Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Verastem, Inc. | tm2017756d1_8k.htm |
| EX-99.2 - EXHIBIT 99.2 - Verastem, Inc. | tm2017756d1_ex99-2.htm |
Exhibit 99.1

PROPERTY OF VERASTEM, INC. 1 Addressing RAS Pathway Blockade & Resistance VS - 6766 & Defactinib Combination Data in KRAS Mutant Solid Tumors Investor Conference Call and Webcast April 27, 2020 NASDAQ: VSTM

PROPERTY OF VERASTEM, INC. 2 2 Speakers Verastem Oncology Brian Stuglik CEO Dan Paterson COO Rob Gagnon CFO Jon Pachter CSO Lead Investigator Udai Banerji, MBBS, MD, DNB, PhD, FRCP Professor Udai Banerji is the deputy head of the Drug Development Unit where he is involved in running the portfolio of more than 40 Phase I trials. He plays a key role bridging pre - clinical and clinical drug discovery by designing and conducting Phase I studies. In addition to clinical trials, Professor Banerji leads the Clinical Pharmacodynamics Biomarker Group and the Clinical Pharmacology – Adaptive Therapy Groups at The Institute of Cancer Research. His laboratory interests include anticancer drug resistance and pharmacological aspects of cancer evolution. Professor Banerji holds a PhD from The Institute of Cancer Research and completed his medical oncology training at The Royal Marsden Hospital.

PROPERTY OF VERASTEM, INC. 3 3 Agenda Topic Presenter • Introduction • Brian Stuglik • RAS Pathway: Current Approaches and Unmet Needs • RAS Pathway Blockade: Bypass Mechanisms and Resistance • VS - 6766 and Defactinib • Jon Pachter • Phase 1 Combination Data • Udai Banerji • Next Steps • Concluding Remarks • Dan Paterson

PROPERTY OF VERASTEM, INC. 4 4 Safe Harbor Statement This presentation includes forward - looking statements about, among other things, Verastem Oncology’s products and product candidates, including anticipated regulatory submissions, approvals, performance and potential benefits of Verastem Oncology products and product candidates, that are subject to substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Additional information regarding these factors can be found in Verastem Oncology’s Annual Report on Form 10 - K for the fiscal year ended December 31, 2019 and in any subsequent filings with the SEC, including in the sections thereof captioned “Risk Factors” and “Forward - Looking Information and Factors that May Affect Future Results,” as well as in our subsequent reports on Form 8 - K, all of which are filed with the U.S. Securities and Exchange Commission (SEC) and available at www.sec.gov and www.verastem.com. The forward - looking statements in this presentation speak only as of the original date of this presentation, and we undertake no obligation to update or revise any of these statements.

5 Strong balance sheet and investor syndicate New lead clinical program has best - in - class potential Significant downstream market opportunity and blockbuster potential Revenue - generating commercial asset with multiple planned indication expansion opportunities VS - 6766 (RAF/MEK) and defactinib (FAK) inhibition clinically active against KRAS mutant variants, especially KRAS G12V & G12D 30% of all human cancers are driven by mutations in RAS family of genes; VS - 6766 combinations poised to fuel the future pipeline COPIKTRA ® ( duvelisib ) generated $12.3M in 2019 and $5.0M in 1Q20 in approved indications; actively working toward label expansions in PTCL and other hematologic malignancies Cash runway into the fourth quarter of 2021; recent financing funded by several premier life science investors Rapid development pathway to market Clinical proof - of - concept achieved in KRAS mutant low - grade serous ovarian cancer (LGSOC); goal to initiate registration - directed trial in 2020 We are a biopharmaceutical company committed to developing and commercializing new medicines for patients battling cancer

PROPERTY OF VERASTEM, INC. 6 6 Key Pipeline Programs Aligned with New Strategic Direction PRECLINICAL PHASE 1 / 1B PHASE 2 PHASE 3 MARKET VS - 6766 In combination with FAK inhibition Advanced solid tumors (LGSOC, NSCLC, CRC)* VS - 6766 + defactinib Advanced solid tumors (KRASm lung)* VS - 6766 + everolimus DEFACTINIB In combination with PD - 1 inhibitors R/R pancreatic ductal adenocarcinoma* Defactinib + pembrolizumab + gemcitabine NSCLC, pancreatic, mesothelioma* Defactinib + pembrolizumab COPIKTRA (duvelisib) Monotherapy R/R CLL/SLL (following two prior therapies) R/R FL (following two prior systemic therapies) R/R PTCL (registration directed) Combinations R/R CLL/SLL* duvelisib + venetoclax R/R PTCL* duvelisib + romidepsin HNSCC duvelisib + pembrolizumab *Investigator - sponsored study

PROPERTY OF VERASTEM, INC. 7 Jon Pachter, PhD RAS Pathway: Current Approaches and Unmet Needs

PROPERTY OF VERASTEM, INC. 8 8 Breadth of potential opportunity ▪ 30% of all human cancers are driven by mutations of the RAS family of genes Established prognostic significance ▪ Patients with mutations of the RAS family have an overall worse prognosis Challenges with conventional approaches ▪ Modest progress; limited number of approved therapies ▪ Single agent therapies (e.g. MEK inhibitors) associated with resistance ▪ Tolerable combination regimens with MEK inhibitors have been challenging High Unmet Needs in RAS/RAF/MEK/ERK - Driven Cancers References: McCormick F Clin Cancer Res 15April2015 Adderley H et al. EBioMedicine 01Mar2019 Papke B et al. Science 17Mar2017 Ryan M et al. Nature Reviews Clinical Oncology 01Oct2018 NIH cancer.gov/research/key - initiatives/ras KRAS - mutant Cancers 1 NSCLC Colorectal Pancreatic Uterine Endometrioid 31% 45% 98% 21% NRAS - mutant Cancers 1 Melanoma Multiple Myeloma 28% BRAF - mutant Cancers 2 Melanoma Ovarian 60% 35 - 60% Papillary Thyroid 30 - 80% 20% 194K Incidence 3,5 : Ovarian Incidence 5 : Incidence 5 : Incidence 5 : Incidence 5 : Incidence 5 : Incidence 5,6 : Incidence 5 : Incidence 5 : Incidence 4,5 : 59K Incidence Sources: 1 Reference for RAS mt frequencies – Cox et al. Nature Reviews 13: 828, 2014 2 Reference for BRAF mt frequencies – Turski et al. Mol Cancer Ther 15: 533, 2016 3 85% of lung cancer is NSCLC (Lu et. al. Cancer Manag Res. 2019) 4 90% of all uterine cancers are of the endometrial type (ACS) 5 Cancer Statistics 2020, Siegel et. al. CA Cancer J Clin 2020;70:7 - 30 6 8 out of 10 thyroid cancers are of the papillary type (ACS) 5% 58K 105K 22K 108K 32K 108K 22K 42K

