Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CAPRICOR THERAPEUTICS, INC. | tm2019558d1_8k.htm |
Exhibit 99.1

A Study of CAP - 1002 in Ambulatory and Non - Ambulatory Patients with Duchenne Muscular Dystrophy [HOPE - 2] 12 - month Top - Line Final Study Results 1 May 13, 2020 Conference Call NASDAQ: CAPR

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Forward - Looking Statements 2 Statements in this presentation regarding the efficacy, safety, and intended utilization of Capricor's product candidates ; the initiation, conduct, size, timing and results of discovery efforts and clinical trials ; the pace of enrollment of clinical trials ; plans regarding regulatory filings, future research and clinical trials ; regulatory developments involving products, including the ability to obtain regulatory approvals or otherwise bring products to market ; plans regarding current and future collaborative activities and the ownership of commercial rights ; scope, duration, validity and enforceability of intellectual property rights ; future royalty streams, revenue projections ; expectations with respect to the expected use of proceeds from the recently completed offerings and the anticipated effects of the offerings, and any other statements about Capricor's management team's future expectations, beliefs, goals, plans or prospects constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Any statements that are not statements of historical fact (including statements containing the words "believes," "plans," "could," "anticipates," "expects," "estimates," "should," "target," "will," "would" and similar expressions) should also be considered to be forward - looking statements . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . More information about these and other risks that may impact Capricor's business is set forth in Capricor's Annual Report on Form 10 - K for the year ended December 31 , 2019 as filed with the Securities and Exchange Commission on March 27 , 2020 . All forward - looking statements in this press release are based on information available to Capricor as of the date hereof, and Capricor assumes no obligation to update these forward - looking statements . CAP - 1002 is an Investigational New Drug and is not approved for any indications . None of Capricor’s exosome - based candidates have been approved for clinical investigation .

