Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Blueprint Medicines Corp | ex-99d1.htm |
| 8-K - 8-K - Blueprint Medicines Corp | f8-k.htm |
Exhibit 99.2
| Avapritinib, a Potent and Selective Inhibitor of KIT D816V, Improves Symptoms of Advanced Systemic Mastocytosis (AdvSM) American Society of Hematology Annual Meeting San Diego, CA, 2 Dec 2018 Analyses of Patient Reported Outcomes (PROs) from the Phase 1 (EXPLORER) Study Using the AdvSM Symptom Assessment Form (AdvSM-SAF), a New PRO Questionnaire for AdvSM Jason Gotlib, Deepti Radia, Daniel J. DeAngelo, Prithviraj Bose, Mark W Drummond, Elizabeth O. Hexner, William A. Robinson, Maureen G. Conlan, Ronny Oren, Hongliang Shi and Michael W. Deininger |
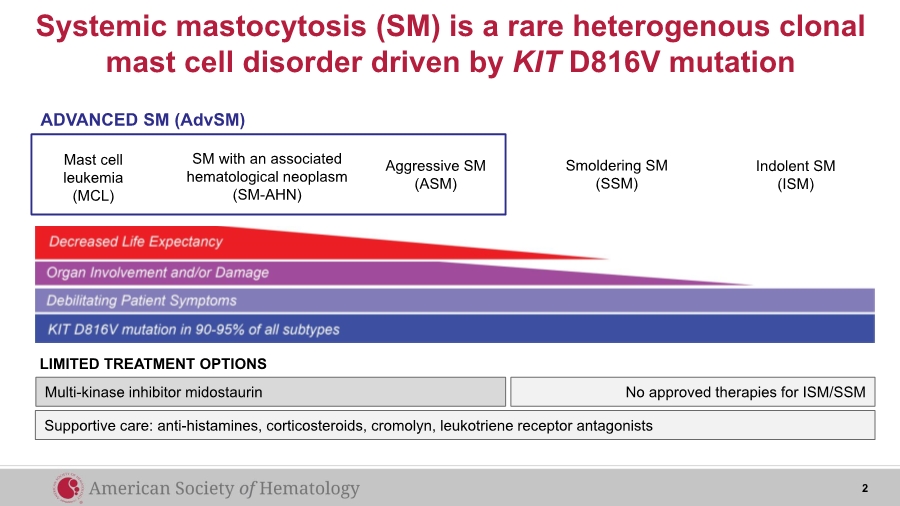
| Systemic mastocytosis (SM) is a rare heterogenous clonal mast cell disorder driven by KIT D816V mutation Multi-kinase inhibitor midostaurin LIMITED TREATMENT OPTIONS No approved therapies for ISM/SSM Mast cell leukemia (MCL) SM with an associated hematological neoplasm (SM-AHN) Aggressive SM (ASM) ADVANCED SM (AdvSM) Smoldering SM (SSM) Indolent SM (ISM) Supportive care: anti-histamines, corticosteroids, cromolyn, leukotriene receptor antagonists 2 |
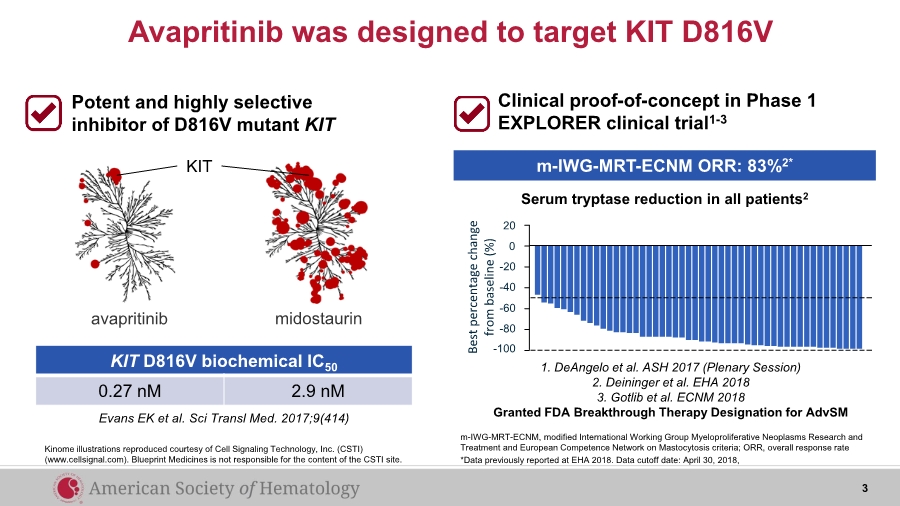
| avapritinib midostaurin KIT Evans EK et al. Sci Transl Med. 2017;9(414) Potent and highly selective inhibitor of D816V mutant KIT KIT D816V biochemical IC50 0.27 nM 2.9 nM Avapritinib was designed to target KIT D816V Kinome illustrations reproduced courtesy of Cell Signaling Technology, Inc. (CSTI) (www.cellsignal.com). Blueprint Medicines is not responsible