Attached files
| file | filename |
|---|---|
| EX-32 - EX 32.2 SEC 906 CERTIFICATION-CFO - PARALLAX HEALTH SCIENCES, INC. | ex322sect906certcfo123119.htm |
| EX-32 - EX 32.1 SEC 906 CERTIFICATION-CEO - PARALLAX HEALTH SCIENCES, INC. | ex321sect906certceo123119.htm |
| EX-31 - EX 31.2 SEC 302 CERTIFICATION-CFO - PARALLAX HEALTH SCIENCES, INC. | ex312sect302certcfo123119.htm |
| EX-31 - EX 31.1 SEC 302 CERTIFICATION-CEO - PARALLAX HEALTH SCIENCES, INC. | ex311sect302certceo123119.htm |
| EX-23 - EX 23.1 AUDITOR CONSENT - PARALLAX HEALTH SCIENCES, INC. | ex231auditorconsent.htm |
UNITED STATES
SECURITIES AD EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2019
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
Commission file number 000-52534

PARALLAX HEALTH SCIENCES, INC.
(Exact name of registrant as specified in its charter)
Nevada | 46-4733512 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
|
|
1327 Ocean Avenue, Suite B, Santa Monica, CA | 90401 |
(Address of principal executive offices) | (Zip Code) |
|
|
Registrant's telephone number, including area code: | (310) 899-4442 |
Copy of all Communications to:
Peter Hogan, Esq.
Buchalter
1000 Wilshire Blvd., Suite 1500
Los Angeles, CA 90017
(213) 891-0700
□ |
| Accelerated filer | □ | |
Non-accelerated filer | □ |
| Smaller reporting company | □ |
|
|
| Emerging growth company | ☒ |
The aggregate market value of Common Stock held by non-affiliates of the Registrant as of June 30, 2019, was $12,850,866, based on a closing price of $0.14 for the Common Stock on June 30, 2019, the last business day of the Registrant’s most recently completed second fiscal quarter. For purposes of this computation, all executive officers and directors have been deemed to be affiliates. Such determination should not be deemed to be an admission that such executive officers and directors are, in fact, affiliates of the Registrant.
Indicate the number of shares outstanding of each of the registrant’s
classes of Common Stock as of the latest practicable date.
270,723,289 common shares issued and outstanding as of May 15, 2020
Forward-Looking Statements
This Annual Report contains forward-looking statements. These statements relate to future events or the future financial performance of Parallax Health Sciences, Inc. (“Parallax” or the “Company”), and include statements made by the Company regarding insurance reimbursements, state licenses, product development and obtaining FDA clearances. In some cases, forward-looking statements can be identified by terminology such as “may”, “should”, “expects”, “plans”, “anticipates”, “believes”, “estimates”, “predicts”, “potential” or “continue” or negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors” that may cause the Company’s or its industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Except as required by applicable law, including the securities laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results.
Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results.
In this Annual Report, unless otherwise specified, all dollar amounts are expressed in United States Dollars and are prepared in accordance with United States Generally Accepted Accounting Principles. All references to “Common Stock” refer to the common shares; and “Preferred Stock” refer to the preferred shares; of the Company’s capital stock.
As used in this Annual Report, the terms “the Company”, “we”, “us”, “our”, and “Parallax” shall mean Parallax Health Sciences, Inc., and its wholly-owned subsidiaries, Parallax Diagnostics, Inc., Parallax Health Management, Inc., Parallax Behavioral Health, Inc., and Parallax Communications, Inc., unless otherwise indicated. The Company’s former wholly-owned subsidiary, RoxSan Pharmacy, Inc., was derecognized effective May 14, 2018. (See “RoxSan Pharmacy” and “Legal Proceedings” sections contained within this Annual Report.)
CORPORATE OVERVIEW |
Parallax Health Sciences, Inc. is a healthcare company focused on developing products and services that can provide remote communication, diagnosis, treatment, and monitoring of patients on a proprietary platform. Through its innovative technologies, both patented and patent-pending, the Company’s principal mission is to deliver solutions that empower patients, reduce costs, and improve the quality of care.
The Company’s principal executive office is located at 1327 Ocean Avenue, Suite B, Santa Monica, California, 90401, with an additional office located at 28 West 36th Street, 8th Floor, New York, NY 10018. The Company’s telephone number is (310) 899-4442.
The Company’s websites are at www.parallaxcare.com, www.parallaxhealthsciences.com, www.parallaxhealthmanagement.com, www.parallaxdiagnostics.com, and www.goodhealthoutcomes.com.
Parallax is a fully reporting company with its stock traded on the OTC Markets under the symbol “PRLX” (OTC.PRLX).
CORPORATE HISTORY
Formation and Development
The Company was incorporated in the State of Nevada on July 6, 2005. On November 1, 2012, the Company, formerly Endeavor Power Corporation, and its wholly-owned subsidiary, Endeavor Holdings, Inc., a Nevada corporation, entered into an Agreement and Plan of Merger with Parallax Diagnostics, Inc., a Nevada corporation, whereby Parallax Diagnostics became a wholly-owned subsidiary. On January 9, 2014, the Company changed its name to Parallax Health Sciences, Inc.
The Parallax business was founded on the Company’s Point-of-Care diagnostic business, Parallax Diagnostics, Inc., in 2010, when the Company acquired the right, title, and interest, through an exclusive license with Montecito BioSciences, Ltd. (“MBS”), to develop, manufacture and commercialize the Target System, an FDA-cleared 2 desktop point-of-care immunoassay diagnostic testing system. Concurrently, through an Assignment Agreement with MBS, the Company acquired the right, title, and interest to twenty-five (25) FDA-cleared 2 tests in the area of infectious disease, medical conditions, drugs of abuse, cardiac and pregnancy, that are designed to be utilized with the Target System.
2FDA 510(k) clearances do not expire. For additional information on FDA clearance and 510(k) requirements, see “FDA Clearances and Approvals” section.
- 1 -
On August 31, 2016, the Company entered into an agreement with Qolpom®, Inc., an Arizona corporation in the remote healthcare monitoring and telehealth business (“Qolpom®”) and its shareholders (the “Seller”) to purchase 100% of the issued and outstanding shares of Qolpom®’s common stock and its assets, inventory and intellectual property. The agreement was fully executed and the transaction was completed on September 20, 2016. The consideration for the acquisition resulted in a fair market value of $290,000, and goodwill of $785,060. In addition, the agreement included contingent royalties and revenue sharing for future revenues generated from the Qolpom® technology. The Qolpom® name was later changed to Parallax Health Management, Inc. (“PHM”).
On March 22, 2017, the Company formed a wholly-owned subsidiary, Parallax Behavioral Health, Inc. (“PBH”), a Delaware corporation, and on April 26, 2017, completed the asset acquisition of 100% of certain intellectual property (“Intellectual Property”) from ProEventa Inc., a Virginia Corporation (“ProEventa”), in accordance with the Intellectual Property Purchase Agreement between the Company, PBH and ProEventa (the “ProEventa Agreement”). ProEventa has an expertise in the development of behavioral health technologies, and is the wholly-owned subsidiary of Grafton Integrated Health Network, Inc., a non-profit Virginia corporation (“Grafton”). Pursuant to the ProEventa Agreement, the initial consideration for the Intellectual Property was paid to ProEventa in the form of a stock purchase agreement to purchase 2,500,000 shares of the Company’s Common Stock for $2,500, resulting in a net cost for the Intellectual Property of $622,500. In addition, the Agreement included conditional contingent royalties and revenue sharing for future revenues generated from the Intellectual Property.
On September 20, 2018, the Company formed Parallax Communications, Inc, a Delaware corporation and wholly-owned subsidiary of Parallax Health Management, Inc.
On August 28, 2019, the Company entered into a Purchase Agreement (the “Purchase Agreement”) with Global Career Networks Inc., a Delaware corporation, (“GCN”) to acquire a 19% interest in GCN. The Purchase Agreement was fully executed on September 6, 2019, with an effective date of October 15, 2019 (the “Effective Date”). Pursuant to the Purchase Agreement, in exchange for 6,666,667 shares of the Company’s restricted Common Stock, valued at $1,000,000, the Company acquired 760 shares of GCN common stock. In addition, in the event the market value of Parallax Common Stock one (1) year from Effective Date, is greater than $0.075 per share but less than $0.30 per share, the Company is required to issue GCN up to an additional 20,000,000 shares of restricted Common Stock, for an aggregate value of the Company’s Common Stock held by GCN of $2,000,000. At December 31, 2019, the Company recorded an impairment on the investment of $1,000,000.
In December 2019, an outbreak of the COVID-19 virus was reported in Wuhan, China. On March 11, 2020, the World Health Organization (“WHO”) declared the COVID-19 virus a global pandemic, and on March 13, 2020, President Donald J. Trump declared the virus a national emergency in the United States. As of the date of the filing of this Annual Report, the WHO reports over 4 million confirmed COVID-19 cases and over 275,000 deaths worldwide, including over 75,000 in the U.S. This highly contagious disease has spread to most of the countries in the world and throughout the United States, creating a serious impact on customers, workforces and suppliers, disrupting economies and financial markets, and potentially leading to a world-wide economic downturn. It has caused a disruption of the normal operations of many businesses, including the temporary closure or scale-back of business operations and/or the imposition of either quarantine or remote work or meeting requirements for employees, either by government order or on a voluntary basis.
The COVID-19 pandemic may adversely affect the Company’s customers’ operations, its employees and its employee productivity. It may also impact the ability of the Company’s subcontractors, partners, and suppliers to operate and fulfill their contractual obligations, and result in an increase in costs, delays or disruptions in performance. These effects, and the direct effect of the virus and the disruption on the Company’s employees and operations, may negatively impact both the Company’s ability to meet customer demand and its revenue and profit margins. The Company’s employees, in many cases, are working remotely and using various technologies to perform their functions. The Company might experience delays or changes in customer demand, particularly if customer funding priorities change. Further, in reaction to the spread of COVID-19 in the United States, many businesses have instituted social distancing policies, including the closure of offices and worksites and deferring planned business activity. Additionally, the disruption and volatility in the global and domestic capital markets may increase the cost of capital and limit the Company’s ability to access capital. Both the health and economic aspects of the COVID-19 virus are highly fluid and the future course of each is uncertain. For these reasons and other reasons that may come to light if the coronavirus pandemic and associated protective or preventative measures expand, the Company may experience a material adverse effect on its business operations, revenues and financial condition; however, its ultimate impact is highly uncertain and subject to change.
In response to the global need for diagnostics and personal safety during the pandemic, the Company, through its wholly-owned subsidiary, Parallax Diagnostics, Inc., has registered with the Food and Drug Administration (FDA), and has established strategic relationships with wholesale suppliers for global distribution of FDA-approved COVID-19 diagnostic test kits, and various Personal Protective Equipment (“PPE”) that meet current FDA guidelines,3 such as protective masks, sterile gowns, and eye goggles. All products distributed by the Company are inspected through an internal quality control process that ensures the products meet the FDA regulations and guidelines. The Company is also in the process of developing the SPARKS Mobile™ testing device along with a proprietary diagnostic test to be able to identify markers to the Coronavirus. Parallax is profoundly gratified by its ability to help those in need during this crisis.
3 Although FDA-approval is preferred, the FDA has issued various guidelines to follow during the COVID-19 pandemic, with consumer safety as the primary concern. This temporary guidance enables certain products, such as facial protective masks, to be distributed in order to meet the urgent demand, without the time intensive FDA-approval process.
- 2 -
On April 13, 2020, the Company received an Order of Suspension of Trading dated April 10, 2020 (the “Order”) from the United States Securities and Exchange Commission (“SEC”). The temporary suspension period was from 9:30 a.m. EDT on April 13, 2020, through 11:59 p.m. EDT on April 24, 2020. The Order referred to questions raised regarding the accuracy of the Company’s recent press releases in relation to the Company’s development of a rapid screening test for COVID-19, and the Company’s access to “large quantities of COVID-19 diagnostics testing kits and personal protective equipment.”
In an effort to protect the interests of shareholders, the SEC has issued similar orders and suspensions recently to several registrants, with concerns over the validity of claims made in connection with the availability of COVID-19 tests and supplies.
The Company, along with its counsel, is cooperating fully with the SEC to substantiate the Company’s recent public announcements and business endeavors, and is addressing any questions and/or concerns raised regarding the accuracy of the assertions made in the Company’s press releases.
Pursuant to Rule 15c2-11 under the Exchange Act, at the termination of the trading suspension, no quotation may be entered unless and until the Company has strictly complied with all provisions of the rule, including the filing of a new Form 15c2-11 with FINRA.
The Company is required to file its Annual Report with the SEC for the purposes of satisfying its financial reporting requirements. However, in addition to the Company's reporting obligations, the Company must have a FINRA Member Market Maker file a 15c2-11 with FINRA in order for the Company’s shares to resume trading on the OTCQB market. These actions do not impact or otherwise affect the Company's results of operations or disclosures as set out in this Annual Report. The Company believes that this Annual Report fully complies with the requirements of the Securities Exchange Act of 1934, as amended and, in accordance with generally accepted accounting principles, that it fairly presents, in all material respects, the financial condition and results of operations of the Company as at the relevant dates.
As of the date of filing of this Annual Report, the trading suspension period expired, and the Company is in the process compiling the information required for its 15c2-11 submission by a FINRA Member Market Maker. The Company anticipates the filing of a new Form 15c2-11 within the next 30 days. If any party has any questions as to whether the Company has complied with the rule, they should contact the staff in the Division of Trading and Markets, Office of Interpretation and Guidance, at (202) 551-5777.
These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for its common stock. Many brokers may be unwilling to engage in transactions in its common stock because of the added disclosure requirements, thereby making it more difficult for stockholders to dispose of their shares.
Changes in Management
On April 6, 2017, the Company’s board of directors (the “Board”) elected Mr. J. Michael Redmond as Chairman, to serve until the next annual meeting of the shareholders, in accordance with the Company’s bylaws, and/or until his successor is duly appointed, or a resignation is duly tendered.
Effective July 6, 2017, the Board caused the departure of Mr. Redmond from his position as President and Chief Executive Officer of the Company and RoxSan Pharmacy, Inc., and concurrently Mr. Redmond was removed from all board member seats.
Effective July 7, 2017, pursuant to a unanimous Board resolution, Mr. Paul R. Arena was appointed as the Company’s President and Chief Executive Officer, and the Board caused Mr. Arena's election to the Board and the Boards of the Company’s wholly-owned subsidiaries, Parallax Health Management, Inc. and Parallax Behavioral Health, Inc.
On July 26, 2017, Dr. Jorn Gorlach resigned as a member of the Board. This resignation did not involve any disagreement with the Company.
On June 4, 2018, Mr. Anand Kumar resigned as a member of the Board. This resignation did not involve any disagreement with the Company. Mr. Nathaniel T. Bradley, currently serving as Chief Technology Officer, succeeded him, and agreed to serve as a member of the Board until the next annual meeting of the shareholders and/or until his successor is duly appointed.
On May 15, 2019, Mr. David Appell joined the Company as its Chief Operating Officer. On February 2, 2020, Mr. Appell resigned his position. His resignation was not the result of a disagreement with the Company on any matters relating to the Company's operations, policies or practices. Concurrent with his resignation as COO, Mr. Appell will serve as Managing Director of the Company’s wholly-owned subsidiary, Parallax Communications, Inc. (“PCOM”). As Managing Director, Mr. Appell will provide business advisory and strategic planning advice to the Company with PCOM. In exchange for these services, Mr. Appell will be paid $2,000 per month plus medical benefits.
On March 1, 2020, Dr. David L. Stark was appointed as the Company’s President. In connection with the appointment, Mr. Paul R. Arena, who has held the position of President since July 2017, remains the Company’s Chief Executive Officer and Chairman of the Board of Directors, but resigned the position of President to afford Dr. Stark’s appointment.
- 3 -
In 2013, the Company identified an opportunity to acquire RoxSan Pharmacy, Inc. (“RoxSan”), a California corporation, and began the due diligence process. The Company’s initial interest centered on utilizing the acquisition as a means of accelerating the commercialization of the Company’s Target System and diagnostic platform, as RoxSan had access to a nationwide network of doctors and sales representatives. During the due diligence process, the Company became aware of numerous opportunities that RoxSan and its markets represented. On March 21, 2013, the Company entered into a Letter of Intent with Shahla Melamed, RoxSan's sole Shareholder, to acquire RoxSan. Between 2013 and 2015, four (4) amendments were also executed.
As part of the acquisition, the Company was required to obtain licensure from the State of California, and on July 31, 2015, the Company received notice that its pharmacy and sterile compounding licenses were issued by the California State Board of Pharmacy.
On August 13, 2015, (the “Closing Date”), pursuant to a resolution of the Board, the Company entered into an Agreement to Purchase and Sell One Hundred Percent of the Issued and Outstanding Shares of RoxSan Pharmacy, Inc. (“RoxSan” or the “Pharmacy”), and its Assets and Inventory (the “Purchase Agreement”). Pursuant to the Purchase Agreement between Parallax, RoxSan and its sole shareholder, Shahla Melamed (the “Seller” or “Melamed” or “Former Owner”), in exchange for 100% of RoxSan's common stock, and its assets and inventory, Parallax, among other things, issued the Seller a Secured Promissory Note (the “Note”) dated August 13, 2015, in the amount of $20.5 million (the “Acquisition”). The Note bore interest at a rate of 6% per annum, and matured August 13, 2018 (“Maturity”). As a result of the Acquisition, effective August 13, 2015, RoxSan became a wholly-owned subsidiary of Parallax. No change in control occurred as a result of the Acquisition.
In October 2015, shortly following the Company’s acquisition of RoxSan, Shahla Melamed (“Melamed”), initiated two (2) legal actions against the Company in the Superior Court of the State of California, County of Los Angeles, West District, Shahla Melamed v. Parallax Health Sciences, Inc., action numbers SC 124873 and SC 125702. In the matter, action No. SC 124873, Melamed sought rescission of the August 13, 2015, Purchase Agreement. In the Matter, action No. SC125702, Melamed alleges that the Company is in default under the terms of the Purchase Agreement and Secured Note, and the Company’s termination of Melamed’s employment agreement.
The Company also initiated legal action against Melamed and filed a complaint in October 2015, action number SC 124898, in the Superior Court of the State of California, County of Los Angeles, West District, Parallax Health Sciences, Inc., et al. v. Shahla Melamed, et al. The complaint in that action alleges that Melamed breached several obligations under the Purchase Agreement, and the Company sought to reduce the Secured Note due to undisclosed material changes in the business.
In January 2019, Melamed requested mediation, seeking settlement of the pending litigation with the Company, including that which was initiated against the Company by her son, Hootan Melamed (Shahla and Hootan, collectively, the “Melameds”). Through mediation, the Company and the Melameds reached agreeable settlement terms, and on February 19, 2020, the Company received a counter-signed Settlement and Release Agreement (the “Settlement”). Effective February 12, 2020 (the “Effective Date”), the Settlement is by and between Parallax Health Sciences, Inc., RoxSan Pharmacy, Inc., Michael Redmond, Edward Withrow III, Huntington Chase Financial Group, LLC, Calli Bucci and Dave Engert (collectively, “Parallax”), and Shahla Melamed and Hootan Melamed (collectively, the “Melameds”), and resolves all pending lawsuits between the parties in connection with the acquisition of RoxSan Pharmacy.
In consideration of the resolution of all existing and potential claims, including the cancellation of the Note in the principal sum of $20,500,000, and accrued interest of approximately $4,500,000, and without further action or litigation and without admission of liability by either party, the Settlement terms include the following:
●A payment of $4,000,000 (the “Settlement Sum”) to the Melameds, to be paid as follows:
$1,250,000 within 90 days of the Effective Date;
$1,250,000 within one (1) year of the Effective Date;
$1,500,000 within two (2) years of the Effective Date.
●The issuance of ten (10) million shares of the Company’s Common Stock to an entity owned by Shahla Melamed.
In addition, in the event forty percent (40%) or more of the Company and/or its subsidiaries (including by way of merger) is sold within two (2) years of the Effective Date, the Company shall pay the Melameds, within two (2) weeks of receipt of the proceeds from such sale (the “Sale Proceeds”), any outstanding unpaid Settlement Sum plus an additional 10% of the Sale Proceeds received, up to a total of an additional $3,000,000 over and above the Settlement Sum.
In the event the Company fails to cure a breach of timely payment of any portion of the Settlement Sum within thirty (30) days of a notice of default, a Stipulated Judgement may be filed by Melamed in the sum of $20,000,000, less any Settlement Sum amounts previously paid by the Company.
(See ITEM 3. LEGAL PROCEEDINGS section).
- 4 -
On May 14, 2018, pursuant to unanimous resolutions of the boards of directors of RoxSan Pharmacy, Inc. and Parallax Health Sciences, Inc., RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California (the “Court”). Mr. Timothy Yoo was appointed trustee (“Trustee”) on May 15, 2018. In connection with this filing, RoxSan sought to discharge approximately $5 million of liabilities owed to various parties, and intercompany loans in excess of $1 million owed to Parallax. The Chapter 7 bankruptcy proceeding by RoxSan Pharmacy, Inc. was fully discharged, and the case was closed on March 13, 2019, in U.S. Bankruptcy Court, Central District of California.
Due to, among other things, the reduction in RoxSan’s cash flows during 2016 and 2017, RoxSan became delinquent in its payroll tax depository obligations, resulting in a liability owed to federal and state taxing agencies in the aggregate of $1,148,811, which includes $601,148 in taxes withheld from employees (“Trust Fund Taxes”), employer taxes of $183,172, and penalties and interest of $364,491 through December 31, 2018. The liability was included as part of the Chapter 7 bankruptcy petition, and certain portions of the liability may be discharged. However, in accordance with California bankruptcy laws, federal and state Trust Fund Taxes are not dischargeable. The Company has retained a tax resolution specialist and is in communications with the taxing agencies in order to resolve RoxSan’s liability. During the year ended December 31, 2019, payments for Trust Fund Taxes in the amount of $485,498 were made, and $115,650 in Trust Fund Taxes were outstanding at December 31, 2019.
As a result of the loss of financial control of RoxSan, the Company derecognized the subsidiary as of September 30, 2018. The derecognition resulted in a gain of $4,478,268. The Company also extinguished $22,778,281 in debt and accrued interest related to the acquisition of RoxSan.
DESCRIPTION OF BUSINESS |
Overview
The Company’s principal focus is on personalized patient care through remote healthcare services, behavioral health systems, and Point-of-Care diagnostic testing. Parallax’s current family of companies that serve as the foundation for its cross-over business model of operations include:
●Parallax Diagnostics, Inc. (“Parallax Diagnostics” or “PDI”) acquired a proprietary Point-of-Care diagnostic immunoassay testing platform and 25 test cartridges for the areas of infectious diseases, cardiac markers, drugs of abuse and various other medical conditions. PDI has recently become an FDA registered distribution partner for various lateral flow-through COVID-19 IgM/IgG antibody instant tests products. It has also registered and distributed approved Personal Protective Equipment (PPE) through an ecommerce website at GoodHealthOutcomes.com.
●Parallax Health Management, Inc. (“PHM”) develops remote patient monitoring (“RPM”) and telehealth market products and services, and commercializes them, including the Good Health Outcomes™ software platform with Fotodigm® proprietary data capture which allows for systems integration with a number of third-party services and solutions.
●Parallax Behavioral Health, Inc. (“PBH”) acquired the intellectual property known as REBOOT™, the acronym for Reliable Evidence-Based Outcomes Optimization Technologies, as well as the Intrinsic Code™ technology, a software platform specifically designed to improve health treatment outcomes through cloud-based and mobile behavioral technology systems that enable its users and user groups to more effectively achieve goals within a prescribed timeline.
The Company envisions a world where healthcare is accessible, reliable and affordable, without compromising quality and economics of the healthcare industry. Driven by a sincere desire to make people’s lives better and push back on the healthcare industry’s crippling economic outlook, the Good Health Outcomes™ platform was created; the Company’s design is for “outcomes realized through intelligent health.”
The Good Health Outcomes™ system facilitates cost-effective remote diagnosis, treatment and monitoring of patients with chronic diseases. Parallax’s integrated products and services provide Point-of-Care (“POC”) patient testing and monitoring, along with information communicated via cloud-based mobile smartphone applications that are agnostic as to operating systems and utilize sophisticated data analytics. Information is retrieved in real-time by physicians and other healthcare professionals and is integrated into electronic health records. The Company’s digital products and services capitalize on the transformation of healthcare to provide improved compliance, diagnosis, monitoring and support to patients, along with cost savings and efficiencies to healthcare systems.
Good Health Outcomes™ encompasses three separate divisions that can operate independently of one another, or integrate services to meet the various needs of the Company’s clientele: Optimized Outcomes, Connected Health and Smart Data.
Optimized Outcomes | REBOOT™ / COMPASS™ Behavioral modification |
Connected Health | Fotodigm® platform Remote patient monitoring, telehealth, Personal Protective Equipment (PPE) and POC diagnostic testing |
Intrinsic Code™ technology Actionable insights to behavior modification |
- 5 -
Each of the Company’s divisions target a separate vertical market that complement each other and the Company value proposition. In addition, the synergistic operational cross-over affords the Company the ability to use built-in economies of scale across multiple operating platforms.
The Company believes that the solutions lay in the empowerment of the patient, the payer and the provider; creating a model of healthcare that aligns all three interests and creates a singular goal of better health outcomes, at reduced cost. The Good Health Outcomes™ platform involves these areas of focus:
●Behavioral Modification
The Company believes in working to empower the patient to modify their behavior through personal empowerment. The Company has developed and designed a revolutionary technology that will aid in the challenges of behavior modification, and improve adherence to medical regimens, which can lead to lower costs and better health.
REBOOT™, the Company’s patented cloud-based behavioral software technology, along with COMPASS™, the Company’s mobile application that incorporates REBOOT™ with unique mobile phone features, and WIZARD™, which supports scalability of the REBOOT™/COMPASS™ platform, was developed by behavioral specialists at Grafton Integrated Health Network, a multistate behavioral healthcare organization with over 60 years of clinical experience and data in behavioral health.
●Connected Health
Continually increasing healthcare costs, difficulty accessing care, and a greater need for convenience, are driving consumers to demand more value out of their healthcare dollar and seek care that meets their needs and preferences. The market is responding to this growing demand, and non-traditional care models are rapidly expanding, such as:
●Traditional providers (e.g., office-based primary care physicians (PCPs) and specialists, hospitals) are partnering with non-traditional care providers to expand reach.
●New parties, such as consumer product and technology companies, are entering this billion-dollar market.
●Non-traditional care models have the opportunity to complement traditional care models to help improve access and affordability, and to deliver a more personalized healthcare experience.
To meet these changing demands, the Company has developed Good Health Outcomes™, the Company’s proprietary connected health platform that provides remote patient monitoring, telehealth, POC diagnostics and health education products and services on a single platform. Currently in its beta stage, Fotodigm®, the integrated data capture utility, has the capability for systems integration of an unlimited number of third-party biometric measurement products, electronic health and medical record software (EHR, EMR) services and solutions. The Company is continuing the Fotodigm® beta stage to test economic models and delivery modalities in preparation for large-scale deployment of the Fotodigm® platform and the filing of 510k FDA approval of the Fotodigm® system. The Good Health Outcomes™ platform is based on the following:
●Telehealth/Remote Patient Monitoring
Improves digital connectivity among consumers, providers, health plans, and life sciences companies.
Facilitates self-managed care, with the help of technology-enabled solutions.
Provides a secure environment that protects consumer privacy.
Delivers care outside the traditional clinical setting, potentially providing better access to care at a lower cost.
Assists chronic disease management and improves population health outcomes.
Empowers patients by providing a cost-effective tool that connects them with their doctors.
Empowers doctors with improved patient scheduling flexibility and timely communications.
Provides hospitals with a tool to address the problem and economic hardship caused by readmissions.
Provides a virtual management tool for chronic disease management.
●Target System
Allows doctors to test patients in their office.
Requires only a one-time learning process to perform all tests.
Delivers test results in 12 minutes or less.
Provides patients with important information at the time of testing.
Costs less than outside lab-based tests, allowing for a reduction in payer costs and patient co-pays.
Allows doctors to earn additional revenue that they cannot participate in with outside labs and testing not performed in their offices.
Patients can test from their homes and transmit data to doctors.
The Parallax Business Model
In the past 60 years, healthcare has transitioned from a direct relationship between doctor and patient, to one that has patients separated from their doctors by the introduction of a huge number of stakeholders, ranging from health insurers, employers, pharmacy benefit managers, imaging, diagnostic testing, lawyers, specialists and a plethora of others. The patient and healthcare provider both want the same thing: information, quality of service, transparency, value for their hard-earned dollars, and more time in their day.
- 6 -
The Company has developed, acquired and licensed multiple proprietary and exclusive platforms that provide services and products, across the healthcare continuum. These platforms are designed to allow for multiple points of reciprocal consideration, through innovative business models, that provide patients with increased quality of services and products, at reduced cost of time and money. They also provide healthcare providers with increased access to their patients, the ability to deliver better and more efficient service and increase their income from the services they supply. The Company believes the Good Health Outcomes™ system will deliver solutions to problems of health and economics, while providing essential actionable data to pharmaceutical firms, payers and healthcare providers, through our:
●Patented behavioral machine learning technology;
●Patented POC diagnostic testing technology;
●Patent-pending interoperable connected health platform targeting two of healthcare’s biggest problems that, combined, address markets that represent over $1 trillion in costs 4:
Medical nonadherence
Chronic disease management; and
●Smart Data delivered through enhanced patient/disease stratification, in combination with dynamic behavioral data, relating to adherence to pharmacologic and medical therapy regimens designed to:
help patient outcomes
reduce the cost of care associated with nonadherence
deliver actionable data.
Cognitive AI
The Company is working with partners that have developed a cognitive artificial intelligence, (“Cognitive AI”) agent architecture with the interaction of emotion, motivation and cognition of situated agents, mainly based on the Psi theory of Dietrich Dörner. The Psi theory addresses emotion, perception, representation and bounded rationality, but being formulated within psychology, has had relatively little impact on the discussion of agents within computer science. Cognitive AI is a formulation of the original theory in a more abstract and formal way, at the same time enhancing it with additional concepts for memory, building of ontological categories and attention.
Big Data Opportunity
The Company’s real-time data generated from patient-users can be stripped to protect the specific patient-user identity and exchanged for historical data with Center for Disease Control, (“CDC”), National Institute of Health, (“NIH”), various universities and others to provide valuable empirical health related information to the Company’s patient-users using Cognitive AI provided by the Company’s partners and coordinated through various electronic health record organizations for which the Company is agnostic. This empirical data when it becomes available on the Company’s outcomes optimization-based platform will become a valuable tool for determining predictive and supportive diagnostics to its patient-users.
The Company’s endeavors to change the healthcare industry are strengthened by providing solutions to real problems facing healthcare stakeholders today. The Company’s products and services have been developed, and are continuing to be developed, to address these issues now. The Company’s models include revenue from, and are compatible with, both the traditional reimbursement through payers, and the new performance-based compensation and financial incentive for the adoption of healthy, preventative behavior.
The Cross-Over and Cross-Pollination Model
The Company’s business model is built on identifying opportunities represented by one market vertical that provides for a separate vertical to utilize one or more of the Company’s core operations. Although the multiple operations of Good Health Outcomes™ are focused in separate vertical markets, the Company has designed its business model to allow for cross-pollination and reciprocal transfer of value at multiple points in their respective economic food chains.
As an example of how each of the Company’s divisions can support each other utilizing the cross-over and cross-pollination model:
●The Optimized Outcomes division can provide a range of after-care products and services through the Connected Health division.
●The Connected Health division can offer telehealth and remote patient monitoring, and POC testing and diagnostics services directly to the doctors and patients of the Optimized Outcomes division. Further, remote patient monitoring customers can be offered POC testing and diagnostics, and vice versa.
●The Smart Data division can offer the Company’s software and Intrinsic Code™ technology systems to the Optimized Outcomes and/or Connected Health clientele.
4 https://www.milkeninstitute.org/publications/view/910
- 7 -
The term cross-over and cross-pollination is best demonstrated by the manner in which Parallax customers are exposed to products and services they might benefit from other than what they are seeking, and these additional products and services could augment the core product or service they receive from Parallax. The cross-over component comes from the customer, their unique situation and perspective (including socio-economic, demographic and stratified health profile), and their participation in innovation through their individual goals as it relates to their health regimen; the cross-pollination comes through the value represented by the exposure to Parallax’s product and technology offerings. By way of the cross-over and cross-pollination model, the customer is empowered and increases participation in their own health, which is one of Parallax’s core strategies.
An example of the cross-over and cross-pollination model is further illustrated as follows:
●A customer has identified Good Health Outcomes™ as a solution for Remote Monitoring of their health or medical condition (i.e. a chronic disease);
●The customer will be prescribed a medical regimen that includes prescription medication and biometric vitals (i.e. blood pressure, weight, glucose, et al);
●The opportunity is created for Parallax to introduce its Medication Adherence product to the customer.
The customer type will vary, depending upon the sales and marketing approach and target audience, but is primarily comprised of:
●Patients
●Providers: Doctors, Nurses, Clinicians and Caregivers
●Payers: Insurance Companies, Corporations, Government
The Company believes that the current healthcare system is built on unsustainable models and significant challenges for all the stakeholders in the healthcare system. The Company also believes that it can deliver solutions which fill a void in the current market for high quality, high efficacy products and services delivered at reasonable and rational prices. The Company’s business strategy is to expand through organic growth, selective synergistic acquisition, and develop, license and/or acquire, quality products and or services that complement the Good Health Outcomes™ systems.
Products and Services
Parallax believes that its products and services can provide solutions that mitigate rising costs, reduce waste in spending through transparency, reduce the amount of unnecessary services, and increase the health and wellness of patients before they are sick.
Remote healthcare products include patented and patent pending software and mobile apps (to be available for iPhone on Apple App Play Store and Android on Google Play) and other services, as well as electronic kits and devices from third-party licensed platforms that are designated towards a patient’s primary health concern (i.e. diabetes, blood pressure, cardiovascular, general monitoring, etc.), and offer both audio and video options that interface with the patient’s healthcare providers. Prescription medication dosage monitoring is also available.
Behavioral health products include the proprietary behavioral health technology, REBOOT™, which powers decision support that can also be delivered securely to any internet connected device. The software can be used by an individual or an organization of any size, with the potential to transform the cost of treating and managing chronic illnesses such as pulmonary-COPD-asthma, diabetes, and cardiovascular disease by effecting the modification of behavior in patients being treated for these chronic diseases.
Diagnostics products include the Target System, the Company’s proprietary POC diagnostic immunoassay testing platform and test cartridges for the areas of infectious diseases, cardiac markers, drugs of abuse and various other medical conditions, and the patented SPARKS Mobile™, the next-generation handheld mobile analyzer currently under development. The Target System will allow doctors to test patients in their office, with test results delivered in 10 minutes or less. This allows patients to be provided with important information at the time of testing. The costs of the Target System are less than outside lab-based tests, benefiting both payers and patients. In addition, doctors will be able to generate additional revenue that would normally be paid to an outside laboratory, and patients can even perform test from their homes, with results transmitted directly to their doctor. The Company has also recently selected antigens and antibodies to be used as the markers for its rapid Coronavirus (“COVID-19”) screen test, currently in the pre-clinical stage of development, for use with its FDA-cleared Target System platform.
Through the development and design of the Good Health Outcomes™ platform, the Company’s telehealth, RPM and POC products and services are interoperable and interchangeable with any FDA cleared/approved medical device, providing ease of use and connectivity between patient and doctor. The advancement in the Company’s technology is strengthened by the ability to scale its products to meet the demands of both individuals and large groups alike.
- 8 -
Global COVID-19 Pandemic and Pandemic Viruses and Diseases of the Future
Data driven and behavioral technologies are well positioned to counter and deal with the current medical crisis within the U.S. and abroad.
The COVID-19 global pandemic is the cause of:
●Estimated $10s of trillions in economic losses on a global basis from economic closures and compound effects of COVID-19;
●Over 1.4 million reported infected in the U.S. with over 83,000 deaths, and growing.
Medication Nonadherence
Medication nonadherence is a priority public health consideration, affecting health outcomes and overall healthcare costs on a worldwide basis. Increasing adherence to medical regimens leads to better health outcomes in chronic disease and reduces the overall costs to the patient, payer and all of the stakeholders across the healthcare continuum.
Nonadherence to medication regimen in chronic disease management is the cause of 5:
●$300 billion in avoidable costs to the U.S. healthcare system annually;
●125,000 premature deaths in the U.S. annually.
Research from the World Health Organization 6 has shown that better adherence to antihypertensive treatment could prevent 89,000 deaths each year in the U.S., with a projected savings of $106 billion a year.
Chronic Disease Management
Chronic diseases are on the rise in the U.S., leaving healthcare payers with the challenge of covering care for patients with these expensive, long-term conditions. Healthcare spending reached a total of $3.2 trillion in 2015, based upon estimates from the Center for Medicare Services (“CMS”). Spending is expected to grow at an average of 5.5% through 2025, with chronic disease treatment comprising a major portion of that spending.
Based on the latest data from the Center for Disease Control (“CDC”), the top 8 most expensive chronic diseases for healthcare payers to treat are:
●Cardiovascular Disease
Cardiovascular disease (“CVD”) in the U.S. total $317 billion per year, split between $193.7 billion in direct medical costs and $123.5 billion in lost productivity. An adult in the U.S dies from CVD related health conditions every 40 seconds, with CVD deaths accounting for 31% of all U.S. deaths each year.
●Smoking-Related Health Issues
The estimated costs for smoking-related health issues in the U.S. total over $300 billion per year, split between direct healthcare expenses of $170 billion and indirect costs of roughly $156 billion.
●Alcohol-Related Health Issues
In 2010, excessive alcohol use cost the U.S. economy $249 billion, or roughly $2.05 per drink. Alcohol-related deaths totaled 88,000 people per year, and shortened the lives of working adults by an average of 30 years.
●Diabetes
As one of the most prevalent chronic conditions in healthcare, diabetes care costs reached $245 billion in 2016. Seventy-one percent of diabetes treatment costs ($176 billion) were related to direct healthcare expenses. That equates to 20 percent of U.S. healthcare spending.
●Cancer
According to the latest estimates from the CDC and the National Cancer Institute, cancer care costs are roughly $171 billion a year due to healthcare inflation over previous decades.
●Obesity
The United States spends $147 billion on healthcare related to obesity, and roughly $117 billion on costs related to inadequate physical activity. In 2006, healthcare costs for obese patients were $1,429 higher than patients at a normal weight. Obesity is implicated in the development or worsening of many other chronic conditions, including diabetes and cardiovascular disease.
The total cost of arthritis in the U.S. was an estimated $128 billion, split between $81 billion in direct medical expenses and $47 billion in related losses of productivity and care management. Arthritis affects 23 percent of adults in the U.S., or 54 million people, and is expected to rise to 78 million cases by 2040. Arthritis also occurs with other chronic conditions, as many patients are unsure on how to manage their own symptoms.
●Strokes
On its own, strokes in the U.S. create medical expenses of $33 billion annually and accounts for 1 out of 20 deaths in the country, or an estimated 130,000 deaths per year.
These nonadherence and chronic disease numbers are daunting in the best of conditions, but the reality is that the sheer volume of U.S. citizens reaching the age of 60 will impact the cost trajectory. The projected financial impact is not sustainable under the current healthcare system.
5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3234383/#B8
6 https://www.who.int/chp/knowledge/publications/adherence_report/en/
- 9 -
Parallax is led by experienced veterans with backgrounds from the healthcare, medical devices, drug development, technology, FDA regulatory, medical insurance billing and patient management, finance and management of early stage and high growth companies. The Company’s disciplined and organized approach is balanced by its optimism for the future, and the opportunities present in the current healthcare market. The Parallax team is grounded in a belief that success in business is built on a combination of research, planning and execution.
At Parallax, management continually strives to identify solutions to the challenges facing the current healthcare system. The Company and its management team of professionals are committed to delivering the highest quality products and services to patients, payers, healthcare insurers and stakeholders that are accessible and reasonable, and are built upon sound business models and economics that are designed to provide for sustainable growth and increased value to the Company’s shareholders.
Overview
Parallax continues its focus on Point-of-Care diagnostics, with an emphasis on its Target System testing platform, including the Target Antigen Detection System, the flow-through test cartridge, and the FDA-cleared VT-1000 desktop analyzer and novel applications that detect and/or monitor infectious diseases, cardiac markers and drug of abuse assays. The Company holds exclusive licenses, in perpetuity, to a line of proprietary, patented and/or patent-pending, FDA-cleared, Point-of-Care diagnostic tests to be utilized with its single platform diagnostic testing Target System. Parallax, with its products and products in development, offers the potential to transform the diagnostic landscape by transitioning critical tests from the centralized lab directly to the hands of the physician or clinicians.
The Company continues to pursue viable opportunities for the commercialization of its product, including strategic partnerships with third-party companies in order to limit the Company’s capital outlay. Additionally, the Company has sought to identify strategies that would make its proposition more valuable and competitive. The Company has spent the last few years expanding its patent portfolio and further developing potential integration of new applications. The Company, through its license with Montecito BioSciences, Ltd. (“MBS”), has been issued patents on the core technology for its Target System. In addition, in 2014, 2015 and 2017, the Company, through its license with MBS, received patents on its mobile testing device, the SPARKS Mobile™ diagnostic reader, in the United States, China, Hong Kong, Macao, and India. In 2019, the Company engaged a patent attorney to identify and pursue infringements of its licensed patents around the foundational technology and the SPARKS Mobile™ diagnostic reader globally, some of which have already been identified.
The Company is also pursuing the expansion of the Target System’s test cartridges for the diagnoses of additional diseases. The Company has identified a technology previously cleared by the FDA that can be utilized as a platform for a test cartridge that will detect CD4 and CD8 cells, which in turn determine a patient’s immune status. In addition, the Company is in the process of developing a test cartridge for the diagnosis of the COVID-19 virus.
Target System Product Strategy
In recent years, there has been a continuing shift from the use of laboratory-based analyzers to point-of-care (“POC”) tests that can be performed in a matter of minutes. Unlike the centralized clinical laboratory segment of the diagnostic market, which is mature and highly competitive, the POC market is still in its relatively early stages. According to the recent worldwide research reports, however, such as the 2010 Worldwide IVD Market, by the research firm Kalorama Information, the growth rate of the POC market continues to rise. Although certain simple, single analyte diagnostic tests have been developed, such tests have remained incapable of precise and highly sensitive quantitative measurements. As a result, medical tests that require precise quantitation of the target analyte have remained the domain of immunoassay analyzers in the centralized laboratory.
Point-of-Care diagnostic kits typically consist of test strips that the healthcare provider applies a patient’s sample to and then reads the strip either visually or with an instrument in order to determine a result. They are simple to use, fast, disposable and reliable within an acceptable range. More sensitive analytes or tests requiring quantitative analysis and definitive antibody screening needed in most situations, must be sent out to a diagnostic lab, and hours or days later results arrive. These tests are comparatively complex, expensive, and time consuming; only centralized diagnostic facilities can manage sample handling and the cost of instruments and reagents. A POC instrument that has the advantage of a test strip device in terms of ease of use and rapid results along with enzyme-linked immunosorbent assay (ELISA)-like capabilities for major diseases would circumscribe diagnosis routinely within the course of a patient visit. This could disrupt the current model. The Company is planning to develop just such a device that it intends to sell to doctors and healthcare providers.
The commercial success of the current generation of small, simple to use diagnostic devices which provide rapid results in POC applications has been limited by their inability to provide precise, highly sensitive, quantitative measurement. Despite these limitations, the rapid increase in discovery of individual markers of disease processes, coupled with the advancements in rapid detection technologies, has made these tools available to medical professionals on a wide scale and POC diagnostics are quickly becoming a high growth industry.
- 10 -
The Target System (the VT-1000 Desktop Analyzer, the Target Antigen Detection Cartridge and associated reagents) technology addresses these limitations by applying sophisticated immunochemical and optical methods to detect and quantify analytes present in various human specimens, including blood, urine, and feces. Data indicates that sensitivity will be comparable to expensive and complicated laboratory-based analyzers. The Company believes that there is market potential for advanced POC diagnostic products that provide quick and accurate diagnosis during a patient visit, shortening the decision time to medical intervention and minimizing the need for additional patient follow-up, thereby reducing overall healthcare delivery costs.
The Company also believes that there is growth opportunity for the exploitation of its Target System platform in developing nations and regions such as Africa, India, South America, Eastern Europe, Russia and Asia as well as developed markets of North America and Western Europe. One of the first initiatives to be developed for this market will combine the Company’s SPARKS Mobile™ (a portable hand-held diagnostic analyzer based on the VT-1000 Desktop Analyzer), currently in development, with a test for the COVID-19 virus, as well as a test for the monitoring of AIDS/TB patients through the use of a proprietary rapid point-of-care immunoassay CD4-CD8 test called PROMISE CD4, also in development.
The Diagnostics Products
The Company’s assets include a FDA-cleared VT-1000 Desktop Analyzer and more than two dozen FDA 510(k) cleared diagnostic tests. The Desktop Analyzer and immunoassay system incorporates a flow-through rapid antigen test platform configuration that has the ability to produce high-performance quantitative blood test results with the ease of rapid qualitative diagnostic strips. The Company has patents and patent applications related to its current and future products, as well as methods for future test development. The Target VT-1000 Desktop Analyzer is ideally suited for rapid development and commercialization of all new tests that may be introduced, as well as integrating remote patient monitoring and telehealth products and services into the Target System through the SPARKS Mobile™ platform.
VT-1000 Desktop Analyzer: Quantitative and Qualitative Immunoassay
The Company’sVT-1000 Desktop Analyzer is FDA-cleared and is capable of rapidly detecting qualitative and quantitative data for its FDA-cleared Target Platform tests. The VT-1000 Desktop Analyzer is used for all Target System platform Tests, allowing for clinical personnel to be trained once and also gives consistent results for both qualitative and quantitative testing. The Company plans to develop the SPARKS Mobile™, a hand-held analyzer unit, similar in size to a mobile phone, which will be based on the VT-1000 Desktop Analyzer (see “SPARKS Mobile™: The Target System Hand-Held Analyzer).
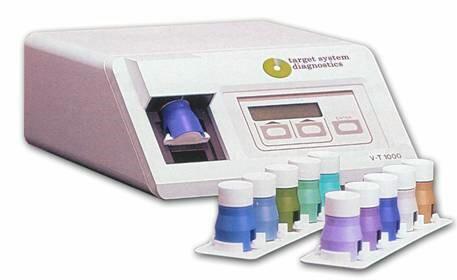
VT-1000 Desktop Analyzer
Target Antigen Detection System (“TADS”)
The Target Antigen Detection System consists of a unique single-use cartridge with reagents capable of testing multiple test markers for qualitative testing and, when used with the VT-1000 Desktop Analyzer, provides quantitative results. The TADS requires a small amount of sample and provides results in minutes. The simplicity of the fully loaded single-use test cartridge and subsequent ease-of-use of the instrument helps to alleviate the technical burden on medical staff and makes patient diagnosis more efficient.
- 11 -
Each individual TADS test cartridge operates in a uniform fashion using a controlled flow-through rapid antigen testing system, utilizing an enzyme-linked immunosorbent assay (“ELISA”). The ELISA method is a technique used to determine if a certain substance is present within a sample. Using special antibodies that attach to the substance, the sample will generate a specific color. The amount of color indicates the amount of substance present. Another set of antibodies are used to “capture” the substance.7 The results can then be measured at specific wavelengths by an ELISA analyzer, such as the VT-1000, in two forms:
●Qualitative: Refers to whether an analyte is present and provides “positive” or “negative” results through color changes, using known positive and negative samples.
●Quantitative: Refers to how much is present and uses a series of standards to measure the unknown amount of analyte.
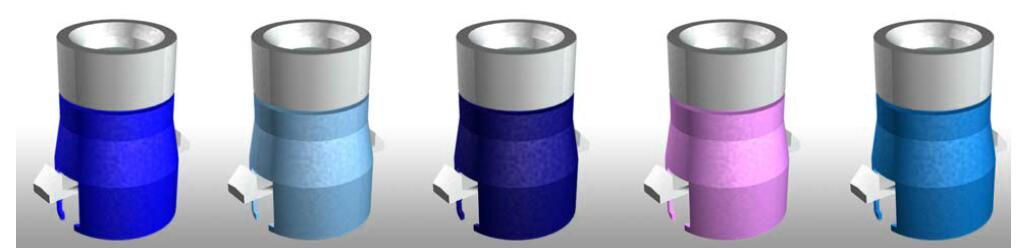
TADS Cartridges
The simplicity of the fully loaded single-use test cartridge, and subsequent ease-of-use of the instrument, helps to alleviate the technical burden on medical staff, and makes patient diagnosis more efficient.
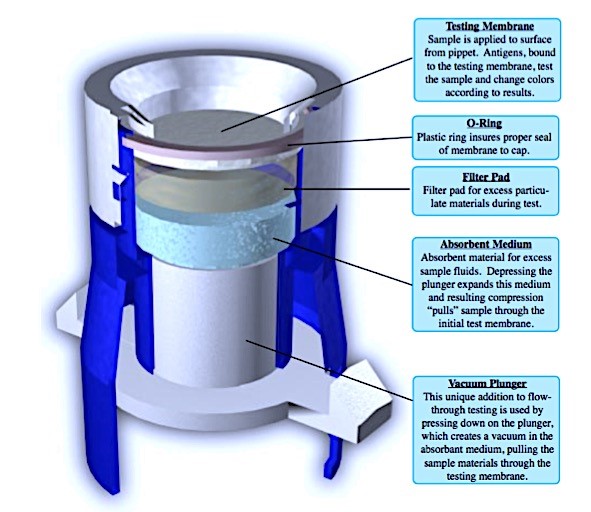
The Company’s Target Antigen Detection System is a departure from the standard devices typical to the rapid testing markets and can allow for physicians to share in revenues. The device is part of the manufacturer’s qualitative and quantitative “Target System Diagnostics Platform,” which offers an array of improved modifications and features to the traditional qualitative and semi-quantitative flow-through immunoassay test. With its platform uniformity, vacuum pump, absorption layer for sample overflow, and complete compatibility with single and multi-light source reflectometer technology, the TADS cartridge is a unique collection of tests for qualitative and quantitative detection diseases and of conditions. The TADS cartridge utilizes a vacuum technology to deposit specimen samples uniformly on test membranes. The Vacuum Control Flow Device provides a vacuum pump action, which reduces test time and ensures maximum contact with the membrane antibodies. This collection device allows for numerous tests to be incorporated. The vacuum specimen filtration and excess specimen absorption is built in.
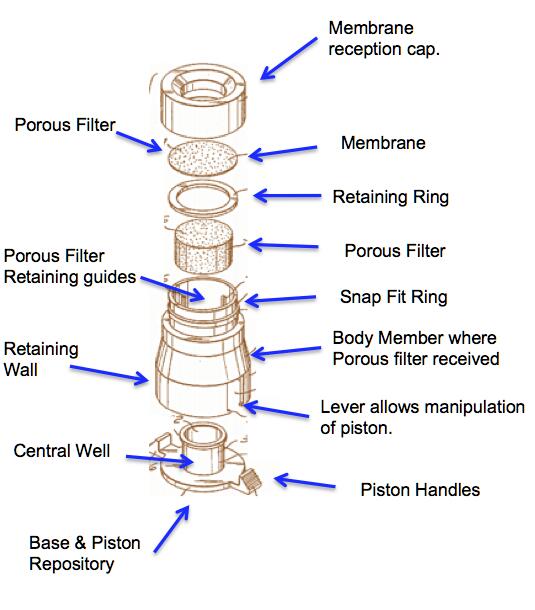
TADS Components
7 “ELISA,” Simple English Wikipedia, [website], March 2013, https://simple.wikipedia.org/wiki/ELISA
- 12 -
TADS 2.0 Flow Through Testing System with Pressure Indicator
On March 3, 2017, the Company, through its licensor, Montecito BioSciences, Inc., was granted patent 9,588,114 (See “INTELLECTUAL PROPERTY SUMMARY”) for the new and improved TADS testing cartridge, which provides an assay device that has an externally manipulatable piston for creating a region of reduced air pressure beneath a membrane containing an analytic compound, preferably a receptor or antibody. The region of reduced pressure causes a fluid sample to be tested to be rapidly drawn through the membrane. To ensure that a sufficient reduction in pressure is achieved, the membrane further includes a pressure sensing means, so the entire sample contacts the analytic compound. The Company believes that obtaining the greatest level of contact where the antibody and antigen meet, is essential when striving for the best possible test results to be consistently achieved.
How the Target Antigen Detection System (“TADS”) Works
The TADS testing system performs immunoassays on analytes for determining the presence and/or amount of an analyte in a sample, and includes:
An immunosorbent membrane;
An absorbent material;
A piston component located below the absorbent material to draw analytes in a sample through the immunosorbent membrane into the absorbent material; and
Discrete groups of pressure-sensitive microcapsules located on the immunosorbent membrane.
Each group of microcapsules has a different predetermined average burst strength; and
Each group of microcapsules includes a dye that is different from the dye of any other of said groups.
The TADS testing system has an immunosorbent membrane with one or more binding agents non-diffusively bound to its upper surface. The immunosorbent membrane refers to a porous support membrane having at least one antibody (polyclonal or monoclonal antibody), antibody fragment or derivative thereof, aptamer, or other non-protein based entity (e.g., a carbohydrate or lipid), which specifically binds to a cognate epitope. In particular, the porous material is a thin disk of material such as nitrocellulose, nylon (e.g., cast from nylon 6,6 polymer) or polyvinylidene difluoride (PDVF).
A sample or analyte solution refers to any sample suspected of containing a particular analyte. It is recognized that a sample may contain no analyte, or, in other words, the test for that ligand (a molecule that binds to another molecule) is negative.
The sample can be of biological or environmental origin.
Examples of biological samples include whole blood, serum, plasma, amniocentesis fluid, pleural fluid, peritoneal fluid, sputum, urine, feces, cerebrospinal fluid, exudates, extracts of skin or tissue specimens, swabs from the throat or wounds and the like.
Examples of environmental samples include water specimens (e.g., drinking water or streams), extracts of soil samples, and swabs of shipping packages, food samples, and the like. In this respect, an “analyte” refers to any material that can be involved in an antibody/antigen reaction.
Typically the analyte will be an antigen, for example, a protein, a carbohydrate, cell walls (e.g., bacterial or fungal cell walls), virus particles and small molecule haptens. Other examples include molecules such as cocaine, morphine, progesterone, luteinizing hormone-releasing hormone, or DNA. It is also possible that the analyte is an antibody that reacts with a bound antigen or an antibody to the antibody.
