Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Liquidia Technologies Inc | ex_176544.htm |
| EX-32.1 - EXHIBIT 32.1 - Liquidia Technologies Inc | ex_176543.htm |
| EX-31.2 - EXHIBIT 31.2 - Liquidia Technologies Inc | ex_176542.htm |
| EX-31.1 - EXHIBIT 31.1 - Liquidia Technologies Inc | ex_176541.htm |
| EX-23.1 - EXHIBIT 23.1 - Liquidia Technologies Inc | ex_177088.htm |
| EX-10.21 - EXHIBIT 10.21 - Liquidia Technologies Inc | ex_176540.htm |
| EX-4.4 - EXHIBIT 4.4 - Liquidia Technologies Inc | ex_176615.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2019
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-38601
LIQUIDIA TECHNOLOGIES, INC.
(Exact Name of Registrant as Specified in Its Charter)
|
Delaware |
|
20-1926605 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) |
|
419 Davis Drive, Suite 100 |
|
27560 |
|
(Address of Principal Executive Offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (919) 328-4400
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
Common stock, $0.001 par value per share |
LQDA |
The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer ☐ |
Accelerated Filer ☒ |
Non-accelerated Filer ☐ |
Smaller Reporting Company ☒ |
|
Emerging Growth Company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of common stock held by non-affiliates of the registrant on June 28, 2019, which was the last business day of the registrant’s most recently completed second fiscal quarter, was $107,845,184 based on a $8.00 closing price per share as reported on the Nasdaq Capital Market.
As of March 9, 2020, there were 28,368,464 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Liquidia Technologies, Inc. Definitive Proxy Statement with respect to the 2020 Annual Meeting of Stockholders to be filed pursuant to Regulation 14A within 120 days after the end of the fiscal year ended December 31, 2019 are incorporated by reference into Part III of this Annual Report on Form 10-K to the extent stated therein. Except with respect to information specifically incorporated by reference in the Form 10-K, each document incorporated by reference herein is deemed not to be filed as part hereof.
LIQUIDIA TECHNOLOGIES, INC.
|
PART I |
3 |
|
|
Item 1. |
Business |
3 |
|
Item 1A. |
Risk Factors |
49 |
|
Item 1B. |
Unresolved Staff Comments |
91 |
|
Item 2. |
Properties |
91 |
|
Item 3. |
Legal Proceedings |
92 |
|
Item 4. |
Mine Safety Disclosures |
92 |
|
PART II |
93 |
|
|
Item 5. |
Market For Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
93 |
|
Item 6. |
Selected Financial Data |
94 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
95 |
|
Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
111 |
|
Item 8. |
Financial Statements and Supplementary Data |
111 |
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
111 |
|
Item 9A. |
Controls and Procedures |
111 |
|
Item 9B. |
Other Information |
113 |
|
PART III |
114 |
|
|
Item 10. |
Directors, Executive Officers and Corporate Governance |
114 |
|
Item 11. |
Executive Compensation |
114 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
114 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
114 |
|
Item 14. |
Principal Accounting Fees and Services |
115 |
|
PART IV |
116 |
|
|
Item 15. |
Exhibits and Financial Statement Schedules |
116 |
|
Item 16. |
Form 10-K Summary |
118 |
This annual report on Form 10-K includes our trademarks, trade names and service marks, such as Liquidia, the Liquidia logo and PRINT, or Particle Replication In Non-wetting Templates, which are protected under applicable intellectual property laws and are the property of Liquidia Technologies, Inc. This annual report also contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. Solely for convenience, trademarks, trade names and service marks referred to in this annual report may appear without the ®, ™ or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the right of the applicable licensor to these trademarks, trade names and service marks. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
Cautionary Note Regarding Forward-Looking Statements
This annual report on Form 10-K contains forward-looking statements. All statements other than statements of historical facts contained in this annual report may be forward-looking statements. The forward-looking statements are contained principally in the sections entitled “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, but are also contained elsewhere in this annual report. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “would,” “intends,” “targets,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
|
● |
our plans to develop and commercialize our product candidates; |
|
● |
our planned clinical trials for our product candidates; |
|
● |
the timing of the availability of data from our clinical trial; |
|
● |
the timing of our planned regulatory filings; |
|
● |
the timing of and our ability to obtain and maintain regulatory approvals for our product candidates, including the January 2020 filing of the New Drug Application, or NDA, for LIQ861 or U.S. Food and Drug Administration, or FDA, acceptance of the NDA submission and potential approval thereof; |
|
● |
our ability to execute on our strategic or financial initiatives; |
|
● |
the clinical utility of our product candidates and their potential advantages compared to other treatments; |
|
● |
our commercialization, marketing and distribution capabilities and strategy; |
|
● |
our ability to establish and maintain arrangements for the manufacture of our product candidates and the sufficiency of our current manufacturing facilities to produce development and commercial quantities of our product candidates; |
|
● |
our ability to establish and maintain collaborations; |
|
● |
our estimates regarding the market opportunities for our product candidates; |
|
● |
our intellectual property position and the duration of our patent rights; |
|
● |
our estimates regarding future expenses, capital requirements and needs for additional financing; and |
|
● |
our expected use of proceeds from prior public offerings and the period over which such proceeds, together with cash, will be sufficient to meet our operating needs. |
You should refer to the “Risk Factors” section of this annual report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. The forward-looking statements in this annual report are only predictions, and we may not actually achieve the plans, intentions or expectations included in our forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements.
These forward-looking statements speak only as of the date of this annual report. While we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this annual report on Form 10-K.
Unless the context otherwise requires, references in this annual report to “we,” “us”, “our” and the “Company” refer to Liquidia Technologies, Inc., a Delaware corporation.
PART I
Item 1. Business.
Overview
We are a late-stage clinical biopharmaceutical company focused on the development and commercialization of novel products utilizing our proprietary PRINT® technology to transform the lives of patients. PRINT is a particle engineering platform that enables precise production of uniform drug particles designed to improve the safety, efficacy and performance of a wide range of therapies. We are currently focused on the development of two product candidates for which we hold worldwide commercial rights: LIQ861 for the treatment of pulmonary arterial hypertension, or PAH, and LIQ865 for the treatment of local post-operative pain. LIQ861, for which we recently filed a New Drug Application, or NDA, with the FDA, is an inhaled dry powder formulation of treprostinil designed to improve the therapeutic profile of treprostinil by enhancing deep-lung delivery and achieving higher dose levels than current inhaled therapies. We have applied our PRINT technology to enable us to deliver LIQ861 through a convenient, palm-sized dry powder inhaler, or DPI. We have also applied our PRINT technology to our second product candidate, LIQ865, for which we have completed two Phase 1 clinical trials and have initiated Phase 2-enabling toxicology studies. LIQ865 is designed to deliver sustained-release particles of bupivacaine, a non-opioid anesthetic, to treat local post-operative pain for three to five days through a single administration. Additionally, we recently initiated a pre-clinical program to develop an inhaled product leveraging the benefits of our PRINT technology to engineer particles with precise, uniform, aerodynamic size and shape for deep lung delivery.
LIQ861 for Pulmonary Arterial Hypertension
In January 2020, we submitted an NDA to the FDA for LIQ861, our lead product candidate, as a potential treatment for patients with PAH. Treprostinil is a synthetic analog of prostacyclin, a vasoactive mediator essential to normal lung function, which is deficient in patients with PAH. We believe that LIQ861 has the potential to improve the therapeutic profile of existing formulations of treprostinil by enhancing deep-lung delivery and achieving higher dose levels than current inhaled therapies. We are developing LIQ861 under the 505(b)(2) regulatory pathway with Tyvaso® (treprostinil, inhaled solution) as the reference listed drug, which allows us to rely in part on the FDA’s previous findings of efficacy and safety of Tyvaso and the active ingredient treprostinil, which has been approved in four different products administered through the oral, inhaled and continuous infusion (parenteral) routes.
PAH is a chronic, progressive disease caused by the hardening and narrowing of the pulmonary arteries that can lead to right heart failure and eventually death. Treprostinil is a synthetic analog of prostacyclin, a vasoactive mediator essential to normal lung function that is deficient in patients with PAH. PAH is a rare disease, with an estimated prevalence in the United States of approximately 30,000 patients. An independent industry research firm estimated that in 2019 products containing treprostinil across its three routes of administration (oral, inhaled and parenteral infusion) may generate revenue that represents about one-quarter of the approximately $3.5 billion U.S. market for pulmonary hypertension drug therapies. The inhaled route of administration, in which medication is inhaled directly into the lungs, helps minimize the off-tissue adverse side effects of systemic delivery. Tyvaso, marketed by United Therapeutics in the United States, is the standard of care among the inhaled therapies, with more than 80% of inhaled prostacyclin sales in the United States. Current inhaled therapies, including Tyvaso, are delivered by a nebulizer, a device that converts a liquid formulation into mist, and require between four and nine doses per day. Nebulizers require regular care and maintenance, including daily cleaning and access to additional parts and supplies, such as distilled water and a power source, all of which compromise the portability of the device and the quality of life of patients.
We believe LIQ861, if approved, will be the first-to-market inhaled dry powder treprostinil that can be delivered using a convenient, palm-sized DPI. We further believe that LIQ861 can overcome the limitations of current inhaled therapies and has the potential to maximize the therapeutic benefits of treprostinil in treating PAH by safely delivering higher doses into the lungs. Based on our in vitro studies we believe that the precise size, trefoil-like shape and uniformity of each LIQ861 particle may provide deep-lung delivery of treprostinil and may reduce deposition in the upper airway where irritation and pain have been observed with nebulized treprostinil.
In August 2019, we completed an open-label Phase 3 clinical trial, INSPIRE, or Investigation of the Safety and Pharmacology of Dry Powder Inhalation of Treprostinil, for LIQ861. The primary objective of the INSPIRE study was to evaluate the long-term safety and tolerability of LIQ861. The study was designed to evaluate patients who have either been under stable treatment with Tyvaso (nebulizer-delivered treprostinil), for at least three months and were transitioned to LIQ861 under the protocol, or Transition patients, or patients who had been under stable treatment with no more than two non-prostacyclin oral PAH therapies for at least three months and then had their treatment regimen supplemented with LIQ861 under the protocol, or Add-On patients. Within the INSPIRE study, 18 Transition patients were evaluated in a one-directional crossover sub-study comparing bioavailability and pharmacokinetics, or PK, of treprostinil following dosing of LIQ861 as compared with Tyvaso.
In March 2019, we reported that we had completed enrollment and met the primary endpoint, which was long-term safety and tolerability, in our INSPIRE trial. LIQ861 was observed to be well-tolerated in 109 patients, with 101 patients (93%) completing at least two months of treatment. During the two-month period, LIQ861 was evaluated at doses up to 159 mcg (clinical trial nomenclature of 150 mcg capsule strength) with no study-drug related serious adverse events. Dosing has exceeded 159 mcg in some patients receiving drug beyond the Month 2 time point. We have not yet determined a maximum tolerated dose of LIQ861. We also reported fully enrolling our one-directional crossover sub-study comparing bioavailability and PK of treprostinil as sub-study patients transitioned from Tyvaso to LIQ861.
In April 2019, we reported further data from these 109 patients in our INSPIRE trial on exploratory endpoints at two months of treatment that demonstrated generally favorable results with respect to six-minute walk distance and quality of life as indicated by the Minnesota Living with Heart Failure Questionnaire, or MLHFQ. In May 2019, we reported further presentation of this data at the American Thoracic Society, or ATS, International Conference 2019.
In June 2019, we reported results from the INSPIRE study indicating that the 79.5 mcg dose of LIQ861 (clinical trial nomenclature of 75 mcg capsule strength) correlates with nine breaths of Tyvaso, the maximum recommended label dose of Tyvaso. Analysis of the data from the PK sub-study in patients showed variability in systemic plasma levels of both LIQ861 and Tyvaso, which is believed to be attributed to variation in severity of disease and has been seen in prior studies of treprostinil in patients. To more accurately characterize the PK of LIQ861, we conducted two additional PK studies in healthy volunteers. In the first of these studies, we observed unexpected variability in PK levels. Post-hoc analysis showed that plasma levels of treprostinil were tightly correlated to the LIQ861 dose delivered. Based upon additional non-clinical and clinical work, we believe the unexpected variability seen in this healthy volunteer study was due to an administration technique unique to the conduct of the study in the Phase 1 setting. In August 2019, we completed a second PK study in healthy volunteers in which the proper administration technique was followed. This study demonstrated significantly reduced variability, and we believe we have established comparative bioavailability to the reference listed drug.
Final enrollment in the pivotal INSPIRE trial included 121 PAH patients to assess safety and tolerability through Month 2, the primary endpoint of the trial. Of the 121 patients enrolled in the study, 55 were Transition patients and 66 were Add-On patients. Add-On patients started on a dose of 26.5 mcg of LIQ861 (clinical trial nomenclature of 25 mcg capsule strength), with most (>80%) titrating to a 79.5 mcg dose or higher within the first two months of treatment. Consistent with preliminary data presented in the second quarter of 2019, LIQ861 was observed to be well-tolerated and treatment-emergent adverse events were mostly mild to moderate in nature at Month 2 up to doses of 159 mcg of LIQ861, the highest dose studied at Month 2. Durability of therapy with LIQ861 appeared to be favorable, with 96% of Transition patients and 91% of Add-On patients remaining on study drug at the Month 2 timepoint.
Initial analysis of the exploratory endpoints from the INSPIRE study indicates that LIQ861 may provide functional and quality-of-life benefits to PAH patients in New York Heart Association, or NYHA, functional classes II and III. More than 90% of all patients who completed two months of treatment maintained or improved their NYHA functional class. Additionally, we observed improvement in six-minute-walk-distance and quality of life as measured by the MLHFQ in both patient groups.
We continued to treat patients who chose to remain on LIQ861 beyond the Month 2 timepoint of the primary endpoint. More than 80% of INSPIRE patients remained on study drug at Month 4 with no significant changes in safety or tolerability observed compared to Month 2. At the completion of the INSPIRE study, the patient with the longest duration of treatment had been on LIQ861 therapy for 18 months. To provide for continuity of treatment, patients from INSPIRE were provided the opportunity to continue receiving treatment in an extension study, which is currently ongoing. In addition, we are enrolling patients in a clinical study at certain investigational sites in Europe to characterize the hemodynamic dose-response relationship to LIQ861. We are also considering conducting other clinical trials to generate additional data on LIQ861, including a clinical trial in pediatric patients. We also continue to conduct development work in support of potential approval and commercialization of LIQ861, including label and patient-use assessments.
LIQ865 for Post-Operative Pain
LIQ865 is our proprietary injectable, sustained-release formulation of bupivacaine, a non-opioid pain medication. We have engineered the size and composition of the LIQ865 PRINT particles to release bupivacaine over three to five days through a single administration for the management of local post-operative pain after a surgical procedure. We completed a Phase 1a clinical trial of LIQ865 in Denmark in 2017 and a Phase 1b clinical trial in the United States in 2018. We initiated Phase 2-enabling toxicology studies in 2019 to assess LIQ865 in multiple non-clinical tissue models. Results from a study to assess incision tensile strength after healing were acceptable and not statistically different from controls. A nonclinical study to examine soft tissue healing was also completed, and the results were acceptable and comparable to vehicle-treated, saline-treated, and Marcaine-treated sites. We believe this data supports progression to Phase 2 hernia repair studies. In a study to assess bone fracture healing, we observed dose-dependent delayed healing at the two LIQ865 doses studied; however, there were no adverse effects noted on surrounding soft tissues. Additional studies have been initiated with lower doses of LIQ865 to determine a no adverse effect level, or NOAEL, on bone healing. We will review the results from these toxicology studies, and if supportive, we intend to initiate Phase 2 proof-of-concept clinical trials, subject to availability of capital and other factors, during 2021. We believe LIQ865, if successfully developed and approved, has the potential to provide significantly longer local post-operative pain relief compared to currently marketed formulations of bupivacaine.
We estimate that there were over 40 million surgeries in our target market, which consists of orthopedic and soft tissue surgeries, performed in the United States in 2016. According to IMS Health, an independent market research firm, the global market for local anesthetics was approximately $761.1 million in 2017. Despite current pain-management protocols, post-operative pain is still undermanaged, with studies showing that approximately 50% of patients self-report inadequate pain relief. Post-operative pain management is becoming more important as surgeries increase in volume and complexity and hospitals seek treatments that support faster recovery and time to discharge. Concurrently, the risk of opioid abuse and diversion has led physicians, payors and the U.S. federal government to prioritize pain management strategies that minimize reliance on opioids. Local anesthetics, such as bupivacaine, provide a well-established, non-opioid option for post-operative pain management, but their duration of efficacy has been limited to eight hours or less. The FDA has approved one long-acting local anesthetic, liposomal bupivacaine, but pain relief typically lasts only 24 to 36 hours, according to physicians, and its use in combination with other local anesthetics can result in an unsafe release of drug.
Our PRINT Technology
Both LIQ861 and LIQ865 are based upon our proprietary PRINT particle engineering technology, which allows us to engineer and manufacture highly uniform drug particles with precise control over their size, three-dimensional geometric shape and chemical composition. By controlling these physical and chemical parameters of particles, PRINT enables us to target and design desirable pharmacological benefits into product candidates, including prolonged duration of drug release, increased drug loading, a more convenient method of administration, novel combination products, enhanced storage and stability and the potential to reduce adverse side effects. We have scaled PRINT manufacturing to meet the demands of clinical development and, we believe, commercial production. Our approach enables us to design and produce custom micro- and nano-particles containing existing or new small molecule drugs or biologics. For example, we have engineered LIQ861 so that each particle has an ideal, uniform, aerodynamic size and shape for deep-lung delivery. Our PRINT particle engineering technology also allows us to design the chemical composition of particles to control drug release ranging from minutes, days, weeks or months as needed to meet a target profile, such as LIQ865’s three to five day release of bupivacaine.
Development, Regulatory and Commercial Strategy
Initially, our internal pipeline is focused on the development of improved and differentiated drug products containing FDA-approved active pharmaceutical ingredients, or APIs, with established efficacy and safety profiles, which we believe are eligible for the 505(b)(2) regulatory pathway to seek marketing approval in the United States. The 505(b)(2) regulatory pathway can be capital efficient and potentially enable a shorter time to approval. We are seeking marketing approval in the United States for LIQ861 under the 505(b)(2) regulatory pathway, which would allow us to rely in part on existing knowledge of the safety and efficacy of the reference listed drugs. The FDA has indicated that it considers LIQ861, which is delivered by a DPI, to be a drug-device combination product and, accordingly, the DPI will be evaluated as part of our NDA filing. We also intend to develop LIQ865 under the 505(b)(2) regulatory pathway. Additionally, we recently initiated a pre-clinical program to develop an inhaled product leveraging the benefits of our PRINT technology to engineer particles with precise, uniform, aerodynamic size and shape for deep lung delivery.
In addition to building our own internal pipeline, we may collaborate with pharmaceutical companies to assist in the development of their product candidates by leveraging our PRINT technology, which we believe has application across a wide range of therapeutic areas, molecule types and routes of administration. If our product candidates receive marketing approval, we plan to commercialize them in the United States either by ourselves or through partnership or licensing arrangements with other pharmaceutical companies. Outside of the United States, we intend to pursue the regulatory approval and commercialization of our product candidates in collaboration with pharmaceutical companies with regional expertise. We intend to manufacture our product candidates using in-house capabilities. Where appropriate, we will rely on contract manufacturing organizations, or CMOs, to produce, package and distribute our approved drug products on a commercial scale.
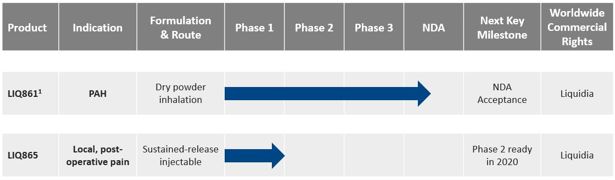
Product Pipeline
The following table summarizes our clinical-stage product candidates being developed using PRINT technology.
Our Strategy
Our goal is to develop and commercialize medicines with improved and differentiated product profiles based on our PRINT particle engineering technology. To achieve this goal, we intend to execute the following key elements of our business strategy:
|
● |
Obtain regulatory approval of LIQ861, our proprietary dry powder inhalation formulation of treprostinil. In January 2020, we submitted an NDA to the FDA for LIQ861, our lead product candidate, as a potential treatment for patients with PAH. Treprostinil is a synthetic analog of prostacyclin, a vasoactive mediator essential to normal lung function, which is deficient in patients with PAH. We believe that LIQ861 has the potential to improve the therapeutic profile of existing formulations of treprostinil by enhancing deep-lung delivery and achieving higher dose levels than current inhaled therapies. LIQ861 is being developed under the 505(b)(2) regulatory pathway with Tyvaso as the reference listed drug, which allows us to rely in part on the FDA’s previous findings of efficacy and safety of Tyvaso and the active ingredient treprostinil, which has been approved in four different products administered through the continuous infusion (parenteral), inhaled and oral routes. Our NDA submission was supported by the favorable results of our pivotal Phase 3 trial, INSPIRE, conducted in 121 patients and completed during the third quarter of 2019. |
|
● |
Advance our local post-operative pain product candidate, LIQ865, through Phase 2-enabling toxicology studies into Phase 2 clinical trials. We completed a Phase 1a clinical trial of LIQ865 in Denmark in 2017 and a Phase 1b clinical trial in the United States in 2018. We initiated Phase 2-enabling toxicology studies in 2019 to assess LIQ865 in multiple non-clinical tissue models. Results from a study to assess incision tensile strength after healing were acceptable and not statistically different from controls. A nonclinical study to examine soft tissue healing was also completed, and the results were acceptable and comparable to vehicle-treated, saline-treated, and Marcaine-treated sites. We believe this data supports progression to Phase 2 hernia repair studies. In a study to assess bone fracture healing, we observed dose-dependent delayed healing at the two LIQ865 doses studied; however, there were no adverse effects noted on surrounding soft tissues. Additional studies have been initiated with lower doses of LIQ865 to determine a NOAEL on bone healing. We will review the results from these toxicology studies, and if supportive, we intend to initiate Phase 2 proof-of-concept clinical trials, subject to availability of capital and other factors, during 2021. We believe LIQ865, if successfully developed and approved, has the potential to provide significantly longer local post-operative pain relief compared to currently marketed formulations of bupivacaine. |
|
● |
Secure regulatory approval and commercialize our products in the United States either ourselves or through partnership or licensing arrangements with other pharmaceutical companies, and globally through licensing arrangements with pharmaceutical companies. We hold worldwide commercialization rights to LIQ861 and LIQ865. We are currently exploring opportunities to commercialize LIQ861 in the United States, subject to receiving regulatory approval, either by ourselves or through partnership or licensing arrangements with other pharmaceutical companies. With respect to LIQ865, after reviewing the results of all of our Phase 2-enabling toxicology studies, and subject to the availability of sufficient funding, we plan to evaluate whether to pursue continued internal development or to explore licensing arrangements with other pharmaceutical companies. Outside of the United States, we intend to pursue the regulatory approval and commercialization of LIQ861 and LIQ865 through licensing arrangements with pharmaceutical companies with regional expertise. |
|
● |
Expand our internal pipeline leveraging our PRINT technology. We intend to continue targeting diseases where we believe our PRINT technology can improve the efficacy, safety and patient experience of current treatments that have been impaired by suboptimal drug product formulation and delivery. We plan to focus initially on the development of improved and differentiated drug products containing FDA-approved active pharmaceutical ingredients, or APIs, with proven efficacy and safety profiles eligible to use the 505(b)(2) regulatory pathway. In addition, we may expand our clinical development of LIQ861 and LIQ865, where appropriate, into broader indications or new applications. |
|
● |
Pursue strategic collaborations to maximize the value of products enabled by PRINT technology. In addition to advancing our own internal product candidates, we have collaborated, and may consider collaborating, with pharmaceutical companies to develop their own product candidates across a wide range of therapeutic areas, molecule types and routes of administration, leveraging our PRINT technology. We believe that collaborating with pharmaceutical companies helps advance new PRINT capabilities, while adding to our intellectual property portfolio. |
Our Competitive Strengths
We believe that we have several key strengths that have contributed to the development of our business and that will help us to realize our goal of becoming a biopharmaceutical company across research, development and commercialization activities. Our competitive strengths include:
|
● |
Our PRINT technology gives us the capability to overcome the constraints of conventional formulation and production methods and can be applied broadly across therapeutic areas, molecule types and routes of administration. Our PRINT technology allows us to precisely engineer drug particles in a wide variety of compositions, sizes and shapes and achieve a high level of control over the physical and chemical characteristics of drug particles, as compared to conventional formulation and production methods. PRINT particles can be designed to address specific pharmacological or therapeutic objectives, such as enhancing the route of administration, improving solubility, enhancing stability or extending therapeutic effects. Using our PRINT technology, we are able to engineer, among others, small molecule and biologic particles, single agent drug and combination drug particles and vaccine particles to improve efficacy, safety and convenience for patients. Our internal pipeline strategy is currently focused on developing proprietary innovations to currently approved drug products in order to minimize development risks and increase speed to market. In particular, we have designed LIQ861 to maximize the therapeutic benefits of treprostinil in treating PAH by safely delivering higher doses into the lungs using a convenient, palm-sized DPI. We believe that this may lead to a more attractive product profile with a more convenient method of administering the drug, as compared to the currently available inhaled therapies. We have also designed LIQ865 with the intention of providing patients with local post-operative analgesia for three to five days. We believe this would provide a longer period of pain relief than the currently available local-acting pain drugs and thereby reduce reliance on opioids and non-steroidal anti-inflammatory drugs, or NSAIDs, for local post-operative pain management. |
|
● |
We have scaled operations with rapid and cost-effective transition to clinical development and commercial production. We believe our research and development operations and PRINT technology allow us to transition rapidly and cost-effectively from laboratory to clinical development and ultimately commercial-scale manufacture of drug particles. Utilizing well-established techniques from other roll-to-roll manufacturing processes, we have scaled PRINT technology to support the quality and quantity needs for clinical and, we believe, commercial production of our product candidates. The manufacturing equipment for the PRINT technology requires a relatively small footprint, low capital investment and minimal operating costs. We believe our manufacturing facilities comply with the FDA’s current good manufacturing practices, or cGMP, requirements. |
|
● |
We have a strong proprietary position through a combination of patents, trade secrets, proprietary know-how and licensing arrangements. We protect our PRINT technology and the resulting engineered particles through a combination of patents, trade secrets, proprietary know-how and licensing arrangements. We have an active patent strategy that covers major geographic markets, including the United States, Europe and Japan. As of December 31, 2019, our patent portfolio, which includes patents and patent applications we own or co-own, as well as patents and patent applications we have licensed from third parties, such as the University of North Carolina at Chapel Hill, or UNC, comprises 127 issued patents and 32 pending patent applications worldwide. As we develop new product candidates, either independently or with collaborators, we will seek additional patent protection. |
|
● |
We have strong capabilities in pharmaceutical research and clinical development. Our research and development team includes 22 employees as of December 31, 2019, led by our senior management, and has extensive experience in clinical development and pharmaceutical research and development activities in our specific areas of research interest. |
|
● |
We have a seasoned management team. Our management team includes industry veterans with significant experience in drug discovery, development and commercialization. Members of our leadership team have worked across different segments of the pharmaceutical industry, including branded and generic pharmaceuticals, medical devices and manufacturing services. Prior to joining us, our Chief Executive Officer and director, Neal Fowler, served as president of Centocor, Inc., a subsidiary of Johnson & Johnson that is focused on the development and commercialization of biomedicines used to treat chronic inflammatory diseases. Additionally, our Chief Operations Officer, Robert Lippe, previously served as executive vice president of operations and chief operations officer at Alexza Pharmaceuticals, Inc. Furthermore, our Senior Vice President, Product Development, Dr. Robert Roscigno, previously served as the president and chief operating officer of Lung Rx, Inc., where he was a member of the team responsible for bringing Tyvaso through Phase 3 development, and held multiple leadership positions at United Therapeutics and its subsidiaries, where he contributed to the successful development and worldwide commercialization of Remodulin™, a parenteral formulation of treprostinil. We believe that the experience of these individuals and other members of our management team enables us to evaluate opportunities and build collaboration arrangements that match the breadth of the potential applications for our PRINT technology. |
Our Product Candidates
LIQ861
Our lead product candidate, LIQ861, is an inhaled dry powder formulation of treprostinil designed using our PRINT technology to enhance deep-lung delivery using a convenient DPI for the treatment of PAH. We believe LIQ861 can overcome the limitations of current inhaled therapies and has the potential to maximize the therapeutic benefits of treprostinil in treating PAH by safely delivering higher doses into the lungs. If approved, we believe LIQ861 will have the potential to increase the number of patients using the inhaled route of administration for PAH by providing the benefits of inhaled prostacyclin therapy earlier in a patient’s disease progression as well as delaying the burden of starting continuously infused agents.
Background on PAH
PAH is a chronic, progressive disease caused by hardening and narrowing of the pulmonary arteries that can lead to right heart failure and eventually death. Prostacyclin is a vasoactive mediator essential to normal lung function that is deficient in patients with PAH. With PAH, the elevated pressure in the pulmonary arteries strains the right side of the heart as it pumps blood to the lungs. The extra stress causes the heart to enlarge and become less flexible, compromising its ability to pump blood through the lungs and to the rest of the body. PAH initially presents as exertional dyspnea, lethargy and fatigue and may be confused with other disease states with similar symptoms. PAH often goes undiagnosed or misdiagnosed until symptoms become severe, with the mean time from onset of symptoms to correct diagnosis being more than two years in the United States. As PAH progresses and right ventricular failure develops, exertional chest pain, or angina, exertional syncope and peripheral edema may develop. Following confirmation of the diagnosis based on hemodynamic parameters, treatment is recommended to lower pulmonary arterial pressures and treat the symptoms of PAH.
PAH is part of a larger classification of pulmonary hypertension, or PH, which is divided into five groups based on the criteria of the World Health Organization, or WHO, as defined at the 5th World Symposium on Pulmonary Hypertension. WHO Group I is comprised of individuals with PAH.
PAH is a rare disease, with an estimated prevalence in the United States of approximately 30,000 patients. The mean age of diagnosis is 50 years according to both French and U.S. registries, with more women being diagnosed with PAH than men. Patients may have idiopathic PAH, in which no underlying cause can be determined, or a heritable form of the disease. A large number of PAH patients also have associated comorbidities such as congenital heart disease, HIV, connective tissue diseases like scleroderma, liver diseases, systemic hypertension, obesity, clinical depression, non-PAH related obstructive airways, sleep apnea and diabetes.
Due to delayed diagnosis, many patients already have an advanced form of PAH, requiring aggressive treatment combining multiple classes of therapy. The severity of PAH may be classified according to the heart failure guidelines of the NYHA based on the degree of limitation of physical activity and described by the American Heart Association as follows:
|
● |
NYHA Class I — No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation or dyspnea, which is shortness of breath. |
|
● |
NYHA Class II — Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation or dyspnea. |
|
● |
NYHA Class III — Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation or dyspnea. |
|
● |
NYHA Class IV — Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases. |
As the disease progresses, these symptoms cause a significant negative impact on quality of life, limiting the ability to perform common daily activities, including work, travel and previous hobbies. Patients also describe the emotional toll of PAH, including fear, frustration, embarrassment and stigma. The burden of care associated with currently available treatments can add further logistical and emotional burden to the patients.
Current Therapies and Their Limitations
There is currently no cure for PAH. The goals of existing treatments are to alleviate symptoms, maintain or improve NYHA functional class, delay disease progression and improve quality of life. Inhaled therapies are generally prescribed for, but not limited to, patients in NYHA Class II and Class III. Approved drugs target three distinct molecular pathways that have been implicated in the disease process: the prostacyclin pathway, the nitric oxide pathway and the endothelin pathway. Drugs targeting each of these pathways are used alone or in combination with each other to treat patients with PAH. Prostacyclin deficiency in the lung is a central dysfunction in PAH, but can be supplemented with prostacyclin analogs. Prostacyclin deficiency can also be managed with a recently approved selective IP prostacyclin receptor agonist, selexipag. Nitric oxide deficiency can be treated with phosphodiesterase-5, or PDE5, inhibitors, which target a specific enzyme, increasing vasodilation. Endothelin overexpression in PAH patients causes vasoconstriction of pulmonary vasculature, but can be treated with endothelin receptor antagonists, or ERAs. Many physicians start their PAH patients on oral PDE5 inhibitors, oral ERAs or both. Drugs targeted to the prostacyclin pathway are usually added to these oral therapies, but can be used alone.
Drugs targeting the prostacyclin pathway are central to PAH therapy. Prostacyclin is essential to normal lung function. In healthy people, prostacyclin, which is a vasoactive mediator, is continually released by the lungs into the pulmonary arterial circulation, where it affects the regulation of vascular tone, including through direct vasodilation of pulmonary arteries, inhibition of the proliferation of smooth muscle cells within arteries and inhibition of platelet aggregation. To supplement the deficiency of prostacyclin in patients with PAH, several prostacyclin analogs have been developed including epoprostenol, which is administered intravenously; treprostinil, which can be administered intravenously, subcutaneously or in nebulized or oral formulations; and iloprost, which can be administered intravenously or in nebulized form. A new class of drugs called selective IP prostacyclin receptor agonists help stimulate some of the mechanisms that would otherwise be promoted by prostacyclin or an analog. Selexipag, an oral agent, is the only approved drug in this new class.
The goal of treatment targeting the prostacyclin pathway is to maximize a patient’s exposure to the highest tolerable level of drug. Prostacyclin analogs, like treprostinil, have been developed for continuous infusion, either intravenously or subcutaneously, inhalation using a nebulizer and oral administration in the form of tablets. The maximal efficacy benefit of any one drug in the prostacyclin pathway is partially limited by its specific safety profile. Drugs exerting their effect through the prostacyclin pathway, including oral treprostinil and IP prostacyclin receptor agonists such as selexipag, are limited by side effects from binding of the drug to receptors in non-targeted tissues, such as the gastrointestinal tract and nerves, which can cause diarrhea, nausea and jaw pain. Nebulized solutions can have side effects including cough, upper airway irritation and pain caused by their topical irritant properties, which limits the amount of drug that can be given to the patient. As the disease progresses, patients require continuous prostacyclin infusion to maximize drug exposure. However, infusion pumps can cause side effects related to infusion site pain and risk of infection, while also adversely affecting quality of life.
Delivering prostacyclin analogs locally to the lungs by inhalation has been effective and causes fewer systemic side effects. Inhalation of prostacyclin analogs supplements the endogenous production of prostacyclin where it is normally synthesized, near the targeted pulmonary arteries. As a result, inhalation of prostacyclin analogs helps avoid side effects related to off-target tissues and takes advantage of binding key prostacyclin receptors that are preferentially expressed in the lung. The only inhaled prostacyclin analogs approved by the FDA are Tyvaso and Ventavis, which both require nebulizers.
Tyvaso (treprostinil) is approved in the United States and Israel, but is not approved in Europe and Japan. Tyvaso is indicated for the treatment of PAH to improve exercise ability. The maximum recommended dose of Tyvaso is 54 mcg, delivered four times daily from a proprietary nebulizer, requiring nine breaths for each dose. In a long-term open-label extension study of Tyvaso, patients continued treatment for a mean duration of 2.3 years, with 89% of patients achieving the target dose of 54 mcg, delivered in nine breaths, and 42% achieving a dose of 72 mcg, delivered in 12 breaths. It has been reported that more than 80% of PAH patients on inhaled therapy in the United States use Tyvaso. United Therapeutics reported approximately $415 million in sales of Tyvaso in 2019.
Ventavis (iloprost) is approved in the United States, Europe and Japan. Ventavis is a synthetic analog of prostacyclin indicated for the treatment of PAH to improve a composite endpoint consisting of exercise tolerance, symptoms and lack of deterioration. Ventavis is administered with a proprietary nebulizer six to nine times per day during waking hours, no more than once every two hours, and takes six to ten minutes to administer per use.
Tyvaso and Ventavis both require the use of proprietary nebulizers. Patients must follow specific instructions to set up and operate the device, clean the device daily, locate a power source or use a battery to operate the device, and carry the device and its associated accessories around in a large carrying case, along with distilled water, to administer the treatment throughout the day. As a result, the use of these approved inhaled prostacyclin therapies is typically limited to patients who have not responded to oral medications that target the three pathways. Current medical practice is to add an inhaled drug to the patient’s existing oral ERA and/or PDE5 treatment regimen, rather than withdrawing the oral drug upon initiation of the inhaled drug.
Potential Benefits of Our Approach
We believe LIQ861 can overcome the limitations of current nebulized therapies and has the potential to maximize the therapeutic benefits of treprostinil in treating PAH by safely delivering higher doses into the lungs using a convenient, palm-sized DPI. In our clinical trials, LIQ861 has been well-tolerated at doses approximately twice as high as the maximum recommended dosage of Tyvaso. These higher doses of inhaled dry powder treprostinil can also be administered in one to four breaths, compared to nine breaths for the maximum recommended dose of Tyvaso. Additionally, we believe LIQ861 may have the potential to improve overall patient adherence by offering the convenience of a discrete, palm-sized DPI. In our market research, patients expressed a preference for a DPI product, noting that it can fit easily into a purse, minimize hassle while traveling and reduce the breaths and time associated with their current nebulized treatments.
The advantages of the LIQ861 product profile are enabled by our PRINT technology. Each LIQ861 particle is designed to enhance delivery and deep-lung penetration. LIQ861 particles are a precise size and highly uniform shape, since particles are formed from mold cavities that exactly match each other. Competing technologies, such as spray-drying, create particles that have a broader variation in size and shape. As a result, particles farther from the mean target size would be too large or too small to reach the intended location in the deep-lung.
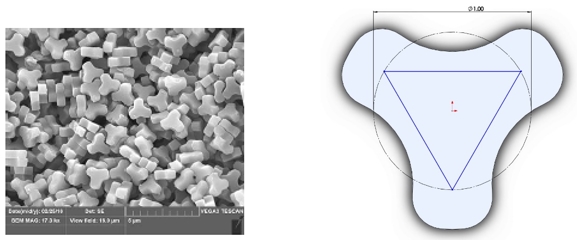
Inspired by a naturally occurring pollen, LIQ861 PRINT particles have a one micrometer trefoil-shape measured by an inscribed one micrometer circle as shown in the figure below. In vitro studies suggest that the uniformity of size and shape allow our inhaled particles to target delivery into the lungs with less deposition in the upper airways. Our independent control of the parameters of drug particles has enabled us to create the first clinically tested therapeutic that stabilizes treprostinil in an inhaled dry powder formulation.
The figures below depict LIQ861, with the figure on the left showing size and shape consistency among particles and the figure on the right showing their trefoil shape:
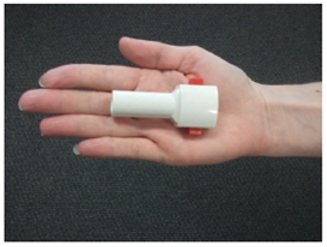
LIQ861 is administered using the RS00 Model 8 DPI, which is manufactured by Plastiape S.p.A. This device and its variants have been used in at least eight marketed products globally since 2001, including Novartis’s Foradil Aerolizer® for the treatment of asthma and chronic obstructive pulmonary disease, or COPD.
The picture below shows the DPI used to administer LIQ861:
Clinical Development
The INSPIRE study was designed to evaluate patients who have either been under stable treatment with nebulizer-delivered treprostinil for at least three months and are transitioned to LIQ861 under the protocol or who have been under stable treatment with no more than two non-prostacyclin oral PAH therapies for at least three months and have their treatment regimen supplemented with LIQ861 under the protocol. The primary objective of the study was to evaluate the long-term safety and tolerability of LIQ861.
In the United States, we submitted an NDA under the 505(b)(2) regulatory pathway in January 2020. The 505(b)(2) pathway allows us to rely, in part, on the FDA’s prior conclusions of efficacy and safety for Tyvaso, and the active ingredient treprostinil (which has been the active ingredient in several different products, in total, approved by the FDA, with routes of administration including continuous infusion, inhaled and oral routes).
Clinical Development
We have developed LIQ861 under the 505(b)(2) regulatory pathway, which allows for an accelerated development program based upon establishing safety, tolerability, and comparative bioavailability to a reference listed drug, which for LIQ861 is Tyvaso. Our clinical development program has consisted of two principal studies. The first of these was a Phase 1 study in healthy volunteers that was designed to assess the safety, tolerability and pharmacokinetic parameters of LIQ861 in healthy volunteers. After an end of Phase 1 meeting with the FDA, we proceeded directly to a pivotal Phase 3 study, without being required to conduct a Phase 2 study. In addition, we conducted two supplementary pharmacokinetic studies in healthy volunteers. The results of these studies, which serve as the basis for our NDA submission, are described below.
Phase 1 Trial
We conducted a randomized, placebo-controlled, double-blind, Phase 1 trial in 57 healthy volunteers to assess safety, tolerability and pharmacokinetics following a single administration of LIQ861 at treprostinil capsule strengths between 25 mcg and 150 mcg. The subjects were enrolled into six dose cohorts. Within each dose cohort, subjects were randomized to receive LIQ861 or a placebo.
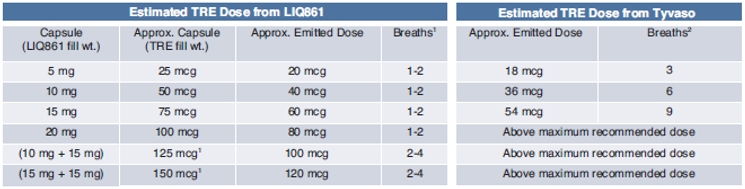
Dose Selection
For the first-in-human study, the initial dose for LIQ861 was chosen based on the indicated dosing for the reference listed drug, Tyvaso. Independent investigations of particle emission using the RS00 Model 8 DPI and simulated inspiration of the bulk powder from a nebulizer led to a projection that a 25 mcg treprostinil capsule strength of LIQ861 dry powder inhalation would result in approximately similar treprostinil administration as three breaths of Tyvaso, or 18 mcg of treprostinil, the lowest approved dose through nebulization. The following table shows the doses of LIQ861 tested along with our estimate of the equivalent Tyvaso dose.
|
(1) |
LIQ861 capsule treprostinil strength doses between 25 mcg and 100 mcg are single capsules. LIQ861 capsule treprostinil strength doses of 125 mcg and 150 mcg are two capsules, but, if approved, they could be developed as single capsules and therefore only require one to two breaths. |
|
(2) |
Tyvaso (treprostinil) full prescribing information: initial dosage: 3 breaths (18 mcg); maximum recommended dosage: 9 breaths (54 mcg). |
Our conclusion from this study is that the capsule strength of 75 mcg of LIQ861 is approximately equivalent to the maximum recommended dose of 54 mcg, or nine breaths, of Tyvaso, and the capsule strength of 150 mcg of LIQ861 is approximately double the maximum recommended dose of Tyvaso.
Safety and Tolerability
In the Phase 1 clinical trial, we escalated the treprostinil capsule strength of LIQ861 progressively from 25 mcg to 150 mcg. There were no dose-limiting toxicities at the highest dose evaluated. We noted no serious adverse events and all reported treatment-emergent adverse events, or TEAEs, related to the treatment were mild. The most frequent adverse event reported by subjects receiving LIQ861 was mild cough and throat irritation.
We did not observe a proportional increase of adverse events as the treprostinil capsule strengths were escalated from 25 mcg to 100 mcg. No adverse events were observed in subjects who received the placebo PRINT particles that contained only excipients.
Pharmacokinetics
In the Phase 1 trial, the LIQ861 plasma levels increased proportionally as the dosage of LIQ861 increased, as shown in the graph below. At higher doses, 50% of subjects receiving LIQ861 had measurable treprostinil levels after four hours, which could indicate the potential to minimize symptoms between dosing cycles.
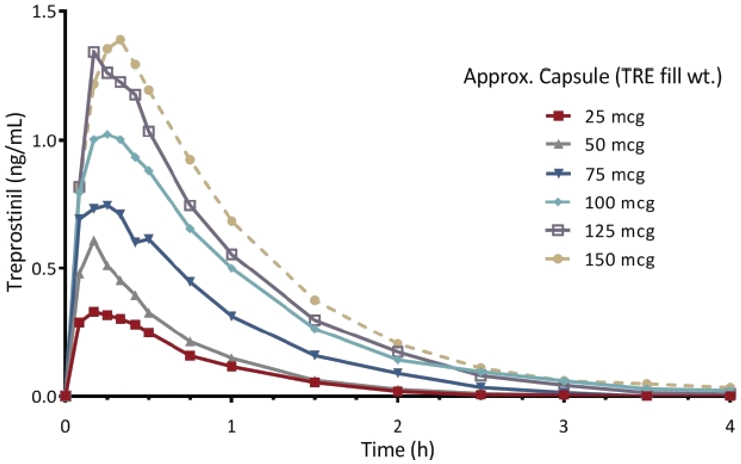
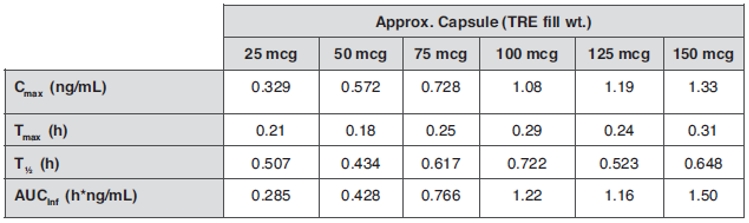
The pharmacokinetic parameters in the table below were estimated from plasma samples. Nominal elapsed time from dosing was used to estimate all individual pharmacokinetic parameters, including:
|
● |
Cmax Maximum observed plasma concentration; |
|
● |
Tmax Time of maximum concentration; |
|
● |
T1/2 Terminal-phase half-life; and |
|
● |
AUCInf Area under the plasma concentration-time curve. |
Plasma levels of LIQ861, as determined by the area under the curve, which is a pharmacokinetic measurement of drug exposure in blood plasma over time, and the maximum concentration were similar to the data used in connection with the approval of Tyvaso, as reported in the FDA Summary Basis of Approval for Tyvaso. LIQ861 also had a half-life in the blood similar to such data. These results suggest that our formulation has not changed the pharmacokinetic profile of inhaled treprostinil.
Non-Clinical Studies
The pharmacology, pharmacokinetics and toxicology of treprostinil are well understood, having previously been characterized to support approval of Remodulin, which is treprostinil administered through subcutaneous or intravenous infusion, Orenitram®, which is treprostinil administered through extended-release tablets, and Tyvaso, which is treprostinil inhaled through a proprietary nebulizer. We plan to rely in part on the data used in support of FDA approval of these treatments, in addition to our own toxicity studies, to support the development and approval of LIQ861.
In October 2016, we completed a 14-day, repeat dose, inhalation toxicity study in rats to support the Phase 1 trial. In August 2017, we completed a 26-week toxicology study in rats. In rats, pharmacokinetic profiles at the end of 14 days of LIQ861 treatment were generally similar to those seen with inhaled nebulized treprostinil delivered at similar treprostinil dose levels. Following 26 weeks of daily dosing, treprostinil exposure was slightly higher in LIQ861-treated rats. The results from this study supported chronic outpatient dosing of LIQ861 in patients with PAH in our Phase 3 trial.
Pivotal Phase 3 INSPIRE Trial
In August 2019, we completed an open-label Phase 3 clinical trial, INSPIRE, or Investigation of the Safety and Pharmacology of Dry Powder Inhalation of Treprostinil, for LIQ861. The study was conducted at multiple sites in the United States. The primary objective of the INSPIRE study was to evaluate the long-term safety and tolerability of LIQ861.
The study was designed to evaluate patients who have either been under stable treatment with Tyvaso (nebulizer-delivered treprostinil), for at least three months and were transitioned to LIQ861 under the protocol, or Transition patients, or patients who had been under stable treatment with no more than two non-prostacyclin oral PAH therapies for at least three months and then had their treatment regimen supplemented with LIQ861 under the protocol, or Add-On patients. Transition patients were initiated at a capsule strength of LIQ861 lower than their current stable treprostinil dose administered four times daily, while Add-on patients were initiated at 26.5 mcg dose, also administered four times daily. In both cases, LIQ861 was uptitrated in 26.5 mcg treprostinil incremental doses to symptom relief or the limit of tolerance. Within the INSPIRE study, 18 Transition patients were evaluated in a one-directional crossover sub-study comparing bioavailability and pharmacokinetics, or PK, of treprostinil following dosing of LIQ861 as compared with Tyvaso.
