Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PhaseBio Pharmaceuticals Inc | a8-kxcorporatepresentation.htm |

Corporate Overview March 4, 2020

Legal Disclaimer This presentation includes forward-looking statements. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of PhaseBio Pharmaceuticals, Inc. (“we,” “us” or “our”) our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2019. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. 2 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

Company Overview Therapeutic Focus Clinical-stage biopharma company focused on the development and commercialization of novel therapies to treat cardiopulmonary indications PB2452 – Novel agent for immediate and sustained reversal of ticagrelor, the preferred antiplatelet therapy of the American College of Cardiology, the American Heart Association and European Society of Cardiology Product Candidates PB1046 – Once-weekly novel treatment for pulmonary arterial hypertension (PAH), based on elastin-like polypeptide (ELP) technology, that is vasodilatory, potentially disease-modifying and complementary to current standard-of-care therapies PB6440 – Oral aldosterone synthase inhibitor in early development for treatment-resistant hypertension ELP Technology Platform Technology • Extends circulating half-life of proteins and peptides, enhances solubility, stability and bioavailability while providing a sustained-release mechanism • Creates product candidates that are straightforward to manufacture and administer 2019 PB2452 Q1 2019 Publication of results of Phase 1 study in New England Journal of Medicine PB2452 Q2 2019 Granted Breakthrough Therapy designation by United States Food and Drug Administration (FDA) PB2452 Q3 2019 Completed Phase 2a trial in older/elderly subjects, and healthy subjects receiving supratherapeutic doses of ticagrelor PB2452 Q4 2019 Initiated Phase 2b trial in older/elderly subjects to support Biologics License Application (BLA) safety database Milestones & Catalysts 2020 PB2452 Q1 2020 Executed PB2452 funding and co-development agreement with SFJ Pharmaceuticals PB2452 Q1 2020 Granted PRIority MEdicines (PRIME) designation by European Medicines Agency (EMA) PB2452 Q1 2020 Expect to initiate Phase 3 trial based on plan to pursue accelerated approval pathway PB1046 Q4 2020 Plan to report Phase 2b data 3 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

A Clinical Stage, Cardiopulmonary Focused Biopharmaceutical Company Program Indication/Therapeutic Area Pre-Clinical Phase 1 Phase 2 Phase 3 WW Commercial Rights Milestones Q1 2020: • Expect to initiate Phase Reversal of Ticagrelor Phase 2b ongoing PB2452 3 trial based on plan to Breakthrough Therapy designation granted in April 2019 Antiplatelet Activity pursue accelerated regulatory pathway Pulmonary Arterial Q4 2020: PB1046 Phase 2b ongoing • Plan to report Phase 2b Hypertension (PAH) trial results IND-enabling 1H 2021: Resistant Hypertension • Plan to initiate first-in- PB6440 activities human clinical trial Partnering Opportunities GLP2-ELP Short Bowel Syndrome Late research CNP-ELP Achondroplasia Late research Early PROPRIETARY LONG ACTING INJECTABLE RECOMBINANT BIOPOLYMERS Programs (Elastin-like Polypeptides – ELPs) 4 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

