Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - GeoVax Labs, Inc. | ex_178179.htm |
| EX-32.1 - EXHIBIT 32.1 - GeoVax Labs, Inc. | ex_178178.htm |
| EX-31.2 - EXHIBIT 31.2 - GeoVax Labs, Inc. | ex_178177.htm |
| EX-31.1 - EXHIBIT 31.1 - GeoVax Labs, Inc. | ex_178176.htm |
| EX-10.7 - EXHIBIT 10.7 - GeoVax Labs, Inc. | ex_178175.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
☑ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
| For the fiscal year ended December 31, 2019 |
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File No. 000-52091
GEOVAX LABS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware (State or other jurisdiction of incorporation or organization)
1900 Lake Park Drive, Suite 380 Smyrna, GA |
87-0455038 (IRS Employer Identification Number)
30080 |
|
(Address of principal executive offices) |
(Zip Code) |
(678) 384-7220
Registrant’s telephone number, including area code:
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock $.001 par value
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ Accelerated filer ☐ Non-accelerated filer ☐ Smaller reporting company ☑ Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of Common Stock held by non-affiliates of the registrant on June 30, 2019, based on the closing price on that date was $416,380.
Number of shares of Common Stock outstanding as of March 23, 2020: 13,791,601
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement to be filed with respect to its 2020 Annual Meeting of Stockholders are incorporated by reference in Part III
Table of Contents
| PART I | 1 | ||
| ITEM 1. | BUSINESS | 1 | |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS | 26 | |
| ITEM 2. | PROPERTIES | 26 | |
| ITEM 3. | LEGAL PROCEEDINGS | 26 | |
| ITEM 4. | MINE SAFETY DISCLOSURES | 26 | |
| PART II | 27 | ||
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 27 | |
| ITEM 6. | SELECTED FINANCIAL DATA | 28 | |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 28 | |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 33 | |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 33 | |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 33 | |
| ITEM 9A. | CONTROLS AND PROCEDURES | 34 | |
| ITEM 9B. | OTHER INFORMATION | 34 | |
| PART III | 35 | ||
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 35 | |
| ITEM 11. | EXECUTIVE COMPENSATION | 35 | |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 35 | |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 35 | |
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES | 36 | |
| PART IV | 36 | ||
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | 36 | |
| ITEM 16. | FORM 10-K SUMMARY | 38 | |
| SIGNATURES | 39 |
This Annual Report (including the following section regarding Management’s Discussion and Analysis of Financial Condition and Results of Operations) contains forward-looking statements regarding our business, financial condition, results of operations and prospects. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates” and similar expressions or variations of such words are intended to identify forward-looking statements but are not the exclusive means of identifying forward-looking statements in this Annual Report. Additionally, statements concerning future matters, including statements regarding our business, our financial position, the research and development of our products and other statements regarding matters that are not historical are forward-looking statements.
Although forward-looking statements in this Annual Report reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include without limitation those discussed under the heading “Risk Factors” below, as well as those discussed elsewhere in this Annual Report. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report. We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this Annual Report. Readers are urged to carefully review and consider the various disclosures made in this Annual Report, which attempt to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
PART I
|
ITEM 1. |
BUSINESS |
Overview
GeoVax Labs, Inc. (“GeoVax” or the “Company”) is a clinical-stage biotechnology company developing immunotherapies and vaccines against cancers and infectious diseases using a novel vector vaccine platform (Modified Vaccinia Ankara-Virus Like Particle or “GV-MVA-VLPTM”). Our current development programs are focused on preventive and therapeutic vaccines against Human Immunodeficiency Virus (HIV); preventive vaccines against hemorrhagic fever viruses (Ebola, Sudan, Marburg, and Lassa fever), Zika virus and malaria; a therapeutic vaccine for chronic hepatitis B virus infections; and immunotherapies for solid tumor cancers. We also recently entered into a letter of intent with BravoVax, an unaffiliated biotechnology company specializing in development of human vaccines based in Wuhan, China, to jointly develop a vaccine for prevention of novel coronavirus (COVID-19) infection.
For our infectious disease vaccines, our recombinant MVA vector expresses target proteins on highly immunogenic VLPs in the person being vaccinated, with the intended result of producing durable immune responses with the safety characteristics of the replication deficient MVA vector and cost-effective manufacturing.
In cancer immunotherapy, we believe that stimulating the immune system to treat or prevent cancers is a compelling concept and that the opportunity for immune-activating technologies is promising, especially in light of advancements such as checkpoint inhibitors leading the way in oncology. Despite drug approvals in limited indications and promising results in clinical trials, there remains a significant need and opportunity for further advancements. We believe our GV-MVA-VLPTM platform is well-suited for delivery of tumor-associated antigens and we plan to pursue development of our platform in this space through our subsidiary, Immutak Oncology, Inc.
Our most advanced vaccine program is focused on prevention of the clade B subtype of HIV prevalent in the regions of the Americas, Western Europe, Japan and Australia; our HIV vaccine candidate, GOVX-B11, will be included in an upcoming clinical trial (HVTN 132) managed by the HIV Vaccine Clinical Trials Network (HVTN) with support from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), which is targeted to begin in late 2020. Additionally, through the efforts of our collaborator, American Gene Technologies International, Inc. (AGT), we expect that our HIV vaccine will enter clinical trials during 2020 in combination with AGT’s gene therapy technology to seek a functional cure for HIV. A similar effort is underway with a consortium led by researchers at the University of California, San Francisco (UCSF), using our vaccine as part of a combinational therapy to induce remission in HIV-positive individuals; we also expect this program to enter clinical trials during 2020.
Our other vaccine and immunotherapy programs are at various other stages of development as described further in the following pages.
Our corporate strategy is to advance, protect and exploit our differentiated vaccine/immunotherapy platform leading to the successful development of preventive and therapeutic vaccines against infectious diseases and various cancers. With our design and development capabilities, we are progressing and validating an array of cancer and infectious disease immunotherapy and vaccine product candidates. Our goal is to advance products through to human clinical testing, and to seek partnership or licensing arrangements for achieving regulatory approval and commercialization. We also leverage third party resources through collaborations and partnerships for preclinical and clinical testing with multiple government, academic and corporate entities.
Our current and recent collaborators and partners include the NIAID/NIH, the HIV Vaccines Trial Network (HVTN), Centers for Disease Control and Prevention (CDC), U.S. Department of Defense (DoD), U.S. Army Research Institute of Infectious Disease (USAMRIID), U.S. Naval Research Laboratory (USNRL), Emory University, University of Pittsburgh, Georgia State University Research Foundation (GSURF), University of Texas Medical Branch (UTMB), the Institute of Human Virology (IHV) at the University of Maryland, the Scripps Research Institute (Scripps), Burnet Institute in Australia, the Geneva Foundation, American Gene Technologies International, Inc. (AGT), ViaMune, Inc., Virometix AG, Enesi Pharma, Leidos, Inc., UCSF, and BravoVax.
We are incorporated in Delaware, and our offices and laboratory facilities are in Smyrna, Georgia (metropolitan Atlanta).
Our Differentiated Vaccine and Immunotherapy Platform
Vaccines typically contain agents (antigens) that resemble disease-causing microorganisms. Traditional vaccines are often made from weakened or killed forms of the virus or from its surface proteins. Many newer vaccines use recombinant DNA (deoxyribonucleic acid) technology to generate vaccine antigens in bacteria or cultured cells from specific portions of the DNA sequence of the target pathogen. The generated antigens are then purified and formulated for use in a vaccine. We believe the most successful of these purified antigens have been non-infectious virus-like particles (VLPs) as exemplified by vaccines for hepatitis B (Merck's Recombivax® and GSK's Engerix®) and Papilloma viruses (GSK's Cervarix®, and Merck's Gardasil®). Our approach uses recombinant DNA and/or recombinant MVA to produce VLPs in the person being vaccinated (in vivo) reducing complexity and costs of manufacturing. In human clinical trials of our HIV vaccines, we believe we have demonstrated that our VLPs, expressed from within the cells of the person being vaccinated, can be safe, yet elicit both strong and durable humoral and cellular immune response.
VLPs can cause the body's immune system to recognize and kill targeted viruses to prevent an infection. VLPs can also train the immune system to recognize and kill virus-infected cells to control infection and reduce the length and severity of disease. One of the biggest challenges with VLP-based vaccines is to design the vaccines in such a way that the VLPs will be recognized by the immune system in the same way as the authentic virus would be. We design our vaccines such that, when VLPs for enveloped viruses like HIV, Ebola, Marburg or Lassa fever are produced in vivo (in the cells of the recipient), they include not only the protein antigens, but also an envelope consisting of membranes from the vaccinated individual's cells. In this way, they are highly similar to the virus generated in a person's body during a natural infection. VLPs produced in vitro (in a pharmaceutical plant), by contrast, have no envelope; or, envelopes from the cultured cells (typically hamster or insect cells) used to produce them. We believe our technology therefore provides distinct advantages by producing VLPs that more closely resemble the authentic viruses. We believe this feature of our immunogens allows the body's immune system to more readily recognize the virus. By producing VLPs in vivo, we believe we also avoid potential purification issues associated with in vitro production of VLPs.
Examples of VLPs
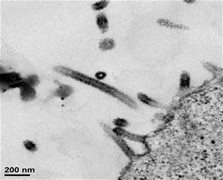 |
 |
| Ebola Virus VLPs | HIV VLPs |
Figure 1. Electron micrographs showing examples of VLPs produced by GeoVax vaccines in human cells. Note that the Ebola virus VLPs on the left self-assemble into the rod-like shape of the actual Ebola virus, while the HIV VLPs shown on the right take on the spherical shape of the actual HIV virus. While below the resolution of these micrographs, both types of VLPs display what we believe to be the native form of their respective viral envelope glycoproteins which we believe is key to generating an effective immune humoral response.
For many viral infectious diseases, natural VLPs are produced by co-assembly of, generally a matrix protein and an envelope protein. These natural VLPs resemble infectious virions but may not contain the virus’ full genetic material and are therefore considered “virus byproducts”. To develop a vaccine for an infectious disease such as Ebola, Zika, HIV, etc., we use viral proteins that naturally form VLPs. For other diseases such as cancer and malaria where there are no natural VLP counterparts, we use an array of other matrix proteins as scaffolds to deliver such antigens as VLPs. Similar approaches have been used for VLPs produced in vitro including the current malaria vaccine (RTS, S) that uses a matrix S protein from the Hepatitis B virus to deliver the malaria liver-stage protective antigen, CSP. We have successfully used viral matrix proteins as scaffolds for our oncology vaccine (MUC1) and malaria (modified CSP) vaccine candidates.
We selected MVA for use as the live viral component of our vaccines because of its well-established safety record and because of the ability of this vector to carry sufficient viral sequences to produce VLPs. MVA was originally developed as a safer smallpox vaccine for use in immune-compromised individuals. It was developed by attenuating the standard smallpox vaccine by passaging it (over 500 passages) in chicken embryos or chicken embryo fibroblasts, resulting in a virus with limited ability to replicate in human cells (thus safe) but with high replication capability in avian cells (thus cost effective for manufacturing). The deletions also resulted in the loss of immune evasion genes which assist the spread of wild type smallpox infections, even in the presence of human immune responses.
Our GV-MVA-VLPTM vaccine platform affords other advantages:
|
● |
Safety: Our HIV vaccines have demonstrated outstanding safety in multiple human clinical trials. Safety for MVA, generally, has been shown in more than 120,000 subjects in Europe, including immunocompromised individuals during the initial development of MVA and more recently with the development of MVA as a safer vaccine against smallpox. |
|
● |
Durability: Our technology raises highly durable (long-lasting) vaccine responses, the most durable in the field of vectored HIV vaccines. We hypothesize that elicitation of durable vaccine responses is conferred on responding B cells by the vaccinia parent of MVA, which raises highly durable responses for smallpox. |
|
● |
Limited pre-existing immunity to vector: Following the eradication of smallpox in 1980, smallpox vaccinations subsequently ended, leaving all but those born before 1980 and selected populations (such as vaccinated laboratory workers and first responders) unvaccinated and without pre-existing immunity to MVA-derived vaccines. A potential interference of pre-existing immunity to a vector may be more problematic with those vectors related to parent viruses used in routine vaccinations (e.g. measles) or constitute common viruses that infect people of all ages (e.g. cytomegalovirus). |
|
● |
Repeated use of the platform for different vaccines used in sequence. In mouse experiments, we have shown that two of our vaccines (e.g. GV-MVA-VLP-Zika followed by GV-MVA-VLP-Ebola) can be given at <4 weeks intervals without any negative impact on their immunogenicity (lack of vector immunity). |
|
● |
No need for adjuvants: MVA generally stimulates strong innate immune responses and does not require the use of adjuvants. |
|
● |
Thermal stability: MVA is stable in both liquid and lyophilized formats (> 6 years of storage). |
|
● |
Genetic stability and manufacturability: If appropriately engineered, MVA is genetically stable and can reliably be manufactured in either the established Chick Embryo Fibroblast cell substrate, or novel continuous cell lines that support scalability as well as greater process consistency and efficiency. |
Our Product Development Pipeline
Our primary focus is to advance, independently and in partnerships, the products developed from our GV-MVA-VLPTM platform. We are currently developing a number of vaccines and immunotherapies for prevention or treatment of infectious diseases and cancer. The table below summarizes the status of our product development programs, which are discussed in greater detail in the following pages.
|
Product Area / Indication |
Stage of Development |
Collaborators / Sponsors |
|
Cancer |
||
|
HPV-related cancers |
Preclinical |
Emory, Virometix |
|
MUC1-expressing tumors |
Preclinical completed |
Univ of Pittsburgh, ViaMune |
|
Cyclin B1-expressing tumors |
Preclinical |
|
|
Checkpoint inhibitors |
Preclinical |
Leidos |
|
Infectious Diseases |
||
|
HIV (preventive) |
Phase 2a completed |
NIH, HVTN, Emory |
|
HIV (immunotherapy) |
Phase 1 |
AGT, UCSF |
|
Zika |
Preclinical completed |
NIH, CDC |
|
Malaria |
Preclinical |
Leidos, Burnet Institute |
|
Ebola, Marburg, Sudan |
Preclinical completed |
NIH, USAMRIID, UTMB |
|
Lassa Fever |
Preclinical |
NIH, DoD, Scripps, IHV, UTMB, USNRL, Geneva Foundation |
|
Hepatitis B (immunotherapy) |
Preclinical |
GSURF |
|
Coronavirus (COVID-19) |
Preclinical |
BravoVax |
|
Novel vaccine delivery |
Preclinical |
Enesi |
We are seeking to develop a broad product pipeline based on our GV-MVA-VLPTM platform and have been very pleased with the results, particularly considering the challenges we have faced in obtaining sufficient capital, and the related relatively small number of scientifically skilled employees we employ. These constraints have made it necessary to set priorities as to our primary focuses, and those will change as opportunities, resources, and other circumstances dictate. During 2019, for example, in addition to working with our collaborators/sponsors, we chose to focus a portion of our management time and budget in the area of immuno-oncology. More recently, the emergence of novel coronavirus (COVID-19) led us to decide to devote our management time and resources, and our platform, to address this epidemic. At times, some of our development programs are paused as we shift our focus due our limited resources.
Our Cancer Immunotherapy Programs
Cancer is the second most common cause of death in the US, exceeded only by heart disease. Its global burden is expected to rise to 22 million new cases per year by 2030. Currently, there is only one FDA approved cancer vaccine, PROVENGE® (sipuleucel-T). PROVENGE® is a personalized therapy for prostate cancer patients, which prolongs survival times by about 4 months. However, the field of immuno-oncology has received new momentum with the discovery and initial launch of monoclonal antibodies (Mabs) called immune checkpoint inhibitors (ICIs). Tumors hijack the body’s natural immune checkpoints by over expressing immune checkpoint ligands (proteins that bind to and activate the inhibitory activity of immune checkpoints), as a mechanism of immune resistance, especially against the T cells that are specific for tumor antigens and can kill cancer cells. ICIs block the interaction of immune checkpoints with their ligands on tumor cells, allowing otherwise poorly functional T cells to resume proliferation, cytokine production and killing of tumor cells.
Unlike conventional therapies (e.g. radiation, chemotherapy, antibody, etc.), therapeutic cancer vaccines have the potential to induce responses that not only result in the control and even clearance of tumors but also establish immunological memory that can suppress and prevent tumor recurrence. Convenience, safety, and low toxicity of cancer vaccines could make them invaluable tools to be included in future immunotherapy approaches for treating tumors. Currently, there are only a few vectored cancer vaccines being tested in combination with ICIs, all of which are in early clinical stages.
Collaborations with University of Pittsburgh and ViaMune – We have established a collaboration with Dr. Olivera Finn, a leading expert in cancer immunotherapy at the University of Pittsburgh. Dr. Finn was the first to show that many tumors express an abnormal form of cell surface-associated Mucin 1 (MUC1) protein that is recognized by the immune system as foreign. Given this, we are developing our GV-MVA-VLPTM vaccine platform to deliver abnormal forms of MUC1 with the goal of raising protective anti-tumor antibodies and T cell responses in cancer patients. Our collaboration with Dr. Finn has shown that a combination of our MVA-VLP-MUC1 vaccine candidate with a 100-mer MUC1 peptide (experiments were performed at the University of Pittsburgh) was capable of breaking tolerance to human MUC1 tumors in huMUC1 transgenic mice and protect them against challenge in a lymphoma tumor model.
We are also collaborating with ViaMune, Inc., which has developed a fully synthetic MUC1 vaccine candidate (MTI). The collaboration will assess each companies’ vaccine platform, separately, and in combination, with the goal of developing a tumor MUC1 vaccine that can produce a broad spectrum of anti-tumor antibody and T cell responses. The resulting MUC1 vaccine would be combined with ICIs as a novel vaccination strategy for cancer patients with advanced MUC1+ tumors. We have produced an MVA-VLP-MUC1 vaccine candidate, demonstrated VLP production by electron microscopy using MUC1 immunogold staining, and showed that the VLPs express a hypo-glycosylated form of MUC1 in human cell lines. Preclinical studies of the combined MTI and MVA-VLP-MUC1 vaccines conducted at the University of North Carolina at Charlotte have shown the combination of our vaccine with MTI and ICI have significantly reduced the tumor burden in a mouse model for colorectal cancer.
Collaboration with Emory Vaccine Center – In July 2018, we began collaborating with Emory University on the development of a therapeutic vaccine for human papillomavirus (HPV) infection, with a specific focus on head and neck cancer (HNC). This is an important research area as there are currently no medical treatments for chronic HPV infections, which can lead to the formation of cancerous tumors. The GeoVax/Emory collaboration will include testing GeoVax’s MVA-VLP-HPV vaccine candidates in therapeutic animal models of HPV in the laboratory of Dr. Rafi Ahmed, Director of the Emory Vaccine Center. Dr. Ahmed, a member of the National Academy of Sciences, is a world-renowned immunologist whose work during the past decade has been highly influential in shaping understanding of memory T cell differentiation and T and B cell-mediated antiviral immunity. We believe our collaboration with Emory on the HPV project is extremely valuable as it was Dr. Ahmed who first discovered in 2006 that the PD-1 pathway could also be exploited by many pathogens to repress normal T cell function during chronic viral infection. This led to development of numerous blockbuster anti-PD1 antibodies currently being used for treatment of various cancers and which hold promise as adjunctive therapy for several chronic infectious diseases. In HIV, Ebola, Zika, and Lassa Fever, our GV-MVA-VLPTM vaccine candidates have demonstrated eliciting strong antigen-specific T cell responses in the host, a response that is critical to fight against HPV infections in HNC patients. To increase the therapeutic efficacy of our HPV vaccine, we intend to apply a combination strategy which could include co-administration of anti-PD1 antibodies and/or other newly discovered immunotherapy drugs to improve a patient’s own anti-cancer immune response.
Collaboration with Virometix – In November 2018, we announced a collaboration with Virometix AG, a company developing next-generation Synthetic Virus-Like Particle (SVLPTM) based vaccines, to develop a therapeutic vaccine for HPV infection. The collaboration will include preclinical animal testing of GeoVax’s MVA-vectored HPV vaccine candidates in combination with Virometix’ synthetic HPV vaccine candidate. This collaboration complements our collaboration with Emory University for HPV-related head and neck cancers in patients who express oncogene products of HPV16, E6 and E7 proteins. Similar to the strategy we are utilizing in our clinical trials for HIV and preclinical testing of our cancer vaccines (e.g. vector and protein combination), we believe the combination of our MVA-vectored HPV vaccines and Virometix’ SVLP-based HPV vaccine could bring a synergy that significantly increases the therapeutic potential over each platform used separately.
