Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Chemomab Therapeutics Ltd. | tm206348d1_8k.htm |
Exhibit 99.1

TARGETING GENETICALLY - DEFINED CANCERS Corporate Presentation February 2020

Safe Harbor Statement This presentation contains forward - looking statements within the meaning of U.S. federal securities laws and Israeli securities laws that involve risks and uncertainties. These forward - looking statements include, but are not limited to, statements about our market opportunities, our strategy, our competition, the further development and potential safety and efficacy of our product candidates, our projected revenue and expense levels and the adequacy of our available cash resources. Some of the information contained herein is based upon or derived from information provided by third - party consultants and other industry sources. We have not independently verified and cannot assure the accuracy of any data obtained by or from these sources. Drug discovery and development involve a high degree of risk. Factors that might cause material differences between expected and actual results include, among others, risks relating to: the successful preclinical development of our product candidates; the completion of clinical trials; the successful completion of the regulatory process with the FDA and other regulatory bodies, including the FDA’s review of any filings we make in connection with treatment protocols; uncertainties related to the ability to attract and retain partners for our technologies and products under development; infringement of our intellectual property; market penetration of competing products; raising sufficient funds needed to support our research and development efforts, and other factors described in our public filings. Although we believe that the expectations reflected in these forward - looking statements are based upon reasonable assumptions, no assurance can be given that such expectations will be attained or that any deviations will not be material. No reliance may be placed for any purpose whatsoever on the information contained in this presentation or on its completeness. No representation or warranty, express or implied, is given by us or on our behalf and/or our subsidiaries or any of our directors, officers or employees or any other person as to the accuracy or completeness of the information or opinions contained in this presentation. Neither we nor any of our subsidiaries, directors, officers, employees or any other person accepts any liability, whatsoever, for any loss howsoever arising, directly or indirectly, from any use of such information or opinions or otherwise arising in connection therewith. This presentation does not constitute or form part of, and should not be construed as constituting or forming part of, any offer or invitation to sell or issue, or any solicitation of any offer to purchase or subscribe for, any of our shares, nor shall any part of this presentation nor the fact of its distribution form part of or be relied on in connection with any contract or investment decision relating thereto, nor does it constitute a recommendation regarding our securities. 2 CORPORATE PRESENTATION |

We Aim to Develop Transformative Therapies Targeting the Genetic Basis of Cancer 3 Anchiano Therapeutics (Nasdaq: ANCN) Pan - RAS Program PDE10/ β - catenin Program • We are a small molecule biopharmaceutical oncology company • We are focused on pan - RAS inhibition • Our team has a record of successful small molecule cancer therapy development leading to FDA and global approvals in their pr ior experience • Mutations in the RAS family of genes (KRAS, HRAS, and NRAS) are the most frequent oncogenic mutations known and are present i n m ore than 30% of all cancer cases • There are currently no approved RAS - directed therapies, and there is an opportunity to target all active forms of RAS • In our lead program, we have identified and are developing a series of molecules that are selective and potent pan - RAS inhibitor s • Genetic alterations in the Wnt /APC/β - catenin pathway occur in approximately 90% of colorectal cancers • We are developing a suite of molecules in this second program which counter the oncogenic activation of Wnt /β - catenin signaling through selective and potent inhibition of PDE10 CORPORATE PRESENTATION |

Executive Team Our team has a track record of oncology development 4 Frank G. Haluska, MD, PhD President and Chief Executive Officer Former Harvard Medical faculty, A riad CMO, led global research team and two oncology drug approvals David Kerstein, MD Chief Medical Officer Spearheaded worldwide approvals for brigatinib in ALK+ NSCLC Ron Knickerbocker, PhD Senior Vice President of Data Sciences Led data science teams for multiple oncology drug and biologic approvals including ponatinib and brigatinib Sean Daly Vice President of Operations Successfully conducted clinical trials supporting two approvals Salar Roshan, MBA, MSF Head of Business Development Expertise in small molecule targeted therapies and corporate development CORPORATE PRESENTATION |

Scientific Advisory Board World authorities on the basic and clinical science of targeting signaling pathways 5 Julian Adams, PhD CEO at Gamida Cell, former Head of R&D at Infinity, and SVP of Drug Discovery at Millennium Pharmaceuticals, expert in signal transduction Neal Rosen, MD, PhD Director, Center for Mechanism - Based Therapeutics, Sloan Kettering Institute, expert in RAS and RAF signaling Ross Camidge , MD, PhD Director of Thoracic Oncology Program, Professor of Medicine - Medical Oncology at University of Colorado, authority in targeted therapies for lung cancer Daniel Von Hoff, MD Physician in Chief, Distinguished Professor at the Translational Genomics Research Institute; CSO of US Oncology and Professor of Medicine at the Mayo Clinic, innovator in the development of multiple cancer therapies CORPORATE PRESENTATION |

