Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d918630dex992.htm |
| 8-K - 8-K - Aldeyra Therapeutics, Inc. | d918630d8k.htm |

COVID-19 Development Update May 20, 2020 Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2020 Systems-Based Approaches for Immunological Disease Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, research and development plans or expectations, political, economic, legal, social and health risks, including the recent COVID-19 outbreak and subsequent public health measures and other responses to it, that may affect Aldeyra’s business or the global economy, the structure, timing and success of Aldeyra’s planned or pending clinical trials, expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. The results of earlier preclinical or clinical trials may not be predictive of future results. As a result of the COVID-19 pandemic, clinical site availability, staffing, and patient recruitment have been negatively affected and the timelines to complete Aldeyra’s clinical trials may be delayed. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development, clinical and regulatory plans or expectations for Aldeyra’s product candidates and systems-based approaches and Aldeyra’s continuing review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in Aldeyra’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission. All of Aldeyra's development plans and timelines may be subject to adjustment depending on funding, recruitment rate, regulatory review, preclinical and clinical results, and other factors any of which could result in changes to Aldeyra’s development plans and programs or delay the initiation, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of May 20, 2020, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.
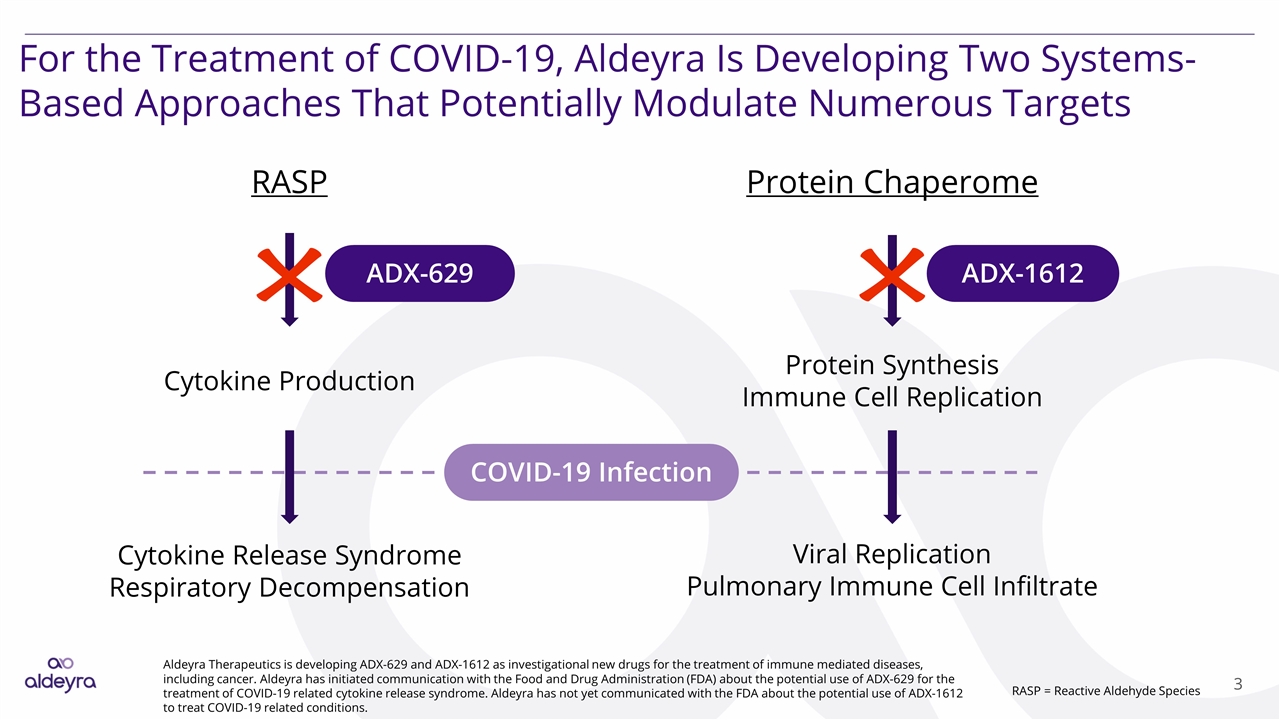
For the Treatment of COVID-19, Aldeyra Is Developing Two Systems-Based Approaches That Potentially Modulate Numerous Targets Aldeyra Therapeutics is developing ADX-629 and ADX-1612 as investigational new drugs for the treatment of immune mediated diseases, including cancer. Aldeyra has initiated communication with the Food and Drug Administration (FDA) about the potential use of ADX-629 for the treatment of COVID-19 related cytokine release syndrome. Aldeyra has not yet communicated with the FDA about the potential use of ADX-1612 to treat COVID-19 related conditions. RASP Cytokine Production ADX-629 ADX-1612 Protein Chaperome Protein Synthesis Immune Cell Replication Cytokine Release Syndrome Respiratory Decompensation Viral Replication Pulmonary Immune Cell Infiltrate COVID-19 Infection RASP = Reactive Aldehyde Species