Collaboration with Leidos – In November 2018, we began collaborating with Leidos, Inc. on a research program evaluating the combination of the companies’ respective technologies in the field of cancer immunotherapy. Currently, there are major limitations on cancer immunotherapies which include high costs (limiting patient access, straining both the healthcare system and the patient’s own finances), the need for multiple injections, and significant side effects. Moreover, monotherapy with one checkpoint inhibitor drug can induce drug resistance in some patients making it necessary to combine with other drugs and treatments, which in turn may further increase toxicity. We have shown that our MVA platform can be safe in humans without any major side effects and hope that delivery of the immune checkpoint inhibitors with or without the tumor-associated antigens may overcome some of the challenges associated with the use of immune checkpoint inhibitors in cancers or other chronic infectious diseases. The GeoVax/Leidos collaboration will include the design, construction, and characterization of multiple immunotherapeutic vaccine candidates using our GV-MVA-VLPTM vaccine platform combined with certain novel peptide PD-1 checkpoint inhibitors developed by Leidos. The vaccine design, construction, and characterization will be performed at GeoVax with further analysis conducted by Leidos. We believe this effort may lead to expanded efforts in cancer immunotherapy, treatments for chronic Hepatitis B infections, or other diseases where an immunological-based therapeutic approach would be beneficial.
Formation of Immutak Oncology, Inc. – In September 2019, we incorporated Immutak Oncology, Inc (Immutak) as a wholly-owned subsidiary of the Company. We established Immutak to focus on the advancement of the immuno-oncology programs developed by GeoVax and to seek additional, complementary technologies and clinical-stage products in the oncology space. We have initiated separate financing efforts in support of these programs through Immutak.
Our Infectious Disease Vaccine Programs
Recent Development – Coronavirus Vaccine Collaboration
In January 2020, in response to the ongoing Coronavirus (COVID-19) epidemic which began in Wuhan, China, we signed a letter of intent with BravoVax, a vaccine developer in Wuhan, China, to collaborate on the develop a vaccine for prevention and/or control of COVID-2019 infection. Under the collaboration, we will use our GV-MVA-VLPTM vaccine platform and expertise to design and construct the vaccine candidate using genetic sequences from the ongoing COVID-2019 outbreak. Upon completion of a definitive agreement, BravoVax will provide testing and manufacturing support, as well as direct interactions with Chinese public health and regulatory authorities, for a parallel regulatory pathway to what GeoVax will pursue in the United States. To date, GeoVax has completed the vaccine construct design and has initiated applications to BARDA (Biomedical Advanced Research and Development Authority) and other entities requesting funding support of our COVID-19 vaccine development efforts. BravoVax has initiated similar funding application requests to entities such as the CNCBD (China National Center for Biotechnology Development).
About COVID-19 – Coronaviruses are common in many species of animals including mammals, avian and bats. In rare occasions these viruses can evolve to cross the animal species and infect humans and quickly spread from person to person resulting in lethal but rare respiratory infections. Recent epidemic with SARS and MERS coronaviruses resulted in 774 and 858 deaths, respectively. Since 2015 there has not been any cases of SARS and MERS reported but in January 2020, WHO identified a novel coronavirus, recently named COVID-19, in the city of Wuhan, China. On January 31, World Health Organization (WHO) declared the novel coronavirus to be a global health emergency, and on March 11, 2020 WHO declared a global pandemic. As of March 18, 2020, in excess of 200,000 people have been infected and more than 8,900 people have died as a result of COVID-19 infections. The situation is fluid, with the infection and death statistics increasing significantly each day.
Our HIV/AIDS Vaccine Programs
About HIV/AIDS. HIV/AIDS is considered by many in the scientific and medical community to be the most lethal infectious disease in the world. An estimated 37 million people are living with HIV worldwide, with approximately 1.8 million newly infected annually. Since the beginning of the epidemic, more than 70 million people have been infected with the HIV virus and about 35 million have died of HIV. The United States currently has an estimated 1.1 million HIV-infected individuals, with approximately 40,000 new infections per year. Gay and bisexual men bear the greatest burden by risk group, representing nearly 70% of new infections in the U.S. African-Americans also bear a disproportionate burden, representing 43% of people living with HIV, yet representing just 12% of the total population.
There are several AIDS-causing HIV virus subtypes, or clades, that are found in different regions of the world. These clades are identified as clade A, clade B and so on. The predominant clade found in Europe, North America, parts of South America, Japan and Australia is clade B, whereas the predominant clades in Africa are clades A and C. In India, the predominant clade is clade C. Genetic differences between the clades may mean that vaccines or treatments developed against HIV of one clade may only be partially effective or ineffective against HIV of other clades. Thus, there is often a geographical focus to designing and developing HIV vaccines.
At present, the standard approach to treating HIV infection is to inhibit viral replication through the use of combinations of drugs. Available drugs include reverse transcriptase inhibitors, protease inhibitors, integration inhibitors and inhibitors of cell entry. However, HIV is prone to genetic changes that can produce strains that are resistant to currently approved drugs. When HIV acquires resistance to one drug within a class, it can often become resistant to the entire class, meaning that it may be impossible to re-establish control of a genetically altered strain by substituting different drugs in the same class. Furthermore, these treatments continue to have significant limitations which include toxicity, patient non-adherence to the treatment regimens and cost. Thus, over time, viruses acquire drug-resistant mutations, and many patients develop intolerance to the medications or simply give up taking the medications due to cost, inconvenience or side effects.
There is no approved vaccine to prevent HIV infection. Prevention of HIV infection remains a worldwide unmet medical need, even in the United States and other first world countries where effective antiretroviral therapies are available. Current antiretroviral therapies (ART) do not eliminate HIV infection, requiring individuals to remain on such drugs for their entire lives. Uptake and successful long-term adherence to therapy is also limited. Only 30% of those infected with HIV in the US ultimately remain in HIV care with their viral load sufficiently suppressed to prevent spread of HIV. Furthermore, the financial burden to the U.S. taxpayer for HIV education, prevention, and treatment costs is borne through multiple federal agencies, totaling over $25 billion annually.
According to the International AIDS Vaccine Initiative (IAVI), the cost and complexity of new treatment advances for HIV/AIDS puts them out of reach for most people in the countries where treatment is most needed. In industrialized nations, where drugs are more readily available, side effects and increased rates of viral resistance have raised concerns about their long-term use. Vaccines are seen by many as the most promising way to end the HIV/AIDS pandemic. We expect that vaccines, once developed, will be used universally and administered worldwide by organizations that provide healthcare services, including hospitals, medical clinics, the military, prisons and schools.
Our Preventive HIV Vaccine Program
Clade B Preventive HIV Vaccine Program. Our most clinically advanced vaccine is GOVX-B11, designed to protect against the clade B subtype of the HIV virus prevalent in the Americas, Western Europe, Japan and Australia. GOVX-B11 consists of a recombinant DNA vaccine used to prime immune responses and a recombinant MVA vaccine (MVA62B) used to boost the primed responses. Both the DNA and MVA vaccines induce the production of non-infectious VLPs by the cells of the vaccinated person.
Phase 1 and phase 2a human clinical trials of GOVX-B11 have been conducted by the HVTN. In these trials, totaling approximately 500 participants, GOVX-B11 was tested at various doses and regimens and was well tolerated. The HVTN is the largest worldwide clinical trials network dedicated to the development and testing of HIV/AIDS vaccines. Support for the HVTN comes from the NIAID, part of the NIH. The HVTN’s HIV Vaccine Trial Units are located at leading research institutions in 27 cities on four continents.
In January 2017 HVTN began the next human clinical trial (HVTN 114) in the path toward pivotal efficacy trials. HVTN 114 enrolled individuals who previously participated in the HVTN 205 Phase 2a trial of the GOVX-B11 vaccine, which concluded in 2012. HVTN 114 tested the ability of late boosts (additional vaccinations) to increase the antibody responses elicited by the GeoVax vaccine regimen. These “late boosts” consist of the GeoVax MVA62B vaccine with or without a gp120 protein vaccine. The gp120 protein, AIDSVAX® B/E, is the same protein used to boost immune responses in the partially protective RV144 trial in Thailand and is being used in HVTN 114 to assess the effect of adding a protein vaccine to GOVX-B11. Participants in HVTN 114 receive either (a) another MVA62B boost, (b) a combined boost of MVA62B and AIDSVAX® B/E, or (c) AIDSVAX® B/E alone. HVTN 114 was completed during 2018 and results were presented during the HIV Research for Prevention (HIVR4P) conference in Madrid, Spain in October. The study demonstrated the most effective boost to be the combination of MVA62B live vector and AIDSVAX B/E proteins, which increased titers of antibodies to the HIV envelope glycoproteins by more than 600-fold.
Following completion of HVTN 114, the HVTN is moving forward with plans for an additional phase 1 trial, designated HVTN 132, which will be a multi-center, randomized, double-blind trial, enrolling up to 70 healthy adults. The primary objectives of HVTN 132 will be to further assess the safety, tolerability and immunogenicity (elicited antibody responses) of a prime-boost regimen of GOVX-B11, in combination with protein boost vaccines. The protein boosts are being tested for their ability to enhance the antibody response elicited by GOVX-B11 to gp120. The protein boosts to be evaluated in the trial were developed by Duke University and by the Institute of Human Virology of the University of Maryland School of Medicine. HVTN 132 will be conducted by the HVTN with support from NIAID and is expected to commence patient enrollment in late-2020.
Clade C Preventive HIV Vaccine Program. We also are developing DNA/MVA vaccines designed for use against the clade C subtype of HIV that predominate in South Africa and India. NIAID has awarded GeoVax Small Business Innovative Research (SBIR) grants in support of this effort, but further development of these vaccines will be dependent upon additional funding support.
Our HIV Immunotherapy Program – Seeking a Cure
Finding a cure for HIV/AIDS remains an elusive goal. Current anti-retroviral therapies (ART), though highly effective at suppressing HIV viral load, are unable to eliminate latent forms of HIV that are invisible to the immune system and inaccessible to antiretroviral drugs. Long-term use of ART can lead to loss of drug effectiveness and can come with severe, debilitating side effects. The lifetime medical costs saved by preventing (or curing) a single HIV infection in the U.S. are estimated to approach $400,000. Therefore, any new treatment regimen that allows patients to reduce, modify, or discontinue their antiretroviral therapy could offer measurable quality of life benefits to the patient and tremendous value to the marketplace.
Collaboration with AGT – In March 2017, we entered into collaboration with American Gene Technologies International, Inc. (AGT) whereby AGT intends to conduct a Phase 1 human clinical trial with our combined technologies, with the ultimate goal of developing a functional cure for HIV infection. In the AGT trial, the GeoVax vaccine will be used to stimulate virus specific CD4+ T cells in vivo, which will then be harvested from the patient, genetically modified ex vivo using AGT’s technology, and reinfused to the patient. The primary objectives of the trial will be to assess the safety and efficacy of the therapy, with secondary objectives to assess the immune responses as a measure of efficacy. In a previous phase 1 clinical trial (GV-TH-01), we demonstrated that our vaccine can stimulate production of CD4+ T cells in HIV infected patients– the intended use of the GV-MVA-VLPTM HIV vaccine in the proposed AGT study. AGT has recently stated their intention to begin the phase 1 trial during mid-2020.
Collaboration with UCSF – In November 2019, we entered into an agreement with the University of California, San Francisco (UCSF), whereby we will participate in a collaborative effort led by researchers at UCSF to develop a combinational therapy aimed at inducing remission in HIV-positive individuals (a “functional cure”). The studies will be conducted with funding from amfAR, The Foundation for AIDS Research. The proposed clinical trial will enroll 20 HIV-infected adults who are on stable and effective anti-retroviral therapy (ART). The therapeutic regimen to be tested involves a combination of vaccines, drugs and biologics. GeoVax will provide its novel boost component (MVA62B) for use in the studies. MVA62B is the boosting component for our preventive HIV vaccine (GOVX-B11) which has successfully completed a Phase 2a clinical trial. The primary objectives of the trial will be to assess the safety and tolerability of the combinational therapy and to determine the viral load “set-point” during a treatment interruption. Secondary objectives will be to assess immune responses and changes in viral reservoir status. Patient enrollment for the clinical trial is expected to commence during 2020.
Our Filovirus (Ebola, Sudan, Marburg) Vaccine Program
Ebola (EBOV, formerly designated as Zaire ebolavirus), Sudan (SUDV), and Marburg viruses (MARV) are the most virulent species of the Filoviridae family. They can cause up to a 90% fatality rate in humans and are epizootic in Central and West Africa with 29 outbreaks since 1976. The 2013-16 Ebola outbreak caused 28,616 cases and 11,310 deaths (case fatality rate of 40%). In August 2018, the Ministry of Health of the Democratic Republic of the Congo declared a new outbreak of Ebola virus disease in North Kivu Province. Despite responses from the Ministry of Health, WHO, and its partners to contain this outbreak, there have been 3,431 cases, 2,253 people have died after contracting the disease and 1,178 patients have recovered (case fatality rate of 66%) as of February 13, 2020. Even after the current outbreak is contained, additional outbreaks are certain in future due to indigenous reservoirs of the virus (e.g. fruit bats), the zoonotic nature of the virus, weak health systems, high population mobility, political unrest, cultural beliefs and burial practices, and for those not at natural risk, the risk of intentional release by a bioterrorist.
We believe an ideal vaccine against major filoviruses must activate both humoral and cellular arms of the immune system. It should include the induction of antibodies to slow the initial rate of infection and a cellular immune response to help clear the infection. Moreover, it should address strain variations by providing broad coverage against potential epizootic filovirus strains, and it should be safe not only in healthy individuals (e.g. travelers or health care workers), but also in immunocompromised persons (e.g., HIV infected) and those with other underlying health concerns.
Despite significant progress being made with some experimental vaccines in clinical trials, none have been fully tested for both safety and efficacy. The replication competent rVSV-ZEBOV showed safety concerns in Phase 1 trials and by virtue of being replication competent could pose threats to immunocompromised individuals, such as those infected with HIV living in West Africa where recent Ebola epidemics started. The less advanced adeno-vectored vaccine candidates may require relatively cumbersome heterologous prime/boost regimens, for example with MVA, to elicit durable protective immunity. The use of Ad5 vectors also has been associated with concerns over increased susceptibility to HIV infection in areas with high HIV incidence. Even with rVSV-ZEBOV showing promise in the 2013-2015 epidemic, the world would benefit by being prepared with a safer and effective vaccine, to prevent or alleviate the effects of the next epidemic.
To address the unmet need for a product that can respond to future filovirus epidemics we are developing innovative vaccines utilizing our GV-MVA-VLPTM platform. We are addressing strain variations, and induction of broad humoral and cellular response through development of monovalent vaccines, which we may also investigate blending together as a single vaccine to provide broad coverage, potentially with a single dose. The MVA vector itself is considered safe, having originally been developed for use in immunocompromised individuals as a smallpox vaccine. We expect our vaccines to not only protect at-risk individuals against EBOV, SUDV and MARV, but also potentially reduce or modify the severity of other re-emerging filovirus pathogens such as Bundibugyo, Ivory Coast, and Reston viruses, based on antigenic cross reactivity and the elicitation of T cells to the more conserved matrix proteins (e.g. VP40 or Z) in addition to standard GP proteins used by us and other manufacturers. Thus, the GeoVax GV-MVA-VLPTM approach could offer a unique combination of advantages to achieve breadth and safety of a pan-filo vaccine. In addition to protecting people in Africa, it is intended to prevent the spread of disease to the US, and for preparedness against terrorist release of any of bio-threat pathogens.
Our initial preclinical studies in rodents and nonhuman primates for our EBOV vaccine candidate have shown 100% protection against a lethal dose of EBOV upon a single immunization. These studies were conducted with support from NIAID and USAMRIID. We have also designed and constructed vaccine candidates for SUDV and MARV. In a recent independent, peer-reviewed paper published by Lazaro Frias et al (J Virol. 2018 Jun 1; 92(11): e00363-18), the authors concluded that the MVA-VLP-Ebola and MVA-VLP-Sudan vaccines are the best-in class vaccine in development.
In July 2019, we reported positive results (100% protection) from preclinical challenge studies of our MARV vaccine candidate. In this study, our MARV vaccine was administered by intramuscular (IM) inoculations to guinea pigs, with a control group receiving saline injections. Eight weeks after inoculation, animals in each group were exposed to a lethal dose of MARV. Within 8 days post-challenge, all animals in the control group had developed moribund conditions and had to be euthanized. At the conclusion of the study (21 days post-challenge), all vaccinated animals survived, with no weight loss or other health issues. The study was conducted in collaboration with researchers at the University of Texas Medical Branch at Galveston. (UTMB).
Further development of our filovirus vaccines will be dependent upon additional funding support.
Our Lassa Fever Vaccine Program
Lassa fever virus (LASV), a member of the Arenaviridae family, causes severe and often fatal hemorrhagic illnesses in an overlapping region with Ebola. Lassa Fever is an acute viral hemorrhagic illness caused by LASV. In contrast to the unpredictable epidemics of filoviruses, LASV is endemic in West Africa with an annual incidence of >300,000 infections, resulting in 5,000-10,000 deaths. Data from a recent independent study suggest that the number of annual Lassa Fever cases may be much higher, reaching three million infections and 67,000 deaths, putting as many as 200 million persons at risk.
Our initial preclinical studies in rodents for our LASV vaccine candidate have shown 100% single-dose protection against a lethal dose of LASV challenge composed of multiple strains delivered directly into the brain. The study was conducted at the Institute of Human Virology at the University of Maryland School of Medicine in Baltimore. Multiple repeats of the study confirmed the findings.
SBIR Grant – Subsequent to these initial findings, in April 2018 NIAID awarded us a $300,000 Small Business Innovative Research (SBIR) grant in support of further advancing our Lassa vaccine development program. The work was performed in collaboration with the Institute of Human Virology at the University of Maryland, The Scripps Research Institute, and the University of Texas Medical Branch.
Defense Department Grant – In September 2018, the U.S. Department of Defense (DoD) awarded us a $2,442,307 cooperative agreement in support of our LASV vaccine development program. The grant was awarded by the U.S. Army Medical Research Acquisition Activity pursuant to the Peer Reviewed Medical Research Program (PRMRP), part of the Congressionally Directed Medical Research Programs (CDMRP). In addition to the grant funds provided directly to GeoVax, DoD will also fund testing of the GeoVax vaccine by U.S. Army scientists at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), under a separate subaward. The project award is supporting generation of immunogenicity and efficacy data for our vaccine candidate in both rodent and nonhuman primate models, as well as manufacturing process development and cGMP production of vaccine seed stock in preparation for human clinical trials. The work is being performed in collaboration with USAMRIID and the Geneva Foundation.
Further development of our Lassa Fever vaccine beyond the work being funded by the U.S. DoD will be dependent upon additional funding and/or partnering support.
Our Zika Vaccine Program
Zika disease is an emerging infectious disease caused by the Zika virus (ZIKV) and has been linked to an increase in microcephaly in infants and Guillain-Barre syndrome (a neurodegenerative disease) in adults. ZIKV is a member of the Flaviviridae family, which includes medically important pathogens such as dengue fever, yellow fever, Japanese encephalitis, tick-borne encephalitis, and West Nile viruses. ZIKV, which was first discovered in 1947 in the Zika forest of Uganda, was considered only a minor public health concern for 60 years. Recently, with its appearance and rapid spread in the Americas, it has emerged as a serious threat with pandemic potential. Symptoms of Zika infection have historically been mild. In the recent epidemic, however, an alarming association between ZIKV infection and fetal brain abnormalities including microcephaly has been observed. No approved preventive or therapeutic products are currently available to fight the Zika epidemic. Public health officials recommend avoiding exposure to ZIKV, delaying pregnancy, and following basic supportive care (fluids, rest, and acetaminophen) after infection. A vaccine is needed to prevent a Zika pandemic.
To address the unmet need for a ZIKV vaccine, we are developing novel vaccine candidates constructed in our MVA live vector platform, which has already shown promise in our HIV, Ebola and Lassa vaccines. We believe that, unlike other vaccines in development, the GeoVax vaccine combines a highly potent, yet safe, replication deficient viral vector (MVA) to deliver novel antigens of ZIKV to develop a single-dose vaccine. MVA has an outstanding safety record, which is particularly important given the need to include women of child-bearing age and newborns among those being vaccinated. Our Zika vaccine does not appear to induce Antibody Dependent Enhancement (ADE) of infection. ADE is a serious side effect induced when a vaccinated individual is bitten a second time by a mosquito carrying a second flavivirus such as dengue, resulting in a more virulent reaction. These features could yield a safe and highly effective vaccine that is well suited to provide potent and durable immunity against ZIKV infection.
Our initial preclinical studies in rodents for our ZIKV vaccine candidate have shown 100% single-dose protection against a lethal dose of ZIKV delivered directly into the brain. The study was conducted and funded by the US Centers for Disease Control and Prevention (CDC), which also provided technical assistance.
SBIR Grant – Subsequent to these initial findings, in June 2017 NIAID awarded us a Small Business Innovative Research (SBIR) grant in support of further advancing our development program. The $600,000 two-year grant supported preclinical testing of our ZIKV vaccine in nonhuman primates in preparation for human clinical trials.
Further development of our ZIKV vaccine will be dependent upon additional funding and/or partnering support.