Additional Tests and Products for Development
The Target System provides the platform for the development of a series of quantitative tests for important diagnostic applications that can provide results at a patient's bedside, in a doctor's office, in the emergency room, in a clinic, in an ambulance, on the battlefield, on-site agri-business locations, rural and economically disadvantaged areas. The Target System expects to meet the POC diagnostic market criteria as follows:
Rapid turnaround time
Direct application of a non-critical volume or placement of sample directly into instrument
Disposable device or minimal maintenance required
Minimal technical expertise required
Positive identification and specimen tracking strategy that eliminates specimen identification errors
Simple strategy for calibration and QC
Transferability of data to the LIS or HIS
Agreement of result with accepted “Gold Standard” tests
Affordable cost
- 13 -
The Company’s testing system is not limited to HIV or AIDS diagnostics. The Company is currently working with Naglreiter, an established medical device development company, to develop the prototype for the SPARKS Mobile™ handheld device in conjunction with a compatible rapid test cartridge for the detection of the COVID-19 virus. The rapid test is being developed using recombinant S1 and S2 glycoproteins reagents that are geared to identify the virus through the “Spike” S1 and S2 antigens in human cells. The COVID-19 Spike proteins play a key role in eliciting potent neutralizing-antibody and T-cell responses. The Spike’s receptor binding domain is the primary determinant of the virus’s ability to infect.
Diseases like COVID-19, malaria, cholera, hepatitis, yellow fever, West Nile virus, and other viral diseases present increasing health threats to large populations around the globe, with the largest problems existing at the stage of proper diagnosis. The Target System test cartridge format is readily adaptable, having been applied to viral and bacterial infections in the past such as Rubella, Rotavirus, and Streptococcus A.
The Company believes that it can adapt the VT-1000 Desktop Analyzer and SPARKS Mobile™ to the rapid, simple POC diagnosis of nearly every disease without the requirement of additional equipment. Further, the Company believes that the combination of a mobile, hand-held testing device along with test cartridges for a host of different diseases can improve disease diagnoses and healthcare in a vast majority of today’s underserved regions. In addition, the Target System platform allows for the monitoring of environmental components influencing the health of populations, such as the presence of toxins in soil and drinking water and contamination of food supply.
SPARKS Mobile™: The Target System Hand-Held Analyzer:
The SPARKS Mobile™ is the Company’s next-generation analyzer. Utilizing re-engineered technology of the VT-1000 Desktop Analyzer, the SPARKS Mobile™ is a handheld device that is intended to utilize the Company’s TADS test cartridges and include a small, rapid testing format, in conjunction with a data acquisition and test reading, with connectivity and features similar to a smartphone device. The SPARKS Mobile™ is currently in the design stage of the development process.

SPARKS Mobile™
Design Concept
Whether searching for markers in the blood stream, or diagnosing a pathogen in urine, the Company’s SPARKS Mobile™ will be a portable tool for rapid diagnostics. The SPARKS Mobile™ will also provide an improvement in POC diagnostics and applications in countries with limited healthcare infrastructures and geographic limitations, both of which are of paramount importance in the combat against infectious diseases and in the fight against proliferation of endemic and pandemic diseases. This innovative SPARKS Mobile™ will allow for a fast (minutes instead of hours or days) performance of tests at the point of care and will only require a test cartridge and a small number of ready-to-use solutions in preformatted quantities. Moreover, the SPARKS Mobile™ will include the ability to store patient information, test data, and QC data, and transmit data through wireless connections.
- 14 -
The SPARKS Mobile™ design goals are intended to:
●achieve a portable monitoring system, which is compatible with proven and reliable ELISA-based target system technology.
●expand readout capabilities to provide a mobile testing and monitoring platform.
●increase the economy of scale and scope of the diagnostics and monitoring platform by the development of additional utility of the device without redundant infrastructure investments (additional data acquisition of patients, additional tests for other, predominant diseases).
The densimeter/multi-light spectrum reflectometer utilizing immobilized enzyme antigens in blood plasma or urine, which is the core testing system of the VT-1000, will not change. The testing technology in the initial SPARKS Mobile™ testing device will be based upon the same FDA. 510(k) cleared technology employed in the Company’s VT-1000 Desktop Analyzer and is compatible with existing TADS test cartridges. However, a number of innovative features will be integrated into the design to meet customer and patient needs, including those included in the Company’s most recent conceptualization of design and functionality and environmental interface:
●High Infrared Light Spectrum;
●Easy Field Upgrades;
●No Change of Equipment;
●Printer Hook-up Capability;
●Low Entry Cost for New Test Development and Analysis;
●Safety, Security and Accuracy by design; and
●Desk to Docking Station: Smart Phone Capability
The SPARKS Mobile™ is being specifically designed to coordinate with the Target System and the TADS cartridges to provide reliable quantitative results within minutes, right at the point-of-care or site of testing. The continuity of the Company’s product and system upgrades and the continuous development of new tests based on an increasing point-of-care market paradigm, points to the VT-1000 Desktop Analyzer and the SPARKS Mobile™ as low cost alternatives to large laboratory analyzers and specialized training of personnel on multiple machinery. The ultimate value to the clinician or the attending physician is the ease of use, reproducibility and the history of accuracy of this type of rapid immunoassay principle in the area of quantitative analysis.
The graphics below represent the Company’s most recent conceptualization of the design components and features for the SPARKS Mobile™, its technology, operational construct and environmental interface:
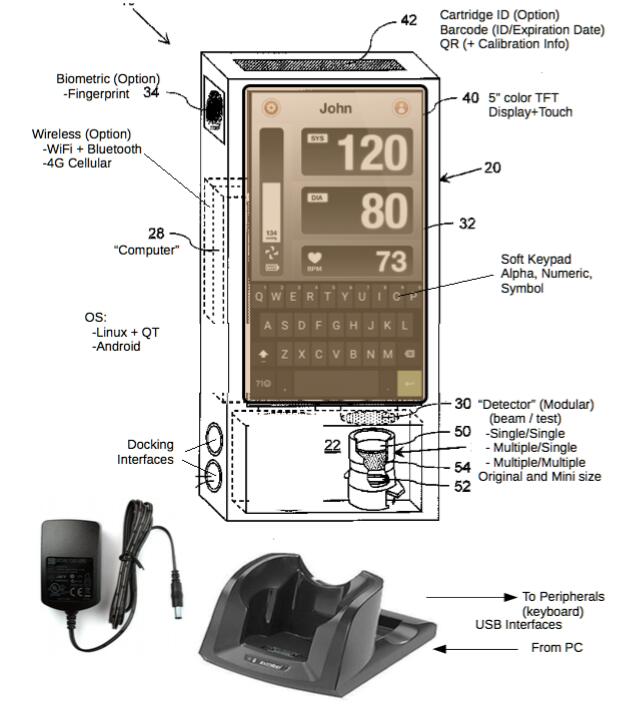
SPARKS Mobile™
- 15 -
The Company has also initiated the development of telehealth, remote patient monitoring and cognitive service offering capabilities into the SPARKS Mobile™ design and business model, for the integration of the Good Health Outcomes™ (Fotodigm® and REBOOT™) and Parallax Communications products and services (see “Additional Tests and Products for Development”).
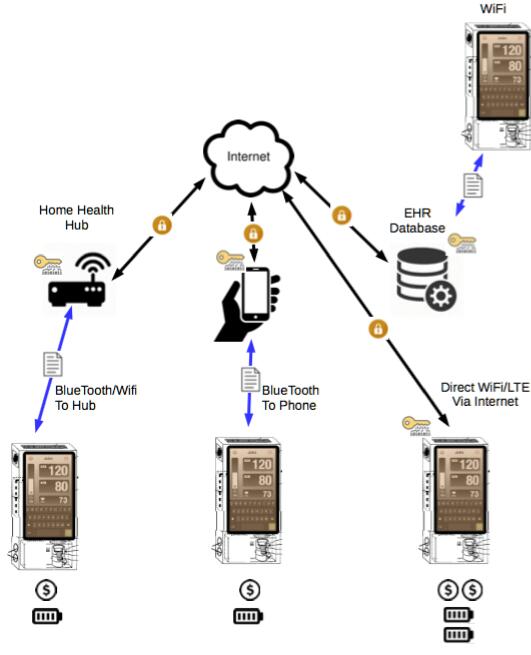
SPARKS Mobile™
Operation Concepts
- 16 -
The Company will continue to develop the design of the SPARKS Mobile™ as well as economic models designed to maximize the value of the SPARKS Mobile™. Although the Company’s 2020 budget does not include 100% of the funding resources to bring the development of the SPARKS Mobile™ to the prototype and beta stages, the Company continues to seek third-party resources, with the goal of obtaining FDA 510(k)-clearance for the SPARKS Mobile™ based upon the FDA 510(k)-cleared VT-1000.
Immunoassays: Defined 8
Immunoassays are chemical tests used to detect or quantify a specific substance, the analyte, in a blood or body fluid sample, using an immunological reaction. Immunoassays are highly sensitive and specific. Their high specificity results from the use of antibodies and purified antigens as reagents. An antibody is a protein (immunoglobulin) produced by B-lymphocytes (immune cells) in response to stimulation by an antigen. Immunoassays measure the formation of antibody-antigen complexes and detect them via an indicator reaction. High sensitivity is achieved by using an indicator system (e.g., enzyme label) that results in amplification of the measured product. Immunoassays may be qualitative (positive or negative) or quantitative (amount measured). An example of a qualitative assay is an immunoassay test for pregnancy. Pregnancy tests detect the presence of human chorionic gonadotropin (hCG) in urine or serum. Highly purified antibodies can detect pregnancy within two days of fertilization. Measuring the signal produced by the indicator reaction performs quantitative immunoassays. This same test for pregnancy can be made into a quantitative assay of hCG by measuring the concentration of product formed.
The purpose of an immunoassay is to measure (or, in a qualitative assay, to detect) an analyte. Immunoassay is the method of choice for measuring analytes normally present at very low concentrations that cannot be determined accurately by other less expensive tests. Common uses include measurement of drugs, hormones, specific proteins, tumor markers, and markers of cardiac injury. Qualitative immunoassays are often used to detect antigens on infectious agents and antibodies that the body produces to fight them. For example, immunoassays are used to detect antigens on Hemophilus, Cryptococcus, and Streptococcus organisms in the cerebrospinal fluid (CSF) of meningitis patients. They are also used to detect antigens associated with organisms that are difficult to culture, such as hepatitis B virus and Chlamydia trichomatis. Immunoassays for antibodies produced in viral hepatitis, HIV, and Lyme disease are commonly used to identify patients with these diseases.
Quantitative Immunoassay Analysis
Immunoassays are powerful techniques for understanding the role of specific components in complex systems. They work on the basis of the recognition of a specific component (target X) by an antibody or equivalent (affibody, RNA aptamer, recombinant antibody, etc.), which results in the production of a detectable signal. In most cases immunoassays are qualitative, providing information in terms of signal intensity. What is really wanted, however, is quantitative assay providing information in absolute chemical terms, namely the concentration of target X.
Quantitative Immunoassays would:
●Allow detection of the absolute concentration of components
●Reduce inter-assay variation in data
●Permit successful statistical analysis of smaller sample sets
●Permit direct comparison of data generated at independent sites or occasions.
Quantitative Immunoassays are simple to construct. They require the simultaneous analysis of experimental (or test) samples and calibration standards. The signal intensity generated by calibration standards of known concentration permits conversion of the signals generated by the test samples into absolute units of concentration.
Calibration curve
A calibration curve (or standard curve) establishes the relationship between the amount of material present and the signal intensity measured. In the case of immunoassays, this would represent the relationship between the epitope concentration and the signal intensity obtained. This relationship is often non-linear, and in many applications displays a dynamic range (or response range) of approximately two orders of magnitude in the concentration of target X.
To perform a Quantitative Immunoassay, a set of "calibration standards" containing the epitope in various concentrations, are deployed in the immunoassay alongside experimental "test samples". Densitometry is performed on all data from the assay, and curve fitting used to define the relationship between epitope concentration and signal intensity. This mathematical relationship is then used to convert the signals generated by experimental samples into concentration of target X, which in the Company’s experience is highly accurate.
8 “Immunoassay tests,” Encyclopedia of Surgery, [website], 2001, https://www.surgeryencyclopedia.com/Fi-La/Immunoassay-Tests.html
- 17 -
Molecular Identity of Calibration Standards
For Western Blot applications, a calibration standard is a molecule which contains the epitope feature of an immunoassay covalently bonded to a protein of known molecular weight. Two configurations of this structure are possible (Figure 1), where the epitope structure is either linked to the amino acid backbone (Fig 1a) in the form of a fusion protein or linked to a side chain of a specific amino acid (Fig 1b).
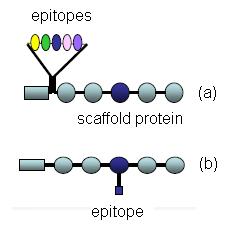
Figure 1: Schematic representation of calibration standard molecules.
A set of calibration standards to common epitope tags (His6, c-myc, HA, FLAG, AU1, AU5, glu-glu,) was analyzed by SDS-PAGE/Western blotting (detected via the His6 tag). A single band of 55kDa was detected, and the intensity of signal decreased with decreasing calibration standard loading as expected (Figure 2).
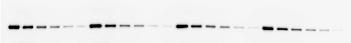
Figure 2: Immunodetection of a serial dilution of His6-calibration standard.
Densitometry of the data was performed and the data plotted to define the relationship between epitope amount and signal intensity (Figure 3). Mathematical fitting of the data was performed, with the best fit achieved by "one site-specific binding" analysis (GraphPad Prism) as shown in Figure 3. An excellent fit of the data was achieved using 6 calibration standard concentrations each analyzed in quadruplicate. Similar excellent fits could also be achieved by analysis of fewer standards, with indistinguishable results obtained from 3 calibration standard samples analyzed in triplicate.
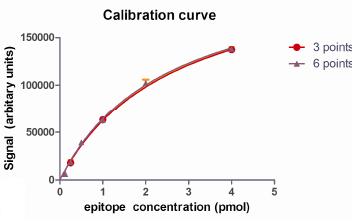
Figure 3: Mathematical description of a calibration curve.
To determine the epitope concentration of an experimental sample, the mathematical description of the calibration curve is rearranged to calculate epitope concentration from raw signal intensity. Figure 4 displays the quantitative measurement of three "test" samples. Test samples of 2pmol and 0.5 pmol were analyzed and the results obtained were 2.153± 0.127 pmol (mean ± standard error, n=4), 0.552± 0.045 pmol (mean ± standard error, n=4), confirming the accuracy of the measure (Figure 4). Samples should only be analyzed which fall within the calibration range, as errors are higher for observations beyond the confines of the calibration curve e.g. 0.125 pmol in this example.
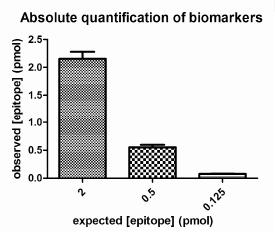
Figure 4: Accuracy of Quantitative Immunoassays.
In summary, Quantitative Immunoassays are easy to construct and offer several valuable benefits to the researcher. They permit calculation of the absolute concentration of the component of study with high accuracy (error <10%) and high reproducibility. This enhances the quality of research results and also the productivity of research programs by facilitating the direct comparison of data obtained on separate occasions.
- 18 -
In recent years, there has been a continuing shift from the use of laboratory-based analyzers to more technologically advanced Point-of-Care tests that can be performed in a matter of minutes. Unlike the centralized clinical laboratory segment, which is mature and highly competitive, the Point-of-Care market is still a relatively early stage market. Although certain simple single analyte diagnostic tests have been developed, such tests have remained incapable of precise and highly sensitive quantitative measurements. As a result, medical tests that require precise quantitation of the target analyte have remained the domain of immunoassay analyzers.
Diagnostic tests performed outside the central laboratory or decentralized testing is generally known as Point-of-Care. Over the years, the increasing introduction of transportable, portable, and handheld instruments has resulted in the migration of POC testing from the hospital environment to a range of medical environments including the workplace, home, disaster care and most recently, convenience clinics. Moreover, POC test devices have contributed significantly to the growth of the overall diagnostics market over the past 10 years. As more diagnostic manufacturers pursue CLIA 9 waiver status for their POC devices and CE Mark 10 for POC or self-use. At the same time, more decentralized test venues invest in non-waived rapid tests and instruments. POC testing appears to be headed for an even bigger role in diagnosis and monitoring patient care. 11
The Global In-Vitro Diagnostics (“IVD”) market is expected to exceed $92 billion by 2025, up from $64 billion in 2017 12, and is expected to continue at a 4.8% compounded annual growth rate over the next eight years. Self-testing is the biggest trend fueling the growth, and the recent advancements in IVD technology has a large influence over the product type segment, which is poised to grow at a compounded annual growth rate of 6.1% from 2018 to 2025 13. The growth is being driven by devices aimed at making acute care more efficient. There is a concerted effort to reduce time spent in expensive intensive care units and in the hospital in general. More tests and technologies have been adapted to serve the needs of physician offices and home testing.
Key Drivers
The two factors that are significant to the rapid growth of POC testing are technology advancements and healthcare economics. The development of new and improved technologies has resulted in the ability to make evidence-based medical decisions that improve patient outcomes and reduce patient acuity, criticality, morbidity and mortality. Quicker diagnosis of infectious agents can also permit the earlier prescription of appropriate medications, thereby potentially shortening the duration of illness. Additionally, the economic climate is driving significant changes in the manner in which patients will be tested and how results are delivered. Recent revisions to government regulations, together with growing patient and insurer pressures on hospitals and physicians have increased incentives to reduce overall patient healthcare costs while providing a higher level of care to a greater number of patients. One cost-cutting measure is to reduce the high cost of diagnostic testing carried out in central laboratory sites.
Limitations
Each of the screening devices described above have limitations in their utility and range of application. Many screening devices have been adopted from their use in clinical laboratories and, when applied to POC application, required special handling of the specimen samples (blood, urine, and feces) and decreased sensitivity and/or specificity.
Competitive Landscape
There are approximately 40 to 50 companies in the Point-of Care diagnostic industry in the U.S. and approximately another 100 outside the U.S. 14. The POC space can be broken down into various sub-sets such as molecular biologist developing reagents, and markers to diagnostic equipment and test development companies, as well as companies who do neither and focus on marketing tests, equipment and assays. Most notably in the POC space are the large pharmaceutical companies such as Bayer, Roche, Abbott Labs, ThermoFisher and others. Parallax’s specific competitive landscape is tied to its patented process involving the SPARKS Mobile™ Analyzer and Target System platform In-Vitro Tests. There are a number of companies developing mobile devices to perform a host of health industry-related services and the Company believes that more companies will enter the mobile diagnostic space in the next few years. The industry has yet to develop a standardized Point-of-Care immunoassay platform for any device to be integrated into. The Company’s SPARKS Mobile™ Analyzer is designed to deliver a device that adds immediate value to health providers, patients and health insurance companies.
9Clinical Laboratory Improvement Amendments (CLIA) of 1988 are United States federal regulatory standards that apply to all clinical laboratory testing performed on humans in the United States, except clinical trials and basic research.
10The letters "CE" are the abbreviation of French phrase "Conformité Européene" which literally means "European Conformity" CE Marking indicates that your medical device complies with the applicable EU regulations and enables the commercialization of your products in 32 European countries.
11“The Worldwide Market for Point-of-Care (POC) Testing (Infectious Disease Testing, POC Cancer Tests, Rapid Coagulation, Urine Testing, Lipid Tests, Pregnancy Testing, Glucose Testing and Other POC)”, Kalorama Information, [website], February 2018, https://kaloramainformation.com/ product/the-worldwide-market-for- point-of-care-poc-testing-infectious-disease- testing-poc-cancer-tests-rapid- coagulation-urine-testing-lipid-tests-pregnancy- testing-glucose-testing-and-other-poc/
12S. Ugalmugale and S. Mupid, “In-vitro Diagnostics Market … Competitive Market Share & Forecast, 2018 – 2024,” Global Market Insights, Inc, [website], August 2018, https://www.gminsights.com/toc/detail/in-vitro-diagnostics-market
13“In Vitro Diagnostics (IVD) Market…Global Opportunity Analysis and Industry Forecast, 2018-2025,” Allied Market Research, [website], December 2018, https://www.alliedmarketresearch.com/ivd-in-vitro-diagnostics-market
14“Top 50 Companies on Point of Care Diagnostic Testing,” Kalorama Information, [website], May 2017, https://www.kaloramainformation.com/product/top-50-companies-in-point-of-care-diagnostic-testing/
- 19 -
The Company’s primary goal is to create a mobile platform that can integrate and utilize the flow-through process of its Target System platform, and offer the healthcare provider a system that is fully interoperable and ubiquitous with a potentially large number of in vitro tests. There are other test platforms in the space, but the Company has filed a patent application on the process of its SPARKS Mobile™ Analyzer and TADS test cartridge. There can be no assurance that the Company’s POC devices will prove to be competitive with the other POC devices under development.
Until the Company secures a minimum of five million dollars ($5,000,000) of additional capital to operate for the next twelve months it will remain highly vulnerable to competition. The Company anticipates the need for a minimum of an additional three million dollars ($3,000,000) of investment capital for it to achieve its goals of developing a commercially viable rapid POC COVID-19 diagnostic test and the SPARKS Mobile™ Analyzer version of the VT-1000 Desktop Analyzer. The Company intends on exploring licensing partners for manufacturing and distribution of its product line. Should adequate funds be available from future funding resources, the Company intends on using a portion of the proceeds for research and development, including the further development of the SPARKS Mobile™ device and COVID-19 test cartridge. There can be no assurance that such amount will prove adequate to develop the Company’s products. Furthermore, the Company’s competition has significantly greater resources that they can deploy and time to head off competition.
In Vitro Diagnostic Sales Leaders
A 2017 report from Kalorama Information ranked the top 10 companies in the In Vitro Diagnostics market, based upon estimated 2017 revenues from clinical diagnostic test instruments, reagents and supplies, as follows:
1.Roche Diagnostics, Switzerland www.roche.com
2.Abbott Diagnostics, Abbott Park, IL www.abbott.com
3.Siemens Medical Solutions Diagnostics, Deerfield, IL www.diagnostics.siemens.com
4.Danaher Corporation, Washington, DC, www.danaher.com
5.Thermo Fisher Scientific, Waltham, MA www.thermofisher.com
6.Sysmex America, Inc. Lincolnshire, IL, www.sysmex.com
7.bioMérieux SA, Marcy l’Etoile, France www.biomerieux.com
8.Ortho Clinical Diagnostics, a division of Johnson & Johnson, Raritan, NJ www.jnj.com
9.Bio-Rad Laboratories Inc., Hercules, CA www.bio-rad.com
10.Beckman Coulter Inc., Fullerton, CA www.beckmancoulter.com
Barriers to Use
The main barriers and constraints to the use of POC rapid diagnostic tests can be put into four main categories:
Acceptability:Rapid tests need to be acceptable to policymakers, clinicians, patients, and the medical community. In the POC rapid test market, tests need to have sufficient sensitivity and specificity, and need to have an adequate predictive value. Ease of use is critical for POC use by clinicians. Culturally appropriate specimens and credible results are vitally important if rapid tests are to be accepted by patients.
Affordability:Many rapid diagnostic tests are more expensive than the tests or syndromic algorithms they are intended to replace. Decreasing per-test costs, carefully designing diagnostic algorithms, and educating end users about the cost-savings of POC testing are important means of maximizing rapid test affordability.
Availability:Rapid diagnostic tests are not always available in developing countries. Most tests have limited shelf lives, and many countries have weak public and private sector procurement and distribution systems. The consistency and quality of imported tests can also cause issues. To address these constraints, local government regulations, quality assurance, shelf life testing, and distribution systems all need to be assessed and improved. The Company, in conjunction with its license partner Montecito BioSciences, Ltd., intends to initially control all of the manufacturing for the Target System Desktop and SPARKS Mobile™ analyzers and test cartridges.
Reimbursement:The ability to gain scale in reimbursement across a wide number of tests is still a challenge for POC diagnostic companies such as Parallax.
Intellectual Property (Diagnostics)
The Company’s products include an FDA-cleared VT-1000 Desktop Analyzer and more than two dozen FDA-cleared tests. The Company acquired the exclusive rights in perpetuity to a number of United States Patent and Trademark Office (“USPTO”) patents and pending patent applications on the Company’s products in the area of infectious diseases, as well as methods for future test development, through a License Agreement with Montecito BioSciences, Ltd. The Company intends to seek intellectual property protection for all supporting products such as novel biomarker candidates, antibodies, proteins, and diagnostic tests surrounding its core indication areas, in order to create a barrier to entry for competitors.
- 20 -
The Target System and certain of its related components were previously issued patents by the USPTO. The following previously-issued patents have expired:
Patent # | Description | Date Filed in US | Date Expired |
US4,748,042 | Target Ringing & Spotting Machine (Method and Apparatus for Imprinting Membrane With Pattern Of Antibody) | May 31, 1988 | May 31, 2008 |
US4,797,260 | Target Cassette (Antibody Testing System) | January 10, 1989 | January 10, 2009 |
US5,137,691 | Target Cassette with Removable Air Gap (Antibody Testing System With Removable Air Gap) | August 11, 1992 | August 11, 2012 |
The Company has retained a team of professionals in the field of patent protection and is continuously seeking out new opportunities for its products and products in development.
Key Patented and Patent-Pending Concepts
●Sample Analysis
●Plurality of Isolated Antibodies to a Plurality of Cognate Antigens
●Identifying Drugs, Targeting Moieties or Diagnostics
●Determining the Immune Status of a Subject
●Flow Through Testing System with Pressure Indicator
●Novel Biomarker Candidates
●Antibodies
●Proteins
●Diagnostic Testing
For additional information on the Company’s patents and patents-pending, see “INTELLECTUAL PROPERTY SUMMARY“ section below.
The FDA-Cleared Tests
The Company acquired, through an Assignment Agreement, the exclusive rights in perpetuity to the following FDA-cleared tests (the “Tests”):
Test/Device Name | 510(k) No. |
| No. | Test/Device Name | 510(k) No. | |
1 | Rotacube (Rotavirus) | K884017 |
| 14 | Target Hcg | K914303 |
2 | Rubella-Cube TM | K892051 |
| 15 | Target Quantitative Hog One Step | K903937 |
3 | Cmv-Cube TM | K884842 |
| 16 | V-Trend Target Rf Test | K904105 |
4 | Target Quantitative Hcg | K890131 |
| 17 | Blue Dot Test for Pregnancy | K884017 |
5 | Target Strep A (Streptococcus Spp.) | K880460 |
| 18 | Target Cocaine Metabolites-R Test | K910122 |
6 | V-Trend Target Im Test (infect mononucleosis) | K890041 |
| 19 | Target Cocaine Metabolites-V Test | K910123 |
7 | First Sign (Pregnancy, Hcg) | K973208 |
| 20 | Target Cannabinoids-R Test | K910893 |
8 | Target Cardiac Ck-Mb | K890295 |
| 21 | Target Cannabinoids-V Test | K910892 |
9 | Target Cardiac Troponin 1 | K972094 |
| 22 | Target Amphetamines / Methamphetamines-R Test | K910739 |
10 | Target C-Reactive Protein Test | K892231 |
| 23 | Target Amphetamines / Methamphetamines-V Test | K910740 |
11 | Target C-Reactive Protein Test | K890423 |
| 24 | Target Opiate-R Test | K890978 |
12 | Target Myoglobin | K963680 |
| 25 | Target Opiate-V Test | K890979 |
13 | Target Aso Test | K910073 |
|
|
|
|
The Company is currently in the pre-clinical testing stage of developing a test with antibodies for COVID-19 detection compatible with the Target System. There can be no assurance that the Company will be successful in developing such tests or in obtaining the required FDA clearance.
For further information on the exclusive rights to the Company’s FDA-cleared Tests, and the complete text of the Assignment Agreement and subsequent Modification, please refer to Exhibits 10.19 and 10.21, respectively, to the Company’s Current Report filed November 15, 2012 on Form 8-K.
It is expected that after successful re-introduction of the Target System, additional tests will be developed and protected by the Company. Generally, the Company will have rights to improvements to the basic technology platform in exclusivity through its licenses with Montecito Bio Sciences, Ltd.
- 21 -
The long legal journey toward medical device regulation began with the Pure Food and Drugs Act of 1906. Medical devices were not included, as no one envisioned how technology would grow increasingly complex, and would ultimately require regulation. The Medical Device Amendments of 1976 gave the FDA authority to ensure the safety and effectiveness of a range of life-saving medical devices, while also protecting the public from fraudulent devices. The Amendments:
●defined a medical device,
●established three device classes (I, II, and III),
●identified pathways to market,
●established Advisory Panels, and
●set clinical investigation requirements.
Subsequent legislation strengthened the FDA’s regulatory authority. The following table identifies the legislation and significance for the Major Medical Device:
Legislation | Significance |
Safe Medical Devices Act of 1990 | ●Established Quality System requirements |
| ●Supported post market surveillance |
| ●Allowed FDA discretion for PMAs brought to panel |
FDA Modernization Act of 1997 | ●Supported for early collaboration, expanded Class I and Class II exemptions |
| ●Set the “least burdensome provision” |
| ●Supported dispute resolution |
| ●Established evaluation of automatic Class III designation (giving the sponsor the opportunity to request lower classification due to a minimal risk device, known as “de novo” review) |
| ●Mandated free and open participation by all interested persons |
Medical Device User Fee and Modernization Act (MDUFMA) of 2002 | ●Established a fee schedule for most types of device submissions to achieve shorter review times |
| ●Requires FDA to include pediatric experts on the panel for a product intended for pediatric use |
FDA Modernization Act of 2007 | ●Reauthorized and expanded MDUFMA |
As part of the regulatory infrastructure, the FDA has established almost 1,700 classifications for generic medical devices. Each one of the generic devices is assigned one of three regulatory Classes, based upon the control level necessary to assure the devices’ safety and effectiveness. The least burdensome provision allows industry and FDA to consider the least burdensome appropriate means of evaluating a device’s effectiveness when there is a reasonable likelihood of its approval. The intent is to help expedite the availability of new device technologies without compromising scientific integrity in the decision-making process or FDA's ability to protect the public health. This provision does not lower the standard for premarket clearance. The FDA maintains a database on its website at www.accessdata.fda.gov for many types of applications, including 510(k) records dating back to 1976, and PMA submissions.
Device classifications depend on the intended use of the device, its indications for use, and risk posed to the patient and/or user.
Class | Risk Level | Type of Controls | Exemptions |
Class I | Low | General Controls | Yes |
Class II | Moderate | General Controls & Special Controls | Yes |
Class III | High | General Controls & Premarket Approval (PMA) | No |
The FDA requires that medical devices obtain either Premarket Notification Clearance (“PNC” or “510(k)”) or Premarket Approval (“PMA”) prior to commercial distribution, unless an exemption is met. Whether to submit a 510(k) or a PMA is determined by the applicant. A 510(k) is normally submitted for a device that can be compared to a similar device already on the market, to illustrate the device’s safety and effectiveness. A PMA is normally submitted for a device that is completely new to the market or is classified as a Class III device.
- 22 -
The term “510(k)” refers to Section 510(k) of the Food, Drug and Cosmetic Act, wherein it provides a pathway to clearance for a device that maintains FDA standards, without the lengthy and costly PMA submission process. A 510(k) submission for clearance must demonstrate that the device is at least as safe and effective, that is, substantially equivalent, to an already legally marketed device. The 510(k) submission must be made at least ninety days in advance of commercial distribution, and if the device meets the requirements, the FDA orders the device as “cleared.”
To further illustrate the 510(k) submission requirement, a 510(k) clearance is required when:
●introducing a device into commercial distribution (marketing) for the first time;
●there is a significant change in the safety or effectiveness of a legally marketed device; or
●a legally marketed device is being marketed for a new or different intended use.
In contrast, a 510(k) is not required when:
●unfinished devices or components are sold for further processing or assembly by other parties, excluding the sale of direct replacement parts to end users;
●the device is not being marketed or commercially distributed;
●distributing another party’s domestically manufactured device;
●repackaging or relabeling, if the condition of the device is not significantly changed;
●the device was commercially distributed prior to May 28, 1976, and no significant changes to design, method, component, method of manufacture or intended use have been made;
●acting as an importer of a foreign made medical device; or
●the device falls into a Class I or II exemption classification; or is a Class III device requiring PMA.
To summarize the 510(k) for Parallax’s VT-1000 Target System, the submission was made to the FDA on December 23, 1988, and received 510(k) clearance on January 27, 1989. In this instance, the clearance process took approximately thirty days. The VT-1000 will not require any further FDA clearance unless there are significant changes in the safety or effectiveness of the device, or its intended use is changed or appended. The same is true for the 25 FDA-cleared tests.
Premarket Approval
Premarket Approval (PMA) is the most stringent type of device submission required by the FDA. A PMA application must be submitted to request FDA approval to market a device. Unlike 510(k) clearance, PMA approval is based upon a determination that there is sufficient valid scientific evidence that the device is safe and effective for its intended use. PMA submissions include extensive research and development, three-phase clinical trials, and a lengthy FDA review process.
To reasonably assure that a device is safe and effective, PMA requires valid scientific evidence that the probable benefits to health from the intended use of a device outweigh the probable risks, and that the device will significantly help a large portion of the target population. Sources of valid scientific evidence may include well controlled investigations, partially controlled studies, historical controls, well documented case histories by qualified experts, and robust human experience. Independence is an important concept for PMAs, meaning that each PMA should establish the safety and effectiveness of the device under review, and that data about one device cannot be used to support another.
The cost to obtain PMA approval on one novel device can be anywhere from $10 million to $1 billion, and can take an average of 3 to 7 years to bring a device to market.15 Examples of PMAs include digital mammography, minimally invasive and non-invasive glucose testing devices, implanted defibrillators, and implantable middle ear devices.
Class III De Novo
As indicated in the FDA Classes chart above, PMA submissions are required for all Class III devices, regardless of any similarity to a previously marketed product. Class III devices are those that support or sustain human life, are of substantial important in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
A “de novo” Class III device is one that was originally submitted for 510(k) clearance, but because the device used for comparison in the 510(k) was “not substantially equivalent” (NSE), the device was automatically denied 510(k) clearance, and was reclassified as being a novel Class III medical device requiring PMA approval.
In the event of a de novo designation of a low to moderate risk device, the applicant may submit a request for consideration to be classified as a Class I or Class II device within thirty days of receiving 510(k) NSE determination. Alternatively, if there is no similar equivalent device for comparison under a 510(k) submission, the applicant may request the FDA to make a risk-based classification of the device as Class I or Class II without ever submitting a 510(k).
Devices that are classified through the de novo process can be marketed and used as predicates for future 510(k) submissions, to be utilized as a “substantially equivalent” device, despite the fact that PMA approval was not provided.
15 https://www.sciencedirect.com/
- 23 -
The FDA can impose requirements at the time of approval of a PMA or HDE, or by regulation afterwards. One requirement may be the need for post-approval studies. The CDRH Post-Approval Studies Program helps ensure that well designed post-approval studies are conducted effectively and efficiently and in the least burdensome manner. Post-approval studies should not be used to evaluate unresolved premarket issues that are important to the initial establishment of device safety and effectiveness.
With post-approval studies, FDA can evaluate device performance and potential problems when the device is used more widely than in clinical trials and over a longer period of time. This allows FDA to build in accountability and gather essential post market information, including:
●longer-term performance of the device (for example, effects of re-treatments and product changes)
●community performance (clinicians and patients)
●effectiveness of training programs
●sub-group performance
●outcomes of concern – real and potential
Summary Comparison of 510(k) and PMA
510(k) Submissions | PMA Submissions |
●primarily for Class II devices (Class I devices are predominantly exempt) | ●primarily for Class III devices |
●a Class I or II pre-amendment or legally marketed device (predicate) exists | ●a Class I or II pre-amendment or legally marketed device (predicate) does not exist |
●third-party review option is available for devices not requiring clinical data | ●device is life supporting and/or has potential risk to patient |
●documented proof of Substantial Equivalence to a predicate is required | ●documented safety and effectiveness data for the device is required |
FDA and COVID-19
In response to the global need for tests to diagnose the COVID-19 virus, the FDA has provided certain flexibilities in its approval process, that will allow for the rapid deployment of certain diagnostic tests whilst ensuring public safety. The following statement was published by the FDA on March 30, 2020, in connection with the review process of diagnostic tests to combat the COVID-19 virus 16:
“The U.S. Food and Drug Administration has been providing unprecedented flexibility to labs and manufacturers to develop and offer COVID-19 tests across the U.S. The FDA’s regulations have not hindered or been a roadblock to the rollout of tests during this pandemic. Every action the FDA has taken during this public health emergency to address the COVID-19 pandemic has balanced the urgent need to make diagnostic tests available with providing a level of oversight that ensures accurate tests are being deployed. Moreover, as in previous emergencies, the FDA has been extremely proactive and supportive of test development by all comers—laboratories, and large and small commercial manufacturers—offering our expertise and support to speed development and to quickly authorize tests that the science supports.
It is not the FDA’s role to develop tests or decide what tests a health care professional uses. Our role is to determine if the tests developed by others provide accurate and reliable results, even when some would prefer that we let tests on the market without evidence that they work. It’s critical that the tests used work. False results can also contribute to the spread of COVID-19. We want our treatments to be tested for effectiveness and reviewed by the FDA. We want the same for our tests—assurances that they are accurate and effective.
Developing a test:
Typically, with an emerging health threat, the Centers for Disease Control and Prevention (CDC) is the first developer of a diagnostic test in the U.S. - Samples of the virus are crucial to confirming the accuracy of the test.
CDC has first access to viral samples that other test developers do not. CDC also manufactures their own tests for distribution to their national network of public health labs. In this pandemic, CDC encountered problems manufacturing their test. FDA assisted CDC in their work to resolve the issue and utilize a commercial manufacturer to make tests for any laboratory, not only public health labs.
Viral samples became commercially available to private sector test developers in later February, when the National Institutes of Health’s partner BEI Resources began selling vials of the virus grown from material provided by CDC.
Laboratories have always had the ability to develop their own tests in the U.S.; the COVID-19 outbreak did not change this. Once a developer has a viral sample, they can confirm the accuracy of their test very quickly, usually in two to three days.
16 “Coronavirus (COVID-19) Update: FDA expedites review of diagnostic tests to combat COVID-19,” Stephen M. Hahn M.D., Commissioner of Food and Drugs - Food and Drug Administration, March 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expedites-review-diagnostic-tests-combat-covid-19
- 24 -
In the future, making viral samples available earlier to commercial developers will be crucial to deploying tests quickly. Moreover, CDC’s test should be manufactured by a commercial entity with the requisite expertise.
Timeline of FDA support for test developers:
Since the beginning of January, the FDA has worked with more than 230 test developers who have or are expected to submit requests for FDA emergency authorization of their tests; to date, 20 authorizations have been granted.
In addition, more than 110 laboratories have notified the FDA that they have begun using their own tests.
For interested developers, the FDA provided recommendations for how to check a test for accuracy as well as a short form to make it easy to share their test information quickly in support of an Emergency Use Authorization (EUA).
Emergency Use Authorization authorities:
An EUA, put into place by Congress, is a relaxed standard that allows tests to be made available based on less data than in non-urgent circumstances and allows for expedited FDA review.
In many cases, the FDA can do this review in as little as a day, which it has done repeatedly.
EUA authority is not a barrier to test availability.
FDA policy updates:
The FDA recognized the urgent need for even faster testing availability. Although laboratories could use the EUA pathway, many were hesitant or didn’t know the pathway was available to them.
To respond to this need, the FDA revised the process to allow labs to begin testing prior to FDA review of their validation data. This policy change was an unprecedented action to expand access to testing. Nevertheless, in the first week, only six laboratories took advantage of this further streamlined process because many laboratories did not have a test, or did not have the viral samples to check the accuracy of their test.
In addition, the FDA implemented another change to empower states to take responsibility for tests developed and used by laboratories in their states without FDA review.
The FDA has and will continue to play a pivotal role in this emergency response. …”
Manufacturing
The Company does not intend to manufacture in house, with the exception of prototype and small batch production of tests for clinical trials and in-house testing. The Company is required to use manufacturers who operate under Good Manufacturing Practices (“GMP”). A GMP is a production and testing practice that helps to ensure a quality product. Many countries have legislated that pharmaceutical and medical device companies must follow GMP procedures, and have created their own GMP guidelines that correspond with their legislation. Basic concepts of all of these guidelines remain more or less similar to the ultimate goals of safeguarding the health of the patient as well as producing good quality medicine, medical devices or active pharmaceutical products. In the U.S. a drug may be deemed adulterated if it has passed all of the specifications tests but is found to be manufactured in a condition which violates current good manufacturing practice guidelines. Therefore, complying with GMP is a mandatory aspect in pharmaceutical manufacturing.
Although there are a number of them, all guidelines follow a few basic principles:
●Manufacturing processes are clearly defined and controlled. All critical processes are validated to ensure consistency and compliance with specifications.
●Manufacturing processes are controlled, and any changes to the process are evaluated. Changes that have an impact on the quality of the drug are validated as necessary.
●Instructions and procedures are written in clear and unambiguous language. (Good Documentation Practices)
●Operators are trained to carry out and document procedures.
●Records are made, manually or by instruments, during manufacture that demonstrate that all the steps required by the defined procedures and instructions were in fact taken and that the quantity and quality of the drug was as expected. Deviations are investigated and documented.
●Records of manufacture (including distribution) that enable the complete history of a batch to be traced are retained in a comprehensible and accessible form.
●The distribution of the drugs minimizes any risk to their quality.
●A system is available for recalling any batch of drug from sale or supply.
●Complaints about marketed drugs are examined, the causes of quality defects are investigated, and appropriate measures are taken with respect to the defective drugs and to prevent recurrence.
●GMP guidelines are not prescriptive instructions on how to manufacture products. They are a series of general principles that must be observed during manufacturing. When a company is setting up its quality program and manufacturing process, there may be many ways it can fulfill GMP requirements. It is the Company’s responsibility to determine the most effective and efficient quality process.
The Company has not yet commenced commercial operations of the Target System, and thus has yet to develop methods of distribution for its diagnostics products beyond the business plan stage.
In order to commercially sell the VT-1000 Desktop Analyzer, the Company must have it manufactured under GMP. The Company can and will provide demonstrations of the VT-1000 Desktop Analyzer capabilities to potential customers.
The Company will need to secure additional capitalization before it can acquire additional antibody markers, produce additional Target System cartridges or produce the VT-1000 Desktop Analyzer under GMP.
Principal Suppliers
The Company has not yet commenced commercial operations of the Target System, and thus has yet to establish principal suppliers of its diagnostics product line.
- 25 -
Overview
Parallax’s primary goal is to deliver Good Health Outcomes™ for patients through a service that allows healthcare providers of all types to reduce costs, increase revenues and provide a better patient experience and satisfaction. Connected Health and the Good Health Outcomes™ platform are a part of the Company’s overall healthcare strategy.
Good Health Outcomes™ features Fotodigm® technology, a proprietary systems integration software component that provides biometric devices and electronic health records to interoperate. The Good Health Outcomes™ platform provides medical practices including nursing, doctors offices, and hospital operations to provide remote patient monitoring (“RPM”), medication adherence, and an intelligent telemedicine delivery system. Featuring distinctive Fotodigm® data capture and analysis capabilities, the Good Health Outcomes™ platform can be promoted directly through hospital and accredited nursing facilities, as well as health and wellness service providers, all seeking to integrate RPM and telehealth services as part of their offerings.
Good Health Outcomes™ integrates remote monitoring solutions to improve a patient’s compliance to therapy, pharmaceutical and other treatment regimens prescribed by their physicians. Through the Good Health Outcomes™ platform, alerts and notifications, including visual, audible and vibratory, are sent to patients through email and SMS. In addition, cloud-based recordings are maintained for continuous patient monitoring and telehealth delivery, benefitting both caregivers and patients. Good Health Outcomes™ is network agnostic and captures data from any data-generating device, insulin meter, or scale, and works with any analog device, providing easy, flexible data management utilizing its Fotodigm® technology.
Parallax’s Connected Health systems will also offer automated medication dispensing through a patented process, which can reduce the costs and risks associated with noncompliance of medication, therapy and treatment. This can, in turn, increase the financial yields and service delivery successes for healthcare providers, ultimately improving the quality of life and peace of mind for the patient, the doctors and the care providers overall.
Biometric Monitoring 17
A biometric monitor is a device that measures different aspects of a person’s behavioral or physiological behavior. It basically measures the biological data of a human being.
There are different kinds of biometric monitors that can be used, depending on the purpose. There are monitors that measure the temperature of the body; the pulse rate or the heart activity; blood pressure; blood glucose level etc. Below are a few common biometric monitors that are often used in households and hospitals:
●Thermometers – One of the popular kinds of thermometer is the mercury thermometer which is used to measure the temperature or fever of the body. The mercury expands when heated, and rises up a glass tube, stopping at the corresponding temperature mark. Most thermometers have both Celsius and Fahrenheit readings for the ease of the user.
●Blood Pressure Monitors – A blood pressure monitor is a device that measures the blood pressure of an individual based on the readings of two values – the systolic pressure, which is the peak pressure in the arteries, over the diastolic pressure, which is the minimum pressure. Blood pressure should be monitored regularly in patients suffering from hypertension or hypotension, and other related conditions.
●Glucose Monitors – Also known as glucometer, glucose monitors measure a person’s blood glucose level. Glucometers are essential to monitor the glucose in the blood of diabetic patients, as an increase in sugar may initiate serious medical conditions. There are two types of glucometers: continuous glucose monitors, which measure the glucose level every few minutes using small sensors; and the fingerstick glucose monitors that uses a sample of blood to read the glucose level.
●Pulse Oximeters – Pulse oximeters are used to determine the amount of oxygen in the blood. It is generally used to detect breathing or respiratory problems, and is also used to monitor oxygen saturation in patients under intensive care. There can be three types of pulse oximeters – stand-alone, handheld, or pocket PC –based pulse oximeters.
●EKG Machines – Electrocardiogram or EKG machines are devices that measure the activity of the heart. It detects the electronic signals sent by the heart in between heartbeats. It is used to detect abnormal activities of the heart induced by sleep, or stress-related problems. EKG machines can be of two types: continuous, which continuously monitors heart activity; or intermittent which records the activity once in a while, but can be used for weeks or months.
●Pedometers – Used mainly for exercise purposes, a pedometer measures the number of steps a person takes while walking to stay fit. By quantifying the number of steps taken, a person can then calculate the amount of calories burned. Many sports item manufacturers are producing pedometers to help motivate fitness enthusiasts.
There are numerous other biometric monitors used for medical or physical training purposes. There are the calorie counters, the body-fat calculators, the cholesterol monitors, fitness monitors – and a number of other devices. Prices of these vary in accordance with the brand, the quality, and the durability of the product.
Many cheap monitors do not give correct readings and can be deadly when monitoring critical patients. Therefore, biometric monitors should be high-quality products that come with a guarantee, rather than inexpensive brand-less ones. Biometric monitors keep a check on health conditions, and a person should never compromise with their health.
17 http://www.ihealthdirectory.com/biometric-monitor/
- 26 -
The Good Health Outcomes™ platform features a full set of modern communications technologies that provide cloud-based Health Insurance Portability and Accountability Act (“HIPAA”) compliant patient data security. The platform was designed specifically for patients’ ease of use, and provides real-time voice and “video conference” communication directly from any mobile or internet-connected device using biometric data collection. The platform, combined with systems integration services that interface with Electronic Health Records (“EHR”) and Electronic Medical Records (“EMR”) technology platforms, enables “virtual doctor visits,” increasing the conveniences for both patients and their doctors and care providers. The Good Health Outcomes™ platform and its systems integration positioning allows for any physician, clinician, nurse, pharmacist, caregiver, or family member to support the treatment of the chronically ill, both acute and post-acute, in a persistently connected real-time environment, on a larger scale and with greater precision and patient satisfaction.
RPM benefits are equally important in a residential environment. Seniors and chronically ill residents have shown to require less support and/or interventions, have higher group participation rates and improved cognitive capabilities, as well as an overall improved health, a more assured time with family, and longer residencies, which results in greater value-added contributions and greater job satisfaction from staff members.
The ability to remotely monitor each resident, from medication compliance and connected sensors, including vital sign, sleep monitoring, and fall detection devices, enables the residential caregiver to provide a significantly enhanced (and commercially desirable) service for optimal health conditions. With the adoption of a centralized monitoring system, residences will also begin to see a marked reduction in the inter-dependencies that can exist between residents, and an improved state of health for every resident, further reducing the support load on staff members.
Medication Monitoring/Compliance/Adherence
Nonadherence with medication is a complex and multidimensional healthcare problem. Patients forget to take their medications, creatively alter their medications, engage in unendorsed polypharmacy, mix their medications, and take medications in combinations that may have dire synergistic interaction effects, such as dizziness and confusion. Estimates of medication nonadherence rates typically range from 20% to 85%, (see figure below) 18. As a result, a substantial number of patients do not benefit optimally from pharmacotherapy and can wind up in emergency situations, hospitalized, or worse. In fact, hospital readmission generated by medical non-compliance and nonadherence was a $41 billion-dollar problem in the United States in prior years and is growing 19.

Medication adherence is a cornerstone of significantly improved quality of life, and the Fotodigm® platform is the cornerstone of medication adherence. A unique device specifically designed for seniors and the chronically ill, the Fotodigm® platform offers enormous potential for patients, their families, their caregivers and for those residences that choose to offer superior services and a superior health environment for their clients.
The Good Health Outcomes™ platform monitors the adherence of treatment and therapy regimens. In addition, with the advent of an intelligent personal medication device with bio-feedback, Good Health Outcomes™ allows, perhaps for the very first time, the quantitative and qualitative feedback of real-time data to pharmacists, physicians and clinicians and, based on the individual patient, enables medication titration to achieve optimal medication therapy.
Product Development
Parallax is in the development stage of acquiring, licensing and developing in-house solutions/products for personalized health monitoring of seniors that will capture a host of a patient’s vital information, including temperature, heart rate, perspiration and movement disorders. All of these sensor products can be connected to the Fotodigm® platform so that the bio-feedback information is directly correlated with medication consumption information, providing clinicians, pharmacists and physicians with real-time, comprehensive data and information on patients’ conditions.
The Company is reducing to practice the claims of its Fotodigm® data capture technology through internal development and through external technology development partnerships. The Fotodigm® system is being developed to utilize a proprietary Machine Face Recognition engine along with proven and existing Optical Character Recognition (“OCR”) technology through third-party license. The technology has been beta tested and utilized in the field by patients within remote patient monitoring systems for the reduction of hospital readmissions.
18 https://www.pillsy.com/articles/medication-adherence-stats
19 https://cvshealth.com/newsroom
- 27 -
Parallax’s customer pilot was able to offer a biometric monitoring capability from devices that were not Internet connected and this lowered the cost of service delivery and sustainability. The pilot for a large hospital group met the requirements of removing the connectivity and cloud-based requirements on biometric measurement devices. The focusing on FDA approved measurement devices connected via Fotodigm® allowed for the reduction of costs of telehealth services delivery. The system has been developed in a mobile application that can be downloaded by Parallax end users at the Company’s remote patient monitoring website at www.goodhealthoutcomes.com.
Product Strategy
Parallax’s product strategy is to eliminate obstructions to consumer adoption of remote patient care systems, so patients can stay out of the hospital longer and have a better quality of care and quality of life.
Remote patient monitoring systems enable efficient healthcare delivery to patients outside conventional hospital or clinical settings by transmitting real-time patient data for remote clinical review. Correlating vitals with medication history and consumption directly informs healthcare providers as to the real-time status of a patient, at home or in residences. Anomalies can be reported or alerted to those in need through a cloud-based network. RPM systems incorporate wireless medical devices and computer-based software applications. The evolution of IoT and IoS technologies is clear, and will offer significant business opportunities for patients, residences and the healthcare system in general. RPM is a cost-effective means of keeping patients out of the hospital and having a better quality of care.
Product Benefits
RPM systems, even within the walls of a single building, can offer countless benefits for the overall healthcare management of clients. There are countless tangible benefits to medication adherence as well, including significant reductions in hospitalizations, enhanced quality of life, and reductions in the effects of both overmedication and undermedication. Eliminating the distribution and administration of scheduled medications exonerates the residence from the liabilities associated with this task. Human error is all but eliminated. Corporate risks are significantly reduced for this activity and liability insurance premiums may be reduced as well. Essentially, increasing medication adherence through automation, empowerment and monitoring improves patient outcomes and achieves benefits for all health system stakeholders.
|
●Better quality of life ●Fewer hospital visits ●Better outcomes ●Reduced travel and health costs ●Increased social interaction with family and friends | Patient Families: ●Better visibility into the quality of life of loved ones ●Fewer hospital visits – fewer interruptions into their work days ●Better outcomes ●Reduced travel costs ●Increased peace of mind – reduced stress and concern |
Residential Providers: ●Better overall quality of life in the residence ●Reduced risk and liability ●Superior service ●Reduced labor costs ●Increased revenues for enhanced services ●Superior reputation / higher desirability / increased ability to charge for core services ●Healthcare professionals and providers deliver better care more efficiently at lower risk | Healthcare Industry: ●Government, insurance and other payers reduce spending ●Patients who are more adherent with medication regimes use fewer health services ●Pharmaceutical companies increase profitability ●and deliver value beyond the pill ●Pharmacy retailers ensure repeat orders, increased fulfillment and enhance brand loyalty ●Physicians have the ability to become proactive, rather than reactive ●Every 1% improvement in medication adherence results in $2 billion in savings to U.S. Healthcare system and a $4 billion revenue increase to pharmaceuticals 20 ●Differentiation in the marketplace for early adopters |
Market Opportunities
The market demand for remote patient care (“RPC”) solutions is at an all-time high and continues to increase due to healthcare insurance reimbursement of the services delivered over RPC systems. Further, the move from “fee-for-services” to outcomes-based payment structures have brought about the need for doctors and all other healthcare providers to increase the efficiency and to reduce the costs of care delivery to patients. Clearly, as with the introduction of any new technology, there is a significant market differentiator for early adopters. Residences, assisted living facilities, and long-term care facilities all exist in a competitive environment in which differentiation between them is based upon higher desirability, which amounts to higher profits for those who are able to offer more. Unlike other service businesses, residences have the ability to attract clients requiring services on a long-term basis. 21 Additional market opportunities exist around hospitals and their management’s needs surrounding the reduction and elimination of patient readmissions.
20 “Adherence, Compliance, Persistence,” MediPENSE, [website], https://medipense.com/en/medication-adherence-compliance/
21 “The Long Term Care Market: Nursing Homes, Home Care, Hospice Care, and Assisted Living,“ Research and Markets, [website], February 2019, https://www.researchandmarkets.com/research/476vtw/united_states?w=12
- 28 -
The global RPM systems market draws immense focus due to strict governmental measures to cut down healthcare expenditure, and reduce hospital stays. Remote patient monitoring serves to minimize hospital readmissions, and reduces the load on physician time, and nursing staff, thus greatly reducing healthcare costs. A rapidly aging population vulnerable to chronic diseases, and a growing desire to live independent lives among the elderly are driving growth in the market. Given the rise in the number of insured patients covered under reforms such as the Affordable Care Act, coupled with stricter reimbursement norms, healthcare providers are under constant pressure to manage wider patient population at lower costs. With the healthcare industry migrating towards an outcome-driven effective healthcare system, remote patient monitoring technology stands optimally positioned for growth.22
Encouraged by the widespread proliferation of high-speed internet, and related services, the adoption of RPM systems is witnessing strong demand. There is growing interest in IoT-driven healthcare services and wearable medical devices that feature sensors, actuators, and other mobile communication methods through which patient data can be continuously transmitted onto a cloud-based platform. Healthcare providers are increasingly adopting cloud computing technologies, which not only offer cost benefits but also allow healthcare organizations to increase operational efficiency.23
The global market for remote monitoring systems is projected to reach over $66 billion by 2020, driven by government measures to reduce healthcare spending against a backdrop widening healthcare budget deficit.24 As stated by the new market research report on Remote Patient Monitoring Devices, North America represents the largest market worldwide, occupying close to 41% of the total market share, and is expected to grow at an exponential compounded annual growth rate of 21.34% during the forecast period.25 Asia-Pacific ranks as the fastest growing market with a compounded annual growth rate of 13% over the forecast period. The growth in the region is driven by the increase in per capita healthcare spending and the need for a cost-effective and sustainable healthcare system along with infrastructure capable of addressing the growing healthcare needs of an expanding population. 26
Misuse of Opioids and Other Addictive Substances
During 2017, there were more than 72,000 overdose deaths in the United States, including 49,068 that involved an opioid, according to a provisional CDC count.27 More than 130 people died every day from opioid-related drug overdoses in 2016 and 2017, according to the US Department of Health & Human Services (HHS).
