Attached files
| file | filename |
|---|---|
| EX-32 - EXHIBIT 32 - RETRACTABLE TECHNOLOGIES INC | tm2031080d1_ex32.htm |
| EX-31.2 - EXHIBIT 31.2 - RETRACTABLE TECHNOLOGIES INC | tm2031080d1_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - RETRACTABLE TECHNOLOGIES INC | tm2031080d1_ex31-1.htm |
| EX-4.(VI) - EXHIBIT 4.(VI) - RETRACTABLE TECHNOLOGIES INC | tm2031080d1_ex4-vi.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-16465
Retractable Technologies, Inc.
(Exact name of registrant as specified in its charter)
| Texas | 75-2599762 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
| 511 Lobo Lane | |
| Little Elm, Texas | 75068-5295 |
| (Address of principal executive offices) | (Zip Code) |
972-294-1010
Registrant’s telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Common | RVP | NYSE American LLC |
Securities registered pursuant to Section 12(g) of the Act:
Preferred Stock
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer ¨ | Accelerated filer ¨ |
| Non-accelerated filer x | Smaller reporting company x |
| Emerging growth company ¨ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ¨ No x
The aggregate market value of the common equity held by non-affiliates as of June 28, 2019, was $9,772,415, assuming a closing price of $0.7245 and outstanding shares held by non-affiliates of 13,488,496.
APPLICABLE ONLY TO REGISTRANTS INVOLVED IN BANKRUPTCY
PROCEEDINGS DURING THE PRECEDING FIVE YEARS:
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13, or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ¨ No ¨
(APPLICABLE ONLY TO CORPORATE REGISTRANTS)
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. As of March 13, 2020, there were 32,682,454 shares of our Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement filed on an even date herewith for the Annual Meeting of Shareholders to be held May 12, 2020 are incorporated by reference into Part III hereof.
RETRACTABLE TECHNOLOGIES, INC.
FORM 10-K
For the Fiscal Year Ended December 31, 2019
TABLE OF CONTENTS
i
FORWARD-LOOKING STATEMENT WARNING
Certain statements included by reference in this filing containing the words “could,” “may,” “believes,” “anticipates,” “intends,” “expects,” and similar such words constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act. Any forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors include, among others, global pandemics, potential tariffs, our ability to maintain liquidity, our maintenance of patent protection, litigation, our ability to maintain favorable third party manufacturing and supplier arrangements and relationships, foreign trade risk, our ability to quickly increase capacity in response to an increase in demand, our ability to access the market, our ability to maintain or decrease production costs, our ability to continue to finance research and development as well as operations and expansion of production, the impact of larger market players, specifically Becton, Dickinson and Company ("BD"), in providing devices to the safety market, and other factors referenced in Item 1A. Risk Factors. Given these uncertainties, undue reliance should not be placed on forward-looking statements.
DESCRIPTION OF BUSINESS
General Development of Business
Retractable Technologies, Inc. was incorporated in Texas in 1994. Our business is the manufacturing and marketing of safety medical products (predominately syringes) for the healthcare industry. We have manufacturing facilities in Little Elm, Texas and use manufacturers in China as well. We have developed several new products in the last few years, including the EasyPoint® needle which can be used with, among other things, prefilled syringes.
Financial Information
We have only one reporting segment. See Item 8 for our financial statements.
Principal Products, Markets, and Distribution
Our goal is to become a leading provider of safety medical products. Our principal products were designed to protect healthcare workers, patients, and others from needlestick injuries, cross-contamination through reuse, and reduce disposal costs. The VanishPoint® products accomplish these goals by retracting the needle when the plunger handle is fully depressed while the needle is still in the patient. This pre-removal activation virtually eliminates exposure to the contaminated needle, reducing the risk of needlestick injuries. Activation is easily accomplished in one step, using one hand. Upon activation of the retraction mechanism, VanishPoint® products are rendered unusable, reducing the risk of disposal-related injuries or reuse.
VanishPoint® syringe sales have historically comprised most of our sales. VanishPoint® syringe sales were 89.9%, 84.9%, and 85.6% of our revenues in 2017, 2018, and 2019.
Our VanishPoint® safety products currently consist of tuberculin, insulin, and allergy antigen VanishPoint® syringes; 0.5mL, 1 mL, 2mL, 3mL, 5mL, and 10mL VanishPoint® syringes; and the VanishPoint® autodisable syringe. We also sell the VanishPoint® IV catheter; the VanishPoint® blood collection tube holder; and the VanishPoint® blood collection set. The Patient Safe® syringe protects patients by reducing the risk of bloodstream infections associated with catheter hub contamination. Our Patient Safe® products currently consist of 3mL, 5mL, 10mL, 20mL, 30mL, 60mL syringes and the Patient Safe® Luer cap.
In the second quarter of 2016, we began selling the EasyPoint® needle. EasyPoint® needles made up 7.2% of revenues in 2019. The EasyPoint® is a retractable needle that can be used with Luer lock syringes, Luer slip
1
syringes, and prefilled syringes to give injections. The EasyPoint® needle can also be used to aspirate fluids and collect blood.
We currently have under development additional safety products that add to or build upon our current product line offering. These products include retractable needles and syringes, glass syringes, dental syringes, IV catheter introducers, and blood collection sets.
Our products are sold to and used by healthcare providers primarily in the U.S. (with 23.4% of revenues in 2019 generated from sales outside the U.S.) which include, but are not limited to, acute care hospitals, alternate care facilities, doctors’ offices, clinics, emergency centers, surgical centers, long-term care facilities, Veterans Administration facilities, military organizations, public health facilities, and prisons.
Under the current supply chain system in the U.S. acute care market, the vast majority of decisions relating to the contracting for and purchasing of medical supplies are made by the representatives of group purchasing organizations (“GPOs”) and purchasing representatives rather than the end-users of the product (nurses, doctors, and testing personnel). The GPOs and larger manufacturers often enter into contracts which can prohibit or limit entry in the marketplace by competitors.
We distribute our products throughout the U.S. through general line and specialty distributors. We also use international distributors. We have developed a national direct marketing network in order to market our products to health care customers and their purchaser representatives. Our marketers make contact with all of the departments that affect the decision-making process for safety products, including the purchasing agents. They call on acute care and alternate care sites and speak directly with the decision-makers of these facilities. We employ trained sales representatives and clinicians, including nurses and/or medical technologists that educate healthcare providers and healthcare workers on the use of safety devices through on-site clinical training, exhibits at related tradeshows, and publications of relevant articles in trade journals and magazines. These employees provide clinical support to customers. In addition to marketing our products, the network demonstrates the safety and cost effectiveness of our products to customers.
The American Journal of Infection Control published an article in its November 2017 issue that estimates that more than 300,000 healthcare workers in the United States suffer sharps injuries (such as needlesticks) annually. The article is the most recent of a series of articles published over the past few years (several of which were published in the AOHP Journal). The data shows that the number of sharps injuries has remained essentially unchanged over the past several years.
Sources and Availability of Raw Materials
Our product components, including needle adhesives and packaging materials, are purchased from various suppliers. There is no current scarcity of such materials or such suppliers.
Intellectual Property
Intellectual property rights, particularly patent rights, are material to our business. The patent rights are jointly owned by the Company and Thomas J. Shaw, our founder and CEO, and have varying expiration dates. Under the terms of an exclusive license agreement that has been in effect since 1995, the Company is exclusively licensed to use the patent rights held by Mr. Shaw, and Mr. Shaw generally receives a five percent (5%) royalty on gross sales of products subject to the license and he receives fifty percent (50%) of the royalties paid to the Company by certain sublicensees of the technology subject to the license.
Recent and expected modifications to our VanishPoint® syringes will effectively cause the modified VanishPoint® syringes products to have extended patent expiration dates, notwithstanding the recent expiration of some patents underlying the old design. Following the expiration of patents related to the old design, competitors may attempt to copy aspects of such prior design, but not the current design. Patents related to recent modifications to the VanishPoint® syringes and core technology of the VanishPoint® syringes will expire during the years 2028 through 2032. Other patent applications covering inventions applicable to the VanishPoint® syringes are pending.
2
The Company has unexpired patents which relate to the EasyPoint® technology and other products as well.
The Company has registered the following trade names and trademarks for our products: VanishPoint®, EasyPoint®, Patient Safe®, VanishPoint® logos, RT and design, the VanishPoint® and design, the spot design and the Company slogans “The New Standard for Safety” ® and “We Make Safety Safe” ®.
Seasonality
Historically, unit sales have increased in the latter part of the year due, in part, to the demand for syringes during the flu season.
Working Capital Items
Our significant accounting policies are set forth in the notes to our financial statements in Item 8. Our inventory practices will vary in response to demand. Order backlog is not currently material to our business.
Dependence on Customers
Although our business has historically derived significant percentages of its revenues from a few customers, we do not believe that the loss of any one of these customers would have a material adverse effect on our business.
Government Approval and Government Regulations
For all products manufactured for sale in the domestic market, we have given notice of intent to market to the FDA, and the devices were shown to be substantially equivalent to the predicate devices for the stated intended use.
For all products manufactured for sale in the domestic market and foreign market, we hold a Quality Management System certification to ISO 13485:2016. Additionally, for all products manufactured for sale into the applicable countries, we hold a Quality Management System certification in compliance with the Medical Device Single Audit Program (MDSAP). For all products manufactured for sale into European Union countries, we hold a Full Quality Assurance System certification to Directive 93/42/EEC Annex II (excluding section 4). All of these certifications are issued by our notified body, BSI, and are reviewed annually.
We will continue to comply with applicable regulations of all countries in which our products are registered for sale.
Competitive Conditions
Major domestic competitors include BD and Medtronic Minimally Invasive Therapies (“Medtronic,” formerly known as Covidien). Terumo Medical Corp., Smiths Medical, and B Braun are additional competitors with smaller market shares. BD and Medtronic have controlling U.S. market share; greater financial resources; larger and more established sales, marketing, and distribution organizations; and greater market influence, including long-term and/or exclusive contracts. Additionally, BD may be able to use its resources to improve its products through research or acquisitions or develop new products which may compete with our products.
We compete primarily on the basis of healthcare worker and patient safety, product performance, and quality. We believe our competitive advantages include, but are not limited to, our leadership in quality and innovation. We believe our products continue to be the most effective safety devices in today’s market. Our syringe products include passive safety activation, require less disposal space, and are activated while in the patient, reducing exposure to the contaminated needle. Our price per unit is competitive or even lower than the competition once all the costs incurred during the life cycle of a syringe are considered. Such life cycle costs include disposal costs, testing and treatment costs for needlestick injuries, and treatment for contracted illnesses resulting from needlestick injuries.
3
EasyPoint® retractable needles offer unique safety benefits not found in other commercially available safety needles. Manually activated safety needles that compete with EasyPoint® must be removed from the patient, exposing the contaminated needle prior to activation of the manual safety mechanism. EasyPoint® needles allow for activation of the automated retraction mechanism while the needle is still in the patient, reducing exposure to the contaminated needle and effectively reducing the risk of needlestick injuries. EasyPoint® retractable needles are compatible with Luer-fitting syringes, including pre-filled syringes. In addition, EasyPoint® retractable needles may be activated with fluid in the syringe, making it applicable for aspiration procedures such as blood collection.
Environmental Compliance
We believe that we do not incur material costs in connection with compliance with environmental laws.
Employees
As of March 2, 2020, we had 140 employees. 135 of such employees were full time employees.
Available Information
We make available, free of charge on our website (www.retractable.com), our Form 10-K Annual Report and Form 10-Q Quarterly Reports and Current Reports on Form 8-K (and any amendments to such reports) as soon as reasonably practical after such reports are filed.
We could be subject to complex and costly regulation. Our business could suffer if we or our suppliers encounter manufacturing problems. We could be subject to risks associated with doing business outside of the U.S. Current or worsening economic conditions may adversely affect our business and financial condition.
You should carefully consider the following material risks facing us. If any of these risks occur, our business, results of operations, or financial condition could be materially affected.
We Compete in a Marketplace Dominated by BD
We operate in a marketplace that is dominated by BD, the major syringe manufacturer in the U.S.
Although we have made limited progress in some areas, such as the alternate care and some international markets, our volumes are not as high as they should be given the nature and quality of our products and the federal and state legislation requiring the use of safe needle devices.
We Have Generally Been Unable to Gain Sufficient Market Access to Achieve Profitable Operations
We have a history of incurring net operating losses. We may experience operating losses in the future. If we are unable to gain sufficient market access and market share, we may be unable to continue to finance research and development as well as support operations and expansion of production.
We Are Challenged by Uncertainties in Obtaining and Enforcing Intellectual Property Rights
Our main competitive strength is our technology. We are dependent on patent rights, and if the patent rights are invalidated or circumvented, our business would be adversely affected. Patent protection is considered, in the aggregate, to be of material importance in the design, development, and marketing of products.
VanishPoint® syringes comprised 85.6% of sales in 2019. Recent and expected modifications to our VanishPoint® syringes will effectively cause the modified VanishPoint® syringes products to have extended patent expiration dates, notwithstanding the recent expiration of some patents underlying the old design. Following the
4
expiration of patents related to the old design, competitors may attempt to copy aspects of such prior design, but not the current design.
When the patents for the former design of the VanishPoint® syringes and other products expire, we may experience a significant and rapid loss of sales, and our competitive position in the marketplace may weaken if other competitors use our technology. Such occurrences could have a material adverse effect on profitability.
We do not maintain patent or trademark protection in all foreign countries, but, where possible, have taken steps to protect our patents and trademarks in those countries where we market our products or where we believe other manufacturers are most likely to attempt to replicate our technology. Our lack of patent and trademark protection in certain foreign countries heightens the risk that our designs may be copied by a competitor in those countries.
We Are Vulnerable to New Technologies
Because we have a narrow focus on particular product lines and technology (currently, predominantly retractable needle products), we are vulnerable to the development of superior competing products and to changes in technology which could eliminate or reduce the need for our products. If a superior technology is created, the demand for our products could greatly diminish.
Our Competitors Have Greater Resources
Our competitors have greater financial resources, larger and more established sales and marketing and distribution organizations, and greater market influence, including long-term contracts. These competitors may be able to use these resources to improve their products through research and acquisitions or develop new products, which may compete more effectively with our products. If our competitors choose to use their resources to create products superior to ours, we may be unable to sell our products and our ability to continue operations would be weakened.
Operations May Be Affected by Foreign Trade Policy
We are subject to risks associated with foreign trade policy. In 2019, we used Chinese manufacturers to produce 82.6% of our products. Trade protection measures, including tariffs, and/or changes to import or export requirements could materially adversely impact our operations. As of the date of this filing, syringes are not included among the Chinese products on which the U.S. has proposed tariffs. We cannot predict the impact of potential changes to U.S. foreign trade policy. Additionally, we derive 23.4% of our revenues from international sales. International sales, particularly in emerging market countries, are further subject to a variety of regulatory, economic, and political risks as well.
