Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Avalo Therapeutics, Inc. | ex-991pressreleasedatedmay.htm |
| 8-K - 8-K - Avalo Therapeutics, Inc. | a8-k002fdaproceed_may2020.htm |

Exhibit 99.2 Patient Inspired Science Establishing a leading, pediatric rare and orphan disease-focused biopharmaceutical company to deliver impactful new medicines to patients May | 2020 Corporate Highlights | COVID-19 ARDS

Forward-Looking Statements This presentation may include forward-looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts. Such forward-looking statements are subject to significant risks and uncertainties that are subject to change based on various factors (many of which are beyond Cerecor, Inc. (“Cerecor”) control, which could cause actual results to differ from the forward-looking statements. Such statements may include, without limitation, statements with respect to Cerecor’s plans, objectives, projections, expectations and intentions and other statements identified by words such as “projects,” “may,” “might,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “aims,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” or similar expressions (including their use in the negative), or by discussions of future matters such as: our 2020 outlook; the development of product candidates or products; potential attributes and benefits of product candidates; strategic alternatives for neurological assets and Millipred; and other statements that are not historical. These statements are based upon the current beliefs and expectations of Cerecor’s management but are subject to significant risks and uncertainties, including: reliance on and integration of key personnel; drug development costs, timing and other risks, including reliance on investigators and enrollment of patients in clinical trials, which might be slowed by the COVID-19 pandemic; regulatory risks; Cerecor's cash position and the need for it to raise additional capital; risks related to potential strategic alternatives for our neurology assets and Millipred; general economic and market risks and uncertainties, including those caused by the COVID-19 pandemic and those other risks detailed in Cerecor’s filings with the Securities and Exchange Commission. Actual results may differ from those set forth in the forward-looking statements. Except as required by applicable law, Cerecor expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Cerecor’s expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based. 2|

Highlights • Recent Merger of Cerecor and Aevi has created a rich pipeline of novel, 1st in class assets all with proven mechanistic rationale • Only known anti-LIGHT mAb in the clinic, offers potential to treat cytokine storm-induced COVID-19 ARDS in the near-term and broader ARDS indication beyond – CERC-002 will enter the clinic in June and is expected to deliver definitive topline POC data in Q4 2020 • CERC-007 (anti-IL-18 mAb), unique molecular target, is expected to deliver initial data in multiple myeloma 4Q 2020 and POC data end of 1Q 2021; initial data in adult onset Still’s Disease by 2Q 2021. • CERC-800 series (substrate replacement therapy for congenital disorders of glycosylation, all orphan designated, Priority Review Vouchers eligible) will release data from CDG-FIRST 1H 2020 – CERC-801 pivotal trial expected start 4Q 2020, top line data expected 4Q 2021 – CERC-802 pivotal trial expected start 4Q 2020, top line data expected 3Q 2021 – CERC-803 pivotal trial expected start 1H 2021, top line data expected 2H 2021 • CERC-006 (dual mTOR inhibitor), topline data expected 2Q 2021 3|

Clinical-Stage Pipeline Core Research & Development Stage Therapeutic Mechanism Development Program Lead Indication Area of Action Areas Preclinical Phase 1 Phase 2 Phase 3 Upcoming Milestone Initial Data Anti-LIGHT mAb ARDS CERC-002 4Q 2020 Initial Data Immunology Inflammation Anti-LIGHT mAb Crohn’s CERC-002 1Q 2021 Initial Data Anti-IL-18 mAb AOSD CERC-007 2Q 2021 Multiple Initial Data Oncology Blood Cancers Anti-IL-18 mAb CERC-007 Myeloma 4Q20/1Q21 Complex Complex Dual mTOR Initial Data Lymphatic Lymphatic CERC-006 inhibitor 2Q 2021 Malformations Malformations D-Galactose PGM1-CDG CERC-801 replacement Rare Genetic Disorders Initial data Congenital D-Mannose from Disorders of CERC-802 MPI-CDG Glycosylation replacement CDG-FIRST 1H20 L-Fucose LADII-CDG CERC-803 replacement 4|