There are many organizations talking about the prevalence of this medical problem; however, those working with the addicted population are segmented and fragmented by a limited or narrow scope of work. The clinics and treatment centers are restricted by the need for an on-site appointment for testing and counseling, the hospital emergency rooms are only able to treat the critical physical problem that presents from misuse or overdose and have difficulty finding treatment resources that are readily available for referral to treatment, and the patients who do enter treatment encounter many challenges including access to treatment, frequent appointment schedules making it difficult to maintain employment and lapses in proper chronic disease and pain management.
The Company believes that bringing together a number of partners and adding the dimension of population health in-home visits will provide improved, measurable outcomes for the patient and their families. Regularly scheduled home visits can provide the link between the provider, the healthcare plans and patients.
It is also the Company’s belief that education, early intervention and remote patient monitoring can have a significant, positive impact on reducing the misuse of opioids, alcohol, tobacco and other addictive substances. The Community Health Workers (“CHW”) have been providing health screenings and educational classes for chronic disease management and healthy living for several years, and have been successful in identifying potential health risks and referrals for care. The CHW have become respected and trusted advisors and regarded as a link to the healthcare system.
Outcomes Optimization Platform monitoring:
●Reduction in number of opioid prescriptions issued;
●Number of individuals attending educational sessions;
●Increase in number of patients finding timely access to treatment facility;
●Reduction in number of patient relapses;
●Reduction in hours spent to recovery independence;
●Number of times transportation arranged for appointments;
●Number of enrollments of uninsured patients;
●Referrals to other public benefits and community resources.
22 S. Kripalani, et al, “Reducing Hospital Readmission: Current Strategies and Future Directions,” Annual Review of Medicine, [website], https://www.annualreviews.org/doi/10.1146/annurev-med-022613-090415
23 I. Liao, “5 Real-Time & Remote Patient Monitoring Trends,” MPO Magazine, [website], August 2018, https://www.mpo-mag.com/contents/view_online-exclusives/2018-08-22/5-real-time-remote-patient-monitoring-trends/7970
24 “Focus On Cost Savings Through Streamlining Clinical Workflow Processes Drives the Healthcare IT Market, According to New Report by Global Industry Analysts, Inc.,” PRWeb, [website], July 2019, https://www.prweb.com/releases/ healthcare_it_market/ electronic_health_record/ prweb11718606.htm
25 “Remote Healthcare (mHealth, Tele-ICUs, & Virtual Health) Market - Global Outlook and Forecast 2018-2023,” Research and Markets, [website], March 2018, https://www.researchandmarkets.com/research/d9cmmq/global_remote?w=4
26 “Asia-Pacific Remote Patient Monitoring Systems Market-Growth, Trends and Forecast (2019-2024)”, Mordor Intelligence, [website], https://www.mordorintelligence.com/industry-reports/asia-pacific-remote-patient-monitoring-system-market-industry
27 “Provisional Drug Overdose Death Counts,” Center for Disease Control, National Center for Health Statistics,” [website], https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 29 -
The lucrative reimbursement policy introduced by the government agencies promoting the use of telemedicine and virtual health services is driving the market in North America. The U.S. and Canada are the major revenue contributors to the market in North America. Further, the increasing investments in research and development and technological innovations will help major players in the North American region occupy a larger market share over the next few years.
Barriers to Entry
Despite the significant potential benefits to outcomes and patient satisfaction that may accrue through the leveraging of patient-generated health data (“PGHD”) in clinical care, data ownership remains a barrier to greater use of PGHD in some circumstances. Though the collection and use of PGHD is intuitive to experienced patients, inclusion of such data represents a shift in the way medicine has been practiced. Taking an active role in the collection and management of data about one’s health status increases patient activation, which is strongly related to better health outcomes in multiple conditions.
Patients’ and providers’ use of mobile health (“mHealth”) apps provide a framework for assessing the role mHealth can play in medicine and as a source of PGHD. Physicians use social media primarily for personal use (60%), though accessing healthcare news (21%), communicating with peers (18%), marketing the practice (11%), and communicating to patients (4%) are also practiced. Among physicians who choose not to use social media, concerns about patient privacy (52%), lack of time (51%), concerns about liability (42%), the belief that social media has little professional value (40%), and lack of familiarity (23%) are cited most frequently. In 2014, two-thirds of physicians surveyed reported using a mobile app to check medication interactions, diagnose a condition, access EHRs, check results, create clinical notes, and prescribe electronically, and more than a third of U.S. physicians recommended their patients use health apps.28
Questions related to provider licensure pose another potential barrier to the routine use of mHealth. When a patient has his or her radiograph read, many current U.S. state laws require that it be read by a physician licensed in the state where the patient is located and had the radiograph. PGHD can raise special issues. For example, if a patient lives in New York and has an mHealth device that continues to monitor certain aspects of his or her health and the patient crosses three states and then travels into the EU, does the patient’s physician need to be licensed in each of the other three states and in the applicable country in the EU when the data is transmitted from that state or country in the EU? Within the United States, most states require that the out-of-state physician receive an unrestricted license in the state in which the initial patient interaction occurred. Some states issue a telemedicine license to facilitate practice across state lines when the physician holds an unrestricted license in another state. In the radiograph situation, the imaging physician is reading the radiograph at one point in time and billing for that service. With the monitoring of an individual’s health information continuously, the clinician may be being paid to manage the patient’s condition or his or her overall care. The special privacy and confidentiality issues can raise important mHealth implications. It is important that vendors and healthcare systems coordinate their efforts to minimize these issues.
Competitive Landscape
Countless smaller companies around the world are innovating in this sector and are now offering everything from Bluetooth connected toothbrushes and bathroom scales (weight loss or gain is a key indicator of health and disease state) to “wearable’s” that capture everything from body temperature, pulse rates, respiratory rates and blood pressure monitoring to game-changing technologies such as non-invasive blood glucometers that sample blood sugar level several times per second – continuously – without requiring lancets and the drawing of blood samples.
Medical clinics, on a global scale, are already adopting technologies that allow them to provide better healthcare to a wider population base. Insurance companies and HMOs also continue to seek the highest quality healthcare with the highest return on investment by leveraging modern technologies to provide better care to a greater population base at lower cost.
There are a large number of companies offering some form of wireless and remote technologies, patient data processing applications and equipment, and electronic medical record data transfer equipment. Competitors supplying advanced patient monitoring and telehealth systems to hospitals are large, established healthcare companies, often working in conjunction with information technology (“IT”) companies on an entire system. The home healthcare and other sectors are much more fragmented and are dominated by privately held companies. In addition, some companies supply innovative products, but only for a small segment of the market; and some companies supply products on a regional basis only.
There is a worldwide interest in “connected health” with major companies such as Philips, Nokia, Apple, IBM, Microsoft and Google, all investing heavily in sensors and sensor systems for healthcare, connectivity and RPM. Traditional telecommunications companies like AT&T, Verizon and a host of others are also investing heavily in healthcare systems and in the RPM market.
Major players in the global RPM market include Abbott Laboratories, Aerotel Medical Systems, AMD Global Telemedicine, Inc., BIOTRONIK SE & Co. KG, Boston Scientific Corporation, GE Healthcare Ltd., Honeywell Life Care Solutions, Intelesens Ltd., LifeWatch AG, Masimo Corporation, Medtronic Plc, Mindray North America, Nihon Kohden Corporation, Omron Healthcare, Inc., Philips Healthcare, Qualcomm Life, Inc., St. Jude Medical Inc., and Welch Allyn, Incorporated.
28Petersen C, DeMuro P. Legal and regulatory considerations associated with use of patient-generated health data from social media and mobile health (mHealth) devices. Appl Clin Inf 2015; 6: 16–26 http://dx.doi.org/10.4338/ACI-2014-09-R-0082
- 30 -
Intellectual Property (Remote Patient Care)
The Company, through Assignment Agreements with La Frontera Community Solutions, Inc., acquired all worldwide rights, title and interest in and to the patent applications for the technology underlying the Fotodigm® platform and systems.
Key Patented and Patent-Pending Concepts
●Diagnostic Monitoring | ●Remote Patient Biometrics | ●Remote Medication Monitoring |
●Data Driven Outcomes | ●Remote Patient Diagnostics | ●Remote Medication Delivery |
●Machine Face Recognition with automated data extraction | ●Remote Patient Monitoring | ●Remote Medication Reconciliation |
●Data systems integration with biometric and electronic health record systems |
|
|
For more information on these patent applications, please see “INTELLECTUAL PROPERTY SUMMARY“ section below.
Government Regulations
The FDA regulates certain medical devices and also certain mobile medical apps. On September 25, 2013, it issued a Final Guidance that defines “mobile medical app,” as a mobile app that (1) meets the definition of a “device” in the Federal Food, Drug and Cosmetic Act and (2) is intended to be used as an accessory to a “regulated medical device” or to transform a platform into a “regulated medical device.” The Guidance grouped mobile medical apps into three categories: 1) apps that are actively regulated, (e.g., a mobile medical app that monitors the patient’s blood glucose levels and calculates the amount of insulin needed based on the patient’s condition, age, weight, etc.); 2) apps that are subject to enforcement discretion, (e.g., a mobile medical app that provides a patient an alert as to when to take his or her medications); and 3) those that are not considered devices and thus are not regulated (e.g., a mobile medical app that merely provides general healthcare information available on the internet, not directed to a specific patient).
The FDA guidance addresses data security because patients and other users may experience severe consequences should the device lack adequate data protection or be hacked. The guidance does not address the protection of privacy. Rather, privacy is protected by Health Insurance Portability and Accountability Act (“HIPAA”), where applicable. When patients’ health information is in the possession of health providers, health plans, business associates, or other covered entities, it is protected under HIPAA; when it is transmitted among individuals or organizations that are not covered entities under HIPAA, it is not protected. Accordingly, health information transmitted via a mobile device by a covered entity is protected under HIPAA privacy and security rules. However, this same information transmitted via a non-covered entity under HIPAA is not protected. HIPAA also does not cover information on an individual’s mobile device.
Patient-generated health data (“PGHD”) is merely data of the patient if a patient checks their blood glucose levels with a mobile device. If, however, he or she uses the device to transmit that information to their clinician for the purpose of monitoring that person’s care and the information becomes part of the patient’s electronic health records (“EHR”), the PGHD then falls under HIPAA.
The Federal Trade Commission (“FTC”) is not hesitant to file complaints against companies that it believes fail to reasonably protect the security of consumers’ personal data, including medical information. In August 2013, FTC filed a complaint against LabMD, Inc., alleging that the medical testing laboratory exposed the personal medical information of more than 9,000 consumers by placing the information on a peer-to-peer file-sharing network. The filing followed the discovery of the personal information of several hundred consumers who used LabMD’s services in the possession of identity thieves. In this case, as in an earlier case against a medical transcription firm that exposed personal medical information on the public internet, FTC is acting to enforce HIPAA’s security requirements.
The Company makes patient security an utmost priority. With the Company’s secure HIPAA-compliant cloud-based system, a patient can have confidence that their personal HER, EMR and PGHD is protected with the highest level of technology available.
FTC also regulates misleading claims. If in the sale or distribution of a mobile medical app or device one makes claims about what the device can do, FTC can bring an action to make the individual or entity cease and desist from making such claims. In 2013, FTC published a written guidance and a short video for mobile app developers that offer advice on creating apps that protect users’ privacy and comply with truth-in-advertising principles.
- 31 -
As of December 31, 2019, the principal suppliers of the medical devices utilized with the Fotodigm® platform were:
●Amazon Web Services, Seattle, WA | HIPAA-compliant secure server environments for hosting and management of Fotodigm® platform |
●La Frontera’s Empact Suicide Prevention Center, Tempe, AZ | Largest CARF (Commission on Accreditation of Rehabilitation Facilities) certified behavioral health suicide prevention call center in United States. |
●Royal Phillips, Inc. 1070 MX Amsterdam, The Netherlands | Primary RPM software and hardware supplier; platform built upon Salesforce.com infrastructure providing clinical, monitoring center, and patient management portals for mobile and desktop access. |
The Company relies upon these suppliers to provide the majority of the delivery of its remote patient monitoring systems and services. The services are web-based and although the Company relies on these vendors, it can also hold them accountable, receive volume-based pricing, discounts and partnership advantages through the competition of its suppliers. The Company has the ability to change vendors at any time in all service and product lines. Further, the Company is working towards the elimination of its reliance on software and hardware providers related to its in-home RPM offerings and services.
PATENTED REBOOT™ TECHNOLOGY |
Overview
On April 26, 2017, the Company completed the acquisition of 100% of certain intellectual property from ProEventa Inc., a Virginia Corporation (“ProEventa”), in accordance with the Intellectual Property Purchase Agreement between the Company and ProEventa (the “ProEventa Agreement”). ProEventa has an expertise in the development of behavioral health technologies, and is the wholly-owned subsidiary of Grafton Integrated Health Network, Inc., a non-profit Virginia corporation (“Grafton”), Pursuant to the ProEventa Agreement, in exchange for 100% of that certain intellectual property, among other things, consideration to ProEventa included:
●a stock purchase agreement to purchase 2,500,000 shares of the Company’s Common Stock; and
●a revenue sharing agreement, providing for a cash earn-out to be paid to the ProEventa shareholders of up to $3,000,000, to be derived from certain net revenue generated by the Company, as defined in the agreement; and
●a royalty agreement, providing for a royalty of 3% of the revenues generated from the intellectual property, ending at such time as the Company has paid ProEventa $25,000,000; and
●a limited license to ProEventa for the use of certain of the Intellectual Property’s technology at Grafton Schools.
On April 26, 2017, in conjunction with the ProEventa Agreement, the Company entered into a consulting agreement with James Gaynor, founder of ProEventa, that, among other things, provides for consideration to Mr. Gaynor as follows:
●a stock purchase agreement to purchase 500,000 shares of the Company’s Common Stock at $0.001 per share; and
●a grant of options to purchase 1,000,000 shares of the Company’s Common Stock at a price of $0.25 per share, vesting annually over a three (3) year period beginning September 1, 2017.
With the completion of this acquisition, the patented technology entitled “Platform for Optimizing Data Driven Outcomes,” is added to the Company’s patent portfolio.
Product History
For over a decade, Grafton School, Inc. (“Grafton”), a Virginia corporation, perfected a manual system of outcomes developed by Dr. Lisa Marshall and James Gaynor, that re-invented utilizing cloud computing and the internet. In March 2014, ProEventa, Inc. (a Latin word meaning, “Good Outcomes”), and wholly-owned subsidiary of Grafton, was formed, and began marketing the software based on rights granted by Grafton. REBOOT™ (“REBOOT™”), an acronym for Reliable Evidence-Based Outcomes Optimization Technology, is the behavioral health platform, and was developed to provide goal obtainment through mastery of skills in a customized and fully automated data analysis and intervention model.
The technology is supported by applied behavioral analytics and over 25 years of efficacy studies and reduction to practice at Grafton, combined with over $3.75 million-dollar invested to transition the system to a cloud-based platform with mobile applications.
- 32 -
Goal Mastery 29
In 2004, Grafton Health’s CEO, Jim Gaynor, asked his leadership team to provide the percentage of goals clients at Grafton mastered annually overall and within specific programs and services. While observations and anecdotal reports from Grafton providers and stakeholders indicated clients were showing improvement in their identified goal areas, there was not a system in place to provide an actual goal mastery rate. Out of this request, Grafton’s “Goal Mastery Initiative” was created.
The goal mastery process was designed to provide a structured and sustainable system to identify, monitor, and evaluate client progress and to embed data-based decision-making into the transdisciplinary treatment and instruction planning. There are several steps to this process: (1) conducting a thorough assessment of the individual’s strengths and needs, (2) writing goals that are functional, measurable, and specific to the needs, (3) collecting data, typically on a weekly basis, (4) graphing data and comparing them to a Minimum Growth Line, (5) determining level of progress according to a specific standard, and (7) providing a reporting mechanism to identify goal mastery rates at all levels within the organization. When the data trend indicates the goal is not on track for mastery, six primary decision-support factors (Six M’s) are explored to help the transdisciplinary team evaluate the situation and make recommendations for changes to treatment and instruction. After an initial baseline goal mastery rate of 35% when this initiative first started and progressive increases over the year, Grafton has maintained a goal mastery rate at or above 80% for the past several years.
The Company has licensed the improved REBOOT™ technology platform for systems integration into the diagnostics and remote patient care systems. The next step in the Company’s commitment to data-based decision-making is the introduction of website and mobile technology to support the goal mastery process. The REBOOT™ technology will be made available to organizations, individual providers, and family members who want to effectively track individuals’ progress and use the Company’s evidence-based process for increasing goal mastery.
Ultimately, through the goal mastery process and the deployment of REBOOT™, the Company will continue to provide individuals with the support they need to make meaningful and sustainable improvements in their functioning and quality of life.
Product Strategy
The patented feature set of the REBOOT™ platform allows it to provide progressive-predictive analytics and goal optimization intelligence on concurrent and compounding goals for an individual or group of people. Its’ utility is broad and has use cases in many vertical growth markets. The company has integrated REBOOT™ into its Good Health Outcomes™ remote patient monitoring platform and through that is offering patented data driven outcomes solutions to its clients within healthcare services provider organizations.
The product strategy is to enter the market through the healthcare initiative of the Company’s Good Health Outcomes™ remote patient care and through its diagnostics divisions. The integration of REBOOT™ is a key differentiating advantage within the remote patient care market. The software can be harnessed to assess and prioritize patient goals, provide interventions, track behavioral data and provide meaningful feedback toward goal obtainment, as well as real time decision support with robust progress reporting. The resulting decision and patient support, combined with the remote patient care and diagnostics technologies, make for a technology advantage and a feature set that is designed to take away market share from competitors and capture new client accounts in healthcare field focused on doctors, nursing operations, and hospitals.
The technology can be marketed through user licensing or enterprise licensing contracts within the healthcare market, in both platform and supported mobile applications, including the Company’s application known as COMPASS™ .
The Company believes that healthcare organizations for the first time will have the opportunity to “scale” their patient adherence programs while having visibility into its effectiveness and bottom-line impact. Individual users will have a goal wizard, virtual coach and rules engine that will help create a path toward wellness. With personalized analysis, decision support and “goal” mastery feedback, the individual will be primed, guided and motivated to adhere to their treatment plan.
REBOOT™ is also applicable to many vertical markets outside healthcare. The short-term strategy is to monetize the patented technology in healthcare, and license to non-competitive companies within other market verticals. The Company is assessing and prioritizing the licensing prospects. The management team is also monitoring its patents for possible infringements, and has retained a patent attorney to assess the licensing value for some infringements already identified. (See “Patent Infringement”)
Behavioral Healthcare Industry
Per a Behavioral Health Services 2016 Report by Capstone Partners, the demand for behavioral health services will continue to grow due to increased awareness and affordability of mental health and substance abuse treatment options. According to the Substance Abuse and Mental Health Services Administration (“SAMHSA”), expenditures on mental health and substance abuse treatment are estimated to have reached $239 billion in 2014. By comparison, expenditures only totaled $42 billion in 1986 and $121 billion in 2003. More specifically, revenues generated by the substance abuse treatment industry reached an estimated $29.8 billion in 2015, a 3.5% growth over the prior year and 2.9% annual growth rate since 2006. Industry revenues are expected to grow 2.6% annually to reach $33.9 billion by 2020. There are a variety of factors that impact growth of the substance abuse treatment market, including development of new treatments, drug availability and healthcare reform.
29 Grafton Integrated Health Network®
- 33 -
Increased awareness of the importance of behaviors in combating the COVID-19 global pandemic
Social distancing, washing hands, contact tracing and controlling human behaviors that enable the reduction in the transmission of viruses and disease have captured the attention of the medical community and the general population. The medical reimbursement and payers within the health insurance system have begun to fund mobile and cloud-based technologies designed to assist in behavioral management.
Increased Exposure to Drugs and Alcohol
In the United States, illicit drug use and alcoholism have grown significantly among all age groups due to increased availability of prescription drugs and medication. This has resulted in an unfortunate trend of increased prescription medication abuse by secondary school and college-aged young adults.
Development of New Treatments and Programs
Clinical advancements in therapy and medication management have yielded new and better procedures for both psychological and detoxification treatments. Over the past several years, medication-assisted opioid therapy, which allows patients to rid their system of substance dependence through new-aged medication treatments, has led to an increased demand for treatment. Another driver is the future need for clinics to provide youth, elderly and gender-specific programs.
Healthcare Reform and Affordability
Healthcare in the U.S. has undergone significant changes in recent years that are favorable for the substance abuse treatment industry. Healthcare reform, specifically the Affordable Care Act (“ACA”), the Mental Health Parity and Addiction Equity Act (“MHPAEA”) and the Medicaid Certified Match Substance Abuse Program (“MCMSAP”), has led to more affordable substance abuse treatment.
Growth in Private Insurance
Following the 2014 health insurance exchange implemented by healthcare reform, the number of people with private health insurance increased significantly. According to a study published by the CDC in January 2020, Private health insurance spending increased 5.8% in 2018, which was faster than growth of 4.9% in 2017.
Increased Awareness
One of the biggest challenges for the industry has been the reluctance of substance abusers and addicts to undergo treatment. Individuals afflicted by substance abuse typically refuse treatment due to the social stigma associated with admitting they have a problem or lack of knowledge about the treatments available. However, recent efforts by health and government agencies have resulted in greater public awareness and acceptance of substance abuse as a disease.
Autism Disorder and Treatment Industry
Although the specific causes of autism are still not known, one thing is. The rate is increasing, and the market for treatment is growing.
Based on new government data, as of 2017, 1 in 45 children in the United States, aged 3-17, have autism. This is up from only 1 in 150 children back in 2000.
Research firm, Market Data’s March 2018 report entitled, “The U.S. Autism Treatment Market,” states that market data analysts estimate that there are currently 1.4 million American children with autism. Another 700,000 adults have autism, having “aged out” of children’s programs, and, 81% of autistic children are male.
The total annual costs for children with Autism Spectrum Disorder (“ASD”) in the United States in 2017 were estimated to be between $11.5 billion and $60.9 billion - a significant economic burden.
Insurance coverage is a problem, but the share of children with access to insurance coverage is expected to increase from the 36% level today. In addition, the number of self-funded private employers covering autism treatment continues to grow.
Autism Treatment Options
In the past, it was thought that the best way to treat the symptoms of autism was to medicate. Data suggest that approximately 58% of patients with a diagnosis of childhood autism receive some type of pharmaceutical treatment. However, this segment of the market has been shrinking in value as concerns continue over the side effects of commonly used drugs.
Today, this attitude is changing, as Applied Behavior Analysis (“ABA”) programs have become more widespread and have displayed good outcomes. There are basically three types of ABA program providers: brick and mortar centers, community providers, and in-home therapists.
- 34 -
Many autism treatment organizations, and some of the largest competitors, are located in California. This is due to the fact that funding for treatment programs has been in place there since the 1990s, prior to the insurance mandates that were later put into place.
Market Opportunities
With healthcare costs continuously on the rise, REBOOT™ is positioned to be unique as an evidenced based, cost-effective approach in improving patient care. The selection of the healthcare market as the Company’s focal effort is a choice based upon the market size and market demand for outcomes improvement across the market that is both target rich and highly scalable.
●An estimated one in four adults (about 60 million Americans) experiences mental illness in a given year.30
●About 14 million people live with a serious mental illness.31
●Approximately 20% of youth ages 13 to 18 experience severe mental disorders in a given year. 30
●7% of American adults live with major depression. 31
●An estimated 18% of American adults live with anxiety disorders (e.g., panic disorder, OCD, PTSD). 30
●About 9 million adults have co-occurring mental health and addiction disorders. 30
●20 million Americans suffer from substance abuse. 30
In addition, there is a critical mental health provider shortage creating significant access to care issues:
●Only 40% of Americans with mental illness report receiving treatment. 30
●One mental health provider exists for every 790 individuals. 32
●Approximately 4,000 Mental Health HPSA (professional shortage areas) exist which is based on a psychiatrist to population ratio of 1:30,000 -- meaning it would take approximately 3,000 additional psychiatrists to eliminate the current mental health HPSA designations. 33
●A report to Congress found that 55 percent of the nation’s 3,100 counties have no practicing psychiatrists, psychologists or social workers. 34
There exists an abundance of opportunities for the Company that are focused on the use of the patented REBOOT™ technology. The market opportunities currently being pursued for REBOOT™ include, but are not limited by:
●Integration within Parallax’s remote patient care platform for goal obtainment and mastery within healthcare operations.
●Integration within Parallax’s COMPASS™ mobile smartphone applications targeted at and focused on specific user applications including, but not limited to, remote patient care, doctor decision support, patient empowerment, population health, ASD (Autism), chronic disease management and hospital readmissions reduction.
●Development alongside the SPARKS Mobile™, Parallax’s patented handheld POC analyzer, featuring immunoassay blood testing and FDA-cleared tests, to enable a complete suite of platform-based offerings for medical practitioners, including individual doctor’s offices, doctor groups, accredited nursing, and hospital operations.
Management is seeking additional opportunities with or in the study of the following markets:
●Environmental
●Economic
●Healthcare
●Enterprise licensing
●E-learning applications
●Professional sports
●Fitness
Management is also in the process of identifying the comprehensive patent landscape, and determining the best course and greatest value accretion in the prospective markets that will be approached through the patents and intellectual property licensing, including the market values related to patent infringement and outbound licensing opportunities centered around REBOOT™ as well as the adjacent assets held by Parallax.
Autism Market 35
The U.S. autism treatment market was estimated to be valued at $1.85 billion as of 2016, growing to $1.87 billion last year. Market data forecasts 3.9% average yearly growth, to $2.23 billion by 2022. This could be conservative, as insurance coverage is improving. In addition, venture capital firms are starting to take notice of investment opportunities in this market.
30https://www.nami.org/learn-more/mental-health-by-the-numbers
31http://www.lb7.uscourts.gov/documents/12-cv-1072url2.pdf
32https://khn.org/news
33https://www.kff.org
34https://www.jconline.com/story/news/2014/04/12/dire-shortage-of-psychiatrists-in-greater-lafayette-leaves-patients-waiting-months-for-help/7656745/
35https://blog.marketresearch.com/autism-treatment-programs-are-growing-a-1.8-billion-market-in-the-u.s
- 35 -
The average ABA center grosses about $821,000, and many are non-profit organizations. Many programs now have waiting lists, and there is a shortage of qualified supervisors.
Nine large multi-site ABA program providers operate an estimated 296 brick and mortar centers, and employ thousands of therapists. Together, they account for about $390 million in revenues, representing a 38% market share of ABA programs.
Revenues of ABA programs exceeded $1.91 billion in 2018, and North America continues to dominate the market, outpacing sales of prescription drugs used for autism symptoms. Growing resources for research and development drive the ASD therapeutics market growth, and favorable insurance reimbursement enable accessibility for treatment. 36
Competitive Landscape
Parallax competes with other technology consulting companies, which provides the Company with key technological advantages over the competing companies in the remote patient care and diagnostics markets. Within licensing, Parallax competes with other value-based contracting and technology companies like Oracle, Accenture and IBM, that have taken the same systems integration go-to-market approach.
Intellectual Property (Behavioral Health)
The Company, through an Intellectual Property Purchase Agreement with ProEventa, Inc., acquired all rights, title and interest in and to the patent entitled “Platform for Data Driven Outcomes.” The patent has been granted in the U.S. under patent #10,061,812, and applications have been filed worldwide. It has been published in the UK under filing GB2526749. A third filing is pending issuance in Australia. Along with the patents, the Company acquired the technology platform referred to as Reliable Evidenced Based Outcomes Optimization Technologies, or REBOOT™, a technology platform specifically designed to improve health treatment outcomes using proprietary applied behavioral analytics technology systems.
For more information on these patents and patent applications, please see “INTELLECTUAL PROPERTY SUMMARY” section below.
Government Regulations
Mental health and substance abuse services are subject to many federal, state and local regulations regarding licensing, operations, facility ownership, reimbursement rates and procedures. These regulations and strict licensure requirements create high barriers to entry for the industry. Licensing prerequisites typically relate to the provider’s medical qualifications, personnel and equipment, staff-to-patient ratio, adequate records maintenance, rate-setting and compliance with standard building and safety codes. Expansion of substance abuse facilities are also subject to state regulations. The construction of new facilities; expansion of existing facilities; transfer or change of ownership; and the addition of new beds, services or equipment may be subject to state laws that require prior approval by regulatory agencies under Certificate of Need (“CON”) laws. CON laws generally require that a state agency determine the public need for construction or acquisition of facilities/addition of new services.
The HIPAA regulations also have a unique impact on the Company, as they impact the management and controls of the data it collects and encounter throughout the Parallax operations. Also, the state and local governments’ Medicare and Medicaid payments and reimbursements require consistent and diligent management, both in following the advantageous changes in the move towards connected healthcare and greater reimbursements. There are also regulations and requirements with each approved connected healthcare treatment. Lastly, there are American Medical Association regulations over U.S. medical practices that affect how the Company’s business is operated, requiring both compliance and alignment with its customers, who require unique reporting and other data and service-related processes as a result of these, and other, regulations.
Principal Suppliers
As of December 31, 2019, the principal suppliers for the Behavioral Health platform were:
●Amazon Web Services, Seattle, WA | HIPAA-compliant secure server environments for hosting and management of REBOOT™ and COMPASS™. |
●La Frontera’s Empact Suicide Prevention Center, Tempe, AZ | Largest CARF-certified behavioral health suicide prevention call center in United States. |
The Company relies upon these suppliers to provide the majority of the delivery of its behavioral health platform products and services. The services are web-based and although the Company relies on these vendors, it can also hold them accountable, receive volume-based pricing, discounts and partnership advantages through the competition of its suppliers. The Company has the ability to change vendors at any time in all service and product lines. Further, the Company is working towards the elimination of its reliance on software and hardware providers related to its in-home behavioral health offerings and services.
36 https://www.fortunebusinessinsights.com/industry-reports/autism-spectrum-disorder-therapeutics-market-101207
- 36 -
Mr. Nathaniel T. Bradley, Chief Technical Officer (“CTO”) for the Company, provides Intellectual Property protection recommendations for all of Parallax’s Intellectual Property, open patent applications and products, both domestically and internationally. Mr. Bradley informs the Company and its shareholders of the accurate and current state of the commercial patented coverage and, where possible, identifies the existence of novel and patentable inventions present in the current innovation initiative. Mr. Bradley concluded that the Company has a strong patent portfolio protecting its business, and recommended that the Company aggressively proceed with additional patent applications to protect Parallax’s inventions and innovations (See “Patent Infringement”).
Patents, Patent Applications, Exclusive Licenses and Patent Portfolio Overview
I.The Company, through a License Agreements with Montecito BioSciences, Ltd., acquired the worldwide exclusive rights to sell, have sold, make, have made, develop, have developed, further develop and modify, or to have further developed or modified, within the field of use and under the terms set forth in the License Agreements, the following patents and/or patents pending:
1. Patent 8,920,725 and 9,170,258 (US) - “Portable Apparatus for Improved Sample Analysis”
The present invention is an improved apparatus for sample analysis. The apparatus employs an assay component containing a membrane having one or a plurality of analyte-specific binding agents attached thereto, a means for absorbing liquid, and a piston means for drawing analytes through said membrane into said means for absorbing liquid. The apparatus is configured to be portable and provide a detector for detecting binding of an analyte to an analyte-specific binding agent, a plurality of data acquisition components, and a computer for integrating, analyzing and storing the detected analyte specific binding and acquired data.
This Patent and pending application(s) cover the Company’s SPARKS Mobile™ hand-held analyzer, which is used in conjunction with the Target System test cartridges. The hand-held Target Analyzer device is capable of housing and analyzing two assay cassettes, and optionally features wired or wireless data transfer and multiple data acquisition components including a keypad, a touch-pad, a barcode wand and / or a fingerprint reader. On August 23, 2013, the Company was notified that its Chinese Patent Application No. 200780039901.X “Portable Apparatus for Improved Sample Analysis” had been granted by the States’ Intellectual Property Office of the Peoples Republic of China. Patents were also issued in Hong Kong, Macao, and India.
Following is the family of cases under Patent 8,920,725 and 9,170,258:
ApplicationCountryDate FiledStatusDate GrantedPatent Number
1.60/863,241United States10/27/2006ProvisionalN/AN/A - continued under 1a
1a.11/924,033 [1]United States10/25/2007AbandonedN/AN/A - continued under 1b
1b.13/248,307United States09/29/2011Granted12/30/20148,920,725 B2
1c.14/553,011United States11/25/2014Granted10/27/20159,170,258 B2
1d.CN101558302China10/25/2007Granted08/23/2013CN200780039901.X
1e.HK2010010103654Hong Kong10/25/2007Granted03/28/2014HK1137813
1f.MO J/001298Macau10/25/2007Granted03/12/2014MOJ/001298
1g.IN785/MUMNP/2009India10/25/2007Granted01/30/2017IN279743
[1]Patent Application US11/924,033 is being prosecuted worldwide. The now abandoned EPO Application No. 07854420.2 was filed in the following designee countries: Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Monaco, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Switzerland, Liechtenstein, Turkey and the United Kingdom.
2. Patent 9,573,990 (US) - “Method of Producing a Plurality of Isolated Antibodies to a Plurality of Cognate Antigens”
The present invention relates to a method for producing high affinity antibodies that are antigen-specific. The method involves binding a plurality of antibody-producing B-cells from a mammal to a plurality of cognate antigens; sorting the bound antibody-producing B-cell and cognate antigen; amplifying nucleic acid sequences encoding each antibody, or fragment thereof, from the B-cells; and expressing each antibody in a protein expression system. Antibodies produced in this manner are useful in diagnostic and therapeutic applications. This family of cases covers a technique for obtaining a population of antibodies produced by B-cells, without any a priori information about the population of antibodies, and use of the same in an array for profiling antigen expression.
ApplicationCountryDate FiledStatusDate GrantedPatent Number
2.60/608,526United States09/09/2004ProvisionalN/AN/A - continued under 2a
2a.11/221,252United States09/07/2005AbandonedN/AN/A - continued under 2b
2b13/253,366United States10/05/2011Granted02/21/20179,573,990
3.Patent 9,588,114 (US) – “Flow Through Testing System with Pressure Indicator”
This family of cases covers an improved assay cassette with pressure-sensitive microcapsules for ensuring that a sufficient reduction in pressure is achieved there by maximizing contact between the sample and analytic compound. A device for performing immunoassays on analytes. The device includes an immunosorbent membrane, an absorbent material, a piston component located below said absorbent material to draw analytes in a sample through the immunosorbent membrane into the absorbent material, and discrete groups of pressure-sensitive microcapsules located on the immunosorbent membrane.
ApplicationCountryDate FiledStatusDate GrantedPatent Number
3.61/814,916United States04/23/2013ProvisionalN/AN/A - continued under 3a
3a.14/786,272United States04/23/2014Granted03/07/20179,588,114
3b.PCT14/35073United States10/22/2015ClosedClosed
4. Application 12/769,036 - “Method of Identifying Drugs, Targeting Moieties or Diagnostics”
The present invention relates to a method for identifying a binding agent or epitope for use in drug design, drug targeting or diagnostics. The method employs contacting and sorting binding agents and cognate epitopes from collections thereof, characterizing the binding agent and cognate epitope, detecting the level or location of the epitope in a sample using the binding agent, and correlating the level or location of the epitope in the sample with the presence or stage of a disease or condition to identify novel drugs, targeting moieties, or diagnostic agents. This family of cases covers a technique for obtaining a population of antibodies that specifically binds to a corresponding population of antigens, without any a priori information about either population. The antigens identified by the method are subsequently characterized and correlated with the presence or stage of a disease or condition there by serving as a target for drug design, drug targeting or diagnostics.
ApplicationCountryDate FiledStatusDate GrantedPatent Number
4.60/608,342United States09/09/2004ProvisionalN/AN/A - continued under 4a
4a.11/221,038United States09/07/2005AbandonedN/AN/A - continued under 4b
4b12/769,036United States04/28/2010Pending
5. Application 14/492,641 - “Method for Determining the Immune Status of a Subject”
The present invention, whose application has been abandoned, is a method for using levels of soluble Clusters of Differentiation (CD) proteins, or cell surface-localized CD proteins extracted from T lymphocytes for determining the immune status of a subject. The present invention also a kit containing a CD protein extraction means and at least one antibody which specifically binds a CD protein for use in carrying out the method of the invention. This family of cases covers a technique and kit for assessing immune status, e.g., in HIV patients, based upon the amount of soluble or surface-localized CD3-CD4, and / or CD8 protein present in a patient sample. This method and kit are an alternative to conventional cell sorting technologies and is owned by Montecito BioSciences, Ltd.
ApplicationCountryDate FiledStatusDate GrantedPatent Number
5.60/845,395United States09/18/2006ProvisionalN/AN/A - continued under 2a
5a.11/856,925United States09/18/2007AbandonedN/AN/A
Summary of Open Applications available for continuation filings (from above):
4b. US12/769,036 - “Method of Identifying Drugs, Targeting Moieties or Diagnostics”
5a. US14/492,641 - “Method for Determining the Immune Status of a Subject”
For further information on the exclusive license of the Patents and Patent Applications above, and the complete text of the License Agreement and subsequent Modification, please refer to Exhibits 10.20 and 10.22, respectively, to the Company’s Current Report filed November 15, 2012 on Form 8-K.
II.The Company, through an Assignment Agreements with La Frontera Community Solutions, Inc., acquired all worldwide rights, title and interest in and to the following patent applications and the invention in its entirety:
1.Application 14/979,889 – “Remote User Monitoring System”
A system and method for monitoring a status of a user. One or more biometrics associated with a user in a residence where the user resides are sensed. A status of the user is determined in response to sensing the one or more biometrics. One or more questions about the status to the user are communicated. One or more answers to the one or more questions communicated to the user are received. The status is communicated to an administrator of the residence. The status is communicated in response to one or more of the answers.
ApplicationCountryDate FiledStatusDate GrantedPatent Number
1.14/979,889United States12/28/2015Pending
2.Application 14/979,742 – “Remote Medication Delivery Systems”
A system and method for medication delivery. Information is received indicating a user is scheduled to receive medication. A route between a dispensary storing the medication and a location of the user is determined. The medication is sent in a container from the dispensary to the location utilizing the route.
ApplicationCountryDate FiledStatusDate GrantedPatent Number
2.14/979,742United States06/29/2017Pending
For further information on the exclusive license of the Patent Applications above, and the complete text of the Intellectual Property Purchase Agreement, please refer to Exhibit 10.33 to the Company’s Current Report filed September 26, 2016 on Form 8-K.
- 37 -
III.The Company, through a License Agreements with ProEventa, Inc., acquired all rights, title and interest in and to the following patent and patent applications and the invention in its entirety:
1.Patent 10,061,812 (US)– “Platform for Optimizing Data Driven Outcomes”
A computer-based method for tracking outcome specific data specifically for optimizing, managing, and tracking data driven outcomes. Process utilizes a multi-dimensional platform to facilitate data-driven outcomes processes through assessment, goal development, data tracking, graphing, and re-evaluation. The process allows for optimization of best practices, successful actions, and success-based plan execution, which is identified through automatic data-mining and analysis as well as user specified parameters, algorithms, and analytics. The process can be utilized by a client, patient, student, service provider, program, product, service, device, organization, business, department, or so forth. The tool can be utilized across various industries for client behavior management, educational instruction, school improvement activities, program evaluation, organizational key performance indicators, financial management, weight management, tracking insurance claims, or so forth.
This patent application covers the intellectual property known as “REBOOT™”, which stands for “Reliable Evidence Based Outcomes Optimization Technologies,” a structured, scalable and sustainable software system used to identify, monitor, and evaluate a single user or an entire organization's progress towards mastery of any achievable task, objective or goal.
ApplicationCountryDate FiledStatusDate GrantedPatent Number
1.61/791,218United States03/15/2013ProvisionalN/AN/A - continued under 1a
1a.14/212,429United States03/14/2014Granted08/28/201810,061,812
1b.GB1517371.9United Kingdom03/14/2014Published12/02/2015Publication GB2526749
1c.2014228758Australia03/14/2014PublishedPublication 2014228758
1d.2014144749A1Worldwide09/18/2014Pending
For further information on the exclusive license of the Patent Application above, and the complete text of the Intellectual Property Purchase Agreement, please refer to Exhibit 10.33 to the Company’s Current Report filed May 4, 2017 on Form 8-K.
- 38 -
There can be no assurance that the Company will be granted patents for any of the patent applications it has filed with the USPTO or other patent organization worldwide.
The Company’s intellectual property portfolio rights will be protected and enforced.
A detailed third-party validity report is pending from a highly respected IP valuation group to identify infringement claims and possible damages within the Human Diagnostics and Behavioral Healthcare markets. The Company is reviewing various strategies to best monetize value for its shareholders through a variety of possible licensing strategies, which could include litigation. The Company is focused on possible non-dilutive litigation financing, as well as various hybrid contingency proposals, for a patent enforcement initiative that will begin to address damages stemming from infringement.
Expired Patents
The Target System and certain of its related components were previously issued patents by the USPTO. The following previously-issued patents have expired:
Patent # | Description | Date Filed in US | Date Expired |
US4,748,042 | Target ringing and spotting machine (method and apparatus for imprinting membrane with pattern of antibody) | May 31, 1988 | May 31, 2008 |
US4,797,260 | Target cassette (antibody testing system) | January 10, 1989 | January 10, 2009 |
US5,137,691 | Target cassette with removable air gap (antibody testing system with removable air gap) | August 11, 1992 | August 11, 2012 |
Trademarks
The Company will also utilize trademark applications to protect its Intellectual Property that may not be suitable for patent protection. Unlike patent applications, which in many cases must be filed in advance of a particular date, there is no specific date by which a trademark application must be filed. Instead, the time constraint is in a different direction. In the United States, an ordinary so-called “use” trademark application can only be filed after the goods or services have been in interstate commerce.
On January 2, 2016, trademark application 86/863,854 was filed with the USPTO for the word mark, “QOLPOM®”. On September 5, 2017, the trademark was registered, registration number 5279137.
On December 19, 2017, trademark application 87/726,201 was filed with the USPTO for the word mark, “Fotodigm®”. On September 25, 2018, the trademark was registered, registration number 5572183.
Facilities
The Company’s principal executive office is located at 1327 Ocean Avenue, Suite B, Santa Monica, California, 90401, with an additional office located at 28 West 36th Street, 8th Floor, New York, NY 10018. The Company’s telephone number is (310) 899-4442 (phone) and (888) 899-3966 (fax).
For additional information on the leased properties, see “ITEM 2. PROPERTIES“ section contained within this Annual Report.
Employees
As of December 31, 2019, the Company had 9 employees, inclusive of 4 executive officers. All 9 employees were full-time employees.
The Company currently has 9 full-time employees, inclusive of 4 executive officers. All 9 employees are full-time employees.
Research and Development
The Company incurred $76,617 and $74,000 in expenditures during 2019 and 2018, respectively, relating to the research and development of its proprietary medical technology, including costs for patent consultants and filings, and costs incurred to develop new products.
- 39 -
The Company is not required to deliver an Annual Report to its stockholders, but will voluntarily send an Annual Report, together with its annual audited financial statements upon request. The Company is required to file annual, quarterly and current reports, proxy statements, and other information with the Securities and Exchange Commission. The Company’s Securities and Exchange Commission filings are available to the public over the internet at the SEC's website at www.sec.gov.
The public may read and copy any of the Company’s materials filed with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The Company is an electronic filer. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The internet address of the site is www.sec.gov.
Although the Company is a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and is not required to provide the information under this item, the following information is being provided:
COVID-19 Pandemic
In December 2019, an outbreak of the COVID-19 virus was reported in Wuhan, China. On March 11, 2020, the World Health Organization (“WHO”) declared the COVID-19 virus a global pandemic, and on March 13, 2020, President Donald J. Trump declared the virus a national emergency in the United States. As of the date of the filing of this Annual Report, the WHO reports over 4 million confirmed COVID-19 cases and over 275,000 deaths worldwide, including over 75,000 in the U.S. This highly contagious disease has spread to most of the countries in the world and throughout the United States, creating a serious impact on customers, workforces and suppliers, disrupting economies and financial markets, and potentially leading to a world-wide economic downturn. It has caused a disruption of the normal operations of many businesses, including the temporary closure or scale-back of business operations and/or the imposition of either quarantine or remote work or meeting requirements for employees, either by government order or on a voluntary basis.
The COVID-19 pandemic may adversely affect the Company’s customers’ operations, its employees and its employee productivity. It may also impact the ability of the Company’s subcontractors, partners, and suppliers to operate and fulfill their contractual obligations, and result in an increase in costs, delays or disruptions in performance. These effects, and the direct effect of the virus and the disruption on the Company’s employees and operations, may negatively impact both the Company’s ability to meet customer demand and its revenue and profit margins. The Company’s employees, in many cases, are working remotely and using various technologies to perform their functions. The Company might experience delays or changes in customer demand, particularly if customer funding priorities change. Further, in reaction to the spread of COVID-19 in the United States, many businesses have instituted social distancing policies, including the closure of offices and worksites and deferring planned business activity. Additionally, the disruption and volatility in the global and domestic capital markets may increase the cost of capital and limit the Company’s ability to access capital. Both the health and economic aspects of the COVID-19 virus are highly fluid and the future course of each is uncertain. For these reasons and other reasons that may come to light if the coronavirus pandemic and associated protective or preventative measures expand, the Company may experience a material adverse effect on its business operations, revenues and financial condition; however, its ultimate impact is highly uncertain and subject to change.
None.
The Company’s principal executive office is located at 1327 Ocean Avenue, Suite B, Santa Monica, CA 90401. On May 1, 2019, the Company leased an additional office located at 28 West 36th Street, 8th Floor, New York, NY 10018. As of December 31, 2019, the Company sub-leases the Santa Monica office for approximately $5,600 a month, and sub-leases the New York office for approximately $8,900 a month. This space is sufficient to meet its needs at December 31, 2019. However, once the Company expands its business to a significant degree, it will require additional space. The Company does not currently own any real estate.
The pharmacy operations located at 465 N. Roxbury Drive, Beverly Hills, CA 90210 ceased in December 2017, and the space was vacated in January 2018.
Dispute with Landlord-Beverly Hills, CA
Upon the completion of the acquisition of RoxSan Pharmacy, Inc., in August 2015, the Company became aware that the former owner, Shahla Melamed (“Melamed”), among other things, failed to properly notify the landlord or the Roxbury Drive property owners (the “Lessors”) of the change in ownership of the pharmacy, as required in the lease agreements. Subsequently, in an effort to unwind the acquisition of the pharmacy, Melamed attempted to undermine the Company’s efforts to obtain any lease assignment or new lease from the Lessors. As a result, no lease assignments or lease agreements were made as of December 31, 2017, and the Company vacated the space in January 2018.
- 40 -
From time to time, the Company may be involved in litigation relating to claims arising out of its operations in the normal course of business. The Company knows of no material, existing or pending legal proceedings against it, nor are the Company involved as a plaintiff in any material proceeding or pending litigation, beyond those defined below. There are no proceedings in which any of the Company’s directors, officers or affiliates, or any registered or beneficial shareholder, is an adverse party or has a material interest that is adverse to the Company’s interests.
Dispute with Former Owner of RoxSan
In October 2015, shortly following the Company’s acquisition of RoxSan, Shahla Melamed (“Melamed”), initiated two (2) legal actions against the Company in the Superior Court of the State of California, County of Los Angeles, West District, Shahla Melamed v. Parallax Health Sciences, Inc., action numbers SC 124873 and SC 125702. In the matter, action No. SC 124873, Melamed sought rescission of the August 13, 2015, Purchase Agreement. In the Matter, action No. SC125702, Melamed alleges that the Company is in default under the terms of the Purchase Agreement and Secured Note, and the Company’s termination of Melamed’s employment agreement.
The Company also initiated legal action against Melamed and filed a complaint in October 2015, action number SC 124898, in the Superior Court of the State of California, County of Los Angeles, West District, Parallax Health Sciences, Inc., et al. v. Shahla Melamed, et al The complaint in that action alleges that Melamed breached several obligations under the Purchase Agreement, and the Company sought to reduce the Secured Note due to undisclosed material changes in the business.
In January 2019, Melamed requested mediation, seeking settlement of the pending litigation with the Company, including that which was initiated against the Company by her son, Hootan Melamed (Shahla and Hootan, collectively, the “Melameds”). Through mediation, the Company and the Melameds reached agreeable settlement terms, and on February 19, 2020, the Company received a counter-signed Settlement and Release Agreement (the “Settlement”). Effective February 12, 2020 (the “Effective Date”), the Settlement is by and between Parallax Health Sciences, Inc., RoxSan Pharmacy, Inc., Michael Redmond, Edward Withrow III, Huntington Chase Financial Group, LLC, Calli Bucci and Dave Engert (collectively, “Parallax”), and Shahla Melamed and Hootan Melamed (collectively, the “Melameds”), and resolves all pending lawsuits between the parties in connection with the acquisition of RoxSan Pharmacy.
In consideration of the resolution of all existing and potential claims, including the cancellation of the Company’s contingent liability in the principal sum of $20,500,000, and accrued interest of approximately $4,500,000, and without further action or litigation and without admission of liability by either party, the Settlement terms include the following:
●A payment of $4,000,000 (the “Settlement Sum”) to the Melameds, to be paid as follows:
$1,250,000 within 90 days of the Effective Date;
$1,250,000 within one (1) year of the Effective Date;
$1,500,000 within two (2) years of the Effective Date.
●The issuance of ten (10) million shares of the Company’s Common Stock to an entity owned by Shahla Melamed.
In addition, in the event forty percent (40%) or more of the Company and/or its subsidiaries (including by way of merger) is sold within two (2) years of the Effective Date, the Company shall pay the Melameds, within two (2) weeks of receipt of the proceeds from such sale (the “Sale Proceeds”), any outstanding unpaid Settlement Sum plus an additional 10% of the Sale Proceeds received, up to a total of an additional $3,000,000 over and above the Settlement Sum.
In the event the Company fails to cure a breach of timely payment of any portion of the Settlement Sum within thirty (30) days of a notice of default, a Stipulated Judgement may be filed by Melamed in the sum of $20,000,000, less any Settlement Sum amounts previously paid by the Company.
RoxSan Dissolution
On May 14, 2018, pursuant to unanimous resolutions of the boards of directors of RoxSan Pharmacy, Inc. and Parallax Health Sciences, Inc., RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California (the “Court”). Mr. Timothy Yoo was appointed trustee (“Trustee”) on May 15, 2018. In connection with this filing, RoxSan seeks to discharge approximately $5 million of liabilities owed to various parties, and intercompany loans in excess of $1 million owed to Parallax. The Chapter 7 bankruptcy proceeding by RoxSan Pharmacy, Inc. was fully discharged and the case was closed on March 13, 2019, in U.S. Bankruptcy Court, Central District of California.
Due to, among other things, the reduction in RoxSan’s cash flows during 2016 and 2017, RoxSan became delinquent in its payroll tax depository obligations, resulting in a liability owed to federal and state taxing agencies in the aggregate of $1,148,811, which includes $601,148 in taxes withheld from employees (“Trust Fund Taxes”), employer taxes of $183,172, and penalties and interest of $364,491 through December 31, 2018. The liability was included as part of the Chapter 7 bankruptcy petition, and certain portions of the liability may be discharged. However, in accordance with California bankruptcy laws, federal and state Trust Fund Taxes are not dischargeable. The Company has retained a tax resolution specialist and is in communications with the taxing agencies in order to resolve RoxSan’s liability. During the year ended December 31, 2019, payments for Trust Fund Taxes in the amount of $485,498 were made, and $115,650 in Trust Fund Taxes were outstanding at December 31, 2019.
Disputes with Former Executives
●Action No. CV2017-052804
On March 9, 2017, Mr. Dave Engert filed a lawsuit in Arizona and then later changed the venue to Federal Court in Southern California claiming, among other issues, that monies are owed to him under his Consulting Agreement and that his termination was without cause. On October 23, 2017, the Company filed a response and counterclaims against Mr. Engert for an amount exceeding $100,000. The counterclaims include possible fraud and negligence committed by Mr. Engert and Mr. J. Michael Redmond, former successor Chairman of Mr. Engert, director, President and Chief Executive Officer of the Company and former President, Chief Executive Officer, Chairman and director of RoxSan Pharmacy, Inc.
On October 8, 2018, a settlement was reached between Mr. Engert and the Company (the “Engert Settlement”). The Engert Settlement includes, among other things, a cash payment to Mr. Engert in the amount of $139,000, and the cancellation of all of Mr. Engert’s equity holdings in the Company. The Engert Settlement resulted in a net loss to the Company of $33,272. On April 10, 2019, a stipulation for dismissal was filed, and the matter has been fully resolved.
●Action No. BC700070
On March 28, 2018, Mr. J. Michael Redmond filed a lawsuit against the Company and RoxSan Pharmacy, Inc. in the United States District Court, Central District of California for an amount exceeding $75,000. The Company intends to vigorously defend against this action. There are counterclaims that include possible fraud and negligence committed by Mr. Redmond, former President, Chief Executive Officer, Chairman and director of the Company and of RoxSan Pharmacy, Inc. An Arbitration is still pending.
Disputes with Lenders
●On March 18, 2020, the Company initiated legal action and filed a complaint against EMA Financial, LLC (“EMA”) a third-party lender, in the US District Court, Southern District of New York, case index number 20-cv-02375, citing fraud, unjust enrichment, and securities law violations, among other things. On April 27, 2020, EMA filed an answer substantially denying the Company’s claims, and filed counterclaims against the Company, including damages in excess of $2 million. The matter is currently pending.
There are two (2) legal matters currently pending.
Not applicable.
- 41 -
ITEM 5.MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS |
The Company’s Common Stock is quoted on the OTC Markets under the trading symbol PRLX.QB. The following table sets forth the high and low bid prices for the Company’s Common Stock per quarter as reported by the OTCQB for the last two years. These prices represent quotations between dealers without adjustment for retail mark-up, markdown or commission and may not represent actual transactions.
