Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - Mersana Therapeutics, Inc. | tm201744d1_ex99-2.htm |
| 8-K - FORM 8-K - Mersana Therapeutics, Inc. | tm201744-1_8k.htm |
Exhibit 99.1

Accelerating ADC Innovation …because patients are waiting January 2020

Legal Disclaimer This presentation contains “forward - looking” statements within the meaning of federal securities laws . These forward - looking statements are not statements of historical facts and are based on management’s beliefs and assumptions and on information currently available to management . Forward - looking statements include information concerning the Company’s business strategy and the design, progression and timing of its clinical trials . Forward - looking statements generally can be identified by terms such as “expects,” “anticipates,” “believes,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms . The Company’s operations involve risks and uncertainties, many of which are outside its control, and any one of which, or combination of which, could materially affect its results of operations and whether the forward - looking statements ultimately prove to be correct . Factors that may materially affect the Company’s results of operations and whether these forward - looking statements prove to be correct include, among other things, that preclinical testing may not be predictive of the results or success of ongoing or later preclinical or clinical trials, that the development of the Company’s product candidates and new platforms will take longer and/or cost more than planned and that the identification of new product candidates will take longer than planned, as well as those listed in our Annual Report on Form 10 - K filed on March 8 , 2019 , with the Securities and Exchange Commission (“SEC”) and subsequent SEC filings . Except as required by law, the Company assumes no obligation to update these forward - looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward - looking statements, even if new information becomes available in the future . Copies of the Company’s Annual Report on Form 10 - K and our other SEC filings are available by visiting EDGAR on the SEC website at http : //www . sec . gov . 2

Mersana is Poised for a Transformational 2020 3 1 Excluding Brazil 2 Cash, Cash Equivalents, and Marketable Securities as of September 30, 2019 XMT - 1536 • First - in - class asset • Clinically - Validated • Wholly - Owned 1 • Fast - to - market strategy On Track for Near - Term Proof of Concept First - In - Class Pipeline • Addressing unmet patient needs • Fast - to - market strategies 1 IND and 2 Development Candidates in 2020 Innovative Platforms • Multiple partnering opportunities • Efficient product engines DolaLock (Dolaflexin, Dolasynthen) and Immunosynthen Strong Foundation • Experienced team • Runway to mid - 2021 $112M in Cash 2 +$15M Credit Facility

2020 Will Be a Data Rich Year 4 o Establish proof of concept o Rapid dose escalation o Select first development candidate o Progress into IND - enabling studies 2019 ACCOMPLISHMENTS 2020 PRIORITIES x Established proof of activity & tolerability x Established preclinical proof of concept x Advanced through discovery x Advanced through discovery XMT - 1536 IND Candidate (XMT - 1592) Immunosynthen Development Candidate DolaLock Development Candidate
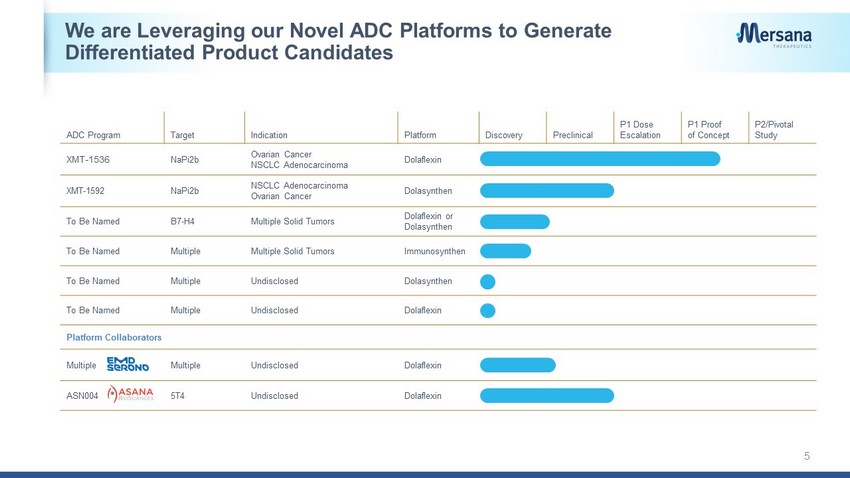
We are Leveraging our Novel ADC Platforms to Generate Differentiated Product Candidates 5 ADC Program Target Indication Platform Discovery Preclinical P1 Dose Escalation P1 Proof of Concept P2/Pivotal Study XMT - 1536 NaPi2b Ovarian Cancer NSCLC Adenocarcinoma Dolaflexin XMT - 1592 NaPi2b NSCLC Adenocarcinoma Ovarian Cancer Dolasynthen To Be Named B7 - H4 Multiple Solid Tumors Dolaflexin or Dolasynthen To Be Named Multiple Multiple Solid Tumors Immunosynthen To Be Named Multiple Undisclosed Dolasynthen To Be Named Multiple Undisclosed Dolaflexin Platform Collaborators Multiple Multiple Undisclosed Dolaflexin ASN004 5T4 Undisclosed Dolaflexin

