Attached files
| file | filename |
|---|---|
| EX-4.2 - DESCRIPTION OF THE REGISTRANT'S SECURITIES REGISTERED PURSUANT TO SECTION 12 OF - ReWalk Robotics Ltd. | f10k2019ex4-2_rewalk.htm |
| EX-32.2 - CERTIFICATION - ReWalk Robotics Ltd. | f10k2019ex32-2_rewalk.htm |
| EX-32.1 - CERTIFICATION - ReWalk Robotics Ltd. | f10k2019ex32-1_rewalk.htm |
| EX-31.2 - CERTIFICATION - ReWalk Robotics Ltd. | f10k2019ex31-2_rewalk.htm |
| EX-31.1 - CERTIFICATION - ReWalk Robotics Ltd. | f10k2019ex31-1_rewalk.htm |
| EX-23.1 - CONSENT OF KOST FORER GABBAY & KASIERER, A MEMBER OF ERNST & YOUNG GLOBAL LIMITE - ReWalk Robotics Ltd. | f10k2019ex23-1_rewalk.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2019
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____ to_____
Commission File Number: 001-36612

ReWalk Robotics Ltd.
(Exact name of registrant as specified in charter)
| Israel | Not applicable | |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S.
employer identification no.) | |
| 3 Hatnufa Street, Floor 6, Yokneam Ilit, Israel | 2069203 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: +972.4.959.0123
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered | ||
| Ordinary Shares, par value NIS 0.25 per share | RWLK | The Nasdaq Stock Market LLC |
Securities Registered Pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by a check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the Ordinary Shares held by non-affiliates of the Registrant based upon the closing price of the Ordinary Shares as reported by the Nasdaq Capital Market on June 30, 2019 (the last business day of the Registrant’s most recently completed second fiscal quarter) was $30,393,283.
As of February 20, 2020, the Registrant had outstanding 12,753,903 Ordinary Shares, par value NIS 0.25 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of our proxy statement for our 2020 Annual Meeting of Shareholders, which is to be filed within 120 days after the end of our 2019 fiscal year, are incorporated by reference into Part III of this annual report on Form 10-K.
REWALK ROBOTICS LTD.
FORM 10-K FOR THE YEAR ENDED DECEMBER 31, 2019
TABLE OF CONTENTS
i
Definitions and Introduction
Our legal and commercial name is ReWalk Robotics Ltd. We are a company limited by shares organized under the laws of the State of Israel and were founded in 2001. In September 2014, we listed our shares on the Nasdaq Global Market, and in May 2017, we transferred our listing to the Nasdaq Capital Market. We have irrevocably appointed ReWalk Robotics, Inc. as our agent to receive service of process in any action against us in any United States federal or state court. The address of ReWalk Robotics, Inc. is 200 Donald Lynch Blvd., Marlborough, Massachusetts 01752. As used herein, and unless the context clearly indicates otherwise, the terms “ReWalk”, “the Company”, “we”, “us”, “our” or “ours” refer to ReWalk Robotics Ltd. and its subsidiaries.
Special Note Regarding Forward-Looking Statements
This annual report on Form 10-K, or annual report, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, that are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements may include projections regarding our future performance and, in some cases, can be identified by words like “anticipate,” “assume,” “believe,” “could,” “seek,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “future,” “should,” “will,” “would” or similar expressions that convey uncertainty of future events or outcomes and the negatives of those terms. These statements may be found in this section of this quarterly report titled “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this quarterly report. These statements include, but are not limited to, statements regarding:
| ● | our management’s conclusion, and our independent registered public accounting firm’s statement in its opinion relating to our consolidated financial statements for the fiscal year ended December 31, 2019, that there is a substantial doubt as to our ability to continue as a going concern; | |
| ● | our ability to have sufficient funds to meet certain future capital requirements, which could impair our efforts to develop and commercialize existing and new products; | |
| ● | our ability to maintain compliance with the continued listing requirements of the Nasdaq Capital Market and the risk that our ordinary shares will be delisted if we cannot do so; | |
| ● | our ability to establish a pathway to commercialize our products in China; | |
| ● | our ability to maintain and grow our reputation and the market acceptance of our products; | |
| ● | our ability to achieve reimbursement from third-party payors for our products; | |
| ● | our limited operating history and our ability to leverage our sales, marketing and training infrastructure; | |
| ● | our expectations as to our clinical research program and clinical results; | |
| ● | our expectations regarding future growth, including our ability to increase sales in our existing geographic markets and expand to new markets; | |
| ● | our ability to obtain certain components of our products from third-party suppliers and our continued access to our product manufacturers; | |
| ● | our ability to repay our secured indebtedness; |
ii
| ● | our ability to improve our products and develop new products; | |
| ● | the outcome of ongoing shareholder class action litigation relating to our initial public offering (“IPO”); | |
| ● | our compliance with medical device reporting regulations to report adverse events involving our products, which could result in voluntary corrective actions or enforcement actions such as mandatory recalls, and the potential impact of such adverse events on ReWalk’s ability to market and sell its products; | |
| ● | our ability to gain and maintain regulatory approvals; | |
| ● | our expectations as to the results of the FDA, potential regulatory developments with respect to our mandatory 522 postmarket surveillance study; | |
| ● | our ability to maintain adequate protection of our intellectual property and to avoid violation of the intellectual property rights of others; | |
| ● | the risk of a cybersecurity attack or breach of our IT systems significantly disrupting our business operations; | |
| ● | the impact of substantial sales of our shares by certain shareholders on the market price of our ordinary shares; | |
| ● | our ability to use effectively the proceeds of our offerings of securities; | |
| ● | the risk of substantial dilution resulting from the periodic issuances of our ordinary shares; and | |
| ● | the impact of the market price of our ordinary shares on the determination of whether we are a passive foreign investment company. |
The preceding list is not intended to be an exhaustive list of all of our statements. The statements are based on our beliefs, assumptions and expectations of future performance, taking into account the information currently available to us. These statements are only predictions based upon our current expectations and projections about future events. There are important factors that could cause our actual results, levels of activity, performance or achievements to differ materially from the results, levels of activity, performance or achievements expressed or implied by the statements. In particular, you should consider the risks provided under “Part I. Item 1A. Risk Factors” in this annual report.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be achieved or will occur.
These statements may also be found in the sections of this annual report titled “Part I. Item 1. Business,” “Part I. Item 1A. Risk Factors,” “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this annual report.
You should not put undue reliance on any forward-looking statements. Any forward-looking statement in this annual report speaks only as of the date hereof. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this annual report, to conform these statements to actual results or to changes in our expectations.
Where You Can Find Other Information
Our principal executive offices are located at 3 Hatnufa Street, Floor 6, Yokneam Ilit 2069203, Israel, and our telephone number is +972 (4) 959-0123. Our website is www.rewalk.com. Information contained, or that can be accessed through, our website does not constitute a part of this annual report and is not incorporated by reference herein. We have included our website address in this annual report solely for informational purposes. Information that we furnish with or file with the Securities and Exchange Commission, or the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to, or exhibits included in, these reports are available for download, free of charge, on our website as soon as reasonably practicable after such materials are filed or furnished with the SEC. Our SEC filings, including exhibits filed or furnished therewith, are also available on the SEC’s website at SEC.gov. You may request copies of these documents, upon payment of a duplicating fee, by writing to the SEC at its principal office at 100 F Street, NE, Room 1580, Washington, D.C. 20549.
iii
PART I
Overview
We are an innovative medical device company that is designing, developing and commercializing robotic exoskeletons that allow individuals with mobility impairments or other medical conditions the ability to stand and walk once again. We have developed and are continuing to commercialize our ReWalk Personal and ReWalk Rehabilitation devices for individuals with Spinal Cord Injury (“SCI Products”), which are exoskeletons designed for individuals with paraplegia that use our patented tilt-sensor technology and an on-board computer and motion sensors to drive motorized legs that power movement. We have also developed and began commercializing our ReStore device in June 2019. ReStore is a powered, lightweight soft exo-suit intended for use in the rehabilitation of individuals with lower limb disability due to stroke. Our principal markets are the United States and Europe. In Europe, we have a direct sales operation in Germany and the United Kingdom and work with distribution partners in certain other major countries. We have offices in Marlborough, Massachusetts, Berlin, Germany and Yokneam, Israel, where we operate our business from.
ReWalk Personal and ReWalk Rehabilitation Products
Development of our SCI Products took over a decade and was spurred by the experiences of our founder, Dr. Amit Goffer, who became a quadriplegic due to an accident. Current ReWalk designs are intended for people with paraplegia, a spinal cord injury resulting in complete or incomplete paralysis of the legs, who have the use of their upper bodies and arms. We currently offer two products: ReWalk Personal and ReWalk Rehabilitation.
ReWalk Personal is a breakthrough product that seeks to fundamentally change the health and life experiences of users. Designed for all-day use, the device is battery-powered and consists of a light, wearable exoskeleton with integrated motors at the joints, an array of sensors and a computer-based control system to power knee and hip movement. The device controls movement using subtle shifts in the user’s center of gravity. A forward tilt of the upper body is sensed by the system, which initiates the first step. Repeated body shifting generates a sequence of steps that results in a functional walking speed. Because the exoskeleton supports its own weight and facilitates the user’s gait, users do not expend unnecessary energy while walking. While ReWalk does not allow side-to-side actuation, users are able to turn by shifting their weight to the side. The ReWalk Personal also allows users to sit, stand and, depending on local regulatory approvals, climb and descend stairs. Use on stairs is not cleared by the FDA in the United States. Upon completion of training, which generally consists of approximately 15 one-hour sessions, most users are able to put on and remove the device by themselves while sitting, typically in less than 15 minutes, to operate the device independently and most are able to put on and remove the devices by themselves. Safety measures include crutches, which provide additional stability, fall protection, which lowers users slowly and safely in the event of a malfunction, and the secure “stand” mode, which automatically initiates if the user does not begin walking within two seconds. ReWalk is also equipped with maintenance alarms, warnings and backup batteries. The rechargeable batteries are easily accessible and can be recharged in any standard power outlet. Our safety guidelines and FDA specifications, however, require users to be accompanied by a trained companion at all times when using the ReWalk Personal.
1
| ReWalk Personal 6.0 | ||
|
● ReWalk Personal: intended for everyday use at home, at work or in the community. We began marketing ReWalk Personal in Europe with CE mark clearance at the end of 2012. We received FDA clearance to market ReWalk Personal in the United States in June 2014. ReWalk Personal units are all manufactured according to the same mechanical specifications. Each unit is then permanently sized to fit the individual user and the software is configured for the user’s specifications by the rehabilitation center, clinic or distributor.
● ReWalk Rehabilitation: designed for the clinical rehabilitation environment, ReWalk Rehabilitation has adjustable sizing enabling multiple patient use. ReWalk Rehabilitation provides a valuable means of exercise and therapy. It also enables individuals to evaluate their capacity for using ReWalk Personal in the future. We began marketing ReWalk Rehabilitation for use in hospitals, rehabilitation centers and stand-alone training centers in the United States and Europe in 2011. ReWalk Rehabilitation units are all manufactured according to the same mechanical specifications and are equipped with adjustable sizing for multi-patient use and software that can be configured for the user’s specifications. |
 |
Additionally, we have received regulatory approval to sell the ReWalk device in other countries. In the future we intend to seek approval from the applicable regulatory agencies in other jurisdictions where we may seek to market ReWalk.
Overview of Spinal Anatomy and Spinal Cord Injury
Spinal Anatomy
The spine is the central core of the human skeleton and provides structural support, alignment and flexibility to the body. It consists of 24 interlocking bones, called vertebrae, which are stacked on top of one another. The spine is comprised of five regions, of which there are three primary regions: cervical, thoracic and lumbar. In addition, there is also the sacral region, or sacrum, a triangular-shaped bone and the coccyx, or “tailbone,” the bottom portion of the spine.
The spinal cord, housed inside the bony spinal column, is a complex bundle of nerves serving as the main pathway for information connecting the brain and nervous system. The spinal cord is divided into 31 segments that feed sensory impulses into the spinal cord, which in turn relays them to the brain. Conversely, motor impulses generated in the brain are relayed by the spinal cord to the spinal nerves, which pass the impulses to muscles and glands. The spinal cord mediates the reflex responses to some sensory impulses directly, without recourse to the brain, for example, when a person’s leg is tapped, producing the knee jerk reflex.
2
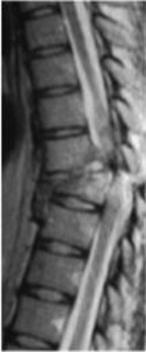
| Spinal Cord Injury | Image
of Separated Spinal Cord of an Adult | |
Spinal cord injury is the result of a direct trauma to the nerves themselves or damage to the surrounding bones and soft tissues which ultimately impacts the spinal cord. Spinal cord damage results in a loss of function, such as mobility or feeling. In most people who have spinal cord injury, the spinal cord is intact. Spinal cord injury is not the same as back injury, which may result from pinched nerves or ruptured disks. Even when a person sustains a break in a vertebra or vertebrae, there may not be any spinal cord injury if the spinal cord itself is not affected. There are two types of spinal cord injury – complete and incomplete. In a complete injury, a person loses all ability to feel and voluntarily move below the level of the injury. In an incomplete injury, there is some functioning below the level of the injury.
Upon examination, a patient is assigned a level of injury depending on the location of the spinal cord injury. Cervical level injuries cause paralysis or weakness in both arms and legs and is referred to as quadriplegia. Sometimes this type of injury is accompanied by loss of physical sensation, respiratory issues, bowel, bladder, and sexual dysfunction. Thoracic level injuries can cause paralysis or weakness of the legs (paraplegia) along with loss of physical sensation, bowel, bladder, and sexual dysfunction. In most cases, arms and hands are not affected. Lumbar level injuries result in paralysis or weakness of the legs (paraplegia). Loss of physical sensation, bowel, bladder, and sexual dysfunction can occur. The shoulder, arm, and hand functions are usually unaffected. Sacral level injuries primarily cause loss of bowel and bladder function as well as sexual dysfunction. |
 |
3
Clinical evidence
Published clinical studies indicate ReWalk Personal’s ability to deliver a functional walking speed. In addition, our experience working with healthcare practitioners and ReWalk users, including reports by study participants, as well as multiple clinical studies, some of which are published in peer-reviewed journals, have been carried out to establish the effectiveness and the potential secondary health benefits achieved by using the SCI Products for individuals with spinal cord injuries. Certain of the benefits suggested include:
| ● | reduced pain; |
| ● | improved bowel and urinary tract function; |
| ● | reduced spasticity; |
| ● | increases in joint range of motion for the hip and ankle joints; |
| ● | improved sleep and reduced fatigue; |
| ● | increase in oxygen uptake and heart rate as a result of walking as opposed to sitting and standing; |
| ● | ability to ambulate at a speed greater than 0.4 meters per second, which is considered to be conducive to outdoor related community ambulation; and |
| ● | reduced hospitalizations. |
Although study participants and other ReWalk users have reported the secondary health benefits listed above, currently there is no conclusive clinical data establishing any secondary health benefits of ReWalk. We believe that using our SCI Products may have the ability to reduce the lifetime healthcare costs of individuals with spinal cord injuries, which we believe will make it economically attractive for individuals and third-party payors. While we believe that using the SCI Products could potentially offer significant advantages over competing technologies and therapies, disadvantages include the time it takes for a user to put on the device, the slower pace of the device compared to a wheelchair, the weight of it when carried, which makes it more burdensome for a companion to transport than a wheelchair, and the requirement that users be accompanied by a trained companion.
Market Opportunity
ReWalk’s current and near-term market opportunities include providing a solution for persons with spinal cord injury that can be used in the clinic and/or home settings, and a solution for therapists to use during stroke rehabilitation in their clinics. For persons with spinal cord injury, confinement to a wheelchair can cause severe physical and psychological deterioration, resulting in bad health, poor quality of life, low self-esteem and high medical expenses. In addition, the secondary medical consequences of paralysis can include difficulty with bowel and urinary tract function, osteoporosis, loss of lean mass, gain in fat mass, insulin resistance, diabetes and heart disease. The cost of treating these conditions is substantial. The National Spinal Cord Injury Statistical Center, or the NSCISC, estimates that complications related to paraplegia cost, excluding indirect costs such as losses in wages, fringe benefits and productivity, approximately $500,000 in the first year post-injury and significant additional amounts over the course of an individual’s lifetime. Further, secondary complications related to spinal cord injury can reduce life expectancies for spinal cord injury, or SCI, patients. The young average age at time of injury and significant remaining life expectancy, the likelihood of living at home and lifetime cost of treatment highlight the need for an out-of-hospital solution with demonstrated health and social benefits.
The NSCISC estimates as of 2019 that there were 291,000 people in the United States living with spinal cord injury or SCI, with an annual incidence of approximately 17,730 new cases per year. Approximately 44,000 of such patients are veterans, and are eligible for medical care and other benefits from the U.S. Department of Veterans Affairs (the “VA”). With 25 VA spinal cord injury centers, the VA has the largest single network of spinal cord injury care in the United States.
The University of Alabama-Birmingham Department of Physical Medicine and Rehabilitation operates the NSCISC, which maintains the world’s largest database on spinal cord injury research. Since 2015, motor vehicle crashes have been the leading cause of reported spinal cord injury cases (39%), followed by falls (32%), acts of violence (14%) and sports injuries (8%). 78% of spinal cord injuries occur among the male population. According to NSCISC data, upon hospital discharge, 87% of persons with spinal cord injuries are sent to private, non-institutional residence (in most cases, their homes prior to injury).
4
Based on information from a 2017 report by the NSCISC, 40.4% of the total U.S. population of SCI patients suffered injuries between levels T4 and L5. Three published ReWalk trials for SCI patients had an aggregate screening acceptance rate of 79% considering all current FDA limitations, resulting in an estimated 32% of the total population of SCI patients being candidates for current ReWalk products. For important qualifying information about this determination, see “Part I, Item 1A. Risk Factors—Risks Related to our Business and our Industry—The market for medical exoskeletons, including soft suit devices, remains relatively new and unproven, and important assumptions about the potential market for our current and future products may be inaccurate.”
Sales and Marketing activities
Our initial commercialization efforts focused on penetrating rehabilitation centers, hospitals and similar facilities that treat patients with spinal cord injuries to become an integral part of their rehabilitation programs and to develop a broad based training network with these facilities to prepare users for home and community use. As our business has developed, we have shifted our commercialization efforts to marketing ReWalk Personal with insurance companies, physicians and physiotherapists as a standard of care that can be used routinely at home, work or in the community.
We market and sell our products directly to third party payers, institutions, including rehabilitation centers, individuals and through third-party distributors. We sell our products directly in Germany, the United Kingdom and the United States and primarily through distributors in our other markets. In our direct markets, we have established relationships with rehabilitation centers and the spinal cord injury community, and in our indirect markets, our distributors maintain these relationships. Sales of ReWalk Personal are generated primarily from the patient base at our rehabilitation centers, referrals through the spinal cord injury community and direct inquiries from potential users.
As of December 31, 2019, we had placed 119 units in use at rehabilitation centers and 453 personal units in a home or community use, compared to 119 units and 399 units, respectively, as of December 31, 2018. In the near future, we intend to continue focusing on our reimbursement efforts, pursuing insurance claims on a case-by-case basis, managing claims through the review process, and investing in efforts to expand commercial reimbursement coverage.
Although we cannot predict the time it will take to achieve higher acceptance rates of our SCI Products, we believe that further clinical evidence supporting the benefits of using the device will be a key element to accelerate it.
Third-Party Reimbursements
United States
In the United States rehabilitation centers generally purchase the ReWalk Rehabilitation unit and then charge patients for ReWalk therapy on a per-session basis. These institutions may then seek reimbursement from insurance companies for each session.
In December 2015, the VA issued a national policy for the evaluation, training and procurement of ReWalk Personal exoskeleton systems for all qualifying veterans across the United States. The VA policy is the first national coverage policy in the United States for qualifying individuals who have suffered spinal cord injury. In June 2018 the VA has updated this policy to include more training options for individuals who could not complete the training due to distance from a VA site. As of December 31, 2019, we had placed 24 units as part of the VA policy. The VA accounted for 15.0% of our total revenues for the year ended December 31, 2019. We continue to work with the VA to accelerate the pace of implementation of the VA policy.
While no broad uniform policy of coverage and reimbursement for electronic exoskeleton medical technology exists among commercial insurance payors in the United States, reimbursement may be achieved on a case-by-case basis. To date, payments for the ReWalk Personal device have been made primarily through case-by-case determinations by third-party payors, including commercial insurers in the United States, by self-payors and donations and, to a lesser extent, through the use of funds from insurance and/or accident settlements.
As of December 31, 2019, we had 40 cases pending in the United States for insurance coverage decisions. For more information, see “Part I, Item 1A. Risk Factors—Risks Related to our Business and our Industry— We may fail to secure or maintain adequate insurance coverage or reimbursement for our products by third-party payors, which risk may be heightened if insurers find the products to be investigational or experimental or if new government regulations change existing reimbursement policies. Additionally, such coverage or reimbursement, even if maintained, may not produce revenues that are high enough to allow us to sell our products profitably.”
5
According to a 2017 report published by the Centers for Medicare and Medicaid Services, or CMS, approximately 55% of the spinal cord injury population which are at least five years post their injury date are covered by CMS. In December 2019, we submitted the first application for code issuance for ReWalk Personal 6.0, which might later be followed by coverage policy of CMS. While we believe that a positive response from CMS may broaden coverage by private insurers, we cannot currently predict how long it would take for us to receive a decision from CMS nor can we predict other business elements that will be decided by CMS, such as price per unit or product labeling. For more information, see “Part I, Item 1A. Risk Factors—Risks Related to our Business and our Industry— We may fail to secure or maintain adequate insurance coverage or reimbursement for our products by third-party payors, which risk may be heightened if insurers find the products to be investigational or experimental or if new government regulations change existing reimbursement policies. Additionally, such coverage or reimbursement, even if maintained, may not produce revenues that are high enough to allow us to sell our products profitably.”
As part of our plan for growth, we intend to continue working with both national and regional commercial insurance companies, health care practitioners, physicians, researchers, and the SCI community to support efforts to demonstrate the benefits and the case to secure potential coverage policies based on supportive data and appeal rulings that have deemed exoskeleton devices a “medically necessary” standard of care for individuals with SCI. Our efforts in the future will be focused on continued education of insurers through data application, supporting clinical trials to demonstrate the clinical benefits of using the SCI Products, working with advocacy groups, ongoing communication as well as trying to obtain CMS coverage for the ReWalk Personal 6.0 device.
Western Europe
Reimbursement for ReWalk in Europe varies by country and historically certain third-party payors have provided reimbursement for our products in certain cases in Germany and Italy.
We initially focused our efforts in Europe in Germany where we continue to make progress toward achieving ReWalk coverage from the various government, private and worker’s compensation payers. Specifically:
| ● | In September 2017, Barmer confirmed it will provide ReWalk systems to all qualifying beneficiaries. Barmer provides insurance coverage for nearly ten million people in Germany, as a member of the German Statutory Health Insurance (“SHI”) network and one of the most significant national insurers in the country. Exoskeletons will be provided to users that meet certain inclusion criteria and assessment by the German Health Insurance Medical Service (Medizinischer Dienst der Krankenversicherungen) before and after training. Barmer has already begun processing claims with users entering training for in-home use of an exoskeleton. |
| ● | In September 2017 Germany’s national social accident insurance provider, DGUV, indicated that the DGUV’s member payers, including the health insurance association Berufsgenossenschaft (also known as BG) and state insurers, will approve the supply of exoskeleton systems for qualifying beneficiaries on a case-by-case basis. DGUV is comprised of 36 different insurers, which provide coverage for more than 80 million individuals in Germany. Per the agreement, eligible individuals will go to BG clinics for evaluation as a part of the procurement. |
| ● | In February 2018, the GKV confirmed its decision to list the ReWalk Personal system in the German MDD, a comprehensive list of all medical devices which are principally and regularly reimbursed by German SHI providers. The ReWalk Personal was added to the official German list of medical aids, code number 23.29.01.2001, in June 2018. This decision means that ReWalk Personal will be listed among all medical devices for compensation, which SHI providers can procure for any approved beneficiary on a case-by-case basis. |
Patients who are covered under these policies must be medically evaluated for their eligibility to use the ReWalk Personal device. If medically qualified, the patient, along with his or her physician, must apply for coverage of the device. We are currently working with several SHIs and German Private Health Insurer (“PHI”) on securing a formal operating contract that will establish the process of obtaining a ReWalk Personal 6.0 device for their beneficiaries within their system. Some of these contracts are in an advanced stage; subject to the timeline for the completion of internal governmental approvals and other processes, we expect certain of these contracts to be finalized in the coming months with payors in key European markets where we sell our products.
As of December 31, 2019, there were 105 insurance cases pending in Germany. We believe that our recent coverage decisions and the existing claims will eventually lead other German insurers, including additional PHIs, to provide coverage on a broad scale. For more information, see “Part I, Item 1A. Risk Factors—Risks Related to our Business and our Industry— We may fail to secure or maintain adequate insurance coverage or reimbursement for our products by third-party payors….”
6
We continue to support clinical research and academic publications, which we believe will further support the case for coverage.
We are also pursuing reimbursement by private insurers and worker’s compensation in various European countries and one of the examples of success was achieved in March 2018, when the Italian Ministry of Labor and Social Policy’s statutory insurance corporation put in place a coverage policy that will provide exoskeleton systems for all qualifying beneficiaries. This policy, the first of its kind in Italy, will provide individuals with spinal cord injury access to obtain their own ReWalk Personal device so that they can stand and walk again. Since the initiation of coverage, we have supplied 7 units through our Italian distributor to individuals covered by this policy.
Other Funding Sources
In addition to being funded by third-party payors, including private insurance plans, government programs such as the VA, and Worker’s Compensation, ReWalk Personal is also funded by self-payers. This includes individuals who purchase ReWalk with funds from legal settlements with insurance companies or third parties.
ReStore
In June 2017 we unveiled our lightweight exo-suit ReStore system designed initially for rehabilitation of stroke patients. The patented soft exo-suit technology was originally developed at Harvard University’s Wyss Institute for Biologically Inspired Engineering, or Harvard, where it also underwent initial clinical testing that demonstrated its potential to improve walking for stroke survivors. ReWalk and Harvard entered into a multi-year research collaboration agreement in 2016 which provides ReWalk license to intellectual property relating to lightweight exo-suit system technologies for lower limb disabilities and provides access to future innovations that emerge from this collaboration and may be relevant to additional stroke products or other therapies. The development and regulatory approval of ReStore took us approximately three years and in June 2019 we received FDA clearance following CE clearance that was obtained in May 2019. Following the regulatory approvals, we have started to commercialize the ReStore product. For more information on the collaboration with Harvard, see “Research and Development-Research and Development Collaborations.”. |
ReStore exo-suit |
The ReStore product is comprised of a soft, fabric-based design which connects to a lightweight waist pack and mechanical cables that help lift the patient’s affected leg in synchronized timing with their natural walking pattern. The lightweight structure wraps around the waist and supports an actuator with a motor, computer and cable, along with sensors attached to a stable point on the user’s calf and footplate in the user’s shoe. This design transfers forces in a controlled manner and targeted assistance to the patient ankle during forward propulsion (plantarflexion) and ground clearance (dorsiflexion), two key phases of the gait cycle. The ReStore system is designed to provide advantages to stroke rehabilitation clinics and therapists as compared to other traditional therapies and devices by improving the quality and pace of care, supplying real-time analytics to optimize session productivity and generating ongoing data reports to assist with tracking patient progress. We expect the device may also provide other secondary benefits for rehabilitation clinics, including reducing staffing and/ or equipment requirements, staff fatigue and the risk for potential staff injuries.
Published clinical trials that were conducted at Harvard using the soft-suit design on stroke patients have shown varying levels of improvements, with the main ones being improved forward propulsion, reductions in compensatory behaviors including paretic hip hiking and circumduction as well as reduction in metabolic burden associated with post stroke walking. The Company is currently supporting additional clinical trials using the ReStore device.
The main market for ReStore is rehabilitation clinics with a stroke therapy program or clinics that would like to broaden their stroke presence. This product is marketed and sold directly to rehabilitation clinics for use during the course of the treatment of their patients which is generally reimbursed by private payors as well as statutory health insurers and CMS. In 2020 our sales and marketing priority is to educate both clinical and executive managers within the clinic on the clinical and economic benefits of using the device through their stroke rehabilitation program. During the second half of 2019 we have expended our sales and marketing presence in the U.S. in order to accelerate the product penetration. Geographically we see our priorities as the United States and Europe.
Stroke incidence rate in the United States is 795,000 incidences per year and the survival rate is approximately 80%. Of this stroke population, 80% are left with some type of lower limb disability. This patient population seeks treatment in one of the thousands inpatient, outpatient, skilled nursing facilities and rehabilitation clinics providing therapy. With the clinical evidence we have to date on ReStore, its unique design and the cost effective offer compared to other products, we believe the ReStore soft-suit has an opportunity to be considered as a standard of care among clinics during their stroke patients therapy, but we also recognize that the process to achieve that might be long. We believe that in order to accelerate adoption, further clinical evidence is required as well as continued education on the new ReStore design and its unique advantages compared to current therapies and products.
As of December 31, 2019 we have placed 16 ReStore units in clinics.
7
Competition
The market in which we operate is characterized by active competition and rapid technological change, and we expect competition to increase. Competition arises from providers of other mobility systems and prosthetic devices used in the clinic and/or home settings.
We are aware of a number of other companies developing competing technology and devices, and some of these competitors may have greater resources, greater name recognition, broader product lines, or larger customer bases than we do.
Our principal competitors in the medical exoskeleton market consist of Ekso Bionics (NASDAQ: EKSO), Rex Bionics Pty, Cyberdyne (Tokyo Stock Exchange: 7779), Parker Hannifin (NYSE: PH), FREE Bionics, Hocoma, AlterG, and Bioness. These products may also compete with the ReStore exo-suit, as well as manual forms of gait training which do not involve robotic assistive devices.
We believe that our ReWalk Personal device possesses key competitive advantages over these companies, such as our tilt-sensor technology that provides a self-initiated walking experience, more natural gait and faster functional walking speed, the ability to support its own weight and broad user specifications. ReWalk Personal is the first medical exoskeleton cleared by the FDA for personal use in the United States.
We believe that our ReStore soft exo-suit device will have key competitive advantages over the products of our competitors, including a design that facilitates a natural, functional walking pattern through flexible materials, sensors, and powered plantarflexion as well as dorsiflexion, making it the only solution of its type of which we are aware of that supports such movements, achieving that with a lower cost and weight than rigid skeletal devices.
In addition, we compete with alternative devices and alternative therapies, including treadmill-based gait therapies, such as those offered by Hocoma, AlterG, Aretech and Reha Technology. Other medical device or robotics companies, academic and research institutions, or others may develop new technologies or therapies that provide a superior walking experience, are more effective in treating the secondary medical conditions that we target or are less expensive than our current or future products. Our technologies and products could be rendered obsolete by such developments.
We may also compete with other treatments and technologies that address the secondary medical conditions that ReWalk seeks to mitigate.
Community Engagement and Education
We devote significant resources to engagement with and education of the spinal cord injury community with respect to the benefits of our SCI Products. We actively seek opportunities to partner with hospitals, rehabilitation centers and key opinion leaders to engage in research and development and clinical activities. We also seek to educate and gain support from organizations such as patient advocacy groups and clinician societies with the goal of promoting adoption of exoskeleton technology from patient, clinician and payor communities. We believe that our success has been, and will continue to be driven in part by our reputation and acceptance within the spinal cord injury community. We are also looking into ways to promote the ReStore device through different advocacy groups to accelerate adoption and support the uniqueness of this technology when compared to current therapies and products
To date, multiple advocacy groups have issued public endorsement to cover the ReWalk Personal device, including leading United States-based national organizations such as the United Spinal Association and the Dana and Christopher Reeves Foundation, as well as others.
Services and Customer Support
Our centers of operations in Marlborough, Massachusetts and Berlin, Germany coordinate all customer support and product service functions for North America and Europe, respectively, through dedicated technical service personnel who provide product services and customer support through training to healthcare providers and support to product users.
8
Research and Development
We are committed to investing in a robust research and development program to enhance our current product line and to develop our pipeline of new and complementary products, and we believe that ongoing research and development efforts are essential to our success. Our research and development team consists of both in-house and external staff, including engineers, machinists, researchers and marketing, quality, manufacturing, regulatory and clinical personnel, which we employ in the most efficient way we can and see fit to our current and future needs, who work closely together to design, enhance and validate our technologies. This research and development team conceptualizes technologies and then builds and tests prototypes before refining and/or redesigning as necessary. Our regulatory and clinical personnel work in parallel with engineers and researchers, allowing us to anticipate and resolve potential issues at early stages in the development cycle. Our level of research and development investment depend on our available resources and future needs, For more information on see “Part I, Item 1A. Risk Factors — Risks Related to Our Business and Our Industry — Our future growth and operating results will depend on our ability to develop, receive regulatory clearance for and commercialize new products and penetrate new product and geographic markets.
We plan to focus our research and development efforts in the future by continually improving and expanding our functional technological platform, by expanding the indication of use of our lightweight “soft suit” exoskeleton to other medical conditions as well as home therapy, and in the longer term by developing our next generation of SCI Products with design improvement. New medical indications that affect the ability to walk may include multiple sclerosis, cerebral palsy, Parkinson’s disease and elderly assistance.
We conduct our research and development efforts at our facility in Yokneam, Israel. We believe that the close interaction among our research and development and manufacturing groups allows for timely and effective realization of our new product concepts.
Our research and development efforts have been financed, in part, through funding from the Israel Innovation Authority, or the IIA (formerly known as Office of the Chief Scientist in the Israel Ministry of Economy), and from the Israel-U.S. Binational Industrial Research and Development, or BIRD Foundation. From our inception through December 31, 2019, we received funding totaling $1.97 million from the IIA and $500 thousand from the BIRD Foundation. For more information regarding our research and development financing arrangements, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” and “—Grants and Other Funding.”
Research and Development Collaborations
On May 16, 2016, we entered into the Research Collaboration Agreement, or Collaboration Agreement, and the Exclusive License Agreement, or Harvard License Agreement, with Harvard. Under the Collaboration Agreement, we and Harvard agreed to collaborate on research regarding the development of lightweight soft suit exoskeleton system technologies for lower limb disabilities, which are intended to treat stroke, multiple sclerosis, mobility limitations for the elderly and other medical applications. Under the Collaboration Agreement, we must pay Harvard quarterly installment payments to help fund the research. Subject to the terms of the Collaboration Agreement, we and Harvard are required to report our respective research results and findings to each other on a regular basis. The Collaboration Agreement governs ownership of the research results and inventions generated in performance of the research collaboration and provides us the option to negotiate with Harvard for a license to certain new inventions of Harvard conceived in performance of the collaboration.
The Collaboration Agreement was amended on April 1, 2018 to extend the term of the Collaboration Agreement by one year to May 16, 2022 and reallocate the Company’s quarterly installment payments to Harvard through such date, and to make certain technical changes. Subject to payment of a minimum funding commitment which were already paid as of December 31, 2019, under applicable circumstances, we may terminate the agreement if there is a loss of Harvard’s principal investigator or if we do not believe that we have or can secure sufficient funding to proceed. The Collaboration Agreement may also be terminated by either Harvard or us due to a material uncured breach by the other party or upon termination of the Harvard License Agreement. If such termination occurs it does not affect the Harvard License Agreement.
Under the Harvard License Agreement, we are granted an exclusive, worldwide royalty-bearing license under certain patents of Harvard relating to lightweight “soft suit” exoskeleton system technologies for lower limb disabilities, a royalty-free license under certain related know-how and the option to obtain a license under certain inventions conceived under our joint research collaboration. Harvard retains the right to practice the patents for research, educational and scholarly purposes. We are required to use commercially reasonable efforts to develop products under the license in accordance with an agreed-upon development plan and to introduce and market such products commercially. In addition to an upfront fee and royalties on net sales, we are obligated to pay Harvard certain milestone payments upon the achievement of certain product development and commercialization milestones. We also agreed to reimburse Harvard for expenses incurred in connection with the filing, prosecution and maintenance of the licensed patents.
The Harvard License Agreement will continue in full force and effect until the expiration of the last-to-expire valid claim of the licensed patents. We may terminate the License Agreement for any reason upon 60 days’ prior written notice, while Harvard may terminate the License Agreement if we do not obtain requisite insurance or become insolvent. The Harvard License Agreement may also be terminated by Harvard or us due to the other party’s material uncured breach.
9
The Collaboration Agreement and Harvard License Agreement contain, as applicable, customary representations and warranties and customary enforcement, indemnification and insurance provisions. For further discussion of the Collaboration Agreement and Harvard License Agreement, see Note 9 to our consolidated financial statements for the fiscal year ended December 31, 2019.
In September 2013, we entered into a strategic alliance with Yaskawa Electric Corporation (“Yaskawa”), pursuant to which, among other arrangements, Yaskawa can apply its expertise in product and quality improvements to ReWalk and assist in marketing, distributing and commercializing on an exclusive basis our products in Japan, China and other East Asian countries. Yaskawa is a global leader in the fields of industrial robotics and automation. While we have not engaged in joint initiatives with Yaskawa to date, we believe that this relationship may provide us with opportunities for product improvement and increased product offerings in the future. In connection with the closing of the first tranche of the private placement of our ordinary shares to Timwell, we amended our exclusive distribution agreement with Yaskawa on May 15, 2018 to terminate the distribution rights granted to Yaskawa in China (including Hong Kong and Macau). For more information on the Timwell private placement, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Timwell Private Placement.”
Intellectual Property
Protection of our intellectual property is important to our business. We seek to protect our intellectual property through a combination of patents, trademarks, confidentiality and assignment agreements with our employees and certain of our contractors and confidentiality agreements with certain of our consultants, scientific advisors and other vendors and contractors. In addition, we rely on trade secrets law to protect our proprietary software and product candidates/products in development.
In addition to ReWalk’s portfolio of issued patents and pending patent applications, the Company licenses certain patented and patented pending technology from a third party as described above under the “Research and Development” section.
As of December 31, 2019, we have eight issued patents in the United States and ten issued patents outside of the United States, as well as 15 pending patent applications in various countries around the world for our technology including the United States and Europe. As such, we have apparatus patent claims in the United States and Europe covering aspects of ReWalk and similar devices which use a plurality of sensors to empower tilt-sensor technology. In addition, in the United States and Europe, we have method patent claims covering certain methods of user activation and control of systems such as ReWalk, including by sensing the user’s torso lean or weight shifts. While our apparatus claims focus on protecting ReWalk in terms of its physical and structural characteristics, we believe that our method claims, which protect the process behind how ReWalk is controlled by the user, provide additional protection for our tilt sensor technology. We do not currently license any of the technology contained in our currently commercialized products other than with respect to technology that is generally publicly available, but we may do so in the future.
Patents filed both in the United States and Europe generally have a life of 20 years from the filing date. As the oldest of our issued patents relating to our tilt-sensor technology was filed in May 2001, our patents on that technology do not begin to expire until May 2021.
We currently hold a registered trademark in Israel and the Unites States for the mark “ReWalk”. We currently hold a registered trademark in Europe and an allowed trademark in the United States for the mark “Restore”.
The employment agreement of our founder and former President and Chief Technology Officer, Dr. Amit Goffer, provides that a patent pending relating to a standing wheelchair is his individual property and that he may independently engage in the development of a standing wheelchair. The agreement also provides that we and any of our affiliates or successors have the royalty-free right to the exclusive use in the field of exoskeletons of any intellectual property developed by Dr. Goffer, alone or jointly with others (whether or not as part of the development of a standing wheelchair and whether or not developed through a company), while he is our employee, consultant or board member and for three years thereafter. Mr. Goffer retired from serving as our President and Chief Technology Officer on November 18, 2015, and as a member of our board of directors on December 3, 2015.
We cannot be sure that our intellectual property will provide us with a competitive advantage or that we will not infringe on the intellectual property rights of others. In addition, we cannot be sure that any patents will be granted in a timely manner or at all with respect to any of our patent pending applications. For a more comprehensive discussion of the risks related to our intellectual property, see “Part I, Item 1A. Risk Factors—Risks Related to Our Intellectual Property.”
10
Government Regulation
U.S. Regulation
Our medical products and manufacturing operations are regulated by the FDA and other federal and state agencies. Our products are regulated as medical devices in the United States under the Federal Food, Drug, and Cosmetic Act, or the FFDCA, as implemented and enforced by the FDA. The FDA regulates the development, testing, manufacturing, labeling, storage, installation, servicing, advertising, promotion, marketing, distribution, import, export, and market surveillance of our medical devices.
Premarket Regulatory Requirements
Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) premarket notification, approval of a premarket approval application (PMA), or issuance of a de novo order. Under the FFDCA, medical devices are classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurance of safety and effectiveness. Classification of a device is important because the class to which a device is assigned determines, among other things, the necessity and type of FDA review required prior to marketing the device. Class I devices are those for which reasonable assurance of safety and effectiveness can be assured by adherence to general controls that include compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. Class I also includes devices for which there is insufficient information to determine that general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device or to establish special controls to provide such assurance, but that are not life-supporting or life-sustaining or for a use which is of substantial importance in preventing impairment of human health, and that do not present a potential unreasonable risk of illness of injury.
Class II devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish “special controls.” These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents. While most Class I devices are exempt from the 510(k) premarket notification requirement, most Class II devices require a 510(k) premarket notification to be marketed in the U.S. As a result, manufacturers of most Class II devices are required to submit to the FDA premarket notifications under Section 510(k) of the FFDCA requesting classification of their devices in order to market or commercially distribute those devices. To obtain a 510(k), a substantial equivalence determination for their devices, manufacturers must submit to the FDA premarket notifications demonstrating that the proposed device is “substantially equivalent” to a predicate device already on the market. A predicate device is a legally marketed device that is not subject to premarket approval, or PMA, meaning, (i) a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, (ii) a device that has been reclassified from Class III to Class II or I, or (iii) a device that was found substantially equivalent through the 510(k) process. If the FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant 510(k) clearance to commercially market the device. If the device is not “substantially equivalent” to a previously cleared device, the device is automatically a Class III device. The device sponsor must then fulfill more rigorous premarket approval requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process, which is a route to market for medical devices that are low to moderate risk, but are not substantially equivalent to a predicate device.