CERC-002 Anti-LIGHT monoclonal antibody in clinical studies for COVID-19 ARDS

The Impact of Cytokine Storm Induced COVID-19 ARDS The outbreak of coronavirus disease 2019 (COVID-19) has created a global health crisis The viral infection triggers a hyperactive Approximately 1,500* people in immune response leading to cytokine the United States die each day storm and Acute Respiratory Distress from COVID-19 Syndrome (ARDS), a leading cause of death in COVID-19 patients There is currently no effective Our data implicate the inflammatory cytokine, LIGHT, as a for treatment Cytokine Storm potential key driver of cytokine storm induced COVID-19 ARDS leading to ARDS and death CERC-002 is the only know therapeutic currently in clinical development that inhibits the inflammatory cytokine LIGHT 6| *Data from Bloomberg COVID Tracker April 2020

LIGHT is a Potential Key Driver of Inflammation in the Lung LIGHT Impacts Lung Function Recent peer-reviewed publications implicate LIGHT in viral pneumonia and acute lung fibrosis 7|

Hyperactive Immune Response Leads to Cytokine Storm Cytokine storm induced ARDS is a major driver of poor COVID-19 outcomes Protective Immune Response Hyperactive Immune Response Immune cells arrive at the site of Excessive cytokines lead to over-recruitment of infection but do not overwhelm immune cells and hyperinflammation Alveoli Air sacs where oxygen transfers from the lungs into the blood stream Oxygen transfer is not impacted Excessive immune response leads to by immune response cell death and respiratory failure Figure adapted from AFP Graphics 8| https://twitter.com/AFPgraphics/status/1246330114171961358/photo/1

LIGHT is Potentially a Key Driver of the Inflammatory Response in Cytokine Storm in ARDS LIGHT Releases Inflammatory Cytokines and Activates both T Cells and B Cells FasL LIGHT TL1A HVEM-mediated LTβR-mediated Signaling Pathways Signaling Pathways • Highly expressed in neutrophils and macrophages and induces airway inflammation. It also appears to Fas HVEM DcR3 LTβR DR3 exacerbate pulmonary fibrosis in Overactivation of Immune Response May Lead to Disease patients who recover from ARDS Pathology • A critical factor in COVID-19 cytokine Activation and Proliferation Upregulation of of Immune Cells Inflammatory Molecules storm, pulmonary failure and longer- IL-1 GM-CSF term pulmonary fibrosis and in IL-6 CXCL5 broader ARDS etiologies IL-10 Myeloid Macro- T-cell Cells phage Pro-inflammatory Mediators Cytokine and Cytokines Storm Recent biomarker data from hospitalized COVID-19 patients demonstrates elevated LIGHT levels, implicating its role in COVID-19 ARDS 9|

LIGHT Linked to Ventilation and Mortality in Hospitalized COVID-19 Patients Very Recent Data Implicate LIGHT in COVID-19 ARDS • Free LIGHT levels are significantly elevated in the serum of hospitalized patients with COVID-19, with the greatest statistical significance in patients who are ventilated • Elevated free LIGHT levels are associated with mortality in ventilated patients infected with COVID-19 • The association between LIGHT levels and mortality is strongest among hospitalized patients over 60, a high-risk group for negative outcomes related to COVID-19 Reducing LIGHT levels using CERC-002 may prevent severe ARDS and reduce the extent of mechanical ventilation required in patients that develop ARDS 10|