In March 2019, we reported that we had completed enrollment and met the primary endpoint, which was long-term safety and tolerability, in our INSPIRE trial. LIQ861 was observed to be well-tolerated in 109 patients, with 101 patients (93%) completing at least two months of treatment. During the two-month period, LIQ861 was evaluated at doses up to 159 mcg with no study-drug related serious adverse events. Dosing has exceeded 159 mcg in some patients receiving drug beyond the Month 2 time point. We have not yet determined a maximum tolerated dose of LIQ861. We also reported fully enrolling our one-directional crossover sub-study comparing bioavailability and PK of treprostinil as sub-study patients transitioned from Tyvaso to LIQ861.
In April 2019, we reported further data from these 109 patients in our INSPIRE trial on exploratory endpoints at two months of treatment that demonstrated generally favorable results with respect to six-minute walk distance and quality of life as indicated by the Minnesota Living with Heart Failure Questionnaire, or MLHFQ. In May 2019, we reported further presentation of this data at the ATS International Conference 2019.
In June 2019, we reported results from the INSPIRE study indicating that the 79.5 mcg dose of LIQ861 correlates with the 54 mcg dose of Tyvaso (nine breaths), the maximum recommended label dose of Tyvaso. Analysis of the data from the PK sub-study in patients showed variability in systemic plasma levels of both LIQ861 and Tyvaso, which is believed to be attributed to variation in severity of disease and has been seen in prior studies of treprostinil in patients. To more accurately characterize the PK of LIQ861, we conducted two additional PK studies in healthy volunteers. In the first of these studies, we observed unexpected variability in PK levels. Post-hoc analysis showed that plasma levels of treprostinil were tightly correlated to the LIQ861 dose delivered. Based upon additional non-clinical and clinical work, we believe the unexpected variability seen in this healthy volunteer study was due to an administration technique unique to the conduct of the study in the Phase 1 setting. In August 2019, we completed a second PK study in healthy volunteers in which the proper administration technique was followed. This study demonstrated significantly reduced variability, and we believe we have established comparative bioavailability to the reference listed drug. The results of this second supplementary PK study in healthy volunteers are described more fully below.
Final enrollment in the pivotal INSPIRE trial included 121 PAH patients to assess safety and tolerability through Month 2, the primary endpoint of the trial. Of the 121 patients enrolled in the study, 55 were Transition patients and 66 were Add-On patients. Add-On patients started on a dose of 26.5 mcg of LIQ861, with most (>80%) titrating to a 79.5 mcg dose or higher within the first two months of treatment. Consistent with preliminary data presented in the second quarter of 2019, LIQ861 was observed to be well-tolerated and treatment-emergent adverse events were mostly mild to moderate in nature at Month 2 up to doses of 159 mcg of LIQ861, the highest dose studied at Month 2. Durability of therapy with LIQ861 appeared to be favorable, with 96% of Transition patients and 91% of Add-On patients remaining on study drug at the Month 2 timepoint.
Initial analysis of the exploratory endpoints from the INSPIRE study indicates that LIQ861 may provide functional and quality-of-life benefits to PAH patients in New York Heart Association, or NYHA, functional classes II and III. More than 90% of all patients who completed two months of treatment maintained or improved their NYHA functional class. Additionally, we observed improvement in six-minute-walk-distance and quality of life as measured by the MLHFQ in both patient groups.
We continued to treat patients who chose to remain on LIQ861 beyond the Month 2 timepoint of the primary endpoint. More than 80% of INSPIRE patients remained on study drug at Month 4 with no significant changes in safety or tolerability observed compared to Month 2. At the completion of the INSPIRE study, the patient with the longest duration of treatment had been on LIQ861 therapy for 18 months. To provide for continuity of treatment, patients from INSPIRE were provided the opportunity to continue receiving treatment in an extension study, which is currently ongoing.
Supplemental Pharmacokinetic Studies in Healthy Volunteers
To complement the pharmacokinetic data from our Phase 3 study, we conducted two additional PK studies in healthy volunteers. In the first of these studies, we observed unexpected variability in PK levels. Post-hoc analysis showed that plasma levels of treprostinil were tightly correlated to the LIQ861 dose delivered. Based upon additional non-clinical and clinical work, we believe the unexpected variability seen in this healthy volunteer study was due to an administration technique unique to the conduct of the study in the Phase 1 setting. In August 2019, we completed a second PK study in healthy volunteers in which the proper administration technique was followed. This study demonstrated significantly reduced variability, and we believe we have established comparative bioavailability to the reference listed drug.
Subjects in this second PK study, an open-label, crossover study in healthy volunteers age 18 to 45 years, inclusive, were randomized to one of three treatment sequences, with each sequence consisting of two periods. Each period and dose of LIQ861 and Tyvaso were separated by at least 24 hours. The three treatment sequences, and the number of patients in each, are shown in the table below:
|
Treatment Sequence |
Number of Subjects |
Period 1 - Day 1 |
Period 2 - Day 2 |
|
Sequence 1 |
16 |
LIQ861 |
LIQ861 |
|
Sequence 2 |
4 |
Tyvaso |
LIQ861 |
|
Sequence 3 |
4 |
LIQ861 |
Tyvaso |
Sequence 1 assessed the reproducibility of plasma treprostinil levels following dosing with 79.5 mcg of LIQ861. Analysis of the results of sequence 1 demonstrated nearly identical PK parameters between the two periods with low variability. Cmax, mean AUCinf and the median time to Cmax (Tmax) following a single dose of 79.5 mcg of LIQ861 were 1.25 ng/ml, 1.01 hr*ng/ml, and 0.17 hours, respectively.
Sequences 2 and 3 evaluated the rate and extent of treprostinil exposure following administration of a 79.5 mcg dose of LIQ861 compared with nine breaths of Tyvaso. The absorption rate was comparable between LIQ861 and Tyvaso, with peak concentrations achieved at approximately 0.13 and 0.17 hours (median Tmax) post inhalation for LIQ861 and Tyvaso, respectively. Following peak concentrations, mean plasma concentrations of treprostinil decreased in a monophasic manner with a similar rate of elimination for both treatments (approximate mean half-life of 0.5 hours for LIQ861 and Tyvaso). The comparative bioavailability of treprostinil, as assessed by the geometric mean ratios (LIQ861/Tyvaso) were 0.923, 0.941, and 0.931 for AUCinf, AUClast and Cmax, respectively, and the 90% confidence intervals for these ratios were within the acceptable equivalence limits of 0.80 to 1.25:
|
Parameter |
GMR |
90% Confidence Interval |
Within subject % Coefficient of Variation |
|
AUCinf |
0.923 |
0.802, 1.064 |
14.6 |
|
AUClast |
0.947 |
0.812, 1.103 |
15.8 |
|
Cmax |
0.931 |
0.819, 1.059 |
13.3 |
This study provided additional data to support our belief that the bioavailability and systemic exposures of treprostinil following a 79.5 mcg dose of LIQ861 and nine breaths of Tyvaso are comparable.
NDA Submission
Based upon the encouraging results of the INSPIRE trial and the supplemental healthy volunteer pharmacokinetics study, we submitted an NDA for LIQ861 to the FDA in January 2020. Prior to submitting the NDA, we participated in a pre-NDA meeting with the FDA with respect to the chemistry, manufacturing and controls, or CMC, aspects of the NDA and received no new CMC requirements from the FDA for the NDA submission. We had also requested a pre-NDA meeting with the FDA focused on clinical and nonclinical contents of the NDA. Based on FDA’s responses to our questions in advance of the meeting, or the preliminary meeting comments, we no longer considered the meeting necessary, as we believe that the responses provided sufficient feedback to inform our NDA submission plans.
Additional Clinical Trials
We intend to conduct additional clinical work during and beyond the NDA review period in order to generate additional data on LIQ861 and to support our marketing and commercial activities in advance of a potential launch of LIQ861, including maintaining patients on LIQ861 up to our U.S. launch and conducting a clinical trial in pediatric patients. We also continue to conduct development work in support of potential approval and commercialization of LIQ861, including label and patient-use assessments.
We are also currently conducting an additional clinical trial in Europe to study the hemodynamic effects of LIQ861 in PAH patients. Although the FDA has not requested that we undertake this clinical trial, the data may help assess the effects of LIQ861 on acute and chronic hemodynamic measurements and right heart function. Data from this clinical trial may also add to our understanding of safety, tolerability and pharmacokinetics of LIQ861.
Commercial Opportunity
An independent industry research firm estimated that sales for all major pulmonary hypertension drugs in 2019 would exceed $3.5 billion in the United States. Approved therapies in the prostacyclin pathway may generate approximately $1.7 billion in sales in 2019, more than half of which would include the prostacyclin analog treprostinil generated approximately $915 million from therapies formulated for continuous infusion, inhalation using a nebulizer and oral delivery.
If approved, we believe LIQ861 would be the first inhaled dry powder formulation of treprostinil delivered using a convenient, palm-sized DPI. The dosing regimens and patient experience for the two approved inhaled therapies compared to the expected product profile of LIQ861 are shown in the following table.
|
|
Ventavis (iloprost) |
|
Tyvaso (treprostinil) |
|
LIQ861 (treprostinil) |
|
|
Regulatory status |
FDA approved, 2004 |
FDA approved, 2009 |
NDA submitted, January 2020 |
|||
|
Method of administration |
Proprietary nebulizer |
Proprietary nebulizer |
Dry powder inhaler |
|||
|
Frequency |
6 to 9 times daily |
4 times daily |
4 times daily |
|||
|
Dose range |
2.5 to 5 mcg |
18 to 72 mcg; (max recommended is 54 mcg) |
26.5 to 212 mcg |
|||
|
Time or breaths per dose |
4 to 10 minutes depending on breathing pattern |
9 breaths (6 mcg/breath) |
1-2 breaths per capsule, with 1 or 2 capsules per dose |
| Ventavis (iloprost) inhalation solution |
Tyvaso (treprostinil) inhalation solution |
LIQ861 (treprostinil) dry powder for inhalation (expected) |
||||
|
Supplies required |
▪ Ventavis Inhalation System
▪ Power supply
▪ Distilled water
▪ 2 medication chamber assemblies
▪ Washing basket
▪ Battery charger
▪ I-neb pouch
▪ Carry bag
▪ Power cord for charger
▪ 2 Spare discs |
▪ Tyvaso Inhalation System
▪ Rechargeable battery
▪ 12V DC adapter
▪ AC wall plug
▪ 16 Medicine cups
▪ Filter membranes
▪ Plugs
▪ Filter shell
▪ Dome assembly with baffle plate
▪ Inhalation piece
▪ Mouthpiece
▪ Water level cup
▪ Carrying case
▪ Distilled water carrier |
▪ Dry powder inhaler
▪ Carrying pouch
▪ Daily blister pack
▪ Small cleaning brush |
|||
| Picture | ||||||
 |
 |
Preferred choice within inhaled options. As reported in our market research, physicians and patients expressed a clear preference for the expected product profile of LIQ861 over current nebulized therapies, primarily due to the ease and convenience of administration of LIQ861. Nebulized therapies require more time and breaths than LIQ861, as well as daily and weekly assembly, disassembly and cleaning.
Attractive switch from orals. The ease and range of dosing of LIQ861 may be attractive to patients who are in earlier stages of the disease, but poorly managed on oral prostacyclin products. Local delivery of treprostinil to the lung offers fewer systemic side effects. However, we believe some of these patients are hesitant to switch to more burdensome nebulized options.
Delay transition to continuous infusion. We are investigating a wide range of LIQ861 doses in order to maximize patient exposure to treprostinil, a key factor in the efficacy of prostacyclin analogs. In our clinical trials, LIQ861 was well tolerated at levels that we estimate are approximately twice the maximum recommended dose of Tyvaso. We believe the dose range enabled by LIQ861 would allow patients to titrate to higher levels of treprostinil and potentially extend the time on inhaled therapy, delaying the eventual transition to continuous infusion.
Expand beyond WHO Group I patients (PAH). Prostacyclin based therapies have only been approved for WHO Group I patients. However, prostacyclin analogs may have utility in the treatment of PH in other categories, as suggested by current off-label use in WHO Group III, which includes individuals with pulmonary hypertension secondary to lung diseases or hypoxemia, and WHO Group IV, which includes individuals with chronic thromboembolic pulmonary hypertension. Although we have no current plans to study LIQ861 in PH patients outside of WHO Group I, we will continue to monitor the investigations conducted by other companies and independent investigators of prostacyclin analogs, especially Tyvaso, and, if appropriate, may consider initiating studies with LIQ861 in patient populations where such agents have been shown to have a therapeutic benefit. In one such recently reported study, United Therapeutics reported positive data in a Phase 3 trial of Tyvaso in a subpopulation of WHO Group III patients with interstitial lung disease, with an estimated prevalence of 30,000 patients in the United States. By 2025, the diagnosed prevalence of all WHO Group III sub-types is expected to grow to over 250,000 patients in the United States, 5EU and Japan. WHO Group IV includes patients diagnosed with chronic thromboembolic pulmonary hypertension, or CTEPH. While considered underdiagnosed and undertreated, the current estimates for diagnosed prevalence of CTEPH are between 2,000 and 6,500 patients in the United States and more than 10,000 patients in the 5EU and Japan.
Competition in PAH
If approved for marketing, we expect that LIQ861 will face competition from the following inhaled treprostinil therapies that are either currently marketed or in clinical development:
|
● |
Tyvaso, or inhaled treprostinil, marketed by United Therapeutics, has been approved for the treatment of PAH in the United States since 2009. Tyvaso is administered via a proprietary nebulizer four times per day. Tyvaso is the reference listed drug in our NDA for LIQ861. Following patent litigation, United Therapeutics and Watson Pharmaceuticals, Inc., or Watson Pharmaceuticals, reached a settlement whereby Watson Pharmaceuticals will be permitted to enter the market with a generic version of Tyvaso beginning on January 1, 2026. |
|
● |
Ventavis, or inhaled iloprost, marketed in the United States by Actelion Pharmaceuticals Ltd, or Actelion, a division of Johnson & Johnson, and in Europe by Bayer Schering Pharma AG., has been approved for the treatment of PAH in the United States since 2004. Ventavis is administered via a proprietary nebulizer six to nine times per day. |
|
● |
TreT, an inhaled formulation of treprostinil which United Therapeutics licensed from MannKind Corporation, or MannKind, is currently in late-stage clinical development in the United States for the treatment of PAH. Under the license agreement, United Therapeutics is responsible for global development, regulatory and commercial activities. MannKind will manufacture clinical supplies and initial commercial supplies of the product while long-term commercial supplies will be manufactured by United Therapeutics. In September 2019, United Therapeutics commenced a clinical study (BREEZE) to evaluate the safety and pharmacokinetics of switching PAH patients from Tyvaso to TreT and announced plans to commence a second clinical study during the first half of 2020 to compare the pharmacokinetics of TreT to Tyvaso in healthy volunteers. United Therapeutics further reported that the two studies, if successful, are the only clinical studies necessary to support FDA approval. |
In addition to these other inhaled treprostinil therapies, we expect that LIQ861 will also face competition from other treprostinil-based drugs, including Orenitram, which is administered orally, and Remodulin, which is administered parenterally, both of which are marketed by United Therapeutics. Other agents that utilize the prostacyclin pathway include parenteral epoprostenol, which is marketed by multiple companies as generic and branded products. Because parenteral agents are considered to offer the greatest efficacy, but also carry the most significant side effects related to infusion site pain, risk of infection, and significant limitations on quality of life, they are usually reserved for patients later in the course of the disease.
In addition to therapies based upon prostacyclin analogues, other classes of therapeutic agents for the treatment of PAH, all of which are delivered orally, include the following:
|
● |
IP-agonists, such as selexipag, marketed by Actelion, and ralinepeg, licensed from Arena Pharmaceuticals by United Therapeutics, which is currently in clinical development; |
|
● |
PDE-5 inhibitors, such as tadalafil, marketed by United Therapeutics, and sildenafil, marketed by Pfizer. Generic versions of both tadalafil and sildenafil are currently available. |
|
● |
Endothelin receptor antagonists, such as bosentan and macitentan, both marketed by Actelion, and ambrisentan, marketed by Gilead Sciences, Inc. Generic version of bosentan and ambrisentan are currently available. |
|
● |
Soluble guanylate cyclase (sGC) stimulator, such as riociguat marketed by Bayer. |
In addition, we are also aware of several agents currently in clinical development in the United States for the treatment of PAH, including those in development by Insmed Inc. and Acceleron Pharma, Inc.
In addition to use in patients who have typically been treated with inhaled agents, we believe that LIQ861 may provide an attractive therapeutic option for patients who are in earlier stages of the disease, but poorly managed on oral prostacyclin analogues. Additionally, we believe that because of the ability to provide higher doses of treprostinil with LIQ861, patients may be able to remain on LIQ861 longer before initiating parenteral treprostinil therapy than might be the case with other inhaled treprostinil therapies.
LIQ865
Our second product candidate, LIQ865, which is designed using PRINT technology, is an injectable, sustained-release formulation of bupivacaine, a non-opioid pain medication for the management of local post-operative pain for three to five days through a single administration after a surgical procedure. If approved, we believe LIQ865 would have the potential to provide significantly longer local post-operative pain relief compared to currently marketed formulations of bupivacaine.
Background on Post-Operative Pain
The treatment of post-operative pain typically involves multi-modal therapy including the administration of local anesthetics after surgery. Although local anesthetics provide a well-established, safe and efficacious option for post-operative pain management, the duration of efficacy for conventional local anesthetics, including bupivacaine and lidocaine, is limited, with pain relief typically lasting for eight hours or less. Because post-operative pain may continue to be severe for several days following surgery, additional therapies are required. These therapies include morphine and other opioids administered orally or parenterally, as well as various non-opioids, including acetaminophen and NSAIDs, such as ibuprofen and ketorolac.
Current Therapies and Their Limitations
Opioids are the mainstay of post-operative pain management, but they are associated with a variety of potentially serious or life-threatening side effects such as sedation, nausea, constipation, cognitive impairment, and respiratory depression. In addition, opioids may be administered through patient-controlled analgesia systems, which may interfere with or delay patient ambulation and require significant hospital resources to implement and monitor. Furthermore, exposure to opioids for as little as four days can lead to increased risk of chronic opioid use and addiction. The risk of opioid abuse has led physicians, payors and the U.S. federal government to prioritize pain management strategies that minimize the use of opioids.
NSAIDs and other non-opioids for pain relief in the post-operative period are also associated with various side effects, including bleeding and gastrointestinal and renal complications. Acetaminophen can cause liver injury or failure with excessive dosing. As a result, we believe there is demand from healthcare providers and patients for post-operative pain relief therapies that can help prevent these issues.
Local anesthetics such as bupivacaine hydrochloride, or Marcaine, and lidocaine have been safely used for post-operative pain for decades, but have a duration of effect limited to less than eight hours. Approved in 2011, EXPAREL is a long-acting local anesthetic that involves an injection of bupivacaine in a multivesicular liposome carrier at the surgical site and is marketed in the United States by Pacira Pharmaceuticals, Inc. Physicians report that EXPAREL typically provides post-surgical analgesia for only 24 to 36 hours in practice, and market research we conducted suggests that physicians desire longer duration of effect to better manage local post-operative pain. In addition, because the interactions between the liposomal formulation of EXPAREL and co-administered local anesthetics can result in rapid release of bupivacaine, co-administration of other local anesthetics is inadvisable.
Potential Benefits of LIQ865
Using our PRINT technology, we have developed a particle formulation of bupivacaine designed to improve the management of local post-operative pain. We engineered the size and composition of LIQ865 particles to slowly release bupivacaine with the goal of providing patients with local pain relief for three to five days through a single administration, which we believe would provide significantly longer local post-operative pain relief compared to currently marketed formulations of bupivacaine. The figure below depicts LIQ865, showing size consistency among particles.
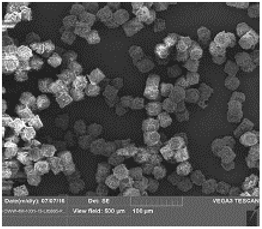
LIQ865 is administered as a suspension and is easily injected at the surgical site. Because the molded drug particles are highly stable, we believe the potential for dose dumping, the unintended rapid drug release of bupivacaine from the carrier, would be minimized with LIQ865. In a non-clinical study, co-administration of LIQ865 with lidocaine did not cause early release of bupivacaine or otherwise negatively affect the pharmacokinetic profile of LIQ865. LIQ865 was engineered to be rapidly reconstituted and administered by injection. Unlike other sustained-release formulations, we do not expect LIQ865 will be constrained by a specific ratio of drug to diluting agent, so its reconstitution volume can be adjusted based on the volume needs of a particular procedure. Furthermore, because particle-to-particle uniformity in size and composition is key to determining drug release rates, the uniformity of our LIQ865 particles creates consistent release rates.
Non-Clinical Efficacy Studies
We commissioned an animal efficacy study of two formulations of LIQ865 in a rat perineural sciatic model, which was completed in January 2016. LIQ865 showed an extended pharmacokinetic profile and duration of nerve sensory block and the potential for extended post-operative pain management. Additionally, we evaluated the safety and tolerability of LIQ865 in a rat toxicology study in 2016. The results of this study supported advancing LIQ865 into human clinical trials.
Phase 1 Trials
Our Phase 1a trial, completed in March 2017, was a randomized, double-blind, controlled, single ascending dose trial of two different PRINT formulations of bupivacaine, LIQ865A and LIQ865B. The trial was conducted in 28 healthy male volunteers at a single site in Copenhagen, Denmark. LIQ865A consists of particles combining bupivacaine and polylactic-glycolic acid, a polymer widely used in sustained-release drug products and surgical sutures. LIQ865B consists of particles of bupivacaine alone, in a proprietary diluting agent. The study design included dosing multiple cohorts, or groups, each receiving increasing bupivacaine doses as either LIQ865A or LIQ865B: 150 mg, 225 mg, 300 mg, 450 mg or 600 mg. The LIQ865 formulation was injected into the upper calf in one leg, and the other leg received the diluting agent without LIQ865 particles. The primary objective of this Phase 1a clinical trial was to evaluate the safety and tolerability profile of the two formulations of LIQ865. We also assessed bupivacaine pharmacokinetic and pharmacodynamic responses.
We observed a dose-response relationship in this trial, and all doses were well-tolerated. The results from this trial helped inform our selection of LIQ865A for further investigation in the United States, and all of our references to LIQ865 are to this formulation. All adverse events were mild to moderate in severity, and most adverse events were limited locally at the site of injection, with most related to sensory block of underlying sensory branches of the saphenous nerve in the leg.
Mean plasma concentrations of bupivacaine over 120 hours comparing the 150 mg, 225 mg, 300 mg, 450 mg and 600 mg dose cohorts of LIQ865A formulation, expressed on a logarithmic, or log, scale for the 16 volunteers who received LIQ865A are shown below:
LIQ865A Log Linear Mean Concentration Over Time
At the 450 mg dose of LIQ865, all subjects had maximum concentration values below 800 ng/ml, which is well below the reported thresholds for neurotoxicity and cardiac toxicity of 2,000 and 4,000 ng/mL, respectively. The pharmacokinetic and pharmacodynamic profile for this dose suggested a sustained duration of effect, with nearly all subjects receiving this dose reporting at least three days of sensory blunting in response to quantitative sensory testing. LIQ865 also showed rapid onset of action at the one-hour time point in all subjects, even at the lowest dose of 150 mg. Additionally, we observed a block of the distal sensory branches of the saphenous nerve below the knee in eight of nine subjects who received a 450 mg dose of LIQ865. This sensory block lasted at least three days, which we believe further supports the duration profile of LIQ865.
In March 2017, we held a pre-IND meeting with the FDA and verified that the CMC and preclinical package were “phase-appropriate” and sufficient to support our initial U.S. Phase 1 trial. Following our submission of the IND for LIQ865, we initiated our U.S. Phase 1b clinical trial in September 2017, which was completed in April 2018. This trial used an experimental pain model in healthy male and female subjects with quantitative sensory testing after an injection of LIQ865 at doses of 150 mg, 300 mg and 450 mg. The experimental pain model was designed to simulate post-operative pain for up to five days through a combination of localized ultraviolet B burn and mini-incision. Additionally, the trial included a cross-over design to compare LIQ865 to EXPAREL. We observed that LIQ865 was well-tolerated across the range of doses. All adverse events were mild to moderate, and no dose-limiting toxicities were noted. The pharmacokinetic profile was similar to that observed in the Phase 1a trial. Pharmacodynamic effects were highly variable and inconclusive, which we associated with the experimental design of the pain model used in this Phase 1b trial.
Plans for Phase 2 Development
At our pre-IND meeting in March 2017, the FDA requested additional toxicology studies prior to the initiation of Phase 2 trials. Accordingly, we initiated Phase 2-enabling toxicology studies in 2019 to assess LIQ865 in multiple non-clinical tissue models. Results from a study to assess incision tensile strength after healing were acceptable and not statistically different from controls. A nonclinical study to examine soft tissue healing was also completed, and the results were acceptable and comparable to vehicle-treated, saline-treated, and Marcaine-treated sites. We believe this data supports progression to Phase 2 hernia repair studies. In a study to assess bone fracture healing, we observed dose-dependent delayed healing at the two LIQ865 doses studied; however, there were no adverse effects noted on surrounding soft tissues. Additional studies have been initiated with lower doses of LIQ865 to determine a NOAEL on bone healing. We will review the results from these toxicology studies, and if supportive, we intend to initiate Phase 2 proof-of-concept clinical trials, subject to availability of capital and other factors, during 2021. In the United States, we plan to pursue the 505(b)(2) regulatory pathway for our development of LIQ865, which would allow us to rely on the FDA’s prior determinations of safety and efficacy for other products containing bupivacaine, such as Marcaine and EXPAREL.
Competition
The primary competitor for LIQ865, if approved, would be liposomal bupivacaine, marketed as EXPAREL by Pacira Pharmaceuticals, Inc. Generic equivalents of EXPAREL may also enter the market when EXPAREL loses patent protection, potentially as early as December 2021. While EXPAREL is currently the only direct competitor to LIQ865 on the market, in October 2018 Heron Therapeutics, Inc., or Heron, announced the submission of its NDA to the FDA for HTX-011, an investigational long-acting, extended-release formulation of the local anesthetic bupivacaine in a fixed-dose combination with the anti-inflammatory meloxicam for the management of post-operative pain. HTX-011 was granted both breakthrough therapy and fast track designations, as well as priority review, by the FDA. In May 2019, Heron announced that it received a complete response letter, or CRL, for HTX-011 from the FDA. In October 2019, Heron announced that it had resubmitted its NDA for HTX-011 to the FDA and expected a six-month review period. In addition to Heron, Durect Corporation and Innocoll Holdings plc each also have products in clinical development that are potential competitors to LIQ865. In addition to long-acting local anesthetics, there are a number of indirect competitors in various stages of research and development, including opioids and other molecules that target the treatment of pain through alternative pathways.
Our PRINT Technology
Both LIQ861 and LIQ865 are being developed using our proprietary PRINT particle engineering technology, which allows us to engineer and manufacture highly uniform drug particles with precise control over the size, three-dimensional geometric shape and chemical composition of the particles. By controlling these physical and chemical parameters of particles, PRINT enables us to engineer desirable pharmacological benefits into product candidates, including prolonged duration of drug release, increased drug loading, more convenient routes of administration, the ability to create novel combination products, enhanced storage and stability and the potential to reduce adverse side effects. Precisely controlling the physical and chemical characteristics of drug particles enables us to research, identify and pursue the improvement of existing therapies and creation of new therapies from existing drugs or new chemical entities, including small molecules and biologics.
Our ability to design and control these features of drug particles has the potential to provide significant benefits across the breadth of pharmaceutical applications. Product characteristics and features can be tuned depending on the need of a particular application, drug substance, delivery route and other such considerations. Based on our research to date, we anticipate the ability to:
|
● |
enhance inhaled delivery through the highly uniform geometric size, shape and composition of each drug particle; |
|
● |
design desired drug release profiles ranging from minutes post-delivery to days, weeks, or months depending on the objective of a target therapy, by controlling the chemical composition of the drug particles and the surface area-to-volume ratio of the particles; |
|
● |
enable combination products where one or more of the chemical constituents can destabilize or interact by encapsulating the desired constituent in a particle to shield it from another constituent during packaging and storage; and |
|
● |
enhance the deposition and retention of topically delivered products by designing particles with a desired charge and/or Young’s modulus. |
Our molding approach, which we branded as “PRINT” or Particle Replication In Non-wetting Templates, combines the precision of the semi-conductor industry with the high throughput of roll-to-roll manufacturing to make highly uniform micro- and nano-particles at a commercially viable scale. Our manufacturing equipment and materials used in the production of our drug particles are proprietary and protected by our patent portfolio and trade secret know-how. Our PRINT equipment is also modular, scalable and cost-effective.
Our PRINT Process
We begin our particle design by procuring a custom designed master template etched with three-dimensional structures, or posts, that will become the eventual shape and size of our drug particles. These three-dimensional structures are then replicated in negative form, through our proprietary processing, into flexible rolls of polymeric PRINT molds. Our PRINT molds consist of thousands of linear feet of thin flexible molds up to twenty-four inches wide. We then design and formulate our desired drug particle composition and apply that to our PRINT molds in our high-throughput roll-to-roll processing equipment, with each particle mimicking the shape of the mold cavity from which it was molded, thus taking the shape of the original master template structures.
The general components and steps of our PRINT molding are as follows:
|
● |
Step A: Etch a master template with the three-dimensional geometric structures of the desired particle size and shape; |
|
● |
Step B: Apply our proprietary polymeric mold material over the master template; |
|
● |
Step C: Cure the polymeric material to form our PRINT molds with discrete molding cavities that replicate the structures of the master template; |
|
● |
Step D: Design the chemical composition of the drug particle of interest; |
|
● |
Step E: Apply the drug particle composition to the cavities in the mold to fill the cavities; |
|
● |
Step F: Form the drug particles in the cavities of the mold that mimic the size and shape of the mold cavities; |
|
● |
Step G: Remove the drug particles from the mold cavities on a harvesting film; and |
|
● |
Step H: Remove the particles from the harvesting film for further functionalization, purification or packaging to be included in the final drug particle product. |
The diagram below shows the general steps involved in producing drug particles using our PRINT technology:
We have translated the PRINT process into a continuous, roll-to-roll manufacturing process that we believe is compliant with cGMP and scaled to support clinical and commercial production, when required. One of our current manufacturing lines is shown below:

Manufacturing and Supply
Our facilities occupy approximately 45,000 square feet and are located in Morrisville, North Carolina. Within these premises, there are office space, research and development laboratories and equipment, analytical development and quality control laboratories, research, development and mold production facilities, research and development particle fabrication equipment, including three operational PRINT particle fabrication lines, all of which we believe are cGMP-compliant, as well as appropriate staging, storage and stability facilities. These three operational PRINT particle fabrication lines are located within class ISO7 clean rooms that operate under applicable ISO and cGMP air quality and environmental requirements.
We currently produce in this facility the product candidates for our preclinical studies and clinical trials. Our current operational PRINT particle fabrication lines are scaled and capable of producing the necessary materials to support our ongoing operations and planned studies and clinical trials and, we believe, ultimately our initial commercial scale manufacturing. The production capacity for each PRINT particle fabrication line within our production facility varies depending on the drug particle that is being produced.
We depend on third-party suppliers for clinical supplies, including active pharmaceutical ingredients which are used in our product candidates. For example, we currently rely on a sole supplier, LGM Pharma, LLC, or LGM Pharma, for treprostinil, the active pharmaceutical ingredient of LIQ861, and we currently rely on a sole supplier, Plastiape S.p.A., or Plastiape, for RS00 Model 8 DPI, the DPI used to administer LIQ861. We also rely on a sole supplier, Xcelience LLC (now a Lonza Group Ltd company), or Xcelience, for encapsulation and packaging services. If and when we receive marketing approval for our product candidates, we may, from time to time, rely on third-party CMOs to produce, package and distribute some or all of our approved drug products on a commercial scale.
Our Collaboration and Licensing Agreements
In addition to advancing our own product candidates, LIQ861 and LIQ865, we have collaborated, and may consider collaborating, with pharmaceutical companies to develop their own product candidates across a wide range of therapeutic areas, molecule types and routes of administration, leveraging our PRINT technology. These collaborations are intended to help advance new PRINT capabilities and build upon our competitive advantage in the pharmaceutical industry, while adding to our intellectual property portfolio.
We have exclusively licensed our PRINT technology to GSK for applications broadly across inhaled delivery of their small molecule and biologic chemical entities, although we retained the ability to develop LIQ861. As discussed below, in June 2019, we amended our Inhaled Collaboration and Option Agreement, or the GSK ICO Agreement, with GSK, to secure rights to develop and commercialize three additional inhaled therapeutics, subject to milestone and royalty payments to GSK, and to establish a mechanism by which we may acquire rights to develop and commercialize further molecular entities for inhaled applications.
We have also exclusively licensed our PRINT technology to Aerie Pharmaceuticals, Inc., which in 2017 acquired most of the assets of Envisia Therapeutics, Inc., an entity which we formed in 2013, for broad usage in the design and commercialization of small molecule and biologic ophthalmic therapies.
GlaxoSmithKline
Previously, we had collaborated with GSK on the use of our PRINT technology in respiratory disease. In June 2012, we entered into the GSK ICO Agreement with GSK to collaborate on research regarding the application of our PRINT technology to specified inhaled therapies. Pursuant to the GSK ICO Agreement, we granted GSK exclusive options and licenses to further develop and commercialize such inhaled therapies using our PRINT technology. In partial consideration of the rights granted to GSK under the GSK ICO Agreement, we received a one-time up-front payment of $4.0 million. We also entered into a stock purchase agreement with GSK pursuant to which GSK purchased 4,765,248 shares of our Series C-1 convertible preferred stock for an aggregate purchase price of $3.8 million.
In September 2015, GSK exercised its option to obtain an exclusive, worldwide license to certain of our know-how and patents relating to our PRINT technology, for the purpose of, among others, preclinical studies of inhaled therapeutics developed, manufactured or otherwise produced using our PRINT technology. In connection with the grant of this license, we received a one-time option exercise fee of $15.0 million. Under the terms of the GSK ICO Agreement, we were also entitled to continued research and development funding and certain milestone payments aggregating up to $158 million upon the achievement of specified events for new non-rescue therapeutic products. Rescue therapeutic products are therapeutics that GSK develops with our PRINT technology that had previously been discontinued from development. We are also entitled to tiered royalties on the worldwide sales of the licensed products at percentages ranging from the mid-single digits to low-single digits depending on the total number of products developed and other royalty step-down events with a fixed low-single digit royalty floor. In February 2016, we received a $3.0 million payment from GSK upon the achievement of a clinical development milestone related to the development of an inhaled antiviral for viral exacerbations in chronic obstructive pulmonary disease. However, in July 2018, GSK notified us of its plans to discontinue development of this compound after completion of the related Phase 1 clinical trial.
GSK has the right to terminate the GSK ICO Agreement in its entirety or on a product-by-product basis upon a specified period of prior written notice. Upon termination of the GSK ICO Agreement, each party will continue to have the right to practice and/or license its interest in any know-how developed during the collaboration without seeking the consent of, or accounting to, the other party.
In June 2019, we and GSK executed the third amendment to the collaboration agreement providing us with rights to develop and commercialize three specified molecular entities for application in inhaled programs using our PRINT technology at our sole expense. This amendment also provides a mechanism for us to acquire rights to develop and commercialize further molecular entities for inhaled applications. New inhaled programs developed under this amendment would carry milestone and royalty payments due to GSK upon initiation of Phase 3 studies and subsequent commercialization, respectively. This amendment, among other factors, including the lack of continued performance anticipated by the Company and GSK under the original agreement, led the Company to the belief that no further research and development services will be provided to GSK under the collaboration agreement. Accordingly, in January 2020, we notified GSK of our intent to terminate the GSK ICO Agreement based upon GSK's lack of performance under the agreement, which we believe constitutes a material breach of the agreement. In February 2020, we received a letter from GSK disputing our basis for termination. The parties are currently attempting to resolve the dispute pursuant to the terms of the GSK ICO Agreement.
The University of North Carolina at Chapel Hill
In December 2008, we entered into the Amended and Restated License Agreement with UNC for the use of certain patent rights and technology relating to initial innovations of our PRINT technology, or the UNC License. Under the terms of the UNC License, we have an exclusive license to such patent rights and technology for our drug products. The UNC License grants us the right to grant sublicenses to the technology as well as control the litigation of any infringement claim instituted by or against us in respect of the licensed patent rights. We are also responsible for the costs of all expenses associated with the prosecution and maintenance of the patents and patent applications. Such filings and prosecution will be carried out by UNC and in UNC’s name but under our control.
Under the UNC License, we are required to pay UNC royalties equal to a low single digit percentage of all net sales of our drug products whose manufacture, use or sale includes any use of the technology or patent rights covered by the UNC License, as well as tiered royalty percentages ranging in the low single digits of sales by our sublicensees for any product covered by rights under a sublicense agreement granted pursuant to the UNC License. Under the UNC License, we are also required to pay UNC 20% of all fees other than royalties that we collect and are attributable to UNC sublicensed intellectual property. As consideration for the UNC License, we paid UNC a license issue fee in the form of 196,469 shares of our Class B non-voting common stock in 2004. During the term of the UNC License, we have also paid approximately $2.9 million in the aggregate to UNC pursuant to a Supported Research Agreement, or the SRA. In connection therewith, we may exclusively license resulting inventions under the SRA for a $5,000 up-front license fee per invention. We have also paid aggregate consideration of $5.7 million in sublicense fees to UNC pursuant to the UNC License, for our sublicenses of our PRINT technology to GSK and G&W Laboratories, Inc., a former licensee. We also reimburse UNC for its costs of procuring and maintaining the patents we license from UNC. Such reimbursements amounted to $142,531 for the year ended December 31, 2019. Effective November 2017, we satisfied all substantive milestones associated with our UNC License other than semi-annual and annual reporting-based milestones that continue through the term of the UNC License. The UNC License expires (i) on the expiration of the last to expire patent included in the patent rights or (ii) if no patents mature from such patent rights, in December 2028.
We have the right to terminate the UNC License upon a specified period of prior written notice. UNC may terminate the UNC License in certain circumstances, including if we fail to pay royalty or other payments on time or if we fail to sublicense in accordance with the terms of the UNC License. Upon termination of the UNC License, we must pay any royalty obligations due upon termination.
Intellectual Property
The proprietary nature and protection of our product candidates, their methods of use and our platform technology that enables our product candidates are an important part of our business strategy of rapidly developing and commercializing new medicines that address areas of significant unmet medical needs.
Our policy is to seek patent protection of our proprietary product candidates and technology by filing U.S., international and certain foreign patent applications covering certain of our proprietary technology, inventions, improvements and product candidates that are important to the growth and protection of our business. We also rely on a combination of trade secrets, know-how, trademarks and contractual restrictions to protect aspects of our business that are not amenable to patent protection or where we do not consider patent protection to be adequate or applicable.
Our success depends, in part, on our ability to obtain and maintain patent and other protection for our product candidates, enabling technology, inventions and know-how and our ability to defend and enforce these patents, preserve the proprietary nature of our trade secrets and trademarks and operate our business without infringing valid and enforceable patent and other proprietary rights of third parties. We pursue both composition-of-matter patents and method-of-use patents for our product candidates. We are also pursuing patents covering our proprietary PRINT micro- and nano-particle fabrication technology.
The term of individual patents depends upon the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest filing date of a non-provisional patent application to which the patent claims priority in the applicable country. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment, or PTA, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office, or the USPTO, in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over a commonly owned patent or a patent naming a common inventor and having an earlier expiration date. The Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Act, permits a patent term extension, or PTE, of up to five years beyond the expiration date of a U.S. patent as partial compensation for the length of time the drug is under regulatory review while the patent is in force. A PTE cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, and only one patent applicable to each regulatory review period may be extended. Further, only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended and the extension only applies to the approved drug, method for using it or method for manufacturing it for which the extension was obtained. Similar provisions are available in Europe and certain other foreign jurisdictions to extend the term of a patent that covers an approved drug.
We are the owner or exclusive licensee of patents and applications relating to our proprietary technology platform and our product candidates, and are pursuing additional patent protection for these and for our other product candidates and technology developments.
We have a total of 159 patents and pending patent applications in our patent portfolio. As of December 31, 2019, we were the sole owner of 14 patents in the United States and 41 patents in foreign jurisdictions, as well as approximately 16 additional pending patent applications, including provisional patent applications, in the United States, Europe, Japan and other jurisdictions. In addition to the patents and patent applications owned solely by us, our patent portfolio also includes 72 patents and 16 patent applications licensed from third parties. As of December 31, 2019, we had an exclusive, worldwide license from UNC to 18 U.S. patents and 53 foreign patents, as well as six additional patent applications in the United States or selected foreign jurisdictions. Five of the patents and two of the patent applications in the portfolio licensed from UNC are jointly owned by us.
With regard to our LIQ861 product candidate, as of December 31, 2019 our owned or in-licensed patents and patent applications that are directed to aspects of the PRINT technology utilized in LIQ861 include:
|
● |
U.S. Patent No. 8,263,129, which includes claims directed to methods of forming substantially uniform particles and is expected to expire on January 14, 2029, including 1,486 days of PTA and assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 8,420,124, which includes claims directed to a plurality of monodisperse particles and is expected to expire on August 19, 2028, including 1,338 days of PTA and assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 9,877,920, which includes claims directed to a plurality of particles and is expected to expire on December 20, 2024, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 10,517,824, which includes claims directed to a method of making a plurality of particles and is expected to expire on December 20, 2024, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 8,439,666, which includes claims directed to laminate molds and is expected to expire on December 4, 2026, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 8,662,878, which includes claims directed to molds and mold systems and is expected to expire on December 4, 2026, assuming payment of all maintenance fees; |
|
● |
U.S. Patent Nos. 8,945,441 and 9,662,809, which include claims directed to methods of making laminate molds and are each expected to expire on December 4, 2026, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 7,976,759, which includes claims directed to methods of forming nanoparticles and is expected to expire on October 13, 2028, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 9,545,737, which includes claims directed to methods of forming pharmaceutical particles and is expected to expire on April 22, 2029, including 191 days of PTA and assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 8,444,907, which includes claims directed to methods for fabricating a substantially seamless pattern and is expected to expire on June 28, 2031, including 572 days of PTA and assuming payment of all maintenance fees; and |
|
● |
U.S. Patent No. 9,744,715, which includes claims directed to methods for fabricating a substantially seamless pattern and is expected to expire on December 3, 2029, assuming payment of all maintenance fees. |
As of December 31, 2019, we were sole owner of one international patent application, PCT/US17/31301, specifically directed to our LIQ861 product candidate, which has been entered into the national/regional stage in Australia, Canada, Europe, Israel, Japan and the United States. PCT/US17/31301 includes claims directed to dry powder inhalation compositions, methods of using such compositions treating a patient with PAH and methods of making such compositions. Any patents that may issue from PCT/US17/31301 are expected to expire on May 5, 2037, absent any terminal disclaimers, patent term adjustments or extensions and assuming payment of all maintenance fees.
With regard to our LIQ865 product candidate, as of December 31, 2019, our owned or in-licensed patents and patent applications that cover aspects of the PRINT technology utilized in LIQ865 include:
|
● |
U.S. Patent No. 8,263,129, which includes claims directed to methods of forming substantially uniform particles and is expected to expire on January 14, 2029, including 1,486 days of PTA and assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 8,420,124, which includes claims directed to a plurality of monodisperse particles and is expected to expire on August 19, 2028, including 1,338 days of PTA and assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 9,877,920, which includes claims directed to a plurality of particles and is expected to expire on December 20, 2024, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 10,517,824, which includes claims directed to a method of making a plurality of particles and is expected to expire on December 20, 2024, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 8,662,878, which includes claims directed to molds and mold systems and is expected to expire on December 4, 2026, assuming payment of all maintenance fees; |
|
● |
U.S. Patent Nos. 8,945,441 and 9,662,809, which include claims directed to methods of making laminate molds and are each expected to expire on December 4, 2026, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 7,976,759, which includes claims directed to methods of forming nanoparticles and is expected to expire on October 13, 2028, assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 9,545,737, which includes claims directed to methods of forming pharmaceutical particles and is expected to expire on April 22, 2029, including 191 days of PTA and assuming payment of all maintenance fees; |
|
● |
U.S. Patent No. 8,444,907, which includes claims directed to methods for fabricating a substantially seamless pattern and is expected to expire on June 28, 2031, including 572 days of PTA and assuming payment of all maintenance fees; and |
|
● |
U.S. Patent No. 9,744,715, which includes claims directed to methods for fabricating a substantially seamless pattern and is expected to expire on December 3, 2029, assuming payment of all maintenance fees. |
As of December 31, 2019, we were sole owner of one international patent application, PCT/US17/31397, specifically directed to our LIQ865 product candidate, which has been entered into the national/regional stage in Europe, Japan and the United States. PCT/US17/31397 includes claims directed to particulate compositions comprising an amino amide anesthetic and Poly(lactide-co-glycolide) polymer, formulations comprising such compositions, methods of using such compositions for inducing extended analgesia and methods of forming such compositions. Any patents that may issue from PCT/US17/31397 are expected to expire on May 5, 2037, absent any patent term adjustments or extensions and assuming payment of all maintenance fees.
We hold multiple U.S. trademark registrations and have numerous pending trademark applications. Issuance of a federally registered trademark creates a rebuttable presumption of ownership of the mark; however, it is subject to challenge by others claiming first use in the mark in some or all the areas in which it is used. Federally registered trademarks have a perpetual life so long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We believe our patents and trademarks are valuable and would provide us certain benefits in marketing our products.
Sales and Marketing
We hold worldwide commercialization rights to LIQ861 and LIQ865. We are currently exploring opportunities to commercialize LIQ861 in the United States, subject to receiving regulatory approval, either by ourselves or through partnership or licensing arrangements with other pharmaceutical companies. With respect to LIQ865, after reviewing the results of all of our Phase 2-enabling toxicology studies, and subject to the availability of sufficient funding, we plan to evaluate whether to pursue continued internal development or to explore licensing arrangements with other pharmaceutical companies. Outside of the United States, we intend to pursue the regulatory approval and commercialization of LIQ861 and LIQ865 through licensing arrangements with pharmaceutical companies with regional expertise. We have not yet established a substantial commercial organization or distribution capabilities.
If we decide to commercialize LIQ861, our lead product candidate, ourselves, we intend to focus our commercial efforts initially on the U.S. market, which we believe represents the largest market opportunity. Within the United States, we believe that we can effectively commercialize LIQ861, if approved, with an initial specialty field team of approximately 50 individuals. We intend to initially pursue a highly concentrated target market of PAH centers of excellence and high prescribers of PAH therapies. Our physician call points within these sites of care will include cardiologists, pulmonologists and their supporting staff. We expect to supplement our field team with medical science liaisons and reimbursement specialists to support the proper training and utilization of LIQ861. As part of our commercialization strategy, we plan to educate physician specialists, healthcare practitioners, patients and caregivers of the benefits of LIQ861 and its proper use. We plan to work with national associations, such as the Pulmonary Hypertension Association, and patient advocacy groups to update treatment guidelines to include LIQ861.
Competition
The pharmaceutical industry is intensely competitive, subject to rapid and significant technological change and places emphasis on the value of proprietary products. While we believe that our technologies and experience provide us with a competitive advantage, our competitors include organizations such as major multinational pharmaceutical companies, established biotechnology companies, biopharmaceutical companies and generic drug companies. Many of our competitors have greater financial and other resources than we have, such as more commercial resources, larger research and development staffs and more extensive marketing and manufacturing organizations. As a result, these companies may obtain marketing approval more rapidly than we are able and may be more effective in selling and marketing their products. Smaller or early stage companies may also prove to be significant competitors, particularly through collaboration arrangements with large, established companies.
Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. Our competitors may succeed in developing, acquiring or licensing, on an exclusive basis, technologies and drug products that are more effective or less costly than products that we are currently developing or that we may develop, which could render our products obsolete and non-competitive. We expect any products that we develop and commercialize to compete on the basis of, among others, efficacy, safety, convenience of administration and delivery, price and the availability of reimbursement from government and other third-party payors. We also expect to face competition in our efforts to recruit and retain qualified personnel, establish clinical trial sites and secure patient enrollment in our clinical trials, and identify appropriate collaborators to help commercialize any approved products in our target commercial markets.
Employees
As of December 31, 2019, we had 64 total employees, all of which are full-time, including seven employees in management (including our executive officers), 22 employees in research and development, 16 employees in manufacturing and technical operations, seven employees in regulatory and quality and 12 employees in general and administration. All of our employees are employed in the United States.
