Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Ra Medical Systems, Inc. | rmed-ex991_6.htm |
| 8-K - 8-K - Ra Medical Systems, Inc. | rmed-8k_20181113.htm |

Ra Medical Systems, Inc. NYSE: RMED Corporate Presentation November 2018 Exhibit 99.2

Disclaimer This presentation (the “Presentation”) includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are based on current expectations, estimates and projections based on information currently available to management. These forward-looking statements include, among others, statements regarding growth strategies for DABRA and Pharos, including pursuing regulatory approval of additional indications including coronary artery disease, in-stent restenosis, atherectomy, and other vascular occlusions indications for DABRA and a cutaneous T-cell lymphoma indication for Pharos; the benefits of DABRA and Pharos, including the potential economic benefits to providers; statements regarding our industry and competitors; the size of our markets and patient populations; sales strategies, including increasing recurring revenues, expansion of our salesforce, raising patient and provider awareness, expanding into hospital catheterization clinics and geographically; and regarding our production capacity. Forward-looking statements are typically identified by words like “believe,” “anticipate,” “could,” “should,” “estimate,” “expect,” “intend,” “plan,” “project,” “will,” “forecast,” “budget,” “pro forma,” and similar terms. Factors that could cause the Company’s results and expectations to differ materially from those expressed in forward-looking statements include, without limitation, successfully marketing and selling DABRA; gaining market acceptance for DABRA by physicians and patients; maintaining relationships with physicians; physicians understanding how to use the Company’s products; the risk of manufacturing delays; the Company’s ability to compete successfully; the Company’s ability to attract and retain highly qualified personnel; risks related to doing business internationally; successful protecting our intellectual property; remediating the material weakness in our internal control over financial reporting and maintaining effective internal controls; the ability of providers to obtain adequate reimbursement from payors for procedures performed using our products; and general market, political, economic, and business conditions. Forward-looking statements represent our management’s beliefs and assumptions only as of our November 13, 2018 press release announcing results for the quarter ended September 30, 2018. You should read our final prospectus filed with the SEC on September 27, 2018 relating to our Registration Statement on Form S-1 (File No. 333-226191), our Quarterly Report on Form 10-Q for the quarter ended September 30, 2018 to be filed with the SEC, and our subsequent reports filed with the SEC, including the Risk Factors set forth therein, completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.
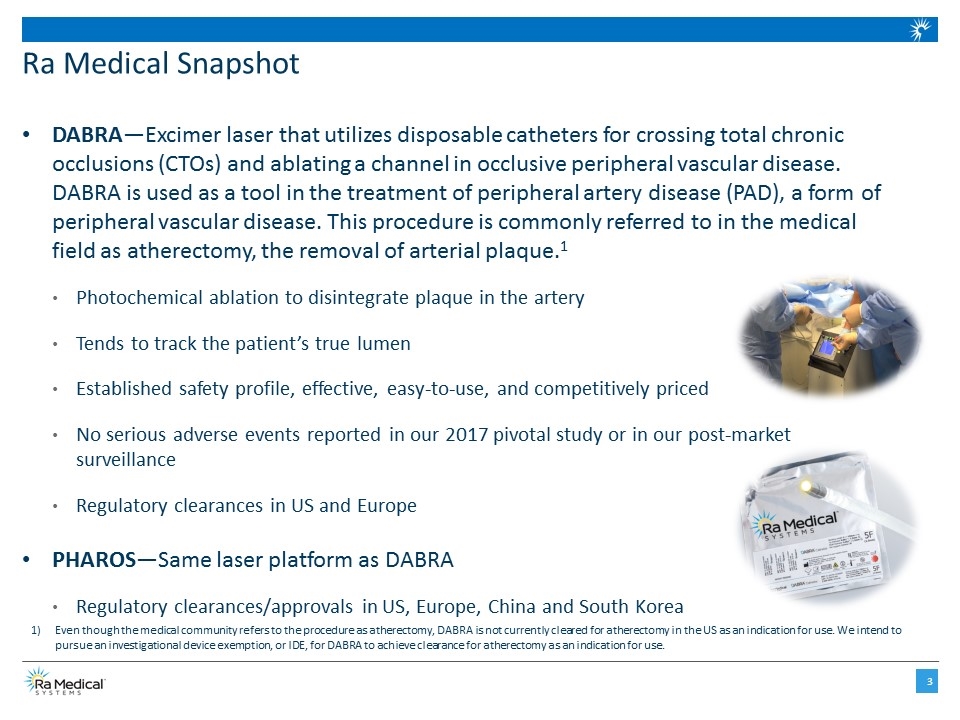
DABRA—Excimer laser that utilizes disposable catheters for crossing total chronic occlusions (CTOs) and ablating a channel in occlusive peripheral vascular disease. DABRA is used as a tool in the treatment of peripheral artery disease (PAD), a form of peripheral vascular disease. This procedure is commonly referred to in the medical field as atherectomy, the removal of arterial plaque.1 Photochemical ablation to disintegrate plaque in the artery Tends to track the patient’s true lumen Established safety profile, effective, easy-to-use, and competitively priced No serious adverse events reported in our 2017 pivotal study or in our post-market surveillance Regulatory clearances in US and Europe PHAROS—Same laser platform as DABRA Regulatory clearances/approvals in US, Europe, China and South Korea Ra Medical Snapshot Even though the medical community refers to the procedure as atherectomy, DABRA is not currently cleared for atherectomy in the US as an indication for use. We intend to pursue an investigational device exemption, or IDE, for DABRA to achieve clearance for atherectomy as an indication for use.
