Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AGENUS INC | d924651dex991.htm |
| 8-K - 8-K - AGENUS INC | agen-8k_20200507.htm |

May 2020 Exhibit 99.2

Forward-Looking Statements This presentation contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws, including statements regarding Agenus and AgenTus' clinical development and regulatory plans and timelines, expected timing for clinical trials and releasing clinical data, anticipated milestones from partnership transactions, goals for 2020 and anticipated commercial market opportunities. These forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially. These risks and uncertainties include, among others, the factors described under the Risk Factors section of our most recent Quarterly Report on Form 10-Q or Annual Report on Form 10-K filed with the Securities and Exchange Commission filed with the Securities and Exchange Commission and made available on our website at www.agenusbio.com. Agenus cautions investors not to place considerable reliance on the forward-looking statements contained in this presentation. These statements speak only as of the date of this presentation, and Agenus undertakes no obligation to update or revise the statements, other than to the extent required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
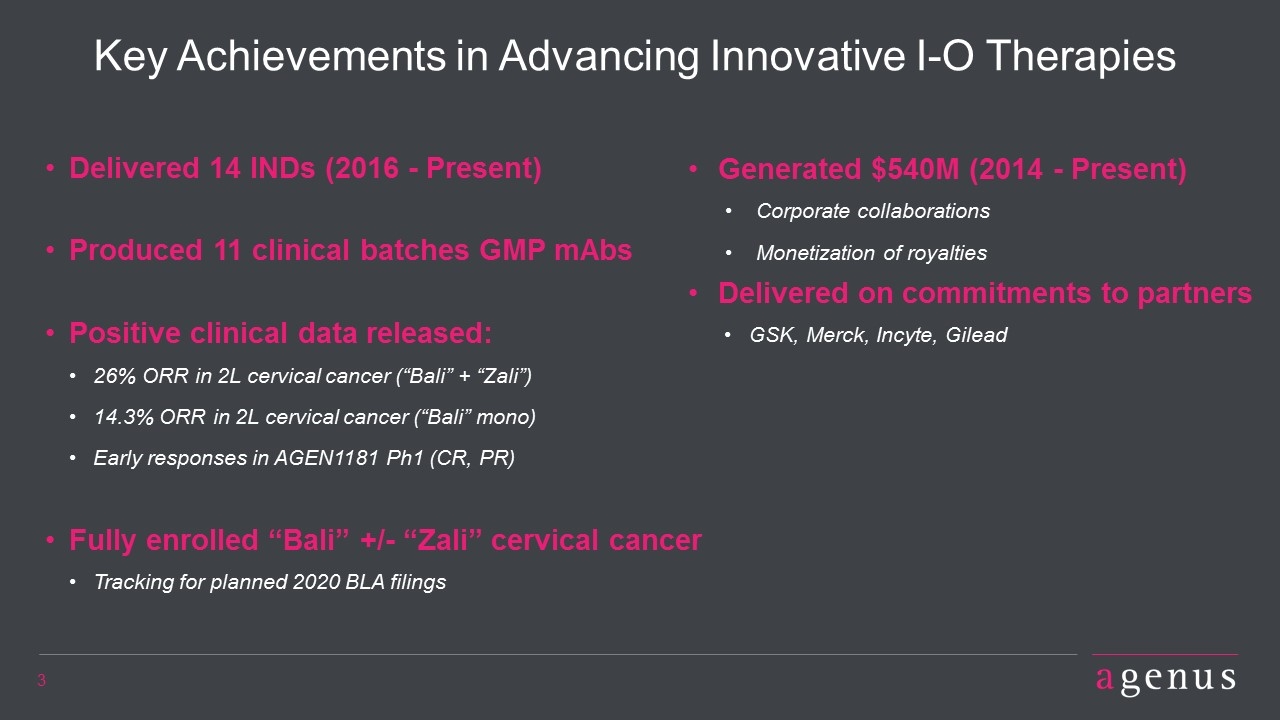
Key Achievements in Advancing Innovative I-O Therapies Delivered 14 INDs (2016 - Present) Produced 11 clinical batches GMP mAbs Positive clinical data released: 26% ORR in 2L cervical cancer (“Bali” + “Zali”) 14.3% ORR in 2L cervical cancer (“Bali” mono) Early responses in AGEN1181 Ph1 (CR, PR) Fully enrolled “Bali” +/- “Zali” cervical cancer Tracking for planned 2020 BLA filings Generated $540M (2014 - Present) Corporate collaborations Monetization of royalties Delivered on commitments to partners GSK, Merck, Incyte, Gilead
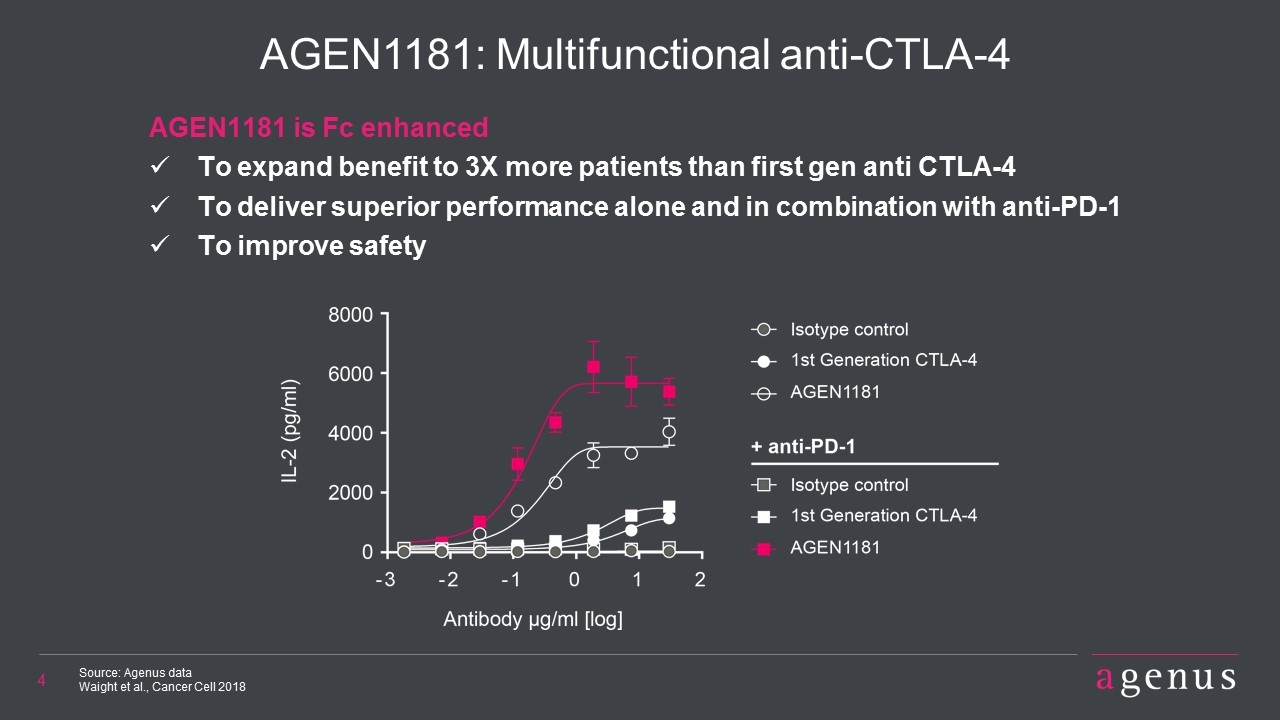
AGEN1181: Multifunctional anti-CTLA-4 Source: Agenus data Waight et al., Cancer Cell 2018 AGEN1181 is Fc enhanced To expand benefit to 3X more patients than first gen anti CTLA-4 To deliver superior performance alone and in combination with anti-PD-1 To improve safety

