Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - LUMOS PHARMA, INC. | lumo-2020q1x8kxex991.htm |
| 8-K - 8-K - LUMOS PHARMA, INC. | lumo-2020q1erx8k.htm |

First Quarter 2020 Financial Results May 28, 2020

Lumos Pharma Q1 2020 Conference Call • Lisa Miller, Director of Investor Relations • Rick Hawkins, CEO Agenda • John McKew, PhD, COO & CSO • Eugene Kennedy, MD, CMO • Carl Langren, CFO 2

Forward Looking Statements This presentation contains forward-looking statements of Lumos Pharma, Inc. that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation are forward-looking statements, within the meaning of The Private Securities Litigation Reform Act of 1995. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "target," "potential," "will," "could," "should," "seek" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements regarding the expected initiation of a Phase 2b clinical trial, the sufficiency of funding for such trial, the potential of an orally administered treatment regimen for PGHD and other indications, projected cash position and its sufficiency to fund the company’s operations through data read-out for the Phase 2b trial of LUM-201 in PGHD; impact of regulatory feedback to clinical timelines and costs, results of its clinical trials for product candidates; its timing of release of data from ongoing clinical studies; its plans related to execution of clinical trials; plans related to moving additional indications into clinical development; future priority review voucher (PRV) monetization, anticipated funds from monetization of the PRV, milestones or other economic interests, Lumos Pharma’s financial guidance for 2020 and beyond; and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that Lumos Pharma makes due to a number of important factors, including the effects of pandemics or other widespread health problems such as the ongoing COVID-19 pandemic and those risks discussed in "Risk Factors" and elsewhere in Lumos Pharma’s Annual Report on Form 10-K for the year ended December 31, 2019, the proxy statement on Form DEFR14A filed on February 13, 2020, and other reports filed with the U.S. Securities and Exchange Commission (SEC). The forward-looking statements in this presentation represent Lumos Pharma’s views as of the date of this presentation. Lumos Pharma anticipates that subsequent events and developments will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing Lumos Pharma’s views as of any date subsequent to the date of this presentation. 3

Overview of Company • Late-stage novel therapeutic asset, LUM-201, with validating Phase 2b trial in Pediatric Growth Hormone Deficiency (PGHD) anticipated to begin prior to the end of 2020 • Established and sizable overall market targeted of over $1B*, with potential to disrupt current treatment regimen for significant subset of patients • Experienced management team with ability to expand pipeline through addition of other rare disease assets • Cash on hand expected to support current operations through planned Phase 2b read-out • Additional non-dilutive funds expected from 60% PRV ownership available to expand portfolio * USA, Germany, France, Italy, Spain, UK, Japan (Global Data Opportunity Analyzer: Growth 4 Hormone Deficiency Opportunity Analysis and Forecasts to GDHC069POA, May 2017)

Experienced Management Experienced management team with significant clinical development and commercial experience • Richard Hawkins – Chairman, CEO & President of Lumos Pharma, developer of Growth Hormone (GH) Receptor Antagonist for Acromegaly at Sensus (sold to Pfizer). Built one of the first contract recombinant protein manufacturing facilities (Covance Biotechnology). Co-founded Pharmaco, a contract research organization (merged with PPD). • John McKew – COO & CSO of Lumos Pharma, former Richard Hawkins John McKew, PhD Carl Langren Scientific Dir, NIH - National Center for Advancing Translational Chairman, CEO & President COO & CSO CFO Science (NCATS) and Therapeutics for Rare and Neglected Diseases (TRND). Director level, Wyeth Research Genetics Institute. • Carl Langren – CFO of Lumos Pharma, former CFO of BioProtection Systems, Housby Mixer Group, Equity Dynamics, Inc., and Tax Manager with McGladrey Pullen & Co. • Eugene Kennedy - CMO of Lumos Pharma, former Associate Professor of Surgery and Chief of the Section of Pancreaticobiliary Surgery Thomas Jefferson University (Philadelphia), former faculty Johns Hopkins Hospital. • Aaron Schuchart - CBO of Lumos Pharma, former CBO of Eugene Kennedy, MD Aaron Schuchart Aeglea BioTherapeutics, former leadership roles in business CMO CBO development and licensing at Coherus Biosciences, Novartis Diagnostics/Grifols, and Amgen. 5

