Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Nemaura Medical Inc. | ex99x1.htm |
| 8-K - FORM 8-K - Nemaura Medical Inc. | nmra_8k.htm |
Exhibit 99.2

Better Diagnostics for Life Management of Type 2 Diabetes using non - invasive CGM 11 th May 2020

2 Authors Dr R K Ibrahim Head of data analytics Mr Steve Willmor Head of Regulatory
3 Summary of Presentation There is compelling evidence suggesting Type 2 diabetes may be managed by use of a Continuous Glucose Monitor (CGM) on non - consecutive days, leading to clinically significant outcomes. Invasive CGMs are generally designed for wear periods of up to 14 consecutive days, with the associated cost. The additional costs are not justified where significant clinical outcomes can potentially be achieved by substantially reduced CGM usage frequency e.g. monitoring on non - consecutive days a or a few (3 to 4) consecutive days per month. sugarBEAT ® is ideally positioned to cater for the Type 2 Diabetic population given it allows monitoring on any day the user chooses, making it an affordable solution. sugarBEAT ® accuracy appears comparable to at least one major incumbent invasive CGM.
4 Volunteer User Feedback Objectives Primary objective: To establish whether use of continuous glucose monitoring (CGM) a few days a week or month could lead to the same long term clinical outcome in terms of reduction in HbA1C and improved quality of life, as continuous wear of a CGM for up to 14 consecutive days at a time. The study would verify anecdotal evidence in literature based on intensive but intermittent glucose monitoring using finger prick testing. [Outcome from this portion will be reported periodically over the course of the next 12 months]. Secondary objective: To determine sugarBEAT® accuracy in real - life settings compared to the incumbent invasive CGMs such as the Dexcom® and Abbott Libre® which are known to exhibit high levels of accuracy. [Preliminary outcome reported in this presentation]. Rationale: If we can achieve the same long term clinical outcome using a CGM on non - consecutive days, we can dramatically reduce the cost of managing Type 2 diabetes, as well as increasing the number of people that can be treated and managed.

5 The Benefits of using CGM in Type 2 Diabetes on intermittent or non - consecutive days Currently there are no known devices other than sugarBEAT® that allow non - consecutive day use of a CGM. Invasive CGMs such as the Abbott Libre® and Dexcom® can be used by persons with Type 2 diabetes though the sensor wear time is up to 14 days and therefore the costs are currently commensurate with the 10 - 14 day sensor life. sugarBEAT® is a daily wear sensor and so the cost per use is limited to the cost of a single day sensor. The use of intensive finger - stick testing on non - consecutive days is considered to resemble the use of a CGM on non - consecutive days. It is on this basis that it is postulated that sugarBEAT® has the potential to provide a superior mode of measurement, and tool with which to manage Type 2 diabetes, a condition which constitutes approximately 90% of the total population with diabetes. The market opportunity is therefore substantial and use of CGM on non - consecutive days will potentially lead to a dramatic reduction in the costs associated with CGM usage in this population. Furthermore users need not suffer the inconvenience of piercing their arm with a sensor filament, nor be troubled with having to keep a device on their body for long periods of up to 2 weeks at a time.
6 Evidence for intensive Finger stick testing in Type 2 diabetes leading to positive long term outcomes 1. 6 - point glucose profiles two days per week. Counselling provided on diet and exercise [1] . Outcome: Significantly greater reduction in HbA1c. Marked improvement in general well - being, with significant improvements in depression and those with a lack of well - being. 2. 4 - point glucose profiles every 2 weeks. All patients instructed on lifestyle interventions [2] . Outcome: Significantly higher rates of regression and remission in experimental subjects. Significantly greater reductions in median HbA1c and BMI. Significantly improved lifestyle score. Treatment changes occurred earlier and more frequently. 3. 7 - point glucose profiles every 4 weeks. Patients received guidance for diet and exercise adjustments based on Self Monitoring Blood Glucose (SMBG) [3] . Outcome: Significant reductions in HbA1c, weight, BMI, systolic BP, diastolic BP, and LDL Cholesterol

7 Evidence for intensive Finger stick testing in Type 2 leading to positive long term outcomes cnt’d 4. 7 - point glucose profiles over 3 consecutive days per month. Education on device use and data collection using a paper tool. Basic education on use of SMBG to alter diet and physical activity [4] . Outcome: Significant reductions in HbA1c and mean, fasting, and postprandial glucose. 5. 7 - point glucose profiles over 3 consecutive days per month. Treatment adjustments made by clinicians based on SMBG [5] Outcome: Significant reductions in HbA1c. 6. 7 - point glucose profiles over 3 consecutive days, every 3 months [6] . Outcome: Significantly improved HbA1c. Treatment changes occurred earlier and more frequently.