ADX-1612: A Potential COVID-19 Antiviral with Nanomolar Potency COVID-19 DEVELOPMENT UPDATE May 2020 Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2020
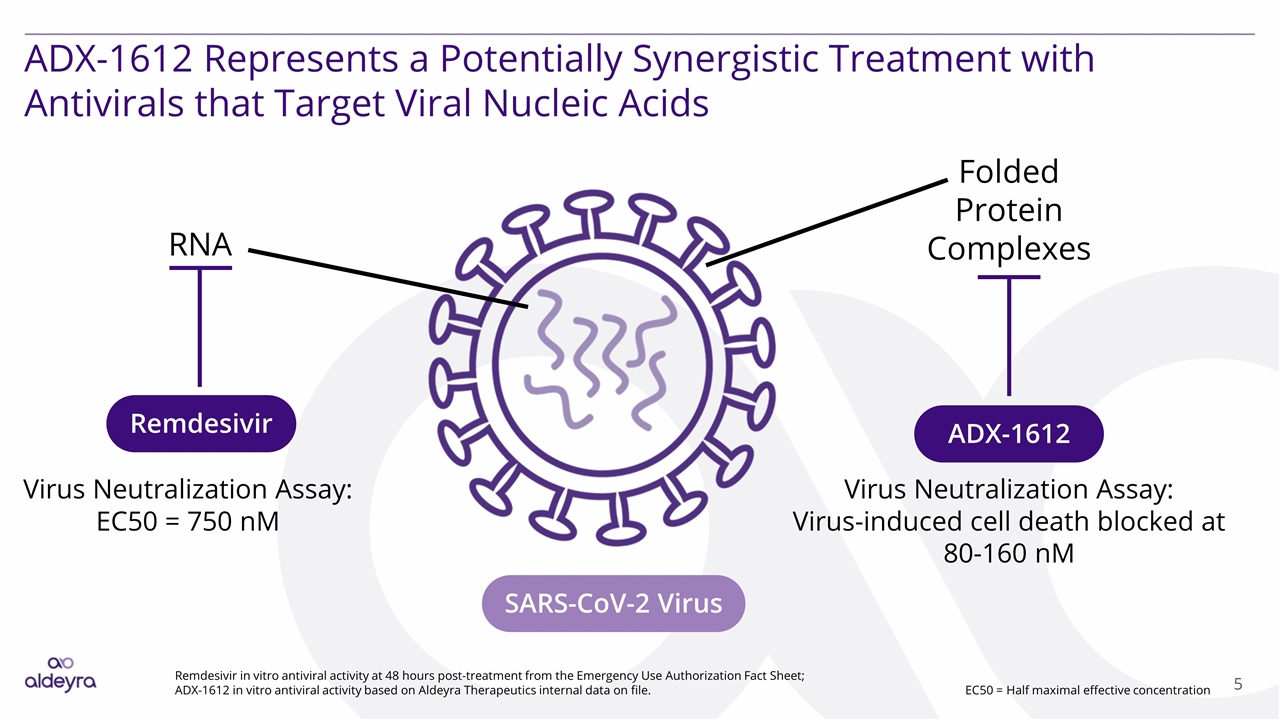
ADX-1612 Represents a Potentially Synergistic Treatment with Antivirals that Target Viral Nucleic Acids Folded Protein Complexes RNA SARS-CoV-2 Virus ADX-1612 Remdesivir Virus Neutralization Assay: EC50 = 750 nM Virus Neutralization Assay: Virus-induced cell death blocked at 80-160 nM Remdesivir in vitro antiviral activity at 48 hours post-treatment from the Emergency Use Authorization Fact Sheet; ADX-1612 in vitro antiviral activity based on Aldeyra Therapeutics internal data on file. EC50 = Half maximal effective concentration
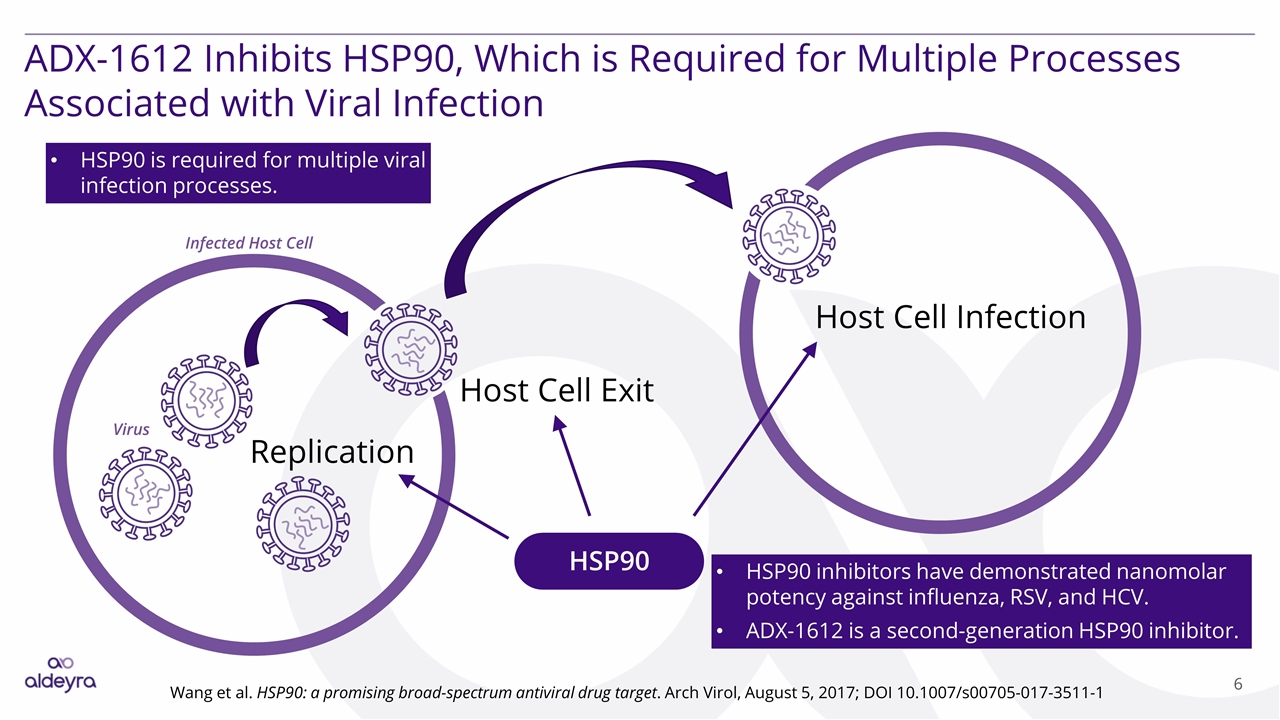
ADX-1612 Inhibits HSP90, Which is Required for Multiple Processes Associated with Viral Infection Wang et al. HSP90: a promising broad‑spectrum antiviral drug target. Arch Virol, August 5, 2017; DOI 10.1007/s00705-017-3511-1 HSP90 Replication Host Cell Exit Host Cell Infection HSP90 inhibitors have demonstrated nanomolar potency against influenza, RSV, and HCV. ADX-1612 is a second-generation HSP90 inhibitor. Infected Host Cell Virus HSP90 is required for multiple viral infection processes.