PGHD and Standard of Care • PGHD occurs due to inadequate secretion of growth hormone by the pituitary gland during childhood • PGHD can be either hereditary or acquired, although the majority of cases have unknown causes (idiopathic) versus - Lack of physical growth is the most obvious manifestation; but numerous metabolic processes are also affected • PGHD incidence in U.S. approximately 1 in 3500 children1 • Standard of care consists of daily, subcutaneous injections of recombinant human growth hormone (rhGH) - Can be painful, potentially leading to missed doses and sub-optimal growth2,3 - ~2500 injections over years of treatment Robust, established market primed for an oral alternative 1 GlobalData EpiCast Report for Growth Hormone Deficiency Epidemiology forecast to 2026 2 Rosenfeld 2008 Endocrine Practice 6 3 Cutfield 2011 PLOS ONE
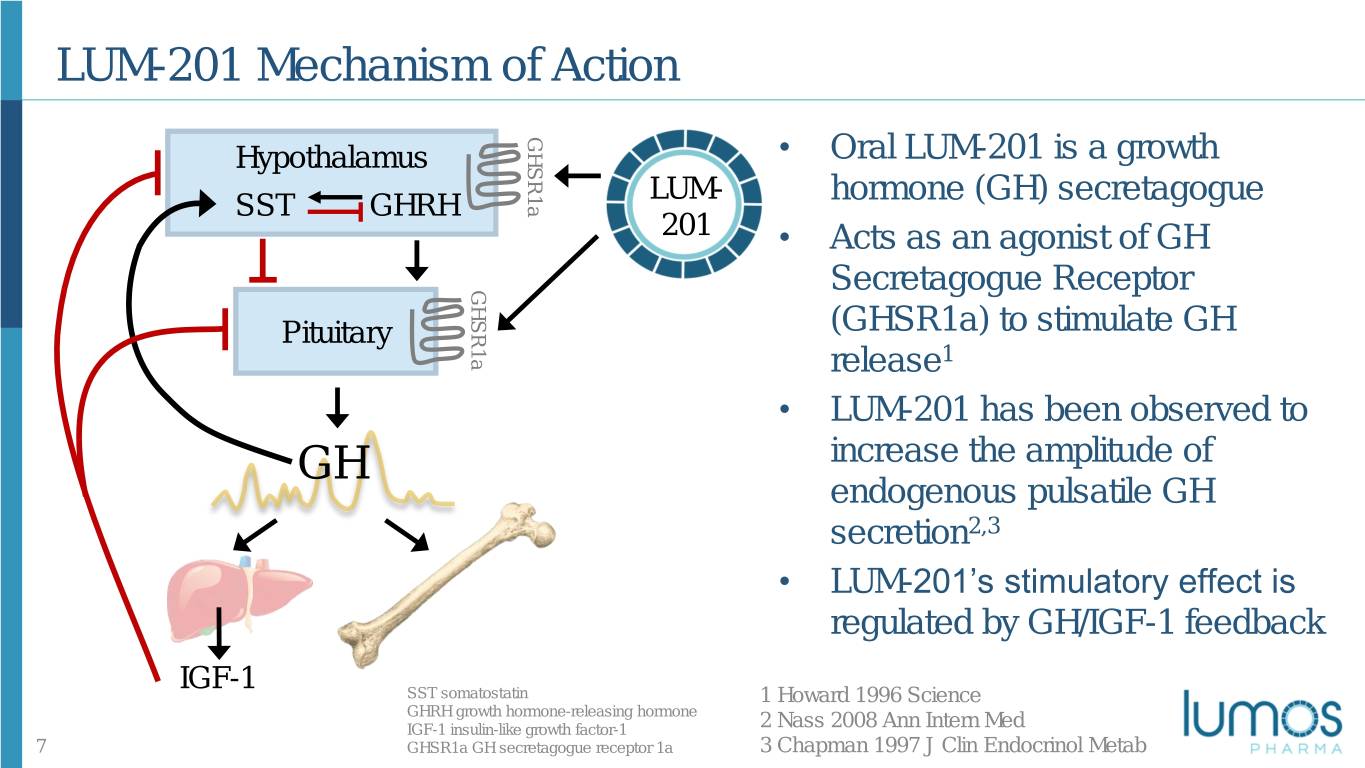
LUM-201 Mechanism of Action GHSR1a Hypothalamus • Oral LUM-201 is a growth LUM- hormone (GH) secretagogue SST GHRH 201 • Acts as an agonist of GH GHSR1a Secretagogue Receptor Pituitary (GHSR1a) to stimulate GH release1 • LUM-201 has been observed to GH increase the amplitude of endogenous pulsatile GH secretion2,3 • LUM-201’s stimulatory effect is regulated by GH/IGF-1 feedback IGF-1 SST somatostatin 1 Howard 1996 Science GHRH growth hormone-releasing hormone IGF-1 insulin-like growth factor-1 2 Nass 2008 Ann Intern Med 7 GHSR1a GH secretagogue receptor 1a 3 Chapman 1997 J Clin Endocrinol Metab
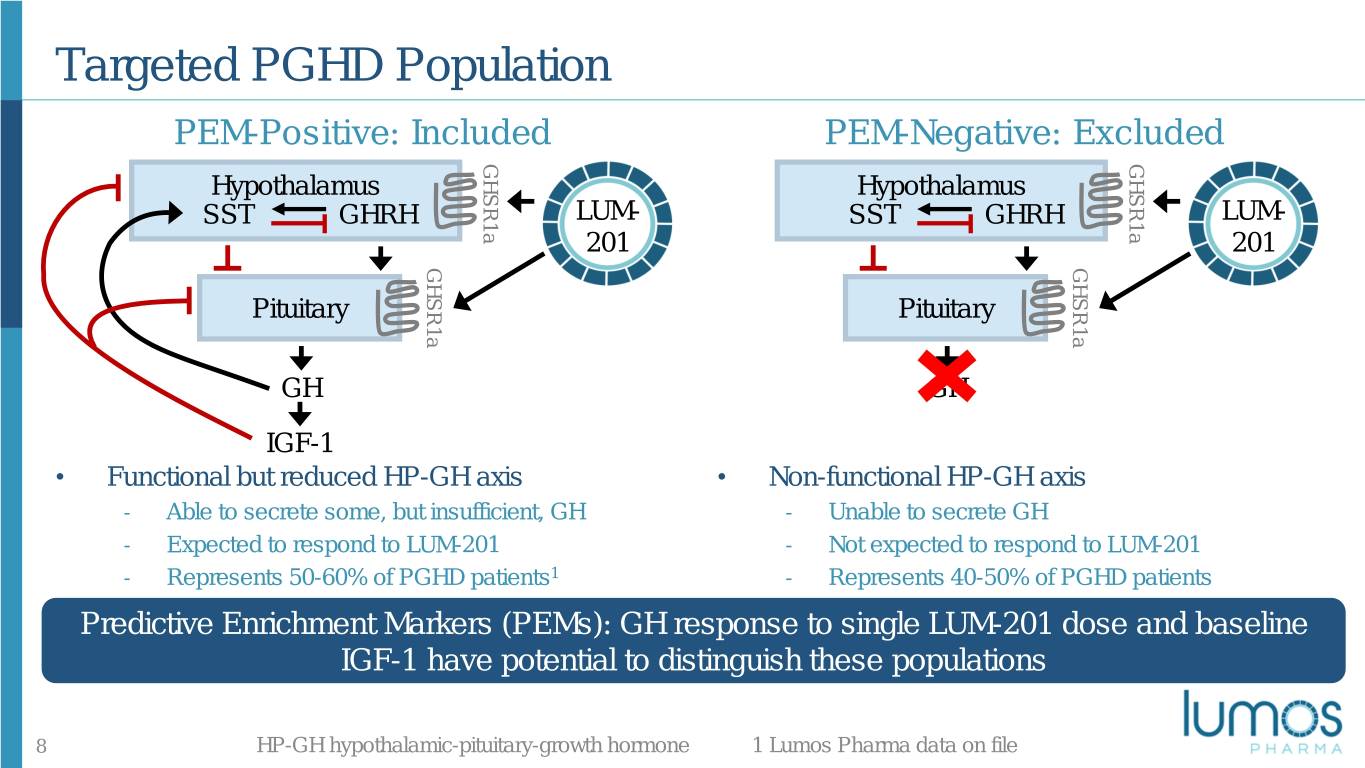
Targeted PGHD Population PEM-Positive: Included PEM-Negative: Excluded GHSR1a GHSR1a Hypothalamus HypothalamusIGF-1 SST GHRH LUM- SST GHRH LUM- 201 201 GHSR1a GHSR1a Pituitary Pituitary GH GH IGF-1 • Functional but reduced HP-GH axis • Non-functional HP-GH axis - Able to secrete some, but insufficient, GH - Unable to secrete GH - Expected to respond to LUM-201 - Not expected to respond to LUM-201 - Represents 50-60% of PGHD patients1 - Represents 40-50% of PGHD patients Predictive Enrichment Markers (PEMs): GH response to single LUM-201 dose and baseline IGF-1 have potential to distinguish these populations 8 HP-GH hypothalamic-pituitary-growth hormone 1 Lumos Pharma data on file