Facilities
Our corporate headquarters are located in Morrisville, North Carolina, and consist of approximately 45,000 square feet of space under a lease that expires on October 31, 2026 and includes an option for us to renew for an additional five years through October 31, 2031, as amended. The primary use of this location is general office, laboratory, research and development and light manufacturing. We believe that our facilities are adequate for our current needs and for the foreseeable future; however, we will continue to seek additional space as needed to accommodate our growth.
Corporate Information
We were incorporated in Delaware on June 8, 2004. Our principal executive offices are located at 419 Davis Drive, Suite 100, Morrisville, North Carolina 27560 and our telephone number is (919) 328-4400. Our website is www.liquidia.com. The information on or that can be accessed through our website is not incorporated by reference into this annual report, and you should not consider any such information as part of this annual report or in deciding whether to purchase our common stock. This annual report and all of our filings under the Securities Exchange Act of 1934, as amended, or the Exchange Act, including copies of annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, are available free of charge through our website on the date we file those materials with, or furnish them to, the U.S. Securities and Exchange Commission, or the SEC. Such filings are also available to the public on the internet at the SEC’s website at www.sec.gov.
Government Regulation
Government Regulation and Product Approval
Government authorities in the United States at the federal, state and local level and in other countries, extensively regulate, among other things, the research, development, testing, manufacture, (including manufacturing changes), quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing, export and import of products such as those we are developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
U.S. Drug Development Process
In the United States, the FDA regulates drugs under the United States Federal Food, Drug, and Cosmetic Act, or the FDCA, and the FDA’s implementing regulations.
Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. The process required by the FDA before a drug may be marketed in the United States generally involves the following:
|
● |
completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices regulations; |
|
● |
submission to the FDA of an Investigational New Drug application, or IND, which must become effective before human clinical studies may begin; |
|
● |
approval by an independent IRB at each clinical site before each trial may be initiated; |
|
● |
performance of adequate and well-controlled human clinical studies according to Good Clinical Practice, or GCP, regulations, to establish the safety and efficacy of the proposed drug for its intended use; |
|
● |
preparation and submission to the FDA of an NDA, containing the results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the drug product, proposed labeling and other relevant information, to request approval to market the drug product; |
|
● |
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug product, or components thereof, are produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
|
● |
satisfactory completion of FDA audits of clinical trial sites to assure compliance with GCPs and the integrity of clinical data; |
|
● |
FDA review and approval of the NDA; |
|
● |
payment of fees, including annual program fees for each drug product on the market; and |
|
● |
ongoing compliance with any post approval requirements, including risk evaluation and mitigation strategy, or REMS, and post approval studies required by the FDA. |
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all.
Once a pharmaceutical product candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity, formulation and stability, as well as animal studies. When a sponsor wants to proceed to test the product candidate in humans, it must submit an IND in order to conduct clinical trials.
An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, to the FDA as part of the IND. The sponsor must also include a protocol detailing, among other things, the objectives of the initial clinical study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated if the initial clinical study lends itself to an efficacy evaluation. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions related to a proposed clinical study and places the study on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical study can begin. Clinical holds also may be imposed by the FDA at any time before or during clinical studies due to safety concerns or non-compliance, and may be imposed on all product candidates within a certain pharmaceutical class. The FDA also can impose partial clinical holds, for example, prohibiting the initiation of clinical studies of a certain duration or for a certain dose.
All clinical studies must be conducted under the supervision of one or more qualified investigators in accordance with GCP regulations. These regulations include the requirement that all research subjects provide informed consent in writing before their participation in any clinical study. Further, an IRB must review and approve the plan for any clinical study before it commences at any institution, and the IRB must conduct continuing review and reapprove the study at least annually. An IRB considers, among other things, whether the risks to individuals participating in the clinical study are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the information regarding the clinical study and the consent form that must be provided to each clinical study subject or his or her legal representative and must monitor the clinical study until completed.
Each new clinical protocol and any amendments to the protocol must be submitted for FDA review, and to the IRBs for approval. Protocols detail, among other things, the objectives of the clinical study, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor subject safety.
Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on their ClinicalTrials.gov website.
Human clinical studies are typically conducted in three sequential phases that may overlap or be combined:
|
● |
Phase 1. The product is initially introduced into a small number of healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, especially when the product is suspected or known to be unavoidably toxic, the initial human testing may be conducted in patients. |
|
● |
Phase 2. Involves clinical studies in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule. |
|
● |
Phase 3. Clinical studies are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These clinical studies are intended to establish the overall risk/benefit relationship of the product and provide an adequate basis for product labeling. |
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and safety reports must be submitted to the FDA and the investigators for serious and unexpected suspected adverse events. Phase 1, Phase 2 and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical study at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical study at its institution if the clinical study is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
There are FDA-imposed limitations on communications about investigational drugs. The FDA prohibits companies from making promotional claims of safety or effectiveness of the drug for a use for which it is under investigation, and from “commercialization” of the drug before it is approved for commercial distribution.
Concurrent with clinical studies, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the product and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
Assuming successful completion of the required clinical testing, the results of product development, preclinical studies and clinical studies, along with descriptions of the manufacturing process, analytical tests conducted on the drug, proposed labeling and other relevant information, are submitted to the FDA as part of an NDA for a new drug, requesting approval to market the product.
The submission of an NDA is subject to the payment of a substantial application user fee although a waiver of such fee may be obtained under certain limited circumstances. For example, the agency will waive the application fee for the first human drug application that a small business or its affiliate submits for review. The sponsor of an approved NDA is also subject to annual program user fees.
In addition, under the Pediatric Research Equity Act of 2003, or PREA, an NDA application (or a supplement to an application) for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration must contain a Pediatric Assessment. If so, the submission must contain data from pediatric studies that are adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective, unless the applicant has obtained a waiver or deferral. PREA applies only to products developed for diseases that occur in both adult and pediatric populations, and generally does not apply to products with Orphan Drug Designation or to ANDAs for generic drugs.
A sponsor who is planning to submit a marketing application for a drug product that is subject to the PREA requirements must submit an initial Pediatric Study Plan, or PSP. The FDA encourages all applications to submit the PSP as soon as possible in the drug development process, and to discuss the plan with FDA at critical points in the development process. For products intended for life-threatening or severely debilitating illnesses, applicants are encouraged to discuss the PSP at the Pre-IND meeting and End-of-Phase 1 meeting. For products not intended for such illnesses, the FDA recommends that sponsors submit and discuss the PSP no later than the End-of-Phase 2, or EOP2, meeting. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information. The FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from preclinical studies, early phase clinical studies or other clinical development programs. The sponsor may submit a request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of data or full or partial waivers. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of data or full or partial waivers. It is critical that sponsors are in compliance with the PREA, as non-compliance may result in the FDA considering the drug product misbranded solely on that basis.
The FDA also may require submission of a REMS to mitigate any identified or suspected serious risks. The REMS could include medication guides, physician communication plans, assessment plans and elements to assure safe use, such as restricted distribution methods, patient registries or other risk minimization tools.
The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an application for filing. In this event, the application must be re-submitted with the additional information. The re-submitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review.
The FDA reviews an NDA to determine whether a product is safe and effective for its intended use, which includes assessment of preclinical and clinical data; proposed labeling; CMC data; and an assessment of whether the manufacturing processes and facilities meet the appropriate requirements and comply with the applicable regulations (including cGMP requirements and adequate assurance for consistent commercial production of the product within required specifications). There are numerous FDA personnel assigned to review different aspects of an NDA, exercising judgment, discretion, and interpretation of data relative to the review process.
The FDA may approve an NDA only if, among other things, the methods used in, and the facilities and controls used for, the manufacture processing, packing and testing of the product are adequate to ensure and preserve its identity, strength, quality and purity.
Before approving an NDA, the FDA often will inspect the facility or facilities where the product is or will be manufactured.
The FDA may refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. An advisory committee is a panel of experts, including clinicians and other scientific experts, who provide advice and recommendations when requested by the FDA. The FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations when making decisions.
Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure clinical data supporting the submission were developed in compliance with GCP.
The approval process is lengthy and difficult, and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied, or may require additional preclinical, clinical or CMC data or other data and information. Even if such data and information are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical studies, as well as other types of supporting data, are not always conclusive and the FDA may interpret data differently than an applicant interprets the same data.
After the FDA’s evaluation of an application, the FDA may issue an approval letter or a complete response letter to indicate that the review cycle is complete and that the application is not ready for approval. A complete response letter generally contains a statement of specific conditions that must be met to secure final approval of the application and may require additional clinical or preclinical testing for the FDA to reconsider the application. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical studies. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the application, addressing all of the deficiencies identified in the letter, or withdraw the application, or request an opportunity for a hearing.
Even with submission of additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require post-approval studies, including Phase 4 clinical studies, to further assess safety and effectiveness after approval and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
New Drug Applications
Most drug products obtain FDA marketing approval pursuant to an NDA (described above) for innovator products, or an abbreviated new drug application, or ANDA, for generic products. Relevant to ANDAs, the Hatch-Waxman Act amendments to the FDCA established a statutory procedure for submission and FDA review and approval of ANDAs for generic versions of branded drugs previously approved by the FDA (such previously approved drugs are also referred to as listed drugs). Because the safety and efficacy of listed drugs have already been established by the brand company (sometimes referred to as the innovator), the FDA does not require new human clinical trials to establish safety and efficacy of generic products. Rather, a generic manufacturer is typically required to conduct bioequivalence studies of its test product against the listed drug. The bioequivalence studies for orally administered, systemically available drug products assess the rate and extent to which the active pharmaceutical ingredient is absorbed into the bloodstream from the drug product and becomes available at the site of action. Bioequivalence is established when there is an absence of a significant difference in the rate and extent for absorption of the generic product and the listed drug. For some drugs, including locally acting drugs such as topical anti-fungals, other means of demonstrating bioequivalence may be required by the FDA, especially where rate and/or extent of absorption are difficult or impossible to measure. In addition to the bioequivalence data, an ANDA must contain patent certifications and chemistry, manufacturing, labeling and stability data.
A third alternative is a special type of NDA, commonly referred to as a 505(b)(2) NDA, which enables the applicant to rely, in part, on the FDA’s findings of safety and efficacy of an existing product, or published literature, in support of its application. 505(b)(2) NDAs often provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon the FDA’s findings with respect to certain preclinical or clinical studies conducted for an approved product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the 505(b)(2) applicant.
In seeking approval for a drug through an NDA, including a 505(b)(2) NDA, applicants are required to list with the FDA certain patents of the applicant or that are held by third parties whose claims cover the applicant’s product. Upon approval of an NDA, each of the patents listed in the application for the drug is then published in the Orange Book. Any subsequent applicant who files an ANDA seeking approval of a generic equivalent version of a drug listed in the Orange Book or a 505(b)(2) NDA referencing a drug listed in the Orange Book must make one of the following certifications to the FDA concerning patents: (1) the patent information concerning the reference listed drug product has not been submitted to the FDA; (2) any such patent that was filed has expired; (3) the date on which such patent will expire; or (4) such patent is invalid, unenforceable or will not be infringed upon by the manufacture, use or sale of the drug product for which the application is submitted. This last certification is known as a paragraph IV certification. A notice of the paragraph IV certification must be provided to each owner of the patent that is the subject of the certification and to the holder of the approved NDA to which the ANDA or 505(b)(2) application refers. The applicant may also elect to submit a “section viii” statement certifying that its proposed label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent.
If the reference NDA holder or patent owners assert a patent challenge directed to one of the Orange Book listed patents within 45 days of the receipt of the paragraph IV certification notice, the FDA is prohibited from approving the application until the earlier of 30 months from the receipt of the paragraph IV certification expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the applicant. The ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the branded reference drug has expired as described in further detail below. Thus approval of a 505(b)(2) NDA or ANDA can be prevented until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA or 505(b)(2) applicant.
Combination Products
Medical products containing a combination of new drugs, biological products, or medical devices are regulated as “combination products” in the United States. A combination product generally is defined as a product comprised of components from two or more regulatory categories, such as drug/device, device/biologic or drug/biologic. The term combination product includes: (i) a product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic or drug/device/biologic, that are physically, chemically or otherwise combined or mixed and produced as a single entity); (ii) two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products or biological and drug products; (iii) a drug, device or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device or biological product where both are required to achieve the intended use, indication or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, such as to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or (iv) any investigational drug, device or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication or effect.
Each constituent part of a combination product is subject to the requirements established by the FDA for that type of constituent part, whether a new drug, biologic or device. In order to facilitate pre-market review of combination products, the FDA designates one of its centers to have primary jurisdiction for the pre-market review and regulation of the overall product based upon a determination by FDA of the primary mode of action of the combination product, and typically one application, such as for a drug/device combination product assigned to the FDA’s Center for Drug Evaluation and Research, or CDER, an NDA, will be made.
A device with the primary purpose of delivering or aiding in the delivery of a drug and distributed containing a drug (i.e., a “prefilled delivery system”) is typically evaluated by CDER using drug authorities and device authorities, as necessary.
A device with the primary purpose of delivering or aiding in the delivery of a drug and that is distributed without the drug (i.e., unfilled) is typically evaluated by the FDA’s Center for Devices and Radiological Health and CDER, respectively, unless the intended use of the two products, through labeling, creates a combination product.
The FDA has indicated that dry powder inhalers, such as our lead product candidate, LIQ861, are drug/device combination products.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to extensive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping (including certain electronic record and signature requirements), periodic reporting, drug supply chain security surveillance and tracking requirements, product sampling and distribution, advertising and promotion and reporting of certain adverse experiences, deviations and other problems with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There are also, under The Prescription Drug User Fee Act, continuing, annual FDA “program fee” requirements for products once they are approved, as well as new application fees for supplemental applications with clinical data.
The FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market. Products may be promoted only for the approved indications and in accordance with the provisions of the approved label. Further, manufacturers must continue to comply with cGMP requirements, which are extensive and require considerable time, resources and ongoing investment to ensure compliance. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
Manufacturers and certain other entities involved in the manufacturing and distribution of approved products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. The cGMP requirements apply to all stages of the manufacturing process, including the production, processing, sterilization, packaging, labeling, storage and shipment of the product. Manufacturers must establish validated systems to ensure that products meet specifications and regulatory standards, and test each product batch or lot prior to its release. Combination products are subject to FDA regulation to ensure the quality of both the constituent parts and the finished product.
Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance.
The FDA may impose a number of post-approval requirements as a condition of approval of an application. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
The FDA may withdraw a product approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, problems with manufacturing processes, or failure to comply with regulatory requirements, may result in restrictions on the product or even complete withdrawal of the product from the market.
Potential implications include required revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
|
● |
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
|
● |
warning letters or holds on post-approval clinical trials; |
|
● |
refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
|
● |
product seizure or detention, or refusal to permit the import or export of products; or |
|
● |
injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. As a compliance best practice and risk mitigation measure, pharmaceutical companies typically train their sales force regarding the limitations on promotion of products relative to their approved indications for use and concerns regarding potential “off-label promotion.” However, a physician may use products off-label, when in the physician’s independent professional medical judgment he or she deems it appropriate. Recent court decisions have impacted FDA’s enforcement activity regarding off-label promotion in the light of First Amendment considerations; however, there are still significant risks in this area in part due to the potential for False Claims Act exposure. Further, the FDA as not materially changed its position on off-label promotion following legal setbacks on First Amendment grounds and the U.S. Department of Justice has consistently asserted in False Claims Act briefings that “speech serves as a conduit for violations of the law is not constitutionally protected.”
The distribution of prescription drugs is subject to the Drug Supply Chain Security Act, or DSCSA, which regulates the distribution of the products at the federal level, and sets certain standards for federal or state registration and compliance of entities in the supply chain and regulation of manufacturers and repackagers, wholesale distributors, third-party logistics providers, and dispensers. The DSCSA preempts certain previously enacted state pedigree laws and upon taking effect superseded the pedigree requirements of the Prescription Drug Marketing Act, or PDMA. Trading partners within the drug supply chain must now ensure certain product tracing requirements are met, and are required to exchange transaction information, transaction history, and transaction statements. Further, the DSCSA limits the distribution of prescription pharmaceutical products and imposes requirements to ensure overall accountability and security in the drug supply chain. Product identifier information (an aspect of the product tracing scheme) is also now required. The DSCSA requirements, development of standards, and the system of product tracing have been and will continue to be phased in over a period of years through 2023, and subject companies will need to continue their implementation efforts. Many states still have in place licensure and other requirements for manufacturers and distributors of drug products. The distribution of product samples continues to be regulated under the PDMA, and some states also impose regulations on drug sample distribution.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations, guidance and policies are often revised or reinterpreted by the agency in ways that may significantly affect our business and our product candidates. It is impossible to predict whether further legislative or FDA regulation or policy changes will be enacted or implemented and what the impact of such changes, if any, may be.
Patent Term Restoration
Depending upon the timing, duration and specifics of FDA approval of the use of our product candidates, some of our U.S. patents may be eligible for limited PTE under the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to five years as compensation for patent term effectively lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug is eligible for the extension. Extensions are not granted as a matter of right and the extension must be applied for prior to expiration of the patent and within a sixty day period from the date the product is first approved for commercial marketing. The USPTO, in consultation with the FDA, reviews and approves the application for any PTE or restoration. In the future, we may apply for PTEs, defined as the length of the regulatory review of products covered by our granted patents, for some of our currently owned or licensed applications and patents to add patent life beyond their current expiration dates. Such extensions will depend on the length of the regulatory review; however, there can be no assurance that any such extension will be granted to us.
Marketing Exclusivity
Market exclusivity provisions under the FDCA can also delay the submission or the approval of certain applications. The specific scope varies, but fundamentally the FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving applications for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical studies necessary to demonstrate safety and effectiveness.
Pediatric exclusivity is another type of exclusivity in the United States. Pediatric exclusivity, if granted, provides an additional six months to the term of any existing regulatory exclusivity, including the non-patent exclusivity periods described above. This six-month exclusivity may be granted based on the voluntary completion of a pediatric clinical study that “fairly responds” to an FDA-issued “Written Request” for such a clinical study.
Pharmaceutical Coverage, Pricing and Reimbursement
In the United States, sales of any products for which we may receive regulatory approval for commercial sale will depend in part on the availability of coverage and reimbursement from third-party payors. Third-party payors include government authorities, managed care providers, private health insurers and other organizations.
Significant uncertainty exists as to the coverage and reimbursement status of any products for which we may obtain regulatory approval. Some of the additional requirements and restrictions on coverage and reimbursement levels imposed by third-party payors influence the purchase of healthcare services and products. The process for determining whether a third-party payor will provide coverage for a product may be separate from the process for establishing the reimbursement rate that such a payor will pay for the product. Third-party payors may limit coverage to specific drugs on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication, or place drugs at certain formulary levels that result in lower reimbursement levels. Moreover, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Further, one payor’s determination to provide coverage does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement may differ significantly from payor to payor as there is no uniform policy of coverage and reimbursement for drug products among third-party payors.
Reimbursement may also impact the demand for drug products that obtain marketing approval. If coverage for a drug product is obtained by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. Further, third party payors require onerous prior approvals or implement other forms of restricted access that make it difficult for patients to utilize our drug products. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Prescribing physicians are unlikely to use or prescribe drug products unless coverage is provided and reimbursement is adequate to cover all or a significant portion of the cost of those drug products. If reimbursement is not available, or is available only to limited levels, a drug product which has obtained marketing approval may not be successfully commercialized.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain and maintain coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of any products, in addition to the costs required to obtain regulatory approvals. Our product candidates may not be considered medically necessary or cost-effective. If third-party payors do not consider a product to be cost-effective compared to other available therapies, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit.
The U.S. government and state legislatures have shown significant interest in implementing cost containment programs to limit the growth of government-paid healthcare costs, including price controls, restrictions on reimbursement and coverage and requirements for substitution of generic products for branded prescription drugs. There has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. For example, U.S. federal prosecutors have issued subpoenas to pharmaceutical companies seeking information about pricing practices in connection with an investigation into pricing practices being conducted by the DOJ. Several state attorneys general also have commenced drug pricing investigations and filed lawsuits against pharmaceutical companies, and the U.S. Senate has publicly investigated a number of pharmaceutical companies relating to price increases and pricing practices. Proposed legislation has been designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. Recent federal budget proposals have included measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. The U.S. Congress and the Trump Administration have indicated that they will continue to seek new legislative and administrative measures to control drug costs, including by addressing the role of pharmacy benefit managers, or PBMs, in the supply chain. Drug pricing is and will remain a key bipartisan issue in the coming year. If drug pricing reform is not meaningfully addressed before the 2020 election, policies to be pursued in the future may be more aggressive, regardless of which party controls the White House. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Adoption of government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could exclude or limit our drugs and product candidates from coverage and limit payments for pharmaceuticals. We anticipate that current and future U.S. federal and state legislative proposals may result in additional downward pressure on drug pricing and reimbursement, which could have a significant impact on our business.
In addition, we expect that the increased emphasis on managed care and cost containment measures in the United States by third-party payors and government authorities to continue and will place pressure on pharmaceutical pricing and coverage. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
Healthcare providers, physicians and third-party payors often play a primary role in the recommendation and prescription of any currently marketed products and product candidates for which we may obtain marketing approval. Our current and future arrangements with healthcare providers, physicians, third-party payors and customers, and our sales, marketing and educational activities, may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations (at the federal and state level) that may constrain our business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval.
In addition, we may be subject to transparency laws and patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include the following:
|
● |
The federal Anti-Kickback Statute, which prohibits, among other things, persons and entities including pharmaceutical manufacturers from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order or recommendation of, an item or service reimbursable, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. This statute has been interpreted broadly to apply to, among other things, arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other hand. The term “remuneration” expressly includes kickbacks, bribes or rebates and also has been broadly interpreted to include anything of value, including, for example, gifts, discounts, waivers of payment, ownership interest and providing anything at less than its fair market value. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny. The failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the federal Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Our practices may not meet all of the criteria for safe harbor protection from federal Anti-Kickback Statute liability in all cases. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it to have committed a violation. In addition, the Affordable Care Act codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a per se false or fraudulent claim for purposes of the federal civil False Claims Act. |
|
● |
The federal civil and criminal false claims laws and civil monetary penalty laws, including the civil False Claims Act, which prohibits individuals or entities from, among other things, knowingly presenting, or causing to be presented, claims for payment to, or approval by, the federal government that are false, fictitious or fraudulent or knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim to avoid, decrease or conceal an obligation to pay money to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the federal government. Although we do not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers, promoting a product off-label, marketing products of sub-standard quality, or, as noted above, paying a kickback that results in a claim for items or services. In addition, our activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state and third-party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, several pharmaceutical and other healthcare companies have faced enforcement actions under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. The False Claims Act also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the False Claims Act and to share in any monetary recovery. In addition, federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, may also constitute a violation of the False Claims Act. Although the False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes. Additionally, the federal government has pursued electronic health record, or HER, vendors and pharmaceutical manufacturers for remunerative relationships involving the EHR platform’s recommendation of particular drugs and “prompting” technology to increase prescribing of particular drugs. |
|
● |
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up by trick, scheme or device, a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. |
|
● |
The Criminal Healthcare Fraud statute, 18 U.S.C. § 1347, prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payers. Federal criminal law at 18 U.S.C. § 1001, among other sections, prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. These statutes are not limited to items and services reimbursed by a governmental health care program and have been used to prosecute commercial insurance fraud as well. |
|
● |
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. |
|
● |
The exclusion statute requires the exclusion of entities and individuals who have been convicted of federal-program related crimes or health care felony fraud or controlled substance charges. The statute also permits the exclusion of those that have been convicted of any form of fraud, the Anti-Kickback Statute, for obstructing an investigation or audit, misdemeanor controlled substance charges, those whose health care license has been revoked or suspended, and those who have filed claims for excessive charges or unnecessary services. If a company were to be excluded, its products would be ineligible for reimbursement from any federal programs, including Medicare and Medicaid, and no other entity participating in those programs would be permitted to enter into contracts with the company. Further, employment or contracting with an individual or entity that has been excluded from participation in federal healthcare programs could serve as a basis to invalidate claims for items or services submitted by that entity and to exclude that entity from participation in such programs as well. In order to preserve access to beneficial drugs, the government may elect to exclude officers and key employees of manufacturers, rather than excluding the organization. Such enforcement actions would prohibit us from engaging those individuals, which could adversely affect operations. |
|
● |
We may be subject to data privacy and security regulations by both the federal government and the states in which we conduct business. We are not a covered entity under HIPAA and have not functioned as a business associate under HIPAA that would cause the HIPAA Security Rule and provisions of the Privacy Rule to apply directly to us as a business associate. To the extent that we ever function in a business associate capacity, however, HIPAA, as amended by as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, including the Final Omnibus Rule published on January 25, 2013, impose, among other things, obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal court to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. The U.S. Department of Health and Human Services, or HHS, Office for Civil Rights, or the OCR, has increased its focus on compliance and continues to train state attorneys general for enforcement purposes. The OCR has recently increased both its efforts to audit HIPAA compliance and its level of enforcement, with one recent penalty exceeding $5 million. In addition, state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways, and may apply more broadly thus complicating compliance efforts (for example, California recently enacted legislation — the California Consumer Privacy Act, or CCPA — which went into effect on January 1, 2020 and among other things, created new data privacy obligations for covered companies and provided new privacy rights to California residents, including the right to opt out of certain disclosures of their information, and created a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach; the California Attorney General will issue final regulations, and although the law includes limited exceptions, including for certain information collected as part of clinical trials as specified in the law, it may regulate or impact our processing of personal information depending on the context, and it remains unclear what language the final Attorney General regulations will contain or how the statute and the regulations will be interpreted. |
|
● |
The federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act,” created under the U.S. Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA, and its implementing regulations, which requires applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program (with certain exceptions) to annually report to the Centers for Medicare & Medicaid Services, or CMS, information related to certain payments or other transfers of value made or distributed to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Federal legislation enacted in 2018 has extended the scope of reporting requirements to apply to payments and transfers of value to not only physicians, but also physician assistants, nurse practitioners, and other mid-level practitioners (with reporting requirements going into effect in 2022 for payments made in 2021). |
|
● |
According to the U.S. Federal Trade Commission, or the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act, or the FTCA, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Medical data is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule. |
|
● |
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to items or services reimbursed by any third-party payor, including commercial insurers, and in some cases may apply regardless of payor (i.e., even for self-pay scenarios). Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report pricing and marketing information, including, among other things, information related to payments to physicians and other healthcare providers or marketing expenditures, requirements related to drug sample distribution, state and local laws that require the registration of pharmaceutical sales representatives, and state laws governing the privacy and security of health information and the use of prescriber-identifiable data in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
|
● |
Price reporting laws that require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursements or discounts on our drug products. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that certain business activities could be subject to challenge under one or more of such laws. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Ensuring that business arrangements with third parties comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert management’s attention from the business, even if the government ultimately finds that no violation has occurred.
If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, individual imprisonment, possible exclusion from participation in government healthcare programs, injunctions, private qui tam actions brought by individual whistleblowers in the name of the government and the curtailment or restructuring of our operations, as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, any of which could adversely affect our ability to operate our business and our results of operations.
Healthcare Reform
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. There have been a number of federal and state proposals during the last few years regarding the pricing of pharmaceutical and biopharmaceutical products, limiting coverage and reimbursement for drugs and other medical products, government control and other changes to the healthcare system in the United States. By way of example, in March 2010, the Patient Protection and Affordable Care Act, or ACA, contained several provisions affecting the pharmaceutical industry:
|
● |
the Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of HHS, as a condition of Medicare Part B and Medicaid coverage of the manufacturer’s outpatient drugs furnished to Medicaid patients; |
|
● |
in order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B Drug Pricing Program. The required 340B discount on a given product is calculated based on the average manufacturer price, or AMP, and Medicaid rebate amounts reported by the manufacturer; |
|
● |
the ACA imposed a requirement on manufacturers of branded drugs to provide a 70% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., the donut hole); |
|
● |
the ACA imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications; |
|
● |
the ACA implemented the Physician Payments Sunshine Act; |
|
● |
the ACA requires annual reporting of drug samples that manufacturers and distributors provide to physicians; |
|
● |
the ACA expanded healthcare fraud and abuse laws in the United States, including the False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for non-compliance; |
|
● |
the ACA established a licensing framework for follow-on biologics; and |
|
● |
the ACA established the new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with the funding for such research. |
The Trump Administration and the Congressional Republicans have proposed several efforts to repeal and replace the ACA. President Trump has also signed Executive Orders and other directives designed to delay the implementation of certain provisions of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. Additionally, on December 15, 2019, a federal district court in Texas struck down the ACA in its entirety, finding that the Tax Cuts and Jobs Act of 2017 (TCJA) rendered the individual mandate unconstitutional. The judge further concluded in Texas v. Azar that since the individual mandate is “essential” to the ACA, it could not be severed from the rest of the ACA and therefore, the entire ACA was unconstitutional. Despite its decision, however, the court did not issue an injunction and therefore, immediate compliance is not required. In addition, the Trump Administration announced that it will continue to administer the law until a formal decision is made by the U.S. Supreme Court. The Supreme Court recently announced that it will hear a challenge in Texas v. United States, though arguments have not yet been set. It is likely that the case will be scheduled for arguments early in the next term that starts in October 2020. Apart from Texas v. United States, ACA litigation continues across the country in district and appellate courts, and before the Supreme Court. The Supreme Court will issue at least two ACA-related decisions before the end of its current term: one on the risk corridors program (Maine Community Health Options v. United States) and the other on religious or moral exemptions to the contraceptive mandate (Trump v. Pennsylvania and Little Sisters of the Poor v. Pennsylvania). Both decisions are expected before July 2020. It is unclear how the eventual decisions from the Supreme Court and the various other courts across the country to repeal and replace the ACA will impact the ACA and our business. It is also unclear how regulations and sub-regulatory policy, which fluctuate continually, may affect interpretation and implementation of the ACA and its practical effects on our business, particularly entering an election year.
In the future, there may continue to be additional proposals relating to the reform of the U.S. healthcare system, some of which could further limit the prices we will be able to charge for our product candidates, or the amounts of reimbursement available for our product candidates. If future legislation were to impose direct governmental price controls or access restrictions, it could have a significant adverse impact on our business. Managed care organizations, as well as Medicaid and other government agencies, continue to seek price discounts. Some states have implemented, and other states are considering, measures to reduce costs of the Medicaid program, and some states are considering implementing measures that would apply to broader segments of their populations that are not Medicaid-eligible. Due to the volatility in the current economic and market dynamics, we are unable to predict the impact of any unforeseen or unknown legislative, regulatory, payor or policy actions, which may include cost containment and healthcare reform measures. Such policy actions could have a material adverse impact on our profitability.
These and other healthcare reform initiatives may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our financial operations. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Additionally, on May 30, 2018, the Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017, or Right to Try Act, was signed into law. The law, among other things, provides a federal framework for patients to access certain investigational new drug products that have completed a Phase I clinical trial. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA approval under the FDA expanded access program. There is no obligation for a pharmaceutical manufacturer to make its drug products available to eligible patients as a result of the Right to Try Act.
Foreign Regulation of Drugs
In order to market any product outside of the United States, we will need to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding development, approval, commercial sales and distribution of our products, and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products, if approved. Whether or not we obtain FDA approval for a product, we must obtain the necessary approvals by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Item 1A. Risk Factors
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, as well as the other information in this Annual Report on Form 10-K, including our financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before deciding whether to invest in our common stock. The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the market price of our common stock could decline and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
Risks Related to our Financial Position and Need for Additional Capital
Our net losses and significant cash used in operating activities have raised substantial doubt regarding our ability to continue as a going concern.
Our financial statements for the year ended December 31, 2019 include a statement that our recurring losses and cash outflows from operations, our accumulated deficit and our debt maturing within twelve months raise substantial doubt about our ability to continue as a going concern. As of December 31, 2019, we had $55.8 million of cash. We believe that our existing cash will enable us to fund our operating expenses and capital expenditure requirements, make payments of interest and principal on our term loan facility with Pacific Western Bank, or PWB, and remain in compliance with the minimum cash covenant of $8.5 million pursuant to this term loan facility, through August 2020. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect.
If we are unable to obtain sufficient funding or execute on strategic initiatives to generate sufficient cash, our business, prospects, financial condition and results of operations will be materially and adversely affected, and we may be unable to continue as a going concern. If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our audited financial statements, and it is likely that investors will lose all or a part of their investment. Future financial statements may also include statements expressing substantial doubt about our ability to continue as a going concern. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all.
We have a history of losses, have not commenced commercial operations to date and our future profitability is uncertain.
We have incurred net losses of $47.6 million, $53.1 million and $29.2 million for the years ended December 31, 2019, 2018 and 2017, respectively. We also had negative operating cash flows for each of these years. As of December 31, 2019 and 2018, we had an accumulated deficit of $215.2 million and $167.1 million, respectively.
Since our incorporation, we have invested heavily in the development of our product candidates and technologies, as well as in recruiting management and scientific personnel. To date, we have not commenced the commercialization of our product candidates and all of our revenue has been derived from up-front fees and milestone payments made to us in connection with licensing and collaboration arrangements we have entered into. These up-front fees and milestone payments have been, and may continue to be, insufficient to match our operating expenses. We expect to continue to devote substantial financial and other resources to the clinical development of our product candidates and, as a result, must generate significant revenue to achieve and maintain profitability. We may continue to incur losses and negative cash flow and may never transition to profitability or positive cash flow.
We expect that we will need further financing for our existing business and future growth, which may not be available on acceptable terms, if at all. Failure to obtain funding on acceptable terms and on a timely basis may require us to curtail, delay or discontinue our product development efforts or other operations. The failure to obtain further financing may also prevent us from capitalizing on other potential product candidates or indications which may be more profitable than LIQ861 and LIQ865 or for which there may be a greater likelihood of success.
We anticipate that we will need to raise additional funds to meet our future funding requirements for the continued research, development and commercialization of our product candidates and technology. In the event that funds generated from our operations are insufficient to fund our future growth, we may raise additional funds through the issuance of equity or debt securities or by borrowing from banks or other financial institutions. We cannot assure you that we will be able to obtain such additional financing on terms that are acceptable to us, or at all. Global and local economic conditions could negatively affect our ability to raise funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of such securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Such financing, even if obtained, may be accompanied by restrictive covenants that may, among others, limit our ability to pay dividends or require us to seek consent for payment of dividends, or restrict our freedom to operate our business by requiring consent for certain actions.
If we fail to obtain additional financing on terms that are acceptable to us, we will not be able to implement our growth plans, and we may be required to significantly curtail, delay or discontinue one or more of our research, development or manufacturing programs or the commercialization of any approved product. Furthermore, if we fail to obtain additional financing on terms that are acceptable to us, we may forgo or delay the pursuit of opportunities presented by other potential product candidates or indications that may later prove to have greater commercial potential than the product candidates and indications that we have chosen to pursue.
We are evaluating potential strategic alternatives that could significantly impact our future operations and financial position.
Our primary objective has been to pursue marketing approval of LIQ861 and commercialize such product if approved by FDA. We will need to raise substantial additional capital to continue our business operations and remain in compliance with the minimum cash covenant on our debt during and beyond the third quarter of 2020, in addition to commercializing LIQ861, if approved. Such capital may not be available to us on a timely basis, on terms that are favorable to us, or at all. Alternatively, in light of the Company’s current limited cash resources, the recent trading price of our common stock, outstanding debt and associated minimum cash covenant, and based on a review of the status of our programs, resources and capabilities, we continue to explore a wide range of strategic alternatives with the support of our financial advisor, Jefferies LLC, that could maximize stockholder value. Our efforts have been and continue to be focused primarily upon the potential formation of a partnership or a licensing transaction with respect to our lead program, LIQ861, for the treatment of PAH. Strategic alternatives may also include the sale of some of our assets or proprietary technologies, or a potential merger or sale of the Company. There can be no assurance that we will be able to enter into such a transaction or transactions on a timely basis, on terms that are favorable to us, or at all.
We may acquire businesses, products or product candidates, or form strategic alliances or create joint ventures, in the future, and we may not realize the benefits of such transactions.
We may acquire additional businesses, products or product candidates, form strategic alliances or create joint ventures with third parties that we believe will complement or augment our existing business, although we have no current agreements, commitments or understandings to do so. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new products or product candidates resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, strategic alliance or joint venture, we will achieve the expected synergies to justify the transaction.
Our credit facility with Pacific Western Bank, or PWB, contains operating and financial covenants that restrict our business and financing activities, and is subject to acceleration in specified circumstances, which may result in PWB taking possession and disposing of any collateral.
Our credit facility contains restrictions that limit our flexibility in operating our business. Under the terms of the amended and restated loan and security agreement dated as of October 26, 2018, as amended, or the A&R LSA, with PWB, pursuant to which PWB extended a $16.0 million term loan facility to us, of which $11.0 million was received in October 2018 in an initial tranche and $5.0 million was received in May 2019, we may not, among others, without the prior written consent of PWB, (a) pay any dividends or make any other distribution or payment on account of or in redemption, retirement or purchase of any capital stock except in certain prescribed circumstances, (b) create, incur, assume, guarantee or be or remain liable with respect to any indebtedness except certain permitted indebtedness or prepay any indebtedness, (c) replace or suffer the departure of our Chief Executive Officer or Chief Financial Officer without delivering written notification to PWB within ten days of such change or (d) suffer a change on our board of directors, or Board, which results in the failure of at least one partner of Canaan Partners or their respective affiliates to serve as a voting member, without having used best efforts to deliver at least 15 days’ prior written notification to PWB. Our facility with PWB is collateralized by all of our assets excluding our intellectual property, on which we have granted a negative pledge.
We have, in the past, breached multiple covenants in our loan and security agreement dated as of January 6, 2016, as amended, with PWB related to cash levels, reporting requirements and required periodic deliverables to PWB, but have obtained waivers from PWB in relation to all such breaches. If we breach certain of our debt covenants and are unable to cure such breach within the prescribed period or are not granted waivers in relation to such breach, it may constitute an event of default under our facility agreements, giving lenders the right to require us to repay the then outstanding debt immediately, and the lenders could, among other things, foreclose on the collateral granted to them to collateralize such indebtedness, which excludes our intellectual property, if we are unable to pay the outstanding debt immediately. A breach of covenants in the A&R LSA and the acceleration of our repayment obligations by PWB could have a material adverse effect on our business, financial condition, results of operations and prospects.
Although we have historically depended on GSK for a significant portion of our revenue, we do not expect to receive any additional revenue from our GSK collaboration.
We are party to a licensing agreement with GSK pursuant to which GSK has exercised an option to exclusively license our PRINT technology for applications in certain inhaled therapies, or the GSK ICO Agreement. We previously entered into a separate licensing agreement with GSK relating to the field of vaccines, which lapsed in April 2016. We have historically received a significant portion of our revenue from GSK pursuant to these licensing agreements. For the years ended December 31, 2019, 2018 and 2017, our revenue attributable to our collaboration and licensing arrangements with GSK, which included a combination of billings for particle formulations, manufacturing, milestone payments and amortization of deferred revenue from up-front fees, accounted for 100%, 16% and 84%, respectively, of our total revenue.
During the second quarter of 2019 we concluded that no further research and development services will be provided to GSK under the collaboration agreement and the earnings process related to the license fees previously received under the collaboration agreement has been completed under the proportional performance model. Therefore, the remaining deferred revenue of $8.1 million was recognized as revenue during the second quarter of 2019, and we do not expect to receive any additional revenue from GSK pursuant to our collaboration. Because GSK is no longer actively advancing any programs under our collaboration, we entered into the Third Amendment to the GSK ICO Agreement during the second quarter of 2019, pursuant to which we have the right to develop three products for delivery via inhalation, subject to specified milestone payments and royalties due to GSK. Additionally, under certain circumstances GSK has a right of first negotiation with respect to these programs. Although a large proportion of our revenue has historically been obtained from our collaboration with GSK, we do not expect this collaboration to continue. To that end, we recently notified GSK of our intent to terminate the collaboration because we believe that GSK's inactivity with respect to the collaboration constitutes a material breach and GSK has rebutted our notice of termination. We are currently attempting to resolve the dispute with GSK pursuant to the terms of the GSK ICO Agreement.
Our management has broad discretion in using the net proceeds from prior equity offerings and may not use them effectively.
We expect to use the net proceeds of prior equity offerings to conduct additional clinical studies for LIQ861, conduct toxicology studies for LIQ865 and advance LIQ865 towards one or more Phase 2 proof-of-concept clinical trials, fund operations supporting the development of, and commercial activities for, LIQ861 and LIQ865, and for working capital and general corporate purposes. Our management has broad discretion in the application of such proceeds and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our equity. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, diminish cash flows available to service our debt, cause the value of our equity to decline and delay the development of our product candidates. Pending their use, we may invest such proceeds in short-term, investment-grade, interest-bearing securities, which may not yield favorable returns.
Our ability to use our net operating loss carry forwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an “ownership change”, generally defined as a greater than 50.0% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes, such as research tax credits, to offset its post-change income may be limited. With our December 2019 private placement and recent issuances under our ATM facility, our March 2019 follow-on equity offering and our July 2018 initial public offering, as well as other past transactions, we believe that we have triggered an “ownership change” limitation. We have not completed a formal study to determine if any “ownership changes” within the meaning of IRC Section 382 have occurred. If “ownership changes” within the meaning of Section 382 of the Code have occurred, and if we earn net taxable income, our ability to use our net operating loss carryforwards and research and development tax credits generated since inception to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us and could require us to pay U.S. federal income taxes earlier than would be required if such limitations were not in effect. Similar rules and limitations may apply for state income tax purposes.
We are a late-stage clinical biopharmaceutical company with no approved products and no historical product revenue, which may make it difficult for you to evaluate our business, financial condition and prospects.
We are a late-stage clinical biopharmaceutical company with no history of commercial operations upon which you can evaluate our prospects. Drug product development involves a substantial degree of uncertainty. Our operations to date have been limited to developing our PRINT technology, undertaking preclinical studies and clinical trials for our product candidates and collaborating with pharmaceutical companies, including GlaxoSmithKline plc and/or its subsidiaries, collectively, GSK, to expand the applications for our PRINT technology through licensing as well as joint product development arrangements. We have not obtained marketing approval for any of our product candidates and, accordingly, have not demonstrated an ability to generate revenue from pharmaceutical products or successfully overcome the risks and uncertainties frequently encountered by companies undertaking drug product development. Consequently, your ability to assess our business, financial condition and prospects may be significantly limited. Further, the net losses that we incur may fluctuate significantly from quarter-to-quarter and year-to-year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. Other unanticipated costs may also arise.
The TCJA could adversely affect our business and financial condition.
On December 22, 2017, the TCJA was enacted into law. The TCJA includes significant changes to the U.S. corporate income tax system, including a permanent reduction in the corporate income tax rate from 35% to 21%, limitations on the deductibility of interest expense and executive compensation and the transition of U.S. international taxation from a worldwide system to a territorial tax system. For taxpayers with revenues over a certain threshold, the TCJA also limits interest expense deductions to 30% of taxable income before interest, depreciation and amortization from 2018 to 2021 and then taxable income before interest thereafter. The TCJA permits disallowed interest expense to be carried forward indefinitely. We calculated our best estimate of the impact of the TCJA in our income tax provision for the year ended December 31, 2017 in accordance with our understanding of the TCJA and guidance available at the time. The overall impact of the TCJA resulted in a decrease to the deferred tax assets and a corresponding decrease to the valuation allowance of $14.1 million. We completed our accounting for the TCJA during the fourth quarter of 2018. No changes to the provisional amounts as of December 31, 2017 were recorded. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the TCJA is uncertain and our business and financial condition could be adversely affected. The impact of this tax reform on holders of our common stock is also uncertain and could be adverse. We urge our stockholders to consult with their legal and tax advisors with respect to such legislation and the potential tax consequences of investing in our common stock.
Risks Related to the Commercialization of our Product Candidates
We face significant competition from large pharmaceutical companies, among others, and our operating results will suffer if we are unable to compete effectively.
We face significant competition from industry players worldwide, including large multi-national pharmaceutical companies, other emerging or smaller pharmaceutical companies, as well as universities and other research institutions. Many of our competitors have substantially greater financial, technical and other resources, such as a larger research and development staff, and more experience in manufacturing and marketing, than we do. As a result, these companies may obtain marketing approval for their product candidates more quickly than we are able to and be more successful in commercializing their products than us. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaboration arrangements with large, established companies. We may also face competition as a result of advances in the commercial applicability of new technologies and greater availability of capital for investment in such technologies. Our competitors may also invest heavily in the discovery and development of novel drug products that could make our product candidates less competitive or may file FDA citizen petitions which may delay the approval process for our product candidates. Furthermore, our competitors may succeed in developing, acquiring or licensing, on an exclusive basis, pharmaceutical products that are easier to develop, more effective or less costly than any product candidates that we are currently developing or that we may develop. Our competitors may also succeed in asserting existing patents or developing new patents to which we do not have a license in an attempt to prevent us from marketing our products.
Any new drug product that competes with a prior approved drug product must demonstrate advantages in safety, efficacy, tolerability or convenience in order to overcome price competition and to be commercially successful. Our products, if and when approved, are expected to face competition from drug products that are already on the market, as well as those in our competitors’ development pipelines. We expect that our lead program, LIQ861, an inhaled treprostinil therapy for the treatment of PAH, will face competition from the following inhaled treprostinil therapies that are either currently marketed or in clinical development:
|
● |
Tyvaso, marketed by United Therapeutics, has been approved for the treatment of PAH in the United States since 2009. Tyvaso is the reference listed drug in our NDA for LIQ861. Following patent litigation, United Therapeutics and Watson Pharmaceuticals reached a settlement whereby Watson Pharmaceuticals will be permitted to enter the market with a generic version of Tyvaso beginning on January 1, 2026. |
|
● |
Ventavis, marketed by Actelion, a division of Johnson & Johnson, has been approved for the treatment of PAH in the United States since 2004. |
|
● |
TreT, licensed from MannKind, by United Therapeutics, is currently in late-stage clinical development in the United States for the treatment of PAH. Under the license agreement, United Therapeutics is responsible for global development, regulatory and commercial activities. MannKind will manufacture clinical supplies and initial commercial supplies of the product while long-term commercial supplies will be manufactured by United Therapeutics. In September, 2019, United Therapeutics commenced a clinical study (BREEZE) to evaluate the safety and pharmacokinetics of switching PAH patients from Tyvaso to TreT and announced plans to commence a second clinical study during the first half of 2020 to compare the pharmacokinetics of TreT to Tyvaso in healthy volunteers. United Therapeutics further reported that the two studies, if successful, are the only clinical studies necessary to support FDA approval. |
In addition to these other inhaled treprostinil therapies, we expect that LIQ861 will also face competition from other treprostinil-based drugs, including Orenitram, which is administered orally, and Remodulin, which is administered parenterally, both of which are marketed by United Therapeutics.
In addition to treprostinil-based therapies, other classes of therapeutic agents for the treatment of PAH include the following:
|
● |
IP-agonists, such as selexipag, marketed by Actelion, and ralinepeg, licensed from Arena Pharmaceuticals by United Therapeutics, which is currently in clinical development; |
|
● |
Endothelin receptor antagonists, such as bosentan and macitentan, both marketed by Actelion, and ambrisentan, marketed by Gilead. Generic version of bosentan and ambrisentan are currently available. |
|
● |
PDE-5 inhibitors, such as tadalafil, marketed by United Therapeutics, and sildenafil, marketed by Pfizer. Generic versions of both tadalafil and sildenafil are currently available. |
|
● |
Soluble guanylate cyclase (sGC) stimulator, such as riociguat marketed by Bayer. |
In addition, we are also aware of several other agents currently in clinical development in the United States for the treatment of PAH, including those in development by Insmed and Acceleron.
We expect LIQ865 to face competition from EXPAREL®, an existing injectable version of bupivacaine. The early success of EXPAREL may make it difficult for us to convince physicians, patients and other members of the medical community to accept and use LIQ865 over EXPAREL. Generic equivalents of EXPAREL may also enter the market following the expiry of EXPAREL’s patent in 2021.
While EXPAREL is currently the only direct competitor to LIQ865 on the market, in October 2018 Heron Therapeutics, Inc., or Heron, announced the submission of its NDA to the FDA for HTX-011, an investigational long-acting, extended-release formulation of the local anesthetic bupivacaine in a fixed-dose combination with the anti-inflammatory meloxicam for the management of postoperative pain. HTX-011 was granted both breakthrough therapy and fast track designations from the FDA as well as priority review by the FDA. On May 1, 2019, Heron announced that it received a complete response letter, or CRL, for HTX-011 from the FDA. On October 1, 2019, Heron announced that it had resubmitted its NDA for HTX-011 to the FDA and expected a six-month review. In addition to Heron, Durect Corporation and Innocoll Holdings plc each also have products in clinical development that are potential competitors to LIQ865.
