Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - CASTLE BIOSCIENCES INC | exhibit991q42019earnin.htm |
| 8-K - 8-K - CASTLE BIOSCIENCES INC | a8-kxearningsreleaseq4.htm |

The Skin Cancer Diagnostics Company COMPANY PRESENTATION March 10, 2020 NASDAQ:CSTL

DISCLAIMERS › Forward-Looking Statements The information in this presentation contains forward-looking statements and information within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are subject to the “safe harbor” created by those sections. These forward-looking statements include, but are not limited to, statements concerning the anticipated milestones, including expected commercial availability of our pipeline products, estimated total addressable market attributable to our pipeline products, and our plans for commercial expansion, including anticipated number of sales territories. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions, or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward- looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make. These forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from those in the forward- looking statements, including, without limitation, the risks set forth in our final prospectus filed with the SEC on July 26, 2019 relating to our Registration Statements on Form S-1 (File Nos. 333-232369 and 333-232796) and our Annual Report on Form 10-K for the year ended December 31, 2019, and in our other filings with the SEC. The forward-looking statements are applicable only as of the date on which they are made, and we do not assume any obligation to update any forward-looking statements, except as may be required by law. DecisionDx, DecisionDx-UM, DecisionDx-Melanoma, and the Castle Biosciences logo are the registered trademarks of Castle Biosciences, Inc. This presentation may also contain trademarks and trade names that are the property of their respective owners. 2

CASTLE BIOSCIENCES Improving Health Outcomes Of Patients With Skin Cancer Through Innovative, Clinically Actionable, Cost-Effective Diagnostics › INNOVATIVE PRODUCTS › Two commercialized products that improve treatment decisions for patients with melanoma › 95% of commercial emphasis currently on skin melanoma (DecisionDx®-Melanoma) › Pipeline products → Two additional skin cancer products expected to launch in second half of 2020 › DecisionDx-SCC › Suspicious Pigmented Lesions › Combined, expected to increase estimated U.S. total addressable market size by $1.4B to $2.0B › HIGH VOLUME GROWTH and STRONG FINANCIALS › 4Q2019 DecisionDx-Melanoma volume increased by 37% over 4Q2018 › 2019 Revenue: $52 million vs $23 million in 2018 › 2019 Gross Margin: 86% vs 77% in 2018 › Cash at December 31, 2019, of $99 million › ADVANCING EVIDENCE DEVELOPMENT › 22 peer-reviewed publications for DecisionDx-Melanoma › Both DecisionDx-Melanoma and DecisionDx-UM achieved Category I MAAA CPT code status 3