Highlights Single-Use Catheters $1.08B+ Targeted US Annual TAM Among ~17.6M PAD Sufferers in the U.S. Novel Therapeutic Solution with Positive Clinical Study Results Existing US Reimbursement Structure Cost Benefits to Outpatient-Based Catheterization Laboratories and Hospitals Time Efficient and Easy-to-Use Solution Highly Experienced Executive Team
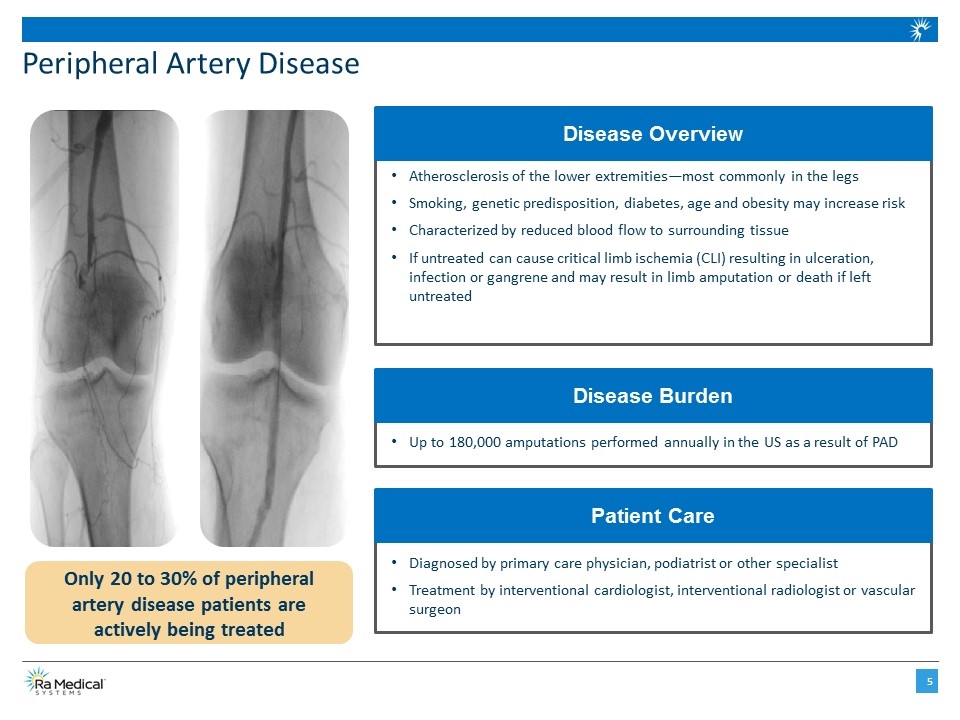
Up to 180,000 amputations performed annually in the US as a result of PAD Disease Burden Peripheral Artery Disease Atherosclerosis of the lower extremities—most commonly in the legs Smoking, genetic predisposition, diabetes, age and obesity may increase risk Characterized by reduced blood flow to surrounding tissue If untreated can cause critical limb ischemia (CLI) resulting in ulceration, infection or gangrene and may result in limb amputation or death if left untreated Disease Overview Diagnosed by primary care physician, podiatrist or other specialist Treatment by interventional cardiologist, interventional radiologist or vascular surgeon Patient Care Only 20 to 30% of peripheral artery disease patients are actively being treated
Current Treatments and Limitations Non-Invasive Management Lifestyle Changes Often hard to sustain and do not treat underlying cause of disease Pharmacotherapy Generally prescribed for life and do not treat the obstruction, making them ineffective for many More aggressive treatments often required Interventional Procedures Angioplasty Trauma due to balloon inflation may cause vessel to reocclude Possible dissection May damage