Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - Biohaven Pharmaceutical Holding Co Ltd. | exhibit231s-1.htm |
| EX-5.1 - EXHIBIT 5.1 - Biohaven Pharmaceutical Holding Co Ltd. | exhibit51s-1.htm |
| EX-1.1 - EXHIBIT 1.1 - Biohaven Pharmaceutical Holding Co Ltd. | exhibit11s-1.htm |
As filed with the Securities and Exchange Commission on December 10, 2018
Registration No. 333‑
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S‑1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Biohaven Pharmaceutical Holding Company Ltd.
(Exact name of registrant as specified in its charter)
British Virgin Islands (State or other jurisdiction of incorporation or organization) | 2834 (Primary Standard Industrial Classification Code Number) | Not applicable (I.R.S. Employer Identification Number) | ||
c/o Biohaven Pharmaceuticals, Inc.
215 Church Street
New Haven, Connecticut 06510
(203) 404‑0410
(Address, including zip code, and telephone number, including
area code, of registrant’s principal executive offices)
Vlad Coric, M.D.
Chief Executive Officer
Biohaven Pharmaceutical Holding Company Ltd.
215 Church Street
New Haven, Connecticut 06510
(203) 404‑0410
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies to: | |
Darren K. DeStefano Brian F. Leaf Madison A. Jones Cooley LLP 11951 Freedom Drive Reston, VA 20190‑5656 (703) 456‑8000 | Patrick O’Brien Robert Hatfield Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, MA 02199 (617) 951‑7000 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post‑effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post‑effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b‑2 of the Exchange Act.
Large accelerated filer ¨ | Accelerated filer ¨ | Non‑accelerated filer x (Do not check if a smaller reporting company) | Smaller reporting company ¨ Emerging growth company x |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. x
CALCULATION OF REGISTRATION FEE
Title of Each Class of Securities to be Registered | Amount to be Registered(1) | Proposed Maximum Aggregate Offering Price Per Share | Proposed Maximum Aggregate Offering Price(2) | Amount of Registration Fee |
Common Shares, no par value | 3,450,000 | $39.44 | $136,068,000 | $16,490 |
(1) | Includes 450,000 common shares that the underwriters have the option to purchase. |
(2) | Estimated solely for purposes of calculating the amount of the registration fee pursuant to Rule 457(c) under the Securities Act on the basis of the average of the high and low prices of the Registrant’s common shares as reported on the New York Stock Exchange on December 3, 2018. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS (Subject to Completion)
Dated December 10, 2018
3,000,000 Shares

COMMON SHARES
Biohaven Pharmaceutical Holding Company Ltd. is offering 3,000,000 of its common shares. Our common shares are listed on the New York Stock Exchange under the symbol “BHVN.” The last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018 was $40.67 per share. The final public offering price will be determined through negotiation between us and the lead underwriters in the offering and the recent market price used throughout the prospectus may not be indicative of the final offering price.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. See “Prospectus Summary—Implications of Being an Emerging Growth Company.”
Investing in our common shares involves risks. Please see “Risk Factors” beginning on page 17 as well as in the documents incorporated by reference into this prospectus.
PRICE $ A SHARE
Price to Public | Underwriting Discounts and Commissions(1) | Proceeds, Before Expenses, to Biohaven | |||
Per Share | $ | $ | $ | ||
Total | $ | $ | $ | ||
________________
(1) | We have agreed to reimburse the underwriters for certain expenses in connection with this offering. See “Underwriting” in this prospectus for a description of compensation payable to the underwriters. |
We have granted the underwriters an option to purchase up to 450,000 additional common shares at the public offering price less the underwriting discount. The underwriters can exercise this option at any time within 30 days after the date of this prospectus.
The underwriters expect to deliver the common shares to purchasers on or about , 2018.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
GOLDMAN SACHS & CO. LLC | PIPER JAFFRAY | |
, 2018
TABLE OF CONTENTS
PAGE | |
Neither we nor the underwriters have authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of our common shares. Our business, financial condition, results of operations, and prospects may have changed since that date.
For investors outside the United States: neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus and any such free writing prospectus outside of the United States.
PROSPECTUS SUMMARY
The following summary highlights some of the information contained elsewhere in or incorporated by reference into this prospectus. Because this is only a summary, however, it does not contain all of the information that may be important to you. You should carefully read this entire prospectus, including the information incorporated by reference herein, especially the matters discussed under “Risk Factors” beginning on page 17 of this prospectus and the “Risk Factors” section of our Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2018, along with our consolidated financial statements and notes thereto, before making an investment decision. All references to “Biohaven,” “we,” “us,” “our” or the “Company” mean Biohaven Pharmaceutical Holding Company Ltd. and its subsidiaries, except where it is made clear that the term means only the parent company.
Biohaven Pharmaceutical Holding Company Ltd.
Overview
We are a clinical‑stage biopharmaceutical company with a portfolio of innovative, late‑stage product candidates targeting neurological diseases, including rare disorders. Our product candidates are small molecules based on three distinct mechanistic platforms — calcitonin gene‑related peptide, or CGRP, receptor antagonists, glutamate modulators and myeloperoxidase, or MPO, inhibition — which we believe have the potential to significantly improve existing treatment approaches across a diverse set of neurological indications with high unmet need in both large markets and orphan indications. Our programs include the following:
Product | Platform | Indication | Development Stage | |||
Rimegepant | CGRP | Acute treatment and prevention of migraine | Three pivotal Phase 3 trials for acute treatment complete; long‑term safety ongoing. Trial for prevention initiated in the fourth quarter of 2018. NDA submission for acute treatment anticipated in the first half of 2019. | |||
BHV‑3500 | CGRP | Acute treatment and prevention of migraine | Phase 1 trial initiated in fourth quarter of 2018. Phase 2/3 trial expected to begin in 2019. | |||
Troriluzole | Glutamate | Ataxias | Phase 2/3 trial in SCA complete; Extension trial ongoing. Second Phase 2/3 trial expected to begin in the first quarter of 2019. | |||
Troriluzole | Glutamate | Obsessive Compulsive Disorder | Phase 2/3 trial ongoing. | |||
Troriluzole | Glutamate | Alzheimer’s disease | Phase 2/3 trial ongoing. | |||
Troriluzole | Glutamate | Generalized Anxiety Disorder | Phase 2/3 trial expected to begin first quarter of 2019 | |||
BHV‑0223 | Glutamate | Amyotrophic Lateral Sclerosis | NDA filed with FDA in fourth quarter of 2018. PDUFA date of July 21, 2019. | |||
BHV‑5000 | Glutamate | Neuropsychiatric disorders | Phase 1 trial completed 2018; Additional nonclinical studies and Phase 1 trial anticipated for 2019. | |||
BHV‑3241 | MPO | Neuroinflammation | Phase 3 trial for the treatment of multiple system atrophy expected to begin in mid‑2019. | |||
1
CGRP Platform
In July 2016, we acquired exclusive, worldwide rights to our CGRP receptor antagonist platform, including rimegepant and BHV‑3500, through a license agreement with Bristol‑Myers Squibb Company, or BMS, which was amended in March 2018.
Rimegepant
Study 301/Study 302
The most advanced product candidate from our CGRP receptor antagonist platform is rimegepant, an orally available, potent and selective small molecule human CGRP receptor antagonist that we are developing for the acute and preventive treatment of migraine. In March 2018, we announced positive topline data from our first two pivotal Phase 3 trials (Study 301 and Study 302) for the acute treatment of migraine. In each trial, treatment with a single 75 mg dose of rimegepant met the co‑primary efficacy endpoints of the trial, which were superior to placebo, at two hours post‑dose, on measures of pain freedom and freedom from the patient’s most bothersome symptom. In addition to achieving both co‑primary endpoints in each of the trials, rimegepant also was observed to be generally well‑tolerated in the trials, with a safety profile similar to placebo.
Study 303
On December 3, 2018, we announced positive topline data from a randomized, controlled Phase 3 clinical trial (BHV3000-303 or Study 303) evaluating the efficacy and safety of our Zydis® orally dissolving tablet (ODT) formulation of rimegepant for the acute treatment of migraine.
In Study 303, rimegepant Zydis ODT statistically differentiated from placebo on the two co-primary endpoints as well as the first 21 consecutive primary and secondary outcome measures that were pre-specified in hierarchical testing. Consistent with the two previous Phase 3 clinical trials, Study 303 met its co-primary endpoints of pain freedom and freedom from the most bothersome symptom (MBS) at 2 hours using a single dose (Table 1). Importantly, patients treated with the rimegepant Zydis ODT formulation began to numerically separate from placebo on pain relief as early as 15 minutes, and this difference was statistically significant at 60 minutes (p < 0.0001) (Figure 1). Additionally, a significantly greater percentage of patients treated with rimegepant Zydis ODT returned to normal functioning within 60 minutes post-dose as compared to placebo (p < 0.0025). Rimegepant Zydis ODT was superior to placebo on the endpoints of sustained freedom from pain (p < 0.001), sustained pain relief (p < 0.001), sustained freedom from the most bothersome symptom (p < 0.0018), and sustained freedom from functional disability (p < 0.0001) from 2 to 48 hours. Superiority over placebo was also demonstrated in multiple other secondary endpoints. The vast majority of rimegepant Zydis ODT treated patients (86%) did not use any rescue medications.
Table 1: Met Co-Primary Endpoints of Pain Freedom & Freedom from Most Bothersome Symptom
2 Hour Endpoint | Rimegepant (N=669) | Placebo (N=682) | Difference | p-value | ||||||
Pain Freedom | 21.2 | % | 10.9 | % | 10.3 | % | < 0.0001 | |||
Freedom from MBS (1) | 35.1 | % | 26.8 | % | 8.3 | % | 0.0009 | |||
________________
(1) | Most Bothersome Symptom of Photophobia, Phonophobia or Nausea |
2
Figure 1: Kaplan-Meier Pain Relief Curve Through 2 Hours after Single Dose of Rimegepant 75 Mg Zydis ODT
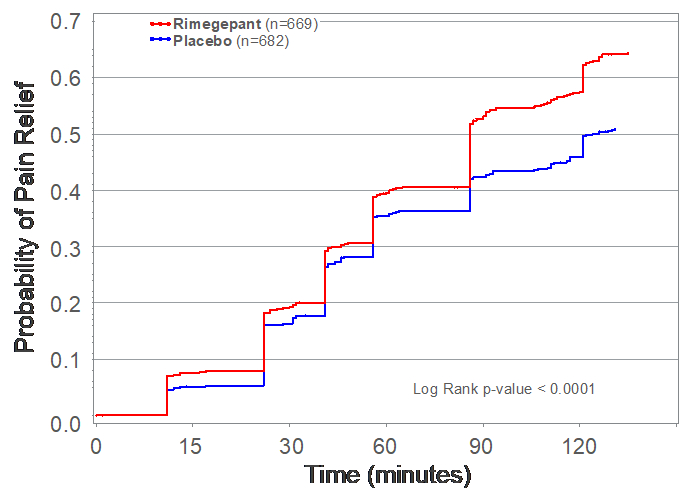
________________
Figure 1 shows the percentage of patients reporting pain relief between 0 and 2 hours after dosing for patients who took a single dose of rimegepant Zydis ODT 75 mg or placebo. Data are Kaplan-Meier estimates of the time to first report of pain relief (report of no pain, or mild pain). Subjects were censored who took rescue medication or were lost to follow-up.
The safety and tolerability observations of rimegepant in Study 303 were consistent with the profile previously observed in Studies 301 and 302. Table 2 shows the pooled safety data across all three trials. In Study 303, no single adverse event (AE) occurred in the rimegepant group with an incidence higher than 1.6% and overall rates of AEs were similar to placebo. With regard to liver function tests, one patient treated with placebo and one patient treated with rimegepant showed LFTs (ALT or AST) > 3x ULN in Study 303. Pooled liver function test results across the three pivotal trials (n=3,556) performed to date showed that rimegepant was similar to placebo with regard to aminotransferase (ALT or AST) levels above the upper limit of normal (ULN) and no patients experienced elevations in bilirubin > 2x ULN (Table 3).
3
Table 2: Pooled Adverse Event (AE) Safety Data: Rimegepant was Similar to Placebo Across Studies
AEs from Studies 301, 302 and 303 with an incidence ≥ 1% | ||||
Adverse Event | Rimegepant N=1,771 | Placebo N=1,785 | ||
≥ 1 On-Study AE (1) | 252 (14.2)% | 209 (13.2)% | ||
Nausea | 26 (1.5)% | 15 (0.8)% | ||
UTI | 21 (1.2)% | 12 (0.7)% | ||
SAEs (2) | 3 (0.2)% | 3 (0.2)% | ||
________________
(1) | No other individual AEs ≥ 1% in rimegepant treated subjects than those listed in table. Includes all AEs without attribution to drug relatedness. |
(2) | No drug-related Serious Adverse Events (SAEs). Two of the subjects with SAE in rimegepant group and one in placebo group had not been dosed before onset of SAE. |
Table 3: Pooled Liver Function Test (LFT) Profile: Rimegepant was Similar to Placebo Across Studies
Pooled LFT Results from Studies 301, 302, and 303* | ||||
ALT or AST | Rimegepant N=1,771 | Placebo N=1,785 | ||
> ULN (1) | 48 (2.7%) | 52 (2.9%) | ||
> 3x ULN | 2 (0.1%) | 2 (0.1%) | ||
> 5x ULN | 1 (0.06%)(2) | 0 | ||
> 10x ULN | 0 | 0 | ||
> 20x ULN | 0 | 0 | ||
________________
(1) | Upper limit of normal; ALT alanine aminotransferase; AST aspartate aminotransferase |
(2) | AST elevation, Not Drug-Related as deemed by the investigator: subject newly initiated weight-lifting with laboratory results consistent with muscle injury |
* | AST/ALT Categories are not mutually exclusive; No bilirubin elevations > 2x ULN across Studies 301, 302 and 303 |
Additional secondary and exploratory outcome measures from this study are anticipated to be presented at upcoming scientific meetings in 2019.
The co‑primary endpoints achieved in the Phase 3 trials are consistent with regulatory guidance from the U.S. Food and Drug Administration, or the FDA, and provide the basis for a planned submission of a new drug application, or NDA, to the FDA in 2019. If the NDA is approved by the FDA, we expect to launch rimegepant in 2020.
Long-term Safety Study
On December 10, 2018, we announced the results of an interim analysis from our ongoing long-term safety study (BHV3000-201 or Study 201). Study 201 is a multicenter, open-label safety study with 1,780 U.S. adult patients with migraine at the time of the November 21, 2018 interim data analysis cutoff. Eligible patients were required to have a history of two to 14 migraine attacks of moderate to severe intensity per month. Patients in the study are allowed to treat migraine attacks of all severities (mild to severe) with rimegepant 75mg up to once per day for up to 52 weeks. There was not a limit on the number of attacks per month that could be treated. At the time of the interim data analysis, approximately 473 patients received near daily dosing (15 or more doses a month) of rimegepant 75mg for a duration of 1-12 months.
4
Data from the interim analysis suggests that it appears that rimegepant 75mg may be safe and well tolerated after long-term dosing in patients with migraine as of the database cutoff date of November 21, 2018. The most common individual AEs (occurring ≥5%) in Study 201 were upper respiratory tract infection and viral respiratory tract infection. There were low rates of discontinuation due to AEs in the treatment period (2.6%). Interim hepatic data from the study were reviewed by an external independent panel of liver experts on December 7, 2018. The panel provided a consensus opinion based upon the Drug-Induced Liver Injury Network (DILIN) causality assessment. The panel did not assess any liver cases as probably related to study drug and there were no "Hy’s Law" (a common measure of liver toxicity) cases identified. The panel concluded that there was no liver safety signal detected through the data analysis cut-off date. In aggregate, the panel noted that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver laboratory abnormalities (1.0% incidence of serum ALT or AST > 3xULN).