9 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION Jon Pachter, PhD RAS Pathway Blockade: Bypass Mechanisms and Resistance

10 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 10 Overcoming Key Resistance Mechanisms to MEK Inhibitors RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G; q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P References: Banerji, BTOG Dublin, Jan 23, 2019 Slack - Davis, JCB 162 :281, 2003 Feng, Cancer Cell, 2019 Konstantinou, Cancer Discovery 3 :444, 2013 Hirata, Cancer Cell 27 :574, 2015

11 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 11 Overcoming Key Resistance Mechanisms to MEK Inhibitors RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P ▪ BRAF inhibition induces compensatory activation of pFAK 1 ▪ MEK inhibition induces compensatory activation of pFAK preclinically and clinically 2 o Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines o Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal = Feedback Reactivation References: 1. Chen, Mol Cancer Res 2018 2. Banerji, BTOG Dublin, Jan 23, 2019

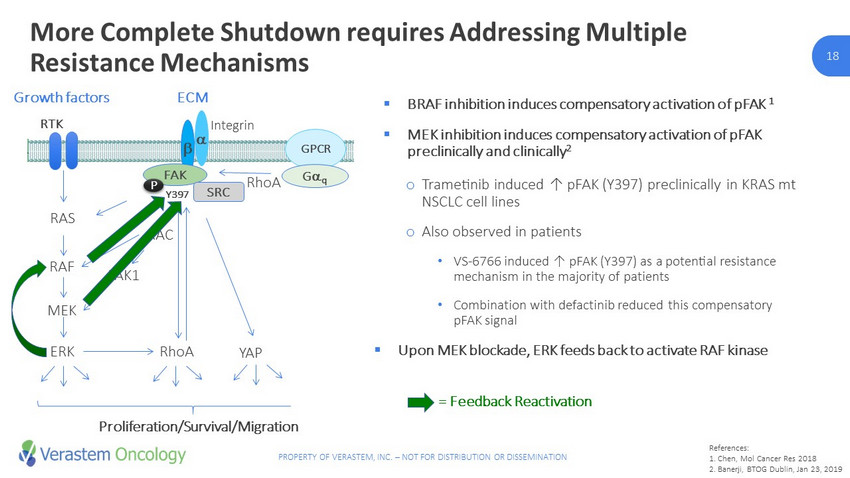
12 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 12 More Complete Shutdown requires Addressing Multiple Resistance Mechanisms RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P ▪ BRAF inhibition induces compensatory activation of pFAK 1 ▪ MEK inhibition induces compensatory activation of pFAK preclinically and clinically 2 o Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines o Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal ▪ Upon MEK blockade, ERK feeds back to activate RAF kinase 3 = Feedback Reactivation References: 1 Chen, Mol Cancer Res 2018 2 Banerji, BTOG Dublin, Jan 23, 2019 3 Ishii et al., Cancer Res , 2013

13 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION VS - 6766 and Defactinib Jon Pachter, PhD

14 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 14 ▪ VS - 6766 inhibits both MEK & RAF kinase activities ▪ Standard MEK inhibitors paradoxically induce MEK phosphorylation ( pMEK ) by relieving ERK - dependent feedback inhibition of RAF ▪ By inhibiting RAF phosphorylation of MEK, VS - 6766 has advantage of not inducing pMEK ▪ VS - 6766 inhibits ERK signaling more completely; may confer enhanced therapeutic activity VS - 6766 is a Unique Small Molecule RAF/MEK Inhibitor Reference: Ishii et al., Cancer Res , 2013; Lito et al., Cancer Cell, 2014; Blasco, R. B. et al. Cancer Cell (2011); Sanclemente , M. et al. Cancer Cell (2018) RAS RAF MEK ERK Proliferation & Survival

15 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 15 ▪ VS - 6766 inhibits both MEK & RAF kinase activities ▪ Standard MEK inhibitors paradoxically induce MEK phosphorylation ( pMEK ) by relieving ERK - dependent feedback inhibition of RAF ▪ By inhibiting RAF phosphorylation of MEK, VS - 6766 has advantage of not inducing pMEK ▪ VS - 6766 inhibits ERK signaling more completely; may confer enhanced therapeutic activity VS - 6766 is a Unique Small Molecule RAF/MEK Inhibitor Reference: Ishii et al., Cancer Res , 2013; Lito et al., Cancer Cell, 2014; Blasco, R. B. et al. Cancer Cell (2011); Sanclemente , M. et al. Cancer Cell (2018) RAS RAF MEK ERK Proliferation & Survival

