Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - Motus GI Holdings, Inc. | s114581_ex23-1.htm |
| EX-5.1 - EXHIBIT 5.1 - Motus GI Holdings, Inc. | s114581_ex5-1.htm |
| EX-1.1 - EXHIBIT 1.1 - Motus GI Holdings, Inc. | s114581_ex1-1.htm |
As filed with the Securities and Exchange Commission on December 17, 2018.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
Motus GI Holdings, Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 3841 | 81-4042793 | ||
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
1301 East Broward Boulevard, 3rd Floor
Ft. Lauderdale, FL, 33301
Telephone: (954) 541-8000
(Address, including zip code, and telephone number,
including area code, of principal executive offices)
Timothy P. Moran
Chief Executive Officer
Motus GI Holdings, Inc.
1301 East Broward Boulevard, 3rd Floor
Ft. Lauderdale, FL 33301
Telephone: (954) 541-8000
(Address, including zip code, and telephone number,
including area code, of agent for service)
Copies to:
|
Steven M. Skolnick, Esq. Michael J. Lerner, Esq. Lowenstein Sandler LLP 1251 Avenue of the Americas New York, New York 10020 Telephone: (212) 262-6700 |
Michael D. Maline, Esq. Seo Salimi, Esq. Goodwin Procter LLP 620 Eighth Avenue New York, New York 10018 Telephone: (212) 813-8800 |
Approximate date of proposed sale to public: As soon as practicable on or after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ |
| Non-accelerated filer x | Smaller reporting company x |
| Emerging growth company x |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. x
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to Be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee | ||||||
| Common Stock, par value $0.0001 per share | $ | 18,572,500 | $ | 2,250.99 | ||||
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended. |
| (2) | Includes the aggregate offering price of the additional shares that the underwriters have the option to purchase to cover over allotments, if any. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| Preliminary Prospectus | Subject to Completion, dated December 17, 2018 |
Motus GI Holdings, Inc.

5,000,000 Shares
Common Stock
We are offering 5,000,000 shares of our common stock.
Our common stock is listed on the Nasdaq Capital Market under the symbol “MOTS.” On December 13, 2018, the last reported sale price of our common stock as reported on the Nasdaq Capital Market was $3.23 per share.
We are an “emerging growth company” as defined under the federal securities laws.
Investing in our common stock involves risks that are described in the “Risk Factors” section beginning on page 8 of this prospectus.
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discount(1) | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ |
| (1) | We refer you to “Underwriting” beginning on page 38 of this prospectus for additional information regarding total underwriter compensation. |
The underwriters may also exercise their option to purchase up to an additional 750,000 shares from us at the public offering price, less the underwriting discount, for 30 days after the date of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares against payment therefor on or about , 2018.
Piper Jaffray
The date of this prospectus is , 2018.
TABLE OF CONTENTS
You should rely only on the information contained or incorporated by reference in this prospectus and any related free writing prospectus that we may provide to you in connection with this offering. We have not, and the underwriters have not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing or incorporated by reference in this prospectus is accurate only as of the date on the front cover of this prospectus or the date of the applicable document incorporated by reference. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: neither we nor any of the underwriters has done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and the distribution of this prospectus and any such free writing prospectus outside of the United States.
In this prospectus, we rely on and refer to information and statistics regarding our industry. We obtained this statistical, market and other industry data and forecasts from publicly available information.
This summary highlights information contained in other parts of this prospectus and in the documents incorporated by reference. Because it is a summary, it does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this prospectus and the documents incorporated by reference in their entirety including “Risk Factors” included in this prospectus at page 8 and incorporated by reference and “Management’s Discussion and Analysis of Financial Condition and Results of Operation” and the financial statements and the notes to those financial statements incorporated by reference in this prospectus before investing in our common stock.
When used herein, unless the context requires otherwise, references to the “Company,” “Holdings,” “we,” “our” and “us” refer to Motus GI Holdings, Inc., a Delaware corporation, collectively with our direct wholly-owned subsidiaries, Motus GI Medical Technologies, Ltd., an Israeli corporation, and Motus GI, Inc., a Delaware corporation.
All trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Our Company
General
We have developed the Pure-Vu System (the “Pure-Vu System”), a medical device that has received 510(k) clearance from the U.S. Food and Drug Administration (the “FDA”) and CE mark approval in the European Economic Area. The Pure-Vu System is indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard and slim colonoscopes to enable safe and rapid cleansing during the procedure while preserving established procedural workflow and techniques by irrigating the colon and evacuating the irrigation fluid (water), feces and other bodily fluids and matter. Challenges with bowel preparation for inpatient colonoscopy represent a significant area of unmet need that directly affects clinical outcomes and increases the cost of care for a hospital in a market segment where most of the reimbursement is under a bundle payment based on a Diagnostic Related Group (a “DRG”), comprising of approximately 1.5 million annual procedures in the U.S. and approximately 3.8 million annual procedures worldwide. The Pure-Vu System does not currently have a unique reimbursement code with any private or governmental third-party payors in any country. To date, as part of the limited market development launch, we have focused on collecting additional clinical and health economic data, as exemplified by the recently initiated Reliable Endoscopic Diagnosis Utilizing Cleansing Enhancement Study (the “REDUCE Study”), along with garnering valuable experience in key hospitals on the use of the Pure-Vu System to support a planned full launch in the United States inpatient colonoscopy market in 2019. We do not expect to generate significant revenue from product sales unless and until we expand our commercialization efforts.
| 1 |
Recent Developments
Clinical Data & Safety
In a recent clinical study performed in the United States, the Pure-Vu System demonstrated safe and effective colonic cleaning in 46 patients receiving a reduced prep regimen. The study was initially designed to compare two different minimal bowel preparation regimens. Initially patients were randomized to receive one of two minimal bowel preparations: three doses of 17 gr. MiraLAX each mixed in 8.5 oz. of clear liquids or two doses of 7.5 oz. magnesium citrate (MgC) each taken with 19.5 oz. of clear liquid. A study amendment early on replaced the MiraLAX arm, due to obvious inferior Boston Bowel Preparation Scale (“BBPS”), a validated assessment instrument, scoring from the outset. The replacement arm consisted of two doses of 5 oz. MgC taken with 16 oz. of clear liquid. All patients were allowed to eat a low residue diet on the day prior and were asked to avoid seeds and nuts for five days prior to their procedure. Study objectives evaluated for each study arm included: (1) improvement of colon cleansing from presentation baseline to completion of the procedure (as assessed by the BBPS) through the use of the Pure-Vu System, (2) time required to reach the cecum, (3) total procedure time, and (4) safety. No significant differences were found between the three groups with regard to demographics or indication for colonoscopy. No serious adverse events related to the device were reported. The use of the Pure-Vu System enabled successful intraprocedural cleansing of the colon and ensured successful completion of all colonoscopies performed (100% success rate). Although there were only 46 patients in the study, there was a highly significant difference in the study population (p value <0.0001) between the baseline preparation and that seen post cleansing with the Pure-Vu System. The use of the Pure-Vu System added some time to the procedure, but the total procedure time was approximately 25 minutes in this study.
The clinical data showing performance of the Pure-Vu System in this study using the BBPS is shown below. The clinical results from the study were presented at the 2018 American College of Gastroenterology (“ACG”) Annual Meeting in October 2018.
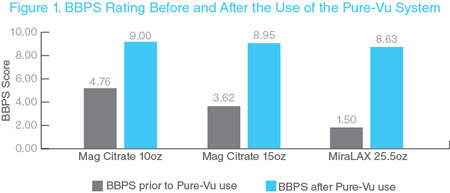
REDUCE Study
Published studies have found that the inpatient population experiences rates of insufficiently prepped colons at the time of colonoscopy as high as 55%. This has been shown to lead directly to significantly longer hospital stays and other additional costs due to the need for repeated preps, repeated colonoscopies and additional diagnostic procedures. This is exemplified in a recently published study from Northwestern University Hospital System which showed an average hospital stay extension of two days and cost increase of as much as $8,000 per patient as a result of challenges associated with bowel preparation. We believe the Pure-Vu System may improve outcomes and lower costs for hospitals by reducing the time to a successful colonoscopy, minimizing delayed and incomplete procedures, and improving the quality of an exam.
On May 23, 2018, we announced the initiation of the Reliable Endoscopic Diagnosis Utilizing Cleansing Enhancement Study (the “REDUCE Study”). The REDUCE Study is a multi-center inpatient prospective trial designed to evaluate the Pure-Vu System’s ability to consistently and reliably cleanse the colon to facilitate a successful colonoscopy in a timely manner in patients who are indicated for a diagnostic colonoscopy. The study will enroll approximately 100 subjects in at least five hospital centers in the United States and Europe.
We expect to announce a top-line readout of the REDUCE Study data in the first quarter of 2019. The primary endpoint of the study is to determine the Pure-Vu System’s rate of improved bowel cleansing level using the BBPS index. Other key data being collected in the study includes the proportion of patients who receive a successful colonoscopy for the intended indication in the first attempt, which impacts the quality of the exam as well as hospital length of stay and costs required for the episode of care. The savings to the hospital can be meaningful as National Inpatient Sample (“NIS”) and other literature sources note that the cost for a standard hospital bed averages $2,298 and the cost for an intensive care unit (“ICU”) bed averages $6,546 per day in the U.S.
| 2 |
FDA Clearance to Market Pure-Vu Slim Sleeve
On September 13, 2018, we announced that we received special 510(k) clearance from FDA for the Pure-Vu Slim Sleeve (the “Pure-Vu Slim Sleeve”), a compatible extension to the Pure-Vu System for slim colonoscopes. The Pure-Vu Slim Sleeve design allows the Pure-Vu System access to the full range of procedures in the colonoscopy market as we estimate, through consultation with colonoscope manufacturing companies, approximately 30% of procedures are performed with a slim colonoscope. The Pure-Vu Slim Sleeve has the same cleansing performance as the standard Pure-Vu System sleeve, and both versions work with the same Pure-Vu workstation control system. The Pure-Vu Slim Sleeve has been designed to be compatible with smaller diameter and more flexible slim colonoscopes with additional enhancements to our low friction lubricious coating technology to aid in navigation through the colon. The first successful clinical cases using the Pure-Vu Slim Sleeve were completed in October 2018.
Second Generation of Pure-Vu
We expect to submit a Special 510(k) Notice to FDA in the first quarter of 2019 for the Second-Generation (“Gen 2”) of the Pure-Vu System. This premarket notification is a premarket submission used to review with FDA modifications to our devices that can be validated within our Quality Systems and which do not affect the device’s intended use or alter the device’s fundamental scientific technology.
The Gen 2 Pure-Vu System has been designed to improve the mobility and logistics in the setup of the system and will retain all the same functionality as the current generation of the Pure-Vu System in terms of how it cleanses the colon. The Gen 2 Pure-Vu System Workstation will have a reduced footprint and be mounted on a roll stand to allow nursing staff to easily move the Gen 2 Pure-Vu System to different procedure rooms or to the ICU as needed. The Gen 2 Pure-Vu System also has improvements that reduce the number of steps to set up the system and simplifies the loading process onto the colonoscope.
Additional Clinical Studies and Market Expansion Opportunities
We expect to initiate the EXPEDITE Study (the “EXPEDITE Study”) in the first quarter of 2019. The EXPEDITE Study is a planned feasibility study in hospitalized patients which will be designed to analyze the Pure-Vu System’s ability to minimize the preparation in order to shorten the time to a successful colonoscopy in the inpatient population. We are also working with key centers to generate clinical data on outpatient populations that have difficulty with the pre-procedural preparation to study the Pure-Vu System’s ability to allow these patients to have a successful exam. This data is expected to lay the ground work for future expansion into high need outpatient populations.
Our resources are currently focused on the planned full launch of the Pure-Vu System in the United States inpatient colonoscopy market in 2019. However, we have identified two follow-on market expansion opportunities we may explore in the future. These include the inpatient upper gastrointestinal bleed (“Upper GI”) endoscopy market and the outpatient high medical need colonoscopy market. In the Upper GI bleed endoscopy market, we believe the Pure-Vu System has the potential to be used during endoscopy procedures to remove clots and debris to provide a clear field of view for the endoscopist. Separately, the outpatient high medical need colonoscopy market presents a large potential commercial market opportunity for the Pure-Vu System, as close to 26 million outpatient colonoscopy procedures are performed worldwide. Based on published literature in several peer reviewed journals from 2010 to 2015 and surveys of physicians conducted in 2015, approximately 23% of such colonoscopy patients can have an inadequate preparation, which may lead to repeat procedures earlier than the medical guidelines suggest. We believe use of the Pure-Vu System has the potential to reduce the need for such repeat procedures if used in the outpatient high medical need colonoscopy market. Additionally, if we choose to explore either market, we may be able to leverage our existing hospital and doctor relationships developed through our inpatient colonoscopy sales force to facilitate such expansion.
Intellectual Property Update
Our intellectual property position comprises a highly innovative portfolio covering technologies rooted in systems and methods for cleaning body cavities with or without the use of an endoscope. Currently we have six granted or allowed patents in the U.S., six patents in Asia, four patents in the EU and 27 (11 in the U.S.) pending patent applications in various regions of the world with a focus on the U.S., EU and Japan.
On March 20, 2018, we announced that the European Patent Office (“EPO”) issued Patent No. 3079556 to us titled, “Apparatus and method for coupling between a colonoscope and add-on tubes.” The patent provides intellectual property protection for our use of the Pure-Vu System in the European market through June 2035.
On March 27, 2018, we announced that the U.S. Patent and Trademark Office (“USPTO”) issued U.S. Patent No. 9,895,483 to us titled, “Systems and methods for cleaning body cavities.” The patent provides intellectual property protection for our use of the Pure-Vu System in the United States through January 2031.
On May 21, 2018, we announced that the Chinese Patent Office (“SIPO”) issued Patent No. ZL201580037467.6 to us titled, “Apparatus and method for coupling between a colonoscope and add on tubes.” The patent provides intellectual property protection for our use of the Pure-Vu System in China through June 2035.
