Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Merus N.V. | d902243d8k.htm |
May 2020 Closing In On Cancer Exhibit 99.1

Disclaimer This presentation (including any oral commentary that accompanies this presentation) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the impact our Biclonics® platform can have on cancer, our product candidates' potential to treat certain types of tumors, the timing of regulatory filings and the timing and anticipated data read outs or results from our clinical trials and our collaborations. These forward-looking statements are based on management's current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: we have incurred significant losses, are not currently profitable and may never become profitable; our need for additional funding, which may not be available and which may require us to restrict out operations or require us to relinquish rights to our technologies or bispecific antibody candidates; potential delays in regulatory approval and impacts of the COVID-19 pandemic, which would impact our ability to commercialize our product candidates and affect our ability to generate revenue; the unproven approach to therapeutic intervention of our Biclonics®, and TriclonicsTM technology; our limited operating history; economic, political, regulatory and other risks involved with international operations; the lengthy and expensive process of clinical drug development, which has an uncertain outcome; the unpredictable nature of our early stage development efforts for marketable drugs; potential adverse public reaction to the use of cancer immunotherapies; potential delays in enrollment of patients, which could affect the receipt of necessary regulatory approvals; failure to obtain marketing approval internationally; failure to compete successfully against other drug companies; potential competition from other drug companies if