Efficacy without severe neutropenia, neuropathy, or ocular toxicity DolaLock Dolaflexin Improved therapeutic index vs. other platforms Dolasynthen Homogenous & Customizable Platform Immunosynthen Systemic administration with targeted immuno - stimulatory effect Innovative and Highly Differentiated ADC Technologies and Platforms 6 • Controlled bystander effect • Selectively toxic to rapidly dividing cells • Not a PgP substrate • Induces immunogenic cell death • DolaLock payload • Polymer scaffold • DAR ~10 - 12 • Excellent drug like properties • DolaLock payload • Synthetic scaffold • Site - specific • Precise DAR (2 - 24) • Novel STING agonist • Complete regression with one dose in multiple models • Limited effect on systemic cytokines DAR = Drug - to - antibody ratio STING = Stimulator of Interferon Genes

XMT - 1536: First - in - Class Dolaflexin ADC Targeting NaPi2b

Leader in Targeting NaPi2b, an Ideal and Validated ADC Target • Broadly expressed in ovarian cancer and NSCLC adenocarcinoma ⁃ No detectable expression in squamous NSCLC ⁃ Limited expression in healthy tissues • NaPi2b is a lineage marker (not an oncogene) that transports phosphate into the cell ⁃ The presence of EGFR and KRAS mutations in NSCLC adenocarcinoma correlates with more frequent expression of NaPi2b • Proprietary biomarker assay can distinguish across low, medium, and high expression ⁃ Correlation between biomarker expression and response in preclinical and clinical settings ⁃ Developing companion diagnostic for use in registration enabling study 8 In Ovarian PDX Models, only tumors with an H - score above cutoff had a tumor response >50% Change in Tumor Volume H - score 290 H - score 0 Increasing H - score Mosher et al, AACR - NCI - EORTC International Conference, October 2017 Mitchell et al, AACR - NCI - EORTC International Conference, October 2019 D’Archangelo, et al. Abstract 194P ESMO 2014
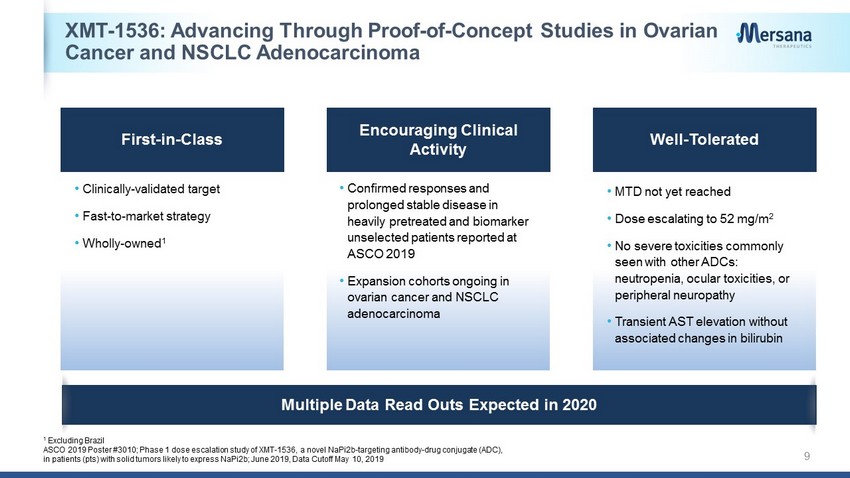
XMT - 1536: Advancing Through Proof - of - Concept Studies in Ovarian Cancer and NSCLC Adenocarcinoma 9 Encouraging Clinical Activity Well - Tolerated • Confirmed responses and prolonged stable disease in heavily pretreated and biomarker unselected patients reported at ASCO 2019 • Expansion cohorts ongoing in ovarian cancer and NSCLC adenocarcinoma • MTD not yet reached • Dose escalating to 52 mg/m 2 • No severe toxicities commonly seen with other ADCs: neutropenia, ocular toxicities, or peripheral neuropathy • Transient AST elevation without associated changes in bilirubin 1 Excluding Brazil ASCO 2019 Poster #3010; Phase 1 dose escalation study of XMT - 1536, a novel NaPi2b - targeting antibody - drug conjugate (ADC), in patients (pts) with solid tumors likely to express NaPi2b; June 2019, Data Cutoff May 10, 2019 • Clinically - validated target • Fast - to - market strategy • Wholly - owned 1 First - in - Class Multiple Data Read Outs Expected in 2020