CASTLE’S DERMATOLOGICAL CANCER PRODUCTS & CURRENT PIPELINE TARGET AN ESTIMATED $2.0B U.S. TOTAL ADDRESSABLE MARKET1 SQUAMOUS CELL SUSPICIOUS PIGMENTED MELANOMA CARCINOMA LESIONS INITIAL CASTLE ~130,000 patients classified ~200,000 patients present ~300,000 patients with an ADDRESSABLE as Stage I, II or III with high-risk features indeterminant biopsy MARKET2 ~$540M Estimated U.S. TAM ~$820M Estimated U.S. TAM ~$600M Estimated U.S. TAM COMPANIES WITH Castle: Castle (proj. 2H2020): Castle (proj. 2H2020): ADRESSABLE OFFERINGS GEP test for Suspicious IN THE U.S. Pigmented Lesions Myriad Genetics: myPath®Melanoma 1U.S. TAM = Total addressable market based on estimated patient population assuming average reimbursement rate among all payors. 2 Annual U.S. incidence for Stage I, II o r III melanoma estimated at 130,000; Annual U.S. incidence for squamous cell carcinoma estimated at 1,000,000 with addressable market limited to carcinomas with one or more high risk features; Annual U.S. incidence for suspicious pigmented lesion biopsies estimated at 2,000,000 with addressable 4 market limited to the 15% with an indeterminant biopsy.
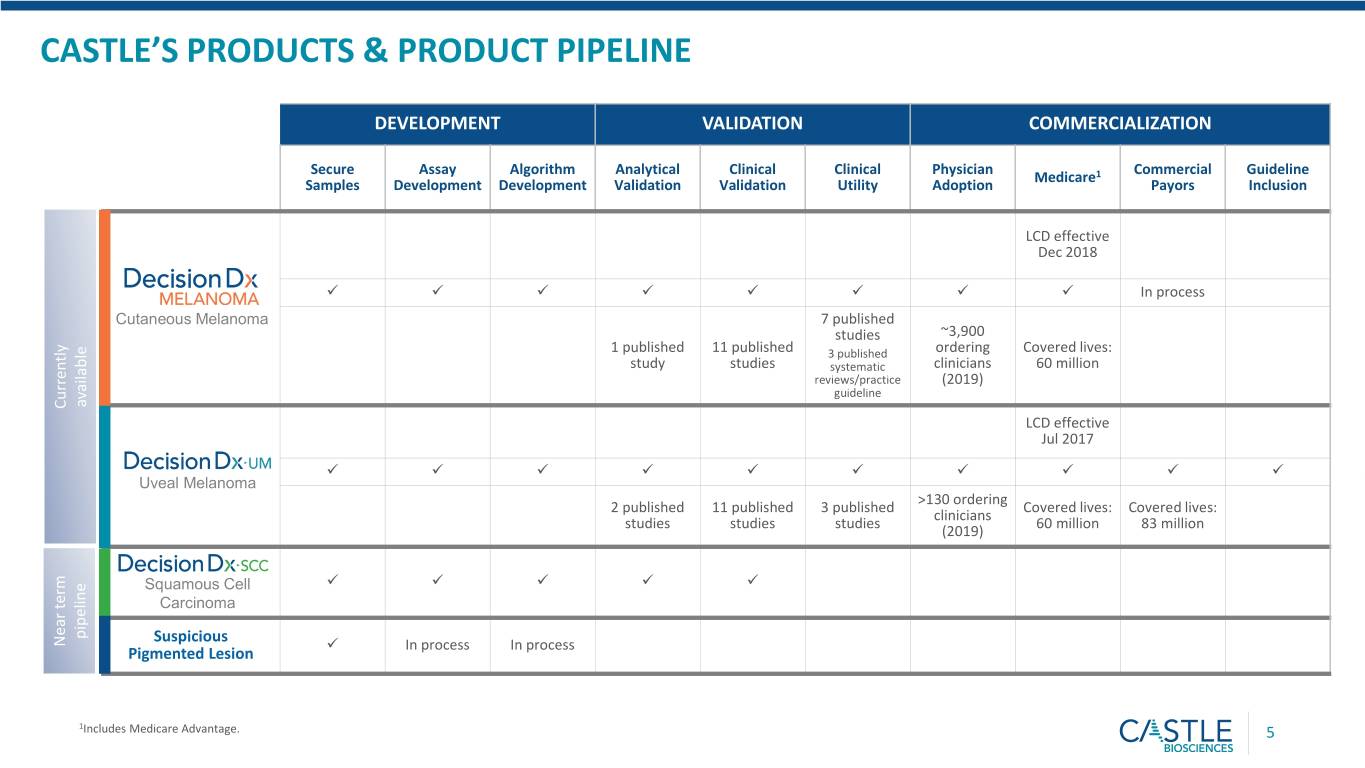
CASTLE’S PRODUCTS & PRODUCT PIPELINE DEVELOPMENT VALIDATION COMMERCIALIZATION Secure Assay Algorithm Analytical Clinical Clinical Physician Commercial Guideline Medicare1 Samples Development Development Validation Validation Utility Adoption Payors Inclusion LCD effective Dec 2018 In process Cutaneous Melanoma 7 published studies ~3,900 1 published 11 published 3 published ordering Covered lives: study studies systematic clinicians 60 million reviews/practice (2019) guideline available Currently LCD effective Jul 2017 Uveal Melanoma >130 ordering 2 published 11 published 3 published Covered lives: Covered lives: clinicians studies studies studies 60 million 83 million (2019) Squamous Cell Carcinoma pipeline Near term Near term Suspicious In process In process Pigmented Lesion 1Includes Medicare Advantage. 5
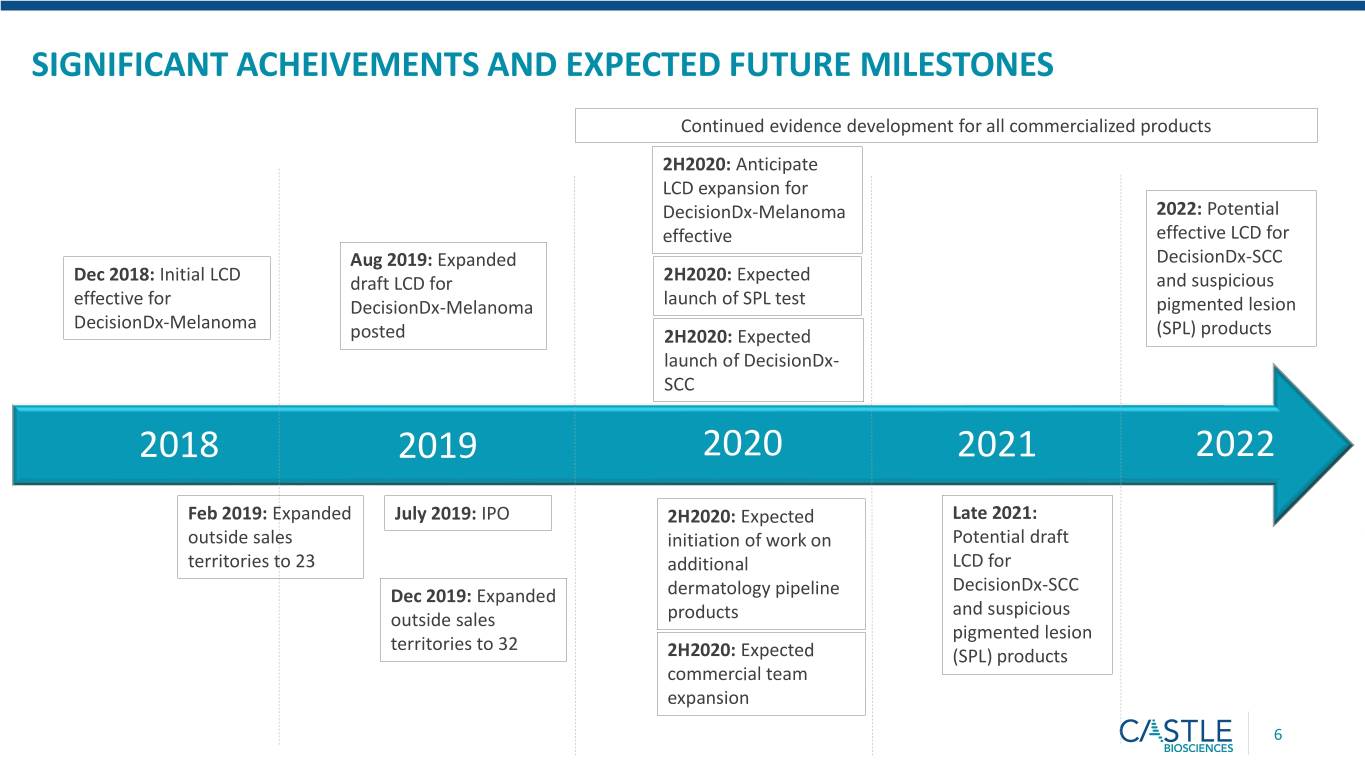
SIGNIFICANT ACHEIVEMENTS AND EXPECTED FUTURE MILESTONES Continued evidence development for all commercialized products 2H2020: Anticipate LCD expansion for DecisionDx-Melanoma 2022: Potential effective effective LCD for Aug 2019: Expanded DecisionDx-SCC Dec 2018: Initial LCD draft LCD for 2H2020: Expected and suspicious effective for DecisionDx-Melanoma launch of SPL test pigmented lesion DecisionDx-Melanoma posted 2H2020: Expected (SPL) products launch of DecisionDx- SCC 2018 2019 2020 2021 2022 Feb 2019: Expanded July 2019: IPO 2H2020: Expected Late 2021: outside sales initiation of work on Potential draft territories to 23 additional LCD for DecisionDx-SCC Dec 2019: Expanded dermatology pipeline and suspicious outside sales products pigmented lesion territories to 32 2H2020: Expected (SPL) products commercial team expansion 6