Our New Products May Not Replace Lost VanishPoint® Sales After 2020
Patents relating to the former design of the VanishPoint® syringes expired in 2020. Following the patent expirations, we may experience declines in sales of VanishPoint® syringes due to third party use of aspects of our former design. Our future success depends on the sale of the modified VanishPoint® syringes and other new products. We have engaged in research and development for many years to develop other commercially successful products. Often, new products take a number of years to develop and sales of a new product may be disappointing.
Foreign Manufacturer May Increase Certain Risks
Most international sales, as well as a substantial portion of domestic sales, are filled by production from Chinese manufacturers. In the event that we become unable to purchase such product from our Chinese manufacturers, we would need to find an alternate manufacturer for the blood collection set, IV catheter, Patient Safe® syringe, 0.5mL insulin syringe, 0.5mL autodisable syringe, and 2mL, 5mL, and 10mL syringes and we would increase domestic production for the 1mL and 3mL syringes. Even with increased domestic production, we may not be able to avoid a disruption in supply. In 2019, the 1mL and 3mL syringes made up 78.6% of our unit sales and
5
76.5% of our revenues. We have a strong relationship with our Chinese manufacturers and we communicate with them frequently.
Other risks of manufacturing outside the United States and foreign sales are also present, including risks associated with global economic, regulatory, or political changes, or health crises. As of March 23, 2020, we believe we have sufficient inventory to fulfill demand despite having experienced a temporary disruption in our supply of products from China due to the recent coronavirus (COVID-19) precautions in that country. Our suppliers in China have resumed production and shipments of products from China have been received.
Fluctuations in Supplies of Inventory Could Temporarily Increase Costs
Fluctuations in the cost and availability of raw materials and inventory and the ability to maintain favorable third-party manufacturing arrangements and relationships could result in the need to manufacture all of our products in the U.S. or find other manufacturers. This could temporarily increase unit costs as we ramp up domestic production.
We Are Controlled by One Shareholder
Thomas J. Shaw, our President and Chief Executive Officer, has investment or voting power over a total of 57.6% of the outstanding Common Stock as of March 13, 2020. Mr. Shaw therefore has the ability to direct our operations and financial affairs and to elect members of our Board of Directors. His interests may not always coincide with the Company’s interests or the interests of other stockholders. This concentration of ownership, for example, may have the effect of delaying, deferring, or preventing a change in control, impeding a merger, consolidation, takeover, or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us, which in turn could materially adversely affect the market price of our Common Stock. Mr. Shaw’s rights under the Technology License Agreement, as the owner of the technology we produce, present similar conflicts of interest.
We Face Inherent Product Liability Risks
As a manufacturer and provider of safety needle products, we face an inherent business risk of exposure to product liability claims. Additionally, our success depends on the quality, reliability, and safety of our products and defects in our products could damage our reputation. If a product liability claim is made and damages are in excess of our product liability coverage, our competitive position could be weakened by the amount of money we could be required to pay to compensate those injured by our products. In the event of a recall, we have recall insurance.
Our Business May Be Affected by Changes in the Health Care Regulatory Environment
In the U.S. and internationally, government authorities may enact changes in regulatory requirements, reform existing reimbursement programs, and/or make changes to patient access to health care, all of which could adversely affect the demand for our products and/or put downward pressure on our prices. Future healthcare rulemaking could affect our business. We cannot predict the timing or impact of any future rulemaking or changes in the law.
We May Experience Losses in Our Investment Account
Our investment portfolio is subject to market risk. As a result, the value and liquidity of our cash equivalents and marketable securities could fluctuate substantially. Likewise, our other income and expenses could vary materially depending on gains or losses realized on the sale or exchange of investments and other factors. Increased volatility in the financial markets and overall economic uncertainty could increase the risk that actual amounts realized on our investments may differ from the fair values currently assigned to them. Because 22% of our liquid assets are invested in the market, fluctuations in market values could have a material adverse impact on our business, financial condition, results of operations, or cash flows.
Epidemic Diseases or The Perception of Their Effects Could Have an Adverse Effect on Our Business
6
Outbreaks of epidemic, pandemic, or contagious diseases, such as the recent coronavirus (COVID-19) outbreak, could divert medical resources and priorities toward the treatment of such disease rather than medical conditions for which our products are typically utilized. An outbreak of contagious disease could also negatively affect hospital admission rates or disrupt our workforce. Pandemics could result in temporary closures of our facilities or the facilities of our suppliers and their suppliers. Any such disruption could impact our sales and operating results. Widespread health crises also negatively affect economies which could affect demand for our products. As of March 23, 2020, we believe we have sufficient inventory to fulfill demand despite having experienced a temporary disruption in our supply of products from China due to the recent coronavirus (COVID-19) precautions in that country. Our suppliers in China have resumed production and shipments of products from China have been received.
Disruption of Critical Information Systems or Material Breaches in The Security of Our Systems Could Harm Our Business, Customer Relations, And Financial Condition
Information technology helps us operate efficiently, interface with customers and suppliers, maintain financial accuracy and efficiency, and accurately produce our financial statements. If we do not allocate and effectively manage the resources necessary to build and sustain the proper technology infrastructure, we could be subject to transaction errors, processing inefficiencies, the loss of customers, business disruptions, or the loss of or damage to intellectual property through security breach. If our data management systems do not effectively collect, store, process, and report relevant data for the operation of our business, whether due to equipment malfunction or constraints, software deficiencies, or human error, our ability to effectively plan, forecast, and execute our business plan and comply with applicable laws and regulations will be impaired, perhaps materially. Any such impairment could materially and adversely affect our financial condition, results of operations, cash flows, and the timeliness with which we report our internal and external operating results. Third parties may attempt to fraudulently induce employees or customers into sensitive information, which may in turn be used to access our information technology systems. In addition, unauthorized persons may attempt to hack into our systems to obtain our confidential or proprietary information or confidential information we hold on behalf of third parties. If the unauthorized persons successfully hack into or interfere with our system, we may experience a negative impact to our business and reputation. We have programs in place to detect, contain, and respond to data security incidents, and we make ongoing improvements to our systems in order to minimize vulnerabilities, in accordance with industry and regulatory standards. However, we may not be able to anticipate and prevent these intrusions or mitigate them when and if they occur. We also rely on external vendors to supply and/or support certain aspects of our information technology systems. The systems of these external vendors may contain defects in design or manufacture or other problems that could unexpectedly compromise information security of our own systems, and we are dependent on these third parties to deploy appropriate security programs to protect their systems. It is possible for such vulnerabilities to remain undetected for an extended period, including several years or longer. The costs to us to eliminate or alleviate network security problems, bugs, viruses, worms, ransomware and other malicious software programs, and security vulnerabilities could be significant. Our efforts to address these problems may not be successful and could result in unexpected interruptions, delays, cessation of service, and harm to our business operations. Depending on the type of breach, we could also be exposed to a risk of loss or litigation and potential liability, which could have a material adverse impact on our business, financial condition, results of operations, or cash flows.
Illegal Distribution and Sale by Third Parties of Counterfeit Versions of Our Products Could Have A Negative Impact
Third parties may illegally distribute and sell counterfeit versions of our products which do not meet our rigorous manufacturing and testing standards. Our reputation and business could suffer harm as a result. In addition, diversion of products into other channels may result in reduced revenues.
Item 1B. Unresolved Staff Comments.
Not applicable and none.
7
Our headquarters are located at 511 Lobo Lane, on 35 acres, which we own, overlooking Lake Lewisville in Little Elm, Texas. The headquarters are in good condition and houses our administrative offices and manufacturing facility. The manufacturing facility produced approximately 17.4% of the units that were manufactured in 2019. In the event that we become unable to purchase product from our Chinese manufacturers, we would need to find an alternate manufacturer for the blood collection set, IV catheter, Patient Safe® syringe, 0.5mL insulin syringe, 0.5mL autodisable syringe, and 2mL, 5mL, and 10mL syringes and we would increase domestic production for the 1mL and 3mL syringes. The 5mL and 10mL syringes are sold principally in the international market. In 2019, we used approximately 20% of our current U.S. productive capacity.
A loan in the original principal amount of approximately $4,210,000 is secured by our land and buildings. See Note 8 to our financial statements for more information.
In the opinion of Management, the property and equipment are suitable for their intended use and are adequately covered by an insurance policy.
On November 7, 2019, we filed a lawsuit in the 44th District Court of Dallas County, Texas (No. DC-19-17946) against Locke Lord, LLP and Roy Hardin in connection with their legal representation in our previous litigation against Becton, Dickinson and Company (“BD”). We allege that the defendants breached their fiduciary duties to us, committed malpractice, and were negligent in their representation. We seek actual and exemplary damages, disgorgement, costs, and interest. The defendants have filed a motion to dismiss and the Court has scheduled a hearing on such motion on April 24, 2020. Trial is currently scheduled for May 24, 2021.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities.
MARKET INFORMATION
Our Common Stock has been listed on the NYSE American (or its predecessor entities) under the symbol “RVP” since May 4, 2001.
SHAREHOLDERS
As of March 13, 2020, there were 32,682,454 shares of Common Stock held by 192 shareholders of record, not including Cede & Co. participants or beneficial owners thereof.
DIVIDENDS
We have not ever declared or paid any dividends on the Common Stock. We have no current plans to pay any cash dividends on the Common Stock. We intend to retain all earnings, except those required to be paid to the holders of the Preferred Stock as resources allow, to support operations and future growth. Dividends on Common Stock cannot be paid so long as preferred dividends are unpaid. As of December 31, 2019, there was an aggregate of $12.3 million in preferred dividends in arrears. As of December 31, 2018, there was an aggregate of $11.8 million in preferred dividends in arrears.
EQUITY COMPENSATION PLAN INFORMATION
The following table sets forth information relating to our equity compensation plans as of December 31, 2019:
8
Equity Compensation Plan Information
| Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column(a)) | ||||||||||
| Plan category | (a) | (b) | (c) | |||||||||
| Equity compensation plans approved by security holders | 639,300 | $ | 2.12 | — | ||||||||
| Total | 639,300 | $ | 2.12 | — | ||||||||
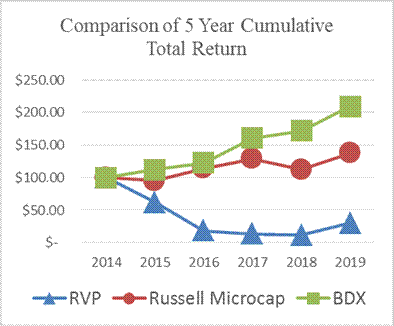
Stock Performance Graph
The following graph compares the cumulative total return for our Common Stock from December 31, 2014 to December 31, 2019, to the total returns for the Russell Microcap® and Becton, Dickinson and Company (or “BDX”), a peer issuer. The graph assumes an investment of $100 in the aforementioned equities as of December 31, 2014, and that all dividends are reinvested.
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Purchases by affiliate(s) during 2019 were not repurchases by or on behalf of the issuer. Based on our review, affiliates properly filed Section 16(a) beneficial ownership reports.
Item 6. Selected Financial Data.
The following selected financial data is qualified by reference to, and should be read in conjunction with, our audited financial statements and the notes to those statements and Management’s Discussion and Analysis of Financial Condition and Results of Operations appearing elsewhere herein. The selected Statements of Operations data presented below for the years ended December 31, 2016 and 2015 and the Balance Sheet data as of December 31, 2017, 2016, and 2015 have been derived from our audited financial statements, which are not included herein.
9
(In thousands except for earnings per share, shares, and percentages)
| As of and for the Years Ended December 31, | ||||||||||||||||||||
| 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||||
| Sales, net | $ | 41,797 | $ | 33,275 | $ | 34,494 | $ | 29,827 | $ | 29,552 | ||||||||||
| Cost of sales | 27,659 | 23,053 | 24,522 | 19,485 | 18,987 | |||||||||||||||
| Gross profit | 14,138 | 10,222 | 9,972 | 10,342 | 10,565 | |||||||||||||||
| Total operating expenses | 11,166 | 11,812 | 13,750 | 13,849 | 13,773 | |||||||||||||||
| Income from insurance proceeds | — | 261 | — | — | — | |||||||||||||||
| Income (loss) from operations | 2,972 | (1,329 | ) | (3,778 | ) | (3,507 | ) | (3,208 | ) | |||||||||||
| Litigation proceeds | — | — | — | — | 7,725 | |||||||||||||||
| Interest and other income | 351 | 153 | 65 | 26 | 25 | |||||||||||||||
| Interest expense | (167 | ) | (177 | ) | (211 | ) | (213 | ) | (220 | ) | ||||||||||
| Income (loss) before income taxes | 3,156 | (1,353 | ) | (3,924 | ) | (3,694 | ) | 4,322 | ||||||||||||
| Provision (benefit) for income taxes | 8 | (13 | ) | (188 | ) | 1 | 8 | |||||||||||||
| Net income (loss) | 3,148 | (1,340 | ) | (3,736 | ) | (3,695 | ) | 4,314 | ||||||||||||
| Preferred Stock dividend requirements | (703 | ) | (705 | ) | (705 | ) | (705 | ) | (709 | ) | ||||||||||
| Deemed capital contribution on extinguishment of preferred stock | — | — | — | — | 2,306 | |||||||||||||||
| Income (loss) applicable to common shareholders | $ | 2,445 | $ | (2,045 | ) | $ | (4,441 | ) | $ | (4,400 | ) | $ | 5,911 | |||||||
| Earnings (loss) per share — basic | $ | 0.07 | $ | (0.06 | ) | $ | (0.14 | ) | $ | (0.15 | ) | $ | 0.21 | |||||||
| Earnings (loss) per share — diluted | $ | 0.07 | $ | (0.06 | ) | $ | (0.14 | ) | $ | (0.15 | ) | $ | 0.20 | |||||||
| Weighted average shares outstanding — basic | 32,672,475 | 32,666,454 | 31,958,121 | 29,354,437 | 27,822,593 | |||||||||||||||
| Weighted average shares outstanding — diluted | 32,672,475 | 32,666,454 | 31,958,121 | 29,354,437 | 29,481,294 | |||||||||||||||
| Current assets | $ | 28,407 | $ | 25,837 | $ | 26,608 | $ | 26,677 | $ | 30,811 | ||||||||||
| Current liabilities | $ | 8,257 | $ | 8,619 | $ | 7,900 | $ | 7,172 | $ | 8,096 | ||||||||||
| Property, plant, and equipment, net | $ | 10,632 | $ | 10,852 | $ | 11,353 | $ | 12,092 | $ | 11,468 | ||||||||||
| Total assets | $ | 39,178 | $ | 36,955 | $ | 38,155 | $ | 38,779 | $ | 42,294 | ||||||||||
| Long-term debt, net of current maturities | $ | 2,378 | $ | 2,640 | $ | 3,081 | $ | 3,498 | $ | 3,417 | ||||||||||
| Stockholders’ equity | $ | 28,542 | $ | 25,614 | $ | 27,174 | $ | 28,108 | $ | 30,781 | ||||||||||
| Redeemable Preferred Stock (in shares) | 772,945 | 781,445 | 781,445 | 781,445 | 781,445 | |||||||||||||||
| Capital leases | — | — | — | — | — | |||||||||||||||
| Cash dividends per common share | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Gross profit margin | 33.8% | 30.7% | 28.9% | 34.7% | 35.8% | |||||||||||||||
Events that could affect the trends indicated above include global pandemics, changes in manufacturing costs, changing average sales prices, changing raw material cost, the gaining of market access, protection of our patents, foreign currency exchange rates, the impact of flu season requirements, new or changing regulations or changes in trade policy, or new products. As our products are made from petroleum products, the changing cost of oil and transportation may have an impact on our costs to the extent increases may not be recoverable through price increases of our products and reductions in oil prices may not quickly affect petroleum product prices.
