Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Cara Therapeutics, Inc. | tm2016471d1_ex99-1.htm |
| 8-K - FORM 8-K - Cara Therapeutics, Inc. | tm2016471d1_8k.htm |
Exhibit 99.2

KORSUVA ™ Injection for Dialysis Patients 1 KALM - 2 Phase 3 Pivotal Topline Results April 21 , 2020

Forward - Looking Statements • This presentation contains certain forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward - looking statements by the words “anticipate,” “believe,” “continue,” “estimate,” “expect,” “objective,” “ongoing,” “plan,” “propose,” “potential,” “projected”, or “up - coming” and/or the negative of these terms, or other comparable terminology intended to identif y statements about the future. Examples of these forward - looking statements in this presentation include, among other things, statements concerning pl ans, strategies and expectations for the future, including statements regarding the expected timing of planned regulatory submissions; the size o f t he potential markets that are potentially addressable for the Company’s product candidates, including the pruritus market; the potential commercializat ion of KORSUVA ™ in the licensed territories; and the potential benefits of license agreements entered by the Company. • These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels o f a ctivity, performance or achievements to be materially different from the information expressed or implied by these forward - looking statements. Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that these statements are b ased on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain. Factors that ma y c ause actual results to differ materially from any future results expressed or implied by any forward - looking statements include the risks described in the “Ri sk Factors” section of the Company’s Annual Report on Form 10 - K for the year ended December 31, 2019, as well as those set forth from time to time in the C ompany’s other SEC filings, available at http://www.sec.gov . Any forward - looking statements speak only as of the date of this presentation. • The Company undertakes no obligation to publicly update any forward - looking statements, whether as a result of new information, futu re events or otherwise except as required by law. 2

Endpoints: Week 12 Primary • Proportion of subjects achieving ≥3 point improvement from baseline in weekly mean of daily worst itching intensity NRS (WI - NRS) Secondary • Proportion of subjects achieving ≥4 point improvement in WI - NRS • Proportion of subjects achieving ≥3 point or ≥4 point improvement in WI - NRS at Weeks 4 & 8 • Change from baseline in itch - related Quality of Life as measured by Skindex - 10 and 5 - D Itch questionnaires Safety assessments 3 KALM - 2: Multicenter Global Pivotal Study Design 12 Weeks INTRAVENOUS BOLUS TREATMENT RUN - IN 7 Days END OF TREATMENT 1:1 RANDOMIZATION SCREEN Placebo after each hemodialysis session KORSUVA 0.5 mcg/kg after each hemodialysis session 52 Week Open - Label Extension Ongoing Subjects Undergoing Hemodialysis With Moderate - to - Severe Pruritus (WI - NRS ≥ 5)

Total Randomized (N= 473* ) 4 Subject Disposition in Double - blind Treatment Period *2 subjects were randomized to KORSUVA but did not receive study drug Completed 223 ( 94.5 %) Discontinued 13 ( 5.5 %) Adverse event 7 Subject withdrew consent 1 Subject non - compliance 2 Eligibility 0 Lost to follow - up 0 Lack of therapeutic efficacy 0 Other 3 Completed 206 ( 87.7 %) Discontinued 29 (12.3%) Adverse event 13 Subject withdrew consent 5 Subject non - compliance 1 Eligibility 2 Lost to follow - up 1 Lack of therapeutic efficacy 1 Other 6 Placebo (N= 236 ) KORSUVA (N= 235 )