If we are unable to maintain our competitive position, our business and prospects will be materially and adversely affected.
If a competitor obtains orphan drug designation from the FDA for the same drug and same indication as we are seeking for a product candidate, and then obtains approval of that drug for that condition before we do, the resulting FDA exclusivity would significantly delay our ability to commercialize that product candidate. Similarly, if a competitor obtains marketing approval for a new condition of use that required new clinical investigations for support, the competitor may obtain three-year marketing exclusivity for that condition of use, and thereby delay our ability to receive marketing approval for that drug product for that condition of use by three years form the date of that approval.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition — generally a disease or condition that affects fewer than 200,000 individuals in the United States or that affects more than 200,000 individuals in the United States and for which there is no reasonable expectation that the costs of research and development of the drug for the indication can be recovered by sales of the drug in the United States. Orphan drug designation must be requested before submitting an NDA.
After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first applicant to receive FDA approval for a particular active ingredient to treat a particular disease or condition with orphan drug designation is entitled to a seven-year exclusive marketing period in the United States for that product in that indication. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
During the exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease or condition, except in limited circumstances, such as if the second applicant demonstrates clinical superiority of its product to the product with orphan drug exclusivity through a demonstration of superior safety, superior efficacy or a major contribution to patient care, or if the manufacturer of the product with orphan exclusivity is not able to assure sufficient quantities of the product. “Same drug” means a drug that contains the same identity of the active moiety if it is a drug composed of small molecules, or of the principal molecular structural features if it is composed of macromolecules and is intended for the same use as a previously approved drug, except that if the subsequent drug can be shown to be clinically superior to the first drug, it will not be considered to be the same drug. Drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition.
The commercial success of our drug products depends on the availability and sufficiency of third-party payor coverage and reimbursement.
Patients in the United States and elsewhere generally rely on third-party payors to reimburse part or all of the costs associated with their prescription drugs. Accordingly, market acceptance of our drug products is dependent on the extent to which third-party coverage and reimbursement is available from government health administration authorities (including in connection with government healthcare programs, such as Medicare and Medicaid in the United States), private healthcare insurers and other healthcare funding organizations.
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we may obtain regulatory approval. Coverage decisions may not favor new drug products when more established or lower-cost therapeutic alternatives are already available. Even if we obtain coverage for a given drug product, the associated reimbursement rate may not be adequate to cover our costs, including research, development, intellectual property, manufacture, sale and distribution expenses, or may require co-payments that patients find unacceptably high. Patients are unlikely to use our products unless reimbursement is adequate to cover all or a significant portion of the cost of our drug products.
Coverage and reimbursement policies for drug products can differ significantly from payor to payor as there is no uniform policy of coverage and reimbursement for drug products among third-party payors in the United States. There may be significant delays in obtaining coverage and reimbursement as the process of determining coverage and reimbursement is often time-consuming and costly, which will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage or adequate reimbursement will be obtained. It is difficult to predict at this time what government authorities and third-party payors will decide with respect to coverage and reimbursement for our drug products.
The market for our product candidates will depend significantly on access to third-party payors’ drug formularies, or lists of medications for which third-party payors provide coverage and reimbursement. Competition to be included in such formularies often leads to downward pricing pressures. In particular, third-party payors may refuse to include a particular drug in their formularies or otherwise restrict patient access to a drug when a less costly generic equivalent or other alternative is available. In particular, given that several therapeutically similar drug products to LIQ861, including inhaled, oral and parenteral prostacyclins, are available on the market, managed care organizations may minimize the utilization of a new to market product and accordingly, we expect that LIQ861, if and when approved, will operate in a highly cost-constrained environment. Similarly, as there are a number of generic and branded therapeutic alternatives to LIQ865 in the post-operative pain market, there is a significant risk that LIQ865 may not be placed on the formularies of key institutions and/or receive favorable reimbursement, if and when approved.
The U.S. government, state legislatures and foreign governmental entities have shown significant interest in implementing cost containment programs to limit the growth of government-paid healthcare costs, including price controls, restrictions on reimbursement and coverage and requirements for substitution of generic products for branded prescription drugs. Adoption of government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could exclude or limit our drug products from coverage and limit payments for pharmaceuticals.
In addition, we expect that the increased emphasis on managed care and cost containment measures in the United States by third-party payors and government authorities will continue and will place pressure on pharmaceutical pricing and coverage. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more drug products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
If we are unable to obtain and maintain sufficient third-party coverage and adequate reimbursement for our drug products, the commercial success of our drug products may be greatly hindered and our financial condition and results of operations may be materially and adversely affected.
Our products may not achieve market acceptance.
We are currently focused on developing drug products that can be approved under abbreviated regulatory pathways in the United States, such as the 505(b)(2) regulatory pathway, which allows us to rely on existing knowledge of the safety and efficacy of the relevant reference listed drugs to support our applications for approval in the United States. While we believe that it will be less difficult for us to convince physicians, patients and other members of the medical community to accept and use our drug products as compared to entirely new drugs, our drug products may nonetheless fail to gain sufficient market acceptance by physicians, patients, other healthcare providers and third-party payors. If any of our drug products fail to achieve sufficient market acceptance, we may not be able to generate sufficient revenue to become profitable. The degree of market acceptance of our drug products, if and when they are approved for commercial sale, will depend on a number of factors, including but not limited to:
|
● |
the timing of our receipt of marketing approvals, the terms of such approvals and the countries in which such approvals are obtained; |
|
● |
the safety, efficacy, reliability and ease of administration of our drug products; |
|
● |
the prevalence and severity of undesirable side effects and adverse events; |
|
● |
the extent of the limitations or warnings required by the FDA or comparable regulatory authorities in other countries to be contained in the labeling of our drug products; |
|
● |
the clinical indications for which our drug products are approved; |
|
● |
the availability and perceived advantages of alternative therapies; |
|
● |
any publicity related to our drug products or those of our competitors; |
|
● |
the quality and price of competing drug products; |
|
● |
our ability to obtain third-party payor coverage and sufficient reimbursement; |
|
● |
the willingness of patients to pay out of pocket in the absence of third-party payor coverage; and |
|
● |
the selling efforts and commitment of our commercialization collaborators. |
If our drug products, if and when approved, fail to receive a sufficient level of market acceptance, our ability to generate revenue from sales of our drug products will be limited, and our business and results of operations may be materially and adversely affected.
The pharmaceutical industry is subject to rapid technological change, which could affect the commercial viability of our products.
The pharmaceutical industry is subject to rapid and significant technological change. Research, discoveries or inventions by others may result in medical insights or breakthroughs that render our products less competitive or even obsolete. Furthermore, there may be breakthroughs of new pharmaceutical technologies which may become superior to our PRINT technology that may result in the loss of our commercial advantage. Our future success will depend in part upon our ability to, among others:
|
● |
develop or license new technologies that address the changing needs of the medical community; and |
|
● |
respond to technological advances and changing industry standards and practices in a cost-effective and timely manner. |
Developing technology entails significant technical and business risks and substantial costs. We cannot assure you that we will be able to utilize new technologies effectively or that we will be able to adapt our existing technologies to changing industry standards in a timely or cost-effective manner, or at all. If we are unable to keep up with advancements in technology, our competitive position may suffer and our business and prospects may be materially and adversely affected.
Disruptions at the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including global pandemics, natural disasters, geopolitical actions, government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the FDA have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, in December 2019, a novel strain of COVID-19, or coronavirus, was reported to have surfaced in Wuhan, China and has become a global pandemic as of the date of this Annual Report on Form 10-K. The full impact of the coronavirus is unknown and rapidly evolving. Additionally, over the last several years, including from December 22, 2018 until January 25, 2019, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government disruption occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, prolonged government disruptions, global pandemics and other natural disasters or geopolitical actions could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Our products may be subject to reduced prices negotiated by certain group purchasing organizations that could adversely impact our product revenue.
Our customers may organize with each other or with third parties, such as distributors, manufacturers or hospitals, to negotiate prices that are lower than we may have been able to obtain from each of them individually. In such event, our ability to generate product revenue, and consequently our results of operations, may be materially and adversely affected.
We may not be able to build our marketing and sales capabilities or enter into agreements with third parties to market and sell our drug products.
In order to market and sell any of our drug products, if and when approved, we will be required to build our marketing and sales capabilities. We cannot assure you that we will be successful in doing so or be able to do so in a cost-effective manner. In addition, we may enter into collaboration arrangements with third parties to market our drug products. We may face significant competition for collaborators. In addition, collaboration arrangements may be time-consuming to negotiate and document. We cannot assure you that we will be able to negotiate collaborations for the marketing and sales of our drug products on acceptable terms, or at all. Even if we do enter into such collaborations, we cannot assure you that our collaborators will be successful in commercializing our products. If we or our collaborators are unable to successfully commercialize our drug products, whether in the United States or elsewhere, our business and results of operations may be materially and adversely affected.
If the FDA or comparable regulatory authorities in other countries approve generic versions of our product candidates, or do not grant our product candidates a sufficient period of market exclusivity before approving their generic versions, our ability to generate revenue may be adversely affected.
Once an NDA is approved, the drug product covered will be listed as a reference listed drug in the FDA’s Orange Book. In the United States, manufacturers of drug products may seek approval of generic versions of reference listed drugs through the submission of abbreviated new drug applications, or ANDAs. In support of an ANDA, a generic manufacturer is generally required to show that its product has the same active pharmaceutical ingredient(s), dosage form, strength, route of administration and conditions of use or labeling as the reference listed drug and that the generic version is bioequivalent to the reference listed drug. Generic drug products may be significantly less expensive to bring to market than the reference listed drug, and companies that produce generic drug products are generally able to offer them at lower prices. Thus, following the introduction of a generic drug product, a significant percentage of the sales of any reference listed drug may be lost to the generic drug product.
The FDA will not approve an ANDA for a generic drug product until the applicable period of market exclusivity for the reference listed drug has expired. The applicable period of market exclusivity varies depending on the type of exclusivity granted. A grant of market exclusivity is separate from the existence of patent protection and manufacturers may seek to launch generic versions of our drug products following the expiry of their respective marketing exclusivity periods, even if our drug products are still under patent protection at the relevant time.
Any competition that our product candidates may face, if and when such product candidates are approved for marketing and commercialized, from generic versions could substantially limit our ability to realize a return on our investment in the development of our product candidates and have a material and adverse effect on our business and prospects.
The off-label use or misuse of our products may harm our image in the marketplace, result in injuries that lead to costly product liability suits, or result in costly investigations and regulatory agency sanctions under certain circumstances if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
We are developing LIQ861 for the treatment of PAH and LIQ865 for the treatment of local post-operative pain. If our product candidates receive marketing approval from the FDA for these specific indications, we may only promote or market our product candidates for their specifically approved indications and make promotional claims consistent with the FDA-required product labeling. We will train our marketing and sales force against promoting our product candidates for “off-label uses” that would be inconsistent with FDA law and guidance. With respect to whether communications are consistent with the FDA-required product labeling, we cannot predict whether the FDA will agree with our assessment. We also cannot prevent a physician from using our products off-label, when in the physician’s independent professional medical judgment he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use our products for uses for which they are not approved. Furthermore, the use of our products for indications other than those approved by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
If the FDA determines that our promotional materials or training constitute promotion of an off-label or other improper use, it could request that we modify our training or promotional materials, or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil or administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, mandatory compliance programs under corporate integrity agreements, debarment, refusal of government contracts, and the curtailment of our operations.
These regulations or codes may limit our ability to effectively market our products, or we could run afoul of the requirements imposed by these regulations, causing reputational harm. These regulations or codes may also impose potentially substantial costs on us.
We may not be able to respond effectively to changing consumer preferences and demand.
Our success depends, in part, on our ability to anticipate and respond to changing consumer trends and preferences in the pharmaceutical industry. We may not be able to respond to these changes in a timely or commercially effective manner or at all. Our failure to accurately predict these trends could negatively impact our inventory levels, sales and reputation. The commercial success of our drug products will depend upon a number of factors, including our ability to, among others:
|
● |
anticipate consumers’ therapeutic needs; |
|
● |
innovate, develop and commercialize new drug products in a timely manner; |
|
● |
competitively price our drug products; |
|
● |
procure and maintain our drug products in sufficient volumes and in a timely manner; and |
|
● |
differentiate our drug products from those of our competitors. |
If we are unable to introduce new drug products, develop improvements to our existing drug products or maintain the appropriate inventory levels to meet our customers’ demand in a timely manner or at all, our business and prospects could be materially and adversely affected.
We may be exposed to claims and may not be able to obtain or maintain adequate product liability insurance.
Our business is exposed to the risk of product liability and other liability risks that are inherent in the development, manufacture, clinical testing and marketing of pharmaceutical products. These risks exist even if a product is approved for commercial sale by the FDA or comparable regulatory authorities in other countries and manufactured in licensed facilities. Our current product candidates, LIQ861 and LIQ865, are designed to affect important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our products could result in injury to a patient or even death.
Claims that are successfully brought against us could have a material and adverse effect on our financial condition and results of operations. Further, even if we are successful in defending claims brought against us, our reputation could suffer. Regardless of merit or eventual outcome, product liability claims may also result in, among others:
|
● |
a decreased demand for our products; |
|
● |
a withdrawal or recall of our products from the market; |
|
● |
a withdrawal of participants from our ongoing clinical trials; |
|
● |
the distraction of our management’s attention from our core business activities to defend such claims; |
|
● |
additional costs to us; and |
|
● |
a loss of revenue. |
Our insurance may not provide adequate coverage against our potential liabilities. Furthermore, we, our collaborators or our licensees may not be able to obtain or maintain insurance on acceptable terms, or at all. In addition, our collaborators or licensees may not be willing to indemnify us against these types of liabilities and may not themselves be sufficiently insured or have sufficient assets to satisfy any product liability claims. To the extent that they are uninsured or uninsurable, claims or losses that may be suffered by us, our collaborators or our licensees may have a material and adverse effect on our financial condition and results of operations.
If our product candidates are approved for commercialization outside of the United States, we may be exposed to a number of risks associated with international business operations.
If our product candidates are approved for commercialization outside of the United States, we may market our drug products ourselves, or we may enter into agreements with third parties to market the aforesaid drug products outside of the United States. In such event, we may be subject to risks related to international business operations, including, but not limited to:
|
● |
varying levels of protection for intellectual property rights; |
|
● |
changes in tariffs and the imposition of trade barriers; |
|
● |
economic weakness, including inflation or political instability in particular foreign economies and markets; |
|
● |
differing payor reimbursement regimes, governmental payors or patient self-pay systems and price controls; |
|
● |
compliance with tax, employment, immigration and labor laws in respect of employees living or traveling abroad; |
|
● |
foreign tax laws; |
|
● |
currency fluctuations; and |
|
● |
business interruptions resulting from geopolitical actions, such as wars and terrorist attacks, among others, or global pandemics or natural disasters, such as fires, floods, earthquakes and hurricanes, among others. |
Risks Related to the Development and Regulatory Approval of our Product Candidates
We are primarily dependent on the success of our lead product candidate, LIQ861, for which we have recently filed an NDA with the FDA, and to a lesser degree, LIQ865, which is still in clinical development, and these product candidates may fail to receive marketing approval or may not be commercialized successfully.
We do not have any products approved for marketing in any jurisdiction and we have never generated any revenue from product sales. Our ability to generate revenue from product sales and achieve profitability depends on our ability, alone or with strategic collaboration partners, to successfully complete the development of, and obtain the regulatory and marketing approvals necessary to commercialize, one or more of our product candidates. We expect that a substantial portion of our efforts and expenditure over the next few years will be devoted to our product candidates, LIQ861, a proprietary inhaled dry powder formulation of treprostinil for the treatment of pulmonary arterial hypertension, or PAH, and LIQ865, a sustained-release formulation of bupivacaine for the management of local post-operative pain. We do not anticipate generating revenue from product sales until 2021 at the earliest, if ever.
LIQ861 is being developed under the 505(b)(2) regulatory pathway with Tyvaso as the reference listed drug. We commenced a Phase 3 clinical trial of LIQ861, which we refer to as INSPIRE, in the first quarter of 2018 and reported completion of enrollment and achievement of the primary endpoint, which was long-term safety and tolerability, in the first quarter of 2019. LIQ861 was observed to be well-tolerated in 109 patients, with 101 patients (93%) completing at least two months of treatment. During the two-month treatment period, LIQ861 was evaluated at doses up to 159 mcg with no study-drug related serious adverse events, with some patients receiving doses up to 212 mcg after the two-month timepoint. Exploratory endpoints of the INSPIRE trial demonstrated generally favorable functional and patient outcomes.
In June 2019, we reported results from the INSPIRE study indicating that the 79.5 mcg dose of LIQ861 correlates with nine breaths of Tyvaso, the maximum recommended label dose of Tyvaso. Analysis of the data from the PK sub-study in patients showed variability in systemic plasma levels of both LIQ861 and Tyvaso, which is believed to be attributed to variation in severity of disease and has been seen in prior studies of treprostinil in patients. To more accurately characterize the PK of LIQ861, we conducted two additional PK studies in healthy volunteers. In the first of these studies, we observed unexpected variability in PK levels. Post-hoc analysis showed that plasma levels of treprostinil were tightly correlated to the LIQ861 dose delivered. Based upon additional non-clinical and clinical work, we believe the unexpected variability seen in this healthy volunteer study was due to an administration technique unique to the conduct of the study in the Phase 1 setting. In August 2019, we completed a second PK study in healthy volunteers in which the proper administration technique was followed. This study demonstrated significantly reduced variability, and we believe we have established comparative bioavailability to the reference listed drug.
In August 2019, one of the clinical investigators in the INSPIRE study reassessed a serious adverse event, preliminarily identified as hypersensitivity pneumonitis, as being possibly related to LIQ861, whereas the investigator had previously, in May and June 2019, characterized the event as not related to LIQ861. Based on the patient’s medical history, two other potential alternative causes of this event noted by the clinical investigator, and the fact that the patient has been taking LIQ861 since October 2018, we do not agree with the clinical investigator’s assessment. However, we reported the event to the FDA, as required, and we will continue to monitor and assess this event for any change.
We completed the pivotal INSPIRE trial in October 2019 and submitted an NDA for LIQ861 to the FDA in January 2020. Final enrollment in the trial included 121 PAH patients to assess safety and tolerability through Month 2, the primary endpoint of the trial. Of the 121 patients enrolled in the study, 55 were Transition patients and 66 were Add-On patients. Add-On patients started on a dose of 26.5 mcg of LIQ861, with most (>80%) titrating to a 79.5 mcg dose or higher within the first two months of treatment. Consistent with preliminary data presented in the second quarter of 2019, LIQ861 was observed to be well-tolerated and treatment-emergent adverse events were mostly mild to moderate in nature at Month 2 up to doses of 159 mcg of LIQ861, the highest dose studied at Month 2. Durability of therapy with LIQ861 appeared to be favorable, with 96% of Transition patients and 91% of Add-On patients remaining on study drug at the Month 2 timepoint.
Initial analysis of the exploratory endpoints from the INSPIRE study indicates that LIQ861 may provide functional and quality-of-life benefits to PAH patients in New York Heart Association, or NYHA, functional classes II and III. More than 90% of all patients who completed two months of treatment maintained or improved their NYHA functional class. Additionally, we observed improvement in six-minute-walk-distance and quality of life as measured by the MLHFQ in both patient groups.
We continued to treat patients who chose to remain on LIQ861 beyond the Month 2 timepoint of the primary endpoint. More than 80% of INSPIRE patients remained on study drug at Month 4 with no significant changes in safety or tolerability observed compared to Month 2. At the completion of the INSPIRE study, the patient with the longest duration of treatment had been on LIQ861 therapy for 18 months. To provide for continuity of treatment, patients from INSPIRE were provided the opportunity to continue receiving treatment in an extension study, which is currently ongoing. In addition, we are enrolling patients in a clinical study at certain investigational sites in Europe to characterize the hemodynamic dose-response relationship to LIQ861. We are also considering conducting other clinical trials to generate additional data on LIQ861, including a clinical trial in pediatric patients. We also continue to conduct development work in support of potential approval and commercialization of LIQ861, including label and patient-use assessments.
With respect to LIQ865, we initiated Phase 2-enabling toxicology studies in March 2019 in both soft tissue and bone models. The soft tissue toxicology study showed favorable results. However, our bone toxicology study showed delayed bone healing at the dose tested and we therefore have recently initiated an additional non-GLP bone toxicology study at lower doses of LIQ865, with results expected during the second half of 2020. Provided that results of this study are favorable, we may then initiate a GLP bone toxicology study. We may also consider developing one or more alternative formulations of LIQ865. Depending upon the results of the further bone toxicology studies and the availability of funding, among other considerations, we may initiate one or more Phase 2 proof-of-concept clinical trials during 2021. We cannot assure you that our toxicology studies or clinical trials, if commenced, will be successful or meet their endpoints, or that the endpoints for any future Phase 3 trials that we may conduct will be sufficient to receive marketing approval.
If we successfully complete the clinical development of LIQ861 and LIQ865, we cannot assure you that they will receive marketing approval. The FDA or comparable regulatory authorities in other countries may delay, limit or deny approval of our product candidates for various reasons. For example, such authorities may disagree with the design, scope or implementation of our clinical trials, or with our interpretation of data from our preclinical studies or clinical trials. Further, there are numerous FDA personnel assigned to review different aspects of an NDA, and uncertainties can be presented by their ability to exercise judgment and discretion during the review process. During the course of review, the FDA may request or require additional preclinical, clinical, CMC (chemistry, manufacturing, and control), or other data and information, and the development and information may be time-consuming and expensive. Status as a combination product, as is the case for LIQ861, may complicate or delay the FDA review process. Product candidates that the FDA deems to be combination products, such as LIQ861, or that otherwise rely on innovative drug delivery systems, may face additional challenges, risks and delays in the product development and regulatory approval process. Moreover, the applicable requirements for approval may differ from country to country.
If we successfully obtain marketing approval for LIQ861 and LIQ865, we cannot assure you that they will be commercialized in a timely manner or successfully, or at all. For example, LIQ861 and LIQ865 may not achieve a sufficient level of market acceptance, or we may not be able to effectively build our marketing and sales capabilities or scale our manufacturing operations to meet commercial demand. The successful commercialization of LIQ861 and LIQ865 will also, in part, depend on factors that are beyond our control. Therefore, we may not generate significant revenue from the sale of such products, even if approved. Any delay or setback we face in the commercialization of LIQ861 or LIQ865 may have a material and adverse effect on our business and prospects, which will adversely affect your investment in our company.
Our preclinical studies and clinical trials may not be successful and delays in such preclinical studies or clinical trials may cause our costs to increase and significantly impair our ability to commercialize our product candidates. Results of previous clinical trials or interim results of ongoing clinical trials may not be predictive of future results.
Before we are able to commercialize our drug products, we are required to undertake extensive preclinical studies and clinical trials to demonstrate that our drug products are safe and effective for their intended uses. However, we cannot assure you that our drug products will, in preclinical studies and clinical trials, demonstrate safety and efficacy as necessary to obtain marketing approval. Due to the nature of drug product development, many product candidates, especially those in early stages of development, may be terminated during development. Although we believe we have completed clinical development for LIQ861, we have not yet obtained approval for or commercialized any product candidates and as a result do not have a track record of successfully bringing product candidates to market. Furthermore, LIQ861 and LIQ865 have, to date, been tested only in relatively small study populations and, accordingly, the results from our earlier clinical trials may be less reliable than results achieved in larger clinical trials. Additionally, the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and preliminary and interim results of a clinical trial do not necessarily predict final results.
Preclinical studies and clinical trials may fail due to factors such as flaws in trial design, dose selection and patient enrollment criteria. The results of preclinical studies and early clinical trials may not be indicative of the results of subsequent clinical trials. Product candidates may, in later stages of clinical testing, fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and earlier clinical trials. Moreover, there may be significant variability in safety or efficacy results between different trials of the same product candidate due to factors including, but not limited to, changes in trial protocols, differences in the composition of the patient population, adherence to the dosing regimen and other trial protocols and amendments to protocols and the rate of drop-out among patients in a clinical trial. If our preclinical studies or clinical trials are not successful and we are unable to bring our product candidates to market as a result, our business and prospects may be materially and adversely affected.
Furthermore, conducting preclinical studies and clinical trials is a costly and time-consuming process. The length of time required to conduct the required studies and trials may vary substantially according to the type, complexity, novelty and intended use of the product candidate. A single clinical trial may take up to several years to complete. Moreover, our preclinical studies and clinical trials may be delayed or halted due to various factors, including, among others:
|
● |
delays in raising the funding necessary to initiate or continue a clinical trial; |
|
● |
delays in manufacturing sufficient quantities of product candidates for clinical trials; |
|
● |
delays in reaching agreement on acceptable terms with prospective contract research organizations and clinical trial sites; |
|
● |
delays in obtaining institutional review board approval at clinical trial sites; |
|
● |
delays in recruiting suitable patients to participate in a clinical trial; |
|
● |
delays in patients’ completion of clinical trials or their post-treatment follow-up; |
|
● |
regulatory authorities’ interpretation of our preclinical and clinical data; and |
|
● |
unforeseen safety issues, including a high and unacceptable severity, or prevalence, of undesirable side effects or adverse events caused by our product candidates or similar drug products or product candidates. |
If our preclinical studies or clinical trials are delayed, the commercialization of our product candidates will be delayed and, as a result, we may incur substantial additional costs or not be able to recoup our investment in the development of our product candidates, which would have a material and adverse effect on our business.
LIQ861, for which we recently submitted an NDA, requires regulatory review, and, subject to feedback from the FDA, may require additional clinical testing and data analysis. LIQ865, for which we have only completed a single Phase 1 study, requires additional clinical testing, data analysis, and regulatory review. Clinical trials and data analysis can be expensive, time-consuming and difficult to design and implement. If we are unsuccessful in obtaining regulatory approval for LIQ861 or LIQ865, or any of our product candidates do not provide positive results, we may be required to delay or abandon development of such product, which would have a material adverse impact on our business.
Continuing product development requires additional and extensive clinical testing. Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is also time-consuming. We cannot provide any assurance or certainty regarding when we might receive regulatory approval for LIQ861 or LIQ865. Furthermore, failure can occur at any stage of the process, and we could encounter problems that cause us to abandon an NDA filed with the FDA or repeat clinical trials. The commencement and completion of clinical trials for any current or future development product candidate may be delayed by several factors, including:
|
● |
unforeseen safety issues; |
|
● |
determination of dosing issues; |
|
● |
lack of effectiveness during clinical trials; |
|
● |
slower than expected rates of patient recruitment; |
|
● |
inability to monitor patients adequately during or after treatment; and |
|
● |
inability or unwillingness of medical investigators to follow our clinical protocols or amendments to our protocols. |
In addition, the FDA or an independent institutional review board, or IRB, may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the FDA finds deficiencies in our IND submissions or the conduct of these trials. Therefore, we cannot provide any assurance or predict with certainty the schedule for future clinical trials. In the event we do not ultimately receive regulatory approval for LIQ861 and LIQ865, we may be required to terminate development of our only product candidates.
The marketing approval processes of the FDA and comparable regulatory authorities in other countries are unpredictable and our product candidates may be subject to multiple rounds of review or may not receive marketing approval.
We have not previously submitted an NDA to the FDA or similar drug approval filings to comparable regulatory authorities in other countries for any product candidate, and we cannot assure you that any of our product candidates will receive marketing approval. Filing an application and obtaining marketing approval for a pharmaceutical product candidate is an extensive, lengthy, expensive and inherently uncertain process, and regulatory authorities may delay, limit or deny approval of our product candidates for many reasons, including, but not limited to, the following:
|
● |
the FDA or comparable regulatory authorities in other countries may refuse to file an NDA or similar drug approval filing if they deem the application to be incomplete; |
|
● |
the FDA or comparable regulatory authorities in other countries may disagree with the number, design, size, conduct or statistical analysis of one or more of our clinical trials; |
|
● |
we may be unable to demonstrate to the satisfaction of the FDA or comparable regulatory authorities in other countries that our product candidate is safe and effective for its proposed indication, or that its clinical and other benefits outweigh its safety risks; |
|
● |
the results of our clinical trials may not meet the level of statistical significance required by the FDA or comparable regulatory authorities in other countries; |
|
● |
the data collected from our clinical trials may not be sufficient to support the submission of an NDA or similar drug approval filing to the FDA or comparable regulatory authorities in other countries; |
|
● |
the FDA or comparable regulatory authorities in other countries may disagree with our interpretation of data from our preclinical studies or clinical trials; |
|
● |
our manufacturing processes and facilities have not been inspected by the FDA, and the FDA or comparable regulatory authorities in other countries may not ultimately conclude that our manufacturing processes or facilities or those of our third-party manufacturers sufficiently demonstrate compliance with cGMP to support NDA approval; |
|
● |
our product candidates may not meet the level of quality and control required by the FDA or comparable regulatory authorities in other countries; |
|
● |
our product candidates may not demonstrate sufficient long-term stability to support an NDA filing or obtain approval, or the product shelf life may be limited by stability results; |
|
● |
the FDA or comparable regulatory authorities in other countries may require development of a costly and extensive risk evaluation and mitigation strategy, or REMS, as a condition of approval; |
|
● |
the success or further approval of competing products approved in indications similar to those of our product candidates may change the standards for approval of our product candidates in their proposed indications; and |
|
● |
the approval policies of the FDA or comparable regulatory authorities in other countries may change in a manner that renders our clinical data insufficient for approval. |
In addition, the FDA or comparable regulatory authorities in other countries may, in their sole discretion, change their views in respect of regulatory pathways they had previously affirmed or clinical trial protocols to which they were previously not opposed. While we have consulted with the FDA on the appropriate regulatory pathway and clinical trial protocols for our product candidates, LIQ861 and LIQ865, we cannot assure you that the FDA will not revise its position significantly at a later date. In the event that this occurs, the clinical development and commercialization of our product candidates may be delayed or even derailed.
Even if we obtain marketing approval, the FDA or comparable regulatory authorities in other countries may approve our product candidates for fewer or more limited indications than those for which we requested approval, or may include safety warnings or other restrictions that may negatively impact the commercial viability of our product candidates. Likewise, regulatory authorities may grant approval contingent on the performance of costly post-marketing clinical trials or the conduct of an expensive REMS, which could significantly reduce the potential for commercial success or viability of our product candidates. We also may not be able to find acceptable collaborators to manufacture our drug products, if and when approved, in commercial quantities and at acceptable prices, or at all.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial potential or result in significant negative consequences following any potential marketing approval.
If our product candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon our development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Any serious adverse or undesirable side effects identified during the development of our product candidates could interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications, and in turn prevent us from commercializing our product candidates and generating revenues from their sale. In addition, if any of our product candidates receive regulatory approval and we or others later identify undesirable adverse effects caused by the product, we could face one or more of the following consequences:
|
● |
regulatory authorities may require the addition of labeling statements, such as a boxed warning or a contraindication, or other safety labeling changes; |
|
● |
regulatory authorities may require a REMS; |
|
● |
regulatory authorities may withdraw their approval of the product; |
|
● |
regulatory authorities may seize the product; |
|
● |
we may be required to change the way that the product is administered; |
|
● |
we may be required to conduct additional clinical trials; |
|
● |
we may be required to recall the product; |
|
● |
we may be subject to litigation or product liability claims, fines, injunctions or criminal penalties; and |
|
● |
our reputation may suffer. |
We may encounter difficulties in enrolling patients in our clinical trials.
We may not be able to commence or complete clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials.
Patient enrollment may be affected by, among others:
|
● |
the severity of the disease under investigation; |
|
● |
the design of the clinical trial protocol and amendments to a protocol; |
|
● |
the size and nature of the patient population; |
|
● |
eligibility criteria for the clinical trial in question; |
|
● |
the perceived risks and benefits of the product candidate under clinical testing, including a high and unacceptable severity, or prevalence, of undesirable side effects or adverse events caused by our product candidates or similar products or product candidates; |
|
● |
the existing body of safety and efficacy data in respect of the product candidate under clinical testing; |
|
● |
the proximity of patients to clinical trial sites; and |
|
● |
the number and nature of competing therapies and clinical trials. |
Any negative results we may report in clinical trials of our product candidates may also make it difficult or impossible to recruit and retain patients in other clinical trials of that same product candidate.
In particular, we will be required to identify and enroll a sufficient number of patients with PAH for our clinical trials and studies of LIQ861. PAH is a rare disease with a relatively small patient population, and our enrollment of clinical trial participants may be slow as a result. Additionally, we expect that if we initiate, as we are currently contemplating, a clinical trial of LIQ861 in pediatric patients, we may encounter difficulties enrolling patients in such a trial because of the limited number of pediatric patients with this disease. Furthermore, we are aware of a number of therapies for PAH that are being developed or that are already available on the market, and we expect to face competition from these investigational drugs or approved drugs for potential subjects in our clinical trials, which may delay enrollment in our planned clinical trials.
Delays or failures in planned patient enrollment or retention may result in increased costs, program delays, or both. We may, as a result of such delays or failures, be unable to carry out our clinical trials as planned or within the timeframe that we expect or at all, and our business and prospects may be materially and adversely affected as a result.
Product candidates that the FDA deems to be combination products, such as LIQ861, or that otherwise rely on innovative drug delivery systems, may face additional challenges, risks and delays in the product development and regulatory approval process.
The FDA has indicated that it considers LIQ861, which is delivered by a DPI, to be a drug-device combination product and, accordingly, the DPI will be evaluated as part of our NDA filing. When evaluating products that utilize a specific drug delivery system or device, the FDA will evaluate the characteristics of that delivery system and its functionality, as well as the potential for undesirable interactions between the drug and the delivery system, including the potential to negatively impact the safety or effectiveness of the drug. The FDA review process can be more complicated for combination products, and may result in delays, particularly if novel delivery systems are involved. We rely on third parties for the design and manufacture of the delivery systems for our products, including the DPI for LIQ861, and in some cases for the right to refer to their data on file with the FDA or other regulators. Quality or design concerns with the delivery system, or commercial disputes with these third parties, could delay or prevent regulatory approval and commercialization of our product candidates.
We are planning to pursue the FDA 505(b)(2) pathway for all of our current product candidates. If we are unable to rely on the 505(b)(2) regulatory pathway to apply for marketing approval of our product candidates in the United States, seeking approval of these product candidates through the 505(b)(1) NDA pathway would require full reports of investigations of safety and effectiveness, and the process of obtaining marketing approval for our product candidates would likely be significantly longer and more costly.
We are currently focused on developing drug products that can be approved under abbreviated regulatory pathways in the United States, such as the 505(b)(2) regulatory pathway, which permits the filing of an NDA where at least some of the information required for approval comes from studies that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Section 505(b)(2), if applicable to us for a particular product candidate, would allow an NDA we submit to the FDA to rely in part on data in the public domain or the FDA’s prior conclusions regarding the safety and effectiveness of approved compounds, which could expedite the development program for a product candidate by potentially decreasing the amount of clinical data that we would need to generate in order to obtain FDA approval. We plan to pursue this pathway for our current product candidates, LIQ861 and LIQ865, and have submitted a 505(b)(2) NDA for LIQ861. Even if the FDA allows us to rely on the 505(b)(2) regulatory pathway, we cannot assure you that such marketing approval will be obtained in a timely manner, or at all.
The FDA may require us to perform additional clinical trials to support any change from the reference listed drug, which could be time-consuming and substantially delay our receipt of marketing approval. Also, as has been the experience of others in our industry, our competitors may file citizens’ petitions with the FDA to contest approval of our NDA, which may delay or even prevent the FDA from approving any NDA that we submit under the 505(b)(2) regulatory pathway. If an FDA decision or action relative to our product candidate, or the FDA’s interpretation of Section 505(b)(2) more generally, is successfully challenged, it could result in delays or even prevent the FDA from approving a 505(b)(2) application for our product candidates. Even if we are able to utilize the 505(b)(2) regulatory pathway, a drug approved via this pathway may be subject to the same post-approval limitations, conditions and requirements as any other drug.
In addition, we may face patent infringement lawsuits in relation to our NDAs submitted under the 505(b)(2) regulatory pathway, which may further delay or prevent the review or approval of our product candidates. The pharmaceutical industry is highly competitive, and 505(b)(2) NDAs are subject to special requirements designed to protect the patent rights of sponsors of previously approved drugs that are referenced in a 505(b)(2) NDA. If the previously approved drugs referenced in an applicant’s 505(b)(2) NDA are protected by patent(s) listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations publication, or the Orange Book, the 505(b)(2) applicant is required to make a claim after filing their its NDA that each such patent is invalid, unenforceable or will not be infringed. The patent holder may thereafter bring suit for patent infringement, which will trigger a mandatory 30-month delay (or the shorter of dismissal of the lawsuit or expiration of the patent(s)) in approval of the 505(b)(2) NDA application. It is not uncommon for a manufacturer of an approved product to file a citizen petition with the FDA seeking to delay approval of, or impose additional approval requirements for, pending competing products. If successful, such petitions can significantly delay, or even prevent, the approval of the new product. However, even if the FDA ultimately denies such a petition, the FDA may substantially delay approval while it considers and responds to the petition.
If the FDA determines that our product candidates do not qualify for the 505(b)(2) regulatory pathway, we would need to reconsider our plans and might not be able to commercialize our product candidates in a cost-efficient manner, or at all. If we were to pursue approval under the 505(b)(1) NDA pathway, we would be subject to more extensive requirements and risks such as conducting additional clinical trials, providing additional data and information or meeting additional standards for marketing approval. As a result, the time and financial resources required to obtain marketing approval for our product candidates would likely increase substantially and further complications and risks associated with our product candidates may arise. Also, new competing products may reach the market faster than ours, which may materially and adversely affect our competitive position, business and prospects.
We may be unable to continually develop a pipeline of product candidates, which could affect our business and prospects.
A key element of our long-term strategy is to continually develop a pipeline of product candidates by developing proprietary innovations to FDA-approved drug products using our PRINT technology. If we are unable to identify off-patent drug products for which we can develop proprietary innovations using our PRINT technology or otherwise expand our product candidate pipeline, whether through licensed or co-development opportunities, and obtain marketing approval for such product candidates within the timeframes that we anticipate, or at all, our business and prospects may be materially and adversely affected.
We have conducted, and may in the future conduct, clinical trials for our product candidates outside the United States and the FDA may not accept data from such trials.
Although the FDA may accept data from clinical trials conducted outside the United States in support of safety and efficacy claims for our product candidates, if not conducted under an IND, this is subject to certain conditions set out in 21 C.F.R. § 312.120. For example, in order for the FDA to accept data from such a foreign clinical trial, the study must have been conducted in accordance with Good Clinical Practice, or GCP, including review and approval by an independent ethics committee and obtaining the informed consent from subjects of the clinical trials. The FDA must also be able to validate the data from the study through an onsite inspection if the agency deems it necessary. In addition, foreign clinical data submitted to support FDA applications should be applicable to the U.S. population and U.S. medical practice. Other factors that may affect the acceptance of foreign clinical data include differences in clinical conditions, study populations or regulatory requirements between the United States and the foreign country.
We conducted the early Phase 1a clinical trial of LIQ865 in Denmark, and not under an IND, we are currently conducting an additional clinical trial in Europe that explores the hemodynamic effects of LIQ861 in PAH patients, and we may, in the future, conduct clinical trials of our product candidates outside the United States. The FDA may not accept such foreign clinical data, and in such event, we may be required to re-conduct relevant clinical trials within the United States, which would be costly and time-consuming, and which could have a material and adverse effect on our ability to carry out our business plans.
Even if we obtain marketing approval for our product candidates in the United States, we or our collaborators may not obtain marketing approval for the same product candidates elsewhere.
We may enter into strategic collaboration arrangements with third parties to commercialize our product candidates outside of the United States. In order to market any product candidate outside of the United States, we or our collaborators will be required to comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Clinical trials conducted in one country may not be recognized or accepted by regulatory authorities in other countries, and obtaining marketing approval in one country does not mean that marketing approval will be obtained in any other country. Approval processes vary among countries and additional product testing and validation, or additional administrative review periods, may be required from one country to the next.
Seeking marketing approval in countries other than the United States could be costly and time-consuming, especially if additional preclinical studies or clinical trials are required to be conducted. We currently do not have any product candidates approved for sale in any jurisdiction, including non-U.S. markets, and we do not have experience in obtaining marketing approval in non-U.S. markets. We currently also have not identified any collaborators to market our products outside of the United States and cannot assure you that such collaborators, even if identified, will be able to successfully obtain marketing approval for our product candidates outside of the United States. If we or our collaborators fail to obtain marketing approval in non-U.S. markets, or if such approval is delayed, our target market may be reduced, and our ability to realize the full market potential of our products will be adversely affected.
Risks Related to Our Dependence on Third Parties
We depend on third parties for clinical and commercial supplies, including single suppliers for the active ingredient, the device, encapsulation and packaging of LIQ861.
We depend on third-party suppliers for clinical and commercial supplies, including the active pharmaceutical ingredients which are used in our product candidates. These supplies may not always be available to us at the standards we require or on terms acceptable to us, or at all, and we may not be able to locate alternative suppliers in a timely manner, or at all. If we are unable to obtain necessary clinical or commercial supplies, our manufacturing operations and clinical trials and the clinical trials of our collaborators may be delayed or disrupted and our business and prospects may be materially and adversely affected as a result.
For example, we currently rely on a sole supplier for treprostinil, the active pharmaceutical ingredient of LIQ861, which sources treprostinil from a manufacturer in South Korea. If our supplier is unable to supply treprostinil to us in the quantities we require, or at all, or otherwise defaults on its supply obligations to us, or if it ceases its relationship with us, we may not be able to obtain alternative supplies of treprostinil from other suppliers on acceptable terms, in a timely manner, or at all. Furthermore, LIQ861 is administered using the RS00 Model 8 DPI, or dry powder inhaler, which is manufactured by Plastiape S.p.A., or Plastiape, which is located in Italy. We also rely on a sole supplier for encapsulation and packaging services. We purchase treprostinil, our DPI supply and encapsulation and packaging services pursuant to purchase orders and do not have long-term contracts with these suppliers. In the event of any prolonged disruption to our supply of treprostinil, the manufacture and supply of RS00 Model 8 DPI or encapsulation and packaging services, our ability to develop and commercialize, and the timeline for commercialization of, LIQ861 may be adversely affected.
Additionally, in December 2019, a novel strain of COVID-19, or coronavirus, was reported to have surfaced in Wuhan, China and has become a global pandemic as of the date of this Annual Report on Form 10-K. The full impact of the coronavirus is unknown and rapidly evolving. Both South Korea, the country from which our supplier sources treprostinil, and Italy, the country in which Plastiape is headquartered, have had significant outbreaks of this disease, which, in the case of Italy, has led to a lockdown of the entire country as of the date of this Annual Report on Form 10-K. The extent to which the coronavirus impacts our ability to procure sufficient supplies for the development and commercialization of our products and product candidates will depend on the severity, location and duration of the spread of the coronavirus, and the actions undertaken to contain the coronavirus or treat its effects.
We rely on third parties to conduct our preclinical studies and clinical trials.
We currently rely on, and plan to continue to rely on, third-party CROs to monitor and manage data for our preclinical studies and clinical trials. However, we are responsible for ensuring that each of our trials is conducted in accordance with the applicable regulatory standards and our reliance on CROs does not relieve us of our regulatory responsibilities.
The CROs on which we rely are required to comply with FDA regulations (and the regulations of comparable regulatory authorities in other countries) regarding GCP. Regulatory authorities enforce GCP standards through periodic inspections. If any of the CROs on which we rely fail to comply with the applicable GCP standards, the clinical data generated in our clinical trials may be deemed unreliable. While we have contractual agreements with these CROs, we have limited influence over their actual performance and cannot control whether or not they devote sufficient time and resources to our preclinical studies and clinical trials. A failure to comply with the applicable regulations in the conduct of the preclinical studies and clinical trials for our product candidates may require us to repeat such studies or trials, which would delay the process of obtaining marketing approval for our product candidates and have a material and adverse effect on our business and prospects.
Some of our CROs have the ability to terminate their respective agreements with us if, among others, it can be reasonably demonstrated that the safety of the patients participating in our clinical trials warrants such termination. If any of our agreements with our CROs is terminated, and if we are not able to enter into agreements with alternative CROs on acceptable terms or in a timely manner, or at all, the clinical development of our product candidates may be delayed and our development expenses could be increased.
If we are unable to establish or maintain licensing and collaboration arrangements with other pharmaceutical companies on acceptable terms, or at all, we may not be able to develop and commercialize additional product candidates using our PRINT technology.
We have collaborated, and may consider collaborating, with, among others, pharmaceutical companies to expand the applications for our PRINT technology through licensing as well as joint product development arrangements. In addition, if we are able to obtain marketing approval for our product candidates from regulatory authorities, we may enter into strategic relationships with collaborators for the commercialization of such products.
Collaboration and licensing arrangements are complex and time-consuming to negotiate, document, implement and maintain. We may not be successful in our efforts to establish collaboration or other alternative arrangements should we so choose to enter into such arrangements. In addition, the terms of any collaboration or other arrangements that we may enter into may not be favorable to us or may restrict our ability to enter into further collaboration or other arrangements with third parties. For example, collaboration agreements may contain exclusivity arrangements which limit our ability to work with other pharmaceutical companies to expand the applications for our PRINT technology, as is the case in our collaboration agreement with GSK.
If we are unable to establish licensing and collaboration arrangements or the terms of such agreements we enter into are unfavorable to us or restrict our ability to work with other pharmaceutical companies, we may not be able to expand the applications for our PRINT technology or commercialize our products, if and when approved, and our business and prospects may be materially and adversely affected.
Our collaboration and licensing arrangements may not be successful.
Our collaboration and licensing arrangements, as well as any future collaboration and licensing arrangements that we may enter into, may not be successful. The success of our collaboration and licensing arrangements will depend heavily on the efforts and activities of our collaborators, which are not within our control. We may, in the course of our collaboration and licensing arrangements, be subject to numerous risks, including, but not limited to, the following:
|
● |
our collaborators may have significant discretion in determining the efforts and resources that they will contribute; |
|
● |
our collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing. For example, in July 2018, GSK notified us of its decision to discontinue development of the inhaled antiviral for viral exacerbations in COPD, part of the GSK ICO Agreement, after completion of its related Phase 1 clinical trial and we do not believe that GSK is currently advancing any program under our collaboration; |
|
● |
our collaborators may independently, or in conjunction with others, develop products that compete directly or indirectly with our product candidates; |
|
● |
we may grant exclusive rights to our collaborators that would restrict us from collaborating with others. For example, we are currently subject to certain restrictions with regard to our ability to enter into collaboration arrangements for the development of inhaled therapeutics based upon our PRINT technology with third parties pursuant to our collaboration with GSK; |
|
● |
our collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; |
|
● |
disputes may arise between us and our collaborators, which may cause a delay in or the termination of our research, development or commercialization activities; |
|
● |
our collaboration and licensing arrangements may be terminated, and if terminated, may result in our need for additional capital to pursue further drug product development or commercialization. For example, our development and licensing agreement with G&W Laboratories, Inc., was mutually terminated in April 2018 and we are currently seeking the termination of our collaboration with GSK; |
|
● |
our collaborators may own or co-own certain intellectual property arising from our collaboration and licensing arrangements with them, which may restrict our ability to develop or commercialize such intellectual property; and |
|
● |
our collaborators may alter the strategic direction of their business or may undergo a change of control or management, which may affect the success of our collaboration arrangements with them. |
Risks Related to Legal Compliance Matters
Even if we obtain regulatory approval for a product candidate, our products and business will remain subject to ongoing regulatory obligations and review.
