Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Clearside Biomedical, Inc. | clsd-ex321_6.htm |
| EX-31.2 - EX-31.2 - Clearside Biomedical, Inc. | clsd-ex312_8.htm |
| EX-31.1 - EX-31.1 - Clearside Biomedical, Inc. | clsd-ex311_9.htm |
| EX-10.2 - EX-10.2 - Clearside Biomedical, Inc. | clsd-ex102_178.htm |
| EX-10.1 - EX-10.1 - Clearside Biomedical, Inc. | clsd-ex101_89.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2020
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number: 001-37783
Clearside Biomedical, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
Delaware |
45-2437375 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer |
|
900 North Point Parkway, Suite 200 Alpharetta, GA |
30005 |
|
(Address of principal executive offices) |
(Zip Code) |
(678) 270-3631
Registrant’s telephone number, including area code
N/A
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
Common Stock, par value $0.001 per share |
CLSD |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 4, 2020, the registrant had 44,868,558 shares of common stock, $0.001 par value per share, outstanding.
|
|
|
Page |
|
|
|
|
|
Item 1. |
2 |
|
|
|
Balance Sheets as of March 31, 2020 (unaudited) and December 31, 2019 |
2 |
|
|
Statements of Operations for the three months ended March 31, 2020 and 2019 (unaudited) |
3 |
|
|
Statements of Stockholders’ Equity for the three months ended March 31, 2020 and 2019 (unaudited) |
4 |
|
|
Statements of Cash Flows for the three months ended March 31, 2020 and 2019 (unaudited) |
5 |
|
|
6 |
|
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
17 |
|
Item 3. |
26 |
|
|
Item 4. |
26 |
|
|
|
|
|
|
Item 1. |
27 |
|
|
Item 1A. |
27 |
|
|
Item 2. |
28 |
|
|
Item 6. |
29 |
|
|
30 |
||
|
|
|
|
1
PART I – FINANCIAL INFORMATION
CLEARSIDE BIOMEDICAL, INC.
(in thousands, except share and per share data)
(unaudited)
|
|
|
March 31, 2020 |
|
|
December 31, 2019 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
20,930 |
|
|
$ |
22,595 |
|
|
Prepaid expenses |
|
|
892 |
|
|
|
1,139 |
|
|
Other current assets |
|
|
1,683 |
|
|
|
1,485 |
|
|
Total current assets |
|
|
23,505 |
|
|
|
25,219 |
|
|
Property and equipment, net |
|
|
551 |
|
|
|
541 |
|
|
Operating lease right-of-use asset |
|
|
627 |
|
|
|
656 |
|
|
Restricted cash |
|
|
360 |
|
|
|
360 |
|
|
Total assets |
|
$ |
25,043 |
|
|
$ |
26,776 |
|
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,633 |
|
|
$ |
1,280 |
|
|
Accrued liabilities |
|
|
1,492 |
|
|
|
2,930 |
|
|
Current portion of long-term debt |
|
|
5,183 |
|
|
|
1,333 |
|
|
Current portion of operating lease liabilities |
|
|
363 |
|
|
|
360 |
|
|
Deferred revenue |
|
|
5,100 |
|
|
|
5,000 |
|
|
Total current liabilities |
|
|
13,771 |
|
|
|
10,903 |
|
|
Long-term debt |
|
|
— |
|
|
|
3,819 |
|
|
Operating lease liabilities |
|
|
832 |
|
|
|
897 |
|
|
Total liabilities |
|
|
14,603 |
|
|
|
15,619 |
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 10,000,000 shares authorized and no shares issued at March 31, 2020 and December 31, 2019 |
|
|
— |
|
|
|
— |
|
|
Common stock, $0.001 par value; 100,000,000 shares authorized at March 31, 2020 and December 31, 2019; 44,868,558 and 44,413,372 shares issued and outstanding at March 31, 2020 and December 31, 2019, respectively |
|
|
45 |
|
|
|
44 |
|
|
Additional paid-in capital |
|
|
250,963 |
|
|
|
248,770 |
|
|
Accumulated deficit |
|
|
(240,568 |
) |
|
|
(237,657 |
) |
|
Total stockholders’ equity |
|
|
10,440 |
|
|
|
11,157 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
25,043 |
|
|
$ |
26,776 |
|
See accompanying notes to the financial statements
2
(in thousands, except share and per share data)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
License and other revenue |
|
$ |
4,097 |
|
|
$ |
45 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
|
3,811 |
|
|
|
10,967 |
|
|
General and administrative |
|
|
3,122 |
|
|
|
4,384 |
|
|
Total operating expenses |
|
|
6,933 |
|
|
|
15,351 |
|
|
Loss from operations |
|
|
(2,836 |
) |
|
|
(15,306 |
) |
|
Other expense |
|
|
(75 |
) |
|
|
(98 |
) |
|
Net loss |
|
$ |
(2,911 |
) |
|
$ |
(15,404 |
) |
|
Net loss per share of common stock — basic and diluted |
|
$ |
(0.07 |
) |
|
$ |
(0.45 |
) |
|
Weighted average shares outstanding — basic and diluted |
|
|
44,753,510 |
|
|
|
34,144,209 |
|
See accompanying notes to the financial statements.
3
Statements of Stockholders’ Equity
(in thousands, except share data)
(unaudited)
|
|
|
Three Months Ended March 31, 2020 |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Stockholders' |
|
||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Paid-In-Capital |
|
|
Deficit |
|
|
Equity |
|
|||||
|
Balance at December 31, 2019 |
|
|
44,413,372 |
|
|
$ |
44 |
|
|
$ |
248,770 |
|
|
$ |
(237,657 |
) |
|
$ |
11,157 |
|
|
Issuance of common shares from at-the-market sales agreement |
|
|
455,186 |
|
|
|
1 |
|
|
|
1,192 |
|
|
|
— |
|
|
|
1,193 |
|
|
Share-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
1,001 |
|
|
|
— |
|
|
|
1,001 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,911 |
) |
|
|
(2,911 |
) |
|
Balance at March 31, 2020 |
|
|
44,868,558 |
|
|
$ |
45 |
|
|
$ |
250,963 |
|
|
$ |
(240,568 |
) |
|
$ |
10,440 |
|
|
|
|
Three Months Ended March 31, 2019 |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Stockholders' |
|
||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Paid-In-Capital |
|
|
Deficit |
|
|
Equity |
|
|||||
|
Balance at December 31, 2018 |
|
|
32,119,227 |
|
|
$ |
32 |
|
|
$ |
230,475 |
|
|
$ |
(206,887 |
) |
|
$ |
23,620 |
|
|
Issuance of common shares from follow-on public offering |
|
|
4,660,966 |
|
|
|
5 |
|
|
|
6,622 |
|
|
|
— |
|
|
|
6,627 |
|
|
Exercise of stock options |
|
|
2,727 |
|
|
|
— |
|
|
|
1 |
|
|
|
— |
|
|
|
1 |
|
|
Share-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
1,247 |
|
|
|
— |
|
|
|
1,247 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(15,404 |
) |
|
|
(15,404 |
) |
|
Balance at March 31, 2019 |
|
|
36,782,920 |
|
|
$ |
37 |
|
|
$ |
238,345 |
|
|
$ |
(222,291 |
) |
|
$ |
16,091 |
|
See accompanying notes to the financial statements.
4
(in thousands)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(2,911 |
) |
|
$ |
(15,404 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
45 |
|
|
|
53 |
|
|
Share-based compensation expense |
|
|
1,001 |
|
|
|
1,247 |
|
|
Non-cash interest expense |
|
|
10 |
|
|
|
46 |
|
|
Accretion of debt discount |
|
|
21 |
|
|
|
15 |
|
|
Amortization and accretion on available-for-sale investments, net |
|
|
— |
|
|
|
(103 |
) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets |
|
|
49 |
|
|
|
1,536 |
|
|
Other assets and liabilities |
|
|
68 |
|
|
|
(29 |
) |
|
Accounts payable and accrued liabilities |
|
|
(1,086 |
) |
|
|
(14 |
) |
|
Net cash used in operating activities |
|
|
(2,803 |
) |
|
|
(12,653 |
) |
|
Investing activities |
|
|
|
|
|
|
|
|
|
Maturities of available-for-sale investments |
|
|
— |
|
|
|
30,950 |
|
|
Acquisition of property and equipment |
|
|
(55 |
) |
|
|
(18 |
) |
|
Net cash (used in) provided by investing activities |
|
|
(55 |
) |
|
|
30,932 |
|
|
Financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from at-the-market sales agreement, net of issuance costs |
|
|
1,193 |
|
|
|
6,627 |
|
|
Proceeds from exercise of stock options |
|
|
— |
|
|
|
1 |
|
|
Net cash provided by financing activities |
|
|
1,193 |
|
|
|
6,628 |
|
|
Net (decrease) increase in cash, cash equivalents and restricted cash |
|
|
(1,665 |
) |
|
|
24,907 |
|
|
Cash, cash equivalents and restricted cash, beginning of period |
|
|
22,955 |
|
|
|
8,403 |
|
|
Cash, cash equivalents and restricted cash, end of period |
|
$ |
21,290 |
|
|
$ |
33,310 |
|
Reconciliation of cash, cash equivalents and restricted cash:
|
|
|
March 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Cash and cash equivalents |
|
$ |
20,930 |
|
|
$ |
32,950 |
|
|
Restricted cash |
|
|
360 |
|
|
|
360 |
|
|
Cash, cash equivalents and restricted cash at end of period |
|
$ |
21,290 |
|
|
$ |
33,310 |
|
See accompanying notes to the financial statements.
5
Notes to the Financial Statements
(unaudited)
1. The Company
Clearside Biomedical, Inc. (the “Company”) is a biopharmaceutical company dedicated to developing and delivering treatments that restore and preserve vision for people with serious back of the eye diseases. The Company’s proprietary SCS Microinjector targeting the suprachoroidal space offers unique access to the macula, retina and choroid where sight-threatening disease often occurs. This suprachoroidal space injection is an inherently flexible, in-office, non-surgical procedure, intended to provide targeted delivery to the site of disease and to work with both established and new formulations of medications, as well as future therapeutic innovations, such as gene therapy. Incorporated in the State of Delaware on May 26, 2011, the Company has its corporate headquarters in Alpharetta, Georgia.
The Company’s activities since inception have primarily consisted of developing product and technology rights and performing research and development activities. The Company has no current source of revenue to sustain present activities, and does not expect to generate meaningful revenue until and unless the Company receives regulatory approval of and successfully commercializes its product candidates, either on its own or with a third party. The Company is subject to a number of risks and uncertainties similar to those of other life science companies at a similar stage of development, including, among others, the need to obtain adequate additional financing, successful development efforts including regulatory approval of products, compliance with government regulations, successful commercialization of potential products, protection of proprietary technology and dependence on key individuals.
Liquidity
The Company had cash and cash equivalents of $20.9 million as of March 31, 2020. The Company has funded its operations primarily through the sale of convertible preferred stock and common stock and the issuance of long-term debt. The Company will continue to need to obtain additional financing to fund future operations, including completing the development, partnering and potential commercialization of its primary product candidates. The Company will need to obtain financing to conduct additional trials for the regulatory approval of its product candidates if requested by regulatory bodies, and completing the development of any product candidates that might be acquired. If such products were to receive regulatory approval, the Company would need to obtain financing to prepare for the potential commercialization of its product candidates, if the Company decides to commercialize the products on its own.