Pipeline 6 Focus on pan - RAS - inhibitor development CORPORATE PRESENTATION |

Human cancers with >5% RAS mutation frequency • Mutations in the RAS family of genes (KRAS, HRAS and NRAS) are the most frequent oncogenic mutations known and are present in over 30% of all cancer • There is a need and opportunity for agents that target all isoforms of RAS and all activating mutations • Investigational compounds that selectively target the KRAS G12C mutation have shown anti - tumor activity in the clinic — validating RAS as a therapeutic target o These drugs are mutation specific — with G12C representing ~11% of KRAS mutations • We have identified a series of molecules that are pan - RAS inhibitors 7 Source: Cox et al., Nat Rev Drug Discov , 2014; Vasan et al., Clinical Cancer Research, 2014. RAS is the Most Frequently Mutated Oncogene in Human Cancer CORPORATE PRESENTATION |

• RAS resides at the center of the key pathways that transduce signals from the surface of the cell to its interior, resulting in survival, proliferation, or other responses to the cellular environment • Most cancers carry activating mutations in this pathway; such mutations can involve an upstream cell - surface tyrosine kinase such as EGFR (shown), or downstream molecules such as RAF or PI3K • The most common mutations are in one of the RAS isoforms themselves • Many successful oncology drugs have been developed to target the mutated proteins: inhibitors of EGFR, PDGFR, PI3K, RAF, and MEK, for example; targeting the mutated proteins is the basis of precision oncology • Until recently, RAS has been refractory to direct inhibition in clinical studies; in the last year success has been achieved in inhibiting RAS proteins specifically 8 RAS is Central to Malignancy CORPORATE PRESENTATION |

9 Amgen and Mirati Have Successfully Demonstrated KRAS G12C Inhibition in Clinical Trials Preclinical data successfully predicted clinical responses with Amgen and Mirati drugs o Data above show that preclinical activity with low nanomolar cellular IC50s against KRAS G12C mutant cell lines, and supporting biochemical and biological data, have translated into clinical activity Our preclinical data also demonstrate low nanomolar cellular IC50s selectively against RAS mutated cell lines, but regardless of isoform or mutation, with supporting biochemical and functional data o A pan - RAS inhibitor could provide an opportunity to extend activity beyond G12C mutations to patients with other isoforms and mutations Source: Hallin et al., AACR 2019; Jänne et al., 2019 AACR - NCI - EORTC Conference; Janes et al., Cell , 2018 The anti - proliferative effects of ARS - 1620 analogs on KRAS G12C mutant cell lines (n = 3) or control cell lines lacking G12C (n = 3) following 5 days of treatment CORPORATE PRESENTATION |

The Pan - RAS Market Opportunity 10 * “Total addressable patients” represents the total treatable advanced/metastatic patient population, and does not consider m ark et dynamics (e.g., therapy line, pref. share, market access, etc.) Source: 2019 SEER Data; Garrido et al., Ther Adv Med Oncol , 2017; Roman et al., Mol Cancer, 2018; Cox et al., Nat Rev Drug Discov, 2014 ; Waters et al., Cold Spring Harb Med, 2018; Prior et al., Cancer Res, 2015. NSCLC NSCLC, CRC and Pancreatic Cancer All Solid Tumors Annual Incidence ~190 K ~395 K ~1.6 M Advanced Patients ~160 K ~265 K ~550 K Patients with RAS Mutations ~55 K total addressable patients* ~130 K total addressable patients* ~165 K total addressable patients* Because RAS is so frequently mutated in multiple cancer types, the market for a RAS - specific therapy is large, relative to other targeted therapies in oncology • In the US, there are ~165 K total addressable patients with RAS mutations annually across all solid tumors, and ~130K total addressable patients with RAS mutations annually across NSCLC, CRC, and pancreatic cancer indications • The global market opportunity is thus likely at least double this By comparison: • The KRAS G12C - specific indication in NSCLC, CRC, and pancreatic cancer has a total addressable population of ~26K in the US • The advanced EGFR mutant NSCLC population in the US is ~16K annually CORPORATE PRESENTATION |

11 Our RAS Inhibitor Discovery Strategy We screened candidate inhibitors from a custom library of compounds having an indene core, as illustrated at left The primary screen involved high - throughput dose - response experiments utilizing cell lines with mutant RAS (HCT - 116) and wild - type (WT) RAS (HT29) Optimization involved engineering COX inhibiting activity out of candidate compounds; resulting compounds are not COX inhibitors Sulindac Indene library Initial hit Primary Screen K - Ras mutant HCT116 vs. WT Ras Raf mutant HT29 ~ 1500 compounds ADT - 004 CORPORATE PRESENTATION |

