Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Osmotica Pharmaceuticals plc | a18-41074_18k.htm |
1 Safe Harbor This presentation contains forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. All statements other than statements of historical facts contained in this presentation, including information concerning the timing of clinical and commercial development and launch plans with respect to our products and product candidates, are forward-looking statements. Forward-looking statements are subject to known and unknown risks, uncertainties and other factors, including that failures of or delays in clinical trials could jeopardize or delay our ability to obtain regulatory approval and commence product sales for new products, as well as the other factors that are described in the “Risk Factors” section of our prospectus relating to our initial public offering that was filed on October 19, 2018. These risks, uncertainties and other factors may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these uncertainties, you should not place undue reliance on any forward-looking statements in this presentation. The forward-looking statements included in this presentation are made only as of the date hereof. We cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, neither we nor our advisors nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. Neither we nor our advisors undertake any obligation to update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as may be required by law. You should read this presentation with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect. Non-GAAP Financial Measures We present Adjusted EBITDA to help us describe our operating performance. Our presentation of Adjusted EBITDA is intended as a supplemental measure of our performance that is not required by, or presented in accordance with, U.S. generally accepted accounting principles (“GAAP”). Adjusted EBITDA should not be considered as an alternative to operating income (loss), net income (loss) or any other performance measures derived in accordance with U.S. GAAP as measures of operating performance or operating cash flows or as measures of liquidity. Our presentation of Adjusted EBITDA should not be construed to imply that our future results will be unaffected by these items. See the appendix to this presentation for a reconciliation of Adjusted EBITDA to net income (loss).

2 Fully integrated and diversified biopharmaceutical company focused on the development and commercialization of specialty products that target markets with unmet medical needs

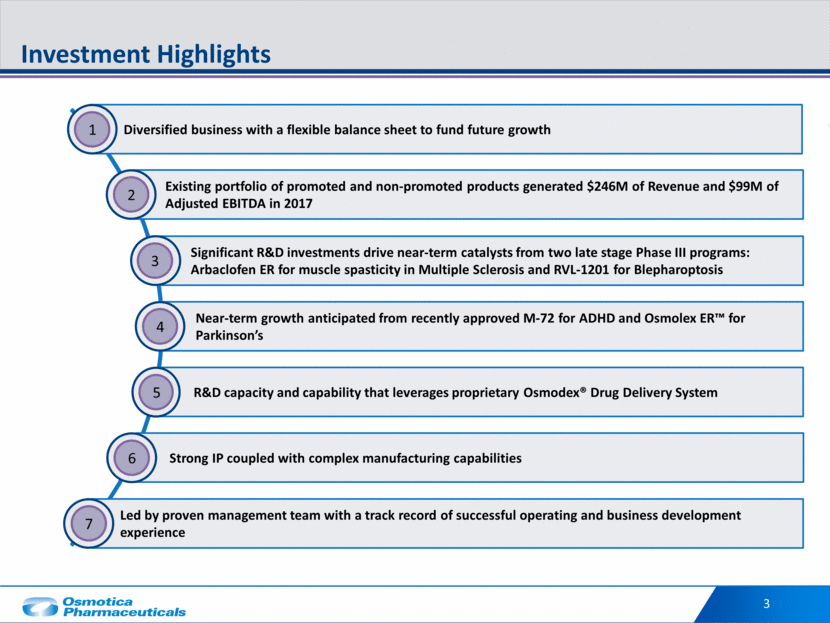
3 Investment Highlights Diversified business with a flexible balance sheet to fund future growth 1 Near-term growth anticipated from recently approved M-72 for ADHD and Osmolex ER™ for Parkinson’s 4 Led by proven management team with a track record of successful operating and business development experience 7 Significant R&D investments drive near-term catalysts from two late stage Phase III programs: Arbaclofen ER for muscle spasticity in Multiple Sclerosis and RVL-1201 for Blepharoptosis 3 R&D capacity and capability that leverages proprietary Osmodex® Drug Delivery System 5 Strong IP coupled with complex manufacturing capabilities 6 Existing portfolio of promoted and non-promoted products generated $246M of Revenue and $99M of Adjusted EBITDA in 2017 2
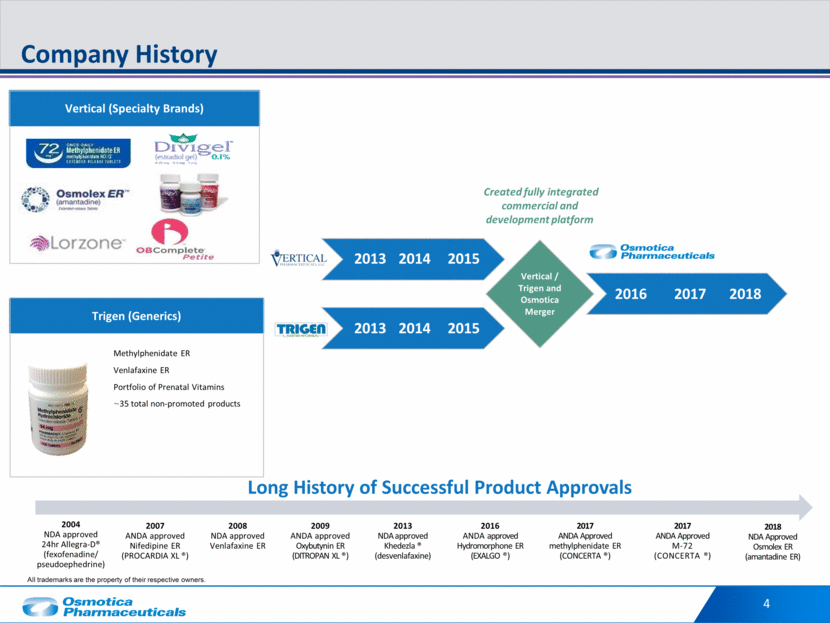
4 Company History 2018 NDA Approved Osmolex ER (amantadine ER) 2017 ANDA Approved methylphenidate ER (CONCERTA ®) 2016 ANDA approved Hydromorphone ER (EXALGO ®) 2013 NDA approved Khedezla ® (desvenlafaxine) 2009 ANDA approved Oxybutynin ER (DITROPAN XL ®) 2008 NDA approved Venlafaxine ER 2007 ANDA approved Nifedipine ER (PROCARDIA XL ®) Long History of Successful Product Approvals 2017 ANDA Approved M-72 (CONCERTA ®) 2004 NDA approved 24hr Allegra-D® (fexofenadine/ pseudoephedrine) Vertical / Trigen and Osmotica Merger 2013 2014 2015 2016 2017 2018 2013 2014 2015 Created fully integrated commercial and development platform Trigen (Generics) Methylphenidate ER Venlafaxine ER Portfolio of Prenatal Vitamins ~35 total non-promoted products Vertical (Specialty Brands) All trademarks are the property of their respective owners.
