Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SUPERNUS PHARMACEUTICALS, INC. | supn-20200428.htm |
| EX-99.1 - EX-99.1 - SUPERNUS PHARMACEUTICALS, INC. | ex99104282020.htm |

Exhibit 99.2 Acquisition of US WorldMeds’ CNS Portfolio April 2020 © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 1

Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking statements within the meaning of the federal securities laws. These statements, among other things, relate to Supernus’ business strategy, goals and expectations concerning its product candidates, ability to integrate the acquired portfolio into its infrastructure, future operations, prospects, plans and objectives of management. The words “anticipate”, “believe”, “could”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict“, “project”, “will“, and similar terms and phrases are used to identify forward-looking statements in this presentation. Supernus’ operations involve risks and uncertainties, many of which are outside its control, including the potential impact of COVID-19, and any one of which, or a combination of which, could materially affect its results of operations and whether the forward-looking statements ultimately prove to be correct. Supernus assumes no obligation to update any forward-looking statements except as required by applicable law. Supernus has filed with the U.S. Securities and Exchange Commission (SEC) reports and other documents required by Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended. Before you purchase any Supernus securities, you should read such reports and other documents to obtain more complete information about the company’s operations and business and the risks and uncertainties that it faces in implementing its business plan. You may get these documents for free by visiting EDGAR on the SEC website at http://www.sec.gov. © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 2

