Attached files
| file | filename |
|---|---|
| EX-10.23 - CITRINE GLOBAL, CORP. | ex10-23.htm |
| EX-32.2 - CITRINE GLOBAL, CORP. | ex32-2.htm |
| EX-32.1 - CITRINE GLOBAL, CORP. | ex32-1.htm |
| EX-31.2 - CITRINE GLOBAL, CORP. | ex31-2.htm |
| EX-31.1 - CITRINE GLOBAL, CORP. | ex31-1.htm |
| EX-23.1 - CITRINE GLOBAL, CORP. | ex23-1.htm |
| EX-10.22 - CITRINE GLOBAL, CORP. | ex10-22.htm |
| EX-10.19 - CITRINE GLOBAL, CORP. | ex10-19.htm |
| EX-10.18 - CITRINE GLOBAL, CORP. | ex10-18.htm |
| EX-10.17 - CITRINE GLOBAL, CORP. | ex10-17.htm |
| EX-10.15 - CITRINE GLOBAL, CORP. | ex10-15.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| [X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
or
| [ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________________ to ________________
Commission file number 000-55680
TECHCARE
CORP.
(Exact Name of Registrant As Specified In Its Charter)
| Delaware | 68-0080601 | |
| (State of Incorporation) | (I.R.S. Employer Identification No.) |
| 3 Hamelacha, Tel Aviv, Israel | 6721503 | |
| (Address of Principal Executive Offices) | (Area Code) |
Registrant’s Telephone Number, Including Area Code: + (972) 73 7600341
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. [ ] Yes [X] No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. [ ] Yes [X] No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. [X] Yes [ ] No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). [X] Yes [ ] No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” or “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | [ ] | Accelerated filer | [ ] | |
| Non-accelerated filer | [X] | Smaller reporting company | [X] | |
| Emerging growth company | [ ] |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. [ ]
Indicate whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) [ ] Yes [X] No
On May 7, 2020, the aggregate market value of the common stock held by non-affiliates of the registrant was approximately $97.5 million based on the closing price of $0.197 per share of the Registrant’s common stock on May 7, 2020.
The registrant had 494,721,815 shares of common stock outstanding as of May 7, 2020.
The registrant is relying on the Securities and Exchange Commission’s Order under Section 36 of the Securities Exchange Act of 1934 Modifying Exemptions from the Reporting and Proxy Delivery Requirements for Public Companies (Securities and Exchange Commission Release No. 34-88465 dated March 25, 2020), which concerns exemptions from certain filing deadlines in light of coronavirus disease 2019 (COVID-19). The registrant could not file this Annual Report on Form 10-K for the fiscal year ended December 31, 2019 on a timely basis because the outbreak of COVID-19 in Israel and attendant restrictions on life in Israel, which included, among others, our team and advisors being required to work from home, combined with the additional workload involved in completing the transactions reported by the Registrant during the first quarter of this year for the issuance and sale of shares in the Registrant and the sale of shares in its subsidiary Novomic Ltd, caused delays in completing the required work.
TABLE OF CONTENTS
| 2 |
Cautionary Statement regarding Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, or the Exchange Act. These forward-looking statements relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” or “continue” or the negative of these terms or other comparable terminology. These forward-looking statements are only predictions and involve known and unknown risks, uncertainties and other factors, any of which may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Forward-looking statements are made based on management’s beliefs, estimates and opinions on the date the forward-looking statements are made, and we undertake no obligation to update forward-looking statements should these beliefs, estimates, and opinions or other circumstances change. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these forward-looking statements to actual results.
Our financial statements are stated in United States dollars, or US$, and are prepared in accordance with United States generally accepted accounting principles, or GAAP. In this Annual Report, unless otherwise specified, all dollar amounts are expressed in United States dollars and all references to “common stock” refer to the shares of our common stock. As used in this Annual Report, the terms “we,” “us,” “our,” “TechCare,” the “Company” and the “Registrant” mean TechCare Corp. and its subsidiaries unless the context clearly requires otherwise.
| 3 |
Recent Developments and Corporate Background
Prior to the Company’s quarterly report for the period ended September 30, 2019 the Company’s Board of Directors was exploring strategic alternatives to enhance stockholder value, which the Company reported in the quarterly report might include future acquisitions, a merger with another company, a potential sale of certain assets, including the Company’s wholly owned subsidiary Novomic Ltd., a private company organized under the laws of the State of Israel (“Novomic”), or the sale of the Company as a public shell company.
Until December 31, 2019, the Company had incurred accumulated losses of approximately $10.6 million, and based on the projected cash flows and Company’s cash balance, the Company’s management was of the opinion that without further fundraising it would not have sufficient resources to enable it to continue advancing its activities including the development, manufacturing, and marketing of its products. The Company’s management had plans including the continued commercialization of the Company’s products, to continue taking cost reduction steps and securing sufficient financing, however, it was also exploring strategic alternatives as detailed above.
During 2019 Company made various efforts to increase sales of its product Novokid® in Europe and Israel, and to receive U.S. Food and Drug Administration (“FDA”) approval to sell in the USA. However, the Company’s sales efforts, including in Israel through Super Pharm, and in the Netherlands, and through Amazon UK, did not yield the forecasted outcomes. The Company’s USA OEM agreement failed to result in FDA approval or USA sales. The Company failed to enter into additional engagements with distributors in Europe and Latin America. The Company also failed to launch its product Shine. In January 2019 the Company entered into a joint venture agreement with a Chinese partner for the formation of a Chinese joint venture intended to focus on the development of a comprehensive and broad range of health, wellness, beauty and home products for customers by utilizing its patented technology of vaporization of natural and plant-based compounds. While a Chinese entity was established in accordance with the joint venture agreement, only part of the amount envisaged to be invested in the Company by ICB Biotechnology Investments Ltd in connection with the joint venture was invested and on September 19, 2019 its nominated director Ningzhou Zhang resigned from the board of directors of the Company.
On November 21, 2019, in light of the above, and absence of any improvement in the situation, and after the board of directors of the Company reached the conclusion that the Company would not be able to successfully commercialize any products or secure sufficient financing, the Company entered into a Memorandum of Understanding with Citrine S A L Investment & Holdings Ltd (“the buyer”) which provided for the issuance and sale of a number of shares of common stock of the Company which after the transaction would result in the buyer holding 95% of the fully diluted capital stock of the Company, and the sale by the Company of 90% of its shares in Novomic.
On January 6, 2020, definitive agreements were executed for the sale of 90% of the shares in Novomic to Traistman Radziejewski Fundacja Ltd, which is expected to be completed during May 2020 (the “Novomic Divestment”), and for the issuance and sale of a number of shares equal after the issuance to 95% of the fully diluted capital stock of the Company to Citrine S A L Investment & Holdings Ltd and a group of related persons and entities (the “TechCare Transaction”). Citrine S A L Investment & Holdings Ltd carried out extensive due diligence appropriate for the acquisition of a public company divesting its activities, and obtained from the Company’s sellers detailed representations and warranties. Traistman Radziejewski Fundacja Ltd is controlled by Oren Traistman, who was a director of the Company until February 27, 2020.
On February 23, 2020, the Company entered into an agreement amending and restating the January 6, 2020 agreement concerning the TechCare Transaction, which provided for the issuance and sale of the shares in stages. Pursuant to this agreement, on February 27, 2020 a number of shares was issued to Citrine S A L Investment & Holdings Ltd which was insufficient to cause a change in control of the Company - which would at that time have invalidated the Company’s Directors and Officers insurance – but which did cause to come into effect the resignation of the board of directors of the Company and the appointment of a new board nominated by Citrine S A L Investment & Holdings Ltd. The board of the directors of the Company has accordingly since February 27, 2020 been composed of three nominees of Citrine S A L Investment & Holdings Ltd, namely Ora Meir Soffer, Ilan Ben-Ishay and Ilanit Halperin. Zviel Gedalihou was appointed as Chief Financial Officer of the Company on March 17, 2020, and Ora Meir Soffer was appointed Chief Executive Officer of the Company on May 7, 2020. The Company has no payroll liabilities at present. On March 5, 2020, additional shares were issued, causing Citrine S A L Investment & Holdings Ltd and its group of related persons and entities to become owners of 90.6% of the fully diluted capital stock of the Company, and resulting in a change of control of the Company. The Company is working to complete the issuance and sale of a number of shares equal after the issuance to up to 95% of the fully diluted capital stock of the Company by amending its Certificate of Incorporation to increase its authorized share capital and then issuing additional shares.
The Company continues to sell the Novokid® product both through online and physical sales channels, including through its own website, Amazon.uk, and Super Pharm’s physical outlets, with its principal markets being the United Kingdom, Europe and and Israel.
| 4 |
At the time of the publication of this annual report, Novomic was a technology company engaged in the design, development and commercialization of a unique delivery platform utilizing vaporization of various natural compounds for multiple health, beauty and wellness applications. Its delivery platform was proprietary and patented. Its product offering included Novokid® - an innovative home use device which vaporizes a natural, plant-based, pesticides, and silicone-free compound that effectively treats head lice and eggs. Following its soft launch of Novokid® in the Netherlands, the Company expanded its distribution network and launched Novokid in Israel during late May 2018 through Super Pharm, Israel’s largest and leading drugstore chain. The launch was accompanied by a radio and digital brand awareness and marketing campaign and supported by Meditrend, its Israeli distributor, specializing in health and wellness products while representing leading brands. The Company showcased Novokid® and met potential additional distributors and partners at CPhI Worldwide, a renowned and leading pharma tradeshow held in Madrid during October 2018.
On February 8, 2016, the Company signed a Merger Agreement (the “Merger Agreement”) with Novomic and a Shareholders’ Agreement with the Novomic’s shareholders (the “Shareholders’ Agreement”). The Merger Agreement was by and between the Company, on the one hand, and Novomic together with YMY Industry Ltd., or YMY, and Microdel Ltd. or Microdel, the latter two of which are hereinafter referred to as the “Novomic Founders,” on the other hand. On August 9, 2016, the Company consummated the merger under the Merger Agreement (the “Merger”) and Novomic became a wholly-owned subsidiary of the Company. Upon closing of the Merger, the former Novomic shareholders owned approximately 73.52% of the Company’s capital stock and TechCare stockholders retained approximately 26.48% of the combined company, on a fully diluted basis. Accordingly, while TechCare was the legal acquirer, Novomic was treated as the acquiring company in the Merger for accounting purposes, and the Merger was accounted for as a reverse merger. In connection with the closing of the Merger Agreement, the Company (i) changed its name from BreedIT Corp. to TechCare Corp.; (ii) subjected the 149,219,173 outstanding shares of the Company’s common stock to a reverse split on a one-for-thirty (1:30) basis, resulting in 4,973,972 outstanding shares of common stock; and (iii) authorized ten million (10,000,000) shares of preferred stock, par value $0.0001, which may be issued in one or more classes or series, having such designations, preferences, privileges and rights as our board of directors may determine.
Novomic was incorporated as a private limited liability Company in Israel in 2009. Since inception, Novomic has been a technology company engaged in the design, development and commercialization of a platform that vaporizes liquids from a contained capsule into a treatment area, utilizing its proprietary intellectual property rights.
Novomic’s Treatment Solutions
Novokid® – Natural, Plant-based and Effective Lice Treatment
Parents and children exposed to head lice are now forced to use standard over-the-counter, or OTC, treatments that are toxic, often ineffective, time consuming and expensive. According to the Journal of Medical Entomology, 98% of lice have developed resistance to existing treatments in the US and they are now referred to as “super-lice”. Most current treatments contain pesticides, alcohol or silicone, which are all associated with a wide variety of hazardous side effects. Novokid® was a non-pesticide, natural, plant-based and eco-friendly solution that eliminated lice and super lice with a 10 minute dry treatment. This compares with current treatments that require 20-40 minutes of shampooing and combing. At the time of publication of this report, Novomic’s treatment was fast, dry, clean, and easily administered at home or on the go. Novokid® could also be used as a maintenance treatment if used regularly. The Company’s products were all based on its proprietary and patented vaporization platform, which was developed over a period of seven years.
Shine – Natural Haircare rejuvenation
Shine used a patented vaporization process and formulation to clean, treat and improve the appearance of the hair and scalp. In addition to removing the residue, the treatments balanced the hair’s pH levels, added body and shine, defined curls, and strengthened and protected hair from further damage. The Company believed that the Shine treatment was user friendly, requiring the user to connect the Shine capsule to a designated tube, place the attached cap on their head and sit for a 10-minute treatment. There was no need to rinse or shampoo following the treatment. The treatment was expected to cleanse the scalp and leave the hair shiny and manageable. According to a report published by Mordor Intelligence, the global hair care was valued at $95.45 billion in 2018 and was expected to reach $116 billion by 2024, registering a compound annual growth rate (CAGR) of 3.35%. The Company’s products were all based on its proprietary and patented vaporization platform, which was developed over a period of seven years.
| 5 |
Sales and Marketing
While the vaporizer for Novokid® was designed to be a one-time purchase, the head cap and the capsules were designed to be sold separately based on the “razor-blade” business model (the initial sale of the introductory kit accompanied by the recurring sales of the capsules and head cap). However, the Company nevertheless failed to meet its sales forecasts.
Production
The Company manufactured its products through third party manufacturers in Israel and China. The Novokid® vaporizer was manufactured in China by a local manufacturer, which also handled assembly, integration and quality assurance for the vaporizer and manufactured the cap and the ancillary components of the Novokid® kit. The Novokid® treatment capsules were manufactured and filled in Israel by third party contractors. The Company’s board of directors decided during 2019 to suspend all all production.
Research and Development
The Company incurred $404 thousand on research and development during the past two years, $115 thousand in 2019 and $289 thousand in 2018. The Company’s board of directors decided during 2019 to suspend all research and development activity.
Intellectual Property
Below is the list of patents registered to Novomic:
| Patents | Jurisdiction | Each patent’s relevance to the program | Date and status of registration | |||
| EP 2 438 830 B1 | EU | Treating lice with gaseous compounds in airtight space. | Approved on July 16, 2014 | |||
| US 9/307820 B2 | U.S. | Treating lice with gaseous compounds in airtight space. | Approved on April 12, 2016 | |||
| US 15/438842 | U.S. | Treating an object with gaseous compounds in an airtight space. | February 22, 2017 * | |||
| US 62661868 | U.S. | A capsule for the vaporization of liquid | April 24, 2018 * |
* Under approval process.
Government Regulation
The Company’s head lice treatment was subject to regulation by and approval from CE (which approval was obtained) and the FDA (which approval was not obtained). The Company failed to obtain FDA approval for its Novokid® product.
Employees
As of December 31, 2019, Novomic engaged 3 employees on a part-time (50%) basis, one of them being Novomic’s Chief Financial Officer Tali Dinar, and and two service providers, one of them being Idan Traistman in the role of Chief Executive Officer, and one being Shlomi Arbel in the role of legal advisor.
Novomic was subject to Israeli labor laws and regulations with respect to its employees located in Israel. These laws and regulations principally concerned matters such as pensions, paid annual vacation, paid sick days, length of the workday and workweek, minimum wages, overtime pay, insurance for work-related accidents, severance pay and other conditions of employment. Novomic’s employees were not represented by a labor union. Novomic considered its relationship with its employees to be good. At the time of the Novomic Divestment, Novomic had not experienced any work stoppages.
| 6 |
The Company has newly appointed directors, Chief Executive Officer and Chief Financial Officer.
Website
The Company’s website address is http://www.techcareltd.com/. It is expected that this will be updated in the near future to www.citrine-global.com.
Description of the New Business – Citrine Global, Corp.

Following the recent change of control over the Company, we started a new business. Our vision is to be a powerhouse for high-growth technology companies via our business and financial expertise. To better align our name with the new business, we decided to change the name of the Company to Citrine Global, Corp. We filed a preliminary Schedule 14(C) in connection with the name change and expect the name change to take effect on or about May 28, 2020. On or about the same time, we expect to also start trading under a new ticker symbol. We will file a current report on Form 8-K once the new name and new ticker symbol become effective.
We are focused on creating value and implementing expansion strategies for growth-stage technology companies.
We aim to support local and global expansion of our client companies. We plan to bolster high-growth technology companies via an array of services, with the ability to customize them to each company’s needs - from assistance with strategic business planning to solving real estate-related and finance issues.
We offer multi-strategy solutions combining strategic marketing, business development, real estate and asset management services and financing solutions. Such wide spectrum of services is targeted at helping create an integrated strategy that supports our client companies in achieving their local and global expansion ambitions.
We seek to work proactively and collaboratively with our clients in order to allow them to scale quickly and achieve their milestones.
We believe the health, wellness and food tech fields are demonstrating high growth potential and are therefore primarily focused on these sectors. We plan on empowering innovative companies to become global leaders and improve the health and quality of life of as many people as possible worldwide.
| 7 |
Strategy
Multi-Strategy Solutions:
We offer a mix of business development services, asset and real estate support and financing solutions to help our client companies achieve their growth potential.
Potential client companies we review will receive financing after successful completion of due diligence and evaluation of legal, IP, financial, technological, business, equity/collateral secured loans, shares, sales, assets and real estate aspects in order to minimize risk.
The Company seeks to work proactively and collaboratively to achieve the best possible results. Through our offices and partners around the world, we believe we have the platform to achieve our goals. Our global network of partners and advisers has vast experience in working with start-ups and growth-stage companies helping them create strategic growth plans and present unique, strategic partnership options using a global professional network.
The Company’s partners and managers are all experienced investors and top-level managers that held high level positions in leading companies. They place at the Company’s disposal their network, experience and expertise and offer deep industry know-how in emerging technology markets, to achieve the growth goals and global success our client companies strive for.
The Company’s services range from assistance with strategic business planning to operational execution and financing, customized for the needs of each client company.
Business Development and Consulting:
Business development, creating synergistic partnerships, assisting managements build a strategy and set milestones, assist in finding M&A targets and in paving the way for a public offering.
We assist our client companies with:
● The Business – assisting the company management in building strategic analysis, business modelling, sales strategies, brand positioning, process development, and milestones for global success.
● Optimize Product Strategy - we bring marketing and industry experts to perfect product strategies.
● Ramp up Sales Force - having scaled businesses globally, our team assists in further sales ramp-up.
● Expand Globally - assisting the company management in building strategy and milestones for global success.
● Preparation for Investment - support with financial valuations, preliminary negotiations for investment, mergers, IPO and more.
● Local and International Networking - market development with the support of our partners and business network, we help our companies access international markets.
● The Board - provide board advisory support and assist in finding the right team.
● The Team - our extensive network allows us to find the right team and recruit top talent.
● Capital Raising - public and private capital raising.
● Public Capital - as a publicly traded company we help with solutions adapted to public trade.
● Streamline Operations - with a strong operational background, we assist in improving operations.
● Scale Infrastructure - we want our businesses to be references in terms of their infrastructure.
● Asset and Real Estate - provide solutions for companies’ real estate and assets to support their local and global expansion.
Real Estate:
Provide solutions for companies’ real estate-related needs, whether it is an office space, a lab or a greenhouse, we will assist the company in finding the right real estate at the right place and provide ongoing management services to these assets - all with the aim to enable our client companies to focus on the core aspects of their business and create value to their shareholders.
Financing:
Assist client companies in finding potential sources of financing for their businesses, whether by connecting them to third party investors or making an investment ourselves, or both.
| 8 |
Selection Criteria and Process:
Growth stage technology companies in the fields of healthcare and wellness with high growth potential.
Selection Criteria:
● The companies’ technology and IP
● The management team
● Financial model, market strategy and growth potential
● Addressable global market, competition and potential for international partnerships, mergers, and strategic investments
● Capability for providing collateral
● Substantial equity and revenue base
● Public companies – an advantage
The Company intends to initially focus on Israeli companies through its Israeli arm, which is being incorporated these days.