Devices that are intended to be life sustaining or life supporting, devices that are implantable, devices that present a potential unreasonable risk of harm or are of substantial importance in preventing impairment of health, and devices that are not substantially equivalent to a predicate device are placed in Class III and generally require approval of a PMA, unless the device is a pre-amendment device not yet subject to a regulation requiring premarket approval. The PMA process is more demanding than the 510(k) premarket notification process. In a PMA, the manufacturer must demonstrate that the device is safe and effective, and the PMA must be supported by extensive data, including data from preclinical studies and clinical trials. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FFDCA to complete its review of a PMA, although in practice, the FDA’s review often takes significantly longer, and can take up to several years.
Clinical trials are almost always required to support PMAs and are sometimes required to support 510(k) submissions. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption, or IDE, regulations that govern investigational device labeling, prohibit promotion of the investigational device, and specify recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk,” as defined by the FDA, the agency requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA denies the application or notifies the company that the investigation is on hold and may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the study, the FDA may permit a clinical trial to proceed under a conditional approval. In addition, the study must be approved by, and conducted under the oversight of, an Institutional Review Board, or IRB, for each clinical site. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still comply with abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
11
In June 2014, the FDA granted our petition for “de novo” classification, which provides a route to market for medical devices that are low to moderate risk, but are not substantially equivalent to a predicate device, and classified ReWalk as Class II subject to special controls. The ReWalk is intended to enable individuals with spinal cord injuries to perform ambulatory functions under supervision of a specially trained companion, and inside rehabilitation institutions. The special controls established in the de novo order include the following: compliance with medical device consensus standards; clinical testing to demonstrate safe and effective use considering the level of supervision necessary and the use environment; non-clinical performance testing, including durability testing to demonstrate that the device performs as intended under anticipated conditions of use; a training program; and labeling related to device use and user training. The special controls of this de novo order also apply to competing products seeking FDA clearance.
In June 2019, the FDA issued a 510(k) clearance for the ReStore which means that the device can be marketed in the U.S The ReWalk ReStore is intended to be used to assist ambulatory functions in rehabilitation institutions under the supervision of a trained therapist for people with hemiplegia or hemiparesis due to stroke. ReStore complies with special controls includes the following: compliance with medical device consensus standards; clinical testing to demonstrate safe and effective use considering the level of supervision necessary and the use environment; non-clinical performance testing, including durability testing, to demonstrate that the device performs as intended under anticipated conditions of use; a training program; and labeling related to device use and user training. In order for us to market ReStore, we must comply with both general controls, including controls related to quality, facility registration, reporting of adverse events and labeling, and the special controls established for the device. Failure to comply with the general and special controls could lead to removal of ReStore from the market, which would have a material adverse effect on our business.
For more information, see “Part I, Item 1A. Risk Factors-Risks Related to Government Regulation-We are subject to extensive governmental regulations relating to the manufacturing, labeling and marketing of our products, and a failure to comply with such regulations could lead to withdrawal or recall of our products from the market.”
Post-market Regulatory Requirements
After a device is cleared for marketing, and prior to marketing, numerous regulatory requirements apply. These include:
| ● | establishment registration and device listing; |
| ● | development of a quality assurance system, including establishing and implementing procedures to design and manufacture devices; |
| ● | labeling regulations that prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; |
| ● | FDA’s Unique Device Identification requirements that call for a unique device identifier (UDI) on device labels and packages and submission of data to the FDA’s Global Unique Device Identification Database (GUDID); |
| ● | medical device reporting regulations that require manufacturers to report to the FDA if a device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and corrections and removal reporting regulations that require manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FFDCA that may present a risk to health; and |
| ● | Post-market surveillance. |
12
ReWalk is required by an FDA order under Section 522 of the FFDCA to conduct a post-market study of the ReWalk Personal device. We launched our post-market surveillance study with Stanford University during the second quarter of 2016. For more information on the post-market surveillance study progress, see “Part I, Item 1A. Risk Factors—Risks Related to Government Regulation.”
Our manufacturing processes are required to comply with the applicable portions of the Quality System Regulation that covers the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. We actively maintain compliance with the FDA’s Quality System Regulation, 21 CFR Part 820, and the European Union’s Quality Management Systems requirements, ENISO 13485:2012.
As a manufacturer, we are subject to periodic scheduled or unscheduled inspections by the FDA. If the FDA believes we or any of our contract manufacturers are not in compliance with the quality system requirements, or other post-market requirements, it has significant enforcement authority. Specifically, if the FDA determines that we failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, which may result in any of the following sanctions:
| ● | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| ● | customer notifications or repair, replacement or refunds; |
| ● | recalls, withdrawals, or administrative detention or seizure of our products; |
| ● | operating restrictions or partial suspension or total shutdown of production; |
| ● | refusing or delaying requests for approval of pre-market approval applications relating to new products or modified products; |
| ● | withdrawing PMA approval; |
| ● | refusal to grant export approvals for our products; or |
| ● | pursuing criminal prosecution. |
Any such action by the FDA would have a material adverse effect on our business. In addition, these regulatory controls, as well as any changes in FDA policies, can affect the time and cost associated with the development, introduction and continued availability of new products. Where possible, we anticipate these factors in our product development processes.
Regulation outside of the U.S.
In addition to the United States regulations, we are subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. In particular, we are subject to regulation in the E.U., which has directives and standards regulating the design, manufacture, clinical trials, labeling and adverse event (i.e. vigilance) reporting for medical devices. Devices that comply with the requirements of a relevant directive are entitled to bear the CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directive and, accordingly, can be commercially distributed throughout Europe. The method of assessing conformity varies depending on the class of the product, but normally involves a combination of self-assessment by the manufacturer and a third party assessment by a “Notified Body.” This third party assessment may consist of an audit of the manufacturer’s quality system or specific testing of the manufacturer’s product. We comply with the E.U. requirements and have received the CE mark for all of our ReWalk systems distributed in the E.U.
Foreign sales outside of the E.U. are subject to the foreign government regulations of the relevant jurisdiction, and we must obtain approval by the appropriate regulatory authorities before we can commence clinical trials or marketing activities in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required to obtain a marketing authorization in the Unites States or the E.U. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
In 2017, the European Union adopted a new Medical Device Regulation, which will repeal and replace the existing directives effective May 26, 2020. The new regulation does not set out a radically new system, but envisages, among other things, stricter controls of medical devices, including strengthening of the conformity assessment procedures, increased expectations with regard to clinical data for devices and pre-market regulatory review of high-risk devices. Under transitional provisions, medical devices with notified body certificates issued under the existing directives prior to May 26, 2020 may continue to be placed on the market for the remaining validity of the certificate, until May 27, 2024 at the latest. After the expiry of any applicable transitional period, only devices that have been CE marked under the new regulation may be placed on the market in the E.U.
The policies of the FDA and foreign regulatory authorities may change and additional government regulations may be enacted that could prevent or delay regulatory approval of our products and could also increase the cost of regulatory compliance. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
13
U.S. Anti-kickback, False Claims and Other Healthcare Fraud and Abuse Laws
In the United States, there are federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in federal healthcare programs. These laws apply to manufacturers of products, such as us, with respect to our financial relationship with hospitals, physicians and other potential purchasers or acquirers of our products. The U.S. government has published regulations that identify “safe harbors” or exemptions for certain practices from enforcement actions under the federal anti-kickback statute, and we will seek to comply with the safe harbors where possible. To qualify for a safe harbor, the activity must fit squarely within the safe harbor. Arrangements that do not meet a safe harbor are not necessarily illegal, but must be evaluated on a case by case basis. Other provisions of state and federal law provide civil and criminal penalties for presenting, or causing to be presented, to third-party payers for reimbursement claims that are false or fraudulent, or for items or services that were not provided as claimed. False claims allegations under federal and some state laws may be brought on behalf of the government by private persons, “whistleblowers,” who then receive a share of any recovery.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, the PPACA, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them. In addition, the PPACA provides that the government may assert that a claim that includes items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act. The PPACA also imposes new reporting and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to physicians and teaching hospitals. Device manufacturers will also be required to report and disclose any investment interests held by physicians and their immediate family members during the preceding calendar year. A number of provisions of PPACA also reflect increased focus on and funding of healthcare fraud enforcement.
In September 2017, members of the U.S. Congress introduced legislation with the announced intention to repeal and replace major provisions of the PPACA. Although this proposed legislation ultimately failed to pass, Congress succeeded in repealing the PPACA’s individual mandate as part of the U.S. Tax Cuts and Jobs Act of 2017. Thus, in light of the stated policies of the new U.S. presidential administration, and actions of certain members of the U.S. Congress, there is uncertainty with respect to the impact, if any, on the provisions of the PPACA affecting us. While any legislative and regulatory changes will likely take time to develop, and may or may not have an impact on the regulatory regime to which we are subject, we cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us.
Environmental Matters
We are subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, noise emissions, the use, transport, management and disposal of chemicals and hazardous materials, the import, export and registration of chemicals, and the cleanup of contaminated sites. Based on information currently available to us, we do not expect environmental costs and contingencies to have a material adverse effect on us. The operation of our business and facilities, however, entails risks in these areas. Significant expenditures could be required in the future to comply with environmental or health and safety laws, regulations or requirements.
In Israel, where our contract manufacturer produces all of our products, businesses storing or using certain hazardous materials (including materials necessary for our manufacturing process) are required, pursuant to the Israeli Dangerous Substances Law 5753-1993, to obtain a toxin permit from the Ministry of Environmental Protection.
In the European marketplace, electrical and electronic equipment is required to comply with the Directive on Waste Electrical and Electronic Equipment, which aims to prevent waste by encouraging reuse and recycling, and the Directive on Restriction of Use of Certain Hazardous Substances, which restricts the use of six hazardous substances in electrical and electronic products. Our products and certain components of such products “put on the market” in the EU (whether or not manufactured in the EU) are subject to these directives. Additionally, we are required to comply with certain laws, regulations and directives, including the Toxic Substances Control Act in the United States and REACH in the EU, governing chemicals. These and similar laws and regulations require the testing, reporting and registration of certain chemicals we use and ship. We believe we are in compliance in all material respects with applicable environmental laws and regulations.
14
Manufacturing
ReWalk includes off-the-shelf and custom-made components produced to our specifications by various third parties, for technical and cost effectiveness. We have contracted with Sanmina Corporation (“Sanmina”), a well-established contract manufacturer with expertise in the medical device industry, for the manufacture of all of our products. Pursuant to this contract, Sanmina manufactures SCI Products and ReStore at its facility in Ma’alot, Israel. All ReWalk Personal units are manufactured pursuant to the same set of specifications, and all ReWalk Rehabilitation units are manufactured pursuant to another set. We place our manufacturing orders with Sanmina pursuant to purchase orders or by providing forecasts for future requirements. We may terminate our relationship with Sanmina at any time upon written notice. Either we or Sanmina may terminate the relationship in the event of a material breach, subject to a 30-day cure period. Our agreement with Sanmina contains a limitation on liability that applies equally to both us and Sanmina.
We believe that this contract manufacturing relationship allows us to operate our business efficiently by focusing our internal efforts on the development and commercialization of our technology and our products and provides us with substantial scale-up capacity. We regularly test quality on-site at Sanmina’s facility and we obtain full quality inspection reports. We maintain a non-disclosure agreement with Sanmina.
We develop certain of the software components internally and license other software components that are generally available for commercial use as open source software.
We manufacture products based upon internal sales forecasts. We deliver products to customers and distributors based upon purchase orders received, and our goal is to fulfill each customer’s order for products in regular production within two weeks of receipt of the order.
Suppliers
We have contracted with Sanmina for the sourcing of all components and raw materials necessary for the manufacture of our products although there are instances that we purchase raw material ourselves. Components of our products and raw materials come from suppliers in the United States, Europe, China and Israel, and we depend on certain of these components and raw materials, including certain electronic parts, for the manufacture of our products. To date, we have not experienced significant volatility in the prices of these components and raw materials. However, such prices are subject to a number of factors, including purchase volumes, general economic conditions, currency exchange rates, industry cycles, production levels and scarcity of supply.
We believe that our and Sanmina’s facilities, our contracted manufacturing arrangement, and our supply arrangements are sufficient to support our potential capacity needs for the foreseeable future.
Employees
As of December 31, 2019, we had 50 employees (including full-time and hourly employees), of whom 19 were located in the United States, 20 were located in Israel and 11 were located in Europe. The majority of our employees are, and have been, engaged in sales and marketing and research and development activities. We do not employ a significant number of temporary or part time employees.
We are subject to labor laws and regulations within our locations mainly in the U.S., Germany and Israel. These laws and regulations principally concern matters such as pensions, paid annual vacation, paid sick days, length of the workday and work week, minimum wages, overtime pay, insurance for work-related accidents, severance pay and other conditions of employment. Our employees are not represented by a labor union. We consider our relationship with our employees to be good. To date, we have not experienced any work stoppages.
15
Financial Information about Geographic Areas and Significant Customer Information
The following table sets forth the geographical breakdown of our revenues for each of the years ended December 31, 2019 and 2018:
| Year Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Revenues based on customer’s location: | ||||||||
| Israel | $ | 2 | — | |||||
| United States | 2,003 | 3,558 | ||||||
| Europe | 2,832 | 2,807 | ||||||
| Asia-Pacific | 36 | 22 | ||||||
| Latin America | — | 58 | ||||||
| Africa | — | 100 | ||||||
| Total revenues | $ | 4,873 | $ | 6,545 | ||||
Additional discussion of financial information by reportable segment and geographic area and sales in excess of 10% of total revenues to certain of our customers is contained in Note 12 to our consolidated financial statements set forth in “Part II. Item 8. Financial Statements and Supplementary Data” of this annual report.
Recent Developments
| ● | 16 ReStore units placed since launch in June 2019; 12 in the U.S., 3 in Europe and 1 in Asia; |
| ● | 15 SCI units and 10 ReStore units were placed during the fourth quarter of 2019; |
| ● | 2019 gross margin improved to 56% compared to 43% in 2018. |
| ● | Completed and pending German insurance contracts establish the implementation procedures for coverage of German spinal cord population |
| ● | Raised $7.0 million in gross proceeds in a “best efforts” offering in February 2020; |
16
Our business faces significant risks. You should carefully consider all of the information set forth in this annual report and in our other filings with the SEC, including the following risk factors which we face and which are faced by our industry. Our business, financial condition and results of operations could be materially and adversely affected by any of these risks. In that event, the trading price of our ordinary shares would likely decline and you might lose all or part of your investment. This report also contains forward-looking statements that involve risks and uncertainties. Our results could materially differ from those anticipated in these forward-looking statements, as a result of certain factors including the risks described below and elsewhere in this report and our other SEC filings. See also “Special Note Regarding Forward-Looking Statements” on page (ii).
Risks Related to Our Business and Our Industry
We have concluded that there are substantial doubts as to our ability to continue as a going concern.
As of December 31, 2019, we had an accumulated deficit in the total amount of approximately $168.5 million, and anticipate further losses in the development of our business. Those factors raise substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern depends upon our obtaining the necessary financing to meet our obligations and timely repay our liabilities arising from normal business operations. The financial statements have been prepared assuming that we will continue to operate as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Our auditors also included an explanatory paragraph to their audit opinion relating to our accompanying consolidated financial statements for the fiscal year ended December 31, 2019 regarding the substantial doubts about the Company’s ability to continue as a going concern. If we are unable to secure additional capital, we may be required to take additional measures to reduce costs in order to conserve our cash in amounts sufficient to sustain operations and meet our obligations. If we become insolvent, investors in our securities may lose the entire value of their investment in our business. The accompanying financial statements do not include any adjustments that may be necessary should we be unable to continue as a going concern, and it is not possible for us to predict at this time the potential success of our business.
We may not have sufficient funds to meet certain future capital requirements, which could impair our efforts to develop and commercialize existing and new products, and may need to take advantage of various forms of capital-raising transactions, future equity financings, strategic transactions or borrowings may also further dilute our shareholders or place us under restrictive covenants limiting our ability to operate.
We intend to finance operating costs over the next 12 months with existing cash on hand, issuances of equity and/or debt securities, and other future public or private issuances of securities, or through a combination of the foregoing. Through equity transactions completed in February 2019, April 2019 and June 2019, we have raised in the aggregate approximately $24.6 million in gross proceeds. However, we will need to seek additional sources of financing if we require more funds than anticipated during the next 12 months or in later periods, including if we cannot make our loan repayments under our loan agreement with Kreos Capital V (Expert Fund) Limited, or Kreos, or if we cannot raise sufficient funds from equity issuances, including through this offering. The alternative capital-raising transactions we may seek may entail significant downsides, due to limitations on use of our Form S-3 and under our at-the-market offering program with Piper Jaffray & Co., or the ATM Offering Program, and our inability to rely on our investment agreement with Timwell Corporation Limited as an ongoing source of liquidity. For more information on our inability to use Form S-3, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Equity Raises.” For more information on our inability to use our investment agreement with Timwell Corporatoin Limited as a liquidity source, see “We no longer believe that we can reach agreement to close the remaining tranches of our Investment Agreement with Timwell, and there is a significant risk that we will not agree to modify the original understandings reflected in the agreement.”
To raise additional capital in the public markets, including taking into account the limitations on our Form S-3 use above, we will likely be required to seek and are currently actively seeking other methods, such as the registration statement on Form S-1 that was filed in January 2020. The preparation of a registration statement on Form S-1 is more time-consuming and costly. We may also conduct fundraising transactions in the form of private placements, potentially with registration rights or priced at a discount to the market value of our ordinary shares, which could require shareholder approval under the rules of Nasdaq, or other equity raise transactions such as equity lines of credit. In addition to entailing increased capital costs, any such transactions could result in substantial dilution of our shareholders’ interests, transfer control to a new investor and diminish the value of an investment in our ordinary shares.
17
We may also need to pursue strategic transactions, such as joint ventures, in-licensing transactions or the sale of our business or all or substantially all of our assets. These private financings and strategic transactions have in the past and could in the future require significant management attention, disrupt our business, adversely affect our financial results, be unsuccessful or fail to achieve the desired results. We are in discussions routinely with such possible sources of additional funding. As another alternative, we may seek to refinance up to a substantial portion of our indebtedness under our Kreos Loan Agreement, which we have considered with Kreos from time to time, including by exchanging our indebtedness with Kreos for new convertible debt from a third-party investor, or to borrow additional funds. Agreements governing any borrowing arrangement may contain covenants that could restrict our operations.
Overall, if we cannot raise the required funds, or cannot raise them on terms acceptable to us or investors, we may be forced to curtail substantially our current operations or cease operations altogether. Further, external perceptions regarding our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations or require us to obtain financing on terms that are more favorable to investors, and could result in the loss of confidence by investors and suppliers. As such, our failure to continue as a going concern could harm our business, operating results and financial position and severely affect the value of your investment.
While we have regained compliance with the quantitative continued listing rules of the Nasdaq Capital Market, we may not be able to maintain the listing of our ordinary shares on the Nasdaq Capital Market going forward, which could adversely affect our liquidity and the trading volume and market price of our ordinary shares.
As previously disclosed, on October 26, 2018, we received a notification letter from Nasdaq stating that, under Nasdaq Rule 5550(b), or Rule 5550(b), we failed to comply with the minimum $35 million market value of listed securities requirement for continued listing on the Nasdaq Capital Market as of October 26, 2018 and did not meet the rule’s alternative $2.5 million shareholders’ equity and $500,000 net income standards as of applicable balance sheet and income statement dates. On February 25, 2019, we completed a follow-on public offering of ordinary shares, and on April 5, 2019, we completed a registered direct offering of ordinary shares and a concurrent private placement of warrants to purchase ordinary shares. As a result of these transactions, Nasdaq informed us on April 25, 2019 that we had regained compliance with Rule 5550(b) regarding the $2.5 million shareholders’ equity requirement for continued listing.
Although we have regained compliance with Rule 5550(b), with shareholders’ equity of approximately $10.8 million as of December 31, 2019, Nasdaq will continue to monitor our ongoing compliance with the shareholders’ equity requirement. If our quarterly or annual report for a subsequent fiscal period does not evidence such compliance, we may become immediately subject to delisting without a cure period. We would be permitted to appeal any delisting determination to a Nasdaq Hearings Panel, and our ordinary shares would remain listed on the Nasdaq Capital Market pending the panel’s decision after the hearing. If we do not appeal the delisting determination or do not succeed in such an appeal, our ordinary shares would be removed from trading on the Nasdaq Capital Market.
As explained in our compliance plan submitted to Nasdaq, in an effort to maintain compliance with Rule 5550(b), we intend to maintain lower expenses and sufficient cash balances. However, if we cannot maintain the requisite cash levels for a compliant amount of shareholders’ equity, our ordinary shares may be at serious risk of immediate delisting. Any delisting determination could seriously decrease or eliminate the value of an investment in our ordinary shares and other securities linked to our ordinary shares. While an alternative listing on an over-the-counter exchange could maintain some degree of a market in our ordinary shares, we could face substantial material adverse consequences, including, but not limited to, the following: limited availability for market quotations for our ordinary shares; reduced liquidity with respect to our ordinary shares; a determination that our ordinary shares are “penny stock” under SEC rules, subjecting brokers trading our ordinary shares to more stringent rules on disclosure and the class of investors to which the broker may sell the ordinary shares; limited news and analyst coverage, in part due to the “penny stock” rules; decreased ability to issue additional securities or obtain additional financing in the future; and potential breaches under or terminations of our agreements with current or prospective large shareholders, strategic investors and banks. The perception among investors that we are at heightened risk of delisting could also negatively affect the market price of our securities and trading volume of our ordinary shares.
In addition, we may not be able to comply with the requirement for continued listing under Nasdaq Listing Rule 5550(a) to maintain a minimum bid price of $1 per share. If our closing bid price is less than $1 per share for 30 consecutive business days, we will be deficient with Rule 5550(a). Our closing bid price has been below $1 per share since February 6, 2020. In case of non-compliance, there can be no assurance that we will be able to regain compliance with the applicable rules.
If we fail to regain compliance with Rule 5550(a) during that rule’s applicable cure period, Nasdaq will notify us that our ordinary shares are subject to delisting. We would then be permitted to appeal any delisting determination to a Nasdaq Hearings Panel. Our ordinary shares would remain listed on the Nasdaq Capital Market pending the panel’s decision after the hearing. If we do not appeal the delisting determination or do not succeed in such an appeal, we may list our ordinary shares on an over-the-counter exchange. Any such delisting determination could seriously decrease or eliminate the value of an investment in our ordinary shares and other securities linked to our ordinary shares. While a listing on an over-the-counter exchange could maintain some degree of a market in our ordinary shares, we could face substantial material adverse consequences, including, but not limited to, the following: limited availability for market quotations for our ordinary shares; reduced liquidity with respect to and decreased trading prices of our ordinary shares; a determination that our ordinary shares are “penny stock” under SEC rules, subjecting brokers trading our ordinary shares to more stringent rules on disclosure and the class of investors to which the broker may sell the ordinary shares; limited news and analyst coverage for our Company, in part due to the “penny stock” rules; decreased ability to issue additional securities or obtain additional financing in the future; and potential breaches under or terminations of our agreements with current or prospective large shareholders, strategic investors and banks. The perception among investors that we are at heightened risk of delisting could also negatively affect the market price of our securities and trading volume of our ordinary shares.
18
We no longer believe that we can reach agreement to close the remaining tranches of our Investment Agreement with Timwell, and there is a significant risk that we will not agree to modify the original understandings reflected in the agreement. This failure to receive the proceeds of the remaining closings under the Investment Agreement could significantly and adversely impact our liquidity and our financial condition.
In March 2018, we entered into the Investment Agreement with Timwell. The first tranche of the investment, representing 160,000 shares, closed in exchange for gross proceeds of $5.0 million in May 2018. The Investment Agreement contemplates prospective issuances of 480,000 remaining ordinary shares to Timwell in exchange for gross proceeds of $15.0 million, subject to specified closing conditions. These proceeds would, represent a significant source of liquidity for the Company. Additionally, to the extent formed, the minimum payments owed by the China JV to us would be expected to provide us with a source of ongoing income to supplement our other then-available capital resources.
While we have pursued actively the steps necessary to fulfill all closing conditions to the remaining two tranches under the Investment Agreement, we have experienced significant delays and difficulties working to form the China JV and to negotiate the required joint venture, license and supply agreement, which would be required for the second tranche closing for proceeds of $10 million. The second tranche closing was initially expected to occur by July 1, 2018 and the third tranche closing was initially expected to occur by December 31, 2018 and no later than April 1, 2019.
In light of the positions taken by Timwell during the negotiations on definitive joint venture and license agreements, we no longer believe that agreement can be reached on the basis of the original understandings reflected in our Investment Agreement with Timwell. We remain in dialogue with RealCan, Timwell’s affiliate, and we are discussing with RealCan various alternative pathways to commercialize our products in China. Due to the various delays in the process and other barriers to closing we also currently see a significant risk that we will not reach agreement with RealCan on a modification of the original agreement. The failure to close any or all of the remaining two tranches could significantly and adversely impact our liquidity and financial condition, requiring us to find additional sources of liquidity on reasonable terms as a replacement. As we continue to view China as a market with key opportunities for products designed for stroke patients, we are also evaluating potential relationships with other groups to penetrate the Chinese market. However, downturns in trade between the United States and China and the impact of public health epidemics like the coronavirus could have an adverse effect on our ability to penetrate the Chinese market and a material impact on the success of any ventures in China. For more information, see “Part I, Item 1A. Risk Factors—Risks Related to Our Business and Our Industry—We are subject to certain regulatory regimes that may affect the way that we conduct business internationally, and our failure to comply with applicable laws and regulations could materially adversely affect our reputation and result in penalties and increased costs.”
To the extent that the non-completion of the second and third tranches causes us to modify or terminate any arrangements with Timwell, we could face further financial losses stemming from threatened or actual claims brought against us and/or reputational harm. Although no such claims have been asserted to date, we cannot make any assurance that we will not face them in the future.
We rely on sales of our ReWalk and ReStore systems and related service contracts and extended warranties for our revenue. We may not be able to achieve or maintain market acceptance of our ReWalk or ReStore systems, or to generate sufficient revenues from these current and future products.
We currently rely, and expect in the future to rely, on sales of our ReWalk and ReStore systems and related service contracts and extended warranties for our revenue. We began marketing in 2019 in the United States and the EU (following the receipt of FDA and CE mark clearance) the ReStore lightweight soft suit exoskeleton, which is designed to support mobility for individuals suffering from other lower limb disabilities. Several factors could negatively affect our ability to achieve and maintain market acceptance of our ReWalk system or our ReStore system, which could in turn materially impair our business, financial condition and operating results.
| ● | ReWalk. We have sold only a limited number of ReWalk systems, and market acceptance and adoption depend on educating people with limited upright mobility and health care providers as to the distinct features, ease-of-use, positive lifestyle impact and other benefits of ReWalk compared to alternative technologies and treatments. ReWalk may not be perceived to have sufficient potential benefits compared with these alternatives. Users may also choose other therapies due to disadvantages of ReWalk, including the time it takes for a user to put on ReWalk, the slower pace of ReWalk compared to a wheelchair, the weight of ReWalk when carried, which makes it more burdensome for a companion to transport than a wheelchair, and the requirement that users be accompanied by a trained companion. Also, we believe that healthcare providers tend to be slow to change their medical treatment practices because of perceived liability risks arising from the use of new products and the uncertainty of third-party reimbursement. Accordingly, healthcare providers may not recommend ReWalk until there is sufficient evidence to convince them to alter the treatment methods they typically recommend, such as prominent healthcare providers or other key opinion leaders in the spinal cord injury community recommending ReWalk as effective in providing identifiable immediate and long-term health benefits. |
19
In addition, we may be unable to sell on a profitable basis current ReWalk systems or other future products for home and community use if third-party payors deny coverage, limit reimbursement or reduce their levels of payment, or if our costs of production increase faster than increases in reimbursement levels. Several private and national insurers in the United States and Europe have provided reimbursement for ReWalk in certain cases to date, the VA maintains its policy of covering the cost of ReWalk devices for qualifying veterans across the United States and German insurers such as Germany’s national social accident insurance provider, Deutsche Gesetzliche Unfallversicherung, or DGUV, indicated that its member payers will approve the supply of exoskeleton systems for qualifying beneficiaries on a case-by-case basis.as the ReWalk device was issued a code in the medical device directory in Germany, however, no broad uniform policy of coverage and reimbursement for electronic exoskeleton medical technology exists among third-party payors in the United States and Germany. Health insurance companies and other third-party payors in the future may also not deliver adequate coverage or reimbursement for our current or future products designed for home and community use. The VA or DGUV may cancel or materially curtail their current policy of providing coverage ReWalk devices in the United States and Germany for qualifying individuals who have suffered spinal cord injury, or we may not place enough units through to make our sales profitable under the their policies. For more information, see “Part I, Item 1A. Risk Factors—Risks Related to our Business and our Industry— We may fail to secure or maintain adequate insurance coverage or reimbursement for our products by third-party payors, which risk may be heightened if insurers find the products to be investigational or experimental or if new government regulations change existing reimbursement policies. Additionally, such coverage or reimbursement, even if maintained, may not produce revenues that are high enough to allow us to sell our products profitably.”
| ● | ReStore. The ReStore system is designed to provide advantages to stroke rehabilitation clinics and therapists as compared to other traditional therapies and devices by minimizing setup time, improving patients’ clinical results during therapy, supplying real-time analytics to optimize session productivity and generating ongoing data reports to assist with tracking patient progress. Other potential secondary benefits for rehabilitation clinics include reducing staffing requirements, staff fatigue and the risk for potential staff injuries. Since the ReStore device is currently being used only in the rehabilitative clinical setting, its market reception will depend heavily on our ability to demonstrate to clinics and therapists the systemic and economic benefits of using the ReStore device, its clinical advantage when compared to other devices or manual therapy, the functionality of the device for a significant portion of the patients that they treat and the overall advantages that the device provides to their patients compared to other technologies. |
As a general matter, achieving and maintaining market acceptance of our current or future products could be negatively impacted by many other factors, including, but not limited to the following: contribution to death or serious injury or malfunction, results of clinical studies relating to our or similar products; claims that our products, or any of their components, infringe on patent or other intellectual property rights of third parties; our ability to support financially and leverage our sales, marketing and training infrastructure, as well as our level of research and development efforts; our ability to enhance and broaden our research and development efforts and product offerings in response to the evolving demands of people with paraplegia and lower limb disability and healthcare providers; our estimates regarding our current or future addressable market; perceived risks associated with the use of our products or similar products or technologies; the introduction of new competitive products or greater acceptance of competitive products; adverse regulatory or legal actions relating to our products or similar products or technologies; and problems arising from the outsourcing of our manufacturing capabilities, or our existing manufacturing and supply relationships. Any or all of these factors could materially and negatively impact our business, financial condition and operating results.
The market for medical exoskeletons, including soft suit devices, remains relatively new and unproven, and important assumptions about the potential market for our current and future products may be inaccurate.
The market for medical exoskeletons, including lightweight exo-suit devices, remains relatively new and unproven. Accordingly, it is difficult to predict the future size and rate of growth of the market. We cannot be certain whether the market will continue to develop or if medical exoskeletons will achieve and sustain a level of market acceptance and demand sufficient for us to continue to generate revenue and achieve profitability.
We obtained FDA clearance for our ReWalk Personal device in June 2014. This clearance permits us to market the device for use by individuals with spinal cord injury at levels T7 to L5 and for use by individuals in rehabilitation institutions with spinal cord injury at levels T4 to L5. The FDA’s clearance requires users of the device to meet the following criteria: healthy hands and shoulders that can support crutches, healthy bone density, no skeletal fractures, in good general health, ability to stand with a stander device, weight of less than 220 pounds/100 kilograms and height between 5 feet 3 inches and 6 feet 2 inches/1.60 meters and 1.88 meters. Additionally, the FDA clearance contraindicates psychiatric or cognitive conditions that could interfere with a user’s proper operation of the device and various other clinical conditions, including pregnancy, severe concurrent medical diseases, a history of severe neurological injuries other than spinal cord injury, impaired joint mobility, unhealed limbs or pelvic fractures or unstable spine, severe spasticity and significant and chronic loss of joint mobility due to structural changes in non-bony tissue.
We obtained FDA clearance for our ReStore system in June 2019. This clearance permits us to market the device to be used to assist ambulatory functions in rehabilitation institutions for people with hemiplegia or hemiparesis due to stroke who can ambulate at least 1.5m (5ft) with no more than minimal to moderate levels of assistance. The FDA’s clearance requires users of the device to meet the following criteria: height between 4 feet 8 inches and 6 feet 3 inches/1.42 meters and 1.92 meters and weight of less than 264 pounds/120 kilograms. Additionally, the FDA clearance contraindicates persons with the following conditions should not use the Restore: serious co-morbidities that may interfere with ability to safely use ReStore, severe peripheral artery disease (PAD), unresolved deep vein thrombosis (DVT), range of motion (ROM) restrictions at the ankle that preclude safe walking, cognitive impairments that may interfere with safe operation of the device, presence of open wounds or broken skin at device locations, urethane allergy or current pregnancy.
20
Future products for those with paraplegia or other mobility impairments or spinal cord injuries, may have the same or other restrictions.
Our business strategy is based, in part, on our estimates of the number of mobility impaired individuals and the incurrence of spinal cord injuries and strokes in our target markets and the percentage of those groups that would be able to use our current and future products. Limited sources exist to obtain reliable market data with respect to the number of mobility-impaired individuals and the incurrence of spinal cord injuries and strokes in our target markets. In addition, there are no third-party reports or studies regarding what percentage of those with limited mobility, spinal cord injuries would be able to use exoskeletons, in general, or our current or planned future products, in particular. Our assumptions may be inaccurate and may change.
The National Spinal Cord Injury Statistical Center, or NSCISC, estimates that as of 2019 there were 291,000 people in the United States living with SCI, and that the annual incidence of SCI cases is approximately 17,730 new cases per year. Based on information from a 2017 report by the NSCISC, 40.6% of the total U.S. population of SCI patients suffered injuries between levels T4 and L5. Three published ReWalk trials with respect to such eligible SCI patients had an aggregate screening acceptance rate of 79% considering all current FDA limitations, resulting in an estimated 33% of the total population of SCI patients being candidates for current ReWalk products under its medical labeling criteria. With regards to our ReStore product for stroke rehabilitation, as the indication of use is currently in rehabilitation clinics our target market is based on the number of current and future clinics who treat stroke patients. Although there are thousands of inpatient, outpatient, skilled nursing facilities and rehabilitation clinics providing therapy in the U.S. for example we believe that only a portion of the clinics will decide to include ReStore in their stroke rehab program. For more information on our expectations regarding these plans, see “-Our future growth and operating results will depend on our ability to develop and commercialize new products and penetrate new markets” below. For more information regarding the potential market for future products, including our lightweight soft suit exoskeleton, see “Business—ReWalk Personal and ReWalk Rehabilitation Products—Market Opportunity.”
We cannot assure you that our estimate regarding our current products is accurate or that our estimate regarding future products will remain the same. FDA or CE mark clearance for such products, if received at all, may contain different limitations from the ones the FDA or EU has placed on the devices we currently market for paraplegia. If our estimates of our current or future addressable market are incorrect, our business may not develop as we expect and the price of our securities may suffer.
We may fail to secure or maintain adequate insurance coverage or reimbursement for our products by third-party payors, which risk may be heightened if insurers find the products to be investigational or experimental or if new government regulations change existing reimbursement policies. Additionally, such coverage or reimbursement, even if maintained, may not produce revenues that are high enough to allow us to sell our products profitably.
We expect that in the future a significant source of payment for ReWalk systems will be private insurance plans and managed care programs, government programs such as the VA, Medicare and Medicaid, worker’s compensation and other third-party payors. We have similar expectations for our ReStore product, although we possess less information regarding the payment by third-party payors for this product as we only began commercializing it in 2019.
In December 2015, the VA issued a national reimbursement policy for the ReWalk system, which entails the evaluation, training and procurement of ReWalk Personal exoskeleton systems for all qualifying veterans across the United States. Additionally, in September 2017, German insurer BARMER GEK (“Barmer”) signed a confirmation and letter of agreement regarding the provision of ReWalk systems for all qualifying beneficiaries and the German national social accident insurance provider DGUV indicated that its member payers will approve the supply of exoskeleton systems for qualifying beneficiaries on a case-by-case basis. However, no broad uniform policy of coverage and reimbursement for electronic exoskeleton medical technology exists among third-party payors in the United States, although reimbursement may be achieved on a case-by-case basis. To date, payments for our products, which are largely for our ReWalk systems, have been made primarily through case-by-case determinations by third-party payors (including several private insurers in the United States), by self-payors and, to a lesser extent, through the use of funds from insurance and/or accident settlements.
Generally, private insurance companies do not cover or provide reimbursement for any medical exoskeleton products for personal use, including ReWalk Personal, and may ultimately provide no coverage at all. For instance, during 2017 we submitted a proposal to a large U.S. national insurance provider for a broader coverage policy for the ReWalk Personal device. While we believe there was support for a change, the insurer was unable to reach internal consensus and therefore elected not change its existing non-coverage policy. Additionally, there is limited clinical data related to the ReWalk and ReStore systems, and third-party payors may consider use of them to be experimental and therefore refuse to cover any or all of them. For example, Aetna has determined that certain lower-limb prostheses, including ReWalk, are experimental and investigational because there is inadequate evidence of their effectiveness. Additionally, the majority of independent medical review decisions made following the denial of ReWalk coverage have determined that ReWalk is experimental and/or investigational, citing a lack of clinical data.
21
Many private third-party payors use coverage decisions and payment amounts determined by the Center for Medicare and Medicaid Services, or the CMS, which administers the Medicare program, as guidelines in setting their coverage and reimbursement policies. We have started the process of obtaining reimbursement coverage from CMS; however, while we believe that a positive response from CMS will broaden coverage by private insurers, we cannot currently predict how long it would take for us to receive a decision from CMS for any of our products nor can we predict other business elements that will be decided by CMS such as the price per unit or product labeling requirements. Even with a positive decision from CMS regarding a product of ours, future action by CMS or other government agencies may diminish possible payments to physicians, outpatient centers and/or hospitals that purchase our products for use by their patients and possible payments to individuals who purchase the ReWalk Personal for their own use. Additionally, a decision by CMS to provide reimbursement could influence other payors, including private insurers. If CMS declines to provide for reimbursements of our products or if its reimbursement price is lower than that of other payors, our products may not be reimbursed at a cost-effective level or at all. Those private third-party payors that do not follow the Medicare guidelines may adopt different coverage and reimbursement policies for purchase of our products or their use in a hospital or rehabilitative setting. In addition, we expect that the purchase of ReWalk Rehabilitation systems and the ReStore system, as it is currently being sold for use in rehabilitative settings, will require the approval of senior management at hospitals or rehabilitation facilities, inclusion in the hospitals’ or rehabilitation facilities’ budget process for capital expenditures, and in the case of ReWalk Personal, fundraising and financial planning or assistance.
Third-party payors are developing increasingly sophisticated methods of controlling healthcare costs. These cost control methods include prospective payment systems, capitated rates, benefit redesigns and an exploration of other cost-effective methods of delivering healthcare. These cost control methods potentially limit the amount that healthcare providers may be willing to pay for electronic exoskeleton medical technology, if they provide coverage at all. We may be unable to sell our products on a profitable basis if third-party payors deny coverage or provide insufficient levels of reimbursement.
Future legislation could result in modifications to the existing public and private health care insurance systems that would have a material adverse effect on the reimbursement policies discussed above. If enacted and implemented, any measures to restrict health care spending could result in decreased revenue from our products and decrease potential returns from our research and development initiatives.
We have a limited operating history upon which you can evaluate our business plan and prospects.
Although we were incorporated in 2001, we did not begin selling ReWalk Rehabilitation until 2011, and we did not begin selling ReWalk Personal in Europe until 2012. We began selling ReWalk Personal in the United States in the third quarter of 2014, as we received FDA clearance to do so in June 2014. We began selling our ReStore product in the United States and Europe in June 2019 following receipt of FDA and CE mark clearance, respectively. Therefore, we have limited operating history upon which you can evaluate our business plan and prospects. Our business plan and prospects must be considered in light of the potential problems, delays, uncertainties and complications encountered in connection with a more newly established business. The risks include, but are not limited to, that:
| ● | a market will not sufficiently develop for our products; | |
| ● | we will not be able to develop scalable products and services, or that, although scalable, our products and services will not be economical to market; | |
| ● | we will not be able to establish brand recognition and competitive advantages for our products; | |
| ● | we will not receive necessary regulatory clearances or approvals for our products; and | |
| ● | our competitors market an equivalent or superior product or hold proprietary rights that preclude us from marketing our products. |
There are no assurances that we can successfully address these challenges. If we are unsuccessful, our business, financial condition and operating results could be materially and adversely affected.
22
If we are unable to leverage our sales, marketing and training infrastructure, including in light of our reduced corporate spending, we may fail to increase our sales.
A key element of our long-term business strategy is the continued leveraging of our sales, marketing, training and reimbursement infrastructure, through the training, retaining and motivating of skilled sales and marketing representatives and reimbursement personnel with industry experience and knowledge. Our ability to derive revenue from sales of our products depends largely on our ability to market the products and obtain reimbursements for them. In order to continue growing our business efficiently, we must therefore coordinate the development of our sales, marketing, training and reimbursement infrastructure with the timing of regulatory approvals, decisions regarding reimbursements, limited resources consideration and other factors in various geographies. Managing and maintaining our sales and marketing infrastructure is expensive and time consuming, and an inability to leverage such an organization effectively, or in coordination with regulatory or other developments, could inhibit potential sales and the penetration and adoption of our products into both existing and new markets. However, certain decisions we make regarding staffing in these areas in our efforts to maintain an adequate spending level could have unintended negative effects on our revenues, such as by weakening our sales infrastructure, impairing our reimbursement efforts and/or harming the quality of our customer service. As we have done throughout the past several years, we intend to continue to evaluate our spending throughout 2020 and focus our resources in areas we believe will support our growth.
