Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Kaleido Biosciences, Inc. | kldo-8k_20200514.htm |
| EX-99.3 - EX-99.3 - Kaleido Biosciences, Inc. | kldo-ex993_96.htm |
| EX-99.1 - EX-99.1 - Kaleido Biosciences, Inc. | kldo-ex991_8.htm |
Exhibit 99.2

1 ©2020 KALEIDO™ ©2020 KALEIDO™ Company Overview May 2020

2 ©2020 KALEIDO™ Forward - Looking Statements This presentation also contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding our strategy, business plans and focus, including the therapeutic potential of our Microbiome Metabolic Therapy (MMT) candidates, the timing of initiation, completion and reporting of results of our clinical studies and our strategy, business plans and focus. The words “may,” “will,” “could,” “would,” “should,” “expect,” “ plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expression s a re intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. Any forward - looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward - looking statements contained in this presentation, including, without limitation, those related to planned clinical and preclinical studies and areas of interest, the preclinical and clinical development and safety profile of our MMT candidates and timelines associated with the programs for such MMT candidates, whether and when, if at all, our MMT candidates will receive approval from the U.S. Food and Drug Administration or other applicable regulatory agencies, if any, competition from other biotechnology companies, and other risks identified in our SEC filings, including the most recently filed 10 - K. We caution you not to place undue reliance on any forward - looking statements, which speak only as of the date they are made. We disclaim any obligation to update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward - looking statements.

3 ©2020 KALEIDO™ Kaleido : A Differentiated Product Platform Company Broad Pipeline to Address Significant Unmet Patient Needs Rapid, Cost - Efficient Discovery & Development Model Proprietary Product Platform Chemistry targeted at changing the metabolic output and construct of the microbiome Transforming product discovery, development and delivery Redefining how we treat disease and driving beneficial outcomes to meaningfully improve health

4 ©2020 KALEIDO™ Microbiome Drives the Progression of a Broad Range of Diseases Image Picture 41 • Cardiometabolic and Liver Diseases • Chronic Kidney Disease Metabolic Health • Urea Cycle Disorders • Hepatic Encephalopathy Nitrogen Metabolism • Pathogens • GvHD Microbial Ecology • Immuno-oncology • IBD • Viral Infections • Multiple sclerosis Immune Modulation Picture 36 Anticancer immunotherapy by CTLA - 4 blockade relies on the gut microbiota Picture 48 The severity of NAFLD is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota Picture 50 Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopment Disorders Picture 56 Gut microbiome modulates response to anti - PD - 1 immunotherapy in melanoma patients Note: 1. Bolded text indicates areas where Kaleido currently has programs A screenshot of a computer Description automatically generated

5 ©2020 KALEIDO™ Source: Koropatkin et al., Nature Reviews Microbiology , 10 , 323 – 335 (2012) Glycans Play a Major Role in Shaping the Gut Microbiome and Drive Metabolites that Modulate Disease Pathways Natural glycans from multiple sources e.g. plant, mucus, meat, microbial, milk Humans: 17 glycosidases Microbiome: >3000 carbohydrate - active enzymes ( CAZYmes ) Advancing Chemistry to Create Novel Synthetic Glycans Targeted at Multiple Pathways

6 ©2020 KALEIDO™ Synthetic Glycan Chemistry enables Extensive Array of Microbiome Metabolic Therapies (MMTs) • Target enzymes across taxa rather than only in specific species, driving ecosystem and metabolic output changes • Related to a class of compounds that is Generally Recognized as Safe (GRAS), enabling rapid advancement into human clinical studies • Extensive IP; NCEs when developed as drugs • Orally administered with limited systemic exposure • Diversity of glycan library enables understanding of structure and disease pathways MICROBIOME METABOLIC THERAPIES (MMT™): Synthetic glycans that are structurally diverse and complex ensembles Chain Length Mono - Oligo - Poly - saccharide Our library of 1,500+ MMTs : + Multiple bond types + B ranching Starting Monomers Almost unlimited diversity of chemical structures in this class

7 ©2020 KALEIDO™ MMTs Targeted at Multiple Metabolites and Pathways of Disease Further Expansion into Immune Modulation - Multiple pathways - I/O, Viral infections (COVID - 19), IBD, other immune - mediated disorders - HE program to . both ammonia and MDR pathogens Platform Validating: Single Metabolite Focus - 4 clinical studies with 2 MMTs show consistent reduction in biomarker of microbiome ammonia production - Phase 2 program in urea cycle disorders BLOOD BRAIN BARRIER Metabolite and Microbiome Construct

8 ©2020 KALEIDO™ Significant Progress: Validating the Platform and Advancing the Technology. Established I - O collaboration with Gustave Roussy, Europe’s largest cancer treatment center. Initiated in vivo mechanistic work for lead MMTs in cardiometabolic/liver diseases. Initiated collaboration with Janssen to explore potential of MMTs to prevent the onset of childhood allergy and other atopic, immune and metabolic conditions. Initiated research collaboration with Dr. Jeffrey Gordon, Washington University, founder of the modern microbiome field Progressed Pipeline A picture containing drawing Description automatically generated Advanced Science. Successfully scaled manufacturing. Confirmation of regulatory pathway for efficient clinical development model. IND cleared and 9 CTAs approved for KB195. FDA feedback allowing for initiation of Phase 3 pivotal trial upon decision to file an IND. Continued to build robust IP portfolio; currently 11 patents issued and 100+ pending worldwide Validated Platform. 5 clinical studies initiated for 3 MMT candidates in 3 different patient populations. Positive results for 2 MMTs in 2 patient populations (KB195 in patients with UCD and KB174 in patients with cirrhosis). Efficient clinical model demonstrated with lead MMT, KB195. Ex vivo assay shown to be predictive of human results. Validated through consistent and clinically relevant reduction in a biomarker of microbiome ammonia production observed in 4 clinical studies, with 2 MMTs

