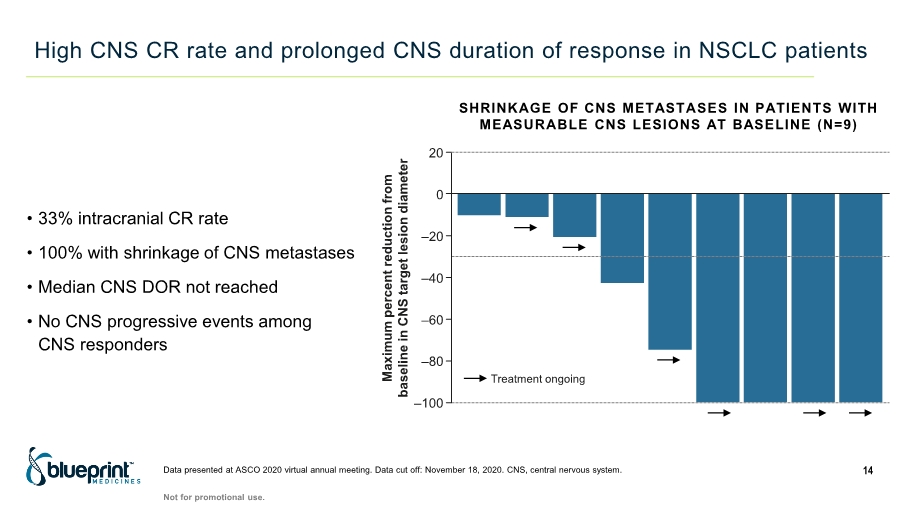
| High CNS CR rate and prolonged CNS duration of response in NSCLC patients 14 • 33% intracranial CR rate • 100% with shrinkage of CNS metastases • Median CNS DOR not reached • No CNS progressive events among CNS responders Maximum percent reduction from baseline in CNS target lesion diameter 20 0 –20 –40 –60 –80 –100 Treatment ongoing Data presented at ASCO 2020 virtual annual meeting. Data cut off: November 18, 2020. CNS, central nervous system. SHRINKAGE OF CNS METASTASES IN PATIENTS WITH MEASURABLE CNS LESIONS AT BASELINE (N=9) Not for promotional use. |
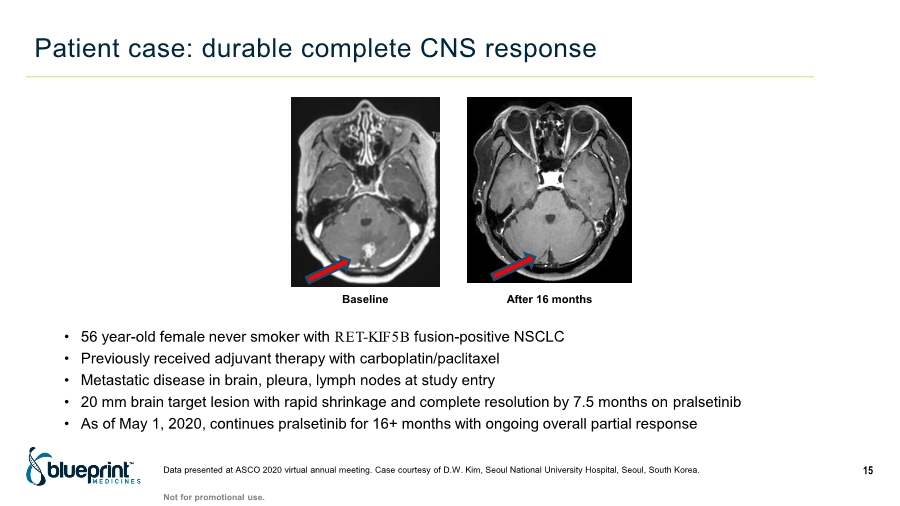
| Baseline After 16 months • 56 year-old female never smoker with RET-KIF5B fusion-positive NSCLC • Previously received adjuvant therapy with carboplatin/paclitaxel • Metastatic disease in brain, pleura, lymph nodes at study entry • 20 mm brain target lesion with rapid shrinkage and complete resolution by 7.5 months on pralsetinib • As of May 1, 2020, continues pralsetinib for 16+ months with ongoing overall partial response Patient case: durable complete CNS response 15 Data presented at ASCO 2020 virtual annual meeting. Case courtesy of D.W. Kim, Seoul National University Hospital, Seoul, South Korea. Not for promotional use. |
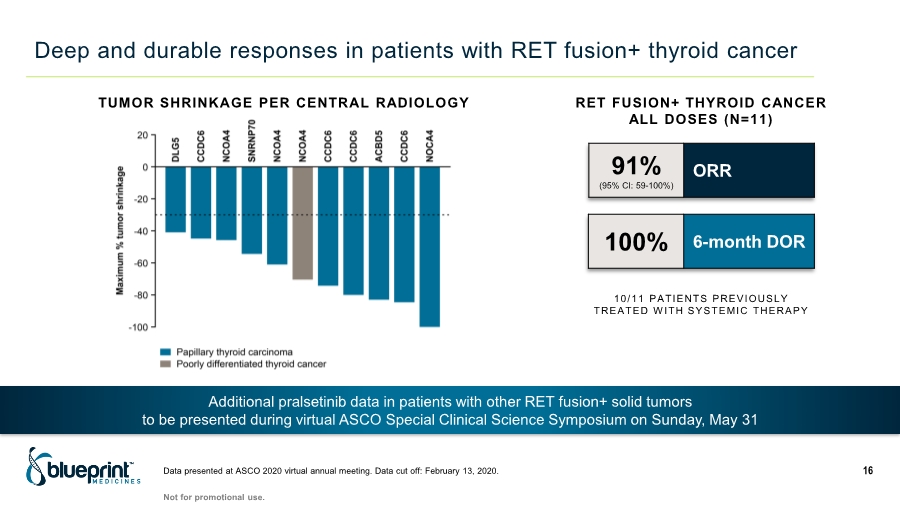
| Deep and durable responses in patients with RET fusion+ thyroid cancer Data presented at ASCO 2020 virtual annual meeting. Data cut off: February 13, 2020. 16 TUMOR SHRINKAGE PER CENTRAL RADIOLOGY Not for promotional use. Additional pralsetinib data in patients with other RET fusion+ solid tumors to be presented during virtual ASCO Special Clinical Science Symposium on Sunday, May 31 91% (95% CI: 59-100%) ORR 10/11 PATIENTS PREVIOUSLY TREATED WITH SYSTEMIC THERAPY 100% 6-month DOR RET FUSION+ THYROID CANCER ALL DOSES (N=11) |