we fail to obtain orphan drug designation or maintain orphan drug exclusivity for our products; our reliance on third parties to conduct our clinical trials and the potential for those third parties to not perform satisfactorily; our reliance on third parties to manufacture our product candidates, which may delay, prevent or impair our development and commercialization efforts; protection of our proprietary technology; our patents being found invalid or unenforceable; potential lawsuits for infringement of third-party intellectual property; our ability to attract and retain key personnel; managing our growth could result in difficulties; and we may lose our foreign private issuer status and incur significant expenses as a result. These and other important factors discussed under the caption “Risk Factors” in our in our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2020 filed with the Securities and Exchange Commission, or SEC, on May 11, 2020, and our other reports filed with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management's estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change.
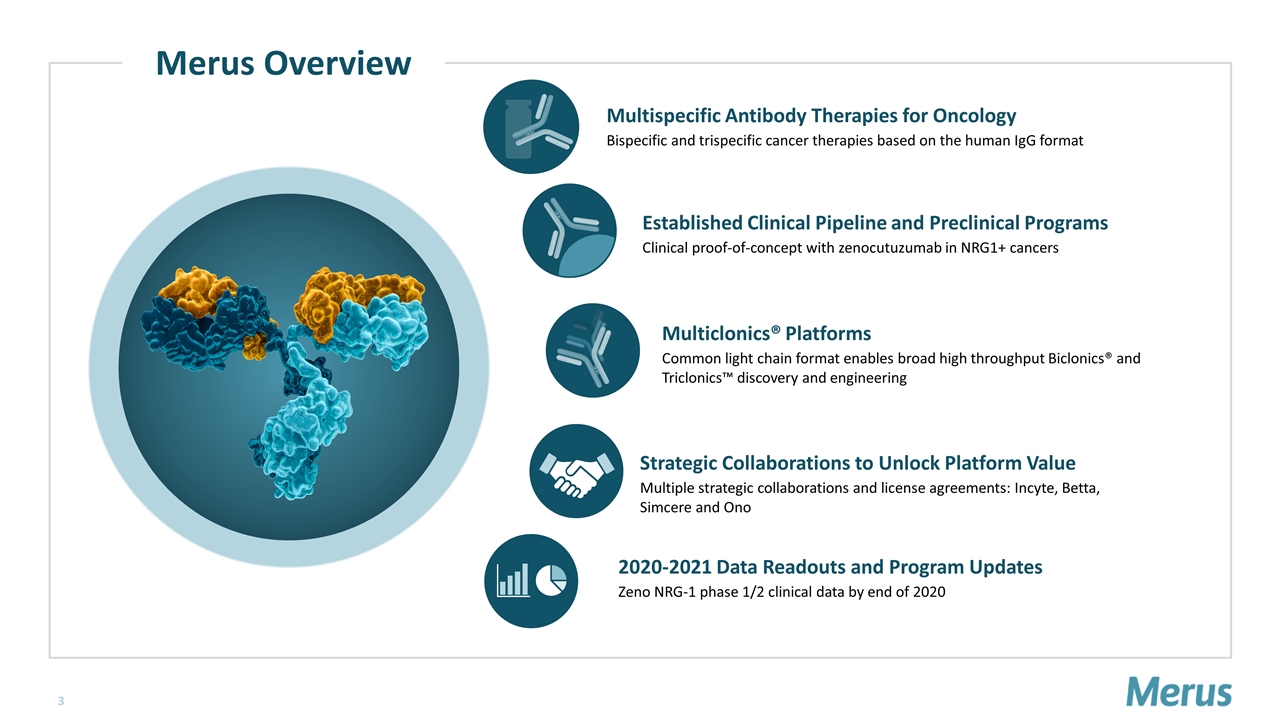
Established Clinical Pipeline and Preclinical Programs Clinical proof-of-concept with zenocutuzumab in NRG1+ cancers Multispecific Antibody Therapies for Oncology Bispecific and trispecific cancer therapies based on the human IgG format 2020-2021 Data Readouts and Program Updates Zeno NRG-1 phase 1/2 clinical data by end of 2020 Multiclonics® Platforms Common light chain format enables broad high throughput Biclonics® and Triclonics™ discovery and engineering Strategic Collaborations to Unlock Platform Value Multiple strategic collaborations and license agreements: Incyte, Betta, Simcere and Ono Merus Overview
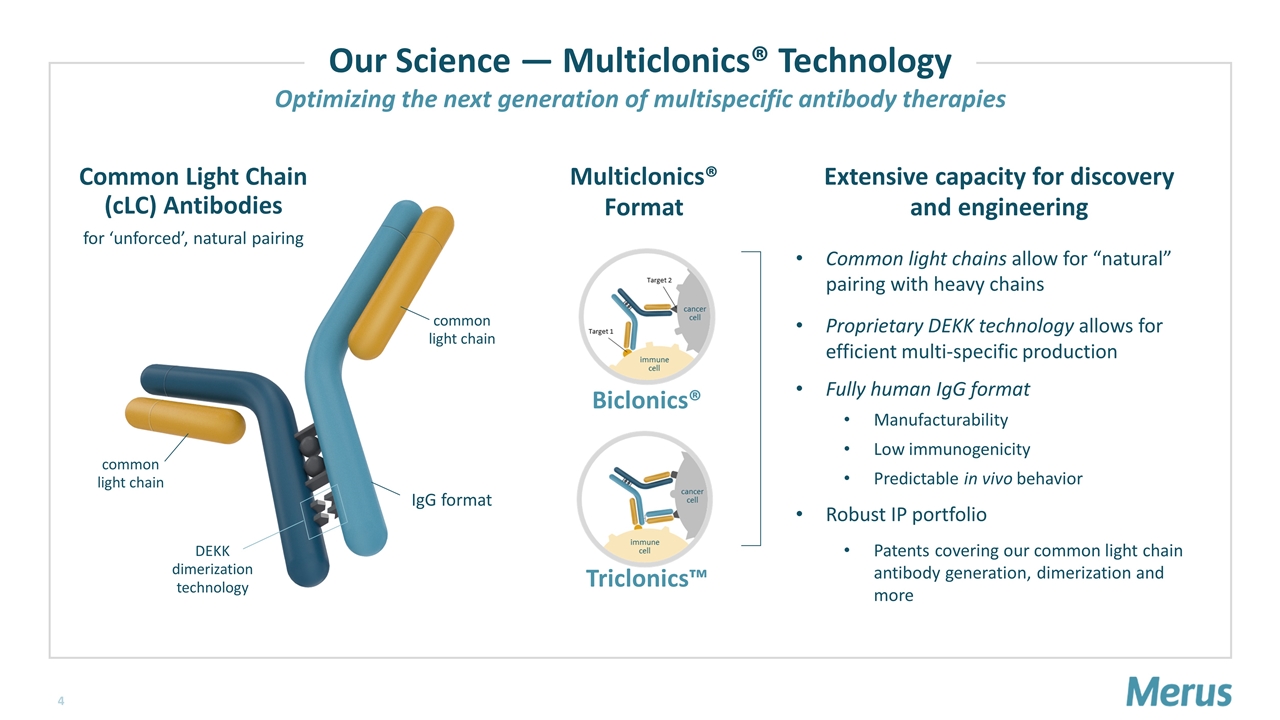