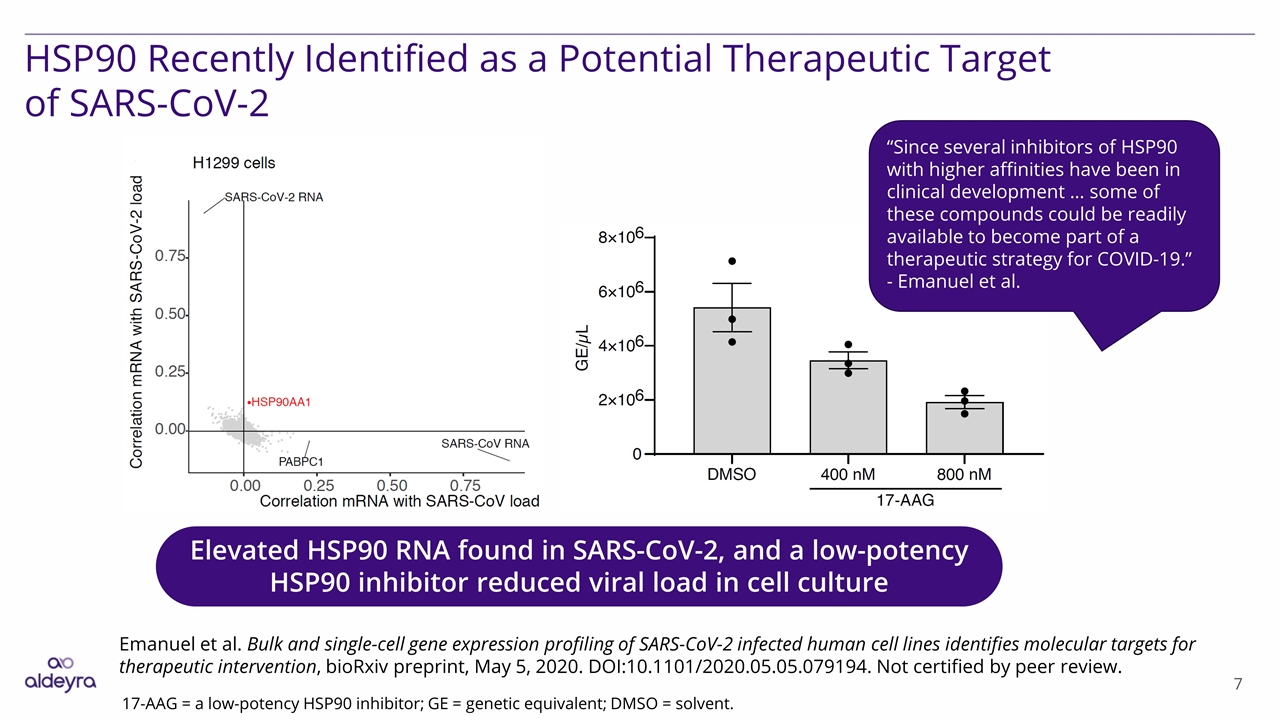
HSP90 Recently Identified as a Potential Therapeutic Target of SARS-CoV-2 Elevated HSP90 RNA found in SARS-CoV-2, and a low-potency HSP90 inhibitor reduced viral load in cell culture Emanuel et al. Bulk and single-cell gene expression profiling of SARS-CoV-2 infected human cell lines identifies molecular targets for therapeutic intervention, bioRxiv preprint, May 5, 2020. DOI:10.1101/2020.05.05.079194. Not certified by peer review. 17-AAG = a low-potency HSP90 inhibitor; GE = genetic equivalent; DMSO = solvent. “Since several inhibitors of HSP90 with higher affinities have been in clinical development … some of these compounds could be readily available to become part of a therapeutic strategy for COVID-19.” - Emanuel et al.