Characteristics Expected with Direct Pan - RAS Inhibition: Comparison with AMG 510 12 * Data not shown Characteristic ANC 007 AMG 510 Potently inhibits KRAS, NRAS, and HRAS mutant cell lines (across multiple mutations) in monolayer and spheroid cultures Potently inhibits KRAS G12C mutant cell lines Potently inhibits cell lines with RAS signaling activated by upstream mutations (e.g. EGFR, PDGFR, NF1), but not downstream mutations (e.g. RAF) Selectivity: lack of potency in cell lines without RAS activation Inhibits downstream signaling through both RAF and PI3K pathways (consistent with direct RAS inhibition as opposed to other nodes in the pathway) Activates anti - tumor immunity and exhibits synergistic effect when combined with checkpoint inhibitors* Exhibits in vivo anti - tumor activity in RAS - mutant tumor models Cell - free biochemical assays, such as RBD pulldown and MANT - GTP guanine nucleotide exchange, showing inhibition of GTP binding to RAS, indicating direct binding to RAS Protein NMR studies indicate direct binding to RAS* Not Reported X - ray crystallography showing specific binding mechanism and site of compound Studies Ongoing ANC 007 has demonstrated the characteristics of a direct, broadly acting, pan - RAS inhibitor with the potential to treat RAS - driven cancers regardless of RAS isoform or mutation CORPORATE PRESENTATION |