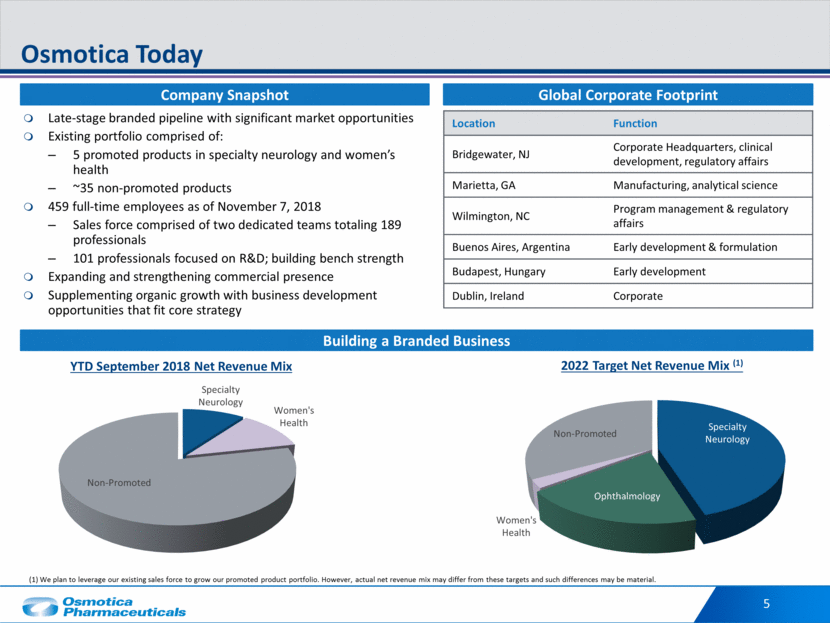
5 Osmotica Today Late-stage branded pipeline with significant market opportunities Existing portfolio comprised of: 5 promoted products in specialty neurology and women’s health ~35 non-promoted products 459 full-time employees as of November 7, 2018 Sales force comprised of two dedicated teams totaling 189 professionals 101 professionals focused on R&D; building bench strength Expanding and strengthening commercial presence Supplementing organic growth with business development opportunities that fit core strategy Location Function Bridgewater, NJ Corporate Headquarters, clinical development, regulatory affairs Marietta, GA Manufacturing, analytical science Wilmington, NC Program management & regulatory affairs Buenos Aires, Argentina Early development & formulation Budapest, Hungary Early development Dublin, Ireland Corporate Company Snapshot Global Corporate Footprint Building a Branded Business YTD September 2018 Net Revenue Mix 2022 Target Net Revenue Mix (1) (1) We plan to leverage our existing sales force to grow our promoted product portfolio. However, actual net revenue mix may differ from these targets and such differences may be material. Specialty Neurology Women's Health Non - Promoted Specialty Neurology Ophthalmology Women's Health Non - Promoted
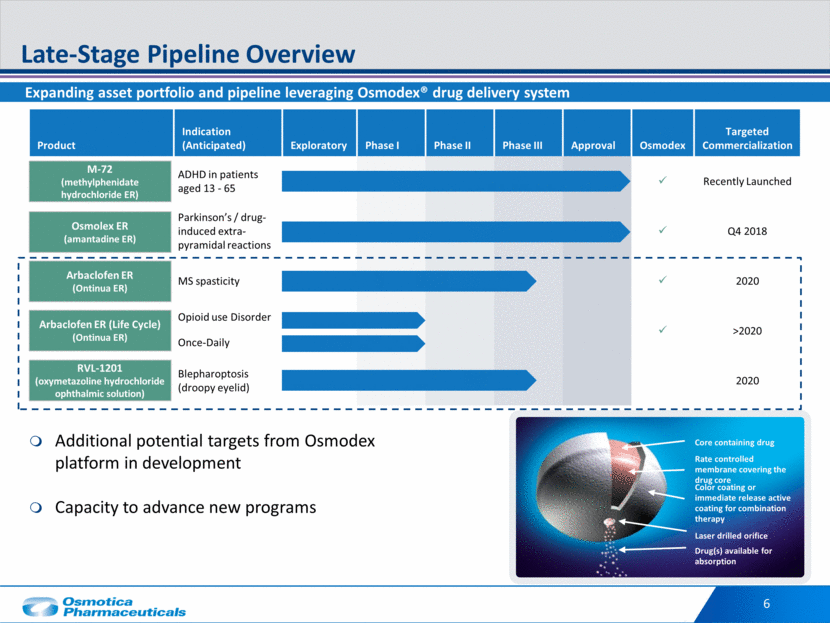
Color coating or immediate release active coating for combination therapy Rate controlled membrane covering the drug core Drug(s) available for absorption Laser drilled orifice Core containing drug Product Indication (Anticipated) Exploratory Phase I Phase II Phase III Approval Osmodex Targeted Commercialization ADHD in patients aged 13 - 65 Recently Launched Parkinson’s / drug-induced extra-pyramidal reactions Q4 2018 MS spasticity 2020 Opioid use Disorder Once-Daily >2020 Blepharoptosis (droopy eyelid) 2020 Late-Stage Pipeline Overview 6 Expanding asset portfolio and pipeline leveraging Osmodex® drug delivery system M-72 (methylphenidate hydrochloride ER) Osmolex ER (amantadine ER) Arbaclofen ER (Ontinua ER) Arbaclofen ER (Life Cycle) (Ontinua ER) Additional potential targets from Osmodex platform in development Capacity to advance new programs RVL-1201 (oxymetazoline hydrochloride ophthalmic solution)
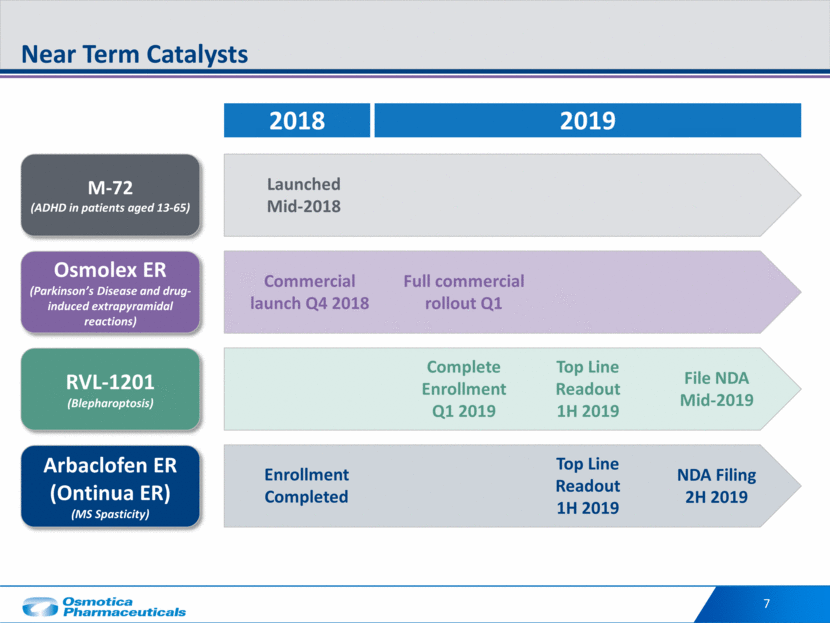
7 Near Term Catalysts Complete Enrollment Q1 2019 Top Line Readout 1H 2019 File NDA Mid-2019 Enrollment Completed Top Line Readout 1H 2019 NDA Filing 2H 2019 2018 2019 M-72 (ADHD in patients aged 13-65) Launched Mid-2018 Osmolex ER (Parkinson’s Disease and drug-induced extrapyramidal reactions) Commercial launch Q4 2018 Full commercial rollout Q1 RVL-1201 (Blepharoptosis) Arbaclofen ER (Ontinua ER) (MS Spasticity)
Arbaclofen ER
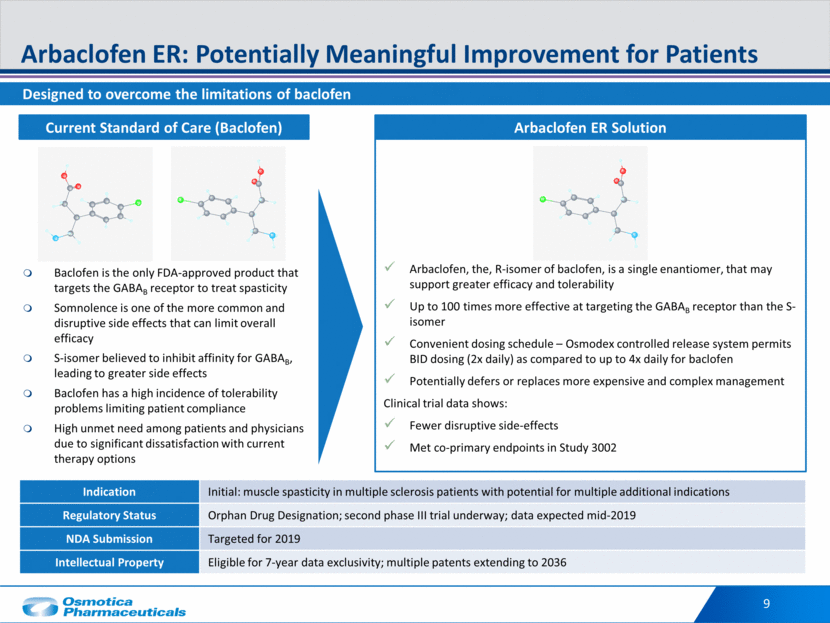
Indication Initial: muscle spasticity in multiple sclerosis patients with potential for multiple additional indications Regulatory Status Orphan Drug Designation; second phase III trial underway; data expected mid-2019 NDA Submission Targeted for 2019 Intellectual Property Eligible for 7-year data exclusivity; multiple patents extending to 2036 Designed to overcome the limitations of baclofen Arbaclofen ER: Potentially Meaningful Improvement for Patients 9 Baclofen is the only FDA-approved product that targets the GABAB receptor to treat spasticity Somnolence is one of the more common and disruptive side effects that can limit overall efficacy S-isomer believed to inhibit affinity for GABAB, leading to greater side effects Baclofen has a high incidence of tolerability problems limiting patient compliance High unmet need among patients and physicians due to significant dissatisfaction with current therapy options Arbaclofen, the, R-isomer of baclofen, is a single enantiomer, that may support greater efficacy and tolerability Up to 100 times more effective at targeting the GABAB receptor than the S-isomer Convenient dosing schedule – Osmodex controlled release system permits BID dosing (2x daily) as compared to up to 4x daily for baclofen Potentially defers or replaces more expensive and complex management Clinical trial data shows: Fewer disruptive side-effects Met co-primary endpoints in Study 3002 Arbaclofen ER Solution Current Standard of Care (Baclofen)
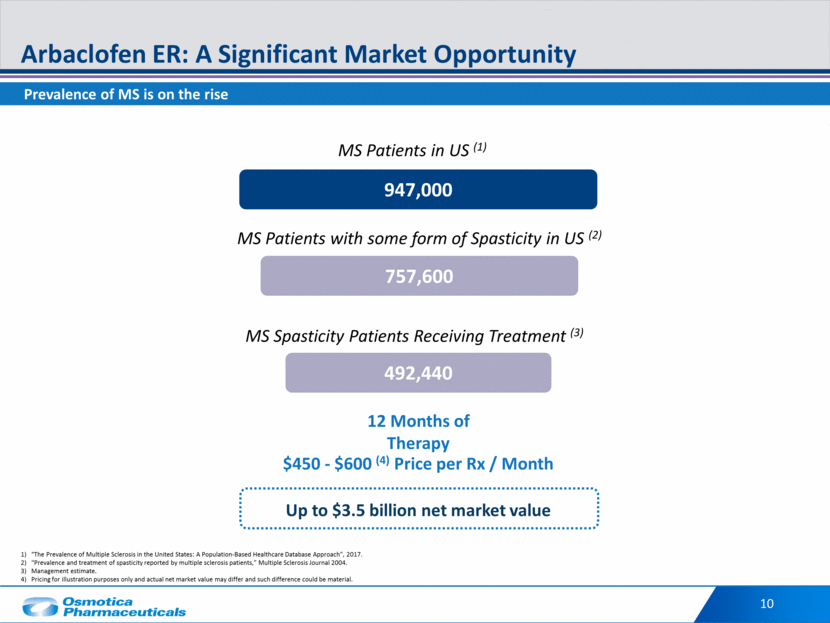