Clinical Development Outline for PGHD • Two main goals set for Phase 2b - Prospectively confirm the utility of PEM strategy - Determine the optimal dose for Phase 3 registration trial • Phase 2b PGHD clinical trial design - Three dose levels of LUM-201 (0.8, 1.6, 3.2 mg/kg) - Positive control arm of daily rhGH injections - Treatment-naïve, age-matched cohorts; 6-month dosing - Primary outcome measure: annualized growth height velocity • Anticipate initiation of Phase 2b trial prior to the end of 2020 Generate safety and efficacy data to move on to Phase 3 study 9
LUM-201: Other Potential Rare Endocrine Disorders • Beyond PGHD, Prader-Willi Syndrome Small for Gestational Age rhGH FDA approved in 2000 rhGH FDA approved in 2001 Lumos Pharma also plans to investigate LUM-201 for other rare endocrine disorders, for which rhGH has been approved Idiopathic Short Stature Turner Syndrome rhGH FDA approved in 2003 rhGH FDA approved in 1996 Significant opportunities with established regulatory pathways 10 GARD: Genetic and Rare Diseases Information Center
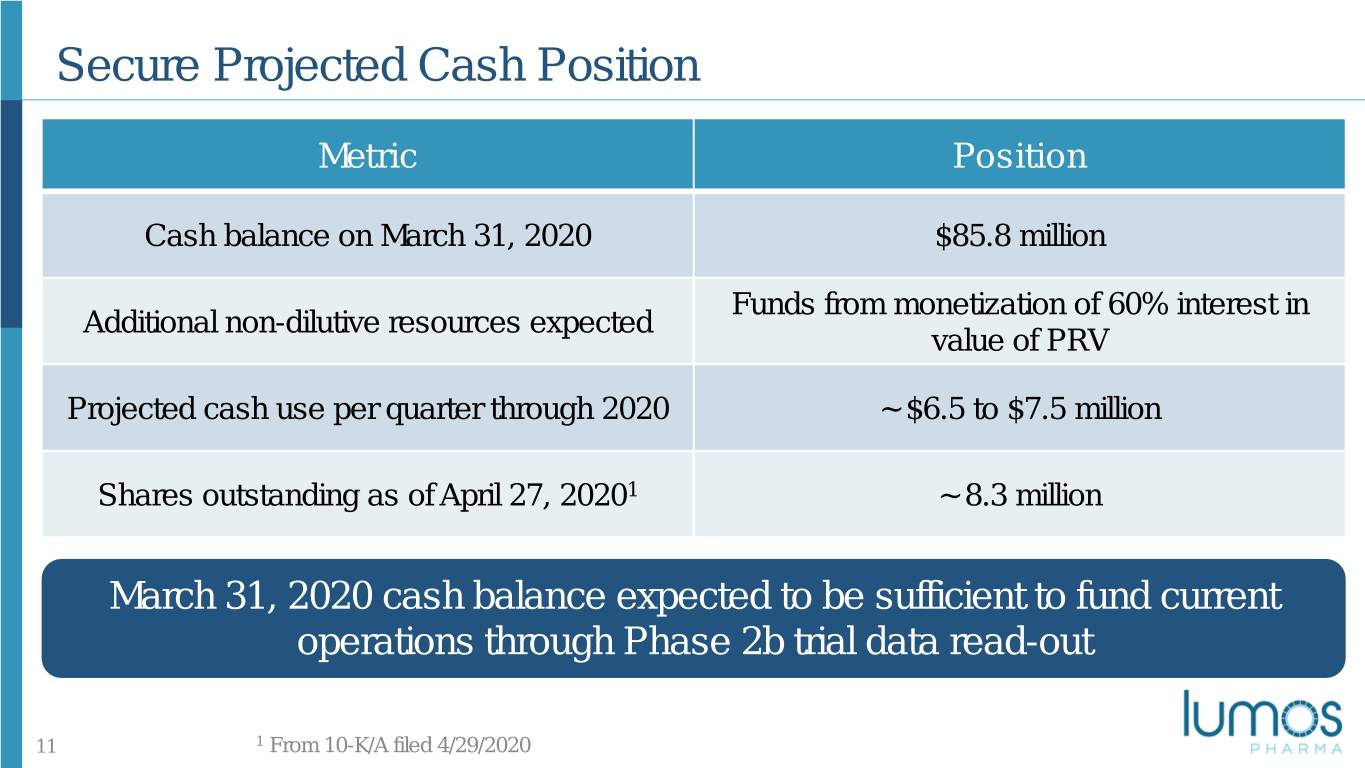
Secure Projected Cash Position Metric Position Cash balance on March 31, 2020 $85.8 million Funds from monetization of 60% interest in Additional non-dilutive resources expected value of PRV Projected cash use per quarter through 2020 ~ $6.5 to $7.5 million Shares outstanding as of April 27, 20201 ~ 8.3 million March 31, 2020 cash balance expected to be sufficient to fund current operations through Phase 2b trial data read-out 11 1 From 10-K/A filed 4/29/2020

Lumos Pharma: Summary of Investment Thesis Lumos Pharma • Lead program, LUM-201, with potential to be the (NASDAQ:LUMO) first oral growth hormone secretagogue therapy Transforming Lives for PGHD with Rare Focus • Opportunity to disrupt established and sizable market • Management team with extensive experience in the clinical advancement of rare disease therapeutics • Cash on hand expected to support current operations through planned Phase 2b read-out, with additional non-dilutive PRV funding available to expand portfolio Potential to significantly increase shareholder value 12