Our Malaria Vaccine Programs
Malaria is a mosquito-borne disease caused by Plasmodium parasites. Symptoms are fever, chills, sweating, vomiting and flu-like illness. If untreated, severe complications (severe anemia, cerebral malaria and organ failure) will lead to death. Over 3 billion people in 106 countries and territories live at risk of malaria infection. According to the latest estimates from the World Health Organization (WHO), 214 million new cases of malaria were recorded worldwide in 2015, resulting in 438,000 deaths. There are 1,500 cases in the US each year (travelers returning home). Children under five years of age are particularly susceptible to malaria illness, infection, and death. In 2015, malaria killed an estimated 306,000 children. Current treatments include bed net distributions, drug treatment and mosquito spraying. Malaria parasites develop resistance to drugs and insecticides. Even though vaccines have shown to be the most cost-effective ways to fight and eliminate infectious diseases (Smallpox, polio, etc.), and after many decades of research and development, there is no commercial malaria vaccine at the present time. Even a vaccine with efficacy of 30-50% will prevent hundreds of thousands of deaths annually. Current vaccine candidates generally consist of subunit proteins, are poorly immunogenic, based on limited number of antigens (generally 4-5 antigens), do not target multiple stages of parasite life cycle, and do not induce strong durable functional antibodies and T cell responses. Therefore, identification of appropriate antigens and vaccine technologies is critical for development of an effective malaria vaccine.
An ideal malaria vaccine candidate should contain antigens from multiple stages of the malaria parasite’s life cycle, and should induce both functional antibodies (predominantly IgG1 and IgG3 subtypes shown to be associated with protection) and strong cell mediated immunity (e.g. Th1 biased CD4+ ad CD8+) to reduce parasitemia by clearing infected cells (liver cells or erythrocytes). We have shown (in animal models and humans) that GV-MVA-VLPTM vaccines for non-malarial disease targets can induce a Th1 biased response with both durable functional antibodies (IgG1 and IgG3) and CD4+ and CD8+ T cell responses, both of which are hallmarks of an ideal malaria vaccine.
Collaboration with Burnet Institute – We have established a collaboration with the Burnet Institute, a leading infectious diseases research institute in Australia, for the development of a vaccine to prevent malaria infection. The project includes the design, construction, and characterization of multiple malaria vaccine candidates using GeoVax’s GV-MVA-VLPTM vaccine platform combined with malaria Plasmodium falciparum and Plasmodium vivax sequences identified by the Burnet Institute. The vaccine design, construction, and characterization will be performed at GeoVax with further characterization and immunogenicity studies in animal models conducted at Burnet Institute using their unique functional assays that provide key information on vaccine efficacy.
Collaboration with Leidos – In February 2019, we began a collaboration with Leidos, Inc. to develop malaria vaccine candidates. The work is supported under a contract to Leidos from the United States Agency for International Development (USAID) Malaria Vaccine Development Program (MVDP). Leidos has been tasked by USAID to advance promising vaccine candidates against P. falciparum malaria and selected the GeoVax GV-MVA-VLPTM platform as part of this development effort. The new collaboration with Leidos complements our ongoing malaria vaccine development project with Burnet Institute and offers a separate opportunity for success. The collaboration also expands upon our existing relationship with Leidos for our cancer immunotherapy program (see below).
Our Hepatitis B Vaccine Program
Hepatitis B is a contagious liver disease caused by the Hepatitis B virus (HBV). It is transmitted person-to-person by blood, semen, or other bodily fluids. This can happen through sexual contact, needle sharing, or mother to infant transmission during birth. For some people, Hepatitis B is an acute (or short-term) illness; but for others, it can become a long-term, chronic infection that may lead to serious health issues like cirrhosis or liver cancer. The risk of chronic infection is related to age at infection. Approximately 90% of infected infants will develop chronic infections. As a child gets older, the risk decreases. Approximately 25%–50% of children infected between the ages of 1 and 5 years will develop chronic hepatitis. The risk drops to 6%–10% when a person is infected at over 5 years of age. Worldwide, most people with chronic Hepatitis B were infected at birth or during early childhood.
The CDC estimates that between 700,000 to 1.4 million people in the United States have chronic HBV infections, with an estimated 20,000 new infections every year. Many people are unaware that they are infected or may not show any symptoms. Therefore, they never seek the attention of medical or public health officials. Globally, chronic Hepatitis B affects more than 240 million people and contributes to nearly 686,000 deaths worldwide each year. Even though a preventive HBV vaccine is available, less than 5% of chronic HBV infections are cured through currently available therapies.
There is a clear medical need to treat chronic HBV infections, which affect hundreds of millions of people around the world, many of whom die due to complications of HBV including cirrhosis and cancer. Multiple vaccines exist to protect against HBV infection, but they cannot help patients already diagnosed with the disease. Although chronic HBV can be treated with drugs, the treatments do not cure 95% of patients; they cannot induce strong neutralizing antibodies and cellular responses needed to break tolerance to HBV antigens and clear infections, but only suppress the replication of the virus. Therefore, most people who start treatments must continue with them for life. Moreover, diagnosis and treatment options are very limited in resource/low income-constrained populations, which leads to many patients succumbing within months of diagnosis.
Our combination therapeutic vaccine strategy is comprised of multivalent vaccine antigens delivered by DNA and GV-MVA-VLPTM in combination with the standard-of-care treatment to induce functional antibodies and CD4+, CD8+ T cell responses to clear infection and break tolerance needed toward a functional cure. Our goal is to significantly increase the current cure rate of HBV infections while reducing the duration of drug therapy, overall treatment costs, side effects, and potential drug resistance.
Collaboration with GSURF – Given the challenges and difficulties of developing an effective therapy for chronic HBV infections, our strategy is to engage with multiple collaborators for combination therapies to increase our chances of success. We are collaborating with Georgia State University Research Foundation (GSURF) on a project that includes the design, construction, characterization and animal testing of multiple vaccine candidates using our GV-MVA-VLPTM vaccine platform. Vaccine antigens include both GeoVax and GSURF’s proprietary designed sequences. This project is ongoing.
Further development of our Hepatitis B vaccine will be dependent upon additional funding and/or partnering support.
Novel Vaccine Delivery Evaluation
Given that several of our programs involve infectious disease targets (e.g. EBOV, LASV, etc.) prevalent in third world countries, we are exploring a novel vaccine delivery platform that may simplify vaccine administration and/or reduce storage and distribution costs.
Collaboration with Enesi – In January 2019, we announced a collaboration with Enesi Pharma, an innovative pharmaceutical company developing unique injectable solid-dose drug-device vaccine products, to develop solid-dose needle-free vaccine formulations utilizing our GV-MVA-VLPTM vaccine platform in combination with Enesi’s ImplaVax® device and formulation technology. The collaboration is expected to include development of thermostable solid-dose needle-free vaccines for a variety of infectious diseases and evaluation of the potential to generate improved vaccine responses with simplified administration and reduced storage and distribution costs. Enesi’s proprietary ImplaVax® solid-dose formulation and needle-free device technology comprises three main components: a single solid-dose Universal Vaccine Implant (UVI) containing the vaccine construct, a separate single-use disposable unit dose cassette pre-loaded with a single solid UVI and a reusable handheld spring-powered actuator. The benefits could include assured consistency with dosing, better product stability and ease of use as well as the potential to minimize vaccination pain and stress, and to eliminate needle disposal and needle stick injuries. We have successfully formulated our GV-MVA-VLPTM-Ebola in the ImplaVax device and completed vaccination of mice. We are currently analyzing the immune responses compared to that of the standard of syringe and needles.
Support from the United States Government
Grants and Contracts. We have been the recipient of multiple federal grants and contracts in support of our vaccine development programs. Our most recent awards are as follows:
Lassa DoD Grant. In September 2018, the U.S. Department of Defense (DoD) awarded us a $2,442,307 cooperative agreement in support of our LASV vaccine development program. The grant was awarded by the U.S. Army Medical Research Acquisition Activity pursuant to the Peer Reviewed Medical Research Program (PRMRP), part of the Congressionally Directed Medical Research Programs (CDMRP). In addition to the grant funds provided directly to GeoVax, DoD will also fund testing of our vaccine by U.S. Army scientists under a separate subaward. The award, entitled “Advanced Preclinical Development and Production of Master Seed Virus of GEO-LM01, a Novel MVA-VLP Vaccine Against Lassa Fever”, will support generation of immunogenicity and efficacy data for our vaccine candidate in both rodent and nonhuman primate models, as well as manufacturing process development and cGMP production of vaccine seed stock in preparation for human clinical trials.
Lassa SBIR Grant. In April 2018, NIAID awarded us a $300,000 SBIR grant entitled “Construction and efficacy testing of novel recombinant vaccine designs for eliciting both broadly neutralizing antibodies and T cells against Lassa virus.”
Malaria Contract with Leidos – In March 2019, we entered into a $196,126 subcontract with Leidos, Inc., supported by a contract to Leidos from the United States Agency for International Development (USAID) Malaria Vaccine Development Program (MVDP). Leidos has been tasked by USAID to advance promising vaccine candidates against P. falciparum malaria and selected the GeoVax GV-MVA-VLPTM platform as part of this development effort. In January 2020, the work was extended through an additional subcontract for $385,193.
Zika SBIR Grant. In June 2017, NIAID awarded us a SBIR grant entitled “Advanced Preclinical Testing of a Novel Recombinant Vaccine Against Zika Virus.” The initial grant award was $300,000 for the first year of a two-year project period beginning June 24, 2017, with a total project budget of $600,000. In May 2018, the second-year grant of $300,000 was awarded to us.
HIV Staged Vaccine Development Contract. In August 2016, NIAID awarded us a Staged Vaccine Development contract to produce our preventive HIV vaccine for use in future clinical trials. The award included a base contract of $199,442 for the initial period from August 1, 2016 to December 31, 2017 (the “base period”) to support process development, as well as $7.6 million in additional development options that can be exercised by NIAID. Prior to the end of the base period NIAID notified us that it did not plan to exercise the additional development option under the contract due to funds availability and NIAID’s programmatic needs. We do not expect this to have an impact on the human clinical trials of our preventive HIV vaccine currently being conducted by the HVTN, or future trials being planned.
HIV SBIR Grant. In April 2016, NIAID awarded us a SBIR grant entitled “Enhancing Protective Antibody Responses for a DNA/MVA HIV Vaccine.” The initial grant award was $740,456 for the first year of a two-year project period beginning April 15, 2016, with a total project budget of $1,398,615. In March 2017, NIAID awarded us $658,159 for the second year of the project period to test the effects of adding two proteins to our vaccine regimen, and we subsequently received a one-year no-cost extension of the project period, which was completed during 2019.
Clinical Trial Support. All our human clinical trials to date for our preventive HIV vaccines, including the recently completed HVTN 114 trial and the HVTN 132 trial currently planned, have been or will be conducted by the HVTN and funded by NIAID. This financial support has been provided by NIAID directly to the HVTN, so has not been recognized in our financial statements, and we do not know the cost of these trials. See “Our Preventive HIV Vaccine Program” above for the current status of our human clinical trials.
Other Federal Support. We have been the recipient of additional in-kind federal support through collaborative and intramural arrangements with CDC for our Zika vaccine program, the Rocky Mountain Laboratory facility of NIAID for our hemorrhagic fever virus vaccine program, and the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) for our hemorrhagic fever virus vaccine program. This support generally has been for the conduct or support of preclinical animal studies on our behalf.
If we are unable to obtain new grants, or if grants that have been awarded are terminated, or if clinical trial and other support becomes unavailable, it could have a material adverse effect on our business.
Government Regulation
Regulation by governmental authorities in the United States and other countries is a significant factor in our ongoing research and development activities and in the manufacture of our products. Complying with these regulations involves considerable expertise, time and expense.
In the United States, drugs and biologics are subject to rigorous federal and state regulation. Our products are regulated under the Federal Food, Drug and Cosmetic Act, the Public Health Service Act, and the regulations promulgated under these statutes, and other federal and state statutes and regulations. These laws govern, among other things, the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of medications and medical devices. Product development and approval within this regulatory framework is difficult to predict, takes several years and involves great expense. The steps required before a human vaccine may be marketed in the United States include:
|
● |
Preclinical laboratory tests, in vivo preclinical studies and formulation studies; |
|
● |
Manufacturing and testing of the product under strict compliance with current Good Manufacturing Practice (cGMP) regulations; |
|
● |
Submission to the FDA of an Investigational New Drug application for human clinical testing which must become effective before human clinical trials can commence; |
|
● |
Adequate and well-controlled human clinical trials to establish the safety and efficacy of the product; |
|
● |
The submission of a Biologics License Application to the FDA, along with the required user fees; and |
|
● |
FDA approval of the BLA prior to any commercial sale or shipment of the product |
Before marketing any drug or biologic for human use in the United States, the product sponsor must obtain FDA approval. In addition, each manufacturing establishment must be registered with the FDA and must pass a pre-approval inspection before introducing any new drug or biologic into commercial distribution.
Because GeoVax does not manufacture vaccines for human use within our own facilities, we must ensure compliance both in our own operations and in the outsourced manufacturing operations. All FDA-regulated manufacturing establishments (both domestic establishments and foreign establishments that export products to the United States) are subject to inspections by the FDA and must comply with the FDA’s cGMP regulations for products, drugs and devices.
FDA determines compliance with applicable statutes and regulations through documentation review, investigations, and inspections. Several enforcement mechanisms are available to FDA, ranging from a simple demand to correct a minor deficiency to mandatory recalls, closure of facilities, and even criminal charges for the most serious violations.
Even if FDA regulatory clearances are obtained, a marketed product is subject to continual review, and later discovery of previously unknown problems or failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions.
Whether or not the FDA has approved the drug, approval of a product by regulatory authorities in foreign countries must be obtained prior to the commencement of commercial sales of the drug in such countries. The requirements governing the conduct of clinical trials and drug approvals vary widely from country to country, and the time required for approval may be longer or shorter than that required for FDA approval.
We also are subject to various federal, state and local laws, regulations, and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals, and the use and disposal of hazardous or potentially hazardous substances used in connection with our research. The extent of government regulation that might result from any future legislation or administrative action cannot be accurately predicted.
Manufacturing
We do not have the facilities or expertise to manufacture any of the clinical or commercial supplies of any of our products. To be successful, our products must be manufactured in commercial quantities in compliance with regulatory requirements and at an acceptable cost. To date, we have not commercialized any products, nor have we demonstrated that we can manufacture commercial quantities of our product candidates in accordance with regulatory requirements. If we cannot manufacture products in suitable quantities and in accordance with regulatory standards, either on our own or through contracts with third parties, it may delay clinical trials, regulatory approvals and marketing efforts for such products. Such delays could adversely affect our competitive position and our chances of achieving profitability. We cannot be sure that we can manufacture, either on our own or through contracts with third parties, such products at a cost or in quantities that are commercially viable.
We currently rely and intend to continue to rely on third-party contract manufacturers to produce vaccines needed for research and clinical trials. We have arrangements with third party manufacturers for the supply of our DNA and MVA vaccines for use in our planned clinical trials. These suppliers operate under the FDA’s Good Manufacturing Practices and (in the case of European manufacturers) similar regulations of the European Medicines Agency. We anticipate that these suppliers will be able to provide sufficient vaccine supplies to complete our currently planned clinical trials. Various contractors are generally available in the United States and Europe for manufacture of vaccines for clinical trial evaluation, however, it may be difficult to replace existing contractors for certain manufacturing and testing activities and costs for contracted services may increase substantially if we switch to other contractors.
The MVA component of our vaccine is currently manufactured in cells that are cultured from embryonated eggs. We are exploring a number of approaches to growing MVA in continuous cell lines that can be grown in bioreactors more suitable for commercial-scale manufacturing.
Competition
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be competitive with our products. As we develop and seek to ultimately commercialize our product candidates, we face and will continue to encounter competition with an array of existing or development-stage drug and immunotherapy approaches targeting diseases we are pursuing. We are aware of various established enterprises, including major pharmaceutical companies, broadly engaged in vaccine/immunotherapy research and development. These include Janssen Pharmaceuticals, Sanofi-Aventis, GlaxoSmithKline, Merck, Pfizer, and MedImmune. There are also various development-stage biotechnology companies involved in different vaccine and immunotherapy technologies including Aduro Biotech, Advaxis, BioNTech, Curevac, Dynavax, Juno, Moderna, and Novavax. If these companies are successful in developing their technologies, it could materially and adversely affect our business and our future growth prospects. The number of companies seeking to develop products and therapies for the treatment of unmet needs in these indications is likely to increase. Some of these competitive products and therapies are based on scientific approaches that are similar to our approaches, and others are based on entirely different approaches.
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Our competitors’ products may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. We expect any products that we develop and commercialize to compete based on, among other things, efficacy, safety, convenience of administration and delivery, price, the level of generic competition and the availability of reimbursement from government and other third-party payers.
There are currently no FDA licensed and commercialized HIV vaccines, Zika vaccines, or hemorrhagic fever virus vaccines (other than for Ebola) available in the world market. We are aware of several development-stage and established enterprises, including major pharmaceutical and biotechnology firms, which are actively engaged in vaccine research and development in these areas. For hemorrhagic fever viruses, these include NewLink Genetics and Merck, Johnson & Johnson, Novavax, Profectus Biosciences, Protein Sciences, Inovio and GlaxoSmithKline. For HIV, these include Sanofi, GlaxoSmithKline, and Johnson & Johnson. Other HIV vaccines are in varying stages of research, testing and clinical trials including those supported by the NIH Vaccine Research Center, the U.S. Military, IAVI, the European Vaccine Initiative, and the South African AIDS Vaccine Initiative. For Zika, these include NewLink Genetics, Inovio, Merck, Butantan Institute and NIH (NIAID). In December 2019, the FDA approved the first vaccine (Ervebo) for prevention of Ebola, developed by Merck.
There are numerous FDA-approved treatments for HIV, primarily antiretroviral therapies, marketed by large pharmaceutical companies. Currently, there are no approved therapies for the eradication of HIV. We expect that major pharmaceutical companies that currently market antiretroviral therapy products or other companies that are developing HIV product candidates may seek to develop products for the eradication of HIV.
There are currently no commercialized vaccines to treat chronic HBV infection. Multiple vaccines exist to protect against HBV infection, but they cannot help patients already diagnosed with the disease. Although chronic HBV can be treated with drugs, the treatments do not cure 95% of patients; they cannot induce strong neutralizing antibodies and cellular responses needed to break tolerance to HBV antigens and clear infections, but only suppress the replication of the virus.
There are currently no commercialized vaccines to prevent malaria infection. A first generation infection-blocking malaria vaccine, RTS,S, is under regulatory review. It requires 4 doses and has been recommended by the WHO for pilot implementation studies. Since this vaccine is based on a single antigen and has modest efficacy (30-40%, depending on the age of subjects), the WHO has defined a Road Map for developing and licensing of next generation malaria vaccines. These vaccines are expected to contain multiple antigens designed to block both infection and transmission of malaria with at least a 75% efficacy rate.
A number of companies are developing various types of therapeutic vaccines or other immunotherapy approaches to treat cancer including Advaxis, Immune Design, Oncothyreon, Bavarian Nordic, Roche Pharmaceuticals, Merck & Co, Bristol Myers Squibb, AstraZeneca plc, and Medimmune, LLC.
Our Intellectual Property
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary rights are described by valid and enforceable patents or are effectively maintained as trade secrets. Accordingly, we are pursuing and will continue to pursue patent protection for our proprietary technologies obtained or developed through our collaborations or developed by us alone. Our patent portfolio includes applications directed to DNA and MVA-based HIV vaccines, their genetic inserts expressing multiple HIV protein components, composition, structure, claim of immunization against multiple subtypes of HIV, routes of administration, safety and other related factors and methods of therapeutic and prophylactic use thereof including administration regimes. Also included are applications directed to preventive vaccines against hemorrhagic fever viruses (Ebola, Sudan, Marburg and Lassa), Zika virus and malaria, and use thereof; immuno-oncology vaccine compositions and methods of use thereof; and therapeutic vaccines against HBV and use thereof. We are the licensee of at least nine issued or allowed U.S. patents and at least twenty-three issued or allowed non-U.S. patents. We are actively pursuing two U.S. provisional applications, two non-U.S. and two international patent applications as the owner of record, in addition to at least two non-U.S. patent applications under license.
We are the exclusive, worldwide licensee of several patents and patent applications, which we refer to as the Emory Technology, owned, licensed or otherwise controlled by Emory University for HIV or smallpox vaccines pursuant to a license agreement originally entered into on August 23, 2002 and restated on June 23, 2004 (the “Emory License”). Through the Emory License we are also a non-exclusive licensee of four issued United States patents owned by the NIH related to the ability of our MVA vector vaccine to operate as a vehicle to deliver HIV virus antigens, and to induce an immune response in humans.
We are not a party to any litigation, opposition, interference, or other potentially adverse proceeding with regard to our patent positions. However, if we become involved in litigation, interference proceedings, oppositions or other intellectual property proceedings, for example as a result of an alleged infringement or a third-party alleging an earlier date of invention, we may have to spend significant amounts of money and time and, in the event of an adverse ruling, we could be subject to liability for damages, invalidation of our intellectual property and injunctive relief that could prevent us from using technologies or developing products, any of which could have a significant adverse effect on our business, financial conditions or results of operations. In addition, any claims relating to the infringement of third-party proprietary rights, or earlier date of invention, even if not meritorious, could result in costly litigation, lengthy governmental proceedings, divert management’s attention and resources and require us to enter royalty or license agreements which are not advantageous if available at all.
In addition to patent protection, we also attempt to protect our proprietary products, processes and other information by relying on trade secrets and non-disclosure agreements with our employees, consultants and certain other persons who have access to such products, processes and information. Under these agreements, all inventions conceived by employees are our exclusive property. Nevertheless, there can be no assurance that these agreements will afford significant protection against misappropriation or unauthorized disclosure of our trade secrets and confidential information.
