Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OPGEN INC | opgen_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - OPGEN INC | ex99x1.htm |
Exhibit 99.2

OpGen Corporate Overview April 2020

FORWARD LOOKING STATEMENTS DISCLAIMER 2 ©2020 OpGen, Inc. This presentation contains forward - looking statements that are subject to many risks and uncertainties. These statements, among other things, relate to our business strategy, goals and expectations concerning our products, future operations, prospects, plans and objectives of management. The words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict ,” “project,” “will” and similar terms and phrases are used to identify forward - looking statements in this presentation. These statements and other statements regarding our future plans constitute “forward - looking statements” within the meaning of Section 27A of the Securitie s Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and are intended to qualify for the saf e harbor from liability established by the Private Securities Litigation Reform Act of 1955. Such statements are subject to ri sks and uncertainties that are often difficult to predict, are beyond OpGen’s control, and that may cause results to differ materially from expectations. Factors that could cause results to differ materially from those described include, but are not limited to, our ability to su cce ssfully, timely and cost - effectively develop, seek and obtain regulatory clearance for and commercialize our product and service offerings, the rate of adoption of our products and services by hospitals and other healthcare providers, the realization of expected synergies from ou r business combination transaction with Curetis GmbH, the successful integration of our company with the operations and business of Curetis GmbH and its subsidiaries and the implementation of the combined company’s strategic and business goals and objectives, the ability to comply with the complexities of operating a global business, the success of our commercialization efforts, the eff ect on our business of existing and new regulatory requirements, and other economic and competitive factors. For a discussion of the mos t significant risks and uncertainties associated with OpGen's business, please review our filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward - looking statements, which are based on our expectatio ns as of the date of this presentation and speak only as of the date of this presentation. We undertake no obligation to publicl y u pdate or revise any forward - looking statement, whether as a result of new information, future events or otherwise.

OPGEN AND CURETIS COMPLETE BUSINESS COMBINATION 3 ©2020 OpGen, Inc.
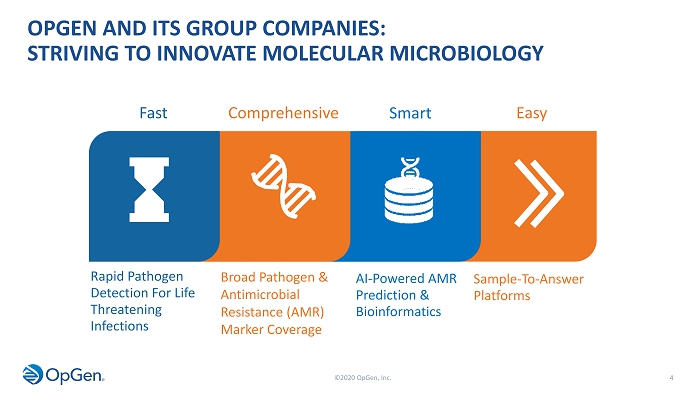
OPGEN AND ITS GROUP COMPANIES: STRIVING TO INNOVATE MOLECULAR MICROBIOLOGY 4 Fast Comprehensive AI - Powered AMR Prediction & Bioinformatics Rapid Pathogen Detection For Life Threatening Infections Broad Pathogen & Antimicrobial Resistance (AMR) Marker Coverage Smart Easy Sample - To - Answer Platforms ©2020 OpGen, Inc.

COMBINED COMPANY’S PORTFOLIO: SYNERGISTIC PRODUCTS & CAPABILITIES Unyvero Platform & Syndromic Tests Acuitas Tests & Acuitas Lighthouse Direct sales in U.S., European and China distribution with partners; 18 distributors covering 43 countries; CoV - 2 test kit distribution in EMEA Unyvero FDA - cleared platform and lower respiratory tract infection (LRT & LRT BAL) as well as 5 CE IVD tests; Unyvero A30 RQ platform in development Acuitas AMR Gene Panel tests in clinical trials (Urine) and pending FDA clearance (isolates) to improve antibiotic decision making; Lighthouse® knowledge base deployed for public health use Global Commercial Presence Ares Genetics NGS & Bioinformatics Ares Technology for AI - powered AMR Prediction combining ARESdb with NGS; Strategic Partnerships with globally leading IVD & pharma companies ©2020 OpGen, Inc. 5

STRATEGIC RATIONALE AND BENEFITS Well positioned to capitalize on global opportunities in infectious disease and rapid AMR detection Proprietary molecular diagnostic tests and platforms Premier AI - powered bioinformatics solutions for multi - drug resistance diagnostics Global commercial channel capabilities & partners Financial leverage, operational synergies, and positive growth - driven business outlook ©2020 OpGen, Inc. 6