Quarter Ended | High | Low |
December 31, 2019 | $0.08 | $0.07 |
September 30, 2019 | $0.16 | $0.13 |
June 30, 2019 | $0.10 | $0.10 |
March 31, 2019 | $0.06 | $0.06 |
December 31, 2018 | $0.11 | $0.08 |
September 30, 2018 | $0.13 | $0.13 |
June 30, 2018 | $0.21 | $0.15 |
March 31, 2018 | $0.24 | $0.16 |
The Company’s Common Stock is subject to rules adopted by the Commission regulating broker dealer practices in connection with transactions in “penny stocks.” Those disclosure rules applicable to “penny stocks” require a broker dealer, prior to a transaction in a “penny stock” not otherwise exempt from the rules, to deliver a standardized list disclosure document prepared by the Securities and Exchange Commission. That disclosure document advises an investor that investment in “penny stocks” can be very risky and that the investor’s salesperson or broker is not an impartial advisor but rather paid to sell the shares. The disclosure contains further warnings for the investor to exercise caution in connection with an investment in “penny stocks,” to independently investigate the security, as well as the salesperson with whom the investor is working and to understand the risky nature of an investment in this security. The broker dealer must also provide the customer with certain other information and must make a special written determination that the “penny stock” is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. Further, the rules require that, following the proposed transaction, the broker provide the customer with monthly account statements containing market information about the prices of the securities.
Record Holders
The Company’s Common Stock is issued in registered form. At December 31, 2019, the registrar and transfer agent for the Company’s Common Stock was Action Stock Transfer Corp., 2469 E. Fort Union Blvd., Suite 214, Salt Lake City, UT 84121 (Telephone 801-274-1088, Facsimile 801-274-1099).
Effective April 1, 2020, the registrar and transfer agent for the Company’s Common Stock is ClearTrust, LLC, 16540 Pointe Village Drive, Suite 205, Lutz, FL 33558 (Telephone: 813-235-4490, Facsimile: 813-388-4549).
As of December 31, 2019, an aggregate of 250,124,755 shares of the Company’s Common Stock were issued and outstanding. Pursuant to Action Stock Transfer Corp., the Company’s shareholders' list at December 31, 2019, showed 153 registered shareholders and 220,849,503 shares of Common Stock issued and outstanding. This total does not include 29,275,252 issued shares pending stock certificates, of which 2,605,252 are in Common Stock awards and purchases, 21,620,000 are debt conversions, 300,000 are in connection with debt settlements, and 4,750,000 which were unvested at December 31, 2019.
As of December 31, 2019, an aggregate of 977,352 shares of the Company’s Preferred Stock were issued and outstanding and are held by 9 shareholders. The 823,691 shares of Series A Preferred Stock and 40,000 shares of Series B Preferred Stock are convertible into the Company’s Common Stock at a conversion rate of 20 shares of Common Stock for each share of Preferred Stock held. The 150,000 shares of Series C Preferred Stock are convertible into the Company’s Common Stock at a conversion rate of 41.67 shares of Common Stock for each share of Preferred Stock held. Shares of Series B and Series C Preferred Stock include 50% warrant coverage (see Warrants).
Trading Suspension
On April 13, 2020, the Company received an Order of Suspension of Trading dated April 10, 2020 (the “Order”) from the United States Securities and Exchange Commission (“SEC”). The temporary suspension period is from 9:30 a.m. EDT on April 13, 2020, through 11:59 p.m. EDT on April 24, 2020. The Order refers to questions raised regarding the accuracy of the Company’s recent press releases in relation to the Company’s development of a rapid screening test for COVID-19, and the Company’s access to “large quantities of COVID-19 diagnostics testing kits and personal protective equipment.”
In an effort to protect the interests of shareholders, the SEC has issued similar orders and suspensions recently to several registrants, with concerns over the validity of claims made in connection with the availability of COVID-19 tests and supplies.
- 42 -
The Company, along with its counsel, is cooperating fully with the SEC to substantiate the Company’s recent public announcements and business endeavors, and is addressing any questions and/or concerns raised regarding the accuracy of the assertions made in the Company’s press releases.
Pursuant to Rule 15c2-11 under the Exchange Act, at the termination of the trading suspension, no quotation may be entered unless and until the Company has strictly complied with all provisions of the rule, including the filing of a new Form 15c2-11 with FINRA.
The Company is required to file its Annual Report with the SEC for the purposes of satisfying its financial reporting requirements. However, in addition to the Company's reporting obligations, the Company must have a FINRA Member Market Maker file a 15c2-11 with FINRA in order for the Company’s shares to resume trading on the OTCQB market. These actions do not impact or otherwise affect the Company's results of operations or disclosures as set out in this Annual Report. The Company believes that this Annual Report fully complies with the requirements of the Securities Exchange Act of 1934, as amended and, in accordance with generally accepted accounting principles, that it fairly presents, in all material respects, the financial condition and results of operations of the Company as at the relevant dates.
As of the date of filing of this Annual Report, the trading suspension period expired, and the Company is in the process compiling the information required for its 15c2-11 submission by a FINRA Member Market Maker. The Company anticipates the filing of a new Form 15c2-11 within the next 30 days. If any party has any questions as to whether the Company has complied with the rule, they should contact the staff in the Division of Trading and Markets, Office of Interpretation and Guidance, at (202) 551-5777.
These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for its common stock. Many brokers may be unwilling to engage in transactions in its common stock because of the added disclosure requirements, thereby making it more difficult for stockholders to dispose of their shares.
Dividends
The Company has never declared or paid dividends on its Common Stock, nor does the Company anticipate paying dividends on its Common Stock in the foreseeable future. Instead, the Company anticipates that all of its earnings, if any, will be used or the operation and growth of its business. Further, any credit agreement the Company may expect to enter into may restrict its ability to pay dividends or make distributions to its stockholders. Any future determination to pay dividends will be at the discretion of the Board and will depend on the financial position, results of operations, cash flows, capital requirements, debt covenants, applicable law and other factors as the Board deems relevant.
Dividends are payable semi-annually on the Company’s Series A Preferred Stock at a rate of 7% per annum, 10% per annum on Series B Preferred Stock, and 8% per annum on Series C Preferred Stock. Dividends may be paid in kind, at the option of Parallax, to the extent that if the Company is not legally permitted to distribute cash dividends, the Company shall pay dividends in the form of shares of Preferred Stock equal to the amount of the dividend. No dividends have been declared on the Company’s Preferred Stock. Dividends in the amount of $422,761, representing cumulative dividends on Parallax’s Preferred Stock, were earned as of December 31, 2019, and are issuable in kind into 13,418,550 shares of Common Stock.
Equity Compensation Plan Information
In June 2019, pursuant to a resolution of the board of directors, the Company adopted the 2019 Stock Incentive Plan (the “2019 Plan”), wherein forty million (40,000,000) shares of the Company’s restricted Common Stock were reserved for issuance. The 2019 Plan was intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. The 2019 Plan is currently administered by the Company's Board. Subject to the provisions of the plan, the board will determine who shall receive options, the number of shares of Common Stock that may be purchased under the options. On June 17, 2019, the Company filed a Registration Statement on form S-8 for the registration of shares reserved under the 2019 Plan.
As of December 31, 2019, the Company has granted options to purchase an aggregate of 26,685,000 shares of its Common Stock, net of options exercised, expired and forfeited. In connection with the options granted, a total of $3,927,006 has been recorded as deferred stock option compensation, of which $1,655,840 was expensed in prior years, and $718,392 was expensed in 2019. There remains $1,552,774 in deferred stock option compensation to be expensed over the next twenty-one (18) months.
Warrants
In connection with 150,000 shares of Series C Preferred Stock issued in 2018, the Company issued warrants to purchase 3,125,000 shares of Common Stock at an exercise price of $0.25 per share for a period of three (3) years.
In connection with certain convertible promissory notes issued in 2018, the Company issued warrants to purchase 25,000 shares of Common Stock at an exercise price of $0.25 per share for a period of three (3) years.
In connection with certain consulting agreements entered into in 2018, the Company issued warrants to purchase 450,000 shares of Common Stock at an exercise price of $0.25 per share for a period of three (3) years.
- 43 -
In connection with certain convertible promissory notes issued in 2018, as amended, the Company issued warrants to purchase 9,377,500 shares of Common Stock, including 3,377,500 issued to related parties, at an exercise price of $0.10 per share for a period of three (3) years. In 2019, 4,250,000 of the warrants were cancelled in connection with a debt settlement. There remains 5,127,500 warrants exercisable.
In connection with a certain consulting agreement entered into in 2018, the Company issued warrants to purchase 300,000 shares of Common Stock at an exercise price of $0.001 for a period of five (5) years.
In connection with a certain consulting agreement entered into in 2018, the Company issued warrants to purchase 125,000 shares of Common Stock at an exercise price of $0.25 for a period of three (3) years.
In connection with certain convertible debentures issued in 2018, the Company issued warrants to purchase 600,000 shares of Common Stock at an exercise price of $0.15 per share for a period of five (5) years. The warrants were subsequently cancelled in 2019 in connection with a debt settlement.
In connection with a certain consulting agreement entered into in 2018, the Company issued warrants to purchase 75,000 shares of Common Stock at an exercise price of $0.01 for a period of two (2) years.
In connection with 40,000 shares of Series B Preferred Stock, the 400,000 underlying warrants have expired.
In connection with certain convertible promissory notes issued in 2019, the Company issued warrants to purchase 3,200,000 shares of Common Stock at an exercise price of $0.15-$0.20 for a period of five (5) years.
In connection with a certain 2019 Securities Purchase Agreement, the Company issued warrants to purchase up to 13,500,000 shares of Common Stock at an exercise price of $0.25 per share for a period of two (2) years.
In connection with a certain Simple Agreement Future Equity (“SAFE”) offering, the Company issued warrants to purchase 18,000,000 shares of Common Stock at an exercise price of $0.25 for a period of three (3) years.
In connection with a certain consulting agreement entered into in 2019, the Company issued warrants to purchase 250,000 shares of Common Stock at an exercise price of $0.25 for a period of three (3) years.
In connection with a certain promissory note issued in 2019, the Company issued warrants to purchase 2,528,413 shares of Common Stock at an exercise price of $0.1046 for a period of five (5) years.
In connection with certain consulting agreements entered into in 2019, the Company issued warrants to purchase 500,000 shares of Common Stock at an exercise price of $0.01 for a period of three (3) years.
In connection with a certain promissory note issued in 2019, the Company issued warrants to purchase 2,500,000 shares of Common Stock at an exercise price of $0.10 for a period of five (5) years.
In connection with a certain promissory note issued in 2019, the Company issued warrants to purchase 100,000 shares of Common Stock at an exercise price of $0.12 for a period of three (3) years.
In connection with a certain 2019 Securities Purchase Agreement, the Company issued warrants to purchase 125,000 shares of Common Stock at an exercise price of $0.12 for a period of two (2) years.
As of December 31, 2019, the Company had 56,785,913 warrants issued and outstanding. The number of shares of Common Stock underlying the warrants and the exercise price are subject to adjustment upon certain events.
Purchase of Equity Securities by the Issuer and Affiliated Purchasers
The Company did not purchase any shares of its Common Stock or other securities registered by the Company during the year ended December 31, 2019.
Recent Sales of Unregistered Securities
The following represents all unregistered securities issued by the registrant during the current period, including sales of reacquired securities, as well as new issues, securities issued in exchange for property, services, or other securities, and new securities resulting from the modification of outstanding securities:
On January 24, 2019, in connection with a certain senior secured promissory note, the Company issued 150,000 shares of its restricted Common Stock to the note holders as a form of interest. The shares were valued at $15,000.
On January 30, 2019, in connection with a certain convertible debenture, the holder elected to convert $175,000 into 1,750,000 shares of its restricted Common Stock at a conversion rate of $0.10 per share.
On January 31, 2019, in connection with a certain consulting agreement, the Company issued 1,666,667 shares of its restricted Common Stock to the consultant for services valued at $200,000.
- 44 -
On January 31, 2019, in connection with a Simple Agreement Future Equity (“SAFE”) offering, the Company issued 800,000 shares of its restricted Common Stock at $0.0625 per share for $50,000 cash.
On February 6, 2019, in connection with certain convertible debt in the amount of $20,000 and accrued interest in the amount of $2,000, the Company issued 220,000 shares of its restricted Common Stock at a conversion rate of $0.10 per share.
In 2019, in connection with a certain senior secured promissory note, the Company issued 180.000 shares of its restricted Common Stock to the note holders as a form of interest. The shares were valued at $18,000.
On February 25, 2019, in connection with a SAFE offering, the Company issued 6,000,000 shares of its restricted Common Stock at $0.0625 per share for $375,000 cash.
In 2019, in connection with a certain consulting agreement, the Company issued 172,656 shares of its restricted Common Stock to the consultant for services valued at $18,750.
On February 27, 2019, in connection with a certain consulting agreement, the Company issued 1,000,000 shares of its restricted Common Stock to the consultant for services valued at $120,000.
In March 2019, in connection with a certain convertible debenture, the holder elected to convert $95,000 into 1,719,328 shares of the Company’s restricted Common Stock.
On March 25, 2019, in connection with a SAFE offering, the Company issued 1,200,000 shares of its restricted Common Stock at $0.0625 per share for $75,000 cash.
In April 2019, in connection with a certain convertible debenture, the holder elected to convert $105,000 into 2,340,410 shares of the Company’s restricted Common Stock.
On April 5, 2019, in connection with a certain services agreement, the Company issued 600,000 shares of its restricted Common Stock for services valued at $44,160.
On April 15, 2019, the Company issued 400,000 shares of its restricted Common Stock for services valued at $50,000.
On April 25, 2019, in connection with a SAFE offering, the Company issued 3,200,000 shares of its restricted Common Stock at $0.625 per share for $200,000 cash.
On April 26, 2019, in connection with a certain stock purchase agreement, the Company issued 400,000 shares of its restricted Common Stock, valued at $28,000, for cash in the amount of $400.
On April 29, 2019, in connection with a certain private placement agent agreement, the Company issued 1,000,000 shares of its restricted Common Stock to the consultant for services valued at $71,000.
On May 2, 2019, in connection with a SAFE offering, the Company issued 800,000 shares of its restricted Common Stock at $0.0625 per share for $50,000 cash and $15,000 in services.
On May 6, 2019, in connection with an equity funding, the Company issued 12,000,000 shares of its restricted Common Stock for cash in the amount of $1,000,000. The shares were included in the Company’s recent Registration Statement filed on Form S-1, with an effective date of December 20, 2019.
On May 8, 2019, in connection with a certain convertible promissory note in the principal sum of $20,000, the Company issued 289,017 shares of its restricted Common Stock.
On May 15 2019, in connection with the conversion of 57,500 Series A Preferred Stock valued at $69,000, the Company issued 1,150,000 shares of its restricted Common Stock at a conversion ratio of 20 shares of Common Stock for each share of Series A Preferred Stock held.
On May 15, 2019, in connection with an employment agreement, the Company issued 125,000 shares of the Company’s restricted Common Stock, valued at $8,388, for cash in the amount of $125.
On May 15, 2019, in connection with the executive employment agreement, the Company issued David Appell, the Chief Operating Officer, 3,000,000 shares of the Company’s restricted Common Stock for cash in the amount of $3,000. The shares were valued at $201,300, of which 25% vest immediately, and the remaining vest when the Company achieves certain earnings goals.
In 2019, in connection with a certain convertible debenture, the holder elected to convert $95,142 into 2,983,970 shares of the Company’s restricted Common Stock.
On June 11, 2019, in connection with a settlement for the retirement of 3,666,670 warrants (4,401,760 warrants, as adjusted under anti-dilution provisions), the Company issued 1,000,000 shares of the Company’s restricted Common Stock, valued at $71,000.
- 45 -
On June 20, 2019, in connection with the cashless exercise of certain warrants, the Company issued 600,000 shares of the Company’s restricted Common Stock, valued at $63,600.
On June 20, 2019, in connection with a certain consulting agreement, the Company issued 2,000,000 shares of its restricted Common Stock to the consultant for services valued at $212,000.
In July 2019, in connection with certain promissory notes in the amount of $220,000, the Company issued 1,200,000 shares of its restricted Common Stock, valued at $190,200.
In July 2019, in connection with the settlement of certain related party convertible debentures, the Company issued 1,380,811 shares of its restricted Common Stock, valued at $131,315.
In August 2019, in connection with an investment in Global Career Network, Inc., the Company issued 6,666,667 shares of its restricted Common Stock for $0.15 per share, valued at $1,000,000.
In September 2019, in connection with the conversion of certain convertible debt in the amount of $437,000, the Company issued 4,370,000 shares of its restricted Common Stock to Huntington Chase, LLC, a beneficial owner.
In September 2019, in connection with the SAFE offering, the Company issued 6,000,000 shares of its restricted Common Stock to four officers/directors in exchange for the reduction of accrued compensation in the amount of $375,000.
In 2019, in connection with certain consulting agreements, the Company issued 1,392,596 shares of its restricted Common Stock for services valued at $122,250.
In 2019, in connection with certain stock subscriptions, the Company issued 500,000 shares of its restricted Common Stock for cash in the amount of $27,500.
In 2019, in connection with the conversion of certain convertible debt in the amount of $722,583, the Company issued 11,654,492 shares of its restricted Common Stock.
In December 2019, the Company issued 100,000 shares of its restricted Common Stock for services valued at $5,000.
In December 2019, in connection with the conversion of certain accrued compensation, the Company issued 12,000,000 shares of its restricted Common Stock in exchange for the reduction of accrued compensation in the amount of $600,000, including 8,000,000 shares issued to related parties for accrued compensation in the amount of $400,000.
As of December 31, 2019, a total of $3,469,313 in deferred stock compensation was recorded, of which $2,123,691 was expensed in prior years, and $685,553 was expensed in 2019. There remains $660,069 in deferred stock compensation as of December 31, 2019, to be expensed over the next eighteen (18) months.
Exemption From Registration. None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. Unless otherwise stated, the sales of the above securities were deemed to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act (and Regulation D or Regulation S promulgated thereunder) as transactions by an issuer not involving any public offering. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to, or for sale in connection with, any distribution thereof, and appropriate legends were placed on the share certificates issued in these transactions. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
The Company is a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and is not required to provide the information under this item.
Item 7.management’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Forward-Looking Statements
This Annual Report contains forward-looking statements. These statements relate to future events or the future financial performance of Parallax Health Sciences, Inc. (“Parallax” or the “Company”), and include statements made by the Company regarding pharmaceutical insurance reimbursements, state licenses, product development and obtaining FDA clearances. In some cases, forward-looking statements can be identified by terminology such as “may”, “should”, “expects”, “plans”, “anticipates”, “believes”, “estimates”, “predicts”, “potential” or “continue” or negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors” that may cause the Company’s or its industry’s actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Except as required by applicable law, including the securities laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results.
- 46 -
Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results.
Unless otherwise specified, all dollar amounts are expressed in United States Dollars and are prepared in accordance with United States Generally Accepted Accounting Principles. All references to “Common Stock” refer to the common shares; and “Preferred Stock” refer to the preferred shares; of the Company’s capital stock.
You should read the following discussion of the Company’s results of operations and financial condition with the consolidated financial statements and related notes included elsewhere in this Annual Report. The Company intends for this discussion to provide you with information that will assist you in understanding the Company’s financial statements, the changes in certain key items in those financial statements from year to year, and the primary factors that accounted for those changes.
NOTE: The following sections of this report and any further reference made to “the Company”, “we”, “us”, “our” and “Parallax “ shall mean Parallax Health Sciences, Inc., and its wholly-owned subsidiaries, Parallax Diagnostics, Inc., Parallax Health Management, Inc., Parallax Behavioral Health, Inc., and Parallax Communications, Inc., unless otherwise indicated. The Company’s former wholly-owned subsidiary, RoxSan Pharmacy, Inc., was derecognized effective May 14, 2018. (See “RoxSan Pharmacy” and “Legal Proceedings” sections contained within this Annual Report.)
Corporate History
The Company was incorporated in the State of Nevada on July 6, 2005. On November 1, 2012, we, formerly Endeavor Power Corporation, and its wholly-owned subsidiary Endeavor Holdings, Inc., a Nevada corporation, entered into an Agreement and Plan of Merger with Parallax Diagnostics, Inc., a Nevada corporation (“Parallax Diagnostics”), whereby Parallax Diagnostics became a wholly-owned subsidiary. On January 9, 2014, the Company changed its name to Parallax Health Sciences, Inc. (“Parallax”). (OTCQB.PRLX)
The Parallax business was founded on the Company’s point of care diagnostic business, Parallax Diagnostics, Inc., in 2010, when the Company acquired the right, title, and interest, through an exclusive license with Montecito BioSciences, Ltd. (“MBS”), to develop, manufacture and commercialize the Target System, an immunoassay point-of-care diagnostic testing system. Concurrently, through an Assignment Agreement with MBS, the Company acquired the right, title, and interest to twenty-five (25) FDA-cleared tests in the area of infectious disease, medical conditions, drugs of abuse, cardiac and pregnancy, that are designed to be utilized with the Target System.
In August 2015, the Company acquired RoxSan Pharmacy, Inc. (“RoxSan” or the “Pharmacy”). In December 2017, the Pharmacy ceased operations, and in May 2018, RoxSan filed a Chapter 7 bankruptcy petition. See “RoxSan Dissolution” below.
On August 31, 2016, the Company entered into an agreement with Qolpom®, Inc., an Arizona corporation in the remote healthcare monitoring and telehealth business (“Qolpom®”) and its shareholders (the “Seller”) to purchase 100% of the issued and outstanding shares of Qolpom®’s common stock and its assets, inventory and intellectual property. The agreement was fully executed on September 20, 2016, and the transaction was completed. The consideration for the acquisition resulted in a fair market value of $290,000, and goodwill of $785,060. In addition, the agreement included contingent royalties and revenue sharing for future revenues generated from the Qolpom® technology. The Qolpom® name was later changed to Parallax Health Management, Inc. (“PHM”).
On March 22, 2017, the Company formed Parallax Behavioral Health, Inc. (“PBH”), a Delaware corporation and wholly-owned subsidiary, and on April 26, 2017, completed the asset acquisition of 100% of certain intellectual property (“Intellectual Property”) from ProEventa Inc., a Virginia Corporation (“ProEventa”), in accordance with the Intellectual Property Purchase Agreement between the Company, PBH and ProEventa (the “ProEventa Agreement”). ProEventa has an expertise in the development of behavioral health technologies, and is the wholly-owned subsidiary of Grafton Integrated Health Network, Inc., a non-profit Virginia corporation (“Grafton”). Pursuant to the ProEventa Agreement, the initial consideration for the Intellectual Property was paid to ProEventa in the form of a stock purchase agreement to purchase 2,500,000 shares of the Company’s Common Stock for $2,500, resulting in a net cost for the Intellectual Property of $622,500. In addition, the Agreement included conditional contingent royalties and revenue sharing for future revenues generated from the Intellectual Property.
On September 20, 2018, the Company formed Parallax Communications, Inc, a Delaware corporation and wholly-owned subsidiary of Parallax Health Management, Inc.
Effective December 24, 2018, pursuant to a majority shareholder consent, the Company increased its authorized Common Stock from 250,000,000 shares to 500,000,000 shares, with a par value of $0.001 per share. On January 28, 2019, the amended articles of incorporation were filed with the state of Nevada.
On August 28, 2019, the Company entered into a Purchase Agreement (the “Purchase Agreement”) with Global Career Networks Inc., a Delaware corporation, (“GCN”) to acquire a 19% interest in GCN. The Purchase Agreement was fully executed on September 6, 2019, with an effective date of October 15, 2019 (the “Effective Date”). Pursuant to the Purchase Agreement, in exchange for 6,666,667 shares of the Company’s restricted Common Stock, valued at $1,000,000, the Company acquired 760 shares of GCN common stock. In addition, in the event the market value of Parallax Common Stock one (1) year from Effective Date, is greater than $0.075 per share but less than $0.30 per share, the Company is required to issue GCN up to an additional 20,000,000 shares of restricted Common Stock, for an aggregate value of the Company’s Common Stock held by GCN of $2,000,000. At December 31, 2019, the Company recorded an impairment on the investment of $1,000,000.
- 47 -
In April 2019, the Company entered into an employment agreement with Mr. David Appell to serve as the Company’s Chief Operating Officer. The agreement commenced May 15, 2019, is for an initial term of two (2) years, and provides a base compensation of $250,000 year one, and $275,000 in year two, as well as various performance bonuses, and customary employee benefits. In addition, the agreement includes a grant to purchase 3,000,000 restricted common shares, valued at $201,300, for cash in the amount of $3,000, of which 25% vest immediately, and the remainder vest when certain earnings goals are met; as well as options granted to purchase 3,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options, valued at $195,600 using the Black-Scholes method, are for a period of five (5) years, and vest annually over the term of the agreement, with an initial vesting of 25%. The assumptions used in valuing the options were: expected term 4.75 years, expected volatility 2.21, risk free interest rate 2.15%, and dividend yield 0%. On February 2, 2020, Mr. Appell resigned as the Company’s Chief Operating Officer. His resignation was not the result of a disagreement with the Company on any matters relating to the Company's operations, policies, or practices. Concurrent with his resignation as COO, Mr. Appell will serve as Managing Director of the Company’s wholly-owned subsidiary, Parallax Communications, Inc. (“PCOM”). As Managing Director, Mr. Appell will provide business advisory and strategic planning advice to the Company with PCOM. In exchange for these services, Mr. Appell will be paid $2,000 per month plus medical benefits.
In May 2019, the Company established a second location at 28 West 36th Street, 8th Floor, New York, NY 10018.
In June 2019, the Company filed a Registration Statement on form S-1 for the registration, as amended, of 32,583,436 shares of Common Stock, and, after resolving the comments and questions from the SEC in relation to the filing, the Company received an effective date from the SEC of December 20, 2019.
In June 2019, pursuant to a resolution of the board of directors, the Company adopted the 2019 Stock Incentive Plan (the “2019 Plan”), wherein forty million (40,000,000) shares of the Company’s restricted Common Stock were reserved for issuance. The 2019 Plan was intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. The 2019 Plan is currently administered by the Company's Board. Subject to the provisions of the plan, the board will determine who shall receive options, the number of shares of Common Stock that may be purchased under the options. On June 17, 2019, the Company filed a Registration Statement on form S-8 for the registration of shares reserved under the 2019 Plan.
Effective February 12, 2020 (the “Effective Date”), the Company and Shahla Melamed and Hootan Melamed (collectively the “Melameds”) reached agreeable settlement terms in connection with the pending legal actions between the parties. The Settlement is by and between Parallax Health Sciences, Inc., RoxSan Pharmacy, Inc., Michael Redmond, Edward Withrow III, Huntington Chase Financial Group, LLC, Calli Bucci and Dave Engert (collectively, “Parallax”), and the “Melameds, and resolves all pending lawsuits between the parties in connection with the acquisition of RoxSan Pharmacy. In consideration of the resolution of all existing and potential claims, including the cancellation of the Note in the principal sum of $20,500,000, and accrued interest of approximately $4,500,000, and without further action or litigation and without admission of liability by either party, the Settlement terms include the following:
●A payment of $4,000,000 (the “Settlement Sum”) to the Melameds, to be paid as follows:
$1,250,000 within 90 days of the Effective Date;
$1,250,000 within one (1) year of the Effective Date;
$1,500,000 within two (2) years of the Effective Date.
●The issuance of ten (10) million shares of the Company’s Common Stock to an entity owned by Shahla Melamed.
In addition, in the event forty percent (40%) or more of the Company and/or its subsidiaries (including by way of merger) is sold within two (2) years of the Effective Date, the Company shall pay the Melameds, within two (2) weeks of receipt of the proceeds from such sale (the “Sale Proceeds”), any outstanding unpaid Settlement Sum plus an additional 10% of the Sale Proceeds received, up to a total of an additional $3,000,000 over and above the Settlement Sum.
In the event the Company fails to cure a breach of timely payment of any portion of the Settlement Sum within thirty (30) days of a notice of default, a Stipulated Judgement may be filed by Melamed in the sum of $20,000,000, less any Settlement Sum amounts previously paid by the Company.
On April 7, 2020, the Company executed an Executive Agreement (the “Executive Agreement”) for the appointment of Dr. David L. Stark as the Company’s President, with an effective date of March 1, 2020. The Executive Agreement replaces any other agreement between Dr. Stark and the Company or any of its subsidiaries, and provides a base compensation of $225,000 in year one, $250,000 in year two and $275,000 in year three, commencing after the initial term estimated at approximately six (6) months, during which time Dr. Stark will devote fifty percent (50%) of his time at a compensation proportionate to the year one base compensation. The Executive Agreement also provides for various performance bonuses, and customary employee benefits, as well as (i) a grant to purchase 2,000,000 restricted common shares at $0.001 per share, of which 25% vest immediately, and the remainder vest upon the Company’s achievement of certain earnings goals; and (ii) stock options to purchase 3,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share for a period of five (5) years, of which 25% vest immediately, and the remainder vest quarterly in equal amounts over the term of the Agreement.
- 48 -
In December 2019, an outbreak of the COVID-19 virus was reported in Wuhan, China. On March 11, 2020, the World Health Organization (“WHO”) declared the COVID-19 virus a global pandemic, and on March 13, 2020, President Donald J. Trump declared the virus a national emergency in the United States. As of the date of the filing of this Annual Report, the WHO reports over 4 million confirmed COVID-19 cases and over 275,000 deaths worldwide, including over 75,000 in the U.S. This highly contagious disease has spread to most of the countries in the world and throughout the United States, creating a serious impact on customers, workforces and suppliers, disrupting economies and financial markets, and potentially leading to a world-wide economic downturn. It has caused a disruption of the normal operations of many businesses, including the temporary closure or scale-back of business operations and/or the imposition of either quarantine or remote work or meeting requirements for employees, either by government order or on a voluntary basis.
The COVID-19 pandemic may adversely affect the Company’s customers’ operations, its employees and its employee productivity. It may also impact the ability of the Company’s subcontractors, partners, and suppliers to operate and fulfill their contractual obligations, and result in an increase in costs, delays or disruptions in performance. These effects, and the direct effect of the virus and the disruption on the Company’s employees and operations, may negatively impact both the Company’s ability to meet customer demand and its revenue and profit margins. The Company’s employees, in many cases, are working remotely and using various technologies to perform their functions. The Company might experience delays or changes in customer demand, particularly if customer funding priorities change. Further, in reaction to the spread of COVID-19 in the United States, many businesses have instituted social distancing policies, including the closure of offices and worksites and deferring planned business activity. Additionally, the disruption and volatility in the global and domestic capital markets may increase the cost of capital and limit the Company’s ability to access capital. Both the health and economic aspects of the COVID-19 virus are highly fluid and the future course of each is uncertain. For these reasons and other reasons that may come to light if the coronavirus pandemic and associated protective or preventative measures expand, the Company may experience a material adverse effect on its business operations, revenues and financial condition; however, its ultimate impact is highly uncertain and subject to change.
In response to the global need for diagnostics and personal protective gear and equipment, the Company, through its wholly-owned subsidiary, Parallax Diagnostics, Inc., has registered with the Food and Drug Administration (FDA), and has established strategic relationships with wholesale suppliers of various FDA-approved diagnostic tests and Personal Protective Equipment (“PPE”) for distribution to US medical practices, hospitals, nursing operations, emergency centers, and nursing homes. Included in the products available are COVID-19 diagnostic test kits, PPE such as protective masks, sterile gowns, and eye goggles, as well as ventilators and other medical grade equipment, The Company also intends on adapting its own proprietary diagnostics test and SPARKS Mobile™ testing device to be able to identify markers to the coronavirus. Parallax is profoundly gratified by its ability to help those in need during this crisis.
Trading Suspension
On April 13, 2020, the Company received an Order of Suspension of Trading dated April 10, 2020 (the “Order”) from the United States Securities and Exchange Commission (“SEC”). The temporary suspension period is from 9:30 a.m. EDT on April 13, 2020, through 11:59 p.m. EDT on April 24, 2020. The Order refers to questions raised regarding the accuracy of the Company’s recent press releases in relation to the Company’s development of a rapid screening test for COVID-19, and the Company’s access to “large quantities of COVID-19 diagnostics testing kits and personal protective equipment.”
In an effort to protect the interests of shareholders, the SEC has issued similar orders and suspensions recently to several registrants, with concerns over the validity of claims made in connection with the availability of COVID-19 tests and supplies.
The Company, along with its counsel, is cooperating fully with the SEC to substantiate the Company’s recent public announcements and business endeavors, and is addressing any questions and/or concerns raised regarding the accuracy of the assertions made in the Company’s press releases.
Pursuant to Rule 15c2-11 under the Exchange Act, at the termination of the trading suspension, no quotation may be entered unless and until the Company has strictly complied with all provisions of the rule, including the filing of a new Form 15c2-11 with FINRA.
The Company is required to file its Annual Report with the SEC for the purposes of satisfying its financial reporting requirements. However, in addition to the Company's reporting obligations, the Company must have a FINRA Member Market Maker file a 15c2-11 with FINRA in order for the Company’s shares to resume trading on the OTCQB market. These actions do not impact or otherwise affect the Company's results of operations or disclosures as set out in this Annual Report. The Company believes that this Annual Report fully complies with the requirements of the Securities Exchange Act of 1934, as amended and, in accordance with generally accepted accounting principles, that it fairly presents, in all material respects, the financial condition and results of operations of the Company as at the relevant dates.
As of the date of filing of this Annual Report, the trading suspension period expired, and the Company is in the process compiling the information required for its 15c2-11 submission by a FINRA Member Market Maker. The Company anticipates the filing of a new Form 15c2-11 within the next 30 days. If any party has any questions as to whether the Company has complied with the rule, they should contact the staff in the Division of Trading and Markets, Office of Interpretation and Guidance, at (202) 551-5777.
- 49 -
These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for its common stock. Many brokers may be unwilling to engage in transactions in its common stock because of the added disclosure requirements, thereby making it more difficult for stockholders to dispose of their shares.
Changes in Management
On April 6, 2017, the Company’s board of directors (the “Board”) elected Mr. J. Michael Redmond as Chairman, to serve until the next annual meeting of the shareholders, in accordance with the Company’s bylaws, and/or until his successor is duly appointed, or a resignation is duly tendered.
Effective July 6, 2017, the Board caused the departure of Mr. Redmond from his position as President and Chief Executive Officer of Parallax and RoxSan Pharmacy, Inc., and concurrently Mr. Redmond was removed from all board member seats.
Effective July 7, 2017, pursuant to a unanimous Board resolution, Mr. Paul R. Arena was appointed as the Company’s President and Chief Executive Officer, and the Board caused Mr. Arena's election to the Board and the board of directors of the Company’s wholly-owned subsidiaries, Parallax Health Management, Inc. and Parallax Behavioral Health, Inc.
On July 26, 2017, Dr. Jorn Gorlach resigned as a member of the Board. This resignation did not involve any disagreement with the Company.
On June 4, 2018, Mr. Anand Kumar resigned as a member of the Board. This resignation did not involve any disagreement with the Company. Mr. Nathaniel T. Bradley, currently serving as Chief Technology Officer, succeeded him; and agreed to serve as a member of the Board until the next annual meeting of the shareholders and/or until his successor is duly appointed.
On May 15, 2019, Mr. David Appell joined the Company as its Chief Operating Officer. On February 2, 2020, Mr. Appell resigned his position. His resignation was not the result of a disagreement with the Company on any matters relating to the Company's operations, policies or practices. Concurrent with his resignation as COO, Mr. Appell will serve as Managing Director of the Company’s wholly-owned subsidiary, Parallax Communications, Inc. (“PCOM”). As Managing Director, Mr. Appell will provide business advisory and strategic planning advice to the Company with PCOM. In exchange for these services, Mr. Appell will be paid $2,000 per month plus medical benefits.
On March 1, 2020, Dr. David L. Stark was appointed as the Company’s President. In connection with the appointment, Mr. Paul R. Arena, who has held the position of President since July 2017, remains the Company’s Chief Executive Officer and Chairman of the Board of Directors, but resigned the position of President to afford Dr. Stark’s appointment.
Dispute with Former Owner of RoxSan
On August 13, 2015, the Company completed the acquisition of RoxSan Pharmacy, Inc. (“RoxSan” or the “Pharmacy”). Shortly thereafter, the Company's management and the former owner (“Former Owner” or “Melamed”) clashed over control of the RoxSan Pharmacy business operations and bank accounts.
In October 2015, shortly following the Company’s acquisition of RoxSan, Melamed initiated two (2) legal actions against the Company in the Superior Court of the State of California, County of Los Angeles, West District, Shahla Melamed v. Parallax Health Sciences, Inc., action numbers SC 124873 and SC 125702. In the matter, action No. SC 124873, Melamed sought rescission of the August 13, 2015, Purchase Agreement. In the Matter, action No. SC125702, Melamed alleges that the Company is in default under the terms of the Purchase Agreement and Secured Note, and the Company’s termination of Melamed’s employment agreement.
The Company also initiated legal action against Melamed and filed a complaint in October 2015, action number SC 124898, in the Superior Court of the State of California, County of Los Angeles, West District, Parallax Health Sciences, Inc., et al. v. Shahla Melamed, et al. The complaint in that action alleges that Melamed breached several obligations under the Purchase Agreement, and the Company sought to reduce the Secured Note due to undisclosed material changes in the business.
Settlement Reached:
In January 2019, Melamed requested mediation, seeking settlement of the pending litigation with the Company, including that which was initiated against the Company by her son, Hootan Melamed (Shahla and Hootan, collectively, the “Melameds”). Through mediation, the Company and the Melameds reached agreeable settlement terms, and on February 19, 2020, the Company received a counter-signed Settlement and Release Agreement (the “Settlement”). Effective February 12, 2020 (the “Effective Date”), the Settlement is by and between Parallax Health Sciences, Inc., RoxSan Pharmacy, Inc., Michael Redmond, Edward Withrow III, Huntington Chase Financial Group, LLC, Calli Bucci and Dave Engert (collectively, “Parallax”), and the Melameds, and resolves all pending lawsuits between the parties in connection with the acquisition of RoxSan Pharmacy.
In consideration of the resolution of all existing and potential claims, including the cancellation of the Note in the principal sum of $20,500,000, and accrued interest of approximately $4,500,000, and without further action or litigation and without admission of liability by either party, the Settlement terms include the following:
●A payment of $4,000,000 (the “Settlement Sum”) to the Melameds, to be paid as follows:
$1,250,000 within 90 days of the Effective Date;
$1,250,000 within one (1) year of the Effective Date;
$1,500,000 within two (2) years of the Effective Date.
●The issuance of ten (10) million shares of the Company’s Common Stock to an entity owned by Shahla Melamed.
- 50 -
In addition, in the event forty percent (40%) or more of the Company and/or its subsidiaries (including by way of merger) is sold within two (2) years of the Effective Date, the Company shall pay the Melameds, within two (2) weeks of receipt of the proceeds from such sale (the “Sale Proceeds”), any outstanding unpaid Settlement Sum plus an additional 10% of the Sale Proceeds received, up to a total of an additional $3,000,000 over and above the Settlement Sum.
In the event the Company fails to cure a breach of timely payment of any portion of the Settlement Sum within thirty (30) days of a notice of default, a Stipulated Judgement may be filed by Melamed in the sum of $20,000,000, less any Settlement Sum amounts previously paid by the Company.
On May 14, 2018, pursuant to unanimous resolutions of the boards of directors of RoxSan Pharmacy, Inc. and Parallax Health Sciences, Inc., RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California (the “Court”). Mr. Timothy Yoo was appointed trustee (“Trustee”) on May 15, 2018. In connection with this filing, RoxSan seeks to discharge approximately $5 million of liabilities owed to various parties, and intercompany loans in excess of $1 million owed to Parallax. The Chapter 7 bankruptcy proceeding by RoxSan Pharmacy, Inc. was fully discharged and the case was closed on March 13, 2019, in U.S. Bankruptcy Court, Central District of California.
Due to, among other things, the reduction in RoxSan’s cash flows during 2016 and 2017, RoxSan became delinquent in its payroll tax depository obligations, resulting in a liability owed to federal and state taxing agencies in the aggregate of $1,148,811, which includes $601,148 in taxes withheld from employees (“Trust Fund Taxes”), employer taxes of $183,172, and penalties and interest of $364,491 through December 31, 2018. The liability was included as part of the Chapter 7 bankruptcy petition, and certain portions of the liability may be discharged. However, in accordance with California bankruptcy laws, federal and state Trust Fund Taxes are not dischargeable. The Company has retained a tax resolution specialist and is in communications with the taxing agencies in order to resolve RoxSan’s liability.
As a result of the loss of financial control of RoxSan, the Company derecognized the subsidiary as of September 30, 2018. The derecognition resulted in a gain of $4,478,268. The Company also extinguished $22,778,281 in debt and accrued interest related to the acquisition of RoxSan.
Disputes with Former Executives
On March 9, 2017, Mr. Dave Engert filed a lawsuit in Arizona and then on or about May 5, 2017, Mr. Engert, changed the venue and filed suit against the Company and RoxSan Pharmacy, Inc. in the United States District Court, Central District of California for an amount exceeding $75,000. The Company filed an answer and counterclaims against Mr. Engert for an amount exceeding $100,000. The counterclaims included possible fraud and negligence committed by Mr. Engert and Mr. J. Michael Redmond, former successor Chairman of Mr. Engert, director, President and Chief Executive Officer of the Company and former President, Chief Executive Officer, Chairman and director of RoxSan Pharmacy, Inc. On October 8, 2018, a settlement was reached between Mr. Engert and the Company (the “Engert Settlement”). The Engert Settlement includes, among other things, a cash payment to Mr. Engert in the amount of $139,000, and the cancellation of all of Mr. Engert’s equity holdings in the Company. The Engert Settlement resulted in a net loss to the Company of $33,272. On April 10, 2019, a stipulation for dismissal was filed, and the matter has been fully resolved.
On March 28, 2018, Mr. J. Michael Redmond filed a lawsuit against the Company and RoxSan Pharmacy, Inc. in the United States District Court, Central District of California for an amount exceeding $75,000. The Company intends to vigorously defend against this action. There are counterclaims that include possible fraud and negligence committed by Mr. Redmond, former President, Chief Executive Officer, Chairman and director of the Company and of RoxSan Pharmacy, Inc. An Arbitration is still pending.
Disputes with Lenders
On March 18, 2020, the Company initiated legal action and filed a complaint against EMA Financial, LLC (“EMA”) a third-party lender, in the US District Court, Southern District of New York, case index number 20-cv-2375, citing fraud, unjust enrichment, and securities law violations, among other things. On April 27, 2020, EMA filed an answer substantially denying the Company’s claims, and filed counterclaims against the Company, including damages in excess of $2 million. The matter is currently pending.
There are currently two (2) legal matters pending. For additional information on these proceedings, see “ITEM 3. LEGAL PROCEEDINGS“ section contained within this Annual Report.
Extinguishment of Debt
As a result of the loss of financial control and derecognition of the subsidiary, RoxSan Pharmacy, Inc., the Company extinguished $22,778,281 in debt and accrued interest related to the acquisition of RoxSan.
In addition, management determined that there is no future sacrifice of economic benefit arising from certain debt previously recognized by the Company to transfer assets or provide services in the future. As a result, certain notes and loans payable in the amount of $95,975, accrued interest in the amount of $56,892, and accounts payable in the amount of $284,714, were extinguished, and a loss of $357,853 was recognized from a debt exchange transaction.
In 2019, the Company recognized losses in the amount of $1,028,766 resulting from the exchange and conversion of certain debt instruments. In addition, a gain of $101,367 was recognized in connection with the cancellation of certain vendor debt.
The total gain (loss) on extinguishment of debt for the years ended December 31, 2019 and 2018, respectively was ($927,399) and $22,858,009.
- 51 -
Parallax Health Sciences, Inc. is a healthcare company focused on developing products and services that can provide remote communication, diagnosis, treatment, and monitoring of patients on a proprietary platform. Through its innovative technologies, both patented and patent-pending, the Company’s principal mission is to deliver solutions that empower patients, reduce costs, and improve the quality of care.
The Company has developed, acquired and licensed multiple platforms, proprietary and exclusive, that provide services and products across the healthcare continuum. These platforms are designed to allow for multiple points of reciprocal consideration through innovative business models that provide patients with increased quality of services and products at reduced cost of time and money. They also provide healthcare providers with increased access to their patients, the ability to deliver better and more efficient service and increase their income from the services they supply.
The Company believes that its products and services can provide solutions that mitigate rising costs, reduce waste in spending and the amount of unnecessary services, and increase the health and wellness of patients. The Company’s endeavors to change the healthcare industry are strengthened by providing solutions to real problems facing healthcare stakeholders today. The Company’s products and services have been developed, and are continuing to be developed, to address these issues now.
The Good Health Outcomes™ technology-enabled digital healthcare system is structured with three separate divisions that can operate independently of one another, or integrate services to meet the various needs of the Company’s clientele: Optimized Outcomes, Connected Health and Smart Data. Each of these divisions target a separate vertical market that are synergistic, compliment, and strengthen each other and the Company’s value proposition as a whole.
●Optimized Outcomes
The Company’s REBOOT™ technology, an acronym for “Reliable Evidence-Based Outcome Optimization Technology,” is at the heart of the Company’s behavioral technology provides reliable evidence-based outcome optimization through a patented machine learning platform. REBOOT™ has been specifically designed to improve health treatment outcomes through internet-based and mobile behavioral technology systems that enable its users and user groups to more effectively achieve goals within a prescribed timeline, with the potential to transform the cost of treating and managing chronic illnesses such as pulmonary-COPD-asthma, diabetes, and cardiovascular disease by effecting the modification of behavior in patients being treated for these chronic diseases. The REBOOT™ technology, developed and commercially tested for over 5 years at a cost exceeding $4,000,000, provides reliable evidence-based outcome optimization through a patented machine learning platform delivered through:
●A cloud-based software system, scalable for use from one patient to over 100 million;
●A mobile application, COMPASS™, that is interchangeable from one disease to another, and one patient to another; and
●A stratification tool, WIZARD™, which was developed specifically to support scalability of the REBOOT™/ COMPASS™ platform.
REBOOT™ can be sold as a product line into certain defined verticals, independent of, or in combination with, the Company’s connected healthcare and data platforms, products and services. The REBOOT™ technology is currently protected by patents issued in U.S., China, India, and Hong Kong and Macao.
●Connected Health
Fotodigm®
Fotodigm® is the Company’s patent-pending, integrated, interoperable, cloud-based platform, that allows for ease of use of the Company’s proprietary products and services and third-party Plug-n-Play interfaces. Designed with increased accessibility and accelerated adoption in mind, Fotodigm® enables patients and doctors to use a singular, integrated, interoperable platform for:
●Telehealth;
●Remote Patient Monitoring (“RPM”);
●Point-of-Care (“POC”) testing;
●Personal Protective Equipment (“PPE”)
●Healthcare education services.
Fotodigm® provides simple, cost-effective, accessible and affordable products and services that deliver industry breakthrough advantages. The Fotodigm® construct was developed to provide payers, patients and providers with the ability to choose and interchange their services, and be able to interchange where the healthcare practitioner deems it the best course of action. The Fotodigm® system is being developed to utilize a proprietary Machine Face Recognition engine along with proven and existing Optical Character Recognition (“OCR”) technology through third-party license. The technology has been beta tested and utilized in the field by patients within remote patient monitoring systems for the reduction of hospital readmissions.
- 52 -
The Target System is the Company’s proprietary diagnostic immunoassay testing platform and test cartridges designed for twenty-five specific FDA-cleared tests in areas of infectious diseases, cardiac markers, drugs of abuse and various other medical conditions. The Target System is comprised of:
●the VT-1000, an FDA-cleared, clinically and commercially proven bench-top quantitative and qualitative immunoassay testing system specifically designed to reside in the primary care physician office;
●the SPARKS Mobile™, a patented (US, China, India, Hong Kong, Macao, and India) mobile testing system, currently in development, that incorporates the VT-1000 feature set with smartphone capabilities (to be completed before commercialization, includes design and build, then certification);
●25 FDA-cleared rapid tests in the areas of:
Cardiac;
Infectious diseases;
Medical conditions;
Drugs of abuse;
Pregnancy.
●the Target Antigen Detection System (“TADS”), a unique, patented single-use device cartridge that allows for positive, controlled sample processing, a system of specific immune reactions to detect individual disease conditions, and a quick response diagnostic system that provides answers to specific screening problems under ten minutes.
The Target System has the capacity to test hundreds of conditions, and is to be offered to doctors for use at the doctors’ offices and at patients’ homes, or in triage and ambulatory environments.
The Target System is not commercially available at this time, as the product is currently in redesign and development, with a primary focus on developing the SPARKS Mobile™, the patented handheld mobile version of the VT-1000 desktop analyzer.
●Smart Data
Parallax’s Intrinsic Code™ is the Company’s unique Smart Data patient data collection and repository system. Intrinsic Code™ not only identifies the traditional data from patients, but is also designed to provide actionable insights into the behavioral changes in patients, which has resulted in increased adherence to their medical regimens and pharmacologic therapies. These insights are extremely valuable to pharmaceutical companies, as medication nonadherence creates false efficacy results of therapies in the manner in which they were designed, tested and provided regulatory approval upon. Payers are deeply concerned with the data on adherence to medical and medication therapy regimens, as it directly affects their financial performance.
Management
Parallax is led by experienced veterans from the healthcare, technology, finance and management fields. The Company's disciplined and organized approach is balanced by its optimism for the future, and the opportunities present in the current healthcare market. The Parallax team is grounded in a belief that success in business is built on a combination of research, planning and execution.
Operating Segments
The Company’s current operations include the following business segments for financial statement presentation: Remote Patient Monitoring (RPM), Behavioral Health Services (BHS), and Corporate.
●Remote Patient Monitoring
Parallax has developed a distinctive technology platform that provides for the complete remote patient care delivery system: the patent-pending Fotodigm® platform, which utilizes proprietary software and technology to bridge clinical behavioral science with technology and logistics for payers, providers and clinical professionals across a variety of wellness and clinical devices, including both fitness and clinical applications. Fotodigm® is a secure and scalable platform for collecting, transmitting and analyzing biometric, pharmaceutical, and health data to healthcare providers, primarily hospitals, accredited nursing operations, and physicians using optical character recognition, otherwise known as “Machine Face Recognition” technology that is licensed from others.
The RPM segment generates revenues through fees charged for the license and utilization of its proprietary system that provides software integrations of the Fotodigm® platform. Additionally, the RPM segment generates incremental revenues through the delivery of acute, post-acute and chronic health patient management software systems that enable Parallax customers to bill for and collect payments from patients and third-party payers for telemonitoring and remote services that they deliver.
- 53 -
In April 2017, the Behavioral Health Services segment commenced with the acquisition of the REBOOT™ and Intrinsic Code™ technologies. The BHS segment will generate revenues primarily through licensing and subscription of software and systems. As of December 31, 2019, the BHS segment had not yet begun full operations, generating limited test market sales.
●Diagnostics/Corporate
The Diagnostics/Corporate Segment supports the costs and operating expenses related to the continued development and exploitation of the Company’s proprietary Target System POS medical diagnostic and monitoring platform and processes. In addition, the Diagnostics/Corporate Segment provides management and administrative services to support the Company and consists of certain aspects of the Company’s executive management, corporate relations, legal, compliance, human resources, and corporate information technology and finance departments.
The following summary of the Company’s financial condition and results of operations should be read in conjunction with the Company’s audited consolidated financial statements for the years ended December 31, 2019 and 2018, which are included herein. The financial information of Parallax Health Sciences, Inc., and its wholly-owned subsidiaries, Parallax Diagnostics, Inc., Parallax Health Management, Inc., Parallax Behavioral Health, Inc., and Parallax Communications, Inc. is provided below on a consolidated basis, unless otherwise indicated. All significant intercompany accounts and transactions have been eliminated.
Balance Sheet
As of December 31, 2019, the Company had total assets of $1,359,896 compared with total assets of $1,364,357 at December 31, 2018. The decrease in total assets of $4,461 is attributable to an increase in cash of $6,183, an increase in operating lease asset of $52,176, an increase in investments, net of impairment, of $50,000, $120,620 of amortization related to intangible assets, and an increase in deposits of $7,800.
As of December 31, 2019, the Company had total liabilities of $11,614,479 compared with total liabilities of $7,314,811 at December 31, 2018. The increase in total liabilities of $4,299,668 is attributable to a decrease in accounts payable and accrued expenses of $572,160, an increase in operating lease liability of $52,176, an increase in short-term settlements payable of $2,824,200, an increase in short-term derivative liability of $38,875, a decrease in short-term convertible debentures of $724,903, a decrease in short-term related party convertible debentures of $411,006, an increase in short-term notes payable of $345,000, an increase in short-term note payable, bank of $18,616, an increase in short-term related party notes payable of $126,152, an increase in short-term convertible notes payable of $806,364, an increase in related party payables of $103,760, an increase in long-term settlements payable of $1,240,000, an increase in license fees payable of $12,000, an increase in royalties payable of $15,165, a decrease in long-term derivative liability of $34,000, a decrease in long-term debentures of $184,870, a decrease in long-term notes payable, bank of $28,995, an increase in related party note payable of $633,294, and an increase in convertible notes payable of $40,000.
Results of Operations
The year ended December 31, 2019, compared to the year ended December 31, 2018
| For the year ended |
| ||||
| December 31, 2019 |
| December 31, 2018 |
| ||
Revenue | $ | 128,600 |
| $ | 11,739 |
|
Cost of sales | $ | 16,827 |
| $ | 20,339 |
|
Gross profit (loss) | $ | 111,773 |
| $ | (8,600 | ) |
General and administrative expenses | $ | 6,505,461 |
| $ | 6,626,063 |
|
Operating loss | $ | (6,393,688 | ) | $ | (6,634,663 | ) |
Gain on disposal of subsidiary | $ | –– |
| $ | 4,478,268 |
|
Gain (loss) on extinguishment of debt | $ | (927,399 | ) | $ | 22,858,009 |
|
Gain (loss) on fair value adjustments | $ | 194,511 |
| $ | (123,875 | ) |
Loss on settlements | $ | (4,134,972 | ) | $ | –– |
|
Impairment losses | $ | (1,002,276 | ) | $ | –– |
|
Discount amortization | $ | (20,000 | ) | $ | (2,805,000 | ) |
Interest expense | $ | (582,508 | ) | $ | (2,164,530 | ) |
Net income (loss) – continuing operations | $ | (12,866,332 | ) | $ | 15,608,209 |
|
Net income (loss) – discontinued operations | $ | –– |
| $ | (824,398 | ) |
Net income (loss) | $ | (12,866,332 | ) | $ | 14,783,811 |
|
- 54 -
Revenue in the amount of $128,600 for the year ended December 31, 2019, consists of license fees in the amount of $125,000 in connection with the Company’s intellectual property, contract fees and equipment sales related to the Company’s remote health care systems in the amount of $1,800, and subscription fees related to the Company’s behavioral health services in the amount of $1,800.
Revenue in the amount of $11,739 for the year ended December 31, 2018, consists contract fees and equipment sales related to the Company’s remote health care systems in the amount of $9,939, and subscription fees related to the Company’s behavioral health services in the amount of $1,800.
The Company’s behavioral health services segment had not yet begun full operations, generating limited test market sales. The Company has not yet fully launched the medical diagnostics and testing activities of the Company’s Connected Health division.
Cost of sales
Costs of sales in the amount of $16,827 for the year ended December 31, 2019, consists of equipment and other costs related to the Company’s remote health care systems.
Costs of sales in the amount of $20,339 for the year ended December 31, 2018, consists of equipment and other costs related to the Company’s remote health care systems.
The Company’s behavioral health services segment had not yet begun full operations, generating limited test market sales. The Company has not yet fully launched the medical diagnostics and testing activities of the Company’s Connected Health division.