Presenters Jack Khattar President and CEO, Director Bryan Roecklein Vice President of Corporate Development Greg Patrick Sr. Vice President, CFO © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 3
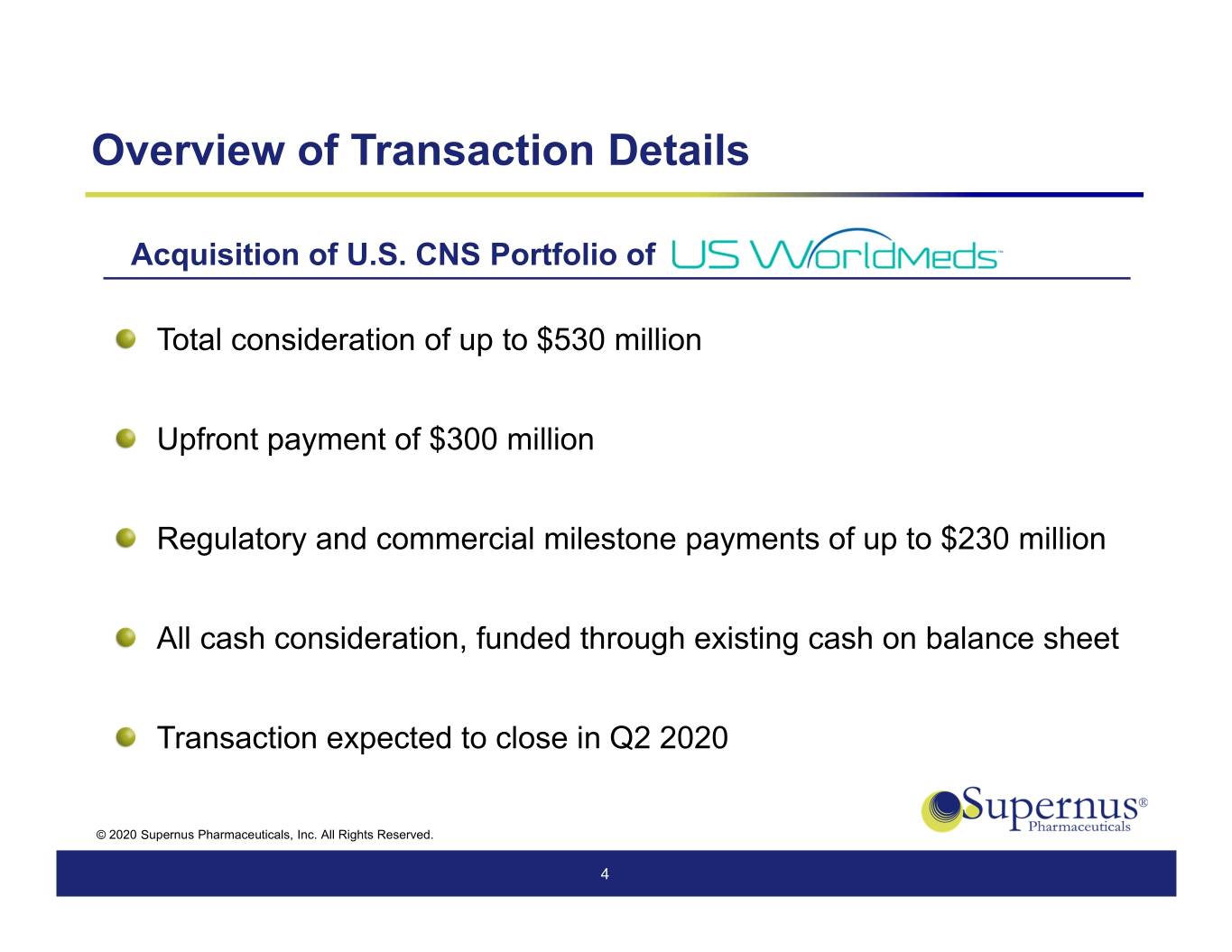
Overview of Transaction Details Acquisition of U.S. CNS Portfolio of Total consideration of up to $530 million Upfront payment of $300 million Regulatory and commercial milestone payments of up to $230 million All cash consideration, funded through existing cash on balance sheet Transaction expected to close in Q2 2020 © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 4
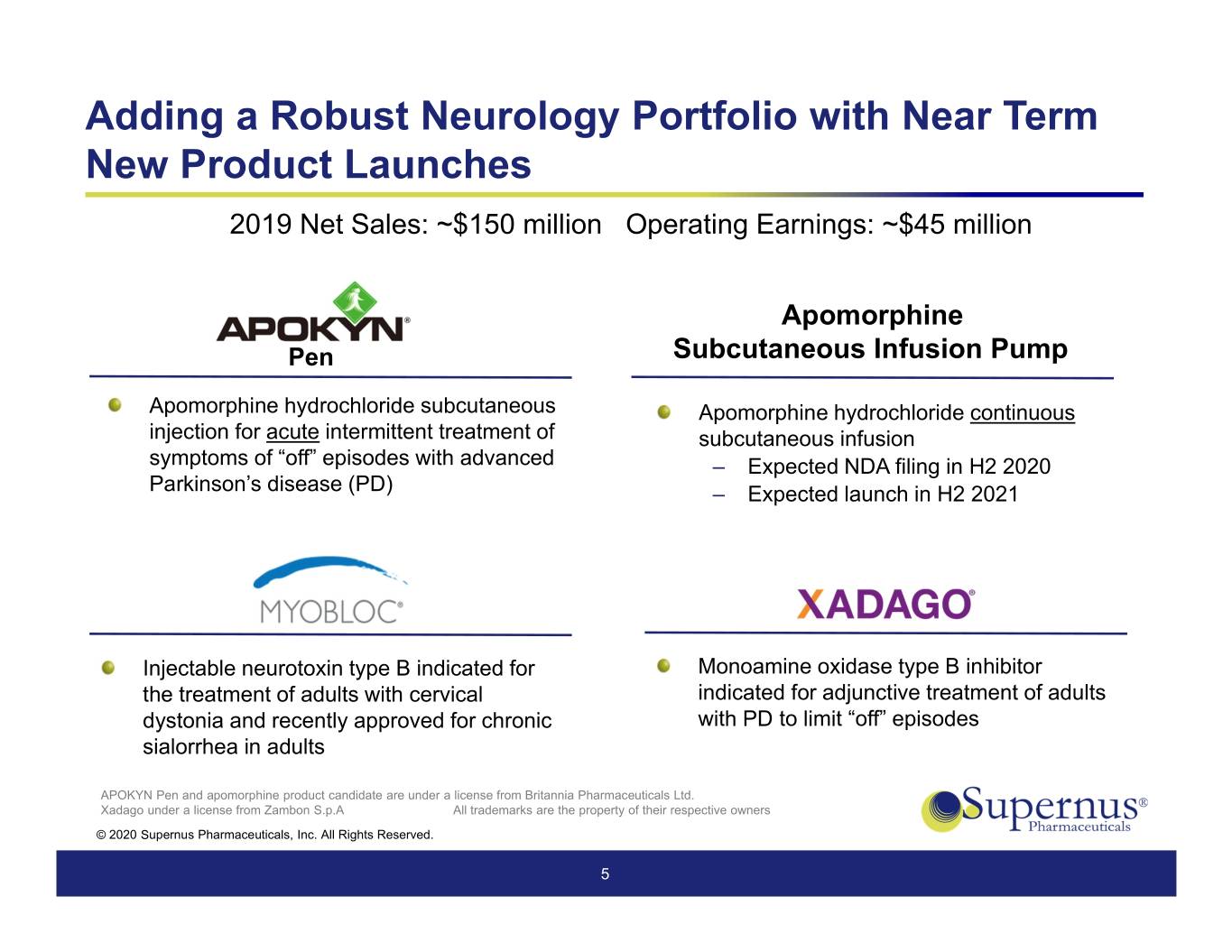
Adding a Robust Neurology Portfolio with Near Term New Product Launches 2019 Net Sales: ~$150 million Operating Earnings: ~$45 million Apomorphine Pen Subcutaneous Infusion Pump Apomorphine hydrochloride subcutaneous Apomorphine hydrochloride continuous injection for acute intermittent treatment of subcutaneous infusion symptoms of “off” episodes with advanced ‒ Expected NDA filing in H2 2020 Parkinson’s disease (PD) ‒ Expected launch in H2 2021 Injectable neurotoxin type B indicated for Monoamine oxidase type B inhibitor the treatment of adults with cervical indicated for adjunctive treatment of adults dystonia and recently approved for chronic with PD to limit “off” episodes sialorrhea in adults APOKYN Pen and apomorphine product candidate are under a license from Britannia Pharmaceuticals Ltd. Xadago under a license from Zambon S.p.A All trademarks are the property of their respective owners © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 5