9 ©2020 KALEIDO™ Opportunity to Deliver Key Data Readouts in 2020 and 2021 COVID - 19 impacting healthcare system, including execution of current and new clinical studies Kaleido leveraging platform to progress pipeline activities Opportunity in COVID - 19 Environment to Deliver Clinical Data & Advance Science in Immune & Inflammatory Disease Pathways A picture containing drawing Description automatically generated Pre - Clinical Programs Clinical Studies New: COVID - 19 results anticipated in Q4’20 UC results expected mid - ’21 New: Immune - mediated disease models expected in 1H'21

10 ©2020 KALEIDO™ Note: 1. Our human clinical studies of our MMT candidates are conducted under regulations supporting research with food, evaluating safety, tolerability and potential markers of effect in human subjects. For MMT candidates that are further developed as therapeutics, the Company conducts clinical trials under an Investigational New Drug (IND) or regulatory equivalent outside the U.S., and in Phase 2 or later development. 2. Initiation gated on partner 3. VITORA study of KB109 paused due to COVID - 19 PRODUCT CANDIDATE PROGRAM CLINICAL DEVELOPMENT STATUS RESULTS EXPECTED KB195 Urea Cycle Disorders 2H 2021 KB174 Hepatic Encephalopathy KB109 COVID - 19 New Pathogens 3 Q4 2020 KB295 Inflammatory Bowel Disease (IBD) New Mid - 2021 Phase 2 clinical trial (UNLOCKED) under IND/CTAs Clinical study 1 in patients Completed Clinical Study Clinical Study or Trial in Progress Clinical Study Planned Clinical study 1 in healthy volunteers Clinical study 1 in patients with cirrhosis Clinical study 1 in healthy volunteers Clinical study 1 in patients with HE 2 Clinical studies 1 in patients with mild - moderate SARS - CoV - 2 Patients at high risk for MDR infections Clinical study 1 in patients with ulcerative colitis Potential Next Clinical Study Progressing a Broad Pipeline of MMT Candidates 4 MMTs in Clinical Development

11 ©2020 KALEIDO™ Preclinical Program Collaborator Clinical - Ready Lead MMT Expected in 2021 Immuno - oncology Cardiometabolic & Liver Diseases Immune - mediated Diseases New Prevention of Childhood - Onset Atopic & Immune Conditions Advancing Our MMTs for Lead Identification Poised to Deliver Multiple Clinical - Ready Programs in 2021 Image result for images gustave roussy logo A picture containing drawing, table Description automatically generated Image result for washington university school of medicine logo Jeffrey Gordon, MD Glycan chemistry & utilization Gnotobiotic preclinical models combined with computational methods to further identify molecular pathways of how MMTs can modify functional configuration of the gut microbiome and its metabolic outputs

12 ©2020 KALEIDO™ Phase 2 clinical trial results of KB195 in patients with UCD (2H’21) cardiometabolic /liver diseases (Q4’20) Key Expected Upcoming Milestones 2020 immuno-oncology (Q4’20) immune - mediated disease (1H’21) Clinical study results of KB295 in UC (mid - ’21) 2021 Clinical study results of KB109 in COVID - 19 (Q4’20) Clinical Results 1. COVID - 19 study Q4’20 2. Clinical study in UC mid - ’21 3. Phase 2 trial results in UCD in 2H’21 Clinical - Ready Lead MMTs Identified from Preclinical Data