U.S. And European Markets With ~10 Million Hospitalized Patients Annually Addressed Through Hospital - Focused Sales Channels COMBINED COMPANY TO ADDRESS UNMET CLINICAL NEEDS AND LARGE AVAILABLE MARKET OPPORTUNITIES The current Unyvero portfolio and pipeline of cartridges according to management estimates target about 10 million patients annually in EU and U.S. with additional upside in Asia / Pacific and ROW markets ∼ 10M cases per year ©2020 OpGen, Inc. 7
STRATEGIC RATIONALE AND BENEFITS ©2020 OpGen, Inc. Well positioned to capitalize on global opportunities in infectious disease and rapid AMR detection Proprietary molecular diagnostic tests and platforms Premier AI - powered bioinformatics solutions for multi - drug resistance diagnostics Global commercial channel capabilities & partners Financial leverage, operational synergies, and positive growth - driven business outlook 8

Innovating Molecular Microbiology Through Proprietary Platforms And Content SAMPLE - TO - ANSWER HIGH - THROUGHPUT TESTING CAPABILITIES * Unyvero A30 RQ Analyzer in development, latest design concept; final product may differ Striving for Molecular Microbiology Innovation MDx Platforms MDx Content Low - to High - Plex PCR Broad Range of Sample Types Proprietary PCR & NGS Applications Based on Leading AI - Powered AMR Knowledgebases Unyvero A50 High - Plex PCR Unyvero A30 RQ* Low - to Mid - Plex PCR ©2020 OpGen, Inc. **Pending 510(k), not for diagnostic use. † ** 9 ARESdb MDx Content & NGS Applications
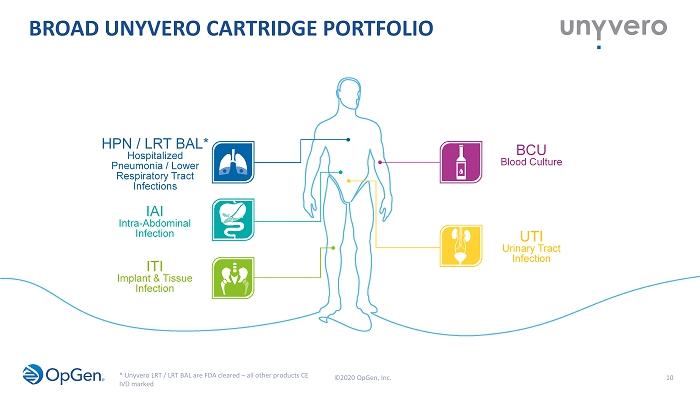
BROAD UNYVERO CARTRIDGE PORTFOLIO 10 HPN / LRT BAL* Hospitalized Pneumonia / Lower Respiratory Tract Infections ITI Implant & Tissue Infection IAI Intra - Abdominal Infection BCU Blood Culture UTI Urinary Tract Infection ©2020 OpGen, Inc. * Unyvero LRT / LRT BAL are FDA cleared – all other products CE IVD marked

Cartridge Indication area Number of targets Sample types Clearance status HPN** Severe cases of Pneumonia 48 targets****, pathogens (29) and antibiotic resistance markers (19) Sputum, broncho - alveolar lavage, tracheal aspirate CE - IVD marked Singapore (HAS) Thailand Malaysia LRT & LRT BAL Lower Respiratory Tract Infections LRT (LRT BAL): 46 (47) targets****, pathogens 36 (37) and antibiotic resistance markers 10 (10) LRT: Tracheal aspirates LRT BAL: Bronchoalveolar Lavage (BAL) LRT: FDA cleared (4/2018) LRT BAL: FDA cleared (12/2019) ITI Severe cases of Implant and Tissue Infections 102 targets, pathogens (85) and antibiotic resistance markers (17) Sonication fluid, swabs, striche, tissue, pus, aspirate/exudate, etc. CE - IVD marked BCU*** Bloodstream infections 103 targets, pathogens (86) and antibiotic resistance markers (17) Positively flagged blood cultures CE - IVD marked Singapore (HAS) Thailand IAI Severe Intra - Abdominal Infections 130 targets, pathogens (105), toxins (3) and antibiotic resistance markers (22) Paracentesis fluids, biliary fluids, peritoneal fluids, drainage fluids, retroperitoneal fluids, pus, swabs, samples from positively flagged blood culture bottles inoculated with other fluids than blood (IAI fluids such as ascites) CE - IVD marked UTI Severe cases of U rinary Tract Infections 103 targets, pathogens (88) and antibiotic resistance markers (15) Midstream urine, suprapubic aspiration, tissue CE - IVD marked UNIQUE AND DIFFERENTIATED SYNDROMIC PANELS 11 **HPN: Hospitalized Pneumonia ***BCU: Blood Culture Application ****Difference between HPN and LRT (BAL) due to different rep ort ing requirements between CE - IVD and U.S. FDA - cleared products ©2020 OpGen, Inc.