On July 30, 2018, we announced that the Japanese Patent Office (“JPO”) issued Patent No. 6,362,640 to us titled, “System and method for cleaning body cavities.” The patent provides intellectual property protection for our use of the Pure-Vu System in Japan through June 2031.
| 3 |
Our Risks
Investing in our common stock involves a high degree of risk. You should carefully consider all of the information in this prospectus and in the documents incorporated by reference prior to investing in our common stock. These risks are discussed more fully in the section titled “Risk Factors” beginning on page 8 herein and in our Annual Report on Form 10-K for the year ended December 31, 2017, as updated by our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2018, June 30, 2018 and September 30, 2018, which are incorporated by reference in this prospectus. These risks and uncertainties include, but are not limited to, the following:
| ● | We have incurred substantial operating losses in each year since our inception and expect to continue to incur substantial losses for the foreseeable future. We may never become profitable or, if achieved, be able to sustain profitability; | |
| ● | If this offering is not successful, there is substantial doubt about our ability to continue as a going concern; |
| ● | We may need to raise additional capital in the future; | |
| ● | We may be unable to obtain or maintain governmental approvals to market our Pure-Vu System outside the United States, including the European Union countries; | |
| ● | Our Pure-Vu System is not currently reimbursable through private or governmental third-party payors, which could limit market acceptance; | |
| ● | The Pure-Vu System may not be accepted by physicians and patients; | |
| ● | Our Pure-Vu System is currently our sole product and we are completely dependent on the successful marketing and sale of this product. There is no assurance that we will be able to develop any additional products; | |
| ● | We may be unable to protect our intellectual property rights or may infringe on the intellectual property rights of others; | |
| ● | The manufacture of our Pure-Vu System, and the technology developed thereunder, is subject to certain Israeli government regulations which may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel; |
| 4 |
| ● | We may face competition from other medical device companies in the future and our operating results will suffer if we fail to compete effectively; and | |
| ● | We currently have a limited sales and marketing organization. If we are unable to secure a sales and marketing partner and/or establish satisfactory sales and marketing capabilities, we may not successfully commercialize our Pure-Vu System. |
Implications of Being an Emerging Growth Company
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and, for as long as we continue to be an “emerging growth company,” we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies,” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, (the “Sarbanes-Oxley Act”), reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years from the date of our initial public offering in February 2018, or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.07 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three-year period. We intend to take advantage of these reporting exemptions described above until we are no longer an “emerging growth company.” Under the JOBS Act, “emerging growth companies” can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we are subject to the same new or revised accounting standards as other public companies that are not “emerging growth companies.”
Corporate History and Information
We are a Delaware corporation formed in September 2016 under the name Eight-Ten Merger Corp. In November 2016, we changed our name to Motus GI Holdings, Inc. We are the parent company of Motus GI Medical Technologies Ltd., an Israeli corporation, and Motus GI, Inc. a Delaware corporation.
On February 16, 2018, we closed our initial public offering (the “IPO”) in which we sold 3,500,000 shares of our common stock at a public offering price of $5.00 per share. In connection with the closing of the IPO, we received net proceeds of approximately $15 million after deducting underwriting discounts and commissions of approximately $1.4 million and other offering expenses of approximately $1.1 million. In addition, on March 12, 2018, we closed the sale of an additional 56,000 shares of our common stock pursuant to a partial exercise of the underwriters’ 30-day option to purchase up to an additional 525,000 shares of our common stock in connection with the IPO, resulting in net proceeds to us of approximately $258,000 after deducting underwriting discounts and commissions and other offering expenses. Shares of our common stock commenced trading on the Nasdaq Capital Market under the symbol “MOTS” on February 14, 2018.
Our principal executive offices are located at 1301 East Broward Boulevard, 3rd Floor, Ft. Lauderdale, FL, 33301. Our phone number is (954) 541-8000 and our web address is http://www.motusgi.com. Information contained in or accessible through our web site is not, and should not be deemed to be, incorporated by reference in, or considered part of, this prospectus.
“Motus GI,” “Pure-Vu,” and our other registered or common law trademarks, service marks or trade names appearing herein are the property of Motus GI Holdings, Inc. Some trademarks referred to in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
| 5 |
The following summary contains basic information about this offering. The summary is not intended to be complete. You should read the full text and more specific details contained elsewhere in this prospectus and in the documents incorporated by reference.
| Common Stock offered by us | 5,000,000 shares | |
| Common Stock to be outstanding after this offering | 20,690,151 shares (21,440,151 shares, if the underwriters exercise their option to purchase additional shares in full) | |
| Underwriters’ option to purchase additional shares from us | 750,000 shares | |
| Use of Proceeds |
We estimate that we will receive net proceeds from this offering of approximately $14.57 million, or approximately $16.82 million if the underwriters exercise their overallotment option in full, based upon an assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) and after deducting the underwriting discounts and commissions and estimating offering expenses payable by us.
We intend to use the net proceeds from this offering to fund commercialization activities, research and development activities, including clinical and regulatory development and the continued development and enhancement of our Pure-Vu System, and for working capital and other general corporate purposes. See the section titled “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. | |
| Dividend Policy | We have never declared or paid any cash dividends on our common stock, and currently do not plan to declare cash dividends on shares of our common stock in the foreseeable future. We expect that we will retain all of our available funds and future earnings, if any, for use in the operation and expansion of our business. See the section titled “Dividend Policy” for a more complete description of our dividend policy. | |
| Risk Factors | Investing in our common stock involves a high degree of risk. See the section titled “Risk Factors” and other information contained or incorporated by reference in this prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. | |
| Nasdaq Capital Market symbol | “MOTS” |
The number of shares of our common stock to be outstanding upon completion of this offering is based on 15,690,151 shares of our common stock outstanding as of September 30, 2018 and excludes:
| ● | 1,964,454 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan as of September 30, 2018, with a weighted average exercise price of $4.48 per share, and 560,000 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan after September 30, 2018, with a weighted average exercise price of $3.78 per share; |
| 6 |
| ● | 653,272 additional shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan as of September 30, 2018, reduced by 560,000 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan after September 30, 2018 as described in the bullet above; | |
| ● | 2,586,551 shares of our common stock issuable upon the exercise of outstanding warrants with a weighted average exercise price of $5.20 per share as of September 30, 2018; | |
| ● | 42,917 shares of our common stock issuable upon the exercise of warrants issued subsequent to September 30, 2018 with a weighted average exercise price of $7.48 per share; and | |
| ● | any automatic increases in the number of shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan. |
Unless otherwise indicated, all information in this prospectus reflects or assumes the following:
| ● | no issuance or exercise of stock options or warrants on or after September 30, 2018; and | |
| ● | no exercise by the underwriters of their option to purchase additional shares of common stock in this offering. |
| 7 |
An investment in our common stock is speculative and illiquid and involves a high degree of risk including the risk of a loss of your entire investment. You should carefully consider the following risk factors, as well as those set forth under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2017, as updated by our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2018, June 30, 2018 and September 30, 2018, which are incorporated by reference in this prospectus. These risk factors contain, in addition to historical information, forward looking statements that involve risks and uncertainties. Our actual results could differ significantly from the results discussed in the forward looking statements. The order in which the following risks are presented is not intended to reflect the magnitude of the risks described. The occurrence of any of the following adverse developments described in the following risk factors and in the documents incorporated by reference could materially and adversely harm our business, financial condition, results of operations or prospects. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of the money that you pay for our common stock. In addition, the risks and uncertainties discussed below and in the documents incorporated by reference are not the only ones we face. Our business, financial condition, results of operations or prospects could also be harmed by risks and uncertainties not currently known to us or that we currently do not believe are material, and these risks and uncertainties could results in a complete loss of your investment. In assessing the risks and uncertainties described below, you should also refer to the other information contained in this prospectus (as supplemented or amended) and the documents incorporated by reference in this prospectus.
Risks Related to Our Financial Position and Need for Capital
There is substantial doubt about our ability to continue as a going concern, which will affect our ability to obtain future financing and may require us to curtail our operations.
Our financial statements as of December 31, 2017 were prepared under the assumption that we will continue as a going concern. The independent registered public accounting firm that audited our 2017 financial statements, in their report, included an explanatory paragraph referring to our recurring losses since inception and expressing substantial doubt in our ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our ability to continue as a going concern depends on our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. We cannot assure you, however, that we will be able to achieve any of the foregoing.
Risks Related to this Offering and Ownership of our Common Stock
After this offering, our officers, directors, and principal stockholders will continue to exercise significant control over our Company, and will control our company for the foreseeable future, including the outcome of matters requiring stockholder approval.
When this offering is completed our officers, directors, entities controlled by our officers and directors, and principal stockholders who beneficially own more than 5% of our common stock before this offering will in the aggregate, beneficially own shares representing approximately 47.76% of our outstanding capital stock immediately after this offering (based upon an assumed public offering price of $3.23 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018). As a result, such entities and individuals have the ability, acting together, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of our company, (ii) a sale of all or substantially all of our assets, and (iii) amendments to our certificate of incorporation and bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those entities and individuals. These individuals also have significant control over our business, policies and affairs as officers and directors of our company. Therefore, you should not invest in reliance on your ability to have any control over our company.
Investors in this offering will pay a higher price than the book value of our common stock.
If you purchase common stock in this offering, you will pay more for your shares than the amounts paid by existing stockholders for their shares. You will incur immediate and substantial dilution of $2.07 per share, representing the difference between our net tangible book value per share after giving effect to this offering and an assumed public offering price of $3.23 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018. To the extent any outstanding options or warrants are ultimately converted or exercised, you will sustain further dilution. For further information, see the section entitled “Dilution.”
| 8 |
If we sell shares of our common stock in future financings, stockholders may experience immediate dilution and, as a result, our stock price may decline.
We may from time to time issue additional shares of common stock at a discount from the current market price of our common stock. As a result, our stockholders would experience immediate dilution upon the purchase of any shares of our common stock sold at such discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preferred stock or common stock. If we issue common stock or securities convertible into common stock, our common stockholders would experience additional dilution and, as a result, our stock price may decline.
We will have broad discretion in how we use the net proceeds of this offering. We may not use these proceeds effectively, which could affect our results of operations and cause our stock price to decline.
We will have considerable discretion in the application of the net proceeds of this offering, including for any of the purposes described in the section entitled “Use of Proceeds.” We intend to use the net proceeds from this offering for commercialization activities, research and development activities, including clinical and regulatory development and the continued development and enhancement of our Pure-Vu System, and for working capital and other general corporate purposes. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions for the use of the balance of the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
An active trading market for our common stock may not be sustained.
Prior to the closing of our IPO, there had been no public market for our common stock. Although our common stock is listed on the Nasdaq Capital Market, the market for our shares has demonstrated varying levels of trading activity. Furthermore, the current level of trading may not be sustained in the future. The lack of an active market for our common stock may impair investors’ ability to sell their shares at the time they wish to sell them or at a price that they consider reasonable, may reduce the fair market value of their shares and may impair our ability to raise capital to continue to fund operations by selling shares and may impair our ability to acquire additional intellectual property assets by using our shares as consideration.
Our share price may be volatile, which could subject us to securities class action litigation and prevent you from being able to sell your shares at or above the offering price.
You may be unable to sell your shares of common stock at or above the offering price. The market price for our common stock has been and may continue to be volatile and subject to wide fluctuations in response to factors including the following:
| ● | actual or anticipated fluctuations in our quarterly or annual operating results; | |
| ● | actual or anticipated changes in our growth rate relative to our competitors; | |
| ● | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; | |
| ● | issuance of new or updated research or reports by securities analysts; | |
| ● | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; additions or departures of key management or other personnel; | |
| ● | disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; | |
| ● | announcement or expectation of additional debt or equity financing efforts; | |
| ● | sales of our common stock by us, our insiders or our other stockholders; and | |
| ● | general economic, market or political conditions in the United States or elsewhere. |
| 9 |
In particular, the market prices of early commercial-stage companies like ours have been highly volatile due to factors, including, but not limited to:
| ● | any delay or failure to conduct a clinical trial for our product or receive approval from the FDA and other regulatory agents; | |
| ● | developments or disputes concerning our product’s intellectual property rights; | |
| ● | our or our competitors’ technological innovations; | |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; | |
| ● | announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies or patents; | |
| ● | failure to complete significant transactions or collaborate with vendors in manufacturing our product; and | |
| ● | proposals for legislation that would place restrictions on the price of medical therapies or devices. |
These and other market and industry factors may cause the market price and demand for our common stock to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, the stock market in general, and the Nasdaq Capital Market and emerging growth companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. In the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
Shareholders will experience dilution by exercises of outstanding warrants and options.
As of September 30, 2018, there were 2,586,551 shares of our common stock issuable upon the exercise of outstanding warrants, with a weighted average exercise price of $5.20 per share, and options to purchase an aggregate of up to 1,964,454 shares of our common stock, with a weighted average exercise price of $4.48 per share. Since September 30, 2018, we have issued (i) an additional 560,000 options to purchase shares of our common stock pursuant to our equity incentive plan, with a weighted average exercise price of $3.78 per share, and (ii) an additional 42,917 warrants to purchase shares of our common stock with a weighted average exercise price of $7.48 per share.
The exercise of such warrants and options will result in dilution of your investment. As a result of this dilution, you may receive significantly less than the full purchase price you paid for our securities in the event of liquidation.
We do not currently intend to pay dividends on our common stock in the foreseeable future, and consequently, any gains from an investment in our common stock will likely depend on appreciation in the price of our common stock.
We have never declared or paid cash dividends on our common stock and do not anticipate paying any cash dividends to holders of our common stock in the foreseeable future. Consequently, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
| 10 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference in this prospectus contain, and our officers and representatives may from time to time make, “forward-looking statements,” which include information relating to future events, future financial performance, financial projections, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “goal,” “seek,” “project,” “strategy,” “likely,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements are neither historical facts, nor should they be read as a guarantee of future performance or results and may not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| ● | our limited operating history; | |
| ● | our history of substantial operating losses in each year since inception and expectation that we will continue to incur substantial operating losses for the foreseeable future; | |
| ● | our current and future capital requirements to support our development and commercialization efforts for the Pure-Vu System and our ability to satisfy our capital needs; | |
| ● | our dependence on the Pure-Vu System, our sole product candidate, which is still in development; | |
| ● | our ability to obtain approval from regulatory agents in different jurisdictions for the Pure-Vu System; | |
| ● | our Pure-Vu System and the procedure to cleanse the colon in preparation for colonoscopy are not currently reimbursable through private or governmental third-party payors; | |
| ● | our lack of a developed sales and marketing organization and our ability to commercialize the Pure-Vu System; | |
| ● | our dependence on third-parties to manufacture the Pure-Vu System; | |
| ● | our ability to maintain or protect the validity of our patents and other intellectual property; | |
| ● | our ability to retain key executives and medical and science personnel; | |
| ● | our ability to internally develop new inventions and intellectual property; | |
| ● | interpretations of current laws and the passages of future laws; | |
| ● | acceptance of our business model by investors; | |
| ● | the accuracy of our estimates regarding expenses and capital requirements; and | |
| ● | our ability to adequately support growth. |
The foregoing does not represent an exhaustive list of matters that may be covered by the forward-looking statements contained herein and in the documents incorporated by reference herein or risk factors that we are faced with that may cause our actual results to differ from those anticipate in our forward-looking statements. Please see “Risk Factors” beginning on page 8 for additional risks which could adversely impact our business and financial performance.