In addition to the interim safety analysis, preliminary exploratory open-label efficacy data from Study 201 suggest that rimegepant may be associated with a reduction in migraine days per month (30 days) compared to the observational lead-in period, suggesting a potential preventive effect that warrants further study. In an exploratory analysis, patients who experienced ≥15 migraine days/month (N=172) during the standard of care observation period demonstrated a mean reduction of four migraine days/month by 12 weeks of intermittent dosing with rimegepant. Approximately 40% of patients who had ≥15 migraine days/month during the observation period showed at least a 30% or more reduction in their monthly number of migraine days by 12 weeks of treatment with rimegepant. Reduction from baseline in the mean number of migraine days per month in patients with ≥ 15 migraine days per month during the observation period was observed beginning as early as the first month and continued in subsequent months of therapy. Biohaven initiated a double-blind, placebo-controlled trial examining regularly scheduled dosing of rimegepant 75mg for the preventive treatment of migraine in November 2018.
Subjects will continue to participate in Study 201 with additional data analyses to be submitted to the FDA in connection with the planned filing, including the required 120-day safety update.
Additional Clinical Trials
In November 2017, the FDA agreed to our initial acute treatment pediatric study plan. In February 2018, a request for scientific advice for rimegepant was submitted to the Committee for Medicinal Products for Human Use, or CHMP, a committee of the European Medicines Agency, or EMA, and feedback was received in June 2018. Based on this feedback, we believe we have several potential pathways to approval.
A fourth Phase 3 clinical trial to evaluate the efficacy and safety of rimegepant as a preventive therapy for migraine was initiated in the fourth quarter 2018.
BHV‑3500
In October 2018, we initiated a Phase 1 clinical trial for BHV‑3500, our third‑generation CGRP receptor antagonist. In the trial, we are evaluating a range of doses using intranasal administration. If the trial is successful, we intend to initiate a Phase 2/3 clinical trial in 2019, for which we would expect to report topline results in the fourth quarter of 2019.
Glutamate Platform
We are developing three product candidates that modulate the body’s glutamate system. Two of these product candidates, troriluzole, previously referred to as trigriluzole or BHV‑4157, and BHV‑0223, act as glutamate transporter modulators, while our product candidate BHV‑5000 is an antagonist of the glutamate N‑methyl‑D‑aspartate, or NMDA, receptor.
5
Troriluzole for Ataxias
We are developing troriluzole for the treatment of ataxias; our initial focus has been spinocerebellar ataxia, or SCA. We have received both orphan drug designation and fast track designation from the FDA for troriluzole for the treatment of SCA. In October 2017, we reported topline data from the 8‑week randomization period from the Phase 2/3 clinical trial in SCA. At the eight‑week time point, troriluzole did not statistically differentiate from placebo. Subsequent to our announcement of the topline results from this trial, we engaged in discussions with the FDA regarding the potential for further development of troriluzole in ataxias, and the FDA expressed willingness to consider a modification of our trial’s primary endpoint, the Scale for Assessment and Rating of Ataxia, or SARA, as an acceptable registrational endpoint. The SARA is a scale consisting of an eight‑item, semi‑quantitative performance‑based assessment of cerebellar ataxia symptoms that measures impairment on a scale of zero to 40, with a higher score indicating more severe ataxia. Subsequent post‑hoc analyses of the data from the Phase 2/3 trial has shown trends for therapeutic benefit in certain patient subgroups (for example, those who are projected to have higher drug exposures, those with certain genotypes, and those with SARA assessments with more consistent levels of rater reliability). Further, item‑specific analyses of the SARA scale data suggest that certain of the eight items measured by SARA were strongly susceptible to a placebo effect. Based upon our analysis of this data, we proposed the use of a modified SARA scale in future clinical evaluation of troriluzole to the FDA. In November 2018, the FDA advised us that it agreed to a modified SARA scale, including a reduction in the number of domains measured.
The following chart shows preliminary data from the long term, open‑label extension phase of the Phase 2/3 trial after applying the modified SARA scale that we proposed to the FDA as compared to data from a natural history reference cohort, to which we applied the same modified SARA scale. A natural history study follows a group of people over time who have a specific medical condition. We utilized data from a published natural history study that enrolled 345 patients with SCA in the United States, and observed the progression of the patients’ disease using the SARA scale for up to two years.
In the chart below, interim results from the extension phase (labeled as “BHV‑4157 OL”) showed sustained improvements in modified SARA scores at all four time periods (extension week 8, 16, 24 and 48) in the troriluzole treated participants, meaning that the mean change in the modified SARA scale was negative at each time point, indicating an improvement or stabilization in ataxia symptoms. In contrast, the natural history reference cohort showed an increase in the modified SARA score, indicating a worsening in ataxia symptoms, when measured at six months and one year. At one year, the mean change in the modified SARA score in the natural history reference cohort was an increase of +0.79, compared to a mean change in the troriluzole cohort of a decrease of −0.44. While the modified SARA scale that we utilized for the analysis includes the same domains as those recommended by the FDA for the planned Phase 2/3 clinical trial, the response categories differ from the modified SARA scale recommended by FDA, and results from the extension analyses may not be indicative of results utilizing the FDA‑recommended modified SARA scale. We believe these observed changes with troriluzole treatment suggest a clinically meaningful benefit relative to the natural history reference cohort.
6
Longitudinal Change on Total Modified SARA Scale
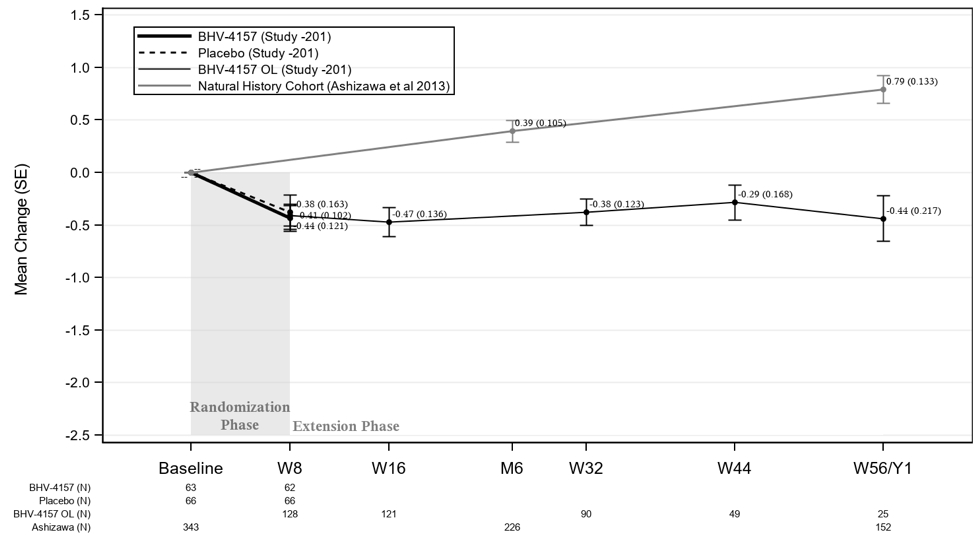
Because we believe that troriluzole may offer therapeutic benefit to patients with SCA and since comparisons to historical controls are generally not accepted by the FDA as the basis for approval, we plan to initiate a second randomized, controlled trial in the first quarter of 2019 to further evaluate the efficacy of troriluzole in SCA. The clinical observations from our Phase 2/3 trial and open‑label extension phase in SCA support our decision to advance troriluzole into an additional randomized, controlled trial that could provide the data needed to serve as the basis for an NDA. This second trial will incorporate trial design modifications based upon our post‑hoc analyses of the initial Phase 2/3 trial, and will include: (1) utilization of the modified SARA scale based on the feedback that we have received from the FDA in November 2018; (2) enrichment of the trial population with particular genotypes; (3) enhanced rater training procedures designed to ensure higher levels of scale reliability; (4) modified dosing regimen; and (5) extension of measurement of the primary endpoint, improvement in the patient’s modified SARA scale, to one year.
Troriluzole for Other Indications
A Phase 2/3 double‑blind, randomized, controlled trial to assess the efficacy of troriluzole in obsessive compulsive disorder, or OCD, commenced in December 2017. We expect to complete the randomization of this trial by the end of 2019. In addition, a Phase 2/3 double‑blind, randomized, controlled trial of troriluzole in the treatment of mild‑to‑moderate Alzheimer’s disease has advanced with the Alzheimer’s Disease Cooperative Study, a consortium of sites funded by the National Institutes of Health. In July 2018, we received authorization to proceed from the FDA and subsequently commenced the trial. We expect to complete enrollment and announce interim futility results for this trial in the third quarter of 2019. We also plan to initiate a Phase 2/3 clinical trial of troriluzole in general anxiety disorder, or GAD, in the first quarter of 2019. We expect to complete enrollment of this trial by the end of 2019.
BHV‑0223
We are developing BHV‑0223 for the treatment of Amyotrophic Lateral Sclerosis, or ALS. In December 2016, we received orphan drug designation from the FDA for BHV‑0223 to treat ALS. In January 2018, we announced positive results of a bioequivalence study with BHV‑0223 and marketed riluzole, thus providing
7
pivotal data that we believe are sufficient for the filing of an NDA with the FDA, allowing us to pursue the regulatory approval of BHV‑0223 for ALS under Section 505(b)(2) of the U.S. Federal Food, Drug, and Cosmetic Act. In addition, we submitted an IND to the FDA in late 2017, with the first trial assessing tolerability in dysphagic patients with ALS. This trial completed dosing in April 2018. We also started an additional tolerability study with 8‑week dosing in ALS patients in the first quarter of 2018, and initiated a trial in healthy volunteers to characterize swallowing in the third quarter of 2018. The NDA was filed in the fourth quarter of 2018.
In the first half of 2017, a Phase 2 investigator‑initiated, double‑blind, proof of concept trial examining the effects of BHV‑0223 on public speaking in social anxiety disorder was initiated. We reported topline results in the third quarter of 2018. The investigator‑initiated trial was performed at Yale University School of Medicine and treated 21 subjects who had a diagnosis of social anxiety disorder and participated in an anxiety‑provoking speech task in a double‑blind, crossover design. In the pre‑specified, primary analysis, BHV‑0223 reduced anxiety by 8.3 points relative to placebo on the 100‑point Visual Analogue Scale (VAS). The observed reduction in anxiety was significant (p=0.056), relative to the protocol specified level of p= 0.10. A likelihood‑based analysis, that analyzed the change in the VAS from the pre‑speech baseline, found that BHV‑0223 had a 14.4‑point advantage relative to placebo (p=0.0259). A p‑value is a statistical calculation that relates to the probability that a difference between groups happened by chance. Typically, a p‑value less than 0.05 represents statistical significance.
BHV‑5000
We are also developing BHV‑5000, an orally available, first‑in‑class, low‑trapping NMDA receptor antagonist, for the treatment of neuropsychiatric diseases. One potential target indication includes Complex Regional Pain Syndrome, or CRPS. CRPS is a rare, chronic pain condition typically affecting limbs and triggered by traumatic injury. Accompanying symptoms also include chronic inflammation and reduced mobility in the affected areas. Other disorders of interest include treatment‑resistant major depressive disorder and Rett syndrome. Rett syndrome is a rare and severe genetic neurodevelopmental disorder for which no approved treatments are currently available. We acquired worldwide rights to BHV‑5000 under an exclusive license agreement with AstraZeneca AB in October 2016. We selected a lead formulation at the end of 2017 and completed single dosing in a Phase 1 clinical trial of BHV‑5000 in January 2018 to evaluate its pharmacokinetic properties. Nonclinical studies are ongoing to support future trials.
MPO Platform
We are developing BHV‑3241, an oral myeloperoxidase inhibitor for the treatment of multiple system atrophy, or MSA, a rare, rapidly progressive and fatal neurodegenerative disease with no cure or effective treatments. BHV‑3241 was progressed through Phase 2 clinical trials by AstraZeneca AB. We have entered into an exclusive license agreement with AstraZeneca AB for the product candidate and, after reactivating the IND, plan to conduct a Phase 3 clinical trial of BHV‑3241 for the treatment of MSA in 2019.
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making a decision to invest in our common shares. These risks are discussed more fully in the “Risk Factors” section of this prospectus and the documents incorporated herein by reference. These risks include the following:
• | We have incurred significant operating losses since inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future and may never achieve or maintain profitability. |
• | Clinical trials are very expensive, time‑consuming and difficult to design and implement and involve uncertain outcomes. Furthermore, results of earlier preclinical studies and clinical trials may not be predictive of results of future preclinical studies or clinical trials. |
8
• | The currently reported results of our Zydis ODT Phase 3 trial of rimegepant are based on topline data for the trial and may differ from complete trial results once additional data are received and evaluated. Also, our long-term safety study of rimegepant is ongoing and could result in adverse safety data in the future. in addition, the FDA may disagree with the interpretation of the results of, or the sufficiency of the data from, our clinical trials of rimegepant. There can be no assurance that our NDA for rimegepant will be submitted in the time frame that we anticipate or that, if accepted for review, the NDA will be approved by FDA. |
• | If we fail to comply with our obligations under our existing and any future intellectual property licenses with third parties, we could lose license rights that are important to our business. |
• | We rely in part on third parties to conduct our preclinical studies and clinical trials and if these third parties perform in an unsatisfactory manner, our business could be substantially harmed. |
• | We currently rely on third parties for the production of our clinical supply of our product candidates and we intend to continue to rely on third parties for our clinical and commercial supply. |
• | We have never commercialized a product candidate and we may lack the necessary expertise, personnel and resources to successfully commercialize any of our products that receive regulatory approval on our own or together with collaborators. |
• | We currently have no marketing, sales or distribution infrastructure. If we are unable to develop sales, marketing and distribution capabilities on our own or through collaborations, or if we fail to achieve adequate pricing or reimbursement, we will not be successful in commercializing our product candidates, if approved. |
• | If we are unable to obtain and maintain patent protection for our technology and product candidates, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets. |
• | An active trading market for our common shares may not continue to develop or be sustained, or be liquid enough for investors to resell our common shares quickly or at the market price. |
• | We are dependent on licensed intellectual property. If we were to lose our rights to licensed intellectual property, we may not be able to continue developing or commercializing our product candidates, if approved. If we breach any of the agreements under which we license the use, development and commercialization rights to our product candidates or technology from third parties or, in certain cases, we fail to meet certain development deadlines, we could lose license rights that are important to our business. |
• | We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common shares less attractive to investors. |
• | You may have fewer protections as a shareholder of our company, as the rights of shareholders under British Virgin Islands law differ from those under U.S. law. |
• | Our independent registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited financial statements included in our Annual Report on Form 10‑K for the year ended December 31, 2017, which is incorporated by reference into this prospectus. |
• | We have identified material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, we may not be able to accurately or timely report our financial condition or results of operations, |
9
which may adversely affect investor confidence in us and, as a result, the value of our common shares.
Corporate Information
We were incorporated as a business company limited by shares organized under the laws of the British Virgin Islands in September 2013. Our registered office is located at P.O. Box 173, Road Town, Tortola, British Virgin Islands and our telephone number is +1 (284) 852‑3000. Our U.S. office and the office of our U.S. subsidiary is located at 215 Church Street, New Haven, Connecticut 06510 and our telephone number is (203) 404‑0410. Our website address is www.biohavenpharma.com. The information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common shares.
We have three wholly owned subsidiaries, including Biohaven Pharmaceuticals, Inc., a Delaware corporation. We also expect to form one or more additional subsidiaries that will be incorporated under the laws of Ireland and resident for tax purposes in Ireland. We expect that an Irish subsidiary will be the principal operating company for conducting our business and the entity that will hold our intellectual property rights in certain of our product candidates. As a result, we expect that we will become subject to taxation in Ireland in the future.
