Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Axsome Therapeutics, Inc. | tm2017765d1_ex99-1.htm |
| 8-K - FORM 8-K - Axsome Therapeutics, Inc. | tm2017765-1_8k.htm |
Exhibit 99.2
 |
NASDAQ: AXSM ADVANCE-1 Phase 2/3 Trial of AXS-05 in Alzheimer’s Disease Agitation Topline Results Conference Call April 27, 2020 © Axsome Therapeutics, Inc. |
 |
Agenda AXS-05 in Alzheimer’s Disease (AD) Agitation ADVANCE-1 Phase 2/3 Trial Topline Results Clinical Development & Medical Affairs Dave Marek, Chief Commercial Officer © Axsome Therapeutics, Inc. 2 Introduction Mark Jacobson, Chief Operating Officer Overview and Summary Herriot Tabuteau, MD, Chief Executive Officer ADVANCE-1 Trial Design & Results Cedric O’Gorman, MD, Senior Vice President, Q&A Presenters, Nick Pizzie, Chief Financial Officer and Concluding Remarks Herriot Tabuteau, MD, Chief Executive Officer |
 |
FLS Forward-Looking Statements & Safe Harbor Certain information contained in this presentation may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of our ongoing clinical trials and the number or type of studies or nature of results necessary to support the filing of a new drug application for any of our current product candidates; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates (including, but not limited to, FDA’s agreement with the Company’s discontinuation of the bupropion treatment arm of the ADVANCE-1 study in accordance with the independent data monitoring committee’s recommendations); the Company’s ability to obtain additional capital necessary to fund its operations; the Company’s ability to generate revenues in the future; the potential for the MOMENTUM clinical trial to provide a basis for approval of AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to our special protocol assessment; the potential for the ASCEND clinical trial, combined with the GEMINI clinical trial results, to provide a basis for