LIGHT is Significantly Elevated in Hospitalized COVID-19 Patients LIGHT Levels in Hospitalized LIGHT Levels in Both Non-Ventilated COVID-19 Patients and Ventilated Patients P < 0.0001 P < 0.0001 P < 0.0001 Free LIGHT levels are significantly elevated in serum of hospitalized patients with COVID-19, suggesting that it plays a key role in underlying disease pathophysiology 11|
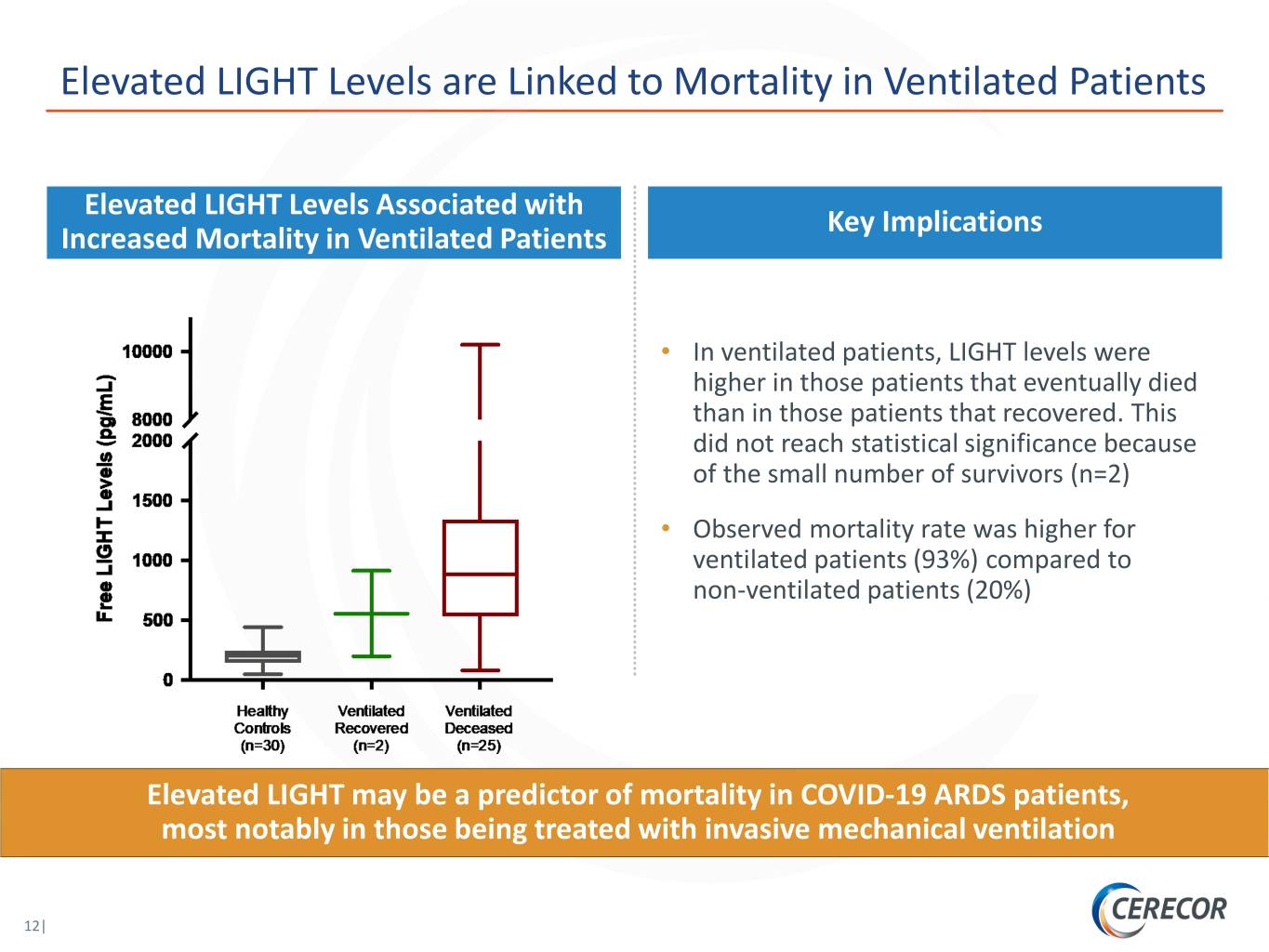
Elevated LIGHT Levels are Linked to Mortality in Ventilated Patients Elevated LIGHT Levels Associated with Key Implications Increased Mortality in Ventilated Patients • In ventilated patients, LIGHT levels were higher in those patients that eventually died than in those patients that recovered. This did not reach statistical significance because of the small number of survivors (n=2) • Observed mortality rate