arterial walls Does not remove plaque Not well suited for highly calcified lesions and bifurcations Often requires stenting Stenting Subject to fractures Cannot be removed Atherectomy Other devices can damage vessel walls due to potential mechanical and thermal trauma Surgical Procedures Bypass Surgery Invasive procedure Requires general anesthesia Multi-day hospital stay Amputation Life altering procedure
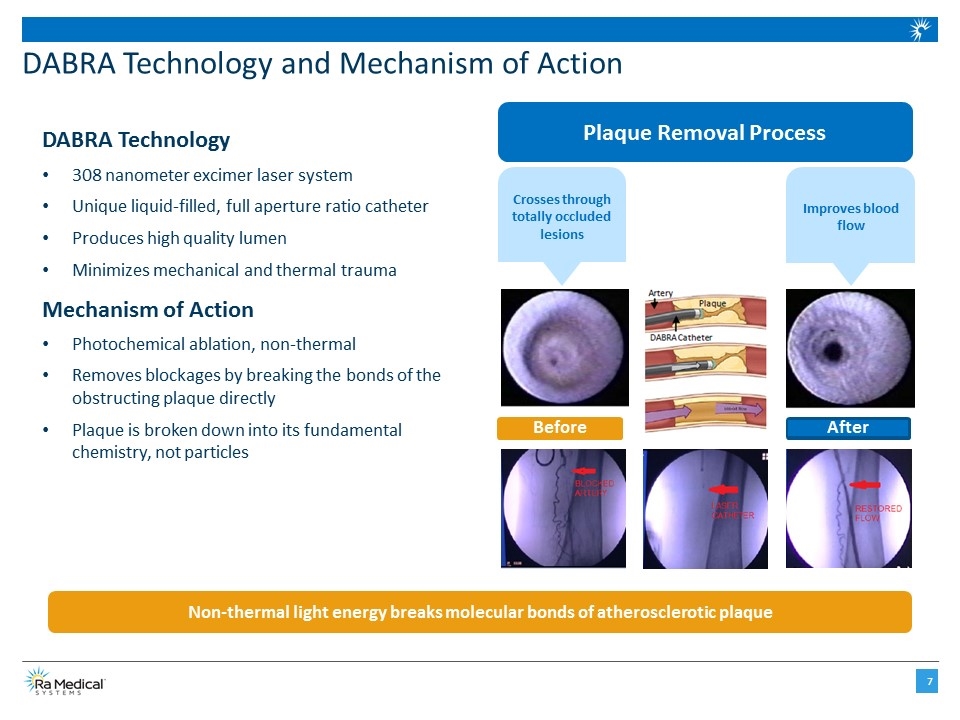
DABRA Technology and Mechanism of Action DABRA Technology 308 nanometer excimer laser system Unique liquid-filled, full aperture ratio catheter Produces high quality lumen Minimizes mechanical and thermal trauma Mechanism of Action Photochemical ablation, non-thermal Removes blockages by breaking the bonds of the obstructing plaque directly Plaque is broken down into its fundamental chemistry, not particles Non-thermal light energy breaks molecular bonds of atherosclerotic plaque Before After Plaque Removal Process Crosses through totally occluded lesions Improves blood flow

Our Solution: DABRA Portable Easy to store Intuitive interface Easy calibration Proprietary catheter Features DABRA Can cross and de-bulk wide variety of plaque, unlike some competitors Soft thrombus to hard calcified plaque Tool used by physicians to treat Chronic Total Occlusions (CTOs), unlike some competitors, prior to other alternative treatments Ability to use Above-the-Knee (ATK) and Below-the-Knee (BTK) Monotherapy or adjunct to angioplasty or other treatments Therapy Programs available that do not require a capital equipment purchase Tracks the true lumen Average of approximately two and a half minutes of lasing time Low cost Cost and Time Efficient