10
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation.
FORWARD-LOOKING STATEMENT WARNING
Certain statements included by reference in this filing containing the words “could,” “may,” “believes,” “anticipates,” “intends,” “expects,” and similar such words constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act. Any forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors include, among others, global pandemics, potential tariffs, our ability to maintain liquidity, our maintenance of patent protection, litigation, our ability to maintain favorable third party manufacturing and supplier arrangements and relationships, foreign trade risk, our ability to quickly increase capacity in response to an increase in demand, our ability to access the market, our ability to maintain or decrease production costs, our ability to continue to finance research and development as well as operations and expansion of production, the impact of larger market players, specifically BD, in providing devices to the safety market, and other factors referenced in Item 1A. Risk Factors. Given these uncertainties, undue reliance should not be placed on forward-looking statements.
Overview
We have been manufacturing and marketing our products since 1997. VanishPoint® syringes comprised 85.6% of our sales in 2019. We also manufacture and market the EasyPoint® needle, blood collection tube holder, IV safety catheter, and VanishPoint® Blood Collection Set. We currently provide other safety medical products in addition to safety products utilizing retractable technology. One such product is the Patient Safe® syringe, which is uniquely designed to reduce the risk of bloodstream infections associated with catheter hub contamination.
In the second quarter of 2016, we began selling the EasyPoint® needle. EasyPoint® needles made up 7.2% of revenues in 2019. The EasyPoint® is a retractable needle that can be used with Luer lock syringes, Luer slip syringes, and prefilled syringes to give injections. The EasyPoint® needle can also be used to aspirate fluids and collect blood.
Historically, unit sales have increased in the latter part of the year due, in part, to the demand for syringes during the flu season.
Our products have been and continue to be distributed nationally and internationally through numerous distributors. Although we have made limited progress in some areas, such as the alternate care market, our volumes are not as high as they should be given the nature and quality of our products and the federal and state legislation requiring the use of safe needle devices. The alternate care market is composed of facilities that provide long-term nursing and out-patient surgery, emergency care, physician services, health clinics, and retail pharmacies.
We continue to pursue various strategies to have better access to the hospital market, as well as other markets, including attempting to gain access to the market through our sales efforts, our innovative technology, introduction of new products, and, when necessary, litigation.
The Further Consolidated Appropriations Act, 2020, signed into law on December 20, 2019, has permanently repealed the 2.3% medical device excise tax. Prior to the repeal, the tax was on a 4-year moratorium. As a result of the repeal and the prior moratorium, sales of taxable medical devices after December 31, 2015, are not subject to the tax.
Product purchases from our Chinese manufacturers have enabled us to increase manufacturing capacity with little capital outlay and have provided a competitive manufacturing cost. In 2019, our Chinese manufacturers produced approximately 82.6% of our products. As of March 23, 2020, we believe we have sufficient inventory to fulfill demand despite having experienced a temporary disruption in our supply of products from China due to the recent coronavirus (COVID-19) precautions in that country. Our suppliers in China have resumed production and shipments of products from China have been received. In the event that we become unable to purchase products from our Chinese manufacturers, we would need to find an alternate manufacturer for the blood collection set, IV
11
catheter, Patient Safe® syringe, 0.5mL insulin syringe, 0.5mL autodisable syringe, and 2mL, 5mL, and 10mL syringes and we would increase domestic production for the 1mL and 3mL syringes.
In 1995, we entered into a license agreement with Thomas J. Shaw for the exclusive right to manufacture, market, and distribute products utilizing his patented automated retraction technology and other patented technology. This technology is the subject of various patents and patent applications owned by Mr. Shaw. The license agreement generally provides for quarterly payments of a 5% royalty fee on gross sales of products subject to the license and he receives fifty percent (50%) of the royalties paid to the Company by certain sublicensees of the technology subject to the license.
With increased volumes, our manufacturing unit costs have generally tended to decline. Factors that could affect our unit costs include increases in costs by third party manufacturers, changing production volumes, costs of petroleum products, and transportation costs. Increases in such costs may not be recoverable through price increases of our products.
RESULTS OF OPERATIONS
The following discussion contains trend information and other forward-looking statements that involve a number of risks and uncertainties. Our actual future results could differ materially from our historical results of operations and those discussed in the forward-looking statements. All period references are to our fiscal years ended December 2019 and 2018. Dollar amounts have been rounded for ease of reading.
Comparison of Year Ended
December 31, 2019 and Year Ended December 31, 2018
Domestic sales accounted for 76.6% and 86.1% of the revenues in 2019 and 2018, respectively. Domestic revenues increased 11.3% principally due to increased volumes, including sales of VanishPoint® products. Domestic unit sales increased 4.9%. Domestic unit sales were 67.9% of total unit sales for 2019. International revenues, excluding product licensing fees, increased from $4.6 million in 2018 to $9.7 million in 2019, primarily due to higher unit sales. Overall unit sales increased 25%. Our international orders may be subject to significant fluctuation over time. Such orders may fluctuate due to health initiatives at various times as well as economic conditions.
Cost of manufactured product increased $4.1 million principally due to an increase in units sold. Royalty expense increased $506 thousand due to increased gross sales. Gross profit margins increased from 30.7% in 2018 to 33.8% in 2019 principally due to an overall increase in sales.
Operating expenses decreased 5.5% from the prior year due to cost cutting measures in the fourth quarter of 2018. These decreases were partially offset by increases in other operating costs.
The loss from operations was $1.3 million in 2018 compared to income from operations of $3.0 million in 2019.
Cash flow from operations was $2.2 million in 2019 due to our net income for the year. The increase in cash was offset by an increase in accounts receivable.
In 2019, we transferred $4.5 million from our cash accounts into investments in high-grade exchange-traded and closed-end funds (ETFs) and mutual funds. This transfer principally caused our net decrease in cash in 2019.
A comparison of the results of operations for the years ended December 31, 2018 and December 31, 2017 is omitted from this discussion. Such comparison was included in our Annual Report on Form 10-K filed with the SEC on March 28, 2019 in Item 7 of Part II thereof.
LIQUIDITY AND CAPITAL RESOURCES
12
At the present time, Management does not intend to publicly raise equity capital. Due to the funds received from prior litigation and direct purchase of stock, we have sufficient cash reserves and intend to rely on operations, cash reserves, and debt financing, when available, as the primary ongoing sources of cash. Our ability to obtain additional funds through loans is uncertain.
In 2019, we transferred $4.5 million from our cash accounts into investments in certificates of deposit, exchange-traded and closed-end funds (ETFs), and mutual funds. This transfer principally caused our net decrease in cash in 2019. The securities may increase investment income in the future. However, the investment of 22% of our liquid assets in an investment account presents substantial risk as well. In 2019, a portfolio rebalancing caused a significant nonrecurring cash event whereby we recognized $2.7 million from sales of investments.
Historical Sources of Liquidity
We have historically funded operations primarily from the proceeds from revenues, private placements, litigation settlements, and loans.
Internal Sources of Liquidity
Margins and Market Access
To routinely achieve positive or break-even quarters, we need increased access to hospital markets which has been difficult to obtain. We will continue to attempt to gain access to the market through our sales efforts, innovative technology, the introduction of new products, and, when necessary, litigation.
We continue to focus on methods of upgrading our manufacturing capability and efficiency in order to reduce costs.
Fluctuations in the cost and availability of raw materials and inventory and our ability to maintain favorable manufacturing arrangements and relationships could result in the need to manufacture all (as opposed to 17.4%) of our products in the U.S. or find other manufacturers. This could temporarily increase unit costs as we ramp up domestic production.
The mix of domestic and international sales affects the average sales price of our products. Generally, the higher the ratio of domestic sales to international sales, the higher the average sales price will be. Some international sales of our products are shipped directly from China to the customer. The number of units produced by us versus manufactured in China can have a significant effect on the carrying costs of Inventory as well as Cost of sales. We will continue to evaluate the appropriate mix of products manufactured domestically and those manufactured in China to achieve economic benefits as well as to maintain our domestic manufacturing capability.
Cash Requirements
Due to funds received from prior litigation, we have sufficient cash reserves and intend to rely on operations, cash reserves, and debt financing, when available, as the primary ongoing sources of cash. We also have access to our investments, which may be liquidated in the event that the Company needs to access the funds for operations. We have taken steps to decrease our legal costs and we continue to evaluate these costs. In the future, we may reduce the number of units being produced, reduce the workforce, reduce the salaries of officers and other employees, and/or defer royalty payments.
External Sources of Liquidity
We have obtained several loans since our inception, which have, together with the proceeds from the sales of equities and litigation efforts, enabled us to pursue development and production of our products. Our ability to obtain additional funds through loans is uncertain. Due to the current market price of our Common Stock, it is unlikely we would choose to raise funds by the public sale of equity.
In 2019, a portfolio rebalancing caused a significant nonrecurring cash event whereby we recognized $2.7 million from sales of investments.
13
Capital Resources
There were no material commitments for capital projects as of December 31, 2019.
OFF-BALANCE SHEET ARRANGEMENTS
None.
CONTRACTUAL OBLIGATIONS
Contractual Obligations and Commercial Commitments
The following chart summarizes our material obligations and commitments to make future payments under contracts for long-term debt and operating leases as of December 31, 2019:
| Payments Due by Period | ||||||||||||||||||||
| Total | Less than 1 Year | 1-3 Years | 3-5 Years | More than 5 Years | ||||||||||||||||
| Contractual Obligations | ||||||||||||||||||||
| Long-term debt | $ | 3,242,166 | $ | 389,041 | $ | 778,083 | $ | 778,083 | $ | 1,296,959 | ||||||||||
| Operating leases | 84,155 | 84,155 | — | — | — | |||||||||||||||
| Total | $ | 3,326,321 | $ | 473,196 | $ | 778,083 | $ | 778,083 | $ | 1,296,959 | ||||||||||
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
Not applicable to smaller reporting companies.
14
Item 8. Financial Statements and Supplementary Data.
RETRACTABLE TECHNOLOGIES, INC.
FINANCIAL STATEMENTS AND
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
DECEMBER 31, 2019 AND 2018
F-1
F-2
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of
Retractable Technologies, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Retractable Technologies, Inc. (the “Company”) as of December 31, 2019 and 2018, the related statements of operations, changes in stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2019, and the related notes and schedules (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Change in Accounting Principle
As discussed in Note 2 to the financial statements, in 2019, the Company changed its method of accounting for leases due to the adoption of Accounting Standards Codification No. 842.
Basis for Opinion
These financial statements are the responsibility of the Company’s Management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by Management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Moss Adams LLP
Dallas, TX
March 30, 2020
We have served as the Company’s auditor since 2016.
F-3
RETRACTABLE TECHNOLOGIES, INC.
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 5,934,749 | $ | 9,647,292 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $146,832 and $149,665, respectively | 6,564,371 | 4,912,356 | ||||||
| Investments in debt and equity securities, at fair value | 7,771,660 | 2,986,156 | ||||||
| Inventories, net | 7,450,592 | 7,545,094 | ||||||
| Income taxes receivable | 50,392 | 100,887 | ||||||
| Other current assets | 635,201 | 644,803 | ||||||
| Total current assets | 28,406,965 | 25,836,588 | ||||||
| Property, plant, and equipment, net | 10,632,057 | 10,851,747 | ||||||
| Income taxes receivable | 50,393 | 100,835 | ||||||
| Other assets | 88,315 | 165,856 | ||||||
| Total assets | $ | 39,177,730 | $ | 36,955,026 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 5,007,604 | $ | 5,369,677 | ||||
| Current portion of long-term debt | 260,939 | 406,361 | ||||||
| Accrued compensation | 607,339 | 540,852 | ||||||
| Dividends payable | 54,800 | 55,113 | ||||||
| Accrued royalties to shareholder | 921,445 | 769,324 | ||||||
| Other accrued liabilities | 1,387,149 | 1,467,935 | ||||||
| Income taxes payable | 17,944 | 10,025 | ||||||
| Total current liabilities | 8,257,220 | 8,619,287 | ||||||
| Other long-term liabilities | — | 82,359 | ||||||
| Long-term debt, net of current maturities | 2,378,055 | 2,639,647 | ||||||
| Total liabilities | 10,635,275 | 11,341,293 | ||||||
| Commitments and contingencies – See Note 9 | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred Stock, $1 par value: | ||||||||
| Class B; authorized: 5,000,000 shares | ||||||||
| Series I, Class B; outstanding: 96,000 shares at December 31, 2019 and 98,500 shares at December 31, 2018 (liquidation preference of $600,000) | 96,000 | 98,500 | ||||||
| Series II, Class B; outstanding: 171,200 shares at December 31, 2019 and 2018 (liquidation preference of $2,140,000) | 171,200 | 171,200 | ||||||
| Series III, Class B; outstanding: 129,245 shares at December 31, 2019 and 2018 (liquidation preference of $1,615,563) | 129,245 | 129,245 | ||||||
| Series IV, Class B; outstanding: 342,500 shares at December 31, 2019 and 2018 (liquidation preference of $3,767,500) | 342,500 | 342,500 | ||||||
| Series V, Class B; outstanding: 34,000 shares at December 31, 2019 and 40,000 shares at December 31, 2018 (liquidation preference of $149,600) | 34,000 | 40,000 | ||||||
| Common Stock, no par value; authorized: 100,000,000 shares; outstanding: 32,674,954 shares at December 31, 2019 and 32,666,454 shares at December 31, 2018 | — | — | ||||||
| Additional paid-in capital | 61,660,744 | 61,871,756 | ||||||
| Accumulated deficit | (33,891,234 | ) | (37,039,468 | ) | ||||
| Total stockholders’ equity | 28,542,455 | 25,613,733 | ||||||
| Total liabilities and stockholders’ equity | $ | 39,177,730 | $ | 36,955,026 | ||||
See accompanying notes to financial statements
F-4
RETRACTABLE TECHNOLOGIES, INC.
