Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - HELIUS MEDICAL TECHNOLOGIES, INC. | hsdt-ex992_6.htm |
| 8-K - 8-K - HELIUS MEDICAL TECHNOLOGIES, INC. | hsdt-8k_20200323.htm |

A Revolution in Mind March 2020 Exhibit 99.1

Legal Disclaimers www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM This presentation contains forward-looking statements, including statements about: uncertainties regarding the FDA regulatory approval process, including whether the results of our clinical trials will be sufficient to support an FDA, CE Mark or TGA approval of the PoNS™ device for marketing or whether the agencies may require that the Company conduct future clinical trials; future economic, competitive, reimbursement and regulatory conditions; new product introductions; ability to commercialize its PoNS Treatment™; demographic trends; the intellectual property landscape; financial market conditions; continued availability of capital and financing, including its ability to continue as a going concern; and future business decisions made by the Company and its competitors. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward looking statements include the risks described in the “Risk Factors” section of Company’s Annual Report on Form 10-K for the period ended December 31, 2019 and the Company’s Quarterly Report on From 10-Q for the period ended September 30, 2019, as well as those set forth from time to time in the Company’s other SEC filings, available at http://www.sec.gov. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Certain data in this presentation was obtained from various external sources. Neither the Company nor its affiliates, advisers or representatives have verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or representatives make any representations as to the accuracy or completeness of that data or commits to update such data after the date of this presentation. Such data involves risks and uncertainties and is subject to change based on various factors. The Company’s first product, known as the Portable Neuromodulation Stimulator (“PoNS"TM), is an authorized class II, non-implantable medical device authorized for sale in Canada. PoNS is intended as a short term treatment (14 weeks) of chronic balance deficit due to mild-to-moderate traumatic brain injury (“mmTBI”) and is to be used in conjunction