Strategic Fit & Rationale This acquisition fits squarely with Supernus’ corporate development strategy of adding commercial and late stage neurology assets Creates Leading 1 2 Adds New CNS Portfolio Growth Catalysts Five Marketed Apomorphine Products Infusion Pump H2 2021 Strong Strategic Fit MYOBLOC® in Additional Late-Stage Neurological Pipeline Disorders © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 6
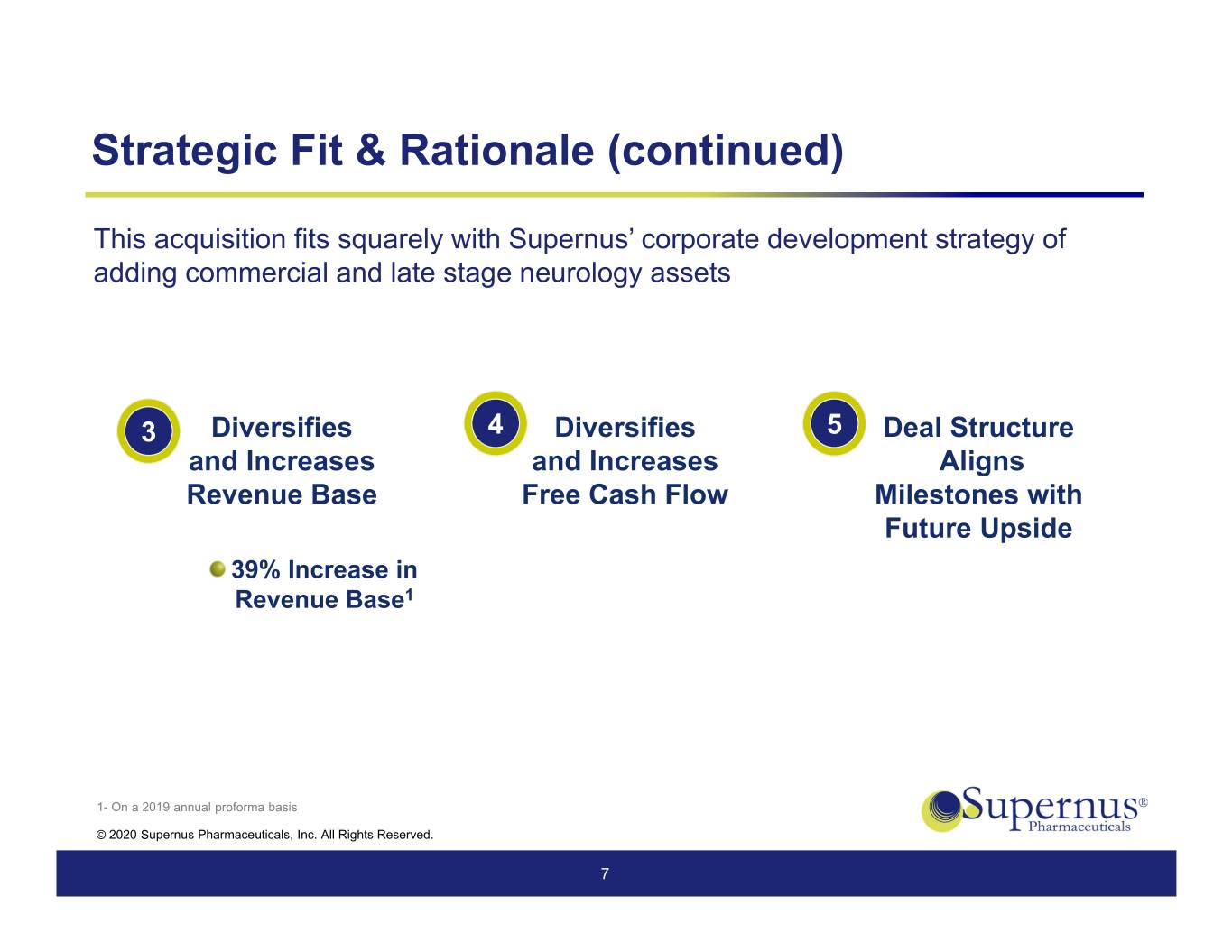
Strategic Fit & Rationale (continued) This acquisition fits squarely with Supernus’ corporate development strategy of adding commercial and late stage neurology assets 3 Diversifies 4 Diversifies 5 Deal Structure and Increases and Increases Aligns Revenue Base Free Cash Flow Milestones with Future Upside 39% Increase in Revenue Base1 1- On a 2019 annual proforma basis © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 7

Parkinson’s Disease (PD) Market US PD Market is anticipated to grow from $1.5B to $6.2B by 20261 Second most common chronic progressive neurodegenerative disorder, affecting 1-2% of individuals 65 years and older2 Number of U.S. PD Patients in 2020 is ~1M with an annual growth rate of approximately 2.5%1 PD occurs when cells in the brain, which produce dopamine, become impaired or die The mainstay for therapy is levodopa with effectiveness wearing off resulting in “OFF” periods 1. Global Data Parkinson’s Disease Global Drug Forecast and Market Analysis 2026 2. Saxton JM. Exercise and Chronic Disease: an Evidence-Based Approach. London, Routledge, 2011 © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 8
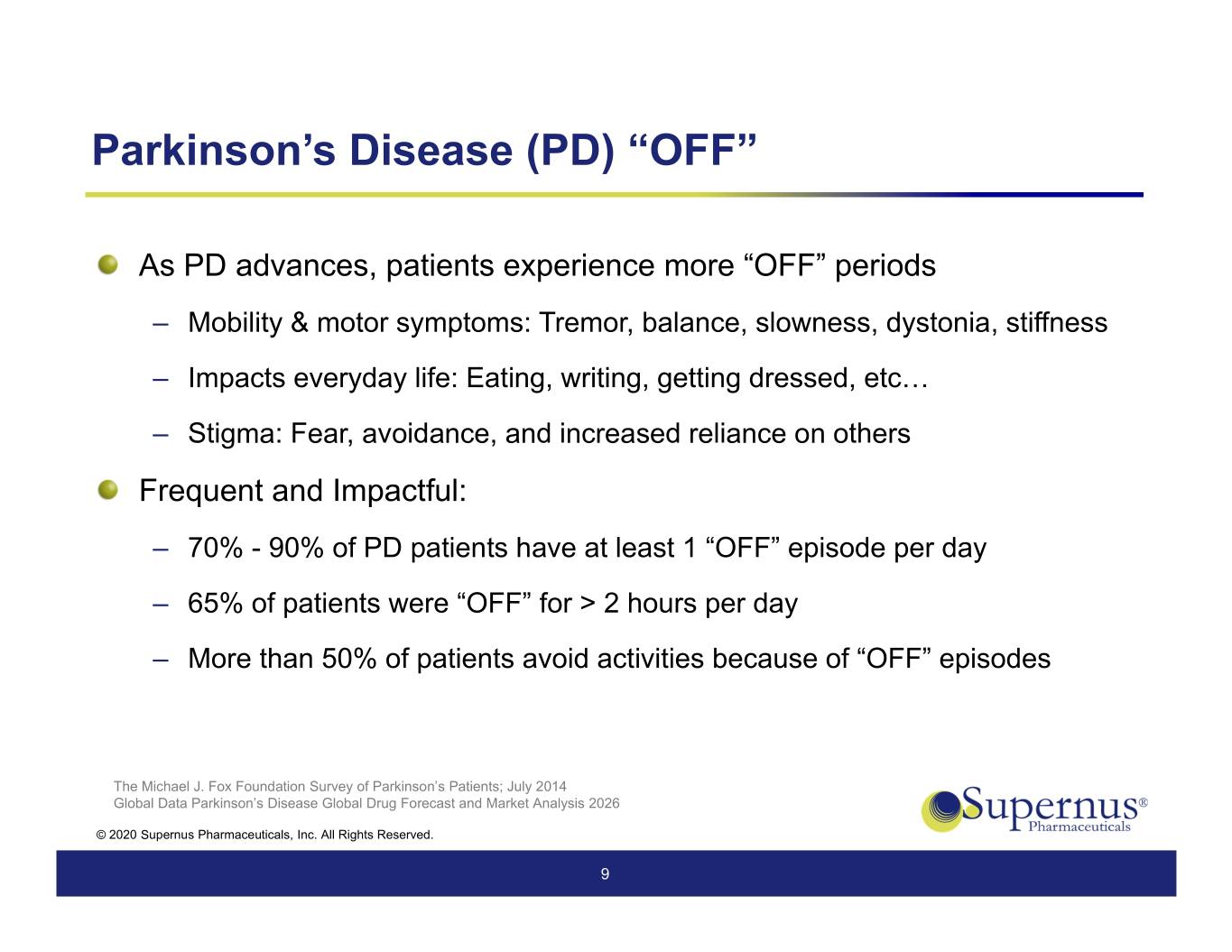
Parkinson’s Disease (PD) “OFF” As PD advances, patients experience more “OFF” periods ‒ Mobility & motor symptoms: Tremor, balance, slowness, dystonia, stiffness ‒ Impacts everyday life: Eating, writing, getting dressed, etc… ‒ Stigma: Fear, avoidance, and increased reliance on others Frequent and Impactful: ‒ 70% - 90% of PD patients have at least 1 “OFF” episode per day ‒ 65% of patients were “OFF” for > 2 hours per day ‒ More than 50% of patients avoid activities because of “OFF” episodes The Michael J. Fox Foundation Survey of Parkinson’s Patients; July 2014 Global Data Parkinson’s Disease Global Drug Forecast and Market Analysis 2026 © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 9
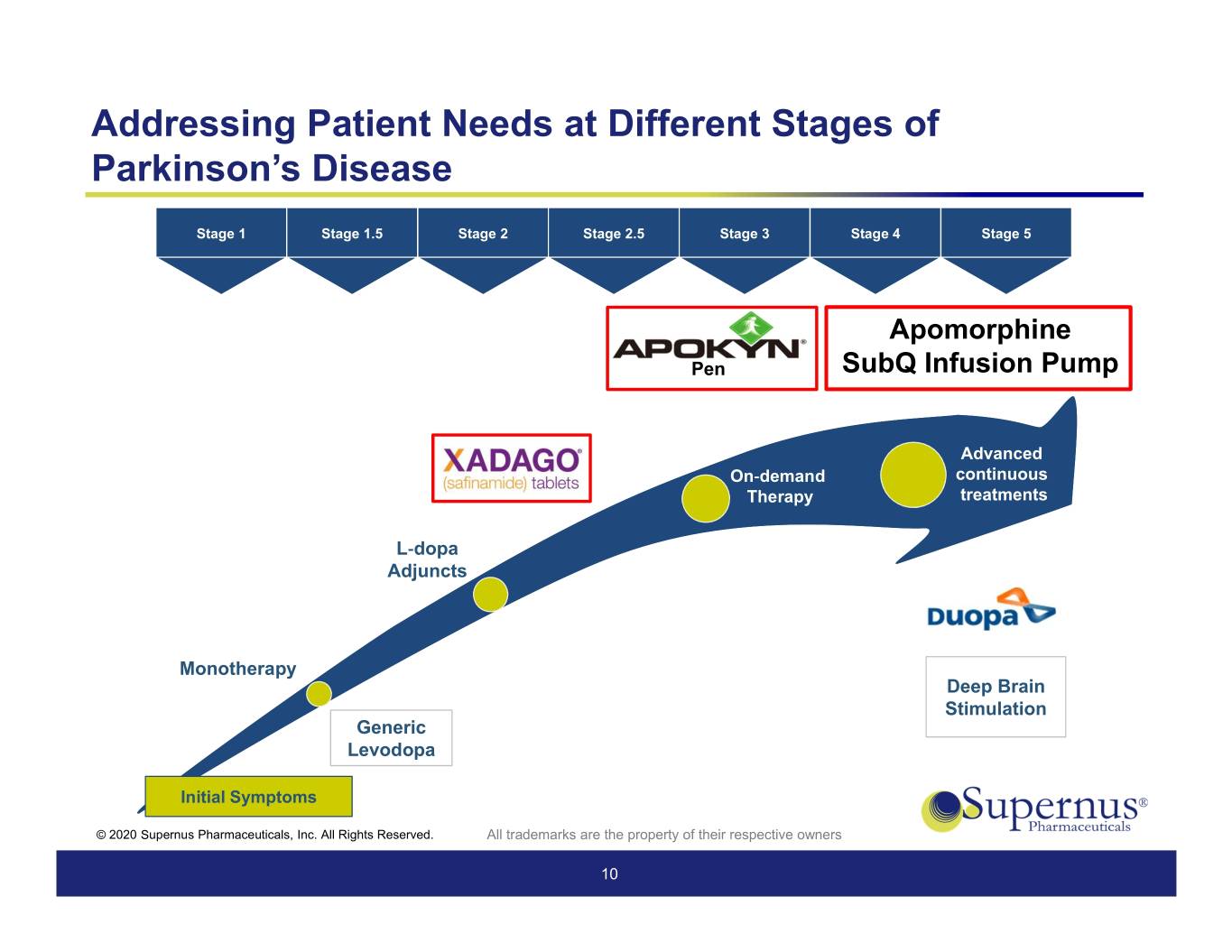
Addressing Patient Needs at Different Stages of Parkinson’s Disease Stage 1 Stage 1.5 Stage 2 Stage 2.5 Stage 3 Stage 4 Stage 5 Apomorphine Pen SubQ Infusion Pump Advanced On-demand continuous Therapy treatments L-dopa Adjuncts Monotherapy Deep Brain Stimulation Generic Levodopa Initial Symptoms © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. All trademarks are the property of their respective owners 10

APOKYN® Pen APOKYN Pen: Apomorphine delivered through a subcutaneous injection ‒ Well established product, with $118.9 million in sales in 2019 ‒ Best-in-class therapy for acute, rapid and reliable treatment of “OFF” Episodes in Parkinson’s Disease ‒ Successfully treats 95% of OFF episodes by 20 minutes1 ‒ A high unmet need with significant market opportunity 1 - Dewey RB Jr, Hutton JT, LeWitt PA, Factor SA. A randomized, double-blind, placebo-controlled trial of subcutaneously injected apomorphine for parkinsonian off-state events. Arch Neurol. 2001;58(9):1385–1392. © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 11
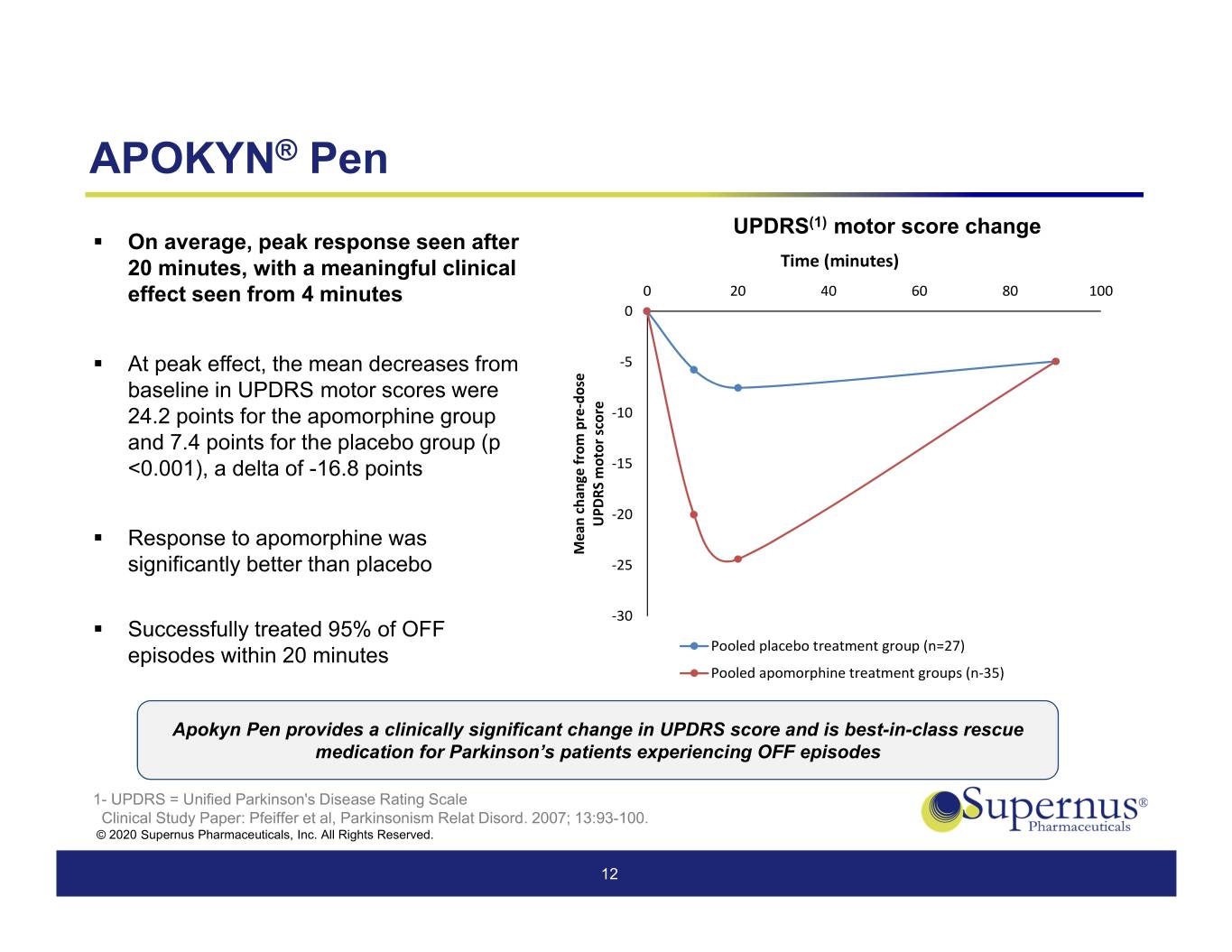
APOKYN® Pen UPDRS(1) motor score change . On average, peak response seen after 20 minutes, with a meaningful clinical Time (minutes) 0 20406080100 effect seen from 4 minutes 0 . At peak effect, the mean decreases from ‐5 baseline in UPDRS motor scores were dose ‐ ‐10 24.2 points for the apomorphine group