Our Compounds Demonstrate Potent and Selective Pan - RAS Inhibition Source: Keeton et al., AACR 2019; Mattox et al. AACR 2019; McConnell et al. AACR 2017; Data on File 13 Mutation Status IC 50 , Cell - based Assay (nM) Cell Line Tumor Origin Genotype KRAS G12C 2.1 Mia PaCa - 2 Pancreatic Mutant RAS KRAS G12D 2.4 PANC - 1 Pancreatic KRAS G12D 6.3 SW - 1990 Pancreatic KRAS G12S 6.8 A549 Lung KRAS G12V 5.8 CFPAC - 1 Pancreatic KRAS G12V 6.5 SW480 Colon KRAS G13D 3.6 MDA - MB - 231 Breast KRAS G13D 4.7 HCT116 Colon HRAS G12D 3.9 Hs578 Breast NRAS Q61K 2.1 SK - MEL - 2 Melanoma NRAS Q61K 2.4 H1299 Lung WT RAS / mut. EGRF 3.9 H1975 Lung RAS activated upstream WT RAS / mut. PDGFR 5.8 B16 Melanoma WT RAS / mut. NF1 6.7 SKOV3 Ovarian WT RAS 350 OV90 Ovarian WT RAS WT RAS 9600 H3322 Lung WT RAS 19100 NHAEC Normal Lung WT RAS >25000 SK - MEL - 28 Melanoma WT RAS / mut. RAF 512 HT29 Colon RAS pathway activated downstream WT RAS / mut. RAF 2500 BXPC3 Pancreatic The strategy resulted in a series of compounds with potent inhibitory activity against lines carrying RAS mutations As illustrated at left for one lead compound, we observed <10 nM cellular potency in monolayer cultures of tumor cell lines harboring mutant KRAS, HRAS, and NRAS Similar IC50s are obtained in spheroid cultures Cell lines with WT RAS and upstream mutations (e.g. EGFR) that signal through RAS display similar IC50s to cell lines with mutated RAS Cell lines with WT RAS in the absence of upstream activation are less sensitive (>100 - fold selectivity), regardless of downstream activating mutations Taken together these observations are consistent with the inhibition of RAS regardless of isoform, and regardless of the mutations tested; in addition, they suggest that the activation of RAS (by mutation or upstream signaling) is a key feature of selectivity CORPORATE PRESENTATION |
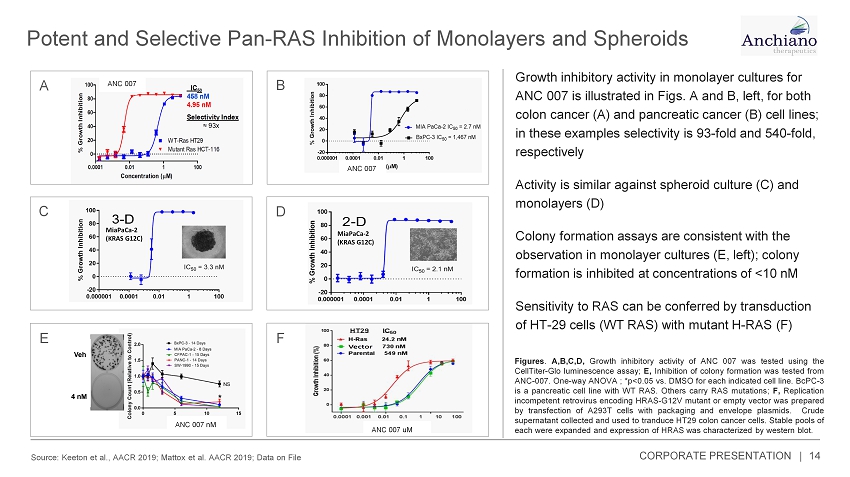
Potent and Selective Pan - RAS Inhibition of Monolayers and Spheroids Source: Keeton et al., AACR 2019; Mattox et al. AACR 2019; Data on File 14 A C E B D Growth inhibitory activity in monolayer cultures for ANC 007 is illustrated in Figs. A and B, left, for both colon cancer (A) and pancreatic cancer (B) cell lines; in these examples selectivity is 93 - fold and 540 - fold, respectively Activity is similar against spheroid culture (C) and monolayers (D) Colony formation assays are consistent with the observation in monolayer cultures (E, left); colony formation is inhibited at concentrations of <10 nM Sensitivity to RAS can be conferred by transduction of HT - 29 cells (WT RAS) with mutant H - RAS (F) MiaPaCa - 2 (KRAS G12C) MiaPaCa - 2 (KRAS G12C) ANC 007 ANC 007 ANC 007 uM ANC 007 nM Figures . A,B,C,D, Growth inhibitory activity of ANC 007 was tested using the CellTiter - Glo luminescence assay ; E, Inhibition of colony formation was tested from ANC - 007 . One - way ANOVA ; *p< 0 . 05 vs . DMSO for each indicated cell line . BcPC - 3 is a pancreatic cell line with WT RAS . Others carry RAS mutations ; F, Replication incompetent retrovirus encoding HRAS - G 12 V mutant or empty vector was prepared by transfection of A 293 T cells with packaging and envelope plasmids . Crude supernatant collected and used to tranduce HT 29 colon cancer cells . Stable pools of each were expanded and expression of HRAS was characterized by western blot . F CORPORATE PRESENTATION |
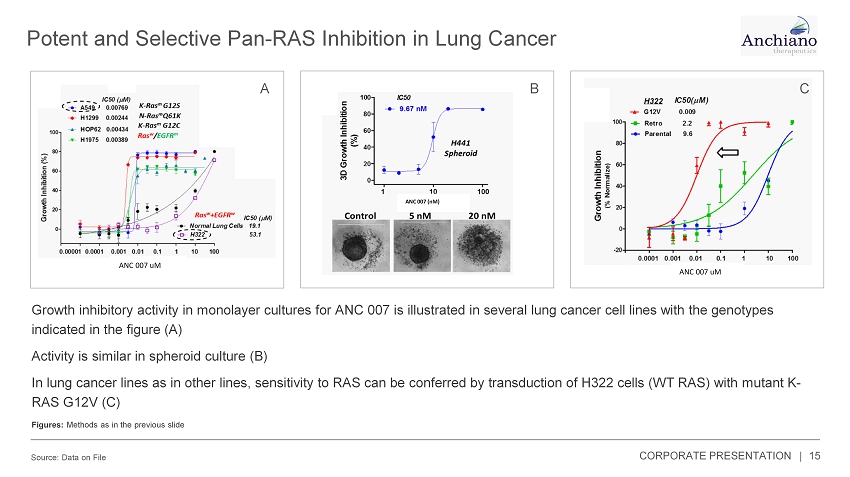
Potent and Selective Pan - RAS Inhibition in Lung Cancer Source: Data on File 15 ANC 007 uM B ANC 007 uM A Growth inhibitory activity in monolayer cultures for ANC 007 is illustrated in several lung cancer cell lines with the genoty pes indicated in the figure (A) Activity is similar in spheroid culture (B) In lung cancer lines as in other lines, sensitivity to RAS can be conferred by transduction of H322 cells (WT RAS) with mutan t K - RAS G12V (C) Figures: Methods as in the previous slide C ANC 007 (nM) CORPORATE PRESENTATION |

Inhibition of Downstream Signaling Through RAF and PI3K Pathways In Vitro and In Vivo 16 Source: Keeton et al., AACR 2019; Mattox et al. AACR 2019 B A Treatment of MIA - PaCa - 2 pancreatic cancer cells with KRAS G12C results in inhibition of signaling of the MAPK pathway as evidenced by inhibition of phosphorylation of CRAF, MEK, and ERK; AKT phosphorylation is also inhibited (A) MAPK signaling in HCT - 116 cells is inhibited in vivo as well (B); each lane in this case corresponds to an individual animal ANC 007 nM ANC 007 Figures : A, ANC 007 reduced RAS - GTP levels and inhibits activation of downstream RAS signaling in MIA - PaCa - 2 pancreatic cancer cells . Cells were treated with vehicle or ANC 007 for 24 hours in serum - free media and subsequently stimulated with 30 ng/mL EGF for 10 minutes . RAS - GTP levels after treatment were determined by the active RAS pull - down using GST - RAF 1 - RBD/glutathione agarose and detection by Western blotting . Detection of phospho - protein levels was performed by Western blot . B, ANC 007 inhibits activation of downstream RAS signaling and activates anti - tumor immunity in murine RAS mutant colon cancer model Mice were implanted in the right flank with 10 million HCT - 116 tumor cells per mouse . ANC 007 - treated mice received 5 mg/kg ANC 007 twice daily by peritumoral administration . Vehicle - treated mice received 5 % DMSO/ 5 % cremophor EL/ 90 % water once daily by peritumoral administration . RAS - GTP levels in tumor lysates were determined by the active RAS pull - down and detection by Western blotting . Levels of MAPK proteins and immune markers were determined by Western blotting with the whole tumor lysate . CORPORATE PRESENTATION |