Additionally, we expect to face significant challenges as we manage and continue to improve our sales and marketing infrastructure and work to retain the individuals who make up those networks. Newly hired sales representatives require training and take time to achieve full productivity. If we fail to train new hires adequately, or if we experience high turnover in our sales force in the future, we cannot be certain that new hires will become as productive as may be necessary to maintain or increase our sales. In addition, if we are not able to retain, subject to our plans to cut operating expenses, and continue to recruit our network of internal trainers, we may not be able to successfully train customers on the use of ReWalk or ReStore, which could inhibit new sales and harm our reputation. If we are unable to expand our sales, marketing and training capabilities, we may not be able to effectively commercialize our products, or enhance the strength of our brand, which could have a material adverse effect on our operating results.
The health benefits of our products have not been substantiated by long-term clinical data, which could limit sales.
Although study participants and other ReWalk users have reported the secondary health benefits of our ReWalk products such as a reduction in pain and spasticity, improved bowel and urinary tract functions and emotional and psychosocial benefits, among others, currently there is no conclusive clinical data establishing any secondary health benefits of ReWalk. There is also a lack of conclusive clinical data for such health benefits of the ReStore specifically its long-term benefits following the usage of the product within the clinic and the trials conducted to date using this product are limited.
As a result, potential customers and healthcare providers may be slower to adopt or recommend ReWalk or ReStore and third-party payors may not be willing to provide coverage or reimbursement for our products. In addition, future studies or clinical experience may indicate that treatment with our current or future products is not superior to treatment with alternative products or therapies. Such results could slow the adoption of our products and significantly reduce our sales.
We depend on a single third party to manufacture our products, and we rely on a limited number of third-party suppliers for certain components of our products.
We have contracted with Sanmina Corporation, a well-established contract manufacturer with expertise in the medical device industry, for the manufacture of all of our products and the sourcing of all of our components and raw materials. Pursuant to this contract, Sanmina manufactures ReWalk and ReStore, pursuant to our specifications, at its facility in Ma’alot, Israel. We may terminate our relationship with Sanmina at any time upon written notice. In addition, either we or Sanmina may terminate the relationship in the event of a material breach, subject to a 30-day cure period. For our business strategy to be successful, Sanmina must be able to manufacture our products in sufficient quantities, in compliance with regulatory requirements and quality control standards, in accordance with agreed upon specifications, at acceptable costs and on a timely basis. Increases in our product sales, whether forecasted or unanticipated, could strain the ability of Sanmina to manufacture an increasingly large supply of our current or future products in a manner that meets these various requirements. In addition, although we are not restricted from engaging an alternative manufacturer, and potentially have the capabilities to manufacture our products in-house, the process of moving our manufacturing activities would be time consuming and costly, and may limit our ability to meet our sales commitments, which could harm our reputation and could have a material adverse effect on our business.
We also rely on third-party suppliers, which contract directly with Sanmina, to supply certain components of our products, and in some cases we purchase these components ourselves. Sanmina does not have long-term supply agreements with most of its suppliers and, in many cases, makes purchases on a purchase order basis. Sanmina’s ability to secure adequate quantities of such products may be limited. Suppliers may encounter problems that limit their ability to manufacture components for our products, including financial difficulties or damage to their manufacturing equipment or facilities. If Sanmina fails to obtain sufficient quantities of high quality components to meet demand on a timely basis, we could lose customer orders, our reputation may be harmed and our business could suffer.
23
Sanmina generally uses a small number of suppliers for ReWalk and ReStore. Depending on a limited number of suppliers exposes us to risks, including limited control over pricing, availability, quality and delivery schedules. If any one or more of our suppliers ceases to provide sufficient quantities of components in a timely manner or on acceptable terms, Sanmina would have to seek alternative sources of supply. It may be difficult to engage additional or replacement suppliers in a timely manner. Failure of these suppliers to deliver products at the level our business requires would limit our ability to meet our sales commitments, which could harm our reputation and could have a material adverse effect on our business. Sanmina also may have difficulty obtaining similar components from other suppliers that are acceptable to the FDA or other regulatory agencies, and the failure of Sanmina’s suppliers to comply with strictly enforced regulatory requirements could expose us to regulatory action including warning letters, product recalls, termination of distribution, product seizures or civil penalties. It could also require Sanmina to cease using the components, seek alternative components or technologies and we could be forced to modify our products to incorporate alternative components or technologies, which could result in a requirement to seek additional regulatory approvals. Any disruption of this nature or increased expenses could harm our commercialization efforts and adversely affect our operating results.
Our future growth and operating results will depend on our ability to develop, receive regulatory clearance for and commercialize new products and penetrate new product and geographic markets.
We are currently engaged in research and development efforts to address the needs of patients with mobility impairments besides paraplegia, such as stroke, and, in the future, we may engage in efforts to address these needs in patients with other conditions such as multiple sclerosis, cerebral palsy Parkinson’s disease and elderly assistance. We also began commercializing in 2019 our first product for stroke patients, the ReStore. For more information, see “Business—ReStore Products.” In addition to other research and development projects, we collaborate with Harvard University’s Wyss Institute for Biologically Inspired Engineering to design, research and develop lightweight exoskeleton system technologies for lower limb disabilities intended to treat stroke, multiple sclerosis, mobility limitations for the elderly and other medical applications. As part of the collaboration, Harvard has also licensed to us certain of its intellectual property relating to lightweight exoskeleton system technologies for lower limb disabilities. We are obligated to use commercially reasonable efforts to develop products under the license in accordance with an agreed-upon development plan and to introduce and market such products commercially.
We expect that a portion of our revenues will be derived, in the next few years, from the ReStore soft suit exoskeleton product and, in later years, from other new products such as a home use device for stroke patients or new products of ours aimed at addressing other medical indications which affect the ability to walk, including multiple sclerosis, cerebral palsy, Parkinson’s disease and elderly assistance. As such, our future results will depend on our ability to successfully develop and commercialize such new products. We cannot ensure you that we will be able to introduce new products, products currently under development and products contemplated for future development for additional indications in a timely manner, or at all as it depends on our available resources to fund such projects While we received governmental clearance to market our ReStore product on the anticipated timetable in 2019, obtaining clearance for any other soft suit exoskeleton products we may develop could involve an extensive, costly and time-consuming process, which would delay any planned commercialization. For more information on the clearance processes, see “Business—Government Regulation.”
Harvard may also terminate its license agreement with us if we fail to obtain the requisite insurance or become insolvent. Any such termination of this aspect of the collaboration with Harvard could impair our research and development efforts into lightweight soft suit exoskeleton system technologies for lower limb disabilities. In addition, we may not be able to clinically demonstrate the medical benefits of our products for new indications. We have limited clinical data demonstrating the benefits of our products and we might not be able to support the economic benefits our products have for the customer. We may also be unable to gain necessary regulatory approvals to enable us to market new products for additional indications or the regulatory process may be more costly and time-consuming than expected, which could adversely impact us given our cash position and ongoing capital requirements. We might also terminate or change our research collaboration agreement with Harvard if we see limited market to the current developed products or seek to focus our available resources to other areas of the business.
Even if we are successful in the design and development of new products, our growth and results of operations will depend on our ability to penetrate new markets and gain acceptance by non-SCI markets such as the stroke rehabilitation market, and, in the longer term, the home use device market for stroke-caused lower limb disability, multiple sclerosis, elderly assist and cerebral palsy patients. We may not be able to gain such market acceptance in these communities in a timely manner, or at all.
While our new products currently under development will share some aspects of the core technology platform in our current products, their design features and components may differ from our current products. Accordingly, these products will also be subject to the risks described under “We rely on sales of our ReWalk and ReStore systems and related service contracts and extended warranties for our revenue. We may not be able to achieve or maintain market acceptance or generate sufficient revenues from such contracts.” To the extent we are unable to successfully develop and commercialize products to address indications other than paraplegia, we will not meet our projected results of operations and future growth.
24
We operate in a competitive industry that is subject to rapid technological change, and we expect competition to increase.
There are several other companies developing technology and devices that compete with our products. Our principal competitors in the medical exoskeleton market consist of Ekso Bionics, Parker Hannifin, FREE Bionics, Rex Bionics, Cyberdyne, and others. These companies have products currently available for institutional use and in some cases personal use. We expect some of such products to become available for personal use in the next few years. In addition, we compete with alternative devices and alternative therapies, including treadmill-based gait therapies, such as those offered by Hocoma, AlterG, Aretech, Reha Technology and Bioness. Our competitor base may change or expand as we continue to develop and commercialize our soft suit exoskeleton product in the future. These or other medical device or robotics companies, academic and research institutions, or others, may develop new technologies or therapies that provide a superior walking experience, are more effective in treating the secondary medical conditions that we target or are less expensive than ReWalk, ReStore or future products. Our technologies and products could be rendered obsolete by such developments. We may also compete with other treatments and technologies that address the secondary medical conditions that our products seek to mitigate.
Our competitors may respond more quickly to new or emerging technologies, undertake more extensive marketing campaigns, have greater financial, marketing and other resources than we do or may be more successful in attracting potential customers, employees and strategic partners. In addition, potential customers, such as hospitals and rehabilitation centers, could have long-standing or contractual relationships with competitors or other medical device companies. Potential customers may be reluctant to adopt ReWalk or ReStore, particularly if it competes with or has the potential to compete with or diminish the need/utilization of products or treatments supported through these existing relationships. If we are not able to compete effectively, our business and results of operations will be negatively impacted.
In addition, because we operate in a new market, the actions of our competitors could adversely affect our business. Adverse events such as product defects or legal claims with respect to competing or similar products could cause reputational harm to the exoskeleton market on the whole. Further, adverse regulatory findings or reimbursement-related decisions with respect to other exoskeleton products could negatively impact the entire market and, accordingly, our business.
In the event that we default under the Loan Agreement with Kreos, Kreos could foreclose on its lien and take possession over all of our assets.
On December 30, 2015, we entered into the Loan Agreement with Kreos, pursuant to which Kreos extended a line of credit to us in the amount of $20.0 million. On January 4, 2016, we drew down $12.0 million and on December 28, 2016, we drew down the remaining $8.0 million. The principal amount of each drawdown was initially repayable monthly over a period of 24 months commencing 12 months after the applicable drawdown date, which period would be extended to 36 months if we raise $20.0 million or more in connection with the issuance of shares of our capital stock (including debt convertible into shares of our capital stock) before the respective 24-month period expires. Interest on each drawdown is payable monthly in arrears at a rate of 10.75% per year from the applicable drawdown date through the date on which all such principal is repaid. In mid-2017, the Company had raised more than $20.0 million and therefore the repayment period was extended by an additional 12 months to 36 months. On November 20, 2018, the Company and Kreos amended the Loan Agreement, where the Company repaid Kreos the $3.6 million covering a convertible note under the loan and “end of loan” payments by issuing equity interest to Kreos as part of the Company’s public offering. The Company and Kreos also agreed to revise the principal and the repayment schedule under the Kreos Loan Agreement to account for payment deferrals, for total deferred payments of $3.9 million compared to the prior repayment schedule. As of December 31, 2019, the outstanding principal amount under the Kreos Loan Agreement was $7.0 million. We may in the future choose to refinance up to a substantial portion of our remaining indebtedness under the Kreos Loan Agreement, including by tying our repayment obligations and amortization schedule to the achievement of certain business milestones, which we have considered with Kreos from time to time.
Pursuant to the Loan Agreement, we granted Kreos a first priority security interest over all of our assets, including certain intellectual property and equity interests in our subsidiaries, subject to certain permitted security interests. For more information, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” and our consolidated financial statements and the related notes thereto in this annual report. In the event that we are unable to make the interest payments when due under the Loan Agreement or to pay the outstanding principal amount following the termination of the Loan Agreement, Kreos could take actions under the Loan Agreement and seek to take possession of or sell our assets to satisfy our obligations thereunder. Any of these actions would have an immediate material adverse effect on our business, operating results and financial condition.
25
We utilize independent distributors who are free to market products that compete with ours.
While we expect that the percentage of our sales generated from independent distributors will decrease over time as we continue to focus our resources on achieving reimbursement within our direct markets in the United States and Europe, we believe that a meaningful percentage of our sales will continue to be generated by independent distributors in the future. None of our independent distributors has been required to sell our products exclusively. Our distributor agreements generally have one-year initial terms and automatic renewals for an additional year. If any of our key independent distributors were to cease to distribute our products, our sales could be adversely affected. In such a situation, we may need to seek alternative independent distributors or increase our reliance on our other independent distributors or our direct sales representatives, which may not prevent our sales from being adversely affected. Additionally, to the extent that we enter into additional arrangements with independent distributors to perform sales, marketing, or distribution services, the terms of the arrangements could cause our product margins to be lower than if we directly marketed and sold our products.
We are dependent on a single facility for the manufacturing and assembly of our products.
All manufacturing and assembly of our products is conducted at a single facility of our contract manufacturer, Sanmina, located in Ma’alot, Israel. Accordingly, we are highly dependent on the uninterrupted and efficient operation of this facility. If operations at this facility were to be disrupted as a result of equipment failures, earthquakes and other natural disasters, fires, accidents, work stoppages, power outages, acts of war or terrorism or other reasons, our business, financial condition and results of operations could be materially adversely affected. In particular, this facility is located in the north of Israel within range of rockets that have from time to time been fired into the country during armed conflicts with Hezbollah and other armed groups in Lebanon, Syria or other countries in the region. Although our manufacturing and assembly operations could be transferred elsewhere, either in-house or to an alternative Sanmina facility, the process of relocating these operations would cause delays in production. Lost sales or increased costs that we may experience during the disruption, or a forced relocation, of operations may not be recoverable under our insurance policies, and longer-term business disruptions could result in a loss of customers. If this were to occur, our business, financial condition and operations could be materially negatively impacted. Additionally, our reliance on Sanmina as a contract manufacturer or any other contract manufacturer makes us vulnerable to possible capacity constraints and reduced control over component availability, delivery schedules, manufacturing yields and costs.
We may receive a significant number of warranty claims or our ReWalk and ReStore systems may require significant amounts of service after sale.
Sales of ReWalk generally include a two-year warranty for parts and services, other than for normal wear and tear. We also provide customers with the option to purchase an extended warranty for up to an additional three years. In the beginning of 2018 we updated our service policy for new devices sold to include a 5-year warranty. Our ReStore product offering includes a two-year warranty for parts and services. If product returns or warranty claims are significant or exceed our expectations, we could incur unanticipated expenditures for parts and services, which could have a material adverse effect on our operating results.
Defects in our products or the software that drives them could adversely affect the results of our operations.
The design, manufacture and marketing of our products involve certain inherent risks. Manufacturing or design defects, unanticipated use of ReWalk or ReStore, or inadequate disclosure of risks relating to the use of our products can lead to injury or other adverse events. In addition, because the manufacturing of our products is outsourced to Sanmina, our original equipment manufacturer, we may not be aware of manufacturing defects that could occur. Such adverse events could lead to recalls or safety alerts relating to our products (either voluntary or required by the FDA or similar governmental authorities in other countries), and could result, in certain cases, in the removal of our products from the market. A recall could result in significant costs. To the extent any manufacturing defect occurs, our agreement with Sanmina contains a limitation on Sanmina’s liability, and therefore we could be required to incur the majority of related costs. Product defects or recalls could also result in negative publicity, damage to our reputation or, in some circumstances, delays in new product approvals.
When an exoskeleton is used by a paralyzed individual to walk, the individual relies completely on the exoskeleton to hold him or her upright. In addition, our products incorporate sophisticated computer software. Complex software frequently contains errors, especially when first introduced. Our software may experience errors or performance problems in the future. If any part of our product’s hardware or software were to fail, the user could experience death or serious injury. Additionally, users may not use our products in accordance with safety protocols and training, which could enhance the risk of death or injury. Any such occurrence could cause delay in market acceptance of our products, damage to our reputation, additional regulatory filings, product recalls, increased service and warranty costs, product liability claims and loss of revenue relating to such hardware or software defects.
The medical device industry has historically been subject to extensive litigation over product liability claims. We have been, and anticipate that as part of our ordinary course of business we may be, subject to product liability claims alleging defects in the design, manufacture or labeling of our products. A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs and high punitive damage payments. Although we maintain product liability insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims. Additionally, we may be unable to maintain our existing product liability insurance in the future at satisfactory rates or adequate amounts.
26
We may not be able to enhance our product offerings through our research and development efforts.
In order to increase our sales and our market share in the exoskeleton market, it is best to enhance and broaden our research and development efforts and product offerings in response to the evolving demands of people with paraplegia or paralysis and healthcare providers, as well as competitive technologies. We are also currently involved in ongoing research and development efforts directed to the needs of patients with other mobility impairments, such as stroke, and began commercializing our ReStore product for stroke patients in 2019. Depending on our future resources and business focus, we plan to address these needs in patients with other conditions or devices for stroke patients to be used at home, improving our current products, or developing products to address additional medical conditions such as multiple sclerosis, Parkinson’s disease or cerebral palsy and support elderly assistance. We may not be successful in developing, obtaining regulatory approval for, or marketing our currently proposed products and products proposed to be created in the future. In addition, notwithstanding our market research efforts, our future products may not be accepted by consumers, their caregivers, healthcare providers or third-party payors who reimburse consumers for our products. The success of any proposed product offerings will depend on numerous factors, including our ability to:
| ● | identify the product features that people with paraplegia or paralysis, their caregivers and healthcare providers are seeking in a medical device that restores upright mobility and successfully incorporate those features into our products; | |
| ● | identify the product features that people with stroke, multiple sclerosis or other similar indications require while the products are used at home as well as what items are valuable to the clinics that provide them rehabilitation | |
| ● | develop and introduce proposed products in sufficient quantities and in a timely manner; | |
| ● | adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third-parties; | |
| ● | demonstrate the safety, efficacy and health benefits of proposed products; and | |
| ● | obtain the necessary regulatory approvals for proposed products. |
If we fail to generate demand by developing products that incorporate features desired by consumers, their caregivers or healthcare providers, or if we do not obtain regulatory clearance or approval for proposed products in time to meet market demand, we may fail to generate sales sufficient to achieve or maintain profitability. We have in the past experienced, and we may in the future experience, delays in various phases of product development, including during research and development, manufacturing, limited release testing, marketing and customer education efforts. Such delays could cause customers to delay or forgo purchases of our products, or to purchase our competitors’ products. Even if we are able to successfully develop proposed products when anticipated, these products may not produce sales in excess of the costs of development, and they may be quickly rendered obsolete by changing consumer preferences or the introduction by our competitors of products embodying new technologies or features.
We may enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships with third-parties that may not result in the development of commercially viable products or the generation of significant future revenues.
In the ordinary course of our business, we may enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships to develop our products and to pursue new geographic or product markets. Proposing, negotiating and implementing collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships may be a lengthy and complex process. We may not identify, secure, or complete any such transactions or arrangements in a timely manner, on a cost-effective basis, on acceptable terms or at all. We have limited institutional knowledge and experience with respect to these business development activities, and we may also not realize the anticipated benefits of any such transaction or arrangement. In particular, these collaborations may not result in the development of products that achieve commercial success or result in significant revenues and could be terminated prior to developing any products. For example, we have entered into arrangements with Yaskawa for the distribution of our products in certain Asian markets. We also collaborate with Harvard University’s Wyss Institute for Biologically Inspired Engineering for the research, design, development and commercialization of lightweight exoskeleton system technologies for lower limb disabilities, aimed to treat stroke, multiple sclerosis, mobility limitations for the elderly and other medical applications.
Additionally, we previously agreed to collaborate with RealCan, an affiliate of Timwell, in forming a joint venture in China for the purposes of assembly, registration, operations, sales, and marketing of our products in China (including Hong Kong and Macau), and to grant to the joint venture, an exclusive license for certain of our patent rights marks and a non-exclusive sublicense for certain Company-controlled know-how. We do not believe we will attain these milestones on the basis of the original understandings reflected in our Investment Agreement. Nevertheless, we remain in dialogue with RealCan, and we are discussing with RealCan various alternative pathways to commercialize our products in China, although we see a significant risk that we will not reach an agreement with Timwell and RealCan on a modification of the original agreement. For more information, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Timwell Private Placement.” Our arrangements with Yaskawa Electric Corporation, or Yaskawa, and Harvard, and any arrangement to commercialize products in China, to the extent developed, may not be as productive or successful as we hope.
27
Additionally, as we pursue these arrangements and choose to pursue other collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships in the future, we may not be in a position to exercise sole decision making authority regarding the transaction or arrangement. This could create the potential risk of creating impasses on decisions, and our collaborators may have economic or business interests or goals that are, or that may become, inconsistent with our business interests or goals. It is possible that conflicts may arise with our collaborators. Our collaborators may act in their self-interest, which may be adverse to our best interest, and they may breach their obligations to us. Any such disputes could result in litigation or arbitration which would increase our expenses and divert the attention of our management. Further, these transactions and arrangements are contractual in nature and may be terminated or dissolved under the terms of the applicable agreements.
Exchange rate fluctuations between the U.S. dollar, the Euro and the NIS may negatively affect our earnings.
The U.S. dollar is our functional and reporting currency. Since 2015, most of our revenues were denominated in U.S. dollars and the remainder of our revenues was denominated in euros and British pound, and most of our expenses were denominated in U.S. dollars and the remaining expenses were denominated in NIS and euros. Accordingly, any appreciation of the NIS or Euro relative to the U.S. dollar would adversely impact our net loss or net income, if any. For example, we are exposed to the risks that the shekel may appreciate relative to the dollar, or, if the shekel instead devalues relative to the dollar, that the inflation rate in Israel may exceed such rate of devaluation of the shekel, or that the timing of such devaluation may lag behind inflation in Israel. In any such event, the dollar cost of our operations in Israel would increase and our dollar-denominated results of operations would be adversely affected.
We cannot predict any future trends in the rate of inflation in Israel or the rate of devaluation (if any) of the shekel against the dollar. For example, while the shekel appreciated against the dollar at a rate of approximately 8% during the fiscal year of 2019, the rate of devaluation of the shekel against the dollar was approximately 7% in 2017. This had the effect of increasing the dollar cost of our operations in Israel. If the dollar cost of our operations in Israel increases once again, our dollar-measured results of operations will be adversely affected. Our operations also could be adversely affected if we are unable to effectively hedge against currency fluctuations in the future.
We have in the past engaged in limited hedging activities, and we may enter into other hedging arrangements with financial institutions from time to time. Any hedging strategies that we may implement in the future to mitigate currency risks, such as forward contracts, options and foreign exchange swaps related to transaction exposures may not eliminate our exposure to foreign exchange fluctuations. For further information, see “Part I, Item 1A. Risk Factors—The economic effects of ‘Brexit’ may affect relationships with existing and future customers and could have an adverse impact on our business and operating results.”
We are subject to certain regulatory regimes that may affect the way that we conduct business internationally, and our failure to comply with applicable laws and regulations could materially adversely affect our reputation and result in penalties and increased costs.
We are subject to a complex system of laws and regulations related to international trade, including economic sanctions and export control laws and regulations. We also depend on our distributors and agents for compliance and adherence to local laws and regulations in the markets in which they operate. Significant political or regulatory developments in the jurisdictions in which we sell our products, such as those stemming from the presidential administration in the United States or the U.K.’s exit from the E.U. (known as “Brexit”), are difficult to predict and may have a material adverse effect on us. For example, in the United States, the presidential administration has imposed tariffs on imports from China, Mexico, Canada and other countries, and has expressed support for greater restrictions on free trade and increase tariffs on goods imported into the United States. Changes in U.S. political, regulatory and economic conditions or in its policies governing international trade and foreign manufacturing and investment in the United States could adversely affect our sales in the United States.
We are also subject to the U.S. Foreign Corrupt Practices Act and may be subject to similar worldwide anti-bribery laws that generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Despite our compliance and training programs, we cannot be certain that our procedures will be sufficient to ensure consistent compliance with all applicable international trade and anti-corruption laws, or that our employees or channel partners will strictly follow all policies and requirements to which we subject them. Any alleged or actual violations of these laws may subject us to government scrutiny, investigation, debarment, and civil and criminal penalties, which may have an adverse effect on our results of operations, financial condition and reputation.
28
The economic effects of Brexit may affect relationships with existing and future customers and could have an adverse impact on our business and operating results.
On June 23, 2016, the United Kingdom, or the U.K., held a referendum in which voters approved Brexit. On January 31, 2020, the U.K. formally ceased to be part of the EU. Although negotiations between the U.K. and EU regarding Brexit began in June 2017 and the UK has passed legislation regarding the immediate impact of the U.K.’s withdrawal from the EU, it is still unclear what terms, if any, may be agreed within the U.K. and between the U.K. and other countries on many aspects of fiscal policy, cross-border trade and international relations, both in the final outcome and for any transitional period. The withdrawal of the U.K from the E.U. could potentially disrupt the free movement of goods, services and people between the U.K. and the E.U., undermine bilateral cooperation in key geographic areas and significantly disrupt trade between the U.K. and the E.U. or other nations as the U.K. pursues independent trade relations. Because this is an unprecedented event, it is unclear what long-term economic, financial, trade and legal implications Brexit would have and how it would affect the regulation applicable to our business globally and in the region. The impact on us will depend, in part, on the outcome of tariff, trade, regulatory and other negotiations. Any of these developments, along with any political, economic and regulatory changes that may occur, could cause political and economic uncertainty in Europe and internationally and could adversely affect our sales in Europe. The impact on us from Brexit will depend, in part, on the outcome of tariff, trade, regulatory and other negotiations.
As a result of Brexit, the global markets and currencies have been adversely impacted, including a decline in the value of the British pound as compared to the U.S. dollar. A potential devaluation of the local currencies of our international buyers relative to the U.S. dollar may impair the purchasing power of our international buyers and could cause international buyers to decrease their participation in our marketplaces or use of our products. Further, volatility in exchange rates resulting from Brexit is expected to continue in the short term as the U.K. negotiates its exit from the E.U. We translate sales and other results of our activities in the U.K. denominated in British pounds into U.S. dollars for our financial statements. During periods of a strengthening dollar, our reported international sales and earnings could be reduced because foreign currencies may translate into fewer U.S. dollars. Finally, Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the U.K. determines which E.U. laws to replace or replicate, and those laws and regulations may be cumbersome, difficult or costly in terms of compliance. In addition, Brexit may lead other E.U. member countries to consider referendums regarding their E.U. membership. Any of these effects of Brexit, among others, could adversely affect our business, financial condition, operating results and cash flows.
Our business may be materially affected by changes to fiscal and tax policies. Potentially negative or unexpected tax consequences of these policies, or the uncertainty surrounding their potential effects, could adversely affect our results of operations and share price.
The U.S. Tax Cuts and Jobs Act of 2017 (the “TCJA”) was approved by Congress on December 20, 2017 and signed into law by President Donald J. Trump on December 22, 2017. This legislation makes significant changes to the U.S. Internal Revenue Code of 1986, as amended (the “IRC”). Such changes include a reduction in the corporate tax rate from a top marginal rate of 35% to a flat rate of 21% and limitations on certain corporate deductions and credits, among other changes. In addition, the TCJA requires complex computations to be performed that were not previously required in U.S. tax law, significant judgments to be made in interpretation of the provisions of the TCJA and significant estimates in calculations, and the preparation and analysis of information not previously relevant or regularly produced.
While to date we believe the effect of the TCJA in our Consolidated Financial Statements the application of accounting guidance for various items, and the ultimate impact of the TCJA on our business are not material, the final impacts of the TCJA could be materially different from our analysis. For example, adverse changes in the underlying profitability and financial outlook of our operations or changes in tax law could lead to changes in our valuation allowances against deferred tax assets on our consolidated balance sheets, which could materially affect our results of operations. The U.S. Treasury Department, the Internal Revenue Service (the “IRS”), and other standard-setting bodies could interpret or issue guidance on how provisions of the TCJA will be applied or otherwise administered that is different from our interpretation which may materially affect our results of operations. Finally, foreign governments may enact tax laws in response to the TCJA that could result in further changes to global taxation and materially affect our financial position and results of operations. The uncertainty surrounding the effect of the reforms on our financial results and business could also weaken confidence among investors in our financial condition. This could, in turn, have a materially adverse effect on the price of our ordinary shares.
29
We may seek to grow our business through acquisitions of complementary products or technologies, and the failure to manage acquisitions, or the failure to integrate them with our existing business, could have a material adverse effect on our business, financial condition and operating results.
From time to time, we may consider opportunities to acquire or license other products or technologies that may enhance our product platform or technology, expand the breadth of our markets or customer base, or advance our business strategies. Potential acquisitions involve numerous risks, including:
| ● | problems assimilating the acquired products or technologies; | |
| ● | issues maintaining uniform standards, procedures, controls and policies; | |
| ● | unanticipated costs associated with acquisitions; | |
| ● | diversion of management’s attention from our existing business; | |
| ● | risks associated with entering new markets in which we have limited or no experience; and | |
| ● | increased legal and accounting costs relating to the acquisitions or compliance with regulatory matters. |
We have no current commitments with respect to any acquisition or licensing. We do not know if we will be able to identify such acquisitions or licensing we deem suitable, whether we will be able to successfully complete any such transactions on favorable terms or at all, or whether we will be able to successfully integrate any acquired products or technologies. Our potential inability to integrate any acquired products or technologies effectively may adversely affect our business, operating results and financial condition.
If there are significant disruptions in our information technology systems, our business, financial condition and operating results could be adversely affected.
The efficient operation of our business depends on our information technology systems. We rely on our information technology systems to effectively manage sales and marketing data, accounting and financial functions, inventory management, product development tasks, research and development data, customer service and technical support functions. Our information technology systems are vulnerable to damage or interruption from earthquakes, fires, floods and other natural disasters, terrorist attacks, attacks by computer viruses or hackers, power losses, and computer system or data network failures. In addition, our data management application is hosted by a third-party service provider whose security and information technology systems are subject to similar risks, and our products’ systems contain software which could be subject to computer virus or hacker attacks or other failures.
The failure of our or our service providers’ information technology systems or our products’ software to perform as we anticipate or our failure to effectively implement new information technology systems could disrupt our entire operation or adversely affect our software products and could result in decreased sales, increased overhead costs, and product shortages, all of which could have a material adverse effect on our reputation, business, financial condition and operating results.
If we fail to properly manage our anticipated growth, our business could suffer.
Our growth and product expansion has placed, and we expect that it will continue to place, a significant strain on our management team and on our financial resources. Failure to manage our growth effectively could cause us to misallocate management or financial resources, and result in losses or weaknesses in our infrastructure, which could materially adversely affect our business. Additionally, our anticipated growth will increase the demands placed on our suppliers, resulting in an increased need for us to manage our suppliers and monitor for quality assurance. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our business objectives.
We depend on the knowledge and skills of our senior management.
We have benefited substantially from the leadership and performance of our senior management. For example, we depend on our Chief Executive Officer’s experience successfully scaling an early stage medical device company, as well as the experience of other members of management. Our success will depend on our ability to retain our current management. Competition for senior management in our industry is intense and we cannot guarantee that we will be able to retain our personnel. Additionally, we do not carry key man insurance on any of our current executive officers. The loss of the services of certain members of our senior management could prevent or delay the implementation and completion of our strategic objectives, or divert management’s attention to seeking qualified replacements.
30
Following the dismissal of several securities class lawsuits against us, we are currently subject to one securities class action lawsuit against us, which may result in an adverse outcome.
Between September 2016 and January 2017, eight putative class actions on behalf of alleged shareholders that purchased or acquired our ordinary shares pursuant and/or traceable to our registration statement on Form F-1 (File No. 333-197344) used in connection with our IPO, were commenced in the following courts: (i) the Superior Court of the State of California, County of San Mateo; (ii) the Superior Court of the Commonwealth of Massachusetts, Suffolk County; (iii) the United States District Court for the Northern District of California; and (iv) the United States District Court for the District of Massachusetts. As of November 14, 2018, the California state and federal cases and the case in Massachusetts Superior Court have been dismissed with no further right to appeal, and the case in the United States District Court for the District of Massachusetts has been partially dismissed. The dismissal is currently on appeal in the United States Court of Appeals for the First Circuit. The action alleges violations of Sections 11 and 15 of the Securities Act and Sections 10(b) and 20(a) of the Exchange Act. For more information, see “Business—Legal Proceedings.”
We are generally required, to the extent permitted by Israeli law, to indemnify our current and former directors and officers who are named as defendants in these types of lawsuits. We also have certain contractual indemnification obligations to the underwriters of our IPO regarding the securities class action lawsuits. While a certain amount of insurance coverage is available for expenses or losses associated with these lawsuits, this coverage may not be sufficient. Based on information currently available, we are unable to reasonably estimate a possible loss or range of possible losses, if any, with regard to the remaining lawsuit; therefore, no litigation reserve has been recorded in our consolidated balance sheets. Although we plan to defend against the remaining lawsuit vigorously, there can be no assurances that a favorable final outcome will be obtained. This lawsuit or future litigation may require significant attention from management and could result in significant legal expenses, settlement costs or damage awards that could have a materially adverse impact on our financial position, results of operations and cash flows.
Risks Related to Government Regulation
We have submitted medical device reports, or MDRs, to the FDA (and equivalent authorities outside of the United States) for numerous serious injuries relating to use of the ReWalk Personal system, and conducted a voluntary correction related to certain use instructions in the device’s labeling, which the FDA classified as a Class II recall. If our product may have caused or contributed to a death or a serious injury, or if our product malfunctioned and the malfunction’s recurrence would be likely to cause or contribute to a death or serious injury, we must comply with the FDA’s MDR regulations (and equivalent authorities outside of the United States), which could result in voluntary corrective actions or enforcement actions, such as mandatory recalls.
Under the FDA’s MDR regulations, we are required to report to the FDA information that reasonably suggests a product we market may have caused or contributed to a death or serious injury or malfunctioned and our product or a similar device marketed by us would be likely to cause or contribute to death or serious injury if the malfunction were to recur. In addition, all manufacturers placing medical devices on the market in the European Union are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the relevant authority in whose jurisdiction the incident occurred. Between 2013 and 2017, we submitted a number of MDRs to the FDA to report incidents in which ReWalk Personal users sustained falls or fractures. The FDA sent us letters requesting additional information relating to these MDRs submitted in 2017, including a request for a failure analysis. In August 2017, we initiated a voluntary correction for the ReWalk device that related to certain use instructions to reduce the risk of tibia/fibula fractures and submitted a report to the FDA under 21 CFR Part 806. Under Part 806, manufacturers and importers are required to make a report to the FDA of any correction or removal of a device if the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the U.S. Federal Food, Drug, and Cosmetic Act caused by the device that may present a risk to health.
In June 2018, we received a letter from the FDA agreeing with our decision to initiate a corrective action for the ReWalk, classifying the recall action as a Class II recall, and requesting that we make regular status reports to the FDA regarding our progress. While the FDA has statutory authority to require a recall, most recalls are undertaken voluntarily when a medical device is defective, when it could present a risk to health, or when it is both defective and presents a risk to health. In January 2019, we submitted a recall termination request to the FDA. In November 2019 the FDA informed us that it considered the recall action terminated. In September 2018, we submitted to the FDA revised labeling that incorporates the revised use instructions intended to prevent the tibia/fibula fractures as a special 510(k). The special 510(k) was not accepted by FDA because it was administratively incomplete, and we withdrew the submission. In January 2020 we submitted a new 510(k) to the FDA for both the revised labeling/use instructions and additional changes to the device. This new 510(k) was not accepted by FDA because it was administratively incomplete and, accordingly, FDA notified ReWalk on January 22, 2020 of the Refuse-to-Accept (RTA) designation. The company requested a meeting with FDA to seek the agency’s feedback regarding its planned RTA response through a Submission Issue Request (SIR). The 510(k) submission will be deemed withdrawn if a response to the RTA notification is not received by the FDA within 180 days of the date of the RTA notification. In September 2019, we also submitted a revised technical file with the additional device changes to the EU notified body and were notified in December 2019 that the extension of our certification had been granted.
In 2018, we submitted additional MDRs for tibia/fibula fractures that occurred in foreign countries between 2015 and 2018. In addition, in 2018 and 2019 we submitted MDRs for tibia/fibula fractures that occurred in the United States and Europe. In 2020 we submitted an MDR for tibial fractures that occurred in the United States. Additional fractures or other adverse events may occur in the future that may require us to report to the FDA pursuant to the MDR regulations (or other governmental authorities pursuant to equivalent outside of the United States regulations), and/or to initiate a removal, correction, or other action. Any adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer letters, or in an FDA enforcement action, such as a mandatory recall, notification to healthcare professionals and users, warning letter, seizure, injunction or import alert. In addition, failure to report such adverse events to appropriate government authorities on a timely basis, or at all, could result in enforcement action against us. Any action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require financial resources and distract management, and may harm our reputation and financial results.
31
U.S. healthcare reform measures and other potential legislative initiatives could adversely affect our business.
Recent political changes in the United States could result in significant changes in, and uncertainty with respect to, legislation, regulation, global trade and government policy that could substantially impact our business and the medical device industry generally. Certain proposals, if enacted into law, could impose limitations on the prices we will be able to charge for our ReWalk system or any products we may develop and offer in the future, or the amounts of reimbursement available for such products from governmental agencies or third-party payers. Additionally, any reduction in reimbursement from Medicare or other government-funded federal programs, including the VA, or state healthcare programs could lead to a similar reduction in payments from private commercial payors. The FDA’s policies may also change and additional government regulations may be issued that could prevent, limit or delay regulatory approval of our future products, or impose more stringent product labeling and post-marketing testing and other requirements. For instance, in September 2017, members of the U.S. Congress introduced legislation with the announced intention to repeal and replace major provisions of the PPACA. Although this proposed legislation ultimately failed to pass, Congress succeeded in repealing the PPACA’s individual mandate as part of the U.S. Tax Cuts and Jobs Act of 2017.
The implementation of cost containment measures or other healthcare reforms may thus prevent us from being able to generate revenue, attain profitability or further commercialize our existing ReWalk systems or future ReWalk products. We are currently unable to predict what additional legislation or regulation, if any, relating to the health care industry may be enacted in the future or what effect recently enacted federal legislation or any such additional legislation or regulation would have on our business. The pendency or approval of such proposals or reforms could result in a decrease in our stock price or limit our ability to raise capital or to enter into collaboration agreements for the further development and commercialization of our programs and products.
Shutdowns of the U.S. federal government could materially impair our business and financial condition.
Development of our product candidates and/or regulatory approval may be delayed for reasons beyond our control. For example, over the last several years the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown or budget sequestration occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets, such as through the declaration of effectiveness of registration statements, and obtain necessary capital in order to properly capitalize and continue our operations.
While we addressed the observations that the FDA cited in a 2015 warning letter related to our mandatory post-market surveillance study and initiated the study, we are currently experiencing enrollment issues that make our study progress inadequate and our modified protocol (intended to overcome the enrollment issues so that we may complete the study, as required) has not yet been approved by FDA. Going forward, if we cannot meet certain FDA requirements and enrollment criteria for the study or otherwise satisfy FDA requests promptly, or if our study produces unfavorable results, we could be subject to additional FDA warnings letters or more significant enforcement action, which could materially and adversely affect our commercial success.
We are conducting an ongoing mandatory FDA postmarket surveillance study on our ReWalk Personal 6.0, which began in June 2016. Before we began the current study, the FDA sent us a warning letter on September 30, 2015, (“the September 2015 Warning Letter”), threatening potential regulatory action against us for violations of Section 522 of the U.S. Federal Food, Drug, and Cosmetic Act, based on our failure to initiate a postmarket surveillance study by the September 28, 2015 deadline, our allegedly deficient protocol for that study, and the lack of progress and communication regarding the study. Between June 2014 and our receipt of the September 2015 Warning Letter, we had responded late to certain of the FDA’s requests related to our study protocol. In February 2016, the FDA sent us an additional information request, or the February 2016 Letter, requesting additional changes to our study protocol and asking that we amend the study within 30 days. This letter also discussed the FDA’s request, as further discussed in later communications with the FDA, for a new premarket notification for our ReWalk device, or a special 510(k), linked to what the FDA viewed as changes to the labeling and the device, including to a computer included with the device. In late March 2016, following multiple discussions with the FDA, including an in-person meeting, the FDA confirmed that the agency would permit the continued marketing of the ReWalk device conditioned upon our timely submitting a special 510(k) and initiating our postmarket surveillance study by June 1, 2016. The special 510(k) was timely submitted on April 8, 2016, and the FDA’s substantial equivalence determination was received by us on July 22, 2016, granting us permission to continue marketing the ReWalk device. Additionally, we submitted a protocol to the FDA for the postmarket surveillance study that was approved by the FDA on May 5, 2016.
32
We began the study on June 13, 2016, with Stanford University as the lead investigational site. In August 2016, the FDA sent us a letter stating that, based on its evaluation of our corrective and preventive actions in response to the September 2015 Warning Letter, it appeared we had adequately addressed the violations cited in the September 2015 Warning Letter. As part of our study, we have provided the FDA with the required periodic reports on the study’s progress, in a few cases with delay, and we intend to continue providing the FDA with periodic reports as required. Through these reports, we have made the FDA aware that due to enrollment issues, we are currently unable to satisfy the target enrollment specified in the study protocol.
As of December 31, 2019, we had four active centers participating in the study (a fifth site is on hold), but only two sites have successfully enrolled patients. Twelve subjects have enrolled in the study, two have completed the study, and three are using the device in the community. This is substantially below the required number of patients included in our study protocol, currently leading the FDA to label our progress as “inadequate.” We are in ongoing communications with the FDA regarding how to address the inadequate progress. However, there can be no assurance that we will be able to satisfy the post-market study requirements. If we cannot meet FDA requirements for the post-market study or timely address requests from the FDA related to the study, or if the results of the study are not as favorable as we expect, the FDA may issue additional warning letters to us, impose limitations on the labeling of our device or require us to stop marketing the ReWalk Personal device in the United States. We derived 41.4% of our revenues in the year ended December 31, 2019 from sales of the ReWalk device in the United States and, if we are unable to market the ReWalk device in the United States, we expect that these sales would be adversely impacted, which could materially adversely affect our business and overall results of operations.
Our devices are subject to the FDA’s regulations pertaining to marketing and promotional communications, among others. Failure to comply with such regulations may give rise to a number of potential FDA enforcement actions, any of which could have a material adverse effect on our business.
Our sales and marketing efforts, as well as promotions, are subject to various laws and regulations. Medical device promotions must be consistent with and not contrary to labeling, be truthful and not false or misleading, and be adequately substantiated. In addition to the requirements applicable to 510(k)-cleared products, we may also be subject to enforcement action in connection with any promotion of an investigational new device. A sponsor or investigator, or any person acting on behalf of a sponsor or investigator, may not represent in a promotional context that an investigational new device is safe or effective for the purposes for which it is under investigation or otherwise promote the device.