with physical therapy (“PoNS Treatment"TM) and indicated as a short term treatment (14 weeks) of gait deficit due to mild and moderate symptoms from MS and is to be used in conjunction with physical therapy. It is an investigational medical device in the United States, the European Union (“EU”), and Australia (“AUS”), and it is currently under review for clearance by the AUS Therapeutic Goods Administration. PoNS Treatment™ is not currently commercially available in the United States, the European Union or Australia. The Company has withdrawn its application from the EU marketing process due to uncertainty in Europe due to the switch from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) and the withdrawal of Lloyd’s Register Quality Assurance, the Company’s notified body, from the notified body business. The Company will reconsider submitting to the EU when conditions stabilize.

30+ years in the health sciences industry Former CEO at MediMedia Health Former President and CEO at GSW Worldwide (Division of inVentiv Health) Former Director of Neuroscience Marketing at Bristol-Myers Squibb Philippe Deschamps President, Chief Executive Officer & Chairman Dr. Jonathan Sackier Chief Medical Officer Joyce LaViscount Chief Financial Officer and Chief Operating Officer 17+ years in the medical devices industry Sales and Marketing Director, Boston Scientific Canada National Sales Manager, Canada Johnson & Johnson Former Media Relations and Marketing executive for Blue Jays & NHL Mark Leno VP, General Manager, Canadian Operations 30+ years in the health sciences industry Trained surgeon and pioneer of new medical technologies Has helped build several companies including medical technology, research and product- design and medical contract sales organizations 30+ years in the health sciences industry Accomplished pharmaceutical/healthcare public company CAO Former COO and CFO at MM Pharmaceutical Solutions Former Executive Director/Group Controller at Aptalis Pharmaceuticals Helius Leadership Team: Experienced Leadership With Healthcare and Commercialization Expertise www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
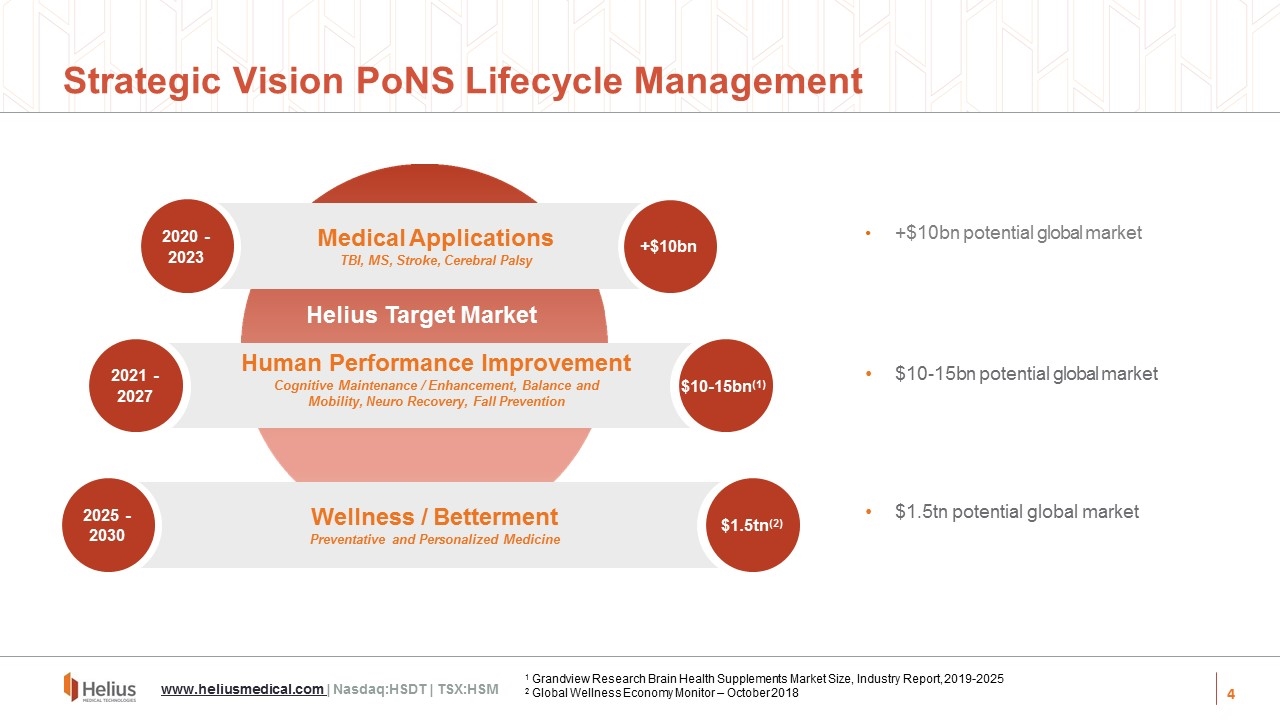