PROPERTY OF VERASTEM, INC. 16 16 VS - 6766 inhibits CRAF A central mediator of KRAS - G12V driven NSCLC Source: Ishii et al. Cancer Res (2013), Blasco, R. B. et al. Cancer Cell (2011), Lito, P. et al. Cancer Cell (2014), Sanclemente, M. et al. Cancer Cell (2018) CRAF, but not BRAF, ablation improves survival of mice with KRAS G12 induced lung tumor formation across two different models CRAF drives KRAS G12V NSCLC 1,3 CRAF KO CRAF KO vs. WT CRAF WT BRAF KO vs. WT 0% ORR 62% ORR +83% OS
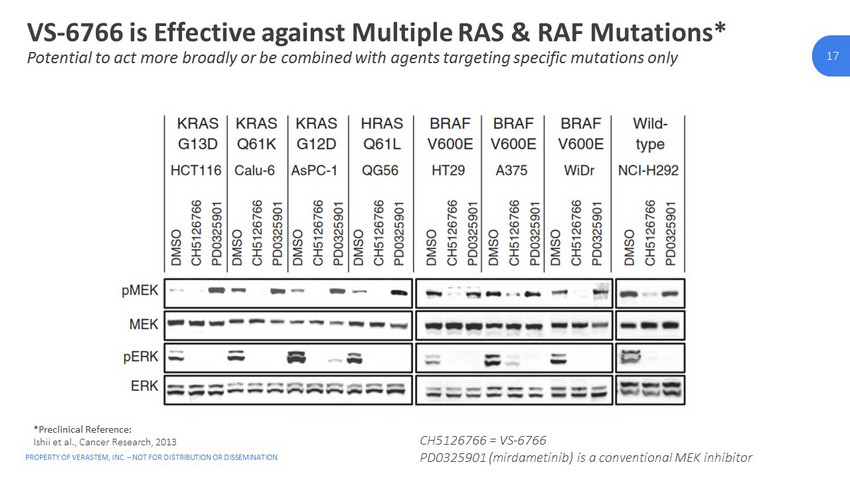
PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 17 17 VS - 6766 is Effective against Multiple RAS & RAF Mutations* Potential to act more broadly or be combined with agents targeting specific mutations only *Preclinical Reference: Ishii et al., Cancer Research, 2013 CH5126766 = VS - 6766 PD0325901 (mirdametinib) is a conventional MEK inhibitor
18 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 18 More Complete Shutdown requires Addressing Multiple Resistance Mechanisms RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P ▪ BRAF inhibition induces compensatory activation of pFAK 1 ▪ MEK inhibition induces compensatory activation of pFAK preclinically and clinically 2 o Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines o Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal ▪ Upon MEK blockade, ERK feeds back to activate RAF kinase = Feedback Reactivation References: 1. Chen, Mol Cancer Res 2018 2. Banerji, BTOG Dublin, Jan 23, 2019

19 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 19 More Complete Shutdown requires Addressing Multiple Resistance Mechanisms RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P ▪ BRAF inhibition induces compensatory activation of pFAK 1 ▪ MEK inhibition induces compensatory activation of pFAK preclinically and clinically 2 o Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines o Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal ▪ Upon MEK blockade, ERK feeds back to activate RAF kinase = Feedback Reactivation References: 1. Chen, Mol Cancer Res 2018 2. Banerji, BTOG Dublin, Jan 23, 2019 VS - 6766

20 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 20 More Complete Shutdown requires Addressing Multiple Resistance Mechanisms RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P ▪ BRAF inhibition induces compensatory activation of pFAK 1 ▪ MEK inhibition induces compensatory activation of pFAK preclinically and clinically 2 o Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines o Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal ▪ Upon MEK blockade, ERK feeds back to activate RAF kinase = Feedback Reactivation References: 1. Chen, Mol Cancer Res 2018 2. Banerji, BTOG Dublin, Jan 23, 2019 VS - 6766 Defactinib

21 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 21 More Complete Shutdown requires Addressing Multiple Resistance Mechanisms RTK RAS RAF MEK ERK RhoA Growth factors PAK1 b a Y397 Integrin FAK ECM SRC RAC GPCR G q YAP RhoA Proliferation/Survival/Migration PLC Ca 2+ /DAG PKC ERK P ▪ BRAF inhibition induces compensatory activation of pFAK 1 ▪ MEK inhibition induces compensatory activation of pFAK preclinically and clinically 2 o Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines o Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal ▪ Upon MEK blockade, ERK feeds back to activate RAF kinase = Feedback Reactivation References: 1. Chen, Mol Cancer Res 2018 2. Banerji, BTOG Dublin, Jan 23, 2019 VS - 6766 Defactinib

22 Background Presented by: Maxime Chénard - Poirier, MD • In view of promising activity, a different trial design was investigated to mitigate toxicity • Mean terminal t 1/2 of ≈ 60 hours • 2x - weekly and 3x - weekly scheduling, in 4 week cycles • Led by the Drug Development Unit at RMH/ICR 4.0mg 2x week n=8 4.0mg 3x week n=7 RP2D 4.0mg 2x week 3.2mg 3x week n=7 2 DLTs 1 DLTs Dose escalation n=22 Martinez - Garcia et al. ClinCancerRes. 2012 Sep1;18(17):4806 - 19 VS - 6766 Monotherapy

23 Adverse Events Presented by: Maxime Chénard - Poirier, MD Adverse event details Expansion: 4mg 2x weekly n=26 Martinez - Garcia et al. CCR 2012 Patient treated at OD MTD n=6 All grades ≥ Gr. 3 ≥ Gr. 3 Rash - related 22 (84.6 %) 5 ( 19.2 % ) 3 ( 50.0 % ) CK elevation 15 (57.7 %) 2 ( 7.6% ) 1 ( 16.7 % ) Blurred vision 13 (50 %) 0 0 Peripheral oedema 10 (38.5 %) 0 0 Diarrhoea 9 (34.1 %) 1 (3.8 %) 0 Mucositis/Mouth ulcer 8 ( 30.8 %) 1 (3.8 %) 0 Fatigue 6 (23.1 %) 1 (3.8 %) 0 Nausea 5 (19.2 %) 0 0 Martinez - Garcia et al. Clin Cancer Res. 2012 Sep 1;18(17):4806 - 19 VS - 6766 Monotherapy