Moreover, new risks regularly emerge and it is not possible for our management to predict or articulate all risks we face, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended, do not protect any forward-looking statements that we make in connection with this offering. All forward-looking statements included in this prospectus and in the documents incorporated by reference in this prospectus are based on information available to us on the date of this prospectus or the date of the applicable document incorporated by reference. Except to the extent required by applicable laws or rules, we undertake no obligation to publicly update or revise any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future events or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained above and throughout this prospectus and in the documents incorporated by reference in this prospectus. We qualify all of our forward-looking statements by these cautionary statements.
| 11 |
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY OUR MANAGEMENT. IN REVIEWING THIS PROSPECTUS AND THE DOCUMENTS INCORPORATED BY REFERENCE IN THIS PROSPECTUS, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT THERE MAY BE OTHER POSSIBLE RISKS THAT COULD BE IMPORTANT.
| 12 |
We estimate that we will receive net proceeds of approximately $14.57 million from the sale of the shares of common stock offered in this offering, or approximately $16.82 million if the underwriters exercise their over-allotment option in full, based on an assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase (decrease) in the assumed public offering price of $3.23 per share (last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $4.65 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $3.00 million, assuming the public offering price stays the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018), would increase the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $8.58 million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018), would decrease the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $6.72 million. We do not expect that a change in the offering price or the number of shares by these amounts would have a material effect on our intended uses of the net proceeds from this offering, although it may impact the amount of time prior to which we may need to seek additional capital.
We expect to use the net proceeds from this offering for commercialization activities, research and development activities, including clinical and regulatory development and the continued development and enhancement of our Pure-Vu System, and for working capital and other general corporate purposes. Although we currently anticipate that we will use the net proceeds from this offering as described above, there may be circumstances where a reallocation of funds is necessary. The amounts and timing of our actual expenditures will depend upon numerous factors, including our sales and marketing and commercialization efforts, demand for our products, our operating costs and the other factors described under “Risk Factors” in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
Although we may use a portion of the net proceeds of this offering for the acquisition or licensing, as the case may be, of additional technologies, other assets or businesses, or for other strategic investments or opportunities, we have no current understandings, agreements or commitments to do so.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
| 13 |
We have never paid any cash dividends on our common stock. We anticipate that we will retain funds and future earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future following this offering. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors that our board of directors deems relevant. In addition, the terms of any future debt or credit financings may preclude us from paying dividends.
In addition, the ability of Motus GI Medical Technologies Ltd., an Israeli Company (“Opco”), our direct wholly-owned operating subsidiary, to distribute dividends may be limited by Israeli law. The Israeli Companies Law, 1999, or the Israeli Companies Law, restricts Opco’s ability to declare dividends. Unless otherwise approved by a court, Opco can distribute dividends only from “profits” (as defined by the Israeli Companies Law). Dividends may be paid with the approval of a court, at a company’s request, provided that there is no reasonable concern that payment of the dividend will prevent the company from satisfying its current and foreseeable obligations, as they become due.
The payment of dividends by Opco to Holdings may be subject to Israeli withholding taxes.
| 14 |
The following table sets forth our cash and cash equivalents and short-term investments balance and capitalization as of September 30, 2018:
| ● | on an actual basis; and | |
| ● | on an as-adjusted basis to reflect the issuance and sale by us of 5,000,000 shares of our common stock in this offering at the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018), after deducting underwriting discounts and commissions and estimated offering expenses payable by us and the receipt by us of the proceeds of such sale. |
You should read the selected data in the table below together with the sections titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2017 and our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2018, June 30, 2018 and September 30, 2018 which are incorporated by reference in this prospectus, and our consolidated financial statements and related notes incorporated by reference in this prospectus.
| As of September 30, 2018 | ||||||||
| Actual | As Adjusted | |||||||
| (unaudited) | ||||||||
| (in thousands, except per share data) |
||||||||
| Cash and cash equivalents and short-term investments | $ | 11,631 | $ | 26,201 | ||||
| Long-term liabilities | $ | 1,990 | $ | 1,990 | ||||
| Stockholders’ equity: | ||||||||
Common stock, $0.0001 par value; 50,000,000 shares authorized, 15,690,151 shares issued and outstanding (actual); 20,690,151 shares issued and outstanding (as adjusted) |
2 | 2 | ||||||
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized, zero shares issued and outstanding (actual), 10,000,000 shares authorized, zero shares issued and outstanding (as adjusted) | — | — | ||||||
| Additional paid-in capital | 65,251 | 79,821 | ||||||
| Accumulated deficit | (55,811 | ) | (55,811 | ) | ||||
| Total shareholders’ equity | 9,442 | 24,012 | ||||||
| Total capitalization | $ | 11,432 | $ | 26,002 | ||||
Each $1.00 increase (decrease) in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase (decrease) the amount of cash and cash equivalents and short-term investments, additional paid-in capital, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $4.65 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million shares offered by us would increase (decrease) cash and cash equivalents and short-term investments, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $3.00 million, assuming the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Each one million share increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase each of cash and cash equivalents and short-term investments and total stockholders’ equity by approximately $8.58 million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, each one million share decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would decrease each of cash and cash equivalents and short-term investments and total stockholders’ equity by approximately $6.72 million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
| 15 |
The number of shares of our common stock to be outstanding upon completion of this offering is based on 15,690,151 shares of our common stock outstanding as of September 30, 2018 and excludes:
| ● | 1,964,454 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan as of September 30, 2018, with a weighted average exercise price of $4.48 per share, and 560,000 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan after September 30, 2018, with a weighted average exercise price of $3.78 per share; |
| ● | 653,272 additional shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan as of September 30, 2018, reduced by 560,000 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan after September 30, 2018 as described in the bullet above; |
| ● | 2,586,551 shares of our common stock issuable upon the exercise of outstanding warrants with a weighted average exercise price of $5.20 per share as of September 30, 2018; |
| ● | 42,917 shares of our common stock issuable upon the exercise of warrants issued subsequent to September 30, 2018 with a weighted average exercise price of $7.48 per share; and |
| ● | any automatic increases in the number of shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan. |
| 16 |
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after this offering.
Our historical net tangible book value is the amount of our total tangible assets less our total liabilities. Net historical tangible book value per share is our historical net tangible book value divided by the number of shares of common stock outstanding as of September 30, 2018. Our historical net tangible book value as of September 30, 2018 was $9.4 million, or $0.60 per share of common stock.
As adjusted net book value is our net tangible book value, plus the effect of the sale of shares of our common stock in this offering at the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Our as adjusted net book value as of September 30, 2018 would have been approximately $24.01 million, or $1.16 per share. This amount represents an immediate increase in as adjusted net tangible book value of $0.56 per share to our existing stockholders, and an immediate dilution of $2.07 per share to new investors participating in this offering. Dilution per share to new investors is determined by subtracting as adjusted net tangible book value per share after this offering from the public offering price per share paid by new investors.
The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share | $ | 3.23 | ||||||
| Historical net tangible book value per share as of September 30, 2018 | $ | 0.60 | ||||||
| Increase in net tangible book value per share as of September 30, 2018, attributable to new investors | $ | 0.56 | ||||||
| As adjusted net tangible book value per share, after giving effect to this offering | 1.16 | |||||||
| Dilution of as adjusted net tangible book value per share to new investors | $ | 2.07 |
Each $1.00 increase (decrease) in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase (decrease) the as adjusted net tangible book value, by $0.22 per share and would decrease (increase) the dilution to new investors by $0.22 per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated expenses payable by us. Each increase of one million shares offered by us would increase the as adjusted net tangible book value by $0.08 per share and decrease the dilution to new investors by $0.08 per share, assuming the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) remains the same and after deducting underwriting discounts and commissions and estimated expenses payable by us. Similarly, each decrease of one million shares offered by us would decrease the as adjusted net tangible book value by $0.09 per share and increase the dilution to new investors by $0.09 per share, assuming the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) remains the same and after deducting underwriting discounts and commissions and estimated expenses payable by us. Each one million share increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase the as adjusted net tangible book value by $0.34 per share and decrease the dilution to new investors by $0.34 per share, after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, each one million share decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would decrease the as adjusted net tangible book value by $0.28 per share and increase the dilution to new investors by $0.28 per share, after deducting underwriting discounts and commissions and any estimated offering expenses payable by us.
| 17 |
If the underwriters exercise their over-allotment option in full, the as adjusted net tangible book value per share after giving effect to this offering would be $1.23 per share, which amount represents an immediate increase in the as adjusted net tangible book value of $0.62 per share of our common stock to existing stockholders and an immediate dilution in net tangible book value of $2.00 per share of our common stock to new investors purchasing shares of common stock in this offering.
If any shares are issued upon the exercise of outstanding options or warrants, you will experience further dilution. The above discussion and table are based on 15,690,151 shares of our common stock outstanding as of September 30, 2018 and excludes:
| ● | 1,964,454 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan as of September 30, 2018, with a weighted average exercise price of $4.48 per share, and 560,000 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan after September 30, 2018, with a weighted average exercise price of $3.78 per share; |
| ● | 653,272 additional shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan as of September 30, 2018, reduced by 560,000 shares of our common stock issuable upon the exercise of outstanding stock options issued under our equity incentive plan after September 30, 2018 as described in the bullet above; | |
| ● | 2,586,551 shares of our common stock issuable upon the exercise of outstanding warrants with a weighted average exercise price of $5.20 per share as of September 30, 2018; | |
| ● | 42,917 shares of our common stock issuable upon the exercise of warrants issued subsequent to September 30, 2018 with a weighted average exercise price of $7.48 per share; and | |
| ● | any automatic increases in the number of shares of our common stock reserved for future issuance under our 2016 Equity Incentive Plan. |
| 18 |
The following table sets forth information regarding the beneficial ownership of our common stock as of September 30, 2018 by:
| ● | each of our stockholders who is known by us to beneficially own 5% or more of our common stock; | |
| ● | each of our named executive officers; | |
| ● | each of our directors; and | |
| ● | all of our directors and current officers as a group. |
Beneficial ownership is determined based on the rules and regulations of the SEC. A person has beneficial ownership of shares if such individual has the power to vote and/or dispose of shares. This power may be sole or shared and direct or indirect. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of our common stock that are subject to options or warrants held by that person and exercisable as of, or within 60 days of, September 30, 2018 are counted as outstanding. These shares, however, are not counted as outstanding for the purposes of computing the percentage ownership of any other person(s). Except as may be indicated in the footnotes to this table and pursuant to applicable community property laws, each person named in the table has sole voting and dispositive power with respect to the shares of our common stock set forth opposite that person’s name. Unless indicated below, the address of each individual listed below is c/o Motus GI Holdings, Inc., 1301 East Broward Boulevard, 3rd Floor, Ft. Lauderdale, FL 33301.
Applicable percentage ownership in the following table is based on 15,690,151 shares of our common stock outstanding as of September 30, 2018 and also lists applicable percentage ownership based on shares of our common stock assumed to be outstanding after completion of the offering, assuming no exercise by the underwriters of their option to purchase additional shares of our common stock.
| 19 |
Beneficial ownership representing less than 1% is denoted with an asterisk (*).