pre score and 7.4 points for the placebo group (p from ‐15 <0.001), a delta of -16.8 points motor change ‐20 UPDRS . Response to apomorphine was Mean significantly better than placebo ‐25 ‐30 . Successfully treated 95% of OFF episodes within 20 minutes Pooled placebo treatment group (n=27) Pooled apomorphine treatment groups (n‐35) Apokyn Pen provides a clinically significant change in UPDRS score and is best-in-class rescue medication for Parkinson’s patients experiencing OFF episodes 1- UPDRS = Unified Parkinson's Disease Rating Scale Clinical Study Paper: Pfeiffer et al, Parkinsonism Relat Disord. 2007; 13:93-100. © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 12
New Product Candidate Apomorphine Continuous Subcutaneous Infusion Expected launch in H2 2021 ‒ Eligible for Orphan Drug Designation and 7 year exclusivity ‒ The only non-invasive continuous, dopaminergic stimulation therapy to reduce “OFF” and maximize “ON” time in PD Less invasive than currently available options ‒ Gastro-intestinal surgically implanted levodopa/carbidopa infusion ‒ Deep Brain Stimulation Potential peak revenue of $100-175 million © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 13
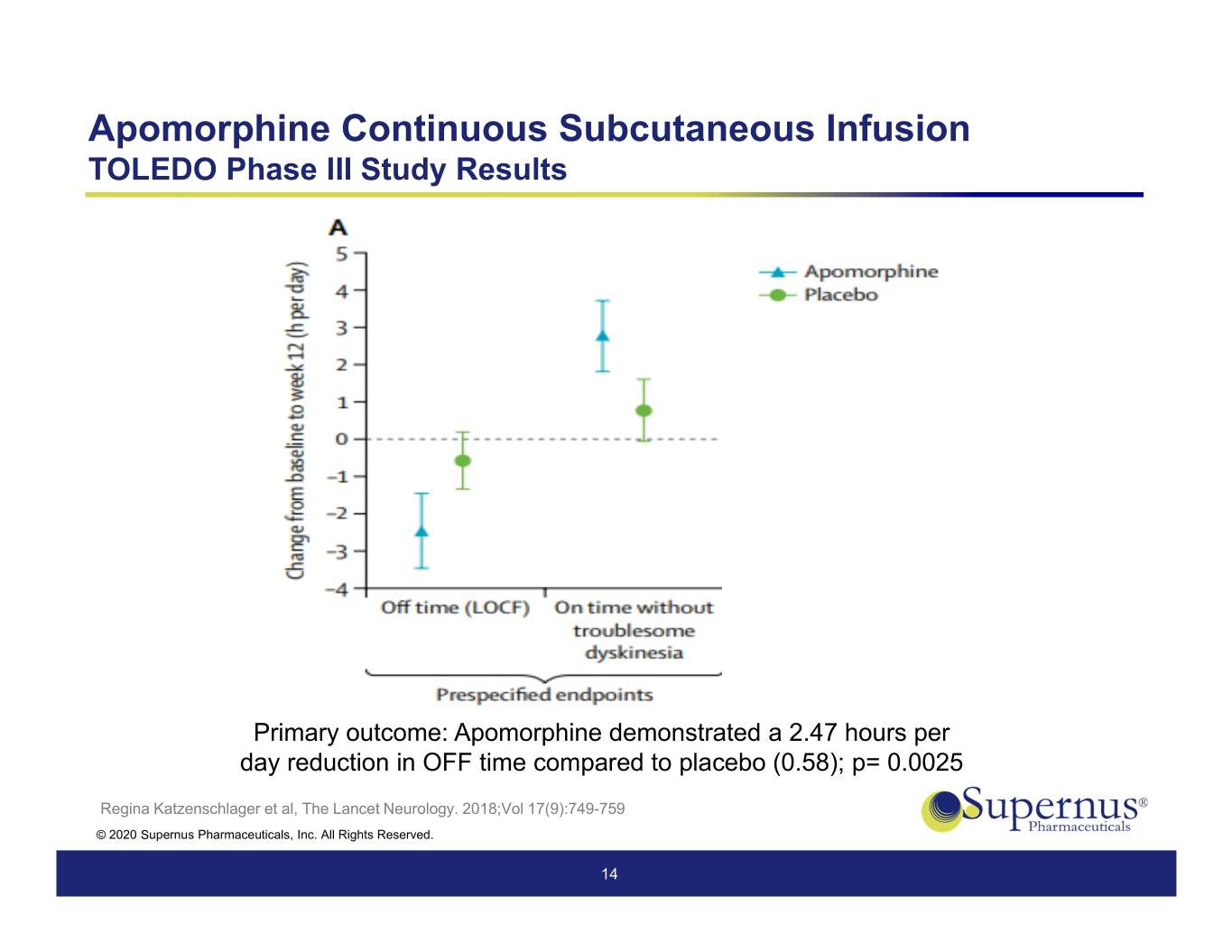
Apomorphine Continuous Subcutaneous Infusion TOLEDO Phase III Study Results Primary outcome: Apomorphine demonstrated a 2.47 hours per day reduction in OFF time compared to placebo (0.58); p= 0.0025 Regina Katzenschlager et al, The Lancet Neurology. 2018;Vol 17(9):749-759 © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 14
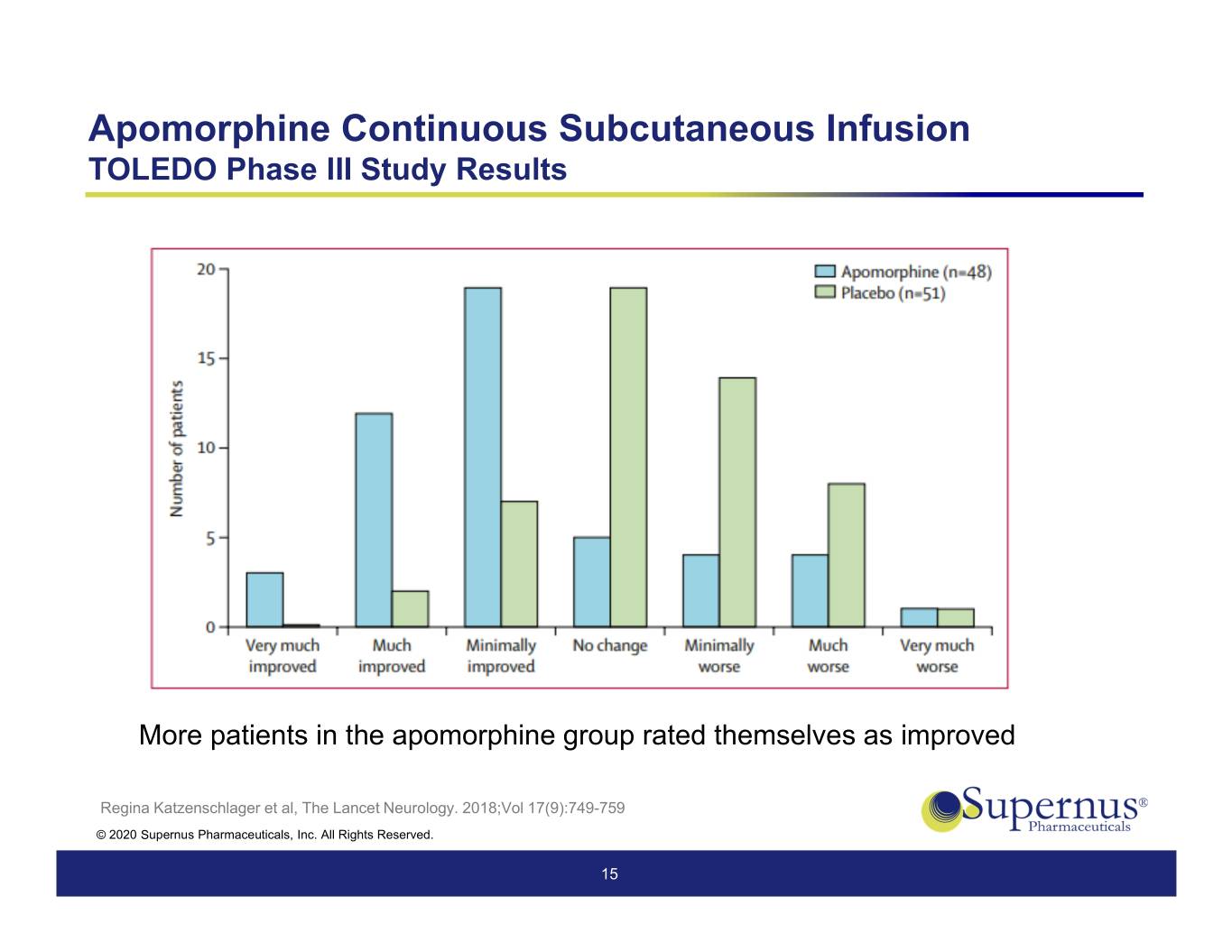
Apomorphine Continuous Subcutaneous Infusion TOLEDO Phase III Study Results More patients in the apomorphine group rated themselves as improved Regina Katzenschlager et al, The Lancet Neurology. 2018;Vol 17(9):749-759 © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 15