Strategic Vision PoNS Lifecycle Management +$10bn potential global market $10-15bn potential global market $1.5tn potential global market 2020 - 2023 +$10bn 2021 - 2027 2025 - 2030 $1.5tn(2) Wellness / Betterment Preventative and Personalized Medicine Human Performance Improvement Cognitive Maintenance / Enhancement, Balance and Mobility, Neuro Recovery, Fall Prevention Medical Applications TBI, MS, Stroke, Cerebral Palsy Helius Target Market $10-15bn(1) 1 Grandview Research Brain Health Supplements Market Size, Industry Report, 2019-2025 2 Global Wellness Economy Monitor – October 2018 www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
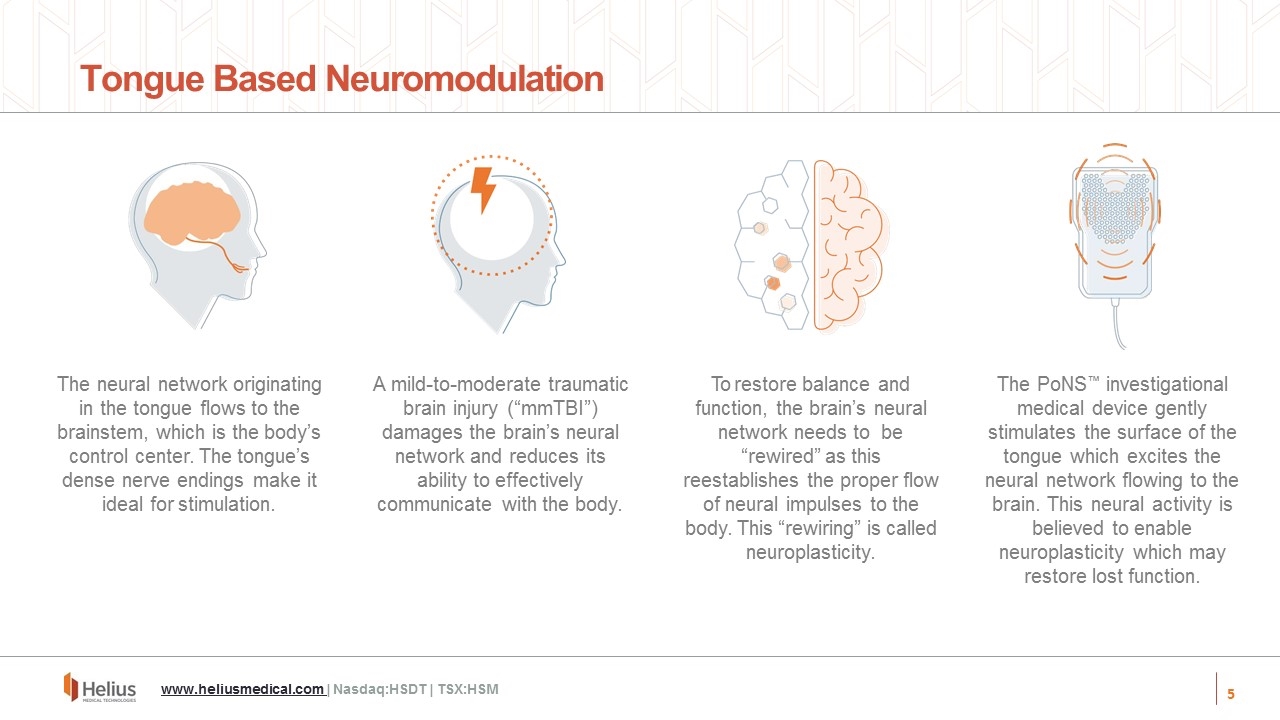
The PoNS™ investigational medical device gently stimulates the surface of the tongue which excites the neural network flowing to the brain. This neural activity is believed to enable neuroplasticity which may restore lost function. Tongue Based Neuromodulation The neural network originating in the tongue flows to the brainstem, which is the body’s control center. The tongue’s dense nerve endings make it ideal for stimulation. A mild-to-moderate traumatic brain injury (“mmTBI”) damages the brain’s neural network and reduces its ability to effectively communicate with the body. To restore balance and function, the brain’s neural network needs to be “rewired” as this reestablishes the proper flow of neural impulses to the body. This “rewiring” is called neuroplasticity. www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM

Smart Device The PoNS™ is a smart device that tracks frequency, duration and intensity of use PoNS - One Smart Device Data Intelligence Capabilities Data captured is uploaded to the cloud for analysis, the results of which support treatment decisions and also provide compliance profiles for payer reimbursement opportunities www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM Treatment Hypothesis Researchers believe that targeted physical therapy performed during neurostimulation may promote neural network changes including rebuilding and reorganization (neuroplasticity) thereby restoring communication with the body and improving functionality such as balance