approval of AXS-05 for the treatment of major depressive disorder and accelerate its development timeline and commercial path to patients; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the enforceability of the Company’s license agreements; the acceptance by the market of the Company’s product candidates, if approved; the Company’s anticipated capital requirements, including the Company’s anticipated cash runway; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. These factors could cause actual results and developments to be materially different from those expressed in or implied by such statements. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. © Axsome Therapeutics, Inc. 3 |
 |
Summary and Overview Herriot Tabuteau, MD Chief Executive Officer Axsome Therapeutics, Inc. © Axsome Therapeutics, Inc. 4 |
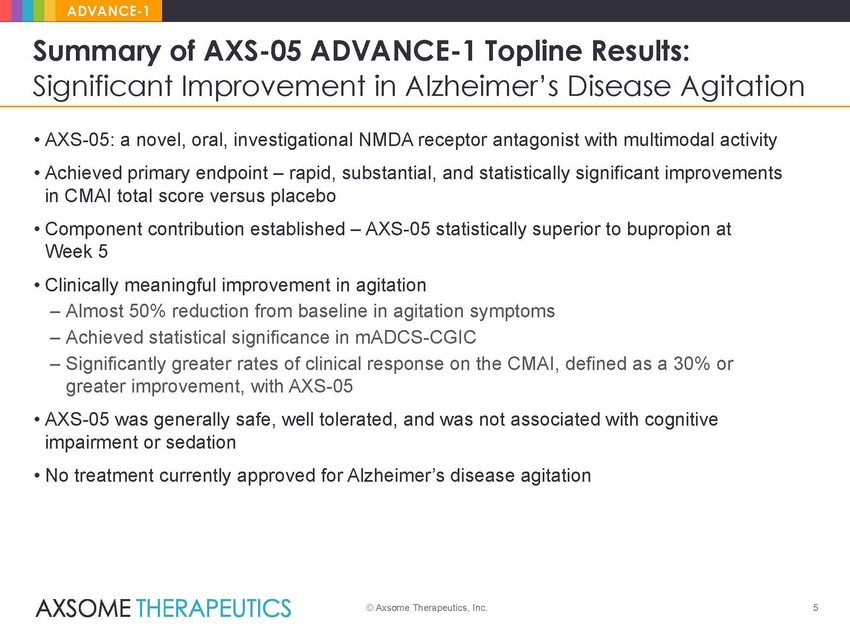 |
ADVANCE-1 Summary of AXS-05 ADVANCE-1 Topline Results: Significant Improvement in Alzheimer’s Disease Agitation • AXS-05: a novel, oral, investigational NMDA receptor antagonist with multimodal activity • Achieved primary endpoint – rapid, substantial, and statistically significant improvements in CMAI total score versus placebo • Component contribution established – AXS-05 statistically superior to bupropion at Week 5 • Clinically meaningful improvement in agitation – Almost 50% reduction from baseline in agitation symptoms – Achieved statistical significance in mADCS-CGIC – Significantly greater rates of clinical response on the CMAI, defined as a 30% or greater improvement, with AXS-05 • AXS-05 was generally safe, well tolerated, and was not associated with cognitive impairment or sedation • No treatment currently approved for Alzheimer’s disease agitation © Axsome Therapeutics, Inc. 5 |
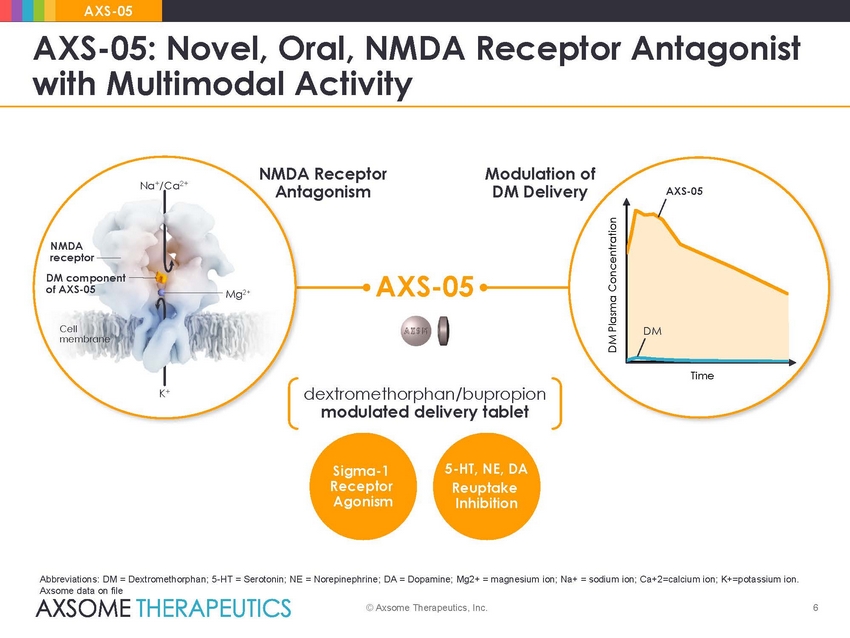 |