AGEN1181: Unprecedented Clinical Responses in Early Phase 1 Yervoy In >1000 patients with solid tumors outside of melanoma, only 4 CRs observed at high doses of 3 or 10mg/kg AGEN1181 >70% disease control rate among 22 patients treated with monotherapy or balstilimab combination 1 CR and 1 PR in aggressive cancer with poor prognosis (endometrial) at low dose levels (1mg/kg or lower) Responders had genetic polymorphism that AGEN1181 was designed to benefit Shows potential for AGEN1181 to benefit an additional 40% of patients unresponsive to Yervoy® due to genetics Source: Agenus data
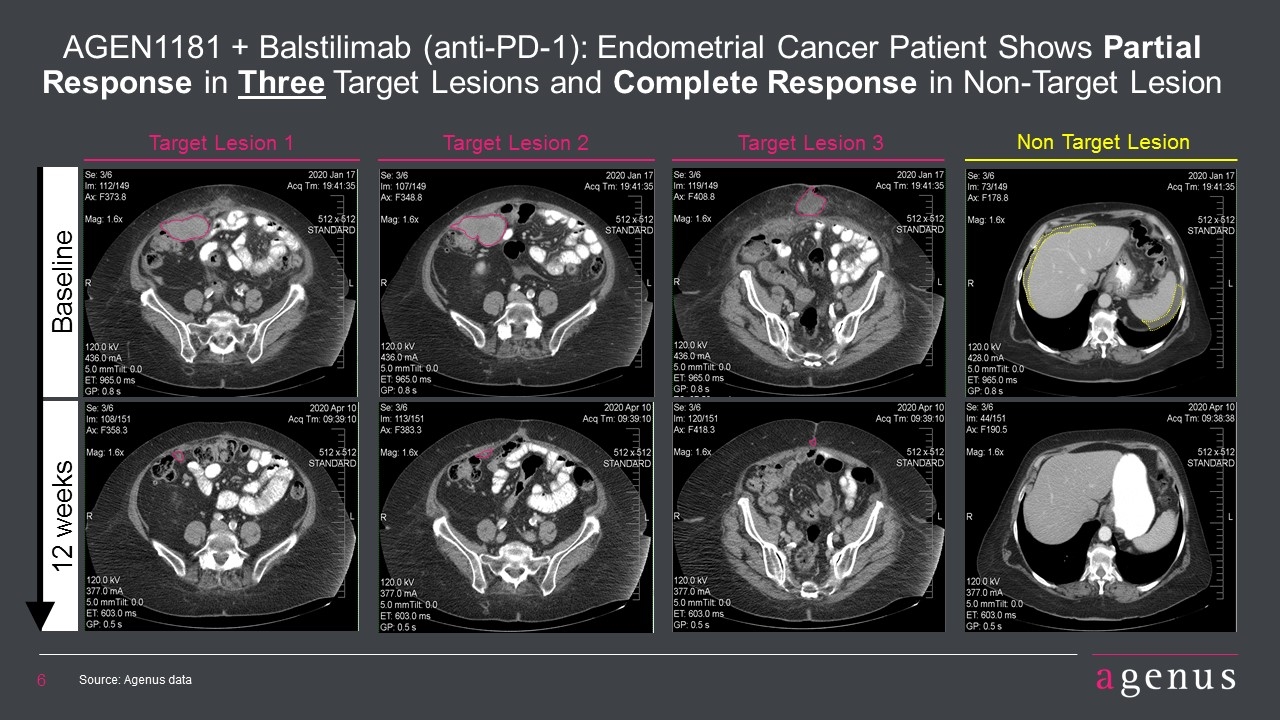
Target Lesion 1 Target Lesion 2 AGEN1181 + Balstilimab (anti-PD-1): Endometrial Cancer Patient Shows Partial Response in Three Target Lesions and Complete Response in Non-Target Lesion Target Lesion 3 Non Target Lesion Baseline 12 weeks Source: Agenus data
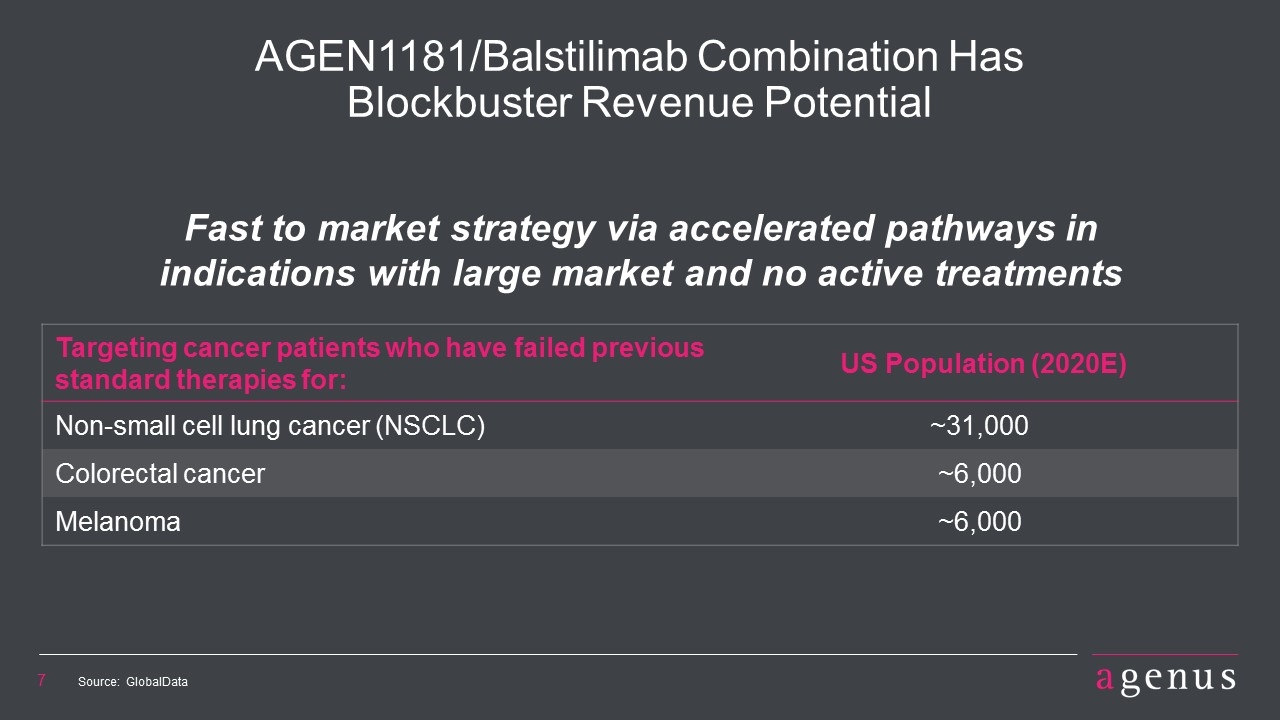
Targeting cancer patients who have failed previous standard therapies for: US Population (2020E) Non-small cell lung cancer (NSCLC) ~31,000 Colorectal cancer ~6,000 Melanoma ~6,000 AGEN1181/Balstilimab Combination Has Blockbuster Revenue Potential Fast to market strategy via accelerated pathways in indications with large market and no active treatments Source: GlobalData