Common Light Chain (cLC) Antibodies for ‘unforced’, natural pairing common light chain DEKK dimerization technology Biclonics® Triclonics™ IgG format Extensive capacity for discovery and engineering Our Science — Multiclonics® Technology common light chain Optimizing the next generation of multispecific antibody therapies Multiclonics® Format Common light chains allow for “natural” pairing with heavy chains Proprietary DEKK technology allows for efficient multi-specific production Fully human IgG format Manufacturability Low immunogenicity Predictable in vivo behavior Robust IP portfolio Patents covering our common light chain antibody generation, dimerization and more
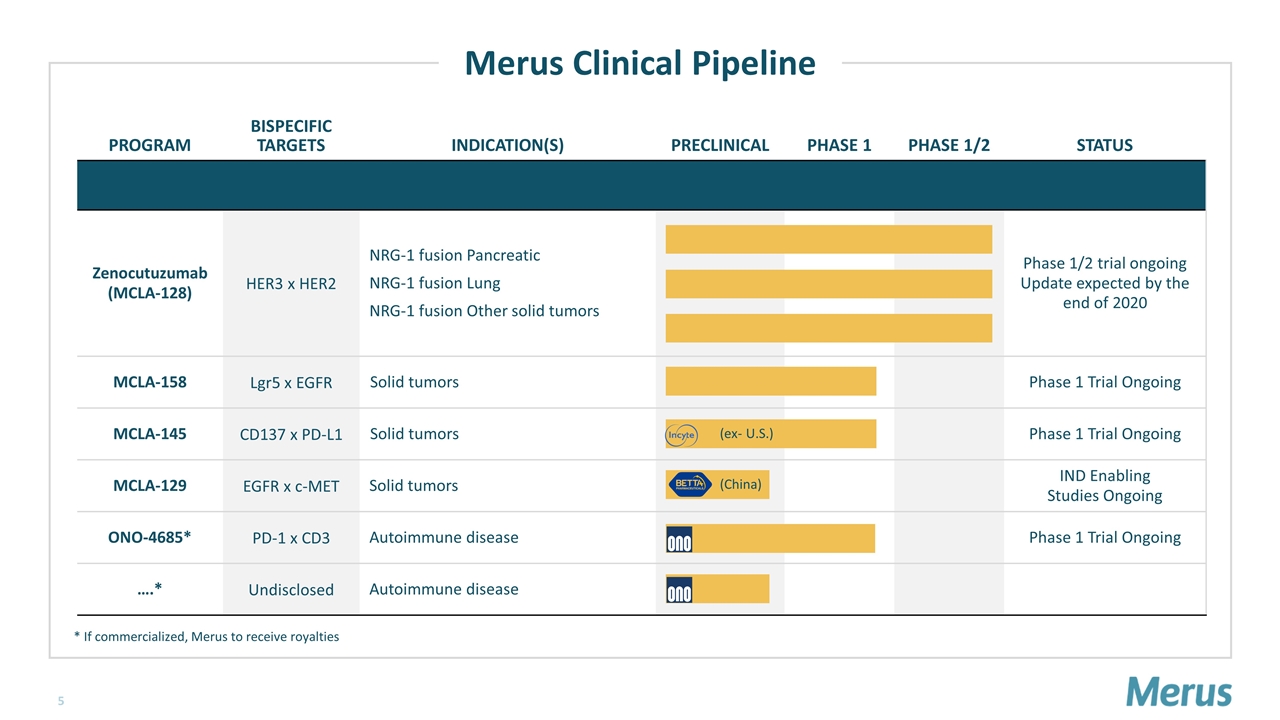
PROGRAM BISPECIFIC TARGETS INDICATION(S) PRECLINICAL PHASE 1 PHASE 1/2 STATUS Zenocutuzumab (MCLA-128) HER3 x HER2 NRG-1 fusion Pancreatic NRG-1 fusion Lung NRG-1 fusion Other solid tumors Phase 1/2 trial ongoing Update expected by the end of 2020 MCLA-158 Lgr5 x EGFR Solid tumors Phase 1 Trial Ongoing MCLA-145 CD137 x PD-L1 Solid tumors Phase 1 Trial Ongoing MCLA-129 EGFR x c-MET Solid tumors IND Enabling Studies Ongoing ONO-4685* PD-1 x CD3 Autoimmune disease Phase 1 Trial Ongoing ….* Undisclosed Autoimmune disease (ex- U.S.) (China) * If commercialized, Merus to receive royalties Merus Clinical Pipeline
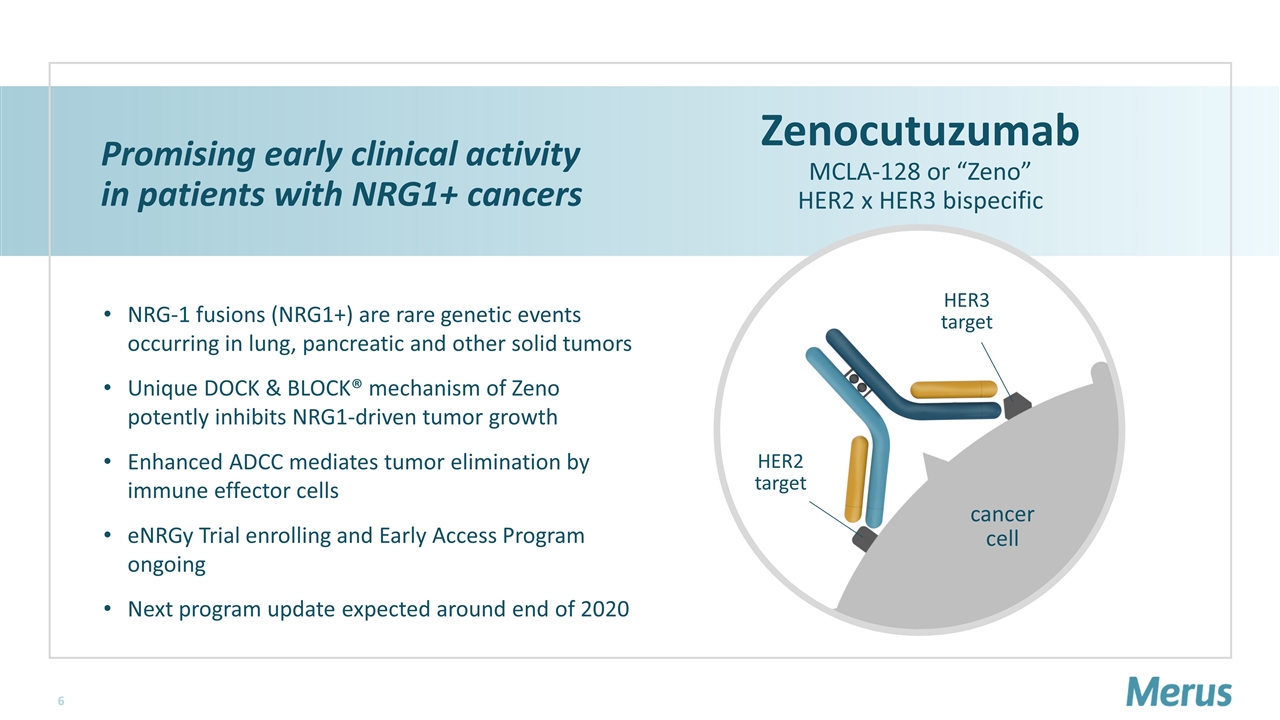
Promising early clinical activity in patients with NRG1+ cancers NRG-1 fusions (NRG1+) are rare genetic events occurring in lung, pancreatic and other solid tumors Unique DOCK & BLOCK® mechanism of Zeno potently inhibits NRG1-driven tumor growth Enhanced ADCC mediates tumor elimination by immune effector cells eNRGy Trial enrolling and Early Access Program ongoing Next program update expected around end of 2020 Zenocutuzumab MCLA-128 or “Zeno” HER2 x HER3 bispecific cancer cell HER3 target HER2 target
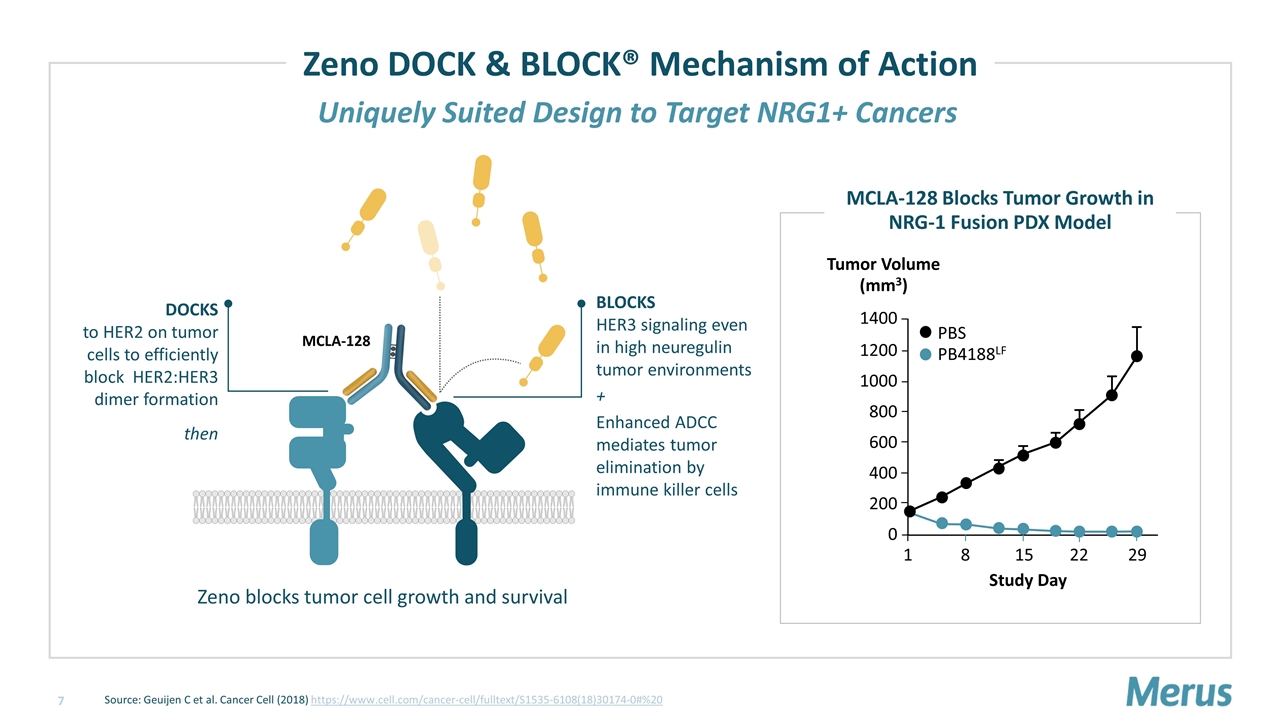