Apomorphine Franchise Combination product/device development requirements are challenging ‒ Patient specific human factor studies required for registration ‒ Infusion pump has the potential for Orphan Drug Exclusivity Additional support is needed to initiate and maintain patients on therapy ‒ Specialty Pharmacy ‒ Fulfillment Hub ‒ Nurse Network © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 16
MYOBLOC® (rimabotulinumtoxinB Injection) Approved in the U.S for adults with Cervical Dystonia (CD) New indication in November 2019 for chronic sialorrhea in adults ‒ 600,000 adult patients in the U.S. suffer from chronic sialorrhea1 ‒ Up to 74% of Parkinson's patients have sialorrhea2 Global rights, except Japan Only Type B toxin with demonstrated efficacy in multiple clinical trials 1 - Based on epidemiology data, prevalanece of Parkinson’s Disease and prevalence of sialorrhea in PD and other neurodegenerative diseases. 2 - Kalf JG, de Swart BJ, Borm GF, Bloem BR, Munneke M. Prevalence and definition of drooling in Parkinson’s disease: a systematic review. J Neurol. 2009;256(9):1391-1396. © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 17
XADAGO® Oral treatment of PD in adults who are having “OFF” episodes Monoamine oxidase type B (MAO-B) inhibitor that is adjunctive to levodopa/carbidopa XADAGO helps block MAO-B from breaking down dopamine in the brain Exclusive license from Zambon S.p.A in U.S territories Launched in the U.S in 2017 Patent protection through at least 2027 © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 18
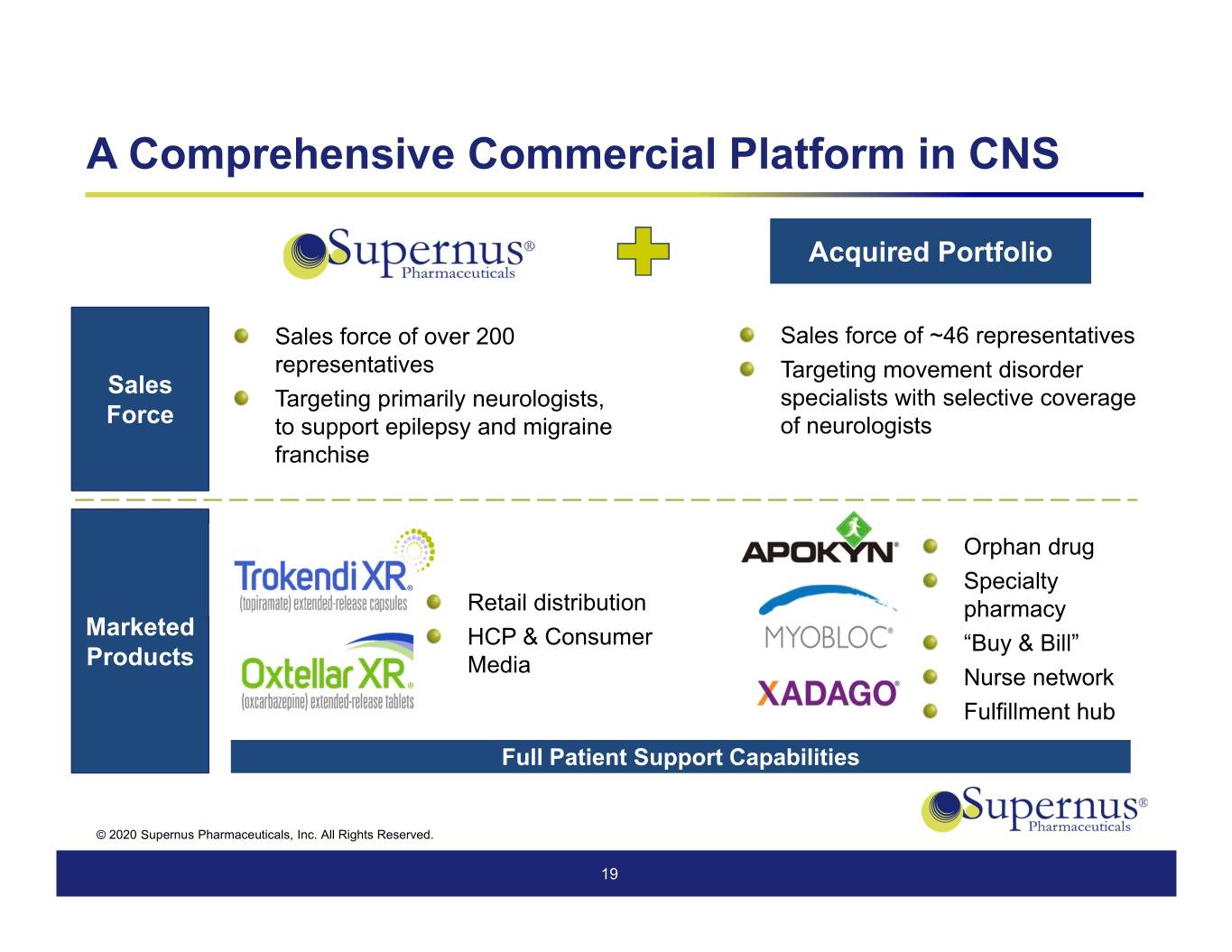
