Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Theravance Biopharma, Inc. | tm2015277d1_ex99-1.htm |
| 8-K - FORM 8-K - Theravance Biopharma, Inc. | tm2015277-1_8k.htm |
Exhibit 99.2

THERAVANCE ® , the Cross/Star logo, and MEDICINES THAT MAKE A DIFFERENCE ® are registered trademarks of the Theravance Biopharma group of companies. All third party trademarks used herein are the property of their respective owners. © 2020 Theravance Biopharma. All rights reserved. TD - 0903: Nebulized pan - JAKi for Acute Lung Injury Associated with COVID - 19 April 9, 2020

Forward looking statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors th at any forward - looking statements or projections made by the company are subject to risks and uncertainties that may cause actual resul ts to differ materially from the forward - looking statements or projections. Examples of forward - looking statements in this presentation may include the Company’s strategies, plans and objectives, the Comp any’s regulatory strategies and timing of clinical studies (including the data therefrom), the potential characteristics, benefits and mechanisms of action of the Company’s product and product candidates, the potential that the Company’s research programs will progress prod uct candidates into the clinic, the Company’s expectations for product candidates through development and potential regulatory ap pro val and commercialization (including their differentiation from other products or potential products), the Company's expectations reg ard ing its allocation of resources, product sales or profit share revenue, the repayment of its notes, expected future commercial perfor man ce of Trelegy Ellipta and the Company’s expectations for its 2020 operating loss, excluding share - based compensation and other financial resul ts. The company’s forward - looking statements are based on the estimates and assumptions of management as of the date of this present ation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, su ch as risks related to impacts of the COVID - 19 global pandemic on our business, delays or difficulties in commencing, enrolling or completin g clinical studies, the potential that results from clinical or non - clinical studies indicate the Company’s compounds or product candidates are unsafe or ineffective, risks that product candidates do not obtain approval from regulatory authorities, the feasibility of undertaking fu ture clinical trials for our product candidates based on policies and feedback from regulatory authorities, dependence on third parties to conduct cli nic al studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, risks associated with establishing and maintaining sales, marketin g a nd distribution capabilities with appropriate technical expertise and supporting infrastructure, potential future disagreements wit h Innoviva, Inc. and TRC LLC, the uncertainty of arbitration and litigation and the possibility that an arbitration award or litigation resul t c ould be adverse to the Company. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the Company’s Form 10 - K filed with the SEC on February 27, 2020, and other periodic reports filed with the SEC. 2 2