AXS-05 AXS-05: Novel, Oral, NMDA with Multimodal Activity Receptor Antagonist Modulation of DM Delivery NMDA Receptor Antagonism Na+/Ca2+ AXS-05 NMDA receptor DM component of AXS-05 AXS-05 Mg2+ Cell membrane Time dextromethorphan/bupropion modulated delivery tablet K+ 5-HT, NE, DA Reuptake Inhibition Sigma-1 Receptor Agonism Abbreviations: DM = Dextromethorphan; 5-HT = Serotonin; NE = Norepinephrine; DA = Dopamine; Mg2+ = magnesium ion; Na+ = sodium ion; Ca+2=calcium ion; K+=potassium ion. Axsome data on file © Axsome Therapeutics, Inc. 6 DM Plasma Concentration DM |
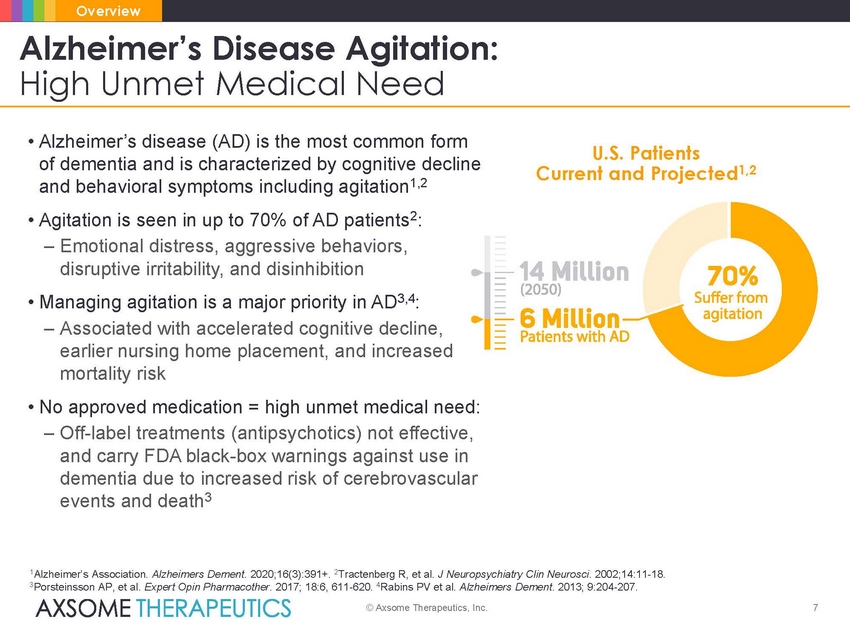 |
Overview Alzheimer’s Disease Agitation: High Unmet Medical Need • Alzheimer’s disease (AD) is the most common form of dementia and is characterized by cognitive decline and behavioral symptoms including agitation1,2 • Agitation is seen in up to 70% of AD patients2: – Emotional distress, aggressive behaviors, disruptive irritability, and disinhibition • Managing agitation is a major priority in AD3,4: – Associated with accelerated cognitive decline, earlier nursing home placement, and increased mortality risk • No approved medication = high unmet medical need: – Off-label treatments (antipsychotics) not effective, and carry FDA black-box warnings against use in dementia due to increased risk of cerebrovascular events and death3 U.S. Patients Current and Projected 1,2 1Alzheimer’s Association. Alzheimers Dement. 2020;16(3):391+. 2Tractenberg R, et al. J Neuropsychiatry Clin Neurosci. 2002;14:11-18. 3Porsteinsson AP, et al. Expert Opin Pharmacother. 2017; 18:6, 611-620. 4Rabins PV et al. Alzheimers Dement. 2013; 9:204-207. © Axsome Therapeutics, Inc. 7 |
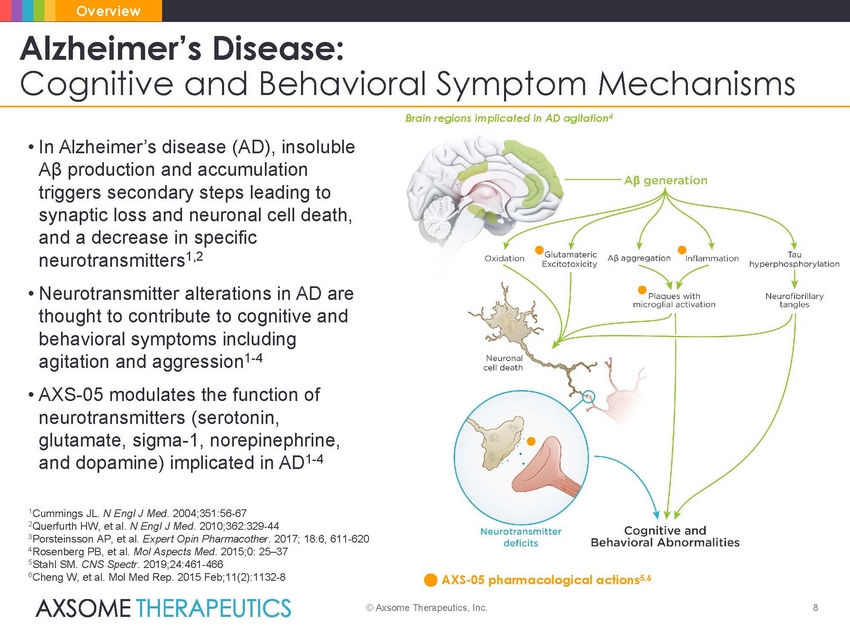 |
Overview Alzheimer’s Disease: Cognitive and Behavioral Symptom Mechanisms Brain regions implicated in AD agitation4 • In Alzheimer’s disease (AD), insoluble Aβ production and accumulation triggers secondary steps leading to synaptic loss and neuronal cell death, and a decrease in specific