Induction of Cell Cycle Arrest and Apoptosis 17 Source: Keeton et al., AACR 2019; Mattox et al. AACR 2019 ANC 007 selectively induces mitotic (M) arrest of HCT - 116 mutant KRAS tumor cells but not RAS WT HT29 cells (A) ANC 007 also induces apoptosis as evidenced by cleaved caspase 3 (B) ANC 007 nM ANC 007 nM ANC 007 nM Figures : A , Mutant KRAS HCT 116 or WT RAS HT 29 colon tumor cells were serum - starved overnight to synchronize in the G 0 /G 1 phase of the cell cycle . Cell cycle distribution was determined following 24 hours of treatment with ANC 007 in the presence of 5 % FBS to induce cell cycle progression . DNA content of the cells was measured by fluorescence intensity quantified using a high - content imager following staining with DAPI (top) . Stacked bar graphs represent percentages of cells in corresponding cell cycle phase for each treatment dose . Representative histograms of DNA content and corresponding thresholds for cell cycle phase assignment from cells treated with 20 nM ANC 007 is presented (bottom) . B, RAS mutant HCT 116 colon tumor cells were treated with vehicle (DMSO) or ANC 007 for the indicated time . Apoptosis was measured using antibodies specific for cleaved (CL) caspase - 3 by western blotting . Antibodies against uncleaved caspase and GAPDH were used as gel loading controls . A B CORPORATE PRESENTATION |

Inhibition of GTP Binding to Recombinant KRAS Mattox et al. AACR 2019; Data on File 18 The effect of ANC 007 on the binding of GTP to RAS has been tested in several experiments ANC 007 inhibited the GTP loading of recombinant RAS in cell - free biochemical assays (A); when added to nucleotide - free RAS ( nf - RAS) ANC 007 inhibits GTP binding in an RBD pull - down assay The binding of GTP to RAS was tested using the fluorescent analogue of GTP, Mant - GTP. As shown in B, addition of ANC 007 inhibited binding of GTP in this cell free system These data are consistent with the direct binding of ANC 007 to RAS and the displacement of GTP Figures : A, ANC 007 inhibits GTP binding to recombinant nucleotide - free RAS in RBD pull - down experiment . Nucleotide - free recombinant WT - KRAS was prepared by incubation with 20 mM EDTA on ice . ANC 007 or vehicle was incubated 1 h with nucleotide - free WT - KRAS, followed by addition of GTP and an additional 30 min incubation (left) or, ANC 007 was incubated with WT - KRAS after addition of GTP (GTP - bound, right) . RAS - GTP levels after treatment were determined by the active RAS pull - down assay using GST - RAF 1 - RBD/glutathione agarose and detection by Western blotting . B, ANC 007 inhibits GTP binding to recombinant nucleotide - free RAS in guanine nucleotide exchange assay . Nucleotide - free recombinant WT - KRAS was prepared by incubation with 20 mM EDTA on ice . Nucleotide free KRAS was incubated with ANC 007 or vehicle for 1 h on ice, followed by addition of an excess of MgCl 2 with fluorogenic MANT - GTP . Recombinant KRAS not treated with EDTA (GTP - KRAS) was included as a control indicator of intrinsic turnover rate . The development of fluorescence reflecting MANT - GTP binding to KRAS was monitored over the course of a 45 min incubation . MCI - 062 (nM ) MCI - 062 (nM ) Nf - Ras GTP - Ras ANC 007 nM ANC 007 nM 0 1000 2000 3000 0 20 40 60 80 100 Time (s) G T P B i n d i n g ( R e l a t i v e f l u o r e s c e n c e u n i t s ) GTP-KRAS nf-KRAS + Vehicle nf-KRAS + MCI-062 ANC 007 A B CORPORATE PRESENTATION |

Source: Mattox et al. AACR 2019; Data on File 19 Figure : ANC 007 inhibits growth of KRAS mutant HCT - 116 tumor cells in a subcutaneous mouse xenograft model . Athymic nude mice were implanted in the right flank with 10 million tumor cells per mouse . Mice were treated once daily by intratumoral administration . Control mice received vehicle only ( 5 % DMSO/ 5 % cremophor EL/ 90 % water) once daily by intratumoral administration . N= 8 mice for vehicle group, n= 7 mice for 5 mg/kg ANC 007 group, n= 4 mice for 10 mg/kg MCI - 062 group . Time After Tumor implantation (Days) Mean Tumor Volume (mm 3 ) ANC 007 inhibits tumor growth in KRAS mutant HCT - 116 colon tumor xenograft model ANC 007 Exhibits In Vivo Anti - tumor Activity in RAS - driven Tumor Models CORPORATE PRESENTATION |