was higher for ventilated patients (93%) compared to non-ventilated patients (20%) Elevated LIGHT may be a predictor of mortality in COVID-19 ARDS patients, most notably in those being treated with invasive mechanical ventilation 12|
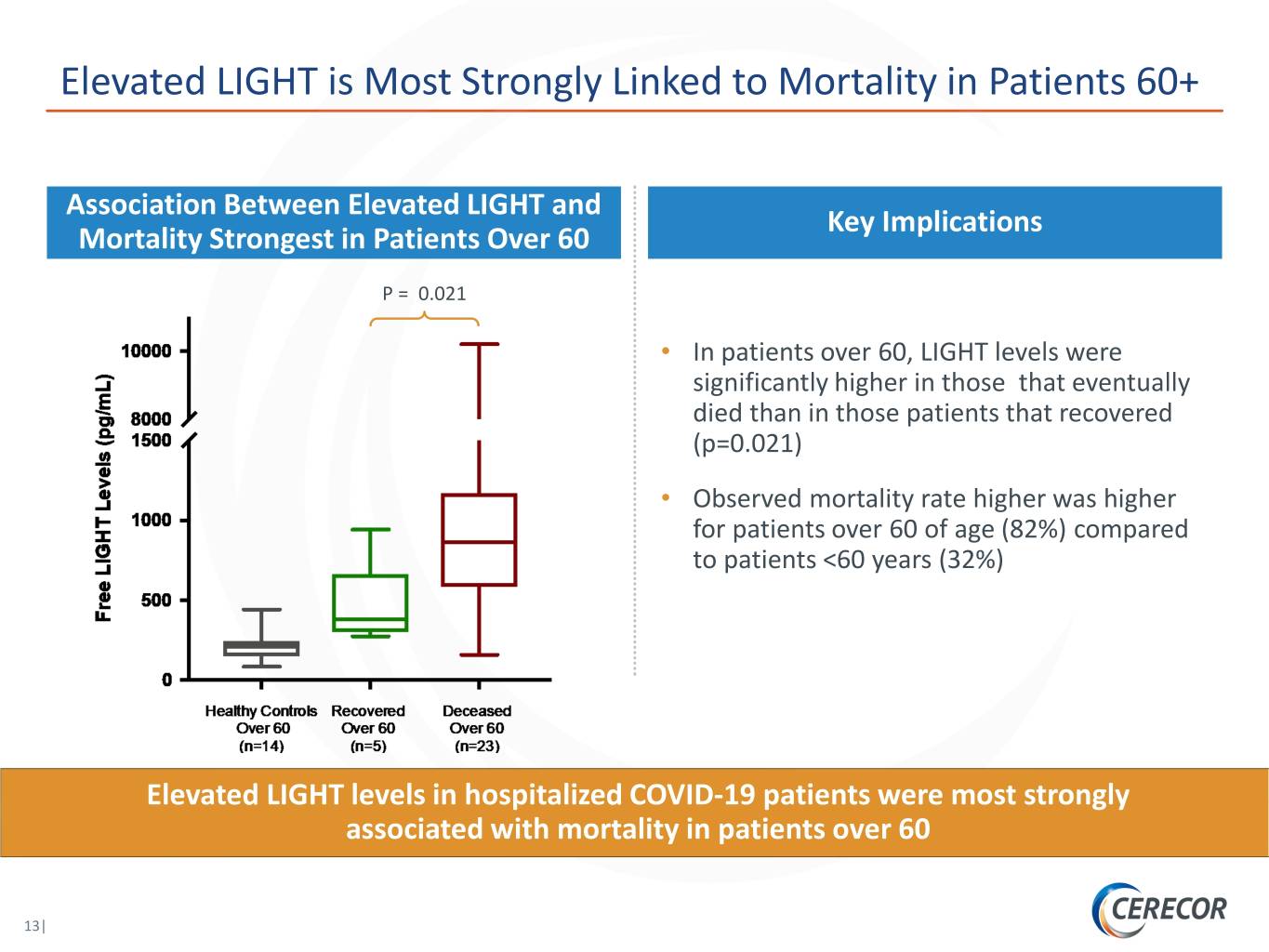
Elevated LIGHT is Most Strongly Linked to Mortality in Patients 60+ Association Between Elevated LIGHT and Key Implications Mortality Strongest in Patients Over 60 P = 0.021 • In patients over 60, LIGHT levels were significantly higher in those that eventually died than in those patients that recovered (p=0.021) • Observed mortality rate higher was higher for patients over 60 of age (82%) compared to patients <60 years (32%) Elevated LIGHT levels in hospitalized COVID-19 patients were most strongly associated with mortality in patients over 60 13|