We cannot be certain that any of the current pending patent applications we have licensed, or any new patent applications we may file or license, will ever be issued in the United States or any other country. Even if issued, there can be no assurance that those patents will be sufficiently broad to prevent others from using our products or processes. Furthermore, our patents, as well as those we have licensed or may license in the future, may be held invalid or unenforceable by a court, or third parties could obtain patents that we would need to either license or to design around, which we may be unable to do. Current and future competitors may have licensed or filed patent applications or received patents and may acquire additional patents or proprietary rights relating to products or processes competitive to ours. In addition, any claims relating to the infringement of third-party proprietary rights, or earlier date of invention, even if not meritorious, could result in costly litigation, lengthy governmental proceedings, divert management’s attention and resources and require us to enter royalty or license agreements which are not advantageous to us, if available at all.
Research and Development
Our expenditures for research and development activities were $1,910,715 and $1,878,652 during the years ended December 31, 2019 and 2018, respectively. As our vaccines continue to go through the process to obtain regulatory approval, we expect our research and development costs to increase. We have not yet formulated any plans for marketing and sales of any vaccine candidate we may successfully develop. Compliance with environmental protection laws and regulations has not had a material effect on our capital expenditures, earnings or competitive position to date.
Scientific Advisors
We seek advice from our Scientific Advisory Board, which consists of a number of leading scientists, on scientific and medical matters. The current members of our Scientific Advisory Board are:
| Name | Position/Institutional Affiliation | |
| Thomas P. Monath, MD | Managing Partner and Chief Scientific Officer at Crozet Biopharma | |
| Stanley A. Plotkin, MD | Professor Emeritus, University of Pennsylvania, | |
| Adjunct Professor, Johns Hopkins University | ||
| Barney S. Graham, MD, PhD | Senior Investigator, Vaccine Research Center, NIAID | |
| Scott C. Weaver, PhD | Director, University of Texas Medical Branch Institute for Human Infections and Immunity | |
| Scientific Director, Galveston National Laboratory | ||
| Olivera J. Finn, PhD | Distinguished Professor of Immunology and Surgery, University of Pittsburgh |
Properties and Employees
We lease approximately 8,400 square feet of office and laboratory space located at 1900 Lake Park Drive, Suite 380, Smyrna, Georgia under a lease agreement which expires on December 31, 2022. We believe this space is adequate for our current needs. We currently have six full-time and one part-time employees. None of our employees are covered by collective bargaining agreements and we believe that our employee relations are good.
Corporate Background
Our primary business is conducted by our wholly owned subsidiary, GeoVax, Inc., which was incorporated under the laws of Georgia in June 2001. In September 2019, we incorporated another wholly owned subsidiary, Immutak, Oncology, Inc., under the laws of Delaware. Our address is 1900 Lake Park Drive, Smyrna, Georgia 30080, and our telephone number at that address is 678-384-7220. The predecessor of our parent company, GeoVax Labs, Inc. (the reporting entity) was originally incorporated in June 1988 under the laws of Illinois as Dauphin Technology, Inc. (“Dauphin”). In September 2006, Dauphin completed a merger with GeoVax, Inc. As a result of the merger, GeoVax, Inc. became a wholly owned subsidiary of Dauphin, and Dauphin changed its name to GeoVax Labs, Inc. In June 2008, the Company was reincorporated under the laws of Delaware. We currently do not conduct any business other than GeoVax, Inc.’s business of developing new products for the treatment or prevention of human diseases. Our principal offices are in Smyrna, Georgia (metropolitan Atlanta).
Available Information
Our website address is www.geovax.com. We make available on this website under “Investors – SEC Reports,” free of charge, our SEC filings, such as proxy statements, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file or furnish such materials to the SEC. We also make available our Code of Ethics on this website under the heading “Investors – Corporate Governance”. Information contained on our website is not incorporated into this Annual Report.
Reverse Stock Split and Outstanding Rights to Acquire our Common Stock
1-for-2000 Reverse Stock Split. In December 2019, we sent notice to our stockholders of a special meeting to be held on January 3, 2020, at which they were asked to approve a 1-for-2000 reverse stock split. The Company had insufficient authorized but unissued shares of common stock available to meet its obligations pursuant to its convertible preferred stock and to issue in connection with additional financing transactions. Unless otherwise indicated, all share and per share amounts in this document reflect the reverse stock split.
On January 21, 2020, we filed a Certificate of Amendment to the Certificate of Incorporation of GeoVax Labs, Inc. effecting a 1-for-2000 reverse stock split pursuant to which each two thousand (2000) shares of the company’s common stock, par value $0.001 per share (“Old Common Stock”), issued and outstanding immediately prior to the filing automatically and without any action on the part of the respective holders thereof, was combined and reclassified into one (1) share of common stock, par value $0.001 per share (the “New Common Stock”) (and such combination and conversion, the “Reverse Stock Split”). No fractional shares of New Common Stock are to be issued in connection with the Reverse Stock Split. Stockholders of record who otherwise would have been entitled to receive fractional shares are entitled, upon surrender to our transfer agent of certificates representing such shares, to a cash payment in lieu thereof equal to the fraction to which the stockholder otherwise would have been entitled multiplied by $1.40, which was the closing price (calculated on a post-split basis) of our common stock as reported by The OTC Market on January 20, 2020. After the reverse stock split, and as adjusted to eliminate fractional shares, as of January 21, 2020, there were 299,835 outstanding shares of our common stock.
Holders of our Convertible Preferred Stock Have the Right to Acquire Significant Amounts of Our Common Stock Upon Conversion of the Convertible Preferred Stock They Own. The current holders of our convertible preferred stock originally invested in GeoVax in March 2012. We issued convertible preferred stock and warrants in exchange for a cash investment. Our first deal with the investors was done at a time when we expected our HIV vaccine clinical program to move on a much faster track. Decisions by the government agencies funding that program (NIH and HVTN) led to the clinical timeline being greatly extended. In response, and despite our limited resources, we expanded our development efforts into other areas such as Ebola, Zika, Lassa, Hepatitis B, malaria, and immuno-oncology. The combination of the extended and uncertain timeline of our HIV clinical program, the early-stage nature of our other assets, and the terms of the preferred stock have made it very difficult for us to attract new investors. We have obtained government funding to support several of our programs and leveraging work done through our collaborators, but it was also necessary to go back to the investors who held our convertible preferred stock. Although we are grateful that these investors have provided critically needed funding, the conversion and other terms for series of preferred stock have been onerous.
The investors own all the outstanding Series J preferred stock. As of March 23, 2020, they still own approximately $300,000 in “stated value,” which is the basis for the number of common shares into which the preferred shares may be converted. The current outstanding preferred stock owned by the investors is convertible into common stock at the lower of $2.00 share or a 20% discount to the 10-day trailing volume-weighted average stock price. So the conversion price is variable, and the number of shares that can be issued is undetermined.
Changes to Outstanding Shares of Capital Stock. Between January 21, 2020, which was the date of the 1-for-2000 reverse stock split, and March 18, 2020, holders of our preferred stock converted approximately 2,386 shares of convertible preferred stock into 13,481,079 shares of our common stock. The following table provides additional details concerning our outstanding capital stock.
|
Class |
January 21, 2020 Outstanding Shares |
March 23, 2020 |
|
Common Stock $0.01 par value |
299,835 |
13,791,601 |
|
Series B Convertible Preferred Stock $0.01 par value |
100 ($100,000 stated value) |
100 ($100,000 stated value) |
|
Series H Convertible Preferred Stock $0.01 par value |
1,686 ($1,686,029 stated value) |
-0- ($-0- stated value) |
|
Series I Convertible Preferred Stock $0.01 par value |
700 ($700,000 stated value) |
-0- ($-0- stated value) |
|
Series J Convertible Preferred Stock $0.01 par value |
-0- ($-0- stated value) |
300 ($300,000 stated value) |
ITEM 1A. RISK FACTORS
Ownership of our securities involves a high degree of risk. You should carefully review and consider the risks, uncertainties and other factors described below before you decide whether to own our securities. Any of these factors could materially and adversely affect our business, financial condition, operating results and prospects and could negatively impact the market price of our common stock, and you may lose some or all of your investment. The risks and uncertainties described below are not the only ones facing our Company. Additional risks and uncertainties that we are unaware of, or that we currently deem immaterial, may also impair our business operations. You should also refer to the other information contained in this Form 10-K, including our financial statements and the related notes.
Risk of Substantial Dilution to Stock Ownership Percentage
The conversion of our preferred stock or exercise of options or warrants may depress our stock price and result in significant dilution to our common stockholders, while also having an adverse effect on our ability to raise funds from other parties.
We have Series B and Series J Convertible Preferred Stock currently outstanding and convertible into our common stock and there are also outstanding warrants to purchase our common stock. The conversion price of the Series J Convertible Preferred Stock is based, in part, on a formula allowing for conversion at 80% of the lowest volume weighted average price of our common stock during the ten trading days immediately preceding the delivery of a notice of conversion. The holders of those securities converted a number of their preferred shares during 2019 into a significant number of shares our common stock and sold the common stock acquired upon conversion of such securities in the open market. They have continued this practice in 2020. It is likely that the holders of these securities will continue to convert their convertible preferred stock into common stock and sell it. Sales of a substantial number of shares of our common stock in the public market by holders of preferred shares have depressed the prevailing market price for our common stock and impaired our ability to raise capital through the future sale of our equity securities, and are likely to continue to do so. Additionally, as the holders of outstanding preferred shares convert those preferred shares, our common stockholders will incur dilution in their relative percentage ownership. The dilution has been significant in the past and may be significant in the future. The prospect of dilution may also impact the price of our common stock.
Risks Related to Our Business
We have a history of operating losses, and we expect losses to continue for the foreseeable future.
We have had no product revenue to date and there can be no assurance that we will ever generate any product revenue. We have experienced operating losses since we began operations in 2001. As of December 31, 2019, we had an accumulated deficit of approximately $42.8 million. We expect to incur additional operating losses and expect cumulative losses to increase as our research and development, preclinical, clinical, manufacturing and marketing efforts expand. Our ability to generate revenue and achieve profitability depends on our ability to successfully complete the development of our product candidates, conduct preclinical tests and clinical trials, obtain the necessary regulatory approvals, and manufacture and market the resulting products, or otherwise commercialize our products. Unless we are able to successfully meet these challenges, we will not be profitable and may not remain in business
We have received a going concern opinion from our auditors.
We have received a "going concern" opinion from our independent registered public accounting firm, reflecting substantial doubt about our ability to continue as a going concern. Our consolidated financial statements contemplate that we will continue as a going concern and do not contain any adjustments that might result if we were unable to continue as a going concern. Our ability to continue as a going concern is dependent upon our ability to raise additional capital and implement our business plan. If we are unable to achieve or sustain profitability or to secure additional financing on acceptable terms, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our stockholders losing their entire investment. There is no guarantee that we will become profitable or secure additional financing on acceptable terms.
Our business will require continued funding. If we do not receive adequate funding, we will not be able to continue our operations.
To date, we have financed our operations principally through the sale of our equity securities and through government grants and clinical trial support. We will require substantial additional financing at various intervals for our operations, including clinical trials, operating expenses, intellectual property protection and enforcement, for pursuit of regulatory approvals, and for establishing or contracting out manufacturing, marketing and sales functions. There is no assurance that such additional funding will be available on terms acceptable to us or at all. If we are not able to secure the significant funding that is required to maintain and continue our operations at current levels, or at levels that may be required in the future, we may be required to delay clinical studies or clinical trials, curtail operations, or obtain funds through collaborative arrangements that may require us to relinquish rights to some of our products or potential markets.
The costs of conducting all of our human clinical trials to date for our preventive HIV vaccine have been borne by the HIV Vaccine Trials Network (HVTN), with funding by NIAID, and we expect NIAID support for additional clinical trials. GeoVax incurs costs associated with manufacturing the clinical vaccine supplies and other study support. We cannot predict the level of support we will receive from the HVTN or NIAID for any additional clinical trials of our HIV vaccines.
Our current operations are also partially supported by a U.S. government grant awarded to us to support our Lassa Fever vaccine program. As of December 31, 2019, there was $1,605,505 of unused grant funds remaining and available for use during 2020. We are pursuing additional support from the federal government for our vaccine programs; however, as we progress to the later stages of our vaccine development activities, government financial support may be more difficult to obtain, or may not be available at all. Furthermore, there is some risk that actual funding for grants could be delayed, cut back, or eliminated due to government budget constraints. Therefore, it will be necessary for us to look to other sources of funding to finance our development activities.
We expect that our current working capital, combined with proceeds from current government grants and committed sources of equity capital will be sufficient to support our planned level of operations into the second quarter of 2020. We will need to raise additional funds to significantly advance our vaccine development programs and to continue our operations. In order to meet our operating cash flow needs we plan to seek sources of non-dilutive capital through government grant programs and clinical trial support. We may also plan additional offerings of our equity securities, debt, or convertible debt instruments. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could have a material adverse effect on our business, operating results, financial condition and prospects.
Significant disruptions of information technology systems or breaches of information security systems could adversely affect our business.
We rely upon a combination of information technology systems and traditional recordkeeping to operate our business. In the ordinary course of business, we collect, store, and transmit confidential information (including, but not limited to, personal information and intellectual property). We have also outsourced elements of our operations to third parties, including elements of our information technology systems and, as a result, we manage a number of independent vendor relationships with third parties who may or could have access to our confidential information. Our information technology and information security systems and records are potentially vulnerable to security breaches, service interruptions, or data loss from inadvertent or intentional actions by our employees or vendors. Our information technology and information security systems and records are also potentially vulnerable to malicious attacks by third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of expertise and motives (including, but not limited to, financial crime, industrial espionage, and market manipulation).
While we have invested, and continue to invest, in our information technology and information security systems, there can be no assurance that our efforts will prevent security breaches, service interruptions, or data losses. Any security breaches, service interruptions, or data losses could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business, and reputational harm to us or allow third parties to gain material, inside information that they may use to trade in our securities.
Our business could be adversely affected by widespread public health epidemics or other catastrophic events beyond our control.
In addition to our reliance on our own employees and facilities, we depend on our collaborators, laboratories and other facilities for the continued operation of our business. Despite any precautions we take for natural disasters or other catastrophic events, events such as pandemic disease, terrorist attack, hurricanes, fire, floods and ice and snowstorms, may result in interruptions in our business.
An outbreak of contagious diseases, and other adverse public health developments, such as the recent novel strain of coronavirus (COVID-19) could impact our operations depending on future developments, which are highly uncertain, largely beyond our control and cannot be predicted with certainty. These uncertain factors, including the duration of the outbreak, new information which may emerge concerning the severity of the disease and the actions to contain or treat its impact, could adversely impact our operations, including among others, conduct of our clinical trials, employee mobility and productiveness, temporary closure of facilities, including clinical trial sites, our manufacturing capabilities, and third party service providers, any of which could have an adverse impact on our business and our financial results.
Risks Related to Development and Commercialization of Product Candidates and Dependence on Third Parties
Our products are still being developed and are unproven. These products may not be successful.
To become profitable, we must generate revenue through sales of our products. However, our products are in varying stages of development and testing. Our products have not been proven in human clinical trials and have not been approved by any government agency for sale. If we cannot successfully develop and prove our products and processes, or if we do not develop other sources of revenue, we will not become profitable and at some point, we would discontinue operations.
We depend upon key personnel who may terminate their employment with us at any time. If we were to lose the services of any of these individuals, our business and operations may be adversely affected.
The success of our business strategy will depend to a significant degree upon the continued services of key management, technical and scientific personnel and our ability to attract and retain additional qualified personnel and managers. Competition for qualified personnel is intense among companies, academic institutions and other organizations. The ability to attract and return personnel is adversely affected by our financial challenges. If we are unable to attract and retain key personnel and advisors, it may negatively affect our ability to successfully develop, test, commercialize and market our products and product candidates.
Regulatory and legal uncertainties could result in significant costs or otherwise harm our business.
To manufacture and sell our products, we must comply with extensive domestic and international regulation. In order to sell our products in the United States, approval from the FDA is required. Satisfaction of regulatory requirements, including FDA requirements, typically takes many years, and if approval is obtained at all, it is dependent upon the type, complexity and novelty of the product, and requires the expenditure of substantial resources. We cannot predict whether our products will be approved by the FDA. Even if they are approved, we cannot predict the time frame for approval. Foreign regulatory requirements differ from jurisdiction to jurisdiction and may, in some cases, be more stringent or difficult to meet than FDA requirements. As with the FDA, we cannot predict if or when we may obtain these regulatory approvals. If we cannot demonstrate that our products can be used safely and successfully in a broad segment of the patient population on a long-term basis, our products would likely be denied approval by the FDA and the regulatory agencies of foreign governments.
We face intense competition and rapid technological change that could result in products that are superior to the products we will be commercializing or developing.
The market for vaccines that protect against or treat human infectious diseases is intensely competitive and is subject to rapid and significant technological change. We have numerous competitors in the United States and abroad, including, among others, large companies with substantially greater resources than us. If any of our competitors develop products with efficacy or safety profiles significantly better than our products, we may not be able to commercialize our products, and sales of any of our commercialized products could be harmed. Some of our competitors and potential competitors have substantially greater product development capabilities and financial, scientific, marketing and human resources than we do. Competitors may develop products earlier, obtain FDA approvals for products more rapidly, or develop products that are more effective than those under development by us. We will seek to expand our technological capabilities to remain competitive; however, research and development by others may render our technologies or products obsolete or noncompetitive, or result in treatments or cures superior to ours.
Our product candidates are based on new medical technology and, consequently, are inherently risky. Concerns about the safety and efficacy of our products could limit our future success.
We are subject to the risks of failure inherent in the development of product candidates based on new medical technologies. These risks include the possibility that the products we create will not be effective, that our product candidates will be unsafe or otherwise fail to receive the necessary regulatory approvals, and that our product candidates will be hard to manufacture on a large scale or will be uneconomical to market.
Many pharmaceutical products cause multiple potential complications and side effects, not all of which can be predicted with accuracy and many of which may vary from patient to patient. Long term follow-up data may reveal previously unidentified complications associated with our products. The responses of potential physicians and others to information about complications could materially affect the market acceptance of our products, which in turn would materially harm our business.
We may experience delays in our clinical trials that could adversely affect our financial results and our commercial prospects.
We do not know whether planned clinical trials will begin on time or whether we will complete any of our clinical trials on schedule, if at all. Product development costs will increase if we have delays in testing or approvals or if we need to perform more or larger clinical trials than planned. Significant delays may adversely affect our financial results and the commercial prospects for our products and delay our ability to become profitable.
We rely heavily on the HVTN, independent clinical investigators, vaccine manufacturers, and other third-party service providers for successful execution of our clinical trials, but do not control many aspects of their activities. We are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Third parties may not complete activities on schedule or may not conduct our clinical trials in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations could delay or prevent the development, approval and commercialization of our product candidates. There is also a risk of changes in clinical trial strategy and timelines due to the HVTN and NIAID altering their trial strategy.
Failure to obtain timely regulatory approvals required to exploit the commercial potential of our products could increase our future development costs or impair our future sales.
None of our vaccines are approved by the FDA for sale in the United States or by other regulatory authorities for sale in foreign countries. To exploit the commercial potential of our technologies, we are conducting and planning to conduct additional pre-clinical studies and clinical trials. This process is expensive and can require a significant amount of time. Failure can occur at any stage of testing, even if the results are favorable. Failure to adequately demonstrate safety and efficacy in clinical trials could delay or preclude regulatory approval and restrict our ability to commercialize our technology or products. Any such failure may severely harm our business. In addition, any approvals we obtain may not cover all of the clinical indications for which approval is sought or may contain significant limitations in the form of narrow indications, warnings, precautions or contraindications with respect to conditions of use, or in the form of onerous risk management plans, restrictions on distribution, or post-approval study requirements.
State pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs and file periodic reports on sales, marketing, pricing and other activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and available guidance is limited. Unless we are in full compliance with these laws, we could face enforcement action, fines, and other penalties and could receive adverse publicity, all of which could harm our business.
Changes in healthcare law and implementing regulations, as well as changes in healthcare policy, may impact our business in ways that we cannot currently predict, and may have a significant adverse effect on our business and results of operations.
In the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates, restrict or regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval. Among policy makers and payors in the United States and elsewhere, including in the European Union, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the Affordable Care Act, substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry. The Affordable Care Act, includes a number of provisions that are intended to lower healthcare costs, including provisions relating to prescription drug prices and government spending on medical products.
Since its enactment, there have also been judicial and Congressional challenges to certain aspects of the Affordable Care Act, as well as efforts by the Trump administration to repeal or replace certain aspects of the statute. We continue to evaluate the effect that the Affordable Care Act and subsequent changes to the statute has on our business. It is uncertain the extent to which any such changes may impact our business or financial condition.
There has also been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products. There have been several Congressional inquiries and proposed bills, as well as state efforts, designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. In June 2017, FDA issued a Drug Competition Action plan intended to lower prescription drug prices by encouraging competition from generic versions of existing products. The Agency announced that it will issue a similar plan intended to promote competition to prescription biologics from biosimilars later this year.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures. For example, in September 2017, the California State Assembly approved SB17, which requires pharmaceutical companies to notify health insurers and government health plans at least 60 days before any scheduled increases in the prices of their products if they exceed 16% over a two-year period, and further requiring pharmaceutical companies to explain the reasons for such increase. Effective in 2016, Vermont passed a law requiring certain manufacturers identified by the state to justify their price increases.