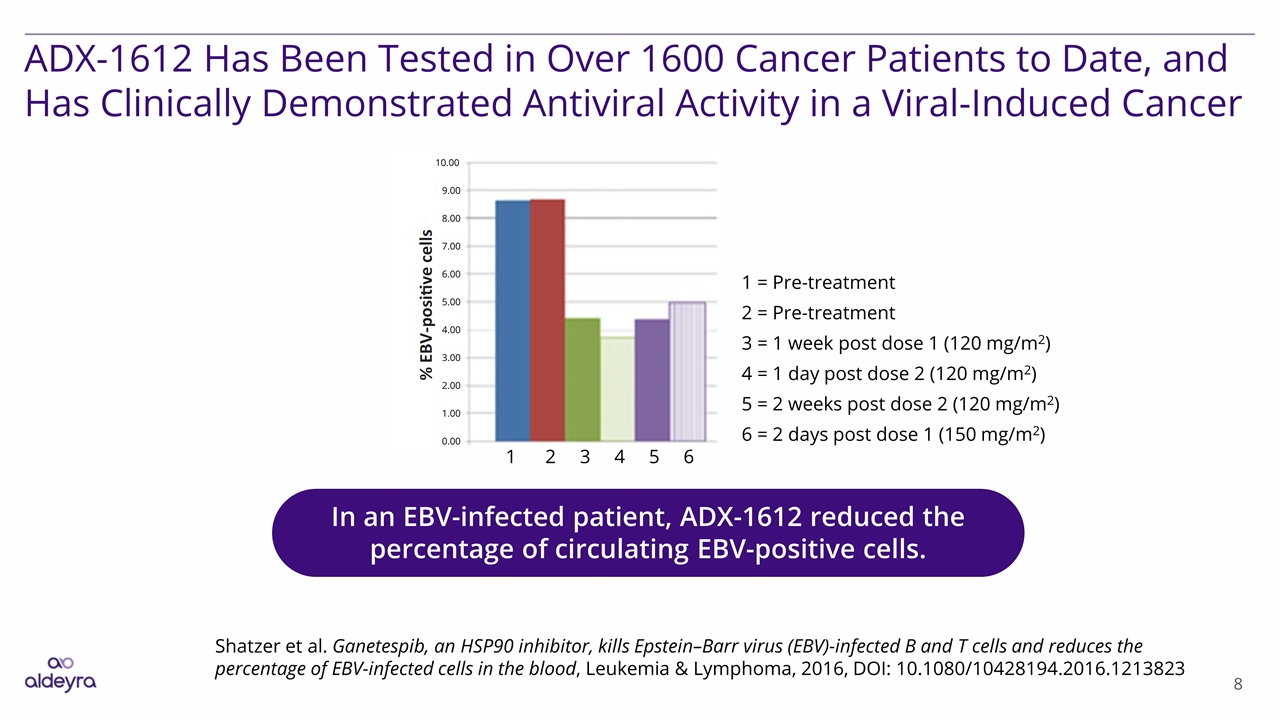
ADX-1612 Has Been Tested in Over 1600 Cancer Patients to Date, and Has Clinically Demonstrated Antiviral Activity in a Viral-Induced Cancer Shatzer et al. Ganetespib, an HSP90 inhibitor, kills Epstein–Barr virus (EBV)-infected B and T cells and reduces the percentage of EBV-infected cells in the blood, Leukemia & Lymphoma, 2016, DOI: 10.1080/10428194.2016.1213823 In an EBV-infected patient, ADX-1612 reduced the percentage of circulating EBV-positive cells. 1 = Pre-treatment 2 = Pre-treatment 3 = 1 week post dose 1 (120 mg/m2) 4 = 1 day post dose 2 (120 mg/m2) 5 = 2 weeks post dose 2 (120 mg/m2) 6 = 2 days post dose 1 (150 mg/m2) 1 2 3 4 5 6 0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 10.00 9.00
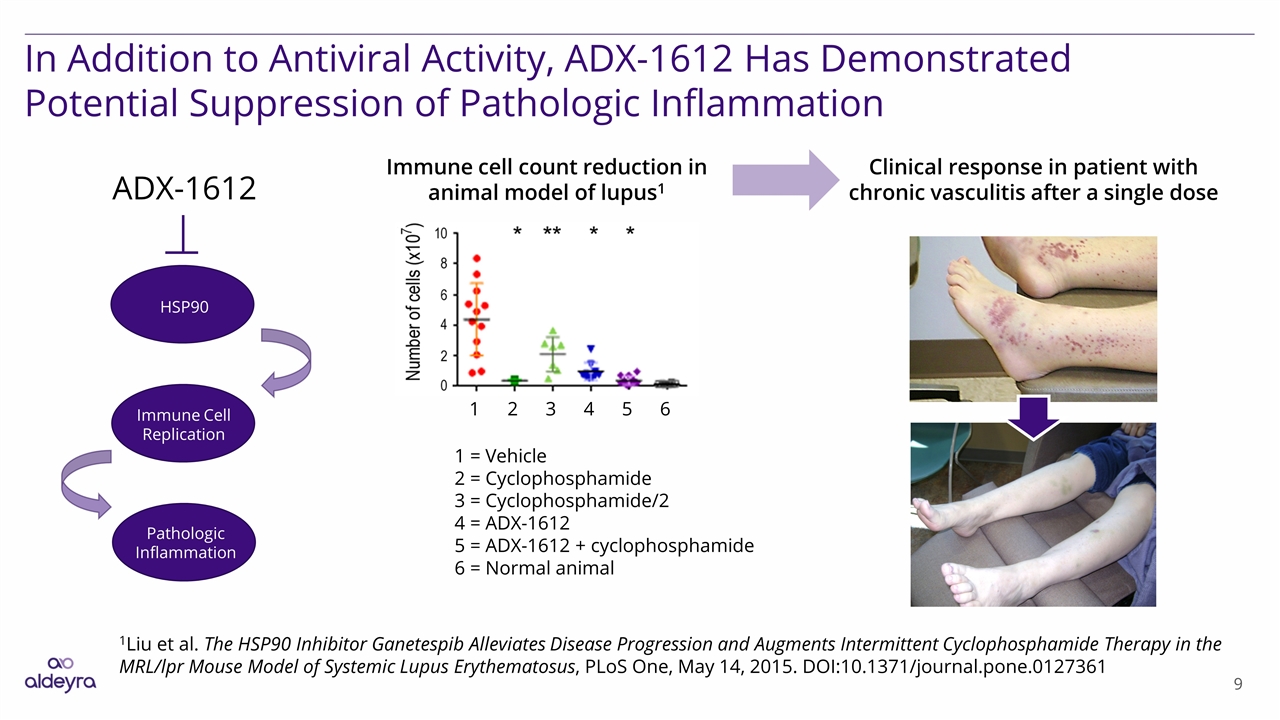