neurotransmitters1,2 • Neurotransmitter alterations in AD are thought to contribute to cognitive and behavioral symptoms including agitation and aggression1-4 • AXS-05 modulates the function of neurotransmitters (serotonin, glutamate, sigma-1, norepinephrine, and dopamine) implicated in AD1-4 1Cummings JL. N Engl J Med. 2004;351:56-67 2Querfurth HW, et al. N Engl J Med. 2010;362:329-44 3Porsteinsson AP, et al. Expert Opin Pharmacother. 2017; 18:6, 611-620 4Rosenberg PB, et al. Mol Aspects Med. 2015;0: 25–37 5Stahl SM. CNS Spectr. 2019;24:461-466 6Cheng W, et al. Mol Med Rep. 2015 Feb;11(2):1132-8 AXS-05 pharmacological actions5,6 © Axsome Therapeutics, Inc. 8 |
 |
ADVANCE-1 Phase 2/3 Trial Design & Results Cedric O’Gorman MD, MBA Senior Vice President, Clinical Development and Medical Affairs Axsome Therapeutics, Inc. © Axsome Therapeutics, Inc. 9 |
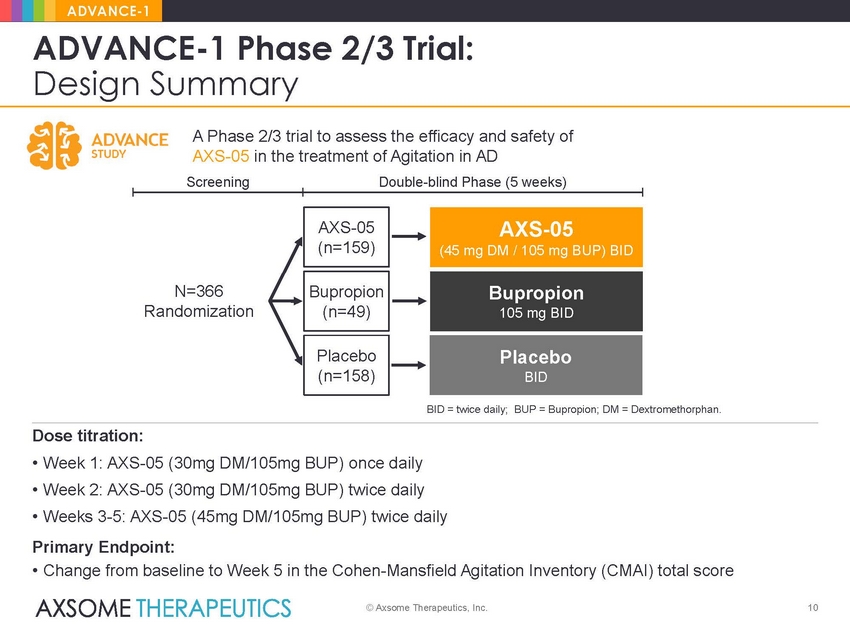 |
ADVANCE-1 ADVANCE-1 Phase 2/3 Trial: Design Summary A Phase 2/3 trial to assess the efficacy and safety of AXS-05 in the treatment of Agitation in AD Screening Double-blind Phase (5 weeks) (45 mg DM / 105 mg BUP) BID N=366 Randomization BID = twice daily; BUP = Bupropion; DM = Dextromethorphan. Dose titration: • Week 1: AXS-05 (30mg DM/105mg BUP) once daily • Week 2: AXS-05 (30mg DM/105mg BUP) twice daily • Weeks 3-5: AXS-05 (45mg DM/105mg BUP) twice daily Primary Endpoint: • Change from baseline to Week 5 in the Cohen-Mansfield Agitation Inventory (CMAI) total score © Axsome Therapeutics, Inc. 10 Placebo BID Bupropion 105 mg BID AXS-05 AXS-05 (n=159) Bupropion (n=49) Placebo (n=158) |
 |
ADVANCE-1 ADVANCE-1 Phase 2/3 Trial: Key Entry Criteria Inclusion criteria included: – Male or female 65-90 years of age inclusive – Diagnosis of probable Alzheimer’s disease, according to the 2011 NIA-AA criteria – Diagnosis of agitation, according to the IPA provisional definition of agitation – MMSE between 10 and 