13 ©2020 KALEIDO™ ©2020 KALEIDO™ COVID - 19
14 ©2020 KALEIDO™ Microbiome Opportunity to Target COVID - 19 with Potential Applicability in Broader Respiratory Viral Infections A picture containing cake, fruit, colored, decorated Description automatically generated Public Health Threat • COVID - 19 infection is associated with inappropriate activation of inflammatory pathways • Large public health need for preventing or dampening the pathology of COVID - 19 infection - Reduce the number of people needing to be hospitalized - Reduce cases of cytokine storm that lead to respiratory failure • Antiviral therapies not yet proven, other treatments under investigation Microbiome impact on Immune Pathways Likely Applicable in Broader Respiratory Viral Infections • Opportunity to support body’s own antiviral immunity and fight infection - Reduce the risk of uncontrolled innate immune response leading to severe inflammation and lung - tissue destruction - Foster appropriate immune response to reduce severity of infection and severe clinical outcomes
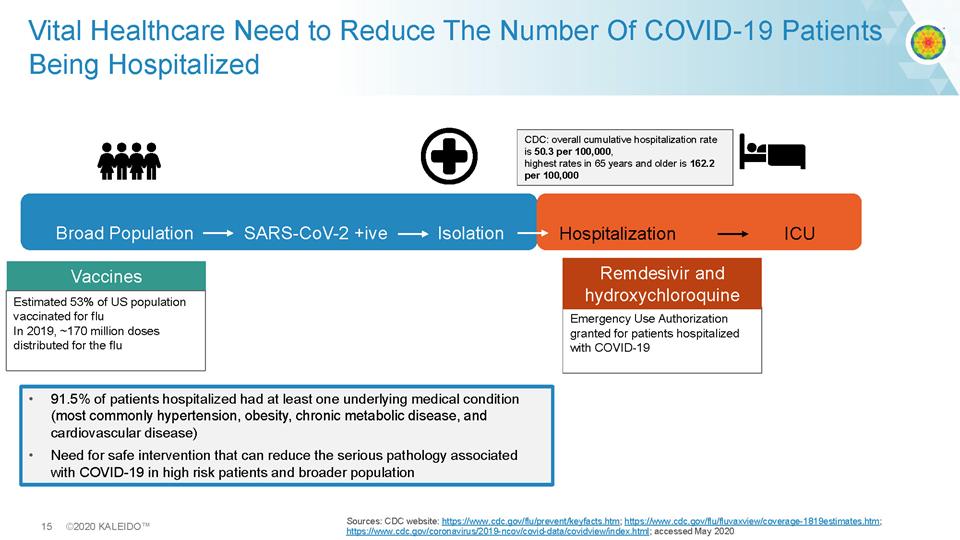
15 ©2020 KALEIDO™ Sources: CDC website: https://www.cdc.gov/flu/prevent/keyfacts.htm ; https://www.cdc.gov/flu/fluvaxview/coverage - 1819estimates.htm ; https://www.cdc.gov/coronavirus/2019 - ncov/covid - data/covidview/index.html ; accessed May 2020 Vital Healthcare Need to Reduce The Number Of COVID - 19 Patients Being Hospitalized Broad Population SARS - CoV - 2 + ive Isolation Vaccines Estimated 53% of US population vaccinated for flu In 2019, ~170 million doses distributed for the flu Children Sleep Medical CDC: overall cumulative hospitalization rate is 50.3 per 100,000 , highest rates in 65 years and older is 162.2 per 100,000 • 91.5% of patients hospitalized had at least one underlying medical condition (most commonly hypertension, obesity, chronic metabolic disease, and cardiovascular disease) • Need for safe intervention that can reduce the serious pathology associated with COVID - 19 in high risk patients and broader population Hospitalization ICU Emergency Use Authorization granted for patients hospitalized with COVID - 19 Remdesivir and hydroxychloroquine

16 ©2020 KALEIDO™ Scientific Rationale for MMTs in Viral Infections • Ever - increasing body of scientific literature elucidating the understanding of gut - lung axis • MMT fermentation end - products by the gut microbiome are short - chain fatty acids (SCFAs) • SCFAs modulate host inflammation, control adaptive immunity, and promote immune tolerance locally as well as systemically • Human and animal data demonstrate that SCFAs and SCFA - producing taxa are linked to reduced risk of acquiring viral infections and protection against effects of infections Source: Anand et al., Front. Microbio. 19, 9, 2147 ). A picture containing map Description generated with very high confidence Coronavirus RSV Influenza

17 ©2020 KALEIDO™ Gut Microbiome Positively Impacts Host Immunity To Mitigate Respiratory Viral Infections High butyrate associated with lower clinical respiratory tract infection, including coronavirus Haak et al, 2018 Blood. Trompette et al, 2018 Immunity Dietary fiber protects against lethal Influenza infection Fig. 2 High fiber diet protects against RSV infection SCFA (a cetate ) reduce impact of antibiotic treatment Antunes et al, 2019 Nature Communications. Fig. 2 Fig. 2 Fig. 2

18 ©2020 KALEIDO™ KB109 Improves Microbiome Characteristics Associated with Immune Response 170AD893-6CF2-406A-BF4A-864DF3510D2B@pexlabs Hours % Pathogen Reduce Pathogens in Healthy Microbiomes KB109 Consistently Induces SCFA Production Across Healthy Microbiomes Microbiome Samples from 13 ICU Patients Pathogen Reduction Relative to Control (%) Reduce Pathogens in ICU Patient Microbiomes Active across healthy and patient microbiomes Different Fecal C ommunities Higher diversity and reduction of pathogens - associated with healthier microbiome and robust immune response Significantly larger reduction of pathogens than FOS (Fructo - oligosaccharides) KB109 support higher microbiome diversity than FOS Data obtained with a healthy community after spike - in with K. pneumonia (left) and Shannon diversity (right) is analyzed based on 16S sequencing.