Key Target Markets
We primarily target growth-stage technology companies focused on health and wellness solutions.
Health and Wellness Market – Overview:
The health and wellness industry is growing consistently and rapidly on a global scale, consisting of over 10% of the global GDP. Health and wellbeing tops consumer agendas creating addressable target markets of trillions of dollars.1 The digital health market is projected to reach over $500 billion at a CAGR of nearly 30% by 2025, the biotech market is expected to reach $775 billion by 2024, and the wellness market is estimated at $4,500 billion.2
The healthcare industry, and specifically the biotech sector, seeks to solve many of the world’s medical problems.
The wellness industry is defined as products and solutions that enable people to incorporate wellness activities and lifestyles into their daily life.
The industry is divided into the following main categories:
● therapeutics
● pharmaceuticals
● biologics
● botanicals
Improvements in health technology and scientific breakthrough innovations are changing treatment paradigms towards directions of:
● preventative
● diagnostic
● holistic patient care
Democratized knowledge is driving demand for innovative health and wellness products and services across all demographics and geographies.
1 https://www.who.int/health_financing/documents/health-expenditure-report-2019.pdf?ua=1
https://www2.deloitte.com/global/en/pages/life-sciences-and-healthcare/articles/global-health-care-sector-outlook.html
2 https://www.gminsights.com/industry-analysis/digital-health-market
https://globalwellnessinstitute.org/industry-research/2018-global-wellness-economy-monitor/
https://www.grandviewresearch.com/industry-analysis/corporate-wellness-market
| 9 |
Changing consumer behavior and disruptive technologies are enabling the rapid consumerization and personalization of healthcare.
There is an evolution from prescription drugs, doctor-administered diagnostics and medical treatments to a new marketplace centered around the well-being of people as individuals not patients, enabling and improving ‘quality of life’ in ways which can be seamlessly integrated into their daily routines.
For these reasons we decided to place our focus on personalized health and wellness.
Many of these innovations are being driven by a new generation of venture-backed, more consumer-orientated companies, often underserved by the traditional medical and pharma VC marketplace. There are also pronounced market asymmetries between the sources of some of the most important wellness innovations in parts of Europe and Israel and the large consumer-driven marketplaces for these innovations globally.
Health and Wellness Markets - Fields:
Medical Food:
● Vitamins and minerals
● Nutritional supplements
● Food allergies
● Personalized nutrition and functional foods
● Digestion and gut health
● Weight management
● Cannabis edibles
● Plant based alternatives
● Neutronics and personalized nutrition
Medical Cannabis:
● Cannabis plant genetics
● Pharmacological cannabis effects
● Cannabis cultivation methods
● Cannabis-infused edibles
● Cannabis-based medications
● Cannabis products development
● Cannabis wellness solutions development
● Cannabis personalized medicine solutions
Physical and Mental Wellbeing:
● Cognitive/brain wellbeing
● Physical therapies
● Injury prevention
● Relaxation management and meditation
● Brain health and neurosciences
● Mood and stress detection and management
● Hypertension
● Anxiety and depression
Anti-Aging and Improved Longevity:
● Skin health/repair
● Bone/joint health
● Personalized fitness and physical mobility
● Lifestyle management
● Fatigue abatement
● Sleep quality
● Pain management
● Mental alertness and dementia abatement
| 10 |
Consumer/Digital Healthcare:
● Preventive and personalized fitness tracking
● Continuous health monitoring and biofeedback
● Point of care testing and screening devices
● Personalized big data and e-health analytics
● Unregulated or minimally regulated wearables, implants
● Post hospital/surgery monitoring
Health and Wellness Markets - Trends and Drivers:
● Health and wellness industry drivers/trends turned into investment opportunities.
● Deregulation of healthcare industry: devolution from hospitals-to-clinics-to-self.
● Technical innovation driving change: consumerization, digitization and democratization of wellbeing.
● Increased awareness of food and nutrition: new generation of functional and personalized foods.
● Cognitive health just as important as physical health: alternative remedies and improved awareness.
● Increased lifespan places huge demands on current systems: anti-aging, lifestyle management, quality of life.
● New consumers’ preferences and behavior: non-surgical, nonprescription, self-administered, self-testing.
● New business models and connected ways of making payments: insurance coverage includes more wellbeing.
● New regulations allowing cannabis infused medications, products and edibles.
● Recent COVID-19 pandemic has brought attention and budgets to developing solutions answering market needs for treatment, prevention and every-day life in this new situation.
Geographies
The Company opens opportunities with an insider’s entree into fast-growing industries with access to strategic investment opportunities. All this under a credible institutional quality platform.
The Company provides solutions to companies from Israel, USA, Canada, Europe and around the world through subsidiaries and local teams and professionals in each region.
The Company’s network of business partners permits access to industry pioneers and leaders allowing for more efficient avenues to create, discover, and assess opportunities.
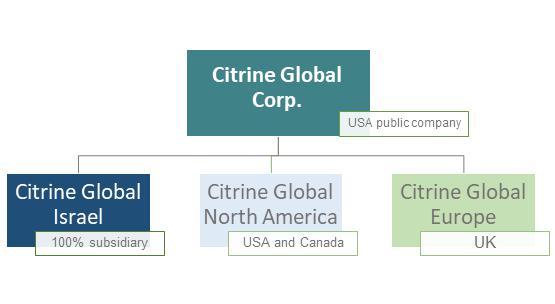
| 11 |
● Israel is truly a ’start-up nation’ and has global leaders in almost any category of technology-driven innovation covered by the Company. 3
● Europe has very active health and wellness innovation clusters in the Netherlands, UK, France, Nordic regions and Germany in particular.
● North America: - the USA is the market leader in health and wellness innovations and leads the world in M&A activity in this area for mature companies with proven revenues.
In December 2019, a strain of novel coronavirus (COVID-19) causing respiratory illness emerged in the city of Wuhan in the Hubei province of China, and in January 2020, the World Health Organization declared the COVID-19 outbreak a public health emergency. The COVID-19 has spread to many countries and is impacting the markets globally. Many countries, states and municipalities have enacted quarantining regulations which severely limit the ability of people to move and travel, and require non-essential businesses and organizations to close their physical offices. The situation created by COVID-19 worldwide has made it difficult and even impossible to meet with different investors, parties and partners. The company managed to adapt to the situation and built an alternative plan in a short time. Since it was difficult and even impossible to travel specifically to New York and Europe, and since the company directors and executives are based in Israel, Citrine Global took the decision to focus on Israel as first step, via its fully-owned subsidiary Citrine Global Israel Ltd.
Citrine Global Israel Ltd
Citrine Global Israel targets Israeli startups and technology companies, and in particular public companies, in the fields of healthcare, wellness, foodtech and medical cannabis.
About Israel - the “Startup Nation” 4:
● Israel has earned the nickname “Startup Nation” for a very good reason: with a population of around 8.5 million, it has the largest number of startups per capita in the world, around one startup per 1,400 people. This phenomenon has caught the eyes of companies with global reach and global aspirations.
● The hi-tech ecosystem in Israel is currently focusing its attention on research and development in the areas of healthcare and biotech, including solutions for COVID-19 and the medical cannabis plant for medical purposes.
● Israel is known for its academic research yielding world renown innovations and Nobel prize winners.
● In addition, the government recognizes the role of the high-tech industry as a main economic catalyst and supports innovations via funding and other models.
● Israel, as small as it may be, has attracted the interest of the world’s major technology companies, which have set up R&D centres in Israel.
Citrine Global Israel Ltd – Strategy:
Citrine Global Israel offers a unique, independent strategy that covers the whole spectrum of services and financing options to ensure the success of its chosen client companies, combining working capital financing, business escort, technological consulting services, real estate and infrastructure services for companies and a global network of experts and business contacts in the relevant fields.
Citrine Global Israel Ltd – Professional Ecosystem:
Citrine Global Israel has a team of serial entrepreneurs and leading business people and a network of top scientists, researchers and industry leaders, targeting to create an eco-system to promote its client companies towards success.
Citrine Global Israel leverages the knowledge and experience of Citrine S A L High Tech and Citrine Biotech investment funds that have been operating for years in the Israeli start up market and have long term experience in investing in and promoting many startup companies.
3 https://apex.aero/2019/05/22/startup-nation-israel-become-silicon-valley
4 ibid.
| 12 |
Citrine Global Israel also leverages the knowledge and experience of WealthStone Group, which specializes in real estate and hedge funds, and Neto Group, which specializes in insurance and financial planning consultancy.
Citrine Global Israel Ltd - Target Market: Medical Foods:
The medical foods market covers fields including: vitamins and minerals; nutritional supplements; food allergies; personalized nutrition and functional foods; digestion and gut health; weight management; cannabis edibles; plant based alternatives; neutronics and personalized nutrition.
The global medical foods market is expected to be worth $30.4 billion by 2027, growing from $18.4 billion in 2019 at a CAGR of 6.3%. 5
Medical food market drivers include:
● Rise in geriatric population
● Growing incidences of chronic diseases
● Increasing awareness regarding clinical nutrition amongst patients and healthcare professionals
In the past, meal replacements were mainly consumed by the elderly or the ill, frequently suffering from nutritional deficiencies. This has changed in recent years, with the marketing of meal replacements increasingly targeting healthy adults.
In addition, we see the emergence of cannabis-enhanced health edibles and drinks, that is expected to continuously grow by more than 250% by 2021. 6
Citrine Global Israel Target Market: Regulated Medical Cannabis:
The medical cannabis market covers fields including: cannabis plant genetics; pharmacological cannabis effects; cannabis cultivation methods; cannabis-infused edibles; cannabis-based medications; cannabis products development; cannabis wellness solutions development; cannabis personalized medicine solutions.
Medical cannabis solutions have been approved for medical use in many countries and have been shown to benefit more than 40 serious medical conditions, including:
● Cancer
● Multiple sclerosis
● Parkinsons
● Epilepsy
● Chronic pain
● Post trauma
● Chronic digestive problems, Crohn’s Disease
● Anxiety and sleep disorders
● Concentration and memory problems
● Tourette Syndrome
Medical cannabis in Israel:
● The State of Israel is currently focusing its attention on research and development on the cannabis plant for medical purposes.
● Research into the cannabis plant began in Israel in the 1960s, when Prof. Rafael Meshulam first discovered the main components of the cannabis plant, a discovery that was a world breakthrough in the study of the plant at the time.
● Research into the cannabis plant in Israel has continued ever since, in academic and research institutions. In recent years, many studies have been conducted in the field of medical cannabis in Israel. Israel is considered a world center in the field of cannabis research and treatment.
5 https://www.grandviewresearch.com/industry-analysis/medical-foods-market
6 https://www.grandviewresearch.com/industry-analysis/medical-foods-market
https://www.grandviewresearch.com/press-release/global-medical-foods-mark
| 13 |
● The cannabis plant is still not permitted for research in the USA, is still restricted under US federal law, and is only recently increasingly studied in European countries.
● As a result, Israel has an advantage in the field, relative to the rest of the world, and in academic knowledge on the medical potential of the cannabis plant in treating diseases such as cancer, epilepsy, and childhood autism.
● The world’s major drug companies have already begun to express an interest in Israeli research, as well as the big challenge involved in registering patents and intellectual property for drugs using the cannabis plant, which is a complex plant with hundreds of active ingredients.
● Around 100 cannabis–related startups are currently operating in Israel.
Medical Cannabis Global Market Size:
● In the world, there are over 40 countries that allow legal use of medical cannabis and the medical cannabis industry is also expanding to the wellness and medical foods sectors with cannabis incorporated into a variety of edibles, pills, spray products, transdermal patches, supplements, salves, ointments and lotions.
● Legal medical cannabis products sales grew 45.7% to $14.9 billion in 2019. This worldwide growth estimate reflects the highest annual growth rate to date. As a result of expected growth ArcView Group has updated their 2024 forecast to $42.7 billion in worldwide legal cannabis sales. 7
● In addition, we see the emergence of the cannabis-enhanced health edibles and drinks market, that is expected to continuously grow by more than 250% by 2021. 8
Asset and real estate services for the health and medical cannabis industry:
● The healthcare and medical cannabis industry creates attractive opportunities to invest in the industrial real estate sector with a focus on regulated medical-use cannabis facilities.
● Healthcare and medical cannabis companies specifically need infrastructure and assets that are licensed and guarded according to various regulations, involve long-term rentals, and more.
● Citrine has built a model adapted to these companies’ needs, covering innovation centers, laboratories, pharmacies, and clinics.
The Company at this time intends to carry out its cannabis-related activity through its Israeli subsidiary only, and not the US parent company, and to be involved, and engage with client companies that are involved, in cannabis-related activities only in countries where the activity has been authorised under all appliable laws. The Company does not at this time intend to be involved, either directly or indirectly, in cannabis-related activity in the United States, in light of the federal-level restrictions in place at this time.
Citrine Global, Corp.’s primary shareholders

7 https://www.businesswire.com/news/home/20200130005274/en/Global-Cannabis-Market-Hit-42.7-Billion-2024
8 https://www.grandviewresearch.com/industry-analysis/medical-foods-market
https://www.grandviewresearch.com/press-release/global-medical-foods-mark
| 14 |
WealthStone Group
WealthStone Group specializes in alternative investments and operates in various fields with extensive financial knowledge and experience. WealthStone Group has real estate funds, hedge funds and technology investments funds and manages more than half a billion US dollars in investments in Israel.
Wealthstone introduces private investors to a world of investments which until now was reserved to an exclusive group, to allow investors to benefit from diversified alternative investments, with strong collateral and attractive returns.
Wealthstone has a variety of products with a wide range of investment periods, risk and security levels, so that each investor is matched with the most suitable investment.
The world of alternative investments is multi-faceted, with a wide range of investment opportunities that tend to be quite confusing for those who are unfamiliar with the field.
The management team and the funds’ GP partners specialize in each fund’s area, covering real estate, technologies, hedge funds and financing, and they are supported by top professional consultants in the respective fields, among them, appraisers, engineering companies, legal advisers, and other experts in each sector.
Wealthstone Real Estate:
Wealthstone Real Estate deals with financing and lending for projects in the real estate sector, urban renewal, removal-construction, and projects requiring equity and senior debt financing. It is one of the largest companies in Israel for financing renewal projects through limited partnerships in which it serves as a general partner. It is ranked by DUN’S 100 among the leading 100 companies in Israel’s real estate sector. DUN’S 100 is a professional, objective, and reliable guide based on fixed, defined, and measurable criteria according to various market sectors.
Citrine S A L technology investment funds
Citrine S A L technology investment funds is part of Wealthstone’s private equity activity for investments in the high-tech and biotech sectors.
Citrine S A L technology funds invest in high potential Israeli startup companies that own transformational technologies, leading a unique, independent investment strategy with a professional team and a global network of first-class partners and advisors.
Citrine S A L operates through limited partnerships, including Citrine S A L High Tech Ltd and Citrine S A L Biotech Ltd, offering a wide array of investment opportunities to private investors, for a range of investment periods.
The funds operate in various fields of technology investment including:
● Partnerships for investing in high-tech companies.
● Partnerships for investing in biotech companies.
● Partnerships for investing in companies designed for an IPO.
● Along with the financial investment, Citrine S A L provides assistance in building strategies, finding business partners, giving support in financial processes, mergers and acquisitions.
Citrine S AL funds have already invested in several promising Israeli companies including: Nicast, NanoMedic, WellBe, Biocep, Improdia, Intelicanna, IBOT, Cannbit, Novomic, Dario, BSP Medical, ICB Israel-China Fund and more.
The Citrine S A L - ICB Israel-China Fund partnership targets strategic international and Chinese partners interested in investment and commercial cooperation with technology companies. The collaboration covers investment in medical and biomedical companies in order to bring them to China as part of joint ventures.
| 15 |
Additionally, Citrine has models and investments in partnerships that are designed for institutional investors, foreign investors and designated investment groups.
Neto Financial Planning
Neto Financial Planning was founded over 27 years ago and is one of the largest companies in the Israeli private and business financial planning and insurance industry.
Neto has thousands of loyal customers, which it has been accompanying for many years, providing financial advisory services in respects of products with a market worth of over $3 billion.
Neto Financial Planning (Neto) is Israel’s largest financial planning private company. DUN’S 100 has ranked Neto among the leading 100 companies in Israel’s financial planning sector each year since 2018. Neto is the only Israeli broker included in the DUN’S 100 rankings. Neto provides holistic (comprehensive) financial planning for thousands of clients across Israel, through a network which includes financial planners who are licensed pension advisors and an administrative and professional support team.
Neto’s significant scale and experience enable its clients to benefit from a wide variety of investment opportunities, income tax planning and reduction, handling retirees, wills, medical committees, loans, mortgages, review and analysis of their insurance files, elementary insurance, lower costs and access to current and comprehensive knowledge and technologies, in the management of their entire financial lives.
Neto financial planning encompasses the full range of financial needs of every household in Israel including Neto - Financial Planning, Neto - Financial Protection, Neto - Savings and Investment Portfolios for Retirement Planning and Neto -Alternative Investments.
Neto - Alternative Investments: Neto offers its clients a variety of alternative investments that are not directly sensitive to capital markets swings in Israel and globally. The operations in this area are conducted through Wealthstone group (which is owned 50% by Neto), which serves as Neto’s alternative investments arm.
Revenues
We plan on generating revenues from consulting fees, brokering fees, leasing and management services for real-estates assets we own, taking advantage of favorable market conditions to sell real-estate assets, company value appreciation, interest income, investments and more.
Competition
We compete with other more established consulting firms, investment bankers, brokers, real-estate funds, investment firms, online lending sites and tech incubators.
Our business competes primarily in Israel, Europe and North America. We mainly target clients with whom we have existing relationships, either directly or via our partners.
We believe that the experience and contacts of our shareholders, directors and officers and the fact that we offer a wide range of services under one roof will contribute to our competitiveness.
Regulatory Environment
We may need to obtain various regulatory approvals and licenses for real-estate assets we acquire, which may be used by companies engaged in businesses that require certain approvals and licenses for the premises in which they operate (such as laboratories).
| 16 |
Coronavirus Disease 2019 (COVID-19)
In December 2019, a strain of novel coronavirus (COVID-19) causing respiratory illness emerged in the city of Wuhan in the Hubei province of China, and in January 2020, the World Health Organization declared the COVID-19 outbreak a public health emergency. COVID-19 has spread to many countries and is impacting the markets globally. Many countries, states and municipalities have enacted quarantining regulations which severely limit the ability of people to move and travel, and require non-essential businesses and organizations to close their physical offices. We are actively monitoring the situation and we have and will continue to monitor and take actions to abide with all regulatory requirements. We will continue to closely track developments and may take further actions based on regulatory mandates, or that we determine are in the best interests of our team, partners and shareholders. COVID-19 is contributing to a general slowdown in the global economy and may affect our business, results of operations, financial condition and our future strategic plans. Neither the duration nor the spread of the COVID-19 virus can be predicted. At this time, the extent to which the COVID-19 may impact our financial condition or results of operations is uncertain.
Future deterioration or prolonged difficulty in economic conditions could have a material adverse impact on our business, financial position and liquidity.
Future deterioration or prolonged difficulty in economic conditions, as a result of COVID-19 or otherwise, could have a material adverse impact on our business, financial position and liquidity. For example, it could adversely affect our ability to access the liquidity that is necessary to fund operations on terms that are acceptable to us or at all, and could reduce our ability to finance future projects. Financial or other difficulties at our affiliates and partners could negatively affect availability of credit to us in the future.
The Company’s bylaws provide for indemnification of its directors and officers and the purchase of directors and officers insurance at the Company’s expense. This will limit the potential liability of the Company’s directors and officers at a major cost to the Company and hurt the interests of its stockholders.
