Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Stoke Therapeutics, Inc. | d876521dex991.htm |
| 8-K - 8-K - Stoke Therapeutics, Inc. | d876521d8k.htm |
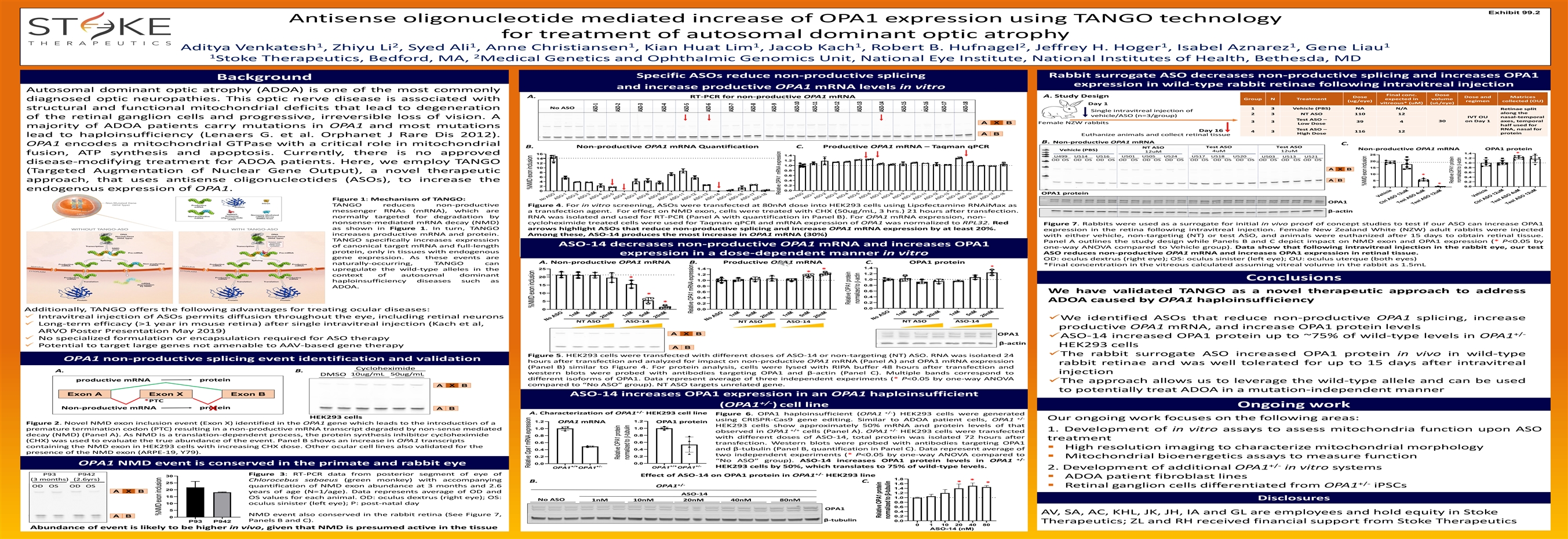
Exhibit 99.2 Antisense oligonucleotide mediated increase of OPA1 expression using TANGO technology for treatment of autosomal dominant optic atrophy 1 2 1 1 1 1 2 1 1 1 Aditya Venkatesh , Zhiyu Li , Syed Ali , Anne Christiansen , Kian Huat Lim , Jacob Kach , Robert B. Hufnagel , Jeffrey H. Hoger , Isabel Aznarez , Gene Liau 1 2 Stoke Therapeutics, Bedford, MA, Medical Genetics and Ophthalmic Genomics Unit, National Eye Institute, National Institutes of Health, Bethesda, MD Rabbit surrogate ASO decreases non-productive splicing and increases OPA1 Specific ASOs reduce non-productive splicing Background expression in wild-type rabbit retinae following intravitreal injection and increase productive OPA1 mRNA levels in vitro Autosomal dominant optic atrophy (ADOA) is one of the most commonly Final conc. Dose A. Study Design A. Dose Dose and Matrices RT-PCR for non-productive OPA1 mRNA Group N Treatment expected in volume diagnosed optic neuropathies. This optic nerve disease is associated with (ug/eye) regimen collected (OU) vitreous* (uM) (uL/eye) Day 1 No ASO 1 3 Vehicle (PBS) NA N/A structural and functional mitochondrial deficits that lead to degeneration Retinae split Single intravitreal injection of along the 2 3 NT ASO 110 12 vehicle/ASO (n=3/group) IVT OU nasal-temporal of the retinal ganglion cells and progressive, irreversible loss of vision. A Test ASO – 30 on Day 1 axes, temporal 3 3 39 4 Female NZW rabbits A X B Low Dose half used for majority of ADOA patients carry mutations in OPA1 and most mutations RNA, nasal for Test ASO – Day 16 4 3 116 12 protein High Dose A B Euthanize animals and collect retinal tissue lead to haploinsufficiency (Lenaers G. et al. Orphanet J Rare Dis 2012). B. Non-productive OPA1 mRNA C. OPA1 encodes a mitochondrial GTPase with a critical role in mitochondrial B. Non-productive OPA1 mRNA Quantification C. Productive OPA1 mRNA – Taqman qPCR Test ASO Test ASO NT ASO Non-productive OPA1 mRNA OPA1 protein Vehicle (PBS) 4uM 12uM 12uM 1.4 * fusion, ATP synthesis and apoptosis. Currently, there is no approved 16 25 1.4 U499 U514 U516 U501 U505 U524 U517 U518 U520 U503 U513 U521 1.2 14 OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS 1.2 20 1.0 disease-modifying treatment for ADOA patients. Here, we employ TANGO 12 1.0 15 0.8 10 A X B 0.8 (Targeted Augmentation of Nuclear Gene Output), a novel therapeutic 0.6 8 10 * 0.6 0.4 6 0.4 A B 5 approach, that uses antisense oligonucleotides (ASOs), to increase the 4 0.2 * 0.2 2 0.0 0 endogenous expression of OPA1. 