for the content of the CSTI site. Clinical proof-of-concept in Phase 1 EXPLORER clinical trial1-3 m-IWG-MRT-ECNM, modified International Working Group Myeloproliferative Neoplasms Research and Treatment and European Competence Network on Mastocytosis criteria; ORR, overall response rate *Data previously reported at EHA 2018. Data cutoff date: April 30, 2018, 1. DeAngelo et al. ASH 2017 (Plenary Session) 2. Deininger et al. EHA 2018 3. Gotlib et al. ECNM 2018 Granted FDA Breakthrough Therapy Designation for AdvSM 20 -100 -80 -60 -40 -20 0 Best percentage change from baseline (%) Serum tryptase reduction in all patients2 m-IWG-MRT-ECNM ORR: 83%2* 3 |
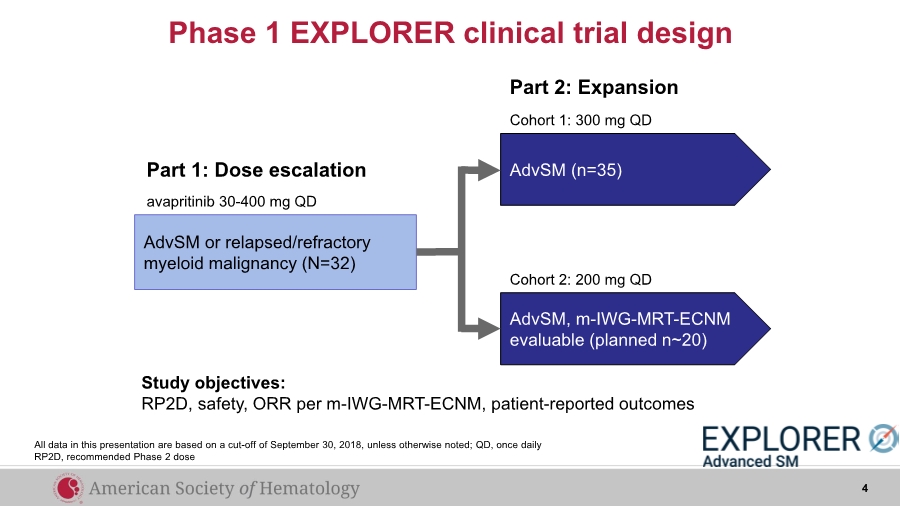
| Phase 1 EXPLORER clinical trial design AdvSM (n=35) AdvSM, m-IWG-MRT-ECNM evaluable (planned n~20) Cohort 1: 300 mg QD Cohort 2: 200 mg QD Part 1: Dose escalation Part 2: Expansion avapritinib 30-400 mg QD Study objectives: RP2D, safety, ORR per m-IWG-MRT-ECNM, patient-reported outcomes AdvSM or relapsed/refractory myeloid malignancy (N=32) 4 All data in this presentation are based on a cut-off of September 30, 2018, unless otherwise noted; QD, once daily RP2D, recommended Phase 2 dose |
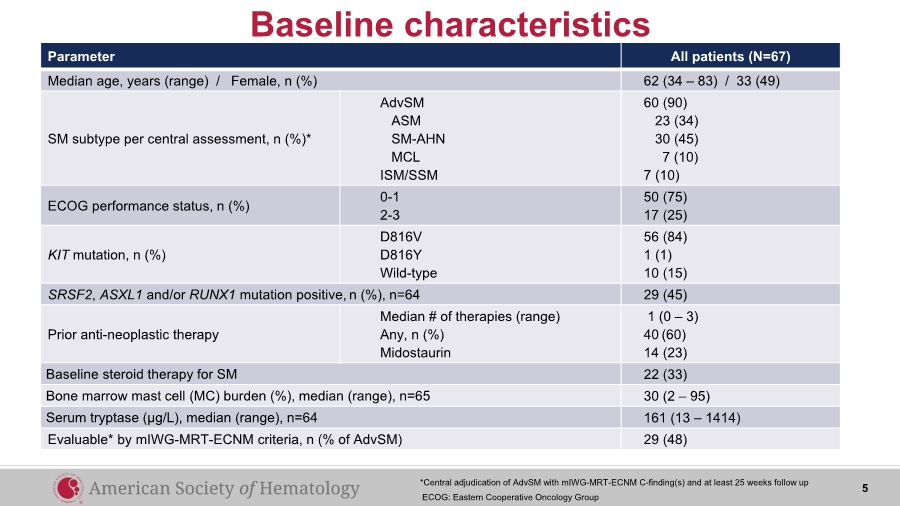
| Parameter All patients (N=67) Median age, years (range) / Female, n (%) 62 (34 – 83) / 33 (49) SM subtype per central assessment, n (%)* AdvSM ASM SM-AHN MCL ISM/SSM 60 (90) 23 (34) 30 (45) 7 (10) 7 (10) ECOG performance status, n (%) 0-1 2-3 50 (75) 17 (25) KIT mutation, n (%) D816V D816Y Wild-type 56 (84) 1 (1) 10 (15) SRSF2, ASXL1 and/or RUNX1 mutation positive, n (%), n=64 