PB2452 – Reversal Agent for Ticagrelor
PB2452 - Novel reversal agent for Brilinta (ticagrelor) • Within the P2Y12 antagonist class of oral antiplatelet therapies, ticagrelor has proven superiority vs. clopidogrel, and a unique reversible binding profile − Clopidogrel and prasugrel, the other members of the oral P2Y12 antagonist class, both permanently bind to the receptor and cannot be reversed • PB2452 is the only specific reversal agent in development for ticagrelor − PB2452 clinical data to date have demonstrated both immediate (<5 min) and sustained reversal (~24 hours) of ticagrelor antiplatelet effects • Approval would differentiate ticagrelor on safety vs. other oral antiplatelet agents − Expect further differentiation of ticagrelor vs. other P2Y12 agents to drive increased demand Significant unmet need for antiplatelet agent reversal Major Bleeding Urgent Surgery or Intervention • Intracranial Haemorrhage (ICH), GI, Trauma • Currently oral P2Y12 agents, including ticagrelor, require a 5 day washout prior to surgery1,2 • All oral antiplatelet agents have the potential to cause major bleeding, which can be severe or even fatal . Urgent surgery often cannot wait 5 days . Higher thrombotic risk during washout • PB2452 immediately and sustainably reverses the antiplatelet effects of ticagrelor • In Phase 1 and Phase 2a studies, PB2452 immediately and sustainably reversed ticagrelor inhibition of platelet activation . Enables immediate surgery 1. Plavix/clopidogrel Prescribing Information: https://packageinserts.bms.com/pi/pi_plavix.pdf, https://www.ema.europa.eu/en/documents/product-information/plavix-epar-product-information_en.pdf 6 2. Brilinta/Brilique/ticagrelor Prescribing Information: https://www.azpicentral.com/brilinta/brilinta.pdf#page=1, https://www.ema.europa.eu/en/documents/product-information/brilique-epar-product-information_en.pdf Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved
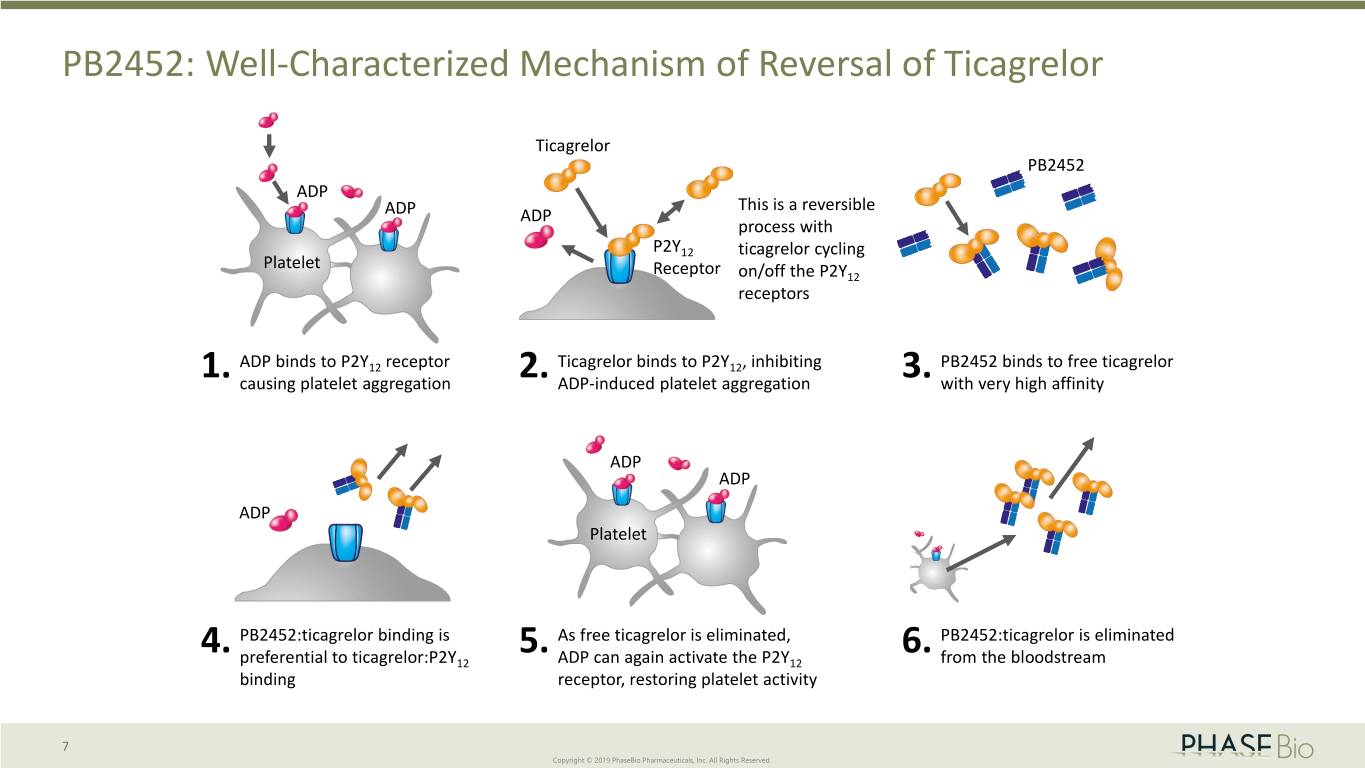
PB2452: Well-Characterized Mechanism of Reversal of Ticagrelor Ticagrelor PB2452 ADP This is a reversible ADP ADP process with P2Y12 ticagrelor cycling Platelet Receptor on/off the P2Y12 receptors ADP binds to P2Y12 receptor Ticagrelor binds to P2Y12, inhibiting PB2452 binds to free ticagrelor 1. causing platelet aggregation 2. ADP-induced platelet aggregation 3. with very high affinity ADP ADP ADP Platelet 4. PB2452:ticagrelor binding is 5. As free ticagrelor is eliminated, 6. PB2452:ticagrelor is eliminated preferential to ticagrelor:P2Y12 ADP can again activate the P2Y12 from the bloodstream binding receptor, restoring platelet activity 7 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