Canada PoNS is an authorized class II medical device. The device is intended for use as a short term treatment (14 weeks) of chronic balance deficit due to mild-to-moderate traumatic brain injury and is to be used in conjunction with physical therapy. The device is intended for use as a short term treatment (14 weeks) of gait deficit due to mild and moderate symptoms from MS and is to be used in conjunction with physical therapy. US Prioritizing using MS indication as the pathway to pursue for our first US clearance of the PoNS device based on: The quality of the data included in our MS submission package to Health Canada The existing published data Real-world evidence gathered in Canada High unmet medical need We plan to submit to the FDA for this high unmet medical need in the second half of 2020. Regulatory Status www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
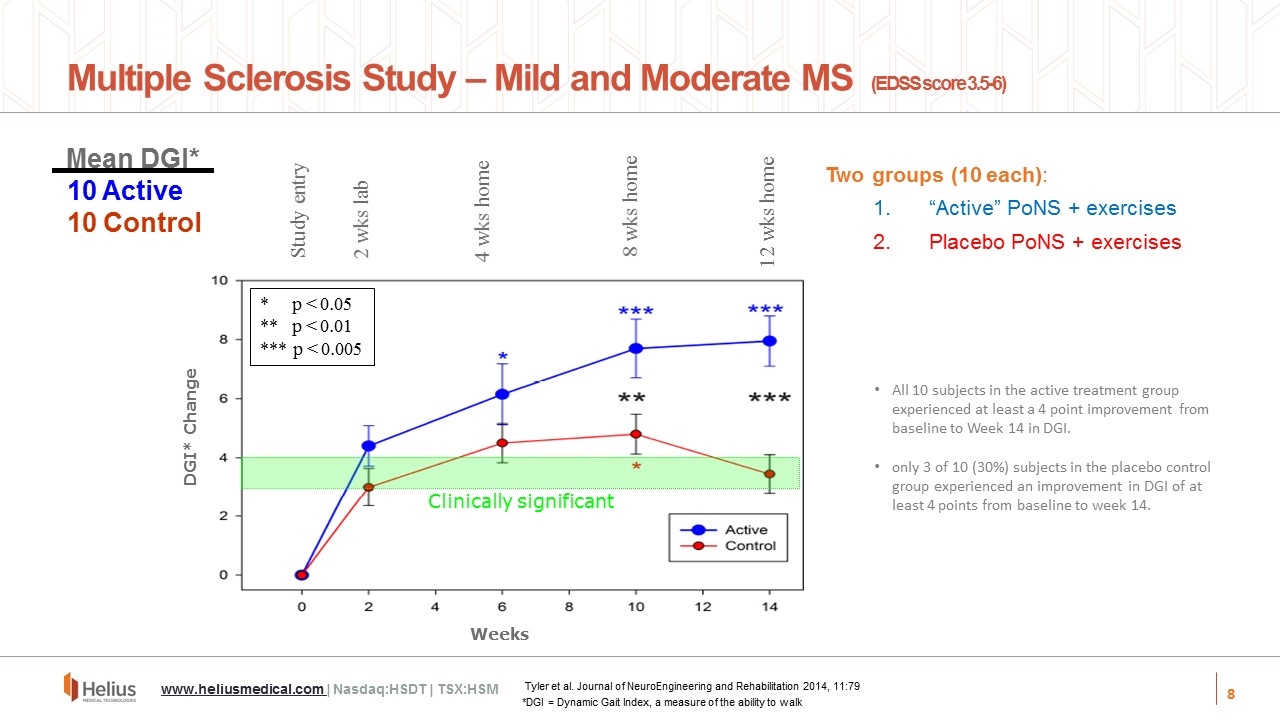
Study entry 2 wks lab 4 wks home 8 wks home 12 wks home Weeks DGI* Change *p < 0.05 **p < 0.01 *** p < 0.005 Clinically significant Two groups (10 each): “Active” PoNS + exercises Placebo PoNS + exercises Multiple Sclerosis Study – Mild and Moderate MS (EDSS score 3.5-6) Tyler et al. Journal of NeuroEngineering and Rehabilitation 2014, 11:79 www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM All 10 subjects in the active treatment group experienced at least a 4 point improvement from baseline to Week 14 in DGI. only 3 of 10 (30%) subjects in the placebo control group experienced an improvement in DGI of at least 4 points from baseline to week 14. Mean DGI* 10 Active 10 Control *DGI = Dynamic Gait Index, a measure of the ability to walk
14 Data on file. Helius Medical Technologies Treatment outcomes for patients treated in Canada are captured in the company developed validated data capture system. 39 patients with MS were treated with PoNS in Canada between March 2019 and September 2019. Using all available data from the treated MS patients, the mean improvement from baseline to Week 14 in the FGA (functional gait assessment) was 4.53 (95% CI 3.35 to 5.72). Based on observed data, the median improvement was 5 points. 56.7% had an improvement at Week 14 greater than or equal to 4 points, the minimum detectable change. Given the safety profile to date, we believe these data support a positive benefit risk ratio in the real-world setting. Summary of Real-World Evidence in MS Patients Treated with PoNS in Canada www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