29 (45) Prior anti-neoplastic therapy Median # of therapies (range) Any, n (%) Midostaurin 1 (0 – 3) 40 (60) 14 (23) Baseline steroid therapy for SM 22 (33) Bone marrow mast cell (MC) burden (%), median (range), n=65 30 (2 – 95) Serum tryptase (µg/L), median (range), n=64 161 (13 – 1414) Evaluable* by mIWG-MRT-ECNM criteria, n (% of AdvSM) 29 (48) Baseline characteristics 5*Central adjudication of AdvSM with mIWG-MRT-ECNM C-finding(s) and at least 25 weeks follow up ECOG: Eastern Cooperative Oncology Group |
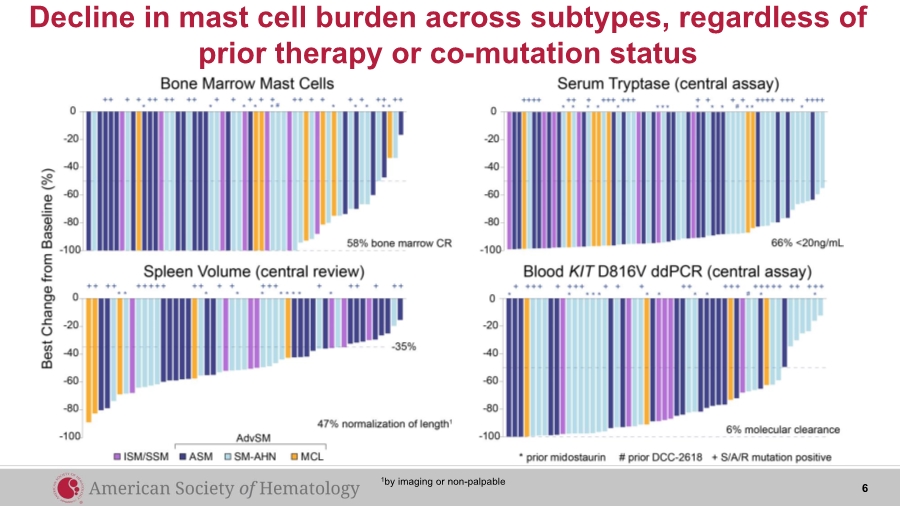
| Decline in mast cell burden across subtypes, regardless of prior therapy or co-mutation status 6 1by imaging or non-palpable |
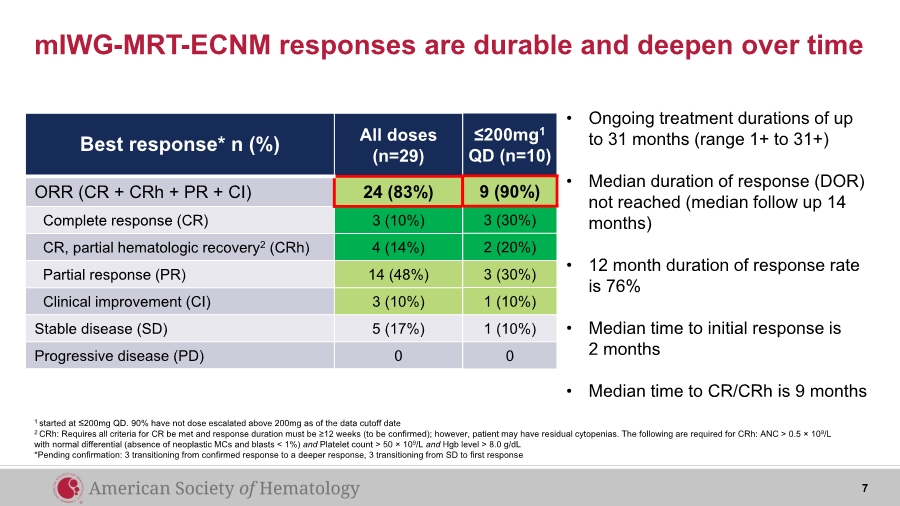
| mIWG-MRT-ECNM responses are durable and deepen over time Best response* n (%) All doses (n=29) ORR (CR + CRh + PR + CI) 24 (83%) Complete response (CR) 3 (10%) CR, partial hematologic recovery2 (CRh) 4 (14%) Partial response (PR) 14 (48%) Clinical improvement (CI) 3 (10%) Stable disease (SD) 5 (17%) Progressive disease (PD) 0 1 started at ≤200mg QD. 90% have not dose escalated above 200mg as of the data cutoff date 2 CRh: Requires all criteria for CR be met and response duration must be ≥12 weeks (to be confirmed); however, patient may have residual cytopenias. The following are required for CRh: ANC > 0.5 × 109/L with normal differential (absence of neoplastic MCs and blasts < 1%) and Platelet count > 50 × 109/L and Hgb level > 8.0 g/dL *Pending confirmation: 3 transitioning from confirmed response to a deeper response, 3 transitioning from SD to first response ≤200mg1 QD (n=10) 