The Skin Cancer Diagnostics Company
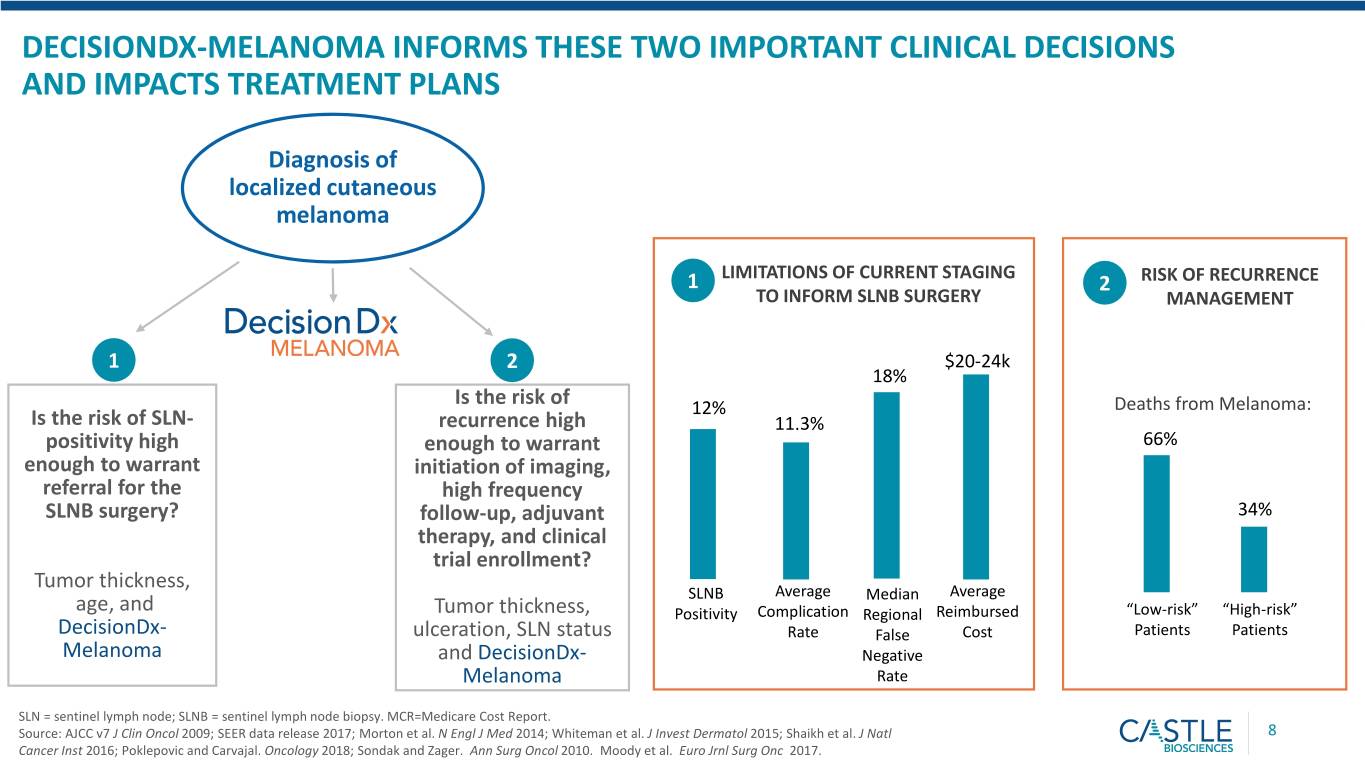
DECISIONDX-MELANOMA INFORMS THESE TWO IMPORTANT CLINICAL DECISIONS AND IMPACTS TREATMENT PLANS Diagnosis of localized cutaneous melanoma LIMITATIONS OF CURRENT STAGING 1 2 RISK OF RECURRENCE TO INFORM SLNB SURGERY MANAGEMENT 1 2 $20-24k 18% Is the risk of 12% Deaths from Melanoma: Is the risk of SLN- recurrence high 11.3% positivity high enough to warrant 66% enough to warrant initiation of imaging, referral for the high frequency SLNB surgery? follow-up, adjuvant 34% therapy, and clinical trial enrollment? Tumor thickness, SLNB Average Median Average age, and Tumor thickness, Positivity Complication Regional Reimbursed “Low-risk” “High-risk” DecisionDx- ulceration, SLN status Rate False Cost Patients Patients Melanoma and DecisionDx- Negative Melanoma Rate SLN = sentinel lymph node; SLNB = sentinel lymph node biopsy. MCR=Medicare Cost Report. Source: AJCC v7 J Clin Oncol 2009; SEER data release 2017; Morton et al. N Engl J Med 2014; Whiteman et al. J Invest Dermatol 2015; Shaikh et al. J Natl 8 Cancer Inst 2016; Poklepovic and Carvajal. Oncology 2018; Sondak and Zager. Ann Surg Oncol 2010. Moody et al. Euro Jrnl Surg Onc 2017.