If our product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, drug supply chain security surveillance and tracking, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies and submission of safety, efficacy and other post-market information, including both federal and state requirements in the United States and comparable requirements outside of the United States. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control. Any regulatory approvals that we may receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a REMS as a condition of approval of our product candidates, which could include requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. We will also be required to report certain adverse reactions and production problems, if any, to the FDA or other regulatory agencies and to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we may not promote our products for indications or uses for which they do not have FDA or other regulatory agency approval. The holder of an approved NDA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling, or manufacturing process. We could also be asked to conduct post-marketing clinical studies to verify the safety and efficacy of our product candidates in general or in specific patient subsets. An unsuccessful post-marketing study or failure to complete such a clinical study could result in the withdrawal of marketing approval. Furthermore, any new legislation addressing drug safety issues could result in delays in product development or commercialization or increased costs to assure compliance. Foreign regulatory authorities impose similar requirements. If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or disagrees with the promotion, marketing or labeling of a product, such regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
|
● |
issue warning letters asserting that we are in violation of the law; |
|
● |
seek an injunction or impose civil or criminal penalties or monetary fines; |
|
● |
suspend or withdraw regulatory approval; |
|
● |
suspend any of our ongoing clinical trials; |
|
● |
refuse to approve pending applications or supplements to approved applications submitted by us or our strategic partners; |
|
● |
restrict the marketing or manufacturing of our products; |
|
● |
seize or detain products, or require a product recall; |
|
● |
refuse to permit the import or export of our product candidates; or |
|
● |
refuse to allow us to enter into government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our product candidates. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be adversely affected.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations.
The terms of approvals, ongoing regulations and post-marketing restrictions for our products may limit how we manufacture and market our products, which could materially impair our ability to generate revenue.
Once marketing approval has been granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling and regulatory requirements. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use, and if we do not restrict the marketing of our products only to their approved indications, we may be subject to enforcement action for off-label marketing.
The FDA applies a heightened level of scrutiny to comparative claims when applying its statutory standards for advertising and promotion, including with regard to its requirement that promotional labeling be truthful and not misleading. Any claim of effectiveness made in prescription drug promotion, including comparative effectiveness, must be supported by substantial evidence or substantial clinical experience.
In addition, making comparative claims may draw concerns from our competitors. Where a company makes a claim in advertising or promotion that its product is superior to the product of a competitor (or that the competitor’s product is inferior), this creates a risk of a lawsuit by the competitor under federal and state false advertising or unfair and deceptive trade practices law, and possibly also state libel law. Such a suit may seek injunctive relief against further advertising, a court order directing corrective advertising, and compensatory and punitive damages where permitted by law.
We, and any potential collaborators we may have in the future, must therefore comply with requirements concerning advertising and promotion for any of our products for which we or our collaborators obtain marketing approval. Thus, if either of our current product candidates receive marketing approval, the accompanying label may limit the approved use of our product, which could limit sales of the product.
In addition, manufacturers of approved products and those manufacturers’ facilities are required to comply with extensive FDA requirements, such as ensuring that quality control and manufacturing procedures conform to cGMP applicable to drug manufacturers, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, any contract manufacturers we may engage in the future, our future collaborators, licensees and their contract manufacturers will also be subject to other regulatory requirements, including submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements regarding the distribution of samples to clinicians, recordkeeping and costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product such as the requirement to implement a risk evaluation and mitigation strategy.
Our drug products may be subject to recalls, withdrawals, seizures or other enforcement actions by the FDA or comparable regulatory authorities in other countries if we fail to comply with regulatory requirements or previously unknown problems with our drug products are discovered after they reach the market.
The FDA or comparable regulatory authorities in other countries may withdraw approval of our drug products if we fail to maintain compliance with regulatory requirements or if problems occur after our drug products reach the market. The discovery of previously unknown problems with a drug product, including adverse events of unanticipated severity or frequency, problems with manufacturing processes or failure to comply with regulatory requirements, including the requirement to promote a drug product only for its approved indications and in accordance with the provisions of its approved label, may result in, among others:
|
● |
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
|
● |
warning letters or holds on post-approval clinical trials; |
|
● |
refusal of the FDA to approve pending NDAs or supplements to approved NDAs or comparable regulatory authorities refusing to approve any pending marketing applications, or suspension or revocation of product approvals; |
|
● |
product seizure or detention, or refusal to permit the import or export of the product; or |
|
● |
injunctions or the imposition of civil or criminal penalties. |
In the event that our drug products are subject to recalls, withdrawals, seizures or other enforcement actions by the FDA or comparable regulatory authorities, our reputation and demand for our drug products could be materially and adversely affected. In addition, we may incur significant and unexpected expenditures and management attention may be diverted in connection with any such recall, withdrawal, seizure or other enforcement action or any corrective action required to be taken, which could have a material and adverse impact on our business and financial condition.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. From time to time and in the future, our operations may involve the use of hazardous and flammable materials, including chemicals and biological materials, and may also produce hazardous waste products. Even if we contract with third parties for the disposal of these materials and waste products, we cannot completely eliminate the risk of contamination or injury resulting from these materials. In the event of contamination or injury resulting from the use or disposal of our hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
We maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, but this insurance may not provide adequate coverage against potential liabilities. However, we do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. Current or future environmental laws and regulations may impair our research, development or production efforts. In addition, failure to comply with these laws and regulations may result in substantial fines, penalties or other sanctions.
Risks Related to our Intellectual Property
Our commercial success depends largely on our ability to protect our intellectual property.
Our commercial success depends, in large part, on our ability to obtain and maintain patent protection and trade secret protection in the United States and elsewhere in respect of our product candidates and PRINT technology. If we fail to adequately protect our intellectual property rights, our competitors may be able to erode, negate or preempt any competitive advantage we may have. To protect our competitive position, we have filed and will continue to file for patents in the United States and elsewhere in respect of our product candidates and PRINT technology. The process of identifying patentable subject matter and filing a patent application is expensive and time-consuming. We cannot assure you that we will be able to file the necessary or desirable patent applications at a reasonable cost, in a timely manner, or at all. Further, since certain patent applications are confidential until patents are issued, third parties may have filed patent applications for subject matters covered by our pending patent applications without us being aware of such applications, and our patent applications may not have priority over patent applications of others. In addition, we cannot assure you that our pending patent applications will result in patents being obtained. The standards that patent offices in different jurisdictions use to grant patents are not always applied predictably or uniformly and may change from time to time.
Even if we have been or are able to obtain patent protection for our product candidates or PRINT technology, if the scope of such patent protection is not sufficiently broad, we may not be able to rely on such patent protection to prevent third parties from developing or commercializing product candidates or technology that may copy our product candidates or technology. The enforceability of patents in the pharmaceutical industry involves complex legal and scientific questions and can be uncertain. Accordingly, we cannot assure you that third parties will not successfully challenge the validity, enforceability or scope of our patents. A successful challenge to our patents may lead to generic versions of our drug products being launched before the expiry of our patents or otherwise limit our ability to stop others from using or commercializing similar or identical products and technology. A successful challenge to our patents may also reduce the duration of the patent protection of our drug products or technology. If any of our patents are narrowed or invalidated, our business and prospects may be materially and adversely affected. In addition, we cannot assure you that we will be able to detect unauthorized use or take appropriate, adequate and timely actions to enforce our intellectual property rights. If we are unable to adequately protect our intellectual property, our business, competitive position and prospects may be materially and adversely affected.
Even if our patents or patent applications are unchallenged, they may not adequately protect our intellectual property or prevent third parties from designing around our patents or other intellectual property rights. If the patent applications we file or may file do not lead to patents being granted or if the scope of any of our patent applications is challenged, we may face difficulties in developing our product candidates, companies may be dissuaded from collaborating with us, and our ability to commercialize our product candidates may be materially and adversely affected. We are unable to predict which of our patent applications will lead to patents or assure you that any of our patents will not be found invalid or unenforceable or challenged by third parties. The patents of others may prevent the commercialization of product candidates incorporating our technology. In addition, given the amount of time required for the development, clinical testing and regulatory review of new product candidates, any patents protecting our product candidates may expire before or shortly after such product candidates might become approved for commercialization.
Moreover, the issuance of a patent is not conclusive as to the inventorship of the patented subject matter, or its scope, validity or enforceability. We cannot assure you that all of the potentially relevant prior art, that is, any evidence that an invention is already known, relating to our patents and patent applications, has been found. If such prior art exists, it may be used to invalidate a patent or may prevent a patent from being issued.
In addition, we, our collaborators or our licensees may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. As a result, we may miss potential opportunities to seek patent protection or strengthen our patent position.
If we are unable to protect our trade secrets, the value of our PRINT technology and product candidates may be negatively impacted, which would have a material and adverse effect on our competitive position and prospects.
In addition to patent protection, we rely on trade secret protection to protect certain aspects of our intellectual property. While we require parties who have access to any portion of our trade secrets, such as our employees, consultants, advisers, CROs, CMOs, collaborators and other third parties, to enter into non-disclosure and confidentiality agreements with us, we cannot assure you that these parties will not disclose our proprietary information, including our trade secrets, in breach of their contractual obligations. Enforcing a claim that a party has illegally disclosed or misappropriated a trade secret is difficult, costly and time-consuming, and we may not be successful in doing so. If the steps we have taken to protect our trade secrets are deemed by the adjudicating court to be inadequate, we may not be able to obtain adequate recourse against a party for misappropriating our trade secrets.
Trade secrets can be difficult to protect as they may, over time, be independently discovered by our competitors or otherwise become known despite our trade secret protection. If any of our trade secrets were to be lawfully obtained or independently developed by our competitors, we would have no right to prevent such competitors, or those to whom they communicate such technology or information, from using that technology or information to compete with us. Such competitors could attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights.
If our trade secrets were to be disclosed to or independently developed by our competitors, our competitors may be able to exploit our PRINT technology to develop competing product candidates, and the value of our PRINT technology and our product candidates may be negatively impacted. This would have a material and adverse effect on our competitive position and prospects.
We rely on licenses to intellectual property that are owned by third parties.
We have entered and may, in the future, enter into license agreements with third parties to license the rights to use their technologies in our research, development and commercialization activities. License agreements generally impose various diligence, milestone payments, royalty, insurance and other obligations on us, and if we fail to comply with these obligations, our licensors may have the right to terminate these license agreements. Termination of these license agreements or the reduction or elimination of our licensed rights or the exclusivity of our licensed rights may have an adverse impact on, among others, our ability to develop and commercialize our product candidates. We cannot assure you that we will be able to negotiate new or reinstated licenses on commercially acceptable terms, or at all.
In addition, we license certain patent rights for our PRINT technology from The University of North Carolina at Chapel Hill, or UNC, under the UNC Amended and Restated License Agreement, dated as of December 15, 2008, as amended, or the UNC license. Under the UNC License, UNC has the right to terminate our license if we materially breach the agreement and fail to cure such breach within the stipulated time. In the event that UNC terminates our license and we have a product that relies on that license, it may bring a claim against us, and if they are successful, we may be required to compensate UNC for the unauthorized use of their patent rights through the payment of royalties.
Also, the agreements under which we license patent rights may not give us control over patent prosecution or maintenance, so that we may not be able to control which claims or arguments are presented and may not be able to secure, maintain or successfully enforce necessary or desirable patent protection from those patent rights. We do not have primary control over patent prosecution and maintenance for certain of the patents we license, and therefore cannot assure you that these patents and applications will be prosecuted or maintained in a manner consistent with the best interests of our business. We also cannot assure you that patent prosecution and maintenance activities by our licensors, if any, will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents.
Pursuant to the terms of some of our license agreements with third parties, some of our third-party licensors have the right, but not the obligation, in certain circumstances, to control the enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents. Even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors, and we cannot assure you that we will receive such cooperation on commercially acceptable terms, or at all. We also cannot assure you that our licensors will allocate sufficient resources or prioritize their or our enforcement of these patents or defense of these claims to protect our interests in the licensed patents. If we cannot obtain patent protection, or enforce existing or future patents against third parties, our competitive position, business and prospects may be materially and adversely affected.
Further, licenses to intellectual property may not always be available to us on commercially acceptable terms, or at all. In the event that the licenses we rely on are not available to us on commercially acceptable terms, or at all, our ability to commercialize our PRINT technology or product candidates, and our business and prospects, may be materially and adversely affected.
We may become involved in litigation to protect our intellectual property or enforce our intellectual property rights, which could be expensive, time-consuming and may not be successful.
Competitors may infringe our patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, we may engage in litigation to, among others, enforce or defend our intellectual property rights, determine the validity or scope of our intellectual property rights and those of third parties, and protect our trade secrets. Such actions may be time-consuming and costly and may divert our management’s attention from our core business and reduce the resources available for our clinical development, manufacturing and marketing activities, and consequently have a material and adverse effect on our business and prospects, regardless of the outcome.
In addition, in an infringement proceeding, a court may decide that a patent owned by, or licensed to, us is invalid or unenforceable, or may refuse to stop the other party from using the technology in question on the ground that our patents do not cover such technology. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that our confidential information may be compromised by disclosure.
We may be subject to claims that our employees or consultants have wrongfully used or disclosed to us alleged trade secrets of their former employers or other clients.
As is common in our industry, a number of our employees, including our Chief Executive Officer and a number of our executive officers, were formerly employed by other biotechnology or pharmaceutical companies, including our competitors or potential competitors, among others, and may have entered into proprietary rights, non-disclosure and non-competition agreements or similar agreements, in connection with such previous employment. Moreover, we engage the services of scientific advisers and consultants to assist us in the development of our products, many of whom were previously employed at or may have previously been or are currently providing consulting or advisory services to, other biotechnology or pharmaceutical companies, and who may have also entered into proprietary rights, non-disclosure and non-competition (or similar) agreements with such other companies.
While we require that our employees, scientific advisers and consultants do not use the proprietary information or know-how of others in their work for us, we cannot assure you that we will not be subject to claims that we or these employees, scientific advisers or consultants have inadvertently or otherwise used or disclosed the trade secrets or proprietary information of their former employers or former or present clients in their work for us, especially where such former employers or former or present clients are our competitors or potential competitors. Claims brought against us could cause us to incur unexpected and substantial costs, as well as divert our management’s attention from our core business and reduce the resources available for our clinical development, manufacturing and marketing activities. Consequently, our business may be materially and adversely affected.
We may be subject to claims from third parties that our products infringe their intellectual property rights.
The pharmaceutical industry has experienced rapid technological change and obsolescence in the past, and our competitors have strong incentives to stop or delay any introduction of new drug products or related technologies by, among others, establishing intellectual property rights over their drug products or technologies and aggressively enforcing these rights against potential new entrants into the market. We expect that we and other industry participants will be increasingly subject to infringement claims as the number of competitors and drug products grows.
Our commercial success depends in large part upon our ability to develop, manufacture, market and sell our drug products or product candidates without infringing on the patents or other proprietary rights of third parties. It is not always clear to industry participants, including us, what the scope of a patent covers. Due to the large number of patents in issue and patent applications filed in our industry, there is a risk that third parties will claim that our products or technologies infringe their intellectual property rights.
Claims for infringement of intellectual property which are brought against us, whether with or without merit, and which are generally uninsurable, could result in time-consuming and costly litigation, diverting our management’s attention from our core business and reducing the resources available for our drug product development, manufacturing and marketing activities, and consequently have a material and adverse effect on our business and prospects, regardless of the outcome. Moreover, such proceedings could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not being issued. We also may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Uncertainties resulting from the initiation and continuation of litigation or other proceedings could also have a material and adverse effect on our ability to compete in the market. Third parties making claims against us could obtain injunctive or other equitable relief against us, which could prevent us from further developing or commercializing our product candidates.
In particular, we may be required to include a certification of patent invalidity or non-infringement, or a paragraph IV certification, in an NDA submitted under the 505(b)(2) regulatory pathway, to certify that a patent over a reference listed drug is invalid, unenforceable or will not be infringed by the manufacture, use or sale of our product candidate. The holder of such patent may file a patent infringement lawsuit against us after receiving notice of the paragraph IV certification. Any such patent infringement lawsuit, if filed, will trigger a one-time, automatic, 30-month stay of the FDA’s ability to approve our application, unless the patent litigation is resolved in our favor or the patent expires before that time. Accordingly, we may invest a significant amount of time and expense in the development of a product candidate only to be subject to significant delay and incur substantial costs in litigation before such product candidate may be commercialized, if at all. Companies that produce reference listed drugs routinely bring claims for patent infringement against applicants under the 505(b)(2) regulatory pathway that are seeking regulatory approval to manufacture and market generic or reformulated forms of their reference listed drugs.
In the event of a successful infringement claim against us, including an infringement claim filed in response to a paragraph IV certification, we may be required to pay damages, cease the development or commercialization of our drug products or product candidates, re-engineer or redevelop our drug products or product candidates or enter into royalty or licensing agreements, any of which could have a material and adverse impact on our business, financial condition and results of operations. Any effort to re-engineer or redevelop our products would require additional monies and time to be expended and may not ultimately be successful.
Infringement claims may be brought against us in the future, and we cannot assure you that we will prevail in any ensuing litigation given the complex technical issues and inherent uncertainties involved in intellectual property litigation. Our competitors may have substantially greater resources than we do and may be able to sustain the costs of such litigation more effectively than we can.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. While various extensions may be available, the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
We intend to seek extensions of patent terms in the United States and, if available, in other countries where we prosecute patents. In the United States, the U.S. Drug Price Competition and Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Act, permits patent owners to request a patent term extension, based on the regulatory review period for a product, of up to five years beyond the normal expiration of the patent, which is limited to one patent claiming the approved drug product or use in an indication (or any additional indications approved during the period of extension). However, the applicable authorities, including the FDA and the U.S. Patent and Trademark Office, or the USPTO, in the United States, and comparable regulatory authorities in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or grant more limited extensions than we had requested. In such event, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our preclinical and clinical data in their marketing approval applications with the FDA to launch their drug product earlier than might otherwise be the case.
If we fail to comply with various procedural, document submission, fee payment or other requirements imposed by the USPTO or comparable patent agencies in other countries, our patent protection could be reduced or eliminated.
We are required, over the lifetime of an issued patent, to pay periodic maintenance fees to the USPTO and comparable patent agencies in other countries. We are also required by such patent agencies to comply with a number of procedural, documentary, fee payment and other conditions during the patent application process. While an inadvertent lapse can, in many cases, be cured by payment of a late fee or other means in accordance with the applicable rules, there are situations in which non-compliance can result in abandonment or lapse of a patent or patent application, resulting in the partial or complete loss of patent rights in the relevant jurisdiction. Such situations include, but are not limited to:
|
● |
a failure to respond to official actions within the prescribed time limits; |
|
● |
the non-payment of fees; and |
|
● |
a failure to properly legalize and submit formal documents. |
If we or our licensors, which control the prosecution and maintenance of patents which we license, fail to maintain the patents or patent applications covering our product candidates or technology, such rights would be reduced or eliminated and, consequently, our competitive position, business and prospects may be materially and adversely affected.
Changes in patent laws or interpretations of patent laws in the United States or elsewhere may diminish the value of our intellectual property or narrow the scope of protection of our patents.
In September 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law, and many of the substantive changes became effective in March 2013. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including changing the United States patent system from a “first to invent” system to a “first inventor to file” system, expanding the definition of prior art and developing a post-grant review system. The provisions under the Leahy-Smith Act changed the way patent applications are prosecuted and may also affect patent litigation. It may have also weakened our ability to obtain patent protection in the United States for applications filed after March 16, 2013.
Further, the post-grant review and inter partes review proceedings established under the Leahy-Smith Act have been used by certain parties to cause a cancellation of selected or all claims in relation to the issued patents of their competitors. For a patent with an effective filing date of March 16, 2013 or later, a petition for post-grant review can be filed by a third party in a nine-month window from issuance of the patent. A petition for inter partes review can be filed after the nine-month period for filing a post-grant review petition has expired for a patent with an effective filing date of March 16, 2013 or later. Post-grant review proceedings can be brought on any ground of invalidity, whereas inter partes review proceedings can only raise an invalidity challenge based on published prior art and patents. These adversarial actions at the USPTO review patent claims without the presumption of validity afforded to U.S. patents in lawsuits in U.S. federal courts, and use a lower burden of proof than that used in civil actions in the U.S. federal courts. Therefore, it is generally considered easier for a competitor or third party to have a U.S. patent invalidated in a USPTO post-grant review or inter partes review proceeding than invalidated in litigation in a U.S. federal court. We cannot assure you that we, our licensors or our collaborators will be successful in defending any challenge by a third party in a USPTO proceeding, or, conversely, that we, our licensors or our collaborators will be successful in challenging a third party in such a proceeding.
In addition, recent court rulings in the United States have narrowed the scope of patent protection available and weakened the rights of patent owners, particularly in the pharmaceutical industry. In 2012, the Supreme Court of the United States, or the Supreme Court, issued a decision in Mayo Collaborative Services v. Prometheus Laboratories, Inc. invalidating patent claims directed to a process of measuring a metabolic product in a patient to optimize a drug dosage for the patient. In 2013, the Supreme Court issued its decision in Association for Molecular Pathology v. Myriad Genetics, Inc. invalidating patent claims directed to the breast cancer susceptibility genes BRCA1 and BRCA2. In 2017, the Supreme Court issued its decision in TC Heartland v. Kraft Food Group Brands, holding that patentees can only sue alleged infringers in their state of incorporation. These rulings deviated from precedents and, accordingly, have created uncertainty with regard to our ability to obtain patents in the future as well as the value of such patents, once obtained. Depending on future actions by Congress, the U.S. courts, the USPTO and the relevant law-making bodies in other countries, the laws and regulations governing patents could change in unpredictable ways that would affect our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting, enforcing and defending patents on our PRINT technology and our product candidates throughout the world may be prohibitively expensive and may not be financially or commercially feasible. In countries where we have not obtained patent protection, our competitors may be able to use our proprietary technologies to develop competing product candidates.
Also, the legal systems of non-U.S. jurisdictions may not protect intellectual property rights to the same extent or in the same manner as the laws of the United States, and we may face significant difficulty in enforcing our intellectual property rights in these jurisdictions. The legal systems of certain developing countries may not favor the enforcement of patents and other intellectual property rights. We may therefore face difficulty in stopping the infringement or misappropriation of our patents or other intellectual property rights in those countries.
We need to protect our trademark, trade name and service mark rights to prevent competitors from taking advantage of our goodwill.
We believe that the protection of our trademark, trade name and service mark rights, such as Liquidia, the Liquidia logo and PRINT, is an important factor in product recognition, protecting our brand, maintaining goodwill and maintaining or increasing market share. We may expend substantial cost and effort in an attempt to register new trademarks, trade names and service marks and maintain and enforce our trademark, trade name and service mark rights. If we do not adequately protect our rights in our trademarks, trade names and service marks from infringement, any goodwill that we have developed in those trademarks could be lost or impaired.
Third parties may claim that the sale or promotion of our products, when and if approved, may infringe on the trademark, trade name and service mark rights of others. Trademark, trade name and service mark infringement problems occur frequently in connection with the sale and marketing of pharmaceutical products. If we become involved in any dispute regarding our trademark, trade name and service mark rights, regardless of whether we prevail, we could be required to engage in costly, distracting and time-consuming litigation that could harm our business. If the trademarks, trade names and service marks we use are found to infringe upon the trademarks, trade names or service marks of another company, we could be liable for damages and be forced to stop using those trademarks, trade names or service marks, and as result, we could lose all the goodwill that has been developed in those trademarks, trade names or service marks.
Risks Related to the Manufacturing of our Product Candidates
Our product candidates are based on our proprietary, novel technology, PRINT, which has not been the subject of FDA manufacturing inspections, making it difficult to predict the time and cost of development and of subsequently obtaining regulatory approval.
Our future success depends on the successful development of our novel PRINT technology and products based on it, including LIQ861 and LIQ865. To our knowledge, no regulatory authority has granted approval to market or commercialize drugs made using our PRINT technology. Further, manufacturing facilities and processes utilizing our PRINT technology have not been the subject of FDA manufacturing inspections. We may never receive approval to market and commercialize any product candidate that uses our PRINT technology.
Our facilities are subject to extensive and ongoing regulatory requirements and failure to comply with these regulations may result in significant liability.
Our company and our facilities are subject to payment of fees, registration and listing requirements, ongoing review and periodic inspections by the FDA and other regulatory authorities for compliance with quality system regulations, including the FDA’s current good manufacturing practices, or cGMP, requirements. These regulations cover all aspects of the manufacturing, testing, quality control and record-keeping of our drug products. Furthermore, the facilities where our product candidates are manufactured may be subject to inspection by the FDA before we can obtain marketing approval and remain subject to periodic inspection even after our product candidates have received marketing approval. Suppliers of components and materials, such as active pharmaceutical ingredients, used to manufacture our drug products are also required to comply with the applicable regulatory standards.
The manufacture of pharmaceutical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. We and any contract manufacturers that we may engage in the future must comply with cGMP requirements. Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up and validating initial production and contamination controls. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination.
Compliance with these regulatory standards often requires significant expense and effort. If we or our suppliers are unable to comply with the applicable regulatory standards or take satisfactory corrective steps in response to adverse results of an inspection, this could result in enforcement action, including, among others, the issue of a public warning letter, a shutdown of or restrictions on our or our suppliers’ manufacturing operations, delays in approving our drug products and refusal to permit the import or export of our drug products. Any adverse regulatory action taken against us could subject us to significant liability and harm our business and prospects.
Our operations are concentrated in Morrisville, North Carolina and interruptions affecting us or our suppliers due to natural disasters or other unforeseen events could materially and adversely affect our operations.
All of our current operations are concentrated in Morrisville, North Carolina. A fire, flood, hurricane, earthquake or other disaster or unforeseen event resulting in significant damage to our facilities could significantly disrupt or curtail or require us to cease our operations. It would be difficult, costly and time-consuming to transfer resources from one facility to another or to repair or replace our facility in the event that it is significantly damaged. In addition, our insurance may not be sufficient to cover all of our losses and may not continue to be available to us on acceptable terms, or at all. In addition, if one of our suppliers experiences a similar disaster or unforeseen event, we could face significant delays in obtaining our supplies or be required to source supplies from an alternative supplier and may incur substantial costs as a result. Any significant uninsured loss, prolonged or repeated disruption to operations or inability to operate, experienced by us or by our suppliers, could materially and adversely affect our business, financial condition and results of operations.
We may not be able to engage third-party contract manufacturing organizations, or CMOs, to manufacture our drug products, if and when approved, on a commercial scale to meet commercial demand for our drug products.
We may, in the future, need to rely on third-party CMOs or enter into contractual arrangements with third parties to manufacture our drug products, if and when approved, on a commercial scale. However, we cannot assure you that we will be able to contract with such third parties on acceptable terms, if at all, or that such third parties will satisfy our quality standards or meet our supply requirements in a timely manner, if at all. In addition, only a limited number of manufacturers are capable of supplying pharmaceutical products. The manufacturing process for our drug products will be highly regulated, and we will need to contract with manufacturers that can meet the relevant regulatory requirements on an ongoing basis. If the third-party manufacturers with whom we contract fail to perform their obligations, we may not be able to meet commercial demand for our drug products, which would have a material and adverse impact on our business.
System failures may disrupt our business operations and delay our product development programs and commercialization activities.
Our systems, including computer systems, and those of our collaborators, contractors and consultants are vulnerable to, among others, unauthorized access, equipment failure and damage from computer viruses as well as cyber hackers. In the event of a material system failure or security breach of, or significant damage to, our systems, our business operations may be disrupted, and our product development programs and commercialization activities may be delayed. For example, failure of, or damage to, equipment leading to a loss of our clinical trial data could result in delays to the process of obtaining marketing approval for our product candidates, as well as significant and unexpected expenditure to recover or reproduce the lost data. To the extent that any disruption or damage to, or security breach of, the systems of our collaborators, contractors or consultants results in a loss of our data or applications, or the disclosure of our confidential information, our business may be adversely affected.
Risks Related to Healthcare Regulation
We are subject to various laws and regulations, such as healthcare fraud and abuse laws, false claim laws and health information privacy and security laws, among others, and failure to comply with these laws and regulations may have an adverse effect on our business.
Healthcare providers, physicians and third-party payors often play a primary role in the recommendation and prescription of any drug products for which we may obtain marketing approval. Our current and future arrangements with healthcare providers, physicians, third-party payors and customers, and our sales, marketing and educational activities, may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations (at the federal and state level) that may constrain our business or financial arrangements and relationships through which we market, sell and distribute our drug products for which we obtain marketing approval.
In addition, we may be subject to transparency laws and patient privacy regulation by both the federal government and the states in which we conduct our business.
The laws that may affect our ability to operate include, but are not limited to, the following:
|
● |
the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities including pharmaceutical manufacturers from, among other things, knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for or the purchase, lease, order or recommendation of an item or service for which payment may be made, in whole or in part, under federal healthcare programs such as the Medicare and Medicaid programs. This statute has been interpreted broadly to apply to, among other things, arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other hand. The term “remuneration” expressly includes kickbacks, bribes or rebates and also has been broadly interpreted to include anything of value, including, for example, gifts, discounts, waivers of payment, ownership interest and providing anything at less than its fair market value. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny. The failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the federal Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Our practices may not meet all of the criteria for safe harbor protection from federal Anti-Kickback Statute liability in all cases. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act. The U.S. Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA, amended the False Claims Act to provide that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim under the False Claims Act; |
|
● |
the federal civil and criminal false claims laws and civil monetary penalty laws, including the False Claims Act, which prohibits individuals or entities from, among other things, knowingly presenting, or causing to be presented, claims for payment to, or approval by, the federal government that are false, fictitious or fraudulent or knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim to avoid, decrease or conceal an obligation to pay money to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the federal government, directly or indirectly. Although we do not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers, promoting a product off-label, marketing products of sub-standard quality, or, as noted above, paying a kickback that results in a claim for items or services. In addition, our activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state and third-party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, several pharmaceutical and other healthcare companies have faced enforcement actions under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. The False Claims Act also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the False Claims Act and to share in any monetary recovery. Penalties under the False Claims Act include treble damages and per claim penalty amounts ranging from $11,665 to $23,331. The ACA further codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a per se false or fraudulent claim for purposes of the federal False Claims Act; |
|
● |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up by trick, scheme or device, a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services; |
|
● |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, including the Final Omnibus Rule published on January 25, 2013, impose, among other things, obligations, impose requirements relating to the privacy, security and transmission of individually identifiable health information. Following enactment of the HITECH Act, HIPAA’s privacy and security standards now directly apply to business associates of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. We are not a covered entity under HIPAA but in certain situations, we may be considered a business associate. HITECH also created four new tiers of civil monetary penalties, gave state attorneys general new authority to file civil actions for damages or injunctions in federal court to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. The U.S. Department of Health and Human Services Office for Civil Rights, or the OCR, has increased its focus on compliance and continues to train state attorneys general for enforcement purposes. The OCR has recently increased both its efforts to audit HIPAA compliance and its level of enforcement; |
|
● |
even when HIPAA does not apply, according to the U.S. Federal Trade Commission, or the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act, or the FTCA. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Medical data is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule. The FTC’s authority under Section 5 is concurrent with HIPAA’s jurisdiction and with any action taken under state law; |
|
● |
the federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act,” created under the ACA which requires applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program (with certain exceptions) to annually report to the Centers for Medicare and Medicaid Services, or CMS, information related to certain payments or other transfers of value made or distributed to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Federal legislation enacted in 2018 has extended the scope of reporting requirements to apply to payments and transfers of value to not only physicians, but also physician assistants, nurse practitioners, and other mid-level practitioners (with reporting requirements going into effect in 2022 for payments made in 2021); |
|
● |
analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to items or services reimbursed by any third-party payor, including commercial insurers, and in some cases may apply regardless of payor (i.e., even for self-pay scenarios). Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report pricing and marketing information, including, among other things, information related to payments to physicians and other healthcare providers or marketing expenditures, state and local laws that require the registration of pharmaceutical sales representatives, and state laws governing the privacy and security of health information and the use of prescriber-identifiable data in certain circumstances. Many of these state laws differ from each other in significant ways and may not have the same effect, and may apply more broadly than their federal counterparts, thus complicating compliance efforts (for example, California recently enacted legislation — the CCPA, which went into effect on January 1, 2020 and among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information, and creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach; although the law includes limited exceptions, including for certain information collected as part of clinical trials as specified in the law, it may regulate or impact our processing of personal information depending on the context, and it remains unclear what, if any, modifications will be made to this legislation or how it will be interpreted); and |
|
● |
price reporting laws that require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursements or discounts on our drug products. Participation in such programs and compliance with their requirements may subject us to increased infrastructure costs and potentially limit our ability to price our drug products. |
Further, we are subject to a number of environmental and health and safety laws and regulations, including those governing laboratory processes and the handling, use, storage, treatment and disposal of hazardous materials and waste.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that certain business activities could be subject to challenge under one or more of such laws. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between pharmaceutical companies and providers and patients, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Ensuring that business arrangements with third parties comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert management’s attention from the business, even if the government ultimately finds that no violation has occurred.
If our operations are found to be in violation of any of the laws or regulations described above or any other laws or government regulations that apply to us, we may be subject to penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid or other government healthcare programs, injunctions, private qui tam actions brought by individual whistleblowers in the name of the government and the curtailment or restructuring of our operations as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, any of which could adversely affect our ability to operate our business and our results of operations.
Our failure to comply with data protection laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results.
European Union member states and other foreign jurisdictions, including Switzerland, have adopted data protection laws and regulations which impose significant compliance obligations. Moreover, the collection and use of personal health data in the European Union, which was formerly governed by the provisions of the European Union Data Protection Directive, was replaced with the European Union General Data Protection Regulation, or the GDPR, in May 2018. The GDPR, which is wide-ranging in scope, imposes several requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, the security and confidentiality of the personal data, data breach notification and the use of third-party processors in connection with the processing of personal data. The GDPR also imposes strict rules on the transfer of personal data out of the European Union to the United States, provides an enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. The recent implementation of the GDPR has increased our responsibility and liability in relation to personal data that we process, including in clinical trials, and we may in the future be required to put in place additional mechanisms to ensure compliance with the GDPR, which could divert management’s attention and increase our cost of doing business. In addition, new regulation or legislative actions regarding data privacy and security (together with applicable industry standards) may increase our costs of doing business. In this regard, we expect that there will continue to be new proposed laws, regulations and industry standards relating to privacy and data protection in the United States, the European Union and other jurisdictions, and we cannot determine the impact such future laws, regulations and standards may have on our business.
Legislative or regulatory reform of the healthcare system in our target markets may affect our operations and profitability.
In recent years, there have been numerous initiatives on the federal and state levels in the United States for comprehensive reforms affecting the payment for, the availability of and reimbursement for healthcare services. There have been a number of federal and state proposals during the last few years regarding the pricing of pharmaceutical and biopharmaceutical products, limiting coverage and reimbursement for drugs and other medical products, government control and other changes to the healthcare system in the United States. Current and future U.S. legislative healthcare reforms may result in price controls and other restrictions for any approved products, if covered, and could seriously harm our business. Given that drug pricing controls is a key legislative and administration priority, it is likely that additional pricing controls will be enacted and could harm our business, financial condition and results of operations.
The ACA, which was signed into law in the United States in March 2010, contained several provisions affecting the pharmaceutical industry:
|
● |
the Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of HHS, as a condition of Medicare Part B and Medicaid coverage of the manufacturer’s outpatient drugs furnished to Medicaid patients; |
|
● |
the expansion of eligibility criteria for Medicaid programs which potentially increases both the volume of sales and manufacturers’ Medicaid rebate liability; |
|
● |
in order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B Drug Pricing Program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer; |
|
● |
the ACA imposed a requirement on manufacturers of branded drugs to provide a 70% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., the donut hole); |
|
● |
the ACA imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications; |
|
● |
the ACA implemented the Physician Payments Sunshine Act; |
|
● |
the ACA requires annual reporting of drug samples that manufacturers and distributors provide to physicians; |
|
● |
the ACA expanded healthcare fraud and abuse laws in the United States, including the False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for non-compliance; |
|
● |
the ACA established a licensing framework for follow-on biologics; and |
|
● |
the ACA established the new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with the funding for such research. |
The Trump Administration and the Congressional Republicans have proposed several efforts to repeal and replace the ACA. President Trump has also signed Executive Orders and other directives designed to delay the implementation of certain provisions of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. Additionally, on December 15, 2019, a federal district court in Texas struck down the ACA in its entirety, finding that the TCJA renders the individual mandate unconstitutional. The judge further concluded in Texas v. Azar that since the individual mandate is “essential” to the ACA, it could not be severed from the rest of the ACA and therefore, the entire ACA was unconstitutional. Despite its decision, however, the court did not issue an injunction and therefore, immediate compliance is not required. In addition, the Trump Administration announced that it will continue to administer the law until a formal decision is made by the U.S. Supreme Court. The Supreme Court has not yet announced when or whether it will hear a challenge in Texas v. United States, though it is highly anticipated that it will do so next term (beginning October 2020). Apart from Texas v. United States, ACA litigation continues across the country in district and appellate courts, and before the Supreme Court. The Supreme Court will issue at least two ACA-related decisions before the end of its current term: one on the risk corridors program (Maine Community Health Options v. United States) and the other on religious or moral exemptions to the contraceptive mandate (Trump v. Pennsylvania and Little Sisters of the Poor v. Pennsylvania). Both decisions are expected before July 2020. It is unclear how the eventual decisions from the Supreme Court and the various other courts across the country to repeal and replace the ACA will impact the ACA and our business. It is also unclear how regulations and sub-regulatory policy, which fluctuate continually, may affect interpretation and implementation of the ACA and its practical effects on our business, particularly entering an election year.
Further, there has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drugs. Recent federal budget proposals have included measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. The U.S. Congress and the Trump Administration have indicated that they will continue to seek new legislative and administrative measures to control drug costs, including by addressing the role of PBMs in the supply chain. Drug pricing is and will remain a key bipartisan issue in the coming year. If drug pricing reform is not meaningfully addressed before the 2020 election, policies to be pursued in the future may be more aggressive, regardless of which party controls the White House. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. The boom in state laws targeting drug pricing is unprecedented and the requirements are not uniform from state to state, creating additional compliance and commercialization challenges for manufacturers.
We are unable to predict the future course of federal or state healthcare legislation in the United States directed at broadening the availability of healthcare and containing or lowering the cost of healthcare, including drugs and biologics. The fate of the ACA and any further changes in the law or regulatory framework that reduce our revenue or increase our costs could also have a material and adverse effect on our business, financial condition and results of operations.
Healthcare laws and regulations may affect the pricing of our drug products and may affect our profitability.
In certain countries, the government may provide healthcare at a subsidized cost to consumers and regulate prices, patient eligibility or third-party payor reimbursement policies to control the cost of drug products. Such a system may lead to inconsistent pricing of our drug products from one country to another. The availability of our drug products at lower prices in certain countries may undermine our sales in other countries where our drug products are more expensive. In addition, certain countries may set prices by reference to the prices of our drug products in other countries. Our inability to secure adequate prices in a particular country may adversely affect our ability to obtain an acceptable price for our drug products in existing and potential markets. If we are unable to obtain a price for our drug products that provides an appropriate return on our investment, our profitability may be materially and adversely affected.
Risks Related to our Employees
We depend on skilled labor, and our business and prospects may be adversely affected if we lose the services of our skilled personnel, including those in senior management, or are unable to attract new skilled personnel.
Our ability to continue our operations and manage our potential future growth depends on our ability to hire and retain suitably skilled and qualified employees, including those in senior management, in the long-term. Due to the specialized nature of our work, there is a limited supply of suitable candidates. We compete with other biotechnology and pharmaceutical companies, educational and research institutions and government entities, among others, for research, technical and clinical personnel. In addition, in order to manage our potential future growth effectively, we will need to improve our financial controls and systems and, as necessary, recruit sales, marketing, managerial and finance personnel. If we are unable to attract and retain skilled personnel, including in particular Neal Fowler, our Chief Executive Officer, our business and prospects may be materially and adversely affected.
Our employees and our independent contractors, principal investigators, CROs, CMOs, consultants or commercial collaborators, as well as their respective subcontractors, if any, may engage in misconduct or fail to comply with certain regulatory standards and requirements, which could expose us to liability and adversely affect our reputation.
Our employees and our independent contractors, principal investigators, CROs, CMOs, consultants or commercial collaborators, as well as their respective subcontractors, if any, may engage in fraudulent conduct or other illegal activity, which may include intentional, reckless or negligent conduct that violates, among others, (a) FDA laws and regulations, or those of comparable regulatory authorities in other countries, including those laws that require the reporting of true, complete and accurate information to the FDA, (b) manufacturing standards, (c) healthcare fraud and abuse laws or (d) laws that require the true, complete and accurate reporting of financial information or data. For example, such persons may improperly use or misrepresent information obtained in the course of our clinical trials, create fraudulent data in our preclinical studies or clinical trials or misappropriate our drug products, which could result in regulatory sanctions being imposed on us and cause serious harm to our reputation. It is not always possible for us to identify or deter misconduct by our employees and third parties, and any precautions we may take to detect or prevent such misconduct may not be effective. Any misconduct or failure by our employees and our independent contractors, principal investigators, CROs, CMOs, consultants or commercial collaborators, as well as their respective subcontractors, if any, to comply with the applicable laws or regulations may subject us to enforcement action or otherwise expose us to liability or compliance costs, which, depending on the nature of the violation, may include but not necessarily be limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid or other government healthcare programs, injunctions, private qui tam actions brought by individual whistleblowers in the name of the government and the curtailment or restructuring of our operations as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws. If any action is instituted against us as a result of the alleged misconduct of our employees or other third parties, regardless of the final outcome, our reputation may be adversely affected and our business may suffer as a result. If we are unsuccessful in defending against any such action, we may also be liable to significant fines or other sanctions, which could have a material and adverse effect on us.
Risks Related to our Common Stock
An active trading market for our common stock may not be sustainable. If an active trading market is not sustained, our ability to raise capital in the future may be impaired.
We completed our initial public offering in July 2018. Prior to this time, there was no public market for our common stock. Although our common stock is listed on the Nasdaq Capital Market, an active trading market for our shares may not be sustained. If an active market for our common stock is not sustained, it may be difficult for you to sell shares of our common stock without depressing the market price for the shares or at all. An inactive trading market may also impair our ability to raise capital to continue to fund operations by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
Future sales of our common stock or securities convertible into our common stock in the public market could cause our stock price to fall.
Our stock price could decline as a result of sales of a large number of shares of our common stock or the perception that these sales could occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
As of March 1, 2020, 28,365,093 shares of our common stock were outstanding, of which 23,041,325 shares of common stock, or 81.2% of our outstanding shares as of March 1, 2020, are freely tradable without restriction or further registration under the Securities Act of 1933, as amended, or the Securities Act, unless held by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, or Rule 144. The resale of the remaining 5,323,768 shares held by our stockholders is currently prohibited or otherwise restricted as a result of securities law provisions. Shares issued upon the exercise of stock options outstanding under our equity incentive plans or pursuant to future awards granted under those plans will become available for sale in the public market to the extent permitted by the provisions of applicable vesting schedules, any applicable market standoff and lock-up agreements, and Rule 144 and Rule 701 under the Securities Act.
As of March 1, 2020, the holders of approximately 5.1 million shares, or 18.0%, of our outstanding shares as of March 1, 2020, have rights, subject to some conditions, to require us to file registration statements covering the sale of their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We have also registered the offer and sale of all shares of common stock that we may issue under our equity compensation plans, including the employee stock purchase plan. Once we register the offer and sale of shares for the holders of registration rights, they can be freely sold in the public market upon issuance or resale (as applicable), subject to lock-up agreements, if any.
We are party to an Open Market Sale Agreement℠ with Jefferies LLC, as sales agent and/or principal, pursuant to which we may offer shares of our common stock from time to time through “at-the-market” offerings. We are not obligated to make or continue to make any sale of shares of our common stock under the “at-the-market” offerings. Although any sale of securities pursuant to the “at-the-market” offerings will result in a concomitant increase in cash for each share sold, it may result in stockholder dilution and may cause our share price to fall.
We expect that the market price of our common stock may be volatile, and you may lose all or part of your investment.
The trading prices of the securities of pharmaceutical and biotechnology companies have been highly volatile. As such, the trading price of our common stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. The market price for our common stock may be influenced by many factors, including:
|
● |
results of clinical trials of LIQ861, LIQ865 or any product candidate we may develop, or those of our competitors; |
|
● |
our cash resources; |
|
● |
the success of competitive products or technologies; |
|
● |
potential approvals of any product candidate we may develop for marketing by the FDA or equivalent foreign regulatory authorities or any failure to obtain such approvals; |
|
● |
our involvement in significant lawsuits, including stockholder or patent litigation, including inter partes review proceedings; |
|
● |
regulatory or legal developments in the United States and other countries; |
|
● |
the results of our efforts to commercialize any product candidate we may develop; |
|
● |
developments or disputes concerning patents or other proprietary rights; |
|
● |
the recruitment or departure of key personnel; |
|
● |
the level of expenses related to any of our product candidates or clinical development programs; |
|
● |
the results of our efforts to discover, develop, acquire or in-license additional product candidates or products; |
|
● |
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
|
● |
variations in our financial results or those of companies that are perceived to be similar to us; |
|
● |
changes in the structure of healthcare payment systems; |
|
● |
market conditions in the pharmaceutical and biotechnology sectors and issuance of new or changed securities analysts’ reports or recommendations; |
|
● |
general economic, industry and market conditions; and |
|
● |
the other factors described in this “Risk Factors” section. |
The stock market in general, and market prices for the securities of pharmaceutical companies like ours in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance. Stock prices of many pharmaceutical companies have fluctuated in a manner unrelated or disproportionate to the operating performance of those companies. In several recent situations when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and harm our operating results.
Future sales and issuances of equity securities, convertible securities or other securities could result in additional dilution of the percentage ownership of holders of our common stock.
Our stockholders may experience dilution upon future equity issuances, including any other convertible debt or equity securities we may issue in the future, the exercise of stock options to purchase common stock granted to our employees, consultants and directors, including options to purchase common stock granted under our stock option and equity incentive plans, the issuance of common stock in settlement of previously issued awards under our stock option and equity incentive plans that may vest in the future or the issuance of common stock pursuant to our employee stock purchase plan.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell equity securities, convertible securities or other securities in one or more transactions at prices and in a manner we determine from time to time. If we sell equity securities, convertible securities or other securities, current investors may be materially diluted by such subsequent sales. We may also need our stockholders to authorize the issuance of additional shares of common stock under our amended and restated certificate of incorporation if we do not have sufficient authorized shares to raise such additional capital or issue future awards under our incentive plan. New investors could also gain rights, preferences and privileges senior to those of holders of our existing equity securities.
Our principal stockholders and management own a significant percentage of our stock and will be able to exercise significant influence over matters subject to stockholder approval.
Our executive officers, directors and principal stockholders, together with their respective affiliates, beneficially owned 39.5% of our capital stock as of March 1, 2020, of which 3.2% are beneficially owned by our executive officers and directors. Accordingly, our executive officers, directors and principal stockholders have significant influence in determining the composition of the Board, and voting on all matters requiring stockholder approval, including mergers and other business combinations, and continue to have significant influence over our operations. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us that you may believe are in your best interests as one of our stockholders. This in turn could have a material adverse effect on our stock price and may prevent attempts by our stockholders to replace or remove the Board or management.
If securities or industry analysts do not publish research reports about our business, or if they issue an adverse opinion about our business, our stock price and trading volume could decline.
The trading market for our common stock may be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of these analysts ceases research coverage of us, fails to regularly publish reports on us or issues an adverse opinion about our business, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
As a public company, we are obligated to develop and maintain proper and effective internal control over financial reporting and any failure to do so may adversely affect investor confidence in us and, as a result, the trading price of our shares. The results of our 2019 assessment of the effectiveness of internal control over financial reporting, or ICFR, indicate that we have multiple material weaknesses.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock. In addition, any future testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement.
As required by the Sarbanes Oxley Act of 2002 and commencing with the fiscal year ended December 31, 2019, we are required to furnish a report by management on, among other things, the effectiveness of our ICFR for the fiscal year ended December 31, 2019. In connection with the assessment of the effectiveness of our ICFR, our management identified the following material weaknesses that existed as of December 31, 2019:
During 2019, we experienced significant turnover in finance personnel that reduced the complement and skill of the resources within the Company. As a result, we did not maintain an effective control environment as we lacked a sufficient complement of resources with an appropriate level of knowledge, experience and training to design, maintain and monitor our ICFR commensurate with our financial reporting requirements. As a result, this material weakness contributed to the following material weaknesses:
|
● |
We did not design and maintain controls to ensure adequate segregation of duties within our financial reporting function, including the preparation and review of journal entries. Specifically, some key accounting personnel had the ability to both prepare and post journal entries without an independent review by someone without the ability to prepare and post journal entries. |
|
● |
We did not design and maintain effective controls over certain information technology general controls for information systems that are relevant to the preparation of our financial statements. Specifically, we did not design and maintain effective user access controls to ensure appropriate segregation of duties and that adequately restrict user and privileged access to financial applications and data to appropriate Company personnel. |
These material weaknesses did not result in a material misstatement of the annual or interim financial statements. Additionally, these material weaknesses could result in a misstatement of the relevant account balances or disclosures that would result in a material misstatement to the annual or interim consolidated financial statements that would not be prevented or detected.