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside • Linda Marban, Ph.D. – Chief Executive Officer, Capricor Therapeutics, Inc. • Craig McDonald, M.D., Professor and Chair of the Department of Physical Medicine and Rehabilitation and Director of the Neuromuscular Disease Clinics at the University of California, Davis. Dr. McDonald is an internationally recognized expert in the clinical management and rehabilitation of neuromuscular diseases including DMD. He is the national PI of the Capricor HOPE - 2 Trial. • AJ Bergmann , Chief Financial Officer, Capricor Therapeutics, Inc. Call Participants 3
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Capricor’s Regulatory Designations - DMD Similar to breakthrough therapy designation: • RMAT provides benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate • Eligibility for rolling review and priority review Products may also be eligible for accelerated approval • On the basis of a surrogate or intermediate endpoint reasonably likely to predict long - term clinical benefit • Reliance upon data obtained from a meaningful number of sites GOAL OF FDA’S RMAT DESIGNATION To facilitate efficient development and expedite review of a drug Rare Pediatric Disease Designation Orphan Drug Designation RMAT Designation 4
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside CAP - 1002 Mechanism of Action Immunomodulation ‒ Allogeneic cardiosphere - derived cells (CDCs) ‒ MOA: cells secrete exosomes: ‒ Contain miRNAs, other non - coding RNAs and proteins ‒ Internalized by target cells ‒ Strongly immunomodulatory ‒ 3 known miRNAs drive CAP - 1002 potency ‒ Strong safety record in more than 150 subjects ‒ Recent peer reviewed publication: COVID - 19 5
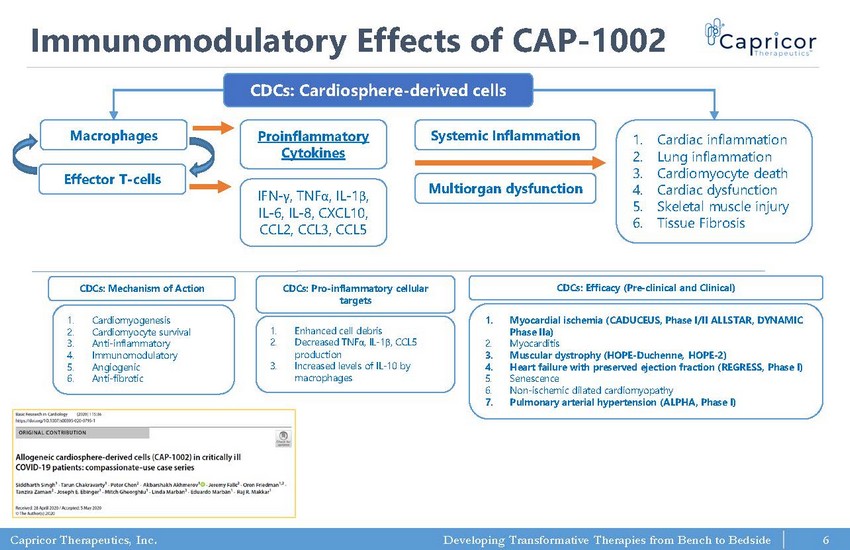
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Immunomodulatory Effects of CAP - 1002 CDCs: Cardiosphere - derived cells Macrophages Effector T - cells Proinflammatory Cytokines IFN - γ , TNF α , IL - 1 β , IL - 6, IL - 8, CXCL10, CCL2, CCL3, CCL5 Systemic Inflammation Multiorgan dysfunction 1. Cardiac inflammation 2. Lung inflammation 3. Cardiomyocyte death 4. Cardiac dysfunction 5. Skeletal muscle injury 6. Tissue Fibrosis 1. Cardiomyogenesis 2. Cardiomyocyte survival 3. Anti - inflammatory 4. Immunomodulatory 5. Angiogenic 6. Anti - fibrotic CDCs: Mechanism of Action 1. Enhanced cell debris 2. Decreased TNF α , IL - 1 β , CCL5 production 3. Increased