We have proprietary rights to a number of trademarks used in this prospectus which are important to our business, including the Biohaven logo. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. All other trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion in revenues during our last fiscal year, we qualify as an emerging growth company as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, enacted in 2012. As an emerging growth company, we expect to take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
• | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes‑Oxley Act of 2002, as amended; |
• | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
• | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We may use these provisions until December 31, 2018. However, based on our public float as of June 30, 2018, we expect to become a “large accelerated filer” and cease to be an emerging growth company as of December 31, 2018. As a result, our independent registered public accounting firm will be required to issue a report on the effectiveness of our internal control over financial reporting, which report will be included in our Annual Report on Form 10-K for the year ending December 31, 2018.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part. As a result, the information that we provide to our shareholders may be different than you might receive from other public reporting companies in which you hold equity interests.
10
The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. We have irrevocably elected not to avail ourselves of this exemption and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
11
THE OFFERING
Common shares offered | 3,000,000 shares |
Common shares outstanding after this offering | 43,261,750 (43,711,750 shares if the underwriters exercise their option to purchase additional shares in full) |
Option to purchase additional shares | We have granted the underwriters the option, exercisable for 30 days from the date of this prospectus, to purchase up to 450,000 additional common shares. |
Use of proceeds | We estimate that the net proceeds to us from this offering, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, will be approximately $114.0 million, based on the assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018. We anticipate that the net proceeds from this offering, together with our existing cash, will be used to: • advance and expand the development of our CGRP receptor antagonist platform, including our planned NDA filing in 2019;• advance and expand the development of glutamate modulation platform product candidates and begin development of our myeloperoxidase platform; and• for working capital and other corporate purposes, including satisfaction of any of our milestone payment obligations under our license agreements. See “Use of Proceeds” on page 22 for additional information. |
Risk Factors | See “Risk Factors” on page 17 of this prospectus and the “Risk Factors” section included our most recent Quarterly Report on Form 10‑Q, which is incorporated by reference herein, for a discussion of factors to consider carefully before deciding to invest in our common shares. |
NYSE symbol | BHVN |
The number of common shares that will be outstanding after this offering is based on 40,261,750 common shares outstanding as of September 30, 2018, and excludes:
• | 5,982,134 common shares issuable upon the exercise of stock options outstanding as of September 30, 2018, at a weighted average exercise price of $11.62 per share; |
• | 221,751 common shares issuable upon the exercise of warrants outstanding as of September 30, 2018, at a weighted average exercise price of $9.68 per share; and |
• | 2,208,631 common shares reserved for future issuance under our 2017 Equity Incentive Plan, or the 2017 Plan, as of September 30, 2018 (of which we granted stock options to purchase an aggregate of 1,582,500 common shares, at a weighted average exercise price of $32.66 per share, subsequent to September 30, 2018); and |
• | 699,716 common shares reserved for future issuance under our 2017 Employee Share Purchase Plan, or ESPP, as of September 30, 2018 as well as any automatic increases in the number of common shares reserved for issuance under the 2017 Plan and the ESPP after the date of this prospectus. |
12
Except as otherwise indicated herein, all information in this prospectus, including the number of shares that will be outstanding after this offering, assumes:
• | no exercise of the outstanding options and warrants described above; and |
• | no exercise of the underwriters’ option to purchase additional shares. |
13
SUMMARY CONSOLIDATED FINANCIAL DATA
The following tables set forth our summary consolidated financial data as of and for the periods presented. We have derived the consolidated statement of operations data for the years ended December 31, 2015, 2016 and 2017 from our audited consolidated financial statements included in our most recent Annual Report on Form 10‑K, which is incorporated by reference into this prospectus. We have derived the consolidated statement of operations data for the nine months ended September 30, 2017 and 2018 and the consolidated balance sheet data as of September 30, 2018 from our unaudited condensed consolidated financial statements included in our most recent Quarterly Report on Form 10-Q, which is incorporated by reference into this prospectus. Our historical results are not necessarily indicative of the results to be expected in the future. This information is only a summary and should be read together with our consolidated financial statements and accompanying notes and Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our most recent Annual Report on Form 10‑K and our most recent Quarterly Report on Form 10-Q, which are incorporated by reference into this prospectus, as well as the “Selected Consolidated Financial Data” section of this prospectus.
14
Year Ended December 31, | Nine Months Ended September 30, | ||||||||||||||||||
2015 | 2016 | 2017 | 2017 | 2018 | |||||||||||||||
(in thousands, except share and per share data) | |||||||||||||||||||
Consolidated Statement of Operations Data: | |||||||||||||||||||
Operating expenses: | |||||||||||||||||||
Research and development | $ | 7,559 | $ | 55,529 | $ | 89,441 | $ | 66,755 | $ | 151,993 | |||||||||
General and administrative | 2,137 | 5,109 | 18,141 | 12,527 | 24,495 | ||||||||||||||
Total operating expenses | 9,696 | 60,638 | 107,582 | 79,282 | 176,488 | ||||||||||||||
Loss from operations | (9,696 | ) | (60,638 | ) | (107,582 | ) | (79,282 | ) | (176,488 | ) | |||||||||
Other income (expense): | |||||||||||||||||||
Interest expense | — | (385 | ) | (906 | ) | (906 | ) | (33 | ) | ||||||||||
Non-cash interest expense on liability related to sale of future royalties | — | — | — | — | (6,134 | ) | |||||||||||||
Change in fair value of warrant liability | — | 154 | (3,241 | ) | (5,509 | ) | (1,182 | ) | |||||||||||
Change in fair value of derivative liability | (370 | ) | (65 | ) | 512 | 512 | — | ||||||||||||
Change in fair value of contingent equity liability | — | (2,263 | ) | (13,082 | ) | (13,082 | ) | — | |||||||||||
Loss from equity method investment | — | (247 | ) | (1,885 | ) | (1,204 | ) | (2,066 | ) | ||||||||||
Other | — | — | — | 10 | 4 | ||||||||||||||
Total other income (expense), net | (370 | ) | (2,806 | ) | (18,602 | ) | (20,179 | ) | (9,411 | ) | |||||||||
Loss before provision for income taxes | (10,066 | ) | (63,444 | ) | (126,184 | ) | (99,461 | ) | (185,899 | ) | |||||||||
Provision for income taxes | — | 90 | 1,006 | 647 | 273 | ||||||||||||||
Net loss | (10,066 | ) | (63,534 | ) | (127,190 | ) | (100,108 | ) | (186,172 | ) | |||||||||
Less: Net (income) loss attributable to non‑controlling interests | (4 | ) | 143 | — | — | — | |||||||||||||
Accretion of beneficial conversion feature on Series A preferred shares | — | — | (12,006 | ) | (12,006 | ) | — | ||||||||||||
Net loss attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd. | $ | (10,062 | ) | $ | (63,677 | ) | $ | (139,196 | ) | $ | (112,114 | ) | $ | (186,172 | ) | ||||
Net loss per share attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd.—basic and diluted (1) | $ | (0.91 | ) | $ | (5.05 | ) | $ | (5.00 | ) | $ | (4.47 | ) | $ | (4.82 | ) | ||||
Weighted average common shares outstanding—basic and diluted (1) | 11,009,277 | 12,608,366 | 27,845,576 | 25,102,920 | 38,636,072 | ||||||||||||||
________________
(1) | See Note 15 to our audited consolidated financial statements included in our most recent Annual Report on Form 10‑K and Note 11 to our unaudited condensed consolidated financial statements included in our most recent Quarterly Report on Form 10‑Q, which are incorporated by reference into this prospectus, for further details on the calculation of basic and diluted net loss per share attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd. |
15
As of September 30, 2018 | |||||||
Actual | As Adjusted(2) | ||||||
(in thousands) | |||||||
Consolidated Balance Sheet Data: | |||||||
Cash | $ | 168,185 | $ | 282,224 | |||
Working capital (1) | 167,716 | 281,755 | |||||
Total assets | 195,091 | 309,130 | |||||
Liability related to sale of future royalties, net | 111,919 | 111,919 | |||||
Total shareholders’ equity | 65,813 | 179,852 | |||||
________________
(1) | We define working capital as current assets less current liabilities. |
(2) | The as adjusted balance sheet data give effect to our issuance and sale of 3,000,000 common shares in this offering at an assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
A $1.00 increase (decrease) in the assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, would increase or decrease each of cash, working capital, total assets and total shareholders’ equity on an as adjusted basis by $2.8 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions. An increase (decrease) of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) each of cash, working capital, total assets and total shareholders’ equity on an as adjusted basis by $19.1 million, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions.
16
RISK FACTORS
Investing in our common shares involves a high degree of risk. Before you invest in our common shares, you should carefully consider the following risks, as well as general economic and business risks, including those set forth under the heading “Risk Factors” in our Quarterly Report on Form 10‑Q for the quarter ended September 30, 2018 incorporated by reference herein, and all of the other information contained in this prospectus and in the documents incorporated by reference herein. Any of the following risks as well as the risks discussed in the documents incorporated by reference herein, could have a material adverse effect on our business, operating results and financial condition and cause the trading price of our common shares to decline, which would cause you to lose all or part of your investment. When determining whether to invest, you should also refer to the other information contained or incorporated by reference in this prospectus, including our financial statements and the related notes thereto. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us may also adversely affect our business.
Risks related to this offering and ownership of our common shares
If you purchase common shares in this offering, you will suffer immediate dilution of your investment.
The assumed public offering price of our common shares is substantially higher than the net tangible book value per share. Therefore, if you purchase common shares in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. Based on an assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, you will experience immediate dilution of $36.51 per share, representing the difference between our as adjusted net tangible book value per share after giving effect to this offering and the assumed public offering price.
In addition, as of September 30, 2018, we had outstanding stock options to purchase an aggregate of 5,982,134 common shares at a weighted average exercise price of $11.62 per share and outstanding warrants to purchase an aggregate of 221,751 common shares at a weighted average exercise price of $9.68 per share. In addition, we issued stock options to purchase an additional 1,582,500 common shares, at a weighted average exercise price of $32.66 per share, subsequent to September 30, 2018. To the extent these outstanding options or warrants are exercised, there will be further dilution to investors in this offering.
Sales of a substantial number of our common shares in the public market could occur at any time. This could cause the market price of our common shares to drop significantly, even if our business is doing well.
Sales of a substantial number of our common shares in the public market could occur at any time, subject to the restrictions and limitations described below. If our shareholders sell, or the market perceives that our shareholders intend to sell, substantial amounts of our common shares in the public market, the market price of our common shares could decline significantly.
Upon the closing of this offering, based upon the number of shares outstanding as of September 30, 2018, we will have 43,261,750 outstanding common shares. Of these shares, approximately 42,150,639 common shares, including the 3,000,000 shares sold in this offering, will be freely tradable, subject, in the case of our affiliates, to the conditions of Rule 144 under the Securities Act.
Of these outstanding shares, approximately 11 million shares are subject to contractual lock‑up agreements with the underwriters for this offering for 45 days following this offering. The lead underwriters of this offering may release these shareholders from their lock‑up agreements at any time and without notice, which would allow for earlier sales of shares in the public market subject to the conditions of Rule 144 under the Securities Act.
17
In addition, we have filed a registration statement on Form S‑8 registering the issuance of 12,849,968 common shares subject to options or other equity awards issued or reserved for future issuance under our equity incentive plans. Shares registered under this registration statement on Form S‑8 are available for sale in the public market subject to vesting arrangements and exercise of options, the lock‑up agreements described above and, in the case of our affiliates, the restrictions of Rule 144.
Additionally, the holders of an aggregate of approximately 7.9 million common shares, or their transferees, have rights, subject to some conditions, to require us to file one or more registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other shareholders. If we were to register the resale of these shares, they could be freely sold in the public market without limitation. If these additional shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common shares could decline.
We will have broad discretion in the use of our existing cash, including the proceeds from this offering, and may invest or spend our cash in ways with which you do not agree and in ways that may not increase the value of your investment.
We will have broad discretion over the use of our cash, including the proceeds from this offering. You may not agree with our decisions, and our use of cash may not yield any return on your investment. We expect to use the net proceeds from this offering, together with our existing cash, to advance and expand the development of our CGRP receptor antagonist platform, including our planned NDA filing in 2019, and glutamate modulation platform product candidates, begin development of our myeloperoxidase platform and for working capital and general corporate purposes, including satisfaction of any of our milestone payment obligations under our license agreements. In addition, we may use a portion of the proceeds from this offering to pursue our strategy to in‑license or acquire additional drug candidates. Our failure to apply the net proceeds from this offering effectively could compromise our ability to pursue our growth strategy and we might not be able to yield a significant return, if any, on our investment of these net proceeds. You will not have the opportunity to influence our decisions on how to use our net proceeds from this offering.
The currently reported results of our Zydis ODT Phase 3 trial of rimegepant are based on topline data for the trial and may differ from complete trial results once additional data are received and evaluated. Also, our long-term safety study of rimegepant is ongoing and could result in adverse safety data in the future. In addition, the FDA may disagree with the interpretation of the results of, or the sufficiency of the data from our clinical trials of rimegepant. There can be no assurance that our NDA for rimegepant will be submitted in the time frame that we anticipate or that, if accepted for review, the NDA will be approved by the FDA.
The reported results of our ODT Phase 3 trial of rimegepant consist of only topline data on the co‑primary endpoints and certain secondary endpoints as well as safety and tolerability data. Topline data are based on a preliminary analysis of currently available efficacy and safety data, and therefore these results are subject to change following a comprehensive review of the more extensive data we expect to receive when available. These data are based on important assumptions, estimations, calculations and information currently available to us, and we have not received or had an opportunity to evaluate all of the data from this trial, including data with respect to all of the secondary and other endpoints from this trial. As a result, we may have additional, different or conclusions that may qualify the to‑line results, once the complete data have been received and fully evaluated.
In addition, we cannot be certain that we will submit our NDA for rimegepant to the FDA within the timeframe we currently expect. Additionally, while we currently intend to submit the initial NDA for the Zydis ODT formulation followed by an NDA for the tablet formulation, we cannot be certain whether the NDA we submit will be for the Zydis ODT formulation, the tablet formation or both. Prior to submitting any NDA, in addition to completing our analysis of the data from the Zydis ODT trial and the data from our ongoing long-term safety study of rimegepant, we also must provide information relating to the manufacture of rimegepant and other modules of any NDA. In addition, our long-term safety study is ongoing and there
18
can be no assurance that we will not receive adverse safety data from this study in the future. In addition, there can be no assurance that the FDA will agree with our conclusions or the conclusions of our investigators or independent liver panel with the regard to whether the adverse events observed in the clinical trials were related to rimegepant or with regard to the safety data generally. For example, the FDA has indicated its desire to see data from our safety study in which patients receive daily or near-daily dosing of rimegepant, but did not specify how much data would be sufficient. While we have designed our long-term safety study of rimegepant to generate this data, there can be no assurance that a sufficient number of patients in the trial have taken or will take rimegepant frequently enough to adequately address the FDA’s request.
If any of the additional information we must generate is not positive or is delayed or if future safety data from our ongoing long-term safety study is not positive, we may not be able to submit any of our NDAs for rimegepant within the timeframe we currently anticipate or at all. There also can be no assurance that once submitted, the FDA will accept any of our NDAs for filing and review.
Even if our NDA is accepted by the FDA for review, clinical trial results and other aspects of the information in our NDA will be subject to interpretation and we cannot be certain that the clinical trial results and other information in our NDA for rimegepant will be sufficient to support approval of the NDA. Among other things, the FDA may, despite prior published guidance and advice, decide that the clinical and non‑clinical data from our rimegepant development program are not sufficient to support regulatory approval.
If we are unable to obtain or are delayed in obtaining FDA approval for rimegepant it would materially adversely affect our business, financial condition, results of operations and prospects and the value of our common shares.
Sales of our common shares through this or other equity offerings could trigger a limitation on our ability to use our net operating losses and tax credits in the future.