24 Results: KRAS mut NSCLC - Adenocarcinoma Presented by: Maxime Chénard - Poirier, MD Progression Free Survival Best response by RECIST v1.1 . . . . . . . . . . -80 -60 -40 -20 0 20 19 0 0 -22 -38 -44 -68 -8 -14 5 B e s t R e s p o n s e % c h a n g e f r o m b a s e l i n e KRAS mut NSCLC 0 10 20 30 . . . . . . . . . . 55 65 120140 PFS (weeks) K R A S m u t N S C L C Partial Response Stable Disease 24 Ongoing Best Response Reason off study PD Toxicity Withdrew consent * # + * # + * * * * + Deteriorating performance ^ ^ VS - 6766 Monotherapy

25 Results: Gynaecological cancers Presented by: Maxime Chénard - Poirier, MD Progression Free Survival Best response by RECIST v1.1 . . . . . -80 -60 -40 -20 0 20 4 1 -30 -48 -65 B e s t R e s p o n s e % c h a n g e f r o m b a s e l i n e O v a r i a n ( K R A S m u t ) O v a r i a n ( B R A F m u t ) E n d o m e t r i a l ( K R A S m u t ) O v a r i a n ( K R A S m u t ) E n d o m e t r i a l ( K R A S m u t ) 0 10 20 30 40 50 . . . . . PFS (weeks) Partial Response Stable Disease Progressive Disease Best Response Reason off study Ovarian (KRAS mut ) PD * Ovarian (BRAF mut ) Endometrial (KRAS mut ) Ovarian (KRAS mut ) Endometrial (KRAS mut ) * * * * * 24 VS - 6766 Monotherapy

26 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 26 Defactinib: Selective FAK inhibitor Focal Adhesion Kinase (FAK) ▪ Non - receptor tyrosine kinase: Mediates signaling downstream of integrins & growth factor receptors ▪ Key roles in drug resistance o RAF & MEK inhibitors o Chemotherapy ▪ Immuno - Oncology/Tumor Microenvironment o FAK inhibition reduces stromal density: ↑ entry of cytotoxic T cells into tumor o FAK inhibition reduces immuno - suppressive Tregs, M2 macrophages & MDSCs Defactinib (VS - 6063) ▪ Selective inhibitor of FAK & related kinase PYK2 ▪ Good pFAK target inhibition in tumors of patients following oral defactinib administration ▪ Early signs of clinical efficacy ▪ Studied in 500+ patients with good safety profile observed to date o Only ≥Gr 3 toxicity over 2.5% was hyperbilirubinemia – Not associated liver AEs ▪ Preliminary results show it is generally well - tolerated in combination o MEK/RAF, PD - 1, Chemo Defactinib Reference: Jones, Invest New Drugs, 2015; Kang, J Natl Cancer Inst. 2013; Diaz Osterman, Elife 2019; Tong, Respiratory Res 2019, Serrels Cell 2015; Jiang et al Nat Med 20 16; Banerji, BTOG Dublin, Jan 23, 2019; Data on file

27 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 27 Defactinib Monotherapy Shows Clinical Activity in KRAS Mutant NSCLC Control siRNA FAK (2) siRNA FAK (1) Mutant INK4a/ARF NSCLC Oncogenic KRAS wt KRAS Mutant p53 NSCLC Oncogenic KRAS wt KRAS KRAS mt is necessary for sensitivity to FAK inhibition in NSCLC cell lines Reference: Konstantinidou G et al. Cancer Discovery 2013;3:444 - 57 References: 1. Phase 3 INTEREST, Douillard et al., JCO 2010 2. Phase 3 MISSION, Mok et al., ESMO 2012 3. Phase 2, Blumenschein et al., Ann Oncol 2015 4. Phase 2, Janne et al., Lancet 2013 12 - week PFS rate of experimental agents for KRAS mt NSCLC “VS - 6063 was generally well tolerated and suitable for long - term dosing. In this cohort of heavily pretreated patients, there were signs of single - agent activity comparable to other targeted agents and docetaxel. Future directions include possible combination studies with existing standard and emerging therapies, including checkpoint inhibitors .” — Dr. David Gerber, IASLC 2015; Lung Cancer 2020

PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 28 28 Screen for Synergy with Defactinib Identified MEK Inhibitors (& VS - 6766) as Top Hit TOV - 21G CELLS KRAS - MUTANT OVARIAN CANCER Defactinib Defactinib + Trametinib Loewe Model H441 CELLS KRAS - MUTANT NON - SMALL - CELL LUNG CANCER Defactinib Defactinib + Trametinib Loewe Model 0.01 0.1 1 10 100 0.0 0.5 1.0 1.5 SW982 cells Defactinib (M) R e l a t i v e V i a b i l i t y Defactinib Defactinib x RO-5126766 @ 0.08uM Loewe model SW982 CELLS SARCOMA BRAF:pV600E Defactinib Defactinib + VS - 6766 Loewe Model 0.01 0.1 1 10 100 0.0 0.5 1.0 1.5 Mero-14 cells Defactinib (M) R e l a t i v e V i a b i l i t y Defactinib Defactinib x RO-5126766 @ 0.156uM Loewe model MERO - 14 CELLS MESOTHELIOMA Defactinib Defactinib + VS - 6766 Loewe Model 0.01 0.1 1 10 100 0.0 0.5 1.0 1.5 CAL-51 cells Defactinib (M) R e l a t i v e V i a b i l i t y Defactinib Defactinib x RO-5126766 @ 0.156uM Loewe model CAL - 51 CELLS TRIPLE NEGATIVE BREAST CANCER Defactinib Defactinib + VS - 6766 Loewe Model Verastem issued patent on FAK/MEK inhibitor combinations KRAS - G12V KRAS - G13C Verastem, data on file
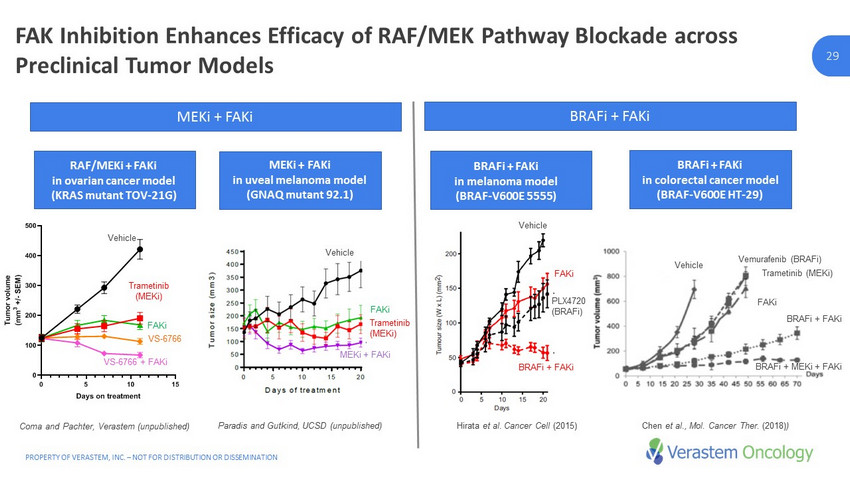
PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 29 29 FAK Inhibition Enhances Efficacy of RAF/MEK Pathway Blockade across Preclinical Tumor Models 0 5 10 15 0 100 200 300 400 500 Tumor growth VS-4718 + CH5126766 Days on treatment T u m o r v o l u m e ( m m 3 + / - S E M ) Vehicle Trametinib VS-4718 CH5126766 VS-4718 + CH5126766 RAF/ MEKi + FAKi in ovarian cancer model (KRAS mutant TOV - 21G) MEKi + FAKi in uveal melanoma model (GNAQ mutant 92.1) BRAFi + FAKi in melanoma model (BRAF - V600E 5555) BRAFi + FAKi in colorectal cancer model (BRAF - V600E HT - 29) Vehicle FAKi Vehicle Vemurafenib ( BRAFi ) FAKi BRAFi + FAKi BRAFi + MEKi + FAKi Trametinib ( MEKi ) FAKi Vehicle Trametinib ( MEKi ) MEKi + FAKi Trametinib ( MEKi ) Vehicle FAKi VS - 6766 VS - 6766 + FAKi Paradis and Gutkind, UCSD (unpublished) Coma and Pachter, Verastem (unpublished) Hirata et al. Cancer Cell (2015) Chen et al., Mol. Cancer Ther. (2018) ) PLX4720 ( BRAFi ) BRAFi + FAKi MEKi + FAKi BRAFi + FAKi

30 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION Udai Banerji, MBBS, MD, DNB, PhD, FRCP Phase 1 VS - 6766 and Defactinib Combination Data in KRAS Mutant Solid Tumors – Initial Results

PROPERTY OF VERASTEM, INC. 31 31 Ongoing Investigator - Sponsored Basket Study of VS - 6766 + Defactinib in KRAS M Cancers Phase I Advanced Solid Cancers • VS - 6766 oral twice wkly x 3 wks every 4 wks • Defactinib oral BID daily x 3 wks q 4 wks • 3 cohorts with increasing doses explored • Cohort 1: VS - 6766 3.2 mg & Defactinib 200 mg • Cohort 2a: VS - 6766 4 mg & Defactinib 200 mg • Cohort 2b: VS - 6766 3.2 mg & Defactinib 400 mg Advanced NSCLC KRAS mutant* (N=20) LGSOC* (N=20) Advanced CRC RAS mutant* (N=10) Advanced Solid Tumors Enriched for RAS* (Biopsy Amenable) (N=6) *Refractory to conventional treatment or for which no conventional treatment exists Dr. Udai Banerji Royal Marsden Hospital 14 enrolled by March 2020 Median prior lines = 2 Majority prior PD - (L)1 treatment 9 enrolled by March 2020 Median prior lines ≥2 Prior MEKi allowed 10 enrolled by March 2020 Median prior lines = 2 - 3 Prior VEGFi allowed 6 enrolled by March, 2020 References: Banerji, AACR VM 1, April 27, 2020, CT143; Data on file