19 ©2020 KALEIDO™ Clinical Plan for KB109 in COVID - 19 High propionate High acetate • 2 studies designed to evaluate the effects of Supportive Self - Care (SSC)+ KB109 compared to SSC in outpatients with mild - to - moderate COVID - 19 • Larger study at multiple centers: ~350 patients (1:1 randomization) . SSC alone (control group) or SSC plus KB109 for two weeks. Up to 3 weeks of follow up • Smaller study (n=~50) with similar design focused on microbiome structure (sequencing) and function (metabolites) • Assessment of clinical outcomes, healthcare utilization, and biomarkers of inflammatory response. Data from common endpoints to be pooled from the 2 studies KB109 in Outpatients with Mild - to - Moderate COVID - 19 Patient Portal Blue Icon PNG Image | Transparent PNG Free Download ... Telemedicine Visit Hospital Icons - Download 33 Free Hospital icons here Influenza Care at Tufts Medical Center Server PNG Images, Server Icon Free Download - Free Transparent ... IVP Toolbox Icon - Safe States Alliance Training Hand with smartphone icon image Royalty Free Vector Image SWS-Flyer Icon 2 | Sahir Web Solutions Patients recruited via outpatient clinical laboratories performing SARS - CoV - 2 testing Consent, COVID - 19 testing, PE and blood draw 1 Suitable patients will be overnight shipped their Kaleido Study Kit and begin reporting daily assessments Diary icon Influenza Care at Tufts Medical Center Patients complete self - assessments, take Study Product (as applicable) for 14 Days and blood draw at the end of the period 1 Patients complete self - assessments for 21 Days of Follow - up Period and blood draw at end of the period 1 Coordinating Center • Data Entry • 24/7 Physician Access • Safety Management • Screening 3 • eConsent 3 Hospital element, medical, nurse, nursing, treatment icon Syringe Injection Icon - Vector injection needle png download Swab Stock Illustrations – 2,635 Swab Stock Illustrations, Vectors ... Diary icon Influenza Care at Tufts Medical Center Cooking, flavoring, food, kitchen, preparation, sachet icon Diary icon Influenza Care at Tufts Medical Center Patient Portal Blue Icon PNG Image | Transparent PNG Free Download ... Telemedicine Visit Hospital element, medical, nurse, nursing, treatment icon Syringe Injection Icon - Vector injection needle png download ... Swab Stock Illustrations – 2,635 Swab Stock Illustrations, Vectors ... Hospital element, medical, nurse, nursing, treatment icon Syringe Injection Icon - Vector injection needle png download ... Swab Stock Illustrations – 2,635 Swab Stock Illustrations, Vectors ... Patients given study information, access to Study Portal & telemedicine visit conducted (if required) 2 1. Blood draws as feasible and only for those patients consenting before COVID - 19 testing 2. Patients will have the option of consenting following testing & discharge; these patients will have a telemedicine visit to conform I/E criteria 3. For those patients consenting following discharge 4. Stool collected in smaller study only (n=50) Patient Portal Blue Icon PNG Image | Transparent PNG Free Download ... Planned Study Framework A picture containing ware Description automatically generated A picture containing ware Description automatically generated 4 4

20 ©2020 KALEIDO™ Potential for KB109 Success in Outpatients with COVID - 19 Positive data in one or more would provide confidence for further development Clinical Data Inflammatory Markers Time to resolution of fever Resolution of symptoms 1 Improvement in seroconversion Decrease in cellular inflammatory markers: WBC Neutrophils (and neutrophil activation) Absolute lymphoid count (<1000 associated with worse response ) Fewer patients with abnormal O 2 sat Fewer unscheduled clinical visits Fewer hospitalizations Decrease in biomarkers: IL - 6 LDH Ferritin D - dimer CRP Decrease persistence of virus Viral Endpoints If positive, these studies position KB109 as a potential option for a range of respiratory viral infections, not just COVID - 19, to improve or even prevent serious outcomes Change in microbiome diversity & metabolites Microbiome Assessments 2 Presence of inflammatory species Notes: 1. For safety assessment in larger study only 2. In the smaller study only

21 ©2020 KALEIDO™ Sources: CDC website: https://www.cdc.gov/flu/prevent/keyfacts.htm ; https://www.cdc.gov/flu/fluvaxview/coverage - 1819estimates.htm ; https://www.cdc.gov/coronavirus/2019 - ncov/covid - data/covidview/index.html ; accessed May 2020 KB109 Potential in COVID - 19 Landscape & Broader Viral Respiratory Infections Emergency Use Authorization granted for patients hospitalized with COVID - 19 Vaccines Estimated 53% of US population vaccinated for flu In 2019, ~170 million doses distributed for the flu KB109 Clinical Study Clinical program focused on outpatients with mild - moderate disease Children Sleep • Relatively few studies ongoing in an outpatient population who test positive for SARS - CoV - 2 • Data from COVID - 19 clinical study of KB109 will provide insights into immune response mechanism applicable across a number of serious viral infections • KB109 is Generally Recognized as Safe (GRAS); convenient oral administration and manufacturing is readily scalable Potential to expand population to prevent infections and/or reduce impact of infection across a range of different viruses KB109 target population CDC: overall cumulative hospitalization rate is 50.3 per 100,000 , highest rates in 65 years and older is 162.2 per 100,000 Broad Population SARS - CoV - 2 + ive Isolation Remdesivir and hydroxychloroquine Hospitalization ICU