0.0 0 OPA1 protein Figure 1: Mechanism of TANGO: OPA1 TANGO reduces non-productive Figure 4. For in vitro screening, ASOs were transfected at 80nM dose into HEK293 cells using Lipofectamine RNAiMax as messenger RNAs (mRNA), which are a transfection agent. For effect on NMD exon, cells were treated with CHX (50ug/mL, 3 hrs.) 21 hours after transfection. β-actin normally targeted for degradation by RNA was isolated and used for RT-PCR (Panel A with quantification in Panel B). For OPA1 mRNA expression, non- nonsense-mediated mRNA decay (NMD) cycloheximide treated cells were used for Taqman qPCR and mRNA expression of OPA1 was normalized to RPL32. Red Figure 7. Rabbits were used as a surrogate for initial in vivo proof of concept studies to test if our ASO can increase OPA1 as shown in Figure 1. In turn, TANGO arrows highlight ASOs that reduce non-productive splicing and increase OPA1 mRNA expression by at least 20%. expression in the retina following intravitreal injection. Female New Zealand White (NZW) adult rabbits were injected increases productive mRNA and protein. Among these, ASO-14 produces the most increase in OPA1 mRNA (30%) with either vehicle, non-targeting (NT) or test ASO, and animals were euthanized after 15 days to obtain retinal tissue. TANGO specifically increases expression Panel A outlines the study design while Panels B and C depict impact on NMD exon and OPA1 expression (* P<0.05 by ASO-14 decreases non-productive OPA1 mRNA and increases OPA1 of canonical target mRNA and full-length one-way ANOVA compared to Vehicle group). Data show that following intravitreal injection in the rabbit eye, our test protein, only in tissues with endogenous ASO reduces non-productive OPA1 mRNA and increases OPA1 expression in retinal tissue. expression in a dose-dependent manner in vitro gene expression. As these events are OD: oculus dextrus (right eye); OS: oculus sinister (left eye); OU: oculus uterque (both eyes) A. Non-productive OPA1 mRNA B. Productive OPA1 mRNA C. OPA1 protein naturally-occurring, TANGO can *Final concentration in the vitreous calculated assuming vitreal volume in the rabbit as 1.5mL * 1.4 1.4 25 upregulate the wild-type alleles in the * * 1.2 1.2 context of autosomal dominant 20 * Conclusions 1.0 1.0 haploinsufficiency diseases such as 15 0.8 0.8 ADOA. 0.6 0.6 We have validated TANGO as a novel therapeutic approach to address 10 * 0.4 0.4 ADOA caused by OPA1 haploinsufficiency 5 * 0.2 0.2 0.0 0.0 0 Additionally, TANGO offers the following advantages for treating ocular diseases: ✓ Intravitreal injection of ASOs permits diffusion throughout the eye, including retinal neurons ✓We identified ASOs that reduce non-productive OPA1 splicing, increase NT ASO ASO-14 NT ASO ASO-14 NT ASO ASO-14 ✓ Long-term efficacy (>1 year in mouse retina) after single intravitreal injection (Kach et al, productive OPA1 mRNA, and increase OPA1 protein levels ARVO Poster Presentation May 2019) A X B OPA1 +/- ✓ASO-14 increased OPA1 protein up to ~75% of wild-type levels in OPA1 ✓ No specialized formulation or encapsulation required for ASO therapy β-actin ✓ Potential to target large genes not amenable to AAV-based gene therapy HEK293 cells A B ✓The rabbit surrogate ASO increased OPA1 protein in vivo in wild-type Figure 5. HEK293 cells were transfected with different doses of ASO-14 or non-targeting (NT) ASO. RNA was isolated 24 OPA1 non-productive splicing event identification and validation hours after