10 Arbaclofen ER: A Significant Market Opportunity “The Prevalence of Multiple Sclerosis in the United States: A Population-Based Healthcare Database Approach”, 2017. “Prevalence and treatment of spasticity reported by multiple sclerosis patients,” Multiple Sclerosis Journal 2004. Management estimate. Pricing for illustration purposes only and actual net market value may differ and such difference could be material. 947,000 757,600 492,440 12 Months of Therapy $450 - $600 (4) Price per Rx / Month MS Patients in US (1) MS Patients with some form of Spasticity in US (2) MS Spasticity Patients Receiving Treatment (3) Up to $3.5 billion net market value Prevalence of MS is on the rise
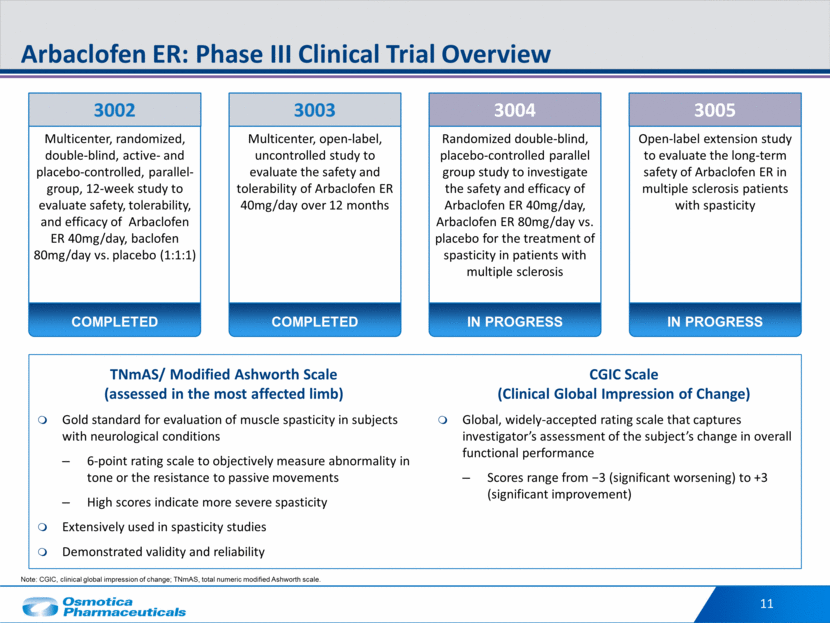
11 Arbaclofen ER: Phase III Clinical Trial Overview Multicenter, randomized, double-blind, active- and placebo-controlled, parallel-group, 12-week study to evaluate safety, tolerability, and efficacy of Arbaclofen ER 40mg/day, baclofen 80mg/day vs. placebo (1:1:1) 3002 COMPLETED CGIC Scale (Clinical Global Impression of Change) Global, widely-accepted rating scale that captures investigator’s assessment of the subject’s change in overall functional performance Scores range from -3 (significant worsening) to +3 (significant improvement) Note: CGIC, clinical global impression of change; TNmAS, total numeric modified Ashworth scale. TNmAS/ Modified Ashworth Scale (assessed in the most affected limb) Gold standard for evaluation of muscle spasticity in subjects with neurological conditions 6-point rating scale to objectively measure abnormality in tone or the resistance to passive movements High scores indicate more severe spasticity Extensively used in spasticity studies Demonstrated validity and reliability Multicenter, open-label, uncontrolled study to evaluate the safety and tolerability of Arbaclofen ER 40mg/day over 12 months 3003 COMPLETED Randomized double-blind, placebo-controlled parallel group study to investigate the safety and efficacy of Arbaclofen ER 40mg/day, Arbaclofen ER 80mg/day vs. placebo for the treatment of spasticity in patients with multiple sclerosis 3004 IN PROGRESS Open-label extension study to evaluate the long-term safety of Arbaclofen ER in multiple sclerosis patients with spasticity 3005 IN PROGRESS
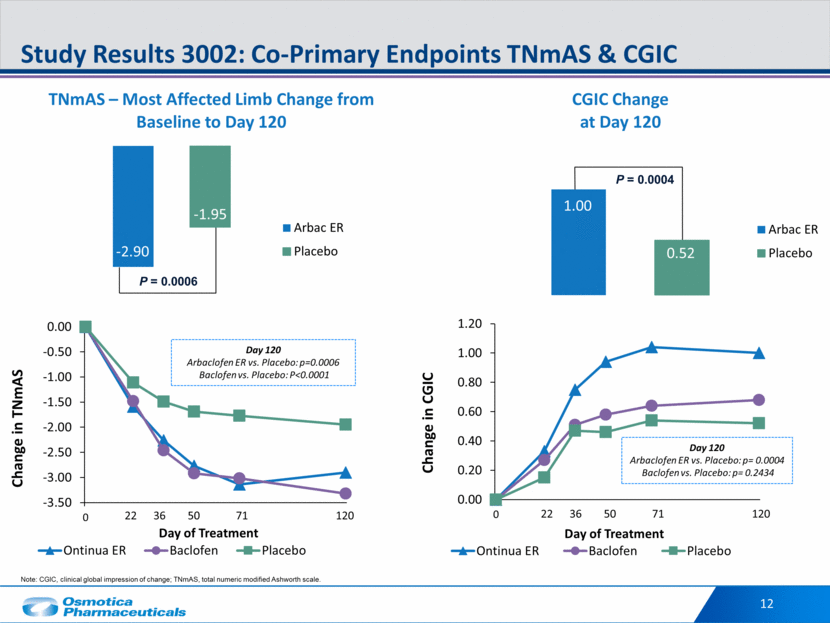
P = 0.0004 P = 0.0006 12 Study Results 3002: Co-Primary Endpoints TNmAS & CGIC -2.90 -1.95 1.00 0.52 Day 120 Arbaclofen ER vs. Placebo: p=0.0006 Baclofen vs. Placebo: P<0.0001 Day 120 Arbaclofen ER vs. Placebo: p= 0.0004 Baclofen vs. Placebo: p= 0.2434 22 36 50 71 120 22 36 50 71 120 Note: CGIC, clinical global impression of change; TNmAS, total numeric modified Ashworth scale. CGIC Change at Day 120 Arbac ER Placebo -3.50 -3.00 -2.50 -2.00 -1.50 -1.00 -0.50 0.00 0 Change in TNmAS Day of Treatment Ontinua ER Baclofen Placebo 0.00 0.20 0.40 0.60 0.80 1.00 1.20 0 Change in CGIC Day of Treatment Ontinua ER Baclofen Placebo
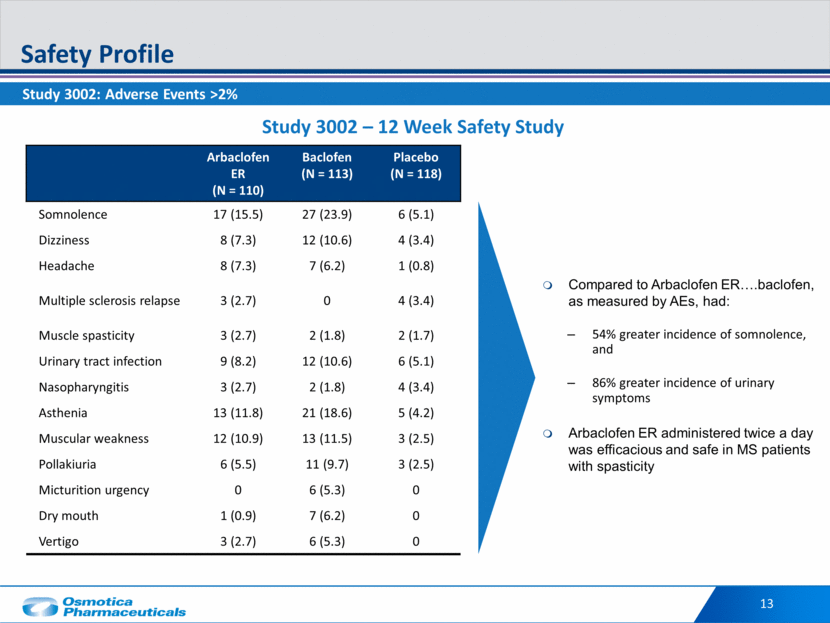
Study 3002: Adverse Events >2% Safety Profile 13 Arbaclofen ER (N = 110) Baclofen (N = 113) Placebo (N = 118) Somnolence 17 (15.5) 27 (23.9) 6 (5.1) Dizziness 8 (7.3) 12 (10.6) 4 (3.4) Headache 8 (7.3) 7 (6.2) 1 (0.8) Multiple sclerosis relapse 3 (2.7) 0 4 (3.4) Muscle spasticity 3 (2.7) 2 (1.8) 2 (1.7) Urinary tract infection 9 (8.2) 12 (10.6) 6 (5.1) Nasopharyngitis 3 (2.7) 2 (1.8) 4 (3.4) Asthenia 13 (11.8) 21 (18.6) 5 (4.2) Muscular weakness 12 (10.9) 13 (11.5) 3 (2.5) Pollakiuria 6 (5.5) 11 (9.7) 3 (2.5) Micturition urgency 0 6 (5.3) 0 Dry mouth 1 (0.9) 7 (6.2) 0 Vertigo 3 (2.7) 6 (5.3) 0 Study 3002 – 12 Week Safety Study Compared to Arbaclofen ER .baclofen, as measured by AEs, had: 54% greater incidence of somnolence, and 86% greater incidence of urinary symptoms Arbaclofen ER administered twice a day was efficacious and safe in MS patients with spasticity
Safety Profile 14 Adverse Event All Subjects (1) (N = 184) CNS disorders, n (%) 11 (6.0) Somnolence 5 (2.7) Dizziness 1 (0.5) Renal and urinary disorders, n (%) 5 (2.7) Pollakiuria 3 (1.6) Micturition urgency 2 (1.1) Urinary incontinence 2 (1.1) Polyuria 1 (0.5) Arbaclofen ER AEs were comparable to those observed with placebo in Study 3002 Study 3003 – Long Term (12 month) Safety Study Study 3003: Adverse Events Leading to Drug Interruption or Discontinuation Comprised of rollover subjects from Study 3002 and de novo subjects.