General and Administrative Expenses
| For the year ended |
|
|
| |||||
| December 31, 2019 |
| December 31, 2018 |
| Variances |
| |||
Legal, accounting, and management services | $ | 2,020,488 |
| $ | 2,166,590 |
| $ | (146,102 | ) |
Stock compensation/stock option amortization |
| 2,782,426 |
|
| 3,513,710 |
|
| (731,284 | ) |
Professional fees and outside services |
| 586,422 |
|
| 210,191 |
|
| 376,231 |
|
Salaries, and related taxes |
| 167,239 |
|
| 181,656 |
|
| (14,417 | ) |
Benefits and allowances |
| 180,213 |
|
| 126,070 |
|
| 54,143 |
|
Marketing |
| 255,507 |
|
| 8,455 |
|
| 247,052 |
|
Product development |
| 76,617 |
|
| 73,896 |
|
| 2,721 |
|
Depreciation and amortization |
| 120,972 |
|
| 120,620 |
|
| 352 |
|
Rent expense-office |
| 139,520 |
|
| 73,351 |
|
| 66,169 |
|
Travel, meals and entertainment |
| 49,893 |
|
| 50,767 |
|
| (874 | ) |
Office supplies and miscellaneous expenses |
| 126,164 |
|
| 100,757 |
|
| 25,407 |
|
Total general and administrative expenses | $ | 6,505,461 |
| $ | 6,626,063 |
| $ | (120,602 | ) |
General and administrative expenses in the amount of $6,505,461 for the year ended December 31, 2019, were comprised of $2,020,488 in legal, accounting and management fees, $2,782,426 in stock compensation/stock option amortization, $584,422 in professional fees and outside services, $167,239 in salaries and related taxes, $180,213 in benefits and allowances, $255,507 in marketing, $76,617 in product development, $120,972 in depreciation and amortization, $139,520 in rent expense, $49,893 in travel, meals and entertainment, and $126,164 in office overhead and other general and administrative expenses.
General and administrative expenses in the amount of $6,626,063 for the year ended December 31, 2018, were comprised of $2,166,590 in legal, accounting and management fees, $3,513,710 in stock compensation/stock option amortization, $210,191 in professional fees and outside services, $181,656 in salaries and related taxes, $126,070 in benefits and allowances, $8,455 in marketing, $73,896 in product development, $120,620 in depreciation and amortization, $73,351 in rent expense, $50,767 in travel, meals and entertainment, and $100,757 in office overhead and other general and administrative expenses.
General and administrative expenses for the year ended December 31, 2019, were $6,505,461 as compared to $6,626,063 for the year ended December 31, 2018, which resulted in a decrease in general and administrative expenses for the current year of $120,602.
- 55 -
Significant changes in general and administrative expenses of $120,602 during the year 2019 compared to 2018 were attributable to the following items:
●a decrease in legal, accounting and management services of $146,102, primarily due to a decrease in legal costs of $372,253 resulting from the settlement of pending litigation; a decrease in the $250,000 reserve established in prior year for anticipated future legal costs related to pending litigation; an increase in accounting and audit fees of $128,016 due to additional services required as a result of a financial statement restatement and the Company’s S-1 filing; an increase management consulting fees of $203,218 resulting from changes in management; an increase of $106,000, resulting from the establishment of director compensation plan; and an increase in miscellaneous management fees of $38,917; and
●a decrease in stock compensation/stock option amortization of $731,284, primarily due to a decrease in stock compensation of $440,976; a decrease in amortization of deferred stock award compensation of $409,640; an increase in amortization of stock option compensation $145,522; and a decrease in amortization of deferred warrant compensation of $26,190; and
●an increase in professional fees and outside services of $376,231, primarily due to an increase in investing and financing advisory services and due diligence costs; and
●a decrease in salaries and related taxes of $14,417, primarily due to a decrease in compensation of $55,779 resulting from a decrease in staff; and an increase in payroll tax expense of $41,362 resulting from a reduction in accrued compensation and related payroll taxes in the prior year; and
●an increase in benefits and allowances of $54,143, primarily due to an increase in insurance benefits of $2,943; and an increase in contractual expense allowances of $51,200 resulting from a full year of expense in the current year compared to a partial year of expense for the prior year; and
●an increase in marketing of $247,052, primarily due to the engagement of marketing firms, resulting in an increase of $198,000; and an increase in other marketing fees of $49,052; and
●an increase in product development of $2,721, primarily due to a decrease in development costs of $5,219; and an increase in patent costs of $7,940; and
●an increase in depreciation and amortization of $352, primarily due to office equipment acquired in the current year, resulting in additional depreciation of $352; and
●an increase in rent expense for office space of $66,169, primarily due to additional office space leased in the current year; and
●a decrease in travel, meals and entertainment of $874, primarily due to a decrease in travel costs of $5,065; and an increase in meals and entertainment of $4,191; and
●an increase in office supplies and miscellaneous expenses of $25,407, due to increases in automobile expense of $17,142, computer and internet costs of $5,557, repairs and maintenance of $7,419, royalties of $7,165; taxes, licenses and permits of $4,644, telephone expense of $5,747, transfer agent fees of $4,787, and other general office and administrative expenses of $3,781; and decreases in insurance expense of $6,654 and storage and moving of $24,181.
General and administrative expenses for both 2019 and 2018 were incurred for the purpose of advancing the Company closer to its financing and operating goals in the bio-medical and digital healthcare sectors.
Net Income (Loss)
During the year ended December 31, 2019, the Company generated a net loss from continuing operations of $12,866,332, compared with net income from continuing operations of $15,608,209 for the year ended December 31, 2018. The decrease in net income from continuing operations of $28,474,541 is attributable to an increase in gross profits of $120,373, a decrease in general and administrative expenses of $120,602, a decrease in the gain from the disposal of a subsidiary of $4,478,268, a decrease in the gains from extinguishment of debt of $23,785,408, an increase in gains from fair value adjustments of $318,386, an increase in losses on settlements of $4,134,972, an increase in impairment losses of $1,002,276, a decrease in discount amortization of $2,785,000, and a decrease in interest expense of $1,582,022.
Liquidity and Capital Resources
Working Capital |
|
| Increase |
| |||||
| December 31, 2019 |
| December 31, 2018 |
| (Decrease) |
| |||
Current assets | $ | 58,621 |
| $ | 262 |
| $ | 58,359 |
|
Current liabilities |
| 7,722,766 |
|
| 5,115,692 |
|
| 2,607,074 |
|
Working capital (deficit) | $ | (7,664,145 | ) | $ | (5,115,430 | ) | $ | (2,548,715 | ) |
As of December 31, 2019, the Company had cash in the amount of $6,445 compared to $262 as of December 31, 2018.
The Company had a working capital deficit of $7,664,145 as of December 31, 2019, compared to a working capital deficit of $5,115,430 at December 31, 2018. The decrease in working capital deficit of $2,548,715 is primarily attributable to increases in cash of $6,183, and operating lease asset of $52,176; increases in operating lease liability of $52,176, short-term settlements payable of $2,824,200, short-term derivative liability of $38,875, notes payable of $345,000, notes payable to bank of $18,616, related party note payable of $126,152, convertible notes payable of $806,364, and related party payable of $103,760; and decreases in accounts payable and accrued expenses of $572,160, short-term debentures of $724,903, and related party short-term debentures of $411,006.
- 56 -
Cash Flows | For the year ended |
| Increase |
| |||||
| December 31, 2019 |
| December 31, 2018 |
| (Decrease) |
| |||
Net cash used by operating activities | $ | (2,945,573 | ) | $ | (1,291,984 | ) | $ | 1,653,589 |
|
Net cash used by investing activities |
| (2,628 | ) |
| –– |
|
| 2,628 |
|
Net cash provided by financing activities |
| 2,954,384 |
|
| 1,332,005 |
|
| 1,622,379 |
|
Net cash provided (used) by continuing operations | $ | 6,183 |
| $ | 40,021 |
| $ | 33,838 |
|
Net cash provided (used) by discontinued operations |
| –– |
|
| (39,942 | ) |
| 39,942 |
|
Net increase (decrease) in cash | $ | 6,183 |
| $ | 79 |
| $ | 6,104 |
|
Cash Flows from Operating Activities
During the year ended December 31, 2019, the Company used $2,945,573 of cash flow for operating activities of continuing operations, compared with $1,291,984 for the year ended December 31, 2018. The increase in cash used by operating activities of $1,653,589 is primarily attributable to a decrease in net income from continuing operations of $28,474,541; increases in depreciation and amortization of $66,761, securities issued in exchange for licenses sold of $50,000, losses on settlements of $4,134,972, and impairment losses of $1,002,276; decreases in stock compensation/stock option amortization of $731,284, discount amortization of $2,780,209, and allowance for bad debt of $236; decreases in gains from disposal of subsidiary of $4,478,268, and extinguishment of debt of $23,785,408; decreases in losses from fair value adjustments of $318,386, and debt accretion of $924,153; increases in deposits of $7,800, royalties payable of $7,165, and related party payables of $96,398; and decreases in accounts receivable of $3,039, and accounts payable and accrued expenses of $1,935,189.
Cash Flows from Investing Activities
During the year ended December 31, 2019, the Company used $2,628 of cash flow for investing activities, compared with none for the year ended December 31, 2018. The increase in cash used by investing activities is attributable to the purchase of professional equipment in the amount of $2,628.
Cash Flows from Financing Activities
During the year ended December 31, 2019, the Company was provided with $2,954,384 in cash flows from financing activities of continuing operations, compared to $1,332,005 during the year ended December 31, 2018. The increase in cash flows provided by financing activities of $1,622,379 is attributable to increases in lease payments of $71,200, proceeds from notes and loans payable of $330,000, repayment of notes and loans payable of $141,134, proceeds from convertible notes payable of $767,280, repayment of convertible notes payable of $348,580, repayment of debentures of $754,369, and proceeds from the issuance of common shares of $2,296,382; and decreases in the proceeds from the issuance of debentures of $225,000, and proceeds from the issuance of preferred shares of $231,000.
As of December 31, 2019, related parties are due a total of $2,359,026, consisting of $796,175 in accrued compensation owed to related parties; $312,305 in accrued benefits owed to related parties, and cash advances from related parties for operating expenses; $759,446 in promissory notes, and $491,100 in convertible promissory notes.
As of December 31, 2019, a convertible promissory note is payable to a related party in the aggregate sum of $491,100, representing cash loans and unpaid compensation. The note bears interest at a rate of 7% per annum, matures December 31, 2023, and contains a repayment provision to convert the debt into restricted shares of the Company’s Common Stock. On December 31, 2019, the note was modified to change the conversion rate from $0.10 to $0.08 per share. During the year ended December 31, 2019, interest in the amount of $33,663 was expensed, and $98,642 was converted to Common Stock. As of December 31, 2019, a total of $9,795 in interest remains accrued.
During the year ended December 31, 2019, the Company exchanged related party convertible debentures in the principal sum of $411,006, plus accrued interest of $42,778, to non-convertible Senior Secured Promissory Notes (the “Senior Notes”) in the aggregate principal of $759,446. The Senior Notes bear interest at a rate of 8% per annum, with payments of $126,152 plus interest accrued thereon due December 31, 2019; $300,000 due December 31, 2020; and the remaining principal and accrued interest due December 31, 2021. In connection with the exchange, the Company issued the following in favor of the lender: 1,380,811 shares of the Company’s restricted Common Stock, valued at $131,315; and warrants, valued at $92,150, to purchase 2,528,413 shares of the Company’s Common Stock for a period of five (5) years at an exercise price of $0.10461. As a result, the Company recognized a net loss on the exchange in the amount of $434,837, net of $2,140 in derivative liability remaining from the warrants issued with the debentures. As of December 31, 2019, no accrued interest remains.
The Company’s principal sources of funds have been from the Company’s sales of its Preferred and Common Stock, and loans from related parties and third-party lenders.
- 57 -
The following represents the obligations previously in default as a result of cash flow constraints, which have been cured to the satisfaction of the noteholder:
Debt Type |
| Principal |
| Default |
| Maturity |
| Date Cured |
| Aggregate Settlement |
| ||
Convertible Promissory Notes |
| $ | 100,000 |
| 20% |
| 05/2018 |
| 08/2019 |
| $ | 435,000 | [1] |
[1]Consists of $125,000 in new non-convertible promissory note, $30,000 in cash and 2,400,000 shares of Common Stock, valued at $280,000.
A convertible promissory note in the principal sum of $100,000 matured in May 2018. Due to cash flow constraints, the note went into default. In August 2019, a settlement was reached with the noteholder, the terms of which include the issuance of a new non-convertible promissory note in the amount of $125,000, a cash payment in the amount of $30,000, and 2,400,000 shares of the Company’s Common Stock, valued at $280,000, at which time the default was cured. The new promissory note bears interest at a rate of 8% and matures August 2020.
In addition to the cured defaults disclosed above, $91,000 of the Company’s short-term indebtedness at December 31, 2019, has been extended by verbal agreement from its original maturity date. Under the verbal agreements, the noteholders have agreed to extend the due date until such time as the Company completes its private placement offering; or the noteholders opt to convert the debt into Common Stock. With the exception of the extension the maturity date, the terms of the notes under verbal agreement remain the same as the original notes, which bear interest at a rate of 10%-12% per annum, and are convertible into restricted shares of the Company’s Common Stock at a conversion rate of $0.10 per share. As of the date of this filing, no demand for payment or request for conversion has been made. For further information on the short-term indebtedness and verbal agreements mentioned above, please refer to Exhibit 4.20 to the Company’s Registration Statement on Form S-1/A filed December 2, 2019.
Future Financings
The Company has suffered recurring losses from operations. The continuation of the Company’s operations is dependent upon the Company’s attaining and maintaining profitable operations and raising additional capital as needed. The Company anticipates that it will have to raise additional funds through private placements of the Company’s equity securities and/or debt financing to complete its business plan.
The Company will require additional financing in order to proceed with its plan of operations, including approximately $5,000,000 over the next 12 months to pay for its ongoing expenses and debt obligations. These cash requirements include working capital, general and administrative expenses, the development of the Company’s product line, the reduction of contractual debt obligations, and the pursuit of acquisitions. These cash requirements are in excess of the Company’s current cash and working capital resources. Accordingly, the Company will require additional financing in order to continue operations and to repay its liabilities. There is no assurance that the financing will be completed as planned or at all. If the Company is unable to secure adequate capital to continue the Company’s planned operations, the Company’s shareholders may lose some or all of their investment and the Company’s business may fail.
In January 2020, the Company issued a short-term non-related party convertible promissory note in the principal sum of $78,000, less original issue discount of $3,000, for proceeds in the amount of $75,000. The note bears interest at a rate of 12% per annum, matures in one (1) year, and is convertible into shares of the Company’s Common Stock at a conversion price equal to the lower of 1) $0.12 per share; or 2) 65% of the average of the lowest three (3) trading prices during the fifteen (15) trading days preceding the conversion date.
In February 2020, the Company made principal repayments on non-related party notes payable of $75,000. As a result, $270,000 in non-related party notes payable remains.
In February 2020, the Company filed a Certificate of Designation (“Designation”) with the secretary of state of Nevada for the designation of 10,000 shares of Series B1 Convertible Preferred Stock. The Series B1 Stock is redeemable at 120% of face value and unpaid dividends; is convertible into Common Stock at a conversion rate of $0.15; carries an annual dividend of 10%; and matures in two (2) years, at which time the Series B1 Stock will automatically convert into Common Stock.
In February 2020, in connection with a $5,000,000 maximum offering of the Company’s Series B1 Convertible Preferred Stock (the “Series B1 Stock”), the Company received a Subscription from an accredited investor (the “Subscription”) for the purchase of 69 shares of Series B1 Stock at a price of $10,000 per share, net of an original issue discount of 15%, or $8,500 per share, for proceeds in the amount of $586,500, pursuant to that certain Securities Purchase Agreement dated February 10, 2020. In addition, the Subscription includes 50% warrant coverage at an exercise price of $0.25 per share for a period of three (3) years.
In March 2020, the Company received subscriptions from accredited investors for the purchase of 36 shares of the Series B1 Stock at a price of $10,000 per share, net of an original issue discount of 15%, or $8,500 per share, for proceeds in the amount of $306,000.
The Company anticipates continuing to rely on equity sales of its Common Stock and Preferred Stock in order to continue to fund its business operations. Issuances of additional shares will result in dilution to the Company’s existing stockholders. There is no assurance that the Company will achieve any additional sales of its equity securities or arrange for debt or other financing to fund its planned business activities.
- 58 -
As of December 31, 2019, the Company had 9 employees, inclusive of 4 executive officers. All 9 employees were full-time employees.
Currently, the Company has 9 employees, inclusive of 4 executive officers. All 9 employees are full-time employees.
Contractual Obligations
The Company is a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and is not required to provide the information under this item.
Going Concern
The Company has incurred losses since inception resulting in an accumulated deficit of $32,057,254, and further losses are anticipated in the development of its business. The Company’s ability to continue as a going concern is dependent upon its ability to generate profitable operations in the future and/or to obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due, which may not be available at commercially reasonable terms. There can be no assurance that the Company will be able to generate profitable operations and/or continue to raise funds, in which case the Company may be unable to meet its obligations and the Company may cease operations. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern.
The audited consolidated financial statements included with this Annual Report have been prepared on the going concern basis which assumes that adequate sources of financing will be obtained as required and that the Company’s assets will be realized and liabilities settled in the ordinary course of business. Accordingly, the audited consolidated financial statements do not include any adjustments related to the recoverability of assets and classification of assets and liabilities that might be necessary should the Company be unable to continue as a going concern.
Off-Balance Sheet Arrangements
The Company has no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on the Company’s financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Critical Accounting Policies
The discussion and analysis of the Company’s financial condition and results of operations are based upon the Company’s audited consolidated financial statements, which have been prepared in accordance with the accounting principles generally accepted in the United States of America. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management’s application of accounting policies. The Company believes that understanding the basis and nature of the estimates and assumptions involved with the following aspects of the Company’s financial statements is critical to an understanding of its consolidated financial statements. The following should be read in conjunction with Note 3 to the Company’s consolidated financial statements, “Summary of Significant Accounting Policies”:
Impairment reviews
Management is required to perform tests annually, or more often if necessary, for impairment of its finite lived and indefinite lived assets, to determine if events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Impairment testing is an area involving management judgement, requiring assessment as to whether the carrying value of assets can be supported by the net present value of future cash flows derived from such assets using cash flow projections, when available, which have been discounted at an appropriate rate. In calculating the net present value of the future cash flows, certain assumptions are required to be made in respect of highly uncertain matters, including management’s expectations of:
●growth in EBITDA, calculated as adjusted operating profit before depreciation and amortization;
●long term growth rates; and
●the selection of discount rates to reflect the risks involved.
For the purpose of determining goodwill impairment, if any, the Company prepares five year projections and uses these as the basis for its impairment reviews. Changing the assumptions selected by management, in particular the discount rate and growth rate assumptions used in the projections, could significantly affect the Company’s impairment evaluation and, hence, results.
The Company’s review for impairment also includes the evaluation of key assumptions related to sensitivity in the projections. Included are estimates for varying levels of growth, including aggressive, median, and conservative. In the Company’s evaluation, the conservative level of growth is utilized. For additional information, see Impairment of Long-Lived Assets under Note 3, Summary of Significant Accounting Policies, in the Notes to the Financial Statements.
Contingent liabilities
The Company’s contingent liabilities require significant judgement in determining potential liabilities resulting from estimated future earnings derived from its intangible assets. Each accounting period, estimated revenues are evaluated and adjusted as necessary, to determine royalty and earn-out contingencies. In addition, potential liabilities are evaluated in connection with pending legal claims. For additional information, see Note 10, Commitments and Contingencies, in the Notes to the Financial Statements.
- 59 -
Item 7A.Quantitative and Qualitative Disclosures About Market Risk |
The Company is a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and is not required to provide the information under this item.
The Company’s audited consolidated financial statements are stated in United States dollars (US$) and are prepared in accordance with United States Generally Accepted Accounting Principles.
The following audited consolidated financial statements are filed as part of this Annual Report:
- 60 -
CERTIFIED PUBLIC ACCOUNTANTS
A PROFESSIONAL CORPORATION
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Stockholders of Parallax Health Sciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Parallax Health Sciences, Inc., and subsidiaries (the “Company”) as of December 31, 2019 and 2018, and the related statements of operations, stockholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 2019, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Notes 1 and 8 to the financial statements, the Company has suffered recurring losses from operations, has a net capital deficiency and has significant contingencies that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are described in Note 1. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinion on the critical audit matter or on the accounts or disclosures to which they relate.
Valuation of Convertible Debt and Warrant Liabilities
The Company issued convertible debt with attached warrants and evaluated the accounting treatment for convertible debt and warrants to determine the impact (if any) of 1) embedded conversion option; 2) beneficial conversion feature; 3) bifurcation; 4) derivative liability; and 5) fair value adjustments and other expenses thereto.
We identified the evaluation of the accounting treatment for convertible debt and warrants as a critical audit matter. Complex auditor judgment was involved in evaluating the Company’s interpretation of applicable accounting rules and the valuation of convertible debt and warrant liabilities.
The primary procedures we performed to address this critical audit matter included the following. We tested the design and implementation of certain internal controls over the Company’s evaluation of the accounting treatment for convertible debt and warrants. We inspected relevant documentation related to the issuance of convertible debt with attached warrants and their accounting treatment and valuation. In addition, we involved valuation specialist, who assisted in evaluating the reasonableness of assumptions, methodologies, and calculations used by management in the determination of the impact (if any) of 1) embedded conversion option; 2) beneficial conversion feature; 3) bifurcation; 4) derivative liability; and 5) fair value adjustments and other expenses thereto related to the issuance of convertible debt and warrants.
/s/ Freedman & Goldberg CPAs
We have served as the Company’s auditor since 2016.
Farmington Hills, Michigan
May 15, 2020
31150 Northwestern Highway, Suite 200, Farmington Hills, Michigan 48334 (248) 626-2400 Fax (248) 626-4298
2444 East Hill Road, Grand Blanc, Michigan 48439 (810) 694-0336 Fax (810) 694-9789
Website: freedmangoldberg.com
- F-1 -
PARALLAX HEALTH SCIENCES, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, 2019 |
| December 31, 2018 |
| ||
|
|
|
| |||
ASSETS |
|
|
|
| ||
Current assets |
|
|
|
|
|
|
Cash and cash equivalents | $ | 6,445 |
| $ | 262 |
|
Operating lease asset |
| 52,176 |
|
| –– |
|
Total current assets |
| 58,621 |
|
| 262 |
|
|
|
|
|
|
|
|
Investments |
| 50,000 |
|
| –– |
|
Goodwill |
| 785,060 |
|
| 785,060 |
|
Intangible assets, net |
| 458,415 |
|
| 579,035 |
|
Deposits |
| 7,800 |
|
| –– |
|
|
|
|
|
|
|
|
TOTAL ASSETS | $ | 1,359,896 |
| $ | 1,364,357 |
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT |
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
Accounts payable and accrued expenses | $ | 2,082,978 |
| $ | 2,655,138 |
|
Operating lease liability |
| 52,176 |
|
| –– |
|
Settlement payable, net of unamortized discount |
| 2,824,200 |
|
| –– |
|
Derivative liability, short-term |
| 62,800 |
|
| 23,925 |
|
Debentures, convertible |
| –– |
|
| 724,903 |
|
Debentures, convertible, related party |
| –– |
|
| 411,006 |
|
Notes payable |
| 345,000 |
|
| –– |
|
Note payable, bank |
| 18,616 |
|
| –– |
|
Note payable, related party |
| 126,152 |
|
| –– |
|
Notes payable, convertible, net of unamortized discount |
| 1,102,364 |
|
| 296,000 |
|
Related party payables |
| 1,108,480 |
|
| 1,004,720 |
|
Total current liabilities |
| 7,722,766 |
|
| 5,115,692 |
|
|
|
|
|
|
|
|
Long-term liabilities |
|
|
|
|
|
|
Settlement payable, net of unamortized discount |
| 1,240,000 |
|
| –– |
|
License fee payable |
| 442,000 |
|
| 430,000 |
|
Royalties payable |
| 325,165 |
|
| 310,000 |
|
Derivative liability, long-term |
| –– |
|
| 34,000 |
|
Debentures, convertible, net of unamortized discount |
| –– |
|
| 184,870 |
|
Note payable, bank |
| –– |
|
| 28,995 |
|
Note payable, related party |
| 633,294 |
|
| –– |
|
Notes payable, convertible |
| 760,154 |
|
| 720,154 |
|
Note payable, convertible, related party |
| 491,100 |
|
| 491,100 |
|
Total long-term liabilities |
| 3,891,713 |
|
| 2,199,119 |
|
Total liabilities |
| 11,614,479 |
|
| 7,314,811 |
|
|
|
|
|
|
|
|
Stockholders' deficit |
|
|
|
|
|
|
Preferred stock, $.001 par, 10,000,000 shares authorized, |
| 978 |
|
| 1,014 |
|
977,352 and 863,691 issued and outstanding |
|
|
|
|
|
|
as of December 31, 2019 and 2018, respectively |
|
|
|
|
|
|
Common stock, $.001 par, 500,000,000 shares authorized, |
| 250,124 |
|
| 158,113 |
|
250,124,755 and 158,113,141 issued and outstanding |
|
|
|
|
|
|
as of December 31, 2019 and 2018, respectively |
|
|
|
|
|
|
Additional paid in capital - preferred |
| 1,599,036 |
|
| 1,699,000 |
|
Additional paid in capital - common |
| 19,952,533 |
|
| 11,382,341 |
|
Accumulated deficit |
| (32,057,254 | ) |
| (19,190,922 | ) |
Total stockholders' deficit |
| (10,254,583 | ) |
| (5,950,454 | ) |
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 1,359,896 |
| $ | 1,364,357 |
|
The accompanying notes are an integral part of these consolidated financial statements
- F-2 -
PARALLAX HEALTH SCIENCES, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the year ended |
| ||||
| December 31, 2019 |
| December 31, 2018 |
| ||
|
|
|
| |||
Revenue | $ | 128,600 |
| $ | 11,739 |
|
Cost of sales |
| 16,827 |
|
| 20,339 |
|
Gross profit (loss) |
| 111,773 |
|
| (8,600 | ) |
|
|
|
|
|
|
|
General and administrative expenses |
| 6,505,461 |
|
| 6,626,063 |
|
|
|
|
|
|
|
|
Operating loss |
| (6,393,688 | ) |
| (6,634,663 | ) |
|
|
|
|
|
|
|
Other expenses |
|
|
|
|
|
|
Gain on disposal of subsidiary |
| –– |
|
| 4,478,268 |
|
Gain (loss) on extinguishment of debt |
| (927,399 | ) |
| 22,858,009 |
|
Gain (loss) on fair value adjustments |
| 194,511 |
|
| (123,875 | ) |
Loss on settlements |
| (4,134,972 | ) |
| –– |
|
Impairment losses |
| (1,002,276 | ) |
| –– |
|
Discount amortization |
| (20,000 | ) |
| (2,805,000 | ) |
Interest expense |
| (582,508 | ) |
| (2,164,530 | ) |
Total other expenses |
| (6,472,644 | ) |
| 22,242,872 |
|
|
|
|
|
|
|
|
Net income (loss) - continuing operations |
| (12,866,332 | ) |
| 15,608,209 |
|
|
|
|
|
|
|
|
Net loss - discontinued operations |
| –– |
|
| (824,398 | ) |
|
|
|
|
|
|
|
Net income (loss) | $ | (12,866,332 | ) | $ | 14,783,811 |
|
|
|
|
|
|
|
|
Net income (loss) per common share - basic |
|
|
|
|
|
|
Continuing operations | $ | (0.064 | ) | $ | 0.105 |
|
Discontinued operations | $ | –– |
| $ | (0.006 | ) |
|
|
|
|
|
|
|
Net income (loss) per common share - diluted |
|
|
|
|
|
|
Continuing operations | $ | (0.049 | ) | $ | 0.072 |
|
Discontinued operations | $ | –– |
| $ | (0.004 | ) |
|
|
|
|
|
|
|
Weighted average common shares outstanding - basic |
| 201,920,901 |
|
| 148,335,736 |
|
Weighted average common shares outstanding - diluted |
| 261,528,105 |
|
| 215,576,153 |
|
The accompanying notes are an integral part of these consolidated financial statements
- F-3 -
PARALLAX HEALTH SCIENCES, INC.
STATEMENT OF STOCKHOLDERS' DEFICIT
JANUARY 1, 2018 TO DECEMBER 31, 2019
| Preferred Stock |
| Common Stock |
| Paid In Capital |
| Deferred |
| Subscriptions |
| Accumulated |
|
|
| ||||||||||||||
| Shares |
| Amount |
| Shares |
| Amount |
| Preferred |
| Common |
| Compensation |
| Receivable |
| Deficit |
| Total |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
863,691 |
| $ | 864 |
| 136,754,530 |
| $ | 136,754 |
| $ | 665,803 |
| $ | 9,637,860 |
| $ | (3,188,092 | ) | $ | (592 | ) | $ | (33,691,386 | ) | $ | (26,438,789 | ) | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of preferred stock for cash | 60,000 |
|
| 60 |
|
|
|
|
|
|
| 299,940 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 300,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of preferred stock for debt-officers/directors | 90,000 |
|
| 90 |
|
|
|
|
|
|
| 449,910 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 450,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for cash-officers/directors |
|
|
|
|
| 6.000,000 |
|
| 6,000 |
|
|
|
|
| 984,000 |
|
|
|
|
|
|
|
|
|
|
| 985,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for cash |
|
|
|
|
| 2,000,000 |
|
| 2,000 |
|
|
|
|
| 238,000 |
|
|
|
|
|
|
|
|
|
|
| 240,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for debt service |
|
|
|
|
| 2,810,000 |
|
| 2,810 |
|
|
|
|
| 278,190 |
|
| (281,000 | ) |
|
|
|
|
|
|
| –– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of debt for common stock |
|
|
|
|
| 6,881,130 |
|
| 6,881 |
|
|
|
|
| 668,733 |
|
|
|
|
|
|
|
|
|
|
| 675,614 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature of debt |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (27,643 | ) |
|
|
|
|
|
|
|
|
|
| (27,643 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant of stock options to consultants |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 539,200 |
|
| (539,200 | ) |
|
|
|
|
|
|
| –– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant of stock options to officers/directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 294,500 |
|
| (294,500 | ) |
|
|
|
|
|
|
| –– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant of stock awards for services |
|
|
|
|
| 1,750,000 |
|
| 1,750 |
|
|
|
|
| 277,360 |
|
| (153,000 | ) |
|
|
|
|
|
|
| 126,110 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant of stock warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 114,603 |
|
| (113,210 | ) |
|
|
|
|
|
|
| 1,393 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of stock options-officers/directors |
|
|
|
|
| 1,071,430 |
|
| 1,071 |
|
|
|
|
| 186,429 |
|
|
|
|
|
|
|
|
|
|
| 187,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of stock options-employees |
|
|
|
|
| 846,051 |
|
| 847 |
|
|
|
|
| 268,479 |
|
|
|
|
|
|
|
|
|
|
| 269,326 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeiture of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (184,373 | ) |
| 184,373 |
|
|
|
|
|
|
|
| –– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 572,869 |
|
|
|
|
|
|
|
| 572,869 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of stock awards |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1,095,193 |
|
|
|
|
|
|
|
| 1,095,193 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of stock warrant |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 73,370 |
|
|
|
|
|
|
|
| 73,370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in derivative liability to equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 750,200 |
|
|
|
|
|
|
|
|
|
|
| 750,200 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 283,347 |
|
|
|
|
|
|
|
| (283,347 | ) |
| –– |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subscriptions received |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5,592 |
|
|
|
|
| 5,592 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 14,783,811 |
|
| 14,783,811 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2018 | 1,013,691 |
| $ | 1,014 |
| 158,113,141 |
| $ | 158,113 |
| $ | 1,699,000 |
| $ | 14,025,538 |
| $ | (2,643,197 | ) | $ | –– |
| $ | (19,190,922 | ) | $ | (5,950,454 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Dividends paid in kind on preferred stock to treasury | 21,161 |
|
| 21 |
|
|
|
|
|
|
| 58,211 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 58,232 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancellation of preferred stock to treasury | (57,500 | ) |
| (57 | ) |
|
|
|
|
|
| (158,175 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
| (158,232 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sale of preferred treasury stock | 57,500 |
|
| 57 |
|
|
|
|
|
|
| 68,943 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 69,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of preferred stock to common stock | (57,500 | ) |
| (57 | ) | 1,150,000 |
|
| 1,150 |
|
| (68,943 | ) |
| 67,850 |
|
|
|
|
|
|
|
|
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for cash |
|
|
|
|
| 24,260,000 |
|
| 24,261 |
|
|
|
|
| 1,738,239 |
|
|
|
|
| (500,000 | ) |
|
|
|
| 1,262,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for cash-officers/directors |
|
|
|
|
| 6,000,000 |
|
| 6,000 |
|
|
|
|
| 369,000 |
|
|
|
|
|
|
|
|
|
|
| 375,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for services |
|
|
|
|
| 240,000 |
|
| 240 |
|
|
|
|
| 14,760 |
|
|
|
|
|
|
|
|
|
|
| 15,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for debt service |
|
|
|
|
| 3,010,811 |
|
| 3,010 |
|
|
|
|
| 356,504 |
|
| (33,000 | ) |
|
|
|
|
|
|
| 326,514 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for investment |
|
|
|
|
| 6,666,667 |
|
| 6,666 |
|
|
|
|
| 993,334 |
|
|
|
|
|
|
|
|
|
|
| 1,000,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of stock warrants |
|
|
|
|
| 1,875,000 |
|
| 1,875 |
|
|
|
|
| 176,825 |
|
|
|
|
|
|
|
|
|
|
| 178,700 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of debt for common stock |
|
|
|
|
| 24,682,217 |
|
| 24,682 |
|
|
|
|
| 1,710,648 |
|
|
|
|
|
|
|
|
|
|
| 1,735,330 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Conversion of debt for common stock- officers/directors |
|
|
|
|
| 12,370,000 |
|
| 12,370 |
|
|
|
|
| 824,630 |
|
|
|
|
|
|
|
|
|
|
| 837,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Grant of stock awards for services |
|
|
|
|
| 8,756,919 |
|
| 8,757 |
|
|
|
|
| 865,791 |
|
| 20,237 |
|
|
|
|
|
|
|
| 894,785 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Grant of stock awards for services-officers/directors |
|
|
|
|
| 3,000,000 |
|
| 3,000 |
|
|
|
|
| 198,300 |
|
| (198,300 | ) |
|
|
|
|
|
|
| 3,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Grant of stock options to consultants |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 584,000 |
|
| (584,000 | ) |
|
|
|
|
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Grant of stock options to officers/directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 291,700 |
|
| (291,700 | ) |
|
|
|
|
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Grant of stock warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 40,730 |
|
|
|
|
|
|
|
|
|
|
| 40,730 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Amortization of stock awards |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 699,803 |
|
|
|
|
|
|
|
| 699,803 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Amortization of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 718,391 |
|
|
|
|
|
|
|
| 718,391 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 6,450 |
|
|
|
|
|
|
|
| 6,450 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Subscriptions received |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 500,000 |
|
|
|
|
| 500,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (12,866,332 | ) |
| (12,866,332 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2019 | 977,352 |
| $ | 978 |
| 250,124,755 |
| $ | 250,124 |
| $ | 1,599,036 |
| $ | 22,257,849 |
| $ | (2,305,316 | ) | $ | –– |
| $ | (32,057,254 | ) | $ | (10,254,583 | ) |
The accompanying notes are an integral part of these consolidated financial statements
- F-4 -
PARALLAX HEALTH SCIENCES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the year ended |
| ||||
| December 31, 2019 |
| December 31, 2018 |
| ||
|
|
|
|
| ||
Cash flows from operating activities: |
|
|
|
|
|
|
Net income (loss) | $ | (12,866,332 | ) | $ | 15,608,209 |
|
Adjustments to reconcile net income (loss) to net cash used by operating activities: |
|
|
|
|
|
|
Depreciation and amortization |
| 187,381 |
|
| 120,620 |
|
Stock compensation/stock option expense |
| 2,782,426 |
|
| 3,513,710 |
|
Securities received in exchange for licenses sold |
| (50,000 | ) |
| –– |
|
Discount amortization |
| 24,791 |
|
| 2,805,000 |
|
Allowance for bad debt |
| –– |
|
| 236 |
|
(Gain) on disposal of subsidiary |
| –– |
|
| (4,478,268 | ) |
(Gain) loss on extinguishment of debt |
| 927,399 |
|
| (22,858,009 | ) |
(Gain) loss on fair value adjustments |
| (194,511 | ) |
| 123,875 |
|
Loss on settlements |
| 4,134,972 |
|
| –– |
|
Impairment loss |
| 1,002,276 |
|
| –– |
|
Debt accretion |
| 183,600 |
|
| 1,107,753 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Decrease in trade and other receivables |
| –– |
|
| 3,039 |
|
Increase in deposits |
| (7,800 | ) |
| –– |
|
Increase in accounts payable and accrued expenses |
| 45,790 |
|
| 1,980,979 |
|
Increase in royalties payable |
| 7,165 |
|
| –– |
|
Increase in related party payables |
| 877,270 |
|
| 780,872 |
|
Net cash used by operating activities |
| (2.945,573 | ) |
| (1,291,984 | ) |
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
Purchase of professional equipment |
| (2,628 | ) |
| –– |
|
Net cash used by investing activities |
| (2,628 | ) |
| –– |
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
Lease payments |
| (71,200 | ) |
| –– |
|
Proceeds from notes and loans payable |
| 330,000 |
|
| –– |
|
Repayment of notes and loans payable |
| (150,379 | ) |
| (9,245 | ) |
Proceeds from convertible notes payable |
| 1,592,280 |
|
| 825,000 |
|
Repayment of convertible notes payable |
| (398,580 | ) |
| (50,000 | ) |
Proceeds from issuance of debentures |
| –– |
|
| 225,000 |
|
Repayment of debentures |
| (754,369 | ) |
| –– |
|
Proceeds from issuance of preferred shares |
| 69,000 |
|
| 300,000 |
|
Proceeds from issuance of common shares |
| 2,337,632 |
|
| 41,250 |
|
Net cash provided by financing activities |
| 2,954,384 |
|
| 1,332,005 |
|
|
|
|
|
|
|
|
Net cash provided by continuing operations |
| 6,183 |
|
| 40,021 |
|
|
|
|
|
|
|
|
Cash flows from discontinued operations: |
|
|
|
|
|
|
Net cash used by operating activities |
| –– |
|
| (39,942 | ) |
Net cash used by discontinued operations |
| –– |
|
| (39,942 | ) |
|
|
|
|
|
|
|
Net increase in cash |
| 6,183 |
|
| 79 |
|
|
|
|
|
|
|
|
Cash - beginning of period |
| 262 |
|
| 183 |
|
|
|
|
|
|
|
|
Cash - end of period | $ | 6,445 |
| $ | 262 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Discounts on long-term liabilities | $ | 24,791 |
| $ | 2,805,000 |
|
Fair value of beneficial conversion feature of convertible promissory notes | $ | –– |
| $ | 347,487 |
|
Fair value of stock warrants | $ | 57,000 |
| $ | 1,393 |
|
Fair value of embedded conversion option of convertible promissory notes | $ | 30,620 |
| $ | 60,350 |
|
Intrinsic value of beneficial conversion feature upon extinguishment of debt | $ | –– |
| $ | 375,100 |
|
Dividends paid in kind on preferred stock returned to treasury | $ | 58,232 |
| $ | –– |
|
Conversion of preferred stock to common stock | $ | 69,000 |
| $ | –– |
|
Cancellation of preferred stock for debt settlement | $ | 100,000 |
| $ | –– |
|
Conversion of accounts payable to convertible note payable | $ | 20,000 |
| $ | –– |
|
Conversion of accounts payable to related party convertible note payable | $ | 20,000 |
| $ | –– |
|
Conversion of accounts payable to related party debentures | $ | –– |
| $ | 128,132 |
|
Conversion of accounts payable into common stock | $ | 200,000 |
| $ | –– |
|
Conversion of related party payables to preferred stock | $ | –– |
| $ | 450,000 |
|
Conversion of related party payable to common stock | $ | 753,510 |
| $ | –– |
|
Conversion of related party convertible notes payable to common stock | $ | 369,815 |
| $ | –– |
|
Conversion of convertible notes payable into common stock | $ | 135,443 |
| $ | 675,614 |
|
Conversion of convertible notes payable to debentures | $ | –– |
| $ | 755,627 |
|
Conversion of convertible debentures to common stock | $ | 785,479 |
| $ | –– |
|
Conversion of related party convertible notes payable to non-related party convertible notes payable | $ | –– |
| $ | 576,154 |
|
Conversion of related party payables to non-related party payables | $ | –– |
| $ | 42,356 |
|
Issuance of common stock for investment | $ | 1,000,000 |
| $ | –– |
|
Securities received in exchange for license fee | $ | 50,000 |
|
| –– |
|
|
|
|
|
|
|
|
SUPPLEMENTAL INFORMATION |
|
|
|
|
|
|
Interest paid: |
|
|
|
|
|
|
Continuing operations | $ | 470,101 |
| $ | 185,937 |
|
Discontinued operations | $ | –– |
| $ | 106 |
|
|
|
|
|
|
|
|
Income taxes paid | $ | –– |
| $ | –– |
|
The accompanying notes are an integral part of these consolidated financial statements
- F-5 -
PARALLAX HEALTH SCIENCES, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2019 and 2018
NOTE 1. OVERVIEW AND NATURE OF BUSINESS
Parallax Health Sciences, Inc. (the “Company”) was incorporated in the State of Nevada on July 6, 2005. The Company’s principal focus is to build and expand an integrated digital healthcare network with products and services that can provide remote communication, diagnosis, treatment, and monitoring of patients on a proprietary platform.
On August 31, 2016, the Company entered into an agreement (the “Qolpom® Agreement”) with Qolpom®, Inc., an Arizona corporation in the remote healthcare monitoring and telehealth business (“Qolpom®”) and its shareholders (the “Seller”) to purchase 100% of the issued and outstanding shares of Qolpom®’s common stock and its assets, inventory and intellectual property. The Qolpom® Agreement was fully executed on September 20, 2016, and the transaction was completed. The consideration for the acquisition resulted in a fair market value of $290,000, and goodwill of $785,060. In addition, the Qolpom® Agreement included contingent royalties and revenue sharing for future revenues generated from the Qolpom® technology. The Qolpom® name was later changed to Parallax Health Management, Inc. (“PHM”).
On March 22, 2017, the Company formed a wholly-owned subsidiary, Parallax Behavioral Health, Inc. (“PBH”), a Delaware corporation and, on April 26, 2017, completed the asset acquisition of 100% of certain intellectual property (“Intellectual Property”) from ProEventa Inc., a Virginia Corporation (“ProEventa”), in accordance with the Intellectual Property Purchase Agreement between the Company, PBH and ProEventa (the “ProEventa Agreement”). ProEventa has an expertise in the development of behavioral health technologies, and is the wholly-owned subsidiary of Grafton Integrated Health Network, Inc., a non-profit Virginia corporation (“Grafton”). Pursuant to the ProEventa Agreement, the initial consideration for the Intellectual Property was paid to ProEventa in the form of a stock purchase agreement to purchase 2,500,000 shares of the Company’s Common Stock for $2,500, resulting in a net cost for the Intellectual Property of $622,500. In addition, the ProEventa Agreement included conditional contingent royalties and revenue sharing for future revenues generated from the Intellectual Property.
On September 20, 2018, the Company formed Parallax Communications, Inc., a Delaware corporation and wholly-owned subsidiary of Parallax Health Management, Inc. to pursue additional opportunities for connected healthcare through the use of telecommunications technology.
In May 2019, the Company established a second location at 28 West 36th Street, 8th Floor, New York, NY 10018.
In June 2019, the Company filed a Registration Statement on form S-1 for the registration, as amended, of 32,583,436 shares of Common Stock, and, after resolving the comments and questions from the SEC in relation to the filing, the Company received an effective date from the SEC of December 20, 2019.
In June 2019, pursuant to a resolution of the board of directors, the Company adopted the 2019 Stock Incentive Plan (the “2019 Plan”), wherein forty million (40,000,000) shares of the Company’s restricted Common Stock were reserved for issuance. The 2019 Plan was intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. The 2019 Plan is currently administered by the Company's Board. Subject to the provisions of the plan, the board will determine who shall receive options, the number of shares of Common Stock that may be purchased under the options. On June 17, 2019, the Company filed a Registration Statement on form S-8 for the registration of shares reserved under the 2019 Plan.
On August 28, 2019, the Company entered into a Purchase Agreement (the “Purchase Agreement”) with Global Career Networks Inc., a Delaware corporation, (“GCN”) to acquire a 19% interest in GCN. The Purchase Agreement was fully executed on September 6, 2019, with an effective date of October 15, 2019. Pursuant to the Purchase Agreement, in exchange for 6,666,667 shares of the Company’s restricted Common Stock, valued at $1,000,000, the Company acquired 760 shares of GCN common stock. In addition, in the event the market value of Parallax Common Stock one (1) year from Effective Date, is greater than $0.075 per share but less than $0.30 per share, the Company is required to issue GCN up to an additional 20,000,000 shares of restricted Common Stock, for an aggregate value of the Company’s Common Stock held by GCN of $2,000,000. At December 31, 2019, the Company recorded an impairment on the investment of $1,000,000.
Business Overview
The Company’s principal focus is on personalized patient care through remote healthcare services, behavioral health systems, and Point-of-Care (“POC”) diagnostic testing. Parallax’s current family of companies that serve as the foundation for its cross-over business model of operations include:
●Parallax Diagnostics, Inc. (“Parallax Diagnostics” or “PDI”) acquired a proprietary Point-of-Care diagnostic immunoassay testing platform and 25 test cartridges for the areas of infectious diseases, cardiac markers, drugs of abuse and various other medical conditions.
●Parallax Health Management, Inc. (“PHM”) develops RPM and telehealth market products and services, and commercializes them, including the Fotodigm® proprietary platform which allows for systems integration with a number of third-party services and solutions.
●Parallax Behavioral Health, Inc. (“PBH”) acquired the intellectual property known as REBOOT™, the acronym for Reliable Evidence-Based Outcomes Optimization Technologies, as well as the Intrinsic Code™ technology, a software platform specifically designed to improve health treatment outcomes through cloud-based and mobile behavioral technology systems that enable its users and user groups to more effectively achieve goals within a prescribed timeline.
- F-6 -
Good Health Outcomes™ is the Company’s technology-enabled digital healthcare system, structured with three separate divisions that can operate independently of one another, or integrate services to meet the various needs of the Company’s clientele: Optimized Outcomes, Connected Health and Smart Data. Each of these divisions target a separate vertical market that are synergistic, compliment, and strengthen each other.
Optimized Outcomes | REBOOT™ / COMPASS™ Behavioral modification |
Connected Health | Fotodigm® platform Remote patient monitoring, telehealth, and POC diagnostic testing |
Smart Data | Intrinsic Code™ technology Actionable insights to behavior modification |
Operating Segments
The Company’s operations include the following operating segments for financial statement presentation: Remote Patient Monitoring (RPM), Behavioral Health Services (BHS), and Diagnostics/Corporate.
●Remote Patient Monitoring
The Company provides a distinctive technology platform that provides for the complete remote patient care delivery system: the patent-pending Fotodigm® platform, which utilizes proprietary software and technology to bridge clinical behavioral science with technology and logistics for payers, providers and clinical professionals across a variety of wellness and clinical devices, including both fitness and clinical applications. Fotodigm® is a secure and scalable platform for collecting, transmitting and analyzing biometric, pharmaceutical, and health data to healthcare providers, primarily hospitals, accredited nursing operations, and physicians.
The RPM segment generates revenues through fees charged for the license and utilization of its proprietary system that provides software integrations of the Fotodigm® platform. Additionally, the RPM segment will generate incremental revenues through the delivery of acute, post-acute and chronic health patient management software systems that enable Parallax customers to bill for and collect payments from patients and third-party payers for telemonitoring and remote services that they deliver.
The BHS segment commenced with the acquisition of the REBOOT™ and Intrinsic Code™ technologies in April 2017. The BHS segment will generate revenues primarily through licensing and subscription of software and systems. As of December 31, 2019, the BHS segment had not yet begun full operations, generating limited test market sales.
●Diagnostics/Corporate
The Diagnostics/Corporate Segment supports the costs and operating expenses related to the continued development and exploitation of the Company’s proprietary Target System POS diagnostic platform and processes. In addition, the Diagnostics/Corporate Segment provides management and administrative services to support the Company and consists of certain aspects of the Company’s executive management, corporate relations, legal, compliance, human resources, corporate information technology and finance departments.
RoxSan Pharmacy
On August 13, 2015, the Company entered into an agreement with RoxSan Pharmacy, Inc., a California corporation (“RoxSan” or the “Pharmacy”), and its sole shareholder, Shahla Melamed (“Melamed” or “Former Owner”), to purchase 100% of the issued and outstanding shares of RoxSan's common stock and its assets and inventory. Pursuant to the agreement, the Company, among other things, issued the Seller a Secured Promissory Note in the amount of $20.5 million. As a result, effective August 13, 2015, RoxSan became the Company's wholly-owned subsidiary. Concurrently, Mrs. Melamed resigned from all positions within RoxSan, and Mr. J. Michael Redmond was appointed RoxSan's President and Chief Executive Officer, and Ms. Calli R. Bucci its Chief Financial Officer. Mr. Redmond and Ms. Bucci were also appointed as Chairman and member, respectively, of RoxSan’s board of directors.
RoxSan provided a full range of pharmacy services including retail, compounding and fertility medications, and generated revenues primarily by dispensing prescription drugs, both through local channels by direct delivery as well as mail order. RoxSan also sold a wide assortment of general merchandise, including over-the-counter drugs, beauty products and cosmetics, seasonal merchandise and convenience foods, through the Company’s pharmacy. The pharmacy was fully licensed and qualified to conduct business in over 40 US States.
In October 2015, shortly following the Company’s acquisition of RoxSan, Melamed, initiated two (2) legal actions against the Company in the Superior Court of the State of California, County of Los Angeles, West District, Shahla Melamed v. Parallax Health Sciences, Inc., action numbers SC 124873 and SC 125702. In the matter, action No. SC 124873, Melamed sought rescission of the August 13, 2015, Purchase Agreement. In the Matter, action No. SC125702, Melamed alleged that the Company was in default under the terms of the Purchase Agreement and Secured Note and Melamed’s employment agreement.
- F-7 -
The Company also initiated legal action against Melamed and filed a complaint in October 2015, action number SC 124898, in the Superior Court of the State of California, County of Los Angeles, West District, Parallax Health Sciences, Inc., et al. v. Shahla Melamed, et al. The complaint in that action alleges that Melamed breached several obligations under the Purchase Agreement, and the Company sought to reduce the Secured Note due to undisclosed material changes in the business.
Since the Company’s acquisition of RoxSan, the deleterious actions against the pharmacy by the Former Owner, including, among other things, interference with management and operations, and attempts to damage and/or divert customer and vendor relationships, had a significant adverse impact on the pharmacy. Furthermore, the discovery of the Former Owner’s alleged involvement in suspected insurance fraud caused RoxSan’s contract with its primary In Vitro Fertilization (“IVF”) drug rebate program to be terminated in August 2016. As a result, RoxSan was no longer eligible to receive incentive rebates for the majority of its IVF drug purchases, which were key to the profitability of the IVF drug sales; and for which without the rebates, RoxSan was unable to provide its customers with comparably priced IVF drugs. This, among other things, caused a precipitous drop of over 90% in RoxSan’s IVF revenues, and ultimate exit from the IVF market in mid-2017. Soon thereafter, in July 2017, RoxSan’s contract with its primary drug supplier was terminated for similar reasons connected to the Former Owner and alleged criminal activities associated with the Melamed family name, despite the Company’s new ownership and management. After careful consideration, the Company determined that RoxSan was unable to generate enough profits to sustain its pharmacy business, and on December 22, 2017, the pharmacy ceased operations and closed the business location in Beverly Hills, CA; however, some residual operations were still ongoing during 2018 to wind down the business.
On May 14, 2018, pursuant to unanimous resolutions of the boards of directors of RoxSan Pharmacy, Inc. and Parallax Health Sciences, Inc., RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California. Mr. Timothy Yoo was appointed trustee on May 15, 2018. In connection with this filing, RoxSan sought to discharge approximately $5 million of liabilities owed to various parties, and intercompany loans in excess of $1 million owed to the Company. The Chapter 7 bankruptcy proceeding by RoxSan Pharmacy, Inc. was fully discharged, and the case was closed on March 13, 2019, in U.S. Bankruptcy Court, Central District of California.
Due to, among other things, the reduction in RoxSan’s cash flows during 2016 and 2017, RoxSan became delinquent in its payroll tax depository obligations, resulting in a liability owed to federal and state taxing agencies in the aggregate of $1,148,811, which includes $601,148 in taxes withheld from employees (“Trust Fund Taxes”), employer taxes of $183,172, and penalties and interest of $364,491 through December 31, 2018. The liability was included as part of the Chapter 7 bankruptcy petition, and certain portions of the liability may be discharged. However, in accordance with California bankruptcy laws, federal and state Trust Fund Taxes are not dischargeable. The Company has retained a tax resolution specialist and is in communications with the taxing agencies in order to resolve RoxSan’s liability. During the year ended December 31, 2019, payments for Trust Fund Taxes in the amount of $485,498 were made, and $115,650 in Trust Fund Taxes were outstanding at December 31, 2019.
As a result of the loss of financial control of RoxSan, the Company derecognized the subsidiary effective May 14, 2018. The derecognition resulted in a gain of $4,478,268. The Company also extinguished $22,778,281 in debt and accrued interest related to the acquisition of RoxSan.
In January 2019, Melamed requested mediation, seeking settlement of the pending litigation with the Company, including that which was initiated against the Company by her son, Hootan Melamed (Shahla and Hootan, collectively, the “Melameds”). Through mediation, the Company and the Melamed reached agreeable settlement terms, and on February 19, 2020, the Company received a counter-signed Settlement and Release Agreement (the “Settlement”). Effective February 12, 2020 (the “Effective Date”), the Settlement is by and between Parallax Health Sciences, Inc., RoxSan Pharmacy, Inc., Michael Redmond, Edward Withrow III, Huntington Chase Financial Group, LLC, Calli Bucci and Dave Engert (collectively, “Parallax”), and the Melameds, and resolves all pending lawsuits between the parties in connection with the acquisition of RoxSan Pharmacy. The Settlement terms include (i) three installment payments, totaling $4,000,000, to the Melameds; (ii) ten (10) million shares of the Company’s Common Stock issued as directed by Melamed; and (iii) 10% of proceeds from the sale or merger of the Company within two (2) years, if any, up to $3,000,000 (See Note 8). In the event the Company fails to cure a breach of timely payment of any portion of the Settlement Sum within thirty (30) days of a notice of default, a Stipulated Judgement may be filed by Melamed in the sum of $20,000,000, less any Settlement Sum amounts previously paid by the Company.
Going Concern
The Company has incurred losses since inception resulting in an accumulated deficit of $32,057,254, and a working capital deficit of $7,664,145, and further losses are anticipated. The Company’s ability to continue as a going concern is dependent upon its ability to generate profitable operations in the future and/or to obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due, which may not be available at commercially reasonable terms. There can be no assurance that the Company will be able to continue to raise funds, in which case the Company may be unable to meet its obligations and the Company may cease operations. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern.