The Company’s bylaws include provisions that eliminate the personal liability of the directors and officers of the Company for monetary damages to the fullest extent possible under the laws of the State of Delaware or other applicable law. These provisions eliminate the liability of directors and officers to the Company and its stockholders for monetary damages arising out of any violation of a director or officer of his fiduciary duty of due care. Under Delaware law, however, such provisions do not eliminate the personal liability of a director or officer for (i) breach of the director’s or officer’s duty of loyalty, (ii) acts or omissions not in good faith or involving intentional misconduct or knowing violation of law, (iii) payment of dividends or repurchases of stock other than from lawfully available funds, or (iv) any transaction from which the director or officer derived an improper benefit. These provisions do not affect a director’s and officer’s liabilities under the federal securities laws or the recovery of damages by third parties.
| 17 |
Failure in the Company’s information technology systems, including by cybersecurity attacks or other data security incidents, could significantly disrupt its operations.
The Company’s operations depend, in part, on the continued performance of its information technology systems. Its information technology systems are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptions. Failure of its information technology systems could adversely affect its business, profitability and financial condition. Although the Company has information technology security systems, a successful cybersecurity attack or other data security incident could result in the misappropriation and/or loss of confidential or personal information, create system interruptions, or deploy malicious software that attacks the Company’s systems. It is possible that a cybersecurity attack might not be noticed for some period of time. The occurrence of a cybersecurity attack or incident could result in business interruptions from the disruption of the Company’s information technology systems, or negative publicity resulting in reputational damage with its shareholders and other stakeholders and/or increased costs to prevent, respond to or mitigate cybersecurity events. In addition, the unauthorized dissemination of sensitive personal information or proprietary or confidential information could expose the Company or other third-parties to regulatory fines or penalties, litigation and potential liability, or otherwise harm its business.
A certain group of the Company’s stockholders may exert significant influence over its affairs, including the outcome of matters requiring stockholder approval.
As of the date of this Annual Report, a certain group of stockholders, including Ora Meir Soffer (directly and through Beezz Home Technologies Ltd and Citrine S A L Investment & Holdings Ltd) and Yaron Pitaru (directly and through WealthStone Private Equity Ltd and Citrine S A L Investment & Holdings Ltd) and others, collectively own a majority of the issued and outstanding shares of the Company. As a result, such individuals will have the ability, acting together, to control the election of the Company’s directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of the Company, (ii) a sale of all or substantially all of its assets, and (iii) amendments to its certificate of incorporation and bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to the Company’s other stockholders and be disadvantageous to the Company’s stockholders with interests different from those individuals. Certain of these individuals also have significant control over the Company’s business, policies and affairs as officers or directors of the Company. Therefore, you should not invest in reliance on your ability to have any control over the Company.
Shares eligible for future sale may adversely affect the market.
From time to time, certain of the Company’s stockholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act, subject to certain limitations. In general, pursuant to Rule 144, non-affiliate stockholders may sell freely after six months subject only to the current public information requirement. Affiliates may sell after six months subject to the Rule 144 volume, manner of sale (for equity securities), and current public information and notice requirements. Any substantial sales of the Company’s common stock pursuant to Rule 144 may have a material adverse effect on the market price of its common stock.
Delaware law contains provisions that could discourage, delay or prevent a change in control of the Company, prevent attempts to replace or remove current management and reduce the market price of its common stock.
Provisions in the Company’s certificate of incorporation and bylaws may discourage, delay or prevent a merger or acquisition involving the Company that its stockholders may consider favorable. For example, the Company’s certificate of incorporation authorizes its board of directors to issue up to fifty million shares of “blank check” preferred stock. As a result, without further stockholder approval, the board of directors has the authority to attach special rights, including voting and dividend rights, to this preferred stock. With these rights, preferred stockholders could make it more difficult for a third party to acquire the Company. The Company is also subject to the anti-takeover provisions of the Delaware General Corporation Law (“DGCL”). Under these provisions, if anyone becomes an “interested stockholder,” the Company may not enter into a “business combination” with that person for three years without special approval, which could discourage a third party from making a takeover offer and could delay or prevent a change in control of the Company. An “interested stockholder” is, generally, a stockholder who owns 15% or more of the Company’s outstanding voting stock or an affiliate of the Company who has owned 15% or more of the Company’s outstanding voting stock during the past three years, subject to certain exceptions as described in the DGCL.
| 18 |
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
Novomic leases from an unaffiliated third party at a monthly rental of approximately $1,600, office space located at 23 Hamelacha Street, Park Afek, Rosh Ha’ain, Israel. The offices consist of approximately 1,300 square feet. Novomic entered a new lease for a period of one year ending on November 30, 2020, for an office space of approximately 500 square feet and monthly rental of approximately $700, with the option to terminate with 90 days’ prior notice. Since the change of control of the Company, its headquarters have been at 3 HaMelacha Street, Tel Aviv, 6721503, Israel. The address of the Company’s registered office in the State of Delaware is c/o Business Filings Incorporated, 108 West 13th St., City of Wilmington, County of Newcastle, Delaware 19801.
The Company knows of no active or pending legal proceedings against the Company, nor of any proceedings that a governmental authority is contemplating against the Company.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
| 19 |
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
The Company’s common stock is traded in the United States on the OTCQB market under the ticker symbol “TECR.” Any over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
Holders of our Common Stock
As of May 7, 2020, the Company had 107 registered stockholders holding 494,721,815 shares of common stock.
Dividends
Since the Company’s inception, it has not declared nor paid any cash dividends on its capital stock and the Company does not anticipate paying any cash dividends in the foreseeable future. Its current policy is to retain any earnings in order to finance its operations. Its Board of directors will determine future declarations and payments of dividends, if any, in light of the then-current conditions it deems relevant and in accordance with applicable corporate law.
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides certain aggregate information with respect to the Company’s shares of common stock that as of December 31, 2019 were issuable under its equity compensation plans in effect as of December 31, 2019. During the first quarter of 2020, in connection with the Techcare Transaction, all options to purchase shares of the Company were waived and cancelled except for options to purchase 311,544 shares of common stock.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (1) | Weighted-average exercise price of outstanding options, warrants and rights (2) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in first column) (3) | |||||||||
| Equity compensation plans approved by security holders | ___ | ___ | ___ | |||||||||
| Equity compensation plans not approved by security holders | 2,363,712 | $ | 0.0001 | |||||||||
| Total | 2,363,712 | $ | 0.0001 | 3,636,288 | ||||||||
| (1) | Represents shares of common stock issuable under our 2017 Employee Incentive Plan and upon exercise of outstanding options to purchase 2,363,712 shares of common stock. |
| (2) | The weighted average remaining term for the expiration of remaining stock options is 2 years. |
| (3) | Represents shares of common stock available for future issuance under equity compensation plans. “Equity Compensation Plan” under Item 11 hereof contains a description of the material features of the 2017 Employee Incentive Plan and the 2018 Stock Incentive Plan. |
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
| 20 |
ITEM 6. SELECTED FINANCIAL DATA
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Liquidity and Capital Resources
The Company’s balance sheet as of December 31, 2019, reflects total assets of approximately $257 thousand consisting mainly of cash and cash equivalents in the amount of approximately $18 thousand, inventory in the amount of approximately $35 thousand, other receivables in the amount of approximately $19 thousand and property and equipment net, in the amount of approximately $156 thousand. As of December 31, 2018, the balance sheet reflects total assets of approximately $1,158 thousand consisting mainly of cash and cash equivalents in the amount of approximately $475 thousand, inventory in the amount of approximately $249 thousand, other receivables in the amount of approximately $177 thousand and property and equipment net, in the amount of approximately $161 thousand. The decrease is related mainly to the decrease in the inventory balance by approximately $214 thousand and the decrease in the cash and cash equivalents balance by approximately $457 thousand.
As of December 31, 2019, the Company had total current liabilities of approximately $360 thousand consisting of accounts payable and accrued expenses in the amount of approximately $224 thousand, and notes payable in the amount of approximately $123 thousand. As of December 31, 2018, the Company had total current liabilities of approximately $385 thousand consisting of approximately $231 thousand in accounts payable and accrued expenses and notes payable in the amount of approximately $80 thousand.
As of December 31, 2019, the Company had negative working capital in the amount of approximately $278 thousand, compared to positive working capital in the amount of approximately $573 thousand at December 31, 2018.
The Company’s total liabilities as of December 31, 2019 and 2018 were approximately $368 thousand and $417 thousand respectively.
During the twelve months ended December 31, 2019, the Company used approximately $1,226 thousand in its operating activities. This resulted in an overall net loss of approximately $1,874 thousand.
During the twelve months ended December 31, 2018, the Company used approximately $2,386 thousand in its operating activities. This resulted in an overall net loss of approximately $2,157 thousand.
During the year ended December 31, 2019, the Company’s investing activities required approximately $11 thousand due to the purchase of property, plant and equipment, approximately $30 thousand for a severance pay fund and approximately $8 thousand in long-term deposit. This compared to approximately $98 thousand due to the purchase of property and approximately $15 thousand for a severance fund during the year ended December 31, 2018.
During the twelve months ended December 31, 2019, the Company’s financing activities provided it with approximately $757 thousand through the issuance of common stock, as compared to approximately $2,372 thousand in the prior year provided through the issuance of common stock.
| 21 |
Results of Operations
Year ended December 31, 2019 as compared to the year ended December 31, 2018
During the twelve months ended December 31, 2019, the Company generated $149 thousand in revenues, compared to $251 thousand in 2018.
The Company’s research and development expenses decreased to $115 thousand comprised of ongoing research and development expenses during the twelve months ended December 31, 2019, compared to approximately $289 thousand during the prior year.
The Company’s marketing, general and administrative expenses during the year ended December 31, 2019, were $1,536 thousand compared to $2,004 thousand during the prior year. The decrease was mainly due to decrease in payroll and consulting.
During the twelve months ended December 31, 2019, the Company incurred financial expenses of $38 thousand, as compared to financial expenses of $30 thousand during the prior year. The increase in financial expenses was mainly due to exchange rates.
As a result of the above, the Company incurred a net loss of approximately $1,874 thousand during the twelve months ended December 31, 2019 as compared to a net loss of approximately $2,157 thousand in 2018.
Year ended December 31, 2018 as compared to the year ended December 31, 2017
During the twelve months ended December 31, 2018, the Company generated $251 thousand in revenues, compared to no revenues in 2017. Revenues were recorded for the first time from the sales of its product, Novokid®.
The Company’s research and development expenses increased to $289 thousand comprised of ongoing research and development expenses during the twelve months ended December 31, 2018, compared to approximately $282 thousand during the prior year, an increase of approximately $6 thousand or 2%.
The Company’s marketing, general and administrative expenses during the year ended December 31, 2018, were $2,003 thousand compared to $2,463 thousand during the prior year. The decrease was mainly due to decrease in payroll and consulting.
During the twelve months ended December 31, 2018, the Company incurred financial expenses of $30 thousand, as compared to financial income of $19 thousand during the prior year. The increase in financial expenses was mainly due to exchange rates.
As a result of the above, the Company incurred a net loss of approximately $2,157 thousand during the twelve months ended December 31, 2018 as compared to a net loss of approximately $2,858 thousand in 2017.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Tabular disclosure of contractual obligations
Not applicable.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
| 22 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
TECHCARE CORP.
CONSOLIDATED FINANCIAL STATEMENTS
| Report of Independent Registered Public Accounting Firm | 24 |
| Consolidated Financial Statements for the Years Ended December 31, 2019 and 2018 | |
| Consolidated Balance Sheets | 25 |
| Consolidated Statements of Operations and Comprehensive Loss | 26 |
| Consolidated Statements of redeemable convertible preferred stock and Stockholders’ Equity (capital deficiency) | 27 |
| Consolidated Statements of Cash Flows | 28 |
| Notes to Consolidated Financial Statements | 29-45 |
| 23 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of TechCare Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of TechCare Corp. and its subsidiary (the “Company”), as of December 31, 2019 and 2018, and the related consolidated statements of operations and comprehensive loss, of redeemable convertible preferred stock and stockholders’ equity (capital deficiency) and of cash flows for the years then ended, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Subsequent Event
As discussed in Note 1 to the consolidated financial statements, the Company has entered into an agreement to sell 90% of its subsidiary’s shares to one of the Company’s existing shareholders.
| /s/ Kesselman & Kesselman | |
| Certified Public Accountants (Isr.) | |
| A member firm of PricewaterhouseCoopers International Limited | |
| Tel Aviv, Israel | |
| May 11, 2020 |
We have served as the Company’s auditor since 2020.
| 24 |
Consolidated Balance Sheets
As of December 31, 2019, and 2018
| December 31, 2019 | December 31, 2018 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 17,636 | $ | 474,715 | ||||
| Inventory | 34,513 | 248,912 | ||||||
| Accounts receivable | 9,141 | 13,462 | ||||||
| Inventory subject to refund | 2,159 | 44,529 | ||||||
| Other receivables | 18,522 | 176,583 | ||||||
| Total current assets | 81,971 | 958,201 | ||||||
| Non-current assets: | ||||||||
| Severance pay fund | - | 27,258 | ||||||
| Right of use asset | 14,502 | - | ||||||
| Long-term deposits | 4,699 | 11,366 | ||||||
| Property and equipment, net | 155,655 | 161,401 | ||||||
| Total non-current assets | 174,856 | 200,025 | ||||||
| Total assets | $ | 256,827 | $ | 1,158,226 | ||||
| Liabilities and Stockholders’ Equity (capital deficiency) | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | 223,841 | $ | 231,311 | ||||
| Notes payable | 123,494 | 80,026 | ||||||
| Refund liability | 4,998 | 73,464 | ||||||
| Current maturities of long-term lease liability | 7,295 | - | ||||||
| Total current liabilities | 359,628 | 384,801 | ||||||
| Non-current liability: | ||||||||
| Lease liability | 7,962 | - | ||||||
| Liability for severance pay | - | 31,971 | ||||||
| Total liabilities | 367,590 | 416,772 | ||||||
| Redeemable convertible preferred stock: | ||||||||
| Redeemable convertible Series A preferred stock, par value $0.0001 per share 12,413,794 shares authorized; 10,344,828 and 0 issued and outstanding at December 31, 2019 and December 31, 2018, respectively | 300,000 | - | ||||||
| Stockholders’ Equity (capital deficiency): | ||||||||
| Common stock, par value $0.0001 per share, 500,000,000 shares authorized; 35,449,398 and 33,212,036 shares issued and outstanding at December 31, 2019 and 2018, respectively | 3,545 | 3,322 | ||||||
| Additional paid-in capital | 10,042,496 | 9,329,419 | ||||||
| Stock to be issued | 30,000 | 30,000 | ||||||
| Preferred stock (excluding redeemable Series A preferred stock), par value $0.0001 per share, 37,586,206 shares authorized at December 31, 2019 and 10,000,000 at December 31, 2018; none issued and outstanding at December 31, 2019 and 2018 | - | - | ||||||
| Accumulated deficit | (10,602,292 | ) | (8,728,157 | ) | ||||
| Accumulated other comprehensive income | 115,488 | 106,870 | ||||||
| Total stockholders’ equity (capital deficiency) | (410,763 | ) | 741,454 | |||||
| Total liabilities and stockholders’ equity (capital deficiency) | $ | 256,827 | $ | 1,158,226 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 25 |
Consolidated Statements of Operations and Comprehensive Loss
For the Years ended December 31, 2019 and 2018
| For the years ended | ||||||||
| December 31, 2019 | December 31, 2018 | |||||||
| Revenues | 149,085 | 251,417 | ||||||
| Cost of revenues | 158,514 | 218,639 | ||||||
| Provision for slow moving inventory | 176,183 | - | ||||||
| Gross profit (loss) | (185,612 | ) | 32,778 | |||||
| Research and development expenses | 114,560 | 288,813 | ||||||
| Marketing, general and administrative expenses | 1,535,576 | 2,003,709 | ||||||
| Change in fair value of option liability | - | (132,470 | ) | |||||
| Operating loss | 1,835,748 | 2,127,274 | ||||||
| Financial expenses ,net | 38,387 | 29,800 | ||||||
| Loss before income taxes | 1,874,135 | 2,157,074 | ||||||
| Net loss | 1,874,135 | 2,157,074 | ||||||
| Accretion of redeemable convertible preferred shares for Beneficial Conversion Feature | 300,000 | - | ||||||
| Net loss attributable to common stockholders | 2,174,135 | 2,157,074 | ||||||
| Net loss per common stock: | ||||||||
| Basic | $ | (0.06 | ) | $ | (0.07 | ) | ||
| Diluted | $ | (0.06 | ) | $ | (0.07 | ) | ||
| Weighted average number of common stock outstanding: | ||||||||
| Basic | 37,473,278 | 32,476,194 | ||||||
| Diluted | 37,473,278 | 32,607,583 | ||||||
| Comprehensive loss: | ||||||||
| Net loss | 1,874,135 | 2,157,074 | ||||||
| Other comprehensive income attributable to foreign currency translation | (8,618 | ) | (2,093 | ) | ||||
| Comprehensive loss | 1,865,517 | 2,154,981 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 26 |
Consolidated Statements of redeemable convertible preferred stock and Stockholders’ Equity (capital deficiency)
Years ended December 31, 2019 and 2018
Redeemable | Accumulated | |||||||||||||||||||||||||||||||||||
convertible | Additional | Stock | Other | Total | ||||||||||||||||||||||||||||||||
preferred stock | Common Stock | Paid-in | to be | Accumulated | Comprehensive | Stockholders’ | ||||||||||||||||||||||||||||||
| stock | amount | stock | amount | Capital | issued | Deficit | Income | equity | ||||||||||||||||||||||||||||
| Balance at December 31, 2017 | 25,835,401 | $ | 2,584 | $ | 6,945,151 | 30,000 | (6,571,083 | ) | $ | 104,777 | $ | 511,429 | ||||||||||||||||||||||||
| Issuance of common stock and warrants | 7,376,635 | 738 | 2,371,262 | - | - | - | 2,372,000 | |||||||||||||||||||||||||||||
| Foreign currency translation differences | - | - | - | - | - | 2,093 | 2,093 | |||||||||||||||||||||||||||||
| Stock-based compensation to non – employees | - | - | 13,006 | - | - | - | 13,006 | |||||||||||||||||||||||||||||
| Net loss for the year | (2,157,074 | ) | - | (2,157,074 | ) | |||||||||||||||||||||||||||||||
| Balance at December 31, 2018 | 33,212,036 | 3,322 | 9,329,419 | 30,000 | (8,728,157 | ) | 106,870 | 741,454 | ||||||||||||||||||||||||||||
| Issuance of Common stock and warrants | 2,237,364 | 223 | 457,232 | - | - | - | 457,455 | |||||||||||||||||||||||||||||
| Waiver of fee by related parties | - | - | 243,377 | - | - | 243,377 | ||||||||||||||||||||||||||||||
| Issuance of redeemable Convertible Preferred Shares and related Beneficial Conversion Feature | 10,344,828 | 300,000 | 300,000 | |||||||||||||||||||||||||||||||||
| Accretion of redeemable preferred shares for Beneficial Conversion Feature | 300,000 | (300,000 | ) | (300,000 | ) | |||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | 12,468 | - | - | - | 12,468 | |||||||||||||||||||||||||||||
| Other comprehensive income | - | - | - | - | - | 8,618 | 8,618 | |||||||||||||||||||||||||||||
| Net loss for the year | - | - | - | - | (1,874,135 | ) | - | (1,874,135 | ) | |||||||||||||||||||||||||||
| Balance at December 31, 2019 | 10,344,828 | 300,000 | 35,449,400 | 3,545 | 10,042,496 | 30,000 | (10,602,292 | ) | 115,488 | (410,763 | ) | |||||||||||||||||||||||||
| 27 |
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2019 and 2018
| For the Years Ended | ||||||||
| December 31, 2019 | December 31, 2018 | |||||||
| Cash flow from operating activities: | ||||||||
| Net loss | $ | (1,874,135 | ) | $ | (2,157,074 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 30,680 | 24,322 | ||||||
| Change in fair value of option liability | - | (132,470 | ) | |||||
| Waiver of fee by related parties | 243,377 | - | ||||||
| Stock-based compensation | 12,468 | 13,006 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Note payable | 42,866 | - | ||||||
| Other receivables | 171,938 | (93,637 | ||||||
| Inventory subject to refund | 44,488 | (47,466 | ||||||
| Inventory | 56,730 | (218,694 | ) | |||||
| Inventory Impairment | 176,183 | |||||||
| Accounts payable and accrued expenses | (23,591 | ) | 138,495 | ) | ||||
| Liability for severance pay | (34,744 | ) | 10,521 | |||||
| Refund liability | (71,837 | ) | 76,561 | |||||
| Net cash used in operating activities | (1,225,577 | ) | (2,386,436 | ) | ||||
| Cash flow from investing activities: | ||||||||
| Purchases of property and equipment | (10,977 | ) | (97,992 | ) | ||||
| Severance pay fund | 30,180 | (14,818 | ) | |||||
| Long-term deposits | 7,739 | - | ||||||
| Net cash provided by (used in) investing activities | 26,942 | (112,810 | ) | |||||
| Cash flow from financing activities: | ||||||||
| Proceeds from issuance of redeemable convertible preferred shares | 300,000 | - | ||||||
| Proceeds from issuance of common stock and warrants | 457,455 | 2,372,000 | ||||||
| Net cash provided by financing activities | 757,455 | 2,372,000 | ||||||
| Effect of exchange rates on cash and cash equivalents | (15,899 | ) | 12,143 | |||||
| Net decrease in cash and cash equivalents | (457,079 | ) | (115,103 | ) | ||||
| Cash and cash equivalents - beginning of year | 474,715 | 589,818 | ||||||
| Cash and cash equivalents - end of year | $ | 17,636 | $ | 474,715 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 28 |
NOTE 1: NATURE OF OPERATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A. Nature of operations
TechCare Corp. (“Techcare” or the “Company”) was incorporated under the laws of the State of Delaware on May 26, 2010. The Company’s common stock is traded in the United States on the OTCQB market under the ticker symbol “TECR.”