DECISIONDX-MELANOMA WORKFLOW: AFTER DIAGNOSIS FOR MORE ACCURATE PREDICTION OF RISK Primary CM Tissue Class 1: 1A (FFPE) Lowest risk Low risk of SLN positivity (eligible T1-T2) Castle CAP/CLIA 1B Low risk of melanoma Low risk Patients with Certified Lab recurrence (T1-T4) Stage I – III Melanoma RNA Isolation Class 2: 2A 31 Gene Expression High-risk Profile Higher risk of SLN positivity (eligible T1-T2) High-risk of melanoma 2B Proprietary recurrence (T1-T4) Highest risk Algorithm Predicts Class 9 Gerami et al. Clin Cancer Res 2015; Gerami et al. JAAD 2015; Zager et al. BMC Cancer 2018; Gastman et al. JAAD 2019

DECISION 1 : SLNB SURGERY RULE OUT IN T1-T2 MELANOMAS Probability of a Positive Sentinel Lymph Node1 DecisionDx- DecisionDx-Melanoma Class 1A identifies a Melanoma All Ages ≥65 years 55-64 years <55 years Result (n=1065) (n=448) (n=247) (n=370) low risk for SLN positivity similar to stage T1a patients (for whom SLNB is not Class 1A 4.6% 1.6% 4.9% 7.6% recommended) Class 1B/2A 10.8% 6.9% 7.7% 19.6% Class 2B 18.8% 11.9% 30.8% 24.0% Class 1A Outcomes (all ages) Median follow-up MSS OS DMFS RFS Class 1A patients show excellent 5-year survival outcomes in >5 years 99.6% 98.2% 95.3% 93.5% long-term archival studies1 Outcomes confirmed in prospective, multi-center study2 3.2 years n/r 99.4% 98.7% 96.6% Data shows DecisionDx-Melanoma could result in 74% fewer SLNB surgeries, potentially saving U.S. healthcare system $250M1,3 1Vetto et al. Future Oncol 2019. 2Hsueh et al. Poster discussion abstract, ASCO 2019. 3Clearview health economic model, data on file. T1-T2 tumors are ≤2.0mm thick (“Breslow’s” thickness or depth). MSS = melanoma specific survival. OS = overall survival. DMFS = distant metastasis free survival. RFS = recurrence free survival. 10 n/r = not reported.
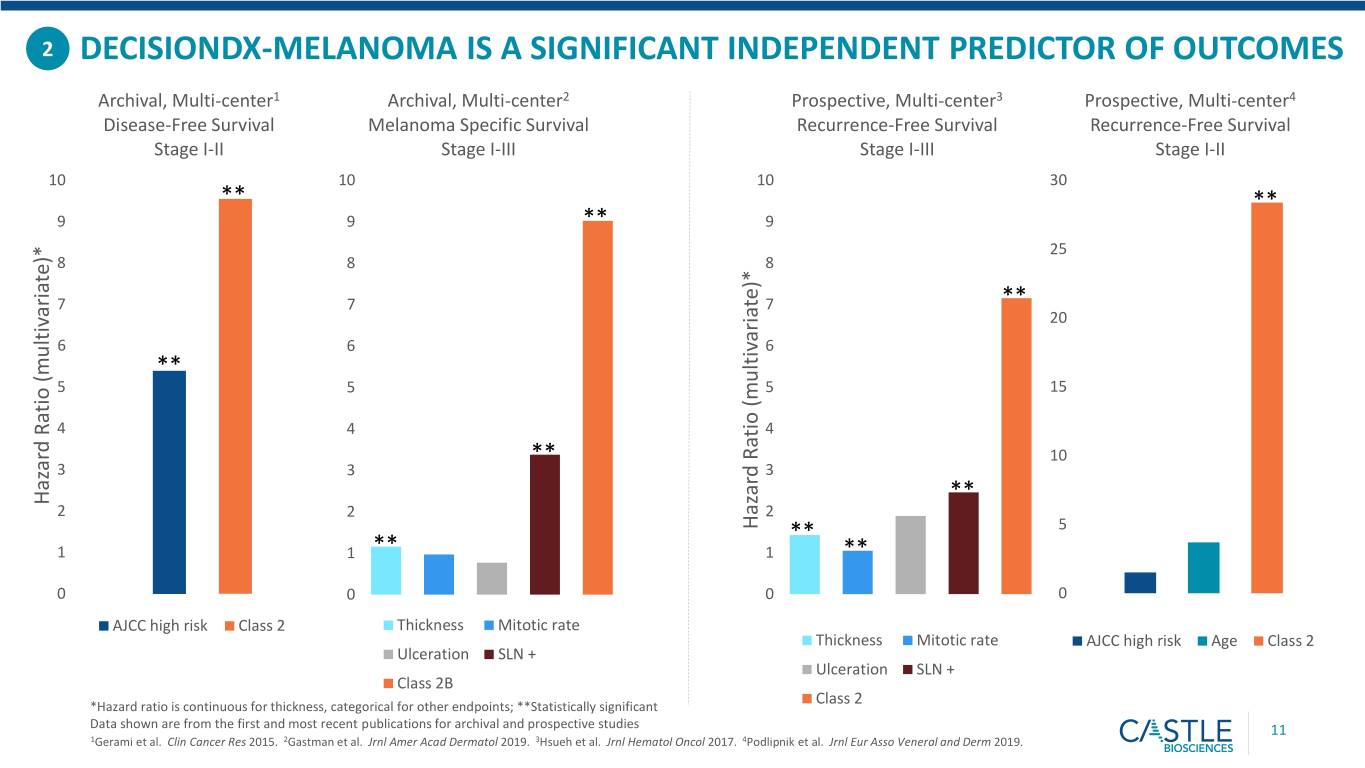
2 DECISIONDX-MELANOMA IS A SIGNIFICANT INDEPENDENT PREDICTOR OF OUTCOMES Archival, Multi-center1 Archival, Multi-center2 Prospective, Multi-center3 Prospective, Multi-center4 Disease-Free Survival Melanoma Specific Survival Recurrence-Free Survival Recurrence-Free Survival Stage I-II Stage I-III Stage I-III Stage I-II 10 10 10 30 ** ** 9 9 ** 9 25 8 8 8 7 7 7 ** 20 6 6 6 ** 5 5 5 15 4 4 4 ** 10 3 3 3 Hazard Ratio Hazard (multivariate)* ** 2 2 2 Hazard Ratio Hazard (multivariate)* ** 5 1 1 ** 1 ** 0 0 0 0 AJCC high risk Class 2 Thickness Mitotic rate Thickness Mitotic rate AJCC high risk Age Class 2 Ulceration SLN + Ulceration SLN + Class 2B *Hazard ratio is continuous for thickness, categorical for other endpoints; **Statistically significant Class 2 Data shown are from the first and most recent publications for archival and prospective studies 11 1Gerami et al. Clin Cancer Res 2015. 2Gastman et al. Jrnl Amer Acad Dermatol 2019. 3Hsueh et al. Jrnl Hematol Oncol 2017. 4Podlipnik et al. Jrnl Eur Asso Veneral and Derm 2019.