We expect that these, and other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and lower reimbursement, and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our drugs, once marketing approval is obtained.
We may not be successful in establishing collaborations for product candidates we seek to commercialize, which could adversely affect our ability to discover, develop, and commercialize products.
We expect to seek collaborations for the development and commercialization of product candidates in the future. The timing and terms of any collaboration will depend on the evaluation by prospective collaborators of the clinical trial results and other aspects of a product’s safety and efficacy profile. If we are unable to reach agreements with suitable collaborators for any product candidate, we will be forced to fund the entire development and commercialization of such product candidates, ourselves, and we may not have the resources to do so. If resource constraints require us to enter into a collaboration agreement early in the development of a product candidate, we may be forced to accept a more limited share of any revenues the product may eventually generate. We face significant competition in seeking appropriate collaborators. Moreover, these collaboration arrangements are complex and time-consuming to negotiate and document. We may not be successful in our efforts to establish collaborations or other alternative arrangements for any product candidate. Even if we are successful in establishing collaborations, we may not be able to ensure fulfillment by collaborators of their obligations or our expectations.
We do not have manufacturing, sales or marketing experience.
We do not have experience in manufacturing, selling, or marketing. To obtain the expertise necessary to successfully manufacture, market, and sell our products, we will require the development of our own commercial infrastructure and/or collaborative commercial arrangements and partnerships. Our ability to execute our current operating plan is dependent on numerous factors, including, the performance of third-party collaborators with whom we may contract.
Our products under development may not gain market acceptance.
Our products may not gain market acceptance among physicians, patients, healthcare payers and the medical community. Significant factors in determining whether we will be able to compete successfully include:
|
● |
the efficacy and safety of our products; |
|
● |
the time and scope of regulatory approval; |
|
● |
reimbursement coverage from insurance companies and others; |
|
● |
the price and cost-effectiveness of our products, especially as compared to any competitive products; and |
|
● |
the ability to maintain patent protection. |
We may be required to defend lawsuits or pay damages for product liability claims.
Product liability is a major risk in testing and marketing biotechnology and pharmaceutical products. We may face substantial product liability exposure in human clinical trials and for products that we sell after regulatory approval. We carry product liability insurance and we expect to continue such policies. However, product liability claims, regardless of their merits, could exceed policy limits, divert management’s attention, and adversely affect our reputation and demand for our products.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance. If there is not sufficient reimbursement for our products, it is less likely that they will be widely used.
Market acceptance of products we develop, if approved, will depend on reimbursement policies and may be affected by, among other things, future healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will cover and establish payment levels. We cannot be certain that reimbursement will be available for any products that we may develop. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for our products. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize products that we develop.
Risks Related to Our Intellectual Property
We could lose our license rights to our important intellectual property if we do not fulfill our contractual obligations to our licensors.
Our rights to significant parts of the technology we use in our products are licensed from third parties and are subject to termination if we do not fulfill our contractual obligations to our licensors. Termination of intellectual property rights under any of our license agreements could adversely impact our ability to produce or protect our products. Our obligations under our license agreements include requirements that we make milestone payments to our licensors upon the achievement of clinical development and regulatory approval milestones, royalties as we sell commercial products, and reimbursement of patent filing and maintenance expenses. Should we become bankrupt or otherwise unable to fulfill our contractual obligations, our licensors could terminate our rights to critical technology that we rely upon.
Other parties may claim that we infringe their intellectual property or proprietary rights, which could cause us to incur significant expenses or prevent us from selling products.
Our success will depend in part on our ability to operate without infringing the patents and proprietary rights of third parties. The manufacture, use and sale of new products have been subject to substantial patent rights litigation in the pharmaceutical industry. These lawsuits generally relate to the validity and infringement of patents or proprietary rights of third parties. Infringement litigation is prevalent with respect to generic versions of products for which the patent covering the brand name product is expiring, particularly since many companies that market generic products focus their development efforts on products with expiring patents. Pharmaceutical companies, biotechnology companies, universities, research institutions or other third parties may have filed patent applications or may have been granted patents that cover aspects of our products or our licensors’ products, product candidates or other technologies.
Future or existing patents issued to third parties may contain patent claims that conflict with those of our products. We expect to be subject to infringement claims from time to time in the ordinary course of business, and third parties could assert infringement claims against us in the future with respect to our current products or with respect to products that we may develop or license. Litigation or interference proceedings could force us to:
|
● |
stop or delay selling, manufacturing or using products that incorporate, or are made using the challenged intellectual property; |
|
● |
pay damages; or |
|
● |
enter into licensing or royalty agreements that may not be available on acceptable terms, if at all. |
Any litigation or interference proceedings, regardless of their outcome, would likely delay the regulatory approval process, be costly and require significant time and attention of our key management and technical personnel.
Any inability to protect intellectual property rights in the United States and foreign countries could limit our ability to manufacture or sell products.
We will rely on trade secrets, unpatented proprietary know-how, continuing technological innovation and, in some cases, patent protection to preserve our competitive position. Our patents and licensed patent rights may be challenged, invalidated, infringed or circumvented, and the rights granted in those patents may not provide proprietary protection or competitive advantages to us. We and our licensors may not be able to develop patentable products. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us. If patents containing competitive or conflicting claims are issued to third parties, we may be prevented from commercializing the products covered by such patents or may be required to obtain or develop alternate technology. In addition, other parties may duplicate, design around or independently develop similar or alternative technologies.
We may not be able to prevent third parties from infringing or using our intellectual property, and the parties from whom we may license intellectual property may not be able to prevent third parties from infringing or using the licensed intellectual property. We generally attempt to control and limit access to, and the distribution of, our product documentation and other proprietary information. Despite efforts to protect this proprietary information, unauthorized parties may obtain and use information that we may regard as proprietary. Other parties may independently develop similar know-how or may even obtain access to these technologies.
The laws of some foreign countries do not protect proprietary information to the same extent as the laws of the United States, and many companies have encountered significant problems and costs in protecting their proprietary information in these foreign countries.
Neither the U.S. Patent and Trademark Office nor the courts have established a consistent policy regarding the breadth of claims allowed in pharmaceutical patents. The allowance of broader claims may increase the incidence and cost of patent interference proceedings and the risk of infringement litigation. On the other hand, the allowance of narrower claims may limit the value of our proprietary rights.
Risks Related To Our Common Stock
The market price of our common stock is highly volatile.
The market price of our common stock has been, and is expected to continue to be, highly volatile. Certain factors, including announcements of new developments by us or other companies, regulatory matters, new or existing medicines or procedures, concerns about our financial position, operating results, litigation, government regulation, developments or disputes relating to agreements, patents or proprietary rights, may have a significant impact on the market price of our stock. In addition, potential dilutive effects of future sales of shares of common stock by us, and subsequent sales of common stock by the holders of warrants and options could have an adverse effect on the market price of our shares. We also believe that conversion of convertible preferred stock into common stock followed by sales of large amounts of our common stock by our primary investors have had and may be expected to have an adverse effect on the market price of our common stock.
Our common stock does not have a vigorous trading market and investors may not be able to sell their securities when desired.
We have a limited active public market for our common shares. A more active public market, allowing investors to buy and sell large quantities of our common stock, may never develop. Consequently, investors may not be able to liquidate their investments in the event of an emergency or for any other reason.
Our common stock is currently subject to the SEC’s “penny stock” rules, which make it more difficult to sell.
Our common stock is currently classified as a “penny stock.” The SEC rules regarding penny stocks may have the effect of reducing trading activity in our shares, making it more difficult for investors to sell. Under these rules, broker-dealers who recommend such securities to persons other than institutional accredited investors must:
|
• |
make a special written suitability determination for the purchaser; |
|
• |
receive the purchaser’s written agreement to a transaction prior to sale; |
|
• |
provide the purchaser with risk disclosure documents which identify certain risks associated with investing in “penny stocks” and which describe the market for these “penny stocks” as well as a purchaser’s legal remedies; |
|
• |
obtain a signed and dated acknowledgment from the purchaser demonstrating that the purchaser has received the required risk disclosure document before a transaction in a “penny stock” can be completed; and |
|
• |
give bid and offer quotations and broker and salesperson compensation information to the customer orally or in writing before or with the confirmation. |
These rules make it more difficult for broker-dealers to effectuate customer transactions and trading activity in our securities and may result in a lower trading volume of our common stock and lower trading prices.
We need additional capital, and the sale of additional shares or other equity securities could result in additional dilution to our stockholders.
In order to meet our operating cash flow needs we plan additional offerings of our equity securities, debt, or convertible debt instruments. The sale of additional equity securities could result in significant additional dilution to our stockholders. Certain equity securities, such as our outstanding convertible preferred stock or warrants, and subsequent issuances, contain or may contain anti-dilution provisions which could result in the issuance of additional shares at lower prices if we sell other shares below specified prices. The incurrence of indebtedness would result in debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure investors that financing will be available in amounts or on terms acceptable to us, if at all.
Certain provisions of our certificate of incorporation which authorize the issuance of additional shares of preferred stock may make it more difficult for a third party to effect a change in control.
Our certificate of incorporation authorizes our Board of Directors to issue up to 10,000,000 shares of preferred stock. We have issued, and there are outstanding, 100 shares of Series B Convertible Preferred Stock and 300 shares of our Series J Convertible Preferred Stock. We believe the conversion terms of these preferred shares could have a substantial impact on the ability of a third party to effect a change in control. The remaining shares of preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our Board of Directors without further action by the stockholders. These terms may include voting rights including the right to vote as a series on particular matters, preferences as to dividends and liquidation, conversion rights, redemption rights and sinking fund provisions. The issuance of any newly issued preferred stock could diminish the rights of holders of our common stock, and therefore could reduce the value of our common stock. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell assets to, a third party. The ability of our Board of Directors to issue preferred stock could make it more difficult, delay, discourage, prevent or make it costlier to acquire or effect a change-in-control, which in turn could prevent the stockholders from recognizing a gain in the event that a favorable offer is extended and could materially and negatively affect the market price of our common stock.
We have never paid dividends and have no plans to do so.
Holders of shares of our common stock are entitled to receive such dividends as may be declared by our Board of Directors. To date, we have paid no cash dividends on our shares of common stock and we do not expect to pay cash dividends on our common stock in the foreseeable future. We intend to retain future earnings, if any, to provide funds for operations of our business. Therefore, any potential return investors may have in our common stock will be in the form of appreciation, if any, in the market value of their shares of common stock.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud.
We are subject to reporting obligations under the United States securities laws. The Securities and Exchange Commission (SEC) as required by the Sarbanes-Oxley Act of 2002, adopted rules requiring every public company to include a management report on such company’s internal controls over financial reporting in its annual report. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent fraud. As a result, our failure to achieve and maintain effective internal controls over financial reporting could result in the loss of investor confidence in the reliability of our financial statements, which in turn could negatively impact the trading price of our stock.
|
ITEM 1B. |
UNRESOLVED STAFF COMMENTS |
None
|
ITEM 2. |
PROPERTIES |
We lease approximately 8,400 square feet of office and laboratory space located at 1900 Lake Park Drive, Suite 380, Smyrna, Georgia under a lease agreement which expires on December 31, 2022. We believe this space is adequate for our current needs.
|
ITEM 3. |
LEGAL PROCEEDINGS |
We are not currently a party to any material legal proceedings. We may from time to time become involved in various legal proceedings such as those arising in the ordinary course of business.
|
ITEM 4. |
MINE SAFETY DISCLOSURES |
Not applicable.
PART II
|
ITEM 5. |
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is currently traded on the OTC Markets “Pink” market under the symbol “GOVX”. Quotations for our common stock reflect inter-dealer prices and do not include retail mark-up, markdown, or commission, and may not necessarily represent actual transactions.
Holders
On March 23, 2020, there were three holders of record of our common stock. The majority of our shares of common stock are held by brokers and other institutions on behalf of stockholders, and we are unable to estimate the total number of stockholders represented by these record holders.
Dividends
We have not paid any dividends since our inception and do not contemplate paying dividends in the foreseeable future. The certificates of designation for our outstanding preferred stock would prohibit the payment of dividends on our common stock if there were any dividends due but unpaid on our preferred stock. Any future determination as to the declaration and payment of dividends, if any, will be at the discretion of our Board of Directors and will depend on then existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our Board of Directors may deem relevant.
Recent Sales of Unregistered Securities
There were no sales of unregistered securities during the period covered by this report that have not previously been reported on Form 10-Q or Form 8-K.
Issuer Purchases of Equity Securities
We did not repurchase any of our equity securities during the fourth quarter of 2019.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth certain information as of December 31, 2019 with respect to compensation plans under which our equity securities are authorized for issuance. As a result of reverse stock splits effected in April 2019 and in January 2020, the number of our equity securities subject to existing equity compensation plans and the number of securities remaining available for future issuance under such plans is de minimis.
|
Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|
Equity compensation plans approved by stockholders |
-0- |
-0- |
-0- |
|
Equity compensation plans not approved by stockholders |
11 |
$33,909 |
5 |
A description of our equity compensation plans can be found in footnote 7 to our 2019 consolidated financial statements.
|
ITEM 6. |
SELECTED FINANCIAL DATA |
The following selected financial data as of and for each of the five years ended December 31, 2019 are derived from our audited consolidated financial statements. The historical results presented below are not necessarily indicative of the results to be expected for any future period. The information set forth below should be read in conjunction with the information contained in “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and our consolidated financial statements and the related notes, beginning on page F-1.
|
Years Ended December 31, |
||||||||||||||||||||
|
2019 |
2018 |
2017 |
2016 |
2015 |
||||||||||||||||
|
Statement of Operations Data: |
||||||||||||||||||||
|
Total revenues |
$ | 1,175,896 | $ | 963,203 | $ | 1,075,270 | $ | 828,918 | $ | 428,081 | ||||||||||
|
Net loss |
(2,370,629 | ) | (2,560,094 | ) | (2,170,162 | ) | (3,271,701 | ) | (2,689,287 | ) | ||||||||||
|
Basic and diluted net loss per common share |
(39.25 | ) | (15,610.33 | ) | (31,451.62 | ) | (78,804.81 | ) | (84,169.60 | ) | ||||||||||
|
As of December 31, |
||||||||||||||||||||
|
2019 |
2018 |
2017 |
2016 |
2015 |
||||||||||||||||
|
Balance Sheet Data: |
||||||||||||||||||||
|
Total assets |
468,880 | 642,064 | 490,235 | 610,217 | 1,331,593 | |||||||||||||||
|
Total stockholders’ equity (deficiency) |
(1,574,556 | ) | (1,022,347 | ) | (321,057 | ) | 240,370 | 1,204,603 | ||||||||||||
|
ITEM 7. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis of our financial condition and results of operations should be read together with “Selected Financial Data” and our consolidated financial statements and the related notes beginning on page F-1. This discussion contains forward-looking statements that involve risks and uncertainties because they are based on current expectations and relate to future events and our future financial performance. Our actual results may differ materially from those anticipated in these forward-looking statements because of many important factors, including those set forth under “Risk Factors” and elsewhere in this Annual Report.
Overview
GeoVax Labs, Inc. (“GeoVax” or the “Company”) is a clinical-stage biotechnology company developing immunotherapies and vaccines against cancers and infectious diseases using a novel vector vaccine platform (Modified Vaccinia Ankara-Virus Like Particle or “GV-MVA-VLPTM”). Our current development programs are focused on preventive and therapeutic vaccines against Human Immunodeficiency Virus (HIV); preventive vaccines against hemorrhagic fever viruses (Ebola, Sudan, Marburg, and Lassa fever), Zika virus and malaria; a therapeutic vaccine for chronic hepatitis B virus infections; and immunotherapies for solid tumor cancers. We also recently entered into a letter of intent to begin a collaboration with BravoVax, based in Wuhan, China, to jointly develop a vaccine for prevention of novel coronavirus (COVID-2019) infection.
For our infectious disease vaccines, our recombinant MVA vector can express target proteins on highly immunogenic VLPs in the person being vaccinated, with the intended result of producing durable immune responses with the safety characteristics of the replication deficient MVA vector and cost-effective manufacturing.
In cancer immunotherapy, we believe that stimulating the immune system to treat or prevent cancers is a compelling concept and that the opportunity for immune-activating technologies is promising, especially in light of advancements such as checkpoint inhibitors leading the way in oncology. Despite drug approvals in limited indications and promising results in clinical trials, there remains a significant need and opportunity for further advancements. We believe our GV-MVA-VLPTM is well-suited for delivery of tumor-associated antigens and we are pursuing development of our platform in this space through our subsidiary, Immutak Oncology, Inc.
Our most advanced vaccine program is focused on prevention of the clade B subtype of HIV prevalent in the regions of the Americas, Western Europe, Japan and Australia; our HIV vaccine candidate, GOVX-B11, will be included in an upcoming clinical trial (HVTN 132) managed by the HIV Vaccine Clinical Trials Network (HVTN) with support from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), which is targeted to begin in late 2020. Additionally, through the efforts of our collaborator, American Gene Technologies International, Inc. (AGT), we expect that our HIV vaccine will enter clinical trials during 2020 in combination with AGT’s gene therapy technology to seek a functional cure for HIV. A similar effort is underway with a consortium led by researchers at the University of California, San Francisco (UCSF), using our vaccine as part of a combinational therapy to induce remission in HIV-positive individuals; we also expect this program to enter clinical trials during 2020. Our other vaccine and immunotherapy programs are at various stages of preclinical development.
Our corporate strategy is to advance, protect and exploit our differentiated vaccine/immunotherapy platform. With our design and development capabilities, we are progressing and validating an array of cancer and infectious disease immunotherapy and vaccine product candidates. Our goal is to advance products through to human clinical testing, and to seek partnership or licensing arrangements for achieving regulatory approval and commercialization. We also leverage third party resources through collaborations and partnerships for preclinical and clinical testing with multiple government, academic and corporate entities.
We have not generated any revenues from the sale of any such products, and we do not expect to generate any such revenues for at least the next several years. Our product candidates will require significant additional research and development efforts, including extensive preclinical and clinical testing. All product candidates that we advance to clinical testing will require regulatory approval prior to commercial use and will require significant costs for commercialization. We may not be successful in our research and development efforts, and we may never generate sufficient product revenue to be profitable.
Critical Accounting Policies and Estimates
This discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. On an ongoing basis, management evaluates its estimates and adjusts the estimates as necessary. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates under different assumptions or conditions.
Our significant accounting policies are summarized in Note 2 to our consolidated financial statements for the year ended December 31, 2019, which are included in this Form 10-K. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements:
Revenue Recognition
In May 2014, the FASB issued Accounting Standards Update 2014-09, Revenue from Contracts with Customers (ASU 2014-09), which created a new Topic, Accounting Standards Codification Topic 606. The standard is principle-based and provides a five-step model to determine when and how revenue is recognized. The core principle is that an entity should recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. We adopted ASU 2014-09 effective January 1, 2018 using the modified retrospective transition method. Under this method, our prior results will remain as reported and starting in 2018 are recognized under the new method. The adoption of ASU 2014-09 had no material impact on the measurement, timing, or recognition of our grant and collaboration revenues, nor on the related research and development expenses.
Grant revenue – We receive payments from government entities under non-refundable grants in support of our vaccine development programs. We record revenue associated with these grants when the reimbursable costs are incurred and we have complied with all conditions necessary to receive the grant funds.
Research collaborations – We are pursuing a strategy of co-developing or licensing our technology for specific vaccine development approaches and/or disease indications. We have entered into multiple collaborative research and development agreements and have received third-party funding for preclinical research under certain of these arrangements. Each agreement is evaluated in accordance with the process defined by ASU 2014-09 and revenue is recognized accordingly.
Stock-Based Compensation
We account for stock-based transactions in which the Company receives services from employees, directors or others in exchange for equity instruments based on the fair value of the award at the grant date. Compensation cost for awards of common stock is estimated based on the price of the underlying common stock on the date of issuance. Compensation cost for stock options or warrants is estimated at the grant date based on each instrument’s fair value as calculated by the Black-Scholes option pricing model. We recognize stock-based compensation cost as expense ratably on a straight-line basis over the requisite service period for the award. See Note 7 to our financial statements for additional stock-based compensation information.
In May 2017, the FASB issued Accounting Standards Update 2017-09, Scope of Modification Accounting (“ASU 2017-09”), which amends Accounting Standards Codification Topic 718, Compensation – Stock Compensation. ASU 2017-09 is an attempt to provide clarity and reduce both (1) diversity in practice and (2) cost and complexity when applying the guidance in Topic 718 Compensation – Stock Compensation, to a change to the terms or conditions of a share-based payment award. We adopted ASU 2017-09 effective January 1, 2018; such adoption had no material impact on our financial statements.
In June 2018, the FASB issued Accounting Standards Update 2018-07, Improvements to Nonemployee Share-Based Payment Accounting (ASU 2018-07), that expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. The guidance is effective for fiscal years beginning after December 15, 2018, including interim reporting periods within that fiscal year. We adopted ASU 2018-07 effective January 1, 2019; such adoption had no material impact on our financial statements.
