Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Akero Therapeutics, Inc. | tm2014246d1_ex99-1.htm |
| 8-K - FORM 8-K - Akero Therapeutics, Inc. | tm2014246-1_8k.htm |
Exhibit 99.2

A Global Disease, A Pioneering Treatment AKR - 001 Phase 2a BALANCED Study Week 12 Efficacy Endpoints Akero Therapeutics, Inc. March 31, 2020

- CONFIDENTIAL - 2 SAFE HARBOR This presentation has been prepared by Akero Therapeutics, Inc. (“we,” “us,” “our,” “Akero” or the “Company”) and is made for in formational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities, nor shall there be any sale of any securities in an y state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Th e i nformation set forth herein does not purport to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this pre sen tation unless stated otherwise, and neither this presentation, nor any sale of securities, shall under any circumstances create an implication that the information contained her ein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring a fte r the date hereof. This presentation may contain “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to current beliefs, expectations and assumptions regarding: the future of our bu siness; future plans and strategies, including our expectations around the therapeutic potential and clinical benefits of AKR - 001; our development plans for AKR - 001; our preclinic al and clinical results, including initial primary efficacy results from our Phase 2a BALANCED study; our plan to report the top - line safety/tolerability, laboratory measures and paired biopsy data from our Phase 2a BALANCED study in the second quarter of 2020; and the potential impact of COVID - 19 on strategy, future operations and clinical t rials. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expr ess ions or words, identify forward - looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncerta int ies. Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new informati on, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward - looking statements are reasonable, we can give no assu rance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward - looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward - looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data ob tai ned from third - party sources and the Company’s own internal estimates and research. While the Company believes these third - party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accur acy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.
- CONFIDENTIAL - Efficacy Measures Blinded Safety & Tolerability AKR - 001 MET ALL WEEK 12 EFFICACY ENDPOINTS 3 • All AKR - 001 dose groups met the primary endpoint, with statistically significant absolute reductions in liver fat of 12 - 14% • Statistically significant relative reductions in liver fat for all AKR - 001 dose groups were observed, with >70% reductions for the 50 mg and 70 mg dose groups • Readout of paired biopsy data is expected in 2Q 2020, with 50 subjects eligible for end - of - study biopsies based on achieving ≥30% relative reductions in liver fat at week 12 • Study is ongoing and remains blinded through completion of the study • Blinded tolerability profile appears generally consistent with results from prior AKR - 001 clinical trials • Adverse events observed most frequently in prior trials were mild/moderate gastrointestinal events and injection site reactions