RVL-1201
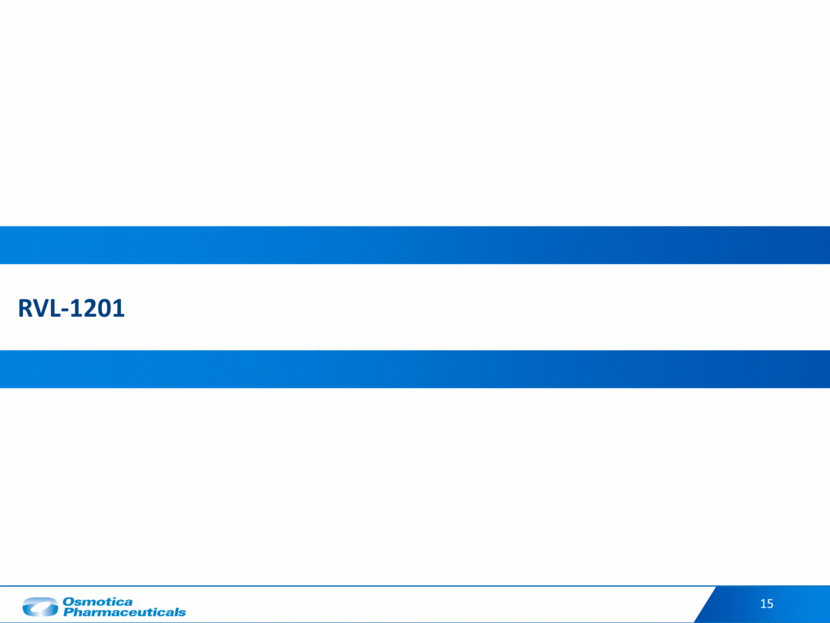
We believe that an estimated 3.1 million patients are observed in US ophthalmology, optometry, dermatology, and plastic surgery offices each year We believe there is a large U.S. prevalence of ptosis, yet only patients with severe ptosis are consistently diagnosed and surgically treated Ptosis: A Large Unmet Need Patient with ptosis of the left eyelid Approximately 1.4 M Diagnosed Patients Approximately 16.8 M Total Ptosis Patients ~150,000 Patients Treated with Surgery (Reimbursed) Ptosis is a drooping of the eyelid that may inhibit a person’s vision Ptosis can be generally classified into two types: congenital (patients born with condition) and acquired (patients that develop the condition) Acquired ptosis can be further categorized by the etiology, aponeurotic (age related ptosis) is the most common kind 16 Approximately 3.1 M Observed Patients Surgery is the only long-term treatment, but few patients have severe enough disease to qualify for insurance coverage Based on Company estimates (1) : While no robust epidemiological studies exploring the prevalence of blepharoptosis in the United States exist, we believe it is a common condition affecting millions of Americans. Although we believe the numbers presented in the graphic above reflect the approximate potential market opportunity in the United States based on our research and available market information, there is no assurance that the market opportunity will not differ from such numbers and such difference could be material. Source: Company research. 10.5% 89.5% Treated Untreated
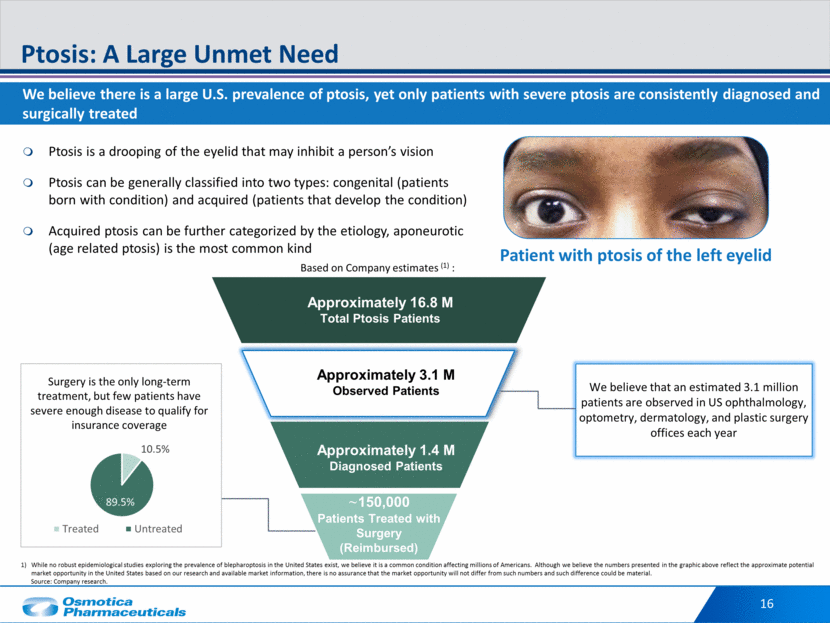
Potential to fulfill significant unmet need and improve vision for many patients RVL-1201: Potentially First Non-Surgical Treatment for Ptosis RVL-1201 Overview Highlights MOA: α-1 and partial α-2 adrenergic receptor targeting the Müller’s muscle Dosing: 1 drop in each affected eye in the morning; chronic safety study in progress Efficacy vs placebo1: 37.6% visual field improvement in Leicester Peripheral Field Test (LPFT); 2mm lift of upper eyelid maximally Safety: AEs were predominantly mild in intensity IP: Patents extending to August 2031 RVL-1201 is a topical prescription eye drop solution in Ph III trials for treatment of acquired blepharoptosis in patients with visual field loss RVL-1201 may offer a non-surgical option to patients who are unwilling or unable to undergo surgery as well as an alternative for those whose ptosis does not qualify for coverage of their surgery Before After Patient A LPFT : Day 1 Prior to RVL-1201 Dosing **3 points seen on top two rows** Patient A LPFT: Day 14 After RVL-1201 Dosing **14 points seen on top two rows** 17 1 - Based on Phase III clinical trial results vs. placebo.