2 DECISIONDX-MELANOMA FURTHER STRATIFIES RISK OF RECURRENCE BEYOND AJCC (8TH Ed.) STAGING Stage NCCN I II III Risk Category 100 99.6% 98% >99% 94.8% Low Risk ≈AJCC IA ≈AJCC IA ≈AJCC IIA Stage I-IIA 90 89.5% 90% ≈AJCC IIIA 84.7% ≈AJCC IIIB 80 High Risk 77% Stage IIB-III AJCC MSS 70 Castle Class 1A MSS Castle Class 2B MSS 61.2% Melanoma - Specific Survival (%) (MSS) ≈AJCC IIIC+ 60 12 Prado et al. SKIN J Cutan Med 2018:suppl 2. n=690
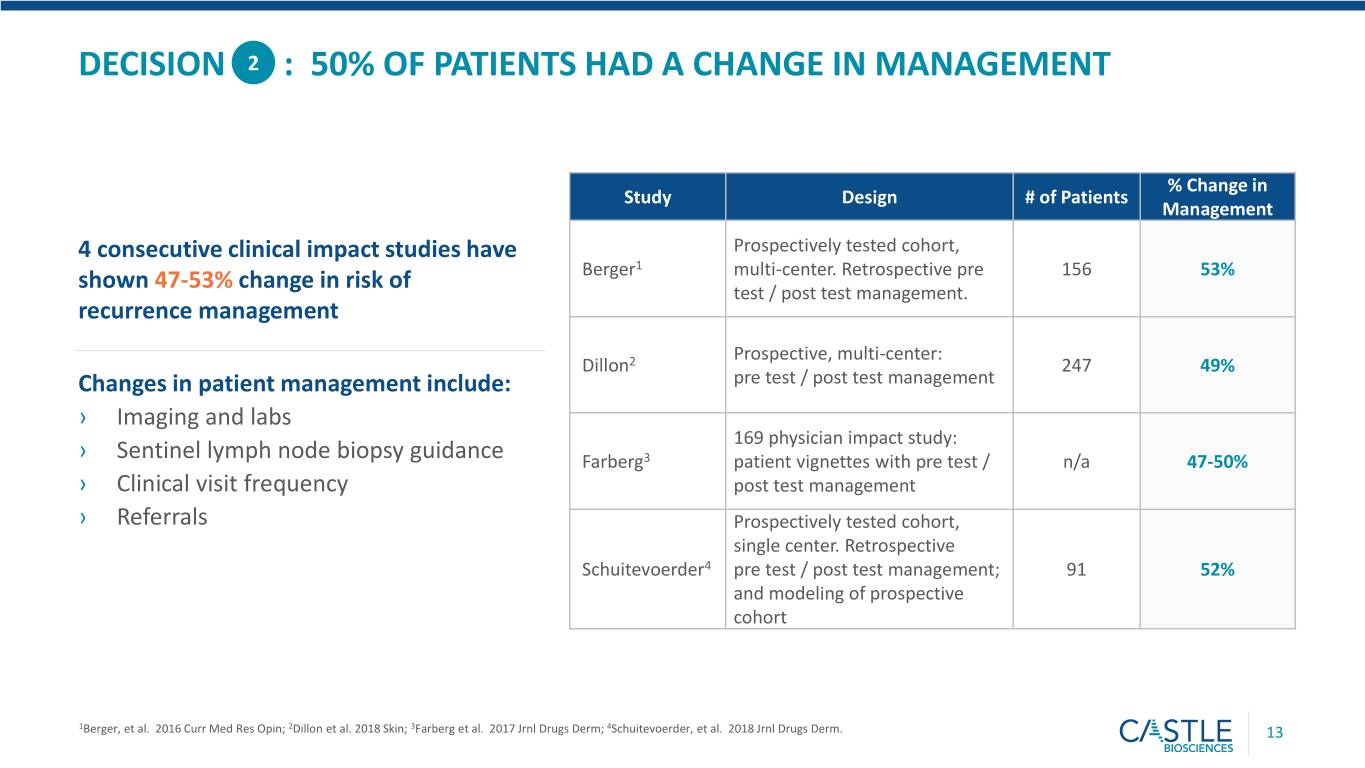
DECISION 2 : 50% OF PATIENTS HAD A CHANGE IN MANAGEMENT % Change in Study Design # of Patients Management 4 consecutive clinical impact studies have Prospectively tested cohort, Berger1 multi-center. Retrospective pre 156 53% shown 47-53% change in risk of test / post test management. recurrence management Prospective, multi-center: Dillon2 247 49% Changes in patient management include: pre test / post test management › Imaging and labs 169 physician impact study: › Sentinel lymph node biopsy guidance Farberg3 patient vignettes with pre test / n/a 47-50% › Clinical visit frequency post test management › Referrals Prospectively tested cohort, single center. Retrospective Schuitevoerder4 pre test / post test management; 91 52% and modeling of prospective cohort 1Berger, et al. 2016 Curr Med Res Opin; 2Dillon et al. 2018 Skin; 3Farberg et al. 2017 Jrnl Drugs Derm; 4Schuitevoerder, et al. 2018 Jrnl Drugs Derm. 13