Clinical Studies PB2452: Development and Regulatory Timelines BLA enabling data 2019 2020 2021 2022 2023 NEJM Phase 2a Phase 2b: Older and elderly subjects N=200, 150 randomized to receive PB2452 Breakthrough FDA Therapy EOP1 Phase 3 Interim Analysis for Accelerated Approval Post-approval completion of Phase 3 EMA PRIME Designation N=100, ~50 major bleeding subjects, ~50 urgent surgery subjects N=100, ~50 major bleeding subjects, ~50 urgent surgery subjects Planned BLA Submission for Accelerated Approval • Phase 2b initiated in October 2019 − N=200 total, evaluation of efficacy and overall safety of PB2452 in older and elderly subjects on DAPT • Phase 3 initiation expected Q1 2020 − N=200 total, evaluation of efficacy in ticagrelor-treated subjects with major bleeding events or requiring urgent surgery − Interim analysis of first 100 patients recommended by FDA for BLA submission for Accelerated Approval NEJM= New England Journal of Medicine, EOP1=End-of-Phase 1 Meeting, DAPT=Dual antiplatelet therapy (ticagrelor + aspirin), BLA=Biologics License Application 8 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

PB2452 Phase 1 Proof-of-Concept Study in Healthy Subjects • Randomized, double-blind, placebo-controlled, single ascending dose (SAD), sequential group study (n=64) • Platelet function evaluated using three well established and commonly used assays: LTA, VerifyNow PRUTest® and VASP − Results from all three assays were highly correlated • Onset of reversal occurred within 5 minutes and was sustained for over 20 hours • Well tolerated with no drug-related serious adverse events Selected as late-breaking oral presentation during featured clinical research session at the • Importantly, no evidence of rebound in platelet activity American College of Cardiology’s Annual Scientific Session – March 17, 20191 after drug cessation Simultaneously published in the New England Journal of Medicine2 LTA = light transmittance aggregometry, VASP = vasodilator stimulated phosphoprotein phosphorylation immunoassay 1. https://www.acc.org/latest-in-cardiology/clinical-trials/2019/03/15/21/37/ticagrelor-reversal-agent 9 2. Bhatt DL, Pollack CV, Weitz JI, et al. Antibody-Based Ticagrelor Reversal Agent in Healthy Volunteers. https://www.nejm.org/doi/full/10.1056/NEJMoa1901778 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved
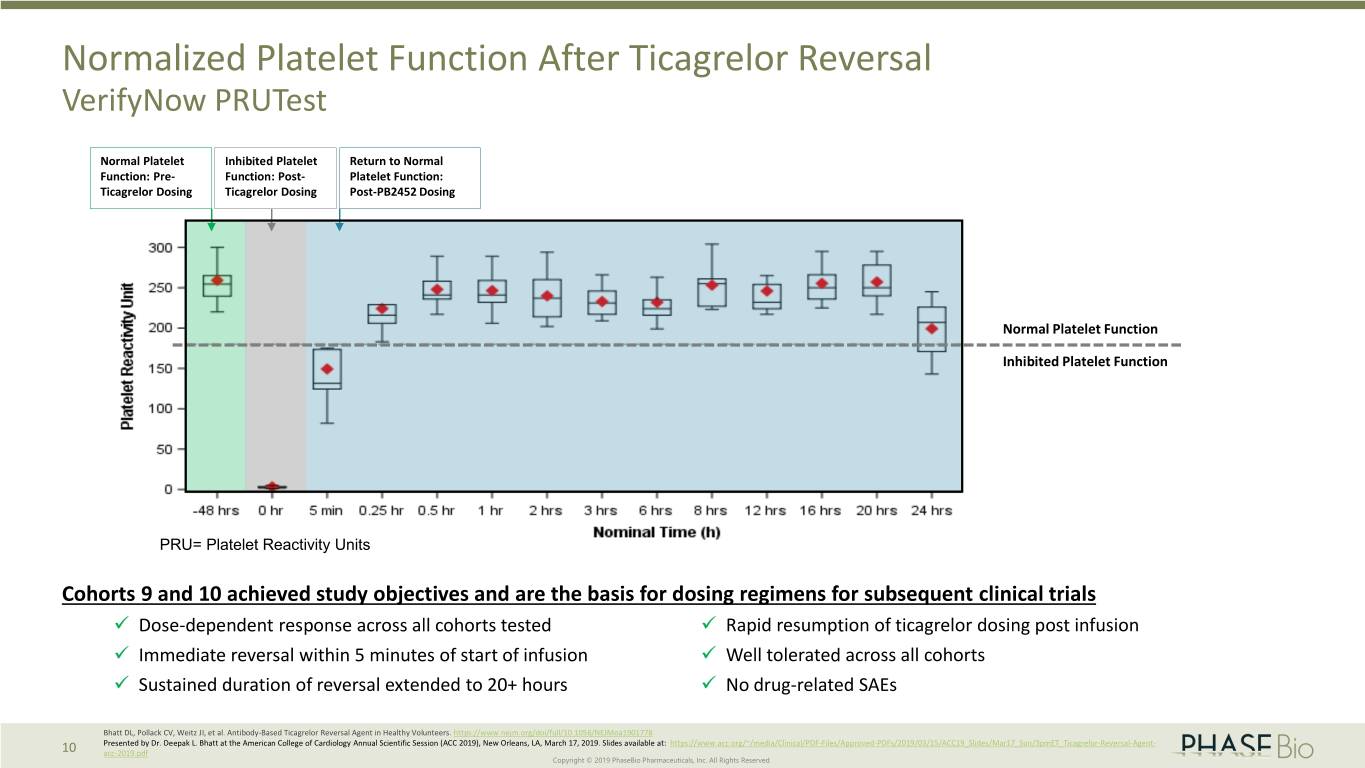