Additionally, we could be subject to regulatory scrutiny, a loss of public and investor confidence, and to litigation from investors and stockholders, all of which could have a material adverse effect on our business and the trading price of our shares. Subsequent to our December 31, 2019 year end, we began taking a number of actions, including designing and implementing new controls and revising existing controls, in order to remediate the material weaknesses described above. See Item 9A. Controls and Procedures in this annual report. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could result in charges by the SEC with violating the books and records and internal control provisions of the federal securities laws which may result in penalties and fines to our company, directors and officers, and also could restrict our future access to the capital markets.
For as long as we are an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012, as amended, or the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404. We could be an emerging growth company for up to an additional four years. An independent assessment of the effectiveness of our internal controls could detect additional problems that our management’s assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur additional remediation expenses.
We are an “emerging growth company,” as defined in the JOBS Act, and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We will take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more, (ii) the last day of 2023, (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
We will continue to incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, we are incurring significant legal, accounting and other expenses that we did not incur as a private company, including costs associated with public company reporting requirements. We have also incurred costs associated with recently adopted corporate governance requirements, including requirements of the U.S. Securities and Exchange Commission and the Nasdaq Stock Market LLC, or Nasdaq. These rules and regulations have increased our legal and financial compliance costs and made some activities more time-consuming and costly. These rules and regulations also make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage that we received as a private company. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our Board or as executive officers. We are currently evaluating and monitoring developments with respect to these rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
When we cease to be an “emerging growth company” and when our independent registered public accounting firm is required to undertake an assessment of our internal control over financial reporting, the cost of our compliance with Section 404 of the Sarbanes-Oxley Act will correspondingly increase. Moreover, if we are not able to comply with the requirements of Section 404 applicable to us in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us difficult, limit attempts by our stockholders to replace or remove our current management and adversely affect our stock price.
Provisions of our amended and restated certificate of incorporation and amended and restated bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our stock. Among other things, the certificate of incorporation and bylaws:
|
● |
permit the Board to issue up to 10 million shares of preferred stock, with any rights, preferences and privileges as they may designate; |
|
● |
provide that the authorized number of directors may be changed only by resolution of our Board; |
|
● |
provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
|
● |
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and may not be taken by written consent; |
|
● |
create a staggered board of directors such that all members of our Board are not elected at one time; |
|
● |
allow for the issuance of authorized but unissued shares of our capital stock without any further vote or action by our stockholders; and |
|
● |
establish advance notice requirements for nominations for election to the Board or for proposing matters that can be acted upon at stockholders’ meetings. |
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or the DGCL, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any stockholder owning in excess of 15% of our outstanding stock for a period of three years following the date on which the stockholder obtained such 15% equity interest in us.
The terms of our authorized preferred stock selected by our Board at any point could decrease the amount of earnings and assets available for distribution to holders of our common stock or adversely affect the rights and powers, including voting rights, of holders of our common stock without any further vote or action by the stockholders. As a result, the rights of holders of our common stock will be subject to, and may be adversely affected by, the rights of the holders of any preferred stock that may be issued by us in the future, which could have the effect of decreasing the market price of our common stock.
Any provision of our certificate of incorporation or bylaws or Delaware corporate law that has the effect of delaying or deterring a change in control could limit opportunities for our stockholders to receive a premium for their shares of common stock, and could also affect the price that investors are willing to pay for our common stock.
Our amended and restated certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our amended and restated certificate of incorporation provides that, to the fullest extent permitted by law, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for: (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors or officers to us or our stockholders; (iii) any action asserting a claim against us arising pursuant to any provision of the DGCL, our amended and restated certificate of incorporation or our amended and restated bylaws; or (d) any action asserting a claim against us governed by the internal affairs doctrine. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock is deemed to have received notice of and consented to the foregoing provisions. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds more favorable for disputes with us or our directors or officers, which may discourage such lawsuits against us and our directors or officers. Alternatively, if a court were to find this choice of forum provision inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial condition, prospects or results of operations.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our equity securities. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of our existing A&R LSA with PWB preclude us, and the terms of any future debt agreement may preclude us, from paying dividends. As a result, capital appreciation, if any, of our equity securities will likely be your sole source of gain for the foreseeable future.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our corporate headquarters are located in Morrisville, North Carolina, and consist of approximately 45,000 square feet of space under a lease that expires on October 31, 2026 and includes an option for us to renew for an additional five years through October 31, 2031, as amended. The primary use of this location is general office, laboratory, research and development and light manufacturing. We believe that our facilities are adequate for our current needs and for the foreseeable future; however, we will continue to seek additional space as needed to accommodate our growth.
Item 3. Legal Proceedings.
We are not currently but may become subject to certain legal proceedings and claims arising in connection with the normal course of our business. In the opinion of management, there are currently no claims that would have a material adverse effect on our consolidated financial position, results of operations or cash flows.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock has been listed on the Nasdaq Capital Market under the symbol “LQDA” since July 26, 2018. Prior to that date, there was no established public trading market for our common stock. As of March 9, 2020, the closing price of our common stock was $3.70 per share.
Holders
As of March 9, 2020, there were 89 record holders of our common stock, based upon information received from our transfer agent. However, this number does not include beneficial owners whose shares were held of record by nominees or broker dealers. We estimate that there are more than 1,000 beneficial owners of our common stock.
Dividend Policy
We have never paid any cash dividends on our capital stock. We anticipate that we will retain earnings, if any, to support operations and to finance the growth and development of our business. In addition, the terms of our A&R LSA with PWB precludes us from paying cash dividends without the prior written consent of PWB. Therefore, we do not expect to pay cash dividends for the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth certain information regarding our equity compensation plans as of December 31, 2019:
|
Plan Category |
|
Number of securities |
|
Weighted-average |
|
Number of securities |
|
|
|
|
|
|
|
|
|
|
|
Equity compensation plans approved by security holders |
|
2,060,469(2) |
|
$ |
9.33 |
|
1,287,561(3) |
|
|
|
|
|
|
|
|
|
|
Equity compensation plans not approved by security holders |
|
— |
|
$ |
— |
|
— |
|
|
|
|
|
|
|
|
|
|
Total |
|
2,060,469(2) |
|
$ |
9.33 |
|
1,287,561 |
(1) Represents the weighted-average exercise price of outstanding stock options only.
(2) Includes 7,493 restricted stock units.
(3) On January 1, 2020, an additional 1,129,250 shares of common stock were automatically added to the shares authorized for issuance under the Liquidia Technologies, Inc. 2018 Long-Term Incentive Plan, or the 2018 Plan, pursuant to an “evergreen” provision contained therein. Pursuant to such provision, on January 1 of each year through 2028, the number of shares authorized for issuance under the 2018 Plan is automatically increased by a number equal to four percent of the outstanding shares of common stock as of the end of our immediately preceding fiscal year, or any lesser number of shares of common stock determined by our Board or Compensation Committee of our Board.
Stock Performance Graph
Not applicable.
Sale of Unregistered Securities
Previously disclosed on a Current Report on Form 8-K filed with the SEC on December 26, 2019.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not repurchase any of our securities during the three months ended December 31, 2019.
Item 6. Selected Financial Data.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing in this Annual Report on Form 10-K. This discussion and other parts of this Annual Report on Form 10-K contain forward-looking statements that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report on Form 10-K, our actual results could differ materially from the results described in, or implied by, the forward-looking statements contained in the following discussion and analysis.
Overview
We are a late-stage clinical biopharmaceutical company focused on the development and commercialization of novel products utilizing our proprietary PRINT® technology to transform the lives of patients. PRINT is a particle engineering platform that enables precise production of uniform drug particles designed to improve the safety, efficacy and performance of a wide range of therapies. We are currently focused on the development of two product candidates for which we hold worldwide commercial rights: LIQ861 for the treatment of pulmonary arterial hypertension, or PAH, and LIQ865 for the treatment of local post-operative pain. LIQ861, for which we recently filed a New Drug Application, or NDA, with the FDA, is an inhaled dry powder formulation of treprostinil designed to improve the therapeutic profile of treprostinil by enhancing deep-lung delivery and achieving higher dose levels than current inhaled therapies. We have applied our PRINT technology to enable us to deliver LIQ861 through a convenient, palm-sized dry powder inhaler, or DPI. We have also applied our PRINT technology to our second product candidate, LIQ865, for which we have completed two Phase 1 clinical trials and have initiated Phase 2-enabling toxicology studies. LIQ865 is designed to deliver sustained-release particles of bupivacaine, a non-opioid anesthetic, to treat local post-operative pain for three to five days through a single administration. Additionally, we recently initiated a pre-clinical program to develop an inhaled product leveraging the benefits of our PRINT technology to engineer particles with precise, uniform, aerodynamic size and shape for deep lung delivery.
Our primary objective has been to pursue marketing approval of LIQ861 and commercialize such product if approved by FDA. We will need to raise substantial additional capital to continue our business operations, remain in compliance with the minimum cash requirement on our debt during and beyond the third quarter of 2020, and to commercialize LIQ861, if approved. Such capital may not be available to us on a timely basis, on terms that are favorable to us, or at all. Alternatively, in light of our current limited cash resources, the recent trading price of our common stock, outstanding debt and associated minimum cash covenant, and based on a review of the status of our programs, resources and capabilities, we continue to explore a wide range of strategic alternatives with the support of our financial advisor, Jefferies LLC, or Jefferies, that could maximize stockholder value. Our efforts have been and continue to be focused primarily upon the potential formation of a partnership or a licensing transaction with respect to our lead program, LIQ861, for the treatment of PAH. Strategic alternatives may also include the sale of some of our assets or proprietary technologies, or a potential merger or sale of the Company. There can be no assurance that we will be able to enter into such a transaction or transactions on a timely basis, on terms that are favorable to us, or at all.
Product Pipeline
We are currently focused on the development of two product candidates for which we hold worldwide commercial rights: LIQ861 for the treatment of PAH and LIQ865 for the treatment of local post-operative pain.
The following table summarizes our clinical-stage product candidates being developed using PRINT technology:
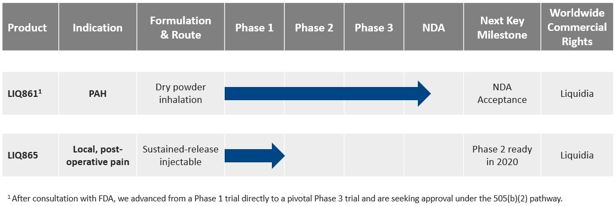
LIQ861
In January 2020, we submitted an NDA to the FDA for LIQ861, our lead product candidate, as a potential treatment for patients with PAH. Treprostinil is a synthetic analog of prostacyclin, a vasoactive mediator essential to normal lung function, which is deficient in patients with PAH. We believe that LIQ861 has the potential to improve the therapeutic profile of existing formulations of treprostinil by enhancing deep-lung delivery and achieving higher dose levels than current inhaled therapies. We are developing LIQ861 under the 505(b)(2) regulatory pathway with Tyvaso as the reference listed drug, which allows us to rely in part on the FDA’s previous findings of efficacy and safety of Tyvaso and the active ingredient treprostinil, which has been approved in four different products administered through the oral, inhaled and continuous infusion (parenteral) routes.
In August 2019, we completed an open-label Phase 3 clinical trial, INSPIRE, or Investigation of the Safety and Pharmacology of Dry Powder Inhalation of Treprostinil, for LIQ861. The primary objective of the INSPIRE study was to evaluate the long-term safety and tolerability of LIQ861. The study was designed to evaluate patients who have either been under stable treatment with Tyvaso (nebulizer-delivered treprostinil), for at least three months and were transitioned to LIQ861 under the protocol, or Transition patients, or patients who had been under stable treatment with no more than two non-prostacyclin oral PAH therapies for at least three months and then had their treatment regimen supplemented with LIQ861 under the protocol, or Add-On patients. Within the INSPIRE study, 18 Transition patients were evaluated in a one-directional crossover sub-study comparing bioavailability and pharmacokinetics, or PK, of treprostinil following dosing of LIQ861 as compared with Tyvaso.
In March 2019, we reported that we had completed enrollment and met the primary endpoint, which was long-term safety and tolerability, in our INSPIRE trial. LIQ861 was observed to be well-tolerated in 109 patients, with 101 patients (93%) completing at least two months of treatment. During the two-month period, LIQ861 was evaluated at doses up to 159 mcg with no study-drug related serious adverse events. Dosing has exceeded 159 mcg in some patients receiving drug beyond the Month 2 time point. We have not yet determined a maximum tolerated dose of LIQ861. We also reported fully enrolling our one-directional crossover sub-study comparing bioavailability and PK of treprostinil as sub-study patients transitioned from Tyvaso to LIQ861.
In April 2019, we reported further data from these 109 patients in our INSPIRE trial on exploratory endpoints at two months of treatment that demonstrated generally favorable results with respect to six-minute walk distance and quality of life as indicated by the Minnesota Living with Heart Failure Questionnaire, or MLHFQ. In May 2019, we reported further presentation of this data at the American Thoracic Society, or ATS, International Conference 2019.
In June 2019, we reported results from the INSPIRE study indicating that the 79.5 mcg dose of LIQ861 correlates with nine breaths of Tyvaso, the maximum recommended label dose of Tyvaso. Analysis of the data from the PK sub-study in patients showed variability in systemic plasma levels of both LIQ861 and Tyvaso, which is believed to be attributed to variation in severity of disease and has been seen in prior studies of treprostinil in patients. To more accurately characterize the PK of LIQ861, we conducted two additional PK studies in healthy volunteers. In the first of these studies, we observed unexpected variability in PK levels. Post-hoc analysis showed that plasma levels of treprostinil were tightly correlated to the LIQ861 dose delivered. Based upon additional non-clinical and clinical work, we believe the unexpected variability seen in this healthy volunteer study was due to an administration technique unique to the conduct of the study in the Phase 1 setting. In August 2019, we completed a second PK study in healthy volunteers in which the proper administration technique was followed. This study demonstrated significantly reduced variability, and we believe we have established comparative bioavailability to the reference listed drug.
Final enrollment in the pivotal INSPIRE trial included 121 PAH patients to assess safety and tolerability through Month 2, the primary endpoint of the trial. Of the 121 patients enrolled in the study, 55 were Transition patients and 66 were Add-On patients. Add-On patients started on a dose of 26.5 mcg of LIQ861, with most (>80%) titrating to a 79.5 mcg dose or higher within the first two months of treatment. Consistent with preliminary data presented in the second quarter of 2019, LIQ861 was observed to be well-tolerated and treatment-emergent adverse events were mostly mild to moderate in nature at Month 2 up to doses of 159 mcg of LIQ861, the highest dose studied at Month 2. Durability of therapy with LIQ861 appeared to be favorable, with 96% of Transition patients and 91% of Add-On patients remaining on study drug at the Month 2 timepoint.
Initial analysis of the exploratory endpoints from the INSPIRE study indicates that LIQ861 may provide functional and quality-of-life benefits to PAH patients in New York Heart Association, or NYHA, functional classes II and III. More than 90% of all patients who completed two months of treatment maintained or improved their NYHA functional class. Additionally, we observed improvement in six-minute-walk-distance and quality of life as measured by the MLHFQ in both patient groups.
We continued to treat patients who chose to remain on LIQ861 beyond the Month 2 timepoint of the primary endpoint. More than 80% of INSPIRE patients remained on study drug at Month 4 with no significant changes in safety or tolerability observed compared to Month 2. At the completion of the INSPIRE study, the patient with the longest duration of treatment had been on LIQ861 therapy for 18 months. To provide for continuity of treatment, patients from INSPIRE were provided the opportunity to continue receiving treatment in an extension study, which is currently ongoing. In addition, we are enrolling patients in a clinical study at certain investigational sites in Europe to characterize the hemodynamic dose-response relationship to LIQ861. We are also considering conducting other clinical trials to generate additional data on LIQ861, including a clinical trial in pediatric patients. We also continue to conduct development work in support of potential approval and commercialization of LIQ861, including label and patient-use assessments.
LIQ865
LIQ865 is our proprietary injectable, sustained-release formulation of bupivacaine, a non-opioid pain medication. We have engineered the size and composition of the LIQ865 PRINT particles to release bupivacaine over three to five days through a single administration for the management of local post-operative pain after a surgical procedure. We completed a Phase 1a clinical trial of LIQ865 in Denmark in 2017 and a Phase 1b clinical trial in the United States in 2018. We initiated Phase 2-enabling toxicology studies in 2019 to assess LIQ865 in multiple non-clinical tissue models. Results from a study to assess incision tensile strength after healing were acceptable and not statistically different from controls. A nonclinical study to examine soft tissue healing was also completed, and the results were acceptable and comparable to vehicle-treated, saline-treated, and Marcaine-treated sites. We believe this data supports progression to Phase 2 hernia repair studies. In a study to assess bone fracture healing, we observed dose-dependent delayed healing at the two LIQ865 doses studied; however, there were no adverse effects noted on surrounding soft tissues. Additional studies have been initiated with lower doses of LIQ865 to determine a no adverse effect level, or NOAEL, on bone healing. We will review the results from these toxicology studies, and if supportive, we intend to initiate Phase 2 proof-of-concept clinical trials, subject to availability of capital and other factors, during 2021. We believe LIQ865, if successfully developed and approved, has the potential to provide significantly longer local post-operative pain relief compared to currently marketed formulations of bupivacaine.
Other
We believe that our PRINT technology can be applied to a wide range of therapeutic areas, molecule types and routes of administration. We are currently focused on developing product candidates that we believe are eligible to be approved under the 505(b)(2) regulatory pathway, which can be capital efficient and potentially enable a shorter time to approval, as it allows us to rely in part on existing knowledge of the safety and efficacy of the relevant reference listed drugs to support our applications for approval in the United States. If any of our product candidates are approved, we intend to conduct initial commercial manufacturing of drug product using in-house capabilities, and to outsource packaging and distribution to third parties. Where appropriate, we may also transition the commercial manufacture of our drug product to third parties. In addition to developing our two product candidates, we have provided specific field-limited licenses to our PRINT technology to pharmaceutical companies seeking to develop their own potential drugs and biological therapies.
Financial Overview
We have not generated any revenue to date from the sale of pharmaceutical products, and we have historically financed our operations in large part with an aggregate of $235.3 million of gross proceeds from sales of our capital stock and convertible promissory notes, $16.0 million in term loans from a bank and a $2.1 million loan from The University of North Carolina at Chapel Hill, or UNC. We do not expect to generate significant product revenue unless and until we obtain marketing approval for and commercialize LIQ861, LIQ865 or one of our other future product candidates.
Since our inception, we have incurred significant operating losses. Our net loss was $47.6 million, $53.1 million and $29.2 million for the years ended December 31, 2019, 2018 and 2017, respectively. As of December 31, 2019, we had an accumulated deficit of $215.2 million. We expect to incur significant expenses and operating losses for the foreseeable future as we advance our product candidates through clinical trials, seek regulatory approval and pursue commercialization of any approved product candidates. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. In addition, we may incur expenses in connection with the in-license or acquisition of additional product candidates.
As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through the sale of equity, debt financings or other capital sources, including potential collaborations with other companies or other strategic transactions. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed, on favorable terms, or at all. If we fail to raise capital or enter into such agreements as, and when, needed, we may have to significantly delay, scale back or discontinue the development and commercialization of one or more of our product candidates or delay our pursuit of potential in-licenses or acquisitions.
As of December 31, 2019, we had $55.8 million of cash. We believe that our existing cash will enable us to fund our operating expenses and capital expenditure requirements, make payments of interest and principal on our term loan facility with Pacific Western Bank, or PWB, and remain in compliance with the minimum cash covenant of $8.5 million pursuant to this term loan facility, through August 2020. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect. See “Liquidity and Capital Resources.”
As of December 31, 2019, our commitments for capital expenditures consisted of a remaining payment obligation of approximately $360,000 related to the build-out of our corporate headquarters which was completed in 2019. As of December 31, 2019, we do not have any other material capital expenditure commitments.
Going Concern
Our financial statements have been prepared assuming we will continue as a going concern, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. Our operations have consisted primarily of developing our technology, developing products, prosecuting our intellectual property and securing financing. We have incurred recurring losses and cash outflows from operations, have an accumulated deficit, and have debt principal payments that commenced in the first quarter of 2020. We expect to continue to incur losses in the foreseeable future and will require additional financial resources to continue to advance our products and intellectual property, in addition to repaying our maturing debt and other obligations. These circumstances raise substantial doubt about our ability to continue as a going concern.
Management’s plans with regard to this matter include continuing attempts to obtain additional financing to sustain our operations. However, there is no assurance that we will be successful in obtaining sufficient financing on terms acceptable to us, and the failure to obtain sufficient funds on acceptable terms, when needed, could have a material adverse effect on our business, results of operations and financial condition. If sufficient financings are not obtained, this may necessitate other actions by us. Alternatively, in light of our current limited cash resources, the recent trading price of our common stock, outstanding debt and associated minimum cash covenant, and based on a review of the status of our programs, resources and capabilities, we continue to explore a wide range of strategic alternatives with the support of our financial advisor, Jefferies LLC, that could maximize stockholder value. Our efforts have been and continue to be focused primarily upon the potential formation of a partnership or a licensing transaction with respect to our lead program, LIQ861, for the treatment of PAH. Strategic alternatives may also include the sale of some of our assets or proprietary technologies, or a potential merger or sale of our Company. Our plans with regard to this matter include continuing attempts to obtain additional financing to sustain our operations. However, there is no assurance that we will be successful in obtaining sufficient financing on terms acceptable to us, and the failure to obtain sufficient funds on acceptable terms, when needed, could have a material adverse effect on our business, results of operations and financial condition. If sufficient financings are not obtained, this may necessitate other actions by us. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Our Collaborations
Our only revenue, which has been derived from collaborating with, and licensing our proprietary PRINT technology to, pharmaceutical companies, amounted to $8.1 and $2.7 million for the years ended December 31, 2019 and 2018, respectively. GlaxoSmithKline plc, or GSK, accounted for $8.1 and $0.4 million of our revenue for the years ended December 31, 2019 and 2018, respectively, or 100% and 15% respectively, of our total revenue during these years. We have received upfront fees for technology access, milestone payments, and fees to develop drug products through research and development services, such as particle formulation and manufacturing.
In addition to advancing our own product candidates, LIQ861 and LIQ865, we have collaborated, and may consider collaborating, with pharmaceutical companies to develop their own product candidates across a wide range of therapeutic areas, molecule types and routes of administration, leveraging our PRINT technology. These collaborations are intended to help advance new PRINT capabilities and build upon our competitive advantage in the pharmaceutical industry, while adding to our intellectual property portfolio.
We have exclusively licensed our PRINT technology to (i) GSK for applications broadly across inhaled delivery of their small molecule and biologic chemical entities, although we retained the ability to develop LIQ861; and (ii) Aerie Pharmaceuticals, Inc., which in 2017 acquired most of the assets of Envisia Therapeutics, Inc., an entity which we formed in 2013, for broad usage in the design and commercialization of small molecule and biologic ophthalmic therapies.
GlaxoSmithKline
Previously, we had collaborated with GSK on the use of our PRINT technology in respiratory disease. In June 2012, we entered into an Inhaled Collaboration and Option Agreement, or the GSK ICO Agreement, with GSK to collaborate on research regarding the application of our PRINT technology to specified inhaled therapies. Pursuant to the GSK ICO Agreement, we granted GSK exclusive options and licenses to further develop and commercialize such inhaled therapies using our PRINT technology. In partial consideration of the rights granted to GSK under the GSK ICO Agreement, we received a one-time up-front payment of $4.0 million.
In September 2015, GSK exercised its option to obtain an exclusive, worldwide license to certain of our know-how and patents relating to our PRINT technology, for the purpose of, among others, preclinical studies of inhaled therapeutics developed, manufactured or otherwise produced using our PRINT technology. In connection with the grant of this license, we received a one-time option exercise fee of $15.0 million. Under the terms of the GSK ICO Agreement, we were also entitled to continued research and development funding and certain milestone payments aggregating up to $158 million upon the achievement of specified events for new non-rescue therapeutic products. Rescue therapeutic products are therapeutics that GSK develops with our PRINT technology that had previously been discontinued from development. We are also entitled to tiered royalties on the worldwide sales of the licensed products at percentages ranging from the mid-single digits to low-single digits depending on the total number of products developed and other royalty step-down events with a fixed low-single digit royalty floor. In February 2016, we received a $3.0 million payment from GSK upon the achievement of a clinical development milestone related to the development of an inhaled antiviral for viral exacerbations in chronic obstructive pulmonary disease. However, in July 2018, GSK notified us of its plans to discontinue development of this compound after completion of the related Phase 1 clinical trial.
GSK has the right to terminate the GSK ICO Agreement in its entirety or on a product-by-product basis upon a specified period of prior written notice. Upon termination of the GSK ICO Agreement, each party will continue to have the right to practice and/or license its interest in any know-how developed during the collaboration without seeking the consent of, or accounting to, the other party.
In June 2019, we and GSK executed the third amendment to the collaboration agreement providing us with rights to develop and commercialize three specified molecular entities for application in inhaled programs using our PRINT technology at our sole expense. This amendment also provided a mechanism for us to acquire rights to develop and commercialize further molecular entities for inhaled applications. New inhaled programs developed under this amendment would carry milestone and royalty payments due to GSK upon initiation of Phase 3 studies and subsequent commercialization, respectively. This amendment, among other factors, including the lack of continued performance anticipated by us and GSK under the original agreement, led us to believe that no further research and development services will be provided to GSK under the collaboration agreement. Accordingly, in January 2020, we notified GSK of our intent to terminate the GSK ICO Agreement based upon GSK's lack of performance under the agreement, which we believe constitutes a material breach of the agreement. In February 2020, we received a letter from GSK disputing our basis for termination. The parties are currently attempting to resolve the dispute pursuant to the terms of the GSK ICO Agreement.
Components of Statements of Operations
Revenue
Our revenue is primarily derived from collaborating and licensing our proprietary PRINT technology to pharmaceutical companies. In the future, we also expect to derive our revenue from our own pharmaceutical products. Up until the fourth quarter of 2018, we managed, reported and evaluated our business in the following two segments: Pharmaceutical Products and Partnering and Licensing. These reportable operating segments were determined in accordance with our internal management structure, which was organized based on operating activities, the manner in which we organized segments for making operating decisions and assessing performance and the availability of separate financial results.
In the fourth quarter of 2018, due to significantly diminished activities pursuant to collaborations, we changed the way we manage and operate the reporting entity and modified our information system to produce financial information for the chief operating decision maker, or CODM, to support the new structure. The changes required us to revise our segment reporting. Management reorganized our operations and reporting structure and began to manage our operations under our new segment structure, resulting in a single reportable segment. The financial statements were adjusted to reflect this change in segment reporting for all periods presented.
All long-lived assets are domiciled and all revenues were earned within the United States.
Cost of Sales
Cost of sales consists of the amortization of license fees owed to UNC upon our receipt of licensing revenues. See “Business — Our Collaboration and Licensing Agreements — The University of North Carolina at Chapel Hill” for further details. We amortize the license fees owed to UNC in a manner consistent with our recognition of the related revenue.
Research and Development Expenses
Research and development expense consists of expenses incurred in connection with the development of our product candidates. We expense research and development costs as incurred. These expenses include:
|
● |
expenses incurred under agreements with CROs as well as investigative sites and consultants that conduct our clinical trials and preclinical studies; |
|
● |
manufacturing process development and scale-up expenses and the cost of acquiring and manufacturing preclinical and clinical trial materials and commercial materials, including manufacturing validation batches; |
|
● |
outsourced professional scientific development services; |
|
● |
employee-related expenses, which include salaries, benefits and stock-based compensation for personnel in research and development functions; |
|
● |
expenses relating to regulatory activities, including filing fees paid to regulatory agencies; |
|
● |
laboratory materials and supplies used to support our research activities; and |
|
● |
allocated expenses for utilities and other facility-related costs. |
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect our research and development expenses to increase significantly over the next several years as we increase personnel costs, including stock-based compensation, conduct our ongoing clinical trial and other development work for LIQ861, continue the development of LIQ865, conduct additional clinical trials, continue manufacturing process development and scale up and prepare for regulatory filings for our product candidates and regulatory inspection of facilities utilizing our PRINT manufacturing process. The successful development of our product candidates is highly uncertain. At this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the remainder of the development of, or when, if ever, material net cash inflows may commence from any of our product candidates. This uncertainty is due to the numerous risks and uncertainties associated with the duration and cost of clinical trials, which vary significantly over the life of a project as a result of many factors, including:
|
● |
the number of clinical sites included in the trials; |
|
● |
the length of time required to enroll suitable patients; |
|
● |
the number of patients that ultimately participate in the trials; |
|
● |
the number of doses patients receive; |
|
● |
the duration of patient follow-up; and |
|
● |
the results of our clinical trials. |
Our expenditures are subject to additional uncertainties, including the terms and timing of regulatory approvals, and the expense of filing, prosecuting, defending and enforcing any patent claims or other intellectual property rights. We may never succeed in achieving regulatory approval for any of our product candidates. We may obtain unexpected results from our clinical trials. We may elect to discontinue, delay or modify clinical trials of some product candidates or focus on others. A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA or other regulatory authorities were to require us to conduct clinical trials beyond those that we currently anticipate, or if we experience significant delays in enrollment in any of our clinical trials, or our ability to manufacture and supply product, we could be required to expend significant additional financial resources and time on the completion of clinical development. Drug commercialization will take several years and millions of dollars in development costs.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for personnel in executive, administrative, finance and legal functions, including stock-based compensation, travel expenses and recruiting expenses. Other general and administrative expenses include facility related costs, patent filing and prosecution costs and professional fees for marketing, legal, auditing and tax services and insurance costs.
We anticipate that our general and administrative expenses will increase as a result of increased personnel costs, including stock-based compensation, expanded infrastructure and higher consulting, legal and tax-related services associated with maintaining compliance with stock exchange listing and SEC requirements, accounting and investor relations costs, and director and officer insurance premiums associated with being a public company. Additionally, when we believe a regulatory approval of a product candidate appears likely, we anticipate an increase in payroll and expense as a result of our preparation for commercial operations, especially as it relates to the sales and marketing of our product candidate.
Other Income (Expense)
Other income (expense) is comprised primarily of interest income and expense. Interest income consists of interest earned on our cash deposits. Interest expense consists of interest charges on leases and debt. These charges include monthly recurring interest on such obligations in addition to non-cash charges. Non-cash charges include the expensing of debt issuance costs and amortization of discounts on long-term debt to interest expense.
Critical Accounting Estimates
This management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those for revenue recognition and accrued research and development expenses. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our financial statements, we believe the following accounting policies to be the most critical to the judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
We derive our revenues primarily from licensing our proprietary PRINT technology and from performing research and development services. Revenues are recognized as services are performed in an amount that reflects the consideration we expect to be entitled to in exchange for those services and technology.
Our research, development and licensing agreements provide for multiple promised goods and services to be provided by us and include a license to our technology in a particular field of study, participation in collaboration committees, performance of certain research and development services and obligations for certain manufacturing services.
The transaction price for these contracts includes non-refundable fees and fees for research and development services. Non-refundable upfront fees, which may include, for example, an initial payment upon effectiveness of the contractual relationship or payment to secure a right for a future license, are recorded as deferred revenue and recognized into revenue over time as we provide the research services under the contract required to advance the products to the point where we are able to transfer control of the licensed technology to the customer, or the Technology Transfer. The contract consideration may also include additional non-refundable payments due to us based on the achievement of research, development, regulatory or commercialization milestone events. In agreements involving multiple goods or services promised to be transferred to customers, we must assess, at the inception of the contract, whether each promise represents a separate performance obligation (i.e., is “distinct”), or whether such promises should be combined as a single performance obligation. As these goods and services are considered to be highly interrelated, they may be considered to represent a single, combined performance obligation. We include an estimate of the probable amount of milestone payments to which we will be entitled in the transaction price. The estimate requires evaluation of factors which are outside of our control and significantly limit our ability to achieve the remaining milestone payments. Therefore, we have not included any future milestone payments in the transaction price allocated to research, development and licensing agreements as of December 31, 2019. We revise the transaction price to include milestone payments once the specific milestone achievement is not considered to be subject to a significant reversal of revenue. At that time, the estimated transaction price is adjusted and a cumulative catch-up adjustment is recorded to adjust the amount of revenue to be recognized from the license inception to the date the milestone was deemed probable of achievement. The milestone is included with other non-refundable upfront fees and recognized into revenue over time as we continue to provide services under the contract through our Technology Transfer. The amount of revenue recognized is based on the proportion of total research services performed to date to the expected services to be provided through the Technology Transfer.
The estimate of the research services to be provided through the Technology Transfer requires significant judgment to evaluate assumptions regarding the level of effort required for us to have performed sufficient obligations for the customer to be able to utilize the licensed technology without requiring further services from us. If the estimated level of effort changes, the remaining deferred revenue is recognized over the revised period in which the expected research services and Technology Transfer are required. Changes in estimates occur for a variety of reasons, including but not limited to (i) research and development acceleration or delays, (ii) customer prioritization of research projects, or (iii) results of research and development activities. We recognize the consideration we are entitled to receive for research and development services, which are primarily billed quarterly in arrears on a time and materials basis, as the services are performed (under a proportional performance model) and collection is reasonably assured. Additionally, any upfront or development milestone payments received are also recognized as revenue, over time, under this same proportional performance model.
Royalties related to product sales will be recognized as revenue when the sale occurs since payments relate directly to products that will have been fully developed and for which we will have satisfied all performance obligations.
Accrued Research and Development Expenses
When preparing our financial statements, we are required to estimate our research and development expenses. This process involves reviewing open contracts and communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. Payments under some of the contracts we have with parties depend on factors, such as successful enrollment of certain numbers of patients, site initiation and the completion of clinical trial milestones. When accruing clinical expenses, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If possible, we obtain information regarding unbilled services directly from our service providers. However, we may be required to estimate the cost of these services based only on information available to us. If we underestimate or overestimate the cost associated with a trial or service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Historically, our estimated research and development expenses have approximated actual expenses incurred.
Examples of estimated accrued expenses include:
|
• |
fees paid to CROs in connection with clinical trials; and |
|
• |
fees paid to investigative sites in connection with clinical trials. |
If the actual timing of the performance of services or the level of effort varies from our estimate, we will adjust the accrual accordingly. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. We do not anticipate the future settlement of existing accruals to differ materially from our estimates.
Year Ended December 31, 2019 Compared to Year Ended December 31, 2018
The following table summarizes our results of operations:
|
Year Ended |
||||||||
|
December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
(in thousands) |
||||||||
|
Revenues |
$ | 8,072 | $ | 2,707 | ||||
|
Costs and expenses: |
||||||||
|
Cost of sales |
807 | 121 | ||||||
|
Research and development |
40,491 | 28,700 | ||||||
|
General and administrative |
13,597 | 8,754 | ||||||
|
Total costs and expenses |
54,895 | 37,575 | ||||||
|
Loss from operations |
(46,823 | ) | (34,868 | ) | ||||
|
Other income (expense): |
||||||||
|
Interest income |
614 | 305 | ||||||
|
Interest expense |
(1,374 | ) | (18,989 | ) | ||||
| Gain on early extinguishment of long-term debt | — | 138 | ||||||
|
Derivative and warrant fair value adjustments |
— | 278 | ||||||
|
Total other income (expense) |
(760 | ) | (18,268 | ) | ||||
|
Net loss |
$ | (47,583 | ) | $ | (53,136 | ) | ||
Revenues
Revenues were $8.1 million for the year ended December 31, 2019, compared with $2.7 million for the year ended December 31, 2018. The increase of $5.4 million, or 198.2%, was due to the full recognition in the second quarter of 2019 of $8.1 million of deferred revenue from the GSK ICO Agreement resulting from the Third Amendment that was entered into in June 2019.
Cost of Sales
Our cost of sales was $0.8 million for the year ended December 31, 2019, compared with $0.1 million for the year ended December 31, 2018. The increase of $0.7 million was due to the increase in revenues. Cost of sales represents sub-licensing fees paid to UNC when licensing revenue is recognized from the use of the intellectual property that we in-licensed from UNC.
Research and Development Expenses
Research and development expenses were $40.5 million for the year ended December 31, 2019, compared with $28.7 million for the year ended December 31, 2018. The increase of $11.8 million, or 41.1%, was primarily due to the ongoing clinical development of LIQ861 which commenced in late December 2017. Research and development expenses for the year ended December 31, 2019 consisted of $31.7 million and $4.0 million attributable to our development of LIQ861 and LIQ865, respectively, and $4.8 million from general research and development that was not directly related to LIQ861 and LIQ865. This compares with $19.6 and $0.7 million which were attributable to our development of LIQ861 and LIQ865, respectively, and $8.4 million from general research and development that was not directly related to either LIQ861 or LIQ865 during the year ended December 31, 2018.
General and Administrative Expenses
General and administrative expenses were $13.6 million for the year ended December 31, 2019, compared with $8.8 million for the year ended December 31, 2018. The increase of $4.8 million, or 55.3% during the year ended December 31, 2019 compared with 2018 was due to an increase in commercialization efforts expenses of $2.4 million, an increase in directors and officers insurance of $0.8 million, an increase in stock-based compensation of $0.7 million, an increase in recruiting fees of $0.5 million, an increase in consulting fees of $0.2 million and an increase in legal fees of $0.2 million. General and administrative expenses consist primarily of personnel expenses, including stock-based compensation, as well as directors and officers insurance, and fees for audit, legal, consulting and other service fees.
Other Income (Expense)
Interest income was $0.6 million for the year ended December 31, 2019, compared with $0.3 million for the year ended December 31, 2018. The increase of $0.3 million was due to an increase in cash balances held in interest-bearing accounts in 2019 compared with 2018.
Interest expense was $1.4 million for the year ended December 31, 2019, compared with $19.0 million for the year ended December 31, 2018. The decrease in interest expense of $17.6 million, or 92.8%, was primarily due to lower levels of debt during the year ended December 31, 2019 and the conversion of $27.4 million of convertible notes into shares of Series D preferred stock in February 2018.
During 2018, our debt refinancing resulted in a non-cash gain of $0.1 million in accordance with ASC 470-50, Debt – Modifications and Extinguishments.
Derivative and warrant fair value adjustments resulted in income of $0 for the year ended December 31, 2019, compared with income of $0.3 million for the year ended December 31, 2018. The decrease of $0.3 million was due to the conversion of the warrants for convertible preferred stock to warrants for common stock at the time of the initial public offering during the year ended December 31, 2018.
Liquidity and Capital Resources
We have financed our growth and operations through a combination of funds generated from our licensing revenues, the issuance of convertible preferred stock and common stock, capital leases, bank borrowings and the issuance of convertible notes. Our principal uses of cash have been for working capital requirements and capital expenditures. As of December 31, 2019, we had a cash balance of $55.8 million, stockholders’ equity of $34.9 million and an accumulated deficit of $215.2 million.
In July 2018, we closed the initial public offering of 4,833,099 shares of common stock at a public offering price of $11.00 per share, including the underwriters’ partial exercise of their over-allotment option in connection therewith, which resulted in aggregate net proceeds of $47.3 million, after underwriting discounts and the payment of other offering expenses.
In March 2019, we closed an underwritten follow-on offering of 3,000,000 shares of our common stock at a public offering price of $11.50 per share. The gross proceeds from the offering were $34.5 million and net proceeds were $31.8 million, after deducting underwriting discounts and commissions and other offering expenses.
In August 2019, we entered into a sales agreement, or the ATM Agreement, with Jefferies to issue and sell shares of our common stock, having an aggregate offering price of up to $40.0 million, from time to time during the term of the ATM Agreement, through an “at-the-market” equity offering program at our sole discretion, under which Jefferies will act as our agent and/or principal. We will pay Jefferies a commission up to 3.0% of the gross proceeds of any common stock sold through Jefferies under the ATM Agreement. During the year ended December 31, 2019, we sold 2,409,356 shares of common stock for gross proceeds of $8.4 million and net proceeds were $8.1 million, after deducting underwriting discounts and other offering expenses under the ATM Agreement.
On December 23, 2019, we entered into a Common Stock Purchase Agreement, or the Purchase Agreement, with certain institutional accredited investors, or the Purchasers, for the sale by us in a private placement, or the Private Placement, of an aggregate of 7,164,534 shares, or the Private Placement Shares, of our common stock, at a purchase price of $3.13 per Private Placement Share. The closing of the Private Placement occurred on December 27, 2019. The gross proceeds from the sale of the Private Placement Shares were $22.4 million and net proceeds were $21.0 million, after placement agent fees and offering expenses.
Prior to our initial public offering, in a series of closings from January 2017 through November 2017, we issued and sold an aggregate of $27.4 million of unsecured subordinated convertible promissory notes, each accruing simple interest at a rate of 8.0% per annum. Also prior to our initial public offering, in February 2018, we issued and sold an aggregate of 91,147,482 shares of Series D preferred stock at a price per share equal to $0.59808. Of the 31 investors that participated in the financing, 10 investors purchased an aggregate of 42,863,825 shares of Series D preferred stock for an aggregate purchase price of $25.6 million and 26 holders of outstanding convertible notes, in the aggregate amount of $28.9 million, converted their notes into an aggregate of 48,283,657 shares of Series D preferred stock.
In addition to raising equity capital, we have financed a portion of our working capital through debt instruments. We maintained a $10.0 million term loan facility with PWB for working capital purposes pursuant to a loan and security agreement, or the LSA. Immediately prior to entry into the A&R LSA (as defined below), we had fully utilized our $10.0 million term loan facility with PWB with a remaining outstanding balance of $8.0 million. The facility was secured by all of our assets other than intellectual property. We could not encumber our intellectual property without the consent of PWB. The outstanding principal amount under the loan facility bore interest at 5.0% per annum. Of the then-current amount outstanding, the loan was to mature with respect to $3.0 million in January 2020, with the remainder being due and payable in October 2020. Beginning in August 2018, the term loan would have required equal monthly payments of principal plus interest each month thereafter until amortized and paid in full. We have, in the past, breached multiple covenants in our LSA related to cash levels and reporting requirements. PWB provided waivers in relation to all such prior breaches.
In October 2018, we and PWB entered into an Amended and Restated Loan and Security Agreement, or the A&R LSA, in which we received an initial tranche of $11.0 million to extinguish our then-current debt of $8.0 million under the LSA, repay in full the outstanding indebtedness under the UNC Promissory Note (as defined below) and to utilize for general corporate purposes. The indebtedness under the A&R LSA bears interest at the greater of the Prime rate or 5% and has a four-year term and maturity. The A&R LSA provided for access to a second tranche of up to $5.0 million, the full amount of which we drew in June 2019. The second tranche became accessible as a result of the full enrollment of the Company’s LIQ861 INSPIRE clinical trial, without observing any materially adverse data through the two week endpoint. Both tranches required payments of interest-only through December 31, 2019. The A&R LSA carries a one-time success fee of $375,000 and a prepayment penalty of 1% if the drawn tranche is prepaid prior to October 27, 2020. The success fee was triggered in December 2019 by the sale of common stock and this was recorded as interest expense of $375,000 during the year ended December 2019. The minimum cash covenant is $8.5 million. In May 2019, we and PWB entered into an amendment to the A&R LSA to, among others, amend our negative covenant related to capitalized expenditures to increase the aggregate amount of capitalized expenditures we are permitted to make without PWB’s prior written consent during the fiscal year ending December 31, 2019 from $1.25 million to $2.5 million.
The A&R LSA with PWB, as amended, contains restrictions that limit our flexibility in operating our business. We may not, among other things, without the prior written consent of PWB, (a) pay any dividends or make any other distribution or payment on account of or in redemption, retirement or purchase of any capital stock except in certain prescribed circumstances, (b) create, incur, assume, guarantee or be or remain liable with respect to any indebtedness except certain permitted indebtedness or prepay any indebtedness, (c) replace or suffer the departure, as defined, of our Chief Executive Officer or Chief Financial Officer without delivering written notification to PWB within ten days of such change or (d) suffer a change on our Board which results in the failure of at least one partner of Canaan Partners or their respective affiliates to serve as a voting member, in each case without having used best efforts to deliver at least 15 days’ prior written notification to PWB. PWB maintains a blanket lien on all assets excluding intellectual property, for which it has been provided a negative pledge.
During most of the year ended December 31, 2018, we had outstanding a promissory note to UNC, or the UNC Promissory Note. The UNC Promissory Note was unsecured and bore interest at a rate equal to one-year LIBOR plus 3%, compounded annually. The UNC Promissory Note was due and payable in full on December 31, 2018. Following the completion of the initial public offering of our common stock in July 2018, we made a payment to UNC of $600,000 in August 2018. We repaid the entire balance outstanding under the UNC Promissory Note, plus accrued interest pursuant to the closing of the A&R LSA with PWB in October 2018.
Cash Flows
The following table summarizes our sources and uses of cash:
|
Year Ended |
||||||||
|
December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
(in thousands) |
||||||||
|
Net cash (used in) provided by: |
||||||||
|
Operating activities |
$ | (48,283 | ) | $ | (31,830 | ) | ||
|
Investing activities |
(1,850 | ) | (871 | ) | ||||
|
Financing activities |
66,394 | 68,817 | ||||||
|
Net (decrease) increase in cash |
$ | 16,261 | $ | 36,116 | ||||
Operating Activities
Net cash used in operating activities increased $16.5 million, to $48.3 million for the year ended December 31, 2019 from $31.8 million for the year ended December 31, 2018. The increase was mainly due to an increase from our research and development expenditures and general and administrative costs during the year ended December 31, 2019 compared with 2018. For the year ended December 31, 2019, the net cash used in operating activities was $48.3 million, which was comprised of operating cash outflows before working capital changes of $40.8 million and net working capital outflows of $7.5 million.
Investing Activities
Net cash used in investing activities increased $1.0 million to $1.9 million for the year ended December 31, 2019 from $0.9 million for the year ended December 31, 2018. The increase was due to a higher level of purchases of property, plant and equipment.
Financing activities
Net cash provided by financing activities decreased $2.4 million to $66.4 million for the year ended December 31, 2019 from $68.8 million for the year ended December 31, 2018. This decrease was primarily due to net proceeds from follow-on offerings of common stock of $63.0 million and the $5.0 million draw under the A&R LSA during the year ended December 31, 2019, compared with the sale of Series D preferred stock of $25.1 million and the sale of our common stock for net proceeds of $47.3 million in our initial public offering, during the year ended December 31, 2018.
Funding Requirements
We plan to focus in the near-term on the development, regulatory approval and potential commercialization of LIQ861 and LIQ865. We anticipate we will incur net losses for the next several years as we complete clinical development of these product candidates and continue research and development of additional product candidates. In addition, we plan to continue to invest in discovery efforts to explore additional product candidates, potentially build commercial capabilities and expand our corporate infrastructure. We may not be able to complete the development and initiate commercialization of these programs if, among others, our clinical trials are not successful or if the FDA does not approve our product candidates arising out of our current clinical trials when we expect, or at all.
Our primary uses of capital are, and we expect will continue to be, compensation and related expenses, clinical costs, manufacturing process development, external research and development services, laboratory and related supplies, legal and other regulatory expenses, administrative and overhead costs and debt service. Our future funding requirements will be heavily determined by the resources needed to support development of our product candidates.
As a publicly traded company we incur significant legal, accounting and other expenses that we were not required to incur as a private company. In addition, the Sarbanes-Oxley Act, as well as rules adopted by the SEC and Nasdaq Stock Market LLC, or Nasdaq, require public companies to implement specified corporate governance practices that previously were inapplicable to us as a private company. We expect these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
We believe that our current cash balance will enable us to fund our operating expenses and capital expenditure requirements into the fourth quarter of 2020. However, there is a minimum cash requirement in conjunction with our term loan facility with PWB of $8.5 million which could be reached by the end of the third quarter of 2020 unless additional funds are raised. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect. We expect that we will require additional capital to complete NDA regulatory review of LIQ861 and commercialize our product candidates, if we receive regulatory approval, and to pursue in-licenses or acquisitions of other product candidates. If we receive regulatory approval for LIQ861 or LIQ865, we expect to incur significant commercialization expenses related to product manufacturing, sales, marketing and distribution, depending on where we choose to commercialize. Additional funds may not be available on a timely basis, on favorable terms, or at all, and such funds, if raised, may not be sufficient to enable us to continue to implement our long-term business strategy. If we are unable to raise sufficient additional capital, we may need to substantially curtail our planned operations and the pursuit of our growth strategy.