| Years Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Sales, net | $ | 41,797,179 | $ | 33,274,702 | $ | 34,493,838 | ||||||
| Cost of Sales | ||||||||||||
| Costs of manufactured product | 24,209,401 | 20,108,798 | 21,658,062 | |||||||||
| Royalty expense to shareholder | 3,449,822 | 2,944,102 | 2,864,188 | |||||||||
| Total cost of sales | 27,659,223 | 23,052,900 | 24,522,250 | |||||||||
| Gross profit | 14,137,956 | 10,221,802 | 9,971,588 | |||||||||
| Operating expenses: | ||||||||||||
| Sales and marketing | 4,217,863 | 4,404,441 | 4,658,548 | |||||||||
| Research and development | 516,095 | 621,365 | 740,567 | |||||||||
| General and administrative | 6,432,158 | 6,786,041 | 8,351,053 | |||||||||
| Total operating expenses | 11,166,116 | 11,811,847 | 13,750,168 | |||||||||
| Income from insurance proceeds | — | 260,514 | — | |||||||||
| Income (loss) from operations | 2,971,840 | (1,329,531 | ) | (3,778,580 | ) | |||||||
| Interest and other income | 351,166 | 153,460 | 65,695 | |||||||||
| Interest expense | (166,897 | ) | (177,190 | ) | (210,761 | ) | ||||||
| Income (loss) before income taxes | 3,156,109 | (1,353,261 | ) | (3,923,646 | ) | |||||||
| Provision (benefit) for income taxes | 7,875 | (13,318 | ) | (187,608 | ) | |||||||
| Net income (loss) | 3,148,234 | (1,339,943 | ) | (3,736,038 | ) | |||||||
| Preferred Stock dividend requirements | (702,618 | ) | (704,996 | ) | (704,996 | ) | ||||||
| Income (loss) applicable to common shareholders | $ | 2,445,616 | $ | (2,044,939 | ) | $ | (4,441,034 | ) | ||||
| Basic earnings (loss) per share | $ | 0.07 | $ | (0.06 | ) | $ | (0.14 | ) | ||||
| Diluted earnings (loss) per share | $ | 0.07 | $ | (0.06 | ) | $ | (0.14 | ) | ||||
| Weighted average common shares outstanding: | ||||||||||||
| Basic | 32,672,475 | 32,666,454 | 31,958,121 | |||||||||
| Diluted | 32,672,475 | 32,666,454 | 31,958,121 | |||||||||
See accompanying notes to financial statements
F-5
RETRACTABLE TECHNOLOGIES, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| Series I Class B | Series II Class B | Series III Class B | Series IV Class B | Series V Class B | Common | |||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2016 | 98,500 | $ | 98,500 | 171,200 | $ | 171,200 | 129,245 | $ | 129,245 | 342,500 | $ | 342,500 | 40,000 | $ | 40,000 | 29,666,454 | $ | — | ||||||||||||||||||||||||||||||
| Issuance of new Common Stock | — | — | — | — | — | — | — | — | — | — | 3,000,000 | — | ||||||||||||||||||||||||||||||||||||
| Dividends | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Stock option compensation | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2017 | 98,500 | 98,500 | 171,200 | 171,200 | 129,245 | 129,245 | 342,500 | 342,500 | 40,000 | 40,000 | 32,666,454 | — | ||||||||||||||||||||||||||||||||||||
| Dividends | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2018 | 98,500 | 98,500 | 171,200 | 171,200 | 129,245 | 129,245 | 342,500 | 342,500 | 40,000 | 40,000 | 32,666,454 | — | ||||||||||||||||||||||||||||||||||||
| Conversion of Preferred Stock into Common Stock | (2,500 | ) | (2,500 | ) | — | — | — | — | — | — | (6,000 | ) | (6,000 | ) | 8,500 | — | ||||||||||||||||||||||||||||||||
| Dividends | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2019 | 96,000 | $ | 96,000 | 171,200 | $ | 171,200 | 129,245 | $ | 129,245 | 342,500 | $ | 342,500 | 34,000 | $ | 34,000 | 32,674,954 | $ | — | ||||||||||||||||||||||||||||||
See accompanying notes to financial statements
F-6
RETRACTABLE TECHNOLOGIES, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| Additional Paid-in Capital | Accumulated Deficit | Total | ||||||||||
| Balance as of December 31, 2016 | $ | 59,290,333 | $ | (31,963,487 | ) | $ | 28,108,291 | |||||
| Issuance of new Common Stock | 2,350,100 | — | 2,350,100 | |||||||||
| Dividends | (220,450 | ) | — | (220,450 | ) | |||||||
| Stock option compensation | 672,223 | — | 672,223 | |||||||||
| Net loss | — | (3,736,038 | ) | (3,736,038 | ) | |||||||
| Balance as of December 31, 2017 | 62,092,206 | (35,699,525 | ) | 27,174,126 | ||||||||
| Dividends | (220,450 | ) | — | (220,450 | ) | |||||||
| Net loss | — | (1,339,943 | ) | (1,339,943 | ) | |||||||
| Balance as of December 31, 2018 | 61,871,756 | (37,039,468 | ) | 25,613,733 | ||||||||
| Conversion of Preferred Stock into Common Stock | 8,500 | — | — | |||||||||
| Dividends | (219,512 | ) | — | (219,512 | ) | |||||||
| Net income | — | 3,148,234 | 3,148,234 | |||||||||
| Balance as of December 31, 2019 | $ | 61,660,744 | $ | (33,891,234 | ) | $ | 28,542,455 | |||||
See accompanying notes to financial statements
F-7
RETRACTABLE TECHNOLOGIES, INC.
| Years Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net income (loss) | $ | 3,148,234 | $ | (1,339,943 | ) | $ | (3,736,038 | ) | ||||
| Adjustments to reconcile net income (loss) to net cash provided (used) by operating activities: | ||||||||||||
| Depreciation and amortization | 852,080 | 886,814 | 834,951 | |||||||||
| Realized gains on investments | (7,925 | ) | — | — | ||||||||
| Net unrealized gains on investments | (129,315 | ) | — | — | ||||||||
| Share based compensation | — | — | 672,223 | |||||||||
| Inventories reserve | — | (297,731 | ) | — | ||||||||
| Provision for doubtful accounts | — | 47,793 | 24,272 | |||||||||
| (Increase) decrease in operating assets: | ||||||||||||
| Accounts receivable | (1,652,015 | ) | 145,407 | (1,861,990 | ) | |||||||
| Inventories | 94,502 | (1,041,202 | ) | 811,063 | ||||||||
| Other current assets | 9,602 | (226,649 | ) | (225,606 | ) | |||||||
| Income taxes receivable | 100,937 | (13,266 | ) | (188,456 | ) | |||||||
| Other assets | 77,541 | — | — | |||||||||
| Increase (decrease) in operating liabilities: | ||||||||||||
| Accounts payable | (362,072 | ) | 411,927 | 485,994 | ||||||||
| Accrued liabilities | 55,150 | 699,030 | ) | (205,342 | ) | |||||||
| Insurance proceeds | — | (466,293 | ) | 466,293 | ||||||||
| Income taxes payable | 7,919 | (1,382 | ) | — | ||||||||
| Net cash provided (used) by operating activities | 2,194,638 | (1,195,495 | ) | (2,922,636 | ) | |||||||
| Cash flows from investing activities: | ||||||||||||
| Purchase of property, plant, and equipment | (632,078 | ) | (382,156 | ) | (91,878 | ) | ||||||
| Purchase of debt and equity securities | (7,360,398 | ) | (2,986,156 | ) | — | |||||||
| Proceeds from the sales of debt and equity securities | 2,712,134 | — | ||||||||||
| Net cash used by investing activities | (5,280,342 | ) | (3,368,312 | ) | (91,878 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Repayments of long-term debt | (407,014 | ) | (446,350 | ) | (436,280 | ) | ||||||
| Proceeds from sale of common stock | — | — | 2,350,100 | |||||||||
| Payment of Preferred Stock dividends | (219,825 | ) | (220,450 | ) | (220,450 | ) | ||||||
| Net cash provided (used) by financing activities | (626,839 | ) | (666,800 | ) | 1,693,370 | |||||||
| Net decrease in cash and cash equivalents | (3,712,543 | ) | (5,230,607 | ) | (1,321,144 | ) | ||||||
| Cash and cash equivalents at: | ||||||||||||
| Beginning of period | 9,647,292 | 14,877,899 | 16,199,043 | |||||||||
| End of period | $ | 5,934,749 | $ | 9,647,292 | $ | 14,877,899 | ||||||
| Supplemental schedule of cash flow information: | ||||||||||||
| Interest paid | $ | 166,897 | $ | 177,190 | $ | 210,761 | ||||||
| Income taxes paid | $ | — | $ | 1,173 | $ | 1,031 | ||||||
| Supplemental schedule of noncash investing and financing activities: | ||||||||||||
| Preferred dividends declared, not paid | $ | 54,800 | $ | 55,113 | $ | 55,113 | ||||||
| Conversion of preferred stock to common stock | $ | 8,500 | $ | — | $ | — | ||||||
See accompanying notes to financial statements
F-8
| 1. | BUSINESS OF THE COMPANY AND BASIS OF PRESENTATION |
Business of the Company
Retractable Technologies, Inc. (the “Company”) was incorporated in Texas on May 9, 1994, and designs, develops, manufactures, and markets safety syringes and other safety medical products for the healthcare profession. The Company began to develop its manufacturing operations in 1995. The Company’s manufacturing and administrative facilities are located in Little Elm, Texas. The Company’s products are the VanishPoint® 0.5mL insulin syringe; 1mL tuberculin, insulin, and allergy antigen syringes; 0.5mL, 1mL, 2mL, 3mL, 5mL, and 10mL syringes; the small diameter tube adapter; the blood collection tube holder; the allergy tray; the IV safety catheter; the Patient Safe® syringes; the Patient Safe® Luer Cap; the VanishPoint® Blood Collection Set; and the EasyPoint® needle. The Company also sells VanishPoint® autodisable syringes in the international market in addition to the Company’s other products.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Accounting estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) requires Management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ significantly from those estimates.
Cash and cash equivalents
For purposes of reporting cash flows, cash and cash equivalents include cash, money market accounts, and investments with original maturities of three months or less.
Accounts receivable
The Company records trade receivables when revenue is recognized. No product has been consigned to customers. The Company’s allowance for doubtful accounts is primarily determined by review of specific trade receivables. Those accounts that are doubtful of collection are included in the allowance. This provision is reviewed to determine the adequacy of the allowance for doubtful accounts. Trade receivables are charged off when there is certainty as to their being uncollectible. Trade receivables are considered delinquent when payment has not been made within contract terms.
The Company requires certain customers to make a prepayment prior to beginning production or shipment of their order. Customers may apply such prepayments to their outstanding invoices or pay the invoice and continue to carry forward the deposit for future orders. Such amounts are included in Other accrued liabilities on the Balance Sheets and are shown in Note 7, Other Accrued Liabilities.
The Company records an allowance for estimated returns as a reduction to Accounts receivable and Gross sales. Historically, returns have been insignificant.
Inventories
Inventories are valued at the lower of cost or net realizable value, with cost being determined using actual average cost. The Company compares the average cost to the net realizable value and records the lower value. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. Management considers such factors as the amount of inventory on hand and in the distribution channel, estimated time to sell such inventory, the shelf
F-9
life of inventory, and current market conditions when determining excess or obsolete inventories. A reserve is established for any excess or obsolete inventories or they may be written off.
Investments in Debt and Equity Securities
The Company holds high-grade exchange-traded and closed-end funds (ETFs), mutual funds, and debt securities as investments. These assets are readily marketable and are carried at fair value as of the date of the Balance Sheets. Net unrealized and realized gains or losses on investments in debt and equity securities are reflected as a component of interest and other income. Realized gains or losses on investments in debt and equity securities are recognized using the specific identification method.
Property, plant, and equipment
Property, plant, and equipment are stated at cost. Expenditures for maintenance and repairs are charged to operations as incurred. Cost includes major expenditures for improvements and replacements which extend useful lives or increase capacity and interest cost associated with significant capital additions. Gains or losses from disposals are included in operations.
The Company's property, plant, and equipment primarily consist of buildings, land, assembly equipment, molding machines, molds, office equipment, furniture, and fixtures. Depreciation and amortization are calculated using the straight-line method over the following useful lives:
| Production equipment | 3 to 13 years | ||
| Office furniture and equipment | 3 to 10 years | ||
| Buildings | 39 years | ||
| Building improvements | 15 years |
Long-lived assets
The Company assesses the recoverability of long-lived assets using an assessment of the estimated undiscounted future cash flows related to such assets. In the event that assets are found to be carried at amounts which are in excess of estimated gross future cash flows, the assets will be adjusted for impairment to a level commensurate with fair value determined using a discounted cash flow analysis or appraised values of the underlying assets.
Fair Value Measurements
For assets and liabilities that are measured using quoted prices in active markets, total fair value is the published market price per unit multiplied by the number of units held without consideration of transaction costs. Assets and liabilities that are measured using significant other observable inputs are valued by reference to similar assets or liabilities, adjusted for contract restrictions and other terms specific to that asset or liability. For these items, a significant portion of fair value is derived by reference to quoted prices of similar assets or liabilities in active markets. For all remaining assets and liabilities, fair value is derived using a fair value model, such as a discounted cash flow model or Black-Scholes model.
Financial instruments
The Company estimates the fair value of financial instruments through the use of public market prices, quotes from financial institutions, and other available information. Judgment is required in interpreting data to develop estimates of fair value and, accordingly, amounts are not necessarily indicative of the amounts that could be realized in a current market exchange. Short-term financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, and other liabilities, consist primarily of instruments without extended maturities, the fair value of which, based on Management's estimates, equals their recorded values. Investments in equity securities consist primarily of exchange-traded and closed-end funds and mutual funds and are reported at their fair value based upon quoted prices in active markets. Investments in U.S.
F-10
Treasury Notes are reported at their fair value based upon quoted prices in active markets. Investments in certificates of deposit (CD) with original maturities of greater than three months are reported at their estimated fair value based upon the duration of the CD and the interest rate earned on the CD versus current interest rates of similar duration CDs. The fair value of long-term liabilities, based on Management’s estimates, approximates their reported values.