The Company has suffered recurring losses and negative cash flows from operations since inception and anticipates incurring additional losses until such time, if ever, that it can obtain regulatory approval to sell, and then generate significant revenue from commercializing its lead product candidate, XIPERE (triamcinolone acetonide suprachoroidal injectable suspension), either on its own or together with a third party. In the absence of product or other revenues, the amount, timing, nature or source of which cannot be predicted, the Company’s losses will continue as it conducts its research and development activities.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date the financial statements are issued. Until the Company can generate sufficient revenue, the Company will need to finance future cash needs through public or private equity offerings, license agreements, debt financings or restructurings, collaborations, strategic alliances and marketing or distribution arrangements.
The Company’s financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result should the Company be unable to continue as a going concern.
2. Significant Accounting Policies
Basis of Presentation
The Company’s financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
6
Unaudited Interim Financial Information
The accompanying balance sheet as of March 31, 2020, statements of operations for the three months ended March 31, 2020 and 2019, statements of stockholders’ equity for the three months ended March 31, 2020 and 2019 and statements of cash flows for the three months ended March 31, 2020 and 2019 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include normal recurring adjustments, necessary for the fair presentation of the Company’s financial position as of March 31, 2020, its results of its operations for the three months ended March 31, 2020 and 2019, its changes in stockholders’ equity for the three months ended March 31, 2020 and 2019 and its cash flows for the three months ended March 31, 2020 and 2019. The financial data and other information disclosed in these notes related to the three months ended March 31, 2020 and 2019 are unaudited. The results for the three months ended March 31, 2020 are not indicative of results to be expected for the year ending December 31, 2020, any other interim periods or any future year or period. These unaudited financial statements should be read in conjunction with the audited financial statements and related footnotes, which are included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2019.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of income and expenses during the reporting periods. Significant items subject to such estimates and assumptions include the accounting for useful lives to calculate depreciation and amortization, clinical expense accruals, share-based compensation expense and income tax valuation allowance. Actual results could differ from these estimates.
The COVID-19 pandemic is expected to result in a global slowdown of economic activity. Estimates and assumptions about future events and their effects cannot be determined with certainty and therefore require the exercise of judgment. As of the date of issuance of these financial statements, we are not aware of any specific event or circumstance that would require us to update our estimates, assumptions and judgments or revise the carrying value of our assets or liabilities. These estimates may change as new events occur and additional information is obtained and are recognized in the consolidated financial statements as soon as they become known. Actual results could differ from those estimates and any such differences may be material to our financial statements.
Revenue Recognition
The Company recognizes revenue from its contracts with customers under Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC Topic 606”). The Company’s primary revenue arrangements are license agreements which typically include upfront payments, regulatory and commercial milestone payments and royalties based on future product sales. The arrangements may also include payments for the Company’s SCS Microinjector devices as well as payments for assistance and oversight of the customer’s use of the Company’s technology. In determining the amount of revenue to be recognized under these agreements, the Company performs the following steps: (i) identifies the promised goods and services to be transferred in the contract, (ii) identifies the performance obligations, (iii) determines the transaction price, (iv) allocates the transaction price to the performance obligations and (v) recognizes revenue as the performance obligations are satisfied.
The Company receives payments from its customers based on billing schedules established in each contract. Upfront and other payments may require deferral of revenue recognition to a future period until the Company performs its obligations under the arrangement. Amounts are recorded as accounts receivable when the Company’s right to consideration is unconditional. The Company does not assess whether a contract has a significant financing component if the expectation at contract inception is such that the period between payment by the customer and the transfer of the promised goods or services to the customer will be one year or less.
Research and Development Costs
Research and development costs are charged to expense as incurred and include:
|
|
• |
employee-related expenses, including salaries, benefits, travel and share-based compensation expense for research and development personnel; |
|
|
• |
expenses incurred under agreements with contract research organizations, contract manufacturing organizations and consultants that conduct clinical trials and preclinical studies; |
|
|
• |
costs associated with preclinical and clinical development activities; |
|
|
• |
costs associated with submitting regulatory approval applications for the Company’s product candidates; |
|
|
• |
costs associated with training physicians on the suprachoroidal injection procedure and educating and providing them with appropriate product candidate information; |
7
|
|
• |
costs for the Company’s research and development facility; and |
|
|
• |
depreciation expense for assets used in research and development activities. |
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information provided to the Company by its vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the patterns of costs incurred, and are reflected in the financial statements as prepaid or accrued expense.
Share-Based Compensation
Compensation cost related to share-based awards granted to employees is measured based on the estimated fair value of the award at the grant date. The Company estimates the fair value of stock options using a Black-Scholes option pricing model. Compensation expense for options granted to non-employees is determined as the fair value of consideration received or the fair value of the equity instruments issued, whichever is more reliably measured. The fair value of restricted stock units granted is measured based on the market value of the Company’s common stock on the date of grant. Share-based compensation costs are expensed on a straight-line basis over the relevant vesting period.
Compensation cost related to shares purchased through the Company’s employee stock purchase plan, which is considered compensatory, is based on the estimated fair value of the shares on the offering date, including consideration of the discount and the look back period. The Company estimates the fair value of the shares using a Black-Scholes option pricing model. Compensation expense is recognized over the six-month withholding period prior to the purchase date.
All share-based compensation costs are recorded in general and administrative or research and development costs in the statements of operations based upon the underlying employees’ roles within the Company.
Cash Equivalents
Cash equivalents consist of short-term, highly liquid investments with an original term of three months or less at the date of purchase.
Concentration of Credit Risk Arising From Cash Deposits in Excess of Insured Limits
The Company maintains its cash in bank deposits that at times may exceed federally insured limits. The Company has not experienced any loss in such accounts. The Company believes it is not exposed to any significant risks with respect to its cash balances.
Recent Accounting Pronouncements
Accounting Pronouncements Recently Adopted
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820-10): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”), which changes the fair value measurement disclosure requirements of ASC Topic 820, Fair Value Measurements and Disclosures. Under this ASU, certain disclosure requirements for fair value measurements are eliminated, amended or added. These changes aim to improve the overall usefulness of disclosures to financial statement users and reduce unnecessary costs to companies when preparing the disclosures. The Company adopted ASU 2018-13 on January 1, 2020, and the adoption did not have a material impact on its financial statements and disclosures.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, which requires the measurement and recognition of expected credit losses for financial assets held at amortized cost, including trade receivables. ASU 2016-13 replaces the existing incurred loss impairment model with an expected loss model that requires the use of forward-looking information to calculate credit loss estimates. The Company adopted ASU 2016-13 on January 1, 2020, and the adoption did not have a material impact on its financial statements and related disclosures.
Recent Accounting Pronouncements Not Yet Adopted
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”), which simplifies the accounting for income taxes by removing certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. The new ASU also simplifies aspects of the accounting for franchise taxes and enacted changes in tax laws or rates. These changes aim to improve the overall usefulness of disclosures to financial statement users and reduce unnecessary costs to companies when preparing the disclosures. The guidance is effective for the Company beginning on January 1,
8
2021 and prescribes different transition methods for the various provisions. Early adoption is permitted. The Company does not expect the adoption of ASU 2019-12 to have a material impact on its financial statements and related disclosures.
3. Property and Equipment, Net
Property and equipment, net consisted of the following (dollar amounts in thousands):
|
|
|
Estimated Useful Lives (Years) |
|
March 31, 2020 |
|
|
December 31, 2019 |
|
||
|
Furniture and fixtures |
|
5 |
|
$ |
337 |
|
|
$ |
337 |
|
|
Machinery and equipment |
|
5 |
|
|
176 |
|
|
|
121 |
|
|
Computer equipment |
|
3 |
|
|
13 |
|
|
|
13 |
|
|
Leasehold improvements |
|
Lesser of useful life or remaining lease term |
|
|
667 |
|
|
|
667 |
|
|
|
|
|
|
|
1,193 |
|
|
|
1,138 |
|
|
Less: Accumulated depreciation |
|
|
|
|
(642 |
) |
|
|
(597 |
) |
|
|
|
|
|
$ |
551 |
|
|
$ |
541 |
|
4. Accrued Liabilities
Accrued liabilities consisted of the following (in thousands):
|
|
|
March 31, |
|
|
December 31, |
|
||
|
|
|
2020 |
|
|
2019 |
|
||
|
Accrued research and development |
|
$ |
185 |
|
|
$ |
359 |
|
|
Accrued employee costs |
|
|
425 |
|
|
|
1,530 |
|
|
Accrued severance |
|
|
531 |
|
|
|
751 |
|
|
Accrued professional fees |
|
|
122 |
|
|
|
58 |
|
|
Accrued expense |
|
|
229 |
|
|
|
232 |
|
|
|
|
$ |
1,492 |
|
|
$ |
2,930 |
|
5. Long-Term Debt
Loan and Security Agreements
On May 14, 2018, the Company entered into a second amended and restated loan and security agreement (the “2nd A&R Loan Agreement”) with SVB, MidCap Funding III Trust and MidCap Financial Trust (together, “MidCap” and collectively with SVB, the “Lenders”), which amended and restated in its entirety the first amended and restated loan agreement. The 2nd A&R Loan Agreement provided for new term loans of up to $20.0 million, with a floating interest rate equal to 6.50% plus the greater of (i) the 30-day U.S. LIBOR, as reported in the Wall Street Journal on the last business day of the month that immediately precedes the month in which the interest will accrue, or (ii) 1.89%. The 2nd A&R Loan Agreement includes, among other things, the ability of the Lenders to accelerate the payment of the term loan in the event of material adverse change and restrictions on the Company’s ability to sell, assign, license, transfer or otherwise dispose of its assets, including intellectual property assets, without the prior written consent of the Lenders.
The Company borrowed an initial tranche of $10.0 million on May 14, 2018, of which $7.0 million was used to repay all amounts outstanding under the first amended and restated loan agreement, including fees associated with the final payment. The prepayment fees were waived. Of the remaining $10.0 million under the 2nd A&R Loan Agreement, the Company elected not to draw $5.0 million and the other $5.0 million is not available for draw.
On October 18, 2019, the Company entered into an amendment to the 2nd A&R Loan Agreement with the Lenders. Pursuant to the amendment, the Company repaid $5.0 million of the outstanding principal balance of the $10.0 million term loan. The Company did not pay any final payment or termination fees in connection with the $5.0 million prepayment. In addition, the Company and the Lenders agreed to modify the term loan repayment schedule. As amended, the term loan repayment schedule provides for interest only payments through October 31, 2020, followed by consecutive equal monthly payments of principal and interest in arrears continuing through the maturity date of October 1, 2022. The Company has the option to prepay the outstanding balance in full, subject to a
9
prepayment fee of 2% of the original principal amount for any prepayment prior to October 1, 2022. A final payment of 5.50% of the aggregate borrowed amount is due at maturity of the loan on October 1, 2022, or upon the prepayment of the facility or the acceleration of amounts due under the facility as a result of an event of default. In addition, the Company agreed that if the Company’s cash and cash equivalents balance with SVB falls below $10.0 million, the Company will transfer to a pledged account an amount of cash and cash equivalents equal to the sum of the then-outstanding principal balance of the term loan plus a final payment fee of $0.3 million.
