Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CAPRICOR THERAPEUTICS, INC. | tm2019802d2_8k.htm |
Exhibit 99.1

Capricor Q1 Earnings Call and Corporate Update 1 May 14, 2020 Earnings Call NASDAQ: CAPR

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Forward - Looking Statements 2 Statements in this presentation regarding the efficacy, safety, and intended utilization of Capricor's product candidates ; the initiation, conduct, size, timing and results of discovery efforts and clinical trials ; the pace of enrollment of clinical trials ; plans regarding regulatory filings, future research and clinical trials ; regulatory developments involving products, including the ability to obtain regulatory approvals or otherwise bring products to market ; plans regarding current and future collaborative activities and the ownership of commercial rights ; scope, duration, validity and enforceability of intellectual property rights ; future royalty streams, revenue projections ; expectations with respect to the expected use of proceeds from the recently completed offerings and the anticipated effects of the offerings, and any other statements about Capricor's management team's future expectations, beliefs, goals, plans or prospects constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Any statements that are not statements of historical fact (including statements containing the words "believes," "plans," "could," "anticipates," "expects," "estimates," "should," "target," "will," "would" and similar expressions) should also be considered to be forward - looking statements . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business is set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2019 as filed with the Securities and Exchange Commission on March 27 , 2020 . All forward - looking statements in this press release are based on information available to Capricor as of the date hereof, and Capricor assumes no obligation to update these forward - looking statements . CAP - 1002 is an Investigational New Drug and is not approved for any indications . None of Capricor’s exosome - based candidates have been approved for clinical investigation .
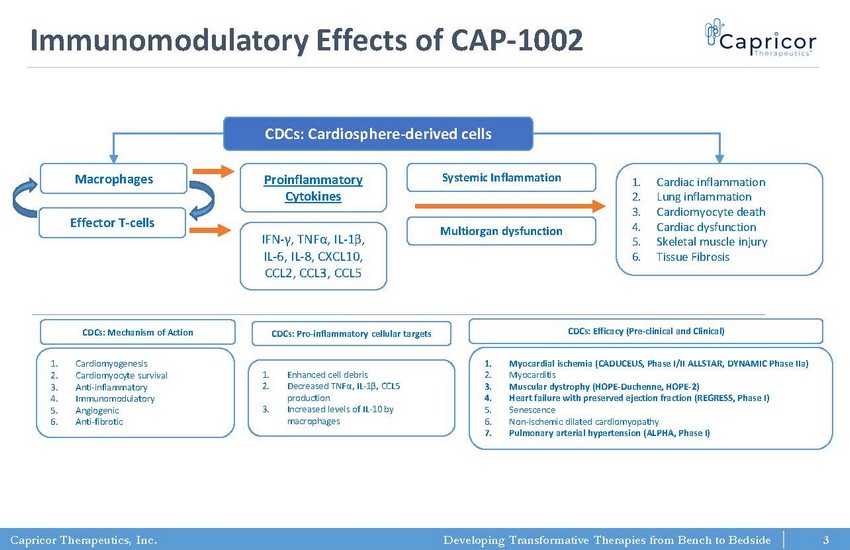
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Immunomodulatory Effects of CAP - 1002 CDCs: Cardiosphere - derived cells Macrophages Effector T - cells Proinflammatory Cytokines IFN - γ , TNF α , IL - 1 β , IL - 6, IL - 8, CXCL10, CCL2, CCL3, CCL5 Systemic Inflammation Multiorgan dysfunction 1. Cardiac inflammation 2. Lung inflammation 3. Cardiomyocyte death 4. Cardiac dysfunction 5. Skeletal