Liquidity and Capital Resources
Our principal uses of cash are to finance our research and development activities. Since inception, we have funded these activities primarily from government grants and clinical trial assistance, and from sales of our equity securities. At December 31, 2019, we had cash and cash equivalents of $283,341 and total assets of $468,880, as compared to $259,701 and $642,064, respectively, at December 31, 2018. At December 31, 2019, we had a working capital deficit of $1,568,929, compared to $1,005,127 at December 31, 2018. Our current liabilities at December 31, 2019 and 2018 include $1,732,702 and $1,220,179, respectively, of accrued management salaries and director fees, payment of which is being deferred as discussed further below.
Net cash used in operating activities was $1,398,497 and $1,543,026 for the years ended December 31, 2019 and 2018, respectively. Generally, the variances between periods are due to fluctuations in our net losses, offset by non-cash charges such as depreciation and stock-based compensation expense, and by net changes in our assets and liabilities. Our net losses generally fluctuate based on expenditures for our research activities, partially offset by government grant revenues. As of December 31, 2019, there is $1,605,505 in approved grant funds available for use during 2020. Of this amount, we expect that $1,053,776 will be used by us to reimburse third parties who will provide services covered by these grants. See “Results of Operations – Grant and Collaboration Revenues” below for additional details concerning our government grants.
Members of our executive management team are deferring receipt of portions of their salaries and members of our board of directors are deferring receipt of all of their fees in order to help conserve the Company’s cash resources. As of December 31, 2019, the accumulated deferrals totaled $1,732,702. We expect the ongoing deferrals of approximately $31,800 per month for the management salaries to continue until such time as a significant financing event (as determined by the board of directors) is consummated.
NIAID has funded the costs of conducting all of our human clinical trials (Phase 1 and Phase 2a) to date for our preventive HIV vaccines, with GeoVax incurring certain costs associated with manufacturing the clinical vaccine supplies and other study support. We expect that NIAID will also fund the cost of the planned Phase 1 trial (HVTN 132) to further evaluate the safety and immunogenicity of adding “protein boost” components to our vaccine, GOVX-B11. We expect HVTN 132 to commence patient enrollment in late 2020. Additionally, we are party to a collaboration with American Gene Technologies International, Inc. (AGT) whereby AGT intends to conduct a Phase 1 human clinical trial with our combined technologies, with the ultimate goal of developing a functional cure for HIV infection. We expect that AGT will begin the phase 1 trial during 2020. A similar effort is underway with a consortium led by researchers at the University of California, San Francisco (UCSF), using our vaccine as part of a combinational therapy to induce remission in HIV-positive individuals. We also expect this program to enter clinical trials during 2020.
Net cash used in investing activities was $7,606 and $-0- for the years ended December 31, 2019 and 2018, respectively. Our investing activities have consisted predominantly of capital expenditures for laboratory equipment.
Net cash provided by financing activities was $1,429,743 and $1,490,000 for the years ended December 31, 2019 and 2018, respectively.
During 2018 we sold shares of our Series E convertible preferred stock ($1,190,000), issued non-interest-bearing Term Promissory Notes (the “Term Notes”) to two current investors for $250,000 in total, and issued a five-year Senior Promissory Note to the Georgia Research Alliance, Inc. for $50,000 (the “GRA Note”). The GRA Note bears an annual interest rate of 5%, payable monthly, with principal repayments which began in March 2019.
During 2019 we sold shares of our Series G and Series I convertible preferred stock for aggregate net proceeds of $1,440,000.
In February 2019, we entered into an agreement for the sale of shares of our Series G convertible preferred stock, which was funded at three different closings. At the first closing, which occurred in February 2019, we issued shares of Series G convertible preferred stock in exchange for net cash proceeds of $240,000 plus the cancellation of Term Notes held by the purchasers in the amount of $250,000. At the second and third closings, which occurred in April and June 2019, we issued additional shares of Series G convertible preferred stock in exchange for the payment of $500,000. In July 2019, we sold shares of our Series I convertible preferred stock for gross proceeds of $700,000. During 2019 we made principal repayments of $10,257 toward the GRA Note.
In January 2020, we sold shares of our Series J convertible preferred stock for gross proceeds of $300,000.
As of December 31, 2019, we had an accumulated deficit of $42.8 million. We expect for the foreseeable future we will continue to operate at a loss. The amount of the accumulated deficit will continue to increase, as it will be expensive to continue our research and development efforts. We will continue to require substantial funds to continue our activities and cannot predict the outcome of our efforts. We have received a “going concern” opinion from our independent registered public accountants reflecting substantial doubt about our ability to continue as a going concern. We believe that our existing cash resources, combined with funding from existing government grants and clinical trial support, and committed sources of equity capital will be sufficient to fund our planned operations into the second quarter of 2020. We will require additional funds to continue our planned operations beyond that date. We are currently seeking sources of capital through additional government grant programs and clinical trial support, and we plan to conduct at least one additional offering of our equity securities. Additional funding may not be available on favorable terms or at all and if we fail to obtain additional capital when needed, we may be required to delay, scale back, or eliminate some or all of our research and development programs as well as reduce our general and administrative expenses.
Net Operating Loss Carryforwards
At December 31, 2019, we had consolidated net operating loss carryforwards for income tax purposes of $65.7 million, of which approximately $62.6 million will expire in 2020 through 2037 if not utilized. We also have research and development tax credits of approximately $1.1 million available to reduce income taxes, if any, which will expire in 2022 through 2039 if not utilized. Section 382 of the Internal Revenue Code contains provisions that may limit our utilization of net operating loss and research tax credit carryforwards in any given year as a result of significant changes in ownership interests that have occurred in past periods or may occur in future periods.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that are likely or reasonably likely to have a material effect on our financial condition or results of operations, other than operating leases.
Results of Operations
We recorded net losses of $2,370,629 and $2,560,094 for the years ended December 31, 2019 and 2018, respectively. Our operating results typically fluctuate due to the timing of activities and related costs associated with our research and development activities and our general and administrative costs, as described below.
Grant and Collaboration Revenues
We recorded grant and collaboration revenues of $1,175,896 and $963,203 for the years ended December 31, 2019 and 2018, respectively.
Grant Revenues – Our grant revenues relate to grants and contracts from agencies of the U.S. government in support of our vaccine development activities, and such revenues were 84% and 97% of our total revenues for 2019 and 2018, respectively. We record revenue associated with these grants as the related costs and expenses are incurred. The variance in our grant revenues from period to period relates to the timing and amount of our expenditures for activities supported by the grants. Additional detail concerning our grant revenues and the remaining funds available for use as of December 31, 2019 is presented in the table below.
|
Grant Revenue Recorded During Year Ended December 31, |
Unused Funds Available at December 31, |
|||||||||||
|
Grant/Contract No. |
2019 |
2018 |
2019 |
|||||||||
|
Lassa Fever – U.S. Army Grant |
$ | 674,179 | $ | 162,563 | $ | 1,605,505 | ||||||
|
Lassa Fever – SBIR Grant |
147,042 | 152,778 | - | |||||||||
|
Zika – NIH SBIR Grant |
162,461 | 363,184 | - | |||||||||
|
HIV – NIH SBIR Grant |
- | 256,050 | - | |||||||||
|
Total |
$ | 983,682 | $ | 934,575 | $ | 1,605,505 | ||||||
Collaboration Revenues – In addition to the grant revenues above, during the years ended December 31, 2019 and 2018, we recorded revenues of $192,214 and $28,628 associated with several research collaborations with third parties. These were 16% and 3% of our revenues for 2019 and 2018, respectively.
Research and Development Expenses
Our research and development expenses were $1,910,715 and $1,878,652 for the years ended December 31, 2019 and 2018, respectively. Research and development expense for these periods includes stock-based compensation expense of $43,801 and $41,998 for 2019 and 2018, respectively (see discussion under “Stock-Based Compensation Expense” below).
Our research and development expenses can fluctuate considerably on a period-to-period basis, depending on the timing of expenditures related to our government grants and other research projects, and other factors. Research and development expenses increased by $32,063, or 1.7% from 2018 to 2019. The fluctuation is primarily due to the timing of expenditures related to our government grants. Our research and development costs do not include costs incurred by the HVTN in conducting clinical trials of our preventive HIV vaccines; those costs are funded directly to the HVTN by NIAID.
We do not disclose our research and development expenses by project, since our employees’ time is spread across multiple programs and our laboratory facility is used for multiple vaccine candidates. We track the direct cost of research and development expenses related to government grant revenue by the percentage of assigned employees’ time spent on each grant and other direct costs associated with each grant. Indirect costs associated with grants are not tracked separately but are applied based on a contracted overhead rate negotiated with the NIH. Therefore, the recorded revenues associated with government grants approximates the costs incurred.
We do not provide forward-looking estimates of costs and time to complete our research programs due to the many uncertainties associated with vaccine development. Due to these uncertainties, our future expenditures are likely to be highly volatile in future periods depending on the outcomes of the trials and studies. As we obtain data from pre-clinical studies and clinical trials, we may elect to discontinue or delay vaccine development programs to focus our resources on more promising vaccine candidates. Completion of preclinical studies and human clinical trials may take several years or more, but the length of time can vary substantially depending upon several factors. The duration and the cost of future clinical trials may vary significantly over the life of the project because of differences arising during development of the human clinical trial protocols, including the number of patients that ultimately participate in the clinical trial; the duration of patient follow-up that seems appropriate in view of the results; the number of clinical sites included in the clinical trials; and the length of time required to enroll suitable patient subjects.
General and Administrative Expenses
Our general and administrative expenses were $1,637,674 and $1,647,268 for the years ended December 31, 2019 and 2018, respectively. General and administrative costs include officers’ salaries, legal and accounting costs, patent costs, and other general corporate expenses. General and administrative expense includes stock-based compensation expense of $283,699 and $427,725 for 2019 and 2018, respectively (see discussion under “Stock-Based Compensation Expense” below). Excluding stock-based compensation expense, general and administrative expenses were $1,353,975 and $1,219,541 for 2019 and 2018, respectively, representing an increase of $134,434, or 11%. The increase from 2018 to 2019 is primarily related to costs associated with the conduct of two special meetings of stockholders and the reverse stock split of our common stock. We expect that our general and administrative costs may increase in the future in support of expanded research and development activities and other general corporate activities.
Stock-Based Compensation Expense
For the two years ended December 31, 2019, the components of stock-based compensation expense were as follows:
|
2019 |
2018 |
|||||||
|
Stock option expense |
$ | 104,420 | $ | 155,304 | ||||
|
Stock issued for non-employee services |
223,080 | 314,419 | ||||||
|
Total stock-based compensation expense |
$ | 327,500 | $ | 469,723 | ||||
In general, stock-based compensation expense is allocated to research and development expense or general and administrative expense according to the classification of cash compensation paid to the employee, consultant or director to whom the stock compensation was granted. For the two years ended December 31, 2019, stock-based compensation expense was allocated as follows:
|
2019 |
2018 |
|||||||
|
General and administrative expense |
$ | 283,699 | $ | 427,725 | ||||
|
Research and development expense |
43,801 | 41,998 | ||||||
|
Total stock-based compensation expense |
$ | 327,500 | $ | 469,723 | ||||
Other Income (Expense)
Interest income was $6,359 and $5,213 for the years ended December 31, 2019 and 2018, respectively. The variances between years are primarily attributable to the cash available for investment and to interest rate fluctuations.
Interest expense was $4,495 and $2,290 for the years ended December 31, 2019 and 2018, respectively, and relates to the note payable issued to the GRA in February 2018 and financing costs associated with insurance premiums.
Impact of Inflation
For the two-year period ended December 31, 2019, we do not believe that inflation and changing prices had a material impact on our operations or on our financial results.
|
ITEM 7A. |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Our exposure to market risk is limited primarily to interest income sensitivity, which is affected by changes in the general level of United States interest rates, particularly because a significant portion of our investments are in institutional money market funds. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income received without significantly increasing risk. Due to the nature of our short-term investments, we believe that we are not subject to any material market risk exposure. We do not have any derivative financial instruments or foreign currency instruments.
|
ITEM 8. |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Our consolidated financial statements and supplemental schedule and notes thereto as of December 31, 2019 and 2018 and for the two year period ended December 31, 2019 together with the independent registered public accounting firm’s report thereon, are set forth on pages F-1 to F-18 of this Annual Report on Form 10-K.
|
ITEM 9. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
There were no disagreements with our accountants on matters of accounting or financial disclosure, or other reportable events requiring disclosure under this Item 9.
|
Item 9A. |
Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures designed to ensure that financial information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, as amended (the Exchange Act), is recorded, processed, summarized, and reported within the required time periods, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow for timely decisions regarding disclosure.
An evaluation was performed by our Chief Executive Officer and Chief Financial Officer of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2019. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were effective as of December 31, 2019 to provide reasonable assurance that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) of the Exchange Act. Management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2019 based on criteria established in Internal Control - Integrated Framework (2013), issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). As a result of this assessment, management concluded that, as of December 31, 2019, our internal control over financial reporting was effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Controls
Management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and fraud. Any control system, no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Company have been detected.
|
Item 9B. |
Other Information |
None.
PART III
|
ITEM 10. |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information required by this Item is included in our definitive proxy statement for our 2020 annual meeting of stockholders to be filed with the SEC under the captions “Directors and Executive Officers” and “Corporate Governance” and is incorporated herein by this reference.
Code of Business Conduct and Ethics
Our Board of Directors has adopted a written Code of Business Conduct and Ethics, a copy of which is available on our website at www.geovax.com. The Company will provide a copy of the Code of Ethics upon request to any person without charge. Such requests may be transmitted by regular mail in the care of the Corporate Secretary. We require all officers, directors and employees to adhere to this code in addressing the legal and ethical issues encountered in conducting their work. The code requires that employees avoid conflicts of interest, comply with all laws and other legal requirements, conduct business in an honest and ethical manner, and otherwise act with integrity and in our best interest. Employees are required to report any conduct that they believe in good faith to be an actual or apparent violation of the code. The Sarbanes-Oxley Act of 2002 requires certain companies to have procedures to receive, retain and treat complaints received regarding accounting, internal accounting controls or auditing matters and to allow for the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters. We have such procedures in place.
The Company will post on its website, www.geovax.com, or will disclose on a Form 8-K filed with the SEC, any amendments to, or waivers from, a provision of the Code of Ethics that applies to the Chief Executive Officer or the Chief Financial Officer, or persons performing similar functions, and that relate to (i) honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; (ii) full, fair, accurate, timely, and understandable disclosure in reports and documents that the Company files with, or submits to, the SEC and in other public communications made by the Company; (iii) compliance with applicable governmental laws, rules and regulations; (iv) the prompt internal reporting of violations of the Code of Ethics to an appropriate person or persons identified in the code; or (v) accountability for adherence to the Code of Ethics. Any waiver granted to an executive officer or a director may only be granted by the Board and will be disclosed, along with the reasons therefor, on a Form 8-K filed with the SEC. No such waivers were granted in 2019.
|
ITEM 11. |
EXECUTIVE COMPENSATION |
The information required by this Item is included in our definitive proxy statement for our 2020 annual meeting of stockholders to be filed with the SEC under the captions “Corporate Governance” and “Executive Compensation” and is incorporated herein by this reference.
|
ITEM 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item is included in our definitive proxy statement for our 2020 annual meeting of stockholders to be filed with the SEC under the captions “Security Ownership of Principal Stockholders, Directors and Executive Officers” and “Securities Authorized for Issuance under Equity Compensation Plans” and is incorporated herein by this reference
|
ITEM 13. |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this Item is included in our definitive proxy statement for our 2020 annual meeting of stockholders to be filed with the SEC under the captions “Corporate Governance” and “Certain Relationships and Related Party Transactions” and is incorporated herein by this reference.
|
ITEM 14. |
PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this Item is included in our definitive proxy statement for our 2020 annual meeting of stockholders to be filed with the SEC under the caption “Ratification of Appointment of the Independent Registered Public Accounting Firm” and is incorporated herein by this reference.
PART IV
|
ITEM 15. |
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| (a) | Documents filed as part of this report: | |
| (1) | Financial Statements | Page |
| Reports of Independent Registered Public Accounting Firms | F-2 | |
| Consolidated Balance Sheets as of December 31, 2019 and 2018 | F-4 | |
| Consolidated Statements of Operations for the years ended December 31, 2019 and 2018 | F-5 | |
| Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2019 and 2018 | F-6 | |
| Consolidated Statements of Cash Flows for the years ended December 31, 2019 and 2018 | F-7 | |
| Notes to Consolidated Financial Statements | F-8 |
|
(2) |
Financial Statement Schedules |
| The following financial statement schedule is set forth on page F-18 of this Annual Report on Form 10-K: | |
| Schedule II—Valuation and Qualifying Accounts for the years ended December 31, 2019 and 2018 |
| All other financial statement schedules have been omitted because they are not applicable or not required or because the information is included elsewhere in the Consolidated Financial Statements or the Notes thereto. | |
|
(3) |
Exhibits Required by Item 601 of Regulation S-K |
|
Exhibit Number |
Description |
|
3.1 |
|
3.1.1 |
|
3.1.2 |
|
3.1.3 |
|
3.1.4 |
|
3.1.5 |
|
3.1.6 |
|
3.1.7 |
|
3.1.8 |
|
3.2 |
|
4.1 |
Form of Stock Certificate representing the Company’s Common Stock, par value $0.001 per share (24) |
|
4.1.1 |
|
4.1.2 |
Form of Stock Certificate for the Series B Convertible Preferred Stock (8) |
|
4.2.1 |
|
4.2.2 |
Form of Stock Certificate for the Series I Convertible Preferred Stock (21) |
|
4.3.1 |
|
4.3.2 |
Form of Stock Certificate for the Series J Convertible Preferred Stock (25) |
|
10.1 ** |
Employment Agreement between GeoVax Labs, Inc. and David A. Dodd (16) |
|
10.2 ** |
Employment Agreement between GeoVax, Inc. and Mark W. Reynolds (3) |
|
10.2.1 ** |
Amendment No. 1 to Employment Agreement between GeoVax Labs, Inc. and Mark W. Reynolds (7) |
|
10.2.2 ** |
Salary Deferral Agreement between GeoVax, Inc. and Mark W. Reynolds (16) |
|
10.2.3 ** |
Amendment to Salary Deferral Agreement between GeoVax, Inc. and Mark W. Reynolds (16) |
|
10.3 ** |
Employment Agreement between GeoVax, Inc. and Harriet Robinson (3) |
|
10.3.1 ** |
Amendment No. 1 to Employment Agreement between GeoVax Labs, Inc. and Harriet Robinson (7) |
|
10.3.2 ** |
Salary Deferral Agreement between GeoVax, Inc. and Harriet Robinson (16) |
|
10.3.3 ** |
Amendment to Salary Deferral Agreement between GeoVax, Inc. and Harriet Robinson (16) |
|
10.4 ** |
Employment Agreement between GeoVax, Inc. and Farshad Guirakhoo (10) |
|
10.4.1 ** |
Amendment No. 1 to Employment Agreement between GeoVax Labs, Inc. and Farshad Guirakhoo (12) |
|
10.4.2 ** |
Salary Deferral Agreement between GeoVax, Inc. and Farshad Guirakhoo (15) |
|
10.5 ** |
GeoVax Labs, Inc. 2016 Stock Incentive Plan, as amended (17) |
|
10.5.1 ** |
|
10.5.2 ** |
|
10.6 |
License Agreement (as amended and restated) between GeoVax, Inc. and Emory University (1) |
|
10.7 * |
Office and Laboratory Lease between UCB, Inc. and GeoVax, Inc. |
|
10.8 |
Summary of the GeoVax Labs, Inc. Director Compensation Plan (3) |
|
10.9 |
Senior Note Purchase Agreement between Georgia Research Alliance, Inc. and GeoVax Labs, Inc. (16) |
|
10.10 |
|
10.11 |
|
10.12 |
|
10.13 |
Form of Securities Purchase Agreement dated January 24, 2020 (25) |
|
14.1 |
|
21.1 |
|
31.1 * |
Certification pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934 |
|
31.2 * |
Certification pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934 |
|
32.1 * |
|
32.2 * |
|
101.INS*** |
XBRL Instance Document |
|
101.SCH*** |
XBRL Taxonomy Extension Schema Document |
|
101.CAL*** |
XBRL Taxonomy Extension Calculation Linkbase Document |
|
101.DEF*** |
XBRL Taxonomy Extension Definition Linkbase Document |
|
101.LAB*** |
XBRL Taxonomy Extension Label Linkbase Document |
|
101.PRE*** |
XBRL Taxonomy Extension Presentation Linkbase Document |
___________________
| * | Filed herewith. |
| ** | Indicates a management contract or compensatory plan or arrangement. |
|
*** |
XBRL (Extensible Business Reporting Language) information furnished hereto are deemed not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections. |
|
(1) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed October 4, 2006. |
|
(2) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed June 23, 2008. |
|
(3) |
Incorporated by reference from the registrant’s Annual Report on Form 10-K filed March 8, 2010. |
|
(4) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed April 14, 2010. |
|
(5) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed April 28, 2010. |
|
(6) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed August 2, 2013. |
|
(7) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed October 23, 2013. |
|
(8) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed December 17, 2013. |
|
(9) |
Incorporated by reference from the registrant’s Current Report on Form 8-K filed May 14, 2015. |
| (10) | Incorporated by reference from the registrant’s Quarterly Report on Form 10-Q filed November 12, 2015. |
| (11) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed June 16, 2016. |
| (12) | Incorporated by reference from the registrant’s Annual Report on Form 10-K filed March 16, 2016. |
| (13) | Incorporated by reference from the registrant’s Quarterly Report on Form 10-Q filed August 5, 2016. |
| (14) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed August 4, 2017. |
| (15) | Incorporated by reference from the registrant’s Annual Report on Form 10-K filed March 23, 2018. |
| (16) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed September 7, 2018. |
| (17) | Incorporated by reference from the registrant’s Quarterly Report on Form 10-Q filed November 8, 2018. |
| (18) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed December 28, 2018. |
| (19) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed February 26, 2019 |
| (20) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed April 30, 2019. |
| (21) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed July 24, 2019. |
| (22) | Incorporated by reference from the registrant’s Annual Report on Form 10-K filed March 26, 2019. |
| (23) | Incorporated by reference from the registrant’s Quarterly Report on Form 10-Q filed November 7, 2019. |
| (24) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed January 21, 2020. |
| (25) | Incorporated by reference from the registrant’s Current Report on Form 8-K filed January 24, 2020. |
|
ITEM 16. |
FORM 10-K SUMMARY |
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
GEOVAX LABS, INC. |
|
|
|
|
|
|
|
|
|
BY: |
/s/ David A. Dodd |
|
|
|
David A. Dodd |
|
|
|
|
President and Chief Executive Officer |
|
|
| (Principal Executive Officer) | |||
| Date: March 24, 2020 | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been duly signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature / Name | Title | Date | ||
| /s/ David A. Dodd | Director | March 24, 2020 | ||
| David A. Dodd | President and Chief Executive Officer | |||
| (Principal Executive Officer) | ||||
| /s/ Mark W. Reynolds | Chief Financial Officer | March 24, 2020 | ||
| Mark W. Reynolds | (Principal Financial and Accounting Officer) | |||
| /s/ Randal D. Chase | Director | March 24, 2020 | ||
| Randal D. Chase | ||||
| /s/ David A. Dodd | Director | March 24, 2020 | ||
| David A. Dodd | ||||
| /s/ Dean G. Kollintzas | Director | March 24, 2020 | ||
| Dean G. Kollintzas | ||||
| /s/ Robert T. McNally | Director | March 24, 2020 | ||
| Robert T. McNally | ||||
| /s/ Harriet L. Robinson | Director | March 24, 2020 | ||
| Harriet L. Robinson | ||||
| /s/ John N. Spencer, Jr. | Director | March 24, 2020 | ||
| John N. Spencer, Jr. |
GEOVAX LABS, INC.