Our marketing and promotional materials are subject to FDA scrutiny to ensure that the device is being marketed in compliance with these requirements. If the FDA investigates our marketing and promotional materials and finds that any of our current or future commercial products were being marketed for unapproved or uncleared uses or in a false or misleading manner, we could be subject to FDA enforcement and/or false advertising consumer lawsuits, each of which could have a material adverse effect on our business.
We are subject to extensive governmental regulations relating to the manufacturing, labeling and marketing of our products, and a failure to comply with such regulations could lead to withdrawal or recall of our products from the market.
Our medical products and manufacturing operations are subject to regulation by the FDA, the European Union, and other governmental authorities both inside and outside of the United States. These agencies enforce laws and regulations that govern the development, testing, manufacturing, labeling, storage, installation, servicing, advertising, promoting, marketing, distribution, import, export and market surveillance of ReWalk.
Our products are regulated as medical devices in the United States under the FFDCA as implemented and enforced by the FDA. Under the FFDCA, medical devices are classified into one of three classes (Class I, Class II or Class III) depending on the degree of risk associated with the medical device, what is known about the type of device, and the extent of control needed to provide reasonable assurance of safety and effectiveness. Classification of a device is important because the class to which a device is assigned determines, among other things, the necessity and type of FDA review required prior to marketing the device. For more information, see “Business—Government Regulation” below.
33
In June 2014, the FDA granted our petition for “de novo” classification, which provides a route to market for medical devices that are low to moderate risk, but are not substantially equivalent to a predicate device, and classified ReWalk as Class II subject to certain special controls. The ReWalk is intended to enable individuals with spinal cord injuries to perform ambulatory functions under supervision of a specially trained companion, and inside rehabilitation institutions. The special controls established in the de novo order include the following: compliance with medical device consensus standards; clinical testing to demonstrate safe and effective use considering the level of supervision necessary and the use environment; non-clinical performance testing, including durability testing to demonstrate that the device performs as intended under anticipated conditions of use; a training program; and labeling related to device use and user training. In order for us to market ReWalk, we must comply with both general controls, including controls related to quality, facility registration, reporting of adverse events and labeling, and the special controls established for the device. Failure to comply with these requirements could lead to an FDA enforcement action, which would have a material adverse effect on our business.
In June 2019, the FDA issued a 510(k) clearance for our ReStore device. ReStore is intended to be used to assist ambulatory functions in rehabilitation institutions under the supervision of a trained therapist for people with hemiplegia or hemiparesis due to stroke who have a specified amount of ambulatory function. In order for us to market ReStore, we must comply with both general controls, including controls related to quality, facility registration, reporting of adverse events and labeling, and the special controls established for the device that include clinical testing, non-clinical performance testing, and a training program. Failure to comply with these requirements could lead to an FDA enforcement action, which would have a material adverse effect on our business.
Following the introduction of a product, the governmental agencies will periodically review our manufacturing processes and quality controls, and we are under a continuing obligation to ensure that all applicable regulatory requirements continue to be met. The process of complying with the applicable good manufacturing practices, adverse event reporting and other requirements can be costly and time consuming, and could delay or prevent the production, manufacturing or sale of our devices. In addition, if we fail to comply with applicable regulatory requirements, it could result in fines or delays of regulatory clearances, closure of manufacturing sites, seizures or recalls of products and damage to our reputation, as well as enforcement actions against us.
For example, the FDA could request that we recall our ReWalk Personal 6.0 device. For more information on certain deficiencies previously identified by the FDA in our mandatory post-market surveillance study on our ReWalk Personal 6.0, see “Part I, Item 1A. Risk Factors—Risks Related to Government Regulation—While we addressed the observations that FDA cited in a 2015 warning letter related to our mandatory post-market surveillance study and initiated the study, we are currently experiencing enrollment issues that make our study progress inadequate. Going forward, if we cannot meet certain FDA requirements and enrollment criteria for the study or otherwise satisfy FDA requests promptly, or if our study produces unfavorable results, we could receive additional FDA warnings, which could materially and adversely affect our commercial success.”
In addition, governmental agencies may impose new requirements regarding registration or labeling that may require us to modify or re-register our products or otherwise impact our ability to market our products in those countries. The process of complying with these governmental regulations can be costly and time consuming, and could delay or prevent the production, manufacturing or sale of our products. In the European Union, for example, a new Medical Device Regulation includes additional premarket and post-market requirements, as well as potential product reclassifications or more stringent commercialization requirements that could adversely affect our CE mark. Penalties for regulatory non-compliance with the Medical Device Regulation could also be substantial, including fines, revocation or suspension of CE mark and criminal sanctions.
If we or our third-party manufacturers fail to comply with the FDA’s Quality System Regulation, or QSR, our manufacturing operations could be interrupted.
We and our manufacturer Sanmina are required to comply with the FDA’s QSR which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. We, Sanmina and our suppliers are also subject to the regulations of foreign jurisdictions regarding the manufacturing process if we or our distributors market our products abroad. We continue to monitor our quality management in order to improve our overall level of compliance. Our facilities are subject to periodic and unannounced inspection by U.S. and foreign regulatory agencies to audit compliance with the QSR and comparable foreign regulations. If our facilities or those of Sanmina or our suppliers are found to be in violation of applicable laws and regulations, or if we, Sanmina or our suppliers fail to take satisfactory corrective action in response to an adverse inspection, the regulatory authority could take enforcement action, including any of the following sanctions:
| ● | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; | |
| ● | customer notifications or repair, replacement or refunds; |
34
| ● | operating restrictions or partial suspension or total shutdown of production; | |
| ● | recalls, withdrawals, or administrative detention or seizure of our products; | |
| ● | refusing or delaying requests for approval of pre-market approval applications relating to new products or modified products; | |
| ● | withdrawing a PMA approval; | |
| ● | refusing to provide Certificates for Foreign Government; | |
| ● | refusing to grant export approval for our products; or | |
| ● | pursuing criminal prosecution. |
Any of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands, and could have a material adverse effect on our reputation, business, results of operations and financial condition. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
We are subject to various laws and regulations, including “fraud and abuse” laws and anti-bribery laws, which, if violated, could subject us to substantial penalties.
Medical device companies such as ours have faced lawsuits and investigations pertaining to alleged violations of numerous statutes and regulations, including anti-corruption laws and health care “fraud and abuse” laws, such as the federal False Claims Act, the federal Anti-Kickback Statute and the U.S. Foreign Corrupt Practices Act, or the FCPA. See “Business-Government Regulation” below. U.S. federal and state laws, including the federal Physician Payments Sunshine Act, or the Sunshine Act, and the implementation of Open Payments regulations under the Sunshine Act, require medical device companies to disclose certain payments or other transfers of value made to healthcare providers and teaching hospitals or funds spent on marketing and promotion of medical device products. It is widely believed that public reporting under the Sunshine Act and implementing Open Payments regulations results in increased scrutiny of the financial relationships between industry, physicians and teaching hospitals. Further, some state laws require medical device companies to report information related to payments to physicians and other health care providers or marketing expenditures. These anti-kickback, anti-bribery, public reporting and aggregate spending laws affect our sales, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, rehabilitation centers, physicians or other potential purchasers or users of ReWalk or ReStore. They also impose additional administrative and compliance burdens on us. In particular, these laws influence, among other things, how we structure our sales offerings, including discount practices, customer support, education and training programs and physician consulting and other service arrangements, including those with marketers and sales agents. We may face significant costs in attempting to comply with these laws and regulations. If we are found to be in violation of any of these requirements or any actions or investigations are instituted against us, those actions could be costly to defend and could have a significant impact on our business, including the imposition of significant criminal and civil fines and penalties, exclusion from federal healthcare programs or other sanctions, and damage to our reputation or business.
The FCPA applies to companies, including ours, with a class of securities registered under the Exchange Act. The FCPA and other anti-bribery laws to which various aspects of our operations may be subject generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. In various jurisdictions, our operations require that we and third parties acting on our behalf routinely interact with government officials, including medical personnel who may be considered government officials for purposes of these laws because they are employees of state-owned or controlled facilities. Other anti-bribery laws to which various aspects of our operations may be subject, including the United Kingdom Bribery Act, also prohibit improper payments to private parties and prohibit receipt of improper payments. Our policies prohibit our employees from making or receiving corrupt payments, including, among other things, to require compliance by third parties engaged to act on our behalf. Our policies mandate compliance with these anti-bribery laws; however, we operate in many parts of the world that have experienced governmental and/or private corruption to some degree. As a result, the existence and implementation of a robust anti-corruption program cannot eliminate all risk that unauthorized reckless or criminal acts have been or will be committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and harm our financial condition, results of operations, cash flows and reputation.
35
If we are found to have violated laws protecting the confidentiality of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
There are a number of federal, state and foreign laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services, or HHS, promulgated patient privacy rules under the Health Insurance Portability and Accountability Act of 1996, or HIPAA. These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. Additionally, the E.U. General Data Protection Regulation (the “GDPR”), which took effect in 2018, imposes more stringent data protection requirements and will provide for greater penalties for noncompliance. Thus with respect to our operations in Europe, the GDPR may increase our responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with the GDPR. This may be onerous and adversely affect our business, financial condition, results of operations and prospects. Additionally, if we or any of our service providers are found to be in violation of the promulgated patient privacy rules under HIPAA or, once enforced, the GDPR, we could be subject to civil or criminal penalties, which could be substantial and could increase our liabilities, harm our reputation and have a material adverse effect on our business, financial condition and operating results.
In addition, a number of U.S. states have enacted data privacy and security laws and regulations that govern the collection, use, disclosure, transfer, storage, disposal, and protection of sensitive personal information, such as social security numbers, financial information and other personal information. For example, several U.S. territories and all 50 states now have data breach laws that require timely notification to individual victims, and at times regulators, if a company has experienced the unauthorized access or acquisition of sensitive personal data. Other state laws include the California Consumer Privacy Act (“CCPA”) which, among other things, contains new obligations for businesses that collect personal information about California residents and affords those individuals new rights relating to their personal information that may affect our ability to use personal information or share it with our business partners. Meanwhile, other states have considered privacy laws like the CCPA. We will continue to monitor and assess the impact of state law developments, which may impose substantial penalties for violations, impose significant costs for investigations and compliance, allow private class-action litigation and carry significant potential liability for our business.
The interpretation and enforcement of the laws and regulations described above are uncertain and subject to change, and may require substantial costs to monitor and implement compliance with any additional requirements. Failure to comply with U.S. or international data protection laws and regulations could result in government enforcement actions (which could include substantial civil and/or criminal penalties), private litigation and/or adverse publicity and could negatively affect our operating results and business.
Compliance with various regulations, including those related to our status as a U.S. public company and the manufacturing, labeling and marketing of our products, may result in heightened general and administrative expenses and costs, divert management’s attention from revenue-generating activities and pose challenges for our management team, which has limited time, personnel and finances to devote to regulatory compliance.
As a U.S. public company, we are subject to various regulatory and reporting requirements, including those imposed by the SEC, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, or the Dodd-Frank Act, the listing requirements of the Nasdaq Capital Market and other applicable securities rules and regulations. Additionally, our medical products and manufacturing operations are regulated by the FDA, the European Union and other governmental authorities both inside and outside of the United States. Compliance with the rules and regulations applicable to us as a publicly traded company in the United States and medical device manufacturer has greatly increased, and may continue to increase, our legal, general and administrative and financial compliance costs and has made, and may continue to make, some activities more difficult, time-consuming or costly. Additionally, these regulatory requirements have diverted, and may continue to divert, management’s attention from revenue-generating activities and may increase demands on management’s already-limited resources.
Our management team consists of few employees, as the majority of our employees are engaged in sales and marketing and research and development activities. For more information, see “Business—Employees.” In light of such constraints on its time, personnel and finances, our management may not be able to implement programs and policies in an effective and timely manner to respond adequately to the heightened legal, regulatory and reporting requirements applicable to us. In the past, for example, we have not always been able to respond on a timely basis to requests from regulators, although we have not to date experienced any long-term material adverse consequences as a result. For more information, see “Part I, Item 1A. Risk Factors—Risks Related to Government Regulation—While we addressed the observations that FDA cited in a 2015 warning letter related to our mandatory post-market surveillance study and initiated the study, we are currently experiencing enrollment issues that make our study progress inadequate. Going forward, if we cannot meet certain FDA requirements and enrollment criteria for the study or otherwise satisfy FDA requests promptly, or if our study produces unfavorable results, we could receive additional FDA warnings, which could materially and adversely affect our commercial success” above. Similar deficiencies, weaknesses or lack of compliance with public company, medical device and other regulations could harm our reputation in the capital markets or for quality and safety, negatively affect our ability to maintain our public company status and to develop, commercialize or continue selling our products on a timely and effective basis, and cause us to incur sanctions, including fines, injunctions and penalties.
In addition, complying with public disclosure rules makes our business more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and operating results could be harmed, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and harm our business and operating results.
36
Uncertainty relating to the LIBOR calculation process and potential phasing out of LIBOR in the future may adversely affect the value of any outstanding debt instruments.
National and international regulators and law enforcement agencies have conducted investigations into a number of rates or indices known as “reference rates.” Actions by such regulators and law enforcement agencies may result in changes to the manner in which certain reference rates are determined, their discontinuance, or the establishment of alternative reference rates. In particular, on July 27, 2017, the Chief Executive of the U.K. Financial Conduct Authority (the “FCA”), which regulates LIBOR, announced that the FCA will no longer persuade or compel banks to submit rates for the calculation of LIBOR after 2021. Such announcement indicates that the continuation of LIBOR on the current basis cannot and will not be guaranteed after 2021. As a result, it appears highly likely that LIBOR will be discontinued or modified by 2021.
At this time, it is not possible to predict the effect that these developments, any discontinuance, modification or other reforms to LIBOR or any other reference rate, or the establishment of alternative reference rates may have on LIBOR, other benchmarks, or LIBOR-based debt instruments. Uncertainty as to the nature of such potential discontinuance, modification, alternative reference rates or other reforms may materially adversely affect the trading market for securities linked to such benchmarks. Furthermore, the use of alternative reference rates or other reforms could cause the interest rate calculated for the LIBOR-based debt instruments to be materially different than expected.
Risks Related to Our Intellectual Property
Our success depends in part on our ability to obtain and maintain protection for the intellectual property relating to or incorporated into our products.
Our success depends in part on our ability to obtain and maintain protection for the intellectual property relating to or incorporated into our products. We seek to protect our intellectual property through a combination of patents, trademarks, confidentiality and assignment agreements with our employees and certain of our contractors, and confidentiality agreements with certain of our consultants, scientific advisors, and other vendors and contractors. In addition, we rely on trade secret law to protect our proprietary software and product candidates/products in development. For more information, see Business—Intellectual Property.
The patent position of robotic and exoskeleton inventions can be highly uncertain and involves many new and evolving complex legal, factual, and technical issues. Patent laws and interpretations of those laws, are subject to change and any such changes may diminish the value of our patents or narrow the scope of our right to exclude others. In addition, we may fail to apply for or be unable to obtain patents necessary to protect our technology or products from competition or fail to enforce our patents due to lack of information about the exact use of technology or processes by third parties. Also, we cannot be sure that any patents will be granted in a timely manner or at all with respect to any of our patent pending applications or that any patents that are granted will be adequate to exclude others for any significant period of time or at all. Given the foregoing and in order to continue reducing operational expenses in the future, we may invest fewer resources in filing and prosecuting new patents and on maintaining and enforcing various patents, especially in regions where we currently do not focus our market growth strategy.
Litigation to establish or challenge the validity of patents, or to defend against or assert against others infringement, unauthorized use, enforceability or invalidity, can be lengthy and expensive and may result in our patents being invalidated or interpreted narrowly and restricting our ability to be granted new patents related to our pending patent applications. Even if we prevail, litigation may be time consuming, force us to incur significant costs, and could divert management’s attention from managing our business while any damages or other remedies awarded to us may not be valuable. In addition, U.S. patents and patent applications may be subject to interference proceedings, and U.S. patents may be subject to re-examination and review proceedings in the U.S. Patent and Trademark Office. Foreign patents may also be subject to opposition or comparable proceedings in the corresponding foreign patent offices. Any of these proceedings may be expensive and could result in the loss of a patent or denial of a patent application, or the loss or reduction in the scope of one or more of the claims of a patent or patent application.
37
In addition, we seek to protect our trade secrets, know-how, and confidential information that is not patentable by entering into confidentiality and assignment agreements with our employees and certain of our contractors and confidentiality agreements with certain of our consultants, scientific advisors, and other vendors and contractors. However, we may fail to enter into the necessary agreements, and even if entered into, these agreements may be breached or otherwise fail to prevent disclosure, third-party infringement, or misappropriation of our proprietary information, may be limited as to their term and may not provide an adequate remedy in the event of unauthorized disclosure or use of proprietary information. Enforcing a claim that a third party illegally obtained or is using our trade secrets without authorization may be expensive and time consuming, and the outcome is unpredictable. Some of our employees or consultants may own certain technology which they license to us for a set term. If these technologies are material to our business after the term of the license, our inability to use them could adversely affect our business and profitability.
We also have taken precautions to initiate reasonable safeguards to protect our information technology systems. However, these measures may not be adequate to safeguard our proprietary information, which could lead to the loss or impairment thereof or to expensive litigation to defend our rights against competitors who may be better funded and have superior resources. In addition, unauthorized parties may attempt to copy or reverse engineer certain aspects of our products that we consider proprietary or our proprietary information may otherwise become known or may be independently developed by our competitors or other third parties. If other parties are able to use our proprietary technology or information, our ability to compete in the market could be harmed. Further, unauthorized use of our intellectual property may have occurred, or may occur in the future, without our knowledge.
If we are unable to obtain or maintain adequate protection for intellectual property, or if any protection is reduced or eliminated, competitors may be able to use our technologies, resulting in harm to our competitive position.
Our patents and proprietary technology and processes may not provide us with a competitive advantage.
Robotics and exoskeleton technologies have been developing rapidly in recent years. We are aware of several other companies developing competing exoskeleton devices for individuals with limited mobility and we expect the level of competition and the pace of development in our industry to increase. For more information, see “Business—Competition” below. While we believe our tilt-sensor technology provides a more natural and superior method of exoskeleton activation, which creates a better user experience, as well as that our licensed technology used in our ReStore device is unique and provides better results when compared to other products, a variety of other activation and control methods exist for exoskeletons, several of which are being developed by our competitors, or may be developed in the future. As a result, our patent portfolio and proprietary technology and processes may not provide us with a significant advantage over our competitors, and competitors may be able to design and sell alternative products that are equal to or superior to our products without infringing on our patents. In addition, upon the expiration of our current patents, we may be unable to adequately develop new technologies and obtain future patent protection to preserve our competitive advantage. If we are unable to maintain a competitive advantage, our business and results of operations may be materially adversely affected.
Even in instances where others are found to infringe on our patents, many countries have laws under which a patent owner may be compelled to grant licenses for the use of the patented technology to other parties. In addition, many countries limit the enforceability of patents against other parties, including government agencies or government contractors. In these countries, a patent owner may have limited remedies, which could diminish the value of a patent in those countries. Further, the laws of some countries do not protect intellectual property rights to the same extent as the laws of the United States, particularly in the field of medical products, and effective enforcement in those countries may not be available. The ability of others to market comparable products could adversely affect our business.
We depend on computer and telecommunications systems we do not own or control and failures in our systems or a cybersecurity attack or breach of our IT systems or technology could significantly disrupt our business operations or result in sensitive customer information being compromised which would negatively materially affect our reputation and/or results of operations.
We have entered into agreements with third parties for hardware, software, telecommunications and other information technology services in connection with the operation of our business. It is possible we or a third party that we rely on could incur interruptions from a loss of communications, hardware or software failures, a cybersecurity attack or a breach of our IT systems or technology, computer viruses or malware. We believe that we have positive relations with our vendors and maintain adequate anti-virus and malware software and controls; however, any interruptions to our arrangements with third parties, to our computing and communications infrastructure, or to our information systems or any of those operated by a third party that we rely on could significantly disrupt our business operations.
38
In the current environment, there are numerous and evolving risks to cybersecurity and privacy, including criminal hackers, hacktivists, state-sponsored intrusions, industrial espionage, employee malfeasance and human or technological error. High-profile security breaches at other companies and in government agencies have increased in recent years, and security industry experts and government officials have warned about the risks of hackers and cyberattacks targeting businesses such as ours. Computer hackers and others routinely attempt to breach the security of technology products, services and systems, and to fraudulently induce employees, customers, or others to disclosure information or unwittingly provide access to systems or data. A cyberattack of our systems or networks that impairs our information technology systems could disrupt our business operations and result in loss of service to customers, including technical support for our ReWalk devices. While we have certain cybersecurity safeguards in place designed to protect and preserve the integrity of our information technology systems, we have experienced and expect to continue to experience actual or attempted cyberattacks of our IT systems or networks. However, none of these actual or attempted cyberattacks has had a material effect on our operations or financial condition.
Additionally, we have access to sensitive customer information in the ordinary course of business. If a significant data breach occurred, our reputation may be adversely affected, customer confidence may be diminished, or we may be subject to legal claims, any of which may contribute to the loss of customers and have a material adverse effect on us. For more information, see “If we are found to have violated laws protecting the confidentiality of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.” above.
We are not able to protect our intellectual property rights in all countries.
Filing, prosecuting, maintaining, and defending patents on each of our products in all countries throughout the world would be prohibitively expensive, and thus our intellectual property rights outside the United States are limited. In addition, the laws of some foreign countries, especially developing countries, such as China, do not protect intellectual property rights to the same extent as federal and state laws in the United States. Also, it may not be possible to effectively enforce intellectual property rights in some countries at all or to the same extent as in the United States and other countries. Consequently, we are unable to prevent third parties from using our inventions in all countries, or from selling or importing products made using our inventions in the jurisdictions in which we do not have (or are unable to effectively enforce) patent protection. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop, market or otherwise commercialize their own products, and we may be unable to prevent those competitors from importing those infringing products into territories where we have patent protection, but enforcement may not be as strong as in the United States. These products may compete with our products and our patents and other intellectual property rights may not be effective or sufficient to prevent them from competing in those jurisdictions. Moreover, strategic partners, competitors or others in the chain of commerce may raise legal challenges against our intellectual property rights or may infringe upon our intellectual property rights, including through means that may be difficult to prevent or detect.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. Proceedings to enforce our patent rights in the United States or foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert patent infringement or other claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights in the United States and around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license from third parties.
We may be subject to patent infringement claims, which could result in substantial costs and liability and prevent us from commercializing our current and future products.
The medical device industry is characterized by competing intellectual property and a substantial amount of litigation over patent rights. In particular, our competitors in both the United States and abroad, many of which have substantially greater resources and have made substantial investments in competing technologies, have been issued patents and filed patent applications with respect to their products and processes and may apply for other patents in the future. The large number of patents, the rapid rate of new patent issuances, and the complexities of the technology involved increase the risk of patent litigation.
Determining whether a product infringes a patent involves complex legal and factual issues and the outcome of patent litigation is often uncertain. Even though we have conducted research of issued patents, no assurance can be given that patents containing claims covering our products, technology or methods do not exist, have not been filed or could not be filed or issued. In addition, because patent applications can take years to issue and because publication schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware and which may result in issued patents that our current or future products infringe. Also, because the claims of published patent applications can change between publication and patent grant, published applications that initially do not appear to be problematic may issue with claims that potentially cover our products, technology or methods.
39
Infringement actions and other intellectual property claims brought against us, whether with or without merit, may cause us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management, and harm our reputation. We cannot be certain that we will successfully defend against any allegations of infringement. If we are found to infringe another party’s patents, we could be required to pay damages. We could also be prevented from selling our infringing products, unless we can obtain a license to use the technology covered by such patents or can redesign our products so that they do not infringe. A license may be available on commercially reasonable terms or none at all, and we may not be able to redesign our products to avoid infringement. Further, any modification to our products could require us to conduct clinical trials and revise our filings with the FDA and other regulatory bodies, which would be time consuming and expensive. In these circumstances, we may not be able to sell our products at competitive prices or at all, and our business and operating results could be harmed.
We rely on trademark protection to distinguish our products from the products of our competitors.
We rely on trademark protection to distinguish our products from the products of our competitors. We have registered the trademark “ReWalk” in Israel and in the United States. The trademark “Restore” is already registered in Europe and allowed in the United States. In jurisdictions where we have not registered our trademark and are using it, and as permitted by applicable local law, we rely on common law trademark protection. Third parties may oppose our trademark applications, or otherwise challenge our use of the trademarks, and may be able to use our trademarks in jurisdictions where they are not registered or otherwise protected by law. If our trademarks are successfully challenged or if a third party is using confusingly similar or identical trademarks in particular jurisdictions before we do, we could be forced to rebrand our products, which could result in loss of brand recognition, and could require us to devote additional resources to marketing new brands. If others are able to use our trademarks, our ability to distinguish our products may be impaired, which could adversely affect our business. Further, we cannot assure you that competitors will not infringe upon our trademarks, or that we will have adequate resources to enforce our trademarks.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at other medical device companies, including our competitors or potential competitors, and we may hire employees in the future that are so employed. We could in the future be subject to claims that these employees, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. If we fail in defending against such claims, a court could order us to pay substantial damages and prohibit us from using technologies or features that are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. If any of these technologies or features that are important to our products, this could prevent us from selling those products and could have a material adverse effect on our business. Even if we are successful in defending against these claims, such litigation could result in substantial costs and divert the attention of management.
Risks Related to an Investment in Our Ordinary Shares
Sales of a substantial number of ordinary shares by us or our large shareholders, certain of whom may have registration rights, or dilutive exercises of a substantial number of warrants by our warrant-holders could adversely affect the value of our ordinary shares.
Sales by us or our shareholders of a substantial number of ordinary shares in the public market, or the perception that these sales might occur, could cause the value of our ordinary shares to decline or could impair our ability to raise capital through a future sale of our equity securities. Additionally, dilutive exercises of a substantial number of warrants by our warrant-holders, or the perception that such exercises may occur, could put downward price on the market price of our ordinary shares.
As of December 31, 2019, 2,853,299 ordinary shares were issuable pursuant to the exercise of warrants, with exercise prices ranging from $5.14 to $118.75 per warrant, issued in private and registered offerings of ordinary shares and warrants in November 2016, November 2018, February 2019, April 2019 and June 2019. There were also 6,679 ordinary shares issuable pursuant to the exercise of warrants granted to Kreos in connection with the Loan Agreement in January and December 2016, with an exercise price that is now set to $7.50 per warrant. For more information, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Loan Agreement with Kreos and Related Warrant to Purchase Ordinary Shares” and “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Equity Raises.”
40
We also entered into a registration rights agreement with Timwell to register under the Securities Act its privately-placed ordinary shares, 160,000 of which are currently outstanding and are registered on our Form S-3. For more information regarding the status of the Timwell transaction, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Timwell Private Placement.” Additionally, 2,522,284 ordinary shares underlying outstanding warrants are registered for resale on a Form S-1.
All shares sold pursuant to an offering covered by a registration statement would be freely transferable. With respect to the outstanding warrants, there may be certain restrictions on the holders to sell the underlying ordinary shares to the extent they are restricted securities, held by “affiliates” or would exceed certain ownership thresholds. Certain of our largest shareholders, may also have limitations under Rule 144 under the Securities Act on the resale of certain ordinary shares they hold unless they are registered for resale under the Securities Act. Despite these limitations, if we, our existing shareholders or their affiliates sell a substantial number of the above-mentioned ordinary shares in the public market, the market price of our ordinary shares could decrease significantly. Shareholders may also incur substantial dilution if holders of our warrants exercise their warrants to purchase ordinary shares, which could lower the market price of our ordinary shares. Any such decrease could impair the value of your investment in us.
U.S. holders of our ordinary shares may suffer adverse U.S. tax consequences if we are characterized as a passive foreign investment company, or a PFIC , under Section 1297(a) of the Code.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets (which may be determined in part by the market value of our ordinary shares, which is subject to change) are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in an offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
The determination of whether we are a PFIC will depend on the nature and composition of our income and the nature, composition and value of our assets from time to time. The 50% passive asset test described above is generally based on the fair market value of each asset, with the value of goodwill and going concern value determined in large part by reference to the market value of our ordinary shares, which may be volatile. If we are characterized as a “controlled foreign corporation,” or a “CFC”, under Section 957(a) of the Code and not considered publicly traded throughout the relevant taxable year, however, the passive asset test may be applied based on the adjusted tax bases of our assets instead of the fair market value of each asset (as described above). Under proposed Treasury Regulations, however, if we are treated as publicly traded for a majority of the relevant taxable year, our assets would generally be required to be measured at their fair market value, even if we are a CFC.
The proposed Treasury Regulations have not yet been adopted as final Treasury Regulations. Until such time as they are adopted as final Treasury Regulations, taxpayers may choose whether or not to apply them, provided (if they choose to apply them) they apply them consistently and in their entirety. The remainder of this discussion ignores the potential application of the proposed Treasury Regulations to the determination of whether we are a PFIC.
Based on our gross income and assets, the market price of our ordinary shares, and the nature of our business, we believe that we may have been a PFIC for the taxable year ended December 31, 2019. However, this determination is subject to uncertainty. In addition, there is a significant risk that we may be a PFIC for future taxable years, unless the market price of our ordinary shares increases or we reduce the amount of cash and other passive assets we hold relative to the amount of non-passive assets we hold. Accordingly, no assurances can be made regarding our PFIC status in one or more subsequent years, and our U.S. counsel expresses no opinion with respect to our PFIC status in the taxable year ended December 31, 2019, and also expresses no opinion with respect to our predictions or past determinations regarding our PFIC status in the past or in the future.
41
If we are characterized as a PFIC, U.S. holders of our ordinary shares may suffer adverse tax consequences, including having gains realized on the sale of our ordinary shares treated as ordinary income, rather than capital gain, the loss of the preferential tax rate applicable to dividends received on our ordinary shares by individuals who are U.S. holders and having interest charges apply to distributions by us and to the proceeds of sales of our ordinary shares. In addition, special information reporting may be required. Certain elections exist that may alleviate some of the adverse consequences of PFIC status and would result in an alternative treatment (such as mark-to-market treatment or being able to make a qualified electing fund election). However, we do not intend to provide the information necessary for U.S. Holders to make qualified electing fund elections if we are classified as a PFIC.
Additionally, if we are characterized as a PFIC, for any taxable year during which a U.S. Holder holds ordinary shares, we generally will continue to be treated as a PFIC with respect to such U.S. holder for all succeeding years during which such U.S. holder holds ordinary shares unless we cease to be a PFIC and such U.S. holder makes a “deemed sale” election with respect to such ordinary shares. If such election is made, such U.S. holder will be deemed to have sold such ordinary shares held by such U.S. holder at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain from such deemed sale would be treated as described above.
Future grants of ordinary shares under our equity incentive plans to our employees, non-employee directors and consultants, or sales by these individuals in the public market, could result in substantial dilution, thus decreasing the value of your investment in our ordinary shares, and certain grants may also require shareholder approval.
We have historically used, and continue to use, our ordinary shares as a means of both rewarding our employees, non-employee directors and consultants and aligning their interests with those of our shareholders. As of December 31, 2019, 149,500 ordinary shares remained available for issuance to our and our affiliates’ respective employees, non-employee directors and consultants under our equity incentive plans, including 137,091 ordinary shares subject to outstanding awards (consisting of outstanding options to purchase 74,713 ordinary shares and 62,378 ordinary shares underlying unvested RSUs. For more information, see Note 8c to our consolidated financial statements for the year ended December 31, 2019.
Additionally, the number of ordinary shares available for issuance under our 2014 Incentive Compensation Plan, or our 2014 Plan, may increase each year due to the operation of an “evergreen” provision previously approved by our shareholders. Pursuant to this provision, the 2014 Plan’s reserve increases on January 1 of each calendar year during the plan’s term by the lesser of (i) 38,880, (ii) 4% of the total number of shares outstanding on December 31 of the immediately preceding calendar year and (iii) an amount determined by our board of directors.
Additionally, to the extent registered on a Form S-8, ordinary shares granted or issued under our equity incentive plans will, subject to vesting provisions, lock-up restrictions and Rule 144 volume limitations applicable to our “affiliates,” be available for sale in the open market immediately upon registration. Sales of a substantial number of the above-mentioned ordinary shares in the public market could result in a significant decrease in the market price of our ordinary shares and have a material adverse effect on an investment in our ordinary shares.
The price of our ordinary shares may be volatile, and you may lose all or part of your investment.
Our ordinary shares were first publicly offered in our initial public offering in September 2014, at a price of $300.00 per share, and our ordinary shares have subsequently traded as high as $1,092.75 per share and as low as $0.82 per share through February 14, 2020. All prices have been adjusted to reflect our 25-to-1 reverse stock split, which we effected in 2019. The market price of our ordinary shares could be highly volatile and may fluctuate substantially as a result of many factors. Moreover, while there is no established public trading market for the warrants offered in our follow-on public offering completed in November 2016, and we do not expect one to develop, our ordinary shares will be issuable pursuant to exercise of these warrants. Because the warrants are exercisable into our ordinary shares, volatility or a reduction in the market price of our ordinary shares could have an adverse effect on the trading price of the warrants. Factors which may cause fluctuations in the price of our ordinary shares include, but are not limited to:
| ● | actual or anticipated fluctuations in our growth rate or results of operations or those of our competitors; | |
| ● | customer acceptance of our products; | |
| ● | announcements by us or our competitors of new products or services, commercial relationships, acquisitions or expansion plans; | |
| ● | announcements by us or our competitors of other material developments; | |
| ● | our involvement in litigation; | |
| ● | changes in government regulation applicable to us and our products; |
42
| ● | sales, or the anticipation of sales, of our ordinary shares, warrants and debt securities by us, or sales of our ordinary shares by our insiders or other shareholders, including upon expiration of contractual lock-up agreements; | |
| ● | developments with respect to intellectual property rights; | |
| ● | competition from existing or new technologies and products; | |
| ● | changes in key personnel; | |
| ● | the trading volume of our ordinary shares; | |
| ● | changes in the estimation of the future size and growth rate of our markets; | |
| ● | changes in our quarterly or annual forecasts with respect to operating results and financial conditions; and | |
| ● | general economic and market conditions. |
In addition, the stock markets have experienced extreme price and volume fluctuations. Broad market and industry factors may materially harm the market price of our ordinary shares, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. If we were involved in any similar litigation, we could incur substantial costs and our management’s attention and resources could be diverted.
If we do not meet the expectations of equity research analysts, if any, if the sole remaining equity analyst following our business does not continue to publish research or reports about our business or if the analyst issues unfavorable commentary or downgrade our ordinary shares, the price of our ordinary shares could decline. Additionally, we may fail to meet publicly announced financial guidance or other expectations about our business, which would cause our ordinary shares to decline in value.
There is currently one equity analyst publishing research reports about our business. If our results of operations are below the estimates or expectations of our sole analyst and investors, our share price could decline. Moreover, the price of our ordinary shares could decline if one or more securities analysts downgrade our ordinary shares or if analysts issue other unfavorable commentary or stop publishing research or reports about us or our business (as has occurred over time, with a decrease in the number of analysts following us from five in 2014 to one in 2020).
From time to time, we have also faced difficulty accurately projecting our earnings and have missed certain of our publicly announced guidance. If our financial results for a particular period do not meet our guidance or if we reduce our guidance for future periods, the market price of our ordinary shares may decline.
We are a “smaller reporting company” and we cannot be certain whether the reduced requirements applicable to smaller reporting companies will make our ordinary shares less attractive to investors.
We are a “smaller reporting company” under the rules of the Securities Act and the Exchange Act. As a result, we may choose to take advantage of certain scaled disclosure requirements available specifically to smaller reporting companies. For example, we are not required to provide market risk disclosures, a contractual obligations table in our management’s discussion and analysis of our financial condition and results of operations or selected financial data in our annual report. Additionally, as long as we continue to be a smaller reporting company, we may continue to use reduced compensation disclosure obligations, and, provided we are also a non-accelerated filer, we will not be obligated to follow the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act.. We will remain a smaller reporting company until the last day of the fiscal year in which the aggregate market value of ordinary shares held by non-affiliated persons and entities (known as our “public float”) was at least $250.0 million or the last day of the fiscal year in which we have at least $100 million in revenue and at least $700 million in public float, and we will remain a non-accelerated filer until the last day of the fiscal year in which our public float was at least $75.0 million (in each case, with respect to public float, as measured as of the last business day of the second quarter of such fiscal year).
We cannot predict or otherwise determine if investors will find our securities less attractive as a result of our reliance on exemptions as a smaller reporting company. If some investors find our securities less attractive as a result, there may be a less active trading market for our ordinary shares and the price of our ordinary shares may be more volatile.
43
We are subject to ongoing costs and risks associated with determining whether our existing internal controls over financial reporting systems are compliant with Section 404 of the Sarbanes-Oxley Act, and if we fail to achieve and maintain adequate internal controls it could have a material adverse effect on our stated results of operations and harm our reputation.
We are required to comply with the internal control, evaluation, and certification requirements of Section 404 of the Sarbanes-Oxley Act and the Public Company Accounting Oversight Board. Once we no longer qualify as a “smaller reporting company,” our independent registered public accounting firm will need to attest to the effectiveness of our internal control over financial reporting under Section 404.
The process of determining whether our existing internal controls over financial reporting systems are compliant with Section 404 and whether there are any material weaknesses or significant deficiencies in our existing internal controls requires the investment of substantial time and resources, including by our Chief Financial Officer and other members of our senior management. This determination and any remedial actions required could divert internal resources and take a significant amount of time and effort to complete and could result in us incurring additional costs that we did not anticipate, including the hiring of outside consultants. We could experience higher than anticipated operating expenses and higher independent auditor fees during and after the implementation of these changes.
Irrespective of compliance with Section 404, any failure of our internal controls could have a material adverse effect on our stated results of operations and harm our reputation. If we are unable to implement any of the required changes to our internal control over financial reporting effectively or efficiently or are required to do so earlier than anticipated, it could adversely affect our operations, financial reporting and/or results of operations and could result in an adverse opinion on internal controls from our management and our independent auditors. Further, if our internal control over financial reporting is not effective, the reliability of our financial statements may be questioned and our share price may suffer.
Risks Related to Our Incorporation and Location in Israel
Our technology development and quality headquarters and the manufacturing facility for our products are located in Israel and, therefore, our results may be adversely affected by economic restrictions imposed on, and political and military instability in, Israel.
Our technology development and quality headquarters, which houses substantially all of our research and development and our core research and development team, including engineers, machinists, researchers, and clinical and regulatory personnel, as well as the facility of our contract manufacturer, Sanmina, are located in Israel. Many of our employees, directors and officers are residents of Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors, Hamas (an Islamist militia and political group in the Gaza Strip), Hezbollah (an Islamist militia and political group in Lebanon) and other armed groups. Any hostilities involving Israel or the interruption or curtailment of trade within Israel or between Israel and its trading partners could materially and adversely affect our business, financial condition and results of operations and could make it more difficult for us to raise capital. In particular, an interruption of operations at the Tel Aviv airport related to the conflict in the Gaza Strip or otherwise could prevent or delay shipments of our components or products. Although we maintain inventory in the United States and Germany, an extended interruption could materially and adversely affect our business, financial condition and results of operations.
Recent political uprisings, social unrest and violence in various countries in the Middle East and North Africa, including Israel’s neighbors Egypt and Syria, are affecting the political stability of those countries. This instability may lead to deterioration of the political relationships that exist between Israel and these countries and has raised concerns regarding security in the region and the potential for armed conflict. Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Any losses or damages incurred by us could have a material adverse effect on our business. In addition, Iran has threatened to attack Israel and is widely believed to be developing nuclear weapons. Iran is also believed to have a strong influence among parties hostile to Israel in areas that neighbor Israel, such as the Syrian government, Hamas in Gaza and Hezbollah in Lebanon. Any armed conflicts, terrorist activities or political instability in the region could materially and adversely affect our business, financial condition and results of operations.
Our operations and the operations of our contract manufacturer, Sanmina, may be disrupted as a result of the obligation of Israeli citizens to perform military service.
Many Israeli citizens are obligated to perform one month, and in some cases more, of annual military reserve duty until they reach the age of 45 (or older, for reservists with certain occupations) and, in the event of a military conflict, may be called to active duty. In response to terrorist activity, there have been periods of significant call-ups of military reservists. For example, the Israeli armed forces called up a significant number of reservists to active duty in connection with the recent conflict in the Gaza Strip. It is possible that there will be additional military reserve duty call-ups in the future in connection with this conflict or otherwise. Some of our executive officers and employees, as well as those of Sanmina, the manufacturer of all of our products, are required to perform annual military reserve duty in Israel and may be called to active duty at any time under emergency circumstances. Although these call-ups have not had a material impact on our operations or on Sanmina’s ability to manufacture our products, our operations and the operations of Sanmina could be disrupted by such call-ups.
44
Our sales may be adversely affected by boycotts of Israel.
Several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies whether as a result of hostilities in the region or otherwise. In addition, there have been increased efforts by activists to cause companies and consumers to boycott Israeli goods based on Israeli government policies. Such actions, particularly if they become more widespread, may adversely impact our ability to sell our products.
The tax benefits that are available to us require us to continue to meet various conditions and may be terminated or reduced in the future, which could increase our costs and taxes.
Some of our operations in Israel, referred to as “Beneficiary Enterprises,” carry certain tax benefits under the Israeli Law for the Encouragement of Capital Investments, 5719-1959, or the Investment Law. Substantially all of our future income before taxes can be attributed to these programs. If we do not meet the requirements for maintaining these benefits or if our assumptions regarding the key elements affecting our tax rates are rejected by the tax authorities, they may be reduced or cancelled and the relevant operations would be subject to Israeli corporate tax at the standard rate. In addition to being subject to the standard corporate tax rate, we could be required to refund any tax benefits that we may receive in the future, plus interest and penalties thereon. Even if we continue to meet the relevant requirements, the tax benefits that our current “Beneficiary Enterprises” receive may not be continued in the future at their current levels or at all. If these tax benefits were reduced or eliminated, the amount of taxes that we pay would likely increase, as all of our Israeli operations would consequently be subject to corporate tax at the standard rate, which could adversely affect our results of operations. Additionally, if we increase our activities outside of Israel, for example, by way of acquisitions, our increased activities may not be eligible for inclusion in Israeli tax benefit programs. For a discussion of our current tax obligations, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
We have received Israeli government grants for certain of our research and development activities and we may receive additional grants in the future. The terms of those grants restrict our ability to manufacture products or transfer technologies outside of Israel, and we may be required to pay penalties in such cases or upon the sale of our company.