We may raise additional capital through licensing activities, other business arrangements or the sale of equity or convertible debt securities. In such an event, your ownership will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of our common stock.
Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceuticals, we are unable to estimate the exact amount of our working capital requirements. Our future funding requirements will depend on many factors, including:
|
● |
the number and characteristics of the product candidates we pursue; |
|
● |
the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical studies and clinical trials; |
|
● |
the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates; |
|
● |
the cost of manufacturing our product candidates and any product we successfully commercialize; |
|
● |
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
|
● |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
|
● |
the timing, receipt and amount of sales of, or milestone payments related to or royalties on, our current or future product candidates, if any. |
See “Risk Factors” for additional risks associated with our substantial capital requirements.
Internal Controls and Procedures
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act). Internal control over financial reporting is a process designed by, or under the supervision of, the issuer’s Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, and effected by the issuer’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the issuer; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the issuer; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the issuer’s assets that could have a material effect on the financial statements.
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, management has assessed the effectiveness of our internal control over financial reporting based on the criteria set forth in the Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations, or COSO, of the Treadway Commission, and concluded that our internal control over financial reporting was not effective as of December 31, 2019 as a result of material weaknesses in our internal control over financial reporting.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company's annual or interim financial statements will not be prevented or detected on a timely basis. In connection with the assessment of the effectiveness of our internal control over financial reporting, our management identified the following material weaknesses that existed as of December 31, 2019:
During 2019, we experienced significant turnover in finance personnel that reduced the complement and skill of the resources within the Company. As a result, we did not maintain an effective control environment as we lacked a sufficient complement of resources with an appropriate level of knowledge, experience and training to design, maintain and monitor our internal control over financial reporting commensurate with our financial reporting requirements. As a result, this material weakness contributed to the following material weaknesses:
|
● |
We did not design and maintain controls to ensure adequate segregation of duties within our financial reporting function, including the preparation and review of journal entries. Specifically, some key accounting personnel had the ability to both prepare and post journal entries without an independent review by someone without the ability to prepare and post journal entries. |
|
|
|
|
|
|
● |
We did not design and maintain effective controls over certain information technology general controls for information systems that are relevant to the preparation of our financial statements. Specifically, we did not design and maintain effective user access controls to ensure appropriate segregation of duties and that adequately restrict user and privileged access to financial applications and data to appropriate Company personnel. |
These material weaknesses did not result in a material misstatement of the annual or interim financial statements. Additionally, these material weaknesses could result in a misstatement of the relevant account balances or disclosures that would result in a material misstatement to the annual or interim consolidated financial statements that would not be prevented or detected.
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm due to an exemption from such requirement for emerging growth companies.
We continue to evaluate the effectiveness of our remediation efforts, including demonstrating that the new or improved controls are designed appropriately and operate effectively for a reasonable period of time. We expect to make further changes to our internal controls. The following actions have been, or are expected to be, taken, to strengthen our controls and organizational structure:
|
• |
To address issues with recent employee turnover, we have hired a new controller, senior accountant, accounting analyst and accounts payable clerk. We also plan to hire or outsource additional accounting personnel to assist with improving the internal control environment, including a manager of accounting or senior accountant and director of SEC reporting and internal control. We expect to continue to evaluate our needs for additional personnel. We plan to leverage the services of consulting firms to assist us with the strengthening and monitoring of our internal controls processes and documentation. We expect to provide enhanced training to existing and new employees in order to enhance the level of communication and understanding of controls with key individuals that provide key information and perform key roles within our financial accounting and reporting group. |
|
• |
We plan to appropriately design, implement and maintain a formal policy that expressly prohibits a manual journal entry from being created and posted by the same employee. Further, we plan to implement a process that includes the creation of a journal entry report that identifies who created and posted each journal entry. The report will be reviewed each month by independent senior accounting personnel so that any exceptions to the policy can be identified on a timely basis and appropriately addressed. |
|
• |
We plan to appropriately design, implement and maintain a formal policy to limit the number of “Super Users”, maintain effective user access controls to ensure appropriate segregation of duties and adequately restrict user and privileged access to financial applications, programs and data to appropriate personnel. |
|
• |
We plan to implement a formal policy that preparation, review and approval of account reconciliations will be performed by qualified accounting personnel independent of those who create and post the related underlying journal entries. |
To assess the effectiveness of internal controls related to the remediation of the 2019 identified material weaknesses, we plan to begin implementing the remediation described herein. Implementation and testing is expected to occur during the year ending December 31, 2020 and we plan to provide an update on the status of our remediation activities on a quarterly basis.
JOBS Act
As an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012, as amended, or the JOBS Act, we can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards when they are required to be adopted by public companies that are not emerging growth companies.
Subject to certain conditions, as an emerging growth company, we rely on certain of these exemptions, including without limitation:
|
● |
reduced disclosure about our executive compensation arrangements; |
|
● |
no advisory votes on executive compensation or golden parachute arrangements; and |
|
● |
exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of 2023; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC. We may choose to take advantage of some but not all of these exemptions. Accordingly, the information contained herein may be different from the information you receive from other public companies in which you hold stock.
Smaller Reporting Company
As a “smaller reporting company,” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, in addition to providing reduced disclosure about our executive compensation arrangements and business developments, among other reduced disclosure requirements available to smaller reporting companies, we present only two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure. Accordingly, the information contained herein may be different from the information you receive from other public companies in which you hold stock.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Not applicable.
Item 8. Financial Statements and Supplementary Data.
Our financial statements required to be filed pursuant to this Item 8 appear in a separate section of this Annual Report on Form 10-K, beginning on page F-1.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
(a) Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act). Internal control over financial reporting is a process designed by, or under the supervision of, the issuer’s Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, and effected by the issuer’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the issuer; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the issuer; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the issuer’s assets that could have a material effect on the financial statements.
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, management has assessed the effectiveness of our internal control over financial reporting based on the criteria set forth in the Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations, or COSO, of the Treadway Commission, and concluded that our internal control over financial reporting was not effective as of December 31, 2019 as a result of material weaknesses in our internal control over financial reporting.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company's annual or interim financial statements will not be prevented or detected on a timely basis. In connection with the assessment of the effectiveness of our internal control over financial reporting, our management identified the following material weaknesses that existed as of December 31, 2019:
During 2019, we experienced significant turnover in finance personnel that reduced the complement and skill of the resources within the Company. As a result, we did not maintain an effective control environment as we lacked a sufficient complement of resources with an appropriate level of knowledge, experience and training to design, maintain and monitor our internal control over financial reporting commensurate with our financial reporting requirements. As a result, this material weakness contributed to the following material weaknesses:
|
● |
We did not design and maintain controls to ensure adequate segregation of duties within our financial reporting function, including the preparation and review of journal entries. Specifically, some key accounting personnel had the ability to both prepare and post journal entries without an independent review by someone without the ability to prepare and post journal entries. |
|
|
|
|
|
|
● |
We did not design and maintain effective controls over certain information technology general controls for information systems that are relevant to the preparation of our financial statements. Specifically, we did not design and maintain effective user access controls to ensure appropriate segregation of duties and that adequately restrict user and privileged access to financial applications and data to appropriate Company personnel. |
These material weaknesses did not result in a material misstatement of the annual or interim financial statements. Additionally, these material weaknesses could result in a misstatement of the relevant account balances or disclosures that would result in a material misstatement to the annual or interim consolidated financial statements that would not be prevented or detected.
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm due to an exemption from such requirement for emerging growth companies.
|
(b) |
Evaluation of Disclosure Controls and Procedures |
Under the supervision of and with the participation of our management, including our Chief Executive Officer, who is our principal executive officer, and our Chief Financial Officer, who is our principal financial officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures as of December 31, 2019, the end of the period covered by this Annual Report on Form 10-K. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by the issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms promulgated by the SEC. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2019, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were not effective due to the material weaknesses in internal control over financial reporting discussed above.
|
(c) |
Changes in Internal Control over Financial Reporting |
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2019 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Subsequent to our December 31, 2019 year end, we began taking a number of actions, including designing and implementing new controls and revising existing controls, in order to remediate the material weaknesses described above. We expect to continue our remediation efforts, including testing of operating effectiveness of new controls, as described below under “Remedial Actions to Address Material Weaknesses” during the year ending December 31, 2020 and we plan to provide an update on the status of our remediation activities on a quarterly basis.
|
(d) |
Remedial Actions to Address Material Weaknesses |
We continue to evaluate the effectiveness of our remediation efforts, including demonstrating that the new or improved controls are designed appropriately and operate effectively for a reasonable period of time. We expect to make further changes to our internal controls. The following actions have been, or are expected to be, taken, to strengthen our controls and organizational structure:
|
● |
To address issues with recent employee turnover, we have hired a new controller, senior accountant, accounting analyst and accounts payable clerk. We also plan to hire or outsource additional accounting personnel to assist with improving the internal control environment, including a manager of accounting or senior accountant and director of SEC reporting and internal control. We expect to continue to evaluate our needs for additional personnel. We plan to leverage the services of consulting firms to assist us with strengthening and monitoring of our internal controls processes and documentation. We expect to provide enhanced training to existing and new employees in order to enhance the level of communication and understanding of controls with key individuals that provide key information and perform key roles within our financial accounting and reporting group. |
|
● |
We plan to appropriately design, implement and maintain a formal policy that expressly prohibits a manual journal entry from being created and posted by the same employee. Further, we plan to implement a process that includes the creation of a journal entry report that identifies who created and posted each journal entry. The report will be reviewed each month by independent senior accounting personnel so that any exceptions to the policy can be identified on a timely basis and appropriately addressed. |
|
● |
We plan to appropriately design, implement and maintain a formal policy to limit the number of “Super Users”, maintain effective user access controls to ensure appropriate segregation of duties and adequately restrict user and privileged access to financial applications, programs and data to appropriate personnel. |
|
● |
We plan to implement a formal policy that preparation, review and approval of account reconciliations will be performed by qualified accounting personnel independent of those who create and post the related underlying journal entries. |
Testing of Internal Control Effectiveness
To assess the effectiveness of internal controls related to the remediation of the 2019 identified material weaknesses, we plan to begin implementing the remediation described herein. Implementation and testing is expected to occur during the year ending December 31, 2020 and we plan to provide an update on the status of our remediation activities on a quarterly basis.
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm due to an exemption from such requirement for emerging growth companies.
Item 9B. Other Information.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Information required to be disclosed by this Item with respect to our executive officers is incorporated into this Annual Report on Form 10-K by reference from the section entitled “Executive Officers and Director and Officer Compensation: Executive Officers” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
Information required to be disclosed by this Item about our Board is incorporated into this Annual Report on Form 10-K by reference from the section entitled “The Class II Director Election Proposal” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
Information required to be disclosed by this Item about the Section 16(a) compliance of our directors and executive officers is incorporated into this Annual Report on Form 10-K by reference from the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
Information required to be disclosed by this Item about our Board, the Audit Committee of our Board, our audit committee financial expert, our code of conduct, as amended, or our Code of Conduct, and other corporate governance matters is incorporated into this Annual Report on Form 10-K by reference from the section entitled “Liquidia Corporate Governance” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
The text of our Code of Conduct, which applies to our directors and employees (including our principal executive officer, principal financial officer, and principal accounting officer or controller, and persons performing similar functions), is posted in the “Corporate Governance” section of the Investors section of our website, http://www.liquidia.com/. A copy of the Code of Conduct can be obtained free of charge on our website. We intend to disclose on our website any amendments to, or waivers from, our Code of Conduct that are required to be disclosed pursuant to the rules of the SEC and The Nasdaq Stock Market.
The information presented on our website is not a part of this Annual Report on Form 10-K and the reference to our website is intended to be an inactive textual reference only.
Item 11. Executive Compensation.
Information required to be disclosed by this Item is incorporated into this Annual Report on Form 10-K by reference from the section entitled “Executive Officers and Director and Officer Compensation” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information required to be disclosed by this Item is incorporated into this Annual Report on Form 10-K by reference from the sections entitled “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required to be disclosed by this Item is incorporated in this Annual Report on Form 10-K by reference from the sections entitled “Certain Relationships and Related Party Transactions” and “Liquidia Corporate Governance” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
Item 14. Principal Accounting Fees and Services.
The information required to be disclosed by this Item is incorporated into this Annual Report on Form 10-K by reference from the section entitled “Principal Accounting Fees and Services” contained in our definitive proxy statement for our 2020 annual meeting of stockholders, which we intend to file within 120 days of the end of our fiscal year ended December 31, 2019.
PART IV
Item 15. Exhibits and Financial Statement Schedules.
Financial Statement Schedules
|
(a) |
The following documents are filed as part of this annual report on Form 10-K: |
|
(1) |
Financial Statements. |
| Report of Independent Registered Public Accounting Firm | F-2 | |
| Balance Sheets as of December 31, 2019 and 2018 | F-3 | |
| Statements of Operations and Comprehensive Loss for the Years Ended December 31, 2019 and 2018 | F-4 | |
| Statements of Stockholders’ Equity (Deficit) for the Years Ended December 31, 2019 and 2018 | F-5 | |
| Statements of Cash Flows for the Years Ended December 31, 2019 and 2018 | F-6 | |
| Notes to Financial Statements | F-7 |
|
(2) |
Financial Statement Schedules. |
|
| Required information is included in the notes to the financial statements. |
|
(3) |
Exhibits. |
|
| See Exhibit Index below. |
|
(b) |
The following exhibits are filed as part of this Annual Report on Form 10-K. |
|
Exhibit No. |
|
Description |
|
3.1 |
||
|
3.2 |
||
|
4.1 |
||
|
4.2 |
||
|
4.3 |
||
|
4.4* |
||
|
10.1# |
||
|
10.2# |
||
|
10.3# |
||
|
10.4 |
|
10.5 |
||
|
10.6 |
||
|
10.7+ |
||
|
10.8+ |
||
|
10.9+ |
||
|
10.10++ |
||
|
10.11+ |
||
|
10.12+ |
||
|
10.13 |
||
|
10.14+ |
||
|
10.15+ |
||
|
10.16# |
||
|
10.17# |
||
|
10.18# |
||
|
10.19# |
||
|
10.20# |
|
10.21* |
||
|
10.22 |
||
|
10.23++ |
||
|
10.24++ |
||
|
23.1* |
Consent of PricewaterhouseCoopers LLP, independent Registered Public Accounting Firm. |
|
|
31.1* |
||
|
31.2* |
||
|
32.1** |
||
|
32.2** |
||
|
101* |
The following materials from Liquidia Technologies, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2019, formatted in eXtensible Business Reporting Language (XBRL): (i) Balance Sheets as of December 31, 2019 and 2018, (ii) Statements of Operations and Comprehensive Loss for the years ended December 31, 2019 and 2018 (iii) Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2019 and 2018, (iv) Statements of Cash Flows for the years ended December 31, 2019 and 2018 and (v) Notes to Financial Statements. |
+ Confidential treatment has been granted with respect as to certain portions of this exhibit. Such portions have been redacted and submitted separately to the SEC.
++ Portions of this exhibit have been redacted in compliance with Regulation S-K Item 601(b)(10). The omitted information is not material and would likely cause competitive harm to the Company if publicly disclosed.
|
* |
Filed herewith. |
|
** |
Furnished herewith. |
|
# |
Indicates management contract or compensatory plan. |
|
(c) |
Not applicable |
Item 16. Form 10-K Summary.
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Liquidia Technologies, Inc. |
||
|
Date: March 16, 2020 |
By: |
/s/ Neal Fowler |
|
Name: |
Neal Fowler |
|
|
Title: |
Chief Executive Officer |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
|
Name |
|
Position |
|
Date |
|
/s/ Neal Fowler |
Director and Chief Executive Officer |
March 16, 2020 |
||
| Neal Fowler | (Principal Executive Officer) | |||
|
/s/ Richard D. Katz, M.D. |
Chief Financial Officer |
March 16, 2020 |
||
| Richard D. Katz, M.D. | (Principal Financial and Accounting Officer) | |||
|
/s/ Dr. Stephen Bloch |
Chairman of the Board of Directors |
March 16, 2020 |
||
| Dr. Stephen Bloch | ||||
|
/s/ Katherine Rielly-Gauvin |
Director |
March 16, 2020 |
||
| Katherine Rielly-Gauvin | ||||
|
/s/ Dr. Joanna Horobin |
Director |
March 16, 2020 |
||
| Dr. Joanna Horobin | ||||
|
/s/ Arthur Kirsch |
Director |
March 16, 2020 |
||
| Arthur Kirsch | ||||
|
/s/ Dr. Seth Rudnick |
Director |
March 16, 2020 |
||
| Dr. Seth Rudnick | ||||
|
/s/ Raman Singh |
Director |
March 16, 2020 |
||
| Raman Singh | ||||
|
/s/ Dr. Ralph Snyderman |
Director |
March 16, 2020 |
||
| Dr. Ralph Snyderman |
LIQUIDIA TECHNOLOGIES, INC.
FINANCIAL STATEMENTS
TABLE OF CONTENTS
| Report of Independent Registered Public Accounting Firm | F-2 |
| Balance Sheets as of December 31, 2019 and 2018 | F-3 |
| Statements of Operations and Comprehensive Loss for the Years Ended December 31, 2019 and 2018 | F-4 |
| Statements of Stockholders’ Equity (Deficit) for the Years Ended December 31, 2019 and 2018 | F-5 |
| Statements of Cash Flows for the Years Ended December 31, 2019 and 2018 | F-6 |
| Notes to Financial Statements | F-7 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Liquidia Technologies, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Liquidia Technologies, Inc. (the “Company”) as of December 31, 2019 and 2018, and the related statements of operations and comprehensive loss, of stockholders’ equity (deficit) and of cash flows for the years then ended, including the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt About the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred recurring losses and cash outflows from operations, has an accumulated deficit and has debt maturing within twelve months that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Changes in Accounting Principles
As discussed in Note 2 to the financial statements, the Company changed the manner in which it accounts for leases in 2019 and the manner in which it accounts for revenues from contracts with customers in 2018.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
Raleigh, North Carolina
March 16, 2020
We have served as the Company’s auditor since 2014.
Liquidia Technologies, Inc.
Balance Sheets
|
December 31, 2019 |
December 31, 2018 |
|||||||
|
Assets |
||||||||
|
Current assets: |
||||||||
|
Cash |
$ | 55,796,378 | $ | 39,534,985 | ||||
|
Accounts receivable, net |
— | 272,557 | ||||||
|
Prepaid expenses and other current assets |
590,251 | 219,057 | ||||||
|
Total current assets |
56,386,629 | 40,026,599 | ||||||
|
Property, plant and equipment, net |
9,253,965 | 8,130,708 | ||||||
|
Operating lease right-of-use assets, net |
2,823,430 | — | ||||||
|
Prepaid expenses and other assets |
378,043 | 1,260,951 | ||||||
|
Total assets |
$ | 68,842,067 | $ | 49,418,258 | ||||
|
Liabilities and stockholders’ equity |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 3,498,043 | $ | 3,235,949 | ||||
|
Accrued compensation |
3,164,687 | 2,515,519 | ||||||
|
Accrued stock offering expenses |
1,289,413 | — | ||||||
|
Other accrued expenses |
1,525,919 | 1,459,182 | ||||||
|
Deferred rent |
— | 268,599 | ||||||
|
Current portion of operating lease liabilities |
566,390 | — | ||||||
|
Current portion of finance lease liabilities |
1,244,229 | 452,703 | ||||||
|
Current portion of long-term debt |
5,585,637 | 316,906 | ||||||
|
Total current liabilities |
16,874,318 | 8,248,858 | ||||||
|
Long-term operating lease liabilities |
5,670,971 | — | ||||||
|
Long-term finance lease liabilities |
1,056,747 | 376,082 | ||||||
|
Long-term deferred rent |
— | 2,406,084 | ||||||
|
Long-term deferred revenue |
— | 8,071,920 | ||||||
|
Long-term debt |
10,292,484 | 11,627,643 | ||||||
|
Total liabilities |
33,894,520 | 30,730,587 | ||||||
|
Commitments and contingencies (Note 9) |
||||||||
|
Stockholders’ equity: |
||||||||
|
Preferred stock — 10,000,000 shares authorized as of December 31, 2019 and December 31, 2018, 0 and 0 issued and outstanding as of December 31, 2019 and December 31, 2018, respectively |
— | — | ||||||
|
Common stock — $0.001 par value, 40,000,000 shares authorized as of December 31, 2019 and December 31, 2018, 28,231,267 and 15,519,469 issued and outstanding as of December 31, 2019 and December 31, 2018, respectively |
28,231 | 15,520 | ||||||
|
Additional paid-in capital |
250,158,766 | 185,726,048 | ||||||
|
Accumulated deficit |
(215,239,450 | ) | (167,053,897 | ) | ||||
|
Total stockholders’ equity |
34,947,547 | 18,687,671 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 68,842,067 | $ | 49,418,258 | ||||
The accompanying notes are an integral part of these financial statements.
Liquidia Technologies, Inc.
Statements of Operations and Comprehensive Loss
|
December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
Revenues |
$ | 8,072,120 | $ | 2,706,981 | ||||
|
Costs and expenses: |
||||||||
|
Cost of sales |
807,192 | 121,391 | ||||||
|
Research and development |
40,491,358 | 28,699,576 | ||||||
|
General and administrative |
13,597,119 | 8,754,088 | ||||||
|
Total costs and expenses |
54,895,669 | 37,575,055 | ||||||
|
Loss from operations |
(46,823,549 | ) | (34,868,074 | ) | ||||
|
Other income (expense): |
||||||||
|
Interest income |
613,716 | 304,981 | ||||||
|
Interest expense |
(1,373,622 | ) | (18,988,176 | ) | ||||
|
Gain on early extinguishment of long-term debt |
— | 137,695 | ||||||
|
Warrant fair value adjustments |
— | 277,715 | ||||||
|
Total other expense, net |
(759,906 | ) | (18,267,785 | ) | ||||
|
Net loss |
(47,583,455 | ) | (53,135,859 | ) | ||||
|
Other comprehensive loss |
— | — | ||||||
|
Comprehensive loss |
$ | (47,583,455 | ) | $ | (53,135,859 | ) | ||
|
Net loss per common share: |
||||||||
|
Basic |
$ | (2.57 | ) | $ | (7.42 | ) | ||
|
Diluted |
(2.59 | ) | (7.51 | ) | ||||
|
Weighted average common shares outstanding: |
||||||||
|
Basic |
18,482,455 | 7,163,304 | ||||||
|
Diluted |
18,371,083 | 7,078,757 | ||||||
The accompanying notes are an integral part of these financial statements.
Liquidia Technologies, Inc.
Statement of Stockholders’ Equity (Deficit)
For the Years Ended December 31, 2019 and 2018
|
Preferred Stock |
Common Stock |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Series A |
Series A-1 |
Series B |
Series C |
Series C-1 |
Series D |
Voting |
Class B Nonvoting |
Additional Paid-In |
Accumulated |
Stockholders' |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Shares |
Amount |
Shares |
Amount |
Shares |
Amount |
Shares |
Amount |
Shares |
Amount |
Shares |
Amount |
Shares |
Amount |
Shares |
Amount |
Capital |
Deficit |
Equity |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Balance as of December 31, 2017 |
1,974,430 | $ | 1,974 | 1,834,862 | $ | 1,835 | 4,496,908 | $ | 4,497 | 17,102,578 | $ | 17,103 | 17,556,178 | $ | 17,556 | — | $ | — | 549,952 | $ | 550 | 19,645 | $ | 20 | $ | 79,677,540 | $ | (113,413,311 | ) | $ | (33,692,236 | ) | ||||||||||||||||||||||||||||||||||||||||||||
|
Adjustment to remove partial shares resulting from reverse split |
— | — | — | — | — | — | — | — | — | — | — | — | (63 | ) | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cumulative adjustment - adoption of ASC 606 |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (504,727 | ) | (504,727 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Exercise of common stock options |
— | — | — | — | — | — | — | — | — | — | — | — | 119,793 | 120 | — | — | 334,591 | — | 334,711 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Exercise of common stock warrants |
— | — | — | — | — | — | — | — | — | — | — | — | 48,836 | 49 | — | — | 773 | — | 822 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Stock-based compensation |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 2,195,075 | — | 2,195,075 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issuance of Series D preferred stock, net |
— | — | — | — | — | — | — | — | — | — | 91,147,482 | 91,147 | — | — | — | — | 53,893,361 | — | 53,984,508 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Initial public offering |
— | — | — | — | — | — | — | — | — | — | — | — | 4,833,099 | 4,833 | — | — | 53,159,256 | — | 53,164,089 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Automatic conversion of preferred stock and Class B common stock |
(1,974,430 | ) | (1,974 | ) | (1,834,862 | ) | (1,835 | ) | (4,496,908 | ) | (4,497 | ) | (17,102,578 | ) | (17,103 | ) | (17,556,178 | ) | (17,556 | ) | (91,147,482 | ) | (91,147 | ) | 9,967,852 | 9,968 | (19,645 | ) | (20 | ) | 124,164 | — | — | |||||||||||||||||||||||||||||||||||||||||||
|
Reclassification of warrant liabilities |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 2,185,144 | — | 2,185,144 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
IPO financing costs |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (5,843,856 | ) | — | (5,843,856 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (53,135,859 | ) | (53,135,859 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Balance as of December 31, 2018 |
— | — | — | — | — | — | — | — | — | — | — | — | 15,519,469 | 15,520 | — | — | 185,726,048 | (167,053,897 | ) | 18,687,671 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cumulative adjustment - adoption of ASC 842 |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (602,098 | ) | (602,098 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Exercise of common stock options |
— | — | — | — | — | — | — | — | — | — | — | — | 32,325 | 32 | — | — | 141,295 | — | 141,327 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Exercise of common stock warrants |
— | — | — | — | — | — | — | — | — | — | — | — | 64,629 | 64 | — | — | 649 | — | 713 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock incentive plan |
— | — | — | — | — | — | — | — | — | — | — | — | 40,954 | 41 | — | — | (41 | ) | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Stock-based compensation |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 3,376,305 | — | 3,376,305 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issuance of common stock, net |
— | — | — | — | — | — | — | — | — | — | — | — | 12,573,890 | 12,574 | — | — | 60,914,510 | — | 60,927,084 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (47,583,455 | ) | (47,583,455 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Balance as of December 31, 2019 |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | 28,231,267 | $ | 28,231 | — | $ | — | $ | 250,158,766 | $ | (215,239,450 | ) | $ | 34,947,547 | |||||||||||||||||||||||||||||||||||||||||||||
Liquidia Technologies, Inc.
Statements of Cash Flows
|
2019 |
2018 |
|||||||
|
Operating activities |
||||||||
|
Net loss |
$ | (47,583,455 | ) | $ | (53,135,859 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Stock-based compensation |
3,376,305 | 2,195,075 | ||||||
|
Depreciation and amortization |
2,567,742 | 1,543,667 | ||||||
|
Non-cash lease expense |
225,537 | - | ||||||
|
Loss on disposal of property and equipment |
6,587 | - | ||||||
|
Amortization of discount and debt issuance costs on long-term debt and convertible notes |
75,364 | 17,550,541 | ||||||
|
Non-cash interest expense |
— | 343,103 | ||||||
|
Non-cash gain on early extinguishment of long-term debt |
— | (137,695 | ) | |||||
|
Warrant fair value adjustment |
— | (277,715 | ) | |||||
|
Non-cash rent (income) expense |
— | (206,498 | ) | |||||
|
Changes in operating assets and liabilities: |
||||||||
|
Accounts receivable – trade and other |
272,557 | 1,349,622 | ||||||
|
Prepaid expenses and other current assets |
(371,194 | ) | (67,154 | ) | ||||
|
Other non-current assets |
807,192 | 2,408,097 | ||||||
|
Accounts payable |
294,514 | (1,281,784 | ) | |||||
|
Accrued expenses |
(108,707 | ) | (1,055,564 | ) | ||||
|
Accrued compensation |
649,168 | 563,013 | ||||||
|
Operating lease liabilities |
(422,364 | ) | — | |||||
|
Deferred revenue |
(8,071,920 | ) | (1,621,384 | ) | ||||
|
Net cash used in operating activities |
(48,282,674 | ) | (31,830,535 | ) | ||||
|
Investing activities |
||||||||
|
Purchases of property, plant and equipment |
(1,850,099 | ) | (870,943 | ) | ||||
|
Net cash used in investing activities |
(1,850,099 | ) | (870,943 | ) | ||||
|
Financing activities |
||||||||
|
Principal payments on finance leases |
(998,687 | ) | (608,154 | ) | ||||
|
Payments for finance lease deposits |
(34,649 | ) | - | |||||
|
Proceeds from issuance of long-term debt |
5,000,000 | 11,000,000 | ||||||
|
Refund of principal payments on long-term debt |
— | 588,889 | ||||||
|
Principal payments on long-term debt |
— | (12,406,010 | ) | |||||
|
Payments for debt issuance costs |
— | (397,000 | ) | |||||
|
Proceeds from issuance of Series D preferred stock, net of issuance costs |
— | 25,106,896 | ||||||
|
Proceeds from sale of common stock, net of underwriting fees and commissions |
63,039,490 | 47,320,233 | ||||||
|
Payments for offering costs |
(754,028 | ) | (2,122,903 | ) | ||||
|
Proceeds from exercise of stock options and warrants |
142,040 | 335,533 | ||||||
|
Net cash provided by financing activities |
66,394,166 | 68,817,484 | ||||||
|
Net increase in cash |
16,261,393 | 36,116,006 | ||||||
|
Cash, beginning of period |
39,534,985 | 3,418,979 | ||||||
|
Cash, end of period |
$ | 55,796,378 | $ | 39,534,985 | ||||
|
Supplemental disclosure of cash flow information |
||||||||
|
Cash paid for interest |
$ | 887,038 | $ | 1,094,532 | ||||
|
Cash paid for operating lease liabilities |
$ | 1,081,582 | $ | — | ||||
|
Right of use assets obtained with finance lease liabilities |
$ | 834,693 | $ | — | ||||
|
Purchase of equipment with leases |
$ | — | $ | 456,517 | ||||
|
Changes in purchases of property and equipment in accounts payable and accrued expenses |
$ | 184,424 | $ | 25,934 | ||||
|
Purchase of build-to-suit asset with deferred financing obligation |
$ | — | $ | 272,656 | ||||
|
Reclassification of deferred financing obligation to long-term debt |
$ | — | $ | 277,009 | ||||
|
Reclassification of financing costs on deferred financing obligation to discount on long-term debt |
$ | — | $ | 1,614,466 | ||||
|
Recording of discount on long-term debt |
— | $ | 168,174 | |||||
|
Leasehold improvements paid by landlord |
$ | 936,104 | $ | — | ||||
|
Conversion of accrued interest to long-term debt |
$ | — | $ | 144,993 | ||||
|
Conversion of convertible notes and accrued interest into Series D preferred stock |
$ | — | $ | 28,877,498 | ||||
|
Deferred offering costs and commissions incurred but not paid |
$ | 1,358,378 | $ | 108,694 | ||||
|
Exercise of stock options through exchange of vested stock options |
$ | — | $ | 162,156 | ||||
The accompanying notes are an integral part of these financial statements.
Liquidia Technologies, Inc.
Notes to Financial Statements
1. Business
Liquidia Technologies, Inc. (“Liquidia” or the “Company”) is a late-stage clinical biopharmaceutical company focused on the development and commercialization of novel products utilizing the Company’s proprietary PRINT technology to transform the lives of patients. PRINT is a particle engineering platform that enables precise production of uniform drug particles designed to improve the safety, efficacy and performance of a wide range of therapies. The Company is currently focused on the development of two product candidates for which it holds worldwide commercial rights: LIQ861 for the treatment of pulmonary arterial hypertension (“PAH”) and LIQ865 for the treatment of local post-operative pain.
The development and commercialization activities are conducted at the Company’s headquarters located in Morrisville, North Carolina. The Company was incorporated under the laws of the state of Delaware in 2004.
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation
The Company has prepared the accompanying financial statements in conformity with generally accepted accounting principles in the United States of America (“GAAP”). Such financial statements reflect all adjustments that are, in management’s opinion, necessary to present fairly, in all material respects, the Company’s financial position, results of operations and cash flows and are presented in U.S. Dollars.
Going Concern
The Company’s financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. The Company's operations have consisted primarily of developing its technology, developing products, prosecuting its intellectual property and securing financing. The Company has incurred recurring losses and cash outflows from operations, has an accumulated deficit, and has debt principal payments that commenced in the first quarter of 2020. The Company expects to continue to incur losses in the foreseeable future and will require additional financial resources to continue to advance its products and intellectual property, in addition to repaying its maturing debt and other obligations. These conditions raise substantial doubt regarding the Company’s ability to continue as a going concern.
The Company believes that its existing cash will enable it to fund its operating expenses and capital expenditure requirements, make payments of interest and principal on its term loan facility with Pacific Western Bank, and remain in compliance with its minimum cash covenant of $8.5 million pursuant to this term loan facility, through August 2020. The Company has based these estimates on assumptions that may prove to be wrong, and it could utilize its available capital resources sooner than it expects. The Company will need to raise substantial additional capital to continue its business operations and remain in compliance with the minimum cash covenant of $8.5 million on its debt during and beyond the third quarter of 2020, in addition to commercializing LIQ861, if approved. Such capital may not be available on a timely basis, on terms that are favorable to the Company, or at all. Alternatively, in light of the Company's current limited cash resources, the recent trading price of the Company's common stock, outstanding debt and associated minimum cash covenant, and based on a review of the status of its programs, resources and capabilities, the Company continues to explore a wide range of strategic alternatives with the support of its financial advisor, Jefferies LLC, that could maximize stockholder value. The Company's efforts have been and continue to be focused primarily upon the potential formation of a partnership or a licensing transaction with respect to its lead program, LIQ861, for the treatment of PAH. Strategic alternatives may also include the sale of some of the Company’s assets or proprietary technologies, or a potential merger or sale of the Company. There can be no assurance that the Company will be able to enter into such a transaction or transactions on a timely basis, on terms that are favorable to the Company, or at all. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Reverse Stock Split
On July 12, 2018 and July 19, 2018, the Company’s Board of Directors and stockholders, respectively, approved an amendment to the Company’s amended and restated certificate of incorporation effecting a 1-for-16.827 reverse stock split of the Company’s issued and outstanding shares of common stock and convertible preferred stock. The reverse stock split was effective on July 19, 2018. The par value of the common and redeemable convertible preferred stock was not adjusted as a result of the reverse stock split. All issued and outstanding share and per share amounts included in the accompanying financial statements have been adjusted to reflect this reverse stock split for all periods presented.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual amounts could differ from those estimates.
Summary of Significant Accounting Policies
Cash
The Company considers all highly liquid investments with a maturity of three months or less, when purchased, to be cash equivalents. The Company had no cash equivalents as of December 31, 2019 and 2018.
Accounts Receivable
Accounts receivable are stated at net realizable value including an allowance for doubtful accounts as of each balance sheet date. The Company has not recorded an allowance for doubtful accounts during the years ended December 31, 2019 and 2018.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash and accounts receivable. The Company is exposed to credit risk, subject to federal deposit insurance, in the event of default by the financial institutions holding its cash to the extent of amounts recorded on the balance sheet. With regard to cash, 100% of the Company’s cash is held on deposit with Pacific Western Bank. With regard to revenues and concentration of credit risk, GlaxoSmithKline plc (“GSK” and “GSK Inhaled”) accounted for $8.1 and $0.4 million of our revenue during the years ended December 31, 2019 and 2018, respectively, or 100% and 15%, respectively, of our total revenue, and $0 or 0% of the Company’s accounts receivable as of December 31, 2019 and 2018.
Leases
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-02, Leases, as amended (Topic 842) (“ASU 2016-02”). The provisions of ASU 2016-02 set out the principles for the recognition, measurement, presentation and disclosure of leases for both lessees and lessors. The new standard requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months regardless of their classification. The Company has elected to account for leases with a term of 12 months or less in a similar manner as under existing guidance for operating leases. ASU 2016-02 supersedes the previous lease standard, Topic 840, Leases. The guidance is effective for public companies with annual periods and interim periods within those annual periods beginning after December 15, 2018. The Company adopted Topic 842, as amended, as of January 1, 2019, using the modified retrospective approach. The modified retrospective approach provides a method for recording existing leases at adoption that approximates the results of a full retrospective approach in the year of adoption. In addition, the Company elected the package of practical expedients permitted under the transition guidance within the new standard, which among others, allowed the Company to carry forward the historical lease classification. Adoption of the new standard resulted in the recording of net lease assets and lease liabilities of approximately $6.4 million and $9.1 million respectively, as of January 1, 2019. The standard had no impact on cash flows. For operating leases, the asset and liability will be expensed over the lease term on a straight-line basis, with all cash flows classified as an operating activity in the Statement of Cash Flows. For finance leases, interest on the lease liability will be recognized separately from the amortization of the right-of-use asset in the Statement of Operations and Comprehensive Loss and the repayment of the principal portion of the lease liability will be classified as a financing activity, while the interest component will be classified as an operating activity in the Statement of Cash Flows.
The net impact of applying Topic 842 was recorded as an adjustment to accumulated deficit of $0.6 million as of January 1, 2019 as follows:
|
Balance at |
Adjustments |
Balance at |
||||||||||
|
December 31, |
Due to |
January 1, |
||||||||||
|
2018 |
Topic 842 |
2019 |
||||||||||
|
Balance Sheet: |
||||||||||||
|
Assets |
||||||||||||
|
Property, plant and equipment, net |
$ | 8,130,708 | $ | (107,734 | ) | $ | 8,022,974 | |||||
|
Operating lease right-of-use assets, net |
— | 3,985,071 | 3,985,071 | |||||||||
|
Liabilities |
||||||||||||
|
Deferred rent |
2,674,683 | (2,674,683 | ) | — | ||||||||
|
Operating lease liabilities |
— | 6,659,725 | 6,659,725 | |||||||||
|
Finance lease liabilities |
828,785 | 1,636,185 | 2,464,970 | |||||||||
|
Long-term debt |
11,944,549 | (1,141,792 | ) | 10,802,757 | ||||||||
|
Stockholders’ equity (deficit) |
||||||||||||
|
Accumulated deficit |
(167,053,897 | ) | (602,098 | ) | (167,655,995 | ) | ||||||
Property, Plant and Equipment
Property, plant and equipment are stated at cost. Depreciation of property, plant and equipment is computed using the straight-line method over the estimated useful lives of the assets beginning when the assets are placed in service. Estimated useful lives for the major asset categories are:
|
Lab and build-to-suit equipment (years) |
5 | - | 7 | ||
|
Office equipment (years) |
5 | ||||
|
Furniture and fixtures (years) |
10 | ||||
|
Computer equipment (years) |
3 | ||||
|
Leasehold improvements |
Lesser of life of the asset or remaining lease term |
||||
Major renewals and improvements are capitalized to the extent that they increase the useful economic life or increase the expected economic benefit of the underlying asset. Maintenance and repairs are charged to operations as incurred. When items of property, plant and equipment are sold or retired, the related cost and accumulated depreciation or amortization is removed from the accounts, and any gain or loss is included in operating expenses in the accompanying Statements of Operations and Comprehensive Loss.
Impairment of Long-Lived Assets
The Company evaluates long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. If the estimated future cash flows (undiscounted and without interest charges) from the use of an asset are less than the carrying value, an impairment is recorded to reduce the related asset to its estimated fair value. To date, no such impairments have occurred.
Deferred Rent
Rent expense is recognized on a straight-line basis over the life of the lease. The difference between rent expense recognized and rental payments, as stipulated in the lease, is reflected as deferred rent in the accompanying Balance Sheets and amortized over the life of the lease. In addition, deferred rent also includes landlord incentives on a portion of the leasehold improvement cost, which is amortized over the life of the lease.
Revenue Recognition
The Company adopted ASU 2014-09, Revenue from Contracts with Customers (“Topic 606”) and all the related amendments as of January 1, 2018. The cumulative effect of the change was an increase of $0.5 million to the balance of accumulated deficit on the Balance Sheet as of January 1, 2018.
The Company derives revenues primarily from licensing its proprietary PRINT technology and from performing research and development services. Revenues are recognized as services are performed in an amount that reflects the consideration the Company expects to be entitled to in exchange for those services and technology.
The Company’s research, development and licensing agreements provide for multiple promised goods and services to be satisfied by the Company and include a license to the Company’s technology in a particular field of study, participation in collaboration committees, performance of certain research and development services and obligations for certain manufacturing services.
The transaction price for these contracts includes non-refundable fees and fees for research and development services. Non-refundable up-front fees which may include, for example, an initial payment upon effectiveness of the contractual relationship or payment to secure a right for a future license, are recorded as deferred revenue and recognized into revenue over time as the Company provides the research services under the contract required to advance the products to the point where the Company is able to transfer control of the licensed technology to the customer (“Technology Transfer”). The contract consideration may also include additional non-refundable payments due to the Company based on the achievement of research, development, regulatory or commercialization milestone events. In agreements involving multiple goods or services promised to be transferred to customers, the Company must assess, at the inception of the contract, whether each promise represents a separate performance obligation (i.e., is “distinct”), or whether such promises should be combined as a single performance obligation. As these goods and services are considered to be highly interrelated, they may be considered to represent a single, combined performance obligation. The Company includes an estimate of the probable amount of milestone payments to which it will be entitled in the transaction price. The estimate requires evaluation of factors which are outside of the Company’s control and significantly limit the Company’s ability to achieve the remaining milestone payments. Therefore, the Company has not included any future milestone payments in the transaction price allocated to research, development and licensing agreements as of December 31, 2018 or December 31, 2019. The Company revises the transaction price to include milestone payments once the specific milestone achievement is not considered to be subject to a significant reversal of revenue. At that time, the estimated transaction price is adjusted and a cumulative catch-up adjustment is recorded to adjust the amount of revenue to be recognized from the license inception to the date the milestone was deemed probable of achievement. The milestone is included with other non-refundable up-front fees and recognized into revenue over time as the Company continues to provide services under the contract through the Company’s Technology Transfer. The amount of revenue recognized is based on the proportion of total research services performed to date to the expected services to be provided through the Technology Transfer.
The estimate of the research services to be provided through the Technology Transfer requires significant judgment to evaluate assumptions regarding the level of effort required for the Company to have performed sufficient obligations for the customer to be able to utilize the licensed technology without requiring further services from the Company. If the estimated level of effort changes, the remaining deferred revenue is recognized over the revised period in which the expected research services and Technology Transfer are required. Changes in estimates occur for a variety of reasons, including but not limited to (i) research and development acceleration or delays, (ii) customer prioritization of research projects, or (iii) results of research and development activities. The Company recognizes the consideration it is entitled to receive for research and development services, which are primarily billed quarterly in arrears on a time and materials basis, as the services are performed (under a proportional performance model) and collection is reasonably assured. Additionally, any up-front or development milestone payments received are also recognized as revenues, over time, under this same proportional performance model.
Royalties related to product sales will be recognized as revenue when the sale occurs since payments relate directly to products that will have been fully developed and for which the Company will have satisfied all of its performance obligations.
Segment Data
Operating segments are defined as components of an enterprise engaging in business activities from which it may earn revenues and incur expenses, for which discrete financial information is available and whose operating results are regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment and all of the Company operations and long-lived assets are in the United States.
Research and Development Expense
Research and development costs are expensed as incurred and include direct costs incurred to third parties related to the salaries of, and stock-based compensation for, personnel involved in research and development activities, contractor fees, grant expenses, administrative expenses and allocations of research-related overhead costs. Administrative expenses and research-related overhead costs included in research and development expense consist of allocations of facility and equipment lease charges, depreciation and amortization of assets and insurance directly related to research and development activities.
Patent Maintenance
The Company is responsible for all patent costs, past and future, associated with the preparation, filing, prosecution, issuance, maintenance, enforcement and defense of United States patent applications. Such costs are recorded as general and administrative expenses as incurred. To the extent that the Company’s licensees share these costs, such benefit is recorded as a reduction of the related expenses.
Stock-Based Compensation
The Company estimates the grant date fair value of its share-based awards and amortizes this fair value to compensation expense over the requisite service period or vesting term (see Note 4).
Net Loss Per Share
Basic net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted average shares outstanding during the period, without consideration of common stock equivalents.
Diluted net loss per share is calculated by adjusting weighted average shares outstanding for the dilutive effect of common stock equivalents outstanding for the period, determined using the treasury-stock method. For purposes of the diluted net loss per share calculation, stock options and warrants are considered to be common stock equivalents but are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive. Due to their anti-dilutive effect, the calculation of diluted net loss per share for the years ended December 31, 2019 and 2018 does not include the following common stock equivalent shares:
|
Year Ended December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
Stock Options |
1,979,411 | 1,658,112 | ||||||
|
Warrants |
111,372 | 170,925 | ||||||
|
Total |
2,090,783 | 1,829,037 | ||||||
For the years ended December 31, 2019 and 2018 the only reconciling item between basic and diluted net loss per share is the impact of the common stock warrants that are included in the calculation of basic net loss per share since their exercise price is de minimis, but excluded from the calculation of diluted net loss per share since the impact of such warrants is antidilutive.
Fair Value of Financial Instruments
The carrying values of cash, accounts receivable, and accounts payable at December 31, 2019 and 2018 approximated their fair value due to the short maturity of these instruments.
The Company’s valuation of financial instruments is based on a three-tiered approach, which requires that fair value measurements be classified and disclosed in one of three tiers. The fair value hierarchy defines a three-level valuation hierarchy for disclosure of fair value measurements as follows:
Level 1 — Quoted prices in active markets for identical assets or liabilities;
Level 2 — Other than quoted prices included in Level 1 inputs that are observable for the asset or liability, either directly or indirectly; and
Level 3 — Unobservable inputs for the asset and liability used to measure fair value, to the extent that observable inputs are not available.
The categorization of a financial instrument within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The following tables present the placement in the fair value hierarchy of financial liabilities measured at fair value as of December 31, 2019 and 2018:
|
Significant |
||||||||||||||||
|
Quoted Prices |
Other |
|||||||||||||||
|
in Active |
Observable |
Significant |
||||||||||||||
|
Markets |
Inputs |
Unobservable |
Carrying |
|||||||||||||
|
December 31, 2019 |
(Level 1) |
(Level 2) |
Inputs (Level 3) |
Value |
||||||||||||
|
Pacific Western Bank note - A&R LSA |
$ | — | $ | 14,094,792 | $ | — | $ | 15,878,121 | ||||||||
|
Significant |
||||||||||||||||
|
Quoted Prices |
Other |
|||||||||||||||
|
in Active |
Observable |
Significant |
||||||||||||||
|
Markets |
Inputs |
Unobservable |
Carrying |
|||||||||||||
|
December 31, 2018 |
(Level 1) |
(Level 2) |
Inputs (Level 3) |
Value |
||||||||||||
|
Pacific Western Bank Tranche I note - A&R LSA |
$ | — | $ | 10,412,650 | $ | — | $ | 10,802,355 | ||||||||
|
CSC build-to-suit equipment financing |
— | 1,311,135 | — | 1,142,194 | ||||||||||||
|
Total |
$ | — | $ | 11,723,785 | $ | — | $ | 11,944,549 | ||||||||
The fair value of debt is measured in accordance with ASU 2016-01, Financial Instruments–Overall: Recognition and Measurement of Financial Assets and Financial Liabilities. The fair value is determined based on the exit price notion using credit spreads and an illiquidity premium for each loan. The credit spread is determined by the credit risk rating, loan rate index, and maturity date. The illiquidity premium is based on the loan’s credit risk rating.
Deferred Offering Costs
The Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process equity financings as deferred offering costs until such equity financings are consummated. After consummation of the equity financing, these costs are recorded in stockholders’ equity as a reduction of proceeds generated as a result of the offering. As of December 31, 2019 and 2018, the Company recorded deferred offering costs relating to its financing activities of $0 and $110,365, respectively, which is included in Prepaid Expenses and Other Assets on the Balance Sheets.
Convertible Instruments
The Company has utilized various types of financing to fund its business needs, including convertible debt and convertible preferred stock, in some cases with corresponding warrants. The Company considered guidance within FASB ASC 470-20, Debt with Conversion and Other Options, (“ASC 470-20”), ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”), when accounting for the issuance of convertible securities. Additionally, the Company reviewed the instruments to determine whether they were freestanding or contain an embedded derivative and, if so, whether they should be classified in permanent equity, mezzanine equity or as a liability at each reporting period until the amount is settled and reclassified into equity.