9 (90%) 3 (30%) 2 (20%) 3 (30%) 1 (10%) 1 (10%) 0 • Ongoing treatment durations of up to 31 months (range 1+ to 31+) • Median duration of response (DOR) not reached (median follow up 14 months) • 12 month duration of response rate is 76% • Median time to initial response is 2 months • Median time to CR/CRh is 9 months 7 |
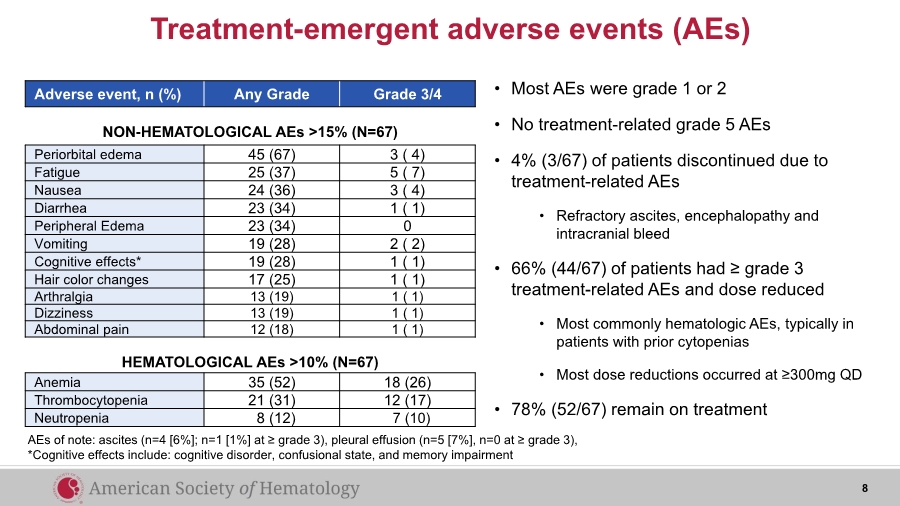
| Treatment-emergent adverse events (AEs) Adverse event, n (%) Any Grade Grade 3/4 Anemia 35 (52) 18 (26) Thrombocytopenia 21 (31) 12 (17) Neutropenia 8 (12) 7 (10) Periorbital edema 45 (67) 3 ( 4) Fatigue 25 (37) 5 ( 7) Nausea 24 (36) 3 ( 4) Diarrhea 23 (34) 1 ( 1) Peripheral Edema 23 (34) 0 Vomiting 19 (28) 2 ( 2) Cognitive effects* 19 (28) 1 ( 1) Hair color changes 17 (25) 1 ( 1) Arthralgia 13 (19) 1 ( 1) Dizziness 13 (19) 1 ( 1) Abdominal pain 12 (18) 1 ( 1) NON-HEMATOLOGICAL AEs >15% (N=67) HEMATOLOGICAL AEs >10% (N=67) AEs of note: ascites (n=4 [6%]; n=1 [1%] at ≥ grade 3), pleural effusion (n=5 [7%], n=0 at ≥ grade 3), *Cognitive effects include: cognitive disorder, confusional state, and memory impairment • Most AEs were grade 1 or 2 • No treatment-related grade 5 AEs • 4% (3/67) of patients discontinued due to treatment-related AEs • Refractory ascites, encephalopathy and intracranial bleed • 66% (44/67) of patients had ≥ grade 3 treatment-related AEs and dose reduced • Most commonly hematologic AEs, typically in patients with prior cytopenias • Most dose reductions occurred at ≥300mg QD • 78% (52/67) remain on treatment 8 |
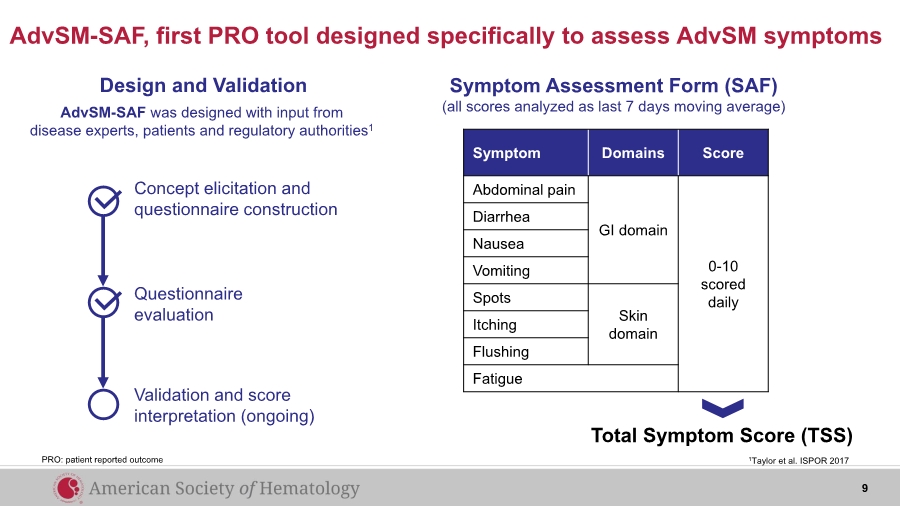