DECISIONDX-MELANOMA INFORMS BOTH PRIMARY TREATMENT DECISIONS, ADDRESSING THE ISSUES OF OVER- AND UNDER-TREATMENT 1 Rule-out SLNB for T1-T2 melanoma Class 1A: <5% SLN positivity and favorable prognosis patients, including T1a with adverse Class 1B-2B: ≥5% SLN positivity and higher rates of metastasis features SLNB decision: T1a with adverse features and T1b-T2 T1a T1a HR T1b T2 T3 T4 Risk of recurrence: Any T ≥0.3 mm Class 1A: lowest risk 2 Guide follow-up, referrals, imaging and adjuvant therapy Class 1B: low risk decisions for patients with any T ≥0.3 mm. Class 2A: high-risk Class 2B: highest risk Vetto et al. Future Oncol 2019; Marks et al SKIN J Cutaneous Med 2019 14

22 STRONG PEER-REVIEWED PUBLICATIONS SINCE 2015 FOR DECISIONDX-MELANOMA Validation & Validation & Prospective Studies Clinical Impact Studies Performance Studies Performance Studies • Vetto et al. Future Oncol 2019 • Keller et al. Cancer Med 2019 • Marks et al. SKIN 2019 • Mirsky et al. J Drugs Dermatol 2018 • Gastman et al. JAAD 2019 • Podlipnik et al. JEADV 2019 • Dillon et al. SKIN 2018 • Gastman et al. Head & Neck 2019 • Greenhaw et al. Dermatol Surg • Schuitevoerder et al. J Drugs 2018 • Zager et al. BMC Cancer 2018 Dermatol 2018 • Hsueh et al. J Hematol Oncol • Ferris et al. JAAD 2017 • Svoboda et al. J Drugs Dermatol 2017 • Gerami et al. CCR 2015 2018 • Gerami et al. JAAD 2015 • Farberg et al. J Drugs Dermatol 2017 • Berger et al. CMRO 2016 • Cook et al. Diagn Pathol 2018 Systematic Reviews and Practice Guidelines • Berman et al. SKIN 2019 • Dubin et al. Am J Clin Dermatol 2019 • Winkelmann et al. Derm Clin 2017 15

CASTLE’S LEAD PRODUCT, DECISIONDX-MELANOMA, INCREASED 29% IN 2019 YEAR-OVER-YEAR FROM 2018 DecisionDx-Melanoma DecisionDx-Melanoma Test DecisionDx-Melanoma Test Report Growth 4Q19 Report Growth New Ordering Clinicians 2019 4480 15,529 3270 12,032 24% 37% 9,300 29% 6,295 2,858 4Q18 4Q19 2015 2016 2017 2018 2019 2018 2019 16