levels of IL - 10 by macrophages CDCs: Pro - inflammatory cellular targets 1. Myocardial ischemia (CADUCEUS, Phase I/II ALLSTAR, DYNAMIC Phase IIa ) 2. Myocarditis 3. Muscular dystrophy (HOPE - Duchenne, HOPE - 2) 4. Heart failure with preserved ejection fraction (REGRESS, Phase I) 5. Senescence 6. Non - ischemic dilated cardiomyopathy 7. Pulmonary arterial hypertension (ALPHA, Phase I) CDCs: Efficacy (Pre - clinical and Clinical) 6
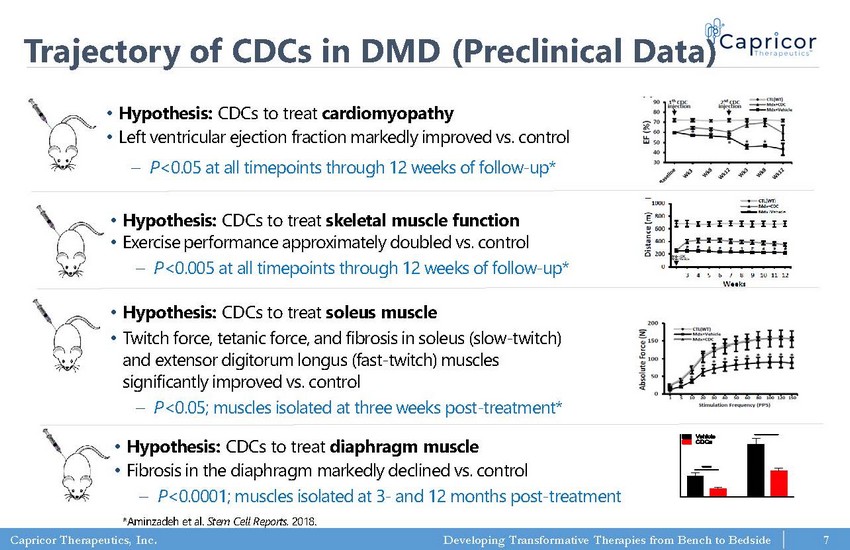
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Trajectory of CDCs in DMD (Preclinical Data) • Hypothesis: CDCs to treat cardiomyopathy • Left ventricular ejection fraction markedly improved vs. control – P <0.05 at all timepoints through 12 weeks of follow - up* • Hypothesis: CDCs to treat skeletal muscle function • Exercise performance approximately doubled vs. control – P <0.005 at all timepoints through 12 weeks of follow - up* • Hypothesis: CDCs to treat soleus muscle • Twitch force, tetanic force, and fibrosis in soleus (slow - twitch) and extensor digitorum longus (fast - twitch) muscles significantly improved vs. control – P <0.05; muscles isolated at three weeks post - treatment* *Aminzadeh et al. Stem Cell Reports. 2018. • Hypothesis: CDCs to treat diaphragm muscle • Fibrosis in the diaphragm markedly declined vs. control – P <0.0001; muscles isolated at 3 - and 12 months post - treatment 7
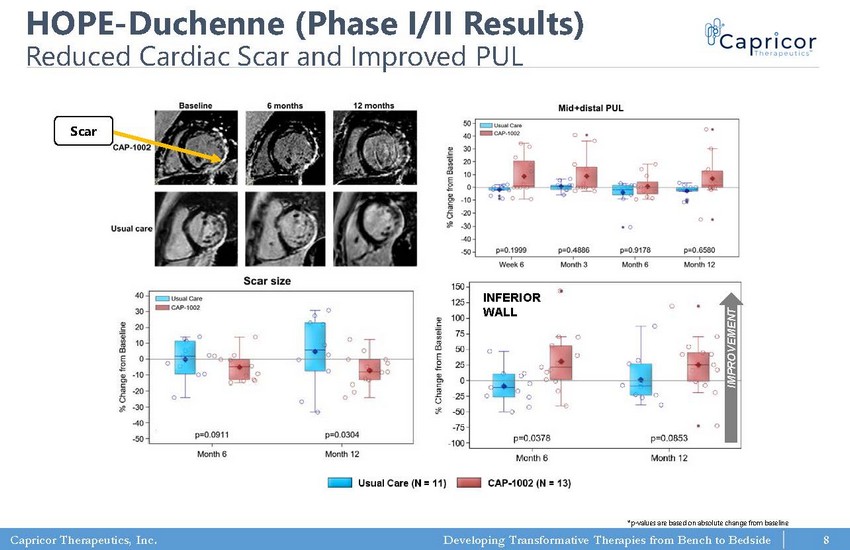
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - Duchenne (Phase I/II Results) Reduced Cardiac Scar and Improved PUL R.G. Victor et al., AHA LBCT 2017; M. Taylor et al., submitted *p - values are based on absolute change from baseline INFERIOR WALL IMPROVEMENT Scar 8

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - 2 12 - Month Top - Line Final Data Dr. Craig McDonald National PI 9
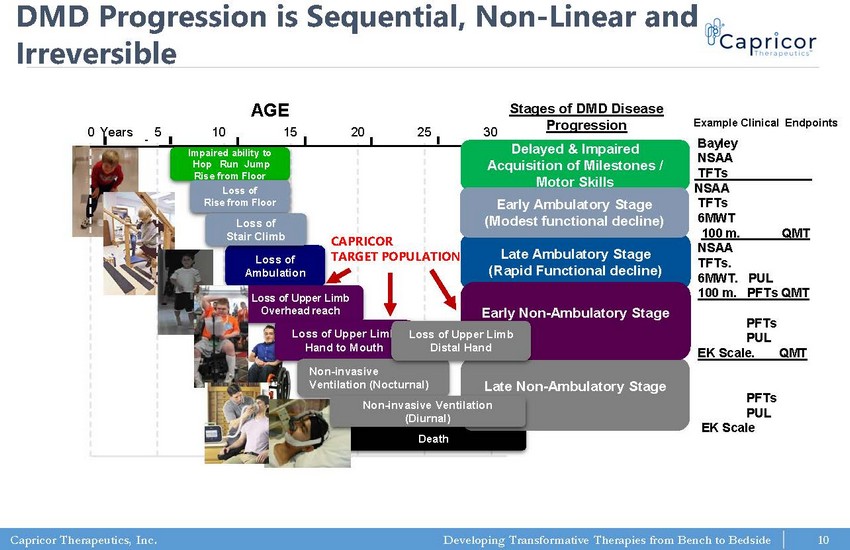
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 0 5 10 15 20 25 30 Years Loss of Ambulation Death Impaired ability to Hop Run Jump Rise from Floor Loss of Rise from Floor Loss of Stair Climb Stages of DMD Disease Progression Loss of Upper Limb Overhead reach Late Ambulatory Stage (Rapid Functional decline) Delayed & Impaired Acquisition of Milestones / Motor Skills Early Ambulatory Stage (Modest functional decline) Late Non - Ambulatory Stage Early Non - Ambulatory Stage Loss of Upper Limb Hand to Mouth Non - invasive Ventilation (Nocturnal) Loss of Upper Limb Distal Hand Non - invasive Ventilation (Diurnal) AGE Bayley NSAA TFTs____________ NSAA TFTs 6MWT 100 m. QMT NSAA TFTs. 6MWT. PUL 100 m. PFTs QMT PFTs PUL EK Scale. QMT PFTs PUL EK Scale Example Clinical Endpoints DMD Progression is Sequential, Non - Linear and Irreversible CAPRICOR TARGET POPULATION 10