The Tax Reform Act of 1986 limits the annual use of net operating loss and tax credit carryforwards in certain situations where changes occur in stock ownership of a company. In the event we have certain changes in ownership the annual utilization of such carryforwards could be limited. This or other equity issuances could trigger a limitation on our ability to use our net operating losses and tax credits in the future under Sections 382 and 383 of the Internal Revenue Code as enacted by the Tax Reform Act of 1986.
19
INFORMATION REGARDING FORWARD‑LOOKING STATEMENTS
This prospectus includes forward‑looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, strategy and plans, and our expectations for future operations, are forward‑looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “design,” “intend,” “expect,” “could,” “plan,” “potential,” “predict,” “seek,” “should,” “would” or the negative version of these words and similar expressions are intended to identify forward‑looking statements. We have based these forward‑looking statements on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short‑ and long‑term business operations and objectives, and financial needs. These forward‑looking statements include, but are not limited to, statements concerning the following:
• | our plans to develop and commercialize our product candidates; |
• | our ongoing and planned clinical trials for our rimegepant, trigriluzole, BHV‑0223 and BHV‑5000 development programs; |
• | the timing of the availability of data from our clinical trials; |
• | our expectation that our planned NDA for rimegepant would be based on the Zydis ODT formulation of rimegepant; |
• | the timing of our planned regulatory filings, including our planned NDA submission for rimegepant; |
• | the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; |
• | the clinical utility of our product candidates; |
• | our commercialization, marketing and manufacturing capabilities and strategy; |
• | our intellectual property position; and |
• | our estimates regarding future revenues, expenses and needs for additional financing. |
These forward‑looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward‑looking statements we may make. In light of these risks, uncertainties and assumptions, the forward‑looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward‑looking statements.
You should not rely upon forward‑looking statements as predictions of future events. Although we believe that the expectations reflected in the forward‑looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward‑looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward‑looking statements. We undertake no obligation to update publicly any forward‑looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the
20
understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
21
USE OF PROCEEDS
We estimate that the net proceeds from our issuance and sale of common shares in this offering will be approximately $114.0 million, or approximately $131.2 million if the underwriters exercise their option to purchase additional shares in full, based upon an assumed public offering price of $40.67 per share, which is the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed public offering price of $40.67 per share would increase or decrease the net proceeds from this offering by approximately $2.8 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 500,000 shares in the number of common shares we are offering would increase or decrease the net proceeds from this offering by $19.1 million, assuming no change in the assumed public offering price.
We currently estimate that we will use the net proceeds from this offering, together with our existing cash to:
• | advance and expand the development of our CGRP receptor antagonist platform, including our planned NDA filing in 2019; |
• | advance and expand the development of glutamate modulation platform product candidates and begin development of our myeloperoxidase platform; and |
• | for working capital and other corporate purposes, including satisfaction of any of our milestone payment obligations under our license agreements. |
In the ordinary course of our business, we expect to from time to time evaluate the acquisition of, investment in or in‑license of complementary products, technologies or businesses, and we could use a portion of the net proceeds from this offering for such activities. We currently do not have any agreements, arrangements or commitments with respect to any potential acquisition, investment or license.
This expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. Predicting the cost necessary to develop product candidates can be difficult and the amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development, the status of and results from clinical trials, any collaborations that we may enter into with third parties for our product candidates and any unforeseen cash needs.
Based on our current operational plans and assumptions, we expect that the net proceeds from this offering, together with our existing cash, will be sufficient to fund our operations into the fourth quarter of 2020. However, our plans and assumptions could be wrong, and we may need to raise additional capital in order to complete our planned and ongoing trials and any potential future trials that may be required by regulatory authorities. We may need to raise additional capital through public and private equity offerings, debt financings, strategic partnerships, alliances and licensing arrangements, or a combination of the above.
Our management will have broad discretion in the application of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds from this offering. The timing and amount of our actual expenditures will be based on many factors, including cash flows from operations and the anticipated growth of our business. Pending these uses, we plan to hold these net proceeds in non‑interest bearing accounts, with the goal of capital preservation and liquidity so that such funds are readily available to fund our operations.
22
MARKET PRICE OF COMMON SHARES
Our common shares commenced trading on the New York Stock Exchange under the symbol “BHVN” on May 4, 2017. As of December 7, 2018, there were 62 holders of record of our common shares. The actual number of shareholders is greater than this number of record holders and includes shareholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include shareholders whose shares may be held in trust by other entities. The last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018 was $40.67 per share.
23
DIVIDEND POLICY
We have never declared or paid any dividends on our common shares. We anticipate that we will retain all of our future earnings, if any, for use in the operation and expansion of our business and do not anticipate paying cash dividends in the foreseeable future.
24
CAPITALIZATION
The following table sets forth our cash and our capitalization as of September 30, 2018:
• | on an actual basis; and |
• | an as adjusted basis to give effect to our issuance and sale of 3,000,000 common shares in this offering at an assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The as adjusted information below is illustrative only of our cash and capitalization following the completion of this offering and will change based on the actual number of shares sold, the public offering price and other terms of this offering determined at pricing. You should read this table together with our consolidated financial statements and accompanying notes and Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our most recent Quarterly Report on Form 10‑Q, which is incorporated by reference into this prospectus.
As of September 30, 2018 | |||||||
Actual | As Adjusted | ||||||
(in thousands, except share and per share data) | |||||||
Cash | $ | 168,185 | $ | 282,224 | |||
Liability related to sale of future royalties, net | $ | 111,919 | $ | 111,919 | |||
Shareholders’ equity: | |||||||
Common shares, no par value; 200,000,000 shares authorized, 40,261,750 shares issued and outstanding, actual; 200,000,000 shares authorized, 43,261,750 shares issued and outstanding, as adjusted | 418,273 | 532,312 | |||||
Additional paid‑in capital | 36,358 | 36,358 | |||||
Accumulated deficit | (388,818 | ) | (388,818 | ) | |||
Total shareholders’ equity | 65,813 | 179,852 | |||||
Total capitalization | $ | 177,732 | $ | 291,771 | |||
A $1.00 increase (decrease) in the assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, would increase (decrease) the as adjusted amount of each of cash, total shareholders’ equity and total capitalization by $2.8 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions. An increase (decrease) of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the as adjusted amount of each of cash, total shareholders’ equity and total capitalization by $19.1 million, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions.
The number of common shares outstanding in the table above does not include:
• | 5,982,134 common shares issuable upon the exercise of stock options outstanding as of September 30, 2018, at a weighted average exercise price of $11.62 per share; |
25
• | 221,751 common shares issuable upon the exercise of warrants outstanding as of September 30, 2018, at a weighted average exercise price of $9.68 per share; and |
• | 2,208,631 common shares reserved for future issuance under our 2017 Equity Incentive Plan, or the 2017 Plan, as of September 30, 2018 (of which we granted stock options to purchase an aggregate of 1,582,500 common shares, at a weighted average exercise price of $32.66 per share, subsequent to September 30, 2018); and |
• | 699,716 common shares reserved for future issuance under our 2017 Employee Share Purchase Plan, or ESPP, as of September 30, 2018 as well as any automatic increases in the number of common shares reserved for issuance under the 2017 Plan and the ESPP after the date of this prospectus. |
26
DILUTION
If you invest in our common shares in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per common share and the as adjusted net tangible book value per common share immediately after this offering.
Our historical net tangible book value as of September 30, 2018 was $65.8 million, or $1.63 per common share. Our historical net tangible book value is the amount of our total tangible assets less our total liabilities. Historical net tangible book value per share represents historical net tangible book value divided by the 40,261,750 common shares outstanding as of September 30, 2018.
After giving effect to the issuance and sale of 3,000,000 common shares in this offering at an assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2018 would have been $179.9 million, or $4.16 per common share. This represents an immediate increase in as adjusted net tangible book value of $2.53 per share to existing shareholders and immediate dilution in as adjusted net tangible book value of $36.51 per share to new investors purchasing common shares in this offering. The dilution information discussed above is for illustrative purposes only and will change based on the actual public offering price. The following table illustrates this per share dilution:
Assumed public offering price per share | $ | 40.67 | |||||
Historical net tangible book value per share as of September 30, 2018 | $ | 1.63 | |||||
Increase in as adjusted net tangible book value per share attributable to this offering | 2.53 | ||||||
As adjusted net tangible book value per share after this offering | 4.16 | ||||||
Dilution per share to new investors purchasing common shares in this offering | $ | 36.51 | |||||
A $1.00 increase (decrease) in the assumed public offering price of $40.67 per share, which was the last reported sale price of our common shares on the New York Stock Exchange on December 7, 2018, would increase (decrease) our as adjusted net tangible book value after this offering by $0.06 per share and the dilution to new investors purchasing common shares in this offering by $0.94 per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discount and commissions. An increase of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase the as adjusted net tangible book value after this offering by $0.39 per share and decrease the dilution to new investors purchasing common shares in this offering by $0.39 per share, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions. A decrease of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would decrease the as adjusted net tangible book value after this offering by $0.40 per share and increase the dilution to new investors purchasing common shares in this offering by $0.40 per share, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions.
If the underwriters exercise their option to purchase additional shares in this offering in full, the as adjusted net tangible book value per share after this offering would be $4.51 per share, and the dilution in as adjusted net tangible book value per share to new investors purchasing common shares in this offering would be $36.16 per share, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
27
The table and discussion above do not include:
• | 5,982,134 common shares issuable upon the exercise of stock options outstanding as of September 30, 2018, at a weighted average exercise price of $11.62 per share; |
• | 221,751 common shares issuable upon the exercise of warrants outstanding as of September 30, 2018, at a weighted average exercise price of $9.68 per share; and |
• | 2,208,631 common shares reserved for future issuance under our 2017 Plan as of September 30, 2018 (of which we granted stock options to purchase an aggregate of 1,582,500 common shares, at a weighted average exercise price of $32.66 per share, subsequent to September 30, 2018); and |
• | 699,716 common shares reserved for future issuance under our ESPP as of September 30, 2018 as well as any automatic increases in the number of common shares reserved for issuance under the 2017 Plan and the ESPP after the date of this prospectus. |
28
SELECTED CONSOLIDATED FINANCIAL DATA
The following tables set forth our selected consolidated financial data as of and for the periods presented. We have derived the consolidated statement of operations data for the years ended December 31, 2015, 2016 and 2017 and the consolidated balance sheet data as of December 31, 2016 and 2017 from our audited consolidated financial statements included in our most recent Annual Report on Form 10‑K, which is incorporated by reference into this prospectus. We have derived the consolidated statement of operations data for the nine months ended September 30, 2017 and 2018 and the consolidated balance sheet data as of September 30, 2018 from our unaudited condensed consolidated financial statements included in our most recent Quarterly Report on Form 10-Q, which is incorporated by reference into this prospectus. Our historical results are not necessarily indicative of results that may be expected in any future period. This information should be read together with our consolidated financial statements and accompanying notes and Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our most recent Annual Report on Form 10‑K and our most recent Quarterly Report on Form 10-Q, which are incorporated by reference into this prospectus.
29
Year Ended December 31, | Nine Months Ended September 30, | ||||||||||||||||||
2015 | 2016 | 2017 | 2017 | 2018 | |||||||||||||||
(in thousands, except share and per share data) | |||||||||||||||||||
Consolidated Statement of Operations Data: | |||||||||||||||||||
Operating expenses: | |||||||||||||||||||
Research and development | $ | 7,559 | $ | 55,529 | $ | 89,441 | $ | 66,755 | $ | 151,993 | |||||||||
General and administrative | 2,137 | 5,109 | 18,141 | 12,527 | 24,495 | ||||||||||||||
Total operating expenses | 9,696 | 60,638 | 107,582 | 79,282 | 176,488 | ||||||||||||||
Loss from operations | (9,696 | ) | (60,638 | ) | (107,582 | ) | (79,282 | ) | (176,488 | ) | |||||||||
Other income (expense): | |||||||||||||||||||
Interest expense | — | (385 | ) | (906 | ) | (906 | ) | (33 | ) | ||||||||||
Non-cash interest expense on liability related to sale of future royalties | — | — | — | — | (6,134 | ) | |||||||||||||
Change in fair value of warrant liability | — | 154 | (3,241 | ) | (5,509 | ) | (1,182 | ) | |||||||||||
Change in fair value of derivative liability | (370 | ) | (65 | ) | 512 | 512 | — | ||||||||||||
Change in fair value of contingent equity liability | — | (2,263 | ) | (13,082 | ) | (13,082 | ) | — | |||||||||||
Loss from equity method investment | — | (247 | ) | (1,885 | ) | (1,204 | ) | (2,066 | ) | ||||||||||
Other | — | — | — | 10 | 4 | ||||||||||||||
Total other income (expense), net | (370 | ) | (2,806 | ) | (18,602 | ) | (20,179 | ) | (9,411 | ) | |||||||||
Loss before provision for income taxes | (10,066 | ) | (63,444 | ) | (126,184 | ) | (99,461 | ) | (185,899 | ) | |||||||||
Provision for income taxes | — | 90 | 1,006 | 647 | 273 | ||||||||||||||
Net loss | (10,066 | ) | (63,534 | ) | (127,190 | ) | (100,108 | ) | (186,172 | ) | |||||||||
Less: Net (income) loss attributable to non‑controlling interests | (4 | ) | 143 | — | — | — | |||||||||||||
Accretion of beneficial conversion feature on Series A preferred shares | — | — | (12,006 | ) | (12,006 | ) | — | ||||||||||||
Net loss attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd. | $ | (10,062 | ) | $ | (63,677 | ) | $ | (139,196 | ) | $ | (112,114 | ) | $ | (186,172 | ) | ||||
Net loss per share attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd.—basic and diluted (1) | $ | (0.91 | ) | $ | (5.05 | ) | $ | (5.00 | ) | $ | (4.47 | ) | $ | (4.82 | ) | ||||
Weighted average common shares outstanding—basic and diluted (1) | 11,009,277 | 12,608,366 | 27,845,576 | 25,102,920 | 38,636,072 | ||||||||||||||
________________
(1) | See Note 15 to our audited consolidated financial statements included in our most recent Annual Report on Form 10‑K and Note 11 to our unaudited condensed consolidated financial statements included in our most recent Quarterly Report on Form 10‑Q, which are incorporated by reference into this prospectus for further details on the calculation of basic and diluted net loss per share attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd. |
30
As of December 31, | As of September 30, | ||||||||||
2016 | 2017 | 2018 | |||||||||
(in thousands) | |||||||||||
Consolidated Balance Sheet Data: | |||||||||||
Cash | $ | 23,565 | $ | 131,468 | $ | 168,185 | |||||
Working capital (1) | 16,093 | 127,236 | 167,716 | ||||||||
Total assets | 27,017 | 146,888 | 195,091 | ||||||||
Notes payable, including to related parties, net of discount | 4,811 | — | — | ||||||||
Warrant liability | 780 | 4,021 | — | ||||||||
Liability related to sale of future royalties, net | — | — | 111,919 | ||||||||
Contingent equity liability | 18,938 | — | — | ||||||||
Total liabilities | 28,780 | 14,917 | 129,278 | ||||||||
Convertible preferred shares classified outside of shareholders’ deficit | 43,270 | — | — | ||||||||
Total shareholders’ equity (deficit) | (45,033) | 131,971 | 65,813 | ||||||||
________________
(1) | We define working capital as current assets less current liabilities. |
31
PRINCIPAL SHAREHOLDERS
The following table sets forth the beneficial ownership of our common shares as of November 30, 2018 for:
• | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common shares; |
• | each of our named executive officers; |
• | each of our directors; and |
• | all of our current executive officers and directors as a group. |
The percentage ownership information shown in the column titled “Before Offering” in the table is based upon 40,327,610 common shares outstanding as of November 30, 2018. The percentage ownership information shown in the column titled “After Offering” in the table is based on 43,327,610 common shares outstanding after this offering, assuming 3,000,000 common shares being sold by us in the offering.