Concentration risks
The Company’s financial instruments exposed to concentrations of credit risk consist primarily of cash, cash equivalents, certificates of deposit, U.S. Treasury Notes, exchange-traded and closed-end funds, mutual funds, and accounts receivable. Cash balances, some of which exceed federally insured limits, are maintained in financial institutions; however, Management believes the institutions are of high credit quality. The majority of accounts receivable are due from companies that are well-established entities. The Company assesses market risk in debt and equity securities through consultation with its outside investment advisors. Management is responsible for directing investment activity based on current economic conditions. As a consequence, Management considers any exposure from concentrations of credit risks to be limited.
The following table reflects our significant customers in 2019, 2018, and 2017:
| Years Ended December 31, | ||||||
| 2019 | 2018 | 2017 | ||||
| Number of significant customers | 3 | 2 | 2 | |||
| Aggregate dollar amount of net sales to significant customers | $19.0 million | $13.1 million | $14.0 million | |||
| Percentage of net sales to significant customers | 45.6% | 39.2% | 40.5% | |||
The Company decreased its allowance for doubtful accounts by approximately $3 thousand in 2019.
The Company manufactures some of its products in Little Elm, Texas, as well as utilizing manufacturers in China. The Company obtained roughly 82.6% of its products in 2019 from its Chinese manufacturers. Purchases from Chinese manufacturers aggregated 85.3% and 82.9% of products in 2018 and 2017, respectively. In the event that the Company becomes unable to purchase products from its Chinese manufacturers, the Company would need to find an alternate manufacturer for its blood collection set, IV catheter, Patient Safe® syringe, 0.5mL insulin syringe, 0.5mL autodisable syringe, and 2mL, 5mL, and 10mL syringes and would increase domestic production for the 1mL and 3mL syringes and EasyPoint® needles.
Revenue recognition
The Company recognizes revenue when it has satisfied all performance obligations to the customer, generally when title and risk of loss pass to the customer. Payments from customers with approved credit terms are typically due 30 days from the invoice date. Under certain contracts, revenue is recorded on the basis of sales price to distributors, less contractual pricing allowances. Contractual pricing allowances consist of: (i) rebates granted to distributors who provide tracking reports which show, among other things, the facility that purchased the products, and (ii) a provision for estimated contractual pricing allowances for products for which the Company has not received tracking reports. Rebates are recorded when issued and are applied against the customer’s receivable balance. Distributors receive a rebate for the difference between the Wholesale Acquisition Cost and the appropriate contract price as reflected on a tracking report provided by the distributor to the Company. If product is sold by a distributor to an entity that has no contract, there is a standard rebate (lower than a contracted rebate) given to the distributor. One of the purposes of the rebate is to encourage distributors to submit tracking reports to the Company. The provision for contractual pricing allowances is recognized in the period the related sales are recognized and is reviewed at the end of each quarter and adjusted for changes in levels of products for which there is no tracking report. Additionally, if it becomes clear that tracking reports will not be provided by individual distributors, the provision is further adjusted. The estimated contractual allowance is included in Accounts payable in the Balance Sheets and deducted from revenues in the Statements of Operations. Accounts payable included estimated contractual
F-11
allowances for $3,586,726 and $3,896,341 as of December 31, 2019 and 2018, respectively. The terms and conditions of contractual pricing allowances are governed by contracts between the Company and its distributors. Revenue for shipments directly to end-users is recognized when title and risk of ownership pass from the Company. End-users do not receive any contractual allowances on their purchases. Any product shipped or distributed for evaluation purposes is expensed.
The Company provides product warranties that: i) the products are fit for medical use as generally defined within the boundaries of United States FDA approval; ii) the products are not defective; and iii) the products will conform to the descriptions set forth in their respective labeling, provided that they are used in accordance with such labeling and the Company’s written directions for use. The Company has historically not incurred significant warranty claims.
The Company’s domestic return policy provides that a customer may return incorrect shipments within 10 days following arrival at the distributor’s facility. In all such cases, the distributor must obtain an authorization code from the Company and affix the code to the returned product.
The Company’s domestic return policy also generally provides that a customer may return product that is overstocked. Overstocking returns are limited to two times in each 12-month period up to 1% of distributor’s total purchase of products for the prior 12-month period. All product overstocks and returns are subject to inspection and acceptance by the Company.
The Company’s international distribution agreements generally do not provide for any returns.
The Company requires certain customers to pay in advance of product shipment. Such prepayments from customers are recorded in Other accrued liabilities and are generally recognized as revenue within 30 to 60 days of receipt at the time product is shipped.
The Company recognizes revenue from licensing agreements when collection of such amounts from third parties is reasonably assured. If the Company licenses its products for sale, the Company is obligated to pay Thomas J. Shaw, the owner of certain patented technology, a certain percentage of such revenue pursuant to the terms of the Technology License Agreement between the Company and Mr. Shaw.
Disaggregated information of revenue recognized from contracts with customers and licensing fees recognized are as follows:
| For the year ended December 31, 2019: | ||||||||||||||||||||
| Geographic Segment | Syringes | Blood Collection Products | EasyPoint® Needles | Other Products | Total Product Sales | |||||||||||||||
| U.S. sales | $ | 26,722,414 | $ | 2,130,767 | $ | 2,970,374 | $ | 74,369 | $ | 31,897,924 | ||||||||||
| North and South America sales (excluding U.S.) | 7,863,796 | 6,313 | 7,996 | 370,885 | 8,248,990 | |||||||||||||||
| Other international sales | 1,052,217 | 578,617 | 635 | 18,796 | 1,650,265 | |||||||||||||||
| Total | $ | 35,638,427 | $ | 2,715,697 | $ | 2,979,005 | $ | 464,050 | $ | 41,797,179 | ||||||||||
| For the year ended December 31, 2018: | ||||||||||||||||||||
| Geographic Segment | Syringes | Blood Collection Products | EasyPoint® Needles | Other Products | Total Product Sales | |||||||||||||||
| U.S. sales | $ | 23,803,483 | $ | 1,365,936 | $ | 3,401,389 | $ | 75,766 | $ | 28,646,574 | ||||||||||
| North and South America sales (excluding U.S.) | 3,521,823 | 8,805 | 252 | 66,564 | 3,597,444 | |||||||||||||||
| Other international sales | 940,740 | 48,101 | 11,768 | 30,075 | 1,030,684 | |||||||||||||||
| Total | $ | 28,266,046 | $ | 1,422,842 | $ | 3,413,409 | $ | 172,405 | $ | 33,274,702 | ||||||||||
F-12
| For the year ended December 31, 2017: | ||||||||||||||||||||
| Geographic Segment | Syringes | Blood Collection Products | EasyPoint® Needles | Other Products | Total Product Sales | |||||||||||||||
| U.S. sales | $ | 23,794,258 | $ | 1,098,667 | $ | 2,065,777 | $ | 57,010 | $ | 27,015,712 | ||||||||||
| North and South America sales (excluding U.S.) | 6,182,952 | 3,859 | — | 193,934 | 6,380,745 | |||||||||||||||
| Other international sales | 1,032,508 | 43,473 | — | 21,400 | 1,097,381 | |||||||||||||||
| Total | $ | 31,009,718 | $ | 1,145,999 | $ | 2,065,777 | $ | 272,344 | $ | 34,493,838 | ||||||||||
Income taxes
The Tax Cuts and Job Act ("the Act") was enacted on December 22, 2017, and the U.S. federal corporate tax rate was reduced from 35% to 21%. U.S. generally accepted accounting principles require companies to account for the effects of changes in income tax rates and laws in the period the change is enacted. Financial results, including provisional amounts, have been calculated for the income tax effects of the change. The U.S. Securities and Exchange Commission issued Staff Accounting Bulletin 118 (SAB 118) allowing companies to use provisional estimates to record the effects of the Act. SAB 118, as codified by Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) 2018-05 “Income Taxes (Topic 740): Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 118 (SEC Update),” allows companies to complete accounting for these effects no later than one year from the enactment date of the Act. During 2018, the Company completed its analysis of the provisional estimates made to record the effects of the Act. On January 14, 2019, the IRS issued a statement saying that alternative minimum tax (“AMT”) refunds for taxable years beginning after December 31, 2017 will not be subject to sequestration. Prior to this statement from the IRS, refundable AMT credits under Section 53(e) were subject to sequestration, as required by the Balanced Budget and Emergency Deficit Control Act of 1985, as amended. The previously recorded AMT receivable was reduced in anticipation of sequestration. Based upon this development, the Company recorded an additional tax benefit of approximately $13 thousand for the year ended December 31, 2018 to reflect the full amount of refundable AMT credits.
The Company evaluates tax positions taken or expected to be taken in a tax return for recognition in the financial statements based on whether it is “more-likely-than-not” that a tax position will be sustained based upon the technical merits of the position. Measurement of the tax position is based upon the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement.
The Company provides for deferred income taxes through utilizing an asset and liability approach for financial accounting and reporting based on the tax effects of differences between the financial statement and tax bases of assets and liabilities, based on enacted rates expected to be in effect when such differences reverse in future periods. Deferred tax assets are periodically reviewed for realizability. The Company has established a valuation allowance for its net deferred tax asset as future taxable income cannot be reasonably assured. Penalties and interest related to income taxes are classified as General and administrative expense and Interest expense, respectively, in the Statements of Operations.
Earnings per share
The Company computes basic earnings per share (“EPS”) by dividing net earnings or loss for the period (adjusted for any cumulative dividends for the period) by the weighted average number of common shares outstanding during the period. Diluted EPS includes the determinants of basic EPS and, in addition, reflects the dilutive effect, if any, of the common stock deliverable pursuant to stock options or common stock issuable upon the conversion of convertible preferred stock. At December 31, 2019, the calculation of diluted EPS excluded 639,300 shares of common stock underlying issued and outstanding stock options, as the exercise prices of the stock options were greater than the average stock prices. The calculation of diluted EPS excluded 1,357,803 and 79,441 shares of Common Stock underlying issued and outstanding stock options at December 31, 2018 and 2017, as their effect was antidilutive. The calculation of diluted EPS also excludes
F-13
the impact of the conversion of convertible preferred stock, as the impact was antidilutive for all periods presented. The potential dilution, if any, is shown on the following schedule:
| Years Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Net income (loss) | $ | 3,148,234 | $ | (1,339,943 | ) | $ | (3,736,038 | ) | ||||
| Preferred dividend requirements | (702,618 | ) | (704,996 | ) | (704,996 | ) | ||||||
| Income (loss) applicable to common shareholders | $ | 2,445,616 | $ | (2,044,939 | ) | $ | (4,441,034 | ) | ||||
| Weighted average common shares outstanding | 32,672,475 | 32,666,454 | 31,958,121 | |||||||||
| Weighted average common and common equivalent shares outstanding - assuming dilution | 32,672,475 | 32,666,454 | 31,958,121 | |||||||||
| Basic earnings (loss) per share | $ | 0.07 | $ | (0.06 | ) | $ | (0.14 | ) | ||||
| Diluted earnings (loss) per share | $ | 0.07 | $ | (0.06 | ) | $ | (0.14 | ) | ||||
Shipping and handling costs
The Company classifies shipping and handling costs as part of Cost of sales in the Statements of Operations.
Self-insured employee benefit costs
Beginning in 2019, the Company self-insures certain health insurance benefits for its employees under certain policy limits. The Company has additional coverage provided by an insurance company for any individual with claims in excess of $60,000 and/or total plan claims in excess of $601,716 for the plan year. A receivable of $445 thousand was recorded as of December 31, 2019 due to claims paid by the Company in 2019 but not yet recouped by the stop-loss policy.
Research and development costs
Research and development costs are expensed as incurred.
Share-based compensation
The Company’s share-based payments are accounted for using the Black-Scholes fair value method. The Company records share-based compensation expense on a straight-line basis over the requisite service period. The Company incurred the following share-based compensation costs:
| Years Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Cost of sales | $ | — | $ | — | $ | 272,811 | ||||||
| Sales and marketing | — | — | 143,255 | |||||||||
| Research and development | — | — | 45,174 | |||||||||
| General and administrative | — | — | 210,983 | |||||||||
| $ | — | $ | — | $ | 672,223 | |||||||
Options awarded to employees in 2016 were amortized over twelve months. The Company amortized four months’ expense for options granted in September 2016 and amortized the remainder in 2017.
Insurance Proceeds
Receipts from insurance, up to the amount of any loss recognized by the Company, are considered recoveries. Any such recoveries are recorded when they are received. Insurance proceeds are not recognized as a component of income (loss) from operations until all repairs are made.
F-14
Recently Adopted Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842)”, as well as several subsequently issued clarifying amendments. Under the ASU, as amended, lessees will be required to recognize the following for all leases (with the exception of short-term leases) at the commencement date: (1) a lease liability, which is a lessee‘s obligation to make lease payments arising from a lease, measured on a discounted basis; and (2) a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term. Under the guidance, lessor accounting is largely unchanged. The lease guidance simplified the accounting for sale and leaseback transactions primarily because lessees must recognize lease assets and lease liabilities. In July 2018, the FASB issued ASU 2018-10, “Codification Improvements to Topic 842, Leases”. This amendment clarifies Topic 842 and corrected unintended application of guidance and is effective concurrent with Topic 842 or upon issuance if Topic 842 was early adopted. In August 2018, the FASB issued ASU 2018-11, “Leases (Topic 842): Targeted Improvements”. This amendment provides additional transition options allowing entities to recognize a cumulative effect adjustment to the opening balance of retained earnings in the period of adoption rather than the earliest period presented and provides a practical expedient to lessors to elect, by class of underlying assets, to account for non-lease and lease components as a single arrangement. The Company adopted the provisions of ASU 2018-11 through a cumulative effect adjustment. Topic 842, and its subsequent amendments, was effective for the Company’s quarter ended March 31, 2019. The Company has completed evaluating the various accounting policy elections associated with this ASU, as amended, including transition methods and practical expedients, identifying contracts for evaluation, and reviewing contracts to determine if they contain leases. The Company completed evaluating the timing and impact of adopting ASU 2016-02, as amended, and recorded lease assets and liabilities of $163,007 on its Balance Sheets, with no impact to its accumulated deficit.