32 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 32 Overcoming Key Resistance Mechanisms to MEK Inhibitors ▪ MEK inhibition induces compensatory activation of pFAK preclinically and clinically P r e d o s e P o s t V S - 6 7 6 6 P o s t c o m b i n a t i o n 0 50 100 150 p-FAK H - S c o r e o Trametinib induced ↑ pFAK (Y397) preclinically in KRAS mt NSCLC cell lines o Also observed in patients • VS - 6766 induced ↑ pFAK (Y397) as a potential resistance mechanism in the majority of patients • Combination with defactinib reduced this compensatory pFAK signal = Feedback Reactivation References: Banerji, BTOG Dublin, Jan 23, 2019 Banerji, AACR VM 1, April 27, 2020, CT143
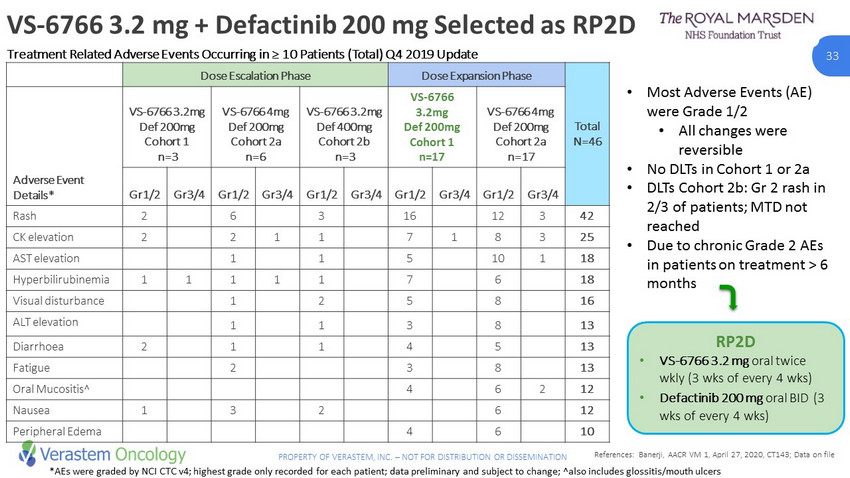
33 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION • Most Adverse Events (AE) were Grade 1/2 • All changes were reversible • No DLTs in Cohort 1 or 2a • DLTs Cohort 2b: Gr 2 rash in 2/3 of patients; MTD not reached • Due to chronic Grade 2 AEs in patients on treatment > 6 months VS - 6766 3.2 mg + Defactinib 200 mg Selected as RP2D *AEs were graded by NCI CTC v4; highest grade only recorded for each patient; data preliminary and subject to change; ^also i ncl udes glossitis/mouth ulcers RP2D • VS - 6766 3.2 mg oral twice wkly (3 wks of every 4 wks) • Defactinib 200 mg oral BID (3 wks of every 4 wks) Dose Escalation Phase Dose Expansion Phase Total N=46 Adverse Event Details* VS - 6766 3.2mg Def 200mg Cohort 1 n=3 VS - 6766 4mg Def 200mg Cohort 2a n=6 VS - 6766 3.2mg Def 400mg Cohort 2b n=3 VS - 6766 3.2mg Def 200mg Cohort 1 n=17 VS - 6766 4mg Def 200mg Cohort 2a n=17 Gr1/2 Gr3/4 Gr1/2 Gr3/4 Gr1/2 Gr3/4 Gr1/2 Gr3/4 Gr1/2 Gr3/4 Rash 2 6 3 16 12 3 42 CK elevation 2 2 1 1 7 1 8 3 25 AST elevation 1 1 5 10 1 18 Hyperbilirubinemia 1 1 1 1 1 7 6 18 Visual disturbance 1 2 5 8 16 ALT elevation 1 1 3 8 13 Diarrhoea 2 1 1 4 5 13 Fatigue 2 3 8 13 Oral Mucositis^ 4 6 2 12 Nausea 1 3 2 6 12 Peripheral Edema 4 6 10 Treatment Related Adverse Events Occurring in ≥ 10 Patients (Total) Q4 2019 Update References: Banerji, AACR VM 1, April 27, 2020, CT143; Data on file

34 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 34 Pharmacokinetic Profiles of VS - 6766 + Defactinib in Combination Similar to that seen in Single Agent Studies Cohort Dose (mg) N Subject AUC 0 - 24h (h*ng/mL) C max (ng/mL) 1 3.2 (with 200mg VS) 3 Mean 6179 354 CV% 32.1 30.4 2a 4 (with 200mg VS) 5 Mean 5353 289 CV% 15.8 16.0 2b 3.2 (with 400mg VS) 1 FRA101 - 007 3302 229 VS - 6766 Cohort Dose (mg) N Subject AUClast (h*ng/mL) Cmax (ng/mL) 1 200 (with 3.2mg RO) 3 Mean 2071 273 CV% 103 80 2a 200 (with 4mg RO) 5 Mean 2252 318 CV% 124 117 2b 400 (with 3.2mg RO) 3 Mean 2807 360 CV% 31 32 Defactinib Reference: Banerji, AACR VM 1, April 27, 2020, CT143

35 Efficacy – Low Grade Serous Ovarian Cancer 0 12 24 36 48 60 72 84 96 108 FRA101015 FRA101012 FRA101007 FRA101019 FRA101014 FRA101009 FRA101002 FRA101001 Time on Treatment (weeks) Previous MEK inhibitor treatment Continuing on treatment * * * * * * Partial response Stable disease • Response rates: LGSOC KRAS M = 67% (4/6); All LGSOC = 50% (4/8) o Also, 1 patient with KRAS mutant mucinous ovarian cancer had PR (> 60% reduction) with > 1 year on therapy • ORR for LGSOC in the current literature is <10 % chemotherapy, 13% letrozole, 26% for trametinib, 24% for binimetinib, 15% for selumetinib G12D G12D G12V G12D G12A G12V WT WT Best response by RECIST Time on treatment F R A 1 0 1 0 0 7 F R A 1 0 1 0 1 4 F R A 1 0 1 0 1 2 F R A 1 0 1 0 1 5 F R A 1 0 1 0 1 9 F R A 1 0 1 0 0 1 F R A 1 0 1 0 0 9 F R A 1 0 1 0 0 2 -70 -60 -50 -40 -30 -20 -10 0 10 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) K R A S G 1 2 A K R A S G 1 2 D K R A S G 1 2 V K R A S G 1 2 D K R A S G 1 2 V N o m u t a t i o n d e t e c t e d N o m u t a t i o n d e t e c t e d K R A S G 1 2 D Previous MEK inhibitor treatment Continuing on treatment * * * * * * - All PRs confirmed with subsequent scan per RECIST References : Annals of Oncology, 10/2019, V30, v897 - 898; Journal of Clinical Oncology 2015 33:15_suppl, TPS5610; Farley, J. et al. Lancet Oncol. (2013); Banerji, AACR VM 1, April 27, 2020, CT143

36 0 12 24 36 48 FRA101017 FRA102005 FRA101018 FRA101020 FRA102004 FRA101010 FRA102009 FRA102008 FRA102007 FRA102006 FRA102002 Time on Treatment (weeks) Continuing on treatment * * Efficacy – KRAS mutant NSCLC Best response by RECIST Time on treatment • 3 patients received treatment for 24 weeks • Median time on treatment for this cohort was approximately 18 weeks (range 4 - 38 weeks) Partial response Stable disease Progressive disease G12D G12D G12C G12D G12V G12D G12C G12A G12D Q61H 14/20 pts enrolled in KRAS mt NSCLC cohort; 1 additional confirmed PR in KRAS - G12V mutant patient G12D F R A 1 0 1 0 1 7 F R A 1 0 1 0 1 8 F R A 1 0 2 0 0 8 F R A 1 0 1 0 2 0 F R A 1 0 2 0 0 7 F R A 1 0 2 0 0 4 F R A 1 0 2 0 0 6 F R A 1 0 2 0 0 2 F R A 1 0 2 0 0 9 F R A 1 0 1 0 1 0 -50 -40 -30 -20 -10 0 10 20 30 40 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Continuing on treatment * * K R A S G 1 2 D K R A S G 1 2 C K R A S G 1 2 C K R A S G 1 2 D K R A S G 1 2 D K R A S Q 6 1 H K R A S G 1 2 D K R A S G 1 2 D K R A S G 1 2 A K R A S G 1 2 V Reference: Banerji, AACR VM 1, April 27, 2020, CT143