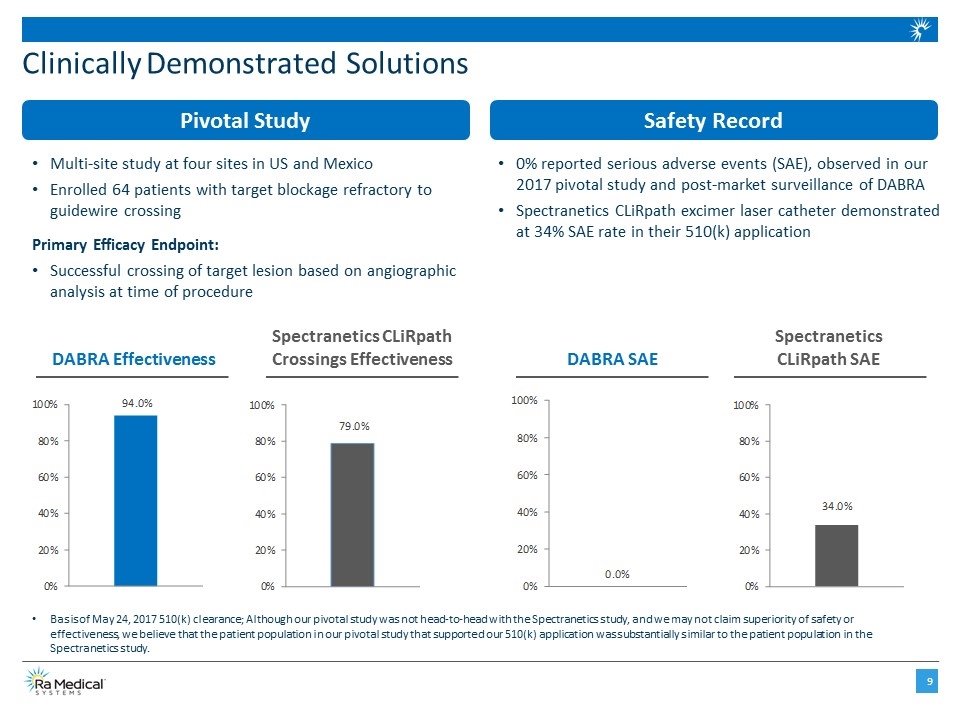
Clinically Demonstrated Solutions Safety Record Pivotal Study Multi-site study at four sites in US and Mexico Enrolled 64 patients with target blockage refractory to guidewire crossing Primary Efficacy Endpoint: Successful crossing of target lesion based on angiographic analysis at time of procedure 0% reported serious adverse events (SAE), observed in our 2017 pivotal study and post-market surveillance of DABRA Spectranetics CLiRpath excimer laser catheter demonstrated at 34% SAE rate in their 510(k) application Basis of May 24, 2017 510(k) clearance; Although our pivotal study was not head-to-head with the Spectranetics study, and we may not claim superiority of safety or effectiveness, we believe that the patient population in our pivotal study that supported our 510(k) application was substantially similar to the patient population in the Spectranetics study. Spectranetics CLiRpath Crossings Effectiveness DABRA Effectiveness Spectranetics CLiRpath SAE DABRA SAE
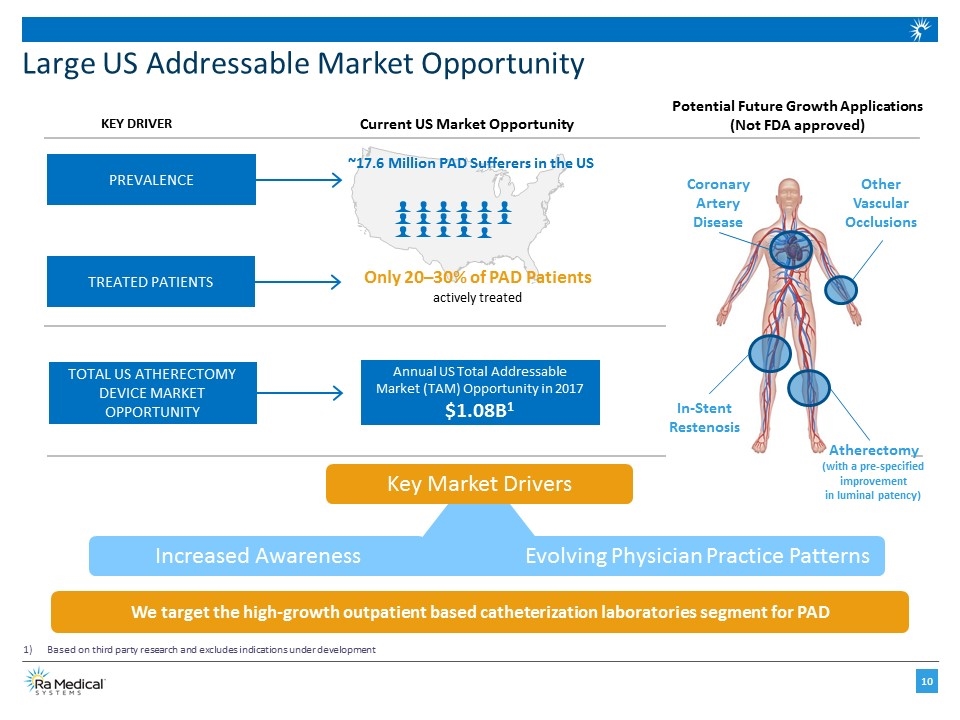
Large US Addressable Market Opportunity PREVALENCE TREATED PATIENTS TOTAL US ATHERECTOMY DEVICE MARKET OPPORTUNITY Current US Market Opportunity KEY DRIVER ~17.6 Million PAD Sufferers in the US Only 20–30% of PAD Patients actively treated Annual US Total Addressable Market (TAM) Opportunity in 2017 $1.08B1 Based on third party research and excludes indications under development