We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include common shares issuable pursuant to the exercise of stock options or warrants that are exercisable on or before January 29, 2019, which is 60 days after November 30, 2018. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws. Except as otherwise noted below, the address for persons listed in the table is c/o Biohaven Pharmaceutical Holding Company Ltd., 215 Church Street, New Haven, CT 06510.
32
Percentage of Shares Beneficially Owned | |||||||||
Name of Beneficial Owner | Number of Shares Beneficially Owned | Before Offering | After Offering | ||||||
Principal Shareholders: | |||||||||
Entities affiliated with Vivo Capital Fund VII, L.P.(1) | 2,049,545 | 5.1 | % | 4.7 | % | ||||
Executive Officers and Directors: | |||||||||
Vlad Coric, M.D.(2) | 1,547,500 | 3.8 | % | 3.6 | % | ||||
Robert Berman, M.D.(3) | 1,194,851 | 3.0 | % | 2.8 | % | ||||
James Engelhart(4) | 194,578 | * | * | ||||||
John Tilton(5) | 83,893 | * | * | ||||||
Charles Conway, Ph.D.(6) | 60,153 | * | * | ||||||
Kimberly Gentile(7) | 93,278 | * | * | ||||||
Eric Aguiar, M.D.(8) | 51,539 | * | * | ||||||
Gregory H. Bailey, M.D.(9) | 2,983,376 | 7.4 | % | 6.9 | % | ||||
Robert Repella | — | — | — | ||||||
John W. Childs(10) | 3,227,713 | 8.0 | % | 7.4 | % | ||||
Declan Doogan, M.D.(11) | 2,137,415 | 5.3 | % | 4.9 | % | ||||
Julia Gregory(12) | 12,000 | * | * | ||||||
All current directors and executive officers as a group (12 persons)(13) | 11,602,257 | 28.8 | % | 26.8 | % | ||||
________________
(1) | This information has been obtained from Vivo Capital VIII, LLC (“Vivo GP”), which is the general partner of both Vivo Capital Fund VIII, L.P. (“VCF”) and Vivo Capital Surplus Fund VIII, L.P. (“VCSF”). Shares beneficially owned prior to this offering consist of (i) 1,800,866 common shares held by VCF and (ii) 248,679 common shares held by VCSF. The voting members of Vivo GP are Frank Kung, Albert Cha, Edgar Engleman, Chen Yu and Shan Fu, none of whom has individual voting or investment power with respect to these shares and each of whom disclaims beneficial ownership of such shares. The principal business address of VCF is 505 Hamilton Avenue, Suite 207, Palo Alto, CA 94301. |
(2) | Consists of (i) 525,000 common shares held by The Vladimir Coric Family Trust 2013, (ii) 490,000 common shares held by The Vladimir Coric Marital Trust 2013 and (iii) 532,500 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(3) | Consists of (i) 550,000 common shares held by The Berman Family Trust 2013, (ii) 234,551 common shares held by The Berman Marital Trust 2013, (iii) 50 shares held by Dr. Berman’s family member and (iv) 410,250 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(4) | Consists of common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(5) | Consists of (i) 1,614 common shares held by JET Ventures LLC, of which Mr. Tilton is a managing member and (ii) 82,279 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(6) | Consists of (i) 4,000 common shares and (ii) 56,153 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(7) | Consists of common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(8) | Consists of (i) 32,500 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018 and (ii) 18,859 common shares underlying options held by Dr. Aguiar's family member that are vested and exercisable within 60 days of November 30, 2018. |
(9) | Consists of (i) 2,475,101 common shares, (ii) 400,775 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018 and (iii) 107,500 common shares underlying an immediately exercisable warrant. |
(10) | Consists of (i) 2,719,438 common shares, (ii) 400,775 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018 and (iii) 107,500 common shares underlying an immediately exercisable warrant. |
(11) | Consists of (i) 1,659,915 common shares and (ii) 477,500 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(12) | Consists of common shares underlying options that are vested and exercisable within 60 days of November 30, 2018. |
(13) | Consists of (i) 8,694,669 common shares, (ii) 2,692,588 common shares underlying options that are vested and exercisable within 60 days of November 30, 2018 and (iii) 215,000 common shares underlying immediately exercisable warrants. |
33
DESCRIPTION OF SHARE CAPITAL
The following descriptions are summaries of the material terms of our amended memorandum and articles of association. Reference is made to the more detailed provisions of, and the descriptions are qualified in their entirety by reference to, the memorandum and articles of association. Please note that this summary is not intended to be exhaustive. For further information, please refer to the full version of our memorandum and articles of association which is included as an exhibit to the registration statement of which this prospectus is part.
General
We are a company incorporated in the British Virgin Islands on September 25, 2013, and our affairs are governed by the provisions of our memorandum of association and articles of association, as amended and restated from time to time, and by the provisions of applicable British Virgin Islands law.
Authorized Share Capital
Our amended and restated memorandum and articles of association authorize us to issue up to 200,000,000 common shares, no par value, and 10,000,000 preferred shares, no par value, all of which preferred shares will be undesignated. Our board of directors may establish the rights and preferences of the preferred shares from time to time. As of December 7, 2018, there were 40,334,956 common shares issued and outstanding, held of record by 62 shareholders.
Common Shares
Holders of common shares are entitled to cast one vote for each share on all matters submitted to a vote of shareholders, including the election of directors.
Holders of common shares are entitled to receive ratably such dividends, if any, as may be declared by the board of directors out of funds legally available therefor, subject to the preferential rights in respect of any preferred shares. See “Dividend Policy.” Holders of common shares do not have any preemptive or other rights to subscribe for additional shares pursuant to our memorandum and articles of association.
All holders of common shares are entitled to share ratably in any assets for distribution to shareholders upon the liquidation, dissolution or winding up of the company, subject to the preferential rights in respect of any preferred shares.
The memorandum and articles of association permit the board of directors to authorize the redemption, purchase or other acquisition of the common shares. All outstanding common shares are fully paid and nonassessable.
Holders of common shares have no conversion or subscription rights and there are no redemption or sinking fund provisions applicable to the common shares. The rights, preferences and privileges of the holders of common shares are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred shares that we may designate in the future.
Preferred Shares
Our board of directors has the authority, without further action by our shareholders, to issue up to 10,000,000 preferred shares in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors may authorize the issuance of preferred shares with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common shares. The
34
purpose of authorizing our board of directors to issue preferred shares and determine its rights and preferences is to eliminate delays associated with a shareholder vote on specific issuances. The issuance of preferred shares, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of us and may adversely affect the market price of our common shares and the voting and other rights of the holders of our common shares. It is not possible to state the actual effect of the issuance of any preferred shares on the rights of holders of common shares until the board of directors determines the specific rights attached to the preferred shares.
Options
Our 2014 Equity Incentive Plan, as amended, or the 2014 Plan, provides for us to sell or issue common shares or restricted common shares, or to grant incentive stock options or nonqualified stock options for the purchase of common shares, to employees, members of the board of directors and consultants. Following the completion of our IPO, we no longer make any grants from the 2014 Plan. Our 2017 Equity Incentive Plan, or the 2017 Plan, provides for us to sell or issue common shares or restricted common shares, or to grant incentive stock options or nonqualified stock options for the purchase of common shares, to employees, members of the board of directors and consultants. As of September 30, 2018, options to purchase an aggregate of 5,982,134 common shares were outstanding under the 2014 Plan and the 2017 Plan. In addition, we issued stock options to purchase an aggregate of 1,582,500 common shares, at a weighted average exercise price of $32.66 per share, subsequent to September 30, 2018.
Warrants
On August 10, 2015, as partial consideration in connection with a license agreement, we issued a warrant to ALS Biopharma LLC, or ALS Biopharma, to purchase 275,000 common shares at an exercise price of $5.60 per share. The warrant was immediately exercisable upon issuance and exercisable for a period of 10 years from the issuance date. This warrant was exercised in January 2018.
On August 10, 2015, as partial consideration in connection with a license agreement, we issued a warrant to ALS Biopharma to purchase 325,000 common shares at an exercise price of $5.60 per share. The warrant became exercisable in May 2016 upon the achievement of a specified regulatory milestone and was exercisable for a period of 10 years from the issuance date. This warrant was exercised in April 2018.
In connection with our credit agreement with Wells Fargo, we issued warrants to two of our directors, John Childs and Gregory Bailey, to purchase 107,500 common shares each, at an exercise price of $9.2911 per share. The warrants were immediately exercisable upon issuance and may be exercised from the issuance date through the second anniversary of our IPO.
On June 6, 2017, as partial consideration issued in connection with an agreement to perform investor relations services with The Trout Group LLC, or Trout, we issued to Trout a warrant to purchase 6,751 common shares at an exercise price of $22.22 per share. The warrant was immediately exercisable upon issuance and expires 10 years from the issuance date.
Registration Rights Under Investors’ Rights Agreement
We and certain of our shareholders have entered into an amended and restated investors’ rights agreement. The registration rights provisions of this agreement provide those holders with demand, piggyback and Form S‑3 registration rights with respect to the common shares currently held by them or acquired by them in the future.
35
Demand Registration Rights
At any time beginning October 28, 2021, the holders of at least 50% of the registrable securities then outstanding have the right to demand that we file a registration statement on Form S‑1 with respect to at least 40% of the registrable securities then outstanding, or a less percent if the anticipated offering price, net of selling expenses, would exceed $10.0 million. These registration rights are subject to specified conditions and limitations, including the right of the underwriters, if any, to limit the number of shares included in any such registration under specified circumstances. Upon such a request, we are required to effect the registration as soon as practicable, but in any event no later than 60 days after the receipt of such request. Because the investor rights agreement terminates in May 2020, the third anniversary of our IPO, no common shares will be entitled to these demand registration rights.
Piggyback Registration Rights
If we propose to register any of our securities under the Securities Act either for our own account or for the account of other stockholders, the holders of registrable securities will each be entitled to notice of the registration and will be entitled to include their common shares in the registration statement. These piggyback registration rights are subject to specified conditions and limitations, including the right of the underwriters to limit the number of shares included in any such registration under specified circumstances. Approximately 7.9 million common shares are entitled to these piggyback registration rights.
Registration on Form S‑3
At any time after we become eligible to file a registration statement on Form S‑3, the holders of at least 10% of the registrable securities then outstanding will each be entitled, upon any such holders’ written request, to have such shares registered by us on a Form S‑3 registration statement at our expense. These Form S‑3 registration rights are subject to other specified conditions and limitations, including the condition that the anticipated aggregate offering price, net of selling expenses, exceeds $5.0 million. Upon receipt of this request, the holders of all registrable securities then outstanding will each be entitled to participate in this registration, and we will be required to effect the registration within 45 days after the receipt of such request from the initiating holders. Approximately 7.9 million common shares are entitled to these S‑3 registration rights.
Expenses of Registration
We will pay all expenses relating to any demand, piggyback or Form S‑3 registration, other than underwriting discounts and commissions, subject to specified conditions and limitations.
Termination of Registration Rights
The registration rights granted under the investors’ rights agreement will terminate upon the earlier of the third anniversary of the closing of our IPO, the closing of a deemed liquidation event as defined in our memorandum and articles of association or at such time as Rule 144 or another similar exemption under the Securities Act is available for the sale of all such holders of registrable securities’ shares without limitation during a three‑month period without registration.
Limitations on the Right to Own Shares
There are no limitations on the right to own our common shares.
Disclosure of Shareholder Ownership
There are no provisions in the memorandum and articles of association governing the ownership threshold above which shareholder ownership must be disclosed.
36
Differences in Corporate Law
The BVI Business Companies Act, or the BVI Act, and the laws of the British Virgin Islands, or the BVI, affecting BVI business companies like us and our shareholders differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of the significant differences between the provisions of the laws of the BVI applicable to us and the laws applicable to companies incorporated in the United States and their shareholders.
Mergers and Similar Arrangements
Under the laws of the BVI, two or more companies may merge or consolidate in accordance with Section 170 of the BVI Act. A merger means the merging of two or more constituent companies into one of the constituent companies and a consolidation means the uniting of two or more constituent companies into a new company. In order to merge or consolidate, the directors of each constituent company must approve a written plan of merger or consolidation, which must be authorized by a resolution of shareholders.
While a director may vote on the plan of merger or consolidation even if he has a financial interest in the plan, the interested director must disclose the interest to all other directors of the company promptly upon becoming aware of the fact that he is interested in a transaction entered into or to be entered into by the company.
A transaction entered into by our company in respect of which a director is interested (including a merger or consolidation) is voidable by us unless the director’s interest was (a) disclosed to the board prior to the transaction or (b) the transaction is (i) between the director and the company and (ii) the transaction is in the ordinary course of the company’s business and on usual terms and conditions.
Notwithstanding the above, a transaction entered into by the company is not voidable if the material facts of the interest are known to the shareholders and they approve or ratify it or the company received fair value for the transaction.
Shareholders not otherwise entitled to vote on the merger or consolidation may still acquire the right to vote if the plan of merger or consolidation contains any provision which, if proposed as an amendment to our memorandum and articles of association, would entitle them to vote as a class or series on the proposed amendment. In any event, all shareholders must be given a copy of the plan of merger or consolidation irrespective of whether they are entitled to vote at the meeting to approve the plan of merger or consolidation.
The shareholders of the constituent companies are not required to receive shares of the surviving or consolidated company but may receive debt obligations or other securities of the surviving or consolidated company, other assets, or a combination thereof. Further, some or all of the shares of a class or series may be converted into a kind of asset while the other shares of the same class or series may receive a different kind of asset. As such, not all the shares of a class or series must receive the same kind of consideration.
After the plan of merger or consolidation has been approved by the directors and authorized by a resolution of the shareholders, articles of merger or consolidation are executed by each company and filed with the Registrar of Corporate Affairs in the BVI.
A shareholder may dissent from a mandatory redemption of his shares, an arrangement (if permitted by the court), a merger (unless the shareholder was a shareholder of the surviving company prior to the merger and continues to hold the same or similar shares after the merger) or a consolidation. A shareholder properly exercising his dissent rights is entitled to a cash payment equal to the fair value of his shares.
37
A shareholder dissenting from a merger or consolidation must object in writing to the merger or consolidation before the vote by the shareholders on the merger or consolidation, unless notice of the meeting was not given to the shareholder. If the merger or consolidation is approved by the shareholders, the company must give notice of this fact to each shareholder within 20 days who gave written objection. These shareholders then have 20 days to give to the company their written election in the form specified by the BVI Act to dissent from the merger or consolidation, provided that in the case of a merger, the 20 days starts when the plan of merger is delivered to the shareholder.
Upon giving notice of his election to dissent, a shareholder ceases to have any shareholder rights except the right to be paid the fair value of his shares. As such, the merger or consolidation may proceed in the ordinary course notwithstanding his dissent.
Within seven days of the later of the delivery of the notice of election to dissent and the effective date of the merger or consolidation, the company must make a written offer to each dissenting shareholder to purchase his shares at a specified price per share that the company determines to be the fair value of the shares. The company and the shareholder then have 30 days to agree upon the price. If the company and a shareholder fail to agree on the price within the 30 days, then the company and the shareholder shall, within 20 days immediately following the expiration of the 30‑day period, each designate an appraiser and these two appraisers shall designate a third appraiser. These three appraisers shall fix the fair value of the shares as of the close of business on the day prior to the shareholders’ approval of the transaction without taking into account any change in value as a result of the transaction.
Shareholders’ Suits
There are both statutory and common law remedies available to our shareholders as a matter of BVI law. These are summarized below:
Prejudiced Members
A shareholder who considers that the affairs of the company have been, are being, or are likely to be, conducted in a manner that is, or any act or acts of the company have been, or are, likely to be oppressive, unfairly discriminatory or unfairly prejudicial to him in that capacity, can apply to the court under Section 184I of the BVI Act, among other things, for an order that his shares be acquired, that he be provided compensation, that the Court regulate the future conduct of the company, or that any decision of the company which contravenes the BVI Act or our memorandum and articles of association be set aside.