On February 8, 2016, the Company signed a Merger Agreement with Novomic Ltd. (“Novomic”), a private company incorporated under the laws of the state of Israel. The closing of the merger took place on August 9, 2016 pursuant to which Novomic became a wholly-owned subsidiary of the Company.
Novomic was incorporated as a private company in Israel in 2009. Since inception, Novomic has been a technology company engaged in the design, development and commercialization of a unique delivery platform utilizing vaporization of various natural compounds for multiple health, beauty and wellness applications. Novomic’s delivery platform is proprietary and patented.
During 2019, the Company’s Board of Directors explored strategic alternatives to enhance stockholder value, which the Company had previously reported that it might include future acquisitions, a merger with another company, a potential sale of certain assets, including the Company’s wholly owned subsidiary Novomic, or the sale of the Company as a public shell company.
Until December 31, 2019, the Company had incurred accumulated losses of approximately $10.6 million, and based on the projected cash flows and Company’s cash balance, the Company’s management was of the opinion that without further fundraising it would not have sufficient resources to enable it to continue advancing its activities including the development, manufacturing, and marketing of its products. The Company’s management had plans including the continued commercialization of the Company’s products, to continue taking cost reduction steps and securing sufficient financing, however, it was also exploring strategic alternatives as detailed above.
During 2019, the Company made various efforts to increase sales of its product Novokid® in Europe and Israel, and to receive U.S. Food and Drug Administration (“FDA”) approval to sell in the USA. However, the Company’s sales efforts, including in Israel through Super Pharm, and in the Netherlands, and through Amazon UK, did not yield the forecasted outcomes. The Company’s USA OEM agreement failed to result in FDA approval or USA sales. The Company failed to enter into additional engagements with distributors in Europe and Latin America. The Company also failed to launch its product Shine. In January 2019, the Company entered into a joint venture agreement with a Chinese partner for the formation of a Chinese joint venture intended to focus on the development of a comprehensive and broad range of health, wellness, beauty and home products for customers by utilizing its patented technology of vaporization of natural and plant-based compounds. While a Chinese entity was established in accordance with the joint venture agreement, only part of the amount envisaged to be invested in the Company by ICB Biotechnology Investments Ltd in connection with the joint venture was invested. On September 19, 2019 its nominated director Ningzhou Zhang resigned from the board of directors of the Company. Refer also to Note 9.
On November 21, 2019, in light of the above, and after the board of directors of the Company reached the conclusion that the Company would not be able to successfully commercialize any products or secure sufficient financing, the Company entered into a Memorandum of Understanding with Citrine S A L Investment & Holdings Ltd., which provided for the issuance and sale of a number of shares resulting in Citrine S A L Investment & Holdings Ltd and/or its designee(s) holding 95% of the fully diluted capital stock of the Company, and the sale by the Company of 90% of its shares in Novomic.
| 29 |
On January 6, 2020, definitive agreements were executed for the sale of 90% of the shares in Novomic to Traistman Radziejewski Fundacja Ltd., which is expected to be completed during May 2020, and for the issuance and sale of a number of shares equal after the issuance to 95% of the fully diluted capital stock of the Company to Citrine S A L Investment & Holdings Ltd. and a group of related persons and entities (the “TechCare Transaction”). Traistman Radziejewski Fundacja Ltd. is controlled by Oren Traistman, which is one of the Company’s shareholders and a director of the Company until February 27, 2020.
On February 23, 2020, the Company entered into an agreement amending and restating the January 6, 2020 agreement concerning the TechCare Transaction, which provided for the issuance and sale of the shares in stages. Pursuant to this agreement, on February 27, 2020 and March 5, 2020, additional shares were issued, causing Citrine S A L Investment & Holdings Ltd and its group of related persons and entities to become owners of 90.6% of the fully diluted capital stock of the Company, and resulting in a change of control of the Company. The Company is working to complete the issuance and sale of a number of shares equal after the issuance to up to 95% of the share capital of the Company by amending its Certificate of Incorporation to increase its authorized share capital and then issuing additional shares.
Citrine S A L Investment & Holdings Ltd. has furnished the Company with an irrevocable letter of obligation to financially support the Company until June 30, 2021 and allow it to be operational as planned and budgeted throughout this period.
During Q1 2020 the Company continued selling the Novokid® product both through online and physical sales channels, including through its own website, Amazon.uk, and Super Pharm’s physical outlets, with its principal markets being the United Kingdom, Europe and Israel.
Novomic is not presented as held for sale, although the held-for-sale criteria are met, because the Company concluded that the disposal of 90% of Novomic’s shares is comprised of substantially almost all of the Company’s net assets and operations and since the separate presentation of such a significant portion of the entity’s net assets as a single line item on the balance sheet would not be meaningful to financial statement users. The amounts of the remaining net assets of the Company, which are not part of the held-for-sale subsidiary, are immaterial to these consolidated financial statements.
At the time of the issuance of these financial statements, Novomic was a technology company engaged in the design, development and commercialization of a unique delivery platform utilizing vaporization of various natural compounds for multiple health, beauty and wellness applications. Its delivery platform was proprietary and patented. Its product offering included Novokid® - an innovative home use device which vaporizes a natural, plant-based, pesticides, and silicone-free compound that effectively treats head lice and eggs. Following its soft launch of Novokid® in the Netherlands, the Company expanded its distribution network and launched Novokid in Israel during late May 2018 through Super Pharm, Israel’s largest and leading drugstore chain. The launch was accompanied by a radio and digital brand awareness and marketing campaign and supported by Meditrend, its Israeli distributor, specializing in health and wellness products while representing leading brands. The Company showcased Novokid® and met potential additional distributors and partners at CPhI Worldwide, a renowned and leading pharma tradeshow held in Madrid during October 2018.
The Company’s new business activity comprises creating value and implementing expansion strategies for growth-stage technology companies. The Company believes the health, wellness and food tech fields are demonstrating high growth potential, and is therefore primarily focused on these sectors. The Company aims to support local and global expansion of such companies via an array of services customized to each company’s needs. The Company offers multi-strategy solutions combining strategic marketing, business development, real estate and asset management services and financing solutions.
B. Summary of significant accounting policies
Basis of Presentation
The consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”).
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Techcare, and its subsidiary, Novomic. All intercompany balances and transactions have been eliminated in consolidation.
| 30 |
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates using assumptions that affect the reported amounts of assets and liabilities and related disclosures at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates, and such differences may have a material impact on the Company’s financial statements. As applicable to these consolidated financial statements, the most significant estimate relates to the assumptions underlying stock-based compensation, refund liability, inventories measurement including inventory subject to refund and accrual for slow moving inventory, and the recoverability of long-lived assets.
Coronavirus Disease 2019 (COVID-19) is a factor which may cause actual results to differ from estimates. COVID-19 is contributing to a general slowdown in the global economy and may affect the Company’s business, results of operations, financial condition and its future strategic plans. At this time, the extent to which the COVID-19 may impact the Company’s financial condition or results of operations is uncertain.
Functional Currency and Foreign Currency Translation and Transactions.
The currency of the primary economic environment in which the operations of the Company and its subsidiary were conducted is the New Israeli Shekel (“NIS”).
The presentation currency of the financial statements is the U.S. dollar. Assets and liabilities are translated at year-end exchange rates, while revenues and expenses are translated at actual exchange rates during the year. Differences resulting from translation are presented in equity, under accumulated other comprehensive income (loss). Gains and losses arising from foreign currency transactions of monetary balances denominated in non-functional currencies are reflected in financial income (expense), net in the consolidated statements of operations and comprehensive loss.
Financial expenses (income), net in the consolidated statements of operations and comprehensive loss comprised mainly of exchange rate differentials.
Cash and Cash Equivalents
All highly liquid investments with original maturities of three months or less when acquired, that are not restricted as to withdrawal or use, are considered to be cash or cash equivalents.
Accounts receivable
The balance of accounts receivable includes amounts due from distributors for products sold in the ordinary course of business, net of commissions earned. If payment is due based on payment terms with one year or less, they are classified as current assets. If not, they are presented as non-current assets.
Inventories
Inventory is measured at the lower of cost or net realizable value. The cost is determined on the “first in-first out” basis. Inventory costs consist of materials, direct labor and overhead. Net realizable value is an estimated selling price in the ordinary course of business less applicable selling expenses. Provisions for potentially obsolete or slow-moving inventory are made based on management’s analysis of inventory levels and historical obsolescence.
The Company periodically assesses inventory slow moving and reduces the carrying value by an amount equal to the differences between its cost and the net realizable value. The net realizable value is primarily estimated based on future demand forecasts, as well as, historical sales trends, product life cycle status and product development plans. The Company provided inventory write-downs for slow moving in amounts of $176 thousands and $ 0 as of December 31, 2019 and 2018, respectively.
Property, plant and Equipment
Property, plant and equipment is stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, or in the case of leasehold improvements, the shorter of the lease term (including any renewal periods, if appropriate) or the estimated useful life of the asset. Repairs and maintenance are charged to expense during the financial period in which they are incurred.
| 31 |
Depreciation lives are as follows:
| Years | ||
| Computers and software | 3 | |
| Electronic equipment | 7 | |
| Office furniture and equipment | 14-15 | |
| Machinery and equipment | mainly 5 |
Leasehold improvements are amortized by the straight line method over the term of the lease, which is shorter than the estimated useful life of the improvements.
Impairment of long-lived assets
Long-lived assets held and used by the Company are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. In the event that the sum of the expected future undiscounted cash flows expected to be generated by the long-lived assets is less than the carrying amount of such assets, an impairment charge would be recognized and the assets would be written down to their estimated fair values. During the years ended 2019 and 2018, no impairment was recorded.
Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset, or paid to transfer a liability, in an orderly transaction between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that are developed using market data, such as publicly available information about actual events or transactions, and that reflect the assumptions that market participants would use when pricing the asset or liability. Unobservable inputs are inputs for which market data are not available and that are developed using the best information available about the assumptions that market participants would use when pricing the asset or liability. The fair value hierarchy categorizes into three levels. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity can access at the measurement date. Level 2 inputs include inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. Level 3 inputs are unobservable inputs for the asset or liability. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities (Level 1 inputs) and the lowest priority to unobservable inputs (Level 3 inputs). Categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
Revenue Recognition
Effective January 1, 2018, the Company adopted a new accounting standard related to the recognition of revenue in contracts with customers. Since the Company had no revenues prior to January 1, 2018, the adoption of the standard did not impact the Company’s financial statements.
The Company derives revenues from sales of its Novokid product directly or indirectly through its distributors in the United Kingdom, Europe and Israel.
The Company determines revenue recognition through the following steps:
| ● | Identification of the contract, or contracts, with a customer. | |
| ● | Identification of the performance obligations in the contract. | |
| ● | Determination of the transaction price. | |
| ● | Allocation of the transaction price to the performance obligations in the contract. | |
| ● | Recognition of revenue when, or as, the Company satisfies a performance obligation. |
| 32 |
Revenue is measured as the amount of consideration expected to be received in exchange for transferring goods to the end customer or to the distributor. The Company also considers products that might be returned mostly based on the terms stipulated in the agreements with its distributors. The Company recognizes the amount received or receivable that is expected to be returned as a refund liability, representing its obligation to return the clients’ consideration. The Company also defers the associated costs of the refund liability and recognize it as inventory subject to refund.
The Company reports revenue net of any revenue based taxes assessed by governmental authorities that are imposed on and concurrent with specific revenue-producing transactions.
Revenue from products are recognized when the customer or the distributor has obtained control of the goods (for the Company’s arrangements, this is at a point in time of revenue recognition) based on the shipping terms. The Company recognizes revenue on sales to distributors upon shipment of the goods, when the distributor has economic substance apart from the Company and the distributor is considered the principal for the transaction with the end-user client.
Research and Development
Research and development expenses are expensed as incurred, and consist primarily of personnel, facilities, equipment and supplies for research and development activities.
Advertising costs
Advertising expenses are expended as incurred and were approximately $99 thousand and $369 thousand for the years ended December 31, 2019 and 2018, respectively.
Loss per Share
Loss per share is based on the loss that is attributed to the stockholders holding common stock divided by the weighted average number of common stock outstanding and fully vested outstanding options granted to employees and non-employees with an exercise price of $0.0001 for the reported periods.
For purposes of the calculation of the diluted loss per share, the Company adjusts the loss that is attributed to the holders of the Company’s common stock, and the weighted average number of common stock assuming conversion of all of the dilutive potential stock using the treasury stock method.
The potential stock are taken into account only if their effect is dilutive (increases loss per share).
Concentration of credit risks
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and accounts receivable. The Company’s cash and cash equivalents were invested with major banks in Israel and the United States. Generally, these investments could be redeemed upon demand and the Company believed that the financial institutions that held the Company’s cash deposits were financially sound and, accordingly, bore minimal risk. The Company’s accounts receivable were mainly derived from sales to its Israeli distributor.
Beneficial Conversion Feature (“BCF”)
When the Company issues redeemable convertible preferred stock, if the stock price is lower than the effective redeemable value on the measurement date, the conversion feature is considered “beneficial” to the holder. If there is no contingency, this difference is treated as issued equity and is credited to additional paid in capital. The resulting discount on the redeemable convertible preferred shares is accreted over the remaining period through the earlier date of redemption of those shares, using the effective yield method.
| 33 |
Stock-Based Compensation
Stock-Based Compensation to employees, officers and directors
The Company measures and recognizes compensation expenses for its equity classified stock-based awards to employees, including stock-based option awards under its plan based on estimated fair values on the grant date. The Company calculates the fair value of stock-based option awards on the grant date using the Black-Scholes option pricing model. The option-pricing model requires a number of assumptions, of which the most significant are the stock price volatility and the expected option term. For the years ended December 31, 2019 and 2018, the volatility was based on the historical stock volatility of several peer companies, as the Company has limited trading history to use the volatility of its own common stock. The expected option term is calculated using the simplified method, as the Company has no historical share option exercise experience which can provide a reasonable basis to estimate its expected option term. The interest rate for periods within the expected term of the award is based on the U.S. Treasury yield curve in effect at the time of the grant. The Company’s expected dividend rate is zero, since the Company does not currently pay cash dividends on its stock and does not anticipate doing so in the foreseeable future. Each of the above factors require the Company to use judgment and make estimates in determining the percentages and time periods used for the calculation. If the Company were to use different percentages or time periods, the fair value of stock-based option awards could be materially different. The Company recognizes stock-based compensation cost for option awards based on the straight line method over the requisite service period.
Stock-Based Compensation to non-employees – Options and Warrants
In June 2018, the Financial Accounting Standards Board (“FASB”) issued ASU 2018-07, “Compensation-Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting” (“ASU 2018-07”). The Company early adopted ASU 2018-07 commencing on January 1, 2018, with no material impact on its consolidated financial statements. Prior to the adoption of ASU 2018-07, stock options issued to consultants and other non-employees, as compensation for services provided to the Company, were accounted for based upon the fair value of the options. The fair value of the options granted were measured on a final basis at the end of the related service period and were recognized over the related service period using the straight line method. After the adoption of ASU 2018-07, the measurement date for non-employee awards is the date of the grant. The compensation expense for non-employees is recognized, without changes in the fair value of the award, over the requisite service period, which is the vesting period of the respective award.
Income Taxes
The Company and its subsidiary were subject to income taxes in the jurisdictions in which they operated. The Company’s provision for income taxes is based on statutory income tax rates in the tax jurisdictions where it operates, permanent differences between financial reporting and tax reporting, and available credits and incentives.
Deferred taxes are determined utilizing the “asset and liability” method based on the estimated future tax effects of temporary differences between the carrying amount and tax bases of assets and liabilities under the applicable tax laws, and on effective tax rates in effect when the deferred taxes are expected to be settled or realized. Deferred taxes for each jurisdiction are presented as a noncurrent net asset or liability, net of any valuation allowances.
The Company may incur an additional tax liability in the event of intercompany dividend distributions by its subsidiary. Such additional tax liability in respect of this foreign subsidiary has not been provided for in these financial statements as it was the Company’s policy to permanently reinvest the subsidiary earnings and to consider distributing dividends only in connection with a specific tax opportunity that may arise.
The Company recognizes liabilities for uncertain tax positions only if it is more likely than not that the tax position will be sustained on examination by the tax authority based on the merits of the position. It is inherently difficult and subjective to estimate such amounts, as the Company has to determine the probability of various possible outcomes. The Company reevaluate these uncertain tax positions based on factors including, but not limited to, changes in facts or circumstances, changes in tax law or an effective settlement of audit issues. Such a change in recognition or measurement would result in the recognition of a tax benefit or an additional charge to a tax provision.
Taxes that would apply in the event of disposal of investments in a foreign subsidiary have not been taken into account in computing the deferred taxes.
| 34 |
Valuation Allowances
Valuation allowances are provided unless it is more likely than not that all or a portion of the deferred tax asset will be realized. In the determination of the appropriate valuation allowances, the Company considers future reversals of existing taxable temporary differences, the most recent projections of future business results, prior earnings history, carryback and carry forward and prudent tax strategies that may enhance the likelihood of realization of a deferred tax asset. Assessments for the realization of deferred tax assets made at a given balance sheet date are subject to change in the future, particularly if earnings of a subsidiary are significantly higher or lower than expected, or if the Company takes operational or tax positions that could impact the future taxable earnings of a subsidiary. Given the Company and subsidiary losses, a full valuation allowance has been provided with respect to its deferred tax assets.
Comprehensive Income (loss)
The Company complies with ASC 220, “Comprehensive Income,” which establishes rules for the reporting and display of comprehensive income (loss) and its components. The Company reports the financial impact of translating its foreign subsidiary financial statements from functional currency to reporting currency as a component of other comprehensive income (loss).
