Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Neon Therapeutics, Inc. | a18-36974_18k.htm |
NEON THERAPEUTICS Directing the Immune System ESMO Congress Conference Call October 22, 2018 Confidential | © 2018 Neon Therapeutics 1

Forward-Looking Statements and Intellectual Property Forward-Looking Statements This presentation may contain forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters; our current and prospective product candidates, current and planned clinical trials and preclinical activities, research and development costs, current and prospective collaborations; the estimated size of the market for our product candidates, the timing and success of our development and commercialization of our anticipated product candidates; and the availability of alternative therapies for our target market. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Intellectual Property Neon Therapeutics, Inc. is the owner of the NEON THERAPEUTICS, RECON, and NEO-STIM trademarks, as well as certain other trademarks, including design versions or some of all of these trademarks. The symbols ™ and ® are not used in connection with the presentation of these trademarks in this presentation and their absence does not indicate a lack of trademark rights. Certain other trademarks used in this presentation are the property of third-party trademark owners and may be presented with or without trademark references. Confidential | © 2018 Neon Therapeutics 2

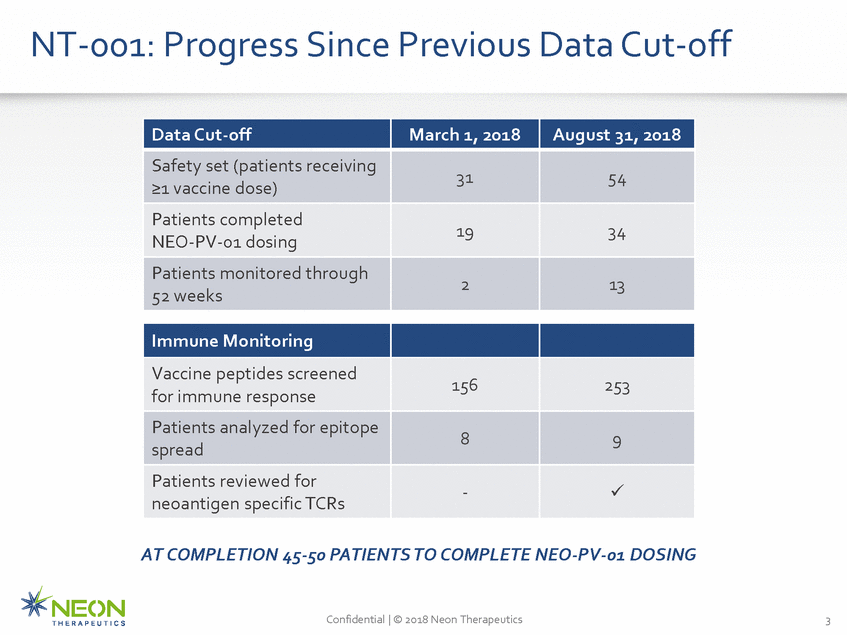
NT-001: Progress Since Previous Data Cut-off AT COMPLETION 45-50 PATIENTS TO COMPLETE NEO-PV-01 DOSING Confidential | © 2018 Neon Therapeutics 3 Data Cut-off March 1, 2018 August 31, 2018 Safety set (patients receiving >1 vaccine dose) 31 54 Patients completed NEO-PV-01 dosing 19 34 Patients monitored through 52 weeks 2 13 Immune Monitoring Vaccine peptides screened for immune response 156 253 Patients analyzed for epitope spread 8 9 Patients reviewed for neoantigen specific TCRs -
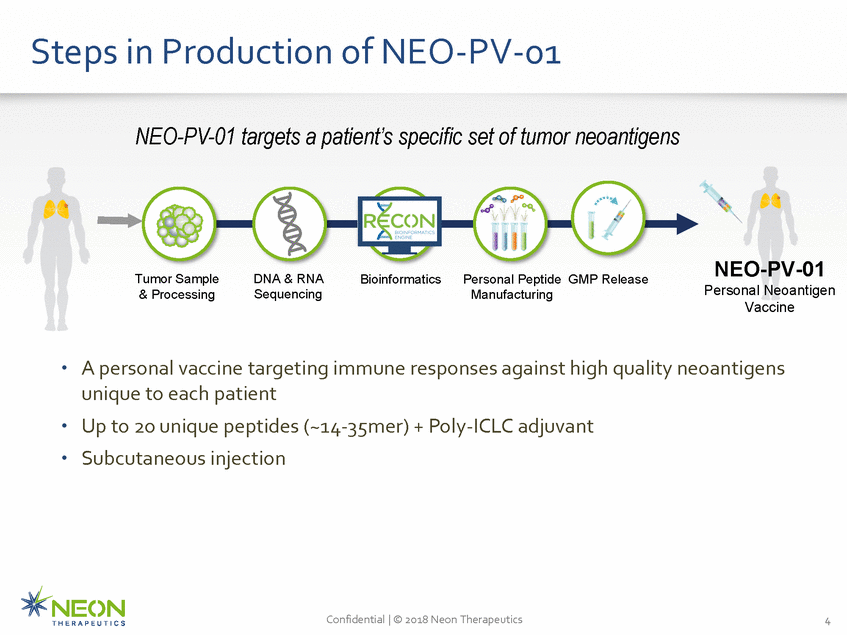