On May 7, 2020, the Company paid $5.0 million principal balance under the 2nd A&R Loan Agreement with the Lenders, plus $0.3 million reflecting the final payment and accrued interest. As a result of the payment, as of March 31, 2020, the Company has reflected in current liabilities the amount of principal it expects to pay within the next 12 months.
The borrowings under the 2nd A&R Loan Agreement are secured by substantially all of the Company’s assets.
Interest expense on the borrowings under the loan agreements described above was $106,000 and $225,000 for the three months ended March 31, 2020 and 2019, respectively. Accretion of the scheduled final payment was $10,000 and $46,000 for the three months ended March 31, 2020 and 2019, respectively. Accretion of the deferred debt issuance costs was $21,000 and $15,000 for the three months ended March 31, 2020 and 2019, respectively.
As of March 31, 2020, the scheduled payments for the 2nd A&R Loan Agreement, as amended, and the scheduled final payment were as follows (in thousands):
|
Year Ending December 31, |
|
Principal |
|
|
Interest and Final Payment |
|
|
Total |
|
|||
|
2020 |
|
$ |
5,000 |
|
|
$ |
382 |
|
|
$ |
5,382 |
|
6. Common Stock
The Company’s amended and restated certificate of incorporation authorizes the Company to issue 100,000,000 shares of $0.001 par value common stock. As of March 31, 2020 and December 31, 2019, there were 44,868,558 and 44,413,372 shares of common stock outstanding, respectively.
7. Stock Purchase Warrants
In September 2016, in connection with a loan agreement, the Company issued warrants to purchase up to 29,796 shares of common stock at a price per share of $10.74. The warrants expire in September 2026, or earlier upon the occurrence of specified mergers or acquisitions of the Company, and are immediately exercisable. The warrants were recorded in equity and had a weighted average remaining life of 6.50 years as of March 31, 2020.
8. Share-Based Compensation
Share-based compensation is accounted for in accordance with the provisions of ASC 718, Compensation-Stock Compensation.
Stock Options
The Company has granted stock option awards to employees, directors and consultants from its 2011 Stock Incentive Plan (the “2011 Plan”) and its 2016 Equity Incentive Plan (the “2016 Plan”). The estimated fair value of options granted is determined as of the date of grant using the Black-Scholes option pricing model. The resulting fair value is recognized ratably over the requisite service period, which is generally the vesting period of the awards.
Share-based compensation expense for options granted under the 2011 Plan and the 2016 Plan is reflected in the statements of operations as follows (in thousands):
|
|
Three Months Ended March 31, |
|
||||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Research and development |
|
$ |
306 |
|
|
$ |
462 |
|
|
General and administrative |
|
|
413 |
|
|
|
775 |
|
|
Total |
|
$ |
719 |
|
|
$ |
1,237 |
|
10
The following table summarizes the activity related to stock options during the three months ended March 31, 2020:
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
Number of |
|
|
Average |
|
||
|
|
|
Shares |
|
|
Exercise Price |
|
||
|
Options outstanding at December 31, 2019 |
|
|
4,104,450 |
|
|
$ |
4.63 |
|
|
Granted |
|
|
903,500 |
|
|
|
2.37 |
|
|
Forfeited |
|
|
(9,845 |
) |
|
|
3.05 |
|
|
Options outstanding at March 31, 2020 |
|
|
4,998,105 |
|
|
|
4.23 |
|
|
|
|
|
|
|
|
|
|
|
|
Options exercisable at December 31, 2019 |
|
|
2,452,764 |
|
|
|
5.44 |
|
|
|
|
|
|
|
|
|
|
|
|
Options exercisable at March 31, 2020 |
|
|
2,782,163 |
|
|
|
5.14 |
|
As of March 31, 2020, the Company had $5.1 million of unrecognized compensation expense related to unvested stock options, which is expected to be recognized over a weighted average period of 2.6 years.
Restricted Stock Units
The Company has granted restricted stock units (“RSUs”) to employees from the 2016 Plan. The shares underlying the RSU awards have vesting terms of eight months to four years from the date of grant subject to the employees’ continuous service and subject to accelerated vesting in specified circumstances.
The fair value of the RSUs granted is measured based on the market value of the Company’s common stock on the date of grant and is recognized ratably over the requisite service period, which is generally the vesting period of the awards.
The following table summarizes the activity related to RSUs during the three months ended March 31, 2020:
|
|
|
|
|
|
|
Weighted Average |
|
|
|
|
|
Number of |
|
|
Grant Date |
|
||
|
|
|
Shares |
|
|
Fair Value |
|
||
|
Non-vested RSUs outstanding at December 31, 2019 |
|
|
1,269,300 |
|
|
$ |
0.87 |
|
|
Granted |
|
|
486,000 |
|
|
|
2.39 |
|
|
Vested |
|
|
(100,000 |
) |
|
|
1.02 |
|
|
Forfeited |
|
|
(6,700 |
) |
|
|
0.91 |
|
|
Non-vested RSUs outstanding at March 31, 2020 |
|
|
1,648,600 |
|
|
|
1.31 |
|
The Company recorded $0.3 million of share-based compensation expense for the three months ended March 31, 2020 for the RSUs. There were no RSUs issued during the same period in the prior year. As of March 31, 2020, the Company had $1.6 million of unrecognized compensation expense related to the RSUs, which is expected to be recognized over a weighted average period of 2.9 years.
Employee Stock Purchase Plan
In January 2016, the Company’s board of directors adopted and approved, and in January 2016 the Company’s stockholders approved, the Clearside Biomedical, Inc. 2016 Employee Stock Purchase Plan (the “2016 ESPP”) which became effective on June 1, 2016. The 2016 ESPP is considered a compensatory plan and the fair value of the discount and the look-back period are estimated using the Black-Scholes option pricing model and expense is recognized over the six month withholding period prior to the purchase date. The Company has recorded $3,000 and $10,000 of share-based compensation expense for the three months ended March 31, 2020 and 2019, respectively, in the statements of operations for the estimated number of shares to be purchased on the next purchase date following the conclusion of the applicable reporting period.
9. Commitments and Contingencies
Lease Commitment Summary
In November 2016, the Company signed an office lease agreement to lease approximately 20,000 square feet of office space in Alpharetta, Georgia for its corporate headquarters. The lease agreement is for a 6.5 year term with a renewal option for one additional five-year term. Rental payments are $35,145 per month subject to an increase of 3% per year. Rent expense under this lease is
11
recognized on a straight-line basis over the term of the lease. In addition, the lease agreement requires payment of the pro-rata share of the annual operating expenses associated with the premises.
The Company’s operating leases included on the balance sheet are as follows (in thousands):
|
|
|
March 31, 2020 |
|
|
|
Operating lease right-of-use asset |
|
$ |
627 |
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
Current portion of operating lease liabilities |
|
$ |
363 |
|
|
Operating lease liabilities |
|
|
832 |
|
|
Total operating lease liabilities |
|
$ |
1,195 |
|
The Company recognizes a right-of-use asset for the right to use the underlying asset for the lease term, and a lease liability, which represents the present value of the Company’s obligation to make payments over the lease term. The renewal option is not included in the calculation of the right-of-use asset and the lease liabilities as the Company has not yet determined if the Alpharetta, Georgia lease will be renewed. The present value of the lease payments is calculated using an incremental borrowing rate as the Company’s leases do not provide an implicit interest rate. At March 31, 2020, the Company’s weighted average discount rate was 11.0% and the weighted average lease term was 3.5 years.
Minimum lease payments were as follows at March 31, 2020 (in thousands):
|
Year Ending December 31, |
|
|
|
|
|
2020 |
|
|
286 |
|
|
2021 |
|
|
392 |
|
|
2022 |
|
|
407 |
|
|
2023 |
|
|
316 |
|
|
Total minimum lease payments |
|
|
1,401 |
|
|
Less imputed interest |
|
|
(206 |
) |
|
Total operating lease liabilities |
|
$ |
1,195 |
|
Equipment leases with an initial term of 12 months or less are not recorded with operating lease liabilities. The Company recognizes expense for these on a straight-line basis over the lease term. The equipment leases were deemed to be immaterial.
Operating lease cost was $62,000 and $100,000 for the three months ended March 31, 2020 and 2019, respectively. Variable lease cost was $24,000 and $30,000 for the three months ended March 31, 2020 and 2019, respectively. Short-term lease cost for the three months ended March 31, 2020 and 2019 was $4,000 and $3,000, respectively. Cash payments included in operating activities on the statement of cash flows for operating lease liabilities were $92,000 and $129,000 for the three months ended March 31, 2020 and 2019, respectively.
Contract Service Providers
In the course of the Company’s normal business operations, it has agreements with contract service providers to assist in the performance of its research and development, clinical research and manufacturing. Substantially all of these contracts are on an as needed basis.
10. License and Other Agreements
Arctic Vision (Hong Kong) Limited
On March 10, 2020, the Company entered into a License Agreement (the “Arctic License Agreement”) with Arctic Vision (Hong Kong) Limited (“Arctic Vision”). Pursuant to the Arctic License Agreement, the Company has granted an exclusive license to Arctic Vision to develop, distribute, promote, market and commercialize XIPERE, the Company’s proprietary suspension of the corticosteroid triamcinolone acetonide formulated for administration to the back of the eye using the Company’s proprietary SCS Microinjector, subject to specified exceptions, in China, Hong Kong, Macau, Taiwan and South Korea (the “Arctic Territory”). Under the terms of the Arctic License Agreement, neither party may commercialize XIPERE in the other party’s territory. Arctic Vision has agreed to use commercially reasonably efforts to pursue development and commercialization of XIPERE for indications associated with uveitis in the Arctic Territory. In addition, upon receipt of the Company’s consent, Arctic Vision will have the right, but not the obligation, to develop and commercialize XIPERE for additional indications in the Arctic Territory.
12
Pursuant to the Arctic License Agreement, Arctic Vision has agreed to pay the Company up to a total of $35.5 million. This amount includes an upfront payment of $4.0 million as well as an aggregate of up to $31.5 million in development milestone payments for specified events prior to and including receipt of approval of XIPERE in the United States and sales milestone payments for achievement of specified levels of net sales. Further, during the applicable royalty term, the Company will also be entitled to receive tiered royalties of ten to twelve percent of net sales based on achieving certain annual net sales thresholds in the Arctic Territory, subject to customary reductions, payable on a product-by-product and country-by-country basis, commencing at launch in such country and lasting until the latest of (i) the date that all valid claims within the licensed patent rights covering XIPERE have expired, (ii) the date of the loss of marketing or regulatory exclusivity of XIPERE in a given country, or (iii) ten years from the first commercial sale of XIPERE in a given country. As of March 31, 2020, it was determined that the Company had completed its performance obligations related to the upfront payment and recognized license revenue of $4.0 million.