Lung selective JAK inhibitor targeted at cytokine storm 3 TD - 0903

Pan - JAK Inhibitor Designed Specifically for Lung Diseases Leveraging >20 years of experience in design of novel respiratory drugs Respiratory experience ‣ Three commercial programs with GSK collaboration* (ANORO ELLIPTA, BREO ELLIPTA, TRELEGY ELLIPTA) ‣ Discovered and developed YUPELRI ® (revefenacin) inhalation solution, the first and only once - daily, nebulized bronchodilator approved for maintenance treatment for COPD Organ - selective therapeutic targets ‣ Driving discovery, development and commercialization of organ - selective small - molecule medicines * Prior to Theravance Biopharma’s spin - off from Innoviva, Inc. in June 2014. COPD, chronic obstructive pulmonary disease; COVID - 19, coronavirus disease 2019; DPI, dry powder inhaler; GSK, GlaxoSmithKline P LC; JAK, Janus kinase inhibitor; LTx, lung transplant; STAT, signal transducer and activator of transcription. 4 Lung - selective pan - JAKi (inhaled) TD - 8236 Pan JAK - inhibition Lung selectivity by design TD - 0903 Dry powder inhaler Asthma Ph 2a Nebulized COVID - 19 JAK - STAT: validated non - steroidal inflammation target
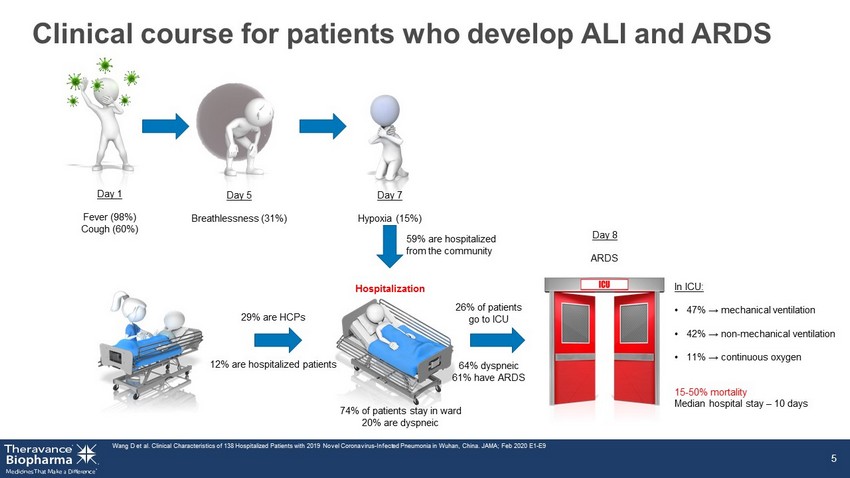
5 26% of patients go to ICU 64% dyspneic 61% have ARDS 29% are HCPs 12% are hospitalized patients Clinical course for patients who develop ALI and ARDS Wang D et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus - Infected Pneumonia in Wuhan, Chi na. JAMA; Feb 2020 E1 - E9 5 Day 1 Fever (98%) Cough (60%) Day 5 Breathlessness (31%) Day 7 Hypoxia (15%) Hospitalization Day 8 ARDS 74% of patients stay in ward 20% are dyspneic In ICU: • 47% → mechanical ventilation • 42% → non - mechanical ventilation • 11% → continuous oxygen 15 - 50% mortality Median hospital stay – 10 days 59% are hospitalized from the community

Host Inflammatory Response to COVID - 19 Drives ALI and ARDS Adapted from Siddiqi HK, et al. J Heart Lung Transplant 2020 Mar 20. 6 6 Clinical symptoms Clinical signs Potential therapies Mild constitutional symptoms Fever >99.6 ° F Dry cough, diarrhea, headache Shortness of breath Hypoxia (PaO 2 /FiO 2 ≤300 mmHg) Lymphopenia, increased prothrombin time, increased D - Dimer and LDH (mild) Abnormal chest imaging Transaminitis Low normal procalcitonin ARDS SIRS/shock Cardiac failure Elevated inflammatory markers (CRP, LDH, IL - 6, D - dimer, ferritin) Troponin, NT - proBNP elevation Severity of Illness Time Course Stage I (Early Infection) Stage II (Pulmonary Phase) Stage III (Hyperinflammation Phase) IIA IIB Corticosteroids, human immunoglobulin, IL - 6 inhibitors, IL - 2 inhibitors, JAK inhibitors Remdesivir, chloroquine, hydroxychloroquine, convalescent plasma transfusions Reduce immunosuppression Viral response phase Host inflammatory response phase

Inhaled pan - JAK inhibitor could suppress dysregulated immune response (“cytokine storm”) in the lung Adapted from Channappanavar R, Perlman S. Seminars in Immunopathology 2017; 39:529 - 39. 7 Inflammatory Response to Pathogenic hCoV Infections CAUSES CONSEQUENCES OUTCOMES • Non - robust virus replication • Early IFN response • ↑↑ Inflammatory monocyte - macrophage & neutrophil infiltration • ↑↑ Proinflammatory cytokines and chemokines • Robust virus replication • Delayed IFN response • ↑↑↑↑ Inflammatory monocyte - macrophage & neutrophil infiltration • ↑↑↑↑ Proinflammatory cytokines and chemokines ALI ARDS Death Protective immunity Host survival Protective/regulated inflammation Pathogenic/dysregulated inflammation • Minimal epithelial & endothelial cell apoptosis • Reduced vascular leakage • Optimal T cell and antibody responses • Effective virus clearance • Enhanced epithelial & endothelial cell apoptosis • Increased vascular leakage • Suboptimal T cell and antibody responses • Impaired virus clearance Key targets for blockade by a lung - selective nebulized pan - JAK inhibitor