From our inception through December 31, 2019, we received a total of $1.97 million from the Israel Innovation Authority, or the IIA. We may in the future apply to receive additional grants from the IIA to support our research and development activities. With respect to such grants we are committed to pay royalties at a rate of 3.0% to 3.5% on sales proceeds up to the total amount of grants received, linked to the dollar and bearing interest at an annual rate of LIBOR applicable to dollar deposits. Even after payment in full of these amounts, we will still be required to comply with the requirements of the Israeli Encouragement of Industrial Research, Development and Technological Innovation Law, 1984, or the R&D Law, and related regulations, with respect to those past grants. When a company develops know-how, technology or products using IIA grants, the terms of these grants and the R&D Law restrict the transfer outside of Israel of such know-how, and of the manufacturing or manufacturing rights of such products, technologies or know-how, without the prior approval of the IIA. Therefore, if aspects of our technologies are deemed to have been developed with IIA funding, the discretionary approval of an IIA committee would be required for any transfer to third parties outside of Israel of know-how or manufacturing or manufacturing rights related to those aspects of such technologies. Furthermore, the IIA may impose certain conditions on any arrangement under which it permits us to transfer technology or development out of Israel or may not grant such approvals at all.
Furthermore, the consideration available to our shareholders in a future transaction involving the transfer outside of Israel of technology or know-how developed with IIA funding (such as a merger or similar transaction) may be reduced by any amounts that we are required to pay to the IIA.
In addition to the above, any non-Israeli citizen, resident or entity that, among other things, (i) becomes a holder of 5% or more of our share capital or voting rights, (ii) is entitled to appoint one or more of our directors or our chief executive officer or (iii) serves as one of our directors or as our chief executive officer (including holders of 25% or more of the voting power, equity or the right to nominate directors in such direct holder, if applicable) is required to notify the IIA and undertake to comply with the rules and regulations applicable to the grant programs of the IIA, including the restrictions on transfer described above. Such notification will be required in connection with the investment being made by an investor.
45
We may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could result in litigation and adversely affect our business.
A significant portion of our intellectual property has been developed by our employees in the course of their employment for us. Under the Israeli Patent Law, 5727-1967, or the Patent Law, and recent decisions by the Israeli Supreme Court and the Israeli Compensation and Royalties Committee, a body constituted under the Patent Law, employees may be entitled to remuneration for intellectual property that they develop for us unless they explicitly waive any such rights, although the validity of any such waivers remains open to judicial review. Although we enter into agreements with our employees pursuant to which they agree that any inventions created in the scope of their employment or engagement are owned exclusively by us, we may face claims demanding remuneration. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current and former employees, or be forced to litigate such claims, which could negatively affect our business.
Provisions of Israeli law and our Articles of Association may delay, prevent or otherwise impede a merger with, or an acquisition of, us, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a tender offer for all of a company’s issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion of the tender offer also requires approval of a majority of the offerees that do not have a personal interest in the tender offer, unless at least 98% of the company’s outstanding shares are tendered. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer (unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek appraisal rights), may, at any time within six months following the completion of the tender offer, petition an Israeli court to alter the consideration for the acquisition.
Our Articles of Association provide that our directors (other than external directors) are elected on a staggered basis, such that a potential acquirer cannot readily replace our entire board of directors at a single annual general shareholder meeting. This could prevent a potential acquirer from receiving board approval for an acquisition proposal that our board of directors opposes.
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers involving an exchange of shares, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred. These and other similar provisions could delay, prevent or impede an acquisition of us or our merger with another company, even if such an acquisition or merger would be beneficial to us or to our shareholders.
We recently amended our articles of association to increase our authorized share capital. There are certain risks associated with this increase.
In late March 2019, following the receipt of shareholder approval, we filed the Third Amended and Restated Articles of Association of the Company with the Registrar of Companies of the State of Israel to increase the Company’s authorized share capital after the effect of the reverse share split (as well as to reflect the reverse share split itself). As a result of the amendment, the total number of ordinary shares the Company is authorized to issue changed from 250,000,000 shares to 60,000,000 shares and the authorized share capital of the Company changed from NIS 2,500,000 to NIS 15,000,000 (representing proportional increases after giving effect to the reverse share split that was effected on April 1, 2019). The objective of the increase in authorized share capital was to maintain our flexibility following the reverse share split to conduct future issuances of our ordinary shares, in the ordinary course from time to time, to fund our operations, consistent with our historical practice of raising financing through equity and debt issuances.
Although the purpose of the increase in authorized share capital was to preserve our capital-raising position, these additional shares may also be issued in the future for other purposes, such as compensation, giving rise to further opportunities for dilution. Future issuances of ordinary shares will dilute the voting power and ownership of our existing shareholders, and, depending on the amount of consideration received in connection with the issuance, could also reduce shareholders’ equity on a per-share basis. Due to the increase in authorized capital, the dilution to the ownership interest of our existing shareholders may be greater than would occur had the increase not been effected.
46
The newly-available authorized shares resulting from the reverse share split may have the potential to limit the opportunity for our shareholders to dispose of their ordinary shares at a premium. We currently do not have any acquisitions or other major transactions planned that would require us to increase our authorized share capital, and our board does not intend to use the increase of the newly-authorized reserve as an anti-takeover device. However, the authorized shares could, in theory, also be used to resist or frustrate a third-party transaction that is favored by a majority of the independent shareholders (for example, by permitting issuances that would dilute the share ownership of a person seeking to effect a change in the composition of the board or management of the Company or contemplating a tender offer or other transaction for the combination of the Company with another company).
It may be difficult to enforce a judgment of a U.S. court against us, our officers and directors, to assert U.S. securities laws claims in Israel or to serve process on our officers and directors.
We are incorporated in Israel. Although the majority of our directors and executive officers reside within the United States and most of the assets of these persons are also likely located within the United States, some of our directors and executive officers reside and may have the majority of their assets outside the United States. Additionally, most of our assets are located outside of the United States. Therefore, a judgment obtained against us, or those of our directors and executive officers residing outside of the United States, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process in the United States on those directors and executive officers residing outside of the United States or to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may be able to collect only limited, or may be unable to collect any, damages awarded by either a U.S. or foreign court.
Your rights and responsibilities as a shareholder will be governed by Israeli law which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
The rights and responsibilities of the holders of our ordinary shares are governed by our Articles of Association and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in U.S.-based corporations. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders, and to refrain from abusing its power in the company, including, among other things, in voting at a general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval. In addition, a shareholder who is aware that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company. There is limited case law available to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our ordinary shares that are not typically imposed on shareholders of U.S. corporations.
Our business could be negatively affected as a result of actions of activist shareholders, and such activism could impact the trading value of our securities.
In recent years, certain Israeli issuers listed on United States exchanges have been faced with governance-related demands from activist shareholders, unsolicited tender offers and proxy contests. Responding to these types of actions by activist shareholders could be costly and time-consuming, disrupting our operations and diverting the attention of management and our employees. Such activities could interfere with our ability to execute our strategic plan. In addition, a proxy contest for the election of directors at our annual meeting would require us to incur significant legal fees and proxy solicitation expenses and require significant time and attention by management and our board of directors. The perceived uncertainties as to our future direction also could affect the market price and volatility of our securities.
47
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
Our corporate headquarters are located in Yokneam, Israel, our U.S. headquarters are located in Marlborough, Massachusetts, and our European headquarters are located in Berlin, Germany.
All of our facilities are leased and we do not own any real property. The table below sets forth details of the square footage of our current leased properties, all of which are fully utilized. We have no material tangible fixed assets apart from the properties described below.
| Square feet(approximate) | ||||
| Marlborough, Massachusetts | 11,850 | |||
| Yokneam, Israel | 11,500 | |||
| Berlin, Germany | 484 | |||
| Total | 23,834 | |||
We believe our facilities are adequate and suitable for our current needs.
48
Occasionally the Company is involved in various claims, lawsuits, regulatory examinations, investigations and other legal matters arising, for the most part, in the ordinary course of business. The outcome of litigation and other legal matters is inherently uncertain. In making a determination regarding accruals, using available information, the Company evaluates the likelihood of an unfavorable outcome in legal or regulatory proceedings to which the Company is a party and records a loss contingency when it is probable a liability has been incurred and the amount of the loss can be reasonably estimated.
Where the Company determines an unfavorable outcome is not probable or reasonably estimable, the Company does not accrue for any potential litigation loss. These subjective determinations are based on the status of such legal or regulatory proceedings, the merits of our defenses and consultation with legal counsel. Actual outcomes of these legal and regulatory proceedings may materially differ from the Company’s current estimates. It is possible that resolution of one or more of the legal matters currently pending or threatened could result in losses material to the Company’s consolidated results of operations, liquidity or financial condition.
As previously disclosed, between September 2016 and January 2017, eight putative class actions on behalf of alleged shareholders that purchased or acquired our ordinary shares pursuant and/or traceable to our registration statement on Form F-1 (File No. 333-197344) used in connection with our initial public offering, or our IPO, were commenced in the following courts: (i) the Superior Court of the State of California, County of San Mateo; (ii) the Superior Court of the Commonwealth of Massachusetts, Suffolk County; (iii) the United States District Court for the Northern District of California; and (iv) the United States District Court for the District of Massachusetts. As of February 11, 2020, seven have been dismissed and one has been partially dismissed. The actions involved or involve claims under various sections of the Securities Act of 1933, or the Securities Act, against us, certain of our current and former directors and officers, the underwriters of our IPO and certain other defendants.
The four actions commenced in the Superior Court of the State of California, County of San Mateo were dismissed in January 2017 for lack of personal jurisdiction, and the action commenced in the United States District Court for the Northern District of California was voluntarily dismissed in March 2017. Additionally, the two actions commenced in the Superior Court of the Commonwealth of Massachusetts, Suffolk County, or the Superior Court, were consolidated in December 2017, and voluntarily dismissed with prejudice in November 2018, after the District Court for the District of Massachusetts partially dismissed the related claims in that court and the parties in the Superior Court entered a stipulation of dismissal with prejudice.
The action commenced in the United States District Court for the District of Massachusetts (the “District Court”), alleging violations of Sections 11 and 15 of the Securities Act and Sections 10(b) and 20(a) of the Exchange Act, was partially dismissed on August 23, 2018. In particular, the District Court granted the motion to dismiss the claims under Sections 11 and 15 of the Securities Act, finding that the plaintiff failed to plead a false or misleading statement in the IPO registration statement. The District Court did not address the claims under Sections 10(b) and 20(a) of the Exchange Act because, as a result of the dismissal of the claims under the Securities Act, the lead plaintiff lacked standing to pursue those claims. Because the action in the District Court was styled as a class action, the District Court permitted the plaintiff to file a supplemental memorandum concerning standing or a motion to appoint a substitute or supplemental plaintiff. On September 10, 2018, the plaintiff sought leave to amend his complaint to add a new plaintiff that purportedly has standing to pursue Exchange Act claims, and the Company opposed the motion to amend on September 24, 2018. On May 16, 2019, the court denied the plaintiff’s motion to amend and the complaint was dismissed. Thereafter, the plaintiff timely appealed to the United States Court of Appeals for the First Circuit. The appeal has been fully briefed and oral arguments have been scheduled for March 2, 2020.
Based on information currently available and the current stage of the litigation, we are unable to reasonably estimate a possible loss or range of possible losses, if any, with regard to the remaining lawsuit in the District Court; therefore, no litigation reserve has been recorded in our consolidated balance sheets as of December 31, 201. We will continue to evaluate information as it becomes known and will record an estimate for losses at the time or times if and when it is probable that a loss will be incurred and the amount of the loss is reasonably estimable For more information, see the information in Note 2s and Note 7e to the Company’s consolidated financial statements set forth in “Part II, Item 8. Financial Statements and Supplementary Data” of this annual report.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
49
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our ordinary shares began trading publicly on The Nasdaq Global Market on September 12, 2014 under the symbol “RWLK” and were transferred for listing on The Nasdaq Capital Market effective May 25, 2017.
Dividend Policy
We have never declared or paid any cash dividends on our ordinary shares. We do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain future earnings, if any, to finance operations and expand our business. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on a number of factors, including future earnings, capital requirements, financial condition and future prospects and other factors our board of directors may deem relevant. The distribution of dividends may also be limited by Israeli law, which permits the distribution of dividends only out of retained earnings or otherwise upon the permission of an Israeli court.
Israeli Taxes Applicable to U.S. Holders
A non-Israeli resident who derives capital gains from the sale of shares in an Israeli resident company that were purchased after the company was listed for trading on a stock exchange outside of Israel will be exempt from Israeli tax so long as the shares were not held through a permanent establishment that the non-resident maintains in Israel. A partial exemption may be available for non-Israeli resident shareholders who acquired their shares prior to the issuer’s initial public offering.
However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents (i) have a controlling interest of more than 25% in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly. Such exemption is not applicable to a person whose gains from selling or otherwise disposing of the shares are deemed to be a business income. Additionally, under the United States-Israel Tax Treaty, or the treaty, the disposition of shares by a shareholder who (i) is a U.S. resident (for purposes of the treaty), (ii) holds the shares as a capital asset, and (iii) is entitled to claim the benefits afforded to such person by the treaty, is generally exempt from Israeli capital gains tax. Such exemption will not apply if: (i) the capital gain arising from the disposition can be attributed to a permanent establishment in Israel; (ii) the shareholder holds, directly or indirectly, shares representing 10% or more of the voting capital during any part of the 12-month period preceding the disposition, subject to certain conditions; or (iii) such U.S. resident is an individual and was present in Israel for 183 days or more during the relevant taxable year. In such case, the sale, exchange or disposition of our ordinary shares should be subject to Israeli tax, to the extent applicable; however, under the treaty, the taxpayer would be permitted to claim a credit for such taxes against the U.S. federal income tax imposed with respect to such sale, exchange or disposition, subject to the limitations under U.S. law applicable to foreign tax credits. The treaty does not relate to U.S. state or local taxes.
50
In some instances where our shareholders may be liable for Israeli tax on the sale of their ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source. If the above exemptions from capital gains tax are not available, individuals will be subject to a 25% tax rate on real capital gains derived from the sale of shares as long as the individual is not a substantial shareholder of the corporation issuing the shares (in which case the individual will be subject to a 30% tax rate), and corporations will be subject to a 23% corporate tax rate. A substantial shareholder is generally a person who alone or together with such person’s relative or another person who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the means of control of the corporation, including the right to vote, receive profits, nominate a director or an executive officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right. A substantial shareholder will be subject to tax at a rate of 30% in respect of capital gains derived from the sale of shares issued by a corporation in which he or she is a substantial shareholder. The determination of whether the individual is a substantial shareholder will be made on the date on which the securities are sold. In addition, the individual will be deemed to be a substantial shareholder if at any time during the 12 months preceding the date of sale he or she was a substantial shareholder.
Dividends paid on publicly traded shares, like our ordinary shares, to non-Israeli residents are generally subject to Israeli withholding tax at a rate of 25%, unless a different rate is provided under an applicable tax treaty, provided that a certificate from the Israeli Tax Authority allowing for a reduced withholding tax rate is obtained in advance. Under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our ordinary shares who is a U.S. resident (for purposes of the treaty) is 25%. The treaty provides for reduced tax rates on dividends if (a) the shareholder is a U.S. corporation holding at least 10% of our issued voting power during the part of the tax year that precedes the date of payment of the dividend and held such minimal percentage during the whole of its prior tax year, and (b) not more than 25% of the Israeli company’s gross income consists of interest or dividends, other than dividends or interest received from subsidiary corporations or corporations 50% or more of the outstanding voting shares of which is owned by the Israeli company. The reduced treaty rate, if applicable, is 15% in the case of dividends paid from income derived from a Beneficiary or Preferred Enterprise (which is entitled to corporate tax benefits) or 12.5% otherwise. We cannot assure you that in the event we declare a dividend we will designate the income out of which the dividend is paid in a manner that will reduce shareholders’ tax liability. If the dividend is attributable partly to income derived from a Beneficiary or Preferred Enterprise and partly to other sources of income, the withholding rate will be a blended rate reflecting the relative portions of the two types of income. U.S. residents who are subject to Israeli withholding tax on a dividend may be entitled to a credit or deduction for U.S. federal income tax purposes in the amount of the taxes withheld.
Individuals who are subject to tax in Israel are also subject to an additional tax at a rate of 3% (as of 2017) on annual income exceeding a certain threshold (NIS 649,560 for 2019, linked to the annual change in the Israeli Consumer Price Index), including, but not limited to income derived from, dividends, interest and capital gains.
If the above exemptions from capital gains tax are not available, corporations will be subject to the corporate tax rate (23%) on capital gains derived from the sale of shares.
51
Stock Performance Graph
The following stock performance graph represents the cumulative total shareholder return for the period September 12, 2014 (the date upon which trading of our ordinary shares commenced) through December 31, 2019 for our ordinary shares, compared to the Nasdaq Composite Index and the Nasdaq Medical Equipment Index. The returns shown in the graph below may not be indicative of future performance.
COMPARISON OF
65 MONTH CUMULATIVE TOTAL RETURN*
Among ReWalk Robotics, the NASDAQ Composite Index
and the NASDAQ Medical Equipment Index
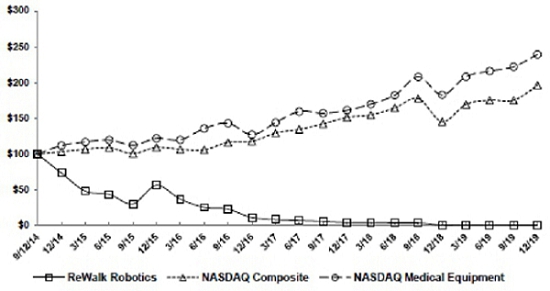
*$100 invested on 9/12/14 in stock
or 8/31/14 in index, including reinvestment of dividends.
Fiscal year ending December 31.
The above stock performance graph shall not be deemed to be soliciting material or to be filed with the SEC under the Securities Act and the Exchange Act except to the extent that we specifically request that such information be treated as soliciting material or specifically incorporate it by reference into a filing under the Securities Act or the Exchange Act.
Recent Sales of Unregistered Securities
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
52
ITEM 6. SELECTED FINANCIAL DATA
The following table presents our selected historical consolidated financial data, which is derived from our consolidated financial statements, which have been prepared in accordance with U.S. Generally Accepted Accounting Principles, or U.S. GAAP. The selected consolidated statements of operations data for the years ended December 31, 2019, 2018, and 2017 and the selected consolidated balance sheet data as of December 31, 2019, and 2018 are derived from our audited consolidated financial statements set forth in “Part II. Item 8. Financial Statements and Supplementary Data” of this annual report. The selected consolidated statement of operations data for the years ended December 31, 2016 and December 31, 2015 and the selected consolidated balance sheet data as of December 31, 2017, 2016 and 2015 has been derived from our audited consolidated financial statements not included in this annual report.
You should read the following selected consolidated financial data in conjunction with “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and it is qualified in its entirety by, reference to our consolidated financial statements and the related notes set forth in “Part II. Item 8. Financial Statements and Supplementary Data” of this annual report. The historical results set forth below are not necessarily indicative of the results to be expected in future periods.
| Year ended December 31 | ||||||||||||||||||||
| (In thousands, except share and per share data) | ||||||||||||||||||||
| 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||||
| Revenues | $ | 4,873 | $ | 6,545 | $ | 7,753 | $ | 5,869 | $ | 3,746 | ||||||||||
| Cost of revenues | 2,147 | 3,720 | 4,652 | 5,133 | 3,532 | |||||||||||||||
| Gross profit | 2,726 | 2,825 | 3,101 | 736 | 214 | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development, net | 5,348 | 7,349 | 6,042 | 9,028 | 5,937 | |||||||||||||||
| Sales and marketing | 6,167 | 7,897 | 11,360 | 13,961 | 13,056 | |||||||||||||||
| General and administrative | 5,259 | 6,793 | 7,691 | 8,188 | 6,395 | |||||||||||||||
| Total operating expenses | 16,774 | 22,039 | 25,093 | 31,177 | 25,388 | |||||||||||||||
| Operating loss | (14,048 | ) | (19,214 | ) | (21,992 | ) | (30,441 | ) | (25,174 | ) | ||||||||||
| Loss on extinguishment of debt | — | — | 313 | — | — | |||||||||||||||
| Financial expenses, net | 1,496 | 2,466 | 2,293 | 2,059 | 188 | |||||||||||||||
| Loss before income taxes | (15,544 | ) | (21,680 | ) | (24,598 | ) | (32,500 | ) | (25,362 | ) | ||||||||||
| Income taxes (tax benefit) | 7 | (5 | ) | 119 | 3 | 53 | ||||||||||||||
| Net loss | $ | (15,551 | ) | $ | (21,675 | ) | $ | (24,717 | ) | $ | (32,503 | ) | $ | (25,415 | ) | |||||
| Net loss per ordinary share, basic and diluted | $ | (2.70 | ) | $ | (14.72 | ) | $ | (30.57 | ) | (61.66 | ) | (52.45 | ) | |||||||
| Weighted average number of shares used in computing net loss per ordinary share, basic and diluted | 5,763,317 | 1,472,499 | 808,557 | 527,096 | 484,602 | |||||||||||||||
| As of December 31, | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||||
| Balance Sheet Data: | ||||||||||||||||||||
| Cash and cash equivalents | $ | 16,253 | $ | 9,546 | $ | 14,567 | $ | 23,678 | $ | 17,689 | ||||||||||
| Total assets | 24,372 | 14,962 | 22,863 | 31,763 | 25,574 | |||||||||||||||
| Accumulated deficit | (168,469 | ) | (152,918 | ) | (131,220 | ) | (106,492 | ) | (73,989 | ) | ||||||||||
| Total shareholders’ equity | $ | 10,780 | $ | 1,945 | $ | 3,707 | $ | 8,260 | $ | 20,920 | ||||||||||
| (1) | Net loss per ordinary share, basic and diluted, is calculated by dividing our net loss excluding dividends accrued on our convertible preferred shares outstanding during the period presented by the weighted average number of shares outstanding during the period presented. See Note 2r to our consolidated financial statements set forth in “Part II. Item 8. Financial Statements and Supplementary Data” of this annual report. |
53
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with “Part I. Item 6. Selected Financial Data” and our consolidated financial statements and the related notes included elsewhere in this annual report. This discussion contains forward-looking statements that are based on our management’s current expectations, estimates and projections for our business, which are subject to a number of risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those set forth under “Special Note Regarding Forward-Looking Statements and “Part I. Item 1A. Risk Factors.”
Overview
We are an innovative medical device company that is designing, developing and commercializing robotic exoskeletons that allow individuals with mobility impairments or other medical conditions the ability to stand and walk once again. We have developed and are continuing to commercialize our SCI Products, ReWalk Personal and ReWalk Rehab devices, these exoskeletons designed for individuals with paraplegia that uses our patented tilt-sensor technology and an on-board computer and motion sensors to drive motorized legs that power movement. Additionally, we have also developed and commercialized our ReStore device in June 2019, ReStore is a powered, lightweight soft exo-suit intended for use in the rehabilitation of individuals with lower limb disability due to stroke.
We have in the past generated and expect to generate in the future revenues from a combination of third-party payors, self-payors, including private and government employers, and institutions. While a broad uniform policy of coverage and reimbursement by third-party commercial payors currently does not exist in the United States for electronic exoskeleton technologies such as ReWalk, we are pursuing various paths of reimbursement and support fundraising efforts by institutions and clinics. In December 2015, the VA issued a national policy for the evaluation, training and procurement of ReWalk Personal exoskeleton units for all qualifying veterans across the United States. The VA policy is the first national coverage policy in the United States for qualifying individuals who have suffered spinal cord injury. As of December 31, 2019, we had placed 24 units as part of the VA policy.
In June 2018, the VA updated its national policy to provide expanded access to ReWalk exoskeletons for veterans in private rehabilitation clinics through the Veterans Choice Program. Under the VA’s revised policy, the exoskeleton evaluation process will have all veterans flow through one of 24 designated spinal cord injury VA centers (which we refer to as “SCI/D”). Once a veteran is determined to be qualified for training and procurement of his/her own exoskeleton unit, the individual may be allowed to pursue training on exoskeleton use, such as use of the ReWalk (i) at the applicable SCI/D hub center; (ii) on a case-by-case basis, at a qualified VA hospital designated by the VA’s “hub & spoke” program; or (iii) on a case-by-case basis, at a qualified private rehabilitation center via the VA’s Veteran’s Choice Program, through which veterans can receive care from a community provider paid for by the VA. Additionally, to date several private insurers in the United States and Europe have provided reimbursement for ReWalk in certain cases. In addition, in September 2017, German insurer Barmer signed a confirmation letter regarding the provision of ReWalk units for all qualifying beneficiaries and the German national social accident insurance provider DGUV indicated that its member payers will approve the supply of exoskeleton systems for qualifying beneficiaries on a case-by-case basis. In February 2018, the head office of German statutory health insurance, or SHI, Spitzenverband (“GKV”) confirmed their decision to list the ReWalk Personal 6.0 Exoskeleton unit in the German Medical Device Directory. This decision means that ReWalk will be listed among all medical devices for compensation, which SHI providers can procure for any approved beneficiary on a case-by-case basis. We are currently working with several SHI’s on securing a formal operating contract that will establish the process of obtaining a ReWalk Personal 6.0 device for their beneficiaries within their system.
We have incurred net losses and negative cash flow from operations since inception and anticipate this to continue in the near term. We will continue to evaluate spending while continuing to focus resources on activities to commercialize the Restore device for stroke patients, achieving additional commercial reimbursement coverage decisions for our ReWalk Personal device, continued research and development activities related mainly to our soft exo-suit design and activities related to our FDA 522 postmarket study.
54
Components of Our Statements of Operations
Revenues
We currently rely, and in the future will rely, on sales and rentals of our ReWalk systems as well as our ReStore device and related service contracts and extended warranties for our revenue. Our revenue is generated from a combination of third-party payors, institutions and self-payors, including private and government employers. Payments for our products by third party payors have been made primarily through case-by-case determinations. Third-party payors include, without limitation, private insurance plans and managed care programs, government programs including the VA, and worker’s compensation payments. We expect that third-party payors will be an increasingly important source of revenue in the future as well as clinics that will be interested in the ReStore device. In December 2015, the VA issued a national policy for the evaluation, training and procurement of ReWalk Personal exoskeleton systems for all qualifying veterans across the United States. The VA policy is the first national coverage policy in the United States for qualifying individuals who have suffered spinal cord injury.
All of our ReWalk Personal and ReWalk Rehabilitation systems sold until the end of 2017 were covered by a two-year warranty from the date of purchase, which is included in the purchase price. We offer customers the ability to purchase, any time during the initial warranty period, an extended warranty for up to three additional years. Both warranties cover all elements of the systems, including the batteries, other than normal wear and tear. In the beginning of 2018 we updated our service policy for new devices sold to include a five-year warranty. Our ReStore device is sold with a two-year warranty.
Revenues are presented net of the amounts of any provision we record for expected future product returns.
Cost of Revenues and Gross Profit
Cost of revenue consists primarily of systems purchased from our outsourced manufacturer, Sanmina, salaries, personnel costs including non-cash share based compensation, associated with manufacturing and inventory management, training and inspection, warranty and service costs, shipping and handling and manufacturing startup and transition costs. Prior to the first quarter of 2014, when we completed the manufacturing transition to Sanmina, cost of revenues also included costs of components, compensation related costs associated with manufacturing and costs to transition manufacturing to Sanmina. Cost of revenues also includes royalties and expenses related to royalty-bearing research and development grants and sales and marketing grants.
Our gross profit and gross margin as a percentage of sales is influenced by a number of factors, including primarily the volume and price of our products sold and fluctuations in our cost of revenues. We expect gross profit as a percentage of sales will improve in the future as we increase our sales volumes and decrease the product manufacturing costs.
Operating Expenses
Research and Development Expenses, Net
Research and development expenses, net consist primarily of salaries, related personnel costs including share-based compensation, supplies, materials and expenses related to product design and development, clinical studies, regulatory submissions, patent costs, sponsored research costs and other expenses related to our product development and research programs. We expense all research and development expenses as they are incurred. We believe that continued investment in research and development is crucial to attaining our strategic product objectives.
Research and development expenses are presented net of the amount of any grants we receive for research and development in the period in which we receive the grant. We previously received grants and other funding from the BIRD Foundation and the Israel Innovation Authority, or “IIA” (formerly known as the Office of the Chief Scientist). Certain of those grants require us to pay royalties on sales of ReWalk systems, which are recorded as cost of revenues. We may receive additional funding from these entities or others in the future. See “Grants and Other Funding” below.
55
Sales and Marketing Expenses
Our sales and marketing expenses consist primarily of salaries, related personnel costs including share-based compensation for sales, marketing and reimbursement personnel, travel, marketing and public relations activities and consulting costs. Also included in the sales and marketing expenses are the costs associated with our reimbursement activities in the United States and Germany.
General and Administrative Expenses
Our general and administrative expenses consist primarily of salaries, related personnel costs including share-based compensation for our administrative, finance, and general management personnel, professional services and insurance.
Financial Income (Expenses), Net
Financial income and expenses consist of bank commissions, foreign exchange gains and losses, interest earned on investments in short term deposits, interest expenses related to the Loan Agreement with kreos..
Interest income consists of interest earned on our cash and cash equivalent balances. Interest expense consists of interest accrued on, and certain other costs with respect to any indebtedness. Foreign currency exchange changes reflect gains or losses related to transactions denominated in currencies other than the U.S. dollar.
On December 30, 2015 we entered into the Loan Agreement with Kreos pursuant to which Kreos extended a line of credit to us in the amount of $20.0 million. In connection with the Loan Agreement we issued to Kreos a warrant to purchase up to 4,771 of our ordinary shares at an exercise price of $241.00 as we drew down $12.0 million under the Loan Agreement, which amount was increased to 6,679 ordinary shares upon an additional drawdown of $8.0 million. On June 9, 2017, $3.0 million of the outstanding principal amount was extended by an additional three years with the same interest rate and became subject to repayment in accordance with, and subject to the terms of a secured convertible promissory note (the “Kreos Convertible Note”). On November 20, 2018, the Company agreed to repay $3.6 million to Kreos in satisfaction of all outstanding indebtedness under the Kreos Convertible Note and other related payments, including prepayment costs and end of loan payments and Kreos agreed to terminate the Kreos Convertible Note. The Company repaid Kreos the $3.6 million by issuing to Kreos 192,000 units and 288,000 pre-funded units at the applicable public offering prices for an aggregate price of $3.6 million (including the aggregate exercise price for the ordinary shares to be received upon exercise of the pre-funded warrants, assuming Kreos exercises all of the pre-funded warrants it purchased as part of the Company’s public offering. The Company and Kreos also agreed to revise the principal and the repayment schedule under the Kreos Loan Agreement. Additionally, Kreos and the Company entered into the Kreos Warrant Amendment, which amended the exercise price of the warrant to purchase 6,679 ordinary shares currently held by Kreos from $241.00 to $7.50.
For further discussion of the Loan Agreement with Kreos, see “-Liquidity and Capital Resources” below and also Note 6 to our audited consolidated financial statements below.
Taxes on Income
As of December 31, 2019, we had not yet generated taxable income in Israel. As of that date, our net operating loss carry forwards for Israeli tax purposes amounted to approximately $152.4 million and our net operating loss carry forwards for U.S. tax purposes amounted to approximately $390 thousands After we utilize our net operating loss carry forwards, we are eligible for certain tax benefits in Israel under the Law for the Encouragement of Capital Investments, 1959. Our benefit period currently ends ten years after the year in which we first have taxable income in Israel provided that the benefit period will not extend beyond 2024.
Our taxable income generated outside of Israel will be subject to the regular corporate tax rate in the applicable jurisdictions. As a result, our effective tax rate will be a function of the relative proportion of our taxable income that is generated in those locations compared to our overall net income.
56
Grants and Other Funding
Israel Innovation Authority (formerly known as Office of the Chief Scientist)
From our inception through December 31, 2019 we have received a total of $1.97 million in funding from the IIA, $1.57 million of which are royalty-bearing grants, while $400 thousand were received in consideration for an investment in our preferred shares. Out of the royalty-bearing grants received, we have paid royalties to the IIA in the total amount of $50 thousand. We may apply to receive additional grants to support our research and development activities in 2017. The agreements with IIA require us to pay royalties at a rate of 3%-3.5% on sales of ReWalk systems and related services up to the total amount of funding received, linked to the dollar and bearing interest at an annual rate of LIBOR applicable to dollar deposits. If we transfer IIA-supported technology or know-how outside of Israel, we will be liable for additional payments to IIA depending upon the value of the transferred technology or know-how, the amount of IIA support, the time of completion of the IIA-supported research project and other factors. As of December 31, 2019, the aggregate contingent liability to the IIA was $1.6 million. For more information, see “Part I, Item 1A. Risk Factors-We have received Israeli government grants for certain of our research and development activities and we may receive additional grants in the future. The terms of those grants restrict our ability to manufacture products or transfer technologies outside of Israel...”
Results of Operations
Year Ended December 31, 2019 Compared to Year Ended December 31, 2018
Revenues
Our revenues for 2019 and 2018 were as follows (dollars in thousands, except unit amounts)
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Personal units placed | 54 | 82 | ||||||
| Rehabilitation units placed | — | 3 | ||||||
| ReStore units placed | 16 | — | ||||||
| Total units placed | 70 | 85 | ||||||
| Personal unit revenues | $ | 4,674 | $ | 6,276 | ||||
| Rehabilitation unit revenues | $ | — | $ | 269 | ||||
| ReStore unit revenues | $ | 199 | $ | — | ||||
| Revenues | $ | 4,873 | $ | 6,545 | ||||
57
Revenues decreased by $1.7 million, or 26%, during 2019 compared to 2018. The decrease in revenue was primarily by lower number of units placed with the VA for its ongoing clinical study.
In the future we expect our growth to be driven by sales of our ReWalk Personal device to third-party payors as we continue to focus our resources on broader commercial coverage policies with third-party payors as well as sales of the ReStore device to rehabilitation clinics.
Gross Profit
Our gross profit for 2019 and 2018 were as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Gross profit | $ | 2,726 | $ | 2,825 | ||||
Gross profit was 56% of revenue for 2019, compared to gross profit of 43% of revenue for 2018. Our gross profit declined due to the reduction of units placed but the reduction was offset by the increase in our margins that was driven by higher average selling price due to change in sales mix of our ReWalk Personal device and less costs related to inventory write-off.
We expect our gross profit to remain at current levels and potentially improve as we increase our sales volumes and decrease the product manufacturing costs. Improvements may be partially offset by the lower margins we expect upon the launch period of our new ReStore device and an increase in the cost of product parts.
Research and Development Expenses, Net
Our research and development expenses, net for 2019 and 2018 were as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Research and development expenses, net | $ | 5,348 | $ | 7,349 | ||||
Research and development expenses, net, decreased by $2.0 million, or 27%, during 2019 compared to 2018. The decrease is attributable to decreased costs associated with the development and clinical study costs of our ReStore soft suit exoskeleton.
We intend to focus our research and development expenses on the “soft suit” exoskeleton for additional indications affecting the ability to walk, including multiple sclerosis, cerebral palsy, Parkinson’s disease and elderly assistance and the next generation of our current ReWalk device.
58
Sales and Marketing Expenses
Our sales and marketing expenses for 2019 and 2018 were as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Sales and marketing expenses | $ | 6,167 | $ | 7,897 | ||||
Sales and marketing expenses decreased by $1.7 million, or 22%, during 2019 compared to 2018. The decrease is driven by lower personnel and personnel-related costs and consulting expenses as result of our cost reduction efforts.
In the near term our sales and marketing expenses are expected to be driven by our efforts to commercialize the ReStore device and to increase reimbursement of the ReWalk Personal device, as we continue to pursue insurance claims on a case-by-case basis and invest in efforts to expand coverage.
General and Administrative Expenses
Our general and administrative expenses for 2019 and 2018 were as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| General and administrative | $ | 5,259 | $ | 6,793 | ||||
General and administrative expenses decreased by $1.5 million, or 23%, during 2019 compared to 2018. The decrease was driven by cash and non-cash compensation recorded in 2018 related to severance accrual for the Company’s former chief financial officer, as well as higher legal expenses related to China market development activities.
Financial Expenses, Net
Our financial expenses, net for 2019 and 2018 were as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Financial expenses, net | $ | 1,496 | $ | 2,466 | ||||
Financial expenses, net, increased by $1.0 million, or 40% during 2019 compared to 2018. The decrease is attributable to decreased interest expenses related to the Loan Agreement, as amended, with Kreos. For further discussion of the Loan Agreement with Kreos, see “-Liquidity and Capital Resources” below and also Note 6 to our audited consolidated financial statements below.
Income Tax
Our income tax for 2019 and 2018 was as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Income tax (tax benefit) | $ | 7 | $ | (5 | ) | |||
Income taxes increased by $12 thousand or 240% during 2019 compared to 2018.
59
Critical Accounting Policies
Our consolidated financial statements are prepared in accordance with United States generally accepted accounting principles. The preparation of our financial statements requires us to make estimates, judgments and assumptions that can affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We base our estimates, judgments and assumptions on historical experience and other factors that we believe to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known. Besides the estimates identified above that are considered critical, we make many other accounting estimates in preparing our financial statements and related disclosures. See Note 2 to our audited consolidated financial statements presented elsewhere in this annual report for a description of the significant accounting policies that we used to prepare our consolidated financial statements. The critical accounting policies that were impacted by the estimates, judgments and assumptions used in the preparation of our consolidated financial statements are discussed below.
Revenue Recognition
On January 1, 2018, we adopted Topic 606 using the modified retrospective method for contracts that were not completed as of January 1, 2018. Under the modified retrospective method, we recognized the cumulative effect of initially applying the new revenue standard as an adjustment to the opening balance of retained earnings. This adjustment did not have a material impact on our consolidated financial statements. Results for reporting periods beginning after January 1, 2018 are presented under Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with our historic accounting under Revenue Recognition (“Topic 605”).
60
The adoption of Topic 606 represents a change in accounting principle that will provide financial statement readers with enhanced revenue recognition disclosures. In accordance with Topic 606, revenue is recognized when obligations under the terms of a contract with our customer are satisfied; generally this occurs with the transfer of control of our products or services. Revenue is measured as the amount of consideration to which we expect to be entitled in exchange for transferring products or providing services. To achieve this core principle, the Company applies the following five steps:
1. Identify the contract with a customer
2. Identify the performance obligations in the contract
3. Determine the transaction price
4. Allocate the transaction price to performance obligations in the contract
5. Recognize revenue when or as the Company satisfies a performance obligation
Provisions are made at the time of revenue recognition for any applicable warranty cost expected to be incurred. The timing for revenue recognition among the various products and customers is dependent upon satisfaction of such criteria and generally varies from either shipment or delivery to the customer depending on the specific shipping terms of a given transaction, as stipulated in the agreement with each customer. Other than pricing terms which may differ due to the different volumes of purchases between distributors and end-users, there are no material differences in the terms and arrangements involving direct and indirect customers. Our products sold through agreements with distributors are non-exchangeable, non-refundable, non-returnable and without any rights of price protection or stock rotation. Accordingly, we consider all the distributors as end-users. We generally do not grant a right of return for our products. There have been a few occasions in which we experienced a return of our products. Therefore, we recorded reductions to revenue for expected future product returns based on our historical experience.
For the majority of sales of Rehabilitation systems, we include training and consider the elements in the arrangement to be a single unit of accounting. In accordance with ASC 606, we have concluded that the training is essential to the functionality of our systems. Therefore, we recognize revenue for the system and training only after delivery, in accordance with the agreement delivery terms, to the customer and after the training has been completed, once all other revenue recognition criteria have been met. For sales of Personal systems to end users, and for sales of Personal or Rehabilitation systems to third party distributors, we do not provide training to the end user as this training is completed by the rehabilitation centers or by the distributor that have previously completed the ReWalk Training program.
Warranties are classified as either assurance type or service type warranty. A warranty is considered an assurance type warranty if it provides the consumer with assurance that the product will function as intended for a limited period of time.
In the beginning of 2018, we updated our service policy to include a five-year warranty compared to a period of two years that were included in the past for parts and services. The first two years are considered as assurance type warranty and the additional period is considered an extended service arrangement, which is a service type warranty. An assurance type warranty is not accounted for as separate performance obligations under the revenue model. A service type warranty is either sold with a unit or separately for units for which the warranty has expired. Revenue is then recognized ratably over the life of the warranty.
The Company also offers a rent-to-purchase option for its ReWalk Personal device. Those transactions provide potential customers the option to use the device for a short term, after which they can choose whether to purchase it. In such cases we recognize revenue ratably according to the agreed rental monthly fee. For units placed, we transfer control and recognize a sale when title has passed to our customer and rental revenue ratably according to the agreed rental monthly fee. Each unit placed is considered an independent, unbundled performance obligation.
Share-Based Compensation – Option Valuations
We account for share-based compensation in accordance with ASC No. 718, “Compensation-Stock Compensation.” ASC No. 718 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an Option-Pricing Model, or OPM. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in our consolidated statements of operations.
We selected the Black-Scholes-Merton option pricing model as the most appropriate method for determining the estimated fair value of options. The resulting cost of an equity incentive award is recognized as an expense over the requisite service period of the award, which is usually the vesting period. We recognize compensation expense over the vesting period using the straight-line method and classify these amounts in the consolidated financial statements based on the department to which the related employee reports.
61
The determination of the grant date fair value of options using the Black-Scholes-Merton option pricing model is affected by estimates and assumptions regarding a number of complex and subjective variables. These variables include the expected volatility of our share price over the expected term of the options, share option exercise and cancellation behaviors, risk-free interest rates and expected dividends, which are estimated as follows:
Risk-free Interest Rate. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the contractual life of the options.
Dividend Yield. We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero.
Expected Volatility. We estimated the expected share price volatility for our ordinary shares by considering the historic price volatility for industry peers based on price observations over a period equivalent to the expected term of the share option grants. Industry peers consist of public companies in the medical device and healthcare industries. We intend to continue to consistently apply this process using the same or similar industry peers until a sufficient amount of historical information regarding the volatility of our ordinary share price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be utilized in the calculation.