In Addition to Antiviral Activity, ADX-1612 Has Demonstrated Potential Suppression of Pathologic Inflammation 1Liu et al. The HSP90 Inhibitor Ganetespib Alleviates Disease Progression and Augments Intermittent Cyclophosphamide Therapy in the MRL/lpr Mouse Model of Systemic Lupus Erythematosus, PLoS One, May 14, 2015. DOI:10.1371/journal.pone.0127361 ADX-1612 HSP90 Immune Cell Replication Pathologic Inflammation 1 = Vehicle 2 = Cyclophosphamide 3 = Cyclophosphamide/2 4 = ADX-1612 5 = ADX-1612 + cyclophosphamide 6 = Normal animal 1 2 3 4 5 6 Immune cell count reduction in animal model of lupus1 Clinical response in patient with chronic vasculitis after a single dose
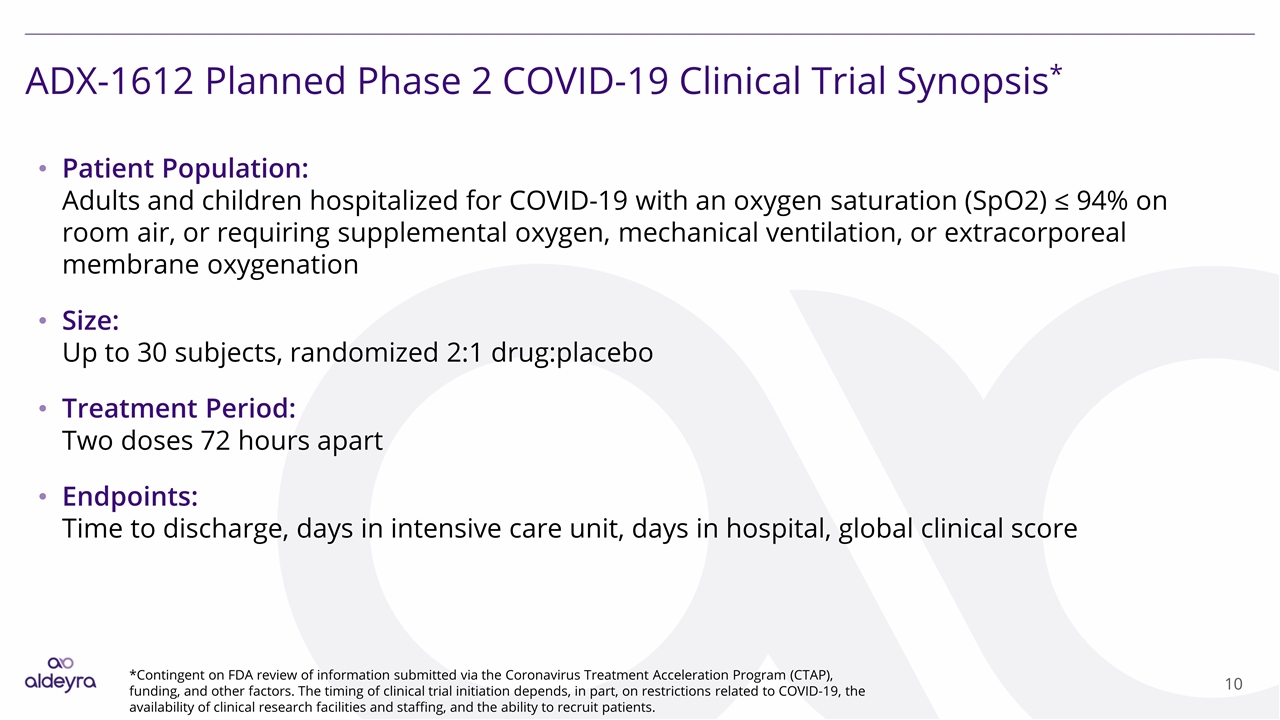
ADX-1612 Planned Phase 2 COVID-19 Clinical Trial Synopsis* Patient Population: Adults and children hospitalized for COVID-19 with an oxygen saturation (SpO2) ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation Size: Up to 30 subjects, randomized 2:1 drug:placebo Treatment Period: Two doses 72 hours apart Endpoints: Time to discharge, days in intensive care unit, days in hospital, global clinical score *Contingent on FDA review of information submitted via the Coronavirus Treatment Acceleration Program (CTAP), funding, and other factors. The timing of clinical trial initiation depends, in part, on restrictions related to COVID-19, the availability of clinical research facilities and staffing, and the ability to recruit patients.

COVID-19 IND submission Q3 2020 Coronavirus Treatment Acceleration Program application May 2020 Expected ADX-1612 Development Milestones and Clinical Plans* Initiation of clinical testing for COVID-19 Q3 2020 Completion of enrollment for ovarian cancer (the Phase 2 EUDARIO Trial) June 2020 *The timing of clinical trial initiation depends, in part, on restrictions related to COVID-19, the availability of clinical research facilities and staffing, and the ability to recruit patients. Contingent on funding, regulatory review, and other factors.