transfection and analyzed for impact on non-productive OPA1 mRNA (Panel A) and OPA1 mRNA expression rabbit retinae and was well tolerated for up to 15 days after intravitreal (Panel B) similar to Figure 4. For protein analysis, cells were lysed with RIPA buffer 48 hours after transfection and Cycloheximide A. B. injection western blots were probed with antibodies targeting OPA1 andβ-actin (Panel C). Multiple bands correspond to 10ug/mL 50ug/mL DMSO different isoforms of OPA1. Data represent average of three independent experiments (* P<0.05 by one-way ANOVA protein productive mRNA ✓The approach allows us to leverage the wild-type allele and can be used compared to“NoASO” group). NT ASO targets unrelated gene. A X B to potentially treat ADOA in a mutation-independent manner Exon A Exon X Exon B ASO-14 increases OPA1 expression in an OPA1 haploinsufficient *PTC +/- (OPA1 ) cell line Ongoing work protein Non-productive mRNA A B X +/- +/- A. Characterization of OPA1 HEK293 cell line Figure 6. OPA1 haploinsufficient (OPA1 ) HEK293 cells were generated HEK293 cells +/- Our ongoing work focuses on the following areas: using CRISPR-Cas9 gene editing. Similar to ADOA patient cells, OPA1 1.2 1.2 OPA1 mRNA OPA1 protein Figure 2. Novel NMD exon inclusion event (Exon X) identified in the OPA1 gene which leads to the introduction of a HEK293 cells show approximately 50% mRNA and protein levels of that 1.0 1.0 premature termination codon (PTC) resulting in a non-productive mRNA transcript degraded by non-sense mediated +/+ +/- 1. Development of in vitro assays to assess mitochondria function upon ASO observed in OPA1 cells (Panel A). OPA1 HEK293 cells were transfected decay (NMD) (Panel A). As NMD is a translation-dependent process, the protein synthesis inhibitor cycloheximide 0.8 0.8 with different doses of ASO-14, total protein was isolated 72 hours after treatment (CHX) was used to evaluate the true abundance of the event. Panel B shows an increase in OPA1 transcripts 0.6 0.6 transfection. Western blots were probed with antibodies targeting OPA1 containing the NMD exon in HEK293 cells with increasing CHX dose. Other ocular cell lines also validated for the ▪ High resolution imaging to characterize mitochondrial morphology andβ-tubulin (Panel B, quantification in Panel C). Data represent average of 0.4 0.4 presence of the NMD exon (ARPE-19, Y79). two independent experiments (* P<0.05 by one-way ANOVA compared to 0.2 0.2 ▪ Mitochondrial bioenergetics assays to measure function +/- “NoASO” group). ASO-14 increases OPA1 protein levels in OPA1 0.0 0.0 OPA1 NMD event is conserved in the primate and rabbit eye +/+ +/- +/+ +/- +/- HEK293 cells by 50%, which translates to 75% of wild-type levels. OPA1 OPA1 OPA1 OPA1 2. Development of additional OPA1 in vitro systems +/- P93 P942 Figure 3: RT-PCR data from posterior segment of eye of Effect of ASO-14 on OPA1 protein in OPA1 HEK293 line 30 ▪ ADOA patient fibroblast lines 1.8 (3 months) (2.6yrs) Chlorocebus sabaeus (green monkey) with accompanying * B. C. * 25 * 1.6 +/- +/- OD OS OD OS quantification of NMD exon