- CONFIDENTIAL - 4 THE BALANCED STUDY TRIAL DESIGN MRI - PDFF Liver Biopsy 12 Weeks Screening Randomization AKR - 001 70 mg (n=20) QW SC Injection AKR - 001 50 mg (n=20) QW SC Injection AKR - 001 28 mg (n=20) QW SC Injection Placebo (n=20) QW SC Injection Liver Biopsy Safety FU – Week 20 Week 6 Screening Weeks 22 - 24 4 Weeks PRIMARY ENDPOINT Absolute change from baseline in hepatic fat fraction (MRI - PDFF) PAIRED BIOPSIES Subjects achieving ≥30% relative reduction of hepatic fat at week 12 are eligible for end - of - study biopsy KEY INCLUSION CRITERIA • F1 - F3 NASH • NAS ≥4 • Liver fat ≥10% KEY SECONDARY EFFICACY ENDPOINTS • Relative Liver Fat • Response Rate • ALT KEY EXPLORATORY EFFICACY ENDPOINTS • Serum Pro - C3 • Fibrosis Improvement • NASH Resolution
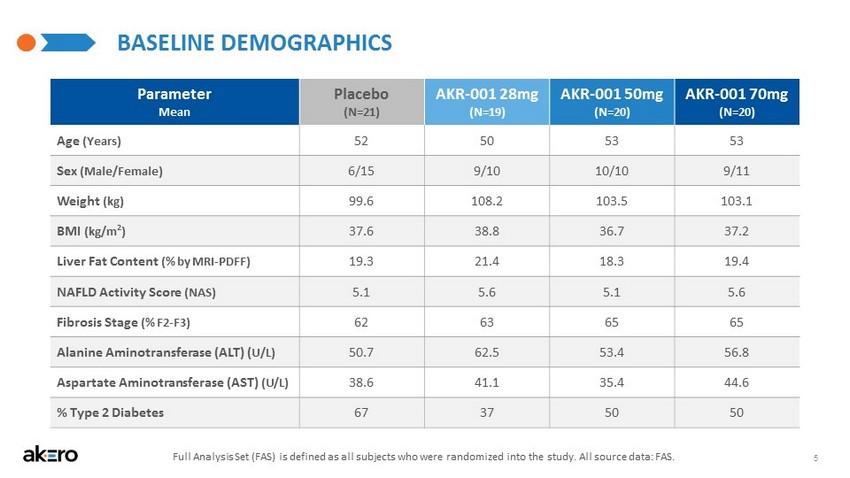
- CONFIDENTIAL - 5 BASELINE DEMOGRAPHICS Parameter Mean Placebo (N=21) AKR - 001 28mg (N=19) AKR - 001 50mg (N=20) AKR - 001 70mg (N=20) Age (Years) 52 50 53 53 Sex (Male/Female) 6/15 9/10 10/10 9/11 Weight (kg) 99.6 108.2 103.5 103.1 BMI (kg/m 2 ) 37.6 38.8 36.7 37.2 Liver Fat Content (% by MRI - PDFF) 19.3 21.4 18.3 19.4 NAFLD Activity Score (NAS) 5.1 5.6 5.1 5.6 Fibrosis Stage (% F2 - F3) 62 63 65 65 Alanine Aminotransferase (ALT) ( U/L) 50.7 62.5 53.4 56.8 Aspartate Aminotransferase (AST) (U/L) 38.6 41.1 35.4 44.6 % Type 2 Diabetes 67 37 50 50 Full Analysis Set (FAS) is defined as all subjects who were randomized into the study. All source data: FAS.

- CONFIDENTIAL - ABSOLUTE REDUCTION IN LIVER FAT: All AKR - 001 Dose Groups Met Primary Endpoint Absolute Reduction in Liver Fat (%) - 0.3 - 12.3 *** - 13.4 *** - 14.1 *** -15 -10 -5 0 Placebo AKR-001 28 mg QW AKR-001 50 mg QW AKR-001 70 mg QW 6 Mean Change From Baseline to Week 12 Normalization of Liver Fat Proportion of subjects with ≤5.0% absolute liver fat at Week 12 ** p=0.004, versus placebo *** p<0.001, versus placebo Placebo 5% 28 mg 21% 50 mg 45% 70 mg 50% **

- CONFIDENTIAL - RELATIVE REDUCTION IN LIVER FAT: All AKR - 001 Dose Groups Met Secondary Endpoint Relative Reduction in Liver Fat 0% - 63% *** - 71% *** - 72% *** -80% -70% -60% -50% -40% -30% -20% -10% 0% Placebo AKR-001 28 mg QW AKR-001 50 mg QW AKR-001 70 mg QW 7 Mean Change From Baseline to Week 12 Response Rate Proportion of subjects with ≥30% relative reduction at Week 12 Placebo 10% 28 mg 84% *** 50 mg 85% *** 70 mg 75% *** *** p<0.001, versus placebo End - of - Study Biopsies 50 subjects eligible

- CONFIDENTIAL - REDUCTION IN ALT: All AKR - 001 Dose Groups Met Secondary Endpoint Reduction in ALT (U/L) - 6 - 24 *** - 30 *** - 32 *** -40 -35 -30 -25 -20 -15 -10 -5 0 Placebo AKR-001 28 mg QW AKR-001 50 mg QW AKR-001 70 mg QW 8 Mean Change From Baseline to Week 12 *** p<0.001, versus placebo An ALT unit decrease of ≥17 U/L may correlate with histologic response Loomba, R (2019) Gastroenterology

- CONFIDENTIAL - AKR - 001 POTENTIAL AS A CORNERSTONE NASH THERAPY 9 x All AKR - 001 dose groups met the primary endpoint for absolute reduction in liver fat x All AKR - 001 dose groups met secondary endpoints for relative reduction in liver fat and ALT reduction x Readout of biopsy data is expected in 2Q 2020, with 50 subjects eligible for end - of - study biopsies

A Global Disease, A Pioneering Treatment NASDAQ: AKRO