Key Market Drivers Increased Awareness Evolving Physician Practice Patterns We target the high-growth outpatient based catheterization laboratories segment for PAD Potential Future Growth Applications (Not FDA approved) Other Vascular Occlusions Coronary Artery Disease In-Stent Restenosis Atherectomy (with a pre-specified improvement in luminal patency)
Payors and Reimbursement Approvals / Clearances DABRA: US FDA 510(k), CE Mark PHAROS: US FDA 510(k), CE Mark, CFDA, KFDA Payor Coverage Reimbursement claims for DABRA procedures typically submitted by providers to Medicare or other third-party payor using established Current Procedural Terminology (CPT) codes. Pharos treatments are reimbursable by Medicare and nearly all major insurance companies under three CPT codes that differ based on the affected area to be treated
Physician Practice Needs We believe our solution expands provider economics Ease of Use Versatility Able to cross and debulk Economics Established Safety and Effectiveness
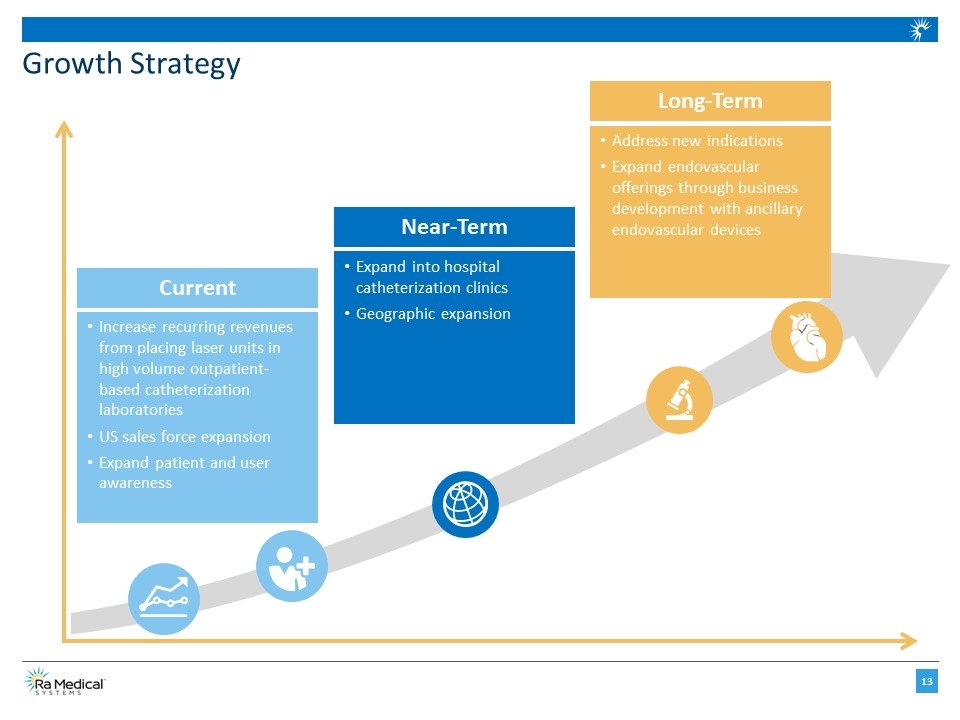
Growth Strategy Increase recurring revenues from placing laser units in high volume outpatient-based catheterization laboratories US sales force expansion Expand patient and user awareness Current Expand into hospital catheterization clinics Geographic expansion Near-Term Address new indications Expand endovascular offerings through business development with ancillary endovascular devices Long-Term
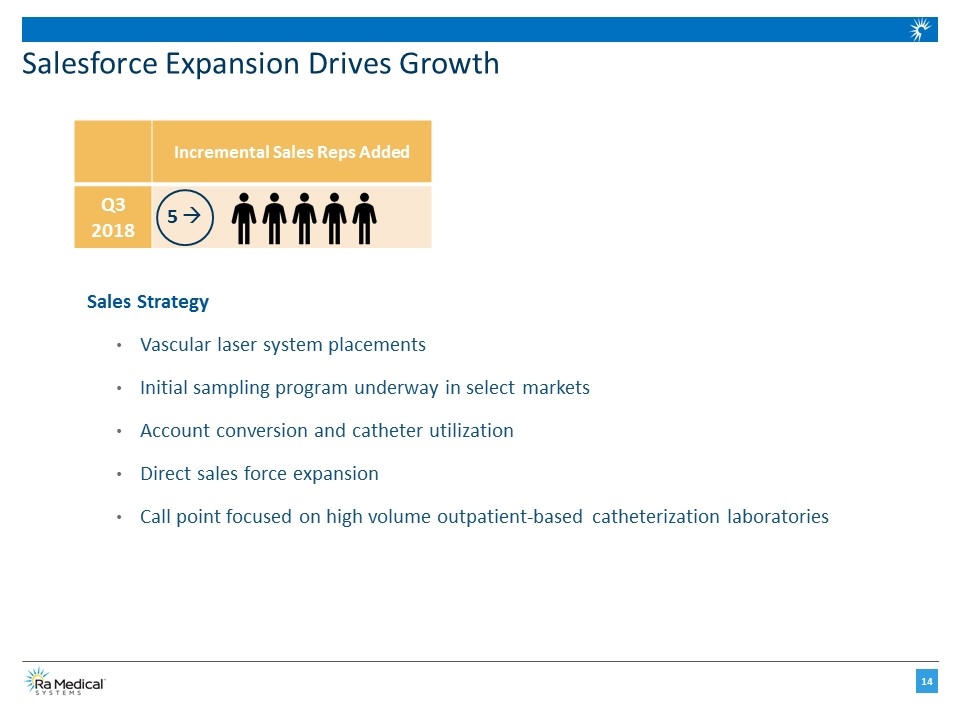