Normalized Platelet Function After Ticagrelor Reversal VerifyNow PRUTest Normal Platelet Inhibited Platelet Return to Normal Function: Pre- Function: Post- Platelet Function: Ticagrelor Dosing Ticagrelor Dosing Post-PB2452 Dosing Normal Platelet Function Inhibited Platelet Function PRU= Platelet Reactivity Units Cohorts 9 and 10 achieved study objectives and are the basis for dosing regimens for subsequent clinical trials Dose-dependent response across all cohorts tested Rapid resumption of ticagrelor dosing post infusion Immediate reversal within 5 minutes of start of infusion Well tolerated across all cohorts Sustained duration of reversal extended to 20+ hours No drug-related SAEs Bhatt DL, Pollack CV, Weitz JI, et al. Antibody-Based Ticagrelor Reversal Agent in Healthy Volunteers. https://www.nejm.org/doi/full/10.1056/NEJMoa1901778 Presented by Dr. Deepak L. Bhatt at the American College of Cardiology Annual Scientific Session (ACC 2019), New Orleans, LA, March 17, 2019. Slides available at: https://www.acc.org/~/media/Clinical/PDF-Files/Approved-PDFs/2019/03/15/ACC19_Slides/Mar17_Sun/3pmET_Ticagrelor-Reversal-Agent- 10 acc-2019.pdf Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved
Positive Preliminary PB2452 Phase 2a Results • Positive preliminary results from the PB2452 Phase 2a trial were disclosed via press release1 − First trial of PB2452 to include older and elderly subjects (ages 50-80) on dual antiplatelet therapy (DAPT) of ticagrelor and low-dose aspirin − Subjects in the trial resembled the patient population most likely to be treated with ticagrelor and potentially benefit from PB2452, if approved • Per FDA request, the Phase 2a trial also explored reversal of supratherapeutic blood levels of ticagrelor that could result from ticagrelor overdosage or drug-drug interactions − PhaseBio believes an appropriate PB2452 regimen has been identified for these patients • Confirmed dosing regimen to be used in the Phase 2b and Phase 3 trials • Results to be presented at an upcoming medical congress Older & Elderly Subjects Supratherapeutic Dose PB2452 Phase 2a Trial on DAPT of Ticagrelor Immediate and sustained reversal of the antiplatelet effects of ticagrelor ✔ ✔ Efficacy demonstrated using same three assays used in Phase 1 trial2 ✔ ✔ Results highly correlated across assays ✔ ✔ Generally well tolerated with only minor adverse events reported ✔ ✔ 1. https://investors.phasebio.com/news-releases/news-release-details/phasebio-announces-completion-phase-2a-clinical-trial-pb2452 11 2. Bhatt, D. L. et al. Antibody-based ticagrelor reversal agent in healthy volunteers. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa1901778 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