Other
The Company has periodically entered into other short-term agreements, generally with performance obligations of one to two months, to evaluate the potential use of its proprietary SCS Microinjector with third-party product candidates for the treatment of various diseases. Funds received from these agreements are recognized as revenue over the term of the agreement. The Company recorded $45,000 of revenue from these agreements during the three months ended March 31, 2019. In addition, the Company recorded $0.1 million of deferred revenue in other current liabilities from these agreements as of March 31, 2020.
11. Fair Value Measurements
The Company records certain financial assets and liabilities at fair value in accordance with the provisions of ASC Topic 820, Fair Value Measurements and Disclosures, on fair value measurements. As defined in the guidance, fair value, defined as an exit price, represents the amount that would be received to sell an asset or pay to transfer a liability in an orderly transaction between market participants. As a result, fair value is a market-based approach that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering these assumptions, the guidance defines a three-tier value hierarchy that prioritizes the inputs used in the valuation methodologies in measuring fair value.
|
|
• |
Level 1—Unadjusted quoted prices in active, accessible markets for identical assets or liabilities. |
|
|
• |
Level 2—Other inputs that are directly or indirectly observable in the marketplace. |
|
|
• |
Level 3—Unobservable inputs that are supported by little or no market activity. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The Company’s material financial instruments at March 31, 2020 and December 31, 2019 consisted primarily of cash and cash equivalents and long-term debt. The fair values of cash and cash equivalents, other current assets and accounts payable approximate their respective carrying values due to the short term nature of these instruments and are classified as Level 1 in the fair value hierarchy. The fair value of long-term debt approximates the carrying value due to variable interest rates that correspond to market rates and is classified as Level 1 in the fair value hierarchy.
There were no significant transfers between Levels 1, 2 and 3 during the three months ended March 31, 2020 and the year ended December 31, 2019.
The following tables summarize the fair value of financial assets that are measured at fair value and the classification by level of input within the fair value hierarchy (in thousands):
|
|
|
March 31, 2020 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Recorded Value |
|
||||
|
Financial Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and money markets |
|
$ |
20,930 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
20,930 |
|
|
Restricted cash money market |
|
|
360 |
|
|
|
— |
|
|
|
— |
|
|
|
360 |
|
|
Total financial assets |
|
$ |
21,290 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
21,290 |
|
13
|
|
|
December 31, 2019 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Recorded Value |
|
||||
|
Financial Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and money markets |
|
$ |
22,595 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,595 |
|
|
Restricted cash money market |
|
|
360 |
|
|
|
— |
|
|
|
— |
|
|
|
360 |
|
|
Total financial assets |
|
$ |
22,955 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,955 |
|
12. Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss by the weighted average number of shares of common stock outstanding for the period, without consideration of the dilutive effect of potential common stock equivalents. Diluted net loss per share gives effect to all dilutive potential shares of common stock outstanding during this period. For all periods presented, the Company’s potential common stock equivalents, which included stock options and stock purchase warrants, have been excluded from the computation of diluted net loss per share as their inclusion would have the effect of reducing the net loss per share. Therefore, the denominator used to calculate both basic and diluted net loss per share is the same in all periods presented. The Company’s potential common stock equivalents that have been excluded from the computation of diluted net loss per share for all periods presented because of their antidilutive effect consisted of the following:
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Outstanding stock options |
|
|
4,998,105 |
|
|
|
4,832,889 |
|
|
Non-vested restricted stock units |
|
|
1,648,600 |
|
|
|
— |
|
|
Stock purchase warrants |
|
|
29,796 |
|
|
|
29,796 |
|
|
|
|
|
6,676,501 |
|
|
|
4,862,685 |
|
13. Subsequent Events
License Agreement Amendment
On April 27, 2020, the Company and Bausch Health Ireland Limited (“Bausch”) entered into an amendment (the “Amendment”) to the Company’s License Agreement with Bausch dated October 22, 2019 (as amended, the “Bausch License Agreement”). Pursuant to the Bausch License Agreement, the Company has granted an exclusive license to Bausch to develop, manufacture, distribute, promote, market and commercialize XIPERE, the Company’s proprietary suspension of the corticosteroid triamcinolone acetonide formulated for administration to the back of the eye using the Company’s proprietary microneedle (the “Device”), as well as specified other steroids, corticosteroids and NSAIDs in combination with the Device (“Other Products”; and together with XIPERE, “Products”), subject to specified exceptions, in the United States and Canada (the “Original Territory”) for the treatment of ophthalmology indications, including non-infectious uveitis. Pursuant to the Amendment, the Company has granted Bausch an exclusive option to develop, manufacture, distribute, promote, market and commercialize XIPERE in one or more of the following regions (the “Option”): (i) the European Union, including the United Kingdom, (ii) Australia and New Zealand and (iii) South America and Mexico (such regions, the “Additional Regions” and together with the Original Territory, the “Territory”). The Option may be exercised any time before the earlier of regulatory approval of XIPERE in the United States and August 31, 2021.
Pursuant to the Bausch License Agreement, Bausch paid the Company an upfront payment of $5.0 million (the “Upfront Payment”) in October 2019, which is subject to a refund if the Bausch License Agreement is terminated in specified circumstances. In addition, Bausch has agreed to make additional payments of up to $15.0 million upon the achievement of specified pre-launch development and regulatory milestones (the “Pre-Launch Milestone Payments”) and up to an aggregate of $57.3 million in additional milestone payments upon the achievement of (i) specified regulatory approvals for specified additional indications of XIPERE (including certain regulatory and commercial milestones if Bausch exercises its Option in the European Union) and (ii) specified levels of annual net sales (as defined in the Bausch License Agreement). Further, during the applicable royalty term, the Company will also be entitled to receive tiered royalties at increasing percentages, from the high-teens to twenty percent, based on XIPERE achieving certain annual net sales thresholds in the Original Territory, as well as a lower royalty on annual net sales of Other Products in the Original Territory and on annual net sales of Xipere in the Additional Regions if Bausch exercises its Option, in each case subject to reductions in specified circumstances; provided that the Company will not receive any royalties on the first $45.0 million of cumulative net sales of all products in the Original Territory.
14
The Company is responsible for all development expenses for XIPERE in the Original Territory until the Company’s New Drug Application (“NDA”) for XIPERE is approved by the U.S. Food and Drug Administration (the “FDA”), subject to specified exceptions, as well as manufacturing costs in connection with the NDA. The Company is also responsible for all clinical and development expenses conducted to satisfy the FDA’s requests in the complete response letter issued on October 18, 2019 related to the NDA and any subsequent complete response letter related to the NDA (the “CRL-related expenses”). If XIPERE is approved by the FDA, Bausch will be responsible for all expenses following such approval; provided that the Company will be responsible for the CRL-related expenses and for half of the costs of any post-approval clinical trials required by the FDA, up to a specified maximum amount.
During the term of the Bausch License Agreement, and in the Territory, the Company has agreed not to (i) develop or commercialize XIPERE alone or in combination with an Other Device (as defined in the Bausch License Agreement) in the licensed field, (ii) develop or commercialize any corticosteroid with the Device or an Other Device in the licensed field, (iii) develop or commercialize the Device or an Other Device with any active pharmaceutical ingredient for non-infectious uveitis or macular edema associated with non-infectious uveitis, including with any Other Drug (as defined in the Bausch License Agreement, which are restricted to those steroids, corticosteroids and non-steroidal anti-inflammatory drugs specifically identified in the Bausch License Agreement), (iv) develop or commercialize any Other Drug in combination with the Device and (v) commercialize any Other Device for achieving non-surgical access to the suprachoroidal space where such device is sold as a stand-alone product, subject to specified exceptions. The Bausch License Agreement will expire upon expiration of the royalty terms for all Products and countries in the Territory, with each royalty term for a given Product and country ending on the latest of (i) the date of expiration of the last-to-expire valid claim of any licensed patent rights covering such Product in such country in the Territory, (ii) the date of the loss of regulatory exclusivity for such Product in such country in the Territory, or (iii) ten years from the later of the first sale of such Product in such country in the Territory. For a specified period of time, Bausch may terminate the Bausch License Agreement immediately and have the Upfront Payment refunded if the FDA has not approved the XIPERE NDA by August 31, 2021. Following the payment of the Pre-Launch Milestone Payments, Bausch may also terminate the Bausch License Agreement for convenience upon 180 days’ written notice. In addition, the Company can terminate the Bausch License Agreement if Bausch commences a legal action challenging the validity, enforceability or scope of any of the licensed patents. If the FDA requires an additional clinical trial prior to approving the NDA for XIPERE and the Company notifies Bausch that the Company will not conduct the trial at the Company’s expense, then Bausch may terminate the Bausch License Agreement and have the Upfront Payment refunded within 60 days of the receipt of such notice from the Company. Both parties may terminate the Bausch License Agreement (i) upon a material breach of the Bausch License Agreement, subject to a specified cure period and specified exceptions, or (ii) if the other party encounters bankruptcy or insolvency. Upon termination (other than for a material breach by or bankruptcy or insolvency event of the Company), all licenses and other rights granted by the Company to Bausch pursuant to the Bausch License Agreement would revert to the Company.
CARES Act Paycheck Protection Program (PPP) Loan
On April 20, 2020, the Company entered into a loan agreement with Silicon Valley Bank (“PPP Lender”) under the terms of which the PPP Lender agreed to make a loan to the Company in an aggregate principal amount of $1.0 million (“PPP Loan”) pursuant to the Paycheck Protection Program under the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”). The PPP Loan is evidenced by a promissory note (the “Note”) containing the terms and conditions for repayment of the PPP Loan.
Under the terms of the Note and the PPP Loan, interest accrues on the outstanding principal at the rate of 1.0% per annum. The term of the Note is two years, though it may be payable sooner in connection with an event of default under the Note. To the extent the loan amount is not forgiven under the PPP, the Company is obligated to make equal monthly payments of principal and interest, beginning seven months from the date of the Note, until the maturity date.
The CARES Act and the PPP provide a mechanism for forgiveness of up to the full amount borrowed. Under the PPP, the Company may apply for forgiveness for all or a part of the PPP Loan. The amount of loan proceeds eligible for forgiveness is based on a formula that takes into account a number of factors, including the amount of loan proceeds used by the Company during the eight-week period after the loan origination for certain purposes including payroll costs, interest on certain mortgage obligations, rent payments on certain leases, and certain qualified utility payments, provided that at least 75% of the loan amount is used for eligible payroll costs; the employer maintaining or rehiring employees and maintaining salaries at certain levels; and other factors. Subject to the other requirements and limitations on loan forgiveness, only loan proceeds spent on payroll and other eligible costs during the covered eight-week period will qualify for forgiveness. The Company intends to use the entire Loan amount for qualifying expenses, though no assurance is provided that the Company will obtain forgiveness of the PPP Loan in whole or in part.