24 – NPI-AA score ≥ 4 – Community-dwelling Exclusion criteria included: – Patient has dementia of non-Alzheimer’s type – Current use of SSRI/SNRI Abbreviations: IPA = International Psychogeriatric Association; MMSE = Mini-mental state evaluation; NIA-AA = National Institute on Aging-Alzheimer Association; NPI-AA = Neuropsychiatric inventory – Agitation / Aggression domain; SNRI = serotonin and norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor. © Axsome Therapeutics, Inc. 11 |
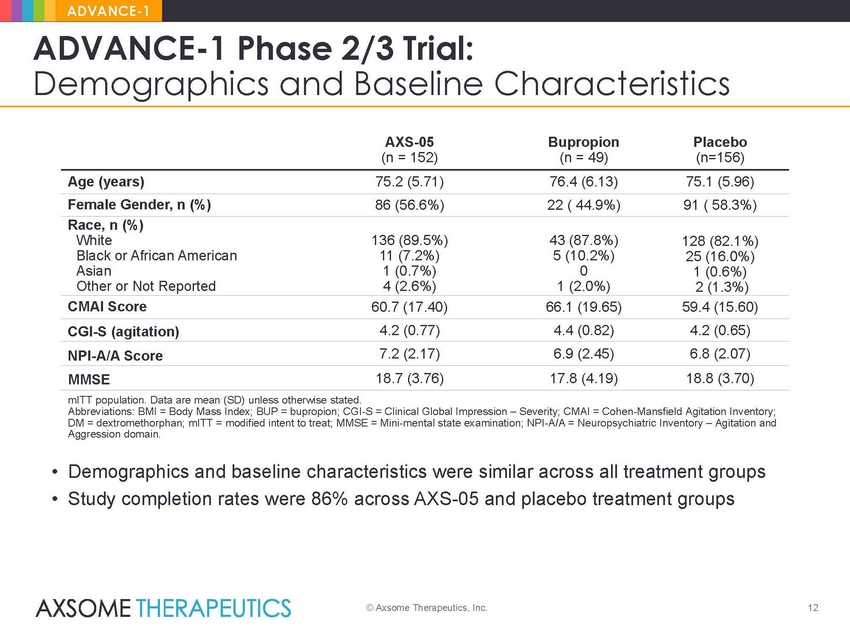 |
ADVANCE-1 ADVANCE-1 Phase 2/3 Trial: Demographics and Baseline Characteristics AXS-05 (n = 152) Bupropion (n = 49) Placebo (n=156) Age (years) 75.2 (5.71) 76.4 (6.13) 75.1 (5.96) Female Gender, n (%) 86 (56.6%) 22 ( 44.9%) 91 ( 58.3%) Race, n (%) White Black or African American Asian Other or Not Reported 136 (89.5%) 11 (7.2%) 1 (0.7%) 4 (2.6%) 43 (87.8%) 5 (10.2%) 0 1 (2.0%) 128 (82.1%) 25 (16.0%) 1 (0.6%) 2 (1.3%) CMAI Score 60.7 (17.40) 66.1 (19.65) 59.4 (15.60) 4.2 (0.77) 4.4 (0.82) 4.2 (0.65) CGI-S (agitation) 7.2 (2.17) 6.9 (2.45) 6.8 (2.07) NPI-A/A Score 18.7 (3.76) 17.8 (4.19) 18.8 (3.70) MMSE mITT population. Data are mean (SD) unless otherwise stated. Abbreviations: BMI = Body Mass Index; BUP = bupropion; CGI-S = Clinical Global Impression – Severity; CMAI = Cohen-Mansfield Agitation Inventory; DM = dextromethorphan; mITT = modified intent to treat; MMSE = Mini-mental state examination; NPI-A/A = Neuropsychiatric Inventory – Agitation and Aggression domain. • Demographics and baseline characteristics were similar across all treatment groups • Study completion rates were 86% across AXS-05 and placebo treatment groups © Axsome Therapeutics, Inc. 12 |
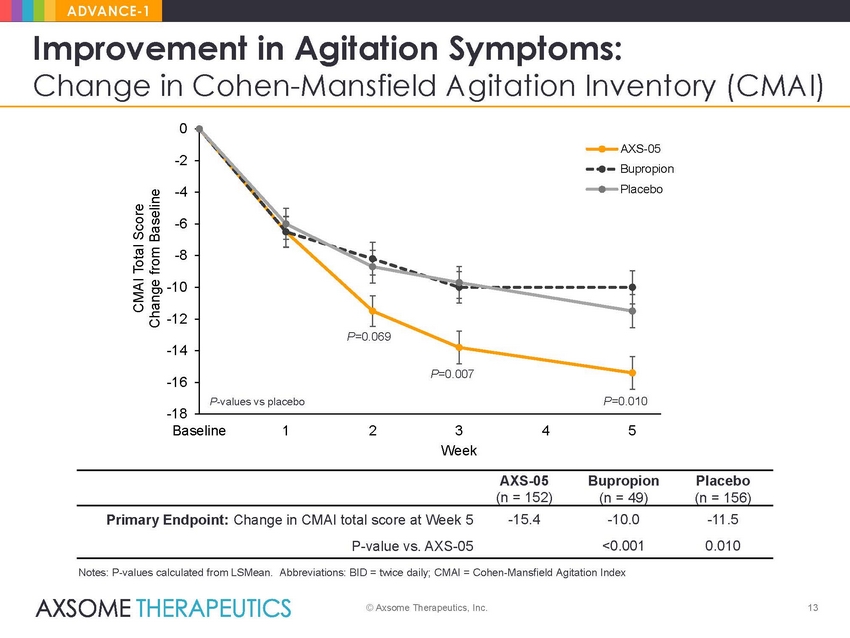 |