PB2452 Regulatory Updates Development plan for PB2452 designed with objective to broadly support global regulatory filings Separate written guidance from FDA and EMA indicates that a single, non-randomized, open-label Phase 3 trial of PB2452 in both surgical and major bleeding populations has the potential to support regulatory filings in the United States and the European Union EMA: • Received written Scientific Advice: February 2020 − Written guidance from the CHMP of the EMA generally agrees with PhaseBio’s proposed development plan for PB2452 • Granted PRIME designation: February 2020 − PRIME designation granted by the EMA to support medicines that demonstrate the potential to address substantial unmet medical need1 − Potentially expedites the review and approval process FDA: • Received meeting minutes from PB2452 End-of-Phase 1 meeting: August 2019 − FDA alignment on development plan and Accelerated Approval regulatory path • PB2452 granted Breakthrough Therapy designation: April 2019 − Breakthrough Therapy designation is designed to expedite the development and review of promising new drugs2 1. The European Medicines Agency. “PRIME: Priority Medicines” Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines#. Accessed February 2020 12 2. The U.S. Food and Drug Administration. “Expedited Programs for Serious Conditions – Drugs and Biologics.” Available at: https://www.fda.gov/downloads/Drugs/Guidances/UCM358301.pdf. Accessed February 2020 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

PB2452 Pivotal Phase 3 Trial Overview • Phase 3 trial is key element of development plan that has garnered FDA Breakthrough Therapy and EMA PRIME designations • Open-label, single-arm study of reversal of the antiplatelet effects of ticagrelor with PB2452 in patients who present with uncontrolled major or life-threatening bleeding or who require urgent surgery or invasive procedure • Total of 200 patients targeted for enrollment − 100 patients from major bleeding population, 100 patients from urgent surgery population − First ~50 patients from each of the respective target populations (total of ~100) will form the basis of accelerated BLA filing in US and MAA in EU • Accelerated BLA endpoint is of platelet function based on VerifyNow® PRUTest® platelet function assay − VerifyNow has been used in the Phase 1, Phase 2a and ongoing Phase 2b trials of PB2452 with consistent results across studies completed to date • Additional clinical endpoints related to hemostasis will also be captured as part of the primary outcome analysis • Trial posted online at https://www.ClinicalTrials.gov and on track to initiate enrollment in Q1 2020 13 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

SFJ Pharmaceuticals Funding and Co-Development Collaboration • Innovative collaboration between SFJ and PhaseBio to support the global development of PB2452 • SFJ will fund up to $120 million to support the clinical development of PB2452 − SFJ has extensive experience in the global clinical development and regulatory approval of numerous pharmaceutical products across multiple indications and therapeutic areas • SFJ will assume a central role in global development and regulatory activities for PB2452 outside the United States − SFJ will lead development and regulatory activities in China and Japan − PhaseBio and SFJ and will work closely on clinical program in EU − PhaseBio will conduct Phase 3 trial in US • PhaseBio retains exclusive worldwide commercial rights to PB2452 14 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

Project Expectation of Continued Long-Term Rx Growth of Ticagrelor PB2452 approval has the potential to drive continued positive momentum • Brilinta/Brilique sales in 2019 were $1.58B and growing (+20% Y/Y) − Patient growth drives Y/Y revenue growth in key regions: strong growth in the United States and Emerging Markets • In February 2019, Brilinta Phase 3 THEMIS1 trial met primary endpoint in patients with established coronary artery disease and type-2 diabetes • In January 2020, Brilinta Phase III THALES2 trial met primary endpoint in patients with acute ischemic stroke or patients with high-risk transient ischemic attack Ticagrelor Differentiation Now Post PB2452 Launch Post LOE of ticagrelor vs. clopidogrel Efficacy ✔ ✔ ✔ Safety ≈ ✔ ✔ (no reversal agent) Price ✔ (branded vs. generic) (branded vs. generic) 1. https://www.astrazeneca.com/media-centre/press-releases/2019/brilintas-phase-iii-themis-trial-met-primary-endpoint-in-patients-with-established-coronary-artery-disease-and-type-2-diabetes-25022019.html 15 2. https://www.astrazeneca.com/media-centre/press-releases/2020/brilinta-met-primary-endpoint-in-phase-iii-thales-trial-in-stroke-27012020.html Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

PB1046 - Once Weekly Vasoactive Intestinal Peptide (VIP) Analog for Pulmonary Arterial Hypertension