In Vivo Anti - tumor Activity in RAS - driven Tumor Models Source: Data on File 20 Day 17 – Tx Start on Day 6 ANC 007 suppressed tumor growth in a CT26 colon tumor mouse xenograft model by subcutaneous delivery Representative control (Left) and MCI - 062 animals (Right) shown in the figure MCI - 062 Figure : Mice were implanted in the right flank with 1 million CT - 26 tumor cells per mouse . MCI - 062 - treated mice received 5 mg/kg ANC 007 twice daily by peritumoral administration . Vehicle - treated mice received 5 % DMSO/ 5 % cremophor EL/ 90 % water once daily by peritumoral administration . Shown on left is control (vehicle - treated) mice and on the right are mice treated with ANC 007 . Animal 1 - 2 Animal 1 - 3 Animal 3 - 19 Animal 3 - 23 CORPORATE PRESENTATION |
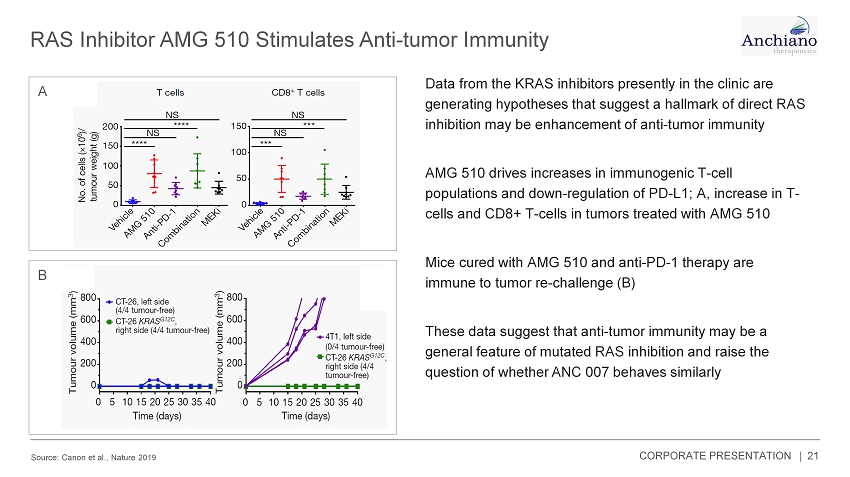
RAS Inhibitor AMG 510 Stimulates Anti - tumor Immunity Source: Canon et al., Nature 2019 21 Data from the KRAS inhibitors presently in the clinic are generating hypotheses that suggest a hallmark of direct RAS inhibition may be enhancement of anti - tumor immunity AMG 510 drives increases in immunogenic T - cell populations and down - regulation of PD - L1; A, increase in T - cells and CD8+ T - cells in tumors treated with AMG 510 Mice cured with AMG 510 and anti - PD - 1 therapy are immune to tumor re - challenge (B) These data suggest that anti - tumor immunity may be a general feature of mutated RAS inhibition and raise the question of whether ANC 007 behaves similarly A B CORPORATE PRESENTATION |

ANC 007 Stimulates Anti - tumor Immunity Similar to AMG 510 Source: Keeton et al., AACR 2019; Mattox et al. AACR 2019 22 In an immune competent mouse tumor model (CT26, colorectal), ANC 007 suppressed tumor growth in association with decreased PD - L1 levels in tumors (figure A, B); decreased PD - 1 in T - cells (C); increased proportion of CD4+ and CD8+ T - cells in tumors(D); and reduced Treg cells in tumors (as evidenced by Foxp3 expression)(E) These observations are consistent with the immune stimulation observed that may be a general characteristic of RAS inhibition Strong rationale exists for evaluating combination of pan - RAS inhibitor with a checkpoint inhibitor c o n t r o l + d r u g 0 20 40 60 80 %Foxp3 in tumor CD4 control +drug Vehicle MCI - 062 % Foxp3+ in Tumor CD4 *** P < .001 vs. vehicle ANC 007 A B C D E Immune competent mouse RAS - driven tumor model (CT26, colorectal cancer) A c o n t r o l + d r u g 0 20 40 60 %PDL1 in tumor control +drug Vehicle MCI - 062 ANC 007 Figures : A, ANC 007 reduces PD - L 1 expression in RAS driven tumor model . Mice were implanted in the right flank with 1 million CT 26 tumor cells per mouse . ANC 007 - treated mice received 5 or 10 mg/kg ANC 007 once daily by intratumoral administration . Vehicle - treated mice received 5 % DMSO/ 5 % cremophor EL/ 90 % water once daily by intratumoral administration . RAS - GTP levels in tumor lysates were determined by the active RAS pull - down and detection by Western blotting . of immune markers was performed on whole tumor lysate . B, C, D, E, ANC 007 reduces PD - 1 , increases proportion of CD 4 + and CD 8 + T - cells, and reduces Tregs . Subcutaneous CT - 26 murine tumors were excised from vehicle or ANC 007 treated mice, and digested for 1 h using the gentle - MACS dissociator and murine tumor digestion protease cocktail . Following digestion, single cell suspensions were recovered by passage through a cell strainer, and cell counts were normalized by quantitation using a hemacytometer . Fluorescently labelled antibodies used to quantitate the indicated cell populations by flow cytometry . CORPORATE PRESENTATION |