abundance at 3 months and 2.6 OPA1 ▪ Retinal ganglion cells differentiated from OPA1 iPSCs 1.4 20 A X B years of age (N=1/age). Data represents average of OD and 1.2 ASO-14 15 1.0 OS values for each animal. OD: oculus dextrus (right eye); OS: Disclosures No ASO 1nM 10nM 20nM 40nM 80nM 0.8 10 oculus sinister (left eye); P: post-natal day 0.6 OPA1 5 0.4 AV, SA, AC, KHL, JK, JH, IA and GL are employees and hold equity in Stoke NMD event also conserved in the rabbit retina (See Figure 7, 0.2 A B 0 β-tubulin Panels B and C). 0.0 P93 P942 Therapeutics; ZL and RH received financial support from Stoke Therapeutics 0 1 10 20 40 80 Abundance of event is likely to be higher in vivo, given that NMD is presumed active in the tissue ASO-14 (nM) No ASO ASO-1 ASO-2 ASO-3 ASO-4 ASO-5 ASO-6 ASO-7 ASO-8 ASO-9 ASO-10 ASO-11 ASO-12 ASO-13 ASO-14 ASO-15 ASO-16 ASO-17 ASO-18 No ASO No ASO ASO-1 ASO-2 1nM ASO-3 5nM ASO-4 ASO-5 20nM ASO-6 1nM ASO-7 ASO-8 5nM ASO-9 20nM ASO-10 ASO-11 ASO-12 ASO-13 ASO-14 ASO-15 ASO-16 ASO-17 ASO-18 No ASO 1nM 5nM 20nM 1nM 5nM 20nM No ASO 1nM 5nM 20nM 1nM 5nM 20nM Vehicle Ctrl ASO 12uM Test ASO 4uM Test ASO 12uM Vehicle Ctrl ASO 12uM Tes ASO 4uM t Test ASO 12uM %NMD exon inclusion RelativeOpa1 mRNA expression %NMD exon inclusion %NMD exon inclusion ASO-1 Relative OPA1 protein ASO-2 normalized tob-tubulin ASO-3 ASO-4 ASO-5 RelativeOPA1 mRNA expression ASO-6 ASO-7 ASO-8 ASO-9 RelativeOPA1 mRNA expression ASO-10 ASO-11 ASO-12 Relative OPA1 protein normalized tob-actin ASO-13 Relative OPA1 protein ASO-14 normalized to β-tubulin ASO-15 ASO-16 ASO-17 ASO-18 %NMD exon inclusion Relative OPA1 protein normalized tob-actinExhibit 99.2 Antisense oligonucleotide mediated increase of OPA1 expression using TANGO technology for treatment of autosomal dominant optic atrophy 1 2 1 1 1 1 2 1 1 1 Aditya Venkatesh , Zhiyu Li , Syed Ali , Anne Christiansen , Kian Huat Lim , Jacob Kach , Robert B. Hufnagel , Jeffrey H. Hoger , Isabel Aznarez , Gene Liau 1 2 Stoke Therapeutics, Bedford, MA, Medical Genetics and Ophthalmic Genomics Unit, National Eye Institute, National Institutes of Health, Bethesda, MD Rabbit surrogate ASO decreases non-productive splicing and increases OPA1 Specific ASOs reduce non-productive splicing Background expression in wild-type rabbit retinae following intravitreal injection and increase productive OPA1 mRNA levels in vitro Autosomal dominant optic atrophy (ADOA) is one of the most commonly Final conc. Dose A. Study Design A. Dose Dose and Matrices RT-PCR for non-productive OPA1 mRNA Group N Treatment expected in volume diagnosed optic neuropathies. This optic nerve disease is associated with (ug/eye) regimen collected (OU) vitreous* (uM) (uL/eye) Day 1 No ASO 1 3 Vehicle (PBS) NA N/A structural and functional mitochondrial deficits that lead to degeneration Retinae split Single intravitreal injection of along the 2 3 NT ASO 110 12 vehicle/ASO (n=3/group) IVT OU nasal-temporal of the retinal ganglion cells and progressive, irreversible loss of vision. A Test ASO – 30 on Day 1 axes, temporal 3 3 39 4 Female NZW rabbits A X B Low Dose