XMT - 1536 was Well - Tolerated with Most AE’s Grade 1 - 2 10 ClinicalTrials.gov: NCT03319628 Dosing: Q3 weeks DL 4A 20 mg/m 2 (0.54 mg/kg) N=9 DL 5A 30 mg/m 2 (0.81 mg/kg) N=8 DL 6A 36 mg/m 2 (0.97 mg/kg) DL 1 3 mg/m 2 (0.081 mg/kg) N=1 DL 2 6 mg/m 2 (0.162 mg/kg) N=1 DL 3 12 mg/m 2 (0.324 mg/kg) N=7 DL 4 20 mg/m 2 (0.54 mg/kg) N=6 DL 5 30 mg/m 2 (0.81 mg/kg) N=4 DL 6 40 mg/m 2 (1.08 mg/kg) N=1 DL 7A 43 mg/m 2 (1.2 mg/kg) Presented at ASCO Dosing: Q4 weeks DL 8A 52 mg/m 2 (1.4 mg/kg) Ongoing No Severe Toxicities Associated with Other ADC Platforms such as Neutropenia, Ocular Toxicities, or Peripheral Neuropathy Data Presented at ASCO with a Data Cutoff of May 10, 2019 Treatment Related Adverse Events in ≥10% of Patients

XMT - 1536 Showed Activity in Heavily Pretreated Patients, Unselected for NaPi2b 11 Data Presented at ASCO with a Data Cutoff of May 10, 2019 Response evaluable All Completed Dose Levels (OC and NSCLC Patients), N=26 Clinically Meaningful Treatment Duration > 16 weeks Clinical Activity at Doses of 20mg/m 2 and Above* Response evaluable Based on objective responses and duration of treatment *As measured by RECIST, version 1.1 Treatment Duration (weeks)

XMT - 1536: Significant Progress Since ASCO 2019 in Maximizing Patient Benefit and Charting Path to Registration 12 • Initiated expansion cohorts in earlier line, more homogeneous populations Earlier, More Homogeneous Patient Population Higher Dose Opportunity for Biomarker Selection • Correlating H - score and efficacy in anticipation of patient selection • Collecting archival and fresh tissue to confirm concordance • Cleared 36 and 43 mg/m 2 with primarily Grade 1 and Grade 2 AEs with no severe neuropathy, neutropenia or ocular toxicities • Increased expansion cohort dose to 43 mg/m 2 • Currently evaluating 52 mg/m 2 FAST - TO - MARKET REGISTRATION STRATEGY