| Name of Beneficial Owner |
Number of Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
||||||||||||||
| Before Offering | After Offering |
|||||||||||||||
| Officers and Directors | ||||||||||||||||
| Timothy P. Moran (1) | 0 | * | * | |||||||||||||
| Mark Pomeranz (2) | 448,491 | 2.78% | 2.12 | % | ||||||||||||
| David Hochman (3)(4)(6)(7)(8)(9) | 3,976,361 | 24.58% | 18.78 | % | ||||||||||||
| Darren Sherman (4)(5)(6)(7)(8)(9) | 3,864,861 | 23.94% | 18.28 | % | ||||||||||||
| Gary Jacobs (10)(11) | 925,498 | 5.83% | 4.43 | % | ||||||||||||
| Samuel Nussbaum (12) | 35,000 | * | * | |||||||||||||
| Shervin Korangy (13) | 52,500 | * | * | |||||||||||||
| Andrew Taylor (14) | 81,999 | * | * | |||||||||||||
| Gary Pruden (15) | 50,000 | * | * | |||||||||||||
| Directors and Officers as a Group (9 persons) | 5,629,149 | 33.12% | 25.59 | % | ||||||||||||
| 5% Stockholders | ||||||||||||||||
| Ascent Biomedical Ventures II, L.P. (16) | 907,364 | 5.67% | 4.32 | % | ||||||||||||
| ABV, LLC (16)(17) | 1,607,163 | 9.99% | 7.62 | % | ||||||||||||
| Geoffrey W. Smith (8) (16)(17) | 3,607,163 | 22.41% | 17.10 | % | ||||||||||||
| Orchestra MOTUS Co-Investment Partners, LLC (7) | 1,345,101 | 8.47% | 6.44 | % | ||||||||||||
| Orchestra Medical Ventures, LLC (7) | 1,345,101 | 8.47% | 6.44 | % | ||||||||||||
| Orchestra BioMed, Inc. (8) | 2,000,000 | 12.75% | 9.67 | % | ||||||||||||
| Jacobs Investment Company LLC (11) | 870,148 | 5.50% | 4.18 | % | ||||||||||||
| Perceptive Life Sciences Master Fund Ltd. (18) | 3,046,596 | 19.12% | 14.55 | % | ||||||||||||
| Perceptive Advisors LLC (17) | 3,046,596 | 19.12% | 14.55 | % | ||||||||||||
| 1. | Does not include 495,000 shares of our common stock issuable upon the exercise of stock options which were awarded to Mr. Moran subsequent to September 30, 2018. Does not include (a) 15,000 shares of our common stock purchased by Mr. Moran in the open market subsequent to September 30, 2018 and (b) 165,000 shares of our common stock pursuant to a restricted stock unit award we anticipate issuing to Mr. Moran pursuant to his employment agreement. |
| 20 |
| 2. | Includes 397,151 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2018. Does not include 181,200 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. |
| 3. | Includes (i) 87,500 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2018, (ii) 300 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018, and (iii) 80,000 shares of common stock held by a family trust of which Mr. Hochman is a co-trustee and sole beneficiary. Does not include 87,500 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. Does not include 15,000 shares of our common stock purchased by Mr. Hochman in the open market subsequent to September 30, 2018. |
| 4. | Includes (i) 50,000 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2018, and (ii) 300 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018. Does not include 50,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. |
| 5. | Includes 215,818 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018, held by Orchestra Medical Ventures II, L.P. The managing members of Orchestra Medical Ventures II GP, LLC, David Hochman and Darren Sherman, exercise sole dispositive and voting power over the shares owned by Orchestra Medical Ventures II, L.P. |
| 6. | Includes 83,352 shares of common stock held by Orchestra Medical Ventures II Reserve, L.P. The managing members of Orchestra Medical Ventures II GP, LLC, David Hochman and Darren Sherman, exercise sole dispositive and voting power over the shares owned by Orchestra Medical Ventures II Reserve, L.P. |
| 7. | Includes 185,133 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018, held by Orchestra MOTUS Co-Investment Partners, LLC. The managing partners of Orchestra Medical Ventures, LLC, David Hochman and Darren Sherman, exercise sole dispositive and voting power over the shares owned by Orchestra MOTUS Co-Investment Partners, LLC. |
| 21 |
| 8. | David Hochman, Darren Sherman and Geoffrey W. Smith are the directors of Orchestra BioMed, Inc. (“OBIO”). David Hochman, Darren Sherman, and Geoffrey W. Smith jointly exercise dispositive and voting power over the shares of common stock owned by OBIO. The principal address for OBIO is 150 Union Square Drive, New Hope, PA 18938. |
| 9. | Includes 51,498 shares of common stock held by Accelerated Technologies, Inc. David Hochman and Darren Sherman share dispositive and voting power over the shares owned by Accelerated Technologies, Inc. |
| 10. | Includes 46,250 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2018. Does not include 46,250 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. |
| 11. | Includes 141,292 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018. Does not include 7,100 shares of our common stock purchased by Jacobs Investment Company LLC in the open market subsequent to September 30, 2018. The managing member of Jacobs Investment Company LLC, Gary Jacobs, exercises sole dispositive and voting power over the shares owned by Jacobs Investment Company LLC. |
| 12. | Includes 25,000 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2018. Does not include 25,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. |
| 13. | Includes 32,500 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2018. Does not include 32,500 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. |
| 14. | Includes 79,999 shares of our common stock issuable upon the exercise of stock options that are exercisable within sixty days of September 30, 2018. Does not include 160,001 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. |
| 15. | Does not include 50,000 shares of our common stock issuable upon the exercise of stock options that are not exercisable within sixty days of September 30, 2018. |
| 16. | Includes 315,883 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018, held by Ascent Biomedical Ventures II, L.P. The managing members of ABV, LLC, Geoffrey W. Smith and Steve Hochberg, exercise sole dispositive and voting power over the shares owned by Ascent Biomedical Ventures II, L.P. The principal address for the entities affiliated with ABV, LLC is 60 East 42nd Street, New York, NY 10165. |
| 17. | Includes 88,558 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018, held by Ascent Biomedical Ventures Synecor, L.P. The managing members of ABV, LLC, Geoffrey W. Smith and Steve Hochberg, exercise sole dispositive and voting power over the shares owned by Ascent Biomedical Ventures Synecor, L.P. The principal address for the entities affiliated with ABV, LLC is 60 East 42nd Street, New York, NY 10165. |
| 18. | Includes 246,055 shares of our common stock issuable upon exercise of warrants that are exercisable within sixty days of September 30, 2018, held by Perceptive Life Sciences Master Fund Ltd. The managing member of Perceptive Advisors LLC, Mr. Joseph Edelman, exercises sole dispositive and voting power over the shares owned by Perceptive Life Sciences Master Fund Ltd. The principal address for the entities affiliated with Perceptive Advisors LLC is 51 Astor Place, 10th Floor New York, NY 10003. |
| 22 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements for our named executive officers and directors, we describe below each transaction or series of similar transactions, since January 1, 2015, to which we were a party or will be a party, in which:
| ● | the amounts involved exceeded or will exceed the lesser of (i) $120,000 or (ii) 1% of the average total assets of the Company at year end for the last two completed fiscal years; and | |
| ● | any of our directors, executive officers, promoters or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
Compensation arrangements for our named executive officers and directors are described in the section titled “Executive Compensation” in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference in this prospectus.
Voting Agreement
Effective on December 1, 2016, Opco, and the holders of all issued and outstanding shares of capital stock of Opco (the “Opco Stockholders”), entered into a share exchange agreement (the “Share Exchange Agreement”) with us. Pursuant to the terms of the Share Exchange Agreement, as a condition of and contemporaneously with the initial closing (the “Initial Closing”) of the private placement offering of units we conducted from December 2016 to February 2017 (the “2017 Private Placement”), the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco (the “Share Exchange Transaction”) and Opco became our direct wholly-owned subsidiary. In connection with the Initial Closing of the 2017 Private Placement, the Opco Stockholders and our stockholders prior to the Share Exchange Transaction and the 2017 Private Placement (the “Formation Stockholders”), including Jacobs Investments Company LLC, an entity in which our director Gary Jacobs is the beneficial owner of the shares held by such entity, and Accelerated Technologies, Inc., Orchestra Medical Ventures II, L.P., Orchestra MOTUS Co-Investment Partners, LLC, and Orchestra Medical Ventures II GP, LLC, entities in which our directors David Hochman and Darren Sherman share beneficial ownership of the shares held by such entities, entered into a Voting Agreement (the “Voting Agreement”). Pursuant to the terms of the Voting Agreement, (i) the Opco Stockholders held the right to nominate four (4) members to our board of directors (the “Opco Stockholders’ Nominees”), (ii) the Formation Stockholders were to vote in favor of the election of the Opco Stockholders’ Nominees, (iii) the Formation Stockholders were to vote in favor of the election of the Placement Agent Nominee (defined below) to our board of directors, (iv) the Opco Stockholders were to vote in favor of the election of the Placement Agent Nominee and (v) the Opco Stockholders and the Formation Stockholders were permitted to vote in favor of up to two additional independent candidates to the board of directors acceptable to the Placement Agent Nominee and the Opco Stockholders’ Nominees. The Voting Agreement expired upon the closing of our IPO and is of no further force or effect.
Board of Directors Composition
Pursuant to the Voting Agreement described above, the placement agent for the 2017 Private Placement (the “Placement Agent”) held a right to appoint one member of our board of directors for a two-year term from the Initial Closing of the 2017 Private Placement (the “Placement Agent Nominee”). However, the Voting Agreement expired upon the closing of our IPO and is of no further force or effect.
Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation
Pursuant to the terms of a convertible note agreement (the “CNA”), as amended, Opco issued convertible notes (the “Convertible Notes”) to certain investors (the “Convertible Holders”) in a series of closings from June 2015 through November 2016. From August 2015 through November 2016, Orchestra Medical Ventures II, L.P., an affiliate of Orchestra Medical Ventures II GP, LLC (an entity in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Members) purchased Convertible Notes in an aggregate principal amount of $1,649,062. On December 22, 2016, Orchestra Medical Ventures II, L.P. exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 299,244 shares of our common stock and (ii) 99,748 shares of our Series A Convertible Preferred Stock. In addition, Orchestra Medical Ventures II, L.P. received five (5) year warrants to purchase an aggregate of 108,838 shares of our common stock at an exercise price of $5.00 per share in an amount equal to thirty-three percent (33%) of the principal amount of such Convertible Note divided by $5.00 (the “Exchange Warrants,” see “Description of Securities – Warrants – Exchange Warrants” for a description of the Exchange Warrants.). Orchestra Medical Ventures II, L.P. and Orchestra Medical Ventures II GP, LLC are no longer beneficial owners of more than five percent of our common stock.
| 23 |
In addition, from August 2015 through November 2016, Orchestra MOTUS Co-Investment Partners, LLC, an affiliate of Orchestra Medical Ventures, LLC (an investment firm in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Partners) purchased Convertible Notes in an aggregate principal amount of $1,047,511. On December 22, 2016, Orchestra MOTUS Co-Investment Partners, LLC exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 195,114 shares of our common stock and (ii) 65,038 shares of our Series A Convertible Preferred Stock. In addition, Orchestra MOTUS Co-Investment Partners, LLC received Exchange Warrants to purchase an aggregate of 69,136 shares of our common stock.
From June 2015 through November 2016, Jacobs Investment Company, LLC, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, purchased Convertible Notes in an aggregate principal amount of $1,044,032. On December 22, 2016, Jacobs Investment Company, LLC exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 189,865 shares of our common stock and (ii) 63,289 shares of our Series A Convertible Preferred Stock. In addition, Jacobs Investment Company, LLC received Exchange Warrants to purchase an aggregate of 68,906 shares of our common stock.
From June 2015 through August 2016, Ascent Biomedical Ventures II, L.P., and from July 2015 through October 2015, Ascent Biomedical Ventures Synecor, L.P. (collectively, the “Ascent Entities”) purchased Convertible Notes in an aggregate principal amount of $2,790,412 (the “Ascent Convertible Notes”). ABV, LLC, a beneficial owner of more than five percent of our common stock, is the beneficial owner of the securities held by the Ascent Entities. On December 22, 2016, the Ascent Entities exchanged the Ascent Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 511,776 shares of our common stock and (ii) 170,593 shares of our Series A Convertible Preferred Stock. In addition, the Ascent Entities received Exchange Warrants to purchase an aggregate of 184,167 shares of our common stock.
On October 27, 2016, Perceptive Life Sciences Master Fund Ltd., and on October 28, 2016, Titan Perc, Ltd. (collectively, the “Perceptive Entities”) purchased Convertible Notes in an aggregate principal amount of $1,000,000 (the “Perceptive Convertible Notes”). Perceptive Advisors LLC, a beneficial owner of more than five percent of our common stock, is the beneficial owner of the securities held by the Perceptive Entities. On December 22, 2016, the Perceptive Entities exchanged the Perceptive Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 169,155 shares of our common stock and (ii) 56,386 shares of our Series A Convertible Preferred Stock. In addition, the Perceptive Entities received Exchange Warrants to purchase an aggregate of 66,000 shares of our common stock. Additionally, the Perceptive entities purchased an aggregate of 800,000 units in our 2017 Private Placement, at a purchase price of $5.00 per unit, comprised of an aggregate of (i) 600,000 shares of our common stock and (ii) 200,000 shares of our Series A Convertible Preferred Stock.
| 24 |
From January 2016 through November 2016 The Peierls Bypass Trust, UD E.F. Peierls for Brian E. Peierls, UD E.F. Peierls for E. Jeffrey Peierls, UD E.S. Peierls for E.F. Peierls et al, UD J.N. Peierls for Brian Eliot Peierls, UD J.N. Peierls for E. Jeffrey Peierls, UW E.S. Peierls for Brian E. Peierls - Accumulation, UW E.S. Peierls for E. Jeffrey Peierls - Accumulation, UW J.N. Peierls for Brian E. Peierls, UW J.N. Peierls for E. Jeffrey Peierls (collectively, the “Peierls Trusts”) and The Peierls Foundation, Inc. and UD Ethel F. Peierls Charitable Lead Trust (collectively, the “Peierls Entities”) purchased Convertible Notes in an aggregate principal amount of $1,448,000 (the “Peierls Convertible Notes”). E. Jeffrey Peierls, a beneficial owner of more than five percent of our common stock prior to the IPO, is the beneficial owner of the securities held by the Peierls Trusts and the Peierls Entities. On December 22, 2016, the Peierls Trusts and the Peierls Entities exchanged the Peierls Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 257,385 shares of our common stock and (ii) 85,801 shares of our Series A Convertible Preferred Stock. In addition, the Peierls Trusts and the Peierls Entities received Exchange Warrants to purchase an aggregate of 95,568 shares of our common stock. Additionally, the Peierls Trusts and the Peierls Entities purchased an aggregate of 100,000 units in our 2017 Private Placement, at a purchase price of $5.00 per unit, comprised of an aggregate of (i) 75,000 shares of our common stock and (ii) 25,000 shares of our Series A Convertible Preferred Stock. As a result of the IPO, E. Jeffrey Peierls is no longer a beneficial owner of more than five percent of our common stock.
On October 27, 2016, AKS Family Partners, LP, a beneficial owner of more than five percent of our common stock prior to the Initial Closing, purchased a Convertible Note in an aggregate principal amount of $250,000. On December 22, 2016, AKS Family Partners, LP exchanged its Convertible Note, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 42,290 shares of our common stock and (ii) 14,097 shares of our Series A Convertible Preferred Stock. In addition, AKS Family Partners, LP received Exchange Warrants to purchase an aggregate of 16,500 shares of our common stock. As a result of the Initial Closing, AKS Family Partners, LP is no longer a beneficial owner of more than five percent of our common stock.
Share Exchange Transaction
Effective on December 1, 2016, Opco, and the Opco Stockholders, entered into the Share Exchange Agreement with us. Pursuant to the terms of the Share Exchange Agreement, the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco and Opco became our wholly owned subsidiary.