Derivative Actions
Section 184C of the BVI Act provides that a shareholder of a company may, with the leave of the Court, bring an action in the name of the company to redress any wrong done to it.
Just and Equitable Winding Up
In addition to the statutory remedies outlined above, shareholders can also petition for the winding up of a company on the grounds that it is just and equitable for the court to so order. Save in exceptional circumstances, this remedy is only available where the company has been operated as a quasi partnership and trust and confidence between the partners has broken down.
38
Indemnification of Directors and Executive Officers and Limitation of Liability
Under our memorandum and articles of association, we indemnify against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with legal, administrative or investigative proceedings for any person who:
• | is or was a party or is threatened to be made a party to any threatened, pending or completed proceedings, whether civil, criminal, administrative or investigative, by reason of the fact that the person is or was our director; or |
• | is or was, at our request, serving as a director or officer of, or in any other capacity is or was acting for, another body corporate or a partnership, joint venture, trust or other enterprise. |
These indemnities only apply if the person acted honestly and in good faith with a view to our best interests and, in the case of criminal proceedings, the person had no reasonable cause to believe that his conduct was unlawful. This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Anti‑takeover Provisions in Our Memorandum and Articles of Association
Some provisions of our memorandum and articles of association may discourage, delay or prevent a change in control of our company or management that shareholders may consider favorable. Yet, under BVI law, our directors may only exercise the rights and powers granted to them under our memorandum and articles of association, as they believe in good faith to be in the best interests of our company.
Directors’ Fiduciary Duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits self‑dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the transaction and that the transaction was of fair value to the corporation.
Under BVI law, our directors owe the company certain statutory and fiduciary duties including, among others, a duty to act honestly, in good faith, for a proper purpose and with a view to what the directors believe to be in the best interests of the company. Our directors are also required, when exercising powers or performing duties as a director, to exercise the care, diligence and skill that a reasonable director would exercise in comparable circumstances, taking into account without limitation, the nature of the company, the nature of the decision and the position of the director and the nature of the responsibilities undertaken. In the exercise of their powers, our directors must ensure neither they nor the company acts in a manner
39
which contravenes the BVI Act or our memorandum and articles of association. A shareholder has the right to seek damages for breaches of duties owed to us by our directors.
Shareholder Action by Written Consent
Under the Delaware General Corporation Law, a corporation may eliminate the right of shareholders to act by written consent by amendment to its certificate of incorporation. BVI law provides that shareholders may approve corporate matters by way of a written resolution without a meeting signed by or on behalf of shareholders sufficient to constitute the requisite majority of shareholders who would have been entitled to vote on such matter at a general meeting; provided that if the consent is less than unanimous, notice must be given to all non‑consenting shareholders.
Shareholder Proposals
Under the Delaware General Corporation Law, a shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings. Our memorandum and articles of association allow our shareholders holding not less than 10% of the votes of the outstanding voting shares to requisition a shareholders’ meeting. We are not obliged by law to call shareholders’ annual general meetings, but our memorandum and articles of association do permit the directors to call such a meeting and we intend to hold annual meetings of shareholders following the completion of this offering. The location of any shareholders’ meeting can be determined by the board of directors and can be held anywhere in the world.
Cumulative Voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director. As permitted under BVI law, our memorandum and articles of association do not provide for cumulative voting. As a result, our shareholders are not afforded any less protections or rights on this issue than shareholders of a Delaware corporation.
Removal of Directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. Under our memorandum and articles of association, directors can be removed from office, with cause, by a resolution of shareholders passed at a meeting called for the purpose of removing the director or for purposes including the removal of the director.
Transactions with Interested Shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or group who or which owns or owned 15% or more of the target’s outstanding voting shares within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two‑tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things,
40
prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware public corporation to negotiate the terms of any acquisition transaction with the target’s board of directors. BVI law has no comparable statute.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board. Under the BVI Act and our memorandum and articles of association, we may appoint a voluntary liquidator by a resolution of the shareholders or by resolution of directors.
Variation of Rights of Shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise.
Amendment of Governing Documents
Under the Delaware General Corporation Law, a corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. As permitted by BVI, our memorandum and articles of association may be amended by a resolution of shareholders and by a resolution of directors. Any amendment is effective from the date it is registered at the Registry of Corporate Affairs in the BVI.
Transfer Agent and Registrar
The transfer agent and registrar for our common shares is American Stock Transfer & Trust Company, LLC. The transfer agent’s address is 6201 15th Avenue, Brooklyn, NY 11219..
Stock Exchange Listing
Our common shares are listed on the New York Stock Exchange under the trading symbol “BHVN.”
41
MATERIAL UNITED STATES FEDERAL INCOME CONSIDERATIONS FOR U.S. HOLDERS
The following is a summary of material U.S. federal income tax considerations relating to the acquisition, ownership and disposition of our common shares by a U.S. holder (as defined below). This summary addresses only the U.S. federal income tax considerations for U.S. holders that are initial purchasers of our common shares pursuant to this offering and that will hold such common shares as capital assets for U.S. federal income tax purposes. This summary does not address all U.S. federal income tax matters that may be relevant to a particular U.S. holder. This summary does not address tax considerations applicable to a U.S. holder of our common shares that may be subject to special tax rules including, without limitation, the following:
• | banks, financial institutions or insurance companies; |
• | brokers, dealers or traders in securities, currencies, commodities, or notional principal contracts; |
• | tax‑exempt entities or organizations, including an “individual retirement account” or “Roth IRA” as defined in Section 408 or 408A of the Code, respectively; |
• | real estate investment trusts, regulated investment companies or grantor trusts; |
• | persons that hold the common shares as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle” for U.S. federal income tax purposes; |
• | partnerships (including entities classified as partnerships for U.S. federal income tax purposes) or other pass‑through entities, or persons that will hold the common shares through such an entity; |
• | certain former citizens or long‑term residents of the United States; |
• | U.S. holders that own directly, indirectly, or through attribution 10% or more of the voting power or value of our common shares; |
• | persons required under Section 451(b) of the Code to conform to the timing of income accruals with respect to our common shares; |
• | U.S. holders that have a “functional currency” for U.S. federal income tax purposes other than the U.S. dollar; and |
• | investors in this offering who are existing shareholders of our company. |
Further, this summary does not address the U.S. federal estate, gift, or alternative minimum tax considerations, or any U.S. state, local, or non‑U.S. tax considerations of the acquisition, ownership and disposition of our common shares.
This description is based on the Code; existing, proposed and temporary U.S. Treasury Regulations promulgated thereunder; and administrative and judicial interpretations thereof, in each case as in effect and available on the date hereof. All the foregoing is subject to change, which change could apply retroactively, and to differing interpretations, all of which could affect the tax considerations described below. No ruling has been or will be obtained from the U.S. Internal Revenue Service, or the IRS, regarding the matters discussed herein, so there can be no assurances that the IRS will not take a contrary or different position concerning the tax consequences of the acquisition, ownership and disposition of our common shares or that such a position would not be sustained. U.S. holders are urged to consult their own tax advisers concerning the U.S. federal, state, local and non‑U.S. tax consequences of acquiring, owning, and disposing of our common shares in their particular circumstances.
42
For the purposes of this summary, a “U.S. holder” is a beneficial owner of our common shares that is (or is treated as), for U.S. federal income tax purposes:
• | an individual who is a citizen or resident of the United States; |
• | a corporation, or other entity that is treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
• | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
• | a trust, (a) if a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of the substantial decisions of such trust or (b) that has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person |
If a partnership (or any other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds our common shares, the U.S. federal income tax consequences relating to an investment in the common shares will depend in part upon the status of the partner and the activities of the partnership. Such a partner or partnership is urged to consult its tax advisor regarding the U.S. federal income tax considerations of acquiring, owning and disposing of our common shares in its particular circumstances.
As indicated below, this discussion is subject to U.S. federal income tax rules applicable to a “passive foreign investment company,” or a PFIC. In addition, U.S. holders that own (directly, indirectly, or constructively) 10% or more of our total combined voting power or value could be subject to adverse U.S. federal income tax consequences pursuant to the controlled foreign corporation rules due to our ownership of one or more U.S. subsidiaries. Such prospective U.S. holders should consult with their tax advisors as to the tax consequences of acquiring, owning and disposing of our common shares.
Persons considering an investment in our common shares are urged to consult their own tax advisors as to the particular tax consequences applicable to them relating to the acquisition, ownership and disposition of our common shares, including the applicability of U.S. federal, state and local tax laws and non‑U.S. tax laws.
Tax Residence
Generally, for U.S. federal tax purposes, a corporation is considered a “domestic corporation” if it is incorporated or organized in the United States, and a “foreign corporation” if it is incorporated or organized in a non‑U.S. jurisdiction. Because we are a British Virgin Islands incorporated entity, we would be classified as a foreign corporation under these general rules. Section 7874 of the Code, or Section 7874, however, contains rules that can result in a foreign corporation being treated as a domestic corporation for U.S. federal tax purposes. Under Section 7874, a foreign corporation will nevertheless be treated as a domestic corporation for U.S. federal tax purposes if (1) the foreign corporation directly or indirectly acquires substantially all of the assets held directly or indirectly by a domestic corporation (including the indirect acquisition of assets by acquisition of all the outstanding shares of a domestic corporation), (2) the shareholders of the acquired domestic corporation hold at least 80% (by either vote or value) of the shares of the acquiring foreign corporation after the acquisition by reason of holding shares in the acquired domestic corporation (including the receipt of the foreign corporation’s shares in exchange for the domestic corporation’s shares) (the “ownership test”), and (3) the foreign corporation’s “expanded affiliated group” does not have substantial business activities in the foreign corporation’s country of organization or incorporation relative to the expanded affiliated group’s worldwide activities. For purposes of Section 7874, “expanded affiliated group” means the foreign corporation and all subsidiaries in which the foreign corporation, directly or indirectly, owns more than 50% of the shares by vote and value.
43
On December 31, 2016, we entered into an agreement with the stockholders of Biohaven Pharmaceuticals, Inc., a Delaware corporation, or BPI, to purchase all of the outstanding capital stock of BPI for an aggregate purchase price of $0.6 million, payable by the issuance of promissory notes to each BPI stockholder (see “Certain Relationships and Related Party Transactions—Transactions with Biohaven Pharmaceuticals, Inc.”). Although we and BPI had certain shareholders in common before December 31, 2016, based on the rules for determining share ownership under Section 7874, we believe the stockholders of BPI owned less than 80% of our shares. Accordingly, we do not believe that this transaction meets the ownership test under Section 7874 and therefore do not believe that we should be treated as a domestic corporation for U.S. federal tax purposes. However, the tax law in this area could be changed, including changed on a retroactive basis, and the application of Section 7874 to our acquisition of BPI could substantially increase our effective tax rate. The remainder of this discussion assumes that we are properly classified as a foreign corporation for U.S. federal tax purposes.
Distributions
Although we do not currently plan to pay dividends, and subject to the discussion under “Passive Foreign Investment Company Considerations,” below, a U.S. holder generally will be required to treat the gross amount of any distribution actually or constructively received with respect to our common shares as a dividend to the extent of the U.S. holder’s pro rata share of our current and accumulated earnings and profits as determined under U.S. federal income tax principles. Distributions in excess of earnings and profits generally will be non‑taxable to the U.S. holder to the extent of, and will be applied against and reduce, the U.S. holder’s adjusted tax basis in the common shares. Distributions in excess of earnings and profits and such adjusted tax basis will generally be taxable to the U.S. holder as either long‑term or short‑term capital gain depending upon whether the U.S. holder has held the common shares for more than one year as of the time such distribution is received. However, since we may not calculate our earnings and profits under U.S. federal income tax principles, a U.S. holder should assume that any distribution with respect to our common shares will constitute ordinary dividend income.
Non‑corporate U.S. holders may qualify for the preferential rates of taxation with respect to dividends on our common shares applicable to long‑term capital gains (i.e., gains from the sale of capital assets held for more than one year) applicable to qualified dividend income (as discussed below) if we are a “qualified foreign corporation” and certain other requirements (discussed below) are met. A non‑U.S. corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation with respect to any dividend it pays on shares of stock that are readily tradable on an established securities market in the United States. Our common shares are, and we expect that they will continue to be, listed and readily tradable on the New York Stock Exchange, which is an established securities market in the United States. However, there can be no assurance that our common shares will be considered readily tradable on an established securities market in the United States in later years. If they are, and subject to the discussion under “Passive Foreign Investment Company Considerations,” below, such dividends paid by us will generally be “qualified dividend income” in the hands of individual U.S. holders, provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the 121‑day period beginning 60 days before the ex‑dividend date) and certain other requirements are met. Any dividend income that a U.S. holder realizes generally will be treated as foreign source income for foreign tax credit limitation purposes and generally will constitute passive category income.
Sale, Exchange or Other Taxable Disposition of our Common Shares
A U.S. holder will generally recognize gain or loss for U.S. federal income tax purposes upon the sale, exchange or other taxable disposition of our common shares in an amount equal to the difference between the U.S. dollar value of the amount realized from such sale or exchange (i.e., the amount of cash plus the fair market value of any property received) and the U.S. holder’s tax basis for those common shares. Subject to the discussion under “Passive Foreign Investment Company Considerations,” below, this gain or loss will generally be a capital gain or loss. The initial tax basis in the common shares
44
generally will be equal to the cost of such common shares. Capital gain from the sale, exchange or other taxable disposition of our common shares by a non‑corporate U.S. holder is generally eligible for a preferential rate of taxation applicable to capital gains, if the non‑corporate U.S. holder’s holding period determined at the time of such sale, exchange or other taxable disposition for such common shares exceeds one year (i.e., such gain is long‑term taxable gain and will be taxable at ordinary income rates if not long‑term capital gain). The deductibility of capital losses for U.S. federal income tax purposes is subject to limitations under the Code. Any such gain or loss that a U.S. holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes. U.S. holders are encouraged to consult their own tax advisors regarding the availability of the U.S. foreign tax credit in their particular circumstances.
Medicare Tax
Certain U.S. holders that are individuals, estates or trusts and whose income exceeds certain thresholds are subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend income and net gains from the disposition of our common shares. Each U.S. holder that is an individual, estate or trust is urged to consult its tax advisors regarding the applicability of the Medicare tax to its income and gains in respect of its investment in our common shares.
Passive Foreign Investment Company Considerations
We do not believe we were a PFIC for our taxable year ended December 31, 2017 and based on the current and anticipated value of our assets and the composition of our income, assets and operations, we do not expect to be a PFIC for the current taxable year or the foreseeable future. However, the application of the PFIC rules is subject to uncertainty in several respects, and therefore we cannot provide any assurance regarding our PFIC status for any past, current or future taxable years. A non‑U.S. corporation will be classified as a PFIC for any taxable year in which, after applying certain look‑through rules, either (i) at least 75% of its gross income is passive income; or (ii) at least 50% of the average quarterly value of its total gross assets (which may be determined in part by reference to the quarterly market value of our common shares, which may be volatile) is attributable to assets that produce passive income or are held for the production of passive income. Moreover, for purposes of determining if a non‑U.S. corporation is a PFIC, if the non‑U.S. corporation owns, directly or indirectly, at least 25%, by value, of the shares of another corporation, it will be treated as if it holds directly its proportionate share of the assets and receives directly its proportionate share of the income of such other corporation.