XMT - 1536: Path to Pivotal Study in High Unmet Need Indications 13 Population Dose Current Standard of Care ORR: 4 - 12% mPFS: 3 - 4 mos mOS: 9 - 12 mos • Prior treatment with a platinum doublet and PD - 1/L1 inhibitor • Prior TKIs if targetable mutation • Up to 2 prior lines of cytotoxic therapy • Adenocarcinoma histology • 36 mg/m 2 dose initiated in Aug 2019 • 43 mg/m 2 dose initiated in Dec 2019 ORR: 14 - 23% mPFS: 3 - 4 mos mOS: 9 - 12 mos Dose Escalation Data in 1H 2020 • Late stage platinum - resistant ovarian cancer • Late stage recurrent NSCLC adenocarcinoma • Evaluating 52 mg/m 2 Ovarian Cancer Expansion Data in 1H & 2H 2020 • 1 - 3 prior lines in platinum resistant • 4 prior lines regardless of platinum status • High grade serous histology • 36 mg/m 2 dose initiated in Aug 2019 • 43 mg/m 2 dose initiated in Dec 2019 NSCLC Adeno Expansion Data in 1H and 2H 2020 Investigational Agent

XMT - 1592 is a Dolasynthen ADC Targeting NaPi2b

XMT - 1592 Shows Four - Fold Greater Efficacy in Lung Tumor Model 15 2X Tumor Exposure of Payload 0 7 14 21 28 35 42 49 56 63 70 0 500 1000 1500 2000 Days on study M e a n T u m o r V o l u m e S E M ( m m 3 ) Vehicle XMT-1536 @ 0.1 mg/kg [2.1 mg/kg mAb] XMT-1592 @ 0.1 mg/kg [3.0 mg/kg mAb] 0.1 mg/kg payload XMT-1592 @ 0.025 mg/kg [0.75 mg/kg mAb] XMT - 1536 XMT - 1592 After single, equal dose of 0.05 mg/kg by payload 4X Greater Activity in Lung PDX 0 7 14 21 28 0 50 100 150 Days Post Dosing n g / g T u m o r T i s s u e P l a s m a T u m o r L i v e r L u n g S p l e e n K i d n e y 0 10000 20000 30000 40000 A U C l a s t Our Success with NaPi2b Makes it an Ideal Target for Evaluation of the Clinical Differentiation of Dolasynthen

Leveraging NaPi2b Experience for Rapid Dose Escalation of XMT - 1592 16 2021 Solidify NaPi2b Leadership Leveraging insights from XMT - 1536 Biomarker Investigators Antibody Payload Patient Populations Target 2020 2020 2021 Inform Product Engine File IND Initiate Dose Escalation Achieve MTD Clinical Proof of Differentiation

First - in - Class B7 - H4 ADC Progressing into IND - Enabling Studies

B7 - H4 Expression Ideally Suited for a DolaLock ADC • B7 - H4 is expressed on both tumor cells and immunosuppressive tumor - associated macrophages (TAMs) ⁃ Limited expression in normal tissues • A DolaLock ADC targeting B7 - H4 can exert its effect through multiple mechanisms of action: ⁃ Uptake by tumor cells and direct cytotoxicity ⁃ Uptake by TAMs to release payload in the tumor microenvironment ⁃ Free payload can activate dendritic cells and a secondary immune response • Expression in PD - L1 negative tumors, provides a potential fast to market opportunities (e.g., triple negative breast cancer) 18 TUMOR CELL CATABOLIZES ADC Immune Response T CELL CHECKPOINT BLOCKADE AF - HPA Diffusion Bystander Effect DENDRITIC CELL ACTIVATION BY AF - HPA BYSTANDER KILLING IMMUNOGENIC CELL DEATH (ICD) ICD B7 - H4 B7 - H4 B7 - H4 B7 - H4 TUMOR KILLING TUMOR KILLING TUMOR - ASSOCIATED MACROPHAGE CATABOLIZES ADC T CELL PRIMING IND - enabling studies in 2020