High Unmet Need in an Orphan Disease Primary Pulmonary Arterial Hypertension (PAH, WHO Group 1 PH) McGlaughlin et al. (2015) J. Am. Coll. Cardiol., 65:18 • High unmet need for novel disease-modifying PAH therapies for greater efficacy − All 3 approved drug classes in PAH are vasodilators: prostacyclin, endothelin, and nitric oxide pathways − Patients inevitably continue to decline and die on current standard of care • VIP addresses PAH vasoconstriction, progressive vascular remodeling, and right heart failure 17 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved
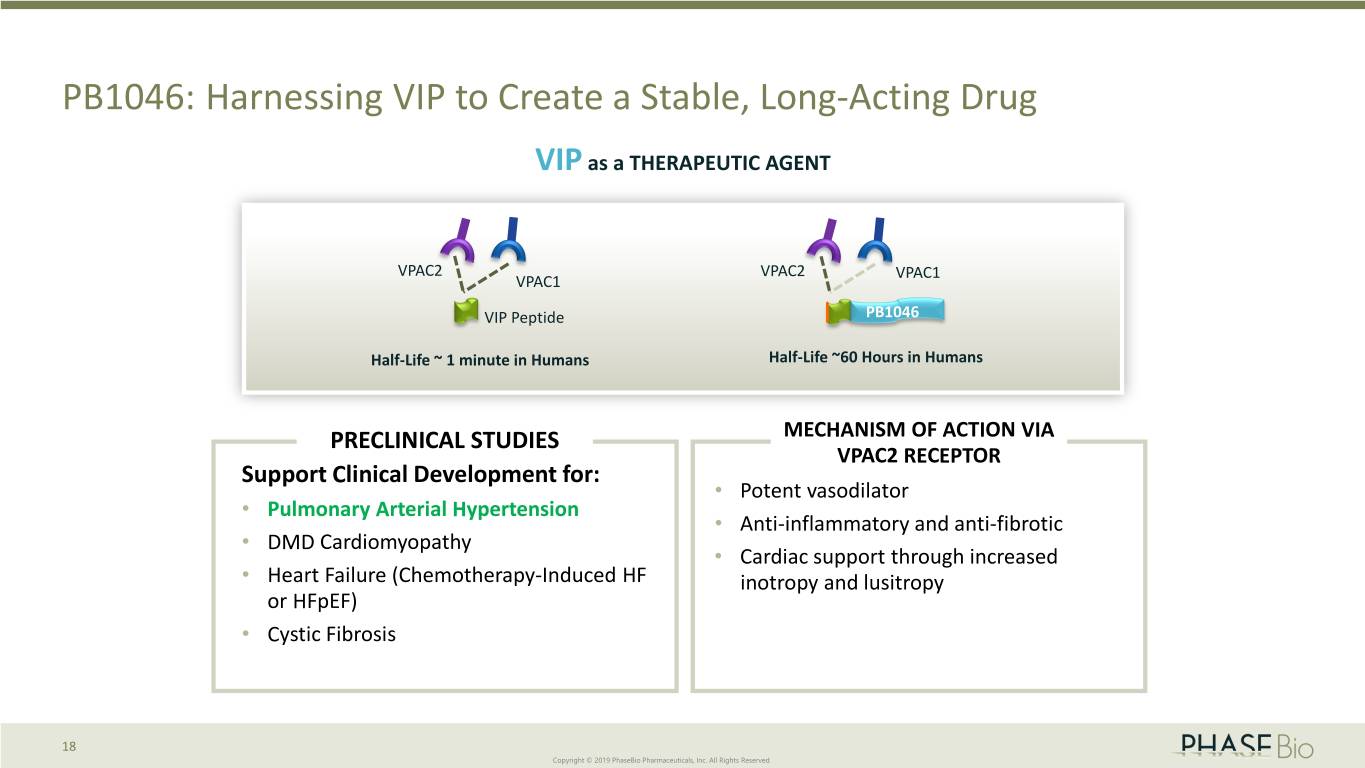
PB1046: Harnessing VIP to Create a Stable, Long-Acting Drug VIP as a THERAPEUTIC AGENT VPAC2 VPAC2 VPAC1 VPAC1 VIP Peptide PB1046 Half-Life ~ 1 minute in Humans Half-Life ~60 Hours in Humans PRECLINICAL STUDIES MECHANISM OF ACTION VIA VPAC2 RECEPTOR Support Clinical Development for: • Potent vasodilator • Pulmonary Arterial Hypertension • Anti-inflammatory and anti-fibrotic • DMD Cardiomyopathy • Cardiac support through increased • Heart Failure (Chemotherapy-Induced HF inotropy and lusitropy or HFpEF) • Cystic Fibrosis 18 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