When multiple instruments were issued in a single transaction, the Company allocated total proceeds from the transaction among the individual freestanding instruments identified. The allocation was made after identifying all the freestanding instruments and the subsequent measurement basis for those instruments. The subsequent measurement basis determines how the proceeds were allocated. Generally, proceeds were allocated based on one of the following methods:
|
• |
Fair value method — The instrument being analyzed is allocated a portion of the proceeds equal to its fair value, with the remaining proceeds allocated to the other instruments as appropriate. |
|
• |
Relative fair value method — The instrument being analyzed is allocated a portion of the proceeds based on the proportion of its fair value to the sum of the fair values of all the instruments covered in the allocation. |
|
• |
Residual value method — The instrument being analyzed is allocated the remaining proceeds after an allocation is made to all other instruments covered in the allocation. |
Generally, when there are multiple instruments issued in a single transaction that have different subsequent measurement bases, the proceeds from the transaction are first allocated to the instrument that is subsequently measured at fair value (i.e., instruments accounted for as derivative liabilities) at its issuance date fair value, with the residual proceeds allocated to the instrument not subsequently measured at fair value. In the event both instruments in the transaction are not subsequently measured at fair value (i.e., equity-classified instruments), the proceeds from the transaction are allocated to the freestanding instruments based on their respective fair values, using the relative fair value method.
After the proceeds are allocated to the freestanding instruments, resulting in an initial discount on the host contract, those instruments were further evaluated for embedded features (i.e., conversion options) that require bifurcation and separate accounting as a derivative financial instrument pursuant to ASC 815. Embedded derivatives were initially and subsequently measured at fair value. Under ASC 815, a portion of the proceeds received upon the issuance of the hybrid contract was allocated to the fair value of the derivative.
The Company accounted for convertible instruments in which it is determined that the embedded conversion options should not be bifurcated from their host instruments in accordance with ASC 470-20. Under ASC 470-20, the Company recorded, when necessary, discounts to convertible notes or convertible preferred stock for the intrinsic value of conversion options embedded in the convertible instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the transaction and the effective conversion price embedded in the convertible instrument, unless limited by the proceeds allocated to such instrument.
Warrant Liabilities
The Company had classified warrants to purchase shares of preferred stock as liabilities on its Balance Sheets as these warrants were freestanding financial instruments that would require the Company to issue convertible securities upon exercise. The warrants were initially recorded at fair value on date of grant, and were subsequently remeasured to fair value at each reporting period. Changes in fair value of the warrants were recognized as a component of other income (expense) in the Statements of Operations and Comprehensive Loss. In conjunction with the Company’s initial public offering (“IPO”) in 2018, the warrants were converted to warrants for common stock. Following that conversion, these warrants no longer met the criteria to be presented as a liability and have been reclassified to additional paid-in capital. The Company will no longer include the warrants as liabilities or recognize changes in their fair value on the Statements of Operations and Comprehensive Loss.
Embedded Derivatives
Embedded derivatives that were required to be bifurcated from the underlying instrument were accounted for and valued as a separate financial instrument. In conjunction with the Company’s convertible notes, embedded derivatives exist associated with the future consummation of a qualified financing event, as defined in the notes, and a subsequent discounted conversion of the instrument to capital stock. The embedded derivatives were bifurcated and classified as derivative liabilities on the Balance Sheets and separately adjusted to their fair values at the end of each reporting period. Changes in fair values of the derivative liabilities are recognized as a component of other income (expense) in the Statements of Operations and Comprehensive Loss. These embedded derivatives were eliminated upon conversion of the underlying convertible notes into Series D preferred stock, $0.001 par value per share (“Series D”) (see Note 3).
Issuance Costs Related to Equity and Debt
The Company allocated issuance costs between the individual freestanding instruments identified on the same basis as proceeds were allocated. Issuance costs associated with the issuance of stock or equity contracts (i.e., equity-classified warrants and convertible preferred stock) were recorded as a charge against the gross proceeds of the offering. Any issuance costs associated with the issuance of liability-classified warrants were expensed as incurred. Issuance costs associated with the issuance of debt (i.e., convertible debt) was recorded as a direct reduction of the carrying amount of the debt liability, but limited to the notional value of the debt. The Company accounted for debt as liabilities measured at amortized cost and amortizes the resulting debt discount to interest expense using the straight-line method over the expected term of the notes pursuant to ASC 835, Interest (“ASC 835”). To the extent that the reduction from issuance costs of the carrying amount of the debt liability would reduce the carrying amount below zero, such excess was recorded as interest expense.
Income Taxes
The asset and liability method is used in the Company’s accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The Company records a valuation allowance against deferred tax assets when realization of the tax benefit is uncertain.
A valuation allowance is recorded, if necessary, to reduce net deferred taxes to their realizable values if management believes it is more likely than not that the net deferred tax assets will not be realized.
The Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement.
On December 22, 2017, the Tax Cuts and Jobs Act (the "TCJA") was enacted into law. This law includes significant changes to the U.S. corporate income tax system, including a permanent reduction in the corporate income tax rate from 35% to 21%, limitations on the deductibility of interest expense and executive compensation and the transition of U.S. international taxation from a worldwide system to a territorial tax system. For taxpayers with revenues over a certain threshold, the TCJA also limits interest expense deductions to 30% of taxable income before interest, depreciation and amortization from 2018 to 2021 and then taxable income before interest thereafter. The TCJA permits disallowed interest expense to be carried forward indefinitely. The Company calculated its best estimate of the impact of the TCJA in its income tax provision for the year ended December 31, 2017 in accordance with its understanding of the TCJA and guidance available at the time. The overall impact of the TCJA resulted in a decrease to the deferred tax assets and a corresponding decrease to the valuation allowance of $14.1 million. Using the guidance issued by the SEC staff in Staff Accounting Bulletin No. 118, the Company completed its accounting for the TCJA during the fourth quarter of 2018.
Recent Accounting Pronouncements
In October 2018, the FASB issued ASU 2018-18, Collaborative Arrangements (Topic 808): Clarifying the Interaction between Topic 808 and Topic 606 ("ASU 2018-17"). The provisions of ASU 2018-18 clarify when certain transactions between collaborative arrangement participants should be accounted for under ASC 606 and incorporates unit-of-account guidance consistent with ASC 606 to aid in this determination. The guidance is effective for annual periods and interim periods within those annual periods beginning after December 15, 2019, with early adoption permitted, and is effective for the Company for the year ending December 31, 2020. The Company is currently evaluating the impact that the implementation of this standard will have on the Company's financial statements.
3. Stockholders’ Equity
Authorized Capital
As of December 31, 2019, the authorized capital of the Company consists of 50,000,000 shares of capital stock, $0.001 par value per share, of which 40,000,000 shares are designated as common stock and 10,000,000 shares are designated as preferred stock.
Preferred Stock
In February 2018, the Company received proceeds of $25.6 million in exchange for the corresponding sale of Series D and related rights offering to new and existing investors. The applicable issue price per share for the Series D was $0.59808, subject to adjustment as provided in the certificate of incorporation. In addition, all outstanding convertible notes, plus accrued interest, totaling $28.9 million were converted into Series D at the same price per share without a discount. Outstanding warrants to purchase shares of Series C-1 preferred stock, $0.001 par value per share (“Series C-1”), were converted to warrants to purchase the equivalent number of shares of Series D. All references herein to these warrants refer to them as warrants to purchase Series D. In total, 91,147,482 shares of Series D were issued. Each share of Series D was convertible at any time into a share of common stock with such conversion ratio subject to future adjustment. Conversion was automatic upon a qualified financing, as defined in the certificate of incorporation. Each series of preferred stock had anti-dilution protection in the event of a dilutive issuance, as defined in the certificate of incorporation. The Series D was senior to all other series of preferred stock.
Common Stock
Upon any voluntary or involuntary liquidation, dissolution or winding up of the affairs of the Company, the holders of the common stock shall be entitled to receive that portion of the remaining funds to be distributed to the stockholders, subject to the liquidation preferences of any outstanding preferred stock, if any. Such funds shall be paid to the holders of common stock on the basis of the number of shares so held by each of them.
In the third quarter of 2018, the Company closed the IPO of 4,833,099 shares of common stock, including the underwriters’ partial exercise of their over-allotment option in connection therewith, which resulted in aggregate net proceeds of $47.3 million, after underwriting discounts and the payment of other offering expenses. In conjunction with the Company’s IPO, all outstanding shares of convertible preferred stock were converted into an aggregate of 9,948,207 shares of common stock and the Class B non-voting common stock, $0.001 par value per share, was converted into shares of voting common stock.
In March 2019, the Company closed an underwritten follow-on offering of 3,000,000 shares of its common stock at a public offering price of $11.50 per share. The gross proceeds from the offering were $34.5 million and net proceeds were $31.8 million, after deducting underwriting discounts and commissions and other offering expenses.
In August 2019, the Company entered into a sales agreement (the “ATM Agreement”) with Jefferies LLC (“Jefferies”) to issue and sell shares of the Company’s common stock, having an aggregate offering price of up to $40.0 million, from time to time during the term of the ATM Agreement, through an “at-the-market” equity offering program at the Company’s sole discretion, under which Jefferies will act as the Company’s agent and/or principal. The Company will pay Jefferies a commission up to 3.0% of the gross proceeds of any common stock sold through Jefferies under the ATM Agreement. During the year ended December 31, 2019, the Company sold 2,409,356 shares of common stock for gross proceeds of $8.4 million and net proceeds were $8.1 million, after deducting underwriting discounts and other offering expenses under the ATM Agreement.
On December 23, 2019, the Company entered into a Common Stock Purchase Agreement (the “Purchase Agreement”) with certain institutional accredited investors (the “Purchasers”) for the sale by the Company in a private placement (the “Private Placement”) of an aggregate of 7,164,534 shares (the “Private Placement Shares”) of common stock, at a purchase price of $3.13 per Private Placement Share. The closing of the Private Placement occurred on December 27, 2019. The Company granted the Purchasers indemnification rights with respect to its representations, warranties, covenants and agreements under the Purchase Agreement. The gross proceeds from the sale of the Private Placement Shares were $22.4 million and net proceeds were $21.0 million, after deducting placement agent fees and offering expenses.
Warrants
Pursuant to the terms of the warrants, upon the conversion of the preferred stock underlying the warrants into common stock, the warrants automatically become exercisable for common stock based upon the conversion ratio of the underlying preferred stock.
Upon closing of the Series D financing, the Company had warrants outstanding to purchase 3,698,128 shares of Series D. In conjunction with the IPO in the third quarter of 2018, these warrants were automatically converted into warrants to purchase 219,761 shares of common stock. During the years ended December 31, 2019 and 2018, 64,629 and 48,836 warrants to purchase shares of common stock were exercised, respectively. As of December 31, 2019, there are outstanding warrants to purchase 106,274 shares of common stock with an exercise price of $0.0168 per share. The warrants expire on December 31, 2026.
4. Stock-Based Compensation
The Company’s 2018 Long-Term Incentive Plan (the “2018 Plan”) was approved by stockholders in July 2018. In addition to stock options, the 2018 Plan provides for the granting of stock appreciation rights, stock awards, stock units, and other stock-based awards. A total of 1,600,000 shares of the Company’s common stock was initially authorized and reserved for issuance under the 2018 Plan. This reserve will automatically increase each subsequent anniversary of January 1 through 2028, by an amount equal to the smaller of (a) 4% of the number of shares of common stock issued and outstanding on the immediately preceding December 31, or (b) an amount determined by the Board of Directors (the “Evergreen Provision”).
On January 1, 2019, the number of shares of common stock available for issuance under the 2018 Plan automatically increased by 620,778 shares from 1,600,000 shares to 2,220,778 shares pursuant to the Evergreen Provision. On January 1, 2020, the number of shares of common stock available for issuance under the 2018 Plan automatically increased by 1,129,250 shares from 1,287,561 shares to 2,416,811 shares pursuant to the Evergreen Provision. The 2018 Plan provides for accelerated vesting under certain change of control transactions. As of December 31, 2019, there were 1,287,561 shares of common stock available for issuance under 2018 Plan.
The 2018 Plan replaced the 2016 and 2004 Plans as the Company’s primary long-term incentive program. The 2016 and 2004 Plans were discontinued following stockholder approval of the 2018 Plan, but the outstanding awards under the 2016 and 2004 Plans will continue to remain in effect in accordance with their terms. Shares that are returned under the 2016 and 2004 Plans upon cancellation, termination or otherwise of awards outstanding under the 2016 and 2004 Plans will not be available for grant under the 2018 Plan. As of December 31, 2019, the Company had reserved for issuance 733,820 shares of common stock under the 2016 Plan and 397,177 shares of common stock under the 2004 Plan representing the remaining outstanding options granted under the 2016 and 2004 Plans.
Stock-Based Compensation Valuation and Expense
The Company accounts for its employee stock-based compensation plans using the fair value method. The fair value method requires the Company to estimate the grant-date fair value of its stock-based awards and amortize this fair value to compensation expense over the requisite service period or vesting term. The fair value of each option grant is estimated using a Black-Scholes option-pricing model.
For restricted stock units (“RSUs”), the grant-date fair value is based upon the market price of the Company’s common stock on the date of the grant. This fair value is then amortized to compensation expense over the requisite service period or vesting term.
Stock-based compensation expense is recognized net of estimated forfeitures such that expense is recognized only for those stock-based awards that are expected to vest. A forfeiture rate is estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures materially differ from initial estimates.
The Company recorded the following stock-based compensation expense:
|
Year Ended December 31, |
||||||||
|
By Expense Category: |
2019 |
2018 |
||||||
|
Research and development |
$ | 1,119,382 | $ | 649,052 | ||||
|
General and administrative |
2,256,923 | 1,546,023 | ||||||
|
Total |
$ | 3,376,305 | $ | 2,195,075 | ||||
|
Year Ended December 31, |
||||||||
|
By Type of Award: |
2019 |
2018 |
||||||
|
Stock Options |
$ | 3,240,376 | $ | 1,881,800 | ||||
|
Restricted Stock Units |
135,929 | 313,275 | ||||||
|
Total |
$ | 3,376,305 | $ | 2,195,075 | ||||
The following table summarizes the unamortized compensation expense and the remaining years over which such expense would be expected to be recognized, on a weighted-average basis, by type of award:
|
As of December 31, 2019 |
||||||||
|
Unamortized Expense |
Weighted Average Remaining Recognition Period (Years) |
|||||||
|
Stock Options |
$ | 7,989,431 | 2.70 | |||||
|
Restricted Stock Units |
$ | 221,462 | 2.62 | |||||
Stock Options
The following table summarizes the assumptions used for estimating the fair value of stock options granted under the Black-Scholes option-pricing model during:
|
Year Ended |
||||||||||
|
December 31, |
||||||||||
|
2019 |
2018 |
|||||||||
|
Expected dividend yield |
—% |
|
—% |
|
||||||
|
Risk-free interest rate |
1.40% | - | 2.40% |
|
2.67% | - | 3.01% |
|
||
|
Expected Volatility |
83% | - | 88% |
|
78% | - | 99% |
|
||
|
Expected life (years) |
6.04 | 6.25 | ||||||||
|
Weighted-average fair value per share |
$8.00 | $7.25 | ||||||||
The following describes each of these assumptions and the Company’s methodology for determining each assumption:
Expected Dividend Yield
The dividend yield percentage is zero because the Company neither currently pays dividends nor intends to do so during the expected option term.
Risk-Free Interest Rate
The risk-free interest rate is based on the U.S. Treasury yield curve approximating the term of the expected life of the award in effect on the date of grant.
Expected Volatility
Expected stock price volatility is based on an average of several peer public companies. For purposes of identifying peer companies, the Company considered characteristics such as industry, length of trading history and similar vesting terms.
Expected Life
The expected life represents the period the awards are expected to be outstanding. The Company’s historical share option exercise experience does not provide a reasonable basis upon which to estimate an expected term because of a lack of sufficient data. Therefore, the Company estimates the expected term by using the simplified method.
The following table summarizes the Company’s stock option activity during the year ended December 31, 2019:
|
Number of |
Weighted |
Weighted |
Aggregate |
|||||||||||||
|
Outstanding as of December 31, 2018 |
1,658,112 | $ | 8.76 | |||||||||||||
|
Granted |
715,108 | $ | 11.39 | |||||||||||||
|
Exercised |
(32,325 |
) |
$ | 4.37 | ||||||||||||
|
Cancelled |
(287,919 |
) |
$ | 11.65 | ||||||||||||
|
Outstanding as of December 31, 2019 |
2,052,976 | $ | 9.33 | 7.70 | $ | 235,669 | ||||||||||
|
Exercisable as of December 31, 2019 |
858,667 | $ | 7.27 | 6.09 | $ | 232,690 | ||||||||||
|
Vested and expected to vest as of December 31, 2019 |
1,865,715 | $ | 9.16 | 7.58 | $ | 235,533 | ||||||||||
The aggregate intrinsic value of stock options in the table above represents the difference between the $4.28 closing price of the Company’s common stock as of December 31, 2019 and the exercise price of outstanding, exercisable, and vested and expected to vest in-the-money stock options.
The following table summarizes information about the Company’s stock options as of December 31, 2019:
|
Exercise Price or Range of Exercise Price |
|
Options Outstanding |
|
Weighted Average |
|
Options Exercisable |
|||||||||||
|
$1.85 |
to |
$5.89 |
|
|
530,657 |
|
|
|
5.55 |
|
|
|
411,949 |
|
|||
|
$7.80 |
to |
$9.01 |
|
|
32,250 |
|
|
|
9.37 |
|
|
|
425 |
|
|||
|
$9.31 |
637,229 |
7.79 |
312,197 |
||||||||||||||
|
$10.04 |
to |
$21.36 |
|
|
852,840 |
|
|
|
8.90 |
|
|
|
134,096 |
|
|||
|
|
|
|
2,052,976 |
|
|
|
7.70 |
|
|
|
858,667 |
|
|||||
Additional information related to our stock options is summarized below:
|
Year Ended December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
Intrinsic value of options exercised |
$ | 266,040 | $ | 2,097,888 | ||||
|
Fair value of options vested |
$ | 4,131,982 | $ | 512,373 | ||||
During the years ended December 31, 2019 and 2018, 32,325 and 119,793 stock options were exercised for the purchase of shares of common stock for total cash proceeds of $141,347 and $334,711, respectively.
Restricted Stock Unit Awards
During the year ended December 31, 2018, the Board of Directors approved grants of 185,768 non-performance-based RSUs to employees. RSUs represent the right to receive shares of common stock of the Company at the end of a specified time period. The RSUs vest over a four-year period similar to stock options. RSUs can only be settled in shares of the Company’s common stock.
A summary of nonvested RSU awards outstanding as of December 31. 2019 and changes during the year then ended is as follows:
|
Number of RSUs |
Weighted Average Grant-Date Fair Value (per RSU) |
|||||||
|
Nonvested as of December 31, 2018 |
185,768 | $ | 11.04 | |||||
|
Granted |
-- | -- | ||||||
|
Vested |
(40,954 | ) | 11.09 | |||||
|
Forfeited |
(137,321 | ) | 10.05 | |||||
|
Nonvested as of December 31, 2019 |
7,493 | $ | 28.87 | |||||
Employee Stock Purchase Plan
On May 8, 2019, the Company’s stockholders approved the Liquidia Technologies, Inc. 2019 Employee Stock Purchase Plan (the “ESPP”). A total of 300,000 shares of the Company’s common stock have been reserved for issuance under the ESPP. Subject to any plan limitations, the ESPP allows eligible employees to contribute through payroll deductions up to $25,000 per year of their earnings for the purchase of the Company’s common stock at a discounted price per share. The offering periods are six months each and begin in March and September of each year, with the initial offering period commencing on September 3, 2019. Unless otherwise determined by the administrator, the Company’s common stock will be purchased for the accounts of employees participating in the ESPP at a price per share that is 85% of the fair market value of the Company’s common stock on the last trading day of the offering period.
As of December 31, 2019, the Company had collected $8,129 in proceeds pursuant to the ESPP. Based upon 85% of the closing price on December 31, 2019 of $4.28, approximately 2,200 shares could be purchased based upon employee withholdings as of December 31, 2019.
5. License Agreements
The Company performs research under a license agreement with The University of North Carolina at Chapel Hill (“UNC”) as amended to date (the “UNC Letter Agreement”). As part of the UNC Letter Agreement, the Company holds an exclusive license to certain research and development technologies and processes in various stages of patent pursuit, for use in its research and development and commercial activities, with a term until the expiration date of the last to expire patent subject to the UNC Letter Agreement, subject to industry standard contractual compliance. Under the UNC Letter Agreement, the Company is obligated to pay UNC royalties equal to a low single digit percentage of all net sales of drug products whose manufacture, use or sale includes any use of the technology or patent rights covered by the UNC Letter Agreement. The Company may grant sublicenses of UNC licensed intellectual property in return for specified payments based on a percentage of any fee, royalty or other consideration received.
6. Revenue From Contracts With Customers
The following tables represent a disaggregation of revenue by each significant research, development and licensing agreement and payment type for the years ended December 31, 2019 and 2018:
|
2019 Revenue Recognized From |
||||||||||||||||
|
Non-Refundable Payments |
Research and |
|||||||||||||||
|
Up front |
Development |
|||||||||||||||
|
Milestones |
Payments |
Services |
Total |
|||||||||||||
|
GSK Inhaled |
$ | 1,345,320 | $ | 6,726,600 | $ | — | $ | 8,071,920 | ||||||||
|
Other |
— | — | 200 | 200 | ||||||||||||
|
Total |
$ | 1,345,320 | $ | 6,726,600 | $ | 200 | $ | 8,072,120 | ||||||||
|
2018 Revenue Recognized From |
||||||||||||||||
|
Non-Refundable Payments |
Research and |
|||||||||||||||
|
Up front |
Development |
|||||||||||||||
|
Milestones |
Payments |
Services |
Total |
|||||||||||||
|
GSK Inhaled |
$ | 45,058 | $ | 225,293 | $ | 168,000 | $ | 438,351 | ||||||||
|
Other |
— | 943,419 | 1,325,211 | 2,268,630 | ||||||||||||
|
Total |
$ | 45,058 | $ | 1,168,712 | $ | 1,493,211 | $ | 2,706,981 | ||||||||
In September 2015, GSK Inhaled exercised the option to permanently license the technology for a non-refundable payment to the Company of $15.0 million. Pursuant to the license provisions of the collaboration agreement, GSK Inhaled is potentially required to pay the Company for certain milestones reached in addition to tiered royalties on the worldwide sales of the licensed products at percentages ranging from the mid-single digits to low-single digits depending on the total number of products developed and other royalty step-down events with a fixed low-single digit royalty floor. In February 2016, GSK Inhaled paid the Company a $3.0 million milestone payment pursuant to the collaboration agreement. The combined $18 million in up-front and milestone payments was subject to deferral pursuant to the adoption of ASC 606 and the revenue policy described herein.
In July 2018, GSK notified the Company of its plans to discontinue development of the inhaled antiviral for viral exacerbations in chronic obstructive pulmonary disease under the GSK Inhaled collaboration agreement after completion of the related Phase 1 clinical trial. In June 2019, the Company and GSK executed the third amendment to the collaboration agreement providing the Company rights to develop and commercialize additional inhaled programs at the Company’s sole cost. This amendment granted the Company the right to develop three additional molecular entities for application in inhaled programs using the Company’s PRINT technology and a mechanism to acquire further molecular entities for inhaled applications. New inhaled programs developed under this amendment would carry milestone and royalty payments due to GSK upon initiation of Phase 3 studies and subsequent commercialization, respectively. This amendment, among other factors including the lack of continued performance anticipated by the Company and GSK under the original agreement, led the Company to the belief that no further research and development services will be provided to GSK under the collaboration agreement and the earnings process related to the up front and development milestone payments previously received under the collaboration agreement was completed under the proportional performance model. Therefore, the remaining deferred revenue of $8.1 million was recognized as revenue during the year ended December 31, 2019. If GSK were to request additional services under the original agreement, which the Company believes is a remote likelihood, the Company does not expect the value of any incremental efforts that the Company might agree to perform to be material. Any potential milestone or royalty payments from the Company to GSK associated with this amendment will be recorded as operating expenses. Accordingly, in January 2020, the Company notified GSK of its intent to terminate the GSK Inhaled collaboration agreement based upon GSK's lack of performance under the agreement, which the Company believes constitutes a material breach of the agreement. In February 2020, the Company received a letter from GSK disputing the Company’s basis for termination. The parties are currently attempting to resolve the dispute pursuant to the terms of the agreement.
In June 2016, the Company entered into a development and license agreement with G&W Laboratories (“G&W”) to develop multiple products for topical delivery in dermatology using the Company’s PRINT technology (the “G&W Agreement”). The first non-refundable up-front fee of $1.0 million was received in June 2016. Research and development services commenced in July 2016 on the first program pursuant to this agreement. In April 2018, the Company and G&W mutually agreed to terminate the G&W Agreement. As a result, during the second quarter of 2018, the remaining unamortized balances in the related deferred revenue and deferred sublicense payments of $0.9 million and $0.1 million, respectively, were fully recorded as Revenues and Cost of Sales, respectively, in the Statement of Operations and Comprehensive Loss for the year ended December 31, 2018.
Deferred Sublicense Payments
Sublicense payments to UNC are considered direct and incremental fulfillment costs of the Company’s research, development and licensing agreements as the PRINT technology resources used by the Company are continually researched by UNC. These costs are deferred and then amortized into Cost of Sales over the same estimated period of benefit as the period of the underlying revenue recognition. In conjunction with the June 2019 amendment to the GSK collaboration agreement, the balance of deferred sublicense payments of $807,192 were expensed to Cost of Sales in the same period. As of December 31, 2019, the balances of these unamortized payments was $0. As of December 31, 2018, the balances of these unamortized payments included in current and long-term prepaid expenses and other assets was $0 and $807,192, respectively.
7. Property, Plant and Equipment
Property, plant and equipment consisted of the following:
|
December 31, |
December 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Lab and build-to-suit equipment |
$ | 7,562,263 | $ | 7,266,895 | ||||
|
Office equipment |
128,669 | 130,460 | ||||||
|
Furniture and fixtures |
237,951 | 205,051 | ||||||
|
Computer equipment |
804,046 | 799,515 | ||||||
|
Leasehold improvements |
11,762,351 | 8,878,361 | ||||||
|
Construction-in-progress |
91,797 | 155,148 | ||||||
|
Total property, plant and equipment |
20,587,077 | 17,435,430 | ||||||
|
Accumulated depreciation and amortization |
(11,333,112 | ) | (9,304,722 | ) | ||||
|
Property, plant and equipment, net |
$ | 9,253,965 | $ | 8,130,708 | ||||
The Company recorded depreciation and amortization expense of $2,567,742 and $1,543,667 for the years ended December 31, 2019 and 2018, respectively. Maintenance and repairs are expensed as incurred and were $220,658 and $153,278, respectively, for the years ended December 31, 2019 and 2018.
In December 2016, the Company executed an agreement with a commercial manufacturer to build a PRINT particle fabrication line for the production of LIQ861. The ultimate cost was approximately $1.6 million. The Company financed this transaction with a third-party vendor, CSC Leasing Company (“CSC”). CSC made payments to the manufacturer per the payment schedule in the agreement as the asset was fabricated. CSC charged the Company a monthly lease rate on the scheduled payments made to the manufacturer as interim financing costs until the asset was completed and placed in service. Upon completion of fabrication, the lease commenced in March 2018 (“CSC Financing”).
In accordance with ASC 840, Leases, for build-to-suit arrangements where the Company is involved in the fabrication of an asset prior to the commencement of the ultimate financing or takes some level of construction risk, the Company is considered the accounting owner of the assets during the fabrication period. Accordingly, during the fabrication phase, the Company recorded a construction-in-progress asset within Property, Plant and Equipment and a corresponding deferred financing obligation liability for contributions by CSC toward fabrication. Upon completion of the fabrication in March 2018, since the Company maintained substantially all of the risk and rewards of ownership of the asset, the Company recorded the transaction as a financing, continuing to record the asset and reclassifying the deferred financing obligation to debt. In accordance with Topic 842, Leases, the CSC Financing was recharacterized as a finance lease in the first quarter of 2019 and accounted for accordingly (see Note 9).
The following table details the activity of Construction in Progress (“CIP”) in 2019 and 2018 and the associated transfer to Leasehold Improvements and Lab Equipment when the assets were placed in service:
|
Leasehold |
Build-to-suit |
Lab |
||||||||||||||
|
Improvements |
Equipment |
Equipment |
Total |
|||||||||||||
|
Balance as of December 31, 2017 |
$ | 1,208,107 | $ | 1,583,054 | $ | 39,246 | $ | 2,830,407 | ||||||||
|
Add: Purchases related to CIP |
425,438 | 82,687 | 114,102 | 622,227 | ||||||||||||
|
Less: Transfer due to being placed in service |
(1,570,194 | ) | (1,665,741 | ) | (61,551 | ) | (3,297,486 | ) | ||||||||
|
Balance as of December 31, 2018 |
63,351 | — | 91,797 | 155,148 | ||||||||||||
|
Add: Purchases related to CIP |
2,820,640 | — | — | 2,820,640 | ||||||||||||
|
Less: Transfer due to being placed in service |
(2,883,991 | ) | — | — | (2,883,991 | ) | ||||||||||
|
Balance as of December 31, 2019 |
$ | - | $ | - | $ | 91,797 | $ | 91,797 | ||||||||
The Construction in Progress balance includes $0 and $3,925 of capitalized interest costs for the years ended December 31, 2019 and 2018, respectively.
8. Income Taxes
No provision for federal and state income tax expense has been recorded for the years ended December 31, 2019 and 2018 due to the valuation allowance recorded against the net deferred tax asset and recurring losses.
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities are as follows at December 31, 2019 and 2018:
|
2019 |
2018 |
|||||||
|
Deferred income tax assets: |
||||||||
|
Tax loss carryforwards |
$ | 42,107,372 | $ | 30,239,898 | ||||
|
Deferred revenue |
— | 1,856,507 | ||||||
|
Research and development credits |
3,016,889 | 2,382,047 | ||||||
|
Stock-based compensation |
973,905 | 489,694 | ||||||
|
Lease liability |
1,508,645 | — | ||||||
|
Compensation |
564,572 | 431,649 | ||||||
|
Fixed assets |
— | 160,784 | ||||||
|
Patent amortization |
86,985 | 97,942 | ||||||
|
Other |
55,972 | 669,151 | ||||||
|
Valuation allowance |
(47,505,967 | ) | (36,327,672 | ) | ||||
|
Total deferred income tax assets |
808,373 | — | ||||||
|
Deferred income tax liabilities: |
||||||||
|
Fixed assets |
158,787 | — | ||||||
|
Operating leases |
649,586 | — | ||||||
|
Total deferred income tax liabilities |
808,373 | — | ||||||
|
Total net deferred tax |
$ | — | $ | — | ||||
As of December 31, 2019 and 2018, the Company established a full valuation allowance against its net deferred tax assets since, at the time, the Company could not assert that it was more likely than not that its deferred tax assets would be realized. As a result, there was an increase in the valuation allowance in 2019 of $11,178,295.
As of December 31, 2019, the Company had federal and state income tax loss carryforwards of $97,268,927 and $184,191,027, respectively, which begin to expire in 2024 for federal purposes and in 2019 for state purposes. As of December 31, 2019, the Company had federal and state income tax loss carryforwards of $85,765,810 and $778,925, respectively, which carryforward indefinitely. In addition, the Company has tax credit carryforwards for federal tax purposes of $3,175,600 as of December 31, 2019, which begin to expire in 2026. The utilization of net operating loss and tax credit carryforwards to reduce future income taxes will depend on the Company’s ability to generate sufficient taxable income prior to the expiration of the loss carryforwards.
The Internal Revenue Code of 1986, as amended, contains provisions which limit the ability to utilize the net operating loss carryforwards in the case of certain events, including significant changes in ownership interests. If the Company’s net operating loss carryforwards are limited, and the Company has taxable income which exceeds the permissible yearly net operating loss carryforwards, the Company would incur a federal income tax liability even though net operating loss carryforwards would be available in future years.
The reasons for the difference between actual income tax expense for the years ended December 31, 2019 and 2018 and the amount computed by applying the statutory federal income tax rate to income before income tax are as follows:
|
2019 |
2018 |
|||||||||||||||
|
% of Pretax |
% of Pretax |
|||||||||||||||
|
Amount |
Earnings |
Amount |
Earnings |
|||||||||||||
|
Income tax benefit at statutory rate |
$ | (10,019,974 | ) | 21.0 |
% |
$ | (11,158,530 | ) | 21.0 |
% |
||||||
|
State income taxes, net of federal tax benefit |
(957,616 | ) | 2.0 | (1,062,492 | ) | 2.0 | ||||||||||
|
Non-deductible expenses |
94,903 | (0.2 | ) | 6,810 | — | |||||||||||
|
Stock-based compensation |
258,338 | (0.5 | ) | 10,925 | — | |||||||||||
|
Non-deductible interest expense |
— | — | 4,074,501 | (7.7 | ) | |||||||||||
|
Derivative and warrant fair value adjustments |
— | — | (63,873 | ) | 0.1 | |||||||||||
|
Credits |
(634,842 | ) | 1.3 | — | — | |||||||||||
|
Change in state rate |
3,887 | — | (2,842 | ) | — | |||||||||||
|
Other |
77,009 | (0.1 | ) | (139,660 | ) | 0.3 | ||||||||||
|
Change in valuation allowance |
11,178,295 | (23.5 | ) | 8,335,161 | (15.7 | ) | ||||||||||
|
Provision for income taxes |
$ | — | 0.0 |
% |
$ | — | 0.0 |
% |
||||||||
The Company has determined that there may be a future limitation on the Company’s ability to utilize its entire federal R&D credit carryover. Therefore, the Company recognized an uncertain tax benefit associated with the federal R&D credit carryover during the years ended December 31, 2019 and 2018, as follows:
|
Balance at December 31, 2017 |
$ | — | ||
|
Increases related to 2018 |
— | |||
|
Increases related to prior periods |
— | |||
|
Balance at December 31, 2018 |
— | |||
|
Increases related to 2019 |
158,710 | |||
|
Increases related to prior periods |
— | |||
|
Balance at December 31, 2019 |
$ | 158,710 |
The Company recognizes the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The Company has determined that it had no other material uncertain tax benefits for the year ended December 31, 2019.The Company’s policy for recording interest and penalties related to uncertain tax provisions is to record them as a component of the provision for income taxes. The Company did not have any accrued interest or penalties associated with any unrecognized tax positions as of December 31, 2019 and 2018, and there were no such interest or penalties recognized during the years ended December 31, 2019 and 2018.
The Company has all tax years open to examination by federal tax and state tax jurisdictions. No income tax returns are currently under examination by taxing authorities.
9. Leases, Commitments and Contingencies
Leases
The Company leases certain lab space, office space, and equipment. Leases with an initial term of 12 months or less are not recorded on the Balance Sheet; the Company recognizes lease expense for these leases on a straight-line basis over the lease term. For lease agreements entered into or reassessed after the adoption of Topic 842, the Company combines lease and non-lease components, if any. Most leases include one or more options to renew. The exercise of lease renewal options is at the Company’s sole discretion. Certain leases also include options to purchase the leased property. Consistent with past practice and current intent, the Company has recognized all such purchase options as part of its right-of-use assets and lease liabilities. The depreciable life of assets and leasehold improvements are limited by the expected lease term, unless there is a transfer of title or purchase option reasonably certain of exercise. The Company’s lease agreements do not contain any material residual value guarantees or material restrictive covenants.
The Company conducts its operations from leased facilities in Morrisville, North Carolina. As of December 31, 2019, the Company’s amended leases for its primary building is for usage of approximately 45,000 square feet of space expiring October 31, 2026. The leases are for general office, laboratory, research and development and light manufacturing space. The lease agreements require the Company to pay property taxes, insurance, common area expenses and maintenance costs. In November 2018, the Company amended the lease of its primary building to expand by 8,264 additional square footage expiring October 31, 2026 in exchange for terminating the Company’s other lease with the same landlord for 4,400 noncontiguous square feet. A tenant allowance of approximately $1.0 million was also made available for use to help fund the build out related to the expansion of the primary building lease. The incremental rent over the terminated lease for the first 12 months of this lease expansion amounts to $0.1 million, subject to lease escalation in subsequent periods. In June 2019, the Company signed a commitment to incur construction costs of up to $3.1 million related to the leasehold improvements for this lease expansion, against which the tenant allowance will be applied. The leasehold improvements were substantially completed in 2019 and the Company took occupancy of the additional square footage in 2019. The total construction costs incurred in 2019 approximated $2.8 million and the remaining estimated costs to be incurred as of December 31, 2019 is not expected to be material.
The Company leases specialized lab equipment under finance leases. The related right-of-use assets are amortized on a straight-line basis over the lesser of the lease term or the estimated useful life of the asset.
The CSC Financing (see Note 7) has a term of three years with equal monthly payments. The CSC Financing is secured by a lien on the related build-to-suit equipment and includes an option to purchase the build-to-suit equipment at maturity at an amount equal to the lesser of fair market value or 23% of the initial financed amount. The right-of-use assets related to finance leases net of amortization is $1,981,002 as of December 31, 2019 and is included in lab equipment, build-to-suit equipment, computer equipment and leasehold improvements within property, plant and equipment in the accompanying balance sheet (see Note 7). The Company does not have access to certain inputs used by its lessors to calculate the rate implicit in its finance leases. As such, the Company utilizes its estimated incremental borrowing rate for the discount rate applied to its finance leases. The incremental borrowing rate used on finance leases was 7.5%.
The Company’s lease cost is reflected in the accompanying Statements of Operations and Comprehensive Loss as follows:
|
Year Ended |
|||||
|
Classification |
December 31, 2019 |
||||
|
Operating lease cost |
General and administrative |
$ | 884,597 | ||
|
Finance lease cost: |
|||||
|
Amortization of lease assets |
General and administrative |
1,316,924 | |||
|
Interest on lease liabilities |
Interest expense |
190,687 | |||
|
Lease cost |
$ | 2,392,208 | |||
Rent expense under prior lease accounting rules (Topic 840) was $953,733 during the year ended December 31, 2018.
The weighted average remaining lease term and discount rates as of December 31, 2019 were as follows:
|
Weighted average remaining lease term (years): |
||||
|
Operating leases |
6.8 | |||
|
Finance leases |
1.6 | |||
|
Weighted average discount rate: |
||||
|
Operating leases |
10.3 |
% |
||
|
Finance leases |
7.7 |
% |
The discount rate for operating leases was estimated based upon market rates of collateralized loan obligations of comparable companies on comparable terms.
The future minimum lease payments as of December 31, 2019 were as follows:
|
Operating |
Finance |
|||||||||||
|
Year ending December 31: |
Leases |
Leases |
Total |
|||||||||
|
2020 |
$ | 1,172,759 | $ | 1,366,026 | $ | 2,538,785 | ||||||
|
2021 |
1,207,708 | 814,360 | 2,022,068 | |||||||||
|
2022 |
1,243,934 | 260,857 | 1,504,791 | |||||||||
|
2023 |
1,283,253 | — | 1,283,253 | |||||||||
|
2024 |
1,316,540 | — | 1,316,540 | |||||||||
|
Thereafter |
2,513,729 | — | 2,513,729 | |||||||||
|
Total minimum lease payments |
8,737,923 | 2,441,243 | 11,179,166 | |||||||||
|
Less: Interest |
(2,500,562 | ) | (140,267 | ) | (2,640,829 | ) | ||||||
|
Present value of lease liabilities |
$ | 6,237,361 | $ | 2,300,976 | $ | 8,538,337 | ||||||
As previously reported in the Company’s Annual Report on Form 10-K for the year ended December 31, 2018 and under legacy lease accounting (ASC 840), future minimum lease payments under non-cancellable leases as of December 31, 2018 are as follows:
|
Operating |
Finance |
|||||||
|
Leases |
Leases |
|||||||
|
2019 |
$ | 1,077,532 | $ | 464,797 | ||||
|
2020 |
1,168,710 | 354,739 | ||||||
|
2021 |
1,203,658 | 33,774 | ||||||
|
2022 |
1,239,885 | — | ||||||
|
2023 |
1,276,356 | — | ||||||
|
Thereafter |
3,818,795 | — | ||||||
|
Total minimum lease payments |
$ | 9,784,936 | 853,310 | |||||
|
Less: Interest |
(24,525 | ) | ||||||
|
Present value of lease liabilities |
$ | 828,785 | ||||||
Other Commitments
In March 2012, the Company entered into an agreement, as amended, with Chasm Technologies, Inc. for manufacturing consulting services related to the Company’s manufacturing capabilities during the term of the agreement. As future contingent consideration under the agreement, the Company agreed to pay $400,000 related to the timing of the Company’s first Phase 3 clinical trial which commenced site initiation in December 2017. The consideration of $400,000 is comprised of initial consideration of $20,000 paid in 2017, $80,000 to be paid upon first dosing of the first patient in the Phase 3 clinical trial which occurred in 2018, and $300,000 due no later than December 31, 2018, which was paid in 2018. In addition, the Company also agreed to pay future contingent royalties on net sales totaling no more than $1,500,000.
Contingencies
The Company from time-to-time is subject to claims and litigation in the normal course of business, none of which the Company believes represent a risk of material loss or exposure.
10. Long-Term Debt
Long-term debt consisted of the following as of December 31, 2019 and 2018:
|
December 31, |
December 31, |
||||||||
|
Maturity Date |
2019 |
2018 |
|||||||
|
Pacific Western Bank note |
October 25, 2022 |
$ | 15,878,121 | $ | 10,802,355 | ||||
|
CSC build-to-suit equipment financing, net of discount |
— | 1,142,194 | |||||||
|
Less current portion |
(5,585,637 | ) | (316,906 | ) | |||||
|
Long-term debt, less current portion |
$ | 10,292,484 | $ | 11,627,643 | |||||
Pacific Western Bank
In January 2016 and October 2016, the Company entered into a Loan and Security Agreement (“LSA”) and an amendment, respectively, with Pacific Western Bank (“Pacific Western”). The LSA provided that the Company may borrow up to $10.0 million in three tranches of a term loan (“Term Loan”) to supplement working capital and finance facility expansion and capital equipment purchases. The Term Loan was collateralized by a lien on all assets of the Company that are not otherwise encumbered, including a negative pledge on intellectual property prohibiting its sale without Pacific Western’s consent. Amounts borrowed under the Term Loan could be repaid at any time without penalty or premium. The Term Loan was interest-only through July 6, 2017, followed by an amortization period of 30 months of equal monthly payments of principal plus interest, beginning on August 6, 2017 and continuing on the same day of each month thereafter until paid in full. Any amounts borrowed under the Term Loan bore interest at 3.75% during the initial 18-month interest-only period. Following the interest-only period, the interest rate increased to 5.00%, which was to be fixed for the duration of the Term Loan. Subsequent to the Company closing its IPO, on August 6, 2018 the Company paid Pacific Western a liquidity event success fee of $400,000, which was recorded as Interest Expense in the Statement of Operations and Comprehensive Loss for the year ended December 31, 2018.
In March 2018, the Company and Pacific Western executed the Ninth Amendment to the LSA (the “Ninth Amendment”). With the Ninth Amendment, new covenants were enacted requiring the Company to (1) at all times maintain a balance of cash at Pacific Western of at least $8.0 million, an increase of $5.5 million from its prior cash balance covenant, and (2) not observe any materially adverse data from its LIQ861 Phase 3 study on or before December 31, 2018. Pursuant to this Ninth Amendment, the interest-only period for the Tranche I loan was amended to include the period from January 7, 2018 to July 6, 2018, and the interest-only period for the Tranche II and Tranche III loans was amended to include the period from January 13, 2018 to July 12, 2018. Prior to executing the amendment, the Company had made principal payments of $0.6 million inside of the defined interest-only period, which were subsequently refunded on the same day. All amendments to the Pacific Western LSA were accounted for as a modification.
In October 2018, the Company and Pacific Western entered into an Amended and Restated Loan and Security Agreement (the “A&R LSA”) in which the Company received an initial tranche of $11.0 million to extinguish its existing debt of $8.0 million under the LSA, repay in full the $1.8 million in outstanding indebtedness under the UNC Promissory Note and for general corporate purposes. The indebtedness under the A&R LSA bears interest at the greater of the Prime rate or 5% and has a four-year term maturing in October 2022. The A&R LSA provided for access to a second tranche of up to $5.0 million available to be drawn at the Company’s option through June 30, 2019. The second tranche became accessible as a result of the full enrollment of the Company’s LIQ861 INSPIRE clinical trial, without observing any materially adverse data through the two week endpoint. The entire second tranche of $5.0 million was drawn by the Company in May 2019 bringing the total amount outstanding to $16.0 million. Both tranches require payments of interest-only through December 31, 2019. As a result of this refinancing, the Company recorded a gain of $0.1 million in accordance with ASC 470-50, Debt – Modifications and Extinguishments.
The A&R LSA carries a one-time success fee of $375,000 and a prepayment penalty of 1% if the drawn tranche is prepaid prior to October 27, 2020. The success fee was triggered in December 2019 by the sale of common stock and this was recorded as interest expense of $375,000 during the year ended December 2019. Accrued interest is included in Other accrued expenses in the Balance Sheet as of December 31, 2019. The minimum cash covenant is $8.5 million. Pacific Western maintains a blanket lien on all assets excluding intellectual property, for which it has been provided a negative pledge. Pursuant to the A&R LSA, the Company is also obligated to comply with various other customary covenants, including, among others, restrictions on its ability to dispose of assets, replace or suffer the departure of the CEO or CFO without delivering ten days’ prior written notification to Pacific Western, suffer a change on the Board of Directors which would result in the failure of at least one partner of Canaan Partners or their respective affiliates to serve as a voting member in each case without having used best efforts to deliver at least 15 days’ prior written notification to Pacific Western, make acquisitions, be acquired, incur indebtedness, grant liens, make distributions to its stockholders, make investments, enter into certain transactions with affiliates or pay down subordinated debt, subject to specified exceptions.
In May 2019, the Company and Pacific Western entered a First Amendment to A&R LSA to provide for a limit of $2.5 million of capital expenditures during the year ended December 31, 2019. As of December 31, 2019, the Company was in compliance with this and all other covenants.
Scheduled annual maturities of long-term debt as of December 31, 2019 are as follows:
|
Year ending December 31: |
||||
|
2020 |
$ | 5,818,182 | ||
|
2021 |
5,818,182 | |||
|
2022 |
4,363,636 | |||
|
Thereafter |
— | |||
|
Total |
16,000,000 | |||
|
Less: Unamortized discount |
(91,520 | ) | ||
|
Less: Unamortized debt issuance costs |
(30,359 | ) | ||
|
Less: Current portion of long-term debt |
(5,585,637 | ) | ||
|
Long-term debt, noncurrent |
$ | 10,292,484 |
11. Warrant Liability Change in Fair Value During Year 2018
The Company’s liability-classified warrants to purchase preferred stock were recorded as a liability at their estimated fair value at the date of issuance, with the subsequent changes in estimated fair value recorded in warrant fair value adjustments in the Company’s Statements of Operations and Comprehensive Loss. The warrants, with a fair value of $4,474,122 at inception, were initially recorded as a warrant liability on the Balance Sheet with a corresponding discount to the notes. The change in the estimated fair value of the warrant liability resulted in a fair value adjustment and is included in warrant fair value adjustments in the Statements of Operations and Comprehensive Loss. In conjunction with the IPO during the year ended December 31, 2018, the warrants automatically converted to warrants to purchase common stock. Therefore, upon the IPO, the warrant liability was marked to fair market value and transferred to additional paid-in capital. Changes in the values of the warrant liability for the years ended December 31, 2019 and 2018 are summarized below:
|
Year Ended December 31, |
||||||||
|
2019 |
2018 |
|||||||
|
Fair value, beginning of period |
$ | — | $ | 2,462,859 | ||||
|
Issuance of warrants |
— | — | ||||||
|
Change in fair value |
— | (277,715 | ) | |||||
|
Transfer to additional paid-in capital |
— | (2,185,144 | ) | |||||
|
Fair value, end of period |
$ | — | $ | — | ||||
12. Defined Contribution Retirement Plan
The Company maintains a defined contribution 401(k) retirement plan for its employees, pursuant to which employees who have completed sixty days of service may elect to contribute a portion of their compensation on a tax-deferred basis up to the maximum amount permitted by the Internal Revenue Code. The Company provides a 4% matching contribution to eligible employee contributions. Matching contributions are paid subsequent to the year to which they relate. The Company’s matching contributions due for the years ended December 31, 2019 and 2018 were $400,378 and $365,988, respectively, and such amounts were included in Accrued Expenses on the Balance Sheets as of December 31, 2019 and 2018, respectively.
F-29