PIPELINE PRODUCTS LEVERAGE OUR EXISTING COMMERCIAL CHANNEL Outside Sales Territories Call Points Product Primary Secondary 401 32 Dermatology (including Mohs), Dermatopathology Surgeons Medical Oncology 23 15 16 15 14 2 Dermatology (including Mohs), Dermatopathology Surgeons Medical Oncology Suspicious 3 Dermatopathology Dermatology 2015 2016 2017 2018 Feb 19 Dec 19 2H20 Pigmented Lesions 1 Expected commercial expansion in 2H20 to at least ~40 outside sales territories 2 Expected commercial launch in 2H20. 3 Expected commercial launch in 2H20 17

PRODUCT PIPELINE The Skin Cancer Diagnostics Company

TREATMENT PATHWAYS IN SQUAMOUS CELL CARCINOMA OF THE SKIN (SCC) ARE BASED UPON RISK OF METASTASIS “Low” Risk Treatment plans may include: 80% of › Curettage and electrodesiccation patients or Mohs or WLE Clinicopathologic factors determine Biopsy high vs low-risk based diagnosis of SCC high-risk is defined as having one or more clinicopathologic factors Treatment plans may include: “High” Risk › Mohs or WLE 20% of › Radiation therapy patients › Chemotherapy › Some centers perform SLNB LIMITATIONS OF CURRENT STAGING SYSTEMS (AJCC [head/neck only], BWH, NCCN): LOW POSITIVE PREDICTIVE VALUE (PPV): AJCC = 14-17%. BWH = 24-38%. NCCN <10%. NCCN Guidelines v2.2019 19

DECISIONDX-SCC APPROPRIATE USE IS TARGETED TO THE HIGH-RISK PATIENT AND PROVIDES FOR DIFFERENTIAL ACTIONS “Low” Risk Treatment plans may include: 80% of › Curettage and electrodesiccation patients or Mohs or WLE Clinicopathologic factors determine Biopsy high vs low risk based diagnosis of SCC High-risk is defined as having one or more clinicopathologic factors Class 2A Treatment plans may include: “High” Risk result › Mohs or WLE 20% of › Radiation therapy patients Class 2B › Chemotherapy result › Some centers perform SLNB Clinical Validation Study presented at Amer Soc Derm Surgery (Oct 2019): • n=321. 93% of patients were staged as “high-risk”. 16% overall metastatic rate. • NPV of Class 1 test result = 91%. 63% of patients were Class 1. • PPV of Class 2B test result = 60%. 20

SUSPICIOUS PIGMENTED LESIONS: PRODUCT CANDIDATE ADJUNCT DIAGNOSTIC SUPPORT FOR HISTOPATHOLOGICALLY CHALLENGING LESIONS Attractive Market Implications of Over- and Undercalling Melanoma ~2 million suspicious pigmented Overcalling can lead to: lesions biopsied annually › Unnecessary surgery potentially including SLNB › Unnecessary follow-up including imaging, and potentially adjuvant ~300,000 biopsies cannot be therapy and/or clinical trial participation confidently confirmed as melanoma or benign lesion Undercalling can miss: through histopathology alone › The most dangerous form of skin cancer Our Development Goal: Improve decision points through increased positive predictive value and identification of low-risk patients actually at high-risk of recurrence 21