At the closing of the Share Exchange Transaction, on December 22, 2016, (i) Orchestra Medical Ventures II, L.P., an affiliate of Orchestra Medical Ventures II GP, LLC (an entity in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Members) and Orchestra MOTUS Co-Investment Partners, LLC, an affiliate of Orchestra Medical Ventures, LLC (an investment firm in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Partners) sold to us, and we acquired, all of the capital stock of Opco held by Orchestra Medical Ventures II, L.P. and Orchestra MOTUS Co-Investment Partners, LLC in exchange for 670,800 and 899,816 shares of our common stock, respectively, (ii) Jacobs Investment Company, LLC, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, sold to us, and we acquired, all of the capital stock of Opco held by Jacobs Investment Company, LLC in exchange for 474,802 shares of our common stock, and (iii) ABV, LLC, a beneficial owner of more than five percent of our common stock, through the Ascent Entities, sold to us, and we acquired, all of the capital stock of Opco held by the Ascent Entities in exchange for an aggregate of 1,520,353 shares of our common stock.
Ten Percent Warrants - Related Party Participation
In connection with the 2017 Private Placement, we entered into a registration rights agreement (the “Registration Rights Agreement”) with the 2017 Private Placement investors. Upon the completion of our IPO in February 2018, we issued warrants to certain of our Series A Convertible Preferred Stock holders, pursuant to an amendment to the Registration Rights Agreement and an amendment to the Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock, to purchase an aggregate of 1,095,682 shares of our common stock (the “Ten Percent Warrants”), including (i) Ten Percent Warrants to purchase 300 shares of our common stock to David Hochman, the Chairman of our Board, (ii) Ten Percent Warrants to purchase 300 shares of our common stock to Darren Sherman, one of our directors, (iii) Ten Percent Warrants to purchase an aggregate of 220,274 shares of our common stock to the Ascent Entities, beneficial owners of more than five percent of our common stock, (iv) Ten Percent Warrants to purchase 106,980 shares of our common stock to Orchestra Medical Ventures II, L.P., a former beneficial owner of more than five percent of our common stock, (v) Ten Percent Warrants to purchase 115,997 shares of our common stock to Orchestra MOTUS Co-Investment Partners, LLC, a beneficial owner of more than five percent of our common stock, (vi) Ten Percent Warrants to purchase 72,386 shares of our common stock to Jacobs Investment Company, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, (vii) Ten Percent Warrants to purchase 180,055 shares of our common stock to Perceptive Life Sciences Master Fund Ltd., a beneficial owner of more than five percent of our common stock, (viii) Ten Percent Warrants to purchase an aggregate of 57,035 shares of our common stock to E. Jeffrey Peierls, including the Peierls Trusts and the Peierls Entities, a former beneficial owner of more than five percent of our common stock. See “Description of Securities – Warrants – Ten Percent Warrants” for a description of the Ten Percent Warrants.
| 25 |
Sales and Marketing Services Arrangement with FreeHold Surgical, Inc.
In August, 2017, we began paying a monthly fee to FreeHold Surgical, Inc., or FreeHold, an entity in which David Hochman, the Chairman of our Board, serves as a Director, and Darren Sherman, one of our Directors, serves as a Director and President. Pursuant to the fee arrangement, we paid FreeHold a monthly amount of approximately $25,000 as all-in compensation for sales and marketing services performed for us, on a part time basis, by two FreeHold sales representatives (the “FreeHold Services”), through June 2018. Effective July 2018, pursuant to an amendment to the fee arrangement, we pay FreeHold a monthly amount of approximately $8,333 as all-in compensation for the FreeHold Services. We have agreed with FreeHold to terminate the fee arrangement effective as of November 30, 2018. As of November 30, 2018 our payment obligations to FreeHold pursuant to the fee arrangement have terminated and all FreeHold Services obligations by FreeHold have ceased.
Participation in the IPO
In addition to the shares issued pursuant to the directed share program described below, all of our directors and executive officers, and certain of our existing stockholders who held greater than 5% of our common stock, including stockholders affiliated with certain of our directors, purchased an aggregate of 1,435,000 shares of our common stock in our IPO, completed February 2018, at the public offering price of $5.00 per share, including (i) Perceptive Life Sciences Master Fund Ltd., a greater than 5% shareholder, which purchased 1,000,000 shares, (ii) Orchestra Medical Ventures II, L.P., a greater than 5% shareholder, which purchased 40,000 shares, (iii) Gary Pruden, one of our directors, who purchased 50,000 shares, (iv) David Hochman, the chairman of our board, who purchased 75,000 shares, (v) Shervin Korangy, one of our directors, who purchased 20,000 shares, (vi) Mark Pomeranz, our president, who purchased 8,000 shares, (vii) Samuel Nussbaum, one of our directors, who purchased 10,000 shares, (viii) Darren Sherman, one of our directors, who purchased 5,000 shares and (ix) Andrew Taylor, our chief financial officer, who purchased 2,000 shares.
Directed Share Program
At our request, the underwriters sold at the IPO price 175,000 shares of our common stock, or five percent (5%) of the shares offered in our IPO, to our employees and other persons associated with us, including Gary Jacobs, one of our Directors, who purchased 5,000 shares of our common stock at the IPO price. The directed share program was arranged through the representative of the underwriters in the IPO.
Indemnification Agreements
We have entered into indemnification agreements with all of our directors and named executive officers. These agreements require us to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. We also intend to enter into indemnification agreements with our future directors and executive officers.
| 26 |
Policies and Procedures for Related Party Transactions
Our board of directors has adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock, any members of the immediate family of any of the foregoing persons and any firms, corporations or other entities in which any of the foregoing persons is employed or is a partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest (collectively “related parties”), are not permitted to enter into a transaction with us without the prior consent of our board of directors acting through the Audit Committee or, in certain circumstances, the chairman of the Audit Committee. Any request for us to enter into a transaction with a related party, in which the amount involved exceeds $100,000 and such related party would have a direct or indirect interest must first be presented to our Audit Committee, or in certain circumstances the chairman of our Audit Committee, for review, consideration and approval. In approving or rejecting any such proposal, our Audit Committee, or the chairman of our Audit Committee, is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, the extent of the benefits to us, the availability of other sources of comparable products or services and the extent of the related party’s interest in the transaction.
Director Independence
Our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that Mr. Hochman, Mr. Sherman, Mr. Jacobs, Dr. Nussbaum, Mr. Korangy and Mr. Pruden do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the Rules of the Nasdaq Market and the SEC.
| 27 |
Our current certificate of incorporation, as amended, authorizes us to issue:
| ● | 50,000,000 shares of common stock, par value $0.0001 per share; and | |
| ● | 10,000,000 shares of preferred stock, par value $0.0001 per share. |
As of September 30, 2018 there were 15,690,151 shares of our common stock outstanding, held of record by approximately 207 stockholders of record, and no shares of preferred stock outstanding.
The following statements are summaries only of provisions of our authorized capital stock and are qualified in their entirety by our certificate of incorporation, as amended. You should review these documents for a description of the rights, restrictions and obligations relating to our capital stock. Copies of our certificate of incorporation may be obtained from the Company upon written request.
Common Stock
Voting. The holders of our common stock are entitled to one vote for each share held of record on all matters on which the holders are entitled to vote (or consent to). When a quorum is present at any meeting of stockholders, any matter before any such meeting (other than an election of a director or directors) shall be decided by a majority of the votes properly cast on such matter, except where a different vote is required by law, by the rules or regulations of any stock exchange applicable to us, or pursuant to any regulation applicable to us or our securities, in which case, such different vote shall apply. A majority in voting power of the shares entitled to vote at the meeting, present in person or represented by proxy, shall constitute a quorum at any meeting of stockholders.
Dividends. The holders of our common stock are entitled to receive, ratably, dividends only if, when and as declared by our board of directors out of funds legally available therefor and after provision is made for each class of capital stock having preference over our common stock.
Liquidation Rights. In the event of our liquidation, dissolution or winding-up, the holders of our common stock are entitled to share, ratably, in all assets remaining available for distribution after payment of all liabilities and after provision is made for each class of capital stock having preference over our common stock.
Conversion Rights. The holders of our common stock have no conversion rights.
Preemptive and Similar Rights. The holders of our common stock have no preemptive or similar rights.
Redemption/Put Rights. There are no redemption or sinking fund provisions applicable to our common stock. All of the outstanding shares of our common stock are fully-paid and non-assessable.
Transfer Restrictions. Shares of our common stock are subject to transfer restrictions. Holders of our common stock may not transfer their securities unless (a) a registration statement is in effect under the Securities Act covering the proposed transfer and such transfer is made in accordance with such registration statement or (b) the securities are transferred in a transaction exempt from the registration requirements of the Securities Act and any related requirements imposed by applicable state securities laws. In the case of any transfer permitted under clause (b), the holder must notify us in writing of the proposed transfer and furnish us with an opinion of counsel, reasonably satisfactory to us, that the transfer will not require registration under the Securities Act or any applicable state securities laws. Each certificate representing a security contains a legend referring to this restriction on transfer and any legends required by state securities laws.
Preferred Stock
We are authorized to issue up to 10,000,000 shares of “blank check” preferred stock, par value $0.0001 per share, with such designations, rights, and preferences as may be determined from time to time by our board of directors. Accordingly, our board of directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock could have the effect of restricting dividends on our common stock, diluting the voting power of our common stock, impairing the liquidation rights of our common stock, or delaying or preventing a change in control of our company. We currently have no plans to issue any shares of preferred stock.
| 28 |
Royalty Payment Rights Certificates
We have issued Royalty Payment Rights Certificates (the “Royalty Payment Rights Certificates”) entitling the holders of such certificates to the following royalty payment rights (the “Royalty Payment Rights”):
Royalties. If and when we generate sales of the current and potential future versions of the Pure-Vu System, including disposables, parts, and services, or if we receive any proceeds from the licensing of the current and potential future versions of the Pure-Vu System, then we will pay to the holders of our Royalty Payment Rights Certificates (the “Holders”) with the allocation of such Royalty Payment Rights between Holders determined as set forth below under “Allocation of Royalty Payments”, a royalty (the “Royalty Amount”) equal to, in the aggregate, in royalty payments in any calendar year for all products:
| The Company Commercializes Product Directly |
The Rights to Commercialize the Product is Sublicensed by the Company to a third-party | |
| 3% of any Net Sales* | 5% of any Licensing Proceeds** |
* Notwithstanding the foregoing, with respect to Net Sales based Royalty Amounts, (a) no Net Sales based Royalty Amount shall begin to accrue or become payable until we have first generated, in the aggregate, since inception, Net Sales equal to $20 million (the “Initial Net Sales Milestone”), and royalties shall only be computed on, and due with respect to, Net Sales generated in excess of the Initial Net Sales Milestone, and (b) the total Net Sales based Royalty Amount due and payable in any calendar year shall be subject to a cap per calendar year of $30 million. “Net Sales” is defined in the Royalty Payment Rights Certificates.
** Notwithstanding the foregoing, with respect to Licensing Proceeds based Royalty Amounts, (a) no Licensing Proceeds based Royalty Amount shall begin to accrue or become payable until we have first generated, in the aggregate, since inception, Licensing Proceeds equal to $3.5 million (the “Initial Licensing Proceeds Milestone”), and royalties shall only be computed on, and due with respect to, Licensing Proceeds in excess of the Initial Licensing Proceeds Milestone and (b) the total Licensing Proceeds based Royalty Amount due and payable in any calendar year shall be subject to a cap per calendar year of $30 million. “Licensing Proceeds” is defined in the Royalty Payment Rights Certificates.
We currently have not licensed any of our products to any third-party and are not in negotiations with respect to any such license. There is no guarantee that we will ever generate sales of, or Licensing Proceeds from, our products. The Holders may never receive any royalty payments and these Royalty Payment Rights may expire worthless.
Timing of Royalty Payments. With respect to products that we commercialize directly, royalty payments, if any, will be paid annually 15 business days after the issuance of our audited financial statements for the prior year in which such Net Sales were generated; for the avoidance of doubt, such payments shall begin only upon achievement of the Initial Net Sales Milestone without regard to whether the Initial Licensing Proceeds Milestone has been met. With respect to products that we sublicense or otherwise dispose of to a third-party, royalty payments, if any, will be paid 10 business days after the end of the applicable quarter in which such Licensing Proceeds are received by us; for the avoidance of doubt, such payments shall begin only upon achievement of the Initial Licensing Proceeds Milestone without regard to whether the Initial Net Sales Milestone has been met.
The royalty will be payable up to the later of (i) the latest expiration date of our patents issued as of December 22, 2016, or (ii) the latest expiration date of any pending patents as of December 22, 2016 that have since been issued or may be issued in the future (which is currently November 2034). Following the expiration of all such patents, the Holders of the Royalty Payment Rights will no longer be entitled to any further royalties for any period following the latest to occur of such patent expiration.
| 29 |
Allocation of Royalty Payment. Once the aggregate Royalty Payment Amount is calculated based on the criteria set forth above under “Royalties,” that amount will be allocated to the holders of the Royalty Payment Rights Certificates by multiplying the aggregate Royalty Payment Amount by the percentage set forth in each Holder’s Royalty Payment Rights Certificate.
Separability. The Royalty Payment Rights Certificates may be transferred, subject to the availability of an exemption from registration under applicable state and federal securities laws.
Unsecured Obligations. The Royalty Payment Rights are unsecured obligations of ours.
Placement Agent Royalty Payment Rights
In connection with completion of the 2017 Private Placement, we issued the Placement Agent royalty payment rights certificates (the “Placement Agent Royalty Payment Rights Certificates”) to receive, in the aggregate, 10% of the amount of payments paid to the holders of the Royalty Payment Rights Certificates. The Placement Agent Royalty Payment Rights Certificates are on substantially similar terms as the Royalty Payment Rights Certificates.
Warrants
Exchange Warrants. In connection with the Share Exchange Transaction and the CNA, on December 22, 2016, we issued warrants to each former Convertible Holder to purchase an aggregate 907,237 shares of our common stock (the “Exchange Warrants”). The Exchange Warrants are exercisable for our common stock at an exercise price equal to $5.00 per share (the “Exercise Price”). The Exchange Warrants are exercisable immediately upon issuance and have a five year term, and provide for cashless exercise. The Exchange Warrants may be exercised at any time in whole or in part at the applicable exercise price until expiration of the Exchange Warrants. No fractional shares will be issued upon the exercise of the Exchange Warrants.
Placement Agent Warrants. In connection with completion of the 2017 Private Placement, on February 24, 2017, we issued the placement agent, and its designees, warrants to purchase 403,632 shares of our common stock at an exercise price of $5.00 as partial compensation (the “Placement Agent Warrants”). These warrants have a five year term and provide cashless exercise.