37 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION 37 Summary: VS - 6766 + Defactinib Rationale ▪ VS - 6766 & defactinib have shown single agent clinical activity in KRAS mt cancer ▪ RAS pathway blockage activates FAK as potential resistance mechanism preclinically & clinically ▪ FAKi and MEKi are synergistic in reducing viability of cancer cell lines in vitro & in vivo in multiple models FRAME study shows promising results & continues to enroll ▪ Most adverse events were grade 1 / 2 with the Intermittent dosing of VS - 6766 + defactinib (no PK interaction observed) ▪ VS - 6766 + defactinib combination shows clinical promise in heavily pre - treated refractory patients with KRAS mt disease o 67% ORR in KRAS mt LGSOC, including patients progressing on prior MEK inhibitors o High rate of disease control and tumor regression in NSCLC with several patients out to 24 weeks o The study continues to enroll with additional responses in LGSOC, NSCLC and colorectal since Nov cut off

38 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION Next Steps and Closing Remarks Dan Paterson / Brian Stuglik

39 PROPERTY OF VERASTEM, INC. – NOT FOR DISTRIBUTION OR DISSEMINATION LGSOC – Strong Proof of Concept, High Unmet Need • LGSOC represents ~4 - 10% of epithelial ovarian cancer 2 • Long survival results in high prevalence rate • RAS pathway mt frequency 50% 3 • No FDA - approved therapy Combination of VS - 6766 + Defactinib offers potential for: • Long duration of therapy • High market share • Speed to market opportunity • Two product revenue streams Incidence 10 y Prevalence Worldwide ~13,000 ~80,000 US ~1,000 ~6,000 *Based on LGSOC representing 5% of epithelial ovarian cancer Am J Pathol. 2016 Apr;186(4):733 - 47 1 http://www.gynecologycancer.org/contact 2 SEER data, 2011 - 2016 3 http://molecularcasestudies.cshlp.org/content/5/6/a004341.full In LGSOC, G12V & G12D are the dominant KRAS mutations, and G12V confers a more aggressive phenotype (Tsang et al., J. Pathol 231 : 449, 2013) 1

PROPERTY OF VERASTEM, INC. 40 40 Clinical Activity in Discrete KRAS Codon 12 Variants (G12V, G12D) Summary: VS - 6766 + Defactinib in KRAS mt ovarian & lung cancers Tumor Type G12V G12D G12A G12C Q61H WT Ovarian # patients 3 3 1 0 0 2 PR 2 (67%) 2 (67%) 1 (100%) 0 (0%) Disease Control 3 (100%) 3 (100%) 1 (100%) 2 (100%) ≥6 months time on therapy 2 (67%) 2 (67%) 1(100%) 0 (0%) Lung # patients 1 6 1 2 1 0 PR 1 (100%) 1 (17%)* 0 (0%) 0 (0%) 0 (0%) Disease Control 1 (100%) 4 (67%) 1 (100%) 2 (100%) 0 (0%) ≥3 months time on therapy 1 (100%) 4 (67%) 1 (100%) 2 (100%) 0 (0%) November 2019 data cut Includes 1 patient with KRAS - G12V mt mucinous ovarian cancer *22% reduction & still on treatment
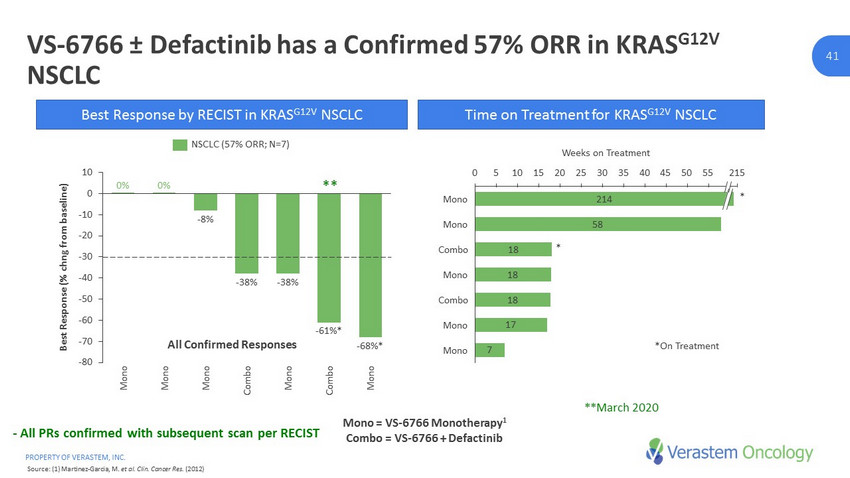
PROPERTY OF VERASTEM, INC. 41 41 VS - 6766 ± Defactinib has a Confirmed 57% ORR in KRAS G12V NSCLC 58 18 18 18 17 7 0 10 25 45 35 215 55 40 20 30 15 50 5 Combo Combo Mono Mono Mono Mono Weeks on Treatment 214 Mono -80 -70 -60 -50 -40 -30 -20 -10 0 10 0% Mono Mono Best Response (% chng from baseline) Combo Mono Mono Mono - 38% Combo 0% - 8% - 38% - 61% * - 68% * Best Response by RECIST in KRAS G12V NSCLC Time on Treatment for KRAS G12V NSCLC *On Treatment NSCLC (57% ORR; N=7) * All Confirmed Responses Mono = VS - 6766 Monotherapy 1 Combo = VS - 6766 + Defactinib Source: (1) Martinez - Garcia, M. et al. Clin. Cancer Res. (2012) - All PRs confirmed with subsequent scan per RECIST **March 2020 ** *