The Note may be prepaid in part or in full, at any time, without penalty. The Note provides for certain customary events of default, including (i) failing to make a payment when due under the Note, (ii) failure to do anything required by the Note or any other loan document, (iii) defaults of any other loan with the PPP Lender, (iv) failure to disclose any material fact or make a materially false or misleading representation to the PPP Lender or SBA, (v) default on any loan or agreement with another creditor, if the PPP Lender believes the default may materially affect the Company’s ability to pay the Note, (vi) failure to pay any taxes when due, (vii) becoming the subject of a proceeding under any bankruptcy or insolvency law, having a receiver or liquidator appointed for any part
15
of the Company’s business or property, or making an assignment for the benefit of creditors, (viii) having any adverse change in financial condition or business operation that the PPP Lender believes may materially affect the Company’s ability to pay the Note, (ix) if the Company reorganizes, merges, consolidates, or otherwise changes ownership or business structure without the PPP Lender’s prior written consent, or (x) becoming the subject of a civil or criminal action that the PPP Lender believes may materially affect the Company’s ability to pay the Note. Upon the occurrence of an event of default, the PPP Lender has customary remedies and may, among other things, require immediate payment of all amounts owed under the Note, collect all amounts owing from the Company, and file suit and obtain judgment against the Company.
Loan Payment
On May 7, 2020, due to various restrictions and other limiting covenants of the 2nd A&R Loan Agreement, the Company elected to make an early payoff of its outstanding $5.0 million principal balance, plus $0.3 million reflecting the final payment fee and accrued interest. The Company intends to pursue a new debt facility to replace some or all of the repaid loan in order to meet future financial needs.
16
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Certain statements contained in this Quarterly Report on Form 10-Q may constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The words or phrases “would be,” “will allow,” “intends to,” “will likely result,” “are expected to,” “will continue,” “is anticipated,” “estimate,” “project,” or similar expressions, or the negative of such words or phrases, are intended to identify “forward-looking statements.” We have based these forward-looking statements on our current expectations and projections about future events. Because such statements include risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Factors that could cause or contribute to these differences include those below and elsewhere in this Quarterly Report on Form 10-Q and our other filings with the Securities and Exchange Commission, or SEC, particularly in Part II – Item 1A, “Risk Factors”. Such risks and uncertainties may be amplified by the COVID-19 pandemic and its potential impact on our business and the global economy. Statements made herein are as of the date of the filing of this Form 10-Q with the SEC and should not be relied upon as of any subsequent date. Unless otherwise required by applicable law, we do not undertake, and we specifically disclaim, any obligation to update any forward-looking statements to reflect occurrences, developments, unanticipated events or circumstances after the date of such statement.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited financial statements and related notes that appear in Item 1 of this Quarterly Report on Form 10-Q and with our audited financial statements and related notes for the year ended December 31, 2019 appearing in our Annual Report on Form 10-K filed with the SEC on March 13, 2020.
We are a biopharmaceutical company dedicated to developing and delivering treatments that restore and preserve vision for people with serious back of the eye diseases. Our proprietary SCS Microinjector targeting the suprachoroidal space, or SCS, offers unique access to the macula, retina and choroid where sight-threatening disease often occurs. When fluid is injected between the choroid and sclera, the elasticity of the SCS allows the fluid to migrate and spread spherically toward the posterior regions of the eye where it is absorbed into adjacent tissue. Our proprietary microinjector is able to precisely administer drugs into the SCS utilizing a needle that is approximately one millimeter in length. This method of administration facilitates more targeted delivery of therapeutic agents to chorioretinal structures.
Our SCS injection platform is an inherently flexible, in-office, non-surgical procedure intended to provide targeted delivery of established and new formulations of medications, as well as future therapeutic innovations such as gene therapy, to the site of disease.
We are leveraging our SCS injection platform by building an internal research and development pipeline, in areas such as novel small molecules and gene therapy, and by creating external collaborations with other companies. Using our suprachoroidal injection technology that can be used in conjunction with proprietary formulations of existing drugs as well as novel therapies, we believe we have created a broad therapeutic platform for developing product candidates to treat serious eye diseases.
Our first candidate, XIPERE, formerly known as CLS-TA, is a proprietary, preservative-free suspension of the corticosteroid triamcinolone acetonide, or TA, formulated for administration via suprachoroidal injection. Corticosteroids are the standard of care in uveitis. They are effective at treating the inflammatory aspect of ocular disease, but when delivered locally, either topically as drops, intravitreally, or by periocular injection, they have been associated with significant side effects, such as cataract formation or exacerbation, and elevated intraocular pressure, or IOP, which can lead to glaucoma.
XIPERE is being developed for the treatment of macular edema associated with uveitis. Uveitis is a set of ocular inflammatory conditions affecting approximately 350,000 patients in the United States and more than one million worldwide. Approximately one-third of uveitis patients develop uveitic macular edema, a build-up of fluid in the macula, the area of the retina responsible for sharp, straight-ahead vision. Macular edema is the leading cause of vision loss and blindness in uveitis patients and can occur from uveitis affecting any anatomic location—anterior, intermediate, posterior or panuveitis.
In December 2018, we submitted a New Drug Application, or NDA, for XIPERE to the Food and Drug Administration, or FDA, for the treatment of macular edema associated with uveitis. In October 2019, we received a complete response letter, or CRL, from the FDA regarding our NDA for XIPERE. The FDA did not identify any efficacy issues, and there were no requests for further clinical efficacy studies. As anticipated, the CRL included the FDA’s request for additional stability data, additional clarifying information on components of the manufacturing process, and reinspection of the drug product manufacturer.
The contract manufacturing organization, or CMO, for XIPERE has been completing certain requalification activities within its facility. While these manufacturing activities are not specifically related to XIPERE, the CMO has advised us that they continue to impact the timing of its production. Although extensive progress has been made, the CMO needs to resolve a final step affecting the proper functioning of its filling line equipment in order to produce the required stability batches to generate the data necessary for the XIPERE NDA resubmission. As a result, and due in part to COVID-19 related challenges that have impacted work schedules, the
17
CMO has informed us that there will be a delay in completing the necessary corrective action. Based on this current information, we expect to resubmit the XIPERE NDA in the fourth quarter of 2020.
Bausch Health, or Bausch, is our commercialization partner for XIPERE in the United States and Canada and, on April 27, 2020, we granted Bausch an exclusive option to develop, manufacture, distribute, promote, market and commercialize XIPERE in one or more of the following regions, or the Option, (i) the European Union, including the United Kingdom, (ii) Australia and New Zealand and (iii) South America and Mexico, or collectively the Additional Regions, in exchange for Bausch extending the deadline by which we must obtain regulatory approval for XIPERE in the United States. The Option may be exercised as to any or all of the Additional Regions any time before the earlier of regulatory approval of XIPERE in the United States and August 31, 2021.
On March 10, 2020, we entered into a license agreement with Arctic Vision (Hong Kong) Limited, or Arctic Vision, and such agreement, the Arctic Vision License Agreement. Pursuant to the Arctic Vision License Agreement, we granted an exclusive license to Artic Vision to develop, distribute, promote, market and commercialize XIPERE, subject to specified exceptions, in China, Hong Kong, Macau, Taiwan and South Korea.
We have research capabilities focused on developing proprietary therapeutic formulations to utilize with our SCS Microinjector. Our internal research and development initiatives are focused on small molecules and gene therapy to address unmet needs in back of the eye diseases.
After evaluation of our prior work and based on recently presented data in the scientific community, we have decided to advance our proprietary suspension of axitinib, a tyrosine kinase inhibitor, or TKI, for suprachoroidal injection, which we refer to as CLS-AX, into further preclinical development. We expect to submit an Investigational New Drug application, or IND, to the FDA, for CLS-AX in mid-2020. This would potentially enable us to initiate a Phase 1/2a clinical trial by the end of 2020.
Our preclinical proof-of-concept studies utilizing suprachoroidal delivery of marker genes using DNA nanoparticles have demonstrated the potential for suprachoroidal administration to deliver genes, so we are now conducting further preclinical work with SCS delivery of therapeutic transgenes in certain inherited retinal disorders.
In addition to growing our internal pipeline, we are also focused on collaborating with other companies to provide access to the suprachoroidal space. Our clinical development partners, including REGENXBIO in gene therapy and Aura Biosciences in ocular cancer, continue to make progress in their programs utilizing our SCS Microinjector. We expect there will be a number of related IND submissions by our partners during 2020.
The current development status of our pipeline, including programs licensed to third parties, is summarized in the chart below:
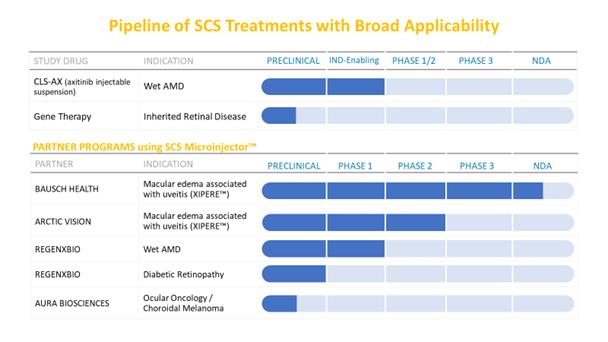
We have incurred net losses since our inception in May 2011. Our operations to date have been limited to organizing and staffing our company, raising capital, conducting preclinical studies and clinical trials and other research and development initiatives. To date, we have not generated any revenue, other than license and other revenue, and we have primarily financed our operations through public offerings and private placements of our equity securities, issuances of convertible promissory notes and loan agreements. As of March 31, 2020, we had an accumulated deficit of $240.6 million. We recorded net losses of $2.9 million and $15.4
18
million for the three months ended March 31, 2020 and 2019, respectively. We anticipate that a substantial portion of our capital resources and efforts in the foreseeable future will be focused on completing the necessary development for and obtaining regulatory approval of our product candidates, as well as discovering compounds and developing proprietary solutions to utilize with our SCS Microinjector.
We expect to continue to incur significant and increasing operating losses at least for the next several years. We do not expect to generate significant product or license and other revenue unless and until we successfully complete necessary development of, obtain regulatory approval for and successfully commercialize one or more of our product candidates, either on our own or together with a third party. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending on the timing of our clinical trials and our expenditures on other research and development activities. Our clinical trial expenses have decreased significantly following our decision to discontinue late-stage clinical trials of XIPERE for indications other than uveitis. However, we will continue our efforts to discover, research and develop additional product candidates and conduct various preclinical and clinical activities to further our pipeline programs. Based on our current research and development plans and expected near-term partnership milestone payments, we expect to have sufficient resources to fund planned operations into the second quarter of 2021.
Recent Developments
As noted above, on April 27, 2020, we and Bausch entered into an Amendment to the Bausch License Agreement. Pursuant to the Bausch License Agreement, we granted an exclusive license to Bausch to develop, manufacture, distribute, promote, market and commercialize XIPERE, our proprietary suspension of the corticosteroid triamcinolone acetonide formulated for administration to the back of the eye using our proprietary microneedle, or Device, as well as specified other steroids, corticosteroids and NSAIDs in combination with the Device, or the Other Products and together with XIPERE, the Products, subject to specified exceptions, in the United States and Canada, or the Original Territory, for the treatment of ophthalmology indications, including non-infectious uveitis. Pursuant to the Amendment, we granted Bausch the Option, which may be exercised any time before the earlier of regulatory approval of XIPERE in the United States and August 31, 2021.