Ten Percent Warrants. In connection with our initial public offering, on February 16, 2018, we issued warrants to purchase 1,095,682 shares of our common stock (the “Ten Percent Warrants”). The Ten Percent Warrants are exercisable for our common stock at an exercise price of $5.00 per share. The Ten Percent Warrants have a five year term, and provide for cashless exercise. No fractional shares will be issued upon the exercise of the Ten Percent Warrants.
Service Provider Warrants. We have additional warrants outstanding, or which are issuable by us or pursuant to contractual obligations, to purchase an aggregate of 257,917 shares of our common stock with a weighted average exercise price of $8.03 per share. We have an additional warrant which is issuable by us pursuant to a contractual obligation to purchase an estimated 6,380 shares of our common stock, subject to adjustment, with an estimated weighted average exercise price of $3.23 per share, subject to adjustment, expected to be issued October 2019.
| 30 |
Registration Rights
Service Provider Warrants. Certain of our other warrants are entitled to piggyback registration rights with respect to the shares issuable upon exercise of such warrants. If we register any of our securities either for our own account or for the account of other security holders, such holders are entitled to include their shares in the registration. In the event we register securities in connection with an underwritten offering, the underwriters will have the right to limit the number of shares included in such offering. The holder of all of our outstanding warrants entitled to piggyback registration rights has executed a notice and waiver of piggyback registration rights, waiving any such piggyback registration rights in connection with this offering.
Lock-Up Agreements
Each of our directors and officers and the holders of substantially all of five percent (5%) or more of our common stock as of the time of the Initial Closing of the 2017 Private Placement have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, which includes any shares of our common stock acquired upon the exercise of any warrants acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending February 13, 2019, without our prior written consent and the prior written consent of the Placement Agent.
In connection with the formation of Motus GI Holdings, Inc. in September 2016, certain affiliates of the Placement Agent and certain other parties not affiliated with us or the Placement Agent subscribed for an aggregate of 1,650,000 shares of our common stock (the “Formation Shares”), for which they paid an aggregate of $82,500 ($0.05 per share). Each of the holders of the Formation Shares have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending February 13, 2019, without our prior written consent and the prior written consent of the Placement Agent.
In March 2017, we signed a consulting service agreement by which we granted the service provider 90,000 shares of our common stock for past services provided. The consultant has agreed that they will not (a) offer, pledge, sell, contract to sell, grant any option or contract to purchase, purchase any option or contract to sell, or otherwise dispose of, directly or indirectly, or (b) transfer title to 45,000 of the subject shares, for a period beginning the effective date of the consulting agreement and ending February 13, 2019, without our prior written consent.
In March 2018, we granted a service provider 15,000 shares of our common stock for services pursuant to a consulting agreement. The consultant has agreed that they will not (a) offer, pledge, sell, contract to sell, grant any option or contract to purchase, purchase any option or contract to sell, or otherwise dispose of, directly or indirectly, or (b) transfer title to the 15,000 shares for a period beginning the effective date of the consulting agreement and ending February 13, 2019, without our prior written consent.
Our officers and directors have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock or securities convertible into or exchangeable for shares of common stock for a period through the date 90 days after the date of this prospectus, except with the prior written consent of Piper Jaffray & Co., as the representative of the underwriters. The representative may in their sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the 90-day period. When determining whether or not to release shares from the lock-up agreements, the representative may consider, among other factors, the stockholder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
| 31 |
Transfer Agent and Registrar
Continental Stock Transfer and Trust, located at 1 State Street 30th Floor, New York, NY 10004, is the transfer agent and registrar for our common stock and preferred stock.
Quotation of Securities
Shares of our common stock are listed on the Nasdaq Capital Market under the trading symbol “MOTS”.
Anti-Takeover Effect of Delaware Law, Certain Charter and Bylaw Provisions
Our certificate of incorporation and bylaws contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change of control of our company. These provisions are as follows:
| ● | they provide that special meetings of stockholders may be called by the board of directors or at the request in writing by stockholders of record owning at least twenty (20%) percent of the issued and outstanding voting shares of our common stock; | |
| ● | they do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in our board of directors; and | |
| ● | they allow us to issue, without stockholder approval, up to 10,000,000 shares of preferred stock that could adversely affect the rights and powers of the holders of our common stock. |
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware, an anti-takeover law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in the following prescribed manner:
| ● | prior to the time of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; | |
| ● | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the stockholder owned at least eighty-five percent (85%) of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding; (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; and | |
| ● | on or subsequent to the time of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least sixty-six and two-thirds percent (66 2/3%) of the outstanding voting stock which is not owned by the interested stockholder. |
| 32 |
Generally, for purposes of Section 203, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns or, within three (3) years prior to the determination of interested stockholder status, owned fifteen percent (15%) or more of a corporation’s outstanding voting securities.
Choice of Forum
Our certificate of incorporation, as amended, provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us, or any of our officers or Directors, arising pursuant to the Delaware General Corporation Law, our certificate of incorporation, as amended, or our bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. This exclusive forum provision may limit the ability of our stockholders to bring a claim in a judicial forum that such stockholders find favorable for the disputes listed above, which may discourage such lawsuits against us, or any of our officers or directors.
| 33 |
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a summary of the material United States federal income tax consequences to non-U.S. holders (as defined below) of their ownership and disposition of our common stock, but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon current provisions of the Internal Revenue Code, or Code, existing and proposed United States Treasury Regulations promulgated thereunder, current administrative rulings, and judicial decisions, all as in effect as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in United States federal tax consequences different from those set forth below. We have not obtained, and do not intend to obtain, any opinion of counsel or ruling from the Internal Revenue Service, or the IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions.
This summary also does not address the tax considerations arising under the laws of any non-United States, state or local jurisdiction or under any non-income tax laws, including United States federal gift and estate tax laws, except to the limited extent set forth below. In addition, this discussion does not address the potential application of the tax on net investment income or the alternative minimum tax. This discussion may not apply, in whole or in part, to particular non-U.S. holders in light of their individual circumstances or to holders subject to special treatment under the United States federal income tax laws, including, without limitation:
| ● | insurance companies, banks or other financial institutions; | |
| ● | tax-exempt organizations; | |
| ● | pension plans; | |
| ● | controlled foreign corporations or passive foreign investment companies; | |
| ● | brokers or dealers in securities or currencies; | |
| ● | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; | |
| ● | persons that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below); | |
| ● | certain former citizens or long-term residents of the United States; | |
| ● | persons that hold our common stock as a position in a hedging transaction, straddle, conversion transaction, synthetic security or other integrated investment; | |
| ● | persons that hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and | |
| ● | persons that do not hold our common stock as a capital asset within the meaning of Section 1221 of the Code. |
In addition, this discussion does not address the tax treatment of partnerships, including any entity or arrangement treated as a partnership for United States federal income tax purposes. Generally, the tax treatment of a person treated as a partner in such an entity will depend on the status of the partner, the activities of the partner and the partnership, and certain determinations made at the partner level. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
THIS SUMMARY IS NOT INTENDED TO BE CONSTRUED AS LEGAL ADVICE. WE RECOMMEND THAT PROSPECTIVE INVESTORS CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES TO THEM OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR FOREIGN TAX LAWS, ANY APPLICABLE INCOME TAX TREATIES, OR ANY OTHER U.S. FEDERAL TAX LAWS (INCLUDING ESTATE AND GIFT TAX LAWS).
| 34 |
Definition of Non-U.S. Holder
For purposes of this discussion, you are a non-U.S. holder if you are a beneficial owner of our common stock that is not, for United States federal income tax purposes:
| ● | an individual citizen or resident of the United States; | |
| ● | a corporation, or other entity taxable as a corporation, created or organized in the United States or under the laws of the United States or any political subdivision thereof; | |
| ● | an estate whose income is subject to United States federal income tax regardless of its source; or | |
| ● | a trust whose administration is subject to the primary supervision of a United States court and which has one or more “United States persons” (as defined in the Code) who have the authority to control all substantial decisions of the trust, or which has made a valid election to be treated as a United States person. |
Distributions to non-U.S. Holders
As described in the section titled “Dividend Policy,” we do not anticipate paying any cash dividends or making distributions of other property on our common stock in the foreseeable future. However, if we do make distributions of cash or property on our common stock, those payments will constitute dividends for United States federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, the excess will constitute a return of capital and will first reduce a non-U.S. holder’s tax basis in our common stock, but not below zero, and then will be treated by a non-U.S. holder as gain from the sale of stock as described below under “Gain on Dispositions of Our Common Stock by Non-U.S. Holders.”
Subject to the discussion below on effectively connected income, any dividend paid to a non-U.S. holder generally will be subject to United States withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. To receive a reduced treaty rate, a non-U.S. holder must provide us with an IRS Form W-8BEN or W-8BEN-E (or applicable successor form) and certify qualification for the reduced rate. If a non-U.S. holder is eligible for a reduced rate of United States withholding tax pursuant to an income tax treaty, such non-U.S. holder may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS. If a non-U.S. holder holds our common stock through a financial institution or other agent acting on such non-U.S. holder’s behalf, appropriate documentation will need to be provided to the agent, which then will be required to provide certification to us or our paying agent, either directly or through other intermediaries.
Dividends received by a non-U.S. holder that are effectively connected with such non-U.S. holder’s conduct of a trade or business in the United States (and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the United States), are generally exempt from the 30% withholding tax if certain certification and disclosure requirements are satisfied. To obtain this exemption, a non-U.S. holder must provide us with an IRS Form W-8ECI (or applicable successor form) properly certifying such exemption. However, such effectively connected dividends, although not subject to withholding tax, generally are taxed at the same United States federal income tax rates applicable to United States persons, net of certain deductions and credits. In addition, dividends received by a corporate non-U.S. holder that are effectively connected with the conduct of a trade or business in the United States may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. Non-U.S. holders should consult with tax advisors regarding any applicable income tax or other treaties that may provide for different rules.
Any documentation provided to an applicable withholding agent may need to be updated in certain circumstances. The certification requirements described above also may require a non-U.S. holder to provide a United States taxpayer identification number.
For additional withholding rules that may apply to dividends, including dividends paid to foreign financial institutions (as specifically defined by the applicable rules) or to certain other foreign entities that have substantial direct or indirect United States owners, see the discussion below under the headings “Information Reporting and Backup Withholding” and “Withholdable Payments to Foreign Financial Institutions and Other Foreign Entities.”
| 35 |
Gain on Disposition of Our Common Stock by Non-U.S. Holders
Subject to the discussion below under the headings “Information Reporting and Backup Withholding” and “Withholdable Payments to Foreign Financial Institutions and Other Foreign Entities,” a non-U.S. holder generally will not be required to pay United States federal income tax or withholding tax on any gain recognized upon the sale, exchange or other taxable disposition of our common stock unless:
| ● | the gain is effectively connected with the conduct of a trade or business by such non-U.S. holder in the United States (and, if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the United States), in which case the non-U.S. holder will be required to pay tax on the net gain derived from the sale or disposition at the rates and in the manner applicable to United States persons, and an additional branch profits tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty) may also apply to a corporate non-U.S. holder; | |
| ● | such non-U.S. holder is a nonresident alien individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met, in which case the non-U.S. holder will be required to pay a flat 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the gain derived from the sale or disposition, which gain may be offset by United States-source capital losses for the taxable year of the sale or disposition; or | |
| ● | our common stock constitutes a United States real property interest by reason of our status as a “United States real property holding corporation”, or USRPHC, for United States federal income tax purposes at any time within the shorter of the five-year period preceding such non-U.S. holder’s disposition of, or holding period for, our common stock, in which case the non-U.S. holder generally will be taxed on net gain derived from the sale or disposition at the rates applicable to United States persons. |
We believe that we are not currently and will not become a USRPHC and the remainder of this discussion so assumes. However, because the determination of whether we are a USRPHC depends on the fair market value of our United States real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as United States real property interests only if a non-U.S. holder actually or constructively holds more than 5% of such regularly traded common stock at any time during the shorter of the five-year period preceding such non-U.S. holder’s disposition of, or holding period for, our common stock. Non- U.S. holders should consult with tax advisors regarding any applicable income tax or other treaties that may provide for different rules.
Information Reporting and Backup Withholding
We (or the applicable paying agent) must report annually to the IRS the amount of dividends on our common stock paid to non-U.S. holders and the amount of tax withheld, if any. A similar report will be sent to each non-U.S. holder. Copies of this information reporting may also be made available under the provisions of a specific income tax treaty or agreement with the tax authorities in a non-U.S. holder’s country of residence.
Non-U.S. holders will generally be subject to backup withholding (at a current rate of 24%) for dividends on our common stock paid to such non-U.S. holders unless an exemption is established such as by, for example, properly certifying non-United States status on an IRS Form W-8BEN or W-8BEN-E (or applicable successor form). Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that a holder of our common stock is a United States person.
| 36 |
Information reporting and backup withholding generally are not required with respect to the amount of any proceeds from the sale or other disposition of our common stock by a non-U.S. holder outside the United States through a foreign office of a foreign broker that does not have certain specified connections to the United States. However, if a non-U.S. holder sells or otherwise disposes of shares of common stock through a United States broker or the United States offices of a foreign broker, the broker will generally be required to report the amount of proceeds paid to such non-U.S. holder to the IRS and also to backup withhold on that amount unless the broker is provided appropriate certification of status as a non-United States person or an exemption is otherwise established. Information reporting will also apply if a non-U.S. holder sells shares of common stock through a foreign broker deriving more than a specified percentage of its income from United States sources or having certain other connections to the United States, unless such broker has documentary evidence in its records that such non-U.S. holder is a non-United States person and certain other conditions are met, or an exemption is otherwise established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment may be refunded or credited against a non-U.S. holder’s United States federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS. Non-U.S. holders should consult with tax advisors regarding the application of the information reporting and backup withholding rules to investment in our common stock.
Withholdable Payments to Foreign Financial Institutions and other Foreign Entities
The Foreign Account Tax Compliance Act, or FATCA, imposes a United States federal withholding tax of 30% on certain payments to “foreign financial institutions” (as specifically defined under these rules) and certain other non-United States persons that fail to comply with certain information reporting and certification requirements pertaining to their direct and indirect United States security holders and/or United States account holders. Such payments include dividends on and, on or after January 1, 2019, gross proceeds from the sale or other disposition of our common stock. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult with tax advisors regarding the possible implications of this legislation and any applicable intergovernmental agreements on investment in our common stock.