Immunosynthen Development Candidate in 2020

Immunosynthen: Strong Rationale for a STING Agonist ADC Approach ADCs are suited to overcome limitations of free agonist: ⁃ Targeted delivery reduces toxicity liabilities ⁃ Improved pharmacokinetics ⁃ Accessibility to metastatic sites ⁃ No restriction on tumor type, location or size 20 Nature Reviews Cancer 4, 11 – 22 (2004) Adaptive Immunity (slow response) Stepping on the gas pedal of the immune system (e.g. STING agonists) Releasing the brakes of the immune system (e.g. checkpoint inhibitors) Innate Immunity (rapid response)
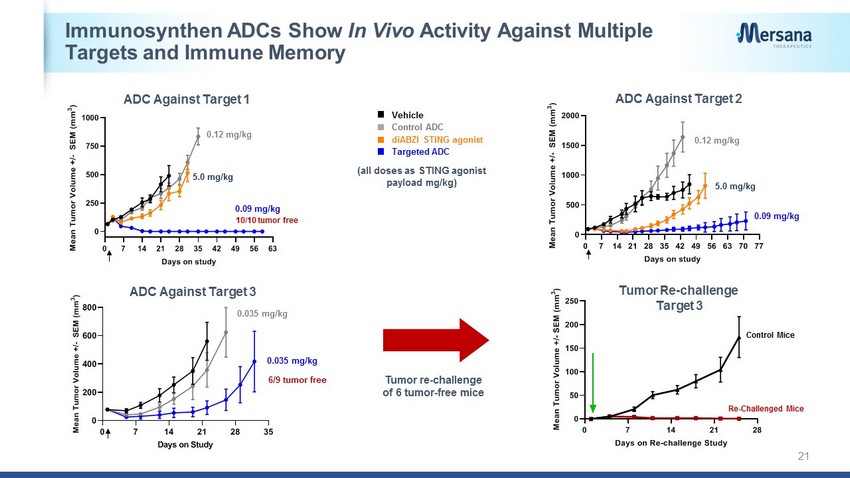
0 7 14 21 28 0 50 100 150 200 250 Days on Re-challenge Study M e a n T u m o r V o l u m e + / - S E M ( m m 3 ) Immunosynthen ADCs Show In Vivo Activity Against Multiple Targets and Immune Memory 21 0 7 14 21 28 35 42 49 56 63 0 250 500 750 1000 Days on study M e a n T u m o r V o l u m e + / - S E M ( m m 3 ) 10/10 tumor free ADC Against Target 1 0.09 mg/kg 0.12 mg/kg 0 7 14 21 28 35 42 49 56 63 70 77 0 500 1000 1500 2000 Days on study M e a n T u m o r V o l u m e + / - S E M ( m m 3 ) 0.12 mg/kg 0.09 mg/kg ADC Against Target 2 5.0 mg/kg 5.0 mg/kg 0 7 14 21 28 35 0 200 400 600 800 Days on Study M e a n T u m o r V o l u m e + / - S E M ( m m 3 ) 0.035 mg/kg 0.035 mg/kg ADC Against Target 3 6/9 tumor free Tumor re - challenge of 6 tumor - free mice diABZI STING agonist (all doses as STING agonist payload mg/kg) Control ADC Vehicle Targeted ADC Control Mice Tumor Re - challenge Target 3 Re - Challenged Mice