The determination of whether we are a PFIC is a fact‑intensive determination made on an annual basis applying principles and methodologies which in some circumstances are unclear and subject to varying interpretation. In particular, the characterization of our assets as active or passive may depend in part on our current and intended future business plans which are subject to change. In addition, for our current and future taxable years, the total value of our assets for PFIC testing purposes may be determined in part by reference to the market price of our shares from time to time, which may fluctuate considerably. Under the income and asset tests, our status as a PFIC depends on the composition of our income and assets which, in our current and future taxable years, we may not be able to control, for example, with respect to income and assets attributed to us from entities owned 25% or more by us. The composition of our income and assets is also affected by how, and how quickly, we spend the cash we raise in any offering, including this offering. Our U.S. counsel expresses no opinion with respect to our PFIC status for our taxable year ended December 31, 2017, and also expresses no opinion with regard to our expectations regarding our PFIC status in the future.
If we are classified as a PFIC for any taxable year during which a U.S. holder owns our common shares, the U.S. holder, absent certain elections (including the Purging Election, Mark‑to‑Market Election, and QEF Election, each described below), generally will be subject to special, adverse tax rules (regardless of whether we continue to be classified as a PFIC) with respect to (a) any “excess distribution” (generally, any distributions received by the U.S. holder on its common shares in a taxable
45
year that are greater than 125% of the average annual distributions received by the U.S. holder in the three preceding taxable years or, if shorter, the U.S. holder’s holding period for its common shares) and (b) any gain realized from a sale or other disposition (including a pledge) of its common shares. Under these special tax rules (i) the excess distribution or gain will be allocated ratably over a U.S. holder’s holding period for the common shares, (ii) the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which we became a PFIC, will be treated as ordinary income, and (iii) the amount allocated to each other year will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. If we are classified as a PFIC in any year with respect to which a U.S. holder owns our common shares, we will, absent certain elections, continue to be treated as a PFIC with respect to such U.S. holder in all succeeding years during which the U.S. holder owns our common shares, regardless of whether we continue to meet the tests described above.
Certain elections may be available to a U.S. holder that would result in alternative treatments. If we are a PFIC at any time when a U.S. holder holds our common shares, we will generally continue to be treated as a PFIC with respect to the U.S. holder for all succeeding years during which the U.S. holder holds our common shares even if we cease to qualify as a PFIC under the income and asset tests described above. However, if we cease to meet these tests, a U.S. holder can avoid the continuing impact of the PFIC rules by making a special election (a “Purging Election”) to recognize gain in the manner described above as if our common shares had been sold on the last day of the last taxable year during which we were a PFIC. Accordingly, a Purging Election may accelerate the recognition of income without a corresponding receipt of cash. In addition, for a U.S. holder making such an election, a new holding period would be deemed to begin for our common shares for purposes of the PFIC rules. After the Purging Election, the common shares with respect to which the Purging Election was made would not be treated as shares in a PFIC unless we were subsequently to qualify as a PFIC. U.S. holders should consult their tax advisers regarding the potential availability of a Purging Election that would allow them to eliminate PFIC status under certain circumstances.
In certain circumstances, a U.S. holder of shares in a PFIC may alleviate some of the adverse tax consequences described above by making a “qualified electing fund” election (a “QEF Election”) to include in income its pro rata share of the corporation’s income on a current basis. Accordingly, a QEF Election would accelerate the recognition of income without a corresponding receipt of cash. However, a U.S. holder may make a QEF Election with respect to our common shares only if we agree to furnish such U.S. holder annually with a PFIC annual information statement as specified in the applicable U.S. Treasury Regulations. We currently do not intend to prepare or provide the information that would enable U.S. holders to make a QEF Election if we are treated as a PFIC for any taxable year, and prospective investors should assume that a QEF Election will not be available.
Alternatively, a U.S. holder of “marketable stock” (as defined below) in a PFIC may make a mark‑to‑market election (the “Mark‑to‑Market Election”) with respect to such stock to elect out of the tax treatment discussed above. A Mark‑to‑Market Election generally is effective for the taxable year in which it is made and all subsequent years and cannot be revoked without the consent of the IRS. An electing U.S. holder generally would take into account as ordinary income, for each year that we are a PFIC, the excess of the fair market value of our common shares held at the end of the taxable year over the U.S. holder’s adjusted tax basis in such common shares at that time. Accordingly, a Mark‑to‑Market Election may accelerate the recognition of income without a corresponding receipt of cash. The U.S. holder would also take into account, as an ordinary loss for each year that we are a PFIC, the excess of the U.S. holder’s adjusted tax basis in such common shares at the end of the taxable year over the fair market value of the shares at that time, but only to the extent of the aggregate of the amounts previously included in income as a result of the Mark‑to‑Market Election. The U.S. holder’s tax basis in our common shares would be adjusted to reflect any income or loss resulting from the Mark‑to‑Market Election. Any gain from a sale, exchange or other disposition of the common shares in any taxable year in which we are a PFIC would be treated as ordinary income and any loss from such sale, exchange or other disposition would be treated first as ordinary loss to the extent of any net mark‑to‑market gains previously included in income and
46
thereafter as capital loss. If, after having been a PFIC for one or more taxable years, we cease to be classified as a PFIC, the U.S. holder would not be required to take into account any latent gain or loss in the manner described above and any realized gain or loss would be classified as a capital gain or loss. A Mark‑to‑Market Election will not apply to our common shares for any taxable year during which we are not a PFIC, but it will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any subsidiary that we own. A Mark‑to‑Market Election is available to a U.S. holder only if the common shares are considered “marketable stock.” Generally, stock will be considered marketable stock if it is “regularly traded” on a “qualified exchange” within the meaning of applicable U.S. Treasury Regulations. A class of stock is regularly traded during any calendar year during which such class of stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. We expect that our common shares will be marketable stock as long as they remain listed on the New York Stock Exchange and are regularly traded. There can be no assurance that our common shares will continue to be so traded. U.S. holders should consult their tax advisers regarding the availability and advisability of making a Mark‑to‑Market Election in their particular circumstances.
Moreover, if we are treated as a PFIC with respect to any taxable year, to the extent any of our subsidiaries are also PFICs, a U.S. holder may be deemed to own shares in such lower‑tier PFICs, and an election for mark‑to‑market treatment would likely not be available with respect to any such subsidiaries. U.S. holders are urged to consult their own tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
If we are a PFIC with respect to a U.S. holder of our common shares, such U.S. holder will generally be required to file an annual information return on IRS Form 8621 with respect to our common shares and the shares of any of our subsidiaries that also qualify as a PFIC. U.S. holders should consult their own tax advisers concerning annual filing requirements.
NO ASSURANCE CAN BE GIVEN REGARDING OUR PFIC STATUS FOR ANY PAST, CURRENT OR FUTURE TAXABLE YEARS. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE OPERATION OF THE PFIC RULES AND RELATED REPORTING REQUIREMENTS IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES, INCLUDING THE ADVISABILITY OF MAKING ANY ELECTION THAT MAY BE AVAILABLE.
Backup Withholding and Information Reporting
U.S. holders generally will be subject to information reporting requirements with respect to dividends on our common shares and on the proceeds from the sale, exchange or disposition of our common shares that are paid within the United States or through U.S.‑related financial intermediaries, unless the U.S. holder is an “exempt recipient.” In addition, U.S. holders may be subject to backup withholding on such payments, unless the U.S. holder provides a taxpayer identification number and a duly executed IRS Form W‑9 or otherwise establishes an exemption. Backup withholding is not an additional tax, and the amount of any backup withholding will be allowed as a credit against a U.S. holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Asset Reporting
Certain U.S. holders (and to the extent provided in IRS guidance, certain non‑U.S. holders) who hold interests in “specified foreign financial assets” (as defined in Section 6038D of the Code) are generally required to file an IRS Form 8938 as part of their U.S. federal income tax returns with information relating to such assets for each taxable year in which the aggregate value of all such assets exceeds $75,000 at any time during the taxable year or $50,000 on the last day of the taxable year (or such higher dollar amount as prescribed by applicable IRS guidance). “Specified foreign financial assets” generally include, among other assets, financial accounts maintained by foreign financial institutions, and our common shares, unless the shares are held through an account maintained by a financial institution. Substantial penalties may apply to any failure to timely file a complete and correct IRS Form 8938. Additionally, in the
47
event an applicable U.S. holder (and to the extent provided in IRS guidance, a non‑U.S. holder) that is required to file IRS Form 8938 either fails to file or fails to report a relevant asset, the statute of limitations on the assessment and collection of U.S. federal income taxes of such holder for the related tax year may not close until three years after the date that the required information is filed. Prospective investors are encouraged to consult with their own tax advisors regarding the possible reporting obligations under these and other disclosure requirements in light of their individual circumstances.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN OUR COMMON SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
48
UNDERWRITING
We and the underwriters named below have entered into an underwriting agreement with respect to the common shares being offered. Subject to certain conditions, each underwriter has severally agreed to purchase the number of common shares indicated in the following table. Goldman Sachs & Co. LLC and Piper Jaffray & Co. are the representatives of the underwriters.
Underwriters | Number of Common Shares |
Goldman Sachs & Co. LLC | |
Piper Jaffray & Co. | |
Total | 3,000,000 |
The underwriters are committed to take and pay for all of the common shares being offered, if any are taken, other than the common shares covered by the option described below unless and until this option is exercised.
The underwriters have an option to buy up to an additional 450,000 common shares from us to cover sales by the underwriters of a greater number of common shares than the total number set forth in the table above. They may exercise that option for 30 days. If any common shares are purchased pursuant to this option, the underwriters will severally purchase common shares in approximately the same proportion as set forth in the table above.
The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters by us. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase 450,000 additional common shares.
No Exercise | Full Exercise | ||||
Per Share | $ | $ | |||
Total | $ | $ | |||
Common shares sold by the underwriters to the public will initially be offered at the public offering price set forth on the cover of this prospectus. Any common shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the public offering price. After the initial offering of the common shares, the representatives may change the offering price and the other selling terms. The offering of the common shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
We, all of our directors and officers and a certain shareholder have agreed that, without the prior written consent of Goldman Sachs & Co. LLC and Piper Jaffray & Co. on behalf of the underwriters, we and they will not, during the period beginning on the date of this prospectus and ending 90 days, in the case of the company, or 45 days, in the case of our directors, officers and such shareholder, after the date of this prospectus, or the restricted period:
• | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any common shares or any securities convertible into or exercisable or exchangeable for common shares; or |
49
• | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common shares or any security convertible into common shares; |
whether any such transaction described above is to be settled by delivery of common shares or such other securities, in cash or otherwise, or publicly disclose the intention to do any of the foregoing. In addition, without the prior written consent of the representatives on behalf of the underwriters, (i) our directors and officers and such shareholder will not, during the restricted period, make any demand for, or exercise any right, or publicly disclose its intention to make any demand or exercise any right, with respect to the registration of any common shares or any security convertible into or exercisable or exchangeable for common shares and (ii) we will not file any registration statement with the Securities and Exchange Commission relating to the offering of any common shares or any securities convertible into or exercisable or exchangeable for common shares. In addition, our directors and officers and such shareholder agreed and consented to the entry of stop transfer instructions with our transfer agent and registrar against the transfer of each such person’s common shares except in compliance with the below restrictions.
The restrictions described in the preceding paragraphs are subject to certain exceptions, including, but not limited to:
• | transactions relating to our common shares or other securities acquired in open market transactions after the completion of this offering, provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period; |
• | transfers of common shares or any security convertible into common shares to an immediate family member or a trust for the direct or indirect benefit of the director, officer or such shareholder or such immediate family member of the director, officer or such shareholder, provided that each transferee signs and delivers a lock-up agreement, and provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period; |
• | transfers of common shares or any security convertible into common shares as a bona fide gift or charitable contribution, provided that each transferee signs and delivers a lock-up agreement, and provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period; |
• | transfers of common shares or any security convertible into common shares by will or intestacy, provided that each transferee signs and delivers a lock-up agreement, and provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period; |
• | if the transferor is a corporation, partnership or other business entity, distributions of common shares or any security convertible into common shares to limited partners, members, stockholders or holders of similar equity interests in the transferor, provided that each distributee signs and delivers a lock-up agreement, and provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period; |
• | if the transferor is a trust, transfers or distributions of common shares or any securities convertible into or exercisable or exchangeable for common shares to a trustor or beneficiary of the trust or to the estate of a beneficiary of such trust, provided that each transferee signs and delivers a lock-up agreement, and provided that no filing under Section 16(a) of the Exchange Act reporting a |
50
reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period;
• | the exercise of a stock option granted under a stock incentive plan or stock purchase plan described in this prospectus, and the receipt from the company of common shares upon such exercise, insofar as such option is outstanding as of the date of the lock-up agreement or the date of this prospectus, provided that the underlying shares shall continue to be subject to the restrictions on transfer set forth in the lock-up agreement, and provided that, if required, any public report or filing under Section 16 of the Exchange Act clearly indicates in the footnotes thereto that the filing relates to the exercise of a stock option, that no common shares were sold by the reporting person and that the common shares received upon exercise of the stock option are subject to a lock-up agreement with the underwriters; |
• | the disposition of common shares to us, or the withholding of common shares by us, in a transaction exempt from Section 16(b) of the Exchange Act solely to the extent required for the payment of taxes due with respect to the vesting or expiration of options or the vesting of restricted stock or restricted stock units granted under a stock incentive plan, stock purchase plan or pursuant to a contractual employment arrangement described in this prospectus, insofar as such options, restricted stock or restricted stock units are outstanding as of the date of the lock-up agreement or the date of this prospectus, provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period; |
• | transfers to us in connection with the repurchase of common shares in connection with the termination of the director or officer’s employment with us pursuant to contractual agreements with us as in effect as of the date of this prospectus, provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period; |
• | transfers of common shares or any security convertible into or exercisable or exchangeable for common shares pursuant to a domestic order, divorce decree or court order, provided that each transferee signs and delivers a lock-up agreement, and provided that, if required, any public report or filing under Section 16 of the Exchange Act shall clearly indicate in the footnotes thereto that the filing relates to the transfer of such securities pursuant to a domestic order, divorce decree or court order, that no such securities were sold by the reporting person and that the securities so transferred are subject to a lock-up agreement with the underwriters; |
• | the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer of common shares, provided that (i) such plan does not provide for the transfer of common shares during the restricted period and (ii) to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made by or on behalf of the director, officer or us regarding the establishment of such plan, such announcement or filing shall include a statement to the effect that no transfer of common shares may be made under such plan during the restricted period; |
• | transfers of common shares made pursuant to a written trading plan established prior to the date of the lock-up agreement in accordance with Rule 10b5-1 under the Exchange Act, provided that, to the extent a public announcement or filing under the Exchange Act is required of or voluntarily made by or on behalf of the director, officer or us regarding a transfer pursuant to such plan, such announcement or filing shall include a statement to the effect that such transfer was made pursuant to such plan; |
• | a merger, consolidation or other similar transaction, occurring after the closing of the offering, in which all holders of the common shares may participate, involving a change of control of the company and approved by our board of directors, provided that, in the event that such change of |
51
control transaction is not completed, the person’s common shares shall remain subject to the restrictions contained in the lock-up agreement and title to the person’s common shares shall remain with the person (for purposes of the foregoing, ‘‘change of control’’ means the transfer (whether by tender offer, merger, consolidation or other similar transaction), in one transaction or a series of related transactions, to a person or group of affiliated persons (other than an underwriter pursuant to this offering), of our voting securities if, after such transfer, such person or group of affiliated persons would hold at least 90% of our or the surviving entity’s outstanding voting securities);
• | transfers of common shares in connection with a pledge thereof by a director or officer existing prior to the date of the lock-up agreement upon foreclosure upon such common shares or any sale or disposition in connection with such foreclosure, provided that, if required, any public report or filing under Section 16 of the Exchange Act shall clearly indicate in the footnotes thereto that the filing relates to the transfer of such securities in connection with such foreclosure pursuant to such existing pledge arrangement; and |
• | transactions relating to common shares or other securities convertible or exercisable into common shares purchased in the offering, provided that no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of common shares or any security convertible into common shares is required or voluntarily made during the restricted period. |
The representatives, in their sole discretion, may release the common shares and other securities subject to the lock-up agreements described above at any time.