8 ▪ A cytokine profile resembling sHLH is associated with COVID - 19 disease severity, characterized by increased IL - 2, IL - 7, GCSF, IP - 10, MCP - 1, MIP1 - α and TNF - α ▪ A multicenter, randomized controlled trial of tocilizumab (IL - 6 receptor blockade, licensed for cytokine release syndrome), has been approved in patients with COVID - 19 pneumonia and elevated IL - 6 in China (ChiCTR2000029765) Mehta P, et al. Lancet 2020; 395:1033 - 1034. GCSF, granulocyte colony stimulating factor; IP - 10, interferon - γ inducible protein 10; MCP - 1, monocyte chemoattractant protein; MIP1 - α , macrophage inflammatory protein 1 - α sHLH, secondary hemophagocytic lymphohistiocytosis; TNF - α , tumor necrosis factor.
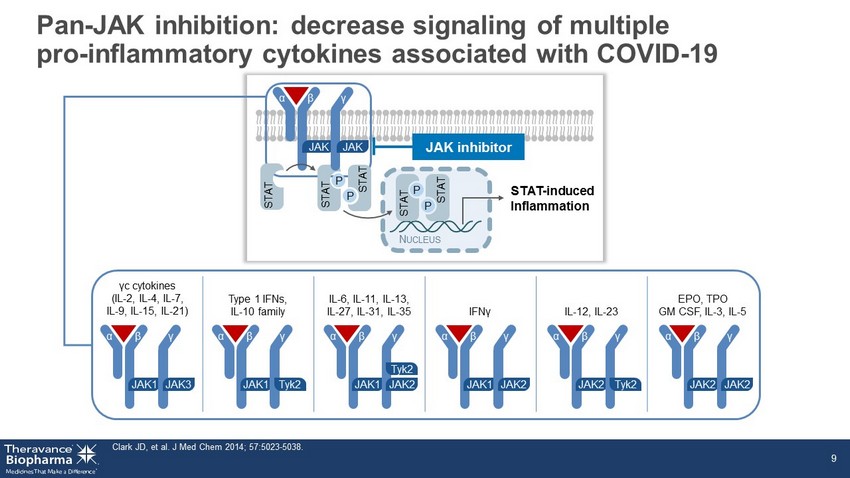
Pan - JAK inhibition: decrease signaling of multiple pro - inflammatory cytokines associated with COVID - 19 Clark JD, et al. J Med Chem 2014; 57:5023 - 5038. 9 STAT - induced Inflammation α N UCLEUS STAT P STAT P STAT P STAT P STAT JAK β γ JAK JAK inhibitor γ c cytokines (IL - 2, IL - 4, IL - 7, IL - 9, IL - 15, IL - 21) Type 1 IFNs, IL - 10 family IL - 6, IL - 11, IL - 13, IL - 27, IL - 31, IL - 35 IFN γ IL - 12, IL - 23 EPO, TPO GM CSF, IL - 3, IL - 5 α JAK1 β γ JAK3 α JAK1 β γ Tyk2 α JAK1 β γ JAK2 Tyk2 α JAK1 β γ JAK2 α JAK2 β γ Tyk2 α JAK2 β γ JAK2
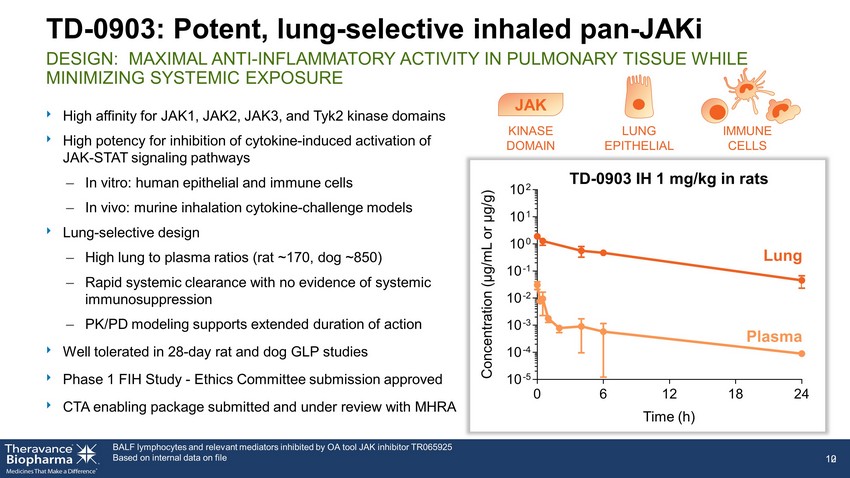