Salesforce Expansion Drives Growth Incremental Sales Reps Added Q3 2018 5 à Sales Strategy Vascular laser system placements Initial sampling program underway in select markets Account conversion and catheter utilization Direct sales force expansion Call point focused on high volume outpatient-based catheterization laboratories

Fully Operational Manufacturing Facility Carlsbad, CA Sizable capacity for laser and catheter production 32,000 sq. ft. Carlsbad, CA with three controlled environments manufacturing facility fully staffed and operational We believe our existing facility is capable of manufacturing over 400 lasers/year and 140,000 catheters/year Fully capitalized with all equipment owned ISO13485 certified, FDA inspected, and CA inspected and licensed Laser Assembly Controlled Environments
Pipeline Indications (Not FDA Approved1) PHAROS (Dermatology) Cutaneous T-Cell Lymphoma (CTCL) DABRA (Vascular) Atherectomy (with a pre-specified improvement in luminal patency) Coronary Artery Disease (CAD) In-Stent Restenosis No application has been made to the FDA as of the date hereof for these indications Other Vascular Occlusions
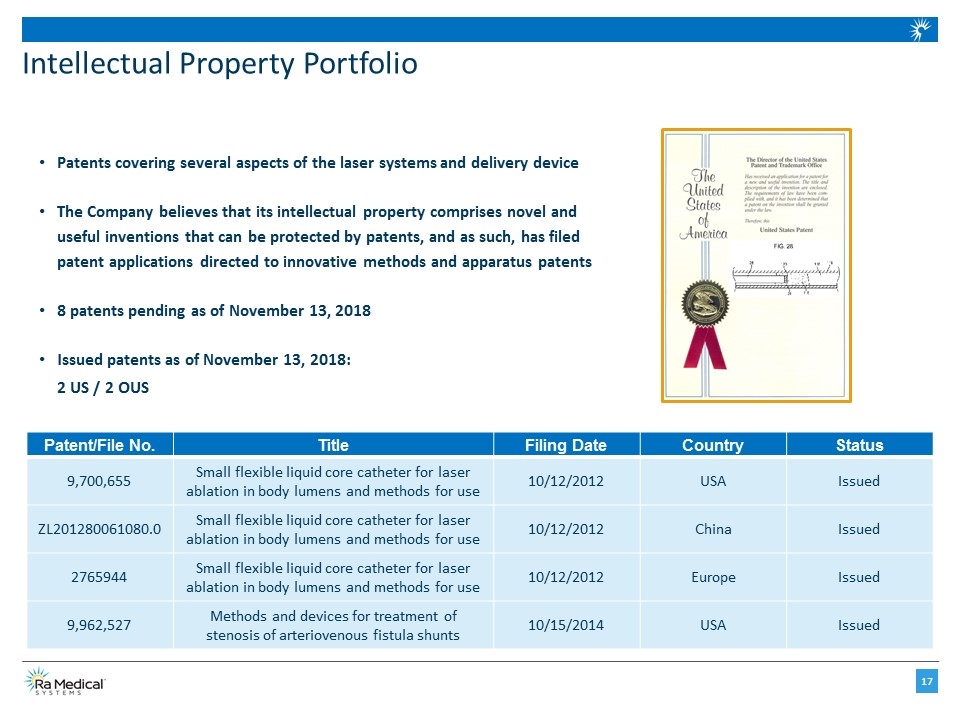
Intellectual Property Portfolio Patents covering several aspects of the laser systems and delivery device The Company believes that its intellectual property comprises novel and useful inventions that can be protected by patents, and as such, has filed patent applications directed to innovative methods and apparatus patents 8 patents pending as of November 13, 2018 Issued patents as of November 13, 2018: 2 US / 2 OUS Patent/File No. Title Filing Date Country