Immunosynthen ADC Activates STING Pathway and Induces Marked Immune Cell Infiltration in Tumors 22 Cytokine expression (qPCR on FFPE samples) 0 5 10 15 20 25 12H 72H mCXCL10 0 0.4 0.8 1.2 1.6 12H 72H mIFN β 0 2 4 6 12H 72H mIL - 6 12 H 12 H 12 H 72 H 72 H mCXCL10 mIL - 6 mIFNβ Vehicle Targeted ADC Control ADC 72 H CD45 Immunohistochemistry Immune cell infiltration Vehicle 12 hrs. 72 hrs. Targeted ADC Control ADC CD45 Immunohistochemistry 12 hrs. 72 hrs. Data shown for Immunosynthen ADC for Target 1 After single dose of 0.09 mg/kg by STING agonist payload
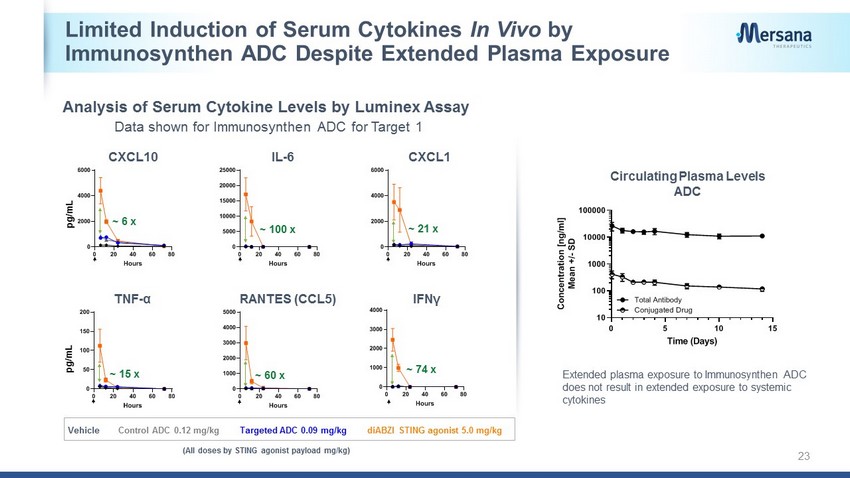
Limited Induction of Serum Cytokines In Vivo by Immunosynthen ADC Despite Extended Plasma Exposure 23 CXCL10 IL - 6 CXCL1 RANTES (CCL5) 0 20 40 60 80 0 1000 2000 3000 4000 Hours 0 20 40 60 80 0 5000 10000 15000 20000 25000 Hours 0 20 40 60 80 0 2000 4000 6000 Hours 0 20 40 60 80 0 1000 2000 3000 4000 5000 Hours 0 20 40 60 80 0 50 100 150 200 Hours TNF - α IFNγ pg/mL 0 5 10 15 10 100 1000 10000 100000 Plasma Concentration Time (Days) C o n c e n t r a t i o n [ n g / m l ] M e a n + / - S D Total Antibody Conjugated Drug Extended plasma exposure to Immunosynthen ADC does not result in extended exposure to systemic cytokines ~ 100 x ~ 15 x pg/mL 0 20 40 60 80 0 2000 4000 6000 Hours ~ 6 x ~ 21 x ~ 60 x ~ 74 x Circulating Plasma Levels ADC (All doses by STING agonist payload mg/kg) diABZI STING agonist 5.0 mg/kg Control ADC 0.12 mg/kg Targeted ADC 0.09 mg/kg Vehicle Analysis of Serum Cytokine Levels by Luminex Assay Data shown for Immunosynthen ADC for Target 1

On Track to Select First Immunosynthen ADC Development Candidate in 2020 24 Payload Molecule Drug Load Per Scaffold Charge Balance Aqueous Solubility Bioconjugation Antibody x Identified proprietary STING payload specifically designed for ADCs x Demonstrated efficacy across multiple targets in a variety of models x Confirmed tolerability in multidose exploratory NHP study o Finalize proprietary STING ADC scaffold (linker, DAR, method and site of bioconjugation) o Select first Immunosynthen ADC from current targets and leads Expect to disclose data package in 2H 2020

2020: A Transformational Year for Mersana with Multiple Data Readouts 25 2020 Goals & Anticipated Milestones XMT - 1536 • Report dose escalation in 1H 2020 • Report interim data from OC and NSCLC expansion cohorts in 1H 2020 • Report more mature data from expansion cohorts in 2H 2020 XMT - 1592 • File IND and initiate clinical study in 1H 2020. Achieve rapid dose escalation B7 - H4 • Advance IND - enabling studies • Disclose development candidate data package in 2H 2020 Immunosynthen • Select first development candidate • Disclose development candidate data package in 2H 2020 Product Engine • Continue to leverage proprietary platforms to expand pipeline Corporate • Proactively evaluate potential for strategic collaborations that maximize value
Positioned to Create Value for Patients and Shareholders 26 • First - in - class NaPi2b ADC • Completion of proof - of - concept studies in 2020 • Fast - to - market registration strategy XMT - 1536 • Extends NaPi2b leadership • Fast to clinical validation of preclinical differentiation XMT - 1592 • First - in - class B7 - H4 and Immunosynthen ADCs • Targeting high unmet medical needs Pipeline • Dolaflexin, Dolasynthen (DolaLock) • Immunosynthen (Novel STING Agonist) • Efficient product engines with multiple partnership opportunities Platforms • Strong team • Strong balance sheet Fundamentals