Steps in Production of NEO-PV-01 NEO-PV-01 targets a patient’s specific set of tumor neoantigens NEO-PV-01 Personal Neoantigen Vaccine Tumor Sample & Processing DNA & RNA Sequencing Bioinformatics Personal Peptide GMP Release Manufacturing A personal vaccine targeting immune responses against high quality neoantigens unique to each patient Up to 20 unique peptides (~14-35mer) + Poly-ICLC adjuvant Subcutaneous injection • • • Confidential | © 2018 Neon Therapeutics 4
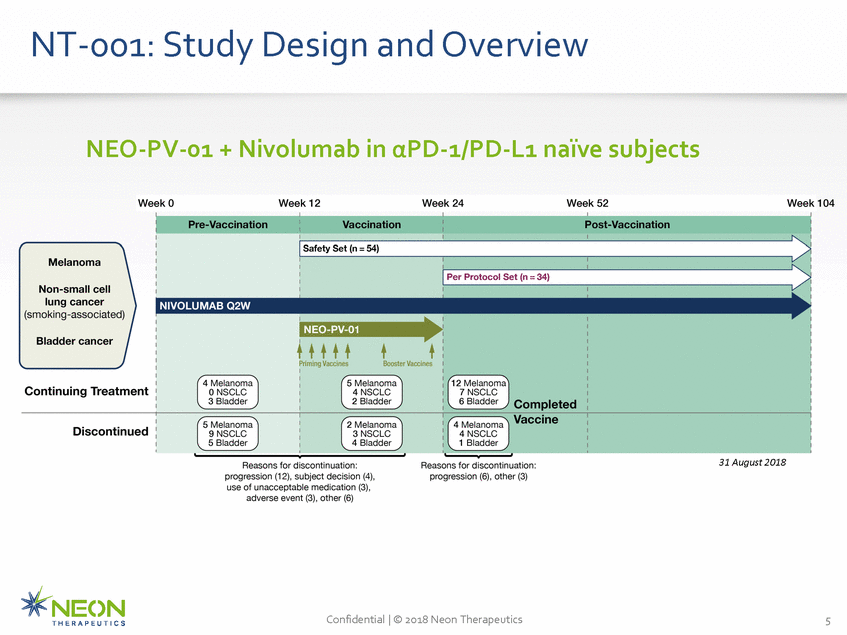
NT-001: Study Design and Overview NEO-PV-01 + Nivolumab in αPD-1/PD-L1 naïve subjects 31 August 2018 Confidential | © 2018 Neon Therapeutics 5
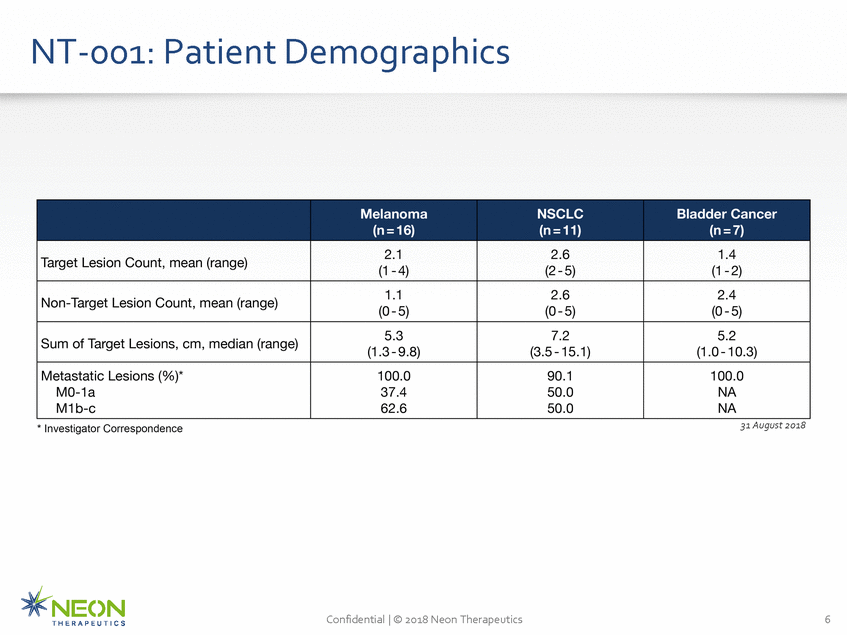
Demogra phics NT-001: Patient NSCLC (1 - 2) (0-5) (1.0 -10.3) Confidential I © 2018 Neon Therapeutics 6 THE RAP EUTI CS Melanoma Bladder Cancer (n = 16) (n = 11) (n=7) Target Lesion Count, mean (range) 2.1 (1-4) 2.6 (2 -5) 1.4 Non-Target Lesion Count, mean (range) 1.1 (0-5) 2.6 (0-5) 2.4 Sum of Target Lesions, em, median (range) 5.3 (1.3-9.8) 7.2 (3.5-15.1) 5.2 Metastatic Lesions (%)* M0-1a M1b-c 100.0 37.4 62.6 90.1 50.0 50.0 100.0 NA NA * Investigator Correspondence 31 August 2018