INDEX TO 2019 CONSOLIDATED FINANCIAL STATEMENTS
| Page | |
| Reports of Independent Registered Public Accounting Firms | F-2 |
| Consolidated Balance Sheets as of December 31, 2019 and 2018 | F-4 |
| Consolidated Statements of Operations for the years ended December 31, 2019 and 2018 | F-5 |
| Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2019 and 2018 | F-6 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2019 and 2018 | F-7 |
| Notes to Consolidated Financial Statements | F-8 |
| Financial Statement Schedule: | |
| Schedule II – Valuation and Qualifying Accounts for the years ended December 31, 2019 and 2018 | F-18 |
 |
235 Peachtree Street NE Suite 1800 Atlanta, GA 30303 |
404 588 4200 wipfli.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of GeoVax Labs, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of GeoVax Labs, Inc. and subsidiary (the “Company”) as of December 31, 2019, and the related consolidated statements of operations, stockholders’ equity (deficiency) and cash flows for the year then ended and the related notes to the consolidated financial statements and schedule (collectively, the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019, and the results of their operations and their cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Emphasis of a Matter - Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations and its total liabilities exceed its total assets. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters also are described in Note 2 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness on the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
We have served as the Company’s auditor since 2019.
Atlanta, Georgia
March 24, 2020

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of GeoVax Labs, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of GeoVax Labs, Inc. and subsidiary (the “Company”) as of December 31, 2018, and the related consolidated statements of operations, stockholders’ equity (deficiency) and cash flows for the year then ended and the related notes to the consolidated financial statements and schedule (collectively, the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018, and the results of their operations and their cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Emphasis of a Matter - Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations and its total liabilities exceed its total assets. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters also are described in Note 2 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness on the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
We have served as the Company’s auditor since 2005.
Atlanta, Georgia
March 8, 2019
|
GEOVAX LABS, INC. |
||||||
|
CONSOLIDATED BALANCE SHEETS |
|
December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 283,341 | $ | 259,701 | ||||
|
Grant funds and other receivables |
68,603 | 121,814 | ||||||
|
Prepaid expenses and other current assets |
95,320 | 238,189 | ||||||
|
Total current assets |
447,264 | 619,704 | ||||||
|
Property and equipment, net (Note 3) |
10,606 | 11,350 | ||||||
|
Deposits |
11,010 | 11,010 | ||||||
|
Total assets |
$ | 468,880 | $ | 642,064 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIENCY) |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 152,653 | $ | 125,859 | ||||
|
Accrued expenses (Note 4) |
1,851,040 | 1,238,552 | ||||||
|
Current portion of notes payable (Note 5) |
12,500 | 260,420 | ||||||
|
Total current liabilities |
2,016,193 | 1,624,831 | ||||||
|
Note payable, net of current portion (Note 5) |
27,243 | 39,580 | ||||||
|
Total liabilities |
2,043,436 | 1,664,411 | ||||||
|
Commitments (Note 6) |
||||||||
|
Stockholders’ equity (deficiency): |
||||||||
|
Preferred stock, $.01 par value (Note 7): Authorized shares – 10,000,000 Issued and outstanding shares – 2,486 and 3,450 at December 31, 2019 and 2018, respectively |
1,932,433 | 1,971,333 | ||||||
|
Common stock, $.001 par value: Authorized shares – 600,000,000 Issued and outstanding shares – 299,835 and 219 at December 31, 2019 and 2018, respectively |
300 | - | ||||||
|
Additional paid-in capital |
39,340,224 | 37,483,204 | ||||||
|
Accumulated deficit |
(42,847,513 | ) | (40,476,884 | ) | ||||
|
Total stockholders’ equity (deficiency) |
(1,574,556 | ) | (1,022,347 | ) | ||||
|
Total liabilities and stockholders’ equity (deficiency) |
$ | 468,880 | $ | 642,064 | ||||
See accompanying notes to consolidated financial statements.
|
GEOVAX LABS. INC. |
||||||
|
CONSOLIDATED STATEMENTS OF OPERATIONS |
|
Years Ended December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
Grant and collaboration revenue |
$ | 1,175,896 | $ | 963,203 | ||||
|
Operating expenses: |
||||||||
|
Research and development |
1,910,715 | 1,878,652 | ||||||
|
General and administrative |
1,637,674 | 1,647,268 | ||||||
|
Total operating expenses |
3,548,389 | 3,525,920 | ||||||
|
Loss from operations |
(2,372,493 | ) | (2,562,717 | ) | ||||
|
Other income (expense): |
||||||||
|
Interest income |
6,359 | 5,213 | ||||||
|
Interest expense |
(4,495 | ) | (2,590 | ) | ||||
|
Total other income (expense) |
1,864 | 2,623 | ||||||
|
Net loss |
$ | (2,370,629 | ) | $ | (2,560,094 | ) | ||
|
Basic and diluted: |
||||||||
|
Loss per common share |
$ | (39.25 | ) | $ | (15,610.33 | ) | ||
|
Weighted average shares outstanding |
60,402 | 164 | ||||||
|
See accompanying notes to consolidate financial statements. |
GEOVAX LABS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIENCY)
|
Total |
||||||||||||||||||||||||||||
|
Preferred Stock (Note 7) |
Common Stock |
Additional Paid-in |
Accumulated |
Stockholders’ Equity |
||||||||||||||||||||||||
|
Shares |
Amount |
Shares |
Amount |
Capital |
Deficit |
(Deficiency) |
||||||||||||||||||||||
|
Balance at December 31, 2017 |
3,670 | $ | 1,899,085 | 107 | $ | - | $ | 35,696,648 | $ | (37,916,790 | ) | $ | (321,057 | ) | ||||||||||||||
|
Sale of convertible preferred stock for cash |
1,200 | 1,190,000 | - | - | - | - | 1,190,000 | |||||||||||||||||||||
|
Conversion of preferred stock to common stock |
(1,420 | ) | (1,117,752 | ) | 95 | - | 1,117,752 | - | - | |||||||||||||||||||
|
Issuance of common stock for services |
- | - | 17 | - | 513,500 | - | 513,500 | |||||||||||||||||||||
|
Stock-based compensation expense |
- | - | - | - | 155,304 | - | 155,304 | |||||||||||||||||||||
|
Net loss for the year ended December 31, 2018 |
- | - | - | - | - | (2,560,094 | ) | (2,560,094 | ) | |||||||||||||||||||
|
Balance at December 31, 2018 |
3,450 | 1,971,333 | 219 | - | 37,483,204 | (40,476,884 | ) | (1,022,347 | ) | |||||||||||||||||||
|
Sale of convertible preferred stock for cash and cancellation of note payable |
1,700 | 1,542,950 | - | - | 147,050 | - | 1,690,000 | |||||||||||||||||||||
|
Conversion of preferred stock to common stock |
(2,664 | ) | (1,581,850 | ) | 296,390 | 296 | 1,581,554 | - | - | |||||||||||||||||||
|
Issuance of common stock for services |
- | - | 3,224 | 3 | 23,997 | - | 24,000 | |||||||||||||||||||||
|
Adjustments and rounding for reverse stock split |
- | - | 2 | 1 | (1 | ) | - | - | ||||||||||||||||||||
|
Stock-based compensation expense |
- | - | - | - | 104,420 | - | 104,420 | |||||||||||||||||||||
|
Net loss for the year ended December 31, 2019 |
- | - | - | - | - | (2,370,629 | ) | (2,370,629 | ) | |||||||||||||||||||
|
Balance at December 31, 2019 |
2,486 | $ | 1,932,433 | 299,835 | $ | 300 | $ | 39,340,224 | $ | (42,847,513 | ) | $ | (1,574,556 | ) | ||||||||||||||
See accompanying notes to consolidated financial statements.
|
GEOVAX LABS. INC. |
|||||||||||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS |
|
Years Ended December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
Cash flows from operating activities: |
||||||||
|
Net loss |
$ | (2,370,629 | ) | $ | (2,560,094 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Depreciation and amortization |
8,350 | 19,801 | ||||||
|
Stock-based compensation expense |
327,500 | 469,724 | ||||||
|
Changes in assets and liabilities: |
||||||||
|
Grant funds and other receivables |
53,211 | (62,056 | ) | |||||
|
Prepaid expenses and other current assets |
(56,211 | ) | 36,480 | |||||
|
Accounts payable and accrued expenses |
639,282 | 553,119 | ||||||
|
Total adjustments |
972,132 | 1,017,068 | ||||||
|
Net cash used in operating activities |
(1,398,497 | ) | (1,543,026 | ) | ||||
|
Cash flows from investing activities: |
||||||||
|
Purchase of property and equipment |
(7,606 | ) | - | |||||
|
Net cash used in investing activities |
(7,606 | ) | - | |||||
|
Cash flows from financing activities: |
||||||||
|
Net proceeds from sale of preferred stock |
1,440,000 | 1,190,000 | ||||||
|
Proceeds from issuance of notes payable |
- | 300,000 | ||||||
|
Principal repayment of notes payable |
(10,257 | ) | - | |||||
|
Net cash provided by financing activities |
1,429,743 | 1,490,000 | ||||||
|
Net increase (decrease) in cash and cash equivalents |
23,640 | (53,026 | ) | |||||
|
Cash and cash equivalents at beginning of period |
259,701 | 312,727 | ||||||
|
Cash and cash equivalents at end of period |
$ | 283,341 | $ | 259,701 | ||||
|
Supplemental disclosure of non-cash financing activities: |
|
|
As discussed in Note 7, during the year ended December 31, 2019, 2,664 shares of preferred stock were converted into 296,390 shares of common stock and during the year ended December 31, 2018, 1,420 shares of preferred stock were converted into 95 shares of common stock. During the year ended December 31, 2019, $250,000 of notes payable were cancelled in exchange for shares of our convertible preferred stock. |
|
|
See accompanying notes to consolidated financial statements. |
GEOVAX LABS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2019 and 2018
1. Description of Business
GeoVax Labs, Inc. (“GeoVax” or the “Company”), is a clinical-stage biotechnology company developing human vaccines and immunotherapies against infectious diseases and cancers using a novel patented Modified Vaccinia Ankara Virus-Like Particle (MVA-VLP) vaccine platform. In this platform, MVA, a large virus capable of carrying several vaccine antigens, expresses proteins that assemble into highly effective VLP immunogens in the person being vaccinated. The MVA-VLP virus replicates to high titers in approved avian cells for manufacturing but cannot productively replicate in mammalian cells. Therefore, the MVA-VLP derived vaccines elicit durable immune responses in the host similar to a live attenuated virus, while providing the safety characteristics of a replication-defective vector.
Our current development programs are focused on preventive vaccines against coronavirus (COVID-19), Human Immunodeficiency Virus (HIV), Zika Virus, hemorrhagic fever viruses (Ebola, Sudan, Marburg, Lassa), and malaria, as well as therapeutic vaccines for chronic Hepatitis B infections and cancers. We believe our technology and vaccine development expertise are well-suited for a variety of human infectious diseases and we intend to pursue further expansion of our product pipeline.
Our corporate strategy is to improve health to patients worldwide by advancing our vaccine platform, using its unique capabilities to design and develop an array of products addressing unmet medical needs in the areas of infectious diseases and oncology. We aim to advance products through to human clinical testing, and to seek partnership or licensing arrangements for achieving regulatory approval and commercialization. We also leverage third party resources through collaborations and partnerships for preclinical and clinical testing with multiple government, academic and corporate entities.
Certain of our vaccine development activities have been, and continue to be, financially supported by the U.S. government. This support has been both in the form of research grants and contracts awarded directly to us, as well as indirect support for the conduct of preclinical animal studies and human clinical trials.
We operate in a highly regulated and competitive environment. The manufacturing and marketing of pharmaceutical products require approval from, and are subject to, ongoing oversight by the Food and Drug Administration (FDA) in the United States, by the European Medicines Agency (EMA) in the European Union, and by comparable agencies in other countries. Obtaining approval for a new pharmaceutical product is never certain, may take many years and often involves expenditure of substantial resources. Our goal is to build a profitable company by generating income from products we develop and commercialize, either alone or with one or more potential strategic partners.
GeoVax is incorporated under the laws of the State of Delaware and our principal offices are located in the metropolitan Atlanta, Georgia area.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of GeoVax Labs, Inc. together with those of our wholly-owned subsidiaries, GeoVax, Inc. and Immutak Oncology, Inc. All intercompany transactions have been eliminated in consolidation.
Basis of Presentation
As described in Notes 7 and 11, effective April 30, 2019, we enacted a one-for-five hundred reverse stock split of our common stock, and effective January 21, 2020, we further enacted a one-for-two thousand reverse split. The accompanying financial statements, and all share and per share information contained herein, have been retroactively restated to reflect the reverse stock splits.
The accompanying consolidated financial statements have been prepared assuming that we will continue as a going concern, which contemplates realization of assets and the satisfaction of liabilities in the normal course of business for the twelve-month period following the date of these consolidated financial statements. We are devoting substantially all of our present efforts to research and development. We have funded our activities to date from government grants and clinical trial assistance, and from sales of our equity securities. We will continue to require substantial funds to continue our research and development activities.
We believe that our existing cash resources and government and other collaborative funding commitments will be sufficient to continue our planned operations into the second quarter of 2020. Due to our history of operating losses and our continuing need for capital to conduct our research and development activities, there is substantial doubt concerning our ability to operate as a going concern beyond that date. We are currently exploring sources of capital through additional government grants and corporate collaborations. We also intend to secure additional funds through sales of our equity securities or by other means. Management believes that we will be successful in securing the additional capital required to continue the Company’s planned operations, but that our plans do not fully alleviate the substantial doubt about the Company’s ability to operate as a going concern. Additional funding may not be available on favorable terms or at all. If we fail to obtain additional capital when needed, we will be required to delay, scale back, or eliminate some or all of our research and development programs as well as reduce our general and administrative expenses.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles (GAAP) requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results may differ from those estimates.
Cash and Cash Equivalents
We consider all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Our cash and cash equivalents consist primarily of bank deposits and money market accounts. The recorded values approximate fair market values due to the short maturities.
Fair Value of Financial Instruments and Concentration of Credit Risk
Financial instruments that subject us to concentration of credit risk consist primarily of cash and cash equivalents, which are maintained by a high credit quality financial institution. The carrying values reported in the balance sheets for cash and cash equivalents approximate fair values.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Expenditures for maintenance and repairs are charged to operations as incurred, while additions and improvements are capitalized. We calculate depreciation using the straight-line method over the estimated useful lives of the assets which range from three to five years. We amortize leasehold improvements using the straight-line method over the term of the related lease.
In February 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update No. 2016-02, Leases (ASU 2016-02). ASU 2016-02 sets out the principles for the recognition, measurement, presentation and disclosure of leases for both parties to a contract (i.e. lessees and lessors). The new standard requires lessees to classify leases as either financing or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification determines whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease, respectively. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months regardless of their classification. Leases with a term of 12 months or less will be accounted for similar to prior guidance for operating leases. We adopted ASU 2016-02 effective January 1, 2019; such adoption had no material impact on our financial statements, given that the noncancelable term of our current lease is less than 12 months (see Note 6).
Impairment of Long-Lived Assets
We review long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the assets to the future net cash flows expected to be generated by such assets. If we consider such assets to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the expected future net cash flows from the assets.
Accrued Expenses
As part of the process of preparing our financial statements, we estimate expenses that we believe we have incurred, but have not yet been billed by our third-party vendors. This process involves identifying services and activities that have been performed by such vendors on our behalf and estimating the level to which they have been performed and the associated cost incurred for such service as of each balance sheet date.
Net Loss Per Share
Basic and diluted loss per common share are computed based on the weighted average number of common shares outstanding. Common share equivalents consist of common shares issuable upon conversion of convertible preferred stock, and upon exercise of stock options and stock purchase warrants. All common share equivalents are excluded from the computation of diluted loss per share since the effect would be anti-dilutive. The weighted average number of common share equivalents which were excluded from the computation of diluted loss per share, totaled 11,157 and 193 shares at December 31, 2019 and 2018, respectively. See Note 7 for more information concerning our outstanding common share equivalents at December 31, 2019 that could potentially dilute earnings per share in the future.
In July 2017, the FASB issued Accounting Standards Update 2017-11, (Part I) Accounting for Certain Financial Instruments with Down Round Features, (Part II) Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception (“ASU 2017-11”), which amends Accounting Standards Codification Topic 260, Earnings Per Share, Topic 480, Distinguishing Liabilities from Equity, and Topic 815, Derivatives and Hedging. ASU 2017-11 changes the classification of certain equity-linked financial instruments (or embedded features) with down round features and clarifies existing disclosure requirements for equity-classified instruments. We adopted ASU 2017-11 effective January 1, 2019; such adoption had no material impact on our financial statements.
Revenue Recognition
In May 2014, the FASB issued Accounting Standards Update 2014-09, Revenue from Contracts with Customers (ASU 2014-09), which created a new Topic, Accounting Standards Codification Topic 606. The standard is principle-based and provides a five-step model to determine when and how revenue is recognized. The core principle is that an entity should recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. We adopted ASU 2014-09 effective January 1, 2018 using the modified retrospective transition method. Under this method, our prior results will remain as reported and starting in 2018 are recognized under the new method. The adoption of ASU 2014-09 had no material impact on the measurement, timing, or recognition of our grant and collaboration revenues, nor on the related research and development expenses.
Grant revenue – We receive payments from government entities under non-refundable grants in support of our vaccine development programs. We record revenue associated with these grants when the reimbursable costs are incurred and we have complied with all conditions necessary to receive the grant funds.
Research collaborations – We are pursuing a strategy of co-developing or licensing our technology for specific vaccine development approaches and/or disease indications. We have entered into multiple collaborative research and development agreements and have received third-party funding for preclinical research under certain of these arrangements. Each agreement is evaluated in accordance with the process defined by ASU 2014-09 and revenue is recognized accordingly.
Research and Development Expense
Research and development expense primarily consists of costs incurred in the discovery, development, testing and manufacturing of our product candidates. These expenses consist primarily of (i) salaries, benefits, and stock-based compensation for personnel, (ii) laboratory supplies and facility-related expenses to conduct development, (iii) fees paid to third-party service providers to perform, monitor and accumulate data related to our preclinical studies and clinical trials, (iv) costs related to sponsored research agreements, and (v) costs to procure and manufacture materials used in clinical trials. These costs are charged to expense as incurred.
Patent Costs
Our expenditures relating to obtaining and protecting patents are charged to expense when incurred and are included in general and administrative expense.