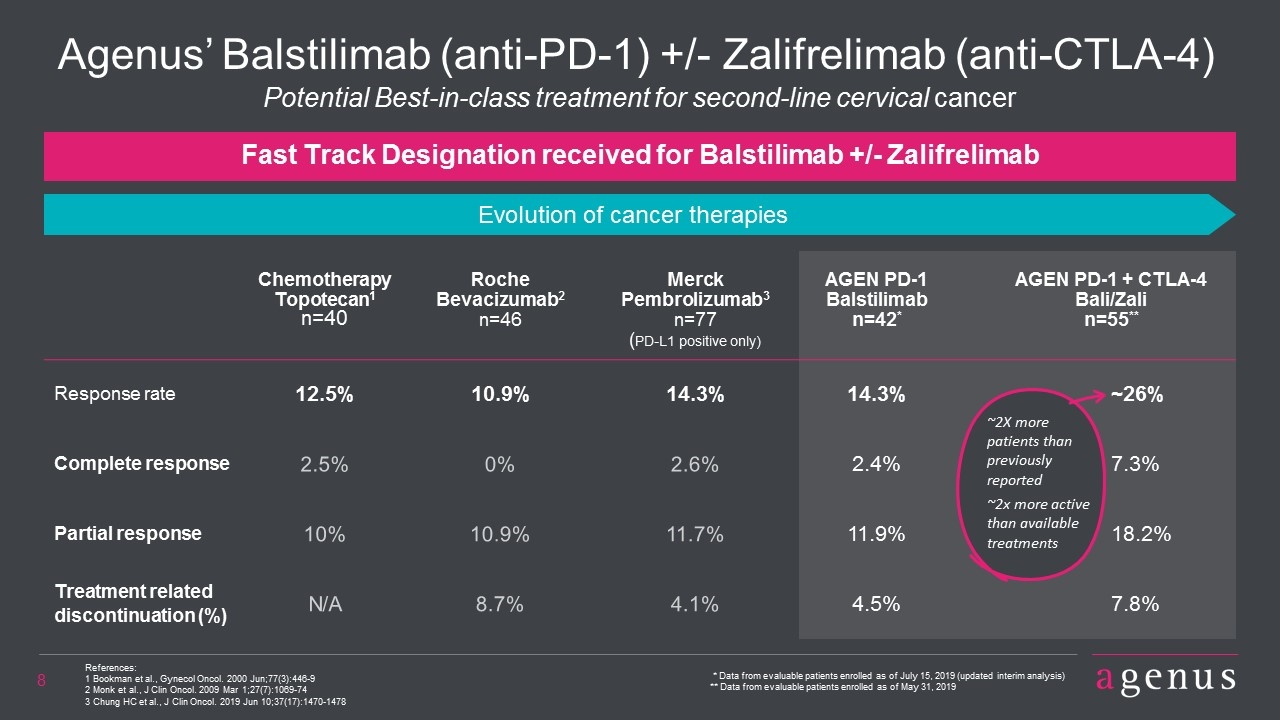
Agenus’ Balstilimab (anti-PD-1) +/- Zalifrelimab (anti-CTLA-4) References: 1 Bookman et al., Gynecol Oncol. 2000 Jun;77(3):446-9 2 Monk et al., J Clin Oncol. 2009 Mar 1;27(7):1069-74 3 Chung HC et al., J Clin Oncol. 2019 Jun 10;37(17):1470-1478 Chemotherapy Topotecan1 n=40 Roche Bevacizumab2 n=46 Merck Pembrolizumab3 n=77 (PD-L1 positive only) AGEN PD-1 Balstilimab n=42* AGEN PD-1 + CTLA-4 Bali/Zali n=55** Response rate 12.5% 10.9% 14.3% 14.3% ~26% Complete response 2.5% 0% 2.6% 2.4% 7.3% Partial response 10% 10.9% 11.7% 11.9% 18.2% Treatment related discontinuation (%) N/A 8.7% 4.1% 4.5% 7.8% Fast Track Designation received for Balstilimab +/- Zalifrelimab Potential Best-in-class treatment for second-line cervical cancer * Data from evaluable patients enrolled as of July 15, 2019 (updated interim analysis) ** Data from evaluable patients enrolled as of May 31, 2019 Evolution of cancer therapies ~2X more patients than previously reported ~2x more active than available treatments