| AdvSM-SAF, first PRO tool designed specifically to assess AdvSM symptoms Symptom Domains Score Abdominal pain GI domain 0-10 scored daily Diarrhea Nausea Vomiting Spots Skin domainItching Flushing Fatigue Total Symptom Score (TSS) Design and Validation Concept elicitation and questionnaire construction Questionnaire evaluation Validation and score interpretation (ongoing) AdvSM-SAF was designed with input from disease experts, patients and regulatory authorities1 1Taylor et al. ISPOR 2017 Symptom Assessment Form (SAF) (all scores analyzed as last 7 days moving average) PRO: patient reported outcome 9 |
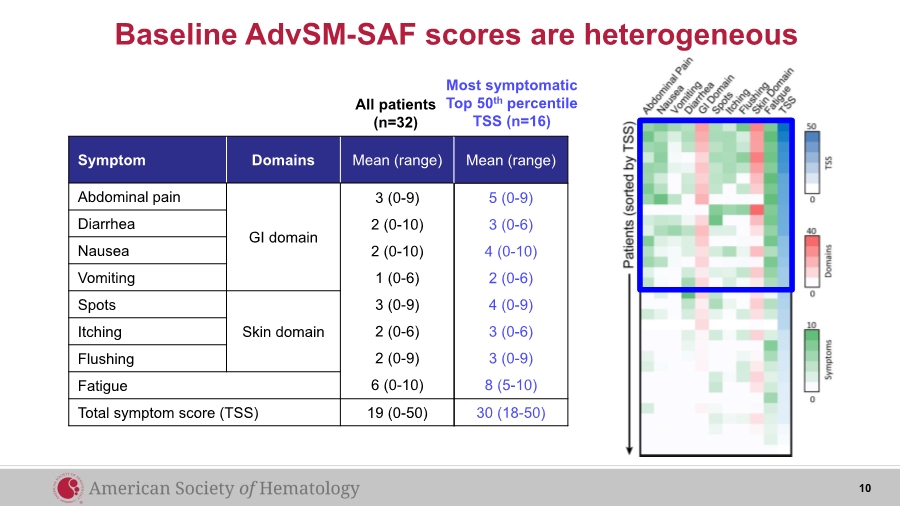
| Baseline AdvSM-SAF scores are heterogeneous Symptom Domains Mean (range) Abdominal pain GI domain 3 (0-9) 2 (0-10) 2 (0-10) 1 (0-6) 3 (0-9) 2 (0-6) 2 (0-9) 6 (0-10) Diarrhea Nausea Vomiting Spots Skin domainItching Flushing Fatigue Total symptom score (TSS) 19 (0-50) All patients (n=32) Most symptomatic Top 50th percentile TSS (n=16) Mean (range) 5 (0-9) 3 (0-6) 4 (0-10) 2 (0-6) 4 (0-9) 3 (0-6) 3 (0-9) 8 (5-10) 30 (18-50) 10 |
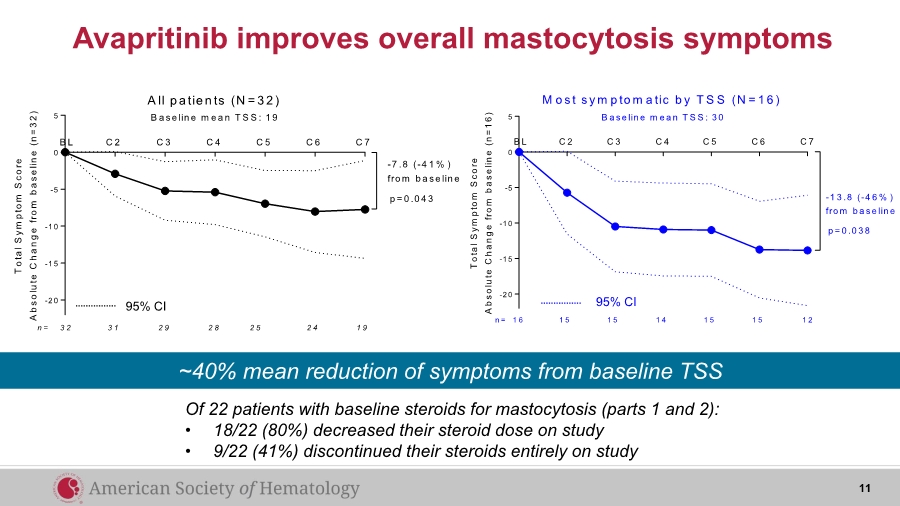
| Avapritinib improves overall mastocytosis symptoms Of 22 patients with baseline steroids for mastocytosis (parts 1 and 2): • 18/22 (80%) decreased their steroid dose on study • 9/22 (41%) discontinued their steroids entirely on study 11 ~40% mean reduction of symptoms from baseline TSS -2 0 -1 5 -1 0 -5 0 5 T o t a l S y m p t o m S c o r e A b s o l u t e C h a n g e f r o m b a s e l i n e ( n = 1 6 ) C 2 C 3 C 4 C 5 C 6 C 7B L p = 0 .0 3 8 1 5 1 5 1 4 1 5 1 5 1 21 6n = M o s t s y m p to m a tic b y T S S (N = 1 6 ) B a se lin e m e a