CURRENT U.S. PRODUCT OFFERINGS: UNYVERO LRT & LRT BAL 12 Providing Clear Direction • FDA - cleared, sample - to - answer, in less than 5 hours with just about 2 min hands - on time • Direct from native specimen, FDA - cleared for bronchoalveolar lavage fluids and tracheal aspirates • Multiplex PCR with array detection • Detects the most clinically relevant pathogens (incl. atypicals ) and antibiotic resistance markers associated with lower respiratory tract infections including pneumonia • Broadest carbapenemase resistance coverage • The only FDA - cleared LRT panel that detects Pneumocystis jirovecii • Critical information for life - saving treatment decisions ©2020 OpGen, Inc.

CURRENT U.S. PRODUCT OFFERINGS: ACUITAS AMR GENE PANEL* 13 IDENTIFIES Up to 47 Resistance Genes, Spanning 9 Antibiotic Classes TESTS Directly from Urine (in clinical trials) or Isolated Colonies (FDA Clearance Decision Pending), Sample - to - Answer Multiplex PCR from Bacterial Isolates (or Native Urine Specimen) in under 3 hours SEMI - QUANTITATIVELY DETECTS MOST DEADLY SUPERBUGS E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, E. faecalis * For Research Use Only. Not for use in diagnostic procedures. ©2020 OpGen, Inc. Available For RUO in Outbreak Monitoring and Epidemiology Settings (FDA Clearance Decision Pending) – And In Clinical Trials for cUTI

UNYVERO A30 RQ RAPID SAMPLE - TO - ANSWER TESTING PLATFORM IN DEVELOPMENT 14 ©2020 OpGen, Inc. Key Design Features • Fully integrated, closed, sample - to - answer MDx platform • Universal real - time PCR technology for low - to mid - plex testing • Flexible cartridge fluidics for numerous chemistries and assay formats • Fast turn - around time of 45 - 90 minutes • Light - weight, stackable benchtop design with small footprint • Modular and scalable from 1 to 8 cartridge slots • Designed for ease - of - use and flexible deployment in labs and near - patient settings • Attractive COGS for instruments and reagents Development Status • First multiplex PCR successfully demonstrated on functional prototypes • Manufacturing aspects fully specified and in development or implementation phase • Curetis aims at having Unyvero A30 RQ platform ready for partnering in 2020 Platform Available For Partnering To Rapidly Create Menu Of Tests And Commercial Channel(s)

STRATEGIC RATIONALE AND BENEFITS ©2020 OpGen, Inc. Well positioned to capitalize on global opportunities in infectious disease and rapid AMR detection Proprietary molecular diagnostic tests and platforms Premier AI - powered bioinformatics solutions for multi - drug resistance diagnostics Global commercial channel capabilities & partners Financial leverage, operational synergies, and positive growth - driven business outlook 15

16 ARES GENETICS & ARESdb * ©2020 OpGen, Inc. Bioinformatics Powerhouse With Industry - Leading Proprietary AI - Powered AMR Knowledgebase for Molecular Microbiology Global ARESdb Database • Unique Knowledgebase on Antibiotic Resistance Markers building on SIEMENS Microbiology Strain Collection • Demonstrated up to > 99% Accuracy for Antibiotic Susceptibility Prediction in evaluation studies • Based on > 50,000 Pathogens and associated Resistance Data for > 100 Antibiotics First RUO applications launched through NGS service laboratory and cloud platform Partners and customers include globally leading IVD & pharma companies † In development; For Research Use Only. Not for use in diagnostic procedures.

Cloud - based bioinformatics platform powers our ability to trace AMR in real - time with the potential to change the landscape of clinical infectious disease management and improve outcomes for patients ACUITAS LIGHTHOUSE®: DIAGNOSTICS DATA MANAGEMENT PLATFORM FOR ANTIBIOTIC RESISTANT PATHOGENS * Rapid Molecular Antibiotic Resistance Prediction * In development; For Research Use Only. Not for use in diagnostic procedures. 17 Successfully Met All Development Milestones Under 1 st Year Contract - Potential State - Wide AMR Surveillance Network ©2020 OpGen, Inc.
STRATEGIC RATIONALE AND BENEFITS Well positioned to capitalize on global opportunities in infectious disease and rapid AMR detection Proprietary molecular diagnostic tests and platforms Premier AI - powered bioinformatics solutions for multi - drug resistance diagnostics Global commercial channel capabilities & partners Financial leverage, operational synergies, and positive growth - driven business outlook^^ 18 ©2020 OpGen, Inc.