Contingent Liabilities
Management applies the guidance in Accounting Standards Codification (“ASC”) 450-20-25 when assessing losses resulting from contingencies. If the assessment of a contingency indicates that it is probable that a loss has been incurred and the amount of the liability can be reasonably estimated, then the Company would record an accrued expense in the Company’s financial statements. If the assessment indicates that a potential loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss, if determinable, is disclosed.
Loss contingencies considered to be remote by management are generally not disclosed unless material or they involve guarantees in which case the guarantee would be disclosed.
Leases
In February 2016, the Financial Accounting Standards Board issued Accounting Standards Update 2016-02 “Leases.” The guidance establishes a right-of-use (“ROU”) model that requires a lessee to recognize an ROU asset and lease liability on the balance sheet for all leases. Leases are classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the income statement. The guidance became effective on January 1, 2019. A modified retrospective transition approach is required, applying the new standard to all leases existing at the date of initial application. The Company adopted the new accounting standard Accounting Standards Codification 842 “Leases,” and all the related amendments, on January 1, 2019 and used the standard’s effective date as the Company’s date of initial application. Consequently, financial information was not updated and the disclosures required under the new standard are not provided for dates and periods before January 1, 2019.
The new lease standard provides practical expedients for an entity’s ongoing accounting. The Company elected the short-term lease recognition exemption for all leases with a term shorter than 12 months. This means, for those leases, the Company does not recognize ROU assets or lease liabilities, including not recognizing ROU assets or lease liabilities for existing short-term leases of those assets in transition. The Company also elected the practical expedient to not separate lease and non-lease components for all of the Company leases.
On January 1, 2019, the Company recognized ROU assets of approximately $83 thousand and lease liabilities of approximately $83 thousand for its operating leases of real estate and vehicles. The adoption of this standard did not have a material impact on the Company’s consolidated statements of income and consolidated statements of cash flows.
| 35 |
NOTE 2: NEW ACCOUNTING PRONOUNCEMENTS
In June 2016, the FASB issued an ASU that supersedes the existing impairment model for most financial assets to a current expected credit loss model. The new guidance requires an entity to recognize an impairment allowance equal to its current estimate of all contractual cash flows the entity does not expect to collect. The ASU also requires that credit losses relating to available-for-sale debt securities will be recorded through an allowance for credit losses. The new guidance is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. Early adoption is permitted. This guidance will be effective on January 1, 2020, and the adoption of this guidance will not have a material impact on the Company’s consolidated financial statements.
NOTE 3: OTHER RECEIVABLES
Other receivables consisted of the following:
| December 31, 2019 | December 31, 2018 | |||||||
| US dollars | ||||||||
| Prepaid expenses | $ | 6,651 | $ | 51,110 | ||||
| VAT Institutions and other | 11,871 | 121,971 | ||||||
| Advanced for suppliers | - | 3,502 | ||||||
| $ | 18,522 | $ | 176,583 | |||||
NOTE 4: PROPERTY PLANT AND EQUIPMENT
Property, plant and equipment, consists of the following:
| December 31, 2019 | December 31, 2018 | |||||||
| US dollars | ||||||||
| Computer and software | $ | 19,396 | $ | 15,840 | ||||
| Electronic equipment | 15,337 | 11,815 | ||||||
| Office furniture and equipment | 10,075 | 9,290 | ||||||
| Leasehold improvements | 5,100 | 4,702 | ||||||
| Machinery and equipment | 207,095 | 185,394 | ||||||
| $ | 257,003 | $ | 227,041 | |||||
| Accumulated depreciation and amortization | (101,348 | ) | (65,640 | ) | ||||
| Property and equipment, net | $ | 155,655 | $ | 161,401 | ||||
Depreciation and amortization expenses were approximately $30 thousand and $24 thousand in the years ended December 31, 2019 and 2018, respectively.
NOTE 5: ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consists of the following:
| December 31, 2019 | December 31, 2018 | |||||||
| US dollars | ||||||||
| Accounts payable | $ | 83,794 | $ | 63,935 | ||||
| Related parties – refer also to Note 14 | 3,547 | 25,506 | ||||||
| Accrued expenses | 15,105 | 39,449 | ||||||
| Professional services | 80,867 | 66,378 | ||||||
| Payroll liabilities | 40,528 | 36,043 | ||||||
| $ | 223,841 | $ | 231,311 | |||||
The carrying amount of accounts payable approximates its fair value.
| 36 |
NOTE 6: NOTES PAYABLE
As of December 31, 2019 and December 31, 2018, notes payable in the aggregate amount of NIS 426,795 and NIS 307,700, respectively ($123,494 and $80,026, respectively) were outstanding. The notes have no stated maturity date and bear no interest but rather are payable immediately upon demand of the lenders.
As of December 31, 2019, the carrying amount of the notes payable approximates their fair value.
NOTE 7: LIABILITY FOR SEVERANCE PAY
Israeli labor laws generally require severance payments upon dismissal of an employee or upon termination of employment in certain other circumstances.
Severance pay liability with respect to Israeli employees is calculated pursuant to the Israeli Severance Pay Law based on the most recent salary of the employees multiplied by the number of years of employment as of the balance sheet date. The Company records an expense for the increase in its severance liability, net of earnings (losses), from the related severance pay fund. The liability is presented on an undiscounted basis as a long-term liability.
The Company’s liability for all of its Israeli employees is covered for by monthly deposits of severance pay funds. The value of the deposited funds is based on the cash surrender value of these policies and includes profits (or losses) accumulated through the balance sheet date. The deposited funds may be withdrawn only upon the fulfillment of the obligations pursuant to the Israeli Severance Pay Law or labor agreements. The amounts funded are presented separately in the balance sheet as a severance pay fund.
NOTE 8: STOCKHOLDERS’ EQUITY
Share capital
Common stock confers upon their holders the right to receive notice to participate and vote in general meetings of the Company, and the right to receive dividends if declared. Also, upon completion of the Merger, the Company’s stockholders approved the authorization of ten million (10,000,000) shares of preferred stock, and in 2019 the authorization of a further forty million (40,000,000) shares of preferred stock, which may be issued in one or more classes or series, having such designations, preferences, privileges and rights as the Board of Directors (the “Board”) may determine. On August 20, 2019, the Company filed the Certificate of Designation with the Delaware Secretary of State to designate the rights and preferences of up to 12,413,794 shares of Series A Redeemable Convertible Preferred Stock. The Company issued a total of 10,344,828 shares of Series A Redeemable Convertible Preferred Stock in 2019 in exchange for an aggregate amount of $300,000, all of which were converted to common stock on a one-for-one basis in the first quarter of 2020 in connection with the TechCare Transaction.
Redeemable convertible preferred stock
Each share of Preferred Stock shall have a par value of $0.0001 per share.
The Redeemable Convertible Preferred Shares confer on the holders thereof all rights accruing to holders of Ordinary Shares in the Company, and in addition bear the following rights:
Dividends
Holders shall be entitled to receive, and the Corporation shall pay, dividends as and when paid to the holders of Common Stock of the Company on an as-converted basis. Dividends are non-cumulative.
Voting Rights
Except as otherwise provided herein or as otherwise required by law, the Preferred Stock shall have no voting rights. However, as long as any shares of Preferred Stock are outstanding, the Corporation shall not, without the affirmative vote of the Holders of a majority of the then outstanding shares of the Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Preferred Stock or alter or amend the Certificate of Designation, (b) authorize or create any class of stock ranking as to dividends, redemption or distribution of assets upon a liquidation, dissolution or winding up of the Corporation, whether voluntary or involuntary, that is senior to the Preferred Stock, (c) amend its certificate of incorporation or other charter documents in any manner that adversely affects any rights of the Holders, (d) increase the number of authorized shares of Preferred Stock, or (e) enter into any agreement with respect to any of the foregoing.
| 37 |
Liquidation
Upon any liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary, and assuming no exercise of the Optional Redemption by the Holder, the Holders shall be entitled to receive distributions out of the assets, whether capital or surplus, of the Corporation on a pari passu basis with the holders of Common Stock. The Corporation shall mail written notice of any such Liquidation, not less than 45 days prior to the payment date stated therein, to each Holder.
Conversion
Each share of Preferred Stock shall be convertible, at any time and from time to time from and after the Original Issue Date at the option of the Holder thereof, into that number of shares of Common Stock determined by dividing the Stated Value of such share of Preferred Stock by the Conversion Price.
Optional Redemption
Holder of Redeemable convertible preferred stock may, under certain terms as specified in the certificate of designation of preferences, deliver irrevocable election to have the Company redeem some or all of the then outstanding Preferred Stock, for cash in an amount equal to the applicable portion of the optional redemption amount. Redemption amount shall not exceed the stated value of the preferred shares in total amount of $300,000. As such, the Company presented the Redeemable convertible preferred stock A as a mezzanine line item on the consolidated balance sheet.
During the year ended December 31, 2018, the Company entered into several agreements with certain investors, pursuant to which the Company raised an aggregate amount of $2,372,000, at purchase price per share ranging from $0.261 to $0.387, with warrants granted with an exercise price of $0.60, which expired during a period ranging from June 17, 2019 to November 13, 2019, as detailed below.
During the year ended December 31, 2019, the Company entered into several agreements with certain investors, pursuant to which the Company raised an aggregate amount of $775,000, as follows:
| (a) | The Company raised $250,000 from the issuance and sale of 957,854 shares of Common Stock at purchase price per share of $0.261. | |
| (b) | In addition, the Company raised $225,000 from the issuance and sale to certain investors of shares of 1,229,508 Common Stock at purchase price per share of $0.183, concurrently with which the Company granted to those investors warrants to purchase 375,001 shares of Common Stock at a price per share of $0.60 for additional aggregate consideration of $225,000. The warrants had expiry date April 20, 2020 but were waived and cancelled in the first quarter of 2020 in connection with the TechCare Transaction | |
| (c) | In addition, the Company raised $300,000 from the issuance and sale to certain investors of 10,344,828 shares of Series A Redeemable Convertible Preferred Stock at purchase price per share of $0.029, concurrently with which the Company granted to those investors warrants to purchase 500 shares of Series A Redeemable Convertible Preferred Stock at a price per share of $0.60 for additional aggregate consideration of $300,000. The warrants to purchase 400 shares of Series A Redeemable Convertible Preferred Stock had expiry date August 29, 2020 and the warrants to purchase the other 100 shares of Series A Redeemable Convertible Preferred Stock had expiry date November 17, 2020; however, all the warrants were waived and cancelled in the first quarter of 2020 in connection with the TechCare Transaction. |
| 38 |
| Warrants granted | Exercise price | Expiration date | ||||
| 70,000 | $ | 0.60 | June 17, 2019 (expired) | |||
| 416,667 | $ | 0.60 | June 27, 2019 (expired) | |||
| 416,667 | $ | 0.60 | August 7, 2019 (expired) | |||
| 83,333 | $ | 0.60 | August 7, 2019 (expired) | |||
| 166,667 | $ | 0.60 | August 7, 2019 (expired) | |||
| 50,000 | $ | 0.60 | August 21, 2019 (expired) | |||
| 416,667 | $ | 0.60 | October 27, 2019 (expired) | |||
| 833,333 | $ | 0.60 | November 13, 2019 (expired) | |||
| 375,001 | $ | 0.60 | April 29, 2020* | |||
| 400,000 | $ | 0.60 | August 29, 2020* | |||
| 100,000 | $ | 0.60 | November 17, 2020* | |||
*cancelled in the first quarter of 2020 in connection with the TechCare Transaction.
Stock-Based Compensation to employees, officers and directors
Stock based awards are accounted for using the fair value method in accordance with ASC 718, “Shared Based Payment.” The Company’s primary type of stock-based compensation consisted of stock options to directors, employees and officers. The Company uses Black-Scholes option pricing model in valuing options.
During 2017, the Company granted to certain employees options to purchase shares of the Company’s common stock for an exercise price of $0.0001. The options granted in 2017 were fully vested on the date of the grant and exercisable into the Company’s common stock at a 1:1 ratio for 2.5 years from the date of the grant.
The following assumptions were applied in determining the options’ fair value on their grant date:
| Risk-free interest rate | 1.54 | % | ||
| Expected shares price volatility | 70 | % | ||
| Expected option term (years) | 2.5 | |||
| Dividend yield | - |
The Company based the risk-free interest rate on the U.S. Treasury yield curve. The expected term in years represents the period of time that the awards granted were expected to be outstanding. The assumption for dividend yield is zero because the Company has not historically paid dividends nor does it expect to do so in the foreseeable future. The volatility was based on the historical stock volatility of several peer companies, as the Company has limited trading history to use the volatility of its own common stock.
A summary of the stock option activity for employees and directors for the years ended December 31, 2019 and 2018:
| Number of Options | Weighted Average Exercise Price | |||||||
| U.S Dollar | ||||||||
| Options outstanding at December 31, 2019 | 1,842,647 | 0.0001 | ||||||
| Options exercisable at December 31, 2019 | 1,842,647 | 0.0001 | ||||||
| Options outstanding at December 31, 2018 | 2,640,334 | 0.0001 | ||||||
| Options exercisable at December 31, 2018 | 2,640,334 | 0.0001 | ||||||
| Options outstanding at December 31, 2017 | 2,640,334 | 0.0001 | ||||||
| Options exercisable at December 31, 2017 | 2,640,334 | 0.0001 | ||||||
| 39 |
Stock-Based Compensation to non-employees – Options and Warrants
The Company early adopted ASU 2018-07 commencing July 1, 2018, with no impact on its consolidated financial statements. Prior to the adoption of ASU 2018-07, stock options issued to consultants and other non-employees, as compensation for services provided to the Company, were accounted for based upon the fair value of the options. The fair value of the options granted were measured on a final basis at the end of the related service period and were recognized over the related service period using the straight line method. After the adoption of ASU 2018-07, the measurement date for non-employee awards is the date of the grant. The compensation expense for non-employees is recognized, without changes in the fair value of the award, over the requisite service period, which is the vesting period of the respective award.
In the second quarter of 2018, as part of consulting agreements, the Company granted options to non-employees, as follows:
| 1) | Options to a related party and a member of the Company’s advisory Board, exercisable to purchase 83,393 shares of common stock of the Company, at an exercise price of $0.0001 per share. The options were to vest as follows: 25% of the options will be exercisable on December 1, 2018, and the remaining 75% will be considered exercisable at the end of each subsequent three-month period thereafter, over the course of 12 quarters. These options expired during 2019 due to termination of the engagement with the related party. | |
| 2) | Options to a related party and member of the Company’s advisory Board, exercisable to purchase 83,393 shares of common stock of the Company, at an exercise price of $0.0001 per share. The options were to vest as follows: 25% of the options will be exercisable on January 1, 2019, and the remaining 75% will be considered exercisable at the end of each subsequent three-month period thereafter, over the course of 12 quarters. These options expired during 2019 due to termination of the engagement with the related party. | |
| 3) | Options to a related party, a member of the Company’s Board and its advisory Board, exercisable to purchase 436,349 shares of common stock of the Company, at an exercise price of $0.387 per share. The options would have become vested in accordance with the following vesting periods: 33.33% of the options will be exercisable on January 1, 2019, and the remaining 66.67% would have been considered exercisable at the end of each subsequent three-month period thereafter, over the course of 8 quarters. The options were waived and cancelled, by mutual consent, on November 14, 2018, following the resignation of the aforesaid related party from the Board. |
The following assumptions were applied in determining the options’ fair value on their grant date:
| Risk-free interest rate | 2.65%-2.85 | % | ||
| Expected shares price volatility | 70 | % | ||
| Expected option term (years) | 5 | |||
| Dividend yield | - |
In 2017, the Company granted options to non-employees, as follows:
| 1) | During January 2017 the Company granted to a non-employee warrants to purchase 100,000 of the Company’s common stock at an exercise price of $1.50 per share, exercisable for a period of 24 months commencing on the date of the agreement, which were fully vested on the date of the grant. The warrants expired on January 21, 2019. | |
| 2) | During March 2017, the Company granted to non-employees options to purchase 521,065 of the Company’s common stock for an exercise price of $0.0001. The options granted were fully vested on the date of the grant and exercisable into the Company’s common stock at a 1:1 ratio for 5 years from the date of the grant. These options were waived and cancelled in the first quarter of 2020 in connection with the TechCare Transaction. |
| 40 |
The following assumptions were applied in determining the options’ fair value on their grant date:
| Risk-free interest rate | 1.54 | % | ||
| Expected shares price volatility | 70 | % | ||
| Expected option term (years) | 2-5 | |||
| Dividend yield | - |
The Company based the risk-free interest rate on the U.S. Treasury yield curve. The expected term in years represents the period of time that the awards granted are expected to be outstanding. The assumption for dividend yield is zero because the Company has not historically paid dividends nor does it expect to do so in the foreseeable future. The volatility was based on the historical stock volatility of several peer companies, as the Company has limited trading history to use the volatility of its own common stock.
A summary of the stock option activity for non-employees for the years ended December 31, 2019 and 2018:
| Number of Options | Weighted Average Exercise Price | |||||||
| U.S Dollar | ||||||||
| Options outstanding at December 31, 2017 | 621,065 | 0.2416 | ||||||
| Granted | 603,135 | 0.2800 | ||||||
| Cancelled | (436,349 | ) | 0.3870 | |||||
| Options outstanding at December 31, 2018 | 787,851 | 0.1905 | ||||||
| Options exercisable at December 31, 2018 | 641,913 | 0.2338 | ||||||
| Cancelled | (266,786 | ) | 0.562 | |||||
| Options outstanding at December 31, 2019 | 521,065 | 0.0011 | ||||||
| Options exercisable at December 31, 2019 | 521,065 | 0.0011 | ||||||
Stock-based compensation expenses in the amount of $12,468 and $13,006 are included in the Company’s statements of operations and comprehensive loss for the years ended December 31, 2019 and 2018, respectively, were recorded in marketing, general and administrative expenses.
All options and warrants granted to employees and non-employees, except for options outstanding to purchase 311,544 shares of common stock of the Company, were waived and cancelled in the first quarter of 2020 in connection with the TechCare Transaction.
On March 28, 2019 the Company filed a Registration Statement on Form S-8 to register 5,328,185 shares of Common Stock of the Company in connection with awards under the 2017 Plan and the 2018 Plan.
NOTE 9: OEM DISTRIBUTION AGREEMENT
On June 23, 2017, the Company entered into an OEM agreement (the “OEM Agreement”) with a medical device and wellness applications company based in the United States (the “OEM Distributor”), according to which the OEM Distributor would manufacture, distribute and sell the Company’s Novokid head lice treatment products in the United States, Canada, Brazil, Argentina, Costa Rica and Colombia, all on an exclusive basis, pursuant to and in accordance with the terms and conditions set forth in the OEM Agreement, including minimum royalties commitments. The OEM Distributor would be solely responsible for obtaining and maintaining approval from the US Food and Drug Administration (the “FDA”) and would bear all costs related to such approval. The Company, through its OEM Distributor, communicated with the FDA regarding Novokid’s designation as a medical device. An application to the FDA Office of Combination (OCP division) was prepared.
On March 25, 2019, the Company received a notice of termination from the OEM Distributor. Accordingly, the Company did not proceed with the OEM Distributor. No FDA approval was obtained, hence, the Company did not generate any revenues from the OEM agreement.
As part of the OEM Agreement, the OEM Distributor paid a royalty advance of $10,000 and an amount of $140,000 was held in an escrow account, until the Company completes certain milestones, as described in the OEM Agreement. The milestones were not achieved and the $140,000 was not paid to the Company.
| 41 |
Also, as part of the OEM Agreement, the Company granted the OEM Distributor an option to purchase up to 9.09% of the Company’s common stock for a total consideration of up to $900,000, exercisable until January 15, 2018. The option expired on January 15, 2018.