Expected Term. The expected term of options granted represents the period of time that options granted are expected to be outstanding, and is determined based on the simplified method in accordance with ASC No. 718-10-S99-1 (SAB No. 110), as adequate historical experience is not available to provide a reasonable estimate. ASC No. 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Income Taxes
As part of the process of preparing our consolidated financial statements, we are required to estimate our taxes in each of the jurisdictions in which we operate. We account for income taxes in accordance with ASC Topic 740, “Income Taxes,” or ASC Topic 740. ASC Topic 740 prescribes the use of an asset and liability method whereby deferred tax asset and liability account balances are determined based on the difference between book value and the tax bases of assets and liabilities and carryforward tax losses. Deferred taxes are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. We exercise judgment and provide a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value if it is more likely than not that some portion or all of the deferred tax asset will not be realized. We have established a full valuation allowance with respect to our deferred tax assets.
ASU 2015-17, “Balance Sheet Classification of Deferred Taxes” provides presentation requirements to classify deferred tax assets and liabilities, along with any related valuation allowance, are classified as non-current on the balance sheet. We account for uncertain tax positions in accordance with ASC 740 and recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. Accordingly, we report a liability for unrecognized tax benefits resulting from uncertain tax positions taken or expected to be taken in a tax return. We recognize interest and penalties, if any, related to unrecognized tax benefits in tax expense.
Recently Issued and Adopted Accounting Pronouncements
A discussion of recent accounting pronouncements is included in Note 2u, New Accounting Pronouncements to our consolidated financial statements in this annual report.
62
Liquidity and Capital Resources
Sources of Liquidity and Outlook
Since inception, we have funded our operations primarily through the sale of certain of our equity securities and convertible notes to investors in private placements, the sale of our ordinary shares in public offerings and the incurrence of bank debt.
As of December 31, 2019, the Company had cash and cash equivalents of $16.2 million. The Company has an accumulated deficit in the total amount of $168.5 million as of December 31, 2019 and further losses are anticipated in the development of its business. Those factors raise substantial doubt about the Company’s ability to continue as a going concern. The ability to continue as a going concern is dependent upon the Company obtaining the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due.
The Company intends to finance operating costs over the next twelve months with existing cash on hand, potential reduction in operating cash burn and future issuances of equity and debt securities, or through a combination of the foregoing. However, we will also need to seek additional sources of financing if we require more funds than anticipated during the next 12 months or in later periods.
We previously considered the Investment Agreement with Timwell as a potential source of ongoing liquidity. We no longer believe that we can reach an agreement with Timwell to close the remaining $15.0 million of issuances on the basis of the original understandings reflected in our Investment Agreement and currently see a significant risk that we will not reach agreement with Timwell and RealCan on a modification of the original agreement. For more information, see “Timwell Private Placement” below.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business. The consolidated financial statements for the year ended December 31, 2019 do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
Our anticipated primary uses of cash are (i) sales, marketing and reimbursement expenses related to market development activities of our ReStore device as well as broadening third-party payor coverage, and (ii) research and development costs related to improving and expanding our functional technological platform by expanding the indication of use of our lightweight “soft suit” exoskeleton to other medical conditions as well as home therapy, and in the longer term by developing our next generation of SCI Products with design improvement. New medical indications that affect the ability to walk may include multiple sclerosis, cerebral palsy, Parkinson’s disease and elderly assistance. Our future cash requirements will depend on many factors, including our rate of revenue growth, the expansion of our sales and marketing activities, the timing and extent of our spending on research and development efforts and international expansion. If our current estimates of revenue, expenses or capital or liquidity requirements change or are inaccurate, we may seek to sell additional equity or debt securities, arrange for additional bank debt financing or refinance our indebtedness. There can be no assurance that we will be able to raise such funds on acceptable terms. For more information, see ““Part I, Item 1A. Risk Factors-We have concluded that there are substantial doubts as to our ability to continue as a going concern.”
63
Loan Agreement with Kreos and Related Warrant to Purchase Ordinary Shares
On December 30, 2015, we entered into the Loan Agreement with Kreos pursuant to which Kreos extended a line of credit to us in the amount of $20 million. On January 4, 2016, we drew down $12.0 million under the Loan Agreement. Under the terms of the Loan Agreement we were entitled to draw down up to an additional $8.0 million until December 31, 2016, if we raised $10.0 million or more in the issuance of shares of our capital stock (including debt convertible into shares of our capital stock) by December 31, 2016. On December 28, 2016, we drew down the remaining $8.0 million available under the Loan Agreement. Interest is payable monthly in arrears on any amounts drawn down at a rate of 10.75% per year from the applicable drawdown date through the date on which all principal is repaid. As of June 30, 2017, the Company raised more than $20 million in connection with the issuance of its share capital and therefore, in accordance with the terms of the Loan Agreement, the repayment period was extended from 24 months to 36 months. The principal was also reduced in connection with the issuance of the Kreos Convertible Note on June 9, 2017. Pursuant to the Loan Agreement, we paid Kreos a transaction fee equal to 1.0% of the total available amount of the line of credit upon the execution of the agreement and we will be required to pay Kreos an “end of loan payment” equal to 1.0% of the amount of each tranche drawn down upon the expiration of each such tranche. Pursuant to the Loan Agreement, we granted Kreos a first priority security interest over all of our assets, including certain intellectual property and equity interests in its subsidiaries, subject to certain permitted security interests.
In connection with the $12.0 million drawdown under the Loan Agreement, we issued to Kreos the warrant to purchase up to 4,771 of our ordinary shares at an exercise price of $241.per share, which represented the average of the closing prices of our ordinary shares for the 30-day calendar period prior to the date of the issuance of the warrant, subject to adjustment as set forth in the warrant. In connection with the $8.0 million drawdown under the Loan Agreement on December 28, 2016, we increased the amount of the warrant from $1.15 million to $1.61 million, or by $460 thousand, such that the warrant represents the right to purchase up to 6,679 of our ordinary shares. The increase was based on the terms of the warrant, which provide that the amount of the warrant will be increased by 5.75% of any additional drawdowns. Subject to the terms of the warrant, the warrant is exercisable, in whole or in part, at any time prior to the earlier of (i) December 30, 2025, or (ii) immediately prior to the consummation of a merger, consolidation, or reorganization of us with or into, or the sale or license of all or substantially all our assets or shares to, any other entity or person, other than a wholly- owned subsidiary of us, excluding any transaction in which our shareholders prior to the transaction will hold more than 50% of the voting and economic rights of the surviving entity after the transaction.
On June 9, 2017, the Company and Kreos entered into the First Amendment (the “First Amendment”). As of that date the outstanding principal amount under the Loan Agreement was $17.2 million. Under the First Amendment, $3.0 million of the outstanding principal under the Loan Agreement is subject to repayment pursuant to the senior secured Kreos Convertible Note issued on June 9, 2017, thus reducing the outstanding principal amount under the Loan Agreement to $14.2 million as of June 9, 2017. This amended outstanding principal amount remains subject to repayment in accordance with the terms and conditions of the Loan Agreement and an amended repayment schedule. Interest on the Kreos Convertible Note is payable monthly in arrears at a rate of 10.75% per year.
Kreos may convert the then-outstanding principal and “end of loan payments” under the Kreos Convertible Note, in whole or in part, on one or more occasions, into up to 100,946 ordinary shares, at a conversion price per share equal to $31.70 per share (subject to customary anti-dilution adjustments) at any time until the earlier of (i) the maturity date of June 9, 2020 or (ii) a “Change of Control,” as defined in the Loan Agreement.
On November 20, 2018, the Company and Kreos entered into the Second Amendment of the Loan Agreement. In the Second Amendment (the “Second Amendment”), the Company agreed to repay $3.6 million to Kreos in satisfaction of all outstanding indebtedness under the Kreos Convertible Note and other related payments, including prepayment costs and end of loan payments and Kreos agreed to terminate the Kreos Convertible Note. The Company repaid Kreos the $3.6 million by issuing to Kreos 192,000 units and 288,000 pre-funded units at the applicable public offering prices for an aggregate price of $3.6 million (including the aggregate exercise price for the ordinary shares to be received upon exercise of the pre-funded warrants, assuming Kreos exercises all of the pre-funded warrants it purchased as part of the Company’s public offering. The Company and Kreos also agreed to revise the principal and the repayment schedule under the Kreos Loan Agreement. The revised repayment schedule, effectively deferred an additional $1.1 million of payments that were due in 2018 and $2.8 million that were due in 2019 under the loan’s prior repayment schedule, for total deferred payments of $3.9 million compared to the prior repayment schedule. Additionally, Kreos and the Company entered into the Kreos Warrant Amendment, which amended the exercise price of the warrant to purchase 6,679 ordinary shares currently held by Kreos from $241 to $7.5. The Second Amendment also made certain changes to the prepayment premiums under the Kreos Loan Agreement, tying them to the date of the Second Amendment.
64
On June 5, 2019 and June 6, 2019, the Company entered into warrant exercise agreements with certain institutional investors of warrants to purchase the Company’s ordinary shares, pursuant to which, Kreos agreed to exercise in cash their November 2018 warrants at the existing exercise price of $7.50 per share. Under the exercise agreements, the Company also agreed to issue to Kreos new warrants to purchase up to 480,000 ordinary shares at an exercise price of $7.50 per share and exercise period of five years.
As of December 31, 2019, the outstanding principal amount under the Kreos Loan Agreement was $6.9 million. Depending on our liquidity needs, we may seek to refinance up to a substantial portion of our indebtedness under our Kreos Loan Agreement, which we have considered with Kreos from time to time, including by exchanging our indebtedness with Kreos for new convertible debt from a third-party investor, or to borrow additional funds.
Equity Raises
Form S-3 Limitations
Since we filed our Form 10-K on February 17, 2017, we have been subject to limitations under the applicable rules of Form S-3, which constrain our ability to secure capital pursuant to our ATM Offering Program or other public offerings pursuant to our effective Form S-3. These rules limit the size of primary securities offerings conducted by issuers with a public float of less than $75 million to no more than one-third of their public float in any 12-month period. Pursuant to these rules, as of February 20, 2020, we may not sell in primary offerings under our Form S-3 more than approximately $10.3 million in any 12-month period, unless and until we are no longer subject to these limitations. We will cease to be subject to these limitations once our public float exceeds $75 million. As of the date of this annual report, we have sold approximately $13.7 million in securities under our Form S-3 during the last 12 months, when we were subject to these restrictions and therefore, we currently cannot sell securities under our Form S-3. We will also recalculate the amount of this limitation if or when we conduct another takedown under Form S-3. Additionally, these limitations do not apply to secondary offerings for the resale of our ordinary shares or other securities by selling shareholders or to the issuance of ordinary shares upon conversion by holders of convertible securities, such as warrants. Our Form S-3 expires on May 23, 2022. With respect to our ATM Offering Program, because we have sold $15.7 million in the program since its inception, we could only raise up to a remaining $9.3 million using the program, subject to the $10.3 million limitation on use of Form S-3.
Because of these limitations to raise capital in securities offerings above the limitation applicable to us for sales under Form S-3 and our ongoing liquidity needs, we may be required to seek and are currently actively exploring other methods of completing primary offerings, including, a registration statement on Form S-1 (which has no such size limitations), the preparation of is be more time-consuming and costly, including due to potential SEC review. We may also conduct such offerings in the form of private placements, potentially with registration rights or priced at a discount to the market value of our ordinary shares, which could require shareholder approval under the rules of the NASDAQ. Any such transactions, including the perception that we will conduct a transaction, could result in substantial dilution of shareholders’ interests.
Initial Public Offering and Follow-on Offerings
Our initial public offering in September 2014 generated $36.3 million in net proceeds. Additionally, on May 9, 2016, the SEC declared effective our Form S-3, pursuant to which we registered up to $100 million of ordinary shares, warrants and/or debt securities and up to 175,525 ordinary shares offered by selling shareholders named therein. On May 10, 2016, we entered into our Equity Distribution Agreement with Piper Jaffray, pursuant to which we may offer and sell, from time to time, ordinary shares having an aggregate offering price of up to $25.0 million through Piper Jaffray acting as our agent. The ordinary shares issued under the Equity Distribution Agreement may be registered under the Securities Act using our Form S-3.
65
Additionally, on November 1, 2016, we closed our follow-on public offering of 130,000 units, each consisting of one ordinary share and 0.75 of a warrant to purchase one ordinary share. The ordinary shares and the warrants underlying the units and the ordinary shares issuable upon exercise of the warrants are registered under the Securities Act on our Form S-3. The warrants became exercisable during the period commencing from the date of original issuance and ending on November 1, 2021, the expiration date of the warrants, at an initial exercise price of $118.75 per ordinary share. Our net aggregate proceeds, after deducting underwriting discounts and commissions and estimated expenses, were $11.1 million. We also granted Oppenheimer & Co. (“Oppenheimer”), as underwriter under the underwriting agreement, an option to purchase up to 19,500 additional units at the public offering price, less the underwriting discount, for 30 days after October 27, 2016, which Oppenheimer did not exercise.
On November 21, 2017, we closed the base portion of our follow-on offering of 274,280 ordinary shares. Each ordinary share was sold to the public at a price of $26.25. On November 22, 2017, National Securities Corporation, as underwriter, exercised in full its option to purchase 41,142 additional ordinary shares at the public offering price of $26.25 per unit, less the underwriting discount. The Company’s net aggregate proceeds of the base offering and over-allotment exercise, after deducting underwriting discounts and commissions and expenses, were $7.2 million.
On November 20, 2018, the Company completed its follow-on public offering in which the Company issued and sold 728,019 units, each consisting of one ordinary share and one warrant to purchase one ordinary share. Each unit was sold to the public at a price of $7.5 per unit, additionally the Company issued and sold 1,050,373 pre-funded units, each unit was sold to the public at a price of $7.25 per unit. Each unit containing one pre-funded warrant with an exercise price of $0.25 per share and one warrant to purchase one ordinary share. The total gross proceeds received from the follow-on public offering, before deducting commissions, discounts and expenses, were $13.1 million (including proceeds from the exercise of 90,691 pre-funded warrants at the closing of the offering). As of December 31, 2018, additional pre-funded warrants to purchase an aggregate 562,466 ordinary shares had been exercised, for additional proceeds of $140,617. During the year ended December 31, 2019 additional pre-funded warrants and warrants to purchase an aggregate 2,048,752 ordinary shares had been exercised, for additional proceeds of $12.4 million. As compensation for their role in the offering, the Company also issued to the underwriters warrants to purchase up to 106,680 ordinary shares, which are immediately exercisable starting on November 20, 2018 until November 15, 2023 at $9.375 per share. See Note 8b (2) below for more information about the Company’s follow-on public offering.
On February 15, 2019, the Company entered into an exclusive placement agent Agreement with H.C. Wainwright, on a reasonable best-efforts basis in connection with a public offering of 760,000 ordinary shares at a price of $5.75 per Share. The total gross proceeds received from the follow-on public offering, before deducting commissions, discounts and expenses, were $4.37 million. The Company also issued to H.C. Wainwright and/or its designees warrants to purchase up to 45,600 ordinary shares, which are immediately exercisable starting on February 25, 2019 until February 21, 2024 at $7.1875 per share.
On April 3, 2019, the Company entered into an exclusive placement agent Agreement with H.C. Wainwright in connection with a registered direct offering of the Company’s ordinary shares, par value NIS 0.25 per share and a concurrent private placement of warrants to purchase ordinary shares. The ordinary shares were offered pursuant to our Form S-3. The Company signed a purchase agreement with certain institutional investors for the issuance and sale of 816,914 ordinary shares at $5.2025 per ordinary share and warrants to purchase up to 408,457 ordinary shares at an exercise price of $5.14. The warrants issued to these purchasers will be exercisable at any time and from time to time, in whole or in part, following the date of issuance and ending five and one-half years from the date of issuance, at an exercise price of $5.14. The Company also issued to H.C. Wainwright and/or its designees warrants to purchase up to 49,015 ordinary shares. The warrants issued to H.C. Wainwright will be exercisable at any time and from time to time, in whole or in part, following the date of issuance and ending five years from the date of the execution of the Purchase Agreement, at a price per share equal to $6.503125. The gross proceeds from the offering, before deducting placement agent fees and offering expenses, were approximately $4.25 million.
66
On June 5, 2019 and June 6, 2019, the Company entered into warrant exercise agreements with certain institutional investors whereby the Company issued warrants to purchase up to 1,464,665 ordinary shares with an exercise price of $7.50 per share, exercisable from June 5, 2019 or June 6, 2019 until June 5, 2024 or June 6, 2024, respectively. Additionally, the Company issued warrants to purchase up to 87,880 ordinary shares, with an exercise price of $9.375 per share, exercisable from June 5, 2019 until June 5, 2024, to certain representatives of H.C. Wainwright as compensation for its role as the placement agent in our June 2019 warrant exercise agreement and concurrent private placement of warrants.
On June 12, 2019, the Company entered into a purchase agreement with certain institutional investors for the issuance and sale of 833,334 ordinary shares, par value NIS 0.25 per share at $6.00 per ordinary share and warrants to purchase up to 416,667 ordinary shares with an exercise price of $6.00 per share, exercisable from June 12, 2019 until December 12, 2024, in a private placement that took place concurrently with our registered direct offering of ordinary shares in June 2019. Additionally, the Company issued warrants to purchase up to 50,000 ordinary shares, with an exercise price of $7.50 per share, exercisable from June 12, 2019 until June 10, 2024, to certain representatives of H.C. Wainwright as compensation for its role as the placement agent in our June 2019 registered direct offering and concurrent private placement of warrants.
ATM Offering Program
On May 10, 2016, we entered into our Equity Distribution Agreement with Piper Jaffray, pursuant to which we may offer and sell, from time to time, ordinary shares having an aggregate offering price of up to $25.0 million through Piper Jaffray acting as our agent. Subject to the terms and conditions of the Equity Distribution Agreement, Piper Jaffray will use its commercially reasonable efforts to sell on our behalf all of the ordinary shares requested to be sold by us, consistent with its normal trading and sales practices. Piper Jaffray may also act as principal in the sale of ordinary shares under the Equity Distribution Agreement. Such sales may be made under our Form S-3 in what may be deemed “at-the-market” equity offerings as defined in Rule 415 promulgated under the Securities Act, directly on or through the Nasdaq Capital Market, to or through a market maker other than on an exchange or otherwise, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, and/or any other method permitted by law, including in privately negotiated transactions.
Piper Jaffray is entitled to compensation at a fixed commission rate of 3% of the gross sales price per share sold through it as agent under the Equity Distribution Agreement. Where Piper Jaffray acts as principal in the sale of ordinary shares under the Equity Distribution Agreement, such rate of compensation will not apply, but in no event will the total compensation of Piper Jaffray, when combined with the reimbursement of Piper Jaffray for the out-of-pocket fees and disbursements of its legal counsel, exceed 8.0% of the gross proceeds received from the sale of the ordinary shares.
We may instruct Piper Jaffray not to sell ordinary shares if the sales cannot be effected at or above the price designated by us in any instruction. We or Piper Jaffray may suspend an offering of ordinary shares under the ATM Offering Program upon proper notice and subject to other conditions, as further described in the Equity Distribution Agreement. Additionally, the ATM Offering Program will terminate on the earlier of (i) the sale of all ordinary shares subject to the Equity Distribution Agreement or (ii) the termination of the Equity Distribution Agreement. The Equity Distribution Agreement may be terminated by Piper Jaffray or us at any time on the close of business on the date of receipt of written notice, and by Piper Jaffray at any time in certain circumstances, including any suspension or limitation on the trading of our ordinary shares on the Nasdaq Capital Market, as further described in the Equity Distribution Agreement. As of December 31, 2019, we had sold 302,092 ordinary shares under the ATM Offering Program for net proceeds to us of $14.5 million (after commissions, fees and expenses). Additionally, as of that date, we had paid Piper Jaffray compensation of $471 thousand and had incurred total expenses of approximately $1.2 million in connection with the ATM Offering Program. Subject to the limitations under Form S-3 due to our public float, we intend to continue using the ATM Offering Program opportunistically to raise additional funds.
67
Timwell Private Placement
On March 6, 2018, we entered into an investment agreement with Timwell Corporation Limited, a Hong Kong corporation (“Timwell”), as amended on May 15, 2018 (the “Investment Agreement”), pursuant to which we agreed, in return for aggregate gross proceeds to us of $20 million, to issue to Timwell an aggregate of 640,000 of our ordinary shares, at a price per share of $31.25. The Investment Agreement contemplates issuances in three tranches, including $5 million for 160,000 shares in the first tranche, $10 million for 320,000 shares in the second tranche and $5 million for 160,000 shares in the third tranche.
The First Tranche, consisting of $5 million for 160,000 shares, closed on May 15, 2018. The net aggregate proceeds after deducting commissions, fees and offering expenses in the amount of approximately $705 thousand were approximately $4.3 million.
The closings of the Second Tranche and Third Tranche are subject to specified closing conditions, including the formation of a joint venture, the signing of a license agreement and a supply agreement and the successful production of certain ReWalk products, among others, with the Third Tranche Closing to occur by December 31, 2018 and no later than April 1, 2019. In light of the positions taken by Timwell during the negotiations on definitive joint venture and license agreements, we no longer believe that agreement can be reached on the basis of the original understandings reflected in our Investment Agreement with Timwell. we remain in dialogue with RealCan, Timwell’s affiliate, on alternative pathways that will allow us to commercialize our products in China through RealCan and its affiliates, and also provide for RealCan or an affiliate to invest in us. Due to various delays in the process and other barriers to closing, there is a significant risk that we will not reach agreement with RealCan on a modification of the original agreement. As we continue to view China as a market with key opportunities for products designed for stroke patients, we continue to evaluate potential relationships with other groups to penetrate the Chinese market.
Cash Flows for the years ended December 31, 2019 and December 31, 2018
| Years Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Net cash used in operating activities | $ | (14,815 | ) | $ | (14,774 | ) | $ | (22,462 | ) | |||
| Net cash provided by used in investing activities | (22 | ) | (13 | ) | (21 | ) | ||||||
| Net cash provided by financing activities | 21,482 | 9,711 | 13,408 | |||||||||
| Net cash flow | $ | 6,645 | $ | (5,076 | ) | $ | (9,075 | ) | ||||
Net Cash Used in Operating Activities
Net cash used in operating activities remained flat at $14.8 million in 2018 and in 2019.
Net Cash Used in Investing Activities
Net cash used in investing activities increased from $13 thousand in 2018 to $22 thousand in 2019, primarily as a result of increased use of cash for the purchase of property and equipment.
Net Cash Provided by Financing Activities
We generated $21.5 million from financing activities in 2019, which reflects $23.2 million net proceeds from the issuance of ordinary shares in the follow-on offering, which was offset by $1.7 million in capital repayments related to the Loan Agreement with Kreos.
68
We generated $9.7 million from financing activities in 2018, which reflects $8.1 million net proceeds from the issuance of ordinary shares in the follow-on offering, $4.3 million net proceeds from the issuance of ordinary shares in the Investment Agreement and $1.1 million net proceeds from the issuance of ordinary shares in the ATM, which was offset by $3.9 million in capital repayments and debt issuance costs related to the Loan Agreement with Kreos.
Obligations and Commercial Commitments
Set forth below is a summary of our contractual obligations as of December 31, 2019.
| Payments due by period (in dollars, in thousands) | ||||||||||||||||||||
| Contractual obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
| Purchase obligations (1) | $ | 2,296 | $ | 2,296 | $ | — | $ | — | $ | — | ||||||||||
| Collaboration Agreement and License Agreement obligations (2) | 2,652 | 964 | 1,688 | — | — | |||||||||||||||
| Operating lease obligations (3) | 2,440 | 685 | 1,297 | 458 | ||||||||||||||||
| Long-term debt obligations (4) | 7,875 | 6,300 | 1,575 | — | — | |||||||||||||||
| Total | $ | 15,263 | $ | 10,245 | $ | 4,560 | $ | 458 | $ | — | ||||||||||
| (1) | The Company depends on one contract manufacturer, Sanmina, for both the ReStore products and the ReWalk products. We place our manufacturing orders with Sanmina pursuant to purchase orders or by providing forecasts for future requirements. Additionally, we have purchase obligations to our raw material vendors related to the ReStore production, which began in the second quarter of 2019 following regulatory clearance. |
| (2) | Our Research Collaboration Agreement is for a period of six years and requires us to pay in quarterly installments for the funding of our joint research collaboration with Harvard, subject to a minimum funding commitment under applicable circumstances. Our License Agreement consists of patent reimbursement expenses payments and of a license upfront fee payment. There are also several milestone payments contingent upon the achievement of certain product development and commercialization milestones and royalty payments on net sales from certain patents licensed to Harvard. These product development milestones have been met as of December 31, 2019. There are commercialization milestones which depend on us reaching certain sales amounts some or all of which may not occur. |
| (3) | Our operating leases consist of leases for our facilities and motor vehicles. |
| (4) | Our long-term debt obligations consist of payments of principal and interest under our Loan Agreement with Kreos. |
We calculated the payments due under our operating lease obligation for our Israeli office that are to be paid in NIS at a rate of exchange of NIS 3.456:$1.00, and the payments due under our operating lease obligation for our German subsidiary that are to be paid in euros at a rate of exchange of €1.00:$1.122, both of which were the applicable exchange rates as of December 31, 2019. We calculated the payments due under our Loan Agreement with Kreos according to the current schedule of repayment of principal and interest, taking into account the two tranches of debt drawn down under the Loan Agreement. For information on this repayment schedule, see “-Liquidity and Capital Resources-Loan Agreement with Kreos and Related Warrant to Purchase Ordinary Shares” above.
Off-Balance Sheet Arrangements
We had no off-balance sheet arrangements or guarantees of third-party obligations during the periods presented.
Trend Information
For information on significant known trends, please see “Part I-Item 1. “Business - Overview” in this annual report.
Subsequent Events
On February 10, 2020, the Company closed a “best efforts” public offering whereby the Company issued an aggregate of 5,600,000 of common units and pre-funded units at a public offering price of $1.25 per common unit and $1.249 per pre-funded unit. As part of the public offering, the Company entered into a securities purchase agreement with certain institutional purchasers. Each common unit consisted of one ordinary share, par value NIS 0.25 per share, and one common warrant to purchase one Ordinary Share. Each pre-funded unit consisted of one pre-funded warrant to purchase one Ordinary Share and one Common Warrant. Additionally the Company issued warrants to purchase up to 336,000 ordinary shares, with an exercise price of $1.5625 per share, to representatives of H.C. Wainwright as compensation for its role as the placement agent in the Company’s February 2020 offering.
69
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Currency Exchange Risk
Our results of operations and cash flows are affected by fluctuations in foreign currency exchange rates. In 2019, 2018 and 2017 most of our revenues were denominated in U.S. dollars and the remainder of our revenues was denominated in euros and British pounds, and most of our expenses were also denominated in U.S. dollars, and the remainder of our expenses were denominated in NIS and euros. Accordingly, changes in the value of the NIS and euro relative to the U.S. dollar in each of the years 2017, 2018 and 2019 impacted amounts recorded on our consolidated statements of operations for those periods. We expect that the denominations of our revenue and expenses in 2020 will be consistent with what we experienced in 2019.
The following table presents information about the changes in the exchange rates of the NIS and euro against the U.S. dollar in 2017, 2018 and 2019:
| Change in Average Exchange Rate | ||||||||
| Period | NIS against the U.S. Dollar (%) | Euro against the U.S. Dollar (%) | ||||||
| 2019 | 0.87 | (5.16 | ) | |||||
| 2018 | 0.11 | 4.57 | ||||||
| 2017 | 6.81 | 1.96 | ||||||
The figures above represent the change in the average exchange rate in the given period compared to the average exchange rate in the immediately preceding period. Negative figures represent depreciation of the U.S. dollar compared to the NIS or the euro. A 10% increase or decrease in the value of the NIS against the U.S. dollar would have decreased or increased our net loss by approximately $430 thousand million in 2019. A 10% increase or decrease in the value of the euro against the U.S. dollar would have decreased or increased our net loss by approximately $70 thousand in 2019.
From time to time, we enter into limited short term hedging arrangements with financial institutions. We do not use derivative financial instruments for speculative or trading purposes.
Other Market Risks
We do not believe that we have material exposure to interest rate risks or to inflationary risks.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required hereunder is set forth under Report of Independent Registered Public Accounting Firm, Consolidated Balance Sheets, Consolidated Statements of Operations, Statements of Changes in Shareholders’ Equity, Consolidated Statements of Cash Flows and Notes to Consolidated Financial Statements included in the Consolidated Financial Statements that are a part of this annual report. Other financial information is included in the Consolidated Financial Statements that are a part of this annual report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
70
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required financial disclosure.
As of the end of the period covered by this Report, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) and Rule 15d-15(e) of the Exchange Act). Based upon, and as of the date of, this evaluation, the Chief Executive Officer and the Chief Financial Officer concluded that our disclosure controls and procedures were effective such that the information required to be disclosed by us in our SEC reports is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP.
Our internal control over financial reporting includes those policies and procedures that:
| ● | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements. |
Management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2019. In making its assessment, management used the criteria described in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Based on management’s assessment, management has concluded that our internal control over financial reporting was effective as of December 31, 2019 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external reporting purposes in accordance with U.S. GAAP.
This annual report does not include an attestation report of our independent registered public accounting firm regarding internal controls over financial reporting because we are exempt from this requirement as a smaller reporting company and non-accelerated filer.
Changes in Internal Control over Financial Reporting
During the fourth quarter of the fiscal year ended December 31, 2019, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
Not applicable
71
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information About Our Executive Officers
The following table sets forth the name, age and position of each of our executive officers as of February 18, 2020:
| Name | Age | Position | ||
| Larry Jasinski | 62 | Chief Executive Officer and Director | ||
| Ori Gon | 38 | Chief Financial Officer | ||
| Ofir Koren | 50 | General
Manager -ReWalk Robotics Ltd. and Vice President, Research & Development and Regulatory |
Larry Jasinski has served as our Chief Executive Officer and as a member of our board since February 2012. From 2005 until 2012, Mr. Jasinski served as the President and Chief Executive Officer of Soteira, Inc., a company engaged in development and commercialization of products used to treat individuals with vertebral compression fractures, which was acquired by Globus Medical in 2012. From 2001 to 2005, Mr. Jasinski was President and Chief Executive Officer of Cortek, Inc., a company that developed next-generation treatments for degenerative disc disease, which was acquired by Alphatec in 2005. From 1985 until 2001, Mr. Jasinski served in multiple sales, research and development, and general management roles at Boston Scientific Corporation. Mr. Jasinski has served on the board of directors of Massachusetts Bay Lines since 2015 and of LeMaitre Vascular, Inc. since 2003. Mr. Jasinski holds a B.Sc. in marketing from Providence College and an MBA from the University of Bridgeport.
Ori Gon became our Chief Financial Officer effective February 22, 2018 From 2015 to 2018, Mr. Gon served as our Corporate Controller. Prior to ReWalk Robotics Mr. Gon served as Corporate Controller at Oti Ltd from 2012 to 2015. Mr. Gon is a Certified Public Accountant in Israel and holds a B.A. in Economics from Hebrew University of Jerusalem.
Ofir Koren has served as our Vice President, Research & Development and Regulatory since February 2017, and was previously our Vice President Research and Development since he joined us in February 2013. Mr. Koren has also served as General Manager of ReWalk Robotics Ltd. in Israel since October 2017. From 2009 to 2013, Mr. Koren served as General Manager of RuggedCOM Israel, a developer of communications equipment. From 2007 to 2009, he served as the Vice President of Research and Development of Alvarion Technologies Ltd., an Israeli provider of wireless services. Mr. Koren holds a B.Sc. in electrical engineering from Tel Aviv University and an MBA from the University of Herriot Watt, Scotland.
The remaining information required by this Item will be included in, and is incorporated herein by reference from, our definitive proxy statement for our 2020 Annual Meeting of Shareholders to be filed with the SEC pursuant to Regulation 14A within 120 days after the end of our fiscal year ended December 31, 2019 (the “Proxy Statement”).
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 will be included in, and is incorporated herein by reference from, our Proxy Statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item will 12 be included in and is incorporated herein by reference from, our Proxy Statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 will be included in and is incorporated herein by reference, from our Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item 14 will be included in and is incorporated herein by reference, from our Proxy Statement.
72
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements.
The Consolidated Financial Statements filed as part of this annual report are identified in the Index to Consolidated Financial Statements on page F-1 hereto.
(a)(2) Financial Statement Schedules.
Financial Statement Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
(a)(3) Exhibits.
See accompanying Exhibit Index included after the signature page of this report for a list of the exhibits filed or furnished with or incorporated by reference in this report.
Not applicable.
73
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ReWalk Robotics Ltd. | ||
| By: | /s/ Larry Jasinski | |
| Name: Larry Jasinski | ||
| Title: Chief Executive Officer | ||
Date: February 20, 2020
KNOW ALL PERSONS BY THESE PRESENT: That the undersigned officers and directors of ReWalk Robotics Ltd. do hereby constitute and appoint Larry Jasinski and Ori Gon the lawful attorney and agent with power and authority to do any and all acts and things and to execute any and all instruments which said attorney and agent determines may be necessary or advisable or required to enable ReWalk Robotics Ltd. to comply with the Securities and Exchange Act of 1934, as amended, and any rules or regulations or requirements of the Securities and Exchange Commission in connection with this report. Without limiting the generality of the foregoing power and authority, the powers granted include the power and authority to sign the names of the undersigned officers and directors in the capacities indicated below to this report or amendments or supplements thereto, and each of the undersigned hereby ratifies and confirms all that said attorneys and agents, or either of them, shall do or cause to be done by virtue hereof. This Power of Attorney may be signed in several counterparts.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Larry Jasinski | Director
and Chief Executive Officer |
February 20, 2020 | ||
| Larry Jasinski | ||||
| /s/ Ori Gon | Chief
Financial Officer |
February 20, 2020 | ||
| Ori Gon | ||||
| /s/ Jeff Dykan | Chairman of the Board | February 20, 2020 | ||
| Jeff Dykan | ||||
| /s/ Yohanan R Engelhardt | Director | February 20, 2020 | ||
| Yohanan R Engelhardt | ||||
| /s/ Dr. John William Poduska | Director | February 20, 2020 | ||
| Dr. John William Poduska | ||||
| /s/ Chunlin Han | Director | February 20, 2020 | ||
| Chunlin Han | ||||
| /s/ Wayne B. Weisman | Director | February 20, 2020 | ||
| Wayne B. Weisman | ||||
| /s/ Yasushi Ichiki | Director | February 20, 2020 | ||
| Yasushi Ichiki | ||||
| /s/ Aryeh Dan | Director | February 20, 2020 | ||
| Aryeh Dan | ||||
| /s/ Peter Wehrly | Director | February 20, 2020 | ||
| Peter Wehrly |
74
EXHIBIT INDEX
75
76
77
| * | Portions of the agreement were omitted and a complete copy of the agreement has been provided separately to the Securities and Exchange Commission pursuant to the Company’s application requesting confidential treatment under, as applicable, Rule 406 of the Securities Act of 1933, as amended and/or Rule 24b-2 of the Securities Exchange Act of 1934, as amended, which application was subsequently granted. |
| ** | Management contract or compensatory plan, contract or arrangement. |
| *** | Furnished herewith. |
| ^ | Portions of this exhibit (indicated by asterisks) have been omitted under rules of the U.S. Securities and Exchange Commission permitting the confidential treatment of select information. |
| # | The schedules to this exhibit have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company agrees to furnish a copy of the omitted schedules to the Securities and Exchange Commission on a supplemental basis upon its request. |
78
CONSOLIDATED FINANCIAL STATEMENTS
U.S. DOLLARS IN THOUSANDS
INDEX
F-1
 |
Kost Forer Gabbay & Kasierer 2 Pal-Yam Blvd. Haifa 3309502, Israel |
Tel:
+972-4-8654000 Fax: +972-3-5633439 ey.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
REWALK ROBOTICS LTD.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Rewalk Robotics Ltd. (the “Company”) as of December 31, 2019 and 2018, and the related consolidated statements of operations, statements of changes in shareholders’ equity and consolidated statement of cash flows for each of the three years in the period ended December 31, 2019, and the related notes (collectively referred to as the “Consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1e to the financial statements, the Company has suffered recurring losses from operations has a working capital deficiency and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1e. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ KOST FORER GABBAY & KASIERER |
| A Member of Ernst & Young Global |
| We have served as the Company’s auditor since 2014. |
| Haifa, Israel |
| February 20, 2020 |
F-2
REWALK ROBOTICS LTD. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 16,253 | $ | 9,546 | ||||
| Trade receivable, net | 794 | 758 | ||||||
| Prepaid expenses and other current assets | 903 | 693 | ||||||
| Inventories | 3,123 | 2,240 | ||||||
| Total current assets | 21,073 | 13,237 | ||||||
| LONG-TERM ASSETS | ||||||||
| Restricted cash and other long term assets | 1,061 | 1,099 | ||||||
| Operating lease right-of-use assets | 1,737 | — | ||||||
| Property and equipment, net | 501 | 626 | ||||||
| Total long-term assets | 3,299 | 1,725 | ||||||
| Total assets | $ | 24,372 | $ | 14,962 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
REWALK ROBOTICS LTD. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands (except share and per share data)
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Current maturities of long term loan | $ | 5,438 | $ | 1,722 | ||||
| Current maturities of operating leases liability | 637 | — | ||||||
| Trade payables | 2,698 | 2,328 | ||||||
| Employees and payroll accruals | 670 | 650 | ||||||
| Deferred revenues | 323 | 237 | ||||||
| Other current liabilities | 402 | 445 | ||||||
| Total current liabilities | 10,168 | 5,382 | ||||||
| LONG-TERM LIABILITIES | ||||||||
| Long term loan, net of current maturities | 1,527 | 6,965 | ||||||
| Deferred revenues | 521 | 431 | ||||||
| Non-current operating leases liability | 1,315 | — | ||||||
| Other long-term liabilities | 61 | 239 | ||||||
| Total long-term liabilities | 3,424 | 7,635 | ||||||
| Total liabilities | 13,592 | 13,017 | ||||||
| COMMITMENTS AND CONTINGENT LIABILITIES | ||||||||
| Shareholders’ equity: | ||||||||
| Share capital | ||||||||
| Ordinary share of NIS 0.25 par value-Authorized: 60,000,000 shares at December 31, 2019 and 10,000,000 shares at December 31, 2018; Issued and outstanding: 7,319,560 and 2,813,087 shares at December 31, 2019 and December 31, 2018, respectively (1) | 504 | 193 | ||||||
| Additional paid-in capital | 178,745 | 154,670 | ||||||
| Accumulated deficit | (168,469 | ) | (152,918 | ) | ||||
| Total shareholders’ equity | 10,780 | 1,945 | ||||||
| Total liabilities and shareholders’ equity | $ | 24,372 | $ | 14,962 | ||||
| (1) | Reflects one-for-twenty-five reverse share split that became effective on April 1, 2019. See Note 8a to these consolidated financial statements. |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
REWALK ROBOTICS LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
U.S. dollars in thousands (except share and per share data)
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Revenues | $ | 4,873 | $ | 6,545 | $ | 7,753 | ||||||
| Cost of revenues | 2,147 | 3,720 | 4,652 | |||||||||
| Gross profit | 2,726 | 2,825 | 3,101 | |||||||||
| Operating expenses: | ||||||||||||
| Research and development, net | 5,348 | 7,349 | 6,042 | |||||||||
| Sales and marketing | 6,167 | 7,897 | 11,360 | |||||||||
| General and administrative | 5,259 | 6,793 | 7,691 | |||||||||
| Total operating expenses | 16,774 | 22,039 | 25,093 | |||||||||
| Operating loss | (14,048 | ) | (19,214 | ) | (21,992 | ) | ||||||
| Loss on extinguishment of debt | — | — | 313 | |||||||||
| Financial expenses, net | 1,496 | 2,466 | 2,293 | |||||||||
| Loss before income taxes | (15,544 | ) | (21,680 | ) | (24,598 | ) | ||||||
| Income taxes (tax benefit) | 7 | (5 | ) | 119 | ||||||||
| Net loss | $ | (15,551 | ) | $ | (21,675 | ) | $ | (24,717 | ) | |||
| Net loss per ordinary share, basic and diluted | $ | (2.70 | ) | $ | (14.72 | ) | $ | (30.57 | ) | |||
| Weighted average number of shares used in computing net loss per ordinary share, basic and diluted | 5,763,317 | 1,472,499 | 808,557 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
REWALK ROBOTICS LTD. AND SUBSIDIARIES
STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
U.S. dollars in thousands (except share data)
| Ordinary Share | Additional paid-in | Accumulated | Total shareholders’ | |||||||||||||||||
| Number | Amount | capital | deficit | equity | ||||||||||||||||
| Balance as of December 31, 2016 | 653,530 | 45 | 114,707 | (106,492 | ) | 8,260 | ||||||||||||||
| Share-based compensation to employees and non-employees | — | — | 3,654 | — | 3,654 | |||||||||||||||
| Issuance of ordinary shares upon exercise of options to purchase ordinary shares and RSUs by employees and non-employees | 6,670 | 1 | 37 | — | 38 | |||||||||||||||
| Issuance of ordinary shares in at-the-market offering, net of issuance expenses in the amount of $467 | 224,524 | 16 | 9,293 | — | 9,309 | |||||||||||||||
| Issuance of ordinary shares in follow-on public offering, net of issuance expenses in an amount of $1,117 | 315,422 | 22 | 7,141 | — | 7,163 | |||||||||||||||
| Cumulative effect to stock based compensation from adoption of a new accounting standard | — | — | 11 | (11 | ) | — | ||||||||||||||
| Net loss | — | — | — | (24,717 | ) | (24,717 | ) | |||||||||||||
| Balance as of December 31, 2017 | 1,200,146 | 84 | 134,843 | (131,220 | ) | 3,707 | ||||||||||||||
| Cumulative effect to accumulated deficit from adoption of a new accounting standard | — | — | — | (23 | ) | (23 | ) | |||||||||||||
| Share-based compensation to employees and non-employees | — | — | 2,766 | — | 2,766 | |||||||||||||||
| Issuance of ordinary shares upon exercise of options to purchase ordinary shares and RSUs by employees and non-employees | 17,181 | * | ) | — | — | — | ||||||||||||||
| Issuance of ordinary shares in investing agreement, net of issuance expenses in an amount of $830 (1) | 164,715 | 12 | 4,283 | — | 4,295 | |||||||||||||||
| Issuance of ordinary shares in at-the-market offering, net of issuance expenses in the amount of $236 (1) | 49,882 | 4 | 1,113 | — | 1,117 | |||||||||||||||
| Issuance of ordinary shares, warrants and pre-funded warrants in follow-on public offering, net of issuance expenses in an amount of $1,505 (1) | 728,019 | 49 | 11,528 | — | 11,577 | |||||||||||||||
| Modification of warrants to purchase ordinary shares (2) | — | — | 18 | — | 18 | |||||||||||||||
| Exercise of pre-funded warrants (1) | 653,144 | 44 | 119 | — | 163 | |||||||||||||||
| Net loss | — | — | — | (21,675 | ) | (21,675 | ) | |||||||||||||
| Balance as of December 31, 2018 | 2,813,087 | 193 | 154,670 | (152,918 | ) | 1,945 | ||||||||||||||
| Share-based compensation to employees and non-employees | — | — | 1,108 | — | 1,108 | |||||||||||||||
| Issuance of ordinary shares upon exercise of options to purchase ordinary shares and RSUs by employees and non-employees | 47,473 | 2 | — | — | 2 | |||||||||||||||
| Issuance of ordinary shares in a “best efforts” offering, net of issuance expenses in the amount of $686 (1) | 760,000 | 52 | 3,632 | — | 3,684 | |||||||||||||||
| Exercise of pre-funded warrants and warrants (1) | 584,087 | 40 | 1,461 | — | 1,501 | |||||||||||||||
| Issuance of ordinary shares in a “Registered Direct” offerings, net of issuance expenses in the amount of $ 1,125 (1) | 1,650,248 | 115 | 8,010 | — | 8,125 | |||||||||||||||
| Issuance of ordinary shares in a “Warrant exercise” agreement, net of issuance expenses in the amount of $ 1,019 (1) | 1,464,665 | 102 | 9,864 | — | 9,966 | |||||||||||||||
| Net loss | — | — | — | (15,551 | ) | (15,551 | ) | |||||||||||||
| Balance as of December 31, 2019 | 7,319,560 | 504 | 178,745 | (168,469 | ) | 10,780 | ||||||||||||||
| *) | Represents an amount lower than $1. |
| (1) | See note 8b. |
| (2) | See note 8e. |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
REWALK ROBOTICS LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Cash flows used in operating activities: | ||||||||||||
| Net loss | $ | (15,551 | ) | $ | (21,675 | ) | $ | (24,717 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 321 | 463 | 642 | |||||||||
| Share-based compensation to employees and non-employees | 1,108 | 2,766 | 3,654 | |||||||||
| Deferred taxes | (57 | ) | (107 | ) | 73 | |||||||
| Loss on extinguishment of debt | — | — | 313 | |||||||||
| Loss on inducement of debt (2) | — | 600 | — | |||||||||
| Financial expenses related to long term loan | — | 224 | 128 | |||||||||
| Changes in assets and liabilities: | ||||||||||||
| Trade receivables, net | (36 | ) | 322 | 151 | ||||||||
| Prepaid expenses, operating lease right-of-use assets and other assets | 64 | 734 | (438 | ) | ||||||||
| Inventories | (1,221 | ) | 1,403 | (582 | ) | |||||||
| Trade payables | 370 | 492 | (1,613 | ) | ||||||||
| Employees and payroll accruals | 20 | (222 | ) | (147 | ) | |||||||
| Deferred revenues | 176 | 283 | 47 | |||||||||
| Other current and long term liabilities | (9 | ) | (57 | ) | 27 | |||||||
| Net cash used in operating activities | (14,815 | ) | (14,774 | ) | (22,462 | ) | ||||||
| Cash flows used in investing activities: | ||||||||||||
| Purchase of property and equipment | (22 | ) | (13 | ) | (21 | ) | ||||||
| Net cash used in investing activities | (22 | ) | (13 | ) | (21 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Issuance of ordinary shares upon exercise of options to purchase ordinary shares by employees and non-employees | — | — | 38 | |||||||||
| Repayment of long term loan | (1,722 | ) | (3,866 | ) | (3,102 | ) | ||||||
| Issuance of ordinary shares in investment agreement, net of issuance expenses in an amount of $830 (1) | — | 4,295 | — | |||||||||
| Issuance of ordinary shares in at-the-market offering, net of issuance expenses paid in the amount of $211 (1) | — | 1,142 | 9,309 | |||||||||
| Issuance of ordinary shares and exercise of pre-funded warrants into ordinary shares in follow-on offering, net of issuance expenses in an amount of $1,505 and net of long term loan conversion in the amount of $3,600 (1) (2) | — | 8,140 | 7,163 | |||||||||
| Issuance of ordinary shares in a “best efforts” offering, net of issuance expenses in the amount of $ 686 (1) | 3,684 | — | — | |||||||||
| Issuance of ordinary shares in a “registered direct” offerings, net of issuance expenses in the amount of $ 1,035 (1) | 8,125 | — | — | |||||||||
| Issuance of ordinary shares in a “warrant exercise” agreement, net of issuance expenses in the amount of $1,019 (1) | 9,966 | — | — | |||||||||
| Exercise of pre-funded warrants and warrants (1) | 1,429 | — | — | |||||||||
| Net cash provided by financing activities | 21,482 | 9,711 | 13,408 | |||||||||
| Increase (decrease) in cash, cash equivalents, and restricted cash | 6,645 | (5,076 | ) | (9,075 | ) | |||||||
| Cash, cash equivalents, and restricted cash at beginning of period | 10,347 | 15,423 | 24,498 | |||||||||
| Cash, cash equivalents, and restricted cash at end of period | $ | 16,992 | $ | 10,347 | $ | 15,423 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
REWALK
ROBOTICS LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Supplemental disclosures of non-cash flow information | ||||||||||||
| Repayment of long term loan by issuance of units and pre-funded units (2) | $ | — | $ | 3,000 | $ | — | ||||||
| At-the-market offering expenses not yet paid (1) | $ | — | $ | 25 | $ | — | ||||||
| Classification of other current assets to property and equipment, net | $ | — | $ | 236 | $ | — | ||||||
| Classification of inventory to other current assets | $ | 164 | $ | — | $ | — | ||||||
| Classification of inventory to property and equipment | $ | 174 | $ | — | $ | 203 | ||||||
| Cashless exercise of pre-funded warrants | $ | 72 | $ | — | $ | — | ||||||
| Initial recognition of operating lease right-of-use assets | $ | 2,099 | $ | — | $ | — | ||||||
| Initial recognition of operating lease liabilities | $ | (2,249 | ) | $ | — | $ | — | |||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Cash and cash equivalents | $ | 16,253 | $ | 9,546 | $ | 14,567 | ||||||
| Restricted cash included in other long term assets | $ | 739 | $ | 801 | $ | 856 | ||||||
| Total Cash, cash equivalents, and restricted cash | $ | 16,992 | $ | 10,347 | $ | 15,423 | ||||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Cash paid for income taxes | $ | 21 | $ | 25 | $ | 21 | ||||||
| Cash paid for interest | $ | 1,499 | $ | 1,501 | $ | 2,300 | ||||||
| (1) | See note 8b. |
| (2) | See note 6. |
The accompanying notes are an integral part of these consolidated financial statements.