Leveraging Synergies From Our Now Combined Commercial Team Structures DUAL COMMERCIAL MODEL Expanding global c ommercial r each though direct sales in U.S. and via global distributors > Direct sales in the U.S. > European distribution through Menarini Diagnostics > China distribution through Beijing Clear Biotech > 18 distributors covering 43 countries in EU, ME, LATAM, and Asia > EMEA distribution and sales of BGI’s CoV - 2 test kits Direct Partners ©2020 OpGen, Inc. ▪ Multiple products to same hospital call points via same sales channel to drive synergies and cost efficiencies 19

Currently 11 EU Countries – Option To Expand Relationship To Further EMEA Markets And Additional Product Lines PAN EUROPEAN DISTRIBUTION VIA MENARINI Menarini Diagnostics & Curetis Collaboration ( since Q1 - 2019) > Covers entire Unyvero A50 product line > Initial countries: BE, CH, DE, ES, FR, IT, LU, NL, PT, UK, GR > Option to expand relationship to further EMEA countries Menarini Diagnostics Other Distributors ©2020 OpGen, Inc. 20

STRATEGIC RATIONALE AND BENEFITS ©2020 OpGen, Inc. Well positioned to capitalize on global opportunities in infectious disease and rapid AMR detection Proprietary molecular diagnostic tests and platforms Premier AI - powered bioinformatics solutions for multi - drug resistance diagnostics Global commercial channel capabilities & partners Financial leverage, operational synergies, and positive growth - driven business outlook 21

FINANCIAL CONSIDERATIONS • Proforma Revenue : • FY2018 revenues of $4.5 • FY2019 revenues of $6.0 million • No revenue guidance for 2020 at this time due to COVID - 19 situation • Cash Position : • March 31, 2020 - $11.5 million • Cash raised via ATM and Warrant exercises YTD 2020 - $14.3 million • Current available ATM gross capacity - $9.3 million • Warrants outstanding – 864k @ avg. exercise price $2.16 – gross available proceeds $1.9 million • Cash Burn – estimated to be approximately $4.5 - $5.5 million per quarter • Capital Structure - Shares outstanding : • Common Stock – 14,746,076 • Warrants – 1,040,107 (864,000 warrants avg. exercise price $2.16) • Convertible – 426,680 • Equity Awards – 158,525 • Fully Diluted Shares Outstanding - 16,371,388 22 ©2020 OpGen, Inc.

FINANCIAL CONSIDERATIONS • Other Key Items • 15,000 sq. ft. FDA registered R&D/ Manufacturing facility in Maryland • 16,000 sq. ft. FDA registered Manufacturing facility in Germany • 15 Acuitas AMR Gene panel system placements • ~ 170 Unyvero Analyzer placements globally (of which ~35 in the U.S.) • Employee count : • Approximately 110 global employees: • ~57 R&D • ~20 Manufacturing, QM /QA / QC & RA • ~18 Sales and Marketing • ~15 General and Administration 23 ©2020 OpGen, Inc.

NEW OPGEN INC. EXECUTIVE LEADERSHIP TEAM AND BOARD Combined Team Has Decades Of Experience In Precision Medicine, Molecular Diagnostics And Capital Markets Chief Executive Officer: Oliver Schacht, Ph.D. Chief Financial Officer: Timothy (Tim) C. Dec Chief Operating Officer: Johannes (Jan) Bacher Board Members: William (Bill) Rhodes (Chairman) Evan Jones Mario Crovetto Don Elsey Prabhavathi Fernandes, Ph.D . Oliver Schacht, PhD (CEO) 24 ©2020 OpGen, Inc.
UPCOMING MILESTONES, NEWSFLOW & CATALYSTS Unyvero & Acuitas ® Rapid Molecular Tests • FDA clearance decision Acuitas ® AMR Gene Panel (isolates) • USA commercial updates on Unyvero LRT / LRT BAL adoption for bacterial co - infections in COVID - 19 ICU patients • Portfolio news on various SARS - CoV - 2 test related programs across OpGen Group • Acuitas ® AMR Gene Panel (urine) clinical trial enrolment completion • FDA submission Acuitas ® AMR Gene Panel for cUTI • Unyvero A30 RQ partnering deal(s) • China NMPA approval and launch for Unyvero HPN test Ares Genetics • Completion of global IVD corporation technology evaluation and R&D program • Further partnering / licensing deal(s) • Publication of clinical data validating ARESdb for NGS - based antibiotic susceptibility testing 25 ©2020 OpGen, Inc.

CONTACT INFO 26 OpGen Inc. (Global HQ) 708 Quince Orchard Road Gaithersburg, MD 20878 USA P +1 301.869.9683 InvestorRelations@opgen.com Curetis GmbH Max - Eyth - Str. 42 71088 Holzgerlingen Germany P +49 (0)7031 49195 - 10 contact@curetis.com ©2020 OpGen, Inc. Ares Genetics GmbH Karl - Farkas - Gasse 18 1030 Wien Austria P +43 (0)1 361 8880 10 contact@ares - genetics.com

Thank You ! 27 ©2020 OpGen, Inc .