202 18 RVL-1201 Clinical Trial Overview 203 201 Randomized, multicenter, double-masked, placebo-controlled study comparing once-daily RVL-1201 0.1% with placebo in patients with acquired blepharoptosis Phase 3, randomized, multicenter, double-masked, placebo-controlled, 6-week study planned to evaluate the safety and efficacy of once-daily treatment with RVL-1201 compared with placebo for the treatment of acquired blepharoptosis Phase 3, randomized, multicenter, double-masked, placebo-controlled, 12-week study planned to evaluate the extended safety of RVL-1201 compared with placebo for the treatment of acquired blepharoptosis A 6-month rabbit toxicology study required by the FDA is complete Primary Endpoints Compare RVL-1201 to placebo on the mean increase from baseline in number of points seen on the top 4 rows of the LPFT in the study eye at: Hour 6 on day 1 Hour 2 on day 14 Secondary Endpoints Mean observed MRD values with RVL-1201 vs placebo assessed at days 1, 14, and 42 Change from baseline MRD values with RVL-1201 vs placebo assessed at days 1, 14, and 42 Design: Randomized, multicenter, double-masked, placebo-controlled study Objective: Compared once-daily RVL-1201 0.1% with placebo in patients with acquired blepharoptosis Randomized, multicenter, double-masked, placebo-controlled study comparing once-daily RVL-1201 0.1% with placebo in patients with acquired blepharoptosis 201 COMPLETED Phase III, randomized, multicenter, double-masked, placebo-controlled, 6-week study planned to evaluate the safety and efficacy of once-daily treatment with RVL-1201 compared with placebo for the treatment of acquired blepharoptosis 202 IN PROGRESS Phase III, randomized, multicenter, double-masked, placebo-controlled, 12-week study planned to evaluate the extended safety of RVL-1201 compared with placebo for the treatment of acquired blepharoptosis 203 IN PROGRESS
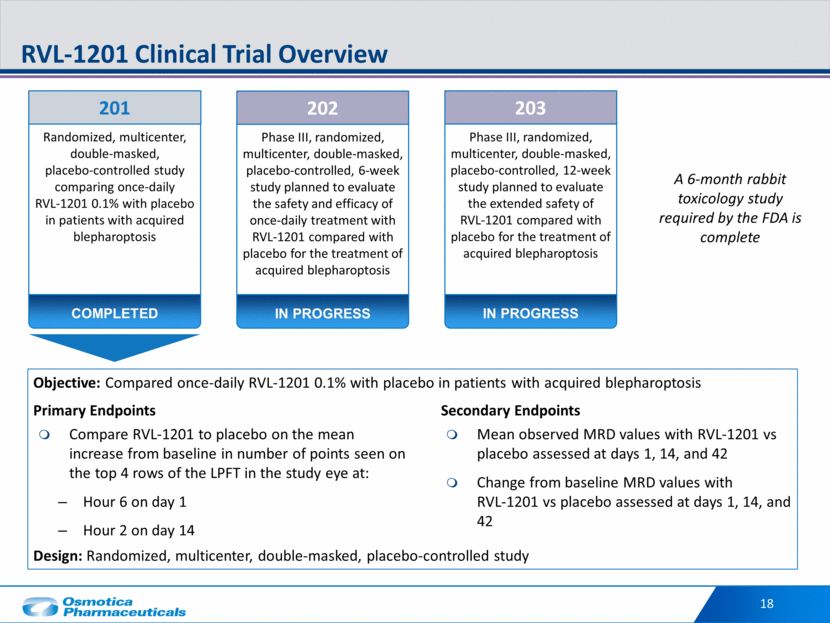
P=0.0003 Study RVL-1201-201 Significant Increase from Baseline in LPFT with RVL-1201 vs Placebo P <0.0001 Prior to RVL-1201 Dosing After RVL-1201 Dosing Points seen in the superior visual field 19
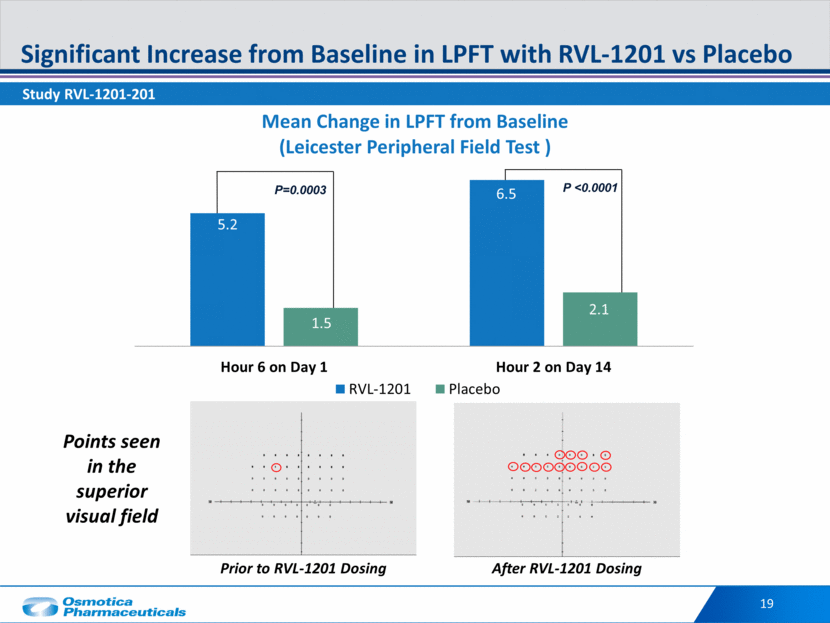
Potential First-in-Class Non-Invasive Ptosis Treatment Today, surgery is the only FDA approved option for treatment of Ptosis Efficacy vs. placebo One Drop Daily Well-Tolerated Additional aesthetic market opportunity for RVL-1201 is under investigation 20 First Phase III Trial 201 confirmed:
Key Promoted Product Overviews
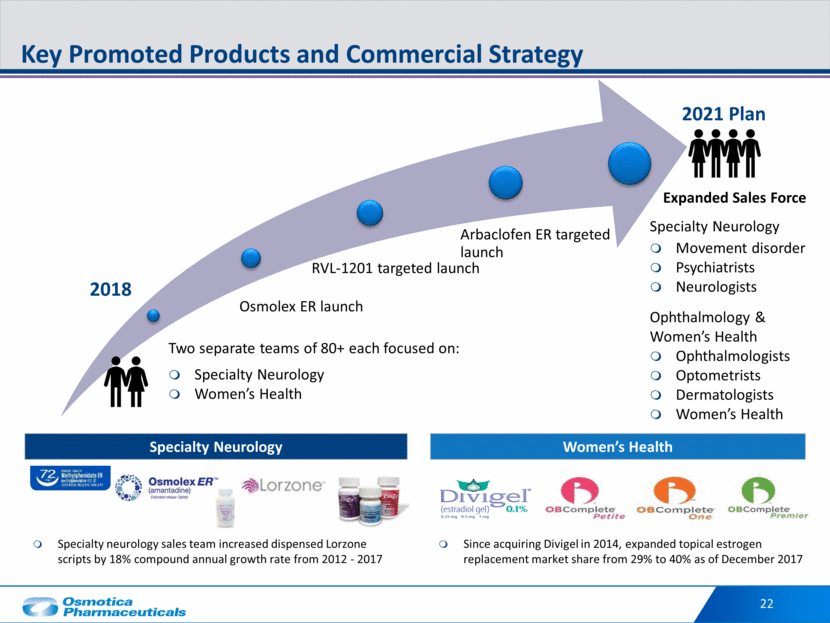
22 Key Promoted Products and Commercial Strategy 2018 2021 Plan Women’s Health Specialty Neurology Specialty neurology sales team increased dispensed Lorzone scripts by 18% compound annual growth rate from 2012 - 2017 Since acquiring Divigel in 2014, expanded topical estrogen replacement market share from 29% to 40% as of December 2017 Two separate teams of 80+ each focused on: Specialty Neurology Women’s Health Specialty Neurology Movement disorder Psychiatrists Neurologists Ophthalmology & Women’s Health Ophthalmologists Optometrists Dermatologists Women’s Health Expanded Sales Force Osmolex ER launch RVL-1201 targeted launch Arbaclofen ER targeted launch
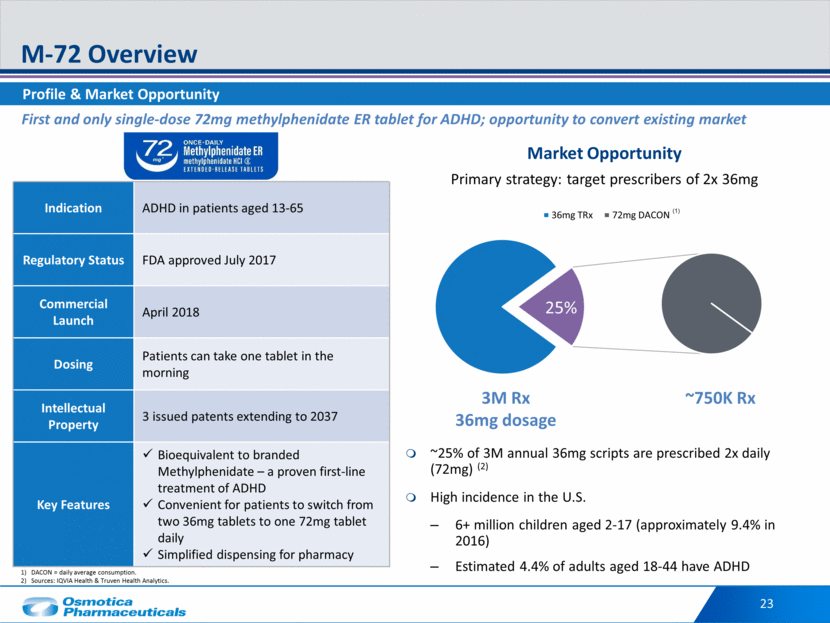
Market Opportunity Primary strategy: target prescribers of 2x 36mg ~25% of 3M annual 36mg scripts are prescribed 2x daily (72mg) (2) High incidence in the U.S. 6+ million children aged 2-17 (approximately 9.4% in 2016) Estimated 4.4% of adults aged 18-44 have ADHD Profile & Market Opportunity M-72 Overview 23 ~750K Rx 25% 3M Rx 36mg dosage First and only single-dose 72mg methylphenidate ER tablet for ADHD; opportunity to convert existing market DACON = daily average consumption. Sources: IQVIA Health & Truven Health Analytics. ` Indication ADHD in patients aged 13-65 Regulatory Status FDA approved July 2017 Commercial Launch April 2018 Dosing Patients can take one tablet in the morning Intellectual Property 3 issued patents extending to 2037 Key Features Bioequivalent to branded Methylphenidate – a proven first-line treatment of ADHD Convenient for patients to switch from two 36mg tablets to one 72mg tablet daily Simplified dispensing for pharmacy (1) 36mg TRx 72mg DACON