PROPERTY OF VERASTEM, INC. 42 42 KRAS G12V Represents a Large Opportunity in NSCLC and across Tumors 21.40% G12V 25.30% G12D 5.10% G12A 13.30% G12C 2.70% Q61H 32.20% Others % Frequency in Total of 780 Cancer Patients with KRAS mts 3 NSCLC Adenocarcinoma 3 G12C G12V G12D G12A G13C G12S G13D 0 5 10 15 NSCLC Adenocarcinoma KRAS Mutation % o f p a t i e n t s Annual Incidence 1,2 : 194K 1 85% of lung cancer is NSCLC (Lu et. al. Cancer Manag Res. 2019) 2 Cancer Statistics 2020, Siegel et. al. CA Cancer J Clin 2020;70:7 - 30 3 TCGA PanCancer Atlas ( cBioPortal analysis)

PROPERTY OF VERASTEM, INC. 43 43 VS - 6766 ± Defactinib has a Confirmed 58% ORR in KRAS G12V Tumors 400 700 1,500 500 600 100 200 0 300 Combo 119 56 Combo 413 Combo 127 Days on Treatment 1,500 Mono 49 810 112 Combo 406 Mono 161 Mono Combo 126 Mono 125 Mono Mono Mono -80 -70 -60 -50 -40 -30 -20 -10 0 10 Mono Combo Best Response (% chng from baseline) Mono Mono Mono Mono Combo Combo Mono Combo Combo Mono 1% 0% 0% - 8% - 18% - 38% - 38% - 68% * - 48% - 53% * - 61% * - 63% Best Response by RECIST in KRAS G12V Tumors Time on Treatment for KRAS G12V Tumors *On Treatment Endometrial (50%; N=2) NSCLC (57% ORR; N=7) OvC (66% ORR; N=3) OvC Endometrial NSCLC * * * All Confirmed Responses Mono = VS - 6766 Monotherapy 1 Combo = VS - 6766 + Defactinib Source: (1) Martinez - Garcia, M. et al. Clin. Cancer Res. (2012) - All PRs confirmed with subsequent scan per RECIST **March 2020 **

PROPERTY OF VERASTEM, INC. 44 44 KRAS G12V and G12D Represent ~50% of KRAS Mutations across Human Cancers 21.40% G12V 25.30% G12D 5.10% G12A 13.30% G12C 2.70% Q61H 32.20% Others Pancreatic Adenocarcinoma 1 G12D G12V G12RQ61H Q61RG12A G12C 0 10 20 30 Pancreatic Cancer KRAS Mutation % o f p a t i e n t s Uterine Endometrioid Carcinoma 1 G12D G12V G13D G12A G12C G13C Q61H 0 2 4 6 8 10 Uterine Endometrioid Carcinoma KRAS Mutation % o f p a t i e n t s 1 TCGA PanCancer Atlas ( cBioPortal analysis) 2 90% of all uterine cancers are of the endometrial type (ACS) 3 Cancer Statistics 2020 (Siegel et al. CA Cancer J Clin 2020; 70:7 - 30) Annual Incidence 3 : 58K Annual Incidence 2 , 3 : 59K % frequency in a total of 780 cancer patients with KRAS mutations 1

PROPERTY OF VERASTEM, INC. 45 45 High Priority Indications Supported by Initial Data • LGSOC 1,2 • KRAS - G12V NSCLC 1 , 2 • Pancreatic 1,2 Focusing on High Priority Indications with Significant Opportunities for Growth VS - 6766 “ Defactinib E xpansion Opportunities • Additional G12V & G12D mt cancers 1 • Uveal Melanoma 2 • BRAF mt melanoma 1,2 • BRAF mt colorectal • BRAF mt prostate 2 Other Mutation Opportunities • GNAQ mutations in uveal melanoma 2 • NF1 mutations in melanoma • MAP3K1 mutations in breast cancer Other Combinations • KRAS G12C inhibitors • EGFR inhibitors • Everolimus 2 • Anti - PD - 1 1 , 2 1 Supported by clinical data 2 Supported by preclinical data

PROPERTY OF VERASTEM, INC. 46 46 Strong Patent Protection for VS - 6766 ц Defactinib ▪ COM for VS - 6766 to 2027 & defactinib to 2028, Hatch Waxman should extend to 2032 ▪ VS - 6766 intermittent dosing regimen until 2038 if granted ▪ FAK/MEK combination to 2035 ▪ VS - 6766/ defactinib combination until 2040 if granted ▪ Method of manufacture for VS - 6766 to 2032 ▪ Other activity related to patent protection is ongoing and will continue into the future
PROPERTY OF VERASTEM, INC. 47 47 Potential Blockbuster Opportunity with VS - 6766 + Defactinib • Potential Best in class RAF/MEK & FAK inhibitors • More complete RAS pathway shut down addressing key resistance mechanisms • Uniquely targeting CRAF to shut down KRAS - G12V • First in class approach to KRAS - G12V & G12D • No approved therapies in LGSOC • 30% of all human cancers driven by RAS family mutations • All - oral combination regimen with non - overlapping safety profile • Initial clinical data with the combination are encouraging including both objective response rate and durability • KRAS - G12V mutant cancers appear to be particularly responsive to VS - 6766 ц defactinib • Goal to initiate LGSOC registration - directed study in 2020 • Complete expansion cohorts in ongoing investigator initiated Phase 1 combination study • KRAS - G12V & G12D expansion cohorts in NSCLC & pancreatic • Explore BRAFm - driven indications • Combinations with KRAS - G12Ci & anti - PD - 1 Key mechanistic attributes Significant commercial potential Early clinical experience Next steps

PROPERTY OF VERASTEM, INC. 48 Q&A