NEO-PV-01 Safety Profile 31 August 2018 -Includes top 5 most frequently occurring AEs that are likely or possibly treatment related -Treated with nivolumab monotherapy = 80 patients -Treated with NEO-PV-01 + nivolumab = 54 patients Confidential | © 2018 Neon Therapeutics 7
NEON THERAPEUTICS Immune Results Monitoring Confidential | © 2018 Neon Therapeutics 8
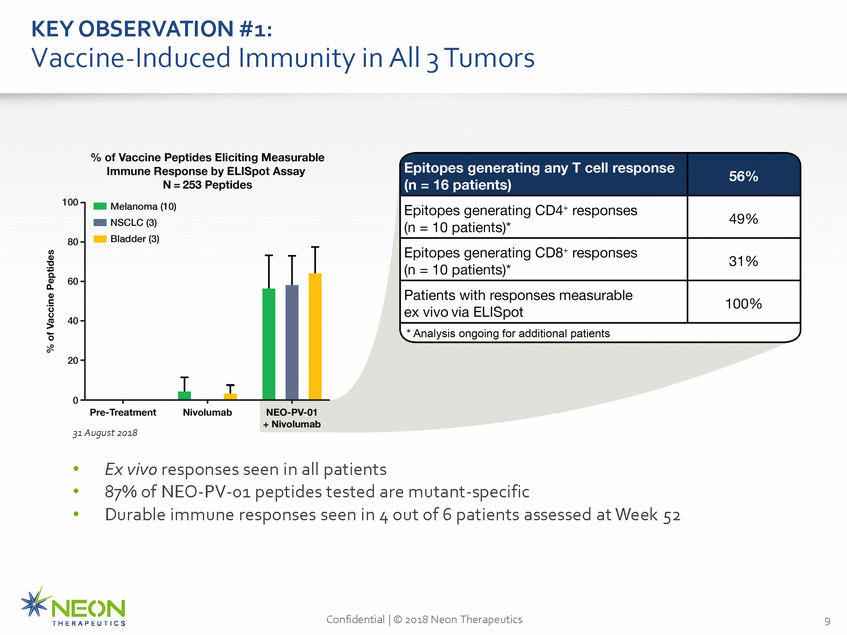
KEY OBSERVATION #1: Vaccine-Induced Immunity in All 3 Tumors 31 August 2018 • • • Ex vivo responses seen in all patients 87% of NEO-PV-01 peptides tested are mutant-specific Durable immune responses seen in 4 out of 6 patients assessed at Week 52 Confidential | © 2018 Neon Therapeutics 9
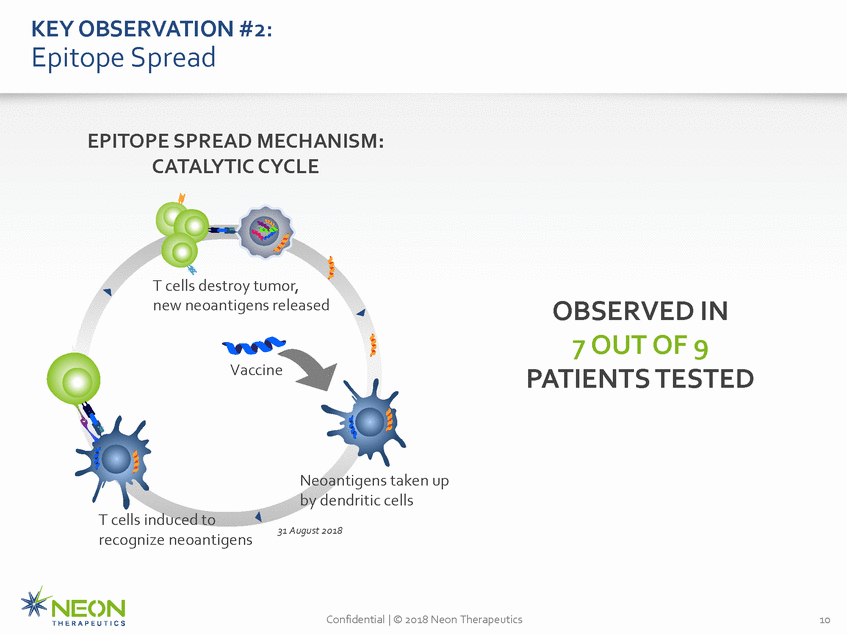
KEY OBSERVATION #2: Epitope Spread EPITOPE SPREAD MECHANISM: CATALYTIC CYCLE T cells destroy tumor, new neoantigens released OBSERVED IN 7 OUT OF 9 PATIENTS TESTED Vaccine Neoantigens taken up by dendritic cells 31 August 2018 T cells induced to recognize neoantigens Confidential | © 2018 Neon Therapeutics 10
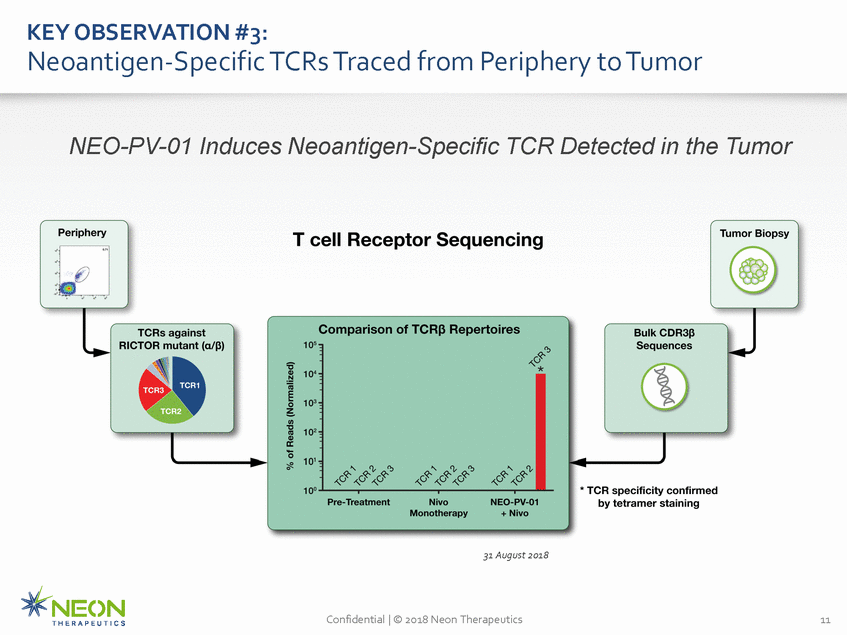