The following table summarizes the impact of the adoption of ASU 2016-02 to the previously reported results:
| Balance Sheet as of December 31, 2018 | As Previously Reported | New Lease Standard Adjustment | As Restated | |||||||||
| Other assets | $ | 2,849 | $ | 163,007 | $ | 165,856 | ||||||
| Other current liabilities | $ | 1,387,287 | $ | 80,648 | $ | 1,467,935 | ||||||
| Other long-term liabilities | $ | — | $ | 82,359 | $ | 82,359 | ||||||
Recently Issued Pronouncements
In June 2016, the FASB issued Accounting Standards Update 2016-13, “Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” as well as subsequent clarifying amendments. Among other things, these amendments require the measurement of all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. Many of the loss estimation techniques applied today will still be permitted, although the inputs to those techniques will change to reflect the full amount of expected credit losses. This ASU is effective for the Company’s quarter ending March 31, 2020 with early application permitted. The Company has considered the potential impact from adoption of ASU 2016-13, as well as the Targeted Transition Relief as provided by ASU 2019-05, “Financial Instruments – Credit Losses (Topic 326) – Targeted Transition Relief.” The Company expects that the adoption of ASU 2016-13, along with the provisions of ASU 2019-05, will not have a material impact on the Company’s financial statements, but may require expanded disclosure.
In August 2018, the FASB issued ASU 2018-15, “Intangibles—Goodwill and Other—Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That is a Service Contract (a Consensus of the FASB Emerging Issues Task Force)”. This amendment requires that implemented costs incurred in a hosting arrangement that is a service contract should be accounted for in accordance with ASC 350-40 Internal-Use Software. Accordingly, costs incurred during the preliminary project and post-implementation stages are expensed and costs associated with the application development phase are capitalized. The amendment also requires that capitalized costs be amortized over the term of the hosting arrangement and that capitalized costs should be evaluated for impairment. The
F-15
amendment is effective for annual periods beginning after December 15, 2019 and interim periods within those annual periods. The Company has completed its assessment of the standard and does not anticipate a material impact on its financial statements or disclosures.
In December 2019, the FASB issued ASU 2019-12, Income Taxes: Simplifying the Accounting for Income Taxes. The new standard intended to simplify the accounting for income taxes by eliminating certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. The new guidance also simplifies aspects of the accounting for franchise taxes and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. The standard is effective for annual periods beginning after December 15, 2020 and interim periods within the annual period, with early adoption permitted. Adoption of the standard requires certain changes to primarily be made prospectively, with some changes to be made retrospectively. The Company is currently evaluating the impact adoption of ASU 2019-12 will have on its financial statements.
| 3. | INVENTORIES |
Inventories consist of the following:
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Raw materials | $ | 1,254,313 | $ | 1,399,543 | ||||
| Finished goods | 6,493,487 | 6,442,759 | ||||||
| 7,747,800 | 7,842,302 | |||||||
| Inventory reserve | (297,208 | ) | (297,208 | ) | ||||
| $ | 7,450,592 | $ | 7,545,094 | |||||
| 4. | FAIR VALUE OF FINANCIAL INSTRUMENTS |
ASC 820, “Fair Value Measurements”, defines fair value, establishes a framework for measuring fair value and requires additional disclosures regarding certain fair value measurements. ASC 820 establishes a three-tier hierarchy for measuring fair value, as follows:
| · | Level 1 – quoted market prices in active markets for identical assets and liabilities |
| · | Level 2 – inputs other than quoted prices that are directly or indirectly observable |
| · | Level 3 - unobservable inputs where there is little or no market activity |
The following tables summarize the values of assets designated as Investments in debt and equity securities:
| December 31, 2019 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Mutual funds and exchange traded funds | $ | 6,708,746 | $ | — | $ | — | $ | 6,708,746 | ||||||||
| Certificates of deposit | — | 1,062,914 | — | 1,062,914 | ||||||||||||
| $ | 6,708,746 | $ | 1,062,914 | $ | — | $ | 7,771,660 | |||||||||
| December 31, 2018 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| US Treasury Notes | $ | — | $ | 1,411,156 | $ | — | $ | 1,411,156 | ||||||||
| Certificates of deposit | — | 1,575,000 | — | 1,575,000 | ||||||||||||
| $ | — | $ | 2,986,156 | $ | — | $ | 2,986,156 | |||||||||
The Company holds high-grade exchange-traded and closed-end funds (ETFs), mutual funds, and debt securities as investments. These assets are readily marketable and are carried at fair value as of the date of the Balance Sheets. The Company intends to hold these assets for possible future operating requirements.
F-16
The following table summarizes gross unrealized gains and losses from Investments in debt and equity securities:
| December 31, 2019 | ||||||||||||||||
| Gross Unrealized | Aggregate | |||||||||||||||
| Cost | Gains | Losses | Fair Value | |||||||||||||
| Mutual funds and exchange traded funds | $ | 6,592,345 | $ | 116,401 | $ | — | $ | 6,708,746 | ||||||||
| Certificates of deposit | 1,050,000 | 12,914 | — | 1,062,914 | ||||||||||||
| $ | 7,642,345 | $ | 129,315 | — | $ | 7,771,660 | ||||||||||
| December 31, 2018 | ||||||||||||||||
| Gross Unrealized | Aggregate | |||||||||||||||
| Cost | Gains | Losses | Fair Value | |||||||||||||
| US Treasury Notes | $ | 1,411,156 | $ | — | $ | — | $ | 1,411,156 | ||||||||
| Certificates of deposit | 1,575,000 | — | — | 1,575,000 | ||||||||||||
| $ | 2,986,156 | $ | — | $ | — | $ | 2,986,156 | |||||||||
| 5. | PROPERTY, PLANT, AND EQUIPMENT |
Property, plant, and equipment consist of the following:
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Land | $ | 261,893 | $ | 261,893 | ||||
| Buildings and building improvements | 11,566,115 | 11,566,115 | ||||||
| Production equipment | 19,903,236 | 19,948,303 | ||||||
| Office furniture and equipment | 3,527,577 | 3,540,846 | ||||||
| Construction in progress | 765,176 | 202,109 | ||||||
| 36,023,997 | 35,519,266 | |||||||
| Accumulated depreciation | (25,391,940 | ) | (24,667,519 | ) | ||||
| $ | 10,632,057 | $ | 10,851,747 | |||||
Depreciation expense for the years ended December 31, 2019, 2018, and 2017 was $851,673; $883,610; and $830,715, respectively.
| 6. | LICENSE AGREEMENT |
In 1995, the Company entered into a license agreement with the Chief Executive Officer of the Company, Thomas J. Shaw, for the exclusive right to manufacture, market, and distribute products utilizing automated retraction technology, which agreement has been amended twice. This technology is the subject of various patents and patent applications owned by Mr. Shaw. The initial licensing fee of $500,000 was amortized over 17 years. The license agreement also provides for quarterly payments of a 5% royalty fee on gross sales. Additionally, if the Company sublicenses the technology and the sublicensee’s customers are not known to the Company, then Mr. Shaw shall be entitled to receive from the Company fifty percent (50%) of the royalties actually paid to the Company by such sublicensee. The royalty fee expense is recognized in the period in which it is earned. Royalty fees of $3,449,822; $2,944,102; and $2,864,188 are included in Cost of sales for the years ended December 31, 2019, 2018, and 2017, respectively. Royalties payable under this agreement aggregated $921,445 and $769,324 at December 31, 2019, and 2018, respectively. Gross sales upon which royalties are based were $67,529,783; $58,882,042; and $57,283,780 for 2019, 2018, and 2017, respectively.
| 7. | OTHER ACCRUED LIABILITIES |
Other accrued liabilities consist of the following:
F-17
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Prepayments from customers | $ | 998,601 | $ | 860,926 | ||||
| Accrued property taxes | — | 170,568 | ||||||
| Accrued professional fees | 263,757 | 294,903 | ||||||
| Other accrued expenses | 124,791 | 141,538 | ||||||
| Total | $ | 1,387,149 | $ | 1,467,935 | ||||
| 8. | LONG-TERM DEBT |
Long-term debt consists of the following:
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Loan from American First National Bank. Maturity date is April 10, 2028. The loan, in the original amount of $4,209,608, provided funding for the expansion of the warehouse, additional office space, and a new Controlled Environment. The loan is secured by the Company’s land and buildings. The interest rate is equal to prime rate plus 0.25%. The interest rate was 5.0% at December 31, 2019. | $ | 2,638,994 | $ | 2,878,980 | ||||
| Note payable to Deutsche Leasing USA, Inc. The interest rate was 4.25%. The original amount of the note was $525,017 with a 36-month maturity ending in November 2019. Beginning December 2016, the loan became payable in equal installments of principal and interest of approximately $15,500. Collateralized by molding machines and ancillary equipment. | — | 167,028 | ||||||
| 2,638,994 | 3,046,008 | |||||||
| Less: current portion | (260,939 | ) | (406,361 | ) | ||||
| $ | 2,378,055 | $ | 2,639,647 | |||||
The fair value of long-term liabilities, based on Management’s estimates, approximates their reported values.
The aggregate maturities of long-term debt as of December 31, 2019, are as follows:
| 2020 | 260,939 | |
| 2021 | 274,858 | |
| 2022 | 289,120 | |
| 2023 | 304,122 | |
| 2024 | 319,688 | |
| Thereafter | 1,190,267 | |
| $ | 2,638,994 |
| 9. | COMMITMENTS AND CONTINGENCIES |
On November 7, 2019, the Company filed a lawsuit in the 44th District Court of Dallas County, Texas (No. DC-19-17946) against Locke Lord, LLP and Roy Hardin in connection with their legal representation of the Company in its previous litigation against Becton, Dickinson and Company (“BD”). The Company alleges that the defendants breached their fiduciary duties, committed malpractice, and were negligent in their representation of the Company. The Company seeks actual and exemplary damages, disgorgement, costs, and interest. The defendants have filed a motion to dismiss and the Court has scheduled a hearing on such motion on April 24, 2020. Trial is currently scheduled for May 24, 2021.
| 10. | INCOME TAXES |
The provision (benefit) for income taxes consists of the following:
F-18
| For the Years Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Current tax provision (benefit) | ||||||||||||
| Federal | $ | — | $ | (13,318 | ) | $ | — | |||||
| State | 7,875 | — | 848 | |||||||||
| Total current provision (benefit) | 7,875 | (13,318 | ) | 848 | ||||||||
| Deferred tax provision (benefit) | ||||||||||||
| Federal | — | — | (188,456 | ) | ||||||||
| State | — | — | — | |||||||||
| Total deferred tax provision (benefit) | — | — | (188,456 | ) | ||||||||
| Total income tax provision (benefit) | $ | 7,875 | $ | (13,318 | ) | $ | (187,608 | ) | ||||
The Company has $23.3 million in tax benefits attributable to net operating losses for federal tax purposes. $21.5 million are net operating losses which were generated before January 1, 2018 and begin to expire in 2028 for federal tax purposes. $1.8 million of net operating losses were generated after January 1, 2018 and have an indefinite carryover period. The Company has state net operating losses of $24.8 million which will begin to expire in 2021. The Company also has credits for alternative minimum taxes (“AMT”) paid of $100 thousand as of December 31, 2019. The alternative minimum tax was repealed with the enactment of the Act. AMT credits carried over may be used to offset regular tax liability for any tax year. Any unused credits are 50% refundable for tax years 2018-2020, and 100% refundable for tax years beginning 2021. The Company has recorded the AMT credit as a tax receivable on its financial statements rather than as a deferred tax asset, as this amount is a refundable credit.
Deferred taxes are provided for those items reported in different periods for income tax and financial reporting purposes. The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and liabilities are presented below:
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Deferred tax assets | ||||||||
| Net operating loss carry forwards | $ | 5,748,724 | $ | 6,668,238 | ||||
| Accrued expenses and reserves | 573,382 | 436,627 | ||||||
| Employee stock option expense | 75,591 | 76,150 | ||||||
| Nonemployee stock option expense | 8,207 | 8,268 | ||||||
| Inventory | 110,455 | 132,114 | ||||||
| Impairment | 111,178 | 112,000 | ||||||
| Unrealized Gain/Loss | 30,434 | — | ||||||
| Deferred tax assets | 6,657,971 | 7,433,397 | ||||||
| Deferred tax liabilities | ||||||||
| Property and equipment | (1,628,133 | ) | (1,281,999 | ) | ||||
| Deferred tax liabilities | (1,628,133 | ) | (1,281,999 | ) | ||||
| Net deferred assets | 5,029,838 | 6,151,398 | ||||||
| Valuation allowance | (5,029,838 | ) | (6,151,398 | ) | ||||
| Net deferred tax assets | $ | — | $ | — | ||||
Utilization of the net operating loss carry forwards and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions.
Deferred income tax calculations reflect the effects of temporary differences between the carrying amounts of assets and liabilities and their tax bases, as well as from net operating loss carry forwards, and are stated at the U.S. tax rate of 21% beginning in 2018. Net operating losses incurred after December 31, 2017 can only offset 80% of taxable income. However, these net operating losses may be carried forward indefinitely instead of limited to twenty years under previous tax law. Carrybacks of these losses are no longer permitted. Deferred income tax assets represent amounts available to reduce income taxes payable on taxable income in future years. The Company has fully reserved these future tax deductions.
F-19
The valuation allowance decreased by $1,121,560 for 2019 and increased by $325,444 for 2018.
A reconciliation of income taxes based on the federal statutory rate and the effective income tax rate is summarized as follows:
| December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Income tax at the federal statutory rate | 21.0 | % | 21.0 | % | 35.0 | % | ||||||
| State tax, net of federal tax | 2.0 | 3.5 | 2.9 | |||||||||
| Change in valuation allowance | (35.6 | ) | (24.3 | ) | (85.9 | ) | ||||||
| Permanent differences | — | (0.3 | ) | 5.7 | ||||||||
| Return-to-provision and other | 12.9 | — | (37.6 | ) | ||||||||
| Tax Reform and Jobs Act tax rate change | — | 0.1 | (81.1 | ) | ||||||||
| Incentive stock options | — | — | (6.0 | ) | ||||||||
| Effective tax rate | 0.3 | % | — | % | 4.8 | % | ||||||
The Company files income tax returns in the U.S. federal jurisdiction and in various state and local jurisdictions. The Company's federal income tax returns for all tax years ended on or after December 31, 2016, remain subject to examination by the Internal Revenue Service. The Company's state and local income tax returns are subject to examination by the respective state and local authorities over various statutes of limitations, most ranging from three to five years from the date of filing.
| 11. | DIVIDENDS |
The Board declared and the Company paid dividends to Series I and Series II Class B Preferred Shareholders in the following amounts: $12,313 and $42,800, respectively, on January 6, 2017, April 24, 2017, July 20, 2017, October 20, 2017, January 19, 2018, April 24, 2018, July 20, 2018, October 23, 2018, January 18, 2019, and April 22, 2019. The Board declared and the Company paid dividends to Series I and Series II Class B Preferred Shareholders in the following amounts: $12,000 and $42,800, respectively, on July 19, 2019, October 21, 2019, and January 22, 2020.
| 12. | STOCKHOLDERS’ EQUITY |
Preferred Stock
The Company is authorized to issue 5,000,000 shares of Preferred Stock Class A with a par value of One Dollar ($1.00) per share; 5,000,000 shares of Preferred Stock Class B with a par value of One Dollar ($1.00) per share; and 5,000,000 shares of Preferred Stock Class C with a par value of One Dollar ($1.00) per share.