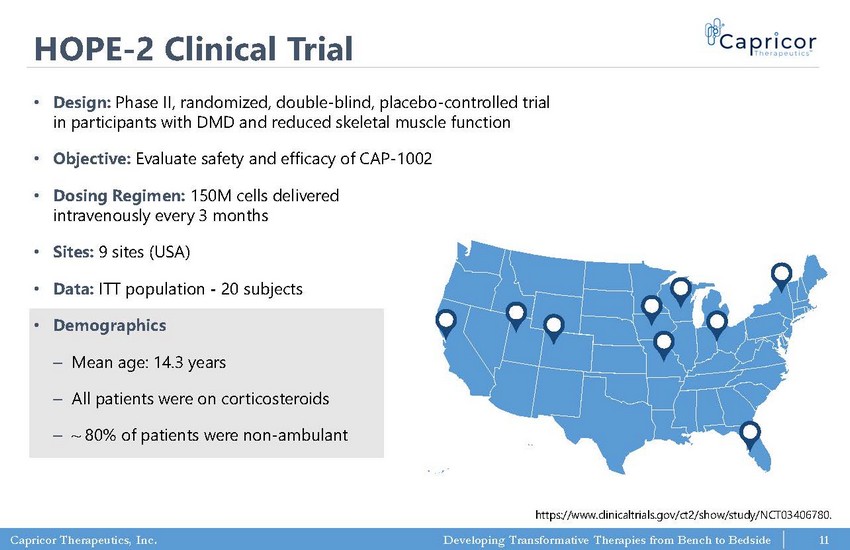
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - 2 Clinical Trial • Design: Phase II, randomized, double - blind, placebo - controlled trial in participants with DMD and reduced skeletal muscle function • Objective: Evaluate safety and efficacy of CAP - 1002 • Dosing Regimen: 150M cells delivered intravenously every 3 months • Sites: 9 sites (USA) • Data: ITT population - 20 subjects • Demographics ‒ Mean age: 14.3 years ‒ All patients were on corticosteroids ‒ ~ 80% of patients were non - ambulant https://www.clinicaltrials.gov/ct2/show/study/NCT03406780. 11
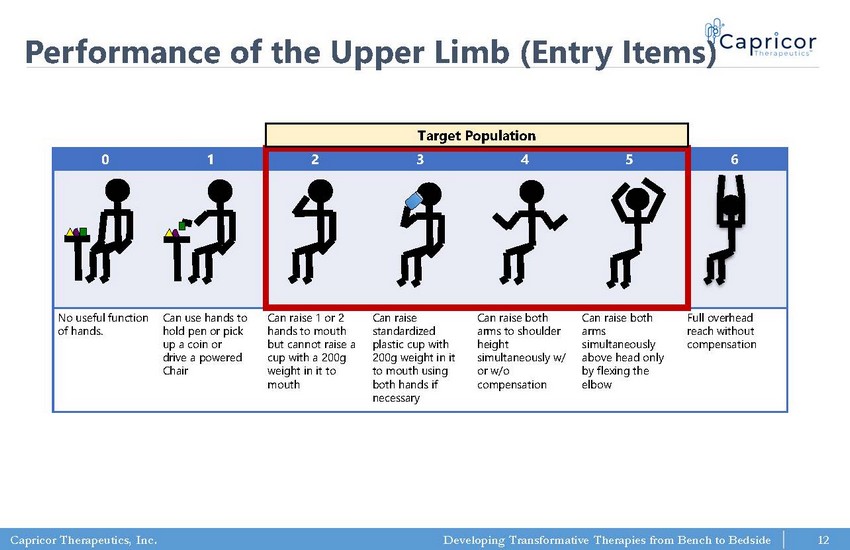
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 0 1 2 3 4 5 6 No useful function of hands. Can use hands to hold pen or pick up a coin or drive a powered Chair Can raise 1 or 2 hands to mouth but cannot raise a cup with a 200g weight in it to mouth Can raise standardized plastic cup with 200g weight in it to mouth using both hands if necessary Can raise both arms to shoulder height simultaneously w/ or w/o compensation Can raise both arms simultaneously above head only by flexing the elbow Full overhead reach without compensation Performance of the Upper Limb (Entry Items) Target Population 12
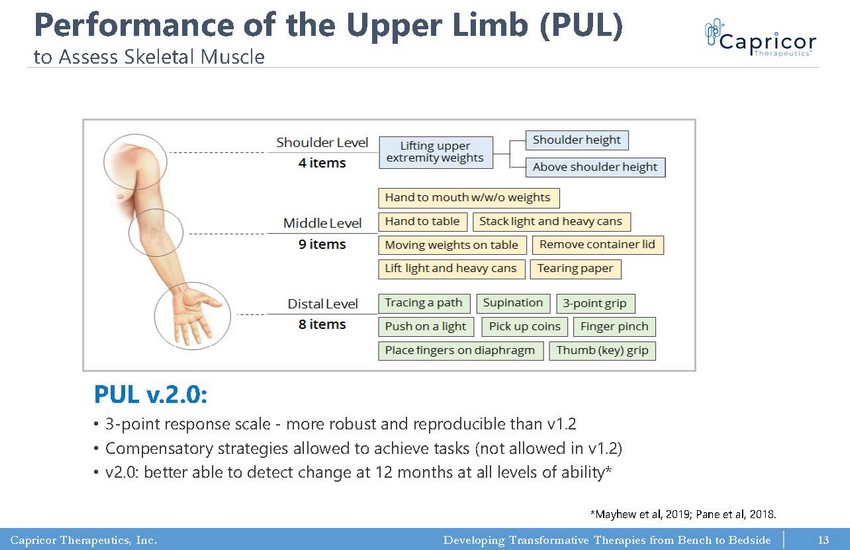
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside PUL v.2.0: • 3 - point response scale - more robust and reproducible than v1.2 • Compensatory strategies allowed to achieve tasks (not allowed in v1.2) • v2.0: better able to detect change at 12 months at all levels of ability* *Mayhew et al, 2019; Pane et al, 2018. Performance of the Upper Limb (PUL) to Assess Skeletal Muscle 13

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Upper - Limb Skeletal Muscle Measured by: Performance of the Upper Limb (PUL) 2.0 Performance of the Upper Limb (PUL) 1.2 14
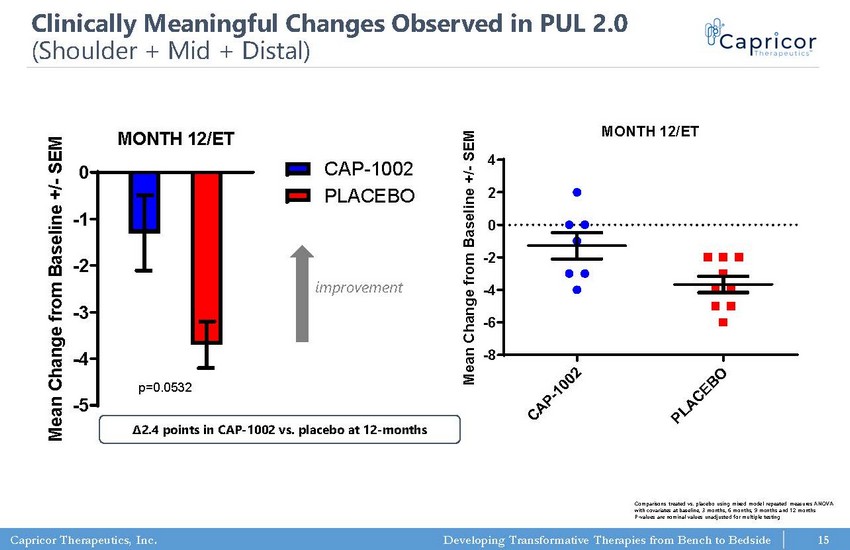