The Company will require additional financing in order to proceed with its plan of operations, including approximately $5,000,000 over the next 12 months to pay for its ongoing expenses and debt obligations. These cash requirements include working capital, general and administrative expenses, the development of the Company’s product line, the reduction of contractual debt obligations, and the pursuit of acquisitions. These cash requirements are in excess of the Company’s current cash and working capital resources. Accordingly, the Company will require additional financing in order to continue operations and to repay its liabilities. There is no assurance that the financing will be completed as planned or at all. If the Company is unable to secure adequate capital to continue the Company’s planned operations, the Company’s shareholders may lose some or all of their investment and the Company’s business may fail.
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and that effect the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
The consolidated financial statements reflect all adjustments consisting of normal recurring adjustments, which, in the opinion of management, are necessary for a fair presentation of the results for the periods shown. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts of and classification of liabilities that might be necessary in the event the Company cannot continue as a going concern.
NOTE: The following notes and any further reference made to “the Company”, “we”, “us”, “our” and “Parallax” shall mean Parallax Health Sciences, Inc., and its wholly-owned subsidiaries, Parallax Diagnostics, Inc., Parallax Health Management, Inc., Parallax Behavioral Health, Inc., and Parallax Communications, Inc., unless otherwise indicated. The Company’s former wholly-owned subsidiary, RoxSan Pharmacy, Inc., was derecognized effective May 14, 2018 (See “RoxSan Pharmacy” section above).
NOTE 2. RESTATEMENT
On October 18, 2019, the Company concluded that the previously issued audited consolidated financial statements as of and for the years ended December 31, 2018 and 2017, should no longer be relied upon. The Company reached its conclusion after consultation with its Audit Committee and a joint discussion with the Company’s independent registered public accounting firm.
The Company has restated its audited consolidated financial statements as of and for the years ended December 31, 2018 and 2017, to reflect adjustments made in connection with the accounting treatment of certain convertible debt (the “Subject Debt”), warrants (the “Subject Warrants”), and convertible preferred stock (the “Subject Preferred Stock”). The adjustments resulted in material overstatements and understatements, the nature and impact of which are further described below.
Valuation of Convertible Debt and Warrant Liabilities:
The Company reviewed the accounting treatment of the Subject Debt, Subject Warrants, and Subject Preferred Stock, and concluded that it was not in accordance with U.S. generally accepted accounting principles. Specifically, the Subject Debt, Subject Warrants and Subject Preferred Stock were not evaluated to determine the impact (if any) of 1) embedded conversion option; 2) beneficial conversion feature; 3) bifurcation; 4) derivative liability; and 5) fair value adjustments and other expenses thereto. A third-party valuation was performed on the Subject Debt, Subject Warrants and Subject Preferred Stock, and the accounting treatment was determined.
The effects of the accounting treatment, all non-cash in nature, resulted in a restatement of convertible debentures and convertible notes payable, additional paid in capital, and accumulated deficit, and the establishment of a derivative liability, resulting in changes to total liabilities and total stockholders’ deficit on the consolidated balance sheets; and a restatement of general and administrative expenses, gain on extinguishment of debt, discount amortization, and interest expense, and the establishment of a loss on fair value adjustments, resulting in changes to net income (loss), net loss per share-basic, and net loss per share-diluted on the consolidated statements of operations; and the restatement of stock compensation/stock option expense, discount amortization, gain on extinguishment of debt, loss on fair value adjustments, debt accretion, and the increase in accounts payable and accrued expenses from operating activities on the consolidated statements of cash flows. The following tables summarize the impacts on the Company’s consolidated financial statements as of and for the years ended December 31, 2018 and 2017. There was no impact from this restatement on the years ended prior to December 31, 2017:
| December 31, 2018 |
| December 31, 2017 |
| ||||||||||||||
| As Previously Reported |
| As Restated |
| Increase (Decrease) |
| As Previously Reported |
| As Restated |
| Increase (Decrease) |
| ||||||
CONSOLIDATED BALANCE SHEETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative liability, short-term |
| –– |
|
| 23,925 |
|
| 23,925 |
|
| –– |
|
| –– |
|
| –– |
|
Debentures, convertible |
| 755,627 |
|
| 724,903 |
|
| (30,724 | ) |
| –– |
|
| –– |
|
| –– |
|
Debentures, convertible, related party |
| 428,132 |
|
| 411,006 |
|
| (17,126 | ) |
| –– |
|
| –– |
|
| –– |
|
Notes payable, convertible, net of discount |
| 296,000 |
|
| 296,000 |
|
| –– |
|
| 741,000 |
|
| 1,706 |
|
| (739,294 | ) |
Total current liabilities |
| 5,139,617 |
|
| 5,115,692 |
|
| (23,925 | ) |
| 4,418,915 |
|
| 3,679,621 |
|
| (739,294 | ) |
Derivative liability, long-term |
| –– |
|
| 34,000 |
|
| 34,000 |
|
| –– |
|
| –– |
|
| –– |
|
Debentures, convertible, net of unamortized discount |
| 226,050 |
|
| 184,870 |
|
| (41,180 | ) |
| –– |
|
| –– |
|
| –– |
|
| 7,345,916 |
|
| 7,314,811 |
|
| (31,105 | ) |
| 28,930,119 |
|
| 28,190,825 |
|
| (739,294 | ) | |
Additional paid in capital - preferred |
| 1,415,653 |
|
| 1,699,000 |
|
| 283,347 |
|
| 665,803 |
|
| 665,803 |
|
| –– |
|
Additional paid in capital - common |
| 9,715,921 |
|
| 11,382,341 |
|
| 1,666,420 |
|
| 5,580,668 |
|
| 6,449,768 |
|
| 869,100 |
|
Accumulated deficit |
| (17,272,260 | ) |
| (19,190,922 | ) |
| 1,918,662 | [1] |
| (33,561,580 | ) |
| (33,691,386 | ) |
| 129,806 |
|
Total stockholders' deficit |
| (5,981,559 | ) |
| (5,950,454 | ) |
| (31,105 | ) |
| (27,178,083 | ) |
| (26,438,789 | ) |
| (739,294 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CONSOLIDATED STATEMENTS OF OPERATIONS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative expenses | $ | 6,552,693 |
| $ | 6,626,063 |
| $ | 73,370 |
| $ | 3,997,512 |
| $ | 4,125,612 |
| $ | 128,100 |
|
Operating loss |
| (6,561,293 | ) |
| (6,634,663 | ) |
| 73,370 |
|
| (4,044,619 | ) |
| (4,172,719 | ) |
| 128,100 |
|
Gain on extinguishment of debt |
| 23,215,862 |
|
| 22,858,009 |
|
| (357,853 | ) |
| –– |
|
| –– |
|
| –– |
|
Loss on fair value adjustments |
| –– |
|
| (123,875 | ) |
| 123,875 |
|
| –– |
|
| –– |
|
| –– |
|
Discount amortization |
| (2,806,050 | ) |
| (2,805,000 | ) |
| (1,050 | ) |
| –– |
|
| –– |
|
| –– |
|
Interest expense, net of income |
| (1,213,069 | ) |
| (2,164,530 | ) |
| 951,461 |
|
| (1,016,773 | ) |
| (1,018,479 | ) |
| (1,706 | ) |
Total other income (expenses) |
| 23,675,011 |
|
| 22,242,872 |
|
| (1,432,139 | ) |
| (6,466,773 | ) |
| (6,468,479 | ) |
| (1,706 | ) |
Net income (loss) - continuing operations |
| 17,113,718 |
|
| 15,608,209 |
|
| (1,505,509 | ) |
| (10,511,392 | ) |
| (10,641,198 | ) |
| 129,806 |
|
Net income (loss) |
| 16,289,320 |
|
| 14,783,811 |
|
| (1,505,509 | ) |
| (13,664,945 | ) |
| (13,794,751 | ) |
| 129,806 |
|
Net income (loss) per common share - continuing operations - basic | $ | 0.115 |
| $ | 0.105 |
| $ | (0.010 | ) | $ | (0.087 | ) | $ | (0.088 | ) | $ | (0.001 | ) |
Net income (loss) per common share - continuing operations - diluted | $ | 0.079 |
| $ | 0.072 |
| $ | (0.007 | ) | $ | (0.059 | ) | $ | (0.060 | ) | $ | 0.001 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CONSOLIDATED STATEMENTS OF CASH FLOWS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) | $ | 17,113,718 |
| $ | 15,608,209 |
| $ | (1,505,509 | ) | $ | (10,511,392 | ) | $ | (10,641,198 | ) | $ | 129,806 |
|
Stock compensation/stock option expense «E2XNCUF7»] |
| 3,440,340 |
|
| 3,513,710 |
|
| 73,370 |
|
| 2,473,510 |
|
| 2,601,608 |
|
| 128,098 |
|
Discount amortization |
| 2,806,050 |
|
| 2,805,000 |
|
| (1,050 | ) |
| –– |
|
| –– |
|
| –– |
|
Gain on extinguishment of debt |
| (23,215,862 | ) |
| (22,858,009 | ) |
| 357,853 |
|
| –– |
|
| –– |
|
| –– |
|
Loss on fair value adjustments |
| –– |
|
| 123,875 |
|
| 123,875 |
|
| –– |
|
| –– |
|
| –– |
|
Debt accretion |
| –– |
|
| 1,107,753 |
|
| 1,107,753 |
|
| –– |
|
| 1,706 |
|
| 1,706 |
|
Increase in accounts payable and accrued expenses |
| 2,137,271 |
|
| 1,980,979 |
|
| (156,292 | ) |
| 1,731,757 |
|
| 1,731,759 |
|
| 2 |
|
Non-Cash Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Discounts on long-term liabilities | $ | 2,806,050 |
| $ | 2,805,000 |
| $ | (1,050 | ) | $ | 5,450,000 |
| $ | 5,450,000 |
| $ | –– |
|
Beneficial conversion feature of convertible promissory notes |
| –– |
|
| 347,487 |
|
| 347,487 |
|
| –– |
|
| 592,582 |
|
| 592,582 |
|
Fair value of stock warrants |
| –– |
|
| 1,393 |
|
| 1,393 |
|
| –– |
|
| 148,418 |
|
| 148,418 |
|
Embedded conversion option of convertible promissory notes |
| –– |
|
| 60,350 |
|
| 60,350 |
|
| –– |
|
| –– |
|
| –– |
|
Supplemental Information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid-continuing operations |
| 342,230 |
|
| 185,937 |
|
| (156,293 | ) |
| 52,566 |
|
| 52,566 |
|
| –– |
|
[1]Includes 2018 and 2017 net increase in accumulated deficit of $1,505,509 and $129,806, respectively, and deemed dividends of $283,347 and $0, respectively.
- F-8 -
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
This summary of significant accounting policies is presented to assist in understanding the Company’s financial statements. These accounting policies conform to accounting principles, generally accepted in the United States of America, and have been consistently applied in the preparation of the financial statements.
The Company’s fiscal year-end is December 31.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated.
Changes in the Company’s ownership interests in subsidiaries that do not result in the Company losing control over the subsidiaries are accounted for as equity transactions. When the Company loses control of a subsidiary, a gain or loss is recognized and is calculated as the difference between:
●the aggregate of the fair value of consideration received and the fair value of any retained interest at the date when control is lost; and
●the carrying amount of the net assets (liabilities) of the subsidiary and any noncontrolling interest.
Upon deconsolidation of a subsidiary, any loans to the former subsidiary made by the Company are measured at fair value at the deconsolidation date. Any difference between the carrying amount of the loan to the subsidiary and its fair value is included as part of the gain or loss calculation upon deconsolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Fair Value Hierarchy
The Company utilizes the three-level valuation hierarchy for the recognition and disclosure of fair value measurements. The categorization of assets and liabilities within this hierarchy is based upon the lowest level of input that is significant to the measurement of fair value. The three levels of the hierarchy consist of the following:
Level 1: Inputs to the valuation methodology are unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
Level 2: Inputs to the valuation methodology are quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active or inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the instrument.
Level 3: Inputs to the valuation methodology are unobservable inputs based upon management’s best estimate of inputs market participants could use in pricing the asset or liability at the measurement date, including assumptions about risk.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents. As of December 31, 2019 and 2018, the Company had no cash equivalents.
Fair Value of Financial Instruments
As of December 31, 2019 and 2018, the carrying values of Company’s Level 1 financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, and short-term debt, approximate fair value. The fair value of Level 3 instruments is calculated as the net present value of expected cash flows based on externally provided or obtained inputs. Certain Level 3 instruments may also be based on sales prices of similar assets. The Company’s fair value calculations take into consideration the credit risk of both the Company and its counterparties as of the date of valuation. See Note 6 for additional information about long-term debt.
●Derivatives of financial instruments:
Derivatives are initially recognized at fair value at the date a derivative contract is entered into and are subsequently remeasured to their fair value at the end of each reporting period, with changes in fair value recognized in profit or loss. A derivative is presented as a non-current asset or a non-current liability if the remaining maturity of the instrument is more than 12 months and it is not expected to be realized or settled within 12 months. Other derivatives are presented as current assets or current liabilities.
●Embedded derivatives:
Derivatives embedded in other financial instruments or other host contracts are treated as separate derivatives when their risks and characteristics are not closely related to those of the host contracts and the host contracts are not measured at fair value with changes in fair value recognized in profit or loss. An embedded derivative is presented as a non-current asset or a non-current liability if the remaining maturity of the hybrid instrument to which the embedded derivative relates is more than 12 months and it is not expected to be realized or settled within 12 months. Other embedded derivatives are presented as current assets or current liabilities.
- F-9 -
The following table represents the Company’s derivative financial instruments:
| December 31, 2019 |
| December 31, 2018 |
| ||
Convertible debentures | $ | –– |
| $ | 23,925 |
|
Convertible promissory notes-embedded conversion option |
| 5,800 |
|
| –– |
|
Warrants |
| 57,000 |
|
| 34,000 |
|
Total derivative liability | $ | 62,800 |
| $ | 57,925 |
|
The following table represents the changes in the Company’s derivative financial instruments:
| December 31, 2019 |
| December 31, 2018 |
| ||
Fair value of derivative liability, beginning | $ | 57,925 |
| $ | –– |
|
Increase in derivative liability-debentures |
| –– |
|
| 60,350 |
|
Increase in derivative liability-convertible promissory notes |
| 30,620 |
|
| –– |
|
Increase in derivative liability-warrants |
| 182,000 |
|
| 623,900 |
|
Fair value adjustment-debentures |
| (26,691 | ) |
| (2,500 | ) |
Fair value adjustment-convertible promissory notes |
| (24,820 | ) |
| –– |
|
Fair value adjustment-warrants |
| (143,000 | ) |
| 126,375 |
|
Reclassification of warrant carrying value due to reset of exercise price |
| –– |
|
| (750,200 | ) |
Reclassification of carrying value due to extinguishment |
| (13,234 | ) |
| –– |
|
Fair value of derivative liability, ending | $ | 62,800 |
| $ | 57,925 |
|
Investments
The Company records investments at cost, net of impairment. During the year ended December 31, 2019, the Company acquired $1,050,000 of equity securities without readily determinable fair value, and recorded an impairment of.$1,000,000. At December 31, 2019, the Company held $50,000 of equity securities without readily determinable fair value, net of impairment.
Property and Equipment
Property and equipment is comprised of office and computer equipment and software, furniture and fixtures, and leasehold improvements, recorded at cost and depreciated using the double declining balance method over the estimated useful lives of 5 to 7 years. Repairs and maintenance costs are charged directly to expense as incurred. Major renewals or replacements that substantially extend the useful life of an asset are capitalized and depreciated. See Note 5 for additional information about property and equipment.
Intangible Assets
Product processes, patents and customer lists are amortized on a straight-line basis over their estimated useful lives between 4 and 20 years. Application development stage costs for significant internally developed software projects are capitalized and amortized on a straight-line basis over the useful life, between 2 and 5 years. Costs to extend and maintain patents and trademarks are charged directly to expense as incurred. See Note 6 for additional information about intangible assets.
Goodwill and other Indefinitely-lived assets
Goodwill and other indefinitely-lived assets are not amortized, but are subject to impairment reviews annually, or more frequently if necessary.
Impairment of Long-Lived Assets
The Company evaluates long-lived assets for impairment whenever events or changes in circumstances indicate that their net book value may not be recoverable. When such factors and circumstances exist, the Company compares the projected undiscounted future cash flows associated with the related asset or group of assets over their estimated useful lives against their respective carrying amount. Impairment, if any, is based on the excess of the carrying amount over the fair value, based on market value when available, or discounted expected cash flows, of those assets and is recorded in the period in which the determination is made.
During the year ended December 31, 2019, the Company recognized $1,002,276 in impairment losses related to its long-lived assets.
The Company believes that future projected cash flows are sufficient for the recoverability of the remainder of its long-lived assets, and no other impairment exists. There can be no assurance, however, that market conditions will not change or demand for the Company’s products and products under development will continue. Either of these could result in future impairment losses.
Convertible Debt
The Company recognizes the advantageous value of conversion rights attached to convertible debt. Such rights give the debt holder the ability to convert debt into Common Stock at a price per share that is less than the trading price to the public on the date of the debt. The beneficial value is calculated as the intrinsic value (the market price of the stock at the commitment date in excess of the conversion rate) of the beneficial conversion feature of the debt, and is recorded as a discount to the related debt and an addition to additional paid in capital. The discount is amortized over the remaining outstanding period of related debt using the interest method.
- F-10 -
Net Income (Loss) Per Common Share
The computation of basic earnings per share ("EPS") is based on the weighted average number of shares that were outstanding during the period, including shares of Common Stock that are issuable at the end of the reporting period. The computation of diluted EPS is based on the number of basic weighted-average shares outstanding plus the number of common shares that would be issued assuming the exercise of all potentially dilutive Common Stock equivalents. Dilutive Common Stock equivalents consist of shares issuable upon conversion of convertible debt, convertible preferred shares and the exercise of the Company’s stock options and warrants.
Comprehensive Loss
As of December 31, 2019 and 2018, the Company has no items that represent comprehensive loss and, therefore, has not included a schedule of comprehensive loss in the financial statements.
Revenue Recognition
The Company recognizes revenue when it satisfies a performance obligation by transferring control over a product or service to a customer. Revenue is measured based on a consideration specified in a contract with a customer, and excludes any amounts collected on behalf of third parties.
Income Taxes
Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. These assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which the temporary differences are expected to reverse.
The Company may have net operating loss carryforwards available to reduce future taxable income. Future tax benefits for these net operating loss carryforwards are recognized to the extent that realization of these benefits is considered more likely than not. To the extent that the Company will not realize a future tax benefit, a valuation allowance is established.
As of December 31, 2019, the Company has not yet filed its 2012 through 2018 annual corporate income tax returns. Due to the Company’s recurring losses, it is anticipated that no corporate income taxes are due for these periods.
Stock-Based Compensation
The Company records stock-based compensation using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued.
Recently Adopted Accounting Standards
The Company evaluates the pronouncements of various authoritative accounting organizations, primarily the Financial Accounting Standards Board (“FASB”), the US Securities and Exchange Commission (“SEC”), and the Emerging Issues Task Force (“EITF”), to determine the impact of new pronouncements on US GAAP and the impact on the Company. The Company has recently adopted the following new accounting standards:
Adopted:
In January 2016, the FASB issued ASU No. 2016-01 (“ASU 2016-01”), Financial Instruments-Overall (Subtopic 825-10) Recognition and Measurement of Financial Assets and Financial Labilities. ASU 2016-01 addresses certain aspects of recognition, measurement, presentation, and disclosure of financial instruments in an effort to enhance the reporting model for financial instruments to provide users of financial statements with more decision-useful information. ASU 2016-01 is effective for the Company for annual periods beginning after December 15, 2017, and interim periods. Early adoption is permitted.
In July 2017, the FASB issued ASU No. 2017-11 (“ASU 2017-11”), Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480), Derivatives and Hedging (Topic 815). ASU 2017-11 addresses the complexity of accounting for certain financial instruments with down round features. Down round features are features of certain equity-linked instruments (or embedded features) that result in the strike price being reduced on the basis of the pricing of future equity offerings. ASU 2017-11 also addresses the difficulty of navigating Topic 480, Distinguishing Liabilities from Equity, because of the existence of extensive pending content in the FASB Accounting Standards Codification®. ASU 2017-11 is effective for the Company for annual periods beginning after December 15, 2018, and interim periods. Early adoption is permitted.
In June 2018, the FASB issued ASU No. 2018-07 (“ASU 2018-07”), Compensation-Stock Compensation (Topic 718), Improvements to Nonemployee Share-Based Payment Accounting. ASU 2018-07 expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. ASU 2018-07 is effective for the Company for annual periods beginning after December 15, 2018, and interim periods. Early adoption is permitted.
In July 2018, the FASB issued ASU No. 2018-11 (“ASU 2018-11”), Leases (Topic 842), Targeted Improvements. ASU 2018-11 addresses certain issues in implementing ASU 2016-02, Leases, which was issued to increase transparency ad comparability by recognizing lease assets and liabilities on the balance sheet and disclosing key information about leasing transaction. ASU 2018-11 clarifies 1) comparative reporting requirements for initial adoption; and 2) for lessors only, separating lease and non-lease components in a contract and allocating the consideration in the contract to the separate components. The amendments in this Update related to separating components of a contract affect the amendments in Update 2016-02, which is effective for the Company for annual periods beginning after December 15, 2018, and interim periods. Early adoption is permitted.
In August 2018, the FASB issued ASU No. 2018-13 (“ASU 2018-13”), Fair Value Measurement (Topic 820), Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement. ASU 2018-13 modifies the disclosure requirements on fair value measurements in Topic 820, Fair Value Measurement, based on the concepts in the Concepts Statement, including the consideration of costs and benefits. ASU 2018-13 is effective for the Company for annual periods beginning after December 15, 2019, and interim periods. Early adoption is permitted.
In August 2018, the FASB issued ASU No. 2018-15 (“ASU 2018-15”), Intangibles-Goodwill and Other Internal-Use Software (Subtopic 350-40). ASU 2018-15 was issued to help entities evaluate the accounting for fees paid by a customer in a cloud computing arrangement (hosting arrangement) by providing guidance for determining when the arrangement includes a software license. ASU 2018-15 is effective for the Company for annual periods beginning after December 15, 2019, and interim periods. Early adoption is permitted.
Not Yet Adopted:
In April 2019, the FASB issued ASU No. 2019-04 (“ASU 2019-04”), Codification Improvements to Topic 326, Financial Instruments-Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments. The amendments to Topic 326 and other Topics in ASU 2019-04 clarify or address stakeholders’ specific issues about certain aspects of the amendments in Update 2016-13. The amendments to Topic 815 in ASU 2019-04 include items related to Update 2017- 12 and clarify certain aspects of Topic 815. The amendments to Topic 321 and other Topics in ASU 2019-04 relate to the amendments in Update 2016-01 and clarify certain aspects of the amendments in Update 2016-01. ASU 2019-04 will be effective for the Company for annual periods beginning after December 15, 2019, and interim periods. Early adoption is permitted. The Company is currently evaluating the impact of the application of this accounting standard update on its financial statements and related disclosures.
In November 2019, the FASB issued ASU No. 2019-10 (“ASU 2019-10”), Financial Instruments-Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842), Effective Dates. ASU 2019-10 was issued to apply changes in how to determine the effective dates for ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (Credit Losses); ASU No. 2017-12, Derivatives and Hedging (Topic 815): Targeted Improvements to Accounting for Hedging Activities (Hedging); and ASU No. 2016-02, Leases (Topic 842) (Leases). Following ASU-2019-10, ASU 2016-13 will be effective for the Company for annual periods beginning after December 15, 2019, and interim periods; and ASU 2017-12 and ASU 2016-01 will be effective for the Company for annual periods beginning after December 15, 2018, and interim periods. Early adoption is permitted. The Company is currently evaluating the impact of the application of this accounting standard update on its financial statements and related disclosures.
In December 2019, the FASB issued ASU No. 2019-12 (“ASU 2019-12”), Income Taxes (Topic 740). ASU 2019-12 was issued to simplify the accounting for income taxes by removing certain exceptions to the general principles in Topic 740. The amendments also improve consistent application of and simplify GAAP for other areas of Topic 740 by clarifying and amending existing guidance. ASU 2019-12 will be effective for the Company for annual periods beginning after December 15, 2020, and interim periods. Early adoption is permitted. The Company is currently evaluating the impact of the application of this accounting standard update on its financial statements and related disclosures.
Recently Issued Accounting Standards Updates:
There were various updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries. None of the updates are expected to a have a material impact on the Company's consolidated financial position, results of operations or cash flows.
- F-11 -
NOTE 4. OPERATING LEASES
The Company entered into a sub-lease agreement with PearTrack Security Systems, Inc. (“PearTrack”), whose principal is a related party, on December 1, 2017, for its headquarters in Santa Monica, California, with a monthly sub-lease payment of $5,600, on a month-to-month basis, consistent with the underlying lease between PearTrack and property owner. Due to the short-term nature of the sub-lease, the Company has elected to not recognize the operating lease asset and liability, and will expense the rent as incurred.
The Company entered into a sub-lease agreement with AI Assist, Inc. (“AI Assist”) on May 1, 2019, for certain office space located in New York, New York, with a monthly sub-lease payment of $8,900 for a term of fourteen (14) months, maturing June 30, 2020. The right-of-use present value of the sub-lease has been calculated as $118,585, utilizing an implied interest rate of 8%. The operating lease asset and liability will be amortized over the term of the lease.
During the year ended December 31, 2019, the Company recognized $66,409 in amortization. The present value of future lease payments at December 31, 2019, was $52,176. As of December 31, 2019, the future minimum lease payments are as follows:
| For the year ended December 31, 2020 |
| |
Operating lease minimum payments | $ | 53,400 |
|
Less: amount treated as interest |
| 1,224 |
|
Present value of minimum lease payments | $ | 52,176 |
|
The total lease costs are summarized as follows:
| December 31, 2019 |
| December 31, 2018 |
| ||
Operating lease costs | $ | 71,200 |
| $ | –– |
|
Short-term lease costs |
| 68,320 |
|
| 73,351 |
|
Total lease costs | $ | 139,520 |
| $ | 73,351 |
|
Lease costs were $139,520 and $73,351 for the years ended December 31, 2019 and 2018, respectively.
NOTE 5. PROPERTY AND EQUIPMENT
Property and equipment, net, consists of the following:
| December 31, 2019 |
| December 31, 2018 |
| ||
Computer equipment | $ | 6,615 |
| $ | 3,987 |
|
Medical devices |
| 45,194 |
|
| 45,194 |
|
Property and equipment, gross |
| 51,809 |
|
| 49,181 |
|
Accumulated depreciation |
| (49,533 | ) |
| (49,181 | ) |
Impairment loss |
| (2,276 | ) |
| –– |
|
Property and equipment, net | $ | –– |
| $ | –– |
|
During the years ended December 31, 2019 and 2018, respectively, the Company recognized $2,276 and $0 in impairment loss related to its property and equipment.
Depreciation expense for the years ended December 31, 2019 and 2018, respectively, was $352 and $0.
NOTE 6. INTANGIBLE ASSETS
The following are the components of finite-lived intangible assets:
December 31, 2019 |
| December 31, 2018 |
| |||
Products and processes | $ | 12,500 |
| $ | 12,500 |
|
Trademarks and patents / technology |
| 150,700 |
|
| 150,700 |
|
Customer lists / relationships |
| 30,000 |
|
| 30,000 |
|
Non-compete agreement |
| 30,000 |
|
| 30,000 |
|
Marketing related |
| 64,000 |
|
| 64,000 |
|
Software |
| 510,300 |
|
| 510,300 |
|
Sub-total |
| 797,500 |
|
| 797,500 |
|
Accumulated amortization |
| (339,085 | ) |
| (218,465 | ) |
Intangible assets, net | $ | 458,415 |
| $ | 579,035 |
|
Amortization expense for the years ended December 31, 2019 and 2018, was $120,620 and $120,620, respectively.
- F-12 -
NOTE 7. ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consist of:
December 31, 2019 |
| December 31, 2018 |
| |||
Accounts payable-vendors | $ | 1,128,381 |
| $ | 830,590 |
|
Credit cards payable |
| 42,552 |
|
| 42,552 |
|
Payroll taxes payable |
| 81,718 |
|
| 78,608 |
|
Accrued interest |
| 376,065 |
|
| 450,187 |
|
Accrued payroll and payroll taxes |
| 338,612 |
|
| 402,053 |
|
Other liabilities |
| 115,650 |
|
| 601,148 |
|
|
| 2,082,978 |
|
| 2,405,138 |
|
Reserve-legal fees |
| –– |
|
| 250,000 |
|
|
|
|
|
|
|
|
Total accounts payable and accrued expenses | $ | 2,082,978 |
| $ | 2,655,138 |
|
Payroll taxes payable includes $17,475 and $17,475 in penalties, and $7,312 and $4,202 in interest, related to unpaid payroll taxes as of December 31, 2019 and 2018, respectively.
Other liabilities consists of certain payroll tax liabilities in the amount of $115,650 and $601,148 owed as of December 31, 2019, and 2018, respectively, owed by the bankrupt entity, RoxSan Pharmacy, Inc., that were not discharged under California bankruptcy laws. The Company has retained a tax resolution specialist to aid the Company in resolving the liability with the taxing agencies on behalf of RoxSan.
During the years ended December 31, 2019 and 2018, respectively, accounts payable and accrued expenses was reduced by $101,367 and $341,606, resulting from the extinguishment of debt consisting of accounts payable-vendors in the amount of $101,367 and $284,714, and accrued interest in the amount of $0 and $56,892.
The Company established an estimated reserve of $0 and $250,000 at December 31, 2019 and 2018, respectively, for legal fees to be incurred in connection with pending legal actions (Note 18).
NOTE 8. SETTLEMENT PAYABLE
On August 13, 2015, the Company issued a secured promissory note in the amount of $20,500,000 (the “Promissory Note”) in connection with the acquisition of RoxSan Pharmacy, Inc. (“RoxSan”) to Shahla Melamed (“Melamed”), the former owner of RoxSan. The Promissory Note bore interest at a rate of 6% per annum, and matured August 13, 2018 (“Maturity”). At the time of issuance, management determined that the Promissory Note did not fairly represent the fair market value for the related acquisition. As a result, a discount of $15,300,000, representing the difference between the face value and the estimated fair market value of the Promissory Note was recorded and has been fully expensed.
On May 14, 2018, pursuant to unanimous resolutions of the boards of directors of RoxSan Pharmacy, Inc. and Parallax Health Sciences, Inc., RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California (the “Court”). Mr. Timothy Yoo was appointed trustee (“Trustee”) on May 15, 2018. In connection with this filing, RoxSan sought to discharge approximately $5 million in liabilities owed to various parties, and intercompany loans in excess of $1 million owed to Parallax. The Chapter 7 bankruptcy proceeding was fully discharged by the Court, and the case was closed on March 13, 2019.
As part of the derecognition of RoxSan resulting from the bankruptcy in 2018, management reevaluated the characteristics of the Promissory Note. Included in the evaluation were the following considerations: 1) the related asset was no longer a part of the parent financial statements due to a loss of financial control; 2) the Company is currently in litigation as a result of material breaches by the note holder, Melamed; 3) the Company has claims against Melamed for losses and damages directly related to the Promissory Note and its underlying assets; 4) there was a high likelihood that no obligation existed. After careful consideration, management determined that the characteristics of the liability were contingent in nature at December 31, 2018, and extinguished the debt of $20,500,000 and related $2,278,281 in accrued interest, resulting in a gain of $22,778,281.
In January 2019, Melamed requested mediation, seeking settlement of the pending litigation with the Company, including that which was initiated against the Company by her son, Hootan Melamed (Shahla and Hootan, collectively, the “Melameds”). Through mediation, the Company and the Melameds reached agreeable settlement terms, and on February 19, 2020, the Company received a counter-signed Settlement and Release Agreement (the “Settlement”). Effective February 12, 2020 (the “Effective Date”), the Settlement is by and between Parallax Health Sciences, Inc., RoxSan Pharmacy, Inc., Michael Redmond, Edward Withrow III, Huntington Chase Financial Group, LLC, Calli Bucci and Dave Engert (collectively, “Parallax”), and the Melameds, and resolves all pending lawsuits between the parties in connection with the acquisition of RoxSan Pharmacy.
In consideration of the resolution of all existing and potential claims between the parties, including the cancellation of the Company’s contingent liability in the principal sum of $20,500,000, and accrued interest of approximately $4,500,000, and without further action or litigation and without admission of liability by either party, the Settlement terms include the following:
●A payment of $4,000,000 (the “Settlement Sum”) to the Melameds, to be paid as follows:
$1,250,000 within 90 days of the Effective Date;
$1,250,000 within one (1) year of the Effective Date;
$1,500,000 within two (2) years of the Effective Date.
●The issuance of ten (10) million shares of the Company’s Common Stock to an entity owned by Shahla Melamed.
In addition, in the event forty percent (40%) or more of the Company and/or its subsidiaries (including by way of merger) is sold within two (2) years of the Effective Date, the Company shall pay the Melameds, within two (2) weeks of receipt of the proceeds from such sale (the “Sale Proceeds”), any outstanding unpaid Settlement Sum plus an additional 10% of the Sale Proceeds received, up to a total of an additional $3,000,000 over and above the Settlement Sum.
In the event the Company fails to cure a breach of timely payment of any portion of the Settlement Sum within thirty (30) days of a notice of default, a Stipulated Judgement may be filed by Melamed in the sum of $20,000,000, less any Settlement Sum amounts previously paid by the Company.
- F-13 -
The following table represents the liabilities recognized in connection with the Settlement:
| December 31, 2019 |
| |
|
|
|
|
Cash settlement, gross | $ | $4,000,000 |
|
|
|
|
|
Less: unamortized present value discount |
| (415,800 | ) |
Net present value of settlement |
| 3,584,200 |
|
|
|
|
|
Common stock, at FMV |
| 480,000 |
|
|
|
|
|
Total settlement | $ | 4,064,200 |
|
|
|
|
|
Due within 12 months | $ | 2,824,200 |
|
Due December 31, 2021 |
| 1,240,000 |
|
| $ | 4,064,200 |
|
NOTE 9. NOTES AND LOANS PAYABLE
Notes and loans payable consists of the following:
December 31, 2019 | December 31, 2018 | |||||
Short-term: |
|
|
|
|
|
|
Debentures, convertible | $ | –– |
| $ | 724,903 |
|
Notes payable |
| 345,000 |
|
| –– |
|
Note payable, bank |
| 18,616 | [1] |
| –– |
|
Notes payable, convertible |
| 1,102,364 |
|
| 296,000 |
|
Total short-term notes payable |
| 1,465,980 |
|
| 1,020,903 |
|
|
|
|
|
|
|
|
Long-term: |
|
|
|
|
|
|
Debentures, convertible, net of unamortized discount |
| –– |
|
| 184,870 |
|
Note payable, bank |
| –– |
|
| 28,995 | [1] |
Notes payable, convertible |
| 760,154 |
|
| 720,154 |
|
Total long-term notes and loans payable |
| 760,154 |
|
| 934,019 |
|
|
|
|
|
|
|
|
Total notes and loans payable | $ | 2,226,134 |
| $ | 1,954,922 |
|
[1] Note payable, bank, maturing in July 2020, has been reclassified as short-term liability at December 31, 2019.
Non-related party convertible debt consist of the following convertible promissory notes:
Holder |
| Principal |
| APR |
| Accrued Interest |
| Conversion Price |
| Term/Due |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-Term: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Investor Group A |
| $ | 91,000 |
| 10% |
| $ | 21,013 |
| $0.10 |
| 02/2020 |
|
Lender Group A |
|
| 20,000 |
| 12% |
|
| 10,000 |
| $0.10 |
| 02/2020 |
|
Lender Group B |
|
| 519,641 |
| 12% |
|
| 59,688 |
| $0.10 to $0.12 |
| 04/2020 to 12/2020 |
|
Lender Group C |
|
| 471,723 |
| 10% |
|
| 5,944 |
| 65% to 70% of Market |
| 10/2020 to 12/2020 |
|
|
| 1,102,364 |
|
|
|
| 96,645 |
|
|
|
|
| |
Long-Term: |
|
|
|
|
|
|
|
|
|
|
|
|
|
The Kasper Group, Ltd. |
|
| 184,000 |
| 7% |
|
| 85,431 |
| $0.10 |
| 12/2021 |
|
Joseph M. Redmond |
|
| 576,154 | [1] | 5% |
|
| 148,358 | [1] | $0.10 |
| 07/2017 |
|
|
|
| 760,154 |
|
|
|
| 233,789 |
|
|
|
|
|
Total convertible debt |
| $ | 1,862,518 |
|
|
| $ | 330,434 |
|
|
|
|
|
[1]As of January 1, 2018, Mr. Joseph M. Redmond, former President and member of the board of directors, is no longer a related party. As a result, $576,154 in convertible promissory notes and accrued interest of $90,742 was reclassified from related party transactions (Note 10) to non-related party transactions. See Note 18 for additional information and legal proceedings related to Mr. Redmond.
During the year ended December 31, 2019, the Company issued four (4) short-term non-related party non-convertible promissory notes (the “Notes”) in the aggregate principal of $375,000, of which repayments in the amount of $30,000 were made. The Notes bear interest at a rate of 8% per annum, and mature between 12/31/2019 to 08/20/2020. As additional consideration, three of the note holders received 400,000 shares of the Company’s restricted Common Stock each, for an aggregate of 1,200,000 shares, valued at $190,200, and one note holder received 2,400,000 shares, valued at $240,000.
- F-14 -
During the years ended December 31, 2019 and 2018, respectively, the Company issued short-term non-related party convertible promissory notes (the “Convertible Notes”) in the aggregate principal of $1,490,000 and $825,000, along with 5,800,000 and 6,025,000 warrants (Note 14). Upon issuance, the Convertible Notes were discounted by a total of $308,840 and $605,000, representing $96,220 and $0 in original issue discount, $0 and $223,607 for beneficial conversion feature, $30,620 and $0 for embedded conversion option, and $182,000 and $381,393 for value of warrants, of which $212,620 and $380,000 was classified as derivative liability. Effective November 14, 2018, $600,000 in principal, plus $55,627 in accrued interest, was exchanged for short-term convertible debentures, at which time $240,000 was reclassified to equity for intrinsic value of warrants, $30,725 was classified as derivative liability for embedded conversion option, and $105,320 was recognized as a loss on extinguishment of debt. The short-term non-related party convertible notes and debentures bear interest at a rate of 10% to 12% per annum, mature in 2020, and are convertible into shares of the Company’s Common Stock at a conversion price equal to the lower of 1) between $0.10 to $0.12 per share; or 2) between 65% to 70% of the 2nd lowest trading prices during the twenty (20) trading days preceding the conversion date. During the years ended December 31, 2019 and 2018, respectively, a total of $173,784 and $1,098,974 in debt accretion was expensed; repayments on Convertible Notes totaling $564,023 and $428,580 and $50,000 was repaid in cash, and $135,443 and $620,000 was converted into the Company’s Common Stock; repayments on debentures totaling $1,169,299 and $0 were made, of which $542,369 and $0 was repaid in cash, and $626,930 and $0 was converted in to the Company’s Common Stock; and a loss on extinguishment of $305,912 and $0 was recognized.
During the year ended December 31, 2018, the Company issued long-term non-related party convertible debentures in the aggregate principal sum of $250,000, along with 600,000 warrants (Note 14). Upon issuance, the long-term convertible debentures were discounted by a total of $67,500, representing $25,000 in original issue discount, $12,500 for embedded conversion option, and $30,000 for value of warrants, of which $42,500 was classified as derivative liability. The debentures maturing in 2021, are convertible into the Company’s Common Stock at a conversion price of $0.10 per share. During the years ended December 31, 2019 and 2018, respectively, a total of $9,816 and $2,370 in debt accretion was expensed, cash repayments of $212,000 and $0 were made; $158,549 and $0 was converted into the Company’s Common Stock, and, a loss on extinguishment of $168,159 and $0 was recognized.
During the years ended December 31, 2019 and 2018, respectively, principal payments of $10,379 and $9,245 in and $1,423 and $632 in interest were made on the note payable to bank. The term loan bears interest at a rate of 7.48%, with monthly payments of principal and interest in the amount of $1,475, maturing July 17, 2020. The principal remaining on the note at December 31, 2019, of $18,616 was reclassified from long-term to short-term.
During the year ended December 31, 2018, the long-term convertible promissory note issued to The Kasper Group was modified to extend the maturity date to October 1, 2019, in exchange for a fee of $40,000. As a result, the principal amount owed was increased from $144,000 to $184,000. Upon maturity, the Kasper Group note was further modified to extend the maturity to December 31, 2021. The long-term non-related party convertible promissory notes bear interest at a rate of 5% to 7% per annum, mature between 2017 to 2021, and are convertible into the Company’s Common Stock at a conversion price of $0.10 per share.
During the year ended December 31, 2018, long-term unsecured non-related party loans and promissory notes in the principal sum of $95,975, and accrued interest in the amount of $56,892, were extinguished, resulting in a gain of $152,867.
During the year ended December 31, 2018, a long-term secured non-related party promissory note in the principal sum of $20,500,000, and accrued interest in the amount of $2,278,281, was extinguished in connection with RoxSan’s Chapter 7 petition filed in May 2018 (Note 18), and recharacterized as a contingent liability (Note 11), resulting in a gain of $22,778,281.
As of December 31, 2019 and 2018, respectively, short-term non-related party debt in the amount $1,465,980 and $1,020,903 consists of $0 and $724,903 in convertible debentures; $345,000 and $0 in notes payable; $18,616 and $0 in note payable, bank, and $1,102,364 and $296,000 in convertible notes payable. During the years ended December 31, 2019 and 2018, respectively, interest in the amount of $201,658 and $270,142 was expensed. As of December 31, 2019 and 2018, respectively, a total of $105,372 and $185,741 in accrued interest remains, and is included as an accrued expense on the accompanying consolidated balance sheet.
As of December 31, 2019 and 2018, respectively, long-term non-related party debt in the amount of $760,154 and $934,019 consists of $0 and $207,500 in convertible debentures, less unamortized discount of $0 and $22,630; $760,154 and $720,154 in convertible notes payable, of which $576,154 and $576,154 is related to pending litigation with a former executive (see Note 18), and subject to compromise; and $0 and $28,995 in notes payable to banks. During the years ended December 31, 2019 and 2018, respectively, interest in the amount of $85,469 and $739,383 was expensed. As of December 31, 2019 and 2018, respectively, a total of $234,432 and $190,386 in accrued interest remains, and is included as an accrued expense on the accompanying consolidated balance sheet.
The future maturities of long-term notes payable are summarized as follows:
Year | Principal | ||
2020 |
| $ | 778,770 |
2021 |
|
| 184,000 |
| $ | 962,770 | |
During the years ended December 31, 2019 and 2018, respectively, interest on non-related party notes and loans payable in the amount of $287,127 and $1,009,525 was expensed. As of December 31, 2019 and 2018, respectively, a total of $339,804 and $376,127 in interest, net of debt extinguishments of $0 and $2,335,173, and conversions to Common Stock of $10,398 and $55,613, remains accrued and is included as part of accrued expenses on the accompanying consolidated balance sheets.
- F-15 -
NOTE 10. RELATED PARTY TRANSACTIONS
Related party transactions consist of the following:
December 31, 2019 | December 31, 2018 | |||||
Related party payables |
|
|
|
|
|
|
Accrued compensation | $ | 796,175 |
| $ | 869,859 | [1] |
Accrued benefits and cash advances |
| 312,305 |
|
| 134,861 |
|
Total related party payables |
| 1,108,480 |
|
| 1,004,720 |
|
|
|
|
|
|
|
|
Debentures, convertible |
| –– |
|
| 411,006 |
|
Notes payable, related party, convertible | 491,100 |
| 491,100 |
| ||
Notes payable, related party |
| 759,446 |
|
| –– |
|
|
|
|
|
|
|
|
Total related party transactions | $ | 2,359,026 |
| $ | 1,906,826 |
|
[1]As of January 1, 2018, Mr. Joseph M. Redmond, former President and member of the board of directors, is no longer a related party. As a result, related party transactions was reduced by $618,510, representing $42,356 in accrued compensation, and $576,154 in convertible promissory notes. As of December 31, 2018, $42,356 is included as part of accounts payable and accrued expenses, and $576,154 is included as part of long-term convertible notes payable, on the accompanying consolidated balance sheets. In addition, accrued interest of $90,742 related to the convertible promissory notes was reclassified from related party to non-related party accrued interest. See Note 18 for additional information and legal proceedings related to Mr. Redmond.
As of December 31, 2019 and 2018, respectively, related parties are due a total of $2,359,026 and $1,906,826, consisting of $796,175 and $869,859 in accrued compensation owed to related parties; $312,305 and $134,861 in accrued benefits owed to related parties, and cash advances from related parties for operating expenses; $0 and $411,006 in convertible debentures, net of embedded conversion option of $0 and $17,125; $759,446 and $0 in promissory notes, and $491,100 and $491,100 in convertible promissory notes.
Related party convertible debt consists of the following:
Note Holder |
| Principal |
| APR |
| Accrued Interest |
| Conversion Price |
| Term/Due |
| ||
|
|
|
|
|
|
|
| ||||||
Convertible promissory notes: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Huntington Chase, Beneficial Owner |
| $ | 491,100 |
| 7% |
| $ | 9,795 |
| $0.08 |
| 12/2023 |
|
During the years ended December 31, 2019 and 2018, respectively, $1,586,724 and $1,371,446 in related party compensation was accrued, $802,500 and $510,500 was paid, $753,510 and $0 was converted to Common Stock; and $0 and $450,000 was converted to Preferred Stock.
During the years ended December 31, 2019 and 2018, respectively, $399,249 and $104,151 in benefits were accrued and cash advances were made to the Company by related parties for overhead requirements, of which $221,805 and $97,587 was paid/repaid to related parties.
In June 2019, $575,132 of related party debt was purchased by non-related-parties (the “Proceeds”). The Proceeds were collected from the non-related parties by the Company on behalf of the related parties. The Proceeds were subsequently loaned to the Company by the related parties for operating expenses, including accrued compensation owed.
Promissory Notes
On September 30, 2015, the Company issued a modified convertible promissory note in the principal sum of $631,100, representing cash loans and unpaid compensation to a related party, of which principal repayments in the aggregate of $100,000 were made in prior years, and $40,000 of which was converted into Common Stock in prior years. The note bears interest at a rate of 7% per annum, and contains a repayment provision to convert the debt into restricted shares of the Company’s Common Stock at a price of $0.10 per share. On December 31, 2018, the note was modified to 1) reduce the principal balance to $491,100; and 2) mature December 31, 2023. During the year ended December 31, 2019, the note holder converted $437,000 in principal into 4,370,000 shares of the Company’s Common Stock. Subsequently, the holder loaned the Company $437,000. As a result, the principal balance was not affected. However, on December 31, 2019, the promissory note was modified to change the conversion price to $0.08.
Between April 26, 2018 and May 8, 2018, the Company issued senior secured convertible promissory notes (the “Notes”) to a related party in the aggregate principal sum of $337,750, along with 3,377,500 warrants (Note 14). Upon issuance, the Notes were discounted by a total of $337,750, representing $123,850 for beneficial conversion feature, and $213,900 for fair value of warrants. During the year ended December 31, 2018, a total of $6,409 in debt accretion was expensed. The Notes bore interest at rate of 12% per annum, contained a repayment provision to convert the Notes into restricted shares of the Company’s Common Stock at a price of $0.10 per share, and included warrant coverage for a period of three (3) years to purchase shares of the Company’s Common Stock at an exercise price of $0.10 per share. Effective November 14, 2018, the Notes and related accrued interest of $34,090 were exchanged into convertible debentures (“Debentures”) in the principal amount of $428,132, at which time $17,125 was reclassified as a derivative liability for embedded conversion option, $135,100 was reclassified to equity for the intrinsic value of warrants issued with the Notes, and $252,533 was recognized as a loss on extinguishment of the Notes. The Debentures bore interest at a rate of 10% per annum, matured February 28, 2019, and were convertible into shares of the Company’s restricted Common Stock at a conversion rate of $0.12 per share. On July 25, 2019, the Debentures in the principal sum of $411,006, plus accrued interest of $42,778, were converted to non-convertible Senior Secured Promissory Notes (the “Senior Notes”) in the aggregate principal of $759,446. The Senior Notes bear interest at a rate of 8% per annum, with payments of $126,152 plus interest accrued thereon due December 31, 2019; $300,000 due December 31, 2020; and the remaining principal and accrued interest due December 31, 2021. In connection with the exchange, the Company issued the following in favor of the lender: 1,380,811 shares of the Company’s restricted Common Stock, valued at $131,315; and warrants, valued at $92,150, to purchase 2,528,413 shares of the Company’s Common Stock for a period of five (5) years at an exercise price of $0.10461. As a result, the Company recognized a net loss on the exchange in the amount of $434,837, net of $2,140 in derivative liability remaining from the warrants issued with the Debentures.
As of December 31, 2019 and 2018, respectively, related party promissory notes in the amount of $1,250,546 and $491,100 are owed, of which $491,100 and $491,100 are convertible.
During the years ended December 31, 2019 and 2018, respectively, interest in the amount of $105,111 and $66,840 was expensed, $0 and $798 was paid to the note holders in cash, $100,132 and $0 was converted to restricted shares of the Company’s Common Stock, and $42,778 and $71,839 was converted to principal. As of December 31, 2019 and 2018, respectively, a total of $36,261 and $74,060 in accrued interest remains and is included as part of accrued expenses on the accompanying consolidated balance sheets.
- F-16 -
On January 11, 2018, pursuant to a resolution of the board of directors, the Company issued 6,000,000 shares of its restricted Common Stock to certain officers and directors. The shares were purchased at par, or $0.001 per share, for cash in the amount of $6,000.
On August 13, 2018, in connection with the exercise of certain employee stock options, the Company issued 1,071,430 shares of its restricted Common Stock at a conversion rate of $0.05 per share. The shares were issued on a cashless basis, resulting in a net value of $187,500.
On September 30, 2018, in connection with the Series C convertible Preferred Stock equity offering (Note 11), three officers of the Company were issued an aggregate of 90,000 Series C preferred shares at a price of $5.00 per share in exchange for accrued compensation in the aggregate of $450,000. Upon issuance, a beneficial conversion feature of $100,578 was recorded as a deemed dividend. In connection with the Series C preferred shares, the officers were also issued an aggregate of 1,250,000 warrants, valued at an aggregate of $75,000, and exercisable for a period of three (3) years at an exercise price of $0.25 per share.
On May 15, 2019, in connection with a certain employment agreement with Mr. David Appell, the Company issued 3,000,000 shares of its restricted Common Stock, valued at $201,300, of which 25% vest immediately, and the remainder vest when certain earnings goals are met.
On July 31, 2019, in connection with the settlement of certain related party convertible debentures, the Company issued 1,380,811 shares of its restricted Common Stock, valued at $131,315. In connection with the settlement, the related parties were also issued an aggregate of 2,528,413 warrants, valued at an aggregate of $92,150, and exercisable for a period of five (5) years at an exercise price of $0.10 per share
On September 18, 2019, in connection with the conversion of certain convertible debt in the amount of $437,000, the, Company issued 4,370,000 shares of its restricted Common Stock, to Huntington Chase, LLC, a beneficial owner.
On September 30, 2019, in connection with a Simple Agreement Future Equity (“SAFE”) offering, the Company issued 6,000,000 shares of its restricted Common Stock to certain officers and directors in exchange for the reduction in accrued compensation in the aggregate of $375,000. In connection with the SAFE offering, the officers and directors were also issued an aggregate of 6,000,000 warrants, valued at an aggregate of $45,600, and exercisable for a period of three (3) years at an exercise price of $0.25 per share.
On December 31, 2019, in connection with debt settlement agreements, the Company issued 8,000,000 shares of its restricted Common Stock to certain officers and directors in exchange for the reduction in accrued compensation in the aggregate of $400,000.
Agreements
On January 1, 2018, the Company entered into an Executive Agreement with its Chief Financial Officer. The agreement replaces any other written agreement with the Company, is for a term of one (1) year, with the option to extend, and includes annual compensation of $216,000 in year 1, as well as a bonus plan and customary executive benefits. In addition, the agreement provides for a non-refundable, fully-vested signing bonus of $100,000, as well as a grant of stock options to purchase 1,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest quarterly over a one (1) year period.
On January 1, 2018, the Company entered into a Consulting Agreement with Huntington Chase, LLC, whose managing member is a related party. The agreement replaces any other written agreement with the Company, is for a term of three (3) years, and includes monthly compensation of $25,000 in year 1; $30,000 in year 2, and $35,000 in year 3, of which the year 2 and 3 increases are deferred until completion of certain development projects, as well as customary expense allowances. In addition, the agreement provides for a grant of stock options to purchase 4,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, of which 25% vest immediately, and the remainder vest when certain market share prices of the Company’s Common Stock are met.
On January 1, 2019, the Company entered into a Consulting Agreement with JL Ogden & Company LLC, for the services of John L. Ogden, a member of the Company’s board of directors, and Chair of the Audit Committee. The agreement is for a term of one (1) year, with the option to extend, and includes annual compensation in the amount of $60,000. In addition, the agreement provides for a non-refundable, fully-vested signing bonus of $50,000, a convertible promissory note in the amount of $20,000 maturing in one (1) year with an annual interest rate of ten percent (10%), a restricted stock award equal to $70,000 worth of Units in the Company’s 2019 private placement offering, as well as a grant of stock options to purchase 1,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest quarterly over a one (1) year period.
On May 15, 2019, the Company entered into an employment agreement with Mr. David Appell to serve as the Company’s Chief Operating Officer. The agreement is for an initial term of two (2) years, and provides a base compensation of $250,000 year one, and $275,000 in year two, as well as various performance bonuses, and customary employee benefits. In addition, the agreement includes a grant to purchase 3,000,000 restricted common shares, valued at $201,300, for cash in the amount of $3,000, of which 25% vest immediately, and the remainder vest when certain earnings goals are met; as well as options granted to purchase 3,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options valued at $195,600 using the Black-Scholes method, are for a period of five (5) years, and vest annually over the term of the agreement, with an initial vesting of 25%. The assumptions used in valuing the options were: expected term 4.75 years, expected volatility 2.21, risk free interest rate 2.15%, and dividend yield 0%.
- F-17 -
NOTE 11. COMMITMENTS AND CONTINGENCIES
On August 31, 2016, as part of the Company’s acquisition of 100% of the issued and outstanding shares of Qolpom®’s common stock and its assets, inventory and intellectual property, the agreement provides for, among other things, the seller to receive up to $2,000,000 through a percentage of revenue generated from RPM business segment (“Revenue Share”), as well as a royalty of 3% (“Royalties”) of certain revenues generated from the Qolpom® intellectual property, as defined in the agreement. As of December 31, 2019 and 2018, respectively, the present value of future Revenue Share was $442,000 and $430,000; and the present value of future Royalties was $318,000 and $310,000.