Uniquely Suited Design to Target NRG1+ Cancers Zeno DOCK & BLOCK® Mechanism of Action BLOCKS HER3 signaling even in high neuregulin tumor environments + Enhanced ADCC mediates tumor elimination by immune killer cells DOCKS to HER2 on tumor cells to efficiently block HER2:HER3 dimer formation then Zeno blocks tumor cell growth and survival MCLA-128 Source: Geuijen C et al. Cancer Cell (2018) https://www.cell.com/cancer-cell/fulltext/S1535-6108(18)30174-0#%20 Study Day 1400 0 1 29 Tumor Volume (mm3) PBS PB4188LF 800 15 1200 1000 600 400 200 8 22 MCLA-128 Blocks Tumor Growth in NRG-1 Fusion PDX Model
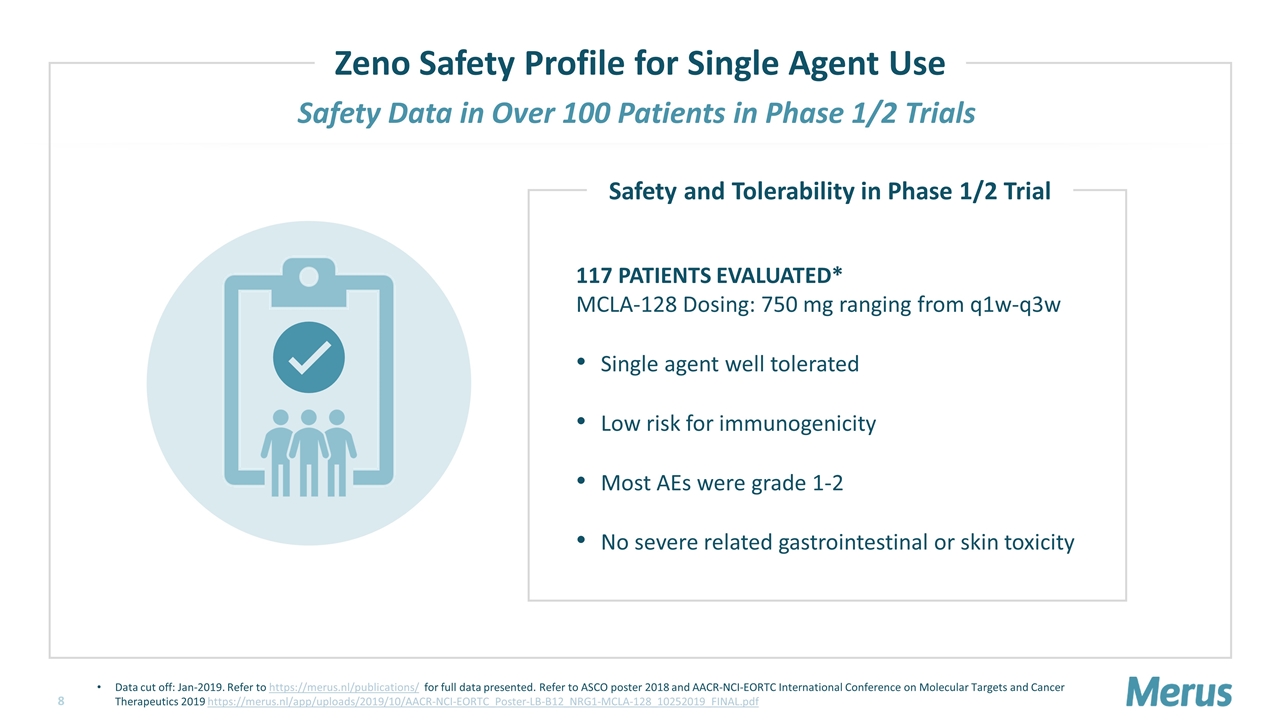
Safety Data in Over 100 Patients in Phase 1/2 Trials 117 PATIENTS EVALUATED* MCLA-128 Dosing: 750 mg ranging from q1w-q3w Single agent well tolerated Low risk for immunogenicity Most AEs were grade 1-2 No severe related gastrointestinal or skin toxicity Safety and Tolerability in Phase 1/2 Trial Zeno Safety Profile for Single Agent Use Data cut off: Jan-2019. Refer to https://merus.nl/publications/ for full data presented. Refer to ASCO poster 2018 and AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2019 https://merus.nl/app/uploads/2019/10/AACR-NCI-EORTC_Poster-LB-B12_NRG1-MCLA-128_10252019_FINAL.pdf
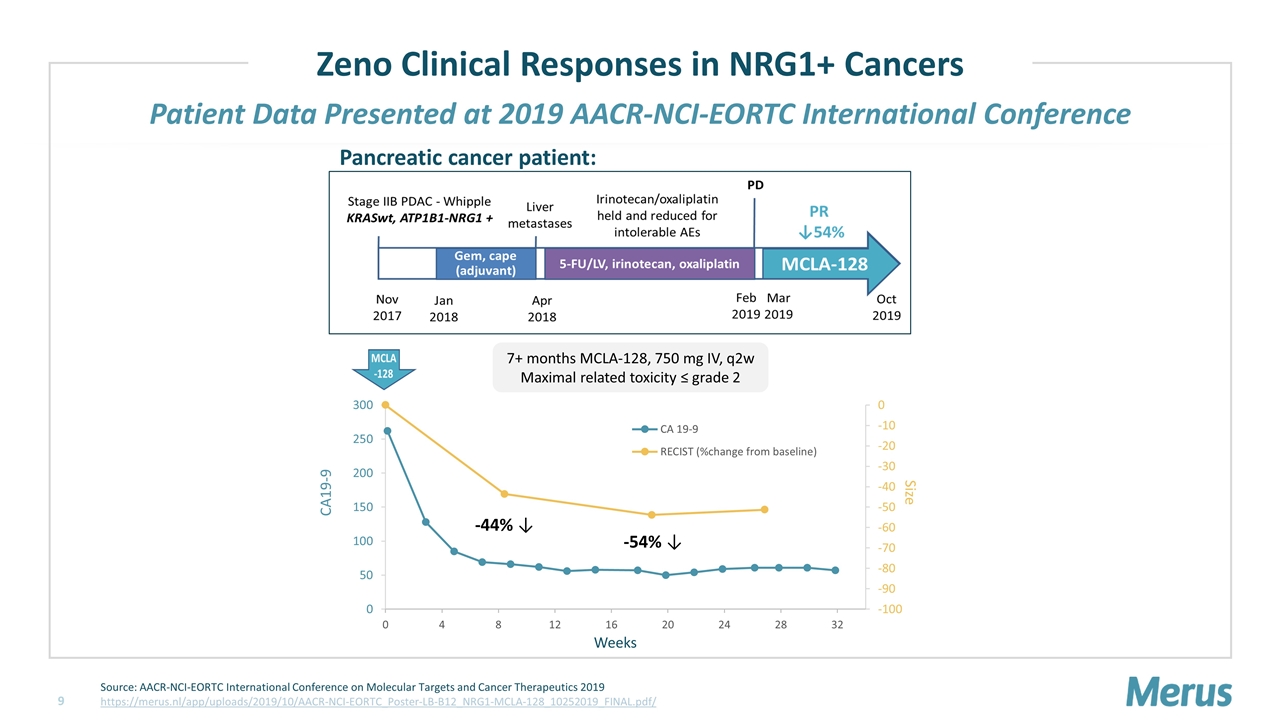
Patient Data Presented at 2019 AACR-NCI-EORTC International Conference 7+ months MCLA-128, 750 mg IV, q2w Maximal related toxicity ≤ grade 2 -44% ↓ -54% ↓ Pancreatic cancer patient: Zeno Clinical Responses in NRG1+ Cancers Source: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2019 https://merus.nl/app/uploads/2019/10/AACR-NCI-EORTC_Poster-LB-B12_NRG1-MCLA-128_10252019_FINAL.pdf/ CA19-9 Weeks Size
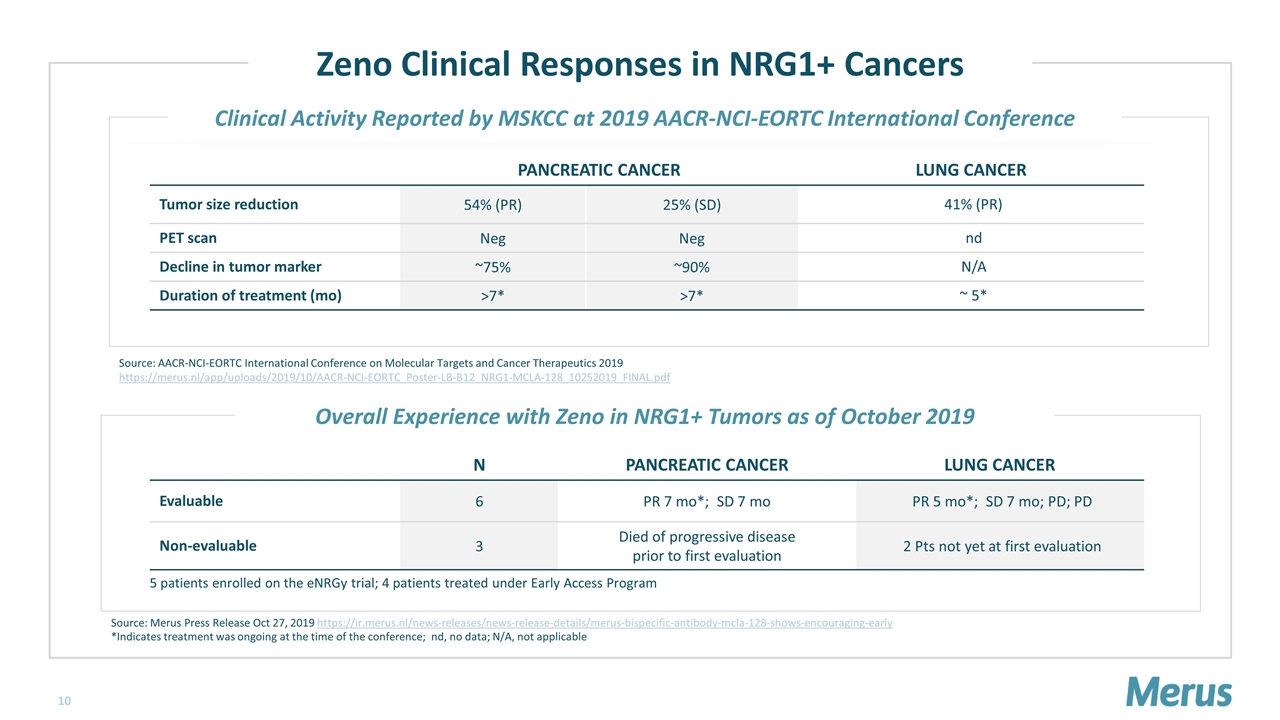