22 ©2020 KALEIDO™ ©2020 KALEIDO™ Inflammatory Bowel Disease (IBD)
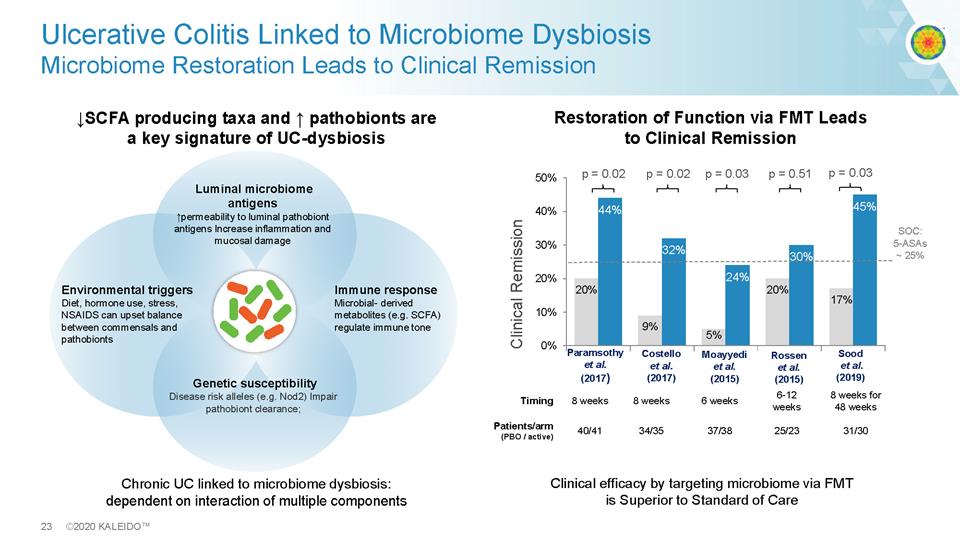
23 ©2020 KALEIDO™ .SCFA producing taxa and . pathobionts are a key signature of UC - dysbiosis Ulcerative Colitis Linked to Microbiome Dysbiosis Microbiome Restoration Leads to Clinical Remission Clinical efficacy by targeting microbiome via FMT is Superior to Standard of Care 20% 9% 5% 20% 17% 44% 32% 24% 30% 45% 0% 10% 20% 30% 40% 50% p = 0.02 p = 0.02 p = 0.03 p = 0.51 Clinical Remission Timing 8 weeks 8 weeks 6 weeks 6 - 12 weeks 8 weeks for 48 weeks Patients/arm (PBO / active) 40/41 34/35 37/38 25/23 31/30 Paramsothy et al . (2017) Costello et al. (2017) Moayyedi et al. (2015) Rossen et al. (2015) SOC: 5 - ASAs ~ 25% Chronic UC linked to microbiome dysbiosis: dependent on interaction of multiple components Sood et al. (2019) p = 0.03 Restoration of Function via FMT Leads to Clinical Remission Luminal microbiome antigens .permeability to luminal pathobiont antigens Increase inflammation and mucosal damage Genetic susceptibility Disease risk alleles (e.g. Nod2) Impair pathobiont clearance; Environmental triggers Diet, hormone use, stress, NSAIDS can upset balance between commensals and pathobionts Immune response Microbial - derived metabolites (e.g. SCFA) regulate immune tone
24 ©2020 KALEIDO™ Downregulation Of Microbiome SCFAs Associated With Ulcerative Colitis Can Be Improved By Glycans SCFA produced by the microbiome are key regulators of gut immune tone SCFA and associated taxa are consistently observed as downregulated in UC patients Adapted from Melhem et al ., 2019 Source: Kumari et al ., 2013 Inulin - glycans improve active ulcerative colitis associated with microbiota changes and increased SCFA levels • Clinical effects of oligofructose - enriched inulin, in mild to moderately active UC for 9 weeks • Treatment significantly increased total SCFA production in the high dose group (126 ± 24 µmol/g at week 9) (P = 0.03) • At the end of the treatment period, only responders (reduction in Mayo score by =3) showed significantly enhanced total SCFA production (135 ± 22 µmol/g stool) (P = 0.02) A screenshot of a computer Description automatically generated A screenshot of a computer Description automatically generated Butyrate Source: Valcheva et al ., 2018 A picture containing text, map Description automatically generated Dietary glycan

25 ©2020 KALEIDO™ KB295 Improves Microbiome Characteristics Associated with Ulcerative Colitis KB295 increases S CFA production across 8 healthy stool communities • Ratio of SCFA is broadly in line with that tested with UC patients in a double - blind, placebo - controlled trial with rectal enemas: 100 ml of 80 mM acetate, 30 mM propionate, and 40 mM butyrate twice a day for 6 weeks) (Breuer et al, 1997). • Results suggest efficacy in subsets of patients with distal ulcerative colitis including those with short active episodes KB295 significantly reduces relative abundance of Enterobacteriaceae Planned Clinical Study • KB295 chosen for butyrate production and suppression of pathobiont growth • Assess potential of KB295 in mild - moderate UC • Single center ~30 patients in single arm – 56 days treatment and 14 days follow - up – Remote monitoring • Evaluate safety and tolerability, Simple Clinical Colitis Activity Index (SCCAI) composite score • Exploratory: Changes in microbiome taxa, SCFA levels in stool and biomarkers of inflammation. Relative abundance of Enterobacteriaceae Decrease in the relative abundance of the family Enterobacteria, spiked into multiple ex vivo communities Different Fecal Communities Different Fecal Communities

26 ©2020 KALEIDO™ ©2020 KALEIDO™ Immuno-oncology

27 ©2020 KALEIDO™ Li et al, 2020 Cell Rep Kaleido preliminary data MMTs and glycans improve tumor control in mice Fiber improves melanoma IO response rates in patients* Fiber improves melanoma IO survivorship* Data Support That Glycans Improve Response to ICI Therapy and Tumor Control * * Source: https://www.umms.org/sjmc/ - /media/files/um - sjmc/health - services/cancer - institute/symposium - powerpoints/role - of - commensal - microdata - in - carcinogenesis - and - cancer - therapy.pdf?upd=20191120214445&la=en&hash=EA5670957E87F12C6434A047801E4E0C632910B6 , Accessed April 2020 aPD - L1 aPD1 No - ICI

28 ©2020 KALEIDO™ Antibiotics Usage Reduces Patient Survival Following Immune Checkpoint Inhibitor (ICI) Treatment Source: Elkrief et al, 2019 • Many cancer patients need antibiotics before and during ICI treatment • But antibiotic treatment reduces survival of cancer patients receiving ICI treatment (aPD - 1) Source: Routy et al, 2018