On April 26, 2017, as part of the Company’s acquisition of 100% of certain intellectual property (“Intellectual Property”) from ProEventa, Inc., a Virginia Corporation (“ProEventa”), the agreement provides for, among other things, ProEventa to receive a revenue sharing cash earn-out of up to $3,000,000 to be derived from certain net revenue generated by the Company; as well as Royalties of 3% of certain revenues generated from the Intellectual Property, ending at such time as the Company has paid ProEventa $25,000,000, as defined in the agreement. As of December 31, 2019 and 2018, respectively, the present value of future Revenue Share was $968,000 and $1,040,000; and the present value of future Royalties was $471,000 and $690,000.
NOTE 12. CONVERTIBLE PREFERRED STOCK
The total number of authorized shares of Preferred Stock that may be issued by the Company is 10,000,000 with a par value of $0.001 per share.
The holders of all series of Preferred Stock have no voting rights. Series A and Series B preferred shares are convertible into the Company’s Common Stock at a rate of 20 shares of Common Stock for each preferred share held. Series C preferred shares are convertible into the Company’s Common Stock at a rate of 41.67 shares of Common Stock for each preferred share held. Series B preferred shares were issued with 50% warrant coverage for a period of two (2) years, to purchase shares of the Company's Common Stock at a price of $0.75 per share. Series C preferred shares were issued with 50% warrant coverage for a period of three (3) years, to purchase shares of the Company's Common Stock at a price of $0.25 per share. The number of shares of Common Stock underlying the warrants and the exercise price are subject to adjustment upon certain events. Dividends are payable semi-annually on the Company’s Series A Preferred Stock at a rate of 7% per annum, 10% per annum on Series B, and 8% per annum on Series C. Dividends may be paid in kind, at the option of the Company, to the extent that if the Company is not legally permitted to distribute cash dividends, it shall pay dividends in the form of preferred shares equal to the amount of the dividend. No dividends have been declared on the Company’s Preferred Stock. In the event of any liquidation, dissolution, winding-up or sale or merger of the Company, whether voluntarily or involuntarily, each holder of Preferred Stock is entitled to receive, in preference to the holders of Common Stock, a per-share amount equal to the original issue price plus all declared but unpaid dividends and dividends in arrears.
In August 2018, the Company established a private placement equity offering for the purchase of Series C convertible Preferred Stock (the “Series C Shares”). The offering provided for, among other thing, the purchase of Series C Shares at a price of $5.00 per share, with a minimum unit of 20,000 shares, or $100,000. All Series C Shares are convertible into Common Stock at a conversion rate of $0.12 per share, or a ratio of 41.67 shares of Common Stock for each Series C Share held (41.67:1) (“Conversion Ratio”) if converted within one (1) year, or at a lesser Conversion Ratio after one year. The shares also include warrants to purchase Common Stock for a period of 3 years at an exercise price of $0.25 per share, of which the number of warrants is determined at 50% of the prevailing Conversion Ratio. The Series C offering is closed to further investors.
During the year ended December 31, 2018, in connection with the Series C Shares equity offering, 150,000 Series C Shares were issued at a price of $5.00 per share, of which 60,000 were issued to accredited investors for cash in the amount of $300,000, and 90,000 were issued to officers of the Company in exchange for debt in the principal sum of $450,000. As a result, $749,850 was recorded to preferred paid in capital, and a beneficial conversion feature of $283,347 was recorded as a deemed dividend, of which $100,578 was in connection with the shares issued to officers of the Company.
On March 31, 2019, in connection with the settlement agreement with Mr. Dave Engert, 36,339 shares of the Company’s Series A Preferred Stock held by Mr. Engert, with a book value of $100,000, were returned to treasury. As a result, preferred paid in capital was reduced by $99,964. On April 1, 2019, dividends owed on the Series A Preferred Stock were paid in kind with the issuance and immediate return to treasury of 21,121 shares of Series A Preferred Stock, resulting in a total of 57,500 shares of Series A Preferred Stock held in treasury (the “Treasury Shares”).
On May 15, 2019, the 57,500 Treasury Shares were reissued for cash in the amount of $69,000. Subsequently, the 57,500 shares of Series A Preferred Stock were converted into 1,150,000 shares of Common Stock at a ratio of 20 shares of Common Stock for each share of Preferred Stock held.
As of December 31, 2019 and 2018, respectively, the Company had 977,352 and 1,013,691 shares of Preferred Stock issued and outstanding.
- F-18 -
The total number of authorized shares of Common Stock that may be issued by the Company at December 31, 2019 and 2018, is 500,000,000 with a par value of $0.001 per share.
During the years ended December 31, 2019 and 2018, respectively, 24,682,217 and 6,881,130 shares of the Company’s restricted Common Stock were issued in connection with the conversion of non-related party debt in the amount of $1,735,330 and $675,614. As a result, $1,710,649 and $668,733 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 12,370,000 and 0 shares of the Company’s restricted Common Stock were issued in connection with the conversion of related party debt in the amount of $837,000 and $0. As a result, $824,630 and $0 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 0 and 846,051 shares of the Company’s restricted Common Stock were issued for the exercise of stock options, and 0 and 1,071,430 were issued for the exercise of related party stock options. As a result, $0 and $454,908 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 8,756,919 and 1,750,000 shares of the Company’s restricted Common Stock were issued in connection with stock awards to non-related parties, valued at $874,548 and $279,110. As a result, $20,237 and $153,000 was deferred, to be amortized over the next nine (9) months, and $865,191 and $277,360 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 3,000,000 and 0 shares of the Company’s restricted Common Stock were issued in connection with stock awards to related parties valued at $201,300 and $0. As a result, $198,300 and $0 was deferred, to be amortized over the next nine (9) months, and $198,300 and $0 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 12,500,000 and 2,000,000 shares of the Company’s restricted Common Stock were issued for cash in the amount of $1,027,500 and $240,000. As a result, $1,015,000 and $238,900 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 3,010,811 and 2,810,000 shares of the Company’s restricted Common Stock were issued in connection with debt and debt service in the amount of $359,514 and $281,000. As a result, $356,504 and $278,190 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 18,000,000 and 0 shares of the Company’s restricted Common Stock were issued in connection with a certain Simple Agreement Future Equity (“SAFE”) offering, including 6,000,000 and 0 shares issued to related parties, for cash in the amount of $735,000 and $0, services valued at $15,000 and $0, and the reduction of related party debt in the amount of $375,000 and $0. As a result, $1,107,000 and $0 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 0 and 6,000,000 shares of the Company’s restricted Common Stock were issued to officers, for cash in the amount of $0 and $6,000. As a result, $0 and $984,000 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 1,150,000 and 0 shares of the Company’s restricted Common Stock were issued in connection with the conversion of Preferred Stock valued at $69,000 and $0. As a result, $67,850 and $0 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 6,666,667 and 0 shares of the Company’s restricted Common Stock were issued in connection with an investment valued at $1,000,000 and $0. As a result, $993,333 and $0 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, 1,875,000 and 0 shares of the Company’s restricted Common Stock were issued in connection with the exercise of warrants valued at $178,700 and $0. As a result, $176,825 and $0 was recorded to paid in capital.
During the years ended December 31, 2019 and 2018, respectively, a total of 92,011,614 and 21,358,611 shares of the Company’s restricted Common Stock were issued. As of December 31, 2019 and 2018, respectively, the Company had 250,124,755 and 158,113,141 common shares issued and outstanding.
- F-19 -
During the years ended December 31, 2019 and 2018, respectively, 3,830,000 and 3,660,000 restricted stock awards were granted, valued at $264,350 and $434,000; and 4,237,595 and 6,827,368 restricted stock awards vested, for which $685,553 and $1,095,193 in deferred stock compensation was expensed. As of December 31, 2019 and 2018, respectively, there remains 4,805,600 and 5,888,195 shares to be vested, and $660,069 and $1,148,809 in deferred stock compensation to be expensed over the next fifteen (15) months.
Restricted Stock Awards Activity | Number of |
| Deferred |
| |
| Shares |
| Compensation |
| |
Outstanding at December 31, 2017 | 9,055,563 |
| $ | 1,810,002 |
|
Granted | 3,660,000 |
|
| 434,000 |
|
Vested | (6,827,368 | ) |
| (1,095,193 | ) |
Outstanding at December 31, 2018 | 5,888,195 |
|
| 1,148,809 |
|
Granted | 3,830,000 |
|
| 264,350 |
|
Vested | (4,237,595 | ) |
| (685,553 | ) |
Forfeited/Canceled | (675,000 | ) |
| (67,537 | ) |
Outstanding at December 31, 2019 | 4,805,600 |
| $ | 660,069 |
|
NOTE 14. WARRANTS AND OPTIONS
As of December 31, 2019 and 2018, respectively, the Company had 56,785,913 and 21,232.500 warrants, and 26,685,000 and 18,060,000 options issued and outstanding.
During the years ended December 31, 2019 and 2018, respectively, 40,703,413 and 14,077,500 warrants were granted, and 300,000 and 100,000 expired, and 4,850,000 and 0 were retired/canceled. The warrants carry an exercise price of between $0.001 to $0.60 per share, expire between 2020 to 2024, and were valued at $498,840 and $851,610, using the Black-Scholes method. The assumptions used in valuing the warrants were: expected term between 2 to 5 years; expected volatility 40% to 45%; risk free interest rate between 1.56% to 2.95%; and a dividend yield of 0%. A total of $40,730 and $113,210 in deferred stock warrant compensation was recorded, and $47,180 and $73,370 was expensed during the years ended December 31, 2019 and 2018, respectively. There remains $92,470 and $98,920 in deferred compensation as of December 31, 2019 and 2018, respectively, to be expensed over the next twelve (12) months.
Warrants Outstanding |
|
|
|
|
|
|
| |||
|
| Number of |
| Remaining |
| Exercise Price |
| Weighted |
| |
|
| Common |
| Contractual Life |
| Times Number |
| Average |
| |
Exercise Price |
| Shares |
| (in years) |
| Of Shares |
| Exercise Price |
| |
|
|
|
|
|
| |||||
$0.001 |
| 300,000 |
| 3.50 |
| $ | 300 |
| $0.17 |
|
$0.01 |
| 500,000 |
| 2.75 |
|
| 5,000 |
| $0.22 |
|
$0.01 |
| 75,000 |
| 1.00 |
|
| 750 |
| $0.18 |
|
$0.10 |
| 5,028,413 |
| 4.75 |
|
| 502,841 |
| $0.21 |
|
$0.10 |
| 62,500 |
| 3.25 |
|
| 6,250 |
| $0.29 |
|
$0.10 |
| 250,000 |
| 1.50 |
|
| 25,000 |
| $0.19 |
|
$0.10 |
| 4,877,500 |
| 1.25 |
|
| 487,750 |
| $0.21 |
|
$0.10 |
| 250,000 |
| 0.75 |
|
| 25,000 |
| $0.32 |
|
$0.12 |
| 100,000 |
| 2.75 |
|
| 12,000 |
| $0.21 |
|
$0.12 |
| 125,000 |
| 2.00 |
|
| 15,000 |
| $0.21 |
|
$0.15 |
| 600,000 |
| 4.25 |
|
| 90,000 |
| $0.19 |
|
$0.15 |
| 1,000,000 |
| 1.00 |
|
| 150,000 |
| $0.28 |
|
$0.17 |
| 62,500 |
| 3.25 |
|
| 10,625 |
| $0.29 |
|
$0.18 |
| 62,500 |
| 3.25 |
|
| 11,250 |
| $0.29 |
|
$0.20 |
| 2,600,000 |
| 4.25 |
|
| 520,000 |
| $0.19 |
|
$0.21 |
| 62,500 |
| 3.25 |
|
| 13,125 |
| $0.29 |
|
$0.21 |
| 100,000 |
| 0.75 |
|
| 21,000 |
| $0.34 |
|
$0.25 |
| 6,000,000 |
| 2.75 |
|
| 1,500,000 |
| $0.20 |
|
$0.25 |
| 1,050,000 |
| 2.50 |
|
| 262,500 |
| $0.21 |
|
$0.25 |
| 10,400,000 |
| 2.25 |
|
| 2,600,000 |
| $0.20 |
|
$0.25 |
| 800,000 |
| 2.00 |
|
| 200,000 |
| $0.21 |
|
$0.25 |
| 3,250,000 |
| 1.75 |
|
| 812,500 |
| $0.18 |
|
$0.25 |
| 13,500,000 |
| 1.25 |
|
| 3,375,000 |
| $0.21 |
|
$0.25 |
| 475,000 |
| 1.00 |
|
| 118,750 |
| $0.27 |
|
$0.25 |
| 3,255,000 |
| 0.75 |
|
| 813,750 |
| $0.32 |
|
$0.25 |
| 1,500,000 |
| 0.50 |
|
| 375,000 |
| $0.39 |
|
$0.35 |
| 250,000 |
| 0.75 |
|
| 87,500 |
| $0.32 |
|
$0.60 |
| 250,000 |
| 0.75 |
|
| 150,000 |
| $0.34 |
|
|
| 56,785,913 |
|
|
| $ | 12,190,891 |
| $0.21 |
|
- F-20 -
|
|
|
|
| |
| Number of |
| Weighted Average |
| |
Shares |
| Exercise Price |
| ||
Outstanding at December 31, 2017 |
| 7,255,000 |
| $0.18 |
|
Issued |
| 54,780,913 |
| $0.20 |
|
Exercised |
| –– |
| –– |
|
Expired / Forfeited |
| (5,250,000 | ) | $0.21 |
|
Outstanding at December 31, 2019 |
| 56,785,913 |
| $0.21 |
|
During the years ended December 31, 2019 and 2018, respectively, 10,000,000 and 6,000,000 stock options were granted, which vest periodically over a two (2) year period, are exercisable for a period of between 3 to 5 years at an exercise price of between $0.05 to $0.25 per share, and were valued at $875,700 and $833,700, using the Black-Scholes method. The assumptions used in valuing the options were: expected term between 3.00 to 4.75 years; expected volatility between 1.82 to 2.29; risk free interest rate between 1.69% to 2.78%; and a dividend yield of 0%.
Options Outstanding |
|
|
|
|
|
|
|
|
| |
|
|
|
| Remaining |
| Exercise Price |
| Weighted |
| |
|
| Number of |
| Contractual Life |
| times Number |
| Average |
| |
Exercise Price |
| Shares |
| (in years) |
| of Shares |
| Exercise Price |
| |
|
|
|
|
|
| |||||
$0.05 |
| 90,000 |
| 2.50 |
| $ | 4,500 |
| $0.14 |
|
$0.05 |
| 1,140,000 |
| 2.25 |
|
| 57,000 |
| $0.09 |
|
$0.05 |
| 100,000 |
| 1.75 |
|
| 5,000 |
| $0.08 |
|
$0.05 |
| 60,000 |
| 1.00 |
|
| 3,000 |
| $0.06 |
|
$0.05 |
| 170,000 |
| 0.75 |
|
| 8,500 |
| $0.12 |
|
$0.09 |
| 5,500,000 |
| 2.50 |
|
| 467,500 |
| $0.20 |
|
$0.10 |
| 500,000 |
| 0.75 |
|
| 50,000 |
| $0.14 |
|
$0.15 |
| 1,000,000 |
| 0.75 |
|
| 150,000 |
| $0.14 |
|
$0.25 |
| 3,125,000 |
| 4.25 |
|
| 781,250 |
| $0.22 |
|
$0.25 |
| 1,000,000 |
| 4.00 |
|
| 250,000 |
| $0.23 |
|
$0.25 |
| 5,000,000 |
| 3.25 |
|
| 1,250,000 |
| $0.16 |
|
$0.25 |
| 7,000,000 |
| 2.50 |
|
| 1,750,000 |
| $0.16 |
|
$0.25 |
| 1,000,000 |
| 0.75 |
|
| 250,000 |
| $0.15 |
|
$0.25 |
| 1,000,000 |
| 0.25 |
|
| 250,000 |
| $0.10 |
|
|
| 26,685,000 |
|
|
| $ | 5,276,750 |
| $0.20 |
|
Options Activity |
|
|
|
|
|
| Number of |
| Weighted Average |
| |
Shares |
| Exercise Price |
| ||
Outstanding at December 31, 2017 |
| 20,675,000 |
| $0.15 |
|
Issued |
| 16,000,000 |
| $0.19 |
|
Exercised |
| (1,973,189 | ) | $0.18 |
|
Expired / Forfeited |
| (3,891,811 | ) | $0.20 |
|
Outstanding at December 31, 2019 |
| 26,685,000 |
| $0.20 |
|
During the years ended December 31, 2019 and 2018, respectively, 10,000,000 and 6,000,000 options were issued, 0 and 1,973,189 options were exercised, 0 and 1,000,000 options expired, and 375,000 and 5,641,811 options were forfeited. A total of $875,700 and $649,327 in deferred stock option compensation was recorded, net of forfeitures, and $718,392 and $572,870 was expensed during the years ended December 31, 2019 and 2018, respectively. There remains $1,552,774 and $1,395,466 in deferred compensation as of December 31, 2019 and 2018, respectively, to be expensed over the next twelve (12) months.
- F-21 -
A reconciliation of the expected statutory federal and state taxes and the total income tax expense (benefit) at December 31, 2019 and 2018 was as follows:
| December 31, 2019 |
| December 31, 2018 |
| ||
|
|
|
|
|
|
|
Income (loss) before taxes | $ | (12,866,332 | ) | $ | 15,608,209 |
|
Statutory rate (Fed & State(s)) |
| 30% |
|
| 30% |
|
|
|
|
|
|
|
|
Computed expected tax payable (recovery) |
| (3,351,800 | ) |
| 4,781,700 |
|
|
|
|
|
|
|
|
Effect of release of net operating loss carryforwards |
| (2,240,900 | ) |
| (2,417,700 | ) |
|
|
|
|
|
|
|
Tax effect of non-deductible expenses: |
|
|
|
|
|
|
Gain on extinguishment of debt-principal |
| –– |
|
| (6,124,000 | ) |
Impairment losses |
| 299,100 |
|
| –– |
|
Stock compensation/amortization of stock options |
| 830,200 |
|
| 1,048,500 |
|
Discount amortization |
| 6,000 |
|
| 837,000 |
|
Other |
| 2,100 |
|
| 1,500 |
|
Total tax effect of non-deductible expenses |
| 1,137,400 |
|
| (4,237,000 | ) |
|
|
|
|
|
|
|
Change in valuation allowance |
| 4,635,300 |
|
| (1,873,000 | ) |
|
|
|
|
|
|
|
Income tax expense | $ | –– |
| $ | –– |
|
|
|
|
|
|
|
|
Reported income taxes: |
|
|
|
|
|
|
Federal | $ | –– |
| $ | –– |
|
State |
| –– |
|
| –– |
|
Total | $ | –– |
| $ | –– |
|
The significant components of deferred income tax assets and liabilities at December 31, 2019 and 2018, are as follows:
| December 31, 2019 |
| December 31, 2018 |
| ||
|
|
|
|
|
|
|
Net operating loss carried forward | $ | 4,666,500 |
| $ | –– |
|
|
|
|
|
|
|
|
Bad debt allowance |
| –– |
|
| –– |
|
|
|
|
|
|
|
|
Officers’ accrued compensation |
| 222,800 |
|
| 243,400 |
|
|
|
|
|
|
|
|
Accrued related party interest |
| 10,100 |
|
| 20,700 |
|
|
|
|
|
|
|
|
Valuation allowance |
| (4,899,400 | ) |
| (264,100 | ) |
|
|
|
|
|
|
|
Net deferred income tax asset | $ | –– |
| $ | –– |
|
During the year ended December 31, 2018, the company realized extinguishment of debt principal in the amount of $20,522,835. Per Internal Revenue Code (“IRC”) Section 108(a) (1) (A) the extinguishment of debt principal is excluded from taxable income for the Company. However, any available tax attributes must be released up and to the amount of the extinguishment. Therefore, net operating loss carryforwards were released for the amount of income excluded from taxable income. The remaining net operating losses available to use toward future taxable income are as follows:
Tax Year |
| Net Operating Loss |
| Expires | |
|
|
|
|
|
|
2016 |
| $ | 1,192,900 |
| 2036 |
2017 |
|
| 2,117,100 |
| 2037 |
2018 |
|
| 548,900 |
| No Expiration |
2019 |
|
| 9,165,700 |
| No Expiration |
|
|
|
|
|
|
Total |
| $ | 13,024,600 |
|
|
The Company is open to examinations for the tax year 2011 through the current tax year.
- F-22 -
NOTE 16. DISCONTINUED OPERATIONS
In December 2017, the Company discontinued all operations related to the Retail Pharmacy Segment involving the Company’s wholly-owned subsidiary, RoxSan Pharmacy, Inc. On May 14, 2018, pursuant to unanimous resolutions of the board of directors of RoxSan Pharmacy, Inc. (“RoxSan”) and Parallax Health Sciences, Inc., RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California. Mr. Timothy Yoo was appointed trustee on May 15, 2018.
The pharmacy operations resulted in an accumulated deficit of $11,582,906 as of May 14, 2018. In addition, certain advances were made to RoxSan Pharmacy, Inc. for the purpose of overhead expenses for which a secured promissory note was issued to the Company. As of May 14, 2018, principal in the amount of $1,280,692 had been disbursed, and interest in the amount of $22,797 had been accrued in connection with the note.
As of May 14, 2018, the assets and liabilities relating to the discontinued operations of RoxSan Pharmacy, Inc. were as follows:
May 14, 2018 | |||
|
|
|
|
Liabilities subject to compromise |
|
|
|
Accounts payable and accrued expenses | $ | 2,942,012 | [1] |
Related party payables | 376,430 | [1] | |
Note payable | 185,000 | [1] | |
Note payable-merchant |
| 974,826 |
|
Total liabilities subject to compromise |
| 4,478,268 |
|
|
|
|
|
Net liabilities of discontinued operations | $ | 4,478,268 |
|
[1]As of January 1, 2018, Mr. Joseph M. Redmond, former President and member of the board of directors, is no longer a related party. As a result, related party payables was reduced by $307,997, representing $185,000 in promissory notes, $119,270 in accrued compensation, and $3,727 in expense advances. As of May 14, 2018, $185,000 is reflected as a note payable, and $122,997 is included in accounts payable and accrued expenses as part of liabilities subject to compromise. In addition, accrued interest of $11,823 related to the promissory note was reclassified from related party to non-related party accrued interest, and is included in accounts payable and accrued expenses as part of liabilities subject to compromise. See Note 18 for additional information and legal proceedings related to Mr. Redmond.
Included in accounts payable and accrued expenses as of May 14, 2018, are $181,580 in unpaid payroll taxes, $296,959 in penalties, and $50,167 in interest related to unpaid payroll taxes.
The results of the discontinued operations of RoxSan Pharmacy, Inc. are summarized as follows:
May 14, 2018 |
| ||
|
|
| |
Revenue | $ | –– |
|
Cost of sales |
| –– |
|
Gross profit |
| –– |
|
Sales, marketing and pharmacy expenses |
| 170,630 |
|
General and administrative expenses |
| 586,993 |
|
Operating loss |
| (757,623 | ) |
Interest expense |
| (56,775 | ) |
Loss on disposal of equipment |
| (10,000 | ) |
Net loss from discontinued operations | $ | (824,398 | ) |
- F-23 -
NOTE 17. SEGMENT REPORTING
The Company currently has three (3) business segments: Remote Care Systems, Behavioral Health Services and Diagnostics/Corporate. The Pharmacy segment ceased operations in December 2017, and was deconsolidated effective May 14, 2018. See Note 1 and 3 for a description of each segment and related significant accounting policies.
The following table is a reconciliation of the Company’s business segments to the consolidated financial statements:
| Remote Care Systems |
| Behavioral Health Services |
| Diagnostics/ Corporate |
| Pharmacy [1] |
| Consolidated Totals |
| |||||
|
|
|
|
|
|
|
|
|
|
| |||||
December 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue | $ | 1,800 |
| $ | 76,800 |
| $ | 50,000 |
| $ | –– |
| $ | 128,600 |
|
Gross profit (loss) |
| 480 |
|
| 61,293 |
|
| 50,000 |
|
| –– |
|
| 111,773 |
|
Operating loss |
| (253,391 | ) |
| (169,485 | ) |
| (5,970,812 | ) |
| –– |
|
| (6,393,688 | ) |
Depreciation and amortization |
| 9,196 |
|
| 109,760 |
|
| 2,016 |
|
| –– |
|
| 120,972 |
|
Interest expense |
| 3,111 |
|
| –– |
|
| 579,397 |
|
| –– |
|
| 582,508 |
|
Gain (loss) on extinguishment of debt |
| 101,367 |
|
| –– |
|
| (1,028,766 | ) |
| –– |
|
| (927,399 | ) |
Gain (loss) on fair value adjustments |
| –– |
|
| –– |
|
| 194,511 |
|
| –– |
|
| 194,511 |
|
Loss on settlements |
|
|
|
|
|
|
| (4,134,972 | ) |
|
|
|
| (4,134,972 | ) |
Impairment loss |
| –– |
|
| –– |
|
| 1,002,276 |
|
| –– |
|
| 1,002,276 |
|
Discount amortization |
| 20,000 |
|
| –– |
|
| –– |
|
| –– |
|
| 20,000 |
|
Total assets |
| 904,757 |
|
| 439,567 |
|
| 125,332 |
|
| –– |
|
| 1,359,896 |
|
Goodwill |
| 785,060 |
|
| –– |
|
|
|
|
| –– |
|
| 785,060 |
|
Additions to property and equipment |
| –– |
|
| –– |
|
| 2,628 |
|
| –– |
|
| 2,628 |
|
|
|
|
|
|
|
|
|
|
|
| |||||
December 31, 2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue | $ | 9,399 |
| $ | 1,800 |
| $ | –– |
| $ | –– |
| $ | 11,739 |
|
Gross profit (loss) |
| (10,400 | ) |
| 1,800 |
|
| –– |
|
| –– |
|
| (8,600 | ) |
Operating loss |
| (365,426 | ) |
| (191,446 | ) |
| (6,077,791 | ) |
| –– |
|
| (6,634,663 | ) |
Depreciation and amortization |
| 9,196 |
|
| 109,760 |
|
| 1,664 |
|
| –– |
|
| 120,620 |
|
Interest expense |
| 2,970 |
|
| –– |
|
| 2,161,560 |
|
| –– |
|
| 2,164,530 |
|
Gain on disposal of subsidiary |
| –– |
|
| –– |
|
| 4,478,268 |
|
| –– |
|
| 4,478,268 |
|
Gain (loss) on extinguishment of debt |
| –– |
|
| –– |
|
| 22,858,009 |
|
| –– |
|
| 22,858,009 |
|
Gain (loss) on fair value adjustments |
| –– |
|
| –– |
|
| (123,875 | ) |
| –– |
|
| (123,875 | ) |
Discount amortization |
| (340,000 | ) |
| –– |
|
| 3,145,000 |
|
| –– |
|
| 2,805,000 |
|
Discontinued operations |
| –– |
|
| –– |
|
| –– |
|
| (824,398 | ) |
| (824,398 | ) |
Total assets |
| 913,636 |
|
| 439,567 |
|
| 11,154 |
|
| –– |
|
| 1,364,357 |
|
Goodwill |
| 785,060 |
|
| –– |
|
| –– |
|
| –– |
|
| 785,060 |
|
Additions to property and equipment |
| –– |
|
| –– |
|
| –– |
|
| –– |
|
| –– |
|
[1]Discontinued operations effective May 14, 2018
NOTE 18. LEGAL MATTERS
Dispute with Former Owner of RoxSan
In October 2015, shortly following the Company's acquisition of RoxSan, Shahla Melamed (“Melamed”), initiated two (2) legal actions against the Company in the Superior Court of the State of California, County of Los Angeles, West District, Shahla Melamed v. Parallax Health Sciences, Inc., action numbers SC 124873 and SC 125702. In the matter, action No. SC 124873, Melamed sought rescission of the August 13, 2015 Purchase Agreement. In the Matter, action No. SC125702, Melamed alleges that the Company is in default under the terms of the Purchase Agreement and Secured Note, and the Company’s termination of Melamed’s employment agreement.
The Company also initiated legal action against Melamed and filed a complaint in October 2015, action number SC 124898, in the Superior Court of the State of California, County of Los Angeles, West District, Parallax Health Sciences, Inc., et al. v. Shahla Melamed, et al. The complaint in that action alleges that Melamed breached several obligations under the Purchase Agreement, and the Company sought to reduce the Secured Note due to undisclosed material changes in the business.
- F-24 -
In January 2019, Melamed requested mediation, seeking settlement of the pending litigation with the Company, including that which was initiated against the Company by her son, Hootan Melamed (Shahla and Hootan, collectively, the “Melameds”). Through mediation, the Company and the Melameds reached agreeable settlement terms, and on February 19, 2020, the Company received a counter-signed Settlement and Release Agreement (the “Settlement”). Effective February 12, 2020 (the “Effective Date”), the Settlement is by and between Parallax Health Sciences, Inc., RoxSan Pharmacy, Inc., Michael Redmond, Edward Withrow III, Huntington Chase Financial Group, LLC, Calli Bucci and Dave Engert (collectively, “Parallax”), and the Melameds, and resolves all pending lawsuits between the parties in connection with the acquisition of RoxSan Pharmacy.
In consideration of the resolution of all existing and potential claims, including the cancellation of the Company’s contingent liability in the principal sum of $20,500,000, and accrued interest of approximately $4,500,000, and without further action or litigation and without admission of liability by either party, the Settlement terms include the following:
●A payment of $4,000,000 (the “Settlement Sum”) to the Melameds, to be paid as follows:
$1,250,000 within 90 days of the Effective Date;
$1,250,000 within one (1) year of the Effective Date;
$1,500,000 within two (2) years of the Effective Date.
●The issuance of ten (10) million shares of the Company’s Common Stock to an entity owned by Shahla Melamed.
In addition, in the event forty percent (40%) or more of the Company and/or its subsidiaries (including by way of merger) is sold within two (2) years of the Effective Date, the Company shall pay the Melameds, within two (2) weeks of receipt of the proceeds from such sale (the “Sale Proceeds”), any outstanding unpaid Settlement Sum plus an additional 10% of the Sale Proceeds received, up to a total of an additional $3,000,000 over and above the Settlement Sum.
In the event the Company fails to cure a breach of timely payment of any portion of the Settlement Sum within thirty (30) days of a notice of default, a Stipulated Judgement may be filed by Melamed in the sum of $20,000,000, less any Settlement Sum amounts previously paid by the Company.
RoxSan Dissolution
On May 14, 2018, pursuant to unanimous resolutions of the boards of directors of RoxSan Pharmacy, Inc. and Parallax Health Sciences, Inc., RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California (the “Court”). Mr. Timothy Yoo was appointed trustee (“Trustee”) on May 15, 2018. In connection with this filing, RoxSan seeks to discharge approximately $5 million of liabilities owed to various parties, and intercompany loans in excess of $1 million owed to Parallax. The Chapter 7 bankruptcy proceeding by RoxSan Pharmacy, Inc. was fully discharged and the case was closed on March 13, 2019, in U.S. Bankruptcy Court, Central District of California.
Due to, among other things, the reduction in RoxSan’s cash flows during 2016 and 2017, RoxSan became delinquent in its payroll tax depository obligations, resulting in a liability owed to federal and state taxing agencies in the aggregate of $1,148,811, which includes $601,148 in taxes withheld from employees (“Trust Fund Taxes”), employer taxes of $183,172, and penalties and interest of $364,491 through December 31, 2018. The liability was included as part of the Chapter 7 bankruptcy petition, and certain portions of the liability may be discharged. However, in accordance with California bankruptcy laws, federal and state Trust Fund Taxes are not dischargeable. The Company has retained a tax resolution specialist and is in communications with the taxing agencies in order to resolve RoxSan’s liability. During the year ended December 31, 2019, payments for Trust Fund Taxes in the amount of $485,498 were made, and $115,650 in Trust Fund Taxes were outstanding at December 31, 2019.
Disputes with Former Executives
On March 9, 2017, Dave Engert former Executive Chairman and director of the Company filed a lawsuit in Arizona and then on or about May 5, 2017, Mr. Engert, changed the venue and filed suit against the Company and RoxSan Pharmacy, Inc. in the United States District Court, Central District of California for an amount exceeding $75,000. On October 23, 2017, the Company filed an answer and counterclaims against Mr. Engert for an amount exceeding $100,000. The counterclaims include possible fraud and negligence committed by Mr. Engert and Mr. J. Michael Redmond, former successor Chairman of Mr. Engert, director, President and Chief Executive Officer of the Company and former President, Chief Executive Officer, Chairman and director of RoxSan Pharmacy, Inc.
On October 8, 2018, a settlement was reached between Mr. Engert and the Company (the “Engert Settlement”). The Engert Settlement includes, among other things, a cash payment to Mr. Engert in the amount of $139,000, and the cancellation of all of Mr. Engert’s equity holdings in the Company. The Engert Settlement resulted in a net loss to the Company of $33,272. On April 10, 2019, a stipulation for dismissal was filed, and the matter has been fully resolved.
●Action No. BC700070
On March 28, 2018, Mr. J. Michael Redmond filed a lawsuit against the Company and RoxSan Pharmacy, Inc. in the United States District Court, Central District of California for an amount exceeding $75,000. The Company intends to vigorously defend against this action. There are counterclaims that include possible fraud and negligence committed by Mr. Redmond, former President, Chief Executive Officer, Chairman and director of the Company and of RoxSan Pharmacy, Inc. An Arbitration is still pending.
Disputes with Lenders
●On March 18, 2020, the Company initiated legal action and filed a complaint against EMA Financial, LLC (“EMA”) a third-party lender, in the US District Court, Southern District of New York, case index number 20-cv-2375, citing fraud, unjust enrichment, and securities law violations, among other things. On April 27, 2020, EMA filed an answer substantially denying the Company’s claims, and filed counterclaims against the Company, including damages in excess of $2 million. The matter is currently pending.
There are two (2) legal matters currently pending.
- F-25 -
The Company has evaluated the events and transactions for recognition or disclosure subsequent to December 31, 2019, through the date of the issuance of the financial statements, and has determined that there have been no events that would require disclosure, except for the following:
On January 22, 2020, the Company issued a short-term non-related party convertible promissory note in the principal sum of $78,000. The note bears interest at a rate of 12% per annum, matures in one (1) year, and is convertible into shares of the Company’s Common Stock at a conversion price equal to the lower of 1) $0.12 per share; or 2) 65% of the average of the lowest three (3) trading prices during the fifteen (15) trading days preceding the conversion date.
On January 15, 2020, in connection with the exercise of certain warrants issued to a beneficial owner, 1,838,217 shares of the Company’s Common Stock were issued. The shares were valued at $108,455.
On January 27, 2020, pursuant to a and resolution of the board of directors, the Company issued 1,351,181 shares of its restricted Common Stock at $0.001 per share, for $1,351, to Nathaniel T. Bradley, a director, in recognition of his contributions and achievements. The shares were valued at $67,559.
On February 2, 2020, Mr. David Appell resigned as the Company’s Chief Operating Officer. His resignation was not the result of a disagreement with the Company on any matters relating to the Company’s operations, policies, or practices. Concurrent with his resignation as COO, Mr. Appell will serve as Managing Director of the Company’s wholly-owned subsidiary, Parallax Communications, Inc. (“PCOM”). As Managing Director, Mr. Appell will provide business advisory and strategic planning advice to the Company with PCOM. In exchange for these services, Mr. Appell will be paid $2,000 per month plus medical benefits. In connection with Mr. Appell’s resignation, the Company canceled 1,450,000 shares of Mr. Appell’s previously granted stock award, and 2,250,000 of unvested stock options. As a result, additional paid in capital was reduced by $207,324.
On February 12, 2020, the Company filed a Certificate of Designation (“Designation”) with the secretary of state of Nevada for the designation of 10,000 shares of Series B1 Convertible Preferred Stock. The Series B1 Stock is redeemable at 120% of face value and unpaid dividends; is convertible into Common Stock at a conversion rate of $0.15; carries an annual dividend of 10%; and matures in two (2) years, at which time the Series B1 Stock will automatically convert into Common Stock.
On February 12, 2020, in connection with a $5,000,000 maximum offering of the Company’s Series B1 Convertible Preferred Stock (the “Series B1 Stock”), the Company received a Subscription from an accredited investor (the “Subscription”) for the purchase of 69 shares of Series B1 Stock at a price of $10,000 per share, net of an original issue discount of 15%, or $8,500 per share, for proceeds in the amount of $586,500, pursuant to that certain Securities Purchase Agreement dated February 10, 2020. In addition, the Subscription includes 50% warrant coverage at an exercise price of $0.25 per share for a period of three (3) years.
Effective February 12, 2020, in connection with the legal settlement between the Company and the Melameds (Note 18), the Company issued 10,000,000 shares of its restricted Common Stock, valued at $480,000.
On February 14, 2020, in connection with a certain consulting agreement, the Company issued 1,000,000 shares of its restricted Common Stock for services, valued at $62,500.
During the first quarter 2020, in connection with the conversion of debt in the amount of $65,000, the Company issued 3,412,292 shares of Common Stock, valued at $178,182.
During the first quarter 2020, in connection with the conversion of debt in the amount of $41,580, the Company issued 2,200,000 shares of Common Stock, valued at $128,000. The Company has initiated legal action against the lender in the US District Court, Southern District of New York. On April 27, 2020, the lender filed an answer and counter claims. The matter is currently pending. (Note 18).
During the first quarter 2020, in connection with a certain consulting agreement, the Company issued 246.844 shares of its restricted Common Stock for services, valued at $9,750.
On or about March 5, 2020, the Company received subscriptions from accredited investors for the purchase of 36 shares of Series B1 Stock at a price of $10,000 per share, net of an original issue discount of 15%, or $8,500 per share, for proceeds in the amount of $306,000.
On April 7, 2020, the Company executed an Executive Agreement (the “Executive Agreement”) for the appointment of Dr. David L. Stark as the Company’s President, with an effective date of March 1, 2020. The Executive Agreement replaces any other agreement between Dr. Stark and the Company or any of its subsidiaries, and provides a base compensation of $225,000 in year one, $250,000 in year two and $275,000 in year three, commencing after the initial term estimated at approximately six (6) months, during which time Dr. Stark will devote fifty percent (50%) of his time at a compensation proportionate to the year one base compensation. The Executive Agreement also provides for various performance bonuses, and customary employee benefits, as well as (i) a grant to purchase 2,000,000 restricted common shares at $0.001 per share, of which 25% vest immediately, and the remainder vest upon the Company’s achievement of certain earnings goals; and (ii) stock options to purchase 3,000,000 shares of the Company’s Common Stock at an exercise price of $0.25 per share for a period of five (5) years, of which 25% vest immediately, and the remainder vest quarterly in equal amounts over the term of the Agreement.
On or about May 12, 2020, in connection with the Melamed Settlement, the initial payment of $1,250,000 was due. Due to cash flow constraints, the payment was not made at that time. Pursuant to the Settlement Agreement, the Company has thirty (30) days to cure the non-payment (See Note 8).
* * * * *
- F-26 -
ITEM 9.CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
(a) | Dismissal of Independent Certifying Accountant |
Effective July 17, 2019, the Company dismissed Freedman & Goldberg, CPA’s (“F&G”) as the Company’s independent registered public accounting firm. This action was approved by the audit committee of the Company’s Board of Directors. F&G served as the Company’s independent registered accounting firm for the fiscal years ended December 31, 2016, 2017 and 2018.
The reports of F&G regarding the Company’s financial statements for the fiscal year ended December 31, 2016, December 31, 2017, and December 31, 2018, did not contain any adverse opinion or disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles, except that the audit reports of F&G on the Company’s financial statements for fiscal years ended December 31, 2016, December 31, 2017, and December 31, 2018, each contained an explanatory paragraph which noted that there was substantial doubt about the Company’s ability to continue as a going concern.
During the years ended December 31, 2016, December 31, 2017, and December 31, 2018, and during the period from January 1, 2019 to July 17, 2019, the date of dismissal, (i) there were no disagreements with F&G on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedures, which disagreements, if not resolved to the satisfaction of F&G would have caused it to make reference to such disagreement in its reports; and (ii) there were no reportable events as defined in Item 304(a)(1)(v) of Regulation S-K.
The Company provided F&G with a copy of the foregoing disclosures and requested that F&G furnish the Company with a letter addressed to the SEC stating whether or not it agrees with the above statements. A copy of such letter is filed as Exhibit 16.1 to the Current Report on Form 8-K July 19, 2019.
On September 27, 2019, the Company, with the approval of Audit Committee of the Company’s Board of Directors, notified Marcum LLP (“Marcum”) that Marcum was being dismissed as the Company’s independent registered public accounting firm effective immediately.
During the Company’s engagement with Marcum, no reports regarding the Company’s financial statements were issued by Marcum. There were no disagreements (as such term is defined in Item 304 of Regulation S-K) with Marcum on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedures, which disagreements, if not resolved to the satisfaction of Marcum would have caused it to make reference to such disagreement in its reports; and (ii) there were no reportable events as defined in Item 304(a)(1)(v) of Regulation S-K except for the following:
1)Marcum advised the Company that there were material weaknesses in the Company’s internal control over financial reporting.
2)Marcum advised Management that the Company did not fully analyze certain agreements for the proper accounting treatment which could have a material impact on previously issued financial statements. However, Marcum was unable to complete its evaluation of the accounting treatment for such agreements as a result of their dismissal.
The Company provided Marcum with a copy of the foregoing disclosures and requested that Marcum furnish the Company with a letter addressed to the SEC stating whether or not it agrees with the above statements. A copy of such letter is filed as Exhibit 16.1 to the Current Report on Form 8-K October 2, 2019.
(b) | Engagement of Independent Certifying Accountant |
Effective July 18, 2019, the Company engaged Marcum, LLP (“Marcum”) as its independent registered public accounting firm. During each of the Company’s two most recent fiscal years and through the interim periods preceding the engagement of Marcum, the Company (a) has not engaged Marcum as either the principal accountant to audit the Company’s financial statements, or as an independent accountant to audit a significant subsidiary of the Company and on whom the principal accountant is expected to express reliance in its report; and (b) has not consulted with Marcum regarding (i) the application of accounting principles to a specific transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements, and no written report or oral advice was provided to the Company by Marcum concluding there was an important factor to be considered by the Company in reaching a decision as to an accounting, auditing or financial reporting issue; or (ii) any matter that was either the subject of a disagreement, as that term is defined in Item 304(a)(1)(iv) of Regulation S-K or a reportable event, as that term is described in Item 304(a)(1)(v) of Regulation S-K. During the short engagement, no reviews were completed by Marcum for the Company’s 2019 quarterly reports.
Effective October 2, 2019, the Company reengaged Freedman & Goldberg, CPA’s (“F&G”) as its independent registered public accounting firm. F&G was previously engaged by the Company for financial years beginning in 2016 through 2018, and has performed the reviews for all three quarters of the 2019 financial year, as well as the 2019 audit.
In connection with the Company’s reappointment of F&G as the Company’s independent registered public accounting firm the Company has not consulted with F&G regarding (i) the application of accounting principles to a specific transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements, and no written report or oral advice was provided to the Company by F&G concluding there was an important factor to be considered by the Company in reaching a decision as to an accounting, auditing or financial reporting issue; or (ii) any matter that was either the subject of a disagreement, as that term is defined in Item 304(a)(1)(iv) of Regulation S-K or a reportable event, as that term is described in Item 304(a)(1)(v) of Regulation S-K.
- 61 -
Management’s Report on Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed in the Company’s reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including the Company’s president, chief executive officer and chief financial officer to allow for timely decisions regarding required disclosure. In designing and evaluating the Company’s disclosure controls and procedures, the Company’s management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and the Company’s management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Because of its inherent limitations, a controls system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As of December 31, 2019, the end of the Company’s fiscal year covered by this report, the Company carried out an evaluation, under the supervision and with the participation of the Company’s president, chief executive officer and chief financial officer, of the effectiveness of the design and operation of the Company’s disclosure controls and procedures. Based on the foregoing, the Company’s president, chief executive officer and chief financial officer concluded that the Company’s disclosure controls and procedures were effective as of the end of the period covered by this Annual Report to provide reasonable assurance that the information required to be disclosed by the Company is recorded, processed, summarized and reported within the time periods specified, and that such information is accumulated and communicated to the Company’s management, including the Company’s president, chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Controls over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting. Responsibility, estimates and judgments by management are required to assess the expected benefits and related costs of the Company’s control procedures. The objectives of internal controls include providing management with reasonable, but not absolute, assurance that assets are safeguarded against loss from unauthorized use or disposition, and that transactions are executed in accordance with management’s authorization and recorded properly to permit the preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2019. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework (2013).
In September 2019, subsequent to the April 2019 filing of the Company’s original 2018 Annual Report (the “Original Report”), and the audited financial statements contained therein, management identified a material weakness in its internal controls. This weakness relates to the lack of controls over the period-end financial reporting process, and the lack of accounting and financial reporting personnel able to implement formal accounting policies with an appropriate level of accounting knowledge to identify and value complex debt and equity instruments. As a result, a misstatement was made of the Company’s financial statements for the years ended December 31, 2018 and 2017.
Specifically, the Company did not adequately analyze and identify certain debt and equity instruments being issued by the Company, to determine, among other things, the instruments’ derivative features. Such derivative instruments had not been recognized in the past, and no internal controls were in place at the time to mitigate the risk of material misstatements in financial reporting processes.
The Company’s management concluded that, due to a material weakness in internal control over financial reporting as of December 31, 2018, the Company’s internal control over financial reporting was not effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the Company generally accepted accounting principles. The Company’s management reviewed the results of their assessment with the Company’s board of directors (the “Board”).
- 62 -
Remediation efforts to address material weakness
The Company remedied the material weaknesses by, among other things, engaging qualified third-party service providers to assist with the financial reporting issues related to accounting for derivatives, and establishing internal processes to ensure the appropriate identification and analysis of derivative instruments. In addition, training was conducted related to analysis of debt and equity instruments, effective internal controls, and key accounting policies for derivative instruments.
As of the date of filing of this Annual Report, the proper controls have been designed and implemented, and management has concluded that they are sufficient and operating effectively. Management has and will continue to enhance the risk assessment process, and the design and implementation of internal controls over financial reporting.
This Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this Annual Report.
Changes in Internal Control over Financial Reporting
During the year ended December 31, 2019, the Company instituted changes in its internal control over financial reporting. The changes were made as a result of a material weakness identified at December 31, 2018, relating to the lack of controls over the period-end financial reporting process, and the lack of accounting and financial reporting personnel able to implement formal accounting policies with an appropriate level of accounting knowledge to identify and value complex debt and equity instruments. As a result of the weakness, a misstatement was made of the Company’s financial statements for the years ended December 31, 2018 and 2017.
In response to the material weaknesses described above, during the year ended December 31, 2019, the Company implemented and evaluated new internal controls over financial reporting. The new controls include, among other things, engaging qualified third-party service providers to assist with the financial reporting issues related to accounting for derivatives, and establishing internal processes to ensure the appropriate identification and analysis of derivative instruments. In addition, training was conducted related to analysis of debt and equity instruments, effective internal controls, and key accounting policies for derivative instruments.
As of December 31, 2019, the end of the period covered by this Annual Report, management believes that the improved processes remediate the material weaknesses, and has concluded that they are sufficient and operating effectively. Management has and will continue to enhance the risk assessment process, and the design and implementation of internal controls over financial reporting.
There have been no other changes in the Company’s internal controls over financial reporting that occurred during the year ended December 31, 2019, that have materially or are reasonably likely to materially affect, the Company’s internal controls over financial reporting, except those disclosed above.
Inherent limitations on effectiveness of controls
Internal control over financial reporting has inherent limitations which include, but is not limited to, the use of independent professionals for advice and guidance, interpretation of existing and/or changing rules and principles, segregation of management duties, scale of organization, and personnel factors. Internal control over financial reporting is a process which involves human diligence and compliance, and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting can also be circumvented by collusion or improper management override. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements on a timely basis. However, these inherent limitations are known features of the financial reporting process, and it is possible to design into the process safeguards to reduce, though not eliminate, this risk. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
None
- 63 -
ITEM 10.DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Identification of Directors and Executive Officers
The following table represents the directors and executive officers of the Company as of the date of the filing of this Annual Rreport:
Name | Position(s) Held | Age | Date first Elected or Appointed |
Paul R. Arena | Chief Executive Officer, Chairman | 62 | July 7, 2017 |
Dr. David L. Stark | President | 62 | March 1, 2020 |
Calli R. Bucci | Chief Financial Officer Corporate Secretary Director | 55 | November 1, 2012 March 31, 2014 December 29, 2016 |
John L. Ogden | Director | 66 | December 29, 2016 |
Capt. E. William Withrow Jr., SC, USN (Ret) | Director | 81 | November 1, 2012 |
Nathaniel T. Bradley | Director | 44 | June 4, 2018 |
Term of Office
The Board elects the Company’s officers, and their terms of office are at the discretion of the Board. Each officer serves until the earlier occurrence of the election of his or her successor; or by death, resignation, or removal by the Board. A director need not be a stockholder.
The members of the Company’s board of directors are elected through a majority vote of the Company’s stockholders, in accordance with the Company’s by-laws. Each director shall hold office until his or her successor has been duly elected and qualified; or by death, resignation, or removal by a majority vote of the Company’s stockholders.
Any director or officer may resign at any time.
Recent Changes in Directors and Executive Officers
On May 15, 2019, Mr. David Appell was appointed the Company’s Chief Operating Officer. On February 2, 2020, Mr. Appell resigned his position. His resignation was not the result of a disagreement with the Company on any matters relating to the Company’s operations, policies, or practices.
On March 1, 2020, Dr. David L. Stark was appointed as the Company’s President. In connection with the appointment, Mr. Paul R. Arena, who has held the position of President since July 2017, remains the Company’s Chief Executive Officer and Chairman of the Board of Directors, but resigned the position of President to afford Dr. Stark’s appointment.
Background and Business Experience
Current Officers and Directors
Paul R. Arena – President, Chief Executive Officer, Chairman of the Board
Mr. Paul R. Arena has over thirty years of executive management experience and has held senior executive positions in a number of publicly traded companies.
Mr. Arena has served as a director and as the Company’s President and Chief Executive Officer since July 2017. Mr. Arena has held the position of Chief Executive Officer of Intellectual Property Network, LLC from April 2017 to present, and is also a shareholder. From May 2016 to present, Mr. Arena founded and is a beneficial owner of ArenaLife, LLC, and from March 1991 to present, Mr. Arena has held the positions of Chairman of the Board, Chief Executive Officer, President and owner of AIM Group, Inc.
Previously, from March 2013 through January 2014, Mr. Arena was a Senior Managing Director of AudioEye, Inc., and then became Executive Chairman from January 2014 through March 2015. From June 2010 to December 2012, he held various executive positions, including Chairman of the Board, Chief Executive Officer, and Principal Financial Officer of Augme Technologies, Inc. and its subsidiary, Hipcricket, Inc. From February 2002 to March 2010, Mr. Arena held various executive positions, including Chairman of the Board, Chief Executive Officer, Principal Financial Officer and founder of Geos Communications (formerly i2 Telecom International) and its subsidiaries. Mr. Arena served in various executive capacities, including Chairman of the Board, Chief Executive Officer, President and founder of Cereus Technology Partners, Inc. and its subsidiaries from May 1991 to April 2000.
The Company believes Mr. Arena is qualified to be the Company’s Chief Executive Officer, Chairman of the board of directors, and President (former), because of his extensive senior executive experience in a multitude of different technology hardware and service markets.
- 64 -
Dr. Stark has 18 years’ experience in the medical and clinical fields, from the toxicology labs to the investigator site, and has been essential to all aspects of clinical and device research. Due to his extensive and broad experiences in the inner workings of the research and regulatory aspects of clinical trials, Dr. Stark brings a unique vision to the industry and the Company as a motivated designer of superior approaches to research challenges. Most importantly, Dr. Stark is highly qualified to manage the development opportunities of the Company.
Formerly the Director of the National Institute of Clinical Research (NICR), Dr. Stark has been responsible for the design, organization and implementation of clinical trials for pharmaceutical and device companies. He has a broad background in designing, conducting, and monitoring clinical trials of new pharmaceuticals and devices. He is also one of the few that has worked in the manufacturing validation of pharmaceuticals, the clinical field, and the regulatory (IRB) arenas, and therefore possesses a big-picture understanding of pharmaceutical development.
Through Dr. Stark’s diverse and devoted networking within the industry, he has assembled a wide network of more than 5,000 physicians throughout the United States and the international community. As part of his experience, he has negotiated a unique Drug Master File (DMF) partnership with drug manufacturers in China.
In addition to his significant accomplishments on the industry side of clinical drug and device development, Dr. Stark has experience with the FDA (major focus on Investigational New Drug (IND), New Drugs (NDA) and 510(k) applications). Prior to his employment at NICR, Dr. Stark was the President and Chief Executive Officer of Powder Ice, Inc a medical products company. Additionally, Dr. Stark is a California state licensed Qualified Medical Examiner and Certified Clinical Research Associate.
The Company believes Dr. Stark is qualified to be the Company’s President because of his extensive background and experience in medical device development, as well as clinical trials, and the implementation of medical and regulatory processes.
Calli R. Bucci – Chief Financial Officer, Corporate Secretary, Director
Ms. Bucci has over thirty years of experience in the field of finance and business management. She joined Parallax in 2010 as its controller, and has served as its Chief Financial Officer since November 2012. Before joining the Company, Ms. Bucci held the position of Chief Financial Officer at InstaSave, Inc., a promotional incentive company, from December 2007 to January 2010, where she was responsible for financial reporting, capital structure strategy and modeling, financial transactions with consumers, consumer product goods companies and retailers, investor relations, audits, payroll and corporate income taxes.
In addition to her public accounting background, Ms. Bucci held the position of Manager/Senior Accountant at Gelfand, Rennert & Feldman, a division of PriceWaterhouseCoopers, from April 1993 to August 1999, where she was responsible for all financial transactions for high net worth clientele, was liaison for annual audits, general ledger reviews and annual tax preparation.
Ms. Bucci held the position of Director of Accounting and Contract Administration at Intercontinental Releasing Corporation (IRC), a Los Angeles based Motion Picture Distribution Company, from April 1989 to April 1993. Ms. Bucci was responsible for all functions within the company’s accounting department, from financial statements and forecasting, to annual audits and corporate taxes. During her tenure with IRC, Ms. Bucci also designed and implemented a custom computerized availabilities system for the film rights of over 35 film properties distributed to foreign territories throughout the world. She was also responsible for the administration and facilitation of all client contracts, dealing heavily in foreign currencies and international import regulations.
Ms. Bucci concurrently holds the position of Chief Financial Officer of PearTrack Security Systems, Inc., a Nevada corporation.
Ms. Bucci attended Santa Monica College and the University of California at Berkley, majoring in Accounting.
The Company believes Ms. Bucci is qualified to be the Company’s Chief Financial Officer, Secretary, and director because of her knowledge of and extensive experience in a multitude of different capacities in corporate finance, business affairs, and public markets.
Nathaniel T. Bradley – Director, Chief Technology Officer
Mr. Bradley has served as the Company’s Chief Technology Officer since January 2016 and as President of the Company’s wholly-owned subsidiary, Parallax Health Management, Inc. (fka Qolpom®, Inc.), since the founding of the company in 2014. He has also served as Chief Technology Officer and Chief Product Officer of Montecito BioSciences, Ltd. From 2011 to present. Mr. Bradley is also founder of Bradley Brothers, LLC and Intellectual Property Network, Inc., both formed in 2012. Mr. Bradley previously served as a member of the board of directors of AudioEye, Inc., from the company’s founding in 2005 to 2015, and as Chief Executive Officer and President between 2007 and 2015.