half used for majority of ADOA patients carry mutations in OPA1 and most mutations RNA, nasal for Test ASO – Day 16 4 3 116 12 protein High Dose A B Euthanize animals and collect retinal tissue lead to haploinsufficiency (Lenaers G. et al. Orphanet J Rare Dis 2012). B. Non-productive OPA1 mRNA C. OPA1 encodes a mitochondrial GTPase with a critical role in mitochondrial B. Non-productive OPA1 mRNA Quantification C. Productive OPA1 mRNA – Taqman qPCR Test ASO Test ASO NT ASO Non-productive OPA1 mRNA OPA1 protein Vehicle (PBS) 4uM 12uM 12uM 1.4 * fusion, ATP synthesis and apoptosis. Currently, there is no approved 16 25 1.4 U499 U514 U516 U501 U505 U524 U517 U518 U520 U503 U513 U521 1.2 14 OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS OD OS 1.2 20 1.0 disease-modifying treatment for ADOA patients. Here, we employ TANGO 12 1.0 15 0.8 10 A X B 0.8 (Targeted Augmentation of Nuclear Gene Output), a novel therapeutic 0.6 8 10 * 0.6 0.4 6 0.4 A B 5 approach, that uses antisense oligonucleotides (ASOs), to increase the 4 0.2 * 0.2 2 0.0 0 endogenous expression of OPA1. 0.0 0 OPA1 protein Figure 1: Mechanism of TANGO: OPA1 TANGO reduces non-productive Figure 4. For in vitro screening, ASOs were transfected at 80nM dose into HEK293 cells using Lipofectamine RNAiMax as messenger RNAs (mRNA), which are a transfection agent. For effect on NMD exon, cells were treated with CHX (50ug/mL, 3 hrs.) 21 hours after transfection. β-actin normally targeted for degradation by RNA was isolated and used for RT-PCR (Panel A with quantification in Panel B). For OPA1 mRNA expression, non- nonsense-mediated mRNA decay (NMD) cycloheximide treated cells were used for Taqman qPCR and mRNA expression of OPA1 was normalized to RPL32. Red Figure 7. Rabbits were used as a surrogate for initial in vivo proof of concept studies to test if our ASO can increase OPA1 as shown in Figure 1. In turn, TANGO arrows highlight ASOs that reduce non-productive splicing and increase OPA1 mRNA expression by at least 20%. expression in the retina following intravitreal injection. Female New Zealand White (NZW) adult rabbits were injected increases productive mRNA and protein. Among these, ASO-14 produces the most increase in OPA1 mRNA (30%) with either vehicle, non-targeting (NT) or test ASO, and animals were euthanized after 15 days to obtain retinal tissue. TANGO specifically increases expression Panel A outlines the study design while Panels B and C depict impact on NMD exon and OPA1 expression (* P<0.05 by ASO-14 decreases non-productive OPA1 mRNA and increases OPA1 of canonical target mRNA and full-length one-way ANOVA compared to Vehicle group). Data show that following intravitreal injection in the rabbit eye, our test protein, only in tissues with endogenous ASO reduces non-productive OPA1 mRNA and increases OPA1 expression in retinal tissue. expression in a dose-dependent manner in vitro gene expression. As these events are OD: oculus dextrus (right eye); OS: oculus sinister (left eye); OU: oculus uterque (both eyes) A. Non-productive OPA1 mRNA B. Productive OPA1 mRNA C. OPA1 