Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - AMICUS THERAPEUTICS, INC. | tm2018913d1_ex99-1.htm |
| 8-K - FORM 8-K - AMICUS THERAPEUTICS, INC. | tm2018913d1_8k.htm |
Exhibit 99.2
 |
1Q20 Financial Results Conference Call & Webcast May 7, 2020 |
 |
Introduction 2 Forward-Looking Statements This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates, commercialization plans, manufacturing and supply plans, financing plans, and the projected revenues and cash position for the Company. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities, and in particular the potential goals, progress, timing, and results of preclinical studies and clinical trials, including as they are impacted by COVID-19 related disruption, are based on current information. The potential impact on operations from the COVID-19 pandemic is inherently unknown and cannot be predicted with confidence and may cause actual results and performance to differ materially from the statements in this release, including without limitation, because of the impact on general political and economic conditions, including as a result of efforts by governmental authorities to mitigate COVID-19, such as travel bans, shelter in place orders and third-party business closures and resource allocations, manufacturing and supply chain disruptions and limitations on patient access to commercial or clinical product. In addition to the impact of the COVID-19 pandemic, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities, including the FDA, EMA, and PMDA, may not grant or may delay approval for our product candidates; the potential that we may not be successful in commercializing Galafold in Europe, Japan, the US and other geographies or our other product candidates if and when approved; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; the potential that we may not be able to manufacture or supply sufficient clinical or commercial products; and the potential that we will need additional funding to complete all of our studies and manufacturing. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. Statements regarding corporate financial guidance and financial goals and the attainment of such goals. With respect to statements regarding projections of the Company's revenue and cash position, actual results may differ based on market factors and the Company's ability to execute its operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2019 and the Quarterly Report filed on Form 10-Q to be filed today. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this news release to reflect events or circumstances after the date hereof. In addition to financial information prepared in accordance with U.S. GAAP, this presentation also contains adjusted financial measures that we believe provide investors and management with supplemental information relating to operating performance and trends that facilitate comparisons between periods and with respect to projected information. These adjusted financial measures are non-GAAP measures and should be considered in addition to, but not as a substitute for, the information prepared in accordance with U.S. GAAP. We typically exclude certain GAAP items that management does not believe affect our basic operations and that do not meet the GAAP definition of unusual or non-recurring items. Other companies may define these measures in different ways. Full reconciliations of GAAP results to the comparable non-GAAP measures for the reported periods appear in the financial tables section of this presentation. When we provide our expectation for non-GAAP operating expenses on a forward-looking basis, a reconciliation of the differences between the non-GAAP expectation and the corresponding GAAP measure generally is not available without unreasonable effort due to potentially high variability, complexity and low visibility as to the items that would be excluded from the GAAP measure in the relevant future period, such as unusual gains or losses. The variability of the excluded items may have a significant, and potentially unpredictable, impact on our future GAAP results. |
 |
Introduction 3 PLATFORM & Glycobiology AT-GAA Phase 3 in Pompe Disease Two Clinical-Stage Gene Therapies $338M+ Cash as of 3/31/20 GLOBAL COMMERCIAL ORGANIZATION Robust R&D Engine Nearly 50+ Lysosomal Disorders and More Prevalent Rare Diseases EMPLYEES in 27ountries Gene Therapy Protein Engineering World Class BIOLOGICS Capabilities A leading fully-integrated, global rare disease biotechnology company |
 |
Introduction 4 2020 Key Strategic Priorities 1 Achieve global product revenue for Galafold of $250M-$260M Complete Pompe Phase 3 PROPEL study, enroll pediatric studies and advance manufacturing to support 2021 BLA and MAA 2 3 4 5 Advance clinical development, manufacturing and regulatory discussions for CLN6 and CLN3 Batten programs Progress Pompe gene therapy towards IND and disclose up to two additional IND candidates Maintain strong financial position |
 |
Galafold®(migalastat) Global Launch… …taking a leadership role in the treatment of Fabry disease “We push ideas as far and as fast as possible” - Amicus Belief Statement |
 |