29 ©2020 KALEIDO™ Collaborating with Gustave Roussy , Pioneer in Immunotherapy and Research in Microbiome and Cancer • Gustave Roussy is a leading center for immunotherapy in Europe – Pioneer in understanding relationship between the microbiome and cancer • Collaboration aimed at improving cancer immunotherapy efficacy by increasing rate of response to ICI – Kaleido : • analyze intestinal microbiome samples from ICI non - responder patients • identify MMTs that drive changes in the microbiome associated with favorable response to treatment – Gustave Roussy : • identify MMTs that stimulate the targeted therapeutic responses in advanced preclinical models Microbiome Dictates a - PD - 1 Outcome Gustave Roussy Utilizes Advanced Preclinical Model with Human Microbiomes of Responder (R) and Non - Responder (NR) Patients Adapted from: Routy , et. al. Science 05 Jan 2018:Vol. 359, Issue 6371, pp. 91 - 97; Gopalakrishnan et. al., Science 05 Jan 2018:Vol. 359, Issue 6371, pp. 97 - 103 Selection of lead compounds based on data from all preclinical work expected in Q4 2020

30 ©2020 KALEIDO™ ©2020 KALEIDO™ Metabolic and Liver Diseases

31 ©2020 KALEIDO™ Platform Validated as Predictive of Human Response for Urea Cycle Disorders & Hepatic Encephalopathy Programs Source: 1. Mean reduction as referenced in: De Preter V. et al., Alimentary Pharmacology & Therapeutics 23, 963 - 974 (2006) Reduction of biomarker of microbiome ammonia production in clinical studies 20% 26% 33% 0% 10% 20% 30% 40% % reduction in urinary 15 N excretion compared to baseline KB195 in healthy subjects KB174 in patients with cirrhosis Lactulose , standard of care in HE, in healthy subjects 1 KB195, KB174 control Lactulose Ex Vivo Screening For Ammonia Reduction in Microbiomes of Healthy Subjects Ammonia Levels Normalized to Control

32 ©2020 KALEIDO™ GUT Ammonia Production LIVER Main Site of Ammonia Clearance Sources: Batshaw , M. L., et. al. (2014). Molecular Genetics and Metabolism , 113(0), 127 - 130; Häberle, J, et al. Journal of Inherited Metabolic Disorders , 2019;1 - 39; Company reports Urea Cycle Disorders: Rare Inborn Errors of Metabolism Resulting in Inability to Clear Ammonia Urea Cycle Disorders (UCD) KB195 • Inability to metabolize ammonia due to inborn deficiency of enzymes in the urea cycle . A significant proportion of ammonia is produced by the microbiome and absorbed from the gut - Results in ammonia accumulation in the brain where it can cause irreversible brain damage, coma and/or death • Rare disease with ~3,000 patients in U.S.; ~4,500 in E.U. – Liver transplant is a practical cure although does not revert preexisting neurological damage; performed in <40 patients per year in U.S. • Limited available treatment options to: – Lower chronic ammonia levels – Reduce the risk of life - threatening hyperammonemic crises A close up of a logo Description automatically generated A picture containing computer, drawing Description automatically generated A close up of a coral Description automatically generated BRAIN Main Site of Ammonia Toxicity NH 3 NH 3
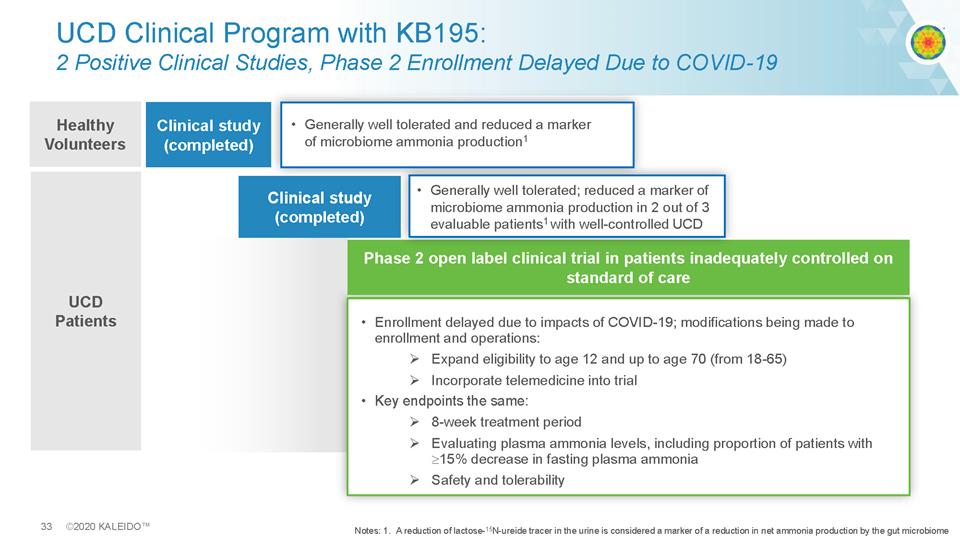
33 ©2020 KALEIDO™ UCD Patients UCD Clinical Program with KB195: 2 Positive Clinical Studies, Phase 2 Enrollment Delayed Due to COVID - 19 Clinical study (completed) • Enrollment delayed due to impacts of COVID - 19; modifications being made to enrollment and operations: . Expand eligibility to age 12 and up to age 70 (from 18 - 65) . Incorporate telemedicine into trial • Key endpoints the same. 8 - week treatment period Evaluating plasma ammonia levels, including proportion of patients with. 15% decrease in fasting plasma ammonia. Safety and tolerability Phase 2 open label clinical trial in patients inadequately controlled on standard of care • Generally well tolerated; reduced a marker of microbiome ammonia production in 2 out of 3 evaluable patients 1 with well - controlled UCD Healthy Volunteers Clinical study (completed) • Generally well tolerated and reduced a marker of microbiome ammonia production 1 Notes: 1. A reduction of lactose - 15 N - ureide tracer in the urine is considered a marker of a reduction in net ammonia production by the gut microbiome