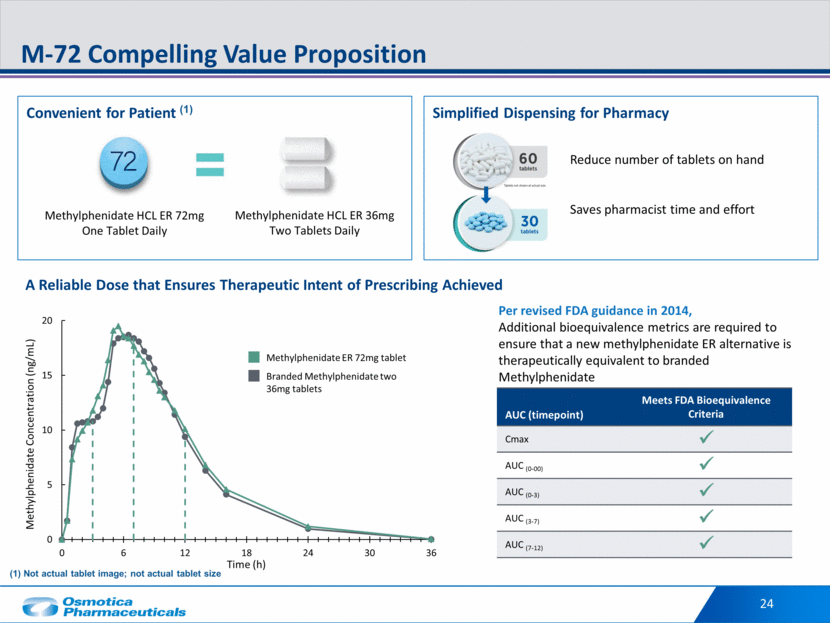
24 M-72 Compelling Value Proposition Convenient for Patient (1) Simplified Dispensing for Pharmacy A Reliable Dose that Ensures Therapeutic Intent of Prescribing Achieved Reduce number of tablets on hand Saves pharmacist time and effort AUC (timepoint) Meets FDA Bioequivalence Criteria Cmax AUC (0-00) AUC (0-3) AUC (3-7) AUC (7-12) Methylphenidate ER 72mg tablet Branded Methylphenidate two 36mg tablets Per revised FDA guidance in 2014, Additional bioequivalence metrics are required to ensure that a new methylphenidate ER alternative is therapeutically equivalent to branded Methylphenidate Methylphenidate HCL ER 72mg One Tablet Daily Methylphenidate HCL ER 36mg Two Tablets Daily (1) Not actual tablet image; not actual tablet size 0 5 10 15 20 0 6 12 18 24 30 36 Methylphenidate Concentration (ng/mL) Time (h)
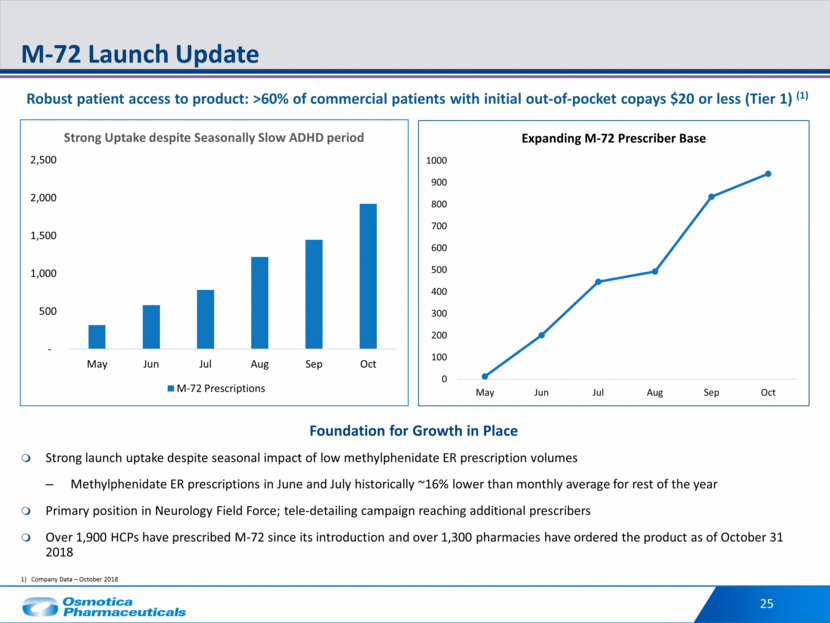
Company Data – October 2018 25 M-72 Launch Update Strong launch uptake despite seasonal impact of low methylphenidate ER prescription volumes Methylphenidate ER prescriptions in June and July historically ~16% lower than monthly average for rest of the year Primary position in Neurology Field Force; tele-detailing campaign reaching additional prescribers Over 1,900 HCPs have prescribed M-72 since its introduction and over 1,300 pharmacies have ordered the product as of October 31 2018 Robust patient access to product: >60% of commercial patients with initial out-of-pocket copays $20 or less (Tier 1) (1) Foundation for Growth in Place - 500 1,000 1,500 2,000 2,500 May Jun Jul Aug Sep Oct Strong Uptake despite Seasonally Slow ADHD period M-72 Prescriptions 0 100 200 300 400 500 600 700 800 900 1000 May Jun Jul Aug Sep Oct Expanding M - 72 Prescriber Base
Profile & Market Opportunity Osmolex ER Overview 26 Sources: IQVIA data, Symphony Health data, and Company estimates Market Opportunity (1) > $400 million market opportunity based on Wholesale Acquisition Cost pricing ~1M amantadine IR Rx annually Estimated 50% Rx written by neurologists and movement disorder specialists in 2017 Estimated 19% of Rx written by psychiatry specialists from Q4 2015 – Q3 2016 Indication Parkinson’s Disease and drug-induced extrapyramidal reactions in adults Regulatory Status Approved February 2018 Commercial Launch Full Launch January 2019 Dosing Immediate-release outer core with extended-release inner core for convenient once-daily dosing in the morning Intellectual Property Two formulation patents, one of which extends to 2030 Key Features Cmax in the middle of the day as the product is administered in the morning Osmotic delivery designed to produce a consistent delivery of amantadine throughout the day, and is available in multiple strengths for added dosing flexibility (129mg, 193mg, 258mg) Amantadine IR Prescribers New once-daily treatment option for Parkinson’s Disease and drug-induced extrapyramidal reactions in adults 50% 19% 31% Neurologists and Movement Specialists Psychiatrists Other
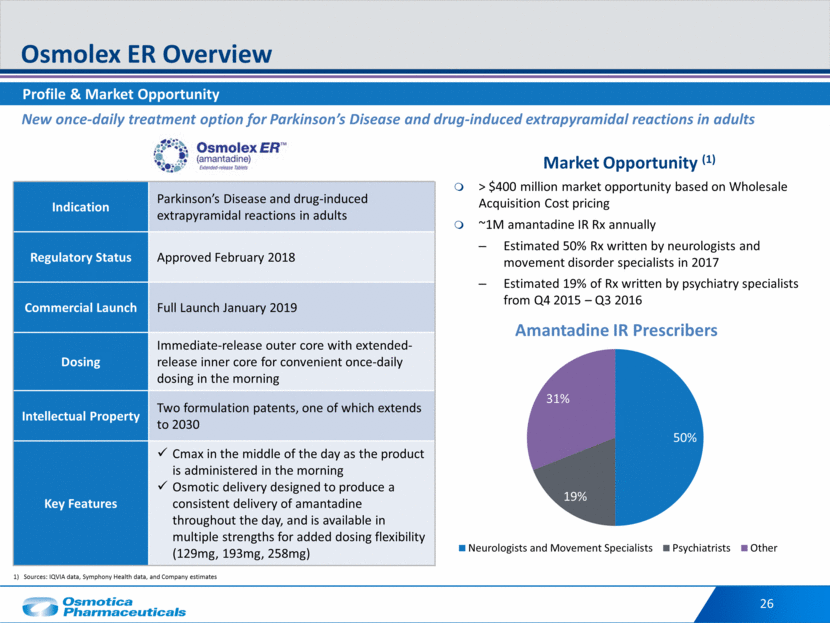
Differentiated Drug-Delivery that Provides Dosing Alternatives Osmolex ER Features 27 The efficacy of Osmolex ER has been established based on bioavailability studies that compared Osmolex ER to immediate-release amantadine. The pharmacokinetics of amantadine in Osmolex ER is dose proportional across the dose range of 129mg to 258mg.