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside PUL 2.0 (full) MONTH 12/ET -5 -4 -3 -2 -1 0 CAP-1002 PLACEBO p=0.0532 M e a n C h a n g e f r o m B a s e l i n e + / - S E M PUL 2.0 (full) MONTH 12/ET C A P - 1 0 0 2 P L A C E B O -8 -6 -4 -2 0 2 4 p=0.0201 (t test; two-tailed) M e a n C h a n g e f r o m B a s e l i n e + / - S E M Clinically Meaningful Changes Observed in PUL 2.0 (Shoulder + Mid + Distal) Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months P - values are nominal values unadjusted for multiple testing 15 improvement Δ2.4 points in CAP - 1002 vs. placebo at 12 - months
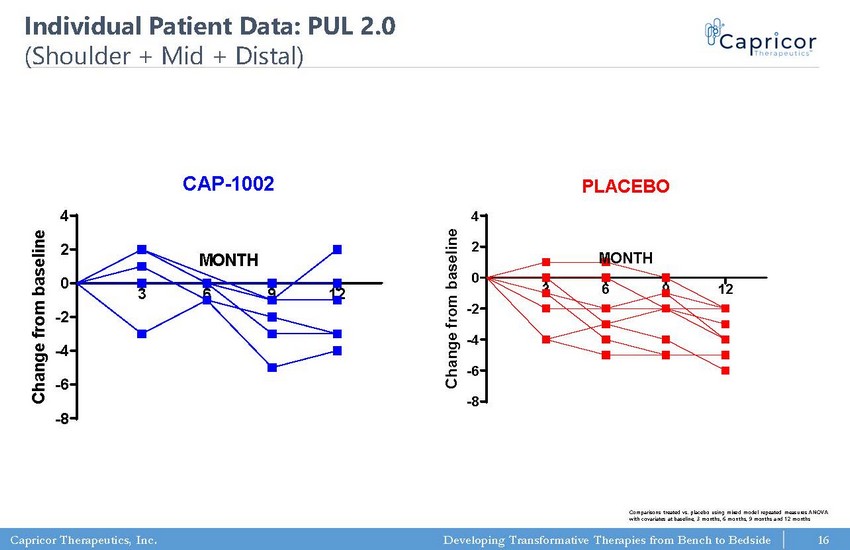
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside PUL 2.0: Combined High-, Mid-, and Distal-Level CAP-1002 3 6 9 12 -8 -6 -4 -2 0 2 4 MONTH C h a n g e f r o m b a s e l i n e PUL 2.0: Combined High-, Mid-, and Distal-Level PLACEBO 3 6 9 12 -8 -6 -4 -2 0 2 4 MONTH C h a n g e f r o m b a s e l i n e 16 Individual Patient Data: PUL 2.0 (Shoulder + Mid + Distal) Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months
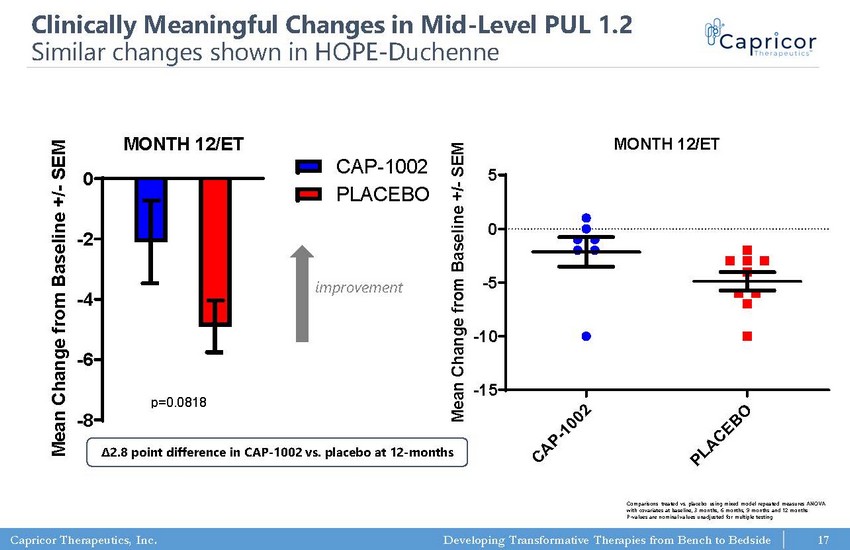
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside PUL 1.2 (mid) MONTH 12/ET -8 -6 -4 -2 0 CAP-1002 PLACEBO p=0.0818 M e a n C h a n g e f r o m B a s e l i n e + / - S E M PUL 1.2 (mid) MONTH 12/ET C A P - 1 0 0 2 P L A C E B O -15 -10 -5 0 5 M e a n C h a n g e f r o m B a s e l i n e + / - S E M p=0.0974 (t test; two-tailed) 17 improvement Clinically Meaningful Changes in Mid - Level PUL 1.2 Similar changes shown in HOPE - Duchenne Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months P - values are nominal values unadjusted for multiple testing Δ2.8 point difference in CAP - 1002 vs. placebo at 12 - months
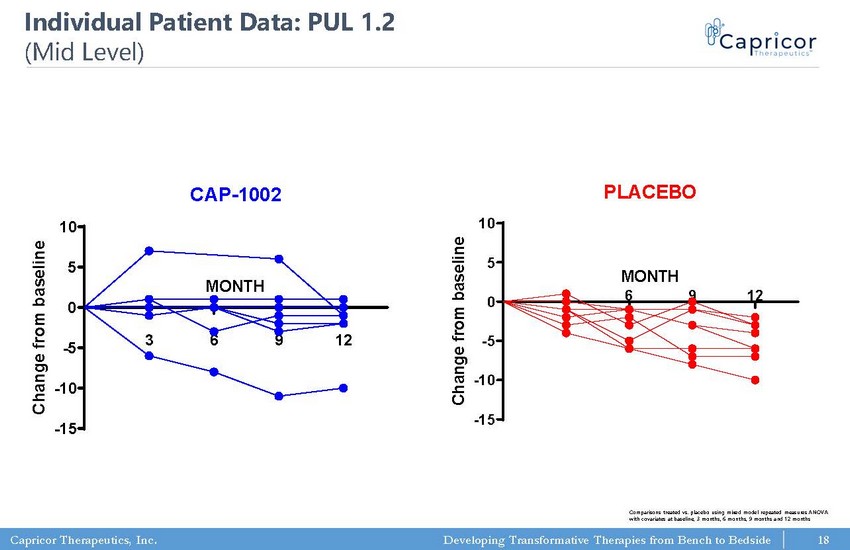
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside PUL 1.2: Mid-Level Elbow Dimension CAP-1002 3 6 9 12 -15 -10 -5 0 5 10 MONTH C h a n g e f r o m b a s e l i n e PUL 1.2: Mid-Level Elbow Dimension PLACEBO 3 6 9 12 -15 -10 -5 0 5 10 MONTH C h a n g e f r o m b a s e l i n e 18 Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months Individual Patient Data: PUL 1.2 (Mid Level)
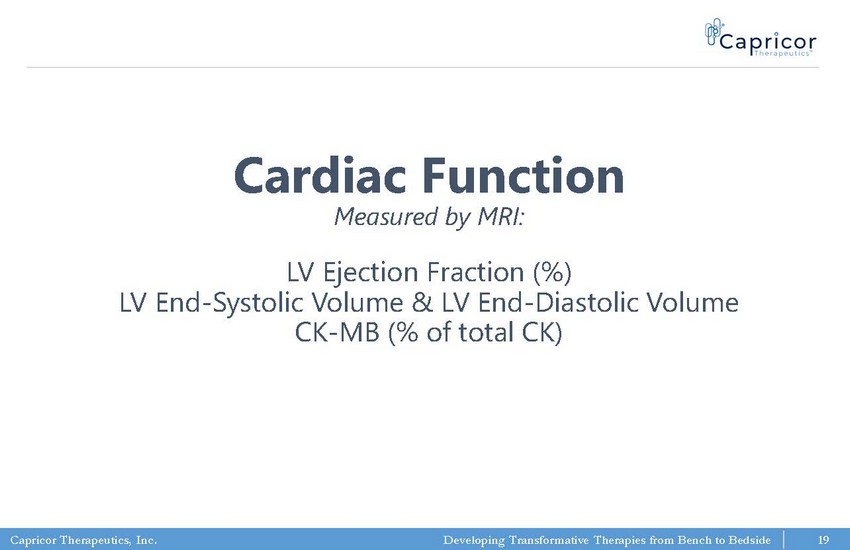
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Cardiac Function Measured by MRI: LV Ejection Fraction (%) LV End - Systolic Volume & LV End - Diastolic Volume CK - MB (% of total CK) 19
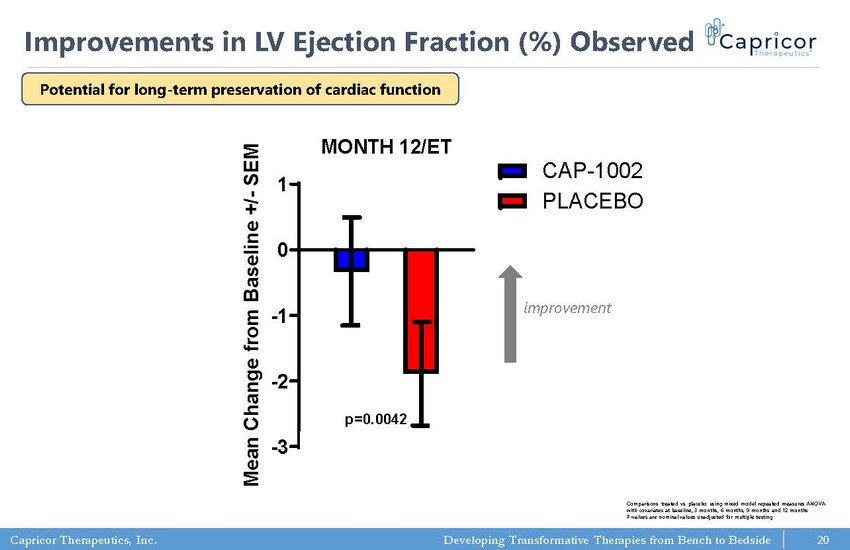