F-8
| a. | ReWalk Robotics Ltd. (“RRL”, and together with its subsidiaries, the “Company”) was incorporated under the laws of the State of Israel on June 20, 2001 and commenced operations on the same date. |
| b. | RRL has two wholly-owned subsidiaries: (i) ReWalk Robotics Inc. (“RRI”) incorporated under the laws of Delaware on February 15, 2012 and (ii) ReWalk Robotics GMBH. (“RRG”) (formerly Argo Medical Technologies GmbH) incorporated under the laws of Germany on January 14, 2013. |
| c. | The Company is designing, developing and commercializing robotic exoskeletons that allow individuals with mobility impairments or other medical conditions the ability to stand and walk once again. The Company has developed and is continuing to commercialize the ReWalk, an exoskeleton designed for individuals with paraplegia that uses its patented tilt-sensor technology and an on-board computer and motion sensors to drive motorized legs that power movement. The ReWalk system consists of a light wearable brace support suit which integrates motors at the joints, rechargeable batteries, an array of sensors and a computer-based control system to power knee and hip movement. There are currently two types of ReWalk products: ReWalk Personal and ReWalk Rehabilitation. ReWalk Personal is designed for everyday use by individuals at home and in their communities and is custom-fitted for each user. ReWalk Rehabilitation is designed for the clinical rehabilitation environment where it provides individuals access to valuable exercise and therapy. Additionally, the Company developed and, in June 2019, started to commercialize the ReStore following receipt of European Union CE mark and United States Food and Drug Administration (“FDA”). The ReStore is a powered, lightweight soft exo-suit intended for use in the rehabilitation of individuals with lower limb disability due to stroke. The Company markets and sells its products directly to institutions and individuals and through third-party distributors. The Company sells its products directly primarily in Germany and the United States, and primarily through distributors in other markets. In its direct markets, the Company has established relationships with rehabilitation centers and the spinal cord injury community, and in its indirect markets, the Company’s distributors maintain these relationships. RRI markets and sells products mainly in the United States. RRG sell the Company’s products mainly in Germany and Europe. |
| d. | The Company depends on one contract manufacturer, Sanmina. Reliance on this vendor makes the Company vulnerable to possible capacity constraints and reduced control over component availability, delivery schedules, manufacturing yields and costs. |
| e. | The Company has an accumulated deficit in the total amount of $168.5 million as of December 31, 2019, negative cash flow from operations of $14.8 million, and further losses are anticipated in the development of its business. Those factors raise substantial doubt about the Company’s ability to continue as a going concern. The ability to continue as a going concern is dependent upon the Company obtaining the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they become due. |
F-9
The Company intends to finance operating costs over the next twelve months with existing cash on hand, potential reduction in operating cash burn and future issuances of equity and debt securities, or through a combination of the foregoing. However, the Company will need to seek additional sources of financing if the Company require more funds than anticipated during the next 12 months or in later periods.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business.
The consolidated financial statements for the year ended December 31, 2019 do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES
The consolidated financial statements are prepared according to United States generally accepted accounting principles (“U.S. GAAP”), applied on a consistent basis, as follows:
| a. | Use of Estimates |
The preparation of the consolidated financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates, judgments and assumptions. The Company’s management believes that the estimates, judgments and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. On an ongoing basis, the Company’s management evaluates estimates, including those related to inventories, fair values of share-based awards and warrants, contingent liabilities, provision for warranty, allowance for doubtful account and sales return reserve. Such estimates are based on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities.
| b. | Financial Statements in U.S. Dollars: |
Most of the revenues and costs of the Company are denominated in United States dollars (“dollars”). Some of the Company’s and its subsidiaries’ revenues and costs are incurred in Euros and New Israeli Shekels (“NIS”), however, the selling prices are linked to the Company’s price list which is determined in dollars, the budget is managed in dollars, financing activities including loans and cash investments, are made in U.S. dollars and the Company’s management believes that the dollar is the primary currency of the economic environment in which the Company and each of its subsidiaries operate. Thus, the dollar is the Company’s and its subsidiaries’ functional and reporting currency.
Accordingly, transactions denominated in currencies other than the functional currency are re-measured to the functional currency in accordance with Accounting Standards Codification (“ASC”) No. 830, “Foreign Currency Matters” at the exchange rate at the date of the transaction or the average exchange rate in the relevant reporting period. At the end of each reporting period, financial assets and liabilities are re-measured to the functional currency using exchange rates in effect at the balance sheet date. Non-financial assets and liabilities are re-measured at historical exchange rates. Gains and losses related to re-measurement are recorded as financial income (expense) in the consolidated statements of operations as appropriate.
| c. | Principles of Consolidation: |
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, RRI and RRG. All intercompany transactions and balances have been eliminated upon consolidation.
F-10
| d. | Cash Equivalents: |
Cash equivalents are short-term highly liquid investments that are readily convertible to cash with original maturities of three months or less, at the date acquired.
| e. | Inventories: |
Inventories are stated at the lower of cost or market value. Inventory reserves are provided to cover risks arising from slow-moving items or technological obsolescence.
The Company periodically evaluates the quantities on hand relative to historical, current and projected sales volume. Based on this evaluation, an impairment charge is recorded when required to write-down inventory to its market value.
Cost is determined as follows:
Finished products - on the basis of raw materials and manufacturing costs on an average basis.
Raw materials - The weighted average cost method.
The Company regularly evaluates the ability to realize the value of inventory based on a combination of factors, including historical usage rates and forecasted sales according to outstanding backlogs. Purchasing requirements and alternative usage are explored within these processes to mitigate inventory exposure. When recorded, the reserves are intended to reduce the carrying value of inventory to its net realizable value. In the years ended December 31, 2019, 2018 and 2017, the Company wrote off inventory in the amount of $64 thousand, $562 thousand and $131 thousand, respectively. The write off inventory were recorded in cost of revenue. If actual demand for the Company’s products deteriorates, or market conditions are less favorable than those projected, additional inventory reserves may be required.
| f. | Related parties transactions and balances: |
The Company has a related party shareholder named Yaskawa Electric Corporation (“YEC”).
In September 2013 the Company entered into a share purchase agreement and a strategic alliance with YEC, pursuant to which YEC has agreed to distribute the Company’s products, in addition to providing sales, marketing, service and training functions, in Japan, China (including Hong-Kong and Macau), Taiwan, South Korea, Singapore and Thailand.
As of December 31, 2019 and 2018 the related party receivable were 0% of trade receivable, net, in both years. Revenues from YEC during the years ended December 31, 2019, 2018 and 2017 amounted to $41 thousand, $13 thousand and $0, respectively.
F-11
| g. | Property and Equipment: |
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets at the following annual rates:
| % | |
| Computer equipment | 20-33 (mainly 33) |
| Office furniture and equipment | 6 - 10 (mainly 10) |
| Machinery and laboratory equipment | 15 |
| Field service units | 50 |
| Leasehold improvements | Over the shorter of the lease term or estimated useful life |
| h. | Impairment of Long-Lived Assets: |
The Company’s long-lived assets are reviewed for impairment in accordance with ASC No. 360, “Property, Plant and Equipment” whenever events or changes in circumstances indicate that the carrying amount of an asset (or asset group) may not be recoverable. Recoverability of assets (or asset group) to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. During the years ended December 31, 2019, 2018 and 2017, no impairment losses have been recorded.
| i. | Restricted cash and Other long term assets: |
Other long term assets include long-term prepaid expenses and restricted cash deposits for offices and cars leasing based upon the term of the remaining restrictions.
| j. | Revenue Recognition: |
The Company generates revenues from sales of products. The Company sells its products directly to end customers and through distributors. The Company sells its products to private individuals (who finance the purchases by themselves, through fundraising or reimbursement coverage from insurance companies), rehabilitation facilities and distributors.
On January 1, 2018, the Company adopted Topic 606 using the modified retrospective method for contracts that were not completed as of January 1, 2018. Under the modified retrospective method, the Company recognized the cumulative effect of initially applying the new revenue standard as an adjustment to the opening balance of retained earnings. This adjustment did not have a material impact on the Company consolidated financial statements. Results for reporting periods beginning after January 1, 2018 are presented under Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with the Company historic accounting under Revenue Recognition (“Topic 605”).
F-12
The adoption of Topic 606 represents a change in accounting principle that will provide financial statement readers with enhanced revenue recognition disclosures. In accordance with Topic 606, revenue is recognized when obligations under the terms of a contract with the Company customer are satisfied; generally this occurs with the transfer of control of the Company products or services. Revenue is measured as the amount of consideration to which the Company expect to be entitled in exchange for transferring products or providing services. To achieve this core principle, the Company applies the following five steps:
1. Identify the contract with a customer
A contract with a customer exists when (i) the Company enters into a written agreement with a customer that defines each party’s rights regarding the products or services to be transferred and identifies the payment terms related to these products or services, (ii) both parties to the contract are committed to perform their respective obligations, (iii) the contract has commercial substance, and (iv) the Company determines that collection of substantially all consideration for products or services that are transferred is probable based on the customer’s intent and ability to pay the promised consideration. The Company applies judgment in determining the customer’s ability and intention to pay, which is based on a variety of factors including the customer’s payment history or, in the case of a new customer, published credit and financial information pertaining to the customer.
2. Identify the performance obligations in the contract
Performance obligations promised in a contract are identified based on the products or services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the product or service either on its own or together with other resources that are readily available from the Company, and are distinct in the context of the contract, whereby the transfer of the products or services is separately identifiable from other promises in the contract.
3. Determine the transaction price
The transaction price is determined based on the consideration to which the Company will be entitled in exchange for transferring products or services to the customer. To the extent the transaction price is variable, revenue is recognized at an amount equal the consideration to which the Company expects to be entitled. This estimate includes customer sales incentives which are accounted for as a reduction to revenue and estimated using either the expected value method or the most likely amount method, depending on the nature of the program.
As a result of the Company’s adoption of this standard, the majority of the amounts that were historically classified as bad debt expense, primarily related to self-payers customers, are now considered an implicit price concession in determining net revenue. Accordingly, the Company recognized uncollectible balances associated with self-payers customers as a reduction of the transaction price and therefore as a reduction in net revenues when historically these amounts were classified as bad debt expense within general and administrative expenses.
Shipping and handling costs charged to customers are included in net sales. Determining the transaction price requires significant judgment, which is discussed by revenue category in further detail below.
In practice, the Company do not offer extended payment terms beyond one year to customers. As such, the Company do not adjust the consideration for financing arrangements.
4. Allocate the transaction price to performance obligations in the contract
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative standalone selling price basis unless a portion of the variable consideration related to the contract is allocated entirely to a performance obligation. The Company determines standalone selling price based on the price at which the performance obligation is sold separately.
F-13
5. Recognize revenue when or as the Company satisfies a performance obligation
The Company generally satisfies performance obligations at a point in time, once the customer has obtained the legal title to the items purchased or service provided.
For systems sold to rehabilitation facilities, the Company includes training and considers the elements in the arrangement to be a single performance obligation. In accordance with ASC 606, the Company has concluded that the training is essential to the functionality of the Company’s systems. Therefore the Company recognizes revenue for the system and training only after delivery in accordance with the agreement delivery terms to the customer and after the training has been completed.
For sales of Personal systems to end users, and for sales of Personal or Rehabilitation systems to third party distributors, the Company does not provide training to the end user as this training is completed by the Rehabilitation centers or by the distributor that have previously completed the ReWalk Training program. Therefore the Company recognizes revenue in such sales upon delivery.
Revenue is recognized based on the transaction price at the time the related performance obligation is satisfied by transferring a promised product or service to a customer.
The Company generally does not grant a right of return for its products. There have been isolated cases in which the Company experienced a return of its products. Therefore, the Company records reductions to revenue for expected future product returns based on the Company’s historical experience.
Disaggregation of Revenues
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 (1) | ||||||||||
| Units placed | $ | 4,385 | $ | 6,237 | $ | 7,490 | ||||||
| Spare parts and warranties | 488 | 308 | 263 | |||||||||
| Total Revenues | $ | 4,873 | $ | 6,545 | $ | 7,753 | ||||||
| (1) | As noted above, prior period amounts have not been adjusted under the modified retrospective method. |
Units placed
the Company currently offer three products: ReWalk Personal, ReWalk Rehabilitation units for Spinal Cord Injury (“SCI Products”) and ReStore soft suit exoskeleton for rehabilitation of individuals suffering from stroke. SCI Products are currently designed for everyday use by paraplegic individuals at home and in their communities, and is custom fitted for each user, as well as for use by paraplegia patients in the clinical rehabilitation environment, where it provides individuals access to valuable exercise and therapy. The ReStore is a powered, lightweight soft exo-suit intended for use in the rehabilitation of individuals with lower limb disability due to stroke in the clinical rehabilitation environment.
Units placed includes revenue from sales of SCI Products and ReStore.
the Company also offer a rent-to-purchase model in which the Company recognize revenue according to the agreed rental monthly fee. For units placed, the Company transfer control and recognize a sale when title has passed to the customer and rental revenue is recognized ratably according to the agreed rental monthly fee. Each unit placed is considered an independent, unbundled performance obligation.
F-14
Spare parts and warranties
Spare parts are sold to private individuals, rehabilitation facilities and distributors. Revenue is recognized when the Company satisfies a performance obligation by transferring control over promised goods or services to the customer. Each part sold is considered an independent, unbundled performance obligation.
Warranties are classified as either assurance type or service type warranty. A warranty is considered an assurance type warranty if it provides the consumer with assurance that the product will function as intended for a limited period of time.
In the beginning of 2018, the Company updated its service policy for SCI Products to include a five- year warranty compared to a period of two years that were included in the past for parts and services. The first two years are considered as assurance type warranty and the additional period is considered an extended service arrangement, which is a service type warranty. An assurance type warranty is not accounted for as separate performance obligations under the revenue model. A service type warranty is either sold with a unit or separately for units for which the warranty has expired. Revenue is then recognized ratably over the life of the warranty.
The ReStore device is offered with two-year warranty which are considered as assurance type warranty.
Contract balances
| December 31, | December 31, | |||||||
| 2019 | 2018 | |||||||
| Trade receivable, net (1) | $ | 794 | $ | 758 | ||||
| Deferred revenues (1) (2) | $ | 844 | $ | 668 | ||||
| (1) | Balance presented net of unrecognized revenues that were not yet collected. |
| (2) | $262 thousand of December 31, 2018 deferred revenues balance were recognized as revenues during the year ended December 31, 2019. |
Typical timing of payment
The timing of satisfaction of the Company performance obligations does not significantly vary from the typical timing of payment. Typical payment terms are based on payment terms as established in the Company’s contracts. For some contracts the Company may be entitled to receive an advance payment.
Transaction price allocated to remaining performance obligations for the year ended December 31, 2019, revenue recognized from performance obligations related to prior periods was not material.
Revenue expected to be recognized in any future year related to remaining performance obligations, excluding revenue pertaining to contracts that have an original expected duration of one year or less, contracts where revenue is recognized as invoiced and contracts with variable consideration related to undelivered performance obligations, is not material.
The Company’s unfilled performance obligations as of December 31, 2019 and the estimated revenue expected to be recognized in the future related to the service type warranty amounts to $876 thousand, which is fulfilled over one to five years.
F-15
| k. | Accounting for Share-Based Compensation: |
The Company accounts for share-based compensation in accordance with ASC No. 718, “Compensation-Stock Compensation” (“ASC No. 718”). ASC No. 718 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an Option-Pricing Model (“OPM”). The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company’s consolidated statements of operations.
The Company recognizes compensation expenses for the value of its awards granted based on the straight-line method over the requisite service period of each of the awards.
Effective as of January 1, 2017, the Company adopted Accounting Standards Update 2016-09, “Compensation-Stock Compensation (Topic 718)” (“ASU 2016-09”) on a modified, retrospective basis. ASU 2016-09 permits entities to make an accounting policy election related to how forfeitures will impact the recognition of compensation cost for stock-based compensation: to estimate the total number of awards for which the requisite service period will not be rendered or to account for forfeitures as they occur. Upon adoption of ASU 2016-09, the Company elected to change its accounting policy to account for forfeitures as they occur. The change was applied on a modified, retrospective basis with a cumulative-effect adjustment to retained earnings of $11 thousand (which increased the accumulated deficit) as of January 1, 2017.
ASU 2016-09 also eliminates the requirement that excess tax benefits be realized as a reduction in current taxes payable before the associated tax benefit can be recognized as an increase in paid in capital. The implementation resulted with no cumulative-effect adjustment to retained earnings as of January 1, 2017.
Additionally, ASU 2016-09 addresses the presentation of excess tax benefits and employee taxes paid on the statement of cash flows. The Company is now required to present excess tax benefits as an operating activity on the statement of cash flows rather than as a financing activity. The Company adopted this change prospectively.
The Company selected the Black-Scholes-Merton option pricing model as the most appropriate fair value method for its share-option awards. The option-pricing model requires a number of assumptions, of which the most significant are the fair market value of the underlying ordinary share, expected share price volatility and the expected option term. Expected volatility was calculated based upon certain peer companies that the Company considered to be comparable. The expected option term represents the period of time that options granted are expected to be outstanding. The expected option term is determined based on the simplified method in accordance with Staff Accounting Bulletin No. 110, as adequate historical experience is not available to provide a reasonable estimate. The simplified method will continue to apply until enough historical experience is available to provide a reasonable estimate of the expected term. The risk-free interest rate is based on the yield from U.S. treasury bonds with an equivalent term. The Company has historically not paid dividends and has no foreseeable plans to pay dividends.
Following the IPO in September 2014, the fair value of ordinary shares is observable as they are publicly traded.
The fair value of Restricted Stock Units (RSUs) granted is determined based on the price of the Company’s ordinary shares on the date of grant.
F-16
The fair value for options granted in 2019, 2018 and 2017 is estimated at the date of grant using a Black-Scholes-Merton option pricing model with the following assumptions:
| December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Expected volatility | 57.5 | % | 57% - 61 | % | 55% - 59 | % | ||||||
| Risk-free rate | 2.22 | % | 2.74% - 2.83 | % | 1.78% - 2.07 | % | ||||||
| Dividend yield | — | % | — | % | — | % | ||||||
| Expected term (in years) | 6.11 | 6.11 | 5.31 - 6.11 | |||||||||
| Share price | $ | 5.37 | $ | 25.5 - $28.75 | $ | 32.5 - $50 | ||||||
The Company accounts for options granted to consultants and other service providers under ASC No. 718 and ASC No. 505, “Equity-based payments to non-employees.” The fair value of these options was estimated using a Black-Scholes-Merton option-pricing model.
The non-cash compensation expenses related to non-employees for the years ended December 31, 2019, 2018 and 2017 amounted to $18 thousand, $90 thousand and $45 thousand, respectively
The non-cash compensation expenses related to employees and non- employees for the years ended December 31, 2019, 2018 and 2017 amounted to $1,108 thousand, $2,766 thousand and $3,654 thousand respectively.
| l. | Research and Development Costs: |
Research and development costs are charged to the consolidated statement of operations as incurred and are presented net of the amount of any grants the company receive for research and development in the period in which the grant was received.
| m. | Income Taxes |
The Company accounts for income taxes in accordance with ASC No. 740, “Income Taxes” (“ASC No. 740”), using the liability method whereby deferred tax assets and liability account balances are determined based on the differences between financial reporting and the tax basis for assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to the amounts that are more likely-than-not to be realized.
ASC No. 740 contains a two-step approach to recognizing and measuring a liability for uncertain tax positions. The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. The Company accrues interest and penalties related to unrecognized tax benefits in its taxes on income. As of December 31, 2019 and 2018, the Company did not identify any significant uncertain tax positions.
F-17
| n. | Warranty: |
In the beginning of 2018 the Company updated its service policy for new SCI Products sold to include a 5-year warranty. Our ReStore product offering includes a two-year warranty for parts and services. The Company records a provision for the estimated cost to repair or replace products under warranty at the time of sale. Factors that affect the Company’s warranty reserve include the number of units sold, historical and anticipated rates of warranty repairs and the cost per repair.
| US Dollars in thousands | ||||
| Balance at December 31, 2018 | $ | 304 | ||
| Provision | 166 | |||
| Usage | (243 | ) | ||
| Balance at December 31, 2019 | 227 | |||
| o. | Concentrations of Credit Risks: |
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash, cash equivalents and trade receivables.
The Company’s cash and cash equivalents are deposited in major banks in Israel, the United States and Germany. Such deposits in the United States may be in excess of insured limits and are not insured in other jurisdictions. The Company maintains cash and cash equivalents with diverse financial institutions and monitors the amount of credit exposure to each financial institution.
Concentration of credit risk with respect to trade receivable is primarily limited to a customer to which the Company makes substantial sales.
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Customer A | 14 | % | * | ) | ||||
| Customer B | 13 | % | * | ) | ||||
| Customer C | 13 | % | * | ) | ||||
| Customer D | 12 | % | * | ) | ||||
| Customer E | 12 | % | * | ) | ||||
| Customer F | 12 | % | * | ) | ||||
| Customer G | 12 | % | * | ) | ||||
| Customer H | * | ) | 28 | % | ||||
| Customer I | * | ) | 15 | % | ||||
| Customer J | * | ) | 14 | % | ||||
| Customer K | * | ) | 13 | % | ||||
| Customer L | * | ) | 12 | % | ||||
| *) | Less than 10% |
F-18
The Company’s trade receivables are geographically diversified and derived primarily from sales to customers in various countries, mainly in the United States and Europe. Concentration of credit risk with respect to trade receivables is limited by credit limits, ongoing credit evaluation and account monitoring procedures. The Company performs ongoing credit evaluations of its distributors based upon a specific review of all significant outstanding invoices. The Company writes off receivables when they are deemed uncollectible and having exhausted all collection efforts. As of December 31, 2019 and 2018 trade receivables are presented net of $31 thousand and $32 thousand allowance for doubtful accounts, respectively, and net of sales return reserve of $86 thousand and $105 thousand, respectively.
| p. | Accrued Severance Pay: |
Pursuant to Israel’s Severance Pay Law, Israeli employees are entitled to severance pay equal to one month’s salary for each year of employment, or a portion thereof. All of the employees of the RRL elected to be included under section 14 of the Severance Pay Law, 1963 (“section 14”). According to this section, these employees are entitled only to monthly deposits, at a rate of 8.33% of their monthly salary, made in their name with insurance companies. Payments in accordance with section 14 release the Company from any future severance payments (under the above Israeli Severance Pay Law) in respect of those employees; therefore, related assets and liabilities are not presented in the balance sheet.
Total Company expenses related to severance pay amounted to $156 thousand, $169 thousand and $185 thousand for the years ended December 31, 2019, 2018 and 2017, respectively.
| q. | Fair Value Measurements: |
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company uses a three-tier fair value hierarchy to classify and disclose all assets and liabilities measured at fair value on a recurring basis, as well as assets and liabilities measured at fair value on a non-recurring basis, in periods subsequent to their initial measurement. The hierarchy requires the Company to use observable inputs when available, and to minimize the use of unobservable inputs when determining fair value. If a financial instrument uses inputs that fall in different levels of the hierarchy, the instrument will be categorized based upon the lowest level of input that is significant to the fair value calculation. The three-tiers are defined as follows:
| ● | Level 1. Observable inputs based on unadjusted quoted prices in active markets for identical assets or liabilities; | |
| ● | Level 2. Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; and | |
| ● | Level 3. Unobservable inputs for which there is little or no market data requiring the Company to develop its own assumptions. |
The carrying amounts of cash and cash equivalents, short term deposits, trade receivables and trade payables approximate their fair value due to the short-term maturity of such instruments.
F-19
| r. | Basic and Diluted Net Loss Per Share: |
Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares of ordinary shares outstanding during the period.
Diluted net loss per share is computed by giving effect to all potential shares of ordinary shares, including stock options, convertible preferred share warrants, to the extent dilutive, all in accordance with ASC No. 260, “Earning Per Share”.
The following table sets forth the computation of the Company’s basic and diluted net loss per ordinary share (in thousands, except share and per share data):
| Year ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Net loss | $ | (15,551 | ) | $ | (21,675 | ) | $ | (24,717 | ) | |||
| Net loss attributable to ordinary shares | (15,551 | ) | (21,675 | ) | (24,717 | ) | ||||||
| Shares used in computing net loss per ordinary shares, basic and diluted | 5,763,317 | 1,472,499 | 808,557 | |||||||||
| Net loss per ordinary share, basic and diluted | $ | (2.70 | ) | $ | (14.72 | ) | $ | (30.57 | ) | |||
Basic and diluted net loss per share was the same for each period presented as the inclusion of all potential shares of ordinary shares outstanding would have been anti-dilutive.
| s. | Contingent liabilities |
The Company accounts for its contingent liabilities in accordance with ASC No. 450, “Contingencies”. A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated.
With respect to legal matters, provisions are reviewed and adjusted to reflect the impact of negotiations, estimated settlements, legal rulings, advice of legal counsel and other information and events pertaining to a particular matter.
See note 7e for further information.
F-20
| t. | Government grants |
Government grants received by the Company relating to categories of operating expenditures are credited to the consolidated statements of operations during the period in which the expenditure to which they relate is charged. Royalty and non-royalty-bearing grants from the Israel Innovation Authority, or the IIA, (formerly known as the Israeli Office of the Chief Scientist), from the Israel-U.S. Binational Industrial Research and Development Foundation (“BIRD”) and from the Israeli Fund for Promoting Overseas Marketing for funding certain approved research and development projects and sales and marketing activities are recognized at the time when the Company is entitled to such grants, on the basis of the related costs incurred, and are included as a deduction from research and development or sales and marketing expenses (see Note 7c).
No royalty-bearings grants were recorded for the year ended December 31, 2019, the Company received royalty-bearing grants in the amount of $198 thousand for the year ended December 31, 2018, as part of the research and development expenses.
During the year ended December 31,2019, $15 thousand were recorded as royalties expenses as part of the cost of revenues. No royalty expenses were recorded for the years ended December 31, 2018, 2017, respectively
| u. | New Accounting Pronouncements |
Recently Implemented Accounting Pronouncements
| i. | Leases: |
In February 2016, the FASB issued Accounting Standard Update, or ASU, No. 2016-02, Leases (Topic 842), to enhance the transparency and comparability of financial reporting related to leasing arrangements. The Company adopted the standard effective January 1, 2019. At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, the Company utilizes its incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment.
Prior to the Company’s adoption of ASU 2016-02, when its lease agreements contained rent payment relief and rent escalation clauses, the Company recorded a deferred rent asset or liability equal to the difference between the rent expense and the future minimum lease payments due. Operating leases are recognized on the balance sheet as right-of-use assets, current maturities of operating leases and noncurrent operating lease liabilities.
The Company used the modified retrospective transition method, under which the Company applied the standard as a cumulative effect adjustment to each lease that had commenced as of the beginning of January 1, 2019 and did not apply the standard to comparative historical periods. In addition, the Company elected to apply the package of practical expedients permitted under the transition guidance, which among other things, allowed the Company to carry forward the historical lease classification. The Company has elected, as of the adoption date, not to reassess whether expired or existing contracts contain leases under the new definition of a lease, not to reassess the lease classification for expired or existing leases, and not to reassess whether previously capitalized initial direct costs would qualify for capitalization under ASC 842.
Leases with an initial term of 12 months or less are not recorded on the balance sheet. The Company recognizes the lease expense for such leases on a straight-line basis in the statement of operations over the lease term. As a result, the Company no longer recognizes deferred rent on the balance sheet.
F-21
Upon adoption of this standard on January 1, 2019, the Company recorded right–of–use assets and corresponding lease liabilities of $2,099 and $2,249, respectively. As of December 31, 2019, the right–of–use assets and corresponding lease liabilities in the Company’s consolidated balance sheets were $1,737 and $1,952, respectively. The adoption of this standard did not have a material impact on the Company’s consolidated statements of operations or cash flows. See also note 7b - Lease commitment.
Recent Accounting Pronouncements Not Yet Adopted
| ii. | Financial Instruments |
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments and subsequent amendments to the initial guidance under ASU 2018-19, ASU 2019-04 and ASU 2019-05, which amends the current approach to estimate credit losses on certain financial assets, including trade and other receivables. Generally, this amendment requires entities to establish a valuation allowance for the expected lifetime losses of these certain financial assets. Upon the initial recognition of such assets, which will be based on, among other things, historical information, current conditions, and reasonable supportable forecasts. Subsequent changes in the valuation allowance are recorded in current earnings and reversal of previous losses are permitted. Currently, U.S. GAAP requires entities to write down credit losses only when losses are probable and loss reversals are not permitted. Originally, ASU 2016-13 was effective for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2019, with early adoption permitted. An entity should apply the standard by recording a cumulative effect adjustment to retained earnings upon adoption. In November 2019, FASB issued ASU No. 2019-10, Financial Instruments – Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842). This ASU defers the effective date of ASU 2016-13 for public companies that are considered smaller reporting companies as defined by the SEC to fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. The Company is planning to adopt this standard in the first quarter of fiscal 2023. The adoption of this standard is not expected to result in a material impact to the Company’s financial statements.
NOTE 3:- PREPAID EXPENSES AND OTHER CURRENT ASSETS
The components of prepaid expenses and other current assets are as follows (in thousands):
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Government institutions | $ | 175 | $ | 167 | ||||
| Prepaid expenses | 318 | 155 | ||||||
| Advances to vendors | 246 | 371 | ||||||
| Other assets | 164 | — | ||||||
| $ | 903 | $ | 693 | |||||
F-22
NOTE 4:- INVENTORIES
The components of inventories are as follows (in thousands):
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Finished products | $ | 2,394 | $ | 2,240 | ||||
| Raw materials | 729 | — | ||||||
| $ | 3,123 | $ | 2,240 | |||||
NOTE 5:- PROPERTY AND EQUIPMENT, NET
The components of property and equipment, net are as follows (in thousands):
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Cost: | ||||||||
| Computer equipment | $ | 723 | $ | 712 | ||||
| Office furniture and equipment | 293 | 293 | ||||||
| Machinery and laboratory equipment | 604 | 593 | ||||||
| Field service units | 1,420 | 1,246 | ||||||
| Leasehold improvements | 333 | 333 | ||||||
| $ | 3,373 | $ | 3,177 | |||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Accumulated depreciation | 2,872 | 2,551 | ||||||
| Property and equipment, net | $ | 501 | $ | 626 | ||||
Depreciation expenses amounted to $321 thousand, $463 thousand and $642 thousand for the years ended December 31, 2019, 2018 and 2017, respectively.
F-23
NOTE 6:- LOAN AGREEMENT WITH KREOS AND RELATED WARRANT TO PURCHASE ORDINARY SHARES
On December 30, 2015, the Company entered into the loan agreement (the “Loan Agreement”) with Kreos Capital V (Expert Fund) Limited (“Kreos”), pursuant to which Kreos extended a line of credit to the Company in the amount of $20 million.
The Loan has a maturity of 36 months and bears annual interest of 10.75%, which is to be paid monthly. The principal of the Loan is to be paid in 24 monthly payments, beginning in January 2017, except for the last loan payment, which was paid in advance on the applicable draw down date. The repayment period will be extended to 36 months if the Company raises $20.0 million or more in connection with the issuance of shares of its capital stock (including debt securities convertible into shares of the Company’s capital stock) prior to the expiration of the respective initial 24-month period.
Repayment of the Loan and payment of all other amounts owed to Kreos are to be made in U.S. dollars.
On June 9, 2017, the Company and Kreos entered into the First Amendment to the Loan Agreement (the “First Amendment”). As of that date the outstanding principal amount under the Loan Agreement was $17.2 million. Under the First Amendment, $3.0 million of the outstanding principal under the Loan Agreement is subject to repayment pursuant to the senior secured Kreos Convertible Note issued on June 9, 2017, thus reducing the outstanding principal amount under the Loan Agreement to $14.2 million as of June 9, 2017. This amended outstanding principal amount remains subject to repayment in accordance with the terms and conditions of the Loan Agreement and an amended repayment schedule. Interest on the Kreos Convertible Note is payable monthly in arrears at a rate of 10.75% per year.
Kreos may convert the then-outstanding principal and “end of loan payments” under the Kreos Convertible Note, in whole or in part, on one or more occasions, into up to 100,946 ordinary shares, at a conversion price per share equal to $31.7 per share (subject to customary anti-dilution adjustments) at any time until the earlier of (i) the maturity date of June 9, 2020 or (ii) a “Change of Control,” as defined in the Loan Agreement.
On November 20, 2018, the Company and Kreos entered into the Second Amendment to the Loan Agreement (the “Second Amendment”). In the Second Amendment, the Company agreed to repay $3.6 million to Kreos in satisfaction of all outstanding indebtedness under the Kreos Convertible Note and other related payments, including prepayment costs and end of loan payments and Kreos agreed to terminate the Kreos Convertible Note. The Company repaid Kreos the $3.6 million by issuing to Kreos 192,000 units and 288,000 pre-funded units at the applicable public offering prices for an aggregate price of $3.6 million (including the aggregate exercise price for the ordinary shares to be received upon exercise of the pre-funded warrants, assuming Kreos exercises all of the pre-funded warrants it purchased as part of the Company’s public offering). The Company and Kreos also agreed to revise the principal and the repayment schedule under the Kreos Loan Agreement. The revised repayment schedule, effectively deferred an additional $1.1 million of payments that were due in 2018 and $2.8 million that were due in 2019 under the loan’s prior repayment schedule, for total deferred payments of $3.9 million compared to the prior repayment schedule. Additionally, Kreos and the Company entered into the Kreos Warrant Amendment, which amended the exercise price of the warrant to purchase 6,679 ordinary shares currently held by Kreos from $241 to $7.5. The Second Amendment also made certain changes to the prepayment premiums under the Kreos Loan Agreement, tying them to the date of the Second Amendment
On June 5, 2019 and June 6, 2019, the Company entered into warrant exercise agreements with certain institutional investors of warrants to purchase the Company’s ordinary shares, pursuant to which, Kreos agreed to exercise in cash their November 2018 warrants at the existing exercise price of $7.50 per share. Under the exercise agreements, the Company also agreed to issue to Kreos new warrants to purchase up to 480,000 ordinary shares at an exercise price of $7.50 per share and exercise period of five years.
As of December 31, 2019, the outstanding principal amount under the Kreos Loan Agreement was $7.0 million. Depending on its liquidity needs, the Company may seek to refinance up to a substantial portion of our indebtedness under our Kreos Loan Agreement, which the Company have considered with Kreos from time to time, including by exchanging its indebtedness with Kreos for new convertible debt from a third-party investor, or to borrow additional funds.
The Company recorded interest expense in the amount of $1.5 million during the fiscal year ended December 31, 2019.