Cytokine Storm Drives ARDS Across Etiologies Patients may progress rapidly and often require invasive mechanical ventilation Pre-ARDS Disease Course ARDS Progression Mild ARDS Moderate ARDS Severe ARDS COVID-19 infection COVID-19 ARDS All etiologies of ARDS have high unmet need, with COVID-19 infection is Cytokine patients presenting at any typically associated with Storm severity and frequently longer duration of progressing rapidly ventilation in severe patients Broader ARDS Injury due to various etiologies (e.g., pneumonia, trauma, aspiration) Potential Opportunity LIGHT Treatment Window Critical Care / ICU Critical Care / ICU ICU Treatment / Typically non-hospitalized for COVID-19 infection and pre-ICU All ARDS patients are candidates for intubation, with the Care Setting for broader ARDS vast majority of moderate and virtually all severe patients requiring invasive mechanical ventilation Reducing LIGHT levels may limit the proportion of patients requiring invasive mechanical ventilation, which drives high cost of treatment and low quality of life in ARDS 14| Source: Physician Interviews; Papazian et al. Ann. Intensive Care 2019; Bhatraju et al. NEJM 2020.

Potential Beyond COVID-19 ARDS CERC-002 may be applicable to the broader ARDS population CERC-002 COVID-19 ARDS High Morbidity and Mortality Broader ARDS Patients High ICU / Ventilation Cost Patients ($90 – $125 K per patient) • Viral / Bacterial Infections • Trauma Rationale for LIGHT as a Target • Aspiration • Sepsis Rapid Development Potential • Pancreatitis Persistent Long-term Incidence CERC-002 has the potential to treat the underlying disease of cytokine storm induced ARDS Source: Chueng. Am J Respir Crit Care Med. 2006; Dasta. Crit Care Med. 2005; Hamel. Am J Med; 2000; Bice. Semin Respir Crit Care 15| Med. 2013; ClearView Analysis. MV: Mechanical Ventilation.

COVID-19 and Broader ARDS Target Populations COVID-19 ARDS provides a potential path to treat a larger patient population in broader ARDS U.S. COVID-19 Related ARDS Patients U.S. Broader ARDS Patients Excluding COVID-19 416 427 392 404 380 367 340 354 19 ARDS Incidence ARDS 19 - COVID Diagnosed Broader ARDS Incidence (K) DiagnosedBroader Time 2019 2020 2021 2022 2023 2024 2025 2026 There is a large market opportunity and high unmet need for effective therapy in cytokine storm induced ARDS beyond COVID-19 16| Source: Rubenfeld et al. N Engl J Med. 2005, 353(16):1685-93. Kissler et al. Science. 2020. UpToDate.