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside LV EF (%) MONTH 12/ET -3 -2 -1 0 1 CAP-1002 PLACEBO p=0.0042 M e a n C h a n g e f r o m B a s e l i n e + / - S E M 20 Improvements in LV Ejection Fraction (%) Observed improvement Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months P - values are nominal values unadjusted for multiple testing Potential for long - term preservation of cardiac function
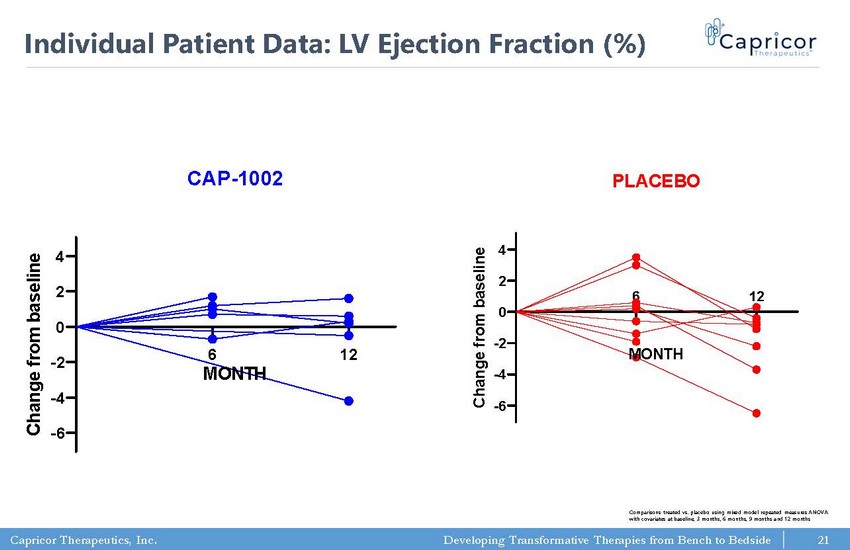
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 6 12 -6 -4 -2 0 2 4 Cardiac MRI: LV Ejection Fraction (%) CAP-1002 MONTH C h a n g e f r o m b a s e l i n e 6 12 -6 -4 -2 0 2 4 Cardiac MRI: LV Ejection Fraction (%) PLACEBO MONTH C h a n g e f r o m b a s e l i n e 21 Individual Patient Data: LV Ejection Fraction (%) Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months
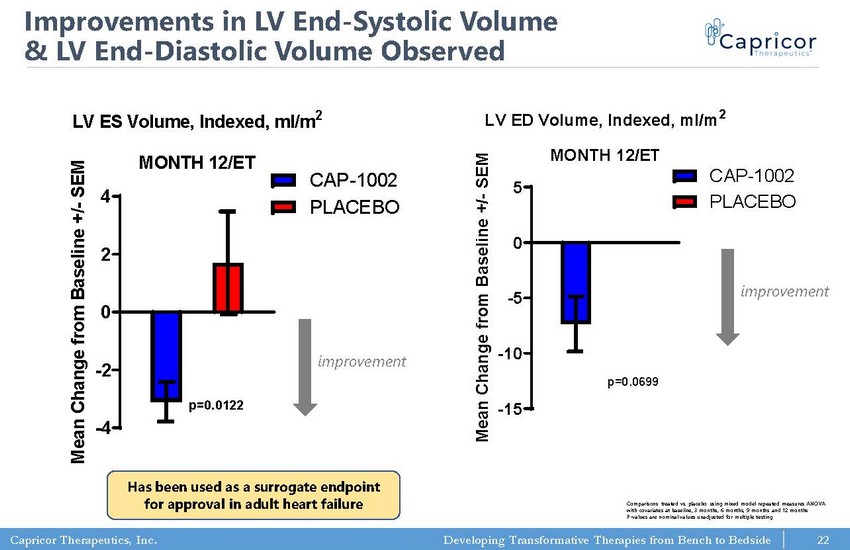
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside LV ES Volume, Indexed, ml/m 2 MONTH 12/ET -4 -2 0 2 4 CAP-1002 PLACEBO p=0.0122 M e a n C h a n g e f r o m B a s e l i n e + / - S E M Has been used as a surrogate endpoint for approval in adult heart failure 22 LV ED Volume, Indexed, ml/m 2 MONTH 12/ET -15 -10 -5 0 5 CAP-1002 PLACEBO p=0.0699 M e a n C h a n g e f r o m B a s e l i n e + / - S E M Improvements in LV End - Systolic Volume & LV End - Diastolic Volume Observed improvement improvement Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months P - values are nominal values unadjusted for multiple testing
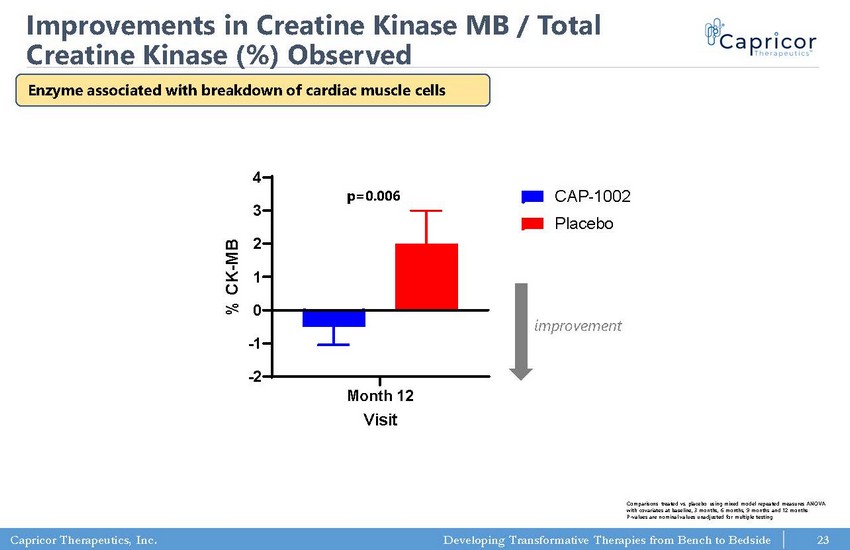
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 23 Improvements in Creatine Kinase MB / Total Creatine Kinase (%) Observed Month 12 -2 -1 0 1 2 3 4 Creatine Kinase MB/Total Creatine Kinase (%) Change From Baseline Visit % C K - M B CAP-1002 Placebo ✱✱✱ Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months P - values are nominal values unadjusted for multiple testing p=0.006 improvement Enzyme associated with breakdown of cardiac muscle cells
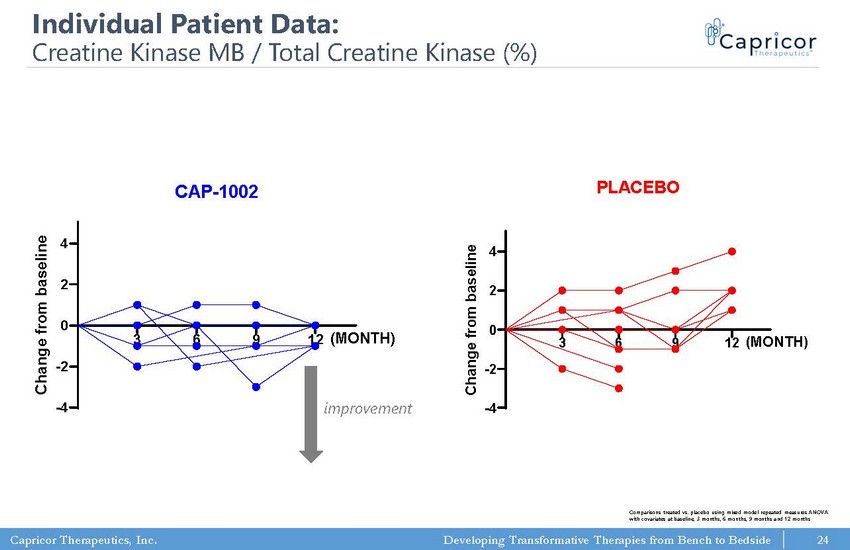
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside 3 6 9 12 -4 -2 0 2 4 Serum Chemistry: Creatine Kinase MB/Total Creatine Kinase (%) CAP-1002 C h a n g e f r o m b a s e l i n e (MONTH) 3 6 9 12 -4 -2 0 2 4 Serum Chemistry: Creatine Kinase MB/Total Creatine Kinase (%) PLACEBO C h a n g e f r o m b a s e l i n e (MONTH) 24 Individual Patient Data: Creatine Kinase MB / Total Creatine Kinase (%) improvement Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Respiratory Function Measured by: Inspiratory Flow Reserve Peak Expiratory Flow (% predicted) 25