A Comprehensive Commercial Platform in CNS Acquired Portfolio Sales force of over 200 Sales force of ~46 representatives representatives Targeting movement disorder Sales Targeting primarily neurologists, specialists with selective coverage Force to support epilepsy and migraine of neurologists franchise Orphan drug Specialty Retail distribution pharmacy Marketed HCP & Consumer “Buy & Bill” Products Media Nurse network Fulfillment hub Full Patient Support Capabilities © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 19

A Robust R&D Pipeline & Platform in CNS SPN-812 ‒ PDUFA date of November 8, Apomorphine 2020, ADHD Infusion Pump ‒ Parkinson’s disease SPN-604 ‒ Launch expected in H2 2021 Pipeline ‒ Phase III, Bipolar disorder & R&D Platform SPN-820 ‒ NV-5138 Phase I, Depression ‒ Potential expansion of SPN-817 Indications to Spasticity & ‒ Phase I, Epilepsy other neurological diseases Small Molecule, Biologics, Device, Drug Delivery Capabilities © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 20

Positioned For Long-Term Growth Diversified Neurology Portfolio Oxtellar XR® , Trokendi XR® , APOKYN®, MYOBLOC®, XADAGO® Innovative Pipeline in CNS SPN-812 Potential Launch in 2020 Apomorphine Infusion Pump Potential Launch in 2021 MYOBLOC Neurological Disorders SPN-604 SPN-817 SPN-820 (NV-5138) © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 21

Q&A © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 22

Appendix © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 23

APOKYN® Pen About APOKYN® (apomorphine hydrochloride) injection: APOKYN is used by injection, as needed, to treat loss of control of body movements in people with advanced Parkinson’s disease (PD). This condition is also called hypomobility or off episodes. An off episode may include symptoms such as muscle stiffness, slow movements, and difficulty starting movements. APOKYN may improve your ability to control your movements when it is used during an off episode. The most common side effects