Galafold: Precision Medicine for Fabry Disease 6 Galafold Snapshot (as of March 31, 2020) One of the Most Successful $250-260M FY20 Global $60.5M 1Q20 Galafold R Rare Disease Launches nce Continue Expansio in 2020 348 ble Variants in U.S. Label 40+ Countries with Regulatory Approvals: Including U.S., EU, Japan, and Other Countries Galafold is indicated for adults with a confirmed diagnosis of Fabry Disease and an amenable mutation/variant. The most common adverse reactions reported with Galafold (≥10%) were headache, nasopharyngitis, urinary tract infection, nausea and pyrexia. For additional information about Galafold, including the full U.S. Prescribing Information, please visit https://www.amicusrx.com/pi/Galafold.pdf. For further important safety information for Galafold, including posology and method of administration, special warnings, drug interactions and adverse drug reactions, please see the European SmPC for Galafold available from the EMA website at www.ema.europa.eu. Galafold is the cornerstone of Amicus’ success.It is an orally delivered small molecule precision medicine with a unique mechanism of action for Fabry patients with amenable variants that replaces the need for intravenously delivered enzyme replacement therapy |
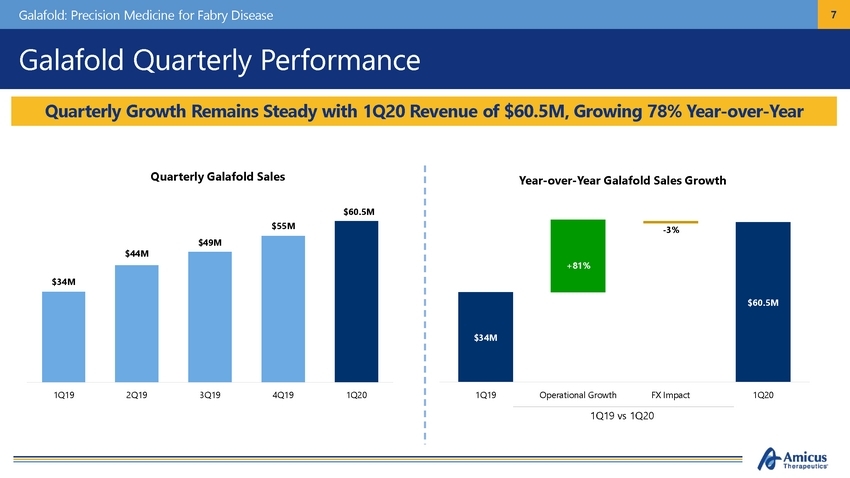 |
Galafold: Precision Medicine for Fabry Disease 7 Galafold Quarterly Performance Quarterly Galafold Sales Year-over-Year Galafold Sales Growth $60.5M 1Q19 2Q19 3Q19 4Q19 1Q20 1Q19 Operational Growth FX Impact 1Q20 1Q19 vs 1Q20 $55M $49M $44M $34M -3% $60.5M $34M +81% Quarterly Growth Remains Steady with 1Q20 Revenue of $60.5M, Growing 78% Year-over-Year |
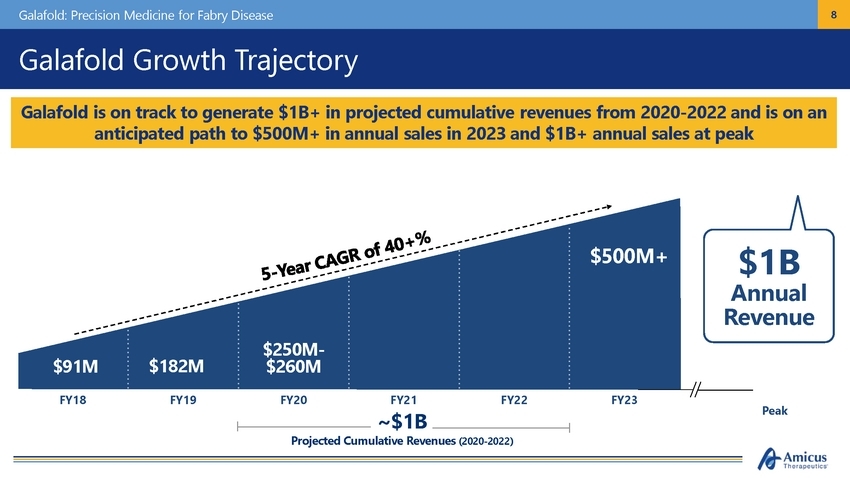 |
Galafold: Precision Medicine for Fabry Disease 8 Galafold Growth Trajectory $1B Annual Revenue $500M+ $250M-$260M $91M $182M FY18 FY19 FY20 FY21 ~$1B FY22 FY23 Peak Projected Cumulative Revenues (2020-2022) Galafold is on track to generate $1B+ in projected cumulative revenues from 2020-2022 and is on an anticipated path to $500M+ in annual sales in 2023 and $1B+ annual sales at peak |
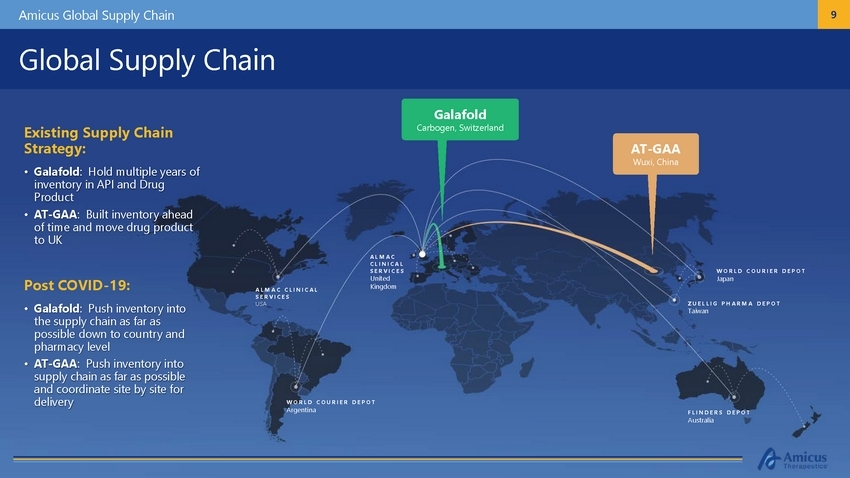 |
Amicus Global Supply Chain 9 Global Supply Chain Galafold Carbogen, Switzerland Existing Supply Chain Strategy: • Galafold: Hold multiple years of inventory in API and Drug Product • AT-GAA: Built inventory ahead of time and move drug product to UK AT-GAA Wuxi, China AL M AC C L IN IC A L S ERV I C ES United Kingdom W OR L D C OU R I E R D E P OT Japan Post COVID-19: • Galafold: Push inventory into the supply chain as far as possible down to country and pharmacy level • AT-GAA: Push inventory into supply chain as far as possible and coordinate site by site for delivery AL M AC C L I N I C AL S ERV I C ES USA Z UE LLI G P H A R M A D E P O T Taiwan W O R L D Argentina C O U R I E R DE P O T F L I N DE R S DE P O T Australia |
 |
AT-GAA:Next Potential Standardof Care for Pompe Disease “We encourage and embrace constant innovation” - Amicus Belief Statement |
 |