Pursuit of clearance for mmTBI indication: Our U.S. regulatory strategy initially focused on pursuing de novo classification and clearance of the PoNS device from the FDA for the treatment of chronic balance deficit due to mmTBI. FDA declined request for de novo classification in April 2019 In reaching its conclusion, the Agency noted: The risk profile of the device is well understood and acceptable FDA did not have sufficient information to discern the relative independent contributions of the PoNS™ device and physical therapy The FDA also noted that the Company could generate additional data to address its concerns and resubmit its application Regulatory Status – US (continued) www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
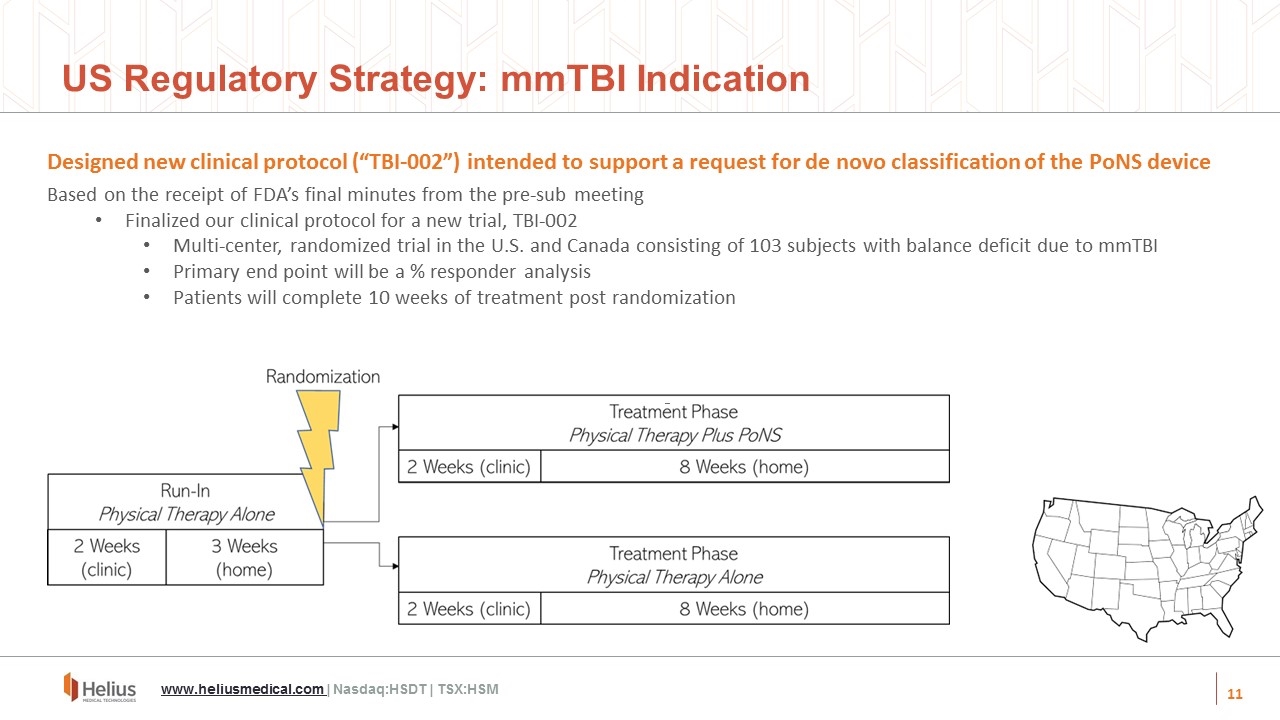
Designed new clinical protocol (“TBI-002”) intended to support a request for de novo classification of the PoNS device Based on the receipt of FDA’s final minutes from the pre-sub meeting Finalized our clinical protocol for a new trial, TBI-002 Multi-center, randomized trial in the U.S. and Canada consisting of 103 subjects with balance deficit due to mmTBI Primary end point will be a % responder analysis Patients will complete 10 weeks of treatment post randomization US Regulatory Strategy: mmTBI Indication www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
Real-world results of initial TBI patients consistent with Helius’ two RCTs in mmTBI: Patients demonstrated the same pattern of positive change over their course of treatment Majority of patients showed improvement in comfortable gait speed, a measure of their ability to walk, with meaningful clinical difference after treatment Focused on driving reimbursement by using health economic data to establish financial ROI for treatment Working with reimbursement specialists to formalize the reimbursement process by targeting multiple insurers Real-World Canadian Clinical Experience www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
Helius – Canadian Market Opportunity www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM Multiple Sclerosis (“MS”) Helius’ target markets in Canada are expected to provide meaningful real world clinical experience in preparation for US launch Multiple Sclerosis (“MS”) Mild-to-Moderate Traumatic Brain Injury (“mmTBI”) 350,000 patients in Canada $9,500 CAD estimated revenue per patient per treatment $25mm CAD market opportunity 2020-2023 93,500 patients in Canada High urgency to treat Less price sensitive Very similar treatment protocol as mmTBI Specialty clinic system