U.S. Federal Estate Tax
Our common stock beneficially owned by an individual who is not a citizen or resident of the United States (as defined for United States federal estate tax purposes) at the time of their death will generally be includable in the decedent’s gross estate for United States federal estate tax purposes and, therefore, may be subject to United States federal estate tax unless an applicable estate tax treaty or other treaty provides otherwise. Investors are urged to consult their own tax advisors regarding the United States federal estate tax consequences of the ownership or disposition of our common stock.
THIS SUMMARY IS NOT INTENDED TO BE CONSTRUED AS LEGAL ADVICE. NON-U.S. HOLDERS ARE URGED TO CONSULT WITH TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE UNITED STATES FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATION, AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK ARISING UNDER THE UNITED STATES FEDERAL ESTATE OR GIFT TAX RULES OR UNDER THE LAWS OF ANY STATE, LOCAL, NON-UNITED STATES OR OTHER TAXING JURISDICTION OR UNDER ANY APPLICABLE TAX TREATY, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
| 37 |
Subject to the terms and conditions of the purchase agreement entered into with Piper Jaffray & Co., as representative of the several underwriters of this offering, we have agreed to sell to the underwriters, and the underwriters have severally agreed to purchase from us the number of shares of common stock indicated in the table below:
| Underwriter | Number of Shares |
|||
| Piper Jaffray & Co. | ||||
| Total | ||||
The underwriters have advised us that they propose to offer the shares of common stock to the public at $ per share of common stock to certain dealers at that price less a concession not in excess of $ per share of common stock. After the offering, this figure may be changed by the underwriters. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The following table shows the per share of common stock and total underwriting discounts to be paid to the underwriters in connection with this offering assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares of common stock:
| Without Option Exercise | With Full Option Exercise | |||||||
| Per Share | $ | $ | ||||||
| Total | $ | $ | ||||||
We estimate that the total fees and expenses payable by us, excluding underwriting discounts and commissions will be approximately $450,000, which includes up to $125,000 that we have agreed to reimburse the underwriters for fees incurred by them in connection with this offering.
In connection with our IPO we granted the representative of the underwriters a right of first refusal during the thirty month period following February 13, 2019, the effective date of our IPO prospectus, to act as our exclusive financial advisor, sole book-running manager, or exclusive placement agent, as the case may be, in connection with any restructuring transaction, any acquisition or disposition transaction, any public offering, any Rule 144A offering or any private placement of securities until the earlier of (i) the expiration of the thirty month period following February 13, 2019, the effective date of our IPO prospectus, or (ii) the aggregate gross proceeds to us in such transactions exceeds $50,000,000, subject to certain specified exceptions. Any such engagement will be on terms and conditions customary to the representative of the underwriters for similar transactions, and will be governed by separate agreement.
We have agreed to indemnify the underwriters against certain liabilities, including civil liabilities under the Securities Act, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
We, each of our directors and officers and certain of our shareholders have entered into lock-up agreements with the underwriters. Under these agreements, we and each of these persons may not, without the prior written approval of the underwriter, subject to limited exceptions, offer, pledge, sell, offer to sell, contract to sell or lend, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock or publicly disclose the intention to make any offer, sale, pledge or disposition, or enter into any swap, hedge or similar arrangements, or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock or such other securities. In addition, subject to limited exceptions, we have agreed not to file any registration statement under the Securities Act for any such transaction or which registers, or offers for sale, our common stock or any securities convertible into or exercisable or exchangeable for our common stock, and each of our directors and officers has agreed not to make any demand for, or exercise any right with respect to, the registration under the Securities Act of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock, in each case without the prior written approval of Piper Jaffray & Co. These restrictions will be in effect for a period of 90 days after the date of this prospectus supplement.
Our shares of common stock are listed on The Nasdaq Capital Market under the symbol “MOTS.”
| 38 |
Price Stabilization and Short Positions
To facilitate the offering being made pursuant to this prospectus supplement or the concurrent preferred stock offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock during and after the offering. Specifically, the underwriters may over-allot or otherwise create a short position in the common stock for its own account by selling more shares of common stock than we have sold to the underwriters. Short sales involve the sale by the underwriters of a greater number of shares than the underwriters is required to purchase in the offering. The underwriters may close out a short position by exercising the over-allotment option or by purchasing shares in the open market.
In addition, the underwriters may attempt to stabilize or maintain the price of the common stock by bidding for or purchasing shares of common stock in the open market and may impose penalty bids. If penalty bids are imposed, selling concessions allowed to other broker-dealers participating in the offering are reclaimed if shares of common stock previously distributed in the offering are repurchased, whether in connection with stabilization transactions or otherwise. The effect of these transactions may be to stabilize or maintain the market price of the common stock at a level above that which might otherwise prevail in the open market. The imposition of a penalty bid may also affect the price of the common stock to the extent that it discourages resales of the common stock. The magnitude or effect of any stabilization or other transactions is uncertain. These transactions may be effected on The Nasdaq Stock Market or otherwise and, if commenced, may be discontinued at any time. The underwriters may also engage in passive market making transactions in our common stock. Passive market making consists of displaying bids on The Nasdaq Stock Market limited by the prices of independent market makers and effecting purchases limited by those prices in response to order flow. Rule 103 of Regulation M promulgated by the SEC limits the amount of net purchases that each passive market maker may make and the displayed size of each bid. Passive market making may stabilize the market price of the common stock at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of shares of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
This prospectus may be made available on a website maintained by the underwriters and the underwriters may distribute this prospectus electronically.
Selling Restrictions
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), each underwriter represents and agrees that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, it has not made and will not make an offer of securities which are the subject of the offering contemplated by this prospectus supplement to the public in that Relevant Member State other than:
| (a) | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| (b) | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives for any such offer; or |
| (c) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive. |
For the purposes of this provision, the expression an “offer to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression Prospectus Directive means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Notice to Prospective Investors in the United Kingdom
Each of the underwriters severally represents, warrants and agrees as follows:
| (a) | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (“FSMA”) received by it in connection with the issue or sale of the securities in circumstances in which Section 21 of the FSMA does not apply to us; and |
| (b) | it has complied with, and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the securities in, from or otherwise involving the United Kingdom. |
| 39 |
Notice to Prospective Investors in Israel
In the State of Israel this prospectus supplement shall not be regarded as an offer to the public to purchase securities under the Israeli Securities Law, 5728 — 1968, which requires a prospectus to be published and authorized by the Israel Securities Authority, if it complies with certain provisions of Section 15 of the Israeli Securities Law, 5728 — 1968, including, inter alia, if: (i) the offer is made distributed or directed to not more than 35 investors, subject to certain conditions, or the Addressed Investors; or (ii) the offer is made, distributed or directed to certain qualified investors defined in the First Addendum of the Israeli Securities Law, 5728 — 1968, subject to certain conditions, or the Qualified Investors. The Qualified Investors shall not be taken into account in the count of the Addressed Investors and may be offered to purchase securities in addition to the 35 Addressed Investors. Our company has not and will not take any action that would require it to publish a prospectus in accordance with and subject to the Israeli Securities Law, 5728 — 1968. We have not and will not distribute this prospectus supplement or make, distribute or direct an offer to subscribe for our securities to any person within the State of Israel, other than to Qualified Investors and up to 35 Addressed Investors.
Qualified Investors may have to submit written evidence that they meet the definitions set out in of the First Addendum to the Israeli Securities Law, 5728 — 1968. In particular, we may request, as a condition to be offered securities, that Qualified Investors will each represent, warrant and certify to us or to anyone acting on our behalf: (i) that it is an investor falling within one of the categories listed in the First Addendum to the Israeli Securities Law, 5728 — 1968; (ii) which of the categories listed in the First Addendum to the Israeli Securities Law, 5728 — 1968 regarding Qualified Investors is applicable to it; (iii) that it will abide by all provisions set forth in the Israeli Securities Law, 5728 — 1968 and the regulations promulgated thereunder in connection with the offer to be issued securities; (iv) that the securities that it will be issued are, subject to exemptions available under the Israeli Securities Law, 5728 — 1968: (a) for its own account; (b) for investment purposes only; and (c) not issued with a view to resale within the State of Israel, other than in accordance with the provisions of the Israeli Securities Law, 5728 — 1968; and (v) that it is willing to provide further evidence of its Qualified Investor status. Addressed Investors may have to submit written evidence in respect of their identity and may have to sign and submit a declaration containing, inter alia, the Addressed Investor’s name, address and passport number or Israeli identification number.
Notice to Prospective Investors in Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption form, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice to Prospective Investors in Hong Kong
The securities may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), or (ii) to “professional investors” as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the securities may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
| 40 |
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”)) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for 6 months after that corporation has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”).
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for 6 months after that trust has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32.
Notice to Prospective Investors in Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended), or the FIEA. The securities may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
| 41 |
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (“ASIC”), in relation to the offering. This offering document does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the “Corporations Act”), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons (the “Exempt Investors”) who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This offering document contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this offering document is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Dubai International Financial Centre
This offering document relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority (“DFSA”). This offering document is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth in this prospectus and has no responsibility for the offering document. The securities to which this offering document relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this offering document you should consult an authorized financial advisor.
Notice to Prospective Investors in Switzerland
We have not and will not register with the Swiss Financial Market Supervisory Authority (“FINMA”) as a foreign collective investment scheme pursuant to Article 119 of the Federal Act on Collective Investment Scheme of 23 June 2006, as amended (“CISA”), and accordingly the securities being offered pursuant to this prospectus have not and will not be approved, and may not be licenseable, with FINMA. Therefore, the securities have not been authorized for distribution by FINMA as a foreign collective investment scheme pursuant to Article 119 CISA and the securities offered hereby may not be offered to the public (as this term is defined in Article 3 CISA) in or from Switzerland. The securities may solely be offered to “qualified investors,” as this term is defined in Article 10 CISA, and in the circumstances set out in Article 3 of the Ordinance on Collective Investment Scheme of 22 November 2006, as amended (“CISO”), such that there is no public offer. Investors, however, do not benefit from protection under CISA or CISO or supervision by FINMA. This prospectus and any other materials relating to the securities are strictly personal and confidential to each offeree and do not constitute an offer to any other person. This prospectus may only be used by those qualified investors to whom it has been handed out in connection with the offer described in this prospectus and may neither directly or indirectly be distributed or made available to any person or entity other than its recipients. It may not be used in connection with any other offer and shall in particular not be copied and/or distributed to the public in Switzerland or from Switzerland. This prospectus does not constitute an issue prospectus as that term is understood pursuant to Article 652a and/or 1156 of the Swiss Federal Code of Obligations. We have not applied for a listing of the securities on the SIX Swiss Exchange or any other regulated securities market in Switzerland, and consequently, the information presented in this prospectus does not necessarily comply with the information standards set out in the listing rules of the SIX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SIX Swiss Exchange.
| 42 |
The validity of the securities offered in this prospectus is being passed upon for us by Lowenstein Sandler LLP, New York, New York. Goodwin Procter LLP, New York, New York has acted as counsel for the underwriters in connection with certain legal matters related to this offering.
The financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2017, have been audited by Brightman Almagor Zohar & Co., a member firm of Deloitte Touche Tohmatsu Limited and an independent registered public accounting firm, as stated in their report, which is incorporated by reference in this prospectus and elsewhere in this registration statement (which report expresses an unqualified opinion on the financial statements and includes an explanatory paragraph referring to the Company’s ability to continue as a going concern). Such financial statements have been incorporated by reference in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus. This prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. For further information pertaining to us and our common stock, reference is made to our SEC filings and the registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
In addition, registration statements and certain other filings made with the SEC electronically are publicly available through the SEC’s web site at http://www.sec.gov. The registration statement, including all exhibits and amendments to the registration statement, has been filed electronically with the SEC.
We are subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934, as amended, and, in accordance with such requirements, will file periodic reports, proxy statements, and other information with the SEC. These periodic reports, proxy statements, and other information will be available for inspection and copying at the web site of the SEC referred to above. We also maintain a website at http://www.motusgi.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
| 43 |
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (File No. 001-38389):
| ● | our Annual Report on Form 10-K for the year ended December 31, 2017, filed with the SEC on March 28, 2018; |
| ● | our Quarterly Reports on Form 10-Q for the periods ended March 31, 2018, June 30, 2018 and September 30, 2018, which were filed with the SEC on May 14, 2018, August 13, 2018 and November 14, 2018 respectively; and |
| ● | our Current Reports on Form 8-K which were filed with the SEC on April 2, 2018 and September 25, 2018; and |
| ● | the description of our common stock contained in our registration statement on Form 8-A filed with the SEC on February 6, 2018, including any amendments or reports filed for the purposes of updating this description. |
In addition, all documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, prior to the termination of the offering (excluding any information furnished rather than filed) shall be deemed to be incorporated by reference into this prospectus.
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the SEC pursuant to the Securities Exchange Act of 1934, as amended shall be incorporated by reference into this prospectus.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Motus GI Holdings, Inc., Attn: Chief Financial Officer, 1301 East Broward Boulevard, 3rd Floor, Ft. Lauderdale, FL, 33301. You may also direct any requests for documents to us by telephone at (954) 541-8000 or e-mail at IR@MotusGI.com.
You also may access these filings on our website at http://www.motusgi.com. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
| 44 |
MOTUS GI HOLDINGS, INC.
5,000,000 Shares
Common Stock
Piper Jaffray
PROSPECTUS
, 2018
| 45 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| ITEM 13. | OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION |
The following table sets forth all fees and expenses to be paid by us, other than estimated underwriting discounts and commissions, in connection with this offering. All amounts shown are estimates except for the SEC registration fee, the FINRA filing fee and the Nasdaq Market initial listing fee.
| Amount | ||||
| SEC Registration Fee | $ | 2,250.99 | ||
| FINRA filing fee | $ | 3,285.88 | ||
| Printing and engraving expenses | $ | 10,000.00 | ||
| Accounting Fees and Expenses | $ | 120,000.00 | ||
| Transfer agent and registrar fees and expenses | $ | 5,000.00 | ||
| Legal Fees and Expenses | $ | 300,000.00 | ||
| Miscellaneous Fees and Expenses | $ | 9,463.13 | ||
| Total Expenses | $ | 450,000.00 | ||
| ITEM 14. | INDEMNIFICATION OF OFFICERS AND DIRECTORS |
Section 145 of the Delaware General Corporation Law (the “DGCL”) provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our certificate of incorporation and bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the DGCL, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any amendment by stockholders or directors resolution.
| II-1 |
Any repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation on the liability of any of our directors or officers existing as of the time of such repeal or modification.