| Pralsetinib commercial strategy Christy Rossi, Chief Commercial Officer |
| Our plan to deliver a best-in-class selective RET inhibitor to patients DIFFERENTIATED CLINICAL PROFILE PATIENT- AND HEALTHCARE PROVIDER-CENTERED APPROACH HIGHLY EXPERIENCED, NIMBLE TEAM Data showing deep responses, long-lasting benefit, tolerability and convenience Tailored support enabling patient identification, ease of prescribing and ongoing patient management Fully-integrated launch-ready team in place, 2/3 with prior lung cancer experience 18 Not for promotional use. |
| Questions & answers |
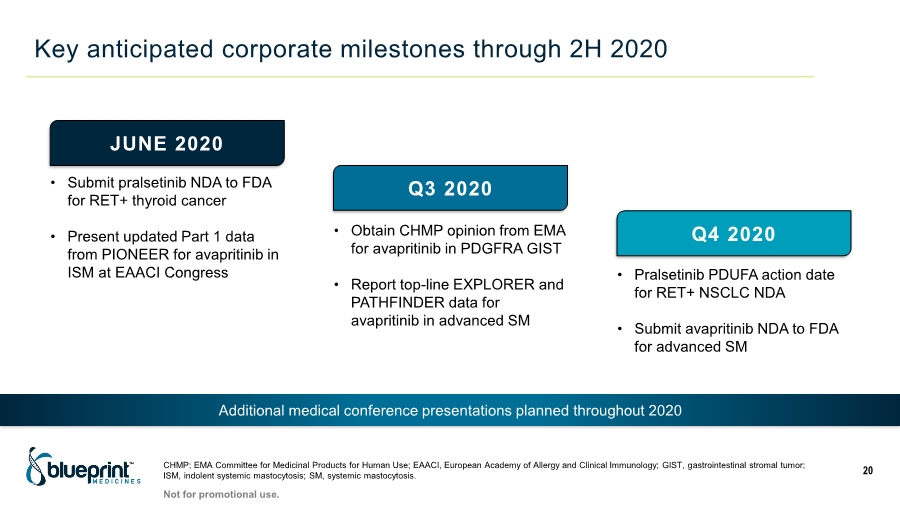
| Key anticipated corporate milestones through 2H 2020 CHMP; EMA Committee for Medicinal Products for Human Use; EAACI, European Academy of Allergy and Clinical Immunology; GIST, gastrointestinal stromal tumor; ISM, indolent systemic mastocytosis; SM, systemic mastocytosis. 20 JUNE 2020 Q3 2020 Q4 2020 • Submit pralsetinib NDA to FDA for RET+ thyroid cancer • Present updated Part 1 data from PIONEER for avapritinib in ISM at EAACI Congress • Obtain CHMP opinion from EMA for avapritinib in PDGFRA GIST • Report top-line EXPLORER and PATHFINDER data for avapritinib in advanced SM • Pralsetinib PDUFA action date for RET+ NSCLC NDA • Submit avapritinib NDA to FDA for advanced SM Additional medical conference presentations planned throughout 2020 Not for promotional use. |
| Thank you |