NOTE 10: CHINA JOINT VENTURE AGREEMENT AND SUBSCRIPTION AGREEMENT
On January 21, 2019 the Company entered into a subscription agreement with ICB Biotechnology Investments Ltd. (“ICB”), and Novomic entered into a joint venture agreement with ICB’s controlling shareholder China-Israel Biological Technology Co. Ltd. (“CIB”). In accordance with the subscription agreement the Company issued and sold 957,854 shares of Common Stock to ICB for $250,000 at price per share of $0.261, and the Company and ICB committed to implement a second share subscription on the same terms upon the achievement of certain milestones, including a transfer by Novomic of intellectual property rights to a joint venture entity to be formed pursuant to the agreement between Novomic and CIB. The joint venture entity was formed, but Novomic did not transfer to it intellectual property, and the second share subscription was not implemented. The Company believes none of the parties thereto intends to take further steps in connection with the China joint venture agreement and subscription agreement, and does not believe they will have any effect on the anticipated Novomic Divestment.
NOTE 11: INCOME TAXES
a. Basis of taxation
The Company and its subsidiary were taxed under the domestic tax laws of the jurisdiction of incorporation of each entity (United States and Israel). Losses before taxes on income for the years ended December 31, 2019 and 2018 were as follows:
| Year ended | ||||||||
| December 31, 2019 | December 31, 2018 | |||||||
| US dollars | ||||||||
| Israeli | 1,623,155 | 2,125,364 | ||||||
| Non-Israeli | 250,980 | 31,710 | ||||||
| $ | 1,874,135 | $ | 2,157,074 | |||||
b. Corporate tax rates
The regular corporate tax rate in Israel was 23% in both 2019 and 2018.
On December 22, 2017, President Trump signed into law the Tax Cuts and Jobs Act (the “TCJA”), which among other things, reduced the federal corporate tax rate from 35% to 21%, effective January 1, 2018.
The TCJA has had no impact on the Company’s consolidated financial statements for the years ended December 31, 2019 and 2018.
c. Deferred Tax Assets
The components of the Company’s deferred tax assets as of December 31, 2019 and 2018 were as follows:
| December 31, 2019 | December 31, 2018 | |||||||
| US dollars | ||||||||
| Individual components giving rise to the deferred tax assets are as follows: | ||||||||
| Tax losses carry forwards | $ | 1,959,070 | $ | 1,346,453 | ||||
| Provision for slow moving inventory | 40,522 | - | ||||||
| Research and Development credit carry forwards | 41,417 | 54,908 | ||||||
| Gross deferred tax assets | $ | 2,041,009 | $ | 1,401,361 | ||||
| Valuation allowance | (2,041,009 | ) | (1,401,361 | ) | ||||
| Total deferred tax assets | $ | - | $ | - | ||||
| 42 |
Change in valuation allowance for the year ended December 31, 2019 and 2018 was $639,648 and $370,175, respectively. The entire change was charged to tax expenses to offset the benefit from the recognition of deferred tax assets.
d. Carryforward tax losses
Carryforward tax losses of the Company in the U.S., as of December 31, 2019, amounted to approximately to $751 thousand. The TCJA also repealed the corporate alternative minimum tax for tax years beginning after December 31, 2017. Losses generated prior to January 1, 2018 will still be subject to the 20-year carryforward limitation and the alternative minimum tax. Carryforward tax losses of the Subsidiary as of December 31, 2019 amounted to approximately to approximately $7,725 thousand with no expiration date for these carryforward tax losses.
NOTE 12: LOSS PER SHARE
The following table sets forth the calculation of basic loss per share for the years indicated:
| Year ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| US dollar | ||||||||
| Numerator: | ||||||||
| Net loss attributable to common stockholders’ | $ | 2,174,135 | $ | 2,157,074 | ||||
| Denominator: | ||||||||
| Weighted average number of common stock outstanding | 35,080,566 | 29,313,081 | ||||||
| Weighted average number of fully vested outstanding options with an excessive price of $0.0001 | 2,392,712 | 3,163,113 | ||||||
| 37,473,278 | 32,476,194 | |||||||
| Net loss per common stock: | ||||||||
| Basic | $ | (0.06 | ) | $ | (0.07 | ) | ||
The following table sets forth the calculation of diluted loss per share for the years indicated:
| Year ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| US dollar | ||||||||
| Numerator: | ||||||||
| Net loss attributable to common stockholders’ | $ | 2,174,135 | $ | 2,157,074 | ||||
| Income resulting from change in fair value of option liability, see also note 9 | - | 132,470 | ||||||
| Loss for the year loss for diluted loss per share | 2,174,135 | 2,289,544 | ||||||
| Denominator: | ||||||||
| Weighted average number of common stock outstanding -Diluted: | 37,473,278 | 32,607,583 | ||||||
| Net loss per common stock: | ||||||||
| Diluted | $ | (0.06 | ) | $ | (0.07 | ) | ||
The computation of diluted net loss per share for the year ended December 31, 2019 excluded 10,344,828 preferred stock, because their inclusion would have an anti-dilutive effect on the diluted net loss per share.
| 43 |
NOTE 13: FAIR VALUE OF FINANCIAL INSTRUMENTS
The carrying amount of the Company’s financial instruments, including cash equivalents, accounts receivable and other current assets, accounts payable and accrued liabilities and note payable approximate their fair value, due to their short term nature and their carrying amounts approximates the amounts expected to be received or paid.
A hierarchy has been established for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. The Company accounts for option liability as Level 3 since its inputs are unobservable inputs for the liability.
The following table is a reconciliation of the change for the financial liability where fair value measurement is estimated utilizing Level 3 inputs:
| 2019 | 2018 | |||||||
| US dollar | ||||||||
| Fair value as of January 1, | $ | - | $ | 132,470 | ||||
| Initial recognition of option liability (see Note 8) recognized in statement of operations and comprehensive loss | - | - | ||||||
| Change in fair value recognized in statement of operations and comprehensive loss | - | (132,470 | ) | |||||
| Fair value as of December 31, | $ | - | $ | - | ||||
NOTE 14: COMMITMENTS
| a. | Novomic leased office and warehouse space, under an operating lease. Novomic entered into a new lease for a period of one year ending on November 30, 2020 with the option to terminate with 90 days’ prior notice, and the option to extend for an additional year by giving 90 days’ notice prior to the expiration of the first year. | |
| Office lease payments for the years ended December 31, 2019 and December 31, 2018, under the above-mentioned agreement, were approximately $18.5 thousand and $23 thousand respectively. | ||
| Effective as of the Novomic Divestment, Novomic Ltd and not TechCare Corp. will be solely responsible for all commitments under the lease. |
NOTE 15: RELATED PARTY TRANSACTIONS
| a. | On November 11, 2014, the Company entered into a consulting agreement with Mr. Yossef De-Levy, a member of the Company’s Board. Pursuant to the consulting agreement, Mr. De-Levy received a gross monthly amount of NIS 5,000, which was updated on May 31, 2015 to 10,000 (approximately $2,900). The foregoing payment was in addition to, and independent of, the fee that Mr. De-Levy was entitled to receive for continued services as a member of the Board. In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018, through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on March 16, 2020 and the monthly retainer from December 31, 2019 was waived. |
| 44 |
| b. | On December 31, 2015, the Company entered into a consulting agreement with Zvi Yemini, and with his affiliated entity Y.M.Y Industry Ltd. (“YMY”). Zvi Yemini served as Chairman of the Board of Directors until August 13, 2019, and as a board member until November 14, 2019, and as Chief Executive Office from February 15, 2019 until November 14, 2019. Pursuant to the consulting agreement, Mr. Yemini received a gross monthly amount of NIS 24,000 (approximately $6,200). The foregoing payment was in addition to, and independent of, the fee that Mr. Yemini was entitled to receive for continued services as a member of the Board. On February 22, 2017, the Company signed an amendment to the original agreement with Mr. Yemini and YMY. Pursuant to the amendment, Mr. Yemini’s monthly payment was increased to NIS 45,000 (approximately $13,000) starting February 2017. In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018 through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on November 14, 2019, which was also the effective date of Zvi Yemeni’s resignation as a director of the Company and of his resignation as an officer of the Company. |
| c. | On July 31, 2016, the Company entered into a consulting agreement with Mr. Oren Traistman, who was a member of the Board until February 27, 2020, including Chairman of the Board from August 13, 2019, and acting principal executive officer from November 14, 2019 until February 27, 2020. Pursuant to the consulting agreement, Mr. Traistman received a gross monthly amount of NIS 10,000 (approximately $2,900). In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018 through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on March 16, 2020 and the monthly retainer from December 31, 2019 was waived. |
| d. | On December 31, 2017, the Company entered into a consulting agreement with Mr. Ran Tuttnauer, a member of the advisory Board. Pursuant to the consulting agreement, Mr. Tuttnauer received a gross monthly amount of $2,000. In March 2019, the Company entered into an amendment to the consulting agreement, pursuant to which the monthly retainer was waived commencing on November 15, 2018. In April 2019 the consulting agreement was terminated. The Company recorded the expense against equity. |
| e. | On April 28, 2019, the Company entered into a form of Securities Purchase Agreement with each of Y.M.Y. Industry Ltd. (“YMY”), Traistman Radziejewski Fundacja Ltd. (“TRF”) and Microdel Ltd. (together with YMY and TRF, the “Investors”) relating to an offering of an aggregate of 1,229,508 shares of the Company’s common stock at a purchase price of $0.183 per share for aggregate gross proceeds of approximately $225,000. In addition, the Company granted the Investors an option, for a period of twelve months, to purchase up to an additional 375,001 shares of common stock at a price per share of $0.60, for additional aggregate consideration of $225,000. The closing of the offering took place on April 29, 2019. |
| f. | On August 20, 2019, the Company entered into an additional form of securities purchase agreement with YMY and TRF, relating to an offering of an aggregate of 8,275,862 shares of the Company’s newly designated Series A Convertible Preferred Stock at a purchase price of $0.029 per share for aggregate gross proceeds of approximately $240,000. In addition, the Company granted YMY and TRF an option, for a period of twelve months, to purchase an additional 400,000 Series A Convertible Preferred Stock, in the aggregate, at a price per share of $0.60, for additional aggregate consideration of $240,000. The closing of the offering took place on August 29, 2019. |
| g. | On October 23, 2019, Novomic appointed Idan Traitsman to serve as the Chief Executive Officer of Novomic, effective immediately. In connection with Mr. Traitsman’s appointment, the Company agreed to pay Mr. Traitsman a monthly salary of NIS 10,000 (approximately $2,800) plus VAT. Idan Traistman is the brother of Oren Traistman, who served as a director of the Company until February 27, 2020. |
| h. | On November 17, 2019, the Company entered into a form of securities purchase agreement with YMY and TRF, relating to an offering of an aggregate of 2,068,966 shares of the Company’s newly designated Series A Convertible Preferred Stock at a purchase price of $0.029 per share for aggregate gross proceeds of approximately $60,000 in 2019 and 931,034 shares of the Company’s newly designated Series A Convertible Preferred Stock at a purchase price of $0.029 per share for aggregate gross proceeds of approximately $27,000 in 2020. In addition, the Company granted YMY and TRF an option, for a period of twelve months, to purchase up to an additional 100,000 Series A Convertible Preferred Stock, in the aggregate, at a price per share of $0.60, for additional aggregate consideration of $60,000 with respect to the 2019 purchase and 45,000 Series A Convertible Preferred Stock, in the aggregate, at a price per share of $0.60, for additional aggregate consideration of $27,000 with respect to the 2020 purchase. |
NOTE 16: SUBSEQUENT EVENT
See Note 1 with regard to the TechCare Transaction.
On April 1, 2020 the Company entered into a Convertible Note Purchase Agreement (the “CL Agreement”) with Citrine S A L Investment & Holdings Ltd, WealthStone Private Equity Ltd, WealthStone Holdings Ltd, Golden Holdings Neto Ltd, Beezz Home Technologies Ltd, Citrine Biotech 5 LP, Citrine High Tech 6 LP, Citrine High Tech 7 LP, Citrine 8 LP, Citrine 9 LP and Citrine Biotech 10 LP (together, the “Buyer”), all of which are affiliated with the Company. Under the CL Agreement, the Buyer agrees to purchase and the Company agrees to issue and sell, for up to an aggregate principal amount of $1,800 thousand, notes convertible into shares of common stock of the Company (the “Notes”), for a period starting on April 1, 2020 and ending upon the earlier of (i) 6 months thereafter and (ii) the consummation of a public offering by the Company. The Notes will bear an annual interest rate of six percent (6%) with respect to amounts paid that are used for working capital purposes of the Company, provided that amounts paid that are used for investment activities of the Company may be subject to different interest rates. The conversion price per share of Common Stock shall equal 85% multiplied by the market price (as defined in the Notes), representing a discount of 15%. Each Note will mature 18 months following the payment date. On April 19, 2020, the Company received an investment amount of $170,000 in connection with this agreement.
| 45 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed in the Company’s reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including the Chair of the board of directors of the Company (Chair) (who is the Company’s principal executive officer) and the Company’s Chief Financial Officer (CFO) (who is the Company’s principal financial officer) to allow for timely decisions regarding required disclosure. In designing and evaluating the Company’s disclosure controls and procedures, the Company’s management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and the Company’s management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Based on the Company’s evaluation of the effectiveness of its disclosure controls and procedures as of December 31, 2019, the Company’s Chair and CFO concluded that due to the material weaknesses described below, the Company’s disclosure controls and procedures were not effective to provide reasonable assurance that information required to be disclosed by the Company in reports that it files or submits under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including its Chair and CFO, to allow timely decisions regarding required disclosure.
| 46 |
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over the Company’s financial reporting. In order to evaluate the effectiveness of internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act of 2002, the Company’s management, with the participation of the Company’s principal executive officer and principal financial officer conducted an assessment, using the criteria in Internal Control - Integrated Framework, issued by the Committee of Sponsoring Organizations of the Tredway Commission (“COSO”) (2013). The Company’s system of internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. This assessment included review of the documentation of controls, evaluation of the design effectiveness of controls, and a conclusion on this evaluation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Based on this evaluation, the Company’s management concluded that its internal control over financial reporting was not effective as of December 31, 2019 as it identified control deficiencies that constituted material weaknesses in the Company’s internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses identified in the Company’s internal control over financial reporting are described below:
(i) Inadequate segregation of duties consistent with control objectives; and
(ii) Ineffective controls over period-end financial reporting and disclosure processes.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
These material weaknesses led to the restatement of the financial statements for the first three quarterly and year-to-date periods in 2017 and the restatement of the financial statements for the three month period ended March 31, 2016, the financial statements for the nine and three month periods ended at September 30, 2016, and the financial statements for the year ended December 31, 2015. In addition, the material weaknesses could result in the misstatement of account balances or disclosure that would result in a material misstatement to the annual or interim financial statements that would not be prevented or detected. The Company is currently reviewing its controls related to these material weaknesses and expects to implement further changes in the next fiscal year, including identifying specific areas within its accounting and financial reporting processes to mitigate these material weaknesses.
The Company’s management will continue to monitor and evaluate the effectiveness of its internal control over financial reporting on an ongoing basis and is committed to taking further action and implementing additional enhancements or improvements, as necessary.
Management believes that despite the material weaknesses set forth above, the Company’s consolidated financial statements as of and for the years ended December 31, 2019 and 2018 are fairly stated, in all material respects, in accordance with US GAAP.
Changes in Internal Control over Financial Reporting
During the three months ended December 31, 2019, there were no changes in the Company’s internal control over financial reporting that have materially affected, or are reasonably likely to materially affect the Company’s internal control over financial reporting.
None.
| 47 |
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT AND CORPORATE GOVERNANCE
The Company’s directors hold office until the next annual general meeting of the stockholders or until their successors are elected and qualified. The Company’s officers are appointed by its board of directors and hold office until the earlier of their death, retirement, resignation, or removal.
The following table sets forth the names and ages of the members of the board of directors and the executive officers and the positions held by each as of May 11, 2020.
| Name | Age | Title | ||
| Ora Meir Soffer | 55 | Chair of the board of directors and chief executive officer | ||
| Zviel Gedalihou | 38 | Chief financial officer | ||
| Ilan Ben-Ishay | 51 | Director | ||
| Ilanit Halperin | 49 | Director |
Ora Meir Soffer, 55, is a prolific entrepreneur, CEO, investor, director and advisory board member with over 30 years of experience in investing and in promoting breakthrough technologies of various high-tech and biotech companies. Ora focuses on startups in the Israeli ecosystem and is an expert in leading and advising the companies in business strategy, positioning, investment, capital raising, financing, M&As and IPOs. Ora serves as director or advisory board member in various companies. Ora is the co-founder and CEO of Citrine SAL http://www.citrineinvestment.com/, an Israeli investment group with funds in various fields of technology, high-tech, and biotech. Citrine SAL funds include Citrine S A L Biotech, which specializes in healthcare, wellness solutions, digital health, medical devices, foodtech, botanical nutraceuticals, and more, and Citrine S A L High-Tech, which specializes in high-tech, cyber, IoT, hardware and software. Citrine SAL is involved in its portfolio companies as active venture capital funds, which combine financial investment with support and assistance for companies’ business strategies, capital raising, financing, M&As and IPOs. Citrine SAL has an experienced professional team and a leading array of consultants worldwide. Citrine SAL’s portfolio includes companies such as Nicast Ltd., Nanomedic Ltd., WellBe Digital Ltd., Biocep Ltd., Improdia Ltd., Intelicanna Ltd., iBOT Israel Botanicals Ltd., Cannbit Pharmaceuticals Ltd., Cannabliss Ltd., Novomic Ltd., Dario Health, BSP Medical, ICB - Israel China Biotechnology, and more. Citrine SAL is part of WealthStone Holdings Group http://www.wealthstone.co.il/, which specializes in alternative investments, real estate, technology and hedge funds. Ora serves as director on the managment boards of Nicast Ltd., Nanomedic Ltd., WellBe Digital Ltd., Biocep Ltd., iBOT Israel Botanicals Ltd., Cannabliss Ltd., Beezhome Technologies, Beyond Blade, and Citrine SAL. Ora is a board member of the Friends’ Association of Shenkar College and Israel’s National Hackathon. Ora is a member of the Peres Center for Peace and Innovation and of Springboard Enterprises, a global organization accelerating women’s leadership in technology and biotech companies. Previously, Ora was founder and CEO of Chip PC Technologies, managing and leading the company, turning it from a startup to a public and international technology company. She is also investor, shareholder and founder in OR1 Investment Ltd., Xseed, Cellergy, Beezhome Technologies, Beyond Blade and more. Ora is based in Israel.