CERC-002: A Novel First-in-Class Anti-LIGHT mAb The only known clinical stage anti-LIGHT antibody Free LIGHT Assay Developed in Collaboration with Myriad RBM Enables a biomarker / precision VH VH medicine development approach NH NH 2 2 CH VL VL Positive Toxicology Profile CL 225 CL 8-week monkey toxicology study was well tolerated up to 231 100 mg/kg per week with NOAEL at 60 mg/kg 263 263 Phase I Trial Successfully Completed 323 323 Up to 1200 mg SQ in healthy volunteers (n=48) 369 369 without significant toxicity 427 427 Phase I/II open-label signal finding study in Crohn’s Discovered at La Jolla Allergy Institute disease currently ongoing (US IND 113264) and Licensed by Cerecor in 2016 One patient completed study and drug was well-tolerated with a significant reduction in LIGHT levels at a low dose with a clinically meaningful improvement 17| SQ: Subcutaneous; NOAEL: No observed adverse effect level.
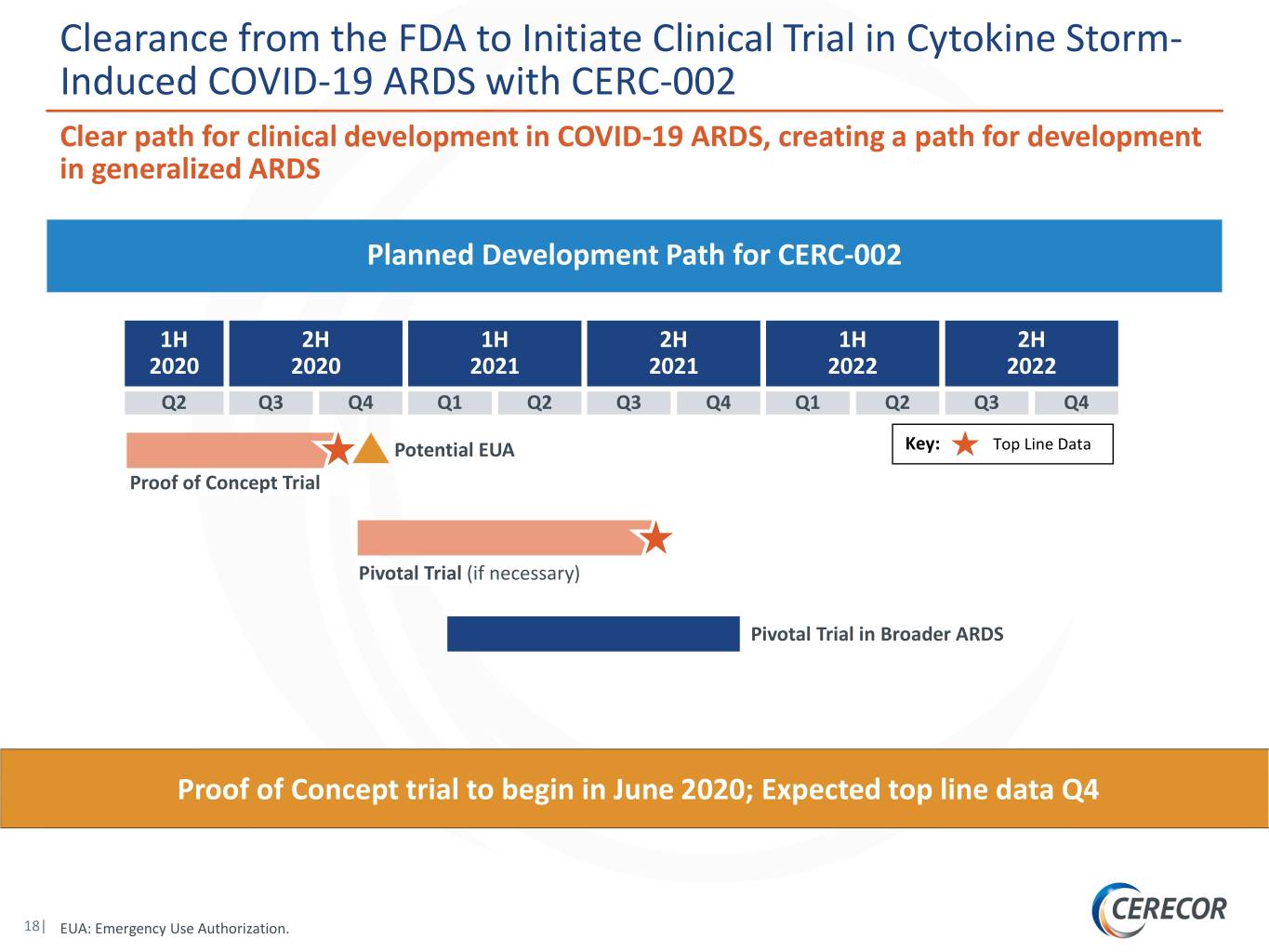
Clearance from the FDA to Initiate Clinical Trial in Cytokine Storm- Induced COVID-19 ARDS with CERC-002 Clear path for clinical development in COVID-19 ARDS, creating a path for development in generalized ARDS Planned Development Path for CERC-002 1H 2H 1H 2H 1H 2H 2020 2020 2021 2021 2022 2022 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Potential EUA Key: Top Line Data Proof of Concept Trial Pivotal Trial (if necessary) Pivotal Trial in Broader ARDS Proof of Concept trial to begin in June 2020; Expected top line data Q4 18| EUA: Emergency Use Authorization.

CERC-002 Treatment of Cytokine Storm-Induced COVID-19 ARDS Primary Endpoint: Respiratory Failure and Mortality Over 28 Days Proof-of-Concept Trial Design Randomized, Double-blind, Placebo-controlled, Multi-Center, Proof-of-Concept Clinical Trial of CERC-002 in Adults with COVID-19 ARDS Inclusion Criteria CERC-002 (16 mg/kg [maximum 1200 mg]) on Day 1 by SQ injection + Standard of Care Hospitalized Patients with Documented COVID-19 Infection and 1:1 Clinical Evidence of Pneumonia with Randomization Acute Lung Injury Placebo-matched SQ injection + Standard of Care Estimated enrollment (N=82) Primary Endpoint Key Secondary / Exploratory Endpoints • The proportion of patients treated with CERC-002 • 1-month mortality compared with placebo in addition to standard of • Change in Pa02/Fi02 ratio care, alive and free of respiratory failure over 28 • Time to and duration of invasive ventilation days • LIGHT levels and other biomarkers of inflammation • Viral load • 80% power to show a 40% ∆ 19|

Physician Perspectives on CERC-002 Front line physicians1 (n=14) with significant experience in COVID-19 and ARDS broadly viewed CERC-002’s mechanism as novel with potential to fill an urgent unmet need Urgent Medical “There are very few options for ARDS patients so I would Need definitely want to use an agent like this.” “This mechanism has higher potential to address ARDS than Novel MOA other cytokine inhibitors.” Broad “This mechanism makes sense for all types of ARDS, not just Anticipated COVID-19.” Utilization Source: Physician Interviews, ClearView Healthcare Partners. 20| 114 physicians and KOLs were interviewed for an hour each for insights into COVID-19 ARDS, broader ARDS, and CERC-002.