Development Timelines Pan - RAS Preclinical Development • Preclinical program focused on development of clinical lead compound • Anticipate total 12 - 18 months (from Q1 2020) to product candidate nomination, followed by approximately 12 months for IND - enabling studies • Anticipate IND and initiation of clinical study by mid - 2022 • For context, there is precedent for accelerated clinical development demonstrated with AMG 510 (in smaller population of KRAS G12C), as well as with other small molecule targeted therapies: o First patient treated with AMG 510 in Phase 1 – August 2018 o First data readout with AMG 510 < 9 months later (ASCO, Jun 2019) o Announced enrollment of Phase 2 cohort < 18 months after first patient treated (Jan 2020, JP Morgan) – initial data expected in 2020 o The timelines for MRTX849 are similar 23 CORPORATE PRESENTATION |

PDE10/ b - catenin Program Overview Source: Li et al., Oncogene, 2015; Data on File 24 Figures : PDE 10 overexpression in colon tumor cells ; inhibition blocks colony formation and B - catenin/ Tcf transcription . A, Differential PDE 10 levels in normal colonocytes (NCM 460 ) and colon tumor cells as measured by Western blot . B, PDE 10 siRNA knockdown (KD) inhibits colony formation of HT 29 colon tumor cells . C, PDE 10 siRNA suppresses Tcf transcription of key regulatory genes (e . g . survivin , cyclin D, myc , etc ) . D, Treatment of HT 29 cells with PDE 10 inhibitor ADT - 094 leads to reduction of B - catenin and products of Tcf transcription as measured by Western blot . A B C D The Wnt /APC/β - catenin pathway is altered in the majority of colon cancers, as well as other types; mutations in the pathway result in impaired degradation of β - catenin and potentiation of Tcf transcription PDE10 was discovered to be overexpressed in colon cancer cells (A) with limited expression in normal colonocytes (NCM460); PDE10 has no known function in peripheral tissues; PDE10 expression is associated with worse prognosis based on analysis of TCGA database Inhibition of PDE10 induces cGMP/PKG signaling to phosphorylate and induce degradation of β - catenin to suppress key proteins essential for tumor cell proliferation and survival (B); PDE10 knockdown (KD) or inhibition with small molecules inhibits growth and colony formation of colon, lung, and breast tumor cells (C) Our orally available small molecule PDE10 inhibitors have unique advantages over known PDE10 inhibitors with potential for development in FAP, colon, lung, liver, breast, and other cancers CORPORATE PRESENTATION |
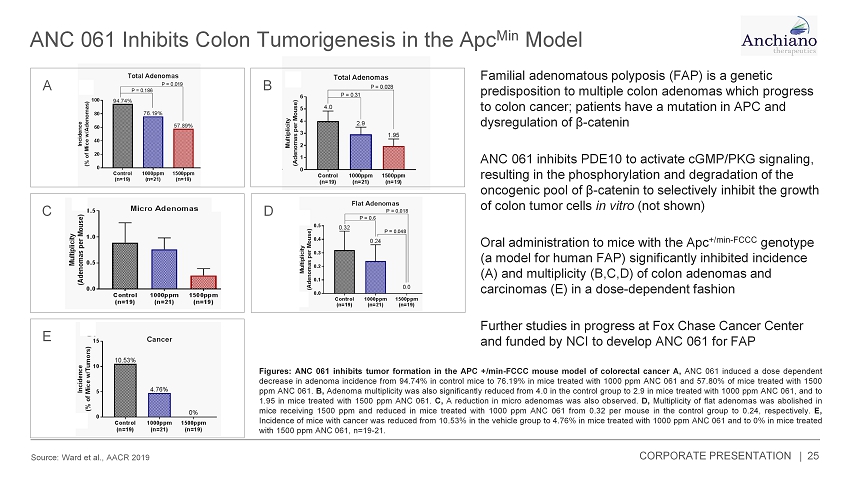
ANC 061 Inhibits Colon Tumorigenesis in the Apc Min Model Source: Ward et al., AACR 2019 25 Familial adenomatous polyposis (FAP) is a genetic predisposition to multiple colon adenomas which progress to colon cancer; patients have a mutation in APC and dysregulation of β - catenin ANC 061 inhibits PDE10 to activate cGMP/PKG signaling, resulting in the phosphorylation and degradation of the oncogenic pool of β - catenin to selectively inhibit the growth of colon tumor cells in vitro (not shown) Oral administration to mice with the Apc +/min - FCCC genotype (a model for human FAP) significantly inhibited incidence (A) and multiplicity (B,C,D) of colon adenomas and carcinomas (E) in a dose - dependent fashion Further studies in progress at Fox Chase Cancer Center and funded by NCI to develop ANC 061 for FAP Figures : ANC 061 inhibits tumor formation in the APC +/min - FCCC mouse model of colorectal cancer A, ANC 061 induced a dose dependent decrease in adenoma incidence from 94 . 74 % in control mice to 76 . 19 % in mice treated with 1000 ppm ANC 061 and 57 . 80 % of mice treated with 1500 ppm ANC 061 . B, Adenoma multiplicity was also significantly reduced from 4 . 0 in the control group to 2 . 9 in mice treated with 1000 ppm ANC 061 , and to 1 . 95 in mice treated with 1500 ppm ANC 061 . C, A reduction in micro adenomas was also observed . D, Multiplicity of flat adenomas was abolished in mice receiving 1500 ppm and reduced in mice treated with 1000 ppm ANC 061 from 0 . 32 per mouse in the control group to 0 . 24 , respectively . E, Incidence of mice with cancer was reduced from 10 . 53 % in the vehicle group to 4 . 76 % in mice treated with 1000 ppm ANC 061 and to 0 % in mice treated with 1500 ppm ANC 061 , n= 19 - 21 . A B C D E CORPORATE PRESENTATION |