KEY OBSERVATION #3: Neoantigen-Specific TCRs Traced from Periphery to Tumor NEO-PV-01 Induces Neoantigen-Specific TCR Detected in the Tumor 31 August 2018 Confidential | © 2018 Neon Therapeutics 11
NEON THERAPEUTICS Clinical Results Confidential | © 2018 Neon Therapeutics 12
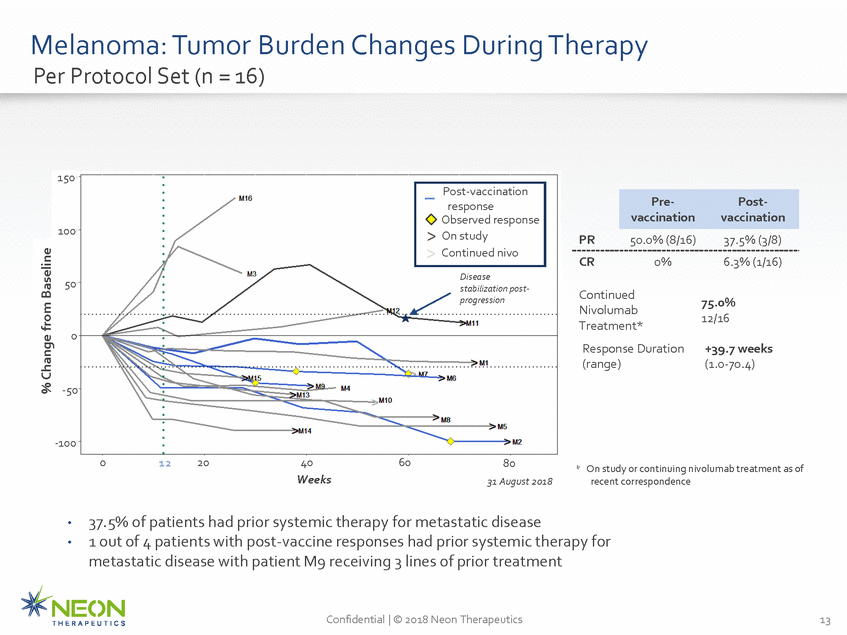
Melanoma: Tumor Burden Changes During Therapy Per Protocol Set (n = 16) 150 vaccination vaccination 100 PR 50.0% (8/16) 37.5% (3/8) CR 0% 6.3% (1/16) Disease stabilization post-progression 50 Continued Nivolumab Treatment* Response Duration (range) 75.0% 12/16 0 +39.7 weeks (1.0-70.4) -50 -100 0 20 40 Weeks 60 12 80 * On study or continuing nivolumab treatment as of 31 August 2018 recent correspondence 37.5% of patients had prior systemic therapy for metastatic disease 1 out of 4 patients with post-vaccine responses had prior systemic therapy for metastatic disease with patient M9 receiving 3 lines of prior treatment • • Confidential | © 2018 Neon Therapeutics 13 % Change from Baseline Pre-Post-Post-vaccination response Observed response > On study > Continued nivo