ADX-629 For The Potential Treatment of Cytokine Release Syndrome COVID-19 DEVELOPMENT UPDATE May 2020 Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2020
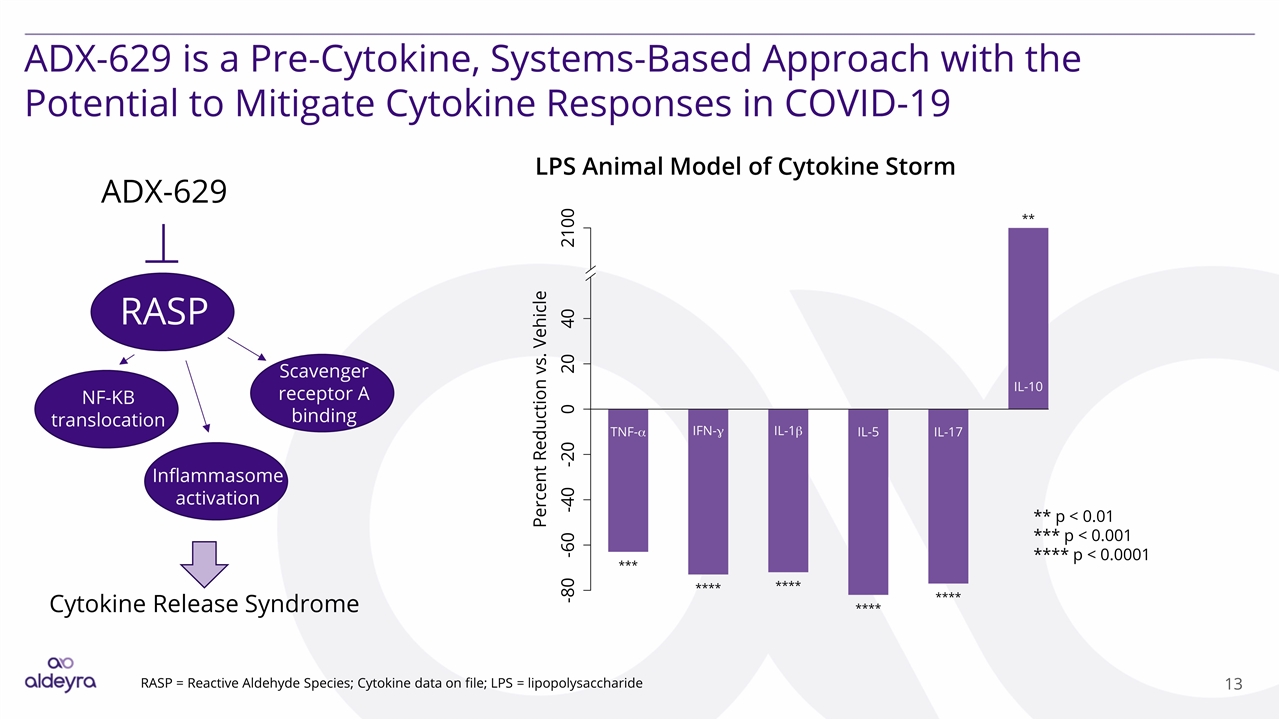
ADX-629 is a Pre-Cytokine, Systems-Based Approach with the Potential to Mitigate Cytokine Responses in COVID-19 RASP LPS Animal Model of Cytokine Storm ADX-629 RASP = Reactive Aldehyde Species; Cytokine data on file; LPS = lipopolysaccharide NF-KB translocation Inflammasome activation Scavenger receptor A binding Cytokine Release Syndrome ** p < 0.01 *** p < 0.001 **** p < 0.0001
ADX-629 Planned Phase 2 COVID-19 Clinical Trial Synopsis* Patient Population: COVID-19 patients recently admitted to hospital for respiratory compromise Dosing: 600mg oral twice daily Size: Up to 30 subjects, randomized 2:1 drug:placebo Treatment Period: Up to 28 days Endpoints: Proportion on mechanical ventilation, time to discharge, cytokine profile *Contingent on FDA review of information submitted via the Coronavirus Treatment Acceleration Program (CTAP), funding, and other factors. The timing of clinical trial initiation depends, in part, on restrictions related to COVID-19, the availability of clinical research facilities and staffing, and the ability to recruit patients.

BARDA CoronaWatch application May 2020 Coronavirus Treatment Acceleration Program (CTAP) application March 2020 Expected ADX-629 Development Milestones and Clinical Plans* BARDA CoronaWatch meeting accepted (to be scheduled) *The timing of clinical trial initiation depends, in part, on restrictions related to COVID-19, the availability of clinical research facilities and staffing, and the ability to recruit patients. Contingent on funding, regulatory review, and other factors. Pre-IND FDA discussion (Pulmonary Division) COVID-19 IND submission June 2020 COVID-19 clinical trial initiation Q3 2020 Psoriasis and atopic asthma clinical trial initiations H2 2020

Systems-Based Approaches for Immunological Disease Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2020