seen in clinical studies with APOKYN were yawning; sleepiness; dyskinesias; dizziness; runny nose; nausea and/or vomiting; hallucinations/confusion; and swelling of hands, arms, legs, and feet. Some patients may notice soreness, redness, bruising, or itching at the injection site. Change the site with each injection. See full Prescribing Information and Pen Instructions for Use/Patient Information at www.apokyn.com. © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 24

MYOBLOC® About MYOBLOC® (rimabotulinumtoxinB) injection: MYOBLOC is a prescription medicine that is: . injected into neck muscles and used to treat the abnormal head position and neck pain that happens with cervical dystonia in adults. . injected into the salivary glands (parotid and submandibular glands) and used to treat chronic sialorrhea in adults. WARNING: DISTANT SPREAD OF TOXIN EFFECT See full prescribing information for complete boxed WARNING. The effects of MYOBLOC® and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms. The most common side effects of MYOBLOC include: . Cervical Dystonia: dry mouth, trouble swallowing, injection site discomfort or pain, headache . Sialorrhea: dry mouth, trouble swallowing See full Prescribing Information, including Boxed WARNING, and Medication Guide at www.myobloc.com © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 25

XADAGO® About XADAGO® (safinamide) tablets: XADAGO is a monoamine oxidase type B (MAO-B) inhibitor. XADAGO is used with levodopa/carbidopa to treat adults with Parkinson's disease (PD) who are having off episodes. The most common side effects seen with XADAGO are uncontrolled movements (dyskinesia), falls, nausea, and insomnia. See full Prescribin g Information and Patient Information at www.xadago.com © 2020 Supernus Pharmaceuticals, Inc. All Rights Reserved. 26