Pursuant to the Bausch License Agreement, in October 2019, Bausch paid us an upfront payment of $5.0 million, or the Upfront Payment, which is subject to a refund if the Bausch License Agreement is terminated in specified circumstances. In addition, Bausch has agreed to make additional payments of up to $15.0 million upon the achievement of specified pre-launch development and regulatory milestones, or the Pre-Launch Milestone Payments, and up to an aggregate of $57.3 million in additional milestone payments upon the achievement of (i) specified regulatory approvals for specified additional indications of XIPERE (including certain regulatory and commercial milestones if Bausch exercises its Option in the European Union) and (ii) specified levels of annual net sales (as defined in the Bausch License Agreement). Further, during the applicable royalty term, we will also be entitled to receive tiered royalties at increasing percentages, from the high-teens to twenty percent, based on XIPERE achieving certain annual net sales thresholds in the Original Territory, as well as a lower royalty on annual net sales of Other Products in the Original Territory and on annual net sales of Xipere in the Additional Regions if Bausch exercises its Option, in each case subject to reductions in specified circumstances; provided that we will not receive any royalties on the first $45.0 million of cumulative net sales of all products in the Original Territory.
We are responsible for all development expenses for XIPERE in the Original Territory until the NDA for XIPERE is approved by the FDA, subject to specified exceptions, as well as manufacturing costs in connection with the NDA. We are also responsible for all clinical and development expenses conducted to satisfy the FDA’s requests in the CRL and any subsequent complete response letter related to the NDA, or the CRL-related expenses. If XIPERE is approved by the FDA, Bausch will be responsible for all expenses following such approval; provided that we will be responsible for the CRL-related expenses and for half of the costs of any post-approval clinical trials required by the FDA, up to a specified maximum amount.
During the term of the Bausch License Agreement, and in the Territory, the we have agreed not to (i) develop or commercialize XIPERE alone or in combination with an Other Device (as defined in the Bausch License Agreement) in the licensed field, (ii) develop or commercialize any corticosteroid with the Device or an Other Device in the licensed field, (iii) develop or commercialize the Device or an Other Device with any active pharmaceutical ingredient for non-infectious uveitis or macular edema associated with non-infectious uveitis, including with any Other Drug (as defined in the Bausch License Agreement, which are restricted to those steroids, corticosteroids and non-steroidal anti-inflammatory drugs which are specifically identified in the Bausch License Agreement), (iv) develop or commercialize any Other Drug in combination with the Device and (v) commercialize any Other Device for achieving non-surgical access to the suprachoroidal space where such device is sold as a stand-alone product, subject to specified exceptions. The Bausch License Agreement will expire upon expiration of the royalty terms for all Products and countries in the Territory, with each royalty term for a given Product and country ending on the latest of (a) the date of expiration of the last-to-expire valid claim of any licensed patent rights covering such Product in such country in the Territory, (b) the date of the loss of regulatory exclusivity for such Product in such country in the Territory, or (c) ten years from the later of the first sale of such Product in such country in the Territory. For a specified period of time, Bausch may terminate the Bausch License Agreement immediately and have the Upfront Payment refunded if the FDA has not approved the XIPERE NDA by August 31, 2021. Following the payment of the Pre-Launch Milestone Payments, Bausch may also terminate the License Agreement for convenience upon 180 days’ written notice.
19
In addition, we can terminate the Bausch License Agreement if Bausch commences a legal action challenging the validity, enforceability or scope of any of the licensed patents. If the FDA requires an additional clinical trial prior to approving the NDA for XIPERE and we notify Bausch that we will not conduct the trial at our expense, then Bausch may terminate the Bausch License Agreement and have the Upfront Payment refunded within 60 days of the receipt of such notice us. Both parties may terminate the Bausch License Agreement (x) upon a material breach of the Bausch License Agreement, subject to a specified cure period and specified exceptions, or (y) if the other party encounters bankruptcy or insolvency. Upon termination (other than for a material breach by us or bankruptcy or insolvency event of ours), all licenses and other rights granted by us to Bausch pursuant to the Bausch License Agreement would revert to the us.
Impact of COVID-19 on Our Business
We have been actively monitoring the novel coronavirus, or COVID-19, situation and its impact globally. Our financial results for the three months ended March 31, 2020 were not impacted by COVID-19, and we currently do not expect any material impact on our financial results for the quarter ending June 30, 2020. We continue to operate normally with the exception of enabling our employees to work productively from home and abiding by travel restrictions issued by federal and local governments. As previously reported, the operations of our CMO for XIPERE, have been impacted and our CMO is limiting daily operation with more restricted work schedules and other limitations and precautions needed to protect its employees. This has impacted the timing of their ability to complete the necessary corrective actions to resolve the issue affecting their filling line equipment, and therefore, the expected timing of our planned NDA resubmission for XIPERE, which is now anticipated to occur in the fourth quarter of 2020.
If the COVID-19 pandemic continues, we may experience other disruptions that could severely impact our business, results of operations and prospects. The extent to which COVID-19 may impact our business, preclinical development and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions and social distancing in the United States and other countries, business closures or business disruptions and the effectiveness of actions taken in the United States and other countries to contain and treat the disease.
Components of Operating Results
Revenue
We have not generated any revenue from the sale of any drugs, and we do not expect to generate any product revenue unless or until we obtain regulatory approval of and commercialize our product candidates, either on our own or with a third party. Our revenue in recent periods has been generated primarily from our license agreements. We are seeking to enter into additional license and other agreements with third parties to evaluate the potential use of our proprietary SCS Microinjector with the third party’s product candidates for the treatment of various eye diseases. These agreements may include payments to us for technology access, upfront license payments, regulatory and commercial milestone payments and royalties.
Research and Development
Since our inception, we have focused on our development programs. Research and development expenses consist primarily of costs incurred for the research and development of our preclinical and clinical product candidates, which include:
|
|
• |
employee-related expenses, including salaries, benefits, travel and share-based compensation expense for research and development personnel; |
|
|
• |
expenses incurred under agreements with contract research organizations, or CROs, as well as contract manufacturing organizations and consultants that conduct clinical trials and preclinical studies; |
|
|
• |
costs associated with nonclinical activities and development activities; |
|
|
• |
costs associated with submitting regulatory approval applications for our product candidates; |
|
|
• |
costs associated with training physicians on the suprachoroidal injection procedure and educating and providing them with appropriate product candidate information; |
|
|
• |
costs associated with technology and intellectual property licenses; |
|
|
• |
costs for our research and development facility; and |
|
|
• |
depreciation expense for assets used in research and development activities. |
We expense research and development costs to operations as incurred. These costs include preclinical activities, such as manufacturing and stability and toxicology studies, that are supportive of a product candidate itself. In addition, there are expenses related to clinical trials and similar activities for each program, including costs associated with CROs. Clinical costs are recognized
20
based on the terms of underlying agreements, as well as an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations and additional information provided to us by our vendors about their actual costs occurred. Expenses related to activities that support more than one development program or activity, such as salaries, share-based compensation and depreciation, are not classified as direct preclinical costs or clinical costs and are separately classified as unallocated.
The following table shows our research and development expenses by program, including those that have been discontinued, for the three months ended March 31, 2020 and 2019 (in thousands).
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
XIPERE (uveitis program) |
|
$ |
613 |
|
|
$ |
1,183 |
|
|
XIPERE (RVO program) |
|
|
17 |
|
|
|
6,802 |
|
|
CLS-AX (wet AMD program) |
|
|
385 |
|
|
|
— |
|
|
Total |
|
|
1,015 |
|
|
|
7,985 |
|
|
Unallocated |
|
|
2,796 |
|
|
|
2,982 |
|
|
Total research and development expense |
|
$ |
3,811 |
|
|
$ |
10,967 |
|
Our expenses related to clinical trials are based on estimates of patient enrollment and related expenses at clinical investigator sites as well as estimates for the services received and efforts expended under contracts with research institutions, consultants and CROs that conduct and manage clinical trials on our behalf. We generally accrue expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity according to the protocol. If future timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, we would modify our estimates of accrued expenses accordingly on a prospective basis.
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. It is difficult to determine with certainty the duration and completion costs of our current or future preclinical programs and clinical trials of our product candidates, or if, when or to what extent we will generate revenues from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates.
The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors that may include, among others:
|
|
• |
the costs associated with process development, scale-up and manufacturing of XIPERE and the SCS Microinjector in support of filings for regulatory approval; |
|
|
• |
the number of trials required for approval and any requirement for extension trials; |
|
|
• |
per patient trial costs; |
|
|
• |
the number of patients that participate in the trials; |
|
|
• |
the number of sites included in the trials; |
|
|
• |
the countries in which the trials are conducted; |
|
|
• |
the length of time required to enroll eligible patients; |
|
|
• |
the number of doses that patients receive; |
|
|
• |
the impact on the timing of our clinical trials due to the COVID-19 pandemic; |
|
|
• |
the drop-out or discontinuation rates of patients; |
|
|
• |
potential additional safety monitoring or other studies requested by regulatory agencies; |
|
|
• |
the duration of patient follow-up; and |
|
|
• |
the efficacy and safety profiles of the product candidates. |
In addition, the probability of success for each product candidate will depend on numerous factors, including competition, manufacturing capability and commercial viability. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success of each product candidate, as well as an assessment of each product candidate’s commercial potential.
21
General and administrative expenses consist primarily of salaries and other related costs, including share-based compensation, for personnel in executive, finance and administrative functions. General and administrative costs historically included commercial pre-launch preparations for XIPERE, and also include facility related costs not otherwise included in research and development expenses, as well as professional fees for legal, patent, consulting, and accounting and audit services.
Other Income (Expense)
Other income consists of interest income earned on our cash and cash equivalents and short-term investments. Interest income is not considered significant to our financial statements.
Other expense consists of interest expense incurred under our loan agreements.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America, or U.S. GAAP. The preparation of these financial statements requires us to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities as of the dates of the balance sheets and the reported amounts of expenses during the reporting periods. In accordance with U.S. GAAP, we evaluate our estimates and judgments on an ongoing basis. Significant estimates include assumptions used in the determination of share-based compensation and some of our research and development expenses. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We define our critical accounting policies as those accounting principles generally accepted in the United States of America that require us to make subjective estimates and judgments about matters that are uncertain and are likely to have a material impact on our financial condition and results of operations, as well as the specific manner in which we apply those principles. During the three months ended March 31, 2020, there were no significant changes to our critical accounting policies disclosed in our audited financial statements for the year ended December 31, 2019, which are included in our Annual Report on Form 10-K, as filed with the SEC on March 13, 2020.
Results of Operations for the Three Months Ended March 31, 2020 and 2019
The following table sets forth our results of operations for the three months ended March 31, 2020 and 2019.
|
|
|
Three Months Ended March 31, |
|
|
Period-to-Period |
|
||||||
|
|
|
2020 |
|
|
2019 |
|
|
Change |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
License and other revenue |
|
$ |
4,097 |
|
|
$ |
45 |
|
|
$ |
4,052 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
3,811 |
|
|
|
10,967 |
|
|
|
(7,156 |
) |
|
General and administrative |
|
|
3,122 |
|
|
|
4,384 |
|
|
|
(1,262 |
) |
|
Total operating expenses |
|
|
6,933 |
|
|
|
15,351 |
|
|
|
(8,418 |
) |
|
Loss from operations |
|
|
(2,836 |
) |
|
|
(15,306 |
) |
|
|
12,470 |
|
|
Other expense |
|
|
(75 |
) |
|
|
(98 |
) |
|
|
23 |
|
|
Net loss |
|
$ |
(2,911 |
) |
|
$ |
(15,404 |
) |
|
$ |
12,493 |
|
Revenue. In the three months ended March 31, 2020, we recognized $4.1 million of revenue associated with our license agreements, primarily the result of the $4.0 million upfront payment from Arctic Vision in March 2020. In the three months ended March 31, 2019, we recognized $45,000 of revenue associated with other agreements.