Mr. Zviel Gedalihou CPA, MBA, 38, has extensive experience in the field. From June 2012 to February 2020 Zviel served as Chief Financial and Operations Officer of the venture capital firm Markstone Capital Group LLC, where he worked with hundreds of investors worldwide, gained board-level experience in a wide variety of companies, and held key management positions in various companies. While serving as Chief Financial and Operations Officer of Markstone Capital Group LLC, Zviel also served as Chief Executive Officer of Tom-Set Ltd., and Chief Financial Officer of Magnolia Silver Jewelry Ltd., Cardboard Technologies Ltd. and EZTD Inc. From June 2011 to June 2012 Zviel worked as Finance Manager of Modus Selective, an investment company owned by First International Bank of Israel. From April 2008 to June 2011 Zviel worked in the Economic Consulting department of Ernst & Young. Zviel obtained his CPA license in 2011. Zviel holds an MBA specializing in Finance (2014) from the Hebrew University of Jerusalem, and a BA in Accounting and Economics (2008), also from the Hebrew University of Jerusalem. On March 1, 2020 Zviel joined WealthStone Holdings Ltd as Chief Financial Officer. WealthStone Holdings Ltd is a member of the WealthStone Holdings Group and is an affiliate of the Registrant. The WealthStone Holdings Group specializes in alternative investments, real estate, technology and hedge funds. Zviel has extensive experience in management, due diligence, finance valuations, M&As and IPOs, debt refinancing and deal structures. Zviel is a Major in the IDF Reserves. Except for WealthStone Holdings Ltd, none of the corporations or organizations Zviel has worked with during the past five years is affiliated with the Registrant.
| 48 |
Ilan Ben-Ishay, 51, is a diligent businessman, director, advisory board member, investor, owner and CEO with over 25 years of experience in taxation, finance and insurance, specialized in advising and leading customers, both private and institutional, on strategy, investment, capital raising, financing, M&As and IPOs. Ilan is a Major in the IDF Reserves. Ilan is CEO and co-founder of Neto Financial Planning, http://www.neto-finance.co.il, which has been operating for over 27 years and is one of the largest companies in the Israeli private and business financial planning and insurance industry. Neto has thousands of loyal customers, which it has been accompanying for many years, providing financial advisory services in respect of products with a market worth of over $3 billion. Ilan is chairman and co-founder of WealthStone Holdings Group (“WealthStone”), http://www.wealthstone.co.il/, a long-standing investment body with extensive financial knowledge and experience. WealthStone specializes in alternative investments, real estate, technology and hedge funds, and manages more than half a billion dollars in investments in Israel alone. In addition, Ilan is co-founder and an active board member of Superb Reality, which integrates machine learning, vision algorithms, AI, imaging optics and 3D into AR and VR imaging devices, and is investor and shareholder in Nicast Ltd., Nanomedic Ltd., and more. Ilan completed accounting studies and is an authorized pension insurance consultant. Ilan is based in Israel.
Ilanit Halperin, CPA, 49, worked for over 21 years in one of the six largest accounting firms in Israel, for the last 11 years as a partner. She then set up her own office providing CPA and financial consulting and management services. For many years Ilanit has accompanied public and private companies in Israel and abroad in diverse sectors, including industrial companies, real estate companies, technology companies, tourism companies and more. Ilanit has extensive experience in auditing and preparing financial statements according to Israeli, international (IFRS) and US GAAP standards. Ilanit specializes in accompanying early and mature stage companies, providing, inter alia, tax advice, general financial consulting, assistance in preparing business plans, and assistance and accompaniment with investors, private placements and IPOs in Israel and the USA. Halperin has many years of experience accompanying NASDAQ and OTC-traded companies.
Family Relationships
There are no family relationships between any members of the Company’s executive management and its directors.
Committees of the Board of Directors
The Company does not presently have a separately constituted audit committee, compensation committee, nominating committee, executive committee or any other committees of its board of directors. As such, its entire board of directors acts as its audit committee.
Audit Committee and Financial Expert, Compensation Committee, Nominations Committee.
The Company does not have any of the above-mentioned standing committees because its corporate financial affairs and corporate governance are simple in nature and each financial transaction is approved by its chief executive officer or board of directors.
Code of Ethics.
The Company does not currently have a Code of Ethics because it has limited business operations and it believes a code of ethics would have limited utility at this stage.
| 49 |
Involvement in Certain Legal Proceedings.
The Company is not aware of any material legal proceedings that have occurred within the past ten years concerning any Director or control person which involved a criminal conviction, a pending criminal proceeding, a pending or concluded administrative or civil proceeding limiting one’s participation in the securities or banking industries, or a finding of securities or commodities law violations.
Section 16(a) Compliance
Section 16(a) of the Securities and Exchange Act of 1934 requires the Company’s directors and executive officers, and persons who own beneficially more than ten percent (10%) of the Company’s Common Stock, to file reports of ownership and changes of ownership with the Securities and Exchange Commission. Copies of all filed reports are required to be furnished to the Company pursuant to Section 16(a). The Company was informed in writing by the previous management of the Company that the Company’s officers and directors and ten percent (10%) stockholders filed all reports required to be filed under Section 16(a) during 2019. The Company’s officers and directors and ten percent (10%) stockholders have filed all reports required to be filed under Section 16(a) so far during 2020.
ITEM 11. EXECUTIVE COMPENSATION
Executive Compensation
The table below provides certain information concerning the compensation for services rendered to us during the years ended December 31, 2019 and 2018 by all individuals who served as our principal executive officer or acted in a similar capacity (the “PEO”) during any part of the year ended December 31, 2019, as well as Tali Dinar, who was the Company’s most highly compensated executive officer other than the PEO who was serving as an executive officer at the end of 2019, as well as Osnat Philipp, who would have been one of the Company’s two most highly compensated executive officers other than the PEO serving as an executive officer at the end of 2019, but for the fact that she was not serving as an executive officer at the end of 2019. The Company had no other individuals who received compensation of more than $100,000 during the year ended December 31, 2019.
Name and Principal Position | Year | Salary ($)(1) | Bonus ($)(2) | Option Awards ($)(3) | All other compensation ($) | Total ($) | ||||||||||||||||||
| Oren Traistman, Former Chairman of the Board of Directors and principal executive officer | 2019 | 37,873 | - | - | - | 37,873 | ||||||||||||||||||
| 2018 | 20,242 | - | - | - | 20,242 | |||||||||||||||||||
| Zvi Yemini, Former Chairman of the Board of Directors and Former Chief Executive Officer | 2019 | 156,263 | - | - | - | 156,263 | ||||||||||||||||||
| 2018 | 140,537 | - | - | - | 140,537 | |||||||||||||||||||
| Doron Biran, Former Chief Executive Officer | 2019 | 28,004 | - | 28,004 | ||||||||||||||||||||
| 2018 | 89,987 | - | 89,987 | |||||||||||||||||||||
| Tali Dinar, Chief Financial Officer | 2019 | 118,883 | - | - | - | 118,883 | ||||||||||||||||||
| 2018 | - | - | - | - | - | |||||||||||||||||||
| Osnat Philipp, Chief Executive Officer of Novomic Ltd | 2019 | 150,071 | - | - | - | 150,071 | ||||||||||||||||||
| 2018 | - | - | - | - | - | |||||||||||||||||||
(1) Represents monthly retainer payments.
(2) Represents one-time discretionary cash bonuses to each of the executive officers.
(3) Represents stock-based compensation.
| 50 |
Executive Services and Employment Agreements
Services Agreement with Oren Traitsman
On July 31, 2016, the Company entered into a consulting agreement with Mr. Oren Traistman, who was a member of the Board until February 27, 2020, including Chairman of the Board from August 13, 2019, and acting principal executive officer from November 14, 2019 until February 27, 2020. Pursuant to the consulting agreement, Mr. Traistman received a gross monthly amount of NIS 10,000 (approximately $2,900). In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018 through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on March 16, 2020 and the monthly retainer from December 31, 2019 was waived.
Services Agreement with Zvi Yemini
On December 31, 2015, the Company entered into a consulting agreement with Zvi Yemini, and with his affiliated entity Y.M.Y Industry Ltd. (“YMY”). Zvi Yemini served as Chairman of the Board of Directors until August 13, 2019, and as a board member until November 14, 2019, and as Chief Executive Office from February 15, 2019 until November 14, 2019. Pursuant to the consulting agreement, Mr. Yemini received a gross monthly amount of NIS 24,000 (approximately $6,200). The foregoing payment was in addition to, and independent of, the fee that Mr. Yemini was entitled to receive for continued services as a member of the Board. On February 22, 2017, the Company signed an amendment to the original agreement with Mr. Yemini and YMY. Pursuant to the amendment, Mr. Yemini’s monthly payment was increased to NIS 45,000 (approximately $13,000) starting February 2017. In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018 through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on November 14, 2019, which was also the effective date of Zvi Yemeni’s resignation as a director of the Company and of his resignation as an officer of the Company.
Services Agreement with Doron Biran
On July 16, 2018, the Board of the Company appointed Mr. Doron Biran as its Chief Executive Officer of the Company and of its wholly-owned subsidiary Novomic. Pursuant to the service agreement signed with Mr. Biran, Mr. Biran was entitled to receive monthly compensation of NIS 52 thousand (approximately $14,300) plus VAT. In the event of a capital raise exceeding $1,000 thousand Mr. Biran was to be entitled to an increase in his compensation to a total of NIS 65 thousand (approximately $17,900). Furthermore, upon the earlier of either 24 months from the effective date of the Agreement, or a capital raise exceeding $5 million and the listing of the Company on the Nasdaq Stock Market, Mr. Biran was to receive a base salary of NIS 60 thousand as well as NIS 5 thousands for automobile expenses (approximately $16,500) and other customary social benefits. On December 20, 2018, the board of directors of the Company and Mr. Biran agreed that Mr. Biran would step down effective as of February 28, 2019. In February 2019 the Company and Mr. Biran agreed that Mr. Biran would step down from his position as Chief Executive Officer effective as of February 15, 2019.
| 51 |
Employment Agreement with Tali Dinar
On January 20, 2019, the Company entered into an employment agreement with Tali Dinar. Pursuant to her agreement, Ms. Dinar was to serve as Chief Financial Officer of the Registrant and of Novomic Ltd and was to be entitled to receive a monthly salary of NIS 23,000 (approximately $6,250). Upon the lapse of the first three months, Ms. Dinar’s monthly salary was to increase to NIS 27,000 (approximately $7,350). Upon the lapse of an additional three months, Ms. Dinar’s monthly salary was to increase to NIS 40,000 (approximately $10,900). In addition, the Company was to contribute certain amounts towards certain pension, severance, disability and tax-advantaged savings for Ms. Dinar. Ms. Dinar was to receive a leased car to be used in accordance with the Company’s policy and also to be entitled to reimbursement of expenses. It was agreed that Ms. Dinar may also be eligible for an annual bonus in an amount equal to up to 1% of any funds raised by the Company in a public offering occurring during Mr. Dinar’s employment. Ms. Dinar was to be entitled to a further bonus equaling 3% of any funds raised by contracts introduced by her to the Registrant. Ms. Dinar was also to be eligible to receive options to purchase shares of common stock constituting 1.2% of the Registrants’ outstanding share capital as of January 20, 2019. The Options were to vest over a period of four years in annual increments, subject to continued provision of services by Ms. Dinar. The agreement contained, among other things, a confidentiality obligation, a covenant not to compete, and an assignment of inventions. Effective December 22, 2019, Ms. Dinar voluntarily resigned from her position as Chief Financial Officer of the Registrant but continued to service as Principal Financial and Accounting Officer of the Registrant. On February 27, 2020, Ms. Dinar voluntarily resigned from her position as the Principal Financial and Accounting Officer of the Registrant, effective immediately. Ms. Dinar is serving as Chief Financial Officer of Novomic.
Employment Agreement with Osnat Philipp
On January 1, 2019, an employment agreement between Novomic Ltd and Osnat Philipp became effective. Pursuant to her agreement, Ms. Philipp was to serve as Chief Executive Officer of Novomic Ltd and was to be entitled to receive a monthly salary of NIS 35,000 (approximately $9,750). In addition, Novomic Ltd was to contribute certain amounts towards certain pension, severance, disability and tax-advantaged savings for Ms. Philipp. Ms. Philipp was to receive a leased car to be used in accordance with the Company’s policy and also to be entitled to reimbursement of expenses. Ms. Philipp was also to be eligible to receive options to purchase shares of common stock constituting 2% of the Registrants’ outstanding share capital as of January 1, 2019. The Options were to vest over a period of four years in annual increments, subject to continued provision of services by Ms. Philipp. The agreement contained, among other things, a confidentiality obligation, a covenant not to compete, and an assignment of inventions. Effective November 1, 2019, Ms. Philipp voluntarily resigned from her position as Chief Executive Officer of Novomic Ltd.
Director’s Compensation
The Company entered into services agreements with Zvi Yemini and Mr. Oren Traistman, as described above, and Mr. Yossef De-Levy. The following table provides certain information concerning the compensation for services rendered in all capacities by each director serving on the Company’s board of directors during the year ended December 31, 2019, other than Mr. Zvi Yemini and Oren Traistman, who did not receive additional compensation for their services as directors and whose compensation is set forth in the summary compensation table above. Mr. Ningzhou Zhang served as a director of the Registrant from March 18, 2019 until September 19, 2019. Mr. Yossef De-Levy served as a director of the Registrant until February 27, 2020.
| Name | Fee Earned or Paid in Cash($) | Option Awards($)(1) | All Other Compensation($)(2) | Total ($) | ||||||||||||
| Ningzhou Zhang | - | - | - | - | ||||||||||||
| Yossef De Levy | - | - | - |
(1) Represents stock-based compensation.
(2) Payments are pursuant to the consulting agreements.
Services Agreement with Yossef De-Levy
On November 11, 2014, the Company entered into a consulting agreement with Mr. Yossef De-Levy, a member of the Company’s Board. Pursuant to the consulting agreement, Mr. De-Levy received a gross monthly amount of NIS 10,000 (approximately $2,900). The foregoing payment was in addition to, and independent of, the fee that Mr. De-Levy was entitled to receive for continued services as a member of the Board. In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018, through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on March 16, 2020 and the monthly retainer from December 31, 2019 was waived.
| 52 |
Golden Parachute Compensation
The Company does not currently have any agreement or understanding, whether written or unwritten, between it and its named executive officers, concerning any type of compensation, whether present, deferred or contingent, that is based on or otherwise relates to an acquisition, merger, consolidation, sale or other disposition of all or substantially all our assets.
Equity Compensation Plan
2018 Stock Incentive Plan
In December 2018, the Company adopted the 2018 Stock Incentive Plan, or the 2018 Plan, which became effective as of December 2, 2018 by the action of its board of directors. The 2018 Plan provides for the grant of stock awards, restricted stock awards and stock options to any employee, director, officer, consultant, or advisor of the Company, or such other persons who provided bona fide services to the Company as shall be determined by a committee designated by the board of directors. If no committee is designated by the board of directors, the 2018 Plan will be administered by the board of directors. As of the date of this annual report the board of directors has not designated a committee to administer the 2018 Plan.
The total number of shares of common stock reserved for issuance under the 2018 Plan, either directly as stock awards or underlying options is 2,000,000 shares of common stock. The total number of shares of common stock reserved for such issuance may be increased only by a resolution adopted by the board of directors and amendment of the 2018 Plan. Awards under the 2018 Plan may be granted until December 2, 2028. The terms of under which a stock award or option is granted under the 2018 Plan shall be set forth in a written agreement, which shall be determined by the committee or the board of directors.
As of March 31, 2020, the total number of shares of common stock issued under the 2018 Plan, either directly as stock awards or underlying options was 0 shares of common stock.
2017 Employee Incentive Plan
In 2017, the Company adopted the 2017 Employee Incentive Plan, or the 2017 Plan, which became effective as of January 1, 2017 by the action of the board of directors. The 2017 Plan provided for the grant of stock awards and stock options to any employee, director, officer, consultant, or advisor of the Company, or such other persons who provided bona fide services to the Company as determined by a committee designated by the board of directors followed by the approval of the board of directors; however, if the committee was composed of a majority of the persons then comprising the board of directors, the approval of the board of directors was not necessary. If no committee was designated by the board of directors, the 2017 was to be administered by the board of directors. The board of directors did not designate a committee to administer the 2017 Plan.
As of March 31, 2020, the total number of shares of common stock issued under the 2017 Plan, either directly as stock awards or underlying options was 311,544 shares of common stock.
| 53 |
Outstanding Equity Awards at Fiscal Year End
The following table sets forth information concerning outstanding equity awards as of December 31, 2019, for each named executive officer:
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Un-exercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options | Option Exercise Price ($) | Option Expiration Date | Number of shares or units of stock that have not vested | Market value of shares of units of stock that have not vested | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested | |||||||||||||||||||||||||||
| Oren Traistman (1) | 521,065 | $ | 0.0001 | 3/13/2022 | - | - | - | - | ||||||||||||||||||||||||||||
| Zvi Yemini (2) | 494,204 | - | $ | 0.0001 | 3/13/2022 | - | - | - | - | |||||||||||||||||||||||||||
| Doron Biran | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Tali Dinar | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Osnat Philipp | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
(1) These options were waived and cancelled in the first quarter of 2020 in connection with the TechCare Transaction.
(2) All the options were held by Y.M.Y Industry Ltd. These options were waived and cancelled in the first quarter of 2020 in connection with the TechCare Transaction.
Compensation Committee Interlocks and Insider Participation
The following former officers of the Company participated during 2019 in deliberations of the Company’s board of directors concerning executive officer compensation: Zvi Yemini and Oren Traitsman.
Compensation Committee Report
To the Company’s present knowledge, during 2019 the board of directors of the Company did not review or discuss with management the Compensation Discussion and Analysis required by Item 402(b) of the Securities and Exchange Commission regulations. The board members of the Company during 2019 were Zvi Yemini, Oren Traitsman, Yossef De-Levie and Ningzhou Zhang.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Certain Beneficial Owners and Management
As of May 7, 2020, there were 494,721,815 shares of common stock outstanding, excluding shares of common stock issuable in connection with the exercise of outstanding warrants or outstanding options. The voting rights of all stockholders are the same.
The following table sets forth certain information as of May 7, 2020, concerning the number of shares of common stock beneficially owned, directly or indirectly, by:
| ● | each person, or group of affiliated persons, known to us to beneficially own more than 5% of our outstanding ordinary shares; | |
| ● | each of our directors; | |
| ● | each of our executive officers; and | |
| ● | all of our directors and executive officers serving as of May 7, 2020, as a group. |
| 54 |
Beneficial ownership is determined in accordance with the rules of the SEC based on voting and investment power with respect to such shares. Shares subject to options or warrants that are currently exercisable or exercisable within 60 days of March 31, 2020, are deemed to be outstanding and to be beneficially owned by the person holding such options or warrants for the purpose of computing the percentage ownership of such person. However, such shares are not deemed to be outstanding and to be beneficially owned for the purpose of computing the percentage ownership of any other person. All information with respect to the beneficial ownership of any principal stockholder has been furnished by such stockholder or is based on our filings with the SEC and, unless otherwise indicated below, we believe that persons named in the table have sole voting and sole investment power with respect to all the shares of common stock as beneficially owned, subject to community property laws, where applicable. Unless otherwise noted below, each shareholder’s address is c/o TechCare Corp. 3 Hamelacha, Tel Aviv, 6721503, Israel.
| Name of Beneficial Owner | Common Stock Beneficially Owned | Percentage of Stock Owned | ||||||
| Principal Stockholders: | ||||||||
| Ora Meir Soffer (1) | 216,498,278 | 43.76 | % | |||||
| Yaron Pitaru (2) | 92,415,806 | 18.68 | % | |||||
| Edan Moshe Katz (3) | 44,768,409 | 9.05 | % | |||||
| Ilan Ben-Ishay (4) | 40,967,999 | 8.28 | % | |||||
| Executive Officers and Directors: | ||||||||
| Ora Meir Soffer | 216,498,278 | 43.76 | % | |||||
| Ilan Ben-Ishay | 40,968,000 | 8.28 | % | |||||
| Ilanit Halperin | 0 | |||||||
| Zviel Gedalihou | 0 | |||||||
| All directors and executive officers as a group (four persons) | 257,466,277 | 52.04 | % | |||||
(1) Includes 85,000,000 shares of common stock owned directly by Ora Meir Soffer, 35,000,000 shares of common stock owned through Beezz Home Technologies Ltd which is 100% owned by Ora Meir Soffer, and 96,498,278 shares of common stock owned through Citrine S A L Investment & Holdings Ltd, which is 50% owned by Beezz Home Technologies Ltd.
(2) Includes 31,666,667 shares of common stock owned directly by Yaron Pitaru, 12,500,000 shares of common stock owned through WealthStone Private Equity Ltd, which is 100% owned by WealthStone Holdings Ltd, which is 50% owned by Yaron Pitaru, and 48,249,139 shares of common stock owned through Citrine S A L Investment & Holdings Ltd, which is 50% owned by WealthStone Private Equity Ltd.