Highlights through 2022 • Multiple catalysts and 3 potential PRV awards from first-in-class medicines for diseases with no approved treatment options CERC-801 CERC-801 TOP LINE DATA Potential NDA APPROVAL CERC-803 CERC-803 TOP LINE DATA Potential NDA APPROVAL Initial Data from CERC-802 CERC-802 CDG FIRST TOP LINE DATA Potential NDA Approval 2020 2021 2022 CERC-002: COVID-19 ARDS CERC-006: Complex CERC-007: Multiple Myeloma 1 TOP LINE DATA Lymphatic Malformations TOP LINE PIVOTAL DATA Potential EUA1 TOP LINE DATA CERC-007: Multiple Myeloma CERC-002: COVID-19 ARDS Proof-of-Concept Data Potential FULL APPROVAL2 CERC-007: AOSD Proof-of-Concept Data CERC-800s CERC-002 ARDS CERC-007 CERC-006 Natural History Study PRV Eligible 1 COVID-19 Related ARDS; additional pivotal study will be run if necessary. 21| 2 Broader ARDS. EUA: Emergency Use Authorization.

NASDAQ:CERC www.cerecor.com

Select Board and Management Team Members Michael F. Cola Chief Executive Officer Garry A. Neil, MD Chief Scientific Officer Sol J. Barer, PhD Chairman 23|

References • LIGHT: Lymphotoxin-like, exhibits Inducible expression, and competes with HSV Glycoprotein D for HVEM, a receptor expressed by T lymphocytes; encoded by TNFSF14 (Tumor Necrosis Factor Superfamily 14). • Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. • Bhatraju PK et al., Covid-19 in Critically Ill Patients in the Seattle Region — Case Series. NEJM. 2020. • Bice T, Cox CE, Carson SS. Cost and health care utilization in ARDS--different from other critical illness?. Semin Respir Crit Care Med. 2013;34(4):529‐536. • Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006. • da Silva Antunes R, Madge L, Soroosh P, Tocker J, Croft M. The TNF Family Molecules LIGHT and Lymphotoxin αβ Induce a Distinct Steroid-Resistant Inflammatory Phenotype in Human Lung Epithelial Cells. J Immunol. 2015;195(5):2429‐2441. • da Silva Antunes R, Mehta AK, Madge L, Tocker J, Croft M. TNFSF14 (LIGHT) Exhibits Inflammatory Activities in Lung Fibroblasts Complementary to IL-13 and TGF-β. Front Immunol. 2018;9:576. • Daher P, Teixeira PG, Coopwood TB, et al. Mild to Moderate to Severe: What Drives the Severity of ARDS in Trauma Patients?. Am Surg. 2018;84(6):808‐812. • Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266‐1271. • Desai P, Tahiliani V, Hutchinson TE, et al. The TNF Superfamily Molecule LIGHT Promotes the Generation of Circulating and Lung-Resident Memory CD8 T Cells following an Acute Respiratory Virus Infection. J Immunol. 2018;200(8):2894‐2904. • Eworuke E, Major JM, Gilbert McClain LI. National incidence rates for Acute Respiratory Distress Syndrome (ARDS) and ARDS cause-specific factors in the United States (2006-2014). J Crit Care. 2018;47:192‐197. • Hamel MB et al., Outcomes and cost-effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am J Med. 2000. • Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243‐252. • Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860‐868. • Papazan L et al., Formal guidelines: management of acute respiratory distress syndrome. Ann. Intensive Care. 2019. • Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685‐1693. • Ware CF. Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev. 2008;223:186‐201. • Xu W, Xu Z, Huang L, et al. Transcriptome Sequencing Identifies Novel Immune Response Genes Highly Related to the Severity of Human Adenovirus Type 55 Infection. Front Microbiol. 2019;10:130. 24|