muscle injury 6. Tissue Fibrosis 1. Cardiomyogenesis 2. Cardiomyocyte survival 3. Anti - inflammatory 4. Immunomodulatory 5. Angiogenic 6. Anti - fibrotic CDCs: Mechanism of Action 1. Enhanced cell debris 2. Decreased TNF α , IL - 1 β , CCL5 production 3. Increased levels of IL - 10 by macrophages CDCs: Pro - inflammatory cellular targets 1. Myocardial ischemia (CADUCEUS, Phase I/II ALLSTAR, DYNAMIC Phase IIa ) 2. Myocarditis 3. Muscular dystrophy (HOPE - Duchenne, HOPE - 2) 4. Heart failure with preserved ejection fraction (REGRESS, Phase I) 5. Senescence 6. Non - ischemic dilated cardiomyopathy 7. Pulmonary arterial hypertension (ALPHA, Phase I) CDCs: Efficacy (Pre - clinical and Clinical) 3
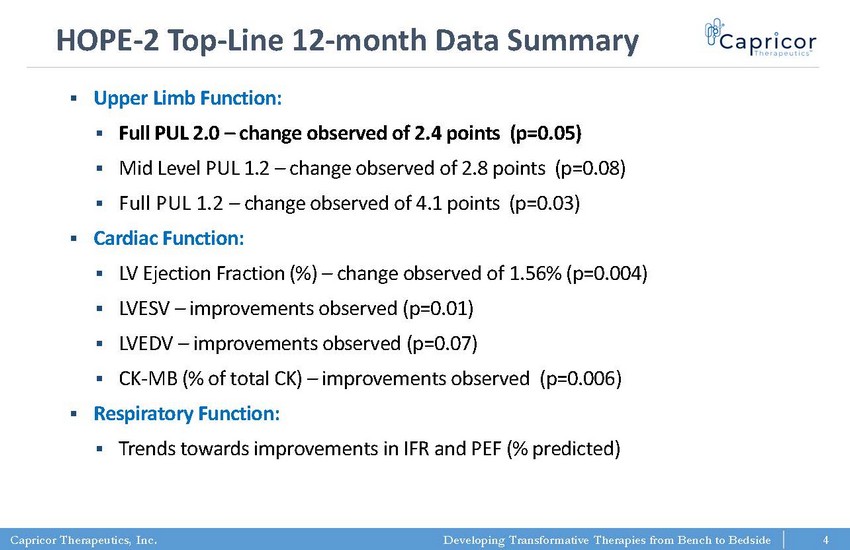
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - 2 Top - Line 12 - month Data Summary 4 ▪ Upper Limb Function: ▪ Full PUL 2.0 – change observed of 2.4 points (p=0.05) ▪ Mid Level PUL 1.2 – change observed of 2.8 points (p=0.08) ▪ Full PUL 1.2 – change observed of 4.1 points (p=0.03) ▪ Cardiac Function: ▪ LV Ejection Fraction (%) – change observed of 1.56% (p=0.004) ▪ LVESV – improvements observed (p=0.01) ▪ LVEDV – improvements observed (p=0.07) ▪ CK - MB (% of total CK) – improvements observed (p=0.006) ▪ Respiratory Function: ▪ Trends towards improvements in IFR and PEF (% predicted) 4 4
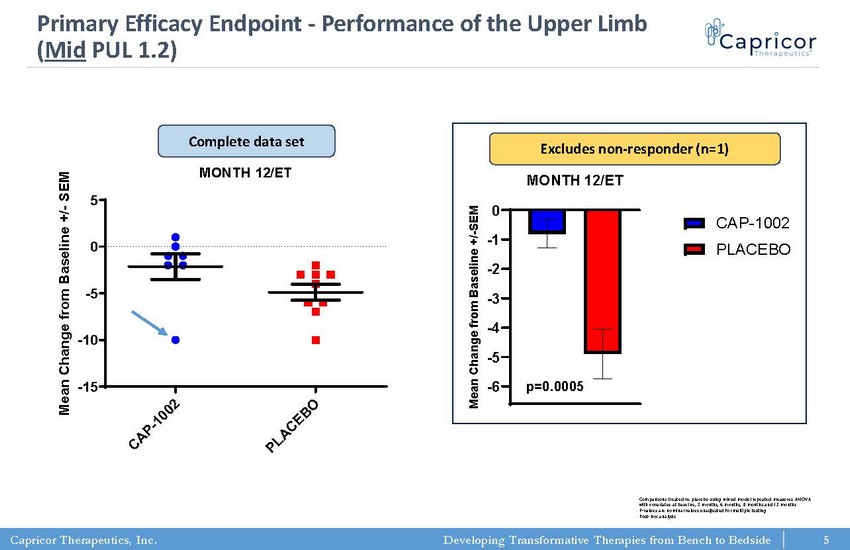
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside PUL 1.2 (mid) MONTH 12/ET C A P - 1 0 0 2 P L A C E B O -15 -10 -5 0 5 M e a n C h a n g e f r o m B a s e l i n e + / - S E M p=0.0974 (t test; two-tailed) 5 Primary Efficacy Endpoint - Performance of the Upper Limb ( Mid PUL 1.2) Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months P - values are nominal values unadjusted for multiple testing Post - hoc analysis -6 -5 -4 -3 -2 -1 0 PUL 1.2 (mid) MONTH 12/ET M e a n C h a n g e f r o m B a s e l i n e + / - S E M CAP-1002 PLACEBO p=0.0005 Excludes non - responder (n=1) Complete data set 5
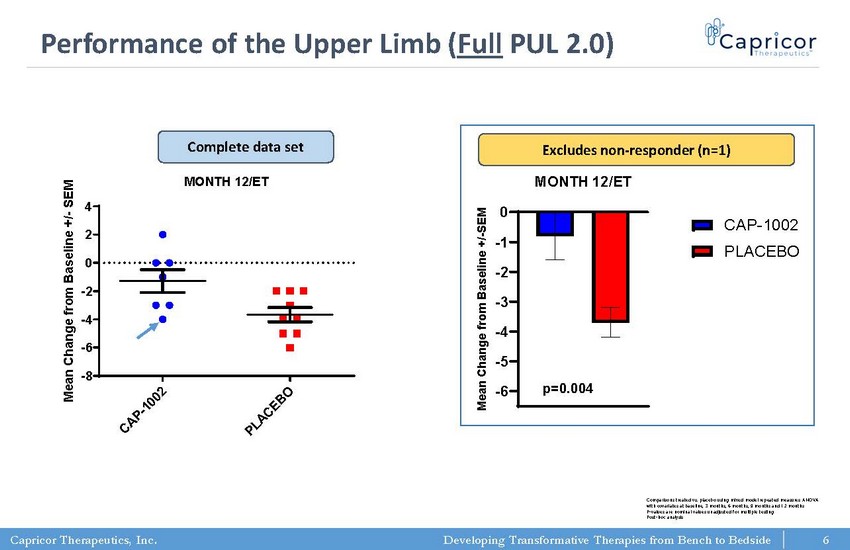
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside -6 -5 -4 -3 -2 -1 0 PUL 2.0 (full) MONTH 12/ET M e a n C h a n g e f r o m B a s e l i n e + / - S E M CAP-1002 PLACEBO p=0.004 PUL 2.0 (full) MONTH 12/ET C A P - 1 0 0 2 P L A C E B O -8 -6 -4 -2 0 2 4 p=0.0201 (t test; two-tailed) M e a n C h a n g e f r o m B a s e l i n e + / - S E M Performance of the Upper Limb ( Full PUL 2.0) Excludes non - responder (n=1) Complete data set 6 Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months P - values are nominal values unadjusted for multiple testing Post - hoc analysis