Status 9,700,655 Small flexible liquid core catheter for laser ablation in body lumens and methods for use 10/12/2012 USA Issued ZL201280061080.0 Small flexible liquid core catheter for laser ablation in body lumens and methods for use 10/12/2012 China Issued 2765944 Small flexible liquid core catheter for laser ablation in body lumens and methods for use 10/12/2012 Europe Issued 9,962,527 Methods and devices for treatment of stenosis of arteriovenous fistula shunts 10/15/2014 USA Issued
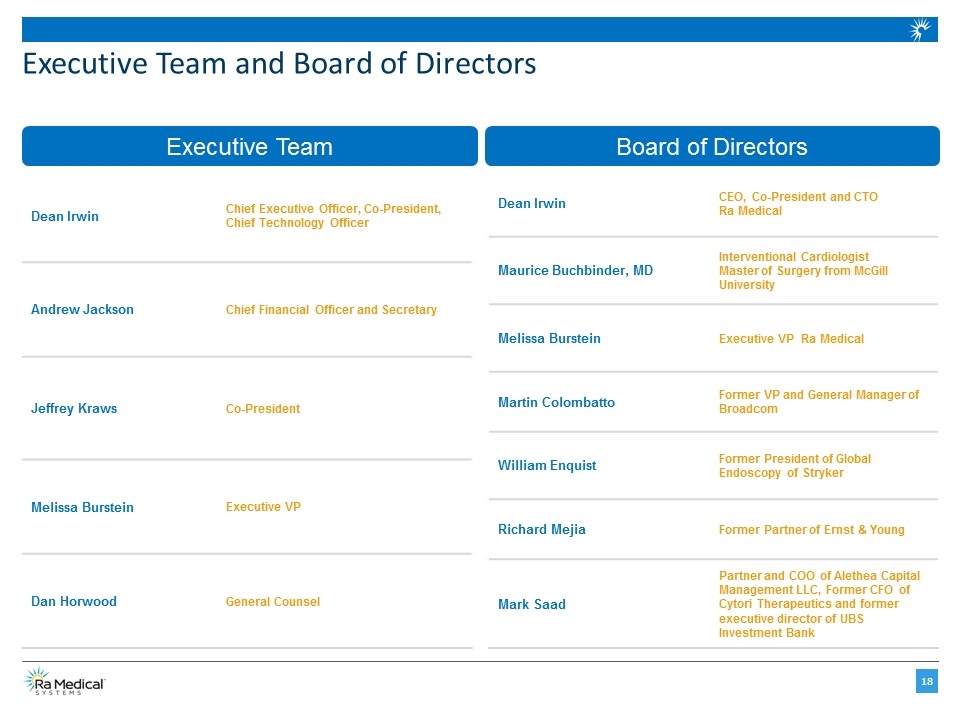
Executive Team and Board of Directors Dean Irwin Chief Executive Officer, Co-President, Chief Technology Officer Andrew Jackson Chief Financial Officer and Secretary Jeffrey Kraws Co-President Melissa Burstein Executive VP Dan Horwood General Counsel Dean Irwin CEO, Co-President and CTO Ra Medical Maurice Buchbinder, MD Interventional Cardiologist Master of Surgery from McGill University Melissa Burstein Executive VP Ra Medical Martin Colombatto Former VP and General Manager of Broadcom William Enquist Former President of Global Endoscopy of Stryker Richard Mejia Former Partner of Ernst & Young Mark Saad Partner and COO of Alethea Capital Management LLC, Former CFO of Cytori Therapeutics and former executive director of UBS Investment Bank Executive Team Board of Directors
Financial Overview
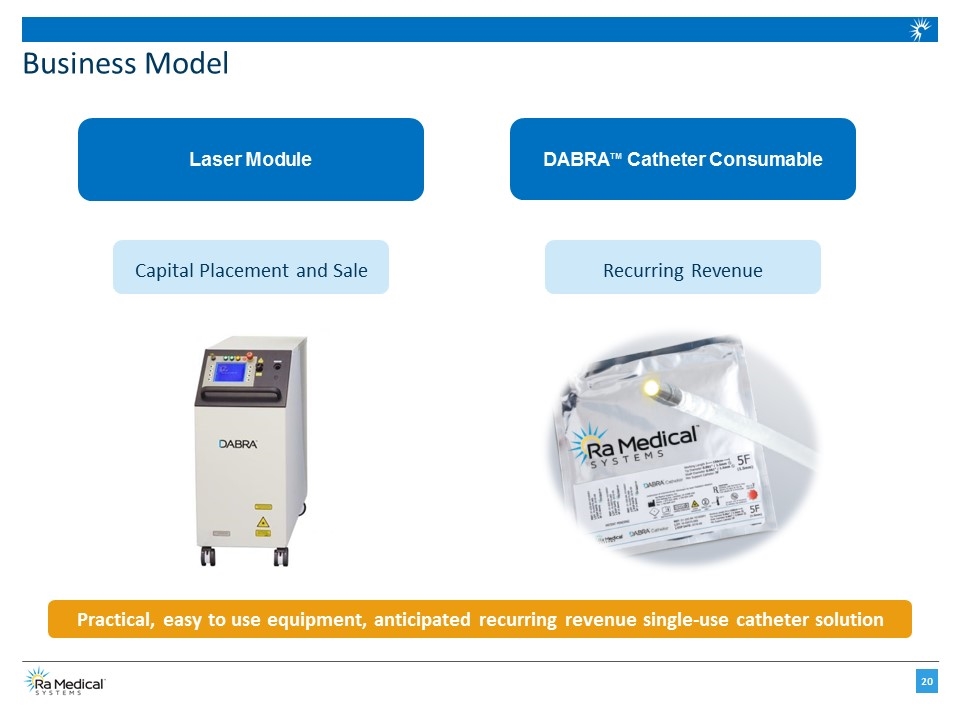
Business Model Laser Module Capital Placement and Sale DABRATM Catheter Consumable Recurring Revenue Practical, easy to use equipment, anticipated recurring revenue single-use catheter solution