BALF lymphocytes and relevant mediators inhibited by OA tool JAK inhibitor TR065925 Based on internal data on file TD - 0903: Potent, lung - selective inhaled pan - JAKi DESIGN: MAXIMAL ANTI - INFLAMMATORY ACTIVITY IN PULMONARY TISSUE WHILE MINIMIZING SYSTEMIC EXPOSURE ‣ High affinity for JAK1, JAK2, JAK3, and Tyk2 kinase domains ‣ High potency for inhibition of cytokine - induced activation of JAK - STAT signaling pathways – In vitro: human epithelial and immune cells – In vivo: murine inhalation cytokine - challenge models ‣ Lung - selective design – High lung to plasma ratios (rat ~170, dog ~850) – Rapid systemic clearance with no evidence of systemic immunosuppression – PK/PD modeling supports extended duration of action ‣ Well tolerated in 28 - day rat and dog GLP studies ‣ Phase 1 FIH Study - Ethics Committee submission approved ‣ CTA enabling package submitted and under review with MHRA 10 12 0 6 12 18 24 10 -5 10 -4 10 -3 10 -2 10 -1 10 0 10 1 10 2 Lung Plasma TD - 0903 IH 1 mg/kg in r ats Time (h) Concentration ( μ g/mL or μ g/g) KINASE DOMAIN LUNG EPITHELIAL IMMUNE CELLS JAK
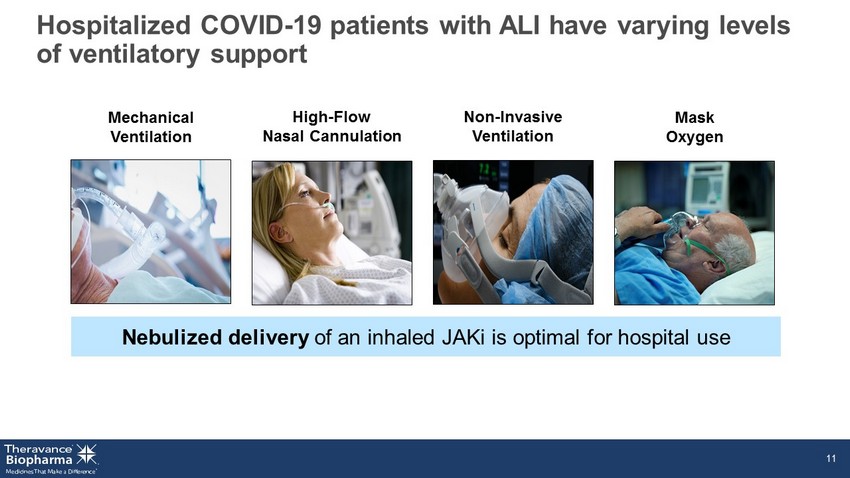
Hospitalized COVID - 19 patients with ALI have varying levels of ventilatory support Nebulized delivery of an inhaled JAKi is optimal for hospital use 11 Mechanical Ventilation High - Flow Nasal Cannulation Non - Invasive Ventilation Mask Oxygen
Apply lung selective JAK inhibition to fight cytokine storm in COVID - 19 Our Mission 12 Transform the treatment of serious diseases through the discovery, development, and commercialization of organ - selective medicines designed to maximize patient benefit while minimizing patient risk