Period-to-Period Comparisons
Our operating results are expected to fluctuate for the foreseeable future. Therefore, period-to-period comparisons should not be relied upon as predictive of the results for future periods.
Income Taxes
We account for income taxes using the liability method. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted rates in effect for the year in which temporary differences are expected to be recovered or settled. Deferred tax assets are reduced by a valuation allowance unless, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will be realized.
Stock-Based Compensation
We account for stock-based transactions in which the Company receives services from employees, directors or others in exchange for equity instruments based on the fair value of the award at the grant date. Stock-based compensation cost for awards of common stock is estimated based on the price of the underlying common stock on the date of issuance. Stock-based compensation cost for stock options or warrants is estimated at the grant date based on each instrument’s fair value as calculated by the Black-Scholes option pricing model. We recognize stock-based compensation cost as expense ratably on a straight-line basis over the requisite service period for the award. See Note 7 for additional stock-based compensation information.
In May 2017, the FASB issued Accounting Standards Update 2017-09, Scope of Modification Accounting (“ASU 2017-09”), which amends Accounting Standards Codification Topic 718, Compensation – Stock Compensation. ASU 2017-09 is an attempt to provide clarity and reduce both (1) diversity in practice and (2) cost and complexity when applying the guidance in Topic 718 Compensation – Stock Compensation, to a change to the terms or conditions of a share-based payment award. We adopted ASU 2017-09 effective January 1, 2018; such adoption had no material impact on our financial statements.
In June 2018, the FASB issued Accounting Standards Update 2018-07, Improvements to Nonemployee Share-Based Payment Accounting (ASU 2018-07), that expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. We adopted ASU 2018-07 effective January 1, 2019; such adoption had no material impact on our financial statements.
Other Recent Accounting Pronouncements
Except as discussed above, there have been no recent accounting pronouncements or changes in accounting pronouncements which we expect to have a material impact on our financial statements, nor do we believe that any recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on our financial statements.
3. Property and Equipment
Property and equipment as shown on the accompanying Consolidated Balance Sheets is composed of the following as of December 31, 2019 and 2018:
|
2019 |
2018 |
|||||||
|
Laboratory equipment |
$ | 534,577 | $ | 530,306 | ||||
|
Leasehold improvements |
115,605 | 115,605 | ||||||
|
Other furniture, fixtures & equipment |
11,736 | 28,685 | ||||||
|
Total property and equipment |
661,918 | 674,596 | ||||||
|
Accumulated depreciation and amortization |
(651,312 | ) | (663,246 | ) | ||||
|
Property and equipment, net |
$ | 10,606 | $ | 11,350 | ||||
Depreciation expense was $8,350 and $19,801 during the years ended December 31, 2019 and 2018, respectively.
4. Accrued Expenses
Accrued expenses as shown on the accompanying Consolidated Balance Sheets is composed of the following as of December 31, 2019 and 2018:
|
2019 |
2018 |
|||||||
|
Accrued management salaries |
$ | 1,323,483 | $ | 924,509 | ||||
|
Accrued directors’ fees |
409,219 | 295,670 | ||||||
|
Other accrued expenses |
118,338 | 18,373 | ||||||
|
Total accrued expenses |
$ | 1,851,040 | $ | 1,238,552 | ||||
5. Notes Payable
On February 28, 2018, we entered into a Senior Note Purchase Agreement with Georgia Research Alliance, Inc. (GRA) pursuant to which we issued a five-year Senior Promissory Note (the “GRA Note”) to GRA in exchange for $50,000. The GRA Note bears an annual interest rate of 5%, payable monthly, with principal repayments beginning in the second year. Future principal repayments are expected to be $12,500 in 2020, 2021 and 2022, and $2,243 in 2023. Interest expense related to the GRA Note was $2,097 and $2,083 for the years ended December 31, 2019 and 2018, respectively.
On December 27, 2018, we issued short-term non-interest-bearing Term Promissory Notes (the “Term Notes”) to two current investors in exchange for an aggregate of $250,000. These notes are presented as current liabilities on the consolidated balance sheet at December 31, 2018. In February 2019, the Term Notes were cancelled in exchange for shares of our convertible preferred stock (see Note 7).
6. Commitments
Lease Agreement
We lease approximately 8,400 square feet of office and laboratory space pursuant to an operating lease which expires on December 31, 2022. Rent expense for the years ended December 31, 2019 and 2018 was $161,673 and $156,939, respectively. Future minimum lease payments total $166,155 in 2020, $171,213 in 2021 and $176,356 in 2022, although the lease may be terminated at any time by either party with ninety days written notice.
Other Commitments
In the normal course of business, we enter into various firm purchase commitments related to production and testing of our vaccine, conduct of research studies, and other activities. As of December 31, 2019, we had approximately $294,000 of unrecorded outstanding purchase commitments to our vendors and subcontractors, all of which we expect will be due in 2020. We expect this entire amount to be reimbursable to us pursuant to currently outstanding government grants.
7. Stockholders’ Equity
Preferred Stock
Summary -- We are authorized to issue up to 10,000,000 shares of our Preferred Stock, $.01 par value, which may be issued in one or more series. The table below presents our issued and outstanding series of preferred stock as of December 31, 2019 and 2018. Each series of our outstanding preferred stock has a stated value of $1,000 per share. Further details concerning each series of preferred stock, and the changes in each series during the years ended December 31, 2019 and 2018 are discussed in the sections that follow the table.
|
December 31, 2019 |
December 31, 2018 |
|||||||||||||||
|
Carrying |
Carrying |
|||||||||||||||
|
Shares |
Value |
Shares |
Value |
|||||||||||||
|
Series B Convertible Preferred Stock |
100 | $ | 76,095 | 100 | $ | 76,095 | ||||||||||
|
Series C Convertible Preferred Stock |
- | - | 2,150 | 705,238 | ||||||||||||
|
Series E Convertible Preferred Stock |
- | - | 1,200 | 1,190,000 | ||||||||||||
|
Series H Convertible Preferred Stock |
1,686 | 1,156,338 | - | - | ||||||||||||
|
Series I Convertible Preferred Stock |
700 | 700,000 | - | - | ||||||||||||
|
Total |
2,486 | $ | 1,932,433 | 3,450 | $ | 1,971,333 | ||||||||||
Series B Convertible Preferred Stock – Our Series B Convertible Preferred Stock, $1,000 stated value (“Series B Preferred Stock”), has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series B Preferred Stock has no voting rights and is not entitled to a dividend. As of December 31, 2019, there were 100 shares of Series B Preferred Stock outstanding, convertible at any time at the option of the holder into shares of common stock at a fixed conversion price of $350,000 per common share. There were no transactions involving our Series B Preferred Stock during the years ended December 31, 2019 and 2018.
Series C Convertible Preferred Stock – Our Series C Convertible Preferred Stock, $1,000 stated value (“Series C Preferred Stock”), has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series C Preferred Stock has no voting rights and is not entitled to a dividend. During 2018, 420 shares of Series C Preferred Stock were converted into 28 shares of common stock. During January and February 2019, 587 shares of our Series C Convertible Preferred Stock (“Series C Preferred Stock”) were converted into 39 shares of our common stock. As discussed below, during February 2019, all remaining outstanding shares of Series C Preferred Stock (1,563 shares) were exchanged for Series F Preferred Stock.
Series D Convertible Preferred Stock – In May 2017, we issued 1,000 shares of our Series D Convertible Preferred Stock, $1,000 stated value (“Series D Preferred Stock”), for net proceeds, after deduction of certain expenses, of $980,000. Our Series D Preferred Stock has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series D Preferred Stock has no voting rights and is not entitled to a dividend. During 2018, all outstanding shares of Series D Preferred Stock were converted into 67 shares of common stock.
Series E Convertible Preferred Stock – In March 2018, we issued 600 shares of our Series E Convertible Preferred Stock, $1,000 stated value, (“Series E Preferred Stock”) for net proceeds, after deduction of certain expenses, of $590,000. In September 2018, we issued an additional 600 shares of Series E Preferred Stock for net proceeds of $600,000. Our Series E Preferred Stock has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series E Preferred Stock has no voting rights and is not entitled to a dividend. As discussed below, during February 2019, all outstanding shares of Series E Preferred Stock (1,200 shares) were exchanged for Series F Preferred Stock.
Series F Preferred Stock – In February 2019, we entered into Exchange Agreements with holders of our Series C and Series E Preferred Stock, pursuant to which the holders exchanged all shares of Series C and Series E Preferred Stock held by them for an aggregate of 2,763 shares of Series F Convertible Preferred Stock (“Series F Preferred Stock”). Our Series F Preferred Stock has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series F Preferred Stock has no voting rights and is not entitled to a dividend. During 2019, 507 shares of Series F Preferred Stock were converted into 191 shares of our common stock. As discussed below, during July 2019, all remaining outstanding shares of Series F Preferred Stock (2,256 shares) were exchanged for Series H Preferred Stock.
Series G Preferred Stock – In February 2019, we entered into a Securities Purchase Agreement with the purchasers identified therein (the “Purchasers”) providing for sale to the Purchasers of an aggregate of up to 1,000 shares of our Series G Convertible Preferred Stock (“Series G Preferred Stock”) and related warrants for gross proceeds of up to $1.0 million, which was funded at three different closings. Our Series G Preferred Stock has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series G Preferred Stock has no voting rights and is not entitled to a dividend. At the first closing, which occurred in February 2019, we issued 500 shares of Series G Preferred Stock in exchange for the payment by the Purchasers of $250,000 in the aggregate, plus the cancellation of Term Notes held by the Purchasers (see Note 5) in the amount of $250,000. At the second and third closings, which occurred in April and June 2019, we issued an aggregate of 500 additional shares of Series G Preferred Stock in exchange for the payment by the Purchasers of a total of $500,000. As discussed below, during July 2019, all outstanding shares of Series G Preferred Stock (1,000 shares) were exchanged for Series H Preferred Stock.
Series H Preferred Stock – In July 2019, we entered into Exchange Agreements with holders of our Series F and Series G Preferred Stock, pursuant to which the holders exchanged all shares of Series F and Series G Preferred Stock held by them for an aggregate of 3,257 shares of Series H Convertible Preferred Stock (“Series H Preferred Stock”). Our Series H Preferred Stock has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series H Preferred Stock has no voting rights and is not entitled to a dividend. The Series H Preferred Stock is convertible at any time at the option of the holders into shares of our common stock, at a conversion price equal to the lesser of (i) $15,000 per common share and (ii) 80% of the lowest volume weighted average price of the common stock during the ten trading days immediately preceding the delivery of a notice of conversion. The Series H Preferred Stock contains price adjustment provisions, which may, under certain circumstances reduce the conversion price to match if we sell or grant options to purchase, including rights to reprice, our common stock or common stock equivalents at a price lower than the then conversion price of the Series H Preferred Stock. During 2019, 1,570 shares of Series H Preferred Stock were converted into 296,160 shares of our common stock.
Series I Preferred Stock – In July 2019, we entered into a Securities Purchase Agreement with the purchasers identified therein (the “Purchasers”) providing for sale to the Purchasers of an aggregate of 700 shares of our Series I Convertible Preferred Stock (“Series I Preferred Stock”) for gross proceeds of $700,000. Our Series I Preferred Stock has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series I Preferred Stock has no voting rights and is not entitled to a dividend. The Series I Preferred Stock is convertible at any time at the option of the holders into shares of our common stock, at a conversion price equal to the lesser of (i) $15,000 per common share and (ii) 80% of the lowest volume weighted average price of the common stock during the ten trading days immediately preceding the delivery of a notice of conversion. The Series I Preferred Stock contains price adjustment provisions, which may, under certain circumstances reduce the conversion price to match if we sell or grant options to purchase, including rights to reprice, our common stock or common stock equivalents at a price lower than the then conversion price of the Series I Preferred Stock. During 2019, there were no conversions of our Series I Preferred Stock.
Common Stock
Reverse Stock Split – Following approval by our shareholders at a meeting held on April 15, 2019, on April 30, 2019, we effected a one-for-five hundred reverse split of our common stock by the filing of an amendment to our certificate of incorporation with the State of Delaware.
During 2019 and 2018 we issued an aggregate of 296,390 and 95 shares of our common stock, respectively, pursuant to the conversion of several series of our Convertible Preferred Stock as discussed above.
During 2019, we issued an aggregate of 3,224 shares of our common stock pursuant to a consulting agreement; for which we recognized $24,000 of expense. During 2018, we issued an aggregate of 17 shares of our common stock pursuant to certain consulting and investment banking agreements. We assigned an aggregate value to these shares of $513,500, $314,419 of which was expensed during 2018. The remaining $199,080 was recorded as a prepaid expense as of December 31, 2018 and recognized as expense during 2019 over the terms of the related agreements.
Stock Options
We have a stock-based incentive plan pursuant to which our Board of Directors may grant stock options to our employees. The exercise price for any option granted may not be less than fair value (110% of fair value for ISO’s granted to certain employees). Options have a maximum ten-year term and generally vest over three years.
There were no grants of stock options during the year ended December 31, 2019. As a result of the reverse stock splits enacted in April 2019 and in January 2020, we have made adjustments and retroactive restatements to all of our outstanding stock options such that the balances as of December 31, 2019 and 2018 are negligible.
We use the Black-Scholes model for determining the grant date fair value of our stock option grants. This model utilizes certain information, such as the interest rate on a risk-free security with a term generally equivalent to the expected life of the option being valued and requires certain other assumptions, such as the expected amount of time an option will be outstanding until it is exercised or expired, to calculate the fair value of stock options granted. The significant assumptions we used in our fair value calculations were as follows:
|
2019 |
2018 |
|||||||
|
Weighted average risk-free interest rates |
N/A | 2.79 | % | |||||
|
Expected dividend yield |
N/A | 0.0 | % | |||||
|
Expected life of option (yrs) |
N/A | 7.0 | ||||||
|
Expected volatility |
N/A | 71.34 | % | |||||
Total employee and director stock-based compensation expense recognized in the consolidated statement of operations for the years ended December 31, 2019 and 2018 was $104,420 and $155,304, respectively, of which $43,801 and $41,998 was included in research and development expenses and $60,619 and $113,306 was included in general and administrative expenses, respectively. As of December 31, 2019, there is $99,975 of unrecognized compensation expense related to employee and director stock-based compensation arrangements.
Stock Purchase Warrants
The following table summarizes our warrants outstanding as of December 31, 2019:
|
Expiration Date |
Exercise Price |
Number of Warrants |
|||||||
|
Series G |
September 2021 |
$ | 25,440 | 48 | |||||
|
Series H |
December 2021 |
1.15 | 217,392 | ||||||
|
Series I |
Aug-Dec 2024 |
15,000 | 48 | ||||||
During 2019, in connection with the sale of our Series G Preferred Stock, we issued Series I Warrants to purchase an aggregate of 48 shares of our common stock with an exercise price of $15,000 per share.
All of the outstanding warrants contain anti-dilution and price adjustment provisions, which may, under certain circumstances reduce the exercise price to match if we sell or grant options to purchase, including rights to reprice, our common stock or common stock equivalents at a price lower than the then exercise price of the warrants. Such provisions as to the Series G and Series H Warrants apply to the exercise price only, with no effect on the number of shares subject to the warrants. Such provisions as to the Series I Warrants apply to both the exercise price and the number of shares subject to the warrants, so that the number of warrants will be increased such that the aggregate exercise price, after taking into account the decrease in the exercise price, will be equal to the aggregate exercise price prior to the adjustment. The Series H Warrants have an additional price adjustment provision requiring a similar adjustment to the exercise price and number of warrants following a reverse stock split of our common stock; such adjustments occurred in connection with our April 30, 2019 reverse stock split and our January 21, 2020 reverse stock split (see Note 11), which is retroactively reflected in the table above.
8. Retirement Plan
We participate in a multi-employer defined contribution retirement plan (the “401k Plan”) administered by a third-party service provider, and the Company contributes to the 401k Plan on behalf of its employees based upon a matching formula. During the years ended December 31, 2019 and 2018 our contributions to the 401k Plan were $25,876 and $23,354, respectively.
9. Income Taxes
At December 31, 2019, we have a consolidated federal net operating loss (“NOL”) carryforward of approximately $65.7 million available to offset against future taxable income of which approximately $62.6 million expires in varying amounts in 2020 through 2037. Additionally, we have approximately $1.1 million in research and development (“R&D”) tax credits that expire in 2022 through 2039 unless utilized earlier. No income taxes have been paid to date. Section 382 of the Internal Revenue Code contains provisions that may limit our utilization of our NOL and R&D tax credit carryforwards in any given year as a result of significant changes in ownership interests that have occurred in past periods or may occur in future periods.
Deferred income taxes reflect the net effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets and liabilities included the following at December 31, 2019 and 2018:
|
2019 |
2018 |
|||||||
|
Deferred tax assets: |
||||||||
|
Net operating loss carryforward |
$ | 15,328,336 | $ | 16,681,908 | ||||
|
Research and development tax credit carryforward |
1,122,536 | 1,063,877 | ||||||
|
Stock-based compensation expense |
1,877,284 | 1,808,509 | ||||||
|
Accrued salaries and directors’ fees |
450,503 | 315,246 | ||||||
|
Depreciation |
8,571 | 8,414 | ||||||
|
Total deferred tax assets |
18,787,230 | 19,879,954 | ||||||
|
Deferred tax liabilities |
- | - | ||||||
|
Net deferred tax assets |
18,787,230 | 19,879,954 | ||||||
|
Valuation allowance |
(18,787,230 | ) | (19,879,954 | ) | ||||
|
Net deferred tax asset after reduction for valuation allowance |
$ | -0- | $ | -0- | ||||
We have established a full valuation allowance equal to the amount of our net deferred tax assets due to uncertainties with respect to our ability to generate sufficient taxable income to realize these assets in the future. A reconciliation of the income tax benefit on losses at the U.S. federal statutory rate to the reported income tax expense is as follows:
|
2019 |
2018 |
|||||||
|
U.S. federal statutory rate applied to pretax loss |
$ | (497,833 | ) | $ | (537,620 | ) | ||
|
Permanent differences |
278 | 549 | ||||||
|
Research and development credits |
(47,053 | ) | (53,884 | ) | ||||
|
Change in valuation allowance |
544,308 | 590,955 | ||||||
|
Reported income tax expense |
$ | -0- | $ | -0- | ||||
10. Grants and Collaboration Revenue
We receive payments from government entities under our grants from the National Institute of Allergy and Infectious Diseases (NIAID) and from the U.S. Department of Defense in support of our vaccine research and development efforts. We record revenue associated with government grants as the reimbursable costs are incurred. During 2019 and 2018, we recorded $983,682 and $934,575, respectively, of revenue associated with these grants. As of December 31, 2019, there is an aggregate of $1,605,505 in remaining grant funds available for use during 2020.
During 2019 and 2018, we recorded $192,214 and $28,628, respectively, of revenues associated with research collaboration agreements with several third parties.
11. Subsequent Events
Reverse Stock Split
Following approval by our shareholders at a meeting held on January 3, 2020, on January 21, 2020, we effected a one-for-two thousand reverse split of our common stock by the filing of an amendment to our certificate of incorporation with the State of Delaware.
Conversions of Preferred Stock to Common Stock
During the first quarter of 2020 (through March 23), all remaining Series H Preferred Stock (1,686 shares) and Series I Preferred Stock (700 shares) were converted into an aggregate of 13,481,349 shares of our common stock.
Issuance of Series J Preferred Stock
On January 24, 2020, we entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with the purchasers identified therein (the “Purchasers”) providing for the issuance and sale to the Purchasers of an aggregate of 300 shares of our Series J Convertible Preferred Stock (“Series G Preferred Stock”) for gross proceeds of $300,000.
Our Series J Preferred Stock has rights and privileges as set forth in the pertinent Certificate of Designation of Preferences, Rights and Limitations, including a liquidation preference equal to the stated value per share. The Series J Preferred Stock has no voting rights and is not entitled to a dividend. The Series J Preferred Stock is convertible at any time at the option of the holders into shares of our common stock, at a conversion price equal to the lesser of (i) $2.00 per share and (ii) 80% of the volume weighted average price of the common stock during the ten trading days immediately preceding the delivery of a notice of conversion. The Series J Preferred Stock contains price adjustment provisions, which may, under certain circumstances reduce the conversion price to match if we sell or grant options to purchase, including rights to reprice, our common stock or common stock equivalents at a price lower than the then conversion price of the Series J Preferred Stock.
GEOVAX LABS, INC.
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS
For the Years Ended December 31, 2019 and 2018
|
Additions (Reductions) |
||||||||||||||||||||
|
Description |
Balance at Beginning Of Period |
Charged to Costs and Expenses |
Charged to Other Accounts |
Deductions |
Balance at End Of Period |
|||||||||||||||
|
Reserve Deducted in the Balance Sheet From the Asset to Which it Applies: |
||||||||||||||||||||
|
Allowance for Deferred Tax Assets |
||||||||||||||||||||
|
Year ended December 31, 2019 |
$ | 19,879,954 | $ | (1,092,724 | ) | $ | -0- | $ | -0- | $ | 18,787,230 | |||||||||
|
Year ended December 31, 2018 |
19,123,959 | 755,995 | -0- | -0- | 19,879,954 | |||||||||||||||
F-18