ADVANCE-1 Improvement in Agitation Symptoms: Change in Cohen-Mansfield Agitation Inventory (CMAI) 0 -2 on -4 -6 -8 -10 -12 -14 -16 -18 Baseline 1 2 3 Week 4 5 AXS-05 Bupropion Placebo (n = 152) (n = 49) (n = 156) -15.4 -10.0 <0.001 -11.5 0.010 Primary Endpoint: Change in CMAI total score at Week 5 P-value vs. AXS-05 Notes: P-values calculated from LSMean. Abbreviations: BID = twice daily; CMAI = Cohen-Mansfield Agitation Index © Axsome Therapeutics, Inc. 13 CMAI Total Score Change from Baseline AXS-05 Bupropi Placebo P=0.069 P=0.007 P-values vs placeboP=0.010 |
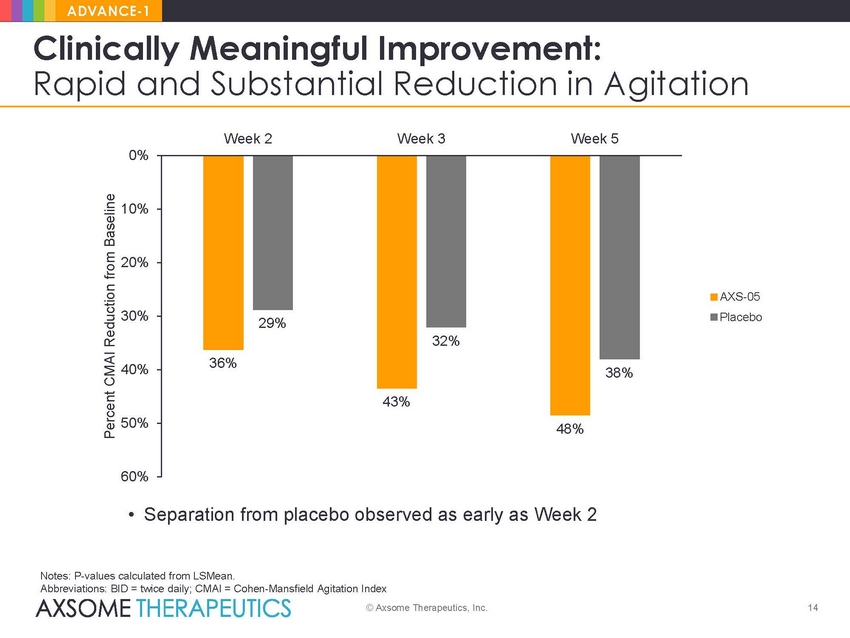 |
ADVANCE-1 Clinically Meaningful Improvement: Rapid and Substantial Reduction in Agitation Week 2 Week 3 Week 5 0% 10% 20% AXS-05 Placebo 30% 40% 50% 60% • Separation from placebo observed as early as Week 2 Notes: P-values calculated from LSMean. Abbreviations: BID = twice daily; CMAI = Cohen-Mansfield Agitation Index © Axsome Therapeutics, Inc. 14 Percent CMAI Reduction from Baseline 29% 32% 36% 38% 43% 48% |
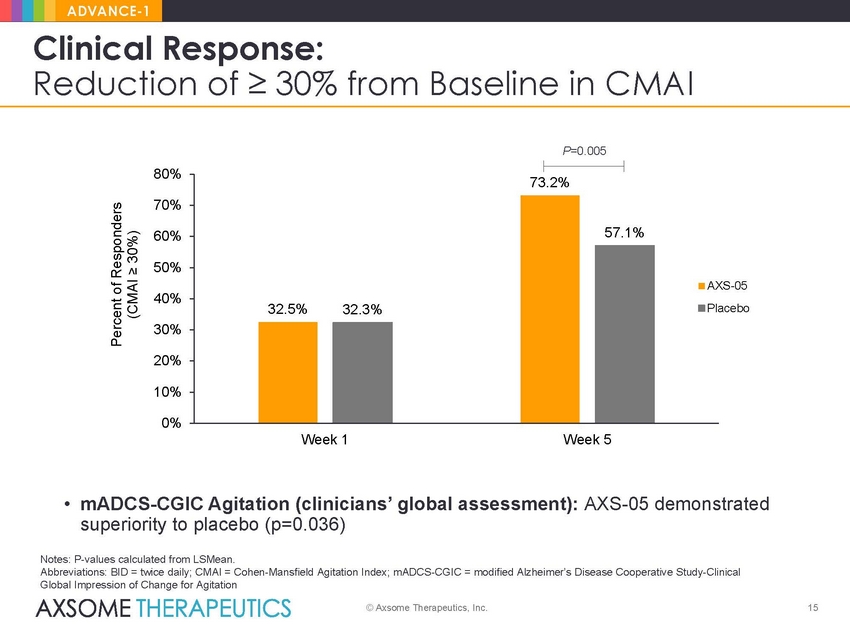 |
ADVANCE-1 Clinical Response: Reduction of ≥ 30% from Baseline in CMAI P=0.005 80% 70% 60% 50% AXS-05 acebo 40% 30% 20% 10% 0% Week 1 Week 5 • mADCS-CGIC Agitation (clinicians’ global assessment): AXS-05 demonstrated superiority to placebo (p=0.036) Notes: P-values calculated from LSMean. Abbreviations: BID = twice daily; CMAI = Cohen-Mansfield Agitation Index; mADCS-CGIC = modified Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change for Agitation © Axsome Therapeutics, Inc. 15 Percent of Responders (CMAI ≥ 30%) 73.2% 32.5%32.3% 57.1% Pl |
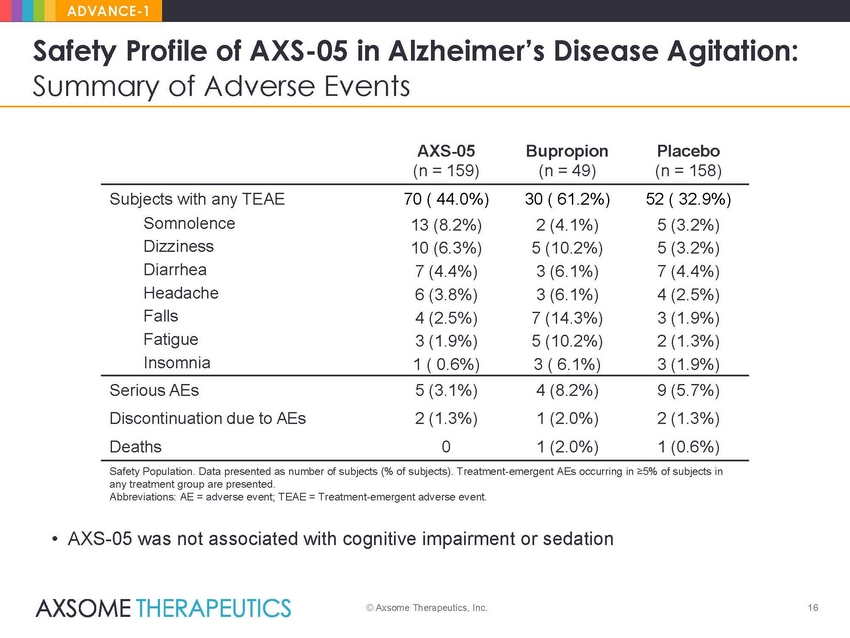 |
ADVANCE-1 Safety Profile of AXS-05 in Alzheimer’s Disease Agitation: Summary of Adverse Events AXS-05 (n = 159) Bupropion (n = 49) Placebo (n = 158) Subjects with any TEAE Somnolence Dizziness Diarrhea Headache Falls Fatigue Insomnia 70 ( 44.0%) 13 (8.2%) 10 (6.3%) 7 (4.4%) 6 (3.8%) 4 (2.5%) 3 (1.9%) 1 ( 0.6%) 30 ( 61.2%) 2 (4.1%) 5 (10.2%) 3 (6.1%) 3 (6.1%) 7 (14.3%) 5 (10.2%) 3 ( 6.1%) 52 ( 32.9%) 5 (3.2%) 5 (3.2%) 7 (4.4%) 4 (2.5%) 3 (1.9%) 2 (1.3%) 3 (1.9%) Serious AEs Discontinuation due to AEs Deaths 5 (3.1%) 2 (1.3%) 0 4 (8.2%) 1 (2.0%) 1 (2.0%) 9 (5.7%) 2 (1.3%) 1 (0.6%) Safety Population. Data presented as number of subjects (% of subjects). Treatment-emergent AEs occurring in ≥5% of subjects in any treatment group are presented. Abbreviations: AE = adverse event; TEAE = Treatment-emergent adverse event. • AXS-05 was not associated with cognitive impairment or sedation © Axsome Therapeutics, Inc. 16 |
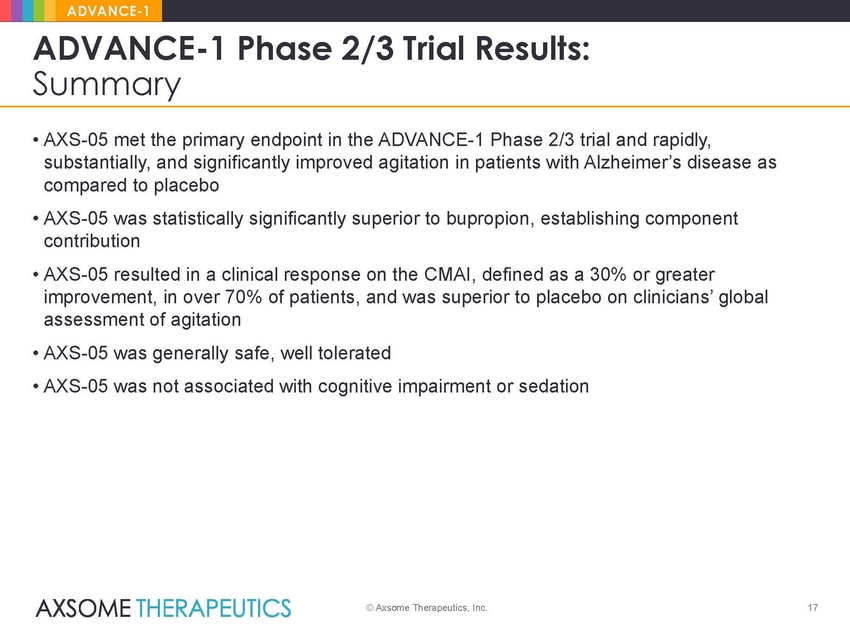 |
ADVANCE-1 ADVANCE-1 Phase 2/3 Trial Results: Summary • AXS-05 met the primary endpoint in the ADVANCE-1 Phase 2/3 trial and rapidly, substantially, and significantly improved agitation in patients with Alzheimer’s disease as compared to placebo • AXS-05 was statistically significantly superior to bupropion, establishing component contribution • AXS-05 resulted in a clinical response on the CMAI, defined as a 30% or greater improvement, in over 70% of patients, and was superior to placebo on clinicians’ global assessment of agitation • AXS-05 was generally safe, well tolerated • AXS-05 was not associated with cognitive impairment or sedation © Axsome Therapeutics, Inc. 17 |
 |
Q&A © Axsome Therapeutics, Inc. 18 |
 |
Concluding Remarks Herriot Tabuteau, MD Chief Executive Officer Axsome Therapeutics, Inc. © Axsome Therapeutics, Inc. 19 |
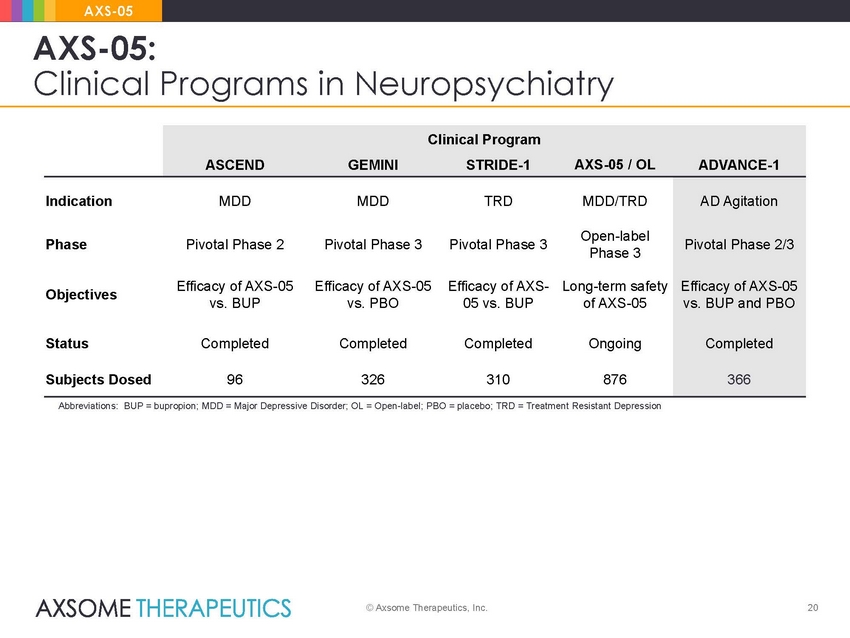 |
AXS-05 AXS-05: Clinical Programs in Neuropsychiatry Phase 3 vs. BUP and PBO Abbreviations: BUP = bupropion; MDD = Major Depressive Disorder; OL = Open-label; PBO = placebo; TRD = Treatment Resistant Depression © Axsome Therapeutics, Inc. 20 Clinical Program ASCENDGEMINISTRIDE-1AXS-05 / OLADVANCE-1 IndicationMDDMDDTRDMDD/TRD PhasePivotal Phase 2Pivotal Phase 3Pivotal Phase 3Open-label ObjectivesEfficacy of AXS-05Efficacy of AXS-05Efficacy of AXS-Long-term safety vs. BUPvs. PBO05 vs. BUPof AXS-05 StatusCompletedCompletedCompletedOngoing Subjects Dosed96326310876 AD Agitation Pivotal Phase 2/3 Efficacy of AXS-05 Completed 366 |