F-24
NOTE 7:- COMMITMENTS AND CONTINGENT LIABILITIES
| a. | Purchase commitment: |
The Company has contractual obligations to purchase goods from its contract manufacturer as well as raw materials from different vendors. Purchase obligations do not include contracts that may be canceled without penalty. As of December 31, 2019, non-cancelable outstanding obligations amounted to approximately $2.3 million.
| b. | Operating lease commitment: |
| (i) | The Company operates from leased facilities in Israel, the United States and Germany. These leases expire between 2019 and 2023. A portion of the Company’s facilities leases is generally subject to annual changes in the Consumer Price Index (CPI). The changes to the CPI are treated as variable lease payments and recognized in the period in which the obligation for those payments was incurred. |
| (ii) | RRL and RRG lease cars for their employees under cancelable operating lease agreements expiring at various dates in between 2019 and 2022. A subset of the Company’s cars leases is considered variable. The variable lease payments for such cars leases are based on actual mileage incurred at the stated contractual rate. RRL and RRG have an option to be released from these agreements, which may result in penalties in a maximum amount of approximately $40 thousand as of December 31, 2019. |
The Company’s future lease payments for its facilities and cars, which are presented as current maturities of operating leases and non-current operating leases liabilities on the Company’s consolidated balance sheets as of December 31, 2019 are as follows (in thousands):
| 2020 | $ | 685 | ||
| 2021 | 675 | |||
| 2022 | 622 | |||
| 2023 | 458 | |||
| Total lease payments | 2,440 | |||
| Less: imputed interest | (488 | ) | ||
| Present value of future lease payments | 1,952 | |||
| Less: current maturities of operating leases | (637 | ) | ||
| Non-current operating leases | $ | 1,315 | ||
| Weighted-average remaining lease term (in years) | 3.54 | |||
| Weighted-average discount rate | 12.9 | % |
Total rent expenses for the years ended December 31, 2019, 2018 and 2017 were $739 thousand, $695 thousand and $682 thousand, respectively.
| c. | Royalties: |
The Company’s research and development efforts are financed, in part, through funding from the IIA and BIRD. Since the Company’s inception through December 31, 2019, the Company received funding from the IIA and BIRD in the total amount of $1.97 million and $500 thousand, respectively. Out of the $1.97 million in funding from the IIA, a total amount of $1.57 million were royalty bearing grants (as of December 31, 2019, the Company paid royalties to the IIA in the total amount of $50 thousand), while a total amount of $400 thousand was received in consideration of 209 convertible preferred A shares, which converted after the Company’s initial public offering in September 2014 into ordinary shares in a conversion ratio of 1 to 1. The Company is obligated to pay royalties to the IIA, amounting to 3%-3.5% of the sales of the products and other related revenues generated from such projects, up to 100% of the grants received. The royalty payment obligations also bear interest at the LIBOR rate. The obligation to pay these royalties is contingent on actual sales of the applicable products and in the absence of such sales, no payment is required.
Additionally, the License Agreement requires the Company to pay Harvard royalties on net sales, See note 9 below for more information about the Collaboration Agreement and the License Agreement.
F-25
During the year ended December 31, 2019, $15 thousand were recorded as royalties expenses in cost of revenues, No royalties expenses recorded for the years ended December 31, 2018 and 2017.
As of December 31, 2019, the contingent liability to the IIA amounted to $1.6 million. The Israeli Research and Development Law provides that know-how developed under an approved research and development program may not be transferred to third parties without the approval of the IIA. Such approval is not required for the sale or export of any products resulting from such research or development. The IIA, under special circumstances, may approve the transfer of IIA-funded know-how outside Israel, in the following cases:
(a) the grant recipient pays to the IIA a portion of the sale price paid in consideration for such IIA-funded know-how or in consideration for the sale of the grant recipient itself, as the case may be, which portion will not exceed six times the amount of the grants received plus interest (or three times the amount of the grant received plus interest, in the event that the recipient of the know-how has committed to retain the R&D activities of the grant recipient in Israel after the transfer); (b) the grant recipient receives know-how from a third party in exchange for its IIA-funded know-how; (c) such transfer of IIA-funded know-how arises in connection with certain types of cooperation in research and development activities; or (d) If such transfer of know-how arises in connection with a liquidation by reason of insolvency or receivership of the grant recipient.
| d. | Liens |
In connection with the Loan Agreement, the Company granted Kreos a first priority security interest over all of its assets, including certain intellectual property and equity interests in its subsidiaries, subject to certain permitted security interests.
The Company’s other long-term assets in the amount of $739 thousand have been pledged as security in respect of a guarantee granted to a third party. Such deposit cannot be pledged to others or withdrawn without the consent of such third party.
| e. | Legal Claims: |
As previously disclosed, between September 2016 and January 2017, eight putative class actions on behalf of alleged shareholders that purchased or acquired the Company ordinary shares pursuant and/or traceable to its registration statement on Form F-1 (File No. 333-197344) used in connection with the initial public offering, or the Company’s IPO, were commenced in the following courts: (i) the Superior Court of the State of California, County of San Mateo; (ii) the Superior Court of the Commonwealth of Massachusetts, Suffolk County; (iii) the United States District Court for the Northern District of California; and (iv) the United States District Court for the District of Massachusetts. February 11, 2020, seven have been dismissed and one has been partially dismissed. The actions involved or involve claims under various sections of the Securities Act of 1933, or the Securities Act, against the Company, certain of its current and former directors and officers, the underwriters of the Company’s IPO and certain other defendants.
The four actions commenced in the Superior Court of the State of California, County of San Mateo were dismissed in January 2017 for lack of personal jurisdiction, and the action commenced in the United States District Court for the Northern District of California was voluntarily dismissed in March 2017. Additionally, the two actions commenced in the Superior Court of the Commonwealth of Massachusetts, Suffolk County, or the Superior Court, were consolidated in December 2017, and voluntarily dismissed with prejudice in November 2018, after the District Court for the District of Massachusetts partially dismissed the related claims in that court and the parties in the Superior Court entered a stipulation of dismissal with prejudice.
The action commenced in the United States District Court for the District of Massachusetts (the “District Court”), alleging violations of Sections 11 and 15 of the Securities Act and Sections 10(b) and 20(a) of the Exchange Act, was partially dismissed on August 23, 2018. In particular, the District Court granted the motion to dismiss the claims under Sections 11 and 15 of the Securities Act, finding that the plaintiff failed to plead a false or misleading statement in the IPO registration statement. The District Court did not address the claims under Sections 10(b) and 20(a) of the Exchange Act because, as a result of the dismissal of the claims under the Securities Act, the lead plaintiff lacked standing to pursue those claims. Because the action in the District Court was styled as a class action, the District Court permitted the plaintiff to file a supplemental memorandum concerning standing or a motion to appoint a substitute or supplemental plaintiff. On September 10, 2018, the plaintiff sought leave to amend his complaint to add a new plaintiff that purportedly has standing to pursue Exchange Act claims, and the Company opposed the motion to amend on September 24, 2018. On May 16, 2019, the court denied the plaintiff’s motion to amend and the complaint was dismissed. Thereafter, the plaintiff timely appealed to the United States Court of Appeals for the First Circuit. The appeal has been fully briefed and oral arguments have been scheduled for March 2, 2020.
Based on information currently available and the current stage of the litigation, the Company are unable to reasonably estimate a possible loss or range of possible losses, if any, with regard to the remaining lawsuit in the District Court; therefore, no litigation reserve has been recorded in the Company’s consolidated balance sheets as of December 31, 2019. the Company will continue to evaluate information as it becomes known and will record an estimate for losses at the time or times if and when it is probable that a loss will be incurred and the amount of the loss is reasonably estimable.
F-26
NOTE 8:- SHAREHOLDERS’ EQUITY
| a. | Reverse share split: |
On March 27, 2019, the Company’s shareholders approved (i) a reverse share split within a range of 1:8 to 1:32, to be effective at the ratio and on a date to be determined by the Board of Directors, and (ii) amendments to the Company’s Articles of Association authorizing an increase in the Company’s authorized share capital (and corresponding authorized number of ordinary shares, proportionally adjusting such number for the reverse share split) by up to NIS 17.5 million. Following the shareholder approval, an authorized committee of the Board of Directors of the Company approved a one-for-twenty-five reverse share split of the Company’s ordinary shares, and the Company filed the Third Amended and Restated Articles of Association of the Company with the Registrar of Companies of the State of Israel to effect the reverse share split and to increase the Company’s authorized share capital after the effect of the reverse share split. The reverse share split became effective on April 1, 2019. Additionally, effective at the same time, the total number of ordinary shares the Company is authorized to issue changed from 250,000,000 shares to 60,000,000 shares, the par value per share of the ordinary shares changed to NIS 0.25 and the authorized share capital of the Company changed from NIS 2,500,000 to NIS 15,000,000. All share and per share data included in these consolidated financial statements, for periods before December 31, 2019, give retroactive effect to the reverse stock split.
Upon the effectiveness of the reverse share split, every twenty-five shares were automatically combined and converted into one ordinary share. Appropriate adjustments were also made to all outstanding derivative securities of the Company, including all outstanding equity awards and warrants.
No fractional shares were issued in connection with the reverse share split. Instead, all fractional shares (including shares underlying outstanding equity awards and warrants) were rounded down to the nearest whole number.
| b. | Equity raise: |
| 1. | At-the-market offering program: |
On May 10, 2016, the Company entered into an equity distribution agreement (the “Equity Distribution Agreement”) with Piper Jaffray & Co. (“Piper Jaffray”), as amended on May 9, 2019, pursuant to which it may offer and sell, from time to time, ordinary shares having an aggregate offering price of up to $25 million, through Piper Jaffray acting as its agent. Subject to the terms and conditions of the Equity Distribution Agreement, Piper Jaffray will use its commercially reasonable efforts to sell on the Company’s behalf all of the ordinary shares requested to be sold by the Company, consistent with its normal trading and sales practices. Piper Jaffray may also act as principal in the sale of ordinary shares under the Equity Distribution Agreement. Sales may be made under the Company’s shelf registration statement on Form S-3, which was declared effective by the SEC on May 9, 2016, or the Company’s shelf registration statement on Form S-3, which was declared effective by the SEC on May 23, 2019 (the “Form S-3”), in what may be deemed “at-the-market” equity offerings as defined in Rule 415 promulgated under the Securities Act of 1933, as amended (the “ATM Offering Program”). Sales may be made directly on or through the NASDAQ Capital Market, the existing trading market for the Company’s ordinary shares, to or through a market maker other than on an exchange or otherwise, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, and/or any other method permitted by law, including in privately negotiated transactions. Piper Jaffray is entitled to compensation at a fixed commission rate of 3.0% of the gross sales price per share sold through it as agent under the Equity Distribution Agreement. Where Piper Jaffray acts as principal in the sale of ordinary shares under the Equity Distribution Agreement, such rate of compensation will not apply, but in no event will the total compensation of Piper Jaffray, when combined with the reimbursement of Piper Jaffray for the out-of-pocket fees and disbursements of its legal counsel, exceed 8.0% of the gross proceeds received from the sale of the ordinary shares. The Company is not required to sell any of its ordinary shares at any time.
From the inception of the ATM Offering Program in May 2016 until December 31, 2019, the Company had sold 302,092 ordinary shares under the ATM Offering Program for gross proceeds of $15.7 million and net proceeds to the Company of $14.5 million (after commissions, fees and expenses). Additionally, as of that date, the Company had paid Piper Jaffray compensation for the fixed commission rate of 3.0% in the aggregated amount of $471 thousand and had incurred total expenses (including such commissions) of approximately $1.2 million in connection with the ATM Offering Program.
F-27
| 2. | Follow-on offerings |
In November 2018, the Company entered into an underwriting agreement with H.C. Wainwright & Co., LLC (“H.C. Wainwright”), in connection with the Company’s follow-on public offering of 496,055 units, each consisting of one ordinary share and one common warrant to purchase one ordinary share with an exercise price of $7.5 per warrant. Each unit was sold to the public at a price of $7.50 per unit. On November 18, 2018, H.C. Wainwright exercised in full its option to purchase 231,964 ordinary shares for $7.25 per share and/or common warrants to purchase up to an additional 231,964 ordinary shares for $0.25 per warrant.
Additionally, the Company issued and sold 1,050,372 pre-funded units at a price to the public of $7.25 per unit. Each unit containing one pre-funded warrant with an exercise price of $0.25 per share and one warrant to purchase one ordinary share with an exercise price of $7.50 per warrant. The total gross proceeds received from the November 2018 follow-on public offering, before deducting commissions, discounts and expenses, were $13.1 million (including proceeds from the exercise of 90,691 pre-funded warrants at the closing of the offering). As of December 31, 2018, additional pre-funded warrants to purchase an aggregate 562,466 ordinary shares had been exercised, for additional proceeds of $140,617. During the year ended December 31, 2019 additional 288,000 pre-funded warrants and 296,087 warrants to purchase an aggregate 584,087 ordinary shares had been exercised, for additional proceeds of $1.5 million. As compensation for their role in the offering, the Company also issued to the Underwriters warrants to purchase up to 106,680 ordinary shares, which became immediately exercisable starting on November 20, 2018 until November 15, 2023 at $9.375 per share.
In February 2019, the Company entered into an exclusive placement agent agreement with H.C. Wainwright, on a reasonable best-efforts basis in connection with a public offering of 760,000 ordinary shares at a price of $5.75 per share.
The total gross proceeds received from the February 2019 follow-on public offering, before deducting commissions, discounts and expenses, were $4.37 million. The Company also issued to H.C Wainwright and/or its designees warrants to purchase up to 45,600 ordinary shares, which are immediately exercisable starting on February 25, 2019 until February 21, 2024 at $7.1875 per share.
In April 2019, the Company entered into securities purchase agreements with certain institutional purchasers whereby the Company issued 816,914 ordinary shares at $5.2025 per ordinary share and warrants to purchase up to 408,457 ordinary shares with an exercise price of $5.14 per share, exercisable from April 5, 2019 until October 7, 2024, in a private placement that took place concurrently with the Company’s registered direct offering of ordinary shares in April 2019. Additionally, the Company issued warrants to purchase up to 49,015 ordinary shares, with an exercise price of $6.503125 per share, exercisable from April 5, 2019 until April 3, 2024, to representatives of H.C. Wainwright as compensation for its role as the placement agent in the Company’s April 2019 registered direct offering and concurrent private placement of warrants.
On June 5, 2019 and June 6, 2019, the Company entered into warrant exercise agreements with certain institutional investors whereby the Company issued warrants to purchase up to 1,464,665 ordinary shares with an exercise price of $7.50 per share, exercisable from June 5, 2019 or June 6, 2019 until June 5, 2024 or June 6, 2024, respectively. Additionally, the Company issued warrants to purchase up to 87,880 ordinary shares, with an exercise price of $9.375 per share, exercisable from June 5, 2019 until June 5, 2024, to certain representatives of H.C. Wainwright as compensation for its role as the placement agent in the Company’s June 2019 warrant exercise agreement and concurrent private placement of warrants.
On June 12, 2019, the Company entered into a purchase agreement with certain institutional investors for the issuance and sale of 833,334 ordinary shares, par value NIS 0.25 per share at $6.00 per ordinary share and warrants to purchase up to 416,667 ordinary shares with an exercise price of $6.00 per share, exercisable from June 12, 2019 until December 12, 2024, in a private placement that took place concurrently with the Company’s registered direct offering of ordinary shares in June 2019. Additionally, the Company issued warrants to purchase up to 50,000 ordinary shares, with an exercise price of $7.50 per share, exercisable from June 12, 2019 until June 10, 2024, to certain representatives of H.C. Wainwright as compensation for its role as the placement agent in the Company’s June 2019 registered direct offering and concurrent private placement of warrants.
F-28
| 3. | Investment agreement |
On March 6, 2018, the Company entered into an investment agreement with Timwell Corporation Limited, a Hong Kong corporation (“Timwell”), as amended on May 15, 2018 (the “Investment Agreement”), pursuant to which the Company agreed to issue to Timwell, in three different tranches, an aggregate of 640,000 ordinary shares in return for aggregate gross proceeds of $20 million. The closing of each tranche is subject to certain closing conditions. The closing of the first tranche (the “First Tranche Closing”) took place on May 15, 2018, upon which Timwell received 160,000 ordinary shares for an aggregate purchase price of $5,000,000, and Timwell and the Company signed a registration rights agreement in the form attached to the Investment Agreement. The net aggregate proceeds of the First Tranche Closing after deducting fees and other related expenses in the amount of approximately $705 thousands were approximately $4.3 million. The remaining investment is to occur in two tranches, including $10 million for the issuance to Timwell of 320,000 ordinary shares (the “Second Tranche”) and $5 million for the issuance to Timwell of 160,000 ordinary shares (the “Third Tranch”). The closing of the second and third tranches is subject to specified closing conditions, including, with respect to the second tranche, the signing of a license agreement and a supply agreement and the formation of the China JV (the “China JV”) based on the JV Framework Agreement, and, with respect to the third tranche, the successful production of certain ReWalk products by the China JV. The second tranche closing was initially expected to occur by July 1, 2018 and the third tranche closing was initially expected to occur by December 31, 2018 and no later than April 1, 2019.
In light of the positions taken by Timwell during the negotiations on definitive joint venture and license agreements, The Companys no longer believe that agreement can be reached on the basis of the original understandings reflected in its Investment Agreement with Timwell. the Company remain in dialogue with RealCan, Timwell’s affiliate, on alternative pathways that will allow to commercialize its products in China through RealCan and its affiliates, and also provide for RealCan or an affiliate to invest in the Company. Due to the various delays in the process and other barriers to closing, there is a significant risk that the Company will not reach agreement with RealCan on a modification of the original agreement. As the Company continue to view China as a market with key opportunities for products designed for stroke patients, the Company continue to evaluate potential relationships with other groups to penetrate the Chinese market.
In May 2018, the Company entered into a fee and release agreement with Canaccord Genuity LLC (“Canaccord Genuity”) requiring the Company to pay to Canaccord Genuity, in connection with a settlement, in addition to certain cash amounts, (i) $125 thousand in ordinary shares of the Company after the First Tranche Closing of the Timwell transaction and (ii) $225 thousand in ordinary shares of the Company after the closing of the Second Tranche of the Timwell transaction (or such lower amount if the Second Tranche Closing is less than $10.0 million). The price per share used for calculation of the number of ordinary shares issued by the Company to Canaccord Genuity is based on the volume weighted average price of the Company’s ordinary shares as reported on the Nasdaq Capital Market for the five consecutive trading days prior to the date of issuance. The Company is also obligated to pay $100 thousand in cash following the closing of the Third Tranche of $5.0 million (or such lower amount if the Third Tranche Closing is less than $5.0 million). Following the First Tranche Closing in May 15, 2018, the Company issued 4,715 ordinary shares to Canaccord Genuity.
| c. | Share option plans: |
On March 30, 2012, the Company’s board of directors adopted the ReWalk Robotics Ltd. 2012 Equity Incentive Plan.
On August 19, 2014, the Company’s board of directors adopted the ReWalk Robotics Ltd. 2014 Incentive Compensation Plan or the “Plan”. The Plan provides for the grant of stock options, stock appreciation rights, restricted stock awards, restricted stock units, cash-based awards, other stock-based awards and dividend equivalents to the Company’s and its affiliates’ respective employees, non-employee directors and consultants.
Starting in 2014, the Company grants to directors and employees also Restricted Stock Units (“RSUs’’) under this Plan. An RSU award is an agreement to issue shares of the company’s ordinary shares at the time the award is vested.
F-29
As of December 31, 2019 and 2018, the Company had reserved 12,409 and 52,298 shares of ordinary shares, respectively, available for issuance to employees, directors, officers and non-employees of the Company.
The share reserve pool will increase on January 1 of each calendar year during the term of the 2014 Plan in an amount equal to the lesser of: (x) 38,880, (y) 4% of the total number of shares outstanding on December 31 of the immediately preceding calendar year, and (z) an amount determined by the Company’s board of directors.
The options generally vest over four years, with certain options granted to non-employee directors during the fiscal year ended December 31, 2019, vesting over one year.
Any option that is forfeited or canceled before expiration becomes available for future grants under the Plan.
A summary of employee share options activity during the fiscal year ended 2019 is as follows:
| Number | Average exercise price | Average remaining contractual life (years) | Aggregate intrinsic value (in thousands) | |||||||||||||
| Options outstanding at the beginning of the year | 72,655 | $ | 47.7 | 6.37 | $ | 114 | ||||||||||
| Granted | 12,425 | 5.37 | — | — | ||||||||||||
| Exercised | — | — | — | — | ||||||||||||
| Forfeited | (10,367 | ) | 40.92 | — | — | |||||||||||
| Options outstanding at the end of the year | 74,713 | $ | 41.6 | 6.34 | $ | 135 | ||||||||||
| Options exercisable at the end of the year | 44,627 | $ | 55.86 | 4.80 | $ | — | ||||||||||
A summary of employee RSUs activity during the fiscal year ended 2019 is as follows:
Number of shares underlying outstanding RSUs | Weighted- average grant date fair value | |||||||
| Unvested RSUs at the beginning of the year | 26,093 | 48.78 | ||||||
| Granted | 88,436 | 4.67 | ||||||
| Vested | (40,793 | ) | 5.78 | |||||
| Forfeited | (11,358 | ) | 17.19 | |||||
| Unvested RSUs at the end of the year | 62,378 | 44.61 | ||||||
F-30
The weighted average grant date fair values of options granted during the fiscal year ended December 31, 2019, 2018 and 2017 were $2.98, $15.25 and $25.5 respectively. The weighted average grant date fair values of RSUs granted during the fiscal year ended December 31, 2019, 2018 and 2017, were $4.67, $26.75 and $36.0, respectively.
The aggregate intrinsic value in the table above represents the total intrinsic value that would have been received by the option holders had all option holders, which hold options with positive intrinsic value, exercised their options on the last date of the exercise period. During the years ended December 31, 2019 and December 31, 2018, no options were exercised. Total intrinsic value of options exercised for the year ended December 31, 2017 was $29 thousand. Total fair value of shares vested during the year ended December 31, 2019, 2018 and 2017 were $1,175 thousand, $2,918 thousand and $3,785 thousand respectively. As of December 31, 2019, there were $857 thousand of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the 2012 and 2014 Plans. This cost is expected to be recognized over a period of approximately 2.0 years.
The number of options and RSUs outstanding as of December 31, 2019 is set forth below, with options separated by range of exercise price:
| Range of exercise price | Options and RSUs Outstanding as of December 31, 2019 | Weighted average remaining contractual life (years) (1) | Options Exercisable as of
December 31, 2019 | Weighted average remaining contractual life (years) (1) | ||||||||||||
| RSUs only | 62,378 | — | — | — | ||||||||||||
| $5.37 | 12,425 | 9.24 | — | — | ||||||||||||
| $20.42- $33.75 | 36,851 | 6.25 | 21,968 | 4.85 | ||||||||||||
| $37.14-$38.75 | 10,194 | 3.99 | 10,194 | 3.99 | ||||||||||||
| $50-$52.5 | 11,395 | 5.69 | 8,621 | 5.13 | ||||||||||||
| $182.5-$524.25 | 3,848 | 5.90 | 3,844 | 5.90 | ||||||||||||
| 137,091 | 6.34 | 44,627 | 4.80 | |||||||||||||
| (1) | Calculation of weighted average remaining contractual term does not include the RSUs that were granted, which have an indefinite contractual term. |
On September 6, 2017, the Company commenced a one-time equity award exchange program (the “Equity Exchange Program”), offering to certain eligible employees, executive officers and consultants the opportunity to cancel certain outstanding “underwater” stock options issued under the 2014 Plan, in exchange for the grant under such plan of a lesser number of RSUs. The Company’s non-employee directors and retirees were not eligible to participate in the Equity Exchange Program. The Company conducted the Equity Exchange Program as a “value-for-value” exchange, pursuant to which the Company issued new RSUs with a value approximately equal to the value of the options that are surrendered, in accordance with the terms approved by the Company’s shareholders at the annual meeting of shareholders held on June 27, 2017. The primary purpose of the Equity Exchange Program was to restore the intended retention and incentive value of certain employee and consultant equity awards. Participation in the Equity Exchange Program was voluntary. The Company used the 52-week high closing price of its ordinary shares (as measured at the commencement of the Equity Exchange Program) as a threshold for options eligible to be exchanged.
On the Equity Exchange Program’s expiration date of October 4, 2017, 46 holders tendered options to purchase an aggregate of 945,416 ordinary shares, representing 96.4% of all options eligible for exchange, and on October 5, 2017, the Company granted to these holders an aggregate of 251,872 new RSUs. 180,167 of these new RSUs were granted to the Company’s executive officers and “named executive officers” (as defined in Item 402 of Regulation S-K of the SEC). Unless the Company’s compensation committee accelerates their vesting, the new RSUs vest over a three-year period, with one-third vesting on the first anniversary of the date of grant and one-third vesting on each of the next two successive anniversaries. Additionally, the forfeiture terms of the new RSUs are substantially the same as those that apply generally to previously-granted RSUs granted under the 2014 Plan.
F-31
The modification was analyzed under ASC No. 718 to determine if the new vesting condition remain probable, as the original. Since the modification also increased the fair value of the Equity Exchange Program, the Company decided to implement one of the two acceptable methods and recognize the incremental compensation amounted to $159 thousand over the new service period, while the unrecognized compensation cost remaining from the original grant will be recognized over the remainder of the original requisite service period.
The stock options exchanged pursuant to the Exchange Program were canceled and the ordinary shares underlying such options became available for issuance under the 2014 Plan.
| d. | Equity compensation issued to consultants: |
The Company granted 6,680 fully vested RSUs during the fiscal year ended December 31, 2019 to non-employee consultants. As of December 31, 2019, there are no outstanding options or RSUs held by non-employee consultants.
| e. | Warrants to purchase ordinary shares: |
The following table summarizes information about warrants outstanding and exercisable as of December 31, 2019:
| Issuance date | Warrants outstanding | Exercise price per warrant | Warrants outstanding and exercisable | Contractual term | ||||||||||
| (number) | (number) | |||||||||||||
| December 31, 2015 (1) | 4,771 | $ | 7.500 | 4,771 | See footnote (1) | |||||||||
| November 1, 2016 (2) | 97,496 | $ | 118.750 | 97,496 | November 1, 2021 | |||||||||
| December 28, 2016 (3) | 1,908 | $ | 7.500 | 1,908 | See footnote (1) | |||||||||
| November 20, 2018 (4) | 126,839 | $ | 7.500 | 126,839 | November 20, 2023 | |||||||||
| November 20, 2018 (5) | 106,680 | $ | 9.375 | 106,680 | November 15, 2023 | |||||||||
| February 25, 2019 (6) | 45,600 | $ | 7.187 | 45,600 | February 21, 2024 | |||||||||
| April 5, 2019 (7) | 408,457 | $ | 5.140 | 408,457 | October 7, 2024 | |||||||||
| April 5, 2019 (8) | 49,015 | $ | 6.503 | 49,015 | April 3, 2024 | |||||||||
| June 5, 2019 and June 6, 2019 (9) | 1,464,665 | $ | 7.500 | 1,464,665 | June 5, 2024 | |||||||||
| June 5, 2019 (10) | 87,880 | $ | 9.375 | 87,880 | June 5, 2024 | |||||||||
| June 12, 2019 (11) | 416,667 | $ | 6.000 | 416,667 | December 12, 2024 | |||||||||
| June 10, 2019 (12) | 50,000 | $ | 7.500 | 50,000 | June 10, 2024 | |||||||||
| 2,859,978 | 2,859,978 | |||||||||||||
| (1) | Represents warrants for ordinary shares issuable upon an exercise price of $7.5 per share, which were granted on December 31, 2015 to Kreos Capital V (Expert) Fund Limited, or Kreos, in connection with a loan made by Kreos to us and are currently exercisable (in whole or in part) until the earlier of (i) December 30, 2025 or (ii) immediately prior to the consummation of a merger, consolidation, or reorganization of us with or into, or the sale or license of all or substantially all the assets or shares of us to, any other entity or person, other than a wholly-owned subsidiary of us, excluding any transaction in which the Company’s shareholders prior to the transaction will hold more than 50% of the voting and economic rights of the surviving entity after the transaction. None of these warrants had been exercised as of December 31, 2019. |
F-32
| (2) | Represents warrants issued as part of the Company’s follow-on offering in November 2016. At any time, the board of directors may reduce the exercise price of the warrants to any amount and for any period of time it deems appropriate. |
| (3) | Represents common warrants that were issued as part of the $8.0 million drawdown under the Loan Agreement which occurred on December 28, 2016. See footnote 1 for exercisability terms. |
| (4) | Represents common warrants that were issued as part of the Company’s follow-on offering in November 2018. As of September 30, 2019, warrants to purchase an aggregate 1,651,537 ordinary shares had been exercised. |
| (5) | Represents common warrants that were issued to the underwriters as compensation for their role in the Company’s follow-on offering in November 2018. |
| (6) | Represents warrants that were issued to the exclusive placement agent as compensation for its role in the Company’s follow-on offering in February 2019. |
| (7) | Represents warrants that were issued to certain institutional purchasers in a private placement in the Company’s registered direct offering of ordinary shares in April 2019. |
| (8) | Represents warrants that were issued to the placement agent as compensation for its role in the Company’s April 2019 registered direct offering. |
| (9) | Represents warrants that were issued to certain institutional investors in a warrant exercise agreement on June 5, 2019 and June 6, 2019, respectively. |
| (10) | Represents warrants that were issued to the placement agent as compensation for its role in the Company’s June 2019 warrant exercise agreement and concurrent private placement of warrants. |
| (11) | Represents warrants that were issued to certain institutional investors in a warrant exercise agreement in June 2019. |
| (12) | Represents warrants that were issued to the placement agent as compensation for its role in the Company’s June 2019 registered direct offering and concurrent private placement of warrants. |
| f. | Share-based compensation expense for employees and non-employees: |
The Company recognized non-cash share-based compensation expense in the consolidated statements of operations as follows:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Cost of revenues | $ | 13 | $ | 16 | $ | 62 | ||||||
| Research and development, net | 204 | 435 | 508 | |||||||||
| Sales and marketing, net | 166 | 467 | 963 | |||||||||
| General and administrative | 725 | 1,848 | 2,121 | |||||||||
| Total | $ | 1,108 | $ | 2,766 | $ | 3,654 | ||||||
F-33
NOTE 9:- RESEARCH COLLABORATION AGREEMENT AND LICENSE AGREEMENT
On May 16, 2016, the Company entered into a Research Collaboration Agreement (“Collaboration Agreement”) and an Exclusive License Agreement (“License Agreement”) with Harvard. The Research Collaboration Agreement was amended on May 1, 2017 and April 1, 2018 (as amended, the “Collaboration Agreement”), and the Exclusive License Agreement was amended on April 1, 2018 (as amended, the “License Agreement”), to extend the term of the Collaboration Agreement by one year to May 16, 2022 and reallocate the Company’s quarterly installment payments to Harvard through such date, and to make certain technical changes.
Under the Collaboration Agreement, Harvard and the Company have agreed to collaborate on research regarding the development of lightweight “soft suit” exoskeleton system technologies for lower limb disabilities, which are intended to treat stroke, multiple sclerosis, mobility limitations for the elderly and other medical applications. The Company has committed to pay in quarterly installments for the funding of this research, subject to a minimum funding commitment under applicable circumstances. The Collaboration Agreement will expire on May 16, 2021.
Under the License Agreement, Harvard has granted the Company an exclusive, worldwide royalty-bearing license under certain patents of Harvard relating to lightweight “soft suit” exoskeleton system technologies for lower limb disabilities, a royalty-free license under certain related know-how and the option to obtain a license under certain inventions conceived under the joint research collaboration.
The License Agreement requires the Company to pay Harvard an upfront fee, reimbursements for expenses that Harvard incurred in connection with the licensed patents, royalties on net sales and several milestone payments contingent upon the achievement of certain product development and commercialization milestones. The Harvard License Agreement will continue in full force and effect until the expiration of the last-to-expire valid claim of the licensed patents. As of December 31, 2019 the Company achieved three of the milestones which represent all development milestones under the License Agreement. The Company continues to evaluate the likelihood that the other milestones will be achieved on a quarterly basis.
The Company’s total payment obligation under the Collaboration Agreement and the Harvard License Agreement is $7.2 million, some of which is subject to a minimum funding commitment under applicable circumstances as indicated above.
The Company has recorded expenses in the amount of $1.6 million which is part of the total payment obligation indicated above, as research and development expenses related to the Harvard License Agreement and to the Collaboration Agreement for the fiscal year ended December 31, 2019. No withholding tax was deducted from the Company’s payments to Harvard in respect of the Collaboration Agreement and License Agreement since this is not taxable income in Israel in accordance with Section 170 of the Israel Income Tax Ordinance 1961-5721.
NOTE 10:- INCOME TAXES
The Company’s subsidiaries are separately taxed under the domestic tax laws of the jurisdiction of incorporation of each entity.
| a. | Corporate tax rates in Israel: |
Presented hereunder are the tax rates relevant to the Company in the years 2017-2019:
The Israeli statutory corporate tax rate and real capital gains were 23% in 2019 and in 2018, and 24% in 2017.
| b. | Income (loss) before taxes on income is comprised as follows: |
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Domestic | $ | (15,599 | ) | $ | (21,784 | ) | $ | (24,728 | ) | |||
| Foreign | 55 | 104 | 130 | |||||||||
| $ | (15,544 | ) | $ | (21,680 | ) | $ | (24,598 | ) | ||||
F-34
| c. | Taxes on income are comprised as follows: |
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Current | $ | 64 | $ | 102 | $ | 46 | ||||||
| Deferred | (57 | ) | (107 | ) | 73 | |||||||
| $ | 7 | $ | (5 | ) | $ | 119 | ||||||
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Domestic | $ | — | $ | — | $ | — | ||||||
| Foreign | 7 | (5 | ) | 119 | ||||||||
| $ | 7 | $ | (5 | ) | $ | 119 | ||||||
| d. | Deferred income taxes: |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company’s deferred tax assets as of December 31, 2019 and 2018 are derived from temporary differences.
In assessing the realization of deferred tax assets, the Company considers whether it is more likely than not that all or some portion of the deferred tax assets will not be realized. Based on the Company’s history of losses, the Company established a full valuation allowance for RRL.
Undistributed earnings of certain subsidiaries as of December 31, 2019 were immaterial. The Company intends to reinvest these earnings indefinitely in the foreign subsidiaries. As a result, the Company has not provided for any deferred income taxes.
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Deferred tax assets: | ||||||||
| Carry forward tax losses | $ | 35,051 | $ | 28,033 | ||||
| Research and development carry forward expenses-temporary differences | 1,294 | 1,567 | ||||||
| Accrual and reserves | 290 | 241 | ||||||
| Right-of-use asset | 433 | — | ||||||
| Total deferred tax assets | 37,068 | 29,841 | ||||||
| Deferred tax liabilities: | ||||||||
| Right-of-use liability | (433 | ) | — | |||||
| Net deferred tax assets | 36,635 | 29,841 | ||||||
| Valuation allowance | (36,392 | ) | (29,655 | ) | ||||
| Net deferred tax assets | $ | 243 | $ | 186 | ||||
The net changes in the total valuation allowance for each of the years ended December 31, 2019, 2018 and 2017, are comprised as follows:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Balance at beginning of year | $ | (29,655 | ) | $ | (26,311 | ) | $ | (22,560 | ) | |||
| Changes due to amendments to tax laws and exchange rate differences | (2,055 | ) | 1,393 | 1,806 | ||||||||
| Adjustment previous year loss | (735 | ) | — | (591 | ) | |||||||
| Additions during the year | (3,947 | ) | (4,737 | ) | (4,966 | ) | ||||||
| Balance at end of year | $ | (36,392 | ) | $ | (29,655 | ) | $ | (26,311 | ) | |||
F-35
| e. | Reconciliation of the theoretical tax expenses: |
A reconciliation between the theoretical tax expense, assuming all income is taxed at the statutory tax rate applicable to income of the Company, and the actual tax expense (benefit) as reported in the consolidated statements of operations is as follows:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Loss before taxes, as reported in the consolidated statements of operations | $ | (15,544 | ) | $ | (21,680 | ) | $ | (24,598 | ) | |||
| Statutory tax rate | 23.0 | % | 23.0 | % | 24.0 | % | ||||||
| Theoretical tax benefits on the above amount at the Israeli statutory tax rate | $ | (3,575 | ) | $ | (4,986 | ) | $ | (5,904 | ) | |||
| Income tax at rate other than the Israeli statutory tax rate | (1 | ) | 5 | 17 | ||||||||
| Non-deductible expenses including equity based compensation expenses and other | 255 | 631 | 878 | |||||||||
| Operating losses and other temporary differences for which valuation allowance was provided | 3,947 | 4,737 | 4,966 | |||||||||
| Permanent differences | (651 | ) | (427 | ) | — | |||||||
| Other | 32 | 35 | 162 | |||||||||
| Actual tax expense | $ | 7 | $ | (5 | ) | $ | 119 | |||||
| f. | Foreign tax rates: |
Taxable income of RRI was subject to tax at the rate of 21% in 2019, 2018 and 2017 and at the rate of 40% in 2016.
The effect of the tax rate change on the Company’s deferred tax expense in 2017 was $58 thousand.
Taxable income of RRG was subject to tax at the rate of 30% in 2019, 2018, and 2017.
| g. | Tax benefits under the Law for the Encouragement of Capital Investments, 1959 (the “Investment Law”): |
Conditions for entitlement to the benefits:
Under the Investment Law, in 2012 the Company elected “Beneficiary Enterprise” status which provides certain benefits, including tax exemptions and reduced tax rates. Income not eligible for Beneficiary Enterprise benefits is taxed at a regular rate.
Income derived from Beneficiary Enterprise from productive activity will be exempt from tax for ten years from the year in which the Company first has taxable income, providing that 12 years have not passed from the beginning of the year of election. In the event of a dividend distribution from income that is exempt from company tax, as aforementioned, the Company will be required to pay tax of 10%- 25% on that income.
In the event of distribution of dividends from the said tax-exempt income, the amount distributed will be subject to corporate tax at the rate ordinarily applicable to the Beneficiary Enterprise’s income. Tax-exempt income generated under the Company’s “Beneficiary Enterprise” program will be subject to taxes upon dividend distribution or complete liquidation.
F-36
The entitlement to the above benefits is conditional upon the Company’s fulfilling the conditions stipulated by the Law and regulations published thereunder.
On December 29, 2010, the Knesset approved an additional amendment to the Law for the Encouragement of Capital Investments, 1959. According to the amendment, a reduced uniform corporate tax rate for exporting industrial enterprises (over 25%) was established. The reduced tax rate will not be program dependent and will apply to the industrial enterprise’s entire income. The tax rates for industrial enterprises have been reduced. In August 2013, the Israeli Knesset approved an amendment to the Investment Law, pursuant to which the rates for development area A will be 9% and for the rest of the country- 16% in 2014 and thereafter. The Amendment also prescribes that any dividends distributed to individuals or foreign residents from a preferred enterprise’s earnings as above will be subject to taxes at a rate of 20% (subject to tax treaty benefits)
In December 2016, the Economic Efficiency Law (Legislative Amendments for Applying the Economic Policy for the 2017 and 2018 Budget Years), 2016 which includes Amendment 73 to the Law for the Encouragement of Capital Investments (“the Amendment”) was published. According to the Amendment, a preferred enterprise located in development area A will be subject to a tax rate of 7.5% instead of 9% effective from January 1, 2017 (and thereafter the tax rate applicable to preferred enterprises located in other areas remains at 16%).
The Company has examined the effect of the adoption of the Amendment on its financial statements, and as of the date of the publication of the financial statements, the Company estimates that it will not apply the Amendment. The Company’s estimate may change in the future.
| h. | Tax assessments: |
RRL has had final tax assessments up to and including the 2014 tax year.
Each RRI and RRG have not had final tax assessment, since its inception.
| i. | Net operating carry-forward losses for tax purposes: |
As of December 31, 2019, RRL has carry-forward losses amounting to approximately $152.4 million, which can be carried forward for an indefinite period, and RRI has carry-forward losses amounting to approximately $390 thousands, which can be carried forward for a period of 20 years.
NOTE 11:- FINANCIAL EXPENSES, NET
The components of financial expenses, net were as follows:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Foreign currency transactions and other | $ | (34 | ) | $ | 42 | $ | (188 | ) | ||||
| Financial expenses related to loan agreement with Kreos | 1,499 | 2,398 | 2,451 | |||||||||
| Bank commissions | 31 | 26 | 30 | |||||||||
| $ | 1,496 | $ | 2,466 | $ | 2,293 | |||||||
F-37
NOTE 12:- GEOGRAPHIC INFORMATION AND MAJOR CUSTOMER AND PRODUCT DATA
Summary information about geographic areas:
ASC 280, “Segment Reporting” establishes standards for reporting information about operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company manages its business on the basis of one reportable segment, and derives revenues from selling systems and services (see Note 1 for a brief description of the Company’s business). The following is a summary of revenues within geographic areas:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Revenues based on customer’s location: | ||||||||||||
| Israel | $ | 2 | $ | — | $ | — | ||||||
| United States | 2,003 | 3,558 | 4,598 | |||||||||
| Europe | 2,832 | 2,807 | 3,094 | |||||||||
| Asia-Pacific | 36 | 22 | 61 | |||||||||
| Latin America | — | 58 | — | |||||||||
| Africa | — | 100 | — | |||||||||
| Total revenues | $ | 4,873 | $ | 6,545 | $ | 7,753 | ||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Long-lived assets by geographic region: | ||||||||
| Israel | $ | 179 | $ | 206 | ||||
| United States | 244 | 330 | ||||||
| Germany | 78 | 90 | ||||||
| $ | 501 | $ | 626 | |||||
| (*) | Long-lived assets are comprised of property and equipment, net. |
Major customer data as a percentage of total revenues:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Customer A | 15.0 | % | 38.0 | % | 35.2 | % | ||||||
NOTE 13:- SUBSEQUENT EVENTS
On February 10, 2020, the Company closed a “best efforts” public offering whereby the Company issued an aggregate of 5,600,000 of common units and pre-funded units at a public offering price of $1.25 per common unit and $1.249 per pre-funded unit. As part of the public offering, the Company entered into a securities purchase agreement with certain institutional purchasers. Each common unit consisted of one ordinary share, par value NIS 0.25 per share and one common warrant to purchase one Ordinary Share. Each pre-funded unit consisted of one pre-funded warrant to purchase one Ordinary Share and one Common Warrant. Additionally the Company issued warrants to purchase up to 336,000 ordinary shares, with an exercise price of $1.5625 per share, to representatives of H.C. Wainwright as compensation for its role as the placement agent in the Company’s February 2020 offering.
F-38