Establish robust network of authorized Clinic locations to deploy PoNS 7 authorized Clinic locations established in 2019 7 additional locations in the first two months of 2020 It takes approximately 6 weeks for the first patients to be treated in a newly authorized clinic Submitted label expansion in MS on February 27, 2020 to Health Canada Gain KOL support through clinical experience programs (CEP) in Canada’s most respected Neuro Treatment Centers Clinical experience program through UHN Toronto at 3 private clinic locations with an opportunity to transition to commercial treatment centers Drive reimbursement by using health economic data to establish financial ROI for treatment Building Access, Credibility and Awareness while we Engage, Train and Authorize Canadian clinics to provide PoNS Treatment™ Commercializing PoNS Treatment™ in Canada Canadian Strategic Focus www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
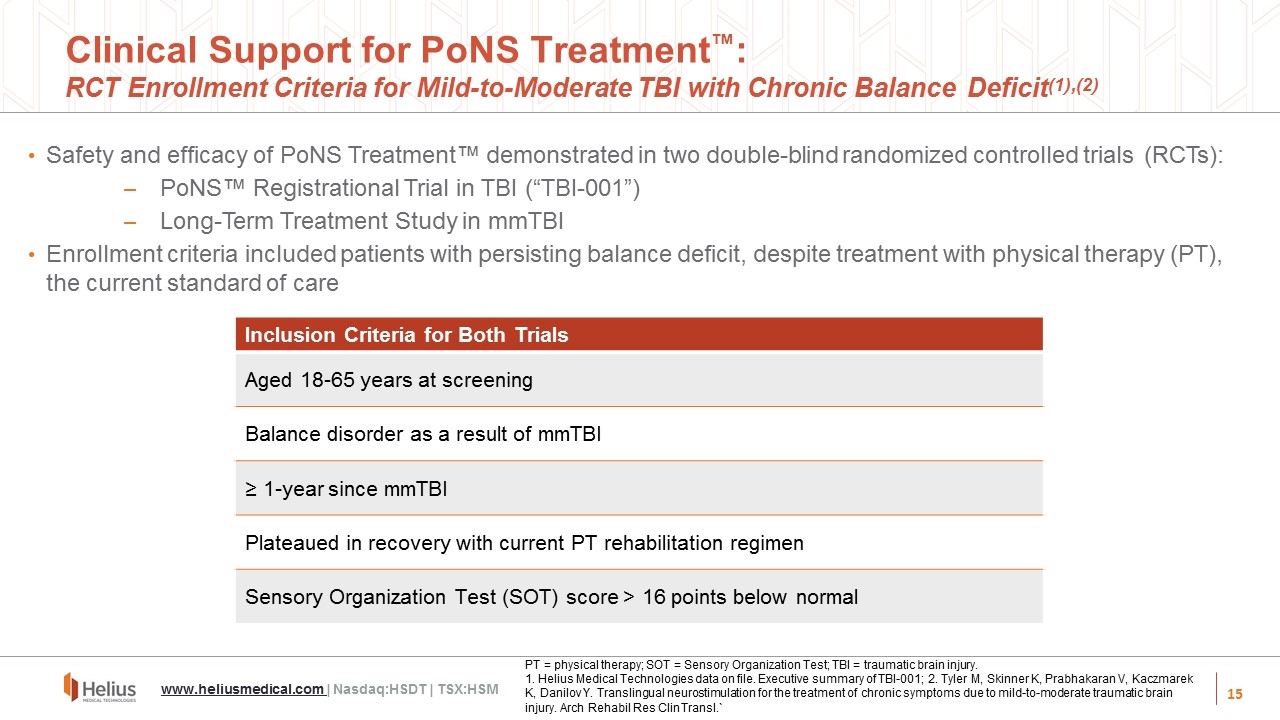
Clinical Support for PoNS Treatment™: RCT Enrollment Criteria for Mild-to-Moderate TBI with Chronic Balance Deficit(1),(2) Safety and efficacy of PoNS Treatment™ demonstrated in two double-blind randomized controlled trials (RCTs): PoNS™ Registrational Trial in TBI (“TBI-001”) Long-Term Treatment Study in mmTBI Enrollment criteria included patients with persisting balance deficit, despite treatment with physical therapy (PT), the current standard of care Inclusion Criteria for Both Trials Aged 18-65 years at screening Balance disorder as a result of mmTBI ≥ 1-year since mmTBI Plateaued in recovery with current PT rehabilitation regimen Sensory Organization Test (SOT) score > 16 points below normal PT = physical therapy; SOT = Sensory Organization Test; TBI = traumatic brain injury. 1. Helius Medical Technologies data on file. Executive summary of TBI-001; 2. Tyler M, Skinner K, Prabhakaran V, Kaczmarek K, Danilov Y. Translingual neurostimulation for the treatment of chronic symptoms due to mild-to-moderate traumatic brain injury. Arch Rehabil Res Clin Transl.` www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
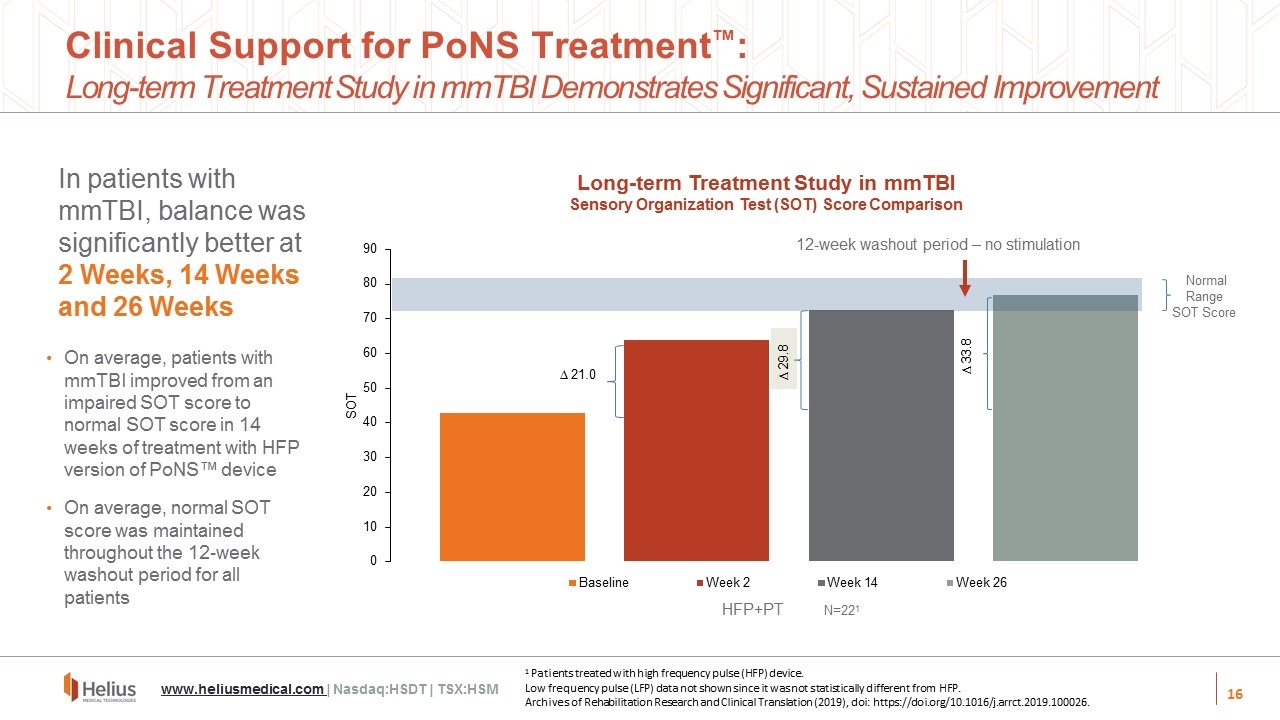