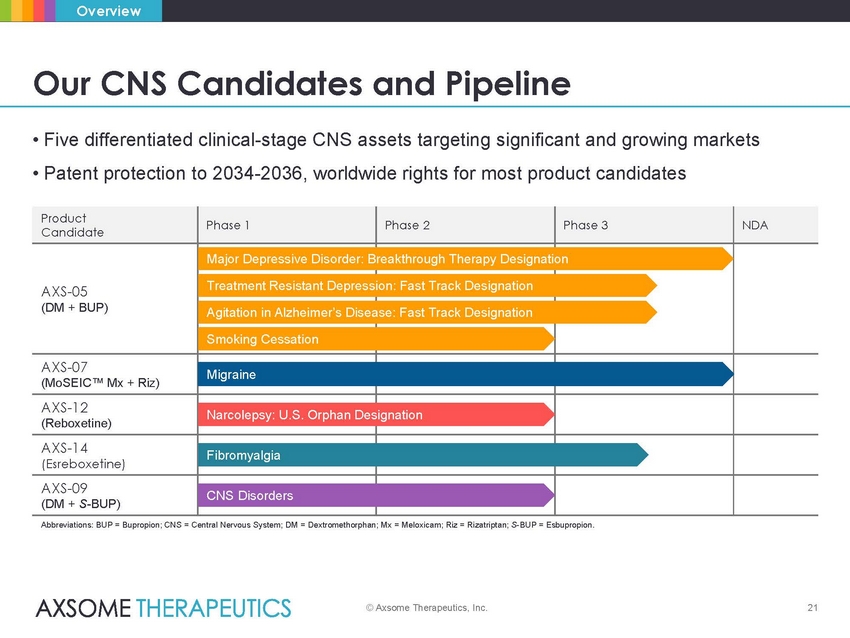 |
Overview Our CNS Candidates and Pipeline • Five differentiated clinical-stage CNS assets targeting significant and growing markets • Patent protection to 2034-2036, worldwide rights for most product candidates ease: Fast Track Designation Abbreviations: BUP = Bupropion; CNS = Central Nervous System; DM = Dextromethorphan; Mx = Meloxicam; Riz = Rizatriptan; S-BUP = Esbupropion. © Axsome Therapeutics, Inc. 21 Product Candidate Phase 1 Phase 2 Phase 3 NDA AXS-05 (DM + BUP) Major Depressive Disorder: B Treatment Resistant Depress Agitation in Alzheimer’s Dis Smoking Cessation reakthrough Therapy Designati ion: Fast Track Designation on AXS-07 (MoSEIC™ Mx + Riz) Migraine AXS-12 (Reboxetine) Narcolepsy: U.S. Orphan Des ignation AXS-14 (Esreboxetine) Fibromyalgia AXS-09 (DM + S-BUP) CNS Disorders |
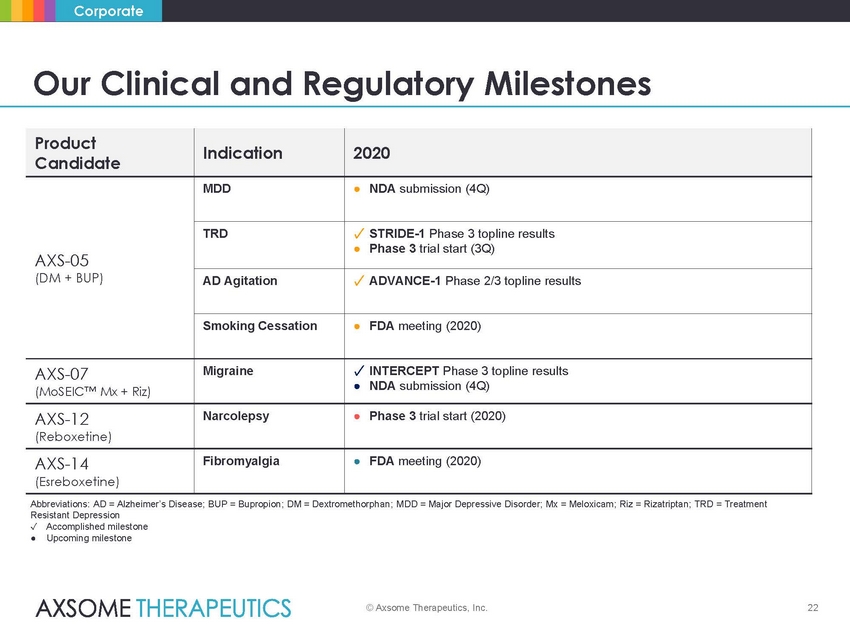 |
Corporate Our Clinical and Regulatory Milestones ● NDA submission (4Q) Abbreviations: AD = Alzheimer’s Disease; BUP = Bupropion; DM = Dextromethorphan; MDD = Major Depressive Disorder; Mx = Meloxicam; Riz = Rizatriptan; TRD = Treatment Resistant Depression ✓ Accomplished milestone ● Upcoming milestone © Axsome Therapeutics, Inc. 22 Product Candidate Indication 2020 AXS-05 (DM + BUP) MDD ● NDA submission (4Q) TRD ✓ STRIDE-1 Phase 3 topline results ● Phase 3 trial start (3Q) AD Agitation ✓ ADVANCE-1 Phase 2/3 topline results Smoking Cessation ● FDA meeting (2020) AXS-07 (MoSEIC™ Mx + Riz) Migraine ✓ INTERCEPT Phase 3 topline results AXS-12 (Reboxetine) Narcolepsy ● Phase 3 trial start (2020) AXS-14 (Esreboxetine) Fibromyalgia ● FDA meeting (2020) |
 |
For more information, please contact Mark Jacobson Chief Operating Officer 212-332-3243 mjacobson@axsome.com axsome.com |