Research and development. Research and development expense decreased by $7.2 million, from $11.0 million for the three months ended March 31, 2019 to $3.8 million for the three months ended March 31, 2020. This was primarily attributable to a $6.8 million decrease due to the closing costs in the prior year of two late-stage clinical trials, SAPPHIRE and TOPAZ, that were part of our RVO program that has been discontinued. Additionally, there was a $0.2 million decrease in expenses related to completion of the PEACHTREE trial, a $0.4 million decrease in costs related to device and drug manufacturing and a $0.6 million decrease in employee-related costs. These decreases were partially offset by a $0.4 million increase in costs for preclinical work on potential product candidates.
22
General and administrative. General and administrative expenses decreased by $1.3 million, from $4.4 million for the three months ended March 31, 2019 to $3.1 million for the three months ended March 31, 2020. This was primarily attributable to a decrease of $1.4 million in marketing-related expenses related to the change of our business strategy to seek partners for XIPERE rather than commercialize XIPERE on our own, partially offset by an increase of $0.2 million in directors and officers insurance premiums.
Other expense. Other expense for each of the three months ended March 31, 2020 and 2019 primarily consisted of interest on long-term debt, the amortization of financing costs, the accretion of warrants and the final payment related to our loan agreements, offset in part by interest income from our short-term investments. The decrease from 2019 to 2020 was the result of lower interest expense due to a $5.0 million principal payment on our long-term debt in October 2019.
Liquidity and Capital Resources
Sources of Liquidity
We have funded our operations primarily through the proceeds of public offerings of our common stock, sales of convertible preferred stock and the issuance of long-term debt. As of March 31, 2020, we had cash and cash equivalents of $20.9 million. We invest any cash in excess of our immediate requirements primarily with a view to liquidity and capital preservation. As of March 31, 2020, our funds were held in cash and money market funds.
On April 20, 2020, we entered into a loan agreement with Silicon Valley Bank under the terms of which Silicon Valley bank loaned us $1.0 million, or the PPP Loan, pursuant to the Paycheck Protection Program under the Coronavirus Aid, Relief, and Economic Security Act, or CARES Act. In accordance with the requirements of the CARES Act, we intend to use the proceeds primarily for payroll costs. The PPP Loan is scheduled to mature on April 20, 2022, has a 1.00% interest rate, and is subject to the terms and conditions applicable to all loans made pursuant to the Paycheck Protection Program as administered by the Small Business Administration, or SBA, under the CARES Act.
On March 10, 2020, we entered into the Arctic License Agreement. Pursuant to the Arctic License Agreement, we granted an exclusive license to Artic Vision to develop, distribute, promote, market and commercialize XIPERE, subject to specified exceptions, in the Arctic Territory. Pursuant to the Arctic License Agreement, Arctic Vision has agreed to pay us up to a total of $35.5 million. This amount includes an upfront payment of $4.0 million as well as an aggregate of up to $31.5 million in development milestone payments for specified events prior to and including receipt of approval of XIPERE in the United States and sales milestone payments for achievement of specified levels of net sales. Further, during the applicable royalty term, we will also be entitled to receive tiered royalties of ten to twelve percent of net sales based on achieving certain annual net sales thresholds in the Arctic Territory, subject to customary reductions.
In October 2019, we received $5.0 million pursuant to the Bausch License Agreement.
On May 14, 2018, we entered into a loan and security agreement with Silicon Valley Bank and MidCap Financial Services, or collectively the Lenders, which amended and restated in its entirety a prior loan and security agreement with the Lenders. The loan and security agreement, as amended to date, or the Loan Agreement, provided for term loans of up to $20.0 million in the aggregate, with a floating interest rate equal to 6.5% plus the greater of (i) the 30-day U.S. LIBOR, as reported in the Wall Street Journal on the last business day of the month that immediately precedes the month in which the interest will accrue or (ii) 1.89%.We borrowed an initial tranche of $10.0 million on May 14, 2018, of which $7.0 million was used to repay all amounts outstanding under the amended and restated loan and security agreement, including the fees payable in connection with the final payment. The prepayment fees were waived. Of the remaining $10.0 million capacity originally contemplated, $5.0 million became available but we elected not to draw it, and the other $5.0 million did not become available for draw. On October 18, 2019, we entered into an amendment to the Loan Agreement and repaid $5.0 million of the outstanding principal balance. We did not pay any final payment or termination fees in connection with the $5.0 million prepayment. On May 7, 2020, due to various restrictions and other limiting covenants of the current Loan Agreement with the Lenders, we elected to make an early payoff of our outstanding $5.0 million principal balance, plus $0.3 million reflecting the final payment fee and accrued interest. We intend to pursue a new debt facility to replace some or all of the repaid loan in order to meet future financial needs.
Under the Loan Agreement, we were required to pay accrued interest only on the $5.0 million remaining outstanding balance through October 31, 2020, followed by consecutive equal monthly payments of principal and interest in arrears continuing through the maturity date of October 1, 2022. We had the option to prepay the outstanding balance in full, subject to a prepayment fee of 2% of the original principal amount for any prepayment prior to October 1, 2022. A final payment of 5.50% of the aggregate borrowed amount would have been due at maturity of the loan on October 1, 2022, or upon the prepayment of the facility or the acceleration of amounts due under the facility as a result of an event of default. In addition, we agreed that if our cash and cash equivalents balance with Silicon Valley Bank falls below $10.0 million, we would transfer to a pledged account an amount of cash and cash equivalents equal to the sum of the then-outstanding principal balance of the term loan plus a final payment fee of $0.3 million.
23
The amounts due under the Loan Agreement were secured by substantially all of our assets.
On June 30, 2017, we entered into an at-the-market sales agreement, or the ATM agreement, with Cowen and Company LLC, or Cowen, under which we may offer and sell, from time to time at our sole discretion, shares of our common stock having an aggregate offering price of up to $50.0 million through Cowen acting as our sales agent. During the three months ended March 31, 2020, we sold 0.5 million shares of our common stock for net proceeds of $1.2 million under the ATM agreement.
Funding Requirements
Our primary uses of capital are, and we expect will continue to be, compensation and related expenses, ongoing costs related to our NDA submission for XIPERE, research and development costs to build our product candidate pipeline, legal and other regulatory expenses and general overhead costs.
The successful development of our product candidates is highly uncertain. As such, at this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the remainder of the development of XIPERE or any future product candidates. We are also unable to predict when, if ever, material net cash inflows will commence from product sales. This is due to the numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
|
|
• |
successful enrollment in, and completion of, clinical trials; |
|
|
• |
receipt of marketing approvals from applicable regulatory authorities; |
|
|
• |
establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers; |
|
|
• |
obtaining and maintaining patent and trade secret protection and regulatory exclusivity for our product candidates; and |
|
|
• |
launching commercial sales of the products, if and when approved, whether alone or in collaboration with others. |
A change in the outcome of any of these variables with respect to the development of any of our product candidates would significantly change the costs and timing associated with the development of that candidate.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings and potential collaboration, license and development agreements. Other than potential payments we may receive under our license and other agreements, we do not currently have any committed external source of funds, though, as described above, we may also be able to sell our common stock under the ATM agreement with Cowen subject to the terms of that agreement and depending on market conditions. We expect that we will require additional capital to fund our ongoing operations. Additional funds may not be available to us on a timely basis, on commercially reasonable terms, or at all. Our ability to raise additional capital may be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility in, the credit and financial markets in the United States and worldwide resulting from the ongoing COVID-19 pandemic. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise funds through additional collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, including any future collaboration or licensing arrangement for XIPERE outside of the territories in which we’ve licensed or granted options to license XIPERE to our existing partners, we may be required to relinquish additional rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our drug development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We also incur costs as a public company, including costs and expenses for fees to members of our board of directors, accounting and finance personnel costs, directors and officers insurance premiums, audit and legal fees, investor relations fees and expenses for compliance with reporting requirements under the Exchange Act and rules implemented by the SEC and Nasdaq.
Outlook
We have suffered recurring losses and negative cash flows from operations since inception and anticipate incurring additional losses until such time, if ever, that we can obtain FDA approval to market and then generate significant revenue from XIPERE. We will need additional financing to fund our operations. These conditions raise substantial doubt about our ability to continue as a going concern within one year after the date of this report. We have plans to mitigate this going concern risk, which primarily consist of raising additional capital, potentially in a combination of equity or debt financings, and by the receipt of future milestone payments from our license agreements.
24
Based on our current plans and forecasted expenses, we expect that our cash and cash equivalents as of March 31, 2020 will enable us to fund our operating expenses and capital expenditure requirements into the second quarter of 2021. This estimate gives effect to additional development milestone payments we might receive under our license agreements. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we expect.
Our financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result should we be unable to continue as a going concern.
Cash Flows
The following is a summary of the net cash flows provided by (used in) our operating, investing and financing activities (in thousands):
|
|
|
Three Months Ended March 31, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Net cash (used in) provided by: |
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(2,803 |
) |
|
$ |
(12,653 |
) |
|
Investing activities |
|
|
(55 |
) |
|
|
30,932 |
|
|
Financing activities |
|
|
1,193 |
|
|
|
6,628 |
|
|
Net change in cash and cash equivalents |
|
$ |
(1,665 |
) |
|
$ |
24,907 |
|
During the three months ended March 31, 2020 and 2019, our operating activities used net cash of $2.8 million and $12.7 million, respectively. The use of cash in each period primarily resulted from our net losses. The decrease in net loss for the three months ended March 31, 2020 as compared to the three months ended March 31, 2019 was primarily attributable to lower research and development expenses as a result of the discontinuation of the SAPPHIRE and TOPAZ trials and commercialization activities. The three months ended March 31, 2020 also included a net cash outflow of $1.1 million from the decrease in our accrued liabilities balance, which was the result of payments we made in connection with accrued employee costs.
During the three months ended March 31, 2020, our net cash used in investing activities was for the purchase of equipment. During the three months ended March 31, 2019, our net cash provided by investing activities was $30.9 million due to maturities of short-term, available-for-sale investments.
During the three months ended March 31, 2020 and 2019, our net cash provided by financing activities was $1.2 million and $6.6 million, respectively. The net cash provided by financing activities for each period was comprised of net proceeds from the sales of shares of common stock under the ATM agreement.
Contractual Obligations
As of March 31, 2020, there were no significant changes to our contractual obligations from those presented as of December 31, 2019 in our Annual Report on Form 10-K. Subsequent to March 31, 2020, the Loan Agreement was paid in full as described above.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules.