1) Patients need affordable access to an extended release amantadine product Osmolex ER™ priced at WAC of $450/month, minimizing risk of placement on a specialty tier and co-pay/coinsurance burden for patients Co-pay assistance for commercial patients Access Osmolex™ for office and patient support, dedicated to assisting patients in gaining access to Osmolex ER™ Contract strategy, where appropriate, to support broad coverage 2) Focused personal promotional effort with dedicated Sales team 32 sales representatives at launch, targeting ~3,500 HCP’s, already hired and trained 3) Complementary multi-channel marketing campaigns 4) Medical support to extend promotional reach Medical Science Liaisons in place to address medical questions Expanding advocacy efforts to advance patient education, preserve patient choice, advance research, and protect appropriate access to innovative therapies Launch Overview Ensuring adult patients with Parkinson’s disease and drug-induced extrapyramidal reactions (EPR), their caregivers and providers, know about, and have access to Osmolex ER 28

Vision Osmolex ER will become the “go-to-amantadine” for PD and drug-induced EPR in adults because of its once daily dosing and affordability. 2018-2019 Strategy Ensure patients have access to Osmolex ER Ensure a positive HCP/Patient experience. Speed to fill Reimbursement support Targeted personal promotion to high decile writers of Amantadine IR. Execute a multi-channel engagement strategy to increase awareness, trial, & adoption KOL engagement Key Levers HCP Patient/Caregiver & Advocacy Payer and Patient Access 2018-2019 Tactics Neuro clinical visual aid Psychiatry flashcard General leave behind MOD video HCP website Table top displays Patient kits Patient brochure Patient website National/District advocacy support Ensure access to medications and support for caregivers and adult patients diagnosed with PD or drug-induced EPR. Partner with select advocacy groups for PD/EPR, and brand, awareness. Payer Presentation Clinical support Distributor Fact Sheet Access Osmolex™ Pharmacy network Access Osmolex™ Office Support Guide Osmolex ER – Strategic Vision 29
Mission: Support the Company’s efforts by establishing awareness of unmet needs in the medical community through data generation, stakeholder engagement, and knowledge dissemination Build upon amantadine history; HCPs are familiar with molecular entity Reactive responses to unsolicited questions surrounding Osmolex ER, payer inquiries, etc. Education on FDA approval pathways (i.e. 505(b)(2) pathway) Education on Osmotica technology Osmolex ER: Pharmacokinetics (consistent blood levels with once daily dosing) and Bioavailablity Osmolex ER: Education on dosing and titration, if needed 1 2 3 4 5 6 Publication Planning Primary Manuscripts Review Manuscripts Abstracts & Posters Advisory Boards AdBoard1: Neuro AdBoard2: Psych Regional Virtual MSL Execution KOL Development Managed Care Advocacy Outreach Investigator Studies and Grants Areas of Interest Education Plan Awareness (i.e. portal/website) Medical Information FAQs Standard Responses Clinical Dossier Key Audiences: Neurologists, Movement disorder specialists, Psychiatry, Allied HCPs (NP, PA, etc.), and Payers Osmolex ER – Medical Strategy 30
Financial Overview 31
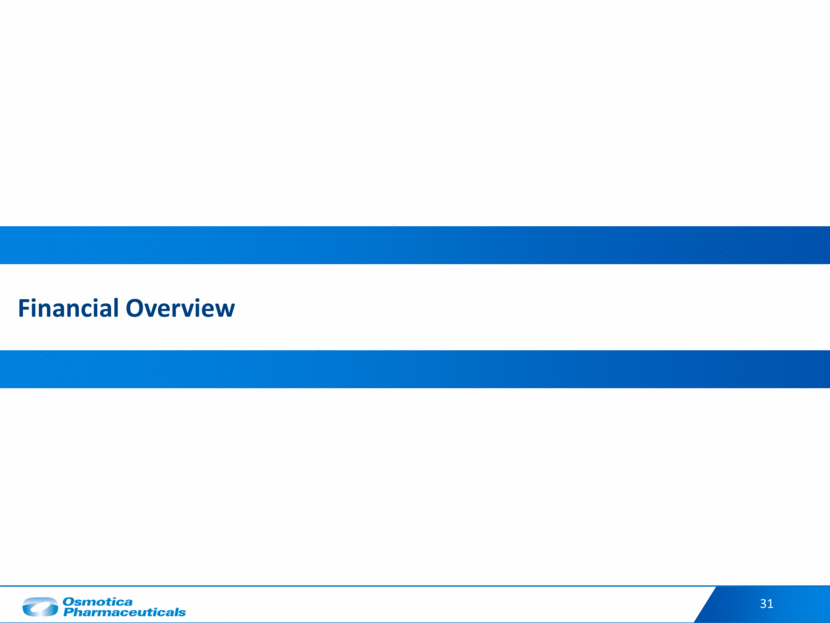
32 Financial Overview ($ in millions) Year Ended December 31, 9 Months Ended Sept. 30, 2016 2017 9/30/2017 9/30/2018 Revenues $218 $246 $169 $198 Gross Profit 93 121 92 99 % of Revenue 42% 49% 54% 50% Adjusted EBITDA(1) 43 99 72 81 % of Revenue 20% 40% 43% 41% Balance Sheet Items Cash & Cash Equivalents $35 $33 $32 Total Debt(2) 328 272 Net Debt / Adjusted EBITDA(1) 3.0x 2.2x(3) See the appendix to this presentation for a reconciliation of Adjusted EBTIDA to net income (loss). Total Debt includes capital lease obligations and is pro-forma for a $50 million prepayment made on Oct. 31, 2018. Calculated as Net Debt as of Sept. 30, 2018 divided by Q3 YTD 2018 EBITDA plus 2017 EBITDA less Q3 YTD 2017 EBITDA.
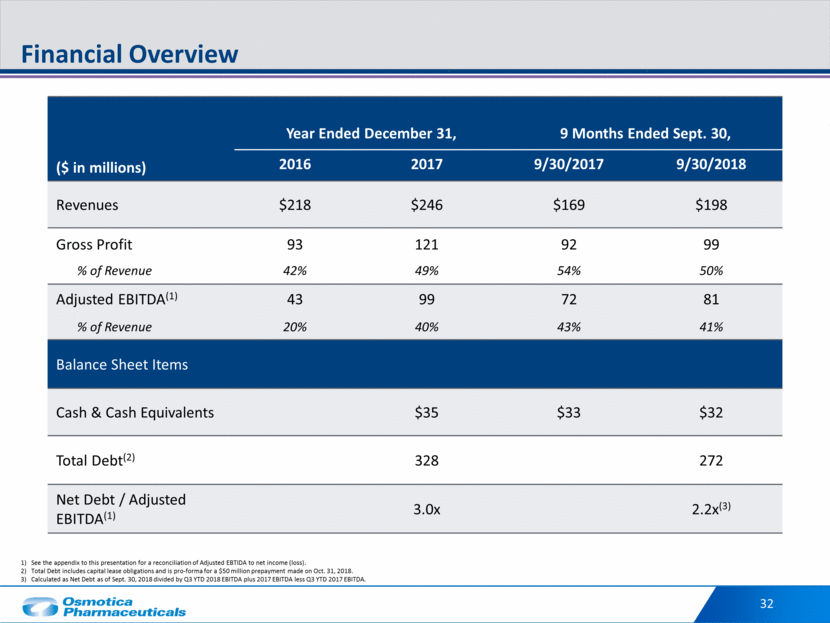
Revenue by Product 33 ( $ in thousands) 3Q YTD 2018 Revenue 3Q YTD 2017 Revenue $50,378 $198,005 $12,537 $15,062 $7,558 $100,573 $11,897 VERT Lorzone Divigel OB Complete Methylphenidate ER Other 1H 2018 Total Revenue $85,292 $169,417 $16,109 $13,171 $7,609 $16,540 $30,696 VERT Lorzone Divigel OB Complete Methylphenidate ER Other 1H 2018 Total Revenue
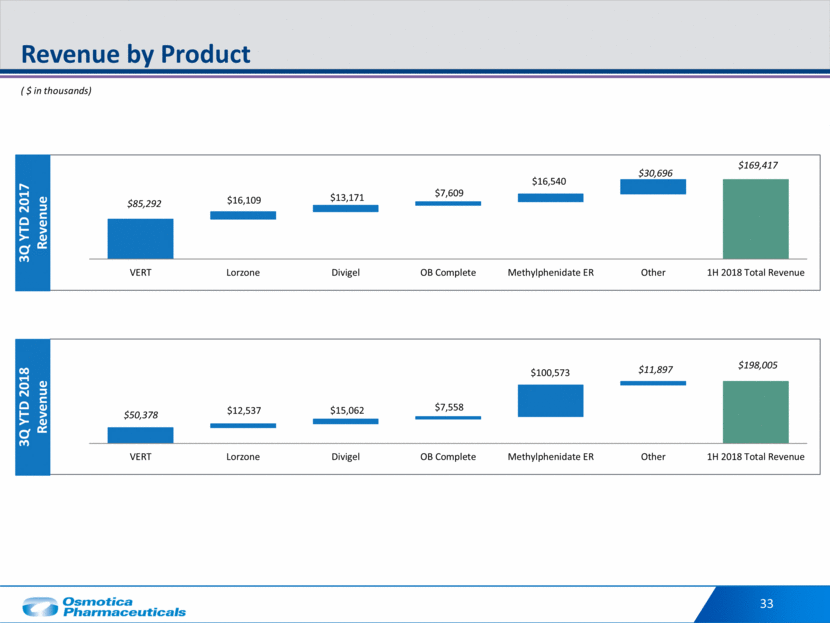