AT-GAA for Pompe Disease 11 AT-GAA: Key Takeaways • PROPEL study timelines are on track with data expected 1H2021 – To date, 97% of the 2,250 planned infusions for the ongoing PROPEL study have been completed on schedule • Breakthrough Therapy Designation and the Promising Innovative Medicine designation highlight unmet need in Pompe disease today U.S. FDA grants rolling BLA submission and company plans to initiate in 2H2020 Expanded Access Program for infantile-onset Pompe patients underway • • • Process performance qualification (PPQ) runs with our partners WuXi have been successfully completed for the drug substance at • Peak revenue potential of $1B-$2B, with exclusivity well into 2030s AT-GAA for Pompe Advances Toward Approval as “Crown Jewel” of Amicus Portfolio |
 |
Next GenerationGene TherapyPlatform “We have a duty to obsolete our own technologies” - Amicus Belief Statement |
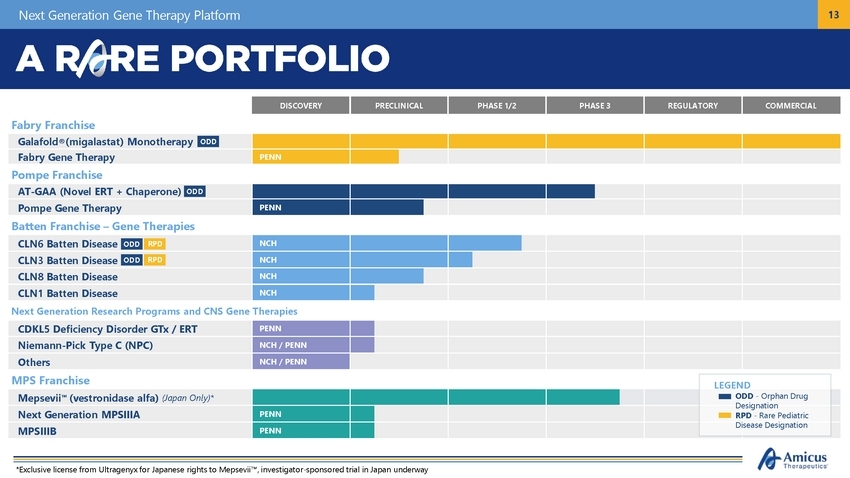 |
Next Generation Gene Therapy Platform 13 sease Designation *Exclusive license from Ultragenyx for Japanese rights to Mepsevii™, investigator-sponsored trial in Japan underway DISCOVERY PRECLINICAL PHASE 1/2 PHASE 3 REGULATORY COMMERCIAL Fabry Franchise Galafold®(migalastat) Monotherapy Fabry Gene Therapy PENN Pompe Franchise AT-GAA (Novel ERT + Chaperone) Pompe Gene Therapy PENN Batten Franchise – Gene Therapies CLN6 Batten Disease NCH CLN3 Batten Disease NCH CLN8 Batten Disease NCH CLN1 Batten Disease NCH Next Generation Research Programs and CNS Gene Therapies CDKL5 Deficiency Disorder GTx / ERT PENN Niemann-Pick Type C (NPC) NCH / PENN Others NCH / PENN MPS Franchise LEGEND ODD - Orphan Drug Designation RPD - Rare Pediatric Mepsevii™ (vestronidase alfa) (Japan Only)* Next Generation MPSIIIA PENN MPSIIIB PENN Di ODD RPD ODD RPD ODD ODD |
 |
Next Generation Gene Therapy Platform 14 Gene Therapy: Updates & Key Takeaways • CLN6 Phase 1/2 interim data show profound impact with potential to become first ever approved gene therapy for fatal brain disease in children Additional patients to be dosed in Phase 1/2 study of CLN3 in 2021 with commercial supply Orphan drug designations granted in U.S. and EU for intrathecal AAV gene therapies for CLN6 and CLN3 Batten disease; CLN3 granted Fast Track designation by U.S. FDA Pompe gene therapy clinical candidate declared to move into IND-enabling studies Penn Collaboration is R&D engine, with rights to 50+ diseases 8 preclinical gene therapies in development • • • • • Portfolio of Gene Therapy Programs and Technologies Provides Foundation for Future |
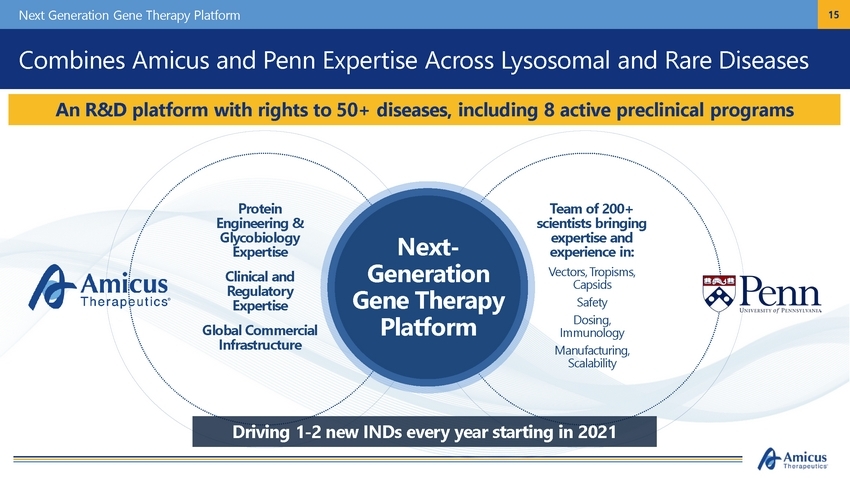 |