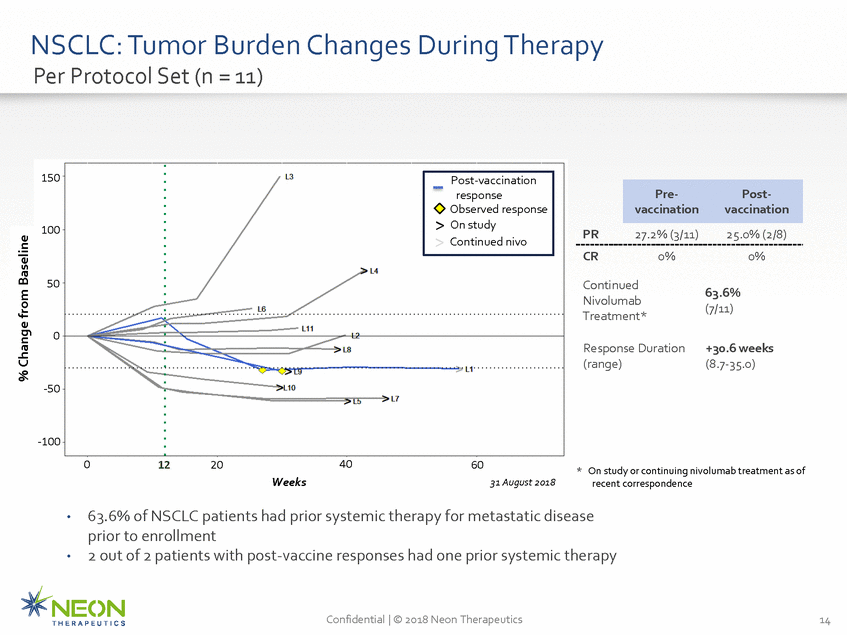
NSCLC: Tumor Burden Changes Per Protocol Set (n = 11) During Therapy 150 100 PR 27.2% (3/11)25.0% (2/8) CR 0% 0% 50 Continued Nivolumab Treatment* 63.6% (7/11) 0 Response Duration (range) +30.6 weeks (8.7-35.0) -50 -100 0 40 12 20 60 * On study or continuing nivolumab treatment as of recent correspondence Weeks 31 August 2018 63.6% of NSCLC patients had prior systemic therapy for metastatic disease prior to enrollment 2 out of 2 patients with post-vaccine responses had one prior systemic therapy • • Confidential | © 2018 Neon Therapeutics 14 % Change from Baseline Pre-Post-vaccinationvaccination Post-vaccination response Observed response > On study > Continued nivo
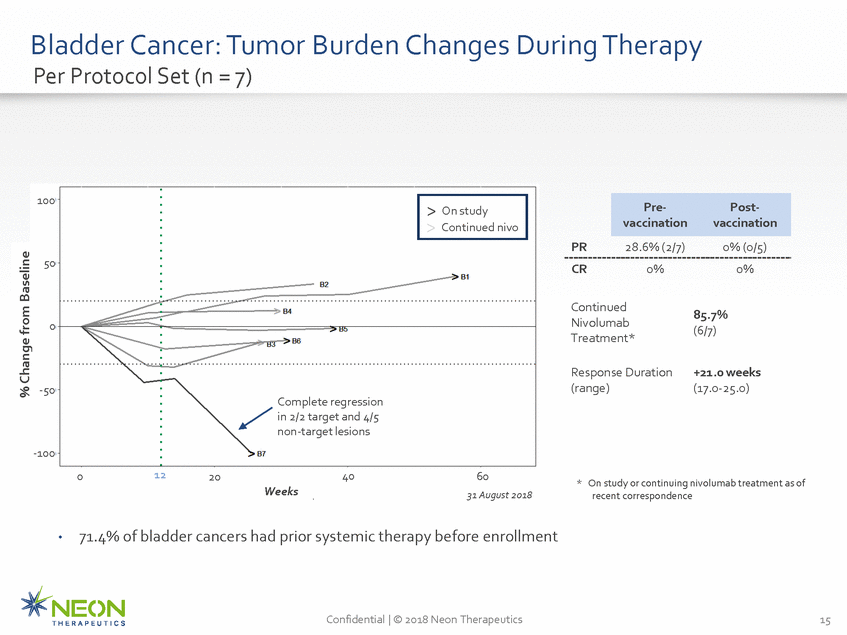
Bladder Cancer: Tumor Per Protocol Set (n = 7) Burden Changes During Therapy 100 PR 28.6% (2/7) 0% (0/5) 50 CR 0% 0% Continued Nivolumab Treatment* 85.7% (6/7) 0 Response Duration (range) +21.0 weeks (17.0-25.0) -50 Complete regression in 2/2 target and 4/5 non-target lesions -100 