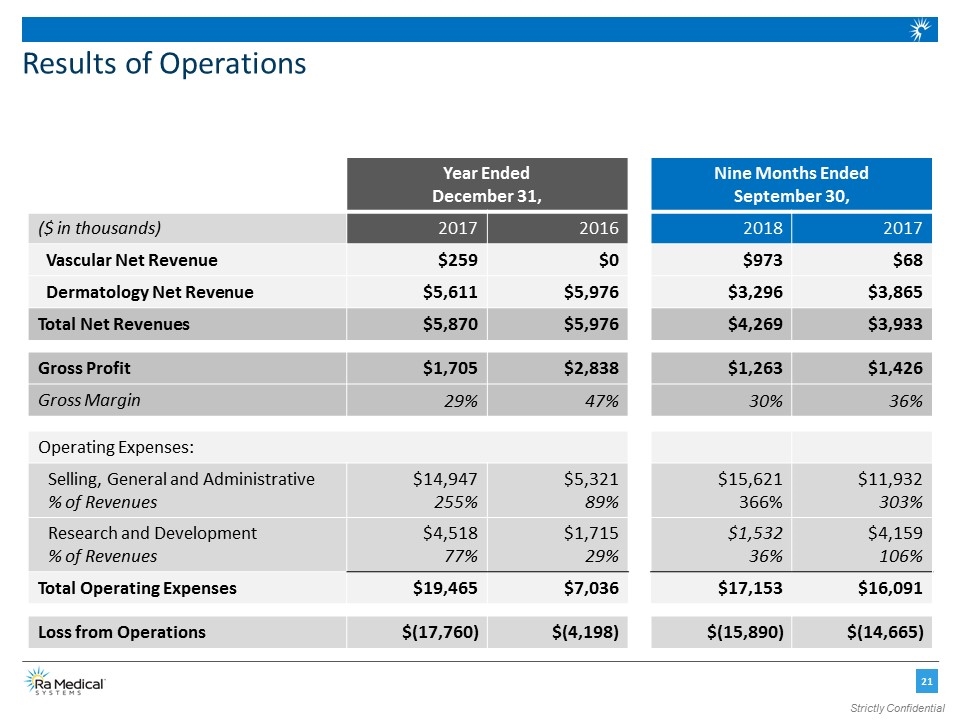
Results of Operations Year Ended December 31, Nine Months Ended September 30, ($ in thousands) 2017 2016 2018 2017 Vascular Net Revenue $259 $0 $973 $68 Dermatology Net Revenue $5,611 $5,976 $3,296 $3,865 Total Net Revenues $5,870 $5,976 $4,269 $3,933 Gross Profit $1,705 $2,838 $1,263 $1,426 Gross Margin 29% 47% 30% 36% Operating Expenses: Selling, General and Administrative % of Revenues $14,947 255% $5,321 89% $15,621 366% $11,932 303% Research and Development % of Revenues $4,518 77% $1,715 29% $1,532 36% $4,159 106% Total Operating Expenses $19,465 $7,036 $17,153 $16,091 Loss from Operations $(17,760) $(4,198) $(15,890) $(14,665) Strictly Confidential
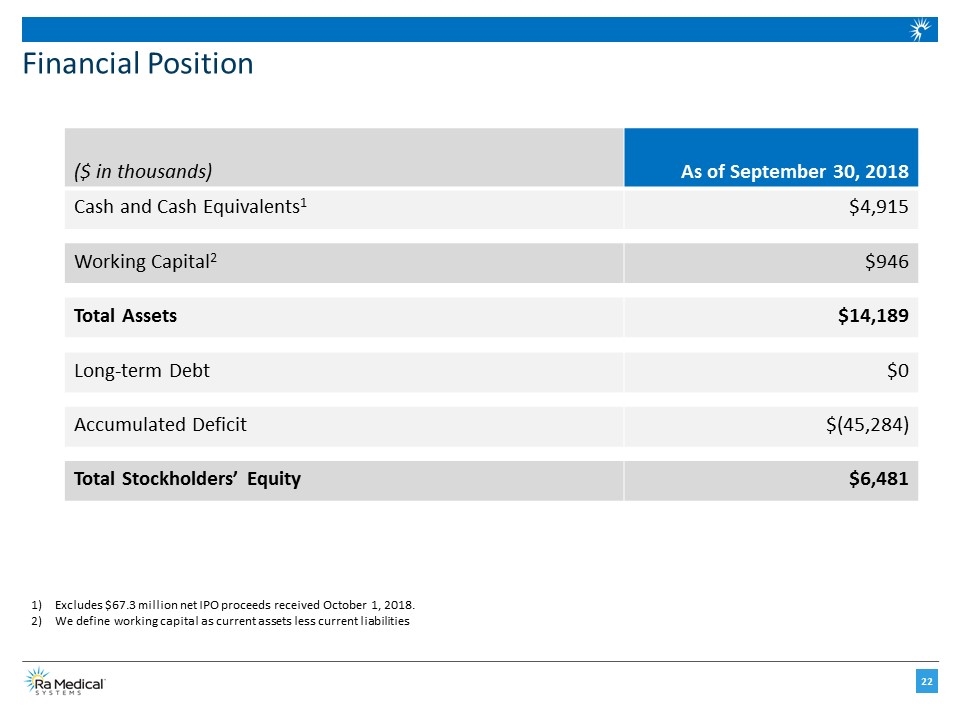
Financial Position ($ in thousands) As of September 30, 2018 Cash and Cash Equivalents1 $4,915 Working Capital2 $946 Total Assets $14,189 Long-term Debt $0 Accumulated Deficit $(45,284) Total Stockholders’ Equity $6,481 Excludes $67.3 million net IPO proceeds received October 1, 2018. We define working capital as current assets less current liabilities
Highlights Single-Use Catheters $1.08B+ Targeted US Annual TAM Among ~17.6M PAD Sufferers in the U.S. Novel Therapeutic Solution with Positive Clinical Study Results Existing US Reimbursement Structure Cost Benefits to Outpatient-Based Catheterization Laboratories and Hospitals Time Efficient and Easy-to-Use Solution Highly Experienced Executive Team

2070 Las Palmas Drive Carlsbad, CA 92011 United States www.ramed.com 760.707.7516 Corporate Presentation NYSE: RMED