Next Generation Gene Therapy Platform 15 Combines Amicus and Penn Expertise Across Lysosomal and Rare Diseases Protein Engineering & Glycobiology Expertise Clinical and Regulatory Expertise Global Commercial Infrastructure Team of 200+ scientists bringing expertise and experience in: Vectors, Tropisms, Capsids Safety Dosing, Immunology Manufacturing, Scalability Next-Generation Gene Therapy Platform Driving 1-2 new INDs every year starting in 2021 An R&D platform with rights to 50+ diseases, including 8 active preclinical programs |
 |
Financial Summary “We are business led and science driven” - Amicus Belief Statement |
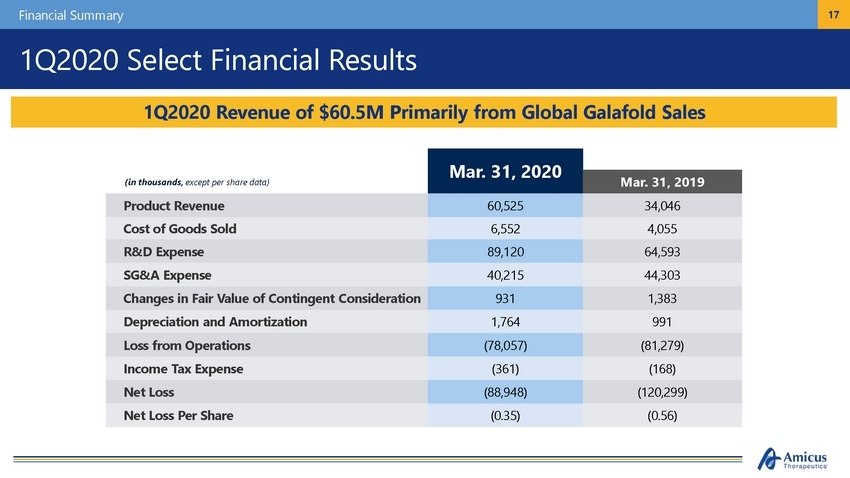 |
Financial Summary 17 1Q2020 Select Financial Results (in thousands, except per share data) Mar. 31, 2020 Mar. 31, 2019 Product Revenue 60,525 34,046 Cost of Goods Sold 6,552 4,055 R&D Expense 89,120 64,593 SG&A Expense 40,215 44,303 Changes in Fair Value of Contingent Consideration 931 1,383 Depreciation and Amortization 1,764 991 Loss from Operations (78,057) (81,279) Income Tax Expense (361) (168) Net Loss (88,948) (120,299) Net Loss Per Share (0.35) (0.56) 1Q2020 Revenue of $60.5M Primarily from Global Galafold Sales |
 |
Financial Summary 18 Cash Runway Now to Well into 2H2022 (~2+ years) $338M+ Cash 1Q2020 Well into ~2+ Years Cash Runway 2H2022 Fully funded through major milestones in portfolio and continued global growth |
 |
Financial Summary 19 Financial Outlook: Key Takeaways • Cash runway now well into 2H2022 – Achieved through continued careful expense management, prioritization of very early stage research programs and more measured capital expenditures • Non-GAAP quarterly operating expense expected to decline throughout 2020 Company fully funded through major milestones in portfolio and continued global growth Cumulative Galafold projected revenue of $1B+ in 2020-2022 offsets significant majority of company spend/investments Only modest additional capital required in the outer years to extend runway into profitability with multiple non-equity sources available as/when needed • • • |
 |
ClosingRemarks “We are business led and science driven” - Amicus Belief Statement |
 |
ThankYou “Our passion for making a difference unites us” -Amicus Belief Statement |
 |
Appendix |
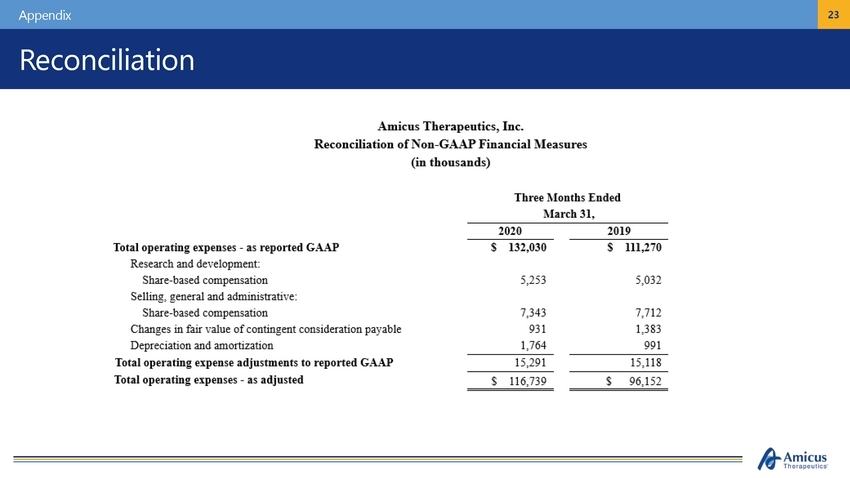 |
Amicus Therapeutics, Inc. Reconciliation of Non-GAAP Financial Measures (in thousands) Three Months Ended March 31, 2020 2019 s Total operating expenses - as reported GAAP Research and development: Share-based compensation Selling, general and administrative: Share-based compensation Changes illfair value of contingent consideration payable Depreciation and amortization Total operating expense adjustments to reported GAAP Total operating expenses - as adjusted $ 132,030 1ll,270 5,253 5,032 7,343 931 1,764 7,712 1,383 991 15,291 s ]}6,739 15,118 $ 96,152 Ll..Amicus {v-Therapeutics- |