12 0 40 20 60 31 August 2018 * On study or continuing nivolumab treatment as of recent correspondence Weeks 40 71.4% of bladder cancers had prior systemic therapy before enrollment • Confidential | © 2018 Neon Therapeutics 15 % Change from Baseline Pre-Post-vaccinationvaccination > On study > Continued nivo
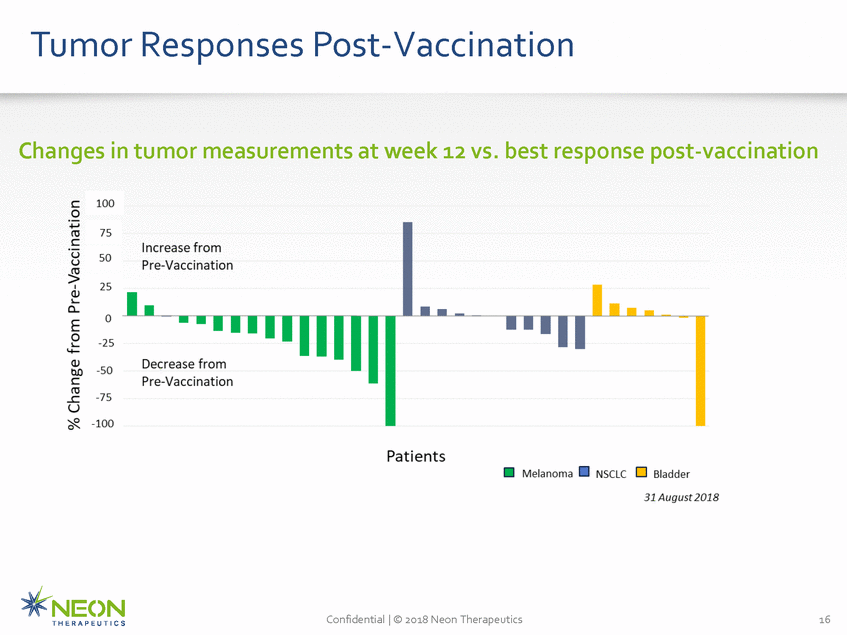
Tumor Responses Post-Vaccination Changes in tumor measurements at week 12 vs. best response post-vaccination Confidential | © 2018 Neon Therapeutics 16
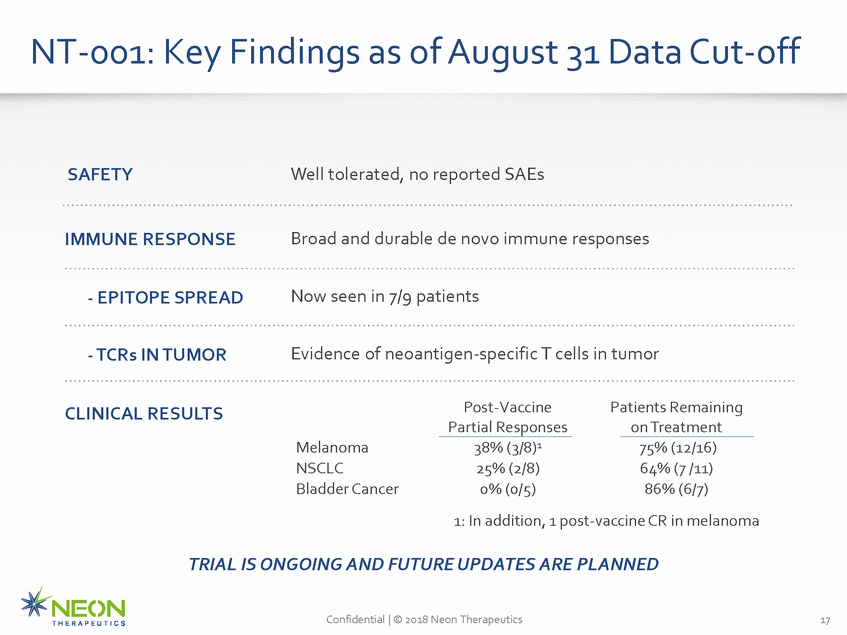
NT-001: Key Findings as of August 31 Data Cut-off SAFETY Well tolerated, no reported SAEs IMMUNE RESPONSE Broad and durable de novo immune responses Now seen in 7/9 patients - EPITOPE SPREAD Evidence of neoantigen-specific T cells in tumor - TCRs IN TUMOR Post-Vaccine Partial Responses Patients Remaining on Treatment CLINICAL RESULTS 38% (3/8)1 25% (2/8) 0% (0/5) Melanoma NSCLC Bladder Cancer 75% (12/16) 64% (7 /11) 86% (6/7) 1: In addition, 1 post-vaccine CR in melanoma TRIAL IS ONGOING AND FUTURE UPDATES ARE PLANNED Confidential | © 2018 Neon Therapeutics 17