Zeno Clinical Responses in NRG1+ Cancers 5 patients enrolled on the eNRGy trial; 4 patients treated under Early Access Program Source: Merus Press Release Oct 27, 2019 https://ir.merus.nl/news-releases/news-release-details/merus-bispecific-antibody-mcla-128-shows-encouraging-early *Indicates treatment was ongoing at the time of the conference; nd, no data; N/A, not applicable Source: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2019 https://merus.nl/app/uploads/2019/10/AACR-NCI-EORTC_Poster-LB-B12_NRG1-MCLA-128_10252019_FINAL.pdf Clinical Activity Reported by MSKCC at 2019 AACR-NCI-EORTC International Conference Overall Experience with Zeno in NRG1+ Tumors as of October 2019 PANCREATIC CANCER LUNG CANCER Tumor size reduction 54% (PR) 25% (SD) 41% (PR) PET scan Neg Neg nd Decline in tumor marker ~75% ~90% N/A Duration of treatment (mo) >7* >7* ~ 5* N PANCREATIC CANCER LUNG CANCER Evaluable 6 PR 7 mo*; SD 7 mo PR 5 mo*; SD 7 mo; PD; PD Non-evaluable 3 Died of progressive disease prior to first evaluation 2 Pts not yet at first evaluation
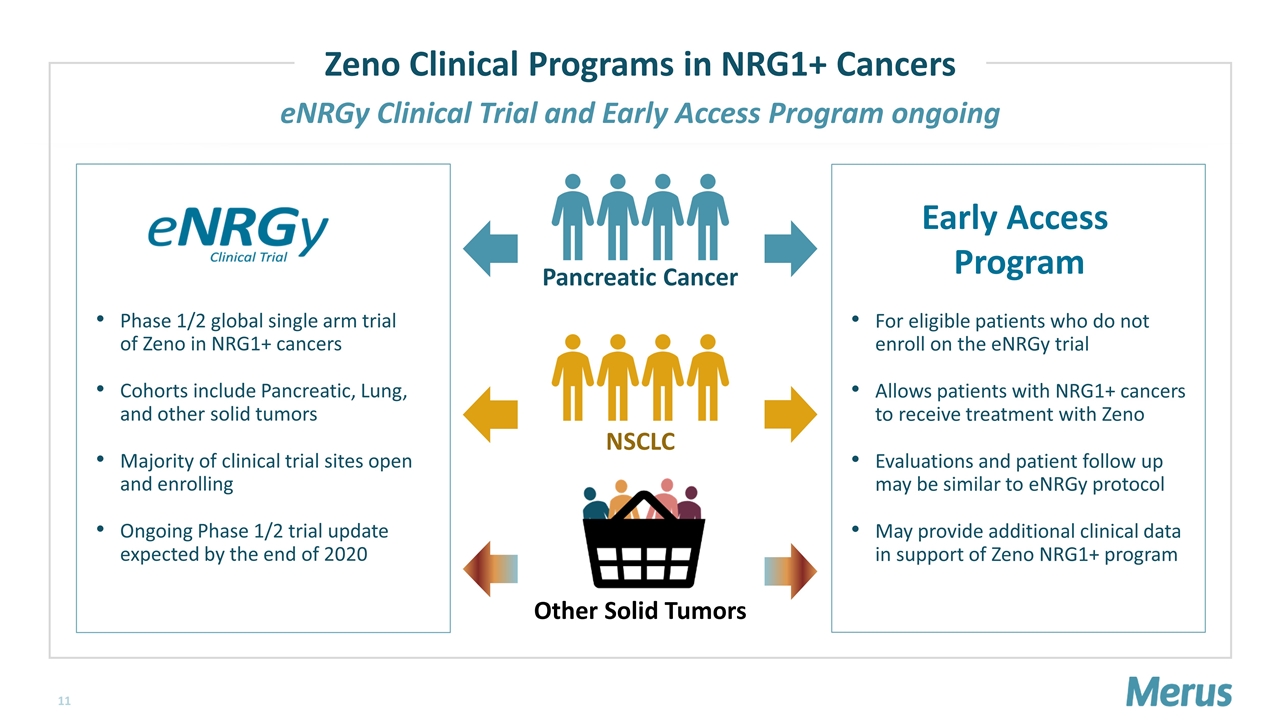
eNRGy Clinical Trial and Early Access Program ongoing Phase 1/2 global single arm trial of Zeno in NRG1+ cancers Cohorts include Pancreatic, Lung, and other solid tumors Majority of clinical trial sites open and enrolling Ongoing Phase 1/2 trial update expected by the end of 2020 Pancreatic Cancer NSCLC Other Solid Tumors For eligible patients who do not enroll on the eNRGy trial Allows patients with NRG1+ cancers to receive treatment with Zeno Evaluations and patient follow up may be similar to eNRGy protocol May provide additional clinical data in support of Zeno NRG1+ program Zeno Clinical Programs in NRG1+ Cancers Early Access Program
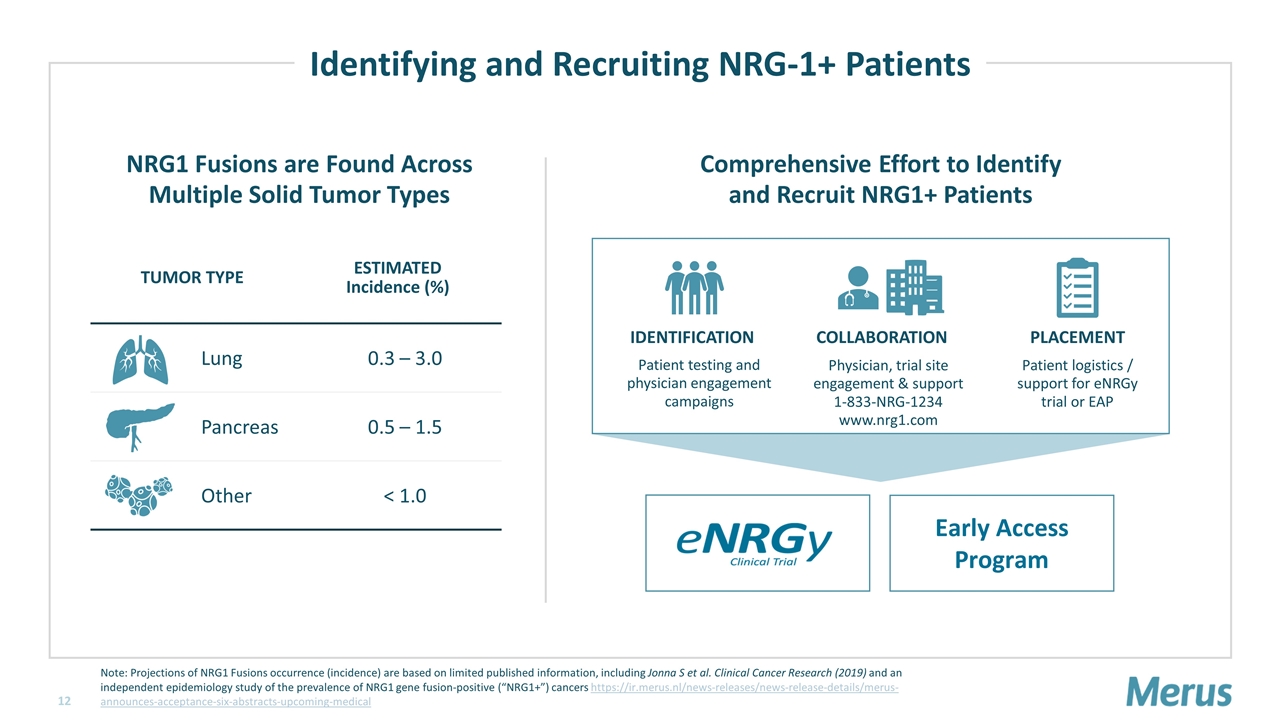
NRG1 Fusions are Found Across Multiple Solid Tumor Types TUMOR TYPE ESTIMATED Incidence (%) Lung 0.3 – 3.0 Pancreas 0.5 – 1.5 Other < 1.0 Early Access Program Comprehensive Effort to Identify and Recruit NRG1+ Patients Identifying and Recruiting NRG-1+ Patients Patient testing and physician engagement campaigns