In patients with mmTBI, balance was significantly better at 2 Weeks, 14 Weeks and 26 Weeks 0 10 20 30 40 50 60 70 80 90 SOT Baseline Week 26 ∆ 29.8 ∆ 21.0 ∆ 33.8 Normal Range SOT Score Week 14 N=221 12-week washout period – no stimulation On average, patients with mmTBI improved from an impaired SOT score to normal SOT score in 14 weeks of treatment with HFP version of PoNS™ device On average, normal SOT score was maintained throughout the 12-week washout period for all patients Week 2 HFP+PT Clinical Support for PoNS Treatment™: Long-term Treatment Study in mmTBI Demonstrates Significant, Sustained Improvement Long-term Treatment Study in mmTBI Sensory Organization Test (SOT) Score Comparison 1 Patients treated with high frequency pulse (HFP) device. Low frequency pulse (LFP) data not shown since it was not statistically different from HFP. Archives of Rehabilitation Research and Clinical Translation (2019), doi: https://doi.org/10.1016/j.arrct.2019.100026. www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
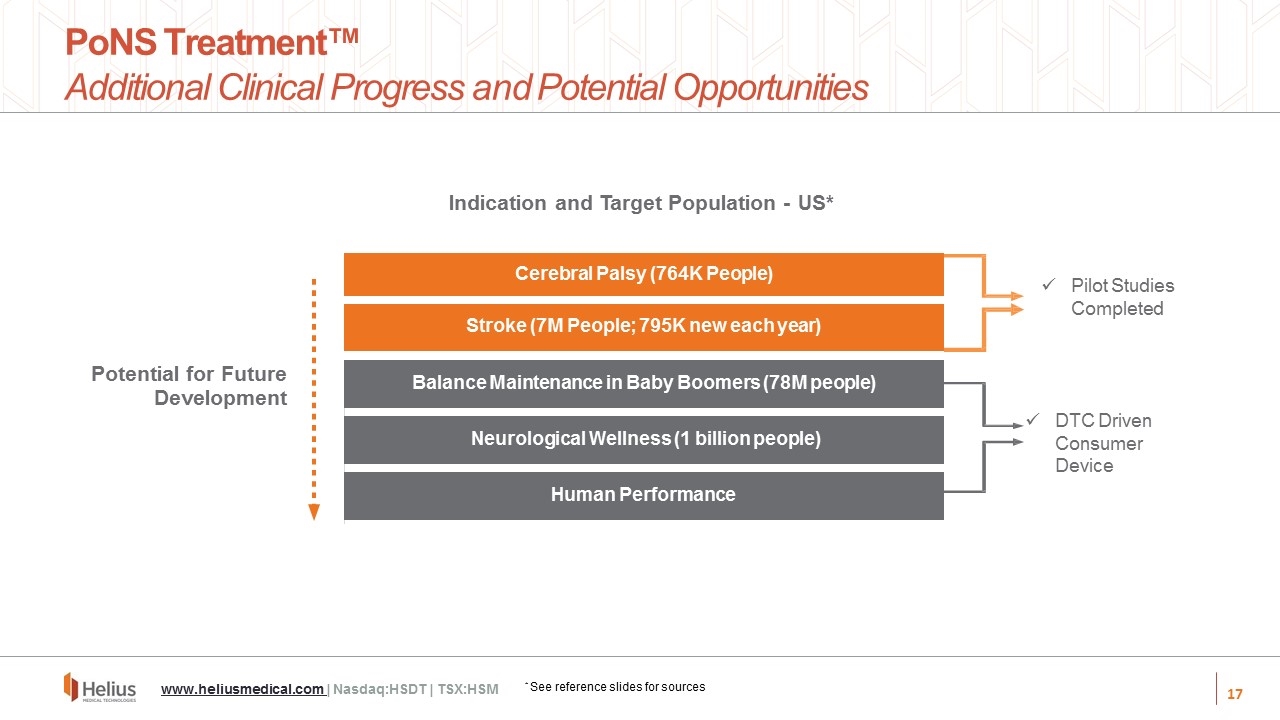
Balance Maintenance in Baby Boomers (78M people) Neurological Wellness (1 billion people) Human Performance Stroke (7M People; 795K new each year) Cerebral Palsy (764K People) Indication and Target Population - US* Potential for Future Development PoNS Treatment™ Additional Clinical Progress and Potential Opportunities www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM Pilot Studies Completed * See reference slides for sources DTC Driven Consumer Device
Extensive IP Portfolio A Significant Barrier to Competitor Entry Exclusively licensed from inventors (4% royalty): 9 US Medical Method Patents Issued Patents expire between 2029 and 2031 Patents owned by Helius (no royalty): 29 US Patents Issued 41 Foreign Patents Issued Patents expire between 2026 and 2040 Helius Patents Transferred to China Medical System Holdings (CMS): 3 Chinese Design Patents Independent Verification of Patents and Freedom to Operate Opinion September 2017 www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM

Investor Contact: Mike Piccinino, CFA 443-213-0500 investorrelations@heliusmedical.com Helius Medical Technologies, Inc. │642 Newtown Yardley Road, Suite 100 │Newtown, PA 18940 T: 215 944-6100 │E: investorrelations@heliusmedical.com │W: www.heliusmedical.com www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM

References Slide 15-16: Clinical Support for PoNS Treatment™ Archives of Rehabilitation Research and Clinical Translation (2019), doi: https://doi.org/10.1016/j.arrct.2019.100026 Slide 19: Disease State Prevalence Multiple Sclerosis - http://www.nationalmssociety.org/About-the-Society/MS-Prevalence Cerebral Palsy: http://www.cerebralpalsy.org/about-cerebral-palsy/prevalence-and-incidence Stroke – http://www.strokeassociation.org/STROKEORG/LifeAfterStroke/Life-After-Stroke_UCM_308546_SubHomePage.jsp–https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_stroke.htm Grandview Research Brain Health Supplements Market Size, Industry Report, 2019-2025 Global Wellness Economy Monitor – October 2018 www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM

Appendix www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM
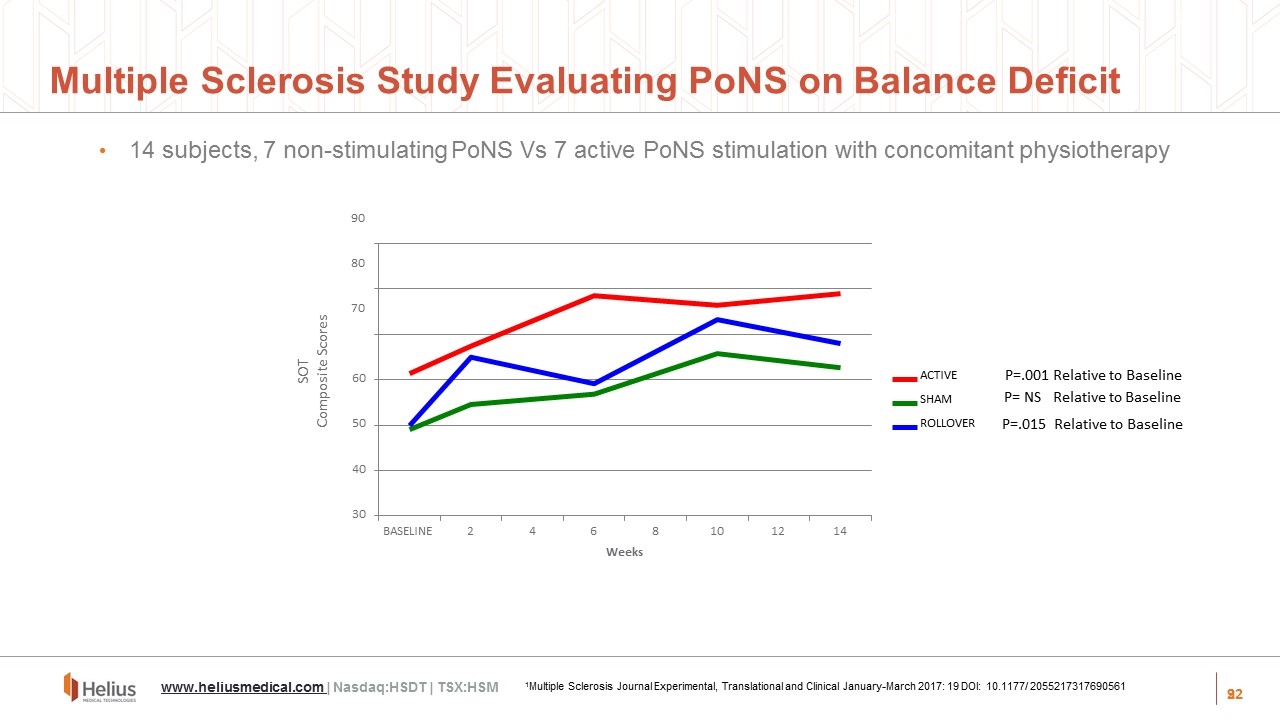
9 14 40 30 50 60 90 80 70 BASELINE 2 4 6 8 10 12 14 Weeks ACTIVE SHAM ROLLOVER SOT Composite Scores www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM 1Multiple Sclerosis Journal Experimental, Translational and Clinical January-March 2017: 19 DOI: 10.1177/ 2055217317690561 P=.015 Relative to Baseline P= NS Relative to Baseline P=.001 Relative to Baseline 14 subjects, 7 non-stimulating PoNS Vs 7 active PoNS stimulation with concomitant physiotherapy Multiple Sclerosis Study Evaluating PoNS on Balance Deficit
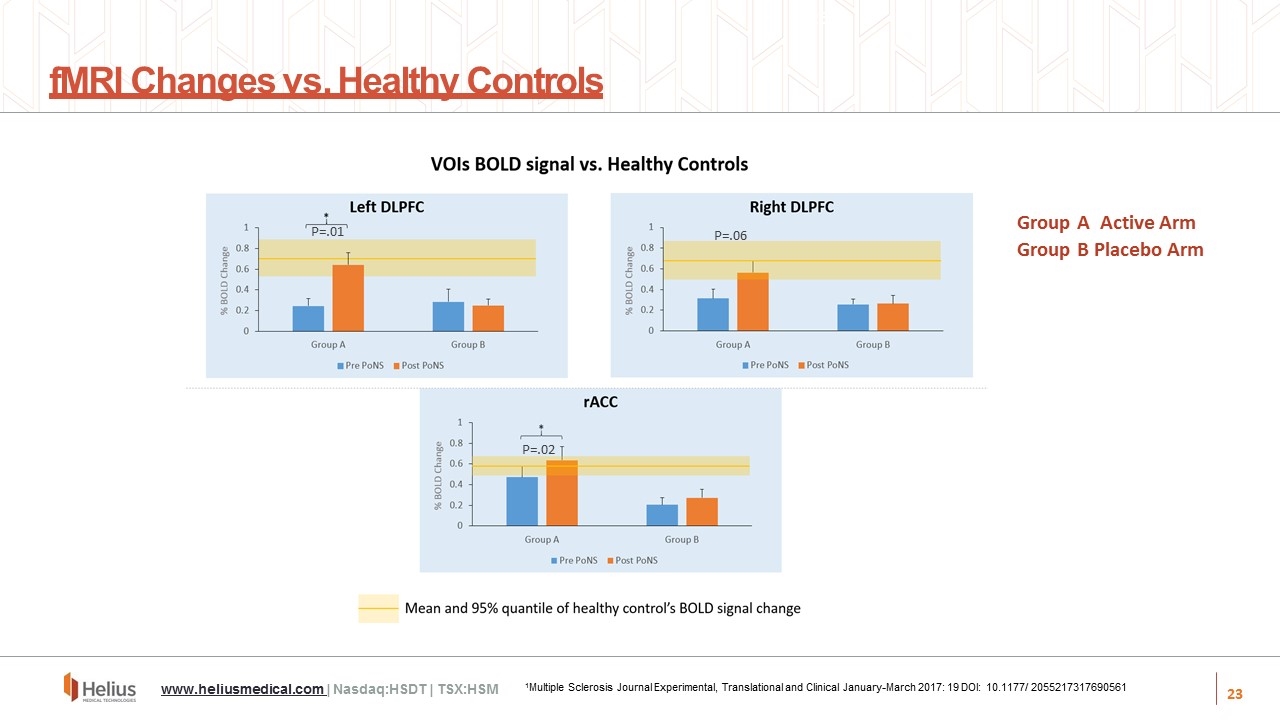
fMRI Changes vs. Healthy Controls 26 Group A Active Arm Group B Placebo Arm Active ArmPlacebo ArmActive Arm Active ArmPlacebo Arm www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM 1Multiple Sclerosis Journal Experimental, Translational and Clinical January-March 2017: 19 DOI: 10.1177/ 2055217317690561 P=.01 P=.02 P=.06
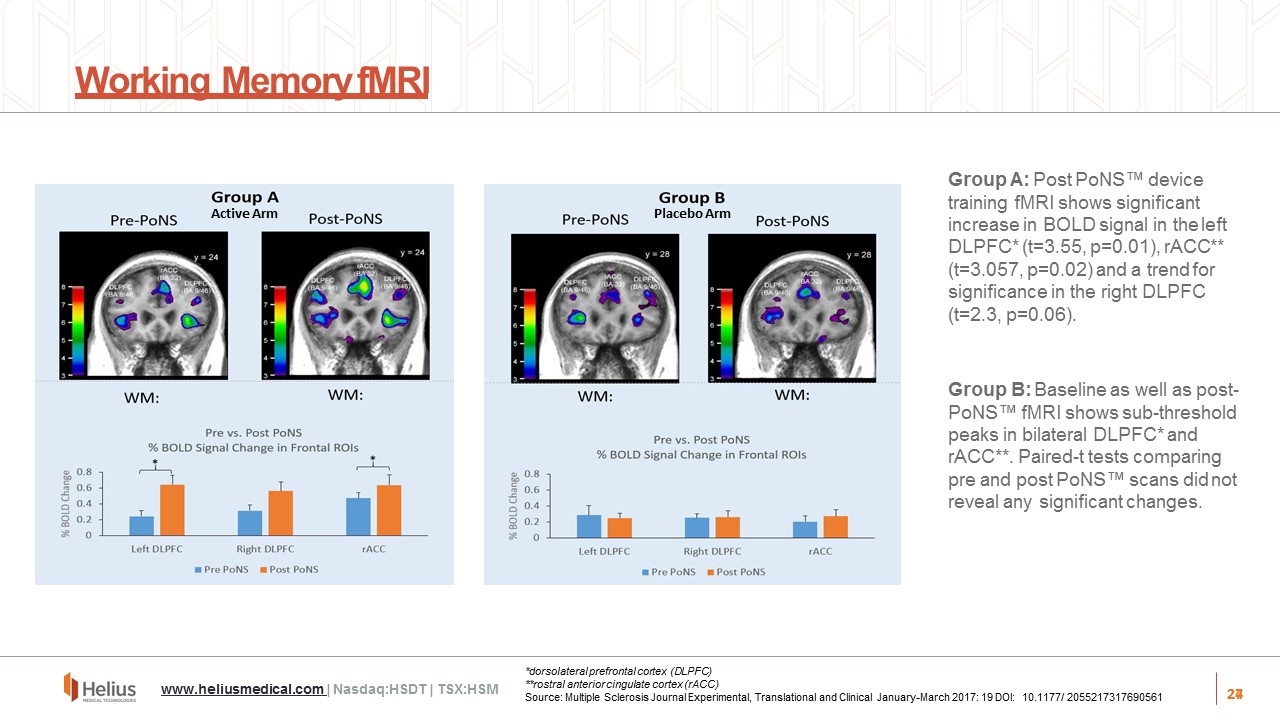
27 Working Memory fMRI Active Arm Placebo Arm Group A: Post PoNS™ device training fMRI shows significant increase in BOLD signal in the left DLPFC* (t=3.55, p=0.01), rACC** (t=3.057, p=0.02) and a trend for significance in the right DLPFC (t=2.3, p=0.06). Group B: Baseline as well as post- PoNS™ fMRI shows sub-threshold peaks in bilateral DLPFC* and rACC**. Paired-t tests comparing pre and post PoNS™ scans did not reveal any significant changes. 27 www.heliusmedical.com | Nasdaq:HSDT | TSX:HSM *dorsolateral prefrontal cortex (DLPFC) **rostral anterior cingulate cortex (rACC) Source: Multiple Sclerosis Journal Experimental, Translational and Clinical January-March 2017: 19 DOI: 10.1177/ 2055217317690561