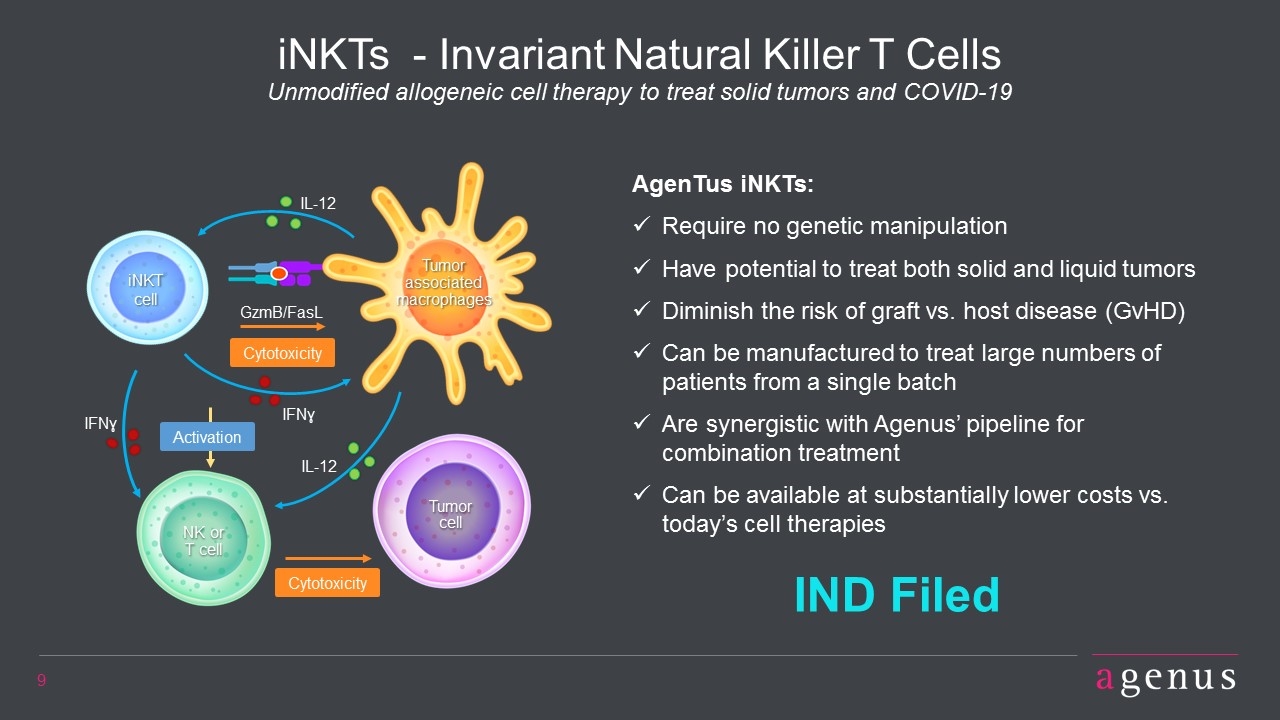
Cytotoxicity GzmB/FasL IFNɣ iNKT cell Tumor cell Cytotoxicity NK or T cell IL-12 IFNɣ Activation iNKTs - Invariant Natural Killer T Cells Unmodified allogeneic cell therapy to treat solid tumors and COVID-19 AgenTus iNKTs: Require no genetic manipulation Have potential to treat both solid and liquid tumors Diminish the risk of graft vs. host disease (GvHD) Can be manufactured to treat large numbers of patients from a single batch Are synergistic with Agenus’ pipeline for combination treatment Can be available at substantially lower costs vs. today’s cell therapies IND Filed IL-12 Tumor associated macrophages
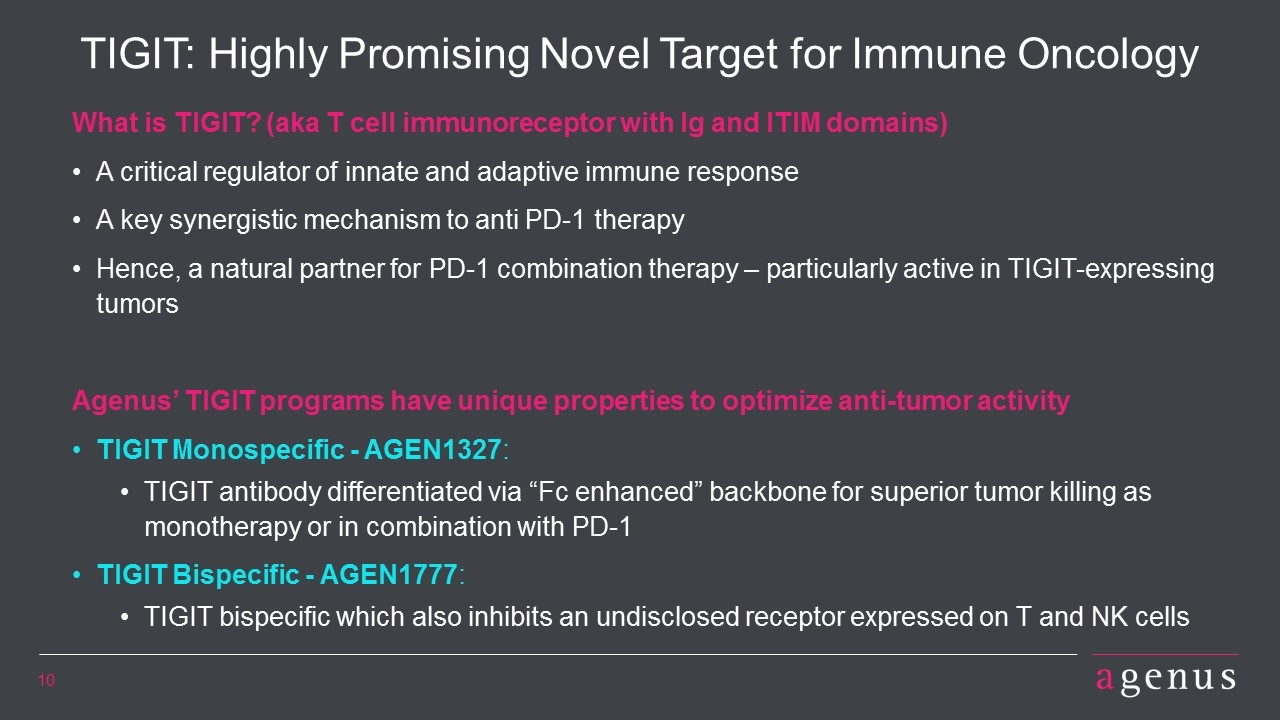
TIGIT: Highly Promising Novel Target for Immune Oncology What is TIGIT? (aka T cell immunoreceptor with Ig and ITIM domains) A critical regulator of innate and adaptive immune response A key synergistic mechanism to anti PD-1 therapy Hence, a natural partner for PD-1 combination therapy – particularly active in TIGIT-expressing tumors Agenus’ TIGIT programs have unique properties