In connection with the offering, the underwriters may purchase and sell common shares in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of common shares than they are required to purchase in the offering, and a short position represents the amount of such sales that have not been covered by subsequent purchases. A “covered short position” is a short position that is not greater than the amount of additional common shares for which the underwriters’ option described above may be exercised. The underwriters may cover any covered short position by either exercising their option to purchase additional common shares or purchasing common shares in the open market. In determining the source of common shares to cover the covered short position, the underwriters will consider, among other things, the price of common shares available for purchase in the open market as compared to the price at which they may purchase additional common shares pursuant to the option described above. “Naked” short sales are any short sales that create a short position greater than the amount of additional common shares for which the option described above may be exercised. The underwriters must cover any such naked short position by purchasing common shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common shares in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common shares made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased common shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of our common shares, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of the common shares. As a result, the price of the common shares may be higher than the price that otherwise might exist in the open market. The underwriters are not required
52
to engage in these activities and may end any of these activities at any time. These transactions may be effected on the New York Stock Exchange, in the over-the-counter market or otherwise.
We may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. In connection with those derivatives, the third parties may sell securities covered by this prospectus, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of common shares, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of common shares. The third party in such sale transactions will be an underwriter or will be identified in a post-effective amendment.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relative Member State”) an offer to the public of our common shares may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of our common shares may be made at any time under the following exemptions under the Prospectus Directive:
• | To any legal entity which is a qualified investor as defined in the Prospectus Directive; |
• | To fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the Bookrunners for any such offer; or |
• | In any other circumstances falling within Article 3(2) of the Prospectus Directive; |
provided that no such offer of our common shares shall result in a requirement for the publication by us or any Brazilian placement agent of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer to public” in relation to our common shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and our common shares to be offered so as to enable an investor to decide to purchase our common shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (as amended), including by Directive 2010/73/EU and includes any relevant implementing measure in the Relevant Member State.
This European Economic Area selling restriction is in addition to any other selling restrictions set out below.
United Kingdom
In the United Kingdom, this prospectus is only addressed to and directed as qualified investors who are (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the Order); or (ii) high net worth entities and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). Any investment or investment activity to which this prospectus relates is available only to relevant persons and will only be engaged with relevant persons. Any person who is not a relevant person should not act or relay on this prospectus or any of its contents.
Canada
The common shares may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as
53
defined in National Instrument 31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the common shares must be made in accordance with an exemption form, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Hong Kong
The common shares may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), or (ii) to "professional investors" as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a "prospectus" as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the common shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to common shares which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the common shares may not be circulated or distributed, nor may the common shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the "SFA")) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the common shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for 6 months after that corporation has acquired the common shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, (3) where no
54
consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”)
Where the common shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferable for 6 months after that trust has acquired the common shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32.
Japan
The common shares have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended), or the FIEA. The securities may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
We estimate that our share of the total expenses of the offering, excluding underwriting discounts and commissions, will be approximately $ .
We have agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act of 1933.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. Certain of the underwriters and their respective affiliates have provided, and may in the future provide, a variety of these services to the issuer and to persons and entities with relationships with the issuer, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the issuer (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with the issuer. The underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
55
LEGAL MATTERS
The validity of the common shares and certain other matters of British Virgin Islands law will be passed upon for us by Maples and Calder, our special BVI counsel. Certain other legal matters will be passed upon for us by Cooley LLP, Reston, Virginia, and for the underwriters by Ropes & Gray LLP, Boston, Massachusetts.
EXPERTS
The financial statements incorporated in this prospectus by reference to the Annual Report on Form 10‑K for the year ended December 31, 2017 have been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company’s ability to continue as a going concern as described in Note 1 to the financial statements) of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
56
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S‑1 under the Securities Act, with respect to the common shares being offered by this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information in the registration statement and its exhibits. For further information with respect to our company and the common shares offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1‑800‑SEC‑0330 for further information on the operation of the public reference facilities.
We are subject to the information reporting requirements of the Exchange Act, and we have filed and will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above. We also maintain a website at www.biohavenpharma.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this prospectus.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (File No. 001‑38080):
• | our Annual Report on Form 10‑K for the fiscal year ended December 31, 2017, filed with the SEC on March 6, 2018; |
• | our definitive Proxy Statement on Schedule 14A, filed with the SEC on March 29, 2018 (excluding those portions that are not incorporated by reference into our Annual Report on Form 10‑K for the fiscal year ended December 31, 2017); |
• | our Quarterly Reports on Form 10‑Q for the quarterly periods ended March 31, 2018, June 30, 2018 and September 30, 2018, filed with the SEC on May 15, 2018, August 14, 2018 and November 14, 2018, respectively; and |
• | our Current Reports on Form 8‑K filed with the SEC on January 11, 2018, March 12, 2018, March 26, 2018, May 18, 2018, June 19, 2018, June 25, 2018, August 31, 2018, September 10, 2018, September 12, 2018, December 3, 2018 and December 10, 2018, in each case to the extent the information in such reports is filed and not furnished. |
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the SEC pursuant to the Exchange Act shall be incorporated by reference into this prospectus.
57
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Biohaven Pharmaceutical Holding Company Ltd., Attn: Corporate Secretary, 215 Church Street, New Haven, Connecticut 06510.
You also may access these filings on our website at www.biohavenpharma.com. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
58
3,000,000 Shares
Biohaven Pharmaceutical Holding Company Ltd.

GOLDMAN SACHS & CO. LLC
PIPER JAFFRAY
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, payable by us in connection with this offering. All amounts shown are estimates except for the SEC registration fee, the Financial Industry Regulatory Authority, or FINRA, filing fee, and the New York Stock Exchange, or NYSE listing fee.
Amount to be Paid | |||
SEC registration fee | $ | 16,490 | |
FINRA filing fee | 20,908 | ||
NYSE listing fee | 16,560 | ||
Printing expenses | 27,000 | ||
Legal fees and expenses | 350,000 | ||
Accountants’ fees and expenses | 200,000 | ||
Transfer agent and registrar fees and expenses | 5,000 | ||
Miscellaneous fees and expenses | 14,042 | ||
Total | $ | 650,000 | |
Item 14. Indemnification of Directors and Officers.
British Virgin Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the British Virgin Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our Amended Memorandum and Articles of Association provide for the indemnification of our directors against all losses or liabilities incurred or sustained by him or her as a director of our company in defending any proceedings, whether civil, criminal, administrative or investigative in which the director acted honestly and in good faith with a view to the best interest of the company and had no reasonable cause to believe that their conduct was unlawful.
Further, we have entered into indemnification agreements with each of our directors and executive officers that may be broader than the specific indemnification provisions contained under British Virgin Islands law. These indemnification agreements require us, among other things, to indemnify our directors and executive officers against liabilities that may arise by reason of their status or service. These indemnification agreements also require us to advance all expenses incurred by the directors and executive officers in investigating or defending any such action, suit, or proceeding. We believe that these agreements are necessary to attract and retain qualified individuals to serve as directors and executive officers.
The limitation of liability and indemnification provisions in our memorandum and articles of association and in indemnification agreements that we enter into with our directors and executive officers may discourage shareholders from bringing a lawsuit against our directors and executive officers for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against our directors and executive officers, even though an action, if successful, might benefit us and other shareholders. Further, a shareholder’s investment may be harmed to the extent that we pay the costs of settlement and damage awards against directors and executive officers as required by these indemnification provisions. At present, we are not aware of any pending litigation or proceeding involving any person who is or was one of our directors, officers, employees or other agents or is or was serving at
II-1
our request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
We have obtained insurance policies under which, subject to the limitations of the policies, coverage is provided to our directors and executive officers against loss arising from claims made by reason of breach of fiduciary duty or other wrongful acts as a director or executive officer, including claims relating to public securities matters, and to us with respect to payments that may be made by us to these directors and executive officers pursuant to our indemnification obligations or otherwise as a matter of law.
The underwriting agreement filed as Exhibit 1.1 to this registration statement provides for indemnification by the underwriters of us and our officers and directors for certain liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, and otherwise.
Item 15. Recent Sales of Unregistered Securities.
Issuances of Capital Stock
The following list sets forth information regarding all unregistered securities sold by us since January 1, 2015 through the date of the prospectus that forms a part of this registration statement.
1. | In July 2015, we issued an aggregate of 867,000 common shares to three investors at a purchase price of $5.60 per share, for aggregate consideration of $4.9 million. |
2. | In August 2015, we issued 50,000 common shares with a value of $0.3 million to a third party in partial settlement of amounts due under a license agreement. |
3. | In August 2015, as part of our license agreement, we issued ALS Biopharma, LLC warrants to purchase an aggregate of 600,000 of our common shares with an exercise price of $5.60 per share. |
4. | In February 2016, we issued an aggregate of 429,000 common shares to two investors at a purchase price of $7.00 per share, for aggregate consideration of $3.0 million. |
5. | In May 2016 and July 2016, we issued an aggregate of 1,090,500 common shares to 10 investors at a purchase price of $7.70 per share, for aggregate consideration of $8.4 million. |
6. | In October 2016, we issued an aggregate of 4,305,209 Series A preferred shares to 45 investors at a purchase price of $9.2911 per share, for aggregate consideration of $40.0 million, 538,150 Series A preferred shares with a value of $5.0 million to a third party in satisfaction of our obligations under a license agreement and 105,010 Series A preferred shares with a value of $1.0 million to two third parties in connection with their placement services with respect to the Series A preferred financing. |
7. | In December 2016, we issued promissory notes in the aggregate principal amount of $0.6 million to the stockholders of Biohaven Pharmaceuticals, Inc. in connection with our purchase of all of the outstanding capital stock of BPI. |
8. | In January 2017, we issued warrants to two of our directors, each to purchase 107,500 of our common shares with an exercise price of $9.2911 per share, in connection with the directors’ guaranties related to our credit agreement with Wells Fargo. |
9. | In February 2017, we issued an aggregate of 4,305,182 Series A preferred shares to 45 investors at a purchase price of $9.2911 per share, for aggregate consideration of $40.0 million and 105,009 Series A preferred shares with a value of $1.2 million to two third parties in connection with their placement services with respect to the Series A preferred financing. |
II-2
10. | In March 2017, we issued 32,500 common shares plus $249,750 in cash to one shareholder in exchange for 500,000 shares of common stock of Kleo Pharmaceuticals, Inc. |
11. | In May 2017, in connection with the closing of our IPO, we issued 538,149 and 1,345,374 common shares to AstraZeneca and BMS, respectively, pursuant to the terms of our license agreement with the respective party, for no additional consideration. |
12. | In June 2017, as partial consideration issued in connection with an agreement to perform investor relations services with The Trout Group LLC, or Trout, we issued to Trout a warrant to purchase 6,751 common shares at an exercise price of $22.22 per share. |
13. | In January 2018, ALS Biopharma exercised 275,000 of the warrants issued in August 2015 through a net share settlement, resulting in an issuance of 228,219 shares. |
14. | In March 2018, we issued and sold an aggregate of 2,000,000 common shares in a private placement to 10 investors at a purchase price of $27.50 per share, for aggregate consideration of $55.0 million. |
15. | In April 2018, ALS Biopharma exercised 325,000 of the warrants issued in August 2015 through a net share settlement, resulting in an issuance of 261,140 shares. |
16. | In September 2018, we issued 109,523 common shares pursuant to a license agreement with a third party as partial consideration for the license and other rights granted to us under the license agreement. |
The offers, sales and issuances of the securities described in the paragraphs above were exempt from registration under Section 4(a)(2) of the Securities Act or Regulation D promulgated under the Securities Act and, in the case of the transactions described in paragraphs 13 and 15 above, Section 3(a)(9) of the Securities Act. Each of the purchasers represented to us that they acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. The purchasers also represented to us that they were accredited investors as defined in Rule 501 promulgated under the Securities Act.
In addition, upon the completion of our IPO in May 2017, all of our Series A preferred shares, including the shares described in paragraphs 6 and 9 above, automatically converted into an aggregate of 9,358,560 common shares. The issuance of such common shares was exempt from registration under Section 3(a)(9) of the Securities Act.
Stock Option Grants
From January 1, 2015 through May 23, 2017, the date on which our registration statement on Form S‑8 was filed and became effective, we granted options under our 2014 Equity Incentive Plan to purchase an aggregate of 2,898,858 common shares to employees, consultants and directors, having exercise prices ranging from $5.60 to $10.82 per share. Of these, through the date of this prospectus, 392,001 common shares were issued upon the exercise of stock options, at a weighted average exercise price of $8.46 per share, for aggregate proceeds of approximately $3.1 million.
The offers, sales and issuances of the securities described in the foregoing paragraph were exempt from registration under Rule 701 promulgated under the Securities Act in that the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of such securities were our employees, directors or consultants and received the securities under our 2014 Equity Incentive Plan. Appropriate legends were affixed to the securities issued in these transactions.
II-3
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
Exhibit Number | Description of Document | |
1.1 | ||
2.1 | ||
2.2 | ||
2.3 | ||
3.1 | ||
4.1 | ||
4.2 | ||
4.3 | ||
4.4 | ||
4.5 | ||
5.1 | ||
10.1# | ||
10.2# | ||
10.3# | ||
II-4
Exhibit Number | Description of Document | |
10.4# | ||
10.5# | ||
10.6# | ||
10.7# | ||
10.8 | ||
10.9# | ||
10.10# | ||
10.11# | ||
10.12# | ||
10.13+ | ||
10.14+ | ||
10.15+ | ||
10.16+ | ||
10.17+ | ||
II-5
Exhibit Number | Description of Document | |
10.18+ | ||
10.19+ | ||
10.20+ | ||
10.21+ | ||
10.22+ | ||
21.1 | ||
23.1 | ||
23.2 | ||
24.1 | ||
________________
# | Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment and have been separately filed with the Securities and Exchange Commission. |
+ | Indicates management contract or compensatory plan. |
(b) Financial Statement Schedules.
No financial statement schedules have been submitted because they are not required or are not applicable or because the information required is included in the consolidated financial statements or the notes thereto.
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
(1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or |
II-6
497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) | For the purpose of determining any liability under the Securities Act, each post‑effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-7
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New Haven, State of Connecticut, on the 10th day of December, 2018.
BIOHAVEN PHARMACEUTICAL HOLDING COMPANY LTD. | |
By: | /s/ VLAD CORIC, M.D. |
Vlad Coric, M.D. Chief Executive Officer | |
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Vlad Coric his or her true and lawful agent, proxy and attorney‑in‑fact, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments (including post‑effective amendments) to this registration statement together with all schedules and exhibits thereto and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, together with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, (iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and (iv) take any and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as he or she might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney‑in‑fact or any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ VLAD CORIC, M.D. | Chief Executive Officer and Director (Principal Executive Officer) | December 10, 2018 | ||
Vlad Coric, M.D. | ||||
/s/ JAMES ENGELHART | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | December 10, 2018 | ||
James Engelhart | ||||
/s/ DECLAN DOOGAN, M.D. | Chairman of the Board of Directors | December 10, 2018 | ||
Declan Doogan, M.D. | ||||
/s/ GREGORY H. BAILEY, M.D. | Director | December 10, 2018 | ||
Gregory H. Bailey, M.D. | ||||
/s/ JOHN W. CHILDS | Director | December 10, 2018 | ||
John W. Childs | ||||
/s/ ROBERT REPELLA | Director | December 10, 2018 | ||
Robert Repella | ||||
/s/ ERIC AGUIAR, M.D. | Director | December 10, 2018 | ||
Eric Aguiar, M.D. | ||||
/s/ JULIA GREGORY | Director | December 10, 2018 | ||
Julia Gregory | ||||