The Company has one class of Preferred Stock outstanding: Class B Convertible Preferred Stock (“Class B Stock”). The Class B Stock has five series: Series I, Series II, Series III, Series IV, and Series V.
The Class B Stock has been allocated among Series I, II, III, IV, and V in the amounts of 96,000; 171,200; 129,245; 342,500; and 34,000 shares, respectively as of December 31, 2019. The remaining 4,227,055 authorized shares had not been assigned a series. Please see “Subsequent Events” for recent information regarding the Preferred Stock.
Series I Class B Stock
There were 96,000 and 98,500 shares of $1 par value Series I Class B Stock outstanding at December 31, 2019 and 2018, respectively. Holders of Series I Class B Stock are entitled to receive a cumulative annual dividend of $0.50 per share, payable quarterly if declared by the Board of Directors. The Company paid dividends of $48,312 in 2019 and $49,250 in 2018 and 2017. At December 31, 2019, no dividends were in arrears.
Series I Class B Stock is redeemable at the option of the Company at a price of $7.50 per share, plus all unpaid dividends. Each share of Series I Class B Stock may, at the option of the stockholder, be converted to
F-20
one share of Common Stock. 2,500 shares of Series I Class B Stock were converted into Common Stock in 2019. No shares of Series I Class B Stock were converted into Common Stock in 2018. In the event of voluntary or involuntary dissolution, liquidation, or winding up of the Company, holders of Series I Class B Stock then outstanding are entitled to $6.25 per share, plus all unpaid dividends prior to any distributions to holders of Series II Class B Stock, Series III Class B Stock, Series IV Class B Stock, Series V Class B Stock, or Common Stock.
Series II Class B Stock
There were 171,200 shares of $1 par value Series II Class B Stock outstanding at December 31, 2019 and 2018. Holders of Series II Class B Stock are entitled to receive a cumulative annual dividend of $1.00 per share, payable quarterly if declared by the Board of Directors. Holders of Series II Class B Stock generally have no voting rights until dividends are in arrears and unpaid for twelve consecutive quarters. In such case, the holders of Series II Class B Stock have the right to elect one-third of the Board of Directors of the Company. The Company paid dividends of $171,200 in each of 2019, 2018, and 2017. At December 31, 2019, no dividends were in arrears.
Series II Class B Stock is redeemable at the option of the Company at a price of $15.00 per share plus all unpaid dividends. Each share of Series II Class B Stock may, at the option of the stockholder, be converted to one share of Common Stock. No shares were converted in 2019 or 2018. In the event of voluntary or involuntary dissolution, liquidation, or winding up of the Company, holders of Series II Class B Stock then outstanding are entitled to $12.50 per share, plus all unpaid dividends, after distribution obligations to holders of Series I Class B Stock have been satisfied and prior to any distributions to holders of Series III Class B Stock, Series IV Class B Stock, Series V Class B Stock, or Common Stock.
Series III Class B Stock
There were 129,245 shares of $1 par value Series III Class B Stock outstanding at December 31, 2019 and 2018. Holders of Series III Class B Stock are entitled to receive a cumulative annual dividend of $1.00 per share, payable quarterly if declared by the Board of Directors. At December 31, 2019, approximately $4,404,000 of dividends which have not been declared were in arrears. Please see “Subsequent Events” for recent information regarding Series III Class B Stock.
Series III Class B Stock is redeemable at the option of the Company at a price of $15.00 per share, plus all unpaid dividends. Each share of Series III Class B Stock may, at the option of the stockholder, be converted to one share of Common Stock. No shares were converted in 2019 or 2018. In the event of voluntary or involuntary dissolution, liquidation, or winding up of the Company, holders of Series III Class B Stock then outstanding are entitled to $12.50 per share, plus all unpaid dividends, after distribution obligations to Series I Class B Stock and Series II Class B Stock have been satisfied and prior to any distributions to holders of Series IV Class B Stock, Series V Class B Stock, or Common Stock.
Series IV Class B Stock
There were 342,500 shares of $1 par value Series IV Class B Stock outstanding at December 31, 2019 and 2018. Holders of Series IV Class B Stock are entitled to receive a cumulative annual dividend of $1.00 per share, payable quarterly, if declared by the Board of Directors. At December 31, 2019, approximately $6,826,000 of dividends which have not been declared were in arrears. Please see “Subsequent Events” for recent information regarding Series IV Class B Stock.
Series IV Class B Stock is redeemable at the option of the Company at a price of $11.00 per share plus all unpaid dividends. Each share of Series IV Class B Stock may, at the option of the stockholder any time, be converted into one share of Common Stock. No shares of Series IV Class B Stock were converted into Common Stock in 2019 or 2018. In the event of voluntary or involuntary liquidation, dissolution, or winding up of the Company, holders of Series IV Class B Stock then outstanding are entitled to receive liquidating distributions of $11.00 per share, plus all unpaid dividends after distribution obligations to Series I Class B
F-21
Stock, Series II Class B Stock, and Series III Class B Stock have been satisfied and prior to any distribution to holders of Series V Class B Stock or Common Stock.
Series V Class B Stock
There were 34,000 and 40,000 shares of $1 par value Series V Class B Stock outstanding at December 31, 2019 and 2018, respectively. Holders of Series V Class B Stock are entitled to receive a cumulative annual dividend of $0.32 per share, payable quarterly, if declared by the Board of Directors. At December 31, 2019, approximately $1,020,000 of dividends which have not been declared were in arrears.
Series V Class B Stock is redeemable at the option of the Company at a price of $4.40 per share plus all unpaid dividends. Each share of Series V Class B Stock may, at the option of the stockholder any time be converted into Common Stock. 6,000 shares of Series V Class B Stock were converted into Common Stock in 2019. No shares of Series V Class B Stock were converted into Common Stock in 2018. In the event of voluntary or involuntary liquidation, dissolution, or winding up of the Company, holders of Series V Class B Stock then outstanding are entitled to receive liquidating distributions of $4.40 per share, plus all unpaid dividends after distribution obligations to Series I Class B Stock, Series II Class B Stock, Series III Class B Stock, and Series IV Class B Stock have been satisfied and prior to any distribution to the holders of the Common Stock.
Common stock
The Company is authorized to issue 100,000,000 shares of no par value Common Stock, of which 32,674,954 and 32,666,454 shares were outstanding at December 31, 2019 and 2018, respectively. Additionally, as of December 31, 2019, a total of 1,412,245 shares of Common Stock were issuable upon the conversion of Preferred Stock and the exercise of stock options.
| 13. | RELATED PARTY TRANSACTIONS |
The Company has a license agreement with the Chief Executive Officer of the Company. See Note 6.
| 14. | STOCK OPTIONS |
Stock options
Options for the purchase of 3,649,508 shares of Common Stock have been issued under the 2008 Stock Option Plan. Options for the purchase of 639,300 shares under the 2008 Stock Option Plan were outstanding as of December 31, 2019. No shares are available for future issuance under the 2008 Stock Option Plan, which expired July 25, 2018.
The Compensation and Benefits Committee administered the Company’s stock option plan prior to its termination.
Director, officer, and employee options
A summary of Director, officer, and employee options granted and outstanding under the 2008 Stock Option Plan is presented below:
F-22
| Years Ended December 31, | ||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||
| Shares | Weighted Average Exercise Price | Shares | Weighted Average Exercise Price | Shares | Weighted Average Exercise Price | |||||||||||||||||||
| Outstanding at beginning of period | 1,300,303 | $ | 1.57 | 1,805,519 | $ | 1.51 | 1,817,919 | $ | 1.52 | |||||||||||||||
| Granted | — | $ | — | — | $ | — | — | $ | — | |||||||||||||||
| Exercised | — | $ | — | — | $ | — | — | $ | — | |||||||||||||||
| Forfeited | (661,003 | ) | $ | (1.05 | ) | (505,216 | ) | $ | (1.36 | ) | (12,400 | ) | $ | (2.75 | ) | |||||||||
| Outstanding at end of period | 639,300 | $ | 2.12 | 1,300,303 | $ | 1.57 | 1,805,519 | $ | 1.51 | |||||||||||||||
| Exercisable at end of period | 639,300 | $ | 2.12 | 1,300,303 | $ | 1.57 | 1,803,119 | $ | 1.51 | |||||||||||||||
No options were issued in 2019, 2018, or 2017 to employees or non-employee directors.
The following table summarizes information about Director, officer, and employee options outstanding under the stock option plan at December 31, 2019:
| Exercise Prices | Shares Outstanding | Weighted Average Remaining Contractual Life | Shares Exercisable | |||||||||||
| $ | 1.05 | 200,000 | 6.99 | 200,000 | ||||||||||
| $ | 1.46 | 50,000 | 3.37 | 50,000 | ||||||||||
| $ | 2.75 | 389,300 | 6.70 | 389,300 | ||||||||||
Non-employee options
A summary of options outstanding and held by non-employees is as follows:
| Years Ended December 31, | ||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||
| Shares | Weighted Average Exercise Price | Shares | Weighted Average Exercise Price | Shares | Weighted Average Exercise Price | |||||||||||||||||||
| Outstanding at beginning of period | 57,500 | $ | 0.81 | 57,500 | $ | 0.81 | 57,500 | $ | 0.81 | |||||||||||||||
| Granted | — | $ | — | — | $ | — | — | $ | — | |||||||||||||||
| Exercised | — | $ | — | — | $ | — | — | $ | — | |||||||||||||||
| Forfeited | (57,500 | ) | $ | (0.81 | ) | — | $ | — | — | $ | — | |||||||||||||
| Outstanding at end of period | — | $ | — | 57,500 | $ | 0.81 | 57,500 | $ | 0.81 | |||||||||||||||
| Exercisable at end of period | — | $ | — | 57,500 | $ | 0.81 | 57,500 | $ | 0.81 | |||||||||||||||
In 2017, the Company recognized stock-based compensation expense of $672 thousand. The Company recorded no stock-based compensation expense in 2018 or 2019. At December 31, 2019, there were 250,000 stock options with exercise prices lower than the closing market price. The intrinsic value of these options at December 31, 2019 was $92,000.
Options Pricing Models – Assumptions
F-23
The expected life is based on the Company’s historical experience with option exercise trends. The assumptions for expected volatility are based on a calculation of volatility over the five-years preceding the grant date. Risk-free interest rates are set using grant-date U.S. Treasury yield curves. In its calculations, the Company assumed no dividends. The Company elected a policy to account for forfeitures as they occur, rather than on an estimated basis.
| 15. | 401(k) PLAN |
The Company implemented an employee savings and retirement plan (the “401(k) Plan”) in 2005 that is intended to be a tax-qualified plan covering substantially all employees. The 401(k) Plan is available to all employees on the first day of the month after 90 days of service. Under the terms of the 401(k) Plan, employees may elect to contribute up to 88% of their compensation, or the statutory prescribed limit, if less. The Company may, at its discretion, match employee contributions. For 2017, 2018, and 2019, the Company matched each participant’s elective deferrals up to 2% of the participant’s compensation for the pay period. The total match was $145,474, $145,146, and $117,917 in 2017, 2018, and 2019, respectively.
| 16. | BUSINESS SEGMENT |
The following is a summary of the Company’s sales and long-lived assets by geography:
| 2019 | 2018 | 2017 | ||||||||||
| U.S. sales | $ | 31,897,924 | $ | 28,646,574 | $ | 27,015,712 | ||||||
| North and South America sales (excluding U.S.) | 8,248,990 | 3,597,444 | 6,380,745 | |||||||||
| Other international sales | 1,650,265 | 1,030,684 | 1,097,381 | |||||||||
| Total sales | $ | 41,797,179 | $ | 33,274,702 | $ | 34,493,838 | ||||||
| Long-lived assets | 2019 | 2018 | ||||||||||
| U.S. | $ | 10,542,688 | $ | 10,738,253 | ||||||||
| International | $ | 89,369 | $ | 113,494 | ||||||||
The Company does not operate in separate reportable segments. The Company has minimal long-lived assets in foreign countries. Shipments to international customers generally require a prepayment either by wire transfer or an irrevocable confirmed letter of credit. The Company does extend credit to international customers on some occasions depending upon certain criteria, including, but not limited to, the credit worthiness of the customer, the stability of the country, banking restrictions, and the size of the order. All transactions are in U.S. currency.
| 17. | STORM DAMAGE AND INSURANCE PROCEEDS |
On March 26, 2017, a hail storm passed through Little Elm, Texas, resulting in damage to the Company’s two buildings. During April 2017, the Company performed an inspection of its facilities and determined that possible roof damage had been sustained. In late April 2017, the Company’s insurance carrier inspected the two buildings and confirmed that damage occurred from the hail storm. This damage was principally to the roofs of the buildings but also many of the HVAC units and a wall alongside one of the buildings were also damaged.
The Company’s insurance carrier assessed damages of $1,009,960 and the Company’s deductible was $5,000. The Company received these funds from its carrier in the second quarter of 2017. Repairs commenced during the third quarter of 2017. All repairs were completed in the fourth quarter of 2018.
During 2017, the Company incurred and recognized $538,667 in repairs due to the storm damage. Repair expense during 2018 was $203,289. This repair expense was offset by the insurance proceeds, resulting in no impact to the Statements of Operations. The remaining insurance proceeds of $261 thousand were recognized as income in the fourth quarter of 2018.
F-24
| 18. | LEASES |
The Company has operating leases for a corporate office and equipment. The leases have a remaining lease term of one year. The Company currently has no finance leases. The right-of-use (“ROU”) asset is determined based on the lease liability adjusted for lease incentives received. Lease expense is recognized on a straight-line basis over the lease term. The leases may include various expenses incidental to the use of the property, such as common area maintenance, property taxes and insurance. These costs are separate from the minimum rent payment and are not considered in the determination of the lease liability and ROU asset. The Company has not noted any material instances in its leases where these costs were combined with the minimum rent payment and has therefore elected the policy to not separate lease from non-lease components if they are combined with the minimum rent payment. The option periods are not included in the determination of the lease liability and right-of-use asset as the Company is not reasonably certain if it will extend at the time of lease commencement.
The operating lease cost component of the lease expense was $80,648 and $79,331 for the years ended December 31, 2019 and 2018, respectively. The cash paid for amounts included in the measurement of lease liabilities as a component of cash flows related to leases was $80,648 and $79,331 for the years ended December 31, 2019 and 2018, respectively.