ANC 030 Inhibits Lung Tumorigenesis in A549 Lung Cancer Model Source: Zhu et al. AACR 2019 26 ANC 030 administered orally inhibits tumor growth in an orthotopic lung cancer mouse model without apparent toxicity ANC 030 significantly extends survival in the A549 mouse model from a median 44 to 77 days with 25% of mice surviving until the end of the experiment ANC 030 was also effective in multiple other mouse models of lung and breast cancer, including models of metastasis Figures : A, The A 549 human lung adenocarcinoma cells (ATCC® CCL - 185 ™) with luciferase tag were generated using lentiviral particles expressing luciferase gene driven by a CMV promoter and a stable A 549 Luc clone was selected . Female athymic nude mice were implanted with 1 x 10 6 A 549 Luc cells intrathoracically . Mice were treated by oral gavage once a day with the vehicle (Maalox) or ANC 030 at doses of 25 , 50 , or 100 mg/kg (n = 7 /group) starting five days before tumor cell implantation . After implantation, treatment continued for 4 additional weeks . Tumor growth was monitored weekly through the detection of the bioluminescence of the A 549 Luc cells using In Vivo Imaging System (IVIS, IVIS Spectrum, Caliper Life Sciences) . Signal intensity was quantified as the average number of photons emitted from a mouse within the chest cavity (Living Image software, version 4 . 3 . 1 . ) B, Female athymic nude mice were implanted with 1 x 10 6 cells per mouse of cultured A 549 human lung adenocarcinoma cells (ATCC® CCL - 185 ™) into the intrathoracic space of the left lung on Day 0 . Animals were randomly assigned to two treatment groups (n= 15 ) on Day 1 and treated with either ANC 030 formulated in Maalox by oral gavage at a dose of 100 mg/kg once daily for 8 weeks or with Maalox using the same schedule starting on Day 1 . All surviving mice were euthanized on Day 151 . A B CORPORATE PRESENTATION |

Intellectual Property Strong IP estate Granted patents cover composition of matter and methods of use in RAS - driven malignancies Patent life through 2034 Sulindac Indene library Initial hit Primary Screen K - Ras mutant HCT116 vs. WT Ras Raf mutant HT29 ~ 1500 compounds ADT - 004 27 CORPORATE PRESENTATION |

E xperienced management has history of successful small molecule targeted therapy development for such programs Data in hand demonstrate pan - RAS nM potency in vitro, inhibition of RAS signaling, inhibition of GTP binding to RAS, in vivo activity, and stimulation of anti - tumor immunity Related compounds inhibit PDE10, with down - regulation of b - catenin in familial polyposis model and several tumor types Company is committed to development of pan - RAS small molecule inhibitors Key Takeaways Strong balance sheet with $23M cash end 3Q2019 and runway to 4Q2020 supporting continued preclinical development on path to development lead compound Potential addressable population with a pan - RAS inhibitor could approach 200,000 patients per year in solid and liquid tumors in US alone 28 CORPORATE PRESENTATION |