Physician, trial site engagement & support 1-833-NRG-1234 www.nrg1.com IDENTIFICATION COLLABORATION PLACEMENT Patient logistics / support for eNRGy trial or EAP Note: Projections of NRG1 Fusions occurrence (incidence) are based on limited published information, including Jonna S et al. Clinical Cancer Research (2019) and an independent epidemiology study of the prevalence of NRG1 gene fusion-positive (“NRG1+”) cancers https://ir.merus.nl/news-releases/news-release-details/merus-announces-acceptance-six-abstracts-upcoming-medical
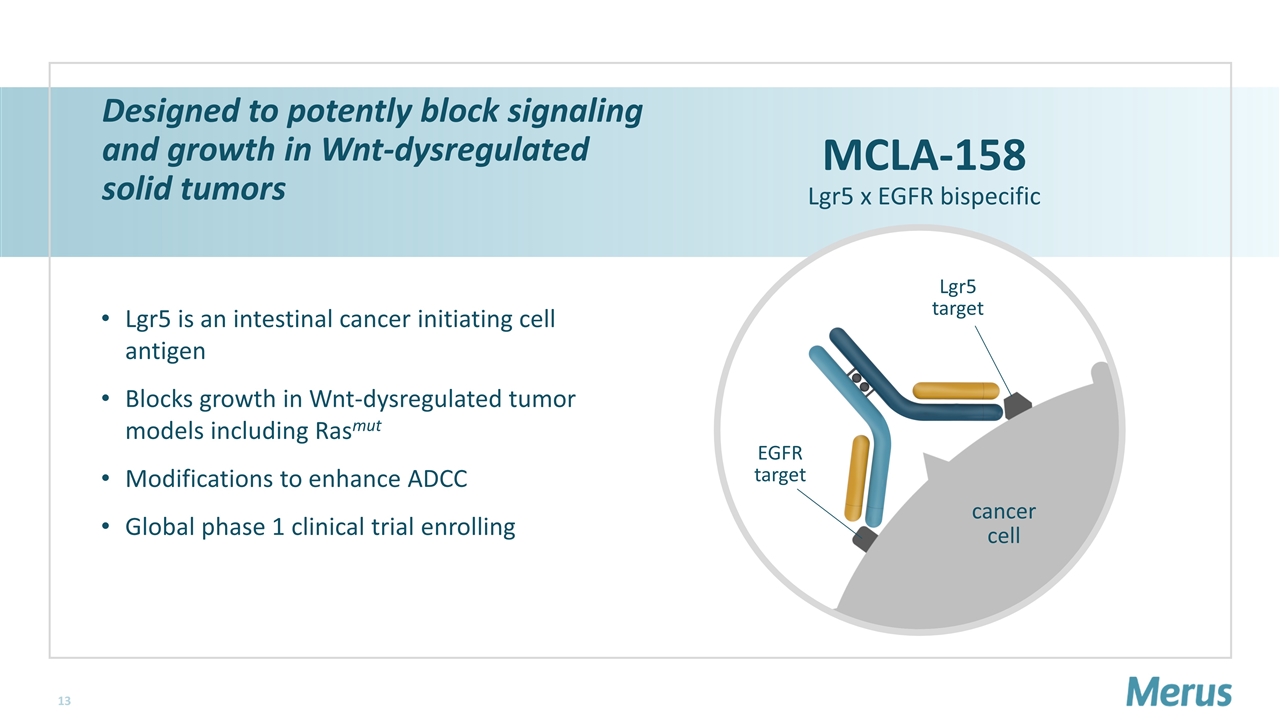
Designed to potently block signaling and growth in Wnt-dysregulated solid tumors Lgr5 is an intestinal cancer initiating cell antigen Blocks growth in Wnt-dysregulated tumor models including Rasmut Modifications to enhance ADCC Global phase 1 clinical trial enrolling MCLA-158 Lgr5 x EGFR bispecific EGFR target Lgr5 target cancer cell
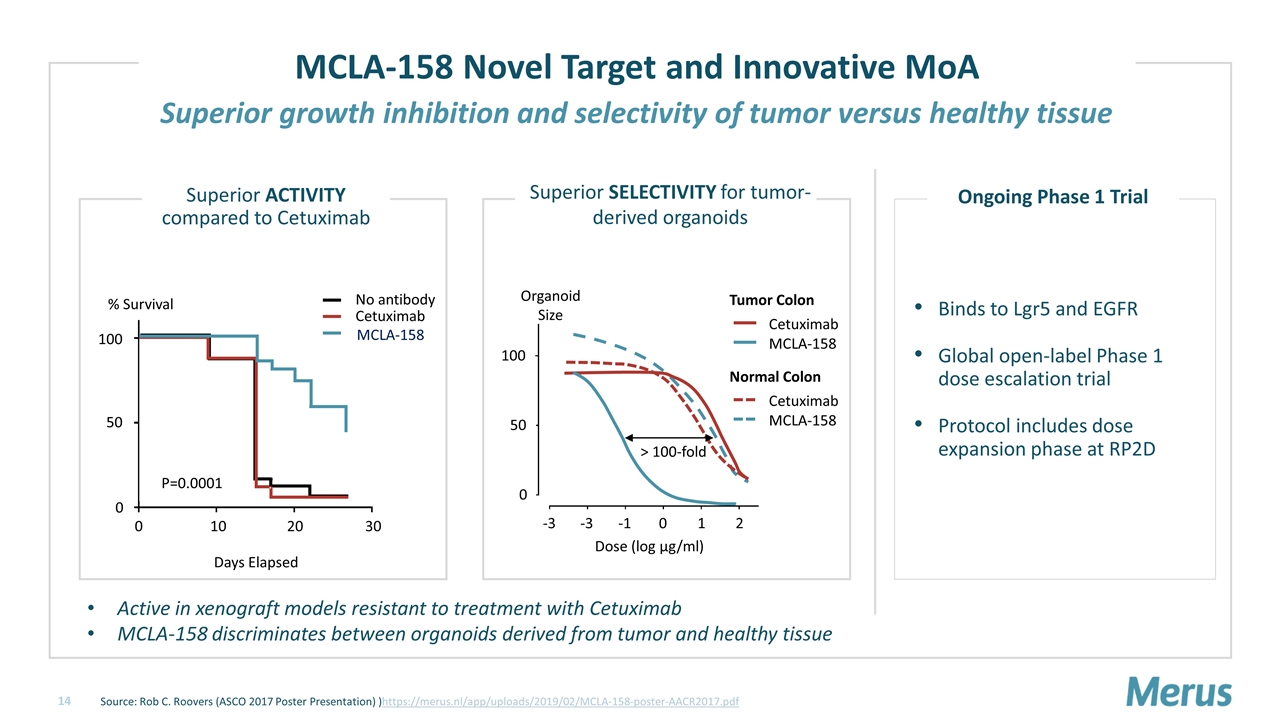
MCLA-158 Novel Target and Innovative MoA Source: Rob C. Roovers (ASCO 2017 Poster Presentation) )https://merus.nl/app/uploads/2019/02/MCLA-158-poster-AACR2017.pdf > 100-fold Dose (log µg/ml) -3 0 50 100 Cetuximab Tumor Colon MCLA-158 Cetuximab Normal Colon MCLA-158 -3 -1 0 1 2 Organoid Size Superior SELECTIVITY for tumor- derived organoids % Survival Days Elapsed P=0.0001 0 10 20 30 0 50 100 Superior ACTIVITY compared to Cetuximab MCLA-158 No antibody Cetuximab Binds to Lgr5 and EGFR Global open-label Phase 1 dose escalation trial Protocol includes dose expansion phase at RP2D Active in xenograft models resistant to treatment with Cetuximab MCLA-158 discriminates between organoids derived from tumor and healthy tissue Superior growth inhibition and selectivity of tumor versus healthy tissue Ongoing Phase 1 Trial
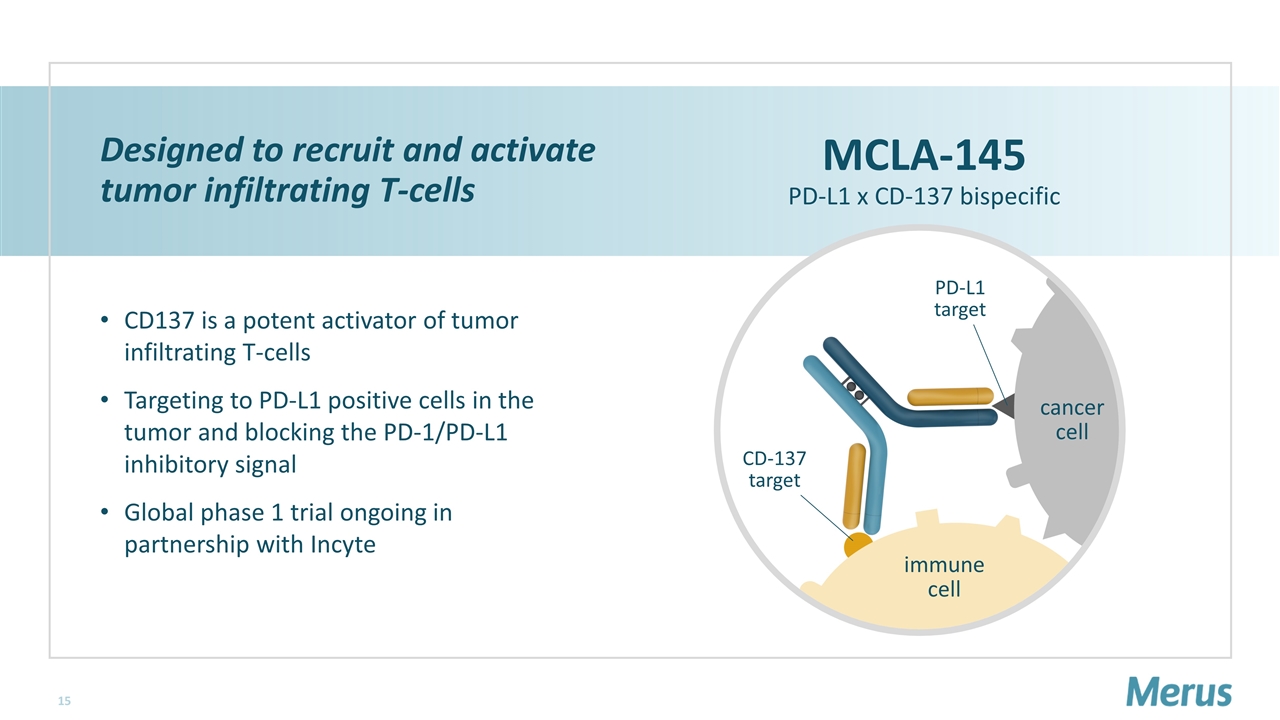