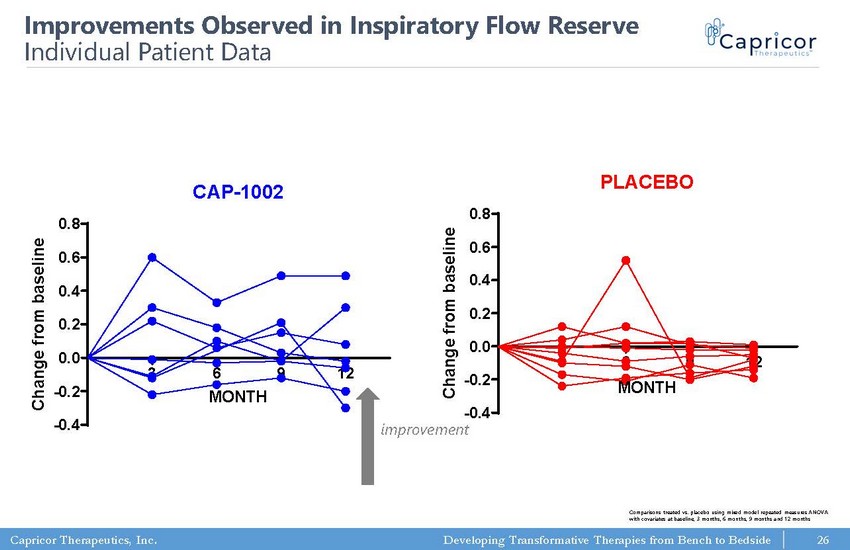
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Pulmonary Function Testing: Inspiratory Flow Reserve CAP-1002 3 6 9 12 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 MONTH C h a n g e f r o m b a s e l i n e Pulmonary Function Testing: Inspiratory Flow Reserve PLACEBO 3 6 9 12 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 MONTH C h a n g e f r o m b a s e l i n e 26 Improvements Observed in Inspiratory Flow Reserve Individual Patient Data improvement Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months
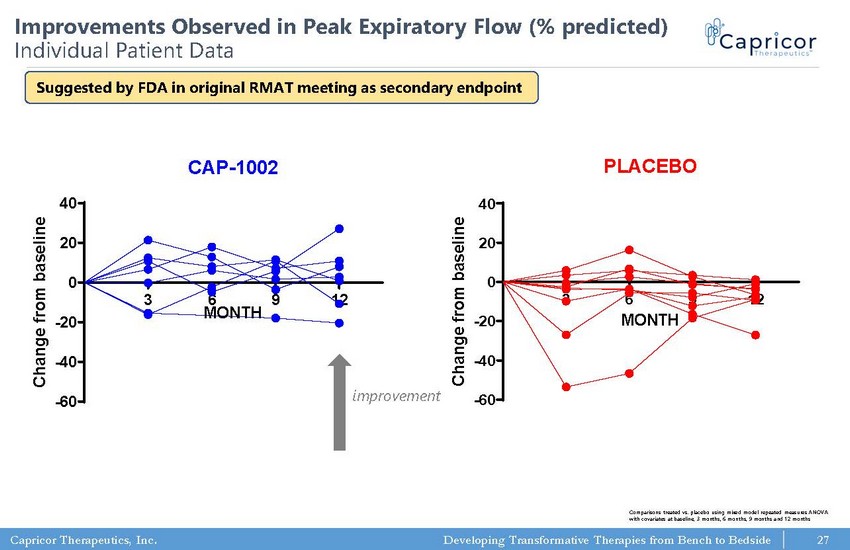
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Pulmonary Function Testing: PEF (% of Predicted) CAP-1002 3 6 9 12 -60 -40 -20 0 20 40 MONTH C h a n g e f r o m b a s e l i n e Pulmonary Function Testing: PEF (% of Predicted) PLACEBO 3 6 9 12 -60 -40 -20 0 20 40 MONTH C h a n g e f r o m b a s e l i n e 27 Improvements Observed in Peak Expiratory Flow (% predicted) Individual Patient Data improvement Comparisons treated vs. placebo using mixed model repeated measures ANOVA with covariates at baseline, 3 months, 6 months, 9 months and 12 months Suggested by FDA in original RMAT meeting as secondary endpoint

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Safety Summary 28

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - 2 Safety Results 29 ▪ A total of 69 infusions (CAP - 1002 or placebo) were performed in HOPE - 2 ▪ Generally safe and well tolerated throughout the study ▪ With the exception of hypersensitivity reactions, no safety signals were identified ▪ In late December 2018, Capricor put a voluntary hold on dosing after two patients in the HOPE trials had a serious adverse event in the form of a hypersensitivity reaction. ▪ Possibly linked to excipients (e.g. DMSO)

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE - 2 Safety Mitigation Efforts 30 ▪ To reduce the risk of such future adverse events, Capricor initiated a commonly used pre - medication strategy including intravenous steroids and antihistamines to prevent or mitigate potential allergic reactions during the administration. ▪ Since the initiation of the pre - treatment regimen, 42 infusions of investigational drug (CAP - 1002 or placebo) were administered with only one hypersensitivity reaction that required an overnight observation of the patient