8 Evidence for intensive Finger stick testing in Type 2 leading to positive long term outcomes cnt’d The above independent study outcomes provide compelling evidence for the use of a CGM on non - consecutive days or a few consecutive days per month, to provide clinically significant outcomes in the management and / or reversal of Type 2 diabetes. sugarBEAT® is ideally positioned to cater for this market over and above the incumbent invasive CGMs which have wear periods of up to 14 consecutive days with associated costs. With sugarBEAT ® it may be possible to provide a CGM to the majority of persons with Type 2 diabetes at an affordable cost point. This has the potential to change the paradigm for the management of Type 2 diabetes on a global scale.
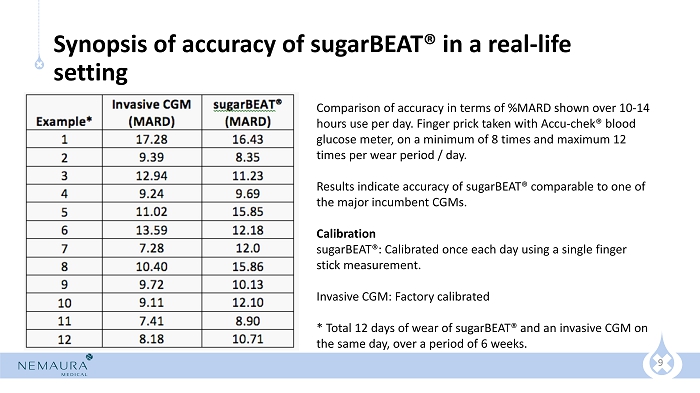
9 Synopsis of accuracy of sugarBEAT® in a real - life setting Comparison of accuracy in terms of %MARD shown over 10 - 14 hours use per day. Finger prick taken with Accu - chek ® blood glucose meter, on a minimum of 8 times and maximum 12 times per wear period / day. Results indicate accuracy of sugarBEAT ® comparable to one of the major incumbent CGMs. Calibration sugarBEAT®: Calibrated once each day using a single finger stick measurement. Invasive CGM: Factory calibrated * Total 12 days of wear of sugarBEAT ® and an invasive CGM on the same day, over a period of 6 weeks.

10 Conclusions There is compelling evidence from a number of studies suggesting Type 2 diabetes may be managed by CGM usage on non - consecutive days, leading to clinically significant outcomes. sugarBEAT ® is ideally positioned to cater for the Type 2 Diabetic population given it allows monitoring on non - consecutive days, making it an affordable solution. sugarBEAT ® accuracy is comparable to at least one major incumbent invasive CGM.
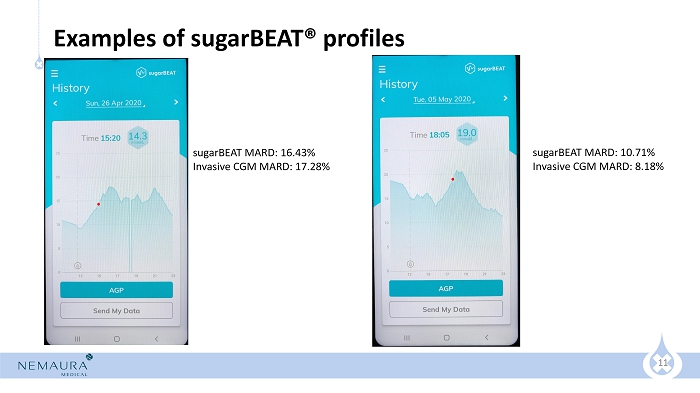
11 Examples of sugarBEAT ® profiles sugarBEAT MARD: 16.43% Invasive CGM MARD: 17.28% sugarBEAT MARD: 10.71% Invasive CGM MARD: 8.18%
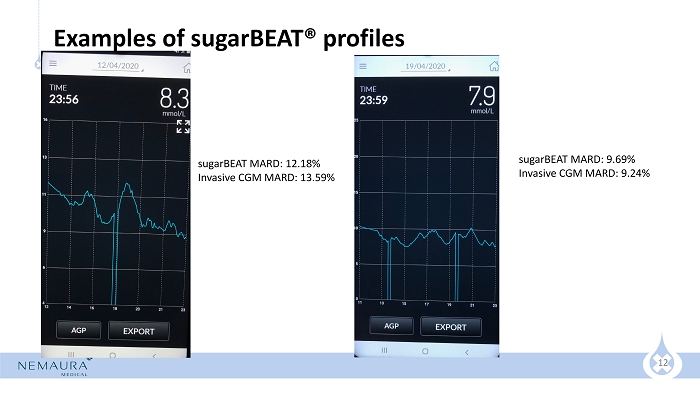
12 Examples of sugarBEAT ® profiles sugarBEAT MARD: 9.69% Invasive CGM MARD: 9.24% sugarBEAT MARD: 12.18% Invasive CGM MARD: 13.59%

13 References 1. Schwedes U, Siebolds M, Mertes G: Meal - related structured self - monitoring of blood glucose: effect on diabetes control in non - insulin - treated type 2 diabetic patients. Diabetes Care 2002;25:1928 – 1932 2. Durán A, Martín P, Runkle I, Pérez N, Abad R, Fernández M, Del Valle L, Sanz MF, Calle - Pascual AL: Benefits of self - monitoring blood glucose in the management of new - onset type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic - based interventional study with parallel groups. J Diabetes 2010;2:203 – 211 3. Kempf K, Kruse J, Martin S: ROSSO - in - praxi : a self - monitoring of blood glucose - structured 12 - week lifestyle intervention significantly improves glucometabolic control of patients with type 2 diabetes mellitus. Diabetes Technol Ther 2010;12:547 – 553 4. Khamseh ME, Ansari M, Malek M, Shafiee G, Baradaran H: Effects of a structured self - monitoring of blood glucose method on patient self - management behavior and metabolic outcomes in type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5:388 – 393 5. Kato NK, Kato M: Use of structured SMBG helps reduce A1c levels in insulin - treated diabetic patients [abstract]. Diabetes 2011;6 0( Suppl 1):A239 6. Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Axel - Schweitzer M, Petersen B, Wagner RS: A structured self - monitoring of blood glucose approach in type 2 diabetes encourages more frequent, intensive, and effective physician interven tio ns: results from the STeP study. Diabetes Technol Ther 2011;13:797 – 802