protein naturally-occurring, TANGO can *Final concentration in the vitreous calculated assuming vitreal volume in the rabbit as 1.5mL * 1.4 1.4 25 upregulate the wild-type alleles in the * * 1.2 1.2 context of autosomal dominant 20 * Conclusions 1.0 1.0 haploinsufficiency diseases such as 15 0.8 0.8 ADOA. 0.6 0.6 We have validated TANGO as a novel therapeutic approach to address 10 * 0.4 0.4 ADOA caused by OPA1 haploinsufficiency 5 * 0.2 0.2 0.0 0.0 0 Additionally, TANGO offers the following advantages for treating ocular diseases: ✓ Intravitreal injection of ASOs permits diffusion throughout the eye, including retinal neurons ✓We identified ASOs that reduce non-productive OPA1 splicing, increase NT ASO ASO-14 NT ASO ASO-14 NT ASO ASO-14 ✓ Long-term efficacy (>1 year in mouse retina) after single intravitreal injection (Kach et al, productive OPA1 mRNA, and increase OPA1 protein levels ARVO Poster Presentation May 2019) A X B OPA1 +/- ✓ASO-14 increased OPA1 protein up to ~75% of wild-type levels in OPA1 ✓ No specialized formulation or encapsulation required for ASO therapy β-actin ✓ Potential to target large genes not amenable to AAV-based gene therapy HEK293 cells A B ✓The rabbit surrogate ASO increased OPA1 protein in vivo in wild-type Figure 5. HEK293 cells were transfected with different doses of ASO-14 or non-targeting (NT) ASO. RNA was isolated 24 OPA1 non-productive splicing event identification and validation hours after transfection and analyzed for impact on non-productive OPA1 mRNA (Panel A) and OPA1 mRNA expression rabbit retinae and was well tolerated for up to 15 days after intravitreal (Panel B) similar to Figure 4. For protein analysis, cells were lysed with RIPA buffer 48 hours after transfection and Cycloheximide A. B. injection western blots were probed with antibodies targeting OPA1 andβ-actin (Panel C). Multiple bands correspond to 10ug/mL 50ug/mL DMSO different isoforms of OPA1. Data represent average of three independent experiments (* P<0.05 by one-way ANOVA protein productive mRNA ✓The approach allows us to leverage the wild-type allele and can be used compared to“NoASO” group). NT ASO targets unrelated gene. A X B to potentially treat ADOA in a mutation-independent manner Exon A Exon X Exon B ASO-14 increases OPA1 expression in an OPA1 haploinsufficient *PTC +/- (OPA1 ) cell line Ongoing work protein Non-productive mRNA A B X +/- +/- A. Characterization of OPA1 HEK293 cell line Figure 6. OPA1 haploinsufficient (OPA1 ) HEK293 cells were generated HEK293 cells +/- Our ongoing work focuses on the following areas: using CRISPR-Cas9 gene editing. Similar to ADOA patient cells, OPA1 1.2 1.2 OPA1 mRNA OPA1 protein Figure 2. Novel NMD exon inclusion event (Exon X) identified in the OPA1 gene which leads to the introduction of a HEK293 cells show approximately 50% mRNA and protein levels of that 1.0 1.0 premature termination codon (PTC) resulting in a non-productive mRNA transcript degraded by non-sense mediated +/+ +/- 1. Development of in vitro assays to assess mitochondria function upon ASO observed in OPA1 cells (Panel