Proprietary Elastin-Like Polypeptide (ELP) Technology Key to optimizing the profile of an injectable VIP product candidate PROLONGED CIRCULATING HALF-LIFE COACERVATION DELIVERS SLOW RELEASE “Biopolymer” Outside Body Inside Body Repeating ↑ Active Moiety Sequence of Temperature Human Elastin Peptides VPGXG Peptide/Protein n (VIP, GLP1, etc.) Highly Soluble ELP Non-Soluble ELP UP TO 200x INCREASE IN ½ LIFE WEEKLY OR MONTHLY DOSING IMPROVING • Pharmacokinetics • Slower rate of bioavailability • Ease of Administration • Patient Compliance 19 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

PB1046 Clinical Development Activities to Date Have Supported Advancement into a Phase 2b PAH Efficacy Study PHASE 1 Studies Completed PAH PHASE 2 Studies SAD study in HTN 4-week MAD study Open-Label Phase 2a Phase 2b PAH Efficacy patients washed in HFrEF patients CardioMEMS in PAH study: Safety 16-Wk Randomized, Controlled off meds on SOC and Hemodynamics Study CardioMEMS Open-Label PAH Study • Well tolerated for a week • Well tolerated across dose range; Phase 2b PAH 16 wk randomized, controlled over broad range of no drug-related SAEs • N = 3 patients, dosed weekly for 8 wks, exposure; no drug-related followed by extension study SAEs • Replicated PK/PD from SAD over 4 • No longer enrolling patients • N = ~60 NYHA class II/III PAH patients, weekly SC injections • Real-time PA pressure and other dosed weekly x 16 weeks • Prolonged PK/PD profile hemodynamic monitoring over 1 week • VIP activity reproduced in HFrEF • Individual dose titration to MTD patients on SOC • Initial data show improved hemodynamics • VIP activity confirmed • Efficacy endpoints PVR via RHC, 6MWD (Systolic and Diastolic BP • One drug-related SAE reported in Extension study to follow lowering) extension portion of open-label pilot study • COMPLETED COMPLETED PHASE 2b STUDY COMPLETED ACTIVELY ENROLLING 20 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