34 ©2020 KALEIDO™ Hepatic Encephalopathy: Cognitive Dysfunction as a Result of Liver Disease, including Cirrhosis GUT Ammonia Production LIVER Main Site of Ammonia Clearance A close up of a logo Description automatically generated A picture containing computer, drawing Description automatically generated A close up of a coral Description automatically generated BRAIN Main Site of Ammonia Toxicity NH 3 NH 3 Hepatic Encephalopathy (HE) KB174 • Clinical spectrum from covert neurocognitive findings to overt neuropsychiatric abnormalities in patients with liver dysfunction – Impact of Health - Related Quality of Life impairment is felt by patients, caregivers and families – Patients at risk for poor clinical outcomes and reduced survival – Patients with liver disease have high rate of infections, and infections are a common precipitating factor for HE events • US: ~700K cirrhosis patients, of which there are up to 500K HE patients , >100K patients with Overt HE (OHE) • EU: > 1 million cirrhosis patients, of which there are up to 800K HE patients, >200K patients with OHE • Two current approved treatments for OHE; unmet needs for patients with OHE: – Reduce OHE episodes and infection risk – More tolerable therapy; lactulose known to have tolerability issues (diarrhea) that impact patient compliance • No current approved treatment for Minimal HE (MHE ); opportunity to reverse MHE and reduce OHE events Sources: Montgomery, J. Y., & Bajaj, J. S. (2011), Current Gastroenterology Reports , 13, 26 - 33; Montagnese , S. & Bajaj, J.S. Drugs (2019) 79(Suppl 1): 11; Flamm, S. American Journal of Managed Care 24:S51 - S61. Company reports

35 ©2020 KALEIDO™ KB174 Clinical Study in Cirrhotic Patients: Positive Top - Line Data Show Reduction in a Biomarker of Ammonia Production • KB174 chosen as lead in HE based on its ability to reduce ammonia & MDR pathogens ex vivo • Patients treated with KB174 had a 26% median reduction in urinary 15 N excretion compared to baseline vs. maltodextrin which had a 7% median increase compared to baseline 1 – Lactulose, an approved product for HE, showed ~20% reduction in a similar study using urinary 15 N tracer model 2 • KB174 was generally well tolerated based on measures that included the GITQ and BSS - No clinically significant or serious treatment - related adverse events were observed • Results support advancing KB174 into study evaluating clinical endpoints in patients with HE KB174 Demonstrated a 26% Median Reduction in Urinary 15 N Excretion Compared to Baseline in Patients with Cirrhosis FDA recent feedback: If Kaleido elects to file an IND, it may do so with a Phase 3 pivotal trial Note: 1. In a subsequent analysis, it was discovered that a patient in the maltodextrin arm had incomplete tracer period 2 da ta which excluded the patient and resulted in the change from decrease of 3% to an increase in 7%. 2. Mean reduction as referenced in: De Preter V. et al., Alimentary Pharmacology & Therapeutics 23, 963 - 974 (2006)
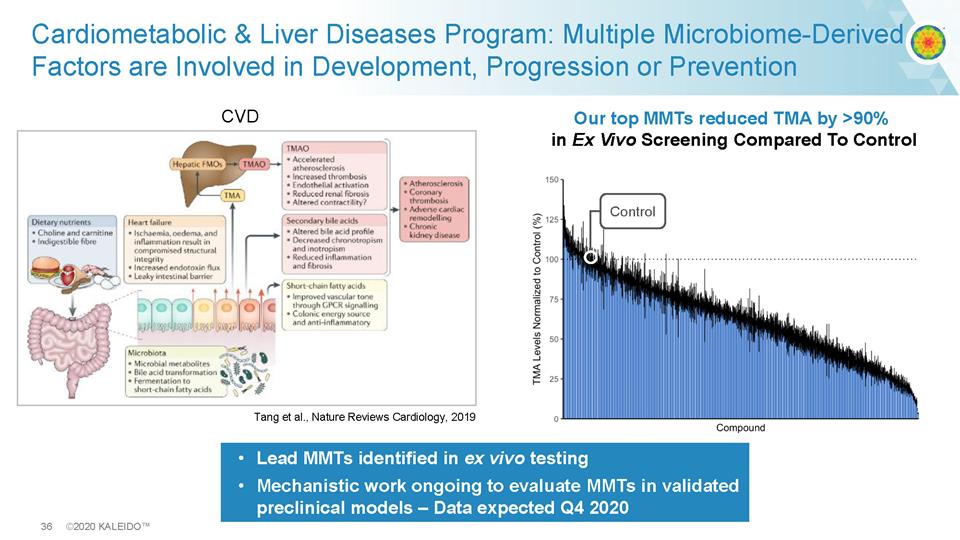
36 ©2020 KALEIDO™ Cardiometabolic & Liver Diseases Program: Multiple Microbiome - Derived Factors are Involved in Development, Progression or Prevention Fig. 1 CVD Tang et al., Nature Reviews Cardiology, 2019 • Lead MMTs identified in ex vivo testing • Mechanistic work ongoing to evaluate MMTs in validated preclinical models – Data expected Q4 2020 Our top MMTs reduced TMA by >90% in Ex Vivo Screening Compared To Control 9DA06818-E968-42AB-BA44-98A9046C1D13@i Control