Recent Accounting Pronouncements
See Item 1, “Financial Statements – Note 2, Significant Accounting Policies” for a discussion of recent accounting pronouncements and their effect on us.
JOBS Act
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Section 107(b) of the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period, and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
25
Item 3. Quantitative and Qualitative Disclosures about Market Risk
The market risk inherent in our financial instruments and in our financial position represents the potential loss arising from adverse changes in interest rates. As of March 31, 2020 and December 31, 2019, we had cash and cash equivalents of $20.9 million and $22.6 million, respectively. We generally hold our cash in interest-bearing money market accounts. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Due to the short-term maturities of our cash equivalents and the low risk profile of our investments, an immediate 100 basis point change in interest rates would not have a material effect on the fair market value of our cash equivalents and short-term investments.
We do not engage in any hedging activities against changes in interest rates. Our outstanding debt instruments carried a floating interest rate that is 6.5% plus the greater of (i) the 30-day U.S. LIBOR, reported in the Wall Street Journal on the last business day of the month that immediately precedes the month in which the interest will accrue or (ii) 1.89%. Based on the current outstanding balance under the Loan Agreement, a 100 basis point increase in the LIBOR rate would result in a $50,000 increase in annual interest expense.
We do not have any foreign currency or other material derivative financial instruments.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), refers to controls and procedures that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that such information is accumulated and communicated to a company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
In designing and evaluating our disclosure controls and procedures, management recognizes that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a control system, misstatements due to error or fraud may occur and not be detected.
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Based on that evaluation, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report at the reasonable assurance level.
Changes in Internal Controls over Financial Reporting
There has been no change in our internal control over financial reporting during the quarter ended March 31, 2020 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. We have not experienced any material impact to our internal controls over financial reporting despite the fact that many of our employees are working remotely due to the COVID-19 pandemic. We are continually monitoring and assessing the COVID-19 situation on our internal controls to minimize the impact on their design and operating effectiveness.
26
From time to time, we may be subject to litigation and claims arising in the ordinary course of business. We are not currently a party to any material legal proceedings and we are not aware of any pending or threatened legal proceeding against us that we believe could have a material adverse effect on our business, operating results, cash flows or financial condition.
Our business is subject to risks and events that, if they occur, could adversely affect our financial condition and results of operations and the trading price of our securities. Such risks may be amplified by the COVID-19 pandemic and its potential impact on our business and the global economy. In addition to the other information set forth in this quarterly report on Form 10-Q, you should carefully consider the factors described below and in “Part I, Item 1A. Risk Factors” of our Annual Report on Form 10-K for the fiscal year ended December 31, 2019, filed with the Securities and Exchange Commission on March 13, 2020. Except as described below, there have been no material changes to the risk factors described in that report.
Our CMO for XIPERE is delayed in completing certain manufacturing activities requested by the FDA which impact its ability to produce data necessary for the resubmission of the NDA for XIPERE. As a result, the timing of regulatory approval for XIPERE is uncertain, and we may never obtain regulatory approval for XIPERE in the United States.
On October 18, 2019, the FDA issued a CRL regarding the NDA for XIPERE, indicating that their review was complete and the NDA was not ready for approval in its present form. In its CRL, the FDA requested additional stability data for the triamcinolone acetonide suspension and re-inspection of the drug manufacturer, among other things.
In November 2019, we were informed by our CMO for XIPERE that the FDA requested that the manufacturer complete certain manufacturing activities not specifically related to XIPERE within its facility. The CMO has informed us that these manufacturing activities continue to impact the timing of its production. Although extensive progress has been made, there remain unresolved issues affecting the proper functioning of the CMO’s filling line equipment which must be resolved in order to produce the stability batches to generate the data necessary for the resubmission of the NDA for XIPERE. As a result, and due in part to COVID-19 related challenges that have impacted work schedules, the CMO has informed us that there will be a delay in completing the necessary corrective action. Based on this current information, we expect to resubmit the NDA for XIPERE in the fourth quarter of 2020.
The timeline for resolution of the issues affecting the CMO’s ability to complete the activities necessary to produce the stability batches which are required for the resubmission of the NDA for XIPERE is uncertain, and the CMO may be unable to resolve these issues. As a result, the timing of regulatory approval for XIPERE is uncertain, and we may never obtain regulatory approval for XIPERE in the United States. If we do not obtain regulatory approval for XIPERE or are further delayed in obtaining such approval, it would have a material adverse effect on our operations and financial condition.
COVID-19 could adversely impact our business, including our clinical trials.
In December 2019, a novel strain of coronavirus, SARS-CoV-2, causing COVID-19, was initially reported and has since been declared a pandemic by the World Health Organization. The COVID-19 pandemic has resulted in travel and other restrictions in order to reduce the spread of the disease, including state and local orders across the country, which, among other things, direct individuals to shelter at their places of residence, direct businesses and governmental agencies to cease non-essential operations at physical locations, prohibit certain non-essential gatherings, and order cessation of non-essential travel. In response to these public health directives and orders, we have implemented work-from-home policies for our employees. The effects of the executive orders, the shelter-in-place orders and our work-from-home policies may negatively impact productivity, disrupt our business and delay our clinical programs and timelines, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. These and similar, and perhaps more severe, disruptions in our operations could negatively impact our business, operating results and financial condition.
Quarantines, shelter-in-place and similar government orders and employer restrictions related to COVID-19 may adversely impact our business operations and the business operations. For example, our CMO for XIPERE is limiting daily operations with more restricted work schedules and other limitations and precautions needed to protect its employees which has contributed to delays in completing the requalification activities within its facility that are necessary to produce the required stability batches to generate the data necessary for the resubmission of the NDA for XIPERE. As a result of these delays, we now expect to resubmit the XIPERE NDA in the fourth quarter of 2020.
27
In addition, our clinical trials and those of our licensing partners may be affected by the COVID-19 pandemic. For example, clinical site initiation and patient enrollment may be delayed due to prioritization of hospital resources toward the COVID-19 pandemic. Some patients may not be able to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, our ability, or that of our licensing partners, to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 may be impaired, which could adversely impact our clinical trial operations or those of our licensing partners and, as a result, negatively affect our ability to receive regulatory and development milestones under our license agreements.
The spread of COVID-19, which has caused a broad impact globally, may materially affect us economically. While the potential economic impact brought by, and the duration of, COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common stock.
The global pandemic of COVID-19 continues to rapidly evolve. The extent to which the COVID-19 pandemic impacts our business, our clinical development and regulatory efforts and those of our licensing partners will depend on future developments that are highly uncertain and cannot be predicted with confidence, such as the duration of the outbreak, travel restrictions, quarantines, social distancing requirements and business closures in the United States and other countries, and business disruptions, and the effectiveness of actions taken in the United States and other countries to contain and treat the disease. Accordingly, we do not yet know the full extent of potential delays or impacts on our business, our clinical and regulatory activities, healthcare systems or the global economy as a whole.
We may not be entitled to forgiveness of our recently received PPP Loan, and our application for the PPP Loan could in the future be determined to have been impermissible or could result in damage to our reputation.
On April 20, 2020, we received proceeds of $1.0 million from a loan under the Paycheck Protection Program of the CARES Act, a portion of which may be forgiven, which we intend to use to retain employees, maintain payroll and make lease and utility payments. The PPP Loan matures on April 20, 2022 and bears annual interest at a rate of 1.0%. Commencing November 20, 2020, we are required to pay the lender equal monthly payments of principal and interest as required to fully amortize by April 20, 2022 any principal amount outstanding on the PPP Loan as of October 20, 2020. A portion of the PPP Loan may be forgiven by the SBA upon our application beginning 60 days but not later than 120 days after loan approval and upon documentation of expenditures in accordance with the SBA requirements. Under the CARES Act, loan forgiveness is available for the sum of documented payroll costs, covered rent payments, covered mortgage interest and covered utilities during the eight week period beginning on the date of loan approval. Not more than 25% of the forgiven amount may be for non-payroll costs. The amount of the PPP Loan eligible to be forgiven is reduced if our full-time headcount declines or if salaries and wages for employees with salaries of $100,000 or less annually are reduced by more than 25%. We will be required to repay any portion of the outstanding principal that is not forgiven, along with accrued interest, in accordance with the amortization schedule described above, and we cannot provide any assurance that we will be eligible for loan forgiveness or that any amount of the PPP Loan will ultimately be forgiven by the SBA.
In order to apply for the PPP Loan, we were required to certify, among other things, that the current economic uncertainty made the PPP Loan request necessary to support our ongoing operations. We made this certification in good faith after analyzing, among other things, our financial situation and access to alternative forms of capital, and believe that we satisfied all eligibility criteria for the PPP Loan, and that our receipt of the PPP Loan is consistent with the broad objectives of the Paycheck Protection Program of the CARES Act. The certification described above does not contain any objective criteria and is subject to interpretation. However, on April 23, 2020, the SBA issued guidance stating that it is unlikely that a public company with substantial market value and access to capital markets will be able to make the required certification in good faith. The lack of clarity regarding loan eligibility under the Paycheck Protection Program has resulted in significant media coverage and controversy with respect to public companies applying for and receiving loans. If, despite our good-faith belief that we satisfied all eligible requirements for the PPP Loan, we are later determined to have violated any of the laws or governmental regulations that apply to us in connection with the PPP Loan, such as the False Claims Act, or it is otherwise determined that we were ineligible to receive the PPP Loan, we may be subject to penalties, including significant civil, criminal and administrative penalties, and could be required to repay the PPP Loan in its entirety. In addition, our receipt of the PPP Loan may result in adverse publicity and damage to our reputation, and a review or audit by the SBA or other government entity or claims under the False Claims Act could consume significant financial and management resources.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
|
(a) |
Sales of Unregistered Securities |
None.
28
|
Exhibit No. |
|
Description |
|
|
|
|
|
3.1 |
|
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
10.1*# |
|
|
|
|
|
|
|
10.2*# |
|
|
|
|
|
|
|
10.3 |
|
|
|
|
|
|
|
10.4 |
|
|
|
|
|
|
|
31.1* |
|
Certification of Principal Executive Officer under Section 302 of the Sarbanes-Oxley Act. |
|
|
|
|
|
31.2* |
|
Certification of Principal Financial Officer under Section 302 of the Sarbanes-Oxley Act. |
|
|
|
|
|
32.1** |
|
|
|
|
|
|
|
101.INS |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
* |
Filed herewith. |
|
** |
These certifications are being furnished solely to accompany this quarterly report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and are not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing. |
|
# |
The schedules and exhibits to this agreement have been omitted, but will be furnished to the Securities and Exchange Commission upon request. |
|
## |
Pursuant to Item 601(b)(10)(iv) of Regulation S-K promulgated by the Securities and Exchange Commission, certain portions of this exhibit have been redacted. The Registrant hereby agrees to furnish supplementally to the Securities and Exchange Commission, upon its request, an unredacted copy of this exhibit. |
29
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
Clearside Biomedical, Inc. |
|
|
|
|
|
|
|
Date: May 8, 2020 |
|
By: |
/s/ Charles A. Deignan |
|
|
|
|
Charles A. Deignan |
|
|
|
|
Chief Financial Officer |
|
|
|
|
(On behalf of the Registrant and as |
30