The Skin Cancer Diagnostics Company
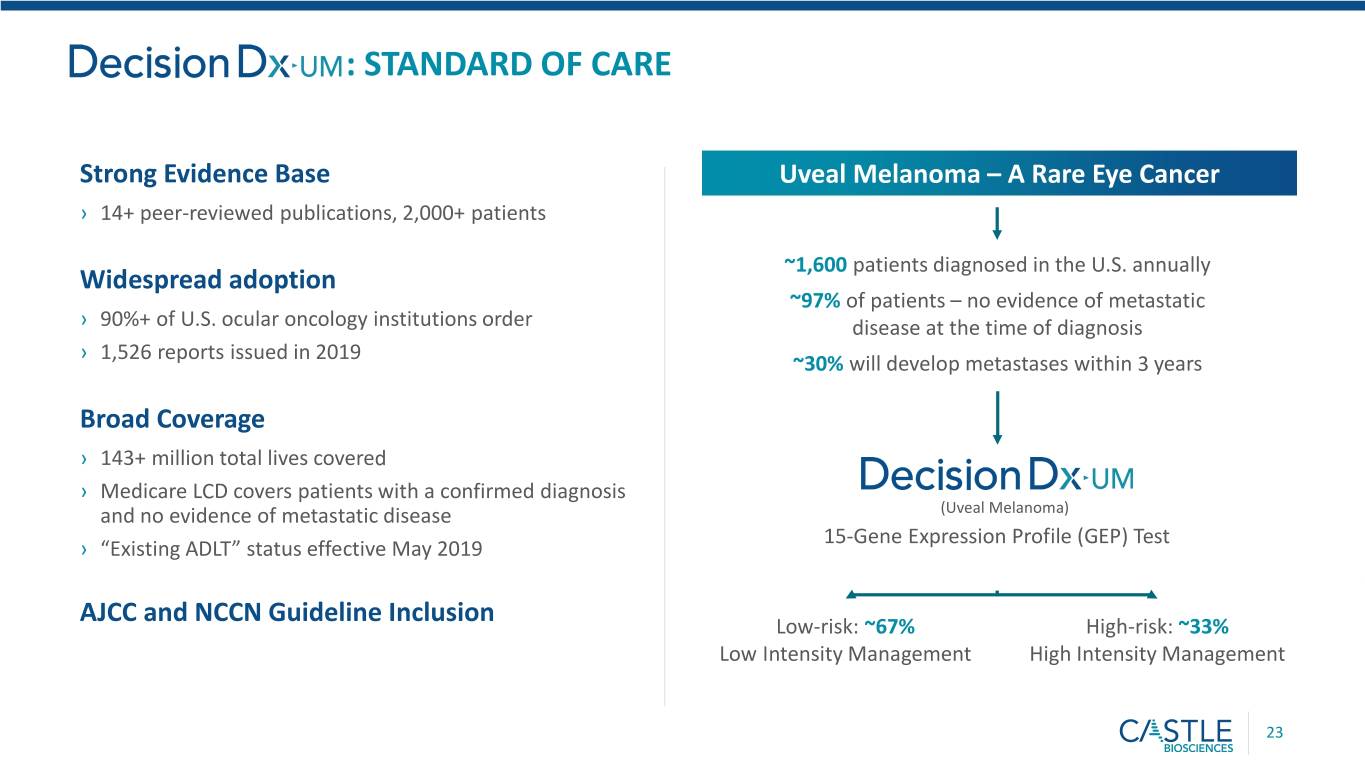
: STANDARD OF CARE Strong Evidence Base Uveal Melanoma – A Rare Eye Cancer › 14+ peer-reviewed publications, 2,000+ patients ~1,600 patients diagnosed in the U.S. annually Widespread adoption ~97% of patients – no evidence of metastatic › 90%+ of U.S. ocular oncology institutions order disease at the time of diagnosis › 1,526 reports issued in 2019 ~30% will develop metastases within 3 years Broad Coverage › 143+ million total lives covered › Medicare LCD covers patients with a confirmed diagnosis and no evidence of metastatic disease (Uveal Melanoma) 15-Gene Expression Profile (GEP) Test › “Existing ADLT” status effective May 2019 AJCC and NCCN Guideline Inclusion Low-risk: ~67% High-risk: ~33% Low Intensity Management High Intensity Management 23

FINANCIAL OVERVIEW The Skin Cancer Diagnostics Company

KEY PERFORMANCE INDICATORS DRIVING GROWTH › 2019: Expanded sales territories from 14 to 32 Report › Further expansion planned for 2H20 from 32 to at least ~40 Volume › 4Q19 vs 4Q18, DecisionDx-Melanoma report volume up 37%. New ordering clinicians up 24% in 2019 vs 2018 Revenue › DecisionDx-Melanoma ASP: Significant growth 2019 vs 2018 Reimbursement › Expanded draft LCD posted August 22, 2019 › DecisionDx-UM ASP: Continued 2019 over 2018 growth. PAMA rate effective Jan 1, 2021 Gross › 86% in 2019 Margin › Expect continued margin expansion driven by increasing ASPs and efficiencies of scale Profitability › 2 pipeline products expected to commercially launch in 2H2020, with additional New Product estimated $1.4B+ U.S. TAM Development Pipeline › Expect to leverage our existing sales channel to support the launch of additional products 25

STRONG 2019 REVENUE AND GROSS MARGIN Net Revenue ($ in millions) Gross Margin ($ in millions) $52 $45 $23 $17 2018 2019 2018 2019 Gross Margin % 77% 86% 26 Source: Company financials.

CASTLE BIOSCIENCES Improving Health Outcomes Of Patients With Skin Cancer Through Innovative, Clinically Actionable, Cost-Effective Diagnostics › INNOVATIVE PRODUCTS › Two commercialized products that improve treatment decisions for patients with melanoma › 95% of commercial emphasis currently on skin melanoma (DecisionDx®-Melanoma) › Pipeline products → Two additional skin cancer products expected to launch in second half of 2020 › DecisionDx-SCC › Suspicious Pigmented Lesions › Combined, expected to increase estimated U.S. total addressable market size by $1.4B to $2.0B › HIGH VOLUME GROWTH and STRONG FINANCIALS › 4Q2019 DecisionDx-Melanoma volume increased by 37% over 4Q2018 › 2019 Revenue: $52 million vs $23 million in 2018 › 2019 Gross Margin: 86% vs 77% in 2018 › Cash at December 31, 2019, of $99 million › ADVANCING EVIDENCE DEVELOPMENT › 22 peer-reviewed publications for DecisionDx-Melanoma › Both DecisionDx-Melanoma and DecisionDx-UM achieved Category I MAAA CPT code status 27

The Skin Cancer Diagnostics Company THANK YOU

Appendix

LEADERSHIP TEAM Board of Directors Name Prior Experience Name Derek Maetzold Founder, Director, Dan Bradbury President and CEO Frank Stokes Derek Maetzold Chief Financial Officer Bernhard Spiess Bonnie Anderson Chief Operating Officer Toby Juvenal Stuart Senior Vice President, Pharmaceuticals Mara Aspinall Sales Kristen Oelschlager, RN, CHC Brad Cole Senior Vice President, Operations Federico Monzon, MD, FCAP Joe Cook, III Chief Medical Officer Robert Cook, PhD Vice President, David Kabakoff Research & Development 30