to optimize anti-tumor activity TIGIT Monospecific - AGEN1327: TIGIT antibody differentiated via “Fc enhanced” backbone for superior tumor killing as monotherapy or in combination with PD-1 TIGIT Bispecific - AGEN1777: TIGIT bispecific which also inhibits an undisclosed receptor expressed on T and NK cells
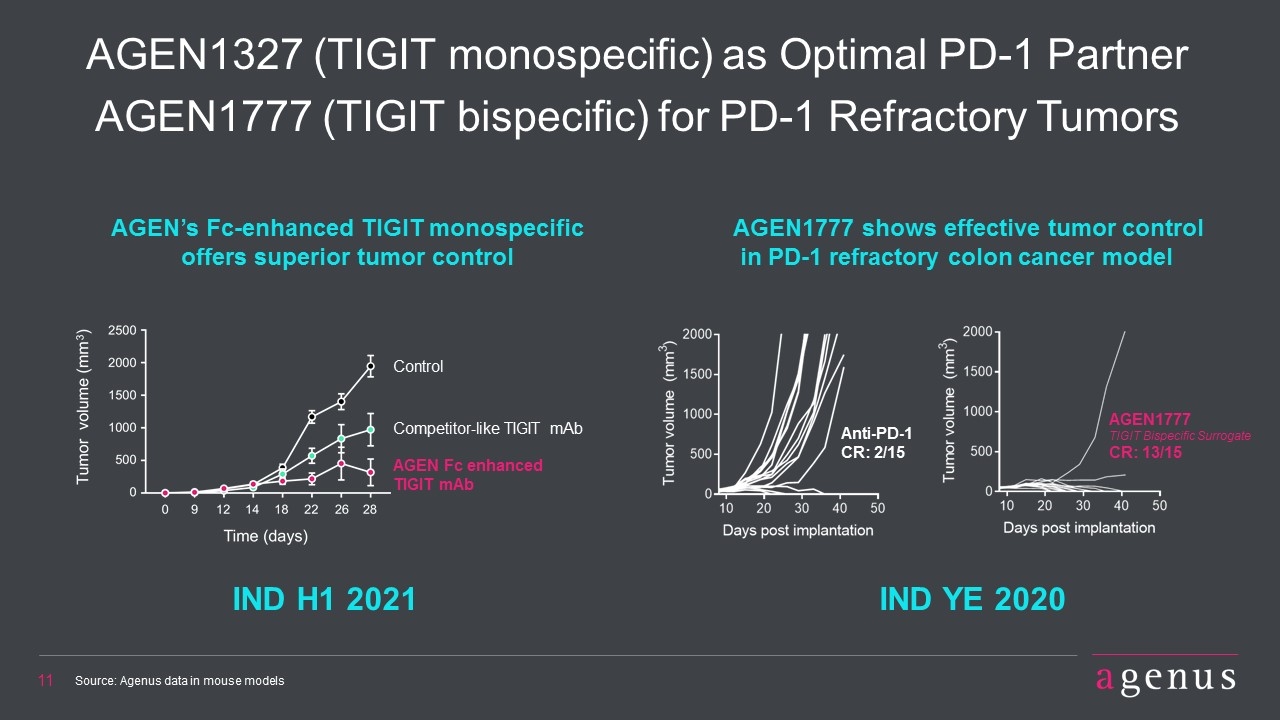
AGEN1327 (TIGIT monospecific) as Optimal PD-1 Partner AGEN1777 (TIGIT bispecific) for PD-1 Refractory Tumors IND H1 2021 Source: Agenus data in mouse models AGEN1777 shows effective tumor control in PD-1 refractory colon cancer model AGEN’s Fc-enhanced TIGIT monospecific offers superior tumor control IND YE 2020 Anti-PD-1 CR: 2/15 AGEN1777 TIGIT Bispecific Surrogate CR: 13/15 Control Competitor-like TIGIT mAb AGEN Fc enhanced TIGIT mAb
Key Achievements in Advancing Innovative I-O Therapies Delivered 14 INDs (2016 - Present) Produced 11 clinical batches GMP mAbs Positive clinical data released: 26% ORR in 2L cervical cancer (“Bali” + “Zali”) 14.3% ORR in 2L cervical cancer (“Bali” mono) Early responses in AGEN1181 Ph1 (CR, PR) Fully enrolled “Bali” +/- “Zali” cervical