n T S S : 3 0 -1 3 .8 (-4 6 % ) fro m b a se lin e 95% CI-2 0 -1 5 -1 0 -5 0 5 T o t a l S y m p t o m S c o r e A b s o l u t e C h a n g e f r o m b a s e l i n e ( n = 3 2 ) B a se lin e m e a n T S S : 1 9 C 2 C 3 C 4 C 5 C 6 C 7B L 3 1 2 9 2 8 2 5 2 4 1 93 2n = A ll p a tie n ts (N = 3 2 ) p = 0 .0 4 3 -7 .8 (-4 1 % ) fro m b a se lin e 95% CI |
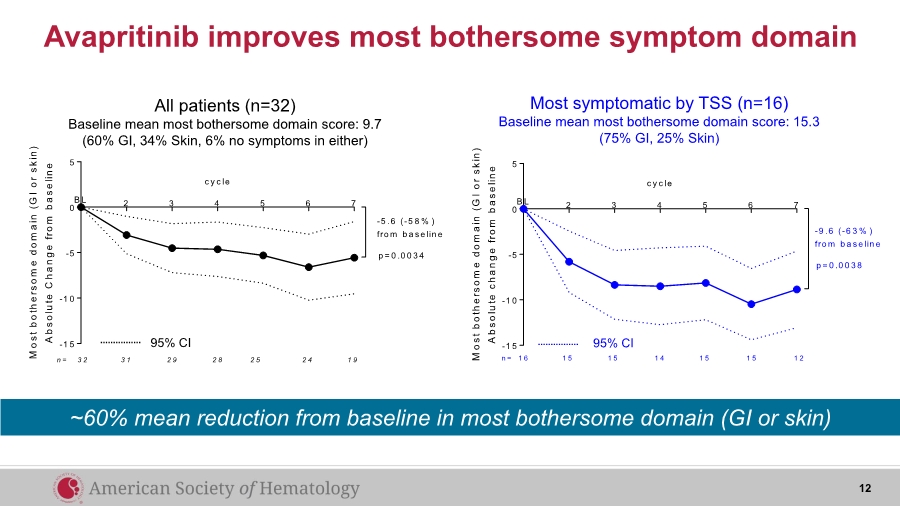
| Avapritinib improves most bothersome symptom domain 2 3 4 5 6 7 -1 5 -1 0 -5 0 5 c y c le M o s t b o t h e r s o m e d o m a i n ( G I o r s k i n ) A b s o l u t e c h a n g e f r o m b a s e l i n e B L -9 .6 (-6 3 % ) fro m b a se lin e p = 0 .0 0 3 8 1 5 1 5 1 4 1 5 1 5 1 21 6n = 2 3 4 5 6 7 -1 5 -1 0 -5 0 5 c y c le M o s t b o t h e r s o m e d o m a i n ( G I o r s k i n ) A b s o l u t e C h a n g e f r o m b a s e l i n e B L -5 .6 (-5 8 % ) fro m b a se lin e p = 0 .0 0 3 4 3 1 2 9 2 8 2 5 2 4 1 93 2n = All patients (n=32) Baseline mean most bothersome domain score: 9.7 (60% GI, 34% Skin, 6% no symptoms in either) Most symptomatic by TSS (n=16) Baseline mean most bothersome domain score: 15.3 (75% GI, 25% Skin) 95% CI 95% CI 12 ~60% mean reduction from baseline in most bothersome domain (GI or skin) |
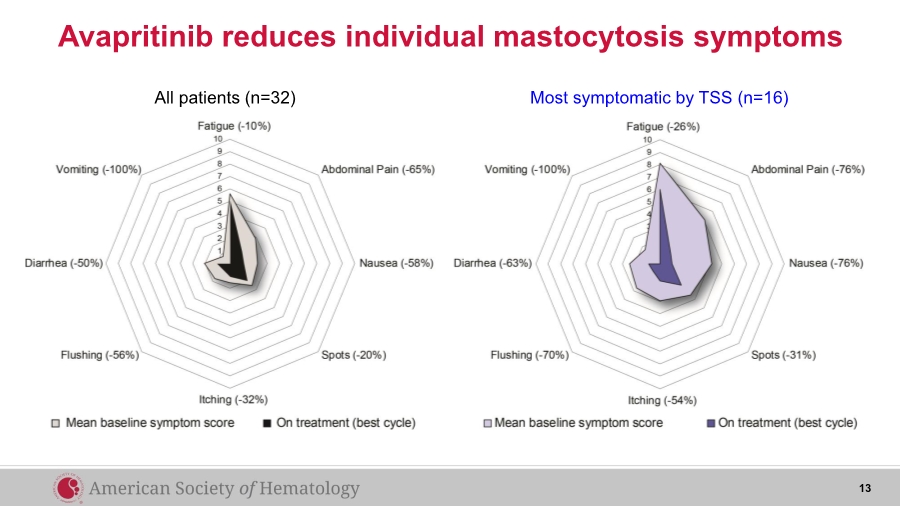
| 13 All patients (n=32) Most symptomatic by TSS (n=16) Avapritinib reduces individual mastocytosis symptoms |
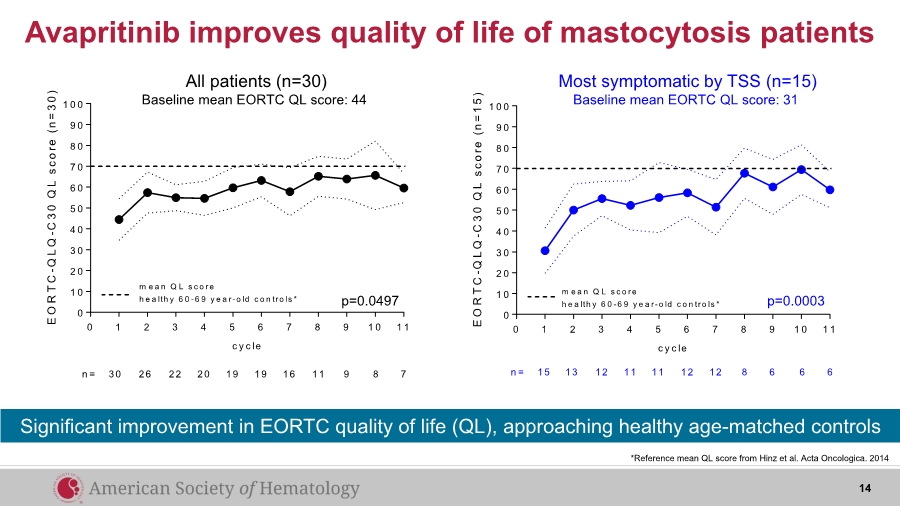