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Conclusions and Future Directions Conclusions: ‒ First placebo - controlled trial showing upper limb functional improvements in non - ambulant DMD patients ‒ Directionally consistent improvements in strength, respiratory and cardiac endpoints ‒ First ever study in DMD that correlates cardiac functional stabilization with reduction of a biomarker of myocardial cell damage ‒ Consistent results shown pre - clinically, Phase I/II and Phase II Moving Forward: ‒ Requested End - of Phase 2 Meeting with FDA to discuss pathway to approval ‒ Engaged global CMO for scale - up of manufacturing of CAP - 1002 ‒ Expeditious initiation of open label extension 31
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside DMD Advisory Board Craig McDonald, M.D. (National PI) University of California at Davis (USA) Michelle Eagle, Ph.D., M.Sc., MCSP Atom International Ltd (UK) Richard Finkel, M.D. Nemours Children's Hospital (USA) Pat Furlong Parent Project Muscular Dystrophy (USA) Kan Hor, M.D. Nationwide Children's Hospital (USA) John Jefferies, M.D. Cincinnati Children's Hospital Medical Center (USA) Oscar Henry Mayer, M.D. Children's Hospital of Philadelphia (USA) Eugenio Mercuri, M.D., Ph.D. Catholic University of the Sacred Heart (Italy) Francesco Muntoni, M.D. University College London (UK) Thomas Voit, M.D. University College London (UK) Lee Sweeney, Ph.D. University of Florida (USA) Michael Taylor, M.D., Ph.D. Cincinnati Children's Hospital Medical Center (USA) 32
Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Acknowledgements ▪ Craig McDonald, MD (UC Davis) ▪ Cuixia Tian, MD (CCHMC) ▪ Russell Butterfield, MD (University of Utah) ▪ Richard Finkel, MD (Nemours Children’s Hospital) ▪ Joanne Janas , MD (Children’s Hospital of Colorado) ▪ Matthew Harmelink, MD (Children’s Hospital of Wisconsin) ▪ Arun Varadhachary, MD (Washington University, Saint Louis Children’s Hospital) ▪ Brenda Wong, MD (University of Massachusetts) ▪ Katherine Mathews, MD (University of Iowa, Children’s Hospital) ▪ All patients and their families who participated in the HOPE - 2 Study ▪ Parent Project Muscular Dystrophy ▪ Coalition Duchenne ▪ CureDuchenne ▪ HOPE - Duchenne (Phase I/II) was funded with the support of CIRM 33

Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Thank you Questions and Answer 34