37 ©2020 KALEIDO™ ©2020 KALEIDO™ Appendix

38 ©2020 KALEIDO™ Discovery & Development Model Enables Rapid Advancement into Human Clinical Studies EX VIVO SCREENING IN HEALTHY MICROBIOME SAMPLES EX VIVO TESTING IN PATIENT MICROBIOME SAMPLES RAPID ADVANCEMENT INTO HUMAN CLINICAL STUDIES 1,2 Patients Healthy Volunteers & Image Image VALIDATED ANIMAL MODELS OF DISEASE (Where informative) MMT DECISION POINT • File IND or global equivalent and Proceed to Phase 2 or later • Pursue Non - Drug Development Pathway/Commercialization Notes: 1. In some cases we will use in vivo models prior to initiating a human clinical study to enhance our understanding of disease mechanisms and biomarkers. 2. Our human clinical studies of our MMT candidates are conducted under regulations supporting research with food, evaluating safety, tolerability and potential markers of effect. For MMT candidates pursuing a drug development pathway, an IND or regulatory equivalent outside the U.S. will be filed; for t hos e that we elect to continue on a non - drug development pathway, INDs or regulatory equivalent outside the U.S. will not be required. Rat >1,500+ MMTs CHEMISTRY LIBRARY

39 ©2020 KALEIDO™ Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Year 7 Kaleido’s Human - Centric Discovery and Development 2 - 3 Years Traditional Pharmaceutical R&D 1 5 - 7 Years Source: 1. DiMasi, Joseph, et al., Journal of Health Economics, 2016, 47, 20 - 23. Platform Enables Rapid, Cost - Efficient Discovery & Development KB195: ~2 years from Ex Vivo Screening to Phase 2 Discovery 1 Year Human Clinical Studies 2 Years Phase 1 ~1.5 Years Start Phase 2 or 3 IND $$ Millions $$$ Millions Start Phase 2 Start Phase 1 Traditional IND. Discovery Data. Animal Data. Scale to Phase 1 Kaleido IND 1 . Discovery (human ex vivo) data. Abbreviated preclinical data. Human clinical safety, tolerability. Biomarkers may also be a surrogate for efficacy. Dose - response. 1/10 the of cost to IND compared to traditional model Start Phase 2 or 3 Discovery, Preclinical ~5 Years IND

40 ©2020 KALEIDO™ Manufacturing is a Core Strategic Advantage: Small - molecule like, Efficient and Scalable Cost - Effective Process Internal Manufacturing Production for ex vivo , toxicology, and human clinical studies 3 rd Party Manufacturers Large scale production for clinical trials and future commercial supply (Thermo Fisher Scientific) Proprietary Methods Standard Small Molecule Unit Operations Scalable and Transferable Internal capability to manufacture 12 MMTs per year; potential to double in 2020 Completed tech transfer to Thermo Fisher in ~6 months, scaled to 1,000 kg with capability to increase by >40X KB195 manufactured for Phase 2 trial and toxicology Efficient and scalable process

41 ©2020 KALEIDO™ Robust Global IP Portfolio 11 Patents Issued and 100+ Pending Worldwide 9 U.S. Patents + 2 EPO Patents : • Glycan pharmaceutical compositions (Europe) • Methods to reduce ammonia (U.S.) • Methods to treat pathogen colonization (U.S.) • Methods to treat diarrhea (U.S.) • Methods to treat dysbiosis (U.S.) • Catalyst compositions (U.S. and Europe) 100+ Non - Provisional Applications Pending, Worldwide in 20+ Regions/Countries • Glycan compositions • Methods to treat immune imbalances (e.g. cancer) • Methods to modulate short chain fatty acids • Methods of making glycans Continued IP Expansion by Filing Patent Applications Directed to Pharmaceutical Compositions, Methods of Treatment and Methods of Manufacture

42 ©2020 KALEIDO™ World - Class Leadership Team Alison Lawton President & Chief Executive Officer Former GM, Genzyme Biosurgery, COO Aura & OvaScience Katharine Knobil, M.D. Chief Medical Officer, Head of R&D Former CMO, GlaxoSmithKline Johan van Hylckama Vlieg , Ph.D. Chief Scientific Officer Former VP for Microbiome & Human Health Innovation, Chr. Hansen William Duke Chief Financial Officer Former CFO, Pulmatrix and Valeritas Clare Fisher Chief Business Officer Former Group VP, Global Head of Transactions & Business Development, Shire Jerald Korn, J.D. General Counsel & Corporate Secretary Former Senior Vice President, Chief Legal and Administrative Officer, TESARO Picture 2 Picture 4 Picture 8 Picture 9 Picture 2 Picture 8 Image result for chrhansen logo Homepage Image result for nizo logo Picture 18 A close up of a sign Description automatically generated COMPANY BUILDING THERAPEUTIC AREA BREADTH & TECHNOLOGY DEPTH CLINICAL DEVELOPMENT & REGULATORY MANUFACTURING Picture 9 A person wearing a suit and tie Description automatically generated A person smiling for the camera Description automatically generated A person wearing a suit and tie smiling at the camera Description automatically generated A person wearing glasses Description automatically generated A person smiling for the camera Description automatically generated A person smiling for the camera Description automatically generated Mike Bonney Executive Chair Former CEO, Cubist Pharmaceuticals Picture 8 A person wearing glasses and smiling at the camera Description automatically generated

43 ©2020 KALEIDO™ ©2020 KALEIDO™