Mr. Bradley is a recognized pioneer and active expert in the new media internet technology sector. He is the named inventor of several internet technology patents and patents-pending with the U.S. Patent and Trademark Office. Over the past decade, Mr. Bradley has been involved in the invention, reduction to practice, commercial licensing, and enforcement of foundational internet and mobile technology patents. Prior to AudioEye, Mr. Bradley was Chairman of the Board of Modavox® from 2006 to 2013, which became Augme Technologies, Inc., and Chief Technology Officer of its subsidiary, Hipcricket, Inc. Mr. Bradley was also founder and managing member of Kino Digital, Kino Communications and Kino Interactive.
The Company believes Mr. Bradley is qualified to be a director of the Company because of his extensive experience in strategic business development, intellectual properties and inventive technologies.
- 65 -
Capt. E. William Withrow Jr., SC, USN (Ret) – Director
Capt. Withrow has nearly twenty years of experience in the financial investment industry, twenty-four years of experience in the logistics field, and twenty years of experience in civic leadership. Since 2004, Capt. Withrow has served as an elected member of the Board of Trustees of the Peralta College District in the San Francisco Bay area, an institution consisting of 2,000 faculty and staff and approximately 30,000 students. From 1997 to 2002, Capt. Withrow served as a financial consultant for Wells Fargo, a provider of personal banking and investing services. From 1993 to 1997, he served as a financial consultant for Merrill Lynch, a financial management and advisory company. From 1987 to 1989, Capt. Withrow. served as a sales manager for Paine Webber, a stock brokerage and asset management firm, and from 1983 to 1987, he served as a financial consultant for Drexel Burnham Lambert, an investment banking firm. As a financial consultant and sales manager for the aforementioned financial institutions, Capt. Withrow examined financial statements, evaluated investment opportunities, provided advice to clients about possible investment opportunities and provided advice to stockbrokers and other individuals attempting to sell securities.
Additionally, Capt. Withrow served twenty-four years on active duty in the U.S. Navy as a professional logistician, retiring with the rank of Captain.
Capt. Withrow has been very active in civic leadership for the past 20 years serving in a number of elected and appointed positions, including Mayor of Alameda, California.
Capt. Withrow received a Bachelor of Business in Finance and Accounting from the University of Colorado in 1959, and in 1972 received a Master’s in Business Administration from Harvard University.
The Company believes Capt. Withrow is qualified to be a director of the Company because of his extensive experience in financial consulting and strategic business development.
John L. Ogden – Director
Mr. Ogden has 40 years’ experience in corporate finance, international negotiations, corporate and asset acquisition, business development and company management. Since 1995, he has been a principal and managing director of Wood Roberts, LLC, an energy corporate financial advisory firm based in Houston, Texas. Between 1985 and 1995, he managed an independent corporate financial consulting business specializing in domestic and international energy issues, providing M&A advice, and strategic corporate financial consulting services. Mr. Ogden graduated from the University of Leeds, England, with a Bachelor of Laws (honors) and is qualified as a Barrister-at-Law in England.
The Company believes Mr. Ogden is qualified to be a director of the Company because of his extensive experience in corporate finance and strategic business development.
Former Officers and Directors
David Appell – Former Chief Operating Officer
Mr. David Appell, age 56, is a highly motivated senior executive with diverse experience and education. Results driven, and extremely dedicated, Mr. Appell has developed long-term relationships with a vast worldwide network. He possesses extensive knowledge in the areas of technology, telecom, manufacturing, financial markets, and management, and is highly skilled in negotiations.
During his career spanning over 25 years, Mr. Appell has held senior executive positions in a multitude of areas, including serving as President of Carbon Capital Corp. since 2000, and Chief Executive Officer of Contour Tech Group from August 2014 to February 2018. From 2008 to 2014, Mr. Appell was Managing Member of MST Acquisitions Group LLC, and from 2001 to 2010, he served as Chief Operating Officer of Digital Products Inc. From 1998 to 2000, Mr. Appell served as Chief Executive Officer of The Regency Group, a full-service brokerage firm on Wall Street. From 1996 to 1998, Mr. Appell was Managing Director of Corporate Finance for Andrew Alexander Wise & Co., and from 1992 to 1997 he was General Partner of HPH Capital Growth LP. From 1992 to 1996, Mr. Appell also served as house counsel and Director for Comart, Inc., and from 1990 to 1992, he worked for Kantrowitz, Goldhamer and Graifman, PC, a law firm serving New York and New Jersey since 1975.
Mr. Appell also maintained his own law practice from 1992 to 2000, providing legal and consulting services to corporate, real estate and commercial clients.
Mr. Appell received his Juris Doctorate from Cardozo Law School in New York City, and a Bachelor’s in Business Administration from Pace University in New York, majoring in Accounting.
Mr. Appell has served as a trustee for the Institute for Educational Achievement (“IEA”) since 2013, a privately run establishment that provides educational services to children and young adults with Autism.
- 66 -
Identification of Significant Consultants
Mr. Appell, the Company’s former Chief Operating Officer, will serve as Managing Director of the Company’s wholly-owned subsidiary, Parallax Communications, Inc. (“PCOM”). As Managing Director, Mr. Appell will provide business advisory and strategic planning advice to the Company with PCOM. In exchange for these services, Mr. Appell will be paid $2,000 per month plus medical benefits.
The Company does not expect any other individuals to make a significant contribution to the Company’s business.
Family Relationships
There are no family relationships among its directors or executive officers.
Involvement in Certain Legal Proceedings
In December 2015, the Company’s Chief Executive Officer, Paul R. Arena, filed for personal bankruptcy in connection with his divorce. The Company does not believe Mr. Arena’s personal bankruptcy has any impact on the Parallax business.
On March 9, 2017, Dave Engert, former Executive Chairman and director of the Company, filed a lawsuit in Arizona and then on or about May 5, 2017, Mr. Engert, changed the venue and filed suit against the Company and RoxSan Pharmacy, Inc. in the United States District Court, Central District of California for an amount exceeding $75,000. On October 23, 2017, the Company filed an answer and counterclaims against Mr. Engert for an amount exceeding $100,000. The counterclaims include possible fraud and negligence committed by Mr. Engert and Mr. J. Michael Redmond, former successor Chairman of Mr. Engert, director, President and Chief Executive Officer of the Company and former President, Chief Executive Officer, Chairman and director of RoxSan Pharmacy, Inc. On October 8, 2018, a settlement was reached between Mr. Engert and the Company subject to the release of the bankruptcy trustee in the RoxSan matter. On October 8, 2018, a settlement was reached between Mr. Engert and the Company (the “Engert Settlement”). The Engert Settlement includes, among other things, a cash payment to Mr. Engert in the amount of $139,000, and the cancellation of all of Mr. Engert’s equity holdings in the Company. The Engert Settlement resulted in a net loss to the Company of $33,272. On April 10, 2019, a stipulation for dismissal was filed, and the matter has been fully resolved.
On March 28, 2018, Mr. J. Michael Redmond filed a lawsuit against the Company and RoxSan Pharmacy, Inc. in the United States District Court, Central District of California for an amount exceeding $75,000. The Company intends to vigorously defend against this action. There are counterclaims that include possible fraud and negligence committed by Mr. Redmond, former President, Chief Executive Officer, Chairman and director of the Company and of RoxSan Pharmacy, Inc. An Arbitration is still pending.
On May 14, 2018, pursuant to unanimous resolutions of the boards of directors of Parallax Health Sciences, Inc and RoxSan Pharmacy, Inc. (“RoxSan”), RoxSan filed a Chapter 7 petition in the United States Bankruptcy Court for the Central District of California. Mr. Timothy Yoo was appointed trustee on May 15, 2018. In connection with this filing, RoxSan seeks to discharge approximately $5 million of liabilities owed to various parties, and intercompany loans in excess of $1 million owed to the Company. The Chapter 7 bankruptcy proceeding by RoxSan Pharmacy, Inc. was fully discharged, and the case was closed on March 13, 2019, in U.S. Bankruptcy Court, Central District of California.
Except as disclosed above, the Company’s directors, executive officers, and control persons, have not been involved in any of the following events during the past five years:
1.any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
2.any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences);
3.being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or
4.being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated.
Audit Committee and Audit Committee Financial Expert
The Company established an audit committee of the Board comprised of John L. Ogden (Chair) and Capt. E. William Withrow Jr., SC, USN (Ret) The audit committee’s duties are to recommend to the Company’s Board the engagement of an independent registered public accounting firm to audit the Company’s financial statements and to review the Company’s accounting and auditing principles. The audit committee will review the scope, timing and fees for the annual audit and the results of audit examinations performed by the internal auditors and independent registered public accounting firm, including their recommendations to improve the system of accounting and internal controls. The audit committee will at all times be composed exclusively of directors who are, in the opinion of the Company’s Board, free from any relationship which would interfere with the exercise of independent judgment as a committee member and who possess an understanding of financial statements and generally accepted accounting principles.
- 67 -
The Company has adopted a Code of Ethics within the meaning of Item 406(b) of Regulation S-K of the Securities Exchange Act of 1934. The Code of Ethics applies to directors and senior officers, such as the principal executive officer, principal financial officer, controller, and persons performing similar functions.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Securities Exchange Act of 1934 requires the Company’s directors and executive officers and persons who beneficially own more than ten percent of a registered class of the Company’s equity securities to file with the SEC initial reports of ownership and reports of change in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent stockholders are required by SEC regulations to furnish the Company with copies of all Section 16(a) forms they file. Based solely upon a review of Forms 3 and 4 and amendments thereto furnished to the Company under Rule 16a-3(e) during the year ended December 31, 2019, Forms 5 and any amendments thereto furnished to the Company with respect to the year ended December 31, 2019, and the representations made by the reporting persons to the Company, the Company believes that during the year ended December 31, 2019, its executive officers and directors and all persons who own more than ten percent of a registered class of the Company’s equity securities complied with all Section 16(a) filing requirements.
Summary Compensation Table
The table below summarizes the compensation paid by the Company to the following persons:
(a)its principal executive officer;
(b)each of the Company’s two most highly compensated executive officers who were serving as executive officers at the end of the years ended December 31, 2019 and 2018; and
(c)up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as the Company’s executive officer at the end of the years ended December 31, 2019 and 2018.
No disclosure is provided for any named executive officer, other than the Company’s principal executive officers, whose total compensation did not exceed $100,000 for the respective fiscal year:
|
| Salary | Bonus | Stock Award | Option Awards | Non-Equity Incentive Plan Compensation | Change in Pension Value and Nonqualified Deferred Compensation Earnings | All Other Compensation | Total | |||||
Name and Principal Position | Year | ($) | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||
Paul R. Arena Chief Executive Officer, President | 2019 | 350,000 | [1] [7] | 75,000 | [1] | None |
| None |
| None | None | 142,815 | [1] [5] | 567,815 |
2018 | 350,000 | [1] [6] | 75,000 | [1] | None |
| None |
| None | None | 55,483 | [1] [5] | 480,483 | |
Calli R. Bucci Chief Financial Officer, Secretary | 2019 | 216,000 | [2] [7] | None |
| None |
| None |
| None | None | 51,355 | [2] [5] | 267,355 |
2018 | 216,000 | [2] [6] | 100,000 | [2] | 820,000 | [2] | 134,500 | [2] | None | None | 8,308 | [2] [5] | 1,278,808 | |
Nathaniel T. Bradley Chief Technology Officer, President, PHM | 2019 | 222,000 | [3] [7] | None |
| None |
| None |
| None | None | 5,655 | [3] [5] | 227,655 |
2018 | 222,000 | [3] [6] | None |
| None |
| 160,000 | [3] | None | None | 20,655 | [3] [5] | 402,655 | |
David Appell Former Chief Operating Officer | 2019 | 156,250 | [4] | None |
| 50,325 | [4] | 97,800 | [4] | None | None | 33,160 | [4] [5] | 310,385 |
2018 | None |
| None |
| None |
| None |
| None | None | None |
| None | |
[1] | Pursuant to Employment Agreement effective July 7, 2017. Includes $146,300 in officer’s compensation, and $87,783 in benefits owed to Executive at December 31, 2019, payment of which has been deferred until the Company reaches certain funding and earnings goals. Included in these goals are a private placement offering for $3-$6 million. |
[2] | Pursuant to Executive Agreement effective January 1, 2018. Includes $63,950 in officer’s compensation, and $66,726 in benefits owed to Executive at December 31, 2019, payment of which has been deferred until the Company reaches certain funding and earnings goals. Included in these goals are a private placement offering for $3-$6 million. Executive Agreement includes 1,000,000 options granted in 2018, all of which vested in 2018, and were valued at $134,500 using the Black Sholes method. The assumptions used in valuing the options were: expected term 3.75 years, expected volatility 1.78, risk free interest rate 2.25%, and dividend yield 0%. Executive also granted stock award in 2018 of 5,000,000 shares, with an aggregate grant date fair value of $820,000, 100% of which vested immediately. |
[3] | Pursuant to Employment Agreement effective August 1, 2017, as amended. Includes $31,172 in officer’s compensation, and $55,014 in benefits owed to Executive at December 31, 2019, payment of which has been deferred until the Company reaches certain funding and earnings goals. Included in these goals are a private placement offering for $3-$6 million. Employment Agreement was amended August 1, 2018, to grant Executive 1,000,000 options, valued at $160,000 using the Black Scholes method. The assumptions used in valuing the options were: expected term 3 years, expected volatility 2.06, risk free interest rate 2.78%, and dividend yield 0%. |
Pursuant to Employment Agreement effective May 15, 2019. Includes $83,750 in officer’s compensation, and $33,160 in benefits owed to Executive at December 31, 2019, payment of which has been deferred until the Company reaches certain funding and earnings goals. Included in these goals are a private placement offering for $3-$6 million. Employment Agreement includes 3,000,000 options granted in 2019, of which 25% vest immediately and the remainder vest over the term of the agreement and were valued at $195,600 using the Black Sholes method. The assumptions used in valuing the options were: expected term 4.75 years, expected volatility 2.21, risk free interest rate 2.15%, and dividend yield 0%. Employment Agreement also includes stock award of 3,000,000 shares, with an aggregate grant date fair value of $201,300, for a cost of $3,000, of which 25% vest immediately and the remainder vest over the term of the agreement. Pursuant to the Separation and Release Agreement dated February 2, 2020, upon Mr. Appell’s resignation,1,500,000 stock options, valued at $97,800, and 2,250,000 shares of the stock award, valued at $150,975, were canceled. As a result, the 2019 compensation has been adjusted to reflect these reductions. | |
[5] | Accrued vacation and benefits. |
[6] | Includes $150,000 paid by executive in connection with the purchase of convertible preferred Series C shares. |
[7] | Includes $100,000 paid by executive in connection with the issuance of 2,000,000 shares of Common Stock at a price of $0.05 per share, as well as $100,000 paid by executive in connection with the 2019 SAFE offering for the issuance of 1,600,000 shares of Common Stock, plus equal warrants to purchase Common Stock, at a price of $0.0625 per unit. The warrants are exercisable at a price of $0.25 per share for a period of three (3) years. |
- 68 -
Employment Contracts and Termination of Employment and Change in Control Arrangements
On July 6, 2017, the Company terminated the August 13, 2015 employment agreement and caused the removal of Mr. Joseph M. Redmond from all positions held in the Company and its subsidiaries (if any).
On July 7, 2017, the board of directors appointed Mr. Paul R. Arena as the Company’s new President and Chief Executive Officer. In connection with the appointment, the Company entered into an Executive Employment Agreement (the “Agreement”) with Mr. Arena dated July 7, 2017, for a period of three (3) years. The Agreement provides a base compensation of $350,000 in year one, of which 30% shall be deferred until certain funding goals are met, $425,000 in year two, and $550,000 in year three, as well as annual bonus compensation equal to 2x base when certain Company earnings are reached. In addition, the Agreement includes a grant to purchase 10,000,000 restricted common shares at $0.001 per share, of which 25% vests immediately; 25% vests in one year; 25% vests after two years; and 25% vests when certain funding goals have been met. The shares were valued at $2,000,000, of which $500,000 was expensed, and $1,500,000 was deferred, to be amortized over the next thirty-six (36) months. The Agreement also includes the grant of 5,000,000 stock options at an exercise price of $0.25 per share. The options are exercisable for a period of five (5) years, and vest when certain market share prices of the Company’s Common Stock are met.
On November 30, 2017, the Company executed an Employment Agreement with Mr. Nathaniel T. Bradley, with an effective date of August 1, 2017, to serve as the Company’s Chief Technology Officer (“CTO”), as well as CTO of Parallax Health Management, Inc. and Parallax Behavioral Health, Inc. The agreement replaces any other agreement between Mr. Bradley and the Company or any of its subsidiaries, is for an initial term of three (3) years, and provides a base compensation in the aggregate of $222,000 year one, $265,000 in year two and $320,000 in year three, as well as various performance bonuses, and customary employee benefits. In addition, the agreement, as amended, includes a grant to purchase 3,000,000 restricted common shares at $0.001 per share, valued at $750,000 and 100% vesting immediately, as well as options granted to purchase 2,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest annually over a three (3) year period, with an initial vesting of 25%.
On January 1, 2018, the Company entered into an Executive Agreement with its Chief Financial Officer. The agreement replaces any other written agreement with the Company, is for a term of one (1) year, with the option to extend, and includes annual compensation of $216,000 in year 1, as well as a bonus plan and customary executive benefits. In addition, the agreement provides for a non-refundable, fully-vested signing bonus of $100,000, as well as a grant of stock options to purchase 1,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest quarterly over a one (1) year period.
On January 1, 2019, the Company entered into a Consulting Agreement with JL Ogden & Company LLC, for the services of John L. Ogden, a member of the Company’s board of directors, and Chair of the Audit Committee. The agreement is for a term of one (1) year, with the option to extend, and includes annual compensation in the amount of $60,000. In addition, the agreement provides for a non-refundable, fully-vested signing bonus of $50,000, a convertible promissory note in the amount of $20,000 maturing in one (1) year with an annual interest rate of ten percent (10%), a restricted stock award equal to $70,000 worth of Units in the Company’s 2019 private placement offering, as well as a grant of stock options to purchase 1,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest quarterly over a one (1) year period.
On May 15, 2019, the Company entered into an employment agreement with Mr. David Appell to serve as the Company’s Chief Operating Officer. The agreement is for an initial term of two (2) years, and provides a base compensation of $250,000 year one, and $275,000 in year two, as well as various performance bonuses, and customary employee benefits. In addition, the agreement includes a grant to purchase 3,000,000 restricted common shares at $0.001 per share, of which 25% vest immediately, and the remainder vest when certain earnings goals are met, as well as options granted to purchase 3,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest annually over the term of the agreement, with an initial vesting of 25%
On February 2, 2020, Mr. David Appell resigned as the Company’s Chief Operating Officer. Mr. Appell’s resignation was not the result of a disagreement with the Company on any matters relating to the Company's operations, policies or practices. In connection with his resignation, Mr. Appell’s May 15, 2019, employment agreement was terminated. Pursuant to the Separation and Release Agreement dated February 2, 2020, 2,250,000 shares of the previously granted stock award of 3,000,000 shares, as well as 1,500,000 stock options of the previously granted 3,000,000 stock options, were canceled.
On April 7, 2020, the Company executed an Executive Agreement (the “Executive Agreement”) for the appointment of Dr. David L. Stark as the Company’s President, with an effective date of March 1, 2020. The Executive Agreement replaces any other agreement between Dr. Stark and the Company or any of its subsidiaries, and provides a base compensation of $225,000 in year one, $250,000 in year two and $275,000 in year three, commencing after the initial term estimated at approximately six (6) months, during which time Dr. Stark will devote fifty percent (50%) of his time at a compensation proportionate to the year one base compensation. The Executive Agreement also provides for various performance bonuses, and customary employee benefits, as well as (i) a grant to purchase 2,000,000 restricted common shares at $0.001 per share, of which 25% vest immediately, and the remainder vest upon the Company’s achievement of certain earnings goals; and (ii) stock options to purchase 3,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share for a period of five (5) years, of which 25% vest immediately, and the remainder vest quarterly in equal amounts over the term of the Agreement.
Each agreement above includes provisions for 1) the accelerated vesting of stock options or similar securities upon a change in control of the Company; and 2) six (6) to twelve (12) months continued benefits and salary at the prevailing rate upon their termination without just cause, excluding the Executive Agreement for Dr. Stark, which is “at will” and does not provide for continued salary or benefits after termination.
There are no other employment contracts, compensatory plans or arrangements, including payments to be received from the Company with respect to any executive officer, that would result in payments to such person because of his or her resignation, retirement or other termination of employment with the Company, or its subsidiaries, any change in control, or a change in the person’s responsibilities following a change in control of the Company.
There are no agreements or understandings for any executive officer to resign at the request of another person. None of the Company’s executive officers acts or will act on behalf of or at the direction of any other person.
- 69 -
In June 2019, the Company adopted and approved the 2019 Stock Incentive Plan (“the 2019 Plan”), wherein forty million (40,000,000) restricted shares of Common Stock were reserved for issuance. The 2019 Plan was intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company’s ownership and growth through the grant of incentive and non-qualified options. The 2019 Plan is currently administered by the Company’s board of directors. Subject to the provisions of the plan, the board will determine who shall receive options, the number of shares of Common Stock that may be purchased under the options. On June 17, 2019, the Company filed a Form S-8 registration statement with the Securities and Exchange Commission for the 40,000,000 shares underlying the 2019 Plan.
As of December 31, 2019, an aggregate of 26,685,000 stock options have been granted under the plans, of which 14,400,278 are vested, and 12,284,722 vest periodically over the next 16 months.
Stock Options/SAR Grants
On January 1, 2018, in connection with an executive agreement, the officer was granted 1,000,000 options to purchase shares of the Company’s Common Stock at $0.25 per share for a period of five (5) years. The options vest annually over a one (1) year period.
On August 1, 2018, in connection with an executive employment agreement, the officer was granted 1,000,000 options to purchase shares of the Company’s Common Stock at $0.25 per share for a period of five (5) years. Of the options granted, 50% vest immediately, and the remainder vest annually over a two (2) year period.
On January 1, 2019, in connection with an executive agreement, the officer was granted 1,000,000 options to purchase shares of the Company’s Common Stock at $0.25 per share for a period of five (5) years. The options vest annually over a one (1) year period.
On May 15, 2019, in connection with an executive employment agreement, the officer was granted 3,000,000 options to purchase shares of the Company’s Common Stock at $0.25 per share for a period of five (5) years. Of the options granted, 25% vested immediately, and the remainder vest quarterly over a three (3) year period.
There were no other stock options granted to directors and officers during the years ended December 31, 2019 or 2018.
Aggregated Option Exercised in Last Fiscal Year
On August 13, 2018, in connection with the exercise of certain employee stock options, the Company issued 1,071,430 shares of its restricted Common Stock at a conversion rate of $0.05 per share.
There were no other options exercised during the years ended December 31, 2019 or 2018, by any officer or director of the Company.
Outstanding Equity Awards at Fiscal Year End
The following tables summarize the outstanding equity awards held by each executive officer and director at December 31, 2019:
|
| Option Awards |
| Stock Awards |
| ||||||||||
Name |
| Total Number of Options Granted |
| Number of Options Vested / Exercisable |
| Number of Options Non-Exercisable |
| Exercise Price |
| Expiration Date |
| Number of Unvested Units |
| Value of Unvested Units |
|
Paul R. Arena |
| 5,000,000 |
| 1,250,000 |
| 3,750,000 |
| $0.25 |
| 07/07/2022 |
| 2,500,000 |
| $500,000 |
|
Nathaniel T. Bradley |
| 2,000,000 |
| 1,500,000 |
| 500,000 |
| $0.25 |
| 08/01/2022 |
| –– |
| –– |
|
Calli R. Bucci |
| 1,000,000 |
| 1,000,000 |
| –– |
| $0.25 |
| 01/01/2022 |
| –– |
| –– |
|
David Appell |
| 3,000,000 |
| 1,312,500 |
| 1,687,500 | [1] | $0.25 |
| 05/13/2024 |
| 2,250,000 |
| $150,975 | [1] |
John L. Ogden |
| 500,000 |
| 500,000 |
| –– |
| $0.05 |
| 10/05/2020 |
| –– |
| –– |
|
| 1,000,000 |
| 1,000,000 |
| –– |
| $0.25 |
| 12/31/2023 |
| –– |
| –– |
| |
Capt. E. William Withrow Jr., SC, USN (Ret) |
| 750,000 |
| 750,000 |
| –– |
| $0.05 |
| 10/05/2020 |
| –– |
| –– |
|
Total |
| 13,250,000 |
| 7,312,500 |
| 5,937,500 |
|
|
|
|
| 4,750,000 |
| $650,975 |
|
[1] Pursuant to the Separation and Release Agreement dated February 2, 2020, upon Mr. Appell’s resignation,1,500,000 stock options and 2,250,000 shares of the stock award, were canceled.
- 70 -
The table below summarizes the compensation paid to the Company’s independent directors for their services as members of the Company’s board of directors for the years ended December 31, 2019, and December 31, 2018:
Director Name |
| Fees earned or paid in cash | Stock awards | Option awards | Non-equity incentive plan | Nonqualified deferred compensation earnings | All other compensation | Total | |||
Capt. E. William Withrow Jr., SC, USN (Ret). Director | 2019 | $53,000 | None | None |
| None |
| None | None |
| $53,000 |
2018 | None | None | None |
| None |
| None | None |
| None | |
John L. Ogden Director | 2019 | $53,000 | None | None |
| None |
| None | None |
| $53,000 |
2018 | None | None | None |
| None |
| None | None |
| None | |
The Company reimburses its directors for expenses incurred in connection with attending board meetings. In addition, certain directors and officers of the Company have received stock options to purchase common shares under the Company’s Employee Stock Option Plan and Incentive Compensation Plan, and may receive additional stock options at the discretion of the Company’s Board.
The Company adopted a compensation and expense reimbursement plan for its independent directors effective January 1, 2019 (the “DC Plan”), to provide the directors with reasonable compensation for the performance of their duties as members of the board of directors and the amount of time spent on official business of the Company. The compensation provided under the DC Plan is as follows:
1.Annual retainer of $24,000 for Board membership, inclusive of all board meeting attendance.
2.Annual retainer of $12,000 for Committee membership, inclusive of all Committee meeting attendance.
3.Annual retainer for service as the Audit and Compensation Committee Chairpersons of $5,000.
4.Annual retainer for service as the Chairperson of any committee established by the Board, other than the Audit or Compensation Committee, of $2,500.
5.Reimbursement for reasonable out-of-pocket expenses actually incurred in connection with participation and/or attendance of Board and Committee meetings.
The Company has not paid any other cash compensation or directors’ fees for services rendered as a director since the Company’s inception to the adoption of the DC Plan.
Pension, Retirement or Similar Benefit Plans
As of December 31, 2019, the Company had no pension plans or compensatory plans or other arrangements which provide compensation in the event of termination of employment or change in control the Company, other than those disclosed above.
There are no arrangements or plans in which the Company provides pension, retirement or similar benefits for directors or executive officers. The Company has no material bonus or profit sharing plans pursuant to which cash or non-cash compensation is or may be paid to its directors or executive officers, except those stock options disclosed above or that may be granted in the future at the discretion of the Board or a committee thereof.
Compensation Committee
The Company established a compensation committee of the Board comprised of John L. Ogden and Capt. E. William Withrow Jr., SC, USN (Ret) (Chair). The compensation committee will oversee and determine the compensation of Parallax’s Chief Executive Officer and other executive officers, including salaries, bonuses, grants of stock options and other forms of equity-based compensation, approve all employment and severance agreements for executive officers, approve significant changes to benefit plans and perform such other functions as the Board may direct. The compensation committee will also approve all other agreements containing compensation and services rendered, such as consulting and other compensatory agreements, and will administer the Company’s stock incentive plans and make recommendations to the Board concerning any director compensation plan.
- 71 -
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth, as of December 31, 2019, certain information with respect to the beneficial ownership of its Common Stock by each stockholder known by the Company to be the beneficial owner of more than 5% of its Common Stock and by each of its current directors and executive officers. Each person has sole voting and investment power with respect to the shares of Common Stock, subject to community property laws where applicable, except as otherwise indicated. In computing the number of shares of Common Stock beneficially owned by a person and the percentage ownership of that person, we deemed to be outstanding all shares of Common Stock subject to restricted stock awards, stock options, warrants, rights or other convertible securities held by that person that are currently exercisable or will be exercisable within 60 days after December 31, 2019. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.:
Address | City, State, Zip | Amount and Nature of Beneficial Ownership [1] | Percentage of Shares of Common Stock | ||
M. Katuska Sandoval | 1327 Ocean Avenue, Suite B | Santa Monica, CA 90401 | 78,130,610 | [2] [4] | 28.75% |
Dr. Jorn & Jennifer Gorlach | 1929 SW Market Street Drive | Portland, OR 97201 | 68,835,544 | [2] [3] | 25.51% |
Montecito BioSciences, Ltd. | 1327 Ocean Avenue, Suite M | Santa Monica, CA 90401 | 38,156,227 | [2] | 15.25% |
Ionic Ventures, LLC | 5328 Yacht Haven Grande, Box # 15 / Suite C201 | St. Thomas, VI 00802 | 25,500,000 | [11] | 9.67% |
Huntington Chase LLC | 1327 Ocean Avenue, Suite B | Santa Monica, CA 90401 | 21,621,238 | [4] | 7.96% |
Hamburg Investment Group LLC | 1929 SW Market Street Drive | Portland, OR 97201 | 15,065,402 | [4] | 5.69% |
|
|
|
|
|
|
Directors and Executive Officers: |
|
|
|
|
|
Paul R. Arena | 28 W. 36th Street, 8th Floor | New York, NY 10018 | 15,925,205 | [5] | 6.31% |
Calli R. Bucci | 1327 Ocean Avenue, Suite B | Santa Monica, CA 90401 | 15,753,197 | [6] | 6.18% |
Nathaniel T. Bradley | 28 W. 36th Street, 8th Floor | New York, NY 10018 | 14,417,440 | [7] | 5.64% |
John L. Ogden | Two Riverway, Suite 1710 | Houston, TX 77056 | 7,216,964 | [8] | 2.85% |
Capt. E. William Withrow Jr., SC, USN (Ret) | 133 Cumberland Way | Alameda, CA 94502 | 2,784,187 | [9] | 1.11% |
David Appell | 28 W. 36th Street, 8th Floor | New York, NY 10018 | 2,062,500 | [10] | 0.83% |
Directors and officers as a group (6 shareholders) |
|
| 58,159,493 |
| 22.92% |
[1]Based upon 250,124,755 shares issued and outstanding at December 31, 2019, and Common Stock deemed outstanding for each beneficial shareholder. The Common Stock deemed outstanding was not deemed outstanding, however, for the purpose of computing the percentage ownership of any other person. The number and percentage of shares beneficially owned is determined under rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose.
[2]38,156,227 shares held by Montecito BioSciences, Ltd., of which 18,200,522 shares are indirectly beneficially owned by M. Katuska Sandoval, and 9,539,057 shares are indirectly beneficially owned by Dr. Jorn Gorlach. See footnote [3] and [4]. Mrs. Sandoval and Dr. Gorlach each disclaim beneficial ownership over the shares held by Montecito BioSciences, Ltd. except to the extent of their proportionate pecuniary interest therein.
[3]Includes direct beneficial ownership of 4,578,747 common shares; and indirect beneficial ownership of (i) 6,111,753 common shares and 4,923,415 warrants to purchase common shares held by Avantgarde LLC, and (ii) 229,368 common shares, 982,498 warrants and 13,853,536 common shares underlying convertible Preferred Stock and dividends, held by Hamburg Investment Group, entities controlled by Dr. Jorn Gorlach; and (iii) 38,156,227 shares of Montecito BioSciences, Ltd., of which 9,539,057 shares are held by Dr. Gorlach. [2]
[4]Includes direct beneficial ownership of 12,631,245 common shares; and indirect beneficial ownership of (i) 5,721,900 common shares held by Withrow Sinclair & Co.; and (ii) 1,000,000 vested stock options, 14,360,051 common shares underlying convertible Preferred Stock and dividends and 6,261,188 common shares underlying convertible debt held by Huntington Chase LLC, entities controlled by M. Katuska Sandoval; and (iii) 38,156,227 shares of Montecito BioSciences, Ltd., of which 18,200,522 shares are held by Mrs. Sandoval. [2]
[5]Includes direct beneficial ownership of 10,975,000 common shares and 1,250,000 stock options, respectively, exercisable within 60 days of December 31, 2019, of 10,000,000 restricted stock award and 5,000,000 stock options granted per Employment Agreement dated July 7, 2017; 2,225,000 warrants to purchase Common Stock; and 1,475,205 common shares underlying convertible Preferred Stock and dividends.
[6]Includes direct beneficial ownership of 11,052,992 common shares; 1,000,000 stock options exercisable within 60 days of December 31, 2019, of 1,000,000 granted per Employment Agreement dated January 1, 2018; 2,225,000 warrants to purchase Common Stock; and 1,475,205 common shares underlying convertible Preferred Stock and dividends.
[7]Includes direct beneficial ownership of 1,888,889 stock options exercisable within 60 days of December 31, 2019, of 2,000,000 granted per Employment Agreement dated August 1, 2017, as amended; 2,225,000 warrants to purchase Common Stock; 1,475,205 common shares underlying convertible Preferred Stock and dividends; and indirect beneficial ownership of 8,828,346 common shares held by Bradley Bros, LLC, an entity controlled by Nathaniel T. Bradley, director.
[8]Includes direct beneficial ownership of 4,516,964 common shares, 1,500,000 vested stock options exercisable within 60 days of December 31, 2019, and 1,200,000 warrants to purchase Common Stock.
[9]Includes direct beneficial ownership of 2,034,187 common shares, and 750,000 vested stock options exercisable within 60 days of December 31, 2019.
[10]Includes direct beneficial ownership of 750,000 common shares and 1,312,500 stock options, respectively, exercisable within 60 days of December 31, 2019, of 3,000,000 restricted stock award and 3,000,000 stock options granted per Employment Agreement dated May 15, 2019.
[11]Includes direct beneficial ownership of 12,000,000 common shares and 13,500,000 warrants to purchase common shares. exercisable within 60 days of December 31, 2019. Ionic Ventures, LLC controlled by Brendan O’Neill, whose address is 5328 Yacht Haven Grande Box # 15 / Suite C201, St. Thomas, VI 00802
Changes in Control
The Company is unaware of any contract or other arrangement or provisions of its Articles or Bylaws the operation of which may at a subsequent date result in a change of control of the Company. There are not any provisions in its Articles or Bylaws, the operation of which would delay, defer, or prevent a change in control of its company.
- 72 -
ITEM 13.CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Related Party Transactions
None of the directors or executive officers of the Company, nor any person who owned of record or was known to own beneficially more than 5% of the Company’s outstanding shares of its Common Stock, nor any associate or affiliate of such persons or companies, has any material interest, direct or indirect, in any transaction that has occurred during the year ended December 31, 2019, or in any proposed transaction, which has materially affected or will affect the Company, with the exception of the following:
Related Parties:
Montecito BioSciences, Ltd. (“MBS”) is a beneficial ownership shareholder of the Company. Capt. E. William Withrow Jr., SC, USN (Ret), a member of the Company’s board of directors, is chairman of the board of directors of MBS, and is the father-in-law of Mrs. M. Katuska Sandoval, a beneficial ownership shareholder of MBS. Mrs. Sandoval is also principal/control person of Withrow Sinclair & Co., a shareholder of the Company, and Huntington Chase, LLC, a beneficial ownership shareholder of the Company.
Dr. Jorn Gorlach, a beneficial ownership shareholder of MBS, is also a beneficial ownership shareholder of the Company. Dr. Gorlach is also principal/control person of Hamburg Investment Company, LLC, a beneficial ownership shareholder of the Company, and AvantGarde LLC, a shareholder of the Company.
Bradley Bros. LLC, a shareholder of the Company, is controlled by Nathaniel T. Bradley, an officer and director of the Company.
Intellectual Property Network, Inc. (“IPN”) is the former controlling shareholder of Parallax Health Management, Inc. (formerly Qolpom, Inc.), the Company’s wholly owned subsidiary since September 2016. IPN is controlled by Paul R. Arena and Nathaniel T. Bradley, officers and directors of the Company.
Stock Issuances:
On January 11, 2018, pursuant to a resolution of the board of directors, the Company issued 6,000,000 shares of its restricted Common Stock to certain officers and directors as follows: 5,000,000 shares to Calli R. Bucci, an officer and director, and 500,000 shares each to John L. Ogden and Capt. E. William Withrow Jr., SC, USN (Ret), directors. The shares were purchased at par, or $0.001 per share, for cash in the amount of $6,000.
On August 13, 2018, in connection with the exercise of certain stock options, the Company issued 1,071,430 shares of its restricted Common Stock at a conversion rate of $0.05 per share to Calli R. Bucci, an officer and director. The shares were issued on a cashless basis, resulting in a net value of $187,500.
On September 30, 2018, in connection with the Series C convertible Preferred Stock equity offering, Paul R. Arena, Calli R. Bucci and Nathaniel T. Bradley, officers and directors of the Company were each issued 30,000 Series C Preferred shares, for an aggregate of 90,000 Series C preferred shares, at a price of $5.00 per share in exchange for accrued compensation in the aggregate of $450,000. Upon issuance, a beneficial conversion feature of $100,578 was recorded as a deemed dividend. In connection with the Series C preferred shares, the officers were also each issued 625,000 warrants, for an aggregate of 1,875,000 warrants, valued at an aggregate of $75000, which are exercisable for a period of three (3) years at an exercise price of $0.25 per share.
On May 15, 2019, in connection with a certain employment agreement with Mr. David Appell, the Company issued 3,000,000 shares of its restricted Common Stock, valued at $201,300, of which 25% vest immediately, and the remainder vest when certain earnings goals are met.
On July 31, 2019, in connection with the settlement of certain related party convertible debentures, the Company issued 1,380,811 shares of its restricted Common Stock, valued at $131,315 to Hamburg Investment Company LLC and AvantGarde LLC, entities controlled by Dr. Jorn Gorlach, a beneficial ownership shareholder. In connection with the settlement, the related parties were also issued an aggregate of 2,528,413 warrants, valued at an aggregate of $92,150, and exercisable for a period of five (5) years at an exercise price of $0.10 per share
On September 18, 2019, in connection with the conversion of certain convertible debt in the amount of $437,000, the, Company issued 4,370,000 shares of its restricted Common Stock, to Huntington Chase, LLC, a beneficial ownership shareholder.
On September 30, 2019, in connection with a Simple Agreement Future Equity (“SAFE”) offering, the Company issued 1,600,000 shares each to Paul R. Arena, Calli R. Bucci Nathaniel T. Bradley, and 1,200,000 shares to John L. Ogden, for a total of 6,000,000 shares of its restricted Common Stock in exchange for the reduction in accrued compensation in the aggregate of $375,000. In connection with the SAFE offering, the officers and directors were also issued an aggregate of 6,000,000 warrants, valued at an aggregate of $45,600, and exercisable for a period of three (3) years at an exercise price of $0.25 per share.
On December 31, 2019, in connection with debt settlement agreements, the Company issued 2,000,000 shares of its restricted Common Stock each to Paul R. Arena, Calli R. Bucci and Nathaniel T. Bradley, all officers and directors of the Company, and 1,000,000 shares of its restricted Common Stock each to John L. Ogden, and Capt. E. William Withrow Jr., SC, USN (Ret), both directors of the Company, for a total of 8,000,000 shares issued at $0.05 per share, in exchange for the reduction in accrued compensation in the aggregate of $400,000.
- 73 -
As of December 31, 2019, Huntington Chase Financial Group, whose principal, M. Katuska Sandoval, is a beneficial shareholder of the Company, holds 399,732 shares of Series A Preferred Stock. Each share of Series A Preferred Stock is convertible into twenty (20) common shares at an average price of $0.27518 per share, for a total of 7,994,638 common shares, if converted. Dividends are payable semi-annually at a rate of 7% per annum, to be paid in cash or in kind, at the option of the Company. As of December 31, 2019, dividend payable totaled $156,443, or 6,365,411 common shares, as converted.
,
As of December 31, 2019, Hamburg Investment Company, LLC, whose principal, Dr. Jorn Gorlach, is a beneficial shareholder of the Company, holds 363,393 shares of Series A and 30,000 shares of Series B convertible Preferred Stock. Each share of Series A Preferred Stock is convertible into twenty (20) common shares at a price of $0.27518 per share, for a total of 7,267,853 common shares, if converted. Each share of Series B Preferred Stock is convertible into twenty (20) common shares at a price of $0.25 per share, for a total of 600,000 common shares, if converted. Warrants on Series A and Series B Preferred Stock have expired. Dividends on Series A and Series B Preferred Stock are payable semi-annually at a rate of 7% and 10% per annum, respectively, to be paid in cash or in kind, at the option of the Company. As of December 31, 2019, dividend payable totaled $124,046, or 5,985,676 common shares, as converted.
As of December 31, 2019, Paul R. Arena, Calli. R. Bucci, and Nathaniel T. Bradley, all officers and directors of the Company, each hold 30,000 shares of convertible Service C Preferred Stock. Each share of Series C Preferred Stock is convertible into 41.67 shares of Common Stock at a conversion price of $0.12 per share, for a total of 1,250,000 common shares each, if converted. The Series C subscriptions include warrants to purchase 625,000 shares of the Company's Common Stock at a price of $0.25 per share for a period of three (3) years. Dividends on Series C Preferred Stock are payable semi-annually at a rate of 8% per annum, to be paid in cash or in kind, at the option of the Company. As of December 31, 2019, dividend payable totaled $5,405 each, or 27,025 common shares each, as converted.
Agreements
On January 1, 2018, the Company entered into an Executive Agreement with Calli R. Bucci, its Chief Financial Officer. The agreement replaces any other written agreement with the Company, is for a term of one (1) year, with the option to extend, and includes annual compensation of $216,000 in year 1, as well as a bonus plan and customary executive benefits. In addition, the agreement provides for a non-refundable, fully-vested signing bonus of $100,000, as well as a grant of stock options to purchase 1,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest quarterly over a one (1) year period.
On January 1, 2018, the Company entered into a Consulting Agreement with Huntington Chase Financial Group, LLC, whose controlling shareholder, M. Katuska Sandoval, is a related party. The agreement replaces any other written agreement with the Company, is for a term of three (3) years, and includes monthly compensation of $25,000 in year 1; $30,000 in year 2 and $35,000 in year 3, of which the year 2 and 3 increases are deferred until completion of certain development projects, as well as customary expense allowances. In addition, the agreement provides for a grant of stock options to purchase 4,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, of which 25% vest immediately, and the remainder vest when certain market share prices of the Company’s Common Stock are met.
On January 1, 2019, the Company entered into a Consulting Agreement with JL Ogden & Company LLC, for the services of John L. Ogden, a member of the Company’s board of directors, and Chair of the Audit Committee. The agreement is for a term of one (1) year, with the option to extend, and includes annual compensation in the amount of $60,000. In addition, the agreement provides for a non-refundable, fully-vested signing bonus of $50,000, a convertible promissory note in the amount of $20,000 maturing in one (1) year with an annual interest rate of ten percent (10%), a restricted stock award equal to $70,000 worth of Units in the Company’s 2019 private placement offering, as well as a grant of stock options to purchase 1,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest quarterly over a one (1) year period.
On May 15, 2019, the Company entered into an employment agreement with Mr. David Appell to serve as the Company’s Chief Operating Officer. The agreement is for an initial term of two (2) years, and provides a base compensation of $250,000 year one, and $275,000 in year two, as well as various performance bonuses, and customary employee benefits. In addition, the agreement includes a grant to purchase 3,000,000 restricted common shares at $0.001 per share, of which 25% vest immediately, and the remainder vest when certain earnings goals are met, as well as options granted to purchase 3,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share. The options are for a period of five (5) years, and vest annually over the term of the agreement, with an initial vesting of 25%.
On February 2, 2020, Mr. David Appell resigned as the Company’s Chief Operating Officer. Mr. Appell’s resignation was not the result of a disagreement with the Company on any matters relating to the Company's operations, policies or practices. In connection with his resignation, Mr. Appell’s May 15, 2019, employment agreement was terminated. Pursuant to the Separation and Release Agreement dated February 2, 2020, 2,250,000 shares of the previously granted stock award of 3,000,000 shares, as well as 1,500,000 stock options of the previously granted 3,000,000 stock options, were canceled.
On April 7, 2020, the Company executed an Executive Agreement (the “Executive Agreement”) for the appointment of Dr. David L. Stark as the Company’s President, with an effective date of March 1, 2020. The Executive Agreement replaces any other agreement between Dr. Stark and the Company or any of its subsidiaries, and provides a base compensation of $225,000 in year one, $250,000 in year two and $275,000 in year three, commencing after the initial term estimated at approximately six (6) months, during which time Dr. Stark will devote fifty percent (50%) of his time at a compensation proportionate to the year one base compensation. The Executive Agreement also provides for various performance bonuses, and customary employee benefits, as well as (i) a grant to purchase 2,000,000 restricted common shares at $0.001 per share, of which 25% vest immediately, and the remainder vest upon the Company’s achievement of certain earnings goals; and (ii) stock options to purchase 3,000,000 shares of the Company's Common Stock at an exercise price of $0.25 per share for a period of five (5) years, of which 25% vest immediately, and the remainder vest quarterly in equal amounts over the term of the Agreement.
- 74 -
Promissory Notes and Accrued Interest:
On September 30, 2015, the Company issued a modified convertible promissory note in the principal sum of $631,100, to Huntington Chase, LLC, whose controlling shareholder, M. Katuska Sandoval, is a beneficial shareholder, representing cash loans and unpaid compensation, of which principal repayments in the aggregate of $100,000 were made in prior years, and $40,000 of which was converted into Common Stock. The note bears interest at a rate of 7% per annum, and contains a repayment provision to convert the debt into restricted shares of the Company’s Common Stock at a price of $0.10 per share. On December 31, 2018, the note was modified to 1) reduce the principal balance to $491,100; and 2) mature December 31, 2023. During the year ended December 31, 2019, the note holder converted $437,000 in principal into 4,370,000 shares of the Company’s Common Stock. Subsequently, the holder loaned the Company $437,000. As a result, the principal balance was not affected. However, on December 31, 2019, the promissory note was modified to reduce the conversion price to $0.08.
Between April 26, 2018 and May 8, 2018, the Company issued senior secured convertible promissory notes (the “Notes”) to Avantgarde, LLC and Hamburg Investment Group LLC, whose principal, Dr. Jorn Gorlach, is a beneficial shareholder, in the aggregate principal sum of $337,750, along with 3,377,500 warrants. The Notes bore interest at rate of 12% per annum, contained a repayment provision to convert the Notes into restricted shares of the Company’s Common Stock at a price of $0.10 per share, and included warrant coverage for a period of three (3) years to purchase shares of the Company’s Common Stock at an exercise price of $0.10 per share. Effective November 14, 2018 (the “Effective Date”), the Notes and related accrued interest of $34,090 were exchanged into convertible debentures (“Debentures”) in the principal amount of $428,132. The Debentures bore interest at a rate of 10% per annum, matured February 28, 2019, and were convertible into shares of the Company’s restricted Common Stock at a conversion rate of $0.12 per share or, if an event of default has occurred or the date of conversion is 120 days after the Effective Date, the lesser of (a) $0.10 or (b) 70% of the second lowest traded price for the 20 trading days immediately preceding the date of conversion.. On July 25, 2019, the Debentures in the principal sum of $411,006, plus accrued interest of $42,778, were converted to non-convertible Senior Secured Promissory Notes (the “Senior Notes”) in the aggregate principal of $759,446. The Senior Notes bear interest at a rate of 8% per annum, with payments of $126,152 plus interest accrued thereon due December 31, 2019; $300,000 due December 31, 2020; and the remaining principal and accrued interest due December 31, 2021. In connection with the exchange, the Company issued the following in favor of the lender: 1,380,811 shares of the Company’s restricted Common Stock, valued at $131,315; and warrants, valued at $92,150, to purchase 2,528,413 shares of the Company’s Common Stock for a period of five (5) years at an exercise price of $0.10461. As a result, the Company recognized a net loss on the exchange in the amount of $434,837.
As of December 31, 2019, related party promissory notes in the amount of $1,250,546 are owed; of which $491,100 are convertible.
During the year ended December 31, 2019, interest in the amount of $105,111 was expensed, $100,132 was converted to restricted shares of the Company’s Common Stock, and $42,778 was converted to principal. As of December 31, 2019, a total of $36,261 in accrued interest remains.
Accrued Compensation and Benefits:
During the year ended December 31, 2019, $1,586,724 in related party compensation was accrued, of which payments were made in the amount of $802,500; and $753,510 was converted to Common Stock; for a net decrease in accrued compensation of $73,686. As of December 31, 2019, accrued compensation is owed to Paul R. Arena in the amount of $195,863; Calli R. Bucci in the amount of $88,874; David Appell in the amount of $89,760; Nathaniel T. Bradley in the amount of $62,187; Huntington Chase LLC, whose principal, M. Katuska Sandoval is a beneficial shareholder, in the amount of $297,000; John L. Ogden in the amount of $59,490 and Capt. E. William Withrow Jr., SC, USN (Ret) in the amount of $3,000; for a total accrued compensation owed in the amount of $796,175 as of December 31 2019.
During the year ended December 31, 2019, accrued benefits increased by $292,464, of which payments were made in the amount of $103,527; and cash advances made by officers and beneficial shareholders to the Company for operating expenses increased by $106,785, of which repayments of cash advances were made in the amount of $118,278; for a net increase in accrued benefits and cash ad advances of $177,445. As of December 31, 2019, accrued benefits and cash advances are owed to Paul R. Arena in the amount of $38,220; Calli R. Bucci in the amount of $32,252; David Appell in the amount of $27,150; Nathaniel T. Bradley in the amount of $27,129; Huntington Chase LLC, whose principal, M. Katuska Sandoval is a beneficial shareholder, in the amount of $109,200; and Intellectual Property Network, whose principals, Paul R. Arena and Nathaniel T. Bradley are officers and directors, in the amount of $78,353; for a total accrued benefits and cash advances owed in the amount of $312,305 as of December 31 2019, as follows:
|
|
|
| For the year ended December 31, 2019 |
|
| ||||||||||||
Related Party |
| Balance 12/31/2018 |
| Accrued Benefits Owed to Related Party |
| Benefits Paid to Related Party |
| Cash Advances to Company |
| Repayments of Cash Advances |
| Balance 12/31/2019 | ||||||
Paul R. Arena |
| $ | 18,525 |
| $ | 123,222 |
| $ | (103,527) |
| $ | 2,500 |
| $ | (2,500) |
| $ | 38,220 |
Calli R. Bucci |
|
| 2,398 |
|
| 40,092 |
|
| –– |
|
| 36,462 |
|
| (46,700) |
|
| 32,252 |
David Appell |
|
| –– |
|
| 27,150 |
|
| –– |
|
| –– |
|
| –– |
|
| 27,150 |
|
| 20,112 |
|
| 24,000 |
|
| –– |
|
| 9,243 |
|
| (26,225) |
|
| 27,130 | |
Huntington Chase, LLC |
|
| 17,753 |
|
| 78,000 |
|
| –– |
|
| 56,000 |
|
| (42,553) |
|
| 109,200 |
Intellectual Property Network |
|
| 76,073 |
|
| –– |
|
| –– |
|
| 2,580 |
|
| (300) |
|
| 78,353 |
Total |
| $ | 134,861 |
| $ | 292,464 |
| $ | (103,527) |
| $ | 106,785 |
| $ | (118,278) |
| $ | 312,305 |
The cash advances made to the Company were used for the purpose of overhead and operating expenses. All accrued benefits and cash advances are non-interest bearing.
As of December 31, 2019, related parties are due a total of $2,359,026, consisting of $796,175 in accrued compensation owed to officers; $312,305 in cash advances from officers and beneficial owners to the Company for operating expenses; $759,446 in promissory notes, and $491,100 in convertible promissory notes.
Director Independence
For purposes of determining director independence, the Company have applied the definitions set out in NASDAQ Rule 5605(a)(2). The OTCQB on which shares of Common Stock are quoted does not have any director independence requirements. The NASDAQ definition of “Independent Officer” means a person other than an Executive Officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company's Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. According to the NASDAQ definition, Paul R. Arena, Calli R. Bucci and Nathaniel T. Bradley are not independent directors of the Company.
The aggregate fees billed or to be billed for the most recently completed fiscal year ended December 31, 2019 and 2018, for professional services rendered by the principal accountant for the audit of its annual financial statements and review of the financial statements included in its quarterly reports on Form 10-Q and services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for these fiscal periods were as follows:
| Year Ended | ||||
| December 31, 2018 |
| December 31, 2018 | ||
Audit Fees | $ | 70,000 |
| $ | 95,000 |
Audit Related Fees (Valuations and Restatements) |
| 0 |
|
| 100,000 |
Tax Fees |
| 0 |
|
| 0 |
All Other Fees |
| 0 |
|
| 0 |
Total | $ | 70,000 |
| $ | 195,000 |
The Company’s board of directors pre-approves all services provided by its independent auditors. All of the above services and fees were reviewed and approved by the board of directors either before or after the respective services were rendered.
The Company’s board of directors has considered the nature and amount of fees billed by its independent auditors and believes that the provision of services for activities unrelated to the audit is compatible with maintaining its independent auditors’ independence.
- 75 -
PART IV
Exhibits required by Item 601 of Regulation S-B.
- 76 -
*Filed herewith.
**Pursuant to Regulation S-T, this interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections.
- 77 -
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| PARALLAX HEALTH SCIENCES, INC. |
|
|
|
|
|
|
|
|
|
| Dated: May 15, 2020 | /s/ Paul R. Arena |
|
|
| Paul R. Arena |
|
|
| President, Chief Executive Officer |
|
|
| (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
| Dated: May 15, 2020 | /s/ Calli R. Bucci |
|
|
| Calli R. Bucci |
|
|
| Chief Financial Officer |
|
|
| (Principal Financial Officer) |
|
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
|
|
|
|
|
|
|
| Dated: May 15, 2020 | /s/ Paul R. Arena |
|
|
| Paul R. Arena |
|
|
| President, Chief Executive Officer |
|
|
| (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
| Dated: May 15, 2020 | /s/ Calli R. Bucci |
|
|
| Calli R. Bucci |
|
|
| Chief Financial Officer |
|
|
| (Principal Financial Officer and Principal Accounting Officer) | |
|
|
|
|
|
|
|
|
| Dated: May 15, 2020 | /s/ Capt. E. William Withrow Jr., SC, USN (Ret) |
|
|
| Capt. E. William Withrow Jr., SC, USN (Ret) |
|
|
| Director |
|
|
|
|
|
|
|
|
|
| Dated: May 15, 2020 | /s/ John L. Ogden |
|
|
| John L. Ogden |
|
|
| Director |
|
|
|
|
|
|
|
|
|
| Dated: May 15, 2020 | /s/ Nathaniel T. Bradley |
|
|
| Nathaniel T. Bradley |
|
|
| Director |
|
- 78 -