Assets and liabilities associated with these leases included in the Balance Sheets are as follows:
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| OPERATING LEASES | ||||||||
| Other assets | $ | 82,359 | $ | 163,007 | ||||
| Other accrued liabilities | $ | 82,359 | $ | 80,648 | ||||
| Other long-term liabilities | — | 82,359 | ||||||
| Total operating lease liabilities | $ | 82,359 | $ | 163,007 | ||||
The weighted average remaining lease term is 1.0 year and the weighted average discount rate is 4.0% at December 31, 2019.
Future minimum payments under non-cancelable operating leases and financing leases consist of the following at December 31, 2019:
| Year ending December 31,2020 | $ | 84,155 | ||
| Less imputed interest | (1,796 | ) | ||
| Total | $ | 82,359 |
| 19. | HELD-TO-MATURITY SECURITIES – CORRECTION OF ERRORS |
The Balance Sheet as of December 31, 2018 has been revised to reflect a correction of an error to the classification of investments previously recorded as Held-to-Maturity. During the quarter ended June 30, 2019, the Company determined that, during 2018, it had incorrectly classified certain investments as Held-to-Maturity that should have been classified as Available-for-Sale. The Company views the correction of the classification error to be immaterial to previously filed financial statements. Nonetheless, the Company has revised the presentation of the Balance Sheet at December 31, 2018 to reflect the reclassification of all previously recorded Held-to-Maturity investments as Available-for-Sale. The effect on the Balance Sheet as of December 31, 2018 is to reclassify $2,986,156 from Held-to-Maturity securities to Investments in debt and equity securities, at fair value. In addition, Total current assets at December 31, 2018 were increased by $1,989,923 as a result of this reclassification. There was no impact to the Statement of Operations for 2018 as a result. The Company will continue to revise its previously-issued financial statements on a prospective basis for this classification error as well as the related disclosures of the fair value of financial instruments.
F-25
| 20. | SUBSEQUENT EVENTS |
Effective January 13, 2020, the Company agreed with two preferred stockholders to purchase outstanding Class B Convertible Preferred Stock (the “Preferred Stock”) for cash and Common Stock. Such preferred stockholders tendered to the Company a total of 2,500 shares of Series III Preferred Stock and 5,000 shares of Series IV Preferred Stock. A total of $75,000 and 7,500 shares of Common Stock were issued as consideration therefor. In accordance with the terms of the agreements, the preferred stockholders agreed to waive all unpaid dividends in arrears associated with their Preferred Stock, which resulted in a waiver of a total of $149,795 in unpaid dividends in arrears.
Subsequent to December 31, 2019, the World Health Organization (WHO) declared the novel coronavirus outbreak a public health emergency. On March 11th, 2020, the WHO Director-General declared that COVID-19 be characterized as a pandemic. Since that time, the situation surrounding the pandemic and the effects on the world economy, public health across the globe and U.S. businesses has been, and continues to be, a fluid situation. The Company is continuing to monitor the situation, including our outsourcing of products from China, the availability of materials and other resources used in production in the United States, the availability of necessary transportation of products from our vendors and to our customers, available labor force needed to continue operations, and the ability to meet the demand requirements of our existing customers. These factors, and numerous other factors which are not yet known, present challenges and uncertainties which the Company cannot quantify at this time. It is possible that these factors may have a materially adverse impact to the Company.
| SELECTED QUARTERLY FINANCIAL DATA - UNAUDITED |
The selected quarterly financial data for the periods ended December 31, 2019, and 2018, have been derived from the Company's unaudited financial statements and include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the results of the interim periods. Certain quarterly amounts may differ from full year totals due to rounding.
| (In thousands, except for per share and outstanding stock amounts) 2019 | ||||||||||||||||
| Quarter 1 | Quarter 2 | Quarter 3 | Quarter 4 | |||||||||||||
| Sales, net | $ | 7,932 | $ | 9,596 | $ | 11,640 | $ | 12,629 | ||||||||
| Cost of sales | 5,442 | 6,665 | 7,872 | 7,680 | ||||||||||||
| Gross profit | 2,490 | 2.931 | 3,768 | 4,949 | ||||||||||||
| Total operating expenses | 2,665 | 2,602 | 2,789 | 3,110 | ||||||||||||
| Income (loss) from continuing operations | (175 | ) | 329 | 979 | 1,839 | |||||||||||
| Interest and other income | 91 | 110 | 91 | 59 | ||||||||||||
| Interest expense | (46 | ) | (44 | ) | (41 | ) | (36 | ) | ||||||||
| Provision (benefit) for income taxes | — | 3 | 4 | 1 | ||||||||||||
| Net income (loss) | (130 | ) | 392 | 1,025 | 1,861 | |||||||||||
| Preferred stock dividend requirements | (176 | ) | (175 | ) | (175 | ) | (177 | ) | ||||||||
| Income (loss) applicable to common shareholders | $ | (306 | ) | $ | 217 | $ | 850 | $ | 1,684 | |||||||
| Basic earnings (loss) per share | $ | (0.01 | ) | $ | 0.01 | $ | 0.03 | $ | 0.05 | |||||||
| Diluted earnings (loss) per share | $ | (0.01 | ) | $ | 0.01 | $ | 0.03 | $ | 0.05 | |||||||
| Earnings (loss) per share from continuing operations | $ | (0.01 | ) | $ | 0.01 | $ | 0.03 | $ | 0.05 | |||||||
| Weighted average common shares outstanding - basic | 32,666,454 | 32,674,954 | 32,674,954 | 32,674,954 | ||||||||||||
| Weighted average common shares outstanding - diluted | 32,666,454 | 32,674,954 | 32,674,954 | 32,710,248 | ||||||||||||
| Gross profit margin | 31.4 | % | 30.5 | % | 32.4 | % | 39.2 | % | ||||||||
F-26
| (In thousands, except for per share and outstanding stock amounts) 2018 | ||||||||||||||||
| Quarter 1 | Quarter 2 | Quarter 3 | Quarter 4 | |||||||||||||
| Sales, net | $ | 7,673 | $ | 7,475 | $ | 9,863 | $ | 8,264 | ||||||||
| Cost of sales | 4,813 | 5,407 | 7,067 | 5,766 | ||||||||||||
| Gross profit | 2,860 | 2,068 | 2,796 | 2,498 | ||||||||||||
| Total operating expenses | 3,017 | 3,007 | 2,856 | 2,932 | ||||||||||||
| Income from insurance proceeds | — | — | — | 261 | ||||||||||||
| Loss from continuing operations | (157 | ) | (939 | ) | (60 | ) | (173 | ) | ||||||||
| Interest and other income | 28 | 35 | 39 | 51 | ||||||||||||
| Interest expense | (50 | ) | (44 | ) | (42 | ) | (41 | ) | ||||||||
| Provision (benefit) for income taxes | — | — | — | (13 | ) | |||||||||||
| Net loss | (179 | ) | (948 | ) | (63 | ) | (150 | ) | ||||||||
| Preferred stock dividend requirements | (176 | ) | (176 | ) | (176 | ) | (177 | ) | ||||||||
| Loss applicable to common shareholders | $ | (355 | ) | $ | (1,124 | ) | $ | (239 | ) | $ | (327 | ) | ||||
| Basic loss per share | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.01 | ) | $ | (0.01 | ) | ||||
| Diluted loss per share | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.01 | ) | $ | (0.01 | ) | ||||
| Loss per share from continuing operations | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.01 | ) | $ | (0.01 | ) | ||||
| Weighted average common shares outstanding - basic | 32,666,454 | 32,666,454 | 32,666,454 | 32,666,454 | ||||||||||||
| Weighted average common shares outstanding - diluted | 32,666,454 | 32,666,454 | 32,666,454 | 32,666,454 | ||||||||||||
| Gross profit margin | 37.3 | % | 27.7 | % | 28.3 | % | 30.2 | % | ||||||||
F-27
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
There were no reportable disagreements with accountants on accounting and financial disclosures.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures
Pursuant to Rule 13a-15(b) under the Securities Exchange Act of 1934 (the "Exchange Act"), Management, with the participation of our President, Chairman, and Chief Executive Officer, Thomas J. Shaw (the “CEO”), and our Vice President and Chief Financial Officer, John W. Fort III (the “CFO”), acting in their capacities as our principal executive and financial officers, evaluated the effectiveness of our disclosure controls and procedures, as defined in Rule 13a-15(e) under the Exchange Act. The term disclosure controls and procedures means controls and other procedures that are designed to ensure that information required to be disclosed by us in our periodic reports is: i) recorded, processed, summarized, and reported within the time periods specified in the Securities and Exchange Commission's (the “SEC”) rules and forms; and ii) accumulated and communicated to our Management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Based upon this evaluation, the CEO and CFO concluded that, as of December 31, 2019, our disclosure controls and procedures were effective.
Management's Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over our financial reporting as defined in Rule 13a-15(f) under the Exchange Act. The term internal control over financial reporting means a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our Board of Directors, Management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets; (ii) provide reasonable assurance that our transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of Management and Directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements. Management used the Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission to evaluate the effectiveness of our internal control over financial reporting as required by paragraph (c) of Rule 13a-15 under the Exchange Act. Management, with the participation of our CEO and CFO, concluded that our internal control over financial reporting as of December 31, 2019, was effective. No material weaknesses in our internal control over financial reporting were identified by Management.
Our Management, including the CEO and CFO, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all instances of fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting during the fourth quarter of 2019 or subsequent to December 31, 2019, which has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
15
Item 10. Directors, Executive Officers and Corporate Governance.
The information in the sections “Proposal – The Election of Three Class 2 Directors” and “Corporate Governance” in the 2020 proxy statement is incorporated herein by reference.
Item 11. Executive Compensation.
The information in the section “Compensation” in the 2020 proxy statement is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information in the section “Security Ownership” in the 2020 proxy statement is incorporated herein by reference. See also Item 5 of Part II of this Annual Report for Equity Compensation Plan Information.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information in the section “Corporate Governance” in the 2020 proxy statement is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services.
The information in the section “Accounting Matters” in the 2020 proxy statement is incorporated herein by reference.
Item 15. Exhibits, Financial Statement Schedules.
| Balance at beginning of period | Additions | Deductions | Balance at end of period | |||||||||||||
| Provision for Inventories | ||||||||||||||||
| Fiscal year ended 2017 | $ | 595,523 | $ | — | $ | 584 | $ | 594,939 | ||||||||
| Fiscal year ended 2018 | $ | 594,939 | $ | — | $ | 297,731 | $ | 297,208 | ||||||||
| Fiscal year ended 2019 | $ | 297,208 | $ | — | $ | — | $ | 297,208 | ||||||||
| Provision for Accounts Receivable | ||||||||||||||||
| Fiscal year ended 2017 | $ | 1,731,985 | $ | 24,272 | $ | 1,654,385 | $ | 101,872 | ||||||||
| Fiscal year ended 2018 | $ | 101,872 | $ | 47,793 | $ | — | $ | 149,665 | ||||||||
| Fiscal year ended 2019 | $ | 149,665 | $ | — | $ | 3,283 | $ | 146,382 | ||||||||
| Deferred Tax Valuation | ||||||||||||||||
| Fiscal year ended 2017 | $ | 9,197,333 | $ | — | $ | 3,371,379 | $ | 5,825,954 | ||||||||
| Fiscal year ended 2018 | $ | 5,825,954 | $ | 325,444 | $ | — | $ | 6,151,398 | ||||||||
| Fiscal year ended 2019 | $ | 6,151,398 | $ | — | $ | 1,121,560 | $ | 5,029,838 | ||||||||
16
| Balance at beginning of period | Additions | Deductions | Balance at end of period | |||||||||||||
| Provision for Rebates | (A) | (B) | (C) | |||||||||||||
| Fiscal year ended 2017 | $ | 38,983,285 | $ | 21,738,072 | $ | 55,927,164 | $ | 4,794,193 | ||||||||
| Fiscal year ended 2018 | $ | 4,794,193 | $ | 24,372,111 | $ | 24,579,457 | $ | 4,586,847 | ||||||||
| Fiscal year ended 2019 | $ | 4,586,847 | $ | 24,212,830 | $ | 24,526,108 | $ | 4,273,569 | ||||||||
| (A) | Represents estimated rebates deducted from gross revenues |
| (B) | Represents rebates credited to the distributor and charge offs against the allowance |
| (C) | Includes $3,586,726; $3,896,341; and $4,115,628 in Accounts payable for 2019, 2018, and 2017, respectively. |
17
(3) Exhibits:
The following exhibits are filed herewith or incorporated herein by reference to exhibits previously filed with the SEC.
(b) Exhibits
| * | Incorporated herein by reference to RTI’s Form 10-Q filed on November 15, 2010 | |
| ** | Incorporated herein by reference to RTI’s Form 8-K filed on May 13, 2010 | |
| *** | Incorporated herein by reference to RTI’s Registration Statement on Form 10-SB filed on June 23, 2000 | |
| **** | Incorporated herein by reference to RTI's Form 10-Q filed on November 14, 2008 |
18
| ***** | Incorporated herein by reference to RTI’s Form 10-K filed on March 31, 2009 | |
| † | Incorporated herein by reference to RTI's Form 10-Q filed on November 14, 2012 | |
| †† | Incorporated herein by reference to RTI's Form 10-Q filed on November 14, 2014 | |
| ◦ | Incorporated herein by reference to RTI’s Schedule TO filed on October 17, 2008 | |
| ◦◦ | Incorporated herein by reference to RTI’s Form 8-K filed on February 19, 2010 | |
| ◦◦◦ | Filed herewith | |
| (c) | Excluded Financial Statement Schedules: None |
None.
19
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| RETRACTABLE TECHNOLOGIES, INC. | ||
| (Registrant) | ||
| By: | /s/ Thomas J. Shaw | |
| THOMAS J. SHAW | ||
| CHAIRMAN, PRESIDENT, AND | ||
| CHIEF EXECUTIVE OFFICER | ||
| March 30, 2020 | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| /s/ John W. Fort III | ||
| JOHN W. FORT III | ||
| VICE PRESIDENT, CHIEF FINANCIAL OFFICER, PRINCIPAL ACCOUNTING OFFICER, TREASURER, AND DIRECTOR | ||
| March 30, 2020 | ||
| /s/ Amy Mack | ||
| AMY MACK | ||
| DIRECTOR | ||
| March 30, 2020 | ||
| /s/ Marco Laterza | ||
| MARCO LATERZA | ||
| DIRECTOR | ||
| March 30, 2020 | ||
| /s/ Walter O. Bigby, Jr. | ||
| WALTER O. BIGBY, JR. | ||
| DIRECTOR | ||
| March 30, 2020 | ||
| /s/ Darren E. Findley | ||
| DARREN E. FINDLEY | ||
| DIRECTOR | ||
| March 30, 2020 |
20