cancer Tracking for planned 2020 BLA filings Generated $540M (2014 - Present) Corporate collaborations Monetization of royalties Delivered on commitments to partners GSK, Merck, Incyte, Gilead
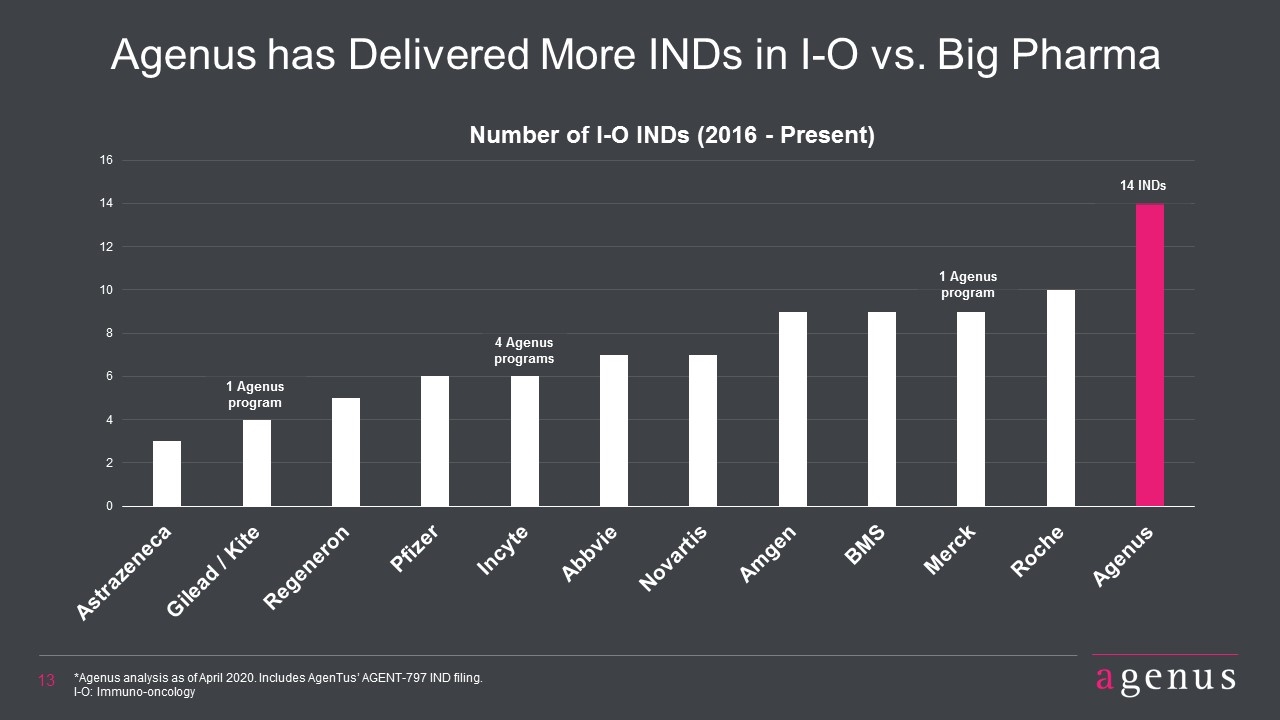
Agenus has Delivered More INDs in I-O vs. Big Pharma *Agenus analysis as of April 2020. Includes AgenTus’ AGENT-797 IND filing. I-O: Immuno-oncology
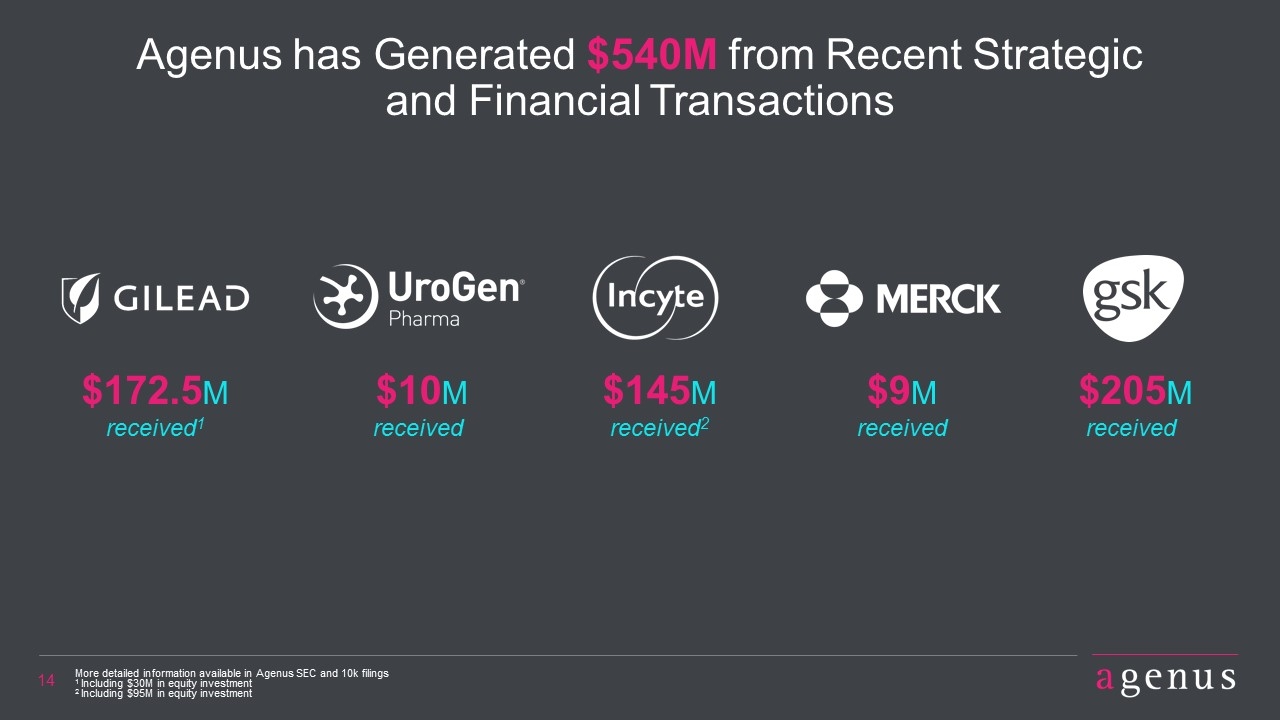
Agenus has Generated $540M from Recent Strategic and Financial Transactions $172.5M received1 $145M received2 $9M received $205M received More detailed information available in Agenus SEC and 10k filings 1 Including $30M in equity investment 2 Including $95M in equity investment $10M received
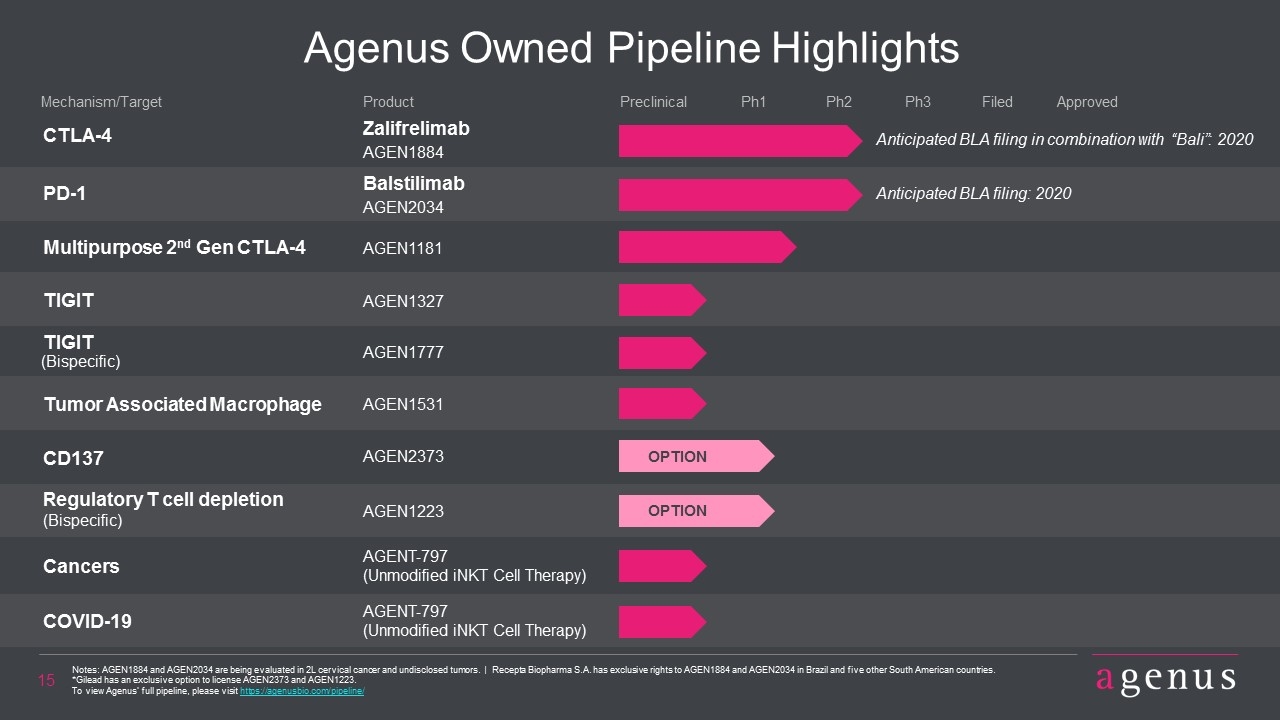
Agenus Owned Pipeline Highlights CTLA-4 Zalifrelimab AGEN1884 PD-1 Balstilimab AGEN2034 Product Mechanism/Target Preclinical Ph1 Ph2 Ph3 Filed Multipurpose 2nd Gen CTLA-4 AGEN1181 TIGIT AGEN1327 TIGIT AGEN1777 (Bispecific) CD137 AGEN2373 Regulatory T cell depletion AGENT-797 (Bispecific) Cancers COVID-19 (Unmodified iNKT Cell Therapy) AGENT-797 (Unmodified