(3) Includes 22,850,387 shares of common stock owned directly by Edan Moshe Katz, about 4,509,945 shares of common stock owned through WealthStone Private Equity Ltd, which is 100% owned by WealthStone Holdings Ltd, which is 50% owned by Golden Holdings Neto Ltd, which is 36.07956% owned by Edan Moshe Katz, and about 17,408,077 shares of common stock owned through through Citrine S A L Investment & Holdings Ltd, which is 50% owned by WealthStone Private Equity Ltd.
(4) Includes 20,910,608 shares of common stock owned directly by Ilan Ben-Ishay, about 4,127,094 shares of common stock owned through WealthStone Private Equity Ltd, which is 100% owned by WealthStone Holdings Ltd, which is 50% owned by Golden Holdings Neto Ltd, which is 33.01675% owned by Ilan Ben-Ishay, and about 15,930,298 shares of common stock owned through through Citrine S A L Investment & Holdings Ltd, which is 50% owned by WealthStone Private Equity Ltd.
Equity Compensation Plan Information
See “Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities – Securities Authorized for Issuance under Equity Compensation Plans.”
| 55 |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTORS INDEPENDENCE
| a. | On November 11, 2014, the Company entered into a consulting agreement with Mr. Yossef De-Levy, a member of the Company’s Board. Pursuant to the consulting agreement, Mr. De-Levy received a gross monthly amount of NIS 10,000 (approximately $2,900). The foregoing payment was in addition to, and independent of, the fee that Mr. De-Levy was entitled to receive for continued services as a member of the Board. In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018, through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on March 16, 2020 and the monthly retainer from December 31, 2019 was waived. |
| b. | On December 31, 2015, the Company entered into a consulting agreement with Zvi Yemini, and with his affiliated entity Y.M.Y Industry Ltd. (“YMY”). Zvi Yemini served as Chairman of the Board of Directors until August 13, 2019, and as a board member until November 14, 2019, and as Chief Executive Office from February 15, 2019 until November 14, 2019. Pursuant to the consulting agreement, Mr. Yemini received a gross monthly amount of NIS 24,000 (approximately $6,200). The foregoing payment was in addition to, and independent of, the fee that Mr. Yemini was entitled to receive for continued services as a member of the Board. On February 22, 2017, the Company signed an amendment to the original agreement with Mr. Yemini and YMY. Pursuant to the amendment, Mr. Yemini’s monthly payment was increased to NIS 45,000 (approximately $13,000) starting February 2017. In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018 through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on November 14, 2019, which was also the effective date of Zvi Yemeni’s resignation as a director of the Company and of his resignation as an officer of the Company. |
| c. | On July 31, 2016, the Company entered into a consulting agreement with Mr. Oren Traistman, who was a member of the Board until February 27, 2020, including Chairman of the Board from August 13, 2019, and acting principal executive officer from November 14, 2019 until February 27, 2020. Pursuant to the consulting agreement, Mr. Traistman received a gross monthly amount of NIS 10,000 (approximately $2,900). In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, according to which the monthly retainer was waived commencing on November 15, 2018 through December 31, 2019. The Company recorded the expense against equity. The consulting agreement was terminated on March 16, 2020 and the monthly retainer from December 31, 2019 was waived. |
| d. | On December 31, 2017, the Company entered into a consulting agreement with Mr. Ran Tuttnauer, a member of the advisory Board. Pursuant to the consulting agreement, Mr. Tuttnauer received a gross monthly amount of $2,000. In March 2019 and April 2019, the Company entered into an amendment to the consulting agreement, pursuant to which the monthly retainer was waived commencing on November 15, 2018 through August 31, 2019. In April 2019 the consulting agreement was terminated. The Company recorded the expense against equity. |
| e. | On June 28, 2018, the Company entered into a subscription agreement with Ran Tuttnauer Family Ltd., pursuant to which it issued 645,995 shares of its Common Stock at a purchase price of $0.387 for a total consideration of $250,000 and warrants to purchase up to 416,667 Common Stock with an exercise price of $0.60, exercisable until June 28, 2019. Ran Tuttnauer has served as an advisory director of the Company. |
| f. | On August 8, 2018, the Company entered into subscription agreements with Traistman Radziejewski Fundacja Ltd., which is controlled by Oren Traistman who served as a director of the Company, pursuant to which the Company issued 258,398 shares of its common stock at a purchase price of $0.387 for a total consideration of $100,000 and warrants to purchase up to 166,667 Common Stock with an exercise price of $0.60, exercisable until August 8, 2019. |
| g. | On August 8, 2018, the Company entered into subscription agreements with YMY Industry Ltd., which is controlled by Zvi Yemini who served as a Chairman of the board of directors of the Company, pursuant to which the Company issued 645,995 shares of its Common Stock at a purchase price of $0.387 for a total consideration of $250,000 and warrants to purchase up to 416,667 shares of Common Stock with an exercise price of $0.60, exercisable until August 8, 2019. This subscription was amended by an amendment dated October 28, 2018, as further detailed below. |
| 56 |
| h. | On October 28, 2018, the Company entered into an amendment to the August 8, 2018 Y.M.Y Industry Ltd. subscription agreement. Pursuant to the amendment, Y.M.Y Industry Ltd. increased its initial investment by an additional amount of $250,000 to a total investment amount of $500,000 in consideration for the issuance of a total of 1,915,708 shares of Common Stock at a price per share of $0.261. In addition, Y.M.Y Industry Ltd. was issued additional warrants to purchase up to 416,667 shares of Common Stock with an exercise price of $0.6, exercisable until October 27, 2019. |
| i. | On November 14, 2018, the Company entered into a subscription agreement with Marius Nacht, pursuant to which the Company issued and sold to Marius Nacht 1,915,708 shares of Common Stock, par value $0.0001 per share, for a price per Share of $0.261, for a consideration of US$500,000. After the issuance and sale of the shares, Marius Nacht held more than 5% of the issued Common Stock of the Company. In addition, the Company granted Marius Nacht an option, for a period of twelve months as of November 14, 2018, to purchase 833,333 additional shares of Common Stock at a price per share of $0.60, for an additional consideration of US$500,000, if and to the extent exercised by Marius Nacht. |
| j. | On January 21, 2019, the Company entered into a subscription agreement with ICB Biotechnology Investments Ltd., pursuant to which the Company agreed to issue and sell to ICB up to 1,915,708 shares of Common Stock, for a price per share of $0.261, and that ICB would maintain the right to nominate one person to serve as a member of the Company’s board of directors for as long as ICB held 2% of the Company’s shares of capital stock on a fully-diluted basis. ICB accordingly nominated Ningzhou Zhang, who joined the Company’s board of directors on March 18, 2019 and resigned from the board on September 19, 2019. Further details are provided above in Item 8 Note 10: China Joint Venture Agreement and Subscription Agreement. |
| k. | On April 28, 2019, the Company entered into a form of Securities Purchase Agreement with each of Y.M.Y. Industry Ltd. (“YMY”), Traistman Radziejewski Fundacja Ltd. (“TRF”) and Microdel Ltd. (together with YMY and TRF, the “Investors”) relating to an offering of an aggregate of 1,229,508 shares of the Company’s common stock at a purchase price of $0.183 per share for aggregate gross proceeds of approximately $225,000. In addition, the Company granted the Investors an option, for a period of twelve months, to purchase up to an additional 375,001 shares of common stock at a price per share of $0.60, for additional aggregate consideration of $225,000. The closing of the offering took place on April 29, 2019 |
| l. | On August 20, 2019, the Company entered into an additional form of securities purchase agreement with YMY and TRF, relating to an offering of an aggregate of 8,275,862 shares of the Company’s newly designated Series A Convertible Preferred Stock at a purchase price of $0.029 per share for aggregate gross proceeds of approximately $240,000. In addition, the Company granted YMY and TRF an option, for a period of twelve months, to purchase an additional 400,000 Series A Convertible Preferred Stock, in the aggregate, at a price per share of $0.60, for additional aggregate consideration of $240,000. |
| m. | On October 23, 2019, Novomic appointed Idan Traitsman to serve as the Chief Executive Officer of Novomic, effective immediately. In connection with Mr. Traitsman’s appointment, the Company agreed to pay Mr. Traitsman a monthly salary of NIS 10,000 (approximately $2,800) plus VAT. Idan Traistman is the brother of Oren Traistman, who served as a director of the Company until February 27, 2020. |
| n. | On November 17, 2019, the Company entered into a form of securities purchase agreement with YMY and TRF, relating to an offering of an aggregate of 2,068,966 shares of the Company’s newly designated Series A Convertible Preferred Stock at a purchase price of $0.029 per share for aggregate gross proceeds of approximately $60,000 in 2019 and 931,034 shares of the Company’s newly designated Series A Convertible Preferred Stock at a purchase price of $0.029 per share for aggregate gross proceeds of approximately $27,000 in 2020. In addition, the Company granted YMY and TRF an option, for a period of twelve months, to purchase up to an additional 100,000 Series A Convertible Preferred Stock, in the aggregate, at a price per share of $0.60, for additional aggregate consideration of $60,000 with respect to the 2019 purchase and 45,000 Series A Convertible Preferred Stock, in the aggregate, at a price per share of $0.60, for additional aggregate consideration of $27,000 with respect to the 2020 purchase. |
| 57 |
| o. | On November 21, 2019, the Company entered into a Memorandum of Understanding with Citrine S A L Investment & Holdings Ltd which provided for the sale of the Company, and the sale by the Company of 90% of its shares in Novomic. |
| p. | On January 6, 2020, definitive agreements were executed for the sale of 90% of the shares in Novomic to Traistman Radziejewski Fundacja Ltd, and for the issuance and sale of a number of shares equal after the issuance to 95% of the share capital of the Company to Citrine S A L Investment & Holdings Ltd and a group of related persons and entities. Citrine S A L Investment & Holdings Ltd carried out extensive due diligence appropriate for the acquisition of a public company divesting its activities, and obtained from the Company’s sellers detailed representations and warranties. Traistman Radziejewski Fundacja Ltd is controlled by Oren Traistman, who was a director of the Company until February 27, 2020. |
| q. | On February 23, 2020, the Company entered into an agreement amending and restating the January 6, 2020 agreement concerning the TechCare Transaction, which provided for the issuance and sale of the shares in stages. Pursuant to this agreement, on February 27, 2020 a number of shares was issued to Citrine S A L Investment & Holdings Ltd which was insufficient to cause a change in control of the Company - which would at that time have invalidated the Company’s Directors and Officers insurance – but which did cause to come into effect the resignation of the board of directors of the Company and the appointment of a new board nominated by Citrine S A L Investment & Holdings Ltd. The board of the directors of the Company has accordingly since February 27, 2020 been composed of three nominees of Citrine S A L Investment & Holdings Ltd, namely Ora Meir Soffer, Ilan Ben-Ishay and Ilanit Halperin. Zviel Gedalihou was appointed as Chief Financial Officer of the Company on March 17, 2020, and Ora Meir Soffer was appointed Chief Executive Officer of the Company on May 7, 2020. The Company has no payroll liabilities at present. On March 5, 2020, additional shares were issued, causing Citrine S A L Investment & Holdings Ltd and its group of related persons and entities to become owners of 90.6% of the fully diluted capital stock of the Company, and resulting in a change of control of the Company. The Company is working to complete the issuance and sale of a number of shares equal after the issuance to up to 95% of the fully diluted capital stock of the Company by amending its Certificate of Incorporation to increase its authorized share capital and then issuing additional shares. |
| r. | On April 1, 2020 the Company entered into a Convertible Note Purchase Agreement (the “CL Agreement”) with Citrine S A L Investment & Holdings Ltd, WealthStone Private Equity Ltd, WealthStone Holdings Ltd, Golden Holdings Neto Ltd, Beezz Home Technologies Ltd, Citrine Biotech 5 LP, Citrine High Tech 6 LP, Citrine High Tech 7 LP, Citrine 8 LP, Citrine 9 LP and Citrine Biotech 10 LP (together, the “Buyer”), all of which are affiliated with the Company. Under the CL Agreement, the Buyer agrees to purchase and the Company agrees to issue and sell, for up to an aggregate principal amount of $1,800,000.00, notes convertible into shares of common stock of the Company (the “Notes”), for a period starting on April 1, 2020 and ending upon the earlier of (i) 6 months thereafter and (ii) the consummation of a public offering by the Company. The Notes will bear interest at a rate of six percent (6%) with respect to amounts paid that are used for working capital purposes of the Company, provided that amounts paid that are used for investment activities of the Company may be subject to different interest rates, in accordance with the Guidelines. The conversion price per share of Common Stock shall equal 85% multiplied by the market price (as defined in the Note), representing a discount of 15%. The payment for each Note must be delivered 14 business days after delivery of the respective draw down notice, and each Note will mature 18 months thereafter. The interval between one draw down and the next must be at least thirty (30) days, provided that the Buyer may waive this requirement. Each draw down notice provided to the Buyer must be for an amount between $50,000 and $350,000, set at the Company’s discretion. The Company must use the amounts paid for the Notes in accordance with guidelines required to be provided by the Buyer within 14 business days following April 1, 2020 (the “Guidelines”). The Buyer shall decide upon and provide to the Company the names of the Buyer parties which will provide the funds to the Company in respect of the Note, including the respective amounts to be transferred to the Company by each such Buyer party. The Buyer shall have the right, from time to time and at its discretion, to add other entities to the list comprising the Buyer. The Buyer may participate alongside the Company in any investment the Company makes for as long as the CL Agreement is in effect. The Company may at any time prepay an outstanding Note (principal and accrued interest) in full by paying the Buyer an amount in cash equal to 115% multiplied by the then outstanding principal amount of the Note, as well as the accrued and unpaid interest on the unpaid principal amount of the Note, provided however that in the event the Company seeks to exercise this right the Buyer will first have the option to fully convert the Note, or any remaining amount outstanding under it, into Common Stock of the Company, and the conversion amount will be equal to the amount the Company would have paid to the Buyer had the Buyer not exercised this option. |
| 58 |
Engagement Agreements with Directors and Officers
There are currently no engagement agreements in effect with any directors or officers. The Company has newly appointed directors, Chief Executive Officer and Chief Financial Officer.
Exculpation, Indemnification and Insurance
The Company’s Bylaws permit it to exculpate, indemnify and insure certain of its directors and officers to the fullest extent permitted under the laws of the State of Delaware or other applicable law. In addition, the Company intends to enter into indemnification agreements with its directors and officers, exculpating them from a breach of their duty of care to the Company to the fullest extent permitted by law and undertaking to indemnify them to the fullest extent permitted by law, subject to certain exceptions, to the extent that these liabilities are not covered by insurance. The Company also maintains directors’ and officers’ liability insurance. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the Company, the Company have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Director Independence
The Company is not required to have independent directors, and as of the date hereof has not determined that any one or more of its directors is independent or not independent.
Parents of smaller reporting company
The immediate parent companies of the Company are Citrine S A L Investment & Holdings Ltd, WealthStone Private Equity Ltd, and Beezz Home Technologies Ltd. Citrine S A L Investment & Holdings Ltd directly holds 192,996,556 shares of Common Stock of the Company, comprising 39.01% of the voting securities of the Company. WealthStone Private Equity Ltd directly holds 25,000,000 shares of Common Stock of the Company, and its total beneficial holding in the Company, including through shares it holds in Citrine S A L Investment & Holdings Ltd, is 24.56% of the voting securities of the Company. Beezz Home Technologies Ltd directly holds 35,000,000 shares of Common Stock of the Company, and its total beneficial holding in the Company, including through shares it holds in Citrine S A L Investment & Holdings Ltd, is 26.58% of the voting securities of the Company. In addition, WealthStone Holdings Ltd, which fully owns WealthStone Private Equity Ltd, beneficially owns 24.56% of the voting securities of the Company, and Golden Holdings Neto Ltd, which owns 50% of WealthStone Holdings Ltd, beneficially owns 12.28% of the voting securities of the Company.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Background
The board of directors and the shareholders of the Company approved the appointment of Kesselman & Kesselman, or Kesselman & Kesselman, a member firm of PricewaterhouseCoopers International Limited, with offices located at Trade Tower, 25 Hamered Street, Tel-Aviv, 6812508 Israel, as the Company’s independent registered public accounting firm on March 3, 2020, having dismissed it on April 25, 2019 and appointed BDO Ziv Haft, which it dismissed on March 3, 2020. The Company has been advised by Kesselman & Kesselman that it is an independent registered public accounting firm with the PCAOB, and complies with the auditing, quality control and independence standards and rules of the PCAOB.
| 59 |
Principal Accounting Fees and Services
The following table presents the fees for professional audit services rendered by Kesselman & Kesselman for the audit of the Registrant’s annual financial statements for the year ended December 31, 2019 and 2018, respectively.
| 2019 | 2018 | |||||||
| ($ in thousands) | ||||||||
| Audit fees (1) | $ | 51 | $ | 71 | ||||
| Audit-related fees (2) | - | - | ||||||
| Tax fees (3) | $ | 9 | 9 | |||||
| All other fees | - | - | ||||||
| Total: | $ | 60 | 80 | |||||
| (1) | Audit fees consist of audit and review services, consents and review of documents filed with the SEC. |
| (2) | Audit-related fees consist of assistance and discussion concerning financial accounting and reporting standards and other accounting issues. |
| (3) | Tax fees consist of preparation of federal and state tax returns, review of quarterly estimated tax payments, and consultation concerning tax compliance issues. |
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a) | Documents filed as part of this report |
| (1) | Financial Statements |
The Consolidated Financial Statements filed as part of this annual report are identified in the Index to Consolidated Financial Statements on page F-1 hereto.
| (2) | Financial Statements Schedules |
Financial Statement Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
| (3) | Exhibits |
The following documents are filed as exhibits to this report on Form 10-K or incorporated by reference herein. Any document incorporated by reference is identified by a parenthetical reference to the SEC filing that included such document.
| 60 |
| 61 |
| + | Management contract or compensatory plan or arrangement |
| * | Filed herewith |
| ** | Furnished herewith |
ITEM 16. SUMMARY
Not Applicable.
| 62 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned.
| TechCare Corp. | ||
| By: | /s/ Ora Meir Soffer | |
| Ora Meir Soffer | ||
| Chair of the Board and Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| Date: | May 11, 2020 | |
| By: | /s/ Zviel Gedalihou | |
| Zviel Gedalihou | ||
| Chief Financial Officer | ||
| (Principal Financial and Principal Accounting Officer) | ||
| Date: | May 11, 2020 | |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENT: That the undersigned officers and directors of TechCare Corp. do hereby constitute and appoint each of Ora Meir Soffer and Zviel Gedalihou as the lawful attorney and agent with power and authority to do any and all acts and things and to execute any and all instruments which said attorney and agent determines may be necessary or advisable or required to enable TechCare Corp. to comply with the Securities and Exchange Act of 1934, as amended, and any rules or regulations or requirements of the Securities and Exchange Commission in connection with this report. Without limiting the generality of the foregoing power and authority, the powers granted include the power and authority to sign the names of the undersigned officers and directors in the capacities indicated below to this report or amendments or supplements thereto, and each of the undersigned hereby ratifies and confirms all that said attorneys and agents, or either of them, shall do or cause to be done by virtue hereof. This Power of Attorney may be signed in several counterparts.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Ora Meir Soffer | Chair of the Board of Directors and Chief Executive Officer | May 11, 2020 | ||
| Ora Meir Soffer | (Principal Executive Officer) | |||
| /s/ Zviel Gedalihou | Chief Financial Officer | May 11, 2020 | ||
| Zviel Gedalihou | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Ilan Ben-Ishay | Director | May 11, 2020 | ||
| Ilan Ben-Ishay | ||||
| /s/ Ilanit Halperin | Director | May 11, 2020 | ||
| Ilanit Halperin |
| 63 |