34 Investment Highlights Diversified business with a flexible balance sheet to fund future growth 1 Existing portfolio of promoted and non-promoted products generated $246M of Revenue and $99M of Adjusted EBITDA in 2017 2 Near-term growth anticipated from recently approved M-72 for ADHD and Osmolex ER for Parkinson’s 4 Led by proven management team with a track record of successful operating and business development experience 7 Significant R&D investments drive near-term catalysts from two late stage Phase III programs: Arbaclofen ER for muscle spasticity in Multiple Sclerosis and RVL-1201 for Blepharoptosis 3 R&D capacity and capability that leverages proprietary Osmodex Drug Delivery System 5 Strong IP coupled with complex manufacturing capabilities 6
Appendix
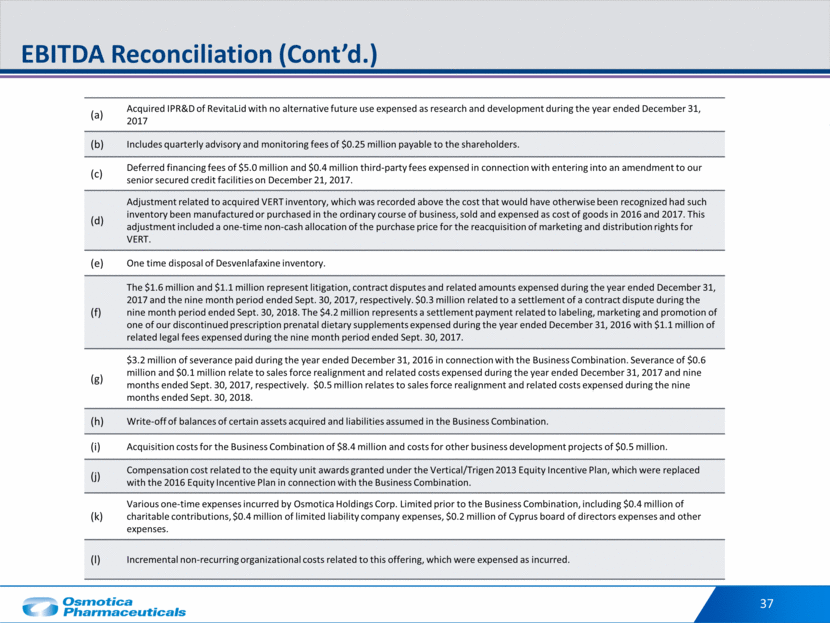
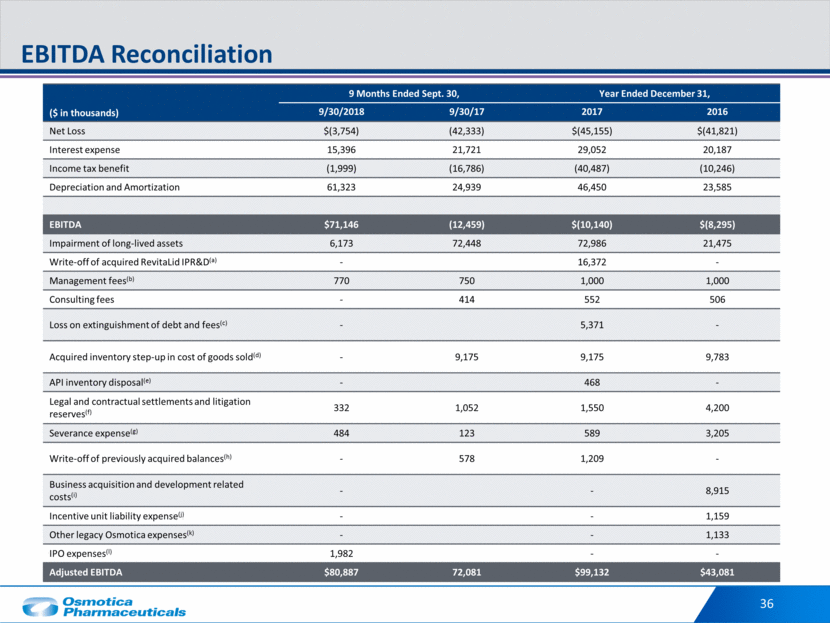
36 EBITDA Reconciliation ($ in thousands) 9 Months Ended Sept. 30, Year Ended December 31, 9/30/2018 9/30/17 2017 2016 Net Loss $(3,754) (42,333) $(45,155) $(41,821) Interest expense 15,396 21,721 29,052 20,187 Income tax benefit (1,999) (16,786) (40,487) (10,246) Depreciation and Amortization 61,323 24,939 46,450 23,585 EBITDA $71,146 (12,459) $(10,140) $(8,295) Impairment of long-lived assets 6,173 72,448 72,986 21,475 Write-off of acquired RevitaLid IPR&D(a) - 16,372 - Management fees(b) 770 750 1,000 1,000 Consulting fees - 414 552 506 Loss on extinguishment of debt and fees(c) - 5,371 - Acquired inventory step-up in cost of goods sold(d) - 9,175 9,175 9,783 API inventory disposal(e) - 468 - Legal and contractual settlements and litigation reserves(f) 332 1,052 1,550 4,200 Severance expense(g) 484 123 589 3,205 Write-off of previously acquired balances(h) - 578 1,209 - Business acquisition and development related costs(i) - - 8,915 Incentive unit liability expense(j) - - 1,159 Other legacy Osmotica expenses(k) - - 1,133 IPO expenses(l) 1,982 - - Adjusted EBITDA $80,887 72,081 $99,132 $43,081
37 EBITDA Reconciliation (Cont’d.) (a) Acquired IPR&D of RevitaLid with no alternative future use expensed as research and development during the year ended December 31, 2017 (b) Includes quarterly advisory and monitoring fees of $0.25 million payable to the shareholders. (c) Deferred financing fees of $5.0 million and $0.4 million third-party fees expensed in connection with entering into an amendment to our senior secured credit facilities on December 21, 2017. (d) Adjustment related to acquired VERT inventory, which was recorded above the cost that would have otherwise been recognized had such inventory been manufactured or purchased in the ordinary course of business, sold and expensed as cost of goods in 2016 and 2017. This adjustment included a one-time non-cash allocation of the purchase price for the reacquisition of marketing and distribution rights for VERT. (e) One time disposal of Desvenlafaxine inventory. (f) The $1.6 million and $1.1 million represent litigation, contract disputes and related amounts expensed during the year ended December 31, 2017 and the nine month period ended Sept. 30, 2017, respectively. $0.3 million related to a settlement of a contract dispute during the nine month period ended Sept. 30, 2018. The $4.2 million represents a settlement payment related to labeling, marketing and promotion of one of our discontinued prescription prenatal dietary supplements expensed during the year ended December 31, 2016 with $1.1 million of related legal fees expensed during the nine month period ended Sept. 30, 2017. (g) $3.2 million of severance paid during the year ended December 31, 2016 in connection with the Business Combination. Severance of $0.6 million and $0.1 million relate to sales force realignment and related costs expensed during the year ended December 31, 2017 and nine months ended Sept. 30, 2017, respectively. $0.5 million relates to sales force realignment and related costs expensed during the nine months ended Sept. 30, 2018. (h) Write-off of balances of certain assets acquired and liabilities assumed in the Business Combination. (i) Acquisition costs for the Business Combination of $8.4 million and costs for other business development projects of $0.5 million. (j) Compensation cost related to the equity unit awards granted under the Vertical/Trigen 2013 Equity Incentive Plan, which were replaced with the 2016 Equity Incentive Plan in connection with the Business Combination. (k) Various one-time expenses incurred by Osmotica Holdings Corp. Limited prior to the Business Combination, including $0.4 million of charitable contributions, $0.4 million of limited liability company expenses, $0.2 million of Cyprus board of directors expenses and other expenses. (l) Incremental non-recurring organizational costs related to this offering, which were expensed as incurred.