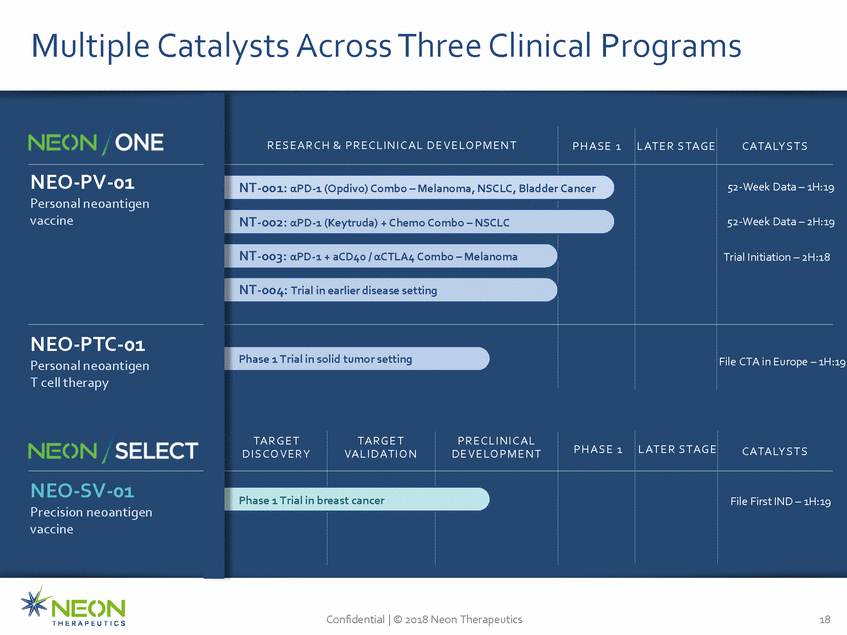
Multiple Catalysts Across Three Clinical Programs :19 19 Precision neoantigen Confidential | © 2018 Neon Therapeutics 18 R E S E A R C H & P R E C L I N I C A L D E V E LO P M E N T P H A S E 1 L AT E R S TA G E C ATA LY S T S NT-001: αPD-1 (Opdivo) Combo – Melanoma, NSCLC, Bladder NT-002: αPD-1 (Keytruda) + Chemo Combo – NSCLC NT-003: αPD-1 + aCD40 / αCTLA4 Combo – Melanoma NT-004: Trial in earlier disease setting Cancer 52-Week Data – 1H:19 52-Week Data – 2H Trial Initiation – 2H:18 NEO-PV-01 Personal neoantigen vaccine Phase 1 Trial in solid tumor setting File CTA in Europe – 1H: NEO-PTC-01 Personal neoantigen T cell therapy TA R G E T D I S C O V E R Y TA R G E T VA L I D AT I O N P R E C L I N I C A L D E V E LO P M E N T P H A S E 1 L AT E R S TA G E C ATA LY S T S Phase 1 Trial in br east cancer File First IND – 1H:19 NEO-SV-01 vaccine
NEON THERAPEUTICS Directing the Immune System ESMO Congress Conference Call October 22, 2018 Confidential | © 2018 Neon Therapeutics 19