PB6440 – Aldosterone Synthase Inhibitor for Resistant Hypertension

PB6440 for Resistant Hypertension • Upwards of 10 million patients in the United States have resistant hypertension and are at risk for serious, costly medical consequences (stroke, heart attack, kidney failure, etc.)1 • Physicians currently prescribe numerous combinations of antihypertensives to lower blood pressure and diminish risk • Blocking aldosterone has been shown to be an effective mechanism for treating resistant hypertension − Currently available aldosterone blockers suffer from poor potency and pharmacokinetics (eplerenone) or poor tolerability (spironolactone) and thus are rarely used • Recent draft guidance from the FDA outlines a streamlined regulatory path for novel drugs to treat resistant hypertension without the need for large outcomes studies2 • Market research indicates that payors aware of high medical costs associated with resistant hypertension Large, growing patient population coupled with a high unmet need creates an attractive opportunity for a novel therapy to help patients and care providers better manage blood pressure 1. Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment‐resistant hypertension in the United States: comparison of the 2008 and 2018 American heart association scientific statements on resistant hypertension. Hypertension. 2019; 73: 424‐ 431. Available at: https://www.ahajournals.org/doi/full/10.1161/HYPERTENSIONAHA.118.12191?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. Accessed February 2020 22 2. U. S. Food and Drug Administration. Center for Drug Evaluation and Research. (2018) Hypertension: Conducting Studies of Drugs to Treat Patients on a Background of Multiple Antihypertensive Drugs Guidance for Industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/hypertension- conducting-studies-drugs-treat-patients-background-multiple-antihypertensive-drugs. Accessed February 2020 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved
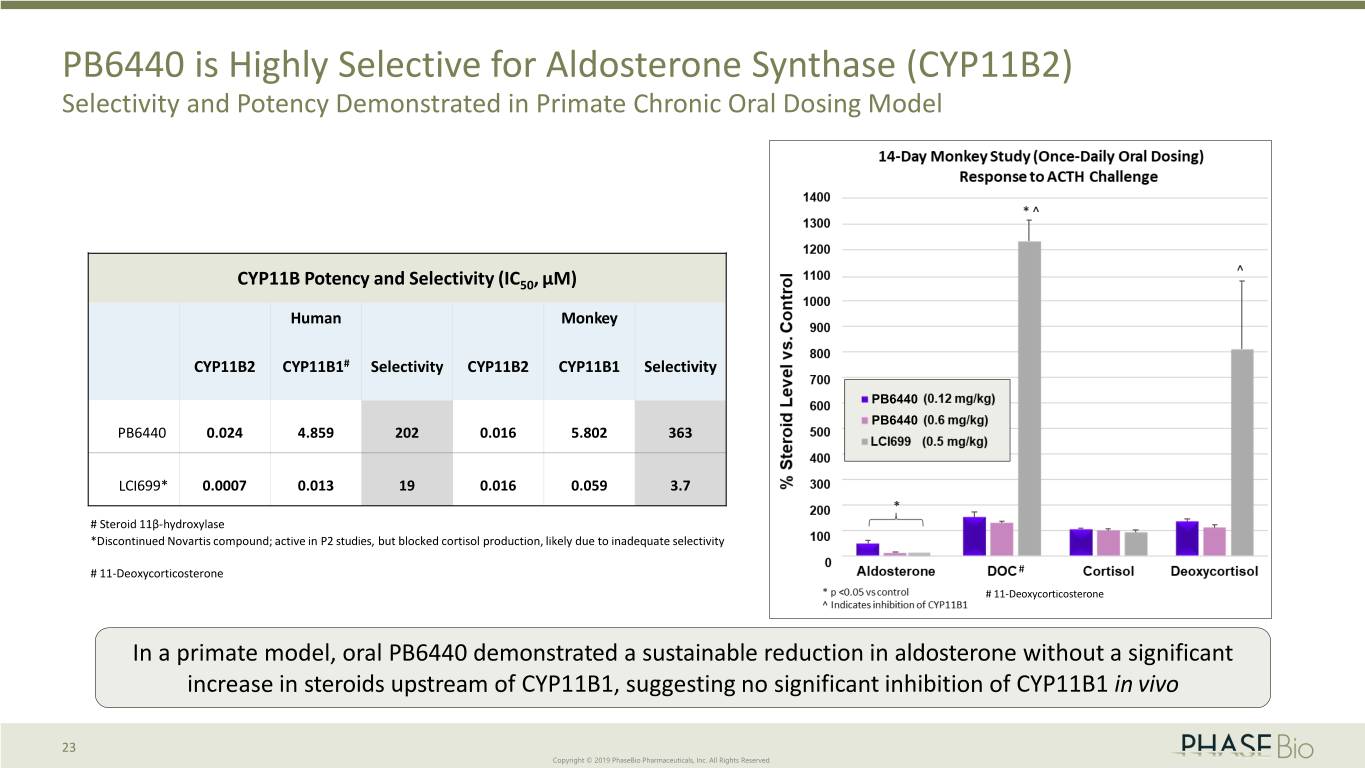
PB6440 is Highly Selective for Aldosterone Synthase (CYP11B2) Selectivity and Potency Demonstrated in Primate Chronic Oral Dosing Model CYP11B Potency and Selectivity (IC50, µM) Human Monkey CYP11B2 CYP11B1# Selectivity CYP11B2 CYP11B1 Selectivity PB6440 PB6440 PB6440 0.024 4.859 202 0.016 5.802 363 LCI699* 0.0007 0.013 19 0.016 0.059 3.7 # Steroid 11β-hydroxylase *Discontinued Novartis compound; active in P2 studies, but blocked cortisol production, likely due to inadequate selectivity # 11-Deoxycorticosterone # # 11-Deoxycorticosterone In a primate model, oral PB6440 demonstrated a sustainable reduction in aldosterone without a significant increase in steroids upstream of CYP11B1, suggesting no significant inhibition of CYP11B1 in vivo 23 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

Closing Remarks

Creating Value for Stakeholders in 2020 • Following a successful 2019, PhaseBio continues a rapid pace of development and execution in 2020 Announced innovative funding and co-development collaboration for PB2452 with SFJ Pharmaceuticals Bolstered pipeline with acquisition of resistant hypertension asset, PB6440 Granted EMA PRIME designation for PB2452 On track to initiate pivotal Phase 3 trial for PB2452 in Q1 2020 On track to report Phase 2b results for PB1046 in Q4 2020 • Experienced management team • Lead program (PB2452) funded through potential approvals in key global markets • Novel, first-in-class program for PAH (PB1046) being developed using ELP platform technology • Evolving focus on high-unmet need areas of cardiovascular disease with new pipeline asset (PB6440) 25 Copyright © 2019 PhaseBio Pharmaceuticals, Inc. All Rights Reserved