cancer cell immune cell Designed to recruit and activate tumor infiltrating T-cells CD137 is a potent activator of tumor infiltrating T-cells Targeting to PD-L1 positive cells in the tumor and blocking the PD-1/PD-L1 inhibitory signal Global phase 1 trial ongoing in partnership with Incyte MCLA-145 PD-L1 x CD-137 bispecific CD-137 target PD-L1 target
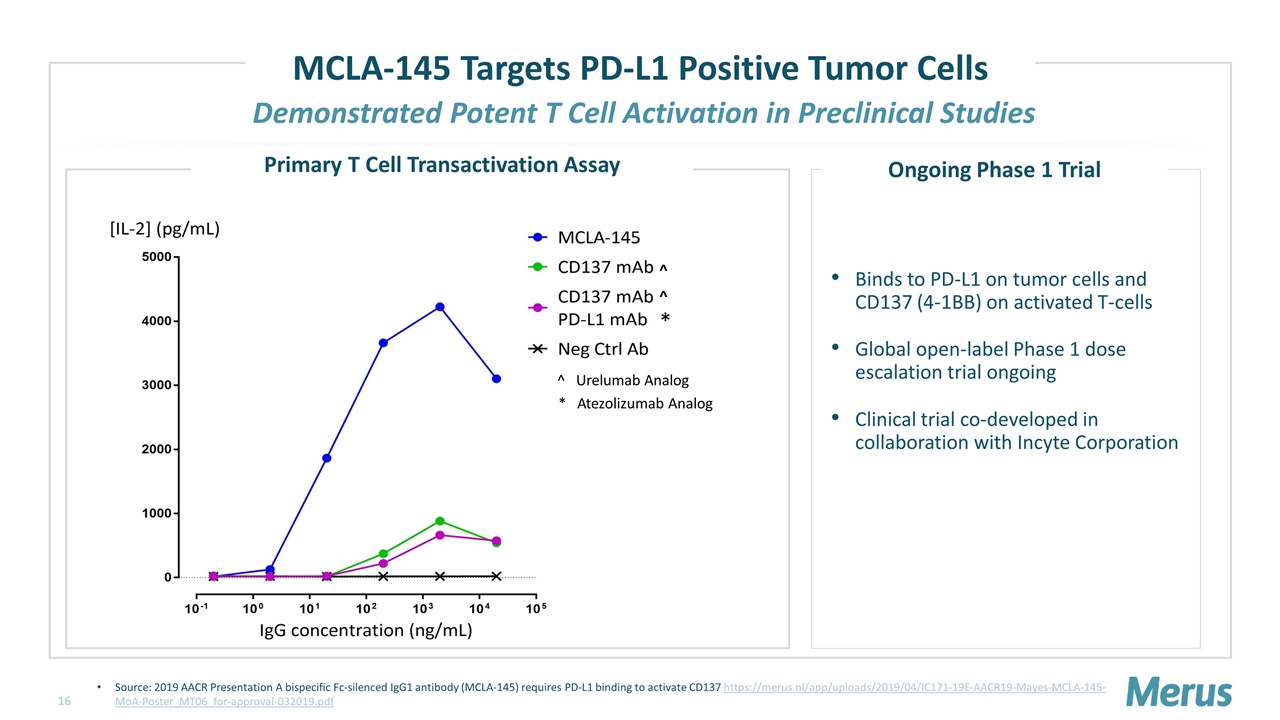
Demonstrated Potent T Cell Activation in Preclinical Studies MCLA-145 Targets PD-L1 Positive Tumor Cells Source: 2019 AACR Presentation A bispecific Fc-silenced IgG1 antibody (MCLA-145) requires PD-L1 binding to activate CD137 https://merus.nl/app/uploads/2019/04/IC171-19E-AACR19-Mayes-MCLA-145-MoA-Poster_MT06_for-approval-032019.pdf ^ Urelumab Analog ^ ^ * * Atezolizumab Analog Primary T Cell Transactivation Assay Binds to PD-L1 on tumor cells and CD137 (4-1BB) on activated T-cells Global open-label Phase 1 dose escalation trial ongoing Clinical trial co-developed in collaboration with Incyte Corporation Ongoing Phase 1 Trial
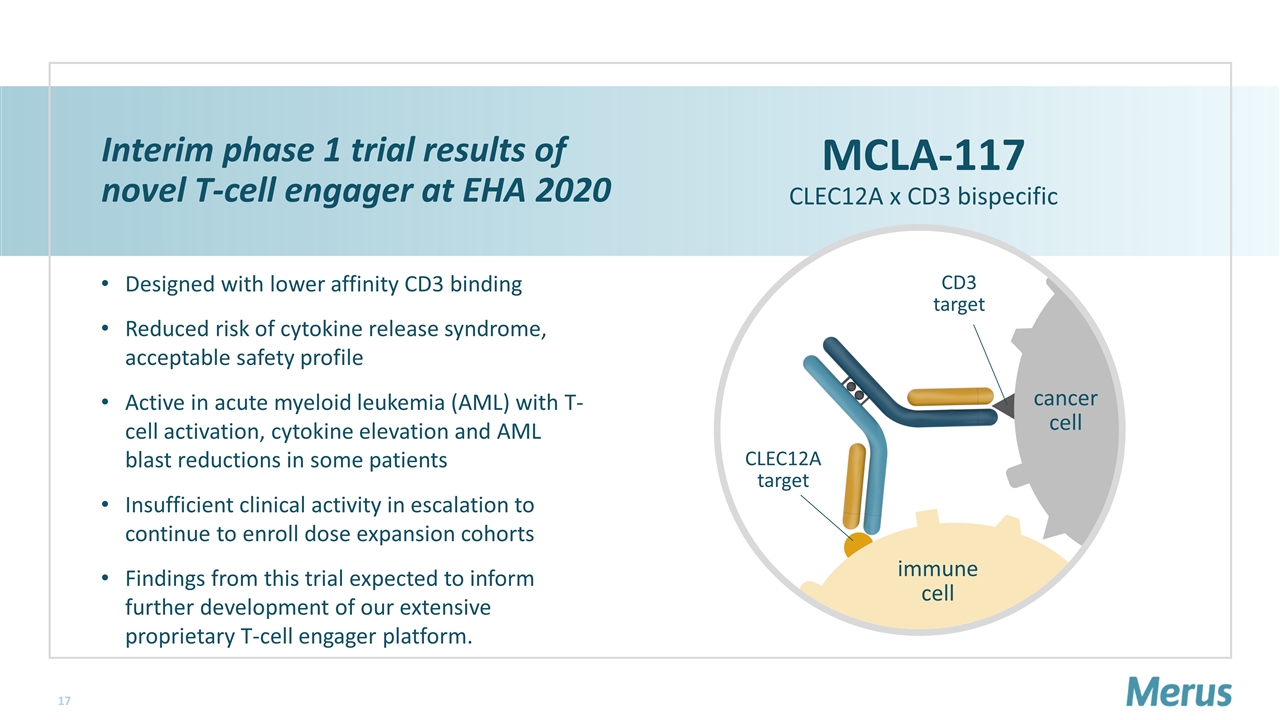
cancer cell immune cell CLEC12A target CD3 target Interim phase 1 trial results of novel T-cell engager at EHA 2020 Designed with lower affinity CD3 binding Reduced risk of cytokine release syndrome, acceptable safety profile Active in acute myeloid leukemia (AML) with T-cell activation, cytokine elevation and AML blast reductions in some patients Insufficient clinical activity in escalation to continue to enroll dose expansion cohorts Findings from this trial expected to inform further development of our extensive proprietary T-cell engager platform. MCLA-117 CLEC12A x CD3 bispecific
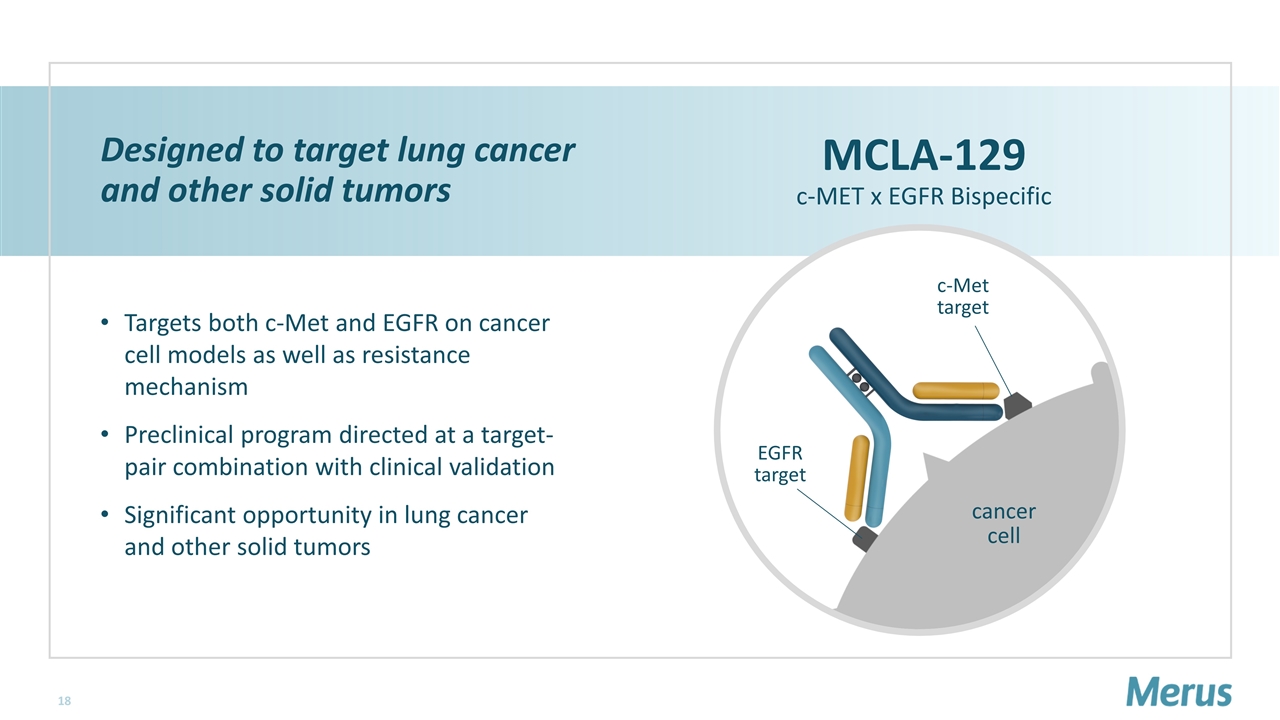
cancer cell Designed to target lung cancer and other solid tumors Targets both c-Met and EGFR on cancer cell models as well as resistance mechanism Preclinical program directed at a target-pair combination with clinical validation Significant opportunity in lung cancer and other solid tumors MCLA-129 c-MET x EGFR Bispecific c-Met target EGFR target