| Avapritinib improves quality of life of mastocytosis patients *Reference mean QL score from Hinz et al. Acta Oncologica. 2014 14 Significant improvement in EORTC quality of life (QL), approaching healthy age-matched controls 0 1 2 3 4 5 6 7 8 9 1 0 1 1 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 9 0 1 0 0 c y c le E O R T C - Q L Q - C 3 0 Q L s c o r e ( n = 1 5 ) m e a n Q L s c o re h e a lth y 6 0 -6 9 y e a r-o ld c o n tro ls * n = 15 13 12 11 11 12 12 8 6 6 6 0 1 2 3 4 5 6 7 8 9 1 0 1 1 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 9 0 1 0 0 c y c le E O R T C - Q L Q - C 3 0 Q L s c o r e ( n = 3 0 ) m e a n Q L s c o re h e a lth y 6 0 -6 9 y e a r-o ld c o n tro ls * n = 30 26 22 20 19 19 16 11 9 8 7 p=0.0497 p=0.0003 Most symptomatic by TSS (n=15) Baseline mean EORTC QL score: 31 All patients (n=30) Baseline mean EORTC QL score: 44 |
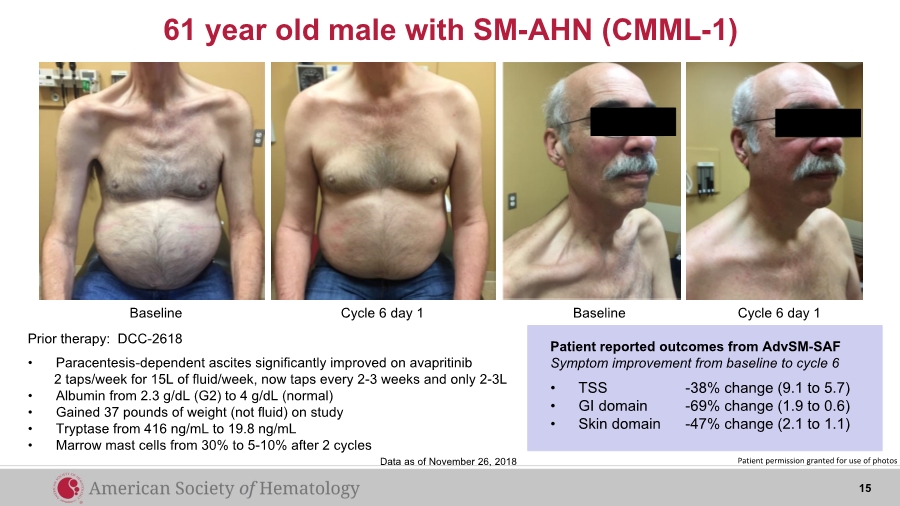
| 61 year old male with SM-AHN (CMML-1) Baseline BaselineCycle 6 day 1 Cycle 6 day 1 Prior therapy: DCC-2618 • Paracentesis-dependent ascites significantly improved on avapritinib 2 taps/week for 15L of fluid/week, now taps every 2-3 weeks and only 2-3L • Albumin from 2.3 g/dL (G2) to 4 g/dL (normal) • Gained 37 pounds of weight (not fluid) on study • Tryptase from 416 ng/mL to 19.8 ng/mL • Marrow mast cells from 30% to 5-10% after 2 cycles Patient reported outcomes from AdvSM-SAF Symptom improvement from baseline to cycle 6 • TSS -38% change (9.1 to 5.7) • GI domain -69% change (1.9 to 0.6) • Skin domain -47% change (2.1 to 1.1) Patient permission granted for use of photosData as of November 26, 2018 15 |
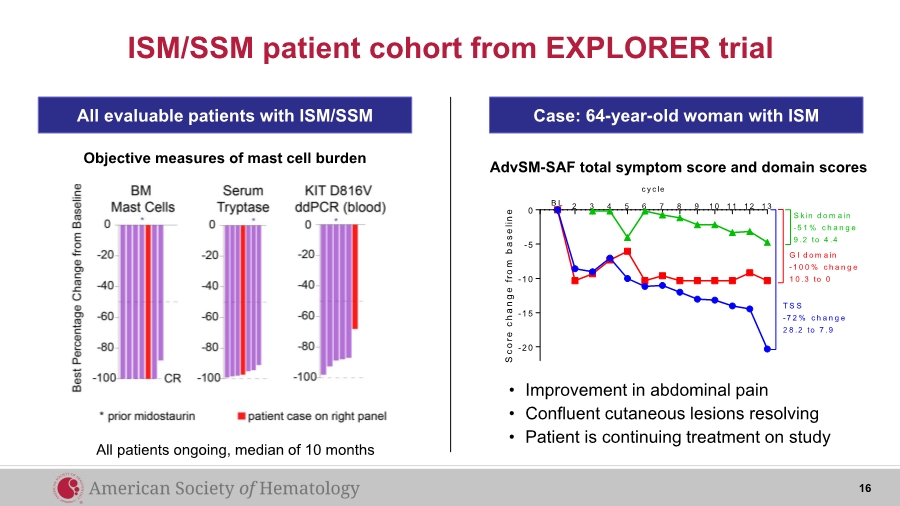