A). OPA1 HEK293 cells were transfected decay (NMD) (Panel A). As NMD is a translation-dependent process, the protein synthesis inhibitor cycloheximide 0.8 0.8 with different doses of ASO-14, total protein was isolated 72 hours after treatment (CHX) was used to evaluate the true abundance of the event. Panel B shows an increase in OPA1 transcripts 0.6 0.6 transfection. Western blots were probed with antibodies targeting OPA1 containing the NMD exon in HEK293 cells with increasing CHX dose. Other ocular cell lines also validated for the ▪ High resolution imaging to characterize mitochondrial morphology andβ-tubulin (Panel B, quantification in Panel C). Data represent average of 0.4 0.4 presence of the NMD exon (ARPE-19, Y79). two independent experiments (* P<0.05 by one-way ANOVA compared to 0.2 0.2 ▪ Mitochondrial bioenergetics assays to measure function +/- “NoASO” group). ASO-14 increases OPA1 protein levels in OPA1 0.0 0.0 OPA1 NMD event is conserved in the primate and rabbit eye +/+ +/- +/+ +/- +/- HEK293 cells by 50%, which translates to 75% of wild-type levels. OPA1 OPA1 OPA1 OPA1 2. Development of additional OPA1 in vitro systems +/- P93 P942 Figure 3: RT-PCR data from posterior segment of eye of Effect of ASO-14 on OPA1 protein in OPA1 HEK293 line 30 ▪ ADOA patient fibroblast lines 1.8 (3 months) (2.6yrs) Chlorocebus sabaeus (green monkey) with accompanying * B. C. * 25 * 1.6 +/- +/- OD OS OD OS quantification of NMD exon abundance at 3 months and 2.6 OPA1 ▪ Retinal ganglion cells differentiated from OPA1 iPSCs 1.4 20 A X B years of age (N=1/age). Data represents average of OD and 1.2 ASO-14 15 1.0 OS values for each animal. OD: oculus dextrus (right eye); OS: Disclosures No ASO 1nM 10nM 20nM 40nM 80nM 0.8 10 oculus sinister (left eye); P: post-natal day 0.6 OPA1 5 0.4 AV, SA, AC, KHL, JK, JH, IA and GL are employees and hold equity in Stoke NMD event also conserved in the rabbit retina (See Figure 7, 0.2 A B 0 β-tubulin Panels B and C). 0.0 P93 P942 Therapeutics; ZL and RH received financial support from Stoke Therapeutics 0 1 10 20 40 80 Abundance of event is likely to be higher in vivo, given that NMD is presumed active in the tissue ASO-14 (nM) No ASO ASO-1 ASO-2 ASO-3 ASO-4 ASO-5 ASO-6 ASO-7 ASO-8 ASO-9 ASO-10 ASO-11 ASO-12 ASO-13 ASO-14 ASO-15 ASO-16 ASO-17 ASO-18 No ASO No ASO ASO-1 ASO-2 1nM ASO-3 5nM ASO-4 ASO-5 20nM ASO-6 1nM ASO-7 ASO-8 5nM ASO-9 20nM ASO-10 ASO-11 ASO-12 ASO-13 ASO-14 ASO-15 ASO-16 ASO-17 ASO-18 No ASO 1nM 5nM 20nM 1nM 5nM 20nM No ASO 1nM 5nM 20nM 1nM 5nM 20nM Vehicle Ctrl ASO 12uM Test ASO 4uM Test ASO 12uM Vehicle Ctrl ASO 12uM Tes ASO 4uM t Test ASO 12uM %NMD exon inclusion RelativeOpa1 mRNA expression %NMD exon inclusion %NMD exon inclusion ASO-1 Relative OPA1 protein ASO-2 normalized tob-tubulin ASO-3 ASO-4 ASO-5 RelativeOPA1 mRNA expression ASO-6 ASO-7 ASO-8 ASO-9 RelativeOPA1 mRNA expression ASO-10 ASO-11 ASO-12 Relative OPA1 protein normalized tob-actin ASO-13 Relative OPA1 protein ASO-14 normalized to β-tubulin ASO-15 ASO-16 ASO-17 ASO-18 %NMD exon inclusion Relative OPA1 protein normalized tob-actin