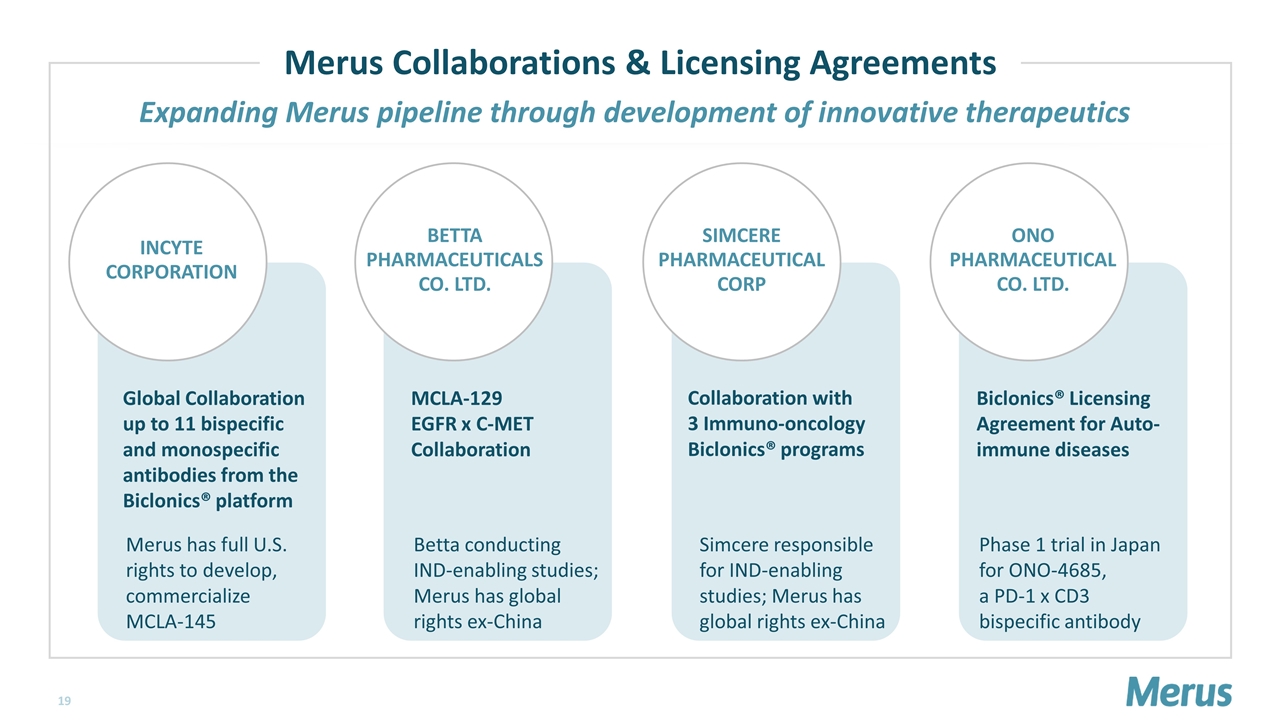
Global Collaboration up to 11 bispecific and monospecific antibodies from the Biclonics® platform MCLA-129 EGFR x C-MET Collaboration Collaboration with 3 Immuno-oncology Biclonics® programs Biclonics® Licensing Agreement for Auto-immune diseases Expanding Merus pipeline through development of innovative therapeutics Merus Collaborations & Licensing Agreements Merus has full U.S. rights to develop, commercialize MCLA-145 Betta conducting IND-enabling studies; Merus has global rights ex-China Simcere responsible for IND-enabling studies; Merus has global rights ex-China Phase 1 trial in Japan for ONO-4685, a PD-1 x CD3 bispecific antibody ONO PHARMACEUTICAL CO. LTD. BETTA PHARMACEUTICALS CO. LTD. SIMCERE PHARMACEUTICAL CORP INCYTE CORPORATION
Extensive landscape of cLC antibodies against targets, multi-specific formats, and ways of killing cancer cells Transgenic Merus Mouse (MeMo®) for human cLC antibody production Sophisticated, automated molecular biology and screening thousands of target combinations of choice Novel insights and unique understanding of multi-specific approaches to killing cancer cells Merus Multiclonics® Antibody Platforms
Established Clinical Pipeline and Preclinical Programs Clinical proof-of-concept with zenocutuzumab in NRG1+ cancers Multispecific Antibody Therapies for Oncology Bispecific and trispecific cancer therapies based on the human IgG format 2020-2021 Data Readouts and Program Updates Zeno NRG-1 phase 1/2 clinical data by end of 2020 Multiclonics® Platforms Common light chain format enables broad high throughput Biclonics® and Triclonics™ discovery and engineering Strategic Collaborations to Unlock Platform Value Multiple strategic collaborations and license agreements: Incyte, Betta, Simcere and Ono Merus Overview