We have director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters arising under the Securities Act.
We have entered into indemnification agreements with all of our directors and named executive officers whereby we have agreed to indemnify those directors and officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of Motus GI Holdings, Inc. (the “Company”), provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed to be in, or not opposed to, the best interests of the Company.
The Underwriting Agreement filed as Exhibit 1.1 to this registration statement provides for indemnification by the underwriters of us, our executive officers and directors, and by us of the underwriters for certain liabilities, including liabilities arising under the Securities Act and otherwise.
| ITEM 15. | RECENT SALES OF UNREGISTERED SECURITIES |
Since September 2016 (date of inception), the Company made sales of the following unregistered securities:
Original Issuances of Stock, Warrants and Payment Rights Certificates
Formation of Holdings
In connection with our formation in September 2016, we sold an aggregate of 1,650,000 shares of our common stock for an aggregate of $82,500 ($0.05 per share), which includes 450,000 shares of our common stock owned by an affiliate of the Placement Agent for the 2017 Private Placement.
2017 Private Placement
From December 2016 through February of 2017, we sold an aggregate of 4,743,311 shares of our common stock and 1,581,128 shares of Series A Convertible Preferred Stock at a price of $5.00 per unit, inclusive of 2,432,808 shares of our common stock and 810,960 shares of Series A Convertible Preferred Stock issued pursuant to the Exchange of Convertible Notes, to 229 accredited investors.
In connection with the 2017 Private Placement, we issued (i) the Placement Agent Warrants to the Placement Agent to purchase 403,632 shares of our common stock with an exercise price of $5.00 per share and (ii) the Placement Agent Royalty Payment Rights Certificates to receive, in the aggregate, 10% of the amount of payments paid to the holders of the Series A Convertible Preferred Stock, or the holders of the Royalty Payment Rights Certificates, upon the conversion of the Series A Convertible Preferred Stock into shares of our common stock.
Share Exchange Transaction
Pursuant to the terms of the Share Exchange Agreement, as a condition of and contemporaneously with the Initial Closing of the 2017 Private Placement, the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco and Opco became our wholly-owned subsidiary. Pursuant to the Share Exchange Transaction (i) the Opco Stockholders received an aggregate of 4,000,000 shares of our common stock in exchange for all of the issued and outstanding shares of capital stock of Opco, (ii) the Convertible Notes and the Convertible Note Warrants (as defined below) were exchanged for our securities, as described below, and (iii) all of the issued and outstanding options to purchase or otherwise acquire shares of Opco capital stock that were outstanding and unexercised as of immediately prior to the Initial Closing were substituted for 125,730 options to acquire shares of our common stock pursuant to our 2016 Equity Incentive Plan.
| II-2 |
Exchange of Convertible Notes
Pursuant to the terms of the CNA, as amended, Opco issued the Convertible Notes in a series of closings from June 2015 through November 2016, in an aggregate amount of $14,596,683 (inclusive of accrued interest through December 22, 2016, the date of the Initial Closing). Certain related parties purchased Convertible Notes pursuant to the CNA, see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation.” At the Initial Closing, Convertible Holders exchanged their Convertible Notes, together with accrued and unpaid interest thereon calculated through the date of the Initial Closing at a rate of 10% per annum, for units of the 2017 Private Placement, at a price of $4.50 per unit. As a result, upon consummation of the Initial Closing, the Convertible Notes were exchanged for units representing (i) 2,432,808 shares of our common stock (inclusive of shares of our common stock resulting from the accrued and unpaid interest of the Convertible Notes through the date of the Initial Closing) and (ii) 810,960 shares of Series A Convertible Preferred Stock (inclusive of shares of Series A Convertible Preferred Stock resulting from the accrued and unpaid interest of the Convertible Notes through the date of the Initial Closing).
Exchange of Convertible Note Warrants
In connection with, and pursuant to the terms of, the CNA, each Convertible Holder also received a seven (7) year warrant (the “Convertible Note Warrants”) to purchase Preferred A Shares of Opco, nominal value in Israeli New Shekel (“NIS”) 0.01 per share, with an exercise price per share of $1.00 (the “Convertible Note Warrant Exercise Price”). Each Convertible Note Warrant entitled the holder to purchase that number of shares of Preferred A Shares of Opco equal to thirty-three percent (33%) of the principal amount of the Convertible Note in connection with which it was issued. Convertible Note Warrants to purchase an aggregate 4,536,188 shares of Preferred A Shares of Opco with an exercise price of $1.00 were issued in connection with the CNA. Certain related parties held Convertible Note Warrants pursuant to the CNA, see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation.” At the Initial Closing, the holders of the Convertible Note Warrants exchanged their Convertible Note Warrants for five (5) year warrants (the “Exchange Warrants”) to purchase an aggregate 907,237 shares of our common stock at an exercise price of $5.00 per share, such amount being equal to thirty-three percent (33%) of the principal amount of the Convertible Notes divided by $5.00.
Service Provider Stock and Warrants
In March 2017, we issued a service provider (a) 90,000 shares of our common stock, subject to a lock-up agreement, and (b) five (5) year warrants to purchase 30,000 shares of our common stock with an exercise price of $8.00 per share (the “May 2017 Consultant Warrant”), as partial payment for services pursuant to a consulting agreement between the service provider and us. In July 2018, we entered into an amendment to this consulting agreement and issued the service provider 30,000 shares of our common stock and a warrant to purchase 90,000 shares of our common stock. The warrants (the “May 2017 Additional Consultant Warrant”) are fully vested, are exercisable at $8.50 per share and expire five years from the date of issuance.
During August, September, and October of 2017, we issued a service provider an aggregate of 4,167 shares, pursuant to the terms of the agreement with such service provider.
On March 27, 2018, we issued a service provider 15,000 shares of our common stock, subject to a lock-up agreement, as partial payment for services pursuant to a consulting agreement between the service provider and us.
In June 2018, we entered into a consultant agreement pursuant to which we (a) issued a warrant, on June 6, 2018, to purchase 10,000 shares of our common stock, with an exercise price of $5.25 per share, (b) issued a warrant, on October 6, 2018, to purchase 10,000 shares of our common stock, with an exercise price of $6.25 per share, and (c) agreed to issue a warrant to purchase 10,000 shares of our common stock, with an exercise price of $7.25 per share, issuable on February 6, 2019, provided the consultant is still engaged at that time, as payment for services pursuant to the consulting agreement. Each warrant (each a “June 2018 Consultant Warrant”) issued or issuable under the consultant agreement has or will have a five year term and a cashless exercise provision.
| II-3 |
In July 2018, we entered into a consulting agreement pursuant to which we (a) issued a warrant on July 2, 2018 to purchase 25,000 shares of our common stock with an exercise price of $7.39 per share, which warrant has an exercise period of 12 months from the date of agreement, (b) issued a warrant on July 2, 2018 to purchase 25,000 shares of our common stock with an exercise price of 7.39 per share, which warrant has an exercise period of 18 months from the date of the agreement, (c) issued a warrant on October 2, 2018 to purchase 25,000 shares of our common stock with an exercise price of 8.75 per share, which warrant has an exercise period of 18 months from the date of the agreement and (d) agreed to issue upon the six month anniversary of the date of the agreement, provided the consultant is still engaged at the respective time of issuance, a warrant to purchase 25,000 shares of our common stock with an exercise price of $10.00 per share, which warrant has an exercise period of 24 months from the date of the agreement. The warrants (each a “July 2018 Consultant Warrant”) issued under this agreement are callable by us.
In July 2018, we entered into a consulting agreement with an executive search firm pursuant to which we issued a warrant on November 8, 2018 to purchase 7,917 shares of our common stock, with an exercise price of $5.00 per share. The warrant (the “November 2018 Consultant Warrant”) issued under the consultant agreement has a three (3) year term. An additional warrant is issuable by us pursuant to the consulting agreement to purchase an estimated 6,380 shares of our common stock, subject to adjustment, with an estimated exercise price of $3.23 per share, subject to adjustment. The warrant (the “Contingent Consultant Warrant”) to be issued under the consulting agreement is expected to have a three (3) year term and is expected to be issued October 2019.
Ten Percent Warrants
Simultaneously with the closing of our IPO, we issued warrants to purchase an aggregate of 1,095,682 shares of our common stock (the “Ten Percent Warrants”) to certain of the holders of our formerly outstanding Series A Convertible Preferred Stock, pursuant to an amendment to the Registration Rights Agreement entered into with the investors in our 2017 Private Placement offering and an amendment to the Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock. The Ten Percent Warrants are exercisable for our common stock at an exercise price of $5.00 per share. The Ten Percent Warrants are exercisable any time on or after the 180 day anniversary of the completion of our IPO, have a five year term, and provide for cashless exercise. No fractional shares will be issued upon the exercise of the Ten Percent Warrants.
Conversion of Series A Convertible Preferred Stock
Simultaneously with the closing of our IPO, all 1,581,128 previously outstanding shares of our Series A Convertible Preferred Stock were converted, on a one-to-one basis, into shares of our common stock. At such time we issued (i) 1,581,128 shares of our common stock and (ii) the Royalty Payment Rights Certificates to each former holder of our Series A Convertible Preferred Stock, entitling the holders of such Royalty Payment Rights Certificates to the same Royalty Payment Rights as were included in the Series A Convertible Preferred Stock.
Issuance Pursuant to Exercise of Stock Option
From September 2016 (date of inception) to March 29, 2018, the date of the filing of our registration statement on Form S-8 (File No. 333-224003), we issued 1,148 shares of our common stock pursuant the exercise of stock options.
Stock Options
From September 2016 (date of inception) to March 29, 2018, the date of the filing of our registration statement on Form S-8 (File No. 333-224003) we granted stock options under our 2016 Equity Incentive Plan to purchase an aggregate of 2,114,769 shares of our common stock, with a weighted average exercise price of $4.51 per share.
Unrestricted Stock Awards
From September 2016 (date of inception) to March 29, 2018, the date of the filing of our registration statement on Form S-8 (File No. 333-224003), we granted unrestricted stock awards under our 2016 Equity Incentive Plan for an aggregate of 5,000 shares of our common stock.
| II-4 |
Securities Act Exemptions
We deemed the offers, sales and issuances of the securities described above under “Original Issuances of Stock, Warrants and Payment Rights Certificates” to be exempt from registration under the Securities Act of 1933, as amended (the “Securities Act”), in reliance on Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options and issuances of our common stock upon exercise of such options, and the restricted share awards, described above to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder and Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
| ITEM 16. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| II-5 |
| II-6 |
| II-7 |
| 23.1 | Consent of Independent Registered Public Accounting Firm | X | ||||||||||
| 23.2 | Consent of Lowenstein Sandler LLP (included in Exhibit 5.1) | X | ||||||||||
| 24.1 | Power of Attorney (contained in the signature page of this registration statement) | X | ||||||||||
| + | As permitted by Item 601(b)(2) of Regulation S-K, certain schedules to this agreement have not been filed herewith. The company will furnish supplementally a copy of any omitted schedule to the SEC upon request. | |||||||||||
| † | Indicates management contract or compensatory plan. | |||||||||||
| # | Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been submitted separately to the SEC. | |||||||||||
| ITEM 17. | UNDERTAKINGS |
(a) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(b) The undersigned registrant hereby undertakes to deliver or cause to be delivered with the prospectus, to each person to whom the prospectus is sent or given, the latest annual report to security holders that is incorporated by reference in the prospectus and furnished pursuant to and meeting the requirements of Rule 14a-3 or Rule 14c-3 under the Securities Exchange Act of 1934; and where interim financial information required to be presented by Article 3 of Regulation S-X are not set forth in the prospectus, to deliver, or cause to be delivered to each person to whom the prospectus is sent or given, the latest quarterly report that is specifically incorporated by reference in the prospectus to provide such interim financial information.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
| II-8 |
(d) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-9 |
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Ft. Lauderdale, State of Florida on December 17, 2018.
| MOTUS GI HOLDINGS, INC. | ||
| By: | /s/ Timothy P. Moran | |
| Name: | Timothy P. Moran | |
| Title: | Chief Executive Officer | |
KNOW ALL MEN BY THESE PRESENTS, that the undersigned officers and directors of Motus GI Holdings, Inc., a Delaware corporation, do hereby constitute and appoint each of Mark Pomeranz and Andrew Taylor as his or her true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments, exhibits thereto and other documents in connection therewith) to this Registration Statement and any subsequent registration statement filed by the registrant pursuant to Rule 462(b) of the Securities Act of 1933, as amended, which relates to this Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons in the capacities and on the dates indicated.
| Person | Capacity | Date | ||
| /s/ Timothy P. Moran | Chief Executive Officer and Director | |||
| Timothy P. Moran | (Principal Executive Officer) | December 17, 2018 | ||
| /s/ Andrew Taylor | Chief Financial Officer | |||
Andrew Taylor
|
(Principal Financial and Accounting Officer) | December 17, 2018 | ||
| /s/ David Hochman | ||||
| David Hochman | Chairman of the Board | December 17, 2018 | ||
| /s/ Mark Pomeranz | ||||
| Mark Pomeranz | President and Director | December 17, 2018 | ||
| /s/ Darren Sherman | ||||
| Darren Sherman | Director | December 17, 2018 | ||
| /s/ Gary Jacobs | ||||
| Gary Jacobs | Director | December 17, 2018 | ||
| /s/ Samuel Nussbaum | ||||
| Samuel Nussbaum | Director | December 17, 2018 | ||
| /s/ Shervin Korangy | ||||
| Shervin Korangy | Director | December 17, 2018 | ||
| /s/ Gary Pruden | ||||
| Gary Pruden | Director | December 17, 2018 |
| II-10 |