iNKT Cell Therapy) AGEN1223 OPTION OPTION Approved Notes: AGEN1884 and AGEN2034 are being evaluated in 2L cervical cancer and undisclosed tumors. | Recepta Biopharma S.A. has exclusive rights to AGEN1884 and AGEN2034 in Brazil and five other South American countries. *Gilead has an exclusive option to license AGEN2373 and AGEN1223. To view Agenus’ full pipeline, please visit https://agenusbio.com/pipeline/ Tumor Associated Macrophage AGEN1531 Anticipated BLA filing in combination with “Bali”: 2020 Anticipated BLA filing: 2020
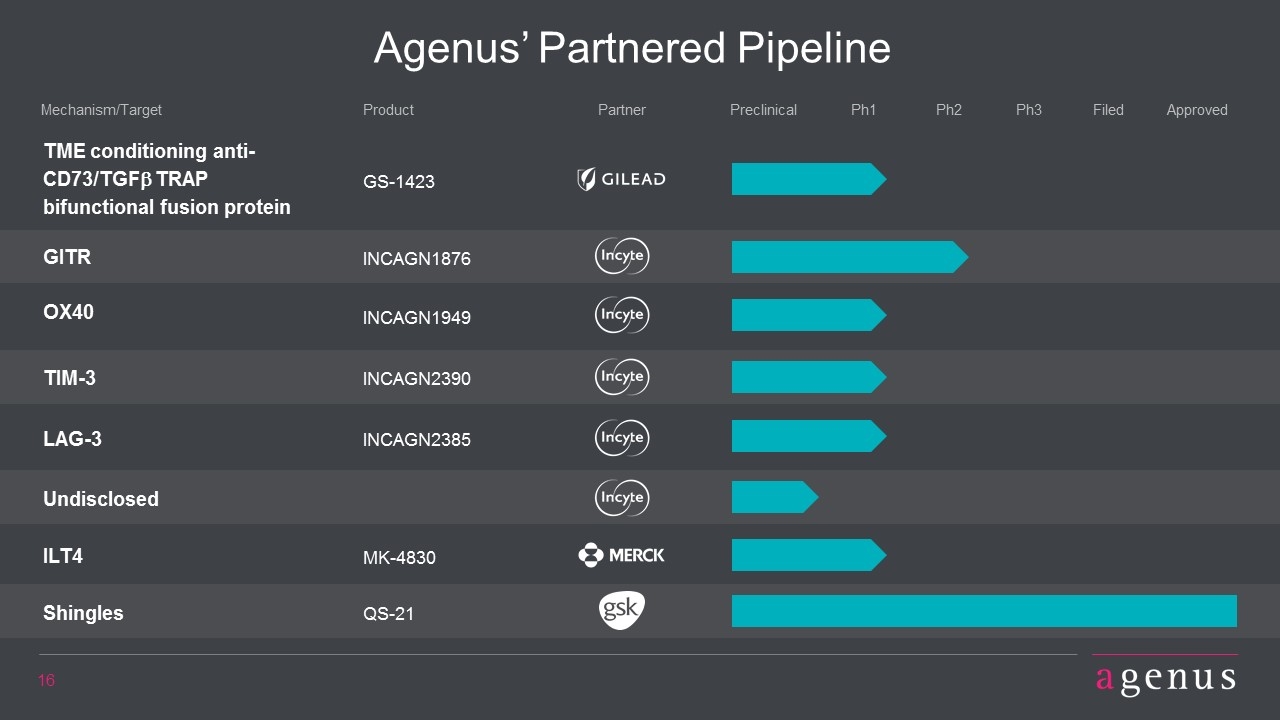
TME conditioning anti-CD73/TGFb TRAP bifunctional fusion protein GS-1423 GITR INCAGN1876 OX40 INCAGN1949 TIM-3 INCAGN2390 LAG-3 INCAGN2385 Undisclosed MK-4830 ILT4 Agenus’ Partnered Pipeline Product Mechanism/Target Preclinical Ph1 Ph2 Ph3 Filed Approved Partner Shingles QS-21

What’s Next? 6 Clinical Data Readouts Zalifrelimab and Balstilimab Balstilimab AGEN1181 AGEN1223 AGEN2373 AgenT-797 2 BLA filings 2 INDs: TIGIT, TIGIT Bispecific Commercial Launch: Cervical More partnerships COVID-19: Protective (QS-21) Therapeutic (iNKT) approaches