| ISM/SSM patient cohort from EXPLORER trial All evaluable patients with ISM/SSM Objective measures of mast cell burden Case: 64-year-old woman with ISM 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 -2 0 -1 5 -1 0 -5 0 c y c le S c o r e c h a n g e f r o m b a s e l i n e B L S k in d o m a in -5 1 % c h a n g e 9 .2 to 4 .4 G I d o m a in -1 0 0 % c h a n g e 1 0 .3 to 0 T S S -7 2 % c h a n g e 2 8 .2 to 7 .9 • Improvement in abdominal pain • Confluent cutaneous lesions resolving • Patient is continuing treatment on study AdvSM-SAF total symptom score and domain scores All patients ongoing, median of 10 months 16 |
| • High response rate and durable clinical benefit in patients with AdvSM – 83% ORR by mIWG-MRT-ECNM (24% CR + CRh) – Median duration of response not reached • Well tolerated with most AEs grade 1 or 2; 78% remain on study, up to 31 months ongoing • First AdvSM specific PRO demonstrates significant improvement in total symptom score • Significant improvement in EORTC quality of life, approaching healthy age-matched controls • Clinical activity and initial PRO data support further evaluation in both AdvSM and ISM/SSM – PATHFINDER trial in AdvSM now enrolling – PIONEER trial in ISM/SSM planned to initiate by end of 2018 17 Avapritinib reduces objective signs and patient symptoms of SM Summary |
| Acknowledgements Stanford Justin Abuel Cheryl Langford Isabel Reyes Cecelia Perkins William Shomali John Baird Vanessa Kennedy Jim Zehnder Anandi Krishnan Phase I Investigators Daniel DeAngelo Michael Deininger Deepti Radia Srdan Verstovsek Prithvi Bose Elizabeth Hexner Albert Quiery William Robinson Mark Drummond Elliott Winton Maria Kremyanskaya Tracy George Patients & Families Charles and Ann Johnson Foundation 18 |