NRS: Numeric Rating Scale (0 to 10) where 0 = no itch and 10 = worst itching imaginable 5 - D Itch score ranges from 0 to 25 Skindex - 10 scale ranges from 0 to 60 Baseline Characteristics Baseline Characteristics Mean (SD) or % Placebo N = 236 KORSUVA N = 235 Years Undergoing Hemodialysis 5.1 (4.33) 4.8 (4.59) Years of Pruritus 3.2 (3.18) 3.2 (4.57) Use of Anti - Itch Medication 36% 37 % Worst Itching Intensity NRS 7.1 (1.4) 7.3 (1.4) 5 - D Itch Total Score 16.2 (3.3) 16.7 (3.5) Skindex - 10 Total Score 34.2 (14.7) 35.5 (15.0) 5
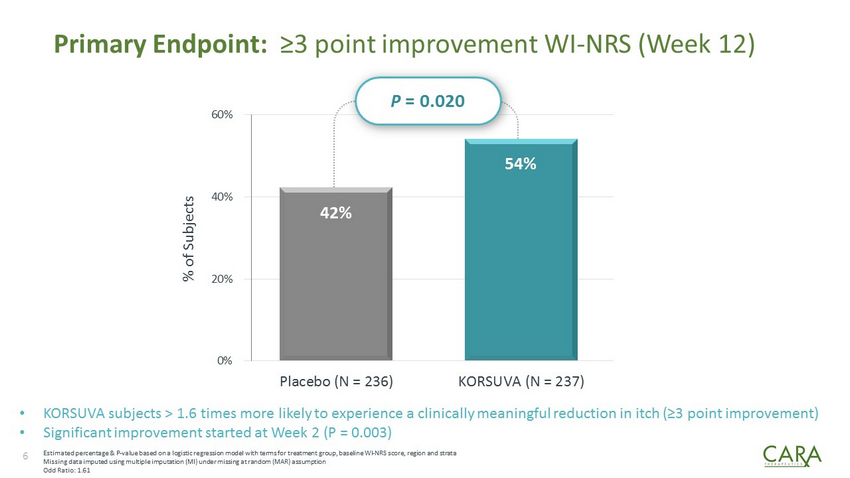
Primary Endpoint: ≥3 point improvement WI - NRS (Week 12) 42% 54% 0% 20% 40% 60% Placebo (N = 236) KORSUVA (N = 237) 6 % of Subjects Estimated percentage & P - value based on a logistic regression model with terms for treatment group, baseline WI - NRS score, regio n and strata Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption Odd Ratio: 1.61 • KORSUVA subjects > 1.6 times more likely to experience a clinically meaningful reduction in itch (≥3 point improvement ) • Significant improvement started at Week 2 (P = 0.003) P = 0.020
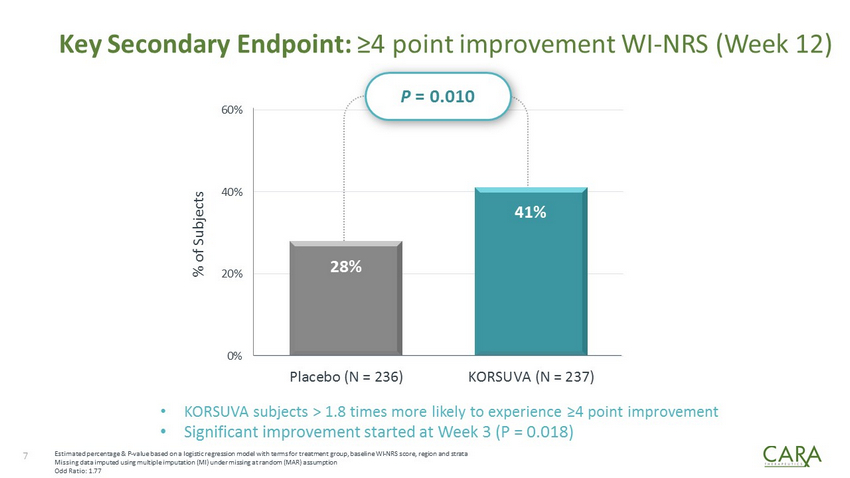
Key Secondary Endpoint: ≥4 point improvement WI - NRS (Week 12) 28% 41% 0% 20% 40% 60% Placebo (N = 236) KORSUVA (N = 237) 7 % of Subjects Estimated percentage & P - value based on a logistic regression model with terms for treatment group, baseline WI - NRS score, regio n and strata Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption Odd Ratio: 1.77 • KORSUVA subjects > 1.8 times more likely to experience ≥4 point improvement • Significant improvement started at Week 3 (P = 0.018) P = 0.010

* P < 0.05, ** P < 0.001 -4 -3 -2 -1 0 * * * ** * ** * * * * * ** Change from Baseline in WI - NRS Over Time LS Means from MMRM with terms for treatment group, week, week by treatment interaction, baseline score, region and strata Missing data imputed using multiple imputation (MI) under missing at random (MAR) assumption Significant differences observed in WI - NRS starting at Week 1 and sustained through treatment period LS Means over time Weeks in Double - blind Treatment Period Change from Baseline Baseline 1 2 3 4 5 6 7 8 9 10 11 12 Placebo (N= 236 ) KORSUVA (N= 237 )

Placebo KORSUVA 0 2 4 6 Change form Baseline 5 - D Placebo KORSUVA 0 5 10 15 20 Change form Baseline Skindex - 10 Other Secondary Endpoints: Skindex - 10 and 5 - D Itch Total Score at Week 12 LS Mean, standard error & P - value based on ANCOVA with terms for treatment group, baseline score, region and strata Missing values imputed using multiple imputation (MI) under MAR assumption # Nominal p value based on sequential statistical analysis 9 29 % improvement over placebo P = 0.171 P = 0.002 # 12% improvement over placebo Itch severity related domains in both scales were significant (P = 0.003 to 0.047) and consistent with WI - NRS improvement

Summary of Adverse Events Treatment - emergent Adverse Events (TEAE) Placebo N = 236 n (%) KORSUVA N = 235 n (%) Subjects with at least one TEAE 145 (61) 160 (68) Subjects with at least one serious TEAE 51 (22) 58 (25) Number of deaths 2 (1) 2 (1) 10 Safety analyses performed in the safety population, defined as all randomized patients who received ≥ 1 dose of study drug based on actual treatment received.

Most Commonly Reported TEAEs Treatment - emergent Adverse Events at ≥5% frequency Placebo N = 236 n (%) KORSUVA N = 235 n (%) Diarrhea 13 (5.5) 19 (8.1) Fall 12 (5.1) 16 (6.8) Dizziness 12 (5.1) 13 (5.5) Vomiting 14 (5.9) 15 (6.4) Nausea 10 (4.2) 15 (6.4) 11 Safety analyses performed in the safety population, defined as all randomized patients who received ≥ 1 dose of study drug based on actual treatment received.

• KALM - 2 Phase 3 trial of KORSUVA™ injection met primary & key secondary endpoints: • Both 3 - point and 4 - point improvement in WI - NRS endpoints achieved • KORSUVA™ Injection provided a rapid and sustained reduction of pruritus • Numerical improvement in itch - related quality of life measures • Safety profile consistent with KALM - 1 and CKD - aP clinical program • Key efficacy results replicated KALM - 1 US Phase 3 pivotal trial (Fishbane et al., N Engl J Med 2020; 382:222 - 232) » Successful outcome of KALM - 2 trial supports NDA submission of KORSUVA™ injection for the treatment of moderate - to - severe CKD - aP in hemodialysis patients planned for 2H, 2020 Executive Summary & Next Steps

Acknowledgement We thank all the investigators and patients who participated in this study and provided support for this program.
